Matrix Effects For Volatiles: HS/SPME Partitioning, Salt Out And Equilibration
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Matrix Effects Background and Objectives
Matrix effects represent a critical consideration in analytical chemistry, particularly in the analysis of volatile compounds. These effects occur when components within a sample matrix influence the partitioning, extraction, and detection of target analytes, potentially leading to significant variations in analytical results. The study of matrix effects for volatiles has evolved considerably over the past three decades, driven by the increasing demand for accurate quantification in complex samples across industries including food science, environmental monitoring, and pharmaceutical development.
Historically, the analysis of volatile compounds faced substantial challenges due to matrix interference, with early techniques providing inconsistent results when applied across different sample types. The development of headspace (HS) and solid-phase microextraction (SPME) techniques in the 1990s represented a significant advancement, offering improved sensitivity and reproducibility. However, these methods remained susceptible to matrix-induced variations in partitioning coefficients and equilibration dynamics.
The phenomenon of matrix effects manifests through several mechanisms, including competitive adsorption, physical entrapment, chemical binding, and modification of phase equilibria. These interactions can significantly alter the apparent concentration of volatile compounds detected during analysis, leading to either enhancement or suppression effects that compromise analytical accuracy and precision.
Salt-out effects, a deliberate manipulation of matrix conditions through the addition of salts like sodium chloride or ammonium sulfate, have emerged as a strategic approach to modify analyte partitioning behavior. This technique exploits the principle of decreasing solubility of non-polar compounds in aqueous solutions with increasing ionic strength, effectively driving volatiles into the headspace or onto SPME fibers.
The primary objective of current research in this field is to develop comprehensive models that accurately predict and account for matrix effects across diverse sample types and analytical conditions. This includes establishing standardized protocols for equilibration time determination, optimizing salt-out procedures for different volatile classes, and creating mathematical corrections for matrix-induced variations.
Additional goals include enhancing the sensitivity and selectivity of volatile analysis through improved understanding of partitioning behavior, reducing analysis time while maintaining accuracy, and developing robust calibration strategies that compensate for matrix effects. The ultimate aim is to establish analytical methodologies that deliver consistent, reliable quantification of volatile compounds regardless of matrix complexity or composition.
Recent technological advances in instrumentation, coupled with sophisticated chemometric approaches, have opened new avenues for addressing these challenges, making this an opportune moment to reassess the state of the art and chart future directions in this critical area of analytical chemistry.
Historically, the analysis of volatile compounds faced substantial challenges due to matrix interference, with early techniques providing inconsistent results when applied across different sample types. The development of headspace (HS) and solid-phase microextraction (SPME) techniques in the 1990s represented a significant advancement, offering improved sensitivity and reproducibility. However, these methods remained susceptible to matrix-induced variations in partitioning coefficients and equilibration dynamics.
The phenomenon of matrix effects manifests through several mechanisms, including competitive adsorption, physical entrapment, chemical binding, and modification of phase equilibria. These interactions can significantly alter the apparent concentration of volatile compounds detected during analysis, leading to either enhancement or suppression effects that compromise analytical accuracy and precision.
Salt-out effects, a deliberate manipulation of matrix conditions through the addition of salts like sodium chloride or ammonium sulfate, have emerged as a strategic approach to modify analyte partitioning behavior. This technique exploits the principle of decreasing solubility of non-polar compounds in aqueous solutions with increasing ionic strength, effectively driving volatiles into the headspace or onto SPME fibers.
The primary objective of current research in this field is to develop comprehensive models that accurately predict and account for matrix effects across diverse sample types and analytical conditions. This includes establishing standardized protocols for equilibration time determination, optimizing salt-out procedures for different volatile classes, and creating mathematical corrections for matrix-induced variations.
Additional goals include enhancing the sensitivity and selectivity of volatile analysis through improved understanding of partitioning behavior, reducing analysis time while maintaining accuracy, and developing robust calibration strategies that compensate for matrix effects. The ultimate aim is to establish analytical methodologies that deliver consistent, reliable quantification of volatile compounds regardless of matrix complexity or composition.
Recent technological advances in instrumentation, coupled with sophisticated chemometric approaches, have opened new avenues for addressing these challenges, making this an opportune moment to reassess the state of the art and chart future directions in this critical area of analytical chemistry.
Market Applications for Volatile Analysis
Volatile analysis technologies have established critical applications across multiple high-value market sectors. The food and beverage industry represents one of the largest application areas, where volatile organic compounds (VOCs) analysis enables quality control, authentication of premium products, detection of adulteration, and flavor profiling. Companies like Nestlé, Unilever, and Coca-Cola have integrated advanced headspace and SPME techniques into their quality assurance protocols, creating a market segment valued at approximately $1.2 billion globally.
Environmental monitoring constitutes another significant application domain, with regulatory agencies and industrial facilities utilizing volatile analysis to detect air pollutants, monitor industrial emissions, and assess indoor air quality. The EPA and similar organizations worldwide have established standardized protocols for volatile analysis, driving demand for increasingly sensitive and selective analytical methods that can overcome matrix effects.
The pharmaceutical sector leverages volatile analysis for residual solvent testing in drug products, raw material verification, and stability studies. With regulatory requirements becoming more stringent, pharmaceutical manufacturers are investing in advanced analytical capabilities that can address complex matrix challenges through optimized salt-out procedures and equilibration protocols.
Forensic science applications represent a specialized but growing market segment, where volatile analysis aids in arson investigation, explosive detection, and biological sample analysis. Law enforcement agencies increasingly rely on portable systems capable of field analysis despite challenging sample matrices.
The cosmetics and personal care industry utilizes volatile analysis for fragrance development, quality control, and regulatory compliance regarding restricted substances. Major players like L'Oréal and Estée Lauder maintain sophisticated analytical laboratories equipped with headspace and SPME capabilities.
Emerging applications in medical diagnostics show particular promise, with breath analysis and disease biomarker detection representing frontier applications. Research indicates certain volatile compounds in breath can serve as indicators for conditions including diabetes, lung cancer, and gastrointestinal disorders. This application area faces significant matrix effect challenges due to the complexity of breath samples and the ultra-trace concentrations of target analytes.
The agricultural sector employs volatile analysis for crop disease detection, ripeness assessment, and soil health monitoring, with precision agriculture technologies incorporating volatile sensing systems to optimize farming practices and increase yields while reducing chemical inputs.
Environmental monitoring constitutes another significant application domain, with regulatory agencies and industrial facilities utilizing volatile analysis to detect air pollutants, monitor industrial emissions, and assess indoor air quality. The EPA and similar organizations worldwide have established standardized protocols for volatile analysis, driving demand for increasingly sensitive and selective analytical methods that can overcome matrix effects.
The pharmaceutical sector leverages volatile analysis for residual solvent testing in drug products, raw material verification, and stability studies. With regulatory requirements becoming more stringent, pharmaceutical manufacturers are investing in advanced analytical capabilities that can address complex matrix challenges through optimized salt-out procedures and equilibration protocols.
Forensic science applications represent a specialized but growing market segment, where volatile analysis aids in arson investigation, explosive detection, and biological sample analysis. Law enforcement agencies increasingly rely on portable systems capable of field analysis despite challenging sample matrices.
The cosmetics and personal care industry utilizes volatile analysis for fragrance development, quality control, and regulatory compliance regarding restricted substances. Major players like L'Oréal and Estée Lauder maintain sophisticated analytical laboratories equipped with headspace and SPME capabilities.
Emerging applications in medical diagnostics show particular promise, with breath analysis and disease biomarker detection representing frontier applications. Research indicates certain volatile compounds in breath can serve as indicators for conditions including diabetes, lung cancer, and gastrointestinal disorders. This application area faces significant matrix effect challenges due to the complexity of breath samples and the ultra-trace concentrations of target analytes.
The agricultural sector employs volatile analysis for crop disease detection, ripeness assessment, and soil health monitoring, with precision agriculture technologies incorporating volatile sensing systems to optimize farming practices and increase yields while reducing chemical inputs.
Current Challenges in HS/SPME Technology
Despite significant advancements in headspace solid-phase microextraction (HS/SPME) technology, several critical challenges persist that limit its analytical performance and reliability. Matrix effects represent one of the most significant obstacles in volatile compound analysis, as sample composition dramatically influences partitioning behavior between phases. The complex interactions between analytes and matrix components create unpredictable extraction efficiencies, particularly in heterogeneous samples like foods, biological fluids, and environmental specimens.
Partitioning equilibrium issues present substantial technical hurdles, as the distribution of volatile compounds between the sample matrix, headspace, and SPME fiber coating is highly dependent on temperature, extraction time, and matrix composition. Achieving true equilibrium conditions often requires extended extraction periods, which conflicts with the need for high-throughput analysis in industrial applications. Furthermore, the kinetics of this partitioning can vary significantly between different analytes in the same sample, complicating multi-analyte quantification.
Salt-out effects, while beneficial for enhancing extraction efficiency of certain compounds, introduce additional complexity and potential variability. The type and concentration of salting-out agents (commonly NaCl, Na₂SO₄, or (NH₄)₂SO₄) significantly impact extraction performance, yet standardized protocols for different matrix types remain underdeveloped. Moreover, high salt concentrations can damage fiber coatings over time, reducing method robustness and fiber longevity.
Equilibration challenges are particularly problematic in routine analysis. Temperature gradients within samples can create micro-environments with different partitioning behaviors, while stirring methods intended to accelerate equilibration may introduce additional variability. The industry lacks consensus on optimal equilibration parameters across diverse sample types, leading to method inconsistencies between laboratories.
Fiber coating selectivity presents another significant limitation, as current commercial coatings exhibit preferential extraction of certain compound classes while performing poorly with others. This selectivity bias creates challenges in comprehensive volatile profiling, particularly for complex samples containing compounds with diverse physicochemical properties. Additionally, coating degradation over multiple extraction cycles alters selectivity patterns, introducing time-dependent variability in analytical results.
Automation compatibility issues persist despite advances in robotic systems. The precise control of equilibration conditions, fiber positioning, and extraction timing remains challenging in automated platforms, leading to higher relative standard deviations compared to manual operations in certain applications. This automation gap particularly affects high-throughput industrial applications where consistency is paramount.
Partitioning equilibrium issues present substantial technical hurdles, as the distribution of volatile compounds between the sample matrix, headspace, and SPME fiber coating is highly dependent on temperature, extraction time, and matrix composition. Achieving true equilibrium conditions often requires extended extraction periods, which conflicts with the need for high-throughput analysis in industrial applications. Furthermore, the kinetics of this partitioning can vary significantly between different analytes in the same sample, complicating multi-analyte quantification.
Salt-out effects, while beneficial for enhancing extraction efficiency of certain compounds, introduce additional complexity and potential variability. The type and concentration of salting-out agents (commonly NaCl, Na₂SO₄, or (NH₄)₂SO₄) significantly impact extraction performance, yet standardized protocols for different matrix types remain underdeveloped. Moreover, high salt concentrations can damage fiber coatings over time, reducing method robustness and fiber longevity.
Equilibration challenges are particularly problematic in routine analysis. Temperature gradients within samples can create micro-environments with different partitioning behaviors, while stirring methods intended to accelerate equilibration may introduce additional variability. The industry lacks consensus on optimal equilibration parameters across diverse sample types, leading to method inconsistencies between laboratories.
Fiber coating selectivity presents another significant limitation, as current commercial coatings exhibit preferential extraction of certain compound classes while performing poorly with others. This selectivity bias creates challenges in comprehensive volatile profiling, particularly for complex samples containing compounds with diverse physicochemical properties. Additionally, coating degradation over multiple extraction cycles alters selectivity patterns, introducing time-dependent variability in analytical results.
Automation compatibility issues persist despite advances in robotic systems. The precise control of equilibration conditions, fiber positioning, and extraction timing remains challenging in automated platforms, leading to higher relative standard deviations compared to manual operations in certain applications. This automation gap particularly affects high-throughput industrial applications where consistency is paramount.
Current Matrix Effect Mitigation Strategies
01 Salt-out effect for volatile compound extraction
The salt-out effect is a technique used to enhance the extraction of volatile compounds from a matrix by adding salts to the solution. This increases the ionic strength of the aqueous phase, reducing the solubility of volatile organic compounds and forcing them into the headspace or organic phase. This technique improves the partitioning coefficient and extraction efficiency of volatile compounds, making it valuable for analytical chemistry applications and sample preparation methods.- Salt-out techniques for volatile compound extraction: Salt-out techniques are used to enhance the partitioning of volatile compounds from aqueous solutions into extraction solvents. The addition of salts like sodium chloride decreases the solubility of volatile organic compounds in water, forcing them into the organic phase. This technique improves extraction efficiency and sensitivity in analytical methods for volatile compounds by reducing their water solubility and increasing their partition coefficients.
- Equilibration methods for volatile analysis: Equilibration methods are critical for accurate analysis of volatile compounds in complex matrices. These methods involve allowing sufficient time for volatile compounds to reach equilibrium between phases before sampling. Proper equilibration ensures representative distribution of volatiles between headspace and liquid phases, leading to more reliable quantitative results. Temperature control during equilibration is essential as it affects partition coefficients and vapor pressures of volatile compounds.
- Matrix modification techniques for volatile partitioning: Matrix modification techniques involve altering the sample matrix to enhance the partitioning of volatile compounds. These modifications include pH adjustment, temperature control, and addition of organic modifiers. By manipulating these parameters, the ionization state and solubility of volatile compounds can be controlled, affecting their partitioning behavior between phases. These techniques are particularly useful in complex biological or environmental samples where matrix effects can significantly impact analytical results.
- Headspace sampling optimization for volatile compounds: Headspace sampling optimization involves adjusting parameters to improve the extraction of volatile compounds from the sample matrix into the gas phase. Key factors include sample volume, headspace volume ratio, agitation methods, and incubation conditions. Proper optimization of these parameters enhances the sensitivity and reproducibility of volatile compound analysis by maximizing the concentration of analytes in the headspace while minimizing interference from the matrix.
- Analytical instrumentation for matrix effect compensation: Advanced analytical instrumentation and methods have been developed to compensate for matrix effects in volatile compound analysis. These include specialized detectors, chromatographic techniques, and data processing algorithms that can identify and correct for matrix-induced variations. Mass spectrometry with internal standardization, multiple reaction monitoring, and matrix-matched calibration are commonly employed approaches to overcome matrix effects and ensure accurate quantification of volatile compounds across different sample types.
02 Equilibration techniques for volatile analysis
Equilibration is a critical step in volatile compound analysis where the system is allowed to reach a steady state between phases before sampling. Proper equilibration time and conditions ensure that volatile compounds distribute between phases according to their partition coefficients, leading to reproducible and accurate analytical results. Techniques include temperature-controlled equilibration, agitation methods, and time-optimized protocols that balance complete equilibration with preventing analyte degradation.Expand Specific Solutions03 Matrix modification for improved volatile partitioning
Various matrix modifications can be employed to enhance the partitioning of volatile compounds during analysis. These modifications include pH adjustment, temperature control, addition of organic modifiers, and use of surfactants. By altering the chemical environment of the sample matrix, the distribution of volatile compounds between phases can be manipulated to improve extraction efficiency and analytical sensitivity, particularly for compounds with challenging physicochemical properties.Expand Specific Solutions04 Headspace sampling optimization for volatile compounds
Headspace sampling is a technique used to analyze volatile compounds by sampling the gas phase above a liquid or solid sample. Optimization strategies include adjusting headspace volume, sample volume ratio, incubation temperature and time, and pressure conditions. These parameters significantly affect the partitioning of volatiles between the sample matrix and headspace, ultimately determining the sensitivity and reproducibility of the analytical method.Expand Specific Solutions05 Computational modeling of volatile partitioning
Computational approaches are increasingly used to model and predict the partitioning behavior of volatile compounds in complex matrices. These models incorporate physicochemical properties, thermodynamic principles, and matrix interactions to simulate how volatile compounds distribute between phases. Advanced algorithms and machine learning techniques help optimize extraction conditions, predict matrix effects, and improve analytical method development for volatile compound analysis.Expand Specific Solutions
Leading Companies in Analytical Chemistry
The matrix effects for volatiles in headspace/solid-phase microextraction (HS/SPME) partitioning represents a maturing field at the intersection of analytical chemistry and environmental science. Currently in a growth phase, this market is expanding as industries seek more precise volatile compound analysis. The global analytical instrumentation market, which includes this technology, is valued at approximately $5-7 billion annually with steady growth projections. Leading organizations like Waters Corporation (through Micromass UK) and Life Technologies are driving technological innovation, while academic institutions including Northwestern University, Rice University, and Fudan University contribute significant research advancements. Industrial players such as S.C. Johnson & Son and Sinopec are applying these technologies in product development and quality control, demonstrating the technique's cross-sector relevance from consumer products to petrochemicals.
S.C. Johnson & Son, Inc.
Technical Solution: S.C. Johnson has developed advanced headspace/solid-phase microextraction (HS/SPME) techniques for volatile compound analysis in consumer products. Their approach focuses on optimizing matrix effects through controlled salt-out procedures that enhance partitioning coefficients of target volatiles. The company employs a proprietary equilibration protocol that reduces matrix interference while maximizing extraction efficiency. Their technology includes specialized fiber coatings that selectively capture volatile organic compounds (VOCs) from complex household product matrices. S.C. Johnson's method incorporates temperature-controlled equilibration chambers that maintain precise conditions during the extraction process, ensuring reproducible results across different product formulations. The company has also developed mathematical models to predict partitioning behavior of volatiles in various product matrices, allowing for rapid method development and optimization.
Strengths: Extensive experience with consumer product matrices provides practical application knowledge; proprietary fiber coatings offer selective extraction capabilities. Weaknesses: Methods may be optimized specifically for household products rather than broader applications; proprietary nature limits scientific sharing and advancement in the field.
Micromass UK Ltd.
Technical Solution: Micromass UK has pioneered integration of HS/SPME techniques with high-resolution mass spectrometry for volatile compound analysis. Their technology platform addresses matrix effects through a multi-phase approach that combines optimized salt-out procedures with advanced equilibration algorithms. The company's system employs automated temperature and pressure controls to maximize partitioning efficiency while maintaining sample integrity. Their proprietary software incorporates matrix-specific calibration models that compensate for varying salt concentrations and matrix compositions. Micromass has developed specialized SPME fiber assemblies with enhanced surface area and selective coatings that improve extraction efficiency for challenging volatile compounds. Their technology includes real-time monitoring of headspace equilibration, allowing for dynamic adjustment of extraction parameters based on matrix behavior. This approach has demonstrated particular success in complex biological and environmental samples where traditional methods struggle with matrix interference.
Strengths: Integration with high-resolution mass spectrometry provides exceptional sensitivity and specificity; automated systems ensure reproducibility across different matrices. Weaknesses: Higher cost compared to conventional methods; requires specialized training and equipment maintenance.
Key Innovations in Partitioning Technology
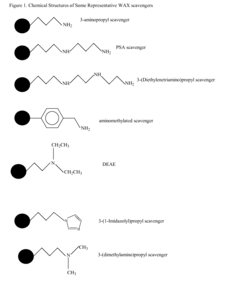
Methods for matrix cleanup and analysis of drugs and metabolites in biological matrices
PatentInactiveUS20080050838A1
Innovation
- The use of weak anion-exchange (WAX) sorbents for adsorption and removal of negative and zwitterionic components from biological matrices, such as phospholipids and carbohydrates, to enhance the extraction of basic and neutral compounds, either as a standalone step or integrated into existing extraction methodologies.
Method Validation Protocols
Method validation protocols for volatile compound analysis in complex matrices require rigorous standardization to ensure reliable and reproducible results. The validation process must specifically address the unique challenges posed by matrix effects on headspace (HS) and solid-phase microextraction (SPME) techniques. A comprehensive validation protocol should begin with linearity assessment across multiple concentration ranges relevant to the target analytes, establishing calibration curves with correlation coefficients exceeding 0.995 for each volatile compound.
Precision validation must include both intra-day and inter-day repeatability studies, with recommended acceptance criteria of relative standard deviation (RSD) values below 15% for high concentration samples and below 20% for samples near the limit of quantification. Matrix-matched calibration standards are essential for accurate quantification, as they account for the differential partitioning behavior of volatiles in various sample matrices.
Recovery studies represent a critical component of method validation, particularly when evaluating salt-out effects and equilibration parameters. These studies should be conducted at multiple concentration levels (low, medium, high) using standard addition techniques, with acceptable recovery rates typically ranging from 70-120% depending on the complexity of the matrix. The validation protocol must also include stability assessments of volatile compounds under various storage conditions to determine optimal sample handling procedures.
Limit of detection (LOD) and limit of quantification (LOQ) determinations require special consideration for volatile compounds due to their susceptibility to matrix effects. Signal-to-noise ratio approaches (S/N of 3:1 for LOD and 10:1 for LOQ) should be supplemented with matrix-specific validation to account for potential suppression or enhancement effects in real samples.
Robustness testing must evaluate the influence of critical method parameters on analytical performance, including equilibration time, extraction temperature, salt concentration, sample volume, and headspace volume. A factorial design approach is recommended to efficiently assess these parameters and their interactions, establishing acceptable ranges for each variable that maintain method performance within validation criteria.
Cross-validation with alternative analytical techniques (such as direct injection GC-MS or LC-MS/MS where applicable) provides additional confidence in method reliability. Finally, the validation protocol should include procedures for ongoing quality control, including the use of internal standards, control charts, and periodic proficiency testing to ensure long-term method stability and comparability of results across different laboratories and analytical platforms.
Precision validation must include both intra-day and inter-day repeatability studies, with recommended acceptance criteria of relative standard deviation (RSD) values below 15% for high concentration samples and below 20% for samples near the limit of quantification. Matrix-matched calibration standards are essential for accurate quantification, as they account for the differential partitioning behavior of volatiles in various sample matrices.
Recovery studies represent a critical component of method validation, particularly when evaluating salt-out effects and equilibration parameters. These studies should be conducted at multiple concentration levels (low, medium, high) using standard addition techniques, with acceptable recovery rates typically ranging from 70-120% depending on the complexity of the matrix. The validation protocol must also include stability assessments of volatile compounds under various storage conditions to determine optimal sample handling procedures.
Limit of detection (LOD) and limit of quantification (LOQ) determinations require special consideration for volatile compounds due to their susceptibility to matrix effects. Signal-to-noise ratio approaches (S/N of 3:1 for LOD and 10:1 for LOQ) should be supplemented with matrix-specific validation to account for potential suppression or enhancement effects in real samples.
Robustness testing must evaluate the influence of critical method parameters on analytical performance, including equilibration time, extraction temperature, salt concentration, sample volume, and headspace volume. A factorial design approach is recommended to efficiently assess these parameters and their interactions, establishing acceptable ranges for each variable that maintain method performance within validation criteria.
Cross-validation with alternative analytical techniques (such as direct injection GC-MS or LC-MS/MS where applicable) provides additional confidence in method reliability. Finally, the validation protocol should include procedures for ongoing quality control, including the use of internal standards, control charts, and periodic proficiency testing to ensure long-term method stability and comparability of results across different laboratories and analytical platforms.
Environmental Impact Assessment
The environmental implications of matrix effects in volatile compound analysis extend far beyond laboratory settings. When considering headspace (HS) and solid-phase microextraction (SPME) techniques, the environmental footprint becomes particularly relevant as these methods are widely used in environmental monitoring and assessment.
The salt-out effect, commonly employed to enhance volatile compound extraction, introduces significant environmental considerations. Traditional salting agents such as sodium chloride, sodium sulfate, and ammonium sulfate contribute to laboratory waste streams that require proper disposal protocols. The discharge of these high-salinity wastes into water systems can adversely affect aquatic ecosystems by altering osmotic conditions for organisms and potentially disrupting natural ionic balances.
Equilibration processes in HS/SPME methodologies typically require temperature control systems that consume substantial energy. The carbon footprint associated with maintaining precise temperature conditions for extended periods represents a notable environmental cost. Modern laboratories are increasingly implementing energy-efficient heating blocks and temperature controllers to mitigate these impacts while maintaining analytical precision.
Sample preparation techniques involving matrix modification also generate organic solvent waste. Though HS/SPME methods generally use fewer solvents than traditional extraction approaches, they still contribute to laboratory chemical waste. The volatile organic compounds (VOCs) potentially released during these processes may contribute to indoor air quality concerns and broader atmospheric pollution if not properly contained and treated.
From a sustainability perspective, the development of green analytical chemistry approaches to matrix effect management shows promising environmental benefits. Innovations include the use of biodegradable salting-out agents, reusable SPME fibers, and automated systems that optimize reagent usage and minimize waste generation. These advancements align with circular economy principles increasingly adopted in analytical laboratories.
The life cycle assessment of matrix effect management techniques reveals opportunities for environmental optimization. By considering the entire analytical process—from sample collection through analysis to waste disposal—laboratories can identify key intervention points to reduce environmental impact while maintaining analytical quality. This holistic approach has led to the development of integrated systems that combine efficient extraction with reduced resource consumption.
Water usage in matrix effect management represents another environmental consideration, particularly in water-scarce regions. Techniques that minimize water consumption during sample preparation and equipment cleaning contribute significantly to sustainable laboratory practices while addressing global water conservation challenges.
The salt-out effect, commonly employed to enhance volatile compound extraction, introduces significant environmental considerations. Traditional salting agents such as sodium chloride, sodium sulfate, and ammonium sulfate contribute to laboratory waste streams that require proper disposal protocols. The discharge of these high-salinity wastes into water systems can adversely affect aquatic ecosystems by altering osmotic conditions for organisms and potentially disrupting natural ionic balances.
Equilibration processes in HS/SPME methodologies typically require temperature control systems that consume substantial energy. The carbon footprint associated with maintaining precise temperature conditions for extended periods represents a notable environmental cost. Modern laboratories are increasingly implementing energy-efficient heating blocks and temperature controllers to mitigate these impacts while maintaining analytical precision.
Sample preparation techniques involving matrix modification also generate organic solvent waste. Though HS/SPME methods generally use fewer solvents than traditional extraction approaches, they still contribute to laboratory chemical waste. The volatile organic compounds (VOCs) potentially released during these processes may contribute to indoor air quality concerns and broader atmospheric pollution if not properly contained and treated.
From a sustainability perspective, the development of green analytical chemistry approaches to matrix effect management shows promising environmental benefits. Innovations include the use of biodegradable salting-out agents, reusable SPME fibers, and automated systems that optimize reagent usage and minimize waste generation. These advancements align with circular economy principles increasingly adopted in analytical laboratories.
The life cycle assessment of matrix effect management techniques reveals opportunities for environmental optimization. By considering the entire analytical process—from sample collection through analysis to waste disposal—laboratories can identify key intervention points to reduce environmental impact while maintaining analytical quality. This holistic approach has led to the development of integrated systems that combine efficient extraction with reduced resource consumption.
Water usage in matrix effect management represents another environmental consideration, particularly in water-scarce regions. Techniques that minimize water consumption during sample preparation and equipment cleaning contribute significantly to sustainable laboratory practices while addressing global water conservation challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!