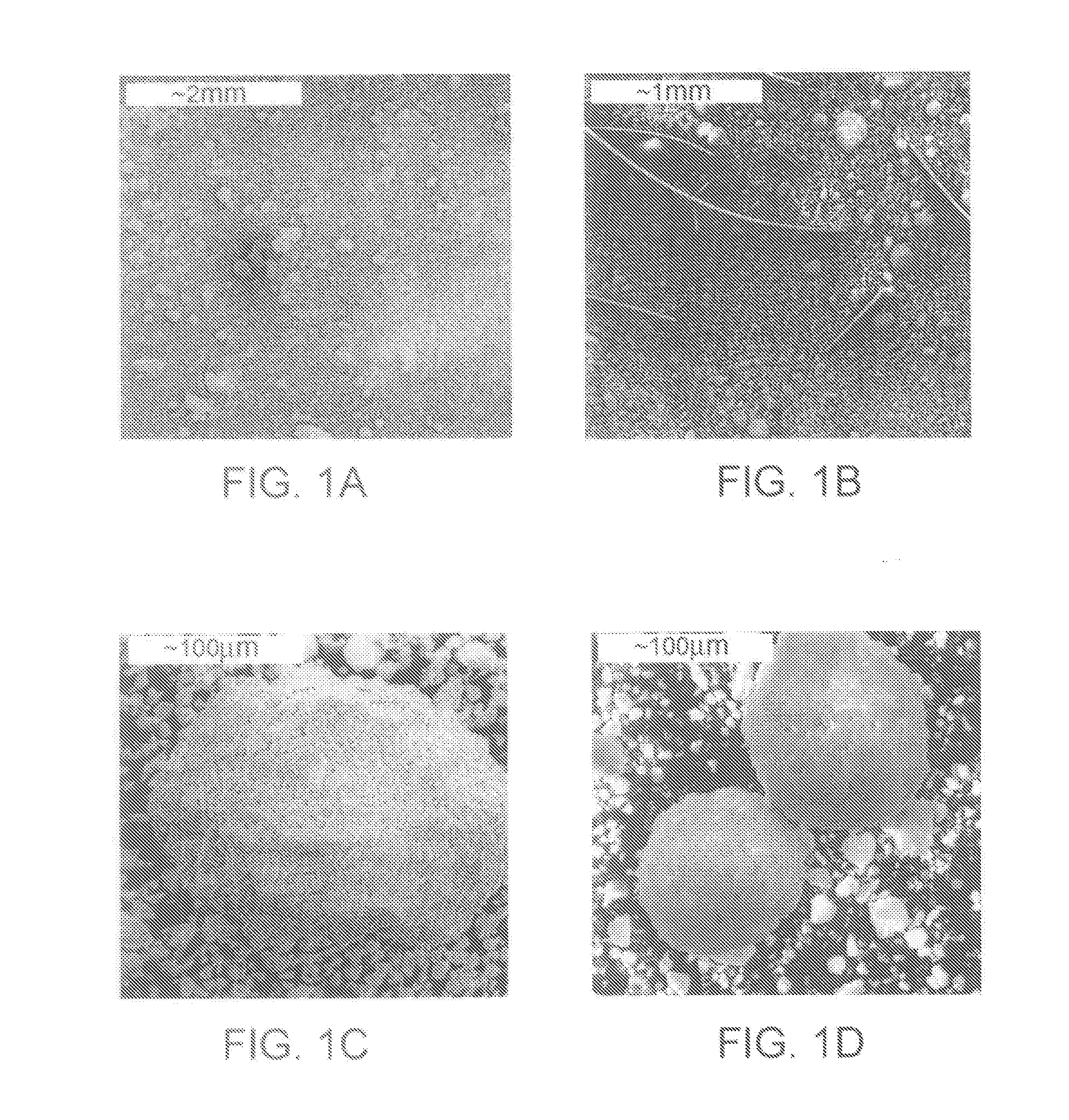
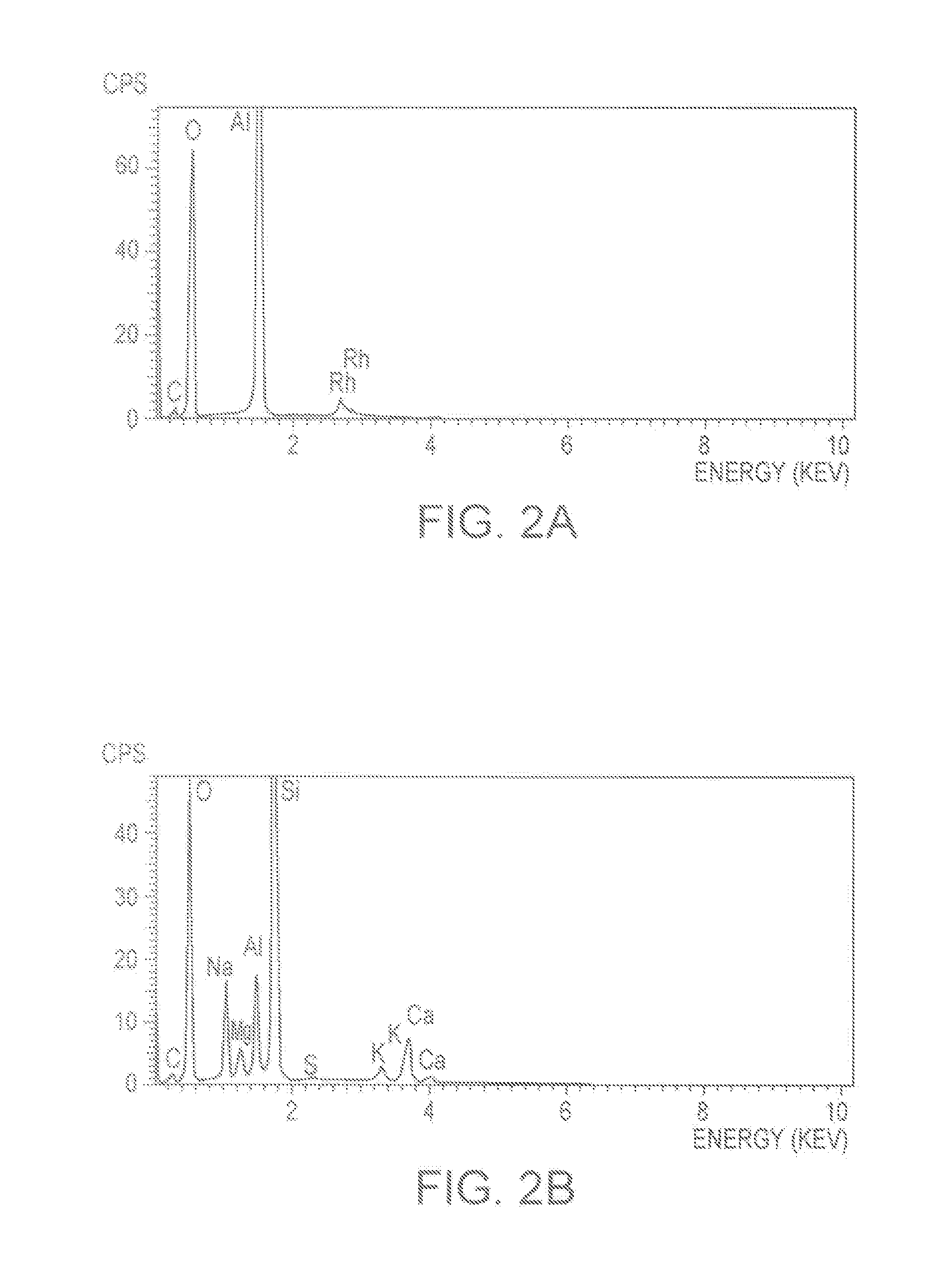
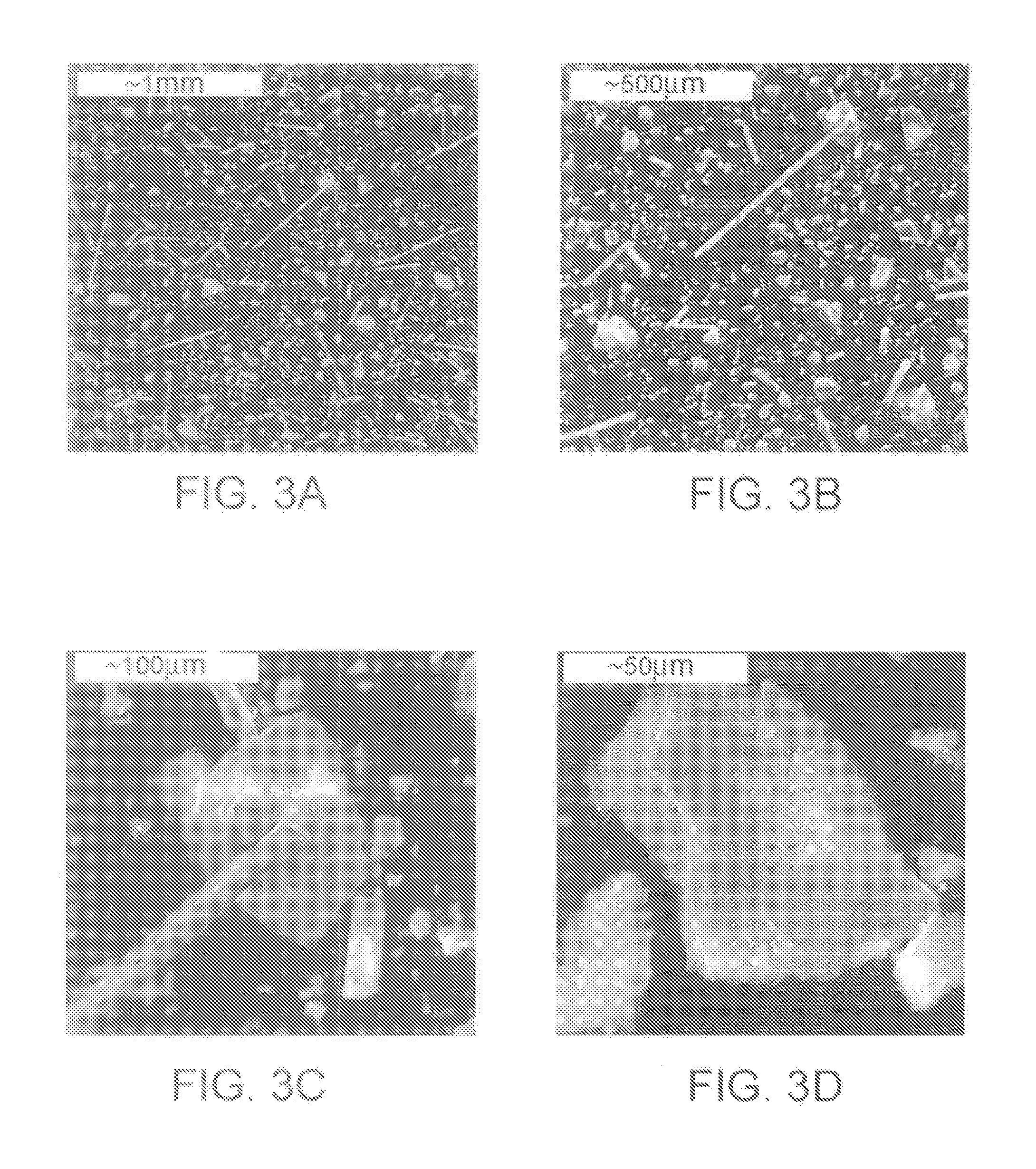
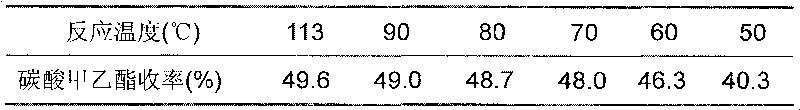
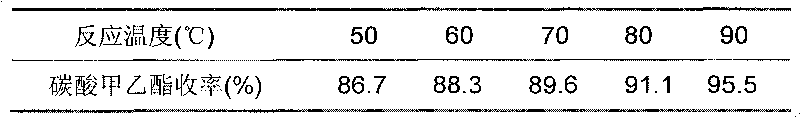
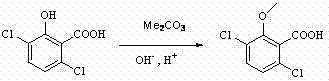Patents
Literature
1869 results about "Dimethyl carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH₃)₂. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent.
Phosphonate additives for nonaqueous electrolyte in rechargeable cells
InactiveUS6200701B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsRechargeable cellPhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphonate compound.
Owner:WILSON GREATBATCH LTD
Phosphate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6203942B1Improve oxidation stabilityImprove dynamic stabilityPrimary cell maintainance/servicingOrganic electrolyte cellsAlkylphosphateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphate compound.
Owner:WILSON GREATBATCH LTD
Nitrate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6136477AImprove oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsNitrateRechargeable cell
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an organic alkyl nitrate compound.
Owner:WILSON GREATBATCH LTD
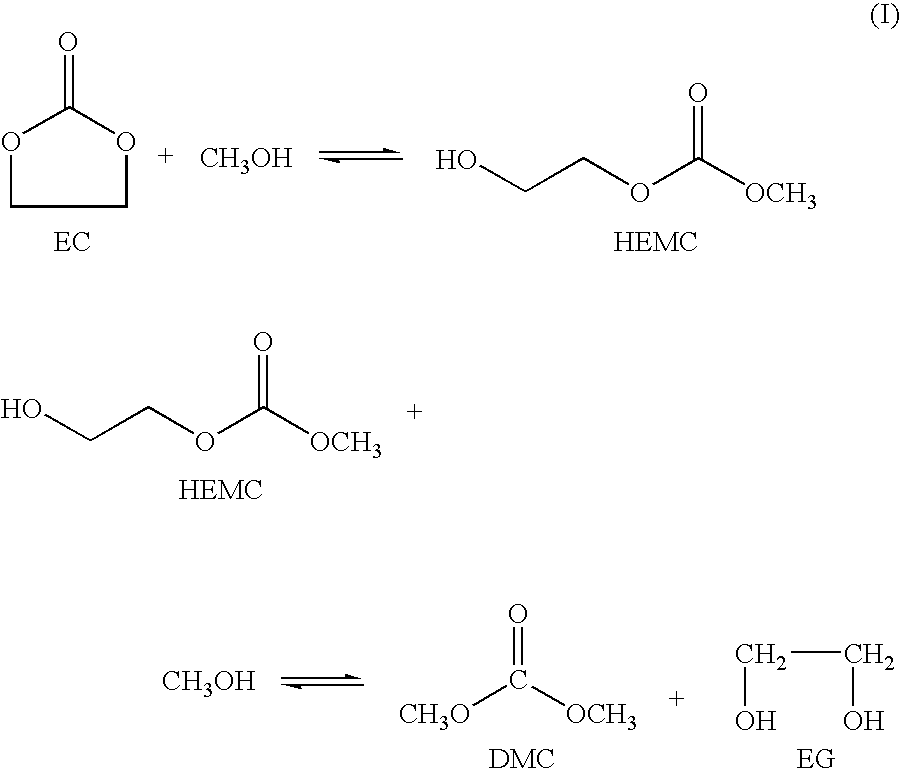
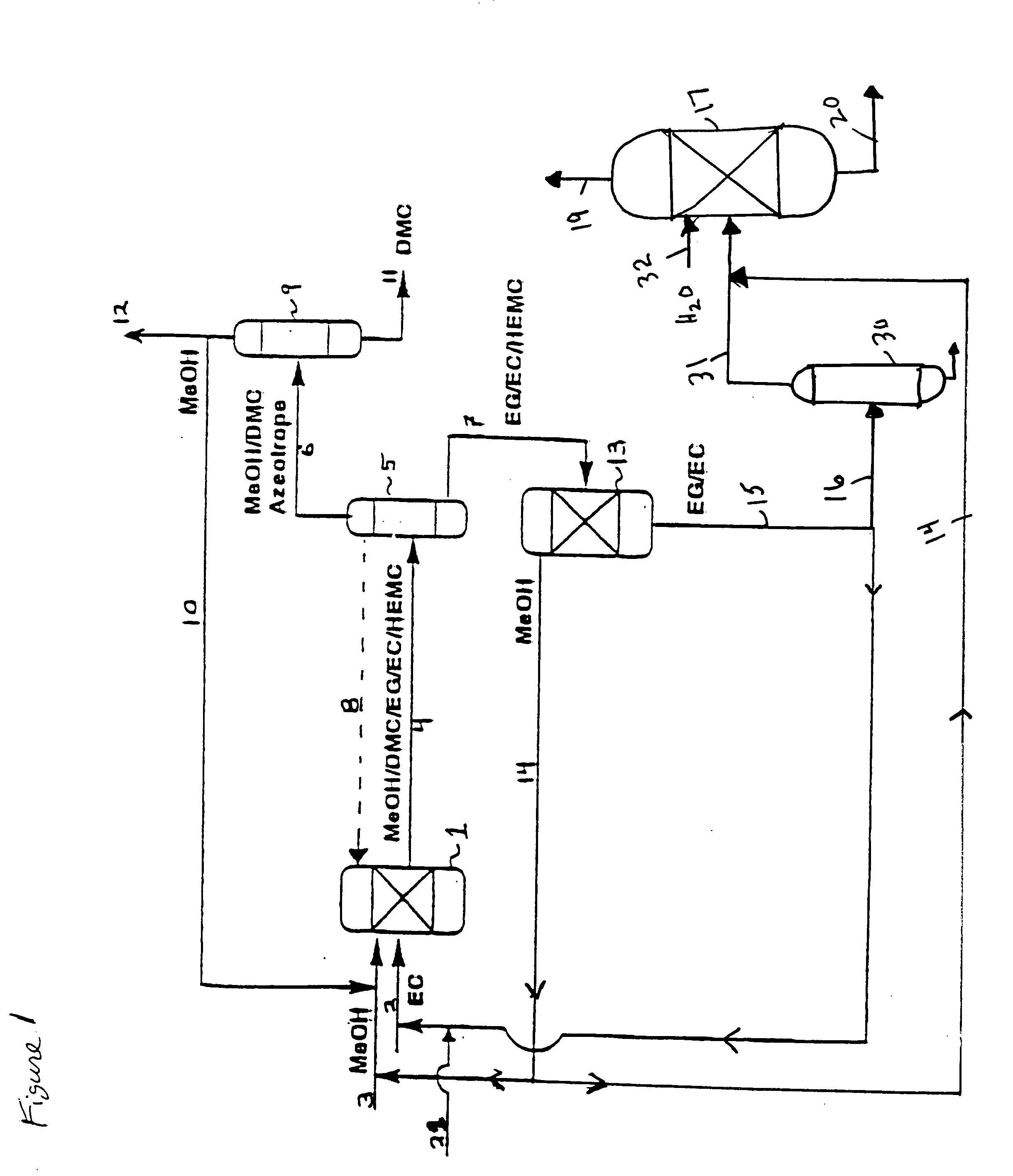
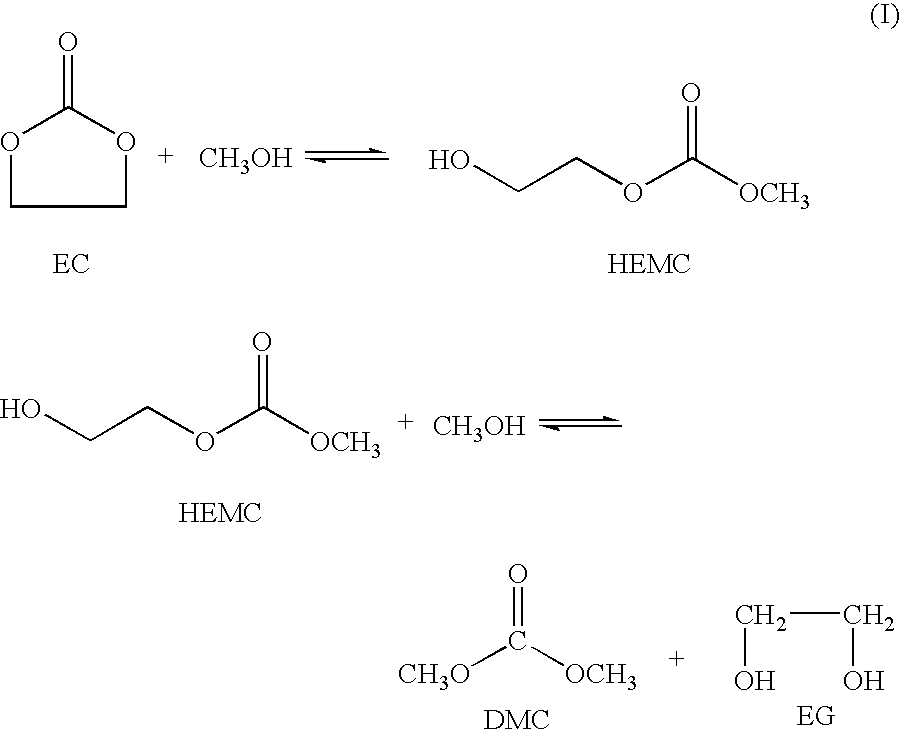
Process for the production of unsymmetric and/or symmetric dialkyl carbonates and diols
InactiveUS6930195B2High yieldOrganic compound preparationHydroxy compound preparationAlcoholTransesterification
A process for the production of a dialkyl carbonate and a diol, such as dimethyl carbonate and ethylene glycol, by reacting a feed containing a cyclic carbonate, a hydroxy alkyl carbonate and an aliphatic monohydric alcohol in the presence of a transesterification catalyst is described. In another aspect, a process is described which is particularly useful for producing unsymmetric dialkyl carbonates, such as ethyl methyl carbonate.
Owner:BADGE LICENSING LLC
Nitrite additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6210839B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSolid electrolyte cellsNitritePhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrite additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl nitrite compound.
Owner:WILSON GREATBATCH LTD
Non-aqueous electrolyte for high-voltage lithium ion batteries
ActiveCN103268956ASimple compositionPromote circulationSecondary cellsHigh voltage batteryPropylene carbonate
The invention relates to a non-aqueous electrolyte for high-voltage lithium ion batteries, which is prepared from the following raw materials in percentage by weight: 70-85% of carbonate, 3-20% of functional additive and 11-17% of lithium hexafluorophosphate. The carbonate is one or mixture of more of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, dipropyl carbonate, methylethyl carbonate, methyl propyl carbonate and methyl butyl carbonate; and the functional additive is one or mixture of more of 0.5-10% of negative pole film-forming additive, 0.5-10% of high-temperature additive, 0.5-10% of positive pole film-forming additive, 0.5-10% of high-voltage additive and 0.001-2% of stability additive. The invention solves the problem of adaptation of the lithium ion battery electrolyte to the 4.35V high-voltage battery positive / negative pole, and provides an electrolyte for high-voltage batteries, which has the advantages of high cycle life, low inflation rate and favorable high-temperature properties.
Owner:广东金光高科股份有限公司
Process for preparing methyl ethyl carbonate by ester exchanging reaction
InactiveCN1900047AHigh yieldQuick responseMetal/metal-oxides/metal-hydroxide catalystsPreparation from organic carbonatesTransesterificationReaction temperature
The transesterification process for preparing methyl ethyl carbonate features that the materials dimehtyl carbonate and ethanol in the molar ratio of 1-4 to 1 produce transesterification reaction at normal pressure and 50-110 deg.c in the presence of binary heterogeneous solid alkali catalyst for 1-8 hr to prepare methyl ethyl carbonate. The solid alkali catalyst has consumption of 5-25 % of ethanol weight and is prepared through soaking process or sol-gel process, and used catalyst may be separated easy from the reaction product for reuse. The transesterification reaction is completed in a reaction-rectification apparatus and has methyl ethyl carbonate yield up to 90 %.
Owner:ZHEJIANG UNIV
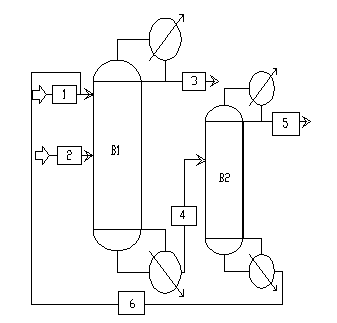
Continuous extractive distillation separation method of dimethyl carbonate-methanol azeotropic mixture
InactiveCN103159586AIncrease relative volatilityAchieve recyclingOxygen-containing compound preparationOrganic compound preparationExtractive distillationTransesterification
The invention discloses a continuous extractive distillation separation method of a dimethyl carbonate-methanol azeotropic mixture, and relates to a continuous extractive distillation method. The technical process of the method is as follows: ethylene glycol is used as an extraction agent at normal pressure; the solvent ratio is 1-3; the separated dimethyl carbonate-methanol azeotropic mixture is fed from the middle part of a tower, the extraction agent is fed from the top of the tower, and the reflux ratio is 2; high-purity methanol is extracted from the tower top of an extractive distillation tower, and the dimethyl carbonate and the extraction agent are extracted from the tower bottom; the fraction at the tower bottom enters an extraction agent recovery tower, and the reflux ratio is 3; and dimethyl carbonate is extracted from the tower top, and the extraction agent extracted from the tower bottom can be recycled. The method disclosed by the invention adopts ethylene glycol as an extraction agent, and improves the separation effect of the dimethyl carbonate-methanol azeotropic mixture; and since ethylene glycol is a co-production product in synthesizing dimethyl carbonate by a transesterification method, the source is conveniently available, and the cost caused by introducing other substances is avoided.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Process for the production of unsymmetric and/or symmetric dialkyl carbonates and diols
InactiveUS20050080287A1High yieldOrganic compound preparationHydroxy compound preparationAlcoholTransesterification
A process for the production of a dialkyl carbonate and a diol, such as dimethyl carbonate and ethylene glycol, by reacting a feed containing a cyclic carbonate, a hydroxy alkyl carbonate and an aliphatic monohydric alcohol in the presence of a transesterification catalyst is described. In another aspect, a process is described which is particularly useful for producing unsymmetric dialkyl carbonates, such as ethyl methyl carbonate.
Owner:BADGE LICENSING LLC
Dicarbonate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6174629B1Improve oxidation stabilityImprove dynamic stabilityAlkaline accumulatorsOrganic electrolyte cellsRechargeable cellPhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one dicarbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl dicarbonate compound.
Owner:WILSON GREATBATCH LTD
Highly conductive and stable nonaqueous electrolyte for lithium electrochemical cells
InactiveUS6844115B2Increase electrolyte conductivityImprove electrochemical stabilitySilver accumulatorsLead-acid accumulatorsPhysical chemistryPropylene carbonate
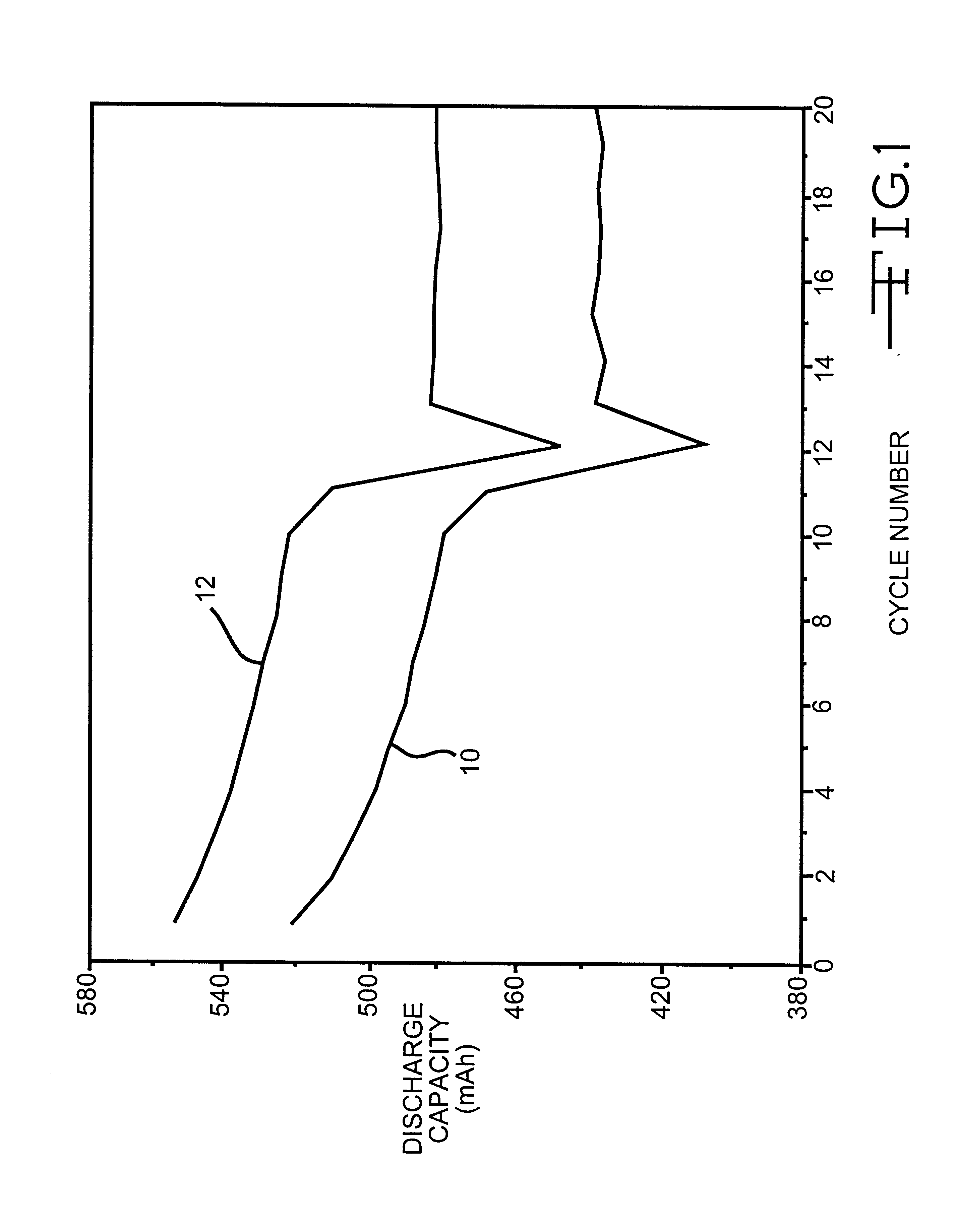
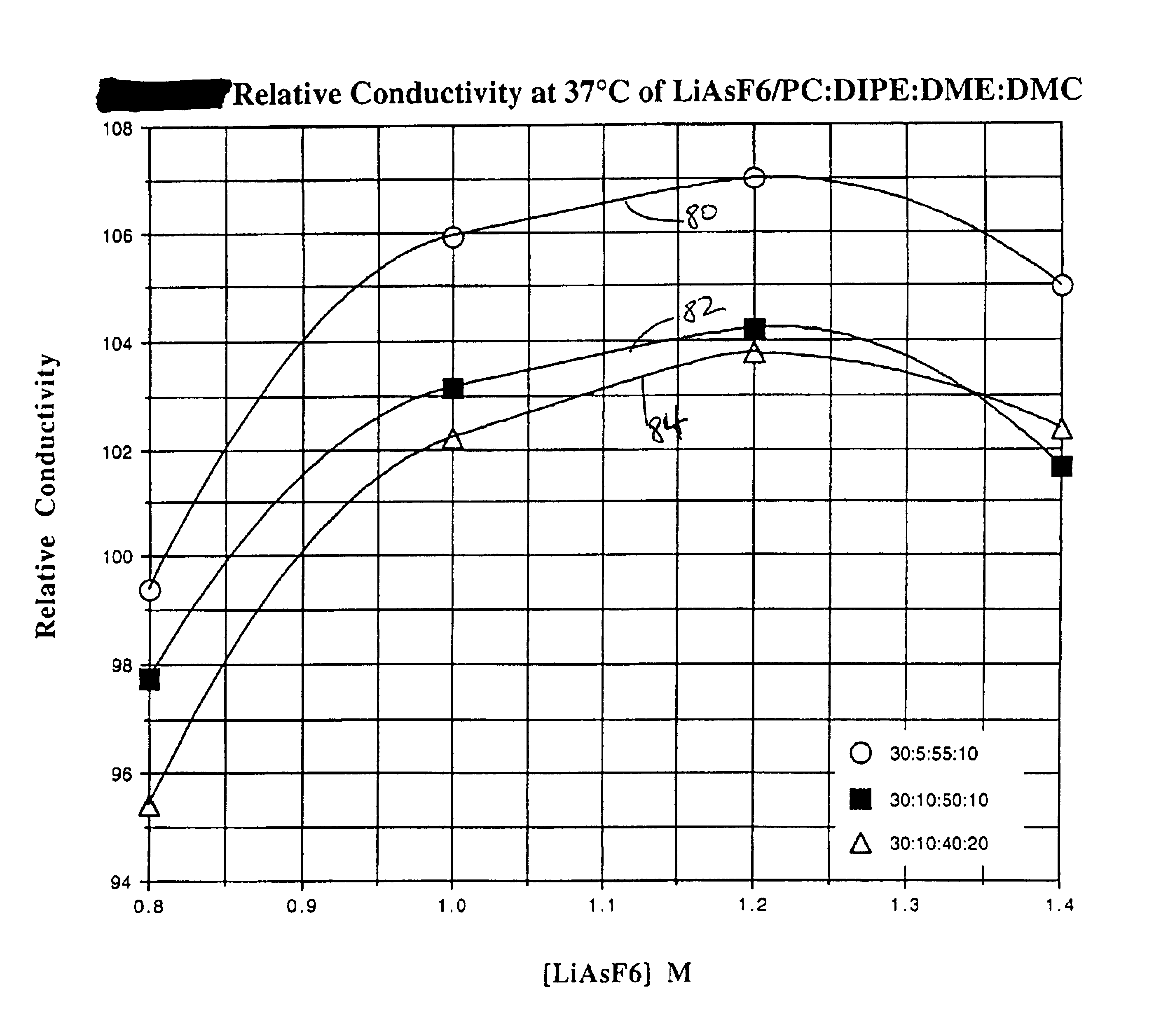
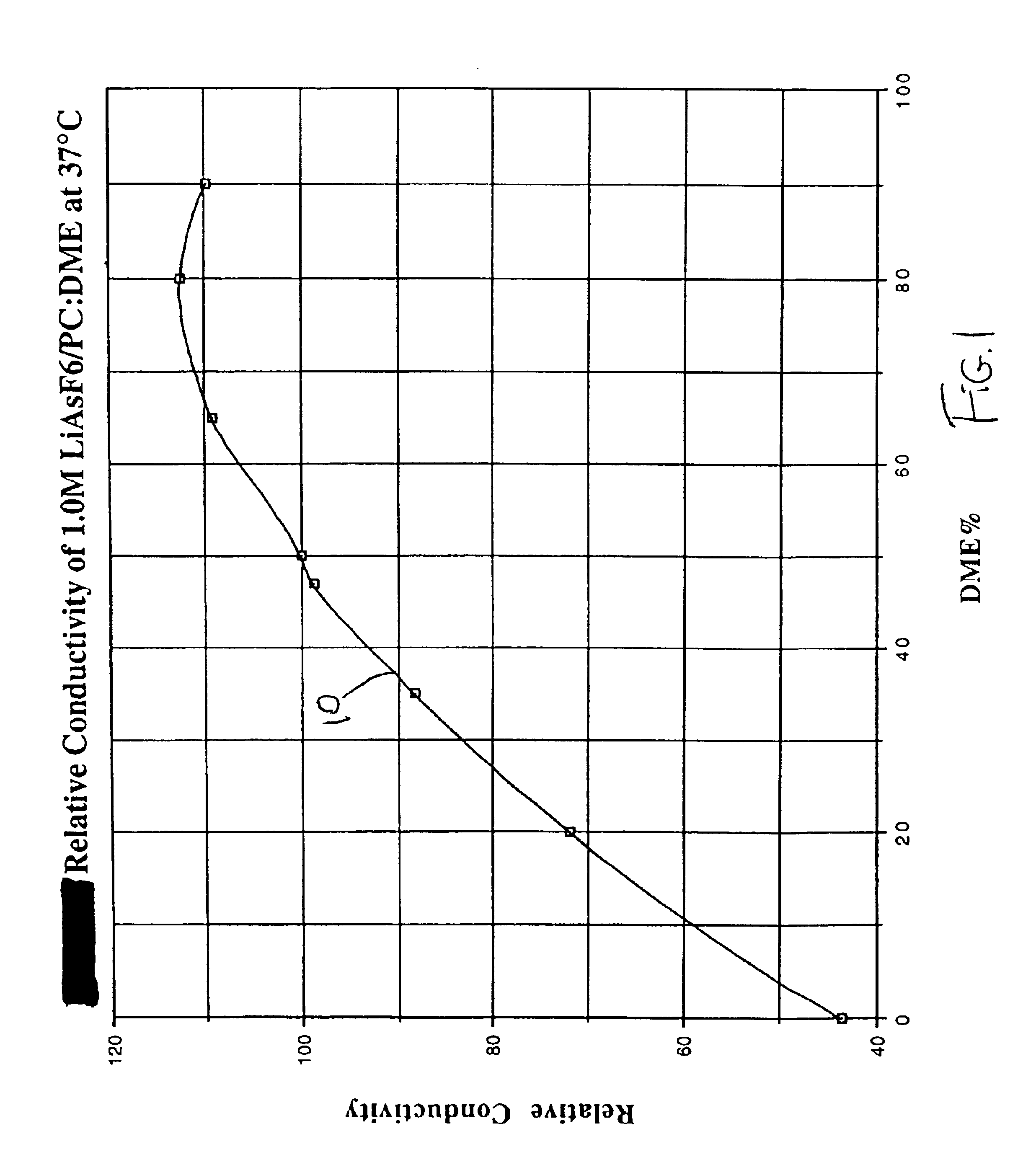
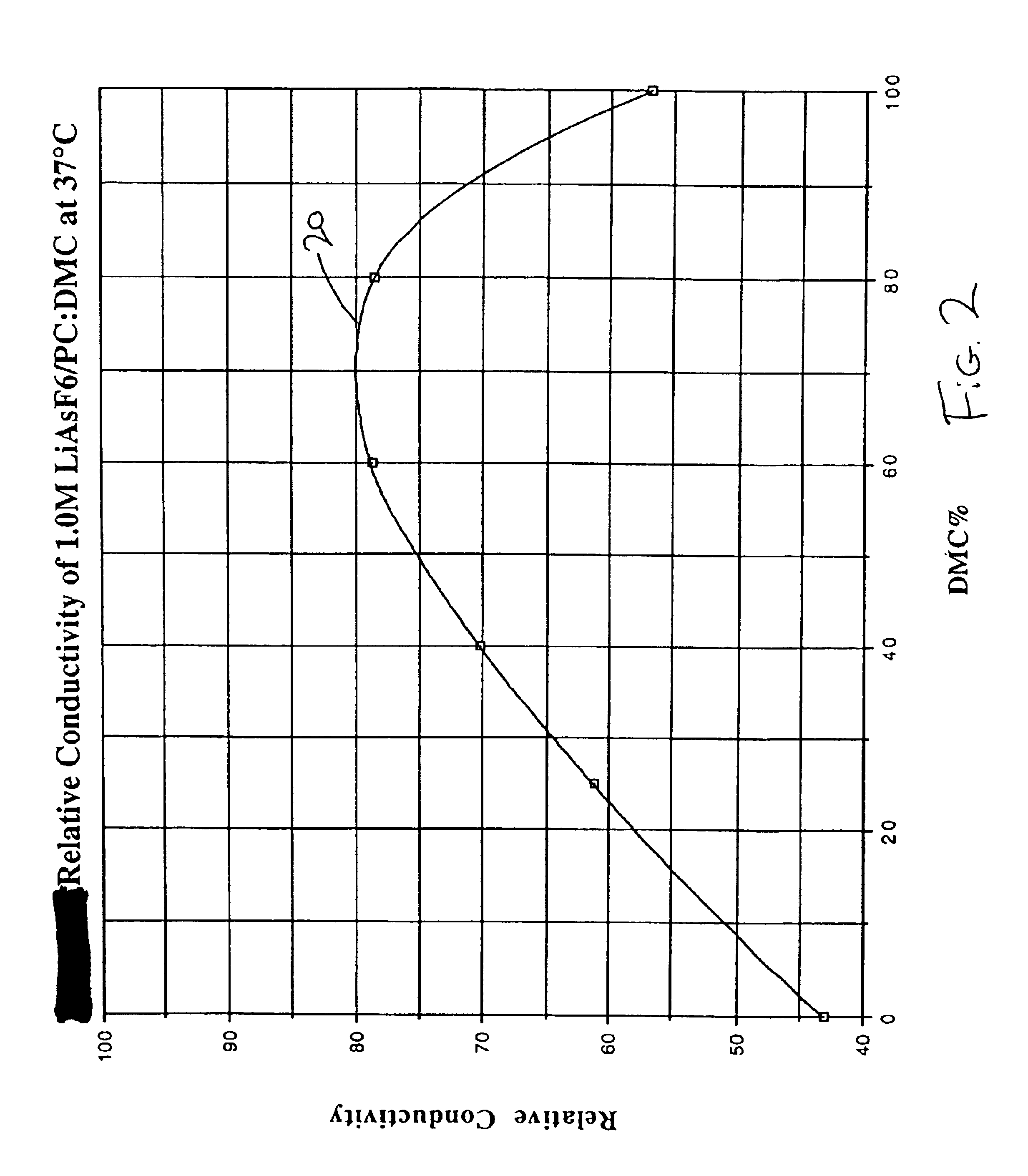
The present invention is directed to at least partially replacing PC and / or DME with a linear carbonate, preferably dimethyl carbonate, and a linear mono-ether, the most preferred being diisopropyl ether, in electrolytes useful for activating alkali metal-containing cells. This electrolyte has improved conductivity and provides electrochemical cells with enhanced discharge performance. A most preferred electrolyte comprises 1,2-dimethoxyethane, propylene carbonate, dimethyl carbonate and diisopropyl ether.
Owner:WILSON GREATBATCH LTD
Synthesis of dimethyl carbonate from carbon dioxide and methanol
InactiveUS20110196167A1Molecular sieve catalystsChemical industryAtmospheric pressureHomogeneous catalysis
A method for producing dimethyl carbonate from methanol and carbon dioxide using a heterogeneous catalyst is described. The heterogeneous catalyst provides both acidic sites and basic sites. The reaction can be carried out at atmospheric pressure and relatively low temperatures.
Owner:KING ABDULAZIZ CITY FOR SCIENCE AND TECHNOLOGY
Preparation process of herbicide dicamba
InactiveCN102125035AReduce pollutionHigh reaction yieldBiocideAnimal repellantsMethoxylaricinolic acidSalicylic acid
The invention relates to a preparation process of herbicide dicamba, in particular to a supercritical preparation process. The preparation process of the herbicide dicamba comprises the following steps of: preparing 2,5-dichlorophenol into corresponding sodium phenolate, and then completing carboxylation reaction under a supercritical condition to obtain 3,6-dichlorosalicylic acid; and then completing O-methylation to prepare a product of 3,6-dichloro-2-methoxy salicylic acid, i.e. the dicamba, by taking dimethyl carbonate as a reagent. The invention changes the heterogeneous gas-solid reaction of the traditional process into homogeneous reaction under the supercritical condition, carries out the O-methylation reaction by using the dimethyl carbonate and has the advantages of high reaction yield, good product quality, reduced environmental pollution, lowered energy consumption, and the like.
Owner:上海力智生化科技有限公司
Method for synthesizing methylethyl carbonate by ester exchange of dimethyl carbonate and diethyl carbonate
ActiveCN102863339AHigh catalytic activityLow costChemical recyclingPreparation from organic carbonatesReaction temperatureIonic liquid
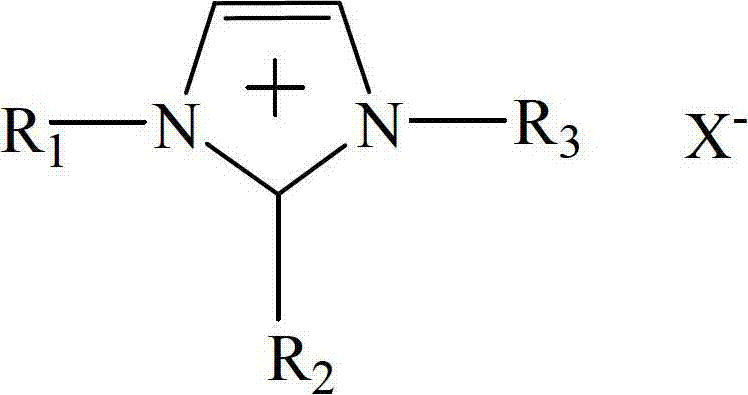
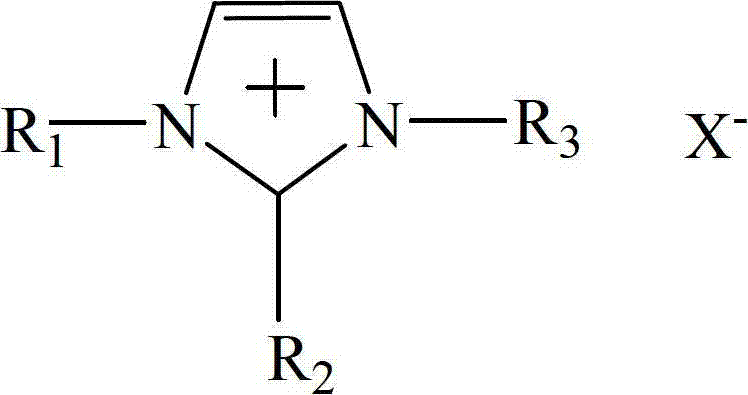
The invention relates to a method for synthesizing methylethyl carbonate by ester exchange of dimethyl carbonate and diethyl carbonate, which is implemented by carrying out synthetic reaction of methylethyl carbonate on raw materials dimethyl carbonate and diethyl carbonate by using an imidazole ionic liquid as a catalyst at certain reaction temperature under ordinary pressure for some reaction time. The catalyst consumption is only 0.2-2 wt% of the dimethyl carbonate; the ionic liquid catalyst has high catalytic activity in the reaction process, the maximum yield of methylethyl carbonate is up to 62.31%, and the selectivity is 100%; and the catalyst can be recycled for cyclic utilization after being subjected to simple treatment after reaction, has the advantages of long service life and no pollution, and greatly lowers the preparation cost of methylethyl carbonate.
Owner:灯塔森佳新能源合伙企业(有限合伙)
Technology and device system for producing dimethyl oxalate by high-pressure carbonylation of industrial synthesis gases and producing ethylene glycol through dimethyl oxalate hydrogenation
ActiveCN104098441AConducive to large-scale productionSmall volume requirementOrganic compound preparationEnergy inputSeparation technologyHigh pressure
The invention relates to a technology and a device system for producing dimethyl oxalate by high-pressure carbonylation of industrial synthesis gases and producing ethylene glycol through dimethyl oxalate hydrogenation. The technology comprises the following steps: adopting industrial NO, O2 and methanol as raw materials for an esterification reaction to produce methyl nitrite; adopting industrial CO and methyl nitrite for a carbonylation reaction in a plate reactor to produce carbonylation products, which mainly include dimethyl oxalate and dimethyl carbonate; separating the carbonylation products to obtain dimethyl carbonate products; subsequently adding hydrogen into dimethyl oxalate in the plate reactor to produce ethylene glycol products; conducting the coupling recovery treatment on waste acids in the esterification reaction and purge gases in the carbonylation reaction for recycling. The device system comprises an esterification reaction system, a carbonylation reaction system, a coupling recovery system for purge gases and waste acids and a hydrogenation reaction system. The technology has the characteristic that device consumption is remarkably reduced, and particularly the nitric acid waste liquid recycling and purge gas recycling technologies as well as the separation technologies thereof are highly coupled; recycling of the raw materials in reaction waste gases is realized, and the effect is remarkable.
Owner:SHANGHAI WUZHENG ENG TECH CO LTD
Supported palladium-based catalyst and preparation method and application thereof
ActiveCN106179506APromote generationHigh activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsGas phaseCarbon nanotube
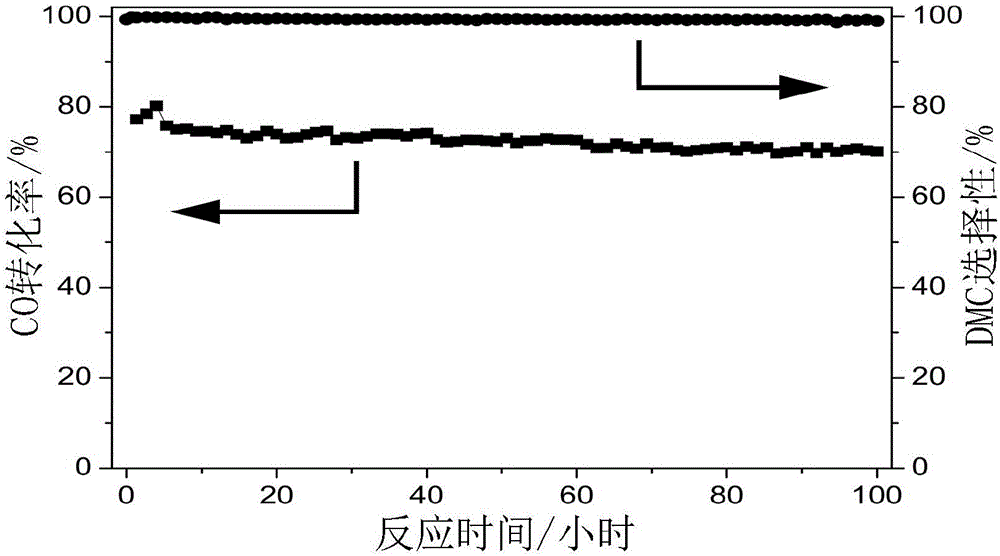
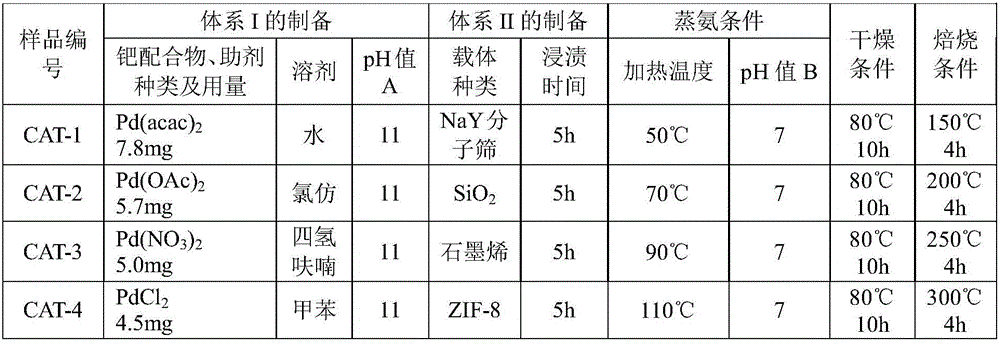
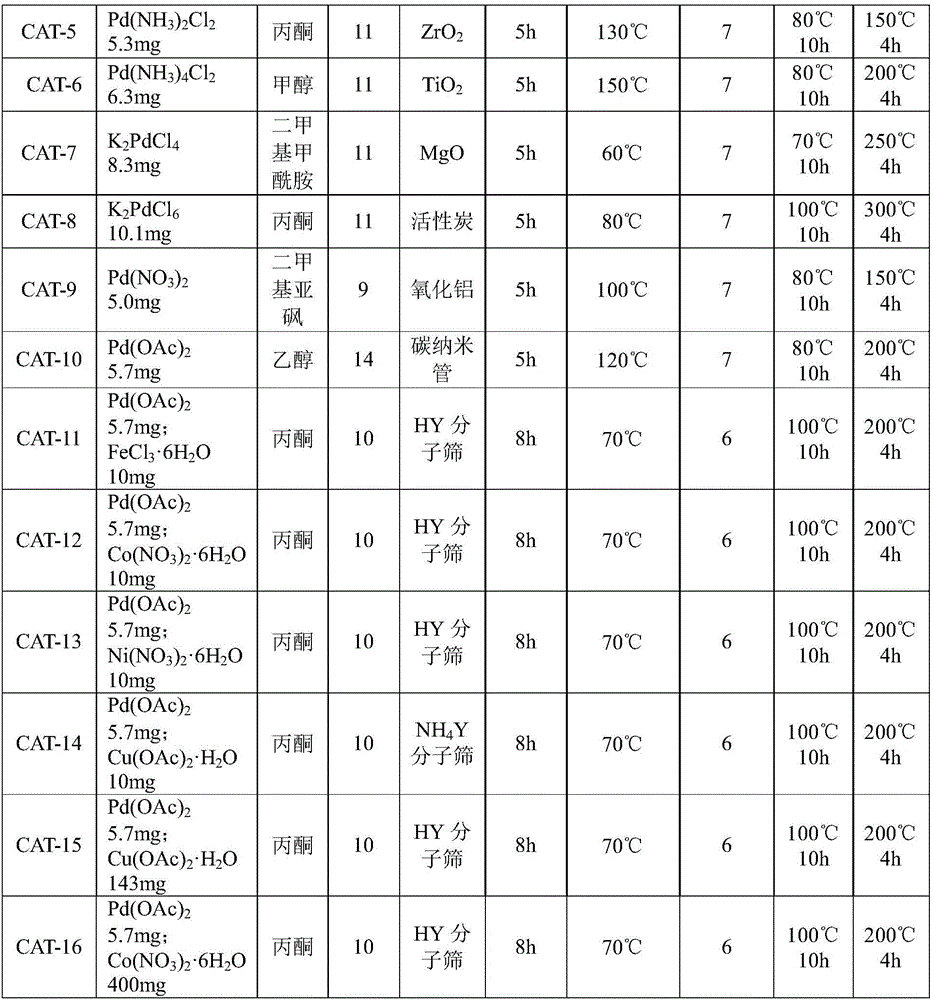
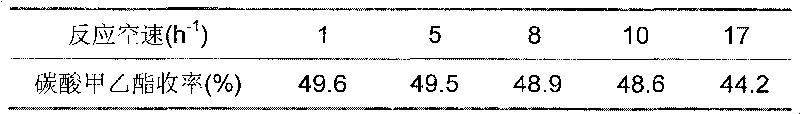
The invention provides a supported palladium-based catalyst and a preparation method and an application thereof. The supported palladium-based catalyst comprises an active ingredient and a carrier, wherein the active ingredient is a palladium-based compound; the carrier is selected from at least one of aluminum oxide, silicon oxide, magnesium oxide, zinc oxide, zirconium oxide, titanium dioxide, metal organic framework compound, activated carbon, molecular sieve, carbon nanotube, and graphene. The supported palladium-based catalyst is applied to the reaction of methyl nitrite vapor-phase carbonylation preparing dimethyl carbonate, and has the advantages that the catalytic activity is high, the product selectivity is high, and the service life is long.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Synthesis method of methyl ethyl carbonate
InactiveCN101704751AStable in natureHigh yieldPreparation from organic carbonatesSynthesis methodsFixed bed
The synthesis method of methyl ethyl carbonate belongs to the technical field of organic compounds synthesized by ester exchange. Dimethyl carbonate and diethyl carbonate, or dimethyl carbonate and ethanol are used as raw materials to perform ester exchange in the presence of catalyst. The reaction mode comprises two kinds of fixed bed continuous reaction and kettle-type reaction. The catalyst issolid alkali catalyst in which the carrier is active carbon, carbon molecular sieve or mesoporous carbon and the active component is Na2O, K2O, MgO, CaO, SrO or BaO. In the process of fixed bed continuous synthesis, the catalyst is stable without inactivation after long-term use, thereby retaining the high yield of the methyl ethyl carbonate, simplifying the production process, and achieving environmental friendliness and energy conservation. In the kettle-type reaction, the catalytic efficiency is high, the catalyst can be recovered by simple filtering and re-sintering, and the target product can be obtained in short time with high yield.
Owner:JILIN UNIV
Method for synthesizing ethyl methyl carbonate through ester exchange
ActiveCN103483200AEasy to makeSimple process conditionsPreparation from organic carbonatesMolecular sieveAlcohol
The invention relates to a method for synthesizing ethyl methyl carbonate through ester exchange. The method comprises the following steps: filling a fixed bed reactor with catalysts, pumping dimethyl carbonate and ethyl alcohol into the fixed bed reactor according to the molar ratio of 0.5-2:1 after nitrogen purging is carried out, reacting at the air speed of 0.5-15h-1, the temperature of 100-240DEG C and the reaction operation pressure of 0-1MPa, and finally the ethyl methyl carbonate is obtained. The catalysts are modified molecular sieve based catalysts. The method is simple in technology condition, easy to control, capable of achieving continuous production due to the gas-solid phase reaction and low in cost of the adopted catalysts. The selectivity of the ethyl methyl carbonate can be higher than 90%, and the yield can be higher than 55%.
Owner:HEBEI UNIV OF TECH
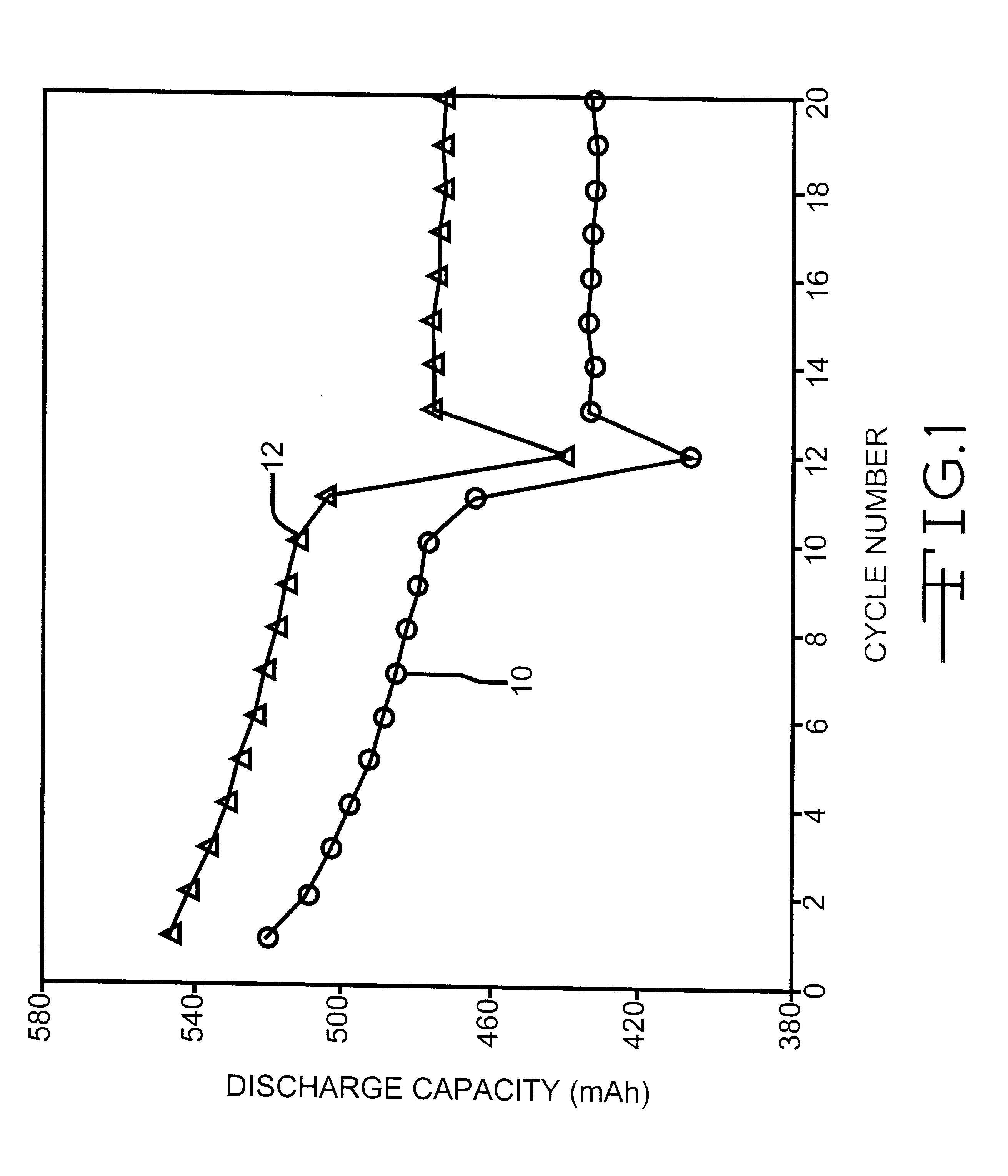
Nonaqueous organic electrolytes for low temperature discharge of rechargeable electrochemical cells
InactiveUS6153338AImprove low temperature charge/discharge performanceImprove cycle lifeElectrolytic capacitorsOrganic electrolyte cellsRoom temperaturePhysical chemistry
An alkali metal secondary electrochemical cell, and preferably a lithium ion cell, activated with a quaternary solvent system, is described. The solvent system comprises a quaternary mixture of dialkyl carbonates and cyclic carbonates, and preferably dimethyl carbonate, diethyl carbonate, ethylmethyl carbonate and ethylene carbonate. Lithium ion cells activated with this electrolyte have good room temperature cycling characteristics and excellent low temperature discharge behavior.
Owner:WILSON GREATBATCH LTD
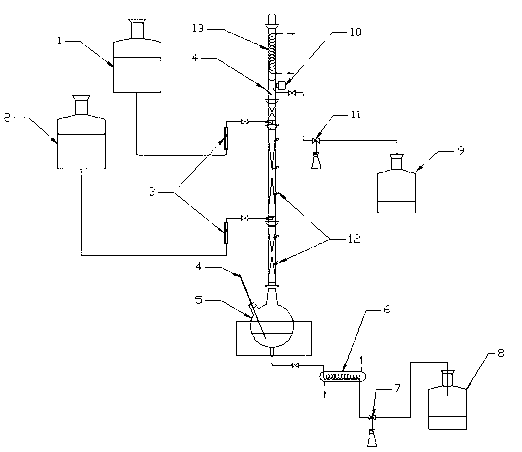
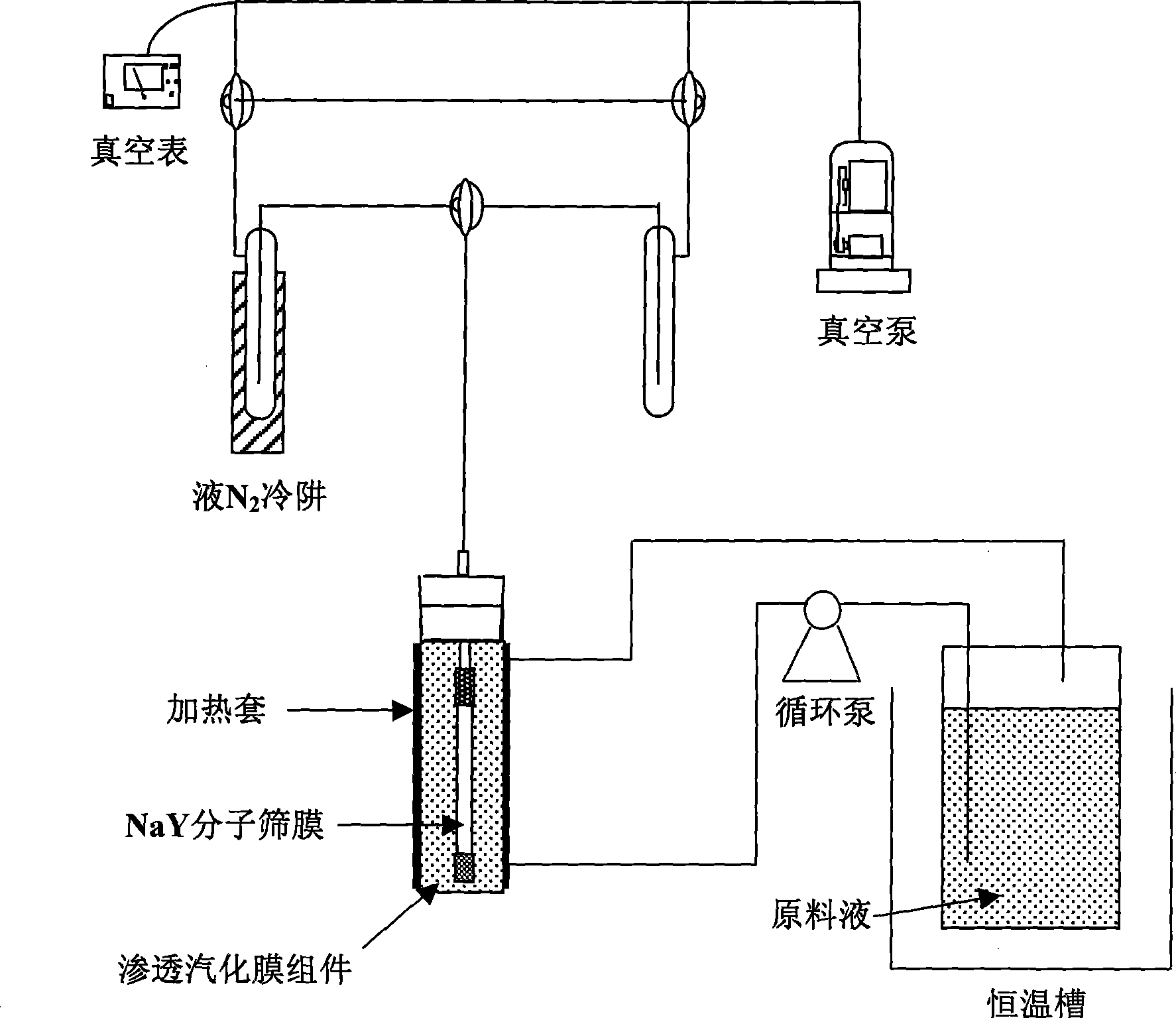
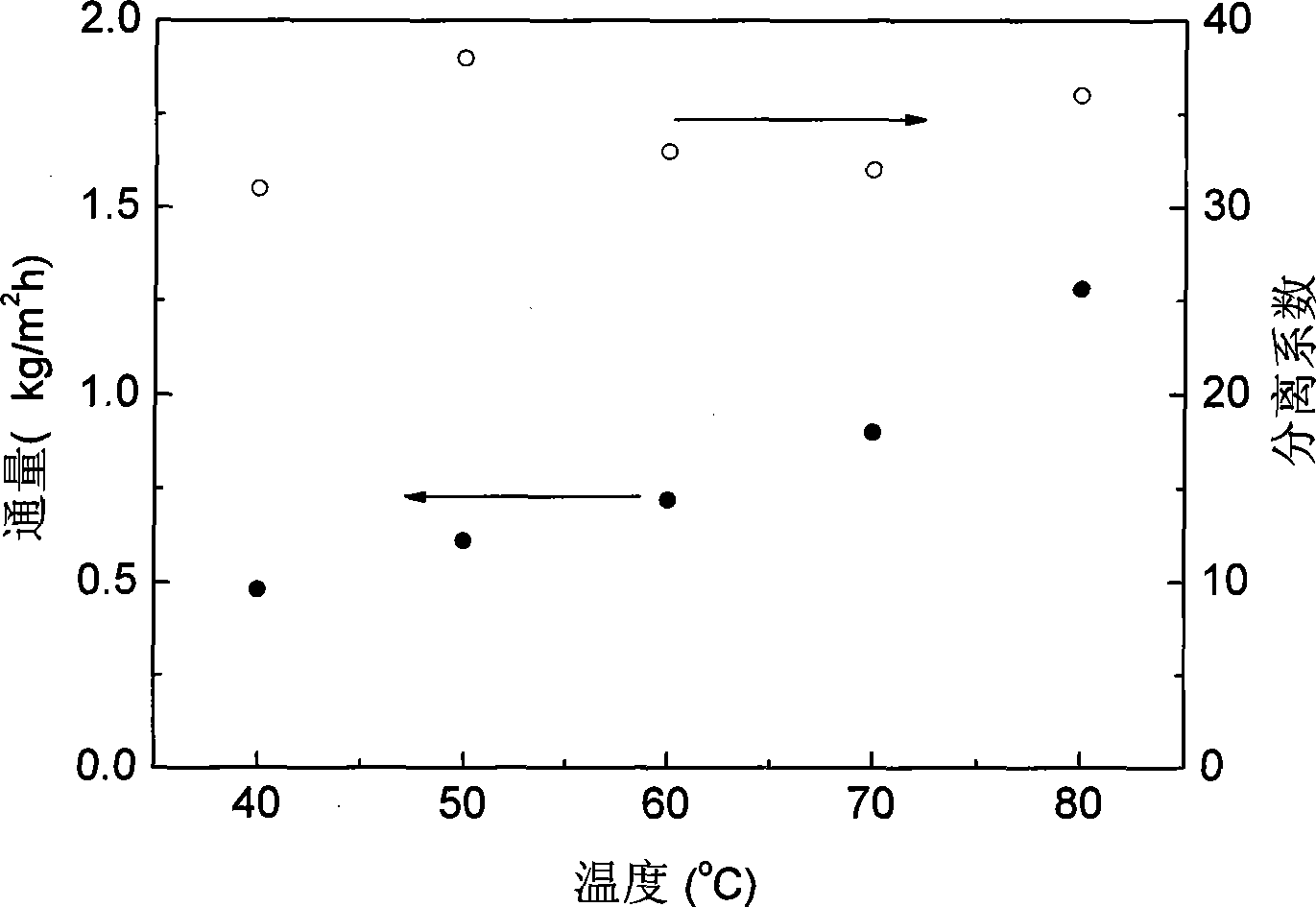
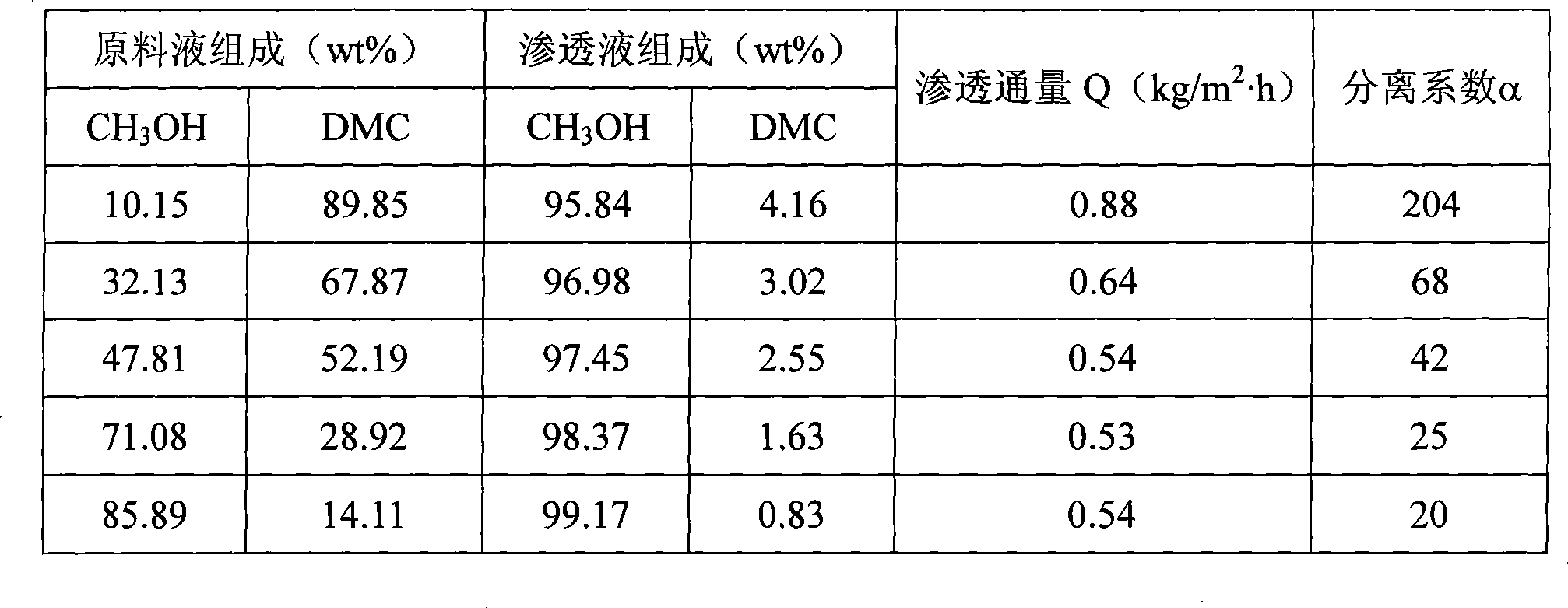
Permeating gasification film used for separation of methanol/dimethyl carbonate azeotropic liquid, and its preparing method
InactiveCN101003002ALow priceReduce energy consumptionDistillationCarbonic/haloformic acid esters purification/separationMethyl carbonateALLYL SUCROSE
A gasifying osmotic film for separating the azeotropic methanol / dimethyl carbonate solution is prepared from polyvinyl alcohol and polyacrylic acid. Its preparing process is also disclosed.
Owner:山东蓝景膜技术工程有限公司
Method for separating methanol and dimethyl carbonate azeotropic mixture
InactiveCN101362694AAchieve separationDistillationHydroxy compound separation/purificationMolecular sieveDesorption
The invention provides a new method for separating azeotropic mixture of methanol and methyl carbonate. The invention is characterized in that the mixed solution of methanol and methyl carbonate is supplied in circulation at the upstream side of molecular sieve membrane, and the downstream side of the molecular sieve membrane is connected to a vacuum system. As the molecular sieve membrane has the very high permselectivity for methanol, methanol in mixed solution preferentially permeates through the molecular sieve membrane, and vaporization and desorption are carried out at the downstream side of membrane, and then methanol is condensed and collected by the vacuum system. After multiple circulations, the mixed solution can achieve the complete separation of methanol and methyl carbonate. Compared with the traditional rectification method, the method of the invention has advantages of low energy consumption, no pollution, compact device, high separation efficiency, and the like, and has a very important meaning for industrial production of methyl carbonate and the preparation of other organic chemical materials by using methyl carbonate.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
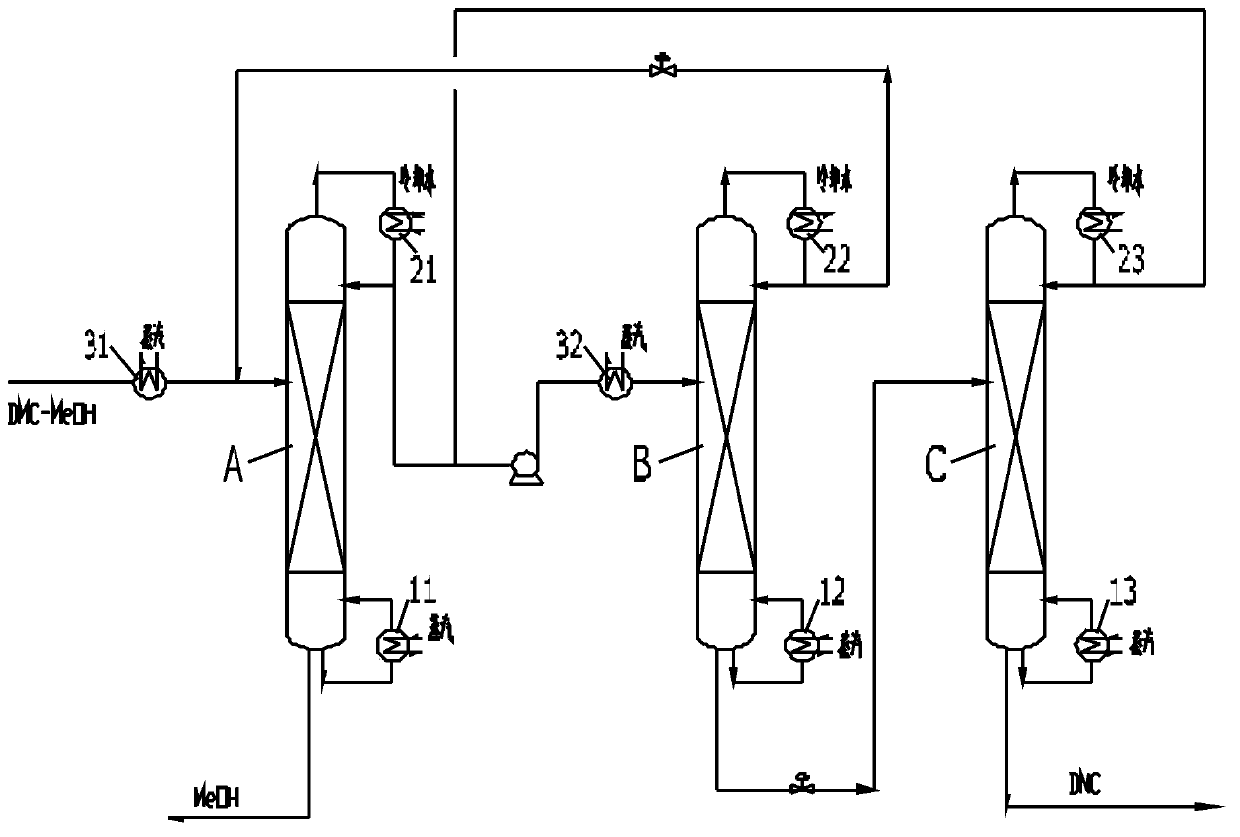
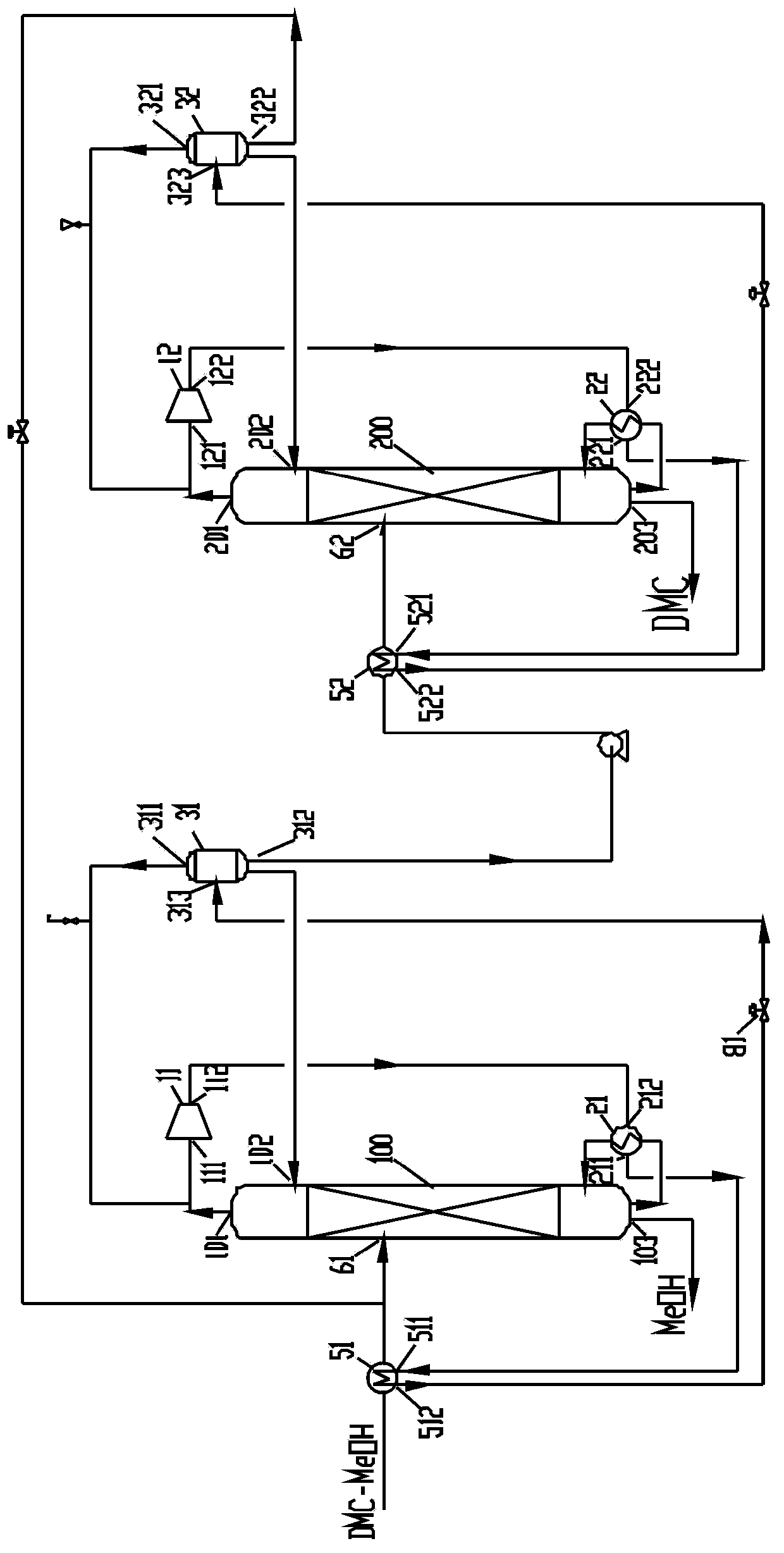
Method for separating dimethyl carbonate and methanol through pressure-swing distillation of heat pump, and apparatus thereof
InactiveCN103626656AReduce energy consumptionLow costCarbamic acid derivatives preparationOrganic compound preparationReboilerSelf-condensation
The invention provides a method for separating dimethyl carbonate and methanol through pressure-swing distillation of heat pump, and an apparatus thereof. In the dimethyl carbonate and methanol through pressure-swing distillation of the heat pump, an atmospheric azeotropic steam material obtained from the top of an atmospheric rectification tower is supercharged and heated by a heat pump compressor, and is introduced to a reboiler at the bottom of the atmospheric rectification tower as a heat source, so a liquid in the atmospheric rectification tower is heated, and the self-condensation of the atmospheric azeotropic steam material is completed. Compared with traditional atmospheric azeotropic steam material condensation through using cooling water and kettle liquid heating realized through an extra external heat source, the method in the invention properly improves the temperature and the pressure of an atmospheric azeotropic steam through the heat pump compressor to make the atmospheric azeotropic steam realize the kettle liquid heating and the reasonable and effective energy cycle as a heat source; and the cost generated by the pump heat compressor is far lower than the cost of traditional cooling water and the external heat source, so a good economic benefit is generated.
Owner:YASHENTECH CORP
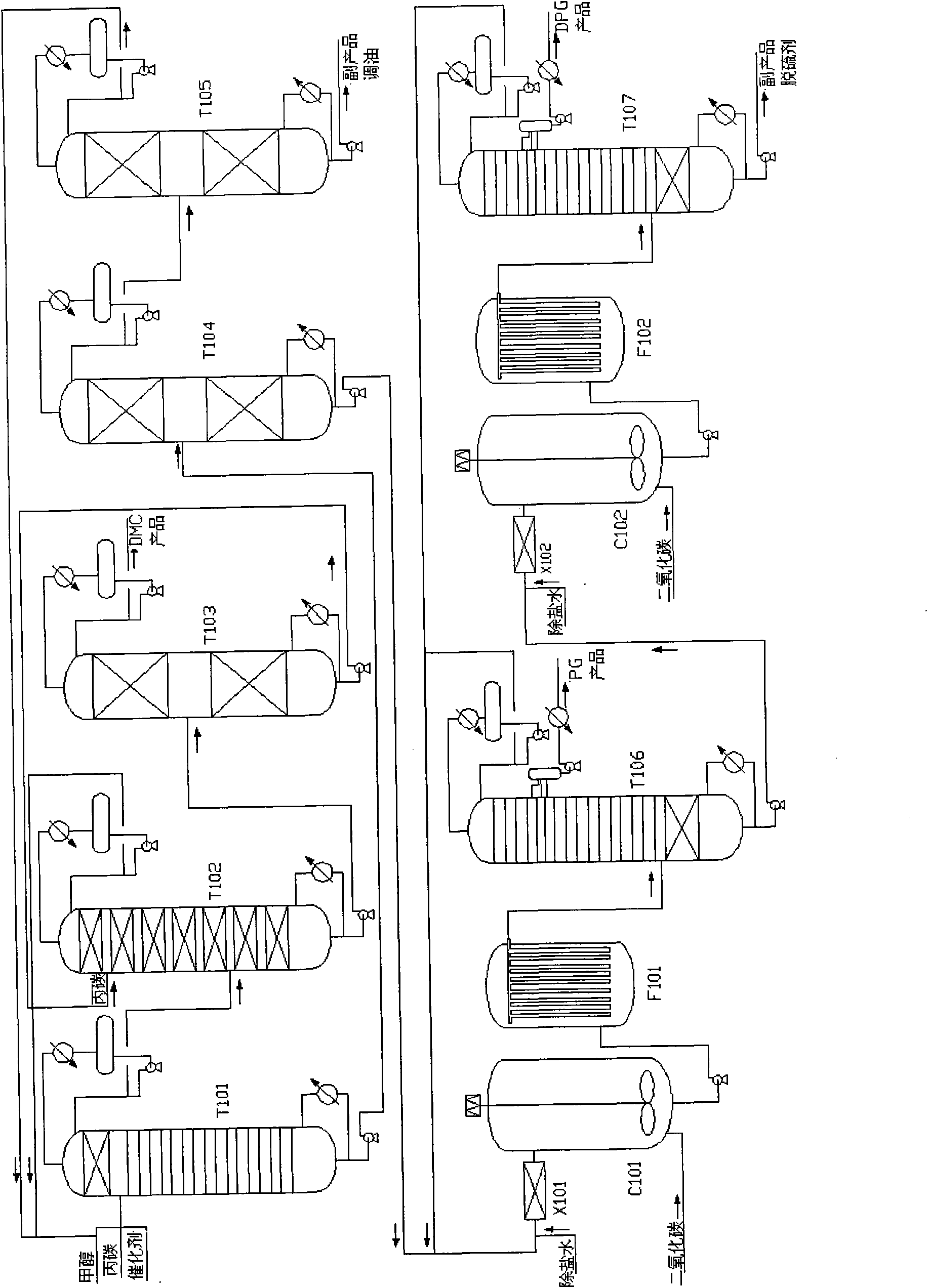
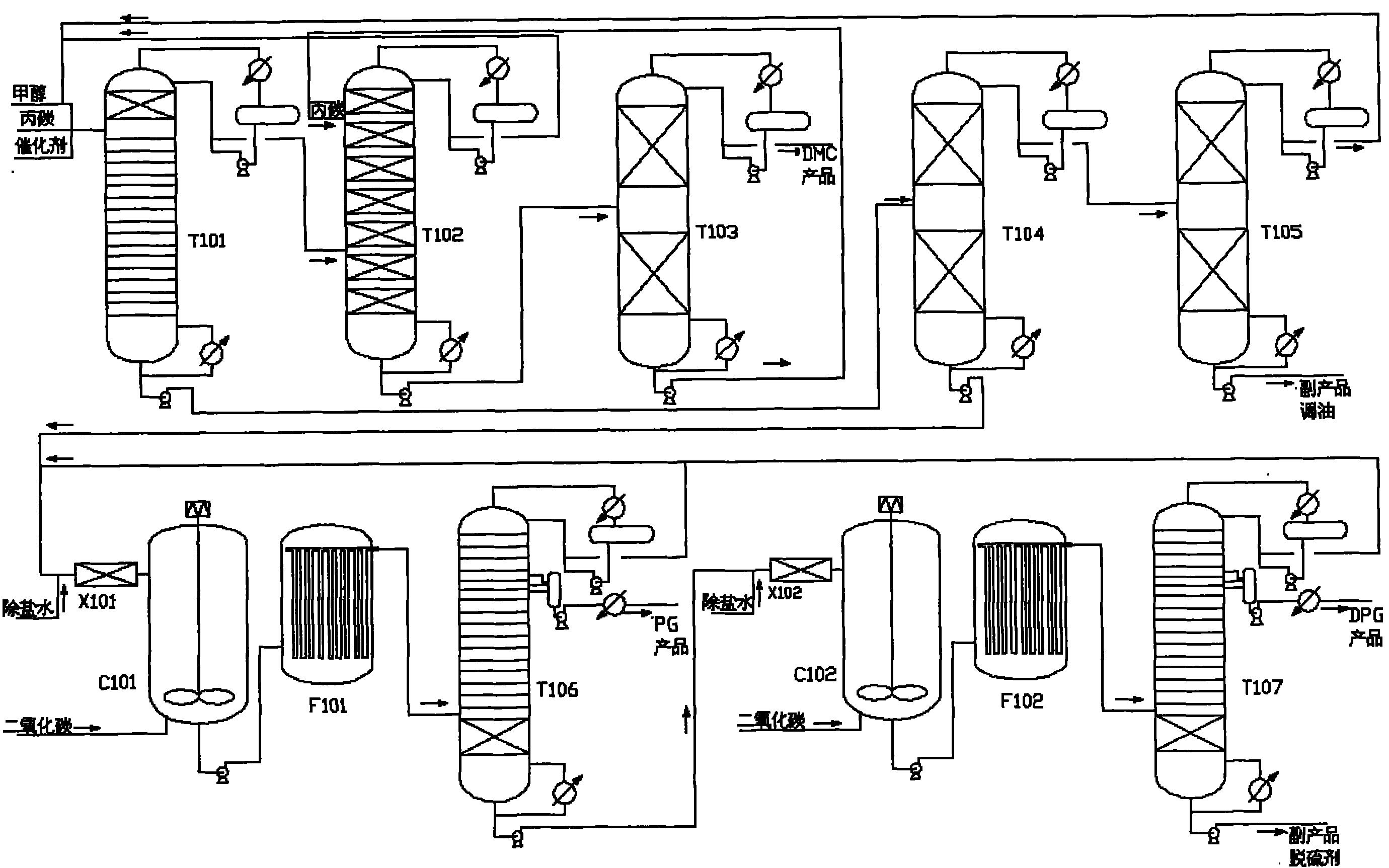
Novel technique for purifying high-quality propylene glycol in production process of dimethyl carbonate
ActiveCN101774888AHigh yieldSolve the emission problemChemical industryHydroxy compound separation/purificationFiltrationExtractive distillation
The invention relates to a technique for coproduction of dimethyl carbonate and propylene glycol, in particular to a novel technique for purifying high-quality propylene glycol in the production process of dimethyl carbonate. The technical scheme includes the steps of reactive distillation, extractive distillation, separation of the dimethyl carbonate, the recovery and refining of carbinol, primary carbonization, first filtration of sodium carbonate crystals and refining of the propylene glycol. The invention solves the problem of low recovery rate of the propylene glycol, and the recovery rate of the propylene glycol can be above 95%. Meanwhile, the propylene glycol has more stable quality, with the purity reaching 99.99% and the chroma less than 10, thereby the propylene glycol totally meets the requirements for medical auxiliary material, daily cosmetics, top grade unsaturated resin and polyether. Meanwhile, the purity of the byproduct dipropylene glycol can be above 99.50%, thus improving the benefit of the equipment.
Owner:DONGYING HI TECH SPRING CHEM IND
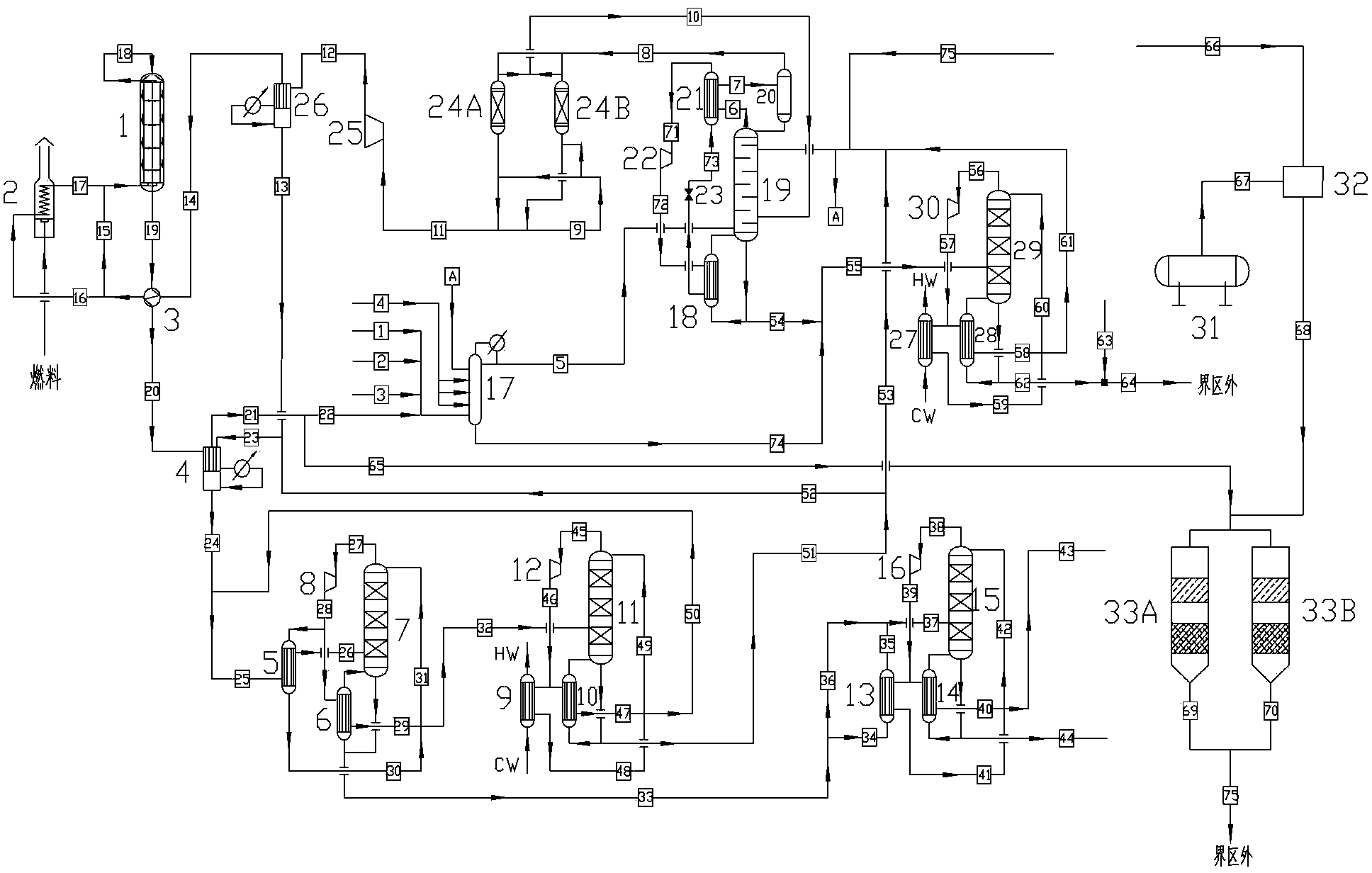
Process for producing dimethyl carbonate from industrial synthetic gas
ActiveCN103408428ANo overheating phenomenonDoes not affect safe operationOrganic compound preparationChemical industryMethyl carbonateGas phase
The invention relates to a process for producing dimethyl carbonate from industrial synthetic gas. According to the invention, O2, CO, N2, NO, and methanol are delivered into an esterification system for esterification; heavy component drawn from the esterification system is subjected to recovery treatment in a wastewater tower; light component drawn from the esterification system passes a compressor II and is subjected to a carbonylation reaction in a carbonylation reactor; the carbonylation reaction product is delivered into a second condensation separation tower, and is subjected to gas-liquid separation; separated liquid phase is refined in a pressurized rectification tower; part of non condensable gas is discharged from the separated gas phase, and the gas phase is continued to be subjected to a reaction in the esterification system; the discharged non-condensable gas is delivered into a denitration reactor; light component at a top of the pressurized rectification tower is subjected to further recovery treatment in a methanol recovery tower; heavy component from the pressurized rectification tower is delivered into a product tower; dimethyl carbonate is drawn from the top of the product tower, and dimethyl oxalate is drawn from the bottom of the product tower. The process has the economical and practical characteristics of low equipment investment, environment friendliness, energy saving, high catalyst efficiency, high raw material utilization rate, and the like.
Owner:SHANGHAI WUZHENG ENG TECH CO LTD
Catalyst used for catalytic synthesizing dimethyl carbonate directly from methanol and carbon dioxide and preparation and using method thereof
The invention discloses a direct catalytic synthesis catalyst to prepare dimethyl carbonate from methanol and carbon dioxide and the preparation and application methods of the catalyst. The catalyst of the invention consists of transitional metal soluble salt, promoter and carrier, with the weight ration from 0.01to 0.5: 0.01to 0.1:1. The preparation method is that: (1) the carrier is impregnated into the transition metal soluble salt solution; (2) the promoter is added into the solution, which is stirred in room temperature, ultrasonically dispersed and stored stationarily in room temperature; (3) the solution is dried, sintered, reduced and activated to produce catalyst. The application method is that: the catalyst is put in high pressure reactor or micro reaction device with the temperature of the catalyst bed controlled between 90 degrees centigrade to 140 degrees centigrade and the reaction pressure between 0.6 to 3.0MPa. The catalyst is applicable in direct catalytic synthesis to prepare dimethyl carbonate from methanol and carbon dioxide. The raw material sources are rich, the cost is low, the preparation method is simple and the operation is easy. The catalyst is easily separated from the products, the reaction conditions are mild and the catalyst can be used repeatedly. The catalyst has high activity and selectivity.
Owner:SUN YAT SEN UNIV
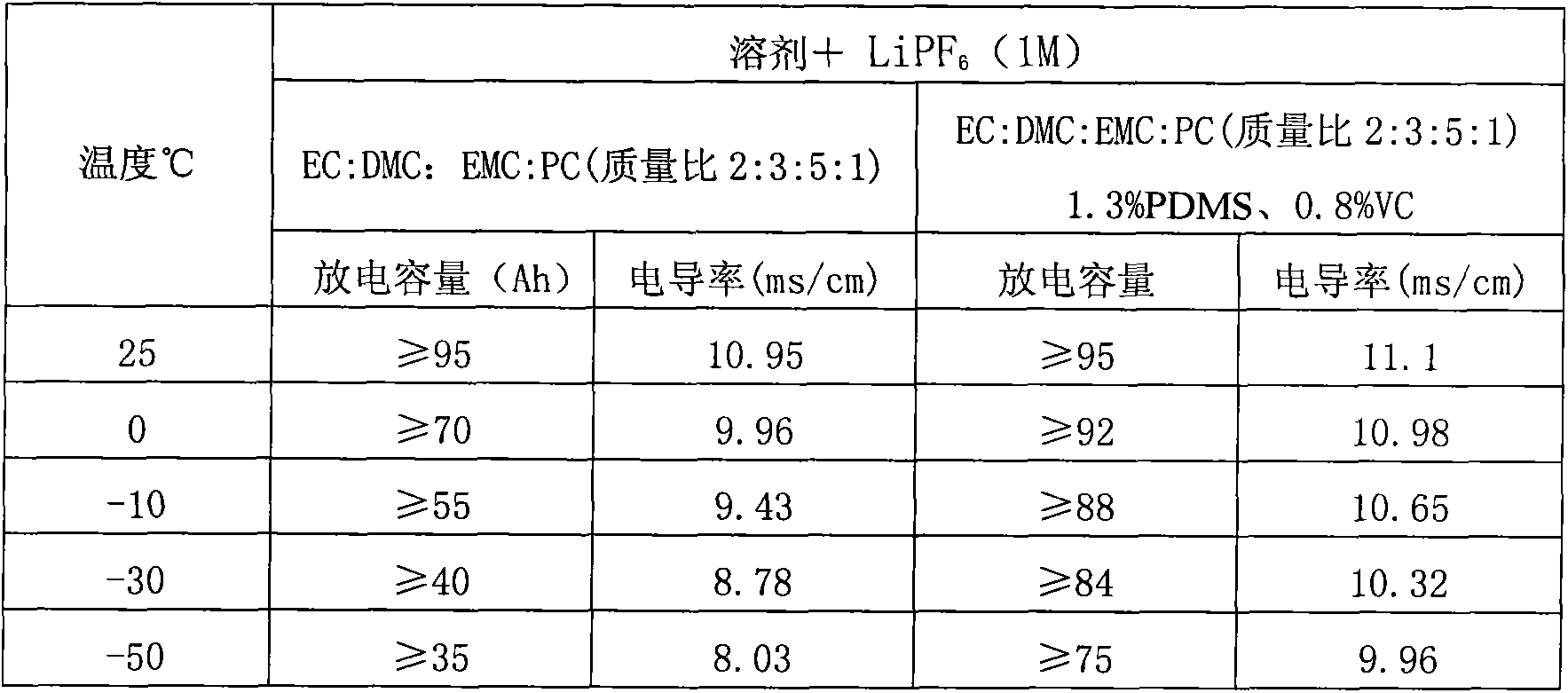
Low-temperature type carbonic ester lithium battery electrolyte
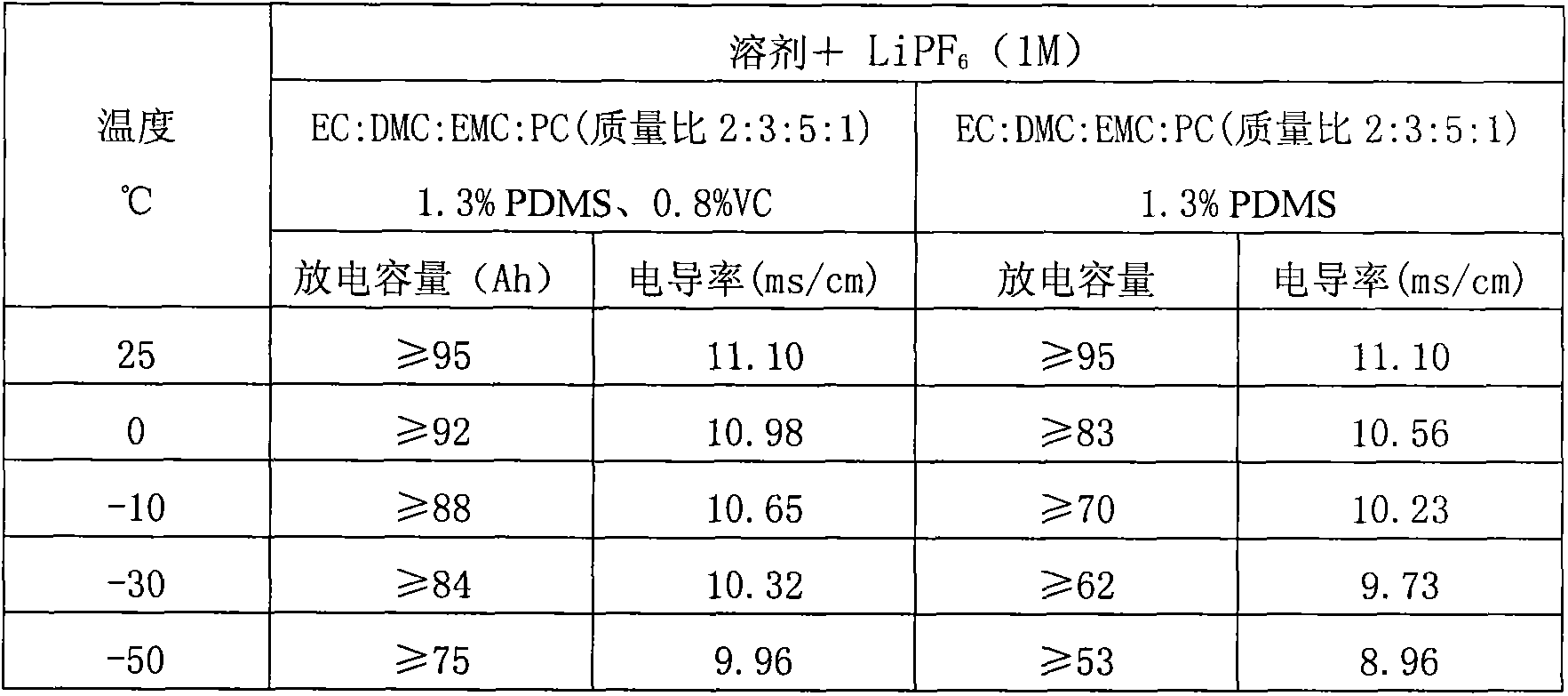
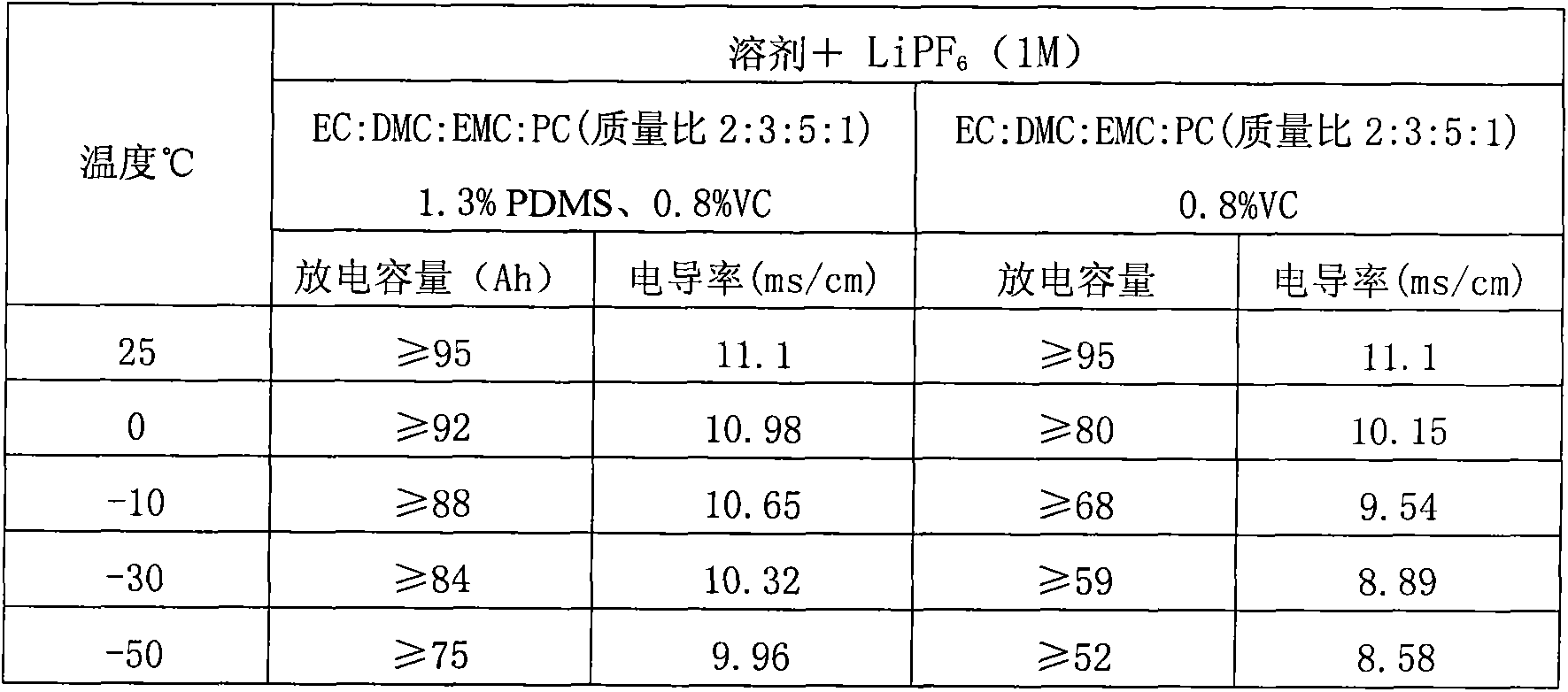
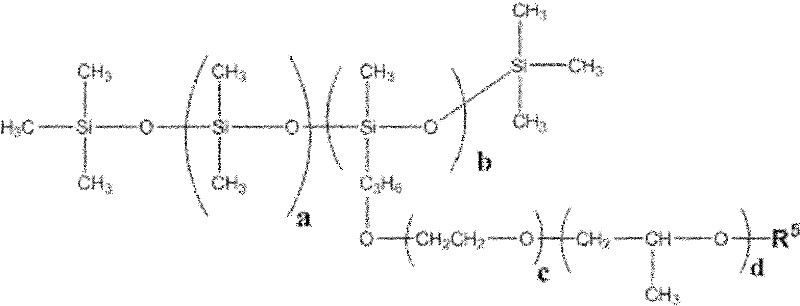
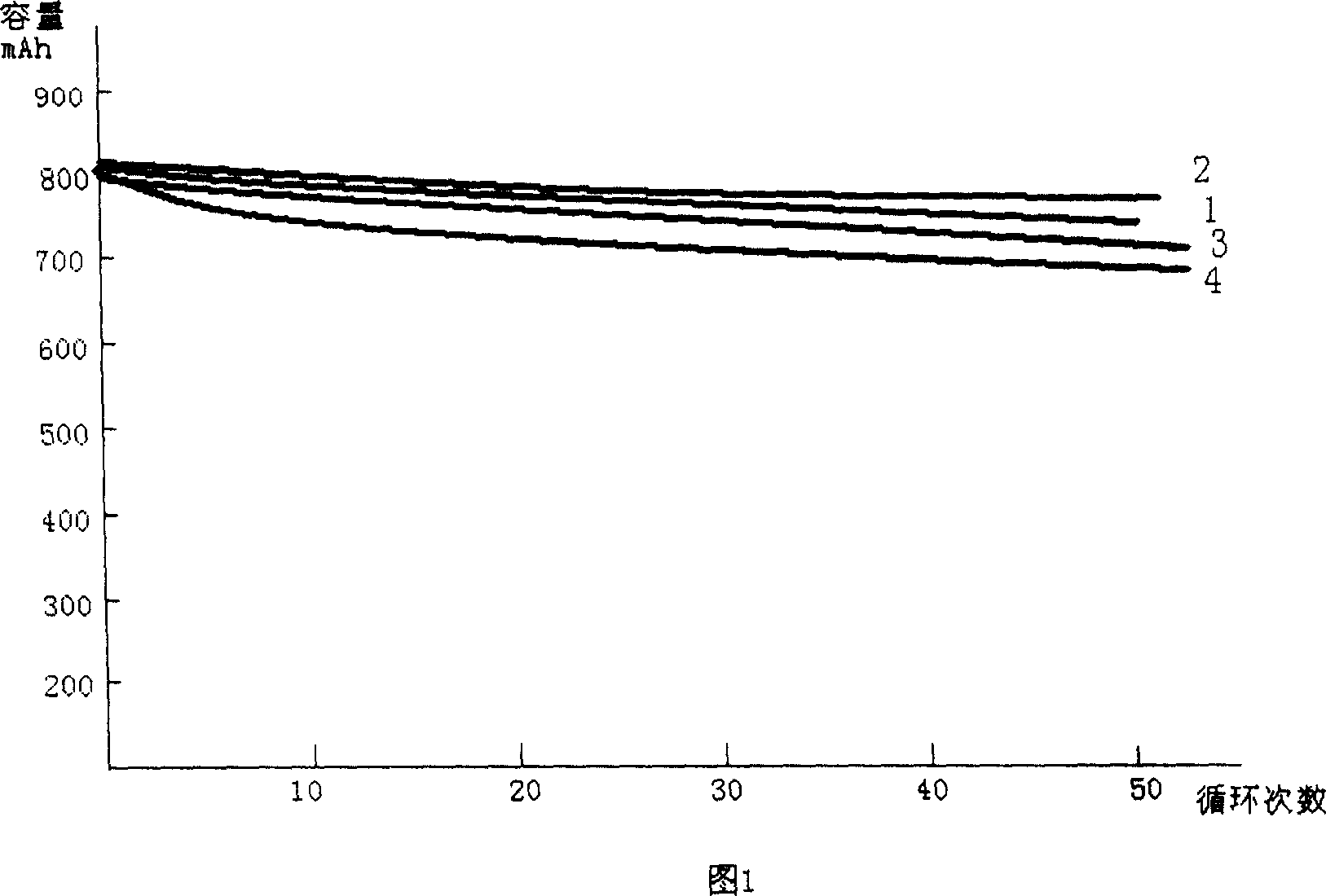
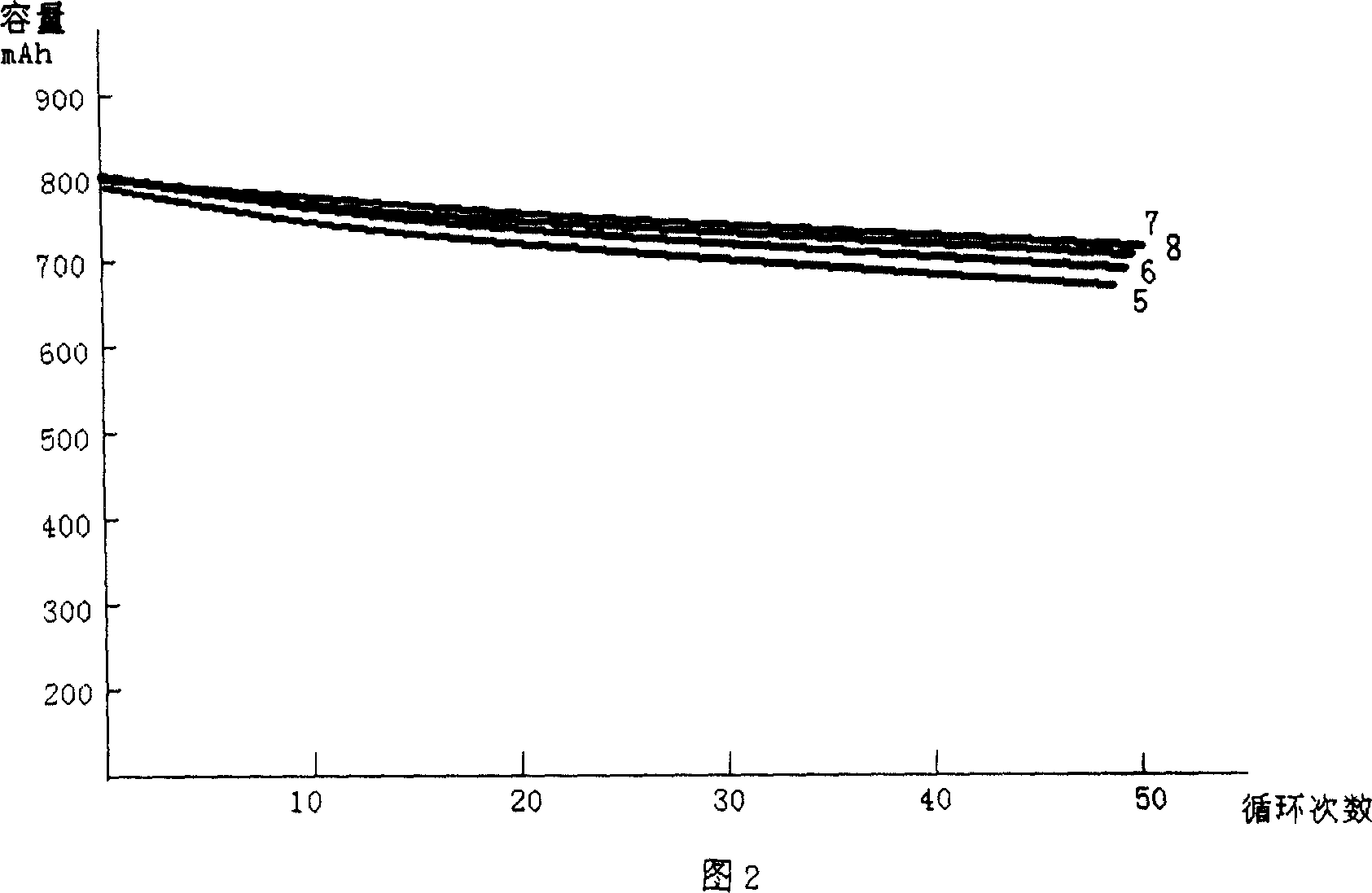
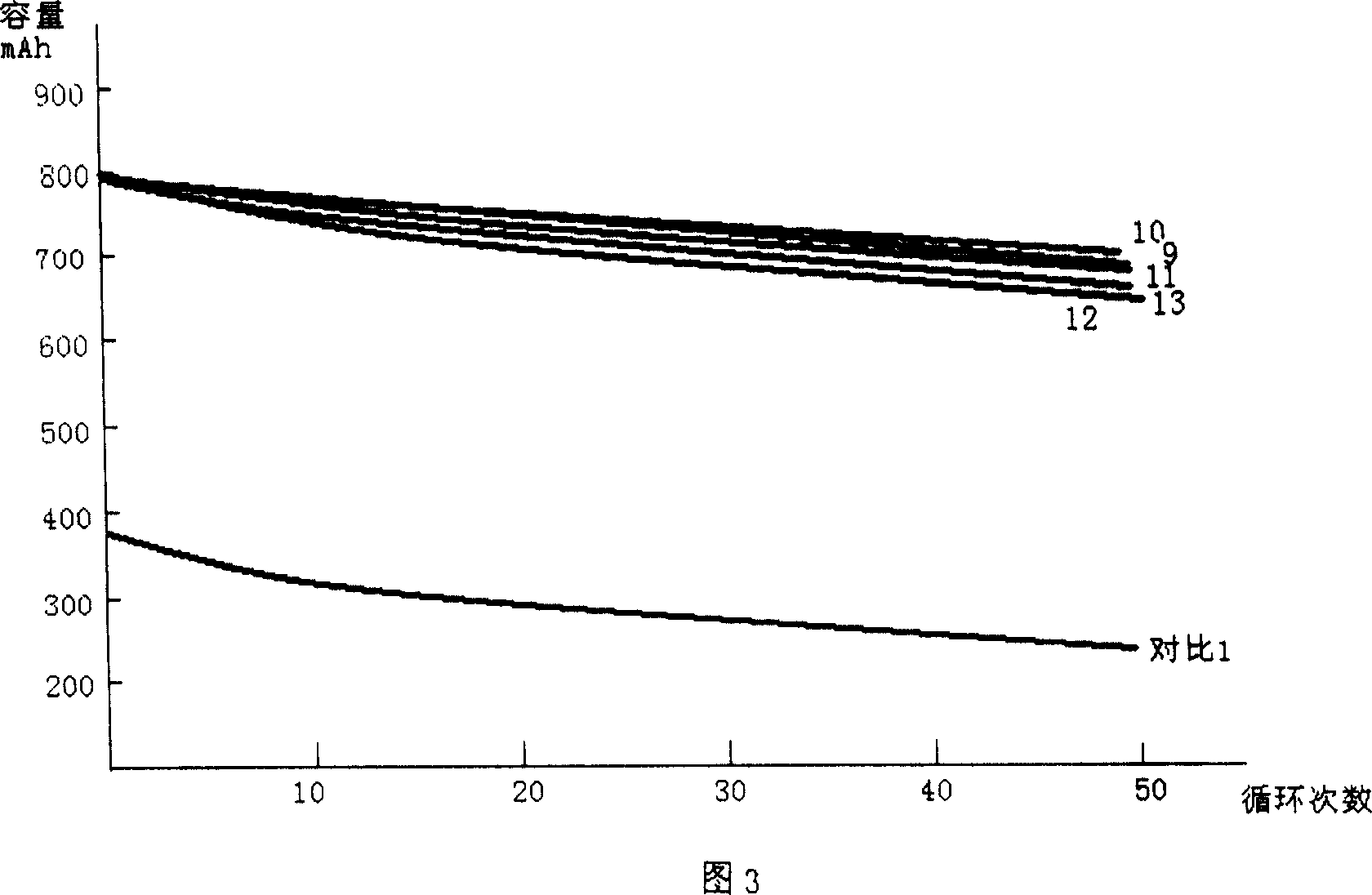
The invention discloses a low-temperature type carbonic ester lithium battery electrolyte. The electrolyte mainly comprises the following components: ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and propylene carbonate as solvents, lithium hexafluorophosphate and a low temperature additive. The four carbonic ester solvents are in the following weight ratio: EC: DMC: EMC: PC=2:3:5:1; the components are prepared into an 1 mol / L electrolyte containing LiPF6, four carbonic ester solvents, the low temperature additive; and the low temperature additive is one selected from polydimethylsiloxane, 1,3- propane sultone, and vinylene carbonate, or a combination thereof. According to the invention, optimization of composition of the carbonic ester solvents and addition of the low temperature additive are employed to increase solubility, degree of dissociation and conductivity of the electrolyte, improve structure of a solid phase interfacial film (SEI membrane) of the lithium ion battery cathode, the integral stability of the battery and battery cycling and prolong usage life. Especially, the invention provides a mainstream electrolyte for lithium ion power batteries used in a lowest temperature of -50 DEG C in most regions in northern China.
Owner:CHAOYANG GUANGDA CHEM
Electrolyte for power lithium ion battery
InactiveCN102544582AImprove wettabilityStable electrochemical propertiesSecondary cellsPolyolefinSolvent
The invention discloses high-wettability and high-pourability electrolyte for a power lithium ion battery. The electrolyte comprises a wetting additive, a lithium salt, a non-aqueous organic solvent and other additives, wherein the wetting additive is a nonionic surfactant, and the wetting additive accounts for 0.001 to 5 percent of the mass of the electrolyte; the non-aqueous solvent is one or a mixture of more of ethylene carbonate, propylene carbonate, methyl ethyl carbonate, dimethyl carbonate, diethyl carbonate and gamma-butyrolactone, and the non-aqueous solvent accounts for 50 to 90 percent of the mass of the electrolyte; the lithium salt at the concentration of 0.6 to 1.5 mol / L is at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(oxalato)borate and lithium trifluoromethanesulfonate; and the other additives accounts for 0.5 to 8 percent of the mass of the electrolyte. The electrolyte has high wettability for a polyolefin diaphragm and electrode active materials, and has long cycle life.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
Preparation method for high-viscosity 107 glue
ActiveCN102558563AHigh yieldSimple preparation processAdhesivesTetramethylammonium hydroxideBoiling point
The invention relates to a preparation method for high-viscosity 107 glue. The preparation method comprises the following steps of: adding a certain amount of DMC (Dimethyl Carbonate) and 107 glue of which the viscosity is 100-1,500 cs or a certain amount of DMC into a reactor provided with a stirrer, a thermometer and a condensation reflowing pipe, starting stirring, heating to a certain temperature, and adding an alkali catalyst, wherein the alkali catalyst comprises one of potassium hydroxide, tetramethylammonium hydroxide pentahydrate, tetrabutyl phosphorus oxychloride or silicon alkoxidealkali gel of the alkalis; undergoing a balanced reaction at the temperature for certain hours, and directly neutralizing or heating for decomposing the alkali catalyst; and adding a phosphoric acid or silanol hydrochloric acid gel neutralizing agent of which the mass is equal to that of the catalyst under the condition that neutralization is performed, or directly heating for decomposing the alkali catalyst under the condition that neutralization is not performed, continuously stirring for 1 hour, and removing low-boiling-point substances. The high-viscosity 107 glue prepared with the methodhas the advantages of simple preparation process, stable viscosity, high product yield and capability of meeting production requirements.
Owner:JIANGXI BLUESTAR XINGHUO SILICONE CO LTD
High rate electrolyte for lithium ion battery
ActiveCN1925207AIncrease dissociationImprove mobilityOrganic electrolyte cellsSecondary cellsHigh ratePhysical chemistry
This invention relates to lithium ion battery electrolyte with high times and discloses the main components as following: vinylene carbonate for 10 to 30 percentage; propylene carbonate for 4 to 20 percentage; dimethyl carbonate for 35 to 50 percentage; methyl carbonate for 10 t0 20 percentage; lithium salt for 12.5 to 16 percentage and B(OR)<3> with R representing carbon atoms less than 4 or crown ether of one or more function addictives for 0.1 to 5.0 percentage.
Owner:GUANGZHOU TINCI MATERIALS TECH
Solid basic catalyst, preparation method of solid basic catalyst and application of solid basic catalyst in ester exchange reaction
InactiveCN102698811AImprove stabilityImprove catalytic performanceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from organic carbonatesOrganic baseSilicon oxide
The invention discloses a solid basic catalyst, a preparation method of the solid basic catalyst and an application of the solid basic catalyst in ester exchange reaction. The solid basic catalyst catalyzes the ester exchange reaction by the reaction of metal organic compound and hydroxyl on a carrier under the moderate conditions to synthesize dimethyl carbonate. The solid basic catalyst provided by the invention consists of metal organic alkali and the carrier. The metal organic alkali is linked to the carrier in bond-forming manner; the metal organic alkali is one or more of lithium methoxide, lithium ethoxide, lithium isopropoxide, lithium n-butoxide, lithium tert-butoxide, sodium methoxide, sodium ethoxide, sodium isopropoxide, sodium n-butoxide, sodium tert-butoxide, potassium methoxide, potassium ethoxide, potassium isopropoxide, potassium n-butoxide and potassium tert-butoxide; the carrier is one or more of silicon oxide, aluminium oxide, titanium oxide, zirconia, mesoporous silicon oxide synthesized by the template method, mesoporous aluminium oxide synthesized by the template method, mesoporous titanium oxide synthesized by the template method, and mesoporous zirconia synthesized by the template method.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com