Sodium silicate interaction in layered double hydroxide synthesis
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LDH Synthesis Background
Layered double hydroxides (LDHs) have garnered significant attention in materials science due to their unique structure and versatile applications. The synthesis of LDHs involves the interaction of various components, with sodium silicate playing a crucial role in many preparation methods. Understanding the background of LDH synthesis is essential for comprehending the importance of sodium silicate interactions in this process.
LDHs, also known as anionic clays, are a class of two-dimensional nanostructured materials composed of positively charged metal hydroxide layers with intercalated anions and water molecules. The general formula for LDHs is [M2+1-xM3+x(OH)2]x+[An-]x/n·mH2O, where M2+ and M3+ represent divalent and trivalent metal cations, respectively, and An- represents the interlayer anions.
The history of LDH synthesis dates back to the discovery of the mineral hydrotalcite in 1842. However, it wasn't until the mid-20th century that researchers began to explore synthetic methods for producing LDHs. The first synthetic LDHs were prepared using coprecipitation techniques, which remain one of the most common methods for LDH synthesis today.
Over the years, various synthesis methods have been developed to produce LDHs with specific compositions and properties. These methods include coprecipitation, ion-exchange, reconstruction, hydrothermal synthesis, and sol-gel techniques. Each method offers unique advantages and challenges, influencing the final properties of the LDHs produced.
The incorporation of sodium silicate in LDH synthesis has emerged as a significant development in recent years. Sodium silicate, also known as water glass, is an aqueous solution of sodium oxide (Na2O) and silicon dioxide (SiO2). Its introduction into LDH synthesis processes has led to improvements in the structural stability, porosity, and functionality of the resulting materials.
The interaction between sodium silicate and LDH precursors during synthesis can result in the formation of silicate-intercalated LDHs or silica-LDH nanocomposites. These materials often exhibit enhanced thermal stability, increased surface area, and improved adsorption properties compared to traditional LDHs. The presence of silicate species can also influence the morphology and crystallinity of the LDH particles.
Understanding the role of sodium silicate in LDH synthesis is crucial for tailoring the properties of these materials for specific applications. The interaction between silicate anions and metal cations during the formation of LDH structures can lead to unique physicochemical properties, opening up new possibilities for their use in catalysis, environmental remediation, drug delivery, and energy storage applications.
LDHs, also known as anionic clays, are a class of two-dimensional nanostructured materials composed of positively charged metal hydroxide layers with intercalated anions and water molecules. The general formula for LDHs is [M2+1-xM3+x(OH)2]x+[An-]x/n·mH2O, where M2+ and M3+ represent divalent and trivalent metal cations, respectively, and An- represents the interlayer anions.
The history of LDH synthesis dates back to the discovery of the mineral hydrotalcite in 1842. However, it wasn't until the mid-20th century that researchers began to explore synthetic methods for producing LDHs. The first synthetic LDHs were prepared using coprecipitation techniques, which remain one of the most common methods for LDH synthesis today.
Over the years, various synthesis methods have been developed to produce LDHs with specific compositions and properties. These methods include coprecipitation, ion-exchange, reconstruction, hydrothermal synthesis, and sol-gel techniques. Each method offers unique advantages and challenges, influencing the final properties of the LDHs produced.
The incorporation of sodium silicate in LDH synthesis has emerged as a significant development in recent years. Sodium silicate, also known as water glass, is an aqueous solution of sodium oxide (Na2O) and silicon dioxide (SiO2). Its introduction into LDH synthesis processes has led to improvements in the structural stability, porosity, and functionality of the resulting materials.
The interaction between sodium silicate and LDH precursors during synthesis can result in the formation of silicate-intercalated LDHs or silica-LDH nanocomposites. These materials often exhibit enhanced thermal stability, increased surface area, and improved adsorption properties compared to traditional LDHs. The presence of silicate species can also influence the morphology and crystallinity of the LDH particles.
Understanding the role of sodium silicate in LDH synthesis is crucial for tailoring the properties of these materials for specific applications. The interaction between silicate anions and metal cations during the formation of LDH structures can lead to unique physicochemical properties, opening up new possibilities for their use in catalysis, environmental remediation, drug delivery, and energy storage applications.
Market Analysis for LDHs
The market for Layered Double Hydroxides (LDHs) has been experiencing significant growth in recent years, driven by their versatile applications across various industries. LDHs, also known as anionic clays or hydrotalcite-like compounds, have gained traction due to their unique structure and properties, making them valuable in catalysis, environmental remediation, and advanced materials development.
In the catalysis sector, LDHs have shown promising results as supports for various catalytic processes, including hydrogenation, oxidation, and polymerization reactions. This has led to increased demand from the petrochemical and fine chemical industries, where efficient and selective catalysts are crucial for process optimization and cost reduction.
The environmental remediation market has also contributed to the growth of LDH demand. These materials have demonstrated excellent adsorption capabilities for various pollutants, including heavy metals, organic contaminants, and phosphates. As global environmental regulations become more stringent, the use of LDHs in water and soil treatment applications is expected to expand further.
In the field of advanced materials, LDHs have found applications in flame retardants, polymer additives, and drug delivery systems. The automotive and construction industries have shown particular interest in LDH-based flame retardants due to their effectiveness and environmentally friendly nature compared to traditional halogenated compounds.
The pharmaceutical and healthcare sectors have also recognized the potential of LDHs as drug delivery vehicles and biocompatible materials. This has opened up new avenues for market growth, especially in the development of controlled-release formulations and targeted drug delivery systems.
Geographically, Asia-Pacific has emerged as the largest market for LDHs, driven by rapid industrialization and increasing environmental concerns in countries like China and India. North America and Europe follow closely, with strong demand from the automotive, pharmaceutical, and chemical industries.
The global LDH market is expected to continue its growth trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is attributed to ongoing research and development efforts, expanding applications, and increasing awareness of the benefits of LDHs across various industries.
However, challenges remain in the widespread adoption of LDHs, including the need for cost-effective large-scale production methods and the development of tailored LDH compositions for specific applications. Overcoming these challenges will be crucial for realizing the full market potential of LDHs and driving further innovation in this field.
In the catalysis sector, LDHs have shown promising results as supports for various catalytic processes, including hydrogenation, oxidation, and polymerization reactions. This has led to increased demand from the petrochemical and fine chemical industries, where efficient and selective catalysts are crucial for process optimization and cost reduction.
The environmental remediation market has also contributed to the growth of LDH demand. These materials have demonstrated excellent adsorption capabilities for various pollutants, including heavy metals, organic contaminants, and phosphates. As global environmental regulations become more stringent, the use of LDHs in water and soil treatment applications is expected to expand further.
In the field of advanced materials, LDHs have found applications in flame retardants, polymer additives, and drug delivery systems. The automotive and construction industries have shown particular interest in LDH-based flame retardants due to their effectiveness and environmentally friendly nature compared to traditional halogenated compounds.
The pharmaceutical and healthcare sectors have also recognized the potential of LDHs as drug delivery vehicles and biocompatible materials. This has opened up new avenues for market growth, especially in the development of controlled-release formulations and targeted drug delivery systems.
Geographically, Asia-Pacific has emerged as the largest market for LDHs, driven by rapid industrialization and increasing environmental concerns in countries like China and India. North America and Europe follow closely, with strong demand from the automotive, pharmaceutical, and chemical industries.
The global LDH market is expected to continue its growth trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is attributed to ongoing research and development efforts, expanding applications, and increasing awareness of the benefits of LDHs across various industries.
However, challenges remain in the widespread adoption of LDHs, including the need for cost-effective large-scale production methods and the development of tailored LDH compositions for specific applications. Overcoming these challenges will be crucial for realizing the full market potential of LDHs and driving further innovation in this field.
Sodium Silicate Challenges
The synthesis of layered double hydroxides (LDHs) using sodium silicate presents several significant challenges that researchers and industry professionals must address. One of the primary issues is the complex interaction between sodium silicate and the metal cations used in LDH formation. Sodium silicate's high alkalinity can lead to uncontrolled precipitation, resulting in poorly defined LDH structures and reduced crystallinity.
Another challenge lies in controlling the incorporation of silicate anions into the LDH interlayer space. While silicate intercalation can enhance the material's properties, excessive silicate content may disrupt the layered structure and compromise the desired functionality of the LDH. Achieving the optimal balance between silicate incorporation and maintaining the LDH's structural integrity remains a critical challenge.
The formation of amorphous silica as a byproduct during LDH synthesis with sodium silicate is another significant obstacle. This unwanted phase can interfere with the LDH crystallization process and affect the purity of the final product. Developing strategies to minimize or eliminate amorphous silica formation while maximizing LDH yield is crucial for improving synthesis efficiency.
Reproducibility and scalability of LDH synthesis using sodium silicate also pose challenges. The sensitivity of the reaction to pH, temperature, and concentration variations can lead to inconsistent results between batches. This variability hinders the large-scale production of LDHs with consistent properties, limiting their potential industrial applications.
Furthermore, the use of sodium silicate in LDH synthesis can impact the material's stability and ion exchange capacity. The strong Si-O bonds formed during the synthesis process may reduce the LDH's ability to exchange anions, potentially limiting its effectiveness in applications such as catalysis and environmental remediation.
Lastly, the environmental impact of using sodium silicate in LDH production is a growing concern. The high alkalinity of sodium silicate solutions can pose handling and disposal challenges, necessitating the development of more sustainable and eco-friendly synthesis methods. Addressing these challenges is essential for advancing LDH technology and expanding its applications across various industries.
Another challenge lies in controlling the incorporation of silicate anions into the LDH interlayer space. While silicate intercalation can enhance the material's properties, excessive silicate content may disrupt the layered structure and compromise the desired functionality of the LDH. Achieving the optimal balance between silicate incorporation and maintaining the LDH's structural integrity remains a critical challenge.
The formation of amorphous silica as a byproduct during LDH synthesis with sodium silicate is another significant obstacle. This unwanted phase can interfere with the LDH crystallization process and affect the purity of the final product. Developing strategies to minimize or eliminate amorphous silica formation while maximizing LDH yield is crucial for improving synthesis efficiency.
Reproducibility and scalability of LDH synthesis using sodium silicate also pose challenges. The sensitivity of the reaction to pH, temperature, and concentration variations can lead to inconsistent results between batches. This variability hinders the large-scale production of LDHs with consistent properties, limiting their potential industrial applications.
Furthermore, the use of sodium silicate in LDH synthesis can impact the material's stability and ion exchange capacity. The strong Si-O bonds formed during the synthesis process may reduce the LDH's ability to exchange anions, potentially limiting its effectiveness in applications such as catalysis and environmental remediation.
Lastly, the environmental impact of using sodium silicate in LDH production is a growing concern. The high alkalinity of sodium silicate solutions can pose handling and disposal challenges, necessitating the development of more sustainable and eco-friendly synthesis methods. Addressing these challenges is essential for advancing LDH technology and expanding its applications across various industries.
Current Synthesis Methods
01 Synthesis and structure of layered double hydroxides
Layered double hydroxides (LDHs) are synthesized through various methods, including coprecipitation and ion-exchange. Their structure consists of positively charged metal hydroxide layers with interlayer anions for charge balance. The composition and synthesis method can be tailored to achieve specific properties and applications.- Synthesis and modification of layered double hydroxides: Various methods for synthesizing and modifying layered double hydroxides (LDHs) are explored. These include coprecipitation, ion-exchange, and hydrothermal synthesis techniques. Modifications can involve the incorporation of different metal ions or organic molecules into the LDH structure, altering their properties and potential applications.
- Intercalation and exfoliation of layered double hydroxides: Processes for intercalating various molecules or ions between the layers of LDHs and methods for exfoliating LDHs into individual nanosheets are investigated. These techniques can enhance the surface area and reactivity of LDHs, making them more suitable for applications in catalysis, adsorption, and nanocomposites.
- Application of layered double hydroxides in catalysis: LDHs are utilized as catalysts or catalyst supports in various chemical reactions. Their tunable composition, high surface area, and ability to stabilize active metal species make them attractive for heterogeneous catalysis applications, including in organic synthesis, environmental remediation, and energy conversion processes.
- Layered double hydroxides in environmental applications: The use of LDHs for environmental remediation and pollution control is explored. Their anion exchange properties and high adsorption capacity make them effective for removing contaminants from water and air. Applications include the removal of heavy metals, organic pollutants, and greenhouse gases.
- Layered double hydroxides in polymer nanocomposites: The incorporation of LDHs into polymer matrices to form nanocomposites is investigated. These nanocomposites can exhibit enhanced mechanical, thermal, and barrier properties compared to the neat polymer. The interaction between LDHs and various polymer systems, as well as methods for improving dispersion and compatibility, are studied.
02 Intercalation and functionalization of LDHs
LDHs can be functionalized through intercalation of various organic and inorganic species in the interlayer space. This process modifies their properties and expands their potential applications. Intercalation can be achieved through ion exchange, coprecipitation, or reconstruction methods, allowing for the design of tailored materials.Expand Specific Solutions03 Catalytic applications of LDHs
LDHs are widely used as catalysts or catalyst supports in various chemical reactions. Their high surface area, tunable composition, and ability to host active species make them versatile catalytic materials. Applications include organic synthesis, environmental remediation, and energy-related processes.Expand Specific Solutions04 LDHs in environmental applications
LDHs are employed in environmental remediation and pollution control. Their anion exchange properties make them effective for removing contaminants from water and air. They can adsorb various pollutants, including heavy metals, organic compounds, and greenhouse gases, making them valuable in wastewater treatment and air purification.Expand Specific Solutions05 LDHs in polymer composites and coatings
LDHs are incorporated into polymer matrices to create nanocomposites with enhanced properties. These materials exhibit improved mechanical strength, thermal stability, and barrier properties. LDHs are also used in coatings to provide corrosion protection, flame retardancy, and UV resistance to various substrates.Expand Specific Solutions
Key LDH Industry Players
The field of sodium silicate interaction in layered double hydroxide synthesis is in a developing stage, with growing market potential due to its applications in various industries. The technology's maturity is advancing, as evidenced by research contributions from institutions like Taiyuan University of Technology and Tokyo Institute of Technology. Companies such as BASF Corp. and LG Chem Ltd. are likely driving commercial applications, while research organizations like the National Institute for Materials Science and IFP Energies Nouvelles are pushing the boundaries of fundamental understanding. The involvement of diverse players, from academic institutions to major chemical companies, indicates a competitive landscape with opportunities for innovation and market growth.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to layered double hydroxide (LDH) synthesis using sodium silicate interaction. Their method involves a controlled precipitation process where sodium silicate is used as both a silica source and pH regulator[1]. This allows for precise control over the LDH structure and composition. The process utilizes a continuous flow reactor system, enabling large-scale production with consistent quality[3]. BASF's technique also incorporates in-situ functionalization of LDHs with organic anions, enhancing their applicability in various fields such as catalysis, adsorption, and polymer composites[5].
Strengths: Scalable production, precise control over LDH properties, versatile functionalization. Weaknesses: May require specialized equipment, potential sensitivity to process parameters.
Clariant (Germany)
Technical Solution: Clariant has pioneered a sustainable approach to LDH synthesis using sodium silicate interaction. Their method employs a bio-based, renewable sodium silicate precursor derived from rice husk ash[2]. This eco-friendly process not only reduces the carbon footprint but also enhances the purity of the resulting LDHs. Clariant's technique involves a two-step synthesis: first, the formation of a silica-rich intermediate, followed by controlled incorporation of metal cations to form the LDH structure[4]. This approach allows for fine-tuning of the LDH's composition and morphology, making it suitable for applications in environmental remediation and advanced materials[6].
Strengths: Sustainable production, high purity products, tailorable LDH properties. Weaknesses: Potentially higher production costs, limited to specific types of LDHs.
Sodium Silicate Innovations
Multilayer Silicate and Method of Making the Same
PatentActiveKR1020210069922A
Innovation
- A method involving precipitation of silicon oxide with alkali or alkaline earth metal hydroxides, hydrothermal reaction, and peeling with a strong oxidizing agent to produce a multilayer silicate with thin unit layers and high corrugation.
Silica adsorbent and method for producing the same
PatentInactiveJP2017119256A
Innovation
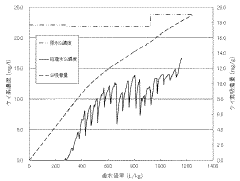
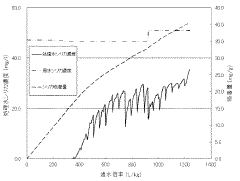
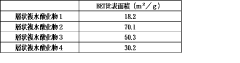
- A silica adsorbent utilizing layered double hydroxides with a specific surface area of 20 m²/g or more, synthesized by mixing divalent and trivalent metal ions with an alkaline solution and silica, and controlled aging to prevent silica scale formation.
Environmental Impact
The synthesis of layered double hydroxides (LDHs) using sodium silicate as a precursor has significant environmental implications that warrant careful consideration. The process involves the interaction of sodium silicate with other metal salts, resulting in the formation of LDH structures. While this method offers certain advantages, it also presents potential environmental challenges that need to be addressed.
One of the primary environmental concerns associated with sodium silicate-based LDH synthesis is the generation of alkaline waste streams. The high pH levels of these effluents can have detrimental effects on aquatic ecosystems if not properly managed. The release of such alkaline waste into natural water bodies may disrupt the pH balance, potentially harming aquatic flora and fauna. To mitigate this issue, appropriate neutralization and treatment processes must be implemented before discharge.
Furthermore, the use of sodium silicate in LDH synthesis may lead to increased sodium levels in the environment. Excessive sodium concentrations can negatively impact soil quality, affecting plant growth and agricultural productivity. This is particularly relevant in areas where LDH production facilities are located near agricultural lands or sensitive ecosystems. Proper waste management and disposal practices are crucial to minimize the risk of soil salinization.
On a positive note, the incorporation of silicate ions into LDHs can enhance their adsorption properties, making them effective materials for environmental remediation applications. LDHs synthesized using sodium silicate have shown promise in removing various pollutants from water and soil, including heavy metals and organic contaminants. This potential for environmental cleanup partially offsets the negative impacts associated with their production.
The energy consumption and carbon footprint of the sodium silicate-based LDH synthesis process also merit consideration. While the process itself may not be particularly energy-intensive, the production of sodium silicate precursors can contribute to greenhouse gas emissions. Efforts to optimize the synthesis process and explore more sustainable precursor sources could help reduce the overall environmental impact of LDH production.
Additionally, the potential for resource recovery from the LDH synthesis process should be explored. The byproducts and waste streams generated during production may contain valuable materials that could be reclaimed and reused. Implementing circular economy principles in LDH manufacturing could not only minimize waste but also improve the overall sustainability of the process.
In conclusion, while sodium silicate interaction in LDH synthesis offers promising applications in environmental remediation, it also presents challenges that require careful management. Balancing the potential benefits with the environmental risks is crucial for the sustainable development and implementation of this technology. Future research should focus on optimizing the synthesis process to minimize waste generation, reduce energy consumption, and explore more environmentally friendly precursor alternatives.
One of the primary environmental concerns associated with sodium silicate-based LDH synthesis is the generation of alkaline waste streams. The high pH levels of these effluents can have detrimental effects on aquatic ecosystems if not properly managed. The release of such alkaline waste into natural water bodies may disrupt the pH balance, potentially harming aquatic flora and fauna. To mitigate this issue, appropriate neutralization and treatment processes must be implemented before discharge.
Furthermore, the use of sodium silicate in LDH synthesis may lead to increased sodium levels in the environment. Excessive sodium concentrations can negatively impact soil quality, affecting plant growth and agricultural productivity. This is particularly relevant in areas where LDH production facilities are located near agricultural lands or sensitive ecosystems. Proper waste management and disposal practices are crucial to minimize the risk of soil salinization.
On a positive note, the incorporation of silicate ions into LDHs can enhance their adsorption properties, making them effective materials for environmental remediation applications. LDHs synthesized using sodium silicate have shown promise in removing various pollutants from water and soil, including heavy metals and organic contaminants. This potential for environmental cleanup partially offsets the negative impacts associated with their production.
The energy consumption and carbon footprint of the sodium silicate-based LDH synthesis process also merit consideration. While the process itself may not be particularly energy-intensive, the production of sodium silicate precursors can contribute to greenhouse gas emissions. Efforts to optimize the synthesis process and explore more sustainable precursor sources could help reduce the overall environmental impact of LDH production.
Additionally, the potential for resource recovery from the LDH synthesis process should be explored. The byproducts and waste streams generated during production may contain valuable materials that could be reclaimed and reused. Implementing circular economy principles in LDH manufacturing could not only minimize waste but also improve the overall sustainability of the process.
In conclusion, while sodium silicate interaction in LDH synthesis offers promising applications in environmental remediation, it also presents challenges that require careful management. Balancing the potential benefits with the environmental risks is crucial for the sustainable development and implementation of this technology. Future research should focus on optimizing the synthesis process to minimize waste generation, reduce energy consumption, and explore more environmentally friendly precursor alternatives.
Scalability Considerations
When considering the scalability of sodium silicate interaction in layered double hydroxide (LDH) synthesis, several key factors come into play. The process of scaling up from laboratory-scale experiments to industrial production presents both opportunities and challenges.
One of the primary considerations is the reactor design. As the synthesis volume increases, maintaining uniform mixing and temperature control becomes more complex. Larger reactors may require advanced agitation systems to ensure homogeneous distribution of reactants and prevent localized concentration gradients. Additionally, heat transfer efficiency must be carefully managed to maintain optimal reaction conditions throughout the scaled-up process.
The choice of raw materials also impacts scalability. While sodium silicate is readily available in bulk quantities, ensuring consistent quality across large batches is crucial. Variations in silicate composition or impurities can affect LDH formation and properties. Implementing robust quality control measures for raw materials becomes increasingly important at larger scales.
Reaction kinetics and mass transfer limitations are another critical aspect of scalability. As reactor volumes increase, the time required for complete reaction and crystallization may change. This can affect product quality and yield. Optimizing reaction parameters such as temperature, pH, and residence time becomes more challenging and may require adjustments from laboratory-scale conditions.
Post-synthesis processing is an often-overlooked aspect of scalability. Separation, washing, and drying of LDH products at industrial scales require significant equipment and energy investments. Filtration and centrifugation processes must be designed to handle larger volumes efficiently while maintaining product quality. Drying techniques need to be optimized to ensure uniform moisture content without compromising the LDH structure.
Environmental and safety considerations also play a crucial role in scaling up sodium silicate-LDH synthesis. Larger-scale production generates more waste and potentially increases the risk of chemical exposure. Implementing effective waste management systems and ensuring proper containment and handling procedures are essential for sustainable and safe large-scale operations.
Economic factors are paramount in scalability assessments. The cost-effectiveness of the process at larger scales must be carefully evaluated, considering factors such as raw material costs, energy consumption, equipment investments, and labor requirements. Economies of scale can potentially reduce per-unit production costs, but this must be balanced against increased complexity and potential quality control challenges.
One of the primary considerations is the reactor design. As the synthesis volume increases, maintaining uniform mixing and temperature control becomes more complex. Larger reactors may require advanced agitation systems to ensure homogeneous distribution of reactants and prevent localized concentration gradients. Additionally, heat transfer efficiency must be carefully managed to maintain optimal reaction conditions throughout the scaled-up process.
The choice of raw materials also impacts scalability. While sodium silicate is readily available in bulk quantities, ensuring consistent quality across large batches is crucial. Variations in silicate composition or impurities can affect LDH formation and properties. Implementing robust quality control measures for raw materials becomes increasingly important at larger scales.
Reaction kinetics and mass transfer limitations are another critical aspect of scalability. As reactor volumes increase, the time required for complete reaction and crystallization may change. This can affect product quality and yield. Optimizing reaction parameters such as temperature, pH, and residence time becomes more challenging and may require adjustments from laboratory-scale conditions.
Post-synthesis processing is an often-overlooked aspect of scalability. Separation, washing, and drying of LDH products at industrial scales require significant equipment and energy investments. Filtration and centrifugation processes must be designed to handle larger volumes efficiently while maintaining product quality. Drying techniques need to be optimized to ensure uniform moisture content without compromising the LDH structure.
Environmental and safety considerations also play a crucial role in scaling up sodium silicate-LDH synthesis. Larger-scale production generates more waste and potentially increases the risk of chemical exposure. Implementing effective waste management systems and ensuring proper containment and handling procedures are essential for sustainable and safe large-scale operations.
Economic factors are paramount in scalability assessments. The cost-effectiveness of the process at larger scales must be carefully evaluated, considering factors such as raw material costs, energy consumption, equipment investments, and labor requirements. Economies of scale can potentially reduce per-unit production costs, but this must be balanced against increased complexity and potential quality control challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!