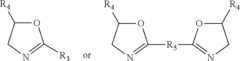
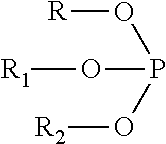
Polycarbonate Applications in Medical Devices
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polycarbonate in Medical Devices: Evolution and Objectives
Polycarbonate has emerged as a crucial material in the medical device industry, revolutionizing healthcare through its unique properties and versatile applications. The evolution of polycarbonate in medical devices can be traced back to the 1960s when it was first introduced as a durable, transparent, and biocompatible alternative to traditional materials. Since then, its usage has expanded significantly, driven by advancements in polymer science and increasing demands for high-performance medical equipment.
The journey of polycarbonate in medical devices has been marked by continuous improvements in material formulations, processing techniques, and application methodologies. Early applications were primarily focused on non-invasive devices such as syringes and IV components. However, as the material's potential became more apparent, its use expanded to more complex and critical applications, including surgical instruments, diagnostic equipment, and implantable devices.
One of the key milestones in the evolution of polycarbonate for medical use was the development of medical-grade polycarbonates in the 1980s. These specialized formulations offered enhanced purity, biocompatibility, and sterilization resistance, opening up new possibilities for long-term implantable devices and sensitive diagnostic equipment. This advancement significantly broadened the scope of polycarbonate applications in the medical field.
The objectives of polycarbonate use in medical devices have evolved in tandem with technological progress and changing healthcare needs. Initially, the primary goals were to provide a clear, strong, and easily moldable material for basic medical tools. As the healthcare industry advanced, the objectives expanded to include enhanced safety, improved functionality, and increased cost-effectiveness.
Currently, the main objectives for polycarbonate applications in medical devices include:
1. Enhancing patient safety through the use of biocompatible, sterilizable, and durable materials.
2. Improving device performance and functionality through tailored material properties.
3. Enabling the development of miniaturized and complex medical devices through advanced molding and fabrication techniques.
4. Supporting the trend towards home healthcare and portable medical devices with lightweight and impact-resistant materials.
5. Meeting stringent regulatory requirements for medical-grade materials.
Looking ahead, the future objectives for polycarbonate in medical devices are likely to focus on sustainability, smart functionalities, and personalized medicine. This includes developing bio-based polycarbonates, incorporating sensor technologies for real-time monitoring, and creating customizable devices for individual patient needs. The ongoing research in nanotechnology and surface modification techniques is expected to further expand the capabilities of polycarbonate in medical applications, potentially leading to breakthroughs in drug delivery systems and regenerative medicine.
The journey of polycarbonate in medical devices has been marked by continuous improvements in material formulations, processing techniques, and application methodologies. Early applications were primarily focused on non-invasive devices such as syringes and IV components. However, as the material's potential became more apparent, its use expanded to more complex and critical applications, including surgical instruments, diagnostic equipment, and implantable devices.
One of the key milestones in the evolution of polycarbonate for medical use was the development of medical-grade polycarbonates in the 1980s. These specialized formulations offered enhanced purity, biocompatibility, and sterilization resistance, opening up new possibilities for long-term implantable devices and sensitive diagnostic equipment. This advancement significantly broadened the scope of polycarbonate applications in the medical field.
The objectives of polycarbonate use in medical devices have evolved in tandem with technological progress and changing healthcare needs. Initially, the primary goals were to provide a clear, strong, and easily moldable material for basic medical tools. As the healthcare industry advanced, the objectives expanded to include enhanced safety, improved functionality, and increased cost-effectiveness.
Currently, the main objectives for polycarbonate applications in medical devices include:
1. Enhancing patient safety through the use of biocompatible, sterilizable, and durable materials.
2. Improving device performance and functionality through tailored material properties.
3. Enabling the development of miniaturized and complex medical devices through advanced molding and fabrication techniques.
4. Supporting the trend towards home healthcare and portable medical devices with lightweight and impact-resistant materials.
5. Meeting stringent regulatory requirements for medical-grade materials.
Looking ahead, the future objectives for polycarbonate in medical devices are likely to focus on sustainability, smart functionalities, and personalized medicine. This includes developing bio-based polycarbonates, incorporating sensor technologies for real-time monitoring, and creating customizable devices for individual patient needs. The ongoing research in nanotechnology and surface modification techniques is expected to further expand the capabilities of polycarbonate in medical applications, potentially leading to breakthroughs in drug delivery systems and regenerative medicine.
Market Demand Analysis for Medical-Grade Polycarbonate
The global market for medical-grade polycarbonate has been experiencing significant growth, driven by the increasing demand for advanced medical devices and equipment. This high-performance thermoplastic material offers a unique combination of properties that make it ideal for various medical applications, including surgical instruments, diagnostic equipment, and drug delivery systems.
The medical device industry has been a key driver of demand for polycarbonate, with the material being widely used in applications such as dialysis machines, blood oxygenators, and intravenous access components. The growing prevalence of chronic diseases and the aging population in many developed countries have contributed to the increased need for medical devices, subsequently boosting the demand for medical-grade polycarbonate.
In recent years, there has been a notable shift towards minimally invasive surgical procedures, which has further propelled the demand for polycarbonate in medical devices. The material's transparency, biocompatibility, and ability to be sterilized using various methods make it an excellent choice for manufacturing endoscopes, laparoscopic instruments, and other minimally invasive surgical tools.
The COVID-19 pandemic has also had a significant impact on the market demand for medical-grade polycarbonate. The sudden surge in demand for personal protective equipment (PPE), ventilators, and diagnostic equipment has led to increased consumption of polycarbonate in the medical sector. This trend is expected to continue in the post-pandemic era as healthcare systems worldwide focus on improving their preparedness for future health crises.
Another factor contributing to the growing demand for medical-grade polycarbonate is the increasing adoption of wearable medical devices and home healthcare equipment. These devices often require materials that are lightweight, durable, and resistant to impact and chemicals, making polycarbonate an ideal choice. The rise of telemedicine and remote patient monitoring has further accelerated this trend, creating new opportunities for polycarbonate applications in portable medical devices.
The market for medical-grade polycarbonate is also benefiting from ongoing technological advancements in material science. Manufacturers are developing new grades of polycarbonate with enhanced properties, such as improved chemical resistance, antimicrobial properties, and radiation stability. These innovations are expanding the potential applications of polycarbonate in the medical field and driving market growth.
In terms of regional demand, North America and Europe continue to be the largest markets for medical-grade polycarbonate, owing to their well-established healthcare infrastructure and high healthcare expenditure. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare investments, rising disposable incomes, and growing awareness of advanced medical technologies in countries like China and India.
The medical device industry has been a key driver of demand for polycarbonate, with the material being widely used in applications such as dialysis machines, blood oxygenators, and intravenous access components. The growing prevalence of chronic diseases and the aging population in many developed countries have contributed to the increased need for medical devices, subsequently boosting the demand for medical-grade polycarbonate.
In recent years, there has been a notable shift towards minimally invasive surgical procedures, which has further propelled the demand for polycarbonate in medical devices. The material's transparency, biocompatibility, and ability to be sterilized using various methods make it an excellent choice for manufacturing endoscopes, laparoscopic instruments, and other minimally invasive surgical tools.
The COVID-19 pandemic has also had a significant impact on the market demand for medical-grade polycarbonate. The sudden surge in demand for personal protective equipment (PPE), ventilators, and diagnostic equipment has led to increased consumption of polycarbonate in the medical sector. This trend is expected to continue in the post-pandemic era as healthcare systems worldwide focus on improving their preparedness for future health crises.
Another factor contributing to the growing demand for medical-grade polycarbonate is the increasing adoption of wearable medical devices and home healthcare equipment. These devices often require materials that are lightweight, durable, and resistant to impact and chemicals, making polycarbonate an ideal choice. The rise of telemedicine and remote patient monitoring has further accelerated this trend, creating new opportunities for polycarbonate applications in portable medical devices.
The market for medical-grade polycarbonate is also benefiting from ongoing technological advancements in material science. Manufacturers are developing new grades of polycarbonate with enhanced properties, such as improved chemical resistance, antimicrobial properties, and radiation stability. These innovations are expanding the potential applications of polycarbonate in the medical field and driving market growth.
In terms of regional demand, North America and Europe continue to be the largest markets for medical-grade polycarbonate, owing to their well-established healthcare infrastructure and high healthcare expenditure. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare investments, rising disposable incomes, and growing awareness of advanced medical technologies in countries like China and India.
Current Challenges in Polycarbonate Medical Applications
Despite the widespread use of polycarbonate in medical devices, several challenges persist in its application. One of the primary concerns is the potential leaching of bisphenol A (BPA), a key component in polycarbonate production. While the FDA has deemed low-level BPA exposure safe, ongoing research and public perception continue to raise questions about its long-term effects on human health, particularly in sensitive medical applications.
Another significant challenge is the material's limited resistance to certain chemicals and sterilization methods. Polycarbonate can be susceptible to stress cracking when exposed to certain organic solvents or aggressive cleaning agents commonly used in medical settings. This vulnerability necessitates careful consideration in device design and maintenance protocols to ensure longevity and safety.
The sterilization process itself poses challenges for polycarbonate medical devices. While the material can withstand some sterilization methods, such as ethylene oxide (EtO) and gamma radiation, repeated exposure to high-temperature steam sterilization can lead to degradation, affecting the material's mechanical properties and potentially compromising device performance.
Achieving consistent quality across large-scale production runs remains a challenge. Variations in molecular weight distribution and residual monomer content can lead to inconsistencies in mechanical properties and long-term stability. This variability can be particularly problematic in critical medical applications where precise performance is essential.
Environmental concerns also present challenges for polycarbonate in medical devices. The material's recyclability is limited, especially when combined with other materials or contaminated with biological substances. As healthcare facilities and regulatory bodies increasingly focus on sustainability, finding effective end-of-life solutions for polycarbonate medical devices becomes crucial.
Lastly, the development of new medical technologies and treatments often requires materials with enhanced properties. While polycarbonate offers a good balance of characteristics, there is a growing demand for materials that can provide improved biocompatibility, antimicrobial properties, or enhanced imaging compatibility. Meeting these evolving requirements while maintaining the beneficial properties of polycarbonate presents an ongoing challenge for material scientists and medical device manufacturers.
Another significant challenge is the material's limited resistance to certain chemicals and sterilization methods. Polycarbonate can be susceptible to stress cracking when exposed to certain organic solvents or aggressive cleaning agents commonly used in medical settings. This vulnerability necessitates careful consideration in device design and maintenance protocols to ensure longevity and safety.
The sterilization process itself poses challenges for polycarbonate medical devices. While the material can withstand some sterilization methods, such as ethylene oxide (EtO) and gamma radiation, repeated exposure to high-temperature steam sterilization can lead to degradation, affecting the material's mechanical properties and potentially compromising device performance.
Achieving consistent quality across large-scale production runs remains a challenge. Variations in molecular weight distribution and residual monomer content can lead to inconsistencies in mechanical properties and long-term stability. This variability can be particularly problematic in critical medical applications where precise performance is essential.
Environmental concerns also present challenges for polycarbonate in medical devices. The material's recyclability is limited, especially when combined with other materials or contaminated with biological substances. As healthcare facilities and regulatory bodies increasingly focus on sustainability, finding effective end-of-life solutions for polycarbonate medical devices becomes crucial.
Lastly, the development of new medical technologies and treatments often requires materials with enhanced properties. While polycarbonate offers a good balance of characteristics, there is a growing demand for materials that can provide improved biocompatibility, antimicrobial properties, or enhanced imaging compatibility. Meeting these evolving requirements while maintaining the beneficial properties of polycarbonate presents an ongoing challenge for material scientists and medical device manufacturers.
Existing Polycarbonate Solutions for Medical Devices
01 Synthesis and modification of polycarbonates
Various methods for synthesizing and modifying polycarbonates are explored, including novel catalysts, reaction conditions, and additives. These techniques aim to improve the properties and performance of polycarbonate materials, such as enhanced thermal stability, optical clarity, and mechanical strength.- Synthesis and modification of polycarbonates: Various methods for synthesizing and modifying polycarbonates are explored, including novel catalysts, reaction conditions, and additives to improve properties such as molecular weight, thermal stability, and optical clarity. These techniques aim to enhance the overall performance and versatility of polycarbonate materials for different applications.
- Polycarbonate blends and composites: Development of polycarbonate blends and composites with other polymers or additives to achieve improved mechanical properties, flame retardancy, or specific functionalities. These formulations expand the range of applications for polycarbonate-based materials in various industries, including automotive, electronics, and construction.
- Polycarbonate processing and manufacturing: Advancements in polycarbonate processing techniques, including extrusion, injection molding, and film formation. These innovations focus on improving production efficiency, reducing defects, and enhancing the quality of final polycarbonate products for diverse applications.
- Polycarbonate surface treatments and coatings: Development of surface treatments and coatings for polycarbonate materials to enhance properties such as scratch resistance, UV stability, and anti-fogging capabilities. These techniques aim to improve the durability and performance of polycarbonate products in various environmental conditions.
- Recycling and sustainability of polycarbonates: Innovations in polycarbonate recycling processes and the development of more sustainable production methods. These advancements focus on reducing environmental impact, improving resource efficiency, and creating circular economy solutions for polycarbonate materials.
02 Polycarbonate blends and composites
Development of polycarbonate blends and composites with other polymers or materials to enhance specific properties. These combinations can result in improved impact resistance, flame retardancy, or other desirable characteristics for various applications in electronics, automotive, and consumer goods industries.Expand Specific Solutions03 Optical applications of polycarbonates
Utilization of polycarbonates in optical applications, such as lenses, displays, and light-guiding components. Research focuses on enhancing optical properties, including transparency, refractive index, and resistance to yellowing, to meet the demands of advanced optical systems and devices.Expand Specific Solutions04 Polycarbonate processing and manufacturing
Advancements in polycarbonate processing and manufacturing techniques, including extrusion, injection molding, and film formation. These innovations aim to improve production efficiency, reduce costs, and enhance the quality of polycarbonate products for various industrial applications.Expand Specific Solutions05 Sustainable and bio-based polycarbonates
Development of sustainable and bio-based polycarbonates as alternatives to traditional petroleum-based materials. Research focuses on using renewable resources, improving biodegradability, and reducing environmental impact while maintaining the desirable properties of conventional polycarbonates.Expand Specific Solutions
Key Players in Medical Polycarbonate Manufacturing
The polycarbonate applications in medical devices market is in a growth phase, driven by increasing demand for durable and biocompatible materials in healthcare. The global market size is projected to reach several billion dollars by 2025, with a compound annual growth rate of around 7-8%. Technologically, polycarbonate applications are maturing, with companies like Covestro, SABIC, and Trinseo leading innovation. These firms, along with medical device manufacturers such as Boston Scientific and Medtronic, are developing advanced polycarbonate formulations for specific medical applications. The technology's maturity is evident in its widespread use across various medical devices, from surgical instruments to implantable components, indicating a high level of industry adoption and ongoing refinement.
Covestro Deutschland AG
Technical Solution: Covestro has developed Makrolon® polycarbonate for medical devices, offering high impact strength and biocompatibility. Their technology focuses on creating grades suitable for various medical applications, including drug delivery systems, surgical instruments, and diagnostic equipment. Covestro's polycarbonate can withstand multiple sterilization cycles without significant property loss[1]. They have also introduced Makrolon® RE, a more sustainable polycarbonate made with up to 71% bio-circular raw materials, maintaining the same performance as fossil-based polycarbonates[2]. This innovation addresses the growing demand for eco-friendly materials in the medical sector.
Strengths: High impact resistance, biocompatibility, and sterilization resistance. Sustainable options available. Weaknesses: May be more expensive than some alternatives, limited high-temperature applications.
SABIC Global Technologies BV
Technical Solution: SABIC has developed LEXAN™ polycarbonate resins specifically for healthcare applications. Their technology focuses on creating materials that meet stringent medical industry requirements, including biocompatibility, chemical resistance, and transparency. SABIC's polycarbonate grades offer solutions for drug delivery devices, surgical instruments, and medical equipment housings. They have introduced antimicrobial-enhanced polycarbonates that inhibit bacterial growth on device surfaces[3]. SABIC also offers custom color matching services for their polycarbonates, allowing medical device manufacturers to create distinctive and brand-consistent products[4].
Strengths: Wide range of specialized grades, antimicrobial options, custom coloring capabilities. Weaknesses: May require specific processing conditions, potential for stress cracking under certain chemicals.
Innovative Polycarbonate Formulations for Medical Use
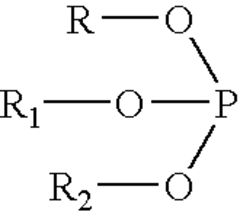
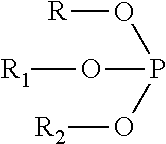
Biocompatible polycarbonate and radiopaque polymer compositions and methods of manufacturing medical devices with same
PatentInactiveUS20100168270A1
Innovation
- A biocompatible radiopaque polymer composition combining poly(bisphenol A carbonate) with polyamide and an inorganic radiopaque filler, along with additives like phosphites and functionalized polyolefins, to enhance melt processability and mechanical properties, allowing for improved visualization and mechanical performance in medical devices.
Biocompatible Polycarbonate and Radiopaque Polymer Compositions and Methods of Manufacturing Medical Devices with Same
PatentInactiveUS20170321054A1
Innovation
- A biocompatible radiopaque polymer composition combining poly(bisphenol A carbonate) with polyamide and an inorganic radiopaque filler, along with additives like phosphites and functionalized polyolefins, to enhance melt processability and mechanical properties, allowing for improved visualization and mechanical strength in medical devices.
Regulatory Framework for Medical-Grade Polycarbonate
The regulatory framework for medical-grade polycarbonate is a critical aspect of its application in medical devices. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those made from polycarbonate materials. The FDA classifies medical devices into three categories based on their risk level and intended use, with Class I being the lowest risk and Class III being the highest.
For polycarbonate used in medical devices, manufacturers must comply with the FDA's Quality System Regulation (QSR), which outlines the requirements for good manufacturing practices. This includes establishing and maintaining quality assurance programs, implementing design controls, and ensuring proper documentation throughout the product lifecycle.
In addition to the FDA regulations, medical-grade polycarbonate must also meet the requirements set forth by international standards organizations. The International Organization for Standardization (ISO) has developed specific standards for medical devices, such as ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) also play a significant role in the regulatory landscape for medical-grade polycarbonate. These regulations establish stringent requirements for safety, performance, and traceability of medical devices sold in the EU market.
Manufacturers must conduct thorough risk assessments and provide extensive documentation to demonstrate compliance with these regulations. This includes providing detailed information on the material composition, manufacturing processes, and sterilization methods used for polycarbonate medical devices.
Specific to polycarbonate, regulatory bodies often require data on the material's resistance to chemical degradation, thermal stability, and potential for leaching of harmful substances. This is particularly important for devices that come into direct contact with patients or biological fluids.
Furthermore, the regulatory framework extends to the entire supply chain, requiring manufacturers to implement robust supplier qualification processes and maintain traceability of raw materials used in medical-grade polycarbonate production.
As the medical device industry continues to evolve, regulatory bodies are increasingly focusing on post-market surveillance and long-term safety data. This trend is likely to impact the regulatory requirements for polycarbonate applications in medical devices, potentially leading to more stringent monitoring and reporting obligations for manufacturers.
For polycarbonate used in medical devices, manufacturers must comply with the FDA's Quality System Regulation (QSR), which outlines the requirements for good manufacturing practices. This includes establishing and maintaining quality assurance programs, implementing design controls, and ensuring proper documentation throughout the product lifecycle.
In addition to the FDA regulations, medical-grade polycarbonate must also meet the requirements set forth by international standards organizations. The International Organization for Standardization (ISO) has developed specific standards for medical devices, such as ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) also play a significant role in the regulatory landscape for medical-grade polycarbonate. These regulations establish stringent requirements for safety, performance, and traceability of medical devices sold in the EU market.
Manufacturers must conduct thorough risk assessments and provide extensive documentation to demonstrate compliance with these regulations. This includes providing detailed information on the material composition, manufacturing processes, and sterilization methods used for polycarbonate medical devices.
Specific to polycarbonate, regulatory bodies often require data on the material's resistance to chemical degradation, thermal stability, and potential for leaching of harmful substances. This is particularly important for devices that come into direct contact with patients or biological fluids.
Furthermore, the regulatory framework extends to the entire supply chain, requiring manufacturers to implement robust supplier qualification processes and maintain traceability of raw materials used in medical-grade polycarbonate production.
As the medical device industry continues to evolve, regulatory bodies are increasingly focusing on post-market surveillance and long-term safety data. This trend is likely to impact the regulatory requirements for polycarbonate applications in medical devices, potentially leading to more stringent monitoring and reporting obligations for manufacturers.
Sustainability in Medical Polycarbonate Production
Sustainability in medical polycarbonate production has become a critical focus for manufacturers and healthcare providers alike. As the demand for polycarbonate-based medical devices continues to grow, the industry is increasingly aware of the environmental impact of their production processes and end-of-life disposal.
One of the primary sustainability challenges in medical polycarbonate production is the energy-intensive nature of the manufacturing process. Traditional methods require high temperatures and pressures, resulting in significant carbon emissions. To address this, manufacturers are exploring more energy-efficient production techniques, such as low-temperature polymerization and the use of catalysts that reduce reaction times and energy requirements.
Material sourcing is another key area for sustainability improvements. Conventional polycarbonate production relies heavily on petroleum-based feedstocks, which are non-renewable and contribute to carbon emissions. Innovative approaches include the development of bio-based polycarbonates derived from renewable resources such as plant-based oils or agricultural waste. These alternatives not only reduce reliance on fossil fuels but also offer the potential for biodegradability, addressing end-of-life concerns.
Waste reduction and recycling initiatives are gaining traction in the medical polycarbonate industry. Closed-loop manufacturing systems are being implemented to recover and reuse production scrap, minimizing material waste. Additionally, advanced recycling technologies are being developed to process end-of-life medical devices, enabling the recovery of high-quality polycarbonate for reuse in new products.
Water conservation is another critical aspect of sustainable polycarbonate production. Manufacturers are implementing water recycling systems and exploring waterless cooling methods to reduce freshwater consumption in their processes. This not only conserves a precious resource but also minimizes the environmental impact of wastewater discharge.
The use of renewable energy sources in polycarbonate production facilities is becoming more prevalent. Solar panels, wind turbines, and other clean energy technologies are being integrated into manufacturing plants to reduce reliance on fossil fuels and decrease overall carbon footprints.
Sustainable packaging solutions for medical polycarbonate products are also being developed. Biodegradable or recyclable packaging materials are replacing traditional plastic packaging, reducing waste and environmental impact throughout the supply chain.
Lastly, the industry is focusing on extending the lifespan of polycarbonate medical devices through improved durability and design for reusability. This approach not only enhances sustainability but also provides economic benefits to healthcare providers and patients.
As the medical device industry continues to prioritize sustainability, these initiatives in polycarbonate production are paving the way for a more environmentally responsible future in healthcare technology.
One of the primary sustainability challenges in medical polycarbonate production is the energy-intensive nature of the manufacturing process. Traditional methods require high temperatures and pressures, resulting in significant carbon emissions. To address this, manufacturers are exploring more energy-efficient production techniques, such as low-temperature polymerization and the use of catalysts that reduce reaction times and energy requirements.
Material sourcing is another key area for sustainability improvements. Conventional polycarbonate production relies heavily on petroleum-based feedstocks, which are non-renewable and contribute to carbon emissions. Innovative approaches include the development of bio-based polycarbonates derived from renewable resources such as plant-based oils or agricultural waste. These alternatives not only reduce reliance on fossil fuels but also offer the potential for biodegradability, addressing end-of-life concerns.
Waste reduction and recycling initiatives are gaining traction in the medical polycarbonate industry. Closed-loop manufacturing systems are being implemented to recover and reuse production scrap, minimizing material waste. Additionally, advanced recycling technologies are being developed to process end-of-life medical devices, enabling the recovery of high-quality polycarbonate for reuse in new products.
Water conservation is another critical aspect of sustainable polycarbonate production. Manufacturers are implementing water recycling systems and exploring waterless cooling methods to reduce freshwater consumption in their processes. This not only conserves a precious resource but also minimizes the environmental impact of wastewater discharge.
The use of renewable energy sources in polycarbonate production facilities is becoming more prevalent. Solar panels, wind turbines, and other clean energy technologies are being integrated into manufacturing plants to reduce reliance on fossil fuels and decrease overall carbon footprints.
Sustainable packaging solutions for medical polycarbonate products are also being developed. Biodegradable or recyclable packaging materials are replacing traditional plastic packaging, reducing waste and environmental impact throughout the supply chain.
Lastly, the industry is focusing on extending the lifespan of polycarbonate medical devices through improved durability and design for reusability. This approach not only enhances sustainability but also provides economic benefits to healthcare providers and patients.
As the medical device industry continues to prioritize sustainability, these initiatives in polycarbonate production are paving the way for a more environmentally responsible future in healthcare technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!