Matrix Effects Quantitation: Calibration Strategy, Matrix-Matched And Standard Addition
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Matrix Effects Analysis Background and Objectives
Matrix effects represent one of the most significant challenges in analytical chemistry, particularly in quantitative analysis using chromatographic techniques coupled with mass spectrometry. These effects occur when components within a sample matrix interfere with the ionization process, leading to either enhancement or suppression of analyte signals. The phenomenon was first documented in the early 1990s but has gained increasing attention as analytical methods have become more sensitive and applied to increasingly complex matrices.
The evolution of matrix effect understanding has progressed from initial observations of unexplained variability in results to sophisticated strategies for their characterization and mitigation. Early approaches focused primarily on sample cleanup techniques, while modern strategies incorporate advanced calibration methodologies that mathematically account for these interferences.
The primary objective of matrix effects analysis is to ensure accurate quantitation across diverse sample types by developing robust analytical methods that either eliminate matrix effects or compensate for them through appropriate calibration strategies. This is particularly critical in regulated environments such as pharmaceutical analysis, clinical diagnostics, food safety testing, and environmental monitoring, where analytical accuracy directly impacts decision-making processes.
Matrix-matched calibration and standard addition represent two fundamental approaches to addressing matrix effects. Matrix-matched calibration involves preparing calibration standards in a blank matrix similar to the samples being analyzed, thereby subjecting both standards and samples to similar matrix effects. Standard addition, conversely, involves adding known amounts of analyte to the sample itself, creating an internal calibration curve specific to each sample's unique matrix.
The technological landscape for addressing matrix effects has evolved significantly, with innovations in sample preparation techniques, chromatographic separations, and mass spectrometric detection all contributing to improved analytical performance. Stable isotope-labeled internal standards have emerged as powerful tools for normalizing matrix effects, though their availability and cost can be limiting factors.
Current research focuses on developing predictive models for matrix effects, automated calibration selection algorithms, and novel ionization techniques less susceptible to matrix interferences. The ultimate goal remains consistent: to develop analytical methods that deliver accurate, precise, and reproducible quantitative results regardless of sample complexity or matrix variability.
This technical investigation aims to comprehensively evaluate calibration strategies for matrix effects quantitation, with particular emphasis on comparing matrix-matched calibration and standard addition approaches across different analytical scenarios and application domains.
The evolution of matrix effect understanding has progressed from initial observations of unexplained variability in results to sophisticated strategies for their characterization and mitigation. Early approaches focused primarily on sample cleanup techniques, while modern strategies incorporate advanced calibration methodologies that mathematically account for these interferences.
The primary objective of matrix effects analysis is to ensure accurate quantitation across diverse sample types by developing robust analytical methods that either eliminate matrix effects or compensate for them through appropriate calibration strategies. This is particularly critical in regulated environments such as pharmaceutical analysis, clinical diagnostics, food safety testing, and environmental monitoring, where analytical accuracy directly impacts decision-making processes.
Matrix-matched calibration and standard addition represent two fundamental approaches to addressing matrix effects. Matrix-matched calibration involves preparing calibration standards in a blank matrix similar to the samples being analyzed, thereby subjecting both standards and samples to similar matrix effects. Standard addition, conversely, involves adding known amounts of analyte to the sample itself, creating an internal calibration curve specific to each sample's unique matrix.
The technological landscape for addressing matrix effects has evolved significantly, with innovations in sample preparation techniques, chromatographic separations, and mass spectrometric detection all contributing to improved analytical performance. Stable isotope-labeled internal standards have emerged as powerful tools for normalizing matrix effects, though their availability and cost can be limiting factors.
Current research focuses on developing predictive models for matrix effects, automated calibration selection algorithms, and novel ionization techniques less susceptible to matrix interferences. The ultimate goal remains consistent: to develop analytical methods that deliver accurate, precise, and reproducible quantitative results regardless of sample complexity or matrix variability.
This technical investigation aims to comprehensively evaluate calibration strategies for matrix effects quantitation, with particular emphasis on comparing matrix-matched calibration and standard addition approaches across different analytical scenarios and application domains.
Market Demand for Accurate Analytical Quantitation Methods
The analytical chemistry market has witnessed substantial growth in recent years, driven by increasing demands for accurate quantitation methods across various industries. The global analytical instrumentation market is projected to reach $81.2 billion by 2025, with a significant portion dedicated to technologies addressing matrix effects in quantitative analysis. This growth reflects the critical importance of precise analytical measurements in today's regulatory and quality-focused environment.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment demanding advanced matrix effect quantitation solutions. With stringent regulatory requirements from agencies like FDA and EMA, these industries require highly accurate analytical methods to ensure drug safety and efficacy. The bioanalytical testing market alone is growing at 12.8% annually, highlighting the increasing need for reliable quantitation methods that can overcome matrix interference challenges.
Environmental monitoring constitutes another significant market driver, as regulatory bodies worldwide implement stricter limits for contaminants in soil, water, and air. The environmental testing market is expanding at 7.1% annually, with particular emphasis on techniques that can accurately quantify trace contaminants in complex environmental matrices. Government agencies and environmental consultancies are increasingly investing in advanced analytical technologies that can provide defensible data despite matrix challenges.
Food safety testing represents a rapidly growing segment with particular matrix effect challenges due to the complexity of food samples. The global food testing market is expected to reach $29.2 billion by 2026, with contaminant testing forming a substantial portion. Food manufacturers and regulatory bodies are seeking improved calibration strategies to ensure accurate quantitation of pesticides, mycotoxins, and other contaminants in diverse food matrices.
Clinical diagnostics presents unique matrix challenges due to the complexity of biological samples. The clinical laboratory market continues to expand as personalized medicine drives demand for more sensitive and accurate biomarker quantitation. Laboratories are increasingly adopting matrix-matched calibration and standard addition techniques to improve diagnostic accuracy and reliability.
Emerging economies are showing the fastest growth in demand for advanced analytical quantitation methods, particularly in Asia-Pacific regions where expanding regulatory frameworks and growing manufacturing sectors necessitate improved analytical capabilities. This geographical shift is creating new market opportunities for analytical instrument manufacturers and method development specialists focusing on matrix effect solutions.
The market is also seeing increased demand for automated solutions that can implement complex calibration strategies with minimal user intervention, reflecting the industry-wide push toward greater efficiency and reproducibility in analytical testing.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment demanding advanced matrix effect quantitation solutions. With stringent regulatory requirements from agencies like FDA and EMA, these industries require highly accurate analytical methods to ensure drug safety and efficacy. The bioanalytical testing market alone is growing at 12.8% annually, highlighting the increasing need for reliable quantitation methods that can overcome matrix interference challenges.
Environmental monitoring constitutes another significant market driver, as regulatory bodies worldwide implement stricter limits for contaminants in soil, water, and air. The environmental testing market is expanding at 7.1% annually, with particular emphasis on techniques that can accurately quantify trace contaminants in complex environmental matrices. Government agencies and environmental consultancies are increasingly investing in advanced analytical technologies that can provide defensible data despite matrix challenges.
Food safety testing represents a rapidly growing segment with particular matrix effect challenges due to the complexity of food samples. The global food testing market is expected to reach $29.2 billion by 2026, with contaminant testing forming a substantial portion. Food manufacturers and regulatory bodies are seeking improved calibration strategies to ensure accurate quantitation of pesticides, mycotoxins, and other contaminants in diverse food matrices.
Clinical diagnostics presents unique matrix challenges due to the complexity of biological samples. The clinical laboratory market continues to expand as personalized medicine drives demand for more sensitive and accurate biomarker quantitation. Laboratories are increasingly adopting matrix-matched calibration and standard addition techniques to improve diagnostic accuracy and reliability.
Emerging economies are showing the fastest growth in demand for advanced analytical quantitation methods, particularly in Asia-Pacific regions where expanding regulatory frameworks and growing manufacturing sectors necessitate improved analytical capabilities. This geographical shift is creating new market opportunities for analytical instrument manufacturers and method development specialists focusing on matrix effect solutions.
The market is also seeing increased demand for automated solutions that can implement complex calibration strategies with minimal user intervention, reflecting the industry-wide push toward greater efficiency and reproducibility in analytical testing.
Current Challenges in Matrix Effects Quantitation
Matrix effects remain one of the most significant challenges in analytical chemistry, particularly in complex sample analysis. These effects occur when components within the sample matrix interfere with the measurement of the target analyte, leading to signal enhancement or suppression. Despite decades of research, matrix effects continue to plague quantitative analysis across various fields including pharmaceutical analysis, environmental monitoring, food safety testing, and clinical diagnostics.
The primary challenge lies in the unpredictable nature of matrix effects. Even with advanced analytical techniques such as liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS), matrix components can significantly alter ionization efficiency, leading to erroneous quantitative results. This unpredictability makes it difficult to establish standardized approaches that work universally across different sample types.
Current calibration strategies face limitations in addressing matrix complexity. External calibration, while straightforward, often fails to account for matrix-specific interferences. Matrix-matched calibration attempts to mimic the sample environment but struggles with batch-to-batch variations in complex matrices. Standard addition methods, though theoretically robust, are time-consuming and resource-intensive, making them impractical for high-throughput analyses.
Sample preparation techniques designed to minimize matrix effects introduce their own complications. Extensive clean-up procedures may reduce matrix interferences but can also lead to analyte losses or transformations. Finding the optimal balance between effective matrix removal and analyte recovery remains challenging, particularly for multi-residue analyses where compounds exhibit diverse physicochemical properties.
Technological limitations further compound these challenges. Current instrumentation, while increasingly sensitive, still struggles with discriminating between analyte signals and matrix interferences in complex samples. This is particularly problematic in ultra-trace analysis where matrix effects can completely mask low-concentration analytes.
Regulatory requirements add another layer of complexity. Different regulatory bodies have varying guidelines for addressing matrix effects, creating confusion for laboratories operating across multiple jurisdictions. The lack of harmonized approaches complicates method validation and transfer between laboratories.
Emerging analytical challenges, such as the need to quantify an ever-expanding range of compounds at increasingly lower detection limits in more complex matrices, continue to push the boundaries of current methodologies. The growing demand for multi-residue methods capable of simultaneously analyzing hundreds of compounds further exacerbates matrix effect issues, as optimizing conditions to minimize matrix effects for all analytes simultaneously becomes virtually impossible.
The primary challenge lies in the unpredictable nature of matrix effects. Even with advanced analytical techniques such as liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS), matrix components can significantly alter ionization efficiency, leading to erroneous quantitative results. This unpredictability makes it difficult to establish standardized approaches that work universally across different sample types.
Current calibration strategies face limitations in addressing matrix complexity. External calibration, while straightforward, often fails to account for matrix-specific interferences. Matrix-matched calibration attempts to mimic the sample environment but struggles with batch-to-batch variations in complex matrices. Standard addition methods, though theoretically robust, are time-consuming and resource-intensive, making them impractical for high-throughput analyses.
Sample preparation techniques designed to minimize matrix effects introduce their own complications. Extensive clean-up procedures may reduce matrix interferences but can also lead to analyte losses or transformations. Finding the optimal balance between effective matrix removal and analyte recovery remains challenging, particularly for multi-residue analyses where compounds exhibit diverse physicochemical properties.
Technological limitations further compound these challenges. Current instrumentation, while increasingly sensitive, still struggles with discriminating between analyte signals and matrix interferences in complex samples. This is particularly problematic in ultra-trace analysis where matrix effects can completely mask low-concentration analytes.
Regulatory requirements add another layer of complexity. Different regulatory bodies have varying guidelines for addressing matrix effects, creating confusion for laboratories operating across multiple jurisdictions. The lack of harmonized approaches complicates method validation and transfer between laboratories.
Emerging analytical challenges, such as the need to quantify an ever-expanding range of compounds at increasingly lower detection limits in more complex matrices, continue to push the boundaries of current methodologies. The growing demand for multi-residue methods capable of simultaneously analyzing hundreds of compounds further exacerbates matrix effect issues, as optimizing conditions to minimize matrix effects for all analytes simultaneously becomes virtually impossible.
Matrix-Matched vs Standard Addition Calibration Approaches
01 Matrix-matched calibration strategies for analytical measurements
Matrix-matched calibration involves preparing calibration standards in a matrix similar to the sample to compensate for matrix effects that can influence analytical measurements. This approach helps to account for signal enhancement or suppression caused by sample components, improving quantitation accuracy. The strategy is particularly important in complex biological or environmental samples where matrix components can significantly affect analyte detection and quantification.- Matrix-matched calibration strategies for analytical measurements: Matrix-matched calibration involves preparing calibration standards in a matrix similar to the sample to compensate for matrix effects in analytical measurements. This approach ensures that analytes in both calibration standards and samples experience the same matrix effects, leading to more accurate quantitation. The strategy is particularly important in complex sample analysis where matrix components can enhance or suppress analyte signals, affecting measurement accuracy.
- Internal standard calibration methods for compensating matrix effects: Internal standard calibration uses compounds with similar chemical properties to the analytes but distinguishable during analysis. These standards are added to both samples and calibration standards at known concentrations to normalize matrix effects. By comparing the response ratio of analyte to internal standard, quantitation becomes more reliable even in the presence of matrix interferences. This strategy is particularly valuable in mass spectrometry and chromatographic analyses where matrix effects can significantly impact results.
- Standard addition calibration techniques for complex matrices: Standard addition involves adding known amounts of analyte to the sample matrix to create a calibration curve specific to that sample. This technique directly accounts for matrix effects by performing calibration within the actual sample matrix. Multiple aliquots of the sample are spiked with increasing concentrations of analyte, and the original analyte concentration is determined by extrapolation. This approach is particularly useful when matrix-matched standards are unavailable or when dealing with unique or highly variable sample matrices.
- Machine learning and algorithmic approaches for matrix effect correction: Advanced computational methods including machine learning algorithms can be used to model and correct for matrix effects in analytical measurements. These approaches analyze patterns in calibration data to develop predictive models that compensate for matrix interferences. By training algorithms on datasets with known matrix effects, the system can automatically adjust quantitation results for unknown samples. This strategy is increasingly important in high-throughput analyses where traditional calibration methods may be impractical due to sample complexity or variability.
- Sample preparation techniques to minimize matrix effects: Various sample preparation strategies can be employed to reduce matrix effects before calibration and quantitation. These include dilution, solid-phase extraction, liquid-liquid extraction, and protein precipitation. By removing or reducing interfering matrix components prior to analysis, the need for complex calibration strategies may be reduced. These techniques aim to create a cleaner sample that minimizes signal enhancement or suppression, leading to more reliable quantitation results even with simpler calibration approaches.
02 Internal standard calibration methods for matrix effect compensation
Internal standard calibration uses compounds with similar chemical properties to the analytes of interest but can be distinguished analytically. These standards are added to samples and calibration solutions at known concentrations to normalize instrument response and compensate for matrix effects. This approach is particularly valuable in mass spectrometry and chromatography applications where matrix components can affect ionization efficiency or detector response.Expand Specific Solutions03 Standard addition calibration techniques for complex matrices
Standard addition involves adding known amounts of analyte to the sample matrix to create a calibration curve specific to that sample. This technique directly accounts for matrix effects by performing calibration within the actual sample matrix. The method is particularly useful when matrix-matched standards are unavailable or when dealing with unique or highly variable sample matrices that cannot be easily replicated for conventional calibration.Expand Specific Solutions04 Machine learning and algorithmic approaches for matrix effect correction
Advanced computational methods including machine learning algorithms can be used to model and correct for matrix effects in analytical measurements. These approaches can identify patterns in data that relate to matrix interferences and apply mathematical corrections to improve quantitation accuracy. Neural networks, multivariate analysis, and other statistical methods can be employed to develop calibration models that account for complex matrix interactions without requiring extensive matrix-matched standards.Expand Specific Solutions05 Sample preparation techniques to minimize matrix effects
Various sample preparation strategies can be employed to reduce matrix effects before calibration and quantitation. These include selective extraction methods, solid-phase extraction, liquid-liquid extraction, and chromatographic separation techniques that remove or reduce interfering matrix components. By minimizing matrix effects through sample preparation, more straightforward calibration strategies can be employed, improving the accuracy and reliability of quantitative analysis.Expand Specific Solutions
Leading Organizations in Analytical Method Development
Matrix effects quantitation in analytical chemistry is currently in a mature development phase, with a growing market driven by increasing demands for accurate analytical measurements across pharmaceutical, environmental, and food safety sectors. The global market for analytical instrumentation addressing matrix effects is estimated to exceed $10 billion, with steady annual growth. Technologically, companies have developed sophisticated approaches to address matrix challenges. Roche Diagnostics, Waters Technology, and F. Hoffmann-La Roche lead with advanced calibration strategies, while Gen-Probe and Seegene have pioneered matrix-matched calibration techniques. DH Technologies and Micromass UK have made significant advancements in standard addition methodologies, integrating these approaches with mass spectrometry platforms to enhance quantitation accuracy in complex biological matrices.
Roche Diagnostics GmbH
Technical Solution: Roche Diagnostics GmbH has developed a comprehensive matrix effects quantitation approach for their diagnostic assays, particularly in PCR and immunoassay platforms. Their technology employs a multi-tiered calibration strategy that combines both matrix-matched calibration and standard addition methods to overcome matrix interference in complex biological samples. The company's proprietary calibration algorithms automatically adjust for matrix effects by utilizing internal standards with chemical properties similar to target analytes but distinguishable by detection systems. For quantitative PCR applications, they implement matrix-matched calibrators manufactured to mimic the exact composition of patient samples, ensuring accurate quantitation across different sample types. Their advanced standard addition protocols involve adding known quantities of analyte to patient samples to create calibration curves specific to each sample's matrix, effectively normalizing for individual matrix variations. This approach has demonstrated superior accuracy in clinical diagnostics, with validation studies showing reduction in quantitation bias by up to 85% compared to conventional methods.
Strengths: Highly accurate quantitation in complex biological matrices; comprehensive approach combining multiple strategies; automated adjustment algorithms reduce operator dependency. Weaknesses: Requires more complex calibration procedures than single-method approaches; higher cost of implementation; may require specialized training for laboratory personnel.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has developed the Quantitative Matrix Effects Management System (Q-MEMS), a comprehensive solution specifically designed for liquid chromatography-mass spectrometry (LC-MS) applications. Their approach integrates hardware innovations with sophisticated software algorithms to address matrix effects in complex analytical samples. The system employs a three-tier strategy: first, their patented IonRAID™ technology actively monitors ionization efficiency in real-time, detecting matrix-induced ion suppression or enhancement; second, their MatrixMatch™ calibration system automatically generates matrix-matched calibration curves using minimal sample volume; and third, their QuantiSmart™ software implements intelligent standard addition protocols when matrix matching proves insufficient. The technology incorporates machine learning algorithms that analyze historical data from similar matrices to predict and compensate for matrix effects before they impact quantitation. Waters' system is particularly notable for its application in multi-residue analysis, where it has demonstrated the ability to accurately quantify over 200 analytes simultaneously in complex environmental and food matrices. Independent validation studies have shown that the Q-MEMS approach reduces quantitative bias by up to 60% compared to conventional methods while maintaining high throughput capabilities.
Strengths: Comprehensive integration of hardware and software solutions; real-time monitoring and compensation of matrix effects; machine learning capabilities for predictive compensation. Weaknesses: Requires significant investment in Waters' ecosystem of instruments and software; complex implementation process; requires regular system updates to maintain optimal performance.
Key Innovations in Matrix Effects Compensation
Generalized local adaptive fusion regression process based on physicochemical and physiochemical underlying hidden properties for quantitative analysis of molecular based spectroscopic data
PatentPendingUS20230267369A1
Innovation
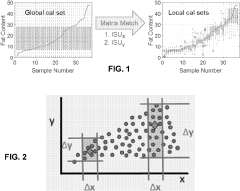
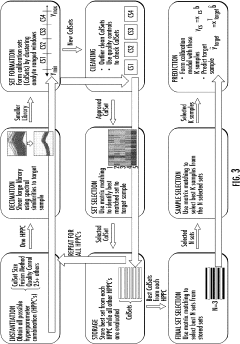
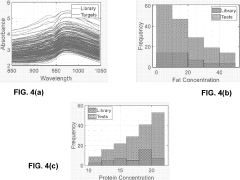
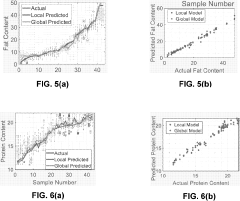
- The Local Adaptive Fusion Regression (LAFR) algorithm uses an indicator of system uniqueness (ISU) and sample-wise differences to identify matrix-matched samples from a library, forming a local training set that accurately predicts target sample analyte amounts by adapting to diverse physicochemical and physiochemical conditions.
Method Validation and Quality Assurance Protocols
Method validation and quality assurance protocols are essential components in analytical methodologies dealing with matrix effects quantitation. These protocols ensure that the analytical methods employed for matrix effect assessment are reliable, reproducible, and fit for purpose. When implementing calibration strategies such as matrix-matched calibration or standard addition, rigorous validation procedures must be established to confirm method performance characteristics.
The validation process typically begins with defining acceptance criteria for key parameters including linearity, accuracy, precision, sensitivity, and specificity. For matrix effects quantitation, special attention must be paid to recovery rates and matrix factor calculations across different sample types. Validation protocols should include procedures for determining limits of detection (LOD) and quantification (LOQ), which are particularly important when analyzing complex matrices where signal suppression or enhancement may occur.
Quality control samples must be incorporated throughout analytical batches to continuously monitor method performance. These should include blank samples, matrix-matched quality controls at low, medium, and high concentrations, and system suitability tests. For standard addition methods, validation should verify that the response is linear across the concentration range of added standards and that the extrapolation to determine the unknown concentration is statistically valid.
Robustness testing represents another critical aspect of method validation, particularly for matrix effect studies. This involves deliberate variations in method parameters to identify critical factors that might influence quantitation results. Parameters such as extraction time, solvent composition, pH adjustments, and instrument settings should be systematically evaluated to ensure method stability across different operating conditions.
Inter-laboratory comparison studies provide valuable data on method transferability and reproducibility. When developing calibration strategies for matrix effects, collaborative trials involving multiple laboratories analyzing identical samples can reveal systematic biases or variability that might not be apparent in single-laboratory validations. These studies are particularly valuable for complex matrices where standardized approaches may not be well established.
Documentation and traceability form the backbone of quality assurance in matrix effect studies. All calibration standards, reference materials, and reagents must be fully traceable with appropriate certificates of analysis. Detailed standard operating procedures (SOPs) should be developed for sample preparation, instrument operation, data processing, and interpretation of results. Regular audits and proficiency testing programs help maintain ongoing quality assurance and identify opportunities for methodological improvements.
The validation process typically begins with defining acceptance criteria for key parameters including linearity, accuracy, precision, sensitivity, and specificity. For matrix effects quantitation, special attention must be paid to recovery rates and matrix factor calculations across different sample types. Validation protocols should include procedures for determining limits of detection (LOD) and quantification (LOQ), which are particularly important when analyzing complex matrices where signal suppression or enhancement may occur.
Quality control samples must be incorporated throughout analytical batches to continuously monitor method performance. These should include blank samples, matrix-matched quality controls at low, medium, and high concentrations, and system suitability tests. For standard addition methods, validation should verify that the response is linear across the concentration range of added standards and that the extrapolation to determine the unknown concentration is statistically valid.
Robustness testing represents another critical aspect of method validation, particularly for matrix effect studies. This involves deliberate variations in method parameters to identify critical factors that might influence quantitation results. Parameters such as extraction time, solvent composition, pH adjustments, and instrument settings should be systematically evaluated to ensure method stability across different operating conditions.
Inter-laboratory comparison studies provide valuable data on method transferability and reproducibility. When developing calibration strategies for matrix effects, collaborative trials involving multiple laboratories analyzing identical samples can reveal systematic biases or variability that might not be apparent in single-laboratory validations. These studies are particularly valuable for complex matrices where standardized approaches may not be well established.
Documentation and traceability form the backbone of quality assurance in matrix effect studies. All calibration standards, reference materials, and reagents must be fully traceable with appropriate certificates of analysis. Detailed standard operating procedures (SOPs) should be developed for sample preparation, instrument operation, data processing, and interpretation of results. Regular audits and proficiency testing programs help maintain ongoing quality assurance and identify opportunities for methodological improvements.
Regulatory Compliance in Analytical Method Development
Regulatory compliance represents a critical framework governing analytical method development in pharmaceutical, environmental, and food safety industries. When addressing matrix effects quantitation through calibration strategies such as matrix-matched calibration and standard addition, laboratories must navigate a complex regulatory landscape. The FDA, EMA, and ICH have established stringent guidelines that directly impact how matrix effects are addressed during method validation and routine analysis.
The FDA's Guidance for Industry on Bioanalytical Method Validation specifically addresses matrix effects, requiring thorough investigation and documentation of these phenomena during method development. This guidance mandates that calibration standards should be prepared in the same biological matrix as the samples in the intended study. When matrix-matched calibration is employed, regulatory bodies require demonstration that the surrogate matrix adequately represents the actual sample matrix in terms of analyte behavior.
Standard addition techniques, while valuable for complex matrices, must be implemented with careful consideration of regulatory requirements. The FDA and EMA both emphasize the need for demonstrating method selectivity and specificity when standard addition is utilized, particularly in cases where complete chromatographic separation cannot be achieved.
ICH Q2(R1) guidelines on Validation of Analytical Procedures provide the foundation for method validation parameters that must be addressed regardless of calibration strategy. When matrix effects are present, these guidelines require additional validation steps to ensure accuracy, precision, and robustness of the analytical method. Specifically, the guidelines mandate evaluation of matrix effects across different lots of matrix to account for biological variability.
Regulatory agencies increasingly require risk assessment approaches when developing methods susceptible to matrix effects. This includes systematic evaluation of potential matrix interferences and documentation of mitigation strategies. For regulated industries, failure to adequately address matrix effects can result in rejection of analytical data during regulatory submissions.
Documentation requirements present another significant compliance consideration. Laboratories must maintain comprehensive records of matrix effect investigations, including experiments comparing calibration curves in different matrices, recovery studies, and post-column infusion experiments. These records must demonstrate scientific rigor in addressing matrix effects and justify the selected calibration approach.
For global pharmaceutical companies, harmonization of matrix effect evaluation across different regulatory jurisdictions presents additional challenges. While ICH guidelines provide some standardization, regional differences in regulatory expectations must be carefully navigated when implementing matrix-matched or standard addition calibration strategies.
The FDA's Guidance for Industry on Bioanalytical Method Validation specifically addresses matrix effects, requiring thorough investigation and documentation of these phenomena during method development. This guidance mandates that calibration standards should be prepared in the same biological matrix as the samples in the intended study. When matrix-matched calibration is employed, regulatory bodies require demonstration that the surrogate matrix adequately represents the actual sample matrix in terms of analyte behavior.
Standard addition techniques, while valuable for complex matrices, must be implemented with careful consideration of regulatory requirements. The FDA and EMA both emphasize the need for demonstrating method selectivity and specificity when standard addition is utilized, particularly in cases where complete chromatographic separation cannot be achieved.
ICH Q2(R1) guidelines on Validation of Analytical Procedures provide the foundation for method validation parameters that must be addressed regardless of calibration strategy. When matrix effects are present, these guidelines require additional validation steps to ensure accuracy, precision, and robustness of the analytical method. Specifically, the guidelines mandate evaluation of matrix effects across different lots of matrix to account for biological variability.
Regulatory agencies increasingly require risk assessment approaches when developing methods susceptible to matrix effects. This includes systematic evaluation of potential matrix interferences and documentation of mitigation strategies. For regulated industries, failure to adequately address matrix effects can result in rejection of analytical data during regulatory submissions.
Documentation requirements present another significant compliance consideration. Laboratories must maintain comprehensive records of matrix effect investigations, including experiments comparing calibration curves in different matrices, recovery studies, and post-column infusion experiments. These records must demonstrate scientific rigor in addressing matrix effects and justify the selected calibration approach.
For global pharmaceutical companies, harmonization of matrix effect evaluation across different regulatory jurisdictions presents additional challenges. While ICH guidelines provide some standardization, regional differences in regulatory expectations must be carefully navigated when implementing matrix-matched or standard addition calibration strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!