Nitrous Acid in Marine Atmospheric Chemistry
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Marine HONO Background
Nitrous acid (HONO) plays a crucial role in marine atmospheric chemistry, significantly influencing the oxidative capacity of the troposphere and contributing to the formation of secondary pollutants. The study of HONO in marine environments has gained increasing attention in recent years due to its potential impact on air quality and climate change.
Marine HONO is primarily formed through heterogeneous reactions on various surfaces, including sea spray aerosols, ship emissions, and marine boundary layer interfaces. These processes are influenced by a complex interplay of factors such as sunlight, humidity, temperature, and the presence of other chemical species. The unique characteristics of the marine environment, such as high salinity and abundant aerosols, create distinct conditions for HONO formation and cycling.
The importance of HONO in marine atmospheric chemistry stems from its ability to rapidly photolyze in the presence of sunlight, producing hydroxyl radicals (OH). These OH radicals are often referred to as the "detergent" of the atmosphere due to their high reactivity and ability to initiate the oxidation of various trace gases. This process significantly affects the atmospheric oxidation capacity and the formation of secondary pollutants, including ozone and particulate matter.
Research on marine HONO has revealed several key aspects of its behavior in the marine atmosphere. Studies have shown that HONO concentrations in marine environments can be significantly higher than previously thought, with levels sometimes comparable to those observed in urban areas. This finding has important implications for understanding the oxidative capacity of the marine boundary layer and its impact on global atmospheric chemistry.
The sources and sinks of marine HONO are diverse and not yet fully understood. While direct emissions from the ocean surface and anthropogenic sources such as ship exhaust contribute to HONO levels, heterogeneous reactions on aerosol surfaces and at the air-sea interface are believed to be major sources. These processes are influenced by factors such as sea surface microlayer composition, aerosol pH, and the presence of photosensitizers.
Understanding the role of HONO in marine atmospheric chemistry is crucial for improving air quality models and climate predictions. The presence of HONO can lead to enhanced ozone formation in coastal areas and shipping lanes, potentially impacting human health and ecosystems. Additionally, HONO chemistry may influence the formation and growth of marine aerosols, which play a significant role in cloud formation and climate regulation.
Recent advancements in measurement techniques, including in-situ and remote sensing methods, have greatly improved our ability to study marine HONO. These developments have enabled more accurate quantification of HONO concentrations and fluxes in marine environments, providing valuable data for model validation and improvement.
Marine HONO is primarily formed through heterogeneous reactions on various surfaces, including sea spray aerosols, ship emissions, and marine boundary layer interfaces. These processes are influenced by a complex interplay of factors such as sunlight, humidity, temperature, and the presence of other chemical species. The unique characteristics of the marine environment, such as high salinity and abundant aerosols, create distinct conditions for HONO formation and cycling.
The importance of HONO in marine atmospheric chemistry stems from its ability to rapidly photolyze in the presence of sunlight, producing hydroxyl radicals (OH). These OH radicals are often referred to as the "detergent" of the atmosphere due to their high reactivity and ability to initiate the oxidation of various trace gases. This process significantly affects the atmospheric oxidation capacity and the formation of secondary pollutants, including ozone and particulate matter.
Research on marine HONO has revealed several key aspects of its behavior in the marine atmosphere. Studies have shown that HONO concentrations in marine environments can be significantly higher than previously thought, with levels sometimes comparable to those observed in urban areas. This finding has important implications for understanding the oxidative capacity of the marine boundary layer and its impact on global atmospheric chemistry.
The sources and sinks of marine HONO are diverse and not yet fully understood. While direct emissions from the ocean surface and anthropogenic sources such as ship exhaust contribute to HONO levels, heterogeneous reactions on aerosol surfaces and at the air-sea interface are believed to be major sources. These processes are influenced by factors such as sea surface microlayer composition, aerosol pH, and the presence of photosensitizers.
Understanding the role of HONO in marine atmospheric chemistry is crucial for improving air quality models and climate predictions. The presence of HONO can lead to enhanced ozone formation in coastal areas and shipping lanes, potentially impacting human health and ecosystems. Additionally, HONO chemistry may influence the formation and growth of marine aerosols, which play a significant role in cloud formation and climate regulation.
Recent advancements in measurement techniques, including in-situ and remote sensing methods, have greatly improved our ability to study marine HONO. These developments have enabled more accurate quantification of HONO concentrations and fluxes in marine environments, providing valuable data for model validation and improvement.
HONO Market Analysis
The market for nitrous acid (HONO) in marine atmospheric chemistry research is experiencing significant growth due to increasing awareness of its role in atmospheric processes and climate change. HONO plays a crucial part in the oxidation capacity of the marine boundary layer, influencing air quality and climate on a global scale. This has led to a surge in demand for HONO detection and measurement technologies in both academic and industrial sectors.
Research institutions and environmental monitoring agencies are the primary consumers of HONO-related products and services. The market is driven by the need for accurate, real-time measurements of HONO concentrations in marine environments. This demand has spurred the development of advanced analytical instruments, including spectroscopic techniques and chemical sensors specifically designed for marine atmospheric applications.
The global market for atmospheric chemistry research equipment, including HONO detection systems, is projected to grow steadily over the next five years. This growth is fueled by increased government funding for climate research, stricter environmental regulations, and a growing emphasis on understanding and mitigating the impacts of air pollution on marine ecosystems.
Key market segments include portable field instruments for on-site measurements, laboratory-grade analytical equipment for detailed studies, and integrated monitoring systems for long-term data collection. There is also a rising demand for data analysis software and cloud-based platforms that can process and interpret the complex datasets generated by HONO measurements in marine environments.
Geographically, North America and Europe currently dominate the market due to their advanced research infrastructure and strong focus on environmental sciences. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing environmental concerns and substantial investments in atmospheric research capabilities.
The competitive landscape is characterized by a mix of established scientific instrument manufacturers and specialized environmental monitoring companies. These firms are investing heavily in research and development to improve the sensitivity, reliability, and portability of HONO detection technologies. Collaborations between industry players and academic institutions are becoming more common, accelerating innovation in this field.
Despite the positive outlook, the market faces challenges such as the high cost of advanced analytical instruments and the complexity of interpreting HONO data in the context of broader atmospheric chemistry. There is a growing need for standardized measurement protocols and improved calibration techniques to ensure consistency across different research studies and monitoring programs.
Research institutions and environmental monitoring agencies are the primary consumers of HONO-related products and services. The market is driven by the need for accurate, real-time measurements of HONO concentrations in marine environments. This demand has spurred the development of advanced analytical instruments, including spectroscopic techniques and chemical sensors specifically designed for marine atmospheric applications.
The global market for atmospheric chemistry research equipment, including HONO detection systems, is projected to grow steadily over the next five years. This growth is fueled by increased government funding for climate research, stricter environmental regulations, and a growing emphasis on understanding and mitigating the impacts of air pollution on marine ecosystems.
Key market segments include portable field instruments for on-site measurements, laboratory-grade analytical equipment for detailed studies, and integrated monitoring systems for long-term data collection. There is also a rising demand for data analysis software and cloud-based platforms that can process and interpret the complex datasets generated by HONO measurements in marine environments.
Geographically, North America and Europe currently dominate the market due to their advanced research infrastructure and strong focus on environmental sciences. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing environmental concerns and substantial investments in atmospheric research capabilities.
The competitive landscape is characterized by a mix of established scientific instrument manufacturers and specialized environmental monitoring companies. These firms are investing heavily in research and development to improve the sensitivity, reliability, and portability of HONO detection technologies. Collaborations between industry players and academic institutions are becoming more common, accelerating innovation in this field.
Despite the positive outlook, the market faces challenges such as the high cost of advanced analytical instruments and the complexity of interpreting HONO data in the context of broader atmospheric chemistry. There is a growing need for standardized measurement protocols and improved calibration techniques to ensure consistency across different research studies and monitoring programs.
HONO Research Challenges
Research on nitrous acid (HONO) in marine atmospheric chemistry faces several significant challenges that hinder our comprehensive understanding of its role and impact. One of the primary obstacles is the complexity of HONO formation mechanisms in marine environments. Unlike terrestrial settings, the ocean surface provides unique conditions that influence HONO production, including sea spray aerosols, marine organic matter, and photochemical reactions at the air-sea interface. These intricate processes make it difficult to accurately quantify HONO sources and sinks in marine atmospheres.
Another major challenge lies in the measurement techniques for HONO in marine settings. The low concentrations of HONO in marine air, coupled with the presence of interfering species such as sea salt aerosols, demand highly sensitive and selective analytical methods. Current instrumentation often struggles to achieve the required detection limits without interference from other nitrogen-containing compounds, leading to potential inaccuracies in HONO quantification.
The spatial and temporal variability of HONO in marine environments presents an additional hurdle. HONO concentrations can fluctuate significantly over short distances and time scales due to factors such as solar radiation, wind patterns, and ocean surface conditions. This variability makes it challenging to obtain representative measurements and develop accurate models of HONO distribution and chemistry in marine atmospheres.
Furthermore, the interaction between HONO and other atmospheric components in marine settings is not fully understood. The presence of halogen species, abundant in marine air, may influence HONO chemistry in ways that are not yet fully elucidated. Additionally, the role of marine aerosols in HONO production and loss processes requires further investigation to determine their impact on overall HONO budgets in marine atmospheres.
The lack of long-term, continuous measurements of HONO in diverse marine environments is another significant challenge. Most studies have been limited to short-term campaigns or specific locations, leaving gaps in our understanding of seasonal variations and global distributions of HONO in marine atmospheres. This scarcity of data hampers efforts to validate and improve atmospheric models that incorporate HONO chemistry.
Lastly, the integration of HONO chemistry into global atmospheric models remains a challenge. The complex interplay between HONO and other atmospheric constituents, coupled with the unique characteristics of marine environments, makes it difficult to accurately parameterize HONO processes in large-scale models. Improving model representations of HONO chemistry in marine atmospheres is crucial for better predictions of air quality, climate impacts, and biogeochemical cycles in these regions.
Another major challenge lies in the measurement techniques for HONO in marine settings. The low concentrations of HONO in marine air, coupled with the presence of interfering species such as sea salt aerosols, demand highly sensitive and selective analytical methods. Current instrumentation often struggles to achieve the required detection limits without interference from other nitrogen-containing compounds, leading to potential inaccuracies in HONO quantification.
The spatial and temporal variability of HONO in marine environments presents an additional hurdle. HONO concentrations can fluctuate significantly over short distances and time scales due to factors such as solar radiation, wind patterns, and ocean surface conditions. This variability makes it challenging to obtain representative measurements and develop accurate models of HONO distribution and chemistry in marine atmospheres.
Furthermore, the interaction between HONO and other atmospheric components in marine settings is not fully understood. The presence of halogen species, abundant in marine air, may influence HONO chemistry in ways that are not yet fully elucidated. Additionally, the role of marine aerosols in HONO production and loss processes requires further investigation to determine their impact on overall HONO budgets in marine atmospheres.
The lack of long-term, continuous measurements of HONO in diverse marine environments is another significant challenge. Most studies have been limited to short-term campaigns or specific locations, leaving gaps in our understanding of seasonal variations and global distributions of HONO in marine atmospheres. This scarcity of data hampers efforts to validate and improve atmospheric models that incorporate HONO chemistry.
Lastly, the integration of HONO chemistry into global atmospheric models remains a challenge. The complex interplay between HONO and other atmospheric constituents, coupled with the unique characteristics of marine environments, makes it difficult to accurately parameterize HONO processes in large-scale models. Improving model representations of HONO chemistry in marine atmospheres is crucial for better predictions of air quality, climate impacts, and biogeochemical cycles in these regions.
Current HONO Detection
01 Production and synthesis of nitrous acid
Nitrous acid can be produced through various chemical processes, including the reaction of nitrogen oxides with water or the reduction of nitric acid. The synthesis methods often involve careful control of temperature, pressure, and reactant concentrations to optimize yield and purity.- Production and applications of nitrous acid: Nitrous acid is a weak and unstable acid with various industrial applications. It can be produced through different methods and is used in chemical processes, particularly in the production of diazonium salts for dye manufacturing and in metal surface treatment.
- Nitrous acid in environmental processes: Nitrous acid plays a role in atmospheric chemistry and environmental processes. It is involved in the formation of nitrogen oxides and can contribute to air pollution and acid rain. Research focuses on understanding its behavior and impact on the environment.
- Use of nitrous acid in material science: Nitrous acid is utilized in various material science applications, including the treatment of polymers, fibers, and other materials. It can modify surface properties, enhance adhesion, or facilitate chemical reactions in material processing.
- Analytical methods involving nitrous acid: Nitrous acid is used in analytical chemistry for various purposes, including as a reagent in spectrophotometric methods, in the detection and quantification of certain compounds, and in the development of sensors for environmental monitoring.
- Safety and handling of nitrous acid: Due to its corrosive and reactive nature, proper safety measures and handling procedures are crucial when working with nitrous acid. This includes appropriate storage, containment, and disposal methods, as well as the use of protective equipment to prevent exposure and accidents.
02 Applications in surface treatment and etching
Nitrous acid is utilized in surface treatment processes, particularly for etching and modifying metal surfaces. It can be employed in the preparation of materials for various industrial applications, including electronics and semiconductor manufacturing.Expand Specific Solutions03 Use in chemical analysis and detection
Nitrous acid plays a role in analytical chemistry, serving as a reagent for detecting and quantifying various compounds. It is used in colorimetric assays and other analytical techniques for environmental monitoring and quality control in industrial processes.Expand Specific Solutions04 Environmental impact and remediation
The environmental effects of nitrous acid, particularly its role in atmospheric chemistry and contribution to air pollution, are subjects of study. Research focuses on understanding its formation, reactions in the atmosphere, and developing methods for its removal or mitigation in industrial emissions.Expand Specific Solutions05 Industrial applications and process improvements
Nitrous acid is used in various industrial processes, including the production of dyes, pharmaceuticals, and other chemicals. Ongoing research aims to improve the efficiency and safety of these processes, focusing on reaction kinetics, catalysis, and process optimization.Expand Specific Solutions
Marine HONO Key Players
The research on nitrous acid in marine atmospheric chemistry is in a developing stage, with growing interest due to its potential impact on air quality and climate change. The market size for related technologies and solutions is expanding, driven by increasing environmental concerns and regulatory pressures. The field is characterized by a mix of academic institutions and private companies, indicating a moderate level of technological maturity. Key players include the Naval Research Laboratory, leading the government research efforts, and universities such as The University of Queensland and the National University of Singapore, contributing significant academic research. Companies like Hach Co. and Kurita Water Industries are developing practical applications and measurement technologies, suggesting a gradual transition from pure research to commercial applications in this niche but growing field.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory (NRL) has been at the forefront of marine atmospheric chemistry research, particularly focusing on nitrous acid (HONO). Their approach involves advanced spectroscopic techniques for in-situ measurements of HONO in marine environments. NRL has developed a high-sensitivity instrument using cavity ring-down spectroscopy (CRDS) for real-time detection of HONO at parts-per-trillion levels[1]. This technology allows for continuous monitoring of HONO in marine boundary layers, providing crucial data on its formation, distribution, and role in atmospheric processes. NRL's research also extends to studying the interactions between HONO and sea-salt aerosols, which is critical for understanding the marine nitrogen cycle[2].
Strengths: Advanced spectroscopic techniques, high-sensitivity measurements, and comprehensive understanding of marine atmospheric chemistry. Weaknesses: Limited to government-funded research, potentially restricting commercial applications.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has made significant contributions to the study of nitrous acid in marine atmospheric chemistry. Their approach combines laboratory experiments with field measurements to elucidate the mechanisms of HONO formation and its impact on marine environments. The institute has developed novel photochemical methods to study HONO production from sea-surface microlayers[3]. They utilize advanced mass spectrometry techniques to identify and quantify HONO precursors in seawater and marine aerosols. Additionally, their research extends to the role of HONO in marine boundary layer oxidation processes, providing insights into its influence on ozone formation and other atmospheric pollutants[4].
Strengths: Comprehensive approach combining lab and field studies, advanced analytical techniques. Weaknesses: Potential limitations in accessing diverse marine environments for global-scale studies.
HONO Formation Mechanisms
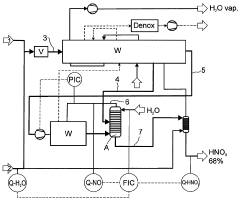
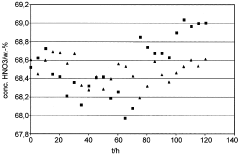
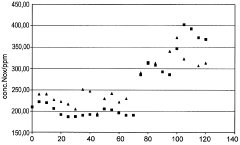
Process for preparing nitric acid having a concentration in the range from 50 to 77.8% by weight
PatentWO2009007355A1
Innovation
- A process that continuously measures and adjusts the water content of the process air and process water fed to the absorber to regulate nitric acid concentration between 50 to 77.8% by weight, while controlling nitrogen oxide levels in the absorber exhaust gas, using catalytic gas-phase oxidation of ammonia with a superstoichiometric air ratio and countercurrent absorption.
Environmental Impact
The environmental impact of nitrous acid (HONO) in marine atmospheric chemistry is significant and multifaceted. HONO plays a crucial role in the formation of hydroxyl radicals (OH), which are often referred to as the "detergent" of the atmosphere due to their ability to oxidize and remove pollutants. In marine environments, HONO can be produced through various pathways, including heterogeneous reactions on sea salt aerosols and photolysis of nitrate in seawater.
The presence of HONO in marine atmospheres contributes to the oxidative capacity of the air, influencing the lifetime and concentration of numerous atmospheric pollutants. This process has implications for air quality in coastal regions and can affect the global climate system. HONO-derived OH radicals can initiate the oxidation of volatile organic compounds (VOCs), leading to the formation of secondary organic aerosols (SOA). These aerosols can impact cloud formation processes and alter the Earth's radiation balance.
Furthermore, HONO chemistry in marine environments can influence the nitrogen cycle in coastal ecosystems. The deposition of HONO and its reaction products can contribute to nitrogen input in marine waters, potentially affecting phytoplankton growth and marine biodiversity. This nitrogen deposition can lead to eutrophication in coastal areas, causing algal blooms and oxygen depletion in marine habitats.
The interaction between HONO and sea salt aerosols also has implications for atmospheric halogen chemistry. HONO can react with chloride ions in sea salt particles, releasing reactive halogen species into the atmosphere. These halogen species can participate in ozone depletion processes, particularly in the marine boundary layer, affecting local and regional air quality.
Research on HONO in marine atmospheric chemistry has revealed its potential impact on the global sulfur cycle. HONO-derived OH radicals can oxidize dimethyl sulfide (DMS), a major biogenic sulfur compound emitted by marine phytoplankton. This oxidation process influences the formation of sulfate aerosols, which play a crucial role in cloud condensation nuclei (CCN) formation and, consequently, affect global climate through cloud-aerosol interactions.
The environmental impact of HONO extends to its role in the formation of nitric acid (HNO3) in marine atmospheres. HNO3 is a major component of acid deposition, which can have detrimental effects on marine ecosystems, including coral reefs and shellfish populations. The acidification of coastal waters through atmospheric deposition can disrupt the delicate balance of marine ecosystems and impact biodiversity.
The presence of HONO in marine atmospheres contributes to the oxidative capacity of the air, influencing the lifetime and concentration of numerous atmospheric pollutants. This process has implications for air quality in coastal regions and can affect the global climate system. HONO-derived OH radicals can initiate the oxidation of volatile organic compounds (VOCs), leading to the formation of secondary organic aerosols (SOA). These aerosols can impact cloud formation processes and alter the Earth's radiation balance.
Furthermore, HONO chemistry in marine environments can influence the nitrogen cycle in coastal ecosystems. The deposition of HONO and its reaction products can contribute to nitrogen input in marine waters, potentially affecting phytoplankton growth and marine biodiversity. This nitrogen deposition can lead to eutrophication in coastal areas, causing algal blooms and oxygen depletion in marine habitats.
The interaction between HONO and sea salt aerosols also has implications for atmospheric halogen chemistry. HONO can react with chloride ions in sea salt particles, releasing reactive halogen species into the atmosphere. These halogen species can participate in ozone depletion processes, particularly in the marine boundary layer, affecting local and regional air quality.
Research on HONO in marine atmospheric chemistry has revealed its potential impact on the global sulfur cycle. HONO-derived OH radicals can oxidize dimethyl sulfide (DMS), a major biogenic sulfur compound emitted by marine phytoplankton. This oxidation process influences the formation of sulfate aerosols, which play a crucial role in cloud condensation nuclei (CCN) formation and, consequently, affect global climate through cloud-aerosol interactions.
The environmental impact of HONO extends to its role in the formation of nitric acid (HNO3) in marine atmospheres. HNO3 is a major component of acid deposition, which can have detrimental effects on marine ecosystems, including coral reefs and shellfish populations. The acidification of coastal waters through atmospheric deposition can disrupt the delicate balance of marine ecosystems and impact biodiversity.
Policy Implications
The research on nitrous acid in marine atmospheric chemistry has significant policy implications that extend beyond the scientific realm. Policymakers and regulatory bodies need to consider the findings of this research when formulating environmental policies and regulations, particularly those related to air quality and marine ecosystem protection.
One of the primary policy implications is the need for more comprehensive monitoring and regulation of nitrous acid emissions in coastal areas. As the research highlights the importance of nitrous acid in marine atmospheric chemistry, policymakers should consider implementing stricter emission controls for industries and activities that contribute to nitrous acid formation. This may include revising existing air quality standards to specifically address nitrous acid levels in coastal regions.
Furthermore, the findings underscore the importance of integrated coastal zone management policies. Policymakers should consider developing and implementing strategies that take into account the complex interactions between land-based activities, atmospheric processes, and marine ecosystems. This holistic approach would help mitigate the potential negative impacts of nitrous acid on marine environments and coastal air quality.
The research also has implications for climate change policies. As nitrous acid plays a role in atmospheric chemistry and potentially affects climate processes, policymakers should incorporate these findings into climate models and mitigation strategies. This may involve reassessing the relative importance of different atmospheric pollutants and adjusting emission reduction targets accordingly.
Additionally, the research highlights the need for increased funding and support for marine atmospheric chemistry studies. Policymakers should consider allocating more resources to this field of research, as it has the potential to inform better environmental management practices and improve our understanding of global atmospheric processes.
International cooperation and policy coordination are also crucial in addressing the implications of this research. As marine atmospheric chemistry is a global issue, policymakers should work towards developing international agreements and standards for monitoring and regulating nitrous acid and related compounds in coastal areas. This may involve strengthening existing environmental treaties or creating new frameworks specifically focused on marine atmospheric chemistry.
Lastly, the research findings should inform educational policies and public awareness campaigns. Policymakers should consider incorporating marine atmospheric chemistry into environmental education curricula and supporting initiatives that raise public awareness about the importance of this field. This would help foster a more informed and engaged citizenry, capable of supporting and implementing necessary environmental policies.
One of the primary policy implications is the need for more comprehensive monitoring and regulation of nitrous acid emissions in coastal areas. As the research highlights the importance of nitrous acid in marine atmospheric chemistry, policymakers should consider implementing stricter emission controls for industries and activities that contribute to nitrous acid formation. This may include revising existing air quality standards to specifically address nitrous acid levels in coastal regions.
Furthermore, the findings underscore the importance of integrated coastal zone management policies. Policymakers should consider developing and implementing strategies that take into account the complex interactions between land-based activities, atmospheric processes, and marine ecosystems. This holistic approach would help mitigate the potential negative impacts of nitrous acid on marine environments and coastal air quality.
The research also has implications for climate change policies. As nitrous acid plays a role in atmospheric chemistry and potentially affects climate processes, policymakers should incorporate these findings into climate models and mitigation strategies. This may involve reassessing the relative importance of different atmospheric pollutants and adjusting emission reduction targets accordingly.
Additionally, the research highlights the need for increased funding and support for marine atmospheric chemistry studies. Policymakers should consider allocating more resources to this field of research, as it has the potential to inform better environmental management practices and improve our understanding of global atmospheric processes.
International cooperation and policy coordination are also crucial in addressing the implications of this research. As marine atmospheric chemistry is a global issue, policymakers should work towards developing international agreements and standards for monitoring and regulating nitrous acid and related compounds in coastal areas. This may involve strengthening existing environmental treaties or creating new frameworks specifically focused on marine atmospheric chemistry.
Lastly, the research findings should inform educational policies and public awareness campaigns. Policymakers should consider incorporating marine atmospheric chemistry into environmental education curricula and supporting initiatives that raise public awareness about the importance of this field. This would help foster a more informed and engaged citizenry, capable of supporting and implementing necessary environmental policies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!