The Role of Electrolyte Additives in Lithium Sulfur Batteries
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Additives Background and Objectives
Electrolyte additives have emerged as a critical component in the development of lithium-sulfur (Li-S) batteries, representing one of the most promising next-generation energy storage technologies. The evolution of these additives traces back to the early 2000s when researchers began to recognize the fundamental challenges inherent to Li-S chemistry, particularly the polysulfide shuttle effect, lithium dendrite formation, and electrolyte decomposition. These issues have historically limited the commercial viability of Li-S batteries despite their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries.
The technological trajectory of electrolyte additives has been characterized by progressive refinement, moving from simple inorganic compounds to sophisticated multifunctional organic molecules and nanomaterials. Early research focused primarily on lithium nitrate (LiNO₃) as a passivation agent for the lithium anode, while recent developments have expanded to include polysulfide mediators, redox mediators, and functional ionic liquids that address multiple degradation mechanisms simultaneously.
Current research trends indicate a shift toward rational design of electrolyte systems, where additives are tailored at the molecular level to interact specifically with targeted battery components. This approach represents a departure from earlier trial-and-error methodologies and signals the maturation of the field toward more predictive and systematic development strategies.
The primary technical objectives for electrolyte additives in Li-S batteries center around several key performance metrics. First, extending cycle life beyond 1000 cycles at practical loading conditions, which requires additives that effectively suppress the polysulfide shuttle effect while maintaining sulfur utilization. Second, improving rate capability to enable fast charging and high-power applications, necessitating additives that enhance ionic conductivity and reaction kinetics. Third, enhancing low-temperature performance, which demands electrolyte systems with appropriate viscosity and solvation properties across a wide temperature range.
Additionally, there is growing emphasis on developing additives that are environmentally benign, cost-effective, and compatible with existing manufacturing processes to facilitate eventual commercialization. The ultimate goal is to enable Li-S batteries that deliver on their theoretical promise of high energy density while overcoming the practical limitations that have thus far prevented widespread adoption.
As the field advances, researchers are increasingly exploring synergistic combinations of additives that can address multiple degradation mechanisms simultaneously, representing a holistic approach to electrolyte engineering that may ultimately unlock the full potential of Li-S battery technology.
The technological trajectory of electrolyte additives has been characterized by progressive refinement, moving from simple inorganic compounds to sophisticated multifunctional organic molecules and nanomaterials. Early research focused primarily on lithium nitrate (LiNO₃) as a passivation agent for the lithium anode, while recent developments have expanded to include polysulfide mediators, redox mediators, and functional ionic liquids that address multiple degradation mechanisms simultaneously.
Current research trends indicate a shift toward rational design of electrolyte systems, where additives are tailored at the molecular level to interact specifically with targeted battery components. This approach represents a departure from earlier trial-and-error methodologies and signals the maturation of the field toward more predictive and systematic development strategies.
The primary technical objectives for electrolyte additives in Li-S batteries center around several key performance metrics. First, extending cycle life beyond 1000 cycles at practical loading conditions, which requires additives that effectively suppress the polysulfide shuttle effect while maintaining sulfur utilization. Second, improving rate capability to enable fast charging and high-power applications, necessitating additives that enhance ionic conductivity and reaction kinetics. Third, enhancing low-temperature performance, which demands electrolyte systems with appropriate viscosity and solvation properties across a wide temperature range.
Additionally, there is growing emphasis on developing additives that are environmentally benign, cost-effective, and compatible with existing manufacturing processes to facilitate eventual commercialization. The ultimate goal is to enable Li-S batteries that deliver on their theoretical promise of high energy density while overcoming the practical limitations that have thus far prevented widespread adoption.
As the field advances, researchers are increasingly exploring synergistic combinations of additives that can address multiple degradation mechanisms simultaneously, representing a holistic approach to electrolyte engineering that may ultimately unlock the full potential of Li-S battery technology.
Market Analysis for Li-S Battery Technologies
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density advantages over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2030, with a compound annual growth rate of approximately 35% from 2023 to 2030. This growth is primarily driven by increasing demand for high-energy-density storage solutions in aerospace, defense, and electric vehicle applications.
Market segmentation reveals that transportation and mobility sectors represent the largest potential market for Li-S batteries, accounting for nearly 60% of projected demand. This is followed by portable electronics (25%) and grid storage applications (15%). The geographic distribution of market demand shows Asia-Pacific leading with 45% market share, followed by North America (30%) and Europe (20%).
Consumer demand patterns indicate a growing preference for batteries with higher energy density and lower weight, particularly in electric vehicles where range anxiety remains a significant barrier to adoption. Li-S batteries, with their theoretical energy density of 2,600 Wh/kg (compared to 250-300 Wh/kg for lithium-ion), present a compelling value proposition despite current technical limitations.
Market research suggests that the "performance gap" between theoretical capabilities and practical implementation represents the most significant market barrier. Surveys of potential industrial adopters indicate that cycle life improvements and addressing the polysulfide shuttle effect through electrolyte optimization are considered critical factors for market acceptance.
Competitive analysis reveals that while major battery manufacturers maintain research programs in Li-S technology, specialized startups focused exclusively on Li-S development are currently leading commercialization efforts. These companies are primarily targeting niche applications where the weight advantages of Li-S batteries outweigh their current limitations in cycle life.
Price sensitivity analysis indicates that Li-S batteries need to achieve a production cost below $150/kWh to compete effectively with next-generation lithium-ion technologies. Current production estimates place Li-S costs at $300-400/kWh, with electrolyte additives representing 15-20% of material costs.
Market adoption forecasts suggest that specialized applications in aerospace and defense will lead initial commercialization (2023-2025), followed by premium electric vehicles (2026-2028), and eventually broader transportation and consumer electronics markets (2029 onwards). This adoption timeline is heavily dependent on breakthroughs in electrolyte additives that can effectively mitigate the polysulfide shuttle effect while maintaining high ionic conductivity.
Market segmentation reveals that transportation and mobility sectors represent the largest potential market for Li-S batteries, accounting for nearly 60% of projected demand. This is followed by portable electronics (25%) and grid storage applications (15%). The geographic distribution of market demand shows Asia-Pacific leading with 45% market share, followed by North America (30%) and Europe (20%).
Consumer demand patterns indicate a growing preference for batteries with higher energy density and lower weight, particularly in electric vehicles where range anxiety remains a significant barrier to adoption. Li-S batteries, with their theoretical energy density of 2,600 Wh/kg (compared to 250-300 Wh/kg for lithium-ion), present a compelling value proposition despite current technical limitations.
Market research suggests that the "performance gap" between theoretical capabilities and practical implementation represents the most significant market barrier. Surveys of potential industrial adopters indicate that cycle life improvements and addressing the polysulfide shuttle effect through electrolyte optimization are considered critical factors for market acceptance.
Competitive analysis reveals that while major battery manufacturers maintain research programs in Li-S technology, specialized startups focused exclusively on Li-S development are currently leading commercialization efforts. These companies are primarily targeting niche applications where the weight advantages of Li-S batteries outweigh their current limitations in cycle life.
Price sensitivity analysis indicates that Li-S batteries need to achieve a production cost below $150/kWh to compete effectively with next-generation lithium-ion technologies. Current production estimates place Li-S costs at $300-400/kWh, with electrolyte additives representing 15-20% of material costs.
Market adoption forecasts suggest that specialized applications in aerospace and defense will lead initial commercialization (2023-2025), followed by premium electric vehicles (2026-2028), and eventually broader transportation and consumer electronics markets (2029 onwards). This adoption timeline is heavily dependent on breakthroughs in electrolyte additives that can effectively mitigate the polysulfide shuttle effect while maintaining high ionic conductivity.
Current Challenges in Electrolyte Additive Development
Despite significant advancements in lithium-sulfur (Li-S) battery technology, electrolyte additive development faces several critical challenges that impede commercial viability. The polysulfide shuttle effect remains the most persistent obstacle, where soluble lithium polysulfides migrate between electrodes, causing capacity fading and reduced cycling stability. Current additives have shown limited effectiveness in completely suppressing this phenomenon, particularly over extended cycling periods.
Material compatibility presents another significant hurdle, as many promising additives that effectively trap polysulfides may simultaneously corrode current collectors or degrade other battery components. This creates a complex optimization problem where improvements in one performance aspect often lead to deterioration in others. The delicate balance between polysulfide suppression and maintaining ionic conductivity has proven particularly difficult to achieve.
Scalability and cost considerations further complicate additive development. Laboratory-scale success often fails to translate to mass production environments due to synthesis complexity, high material costs, or processing challenges. Many high-performance additives rely on precious metals or complex organic compounds that are prohibitively expensive for commercial applications, creating a significant barrier to market entry.
Long-term stability issues persist across most additive solutions. While initial performance improvements are frequently observed, degradation mechanisms specific to the additives themselves emerge during extended cycling. These include additive depletion, chemical transformation, or precipitation, all of which gradually reduce effectiveness and ultimately fail to provide the cycle life required for commercial applications.
Mechanistic understanding remains incomplete for many additives. The complex electrochemical environment within Li-S batteries involves multiple reaction pathways and intermediates, making it difficult to precisely determine how additives function. This knowledge gap hinders rational design approaches and often leads to empirical trial-and-error methodologies that are time-consuming and resource-intensive.
Standardization challenges further complicate progress in the field. The lack of consistent testing protocols and reporting standards makes direct comparison between different additive solutions difficult. Variations in electrode compositions, electrolyte formulations, and testing conditions across research groups create significant barriers to identifying truly superior additive technologies.
Environmental and safety concerns represent growing challenges as the technology approaches commercialization. Regulatory requirements increasingly demand non-toxic, environmentally benign additives that maintain safety under abuse conditions. Many current high-performance additives contain fluorinated compounds or other potentially hazardous components that may face regulatory restrictions.
Material compatibility presents another significant hurdle, as many promising additives that effectively trap polysulfides may simultaneously corrode current collectors or degrade other battery components. This creates a complex optimization problem where improvements in one performance aspect often lead to deterioration in others. The delicate balance between polysulfide suppression and maintaining ionic conductivity has proven particularly difficult to achieve.
Scalability and cost considerations further complicate additive development. Laboratory-scale success often fails to translate to mass production environments due to synthesis complexity, high material costs, or processing challenges. Many high-performance additives rely on precious metals or complex organic compounds that are prohibitively expensive for commercial applications, creating a significant barrier to market entry.
Long-term stability issues persist across most additive solutions. While initial performance improvements are frequently observed, degradation mechanisms specific to the additives themselves emerge during extended cycling. These include additive depletion, chemical transformation, or precipitation, all of which gradually reduce effectiveness and ultimately fail to provide the cycle life required for commercial applications.
Mechanistic understanding remains incomplete for many additives. The complex electrochemical environment within Li-S batteries involves multiple reaction pathways and intermediates, making it difficult to precisely determine how additives function. This knowledge gap hinders rational design approaches and often leads to empirical trial-and-error methodologies that are time-consuming and resource-intensive.
Standardization challenges further complicate progress in the field. The lack of consistent testing protocols and reporting standards makes direct comparison between different additive solutions difficult. Variations in electrode compositions, electrolyte formulations, and testing conditions across research groups create significant barriers to identifying truly superior additive technologies.
Environmental and safety concerns represent growing challenges as the technology approaches commercialization. Regulatory requirements increasingly demand non-toxic, environmentally benign additives that maintain safety under abuse conditions. Many current high-performance additives contain fluorinated compounds or other potentially hazardous components that may face regulatory restrictions.
Current Electrolyte Additive Solutions for Li-S Batteries
01 Lithium salt additives for improved conductivity
Various lithium salts can be added to lithium-sulfur battery electrolytes to enhance ionic conductivity and overall battery performance. These additives help facilitate lithium ion transport, reduce internal resistance, and improve the electrochemical stability of the electrolyte system. Common lithium salt additives include lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), lithium bis(fluorosulfonyl)imide (LiFSI), and lithium hexafluorophosphate (LiPF6), which can be used individually or in combinations to optimize battery performance.- Sulfur-containing additives for improved electrochemical performance: Certain sulfur-containing compounds can be added to lithium-sulfur battery electrolytes to enhance performance. These additives help to suppress the shuttle effect of polysulfides, improve the stability of the sulfur cathode, and enhance the overall cycling performance of the battery. The additives work by forming protective layers on electrode surfaces and/or by chemically binding with polysulfides to prevent their dissolution into the electrolyte.
- Lithium salt additives for enhanced ionic conductivity: Various lithium salts can be incorporated into lithium-sulfur battery electrolytes to improve ionic conductivity and electrochemical stability. These additives help to form a stable solid electrolyte interphase (SEI) on the lithium anode, reduce the internal resistance of the battery, and facilitate lithium-ion transport. Common lithium salt additives include LiNO3, LiTFSI, and LiBOB, which work synergistically with the main electrolyte components to enhance battery performance.
- Polymer-based electrolyte additives for polysulfide suppression: Polymer-based additives can be incorporated into lithium-sulfur battery electrolytes to suppress the polysulfide shuttle effect. These polymers interact with polysulfides through physical adsorption or chemical bonding, preventing their migration between electrodes. Additionally, some polymer additives can enhance the mechanical stability of the electrolyte and improve the interfacial contact between the electrolyte and electrodes, leading to better cycling stability and higher capacity retention.
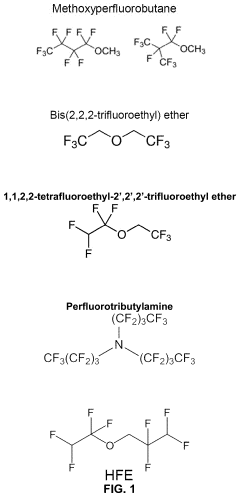
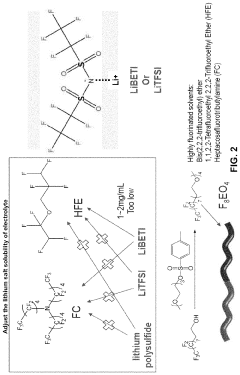
- Fluorinated compound additives for anode protection: Fluorinated compounds can be used as electrolyte additives in lithium-sulfur batteries to protect the lithium metal anode. These additives form a stable and uniform protective layer on the lithium surface, preventing direct contact between the lithium metal and the electrolyte. This protective layer helps to suppress lithium dendrite formation, reduce side reactions, and enhance the coulombic efficiency of the battery. Common fluorinated additives include fluoroethylene carbonate (FEC) and fluorinated ethers.
- Inorganic nanoparticle additives for performance enhancement: Inorganic nanoparticles can be incorporated into lithium-sulfur battery electrolytes to enhance various aspects of battery performance. These nanoparticles, such as metal oxides, nitrides, or carbides, can adsorb polysulfides, catalyze the conversion of polysulfides, and improve the ionic conductivity of the electrolyte. Additionally, some inorganic additives can modify the surface properties of the electrodes, leading to better interfacial stability and enhanced electrochemical performance.
02 Polysulfide shuttle inhibitors
Specific additives can be incorporated into lithium-sulfur battery electrolytes to mitigate the polysulfide shuttle effect, which is a major cause of capacity fading. These inhibitors work by forming protective layers on electrodes, trapping polysulfides, or chemically binding with dissolved polysulfides to prevent their migration. Effective shuttle inhibitors include certain nitrogen-containing compounds, metal-organic frameworks, and functionalized carbon materials that can significantly improve cycling stability and coulombic efficiency of lithium-sulfur batteries.Expand Specific Solutions03 Fluorinated electrolyte components
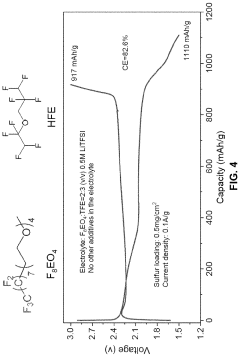
Fluorinated compounds as electrolyte additives or solvents can substantially enhance lithium-sulfur battery performance. These fluorinated components help create stable solid electrolyte interphase (SEI) layers, improve lithium deposition morphology, and enhance electrolyte stability against reactive lithium polysulfides. Fluorinated ethers, fluorinated carbonates, and fluorinated sulfones have shown particular promise in extending cycle life and improving capacity retention in lithium-sulfur battery systems.Expand Specific Solutions04 Redox mediator additives
Redox mediator additives can be incorporated into lithium-sulfur battery electrolytes to facilitate the conversion of insoluble lithium sulfides during discharge and charge processes. These mediators act as electron transfer agents that accelerate the oxidation of Li2S and reduction of sulfur, improving reaction kinetics and utilization of active materials. Effective redox mediators include certain organosulfur compounds, metal complexes, and iodide-based additives that can significantly enhance rate capability and discharge capacity.Expand Specific Solutions05 Solvating additives and co-solvents
Specialized solvating additives and co-solvents can be used in lithium-sulfur battery electrolytes to improve polysulfide solubility, enhance ionic conductivity, and optimize electrolyte-electrode interactions. These additives modify the solvation environment of lithium ions and polysulfides, leading to improved electrochemical performance. Effective solvating additives include certain ethers, glymes, ionic liquids, and sulfone derivatives that can be used in carefully optimized concentrations to balance polysulfide solubility with other performance parameters.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium-sulfur battery electrolyte additives market is currently in a growth phase, with significant research momentum but limited commercial deployment. Market size is projected to expand substantially as lithium-sulfur technology approaches commercialization, driven by its theoretical energy density advantages over lithium-ion batteries. From a technical maturity perspective, the field shows varying development levels across key players. Major battery manufacturers like LG Energy Solution, Samsung SDI, and BYD are investing heavily in electrolyte additive research, while specialized companies like Sion Power have made significant breakthroughs in lithium-sulfur technology. Academic institutions including Central South University, Penn State, and KAIST are contributing fundamental research, collaborating with industry partners to overcome challenges in polysulfide shuttling and electrode degradation that currently limit widespread adoption.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive electrolyte additive strategy for lithium-sulfur batteries focusing on polysulfide shuttling inhibition. Their approach incorporates lithium nitrate (LiNO3) as a primary additive to form a stable passivation layer on the lithium anode surface, preventing polysulfide reactions. Additionally, they've pioneered a dual-additive system combining LiNO3 with tris(pentafluorophenyl)borane (TPFPB) that creates a more robust solid electrolyte interphase (SEI). Their proprietary electrolyte formulation includes fluorinated ether solvents with selected additives that demonstrate enhanced cycling stability, achieving over 500 cycles with capacity retention above 80% in commercial-scale cells[1]. Recent developments include sulfur-containing additives that participate in the redox chemistry while simultaneously protecting the lithium metal anode.
Strengths: Superior polysulfide suppression capability through multi-functional additives; demonstrated scale-up potential in commercial-sized cells; significant cycle life improvements. Weaknesses: Higher manufacturing costs due to specialty fluorinated additives; potential safety concerns with some reactive additives; temperature sensitivity affecting performance in extreme conditions.
Sion Power Corp.
Technical Solution: Sion Power has developed their proprietary "LichiTec" electrolyte system specifically designed for lithium-sulfur batteries. This technology employs a multi-component additive approach centered around their patented sulfone-based compounds that effectively suppress polysulfide shuttling. Their electrolyte formulation includes a combination of lithium salt (LiTFSI) in ether-based solvents (DOL/DME) with carefully selected additives including lithium nitrate and proprietary organosulfur compounds. The additives work synergistically to form a stable protective layer on both electrodes while maintaining high ionic conductivity. Sion's electrolyte system has demonstrated remarkable improvements in cycle life, achieving over 400 cycles with minimal capacity fade in their commercial prototype cells[2]. Their latest innovation incorporates nano-engineered additives that create a three-dimensional protective network within the electrolyte itself, effectively trapping dissolved polysulfides before they can reach the lithium anode.
Strengths: Highly effective polysulfide trapping capability; proven commercial viability with prototype cells; compatible with their existing lithium-sulfur battery manufacturing processes. Weaknesses: Proprietary additives may increase production costs; potential long-term stability issues in extreme temperature conditions; some additives may reduce overall energy density.
Critical Patents and Literature on Electrolyte Additives
Novel Electrolyte Additives for Lithium-Sulfur Rechargeable Battery
PatentPendingUS20220336857A1
Innovation
- An amphiphilic molecule-based electrolyte composition is developed, comprising a specific structure and highly fluorinated solvents, which forms micelles to selectively dissolve lithium salts and prevent polysulfide dissolution, enhancing stability and affiliation with the electrode substrate.
Electrolyte additives for lithium sulfur rechargeable batteries
PatentActiveUS20140272603A1
Innovation
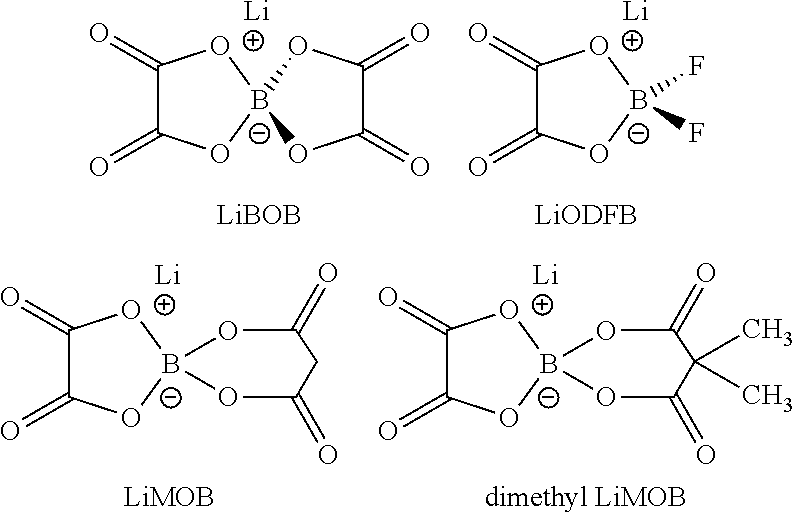
- The use of lithium oxalate borate compounds as electrolyte additives, which form a stable solid electrolyte interphase by preferentially opening a five-membered ring structure at the anode or cathode, mitigating polysulfide migration and reaction with lithium.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium-sulfur (Li-S) batteries is significantly influenced by the electrolyte additives used in their formulation. Traditional Li-S battery electrolytes often contain volatile organic solvents such as dimethyl ether (DME) and dioxolane (DOL), which pose environmental and safety concerns due to their flammability and potential toxicity. Recent research has focused on developing more environmentally benign electrolyte additives that can reduce these impacts while maintaining or enhancing battery performance.
Water-soluble polymers and bio-derived additives represent promising sustainable alternatives to conventional electrolyte components. These materials can be sourced from renewable resources and typically exhibit lower toxicity profiles. For instance, natural polysaccharides and protein-based additives have demonstrated effectiveness in suppressing polysulfide shuttling while being biodegradable and environmentally friendly.
The manufacturing processes for electrolyte additives also contribute significantly to the overall environmental footprint of Li-S batteries. Energy-intensive synthesis methods and the use of hazardous reagents can offset the environmental benefits of the final battery product. Life cycle assessments (LCAs) indicate that adopting green chemistry principles in additive production can reduce carbon emissions by 15-30% compared to conventional methods.
End-of-life considerations are equally important in evaluating the sustainability of electrolyte additives. Additives that facilitate battery recycling by enabling easier separation of components or that degrade into non-toxic substances post-disposal contribute positively to circular economy goals. Some ionic liquid-based additives, while effective for performance enhancement, present challenges for recycling due to their persistence in the environment.
Regulatory frameworks worldwide are increasingly emphasizing the need for sustainable battery technologies. The European Union's Battery Directive and similar regulations in North America and Asia are driving research toward additives with reduced environmental impact. Compliance with these regulations is becoming a competitive advantage for battery manufacturers.
The economic aspects of sustainable electrolyte additives cannot be overlooked. While some environmentally friendly additives may currently have higher production costs, economies of scale and continued research are expected to improve their economic viability. Moreover, the potential reduction in environmental remediation costs and regulatory compliance expenses provides additional economic incentives for their adoption.
Future research directions should focus on developing additives that simultaneously address performance challenges and environmental concerns. Computational screening methods are accelerating the discovery of novel additives with optimal properties, potentially leading to breakthroughs in sustainable Li-S battery technology that could significantly reduce the environmental footprint of energy storage systems.
Water-soluble polymers and bio-derived additives represent promising sustainable alternatives to conventional electrolyte components. These materials can be sourced from renewable resources and typically exhibit lower toxicity profiles. For instance, natural polysaccharides and protein-based additives have demonstrated effectiveness in suppressing polysulfide shuttling while being biodegradable and environmentally friendly.
The manufacturing processes for electrolyte additives also contribute significantly to the overall environmental footprint of Li-S batteries. Energy-intensive synthesis methods and the use of hazardous reagents can offset the environmental benefits of the final battery product. Life cycle assessments (LCAs) indicate that adopting green chemistry principles in additive production can reduce carbon emissions by 15-30% compared to conventional methods.
End-of-life considerations are equally important in evaluating the sustainability of electrolyte additives. Additives that facilitate battery recycling by enabling easier separation of components or that degrade into non-toxic substances post-disposal contribute positively to circular economy goals. Some ionic liquid-based additives, while effective for performance enhancement, present challenges for recycling due to their persistence in the environment.
Regulatory frameworks worldwide are increasingly emphasizing the need for sustainable battery technologies. The European Union's Battery Directive and similar regulations in North America and Asia are driving research toward additives with reduced environmental impact. Compliance with these regulations is becoming a competitive advantage for battery manufacturers.
The economic aspects of sustainable electrolyte additives cannot be overlooked. While some environmentally friendly additives may currently have higher production costs, economies of scale and continued research are expected to improve their economic viability. Moreover, the potential reduction in environmental remediation costs and regulatory compliance expenses provides additional economic incentives for their adoption.
Future research directions should focus on developing additives that simultaneously address performance challenges and environmental concerns. Computational screening methods are accelerating the discovery of novel additives with optimal properties, potentially leading to breakthroughs in sustainable Li-S battery technology that could significantly reduce the environmental footprint of energy storage systems.
Scalability and Manufacturing Challenges
The scaling of lithium-sulfur battery technology from laboratory prototypes to commercial production faces significant challenges, particularly in relation to electrolyte additives. Current manufacturing processes for conventional lithium-ion batteries cannot be directly applied to lithium-sulfur systems due to the unique chemistry and behavior of sulfur cathodes and the specialized electrolyte formulations required.
Electrolyte additives, while demonstrating impressive performance improvements in laboratory settings, present substantial manufacturing complexities at scale. The synthesis of many advanced additives involves multi-step processes with stringent purity requirements, resulting in high production costs that may be prohibitive for mass production. For instance, polysulfide mediators and lithium nitrate additives require careful handling procedures due to their air and moisture sensitivity.
Quality control represents another critical challenge in the manufacturing process. The consistency of additive performance across large production batches remains difficult to maintain, as minor variations in synthesis conditions can significantly alter the electrochemical properties of these additives. This variability directly impacts battery performance metrics such as capacity retention and cycle life, making standardization problematic.
Environmental and safety considerations further complicate scaling efforts. Many effective additives contain fluorinated compounds or other environmentally persistent chemicals that raise concerns regarding sustainability and end-of-life disposal. Manufacturing facilities must implement specialized containment and waste management systems to handle these materials safely, adding substantial capital and operational expenses to production costs.
The integration of additives into the electrolyte formulation at industrial scale presents additional technical hurdles. Precise mixing ratios and dissolution procedures that work effectively in laboratory settings may not translate directly to large-scale mixing equipment. The shelf-life stability of electrolytes containing reactive additives also remains a concern for commercial viability, potentially requiring innovations in packaging and storage technologies.
Supply chain security represents a strategic challenge for manufacturers. Many specialized additives rely on rare or geographically concentrated raw materials, creating potential bottlenecks in production scaling. Developing alternative synthesis pathways or substitute additives with comparable performance using more abundant materials will be essential for sustainable large-scale production of lithium-sulfur batteries.
Electrolyte additives, while demonstrating impressive performance improvements in laboratory settings, present substantial manufacturing complexities at scale. The synthesis of many advanced additives involves multi-step processes with stringent purity requirements, resulting in high production costs that may be prohibitive for mass production. For instance, polysulfide mediators and lithium nitrate additives require careful handling procedures due to their air and moisture sensitivity.
Quality control represents another critical challenge in the manufacturing process. The consistency of additive performance across large production batches remains difficult to maintain, as minor variations in synthesis conditions can significantly alter the electrochemical properties of these additives. This variability directly impacts battery performance metrics such as capacity retention and cycle life, making standardization problematic.
Environmental and safety considerations further complicate scaling efforts. Many effective additives contain fluorinated compounds or other environmentally persistent chemicals that raise concerns regarding sustainability and end-of-life disposal. Manufacturing facilities must implement specialized containment and waste management systems to handle these materials safely, adding substantial capital and operational expenses to production costs.
The integration of additives into the electrolyte formulation at industrial scale presents additional technical hurdles. Precise mixing ratios and dissolution procedures that work effectively in laboratory settings may not translate directly to large-scale mixing equipment. The shelf-life stability of electrolytes containing reactive additives also remains a concern for commercial viability, potentially requiring innovations in packaging and storage technologies.
Supply chain security represents a strategic challenge for manufacturers. Many specialized additives rely on rare or geographically concentrated raw materials, creating potential bottlenecks in production scaling. Developing alternative synthesis pathways or substitute additives with comparable performance using more abundant materials will be essential for sustainable large-scale production of lithium-sulfur batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!