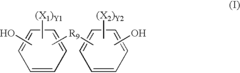
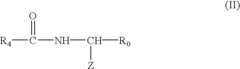
Polycarbonate in Medical Implant Innovations
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polycarbonate in Medical Implants: Background and Objectives
Polycarbonate has emerged as a revolutionary material in the field of medical implants, offering a unique combination of properties that make it ideal for various biomedical applications. The journey of polycarbonate in medical implants began in the late 1960s when it was first introduced as a potential material for orthopedic and dental implants. Since then, its use has expanded significantly, encompassing a wide range of medical devices and implantable structures.
The evolution of polycarbonate in medical implants has been driven by the increasing demand for biocompatible materials that can withstand the harsh environment of the human body while providing long-term stability and functionality. As medical technology advanced, so did the requirements for implant materials, pushing researchers and engineers to explore the full potential of polycarbonate and its derivatives.
One of the key factors contributing to the widespread adoption of polycarbonate in medical implants is its exceptional mechanical properties. The material exhibits high impact strength, dimensional stability, and resistance to deformation under load, making it suitable for load-bearing implants and devices that require structural integrity over extended periods.
Furthermore, polycarbonate's optical clarity has opened up new possibilities in the development of transparent medical devices and implants, particularly in ophthalmology and diagnostic equipment. This unique characteristic allows for the creation of implants that can be easily monitored and assessed post-implantation, enhancing patient care and treatment outcomes.
The biocompatibility of polycarbonate has been a subject of extensive research and development. Over the years, modifications and surface treatments have been developed to enhance its interaction with biological tissues, reduce the risk of rejection, and promote integration with the surrounding tissue. These advancements have significantly expanded the range of applications for polycarbonate in medical implants.
As we look towards the future, the objectives for polycarbonate in medical implant innovations are multifaceted. Researchers aim to further improve the material's biocompatibility, develop new composites that combine the strengths of polycarbonate with other materials, and explore novel manufacturing techniques such as 3D printing to create custom-designed implants with complex geometries.
Additionally, there is a growing focus on developing smart implants using polycarbonate as a base material. These implants would incorporate sensors and other electronic components to monitor patient health, deliver targeted therapies, and provide real-time data to healthcare providers. The versatility of polycarbonate makes it an ideal candidate for such advanced applications, potentially revolutionizing personalized medicine and patient care.
The evolution of polycarbonate in medical implants has been driven by the increasing demand for biocompatible materials that can withstand the harsh environment of the human body while providing long-term stability and functionality. As medical technology advanced, so did the requirements for implant materials, pushing researchers and engineers to explore the full potential of polycarbonate and its derivatives.
One of the key factors contributing to the widespread adoption of polycarbonate in medical implants is its exceptional mechanical properties. The material exhibits high impact strength, dimensional stability, and resistance to deformation under load, making it suitable for load-bearing implants and devices that require structural integrity over extended periods.
Furthermore, polycarbonate's optical clarity has opened up new possibilities in the development of transparent medical devices and implants, particularly in ophthalmology and diagnostic equipment. This unique characteristic allows for the creation of implants that can be easily monitored and assessed post-implantation, enhancing patient care and treatment outcomes.
The biocompatibility of polycarbonate has been a subject of extensive research and development. Over the years, modifications and surface treatments have been developed to enhance its interaction with biological tissues, reduce the risk of rejection, and promote integration with the surrounding tissue. These advancements have significantly expanded the range of applications for polycarbonate in medical implants.
As we look towards the future, the objectives for polycarbonate in medical implant innovations are multifaceted. Researchers aim to further improve the material's biocompatibility, develop new composites that combine the strengths of polycarbonate with other materials, and explore novel manufacturing techniques such as 3D printing to create custom-designed implants with complex geometries.
Additionally, there is a growing focus on developing smart implants using polycarbonate as a base material. These implants would incorporate sensors and other electronic components to monitor patient health, deliver targeted therapies, and provide real-time data to healthcare providers. The versatility of polycarbonate makes it an ideal candidate for such advanced applications, potentially revolutionizing personalized medicine and patient care.
Market Analysis for Polycarbonate-based Medical Implants
The market for polycarbonate-based medical implants has shown significant growth and potential in recent years, driven by the material's unique properties and the increasing demand for advanced medical devices. Polycarbonate, known for its biocompatibility, durability, and transparency, has found widespread applications in various medical implant innovations.
The global market for polycarbonate medical implants is experiencing steady expansion, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and advancements in medical technology. Orthopedic implants, cardiovascular devices, and dental implants are among the key segments driving market demand.
North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, fueled by improving healthcare access and increasing healthcare expenditure.
Key factors influencing market demand include the growing preference for minimally invasive surgical procedures, the need for long-lasting and biocompatible implants, and the increasing focus on patient comfort and aesthetics. Polycarbonate's ability to be sterilized using various methods, its resistance to chemicals and impact, and its capacity for customization make it an attractive choice for medical device manufacturers.
The market is characterized by intense competition among major players, with continuous research and development efforts aimed at improving product performance and expanding applications. Collaborations between medical device companies and material suppliers are becoming more common, driving innovation in polycarbonate-based implants.
Challenges facing the market include stringent regulatory requirements, concerns over long-term biocompatibility, and the emergence of alternative materials. However, ongoing research into surface modifications and composite formulations is addressing these challenges, potentially expanding the use of polycarbonate in more complex medical implant applications.
In conclusion, the market for polycarbonate-based medical implants presents significant opportunities for growth and innovation. As technology advances and healthcare needs evolve, polycarbonate is likely to play an increasingly important role in the development of next-generation medical implants, offering improved patient outcomes and expanding treatment options across various medical fields.
The global market for polycarbonate medical implants is experiencing steady expansion, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and advancements in medical technology. Orthopedic implants, cardiovascular devices, and dental implants are among the key segments driving market demand.
North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, fueled by improving healthcare access and increasing healthcare expenditure.
Key factors influencing market demand include the growing preference for minimally invasive surgical procedures, the need for long-lasting and biocompatible implants, and the increasing focus on patient comfort and aesthetics. Polycarbonate's ability to be sterilized using various methods, its resistance to chemicals and impact, and its capacity for customization make it an attractive choice for medical device manufacturers.
The market is characterized by intense competition among major players, with continuous research and development efforts aimed at improving product performance and expanding applications. Collaborations between medical device companies and material suppliers are becoming more common, driving innovation in polycarbonate-based implants.
Challenges facing the market include stringent regulatory requirements, concerns over long-term biocompatibility, and the emergence of alternative materials. However, ongoing research into surface modifications and composite formulations is addressing these challenges, potentially expanding the use of polycarbonate in more complex medical implant applications.
In conclusion, the market for polycarbonate-based medical implants presents significant opportunities for growth and innovation. As technology advances and healthcare needs evolve, polycarbonate is likely to play an increasingly important role in the development of next-generation medical implants, offering improved patient outcomes and expanding treatment options across various medical fields.
Current Challenges in Polycarbonate Medical Implant Technology
Despite the widespread use of polycarbonate in medical implants, several challenges persist in this field. One of the primary concerns is the long-term biocompatibility of polycarbonate implants. While generally considered safe, there are ongoing studies to assess the potential for adverse reactions or degradation over extended periods inside the human body. This is particularly crucial for implants intended for lifelong use.
Another significant challenge lies in the mechanical properties of polycarbonate. Although it offers good impact resistance and flexibility, there are limitations in its strength and wear resistance compared to some alternative materials. This becomes especially problematic in load-bearing implants or those subject to constant friction, where material fatigue and wear can lead to implant failure.
The sterilization of polycarbonate implants presents another hurdle. Traditional sterilization methods, such as high-temperature autoclaving or gamma radiation, can potentially degrade the material properties of polycarbonate. This necessitates the development of alternative sterilization techniques that maintain the integrity of the implant while ensuring complete sterility.
Surface modification of polycarbonate implants remains a complex issue. Enhancing the surface properties to improve cell adhesion, reduce bacterial colonization, or incorporate drug-delivery capabilities is an area of ongoing research. Current methods often involve complex chemical treatments or coatings, which can be challenging to apply uniformly and may affect the bulk properties of the implant.
The environmental impact of polycarbonate implants is also a growing concern. As the healthcare industry moves towards more sustainable practices, there is a need to develop biodegradable or easily recyclable polycarbonate formulations for implants with shorter lifespans. However, achieving this while maintaining the necessary mechanical and biocompatibility properties is a significant challenge.
Lastly, the regulatory landscape for polycarbonate medical implants is becoming increasingly complex. Stricter regulations regarding material purity, manufacturing processes, and long-term safety data are being implemented. Meeting these evolving standards while keeping implant development economically viable is a considerable challenge for manufacturers and researchers in this field.
Another significant challenge lies in the mechanical properties of polycarbonate. Although it offers good impact resistance and flexibility, there are limitations in its strength and wear resistance compared to some alternative materials. This becomes especially problematic in load-bearing implants or those subject to constant friction, where material fatigue and wear can lead to implant failure.
The sterilization of polycarbonate implants presents another hurdle. Traditional sterilization methods, such as high-temperature autoclaving or gamma radiation, can potentially degrade the material properties of polycarbonate. This necessitates the development of alternative sterilization techniques that maintain the integrity of the implant while ensuring complete sterility.
Surface modification of polycarbonate implants remains a complex issue. Enhancing the surface properties to improve cell adhesion, reduce bacterial colonization, or incorporate drug-delivery capabilities is an area of ongoing research. Current methods often involve complex chemical treatments or coatings, which can be challenging to apply uniformly and may affect the bulk properties of the implant.
The environmental impact of polycarbonate implants is also a growing concern. As the healthcare industry moves towards more sustainable practices, there is a need to develop biodegradable or easily recyclable polycarbonate formulations for implants with shorter lifespans. However, achieving this while maintaining the necessary mechanical and biocompatibility properties is a significant challenge.
Lastly, the regulatory landscape for polycarbonate medical implants is becoming increasingly complex. Stricter regulations regarding material purity, manufacturing processes, and long-term safety data are being implemented. Meeting these evolving standards while keeping implant development economically viable is a considerable challenge for manufacturers and researchers in this field.
Existing Polycarbonate Medical Implant Solutions
01 Synthesis and modification of polycarbonates
Various methods for synthesizing and modifying polycarbonates are explored, including new catalysts, reaction conditions, and additives to improve properties such as molecular weight, thermal stability, and optical clarity. These techniques aim to enhance the performance and versatility of polycarbonate materials for different applications.- Synthesis and modification of polycarbonates: This category focuses on the methods for synthesizing polycarbonates and modifying their properties. It includes techniques for polymerization, copolymerization, and the incorporation of various additives to enhance specific characteristics of the resulting polymer.
- Polycarbonate blends and composites: This area covers the development of polycarbonate blends and composites with other materials to improve performance characteristics. It includes the creation of polymer alloys, reinforced polycarbonates, and multi-component systems for specific applications.
- Optical and electronic applications of polycarbonates: This category encompasses the use of polycarbonates in optical and electronic applications. It includes the development of polycarbonate-based materials for lenses, displays, data storage devices, and other optical or electronic components.
- Flame retardant and heat-resistant polycarbonates: This area focuses on improving the flame retardancy and heat resistance of polycarbonates. It includes the incorporation of flame retardant additives, development of intrinsically flame-resistant polycarbonate structures, and methods to enhance thermal stability.
- Polycarbonate processing and manufacturing techniques: This category covers innovative processing and manufacturing techniques for polycarbonates. It includes methods for molding, extrusion, film formation, and other fabrication processes, as well as techniques to improve production efficiency and product quality.
02 Polycarbonate blends and composites
Development of polycarbonate blends and composites with other polymers or additives to achieve improved mechanical, thermal, or electrical properties. These formulations can include flame retardants, impact modifiers, or other functional materials to tailor the characteristics of the final product for specific uses.Expand Specific Solutions03 Polycarbonate processing techniques
Advancements in processing techniques for polycarbonates, including extrusion, injection molding, and film formation. These methods focus on optimizing processing parameters, reducing defects, and improving the overall quality and consistency of polycarbonate products.Expand Specific Solutions04 Polycarbonate applications in electronics
Utilization of polycarbonates in electronic applications, such as in the manufacture of capacitors, circuit boards, and protective housings. The focus is on developing polycarbonate formulations with enhanced dielectric properties, heat resistance, and dimensional stability for use in electronic components.Expand Specific Solutions05 Recycling and sustainability of polycarbonates
Methods for recycling polycarbonate materials and developing more sustainable production processes. This includes chemical recycling techniques, bio-based polycarbonate alternatives, and strategies to reduce the environmental impact of polycarbonate manufacturing and disposal.Expand Specific Solutions
Key Players in Polycarbonate Medical Implant Industry
The research on polycarbonate in medical implant innovations is in a mature stage, with significant market growth potential. The global medical implants market is projected to reach $153.8 billion by 2026, driven by an aging population and increasing prevalence of chronic diseases. Key players in this field include established companies like Covestro Deutschland AG, Abbott Cardiovascular Systems, and Bayer AG, as well as research institutions such as Wuhan University and Centre National de la Recherche Scientifique. These organizations are focusing on developing advanced polycarbonate materials with enhanced biocompatibility, durability, and functionality for various medical implant applications. The competitive landscape is characterized by ongoing R&D efforts, strategic collaborations, and a growing emphasis on sustainable and bioabsorbable polycarbonate-based solutions.
Covestro Deutschland AG
Technical Solution: Covestro has developed innovative polycarbonate materials specifically designed for medical implants. Their Makrolon® polycarbonate grades offer exceptional biocompatibility, durability, and transparency, making them ideal for various medical applications[1]. The company has focused on creating polycarbonate formulations that can withstand sterilization processes without compromising mechanical properties. Covestro's research has led to the development of polycarbonate materials with enhanced impact resistance and long-term stability, crucial for implantable devices[2]. They have also explored surface modifications to improve the integration of polycarbonate implants with biological tissues, potentially reducing rejection rates and improving patient outcomes[3].
Strengths: Extensive experience in polycarbonate production, strong R&D capabilities, and a wide range of medical-grade materials. Weaknesses: Potential regulatory challenges and competition from alternative biomaterials.
Abbott Cardiovascular Systems, Inc.
Technical Solution: Abbott Cardiovascular Systems has made significant strides in utilizing polycarbonate for cardiovascular implants. Their research focuses on developing polycarbonate-based stents and heart valve components that offer superior mechanical properties and biocompatibility[4]. The company has pioneered the use of polycarbonate in drug-eluting stents, where the polymer serves as both a structural component and a drug delivery platform[5]. Abbott's innovations include the development of bioresorbable polycarbonate stents that gradually dissolve in the body, reducing long-term complications associated with permanent implants[6]. They have also explored surface modifications of polycarbonate to enhance its hemocompatibility and reduce the risk of thrombosis in cardiovascular applications.
Strengths: Specialized expertise in cardiovascular applications, strong clinical research capabilities. Weaknesses: Limited to cardiovascular applications, potential long-term effects of degradable implants still under study.
Core Innovations in Polycarbonate Implant Technology
Radio-opaque polymeric biomaterials
PatentInactiveUS20040037778A1
Innovation
- Development of radio-opaque polycarbonates and polyarylates with iodinated or brominated aromatic rings that degrade through the main polymer chain, maintaining radio-opacity and exhibiting good mechanical properties.
Implantable markers
PatentInactiveEP3019208A1
Innovation
- Development of a biodegradable implant comprising a contrast agent and a slowly degradable polymer that can be injected into tissues, maintaining structure for imaging purposes and degrading harmlessly over time, allowing for non-invasive monitoring and localization of medical conditions without the need for further surgical intervention.
Regulatory Framework for Polycarbonate Medical Implants
The regulatory framework for polycarbonate medical implants is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of these innovative devices. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing medical implants, including those made from polycarbonate materials. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for evaluating and approving medical devices, including implants, through various regulatory pathways.
For polycarbonate medical implants, manufacturers typically need to follow the 510(k) premarket notification process or the more rigorous Premarket Approval (PMA) pathway, depending on the device's classification and intended use. The 510(k) process is applicable for devices that are substantially equivalent to existing approved devices, while novel or high-risk implants may require PMA.
In the European Union, the regulatory landscape for medical implants has undergone significant changes with the implementation of the Medical Device Regulation (MDR) in 2021. This new regulation has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability of medical devices, including polycarbonate implants. Manufacturers must now obtain CE marking under the MDR to market their products in the EU.
The International Organization for Standardization (ISO) also plays a vital role in setting global standards for medical implants. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is particularly relevant for manufacturers of polycarbonate implants. Additionally, ISO 10993 series of standards provides guidelines for the biological evaluation of medical devices, including implants.
Regulatory bodies worldwide are increasingly focusing on the long-term safety and biocompatibility of implant materials. For polycarbonate implants, this includes evaluating potential leaching of bisphenol A (BPA) and other compounds, as well as assessing the material's degradation characteristics over time. Manufacturers must provide comprehensive data on material properties, biocompatibility testing, and clinical performance to meet regulatory requirements.
As the field of medical implants continues to advance, regulatory frameworks are adapting to address emerging technologies and materials. This includes considerations for 3D-printed polycarbonate implants, which may require additional regulatory scrutiny due to their unique manufacturing processes. Regulatory agencies are also placing greater emphasis on post-market surveillance and real-world evidence to monitor the long-term performance and safety of implanted devices.
For polycarbonate medical implants, manufacturers typically need to follow the 510(k) premarket notification process or the more rigorous Premarket Approval (PMA) pathway, depending on the device's classification and intended use. The 510(k) process is applicable for devices that are substantially equivalent to existing approved devices, while novel or high-risk implants may require PMA.
In the European Union, the regulatory landscape for medical implants has undergone significant changes with the implementation of the Medical Device Regulation (MDR) in 2021. This new regulation has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability of medical devices, including polycarbonate implants. Manufacturers must now obtain CE marking under the MDR to market their products in the EU.
The International Organization for Standardization (ISO) also plays a vital role in setting global standards for medical implants. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is particularly relevant for manufacturers of polycarbonate implants. Additionally, ISO 10993 series of standards provides guidelines for the biological evaluation of medical devices, including implants.
Regulatory bodies worldwide are increasingly focusing on the long-term safety and biocompatibility of implant materials. For polycarbonate implants, this includes evaluating potential leaching of bisphenol A (BPA) and other compounds, as well as assessing the material's degradation characteristics over time. Manufacturers must provide comprehensive data on material properties, biocompatibility testing, and clinical performance to meet regulatory requirements.
As the field of medical implants continues to advance, regulatory frameworks are adapting to address emerging technologies and materials. This includes considerations for 3D-printed polycarbonate implants, which may require additional regulatory scrutiny due to their unique manufacturing processes. Regulatory agencies are also placing greater emphasis on post-market surveillance and real-world evidence to monitor the long-term performance and safety of implanted devices.
Biocompatibility and Long-term Safety Considerations
Biocompatibility and long-term safety are critical considerations in the development of polycarbonate-based medical implants. Polycarbonate has gained significant attention in the medical field due to its unique properties, including transparency, durability, and ease of sterilization. However, ensuring its compatibility with the human body and its long-term safety is paramount for successful implant innovations.
The biocompatibility of polycarbonate implants is primarily assessed through in vitro and in vivo studies. These evaluations focus on the material's interaction with living tissues, potential inflammatory responses, and any adverse effects on cellular functions. Research has shown that polycarbonate generally exhibits good biocompatibility, with minimal tissue reactions and low cytotoxicity. However, the specific formulation and manufacturing processes can significantly influence its biological performance.
Long-term safety considerations for polycarbonate implants encompass several key aspects. One primary concern is the potential leaching of bisphenol A (BPA), a component used in polycarbonate synthesis. While modern polycarbonates are designed to minimize BPA release, ongoing research is essential to ensure that trace amounts do not accumulate in the body over extended periods, potentially leading to endocrine disruption or other health issues.
Another critical factor in long-term safety is the material's degradation behavior in the physiological environment. Polycarbonate implants must maintain their structural integrity and mechanical properties over the intended lifespan of the device. Studies have shown that polycarbonate can undergo slow hydrolytic degradation in vivo, which may affect its long-term performance. Researchers are exploring various surface modifications and composite formulations to enhance the material's resistance to degradation and improve its longevity in biological systems.
The immune response to polycarbonate implants is another crucial aspect of long-term safety. While the material is generally well-tolerated, some patients may develop a foreign body response over time. This can lead to the formation of a fibrous capsule around the implant, potentially affecting its function or integration with surrounding tissues. Ongoing research focuses on developing surface treatments and coatings that can modulate the immune response and promote better tissue integration.
Sterilization methods and their impact on polycarbonate's long-term performance are also under scrutiny. Common sterilization techniques, such as gamma irradiation or ethylene oxide treatment, can potentially alter the material's properties or induce the formation of toxic byproducts. Researchers are investigating alternative sterilization methods and optimizing existing processes to ensure the long-term stability and safety of polycarbonate implants.
As medical implant innovations continue to evolve, the development of more sophisticated in vitro models and long-term in vivo studies will be crucial for comprehensively assessing the biocompatibility and safety of polycarbonate-based devices. Additionally, post-market surveillance and real-world data collection will play an increasingly important role in identifying any unforeseen long-term effects and refining the design of future implants.
The biocompatibility of polycarbonate implants is primarily assessed through in vitro and in vivo studies. These evaluations focus on the material's interaction with living tissues, potential inflammatory responses, and any adverse effects on cellular functions. Research has shown that polycarbonate generally exhibits good biocompatibility, with minimal tissue reactions and low cytotoxicity. However, the specific formulation and manufacturing processes can significantly influence its biological performance.
Long-term safety considerations for polycarbonate implants encompass several key aspects. One primary concern is the potential leaching of bisphenol A (BPA), a component used in polycarbonate synthesis. While modern polycarbonates are designed to minimize BPA release, ongoing research is essential to ensure that trace amounts do not accumulate in the body over extended periods, potentially leading to endocrine disruption or other health issues.
Another critical factor in long-term safety is the material's degradation behavior in the physiological environment. Polycarbonate implants must maintain their structural integrity and mechanical properties over the intended lifespan of the device. Studies have shown that polycarbonate can undergo slow hydrolytic degradation in vivo, which may affect its long-term performance. Researchers are exploring various surface modifications and composite formulations to enhance the material's resistance to degradation and improve its longevity in biological systems.
The immune response to polycarbonate implants is another crucial aspect of long-term safety. While the material is generally well-tolerated, some patients may develop a foreign body response over time. This can lead to the formation of a fibrous capsule around the implant, potentially affecting its function or integration with surrounding tissues. Ongoing research focuses on developing surface treatments and coatings that can modulate the immune response and promote better tissue integration.
Sterilization methods and their impact on polycarbonate's long-term performance are also under scrutiny. Common sterilization techniques, such as gamma irradiation or ethylene oxide treatment, can potentially alter the material's properties or induce the formation of toxic byproducts. Researchers are investigating alternative sterilization methods and optimizing existing processes to ensure the long-term stability and safety of polycarbonate implants.
As medical implant innovations continue to evolve, the development of more sophisticated in vitro models and long-term in vivo studies will be crucial for comprehensively assessing the biocompatibility and safety of polycarbonate-based devices. Additionally, post-market surveillance and real-world data collection will play an increasingly important role in identifying any unforeseen long-term effects and refining the design of future implants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!