How Nitrous Acid Shapes Health and Safety Regulations
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrous Acid Overview
Nitrous acid (HNO2) is a weak and unstable inorganic acid that plays a significant role in atmospheric chemistry and various industrial processes. This compound exists in both gaseous and aqueous forms, with the latter being more common due to its high solubility in water. Nitrous acid is formed through the reaction of nitrogen oxides with water or through the reduction of nitric acid.
In the atmosphere, nitrous acid is a crucial intermediate in the nitrogen cycle, contributing to the formation of photochemical smog and acid rain. It is primarily produced through heterogeneous reactions on surfaces, such as building materials and aerosols, and can significantly impact air quality in urban environments. The photolysis of nitrous acid is a major source of hydroxyl radicals, which are essential for the self-cleaning capacity of the atmosphere.
From a health perspective, exposure to nitrous acid can cause irritation to the eyes, skin, and respiratory system. Prolonged exposure may lead to more severe health effects, including lung damage and increased susceptibility to respiratory infections. The compound's ability to form nitrosamines, which are known carcinogens, further underscores its potential health risks.
In industrial settings, nitrous acid is utilized in various applications, including the production of diazo dyes, the synthesis of pharmaceuticals, and as a reducing agent in certain chemical processes. Its corrosive nature and potential to release toxic nitrogen oxides necessitate strict handling and storage protocols to ensure worker safety and environmental protection.
The environmental impact of nitrous acid extends beyond its direct effects on human health. It contributes to the acidification of soil and water bodies, potentially disrupting ecosystems and biodiversity. Additionally, its role in the formation of secondary organic aerosols influences climate change dynamics, as these aerosols can affect cloud formation and radiative forcing.
Given its multifaceted impacts on health and the environment, nitrous acid has become a focal point for regulatory bodies worldwide. Occupational exposure limits have been established to protect workers in industries where nitrous acid is present. Environmental agencies monitor ambient levels of nitrous acid and its precursors as part of air quality management strategies. The compound's involvement in complex atmospheric chemistry also makes it a subject of ongoing research, as scientists seek to better understand its role in pollution formation and climate processes.
In the atmosphere, nitrous acid is a crucial intermediate in the nitrogen cycle, contributing to the formation of photochemical smog and acid rain. It is primarily produced through heterogeneous reactions on surfaces, such as building materials and aerosols, and can significantly impact air quality in urban environments. The photolysis of nitrous acid is a major source of hydroxyl radicals, which are essential for the self-cleaning capacity of the atmosphere.
From a health perspective, exposure to nitrous acid can cause irritation to the eyes, skin, and respiratory system. Prolonged exposure may lead to more severe health effects, including lung damage and increased susceptibility to respiratory infections. The compound's ability to form nitrosamines, which are known carcinogens, further underscores its potential health risks.
In industrial settings, nitrous acid is utilized in various applications, including the production of diazo dyes, the synthesis of pharmaceuticals, and as a reducing agent in certain chemical processes. Its corrosive nature and potential to release toxic nitrogen oxides necessitate strict handling and storage protocols to ensure worker safety and environmental protection.
The environmental impact of nitrous acid extends beyond its direct effects on human health. It contributes to the acidification of soil and water bodies, potentially disrupting ecosystems and biodiversity. Additionally, its role in the formation of secondary organic aerosols influences climate change dynamics, as these aerosols can affect cloud formation and radiative forcing.
Given its multifaceted impacts on health and the environment, nitrous acid has become a focal point for regulatory bodies worldwide. Occupational exposure limits have been established to protect workers in industries where nitrous acid is present. Environmental agencies monitor ambient levels of nitrous acid and its precursors as part of air quality management strategies. The compound's involvement in complex atmospheric chemistry also makes it a subject of ongoing research, as scientists seek to better understand its role in pollution formation and climate processes.
Health Impact Analysis
Nitrous acid (HONO) has emerged as a significant concern in public health and environmental safety, prompting the development and refinement of regulations across various sectors. The health impacts of nitrous acid exposure are multifaceted and can affect both acute and chronic well-being. Inhalation of nitrous acid vapors can cause immediate respiratory irritation, leading to coughing, wheezing, and shortness of breath. Prolonged exposure has been linked to more severe respiratory conditions, including bronchitis and exacerbation of asthma symptoms.
Studies have shown that nitrous acid can react with other atmospheric compounds to form secondary pollutants, such as ozone and fine particulate matter (PM2.5), which are known to have detrimental effects on cardiovascular and respiratory health. This indirect impact of nitrous acid on air quality has led to increased scrutiny in urban and industrial areas where its precursors are more prevalent.
The health effects of nitrous acid are not limited to respiratory issues. Research has indicated potential links between nitrous acid exposure and increased oxidative stress in the body, which may contribute to cellular damage and inflammation. This oxidative stress has been associated with a higher risk of developing certain cancers and neurodegenerative diseases, although more comprehensive studies are needed to establish definitive causal relationships.
Vulnerable populations, including children, the elderly, and individuals with pre-existing respiratory conditions, are particularly susceptible to the adverse effects of nitrous acid. Epidemiological studies have demonstrated higher rates of hospital admissions for respiratory ailments in areas with elevated nitrous acid levels, especially during periods of poor air quality or temperature inversions that trap pollutants near the ground.
The recognition of these health impacts has driven the evolution of safety regulations in various industries. Occupational health standards have been updated to include stricter limits on workplace exposure to nitrous acid and its precursors. Environmental agencies have incorporated nitrous acid monitoring into air quality assessments, leading to more comprehensive pollution control strategies in urban planning and industrial operations.
In response to the growing body of evidence on nitrous acid's health effects, regulatory bodies have begun to revise ambient air quality standards. These revisions aim to address not only direct exposure to nitrous acid but also its role in the formation of secondary pollutants. The implementation of these standards has led to changes in emission control technologies, industrial processes, and urban design practices to mitigate the production and accumulation of nitrous acid in the environment.
Studies have shown that nitrous acid can react with other atmospheric compounds to form secondary pollutants, such as ozone and fine particulate matter (PM2.5), which are known to have detrimental effects on cardiovascular and respiratory health. This indirect impact of nitrous acid on air quality has led to increased scrutiny in urban and industrial areas where its precursors are more prevalent.
The health effects of nitrous acid are not limited to respiratory issues. Research has indicated potential links between nitrous acid exposure and increased oxidative stress in the body, which may contribute to cellular damage and inflammation. This oxidative stress has been associated with a higher risk of developing certain cancers and neurodegenerative diseases, although more comprehensive studies are needed to establish definitive causal relationships.
Vulnerable populations, including children, the elderly, and individuals with pre-existing respiratory conditions, are particularly susceptible to the adverse effects of nitrous acid. Epidemiological studies have demonstrated higher rates of hospital admissions for respiratory ailments in areas with elevated nitrous acid levels, especially during periods of poor air quality or temperature inversions that trap pollutants near the ground.
The recognition of these health impacts has driven the evolution of safety regulations in various industries. Occupational health standards have been updated to include stricter limits on workplace exposure to nitrous acid and its precursors. Environmental agencies have incorporated nitrous acid monitoring into air quality assessments, leading to more comprehensive pollution control strategies in urban planning and industrial operations.
In response to the growing body of evidence on nitrous acid's health effects, regulatory bodies have begun to revise ambient air quality standards. These revisions aim to address not only direct exposure to nitrous acid but also its role in the formation of secondary pollutants. The implementation of these standards has led to changes in emission control technologies, industrial processes, and urban design practices to mitigate the production and accumulation of nitrous acid in the environment.
Current Regulatory Landscape
The current regulatory landscape surrounding nitrous acid is characterized by a complex web of national and international standards aimed at protecting human health and environmental safety. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for nitrous acid in workplace environments. These limits are set at 2 parts per million (ppm) for an 8-hour time-weighted average, with a short-term exposure limit of 4 ppm for 15 minutes.
The Environmental Protection Agency (EPA) regulates nitrous acid under the Clean Air Act and Clean Water Act, setting limits on emissions and discharge into water bodies. The EPA's National Ambient Air Quality Standards (NAAQS) indirectly address nitrous acid through its regulation of nitrogen oxides, which are precursors to nitrous acid formation in the atmosphere.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation includes nitrous acid in its list of substances subject to authorization. This requires manufacturers and importers to assess and manage the risks associated with nitrous acid and its compounds. The World Health Organization (WHO) has also issued guidelines on air quality that indirectly impact nitrous acid regulation through its recommendations on nitrogen dioxide levels.
In the realm of transportation and storage, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those associated with nitrous acid. This system has been adopted by many countries, influencing labeling and safety data sheet requirements for nitrous acid and its derivatives.
The chemical industry has also developed voluntary standards and best practices for handling nitrous acid. Organizations such as the American Chemistry Council and the European Chemical Industry Council provide guidance to their members on safe handling, storage, and transportation of nitrous acid, often going beyond regulatory requirements.
Recent scientific findings on the health impacts of nitrous acid have led to ongoing reviews of existing regulations. There is growing concern about the role of nitrous acid in indoor air quality, particularly in relation to its formation from reactions between nitrogen oxides and surfaces in buildings. This has prompted some jurisdictions to consider new guidelines for indoor air quality that specifically address nitrous acid levels.
As research continues to unveil the complex interactions between nitrous acid and human health, regulatory bodies are faced with the challenge of adapting their frameworks to address emerging concerns. This dynamic regulatory landscape reflects the evolving understanding of nitrous acid's impact on health and safety, necessitating ongoing collaboration between scientists, policymakers, and industry stakeholders to ensure effective and up-to-date regulations.
The Environmental Protection Agency (EPA) regulates nitrous acid under the Clean Air Act and Clean Water Act, setting limits on emissions and discharge into water bodies. The EPA's National Ambient Air Quality Standards (NAAQS) indirectly address nitrous acid through its regulation of nitrogen oxides, which are precursors to nitrous acid formation in the atmosphere.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation includes nitrous acid in its list of substances subject to authorization. This requires manufacturers and importers to assess and manage the risks associated with nitrous acid and its compounds. The World Health Organization (WHO) has also issued guidelines on air quality that indirectly impact nitrous acid regulation through its recommendations on nitrogen dioxide levels.
In the realm of transportation and storage, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those associated with nitrous acid. This system has been adopted by many countries, influencing labeling and safety data sheet requirements for nitrous acid and its derivatives.
The chemical industry has also developed voluntary standards and best practices for handling nitrous acid. Organizations such as the American Chemistry Council and the European Chemical Industry Council provide guidance to their members on safe handling, storage, and transportation of nitrous acid, often going beyond regulatory requirements.
Recent scientific findings on the health impacts of nitrous acid have led to ongoing reviews of existing regulations. There is growing concern about the role of nitrous acid in indoor air quality, particularly in relation to its formation from reactions between nitrogen oxides and surfaces in buildings. This has prompted some jurisdictions to consider new guidelines for indoor air quality that specifically address nitrous acid levels.
As research continues to unveil the complex interactions between nitrous acid and human health, regulatory bodies are faced with the challenge of adapting their frameworks to address emerging concerns. This dynamic regulatory landscape reflects the evolving understanding of nitrous acid's impact on health and safety, necessitating ongoing collaboration between scientists, policymakers, and industry stakeholders to ensure effective and up-to-date regulations.
Existing Safety Measures
01 Exposure limits and safety guidelines
Regulations establish permissible exposure limits for nitrous acid in workplace environments. These guidelines outline safe concentration levels, monitoring requirements, and protective measures to minimize health risks associated with nitrous acid exposure.- Exposure limits and safety guidelines: Regulations establish permissible exposure limits for nitrous acid in workplace environments. These guidelines outline safe concentration levels, monitoring requirements, and protective measures to minimize health risks associated with nitrous acid exposure.
- Personal protective equipment (PPE) requirements: Safety regulations mandate the use of appropriate personal protective equipment when handling nitrous acid. This includes respiratory protection, chemical-resistant gloves, protective clothing, and eye/face protection to prevent direct contact and inhalation of vapors.
- Storage and handling protocols: Specific guidelines are established for the proper storage and handling of nitrous acid. These protocols cover container requirements, ventilation systems, spill containment measures, and procedures for safe transfer and disposal to prevent accidents and environmental contamination.
- Emergency response and first aid procedures: Regulations outline emergency response protocols and first aid procedures for nitrous acid exposure. This includes decontamination methods, eye washing stations, safety showers, and specific medical interventions to mitigate the health effects of accidental exposure.
- Environmental protection and waste management: Health and safety regulations address the environmental impact of nitrous acid, including proper waste management and disposal methods. Guidelines cover neutralization techniques, containment strategies, and reporting requirements for spills or releases to minimize environmental contamination.
02 Personal protective equipment requirements
Safety regulations mandate the use of appropriate personal protective equipment when handling nitrous acid. This includes respiratory protection, chemical-resistant gloves, protective clothing, and eye/face protection to prevent direct contact and inhalation of vapors.Expand Specific Solutions03 Storage and handling protocols
Specific protocols are established for the safe storage and handling of nitrous acid. These include proper containment, ventilation requirements, segregation from incompatible materials, and spill response procedures to prevent accidents and environmental contamination.Expand Specific Solutions04 Waste management and disposal regulations
Regulations govern the proper disposal of nitrous acid and related waste materials. These guidelines outline neutralization procedures, containment requirements, and approved disposal methods to minimize environmental impact and ensure worker safety during waste handling.Expand Specific Solutions05 Emergency response and first aid procedures
Health and safety regulations mandate the establishment of emergency response plans and first aid procedures for nitrous acid incidents. These include decontamination protocols, medical treatment guidelines, and reporting requirements in case of accidental exposure or release.Expand Specific Solutions
Key Stakeholders
The competitive landscape surrounding nitrous acid's impact on health and safety regulations is evolving rapidly. The market is in a growth phase, with increasing awareness of the compound's health implications driving regulatory changes and research initiatives. The global market for nitrous acid-related safety solutions is expanding, fueled by stricter environmental and occupational health standards. Technologically, the field is advancing, with companies like Boehringer Ingelheim, Medtronic Vascular, and 3M Innovative Properties leading research efforts. Academic institutions such as Michigan Technological University and Case Western Reserve University are contributing to the knowledge base, while organizations like SaNOtize Research & Development are exploring innovative applications. This collaborative ecosystem of industry and academia is accelerating the development of detection, mitigation, and treatment technologies related to nitrous acid exposure.
Teledyne Instruments, Inc.
Technical Solution: Teledyne Instruments has developed advanced monitoring and detection systems for nitrous acid and other air pollutants. Their technology includes high-precision spectroscopic instruments that can measure nitrous acid concentrations in real-time with parts-per-billion (ppb) sensitivity. Teledyne's approach combines Cavity Ring-Down Spectroscopy (CRDS) and Quantum Cascade Laser (QCL) technology to achieve accurate and continuous monitoring of nitrous acid levels in various environments[5]. These instruments are designed for use in atmospheric research, industrial emissions monitoring, and regulatory compliance applications. Teledyne's systems can simultaneously measure multiple gas species, including NO, NO2, and HONO, providing comprehensive data for air quality assessment and pollution control strategies[6].
Strengths: High-precision measurement capabilities; real-time monitoring; versatility in measuring multiple gas species. Weaknesses: High initial cost of equipment; complexity requiring specialized training for operation and maintenance; limited to detection and monitoring rather than direct mitigation.
3M Innovative Properties Co.
Technical Solution: 3M has developed advanced filtration and air purification technologies that address the health and safety concerns related to nitrous acid and other airborne pollutants. Their approach includes the use of specialized filter media and adsorbent materials designed to capture and neutralize nitrous acid and its precursors. 3M's technology incorporates multi-layer filtration systems that can remove up to 99.97% of airborne particles as small as 0.3 microns[3]. For nitrous acid specifically, 3M has developed chemical-specific filters that utilize activated carbon and other specialized materials to adsorb and neutralize acidic gases. These filtration systems are designed for both industrial and residential applications, helping to improve air quality and reduce exposure to harmful pollutants[4].
Strengths: Extensive experience in filtration technology; wide range of applications from personal to industrial use; strong brand recognition. Weaknesses: High cost of advanced filtration systems; need for regular maintenance and filter replacement; limited effectiveness against certain gaseous pollutants.
Scientific Advancements
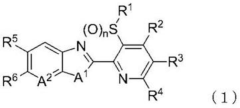
Nitrosamine impurity, varenicline pharmaceutical composition capable of reducing generation of nitrosamine impurities and preparation and use thereof
PatentPendingEP4389748A2
Innovation
- Incorporating a pharmaceutically acceptable acid into the varenicline or its salt, such as varenicline hydrochloride or varenicline tartrate, to inhibit the formation of nitrosamine impurities, thereby stabilizing the compound and maintaining low levels of genotoxic nitrosamine impurities within safe limits.
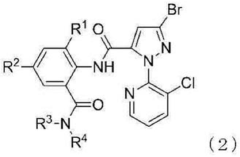
Composition and method for controlling pests
PatentActiveCN112021322A
Innovation
- A composition for controlling pests was developed, which is prepared from a combination of a specific fused heterocyclic compound and a pesticidal compound through an oxidation or condensation reaction to form an efficient pest control agent.
Economic Implications
The economic implications of nitrous acid's impact on health and safety regulations are far-reaching and multifaceted. Industries that produce or utilize nitrous acid, such as chemical manufacturing, agriculture, and pharmaceuticals, face increased operational costs due to stringent safety measures and emission controls. These regulations often necessitate substantial investments in advanced filtration systems, personal protective equipment, and monitoring technologies to ensure compliance with air quality standards and worker safety protocols.
The implementation of stricter regulations has led to a surge in demand for environmental consulting services and specialized equipment manufacturers. This has created new market opportunities and job prospects in the environmental technology sector. However, smaller businesses may struggle to absorb these additional costs, potentially leading to market consolidation or reduced competitiveness in global markets where regulations may be less stringent.
In the agricultural sector, limitations on nitrous acid-based fertilizers have prompted research and development into alternative soil management techniques and more environmentally friendly fertilizer options. This shift has spurred innovation in sustainable agriculture practices, potentially leading to long-term economic benefits through improved soil health and reduced environmental impact.
The healthcare industry has also been significantly affected, with increased focus on occupational health and safety measures for medical professionals exposed to nitrous acid in various clinical settings. This has led to additional training requirements and the adoption of more advanced protective equipment, impacting healthcare costs and resource allocation.
Insurance companies have adjusted their risk assessment models to account for the health hazards associated with nitrous acid exposure, potentially leading to higher premiums for businesses in affected industries. This, in turn, has encouraged proactive risk management strategies and investments in safety measures to mitigate insurance costs.
The regulatory landscape surrounding nitrous acid has also influenced international trade dynamics. Countries with more stringent regulations may face challenges in maintaining competitiveness with nations that have less restrictive policies. This disparity has led to discussions on harmonizing global standards to create a level playing field and prevent regulatory arbitrage.
Overall, while the economic burden of compliance with nitrous acid-related health and safety regulations is significant, it has also driven innovation, created new markets, and potentially led to long-term cost savings through improved public health outcomes and reduced environmental remediation expenses.
The implementation of stricter regulations has led to a surge in demand for environmental consulting services and specialized equipment manufacturers. This has created new market opportunities and job prospects in the environmental technology sector. However, smaller businesses may struggle to absorb these additional costs, potentially leading to market consolidation or reduced competitiveness in global markets where regulations may be less stringent.
In the agricultural sector, limitations on nitrous acid-based fertilizers have prompted research and development into alternative soil management techniques and more environmentally friendly fertilizer options. This shift has spurred innovation in sustainable agriculture practices, potentially leading to long-term economic benefits through improved soil health and reduced environmental impact.
The healthcare industry has also been significantly affected, with increased focus on occupational health and safety measures for medical professionals exposed to nitrous acid in various clinical settings. This has led to additional training requirements and the adoption of more advanced protective equipment, impacting healthcare costs and resource allocation.
Insurance companies have adjusted their risk assessment models to account for the health hazards associated with nitrous acid exposure, potentially leading to higher premiums for businesses in affected industries. This, in turn, has encouraged proactive risk management strategies and investments in safety measures to mitigate insurance costs.
The regulatory landscape surrounding nitrous acid has also influenced international trade dynamics. Countries with more stringent regulations may face challenges in maintaining competitiveness with nations that have less restrictive policies. This disparity has led to discussions on harmonizing global standards to create a level playing field and prevent regulatory arbitrage.
Overall, while the economic burden of compliance with nitrous acid-related health and safety regulations is significant, it has also driven innovation, created new markets, and potentially led to long-term cost savings through improved public health outcomes and reduced environmental remediation expenses.
Environmental Considerations
Nitrous acid plays a significant role in shaping environmental regulations due to its complex interactions with various ecosystems and its potential impact on human health. The environmental considerations surrounding nitrous acid are multifaceted and require a comprehensive approach to regulation and management.
One of the primary environmental concerns related to nitrous acid is its contribution to acid rain formation. When released into the atmosphere, nitrous acid can react with water vapor to form nitric acid, a major component of acid rain. This process has far-reaching consequences for terrestrial and aquatic ecosystems, affecting soil chemistry, water quality, and the health of flora and fauna. Regulatory bodies must consider these impacts when establishing emission standards for industries that produce nitrous acid as a byproduct.
The presence of nitrous acid in the atmosphere also contributes to the formation of ground-level ozone, a key component of photochemical smog. This secondary pollutant can have detrimental effects on human respiratory health and vegetation. As a result, environmental regulations often focus on controlling precursor emissions, including nitrous acid, to mitigate ozone formation in urban and industrial areas.
Water quality is another critical environmental consideration influenced by nitrous acid. When introduced into aquatic systems, nitrous acid can alter pH levels and contribute to the acidification of water bodies. This can have cascading effects on aquatic life, disrupting ecosystems and potentially leading to biodiversity loss. Regulatory frameworks must address both direct discharges of nitrous acid into water sources and atmospheric deposition to protect water resources effectively.
The role of nitrous acid in nitrogen cycling is an important environmental factor that shapes regulatory approaches. As a reactive nitrogen compound, nitrous acid can contribute to eutrophication in aquatic environments when excess nitrogen enters water systems. This process can lead to algal blooms, oxygen depletion, and the degradation of water quality. Regulations aimed at controlling nitrous acid emissions and runoff must consider these broader ecological impacts.
Climate change considerations also influence the regulatory landscape surrounding nitrous acid. While not a direct greenhouse gas, nitrous acid's involvement in atmospheric chemistry can indirectly affect climate processes. Its role in the formation of aerosols and its interactions with other atmospheric compounds make it a subject of interest in climate-related environmental policies.
Biodiversity protection is another key environmental consideration in nitrous acid regulation. The compound's potential to alter habitats through soil and water acidification can threaten sensitive species and ecosystems. Conservation efforts and environmental impact assessments often incorporate measures to mitigate the effects of nitrous acid on biodiversity, influencing the development of regulatory frameworks.
One of the primary environmental concerns related to nitrous acid is its contribution to acid rain formation. When released into the atmosphere, nitrous acid can react with water vapor to form nitric acid, a major component of acid rain. This process has far-reaching consequences for terrestrial and aquatic ecosystems, affecting soil chemistry, water quality, and the health of flora and fauna. Regulatory bodies must consider these impacts when establishing emission standards for industries that produce nitrous acid as a byproduct.
The presence of nitrous acid in the atmosphere also contributes to the formation of ground-level ozone, a key component of photochemical smog. This secondary pollutant can have detrimental effects on human respiratory health and vegetation. As a result, environmental regulations often focus on controlling precursor emissions, including nitrous acid, to mitigate ozone formation in urban and industrial areas.
Water quality is another critical environmental consideration influenced by nitrous acid. When introduced into aquatic systems, nitrous acid can alter pH levels and contribute to the acidification of water bodies. This can have cascading effects on aquatic life, disrupting ecosystems and potentially leading to biodiversity loss. Regulatory frameworks must address both direct discharges of nitrous acid into water sources and atmospheric deposition to protect water resources effectively.
The role of nitrous acid in nitrogen cycling is an important environmental factor that shapes regulatory approaches. As a reactive nitrogen compound, nitrous acid can contribute to eutrophication in aquatic environments when excess nitrogen enters water systems. This process can lead to algal blooms, oxygen depletion, and the degradation of water quality. Regulations aimed at controlling nitrous acid emissions and runoff must consider these broader ecological impacts.
Climate change considerations also influence the regulatory landscape surrounding nitrous acid. While not a direct greenhouse gas, nitrous acid's involvement in atmospheric chemistry can indirectly affect climate processes. Its role in the formation of aerosols and its interactions with other atmospheric compounds make it a subject of interest in climate-related environmental policies.
Biodiversity protection is another key environmental consideration in nitrous acid regulation. The compound's potential to alter habitats through soil and water acidification can threaten sensitive species and ecosystems. Conservation efforts and environmental impact assessments often incorporate measures to mitigate the effects of nitrous acid on biodiversity, influencing the development of regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!