Examining Photodegradation of Nitrous Acid in Natural Waters
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrous Acid Photodegradation Background and Objectives
Nitrous acid (HONO) photodegradation in natural waters has emerged as a critical area of study in environmental chemistry and atmospheric sciences. This process plays a significant role in the nitrogen cycle and atmospheric chemistry, influencing air quality and climate change. The evolution of research in this field has been driven by the growing recognition of HONO's importance in atmospheric processes and its complex behavior in aquatic environments.
Historically, the study of HONO photodegradation began in the late 20th century, with initial focus on atmospheric chemistry. As research progressed, scientists realized the need to understand HONO's behavior in natural waters, given its potential to act as a source of hydroxyl radicals and nitric oxide. This shift in focus has led to a more comprehensive approach in examining HONO's role in both atmospheric and aquatic systems.
The technological advancements in analytical chemistry and spectroscopy have been crucial in advancing our understanding of HONO photodegradation. Improved detection methods and in-situ measurement techniques have allowed researchers to study HONO dynamics in natural waters with unprecedented precision and temporal resolution.
Current research trends in HONO photodegradation are focused on elucidating the mechanisms of its formation and degradation in various aquatic environments. Scientists are particularly interested in understanding how factors such as pH, dissolved organic matter, and the presence of other chemical species influence HONO photochemistry. Additionally, there is growing interest in quantifying the contribution of HONO photodegradation to atmospheric HONO levels and its subsequent impact on air quality.
The primary objectives of studying HONO photodegradation in natural waters are multifaceted. Firstly, researchers aim to develop accurate models that can predict HONO formation and degradation rates in different aquatic ecosystems. This knowledge is crucial for understanding the global nitrogen cycle and improving atmospheric chemistry models.
Secondly, there is a strong focus on elucidating the environmental factors that control HONO photodegradation. This includes investigating the role of sunlight intensity, water chemistry, and biological processes in modulating HONO dynamics. Understanding these factors is essential for predicting how changes in climate and water quality might affect HONO photochemistry in the future.
Lastly, researchers are working towards quantifying the impact of HONO photodegradation on air quality and climate. This involves assessing the contribution of aquatic HONO sources to atmospheric HONO levels and evaluating the subsequent effects on tropospheric ozone formation and other atmospheric processes.
Historically, the study of HONO photodegradation began in the late 20th century, with initial focus on atmospheric chemistry. As research progressed, scientists realized the need to understand HONO's behavior in natural waters, given its potential to act as a source of hydroxyl radicals and nitric oxide. This shift in focus has led to a more comprehensive approach in examining HONO's role in both atmospheric and aquatic systems.
The technological advancements in analytical chemistry and spectroscopy have been crucial in advancing our understanding of HONO photodegradation. Improved detection methods and in-situ measurement techniques have allowed researchers to study HONO dynamics in natural waters with unprecedented precision and temporal resolution.
Current research trends in HONO photodegradation are focused on elucidating the mechanisms of its formation and degradation in various aquatic environments. Scientists are particularly interested in understanding how factors such as pH, dissolved organic matter, and the presence of other chemical species influence HONO photochemistry. Additionally, there is growing interest in quantifying the contribution of HONO photodegradation to atmospheric HONO levels and its subsequent impact on air quality.
The primary objectives of studying HONO photodegradation in natural waters are multifaceted. Firstly, researchers aim to develop accurate models that can predict HONO formation and degradation rates in different aquatic ecosystems. This knowledge is crucial for understanding the global nitrogen cycle and improving atmospheric chemistry models.
Secondly, there is a strong focus on elucidating the environmental factors that control HONO photodegradation. This includes investigating the role of sunlight intensity, water chemistry, and biological processes in modulating HONO dynamics. Understanding these factors is essential for predicting how changes in climate and water quality might affect HONO photochemistry in the future.
Lastly, researchers are working towards quantifying the impact of HONO photodegradation on air quality and climate. This involves assessing the contribution of aquatic HONO sources to atmospheric HONO levels and evaluating the subsequent effects on tropospheric ozone formation and other atmospheric processes.
Environmental Impact and Research Demand
The photodegradation of nitrous acid in natural waters has significant environmental implications and drives substantial research demand. This process plays a crucial role in atmospheric chemistry and water quality, affecting ecosystems and human health. Nitrous acid (HONO) is a key precursor to hydroxyl radicals, which are essential for atmospheric oxidation processes and the self-cleansing capacity of the atmosphere. Understanding its photodegradation in natural waters is vital for accurately modeling atmospheric chemistry and predicting air quality.
The environmental impact of HONO photodegradation extends to both aquatic and terrestrial ecosystems. In natural waters, this process influences the nitrogen cycle, potentially affecting nutrient availability for aquatic organisms. The breakdown of HONO can lead to the formation of other nitrogen-containing compounds, which may have varying effects on water quality and aquatic life. Additionally, the photodegradation of HONO in surface waters can contribute to the emission of nitrogen oxides into the atmosphere, further impacting air quality and climate.
Research demand in this area is driven by the need to better understand the complex interactions between HONO and natural water systems. Scientists are particularly interested in quantifying the rates of photodegradation under various environmental conditions, such as different pH levels, dissolved organic matter concentrations, and light intensities. This information is crucial for developing accurate models of atmospheric chemistry and water quality dynamics.
Furthermore, there is a growing interest in investigating the potential impact of climate change on HONO photodegradation processes. As global temperatures rise and precipitation patterns shift, the chemistry of natural waters may be altered, potentially affecting the rates and mechanisms of HONO breakdown. Understanding these changes is essential for predicting future air and water quality scenarios and developing appropriate mitigation strategies.
The research demand also extends to the development of advanced analytical techniques for measuring HONO concentrations and photodegradation products in natural waters. Improved methods for in-situ monitoring and remote sensing of HONO in aquatic environments are needed to enhance our understanding of its spatial and temporal distribution. This knowledge is crucial for assessing the environmental impact of HONO photodegradation on a larger scale and informing policy decisions related to air and water quality management.
The environmental impact of HONO photodegradation extends to both aquatic and terrestrial ecosystems. In natural waters, this process influences the nitrogen cycle, potentially affecting nutrient availability for aquatic organisms. The breakdown of HONO can lead to the formation of other nitrogen-containing compounds, which may have varying effects on water quality and aquatic life. Additionally, the photodegradation of HONO in surface waters can contribute to the emission of nitrogen oxides into the atmosphere, further impacting air quality and climate.
Research demand in this area is driven by the need to better understand the complex interactions between HONO and natural water systems. Scientists are particularly interested in quantifying the rates of photodegradation under various environmental conditions, such as different pH levels, dissolved organic matter concentrations, and light intensities. This information is crucial for developing accurate models of atmospheric chemistry and water quality dynamics.
Furthermore, there is a growing interest in investigating the potential impact of climate change on HONO photodegradation processes. As global temperatures rise and precipitation patterns shift, the chemistry of natural waters may be altered, potentially affecting the rates and mechanisms of HONO breakdown. Understanding these changes is essential for predicting future air and water quality scenarios and developing appropriate mitigation strategies.
The research demand also extends to the development of advanced analytical techniques for measuring HONO concentrations and photodegradation products in natural waters. Improved methods for in-situ monitoring and remote sensing of HONO in aquatic environments are needed to enhance our understanding of its spatial and temporal distribution. This knowledge is crucial for assessing the environmental impact of HONO photodegradation on a larger scale and informing policy decisions related to air and water quality management.
Current Understanding and Challenges
The current understanding of nitrous acid (HONO) photodegradation in natural waters has advanced significantly in recent years, yet several challenges remain. Research has shown that HONO plays a crucial role in atmospheric chemistry, particularly in the formation of hydroxyl radicals, which are key oxidants in the troposphere. However, the fate of HONO in aquatic environments is less well understood.
Studies have demonstrated that HONO undergoes photolysis in natural waters, with the rate of degradation influenced by various factors such as pH, temperature, and the presence of dissolved organic matter (DOM). The photodegradation process is believed to be primarily driven by direct photolysis and indirect photochemical reactions involving reactive oxygen species generated from DOM photosensitization.
One of the main challenges in studying HONO photodegradation is the complex interplay between different environmental factors. The pH of natural waters can significantly affect HONO stability and its photochemical behavior. Acidic conditions tend to favor HONO formation, while alkaline conditions promote its dissociation. This pH-dependent behavior complicates the understanding of HONO dynamics in diverse aquatic ecosystems.
Another challenge lies in accurately quantifying HONO concentrations in natural waters. The highly reactive nature of HONO and its rapid photolysis make it difficult to measure in situ. Current analytical techniques often require sample pretreatment or have limited sensitivity, potentially leading to underestimation of HONO levels and photodegradation rates.
The presence of DOM in natural waters adds another layer of complexity to HONO photodegradation studies. DOM can act as both a photosensitizer, enhancing HONO degradation, and a scavenger of reactive species, potentially inhibiting the process. The heterogeneous nature of DOM across different water bodies further complicates the development of universally applicable models for HONO photodegradation.
Seasonal variations and diurnal cycles also pose challenges in understanding HONO photodegradation. Solar radiation intensity, water temperature, and biological activity all fluctuate throughout the year and day, influencing HONO formation and degradation rates. Capturing these temporal dynamics requires long-term monitoring and sophisticated experimental designs.
Furthermore, the potential formation of byproducts during HONO photodegradation and their environmental impacts remain poorly understood. Identifying these byproducts and assessing their fate in aquatic ecosystems is crucial for comprehensively evaluating the environmental implications of HONO photochemistry in natural waters.
Studies have demonstrated that HONO undergoes photolysis in natural waters, with the rate of degradation influenced by various factors such as pH, temperature, and the presence of dissolved organic matter (DOM). The photodegradation process is believed to be primarily driven by direct photolysis and indirect photochemical reactions involving reactive oxygen species generated from DOM photosensitization.
One of the main challenges in studying HONO photodegradation is the complex interplay between different environmental factors. The pH of natural waters can significantly affect HONO stability and its photochemical behavior. Acidic conditions tend to favor HONO formation, while alkaline conditions promote its dissociation. This pH-dependent behavior complicates the understanding of HONO dynamics in diverse aquatic ecosystems.
Another challenge lies in accurately quantifying HONO concentrations in natural waters. The highly reactive nature of HONO and its rapid photolysis make it difficult to measure in situ. Current analytical techniques often require sample pretreatment or have limited sensitivity, potentially leading to underestimation of HONO levels and photodegradation rates.
The presence of DOM in natural waters adds another layer of complexity to HONO photodegradation studies. DOM can act as both a photosensitizer, enhancing HONO degradation, and a scavenger of reactive species, potentially inhibiting the process. The heterogeneous nature of DOM across different water bodies further complicates the development of universally applicable models for HONO photodegradation.
Seasonal variations and diurnal cycles also pose challenges in understanding HONO photodegradation. Solar radiation intensity, water temperature, and biological activity all fluctuate throughout the year and day, influencing HONO formation and degradation rates. Capturing these temporal dynamics requires long-term monitoring and sophisticated experimental designs.
Furthermore, the potential formation of byproducts during HONO photodegradation and their environmental impacts remain poorly understood. Identifying these byproducts and assessing their fate in aquatic ecosystems is crucial for comprehensively evaluating the environmental implications of HONO photochemistry in natural waters.
Analytical Methods for Nitrous Acid Detection
01 Photocatalytic degradation of nitrous acid
The process involves using photocatalysts to degrade nitrous acid under light exposure. This method can be effective for removing nitrous acid from various environments, including water and air. The photocatalytic reaction typically involves the generation of reactive oxygen species that break down the nitrous acid molecules.- Photocatalytic degradation of nitrous acid: Photocatalytic processes can be employed to degrade nitrous acid in various environments. This method utilizes light energy and catalysts to break down nitrous acid into less harmful compounds, improving air and water quality.
- UV light-induced decomposition of nitrous acid: Ultraviolet (UV) light can be used to induce the photodegradation of nitrous acid. This process involves the absorption of UV radiation by nitrous acid molecules, leading to their breakdown and the formation of simpler compounds.
- Nitrous acid photodegradation in atmospheric chemistry: The photodegradation of nitrous acid plays a significant role in atmospheric chemistry. This process contributes to the formation of hydroxyl radicals and affects the overall composition and reactivity of the atmosphere.
- Photosensitizers for enhanced nitrous acid degradation: Photosensitizers can be used to enhance the photodegradation of nitrous acid. These compounds absorb light and transfer energy to nitrous acid molecules, increasing the efficiency of the degradation process.
- Monitoring and control of nitrous acid photodegradation: Various techniques and systems can be employed to monitor and control the photodegradation of nitrous acid. These methods help optimize the degradation process and ensure its effectiveness in different applications and environments.
02 UV light-induced decomposition of nitrous acid
Ultraviolet (UV) light can be used to induce the decomposition of nitrous acid. This process involves the absorption of UV radiation by nitrous acid molecules, leading to their breakdown into simpler compounds. The method can be applied in various fields, including water treatment and air purification.Expand Specific Solutions03 Nitrous acid removal in industrial processes
Various industrial processes involve the removal or degradation of nitrous acid as a byproduct or contaminant. These methods may include chemical treatments, adsorption techniques, or specialized equipment designed to handle nitrous acid. The goal is often to reduce environmental impact and improve process efficiency.Expand Specific Solutions04 Photochemical reactions involving nitrous acid
Nitrous acid can participate in various photochemical reactions, which may lead to its degradation or transformation into other compounds. These reactions can be influenced by factors such as light intensity, wavelength, and the presence of other chemical species. Understanding these processes is crucial for developing effective nitrous acid management strategies.Expand Specific Solutions05 Monitoring and analysis of nitrous acid photodegradation
Techniques and methods for monitoring and analyzing the photodegradation of nitrous acid are essential for understanding the process and optimizing treatment strategies. This may involve spectroscopic methods, chemical analysis, or advanced sensing technologies to track the degradation progress and identify reaction products.Expand Specific Solutions
Key Research Institutions and Scientists
The examination of photodegradation of nitrous acid in natural waters is an emerging field in environmental chemistry, currently in its early development stage. The market size for this research area is relatively small but growing, driven by increasing concerns about water quality and environmental pollution. The technology's maturity is still evolving, with key players like Nanjing University, Hach Co., and CSIC leading research efforts. Companies such as Mitsubishi Gas Chemical and Advantage Controls are also contributing to advancements in water treatment technologies. The competitive landscape is characterized by collaboration between academic institutions and industry partners, with universities like Peking University and Arizona State University playing crucial roles in fundamental research. As environmental regulations tighten globally, this field is expected to gain more attention and investment in the coming years.
Nanjing University
Technical Solution: Nanjing University has developed advanced techniques for examining photodegradation of nitrous acid in natural waters. Their approach involves using high-performance liquid chromatography (HPLC) coupled with UV-visible spectroscopy to quantify nitrous acid concentrations and track its degradation over time[1]. They have also implemented a novel photochemical reactor design that simulates natural sunlight conditions, allowing for more accurate representation of environmental processes[3]. Additionally, the university has pioneered the use of isotope-labeling techniques to trace the fate of nitrogen compounds during photodegradation, providing insights into reaction mechanisms and pathways[5].
Strengths: Comprehensive analytical techniques, innovative reactor design, and isotope tracing capabilities. Weaknesses: Potential limitations in scaling up laboratory experiments to field conditions.
Consejo Superior de Investigaciones Científicas
Technical Solution: The Consejo Superior de Investigaciones Científicas (CSIC) has developed a multi-faceted approach to studying nitrous acid photodegradation in natural waters. Their methodology incorporates advanced spectroscopic techniques, including time-resolved laser spectroscopy, to capture the rapid kinetics of photochemical reactions[2]. CSIC researchers have also implemented a unique combination of field measurements and laboratory experiments, using custom-designed floating platforms equipped with in-situ sensors to monitor nitrous acid levels in various aquatic environments[4]. Furthermore, they have developed sophisticated computational models that integrate experimental data with atmospheric chemistry simulations to predict the impact of nitrous acid photodegradation on air quality and climate[6].
Strengths: Cutting-edge spectroscopic techniques, innovative field measurement systems, and advanced modeling capabilities. Weaknesses: Potential challenges in integrating diverse data sources and scaling up to global predictions.
Influential Studies on Nitrous Acid Photolysis
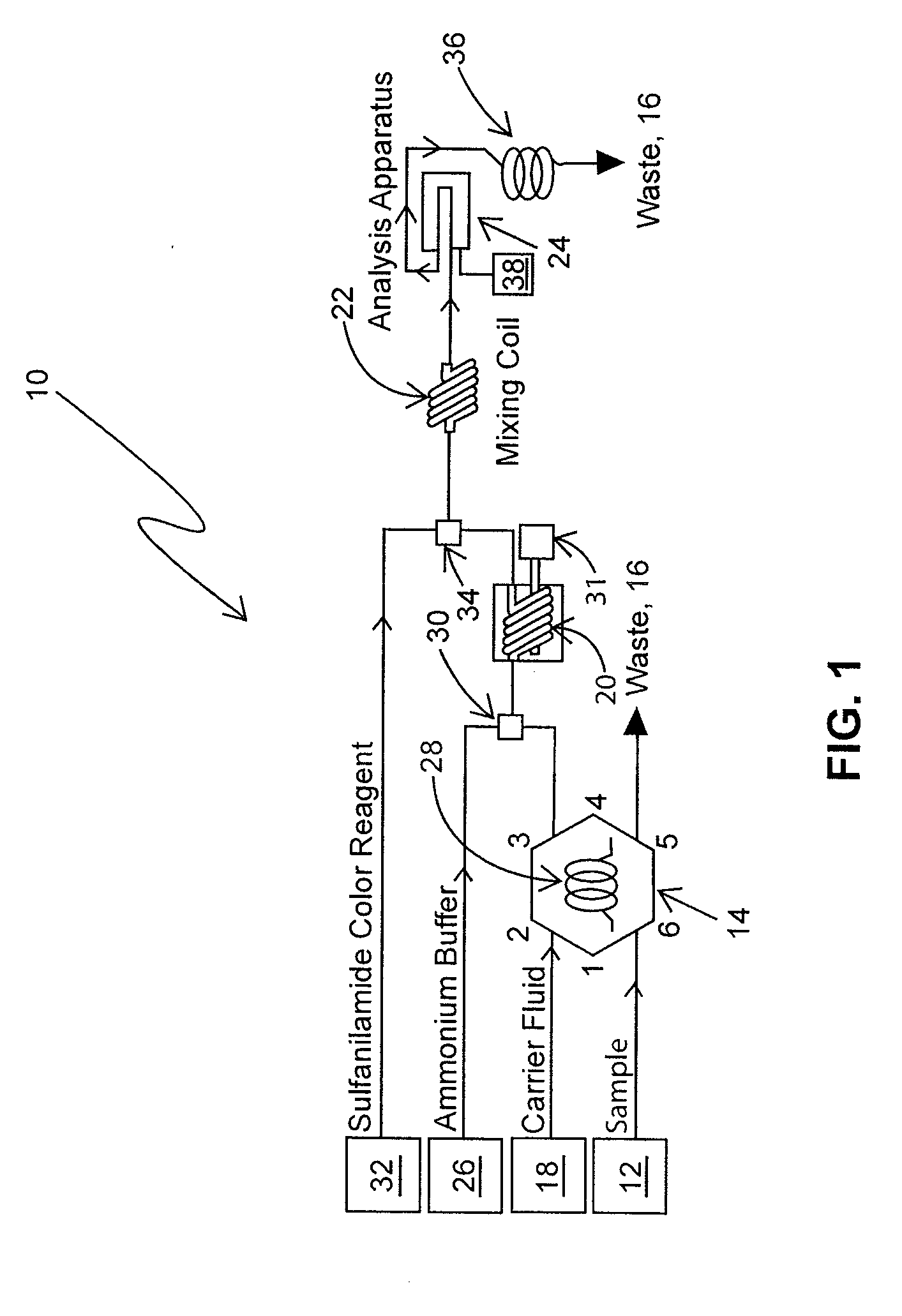
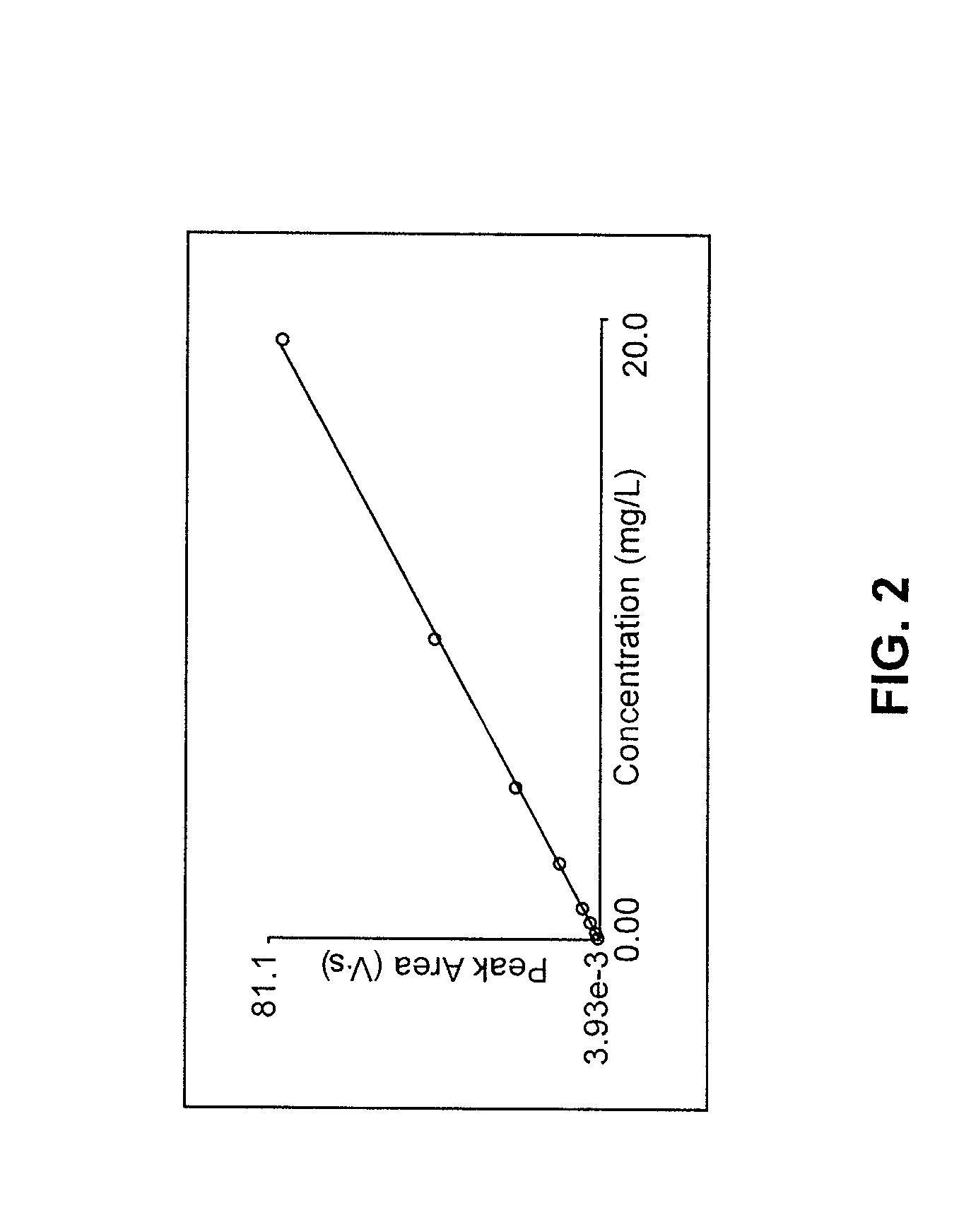
Determination of nitrate/nitrite concentration in water by photochemical reduction
PatentInactiveUS20110212533A1
Innovation
- A method using a buffered aqueous solution with ammonium chloride and ethylenediamine tetraacetic acid, adjusted to a specific pH, for photoreducing nitrates and nitrites to detectable species, achieving approximately 100% reduction efficiency without toxic materials.
determination of the nitrate/nitrite concentration in water by photochemical reduction
PatentInactiveDE112010003216T5
Innovation
- A method involving photoreduction of nitrates and nitrites to detectable species using a buffered aqueous solution with ethylenediaminetetraacetic acid (EDTA) and ammonium chloride, irradiated with UV light, followed by diazotization with sulfanilamide and N-(1-naphthyl)ethylenediamine dihydrochloride for colorimetric analysis, achieving nearly 100% reduction efficiency without toxic compounds.
Ecological Implications of Nitrous Acid Degradation
The photodegradation of nitrous acid in natural waters has significant ecological implications that extend beyond the immediate chemical reactions. This process plays a crucial role in the nitrogen cycle, influencing the availability of nitrogen compounds in aquatic ecosystems and, consequently, affecting the balance of various ecological processes.
One of the primary ecological implications is the alteration of nutrient availability for aquatic organisms. As nitrous acid degrades, it can lead to the formation of nitrite and nitrate ions, which are essential nutrients for phytoplankton and aquatic plants. This transformation can stimulate primary production in water bodies, potentially leading to algal blooms if the nutrient influx is excessive. Such blooms can have cascading effects on the entire aquatic food web, impacting fish populations and other higher-level consumers.
The photodegradation process also influences the pH balance of natural waters. As nitrous acid breaks down, it can contribute to the acidification of aquatic environments, particularly in areas with limited buffering capacity. This pH shift can affect the survival and reproduction of various aquatic species, especially those sensitive to changes in acidity levels, such as mollusks and certain fish species.
Furthermore, the degradation of nitrous acid can impact the biogeochemical cycling of other elements. For instance, it can influence the mobility and bioavailability of trace metals in aquatic systems. Some metals may become more soluble under changing pH conditions, potentially increasing their toxicity to aquatic organisms.
The photodegradation process also has implications for atmospheric chemistry and climate. Nitrous acid is a precursor to hydroxyl radicals, which play a crucial role in the self-cleaning capacity of the atmosphere. The breakdown of nitrous acid in natural waters can affect the flux of these compounds between aquatic and atmospheric systems, influencing regional air quality and potentially contributing to broader climate dynamics.
Additionally, the ecological implications extend to microbial communities in natural waters. The presence and degradation of nitrous acid can shape the composition and activity of microbial populations, favoring certain species that can utilize the resulting nitrogen compounds. This, in turn, can influence the broader ecological functions performed by these microbial communities, such as organic matter decomposition and nutrient cycling.
One of the primary ecological implications is the alteration of nutrient availability for aquatic organisms. As nitrous acid degrades, it can lead to the formation of nitrite and nitrate ions, which are essential nutrients for phytoplankton and aquatic plants. This transformation can stimulate primary production in water bodies, potentially leading to algal blooms if the nutrient influx is excessive. Such blooms can have cascading effects on the entire aquatic food web, impacting fish populations and other higher-level consumers.
The photodegradation process also influences the pH balance of natural waters. As nitrous acid breaks down, it can contribute to the acidification of aquatic environments, particularly in areas with limited buffering capacity. This pH shift can affect the survival and reproduction of various aquatic species, especially those sensitive to changes in acidity levels, such as mollusks and certain fish species.
Furthermore, the degradation of nitrous acid can impact the biogeochemical cycling of other elements. For instance, it can influence the mobility and bioavailability of trace metals in aquatic systems. Some metals may become more soluble under changing pH conditions, potentially increasing their toxicity to aquatic organisms.
The photodegradation process also has implications for atmospheric chemistry and climate. Nitrous acid is a precursor to hydroxyl radicals, which play a crucial role in the self-cleaning capacity of the atmosphere. The breakdown of nitrous acid in natural waters can affect the flux of these compounds between aquatic and atmospheric systems, influencing regional air quality and potentially contributing to broader climate dynamics.
Additionally, the ecological implications extend to microbial communities in natural waters. The presence and degradation of nitrous acid can shape the composition and activity of microbial populations, favoring certain species that can utilize the resulting nitrogen compounds. This, in turn, can influence the broader ecological functions performed by these microbial communities, such as organic matter decomposition and nutrient cycling.
Water Quality Regulations and Compliance
Water quality regulations and compliance play a crucial role in the context of examining photodegradation of nitrous acid in natural waters. These regulations are designed to protect human health and the environment by setting standards for various pollutants, including nitrous acid and its derivatives. In the United States, the Clean Water Act (CWA) serves as the primary federal law governing water pollution, with the Environmental Protection Agency (EPA) responsible for implementing and enforcing these regulations.
The EPA has established National Primary Drinking Water Regulations (NPDWRs) that set legally enforceable standards for contaminants in drinking water. While nitrous acid itself is not directly regulated, its potential breakdown products, such as nitrites and nitrates, are subject to strict limits. The maximum contaminant level (MCL) for nitrates is set at 10 mg/L, while the MCL for nitrites is 1 mg/L. These standards are crucial for water treatment facilities and environmental managers to consider when assessing the photodegradation of nitrous acid in natural waters.
In addition to federal regulations, many states have implemented their own water quality standards that may be more stringent than federal requirements. These state-level regulations often take into account local environmental conditions and specific water body characteristics, which can significantly impact the photodegradation processes of nitrous acid. Compliance with these regulations requires regular monitoring and reporting of water quality parameters, including those related to nitrogen compounds.
The European Union has also established comprehensive water quality regulations through the Water Framework Directive (WFD) and the Drinking Water Directive. These directives set similar standards for nitrates and nitrites, with maximum allowable concentrations of 50 mg/L and 0.5 mg/L, respectively. The WFD takes a holistic approach to water management, considering the entire water cycle and emphasizing the importance of understanding natural processes like photodegradation in maintaining water quality.
Compliance with these regulations necessitates a thorough understanding of the fate and transport of nitrous acid in natural waters, including its photodegradation pathways. Water treatment facilities must implement appropriate treatment technologies to ensure that nitrous acid and its photodegradation products do not exceed regulatory limits. This may involve advanced oxidation processes, UV treatment, or other methods that can effectively remove or transform these compounds.
Research into the photodegradation of nitrous acid in natural waters directly supports regulatory compliance efforts by providing valuable insights into the behavior of this compound under various environmental conditions. This knowledge helps in developing more effective water treatment strategies and in predicting potential water quality issues related to nitrous acid and its derivatives. As regulations continue to evolve based on new scientific findings, ongoing research in this area remains critical for maintaining compliance and protecting water resources.
The EPA has established National Primary Drinking Water Regulations (NPDWRs) that set legally enforceable standards for contaminants in drinking water. While nitrous acid itself is not directly regulated, its potential breakdown products, such as nitrites and nitrates, are subject to strict limits. The maximum contaminant level (MCL) for nitrates is set at 10 mg/L, while the MCL for nitrites is 1 mg/L. These standards are crucial for water treatment facilities and environmental managers to consider when assessing the photodegradation of nitrous acid in natural waters.
In addition to federal regulations, many states have implemented their own water quality standards that may be more stringent than federal requirements. These state-level regulations often take into account local environmental conditions and specific water body characteristics, which can significantly impact the photodegradation processes of nitrous acid. Compliance with these regulations requires regular monitoring and reporting of water quality parameters, including those related to nitrogen compounds.
The European Union has also established comprehensive water quality regulations through the Water Framework Directive (WFD) and the Drinking Water Directive. These directives set similar standards for nitrates and nitrites, with maximum allowable concentrations of 50 mg/L and 0.5 mg/L, respectively. The WFD takes a holistic approach to water management, considering the entire water cycle and emphasizing the importance of understanding natural processes like photodegradation in maintaining water quality.
Compliance with these regulations necessitates a thorough understanding of the fate and transport of nitrous acid in natural waters, including its photodegradation pathways. Water treatment facilities must implement appropriate treatment technologies to ensure that nitrous acid and its photodegradation products do not exceed regulatory limits. This may involve advanced oxidation processes, UV treatment, or other methods that can effectively remove or transform these compounds.
Research into the photodegradation of nitrous acid in natural waters directly supports regulatory compliance efforts by providing valuable insights into the behavior of this compound under various environmental conditions. This knowledge helps in developing more effective water treatment strategies and in predicting potential water quality issues related to nitrous acid and its derivatives. As regulations continue to evolve based on new scientific findings, ongoing research in this area remains critical for maintaining compliance and protecting water resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!