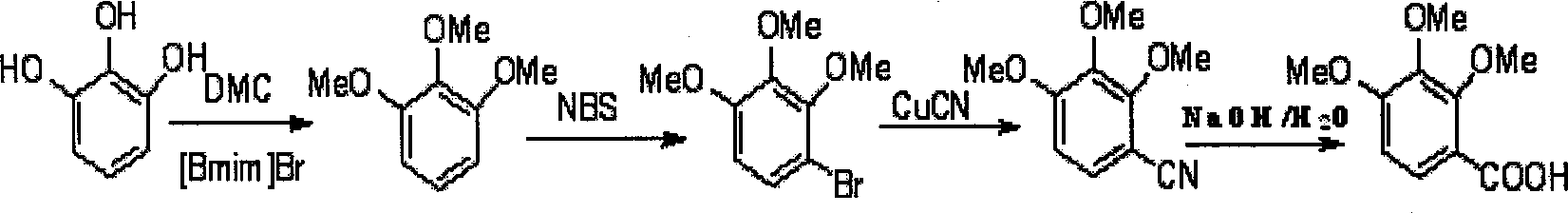
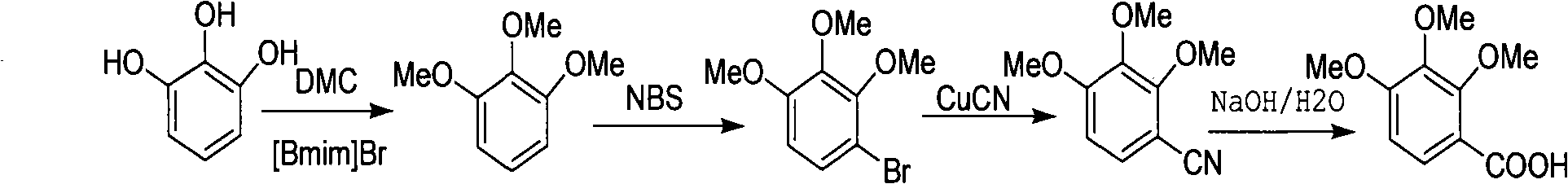
Preparation method of 2,3,4-trimethoxybenzoic acid
A technology of trimethoxybenzoic acid and trimethoxybenzonitrile, which is applied in the field of pharmaceutical intermediates 2, can solve the problems of large pollution and high cost of raw materials, and achieve the effect of cheap raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] ①Weigh 31.5g of pyrogallic acid, 135g of dimethyl carbonate DMC and 54.76g of 1-n-butyl-3-methylimidazolium bromide, place them in a 500mL pressure-resistant reactor, stir them magnetically, and react at 150°C After 7 hours, cool to room temperature, then distill off low-boiling dimethyl carbonate DMC and methanol under reduced pressure, then extract with ether (150mL×3), combine the extracts, distill off part of the ether, and crystallize at low temperature Crude triphenylmethyl ether, dissolve the crude product in 150mL of petroleum ether, add 5g of activated carbon and heat it slightly (about 40°C, 30min), then heat filter, then wash the filter paper with 30mL of petroleum ether, evaporate part of the petroleum ether and put it in the refrigerator After crystallization, 38.9 g of pure white trityl ether was obtained, with a yield of 92.6%.
[0016] ②Add 35mL DMF and 16.8g (0.1mol) trityl ether into a 100mL four-neck flask, stir to dissolve completely, add 18.6g (0.10...
Embodiment 2
[0020] ① Weigh 31.5g of pyrogallic acid, 135g of DMC and 37.38g of 1-n-butyl-3-methylimidazolium bromide, place them in a 500mL pressure-resistant reactor and stir them magnetically, react at 150°C for 7h, finish, and cool to room temperature, then distill off low-boiling DMC and methanol under reduced pressure, then extract with diethyl ether (150mL×3), combine the extracts, distill off part of the diethyl ether, and crystallize at low temperature to obtain the crude trityl ether. Dissolve in 150mL of petroleum ether, add 5g of activated carbon, heat for a while (about 40°C, 30min), then heat filter, then wash the filter paper with petroleum ether, evaporate part of the petroleum ether and put it in the refrigerator to crystallize to obtain white trityl ether The pure product is 38.9g, and the yield is 92.6%.
[0021] ② Add 35ml DMF and 16.8g (0.1mol) trityl ether to a 100mL four-neck flask, stir to dissolve completely, add 18.6g (0.105mol) NBS in batches at no more than 20°C...
Embodiment 3
[0025] ① Weigh 31.5g of pyrogallic acid, 67.5g of DMC and 54.76g of 1-n-butyl-3-methylimidazolium bromide, place them in a 500mL pressure-resistant reactor and stir them magnetically, and react at 150°C for 7 hours. Cool to room temperature, distill off low-boiling DMC and methanol under reduced pressure, then extract with diethyl ether (150mL×3), combine the extracts, evaporate part of the diethyl ether, and then crystallize at low temperature to obtain the crude trityl ether. Dissolve in 150mL of petroleum ether, add 5g of activated carbon, heat for a while (about 40°C, 30min) and heat filter, then wash the filter paper with petroleum ether, evaporate part of the petroleum ether, put it in the refrigerator to crystallize to obtain pure white trityl ether The product was 38.9g, and the yield was 92.6%.
[0026]②Add 35mL DMF and 16.8g (0.1mol) trityl ether into a 100mL four-neck flask, stir to dissolve completely, add 18.6g (0.105mol) NBS in batches at no more than 20°C, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com