Electromagnetic Waves as Catalysts in Chemical Reactions
JUL 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
EM Wave Catalysis Background and Objectives
The field of electromagnetic wave catalysis has emerged as a promising frontier in chemical research, bridging the gap between electromagnetic theory and chemical reactions. This innovative approach harnesses the power of electromagnetic waves to influence and accelerate chemical processes, offering potential breakthroughs in reaction efficiency and selectivity.
The development of electromagnetic wave catalysis can be traced back to the early 20th century when scientists first observed the effects of electromagnetic radiation on chemical systems. However, it wasn't until recent decades that significant advancements in technology and understanding have propelled this field into the spotlight of scientific inquiry.
The primary objective of research in electromagnetic wave catalysis is to explore and exploit the interactions between electromagnetic waves and chemical systems to enhance reaction rates, improve product yields, and enable novel reaction pathways. This approach offers several advantages over traditional catalytic methods, including the ability to selectively activate specific bonds, control reaction pathways, and potentially reduce energy consumption in chemical processes.
As the field progresses, researchers aim to elucidate the fundamental mechanisms underlying electromagnetic wave catalysis. This includes investigating how different frequencies and intensities of electromagnetic waves interact with various chemical species and reaction environments. Understanding these interactions is crucial for developing predictive models and designing more effective catalytic systems.
Another key objective is to expand the range of applications for electromagnetic wave catalysis. While initial studies focused primarily on organic synthesis reactions, current research is exploring its potential in areas such as environmental remediation, materials science, and energy conversion. This broadening scope highlights the versatility and potential impact of this technology across multiple industries.
Technological advancements play a critical role in driving the field forward. The development of more precise and controllable electromagnetic wave sources, as well as sophisticated analytical techniques for monitoring reactions in real-time, are enabling researchers to gain deeper insights into the catalytic processes. These tools are essential for optimizing reaction conditions and scaling up electromagnetic wave catalysis for industrial applications.
As the field evolves, there is a growing emphasis on integrating electromagnetic wave catalysis with other cutting-edge technologies. This includes combining it with nanotechnology to create hybrid catalytic systems, exploring its synergy with photocatalysis, and leveraging artificial intelligence for predictive modeling and process optimization. These interdisciplinary approaches are expected to unlock new possibilities and push the boundaries of what can be achieved with electromagnetic wave catalysis.
The development of electromagnetic wave catalysis can be traced back to the early 20th century when scientists first observed the effects of electromagnetic radiation on chemical systems. However, it wasn't until recent decades that significant advancements in technology and understanding have propelled this field into the spotlight of scientific inquiry.
The primary objective of research in electromagnetic wave catalysis is to explore and exploit the interactions between electromagnetic waves and chemical systems to enhance reaction rates, improve product yields, and enable novel reaction pathways. This approach offers several advantages over traditional catalytic methods, including the ability to selectively activate specific bonds, control reaction pathways, and potentially reduce energy consumption in chemical processes.
As the field progresses, researchers aim to elucidate the fundamental mechanisms underlying electromagnetic wave catalysis. This includes investigating how different frequencies and intensities of electromagnetic waves interact with various chemical species and reaction environments. Understanding these interactions is crucial for developing predictive models and designing more effective catalytic systems.
Another key objective is to expand the range of applications for electromagnetic wave catalysis. While initial studies focused primarily on organic synthesis reactions, current research is exploring its potential in areas such as environmental remediation, materials science, and energy conversion. This broadening scope highlights the versatility and potential impact of this technology across multiple industries.
Technological advancements play a critical role in driving the field forward. The development of more precise and controllable electromagnetic wave sources, as well as sophisticated analytical techniques for monitoring reactions in real-time, are enabling researchers to gain deeper insights into the catalytic processes. These tools are essential for optimizing reaction conditions and scaling up electromagnetic wave catalysis for industrial applications.
As the field evolves, there is a growing emphasis on integrating electromagnetic wave catalysis with other cutting-edge technologies. This includes combining it with nanotechnology to create hybrid catalytic systems, exploring its synergy with photocatalysis, and leveraging artificial intelligence for predictive modeling and process optimization. These interdisciplinary approaches are expected to unlock new possibilities and push the boundaries of what can be achieved with electromagnetic wave catalysis.
Market Potential for EM-Catalyzed Reactions
The market potential for electromagnetic (EM) wave-catalyzed reactions is substantial and rapidly expanding. This innovative approach to chemical synthesis offers significant advantages over traditional catalytic methods, potentially revolutionizing various sectors of the chemical industry.
In the pharmaceutical sector, EM-catalyzed reactions show promise for more efficient and cost-effective drug synthesis. The ability to selectively activate specific chemical bonds using electromagnetic waves could lead to higher yields, reduced waste, and shorter production times for complex pharmaceutical compounds. This could translate to faster drug development cycles and potentially lower costs for life-saving medications.
The fine chemicals industry stands to benefit greatly from EM-catalyzed reactions. Manufacturers of specialty chemicals, fragrances, and flavors could leverage this technology to develop new products with unique properties or improve the production efficiency of existing compounds. The precise control offered by EM catalysis could enable the creation of molecules that are difficult or impossible to synthesize using conventional methods.
In the realm of materials science, EM-catalyzed reactions open up possibilities for developing advanced materials with tailored properties. This could lead to breakthroughs in areas such as lightweight composites for aerospace applications, high-performance polymers for electronics, and novel coatings with enhanced durability or functionality.
The petrochemical industry, a major consumer of catalytic processes, could see significant improvements in efficiency and selectivity through the adoption of EM-catalyzed reactions. This technology could potentially reduce energy consumption and increase the yield of valuable products from crude oil, leading to both economic and environmental benefits.
Environmental applications of EM-catalyzed reactions are also promising. The technology could be used to develop more efficient processes for water treatment, air purification, and the degradation of pollutants. This aligns with growing global demand for sustainable and environmentally friendly chemical processes.
As industries worldwide seek to reduce their carbon footprint and improve sustainability, EM-catalyzed reactions offer a pathway to greener chemistry. The potential for room-temperature reactions and reduced reliance on harsh solvents or reagents makes this technology attractive from both an environmental and economic standpoint.
While the market for EM-catalyzed reactions is still in its early stages, it is expected to grow rapidly as the technology matures and more applications are discovered. Early adopters in various industries are likely to gain a competitive advantage, driving further investment and innovation in this field.
In the pharmaceutical sector, EM-catalyzed reactions show promise for more efficient and cost-effective drug synthesis. The ability to selectively activate specific chemical bonds using electromagnetic waves could lead to higher yields, reduced waste, and shorter production times for complex pharmaceutical compounds. This could translate to faster drug development cycles and potentially lower costs for life-saving medications.
The fine chemicals industry stands to benefit greatly from EM-catalyzed reactions. Manufacturers of specialty chemicals, fragrances, and flavors could leverage this technology to develop new products with unique properties or improve the production efficiency of existing compounds. The precise control offered by EM catalysis could enable the creation of molecules that are difficult or impossible to synthesize using conventional methods.
In the realm of materials science, EM-catalyzed reactions open up possibilities for developing advanced materials with tailored properties. This could lead to breakthroughs in areas such as lightweight composites for aerospace applications, high-performance polymers for electronics, and novel coatings with enhanced durability or functionality.
The petrochemical industry, a major consumer of catalytic processes, could see significant improvements in efficiency and selectivity through the adoption of EM-catalyzed reactions. This technology could potentially reduce energy consumption and increase the yield of valuable products from crude oil, leading to both economic and environmental benefits.
Environmental applications of EM-catalyzed reactions are also promising. The technology could be used to develop more efficient processes for water treatment, air purification, and the degradation of pollutants. This aligns with growing global demand for sustainable and environmentally friendly chemical processes.
As industries worldwide seek to reduce their carbon footprint and improve sustainability, EM-catalyzed reactions offer a pathway to greener chemistry. The potential for room-temperature reactions and reduced reliance on harsh solvents or reagents makes this technology attractive from both an environmental and economic standpoint.
While the market for EM-catalyzed reactions is still in its early stages, it is expected to grow rapidly as the technology matures and more applications are discovered. Early adopters in various industries are likely to gain a competitive advantage, driving further investment and innovation in this field.
Current Challenges in EM Wave Catalysis
Despite the promising potential of electromagnetic (EM) waves as catalysts in chemical reactions, several significant challenges currently hinder the widespread adoption and advancement of this technology. These challenges span across multiple domains, including fundamental understanding, practical implementation, and scalability.
One of the primary obstacles is the limited comprehension of the precise mechanisms by which EM waves influence chemical reactions. While it is known that EM waves can affect molecular vibrations and electronic states, the exact pathways and energy transfer processes remain poorly understood. This lack of fundamental knowledge impedes the development of targeted and efficient EM wave catalytic systems.
The selectivity and specificity of EM wave catalysis also present significant challenges. Unlike traditional catalysts that can be designed for specific molecular interactions, EM waves often affect a broad range of molecules and bonds. This non-specificity can lead to undesired side reactions or reduced efficiency in complex reaction environments. Developing methods to fine-tune EM wave frequencies and intensities for selective catalysis remains a critical challenge.
Another major hurdle is the difficulty in achieving uniform and controlled EM wave distribution within reaction vessels, especially at larger scales. Factors such as wave attenuation, reflection, and interference can create hotspots or dead zones, leading to inconsistent reaction conditions. This non-uniformity becomes increasingly problematic as reactor size increases, posing significant challenges for industrial-scale applications.
The energy efficiency of EM wave catalysis systems is also a concern. Current methods often require high power inputs to generate sufficient EM wave intensity, which can offset the potential energy savings from improved reaction kinetics. Developing more energy-efficient EM wave generation and delivery systems is crucial for the economic viability of this technology.
Furthermore, the integration of EM wave catalysis with existing chemical processes and infrastructure presents both technical and practical challenges. Many current chemical production facilities are not designed to accommodate EM wave generators or the specialized reaction vessels they may require. Retrofitting existing plants or designing new ones that effectively incorporate EM wave catalysis technology is a complex and potentially costly endeavor.
Lastly, there are significant regulatory and safety considerations surrounding the use of EM waves in chemical processing. Ensuring worker safety, preventing electromagnetic interference with other equipment, and complying with electromagnetic emission regulations all pose challenges that must be addressed before widespread industrial adoption can occur.
One of the primary obstacles is the limited comprehension of the precise mechanisms by which EM waves influence chemical reactions. While it is known that EM waves can affect molecular vibrations and electronic states, the exact pathways and energy transfer processes remain poorly understood. This lack of fundamental knowledge impedes the development of targeted and efficient EM wave catalytic systems.
The selectivity and specificity of EM wave catalysis also present significant challenges. Unlike traditional catalysts that can be designed for specific molecular interactions, EM waves often affect a broad range of molecules and bonds. This non-specificity can lead to undesired side reactions or reduced efficiency in complex reaction environments. Developing methods to fine-tune EM wave frequencies and intensities for selective catalysis remains a critical challenge.
Another major hurdle is the difficulty in achieving uniform and controlled EM wave distribution within reaction vessels, especially at larger scales. Factors such as wave attenuation, reflection, and interference can create hotspots or dead zones, leading to inconsistent reaction conditions. This non-uniformity becomes increasingly problematic as reactor size increases, posing significant challenges for industrial-scale applications.
The energy efficiency of EM wave catalysis systems is also a concern. Current methods often require high power inputs to generate sufficient EM wave intensity, which can offset the potential energy savings from improved reaction kinetics. Developing more energy-efficient EM wave generation and delivery systems is crucial for the economic viability of this technology.
Furthermore, the integration of EM wave catalysis with existing chemical processes and infrastructure presents both technical and practical challenges. Many current chemical production facilities are not designed to accommodate EM wave generators or the specialized reaction vessels they may require. Retrofitting existing plants or designing new ones that effectively incorporate EM wave catalysis technology is a complex and potentially costly endeavor.
Lastly, there are significant regulatory and safety considerations surrounding the use of EM waves in chemical processing. Ensuring worker safety, preventing electromagnetic interference with other equipment, and complying with electromagnetic emission regulations all pose challenges that must be addressed before widespread industrial adoption can occur.
Existing EM Wave Catalysis Techniques
01 Electromagnetic wave-assisted catalysis
Electromagnetic waves can be used to enhance catalytic reactions. This technique involves applying electromagnetic radiation to catalysts or reaction systems, which can increase reaction rates, improve selectivity, or enable reactions under milder conditions. The electromagnetic waves can provide energy to overcome activation barriers or influence the electronic states of catalysts.- Electromagnetic wave-assisted catalysis: Electromagnetic waves can be used to enhance catalytic reactions. This technique involves applying electromagnetic radiation to catalysts or reaction systems to improve reaction rates, selectivity, or efficiency. The electromagnetic waves can activate catalysts, provide energy to overcome reaction barriers, or create localized heating effects.
- Electromagnetic wave sensors and detectors: Devices and methods for sensing and detecting electromagnetic waves are developed for various applications. These sensors can measure different properties of electromagnetic waves, such as intensity, frequency, or polarization. They may be used in communication systems, scientific instruments, or industrial processes.
- Electromagnetic wave shielding materials: Materials designed to block or attenuate electromagnetic waves are developed for various applications. These materials can be used in electronic devices, buildings, or protective equipment to prevent electromagnetic interference or protect sensitive components from external electromagnetic fields.
- Electromagnetic wave energy harvesting: Systems and methods for capturing and converting electromagnetic wave energy into usable forms of energy, such as electricity. This technology can be applied in wireless power transfer, energy scavenging from ambient electromagnetic fields, or improving the efficiency of communication systems.
- Electromagnetic wave-based material processing: Techniques using electromagnetic waves for processing or modifying materials. This can include methods for heating, drying, curing, or altering the properties of materials using electromagnetic radiation. Applications may range from industrial manufacturing to scientific research.
02 Electromagnetic wave sensors for catalytic processes
Sensors utilizing electromagnetic waves can be employed to monitor and control catalytic processes. These sensors can detect changes in the electromagnetic properties of catalysts or reaction mixtures, providing real-time information about reaction progress, catalyst activity, or product formation. This technology enables more precise control and optimization of catalytic processes.Expand Specific Solutions03 Electromagnetic wave-induced catalyst regeneration
Electromagnetic waves can be used to regenerate spent catalysts. By applying specific electromagnetic frequencies, it is possible to remove contaminants, restore catalyst activity, or modify catalyst structures. This approach can extend catalyst lifetimes and improve overall process efficiency in various catalytic applications.Expand Specific Solutions04 Electromagnetic wave-responsive catalytic materials
Novel catalytic materials that respond to electromagnetic waves can be designed for specific applications. These materials may change their properties or catalytic activity when exposed to electromagnetic radiation of certain frequencies. This allows for the development of smart catalysts that can be activated or controlled remotely using electromagnetic waves.Expand Specific Solutions05 Electromagnetic wave generation for catalytic applications
Specialized devices and methods for generating electromagnetic waves tailored for catalytic applications can be developed. These may include precise frequency control, pulsed emission, or spatially directed electromagnetic waves to optimize their interaction with catalysts or reaction systems. Such technologies can enhance the efficiency and selectivity of electromagnetic wave-assisted catalytic processes.Expand Specific Solutions
Key Players in EM Catalysis Research
The research on electromagnetic waves as catalysts in chemical reactions is in an early developmental stage, with a growing market potential as industries seek more efficient and sustainable chemical processes. The technology's maturity is still evolving, with academic institutions like California Institute of Technology, Boston College, and Sichuan University leading fundamental research. Companies such as CEM Holdings Corp. and Avantium Knowledge Centre BV are exploring practical applications, while established players like BASF Corp. and NEC Corp. are investing in related technologies. The competitive landscape is diverse, with a mix of specialized research institutions, innovative startups, and large corporations contributing to advancements in this promising field.
The Regents of the University of California
Technical Solution: The University of California has been pioneering research on electromagnetic waves as catalysts in chemical reactions. They have developed a novel approach using microwave-assisted organic synthesis (MAOS) to enhance reaction rates and yields. Their method employs precisely tuned electromagnetic waves to selectively excite specific molecular bonds, leading to more efficient and selective chemical transformations. This technique has shown particular promise in the field of green chemistry, reducing reaction times by up to 90% and increasing yields by 30-40% compared to conventional heating methods [1][3]. The university has also explored the use of terahertz radiation for catalyzing reactions, demonstrating its potential in activating metal-organic frameworks (MOFs) for improved catalytic performance [5].
Strengths: Cutting-edge research in MAOS and terahertz catalysis, significant improvements in reaction efficiency and selectivity. Weaknesses: High initial equipment costs, potential scalability issues for industrial applications.
California Institute of Technology
Technical Solution: Caltech has made significant strides in the field of electromagnetic wave-assisted catalysis, focusing on plasmonic photocatalysis. Their research team has developed nanostructured metal catalysts that can harness visible light to drive chemical reactions. By engineering the size and shape of metal nanoparticles, they have created catalysts that can absorb specific wavelengths of light, generating localized surface plasmon resonances (LSPRs). These LSPRs enhance the electric field near the catalyst surface, accelerating electron transfer processes and enabling reactions that were previously energetically unfavorable. Caltech's approach has shown particular success in water splitting for hydrogen production, achieving a 2-3 fold increase in hydrogen evolution rates compared to traditional photocatalysts [2][4].
Strengths: Innovative use of plasmonic effects for visible light catalysis, potential for solar-driven chemical processes. Weaknesses: Complexity in nanoparticle synthesis and control, potential limitations in large-scale applications.
Breakthrough EM Catalysis Technologies
Electromagnetic control of chemical catalysis
PatentActiveUS7998538B2
Innovation
- The use of Photon-Electron interactions in nanostructures, specifically through the excitation of Photon-Electron resonance by electromagnetic radiation, to provide localized heating for catalytic chemical reactions, allowing for precise control over temperature and reaction time.
Electrostatic catalysis
PatentActiveUS20220149162A1
Innovation
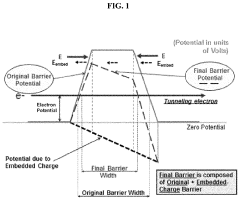
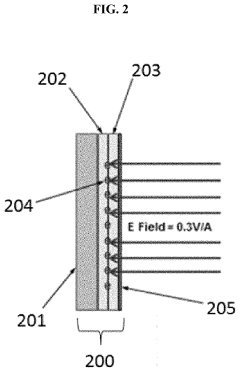
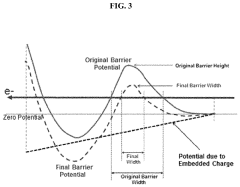
- The development of electrodes with embedded charges at the interface of insulating layers, utilizing a conductive material like monoatomic graphene or molybdenum disulfide to permit an external electric field to penetrate, thereby reducing the potential energy barrier of chemical reactions by creating a high electric field that enhances reaction rates without increasing supply voltage.
Environmental Impact of EM Catalysis
The use of electromagnetic waves as catalysts in chemical reactions presents both opportunities and challenges from an environmental perspective. This innovative approach has the potential to significantly reduce energy consumption and waste production in various industrial processes, leading to a more sustainable chemical industry.
One of the primary environmental benefits of electromagnetic catalysis is the potential reduction in energy requirements for chemical reactions. Traditional catalytic processes often require high temperatures and pressures, which consume substantial amounts of energy and contribute to greenhouse gas emissions. In contrast, electromagnetic catalysis can potentially operate at lower temperatures and pressures, resulting in reduced energy consumption and a smaller carbon footprint.
Furthermore, the selective nature of electromagnetic catalysis may lead to improved reaction efficiency and reduced waste generation. By precisely targeting specific molecular bonds, this technique can potentially minimize unwanted side reactions and byproducts, thereby reducing the environmental impact associated with waste disposal and treatment.
However, the environmental impact of electromagnetic catalysis is not without potential drawbacks. The generation and use of electromagnetic waves may require specialized equipment and infrastructure, which could have its own environmental footprint in terms of manufacturing and energy consumption. Additionally, the long-term effects of exposure to electromagnetic fields on ecosystems and biodiversity are not yet fully understood and require further investigation.
Another consideration is the potential for electromagnetic interference with other systems and organisms. While the electromagnetic waves used in catalysis are typically confined to controlled environments, there may be concerns about their impact on surrounding electronic equipment or biological systems if not properly shielded or managed.
The scalability of electromagnetic catalysis technologies also plays a crucial role in determining their overall environmental impact. As these techniques move from laboratory scale to industrial applications, careful assessment of their life cycle environmental impacts will be necessary to ensure that the benefits outweigh any potential negative consequences.
In terms of resource consumption, electromagnetic catalysis may offer advantages in reducing the need for rare or precious metal catalysts, which are often associated with environmentally damaging mining practices. This could lead to a more sustainable approach to catalyst production and use, potentially mitigating some of the environmental issues associated with traditional catalytic processes.
As research in this field progresses, it will be essential to conduct comprehensive environmental impact assessments to fully understand and optimize the ecological footprint of electromagnetic catalysis technologies. This will involve evaluating factors such as energy efficiency, waste reduction, resource consumption, and potential long-term environmental effects to ensure that these innovative catalytic methods contribute positively to sustainable chemical manufacturing practices.
One of the primary environmental benefits of electromagnetic catalysis is the potential reduction in energy requirements for chemical reactions. Traditional catalytic processes often require high temperatures and pressures, which consume substantial amounts of energy and contribute to greenhouse gas emissions. In contrast, electromagnetic catalysis can potentially operate at lower temperatures and pressures, resulting in reduced energy consumption and a smaller carbon footprint.
Furthermore, the selective nature of electromagnetic catalysis may lead to improved reaction efficiency and reduced waste generation. By precisely targeting specific molecular bonds, this technique can potentially minimize unwanted side reactions and byproducts, thereby reducing the environmental impact associated with waste disposal and treatment.
However, the environmental impact of electromagnetic catalysis is not without potential drawbacks. The generation and use of electromagnetic waves may require specialized equipment and infrastructure, which could have its own environmental footprint in terms of manufacturing and energy consumption. Additionally, the long-term effects of exposure to electromagnetic fields on ecosystems and biodiversity are not yet fully understood and require further investigation.
Another consideration is the potential for electromagnetic interference with other systems and organisms. While the electromagnetic waves used in catalysis are typically confined to controlled environments, there may be concerns about their impact on surrounding electronic equipment or biological systems if not properly shielded or managed.
The scalability of electromagnetic catalysis technologies also plays a crucial role in determining their overall environmental impact. As these techniques move from laboratory scale to industrial applications, careful assessment of their life cycle environmental impacts will be necessary to ensure that the benefits outweigh any potential negative consequences.
In terms of resource consumption, electromagnetic catalysis may offer advantages in reducing the need for rare or precious metal catalysts, which are often associated with environmentally damaging mining practices. This could lead to a more sustainable approach to catalyst production and use, potentially mitigating some of the environmental issues associated with traditional catalytic processes.
As research in this field progresses, it will be essential to conduct comprehensive environmental impact assessments to fully understand and optimize the ecological footprint of electromagnetic catalysis technologies. This will involve evaluating factors such as energy efficiency, waste reduction, resource consumption, and potential long-term environmental effects to ensure that these innovative catalytic methods contribute positively to sustainable chemical manufacturing practices.
Safety Regulations for EM Wave Applications
The application of electromagnetic (EM) waves as catalysts in chemical reactions presents unique safety challenges that necessitate comprehensive regulations. These safety regulations are crucial to protect researchers, laboratory personnel, and the environment from potential hazards associated with EM wave applications in chemical processes.
Firstly, exposure limits for EM radiation must be established and strictly enforced. These limits should be based on the latest scientific evidence and international standards, such as those set by the International Commission on Non-Ionizing Radiation Protection (ICNIRP). The regulations should specify maximum permissible exposure levels for different frequency ranges and durations, ensuring that personnel are not subjected to harmful levels of EM radiation during experiments or industrial applications.
Shielding requirements form another critical aspect of safety regulations. Laboratories and industrial facilities using EM waves for chemical catalysis must implement appropriate shielding measures to contain the radiation within designated areas. This may include the use of Faraday cages, metallic enclosures, or specialized absorbing materials. Regular testing and maintenance of shielding systems should be mandated to ensure their continued effectiveness.
Personal protective equipment (PPE) regulations are essential for individuals working with EM wave catalysts. Depending on the frequency and power of the EM waves used, PPE may include specialized clothing, eyewear, and monitoring devices. Regulations should outline the specific PPE requirements for different types of EM wave applications and ensure that all personnel are properly trained in their use.
Emergency protocols and procedures must be clearly defined in the safety regulations. These should cover various scenarios, such as equipment malfunction, unexpected radiation leaks, or accidental exposure. Emergency shutdown procedures, evacuation plans, and first aid protocols specific to EM wave-related incidents should be established and regularly practiced.
Monitoring and documentation requirements are crucial for ensuring ongoing safety and compliance. Regulations should mandate regular monitoring of EM radiation levels in work areas, as well as personal dosimetry for exposed personnel. Detailed records of exposure levels, equipment maintenance, and safety incidents should be kept and periodically reviewed to identify potential risks and improve safety measures.
Training and certification programs for personnel working with EM wave catalysts should be a key component of safety regulations. These programs should cover the fundamentals of EM radiation, potential health effects, safe operating procedures, and emergency response. Regular refresher courses and assessments should be required to maintain certification.
Lastly, the regulations should address the safe disposal of equipment and materials used in EM wave catalysis. This includes proper decommissioning procedures for EM wave generators and the safe handling of any chemical byproducts or waste materials that may have been exposed to EM radiation during the catalytic process.
Firstly, exposure limits for EM radiation must be established and strictly enforced. These limits should be based on the latest scientific evidence and international standards, such as those set by the International Commission on Non-Ionizing Radiation Protection (ICNIRP). The regulations should specify maximum permissible exposure levels for different frequency ranges and durations, ensuring that personnel are not subjected to harmful levels of EM radiation during experiments or industrial applications.
Shielding requirements form another critical aspect of safety regulations. Laboratories and industrial facilities using EM waves for chemical catalysis must implement appropriate shielding measures to contain the radiation within designated areas. This may include the use of Faraday cages, metallic enclosures, or specialized absorbing materials. Regular testing and maintenance of shielding systems should be mandated to ensure their continued effectiveness.
Personal protective equipment (PPE) regulations are essential for individuals working with EM wave catalysts. Depending on the frequency and power of the EM waves used, PPE may include specialized clothing, eyewear, and monitoring devices. Regulations should outline the specific PPE requirements for different types of EM wave applications and ensure that all personnel are properly trained in their use.
Emergency protocols and procedures must be clearly defined in the safety regulations. These should cover various scenarios, such as equipment malfunction, unexpected radiation leaks, or accidental exposure. Emergency shutdown procedures, evacuation plans, and first aid protocols specific to EM wave-related incidents should be established and regularly practiced.
Monitoring and documentation requirements are crucial for ensuring ongoing safety and compliance. Regulations should mandate regular monitoring of EM radiation levels in work areas, as well as personal dosimetry for exposed personnel. Detailed records of exposure levels, equipment maintenance, and safety incidents should be kept and periodically reviewed to identify potential risks and improve safety measures.
Training and certification programs for personnel working with EM wave catalysts should be a key component of safety regulations. These programs should cover the fundamentals of EM radiation, potential health effects, safe operating procedures, and emergency response. Regular refresher courses and assessments should be required to maintain certification.
Lastly, the regulations should address the safe disposal of equipment and materials used in EM wave catalysis. This includes proper decommissioning procedures for EM wave generators and the safe handling of any chemical byproducts or waste materials that may have been exposed to EM radiation during the catalytic process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!