Nitrous Acid in Electrochemical Synthesis Applications
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrous Acid Synthesis Background and Objectives
Nitrous acid has been a subject of interest in electrochemical synthesis applications for several decades. The evolution of this technology can be traced back to the early 20th century when researchers first began exploring the potential of nitrous acid in various chemical processes. Over time, the focus has shifted towards harnessing the unique properties of nitrous acid in electrochemical reactions, particularly for the synthesis of valuable organic compounds.
The development of nitrous acid in electrochemical synthesis has been driven by the growing demand for more efficient and environmentally friendly production methods in the chemical industry. As traditional synthetic routes often involve harsh conditions and generate significant waste, electrochemical approaches using nitrous acid have emerged as promising alternatives. These methods offer the potential for milder reaction conditions, improved selectivity, and reduced environmental impact.
Recent advancements in electrochemistry and materials science have further accelerated the progress in this field. The introduction of novel electrode materials, improved cell designs, and sophisticated control systems have expanded the scope and efficiency of nitrous acid-mediated electrochemical syntheses. This has opened up new possibilities for the production of pharmaceuticals, fine chemicals, and other high-value products.
The primary objective of current research in this area is to optimize the use of nitrous acid in electrochemical synthesis applications. This includes enhancing reaction rates, improving product yields, and increasing the range of substrates that can be effectively transformed. Additionally, there is a strong focus on developing more sustainable processes by minimizing the use of hazardous reagents and reducing energy consumption.
Another key goal is to elucidate the underlying mechanisms of nitrous acid-mediated electrochemical reactions. Understanding the complex interplay between nitrous acid, electrode surfaces, and target molecules is crucial for designing more efficient and selective synthetic protocols. This knowledge will also facilitate the development of predictive models and computational tools to guide future research and process optimization.
As the field continues to evolve, researchers are exploring the integration of nitrous acid-based electrochemical synthesis with other emerging technologies. This includes the use of flow chemistry systems for continuous production, the application of artificial intelligence for process optimization, and the development of novel reactor designs for improved mass transfer and reaction control. These interdisciplinary approaches are expected to drive further innovation and expand the practical applications of nitrous acid in electrochemical synthesis.
The development of nitrous acid in electrochemical synthesis has been driven by the growing demand for more efficient and environmentally friendly production methods in the chemical industry. As traditional synthetic routes often involve harsh conditions and generate significant waste, electrochemical approaches using nitrous acid have emerged as promising alternatives. These methods offer the potential for milder reaction conditions, improved selectivity, and reduced environmental impact.
Recent advancements in electrochemistry and materials science have further accelerated the progress in this field. The introduction of novel electrode materials, improved cell designs, and sophisticated control systems have expanded the scope and efficiency of nitrous acid-mediated electrochemical syntheses. This has opened up new possibilities for the production of pharmaceuticals, fine chemicals, and other high-value products.
The primary objective of current research in this area is to optimize the use of nitrous acid in electrochemical synthesis applications. This includes enhancing reaction rates, improving product yields, and increasing the range of substrates that can be effectively transformed. Additionally, there is a strong focus on developing more sustainable processes by minimizing the use of hazardous reagents and reducing energy consumption.
Another key goal is to elucidate the underlying mechanisms of nitrous acid-mediated electrochemical reactions. Understanding the complex interplay between nitrous acid, electrode surfaces, and target molecules is crucial for designing more efficient and selective synthetic protocols. This knowledge will also facilitate the development of predictive models and computational tools to guide future research and process optimization.
As the field continues to evolve, researchers are exploring the integration of nitrous acid-based electrochemical synthesis with other emerging technologies. This includes the use of flow chemistry systems for continuous production, the application of artificial intelligence for process optimization, and the development of novel reactor designs for improved mass transfer and reaction control. These interdisciplinary approaches are expected to drive further innovation and expand the practical applications of nitrous acid in electrochemical synthesis.
Market Analysis for Electrochemical Nitrous Acid Applications
The market for electrochemical nitrous acid applications is experiencing significant growth, driven by increasing demand across various industries. The global market size for nitrous acid production and applications is projected to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) exceeding 5% during the forecast period. This growth is primarily attributed to the expanding use of nitrous acid in chemical synthesis, pharmaceuticals, and electronics manufacturing.
In the chemical industry, nitrous acid serves as a crucial intermediate in the production of various compounds, including dyes, pigments, and specialty chemicals. The rising demand for these end products, particularly in emerging economies, is fueling the market growth. Additionally, the pharmaceutical sector is witnessing increased adoption of nitrous acid in drug synthesis processes, contributing to market expansion.
The electronics industry represents another key market segment for electrochemical nitrous acid applications. With the growing demand for advanced electronic devices and semiconductors, the need for high-purity nitrous acid in etching and cleaning processes has surged. This trend is expected to continue as the electronics industry evolves and new technologies emerge.
Geographically, Asia-Pacific dominates the market for electrochemical nitrous acid applications, accounting for a substantial share of global consumption. This is primarily due to the region's robust industrial growth, particularly in China and India. North America and Europe follow, with steady demand from established chemical and pharmaceutical industries.
Environmental regulations and sustainability concerns are shaping market dynamics. There is a growing emphasis on developing eco-friendly production methods and reducing emissions associated with nitrous acid manufacturing. This has led to increased research and development efforts in green chemistry and sustainable production techniques, creating new opportunities for market players.
The market is characterized by the presence of several key players, including major chemical companies and specialized manufacturers. These companies are focusing on expanding their production capacities, improving product quality, and developing innovative applications to gain a competitive edge. Strategic partnerships and collaborations are also becoming more prevalent as companies seek to strengthen their market position and access new technologies.
Looking ahead, the market for electrochemical nitrous acid applications is poised for continued growth. Emerging applications in areas such as water treatment, agriculture, and renewable energy are expected to create new avenues for market expansion. However, challenges such as raw material price volatility and stringent regulatory requirements may impact market growth to some extent.
In the chemical industry, nitrous acid serves as a crucial intermediate in the production of various compounds, including dyes, pigments, and specialty chemicals. The rising demand for these end products, particularly in emerging economies, is fueling the market growth. Additionally, the pharmaceutical sector is witnessing increased adoption of nitrous acid in drug synthesis processes, contributing to market expansion.
The electronics industry represents another key market segment for electrochemical nitrous acid applications. With the growing demand for advanced electronic devices and semiconductors, the need for high-purity nitrous acid in etching and cleaning processes has surged. This trend is expected to continue as the electronics industry evolves and new technologies emerge.
Geographically, Asia-Pacific dominates the market for electrochemical nitrous acid applications, accounting for a substantial share of global consumption. This is primarily due to the region's robust industrial growth, particularly in China and India. North America and Europe follow, with steady demand from established chemical and pharmaceutical industries.
Environmental regulations and sustainability concerns are shaping market dynamics. There is a growing emphasis on developing eco-friendly production methods and reducing emissions associated with nitrous acid manufacturing. This has led to increased research and development efforts in green chemistry and sustainable production techniques, creating new opportunities for market players.
The market is characterized by the presence of several key players, including major chemical companies and specialized manufacturers. These companies are focusing on expanding their production capacities, improving product quality, and developing innovative applications to gain a competitive edge. Strategic partnerships and collaborations are also becoming more prevalent as companies seek to strengthen their market position and access new technologies.
Looking ahead, the market for electrochemical nitrous acid applications is poised for continued growth. Emerging applications in areas such as water treatment, agriculture, and renewable energy are expected to create new avenues for market expansion. However, challenges such as raw material price volatility and stringent regulatory requirements may impact market growth to some extent.
Current Challenges in Nitrous Acid Electrochemical Synthesis
The electrochemical synthesis of nitrous acid faces several significant challenges that hinder its widespread industrial application and efficiency. One of the primary obstacles is the low selectivity of the process, which often results in the formation of unwanted by-products. This issue stems from the complex redox chemistry involved in the electrochemical reduction of nitrate or nitrite to nitrous acid, where competing reactions can lead to the production of other nitrogen-containing compounds.
Another major challenge is the stability of nitrous acid under electrochemical conditions. Nitrous acid is inherently unstable and can readily decompose into nitric oxide and other nitrogen oxides, especially in acidic environments often present in electrochemical cells. This instability not only reduces the yield of the desired product but also complicates the control and optimization of the synthesis process.
The choice of electrode materials presents a significant hurdle in nitrous acid electrochemical synthesis. Many conventional electrode materials suffer from poor catalytic activity or rapid deactivation in the presence of nitrous acid or its precursors. This necessitates the development of novel, highly selective, and durable electrode materials that can withstand the corrosive nature of the reaction medium while maintaining high catalytic efficiency.
Energy efficiency is another critical challenge in the electrochemical production of nitrous acid. The process often requires high overpotentials to drive the desired reactions, leading to increased energy consumption and operational costs. Improving the energy efficiency while maintaining high product yields remains a key focus area for researchers and industry practitioners.
Scale-up and process integration pose significant engineering challenges. Translating laboratory-scale successes to industrial-scale production involves overcoming issues related to heat management, mass transfer limitations, and uniform current distribution across large electrode surfaces. These factors can significantly impact the overall efficiency and economic viability of the process.
Furthermore, the control of reaction parameters such as pH, temperature, and electrolyte composition is crucial for optimizing nitrous acid production. However, maintaining precise control over these parameters in a dynamic electrochemical environment, especially at larger scales, remains challenging. Fluctuations in these conditions can lead to inconsistent product quality and reduced process efficiency.
Lastly, the environmental and safety concerns associated with nitrous acid production and handling present ongoing challenges. The potential for the release of nitrogen oxides and other harmful gases during the synthesis process necessitates robust safety measures and emission control systems, adding complexity and cost to the overall production setup.
Another major challenge is the stability of nitrous acid under electrochemical conditions. Nitrous acid is inherently unstable and can readily decompose into nitric oxide and other nitrogen oxides, especially in acidic environments often present in electrochemical cells. This instability not only reduces the yield of the desired product but also complicates the control and optimization of the synthesis process.
The choice of electrode materials presents a significant hurdle in nitrous acid electrochemical synthesis. Many conventional electrode materials suffer from poor catalytic activity or rapid deactivation in the presence of nitrous acid or its precursors. This necessitates the development of novel, highly selective, and durable electrode materials that can withstand the corrosive nature of the reaction medium while maintaining high catalytic efficiency.
Energy efficiency is another critical challenge in the electrochemical production of nitrous acid. The process often requires high overpotentials to drive the desired reactions, leading to increased energy consumption and operational costs. Improving the energy efficiency while maintaining high product yields remains a key focus area for researchers and industry practitioners.
Scale-up and process integration pose significant engineering challenges. Translating laboratory-scale successes to industrial-scale production involves overcoming issues related to heat management, mass transfer limitations, and uniform current distribution across large electrode surfaces. These factors can significantly impact the overall efficiency and economic viability of the process.
Furthermore, the control of reaction parameters such as pH, temperature, and electrolyte composition is crucial for optimizing nitrous acid production. However, maintaining precise control over these parameters in a dynamic electrochemical environment, especially at larger scales, remains challenging. Fluctuations in these conditions can lead to inconsistent product quality and reduced process efficiency.
Lastly, the environmental and safety concerns associated with nitrous acid production and handling present ongoing challenges. The potential for the release of nitrogen oxides and other harmful gases during the synthesis process necessitates robust safety measures and emission control systems, adding complexity and cost to the overall production setup.
Current Electrochemical Synthesis Methods for Nitrous Acid
01 Production and applications of nitrous acid
Nitrous acid is a weak and unstable inorganic acid that plays a crucial role in various industrial processes. It is commonly produced through the reaction of nitrogen oxides with water or by the reduction of nitric acid. Nitrous acid finds applications in chemical synthesis, metal processing, and environmental studies.- Production and applications of nitrous acid: Nitrous acid is a weak and unstable inorganic acid that plays a crucial role in various industrial processes. It is commonly produced through the reaction of nitrogen oxides with water or by the reduction of nitric acid. Nitrous acid finds applications in chemical synthesis, dye manufacturing, and as an intermediate in the production of other nitrogen compounds.
- Nitrous acid in environmental chemistry: Nitrous acid is an important component in atmospheric chemistry and environmental processes. It contributes to the formation of acid rain and plays a role in the nitrogen cycle. Research focuses on understanding its impact on air quality, ozone depletion, and climate change. Monitoring and controlling nitrous acid emissions are crucial for environmental protection.
- Use of nitrous acid in surface treatment and etching: Nitrous acid is utilized in various surface treatment processes, particularly in the electronics and semiconductor industries. It is employed for etching and cleaning metal surfaces, as well as in the preparation of printed circuit boards. The controlled use of nitrous acid can enhance surface properties and improve adhesion in manufacturing processes.
- Nitrous acid in organic synthesis and chemical reactions: Nitrous acid serves as a versatile reagent in organic synthesis and chemical reactions. It is used in diazotization reactions, nitrosation processes, and the preparation of various organic compounds. The controlled generation and handling of nitrous acid are crucial for achieving desired chemical transformations in laboratory and industrial settings.
- Detection and analysis methods for nitrous acid: Accurate detection and analysis of nitrous acid are essential for environmental monitoring, quality control, and research purposes. Various analytical techniques have been developed, including spectrophotometric methods, electrochemical sensors, and chromatographic approaches. These methods enable the quantification of nitrous acid in different matrices and help in understanding its behavior in various systems.
02 Nitrous acid in surface treatment and etching
Nitrous acid is utilized in surface treatment processes, particularly for metals and semiconductors. It is effective in etching and cleaning surfaces, removing oxides, and preparing materials for further processing or coating. The controlled use of nitrous acid can enhance surface properties and improve adhesion in various manufacturing applications.Expand Specific Solutions03 Environmental impact and atmospheric chemistry
Nitrous acid plays a significant role in atmospheric chemistry and environmental processes. It is involved in the formation of photochemical smog and contributes to acid rain. Understanding the behavior and reactions of nitrous acid in the atmosphere is crucial for air quality management and environmental protection strategies.Expand Specific Solutions04 Analytical methods for nitrous acid detection
Various analytical techniques have been developed for the detection and quantification of nitrous acid in different matrices. These methods include spectrophotometry, chemiluminescence, and electrochemical sensors. Accurate measurement of nitrous acid is essential for monitoring air quality, industrial processes, and environmental studies.Expand Specific Solutions05 Nitrous acid in chemical synthesis and catalysis
Nitrous acid serves as a reagent and catalyst in numerous chemical reactions. It is particularly useful in diazotization reactions, nitrosation processes, and the synthesis of various organic compounds. The controlled generation and use of nitrous acid in chemical synthesis can lead to improved yields and selectivity in many industrial applications.Expand Specific Solutions
Key Industry Players in Electrochemical Nitrous Acid Production
The field of nitrous acid in electrochemical synthesis applications is in a nascent stage of development, with growing interest due to its potential in various industrial processes. The market size is relatively small but expanding, driven by increasing demand for sustainable chemical synthesis methods. Technologically, the field is still evolving, with companies like BASF Corp. and Agilent Technologies leading research efforts. BASF's expertise in chemical manufacturing and Agilent's advanced analytical instruments are contributing to the advancement of this technology. While not yet fully mature, the technology shows promise for future applications in green chemistry and energy-efficient synthesis processes.
BASF Corp.
Technical Solution: BASF has developed an innovative electrochemical synthesis process utilizing nitrous acid as a key reagent. Their approach involves a controlled oxidation of nitrogen oxides to produce nitrous acid in situ, which is then used as an electrophilic nitrosating agent in organic synthesis reactions. This method allows for the efficient production of various nitrogen-containing compounds, including pharmaceuticals and agrochemicals. BASF's process employs specially designed electrodes and optimized electrolyte compositions to enhance the selectivity and yield of desired products[1][3]. The company has also implemented advanced process control systems to maintain precise reaction conditions, ensuring consistent product quality and minimizing waste generation[5].
Strengths: High efficiency in nitrogen-containing compound synthesis, reduced environmental impact compared to traditional methods, scalable for industrial production. Weaknesses: Requires specialized equipment and expertise, potential safety concerns due to the reactive nature of nitrous acid.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced analytical techniques for monitoring and characterizing nitrous acid in electrochemical synthesis applications. Their approach combines high-performance liquid chromatography (HPLC) with mass spectrometry (MS) to provide real-time, in-situ analysis of reaction intermediates and products. This technology enables researchers to optimize reaction conditions and improve process efficiency. Agilent's system incorporates specialized electrochemical flow cells that allow for direct sampling from the reaction mixture, minimizing sample degradation and ensuring accurate results[2]. The company has also developed software algorithms for data analysis and interpretation, facilitating rapid identification of reaction pathways and potential side products[4].
Strengths: High-precision analysis of complex reaction mixtures, real-time monitoring capabilities, integration with existing electrochemical setups. Weaknesses: High initial investment cost, requires specialized training for operation and data interpretation.
Key Innovations in Nitrous Acid Electrochemical Synthesis
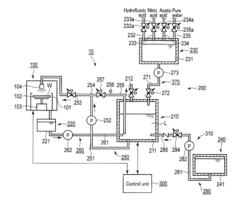
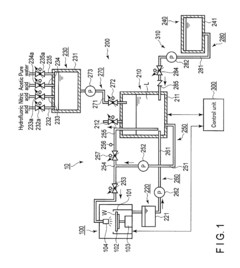
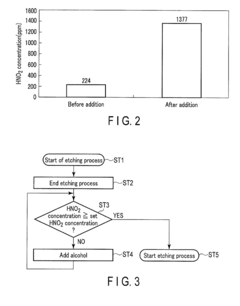
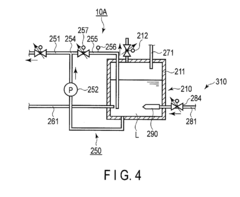
Device and method for treating a substrate with hydrofluoric and nitric acid
PatentActiveUS9972513B2
Innovation
- A substrate treating device and method that involves storing a treatment liquid containing hydrofluoric acid and nitric acid, and efficiently generating nitrous acid by feeding alcohol to the liquid, thereby maintaining a suitable nitrous acid concentration for effective etching through controlled addition of isopropyl alcohol.
A process for the electrochemical synthesis of nitric acid (HNO3) by the oxidation of inert nitrogen gas (N2), and the nitric acid produced thereby.
PatentUndeterminedIN202131029797A
Innovation
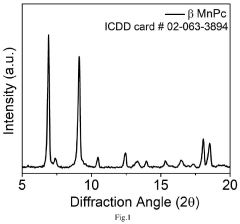
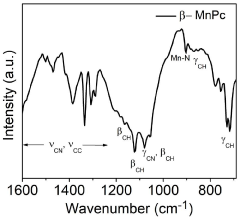
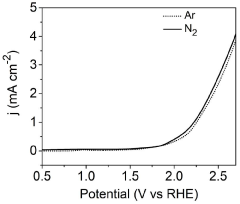
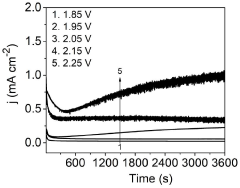
- An electrochemical process using metal phthalocyanine catalysts like MnPc, CuPc, and CoPc for the oxidation of nitrogen gas to produce nitric acid at ambient conditions, reducing energy consumption and environmental impact by operating at room temperature and pressure with low oxidation potential.
Environmental Impact of Nitrous Acid Electrochemical Synthesis
The environmental impact of nitrous acid in electrochemical synthesis applications is a critical consideration for sustainable industrial practices. Nitrous acid, while valuable in various electrochemical processes, poses significant environmental challenges that must be carefully managed.
One of the primary environmental concerns is the potential for nitrous acid to contribute to air pollution. When released into the atmosphere, nitrous acid can participate in photochemical reactions, leading to the formation of ground-level ozone and other harmful air pollutants. These pollutants can have detrimental effects on human health, vegetation, and ecosystems, particularly in urban areas where air quality is already compromised.
Water pollution is another significant environmental risk associated with nitrous acid use in electrochemical synthesis. Improper handling or disposal of nitrous acid-containing waste can lead to contamination of water bodies, affecting aquatic life and potentially entering the human water supply. The acidic nature of nitrous acid can also alter the pH balance of water systems, disrupting delicate ecological equilibria.
Soil contamination is a further environmental concern. Accidental spills or leaks of nitrous acid can result in soil acidification, negatively impacting soil fertility and microbial communities. This can have long-lasting effects on local ecosystems and agricultural productivity.
The production and use of nitrous acid in electrochemical synthesis also contribute to greenhouse gas emissions. The energy-intensive processes involved in its manufacture and application can lead to increased carbon dioxide emissions, exacerbating climate change concerns.
However, it is important to note that the environmental impact of nitrous acid can be mitigated through proper management and technological innovations. Advanced containment systems, efficient recycling processes, and improved synthesis methods can significantly reduce emissions and waste. Additionally, the development of alternative, more environmentally friendly reagents for electrochemical synthesis is an active area of research that holds promise for reducing the reliance on nitrous acid.
Regulatory frameworks play a crucial role in managing the environmental impact of nitrous acid use. Stringent guidelines for handling, storage, and disposal, coupled with regular environmental monitoring, can help minimize the negative effects on ecosystems and human health. Furthermore, industry-wide adoption of best practices and cleaner production technologies can lead to a more sustainable approach to electrochemical synthesis involving nitrous acid.
One of the primary environmental concerns is the potential for nitrous acid to contribute to air pollution. When released into the atmosphere, nitrous acid can participate in photochemical reactions, leading to the formation of ground-level ozone and other harmful air pollutants. These pollutants can have detrimental effects on human health, vegetation, and ecosystems, particularly in urban areas where air quality is already compromised.
Water pollution is another significant environmental risk associated with nitrous acid use in electrochemical synthesis. Improper handling or disposal of nitrous acid-containing waste can lead to contamination of water bodies, affecting aquatic life and potentially entering the human water supply. The acidic nature of nitrous acid can also alter the pH balance of water systems, disrupting delicate ecological equilibria.
Soil contamination is a further environmental concern. Accidental spills or leaks of nitrous acid can result in soil acidification, negatively impacting soil fertility and microbial communities. This can have long-lasting effects on local ecosystems and agricultural productivity.
The production and use of nitrous acid in electrochemical synthesis also contribute to greenhouse gas emissions. The energy-intensive processes involved in its manufacture and application can lead to increased carbon dioxide emissions, exacerbating climate change concerns.
However, it is important to note that the environmental impact of nitrous acid can be mitigated through proper management and technological innovations. Advanced containment systems, efficient recycling processes, and improved synthesis methods can significantly reduce emissions and waste. Additionally, the development of alternative, more environmentally friendly reagents for electrochemical synthesis is an active area of research that holds promise for reducing the reliance on nitrous acid.
Regulatory frameworks play a crucial role in managing the environmental impact of nitrous acid use. Stringent guidelines for handling, storage, and disposal, coupled with regular environmental monitoring, can help minimize the negative effects on ecosystems and human health. Furthermore, industry-wide adoption of best practices and cleaner production technologies can lead to a more sustainable approach to electrochemical synthesis involving nitrous acid.
Safety Protocols in Nitrous Acid Handling and Production
Safety protocols in nitrous acid handling and production are critical aspects of electrochemical synthesis applications. These protocols are designed to mitigate risks associated with the corrosive and potentially hazardous nature of nitrous acid. Proper handling procedures begin with appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing.
Storage of nitrous acid requires specialized containers made of materials resistant to acid corrosion, such as high-density polyethylene or glass. These containers must be kept in well-ventilated areas, away from direct sunlight and heat sources. Proper labeling and segregation from incompatible chemicals are essential to prevent accidental mixing and potential reactions.
During production and handling, engineering controls play a crucial role in maintaining safety. Fume hoods or local exhaust ventilation systems should be employed to minimize exposure to acid vapors. Closed systems and automated processes can further reduce the risk of direct contact with nitrous acid.
Emergency response procedures must be clearly defined and communicated to all personnel working with nitrous acid. This includes the location and proper use of safety showers, eyewash stations, and spill containment equipment. Regular safety drills and training sessions ensure that workers are prepared to respond effectively in case of accidents or spills.
Waste management is another critical aspect of nitrous acid safety protocols. Proper neutralization and disposal methods must be implemented to prevent environmental contamination and comply with regulatory requirements. This may involve the use of specialized waste treatment facilities or on-site neutralization processes.
Monitoring and detection systems are essential for maintaining a safe working environment. Gas detectors and pH monitors can provide early warning of potential leaks or unexpected changes in acid concentration. Regular inspections of equipment, storage areas, and safety systems help identify and address potential hazards before they escalate.
Documentation and record-keeping are integral components of safety protocols. Detailed safety data sheets (SDS) must be readily available, and all handling procedures should be documented in standard operating procedures (SOPs). Incident reporting and investigation processes should be in place to learn from any safety-related events and continuously improve safety measures.
Storage of nitrous acid requires specialized containers made of materials resistant to acid corrosion, such as high-density polyethylene or glass. These containers must be kept in well-ventilated areas, away from direct sunlight and heat sources. Proper labeling and segregation from incompatible chemicals are essential to prevent accidental mixing and potential reactions.
During production and handling, engineering controls play a crucial role in maintaining safety. Fume hoods or local exhaust ventilation systems should be employed to minimize exposure to acid vapors. Closed systems and automated processes can further reduce the risk of direct contact with nitrous acid.
Emergency response procedures must be clearly defined and communicated to all personnel working with nitrous acid. This includes the location and proper use of safety showers, eyewash stations, and spill containment equipment. Regular safety drills and training sessions ensure that workers are prepared to respond effectively in case of accidents or spills.
Waste management is another critical aspect of nitrous acid safety protocols. Proper neutralization and disposal methods must be implemented to prevent environmental contamination and comply with regulatory requirements. This may involve the use of specialized waste treatment facilities or on-site neutralization processes.
Monitoring and detection systems are essential for maintaining a safe working environment. Gas detectors and pH monitors can provide early warning of potential leaks or unexpected changes in acid concentration. Regular inspections of equipment, storage areas, and safety systems help identify and address potential hazards before they escalate.
Documentation and record-keeping are integral components of safety protocols. Detailed safety data sheets (SDS) must be readily available, and all handling procedures should be documented in standard operating procedures (SOPs). Incident reporting and investigation processes should be in place to learn from any safety-related events and continuously improve safety measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!