How Nitrous Acid Forms in Combustion Systems
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Combustion HONO Formation Background and Objectives
The formation of nitrous acid (HONO) in combustion systems has been a subject of increasing interest in recent years due to its significant impact on atmospheric chemistry and air quality. HONO is a crucial intermediate species in combustion processes and plays a vital role in the formation of nitrogen oxides (NOx), which are major air pollutants. Understanding the mechanisms of HONO formation is essential for developing effective strategies to control emissions and improve combustion efficiency.
The study of HONO formation in combustion systems dates back to the 1970s when researchers first recognized its importance in atmospheric chemistry. Since then, significant progress has been made in elucidating the complex pathways leading to HONO production during combustion. The evolution of analytical techniques and computational modeling has greatly enhanced our ability to investigate these processes at a molecular level.
The primary objective of research in this field is to gain a comprehensive understanding of the chemical and physical processes that govern HONO formation under various combustion conditions. This includes identifying the key precursors, reaction pathways, and environmental factors that influence HONO production. By achieving this goal, researchers aim to develop more accurate predictive models and design more efficient combustion systems with reduced HONO emissions.
One of the main challenges in studying HONO formation is the complexity of combustion environments, which involve high temperatures, rapid chemical reactions, and multiple interacting species. Researchers must consider various factors such as fuel composition, combustion temperature, pressure, and residence time to accurately describe HONO formation mechanisms. Additionally, the transient nature of HONO in combustion systems makes it difficult to measure and quantify its concentration in real-time.
Recent technological advancements have enabled more precise measurements and detailed simulations of HONO formation. Advanced laser-based diagnostic techniques, such as cavity ring-down spectroscopy and laser-induced fluorescence, have improved our ability to detect and quantify HONO in combustion environments. Simultaneously, the development of more sophisticated chemical kinetics models and computational fluid dynamics simulations has enhanced our predictive capabilities.
As we look towards the future, the objectives of HONO formation research in combustion systems are expanding. There is a growing emphasis on understanding the role of HONO in the formation of secondary pollutants, such as ozone and particulate matter, in the atmosphere. Additionally, researchers are exploring novel combustion technologies and alternative fuels, necessitating a reevaluation of HONO formation mechanisms under these new conditions.
The study of HONO formation in combustion systems dates back to the 1970s when researchers first recognized its importance in atmospheric chemistry. Since then, significant progress has been made in elucidating the complex pathways leading to HONO production during combustion. The evolution of analytical techniques and computational modeling has greatly enhanced our ability to investigate these processes at a molecular level.
The primary objective of research in this field is to gain a comprehensive understanding of the chemical and physical processes that govern HONO formation under various combustion conditions. This includes identifying the key precursors, reaction pathways, and environmental factors that influence HONO production. By achieving this goal, researchers aim to develop more accurate predictive models and design more efficient combustion systems with reduced HONO emissions.
One of the main challenges in studying HONO formation is the complexity of combustion environments, which involve high temperatures, rapid chemical reactions, and multiple interacting species. Researchers must consider various factors such as fuel composition, combustion temperature, pressure, and residence time to accurately describe HONO formation mechanisms. Additionally, the transient nature of HONO in combustion systems makes it difficult to measure and quantify its concentration in real-time.
Recent technological advancements have enabled more precise measurements and detailed simulations of HONO formation. Advanced laser-based diagnostic techniques, such as cavity ring-down spectroscopy and laser-induced fluorescence, have improved our ability to detect and quantify HONO in combustion environments. Simultaneously, the development of more sophisticated chemical kinetics models and computational fluid dynamics simulations has enhanced our predictive capabilities.
As we look towards the future, the objectives of HONO formation research in combustion systems are expanding. There is a growing emphasis on understanding the role of HONO in the formation of secondary pollutants, such as ozone and particulate matter, in the atmosphere. Additionally, researchers are exploring novel combustion technologies and alternative fuels, necessitating a reevaluation of HONO formation mechanisms under these new conditions.
Market Demand for HONO Reduction in Combustion
The market demand for HONO reduction in combustion systems has been steadily growing due to increasing awareness of its environmental and health impacts. Nitrous acid (HONO) is a significant contributor to air pollution, playing a crucial role in the formation of ground-level ozone and particulate matter. As governments worldwide implement stricter emissions regulations, industries relying on combustion processes are under pressure to reduce HONO emissions.
In the power generation sector, coal-fired power plants are major sources of HONO emissions. With many countries transitioning towards cleaner energy sources, there is a growing demand for technologies that can mitigate HONO formation in existing plants. This demand is particularly strong in developing economies where coal remains a significant part of the energy mix.
The automotive industry is another key market for HONO reduction technologies. As vehicle emission standards become more stringent, manufacturers are seeking innovative solutions to minimize HONO formation in internal combustion engines. This trend is expected to continue, even as the industry shifts towards electric vehicles, due to the ongoing use of combustion engines in hybrid vehicles and heavy-duty transport.
Industrial processes involving high-temperature combustion, such as cement production and waste incineration, also contribute significantly to HONO emissions. These sectors are actively seeking cost-effective methods to reduce HONO formation to comply with environmental regulations and improve their sustainability profiles.
The market for HONO reduction technologies is also driven by public health concerns. Studies have linked exposure to HONO and its secondary pollutants to various respiratory and cardiovascular issues. This has led to increased pressure from communities and environmental groups on industries to address HONO emissions, creating a demand for effective mitigation strategies.
Research institutions and environmental agencies are investing in the development of advanced monitoring and control systems for HONO emissions. This has created a niche market for specialized equipment and analytical tools designed to measure and track HONO levels in combustion systems.
The global focus on climate change and air quality improvement is expected to further drive the demand for HONO reduction technologies in the coming years. As countries work towards meeting their emissions reduction targets under international agreements, the market for innovative solutions in combustion systems is likely to expand significantly.
In the power generation sector, coal-fired power plants are major sources of HONO emissions. With many countries transitioning towards cleaner energy sources, there is a growing demand for technologies that can mitigate HONO formation in existing plants. This demand is particularly strong in developing economies where coal remains a significant part of the energy mix.
The automotive industry is another key market for HONO reduction technologies. As vehicle emission standards become more stringent, manufacturers are seeking innovative solutions to minimize HONO formation in internal combustion engines. This trend is expected to continue, even as the industry shifts towards electric vehicles, due to the ongoing use of combustion engines in hybrid vehicles and heavy-duty transport.
Industrial processes involving high-temperature combustion, such as cement production and waste incineration, also contribute significantly to HONO emissions. These sectors are actively seeking cost-effective methods to reduce HONO formation to comply with environmental regulations and improve their sustainability profiles.
The market for HONO reduction technologies is also driven by public health concerns. Studies have linked exposure to HONO and its secondary pollutants to various respiratory and cardiovascular issues. This has led to increased pressure from communities and environmental groups on industries to address HONO emissions, creating a demand for effective mitigation strategies.
Research institutions and environmental agencies are investing in the development of advanced monitoring and control systems for HONO emissions. This has created a niche market for specialized equipment and analytical tools designed to measure and track HONO levels in combustion systems.
The global focus on climate change and air quality improvement is expected to further drive the demand for HONO reduction technologies in the coming years. As countries work towards meeting their emissions reduction targets under international agreements, the market for innovative solutions in combustion systems is likely to expand significantly.
Current State and Challenges in HONO Formation Research
The current state of HONO formation research in combustion systems reveals significant progress alongside persistent challenges. Recent studies have substantially improved our understanding of the mechanisms behind nitrous acid formation, particularly in high-temperature environments. Advanced analytical techniques, such as laser-induced fluorescence and cavity ring-down spectroscopy, have enabled more precise measurements of HONO concentrations in combustion processes.
One of the key advancements is the identification of multiple pathways for HONO formation. Researchers have established that the reaction between NO and OH radicals is a primary route, but other mechanisms, including heterogeneous reactions on surfaces and the reduction of NO2 by various hydrocarbon species, also contribute significantly. These findings have led to more comprehensive kinetic models that better predict HONO formation under diverse combustion conditions.
Despite these advances, several challenges persist in HONO formation research. The complexity of combustion systems, with their multitude of simultaneous reactions and varying conditions, makes it difficult to isolate and quantify individual HONO formation pathways. This complexity is further compounded by the transient nature of many intermediate species involved in HONO formation, which are often present in trace amounts and have short lifetimes.
Another significant challenge is the accurate measurement of HONO in real-time combustion environments. While analytical techniques have improved, interference from other nitrogen-containing species and the high temperatures involved in combustion processes continue to pose difficulties for precise quantification. This challenge is particularly acute in practical combustion systems, where access for measurement devices is limited.
The role of surface reactions in HONO formation remains a contentious area of research. While laboratory studies have demonstrated the importance of heterogeneous processes, translating these findings to real-world combustion systems, with their diverse and dynamic surface conditions, remains challenging. Understanding how factors such as surface composition, temperature, and residence time affect HONO formation rates is crucial for developing accurate predictive models.
Furthermore, the interplay between HONO formation and other pollutant formation processes, such as NOx and particulate matter, is not fully understood. This knowledge gap hinders the development of comprehensive emission control strategies that can simultaneously address multiple pollutants. Researchers are working to elucidate these complex interactions, but progress is slow due to the multifaceted nature of the problem.
In conclusion, while significant strides have been made in understanding HONO formation in combustion systems, numerous challenges remain. Overcoming these obstacles will require continued innovation in measurement techniques, more sophisticated modeling approaches, and interdisciplinary collaboration to address the complex interplay of chemical and physical processes involved in HONO formation.
One of the key advancements is the identification of multiple pathways for HONO formation. Researchers have established that the reaction between NO and OH radicals is a primary route, but other mechanisms, including heterogeneous reactions on surfaces and the reduction of NO2 by various hydrocarbon species, also contribute significantly. These findings have led to more comprehensive kinetic models that better predict HONO formation under diverse combustion conditions.
Despite these advances, several challenges persist in HONO formation research. The complexity of combustion systems, with their multitude of simultaneous reactions and varying conditions, makes it difficult to isolate and quantify individual HONO formation pathways. This complexity is further compounded by the transient nature of many intermediate species involved in HONO formation, which are often present in trace amounts and have short lifetimes.
Another significant challenge is the accurate measurement of HONO in real-time combustion environments. While analytical techniques have improved, interference from other nitrogen-containing species and the high temperatures involved in combustion processes continue to pose difficulties for precise quantification. This challenge is particularly acute in practical combustion systems, where access for measurement devices is limited.
The role of surface reactions in HONO formation remains a contentious area of research. While laboratory studies have demonstrated the importance of heterogeneous processes, translating these findings to real-world combustion systems, with their diverse and dynamic surface conditions, remains challenging. Understanding how factors such as surface composition, temperature, and residence time affect HONO formation rates is crucial for developing accurate predictive models.
Furthermore, the interplay between HONO formation and other pollutant formation processes, such as NOx and particulate matter, is not fully understood. This knowledge gap hinders the development of comprehensive emission control strategies that can simultaneously address multiple pollutants. Researchers are working to elucidate these complex interactions, but progress is slow due to the multifaceted nature of the problem.
In conclusion, while significant strides have been made in understanding HONO formation in combustion systems, numerous challenges remain. Overcoming these obstacles will require continued innovation in measurement techniques, more sophisticated modeling approaches, and interdisciplinary collaboration to address the complex interplay of chemical and physical processes involved in HONO formation.
Existing HONO Formation Mechanisms in Combustion
01 Chemical synthesis of nitrous acid
Nitrous acid can be synthesized through various chemical reactions, including the reduction of nitric acid or the oxidation of nitrogen compounds. These processes often involve specific catalysts, reaction conditions, and precursor materials to control the formation of nitrous acid.- Chemical reactions for nitrous acid formation: Nitrous acid can be formed through various chemical reactions, including the reaction of nitrogen oxides with water or the reduction of nitric acid. These processes are often utilized in industrial settings and can be controlled to produce nitrous acid for different applications.
- Nitrous acid formation in atmospheric processes: Nitrous acid can be formed in the atmosphere through complex chemical reactions involving nitrogen oxides, water vapor, and other atmospheric components. This process plays a role in air quality and atmospheric chemistry, affecting the formation of other pollutants and contributing to acid rain.
- Nitrous acid formation in soil and water systems: Nitrous acid can be formed in soil and water systems through microbial activity and chemical processes. This formation is important in the nitrogen cycle and can affect soil fertility, water quality, and ecosystem health. Understanding these processes is crucial for environmental management and agricultural practices.
- Industrial applications of nitrous acid formation: The controlled formation of nitrous acid is utilized in various industrial processes, including the production of dyes, pharmaceuticals, and other chemicals. Specific methods and catalysts can be employed to optimize nitrous acid formation for these applications, considering factors such as yield, purity, and safety.
- Detection and measurement of nitrous acid formation: Various analytical techniques and instruments have been developed to detect and measure nitrous acid formation in different environments. These methods are important for monitoring air and water quality, assessing industrial processes, and conducting scientific research on atmospheric chemistry and environmental pollution.
02 Industrial applications of nitrous acid formation
The formation of nitrous acid is crucial in several industrial processes, such as the production of dyes, pharmaceuticals, and other chemical compounds. Controlled nitrous acid formation is utilized in manufacturing processes to achieve desired chemical transformations or as an intermediate in multi-step reactions.Expand Specific Solutions03 Environmental impact and control of nitrous acid formation
Nitrous acid formation in the environment can have significant impacts on air quality and atmospheric chemistry. Research focuses on understanding the mechanisms of nitrous acid formation in various environmental conditions and developing strategies to control or mitigate its formation to reduce pollution and associated health risks.Expand Specific Solutions04 Analytical methods for detecting nitrous acid
Various analytical techniques have been developed to detect and quantify nitrous acid in different matrices. These methods may include spectroscopic techniques, electrochemical sensors, or chemical assays designed to accurately measure nitrous acid concentrations in gas, liquid, or solid samples.Expand Specific Solutions05 Nitrous acid in biological systems
The formation and role of nitrous acid in biological systems, including its involvement in cellular processes and potential impacts on organisms, is an area of ongoing research. Studies investigate the generation of nitrous acid in living tissues, its physiological effects, and its potential applications in biotechnology or medicine.Expand Specific Solutions
Key Players in Combustion Chemistry Research
The formation of nitrous acid in combustion systems represents a complex technological challenge at the intersection of environmental science and industrial processes. The competitive landscape in this field is characterized by a mix of established industrial players and academic institutions, reflecting the ongoing research and development efforts. Companies like Yara International, BASF, and Siemens are actively involved, leveraging their extensive experience in chemical processes and emissions control. The market is still evolving, with potential for significant growth as environmental regulations tighten globally. While the technology is advancing, it has not yet reached full maturity, evidenced by the continued involvement of research institutions such as the University of Sydney and the Norwegian University of Science & Technology, which are contributing to fundamental understanding and practical applications in this domain.
The University of Sydney
Technical Solution: The University of Sydney has conducted extensive research on nitrous acid formation in combustion systems. They have developed a comprehensive model that accounts for both gas-phase and heterogeneous reactions[1]. Their approach combines detailed chemical kinetics with computational fluid dynamics (CFD) simulations to accurately predict HONO formation under various combustion conditions[2]. The model considers factors such as temperature, pressure, and fuel composition, providing insights into the complex interplay between NOx chemistry and surface reactions[3]. This research has significantly contributed to understanding the role of HONO in atmospheric chemistry and its impact on air quality.
Strengths: Comprehensive modeling approach, integration of multiple reaction pathways, and high-resolution simulations. Weaknesses: Computational intensity may limit real-time applications, and model validation in diverse combustion systems is ongoing.
Xiangtan University
Technical Solution: Xiangtan University has focused on experimental studies of nitrous acid formation in combustion systems. Their research utilizes advanced spectroscopic techniques, including cavity ring-down spectroscopy (CRDS) and Fourier transform infrared spectroscopy (FTIR), to measure HONO concentrations in real-time during combustion processes[4]. They have investigated the effects of various fuel additives and combustion parameters on HONO formation, providing valuable empirical data for model validation[5]. Their work has also explored the role of surface-mediated reactions in promoting HONO formation, particularly in the presence of metal oxides commonly found in combustion chambers[6].
Strengths: High-precision experimental measurements, real-time monitoring capabilities, and insights into surface chemistry effects. Weaknesses: Limited to laboratory-scale experiments, which may not fully represent industrial-scale combustion systems.
Core Innovations in HONO Detection and Quantification
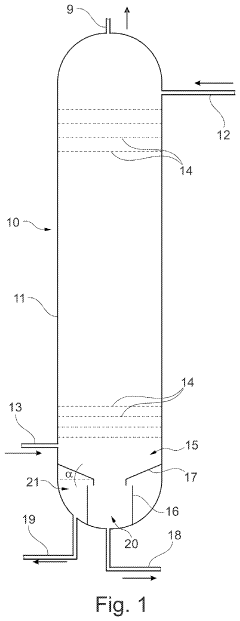
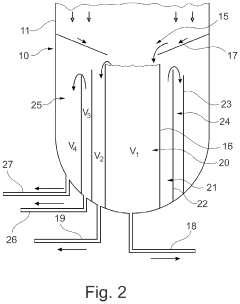
Absorption tower for a nitric acid plant method for producing nitric acid
PatentPendingUS20230074083A1
Innovation
- The absorption tower incorporates a dividing wall at the column bottom, dividing it into radially inner and outer regions, allowing for the collection of concentrated nitric acid in the inner region and less concentrated acid in the outer region, preventing dilution and enabling separate collection and reuse of more concentrated acid for faster startup and improved acid production.
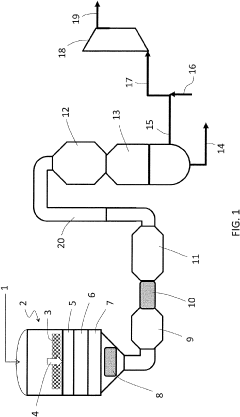
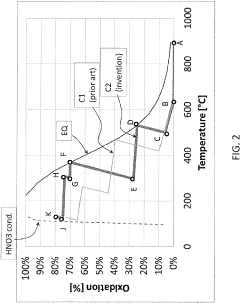
Process for the production of nitric acid
PatentPendingUS20230115002A1
Innovation
- A process using a first catalyst for high-temperature N2O removal and a second catalyst, preferably iron-loaded ferrierite (Fe-FER) or ferrierite, for low-temperature NO oxidation, which can be the same or different, to enhance the conversion of NO to NO2, thereby reducing catalyst costs and improving heat recovery.
Environmental Impact of Combustion-Generated HONO
The formation of nitrous acid (HONO) in combustion systems has significant environmental implications, particularly in terms of air quality and atmospheric chemistry. HONO is a key precursor to hydroxyl radicals (OH), which play a crucial role in the oxidation processes of the atmosphere. The presence of combustion-generated HONO can lead to increased ozone formation in urban areas, contributing to photochemical smog and associated health risks.
In the troposphere, HONO photolysis is a major source of OH radicals during daytime hours. This process accelerates the oxidation of volatile organic compounds (VOCs) and nitrogen oxides (NOx), leading to the formation of secondary pollutants such as ozone and particulate matter. The impact is particularly pronounced in urban environments with high traffic density, where combustion emissions are concentrated.
The environmental effects of combustion-generated HONO extend beyond local air quality issues. HONO can participate in long-range transport processes, influencing atmospheric chemistry on regional and potentially global scales. This transport can lead to the formation of secondary pollutants in areas far from the original combustion sources, complicating air quality management efforts across different jurisdictions.
Furthermore, HONO emissions from combustion systems can contribute to acid deposition. While nitric acid (HNO3) is typically considered the primary contributor to acid rain, HONO can also play a role in this process. The deposition of HONO and its reaction products can lead to soil and water acidification, impacting ecosystems and biodiversity.
The presence of HONO in the atmosphere also affects the nitrogen cycle. As a reactive nitrogen species, HONO contributes to the overall nitrogen budget in the environment. This can lead to eutrophication in aquatic ecosystems when deposited, causing algal blooms and potential disruption of aquatic food webs.
In indoor environments, particularly in areas with poor ventilation and high use of combustion appliances, HONO can accumulate and pose direct health risks. Exposure to elevated levels of HONO has been associated with respiratory irritation and other adverse health effects, highlighting the importance of proper ventilation and emission control in indoor settings.
Understanding the environmental impact of combustion-generated HONO is crucial for developing effective air quality management strategies and emission control technologies. As research continues to elucidate the complex role of HONO in atmospheric chemistry, it becomes increasingly important to consider its formation and impacts in the design and regulation of combustion systems across various sectors, including transportation, industry, and residential energy use.
In the troposphere, HONO photolysis is a major source of OH radicals during daytime hours. This process accelerates the oxidation of volatile organic compounds (VOCs) and nitrogen oxides (NOx), leading to the formation of secondary pollutants such as ozone and particulate matter. The impact is particularly pronounced in urban environments with high traffic density, where combustion emissions are concentrated.
The environmental effects of combustion-generated HONO extend beyond local air quality issues. HONO can participate in long-range transport processes, influencing atmospheric chemistry on regional and potentially global scales. This transport can lead to the formation of secondary pollutants in areas far from the original combustion sources, complicating air quality management efforts across different jurisdictions.
Furthermore, HONO emissions from combustion systems can contribute to acid deposition. While nitric acid (HNO3) is typically considered the primary contributor to acid rain, HONO can also play a role in this process. The deposition of HONO and its reaction products can lead to soil and water acidification, impacting ecosystems and biodiversity.
The presence of HONO in the atmosphere also affects the nitrogen cycle. As a reactive nitrogen species, HONO contributes to the overall nitrogen budget in the environment. This can lead to eutrophication in aquatic ecosystems when deposited, causing algal blooms and potential disruption of aquatic food webs.
In indoor environments, particularly in areas with poor ventilation and high use of combustion appliances, HONO can accumulate and pose direct health risks. Exposure to elevated levels of HONO has been associated with respiratory irritation and other adverse health effects, highlighting the importance of proper ventilation and emission control in indoor settings.
Understanding the environmental impact of combustion-generated HONO is crucial for developing effective air quality management strategies and emission control technologies. As research continues to elucidate the complex role of HONO in atmospheric chemistry, it becomes increasingly important to consider its formation and impacts in the design and regulation of combustion systems across various sectors, including transportation, industry, and residential energy use.
Regulatory Framework for NOx and HONO Emissions
The regulatory framework for NOx and HONO emissions has evolved significantly over the past few decades, driven by increasing awareness of their environmental and health impacts. At the international level, the United Nations Economic Commission for Europe (UNECE) Convention on Long-range Transboundary Air Pollution (CLRTAP) has been instrumental in setting guidelines for NOx emissions since 1979. This convention has been ratified by numerous countries and has led to substantial reductions in NOx emissions across Europe and North America.
In the United States, the Clean Air Act and its subsequent amendments form the backbone of NOx and HONO regulation. The Environmental Protection Agency (EPA) has established National Ambient Air Quality Standards (NAAQS) for nitrogen dioxide (NO2), which indirectly affects HONO formation. These standards are periodically reviewed and updated based on the latest scientific evidence. The EPA also implements the NOx Budget Trading Program and the Cross-State Air Pollution Rule to further reduce NOx emissions from power plants and large industrial sources.
The European Union has implemented stringent regulations through its Air Quality Directive and the Industrial Emissions Directive. These directives set limit values for NO2 concentrations in ambient air and establish emission standards for various industrial sectors. The Euro emission standards for vehicles have also played a crucial role in reducing NOx emissions from the transportation sector, with increasingly stringent limits imposed on new vehicles.
In Asia, countries like China and Japan have introduced their own regulatory frameworks to address NOx and HONO emissions. China's Air Pollution Prevention and Control Action Plan, implemented in 2013, set ambitious targets for reducing NOx emissions from various sources. Japan has long-standing regulations under its Air Pollution Control Law, which includes specific standards for NOx emissions from stationary and mobile sources.
While direct regulation of HONO emissions is less common, many countries have implemented policies that indirectly affect HONO formation. These include regulations on VOC emissions, which can contribute to HONO formation through heterogeneous reactions. Additionally, some jurisdictions have begun to consider HONO in their air quality modeling and assessment processes, recognizing its role in photochemical smog formation.
The regulatory landscape continues to evolve as new scientific understanding of NOx and HONO chemistry emerges. There is a growing trend towards more integrated approaches that consider the complex interactions between different pollutants and their precursors. Future regulations are likely to focus on multi-pollutant strategies that address NOx, HONO, and other related compounds simultaneously, potentially leading to more effective and efficient emission control measures.
In the United States, the Clean Air Act and its subsequent amendments form the backbone of NOx and HONO regulation. The Environmental Protection Agency (EPA) has established National Ambient Air Quality Standards (NAAQS) for nitrogen dioxide (NO2), which indirectly affects HONO formation. These standards are periodically reviewed and updated based on the latest scientific evidence. The EPA also implements the NOx Budget Trading Program and the Cross-State Air Pollution Rule to further reduce NOx emissions from power plants and large industrial sources.
The European Union has implemented stringent regulations through its Air Quality Directive and the Industrial Emissions Directive. These directives set limit values for NO2 concentrations in ambient air and establish emission standards for various industrial sectors. The Euro emission standards for vehicles have also played a crucial role in reducing NOx emissions from the transportation sector, with increasingly stringent limits imposed on new vehicles.
In Asia, countries like China and Japan have introduced their own regulatory frameworks to address NOx and HONO emissions. China's Air Pollution Prevention and Control Action Plan, implemented in 2013, set ambitious targets for reducing NOx emissions from various sources. Japan has long-standing regulations under its Air Pollution Control Law, which includes specific standards for NOx emissions from stationary and mobile sources.
While direct regulation of HONO emissions is less common, many countries have implemented policies that indirectly affect HONO formation. These include regulations on VOC emissions, which can contribute to HONO formation through heterogeneous reactions. Additionally, some jurisdictions have begun to consider HONO in their air quality modeling and assessment processes, recognizing its role in photochemical smog formation.
The regulatory landscape continues to evolve as new scientific understanding of NOx and HONO chemistry emerges. There is a growing trend towards more integrated approaches that consider the complex interactions between different pollutants and their precursors. Future regulations are likely to focus on multi-pollutant strategies that address NOx, HONO, and other related compounds simultaneously, potentially leading to more effective and efficient emission control measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!