How to Increase Alkane Yield in Hydrocracking
JAN 7, 20269 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrocracking Alkane Yield Enhancement Background and Objectives
Hydrocracking represents a critical refining process that converts heavy petroleum fractions into lighter, more valuable products through catalytic cracking under high hydrogen pressure. The technology has evolved significantly since its commercial introduction in the 1960s, driven by increasing demand for transportation fuels and the need to process heavier crude oil feedstocks. As global refineries face stricter environmental regulations and shifting market dynamics toward cleaner fuels, optimizing product selectivity has become paramount.
The primary objective of enhancing alkane yield in hydrocracking centers on maximizing the production of saturated hydrocarbons, particularly in the gasoline and diesel range. Alkanes possess superior fuel properties including high cetane numbers, excellent combustion characteristics, and minimal aromatic content, making them ideal components for meeting stringent fuel specifications. Traditional hydrocracking processes often generate significant quantities of aromatics and naphthenes, which require additional processing or blending adjustments.
Current industry challenges necessitate technological advancement in several key areas. First, achieving selective bond cleavage to favor paraffinic products while minimizing secondary cracking reactions remains technically demanding. Second, balancing catalyst activity and selectivity to optimize alkane formation without compromising overall conversion efficiency presents ongoing difficulties. Third, managing hydrogen consumption economics while maintaining high hydrogenation rates requires careful process optimization.
The strategic importance of this research direction extends beyond immediate operational improvements. Enhanced alkane selectivity directly impacts refinery economics by reducing downstream processing requirements, improving product quality margins, and facilitating compliance with evolving fuel standards. Furthermore, as the industry transitions toward processing opportunity crudes and renewable feedstocks, developing robust hydrocracking technologies with superior alkane selectivity becomes essential for maintaining competitive advantage.
This technical investigation aims to systematically explore catalyst design principles, reaction mechanism optimization, and process parameter manipulation strategies that collectively enable significant improvements in alkane yield. The research encompasses both fundamental understanding of hydrocracking chemistry and practical implementation considerations for industrial-scale operations.
The primary objective of enhancing alkane yield in hydrocracking centers on maximizing the production of saturated hydrocarbons, particularly in the gasoline and diesel range. Alkanes possess superior fuel properties including high cetane numbers, excellent combustion characteristics, and minimal aromatic content, making them ideal components for meeting stringent fuel specifications. Traditional hydrocracking processes often generate significant quantities of aromatics and naphthenes, which require additional processing or blending adjustments.
Current industry challenges necessitate technological advancement in several key areas. First, achieving selective bond cleavage to favor paraffinic products while minimizing secondary cracking reactions remains technically demanding. Second, balancing catalyst activity and selectivity to optimize alkane formation without compromising overall conversion efficiency presents ongoing difficulties. Third, managing hydrogen consumption economics while maintaining high hydrogenation rates requires careful process optimization.
The strategic importance of this research direction extends beyond immediate operational improvements. Enhanced alkane selectivity directly impacts refinery economics by reducing downstream processing requirements, improving product quality margins, and facilitating compliance with evolving fuel standards. Furthermore, as the industry transitions toward processing opportunity crudes and renewable feedstocks, developing robust hydrocracking technologies with superior alkane selectivity becomes essential for maintaining competitive advantage.
This technical investigation aims to systematically explore catalyst design principles, reaction mechanism optimization, and process parameter manipulation strategies that collectively enable significant improvements in alkane yield. The research encompasses both fundamental understanding of hydrocracking chemistry and practical implementation considerations for industrial-scale operations.
Market Demand for High-Quality Alkane Products
The global refining industry is experiencing a structural shift in product demand, with high-quality alkanes emerging as increasingly valuable commodities across multiple sectors. Linear and branched alkanes, particularly those in the C10-C20 range, serve as essential feedstocks for producing premium fuels, specialty chemicals, and high-performance lubricants. This demand evolution is driven by stringent environmental regulations mandating cleaner-burning fuels with lower aromatic content, coupled with growing requirements for high-cetane diesel and sustainable aviation fuel components.
Transportation fuel markets represent the largest consumption segment for hydrocracking-derived alkanes. Modern diesel engines require fuels with cetane numbers exceeding 51, achievable primarily through higher concentrations of normal and iso-alkanes. Similarly, the aviation sector's commitment to reducing carbon emissions has accelerated demand for synthetic paraffinic kerosene, where alkane-rich fractions serve as critical blending components. Regional markets in Asia-Pacific and North America are particularly active, as these regions implement increasingly stringent fuel quality specifications.
The petrochemical industry constitutes another significant demand driver. High-purity alkanes function as essential raw materials for producing detergents, plasticizers, and polymer additives. The shift toward bio-based and circular economy principles has further intensified interest in alkane streams that can be converted into sustainable chemical intermediates. Specialty applications in cosmetics, pharmaceuticals, and food-grade products require exceptionally pure alkane fractions, commanding premium pricing and creating lucrative market opportunities.
Industrial lubricant manufacturers increasingly seek high-viscosity-index base oils derived from alkane-rich hydrocracking products. These materials offer superior thermal stability and oxidation resistance compared to conventional mineral oils, addressing performance demands in automotive, aerospace, and industrial machinery applications. Market analysts observe sustained growth in synthetic and semi-synthetic lubricant segments, directly correlating with demand for high-quality alkane feedstocks.
Emerging applications in energy storage and specialty materials are creating additional market pull. Alkanes serve as phase-change materials in thermal management systems and as dielectric fluids in advanced electrical applications. These niche but growing markets value the chemical stability and predictable physical properties that characterize high-purity alkane products from optimized hydrocracking processes.
Transportation fuel markets represent the largest consumption segment for hydrocracking-derived alkanes. Modern diesel engines require fuels with cetane numbers exceeding 51, achievable primarily through higher concentrations of normal and iso-alkanes. Similarly, the aviation sector's commitment to reducing carbon emissions has accelerated demand for synthetic paraffinic kerosene, where alkane-rich fractions serve as critical blending components. Regional markets in Asia-Pacific and North America are particularly active, as these regions implement increasingly stringent fuel quality specifications.
The petrochemical industry constitutes another significant demand driver. High-purity alkanes function as essential raw materials for producing detergents, plasticizers, and polymer additives. The shift toward bio-based and circular economy principles has further intensified interest in alkane streams that can be converted into sustainable chemical intermediates. Specialty applications in cosmetics, pharmaceuticals, and food-grade products require exceptionally pure alkane fractions, commanding premium pricing and creating lucrative market opportunities.
Industrial lubricant manufacturers increasingly seek high-viscosity-index base oils derived from alkane-rich hydrocracking products. These materials offer superior thermal stability and oxidation resistance compared to conventional mineral oils, addressing performance demands in automotive, aerospace, and industrial machinery applications. Market analysts observe sustained growth in synthetic and semi-synthetic lubricant segments, directly correlating with demand for high-quality alkane feedstocks.
Emerging applications in energy storage and specialty materials are creating additional market pull. Alkanes serve as phase-change materials in thermal management systems and as dielectric fluids in advanced electrical applications. These niche but growing markets value the chemical stability and predictable physical properties that characterize high-purity alkane products from optimized hydrocracking processes.
Current Hydrocracking Technology Status and Yield Limitations
Hydrocracking technology has evolved significantly since its commercial introduction in the 1960s, establishing itself as a critical process in modern petroleum refining. The technology operates under severe conditions, typically at temperatures between 300-450°C and pressures ranging from 35-200 bar, utilizing bifunctional catalysts that combine metal hydrogenation sites with acidic cracking functions. Current industrial hydrocracking units predominantly employ zeolite-based catalysts, particularly Y-zeolite and beta-zeolite formulations, which facilitate the simultaneous breaking of carbon-carbon bonds and hydrogenation of unsaturated intermediates.
Despite substantial technological advancements, contemporary hydrocracking processes face persistent challenges in maximizing alkane yield while maintaining operational efficiency. The primary limitation stems from the inherent trade-off between conversion depth and product selectivity. As conversion rates increase beyond 70-80%, secondary cracking reactions become more prevalent, leading to excessive production of light gases and reducing the yield of valuable middle distillates and alkanes in the desired carbon number range.
Catalyst deactivation represents another critical constraint affecting long-term alkane productivity. Coke deposition on catalyst surfaces, metal poisoning from feed contaminants, and structural degradation of zeolite frameworks progressively diminish catalytic activity. Current catalyst lifetimes typically range from 2-5 years, with gradual increases in operating temperature required to compensate for activity loss, ultimately compromising selectivity toward desired alkane products.
The complexity of feedstock composition further complicates yield optimization. Modern refineries increasingly process heavier and more contaminated crude oils containing higher concentrations of polycyclic aromatics, sulfur compounds, and nitrogen-containing molecules. These challenging feedstocks demand more severe processing conditions, which paradoxically promote undesirable side reactions including excessive hydrogenation, isomerization, and over-cracking that reduce net alkane yields.
Process configuration limitations also constrain performance improvements. Traditional single-stage and two-stage hydrocracking designs struggle to independently optimize the distinct reaction requirements for heteroatom removal, aromatic saturation, and selective cracking. This integrated approach forces operational compromises that prevent achievement of theoretical maximum alkane yields across the full product spectrum.
Despite substantial technological advancements, contemporary hydrocracking processes face persistent challenges in maximizing alkane yield while maintaining operational efficiency. The primary limitation stems from the inherent trade-off between conversion depth and product selectivity. As conversion rates increase beyond 70-80%, secondary cracking reactions become more prevalent, leading to excessive production of light gases and reducing the yield of valuable middle distillates and alkanes in the desired carbon number range.
Catalyst deactivation represents another critical constraint affecting long-term alkane productivity. Coke deposition on catalyst surfaces, metal poisoning from feed contaminants, and structural degradation of zeolite frameworks progressively diminish catalytic activity. Current catalyst lifetimes typically range from 2-5 years, with gradual increases in operating temperature required to compensate for activity loss, ultimately compromising selectivity toward desired alkane products.
The complexity of feedstock composition further complicates yield optimization. Modern refineries increasingly process heavier and more contaminated crude oils containing higher concentrations of polycyclic aromatics, sulfur compounds, and nitrogen-containing molecules. These challenging feedstocks demand more severe processing conditions, which paradoxically promote undesirable side reactions including excessive hydrogenation, isomerization, and over-cracking that reduce net alkane yields.
Process configuration limitations also constrain performance improvements. Traditional single-stage and two-stage hydrocracking designs struggle to independently optimize the distinct reaction requirements for heteroatom removal, aromatic saturation, and selective cracking. This integrated approach forces operational compromises that prevent achievement of theoretical maximum alkane yields across the full product spectrum.
Existing Solutions for Alkane Selectivity Improvement
01 Use of zeolite-based catalysts for hydrocracking
Zeolite-based catalysts are widely employed in hydrocracking processes to enhance alkane yield. These catalysts provide high acidity and shape selectivity, which facilitate the cracking of heavy hydrocarbons into lighter alkanes. The zeolite structure allows for controlled cracking reactions, improving the selectivity towards desired alkane products while minimizing over-cracking and gas formation.- Use of zeolite-based catalysts for hydrocracking to enhance alkane yield: Zeolite-based catalysts are widely employed in hydrocracking processes to improve the selectivity and yield of alkane products. These catalysts possess unique pore structures and acidic properties that facilitate the cracking of heavy hydrocarbons into lighter alkanes. The catalyst composition, including the type of zeolite and metal loading, can be optimized to maximize alkane production while minimizing undesired byproducts. Various zeolite frameworks and their modifications have been developed to enhance catalytic performance in hydrocracking operations.
- Optimization of reaction conditions for maximizing alkane yield: The hydrocracking process parameters, including temperature, pressure, hydrogen-to-oil ratio, and space velocity, significantly influence the yield of alkanes. Operating at optimal temperature ranges ensures sufficient cracking activity while preventing excessive secondary reactions that reduce alkane selectivity. Pressure conditions affect hydrogen availability and reaction kinetics, impacting the degree of saturation and product distribution. Careful control of these parameters enables the maximization of desired alkane products from various feedstocks.
- Two-stage hydrocracking processes for improved alkane production: Two-stage hydrocracking configurations involve sequential reaction zones with different operating conditions or catalysts to enhance alkane yield. The first stage typically focuses on initial cracking and heteroatom removal, while the second stage emphasizes further conversion and product upgrading. This approach allows for better control over product distribution and enables higher overall conversion of heavy feedstocks to valuable alkane products. The staged process can be optimized by adjusting interstage separation and recycling strategies.
- Use of noble metal catalysts to increase alkane selectivity: Noble metal-containing catalysts, particularly those incorporating platinum, palladium, or combinations thereof, demonstrate enhanced hydrogenation activity in hydrocracking processes. These catalysts promote the saturation of aromatic and olefinic intermediates, leading to increased alkane yield. The metal dispersion, support material, and metal-acid balance are critical factors affecting catalyst performance. Proper formulation of noble metal catalysts can significantly improve the selectivity toward paraffinic products while maintaining catalyst stability.
- Feedstock pretreatment and hydrogen management for enhanced alkane yield: Pretreatment of hydrocracking feedstocks through hydrotreating or other purification methods removes catalyst poisons and improves subsequent conversion to alkanes. Effective hydrogen management, including hydrogen purity, distribution, and partial pressure control, is essential for maximizing hydrogenation reactions that produce saturated alkane products. The integration of hydrogen recovery and recycling systems can improve process economics while maintaining optimal hydrogen availability. Feedstock characterization and appropriate pretreatment selection contribute to achieving higher alkane yields in hydrocracking operations.
02 Optimization of reaction conditions
The yield of alkanes in hydrocracking can be significantly improved by optimizing key reaction parameters such as temperature, pressure, and hydrogen-to-oil ratio. Higher pressures and moderate temperatures favor the production of middle distillates and alkanes while suppressing excessive cracking. Proper control of these conditions ensures maximum conversion of feedstock to valuable alkane products.Expand Specific Solutions03 Two-stage hydrocracking process
A two-stage hydrocracking process can be implemented to maximize alkane yield. The first stage focuses on hydrogenation and partial cracking, while the second stage completes the conversion to lighter alkanes. This approach allows for better control over product distribution and enables the production of high-quality alkane fractions with improved yields compared to single-stage processes.Expand Specific Solutions04 Use of noble metal catalysts
Noble metal catalysts, particularly those containing platinum or palladium, are effective in hydrocracking processes for enhancing alkane production. These catalysts provide excellent hydrogenation activity, which helps in saturating aromatic compounds and preventing catalyst deactivation. The combination of noble metals with acidic supports creates bifunctional catalysts that optimize both hydrogenation and cracking functions.Expand Specific Solutions05 Feedstock pretreatment and catalyst regeneration
Pretreatment of feedstock to remove contaminants such as sulfur, nitrogen, and metals is crucial for maintaining catalyst activity and improving alkane yield in hydrocracking. Additionally, periodic catalyst regeneration through controlled oxidation and reduction cycles helps restore catalytic activity and extends catalyst life. These practices ensure sustained high performance and consistent alkane production over extended operational periods.Expand Specific Solutions
Major Players in Hydrocracking Technology and Catalyst Market
The hydrocracking alkane yield enhancement technology operates in a mature, highly competitive market dominated by established players across the global refining industry. Major integrated oil companies like China Petroleum & Chemical Corp., ExxonMobil, and Saudi Arabian Oil Co. lead in both technology development and commercial implementation, leveraging extensive R&D capabilities and operational scale. Specialized technology licensors including UOP LLC, Axens SA, and IFP Energies Nouvelles provide advanced catalyst systems and process designs, while research institutes such as Sinopec Research Institute of Petroleum Processing and SABIC Global Technologies BV drive innovation in catalyst formulation and reactor optimization. The technology has reached commercial maturity with continuous incremental improvements focused on catalyst selectivity, hydrogen efficiency, and feedstock flexibility. Market growth is driven by increasing demand for lighter products and stricter fuel specifications, with Asia-Pacific regions, particularly China through entities like Dalian Petrochemical Research Institute, showing significant investment in capacity expansion and technology upgrades.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has developed comprehensive hydrocracking technologies focusing on heavy oil conversion to maximize middle distillate and alkane production. Their technical approach combines proprietary catalyst systems with optimized reactor configurations, employing two-stage hydrocracking processes where the first stage performs partial conversion and heteroatom removal, while the second stage achieves deep conversion to alkanes. The company utilizes modified Y-zeolite based catalysts with controlled acidity and metal loading to enhance selective ring opening and hydrogenation reactions. Process conditions are optimized with hydrogen circulation ratios of 800-1500 Nm³/m³, operating pressures of 15-20 MPa, and temperature ranges of 360-420°C. Sinopec's technology incorporates advanced fractionation systems to maximize recovery of diesel and kerosene range alkanes while recycling unconverted heavy fractions. Their catalyst formulations emphasize resistance to nitrogen and sulfur poisoning, extending catalyst life in processing challenging feedstocks.
Strengths: Cost-effective solutions tailored for Asian refinery configurations, strong integration with domestic catalyst manufacturing capabilities, extensive experience processing diverse heavy oil feedstocks. Weaknesses: Technology maturity and international market penetration lag behind Western competitors, lower per-pass conversion efficiency compared to leading global technologies.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed the MIDW (Mild Hydrocracking) and MPHC (Maximum Pressure Hydrocracking) technologies that optimize alkane yield through precise control of cracking selectivity and hydrogenation activity. Their catalyst systems employ proprietary zeolite formulations with optimized Si/Al ratios and hierarchical pore structures that facilitate selective cracking of multi-ring aromatics and naphthenes into alkane products. The technology utilizes advanced reactor internals for optimal hydrogen distribution and temperature control, operating at pressures of 1500-2500 psi and temperatures of 350-425°C depending on feedstock characteristics. ExxonMobil's approach emphasizes maximizing diesel yield with high cetane numbers through controlled aromatic saturation and selective cracking pathways. Their process integration includes sophisticated hydrogen management systems and heat recovery networks that improve overall energy efficiency. The catalyst design incorporates resistance to metal deposition and maintains activity over extended cycles of 3-5 years.
Strengths: Superior product quality with high cetane diesel output, excellent catalyst stability and cycle length, comprehensive technical support and process guarantees. Weaknesses: Premium pricing structure for technology licensing and catalysts, stringent feedstock quality requirements, complex process control systems requiring advanced automation.
Core Catalyst and Reaction Mechanism Innovations
Hydrocracking of gas oils with increased distillate yield
PatentActiveEP3077485A1
Innovation
- A multi-stage process involving hydrotreating, separation or fractionation to remove contaminant gases and distillate boiling range components before hydrocracking, followed by dewaxing prior to hydrocracking to reduce severity and prevent further conversion of distillate products, using catalysts with specific metal compositions and molecular sieves to enhance distillate yield.
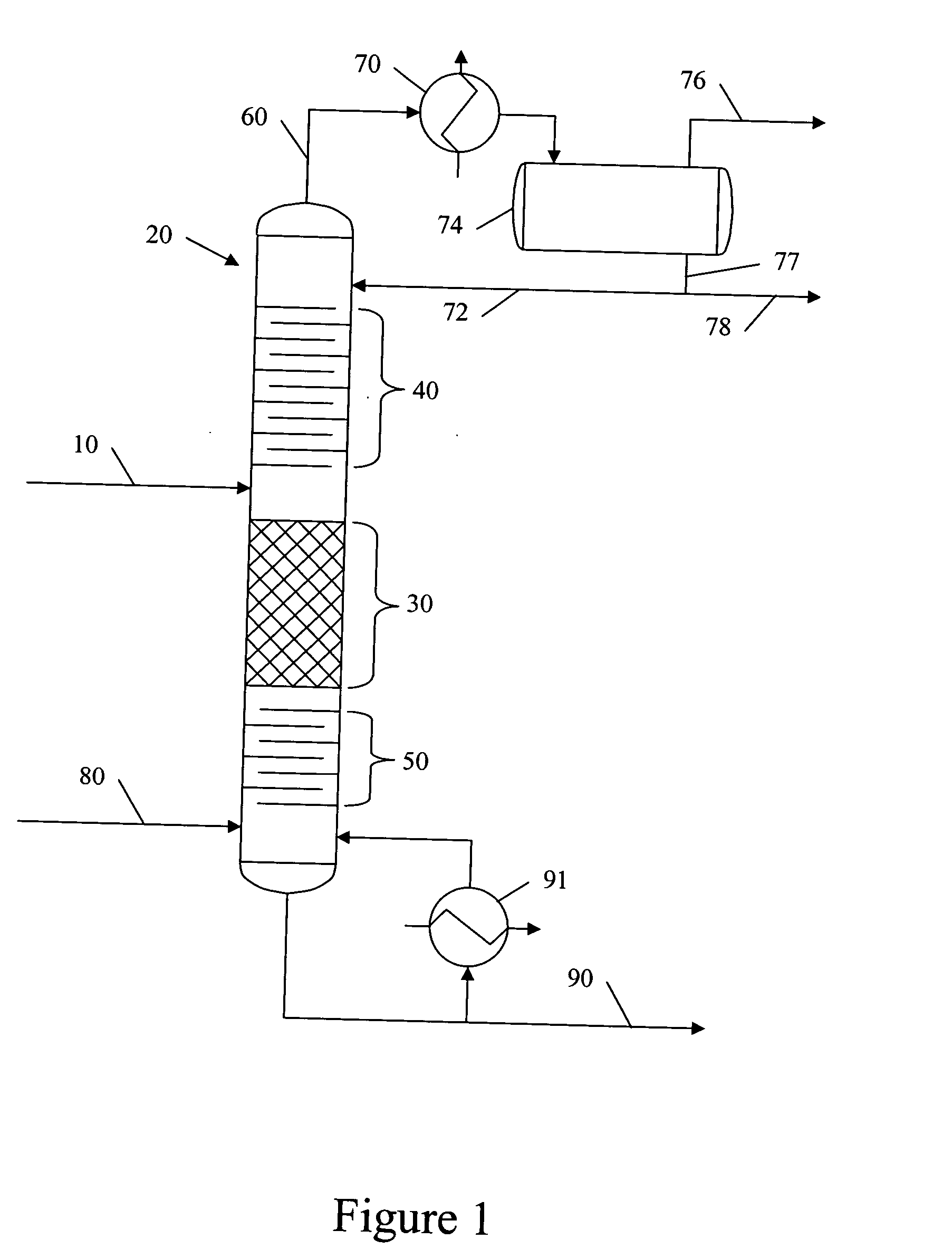
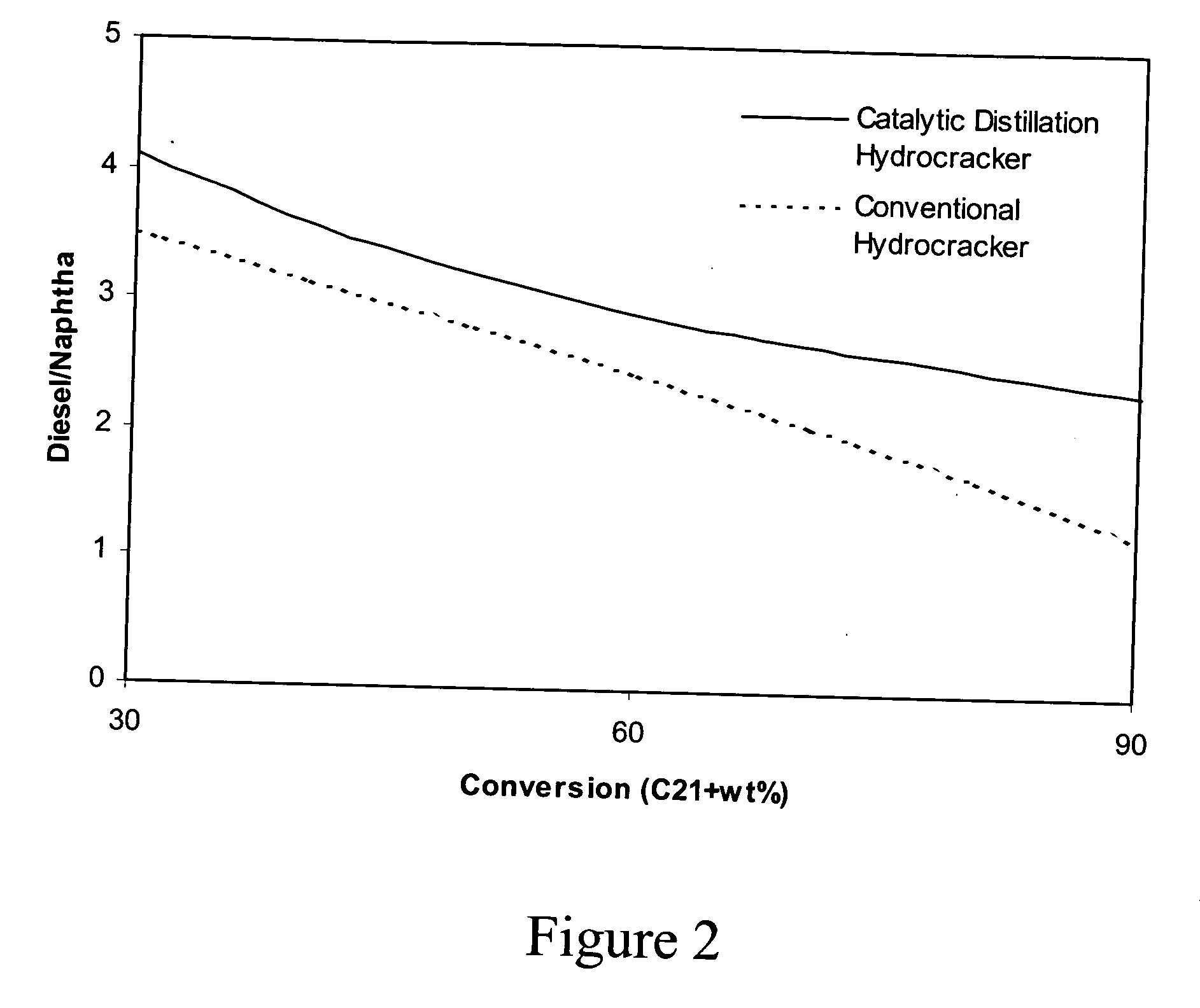
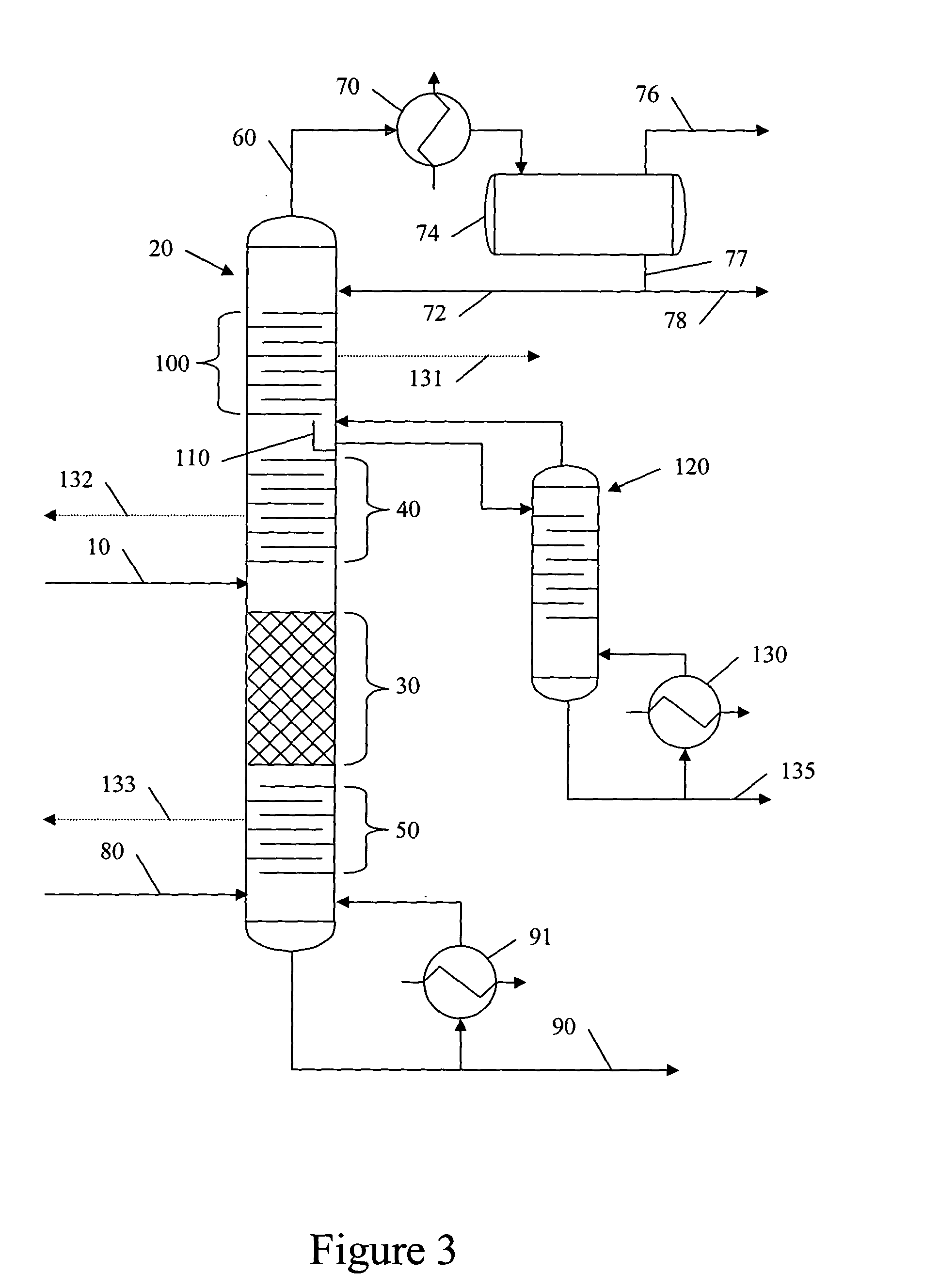
Catalytic distillation hydroprocessing
PatentInactiveUS20050035026A1
Innovation
- Implementing a catalytic distillation system that selectively vaporizes lighter components as they are produced, removing middle distillate and lighter components from the reaction zone to minimize unwanted secondary cracking, thereby optimizing the hydroprocessing unit's efficiency and output.
Environmental Regulations Impact on Hydrocracking Operations
Environmental regulations have become increasingly stringent worldwide, fundamentally reshaping hydrocracking operations and directly influencing strategies to maximize alkane yield. Regulatory frameworks governing emissions, waste management, and product specifications impose operational constraints that require careful consideration when optimizing process parameters for higher alkane production. The balance between achieving desired product yields and maintaining environmental compliance has emerged as a critical operational challenge for refineries.
Emission control regulations significantly impact hydrocracking unit design and operation. Stricter limits on sulfur dioxide, nitrogen oxides, and particulate matter emissions necessitate additional treatment systems that can affect overall process efficiency. These requirements often mandate operating conditions that may not align with optimal alkane yield parameters, forcing operators to find compromise solutions. The installation of scrubbers, selective catalytic reduction systems, and other emission control technologies adds complexity and operational costs while potentially constraining process flexibility.
Product quality specifications driven by environmental mandates directly influence hydrocracking objectives. Ultra-low sulfur diesel and gasoline requirements demand more severe operating conditions and higher hydrogen consumption, which can impact the selectivity toward desired alkane products. Meeting these specifications while maximizing alkane yield requires sophisticated catalyst selection and precise process control strategies that accommodate both environmental and production targets.
Waste stream management regulations affect hydrocracking operations through restrictions on wastewater discharge, spent catalyst disposal, and hazardous waste handling. These requirements influence catalyst selection, regeneration frequency, and overall process economics. The need to minimize waste generation may drive operators toward catalysts and operating conditions that extend cycle lengths, potentially affecting alkane yield optimization strategies.
Carbon emission regulations and greenhouse gas reduction targets are increasingly shaping hydrocracking operations. Pressure to reduce carbon intensity encourages energy efficiency improvements and hydrogen sourcing strategies that can influence process economics and operational parameters. These considerations may affect decisions regarding operating severity, hydrogen partial pressure, and temperature profiles that directly impact alkane selectivity and yield.
Regulatory compliance monitoring and reporting requirements impose additional operational burdens that influence process optimization approaches. The need for continuous emissions monitoring, detailed record-keeping, and periodic reporting affects operational flexibility and may constrain rapid process adjustments aimed at yield optimization. Integration of environmental compliance systems with process control infrastructure has become essential for maintaining both regulatory adherence and production efficiency.
Emission control regulations significantly impact hydrocracking unit design and operation. Stricter limits on sulfur dioxide, nitrogen oxides, and particulate matter emissions necessitate additional treatment systems that can affect overall process efficiency. These requirements often mandate operating conditions that may not align with optimal alkane yield parameters, forcing operators to find compromise solutions. The installation of scrubbers, selective catalytic reduction systems, and other emission control technologies adds complexity and operational costs while potentially constraining process flexibility.
Product quality specifications driven by environmental mandates directly influence hydrocracking objectives. Ultra-low sulfur diesel and gasoline requirements demand more severe operating conditions and higher hydrogen consumption, which can impact the selectivity toward desired alkane products. Meeting these specifications while maximizing alkane yield requires sophisticated catalyst selection and precise process control strategies that accommodate both environmental and production targets.
Waste stream management regulations affect hydrocracking operations through restrictions on wastewater discharge, spent catalyst disposal, and hazardous waste handling. These requirements influence catalyst selection, regeneration frequency, and overall process economics. The need to minimize waste generation may drive operators toward catalysts and operating conditions that extend cycle lengths, potentially affecting alkane yield optimization strategies.
Carbon emission regulations and greenhouse gas reduction targets are increasingly shaping hydrocracking operations. Pressure to reduce carbon intensity encourages energy efficiency improvements and hydrogen sourcing strategies that can influence process economics and operational parameters. These considerations may affect decisions regarding operating severity, hydrogen partial pressure, and temperature profiles that directly impact alkane selectivity and yield.
Regulatory compliance monitoring and reporting requirements impose additional operational burdens that influence process optimization approaches. The need for continuous emissions monitoring, detailed record-keeping, and periodic reporting affects operational flexibility and may constrain rapid process adjustments aimed at yield optimization. Integration of environmental compliance systems with process control infrastructure has become essential for maintaining both regulatory adherence and production efficiency.
Process Integration and Energy Efficiency Strategies
Process integration and energy efficiency strategies represent critical enablers for maximizing alkane yield in hydrocracking operations while maintaining economic viability. The inherently endothermic nature of hydrocracking reactions demands substantial energy input, making thermal management and heat recovery essential considerations. Advanced process integration approaches focus on optimizing heat exchanger networks to capture and redistribute thermal energy across reaction zones, preheating systems, and product fractionation units. By implementing pinch analysis methodologies, refineries can identify minimum energy requirements and design heat integration schemes that reduce external heating demands by 15-25%, thereby allowing more precise temperature control in reactor beds where selective cracking to alkanes occurs.
Energy efficiency improvements extend beyond heat integration to encompass hydrogen management strategies. Since hydrogen partial pressure directly influences hydrogenation rates and alkane formation, optimizing hydrogen circulation systems through advanced compression technologies and membrane separation units enables maintenance of optimal hydrogen-to-hydrocarbon ratios with reduced energy consumption. Variable speed drive systems on recycle gas compressors allow dynamic adjustment to process conditions, ensuring sufficient hydrogen availability for saturation reactions while minimizing compression energy penalties that can account for 20-30% of total hydrocracking unit energy consumption.
Catalyst bed temperature profiling through strategic heat injection or removal between reactor stages offers another dimension of process integration. Inter-stage heating systems utilizing recovered process heat maintain optimal cracking temperatures in subsequent beds, promoting continued alkane production without excessive thermal cracking. This staged approach prevents temperature runaway in initial beds while sustaining activity in downstream zones where heavier intermediates undergo final conversion to desired alkane products.
Advanced process control systems integrating real-time optimization algorithms enable dynamic adjustment of operating parameters based on feed characteristics and product specifications. Model predictive control platforms can simultaneously optimize reactor temperatures, hydrogen flow rates, and separation unit operations to maximize alkane selectivity while minimizing energy consumption per unit of product. These integrated control strategies typically achieve 3-8% improvements in overall energy efficiency compared to conventional regulatory control approaches, directly supporting enhanced alkane yields through more stable and optimized operating conditions across the entire hydrocracking complex.
Energy efficiency improvements extend beyond heat integration to encompass hydrogen management strategies. Since hydrogen partial pressure directly influences hydrogenation rates and alkane formation, optimizing hydrogen circulation systems through advanced compression technologies and membrane separation units enables maintenance of optimal hydrogen-to-hydrocarbon ratios with reduced energy consumption. Variable speed drive systems on recycle gas compressors allow dynamic adjustment to process conditions, ensuring sufficient hydrogen availability for saturation reactions while minimizing compression energy penalties that can account for 20-30% of total hydrocracking unit energy consumption.
Catalyst bed temperature profiling through strategic heat injection or removal between reactor stages offers another dimension of process integration. Inter-stage heating systems utilizing recovered process heat maintain optimal cracking temperatures in subsequent beds, promoting continued alkane production without excessive thermal cracking. This staged approach prevents temperature runaway in initial beds while sustaining activity in downstream zones where heavier intermediates undergo final conversion to desired alkane products.
Advanced process control systems integrating real-time optimization algorithms enable dynamic adjustment of operating parameters based on feed characteristics and product specifications. Model predictive control platforms can simultaneously optimize reactor temperatures, hydrogen flow rates, and separation unit operations to maximize alkane selectivity while minimizing energy consumption per unit of product. These integrated control strategies typically achieve 3-8% improvements in overall energy efficiency compared to conventional regulatory control approaches, directly supporting enhanced alkane yields through more stable and optimized operating conditions across the entire hydrocracking complex.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!