Nitrous Acid's Interaction with Water Vapor in the Atmosphere
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HONO Atmospheric Chemistry
Nitrous acid (HONO) plays a crucial role in atmospheric chemistry, particularly in the troposphere. Its interaction with water vapor significantly influences air quality, climate, and human health. HONO is a key source of hydroxyl radicals (OH), which are often referred to as the "detergent" of the atmosphere due to their ability to oxidize and remove pollutants.
The chemistry of HONO in the atmosphere is complex and multifaceted. In the presence of sunlight, HONO undergoes photolysis, producing OH radicals and nitric oxide (NO). This process is a major source of OH radicals, especially in the early morning hours when other OH sources are less active. The OH radicals then initiate various oxidation processes, affecting the concentrations of numerous atmospheric pollutants.
Water vapor plays a significant role in HONO chemistry. The interaction between HONO and water vapor can lead to the formation of nitrous acid in the gas phase through a reversible reaction. This equilibrium is temperature-dependent and influences the partitioning of HONO between the gas and aqueous phases in the atmosphere. Additionally, water vapor can affect the heterogeneous formation of HONO on surfaces, which is an important source of atmospheric HONO.
The heterogeneous formation of HONO on various surfaces, including aerosols, buildings, and vegetation, is a key aspect of its atmospheric chemistry. These processes are influenced by factors such as relative humidity, temperature, and the presence of other chemical species. For instance, the conversion of nitrogen dioxide (NO2) to HONO on wet surfaces is enhanced in the presence of water vapor, contributing to elevated HONO concentrations in urban environments.
HONO's atmospheric chemistry also involves complex interactions with other nitrogen oxides (NOx) and volatile organic compounds (VOCs). These interactions can lead to the formation of secondary pollutants, such as ozone and particulate matter, which have significant impacts on air quality and human health. The presence of water vapor can modulate these reactions, affecting the overall atmospheric composition and chemistry.
Understanding the atmospheric chemistry of HONO and its interaction with water vapor is crucial for accurately modeling air quality and predicting the formation of secondary pollutants. It also has implications for climate change studies, as HONO chemistry influences the oxidative capacity of the atmosphere and the lifetime of greenhouse gases. Ongoing research in this field continues to reveal new insights into the complex role of HONO in atmospheric processes and its broader environmental impacts.
The chemistry of HONO in the atmosphere is complex and multifaceted. In the presence of sunlight, HONO undergoes photolysis, producing OH radicals and nitric oxide (NO). This process is a major source of OH radicals, especially in the early morning hours when other OH sources are less active. The OH radicals then initiate various oxidation processes, affecting the concentrations of numerous atmospheric pollutants.
Water vapor plays a significant role in HONO chemistry. The interaction between HONO and water vapor can lead to the formation of nitrous acid in the gas phase through a reversible reaction. This equilibrium is temperature-dependent and influences the partitioning of HONO between the gas and aqueous phases in the atmosphere. Additionally, water vapor can affect the heterogeneous formation of HONO on surfaces, which is an important source of atmospheric HONO.
The heterogeneous formation of HONO on various surfaces, including aerosols, buildings, and vegetation, is a key aspect of its atmospheric chemistry. These processes are influenced by factors such as relative humidity, temperature, and the presence of other chemical species. For instance, the conversion of nitrogen dioxide (NO2) to HONO on wet surfaces is enhanced in the presence of water vapor, contributing to elevated HONO concentrations in urban environments.
HONO's atmospheric chemistry also involves complex interactions with other nitrogen oxides (NOx) and volatile organic compounds (VOCs). These interactions can lead to the formation of secondary pollutants, such as ozone and particulate matter, which have significant impacts on air quality and human health. The presence of water vapor can modulate these reactions, affecting the overall atmospheric composition and chemistry.
Understanding the atmospheric chemistry of HONO and its interaction with water vapor is crucial for accurately modeling air quality and predicting the formation of secondary pollutants. It also has implications for climate change studies, as HONO chemistry influences the oxidative capacity of the atmosphere and the lifetime of greenhouse gases. Ongoing research in this field continues to reveal new insights into the complex role of HONO in atmospheric processes and its broader environmental impacts.
Atmospheric Water Vapor Demand
The demand for atmospheric water vapor analysis in relation to nitrous acid (HONO) interactions has grown significantly in recent years due to its crucial role in atmospheric chemistry and climate science. Water vapor is a key component in the atmosphere, influencing various chemical reactions and physical processes. Its interaction with HONO is of particular interest due to the impact on air quality, climate change, and human health.
Atmospheric scientists and environmental researchers have identified a pressing need for accurate measurements and models of water vapor concentrations and their interactions with trace gases like HONO. This demand stems from the complex role water vapor plays in the formation, transformation, and removal of atmospheric pollutants. The heterogeneous reactions between HONO and water vapor on various surfaces, including aerosols and building materials, have become a focal point of research.
The increasing awareness of air quality issues in urban environments has further driven the demand for understanding HONO-water vapor interactions. As HONO is a significant source of hydroxyl radicals, which are crucial for atmospheric self-cleaning processes, its behavior in the presence of water vapor directly affects urban air quality management strategies. This has led to a surge in demand for advanced monitoring technologies and modeling approaches that can accurately capture these interactions.
Climate change research has also contributed to the growing interest in atmospheric water vapor demand related to HONO. As global temperatures rise, changes in water vapor distribution and concentration are expected to alter atmospheric chemistry significantly. Understanding how these changes will affect HONO production and cycling is vital for predicting future air quality scenarios and developing effective mitigation strategies.
The pharmaceutical and agricultural sectors have shown increased interest in atmospheric water vapor and HONO interactions due to their potential impacts on human health and crop yields. This has led to a rise in demand for interdisciplinary research combining atmospheric chemistry with health sciences and agronomy. The need for comprehensive data on how HONO-water vapor interactions affect air quality in both indoor and outdoor environments has become more pronounced.
Technological advancements in remote sensing and in-situ measurement techniques have also fueled the demand for more detailed studies on atmospheric water vapor and its interaction with trace gases like HONO. There is a growing need for high-resolution, real-time data to improve our understanding of these complex atmospheric processes and their implications for various sectors of society and the environment.
Atmospheric scientists and environmental researchers have identified a pressing need for accurate measurements and models of water vapor concentrations and their interactions with trace gases like HONO. This demand stems from the complex role water vapor plays in the formation, transformation, and removal of atmospheric pollutants. The heterogeneous reactions between HONO and water vapor on various surfaces, including aerosols and building materials, have become a focal point of research.
The increasing awareness of air quality issues in urban environments has further driven the demand for understanding HONO-water vapor interactions. As HONO is a significant source of hydroxyl radicals, which are crucial for atmospheric self-cleaning processes, its behavior in the presence of water vapor directly affects urban air quality management strategies. This has led to a surge in demand for advanced monitoring technologies and modeling approaches that can accurately capture these interactions.
Climate change research has also contributed to the growing interest in atmospheric water vapor demand related to HONO. As global temperatures rise, changes in water vapor distribution and concentration are expected to alter atmospheric chemistry significantly. Understanding how these changes will affect HONO production and cycling is vital for predicting future air quality scenarios and developing effective mitigation strategies.
The pharmaceutical and agricultural sectors have shown increased interest in atmospheric water vapor and HONO interactions due to their potential impacts on human health and crop yields. This has led to a rise in demand for interdisciplinary research combining atmospheric chemistry with health sciences and agronomy. The need for comprehensive data on how HONO-water vapor interactions affect air quality in both indoor and outdoor environments has become more pronounced.
Technological advancements in remote sensing and in-situ measurement techniques have also fueled the demand for more detailed studies on atmospheric water vapor and its interaction with trace gases like HONO. There is a growing need for high-resolution, real-time data to improve our understanding of these complex atmospheric processes and their implications for various sectors of society and the environment.
HONO-H2O Interaction Challenges
The interaction between nitrous acid (HONO) and water vapor in the atmosphere presents significant challenges for atmospheric chemistry research. One of the primary difficulties lies in accurately measuring and quantifying HONO concentrations due to its rapid photolysis and heterogeneous formation processes. The presence of water vapor further complicates these measurements, as it can interfere with spectroscopic detection methods and influence HONO's chemical behavior.
Another major challenge is understanding the complex mechanisms of HONO formation and loss in the presence of water vapor. While it is known that heterogeneous reactions on various surfaces play a crucial role in HONO production, the exact pathways and kinetics of these processes in the presence of varying humidity levels remain unclear. This uncertainty extends to both daytime and nighttime chemistry, where different mechanisms may dominate.
The role of aerosols in HONO-H2O interactions poses additional challenges. Aerosol particles can serve as reaction sites for HONO formation and provide surfaces for adsorption and desorption processes. However, the influence of particle composition, size distribution, and water content on these interactions is not fully understood, making it difficult to accurately model HONO chemistry in atmospheric models.
Climate change and its impact on atmospheric water vapor content further complicate the study of HONO-H2O interactions. As global temperatures rise, changes in humidity patterns may alter HONO formation and loss rates, potentially leading to feedback loops that affect air quality and climate. Predicting these long-term effects requires a comprehensive understanding of HONO-H2O chemistry across a wide range of environmental conditions.
The development of advanced measurement techniques and modeling approaches is crucial for addressing these challenges. Improved in situ and remote sensing methods are needed to accurately quantify HONO and water vapor concentrations simultaneously. Additionally, more sophisticated laboratory experiments that can simulate realistic atmospheric conditions are essential for elucidating the fundamental processes governing HONO-H2O interactions.
Integrating HONO-H2O chemistry into atmospheric models presents its own set of challenges. The complexity of the interactions and the wide range of influencing factors make it difficult to develop parameterizations that accurately represent HONO chemistry across different spatial and temporal scales. Bridging the gap between laboratory studies, field measurements, and model simulations remains a significant hurdle in advancing our understanding of this important atmospheric process.
Another major challenge is understanding the complex mechanisms of HONO formation and loss in the presence of water vapor. While it is known that heterogeneous reactions on various surfaces play a crucial role in HONO production, the exact pathways and kinetics of these processes in the presence of varying humidity levels remain unclear. This uncertainty extends to both daytime and nighttime chemistry, where different mechanisms may dominate.
The role of aerosols in HONO-H2O interactions poses additional challenges. Aerosol particles can serve as reaction sites for HONO formation and provide surfaces for adsorption and desorption processes. However, the influence of particle composition, size distribution, and water content on these interactions is not fully understood, making it difficult to accurately model HONO chemistry in atmospheric models.
Climate change and its impact on atmospheric water vapor content further complicate the study of HONO-H2O interactions. As global temperatures rise, changes in humidity patterns may alter HONO formation and loss rates, potentially leading to feedback loops that affect air quality and climate. Predicting these long-term effects requires a comprehensive understanding of HONO-H2O chemistry across a wide range of environmental conditions.
The development of advanced measurement techniques and modeling approaches is crucial for addressing these challenges. Improved in situ and remote sensing methods are needed to accurately quantify HONO and water vapor concentrations simultaneously. Additionally, more sophisticated laboratory experiments that can simulate realistic atmospheric conditions are essential for elucidating the fundamental processes governing HONO-H2O interactions.
Integrating HONO-H2O chemistry into atmospheric models presents its own set of challenges. The complexity of the interactions and the wide range of influencing factors make it difficult to develop parameterizations that accurately represent HONO chemistry across different spatial and temporal scales. Bridging the gap between laboratory studies, field measurements, and model simulations remains a significant hurdle in advancing our understanding of this important atmospheric process.
Current HONO Detection Methods
01 Production and applications of nitrous acid
Nitrous acid is a weak and unstable acid with various industrial applications. It can be produced through different methods and is used in chemical processes, particularly in the production of diazonium salts for dye manufacturing. Its properties and reactivity make it valuable in certain industrial and laboratory settings.- Production and synthesis of nitrous acid: Various methods for producing and synthesizing nitrous acid are described, including chemical reactions and industrial processes. These methods often involve the use of nitrogen oxides or nitrites as precursors.
- Applications in material processing: Nitrous acid is utilized in various material processing applications, such as surface treatment of metals, etching of semiconductors, and modification of polymers. These processes can enhance material properties or create specific surface characteristics.
- Environmental and atmospheric chemistry: The role of nitrous acid in atmospheric chemistry and environmental processes is explored. This includes its formation in the atmosphere, its impact on air quality, and its involvement in various chemical reactions affecting the environment.
- Analytical methods and detection techniques: Various analytical methods and detection techniques for nitrous acid are described. These include spectroscopic methods, electrochemical sensors, and other innovative approaches for measuring nitrous acid concentrations in different media.
- Industrial applications and processes: Nitrous acid is used in various industrial applications and processes, including chemical manufacturing, wastewater treatment, and as a reagent in organic synthesis. These applications leverage the unique chemical properties of nitrous acid.
02 Nitrous acid in water treatment and environmental processes
Nitrous acid plays a role in water treatment and environmental processes. It is involved in the nitrogen cycle and can be found in natural water systems. In water treatment, it can be used for disinfection and in processes related to nitrogen removal. Its presence and reactions are important considerations in environmental chemistry and pollution control.Expand Specific Solutions03 Analytical methods for nitrous acid detection and measurement
Various analytical techniques have been developed for the detection and measurement of nitrous acid in different matrices. These methods are important for monitoring environmental samples, industrial processes, and quality control. Spectrophotometric, electrochemical, and chromatographic techniques are among the approaches used for nitrous acid analysis.Expand Specific Solutions04 Nitrous acid in materials science and surface treatment
Nitrous acid is utilized in certain materials science applications and surface treatment processes. It can be involved in the modification of material surfaces, etching processes, and in the preparation of specialized coatings or films. Its reactivity with various materials makes it useful in specific industrial applications related to surface chemistry and materials engineering.Expand Specific Solutions05 Safety and handling considerations for nitrous acid
Due to its corrosive nature and potential health hazards, proper safety measures and handling procedures are crucial when working with nitrous acid. This includes appropriate storage, personal protective equipment, and disposal methods. Understanding its reactivity and potential decomposition products is important for safe industrial use and laboratory practices.Expand Specific Solutions
Key Atmospheric Chemistry Players
The interaction of nitrous acid with water vapor in the atmosphere represents a complex and evolving field of study. The research landscape is characterized by a mix of academic institutions and industrial players, reflecting the early to mid-stage development of this area. Key academic contributors include the University of Sydney, Chinese Academy of Science Institute of Chemistry, and Michigan Technological University, indicating a strong focus on fundamental research. Industrial involvement from companies like Air Liquide, BASF, and Yara International suggests growing commercial interest, particularly in applications related to atmospheric chemistry and pollution control. The market size is still relatively small but expanding, driven by increasing environmental concerns and regulatory pressures. While the technology is not yet fully mature, ongoing collaborations between academia and industry are accelerating progress towards practical applications.
The University of Sydney
Technical Solution: The University of Sydney has conducted pioneering research on the interaction between nitrous acid and water vapor in the atmosphere, with a particular focus on its role in tropospheric chemistry. Their approach combines advanced laboratory experiments with atmospheric modeling to provide a comprehensive understanding of these complex interactions[14]. They have developed a unique environmental chamber capable of simulating a wide range of atmospheric conditions, allowing for the study of nitrous acid-water vapor interactions under various temperature, pressure, and humidity scenarios[15]. Additionally, they have employed cutting-edge laser-induced fluorescence techniques to detect and quantify nitrous acid with unprecedented sensitivity[16]. Their research has revealed that the presence of water vapor significantly enhances the photolysis of nitrous acid, leading to increased production of hydroxyl radicals, which are key drivers of atmospheric oxidation processes.
Strengths: Advanced environmental chamber technology, high-sensitivity detection methods, integration of experimental and modeling approaches. Weaknesses: Potential challenges in scaling up laboratory findings to global atmospheric processes.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has conducted extensive research on the interaction between nitrous acid and water vapor in the atmosphere. They have developed advanced spectroscopic techniques to study the formation and decomposition of nitrous acid in the presence of water vapor[1]. Their approach involves using high-resolution Fourier transform infrared spectroscopy to monitor the gas-phase reactions in real-time[2]. This method allows for precise measurements of reaction rates and the identification of intermediate species. Additionally, they have employed quantum chemical calculations to elucidate the molecular-level mechanisms of these interactions[3]. Their research has revealed that water vapor plays a crucial role in catalyzing the formation of nitrous acid from nitrogen dioxide, which has significant implications for atmospheric chemistry and air quality[4].
Strengths: Advanced spectroscopic techniques, combination of experimental and theoretical approaches, high-resolution measurements. Weaknesses: Limited field studies, potential challenges in scaling up laboratory findings to atmospheric conditions.
HONO-H2O Reaction Mechanisms
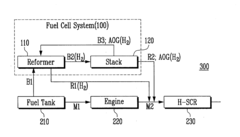
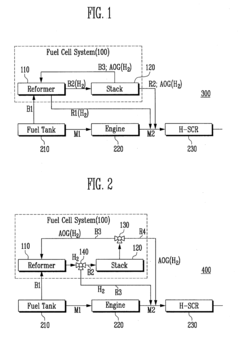
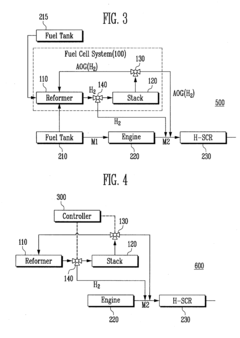
NOx EMISSION REDUCTION SYSTEM AND METHOD
PatentInactiveUS20110167799A1
Innovation
- A vehicular NOx emission reduction system utilizing hydrogen from a fuel cell, which includes a fuel reformer, a fuel cell stack, and a reduction unit with an Ag/Alumina catalyst, where anode off-gas and reformate gas are controlled to facilitate a preferential selective catalytic reduction reaction, reducing NOx emissions using hydrogen as a reducing agent.
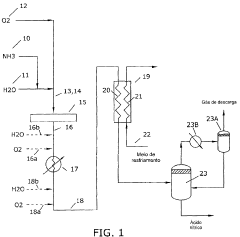
process FOR THE PRODUCTION OF NITRIC ACID
PatentInactiveBR112013013700A2
Innovation
- A process using a gaseous oxidiser feed of ammonia, steam, and oxygen, where ammonia is oxidised to produce nitrogen monoxide and water vapour, then cooled in a heat exchanger to convert nitrogen monoxide to nitric acid, eliminating the need for an absorption tower and reducing nitrogen dioxide levels in the acid.
Climate Impact Assessment
The interaction between nitrous acid (HONO) and water vapor in the atmosphere has significant implications for climate change and global warming. HONO plays a crucial role in atmospheric chemistry, particularly in the formation of hydroxyl radicals (OH), which are often referred to as the "detergent" of the atmosphere due to their ability to oxidize and remove pollutants. The presence of water vapor can significantly influence HONO's behavior and its subsequent impact on climate.
When HONO interacts with water vapor, it can undergo various processes that affect its concentration and distribution in the atmosphere. One key process is the heterogeneous formation of HONO on wet surfaces, including aerosols and cloud droplets. This interaction can lead to increased HONO concentrations, especially in urban areas with high levels of nitrogen oxides (NOx) emissions. The enhanced HONO levels can, in turn, contribute to the formation of ozone and other secondary pollutants, which are known to have warming effects on the climate.
Furthermore, the presence of water vapor can affect the photolysis rate of HONO, which is a primary source of OH radicals in the lower troposphere. Changes in HONO photolysis rates can alter the oxidative capacity of the atmosphere, influencing the lifetime of greenhouse gases such as methane. This indirect effect on methane concentrations can have long-term implications for global warming, as methane is a potent greenhouse gas with a global warming potential significantly higher than that of carbon dioxide.
The interaction between HONO and water vapor also impacts the formation and properties of aerosols in the atmosphere. Aerosols play a complex role in climate change, as they can have both cooling and warming effects depending on their composition and optical properties. HONO's interaction with water vapor can contribute to the formation of secondary organic aerosols (SOA), which can influence cloud formation processes and alter the Earth's radiation balance.
In urban environments, where HONO concentrations are typically higher due to anthropogenic emissions, the interaction with water vapor becomes even more critical. The enhanced HONO levels in these areas can lead to increased production of OH radicals, potentially accelerating the oxidation of volatile organic compounds (VOCs) and the formation of photochemical smog. This can result in localized climate impacts, such as urban heat island effects and changes in precipitation patterns.
Understanding the complex interplay between HONO and water vapor is crucial for improving climate models and predicting future climate scenarios. The accurate representation of these interactions in atmospheric chemistry models can enhance our ability to forecast air quality, assess the effectiveness of emission control strategies, and develop more targeted climate mitigation policies. As research in this area continues to evolve, it will be essential to incorporate new findings into climate impact assessments to better inform decision-making processes related to climate change adaptation and mitigation efforts.
When HONO interacts with water vapor, it can undergo various processes that affect its concentration and distribution in the atmosphere. One key process is the heterogeneous formation of HONO on wet surfaces, including aerosols and cloud droplets. This interaction can lead to increased HONO concentrations, especially in urban areas with high levels of nitrogen oxides (NOx) emissions. The enhanced HONO levels can, in turn, contribute to the formation of ozone and other secondary pollutants, which are known to have warming effects on the climate.
Furthermore, the presence of water vapor can affect the photolysis rate of HONO, which is a primary source of OH radicals in the lower troposphere. Changes in HONO photolysis rates can alter the oxidative capacity of the atmosphere, influencing the lifetime of greenhouse gases such as methane. This indirect effect on methane concentrations can have long-term implications for global warming, as methane is a potent greenhouse gas with a global warming potential significantly higher than that of carbon dioxide.
The interaction between HONO and water vapor also impacts the formation and properties of aerosols in the atmosphere. Aerosols play a complex role in climate change, as they can have both cooling and warming effects depending on their composition and optical properties. HONO's interaction with water vapor can contribute to the formation of secondary organic aerosols (SOA), which can influence cloud formation processes and alter the Earth's radiation balance.
In urban environments, where HONO concentrations are typically higher due to anthropogenic emissions, the interaction with water vapor becomes even more critical. The enhanced HONO levels in these areas can lead to increased production of OH radicals, potentially accelerating the oxidation of volatile organic compounds (VOCs) and the formation of photochemical smog. This can result in localized climate impacts, such as urban heat island effects and changes in precipitation patterns.
Understanding the complex interplay between HONO and water vapor is crucial for improving climate models and predicting future climate scenarios. The accurate representation of these interactions in atmospheric chemistry models can enhance our ability to forecast air quality, assess the effectiveness of emission control strategies, and develop more targeted climate mitigation policies. As research in this area continues to evolve, it will be essential to incorporate new findings into climate impact assessments to better inform decision-making processes related to climate change adaptation and mitigation efforts.
Air Quality Regulations
Air quality regulations play a crucial role in addressing the environmental and health impacts of atmospheric pollutants, including those resulting from the interaction between nitrous acid and water vapor. These regulations have evolved significantly over the past few decades, driven by scientific advancements in understanding atmospheric chemistry and its effects on human health and ecosystems.
In many countries, air quality standards now include specific limits for nitrogen oxides (NOx), which are precursors to nitrous acid formation. The United States Environmental Protection Agency (EPA), for instance, has established National Ambient Air Quality Standards (NAAQS) for nitrogen dioxide (NO2), with primary standards set at 100 parts per billion (ppb) for a 1-hour averaging time and 53 ppb for an annual mean.
The European Union has also implemented stringent air quality directives, including limits for NO2 at 200 μg/m³ for 1-hour mean (not to be exceeded more than 18 times per year) and 40 μg/m³ for annual mean. These regulations indirectly address nitrous acid concentrations by controlling its precursors.
Monitoring and enforcement of these regulations have become increasingly sophisticated. Many urban areas now employ networks of air quality monitoring stations that provide real-time data on various pollutants, including NOx. This data is used to inform public health advisories and to assess compliance with air quality standards.
The recognition of the role of nitrous acid in atmospheric chemistry has led to more comprehensive approaches in air quality management. Regulations now often consider the complex interactions between different pollutants and atmospheric conditions. For example, ozone formation, which is influenced by nitrous acid chemistry, is addressed through regulations on both NOx and volatile organic compounds (VOCs).
International agreements, such as the Convention on Long-range Transboundary Air Pollution, have also been instrumental in addressing air quality issues that transcend national borders. These agreements recognize the transboundary nature of atmospheric pollutants and promote coordinated efforts to reduce emissions.
As research continues to elucidate the intricate relationships between nitrous acid, water vapor, and other atmospheric components, air quality regulations are likely to evolve further. Future regulations may incorporate more specific provisions for secondary pollutants formed through atmospheric reactions, potentially including direct consideration of nitrous acid concentrations or its formation processes.
In many countries, air quality standards now include specific limits for nitrogen oxides (NOx), which are precursors to nitrous acid formation. The United States Environmental Protection Agency (EPA), for instance, has established National Ambient Air Quality Standards (NAAQS) for nitrogen dioxide (NO2), with primary standards set at 100 parts per billion (ppb) for a 1-hour averaging time and 53 ppb for an annual mean.
The European Union has also implemented stringent air quality directives, including limits for NO2 at 200 μg/m³ for 1-hour mean (not to be exceeded more than 18 times per year) and 40 μg/m³ for annual mean. These regulations indirectly address nitrous acid concentrations by controlling its precursors.
Monitoring and enforcement of these regulations have become increasingly sophisticated. Many urban areas now employ networks of air quality monitoring stations that provide real-time data on various pollutants, including NOx. This data is used to inform public health advisories and to assess compliance with air quality standards.
The recognition of the role of nitrous acid in atmospheric chemistry has led to more comprehensive approaches in air quality management. Regulations now often consider the complex interactions between different pollutants and atmospheric conditions. For example, ozone formation, which is influenced by nitrous acid chemistry, is addressed through regulations on both NOx and volatile organic compounds (VOCs).
International agreements, such as the Convention on Long-range Transboundary Air Pollution, have also been instrumental in addressing air quality issues that transcend national borders. These agreements recognize the transboundary nature of atmospheric pollutants and promote coordinated efforts to reduce emissions.
As research continues to elucidate the intricate relationships between nitrous acid, water vapor, and other atmospheric components, air quality regulations are likely to evolve further. Future regulations may incorporate more specific provisions for secondary pollutants formed through atmospheric reactions, potentially including direct consideration of nitrous acid concentrations or its formation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!