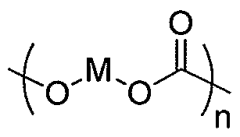
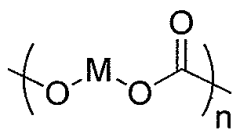
Polycarbonate in Pharmaceutical Packaging: Trends and Techniques
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polycarbonate Packaging Evolution and Objectives
Polycarbonate has emerged as a revolutionary material in pharmaceutical packaging, evolving significantly over the past few decades. Initially introduced in the 1950s, polycarbonate's journey in pharmaceutical packaging began in earnest during the 1970s and 1980s. The primary objective driving its adoption was to overcome the limitations of traditional glass and other plastic materials, particularly in terms of durability, clarity, and chemical resistance.
The evolution of polycarbonate in pharmaceutical packaging has been marked by continuous improvements in material properties and manufacturing techniques. Early applications focused on rigid containers for storing and transporting medications. As technology advanced, the use of polycarbonate expanded to include pre-filled syringes, vials, and other drug delivery systems. This expansion was driven by the growing demand for safer, more convenient, and cost-effective packaging solutions in the healthcare industry.
A significant milestone in polycarbonate's evolution was the development of medical-grade polycarbonate in the 1990s. This specialized grade offered enhanced purity and biocompatibility, making it suitable for a wider range of pharmaceutical applications. The introduction of medical-grade polycarbonate aligned with the industry's increasing focus on patient safety and regulatory compliance.
The 21st century has seen further advancements in polycarbonate technology for pharmaceutical packaging. Key objectives have included improving barrier properties to enhance drug stability, developing antimicrobial formulations to reduce contamination risks, and creating more sustainable and recyclable packaging solutions. These objectives reflect the changing landscape of pharmaceutical manufacturing and distribution, as well as growing environmental concerns.
Recent trends in polycarbonate packaging development have focused on customization and smart packaging solutions. This includes the integration of sensors and indicators to monitor drug quality and usage, as well as the development of tamper-evident and child-resistant features. The goal is to enhance patient compliance, improve drug efficacy, and reduce medication errors.
Looking ahead, the objectives for polycarbonate in pharmaceutical packaging continue to evolve. There is a growing emphasis on developing bio-based and biodegradable polycarbonate alternatives to address environmental concerns. Additionally, research is ongoing to further enhance the material's properties, such as improving its resistance to sterilization processes and extending its compatibility with a broader range of pharmaceutical formulations.
In conclusion, the evolution of polycarbonate in pharmaceutical packaging reflects a journey of continuous innovation driven by the changing needs of the healthcare industry. From its initial adoption to its current sophisticated applications, polycarbonate has consistently aimed to provide safer, more efficient, and increasingly sustainable packaging solutions for pharmaceuticals. The ongoing objectives in this field promise to further revolutionize drug packaging and delivery systems in the years to come.
The evolution of polycarbonate in pharmaceutical packaging has been marked by continuous improvements in material properties and manufacturing techniques. Early applications focused on rigid containers for storing and transporting medications. As technology advanced, the use of polycarbonate expanded to include pre-filled syringes, vials, and other drug delivery systems. This expansion was driven by the growing demand for safer, more convenient, and cost-effective packaging solutions in the healthcare industry.
A significant milestone in polycarbonate's evolution was the development of medical-grade polycarbonate in the 1990s. This specialized grade offered enhanced purity and biocompatibility, making it suitable for a wider range of pharmaceutical applications. The introduction of medical-grade polycarbonate aligned with the industry's increasing focus on patient safety and regulatory compliance.
The 21st century has seen further advancements in polycarbonate technology for pharmaceutical packaging. Key objectives have included improving barrier properties to enhance drug stability, developing antimicrobial formulations to reduce contamination risks, and creating more sustainable and recyclable packaging solutions. These objectives reflect the changing landscape of pharmaceutical manufacturing and distribution, as well as growing environmental concerns.
Recent trends in polycarbonate packaging development have focused on customization and smart packaging solutions. This includes the integration of sensors and indicators to monitor drug quality and usage, as well as the development of tamper-evident and child-resistant features. The goal is to enhance patient compliance, improve drug efficacy, and reduce medication errors.
Looking ahead, the objectives for polycarbonate in pharmaceutical packaging continue to evolve. There is a growing emphasis on developing bio-based and biodegradable polycarbonate alternatives to address environmental concerns. Additionally, research is ongoing to further enhance the material's properties, such as improving its resistance to sterilization processes and extending its compatibility with a broader range of pharmaceutical formulations.
In conclusion, the evolution of polycarbonate in pharmaceutical packaging reflects a journey of continuous innovation driven by the changing needs of the healthcare industry. From its initial adoption to its current sophisticated applications, polycarbonate has consistently aimed to provide safer, more efficient, and increasingly sustainable packaging solutions for pharmaceuticals. The ongoing objectives in this field promise to further revolutionize drug packaging and delivery systems in the years to come.
Pharmaceutical Market Demand Analysis
The pharmaceutical packaging market has been experiencing significant growth, driven by the increasing demand for healthcare products worldwide. Polycarbonate, as a versatile material, has found its place in this expanding market due to its unique properties that align well with the stringent requirements of pharmaceutical packaging.
The global pharmaceutical packaging market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other industries. This growth is primarily attributed to the rising prevalence of chronic diseases, the aging population, and the increasing focus on drug safety and efficacy. As a result, there is a growing demand for high-quality, durable, and safe packaging materials like polycarbonate.
Polycarbonate's market demand in pharmaceutical packaging is particularly strong in certain segments. Pre-filled syringes, a rapidly growing segment, benefit from polycarbonate's clarity, strength, and chemical resistance. The material's ability to withstand sterilization processes makes it ideal for this application. Additionally, polycarbonate is increasingly used in drug delivery devices, such as inhalers and auto-injectors, where its impact resistance and dimensional stability are crucial.
The trend towards biologics and personalized medicine is another factor driving the demand for polycarbonate in pharmaceutical packaging. These sensitive drugs often require specialized packaging that can protect them from light, moisture, and temperature fluctuations. Polycarbonate's barrier properties and customizability make it a suitable choice for these advanced pharmaceutical products.
In the context of sustainability, which is becoming increasingly important in the pharmaceutical industry, polycarbonate offers advantages. Its durability allows for the creation of reusable packaging solutions, aligning with the industry's efforts to reduce waste and environmental impact. However, this aspect also presents challenges, as there is a growing demand for more environmentally friendly and recyclable materials.
The COVID-19 pandemic has further accelerated the demand for polycarbonate in pharmaceutical packaging. The material's use in vaccine vials, syringes, and other medical devices has seen a significant uptick. This sudden increase in demand has also highlighted the need for a robust and flexible supply chain in the polycarbonate industry to meet rapid fluctuations in market needs.
Despite the positive outlook, the market demand analysis also reveals certain challenges. Regulatory scrutiny regarding the safety of polycarbonate, particularly concerns about bisphenol A (BPA), continues to influence market dynamics. This has led to increased research and development efforts to create BPA-free polycarbonate alternatives, which could reshape the market in the coming years.
The global pharmaceutical packaging market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other industries. This growth is primarily attributed to the rising prevalence of chronic diseases, the aging population, and the increasing focus on drug safety and efficacy. As a result, there is a growing demand for high-quality, durable, and safe packaging materials like polycarbonate.
Polycarbonate's market demand in pharmaceutical packaging is particularly strong in certain segments. Pre-filled syringes, a rapidly growing segment, benefit from polycarbonate's clarity, strength, and chemical resistance. The material's ability to withstand sterilization processes makes it ideal for this application. Additionally, polycarbonate is increasingly used in drug delivery devices, such as inhalers and auto-injectors, where its impact resistance and dimensional stability are crucial.
The trend towards biologics and personalized medicine is another factor driving the demand for polycarbonate in pharmaceutical packaging. These sensitive drugs often require specialized packaging that can protect them from light, moisture, and temperature fluctuations. Polycarbonate's barrier properties and customizability make it a suitable choice for these advanced pharmaceutical products.
In the context of sustainability, which is becoming increasingly important in the pharmaceutical industry, polycarbonate offers advantages. Its durability allows for the creation of reusable packaging solutions, aligning with the industry's efforts to reduce waste and environmental impact. However, this aspect also presents challenges, as there is a growing demand for more environmentally friendly and recyclable materials.
The COVID-19 pandemic has further accelerated the demand for polycarbonate in pharmaceutical packaging. The material's use in vaccine vials, syringes, and other medical devices has seen a significant uptick. This sudden increase in demand has also highlighted the need for a robust and flexible supply chain in the polycarbonate industry to meet rapid fluctuations in market needs.
Despite the positive outlook, the market demand analysis also reveals certain challenges. Regulatory scrutiny regarding the safety of polycarbonate, particularly concerns about bisphenol A (BPA), continues to influence market dynamics. This has led to increased research and development efforts to create BPA-free polycarbonate alternatives, which could reshape the market in the coming years.
Current Challenges in Polycarbonate Packaging
Despite the widespread use of polycarbonate in pharmaceutical packaging, several challenges persist in its application. One of the primary concerns is the potential leaching of bisphenol A (BPA), a chemical compound used in polycarbonate production. BPA has been associated with various health risks, including endocrine disruption and potential developmental issues. This has led to increased scrutiny from regulatory bodies and consumers alike, prompting the pharmaceutical industry to seek alternative materials or improved polycarbonate formulations.
Another significant challenge is the environmental impact of polycarbonate packaging. As sustainability becomes a growing concern in the pharmaceutical sector, the non-biodegradable nature of polycarbonate poses a considerable problem. The industry is grappling with the need to balance the excellent barrier properties and durability of polycarbonate with the increasing demand for eco-friendly packaging solutions.
The sterilization process presents yet another hurdle for polycarbonate packaging. While polycarbonate can withstand various sterilization methods, repeated exposure to high temperatures or certain sterilizing agents can lead to degradation of the material. This degradation may compromise the integrity of the packaging, potentially affecting the safety and efficacy of the pharmaceutical products contained within.
Compatibility issues between polycarbonate and certain drug formulations also pose a challenge. Some active pharmaceutical ingredients or excipients may interact with the polycarbonate material, leading to absorption, adsorption, or chemical reactions. These interactions can potentially alter the drug's composition or efficacy, necessitating extensive compatibility studies and, in some cases, limiting the use of polycarbonate for specific pharmaceutical products.
The cost factor remains a persistent challenge in polycarbonate packaging. While the material offers numerous benefits, it is generally more expensive than some alternative packaging materials. This cost consideration becomes particularly significant in the production of generic drugs or in markets where price sensitivity is high.
Lastly, the regulatory landscape surrounding polycarbonate packaging continues to evolve. As new research emerges and public awareness grows, regulatory bodies are implementing stricter guidelines and standards for the use of polycarbonate in pharmaceutical packaging. Staying compliant with these changing regulations while maintaining product quality and cost-effectiveness presents an ongoing challenge for pharmaceutical companies and packaging manufacturers alike.
Another significant challenge is the environmental impact of polycarbonate packaging. As sustainability becomes a growing concern in the pharmaceutical sector, the non-biodegradable nature of polycarbonate poses a considerable problem. The industry is grappling with the need to balance the excellent barrier properties and durability of polycarbonate with the increasing demand for eco-friendly packaging solutions.
The sterilization process presents yet another hurdle for polycarbonate packaging. While polycarbonate can withstand various sterilization methods, repeated exposure to high temperatures or certain sterilizing agents can lead to degradation of the material. This degradation may compromise the integrity of the packaging, potentially affecting the safety and efficacy of the pharmaceutical products contained within.
Compatibility issues between polycarbonate and certain drug formulations also pose a challenge. Some active pharmaceutical ingredients or excipients may interact with the polycarbonate material, leading to absorption, adsorption, or chemical reactions. These interactions can potentially alter the drug's composition or efficacy, necessitating extensive compatibility studies and, in some cases, limiting the use of polycarbonate for specific pharmaceutical products.
The cost factor remains a persistent challenge in polycarbonate packaging. While the material offers numerous benefits, it is generally more expensive than some alternative packaging materials. This cost consideration becomes particularly significant in the production of generic drugs or in markets where price sensitivity is high.
Lastly, the regulatory landscape surrounding polycarbonate packaging continues to evolve. As new research emerges and public awareness grows, regulatory bodies are implementing stricter guidelines and standards for the use of polycarbonate in pharmaceutical packaging. Staying compliant with these changing regulations while maintaining product quality and cost-effectiveness presents an ongoing challenge for pharmaceutical companies and packaging manufacturers alike.
Existing Polycarbonate Packaging Solutions
01 Synthesis and modification of polycarbonates
Various methods for synthesizing and modifying polycarbonates are explored, including novel catalysts, reaction conditions, and additives. These techniques aim to improve the properties and performance of polycarbonate materials, such as thermal stability, impact resistance, and optical clarity.- Synthesis and modification of polycarbonates: Various methods for synthesizing and modifying polycarbonates are explored, including novel catalysts, reaction conditions, and additives to improve properties such as molecular weight, thermal stability, and optical clarity. These techniques aim to enhance the overall performance and versatility of polycarbonate materials for different applications.
- Polycarbonate blends and composites: Development of polycarbonate blends and composites with other polymers or additives to achieve improved mechanical properties, flame retardancy, or specific functionalities. These formulations expand the range of applications for polycarbonate-based materials in various industries, including automotive, electronics, and construction.
- Optical applications of polycarbonates: Utilization of polycarbonates in optical applications, such as lenses, displays, and light-guiding components. Research focuses on improving optical clarity, UV resistance, and scratch resistance of polycarbonate materials for use in eyewear, automotive lighting, and electronic displays.
- Recycling and sustainability of polycarbonates: Development of methods for recycling polycarbonate materials and improving their sustainability. This includes chemical recycling processes, biodegradable polycarbonate formulations, and the use of bio-based monomers in polycarbonate synthesis to reduce environmental impact.
- Polycarbonate surface treatments and coatings: Techniques for modifying polycarbonate surfaces through treatments and coatings to enhance properties such as scratch resistance, chemical resistance, and adhesion. These methods improve the durability and performance of polycarbonate products in various applications, including automotive parts and electronic device housings.
02 Polycarbonate blends and composites
Polycarbonates are often blended with other polymers or reinforced with various materials to create composites with enhanced properties. These blends and composites can offer improved mechanical strength, flame retardancy, or specific functional characteristics for diverse applications.Expand Specific Solutions03 Polycarbonate processing and molding techniques
Advanced processing and molding techniques for polycarbonates are developed to optimize production efficiency and product quality. These methods may include innovative extrusion processes, injection molding techniques, or surface treatment methods to enhance the final product's properties.Expand Specific Solutions04 Polycarbonate applications in electronics and optics
Polycarbonates find extensive use in electronics and optical applications due to their unique properties. Innovations in this area focus on improving the material's performance for specific uses such as LED encapsulation, optical lenses, or electronic device housings.Expand Specific Solutions05 Recycling and sustainability of polycarbonates
Efforts to improve the recyclability and sustainability of polycarbonates are ongoing. This includes developing new recycling processes, creating more environmentally friendly production methods, and exploring bio-based alternatives to traditional polycarbonate materials.Expand Specific Solutions
Key Players in Pharma Packaging Industry
The polycarbonate pharmaceutical packaging market is in a growth phase, driven by increasing demand for high-performance, durable, and transparent packaging solutions. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, polycarbonate packaging is mature, but innovations continue in areas such as enhanced barrier properties and sustainability. Key players like SABIC, Covestro, and Wanhua Chemical are leading the field, investing in R&D to develop advanced formulations and manufacturing techniques. Companies such as LG Chem and Kingfa Sci. & Tech. are also making strides in this sector, focusing on specialized applications and eco-friendly alternatives to traditional polycarbonate packaging.
SABIC Global Technologies BV
Technical Solution: SABIC has developed LEXAN™ HP92AF polycarbonate resin specifically for pharmaceutical packaging applications. This high-performance material offers excellent clarity, impact resistance, and chemical compatibility. SABIC's technology focuses on enhancing the barrier properties of polycarbonate, reducing potential leaching of bisphenol A (BPA), and improving sterilization resistance. They have implemented advanced polymer processing techniques to achieve a balance between transparency and barrier performance[1][3]. SABIC also utilizes proprietary additives to enhance the material's resistance to gamma radiation, making it suitable for radiation sterilization of pharmaceutical packaging[2].
Strengths: Superior clarity and impact resistance, improved chemical compatibility, enhanced barrier properties. Weaknesses: Potential concerns about BPA leaching, higher cost compared to some alternative materials.
Covestro Deutschland AG
Technical Solution: Covestro has developed Makrolon® Rx1805 polycarbonate for pharmaceutical packaging applications. This innovative material is designed to meet the stringent requirements of the healthcare industry. Covestro's technology focuses on enhancing the material's resistance to sterilization processes, including gamma radiation, e-beam, and ethylene oxide. They have implemented a proprietary additive package that improves color stability after sterilization, maintaining the clarity and aesthetics of the packaging[4]. Covestro has also developed a surface treatment technology that reduces protein adsorption and enhances the material's compatibility with various drug formulations[5].
Strengths: Excellent sterilization resistance, improved color stability, enhanced drug compatibility. Weaknesses: May be more expensive than standard polycarbonate grades, limited long-term clinical data on new formulations.
Innovations in Polycarbonate Formulations
Polypropylene compositions with improved sealing and barrier properties
PatentPendingEP4495148A1
Innovation
- A polypropylene composition comprising a propylene-ethylene random copolymer, with optional additives, is developed. This composition has improved mechanical, optical, barrier, and sealing properties, making it suitable for pharmaceutical blister packaging.
Degradable polycarbonates
PatentWO2012051448A1
Innovation
- Development of degradable polycarbonates formed from polyhydroxyl monomers linked by a carbonate moiety, which can undergo hydrolytic degradation to release natural products, reducing dependence on petrochemicals and offering tunable mechanical properties for various applications.
Regulatory Compliance for Pharma Packaging
Regulatory compliance is a critical aspect of pharmaceutical packaging, particularly when it comes to the use of polycarbonate materials. The pharmaceutical industry is subject to stringent regulations aimed at ensuring product safety, efficacy, and quality throughout the supply chain. These regulations are established and enforced by various regulatory bodies, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other national health authorities.
For polycarbonate packaging in pharmaceuticals, manufacturers must adhere to specific guidelines and standards. The FDA's Code of Federal Regulations (CFR) Title 21, Part 177, Subpart B, Section 177.1580 outlines the requirements for polycarbonate resins used in food-contact applications, which also applies to pharmaceutical packaging. This regulation specifies the permissible raw materials, manufacturing processes, and specifications for polycarbonate resins.
In addition to FDA regulations, the International Conference on Harmonisation (ICH) guidelines play a crucial role in establishing global standards for pharmaceutical packaging. The ICH Q7 guideline on Good Manufacturing Practice (GMP) for Active Pharmaceutical Ingredients includes requirements for packaging materials, emphasizing the need for appropriate specifications, testing, and quality control.
Manufacturers must also comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards provide detailed specifications for packaging materials, including polycarbonate, and outline testing methods to ensure their suitability for pharmaceutical use.
Environmental considerations are becoming increasingly important in regulatory compliance. The European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation impacts the use of certain chemicals in polycarbonate production, requiring manufacturers to assess and manage the risks associated with these substances.
To ensure compliance, pharmaceutical companies must implement robust quality management systems and documentation practices. This includes maintaining detailed records of material specifications, supplier qualifications, and quality control test results. Regular audits and inspections by regulatory authorities are conducted to verify compliance with these requirements.
As regulations evolve, manufacturers must stay informed about changes and updates to ensure ongoing compliance. This may involve participating in industry associations, attending regulatory conferences, and maintaining open communication channels with regulatory bodies. Continuous monitoring and adaptation of packaging processes and materials are essential to meet the ever-changing regulatory landscape in pharmaceutical packaging.
For polycarbonate packaging in pharmaceuticals, manufacturers must adhere to specific guidelines and standards. The FDA's Code of Federal Regulations (CFR) Title 21, Part 177, Subpart B, Section 177.1580 outlines the requirements for polycarbonate resins used in food-contact applications, which also applies to pharmaceutical packaging. This regulation specifies the permissible raw materials, manufacturing processes, and specifications for polycarbonate resins.
In addition to FDA regulations, the International Conference on Harmonisation (ICH) guidelines play a crucial role in establishing global standards for pharmaceutical packaging. The ICH Q7 guideline on Good Manufacturing Practice (GMP) for Active Pharmaceutical Ingredients includes requirements for packaging materials, emphasizing the need for appropriate specifications, testing, and quality control.
Manufacturers must also comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards provide detailed specifications for packaging materials, including polycarbonate, and outline testing methods to ensure their suitability for pharmaceutical use.
Environmental considerations are becoming increasingly important in regulatory compliance. The European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation impacts the use of certain chemicals in polycarbonate production, requiring manufacturers to assess and manage the risks associated with these substances.
To ensure compliance, pharmaceutical companies must implement robust quality management systems and documentation practices. This includes maintaining detailed records of material specifications, supplier qualifications, and quality control test results. Regular audits and inspections by regulatory authorities are conducted to verify compliance with these requirements.
As regulations evolve, manufacturers must stay informed about changes and updates to ensure ongoing compliance. This may involve participating in industry associations, attending regulatory conferences, and maintaining open communication channels with regulatory bodies. Continuous monitoring and adaptation of packaging processes and materials are essential to meet the ever-changing regulatory landscape in pharmaceutical packaging.
Sustainability in Polycarbonate Packaging
Sustainability in polycarbonate packaging has become a critical focus in the pharmaceutical industry, driven by increasing environmental concerns and regulatory pressures. The use of polycarbonate in pharmaceutical packaging offers numerous advantages, including durability, transparency, and chemical resistance. However, the environmental impact of traditional polycarbonate production and disposal has led to a push for more sustainable alternatives and practices.
One of the primary sustainability challenges for polycarbonate packaging is its reliance on bisphenol A (BPA) as a key component. While BPA-based polycarbonates have been widely used, concerns about potential health risks and environmental persistence have prompted research into BPA-free alternatives. Manufacturers are exploring novel monomers and polymerization techniques to create polycarbonates with similar performance characteristics but improved environmental profiles.
Recycling initiatives for polycarbonate packaging have gained traction in recent years. Advanced sorting and processing technologies are being developed to efficiently separate and recycle polycarbonate materials from mixed waste streams. Closed-loop recycling systems, where pharmaceutical packaging is collected, processed, and remanufactured into new packaging, are being implemented by forward-thinking companies to reduce waste and conserve resources.
Bio-based polycarbonates represent another promising avenue for sustainability. These materials, derived from renewable resources such as plant-based feedstocks, offer a reduced carbon footprint compared to traditional petroleum-based polycarbonates. Research is ongoing to improve the performance and cost-effectiveness of bio-based alternatives to make them viable for widespread adoption in pharmaceutical packaging.
Energy efficiency in polycarbonate production is another key focus area for sustainability. Manufacturers are investing in more efficient production processes, including the use of catalysts that enable lower reaction temperatures and pressures. Additionally, the integration of renewable energy sources in manufacturing facilities is helping to reduce the overall carbon footprint of polycarbonate packaging production.
Lifecycle assessment (LCA) tools are increasingly being employed to evaluate the environmental impact of polycarbonate packaging throughout its entire lifecycle. These assessments help identify hotspots for improvement and guide decision-making in packaging design and material selection. By considering factors such as raw material sourcing, production energy, transportation, and end-of-life disposal, companies can make more informed choices to enhance the sustainability of their packaging solutions.
As the pharmaceutical industry continues to prioritize sustainability, collaboration between material scientists, packaging engineers, and environmental experts is driving innovation in polycarbonate packaging. The development of new materials, improved recycling technologies, and more efficient production processes are paving the way for a more sustainable future in pharmaceutical packaging, balancing the need for product protection with environmental stewardship.
One of the primary sustainability challenges for polycarbonate packaging is its reliance on bisphenol A (BPA) as a key component. While BPA-based polycarbonates have been widely used, concerns about potential health risks and environmental persistence have prompted research into BPA-free alternatives. Manufacturers are exploring novel monomers and polymerization techniques to create polycarbonates with similar performance characteristics but improved environmental profiles.
Recycling initiatives for polycarbonate packaging have gained traction in recent years. Advanced sorting and processing technologies are being developed to efficiently separate and recycle polycarbonate materials from mixed waste streams. Closed-loop recycling systems, where pharmaceutical packaging is collected, processed, and remanufactured into new packaging, are being implemented by forward-thinking companies to reduce waste and conserve resources.
Bio-based polycarbonates represent another promising avenue for sustainability. These materials, derived from renewable resources such as plant-based feedstocks, offer a reduced carbon footprint compared to traditional petroleum-based polycarbonates. Research is ongoing to improve the performance and cost-effectiveness of bio-based alternatives to make them viable for widespread adoption in pharmaceutical packaging.
Energy efficiency in polycarbonate production is another key focus area for sustainability. Manufacturers are investing in more efficient production processes, including the use of catalysts that enable lower reaction temperatures and pressures. Additionally, the integration of renewable energy sources in manufacturing facilities is helping to reduce the overall carbon footprint of polycarbonate packaging production.
Lifecycle assessment (LCA) tools are increasingly being employed to evaluate the environmental impact of polycarbonate packaging throughout its entire lifecycle. These assessments help identify hotspots for improvement and guide decision-making in packaging design and material selection. By considering factors such as raw material sourcing, production energy, transportation, and end-of-life disposal, companies can make more informed choices to enhance the sustainability of their packaging solutions.
As the pharmaceutical industry continues to prioritize sustainability, collaboration between material scientists, packaging engineers, and environmental experts is driving innovation in polycarbonate packaging. The development of new materials, improved recycling technologies, and more efficient production processes are paving the way for a more sustainable future in pharmaceutical packaging, balancing the need for product protection with environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!