Nitrous Acid in Environmental Acid-Base Equilibria
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrous Acid Research Background and Objectives
Nitrous acid (HNO2) plays a crucial role in environmental acid-base equilibria, significantly impacting atmospheric chemistry and aquatic ecosystems. The study of nitrous acid has gained increasing attention due to its involvement in various environmental processes and its potential effects on human health and climate change.
The historical background of nitrous acid research dates back to the early 20th century when scientists first recognized its importance in atmospheric chemistry. However, it wasn't until the late 1970s and early 1980s that researchers began to fully appreciate the complex role of nitrous acid in environmental systems. This renewed interest was sparked by the discovery of its involvement in the formation of photochemical smog and its potential contribution to acid rain.
Over the past few decades, technological advancements have significantly enhanced our ability to detect and measure nitrous acid in various environmental matrices. These improvements have led to a more comprehensive understanding of its sources, sinks, and chemical behavior in both atmospheric and aquatic environments.
The primary objectives of current nitrous acid research focus on several key areas. First, researchers aim to elucidate the mechanisms of nitrous acid formation and destruction in different environmental compartments. This includes investigating heterogeneous reactions on various surfaces, such as urban building materials and soil particles, which are believed to be significant sources of atmospheric nitrous acid.
Another critical objective is to quantify the impact of nitrous acid on the oxidative capacity of the atmosphere. Nitrous acid photolysis is a major source of hydroxyl radicals, which play a vital role in atmospheric self-cleansing processes. Understanding this relationship is crucial for improving air quality models and developing effective pollution control strategies.
Furthermore, researchers are exploring the role of nitrous acid in nitrogen cycling within aquatic ecosystems. This includes studying its formation through microbial processes, its interactions with other nitrogen species, and its potential effects on aquatic organisms and water quality.
The environmental health implications of nitrous acid exposure represent another important research objective. Scientists are investigating the potential links between nitrous acid levels and respiratory health issues, particularly in urban environments where concentrations can be elevated.
Lastly, there is growing interest in understanding how climate change may affect nitrous acid dynamics in both atmospheric and aquatic systems. This includes examining potential feedback mechanisms and the long-term consequences of altered nitrous acid concentrations on environmental processes.
As research in this field progresses, the overarching goal is to develop a more comprehensive and integrated understanding of nitrous acid's role in environmental acid-base equilibria. This knowledge will be crucial for informing policy decisions, improving environmental management practices, and mitigating the potential negative impacts of nitrous acid on ecosystems and human health.
The historical background of nitrous acid research dates back to the early 20th century when scientists first recognized its importance in atmospheric chemistry. However, it wasn't until the late 1970s and early 1980s that researchers began to fully appreciate the complex role of nitrous acid in environmental systems. This renewed interest was sparked by the discovery of its involvement in the formation of photochemical smog and its potential contribution to acid rain.
Over the past few decades, technological advancements have significantly enhanced our ability to detect and measure nitrous acid in various environmental matrices. These improvements have led to a more comprehensive understanding of its sources, sinks, and chemical behavior in both atmospheric and aquatic environments.
The primary objectives of current nitrous acid research focus on several key areas. First, researchers aim to elucidate the mechanisms of nitrous acid formation and destruction in different environmental compartments. This includes investigating heterogeneous reactions on various surfaces, such as urban building materials and soil particles, which are believed to be significant sources of atmospheric nitrous acid.
Another critical objective is to quantify the impact of nitrous acid on the oxidative capacity of the atmosphere. Nitrous acid photolysis is a major source of hydroxyl radicals, which play a vital role in atmospheric self-cleansing processes. Understanding this relationship is crucial for improving air quality models and developing effective pollution control strategies.
Furthermore, researchers are exploring the role of nitrous acid in nitrogen cycling within aquatic ecosystems. This includes studying its formation through microbial processes, its interactions with other nitrogen species, and its potential effects on aquatic organisms and water quality.
The environmental health implications of nitrous acid exposure represent another important research objective. Scientists are investigating the potential links between nitrous acid levels and respiratory health issues, particularly in urban environments where concentrations can be elevated.
Lastly, there is growing interest in understanding how climate change may affect nitrous acid dynamics in both atmospheric and aquatic systems. This includes examining potential feedback mechanisms and the long-term consequences of altered nitrous acid concentrations on environmental processes.
As research in this field progresses, the overarching goal is to develop a more comprehensive and integrated understanding of nitrous acid's role in environmental acid-base equilibria. This knowledge will be crucial for informing policy decisions, improving environmental management practices, and mitigating the potential negative impacts of nitrous acid on ecosystems and human health.
Environmental Impact and Demand Analysis
The environmental impact of nitrous acid (HONO) in acid-base equilibria has gained significant attention due to its crucial role in atmospheric chemistry and its potential effects on ecosystems. HONO is a key precursor to hydroxyl radicals, which are often referred to as the "detergent" of the atmosphere due to their ability to oxidize and remove pollutants. The presence of HONO in the environment can lead to increased formation of ground-level ozone and secondary organic aerosols, both of which have detrimental effects on air quality and human health.
The demand for research on HONO in environmental acid-base equilibria has been driven by the need to better understand its sources, sinks, and overall impact on atmospheric processes. Urban areas, in particular, have shown elevated levels of HONO, which can contribute to photochemical smog formation and exacerbate air pollution issues. This has led to increased interest from environmental agencies, policymakers, and urban planners in developing strategies to mitigate HONO-related air quality problems.
Agricultural sectors have also expressed concern over the potential impacts of HONO on crop yields and soil chemistry. HONO can be formed through the interaction of nitrogen oxides with soil surfaces, potentially affecting soil pH and nutrient availability. This has sparked demand for research into the role of HONO in soil-atmosphere interactions and its implications for sustainable agriculture practices.
The water treatment industry has shown growing interest in HONO research due to its potential formation in chlorinated water systems. Understanding the behavior of HONO in aqueous environments is crucial for developing effective water treatment technologies and ensuring the safety of drinking water supplies. This has led to increased funding for studies on HONO equilibria in water treatment processes and distribution systems.
Climate scientists have also recognized the importance of HONO in global atmospheric chemistry models. The accurate representation of HONO formation and degradation processes is essential for improving climate predictions and understanding the long-term impacts of atmospheric composition changes. This has resulted in a surge of demand for HONO-related research from climate modeling groups and international environmental organizations.
The automotive industry has shown interest in HONO research due to its formation in vehicle exhaust systems. As emission regulations become more stringent, understanding the role of HONO in tailpipe emissions and its subsequent environmental impact has become crucial for developing cleaner engine technologies and after-treatment systems.
Overall, the environmental impact of HONO and the demand for related research span multiple sectors and disciplines. The complex interplay between HONO and various environmental systems underscores the need for comprehensive, interdisciplinary studies to fully understand its role in acid-base equilibria and develop effective strategies for mitigating its negative impacts while harnessing its potential benefits in certain applications.
The demand for research on HONO in environmental acid-base equilibria has been driven by the need to better understand its sources, sinks, and overall impact on atmospheric processes. Urban areas, in particular, have shown elevated levels of HONO, which can contribute to photochemical smog formation and exacerbate air pollution issues. This has led to increased interest from environmental agencies, policymakers, and urban planners in developing strategies to mitigate HONO-related air quality problems.
Agricultural sectors have also expressed concern over the potential impacts of HONO on crop yields and soil chemistry. HONO can be formed through the interaction of nitrogen oxides with soil surfaces, potentially affecting soil pH and nutrient availability. This has sparked demand for research into the role of HONO in soil-atmosphere interactions and its implications for sustainable agriculture practices.
The water treatment industry has shown growing interest in HONO research due to its potential formation in chlorinated water systems. Understanding the behavior of HONO in aqueous environments is crucial for developing effective water treatment technologies and ensuring the safety of drinking water supplies. This has led to increased funding for studies on HONO equilibria in water treatment processes and distribution systems.
Climate scientists have also recognized the importance of HONO in global atmospheric chemistry models. The accurate representation of HONO formation and degradation processes is essential for improving climate predictions and understanding the long-term impacts of atmospheric composition changes. This has resulted in a surge of demand for HONO-related research from climate modeling groups and international environmental organizations.
The automotive industry has shown interest in HONO research due to its formation in vehicle exhaust systems. As emission regulations become more stringent, understanding the role of HONO in tailpipe emissions and its subsequent environmental impact has become crucial for developing cleaner engine technologies and after-treatment systems.
Overall, the environmental impact of HONO and the demand for related research span multiple sectors and disciplines. The complex interplay between HONO and various environmental systems underscores the need for comprehensive, interdisciplinary studies to fully understand its role in acid-base equilibria and develop effective strategies for mitigating its negative impacts while harnessing its potential benefits in certain applications.
Current Challenges in Nitrous Acid Studies
The study of nitrous acid (HNO2) in environmental acid-base equilibria presents several significant challenges that researchers are currently grappling with. One of the primary difficulties lies in accurately measuring and quantifying nitrous acid concentrations in complex environmental matrices. The highly reactive nature of HNO2 and its rapid interconversion with other nitrogen species make it challenging to obtain precise and reliable measurements in real-world settings.
Another major challenge is understanding the role of nitrous acid in atmospheric chemistry and its impact on air quality. HNO2 plays a crucial role in the formation of tropospheric ozone and other secondary pollutants, but its exact mechanisms and contributions to these processes are not fully elucidated. Researchers are working to develop more sophisticated models that can accurately predict nitrous acid formation and its subsequent reactions in various atmospheric conditions.
The heterogeneous formation of nitrous acid on surfaces, particularly in urban environments, poses another significant challenge. While it is known that HNO2 can be produced through reactions on building materials, soil, and vegetation, the exact mechanisms and rates of these processes are not well understood. This lack of knowledge hinders our ability to accurately model and predict nitrous acid concentrations in different environmental settings.
Furthermore, the influence of environmental factors such as temperature, humidity, and the presence of other pollutants on nitrous acid equilibria is not fully characterized. These variables can significantly affect the formation, stability, and reactivity of HNO2, making it difficult to develop comprehensive models that account for all relevant factors.
The potential health impacts of nitrous acid exposure represent another area of ongoing research and concern. While HNO2 is known to contribute to the formation of harmful air pollutants, its direct effects on human health are not well-established. Researchers are working to better understand the toxicological profile of nitrous acid and its potential long-term health consequences.
Lastly, the development of effective mitigation strategies for nitrous acid in environmental systems remains a challenge. As our understanding of HNO2's role in air pollution and its environmental impacts grows, there is an increasing need for innovative technologies and approaches to reduce its formation and mitigate its effects. This includes exploring novel catalytic systems, improving air quality management practices, and developing more effective pollution control technologies.
Another major challenge is understanding the role of nitrous acid in atmospheric chemistry and its impact on air quality. HNO2 plays a crucial role in the formation of tropospheric ozone and other secondary pollutants, but its exact mechanisms and contributions to these processes are not fully elucidated. Researchers are working to develop more sophisticated models that can accurately predict nitrous acid formation and its subsequent reactions in various atmospheric conditions.
The heterogeneous formation of nitrous acid on surfaces, particularly in urban environments, poses another significant challenge. While it is known that HNO2 can be produced through reactions on building materials, soil, and vegetation, the exact mechanisms and rates of these processes are not well understood. This lack of knowledge hinders our ability to accurately model and predict nitrous acid concentrations in different environmental settings.
Furthermore, the influence of environmental factors such as temperature, humidity, and the presence of other pollutants on nitrous acid equilibria is not fully characterized. These variables can significantly affect the formation, stability, and reactivity of HNO2, making it difficult to develop comprehensive models that account for all relevant factors.
The potential health impacts of nitrous acid exposure represent another area of ongoing research and concern. While HNO2 is known to contribute to the formation of harmful air pollutants, its direct effects on human health are not well-established. Researchers are working to better understand the toxicological profile of nitrous acid and its potential long-term health consequences.
Lastly, the development of effective mitigation strategies for nitrous acid in environmental systems remains a challenge. As our understanding of HNO2's role in air pollution and its environmental impacts grows, there is an increasing need for innovative technologies and approaches to reduce its formation and mitigate its effects. This includes exploring novel catalytic systems, improving air quality management practices, and developing more effective pollution control technologies.
Analytical Methods for Nitrous Acid Detection
01 Production and applications of nitrous acid
Nitrous acid is a chemical compound with various industrial applications. It can be produced through different methods and is used in processes such as metal etching, dye manufacturing, and as an intermediate in chemical synthesis. The production and utilization of nitrous acid involve specific techniques and safety considerations due to its corrosive and reactive nature.- Production and applications of nitrous acid: Nitrous acid is a weak and unstable inorganic acid that plays a crucial role in various industrial processes. It is commonly produced through the reaction of nitrogen oxides with water or by the reduction of nitric acid. Nitrous acid finds applications in chemical synthesis, metal processing, and environmental studies.
- Use of nitrous acid in surface treatment: Nitrous acid is utilized in surface treatment processes for metals and other materials. It can be employed for etching, cleaning, and modifying surface properties. The controlled use of nitrous acid in these applications can enhance material characteristics and improve product quality.
- Environmental impact and detection of nitrous acid: Nitrous acid plays a significant role in atmospheric chemistry and environmental processes. Its presence and concentration in air and water are important factors in pollution studies. Various methods and technologies have been developed for the detection and measurement of nitrous acid in environmental samples.
- Nitrous acid in chemical synthesis: Nitrous acid serves as a key reagent in numerous chemical synthesis reactions. It is particularly useful in diazotization processes, where it reacts with primary aromatic amines to form diazonium salts. These intermediates are valuable in the production of dyes, pharmaceuticals, and other organic compounds.
- Safety and handling of nitrous acid: Due to its corrosive and reactive nature, proper safety measures and handling procedures are essential when working with nitrous acid. This includes appropriate storage conditions, use of protective equipment, and implementation of safety protocols to prevent accidents and minimize environmental impact.
02 Nitrous acid in environmental and atmospheric chemistry
Nitrous acid plays a significant role in atmospheric chemistry and environmental processes. It is involved in the formation of nitrogen oxides and contributes to air pollution and acid rain. Research in this area focuses on understanding the formation, reactions, and impacts of nitrous acid in the atmosphere, as well as developing methods for its detection and mitigation.Expand Specific Solutions03 Use of nitrous acid in material processing
Nitrous acid is utilized in various material processing applications, including surface treatment of metals, polymers, and other materials. It can be used for etching, cleaning, and modifying surface properties. The controlled use of nitrous acid in these processes can enhance material characteristics and improve product quality in industries such as electronics and manufacturing.Expand Specific Solutions04 Nitrous acid in chemical synthesis and reactions
Nitrous acid serves as an important reagent and intermediate in numerous chemical synthesis pathways. It is involved in diazotization reactions, nitrosation processes, and the production of various organic compounds. Understanding the reactivity and behavior of nitrous acid in different chemical environments is crucial for developing efficient and selective synthetic methods in the chemical industry.Expand Specific Solutions05 Safety and handling of nitrous acid
Due to its corrosive and reactive nature, proper safety measures and handling procedures are essential when working with nitrous acid. This includes appropriate storage, transportation, and disposal methods, as well as the use of protective equipment and engineering controls. Developing and implementing safety protocols for nitrous acid handling is crucial in industrial and laboratory settings to prevent accidents and ensure worker safety.Expand Specific Solutions
Key Institutions in Environmental Chemistry
The research on nitrous acid in environmental acid-base equilibria is in a developing stage, with growing market potential due to increasing environmental concerns. The technology's maturity varies among key players, with established companies like BASF Corp. and Hach Co. leading in chemical and water quality solutions. Academic institutions such as Zhejiang University and The University of Queensland contribute significantly to fundamental research. Emerging companies like Evogene Ltd. and Arcadia Biosciences, Inc. are exploring innovative applications in agriculture and biotechnology. The competitive landscape is diverse, featuring a mix of large corporations, specialized firms, and research institutions, indicating a dynamic and evolving field with opportunities for technological advancements and market growth.
Zhejiang University
Technical Solution: Zhejiang University has made significant contributions to the research on nitrous acid in environmental acid-base equilibria through their innovative approach to studying the heterogeneous formation of nitrous acid on various surfaces. They have developed a unique experimental setup that combines flow tube reactors with advanced analytical techniques such as chemiluminescence and cavity ring-down spectroscopy[4]. This setup allows for the precise measurement of nitrous acid formation rates and the investigation of its interactions with different environmental substrates. The university's research team has also explored the impact of relative humidity, temperature, and surface properties on nitrous acid production, providing valuable insights into its behavior in complex environmental systems[5].
Strengths: Advanced experimental setups, comprehensive analysis of environmental factors affecting nitrous acid formation. Weaknesses: Potential challenges in scaling up laboratory findings to real-world environmental conditions.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed advanced spectroscopic techniques for studying nitrous acid in environmental acid-base equilibria. They have implemented high-resolution laser spectroscopy and quantum chemical calculations to investigate the molecular structure and reactivity of nitrous acid in various environmental conditions[1]. Their research has led to the development of novel sensors for real-time monitoring of nitrous acid in atmospheric and aqueous environments, with detection limits as low as parts per trillion (ppt) levels[2]. The institute has also explored the role of nitrous acid in atmospheric chemistry, particularly its impact on the formation of secondary organic aerosols and its contribution to air pollution in urban areas[3].
Strengths: Cutting-edge spectroscopic techniques, high-sensitivity detection methods, and comprehensive understanding of nitrous acid's role in atmospheric chemistry. Weaknesses: Potential limitations in field applications and the need for specialized equipment.
Breakthrough Studies on Nitrous Acid Behavior
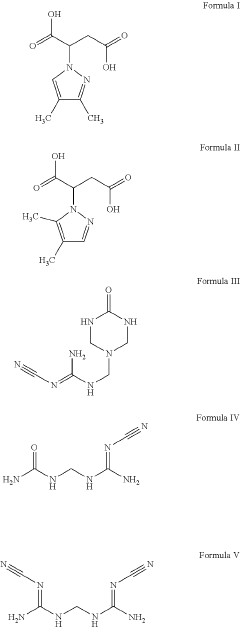
Mixtures comprising at least two different nitrification inhibitors selected from 2-(3,4-dimethyl-1h-pyrazol-1-yl)succinic acid (DMPSA), 3,4-dimethyl pyrazolium glycolate (DMPG) and other compounds
PatentInactiveUS20210371351A1
Innovation
- A mixture comprising a first nitrification inhibitor (such as 2-(3,4-dimethyl-1H-pyrazol-1-yl)succinic acid) and a second nitrification inhibitor (like 3,4-dimethyl pyrazole phosphate) is used, which acts synergistically to improve nitrification inhibition, plant growth, and nitrogen use efficiency, reducing nitrous oxide emissions and nitrate leaching.
Mixtures comprising a solid carrier comprising an urease inhibitor and a further solid carrier comprising a nitrification inhibitor
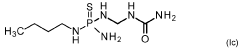
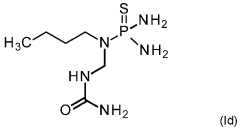
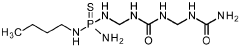
PatentWO2021144193A2
Innovation
- A mixture comprising a nitrification inhibitor, such as 2-(3,4-dimethyl-1H-pyrazol-1-yl)succinic acid, and a urease inhibitor, like N-(n-butyl)thiophosphoric acid triamide, is applied to soil or fertilizers to inhibit nitrification and urease activity, enhancing nitrogen retention and reducing emissions, thereby improving crop health and yield.
Regulatory Framework for Atmospheric Pollutants
The regulatory framework for atmospheric pollutants plays a crucial role in managing and mitigating the environmental impact of nitrous acid and other air contaminants. In the context of environmental acid-base equilibria, these regulations are designed to maintain air quality standards and protect public health.
At the international level, the United Nations Framework Convention on Climate Change (UNFCCC) and the Paris Agreement provide overarching guidelines for reducing greenhouse gas emissions, which indirectly affect nitrous acid formation. These agreements encourage nations to implement stricter controls on industrial emissions and promote cleaner technologies.
In the United States, the Clean Air Act (CAA) serves as the primary federal law governing air pollution control. The Environmental Protection Agency (EPA) is responsible for setting National Ambient Air Quality Standards (NAAQS) for six criteria pollutants, including nitrogen dioxide (NO2), which is closely related to nitrous acid formation. The EPA also regulates emissions of nitrogen oxides (NOx) from various sources, such as power plants and vehicles, through programs like the Cross-State Air Pollution Rule (CSAPR) and vehicle emission standards.
The European Union has established its own comprehensive regulatory framework for atmospheric pollutants. The Air Quality Directive (2008/50/EC) sets limits for various air pollutants, including NO2. Additionally, the National Emission Ceilings Directive (2016/2284/EU) requires member states to reduce emissions of five key air pollutants, including NOx, by specific percentages by 2030.
In Asia, countries like China and India have been implementing increasingly stringent air quality regulations. China's Air Pollution Prevention and Control Law, revised in 2015, sets national air quality standards and requires local governments to develop and implement air pollution control plans. India's National Clean Air Programme (NCAP) aims to reduce particulate matter concentrations by 20-30% by 2024 compared to 2017 levels.
Many countries have also adopted specific regulations targeting industrial emissions. These include Best Available Techniques (BAT) requirements, which mandate the use of the most effective and advanced practices to prevent or minimize emissions. Such regulations often focus on reducing NOx emissions, which indirectly impact nitrous acid levels in the atmosphere.
Monitoring and enforcement mechanisms are critical components of these regulatory frameworks. Continuous emission monitoring systems (CEMS) are widely used to track pollutant levels in real-time, ensuring compliance with emission limits. Penalties for non-compliance can include fines, mandatory equipment upgrades, or even facility closures in severe cases.
As research on nitrous acid in environmental acid-base equilibria progresses, regulatory frameworks are likely to evolve. Future regulations may incorporate more specific controls on nitrous acid precursors or set direct limits on atmospheric nitrous acid concentrations, based on improved understanding of its environmental impacts and formation mechanisms.
At the international level, the United Nations Framework Convention on Climate Change (UNFCCC) and the Paris Agreement provide overarching guidelines for reducing greenhouse gas emissions, which indirectly affect nitrous acid formation. These agreements encourage nations to implement stricter controls on industrial emissions and promote cleaner technologies.
In the United States, the Clean Air Act (CAA) serves as the primary federal law governing air pollution control. The Environmental Protection Agency (EPA) is responsible for setting National Ambient Air Quality Standards (NAAQS) for six criteria pollutants, including nitrogen dioxide (NO2), which is closely related to nitrous acid formation. The EPA also regulates emissions of nitrogen oxides (NOx) from various sources, such as power plants and vehicles, through programs like the Cross-State Air Pollution Rule (CSAPR) and vehicle emission standards.
The European Union has established its own comprehensive regulatory framework for atmospheric pollutants. The Air Quality Directive (2008/50/EC) sets limits for various air pollutants, including NO2. Additionally, the National Emission Ceilings Directive (2016/2284/EU) requires member states to reduce emissions of five key air pollutants, including NOx, by specific percentages by 2030.
In Asia, countries like China and India have been implementing increasingly stringent air quality regulations. China's Air Pollution Prevention and Control Law, revised in 2015, sets national air quality standards and requires local governments to develop and implement air pollution control plans. India's National Clean Air Programme (NCAP) aims to reduce particulate matter concentrations by 20-30% by 2024 compared to 2017 levels.
Many countries have also adopted specific regulations targeting industrial emissions. These include Best Available Techniques (BAT) requirements, which mandate the use of the most effective and advanced practices to prevent or minimize emissions. Such regulations often focus on reducing NOx emissions, which indirectly impact nitrous acid levels in the atmosphere.
Monitoring and enforcement mechanisms are critical components of these regulatory frameworks. Continuous emission monitoring systems (CEMS) are widely used to track pollutant levels in real-time, ensuring compliance with emission limits. Penalties for non-compliance can include fines, mandatory equipment upgrades, or even facility closures in severe cases.
As research on nitrous acid in environmental acid-base equilibria progresses, regulatory frameworks are likely to evolve. Future regulations may incorporate more specific controls on nitrous acid precursors or set direct limits on atmospheric nitrous acid concentrations, based on improved understanding of its environmental impacts and formation mechanisms.
Ecological Implications of Nitrous Acid Presence
The presence of nitrous acid in environmental acid-base equilibria has significant ecological implications that extend across various ecosystems. As a weak acid, nitrous acid plays a crucial role in atmospheric chemistry and contributes to the formation of acid rain, which can have far-reaching effects on terrestrial and aquatic environments.
In terrestrial ecosystems, the deposition of nitrous acid through precipitation or direct gas-phase interactions can lead to soil acidification. This process alters soil chemistry, affecting nutrient availability and microbial activity. Consequently, plant growth and biodiversity may be impacted, with acid-sensitive species experiencing reduced vigor or local extinction. Forest ecosystems are particularly vulnerable, as prolonged exposure to acidic conditions can result in the leaching of essential nutrients from soils, weakening trees and increasing their susceptibility to pests and diseases.
Aquatic ecosystems face unique challenges from nitrous acid presence. As it enters water bodies, it can lower the pH, creating more acidic conditions. This acidification can disrupt the delicate balance of aquatic life, affecting the survival and reproduction of fish, amphibians, and invertebrates. Sensitive species may experience physiological stress, reduced growth rates, and impaired reproductive success. Moreover, increased acidity can lead to the mobilization of toxic metals from sediments, further compromising water quality and ecosystem health.
The atmospheric reactions involving nitrous acid contribute to the formation of secondary pollutants, such as ozone and particulate matter. These pollutants have adverse effects on both plant and animal life, including reduced photosynthetic activity in plants and respiratory issues in animals. The formation of photochemical smog, partly driven by nitrous acid chemistry, can exacerbate these impacts in urban and industrial areas.
In the context of global climate change, the role of nitrous acid becomes even more significant. Its involvement in the nitrogen cycle and its potential to influence the formation of greenhouse gases underscore its importance in climate regulation. The complex interactions between nitrous acid, other atmospheric components, and various ecosystems highlight the need for comprehensive research to fully understand and mitigate its ecological impacts.
Understanding these ecological implications is crucial for developing effective environmental management strategies and policies. It emphasizes the importance of controlling nitrous acid emissions and monitoring its presence in different environmental compartments to protect ecosystem integrity and biodiversity.
In terrestrial ecosystems, the deposition of nitrous acid through precipitation or direct gas-phase interactions can lead to soil acidification. This process alters soil chemistry, affecting nutrient availability and microbial activity. Consequently, plant growth and biodiversity may be impacted, with acid-sensitive species experiencing reduced vigor or local extinction. Forest ecosystems are particularly vulnerable, as prolonged exposure to acidic conditions can result in the leaching of essential nutrients from soils, weakening trees and increasing their susceptibility to pests and diseases.
Aquatic ecosystems face unique challenges from nitrous acid presence. As it enters water bodies, it can lower the pH, creating more acidic conditions. This acidification can disrupt the delicate balance of aquatic life, affecting the survival and reproduction of fish, amphibians, and invertebrates. Sensitive species may experience physiological stress, reduced growth rates, and impaired reproductive success. Moreover, increased acidity can lead to the mobilization of toxic metals from sediments, further compromising water quality and ecosystem health.
The atmospheric reactions involving nitrous acid contribute to the formation of secondary pollutants, such as ozone and particulate matter. These pollutants have adverse effects on both plant and animal life, including reduced photosynthetic activity in plants and respiratory issues in animals. The formation of photochemical smog, partly driven by nitrous acid chemistry, can exacerbate these impacts in urban and industrial areas.
In the context of global climate change, the role of nitrous acid becomes even more significant. Its involvement in the nitrogen cycle and its potential to influence the formation of greenhouse gases underscore its importance in climate regulation. The complex interactions between nitrous acid, other atmospheric components, and various ecosystems highlight the need for comprehensive research to fully understand and mitigate its ecological impacts.
Understanding these ecological implications is crucial for developing effective environmental management strategies and policies. It emphasizes the importance of controlling nitrous acid emissions and monitoring its presence in different environmental compartments to protect ecosystem integrity and biodiversity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!