Nitrous Acid Reactions in Detergent Production Processes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrous Acid in Detergents: Background and Objectives
Nitrous acid reactions have played a significant role in the evolution of detergent production processes. The journey of understanding and harnessing these reactions dates back to the early 20th century when the chemical industry began exploring more efficient ways to produce cleaning agents. Initially, the focus was on developing synthetic detergents to overcome the limitations of traditional soap-based products.
The presence of nitrous acid in detergent production processes was first recognized as a byproduct of certain chemical reactions. However, as research progressed, scientists and engineers began to explore the potential benefits and challenges associated with these reactions. The primary objective of studying nitrous acid reactions in detergent production is to optimize the manufacturing process, enhance product quality, and minimize environmental impact.
One of the key areas of interest has been the role of nitrous acid in the nitrosation of amines, a process that can occur during the production of certain types of detergents. This reaction has both positive and negative implications for the final product, influencing factors such as stability, color, and potential health effects. Understanding and controlling these reactions became crucial for producing safe and effective detergents.
The technological evolution in this field has been driven by the need to meet increasingly stringent environmental regulations and consumer demands for more sustainable products. Researchers have focused on developing methods to either eliminate or utilize nitrous acid reactions in ways that benefit the production process without compromising product quality or safety.
Recent advancements in analytical techniques have allowed for more precise monitoring and control of nitrous acid reactions. This has led to the development of innovative production methods that leverage these reactions for improved efficiency while mitigating potential negative effects. The integration of real-time monitoring systems and advanced process control algorithms has further enhanced the ability to manage these reactions effectively.
Looking ahead, the objectives for future research and development in this area include further optimization of nitrous acid reactions for enhanced detergent performance, exploration of novel catalysts to control reaction pathways, and the development of more environmentally friendly production processes. There is also a growing interest in understanding the potential applications of nitrous acid reactions beyond traditional detergent production, opening up new avenues for innovation in the cleaning products industry.
As the field continues to evolve, interdisciplinary collaboration between chemists, chemical engineers, and environmental scientists will be crucial in addressing the complex challenges associated with nitrous acid reactions in detergent production. The ultimate goal remains to develop more efficient, sustainable, and high-performance cleaning products that meet the ever-changing needs of consumers while minimizing environmental impact.
The presence of nitrous acid in detergent production processes was first recognized as a byproduct of certain chemical reactions. However, as research progressed, scientists and engineers began to explore the potential benefits and challenges associated with these reactions. The primary objective of studying nitrous acid reactions in detergent production is to optimize the manufacturing process, enhance product quality, and minimize environmental impact.
One of the key areas of interest has been the role of nitrous acid in the nitrosation of amines, a process that can occur during the production of certain types of detergents. This reaction has both positive and negative implications for the final product, influencing factors such as stability, color, and potential health effects. Understanding and controlling these reactions became crucial for producing safe and effective detergents.
The technological evolution in this field has been driven by the need to meet increasingly stringent environmental regulations and consumer demands for more sustainable products. Researchers have focused on developing methods to either eliminate or utilize nitrous acid reactions in ways that benefit the production process without compromising product quality or safety.
Recent advancements in analytical techniques have allowed for more precise monitoring and control of nitrous acid reactions. This has led to the development of innovative production methods that leverage these reactions for improved efficiency while mitigating potential negative effects. The integration of real-time monitoring systems and advanced process control algorithms has further enhanced the ability to manage these reactions effectively.
Looking ahead, the objectives for future research and development in this area include further optimization of nitrous acid reactions for enhanced detergent performance, exploration of novel catalysts to control reaction pathways, and the development of more environmentally friendly production processes. There is also a growing interest in understanding the potential applications of nitrous acid reactions beyond traditional detergent production, opening up new avenues for innovation in the cleaning products industry.
As the field continues to evolve, interdisciplinary collaboration between chemists, chemical engineers, and environmental scientists will be crucial in addressing the complex challenges associated with nitrous acid reactions in detergent production. The ultimate goal remains to develop more efficient, sustainable, and high-performance cleaning products that meet the ever-changing needs of consumers while minimizing environmental impact.
Market Analysis for Nitrous Acid-Based Detergents
The market for nitrous acid-based detergents has shown significant growth potential in recent years, driven by increasing demand for effective and environmentally friendly cleaning solutions. The global detergent market, valued at approximately $133 billion in 2020, is expected to reach $177 billion by 2026, with a compound annual growth rate (CAGR) of 4.9%. Within this broader market, nitrous acid-based detergents are carving out a niche due to their unique properties and applications.
Nitrous acid-based detergents offer several advantages over traditional cleaning products, including enhanced stain removal capabilities, improved disinfection properties, and reduced environmental impact. These benefits have led to increased adoption in both household and industrial cleaning applications. The household segment currently dominates the market, accounting for roughly 60% of total sales, while the industrial and institutional sectors make up the remaining 40%.
Geographically, North America and Europe are the largest markets for nitrous acid-based detergents, collectively representing about 55% of global sales. However, the Asia-Pacific region is emerging as the fastest-growing market, with a projected CAGR of 6.5% over the next five years. This growth is primarily attributed to rapid urbanization, increasing disposable incomes, and growing awareness of hygiene and sanitation in countries like China and India.
Key market drivers include the rising consumer preference for eco-friendly products, stringent regulations on chemical usage in cleaning products, and the need for more effective cleaning solutions in healthcare and food processing industries. The COVID-19 pandemic has further accelerated market growth, as consumers and businesses alike have placed greater emphasis on cleanliness and disinfection.
Despite the positive outlook, the market faces challenges such as the high production costs of nitrous acid-based detergents compared to conventional alternatives and concerns about potential health risks associated with prolonged exposure to nitrous acid. These factors may limit market penetration in price-sensitive regions and certain application areas.
Looking ahead, technological advancements in nitrous acid production and formulation are expected to drive down costs and improve product safety, potentially expanding market opportunities. Additionally, the growing trend towards sustainable and biodegradable cleaning solutions aligns well with the properties of nitrous acid-based detergents, suggesting a promising future for this market segment.
Nitrous acid-based detergents offer several advantages over traditional cleaning products, including enhanced stain removal capabilities, improved disinfection properties, and reduced environmental impact. These benefits have led to increased adoption in both household and industrial cleaning applications. The household segment currently dominates the market, accounting for roughly 60% of total sales, while the industrial and institutional sectors make up the remaining 40%.
Geographically, North America and Europe are the largest markets for nitrous acid-based detergents, collectively representing about 55% of global sales. However, the Asia-Pacific region is emerging as the fastest-growing market, with a projected CAGR of 6.5% over the next five years. This growth is primarily attributed to rapid urbanization, increasing disposable incomes, and growing awareness of hygiene and sanitation in countries like China and India.
Key market drivers include the rising consumer preference for eco-friendly products, stringent regulations on chemical usage in cleaning products, and the need for more effective cleaning solutions in healthcare and food processing industries. The COVID-19 pandemic has further accelerated market growth, as consumers and businesses alike have placed greater emphasis on cleanliness and disinfection.
Despite the positive outlook, the market faces challenges such as the high production costs of nitrous acid-based detergents compared to conventional alternatives and concerns about potential health risks associated with prolonged exposure to nitrous acid. These factors may limit market penetration in price-sensitive regions and certain application areas.
Looking ahead, technological advancements in nitrous acid production and formulation are expected to drive down costs and improve product safety, potentially expanding market opportunities. Additionally, the growing trend towards sustainable and biodegradable cleaning solutions aligns well with the properties of nitrous acid-based detergents, suggesting a promising future for this market segment.
Current Challenges in Nitrous Acid Reactions
The production of detergents using nitrous acid reactions faces several significant challenges that hinder efficiency and sustainability. One of the primary issues is the instability of nitrous acid, which readily decomposes into nitrogen oxides and water. This decomposition not only reduces the yield of the desired products but also leads to the formation of potentially harmful byproducts.
Another major challenge is the corrosive nature of nitrous acid, which can damage production equipment and increase maintenance costs. The use of specialized corrosion-resistant materials is often necessary, adding to the overall production expenses. Additionally, the handling and storage of nitrous acid pose safety risks due to its reactive nature, requiring stringent safety protocols and specialized containment systems.
The environmental impact of nitrous acid reactions in detergent production is also a significant concern. The release of nitrogen oxides as byproducts contributes to air pollution and can lead to the formation of acid rain. This necessitates the implementation of costly emission control systems to comply with increasingly stringent environmental regulations.
Furthermore, the precise control of reaction conditions, such as temperature and pH, is crucial for optimal nitrous acid reactions. Maintaining these conditions consistently across large-scale production processes presents technical difficulties, often resulting in variations in product quality and yield.
The limited availability of high-purity nitrous acid for industrial use is another challenge. The production of nitrous acid itself is complex and energy-intensive, which affects the overall cost-effectiveness of detergent manufacturing processes that rely on this reagent.
Researchers and industry professionals are also grappling with the challenge of developing more sustainable alternatives to nitrous acid-based processes. While some progress has been made in exploring greener chemistry approaches, finding economically viable and equally effective substitutes remains a significant hurdle.
Lastly, the optimization of reaction kinetics and mass transfer in nitrous acid reactions presents ongoing challenges. Improving the efficiency of these processes requires advanced modeling and experimental techniques, which demand substantial research and development investments from detergent manufacturers.
Another major challenge is the corrosive nature of nitrous acid, which can damage production equipment and increase maintenance costs. The use of specialized corrosion-resistant materials is often necessary, adding to the overall production expenses. Additionally, the handling and storage of nitrous acid pose safety risks due to its reactive nature, requiring stringent safety protocols and specialized containment systems.
The environmental impact of nitrous acid reactions in detergent production is also a significant concern. The release of nitrogen oxides as byproducts contributes to air pollution and can lead to the formation of acid rain. This necessitates the implementation of costly emission control systems to comply with increasingly stringent environmental regulations.
Furthermore, the precise control of reaction conditions, such as temperature and pH, is crucial for optimal nitrous acid reactions. Maintaining these conditions consistently across large-scale production processes presents technical difficulties, often resulting in variations in product quality and yield.
The limited availability of high-purity nitrous acid for industrial use is another challenge. The production of nitrous acid itself is complex and energy-intensive, which affects the overall cost-effectiveness of detergent manufacturing processes that rely on this reagent.
Researchers and industry professionals are also grappling with the challenge of developing more sustainable alternatives to nitrous acid-based processes. While some progress has been made in exploring greener chemistry approaches, finding economically viable and equally effective substitutes remains a significant hurdle.
Lastly, the optimization of reaction kinetics and mass transfer in nitrous acid reactions presents ongoing challenges. Improving the efficiency of these processes requires advanced modeling and experimental techniques, which demand substantial research and development investments from detergent manufacturers.
Existing Nitrous Acid Reaction Processes
01 Production and applications of nitrous acid
Nitrous acid is a chemical compound with various industrial applications. It can be produced through different methods and is used in processes such as metal etching, dye manufacturing, and as an intermediate in chemical synthesis. The production and utilization of nitrous acid involve specific techniques and safety considerations due to its corrosive nature.- Production and applications of nitrous acid: Nitrous acid is a weak and unstable acid with various industrial applications. It can be produced through different methods and is used in chemical processes, particularly in the production of diazonium salts for dye manufacturing. The compound plays a role in atmospheric chemistry and environmental processes.
- Nitrous acid in material treatment and modification: Nitrous acid is utilized in the treatment and modification of various materials, including textiles, polymers, and metals. It can be employed for surface etching, cleaning, and functionalization processes. The acid's properties allow for controlled chemical reactions that alter material characteristics or prepare surfaces for further processing.
- Environmental and atmospheric implications: Nitrous acid plays a significant role in atmospheric chemistry and environmental processes. It is involved in the formation of photochemical smog and can contribute to acid rain. Research focuses on understanding its formation, reactions, and impact on air quality and ecosystems.
- Analytical methods and detection techniques: Various analytical methods and detection techniques have been developed for measuring nitrous acid concentrations in different environments. These include spectroscopic methods, electrochemical sensors, and chromatographic techniques. Accurate detection is crucial for environmental monitoring and industrial process control.
- Nitrous acid in chemical synthesis and reactions: Nitrous acid is an important reagent in organic synthesis and inorganic reactions. It is used in the preparation of various compounds, including diazo compounds, nitro compounds, and certain dyes. The acid's reactivity and unique properties make it valuable in specific chemical transformations and industrial processes.
02 Nitrous acid in water treatment and environmental processes
Nitrous acid plays a role in water treatment and environmental processes. It is involved in the nitrogen cycle and can be found in wastewater treatment systems. The compound is also relevant in atmospheric chemistry, contributing to the formation of acid rain and other environmental phenomena. Understanding its behavior in aqueous environments is crucial for effective water management and pollution control.Expand Specific Solutions03 Analytical methods for nitrous acid detection and quantification
Various analytical techniques have been developed to detect and quantify nitrous acid in different matrices. These methods may include spectrophotometric analysis, electrochemical sensors, or chromatographic techniques. Accurate measurement of nitrous acid is important in fields such as environmental monitoring, industrial process control, and scientific research.Expand Specific Solutions04 Nitrous acid in materials science and surface treatment
Nitrous acid is utilized in materials science and surface treatment applications. It can be employed in the modification of surface properties, such as in the treatment of metals or polymers. The compound's reactivity makes it useful for etching, cleaning, or activating surfaces in various industrial processes, including semiconductor manufacturing and metal finishing.Expand Specific Solutions05 Safety and handling of nitrous acid
Due to its corrosive and potentially hazardous nature, proper safety measures and handling procedures are essential when working with nitrous acid. This includes appropriate storage, transportation, and disposal methods, as well as the use of personal protective equipment. Understanding the chemical properties and potential risks associated with nitrous acid is crucial for ensuring safe handling in laboratory and industrial settings.Expand Specific Solutions
Key Players in Detergent Chemical Industry
The competitive landscape for nitrous acid reactions in detergent production processes is characterized by a mature market with established players and ongoing technological advancements. The global market size for this sector is substantial, driven by the increasing demand for detergents and cleaning products. Key players like BASF Corp., Covestro Deutschland AG, and Wanhua Chemical Group Co., Ltd. are at the forefront of innovation, investing in research and development to improve process efficiency and sustainability. The technology's maturity is evident, with companies like ThyssenKrupp Uhde GmbH and Casale SA offering specialized engineering solutions. However, there's still room for optimization and environmental improvements, as evidenced by the involvement of research institutions like The University of Sydney and Sichuan Golden-Elephant Sincrity Chemical Co., Ltd.
Covestro Deutschland AG
Technical Solution: Covestro has developed a novel approach to manage nitrous acid reactions in detergent production processes. Their method involves a two-stage reaction system that first converts nitrous acid into nitrogen oxides, followed by a catalytic reduction process to transform these oxides into harmless nitrogen gas[2]. The company has also implemented an advanced monitoring system that uses real-time spectroscopic analysis to precisely control the reaction conditions and prevent the formation of unwanted by-products[4]. Covestro's technology incorporates a closed-loop recycling system that captures and reuses unreacted nitrous acid, significantly improving process efficiency and reducing waste[6].
Strengths: Efficient two-stage reaction system, advanced real-time monitoring, and waste reduction through recycling. Weaknesses: Complex system that may require significant initial investment and ongoing maintenance.
Yara International ASA
Technical Solution: Yara has developed a cutting-edge approach to manage nitrous acid reactions in detergent production processes. Their method utilizes a proprietary absorption technology that effectively removes nitrous acid from process streams, preventing unwanted side reactions and improving product quality[1]. The company has also implemented an innovative catalytic decomposition process that converts captured nitrous acid into nitrogen and oxygen, eliminating the need for hazardous waste disposal[3]. Yara's system incorporates advanced process control algorithms that optimize reaction conditions based on real-time data, resulting in improved energy efficiency and reduced environmental impact[5].
Strengths: Effective nitrous acid removal, environmentally friendly decomposition process, and advanced process optimization. Weaknesses: May require significant modifications to existing production facilities and ongoing catalyst management.
Innovative Nitrous Acid Reaction Mechanisms
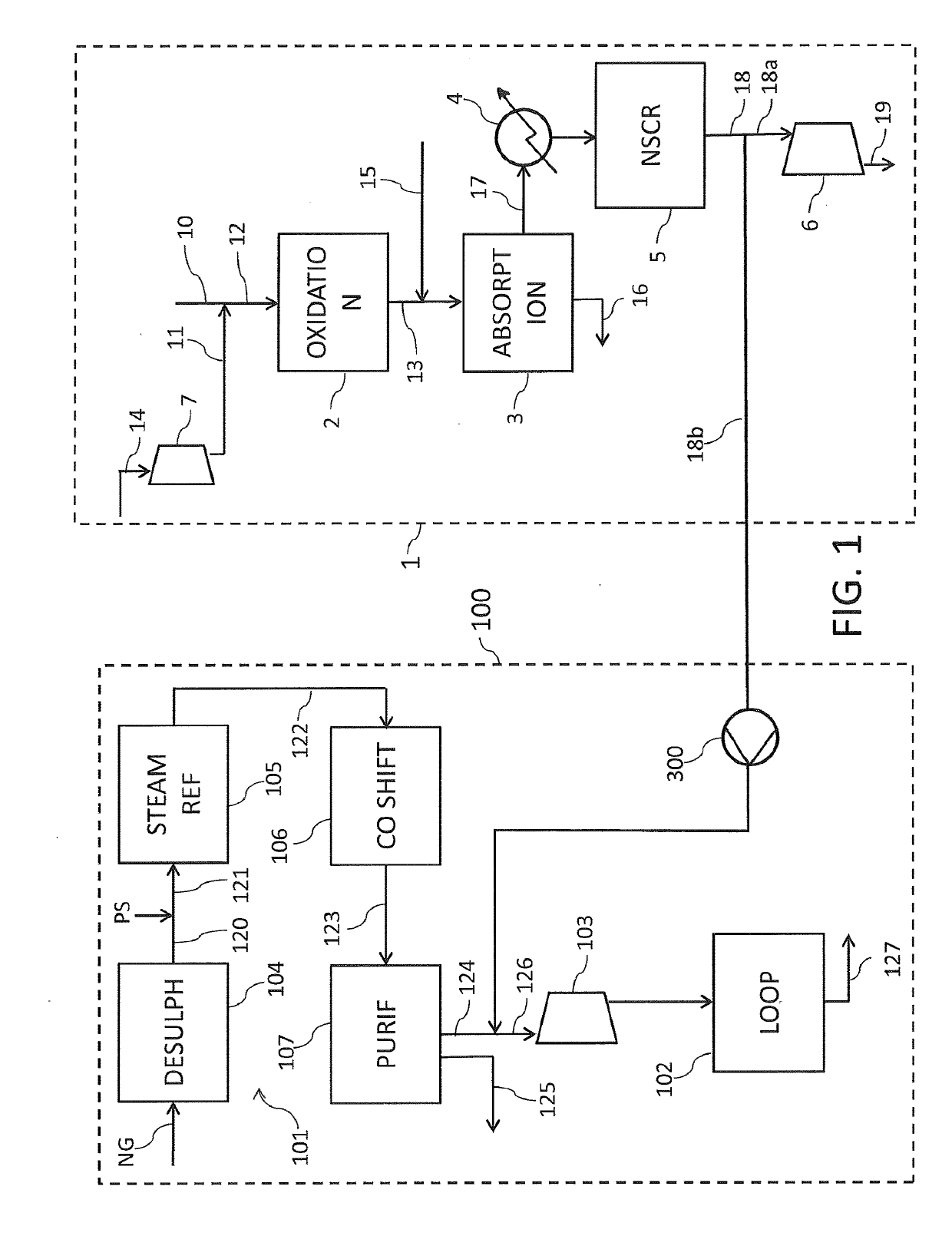
NITRIC ACID PRODUCTION PROCESS AND SYSTEM
PatentActiveVN44990A
Innovation
- Introducing oxygen in liquid or gaseous form into the lower region of the vertical pipeline to enhance oxygen dissolution and reaction with dissolved nitrous oxide.
- Utilizing vertical pipes for compressing and recirculating the aqueous nitric acid solution back to the absorption tower, creating a closed-loop system.
- Strategically positioning the oxygen injection point in the vertical pipeline to optimize the reaction between oxygen and dissolved nitrous oxide.
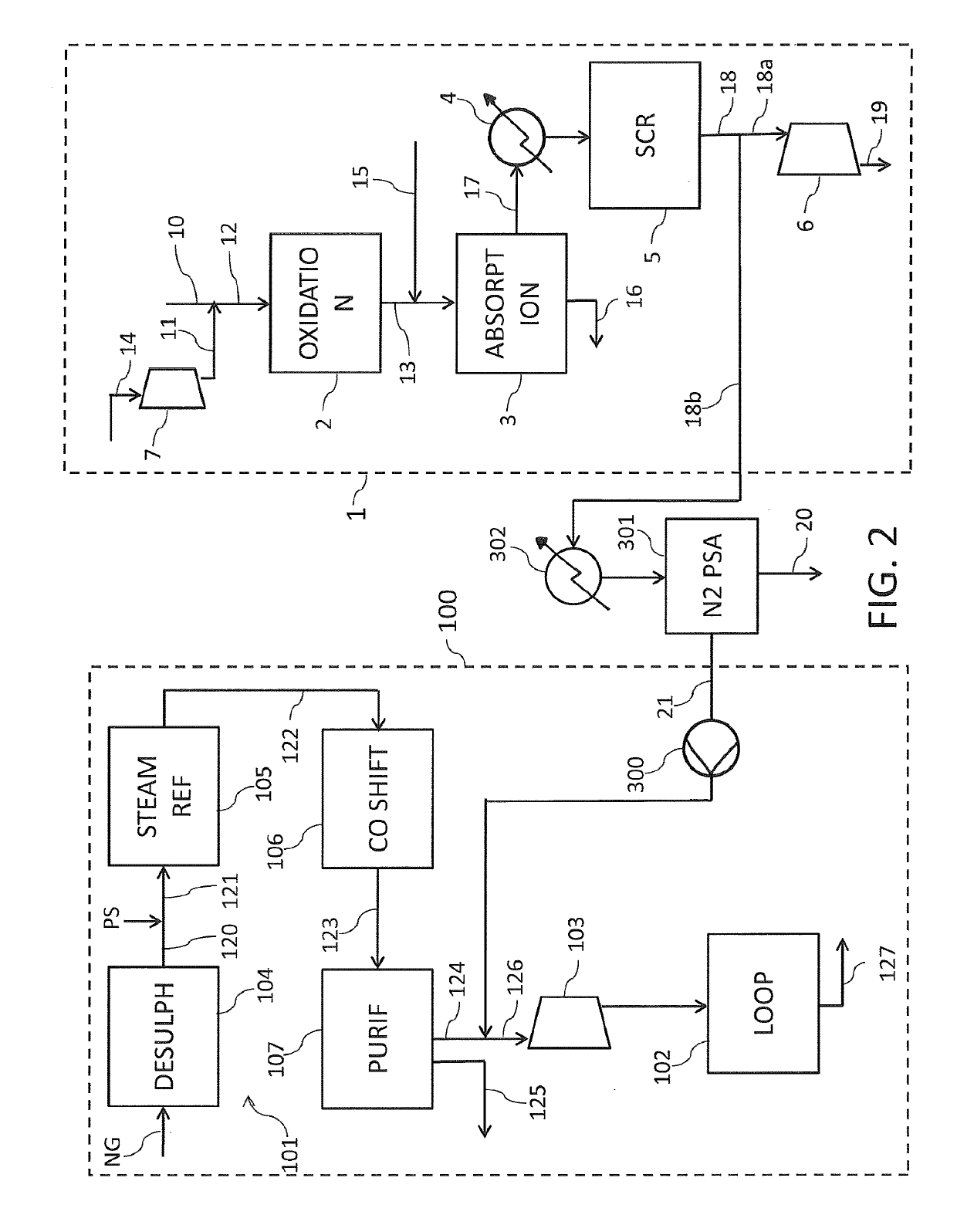
A process for nitric acid production
PatentActiveUS20190284052A1
Innovation
- The process integrates the use of NOx-depleted tail gas as a nitrogen source for ammonia synthesis, eliminating the need for an air separation unit and nitrogen compressor, by splitting the tail gas into portions where one is work-expanded for power generation and the other used directly as a nitrogen source, reducing nitrogen consumption and oxygen content.
Environmental Impact Assessment
The environmental impact of nitrous acid reactions in detergent production processes is a critical consideration for manufacturers and regulatory bodies alike. These reactions, while essential for certain detergent formulations, can have significant implications for air and water quality, as well as overall ecosystem health.
One of the primary environmental concerns is the potential for nitrous acid to contribute to air pollution. When released into the atmosphere, nitrous acid can participate in photochemical reactions, leading to the formation of ground-level ozone and other harmful air pollutants. This can exacerbate urban smog problems and negatively impact respiratory health in surrounding communities.
Water pollution is another key environmental issue associated with nitrous acid reactions in detergent production. Effluents containing nitrous acid or its byproducts can alter the pH balance of receiving water bodies, potentially harming aquatic ecosystems. Furthermore, the nitrogen content in these effluents can contribute to eutrophication, leading to algal blooms and oxygen depletion in water systems.
The production process itself may also pose risks to soil quality if proper containment measures are not in place. Accidental spills or leaks of nitrous acid-containing solutions can lead to soil acidification, impacting local flora and potentially entering groundwater systems.
From a broader perspective, the use of nitrous acid in detergent production raises questions about resource efficiency and sustainability. The energy requirements for these reactions and the potential for greenhouse gas emissions during the production process contribute to the overall carbon footprint of detergent manufacturing.
To mitigate these environmental impacts, many detergent manufacturers are exploring alternative production methods or implementing advanced treatment technologies. Closed-loop systems that recycle and reuse nitrous acid can significantly reduce emissions and effluent discharge. Additionally, the development of low-temperature reaction processes can help minimize energy consumption and associated environmental impacts.
Regulatory frameworks play a crucial role in managing the environmental impact of these processes. Stringent emissions standards, wastewater treatment requirements, and mandatory environmental impact assessments for new production facilities are common measures implemented by governing bodies to ensure responsible manufacturing practices.
As the industry continues to evolve, there is a growing emphasis on green chemistry principles in detergent production. This includes exploring bio-based alternatives to nitrous acid reactions and developing more environmentally benign catalysts that can achieve similar results with reduced ecological impact.
One of the primary environmental concerns is the potential for nitrous acid to contribute to air pollution. When released into the atmosphere, nitrous acid can participate in photochemical reactions, leading to the formation of ground-level ozone and other harmful air pollutants. This can exacerbate urban smog problems and negatively impact respiratory health in surrounding communities.
Water pollution is another key environmental issue associated with nitrous acid reactions in detergent production. Effluents containing nitrous acid or its byproducts can alter the pH balance of receiving water bodies, potentially harming aquatic ecosystems. Furthermore, the nitrogen content in these effluents can contribute to eutrophication, leading to algal blooms and oxygen depletion in water systems.
The production process itself may also pose risks to soil quality if proper containment measures are not in place. Accidental spills or leaks of nitrous acid-containing solutions can lead to soil acidification, impacting local flora and potentially entering groundwater systems.
From a broader perspective, the use of nitrous acid in detergent production raises questions about resource efficiency and sustainability. The energy requirements for these reactions and the potential for greenhouse gas emissions during the production process contribute to the overall carbon footprint of detergent manufacturing.
To mitigate these environmental impacts, many detergent manufacturers are exploring alternative production methods or implementing advanced treatment technologies. Closed-loop systems that recycle and reuse nitrous acid can significantly reduce emissions and effluent discharge. Additionally, the development of low-temperature reaction processes can help minimize energy consumption and associated environmental impacts.
Regulatory frameworks play a crucial role in managing the environmental impact of these processes. Stringent emissions standards, wastewater treatment requirements, and mandatory environmental impact assessments for new production facilities are common measures implemented by governing bodies to ensure responsible manufacturing practices.
As the industry continues to evolve, there is a growing emphasis on green chemistry principles in detergent production. This includes exploring bio-based alternatives to nitrous acid reactions and developing more environmentally benign catalysts that can achieve similar results with reduced ecological impact.
Safety Protocols for Nitrous Acid Handling
Safety protocols for handling nitrous acid in detergent production processes are critical to ensure the well-being of workers and the integrity of the manufacturing environment. These protocols encompass a comprehensive set of guidelines and procedures designed to mitigate risks associated with the use of this highly corrosive and potentially hazardous substance.
Personal protective equipment (PPE) forms the first line of defense against nitrous acid exposure. Workers must wear chemical-resistant gloves, protective eyewear, face shields, and acid-resistant clothing. Respiratory protection, such as a full-face respirator with appropriate cartridges, is essential when working with nitrous acid vapors. Regular inspection and maintenance of PPE are crucial to ensure its effectiveness.
Proper storage and handling procedures are paramount. Nitrous acid should be stored in tightly sealed, corrosion-resistant containers in well-ventilated areas away from direct sunlight and heat sources. Storage areas must be equipped with secondary containment systems to prevent spills from spreading. Clear labeling of containers and storage areas is essential, along with restricted access to authorized personnel only.
Engineering controls play a vital role in minimizing exposure risks. Adequate ventilation systems, including local exhaust ventilation, should be installed in areas where nitrous acid is used or stored. Closed systems for transfer and handling of nitrous acid can significantly reduce the risk of spills and vapor exposure. Emergency eyewash stations and safety showers must be readily accessible in all areas where nitrous acid is handled.
Training and education are fundamental components of safety protocols. All personnel working with or around nitrous acid must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Emergency response procedures must be clearly defined and communicated. This includes protocols for spill containment and cleanup, first aid measures for exposure, and evacuation procedures. Specialized spill kits designed for acid neutralization should be readily available, and personnel must be trained in their use.
Regular safety audits and inspections are essential to ensure compliance with established protocols and identify potential hazards. This includes routine checks of storage areas, handling equipment, and safety systems. Any incidents or near-misses should be thoroughly investigated, and lessons learned should be incorporated into revised safety procedures.
Proper waste management is crucial when dealing with nitrous acid. Neutralization and disposal procedures must comply with local environmental regulations. Dedicated waste streams and treatment systems may be necessary to handle nitrous acid-containing waste safely and responsibly.
By implementing and strictly adhering to these comprehensive safety protocols, detergent manufacturers can significantly reduce the risks associated with nitrous acid handling, ensuring a safer working environment and more sustainable production processes.
Personal protective equipment (PPE) forms the first line of defense against nitrous acid exposure. Workers must wear chemical-resistant gloves, protective eyewear, face shields, and acid-resistant clothing. Respiratory protection, such as a full-face respirator with appropriate cartridges, is essential when working with nitrous acid vapors. Regular inspection and maintenance of PPE are crucial to ensure its effectiveness.
Proper storage and handling procedures are paramount. Nitrous acid should be stored in tightly sealed, corrosion-resistant containers in well-ventilated areas away from direct sunlight and heat sources. Storage areas must be equipped with secondary containment systems to prevent spills from spreading. Clear labeling of containers and storage areas is essential, along with restricted access to authorized personnel only.
Engineering controls play a vital role in minimizing exposure risks. Adequate ventilation systems, including local exhaust ventilation, should be installed in areas where nitrous acid is used or stored. Closed systems for transfer and handling of nitrous acid can significantly reduce the risk of spills and vapor exposure. Emergency eyewash stations and safety showers must be readily accessible in all areas where nitrous acid is handled.
Training and education are fundamental components of safety protocols. All personnel working with or around nitrous acid must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Emergency response procedures must be clearly defined and communicated. This includes protocols for spill containment and cleanup, first aid measures for exposure, and evacuation procedures. Specialized spill kits designed for acid neutralization should be readily available, and personnel must be trained in their use.
Regular safety audits and inspections are essential to ensure compliance with established protocols and identify potential hazards. This includes routine checks of storage areas, handling equipment, and safety systems. Any incidents or near-misses should be thoroughly investigated, and lessons learned should be incorporated into revised safety procedures.
Proper waste management is crucial when dealing with nitrous acid. Neutralization and disposal procedures must comply with local environmental regulations. Dedicated waste streams and treatment systems may be necessary to handle nitrous acid-containing waste safely and responsibly.
By implementing and strictly adhering to these comprehensive safety protocols, detergent manufacturers can significantly reduce the risks associated with nitrous acid handling, ensuring a safer working environment and more sustainable production processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!