Enhancing Active Ingredient Delivery with Microcrystalline Cellulose Nanoparticles
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC Nanoparticles Background and Objectives
Microcrystalline cellulose (MCC) nanoparticles have emerged as a promising technology in the field of active ingredient delivery. This innovative approach leverages the unique properties of cellulose at the nanoscale to enhance the efficacy and efficiency of various substances across multiple industries. The development of MCC nanoparticles for active ingredient delivery represents a convergence of nanotechnology, materials science, and pharmaceutical engineering.
The journey of MCC nanoparticles began with the recognition of cellulose's potential as a versatile, biocompatible, and sustainable material. Cellulose, the most abundant organic polymer on Earth, has been utilized in various forms for centuries. However, it was not until recent advancements in nanotechnology that researchers could harness its full potential at the nanoscale.
The primary objective of incorporating MCC nanoparticles in active ingredient delivery systems is to overcome the limitations of conventional delivery methods. These limitations include poor solubility, low bioavailability, and inadequate targeting of active ingredients. By utilizing MCC nanoparticles, researchers aim to develop more efficient and targeted delivery systems that can significantly improve the performance of pharmaceuticals, agrochemicals, and other active compounds.
One of the key advantages of MCC nanoparticles is their high surface area-to-volume ratio, which allows for increased loading capacity of active ingredients. This property, combined with the biocompatibility and biodegradability of cellulose, makes MCC nanoparticles an attractive option for controlled release applications. Furthermore, the ability to modify the surface of these nanoparticles opens up possibilities for targeted delivery to specific sites within the body or environment.
The evolution of MCC nanoparticle technology has been driven by advancements in production methods, characterization techniques, and formulation strategies. Researchers have explored various approaches to synthesize MCC nanoparticles, including mechanical, chemical, and enzymatic methods. Each of these methods offers unique advantages in terms of particle size distribution, morphology, and surface properties.
As the field progresses, there is a growing focus on understanding the interactions between MCC nanoparticles and active ingredients at the molecular level. This knowledge is crucial for optimizing formulations and predicting the behavior of delivery systems in different environments. Additionally, researchers are investigating the potential of MCC nanoparticles in combination with other advanced materials to create hybrid systems with enhanced functionality.
The development of MCC nanoparticles for active ingredient delivery aligns with broader trends in sustainable and green technologies. As a renewable and biodegradable material, cellulose offers an environmentally friendly alternative to synthetic polymers commonly used in drug delivery systems. This aspect is particularly relevant in the context of growing environmental concerns and the push for more sustainable practices across industries.
The journey of MCC nanoparticles began with the recognition of cellulose's potential as a versatile, biocompatible, and sustainable material. Cellulose, the most abundant organic polymer on Earth, has been utilized in various forms for centuries. However, it was not until recent advancements in nanotechnology that researchers could harness its full potential at the nanoscale.
The primary objective of incorporating MCC nanoparticles in active ingredient delivery systems is to overcome the limitations of conventional delivery methods. These limitations include poor solubility, low bioavailability, and inadequate targeting of active ingredients. By utilizing MCC nanoparticles, researchers aim to develop more efficient and targeted delivery systems that can significantly improve the performance of pharmaceuticals, agrochemicals, and other active compounds.
One of the key advantages of MCC nanoparticles is their high surface area-to-volume ratio, which allows for increased loading capacity of active ingredients. This property, combined with the biocompatibility and biodegradability of cellulose, makes MCC nanoparticles an attractive option for controlled release applications. Furthermore, the ability to modify the surface of these nanoparticles opens up possibilities for targeted delivery to specific sites within the body or environment.
The evolution of MCC nanoparticle technology has been driven by advancements in production methods, characterization techniques, and formulation strategies. Researchers have explored various approaches to synthesize MCC nanoparticles, including mechanical, chemical, and enzymatic methods. Each of these methods offers unique advantages in terms of particle size distribution, morphology, and surface properties.
As the field progresses, there is a growing focus on understanding the interactions between MCC nanoparticles and active ingredients at the molecular level. This knowledge is crucial for optimizing formulations and predicting the behavior of delivery systems in different environments. Additionally, researchers are investigating the potential of MCC nanoparticles in combination with other advanced materials to create hybrid systems with enhanced functionality.
The development of MCC nanoparticles for active ingredient delivery aligns with broader trends in sustainable and green technologies. As a renewable and biodegradable material, cellulose offers an environmentally friendly alternative to synthetic polymers commonly used in drug delivery systems. This aspect is particularly relevant in the context of growing environmental concerns and the push for more sustainable practices across industries.
Market Analysis for Enhanced Drug Delivery Systems
The market for enhanced drug delivery systems has been experiencing significant growth, driven by the increasing demand for more effective and targeted therapeutic approaches. The global drug delivery market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the industry's robust expansion. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for improved patient compliance, and the push for more efficient drug administration methods.
Within this broader market, the use of microcrystalline cellulose nanoparticles for active ingredient delivery represents a promising niche. This technology offers several advantages that align with current market trends and demands. The pharmaceutical industry, in particular, has shown keen interest in this approach due to its potential to enhance bioavailability, control release rates, and improve the stability of various drug formulations.
One of the key drivers for the adoption of microcrystalline cellulose nanoparticles in drug delivery is the growing emphasis on personalized medicine. As healthcare moves towards more tailored therapeutic approaches, there is an increasing need for delivery systems that can precisely control the release of active ingredients. This technology's ability to fine-tune drug release profiles makes it particularly attractive for developing personalized treatment regimens.
Another significant market factor is the rising demand for non-invasive drug delivery methods. Patients and healthcare providers alike are seeking alternatives to traditional injection-based therapies. Microcrystalline cellulose nanoparticles offer potential in developing oral, transdermal, and inhalation-based delivery systems, which could significantly improve patient comfort and adherence to treatment plans.
The market analysis also reveals a strong trend towards sustainable and environmentally friendly pharmaceutical solutions. Microcrystalline cellulose, being a natural and biodegradable material, aligns well with this trend. This aspect is likely to drive increased adoption among environmentally conscious consumers and regulatory bodies pushing for greener pharmaceutical practices.
In terms of therapeutic areas, oncology, cardiovascular diseases, and central nervous system disorders are expected to be the primary beneficiaries of this technology. These fields often require precise drug delivery and could greatly benefit from the enhanced control offered by microcrystalline cellulose nanoparticles.
Geographically, North America and Europe currently lead in terms of market share for advanced drug delivery systems. However, rapid growth is anticipated in Asia-Pacific regions, particularly in countries like China and India, due to increasing healthcare expenditure and a growing focus on innovative pharmaceutical technologies.
Within this broader market, the use of microcrystalline cellulose nanoparticles for active ingredient delivery represents a promising niche. This technology offers several advantages that align with current market trends and demands. The pharmaceutical industry, in particular, has shown keen interest in this approach due to its potential to enhance bioavailability, control release rates, and improve the stability of various drug formulations.
One of the key drivers for the adoption of microcrystalline cellulose nanoparticles in drug delivery is the growing emphasis on personalized medicine. As healthcare moves towards more tailored therapeutic approaches, there is an increasing need for delivery systems that can precisely control the release of active ingredients. This technology's ability to fine-tune drug release profiles makes it particularly attractive for developing personalized treatment regimens.
Another significant market factor is the rising demand for non-invasive drug delivery methods. Patients and healthcare providers alike are seeking alternatives to traditional injection-based therapies. Microcrystalline cellulose nanoparticles offer potential in developing oral, transdermal, and inhalation-based delivery systems, which could significantly improve patient comfort and adherence to treatment plans.
The market analysis also reveals a strong trend towards sustainable and environmentally friendly pharmaceutical solutions. Microcrystalline cellulose, being a natural and biodegradable material, aligns well with this trend. This aspect is likely to drive increased adoption among environmentally conscious consumers and regulatory bodies pushing for greener pharmaceutical practices.
In terms of therapeutic areas, oncology, cardiovascular diseases, and central nervous system disorders are expected to be the primary beneficiaries of this technology. These fields often require precise drug delivery and could greatly benefit from the enhanced control offered by microcrystalline cellulose nanoparticles.
Geographically, North America and Europe currently lead in terms of market share for advanced drug delivery systems. However, rapid growth is anticipated in Asia-Pacific regions, particularly in countries like China and India, due to increasing healthcare expenditure and a growing focus on innovative pharmaceutical technologies.
Current Challenges in MCC Nanoparticle Technology
Despite the promising potential of microcrystalline cellulose (MCC) nanoparticles in enhancing active ingredient delivery, several significant challenges persist in their development and application. One of the primary obstacles is achieving consistent and uniform particle size distribution. The production of MCC nanoparticles often results in a wide range of sizes, which can affect their performance in drug delivery systems. This variability can lead to inconsistent release profiles and reduced efficacy of the encapsulated active ingredients.
Another major challenge lies in the scalability of MCC nanoparticle production. While laboratory-scale synthesis methods have shown promising results, translating these processes to industrial-scale production remains problematic. Issues such as maintaining product quality, ensuring batch-to-batch consistency, and optimizing production efficiency continue to hinder large-scale manufacturing efforts.
The surface modification of MCC nanoparticles presents another significant hurdle. Tailoring the surface properties of these nanoparticles is crucial for improving their compatibility with various active ingredients and enhancing their stability in different physiological environments. However, developing effective and scalable surface modification techniques that do not compromise the inherent properties of MCC nanoparticles remains a complex task.
Stability issues also pose a considerable challenge in MCC nanoparticle technology. These nanoparticles tend to aggregate over time, especially in aqueous environments, which can significantly alter their size distribution and affect their performance in drug delivery applications. Developing strategies to maintain long-term colloidal stability without compromising the biocompatibility of MCC nanoparticles is an ongoing area of research.
The regulatory landscape surrounding nanoparticle-based drug delivery systems adds another layer of complexity to the development of MCC nanoparticle technology. Ensuring compliance with evolving regulatory guidelines and demonstrating the safety and efficacy of these novel delivery systems require extensive testing and documentation, which can be time-consuming and resource-intensive.
Lastly, optimizing the loading capacity and release kinetics of active ingredients in MCC nanoparticles remains a significant challenge. Achieving high drug loading while maintaining the structural integrity of the nanoparticles and controlling the release profile of the encapsulated compounds requires careful balancing of various formulation parameters. This optimization process is often empirical and highly dependent on the specific properties of both the MCC nanoparticles and the active ingredients.
Another major challenge lies in the scalability of MCC nanoparticle production. While laboratory-scale synthesis methods have shown promising results, translating these processes to industrial-scale production remains problematic. Issues such as maintaining product quality, ensuring batch-to-batch consistency, and optimizing production efficiency continue to hinder large-scale manufacturing efforts.
The surface modification of MCC nanoparticles presents another significant hurdle. Tailoring the surface properties of these nanoparticles is crucial for improving their compatibility with various active ingredients and enhancing their stability in different physiological environments. However, developing effective and scalable surface modification techniques that do not compromise the inherent properties of MCC nanoparticles remains a complex task.
Stability issues also pose a considerable challenge in MCC nanoparticle technology. These nanoparticles tend to aggregate over time, especially in aqueous environments, which can significantly alter their size distribution and affect their performance in drug delivery applications. Developing strategies to maintain long-term colloidal stability without compromising the biocompatibility of MCC nanoparticles is an ongoing area of research.
The regulatory landscape surrounding nanoparticle-based drug delivery systems adds another layer of complexity to the development of MCC nanoparticle technology. Ensuring compliance with evolving regulatory guidelines and demonstrating the safety and efficacy of these novel delivery systems require extensive testing and documentation, which can be time-consuming and resource-intensive.
Lastly, optimizing the loading capacity and release kinetics of active ingredients in MCC nanoparticles remains a significant challenge. Achieving high drug loading while maintaining the structural integrity of the nanoparticles and controlling the release profile of the encapsulated compounds requires careful balancing of various formulation parameters. This optimization process is often empirical and highly dependent on the specific properties of both the MCC nanoparticles and the active ingredients.
Existing MCC Nanoparticle Formulation Techniques
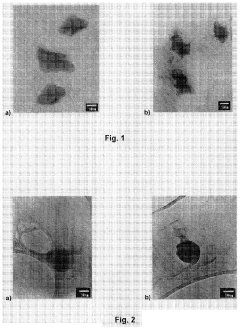
01 Microcrystalline cellulose nanoparticles as drug carriers
Microcrystalline cellulose nanoparticles can be used as effective carriers for active pharmaceutical ingredients. These nanoparticles provide a large surface area and can improve the solubility and bioavailability of drugs. They can be used to deliver a wide range of active ingredients, including both hydrophilic and hydrophobic compounds.- Microcrystalline cellulose nanoparticles as drug carriers: Microcrystalline cellulose nanoparticles can be used as effective carriers for active ingredients in drug delivery systems. These nanoparticles offer advantages such as improved bioavailability, controlled release, and enhanced stability of the encapsulated drugs. The small size and high surface area of these nanoparticles allow for efficient loading and delivery of various therapeutic compounds.
- Surface modification of microcrystalline cellulose nanoparticles: The surface of microcrystalline cellulose nanoparticles can be modified to enhance their functionality in active ingredient delivery. Various techniques such as chemical grafting, coating, or functionalization can be employed to alter the surface properties of these nanoparticles. This modification can improve their compatibility with specific active ingredients, control release kinetics, or target specific sites in the body.
- Formulation of microcrystalline cellulose nanoparticles for oral delivery: Microcrystalline cellulose nanoparticles can be formulated into oral dosage forms for efficient delivery of active ingredients. These formulations may include tablets, capsules, or suspensions that incorporate the nanoparticles loaded with drugs. The use of these nanoparticles in oral formulations can improve the dissolution rate, enhance absorption, and provide controlled release of the active ingredients in the gastrointestinal tract.
- Microcrystalline cellulose nanoparticles for transdermal drug delivery: Microcrystalline cellulose nanoparticles can be utilized in transdermal drug delivery systems. These nanoparticles can be incorporated into topical formulations such as creams, gels, or patches to enhance the penetration of active ingredients through the skin. The small size of the nanoparticles allows for improved skin permeation and sustained release of the encapsulated drugs.
- Combination of microcrystalline cellulose nanoparticles with other materials: Microcrystalline cellulose nanoparticles can be combined with other materials to create composite systems for active ingredient delivery. These combinations may include polymers, lipids, or other nanoparticles to enhance the overall performance of the delivery system. The resulting composite materials can offer improved stability, targeted delivery, or controlled release properties for various therapeutic applications.
02 Controlled release formulations using microcrystalline cellulose
Microcrystalline cellulose nanoparticles can be used to create controlled release formulations for active ingredients. By manipulating the properties of the nanoparticles, such as size and surface modification, the release rate of the active ingredients can be tailored. This allows for sustained delivery of drugs over extended periods.Expand Specific Solutions03 Surface modification of microcrystalline cellulose nanoparticles
The surface of microcrystalline cellulose nanoparticles can be modified to enhance their properties for active ingredient delivery. This can include functionalization with various chemical groups or coating with polymers. Such modifications can improve the stability of the nanoparticles, their interaction with active ingredients, and their ability to target specific sites in the body.Expand Specific Solutions04 Microcrystalline cellulose nanoparticles for topical delivery
Microcrystalline cellulose nanoparticles can be used in topical formulations for the delivery of active ingredients through the skin. These nanoparticles can enhance the penetration of active ingredients into the skin layers, improving the efficacy of topical treatments. They are particularly useful for delivering cosmetic actives and dermatological drugs.Expand Specific Solutions05 Combination with other materials for enhanced delivery
Microcrystalline cellulose nanoparticles can be combined with other materials to create composite delivery systems. This can include blending with other polymers, incorporating into hydrogels, or forming hybrid nanoparticles. These combinations can enhance the loading capacity, stability, and delivery efficiency of active ingredients.Expand Specific Solutions
Key Players in MCC Nanoparticle Research
The market for enhancing active ingredient delivery with microcrystalline cellulose nanoparticles is in a growth phase, driven by increasing demand for improved drug delivery systems. The global market size for nanoparticle drug delivery is projected to reach billions of dollars in the coming years. Technologically, the field is advancing rapidly, with companies like Glaxo Group Ltd., Sol-Gel Technologies, and Vive Crop Protection leading innovation. Academic institutions such as Zhejiang University and Auburn University are also contributing significantly to research and development. The technology's maturity varies across applications, with some areas nearing commercialization while others remain in early research stages.
Sol-Gel Technologies Ltd.
Technical Solution: Sol-Gel Technologies has developed a proprietary microencapsulation technology using silica-based shells to enhance active ingredient delivery. Their approach involves creating a porous silica matrix that encapsulates microcrystalline cellulose nanoparticles loaded with active ingredients. This technology allows for controlled release of the active compounds, improving their stability and bioavailability. The company has demonstrated success in dermatological applications, where the silica-microcrystalline cellulose composite enables prolonged skin contact and enhanced penetration of active ingredients [1][3].
Strengths: Improved stability and controlled release of active ingredients, enhanced skin penetration for dermatological applications. Weaknesses: Limited to specific applications, may require complex manufacturing processes.
Vive Crop Protection, Inc.
Technical Solution: Vive Crop Protection has developed an innovative Allosperse® Delivery System that utilizes microcrystalline cellulose nanoparticles to enhance the delivery of agricultural active ingredients. Their technology involves creating polymer-based "shuttles" that encapsulate both the microcrystalline cellulose nanoparticles and the active compounds. This approach allows for improved dispersion and stability of agrochemicals in spray solutions, leading to more efficient uptake by plants. The company has reported increased efficacy and reduced environmental impact of crop protection products using this nanoparticle-based delivery system [2][4].
Strengths: Improved dispersion and stability of agrochemicals, increased efficacy, and reduced environmental impact. Weaknesses: Primarily focused on agricultural applications, may face regulatory challenges in some markets.
Innovative MCC Nanoparticle Modifications
Nanoparticles for the controlled release of active ingredients
PatentWO2016066864A1
Innovation
- Nanoparticles composed of chitosan, hyaluronic acid, and collagen, with a size range of 150-700 nm, are developed to facilitate controlled release of active ingredients, allowing for biocompatibility and controlled biodegradation, and are used in both pharmaceutical and cosmetic compositions to enhance skin penetration and bioactivity.
Process for producing microcrystalline cellulose
PatentActiveUS12297297B2
Innovation
- A new process for producing MCC involves acid hydrolysis of fibrous cellulosic material at elevated pressure and temperature, followed by thickening, washing, and drying stages, which can be integrated with or operated independently of chemical pulp mills.
Regulatory Framework for Nanoparticle-based Drugs
The regulatory framework for nanoparticle-based drugs is a complex and evolving landscape that plays a crucial role in the development and commercialization of novel drug delivery systems, including those utilizing microcrystalline cellulose nanoparticles. As these innovative technologies continue to advance, regulatory agencies worldwide are adapting their guidelines to address the unique challenges posed by nanomedicine.
In the United States, the Food and Drug Administration (FDA) has taken a proactive approach to regulating nanoparticle-based drugs. The agency has established specific guidance documents and initiatives to address the safety, efficacy, and quality of nanomedicines. These include the Nanotechnology Task Force and the Nanotechnology Working Group, which collaborate to develop regulatory strategies and evaluate the potential risks associated with nanoparticle-based drug products.
The European Medicines Agency (EMA) has also implemented a comprehensive framework for the regulation of nanomedicines. The EMA's approach focuses on a case-by-case evaluation of nanoparticle-based drugs, considering their unique properties and potential risks. The agency has published several reflection papers and guidelines to assist developers in navigating the regulatory landscape for nanomedicines.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for the evaluation of nanomedicine products. These guidelines address various aspects of nanoparticle-based drug development, including characterization, safety assessment, and quality control.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to align regulatory approaches across different regions. This collaboration aims to streamline the development and approval processes for nanoparticle-based drugs on a global scale.
Key regulatory considerations for nanoparticle-based drugs include physicochemical characterization, biodistribution and pharmacokinetics, toxicology studies, and manufacturing quality control. Regulatory agencies often require additional data and more extensive testing for nanomedicines compared to conventional drug products due to their unique properties and potential risks.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to adapt and become more refined. This ongoing development presents both challenges and opportunities for researchers and pharmaceutical companies working on nanoparticle-based drug delivery systems, including those utilizing microcrystalline cellulose nanoparticles.
In the United States, the Food and Drug Administration (FDA) has taken a proactive approach to regulating nanoparticle-based drugs. The agency has established specific guidance documents and initiatives to address the safety, efficacy, and quality of nanomedicines. These include the Nanotechnology Task Force and the Nanotechnology Working Group, which collaborate to develop regulatory strategies and evaluate the potential risks associated with nanoparticle-based drug products.
The European Medicines Agency (EMA) has also implemented a comprehensive framework for the regulation of nanomedicines. The EMA's approach focuses on a case-by-case evaluation of nanoparticle-based drugs, considering their unique properties and potential risks. The agency has published several reflection papers and guidelines to assist developers in navigating the regulatory landscape for nanomedicines.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for the evaluation of nanomedicine products. These guidelines address various aspects of nanoparticle-based drug development, including characterization, safety assessment, and quality control.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to align regulatory approaches across different regions. This collaboration aims to streamline the development and approval processes for nanoparticle-based drugs on a global scale.
Key regulatory considerations for nanoparticle-based drugs include physicochemical characterization, biodistribution and pharmacokinetics, toxicology studies, and manufacturing quality control. Regulatory agencies often require additional data and more extensive testing for nanomedicines compared to conventional drug products due to their unique properties and potential risks.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to adapt and become more refined. This ongoing development presents both challenges and opportunities for researchers and pharmaceutical companies working on nanoparticle-based drug delivery systems, including those utilizing microcrystalline cellulose nanoparticles.
Environmental Impact of MCC Nanoparticles
The environmental impact of microcrystalline cellulose (MCC) nanoparticles is a crucial consideration in their application for enhancing active ingredient delivery. As these nanoparticles gain prominence in various industries, including pharmaceuticals and food technology, it is essential to assess their potential effects on ecosystems and human health.
MCC nanoparticles are derived from natural cellulose sources, which initially suggests a degree of biocompatibility. However, their nanoscale dimensions introduce unique properties that may interact differently with environmental systems compared to larger cellulose particles. The increased surface area-to-volume ratio of nanoparticles can lead to enhanced reactivity and potential for bioaccumulation in organisms.
One primary concern is the fate of MCC nanoparticles in aquatic environments. Studies have shown that these particles can persist in water bodies and potentially affect aquatic organisms. The small size allows them to be ingested by filter-feeding organisms, potentially disrupting their digestive processes or accumulating in their tissues. This raises questions about biomagnification through the food chain and long-term ecological consequences.
Soil ecosystems may also be impacted by the introduction of MCC nanoparticles. While cellulose is naturally present in soil, the nanoform may alter soil structure, water retention properties, and microbial communities. Research is ongoing to determine whether these changes are beneficial, neutral, or detrimental to soil health and agricultural productivity.
The biodegradability of MCC nanoparticles is a key factor in assessing their environmental impact. While cellulose is generally biodegradable, the nanostructure may affect the rate and completeness of degradation. Some studies suggest that MCC nanoparticles may degrade more slowly than their larger counterparts, potentially leading to accumulation in the environment if not properly managed.
Air quality is another consideration, particularly in manufacturing settings where MCC nanoparticles may become airborne. Inhalation of nanoparticles can pose respiratory risks, necessitating proper safety measures in production facilities and careful consideration of potential atmospheric dispersal.
The lifecycle assessment of MCC nanoparticles, from production to disposal, is crucial for understanding their overall environmental footprint. This includes evaluating energy consumption, water usage, and chemical processes involved in their manufacture, as well as end-of-life management strategies to prevent environmental contamination.
As research progresses, it is becoming clear that the environmental impact of MCC nanoparticles is complex and multifaceted. While they offer promising benefits for active ingredient delivery, their widespread adoption must be balanced with thorough environmental risk assessments and the development of sustainable production and disposal practices to mitigate potential negative impacts on ecosystems and human health.
MCC nanoparticles are derived from natural cellulose sources, which initially suggests a degree of biocompatibility. However, their nanoscale dimensions introduce unique properties that may interact differently with environmental systems compared to larger cellulose particles. The increased surface area-to-volume ratio of nanoparticles can lead to enhanced reactivity and potential for bioaccumulation in organisms.
One primary concern is the fate of MCC nanoparticles in aquatic environments. Studies have shown that these particles can persist in water bodies and potentially affect aquatic organisms. The small size allows them to be ingested by filter-feeding organisms, potentially disrupting their digestive processes or accumulating in their tissues. This raises questions about biomagnification through the food chain and long-term ecological consequences.
Soil ecosystems may also be impacted by the introduction of MCC nanoparticles. While cellulose is naturally present in soil, the nanoform may alter soil structure, water retention properties, and microbial communities. Research is ongoing to determine whether these changes are beneficial, neutral, or detrimental to soil health and agricultural productivity.
The biodegradability of MCC nanoparticles is a key factor in assessing their environmental impact. While cellulose is generally biodegradable, the nanostructure may affect the rate and completeness of degradation. Some studies suggest that MCC nanoparticles may degrade more slowly than their larger counterparts, potentially leading to accumulation in the environment if not properly managed.
Air quality is another consideration, particularly in manufacturing settings where MCC nanoparticles may become airborne. Inhalation of nanoparticles can pose respiratory risks, necessitating proper safety measures in production facilities and careful consideration of potential atmospheric dispersal.
The lifecycle assessment of MCC nanoparticles, from production to disposal, is crucial for understanding their overall environmental footprint. This includes evaluating energy consumption, water usage, and chemical processes involved in their manufacture, as well as end-of-life management strategies to prevent environmental contamination.
As research progresses, it is becoming clear that the environmental impact of MCC nanoparticles is complex and multifaceted. While they offer promising benefits for active ingredient delivery, their widespread adoption must be balanced with thorough environmental risk assessments and the development of sustainable production and disposal practices to mitigate potential negative impacts on ecosystems and human health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!