Role of Solid Electrolyte Interphase in Lithium Anode Stability
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SEI Formation Background and Objectives
The Solid Electrolyte Interphase (SEI) represents one of the most critical components in lithium-ion battery systems, particularly for lithium metal anodes. Since its discovery in the 1970s, researchers have recognized that this thin, passive film forms spontaneously on electrode surfaces when exposed to electrolytes, fundamentally altering the electrode-electrolyte interface properties. The SEI layer serves as a crucial protective barrier that prevents continuous electrolyte decomposition while allowing selective lithium-ion transport.
Historical development of SEI understanding has evolved through several distinct phases. Initially considered merely a side reaction product, the SEI is now recognized as an engineerable interface that significantly determines battery performance metrics including capacity retention, cycling stability, and safety characteristics. The evolution of analytical techniques, particularly surface characterization methods like XPS, TOF-SIMS, and cryo-electron microscopy, has enabled increasingly sophisticated understanding of SEI composition, morphology, and formation mechanisms.
Current technological challenges in lithium metal batteries primarily stem from the highly reactive nature of lithium metal and its tendency to form dendrites during cycling. The unstable SEI formation on lithium metal surfaces leads to continuous electrolyte consumption, low Coulombic efficiency, and eventual cell failure. These issues have prevented the widespread commercialization of lithium metal batteries despite their theoretical energy density advantages over conventional lithium-ion systems.
The primary objective of SEI research in lithium metal anodes is to develop strategies for creating stable, uniform, and functional interfaces that can effectively suppress dendrite growth while maintaining high ionic conductivity. This requires fundamental understanding of SEI formation mechanisms, composition-property relationships, and dynamic evolution during battery operation. Researchers aim to design artificial SEI layers or electrolyte additives that can form ideal interfaces with specific properties.
Geographic distribution of SEI research shows concentrated efforts in East Asia (particularly Japan, South Korea, and China), North America, and Europe, with emerging contributions from other regions. The field has seen accelerated growth in patent filings and publications over the past decade, reflecting its strategic importance in next-generation battery development.
The technological trajectory suggests that mastering SEI formation and stability represents a critical bottleneck in realizing practical lithium metal batteries. Success in this domain could enable energy storage systems with significantly higher energy densities than current commercial technologies, potentially revolutionizing applications from portable electronics to electric vehicles and grid-scale storage.
Historical development of SEI understanding has evolved through several distinct phases. Initially considered merely a side reaction product, the SEI is now recognized as an engineerable interface that significantly determines battery performance metrics including capacity retention, cycling stability, and safety characteristics. The evolution of analytical techniques, particularly surface characterization methods like XPS, TOF-SIMS, and cryo-electron microscopy, has enabled increasingly sophisticated understanding of SEI composition, morphology, and formation mechanisms.
Current technological challenges in lithium metal batteries primarily stem from the highly reactive nature of lithium metal and its tendency to form dendrites during cycling. The unstable SEI formation on lithium metal surfaces leads to continuous electrolyte consumption, low Coulombic efficiency, and eventual cell failure. These issues have prevented the widespread commercialization of lithium metal batteries despite their theoretical energy density advantages over conventional lithium-ion systems.
The primary objective of SEI research in lithium metal anodes is to develop strategies for creating stable, uniform, and functional interfaces that can effectively suppress dendrite growth while maintaining high ionic conductivity. This requires fundamental understanding of SEI formation mechanisms, composition-property relationships, and dynamic evolution during battery operation. Researchers aim to design artificial SEI layers or electrolyte additives that can form ideal interfaces with specific properties.
Geographic distribution of SEI research shows concentrated efforts in East Asia (particularly Japan, South Korea, and China), North America, and Europe, with emerging contributions from other regions. The field has seen accelerated growth in patent filings and publications over the past decade, reflecting its strategic importance in next-generation battery development.
The technological trajectory suggests that mastering SEI formation and stability represents a critical bottleneck in realizing practical lithium metal batteries. Success in this domain could enable energy storage systems with significantly higher energy densities than current commercial technologies, potentially revolutionizing applications from portable electronics to electric vehicles and grid-scale storage.
Market Analysis for Lithium Battery Technologies
The global lithium battery market has experienced unprecedented growth, driven primarily by the electric vehicle (EV) revolution and renewable energy storage demands. Currently valued at approximately $46.6 billion in 2022, the market is projected to reach $165.8 billion by 2030, representing a CAGR of 17.3% during the forecast period. This remarkable growth trajectory underscores the critical importance of advancing lithium anode stability technologies, particularly those focused on Solid Electrolyte Interphase (SEI) optimization.
The EV segment dominates market demand, accounting for over 60% of lithium battery consumption. Major automotive manufacturers have committed to electrification targets, with companies like Volkswagen, GM, and Ford pledging billions in EV development. This automotive transition is creating substantial pull for batteries with higher energy density, faster charging capabilities, and improved safety profiles – all factors directly impacted by SEI performance on lithium anodes.
Consumer electronics represents the second-largest application segment, demanding approximately 25% of the market share. Here, the emphasis on longer device runtime and reduced form factors drives interest in lithium metal anodes with stable SEI layers that can enable higher energy densities while maintaining cycle life.
Geographically, Asia-Pacific leads manufacturing capacity, with China controlling nearly 75% of global lithium battery production. However, significant investments are underway in North America and Europe to reduce supply chain dependencies, with the US Inflation Reduction Act allocating $369 billion toward clean energy initiatives, including battery manufacturing.
Investor confidence in advanced battery technologies remains strong, with venture capital funding for battery startups reaching $17 billion in 2022. Technologies specifically addressing lithium anode stability through SEI engineering have attracted particular interest, with several startups securing funding rounds exceeding $100 million.
Market analysis reveals a clear premium pricing potential for batteries incorporating advanced SEI technologies. Batteries demonstrating 30% higher energy density through stable lithium metal anodes can command price premiums of 40-50% in high-value applications such as aerospace, premium EVs, and medical devices.
Customer surveys indicate that safety remains the primary concern among EV consumers, with 78% of potential buyers citing battery safety as "very important" in purchasing decisions. This consumer sentiment directly aligns with the benefits of optimized SEI layers, which can significantly reduce dendrite formation and thermal runaway risks in lithium metal batteries.
The EV segment dominates market demand, accounting for over 60% of lithium battery consumption. Major automotive manufacturers have committed to electrification targets, with companies like Volkswagen, GM, and Ford pledging billions in EV development. This automotive transition is creating substantial pull for batteries with higher energy density, faster charging capabilities, and improved safety profiles – all factors directly impacted by SEI performance on lithium anodes.
Consumer electronics represents the second-largest application segment, demanding approximately 25% of the market share. Here, the emphasis on longer device runtime and reduced form factors drives interest in lithium metal anodes with stable SEI layers that can enable higher energy densities while maintaining cycle life.
Geographically, Asia-Pacific leads manufacturing capacity, with China controlling nearly 75% of global lithium battery production. However, significant investments are underway in North America and Europe to reduce supply chain dependencies, with the US Inflation Reduction Act allocating $369 billion toward clean energy initiatives, including battery manufacturing.
Investor confidence in advanced battery technologies remains strong, with venture capital funding for battery startups reaching $17 billion in 2022. Technologies specifically addressing lithium anode stability through SEI engineering have attracted particular interest, with several startups securing funding rounds exceeding $100 million.
Market analysis reveals a clear premium pricing potential for batteries incorporating advanced SEI technologies. Batteries demonstrating 30% higher energy density through stable lithium metal anodes can command price premiums of 40-50% in high-value applications such as aerospace, premium EVs, and medical devices.
Customer surveys indicate that safety remains the primary concern among EV consumers, with 78% of potential buyers citing battery safety as "very important" in purchasing decisions. This consumer sentiment directly aligns with the benefits of optimized SEI layers, which can significantly reduce dendrite formation and thermal runaway risks in lithium metal batteries.
Current SEI Challenges in Lithium Metal Anodes
Despite significant advancements in lithium-ion battery technology, the Solid Electrolyte Interphase (SEI) layer on lithium metal anodes continues to present formidable challenges that impede the commercialization of high-energy-density lithium metal batteries. The inherent instability and dynamic nature of the SEI layer on lithium metal surfaces create a complex set of interrelated problems that researchers worldwide are striving to overcome.
One of the primary challenges is the inhomogeneous and unstable nature of naturally formed SEI layers. Unlike graphite anodes, lithium metal undergoes continuous volume changes during cycling, causing repeated breaking and reformation of the SEI. This process consumes electrolyte and lithium, leading to capacity fade and shortened battery life. The mechanical instability of conventional SEI layers cannot accommodate the significant volume changes associated with lithium plating and stripping.
The chemical composition and structure of SEI layers present another critical challenge. Current SEI formations typically consist of a heterogeneous mixture of organic and inorganic components with varying ionic conductivities and mechanical properties. This heterogeneity leads to uneven lithium deposition, promoting dendrite formation that can eventually penetrate the separator and cause catastrophic short circuits. The lack of precise control over SEI composition remains a significant hurdle.
Ion transport limitations within the SEI layer constitute another major challenge. Ideally, an SEI should facilitate rapid Li+ transport while blocking electron transfer. However, most naturally formed SEI layers exhibit suboptimal Li+ conductivity, creating concentration gradients that further promote non-uniform lithium deposition. This transport limitation becomes particularly problematic at high current densities required for fast-charging applications.
The reactivity of lithium metal with conventional liquid electrolytes represents a persistent challenge. Even with SEI formation, slow but continuous parasitic reactions occur between lithium and electrolyte components, gradually degrading battery performance. These reactions are particularly aggressive at elevated temperatures, limiting the thermal operating window of lithium metal batteries.
Manufacturing challenges also complicate SEI engineering. Creating consistent, high-quality SEI layers at industrial scales remains difficult due to lithium's high reactivity with atmospheric components. The extreme sensitivity to moisture and oxygen necessitates stringent manufacturing conditions that add significant cost and complexity to production processes.
Finally, analytical limitations hinder progress in SEI research. The thin, chemically complex, and environmentally sensitive nature of SEI layers makes in-situ characterization extremely challenging. This creates obstacles for researchers attempting to understand formation mechanisms and degradation pathways, ultimately slowing the development of effective SEI engineering strategies.
One of the primary challenges is the inhomogeneous and unstable nature of naturally formed SEI layers. Unlike graphite anodes, lithium metal undergoes continuous volume changes during cycling, causing repeated breaking and reformation of the SEI. This process consumes electrolyte and lithium, leading to capacity fade and shortened battery life. The mechanical instability of conventional SEI layers cannot accommodate the significant volume changes associated with lithium plating and stripping.
The chemical composition and structure of SEI layers present another critical challenge. Current SEI formations typically consist of a heterogeneous mixture of organic and inorganic components with varying ionic conductivities and mechanical properties. This heterogeneity leads to uneven lithium deposition, promoting dendrite formation that can eventually penetrate the separator and cause catastrophic short circuits. The lack of precise control over SEI composition remains a significant hurdle.
Ion transport limitations within the SEI layer constitute another major challenge. Ideally, an SEI should facilitate rapid Li+ transport while blocking electron transfer. However, most naturally formed SEI layers exhibit suboptimal Li+ conductivity, creating concentration gradients that further promote non-uniform lithium deposition. This transport limitation becomes particularly problematic at high current densities required for fast-charging applications.
The reactivity of lithium metal with conventional liquid electrolytes represents a persistent challenge. Even with SEI formation, slow but continuous parasitic reactions occur between lithium and electrolyte components, gradually degrading battery performance. These reactions are particularly aggressive at elevated temperatures, limiting the thermal operating window of lithium metal batteries.
Manufacturing challenges also complicate SEI engineering. Creating consistent, high-quality SEI layers at industrial scales remains difficult due to lithium's high reactivity with atmospheric components. The extreme sensitivity to moisture and oxygen necessitates stringent manufacturing conditions that add significant cost and complexity to production processes.
Finally, analytical limitations hinder progress in SEI research. The thin, chemically complex, and environmentally sensitive nature of SEI layers makes in-situ characterization extremely challenging. This creates obstacles for researchers attempting to understand formation mechanisms and degradation pathways, ultimately slowing the development of effective SEI engineering strategies.
Current SEI Engineering Approaches
01 Electrolyte additives for SEI stabilization
Various electrolyte additives can be incorporated into battery systems to enhance the stability of the Solid Electrolyte Interphase (SEI). These additives form a more robust and uniform SEI layer on the electrode surface, preventing continuous electrolyte decomposition and improving the cycling performance of lithium-ion batteries. Common additives include fluorinated compounds, carbonates, and specific salts that contribute to forming a more stable protective layer.- Electrolyte additives for SEI stabilization: Various electrolyte additives can be incorporated into battery systems to enhance the stability of the Solid Electrolyte Interphase (SEI). These additives form a more robust and uniform SEI layer on the electrode surface, preventing continuous electrolyte decomposition and improving cycling performance. Common additives include fluorinated compounds, carbonates, and specific salts that contribute to forming a more stable passivation layer, thereby extending battery life and improving safety.
- Novel electrode materials for improved SEI formation: Advanced electrode materials can be designed to promote the formation of a more stable SEI layer. These materials include modified graphite anodes, silicon-based composites, and metal oxides with tailored surface properties. By controlling the surface chemistry of electrode materials, the SEI formation process can be optimized to create a more compact and durable interface layer that resists cracking during cycling and provides better protection against continuous electrolyte decomposition.
- Protective coatings and surface modifications: Applying protective coatings or surface modifications to electrode materials can significantly enhance SEI stability. These coatings act as artificial SEI layers or templates for more controlled natural SEI formation. Techniques include atomic layer deposition of metal oxides, polymer coatings, and carbon-based protective layers. These modifications create a barrier between the electrode and electrolyte, reducing unwanted side reactions while still allowing lithium ion transport, resulting in more stable cycling performance.
- Advanced electrolyte formulations: Novel electrolyte formulations can be developed to promote the formation of more stable SEI layers. These include concentrated electrolytes, ionic liquid-based systems, and solvent blends with optimized properties. By carefully selecting electrolyte components, the chemical composition and physical properties of the resulting SEI can be tailored to provide better mechanical stability, ionic conductivity, and resistance to degradation mechanisms, leading to improved battery performance and longevity.
- Temperature and cycling condition management: Controlling operating conditions such as temperature ranges and charging protocols can significantly impact SEI stability. Optimized formation cycles, temperature-controlled operation, and tailored current densities during charging and discharging can lead to more favorable SEI properties. Battery management systems can be designed to implement these strategies, avoiding conditions that accelerate SEI degradation while promoting those that enhance its protective qualities, thereby extending battery life and maintaining performance.
02 Novel electrode materials for improved SEI formation
Advanced electrode materials can be designed to promote the formation of a more stable SEI layer. These materials include modified graphite anodes, silicon-based composites, and metal oxides with tailored surface properties. By controlling the surface chemistry of electrode materials, the SEI formation process can be optimized, resulting in enhanced stability during battery cycling and improved capacity retention over extended use.Expand Specific Solutions03 Protective coatings and surface modifications
Applying protective coatings or surface modifications to electrode materials can significantly enhance SEI stability. These coatings act as artificial SEI layers or templates for controlled SEI formation, preventing direct contact between the electrode and aggressive electrolyte components. Various coating materials including metal oxides, polymers, and carbon-based materials can be used to create a more stable interface, reducing capacity fade and extending battery life.Expand Specific Solutions04 Temperature and cycling condition optimization
Controlling operating temperature and cycling conditions plays a crucial role in maintaining SEI stability. Specific temperature ranges and charging protocols can be implemented to minimize SEI degradation and growth. Advanced battery management systems that regulate charging rates, depth of discharge, and operating temperature can significantly improve SEI stability, leading to enhanced battery performance and longevity.Expand Specific Solutions05 Novel electrolyte systems and formulations
Developing new electrolyte systems and formulations can fundamentally improve SEI stability. These include concentrated electrolytes, ionic liquid-based electrolytes, and solid-state electrolytes that form more stable interfaces with electrode materials. By carefully designing the electrolyte composition, the chemical reactions at the electrode-electrolyte interface can be controlled to form a more stable and functional SEI layer, improving overall battery performance and safety.Expand Specific Solutions
Leading Institutions and Companies in SEI Development
The Solid Electrolyte Interphase (SEI) in lithium anode stability represents a critical frontier in battery technology, currently in a growth phase with increasing market adoption. The global market for advanced lithium battery technologies is expanding rapidly, projected to reach significant scale as electric vehicle adoption accelerates. Technical maturity varies across key players, with companies like Samsung SDI, LG Energy Solution, and Nissan demonstrating advanced commercial implementations. Research institutions including ETH Zurich, University of Maryland, and Beijing Institute of Technology are making fundamental breakthroughs in SEI formation mechanisms. Emerging companies such as Enevate and Ion Storage Systems are developing innovative approaches to stabilize lithium anodes through engineered SEI layers, while established manufacturers like Corning and Sharp are integrating these advances into production processes.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed a systematic approach to lithium anode stabilization through advanced SEI engineering called "Functional Interface Architecture." Their technology focuses on creating multi-functional SEI layers with precisely controlled composition and structure. The institute has pioneered the use of molecular design principles to develop electrolyte additives that decompose to form specific SEI components with targeted properties. Their research has demonstrated that incorporating nitrogen-rich heterocyclic compounds and fluorinated aromatics can create SEI layers with superior mechanical properties and lithium ion conductivity. The institute has also developed novel in-situ characterization techniques that allow real-time monitoring of SEI formation and evolution, enabling precise optimization of formation protocols. Their approach includes the use of self-healing polymeric components within the SEI structure that can repair damage during cycling, significantly extending battery lifetime. Recent publications from the institute have shown that their engineered SEI layers can enable lithium metal anodes to achieve over 1000 cycles with minimal capacity degradation.
Strengths: Cutting-edge fundamental research capabilities allowing precise understanding and control of SEI formation mechanisms. Their self-healing SEI concept addresses a critical failure mode in lithium metal batteries. Weaknesses: The sophisticated molecular design approach may face challenges in cost-effective scale-up for commercial applications. Some of their advanced additives may present environmental or safety concerns that require further evaluation.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has pioneered a comprehensive approach to lithium anode stabilization through SEI engineering called "Composite Protection Layer Technology." This system combines ex-situ fabricated artificial SEI layers with in-situ SEI formation strategies. Their technology utilizes a gradient-structured protective layer where the inner layer consists of inorganic components (primarily lithium phosphorus oxynitride and lithium fluorides) that provide mechanical stability, while the outer layer incorporates elastomeric polymers that accommodate volume changes during cycling. Samsung's approach also features electrolyte additives specifically designed to form stable SEI components that resist continuous electrolyte decomposition. They've developed proprietary fluoroethylene carbonate derivatives that decompose at controlled potentials to form LiF-rich SEI layers. Additionally, Samsung employs surface texturing of lithium metal to control SEI formation sites and distribute current density, preventing dendrite nucleation at specific locations.
Strengths: The gradient-structured approach provides both mechanical stability and flexibility, addressing the volume change issues in lithium metal anodes. Their integrated system approach combines multiple stabilization strategies. Weaknesses: The complex multi-layer structure may introduce manufacturing challenges and increase production costs. The technology may still face limitations in extremely fast charging scenarios where dendrite formation is accelerated.
Key Patents and Scientific Breakthroughs in SEI Technology
Lithium anode with solid electrolyte interface
PatentInactiveUS6337159B1
Innovation
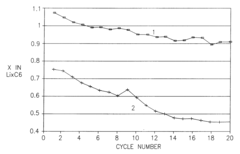
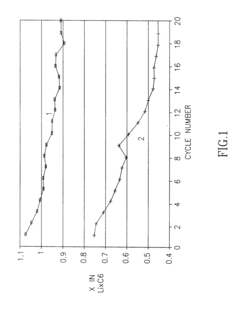
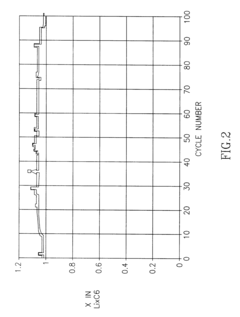
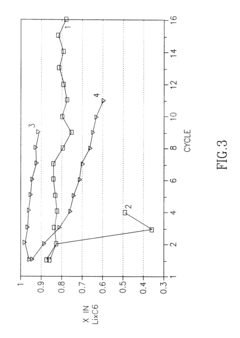
- The development of a non-aqueous battery with carbon-based anodes coated by a thin, chemically bonded Solid Electrolyte Interphase (SEI) composed of alkali or alkaline-earth metal salts and organic binders, which are insoluble in the electrolyte, and partially oxidized to form narrow pores and surface carboxylic groups, enhancing mechanical stability and preventing solvent co-intercalation.
Safety Standards and Testing Protocols for Lithium Anodes
The development of comprehensive safety standards and testing protocols for lithium anodes represents a critical aspect of advancing lithium metal battery technology. Current safety evaluation frameworks primarily focus on conventional lithium-ion batteries with graphite anodes, necessitating significant adaptation to address the unique challenges posed by lithium metal anodes and their Solid Electrolyte Interphase (SEI) layers.
International organizations including the International Electrotechnical Commission (IEC), Underwriters Laboratories (UL), and the Institute of Electrical and Electronics Engineers (IEEE) have begun establishing specialized testing protocols for lithium metal batteries. These standards typically encompass thermal stability assessments, mechanical integrity evaluations, and electrical safety parameters that specifically address lithium metal's reactivity and dendrite formation tendencies.
The thermal runaway risk assessment has emerged as a cornerstone of lithium anode safety testing, with differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) serving as primary analytical methods. These techniques enable researchers to quantify the onset temperature and heat generation during thermal events, providing critical data on SEI stability under thermal stress conditions.
Mechanical testing protocols have evolved to evaluate the robustness of lithium anodes and their SEI layers under various stress conditions. Nail penetration tests, crush tests, and vibration analyses help determine the mechanical integrity of the anode structure and its protective SEI layer, with particular attention to dendrite penetration resistance.
Electrochemical safety testing has become increasingly sophisticated, incorporating cycling stability assessments under various current densities, temperature conditions, and pressure environments. Advanced protocols now include impedance spectroscopy measurements to monitor SEI formation and evolution during cycling, providing early indicators of potential safety issues.
Standardized dendrite growth evaluation methods represent a significant advancement in lithium anode safety protocols. These include time-lapse microscopy techniques, electrochemical impedance spectroscopy (EIS), and post-mortem analyses that quantify dendrite morphology, growth rates, and penetration tendencies through the SEI layer.
The integration of in-situ monitoring technologies into safety testing frameworks has enabled real-time observation of SEI formation and degradation processes. Techniques such as in-situ X-ray diffraction, neutron imaging, and specialized electrochemical cells with observation windows provide unprecedented insights into failure mechanisms and early warning indicators.
Regulatory bodies including the Department of Transportation (DOT), International Air Transport Association (IATA), and various national safety agencies have established increasingly stringent transportation and handling requirements for lithium metal batteries, reflecting the heightened safety concerns associated with these high-energy-density systems compared to conventional lithium-ion technologies.
International organizations including the International Electrotechnical Commission (IEC), Underwriters Laboratories (UL), and the Institute of Electrical and Electronics Engineers (IEEE) have begun establishing specialized testing protocols for lithium metal batteries. These standards typically encompass thermal stability assessments, mechanical integrity evaluations, and electrical safety parameters that specifically address lithium metal's reactivity and dendrite formation tendencies.
The thermal runaway risk assessment has emerged as a cornerstone of lithium anode safety testing, with differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) serving as primary analytical methods. These techniques enable researchers to quantify the onset temperature and heat generation during thermal events, providing critical data on SEI stability under thermal stress conditions.
Mechanical testing protocols have evolved to evaluate the robustness of lithium anodes and their SEI layers under various stress conditions. Nail penetration tests, crush tests, and vibration analyses help determine the mechanical integrity of the anode structure and its protective SEI layer, with particular attention to dendrite penetration resistance.
Electrochemical safety testing has become increasingly sophisticated, incorporating cycling stability assessments under various current densities, temperature conditions, and pressure environments. Advanced protocols now include impedance spectroscopy measurements to monitor SEI formation and evolution during cycling, providing early indicators of potential safety issues.
Standardized dendrite growth evaluation methods represent a significant advancement in lithium anode safety protocols. These include time-lapse microscopy techniques, electrochemical impedance spectroscopy (EIS), and post-mortem analyses that quantify dendrite morphology, growth rates, and penetration tendencies through the SEI layer.
The integration of in-situ monitoring technologies into safety testing frameworks has enabled real-time observation of SEI formation and degradation processes. Techniques such as in-situ X-ray diffraction, neutron imaging, and specialized electrochemical cells with observation windows provide unprecedented insights into failure mechanisms and early warning indicators.
Regulatory bodies including the Department of Transportation (DOT), International Air Transport Association (IATA), and various national safety agencies have established increasingly stringent transportation and handling requirements for lithium metal batteries, reflecting the heightened safety concerns associated with these high-energy-density systems compared to conventional lithium-ion technologies.
Environmental Impact of SEI Components
The environmental impact of SEI components in lithium-ion batteries represents a critical consideration as these energy storage technologies continue to proliferate globally. The formation of the Solid Electrolyte Interphase involves various chemical compounds that may pose significant environmental concerns throughout the battery lifecycle, from manufacturing to disposal.
Primary SEI components such as lithium fluoride (LiF), lithium carbonate (Li₂CO₃), and various organic compounds can enter ecosystems through improper disposal or recycling processes. These materials may contaminate soil and water systems, potentially disrupting local ecological balances. Studies have shown that fluoride compounds in particular can persist in the environment and bioaccumulate in certain organisms, raising long-term toxicity concerns.
Manufacturing processes for SEI precursors often involve fluorinated compounds and organic solvents that contribute to greenhouse gas emissions and air pollution. The production of fluoroethylene carbonate (FEC) and vinylene carbonate (VC), common SEI-forming additives, requires energy-intensive synthesis routes that further increase the carbon footprint of battery production.
Leaching tests conducted on degraded battery materials have demonstrated that SEI decomposition products can release heavy metals and toxic organic compounds. This presents challenges for waste management systems and highlights the need for specialized recycling protocols. Current estimates suggest that less than 5% of lithium battery components are effectively recovered through existing recycling methods, with SEI materials presenting particular separation difficulties.
Recent research has focused on developing environmentally benign SEI components derived from sustainable sources. Bio-based additives and naturally occurring polymers show promise as green alternatives that can form stable interfaces while minimizing environmental impact. These approaches align with circular economy principles and may significantly reduce the ecological footprint of lithium battery technologies.
Regulatory frameworks worldwide are increasingly addressing the environmental implications of battery components, including SEI materials. The European Union's Battery Directive and similar legislation in Asia and North America are establishing more stringent requirements for battery composition disclosure and end-of-life management. These regulations are driving innovation toward more environmentally compatible SEI formulations.
Life cycle assessment (LCA) studies comparing different SEI compositions reveal significant variations in environmental impact. Batteries utilizing silicon-based anodes typically form more complex SEI structures with higher environmental burdens compared to traditional graphite systems. This highlights the importance of considering SEI environmental impacts when developing next-generation battery technologies.
Primary SEI components such as lithium fluoride (LiF), lithium carbonate (Li₂CO₃), and various organic compounds can enter ecosystems through improper disposal or recycling processes. These materials may contaminate soil and water systems, potentially disrupting local ecological balances. Studies have shown that fluoride compounds in particular can persist in the environment and bioaccumulate in certain organisms, raising long-term toxicity concerns.
Manufacturing processes for SEI precursors often involve fluorinated compounds and organic solvents that contribute to greenhouse gas emissions and air pollution. The production of fluoroethylene carbonate (FEC) and vinylene carbonate (VC), common SEI-forming additives, requires energy-intensive synthesis routes that further increase the carbon footprint of battery production.
Leaching tests conducted on degraded battery materials have demonstrated that SEI decomposition products can release heavy metals and toxic organic compounds. This presents challenges for waste management systems and highlights the need for specialized recycling protocols. Current estimates suggest that less than 5% of lithium battery components are effectively recovered through existing recycling methods, with SEI materials presenting particular separation difficulties.
Recent research has focused on developing environmentally benign SEI components derived from sustainable sources. Bio-based additives and naturally occurring polymers show promise as green alternatives that can form stable interfaces while minimizing environmental impact. These approaches align with circular economy principles and may significantly reduce the ecological footprint of lithium battery technologies.
Regulatory frameworks worldwide are increasingly addressing the environmental implications of battery components, including SEI materials. The European Union's Battery Directive and similar legislation in Asia and North America are establishing more stringent requirements for battery composition disclosure and end-of-life management. These regulations are driving innovation toward more environmentally compatible SEI formulations.
Life cycle assessment (LCA) studies comparing different SEI compositions reveal significant variations in environmental impact. Batteries utilizing silicon-based anodes typically form more complex SEI structures with higher environmental burdens compared to traditional graphite systems. This highlights the importance of considering SEI environmental impacts when developing next-generation battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!