CRISPR Base Editing: Exploring Electrolyte Applications in Medicine
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CRISPR Base Editing Background and Objectives
CRISPR base editing technology represents a revolutionary advancement in genome editing, evolving from the original CRISPR-Cas9 system discovered in bacterial adaptive immune systems. Unlike traditional CRISPR-Cas9 which creates double-strand breaks in DNA, base editing enables precise single nucleotide modifications without cleaving the DNA backbone, significantly reducing off-target effects and unwanted mutations.
The technology has progressed through several key developmental phases since its introduction in 2016 by David Liu's laboratory at Harvard University. Initial cytosine base editors (CBEs) could convert C•G to T•A base pairs, while subsequent adenine base editors (ABEs) enabled A•T to G•C conversions. Recent innovations include prime editing, which offers even greater precision for targeted nucleotide replacements, insertions, and deletions.
Base editing technology addresses critical limitations in conventional gene therapy approaches by offering unprecedented precision in genetic modification. This precision is particularly valuable for treating monogenic disorders caused by point mutations, which constitute approximately 58% of known genetic diseases.
The integration of electrolyte applications with CRISPR base editing represents an emerging frontier with significant potential for enhancing delivery efficiency and cellular uptake. Electrolytes, as ionic conductors, can facilitate the transport of editing components across cell membranes through techniques such as electroporation or by forming specialized delivery complexes.
Our technical objectives focus on exploring novel electrolyte formulations that can improve the delivery, stability, and efficiency of base editing components in therapeutic applications. Specifically, we aim to develop biocompatible electrolyte systems that enhance cellular uptake while minimizing cytotoxicity and immunogenicity.
Additionally, we seek to investigate the potential of electrolyte-mediated base editing for treating conditions previously considered challenging for gene therapy approaches, including cardiovascular diseases, neurological disorders, and certain forms of cancer where precise genetic modifications are required.
The long-term vision encompasses the development of personalized medicine platforms where electrolyte-enhanced base editing technologies can be tailored to individual genetic profiles, enabling customized therapeutic interventions with minimal side effects. This approach could revolutionize treatment paradigms for genetic disorders by addressing the root genetic causes rather than merely managing symptoms.
Furthermore, we aim to establish standardized protocols and delivery systems that can translate these advanced technologies from laboratory settings to clinical applications, addressing current challenges in scalability, reproducibility, and regulatory compliance that have limited the broader implementation of gene editing therapies.
The technology has progressed through several key developmental phases since its introduction in 2016 by David Liu's laboratory at Harvard University. Initial cytosine base editors (CBEs) could convert C•G to T•A base pairs, while subsequent adenine base editors (ABEs) enabled A•T to G•C conversions. Recent innovations include prime editing, which offers even greater precision for targeted nucleotide replacements, insertions, and deletions.
Base editing technology addresses critical limitations in conventional gene therapy approaches by offering unprecedented precision in genetic modification. This precision is particularly valuable for treating monogenic disorders caused by point mutations, which constitute approximately 58% of known genetic diseases.
The integration of electrolyte applications with CRISPR base editing represents an emerging frontier with significant potential for enhancing delivery efficiency and cellular uptake. Electrolytes, as ionic conductors, can facilitate the transport of editing components across cell membranes through techniques such as electroporation or by forming specialized delivery complexes.
Our technical objectives focus on exploring novel electrolyte formulations that can improve the delivery, stability, and efficiency of base editing components in therapeutic applications. Specifically, we aim to develop biocompatible electrolyte systems that enhance cellular uptake while minimizing cytotoxicity and immunogenicity.
Additionally, we seek to investigate the potential of electrolyte-mediated base editing for treating conditions previously considered challenging for gene therapy approaches, including cardiovascular diseases, neurological disorders, and certain forms of cancer where precise genetic modifications are required.
The long-term vision encompasses the development of personalized medicine platforms where electrolyte-enhanced base editing technologies can be tailored to individual genetic profiles, enabling customized therapeutic interventions with minimal side effects. This approach could revolutionize treatment paradigms for genetic disorders by addressing the root genetic causes rather than merely managing symptoms.
Furthermore, we aim to establish standardized protocols and delivery systems that can translate these advanced technologies from laboratory settings to clinical applications, addressing current challenges in scalability, reproducibility, and regulatory compliance that have limited the broader implementation of gene editing therapies.
Medical Market Demand for Precision Gene Editing
The precision gene editing market is experiencing unprecedented growth, driven by the revolutionary CRISPR-Cas9 technology and its advanced iterations like base editing. Current market valuations place the global gene editing sector at approximately $5.3 billion as of 2022, with projections indicating a compound annual growth rate of 18.2% through 2030. Within this expanding landscape, base editing technologies are gaining significant traction due to their enhanced precision and reduced off-target effects compared to traditional CRISPR systems.
Healthcare providers and pharmaceutical companies are increasingly seeking more precise genetic modification tools that minimize unintended mutations. This demand stems from the growing recognition that conventional gene editing approaches, while groundbreaking, still present considerable risks when applied to human therapeutics. Base editing, particularly when optimized with electrolyte applications, addresses this critical need by offering single-nucleotide precision without requiring double-strand DNA breaks.
The clinical pipeline for precision gene editing applications has expanded dramatically, with over 75 active clinical trials utilizing various CRISPR technologies worldwide. Notably, treatments for genetic blood disorders like sickle cell disease and beta-thalassemia have progressed furthest, demonstrating the immediate market demand for these technologies. The potential application of electrolyte-enhanced base editing could significantly improve outcomes in these established therapeutic areas.
Investor confidence in precision gene editing technologies is evidenced by substantial funding rounds, with specialized base editing companies securing over $800 million in combined investments during 2021-2022. This financial momentum reflects market recognition of base editing's potential to address previously untreatable genetic conditions with greater safety margins.
Patient advocacy groups are increasingly vocal about the need for more precise genetic interventions, particularly for rare genetic disorders affecting pediatric populations. Market research indicates that approximately 400 million people worldwide suffer from rare diseases, many of which have genetic origins potentially addressable through precision editing technologies.
Regulatory frameworks are evolving to accommodate these advanced therapeutic approaches, with the FDA and EMA developing specialized guidance for gene editing therapies. This regulatory adaptation signals institutional recognition of precision editing's growing importance in the medical landscape and creates pathways for commercial development.
The convergence of these market factors creates a robust demand environment for electrolyte-optimized base editing technologies, particularly in oncology, rare genetic disorders, and infectious disease applications where precision modifications can yield significant therapeutic benefits with minimized risk profiles.
Healthcare providers and pharmaceutical companies are increasingly seeking more precise genetic modification tools that minimize unintended mutations. This demand stems from the growing recognition that conventional gene editing approaches, while groundbreaking, still present considerable risks when applied to human therapeutics. Base editing, particularly when optimized with electrolyte applications, addresses this critical need by offering single-nucleotide precision without requiring double-strand DNA breaks.
The clinical pipeline for precision gene editing applications has expanded dramatically, with over 75 active clinical trials utilizing various CRISPR technologies worldwide. Notably, treatments for genetic blood disorders like sickle cell disease and beta-thalassemia have progressed furthest, demonstrating the immediate market demand for these technologies. The potential application of electrolyte-enhanced base editing could significantly improve outcomes in these established therapeutic areas.
Investor confidence in precision gene editing technologies is evidenced by substantial funding rounds, with specialized base editing companies securing over $800 million in combined investments during 2021-2022. This financial momentum reflects market recognition of base editing's potential to address previously untreatable genetic conditions with greater safety margins.
Patient advocacy groups are increasingly vocal about the need for more precise genetic interventions, particularly for rare genetic disorders affecting pediatric populations. Market research indicates that approximately 400 million people worldwide suffer from rare diseases, many of which have genetic origins potentially addressable through precision editing technologies.
Regulatory frameworks are evolving to accommodate these advanced therapeutic approaches, with the FDA and EMA developing specialized guidance for gene editing therapies. This regulatory adaptation signals institutional recognition of precision editing's growing importance in the medical landscape and creates pathways for commercial development.
The convergence of these market factors creates a robust demand environment for electrolyte-optimized base editing technologies, particularly in oncology, rare genetic disorders, and infectious disease applications where precision modifications can yield significant therapeutic benefits with minimized risk profiles.
Current Challenges in CRISPR Base Editing Technology
Despite significant advancements in CRISPR base editing technology, several critical challenges persist that limit its widespread clinical application. The primary concern remains off-target effects, where unintended edits occur at genomic sites similar to the target sequence. These off-target modifications can potentially lead to unwanted mutations, cellular dysfunction, or even carcinogenesis, raising substantial safety concerns for therapeutic applications.
Delivery efficiency presents another major hurdle, particularly in the context of electrolyte applications. Current delivery systems struggle to transport base editing components effectively across cell membranes while maintaining the delicate electrolyte balance necessary for cellular function. This challenge becomes especially pronounced when targeting specific tissues or organs where the blood-brain barrier or other biological barriers must be overcome.
The limited editing window of conventional base editors restricts their versatility in addressing diverse genetic conditions. Most cytosine base editors (CBEs) and adenine base editors (ABEs) can only edit within a narrow sequence context, typically positions 4-8 of the protospacer, significantly constraining the range of targetable mutations.
Immune responses to CRISPR components represent another significant obstacle. The bacterial origin of Cas proteins can trigger innate and adaptive immune responses in patients, potentially neutralizing the therapeutic effect and causing adverse inflammatory reactions. This immunogenicity is particularly problematic when considering electrolyte-based delivery systems, as immune activation can disrupt ion channel function and membrane potential.
Precision control of editing outcomes remains challenging, especially in the context of maintaining electrolyte homeostasis. Current base editing systems often produce a mixture of edited products rather than a single, desired outcome. This heterogeneity can complicate therapeutic development and regulatory approval processes.
The scalability of production presents economic and technical barriers to widespread implementation. Manufacturing high-quality, clinical-grade base editing components with consistent performance characteristics requires sophisticated bioprocessing capabilities that are currently limited to specialized facilities.
Additionally, the complex regulatory landscape surrounding gene editing technologies creates uncertainty in development pathways. Regulatory frameworks are still evolving to address the unique safety and efficacy considerations of base editing technologies, particularly those involving novel electrolyte applications.
Finally, there exists a significant knowledge gap regarding the long-term effects of base editing interventions on cellular physiology and electrolyte balance. The potential for delayed adverse effects or subtle alterations in cellular function necessitates extensive longitudinal studies before widespread clinical adoption can be realized.
Delivery efficiency presents another major hurdle, particularly in the context of electrolyte applications. Current delivery systems struggle to transport base editing components effectively across cell membranes while maintaining the delicate electrolyte balance necessary for cellular function. This challenge becomes especially pronounced when targeting specific tissues or organs where the blood-brain barrier or other biological barriers must be overcome.
The limited editing window of conventional base editors restricts their versatility in addressing diverse genetic conditions. Most cytosine base editors (CBEs) and adenine base editors (ABEs) can only edit within a narrow sequence context, typically positions 4-8 of the protospacer, significantly constraining the range of targetable mutations.
Immune responses to CRISPR components represent another significant obstacle. The bacterial origin of Cas proteins can trigger innate and adaptive immune responses in patients, potentially neutralizing the therapeutic effect and causing adverse inflammatory reactions. This immunogenicity is particularly problematic when considering electrolyte-based delivery systems, as immune activation can disrupt ion channel function and membrane potential.
Precision control of editing outcomes remains challenging, especially in the context of maintaining electrolyte homeostasis. Current base editing systems often produce a mixture of edited products rather than a single, desired outcome. This heterogeneity can complicate therapeutic development and regulatory approval processes.
The scalability of production presents economic and technical barriers to widespread implementation. Manufacturing high-quality, clinical-grade base editing components with consistent performance characteristics requires sophisticated bioprocessing capabilities that are currently limited to specialized facilities.
Additionally, the complex regulatory landscape surrounding gene editing technologies creates uncertainty in development pathways. Regulatory frameworks are still evolving to address the unique safety and efficacy considerations of base editing technologies, particularly those involving novel electrolyte applications.
Finally, there exists a significant knowledge gap regarding the long-term effects of base editing interventions on cellular physiology and electrolyte balance. The potential for delayed adverse effects or subtle alterations in cellular function necessitates extensive longitudinal studies before widespread clinical adoption can be realized.
Current Electrolyte Applications in CRISPR Base Editing
01 CRISPR base editing systems and components
CRISPR base editing systems comprise modified Cas proteins fused with deaminase enzymes that enable precise single nucleotide changes without creating double-strand breaks. These systems include cytosine base editors (CBEs) that convert C•G to T•A and adenine base editors (ABEs) that convert A•T to G•C. The components typically include a catalytically impaired Cas9 or Cas12 protein, a deaminase domain, and a guide RNA that directs the editing machinery to the target site.- CRISPR base editing systems and components: CRISPR base editing systems comprise modified Cas proteins fused with deaminase enzymes that enable precise single nucleotide changes without creating double-strand breaks. These systems include cytosine base editors (CBEs) that convert C•G to T•A and adenine base editors (ABEs) that convert A•T to G•C. The components typically include a catalytically impaired Cas protein (such as dCas9 or Cas9 nickase), a deaminase domain, and a guide RNA that directs the editing machinery to the target site.
- Therapeutic applications of CRISPR base editing: CRISPR base editing technologies are being developed for treating genetic diseases by correcting disease-causing point mutations. These applications include addressing blood disorders like sickle cell disease and beta-thalassemia, metabolic disorders, and various inherited conditions. The precision of base editing allows for correction of pathogenic mutations without the risks associated with conventional CRISPR-Cas9 gene editing, such as unwanted insertions or deletions, making it particularly valuable for clinical applications where safety is paramount.
- Enhanced specificity and efficiency in base editing: Innovations in CRISPR base editing focus on improving specificity and efficiency through engineered deaminases, optimized guide RNA designs, and modified Cas variants. These advancements reduce off-target effects while increasing on-target editing efficiency. Strategies include the development of high-fidelity base editors with reduced bystander editing, expanded targeting scope through altered PAM requirements, and improved delivery methods to enhance cellular uptake and expression of the base editing machinery.
- Delivery methods for base editing components: Various delivery systems have been developed to introduce base editing components into cells and organisms. These include viral vectors (such as AAV, lentivirus), lipid nanoparticles, and ribonucleoprotein complexes. Each delivery method offers different advantages in terms of efficiency, cell type specificity, immunogenicity, and persistence of the editing machinery. Recent innovations focus on tissue-specific delivery and reducing the size of base editing components to fit within delivery vector capacity constraints.
- Agricultural and industrial applications of base editing: Beyond medical applications, CRISPR base editing is being utilized in agriculture and industrial biotechnology. In agriculture, base editing enables precise modification of crop genomes to enhance traits like disease resistance, drought tolerance, and nutritional content without introducing foreign DNA. In industrial biotechnology, base editing is employed to optimize microorganisms for biofuel production, enzyme manufacturing, and other biotechnological processes by making targeted changes to metabolic pathways.
02 Therapeutic applications of CRISPR base editing
CRISPR base editing technologies are being developed for treating genetic diseases by correcting disease-causing point mutations. These applications include addressing blood disorders like sickle cell disease and beta-thalassemia, metabolic disorders, and various inherited conditions. The precision of base editing allows for correction of pathogenic mutations without the risks associated with conventional CRISPR-Cas9 editing, such as unwanted insertions or deletions at the target site.Expand Specific Solutions03 Delivery methods for CRISPR base editors
Various delivery systems have been developed to introduce base editing components into target cells, including viral vectors (AAV, lentivirus), lipid nanoparticles, and ribonucleoprotein complexes. Ex vivo delivery approaches involve editing cells outside the body before transplantation back into patients, while in vivo delivery systems target specific tissues directly within the organism. These delivery methods aim to maximize editing efficiency while minimizing off-target effects and immunogenicity.Expand Specific Solutions04 Enhanced specificity and efficiency in base editing
Innovations to improve base editing specificity and efficiency include engineered deaminases with reduced off-target activity, modified guide RNA structures, and optimized linkers between Cas and deaminase domains. High-fidelity base editors incorporate mutations in the Cas protein that reduce off-target DNA binding. Additionally, computational tools have been developed to predict and minimize off-target effects, ensuring safer application in research and therapeutic contexts.Expand Specific Solutions05 Novel base editing architectures and applications
Advanced base editing platforms include dual-function editors capable of performing both C-to-T and A-to-G edits simultaneously, prime editing systems that combine base editing with targeted insertions or deletions, and RNA base editors that modify RNA rather than DNA. These technologies expand the scope of genetic modifications possible with base editing and enable applications in agriculture, biofuel production, and synthetic biology beyond human therapeutics.Expand Specific Solutions
Key Players in CRISPR Base Editing Research and Development
CRISPR Base Editing in medicine is evolving rapidly, currently transitioning from early research to clinical application phases. The market is projected to grow significantly, driven by increasing investment in genetic medicine applications. Technologically, leading academic institutions (MIT, Broad Institute, Harvard) are pioneering fundamental research, while companies like Beam Therapeutics, Editas Medicine, and HuidaGene Therapeutics are advancing commercial applications. Beam Therapeutics has established itself as a frontrunner in base editing technology commercialization, while traditional CRISPR players like Editas are expanding their portfolios. The competitive landscape features strong collaboration between academia and industry, with emerging players from China (ShanghaiTech, Jiangnan University) increasingly contributing to innovation, particularly in delivery systems and therapeutic applications.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has developed advanced CRISPR base editing platforms that enable precise nucleotide changes without double-strand breaks, crucial for applications in electrolyte-related disorders. Their technology combines CRISPR-Cas9 with deaminase enzymes to create targeted C-to-T or A-to-G conversions. For electrolyte applications in medicine, they've pioneered techniques to modify genes encoding ion channels, transporters, and regulatory proteins that maintain electrolyte homeostasis. Their research includes developing adenine base editors (ABEs) that can correct mutations in genes like CFTR (cystic fibrosis), SCN5A (cardiac sodium channels), and KCNQ1 (potassium channels). The Broad has also explored novel delivery methods optimized for tissues with complex electrolyte environments, such as engineered viral vectors and lipid nanoparticles designed to function in the ionic conditions of specific target tissues. Their collaborative approach with clinical partners has accelerated translation of these technologies toward therapeutic applications for diseases involving electrolyte imbalances.
Strengths: World-leading expertise in CRISPR technology development; extensive collaborative network with clinical and industry partners; comprehensive intellectual property portfolio. Weaknesses: As a research institute, faces challenges in direct commercialization; complex licensing arrangements may slow clinical translation; base editing efficiency still requires optimization for certain tissue types with unique electrolyte compositions.
Massachusetts Institute of Technology
Technical Solution: MIT has developed cutting-edge CRISPR base editing technologies with significant applications for electrolyte-related medical conditions. Their research teams have engineered highly precise cytosine and adenine base editors that enable targeted C→T and A→G conversions without inducing double-strand breaks, crucial for modifying genes involved in electrolyte regulation. For electrolyte applications in medicine, MIT researchers have focused on optimizing base editors for genes encoding ion channels, transporters, and regulatory proteins that maintain electrolyte homeostasis across various tissues. Their technology incorporates rationally designed deaminases with modified Cas proteins to achieve single-nucleotide precision even in tissues with complex ionic environments. MIT has pioneered innovative delivery systems specifically designed for electrolyte-rich environments, including engineered lipid nanoparticles with tailored surface properties that maintain stability across varying pH and ionic strength conditions. Their collaborative research has demonstrated successful editing of genes like CFTR (cystic fibrosis), SCN5A (cardiac sodium channels), and ATP1A1 (sodium-potassium pump), which are critical in maintaining electrolyte balance and are implicated in numerous diseases involving electrolyte dysregulation.
Strengths: World-class expertise in CRISPR technology development and engineering; strong interdisciplinary approach combining biology, chemistry, and materials science; extensive collaborative network with clinical and industry partners. Weaknesses: As an academic institution, faces challenges in direct commercialization; complex licensing arrangements may slow clinical translation; base editing efficiency still requires optimization for certain tissue types with unique electrolyte compositions.
Core Patents and Innovations in Electrolyte-Enhanced Editing
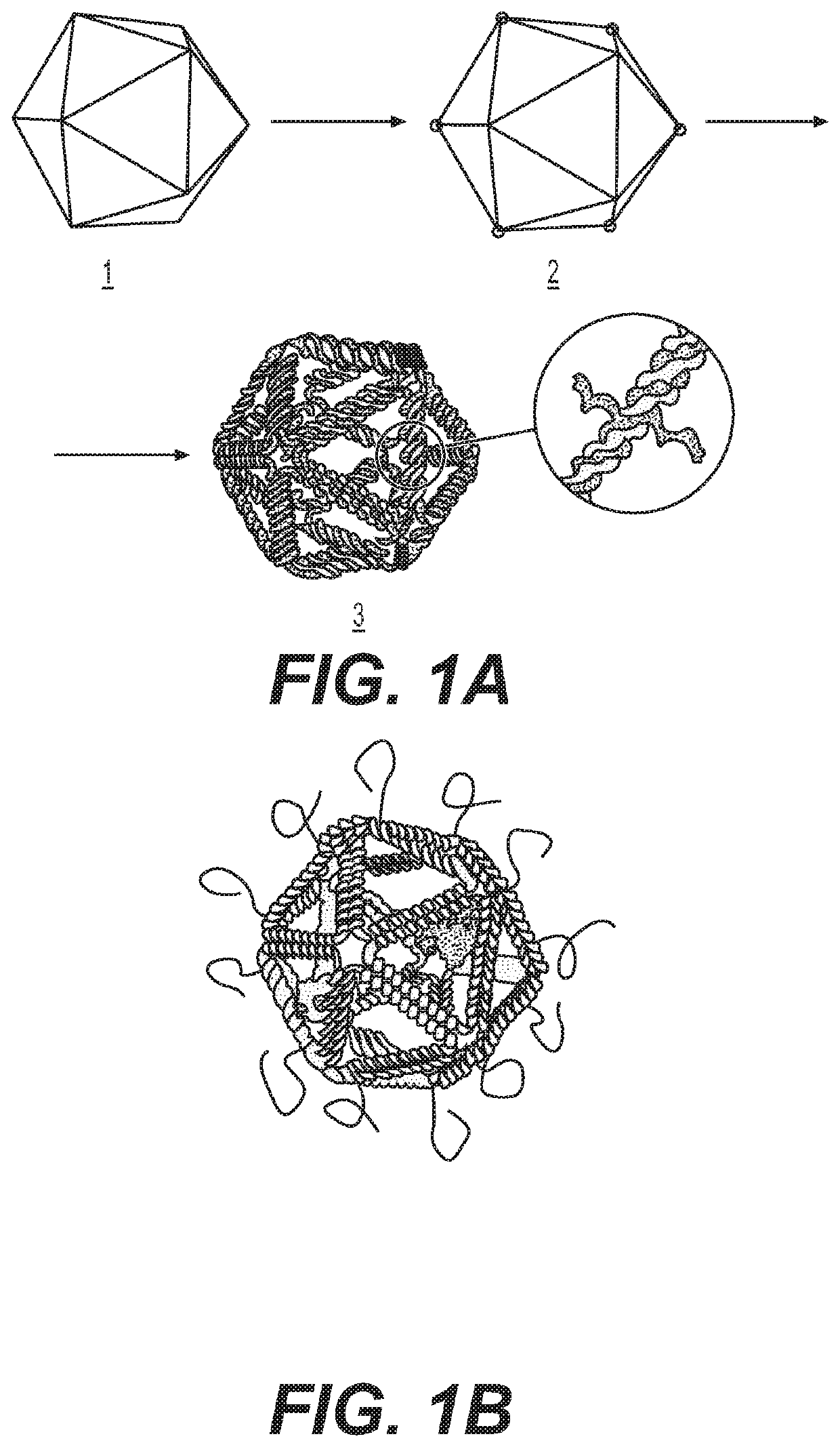
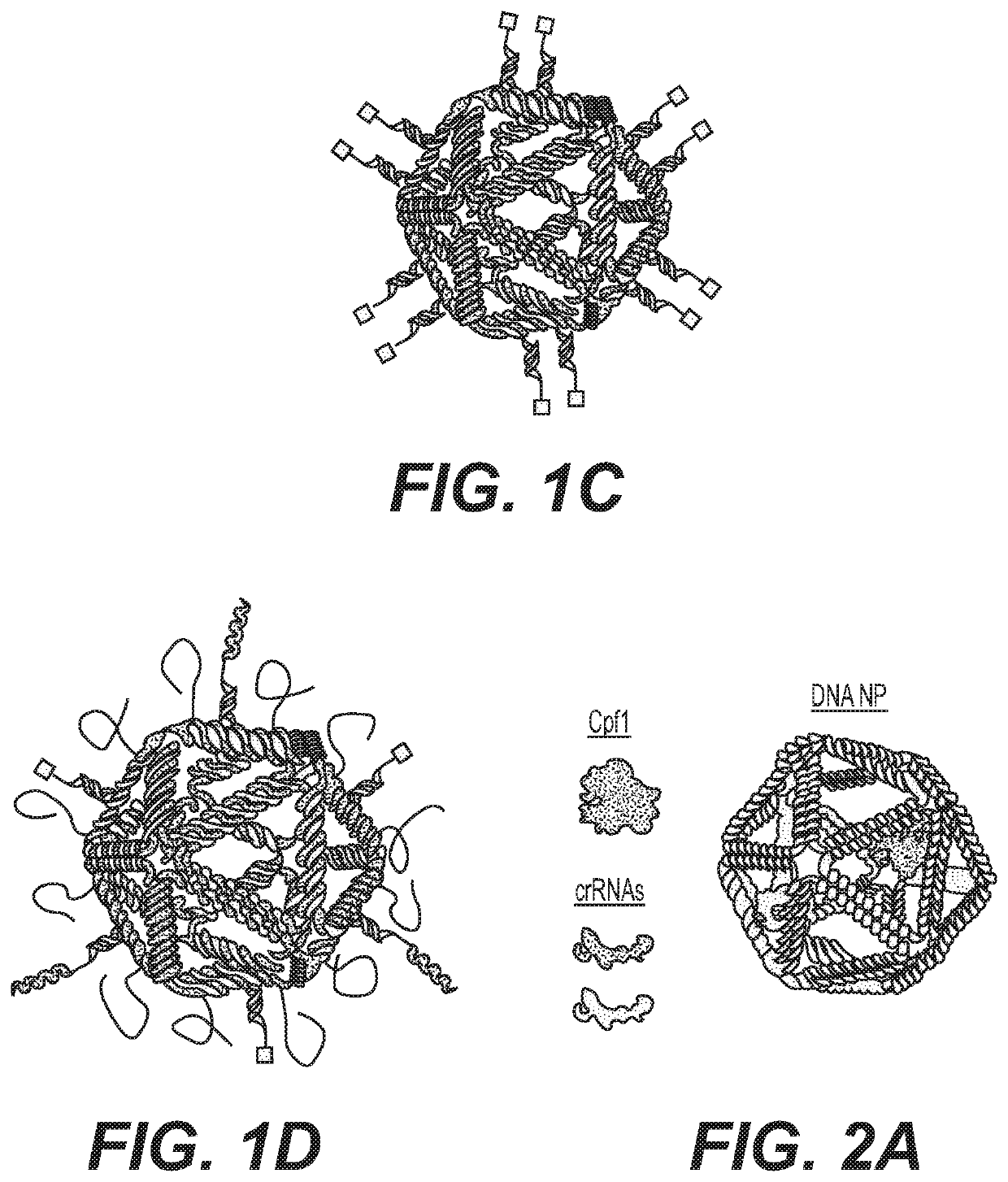
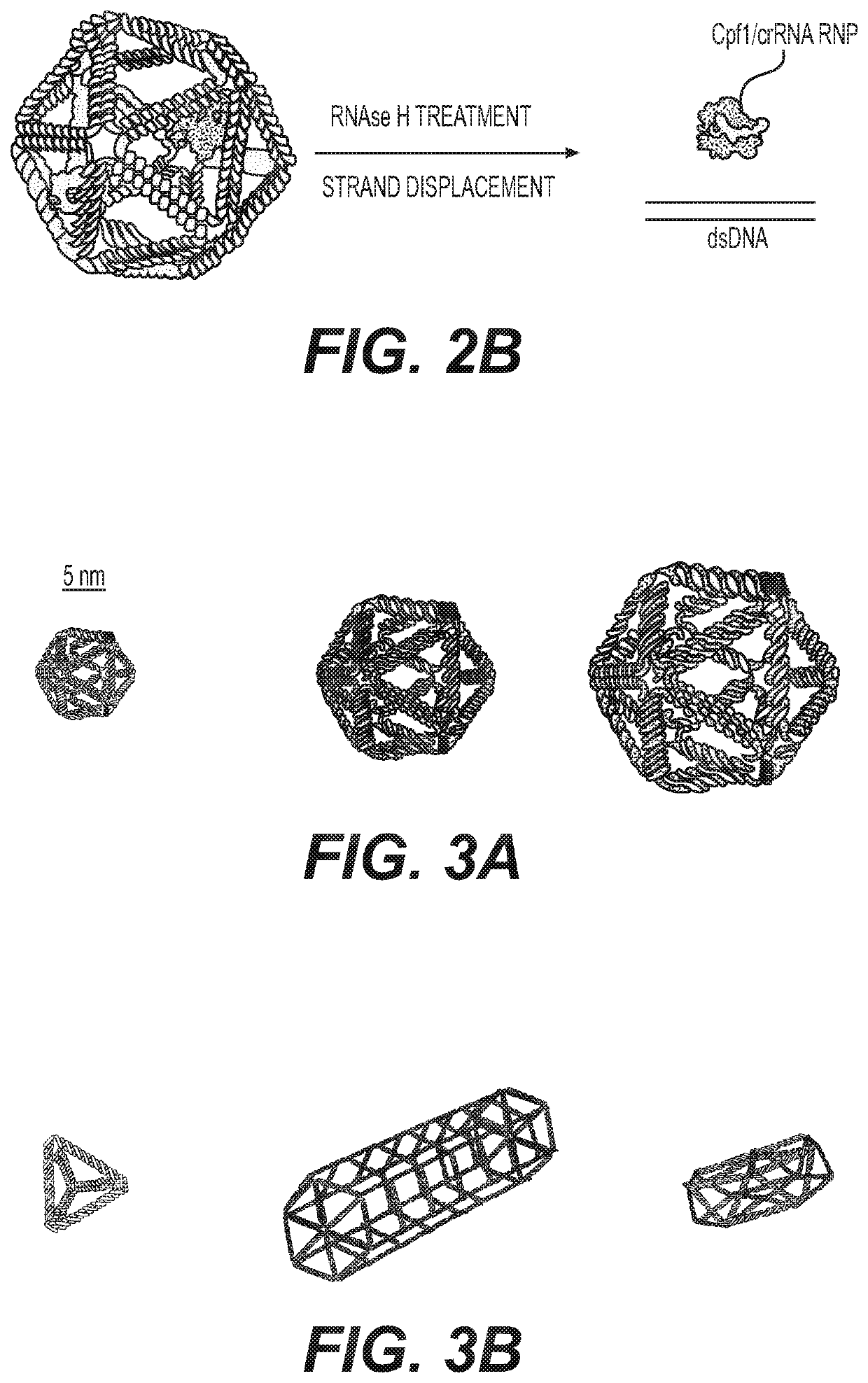
Nucleic acid assemblies for use in targeted delivery
PatentPendingUS20210317479A1
Innovation
- Nucleic acid assemblies that enclose and protect cargo, such as CRISPR-Cas effector proteins and guide molecules, with designed physiochemical properties for targeted delivery, enhanced stability, and reduced immunogenicity, allowing for controlled stoichiometry and intracellular trafficking.
Patent
Innovation
- Development of CRISPR base editing systems utilizing optimized electrolyte compositions to enhance editing efficiency and reduce off-target effects in therapeutic applications.
- Novel delivery methods combining lipid nanoparticles with specialized electrolyte solutions that improve cellular uptake and nuclear localization of base editing components.
- Engineering of electrolyte-responsive base editor variants that exhibit enhanced activity under specific ionic conditions, enabling tissue-specific editing through manipulation of local electrolyte environments.
Regulatory Framework for Gene Editing Therapeutics
The regulatory landscape for CRISPR base editing technologies, particularly those involving electrolyte applications in medicine, presents a complex framework that continues to evolve globally. Current regulatory approaches vary significantly across jurisdictions, with the FDA in the United States establishing a risk-based framework that evaluates gene editing therapeutics based on their mechanism of action, intended use, and potential risks to patients.
In the European Union, the European Medicines Agency (EMA) has developed specific guidelines for advanced therapy medicinal products (ATMPs), which include gene therapy medicinal products. These guidelines emphasize the need for comprehensive preclinical and clinical data, with particular attention to off-target effects and long-term safety monitoring requirements for base editing technologies.
Asian regulatory frameworks, particularly in China and Japan, have shown more accelerated pathways for gene editing therapeutics, though recent developments indicate increasing harmonization with international standards. China's National Medical Products Administration (NMPA) has established specialized review processes for innovative gene therapies, while Japan's PMDA offers expedited review through its Sakigake designation system.
Key regulatory considerations specific to CRISPR base editing with electrolyte applications include characterization of editing precision, assessment of off-target modifications, evaluation of delivery systems, and monitoring of immune responses. Regulatory bodies increasingly require robust analytical methods to detect and quantify these parameters, with particular emphasis on the novel electrolyte components that may influence editing efficiency and safety profiles.
Clinical trial designs for base editing therapeutics face unique regulatory challenges, including requirements for long-term follow-up studies (often 15+ years), specialized risk mitigation strategies, and comprehensive genomic analysis protocols. The FDA's recent guidance documents specifically address the need for sensitive detection methods for unintended genomic alterations resulting from base editing interventions.
Ethical considerations have become increasingly integrated into regulatory frameworks, with requirements for informed consent processes that adequately communicate the novel and potentially irreversible nature of genetic modifications. International organizations such as the WHO have published governance frameworks that emphasize responsible innovation while acknowledging the transformative potential of these technologies.
Looking forward, regulatory convergence efforts through initiatives like the International Council for Harmonisation (ICH) aim to develop standardized approaches to evaluating base editing therapeutics, potentially streamlining global development pathways while maintaining rigorous safety standards. The integration of real-world evidence and patient-reported outcomes is also emerging as a complementary approach to traditional regulatory assessment methods for these innovative therapies.
In the European Union, the European Medicines Agency (EMA) has developed specific guidelines for advanced therapy medicinal products (ATMPs), which include gene therapy medicinal products. These guidelines emphasize the need for comprehensive preclinical and clinical data, with particular attention to off-target effects and long-term safety monitoring requirements for base editing technologies.
Asian regulatory frameworks, particularly in China and Japan, have shown more accelerated pathways for gene editing therapeutics, though recent developments indicate increasing harmonization with international standards. China's National Medical Products Administration (NMPA) has established specialized review processes for innovative gene therapies, while Japan's PMDA offers expedited review through its Sakigake designation system.
Key regulatory considerations specific to CRISPR base editing with electrolyte applications include characterization of editing precision, assessment of off-target modifications, evaluation of delivery systems, and monitoring of immune responses. Regulatory bodies increasingly require robust analytical methods to detect and quantify these parameters, with particular emphasis on the novel electrolyte components that may influence editing efficiency and safety profiles.
Clinical trial designs for base editing therapeutics face unique regulatory challenges, including requirements for long-term follow-up studies (often 15+ years), specialized risk mitigation strategies, and comprehensive genomic analysis protocols. The FDA's recent guidance documents specifically address the need for sensitive detection methods for unintended genomic alterations resulting from base editing interventions.
Ethical considerations have become increasingly integrated into regulatory frameworks, with requirements for informed consent processes that adequately communicate the novel and potentially irreversible nature of genetic modifications. International organizations such as the WHO have published governance frameworks that emphasize responsible innovation while acknowledging the transformative potential of these technologies.
Looking forward, regulatory convergence efforts through initiatives like the International Council for Harmonisation (ICH) aim to develop standardized approaches to evaluating base editing therapeutics, potentially streamlining global development pathways while maintaining rigorous safety standards. The integration of real-world evidence and patient-reported outcomes is also emerging as a complementary approach to traditional regulatory assessment methods for these innovative therapies.
Bioethical Implications of CRISPR Base Editing Applications
The rapid advancement of CRISPR base editing technology, particularly in electrolyte applications for medicine, raises significant bioethical considerations that must be addressed before widespread implementation. These precision genome editing tools offer unprecedented potential for treating genetic disorders by making specific nucleotide changes without double-strand breaks, yet they simultaneously challenge existing ethical frameworks and regulatory systems.
The principle of autonomy stands at the forefront of these considerations, demanding that patients maintain informed consent rights regarding genetic modifications. This becomes particularly complex when considering germline editing applications that could affect future generations who cannot consent to these modifications. The distinction between therapeutic applications and enhancement purposes remains blurry, raising questions about where society should draw ethical boundaries.
Justice and accessibility concerns emerge prominently in the CRISPR base editing discourse. As these technologies develop, ensuring equitable access across socioeconomic divides becomes imperative. Without careful consideration, base editing therapies could exacerbate existing healthcare disparities, creating a genetic divide between those who can afford genetic interventions and those who cannot.
Safety considerations remain paramount, as off-target effects and unintended consequences of base editing could manifest generations after initial application. The current limitations in predicting long-term outcomes of genetic modifications necessitate robust risk assessment frameworks and longitudinal monitoring systems that may span decades or even generations.
Cultural and religious perspectives on genetic modification vary significantly worldwide, complicating the establishment of universal ethical guidelines. Some traditions view genetic intervention as interfering with natural or divine processes, while others may embrace therapeutic applications while rejecting enhancement uses. These diverse viewpoints must be respected in developing regulatory frameworks.
Governance challenges are substantial, as existing regulatory systems were not designed for the precision and permanence of base editing technologies. International harmonization of oversight mechanisms becomes crucial to prevent regulatory arbitrage while respecting cultural differences in ethical perspectives. Transparent stakeholder engagement processes must include diverse voices from patient advocacy groups, religious organizations, indigenous communities, and global south perspectives.
The responsible development of CRISPR base editing in electrolyte applications requires ongoing ethical deliberation that evolves alongside the technology itself. Establishing ethics review boards with interdisciplinary expertise could help navigate these complex issues while maintaining public trust in this transformative technology.
The principle of autonomy stands at the forefront of these considerations, demanding that patients maintain informed consent rights regarding genetic modifications. This becomes particularly complex when considering germline editing applications that could affect future generations who cannot consent to these modifications. The distinction between therapeutic applications and enhancement purposes remains blurry, raising questions about where society should draw ethical boundaries.
Justice and accessibility concerns emerge prominently in the CRISPR base editing discourse. As these technologies develop, ensuring equitable access across socioeconomic divides becomes imperative. Without careful consideration, base editing therapies could exacerbate existing healthcare disparities, creating a genetic divide between those who can afford genetic interventions and those who cannot.
Safety considerations remain paramount, as off-target effects and unintended consequences of base editing could manifest generations after initial application. The current limitations in predicting long-term outcomes of genetic modifications necessitate robust risk assessment frameworks and longitudinal monitoring systems that may span decades or even generations.
Cultural and religious perspectives on genetic modification vary significantly worldwide, complicating the establishment of universal ethical guidelines. Some traditions view genetic intervention as interfering with natural or divine processes, while others may embrace therapeutic applications while rejecting enhancement uses. These diverse viewpoints must be respected in developing regulatory frameworks.
Governance challenges are substantial, as existing regulatory systems were not designed for the precision and permanence of base editing technologies. International harmonization of oversight mechanisms becomes crucial to prevent regulatory arbitrage while respecting cultural differences in ethical perspectives. Transparent stakeholder engagement processes must include diverse voices from patient advocacy groups, religious organizations, indigenous communities, and global south perspectives.
The responsible development of CRISPR base editing in electrolyte applications requires ongoing ethical deliberation that evolves alongside the technology itself. Establishing ethics review boards with interdisciplinary expertise could help navigate these complex issues while maintaining public trust in this transformative technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!