Nitrous Acid in Interfacial Chemistry and Atmospheric Aging Processes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HONO Research Background and Objectives
Nitrous acid (HONO) has emerged as a crucial component in atmospheric chemistry and interfacial processes, playing a significant role in air quality and climate change. The study of HONO has gained increasing attention over the past few decades due to its impact on the formation of hydroxyl radicals (OH), which are often referred to as the "detergent" of the atmosphere. These radicals are key players in the oxidation of various pollutants and the formation of secondary organic aerosols.
The research on HONO in interfacial chemistry and atmospheric aging processes aims to unravel the complex mechanisms governing its formation, transformation, and impact on atmospheric composition. This field of study has evolved from initial observations of unexpectedly high HONO concentrations in urban environments to a comprehensive investigation of its sources, sinks, and chemical pathways in various atmospheric and interfacial settings.
One of the primary objectives of HONO research is to elucidate the heterogeneous formation processes occurring at various interfaces, such as aerosol surfaces, building materials, and vegetation. These interfaces provide unique chemical environments that can significantly enhance HONO production rates compared to homogeneous gas-phase reactions. Understanding these processes is crucial for accurately predicting HONO concentrations and their subsequent effects on atmospheric chemistry.
Another key goal is to investigate the role of HONO in atmospheric aging processes, particularly its involvement in the formation and transformation of secondary organic aerosols. As these aerosols age, they undergo complex chemical and physical changes that can alter their properties and environmental impacts. HONO's participation in these aging processes can influence the optical properties, hygroscopicity, and overall lifetime of aerosols in the atmosphere.
Furthermore, researchers aim to develop more accurate measurement techniques and modeling approaches to better quantify HONO concentrations and fluxes in diverse environments. This includes improving our understanding of HONO's vertical distribution in the atmosphere and its behavior in different layers, from the ground level to the upper troposphere.
The study of HONO also extends to its potential impacts on human health and ecosystems. As a precursor to OH radicals and a contributor to the nitrogen oxide cycle, HONO indirectly affects the formation of other pollutants such as ozone and particulate matter. Therefore, understanding its chemistry is essential for developing effective air quality management strategies and assessing the broader implications of atmospheric pollution.
In the context of global climate change, HONO research seeks to elucidate the compound's role in feedback mechanisms that may amplify or mitigate warming effects. This includes investigating how changes in temperature, humidity, and land use patterns might influence HONO formation and its subsequent impact on atmospheric composition and radiative forcing.
The research on HONO in interfacial chemistry and atmospheric aging processes aims to unravel the complex mechanisms governing its formation, transformation, and impact on atmospheric composition. This field of study has evolved from initial observations of unexpectedly high HONO concentrations in urban environments to a comprehensive investigation of its sources, sinks, and chemical pathways in various atmospheric and interfacial settings.
One of the primary objectives of HONO research is to elucidate the heterogeneous formation processes occurring at various interfaces, such as aerosol surfaces, building materials, and vegetation. These interfaces provide unique chemical environments that can significantly enhance HONO production rates compared to homogeneous gas-phase reactions. Understanding these processes is crucial for accurately predicting HONO concentrations and their subsequent effects on atmospheric chemistry.
Another key goal is to investigate the role of HONO in atmospheric aging processes, particularly its involvement in the formation and transformation of secondary organic aerosols. As these aerosols age, they undergo complex chemical and physical changes that can alter their properties and environmental impacts. HONO's participation in these aging processes can influence the optical properties, hygroscopicity, and overall lifetime of aerosols in the atmosphere.
Furthermore, researchers aim to develop more accurate measurement techniques and modeling approaches to better quantify HONO concentrations and fluxes in diverse environments. This includes improving our understanding of HONO's vertical distribution in the atmosphere and its behavior in different layers, from the ground level to the upper troposphere.
The study of HONO also extends to its potential impacts on human health and ecosystems. As a precursor to OH radicals and a contributor to the nitrogen oxide cycle, HONO indirectly affects the formation of other pollutants such as ozone and particulate matter. Therefore, understanding its chemistry is essential for developing effective air quality management strategies and assessing the broader implications of atmospheric pollution.
In the context of global climate change, HONO research seeks to elucidate the compound's role in feedback mechanisms that may amplify or mitigate warming effects. This includes investigating how changes in temperature, humidity, and land use patterns might influence HONO formation and its subsequent impact on atmospheric composition and radiative forcing.
Atmospheric HONO Demand Analysis
The demand for atmospheric nitrous acid (HONO) research has grown significantly in recent years due to its crucial role in atmospheric chemistry and its impact on air quality and climate change. HONO is a key source of hydroxyl radicals (OH), which are often referred to as the "detergent" of the atmosphere due to their ability to oxidize and remove pollutants. The increasing interest in HONO stems from its complex formation mechanisms and its potential to influence ozone production, particulate matter formation, and overall atmospheric oxidation capacity.
In urban environments, where air pollution is a major concern, understanding HONO chemistry has become essential for developing effective air quality management strategies. The demand for HONO research is driven by the need to accurately model and predict air quality, especially in densely populated areas where human health is directly impacted by atmospheric pollutants. Regulatory agencies and policymakers require comprehensive data on HONO dynamics to establish appropriate emission control measures and air quality standards.
The scientific community has recognized significant gaps in our understanding of HONO chemistry, particularly in the context of interfacial processes and atmospheric aging. These knowledge gaps have spurred demand for advanced research techniques and instrumentation capable of measuring HONO concentrations and fluxes with high temporal and spatial resolution. There is a growing need for field campaigns and laboratory studies that can elucidate the complex interplay between HONO and various surfaces, including aerosols, buildings, and vegetation.
Climate change research has also contributed to the increased demand for HONO studies. As global temperatures rise and precipitation patterns shift, the behavior of HONO in the atmosphere may change, potentially altering atmospheric chemistry on a large scale. Climate scientists and atmospheric modelers require accurate parameterizations of HONO chemistry to improve global climate models and predict future air quality scenarios.
The agricultural sector has shown interest in HONO research due to its potential impact on crop yields and soil chemistry. HONO emissions from fertilized soils and their subsequent atmospheric reactions can affect nitrogen cycling and deposition, which are critical factors in agricultural productivity and ecosystem health. This has led to collaborations between atmospheric scientists and agronomists, further expanding the scope and demand for HONO research.
In the realm of indoor air quality, HONO has gained attention as a potential indoor pollutant. With people spending more time indoors, especially in urban settings, there is a growing demand for studies on HONO formation and mitigation in indoor environments. This research is crucial for developing guidelines for building ventilation and materials selection to ensure healthy indoor air quality.
In urban environments, where air pollution is a major concern, understanding HONO chemistry has become essential for developing effective air quality management strategies. The demand for HONO research is driven by the need to accurately model and predict air quality, especially in densely populated areas where human health is directly impacted by atmospheric pollutants. Regulatory agencies and policymakers require comprehensive data on HONO dynamics to establish appropriate emission control measures and air quality standards.
The scientific community has recognized significant gaps in our understanding of HONO chemistry, particularly in the context of interfacial processes and atmospheric aging. These knowledge gaps have spurred demand for advanced research techniques and instrumentation capable of measuring HONO concentrations and fluxes with high temporal and spatial resolution. There is a growing need for field campaigns and laboratory studies that can elucidate the complex interplay between HONO and various surfaces, including aerosols, buildings, and vegetation.
Climate change research has also contributed to the increased demand for HONO studies. As global temperatures rise and precipitation patterns shift, the behavior of HONO in the atmosphere may change, potentially altering atmospheric chemistry on a large scale. Climate scientists and atmospheric modelers require accurate parameterizations of HONO chemistry to improve global climate models and predict future air quality scenarios.
The agricultural sector has shown interest in HONO research due to its potential impact on crop yields and soil chemistry. HONO emissions from fertilized soils and their subsequent atmospheric reactions can affect nitrogen cycling and deposition, which are critical factors in agricultural productivity and ecosystem health. This has led to collaborations between atmospheric scientists and agronomists, further expanding the scope and demand for HONO research.
In the realm of indoor air quality, HONO has gained attention as a potential indoor pollutant. With people spending more time indoors, especially in urban settings, there is a growing demand for studies on HONO formation and mitigation in indoor environments. This research is crucial for developing guidelines for building ventilation and materials selection to ensure healthy indoor air quality.
HONO in Interfacial Chemistry: Current Status and Challenges
Nitrous acid (HONO) plays a crucial role in atmospheric chemistry and interfacial processes. The current status of HONO research in interfacial chemistry reveals significant progress, yet numerous challenges remain. Recent studies have highlighted the importance of HONO in atmospheric oxidation processes, particularly its role as a major source of hydroxyl radicals (OH) in the lower troposphere.
One of the primary challenges in HONO research is understanding its formation mechanisms at various interfaces. While gas-phase reactions are well-documented, heterogeneous processes occurring on surfaces such as aerosols, buildings, and vegetation are less understood. These interfacial reactions are believed to be a significant source of HONO, especially in urban environments, but quantifying their contributions remains difficult.
Another key challenge is accurately measuring HONO concentrations in different environmental settings. Current analytical techniques often struggle with interference from other nitrogen-containing compounds, leading to potential overestimation or underestimation of HONO levels. This uncertainty hampers efforts to develop accurate atmospheric models and assess the true impact of HONO on air quality.
The role of HONO in atmospheric aging processes presents additional complexities. As aerosols and other particles age, their surfaces undergo chemical and physical changes that can alter HONO formation and uptake rates. Understanding these dynamic processes and their impact on HONO chemistry is crucial for predicting air quality and climate effects.
Furthermore, the interplay between HONO and other atmospheric constituents, such as volatile organic compounds (VOCs) and particulate matter, remains an area of active research. These interactions can lead to complex feedback loops that affect overall atmospheric composition and reactivity.
Recent advancements in spectroscopic techniques and computational modeling have provided new insights into HONO's behavior at interfaces. However, bridging the gap between laboratory studies and real-world atmospheric conditions remains a significant challenge. Field measurements often show discrepancies with model predictions, highlighting the need for improved understanding of HONO chemistry in complex, multi-phase environments.
In conclusion, while substantial progress has been made in understanding HONO's role in interfacial chemistry, significant challenges persist. Addressing these challenges will require interdisciplinary approaches, combining advanced analytical techniques, sophisticated modeling, and comprehensive field studies to unravel the complexities of HONO chemistry in the atmosphere.
One of the primary challenges in HONO research is understanding its formation mechanisms at various interfaces. While gas-phase reactions are well-documented, heterogeneous processes occurring on surfaces such as aerosols, buildings, and vegetation are less understood. These interfacial reactions are believed to be a significant source of HONO, especially in urban environments, but quantifying their contributions remains difficult.
Another key challenge is accurately measuring HONO concentrations in different environmental settings. Current analytical techniques often struggle with interference from other nitrogen-containing compounds, leading to potential overestimation or underestimation of HONO levels. This uncertainty hampers efforts to develop accurate atmospheric models and assess the true impact of HONO on air quality.
The role of HONO in atmospheric aging processes presents additional complexities. As aerosols and other particles age, their surfaces undergo chemical and physical changes that can alter HONO formation and uptake rates. Understanding these dynamic processes and their impact on HONO chemistry is crucial for predicting air quality and climate effects.
Furthermore, the interplay between HONO and other atmospheric constituents, such as volatile organic compounds (VOCs) and particulate matter, remains an area of active research. These interactions can lead to complex feedback loops that affect overall atmospheric composition and reactivity.
Recent advancements in spectroscopic techniques and computational modeling have provided new insights into HONO's behavior at interfaces. However, bridging the gap between laboratory studies and real-world atmospheric conditions remains a significant challenge. Field measurements often show discrepancies with model predictions, highlighting the need for improved understanding of HONO chemistry in complex, multi-phase environments.
In conclusion, while substantial progress has been made in understanding HONO's role in interfacial chemistry, significant challenges persist. Addressing these challenges will require interdisciplinary approaches, combining advanced analytical techniques, sophisticated modeling, and comprehensive field studies to unravel the complexities of HONO chemistry in the atmosphere.
Current Methodologies for HONO Detection and Analysis
01 Production and synthesis of nitrous acid
Nitrous acid is typically produced through the reaction of nitrogen oxides with water or by the reduction of nitric acid. Various methods and processes have been developed to synthesize nitrous acid efficiently and control its concentration for different industrial applications.- Production and applications of nitrous acid: Nitrous acid is a weak and unstable inorganic acid with various industrial applications. It can be produced through different methods and is used in chemical processes, such as diazotization reactions and the production of dyes. The acid plays a role in atmospheric chemistry and environmental processes.
- Nitrous acid in material treatment and manufacturing: Nitrous acid is utilized in various material treatment processes, including surface modification of polymers, etching of metals, and in the production of specialty chemicals. It can enhance material properties or facilitate specific chemical reactions in manufacturing processes.
- Environmental and atmospheric implications of nitrous acid: Nitrous acid plays a significant role in atmospheric chemistry and environmental processes. It is involved in the formation of photochemical smog and can impact air quality. Research focuses on understanding its formation, reactions, and effects on the environment.
- Analytical methods for nitrous acid detection and measurement: Various analytical techniques have been developed for the detection and quantification of nitrous acid in different matrices. These methods are crucial for environmental monitoring, quality control in industrial processes, and research applications related to nitrous acid chemistry.
- Nitrous acid in chemical synthesis and reactions: Nitrous acid is an important reagent in organic synthesis, particularly in diazotization reactions and the production of diazo compounds. It is also involved in various other chemical transformations and can be used as a catalyst or intermediate in the synthesis of specialty chemicals.
02 Applications in chemical processing
Nitrous acid plays a crucial role in various chemical processes, including the production of diazo compounds, nitrosation reactions, and as an intermediate in the manufacture of other chemicals. It is utilized in industries such as dye production, pharmaceuticals, and organic synthesis.Expand Specific Solutions03 Environmental and atmospheric chemistry
Nitrous acid is an important component in atmospheric chemistry, playing a role in the formation of photochemical smog and acid rain. Research focuses on understanding its formation, reactions, and impact on air quality and climate change.Expand Specific Solutions04 Analytical methods and detection techniques
Various analytical methods have been developed to detect and quantify nitrous acid in different matrices. These techniques are essential for monitoring environmental levels, industrial processes, and quality control in chemical manufacturing.Expand Specific Solutions05 Safety and handling considerations
Due to its corrosive and reactive nature, special precautions are required when handling nitrous acid. Research focuses on developing safer handling methods, storage solutions, and protective measures to minimize risks associated with its use in industrial and laboratory settings.Expand Specific Solutions
Key Players in HONO Atmospheric Chemistry Research
The research on nitrous acid in interfacial chemistry and atmospheric aging processes is in a developing stage, with growing market potential due to its relevance in environmental and atmospheric sciences. The field is characterized by a mix of academic institutions and industry players, indicating an emerging competitive landscape. Key players like Chinese Academy of Science Institute of Chemistry, University of Manchester, and Purdue Research Foundation are driving academic research, while companies such as BASF Corp. and Dow Global Technologies LLC are exploring industrial applications. The technology's maturity is progressing, with ongoing research focusing on understanding complex atmospheric processes and developing innovative solutions for environmental challenges.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has conducted extensive research on nitrous acid in interfacial chemistry and atmospheric aging processes. Their approach involves advanced spectroscopic techniques, including surface-enhanced Raman spectroscopy (SERS) and attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), to study the behavior of nitrous acid at various interfaces[1]. They have developed novel nanostructured materials to enhance the detection sensitivity of nitrous acid in atmospheric samples[2]. Their research also focuses on the role of nitrous acid in atmospheric aging of organic aerosols, utilizing environmental chambers to simulate real-world conditions[3]. The institute has made significant contributions to understanding the heterogeneous formation of nitrous acid on urban surfaces and its impact on air quality[4].
Strengths: Advanced analytical techniques, expertise in atmospheric chemistry, and access to state-of-the-art facilities. Weaknesses: Potential limitations in field studies and real-world applications of laboratory findings.
Queen Mary University of London
Technical Solution: Queen Mary University of London has been at the forefront of research on nitrous acid in atmospheric chemistry. Their approach combines laboratory experiments, field measurements, and atmospheric modeling to understand the formation, distribution, and impact of nitrous acid in urban environments[5]. They have developed innovative techniques for measuring nitrous acid in real-time using quantum cascade laser absorption spectroscopy (QCLAS)[6]. Their research has revealed the significant role of nitrous acid in initiating photochemical smog formation and its contribution to secondary organic aerosol (SOA) formation[7]. The university has also investigated the heterogeneous reactions of nitrous acid on various surfaces, including urban building materials and vegetation, providing insights into its sources and sinks in the atmosphere[8].
Strengths: Comprehensive approach combining multiple research methods, strong focus on urban atmospheric chemistry. Weaknesses: May have limited resources compared to larger research institutions for extensive field campaigns.
Breakthrough Studies on HONO Interfacial Reactions
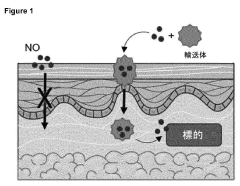
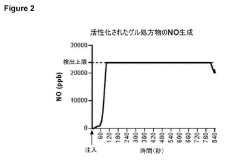
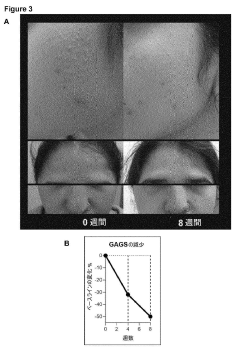
Systems for transport and delivery of nitric oxide
PatentPendingJP2024523533A
Innovation
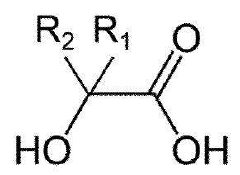
- A delivery system combining alpha-hydroxy acids with a nitric oxide source to enhance penetration through epithelial layers, using alpha-hydroxy acids to capture and transport nitric oxide generated in situ, overcoming the natural barrier of the epidermis.
Improvements in and relating to the Manufacture of Nitric, Nitrous, and Sulphuric Acids and Oxides of Nitrogen.
PatentInactiveGB189904643A
Innovation
- An apparatus comprising a vessel with terminals connected to a dynamo and transformer producing alternating currents, where the terminals are placed to prevent sparking and a master flame is used to initiate a nitrogen flame, allowing oxygen and nitrogen to combine and produce nitric and nitrous vapors, which are then condensed or directed to a sulphuric acid plant for efficient production.
Environmental Impact of HONO in Atmospheric Processes
Nitrous acid (HONO) plays a crucial role in atmospheric chemistry, significantly impacting air quality and climate change. As a key precursor to hydroxyl radicals (OH), HONO contributes substantially to the oxidative capacity of the atmosphere. Its presence in the troposphere has far-reaching consequences for both human health and environmental systems.
In urban environments, HONO concentrations can reach levels that directly affect air quality. The photolysis of HONO during daytime hours leads to the formation of OH radicals, which initiate the oxidation of volatile organic compounds (VOCs) and nitrogen oxides (NOx). This process results in the production of secondary pollutants, including ozone and particulate matter, which are known to have detrimental effects on human respiratory health and ecosystem functioning.
The environmental impact of HONO extends beyond local air quality issues. Its involvement in atmospheric aging processes influences the global climate system. HONO-derived OH radicals contribute to the formation of secondary organic aerosols (SOA), which affect cloud formation and precipitation patterns. These aerosols can alter the Earth's radiative balance by scattering or absorbing incoming solar radiation, thus influencing global temperature trends.
In coastal and marine environments, HONO plays a unique role in the chemistry of sea spray aerosols. Its presence in these aerosols can enhance the oxidation of organic matter, affecting the biogeochemical cycling of nutrients and trace elements in marine ecosystems. This process has implications for marine biodiversity and the ocean's capacity to act as a carbon sink.
The impact of HONO on atmospheric nitrogen cycling is another critical aspect of its environmental significance. HONO serves as a reservoir for reactive nitrogen in the atmosphere, influencing the deposition of nitrogen compounds to terrestrial and aquatic ecosystems. This deposition can lead to eutrophication in water bodies and changes in soil chemistry, affecting plant growth and biodiversity in sensitive ecosystems.
Furthermore, HONO's involvement in heterogeneous reactions on various surfaces, including urban building materials and vegetation, highlights its importance in modifying local atmospheric composition. These reactions can lead to the accumulation of pollutants in urban areas, exacerbating air quality issues during periods of low wind speed and temperature inversions.
In urban environments, HONO concentrations can reach levels that directly affect air quality. The photolysis of HONO during daytime hours leads to the formation of OH radicals, which initiate the oxidation of volatile organic compounds (VOCs) and nitrogen oxides (NOx). This process results in the production of secondary pollutants, including ozone and particulate matter, which are known to have detrimental effects on human respiratory health and ecosystem functioning.
The environmental impact of HONO extends beyond local air quality issues. Its involvement in atmospheric aging processes influences the global climate system. HONO-derived OH radicals contribute to the formation of secondary organic aerosols (SOA), which affect cloud formation and precipitation patterns. These aerosols can alter the Earth's radiative balance by scattering or absorbing incoming solar radiation, thus influencing global temperature trends.
In coastal and marine environments, HONO plays a unique role in the chemistry of sea spray aerosols. Its presence in these aerosols can enhance the oxidation of organic matter, affecting the biogeochemical cycling of nutrients and trace elements in marine ecosystems. This process has implications for marine biodiversity and the ocean's capacity to act as a carbon sink.
The impact of HONO on atmospheric nitrogen cycling is another critical aspect of its environmental significance. HONO serves as a reservoir for reactive nitrogen in the atmosphere, influencing the deposition of nitrogen compounds to terrestrial and aquatic ecosystems. This deposition can lead to eutrophication in water bodies and changes in soil chemistry, affecting plant growth and biodiversity in sensitive ecosystems.
Furthermore, HONO's involvement in heterogeneous reactions on various surfaces, including urban building materials and vegetation, highlights its importance in modifying local atmospheric composition. These reactions can lead to the accumulation of pollutants in urban areas, exacerbating air quality issues during periods of low wind speed and temperature inversions.
HONO Modeling and Simulation Techniques
Modeling and simulation techniques for nitrous acid (HONO) have become increasingly sophisticated in recent years, driven by the need to better understand its role in atmospheric chemistry and air quality. These techniques range from simple box models to complex three-dimensional chemical transport models, each with its own strengths and limitations.
One of the most widely used approaches is the incorporation of HONO chemistry into existing atmospheric models. This involves adding HONO formation and loss processes to the chemical mechanism, as well as including relevant emission sources. The Model of Emissions of Gases and Aerosols from Nature (MEGAN) has been adapted to include HONO emissions from soil and vegetation, providing a more comprehensive representation of natural sources.
For urban environments, computational fluid dynamics (CFD) models have been employed to simulate HONO formation and transport in street canyons and near buildings. These models can capture the complex interactions between HONO and urban surfaces, including heterogeneous reactions on building materials and the influence of urban heat islands on HONO chemistry.
Machine learning techniques have also been applied to HONO modeling, particularly for predicting HONO concentrations based on other atmospheric parameters. Neural networks and random forest algorithms have shown promise in capturing the non-linear relationships between HONO and its precursors, potentially improving short-term forecasting capabilities.
Quantum chemical calculations have been utilized to investigate the molecular-level processes involved in HONO formation and reactions. These simulations provide insights into reaction mechanisms and kinetics, which can then be incorporated into larger-scale atmospheric models to improve their accuracy.
Multi-scale modeling approaches have been developed to bridge the gap between molecular-level simulations and regional-scale atmospheric models. These techniques allow for a more seamless integration of processes occurring at different spatial and temporal scales, providing a more comprehensive understanding of HONO behavior in the atmosphere.
Sensitivity analysis and uncertainty quantification methods have become increasingly important in HONO modeling. These techniques help identify the most critical parameters and processes influencing HONO concentrations, guiding future research efforts and improving model reliability.
As computational power continues to increase, more complex and detailed HONO modeling approaches are becoming feasible. This includes the integration of aerosol microphysics, cloud chemistry, and heterogeneous reactions in a single modeling framework, allowing for a more comprehensive representation of HONO's role in atmospheric processes.
One of the most widely used approaches is the incorporation of HONO chemistry into existing atmospheric models. This involves adding HONO formation and loss processes to the chemical mechanism, as well as including relevant emission sources. The Model of Emissions of Gases and Aerosols from Nature (MEGAN) has been adapted to include HONO emissions from soil and vegetation, providing a more comprehensive representation of natural sources.
For urban environments, computational fluid dynamics (CFD) models have been employed to simulate HONO formation and transport in street canyons and near buildings. These models can capture the complex interactions between HONO and urban surfaces, including heterogeneous reactions on building materials and the influence of urban heat islands on HONO chemistry.
Machine learning techniques have also been applied to HONO modeling, particularly for predicting HONO concentrations based on other atmospheric parameters. Neural networks and random forest algorithms have shown promise in capturing the non-linear relationships between HONO and its precursors, potentially improving short-term forecasting capabilities.
Quantum chemical calculations have been utilized to investigate the molecular-level processes involved in HONO formation and reactions. These simulations provide insights into reaction mechanisms and kinetics, which can then be incorporated into larger-scale atmospheric models to improve their accuracy.
Multi-scale modeling approaches have been developed to bridge the gap between molecular-level simulations and regional-scale atmospheric models. These techniques allow for a more seamless integration of processes occurring at different spatial and temporal scales, providing a more comprehensive understanding of HONO behavior in the atmosphere.
Sensitivity analysis and uncertainty quantification methods have become increasingly important in HONO modeling. These techniques help identify the most critical parameters and processes influencing HONO concentrations, guiding future research efforts and improving model reliability.
As computational power continues to increase, more complex and detailed HONO modeling approaches are becoming feasible. This includes the integration of aerosol microphysics, cloud chemistry, and heterogeneous reactions in a single modeling framework, allowing for a more comprehensive representation of HONO's role in atmospheric processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!