How Effective Is Nitrous Acid in Advanced Oxidation Processes?
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
AOP Background and Objectives
Advanced Oxidation Processes (AOPs) have emerged as a promising technology for water and wastewater treatment, addressing the growing concern over persistent organic pollutants and emerging contaminants. These processes rely on the generation of highly reactive species, particularly hydroxyl radicals, to degrade recalcitrant organic compounds into less harmful or completely mineralized products.
The development of AOPs can be traced back to the 1970s, with significant advancements occurring in the 1990s and early 2000s. The primary objective of AOPs is to achieve rapid and efficient degradation of organic pollutants that are resistant to conventional treatment methods. This technology has gained considerable attention due to its versatility and effectiveness in treating a wide range of contaminants, including pharmaceuticals, personal care products, pesticides, and industrial chemicals.
In recent years, researchers have been exploring various AOP configurations and combinations to enhance treatment efficiency and reduce operational costs. One such area of interest is the use of nitrous acid in AOPs. Nitrous acid (HNO2) is a weak acid that can participate in redox reactions and potentially contribute to the oxidation of organic pollutants.
The effectiveness of nitrous acid in AOPs is a subject of ongoing research and debate. While traditional AOPs primarily rely on hydroxyl radicals, the incorporation of nitrous acid introduces additional reactive species and pathways for contaminant degradation. The potential advantages of using nitrous acid in AOPs include enhanced oxidation rates, improved selectivity for certain pollutants, and the possibility of synergistic effects when combined with other oxidants.
However, the efficacy of nitrous acid in AOPs is influenced by various factors, including pH, temperature, and the presence of other chemical species. Understanding these parameters and their impact on the overall treatment process is crucial for optimizing the use of nitrous acid in AOPs. Additionally, the potential formation of byproducts and the environmental implications of using nitrous acid must be carefully evaluated.
The objectives of investigating the effectiveness of nitrous acid in AOPs are multifaceted. Researchers aim to elucidate the reaction mechanisms involved, quantify the contribution of nitrous acid to the overall oxidation process, and identify the optimal conditions for its application. Furthermore, there is a need to assess the economic feasibility and environmental sustainability of incorporating nitrous acid into existing AOP systems.
As water scarcity and pollution continue to pose significant challenges globally, the development of efficient and cost-effective treatment technologies remains a priority. The exploration of nitrous acid's role in AOPs represents an important step towards expanding the toolbox of available treatment options and improving the overall effectiveness of water and wastewater treatment processes.
The development of AOPs can be traced back to the 1970s, with significant advancements occurring in the 1990s and early 2000s. The primary objective of AOPs is to achieve rapid and efficient degradation of organic pollutants that are resistant to conventional treatment methods. This technology has gained considerable attention due to its versatility and effectiveness in treating a wide range of contaminants, including pharmaceuticals, personal care products, pesticides, and industrial chemicals.
In recent years, researchers have been exploring various AOP configurations and combinations to enhance treatment efficiency and reduce operational costs. One such area of interest is the use of nitrous acid in AOPs. Nitrous acid (HNO2) is a weak acid that can participate in redox reactions and potentially contribute to the oxidation of organic pollutants.
The effectiveness of nitrous acid in AOPs is a subject of ongoing research and debate. While traditional AOPs primarily rely on hydroxyl radicals, the incorporation of nitrous acid introduces additional reactive species and pathways for contaminant degradation. The potential advantages of using nitrous acid in AOPs include enhanced oxidation rates, improved selectivity for certain pollutants, and the possibility of synergistic effects when combined with other oxidants.
However, the efficacy of nitrous acid in AOPs is influenced by various factors, including pH, temperature, and the presence of other chemical species. Understanding these parameters and their impact on the overall treatment process is crucial for optimizing the use of nitrous acid in AOPs. Additionally, the potential formation of byproducts and the environmental implications of using nitrous acid must be carefully evaluated.
The objectives of investigating the effectiveness of nitrous acid in AOPs are multifaceted. Researchers aim to elucidate the reaction mechanisms involved, quantify the contribution of nitrous acid to the overall oxidation process, and identify the optimal conditions for its application. Furthermore, there is a need to assess the economic feasibility and environmental sustainability of incorporating nitrous acid into existing AOP systems.
As water scarcity and pollution continue to pose significant challenges globally, the development of efficient and cost-effective treatment technologies remains a priority. The exploration of nitrous acid's role in AOPs represents an important step towards expanding the toolbox of available treatment options and improving the overall effectiveness of water and wastewater treatment processes.
Market Analysis for AOP Technologies
The Advanced Oxidation Processes (AOP) market has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations regarding water and air pollution. The global AOP market was valued at approximately $5.8 billion in 2020 and is projected to reach $9.2 billion by 2026, growing at a CAGR of 7.8% during the forecast period.
The market for AOP technologies is primarily segmented into water treatment, air purification, and soil remediation applications. Among these, water treatment holds the largest market share, accounting for over 60% of the total market revenue. This dominance is attributed to the growing demand for clean water in industrial processes, municipal water treatment, and the increasing need for wastewater recycling.
Geographically, North America and Europe are the leading markets for AOP technologies, owing to strict environmental regulations and high adoption rates of advanced water treatment solutions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in water infrastructure.
Key market players in the AOP industry include Xylem Inc., Suez SA, Evoqua Water Technologies LLC, and Calgon Carbon Corporation. These companies are focusing on research and development activities to enhance the efficiency of their AOP solutions and expand their product portfolios.
The effectiveness of nitrous acid in AOP has garnered increasing attention from both researchers and industry players. While traditional AOP methods like UV/H2O2 and ozonation remain popular, nitrous acid-based processes are emerging as a promising alternative due to their potential for higher efficiency and lower operational costs.
Market analysis indicates that nitrous acid-based AOP technologies could potentially capture a significant market share in the coming years, especially in industrial wastewater treatment applications. This is due to their ability to effectively degrade recalcitrant organic compounds and their compatibility with existing treatment infrastructure.
However, challenges such as the need for precise pH control and potential formation of harmful byproducts need to be addressed for widespread adoption. As research progresses and these challenges are overcome, nitrous acid-based AOP technologies are expected to play an increasingly important role in the overall AOP market landscape.
The market for AOP technologies is primarily segmented into water treatment, air purification, and soil remediation applications. Among these, water treatment holds the largest market share, accounting for over 60% of the total market revenue. This dominance is attributed to the growing demand for clean water in industrial processes, municipal water treatment, and the increasing need for wastewater recycling.
Geographically, North America and Europe are the leading markets for AOP technologies, owing to strict environmental regulations and high adoption rates of advanced water treatment solutions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in water infrastructure.
Key market players in the AOP industry include Xylem Inc., Suez SA, Evoqua Water Technologies LLC, and Calgon Carbon Corporation. These companies are focusing on research and development activities to enhance the efficiency of their AOP solutions and expand their product portfolios.
The effectiveness of nitrous acid in AOP has garnered increasing attention from both researchers and industry players. While traditional AOP methods like UV/H2O2 and ozonation remain popular, nitrous acid-based processes are emerging as a promising alternative due to their potential for higher efficiency and lower operational costs.
Market analysis indicates that nitrous acid-based AOP technologies could potentially capture a significant market share in the coming years, especially in industrial wastewater treatment applications. This is due to their ability to effectively degrade recalcitrant organic compounds and their compatibility with existing treatment infrastructure.
However, challenges such as the need for precise pH control and potential formation of harmful byproducts need to be addressed for widespread adoption. As research progresses and these challenges are overcome, nitrous acid-based AOP technologies are expected to play an increasingly important role in the overall AOP market landscape.
Nitrous Acid in AOP: Current Status
Nitrous acid (HNO2) has emerged as a promising agent in Advanced Oxidation Processes (AOPs) for water and wastewater treatment. The current status of nitrous acid in AOPs reflects a growing interest in its potential to enhance the efficiency of pollutant degradation and improve overall treatment outcomes.
Recent studies have demonstrated that nitrous acid can act as both an oxidant and a catalyst in various AOP systems. Its ability to generate reactive nitrogen species (RNS) and contribute to the formation of hydroxyl radicals (•OH) has been particularly noteworthy. These reactive species play a crucial role in the degradation of recalcitrant organic pollutants, making nitrous acid an attractive option for addressing challenging water treatment scenarios.
One of the key advantages of nitrous acid in AOPs is its versatility. It can be effectively combined with other oxidation techniques, such as UV irradiation, ozonation, and Fenton processes, to create synergistic effects. For instance, the UV/HNO2 system has shown promising results in the degradation of pharmaceuticals and personal care products (PPCPs) in water, achieving higher removal rates compared to conventional UV treatment alone.
The effectiveness of nitrous acid in AOPs is also attributed to its ability to operate under a wide range of pH conditions. This characteristic allows for greater flexibility in treatment design and implementation, making it suitable for various water matrices and contaminant profiles. Furthermore, the relatively low cost and ease of handling associated with nitrous acid make it an attractive option for large-scale water treatment applications.
However, the current status of nitrous acid in AOPs also reveals certain challenges and limitations. The formation of potentially harmful byproducts, such as nitrosamines, during the treatment process is a concern that requires careful monitoring and control. Additionally, the effectiveness of nitrous acid-based AOPs can be influenced by the presence of scavengers in the water matrix, which may reduce the overall efficiency of the treatment process.
Ongoing research is focused on optimizing the use of nitrous acid in AOPs, with particular emphasis on understanding the reaction mechanisms, improving the selectivity of pollutant degradation, and minimizing the formation of undesirable byproducts. The development of novel catalysts and hybrid systems incorporating nitrous acid is also an active area of investigation, aiming to enhance the overall performance and applicability of these treatment processes.
In conclusion, the current status of nitrous acid in AOPs reflects a promising and evolving field with significant potential for water and wastewater treatment applications. While challenges remain, the unique properties and versatility of nitrous acid continue to drive research and innovation in this area, paving the way for more efficient and sustainable water treatment solutions.
Recent studies have demonstrated that nitrous acid can act as both an oxidant and a catalyst in various AOP systems. Its ability to generate reactive nitrogen species (RNS) and contribute to the formation of hydroxyl radicals (•OH) has been particularly noteworthy. These reactive species play a crucial role in the degradation of recalcitrant organic pollutants, making nitrous acid an attractive option for addressing challenging water treatment scenarios.
One of the key advantages of nitrous acid in AOPs is its versatility. It can be effectively combined with other oxidation techniques, such as UV irradiation, ozonation, and Fenton processes, to create synergistic effects. For instance, the UV/HNO2 system has shown promising results in the degradation of pharmaceuticals and personal care products (PPCPs) in water, achieving higher removal rates compared to conventional UV treatment alone.
The effectiveness of nitrous acid in AOPs is also attributed to its ability to operate under a wide range of pH conditions. This characteristic allows for greater flexibility in treatment design and implementation, making it suitable for various water matrices and contaminant profiles. Furthermore, the relatively low cost and ease of handling associated with nitrous acid make it an attractive option for large-scale water treatment applications.
However, the current status of nitrous acid in AOPs also reveals certain challenges and limitations. The formation of potentially harmful byproducts, such as nitrosamines, during the treatment process is a concern that requires careful monitoring and control. Additionally, the effectiveness of nitrous acid-based AOPs can be influenced by the presence of scavengers in the water matrix, which may reduce the overall efficiency of the treatment process.
Ongoing research is focused on optimizing the use of nitrous acid in AOPs, with particular emphasis on understanding the reaction mechanisms, improving the selectivity of pollutant degradation, and minimizing the formation of undesirable byproducts. The development of novel catalysts and hybrid systems incorporating nitrous acid is also an active area of investigation, aiming to enhance the overall performance and applicability of these treatment processes.
In conclusion, the current status of nitrous acid in AOPs reflects a promising and evolving field with significant potential for water and wastewater treatment applications. While challenges remain, the unique properties and versatility of nitrous acid continue to drive research and innovation in this area, paving the way for more efficient and sustainable water treatment solutions.
Existing Nitrous Acid AOP Solutions
01 Nitrous acid in metal treatment
Nitrous acid is effective in metal surface treatment processes, particularly in etching and passivation of metals. It can be used to modify surface properties, improve corrosion resistance, and prepare metals for further processing or coating applications.- Nitrous acid in metal treatment: Nitrous acid is effective in metal surface treatment processes, particularly in etching and passivation of various metals and alloys. It can be used to modify surface properties, improve corrosion resistance, and prepare metals for further processing or coating applications.
- Nitrous acid in chemical synthesis: Nitrous acid serves as an effective reagent in various chemical synthesis processes. It is particularly useful in diazotization reactions, nitrosation of organic compounds, and as an intermediate in the production of other nitrogen-containing chemicals.
- Nitrous acid in water treatment: The effectiveness of nitrous acid in water treatment applications is notable. It can be used for disinfection, removal of certain contaminants, and in processes related to nitrogen cycle management in wastewater treatment systems.
- Nitrous acid in agricultural applications: Nitrous acid and its derivatives have shown effectiveness in agricultural applications. This includes soil treatment, plant growth regulation, and as a component in certain fertilizers or pesticides formulations.
- Nitrous acid in analytical chemistry: The effectiveness of nitrous acid in analytical chemistry is significant. It is used in various analytical methods and detection techniques, particularly for the analysis of nitrogen-containing compounds and in colorimetric assays.
02 Nitrous acid in chemical synthesis
Nitrous acid serves as an effective reagent in various chemical synthesis reactions. It is particularly useful in diazotization processes, nitrosation reactions, and the production of certain organic compounds. Its reactivity makes it valuable in pharmaceutical and fine chemical manufacturing.Expand Specific Solutions03 Environmental impact and treatment
The effectiveness of nitrous acid in environmental contexts is significant, particularly in atmospheric chemistry and water treatment. It plays a role in the formation of acid rain and can impact air quality. Methods for treating nitrous acid in industrial effluents and reducing its environmental impact are important areas of research.Expand Specific Solutions04 Analytical applications
Nitrous acid is effective in various analytical techniques and methodologies. It can be used as a reagent in spectrophotometric analysis, as part of detection systems for certain compounds, and in the development of sensors for environmental monitoring and industrial process control.Expand Specific Solutions05 Industrial process applications
The effectiveness of nitrous acid in industrial processes extends to areas such as textile dyeing, polymer modification, and certain cleaning applications. It can be used to modify material properties, enhance reactivity, or as part of complex industrial chemical processes.Expand Specific Solutions
Key Players in AOP Industry
The advanced oxidation process (AOP) utilizing nitrous acid is in an early development stage, with a growing market as environmental regulations tighten. The technology's effectiveness is still being researched, indicating low to moderate maturity. Key players like China Petroleum & Chemical Corp., Shell Oil Co., and Air Liquide SA are investing in AOP research, while universities such as Sun Yat-Sen University and Tohoku University are contributing to fundamental studies. The market size is expanding as industries seek more efficient water treatment solutions, but widespread adoption is limited by the need for further optimization and cost reduction.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced oxidation process utilizing nitrous acid for wastewater treatment. Their approach involves generating nitrous acid in-situ through the reaction of nitrite with sulfuric acid. This method has shown high efficiency in degrading recalcitrant organic pollutants in petrochemical wastewater[1]. The process operates at ambient temperature and pressure, making it cost-effective. Sinopec's research indicates that nitrous acid-based advanced oxidation can achieve up to 80% removal of chemical oxygen demand (COD) in complex industrial effluents[2]. The company has also explored combining nitrous acid with other oxidants like hydrogen peroxide to enhance treatment efficacy[3].
Strengths: Cost-effective, operates at ambient conditions, high removal efficiency for recalcitrant pollutants. Weaknesses: Potential for nitrate formation as a by-product, pH-dependent effectiveness, may require post-treatment for nitrate removal.
Shell Oil Co.
Technical Solution: Shell Oil Co. has investigated the use of nitrous acid in advanced oxidation processes for treating produced water from oil and gas operations. Their approach involves a two-step process: first, generating nitrous acid from nitrogen oxides present in flue gases, and then using this nitrous acid for oxidative treatment of organic contaminants[4]. Shell's research has demonstrated that this method can effectively remove up to 70% of dissolved organic carbon from produced water samples[5]. The company has also explored the synergistic effects of combining nitrous acid with UV irradiation, which has shown promise in enhancing the degradation of aromatic hydrocarbons commonly found in oil field wastewater[6].
Strengths: Utilizes waste gas streams, effective for oil and gas industry-specific contaminants, potential for integration with existing operations. Weaknesses: Variability in nitrous acid generation based on flue gas composition, potential corrosion issues, may require specialized materials for equipment.
Core Innovations in Nitrous Acid AOP
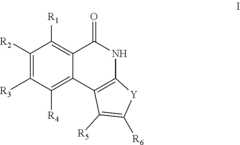
Thieno[2,3-c] isoquinolines for use as inhibitors of parp
PatentInactiveUS20110237617A1
Innovation
- Development of heterocyclic derivatives, specifically thieno[2,3-c]isoquinolin-3-one compounds, which act as non-toxic inhibitors of PARP, capable of forming salts with pharmaceutically acceptable acids and bases, and can be administered in various pharmaceutical forms to prevent and treat tissue damage, degenerative, inflammatory, and tumour diseases.
Environmental Impact Assessment
The environmental impact assessment of nitrous acid in advanced oxidation processes (AOPs) reveals both positive and negative implications for ecosystems and human health. On the positive side, nitrous acid-based AOPs demonstrate high efficiency in degrading recalcitrant organic pollutants, potentially reducing the overall environmental burden of these contaminants. This process can lead to improved water quality in treated effluents, benefiting aquatic ecosystems and reducing risks associated with human exposure to harmful chemicals.
However, the use of nitrous acid in AOPs also presents several environmental concerns. The production and handling of nitrous acid can result in atmospheric emissions of nitrogen oxides (NOx), which contribute to air pollution and the formation of acid rain. These emissions may have adverse effects on local air quality and can potentially impact vegetation and soil chemistry in surrounding areas.
Furthermore, the byproducts generated during the oxidation process require careful consideration. While many organic pollutants are effectively degraded, some intermediate compounds formed during treatment may possess toxicity or persistence that could pose risks to aquatic life or accumulate in the food chain. Comprehensive monitoring and analysis of these byproducts are essential to ensure that the overall environmental impact remains positive.
The energy consumption associated with nitrous acid-based AOPs is another factor to consider in the environmental assessment. The process may require significant energy inputs, potentially contributing to increased greenhouse gas emissions if the energy source is not renewable. This aspect underscores the importance of optimizing process efficiency and exploring integration with sustainable energy sources to minimize the carbon footprint of the treatment system.
Additionally, the potential for nitrate formation during the AOP process raises concerns about nutrient loading in receiving water bodies. Elevated nitrate levels can lead to eutrophication, algal blooms, and subsequent ecosystem imbalances. Proper management and monitoring of effluent quality are crucial to mitigate these risks and maintain the ecological integrity of aquatic environments.
In conclusion, while nitrous acid-based AOPs offer promising solutions for water treatment, their environmental impact is multifaceted. The technology's effectiveness in pollutant removal must be balanced against potential negative consequences such as air emissions, byproduct formation, energy consumption, and nutrient loading. Ongoing research and development efforts should focus on optimizing the process to maximize environmental benefits while minimizing adverse effects, ensuring a sustainable approach to water treatment technologies.
However, the use of nitrous acid in AOPs also presents several environmental concerns. The production and handling of nitrous acid can result in atmospheric emissions of nitrogen oxides (NOx), which contribute to air pollution and the formation of acid rain. These emissions may have adverse effects on local air quality and can potentially impact vegetation and soil chemistry in surrounding areas.
Furthermore, the byproducts generated during the oxidation process require careful consideration. While many organic pollutants are effectively degraded, some intermediate compounds formed during treatment may possess toxicity or persistence that could pose risks to aquatic life or accumulate in the food chain. Comprehensive monitoring and analysis of these byproducts are essential to ensure that the overall environmental impact remains positive.
The energy consumption associated with nitrous acid-based AOPs is another factor to consider in the environmental assessment. The process may require significant energy inputs, potentially contributing to increased greenhouse gas emissions if the energy source is not renewable. This aspect underscores the importance of optimizing process efficiency and exploring integration with sustainable energy sources to minimize the carbon footprint of the treatment system.
Additionally, the potential for nitrate formation during the AOP process raises concerns about nutrient loading in receiving water bodies. Elevated nitrate levels can lead to eutrophication, algal blooms, and subsequent ecosystem imbalances. Proper management and monitoring of effluent quality are crucial to mitigate these risks and maintain the ecological integrity of aquatic environments.
In conclusion, while nitrous acid-based AOPs offer promising solutions for water treatment, their environmental impact is multifaceted. The technology's effectiveness in pollutant removal must be balanced against potential negative consequences such as air emissions, byproduct formation, energy consumption, and nutrient loading. Ongoing research and development efforts should focus on optimizing the process to maximize environmental benefits while minimizing adverse effects, ensuring a sustainable approach to water treatment technologies.
Regulatory Framework for AOP Technologies
The regulatory framework for Advanced Oxidation Processes (AOPs) technologies, including those utilizing nitrous acid, is a complex and evolving landscape. Governments and environmental agencies worldwide have established guidelines and standards to ensure the safe and effective implementation of AOPs in water and wastewater treatment.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating AOP technologies. The EPA's Effluent Guidelines Program sets technology-based regulations for industrial discharges into surface waters and municipal sewage treatment plants. These guidelines often include specific requirements for the use of AOPs, including those involving nitrous acid, in treating industrial wastewater.
The European Union has implemented the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which provide a comprehensive framework for water quality management. These directives indirectly influence the adoption and regulation of AOP technologies by setting stringent water quality standards that often necessitate advanced treatment methods.
Many countries have adopted risk-based approaches to regulating AOPs, considering factors such as the formation of potentially harmful by-products, energy consumption, and overall treatment efficacy. For instance, Health Canada has developed guidelines for drinking water treatment that include considerations for AOPs, emphasizing the need for proper monitoring and control of treatment processes.
International organizations like the World Health Organization (WHO) provide guidance on water quality and treatment technologies, including AOPs. While not legally binding, these guidelines often inform national regulations and industry best practices.
Regulatory bodies typically require extensive testing and validation of AOP technologies before approval for large-scale implementation. This process often involves pilot studies, environmental impact assessments, and ongoing monitoring to ensure compliance with water quality standards and environmental protection goals.
As research continues to demonstrate the effectiveness of nitrous acid in AOPs, regulatory frameworks are likely to evolve. Policymakers and environmental agencies are increasingly recognizing the potential of AOPs to address emerging contaminants and complex water quality issues. This recognition may lead to more specific regulations and guidelines for the use of nitrous acid and other AOP technologies in water treatment processes.
However, the regulatory landscape remains fragmented, with significant variations between countries and regions. This diversity in regulations can pose challenges for technology developers and water treatment facilities seeking to implement AOP solutions across different jurisdictions. As a result, there is a growing call for harmonization of regulatory approaches to facilitate the adoption of effective and sustainable water treatment technologies.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating AOP technologies. The EPA's Effluent Guidelines Program sets technology-based regulations for industrial discharges into surface waters and municipal sewage treatment plants. These guidelines often include specific requirements for the use of AOPs, including those involving nitrous acid, in treating industrial wastewater.
The European Union has implemented the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which provide a comprehensive framework for water quality management. These directives indirectly influence the adoption and regulation of AOP technologies by setting stringent water quality standards that often necessitate advanced treatment methods.
Many countries have adopted risk-based approaches to regulating AOPs, considering factors such as the formation of potentially harmful by-products, energy consumption, and overall treatment efficacy. For instance, Health Canada has developed guidelines for drinking water treatment that include considerations for AOPs, emphasizing the need for proper monitoring and control of treatment processes.
International organizations like the World Health Organization (WHO) provide guidance on water quality and treatment technologies, including AOPs. While not legally binding, these guidelines often inform national regulations and industry best practices.
Regulatory bodies typically require extensive testing and validation of AOP technologies before approval for large-scale implementation. This process often involves pilot studies, environmental impact assessments, and ongoing monitoring to ensure compliance with water quality standards and environmental protection goals.
As research continues to demonstrate the effectiveness of nitrous acid in AOPs, regulatory frameworks are likely to evolve. Policymakers and environmental agencies are increasingly recognizing the potential of AOPs to address emerging contaminants and complex water quality issues. This recognition may lead to more specific regulations and guidelines for the use of nitrous acid and other AOP technologies in water treatment processes.
However, the regulatory landscape remains fragmented, with significant variations between countries and regions. This diversity in regulations can pose challenges for technology developers and water treatment facilities seeking to implement AOP solutions across different jurisdictions. As a result, there is a growing call for harmonization of regulatory approaches to facilitate the adoption of effective and sustainable water treatment technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!