How to Secure Hypertonic Solutions in Emergency Medicine?
Hypertonic Solutions in Emergency Medicine: Background and Objectives
Hypertonic solutions have played a crucial role in emergency medicine for decades, offering a rapid and effective means of addressing various critical conditions. These concentrated solutions, typically containing higher solute concentrations than normal body fluids, have proven invaluable in managing severe dehydration, traumatic brain injury, and hypovolemic shock. The primary objective of utilizing hypertonic solutions in emergency settings is to quickly restore intravascular volume and improve tissue perfusion while minimizing the total fluid volume administered.
The evolution of hypertonic solutions in emergency medicine can be traced back to the mid-20th century when researchers began exploring alternatives to isotonic fluids for resuscitation. Early studies demonstrated the potential of hypertonic saline to rapidly expand plasma volume and improve hemodynamics in shock patients. Over time, the applications of hypertonic solutions expanded to include the management of increased intracranial pressure and the treatment of severe electrolyte imbalances.
As the field of emergency medicine has advanced, so too has the understanding of the physiological effects of hypertonic solutions. These solutions work by creating an osmotic gradient that draws fluid from the intracellular and interstitial spaces into the intravascular compartment. This mechanism not only rapidly expands plasma volume but also reduces tissue edema, particularly in the brain, making hypertonic solutions especially valuable in the management of traumatic brain injury.
The use of hypertonic solutions in emergency medicine has been driven by the need for more efficient and targeted fluid resuscitation strategies. Traditional large-volume isotonic fluid resuscitation, while effective in many scenarios, can lead to complications such as pulmonary edema and compartment syndrome. Hypertonic solutions offer the advantage of achieving similar or superior hemodynamic improvements with significantly less fluid volume, potentially reducing these risks.
Despite their benefits, the use of hypertonic solutions in emergency medicine is not without challenges. Concerns regarding electrolyte imbalances, particularly hypernatremia, have necessitated careful monitoring and dosing protocols. Additionally, the optimal composition and concentration of hypertonic solutions for different clinical scenarios remain subjects of ongoing research and debate within the medical community.
Looking ahead, the objectives for advancing the use of hypertonic solutions in emergency medicine are multifaceted. There is a pressing need to refine protocols for their safe and effective administration, taking into account patient-specific factors and the nature of the emergency. Furthermore, research efforts are focused on developing novel hypertonic formulations that may offer enhanced therapeutic benefits while minimizing potential side effects. The ultimate goal is to establish hypertonic solutions as a cornerstone of emergency fluid therapy, providing clinicians with a powerful tool to rapidly stabilize critically ill patients and improve outcomes in time-sensitive emergency situations.
Market Analysis for Hypertonic Solutions in Emergency Care
The market for hypertonic solutions in emergency medicine has shown significant growth in recent years, driven by the increasing prevalence of trauma cases, sepsis, and other critical conditions requiring rapid fluid resuscitation. The global market for hypertonic saline solutions is expected to reach several billion dollars by 2025, with a compound annual growth rate exceeding 5% over the forecast period.
Emergency departments and intensive care units represent the primary end-users of hypertonic solutions, accounting for the majority of market share. Hospitals, particularly those with Level I and II trauma centers, are the largest consumers of these products. The pre-hospital emergency care segment, including ambulance services and air medical transport, is also experiencing rapid growth in the adoption of hypertonic solutions.
Geographically, North America dominates the market due to its advanced healthcare infrastructure and high healthcare expenditure. Europe follows closely, with countries like Germany, France, and the UK being major contributors. The Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare systems in countries such as China and India, and the rising incidence of traumatic injuries.
The market is characterized by a mix of established pharmaceutical companies and specialized medical fluid manufacturers. Key players include Baxter International, B. Braun Melsungen, Fresenius Kabi, and Hospira (now part of Pfizer). These companies are focusing on product innovations, such as ready-to-use formulations and improved packaging, to gain a competitive edge.
Factors driving market growth include the increasing awareness of the benefits of hypertonic solutions in emergency care, advancements in fluid resuscitation protocols, and the rising number of surgical procedures. However, the market faces challenges such as the risk of adverse effects associated with hypertonic solutions and the availability of alternative fluid therapies.
Emerging trends in the market include the development of balanced hypertonic solutions that combine the benefits of hypertonic saline with other electrolytes, and the exploration of hypertonic solutions for specific indications such as traumatic brain injury. Additionally, there is growing interest in personalized fluid therapy approaches, which may influence future product development and market dynamics in the hypertonic solutions segment.
Current Challenges in Securing Hypertonic Solutions
The current challenges in securing hypertonic solutions for emergency medicine are multifaceted and complex. One of the primary issues is the stability of these solutions over time. Hypertonic solutions, particularly those with high concentrations of sodium chloride, can be prone to crystallization and precipitation, especially when exposed to temperature fluctuations. This instability can compromise the efficacy and safety of the solution, potentially leading to adverse effects in emergency situations.
Another significant challenge is the packaging and storage of hypertonic solutions. These solutions require specialized containers that can withstand the high osmotic pressure and prevent contamination. The materials used for packaging must be carefully selected to ensure they do not interact with the solution or allow for permeation of external substances. Additionally, the packaging must be designed to facilitate quick and easy administration in high-stress emergency environments.
The transportation and distribution of hypertonic solutions present further challenges. These solutions are often needed in various healthcare settings, including ambulances, emergency rooms, and remote medical facilities. Ensuring consistent quality and maintaining the integrity of the solutions during transit, especially in extreme weather conditions or over long distances, is a critical concern. This requires robust supply chain management and specialized transportation protocols.
Dosage accuracy is another crucial challenge in securing hypertonic solutions. In emergency medicine, precise administration is vital to avoid complications such as rapid shifts in electrolyte balance or fluid overload. Developing reliable and user-friendly delivery systems that allow for accurate dosing under pressure is an ongoing challenge for manufacturers and healthcare providers.
Furthermore, there are regulatory challenges associated with hypertonic solutions. Ensuring compliance with varying international standards and regulations for pharmaceutical production, storage, and distribution adds complexity to the securing process. This includes meeting stringent quality control measures, documentation requirements, and adhering to good manufacturing practices across different jurisdictions.
Lastly, the cost-effectiveness of producing and maintaining a secure supply of hypertonic solutions is a significant challenge. The specialized production processes, packaging requirements, and distribution networks contribute to higher costs, which can impact accessibility, especially in resource-limited settings. Balancing the need for high-quality, secure solutions with economic feasibility remains an ongoing challenge for healthcare systems and providers.
Existing Security Protocols for Hypertonic Solutions
01 Medical applications of hypertonic solutions
Hypertonic solutions are used in various medical applications, including treatment of edema, intracranial pressure reduction, and as a component in wound healing therapies. These solutions can help draw excess fluid from tissues and promote osmotic balance in the body.- Medical applications of hypertonic solutions: Hypertonic solutions are used in various medical applications, including treatment of edema, intracranial pressure reduction, and as a component in wound healing therapies. These solutions can help draw excess fluid from tissues and promote osmotic balance in the body.
- Formulation of hypertonic solutions for specific treatments: Hypertonic solutions are formulated with specific concentrations and compositions for targeted treatments. These may include saline solutions, sugar-based solutions, or combinations of electrolytes designed to address particular medical conditions or physiological imbalances.
- Use of hypertonic solutions in cell culture and preservation: Hypertonic solutions play a crucial role in cell culture techniques and preservation of biological samples. They are used to maintain osmotic balance, prevent cell lysis, and optimize conditions for cell growth and storage.
- Hypertonic solutions in ophthalmic applications: Hypertonic solutions are utilized in various ophthalmic treatments, including management of corneal edema and as a component in eye drops. These solutions help maintain proper osmolarity in the eye and can aid in reducing swelling of ocular tissues.
- Development of novel hypertonic solution delivery systems: Research is ongoing to develop innovative delivery systems for hypertonic solutions, including controlled-release mechanisms, specialized applicators, and combination therapies. These advancements aim to improve the efficacy and targeted delivery of hypertonic solutions in various medical applications.
02 Formulation of hypertonic solutions for specific treatments
Hypertonic solutions are formulated with specific concentrations and compositions for targeted treatments. These may include saline solutions, sugar-based solutions, or combinations of electrolytes designed to address particular medical conditions or physiological imbalances.Expand Specific Solutions03 Use of hypertonic solutions in cell culture and preservation
Hypertonic solutions play a crucial role in cell culture techniques and preservation of biological samples. They are used to maintain osmotic balance, prevent cell lysis, and optimize conditions for cell growth and storage.Expand Specific Solutions04 Hypertonic solutions in diagnostic procedures
Hypertonic solutions are utilized in various diagnostic procedures, including contrast-enhanced imaging studies and tests for assessing kidney function. These solutions can help improve visibility of specific structures or evaluate physiological responses.Expand Specific Solutions05 Development of novel hypertonic solution delivery systems
Research is ongoing to develop innovative delivery systems for hypertonic solutions, including controlled-release mechanisms, targeted delivery methods, and combination therapies. These advancements aim to improve the efficacy and safety of hypertonic solution treatments.Expand Specific Solutions
Key Players in Emergency Medicine and Hypertonic Solutions
The field of securing hypertonic solutions in emergency medicine is in a mature stage, with ongoing research and development to enhance safety and efficacy. The market size is substantial, driven by the critical need for these solutions in various emergency scenarios. Technologically, the field is well-developed but continues to evolve. Companies like Gambro Lundia AB, B. Braun Melsungen AG, and Becton, Dickinson & Co. are at the forefront, developing advanced delivery systems and formulations. Research institutions such as The Regents of the University of California and Virginia Commonwealth University contribute significantly to the scientific advancements. Pharmaceutical giants like Merck & Co., Inc. and F. Hoffmann-La Roche Ltd. are also key players, leveraging their extensive R&D capabilities to improve solution stability and safety profiles.
Merck & Co., Inc.
B. Braun Melsungen AG
Innovative Approaches to Hypertonic Solution Security
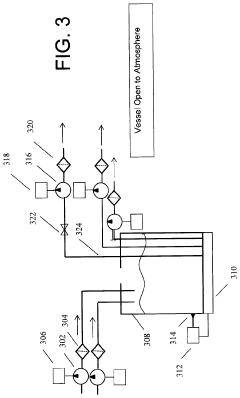
- A portable apparatus and method that includes vacuum hoses, infusion catheters, a reservoir, filters, and pumps to suction blood from a hemorrhaging wound, filter and regulate temperature, and return the blood to the patient via infusion pumps, allowing for on-site treatment of severe hemorrhaging.
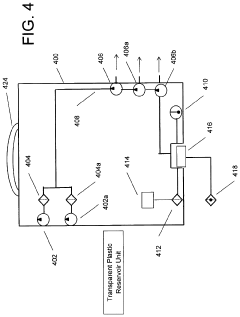
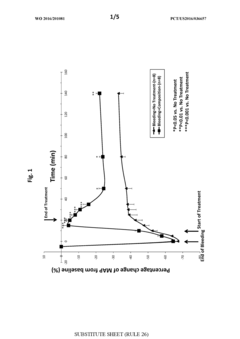
- A composition comprising specific salts, natural or synthetic sugars, and a high molecular weight, non-ionic, hydrophilic polymer, which can be administered as a solid or liquid, effectively increasing intravascular volume and pressure, suitable for both therapeutic and prophylactic use, particularly in conditions like traumatic brain injury and hypovolemia, without causing renal impairment.
Regulatory Framework for Emergency Medicine Solutions
The regulatory framework for emergency medicine solutions, particularly concerning hypertonic solutions, is a critical aspect of ensuring patient safety and treatment efficacy. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, production, and distribution of emergency medical products, including hypertonic solutions.
The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and effectiveness of hypertonic solutions used in emergency medicine. These solutions must undergo rigorous clinical trials and meet stringent quality standards before receiving approval for use in emergency settings. The FDA also mandates that manufacturers adhere to Good Manufacturing Practices (GMP) to ensure consistent product quality and safety.
In addition to FDA regulations, emergency medicine solutions are subject to oversight by other regulatory bodies. The Joint Commission, an independent, non-profit organization, accredits and certifies healthcare organizations and programs in the United States. Their standards include guidelines for the storage, handling, and administration of emergency medications, including hypertonic solutions.
The Drug Enforcement Administration (DEA) also plays a role in regulating certain emergency medications, particularly those with potential for abuse. While hypertonic solutions are not typically controlled substances, the DEA's regulations may impact the overall management of emergency medicine inventories and protocols.
At the state level, boards of pharmacy and medicine often have additional requirements for the handling and administration of emergency medications. These may include specific storage conditions, documentation procedures, and training requirements for healthcare professionals who administer these solutions.
Internationally, regulatory frameworks for emergency medicine solutions vary. The European Medicines Agency (EMA) oversees the evaluation and monitoring of medicines in the European Union, including those used in emergency settings. The World Health Organization (WHO) provides global guidance on essential medicines and emergency health interventions, which can influence national regulatory policies worldwide.
To ensure compliance with these regulatory frameworks, healthcare institutions and emergency medical services must implement robust quality management systems. These systems typically include standard operating procedures for the procurement, storage, and administration of hypertonic solutions, as well as regular audits and staff training programs.
As medical technology and emergency care practices evolve, regulatory frameworks must adapt to address new challenges and opportunities. This includes considerations for novel delivery systems, such as pre-filled syringes or automated dispensing devices, which may require additional regulatory scrutiny to ensure patient safety and treatment efficacy in emergency situations.
Risk Assessment and Mitigation Strategies
The use of hypertonic solutions in emergency medicine presents several risks that require careful assessment and mitigation strategies. One primary concern is the potential for rapid shifts in fluid balance, which can lead to severe electrolyte imbalances, particularly hypernatremia. This risk is especially pronounced in patients with pre-existing renal impairment or those unable to regulate their fluid intake. To mitigate this, healthcare providers must closely monitor serum electrolyte levels and adjust infusion rates accordingly.
Another significant risk is the potential for central pontine myelinolysis, a neurological complication that can occur with rapid correction of hyponatremia. This risk necessitates a gradual approach to administering hypertonic solutions, with careful attention to the rate of sodium correction. Implementing protocols that limit the rate of sodium increase to no more than 8-10 mEq/L in 24 hours can help mitigate this risk.
Vascular complications, including phlebitis and tissue necrosis, pose additional risks when administering hypertonic solutions. These risks are particularly high when solutions are infused through peripheral veins. To address this, central venous access should be prioritized for the administration of hypertonic solutions whenever possible. When peripheral access is necessary, regular monitoring of the infusion site and rotation of access points can help minimize the risk of vascular damage.
The risk of volume overload is another critical consideration, especially in patients with compromised cardiac function. Careful assessment of fluid status and cardiac output is essential before and during the administration of hypertonic solutions. Implementing a goal-directed fluid therapy approach, guided by hemodynamic monitoring, can help optimize fluid management and reduce the risk of pulmonary edema or heart failure exacerbation.
To enhance overall safety, healthcare institutions should develop and implement standardized protocols for the use of hypertonic solutions in emergency settings. These protocols should include clear guidelines for patient selection, dosing, administration rates, and monitoring parameters. Regular training and education of healthcare staff on the risks and proper use of hypertonic solutions can further improve safety and outcomes.
Lastly, the implementation of electronic alert systems within the hospital's medication management software can provide an additional layer of safety. These systems can flag high-risk medications, prompt for necessary monitoring, and alert healthcare providers to potential contraindications or dosing errors, thereby reducing the risk of adverse events associated with hypertonic solution administration in emergency medicine.