Wearable biosensing patches for continuous glucose monitoring
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CGM Patch Technology Background and Objectives
Continuous glucose monitoring (CGM) technology has evolved significantly over the past three decades, transforming from bulky hospital equipment to miniaturized wearable devices. The journey began in the 1990s with the first FDA-approved CGM system, which required professional assistance and offered limited monitoring capabilities. By the early 2000s, the technology progressed to patient-usable systems with improved accuracy but still requiring frequent calibration and featuring short sensor lifespans.
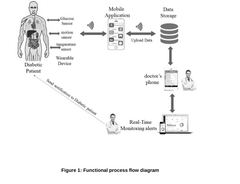
The development of wearable biosensing patches for CGM represents a pivotal advancement in diabetes management technology. These patches integrate sophisticated electrochemical sensors, wireless communication modules, and power management systems into a discreet, user-friendly form factor. The primary technological objective is to provide accurate, real-time glucose measurements through minimally invasive or non-invasive methods while extending wear time beyond the current standard of 7-14 days.
Current CGM patch technology predominantly utilizes subcutaneous sensing methods, where a small filament inserted under the skin measures glucose in interstitial fluid. However, the field is rapidly advancing toward truly non-invasive approaches leveraging techniques such as optical sensing, reverse iontophoresis, and dielectric spectroscopy. These innovations aim to eliminate the discomfort associated with sensor insertion while maintaining or improving measurement accuracy.
A critical technological goal is enhancing sensor longevity and stability. Extended wear periods reduce replacement frequency, improving user experience and decreasing overall system costs. This objective necessitates advancements in biocompatible materials, anti-fouling surface treatments, and improved enzyme stabilization techniques to prevent sensor degradation in the biological environment.
Data accuracy and reliability represent another fundamental objective in CGM patch development. Current systems typically achieve a mean absolute relative difference (MARD) of 9-14% compared to reference blood glucose measurements. The industry is striving to reduce this figure below 8% while simultaneously decreasing calibration requirements, with the ultimate goal of factory-calibrated sensors that maintain accuracy throughout their operational lifetime.
Power efficiency remains a significant challenge, with developers seeking to balance the competing demands of continuous monitoring, wireless data transmission, and compact form factor. Innovations in ultra-low-power electronics, energy harvesting techniques, and optimized algorithms are being pursued to extend battery life or potentially eliminate the need for batteries altogether in future generations of CGM patches.
The overarching technological trajectory points toward fully integrated closed-loop systems, where CGM patches not only monitor glucose levels but also automatically administer appropriate insulin doses, effectively functioning as an artificial pancreas. This represents the culmination of CGM technology development and would revolutionize diabetes management by dramatically reducing patient intervention requirements.
The development of wearable biosensing patches for CGM represents a pivotal advancement in diabetes management technology. These patches integrate sophisticated electrochemical sensors, wireless communication modules, and power management systems into a discreet, user-friendly form factor. The primary technological objective is to provide accurate, real-time glucose measurements through minimally invasive or non-invasive methods while extending wear time beyond the current standard of 7-14 days.
Current CGM patch technology predominantly utilizes subcutaneous sensing methods, where a small filament inserted under the skin measures glucose in interstitial fluid. However, the field is rapidly advancing toward truly non-invasive approaches leveraging techniques such as optical sensing, reverse iontophoresis, and dielectric spectroscopy. These innovations aim to eliminate the discomfort associated with sensor insertion while maintaining or improving measurement accuracy.
A critical technological goal is enhancing sensor longevity and stability. Extended wear periods reduce replacement frequency, improving user experience and decreasing overall system costs. This objective necessitates advancements in biocompatible materials, anti-fouling surface treatments, and improved enzyme stabilization techniques to prevent sensor degradation in the biological environment.
Data accuracy and reliability represent another fundamental objective in CGM patch development. Current systems typically achieve a mean absolute relative difference (MARD) of 9-14% compared to reference blood glucose measurements. The industry is striving to reduce this figure below 8% while simultaneously decreasing calibration requirements, with the ultimate goal of factory-calibrated sensors that maintain accuracy throughout their operational lifetime.
Power efficiency remains a significant challenge, with developers seeking to balance the competing demands of continuous monitoring, wireless data transmission, and compact form factor. Innovations in ultra-low-power electronics, energy harvesting techniques, and optimized algorithms are being pursued to extend battery life or potentially eliminate the need for batteries altogether in future generations of CGM patches.
The overarching technological trajectory points toward fully integrated closed-loop systems, where CGM patches not only monitor glucose levels but also automatically administer appropriate insulin doses, effectively functioning as an artificial pancreas. This represents the culmination of CGM technology development and would revolutionize diabetes management by dramatically reducing patient intervention requirements.
Market Analysis for Continuous Glucose Monitoring Solutions
The global continuous glucose monitoring (CGM) market has experienced substantial growth in recent years, driven primarily by the increasing prevalence of diabetes worldwide. As of 2023, the market was valued at approximately $8.3 billion and is projected to reach $19.4 billion by 2030, representing a compound annual growth rate (CAGR) of 12.8% during the forecast period. This growth trajectory is supported by rising diabetes cases, estimated at 537 million adults globally in 2021, with projections suggesting this number could reach 783 million by 2045 according to the International Diabetes Federation.
The wearable biosensing patches segment within the CGM market is emerging as a particularly promising area, currently accounting for about 24% of the total market share. These non-invasive or minimally invasive solutions address significant patient pain points related to traditional finger-prick methods, offering improved quality of life and treatment adherence. Market research indicates that patient satisfaction rates with wearable CGM patches exceed 85%, significantly higher than traditional monitoring methods.
North America dominates the CGM market with approximately 43% market share, followed by Europe at 28% and Asia-Pacific at 21%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.2% CAGR through 2030, driven by improving healthcare infrastructure, increasing diabetes prevalence, and growing middle-class populations with greater healthcare spending capacity.
The consumer segment for CGM solutions has expanded beyond Type 1 diabetes patients to include Type 2 diabetes patients and even pre-diabetic individuals interested in preventive health monitoring. This expansion has increased the total addressable market by an estimated 300% over the past five years. Additionally, there is growing interest from athletes and health-conscious consumers, representing a potential new market segment estimated at $1.2 billion by 2028.
Reimbursement policies significantly impact market adoption rates, with countries having favorable insurance coverage showing 2.5 times higher adoption rates compared to regions with limited coverage. Recent policy changes in major markets like the US, Germany, and Japan have expanded coverage for CGM devices, potentially accelerating market growth by 18-22% in these regions over the next three years.
The integration of CGM data with digital health platforms and artificial intelligence represents another significant market driver, with approximately 67% of users expressing interest in comprehensive health management solutions that incorporate glucose data alongside other health metrics. This integration capability is increasingly becoming a competitive differentiator among market players, with integrated solutions commanding premium pricing of 15-30% above standalone devices.
The wearable biosensing patches segment within the CGM market is emerging as a particularly promising area, currently accounting for about 24% of the total market share. These non-invasive or minimally invasive solutions address significant patient pain points related to traditional finger-prick methods, offering improved quality of life and treatment adherence. Market research indicates that patient satisfaction rates with wearable CGM patches exceed 85%, significantly higher than traditional monitoring methods.
North America dominates the CGM market with approximately 43% market share, followed by Europe at 28% and Asia-Pacific at 21%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.2% CAGR through 2030, driven by improving healthcare infrastructure, increasing diabetes prevalence, and growing middle-class populations with greater healthcare spending capacity.
The consumer segment for CGM solutions has expanded beyond Type 1 diabetes patients to include Type 2 diabetes patients and even pre-diabetic individuals interested in preventive health monitoring. This expansion has increased the total addressable market by an estimated 300% over the past five years. Additionally, there is growing interest from athletes and health-conscious consumers, representing a potential new market segment estimated at $1.2 billion by 2028.
Reimbursement policies significantly impact market adoption rates, with countries having favorable insurance coverage showing 2.5 times higher adoption rates compared to regions with limited coverage. Recent policy changes in major markets like the US, Germany, and Japan have expanded coverage for CGM devices, potentially accelerating market growth by 18-22% in these regions over the next three years.
The integration of CGM data with digital health platforms and artificial intelligence represents another significant market driver, with approximately 67% of users expressing interest in comprehensive health management solutions that incorporate glucose data alongside other health metrics. This integration capability is increasingly becoming a competitive differentiator among market players, with integrated solutions commanding premium pricing of 15-30% above standalone devices.
Current Challenges in Wearable Biosensing for Glucose Monitoring
Despite significant advancements in wearable biosensing technology for continuous glucose monitoring (CGM), several critical challenges persist that hinder widespread adoption and optimal performance. The current generation of wearable glucose monitoring patches faces substantial biocompatibility issues, with many users reporting skin irritation and allergic reactions after prolonged wear. These adverse reactions stem from adhesives, sensor materials, and chemical components necessary for glucose detection, creating a significant barrier to long-term patient compliance.
Sensor accuracy and reliability remain problematic under real-world conditions. Environmental factors such as temperature fluctuations, humidity, and physical activity can significantly impact measurement precision. Current CGM patches typically demonstrate a mean absolute relative difference (MARD) of 10-15% compared to reference blood glucose measurements, which falls short of the desired <10% threshold for clinical decision-making without confirmatory blood tests.
Power management presents another substantial hurdle. The continuous nature of glucose monitoring demands efficient energy utilization, yet current battery technologies struggle to support extended monitoring periods without increasing device size and weight. Most commercial CGM patches require replacement every 7-14 days, primarily due to power limitations rather than sensor degradation.
Data transmission and connectivity issues frequently compromise the user experience. Bluetooth and NFC technologies employed in current devices suffer from intermittent connection failures, particularly in signal-congested environments. These connectivity problems can result in dangerous data gaps for patients who rely on continuous monitoring for insulin dosing decisions.
Miniaturization challenges persist as manufacturers attempt to balance functionality with user comfort. Current patches remain bulky compared to ideal form factors, increasing the risk of accidental dislodgement and reducing user acceptance. The integration of multiple sensors, power sources, and transmission components into a discrete, low-profile device continues to challenge design engineers.
Calibration requirements represent another significant limitation. Many systems still necessitate periodic finger-prick calibrations to maintain accuracy, undermining the non-invasive promise of wearable technology. The biological and chemical interface between the sensor and interstitial fluid gradually degrades over time, affecting measurement stability and necessitating recalibration or replacement.
Cost barriers severely restrict accessibility, with current CGM systems remaining prohibitively expensive for many potential users. The combination of specialized materials, complex manufacturing processes, and proprietary algorithms results in high unit costs and ongoing expenses for consumable components, limiting adoption particularly in resource-constrained healthcare systems.
Sensor accuracy and reliability remain problematic under real-world conditions. Environmental factors such as temperature fluctuations, humidity, and physical activity can significantly impact measurement precision. Current CGM patches typically demonstrate a mean absolute relative difference (MARD) of 10-15% compared to reference blood glucose measurements, which falls short of the desired <10% threshold for clinical decision-making without confirmatory blood tests.
Power management presents another substantial hurdle. The continuous nature of glucose monitoring demands efficient energy utilization, yet current battery technologies struggle to support extended monitoring periods without increasing device size and weight. Most commercial CGM patches require replacement every 7-14 days, primarily due to power limitations rather than sensor degradation.
Data transmission and connectivity issues frequently compromise the user experience. Bluetooth and NFC technologies employed in current devices suffer from intermittent connection failures, particularly in signal-congested environments. These connectivity problems can result in dangerous data gaps for patients who rely on continuous monitoring for insulin dosing decisions.
Miniaturization challenges persist as manufacturers attempt to balance functionality with user comfort. Current patches remain bulky compared to ideal form factors, increasing the risk of accidental dislodgement and reducing user acceptance. The integration of multiple sensors, power sources, and transmission components into a discrete, low-profile device continues to challenge design engineers.
Calibration requirements represent another significant limitation. Many systems still necessitate periodic finger-prick calibrations to maintain accuracy, undermining the non-invasive promise of wearable technology. The biological and chemical interface between the sensor and interstitial fluid gradually degrades over time, affecting measurement stability and necessitating recalibration or replacement.
Cost barriers severely restrict accessibility, with current CGM systems remaining prohibitively expensive for many potential users. The combination of specialized materials, complex manufacturing processes, and proprietary algorithms results in high unit costs and ongoing expenses for consumable components, limiting adoption particularly in resource-constrained healthcare systems.
Current Technical Solutions for Wearable Glucose Monitoring
01 Wearable patch design for continuous glucose monitoring
Wearable biosensing patches for continuous glucose monitoring incorporate specific design elements to ensure comfort and functionality. These patches are designed to adhere securely to the skin while maintaining flexibility for user movement. The patches typically include a sensor component, data processing unit, and adhesive layer that allows for extended wear time. Design considerations include minimizing skin irritation, optimizing patch size, and ensuring waterproof capabilities for daily activities.- Wearable patch design for continuous glucose monitoring: Wearable biosensing patches for continuous glucose monitoring incorporate specific design elements to ensure comfort, durability, and accurate sensing. These patches are designed to adhere securely to the skin while maintaining flexibility for user comfort during daily activities. The designs often include layered structures with biocompatible materials that minimize skin irritation during extended wear periods. Advanced adhesive technologies enable secure attachment while allowing for moisture management and skin breathability.
- Sensing technologies for glucose detection: Various sensing technologies are employed in wearable glucose monitoring patches, including electrochemical, optical, and impedance-based methods. Electrochemical sensors measure glucose levels through enzymatic reactions that generate electrical signals proportional to glucose concentration. Optical sensors utilize fluorescence or spectroscopic techniques to detect glucose levels without requiring enzyme reactions. These technologies are miniaturized to fit within thin, flexible patch formats while maintaining sensitivity and specificity for glucose detection in interstitial fluid.
- Data processing and wireless communication systems: Wearable glucose monitoring patches incorporate advanced data processing and wireless communication capabilities to transmit readings to smartphones or dedicated receivers. These systems include low-power microprocessors that analyze sensor data, apply calibration algorithms, and prepare information for transmission. Bluetooth Low Energy (BLE) and Near Field Communication (NFC) technologies enable efficient wireless data transfer while minimizing power consumption. Cloud connectivity allows for data storage, trend analysis, and integration with healthcare management systems.
- Power management and energy harvesting solutions: Innovative power management systems are critical for extending the operational life of wearable glucose monitoring patches. These include ultra-low-power circuit designs, optimized duty cycling, and power-efficient sensor operation modes. Some patches incorporate energy harvesting technologies such as photovoltaic cells, thermoelectric generators, or radio frequency energy harvesting to supplement or replace traditional batteries. Advanced battery technologies with high energy density and thin-film designs enable extended monitoring periods while maintaining patch thinness and flexibility.
- Biocompatibility and extended wear technologies: Extended wear capabilities are achieved through biocompatible materials and specialized sensor designs that minimize foreign body responses and sensor drift. Advanced membrane technologies control analyte diffusion to the sensor while blocking interfering substances. Anti-inflammatory and anti-biofouling coatings help maintain sensor performance over extended periods. Moisture management systems prevent signal degradation from sweat or environmental exposure, while skin-friendly adhesives allow for comfortable wear for periods ranging from 7 to 14 days or longer.
02 Sensor technologies for glucose detection
Various sensor technologies are employed in wearable glucose monitoring patches to accurately detect glucose levels in interstitial fluid. These include electrochemical sensors that measure electrical signals generated by glucose oxidation, optical sensors that detect changes in fluorescence or color, and microneedle-based sensors that access interstitial fluid with minimal invasiveness. Advanced sensor materials and coatings are used to improve accuracy, reduce interference from other substances, and extend sensor lifetime while maintaining biocompatibility.Expand Specific Solutions03 Data processing and transmission systems
Wearable glucose monitoring patches incorporate sophisticated data processing and transmission systems to collect, analyze, and communicate glucose readings. These systems include microprocessors that filter and process sensor signals, algorithms that convert raw data into glucose values, and wireless communication modules (Bluetooth, NFC, or proprietary protocols) that transmit data to smartphones or dedicated receivers. Power management systems optimize battery life while maintaining continuous monitoring capabilities, and encryption protocols ensure secure data transmission.Expand Specific Solutions04 Integration with mobile applications and healthcare systems
Continuous glucose monitoring patches are designed to integrate with mobile applications and broader healthcare systems. Mobile apps provide real-time glucose data visualization, trend analysis, and customizable alerts for high or low glucose levels. Cloud-based platforms enable data sharing with healthcare providers, facilitating remote monitoring and telehealth consultations. Some systems incorporate machine learning algorithms to predict glucose trends and provide personalized insights, while others integrate with insulin delivery systems for closed-loop diabetes management.Expand Specific Solutions05 Biocompatible materials and manufacturing techniques
Advanced biocompatible materials and manufacturing techniques are essential for effective wearable glucose monitoring patches. These patches utilize hypoallergenic adhesives that maintain skin contact while minimizing irritation, flexible substrate materials that conform to body contours, and biocompatible sensor coatings that reduce foreign body response and extend sensor life. Manufacturing processes include microfabrication techniques for sensor components, precision assembly methods for electronic integration, and quality control measures to ensure consistent performance across production batches.Expand Specific Solutions
Key Industry Players in Biosensing Patch Development
The wearable biosensing patches for continuous glucose monitoring market is in a growth phase, characterized by rapid technological advancements and expanding applications. The global market size is projected to reach significant value due to increasing diabetes prevalence and demand for non-invasive monitoring solutions. Leading players like DexCom, Abbott Diabetes Care, and Roche Diabetes Care have established strong market positions with FDA-approved devices, while companies such as Samsung Electronics, Huawei, and LG Electronics are leveraging their consumer electronics expertise to enter this space. Emerging players like Laxmi Therapeutic Devices and Bioland Technology are focusing on innovative patch technologies. The technology is maturing rapidly with improvements in accuracy, comfort, and connectivity, though challenges remain in sensor longevity and complete non-invasiveness.
Abbott Diabetes Care, Inc.
Technical Solution: Abbott's FreeStyle Libre system represents a breakthrough in wearable biosensing for glucose monitoring. The latest generation, FreeStyle Libre 3, features an ultra-small sensor (the size of two stacked pennies) that is applied to the back of the upper arm with a simple, virtually painless applicator. The system utilizes a proprietary wired enzyme technology with a glucose oxidase approach that generates an electrical current proportional to the glucose concentration in interstitial fluid. Unlike traditional CGMs, Abbott's technology eliminates the need for routine fingerstick calibrations through factory calibration processes. The sensor continuously measures glucose levels and transmits data in real-time via Bluetooth to a smartphone app, providing minute-by-minute glucose readings, trend arrows, and customizable alarms. The system offers a remarkable 14-day wear period with a 1-hour warm-up time and achieves a MARD of approximately 7.9%, demonstrating exceptional accuracy[3]. The patch is water-resistant, allowing users to shower, bathe, and swim while wearing it.
Strengths: Extended 14-day wear period exceeds industry standard; factory calibration eliminates fingerstick calibrations; exceptionally small and discreet form factor improves user experience; lower cost compared to traditional CGM systems. Weaknesses: Limited integration with automated insulin delivery systems compared to competitors; smartphone dependency for full functionality; occasional skin irritation reported by some users; slightly longer warm-up time compared to newest competitors.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a cutting-edge wearable biosensing platform for continuous glucose monitoring that leverages their expertise in consumer electronics and semiconductor manufacturing. Their approach utilizes a non-invasive or minimally invasive optical sensing technology that employs Raman spectroscopy and photoplethysmography (PPG) sensors integrated into a compact, flexible patch. The system uses advanced algorithms to analyze the spectral data and correlate it with blood glucose levels, eliminating the need for traditional needle-based sensors. Samsung's technology incorporates their proprietary System-on-Chip (SoC) design that integrates sensing, signal processing, and wireless communication components into a single, energy-efficient package. The patch transmits data to Samsung's health ecosystem, including smartphones and smartwatches, providing continuous monitoring with customizable alerts. The system features extended battery life through power optimization techniques and wireless charging capabilities. Samsung has focused on improving user comfort and reducing skin irritation through hypoallergenic adhesives and breathable materials. Clinical evaluations have demonstrated promising accuracy with MARD values approaching 12-15%, though not yet matching the precision of invasive CGM systems[5].
Strengths: Integration with Samsung's extensive consumer electronics ecosystem; potentially non-invasive or minimally invasive approach improves user comfort; advanced semiconductor technology enables miniaturization and power efficiency; strong manufacturing capabilities for mass production. Weaknesses: Lower accuracy compared to established invasive CGM systems; relatively early in development and clinical validation compared to diabetes-focused competitors; potential challenges with optical interference from various skin types and conditions; battery life limitations compared to simpler enzymatic systems.
Core Innovations in CGM Sensor Technology
Ultra-low power wearable biosensor for continuous glucose monitoring
PatentPendingIN202341062730A
Innovation
- An ultra-low power wearable biosensor that leverages advancements in electronic engineering, materials science, and biotechnology to provide continuous, real-time glucose monitoring with extended battery life, biocompatible design, and wireless connectivity for seamless data transmission.
Regulatory Framework for Medical Wearable Devices
The regulatory landscape for wearable biosensing patches for continuous glucose monitoring (CGM) is complex and multifaceted, varying significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies these devices primarily as Class II medical devices, requiring premarket notification (510(k)) or in some cases, premarket approval (PMA) depending on their specific functionality and risk profile. The FDA has established specific guidance for CGM systems, addressing aspects such as accuracy requirements, calibration protocols, and data security standards.
The European Union regulates these devices under the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) with stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. CGM patches must obtain CE marking through conformity assessment procedures conducted by Notified Bodies, with particular emphasis on risk management and clinical evidence.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a stringent review process focusing on quality management systems and clinical data. China's National Medical Products Administration (NMPA) has recently updated its regulations to expedite approval for innovative medical devices while maintaining strict control over foreign-manufactured products.
Regulatory compliance for CGM patches encompasses several critical dimensions beyond initial approval. Data privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient health information collected by these devices. Manufacturers must implement robust data protection measures and transparent privacy policies to ensure compliance.
Interoperability standards represent another significant regulatory consideration. The FDA's guidance on interoperability for medical devices emphasizes the importance of secure data exchange between CGM systems and other healthcare technologies. Standards organizations such as IEEE and ISO have developed specific protocols for medical device communication that manufacturers must adhere to.
Post-market surveillance requirements have become increasingly stringent globally, with regulatory bodies demanding comprehensive adverse event reporting and continuous performance monitoring. The FDA's Unique Device Identification (UDI) system and the EU's EUDAMED database exemplify efforts to enhance traceability and safety monitoring throughout device lifecycles.
Reimbursement pathways constitute a critical aspect of the regulatory framework, with significant implications for market access. In the US, obtaining coverage from the Centers for Medicare & Medicaid Services (CMS) requires demonstrating both clinical utility and cost-effectiveness, while European health technology assessment bodies apply varying criteria across member states.
The European Union regulates these devices under the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) with stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. CGM patches must obtain CE marking through conformity assessment procedures conducted by Notified Bodies, with particular emphasis on risk management and clinical evidence.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a stringent review process focusing on quality management systems and clinical data. China's National Medical Products Administration (NMPA) has recently updated its regulations to expedite approval for innovative medical devices while maintaining strict control over foreign-manufactured products.
Regulatory compliance for CGM patches encompasses several critical dimensions beyond initial approval. Data privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient health information collected by these devices. Manufacturers must implement robust data protection measures and transparent privacy policies to ensure compliance.
Interoperability standards represent another significant regulatory consideration. The FDA's guidance on interoperability for medical devices emphasizes the importance of secure data exchange between CGM systems and other healthcare technologies. Standards organizations such as IEEE and ISO have developed specific protocols for medical device communication that manufacturers must adhere to.
Post-market surveillance requirements have become increasingly stringent globally, with regulatory bodies demanding comprehensive adverse event reporting and continuous performance monitoring. The FDA's Unique Device Identification (UDI) system and the EU's EUDAMED database exemplify efforts to enhance traceability and safety monitoring throughout device lifecycles.
Reimbursement pathways constitute a critical aspect of the regulatory framework, with significant implications for market access. In the US, obtaining coverage from the Centers for Medicare & Medicaid Services (CMS) requires demonstrating both clinical utility and cost-effectiveness, while European health technology assessment bodies apply varying criteria across member states.
Patient Experience and Usability Considerations
Patient experience and usability are critical factors in the adoption and effectiveness of wearable biosensing patches for continuous glucose monitoring (CGM). These devices, worn directly on the skin, must balance technical functionality with comfort and ease of use to ensure patient compliance and satisfaction.
The physical design of CGM patches significantly impacts user experience. Current generations of patches have evolved to be increasingly thin, flexible, and lightweight, with dimensions typically ranging from 2-5 cm in diameter and weighing less than 10 grams. Materials science advancements have enabled the development of hypoallergenic adhesives that maintain secure attachment while minimizing skin irritation, a common complaint in earlier iterations.
Wearability duration represents another crucial consideration. Modern CGM patches offer wear times ranging from 7 to 14 days, reducing the frequency of application and removal procedures. This extended duration must be balanced against potential skin irritation from prolonged contact and adhesive exposure. Studies indicate that over 30% of CGM users report some form of skin reaction, highlighting the need for continued material innovation.
User interface design plays a pivotal role in patient engagement. Most contemporary CGM systems pair the physical patch with smartphone applications that display glucose data through intuitive visualizations and customizable alerts. Research shows that simplified data presentation improves patient understanding and engagement, with color-coded trend indicators being particularly effective for quick interpretation.
The psychological impact of wearing visible medical devices cannot be overlooked. Miniaturization and discreet design options have helped address stigma concerns, particularly important for adolescent and young adult users. Survey data indicates that 65% of users consider device visibility when making CGM selection decisions.
Onboarding and education significantly influence user satisfaction. Simplified application procedures with clear visual instructions have reduced user error rates by approximately 40% compared to earlier CGM generations. Additionally, virtual training programs have demonstrated effectiveness in improving user confidence and reducing technical support requirements.
Future usability enhancements are focusing on personalization features, including customizable alert thresholds, interoperability with other health monitoring systems, and adaptive learning algorithms that provide increasingly relevant insights based on individual patterns. These advancements aim to transform CGM devices from passive monitoring tools to active partners in diabetes management.
The physical design of CGM patches significantly impacts user experience. Current generations of patches have evolved to be increasingly thin, flexible, and lightweight, with dimensions typically ranging from 2-5 cm in diameter and weighing less than 10 grams. Materials science advancements have enabled the development of hypoallergenic adhesives that maintain secure attachment while minimizing skin irritation, a common complaint in earlier iterations.
Wearability duration represents another crucial consideration. Modern CGM patches offer wear times ranging from 7 to 14 days, reducing the frequency of application and removal procedures. This extended duration must be balanced against potential skin irritation from prolonged contact and adhesive exposure. Studies indicate that over 30% of CGM users report some form of skin reaction, highlighting the need for continued material innovation.
User interface design plays a pivotal role in patient engagement. Most contemporary CGM systems pair the physical patch with smartphone applications that display glucose data through intuitive visualizations and customizable alerts. Research shows that simplified data presentation improves patient understanding and engagement, with color-coded trend indicators being particularly effective for quick interpretation.
The psychological impact of wearing visible medical devices cannot be overlooked. Miniaturization and discreet design options have helped address stigma concerns, particularly important for adolescent and young adult users. Survey data indicates that 65% of users consider device visibility when making CGM selection decisions.
Onboarding and education significantly influence user satisfaction. Simplified application procedures with clear visual instructions have reduced user error rates by approximately 40% compared to earlier CGM generations. Additionally, virtual training programs have demonstrated effectiveness in improving user confidence and reducing technical support requirements.
Future usability enhancements are focusing on personalization features, including customizable alert thresholds, interoperability with other health monitoring systems, and adaptive learning algorithms that provide increasingly relevant insights based on individual patterns. These advancements aim to transform CGM devices from passive monitoring tools to active partners in diabetes management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!