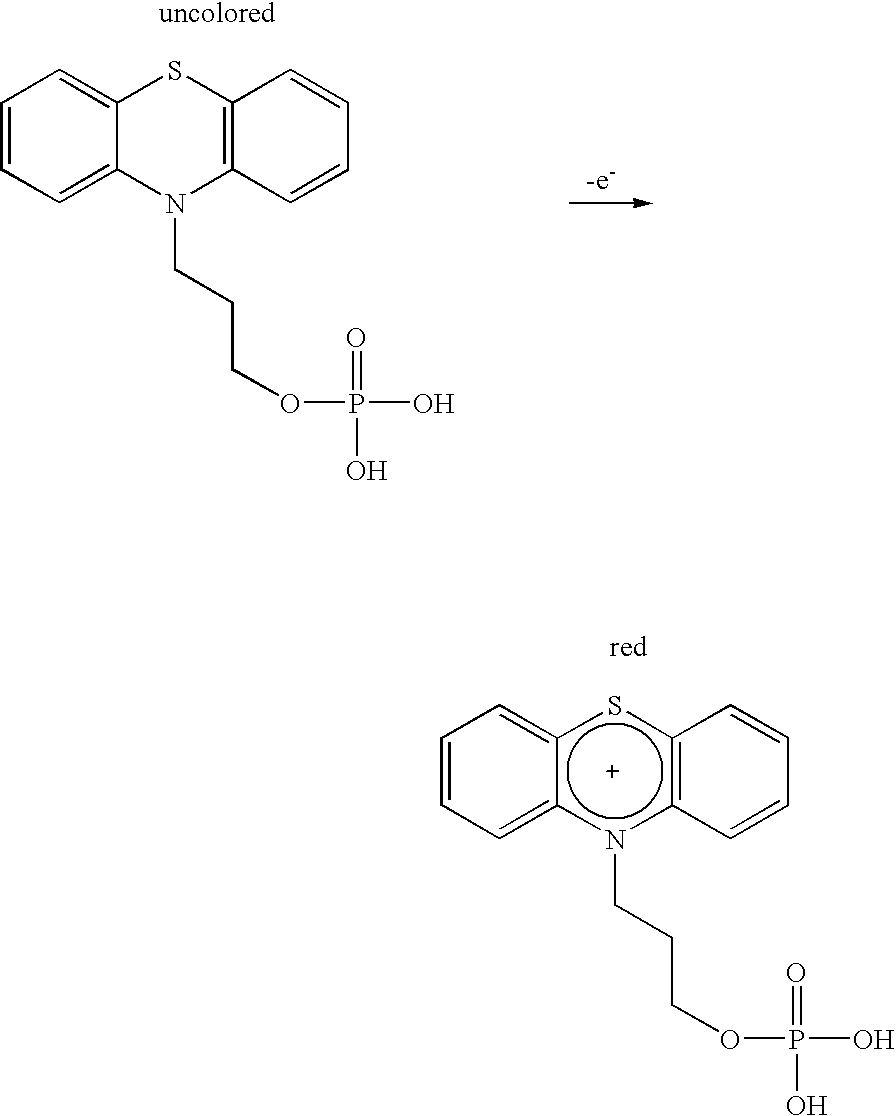
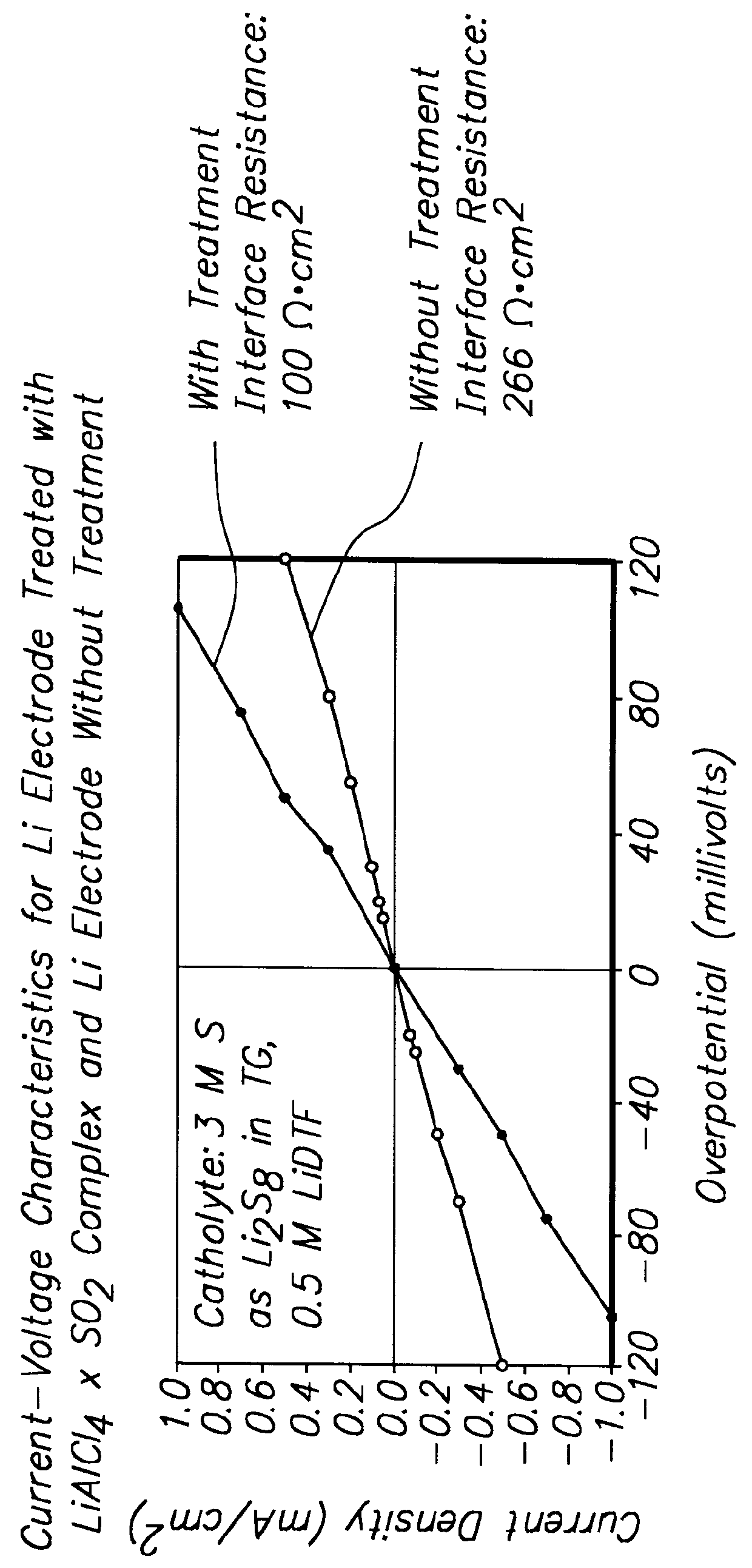
Patents
Literature
5498 results about "Electrochemical cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic cells or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells. A common example of a galvanic cell is a standard 1.5 volt cell meant for consumer use. A battery consists of one or more cells, connected either in parallel, series or series-and-parallel pattern.
Electrochemical sensors including electrode systems with increased oxygen generation
ActiveUS7108778B2Immobilised enzymesBioreactor/fermenter combinationsAnalyteElectrochemical gas sensor
The present invention relates generally to systems and methods for increasing oxygen generation in electrochemical sensors in order to overcome the oxygen limitations. The preferred embodiments employ electrode systems with at least two electrodes in relatively close proximity to each other; wherein at least one electrode is configured to generate oxygen and at least one other electrode is configured to sense an analyte or a product of a reaction indicative of the concentration of analyte. The oxygen generated by the oxygen-generating electrode is available to the catalyst within a membrane system and / or the counter electrode, thereby enabling the electrochemical sensors of the preferred embodiments to function even during ischemic conditions.
Owner:DEXCOM INC
Sample detection to initiate timing of an electrochemical assay
InactiveUS6193873B1Good accuracy and precisionImprove accuracyImmobilised enzymesBioreactor/fermenter combinationsAnalytePotential difference
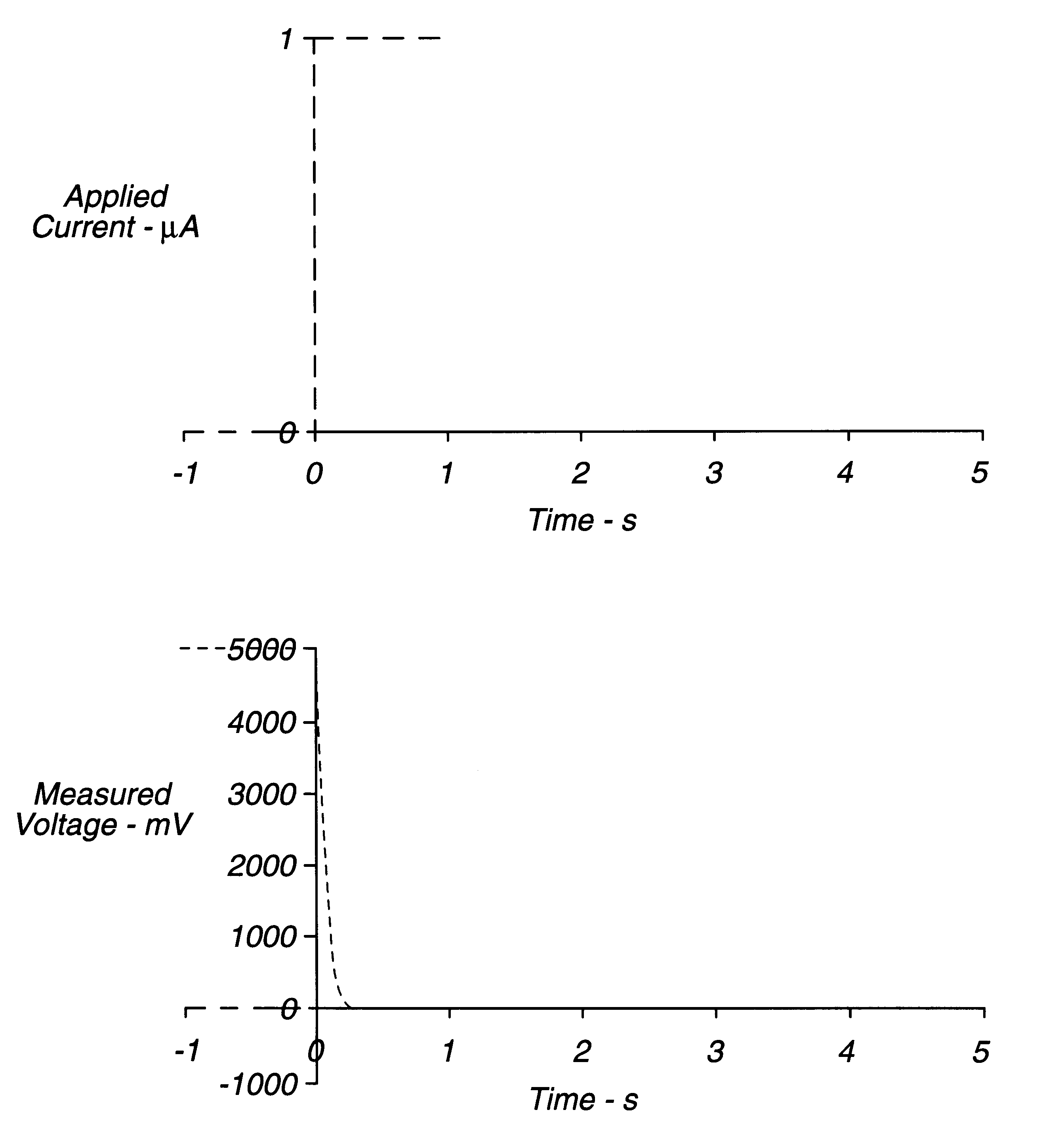
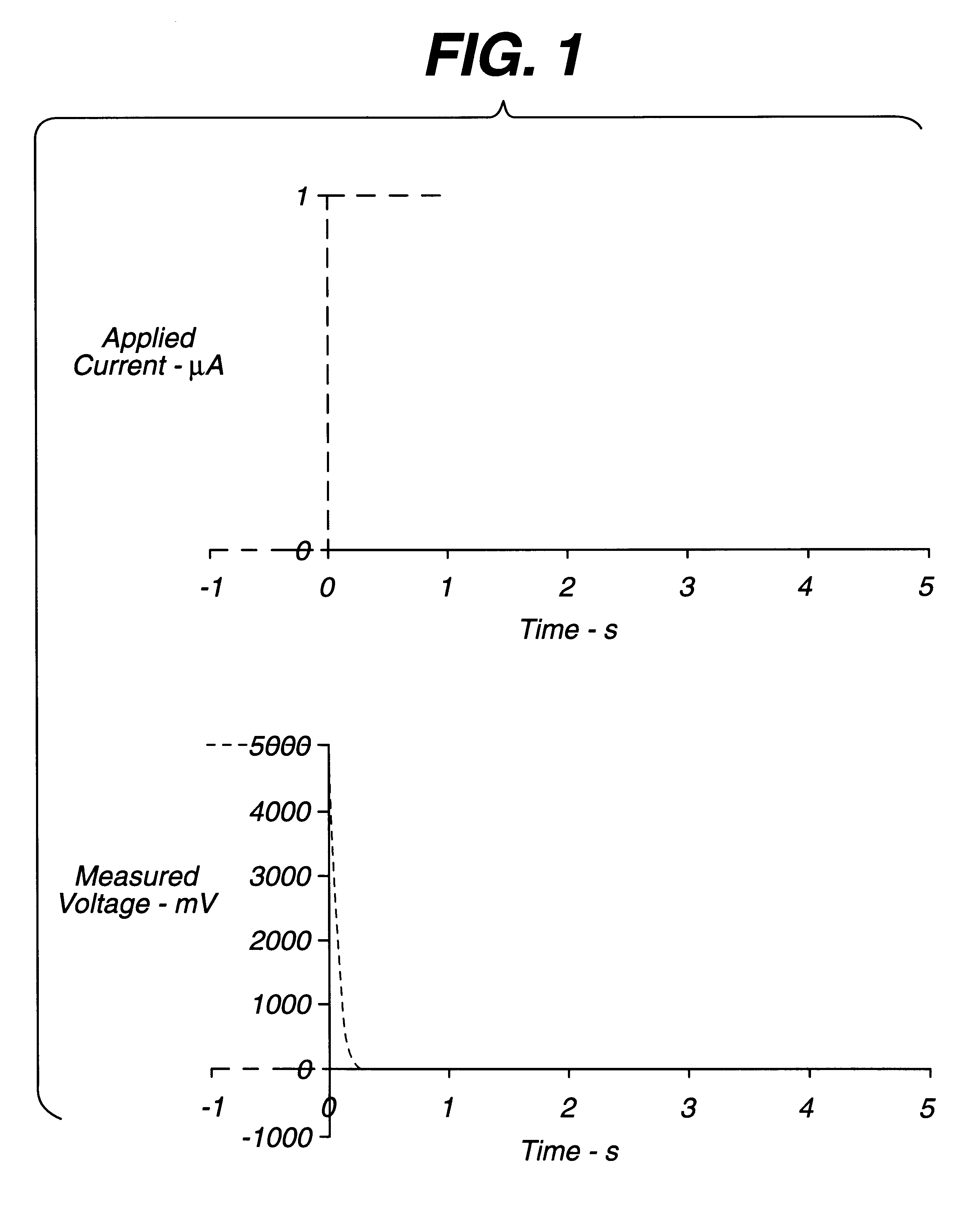
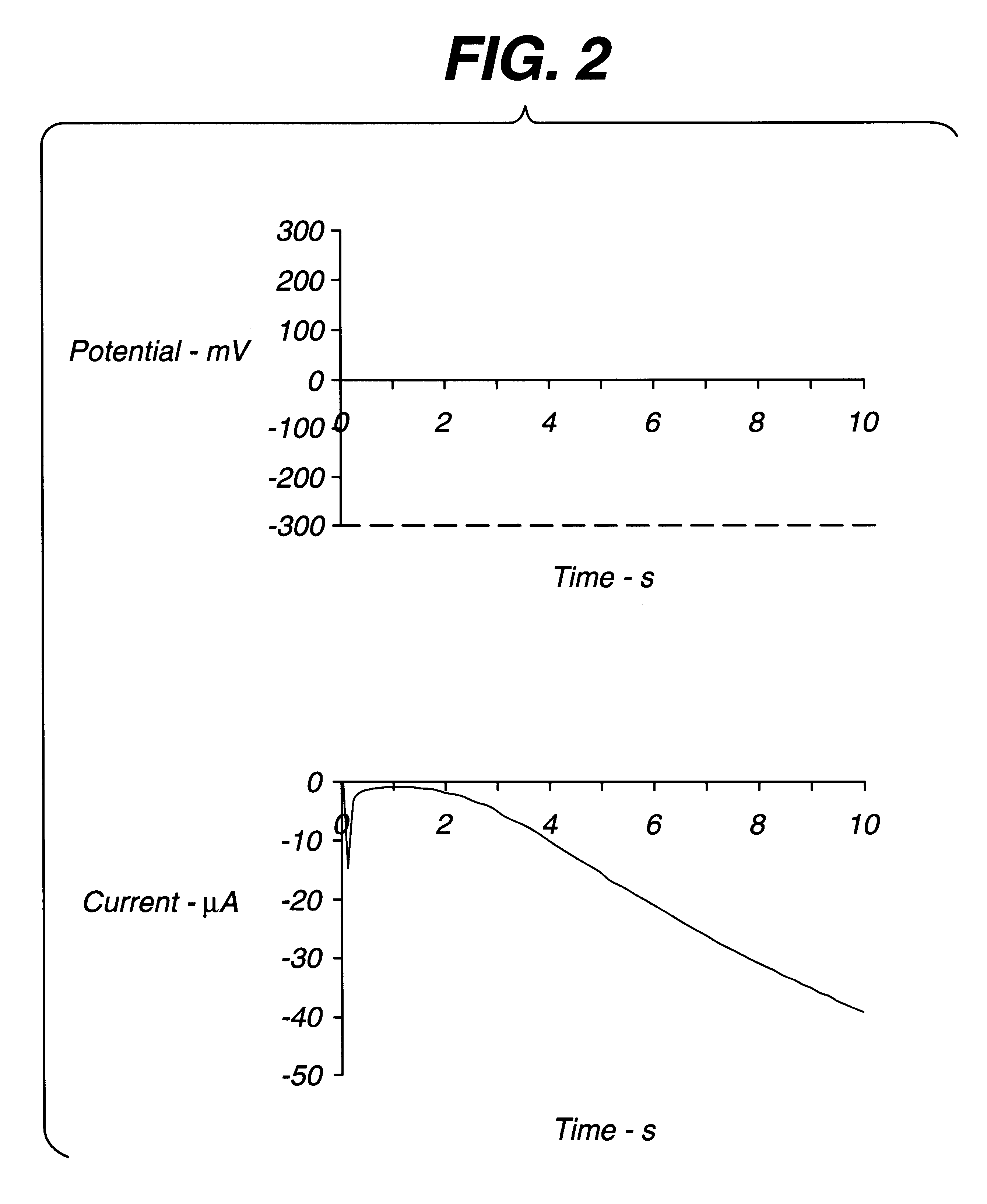
An electrochemical assay includes a method for determining with great accuracy the time at which an applied sample bridges a gap between the electrodes of an electrochemical cell. The method involves applying a constant small current across the gap, while monitoring the potential difference between the electrodes. The time at which the sample bridges the gap is marked by a sharp drop in the potential. A constant voltage is applied after the sample is detected, and the current and / or charge through the sample is monitored over a period of time. From the measured current or charge, the analyte concentration of interest can be calculated.
Owner:LIFESCAN IP HLDG LLC
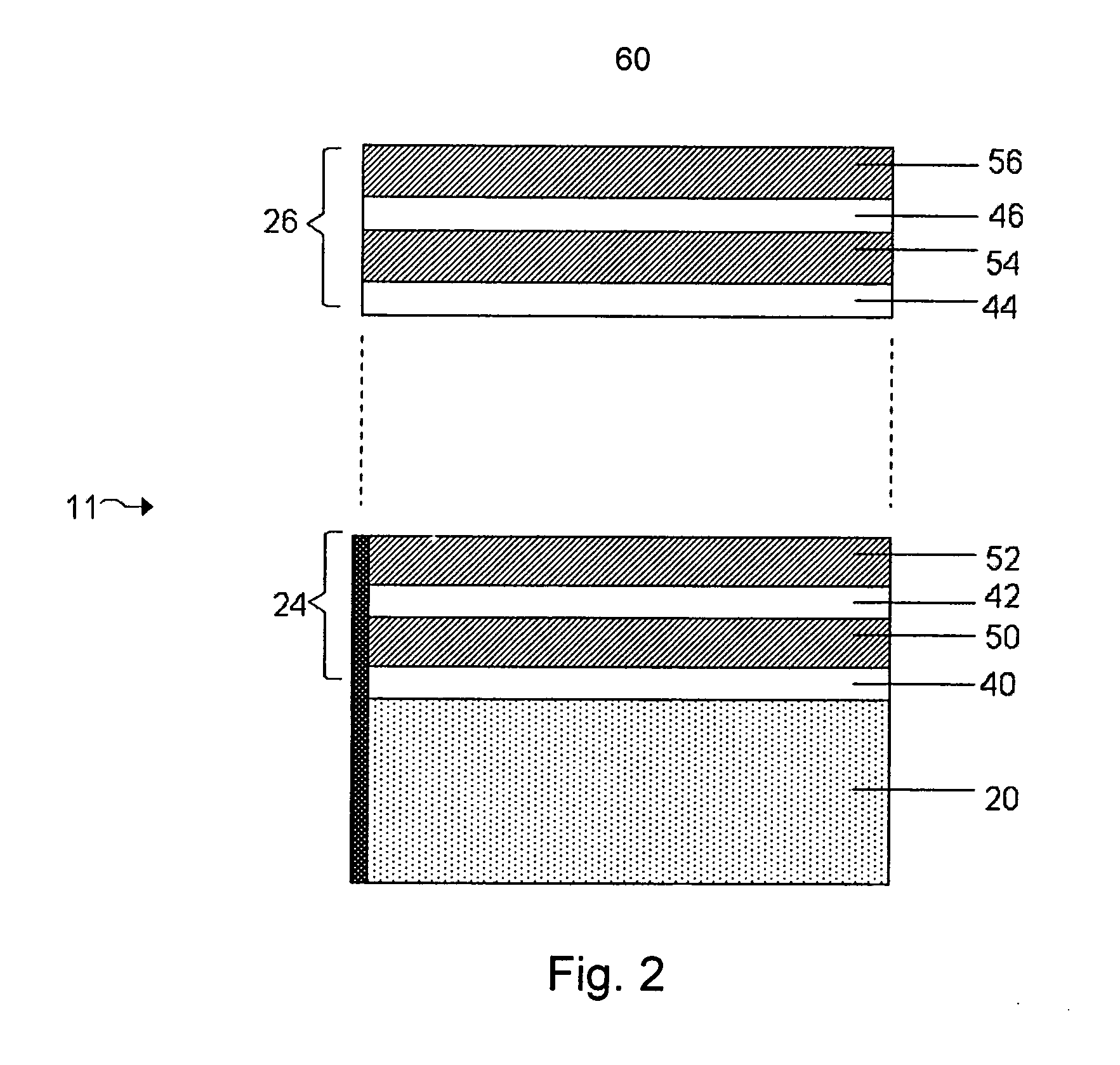
Electrochemical cell
InactiveUS6284125B1Improve accuracyImprove reliabilityImmobilised enzymesBioreactor/fermenter combinationsElectricityDiffusion
A method for determining the concentration of a reduced (or oxidised) form of a redox species in an electrochemical cell of the kind comprising a working electrode and a counter electrode spaced from the working electrode by a predetermined distance, said method comprising the steps of: (1) applying an electric potential difference between the electrodes; (2) selecting the potential of the working electrode such that the rate of electro-oxidation of the reduced form (or electro-reduction of the oxidised form) of the species is diffusion controlled, (3) selecting the spacing between the working electrode and the counter electrode so that reaction products from the counter electrode arrive at the working electrode; (4) determining current as a function of time after application of the potential and prior to achievement of a steady state; (5) estimating the magnitude of the steady state current, and (6) obtaining from the change in current with time and the magnitude of the steady state current, a value indicative of the diffusion coefficient and / or of the concentration of the reduced form (or the oxidised form) of the species. Also disclosed is an apparatus for determining the concentration of a redox species in an electrochemical cell comprising: an electrochemical cell having a working electrode and a counter (or counter / reference) electrode, means for applying and electric potential difference between said electrodes, means for measuring the change in current with time, and characterised in that the working electrode is spaced from the counter electrode by less than 500 mum.
Owner:LIFESCAN INC
Electrochemical cell
InactiveUS6413410B1Improve accuracyImprove reliabilityWeather/light/corrosion resistanceMicrobiological testing/measurementAnalyteElectrochemical biosensor
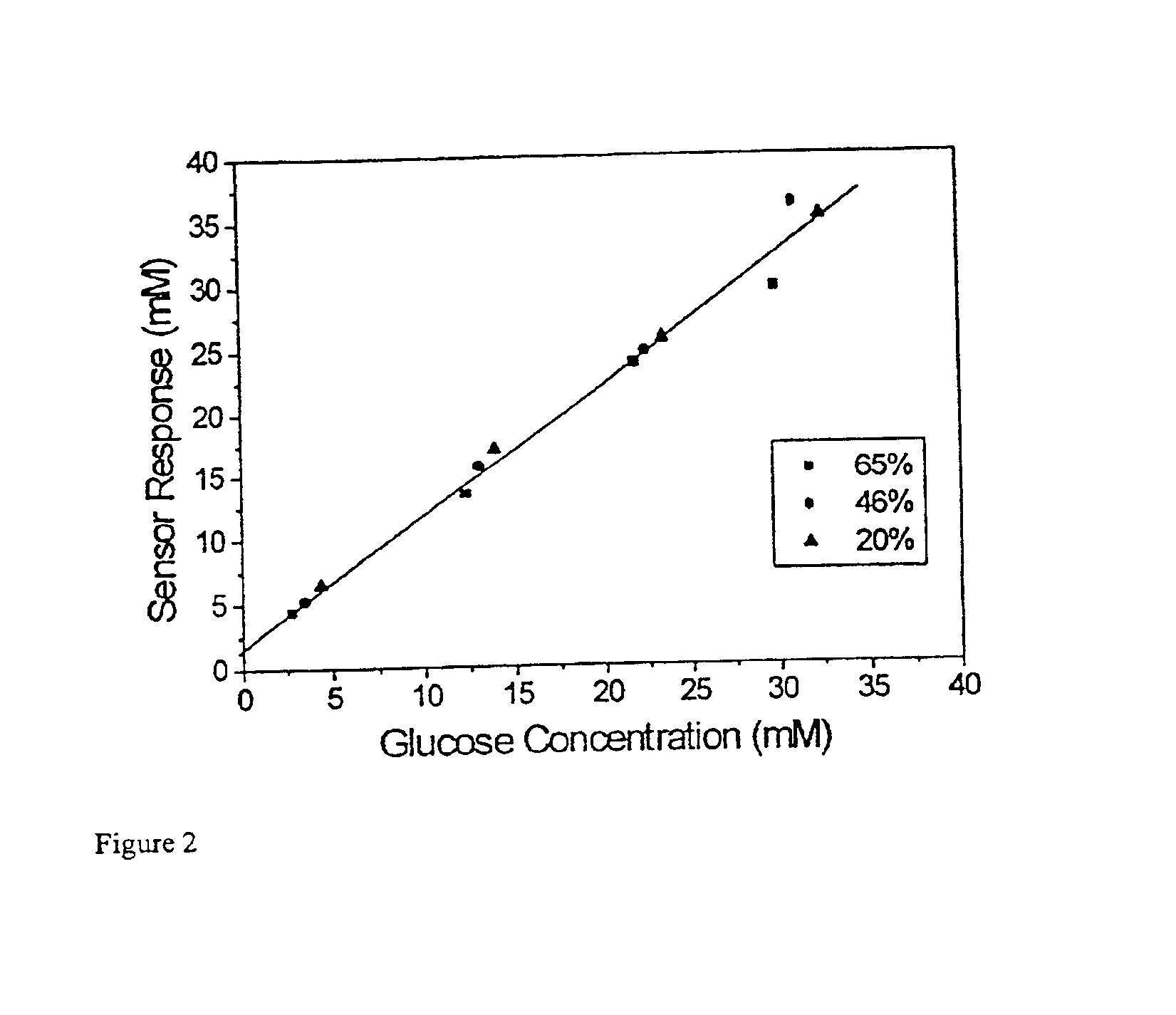
This invention relates to a biosensor and more particularly to an electrochemical biosensor for determining the concentration of an analyte in a carrier. The invention is particularly useful for determining the concentration of glucose in blood and is described herein with reference to that use but it should be understood that the invention is applicable to other analytic determinations.
Owner:LIFESCAN INC
Device and method for wound therapy
A disposable wound-healing device is disclosed that incorporates a housing having a fluid-impermeable material having a cavity and a perimeter that can be sealed in an air-tight manner over a wound region of a patient. The device is capable of producing a negative pressure over the wound region by either removing oxygen from within the cavity, or absorbing fluid into the cavity and then removing the fluid from the cavity. The oxygen may be removed via chemical absorption, by an electrochemical cell or by a chemical reaction that cannibalizes oxygen from the cavity. The fluid may be removed through the use of osmotic or electro-osmotic cells, or through a one-way valve. The negative partial pressure over the wound region promotes healing.
Owner:MICROLIN
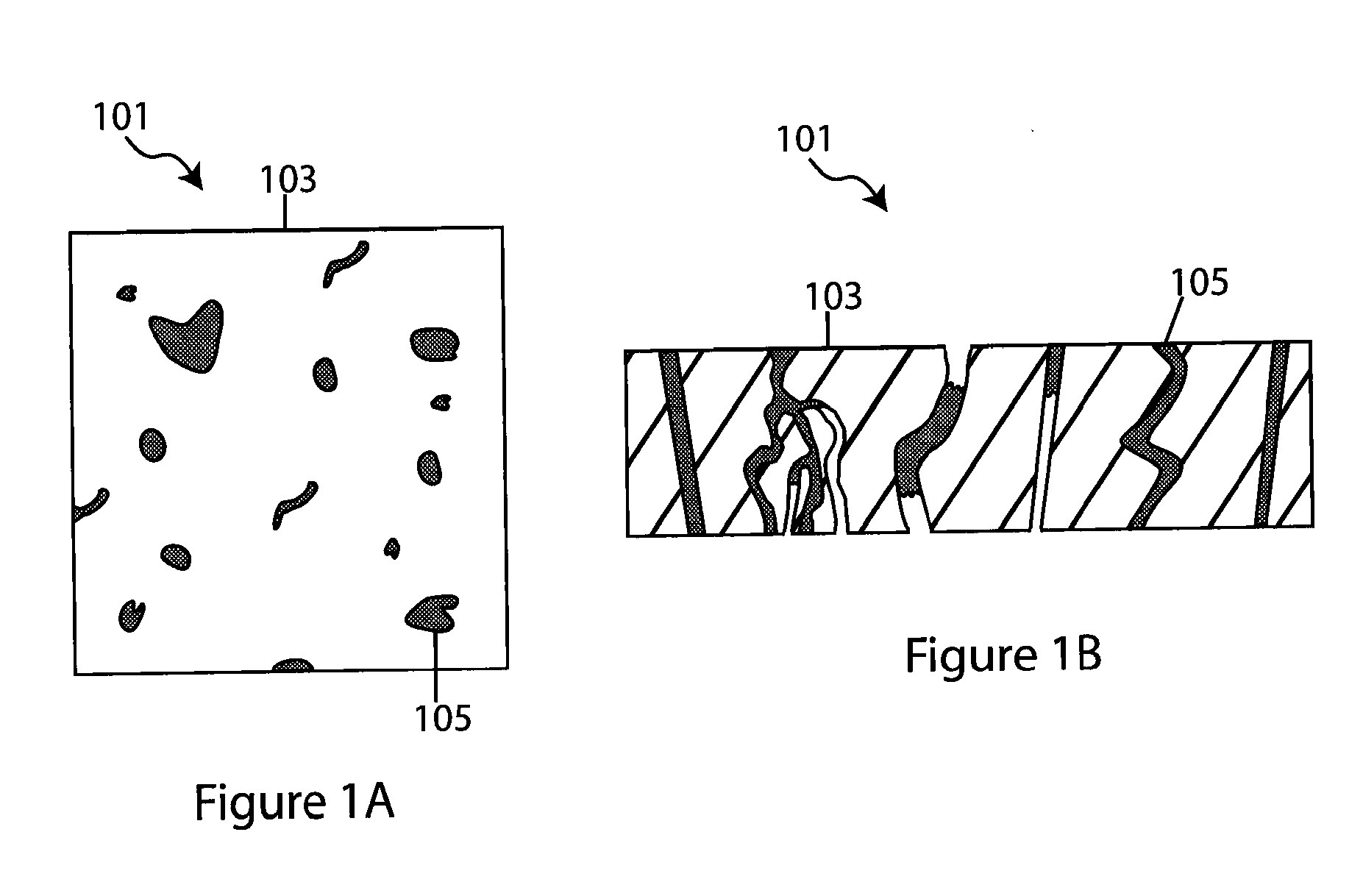
Slotted insulator for unsealed electrode edges in electrochemical cells
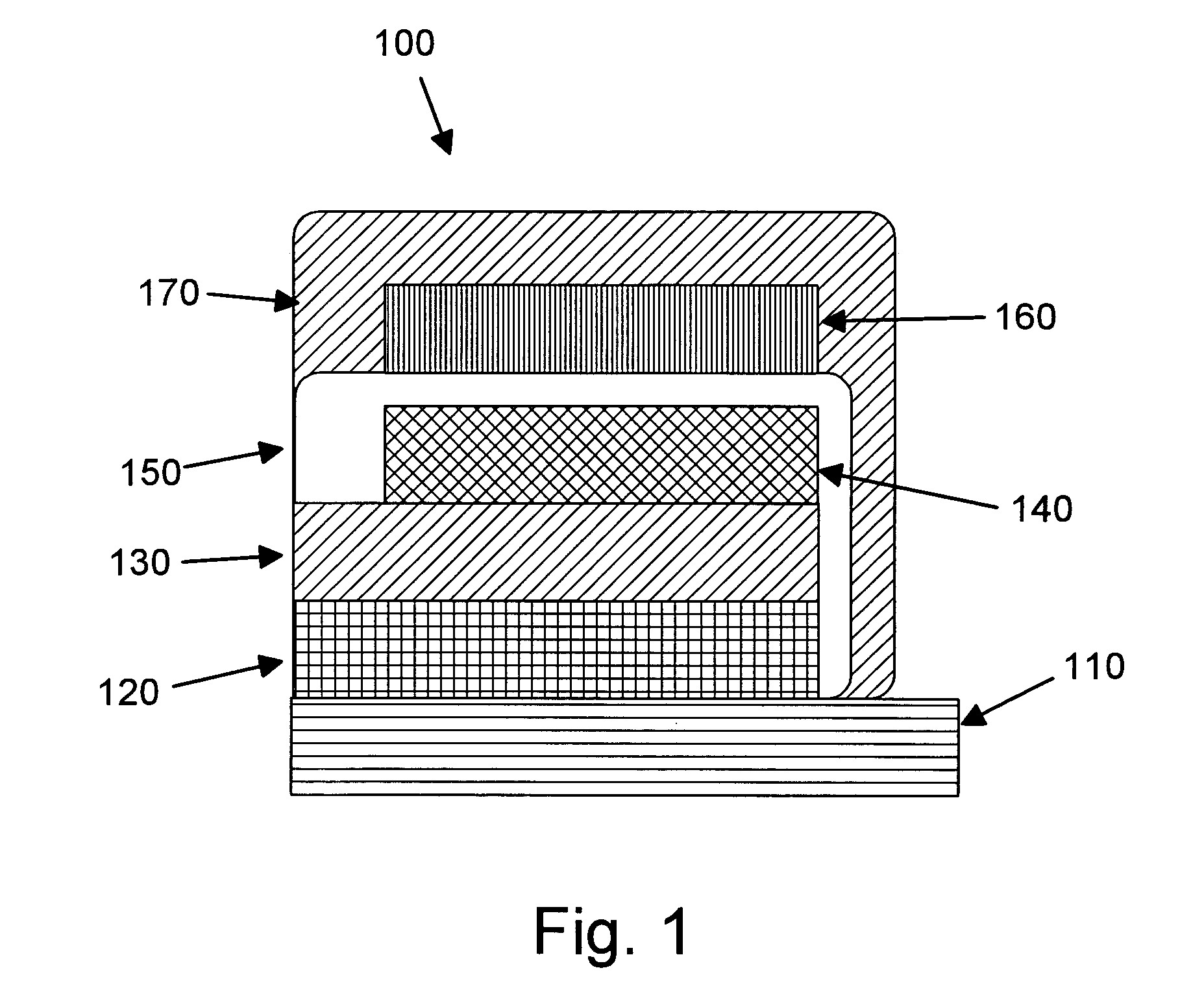
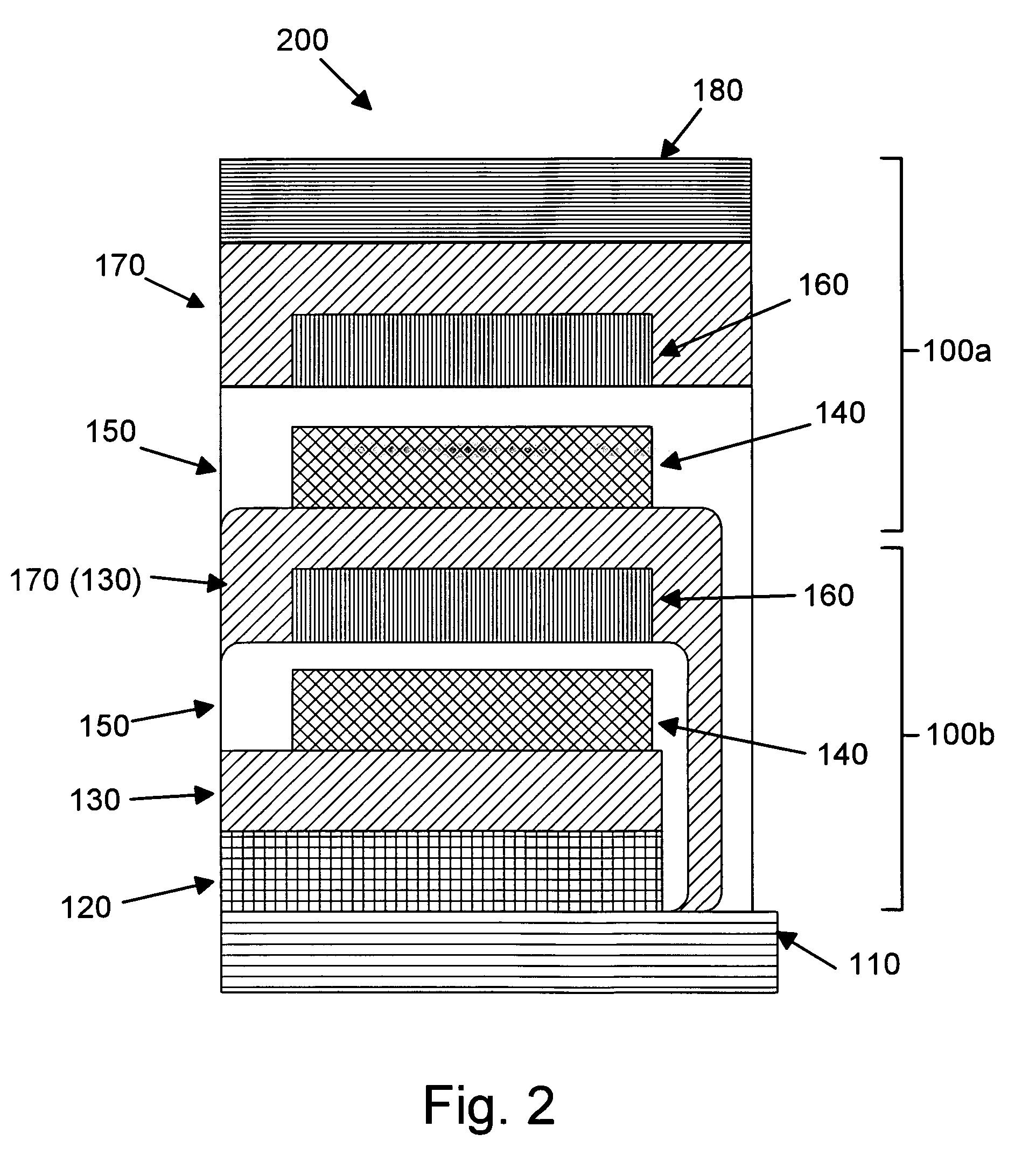
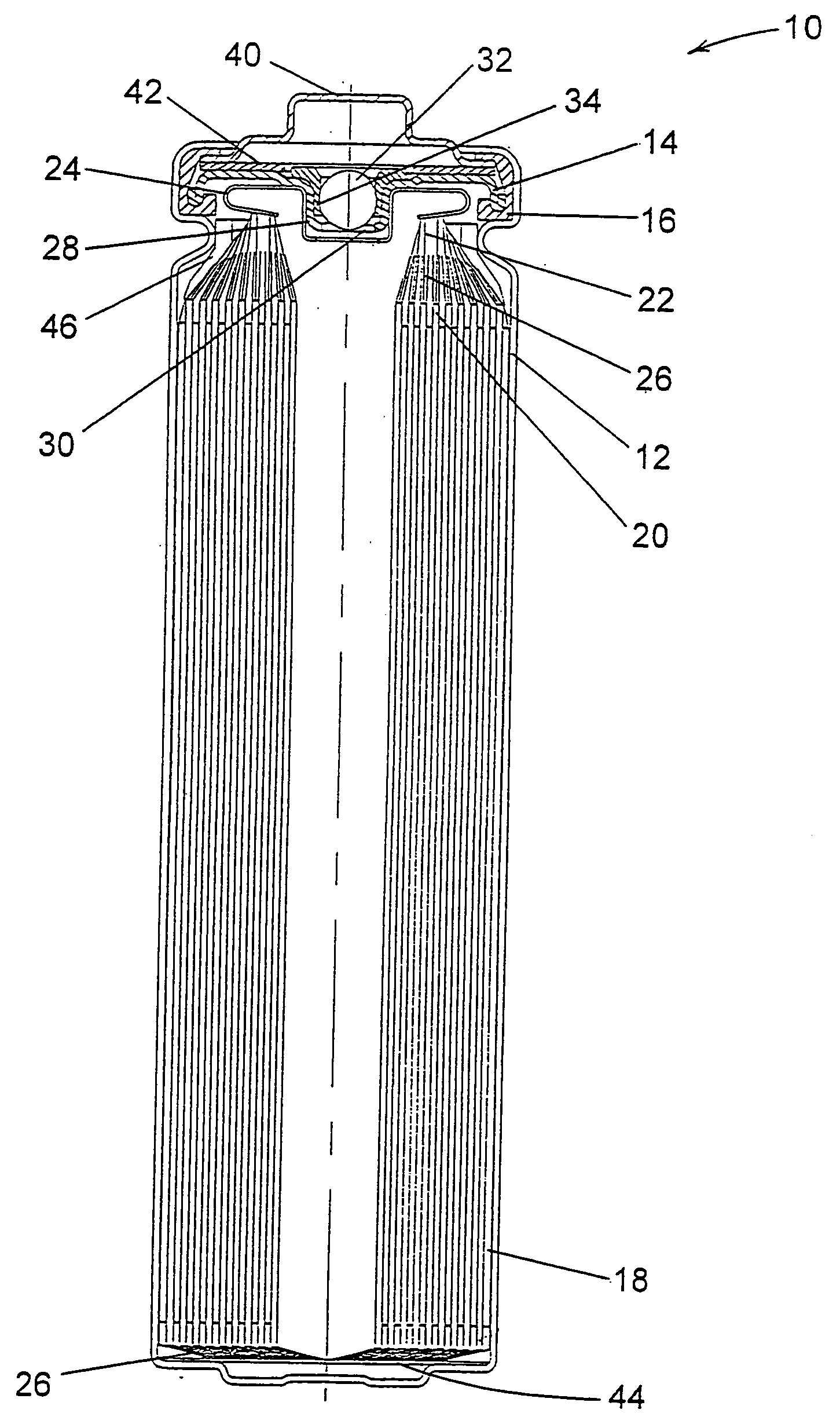
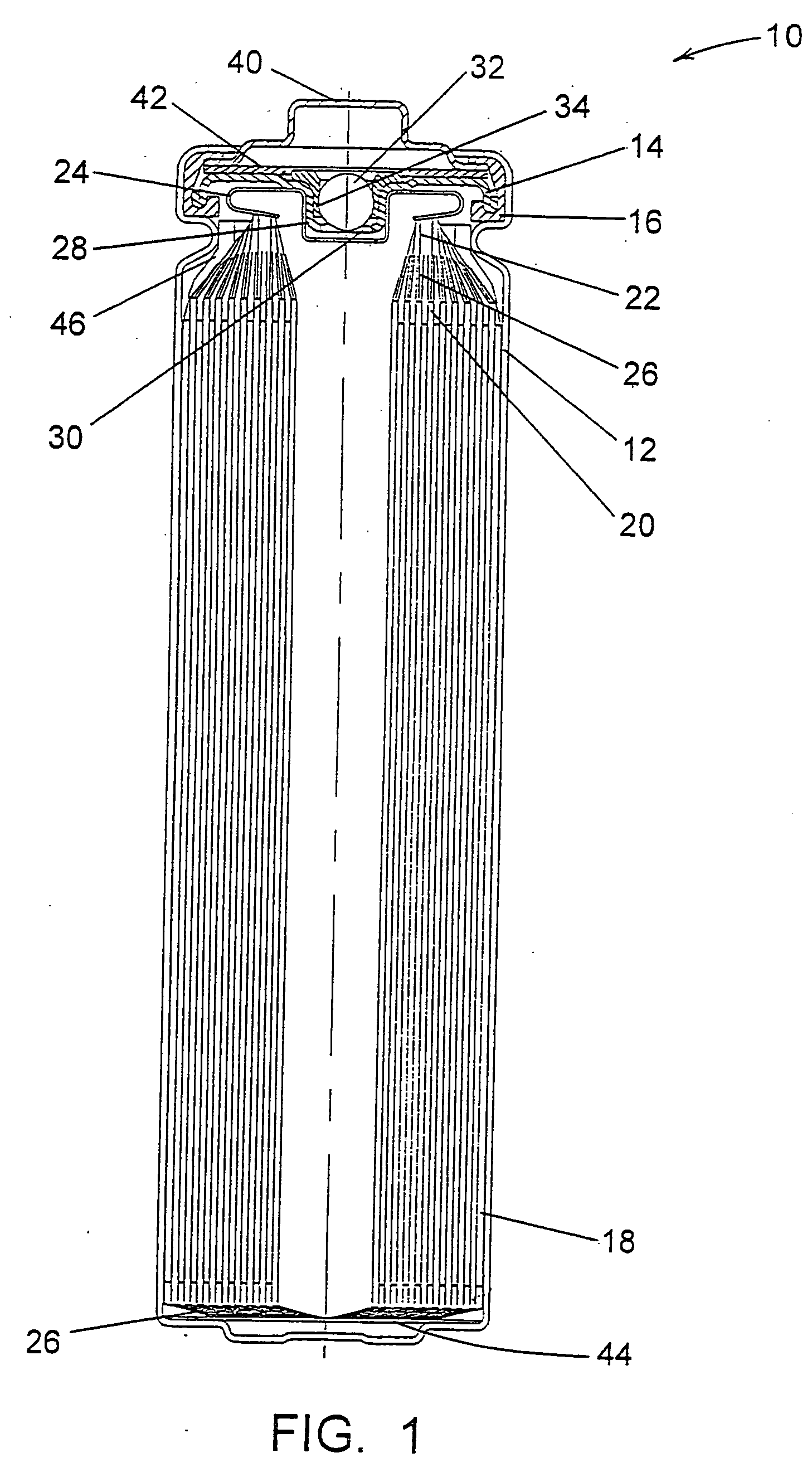
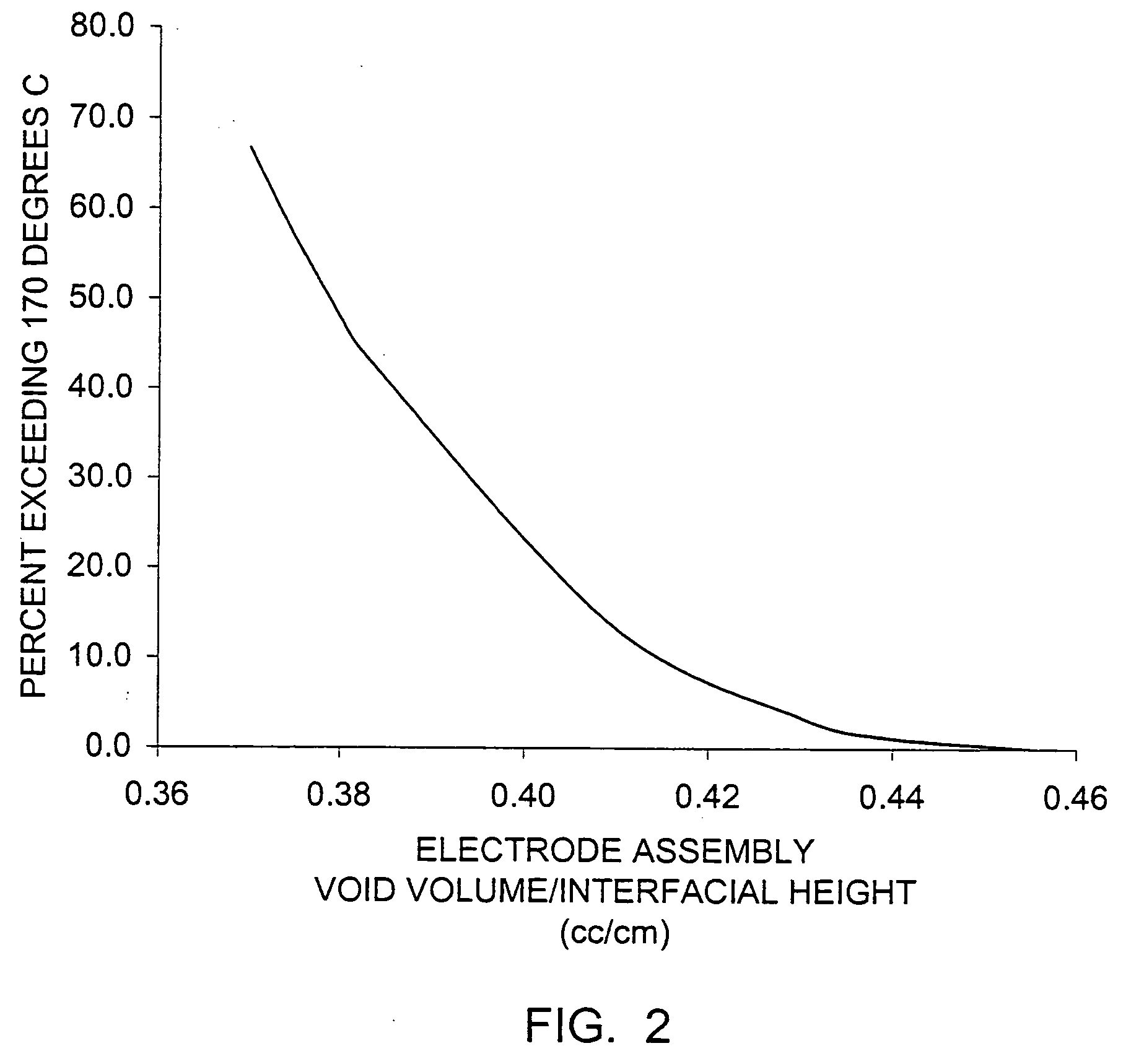
InactiveUS6013113AFinal product manufactureElectrode carriers/collectorsMushroomElectrochemical cell
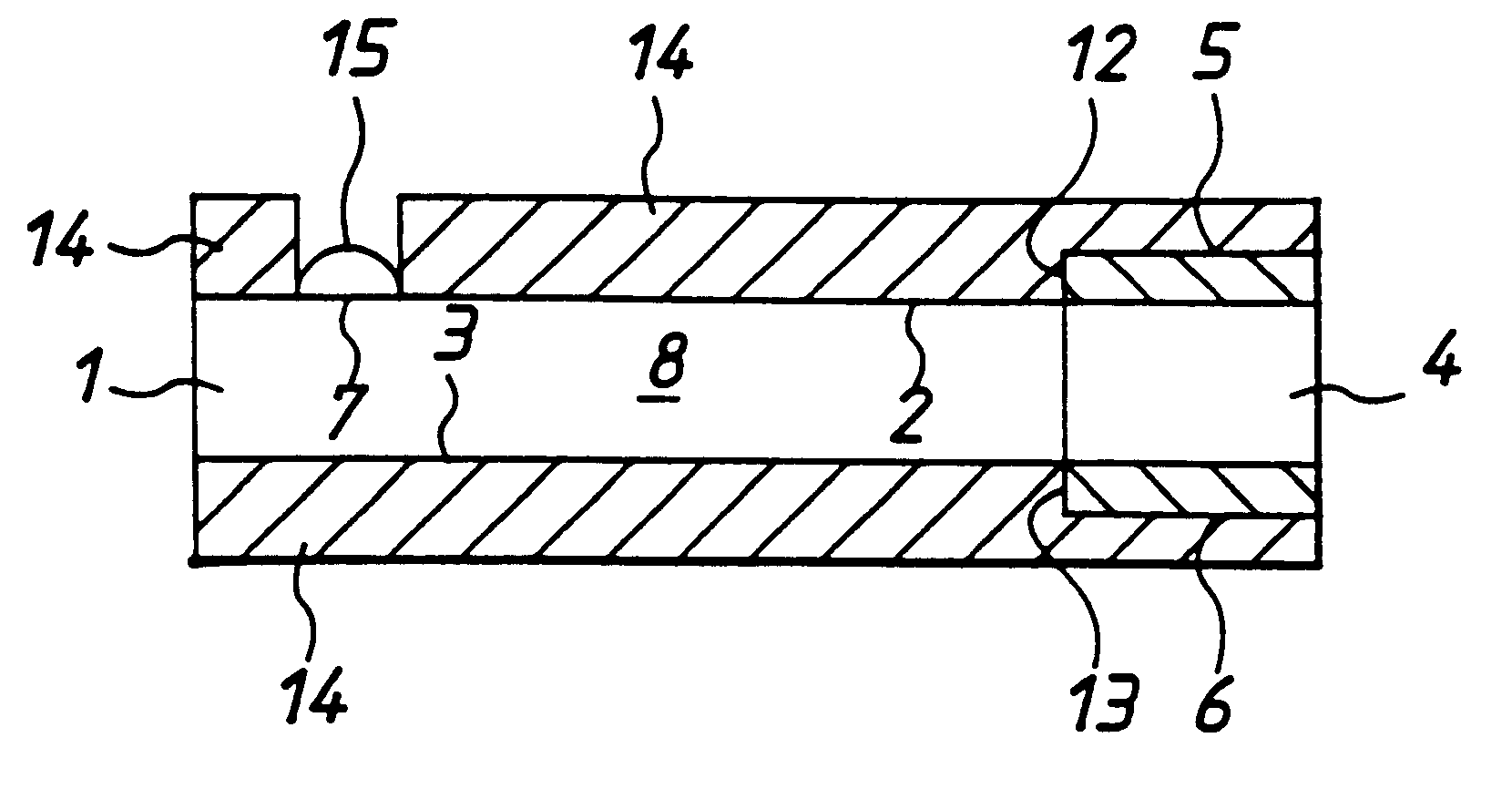
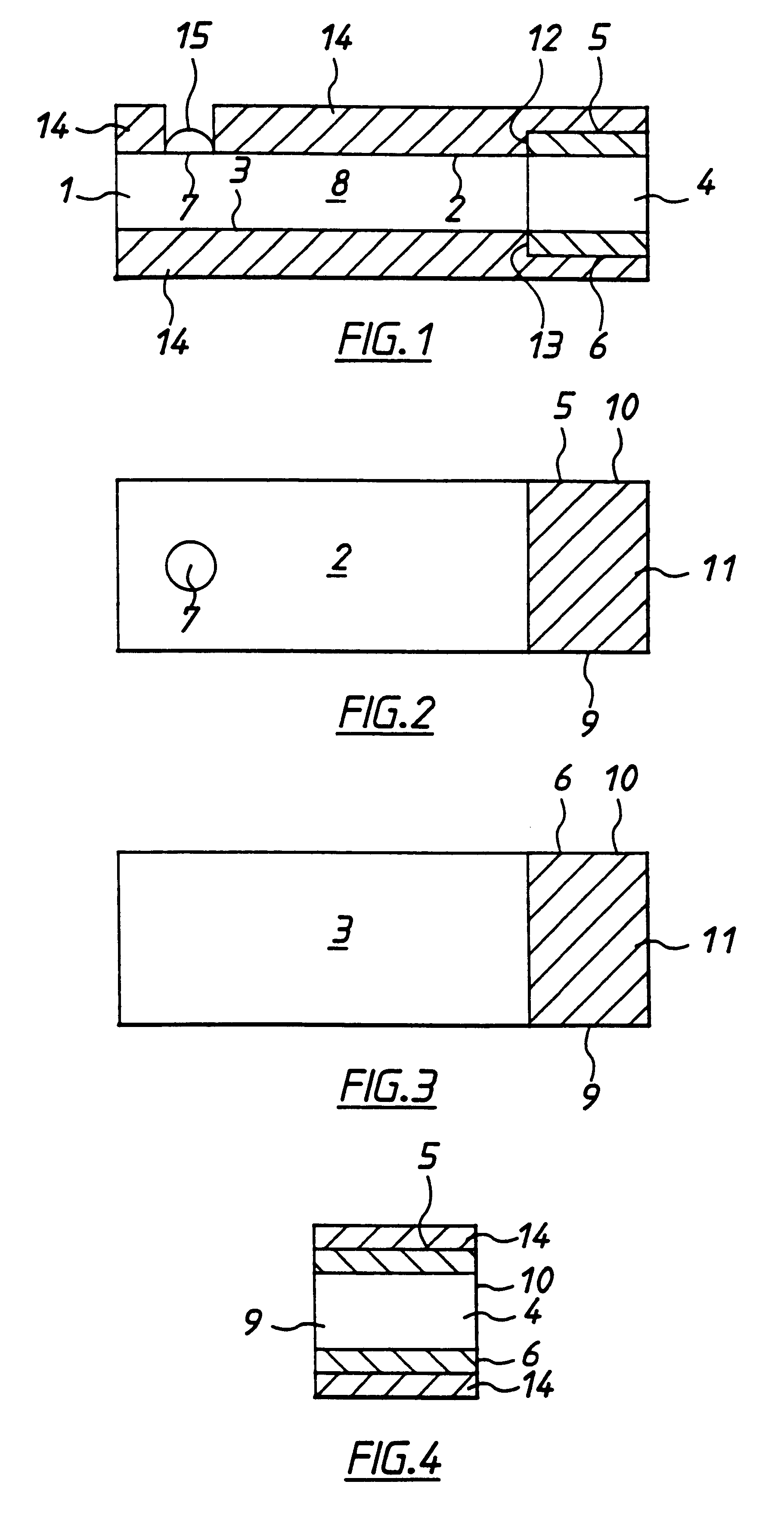
In fabrication of conventional spirally wound cells, a length of separator is provided at least twice as long as one of the electrodes, for example, the cathode, and then folded to cover both sides of the electrode. The separator is also somewhat wider than the covered electrode to extend beyond the upper and lower edges thereof. The cathode assembly is then placed along side a strip of anode material and rolled into a jellyroll configuration. The separator sheet is not sealed at the opposed upper and lower edges of the cathode, and during high shock and vibration conditions the edges tend to mushroom which can lead to short circuit conditions. The insulator of the present invention is a slotted member that covers the upper and lower edges of the other electrode not covered by the separator, for example the anode with the anode leads extending through the slots to shield them from short circuit conditions with the cell casing or other leads if the cell should be subjected to severe shock forces and the like.
Owner:WILSON GREATBATCH LTD
Biofouling self-compensating biosensor
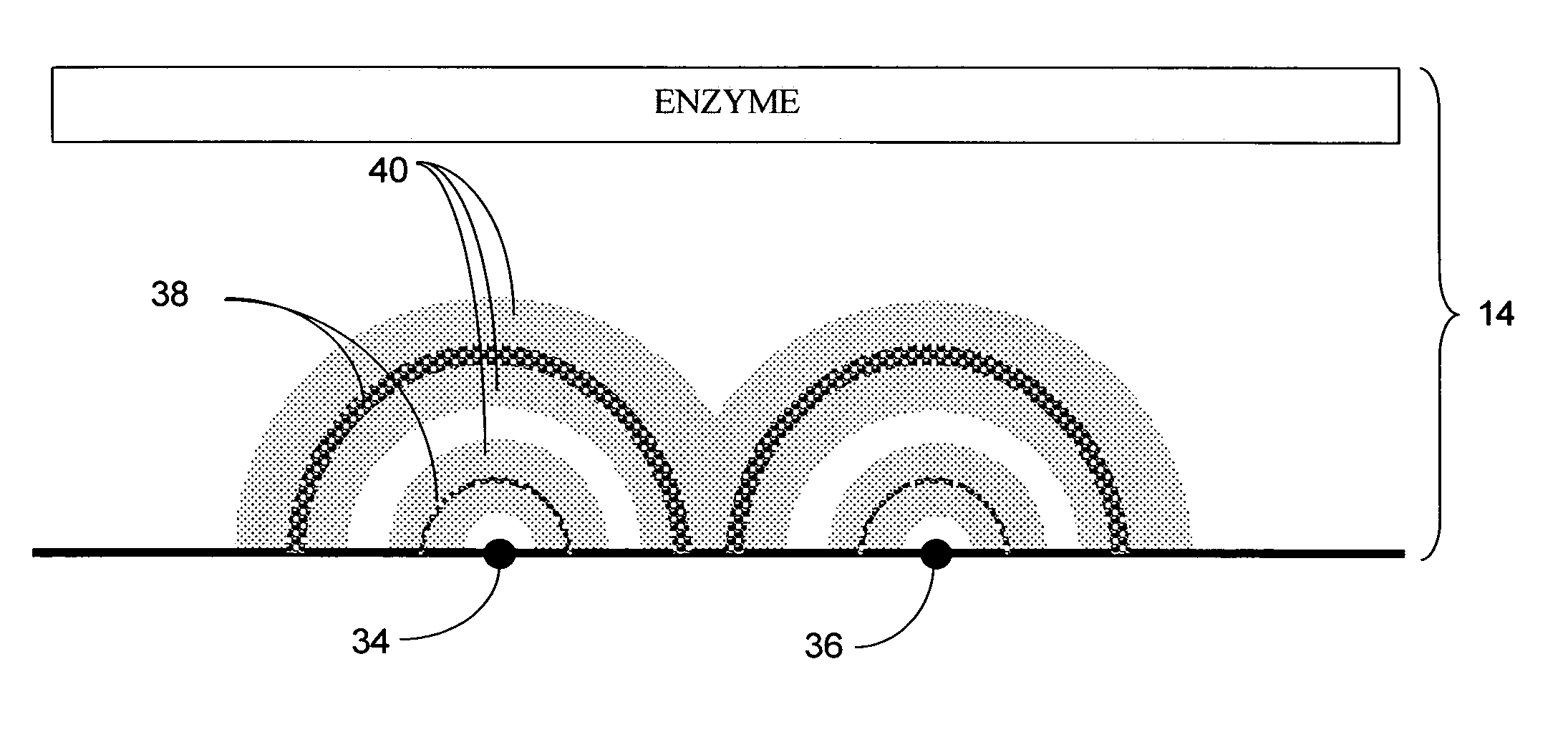
InactiveUS20070299617A1Reduce the burden onPatient compliance is goodImmobilised enzymesBioreactor/fermenter combinationsEngineeringBiofouling
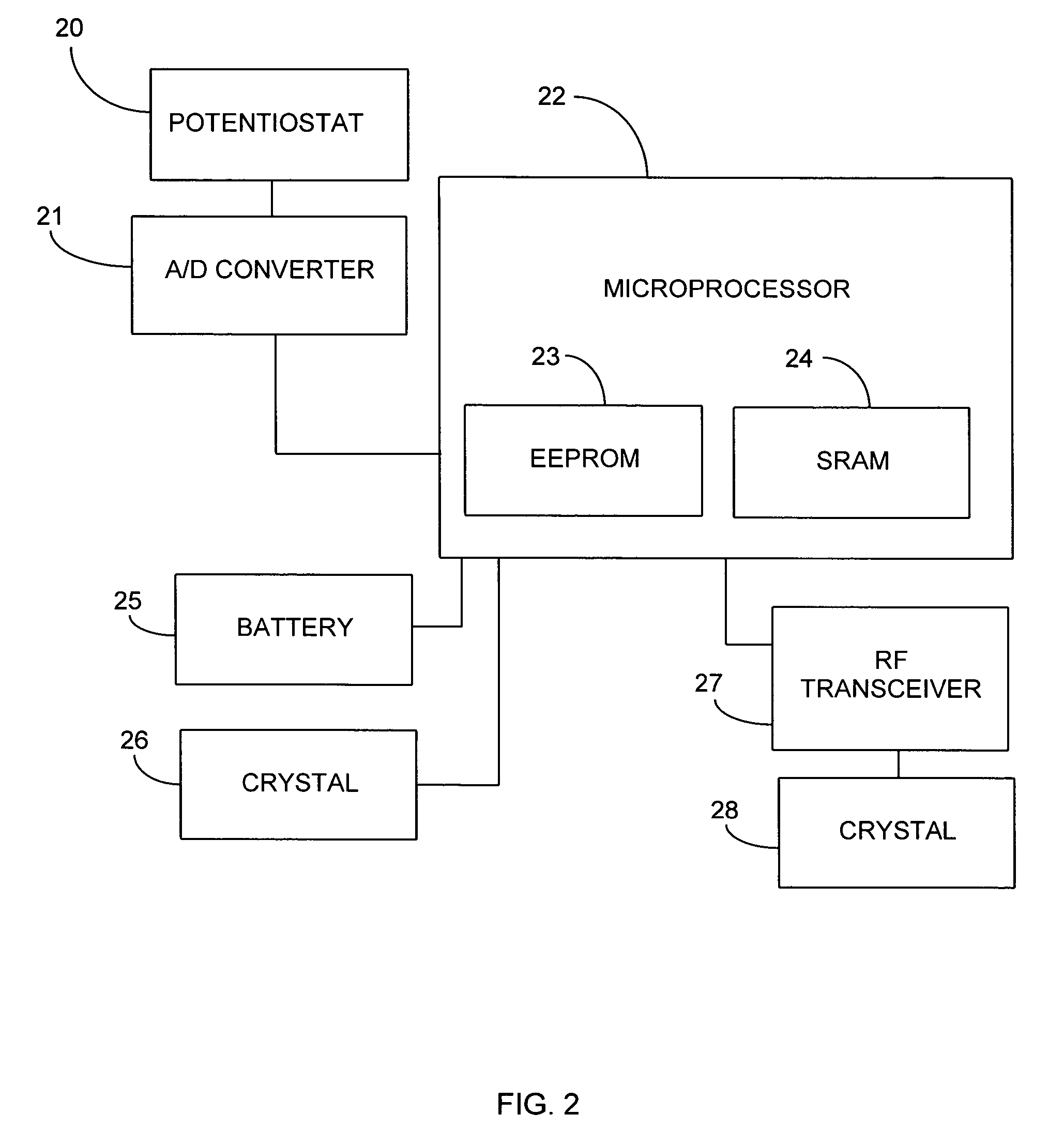
An in vivo biosensor disposed upon a subject comprising an electrochemical cell having a plurality of electrodes and a computer-controlled voltage source incorporating a potentiostat that is generative of a poise potential regime, which computer-controlled voltage source is operationally coupled to a computing device that: computes an output current whose magnitude is proportional to an amount of an analyte in a bodily fluid of the subject; and, adjusts the output current for drift due to biofouling at points in time greater than or equal to an induction period; and, outputs the amount of the analyte by transducing the adjusted output current. Methods and algorithms for adjusting the output current for drift due to biofouling are provided.
Owner:ULTRADIAN DIAGNOSTICS
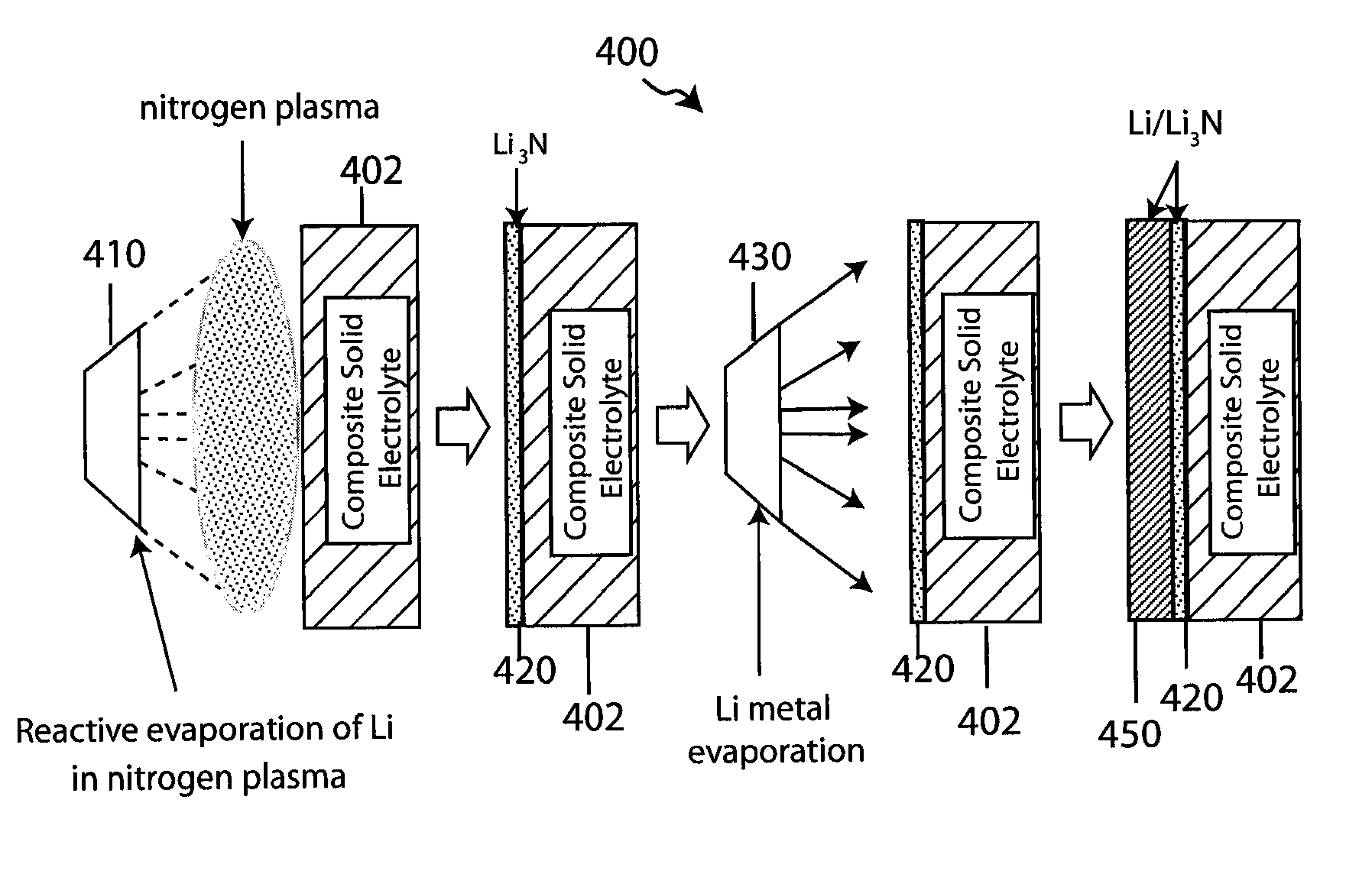
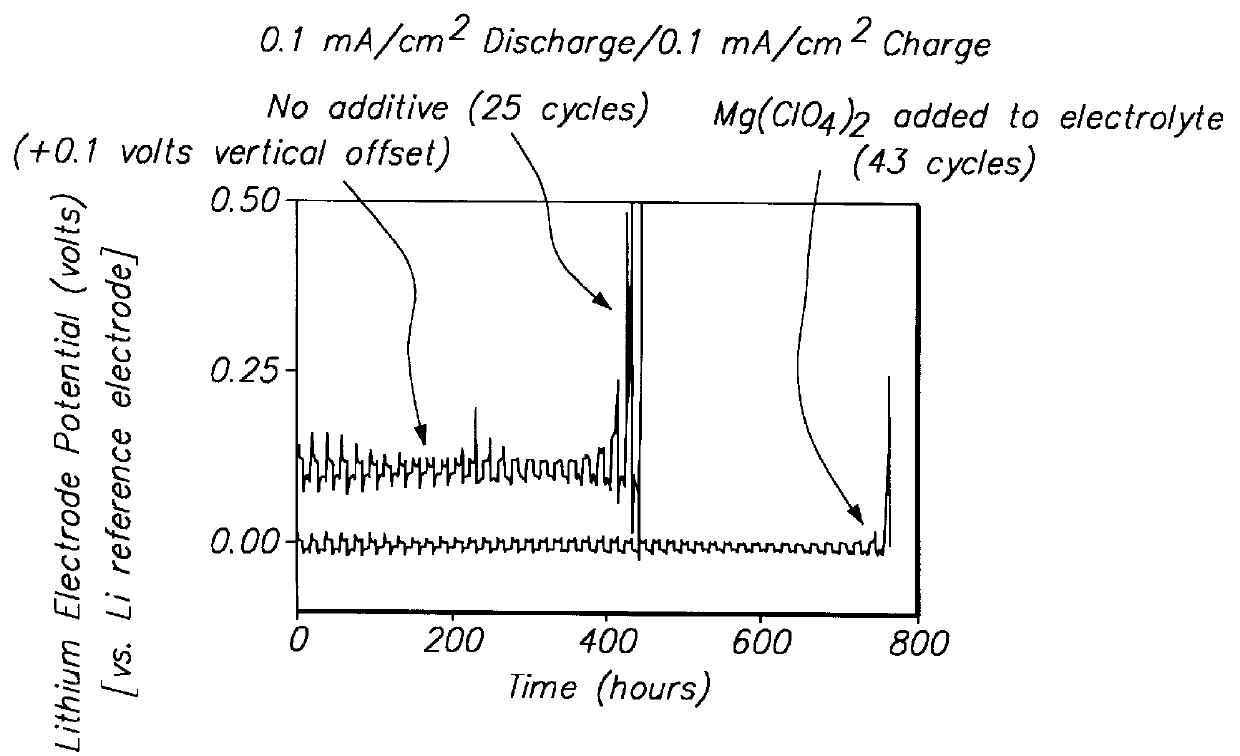
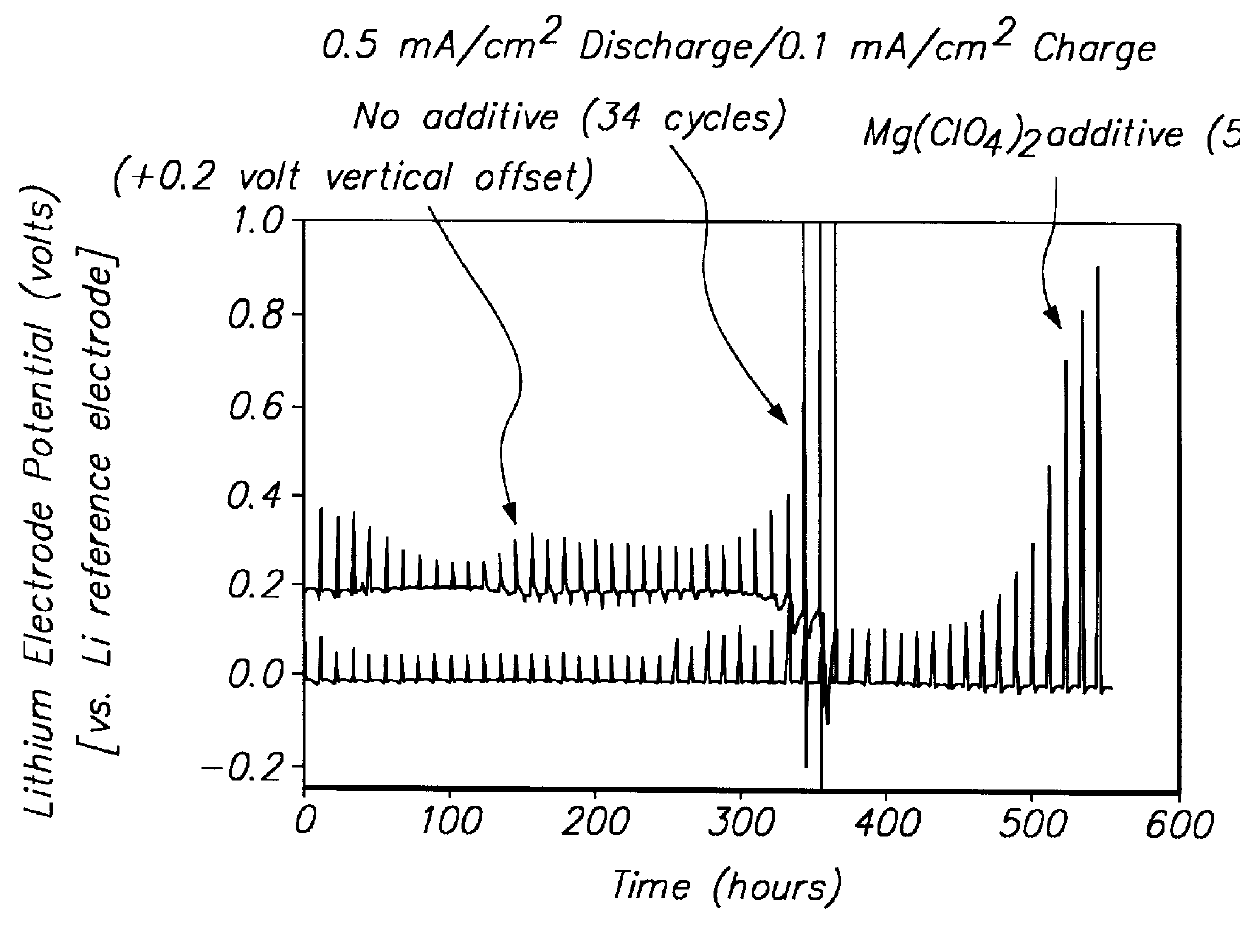
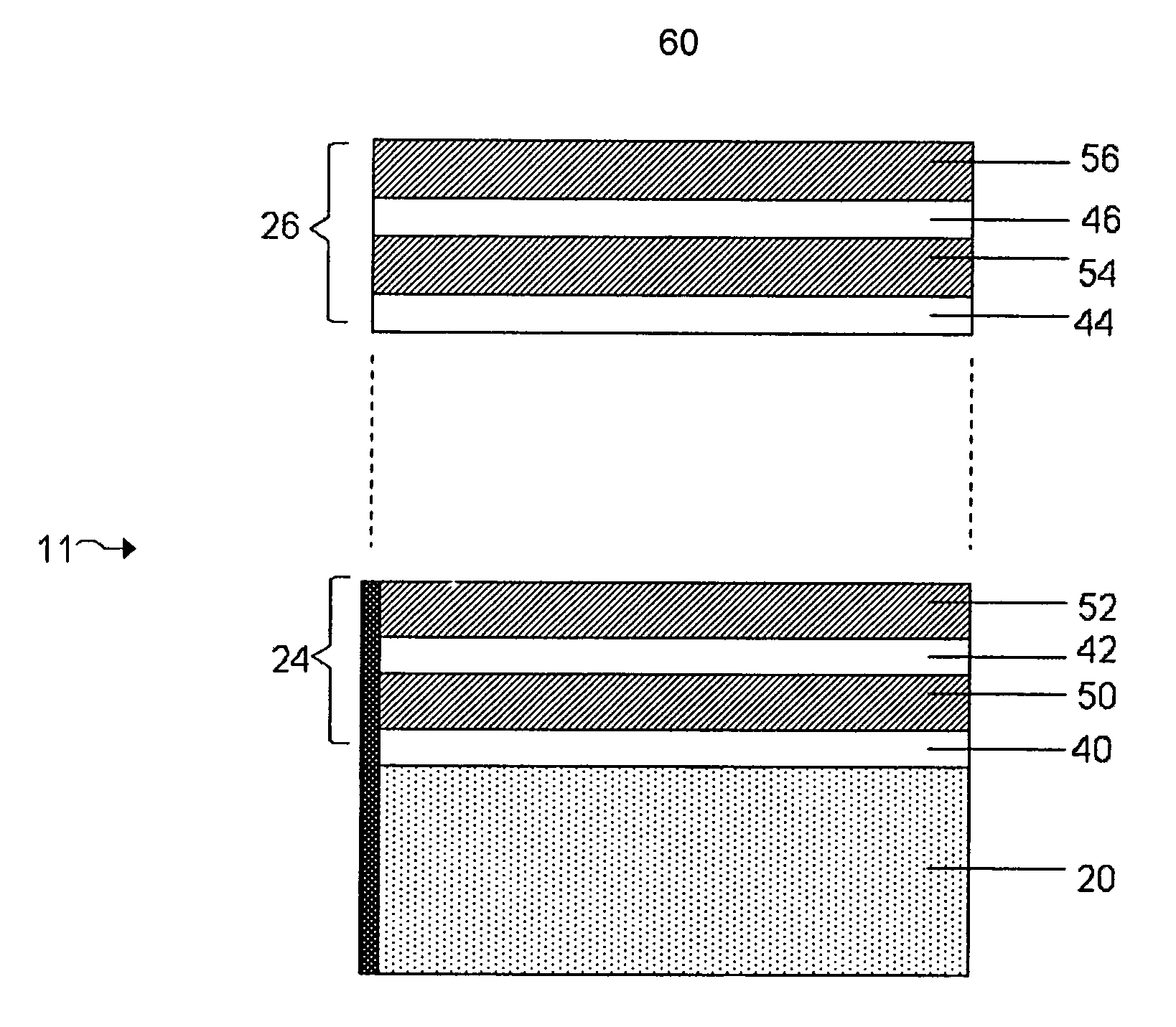
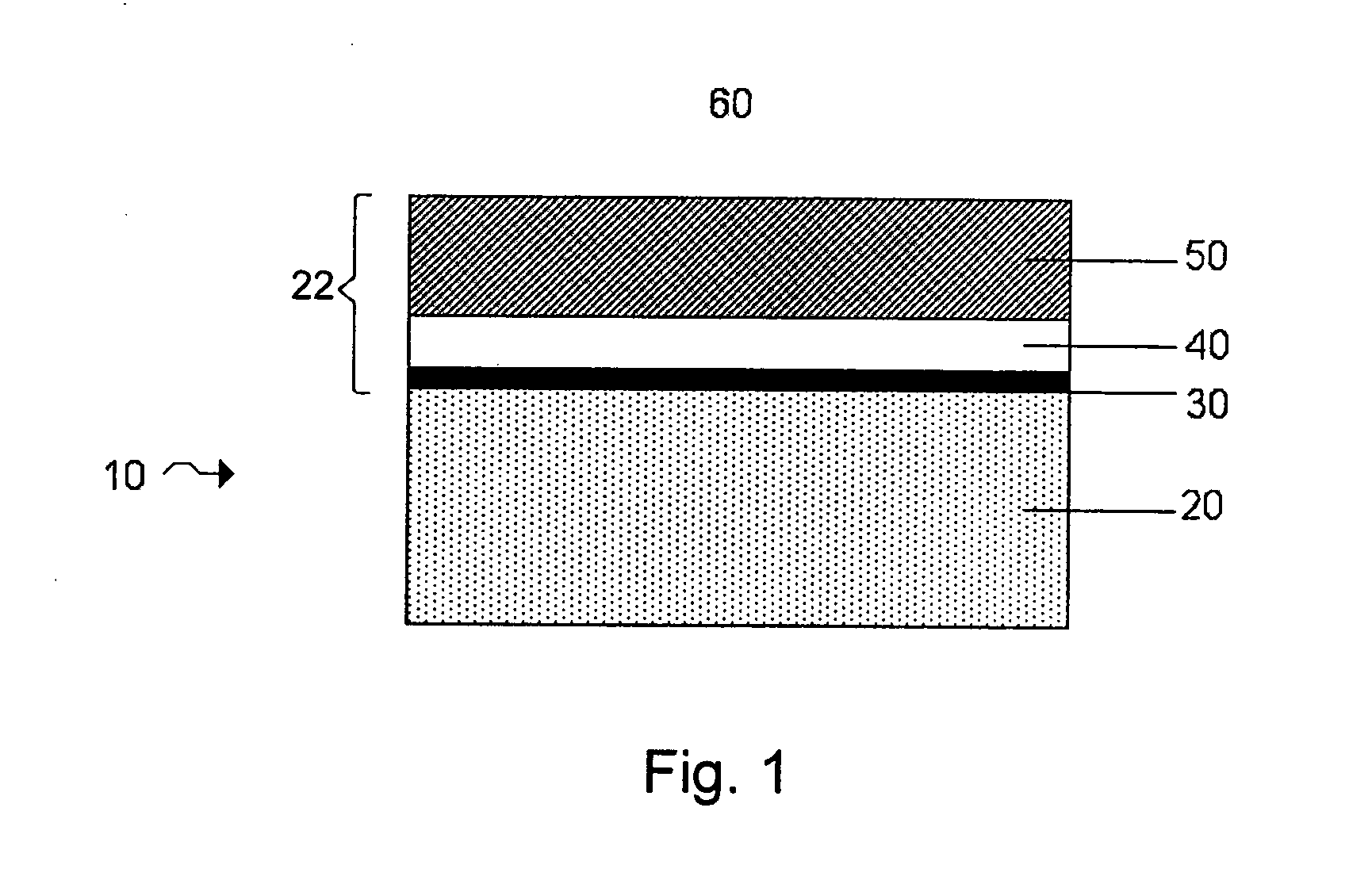
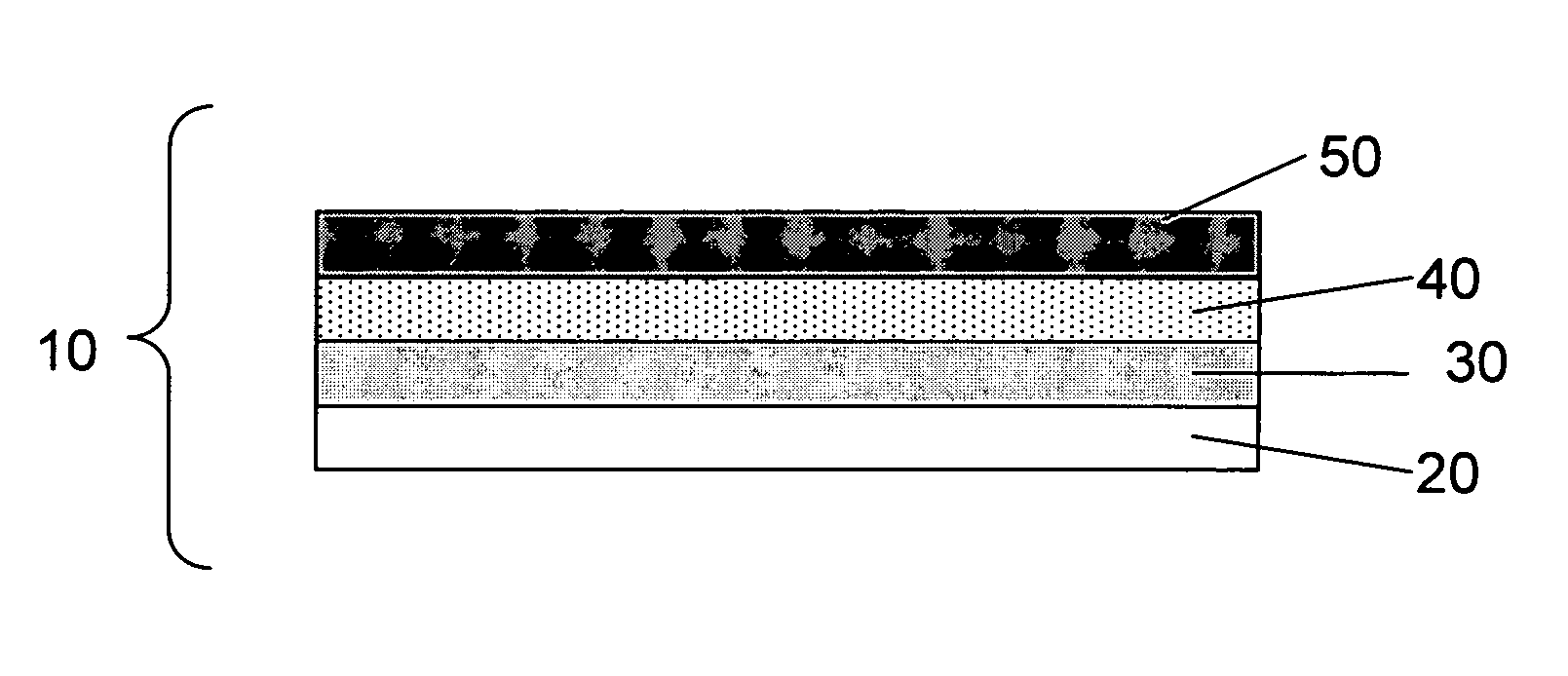
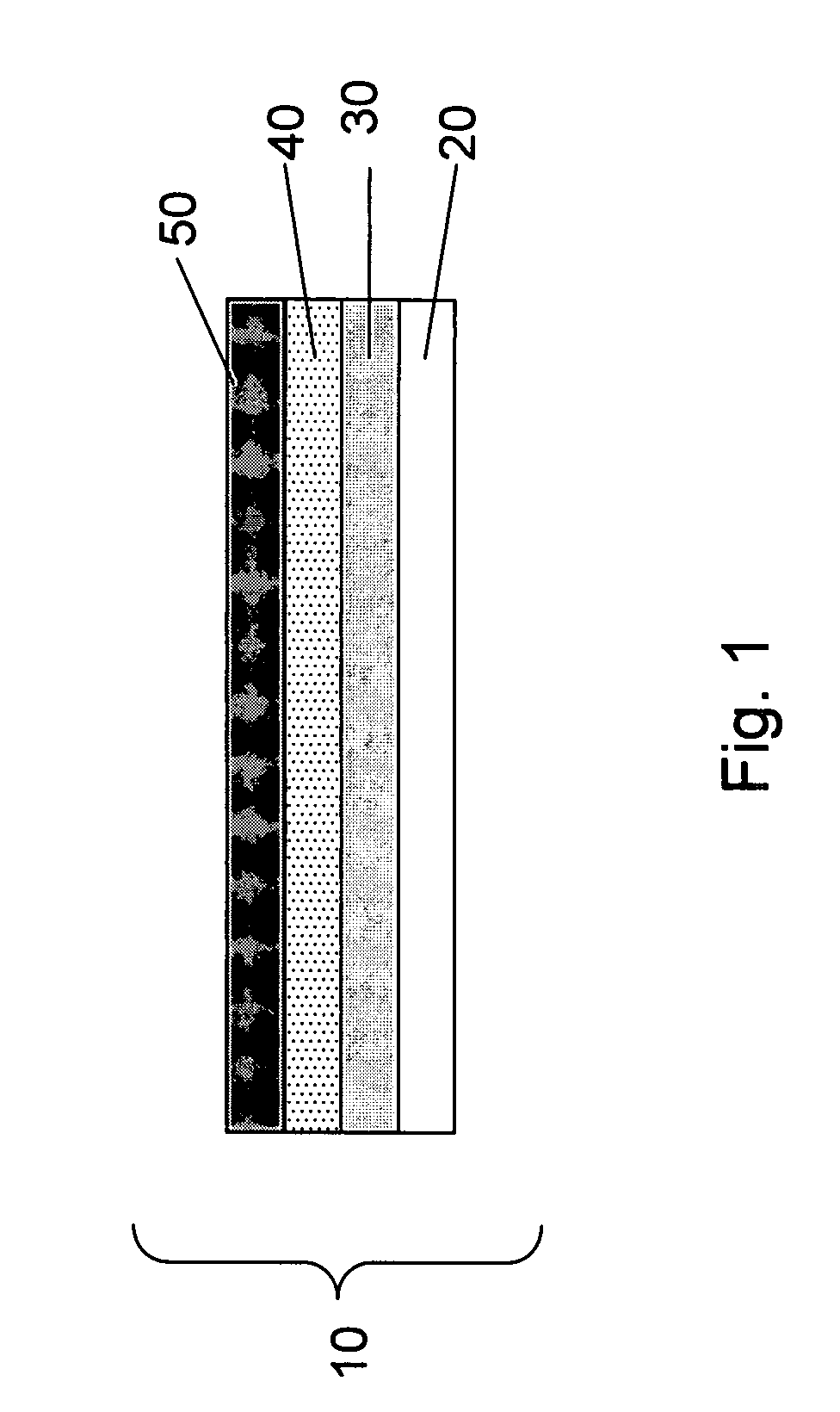
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
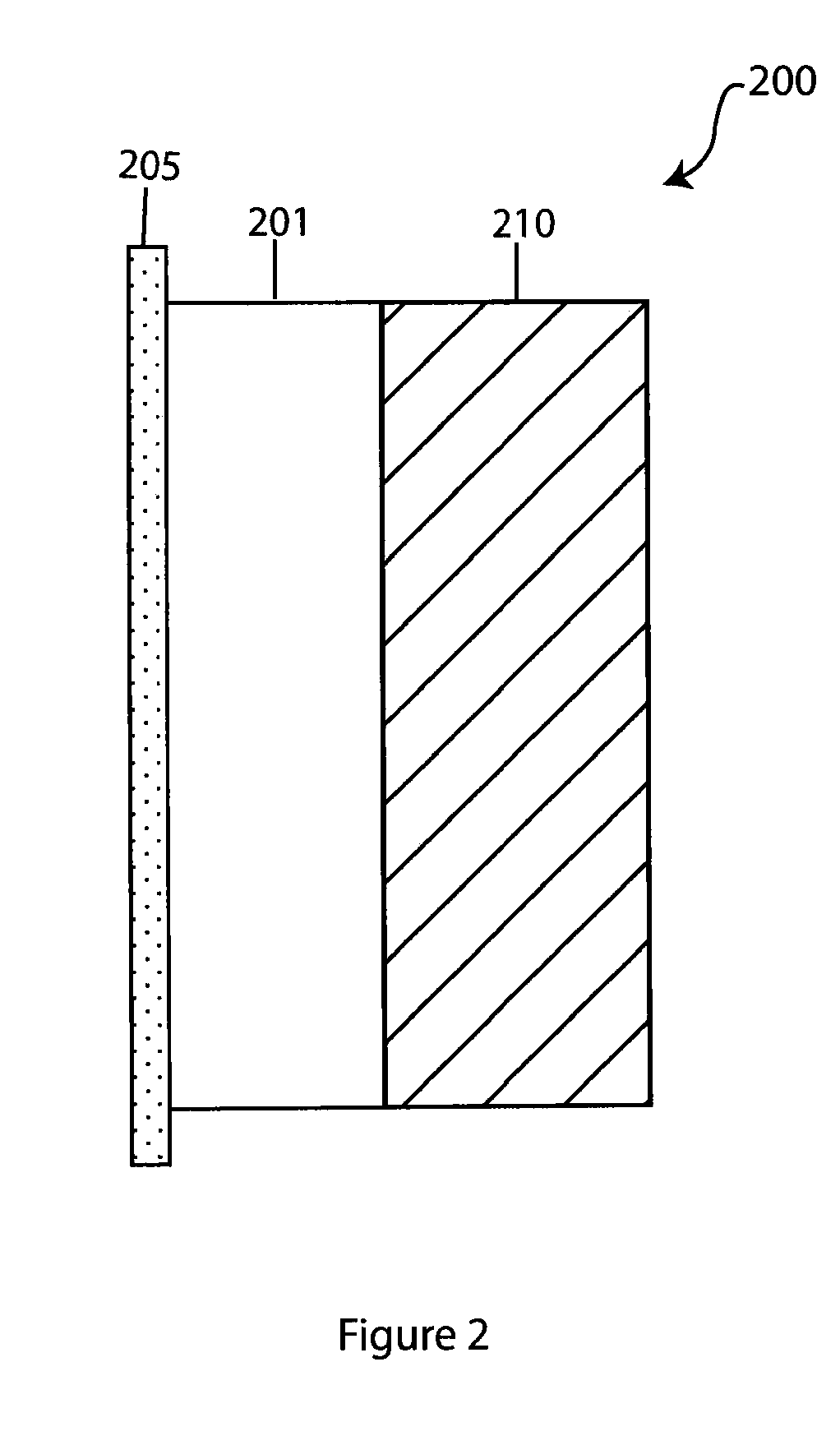
Composite solid electrolyte for protection of active metal anodes
ActiveUS20070172739A1Eliminate through-porosityHigh metal ion conductivityCell electrodesPrimary cellsPorosityTectorial membrane
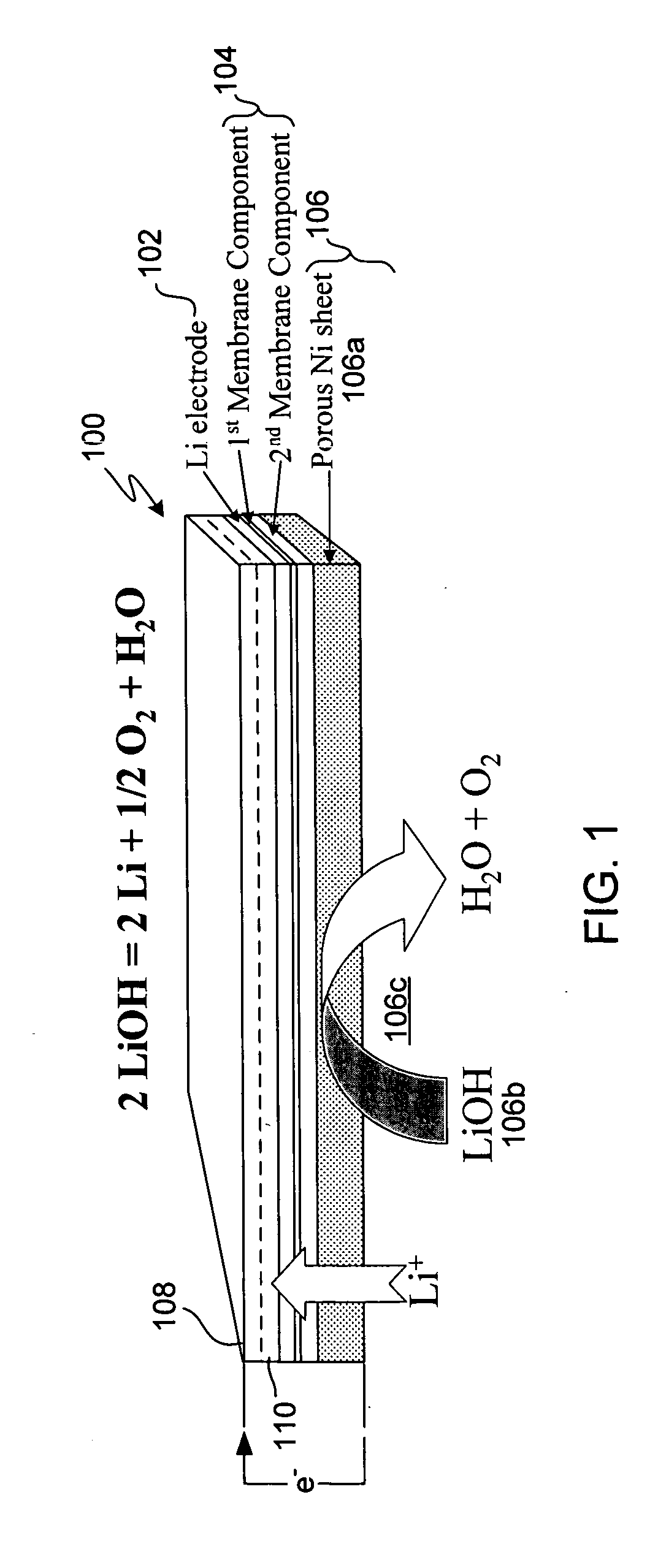
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
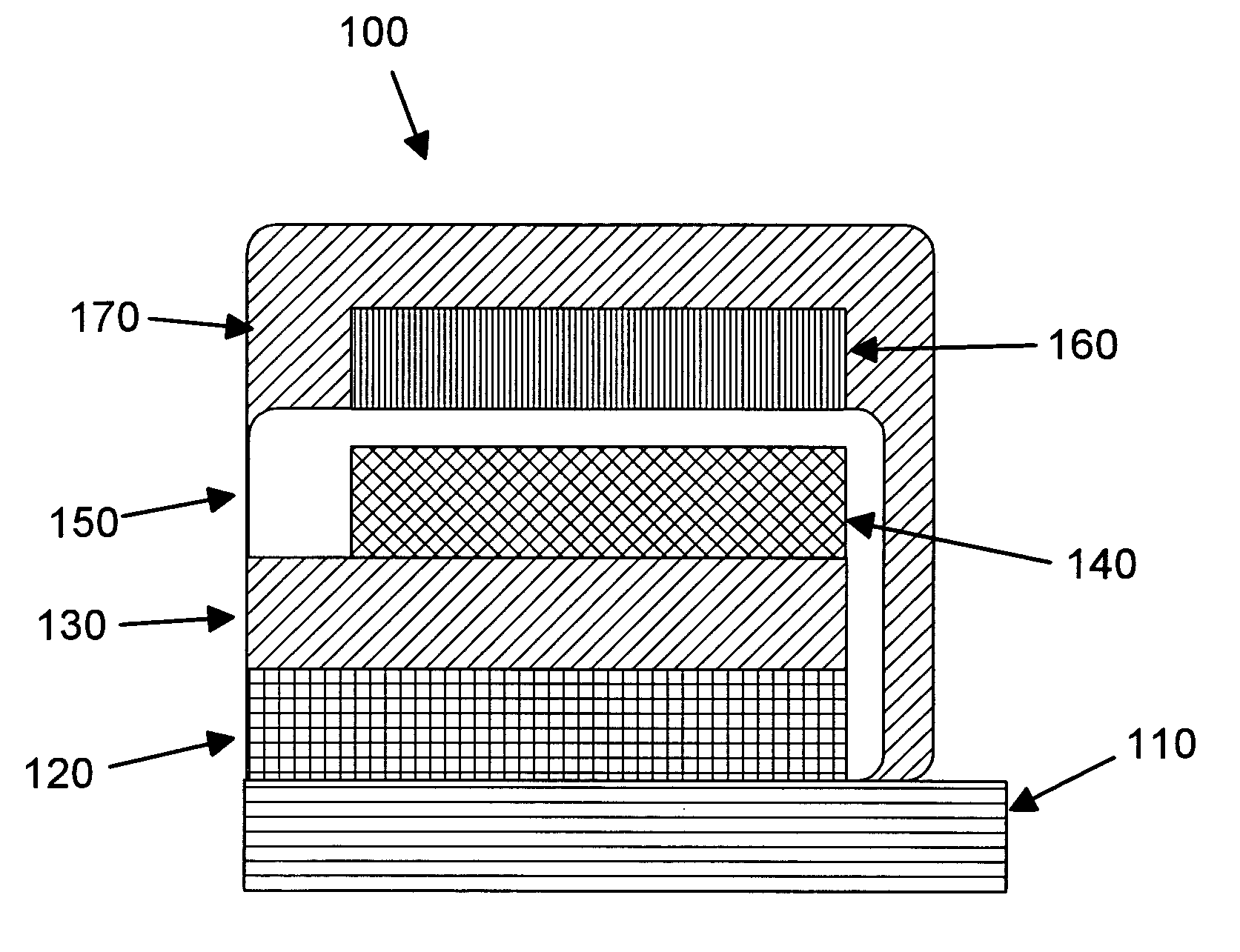
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Electroactive high storage capacity polyacetylene-co-polysulfur materials and electrolytic cells containing same
InactiveUS6117590AHigh storage capacity per unit weightFacilitates electron transportElectrode manufacturing processesNon-aqueous electrolyte accumulatorsElectrochemical cellElectrode material
The present invention relates to novel electroactive energy storing polyacetylene-co-polysulfur (PAS) materials of general formula (C2Sx)n wherein x is greater than 1 to about 100, and n is equal to or greater than 2. This invention also relates to novel rechargeable electrochemical cells containing positive electrode materials comprised of said polyacetylene-co-polysulfur materials with improved storage capacity and cycle life at ambient and sub-ambient temperatures.
Owner:THE BANK OF NEW YORK +1
Mesoporous network electrode for electrochemical cell
InactiveUS20040131934A1Simplifies production of cellFull penetrationMaterial nanotechnologyElectrode manufacturing processesLithiumNanoparticle
A high kinetics rate electrochemical cell in which at least one of the electrodes is composed of a mesostructural electroactive material comprising nanoparticles forming a three-dimensional framework structure of mesoporous texture having a bicontinuous junction of large specific surface area with the electrolyte. A low temperature method of preparation of the electrodes employs a high-speed deposition of the electrically active material in the form of a thin film. The application of said electrodes in high power lithium ion insertion batteries, photovoltaic cells, supercapacitors and fast electrochromic devices is disclosed.
Owner:FRANCOIS SUGNAUX
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Rechargeable lithium/water, lithium/air batteries
InactiveUS20070221265A1Improve protectionEasy to controlFinal product manufacturePV power plantsHigh energyOptoelectronics
Electrochemical cells, and more specifically, rechargeable batteries comprising lithium anodes for use in water and / or air environments, as well as non-aqueous and non-air environments, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
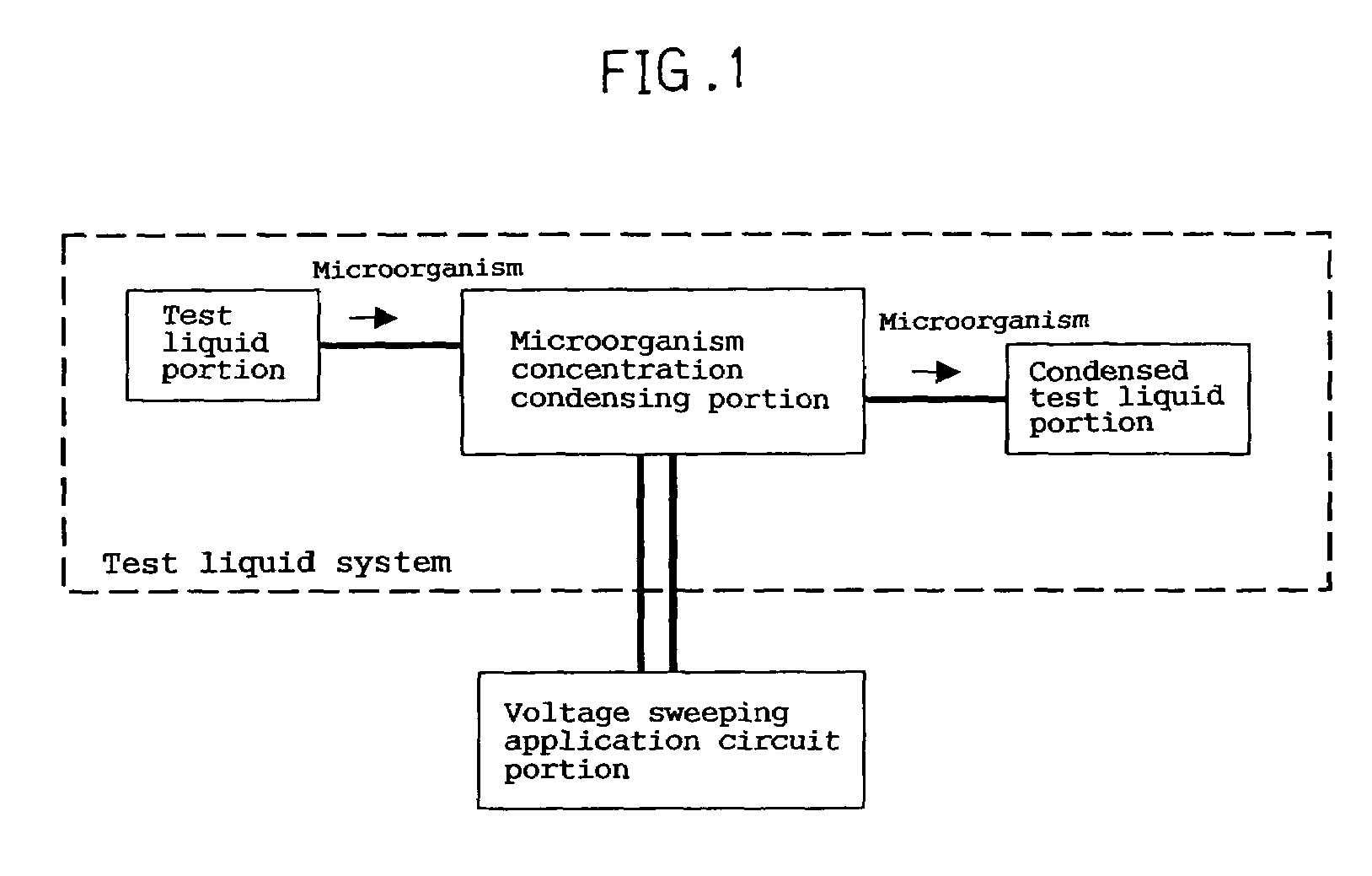
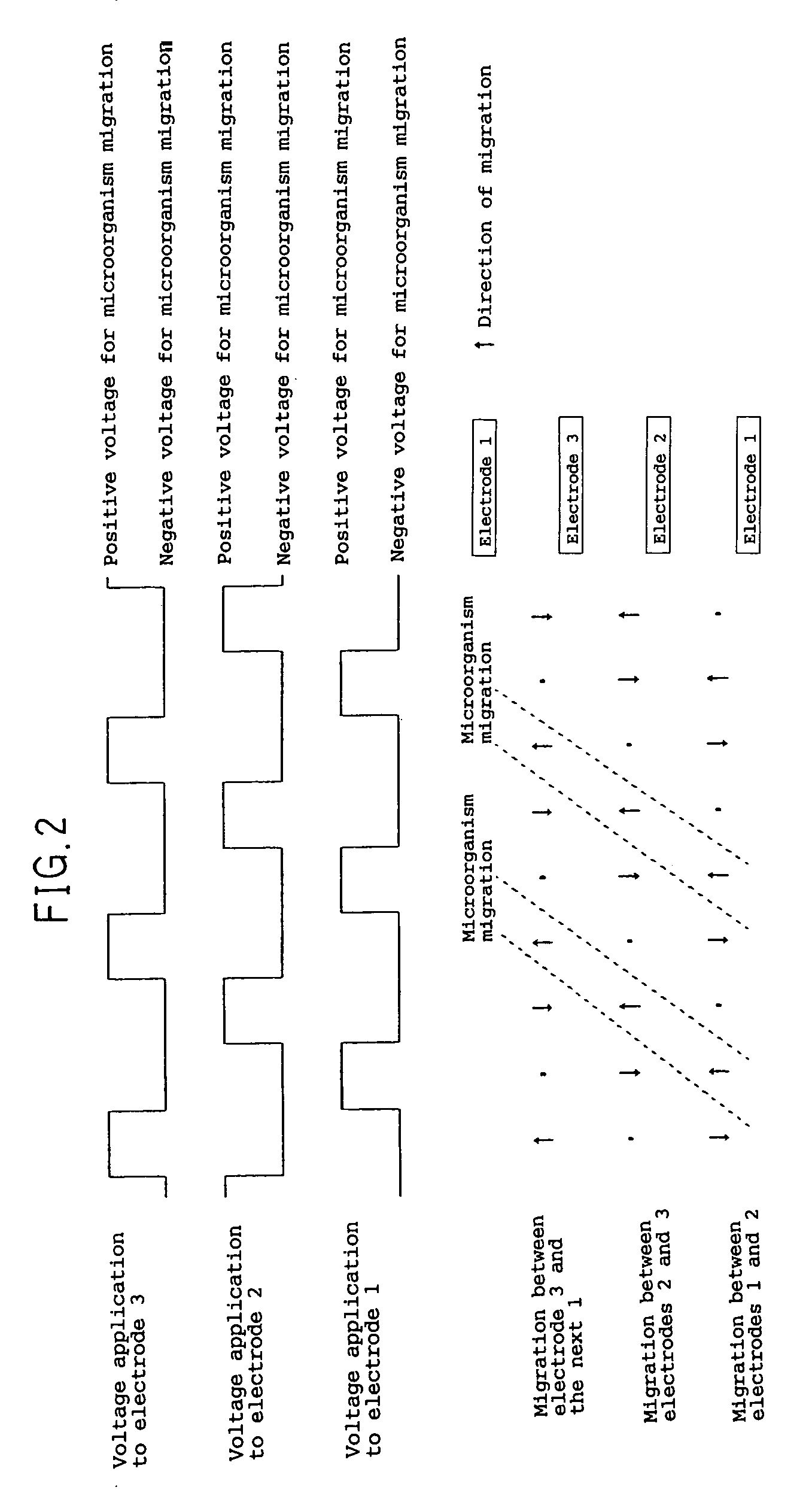
Electrochemical device for moving particles covered with protein
InactiveUS6972080B1Efficient disposalElectrostatic separatorsSludge treatmentBlood componentElectrolysis
An electrochemical device for moving particles covered with a protein is provided. The device includes at least two electrodes that are in contact with a liquid containing the protein-covered particles and a circuit that generates a potential difference in a range that does not cause electrolysis of the liquid between the electrodes. The particles are moved by electrophoresis in the direction of the arrangement of the electrodes. The invention provided herein has numerous applications, including use in a microorganism concentration condensing device, a blood component induction device, and / or a blood component induction method, and / or an electric appliance that decreases the concentration of microorganisms present on the surface of a heat exchanger.
Owner:PANASONIC CORP
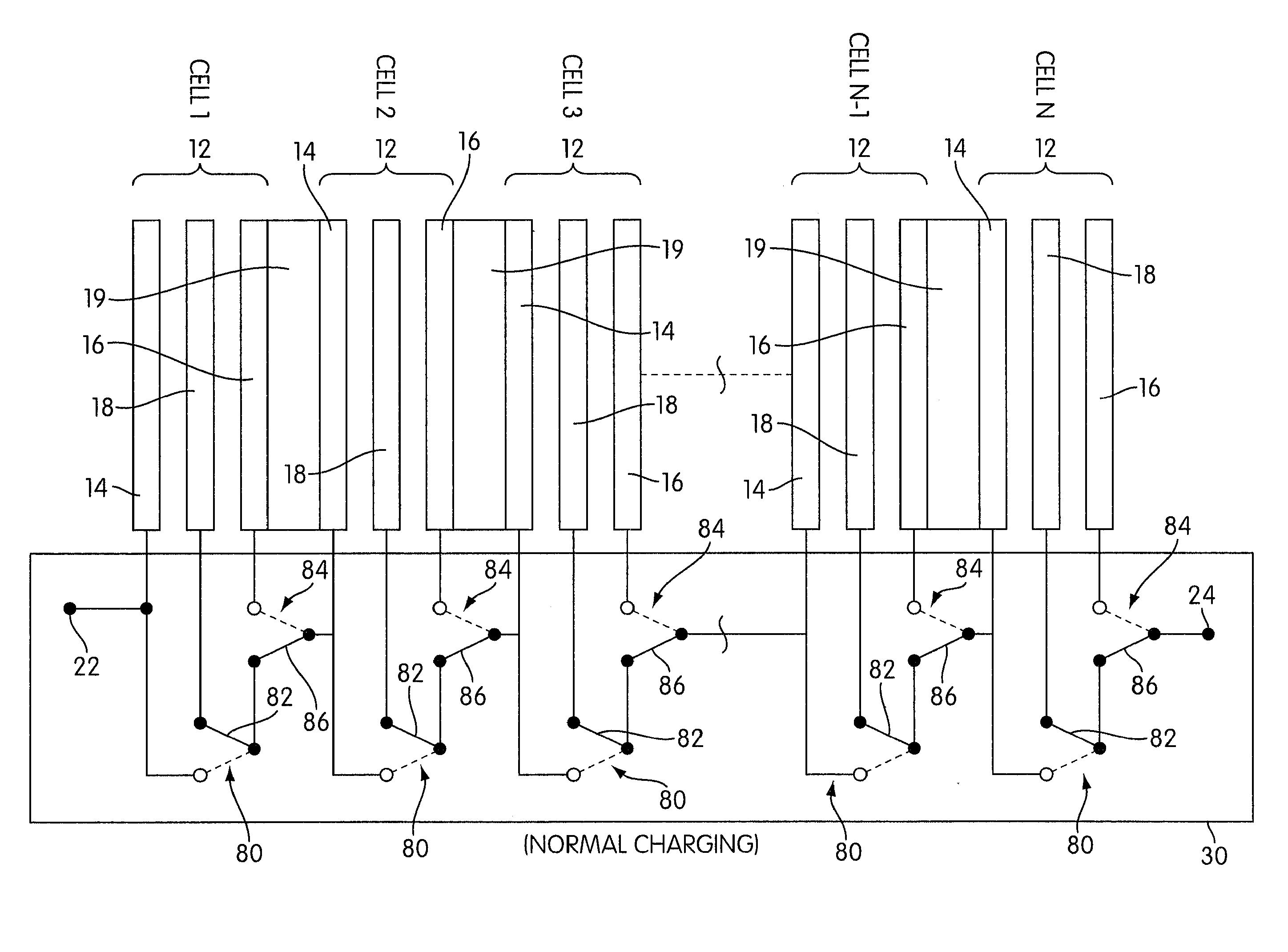
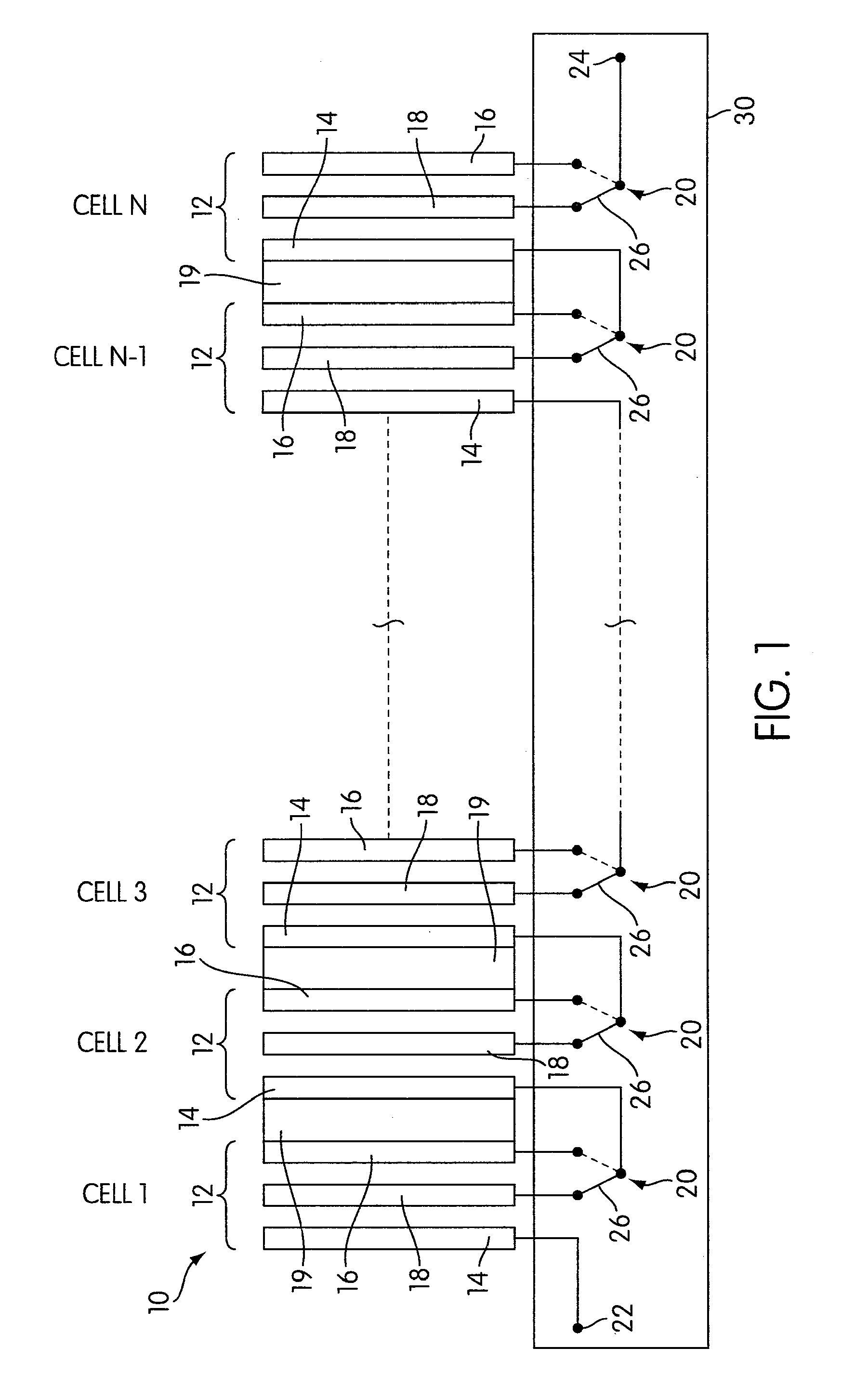
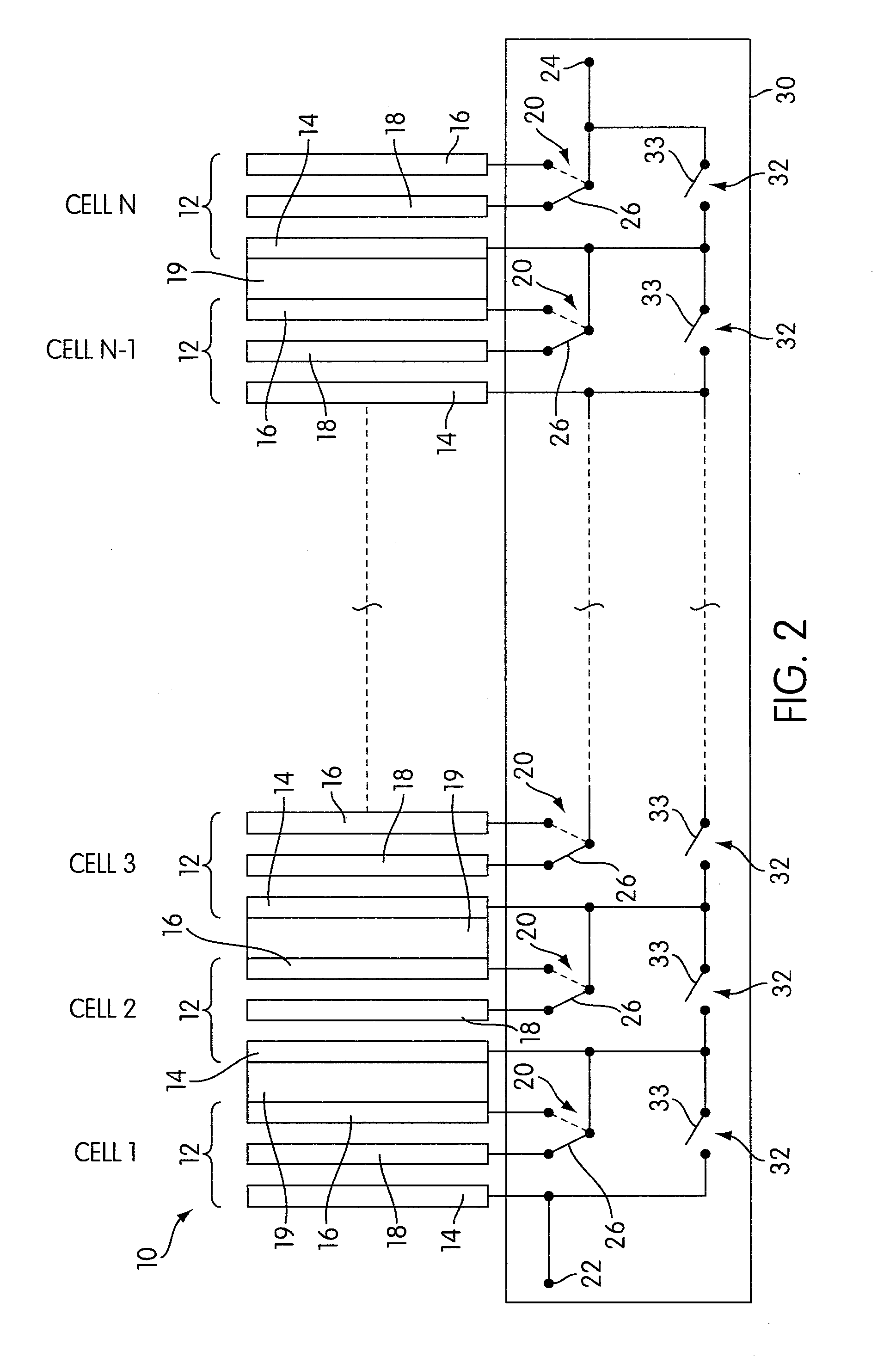
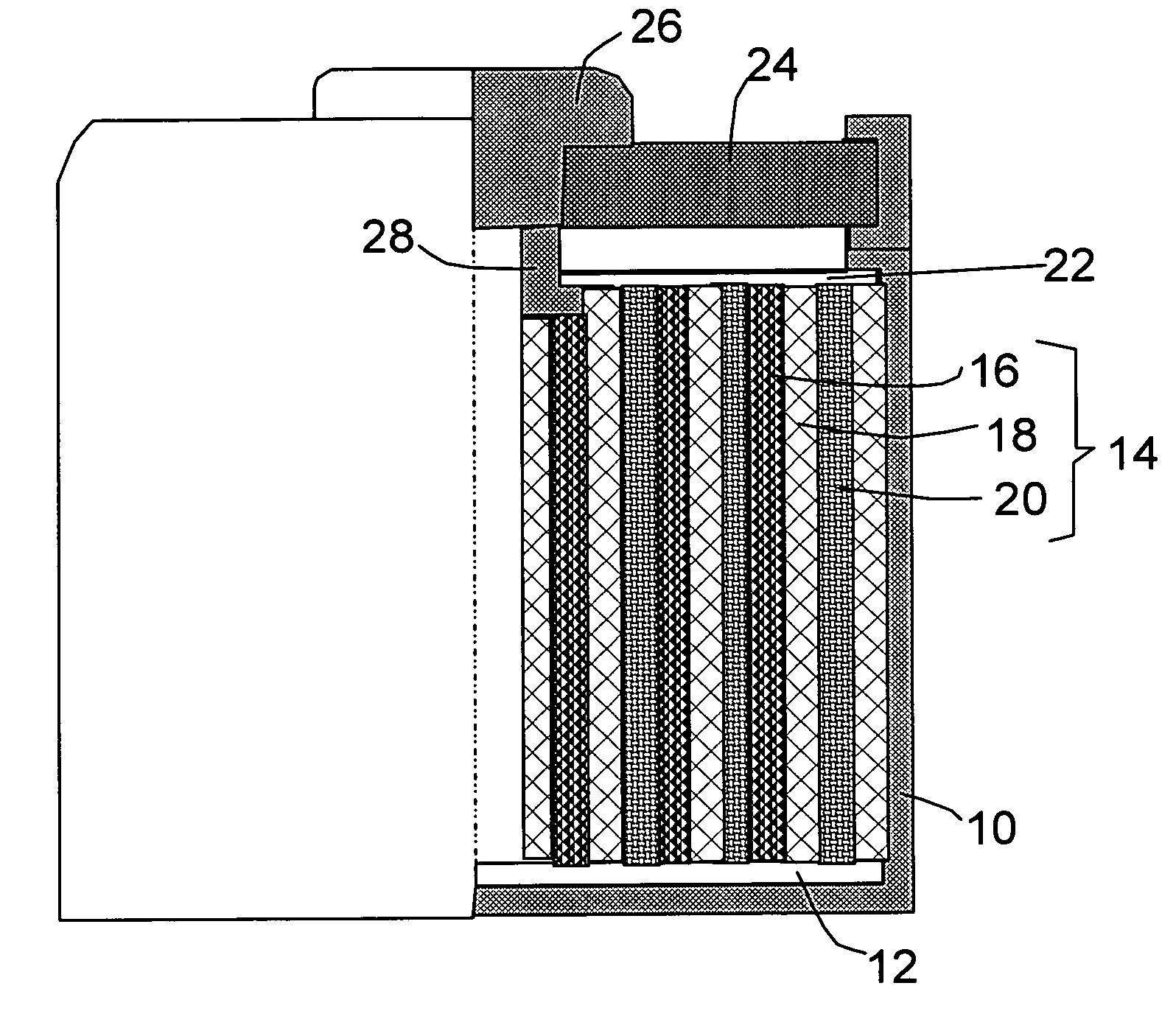
Rechargeable electrochemical cell system with a charging electrode charge/discharge mode switching in the cells
One aspect of the present invention provides a rechargeable electrochemical cell system for generating electrical current using a fuel and an oxidant. The cell system comprises N electrochemical cells each comprising a fuel electrode, an oxidant electrode, a charging electrode, and an ionically conductive medium communicating the electrodes, wherein N is an integer greater than or equal to two. Any number of cells may be used. The cell system includes a plurality of switches that are switcheable to a discharge mode coupling the oxidant electrode of each cell to the fuel electrode of the subsequent cell, a charge mode coupling the charging electrode of each cell to the fuel electrode of the subsequent cell, and a bypass mode coupling charging electrode or the oxidant electrode of a previous cell to the fuel electrode of a subsequent cell.
Owner:FLUIDIC
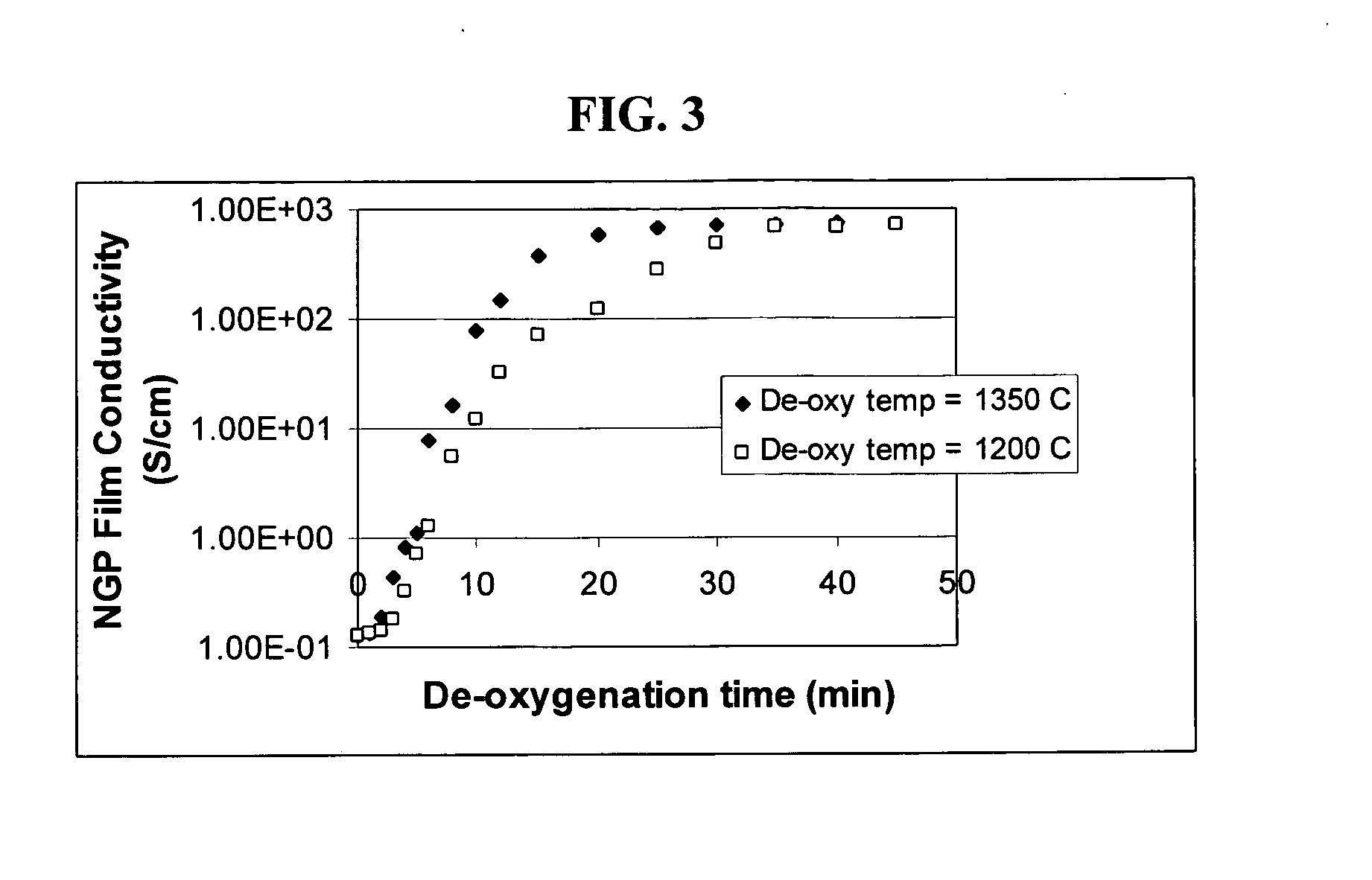
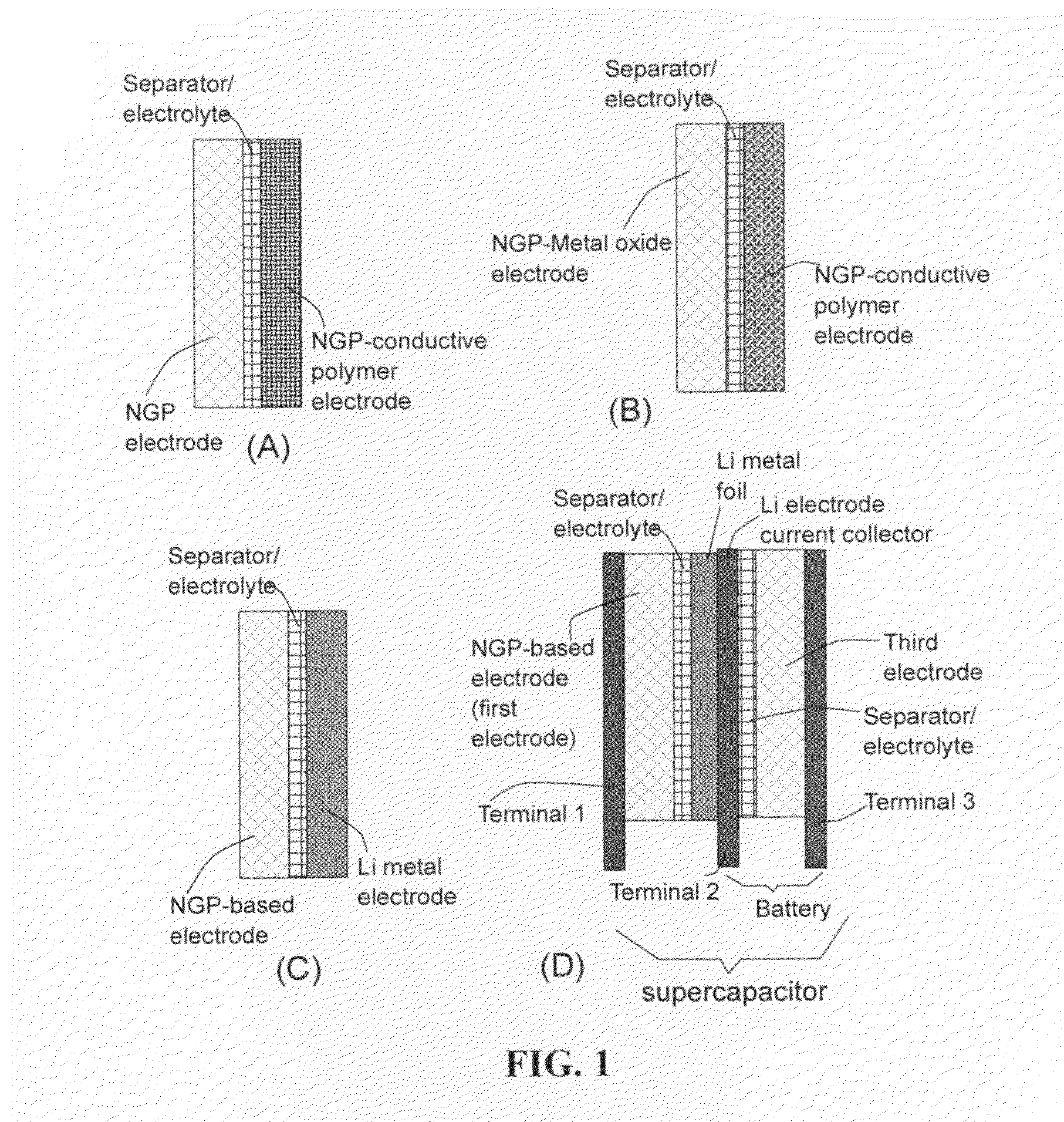
Graphene nanocomposites for electrochemical cell electrodes
ActiveUS20100021819A1High and reversible anode capacityTedious and energy-intensiveMicroscopic fiber electrodesHybrid capacitor electrodesGraphene nanocompositesSolid particle
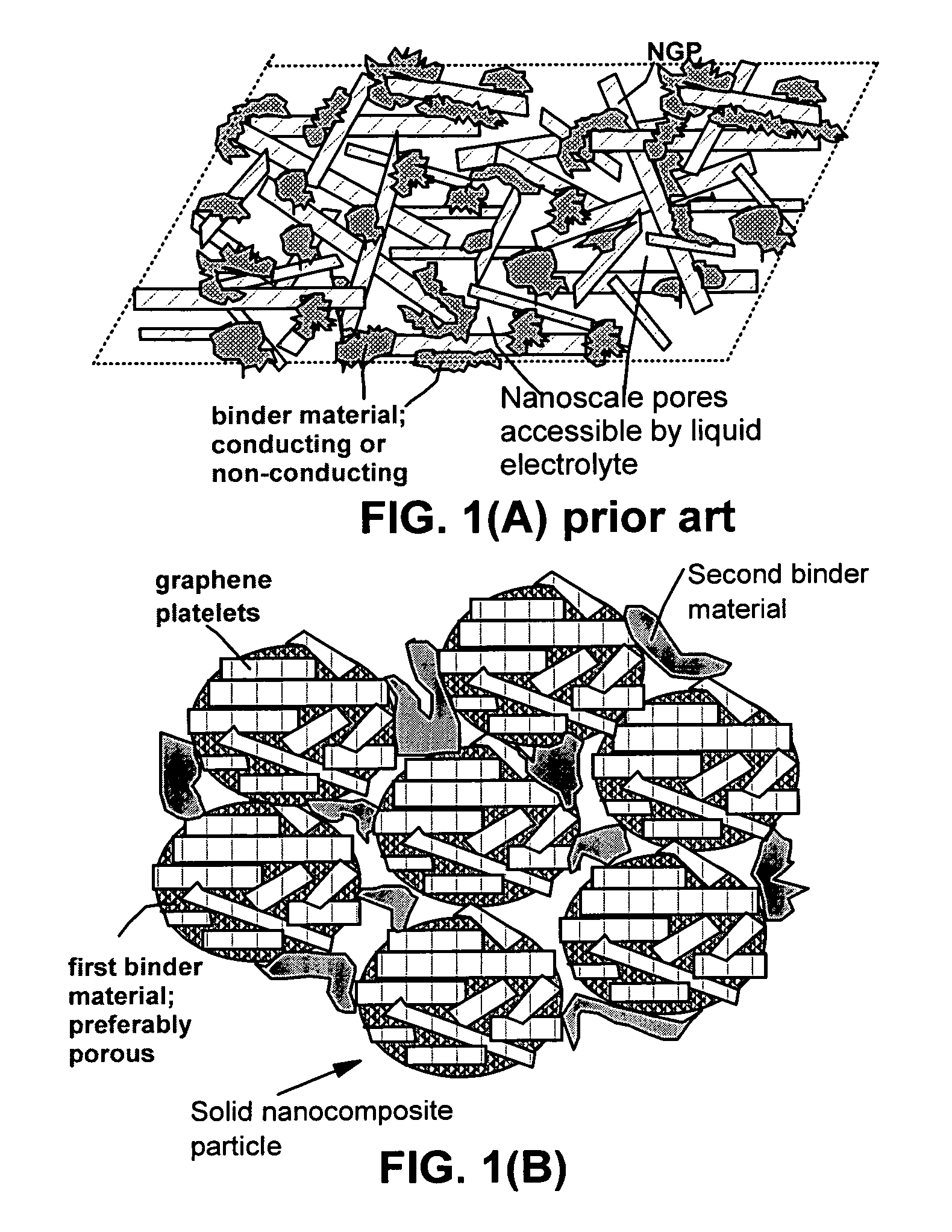
A composite composition for electrochemical cell electrode applications, the composition comprising multiple solid particles, wherein (a) a solid particle is composed of graphene platelets dispersed in or bonded by a first matrix or binder material, wherein the graphene platelets are not obtained from graphitization of the first binder or matrix material; (b) the graphene platelets have a length or width in the range of 10 nm to 10 μm; (c) the multiple solid particles are bonded by a second binder material; and (d) the first or second binder material is selected from a polymer, polymeric carbon, amorphous carbon, metal, glass, ceramic, oxide, organic material, or a combination thereof. For a lithium ion battery anode application, the first binder or matrix material is preferably amorphous carbon or polymeric carbon. Such a composite composition provides a high anode capacity and good cycling response. For a supercapacitor electrode application, the solid particles preferably have meso-scale pores therein to accommodate electrolyte.
Owner:NANOTEK INSTR GRP LLC
Conductive graphene polymer binder for electrochemical cell electrodes
The present invention provides an electrically conductive electrode comprising particles of an electroactive material and a conductive graphene polymer binder that bonds multiple particles of the electroactive material together, wherein the binder is in an amount of from 0.01% to 90% by weight based on the total electrode weight. Also provided are (a) a precursor solution or suspension to the graphene polymer binder for the electrode; (b) a paste containing electroactive particles and a graphene polymer dispersed in a liquid; (c) a method of producing the electrode from the precursor paste; and (d) an electrochemical cell (a battery or supercapacitor) containing such an electrode.
Owner:GLOBAL GRAPHENE GRP INC
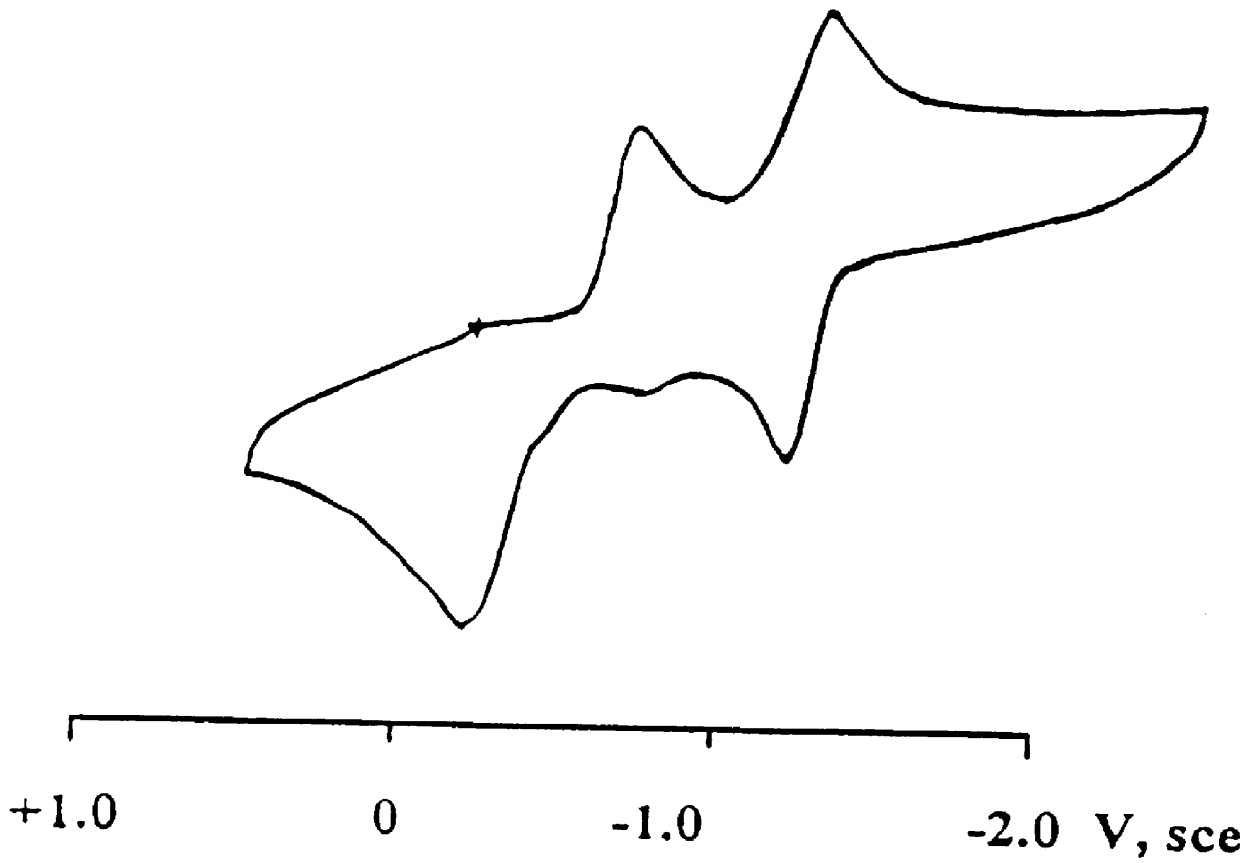
Electrolyte composition, photoelectric conversion device and photo-electrochemical cell
InactiveUS6376765B1Improve rendering capabilitiesIncreased durabilityLight-sensitive devicesOrganic chemistryPhotoelectrochemical cellHydrogen atom
Owner:FUJIFILM HLDG CORP +1
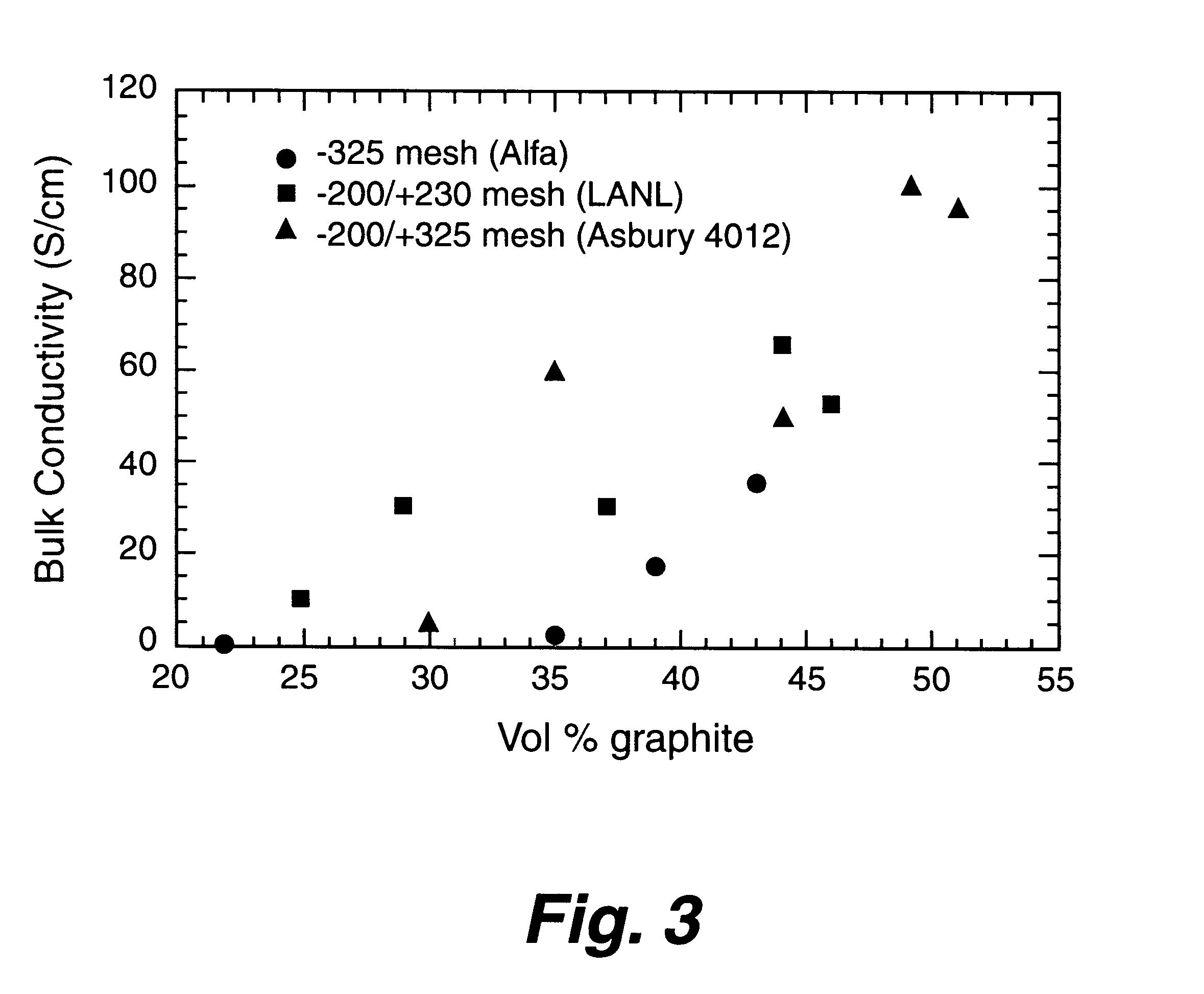
Composite bipolar plate for electrochemical cells
A bipolar separator plate for fuel cells consists of a molded mixture of a vinyl ester resin and graphite powder. The plate serves as a current collector and may contain fluid flow fields for the distribution of reactant gases. The material is inexpensive, electrically conductive, lightweight, strong, corrosion resistant, easily mass produced, and relatively impermeable to hydrogen gas. The addition of certain fiber reinforcements and other additives can improve the properties of the composite material without significantly increasing its overall cost.
Owner:TRIAD NAT SECURITY LLC
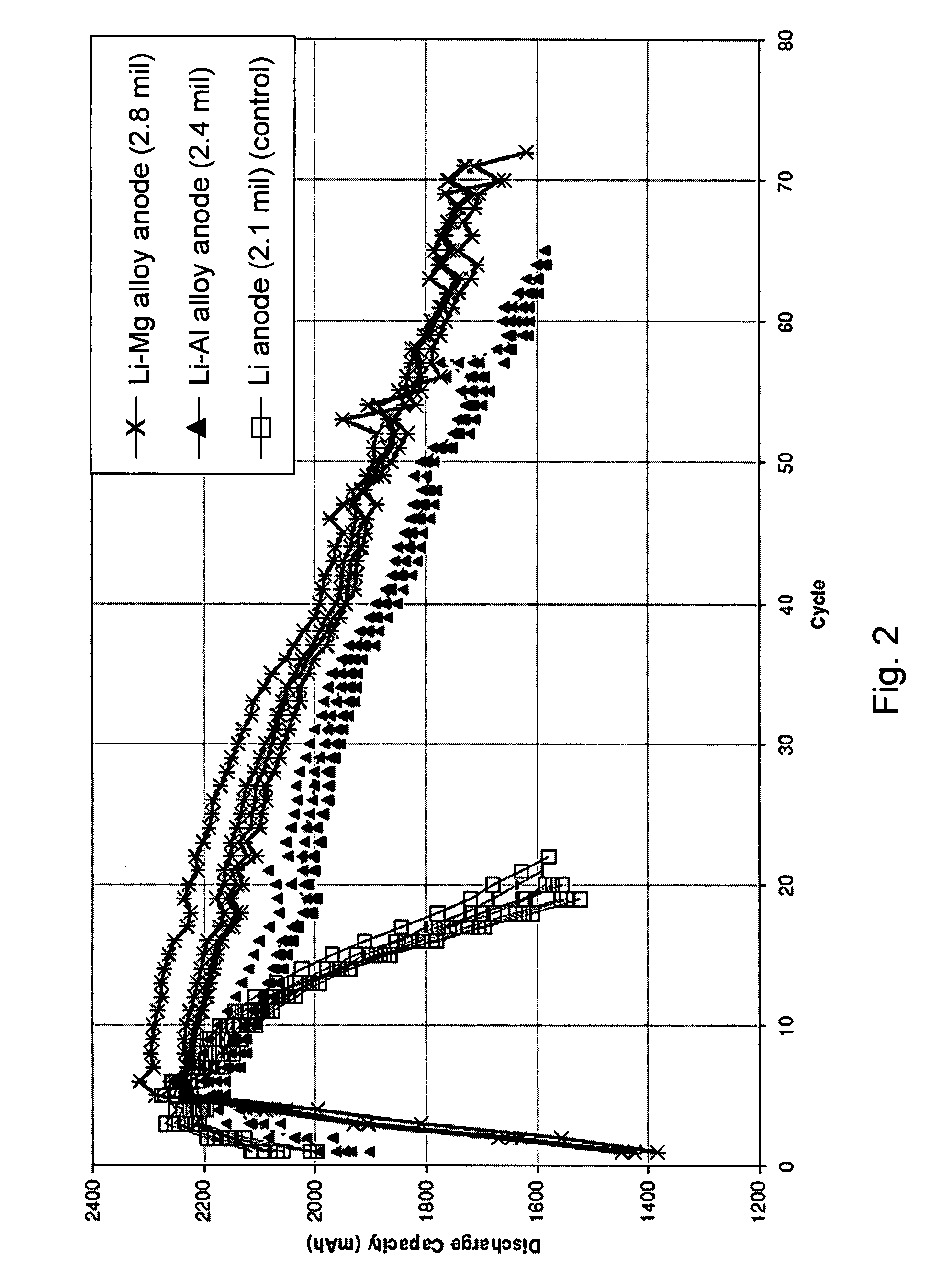
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Lithium alloy/sulfur batteries
Electrochemical cells including anode compositions that may enhance charge-discharge cycling efficiency and uniformity are presented. In some embodiments, alloys are incorporated into one or more components of an electrochemical cell, which may enhance the performance of the cell. For example, an alloy may be incorporated into an electroactive component of the cell (e.g., electrodes) and may advantageously increase the efficiency of cell performance. Some electrochemical cells (e.g., rechargeable batteries) may undergo a charge / discharge cycle involving deposition of metal (e.g., lithium metal) on the surface of the anode upon charging and reaction of the metal on the anode surface, wherein the metal diffuses from the anode surface, upon discharging. In some cases, the efficiency and uniformity of such processes may affect cell performance. The use of materials such as alloys in an electroactive component of the cell have been found to increase the efficiency of such processes and to increase the cycling lifetime of the cell. For example, the use of alloys may reduce the formation of dendrites on the anode surface and / or limit surface development.
Owner:SION POWER CORP
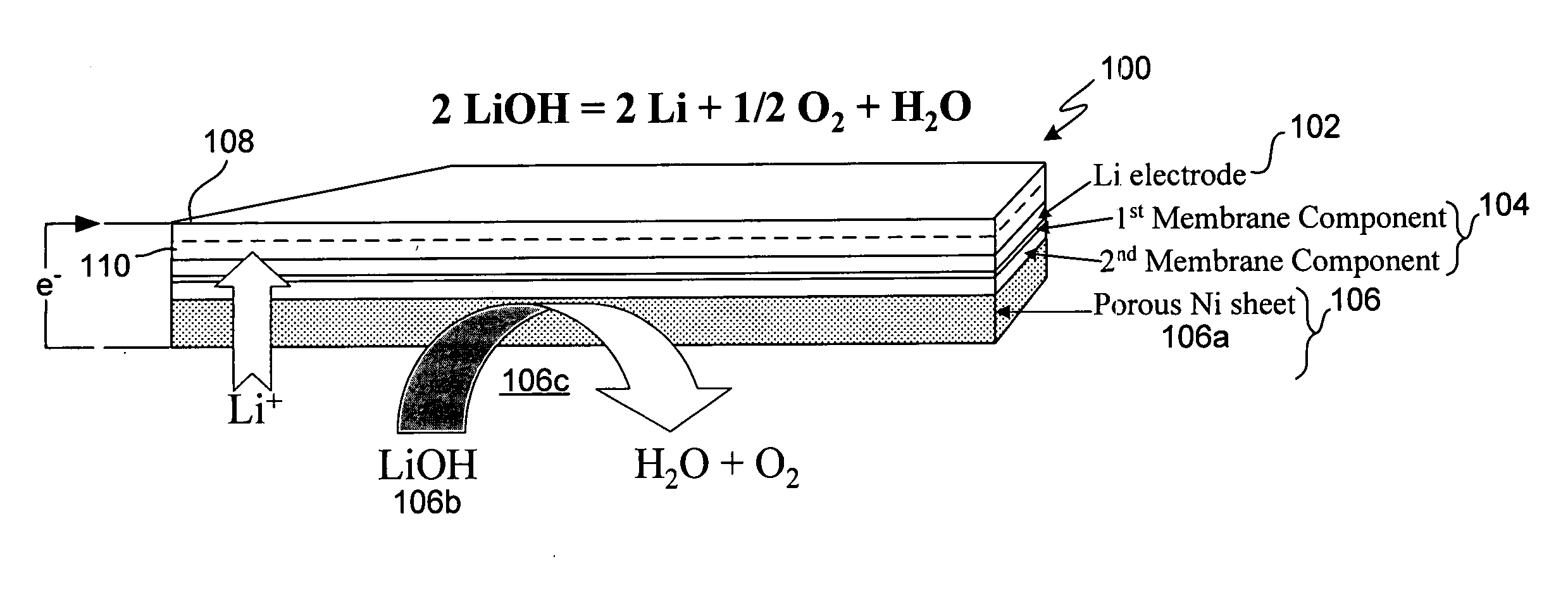
Active metal electrolyzer
InactiveUS20050100793A1Effective isolationReduce environmental pollutionPhotography auxillary processesElectrode manufacturing processesElectrolysisAqueous electrolyte
Electro-winning of active metal (e.g., lithium) ions from a variety of sources including industrial waste, and recycled lithium and lithium-ion batteries is accomplished with an electrolyzer having a protected cathode that is stable against aggressive solvents, including water, aqueous electrolytes, acid, base, and a broad range of protic and aprotic solvents. The electrolyzer has a highly ionically conductive protective membrane adjacent to the alkali metal cathode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the cathode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or anode material in conjunction with the cathode. The electrolyzer can be configured and operated to claim or reclaim lithium or other active metals from such sources.
Owner:POLYPLUS BATTERY CO INC
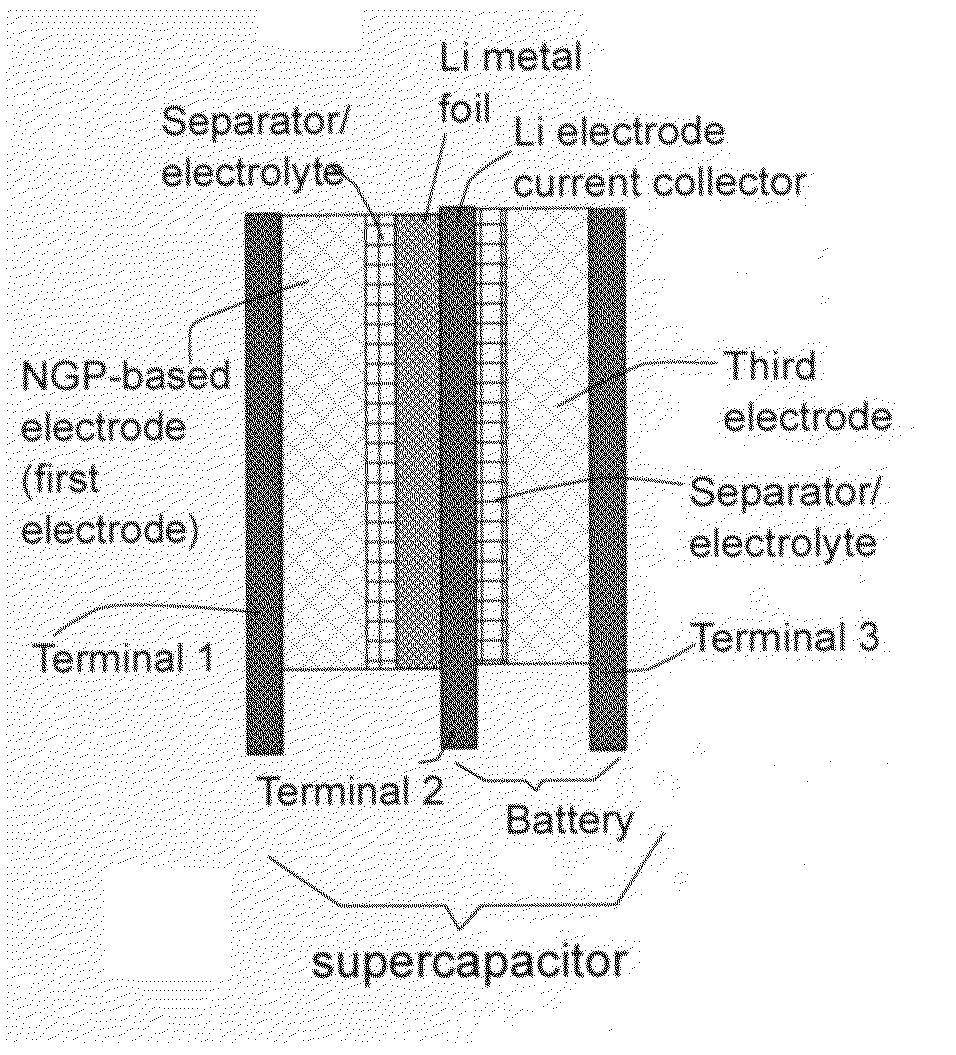
Flexible asymmetric electrochemical cells using nano graphene platelet as an electrode material
InactiveUS20110183180A1Excellent specific capacitanceLarge specific surface areaElectrochemical generatorsHybrid capacitor separatorsPlateletGraphene
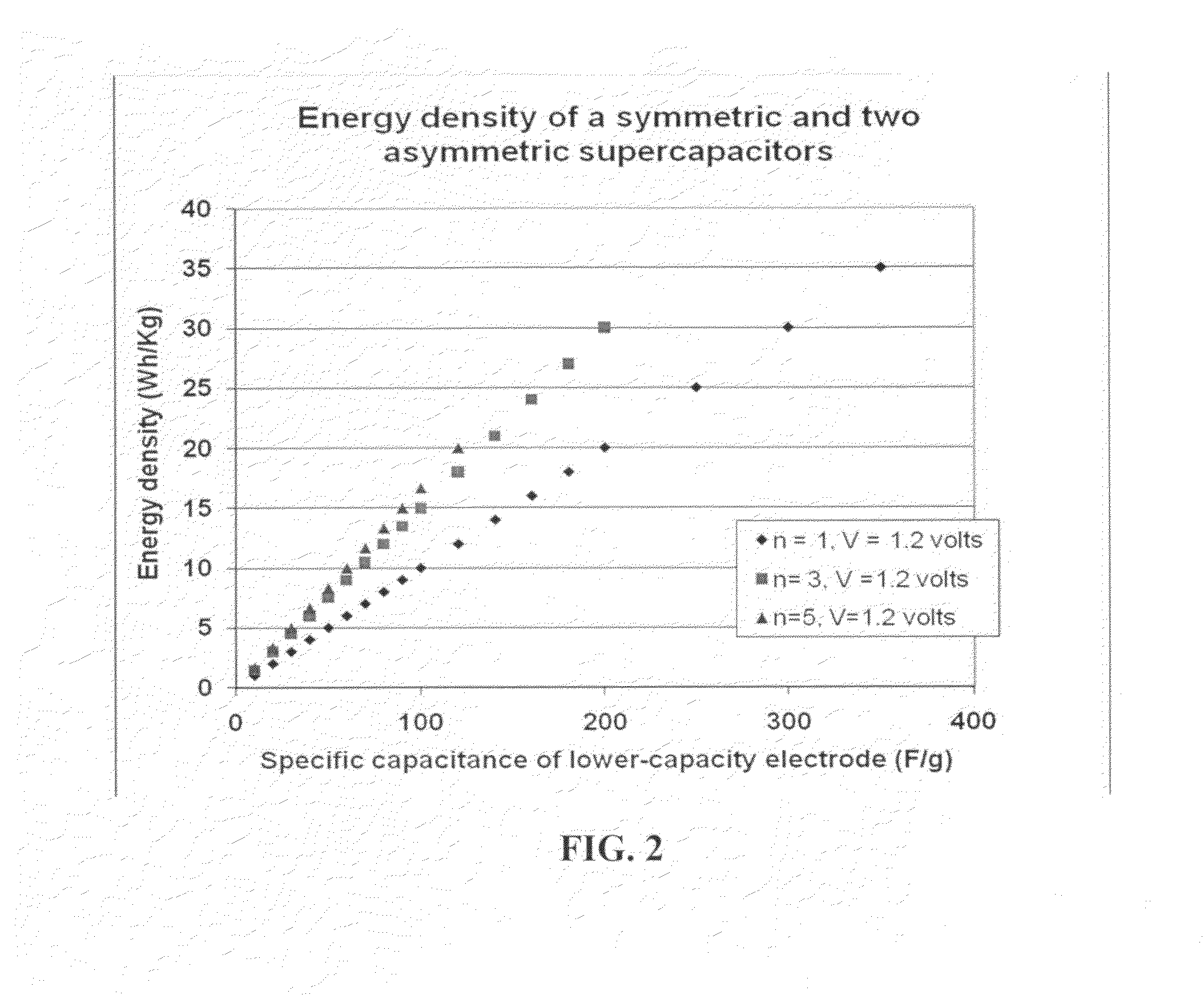
A flexible, asymmetric electrochemical cell comprising: (A) A sheet of graphene paper as first electrode comprising nano graphene platelets having a platelet thickness less than 1 nm, wherein the first electrode has electrolyte-accessible pores; (B) A thin-film or paper-like first separator and electrolyte; and (C) A thin-film or paper-like second electrode which is different in composition than the first electrode; wherein the separator is sandwiched between the first and second electrode to form a flexible laminate configuration. The asymmetric supercapacitor cells with different NGP-based electrodes exhibit an exceptionally high capacitance, specific energy, and stable and long cycle life.
Owner:NANOTEK INSTR GRP LLC
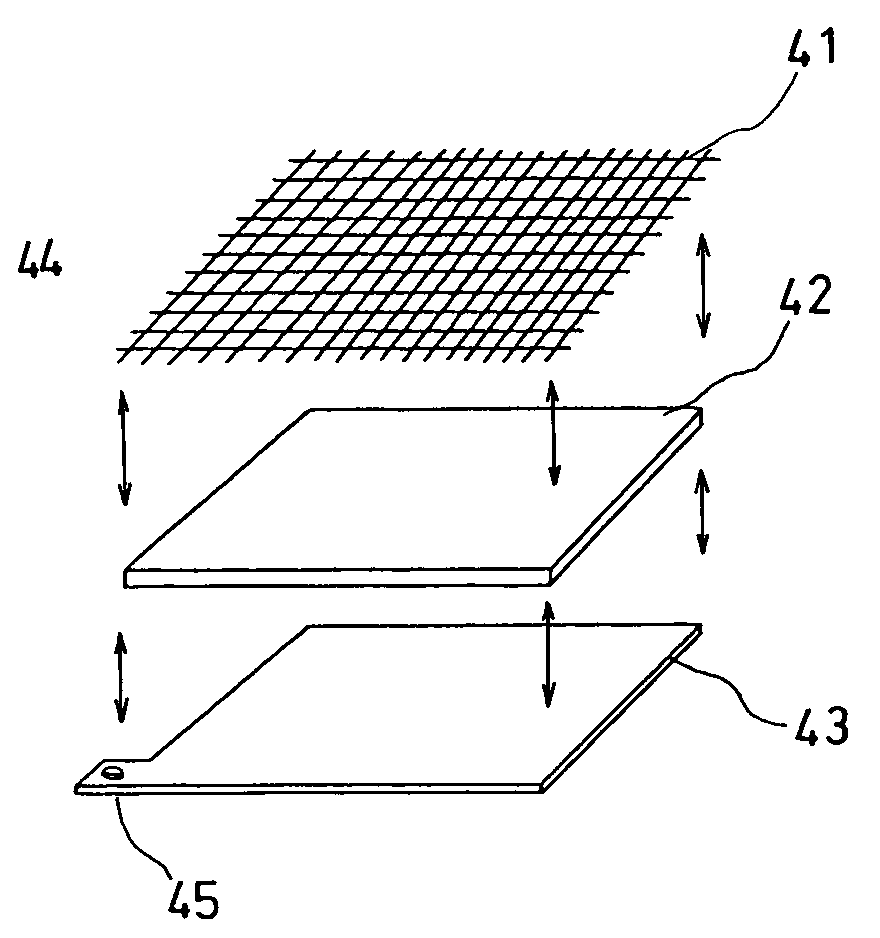
Heated electrochemical cell
The invention provides a method for determining the concentration of an analyte in a sample comprising the steps of heating the sample and measuring the concentration of the analyte or the concentration of a species representative thereof in the sample at a predetermined point on a reaction profile by means that are substantially independent of temperature. Also provided is an electrochemical cell comprising a spacer pierced by an aperture which defines a cell wall, a first metal electrode on one side of the spacer extending over one side of the aperture, a second metal electrode on the other side of the spacer extending over the side of the aperture opposite the first electrode, means for admitting a sample to the cell volume defined between the electrodes and the cell wall, and means for heating a sample contained within the cell.
Owner:LIFESCAN INC
Percutaneous biological fluid sampling and analyte measurement devices and methods
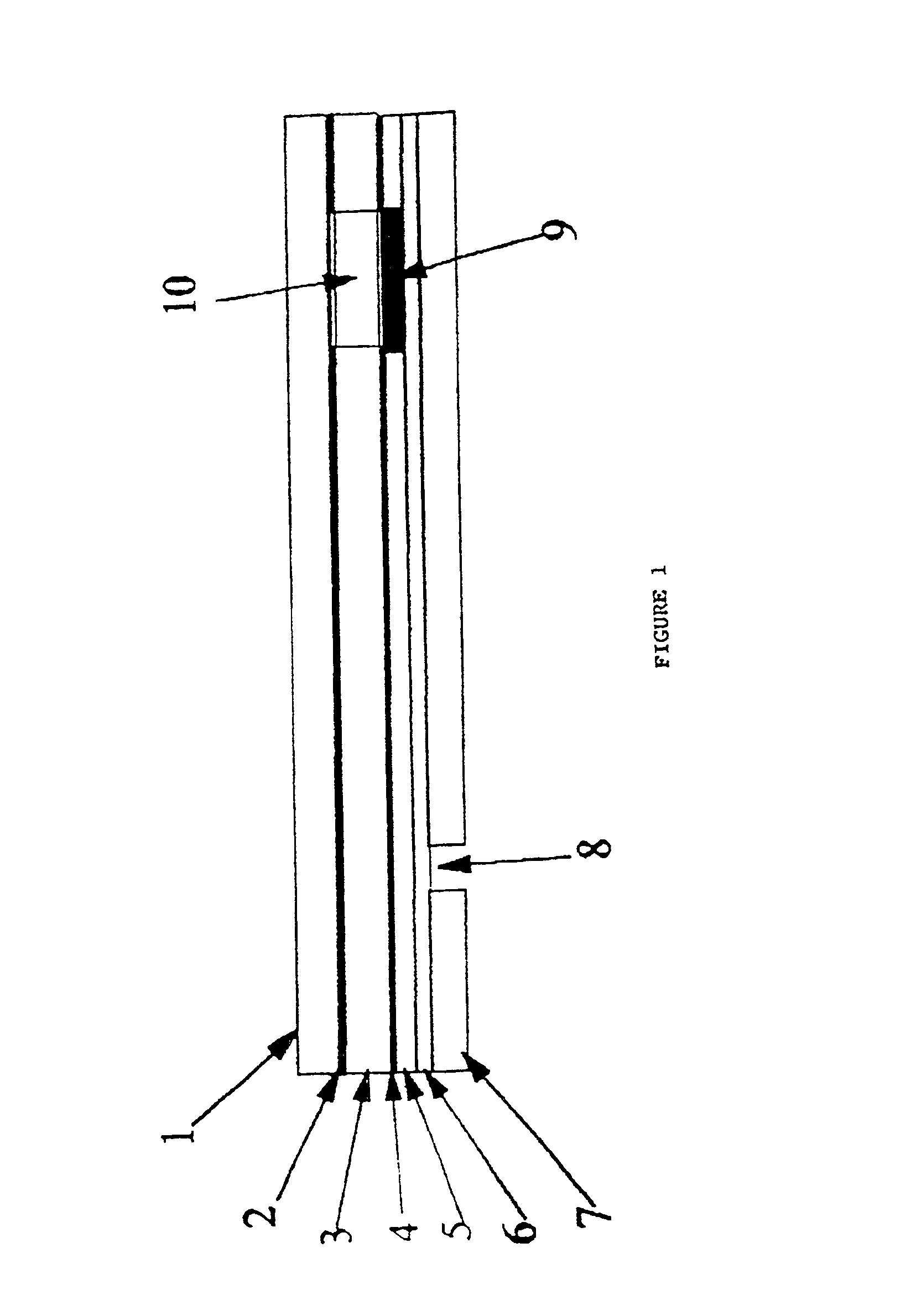
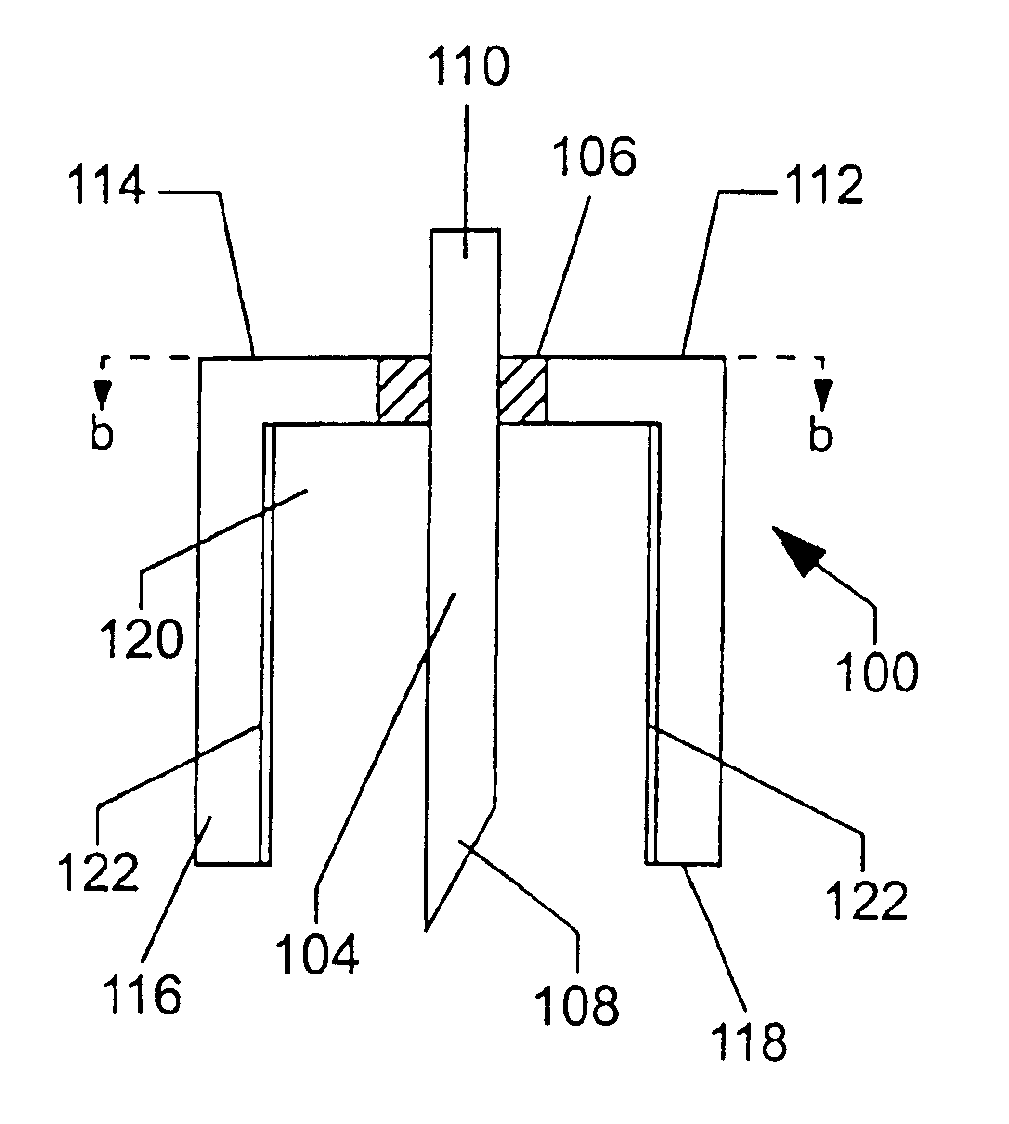
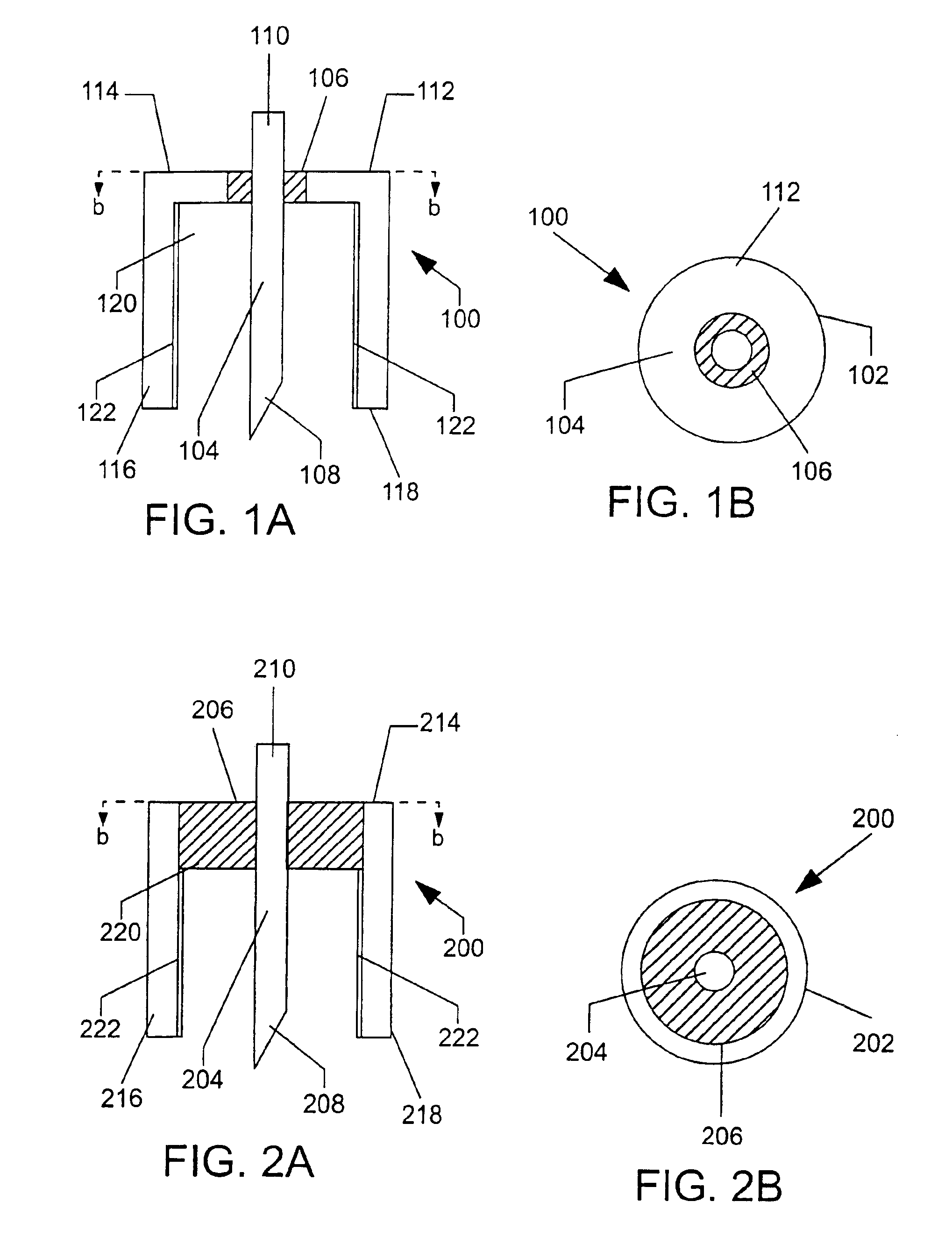
A device for sampling a biological fluid and measuring at least one target constituent within the biological fluid. The device has at least one electrochemical cell having an inner electrode and an outer electrode in a concentrically-spaced relationship. In a preferred embodiment, the outer electrode has a cylindrical configuration having an open distal end and the inner electrode has an elongated configuration positioned co-axially within the outer electrode and a distal end configured to penetrate the skin. The spacing between the electrodes exerts a capillary force on biological fluid present at the open distal end of the outer electrode. A system is also provided which includes a control unit in electrical communication with the electrochemical cell for controlling the selection and measurement of the target constituent. Methods of sampling of biological fluids within the skin and measuring the sampled fluids are also provided, as well as kits comprising one or more of the inventive devices and / or systems.
Owner:LIFESCAN IP HLDG LLC
Application of force in electrochemical cells
ActiveUS20100035128A1Improve performancePrimary cell to battery groupingCell seperators/membranes/diaphragms/spacersRough surfaceChemical reaction
The present invention relates to the application of a force to enhance the performance of an electrochemical cell. The force may comprise, in some instances, an anisotropic force with a component normal to an active surface of the anode of the electrochemical cell. In the embodiments described herein, electrochemical cells (e.g., rechargeable batteries) may undergo a charge / discharge cycle involving deposition of metal (e.g., lithium metal) on a surface of the anode upon charging and reaction of the metal on the anode surface, wherein the metal diffuses from the anode surface, upon discharging. The uniformity with which the metal is deposited on the anode may affect cell performance. For example, when lithium metal is redeposited on an anode, it may, in some cases, deposit unevenly forming a rough surface. The roughened surface may increase the amount of lithium metal available for undesired chemical reactions which may result in decreased cycling lifetime and / or poor cell performance. The application of force to the electrochemical cell has been found, in accordance with the invention, to reduce such behavior and to improve the cycling lifetime and / or performance of the cell.
Owner:SION POWER CORP
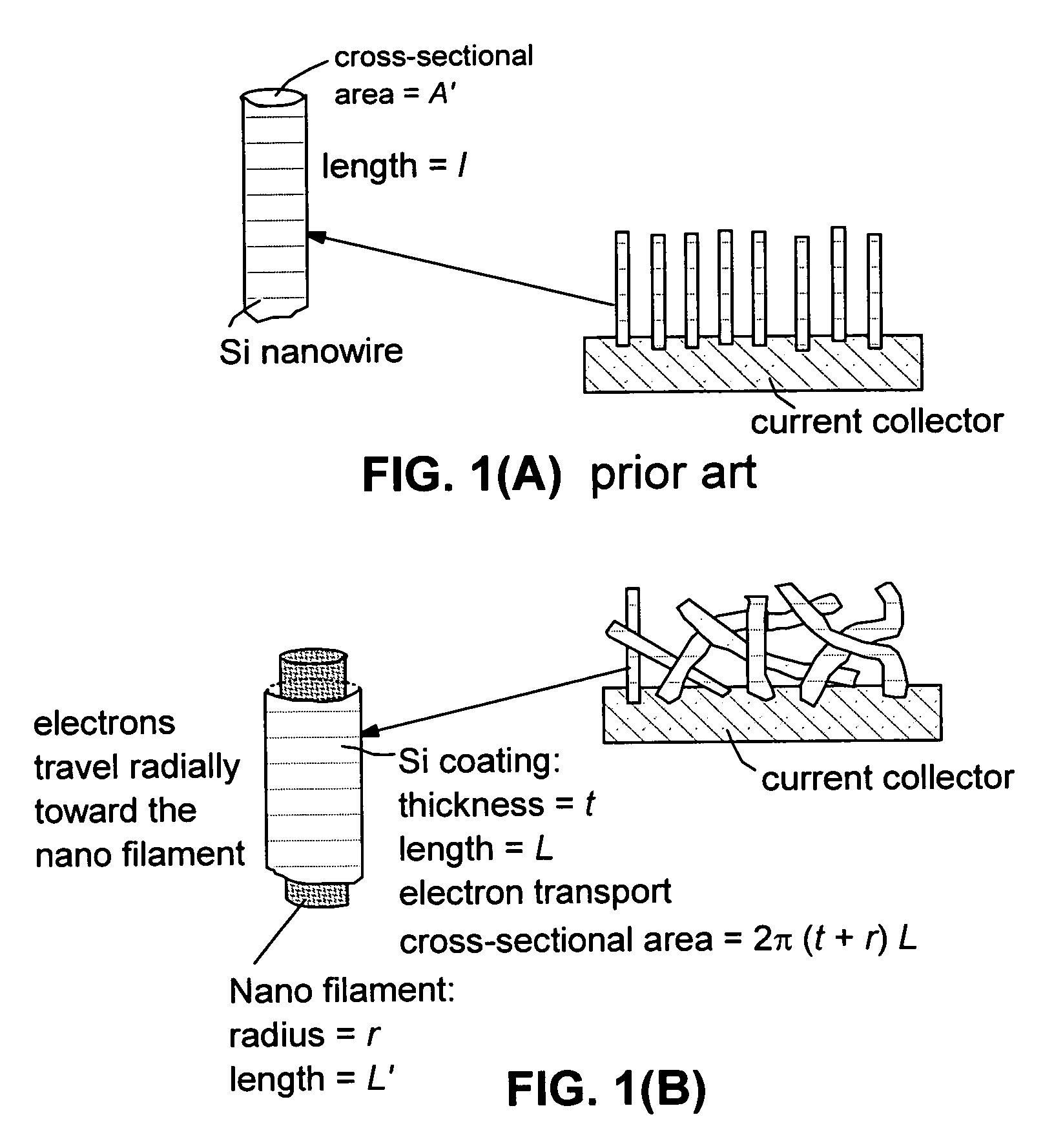
Hybrid nano-filament anode compositions for lithium ion batteries
ActiveUS20090169996A1Superior multiple-cycle behaviorImprove cycle lifeElectrochemical processing of electrodesElectrode thermal treatmentLithium-ion batteryNanometre
This invention provides a hybrid nano-filament composition for use as an electrochemical cell electrode. The composition comprises: (a) an aggregate of nanometer-scaled, electrically conductive filaments that are substantially interconnected, intersected, or percolated to form a porous, electrically conductive filament network comprising substantially interconnected pores, wherein the filaments have an elongate dimension and a first transverse dimension with the first transverse dimension being less than 500 nm (preferably less than 100 nm) and an aspect ratio of the elongate dimension to the first transverse dimension greater than 10; and (b) micron- or nanometer-scaled coating that is deposited on a surface of the filaments, wherein the coating comprises an anode active material capable of absorbing and desorbing lithium ions and the coating has a thickness less than 20 μm (preferably less than 1 μm). Also provided is a lithium ion battery comprising such an electrode as an anode. The battery exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
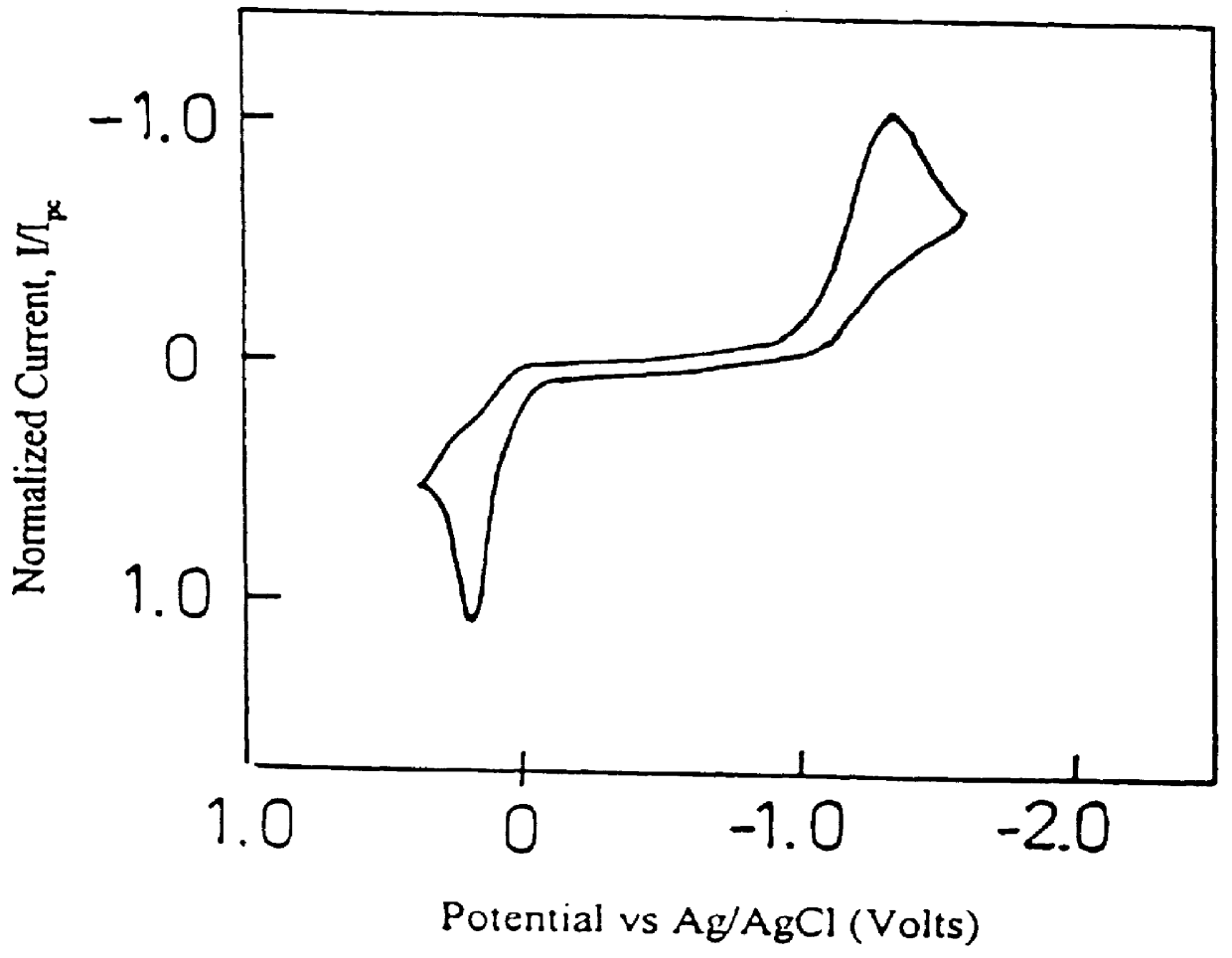
Methods And Apparatus For Analyzing A Sample In The Presence Of Interferents
ActiveUS20070227912A1Minimize impactReduce impactWeather/light/corrosion resistanceMicrobiological testing/measurementAnalytePeak current
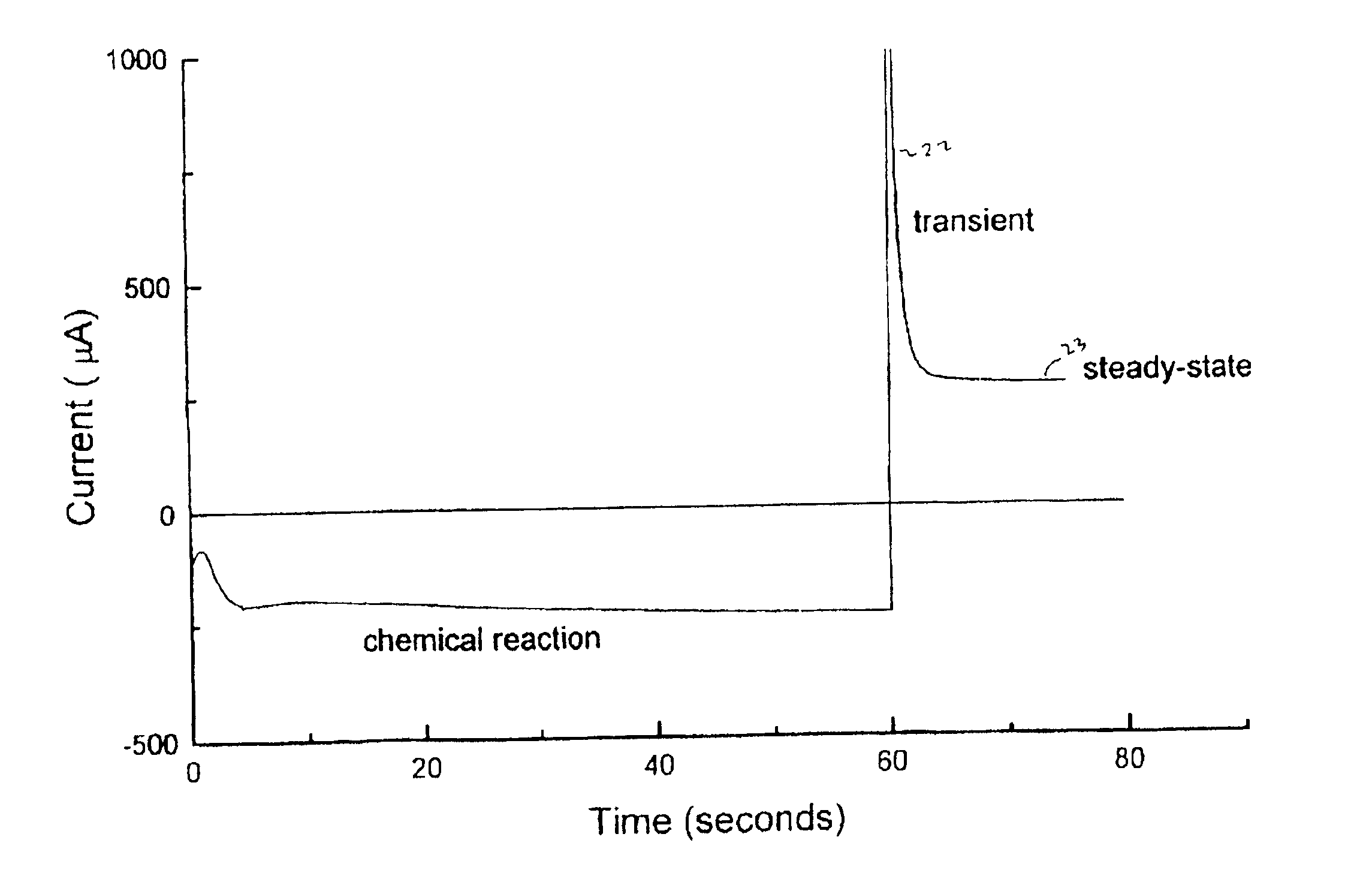
Disclosed herein are methods and apparatus for determining analyte concentration in a rapid and accurate manner. The methods include depositing a physiological sample in an electrochemical cell and finding a first and second current transient. Peak current values are obtained from the first and second peak current values and used to reduce the influence of interferents in a current value. Based on this “corrected” current value, an accurate analyte concentration can be determined.
Owner:LIFESCAN IP HLDG LLC
High discharge capacity lithium battery
InactiveUS20050233214A1Improve discharge performanceIncrease energy densityFinal product manufactureOrganic electrolyte cellsHigh rateIron disulfide
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:EVEREADY BATTERY CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com