Polycarbonate for Cutting-Edge Biocompatibility
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polycarbonate Biocompatibility Evolution and Objectives
Polycarbonate has undergone a remarkable evolution in the field of biocompatibility since its introduction in the 1950s. Initially developed as a durable, transparent thermoplastic, polycarbonate's potential for biomedical applications was quickly recognized due to its excellent mechanical properties and ease of sterilization. The journey of polycarbonate in biocompatibility research has been marked by continuous improvements and adaptations to meet the stringent requirements of medical devices and implants.
In the early stages, polycarbonate was primarily used in non-implantable medical devices due to concerns about its long-term biocompatibility. However, as research progressed, scientists began to explore surface modification techniques and composite formulations to enhance its biocompatibility profile. The 1980s and 1990s saw significant advancements in this area, with the development of plasma treatment methods and the incorporation of bioactive compounds to improve cell adhesion and reduce inflammatory responses.
The turn of the millennium brought about a new era in polycarbonate biocompatibility research, driven by the growing demand for advanced biomaterials in tissue engineering and regenerative medicine. Researchers began to focus on creating polycarbonate-based scaffolds with controlled degradation rates and the ability to support cell growth and differentiation. This period also saw the emergence of novel polycarbonate copolymers designed specifically for biomedical applications, offering improved biocompatibility and tailored mechanical properties.
Recent years have witnessed a surge in cutting-edge research aimed at pushing the boundaries of polycarbonate biocompatibility. The integration of nanotechnology has opened up new possibilities, with nanostructured polycarbonate surfaces showing enhanced protein adsorption and cell interactions. Additionally, the development of biodegradable polycarbonate variants has addressed concerns about long-term implant stability and the need for secondary removal surgeries.
The primary objectives of current polycarbonate biocompatibility research are multifaceted and ambitious. Researchers aim to develop polycarbonate-based materials that not only exhibit excellent biocompatibility but also possess advanced functionalities such as controlled drug release, antimicrobial properties, and the ability to promote tissue regeneration. There is a strong focus on creating "smart" polycarbonate materials that can respond to physiological stimuli and adapt to the dynamic in vivo environment.
Another key objective is to establish standardized testing protocols and long-term in vivo studies to comprehensively evaluate the biocompatibility of novel polycarbonate formulations. This includes assessing their performance under various physiological conditions and their potential for triggering adverse immune responses or toxicity over extended periods. The ultimate goal is to develop polycarbonate-based biomaterials that can seamlessly integrate with the human body, supporting tissue regeneration and functional recovery in a wide range of medical applications.
In the early stages, polycarbonate was primarily used in non-implantable medical devices due to concerns about its long-term biocompatibility. However, as research progressed, scientists began to explore surface modification techniques and composite formulations to enhance its biocompatibility profile. The 1980s and 1990s saw significant advancements in this area, with the development of plasma treatment methods and the incorporation of bioactive compounds to improve cell adhesion and reduce inflammatory responses.
The turn of the millennium brought about a new era in polycarbonate biocompatibility research, driven by the growing demand for advanced biomaterials in tissue engineering and regenerative medicine. Researchers began to focus on creating polycarbonate-based scaffolds with controlled degradation rates and the ability to support cell growth and differentiation. This period also saw the emergence of novel polycarbonate copolymers designed specifically for biomedical applications, offering improved biocompatibility and tailored mechanical properties.
Recent years have witnessed a surge in cutting-edge research aimed at pushing the boundaries of polycarbonate biocompatibility. The integration of nanotechnology has opened up new possibilities, with nanostructured polycarbonate surfaces showing enhanced protein adsorption and cell interactions. Additionally, the development of biodegradable polycarbonate variants has addressed concerns about long-term implant stability and the need for secondary removal surgeries.
The primary objectives of current polycarbonate biocompatibility research are multifaceted and ambitious. Researchers aim to develop polycarbonate-based materials that not only exhibit excellent biocompatibility but also possess advanced functionalities such as controlled drug release, antimicrobial properties, and the ability to promote tissue regeneration. There is a strong focus on creating "smart" polycarbonate materials that can respond to physiological stimuli and adapt to the dynamic in vivo environment.
Another key objective is to establish standardized testing protocols and long-term in vivo studies to comprehensively evaluate the biocompatibility of novel polycarbonate formulations. This includes assessing their performance under various physiological conditions and their potential for triggering adverse immune responses or toxicity over extended periods. The ultimate goal is to develop polycarbonate-based biomaterials that can seamlessly integrate with the human body, supporting tissue regeneration and functional recovery in a wide range of medical applications.
Market Analysis for Biocompatible Polycarbonate Applications
The biocompatible polycarbonate market is experiencing significant growth driven by increasing demand in medical devices, implants, and healthcare applications. This market segment is expected to expand at a robust rate due to the rising prevalence of chronic diseases, an aging population, and advancements in medical technology.
In the medical device sector, biocompatible polycarbonates are widely used in surgical instruments, diagnostic equipment, and drug delivery systems. The material's transparency, durability, and sterilization compatibility make it ideal for these applications. The growing trend towards minimally invasive surgeries and point-of-care diagnostics is further fueling the demand for biocompatible polycarbonate components.
The implantable medical devices market presents a substantial opportunity for biocompatible polycarbonates. These materials are increasingly used in orthopedic implants, cardiovascular devices, and dental applications due to their excellent mechanical properties and long-term stability in the human body. The global implantable medical devices market is projected to grow steadily, with biocompatible polycarbonates playing a crucial role in this expansion.
In the pharmaceutical and biotechnology industries, biocompatible polycarbonates are gaining traction in drug delivery systems and laboratory equipment. The material's chemical resistance and ability to maintain drug efficacy make it suitable for various drug delivery applications, including inhalers, auto-injectors, and transdermal patches.
Geographically, North America and Europe currently dominate the biocompatible polycarbonate market due to their advanced healthcare infrastructure and high adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing healthcare expenditure, and a large patient population.
The market is also influenced by regulatory factors, with stringent approval processes for medical-grade materials. This has led to increased collaboration between material manufacturers and medical device companies to develop and validate biocompatible polycarbonate formulations that meet regulatory requirements.
Emerging trends in the biocompatible polycarbonate market include the development of antimicrobial formulations to reduce healthcare-associated infections and the integration of nanotechnology to enhance material properties. Additionally, there is a growing focus on sustainable and bio-based polycarbonates to address environmental concerns and meet the increasing demand for eco-friendly medical materials.
In the medical device sector, biocompatible polycarbonates are widely used in surgical instruments, diagnostic equipment, and drug delivery systems. The material's transparency, durability, and sterilization compatibility make it ideal for these applications. The growing trend towards minimally invasive surgeries and point-of-care diagnostics is further fueling the demand for biocompatible polycarbonate components.
The implantable medical devices market presents a substantial opportunity for biocompatible polycarbonates. These materials are increasingly used in orthopedic implants, cardiovascular devices, and dental applications due to their excellent mechanical properties and long-term stability in the human body. The global implantable medical devices market is projected to grow steadily, with biocompatible polycarbonates playing a crucial role in this expansion.
In the pharmaceutical and biotechnology industries, biocompatible polycarbonates are gaining traction in drug delivery systems and laboratory equipment. The material's chemical resistance and ability to maintain drug efficacy make it suitable for various drug delivery applications, including inhalers, auto-injectors, and transdermal patches.
Geographically, North America and Europe currently dominate the biocompatible polycarbonate market due to their advanced healthcare infrastructure and high adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing healthcare expenditure, and a large patient population.
The market is also influenced by regulatory factors, with stringent approval processes for medical-grade materials. This has led to increased collaboration between material manufacturers and medical device companies to develop and validate biocompatible polycarbonate formulations that meet regulatory requirements.
Emerging trends in the biocompatible polycarbonate market include the development of antimicrobial formulations to reduce healthcare-associated infections and the integration of nanotechnology to enhance material properties. Additionally, there is a growing focus on sustainable and bio-based polycarbonates to address environmental concerns and meet the increasing demand for eco-friendly medical materials.
Current Challenges in Polycarbonate Biocompatibility
Despite the widespread use of polycarbonate in biomedical applications, several challenges persist in achieving cutting-edge biocompatibility. One of the primary concerns is the potential leaching of bisphenol A (BPA), a key component in polycarbonate synthesis. While the levels of BPA released are generally considered safe by regulatory bodies, there is ongoing debate about its long-term effects on human health, particularly in sensitive applications such as implants and medical devices.
Another significant challenge lies in the surface properties of polycarbonate. The material's inherent hydrophobicity can lead to protein adsorption and subsequent bacterial adhesion, potentially causing infections or adverse immune responses in biological environments. This necessitates the development of effective surface modification techniques to enhance hydrophilicity and reduce biofouling without compromising the bulk properties of the polymer.
The mechanical properties of polycarbonate, while generally favorable, can present challenges in certain biomedical applications. For instance, in load-bearing implants or devices subject to repeated stress, there are concerns about long-term fatigue resistance and potential degradation in physiological conditions. Balancing the need for mechanical strength with flexibility and biocompatibility remains an ongoing challenge for researchers and engineers.
Sterilization processes pose another hurdle in polycarbonate biocompatibility. Common sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving can potentially alter the material's surface chemistry or mechanical properties. This necessitates the development of sterilization protocols that maintain the integrity of polycarbonate while ensuring complete microbial elimination.
The biodegradation of polycarbonate in biological environments is also a concern, particularly for long-term implants. While polycarbonate is generally considered biostable, there is evidence of slow degradation over time, which could lead to the release of potentially harmful byproducts or compromise the structural integrity of the device.
Lastly, the biocompatibility of polycarbonate composites and blends presents unique challenges. As researchers explore ways to enhance the properties of polycarbonate through the incorporation of other materials, ensuring the biocompatibility of these complex systems becomes increasingly difficult. The interactions between different components and their collective impact on biological systems require extensive testing and validation.
Another significant challenge lies in the surface properties of polycarbonate. The material's inherent hydrophobicity can lead to protein adsorption and subsequent bacterial adhesion, potentially causing infections or adverse immune responses in biological environments. This necessitates the development of effective surface modification techniques to enhance hydrophilicity and reduce biofouling without compromising the bulk properties of the polymer.
The mechanical properties of polycarbonate, while generally favorable, can present challenges in certain biomedical applications. For instance, in load-bearing implants or devices subject to repeated stress, there are concerns about long-term fatigue resistance and potential degradation in physiological conditions. Balancing the need for mechanical strength with flexibility and biocompatibility remains an ongoing challenge for researchers and engineers.
Sterilization processes pose another hurdle in polycarbonate biocompatibility. Common sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving can potentially alter the material's surface chemistry or mechanical properties. This necessitates the development of sterilization protocols that maintain the integrity of polycarbonate while ensuring complete microbial elimination.
The biodegradation of polycarbonate in biological environments is also a concern, particularly for long-term implants. While polycarbonate is generally considered biostable, there is evidence of slow degradation over time, which could lead to the release of potentially harmful byproducts or compromise the structural integrity of the device.
Lastly, the biocompatibility of polycarbonate composites and blends presents unique challenges. As researchers explore ways to enhance the properties of polycarbonate through the incorporation of other materials, ensuring the biocompatibility of these complex systems becomes increasingly difficult. The interactions between different components and their collective impact on biological systems require extensive testing and validation.
Existing Biocompatible Polycarbonate Solutions
01 Surface modification for improved biocompatibility
Polycarbonate surfaces can be modified to enhance biocompatibility. This includes techniques such as plasma treatment, chemical functionalization, or coating with biocompatible materials. These modifications can improve cell adhesion, reduce protein adsorption, and minimize inflammatory responses, making polycarbonates more suitable for medical applications.- Surface modification of polycarbonate for biocompatibility: Various techniques are used to modify the surface of polycarbonate materials to enhance their biocompatibility. These methods include plasma treatment, chemical functionalization, and coating with biocompatible materials. Such modifications can improve cell adhesion, reduce protein adsorption, and enhance the overall biocompatibility of polycarbonate for medical applications.
- Polycarbonate composites for biomedical applications: Polycarbonate-based composites are developed by incorporating various additives or blending with other polymers to improve biocompatibility and mechanical properties. These composites can be tailored for specific biomedical applications such as implants, tissue engineering scaffolds, and drug delivery systems.
- Biodegradable polycarbonate derivatives: Research focuses on developing biodegradable polycarbonate derivatives to address the long-term biocompatibility concerns of traditional polycarbonates. These materials are designed to degrade safely in the body over time, reducing the risk of long-term complications and eliminating the need for removal surgeries.
- Biocompatibility testing methods for polycarbonates: Various in vitro and in vivo testing methods are developed and standardized to assess the biocompatibility of polycarbonate materials. These tests evaluate cytotoxicity, genotoxicity, sensitization, and long-term tissue response to ensure the safety and efficacy of polycarbonate-based medical devices and implants.
- Polycarbonate sterilization and its impact on biocompatibility: Different sterilization methods and their effects on the biocompatibility of polycarbonate materials are studied. Techniques such as ethylene oxide treatment, gamma irradiation, and steam sterilization are evaluated to determine their impact on the material properties and long-term biocompatibility of polycarbonate medical devices.
02 Polycarbonate blends and copolymers
Blending polycarbonates with other biocompatible polymers or creating copolymers can enhance overall biocompatibility. These composite materials often combine the mechanical strength of polycarbonates with the biocompatibility of other polymers, resulting in materials suitable for various biomedical applications such as implants or tissue engineering scaffolds.Expand Specific Solutions03 Biocompatible additives and fillers
Incorporating biocompatible additives or fillers into polycarbonate formulations can improve their biological performance. These additives may include antioxidants, antimicrobial agents, or bioactive compounds that enhance cell growth or reduce adverse reactions when in contact with living tissues.Expand Specific Solutions04 Biodegradable polycarbonate derivatives
Development of biodegradable polycarbonate derivatives aims to combine the desirable properties of polycarbonates with the ability to degrade safely in biological environments. These materials are particularly useful for temporary medical devices or drug delivery systems where eventual degradation is beneficial.Expand Specific Solutions05 Biocompatibility testing and evaluation methods
Various methods and techniques have been developed to assess the biocompatibility of polycarbonates. These include in vitro cell culture studies, protein adsorption assays, and in vivo implantation tests. Advanced analytical techniques are also used to characterize surface properties and interactions with biological systems, helping to predict and improve biocompatibility.Expand Specific Solutions
Key Players in Biocompatible Polycarbonate Research
The research on polycarbonate for cutting-edge biocompatibility is in a growth phase, with increasing market size and technological advancements. The global biocompatible materials market is expanding rapidly, driven by rising demand in medical devices and implants. While the technology is maturing, there's still room for innovation. Key players like Boston Scientific Scimed, Wanhua Chemical Group, and Sumitomo Seika Chemicals are actively developing new polycarbonate formulations. Academic institutions such as Wuhan University, University of Sydney, and Fudan University are contributing to fundamental research. The collaboration between industry and academia is accelerating progress in this field, with a focus on enhancing biocompatibility and performance for medical applications.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed advanced polycarbonate-based biocompatible materials for medical devices. Their research focuses on enhancing the surface properties of polycarbonate through plasma treatment and coating technologies[1]. This approach improves the material's hemocompatibility and reduces protein adsorption, making it suitable for long-term implantable devices. The company has also explored the incorporation of bioactive molecules into polycarbonate matrices to promote tissue integration and reduce inflammatory responses[2]. Their latest innovations include the development of polycarbonate-urethane blends with improved mechanical properties and degradation resistance for cardiovascular applications[3].
Strengths: Extensive experience in medical device materials, strong R&D capabilities, and established market presence. Weaknesses: High development costs and lengthy regulatory approval processes for new materials.
SABIC Global Technologies BV
Technical Solution: SABIC has pioneered the development of high-performance polycarbonate resins for healthcare applications. Their LEXAN™ healthcare polycarbonate portfolio includes grades specifically designed for biocompatibility and sterilization resistance[4]. SABIC's research focuses on enhancing the chemical resistance and impact strength of polycarbonate while maintaining its biocompatibility. They have developed proprietary additive packages that improve the material's resistance to lipids and disinfectants commonly used in medical environments[5]. Additionally, SABIC has invested in the development of sustainable polycarbonates derived from renewable resources, aiming to reduce the environmental impact of medical plastics[6].
Strengths: Global scale, diverse product portfolio, and strong material science expertise. Weaknesses: Dependence on petrochemical feedstocks and potential regulatory challenges in different markets.
Core Innovations in Polycarbonate Biocompatibility
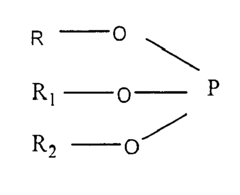
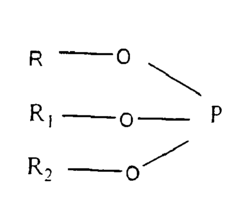
Biocompatible polycarbonate and radiopaque polymer compositions and methods of manufacturing medical devices with same
PatentInactiveEP2204419A2
Innovation
- A biocompatible radiopaque polymer composition is developed, combining poly(bisphenol A carbonate) with polyamide and an inorganic radiopaque filler, along with additives like phosphites and functionalized polyolefins, to enhance mechanical properties and melt processability, allowing for improved visualization and mechanical performance in medical devices.
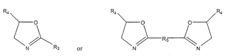
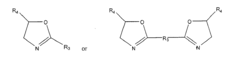
Monomers and polymers for functional polycarbonates and poly(ester-carbonates) and peg-co-polycarbonate hydrogels
PatentActiveUS20180155328A1
Innovation
- Development of novel carbonate-based monomers and their controlled homopolymerization and copolymerization, enabling the creation of cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels through copper-free, strain-promoted azide-alkyne cycloaddition 'click' chemistry, which allows for facile functionalization and crosslinking without external perturbations or cytotoxicity.
Regulatory Framework for Biocompatible Materials
The regulatory framework for biocompatible materials plays a crucial role in ensuring the safety and efficacy of medical devices and implants. In the context of polycarbonate research for cutting-edge biocompatibility, understanding and adhering to these regulations is paramount for successful development and market entry.
At the forefront of this regulatory landscape is the International Organization for Standardization (ISO) 10993 series, which provides a comprehensive set of standards for evaluating the biocompatibility of medical devices. These standards outline the necessary tests and assessments required to demonstrate the safety of materials intended for use in medical applications.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for biocompatible materials. The FDA's guidance document on the use of ISO 10993-1 provides a risk-based approach to determining the biocompatibility testing needed for medical devices. This guidance is particularly relevant for polycarbonate research, as it helps manufacturers identify the appropriate tests based on the intended use and duration of contact with the human body.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) also play a significant role in shaping the regulatory landscape for biocompatible materials. These regulations emphasize the importance of risk management and post-market surveillance, which are critical considerations in the development of advanced polycarbonate materials for biomedical applications.
Japan's Pharmaceutical and Medical Device Agency (PMDA) has its own set of regulations for biocompatible materials, which align closely with international standards but may have specific requirements for certain types of medical devices. Researchers working on polycarbonate biocompatibility must be aware of these nuances when targeting the Japanese market.
Emerging markets, such as China and India, are also developing their regulatory frameworks for biocompatible materials. The National Medical Products Administration (NMPA) in China and the Central Drugs Standard Control Organization (CDSCO) in India have been aligning their regulations with international standards, but may have additional local requirements that need to be considered.
As research on polycarbonate for cutting-edge biocompatibility progresses, it is essential to maintain ongoing dialogue with regulatory bodies. This proactive approach can help identify potential regulatory hurdles early in the development process and ensure that the research aligns with current and anticipated future requirements.
Furthermore, the regulatory landscape for biocompatible materials is continually evolving, driven by advancements in materials science and a growing understanding of long-term biocompatibility effects. Researchers must stay informed about proposed changes to regulations and participate in public consultations to contribute to the development of appropriate standards for novel materials and applications.
At the forefront of this regulatory landscape is the International Organization for Standardization (ISO) 10993 series, which provides a comprehensive set of standards for evaluating the biocompatibility of medical devices. These standards outline the necessary tests and assessments required to demonstrate the safety of materials intended for use in medical applications.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for biocompatible materials. The FDA's guidance document on the use of ISO 10993-1 provides a risk-based approach to determining the biocompatibility testing needed for medical devices. This guidance is particularly relevant for polycarbonate research, as it helps manufacturers identify the appropriate tests based on the intended use and duration of contact with the human body.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) also play a significant role in shaping the regulatory landscape for biocompatible materials. These regulations emphasize the importance of risk management and post-market surveillance, which are critical considerations in the development of advanced polycarbonate materials for biomedical applications.
Japan's Pharmaceutical and Medical Device Agency (PMDA) has its own set of regulations for biocompatible materials, which align closely with international standards but may have specific requirements for certain types of medical devices. Researchers working on polycarbonate biocompatibility must be aware of these nuances when targeting the Japanese market.
Emerging markets, such as China and India, are also developing their regulatory frameworks for biocompatible materials. The National Medical Products Administration (NMPA) in China and the Central Drugs Standard Control Organization (CDSCO) in India have been aligning their regulations with international standards, but may have additional local requirements that need to be considered.
As research on polycarbonate for cutting-edge biocompatibility progresses, it is essential to maintain ongoing dialogue with regulatory bodies. This proactive approach can help identify potential regulatory hurdles early in the development process and ensure that the research aligns with current and anticipated future requirements.
Furthermore, the regulatory landscape for biocompatible materials is continually evolving, driven by advancements in materials science and a growing understanding of long-term biocompatibility effects. Researchers must stay informed about proposed changes to regulations and participate in public consultations to contribute to the development of appropriate standards for novel materials and applications.
Environmental Impact of Biocompatible Polycarbonates
The environmental impact of biocompatible polycarbonates is a critical consideration in the development and application of these materials for medical and biological purposes. As the demand for biocompatible materials increases, it is essential to assess their lifecycle environmental footprint, from production to disposal.
Polycarbonates used in biomedical applications are typically synthesized through processes that involve the use of potentially harmful chemicals, such as bisphenol A (BPA) and phosgene. The production of these materials can lead to the release of toxic substances into the environment if not properly managed. However, advancements in green chemistry have led to the development of more environmentally friendly synthesis methods, including the use of bio-based precursors and catalysts.
During their use phase, biocompatible polycarbonates generally have a minimal direct environmental impact. Their stability and resistance to degradation in biological environments contribute to their longevity and reduced need for frequent replacement. This durability can be seen as a positive attribute in terms of resource conservation.
End-of-life considerations for biocompatible polycarbonates present both challenges and opportunities. Traditional polycarbonates are not biodegradable, which can lead to accumulation in landfills or marine environments if not properly disposed of. However, research into biodegradable and compostable polycarbonates is progressing, with promising results in developing materials that maintain biocompatibility while offering improved end-of-life environmental profiles.
Recycling of biocompatible polycarbonates is another area of focus for reducing environmental impact. While medical-grade polycarbonates often cannot be recycled due to contamination concerns, efforts are being made to develop closed-loop recycling systems for certain applications, such as single-use medical devices.
The carbon footprint associated with the production and transportation of biocompatible polycarbonates is also a significant environmental consideration. As with many synthetic materials, the energy-intensive manufacturing processes contribute to greenhouse gas emissions. However, the long lifespan and potential for recycling or biodegradation of newer polycarbonate formulations may offset some of these impacts over time.
Water usage and pollution are additional environmental factors to consider. The production of polycarbonates requires substantial amounts of water, and there is a risk of water contamination if effluents are not properly treated. Implementing advanced water treatment technologies and closed-loop water systems in manufacturing facilities can help mitigate these impacts.
In conclusion, while biocompatible polycarbonates offer significant benefits in medical applications, their environmental impact remains a complex issue. Ongoing research and development efforts are focused on improving the sustainability of these materials throughout their lifecycle, from greener synthesis methods to enhanced end-of-life options. Balancing the need for high-performance biocompatible materials with environmental stewardship will continue to be a key challenge and opportunity in this field.
Polycarbonates used in biomedical applications are typically synthesized through processes that involve the use of potentially harmful chemicals, such as bisphenol A (BPA) and phosgene. The production of these materials can lead to the release of toxic substances into the environment if not properly managed. However, advancements in green chemistry have led to the development of more environmentally friendly synthesis methods, including the use of bio-based precursors and catalysts.
During their use phase, biocompatible polycarbonates generally have a minimal direct environmental impact. Their stability and resistance to degradation in biological environments contribute to their longevity and reduced need for frequent replacement. This durability can be seen as a positive attribute in terms of resource conservation.
End-of-life considerations for biocompatible polycarbonates present both challenges and opportunities. Traditional polycarbonates are not biodegradable, which can lead to accumulation in landfills or marine environments if not properly disposed of. However, research into biodegradable and compostable polycarbonates is progressing, with promising results in developing materials that maintain biocompatibility while offering improved end-of-life environmental profiles.
Recycling of biocompatible polycarbonates is another area of focus for reducing environmental impact. While medical-grade polycarbonates often cannot be recycled due to contamination concerns, efforts are being made to develop closed-loop recycling systems for certain applications, such as single-use medical devices.
The carbon footprint associated with the production and transportation of biocompatible polycarbonates is also a significant environmental consideration. As with many synthetic materials, the energy-intensive manufacturing processes contribute to greenhouse gas emissions. However, the long lifespan and potential for recycling or biodegradation of newer polycarbonate formulations may offset some of these impacts over time.
Water usage and pollution are additional environmental factors to consider. The production of polycarbonates requires substantial amounts of water, and there is a risk of water contamination if effluents are not properly treated. Implementing advanced water treatment technologies and closed-loop water systems in manufacturing facilities can help mitigate these impacts.
In conclusion, while biocompatible polycarbonates offer significant benefits in medical applications, their environmental impact remains a complex issue. Ongoing research and development efforts are focused on improving the sustainability of these materials throughout their lifecycle, from greener synthesis methods to enhanced end-of-life options. Balancing the need for high-performance biocompatible materials with environmental stewardship will continue to be a key challenge and opportunity in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!