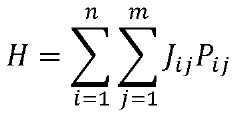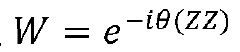Quantum Computing and its Role in Human Genome Decoding
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Computing in Genomics: Background and Objectives
Quantum computing represents a revolutionary paradigm in computational technology, leveraging the principles of quantum mechanics to perform complex calculations at unprecedented speeds. In the context of human genome decoding, this emerging field holds immense potential to transform our understanding of genetic information and accelerate breakthroughs in personalized medicine.
The evolution of genomic research has been closely tied to advancements in computing power. Since the completion of the Human Genome Project in 2003, the volume of genetic data has grown exponentially, presenting significant challenges in storage, processing, and analysis. Traditional computing methods have struggled to keep pace with the increasing complexity of genomic datasets, creating a bottleneck in scientific progress.
Quantum computing offers a promising solution to these challenges by harnessing the unique properties of quantum systems, such as superposition and entanglement. These properties enable quantum computers to perform certain types of calculations exponentially faster than classical computers, making them particularly well-suited for tackling the computational demands of genomic research.
The primary objective of integrating quantum computing into genomics is to accelerate the analysis of vast genetic datasets, enabling researchers to identify patterns, mutations, and correlations that were previously undetectable or too time-consuming to process. This enhanced analytical capability has the potential to revolutionize our understanding of genetic diseases, drug interactions, and personalized treatment strategies.
Furthermore, quantum computing aims to overcome the limitations of current sequencing technologies by improving the accuracy and efficiency of DNA sequencing processes. By leveraging quantum algorithms, researchers hope to develop more sophisticated error correction techniques and enhance the overall fidelity of genomic data.
Another critical goal is to facilitate the development of more accurate predictive models for complex genetic traits and diseases. Quantum machine learning algorithms could potentially uncover intricate relationships within genetic data that are beyond the reach of classical computing methods, leading to more precise risk assessments and treatment recommendations.
As we look towards the future, the integration of quantum computing in genomics is expected to play a pivotal role in advancing personalized medicine. By enabling faster and more comprehensive analysis of individual genetic profiles, quantum technologies could pave the way for tailored therapeutic approaches and preventive strategies based on a person's unique genetic makeup.
In conclusion, the convergence of quantum computing and genomics represents a frontier of scientific exploration with far-reaching implications for human health and our understanding of life itself. As research in this field progresses, we anticipate groundbreaking discoveries that will reshape the landscape of genetic research and medical practice in the coming decades.
The evolution of genomic research has been closely tied to advancements in computing power. Since the completion of the Human Genome Project in 2003, the volume of genetic data has grown exponentially, presenting significant challenges in storage, processing, and analysis. Traditional computing methods have struggled to keep pace with the increasing complexity of genomic datasets, creating a bottleneck in scientific progress.
Quantum computing offers a promising solution to these challenges by harnessing the unique properties of quantum systems, such as superposition and entanglement. These properties enable quantum computers to perform certain types of calculations exponentially faster than classical computers, making them particularly well-suited for tackling the computational demands of genomic research.
The primary objective of integrating quantum computing into genomics is to accelerate the analysis of vast genetic datasets, enabling researchers to identify patterns, mutations, and correlations that were previously undetectable or too time-consuming to process. This enhanced analytical capability has the potential to revolutionize our understanding of genetic diseases, drug interactions, and personalized treatment strategies.
Furthermore, quantum computing aims to overcome the limitations of current sequencing technologies by improving the accuracy and efficiency of DNA sequencing processes. By leveraging quantum algorithms, researchers hope to develop more sophisticated error correction techniques and enhance the overall fidelity of genomic data.
Another critical goal is to facilitate the development of more accurate predictive models for complex genetic traits and diseases. Quantum machine learning algorithms could potentially uncover intricate relationships within genetic data that are beyond the reach of classical computing methods, leading to more precise risk assessments and treatment recommendations.
As we look towards the future, the integration of quantum computing in genomics is expected to play a pivotal role in advancing personalized medicine. By enabling faster and more comprehensive analysis of individual genetic profiles, quantum technologies could pave the way for tailored therapeutic approaches and preventive strategies based on a person's unique genetic makeup.
In conclusion, the convergence of quantum computing and genomics represents a frontier of scientific exploration with far-reaching implications for human health and our understanding of life itself. As research in this field progresses, we anticipate groundbreaking discoveries that will reshape the landscape of genetic research and medical practice in the coming decades.
Market Analysis for Quantum-Enabled Genome Sequencing
The market for quantum-enabled genome sequencing is poised for significant growth, driven by the convergence of quantum computing advancements and the increasing demand for faster, more accurate genomic analysis. This emerging field represents a potential paradigm shift in genomic research and personalized medicine.
Current market estimates suggest that the global genomics market, valued at approximately $23 billion in 2020, is expected to reach $94 billion by 2028, with a compound annual growth rate (CAGR) of around 19%. While quantum-enabled genome sequencing is still in its nascent stages, it is anticipated to capture a growing share of this market as the technology matures.
The primary drivers for this market include the rising prevalence of genetic disorders, increasing investments in genomics research, and the growing adoption of personalized medicine. Healthcare providers, research institutions, and pharmaceutical companies are showing keen interest in quantum-enabled genome sequencing due to its potential to dramatically reduce sequencing time and costs while improving accuracy.
Geographically, North America currently leads the genomics market, followed by Europe and Asia-Pacific. However, the quantum-enabled segment is likely to see more distributed growth, with significant investments and research initiatives emerging in countries with strong quantum computing programs, such as China, Japan, Germany, and Canada.
The market for quantum-enabled genome sequencing is characterized by high barriers to entry due to the complex nature of quantum technology and the substantial investment required. This has led to a landscape dominated by collaborations between established genomics companies, quantum computing firms, and academic institutions.
Key market segments for quantum-enabled genome sequencing include clinical diagnostics, drug discovery and development, agriculture and animal research, and forensics. The clinical diagnostics segment is expected to witness the highest growth rate, driven by the increasing focus on early disease detection and personalized treatment plans.
Despite the promising outlook, several challenges could impact market growth. These include the high cost of quantum computing infrastructure, the need for specialized skills, and regulatory hurdles surrounding the use of quantum technologies in healthcare applications. Additionally, concerns about data privacy and security in quantum systems could influence market adoption rates.
As the technology progresses, we anticipate a gradual shift from traditional sequencing methods to quantum-enabled approaches, particularly for complex genomic analyses that require significant computational power. This transition is likely to create new market opportunities for quantum hardware manufacturers, software developers, and bioinformatics specialists.
Current market estimates suggest that the global genomics market, valued at approximately $23 billion in 2020, is expected to reach $94 billion by 2028, with a compound annual growth rate (CAGR) of around 19%. While quantum-enabled genome sequencing is still in its nascent stages, it is anticipated to capture a growing share of this market as the technology matures.
The primary drivers for this market include the rising prevalence of genetic disorders, increasing investments in genomics research, and the growing adoption of personalized medicine. Healthcare providers, research institutions, and pharmaceutical companies are showing keen interest in quantum-enabled genome sequencing due to its potential to dramatically reduce sequencing time and costs while improving accuracy.
Geographically, North America currently leads the genomics market, followed by Europe and Asia-Pacific. However, the quantum-enabled segment is likely to see more distributed growth, with significant investments and research initiatives emerging in countries with strong quantum computing programs, such as China, Japan, Germany, and Canada.
The market for quantum-enabled genome sequencing is characterized by high barriers to entry due to the complex nature of quantum technology and the substantial investment required. This has led to a landscape dominated by collaborations between established genomics companies, quantum computing firms, and academic institutions.
Key market segments for quantum-enabled genome sequencing include clinical diagnostics, drug discovery and development, agriculture and animal research, and forensics. The clinical diagnostics segment is expected to witness the highest growth rate, driven by the increasing focus on early disease detection and personalized treatment plans.
Despite the promising outlook, several challenges could impact market growth. These include the high cost of quantum computing infrastructure, the need for specialized skills, and regulatory hurdles surrounding the use of quantum technologies in healthcare applications. Additionally, concerns about data privacy and security in quantum systems could influence market adoption rates.
As the technology progresses, we anticipate a gradual shift from traditional sequencing methods to quantum-enabled approaches, particularly for complex genomic analyses that require significant computational power. This transition is likely to create new market opportunities for quantum hardware manufacturers, software developers, and bioinformatics specialists.
Current Challenges in Quantum-Assisted Genomic Analysis
Quantum-assisted genomic analysis faces several significant challenges that hinder its widespread adoption and effectiveness in decoding the human genome. One of the primary obstacles is the current limitation in qubit coherence time. While quantum computers have shown promise in performing complex calculations rapidly, maintaining quantum states for extended periods remains difficult. This constraint restricts the depth and complexity of quantum circuits that can be implemented for genomic analysis, potentially limiting the scope of problems that can be addressed.
Another major challenge lies in the error rates of quantum gates. Despite advancements in quantum error correction techniques, the high error rates of current quantum processors make it challenging to perform long sequences of operations without introducing significant noise. This issue is particularly problematic for genomic analysis, which often requires extensive computational sequences to process large volumes of genetic data accurately.
The scalability of quantum systems presents another hurdle. While small-scale quantum computers have demonstrated potential in solving specific genomic problems, scaling these systems to handle the enormous datasets associated with human genome sequencing remains a formidable task. The need for a large number of high-quality qubits and the associated control systems poses both technical and economic challenges.
Data input and output bottlenecks also pose significant challenges. Efficiently transferring classical genomic data into quantum states and retrieving meaningful results from quantum measurements is a complex process that can limit the overall speed and utility of quantum-assisted genomic analysis. Developing efficient quantum-classical interfaces is crucial for realizing the full potential of quantum computing in genomics.
Furthermore, the development of quantum algorithms specifically tailored for genomic analysis is still in its early stages. While quantum algorithms like Shor's and Grover's have shown theoretical advantages in certain computational tasks, translating these benefits to practical genomic applications requires further research and innovation. The complexity of genomic data and the diverse range of analytical tasks involved in genome decoding necessitate the development of specialized quantum algorithms that can effectively leverage quantum properties.
Lastly, the integration of quantum computing with existing genomic analysis workflows and infrastructure presents logistical and technical challenges. Ensuring compatibility with current bioinformatics tools and databases, as well as training researchers and clinicians in quantum computing principles, are essential steps for the successful implementation of quantum-assisted genomic analysis in real-world scenarios.
Another major challenge lies in the error rates of quantum gates. Despite advancements in quantum error correction techniques, the high error rates of current quantum processors make it challenging to perform long sequences of operations without introducing significant noise. This issue is particularly problematic for genomic analysis, which often requires extensive computational sequences to process large volumes of genetic data accurately.
The scalability of quantum systems presents another hurdle. While small-scale quantum computers have demonstrated potential in solving specific genomic problems, scaling these systems to handle the enormous datasets associated with human genome sequencing remains a formidable task. The need for a large number of high-quality qubits and the associated control systems poses both technical and economic challenges.
Data input and output bottlenecks also pose significant challenges. Efficiently transferring classical genomic data into quantum states and retrieving meaningful results from quantum measurements is a complex process that can limit the overall speed and utility of quantum-assisted genomic analysis. Developing efficient quantum-classical interfaces is crucial for realizing the full potential of quantum computing in genomics.
Furthermore, the development of quantum algorithms specifically tailored for genomic analysis is still in its early stages. While quantum algorithms like Shor's and Grover's have shown theoretical advantages in certain computational tasks, translating these benefits to practical genomic applications requires further research and innovation. The complexity of genomic data and the diverse range of analytical tasks involved in genome decoding necessitate the development of specialized quantum algorithms that can effectively leverage quantum properties.
Lastly, the integration of quantum computing with existing genomic analysis workflows and infrastructure presents logistical and technical challenges. Ensuring compatibility with current bioinformatics tools and databases, as well as training researchers and clinicians in quantum computing principles, are essential steps for the successful implementation of quantum-assisted genomic analysis in real-world scenarios.
Existing Quantum Algorithms for Genome Decoding
01 Quantum computing architectures
Various quantum computing architectures are being developed to improve qubit stability, scalability, and error correction. These include superconducting circuits, trapped ions, topological qubits, and photonic systems. Each architecture has its own advantages and challenges in terms of coherence time, gate fidelity, and integration with classical computing systems.- Quantum computing architectures: This category focuses on the design and implementation of quantum computing systems. It includes innovations in qubit arrangements, circuit layouts, and overall system architecture to improve quantum computation efficiency and scalability.
- Error correction and fault tolerance: This area addresses the challenges of maintaining quantum coherence and reducing errors in quantum computations. It involves techniques for error detection, correction, and fault-tolerant quantum operations to improve the reliability of quantum systems.
- Quantum algorithms and applications: This category covers the development of quantum algorithms for various applications, including optimization, machine learning, cryptography, and simulation of quantum systems. It focuses on leveraging quantum properties to solve complex problems more efficiently than classical computers.
- Quantum-classical hybrid systems: This area explores the integration of quantum and classical computing technologies. It includes methods for interfacing quantum processors with classical systems, optimizing resource allocation, and developing hybrid algorithms that leverage the strengths of both paradigms.
- Quantum hardware and qubit technologies: This category focuses on the physical implementation of quantum bits (qubits) and the development of quantum hardware. It includes advancements in various qubit technologies such as superconducting circuits, trapped ions, photonics, and topological qubits, as well as improvements in quantum gate operations and readout mechanisms.
02 Quantum error correction and fault tolerance
Quantum error correction techniques are crucial for maintaining the integrity of quantum information in the presence of noise and decoherence. This includes the development of quantum error-correcting codes, fault-tolerant quantum gates, and surface code implementations. These methods aim to extend the coherence time of quantum systems and enable large-scale quantum computations.Expand Specific Solutions03 Quantum algorithms and applications
Researchers are developing quantum algorithms that can outperform classical algorithms for specific problems. This includes algorithms for optimization, machine learning, cryptography, and simulation of quantum systems. The focus is on identifying quantum advantage in practical applications across various industries such as finance, chemistry, and materials science.Expand Specific Solutions04 Quantum-classical hybrid systems
Integration of quantum and classical computing systems is being explored to leverage the strengths of both paradigms. This includes the development of hybrid algorithms, quantum-inspired classical algorithms, and interfaces between quantum and classical hardware. Such hybrid approaches aim to enhance the practical utility of quantum computing in the near term.Expand Specific Solutions05 Quantum hardware optimization
Efforts are being made to improve the performance and reliability of quantum hardware components. This includes enhancing qubit coherence times, reducing gate errors, developing more efficient control systems, and improving readout fidelity. Additionally, research is focused on scaling up quantum processors while maintaining or improving their performance metrics.Expand Specific Solutions
Key Players in Quantum Computing and Genomics
The quantum computing landscape in human genome decoding is rapidly evolving, with the industry in its early growth stage. The market size is expanding, driven by increasing investments and potential applications in genomics. Technologically, quantum computing for genome decoding is still in its nascent phase, with varying levels of maturity among key players. Companies like Google, IBM, and D-Wave Systems are at the forefront, developing quantum hardware and algorithms. Origin Quantum and Huawei are making significant strides in China, while startups like IonQ and Universal Quantum are pushing innovation boundaries. Academic institutions such as MIT and Delft University of Technology are contributing crucial research, fostering a competitive and collaborative ecosystem in this transformative field.
Google LLC
Technical Solution: Google's approach to quantum computing in genomics centers around their Sycamore processor and quantum supremacy experiments. They are developing quantum algorithms for simulating molecular systems, which has direct applications in understanding protein folding and gene expression[4]. Google's quantum neural networks show potential for pattern recognition in genomic data, potentially accelerating the identification of disease-causing mutations. Their quantum approximate optimization algorithm (QAOA) is being adapted for genomic sequence alignment problems. Google is also exploring quantum machine learning techniques for predicting gene function and interactions[5]. Their collaboration with pharmaceutical companies aims to use quantum computing for drug discovery based on genomic data. Google's quantum computing platform, Cirq, allows researchers to design and implement quantum algorithms specific to genomic analysis[6].
Strengths: Cutting-edge quantum hardware, strong focus on quantum algorithms, and integration with AI capabilities. Weaknesses: Still in early stages of applying quantum computing to specific genomic problems.
International Business Machines Corp.
Technical Solution: IBM has made significant strides in quantum computing for genomic research. Their quantum systems, such as IBM Q, utilize superconducting qubits to process complex genomic data. IBM's approach focuses on developing quantum algorithms specifically tailored for genomic analysis, including sequence alignment and variant calling. They have demonstrated a 127-qubit processor, Eagle, which shows promise for handling the massive datasets involved in human genome decoding[1]. IBM's quantum computing platform integrates with classical computing resources, allowing for hybrid quantum-classical approaches to genomic data analysis. This integration enables researchers to leverage quantum advantages while still utilizing existing bioinformatics tools[2]. IBM has also developed quantum-inspired algorithms that can run on classical computers, providing immediate benefits to genomic research while full-scale quantum computers are being perfected[3].
Strengths: Advanced quantum hardware, extensive research collaborations, and a hybrid quantum-classical approach. Weaknesses: Still limited in qubit count and coherence times for full-scale genomic analysis.
Breakthrough Quantum Technologies for DNA Sequencing
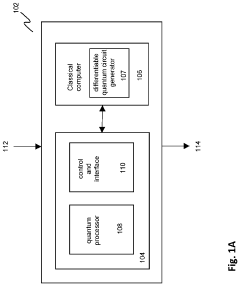
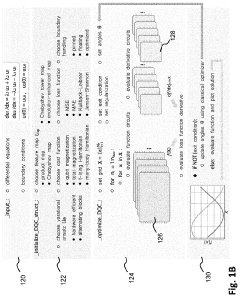
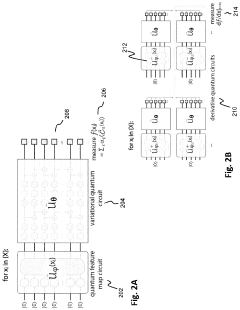
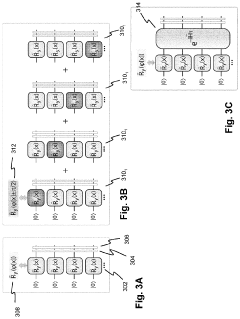
Solving a set of (NON)linear differential equations using a hybrid data processing system comprising a classical computer system and a quantum computer system
PatentPendingUS20230418896A1
Innovation
- A quantum-classical hybrid system that uses differentiable quantum circuits to encode and solve differential equations, allowing for analytical derivatives and efficient encoding of solutions, compatible with near-term quantum hardware, and extensible to fault-tolerant systems, by employing quantum feature maps and variational circuits for optimization.
Quantum computing system and method for time evolution of bipartite hamiltonians on a lattice
PatentWO2021247125A2
Innovation
- A method for evolving a lattice of qubits in a quantum computer by applying quantum gates in parallel to create entangled pairs and swapping qubits according to specific criteria, ensuring full connectivity between bipartitions, thereby reducing the number of operations required for time evolution under a Hamiltonian.
Ethical Implications of Quantum-Enhanced Genomics
The advent of quantum computing in genomics research raises significant ethical concerns that demand careful consideration. As quantum-enhanced genomic analysis becomes more powerful and accessible, issues of privacy, consent, and data security take on new dimensions. The ability to process vast amounts of genetic data at unprecedented speeds could lead to breakthroughs in personalized medicine and disease prevention, but it also increases the risk of unauthorized access to sensitive genetic information.
One of the primary ethical challenges is maintaining individual privacy in an era of quantum-enhanced genomic analysis. The enhanced processing capabilities of quantum computers may make it easier to identify individuals from anonymized genetic data sets, potentially compromising the confidentiality promised to research participants. This raises questions about the adequacy of current consent processes and whether individuals can truly understand the long-term implications of sharing their genetic information in a quantum computing era.
The potential for discrimination based on genetic information becomes more acute with quantum-enhanced genomics. Employers, insurers, and other entities may gain access to detailed genetic profiles, leading to unfair treatment or denial of services based on genetic predispositions. Safeguarding against such misuse of genetic information will require robust legal and ethical frameworks that keep pace with technological advancements.
Quantum computing's role in genomics also raises concerns about global equity and access to healthcare benefits. As quantum-enhanced genomic technologies become more sophisticated, there is a risk of widening the gap between those who can afford advanced genetic treatments and those who cannot. This could exacerbate existing health disparities and create new forms of genetic inequality.
The rapid advancement of quantum-enhanced genomics may outpace our ability to fully understand and regulate its implications. There is a need for ongoing ethical review and public discourse to ensure that the benefits of these technologies are realized while minimizing potential harms. This includes developing guidelines for responsible research practices, establishing international agreements on genetic data sharing, and educating the public about the implications of quantum-enhanced genomic analysis.
Moreover, the potential for quantum computing to break current encryption methods poses a significant threat to the long-term security of genetic data. Ensuring the confidentiality of genomic information over extended periods becomes increasingly challenging, necessitating the development of quantum-resistant encryption methods for genetic data storage and transmission.
One of the primary ethical challenges is maintaining individual privacy in an era of quantum-enhanced genomic analysis. The enhanced processing capabilities of quantum computers may make it easier to identify individuals from anonymized genetic data sets, potentially compromising the confidentiality promised to research participants. This raises questions about the adequacy of current consent processes and whether individuals can truly understand the long-term implications of sharing their genetic information in a quantum computing era.
The potential for discrimination based on genetic information becomes more acute with quantum-enhanced genomics. Employers, insurers, and other entities may gain access to detailed genetic profiles, leading to unfair treatment or denial of services based on genetic predispositions. Safeguarding against such misuse of genetic information will require robust legal and ethical frameworks that keep pace with technological advancements.
Quantum computing's role in genomics also raises concerns about global equity and access to healthcare benefits. As quantum-enhanced genomic technologies become more sophisticated, there is a risk of widening the gap between those who can afford advanced genetic treatments and those who cannot. This could exacerbate existing health disparities and create new forms of genetic inequality.
The rapid advancement of quantum-enhanced genomics may outpace our ability to fully understand and regulate its implications. There is a need for ongoing ethical review and public discourse to ensure that the benefits of these technologies are realized while minimizing potential harms. This includes developing guidelines for responsible research practices, establishing international agreements on genetic data sharing, and educating the public about the implications of quantum-enhanced genomic analysis.
Moreover, the potential for quantum computing to break current encryption methods poses a significant threat to the long-term security of genetic data. Ensuring the confidentiality of genomic information over extended periods becomes increasingly challenging, necessitating the development of quantum-resistant encryption methods for genetic data storage and transmission.
Regulatory Framework for Quantum Genomic Technologies
The regulatory framework for quantum genomic technologies is a complex and evolving landscape that requires careful consideration of ethical, legal, and societal implications. As quantum computing advances and its potential applications in human genome decoding become more apparent, governments and international bodies are working to establish guidelines and regulations to ensure responsible development and use of these technologies.
One of the primary concerns addressed by regulatory frameworks is data privacy and security. Quantum computing's ability to process vast amounts of genomic data at unprecedented speeds raises questions about the protection of sensitive genetic information. Regulatory bodies are implementing stringent data protection measures, including encryption standards specifically designed to withstand potential quantum attacks.
Ethical considerations form another crucial aspect of the regulatory framework. Guidelines are being developed to address issues such as genetic discrimination, informed consent for genomic research, and the potential for unintended consequences of genome editing enabled by quantum computing. These regulations aim to strike a balance between scientific progress and the protection of individual rights and societal values.
Intellectual property rights in the field of quantum genomics are also being carefully examined. Patent offices and regulatory agencies are working to establish clear guidelines for what constitutes patentable innovations in this emerging field, considering both the quantum computing aspects and the genomic applications.
International cooperation plays a significant role in shaping the regulatory landscape. Organizations such as the World Health Organization (WHO) and the Organisation for Economic Co-operation and Development (OECD) are facilitating discussions and developing recommendations for harmonized approaches to regulating quantum genomic technologies across different countries.
Regulatory bodies are also addressing the potential dual-use nature of quantum genomic technologies. Frameworks are being put in place to prevent misuse of these technologies for biological warfare or other malicious purposes while still allowing for legitimate scientific research and medical applications.
As the field rapidly evolves, regulatory agencies are adopting adaptive approaches to keep pace with technological advancements. This includes the establishment of expert advisory committees, regular review processes, and mechanisms for incorporating new scientific findings into existing regulatory frameworks.
One of the primary concerns addressed by regulatory frameworks is data privacy and security. Quantum computing's ability to process vast amounts of genomic data at unprecedented speeds raises questions about the protection of sensitive genetic information. Regulatory bodies are implementing stringent data protection measures, including encryption standards specifically designed to withstand potential quantum attacks.
Ethical considerations form another crucial aspect of the regulatory framework. Guidelines are being developed to address issues such as genetic discrimination, informed consent for genomic research, and the potential for unintended consequences of genome editing enabled by quantum computing. These regulations aim to strike a balance between scientific progress and the protection of individual rights and societal values.
Intellectual property rights in the field of quantum genomics are also being carefully examined. Patent offices and regulatory agencies are working to establish clear guidelines for what constitutes patentable innovations in this emerging field, considering both the quantum computing aspects and the genomic applications.
International cooperation plays a significant role in shaping the regulatory landscape. Organizations such as the World Health Organization (WHO) and the Organisation for Economic Co-operation and Development (OECD) are facilitating discussions and developing recommendations for harmonized approaches to regulating quantum genomic technologies across different countries.
Regulatory bodies are also addressing the potential dual-use nature of quantum genomic technologies. Frameworks are being put in place to prevent misuse of these technologies for biological warfare or other malicious purposes while still allowing for legitimate scientific research and medical applications.
As the field rapidly evolves, regulatory agencies are adopting adaptive approaches to keep pace with technological advancements. This includes the establishment of expert advisory committees, regular review processes, and mechanisms for incorporating new scientific findings into existing regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!