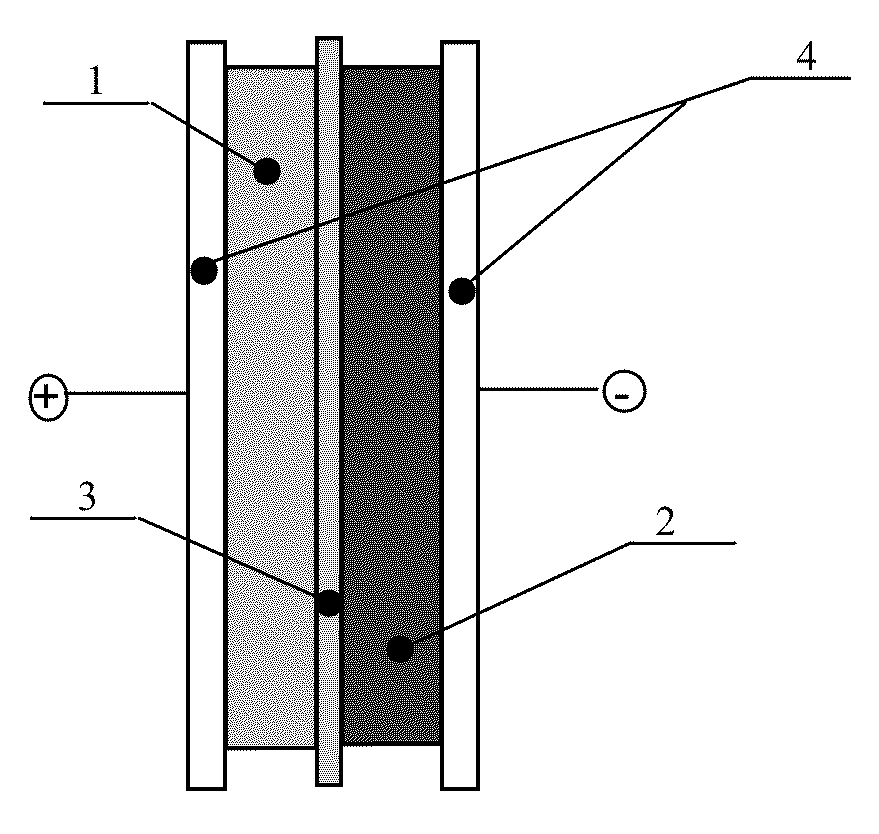
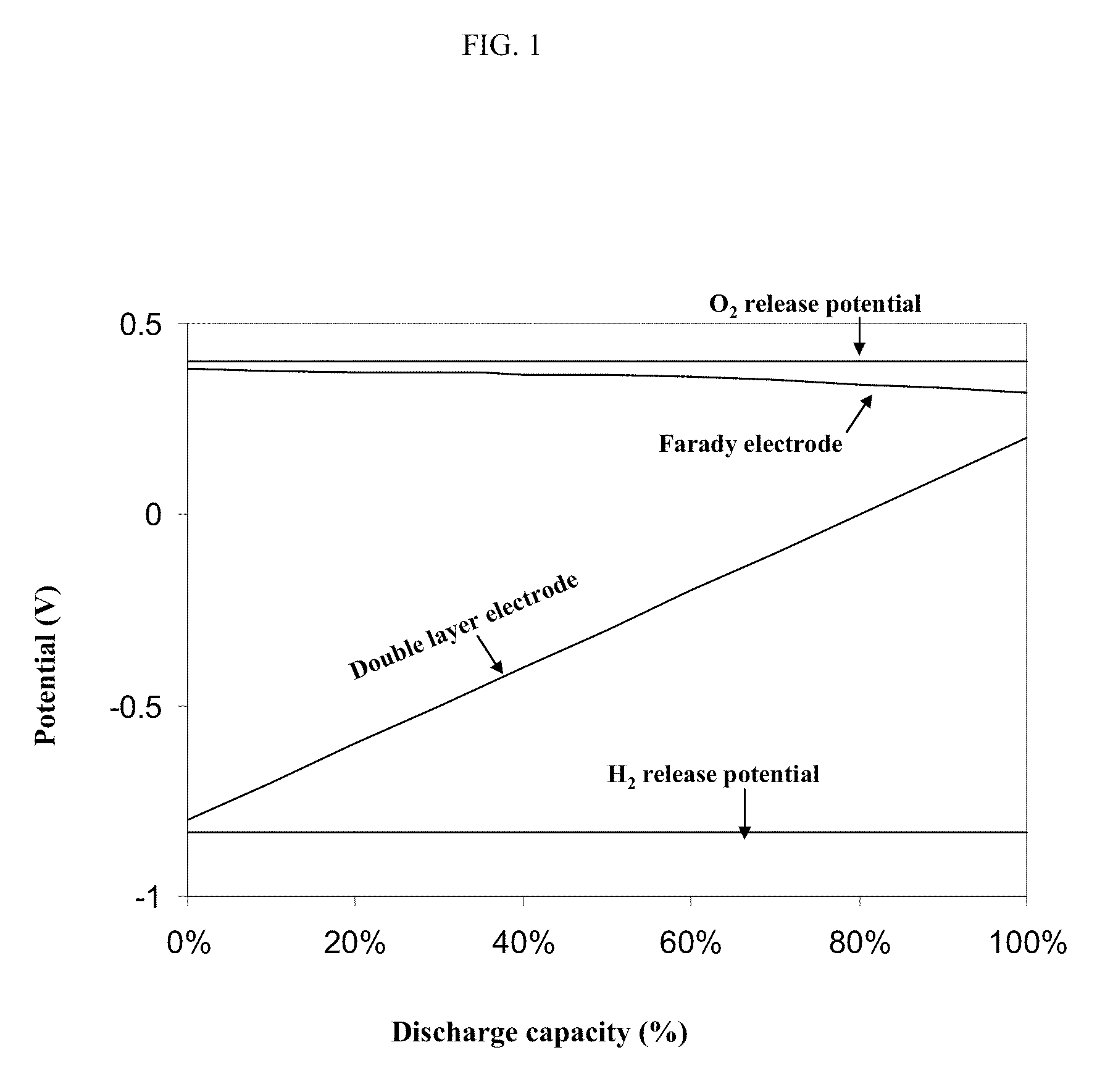
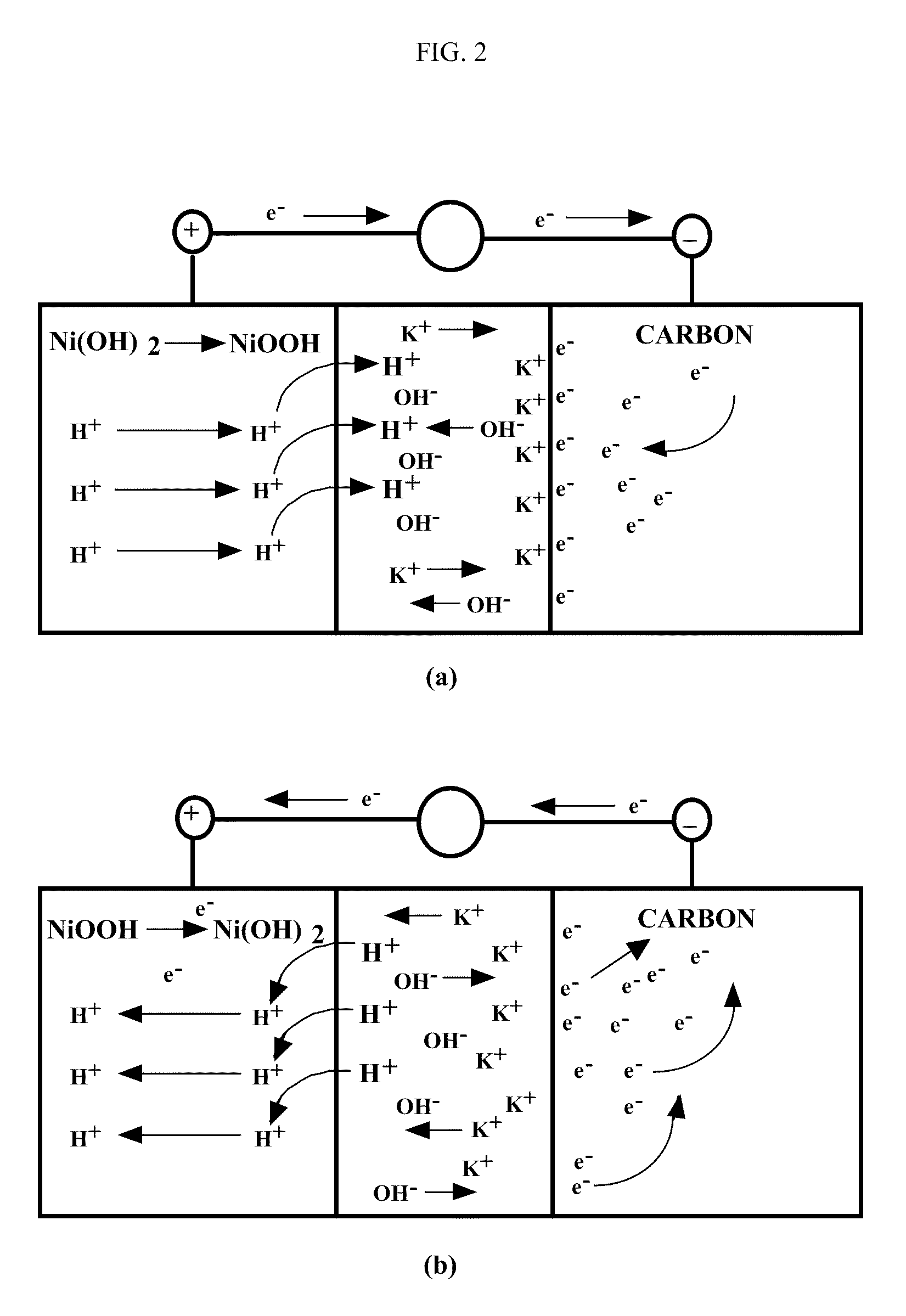
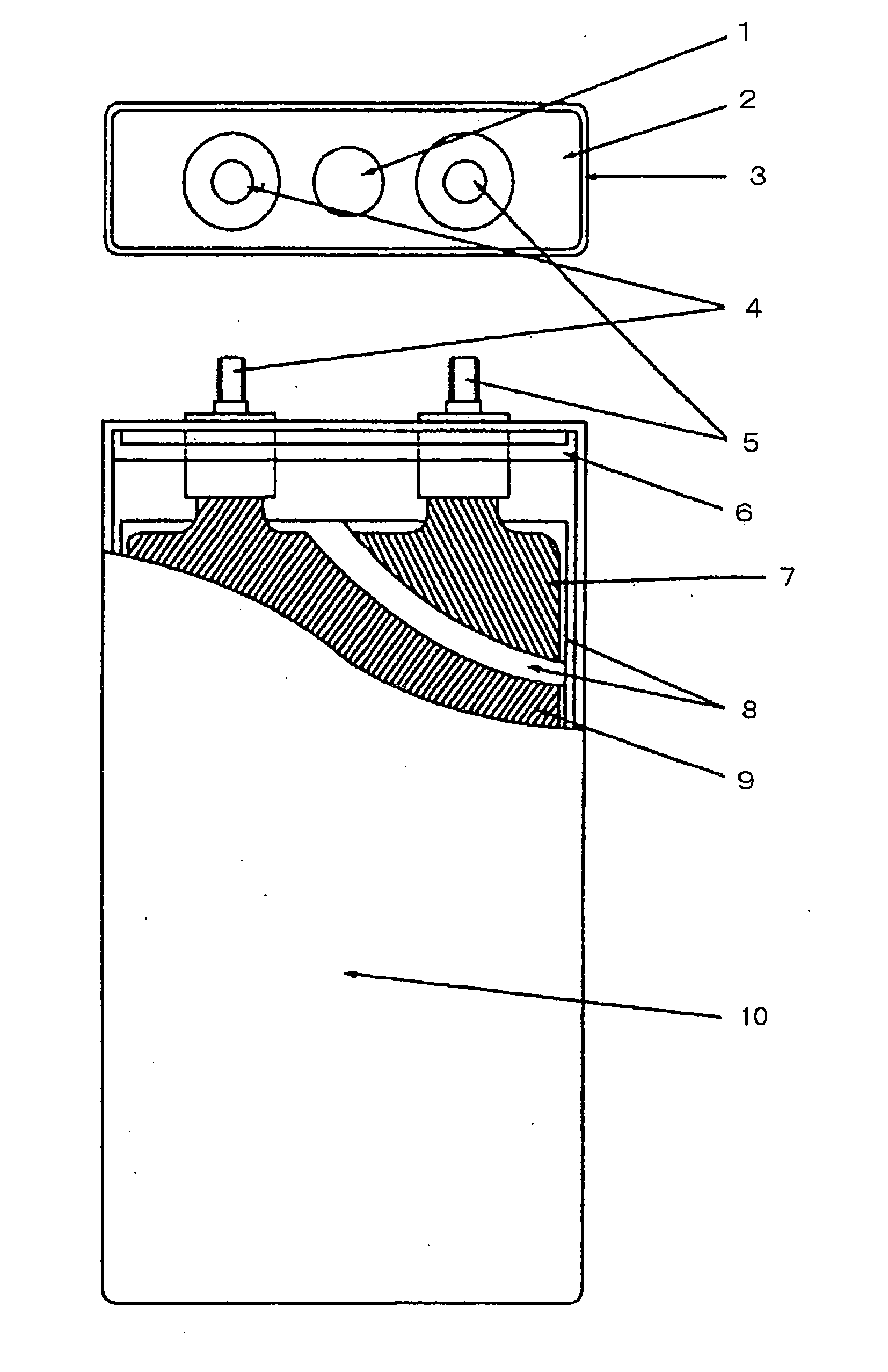
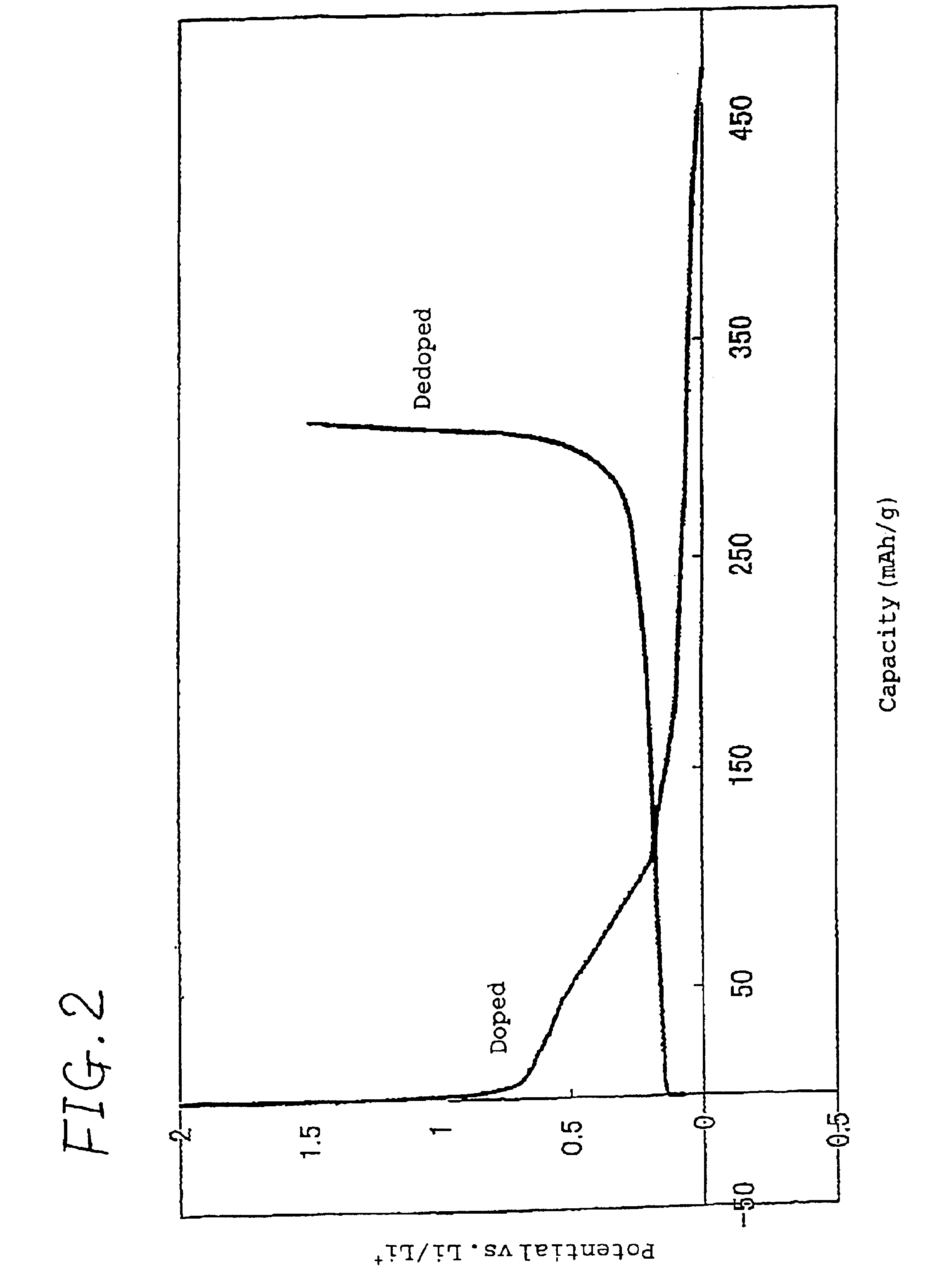
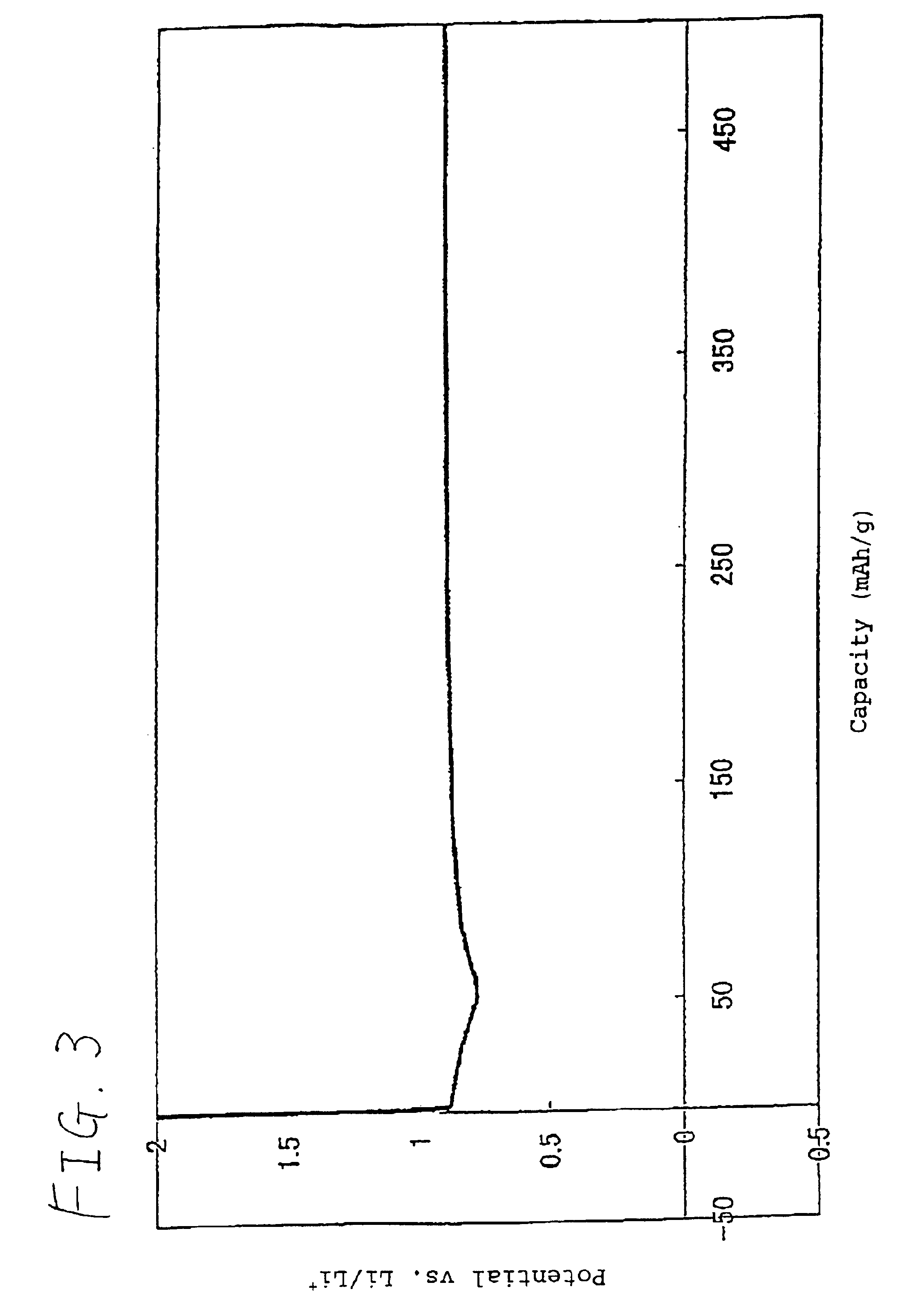
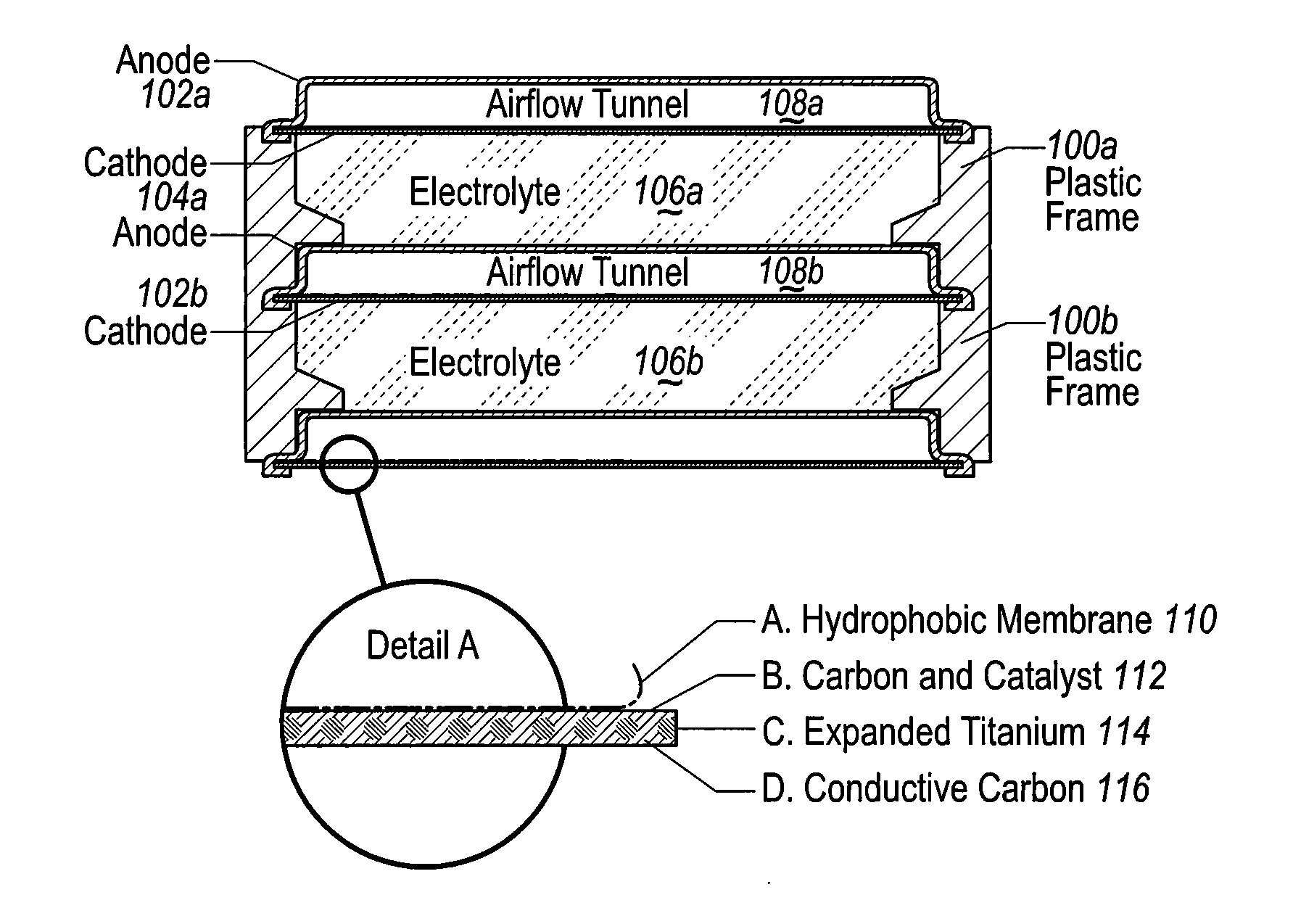
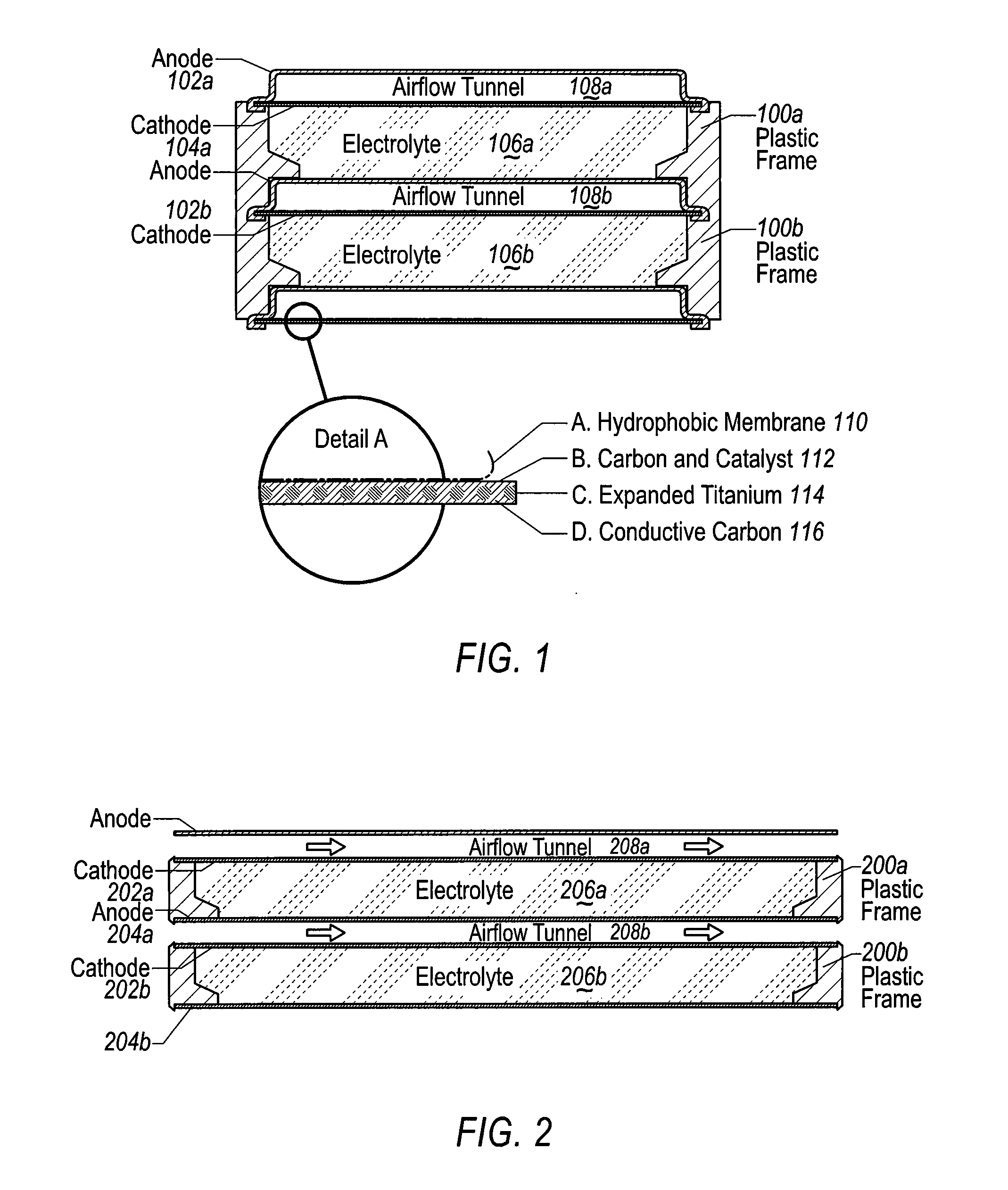
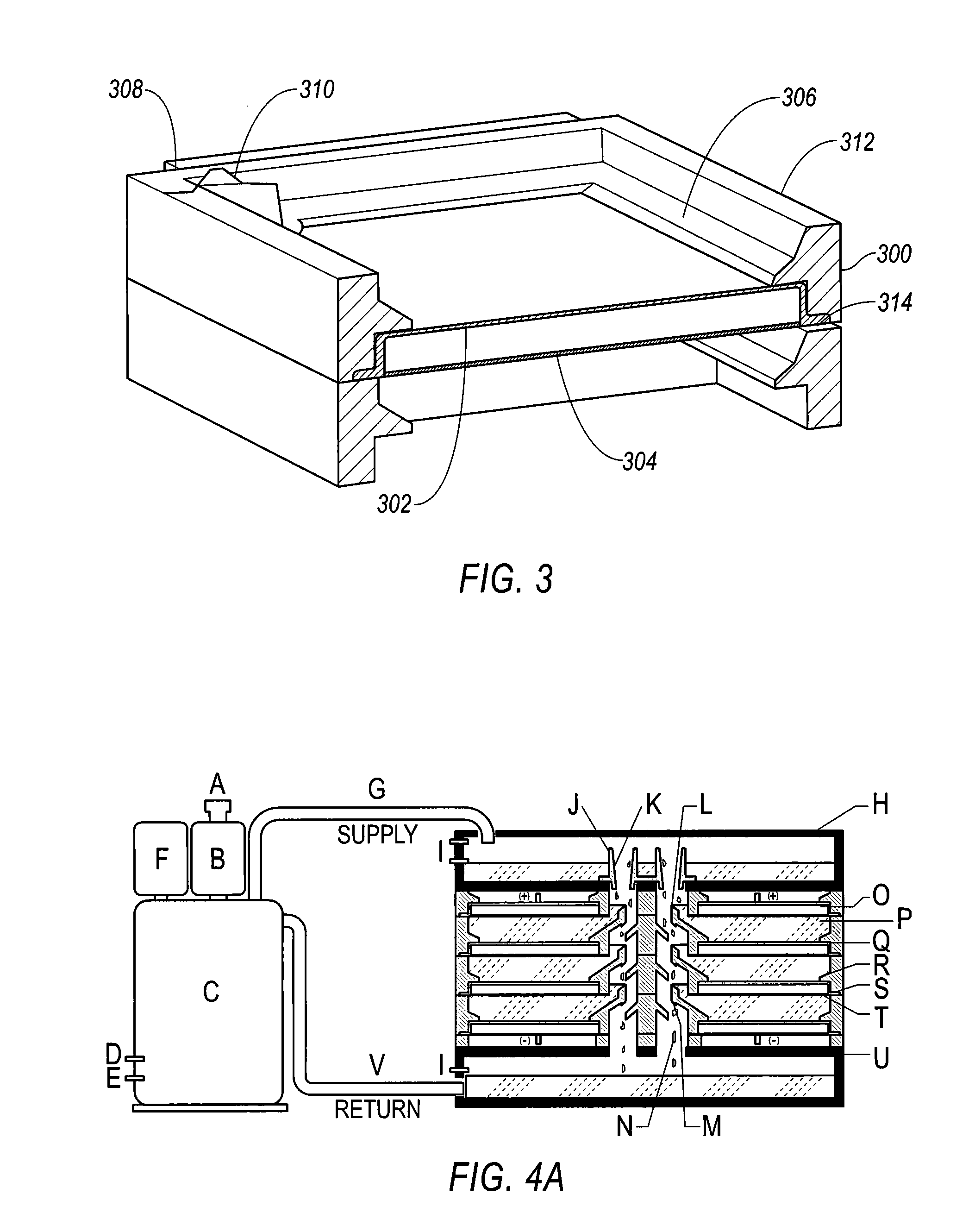
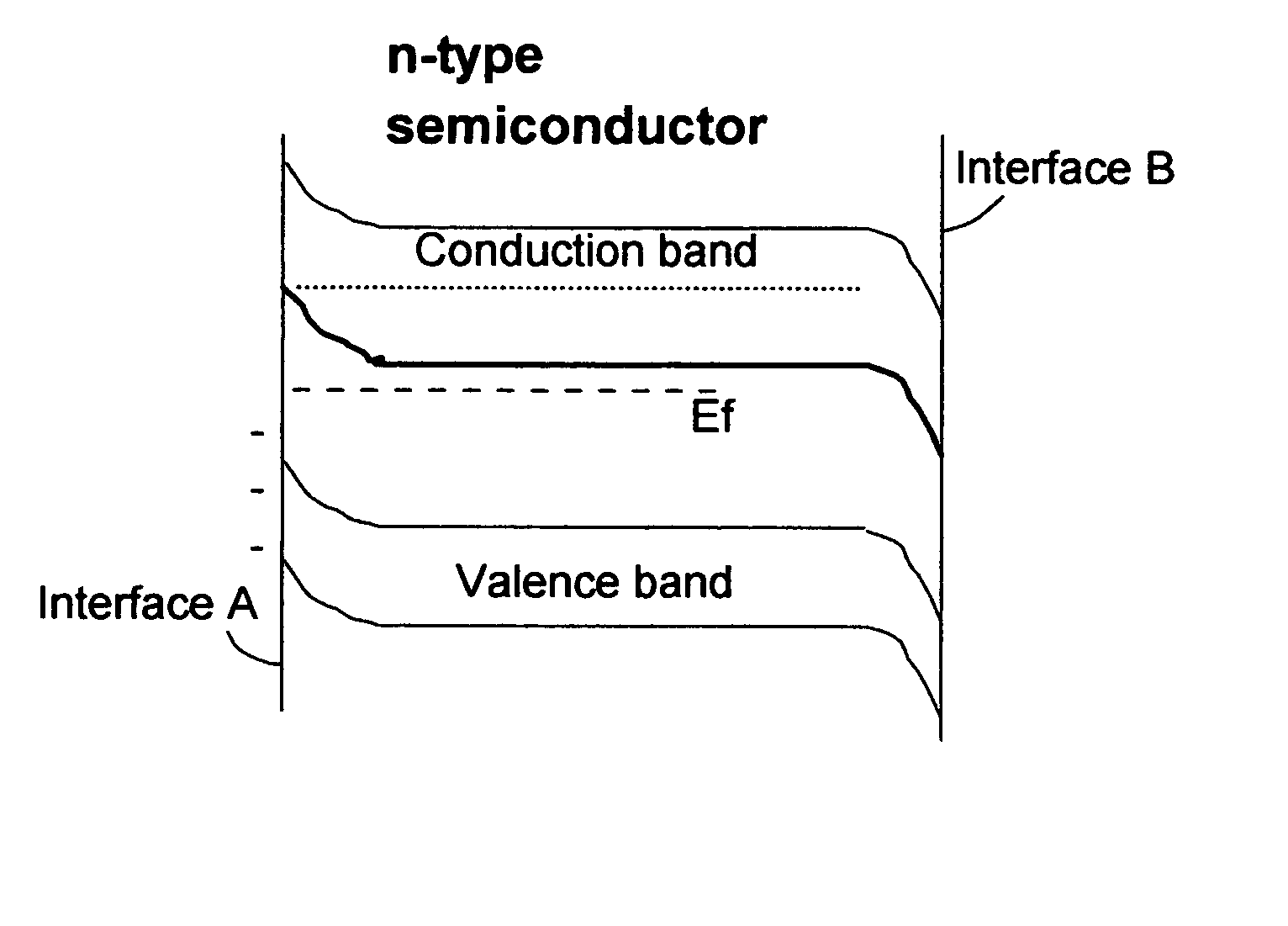
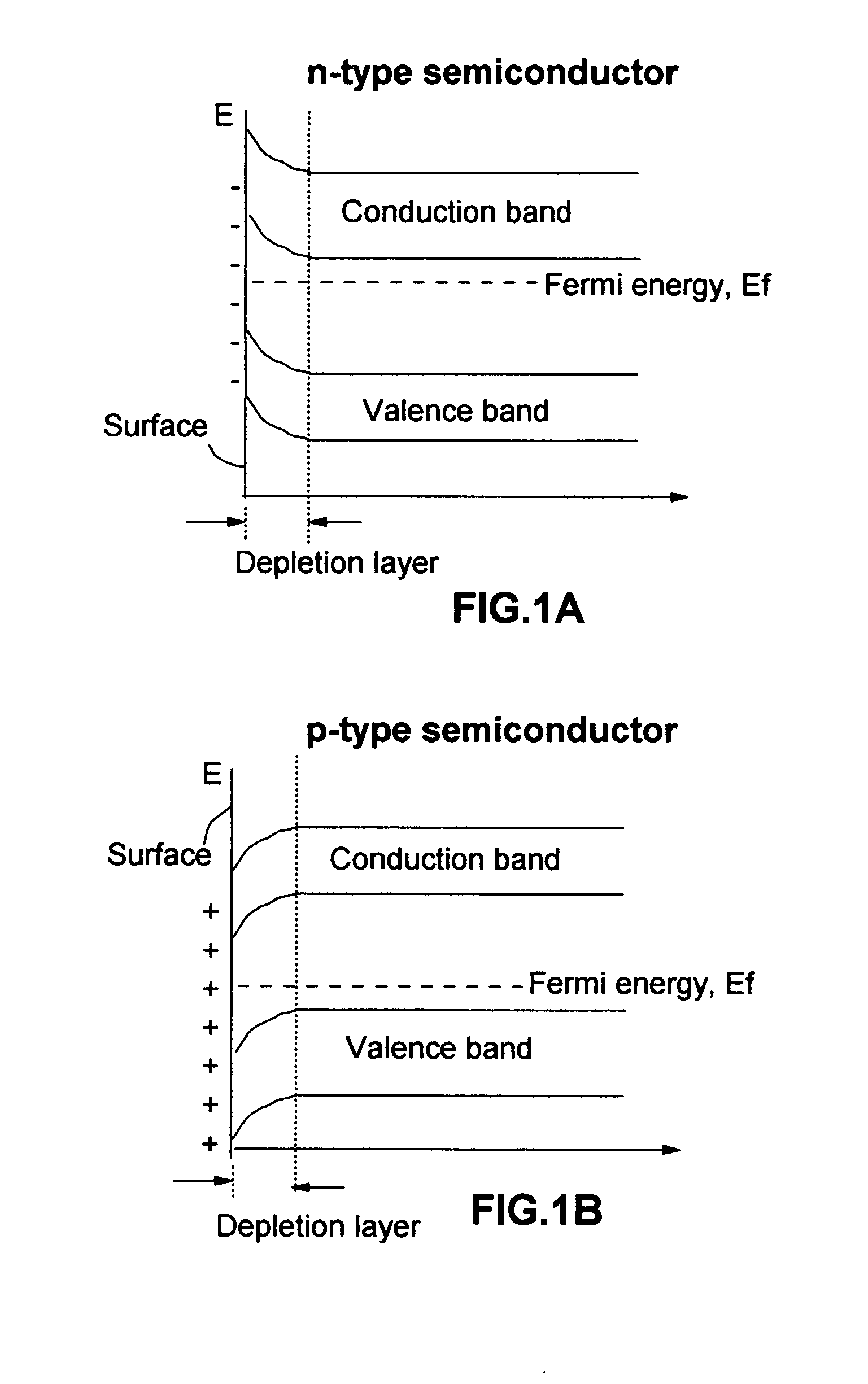
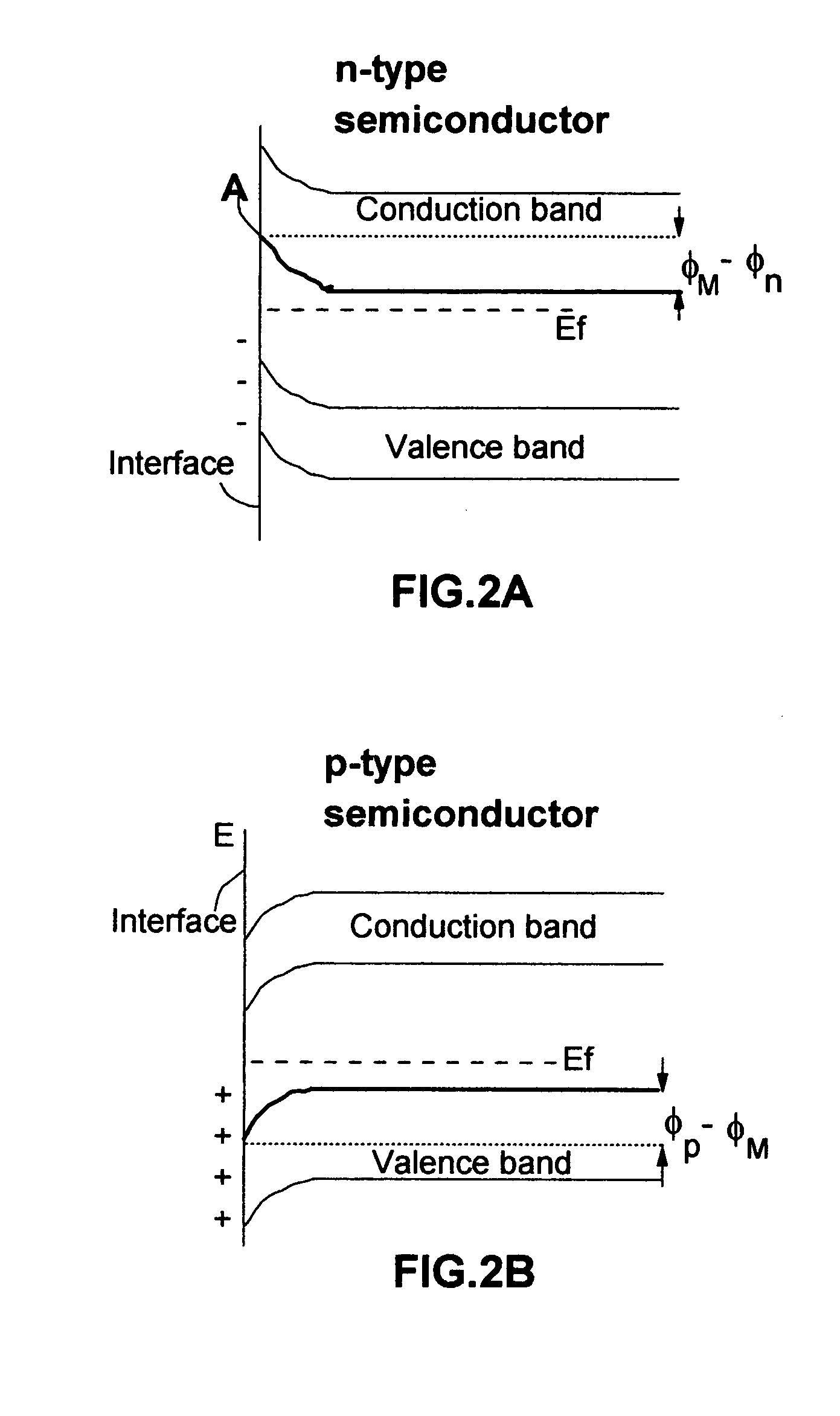
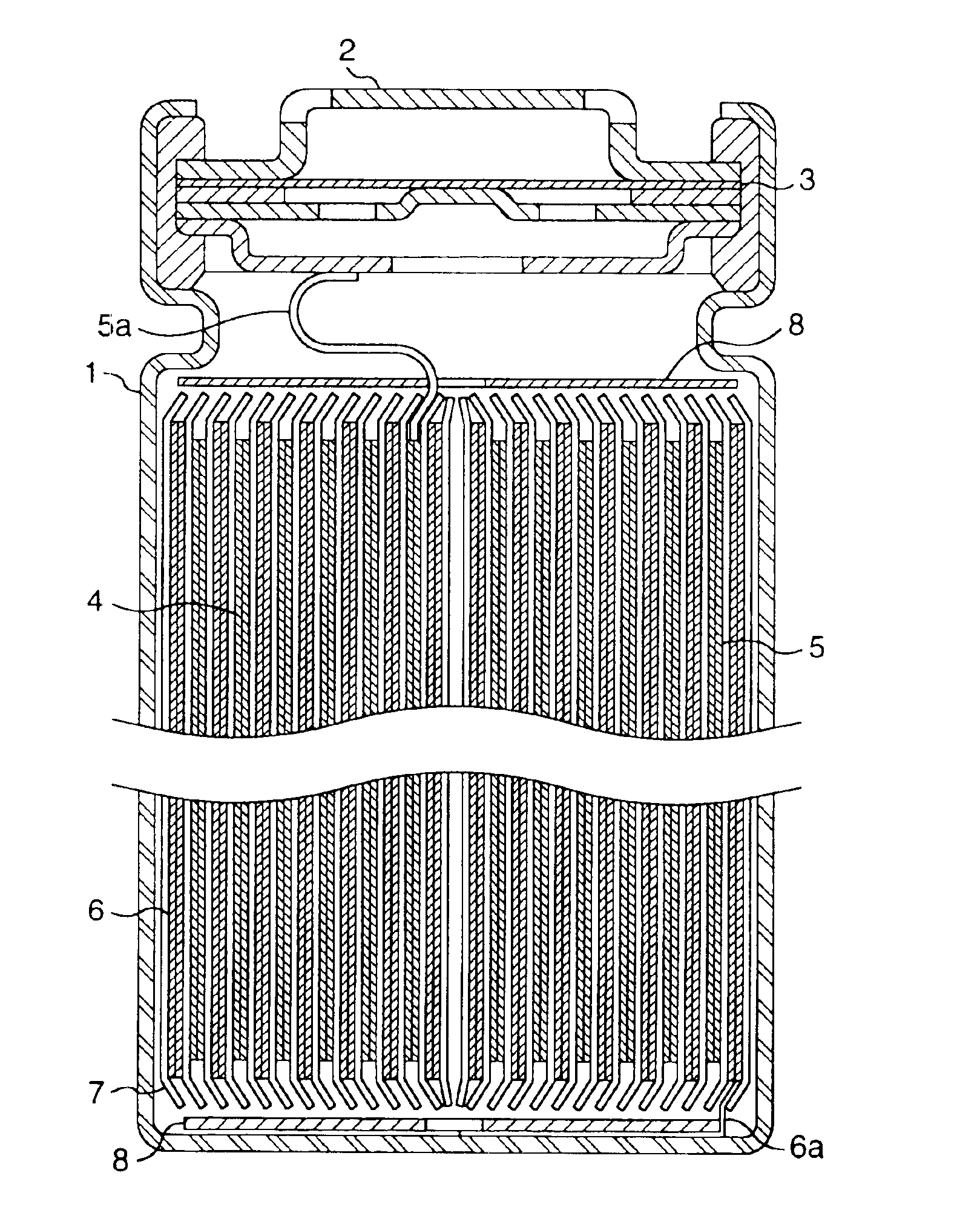
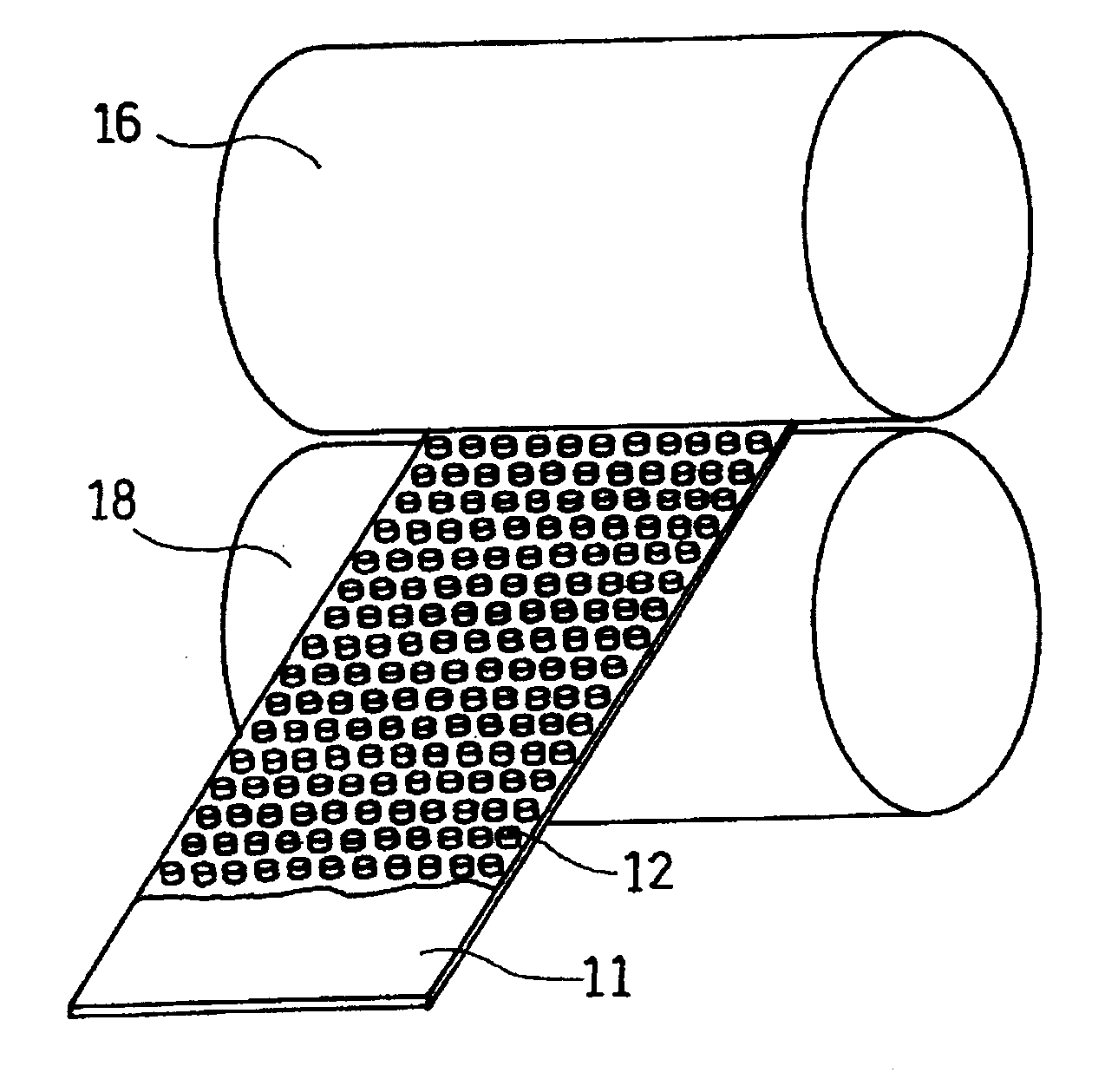
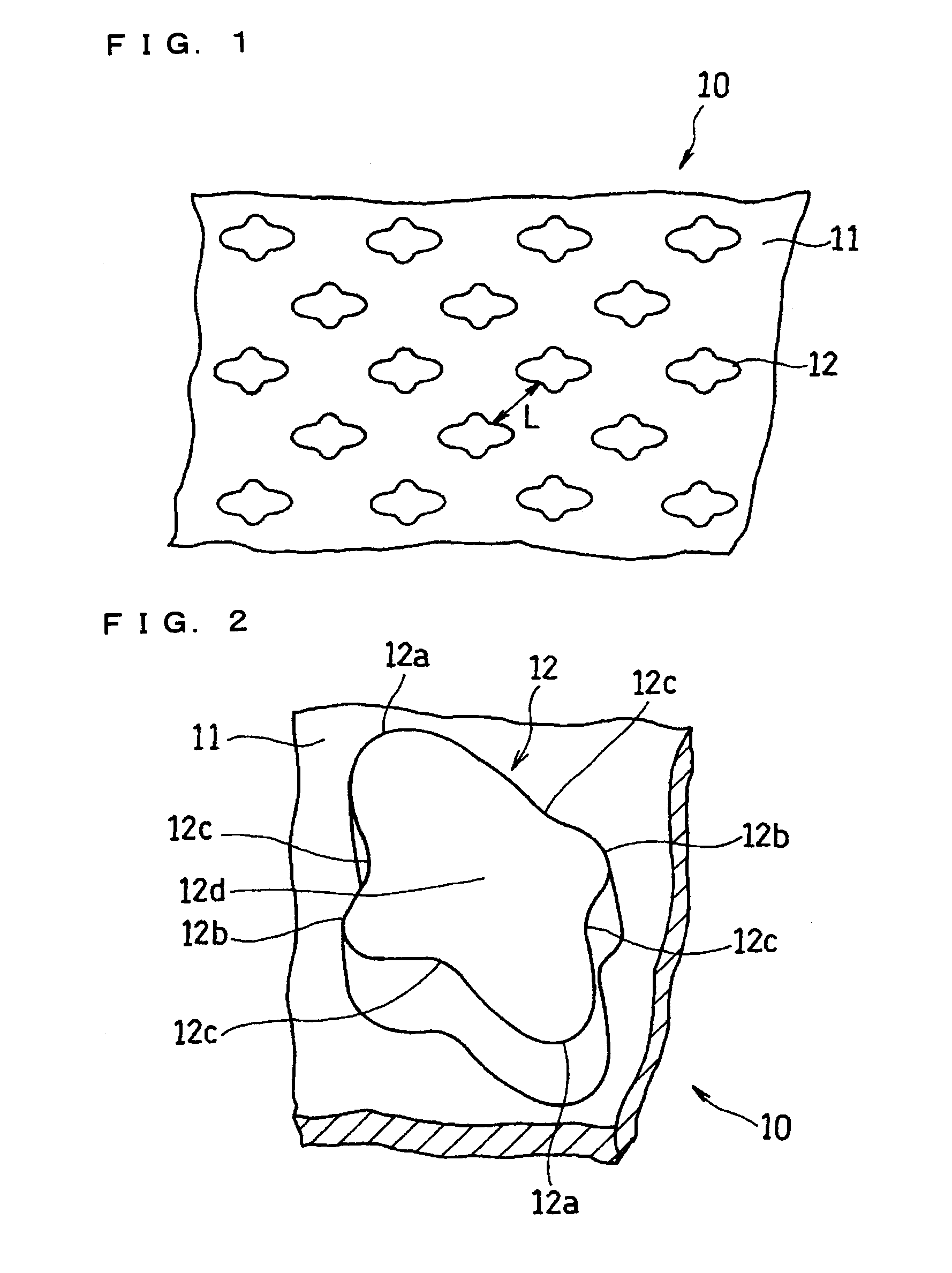
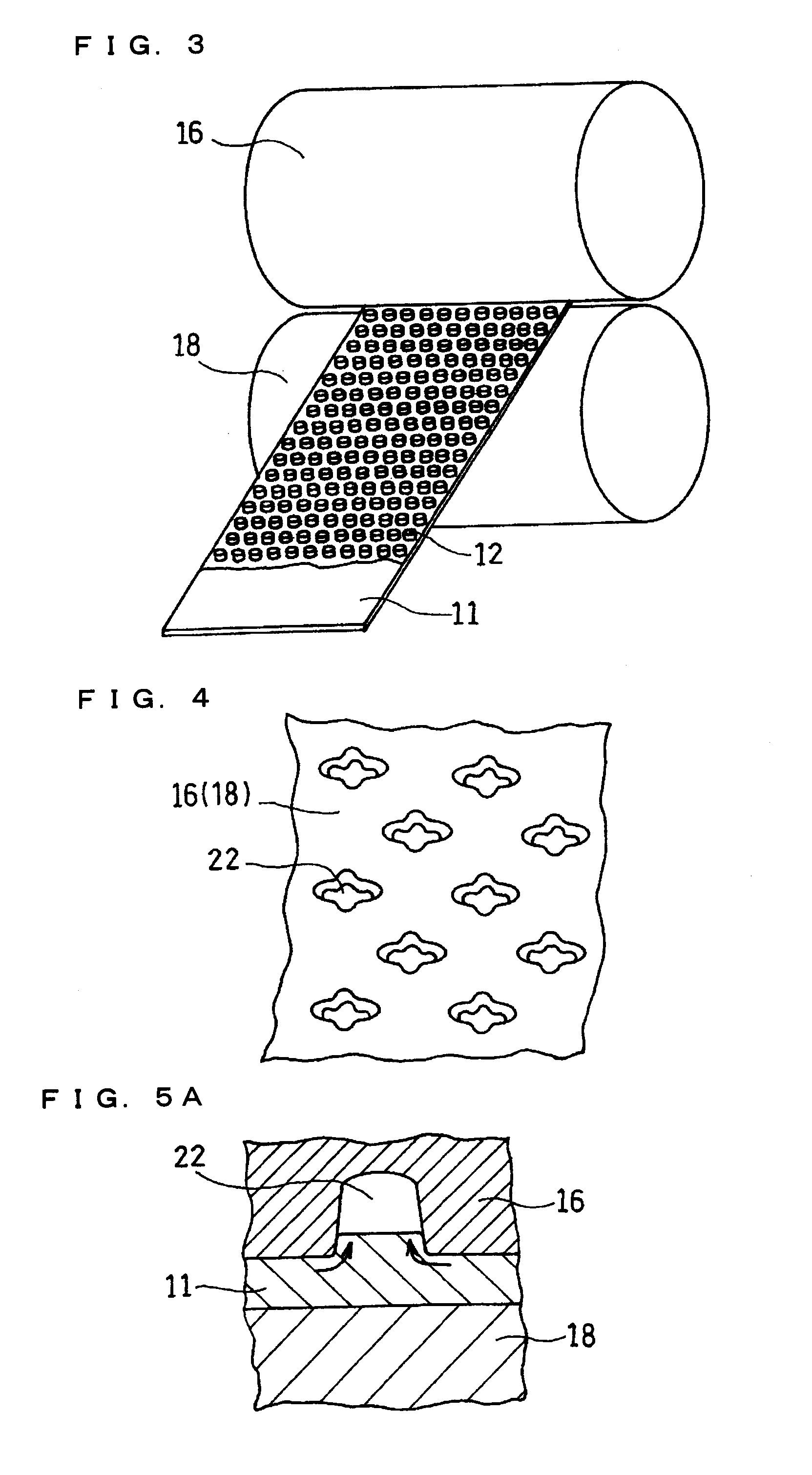
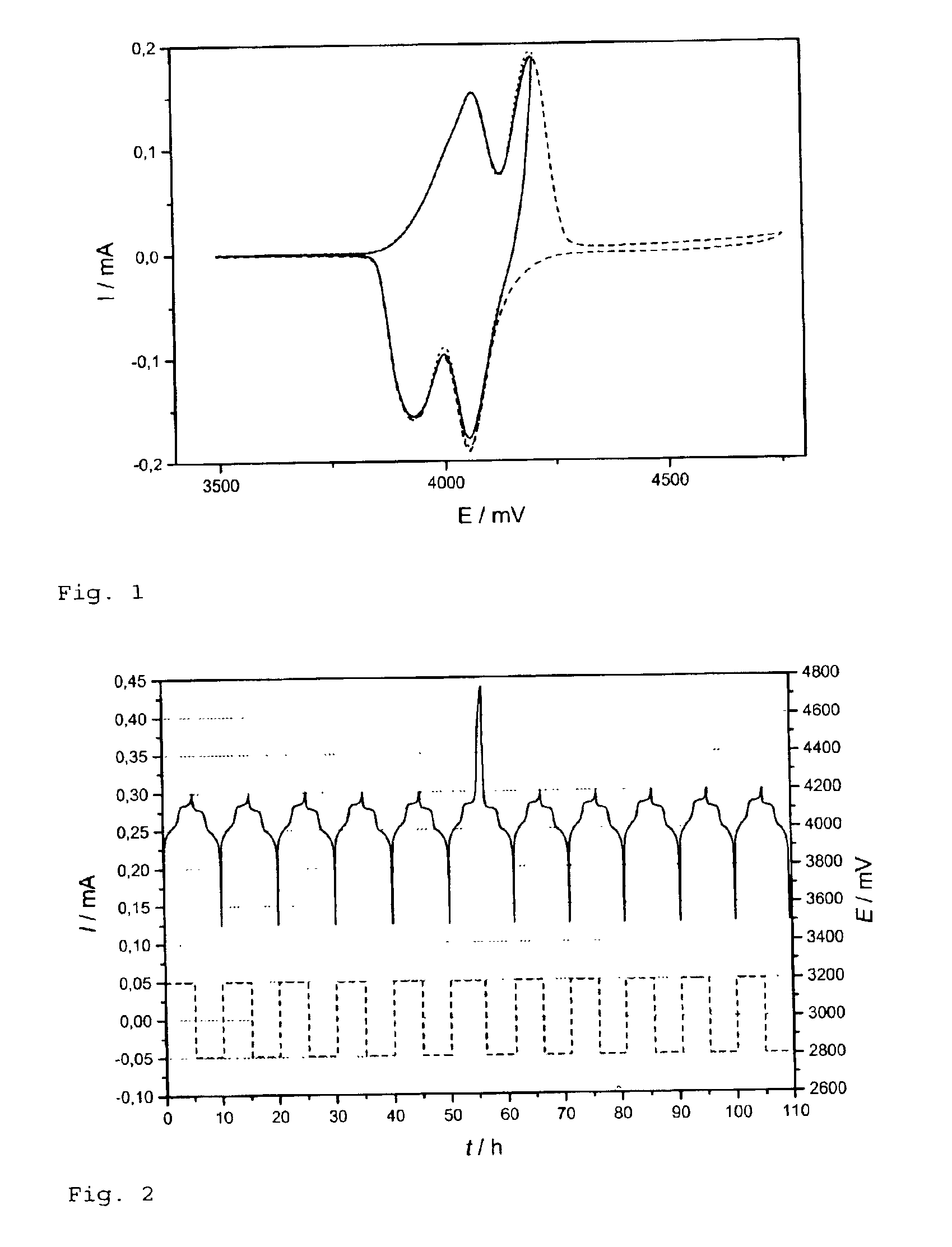
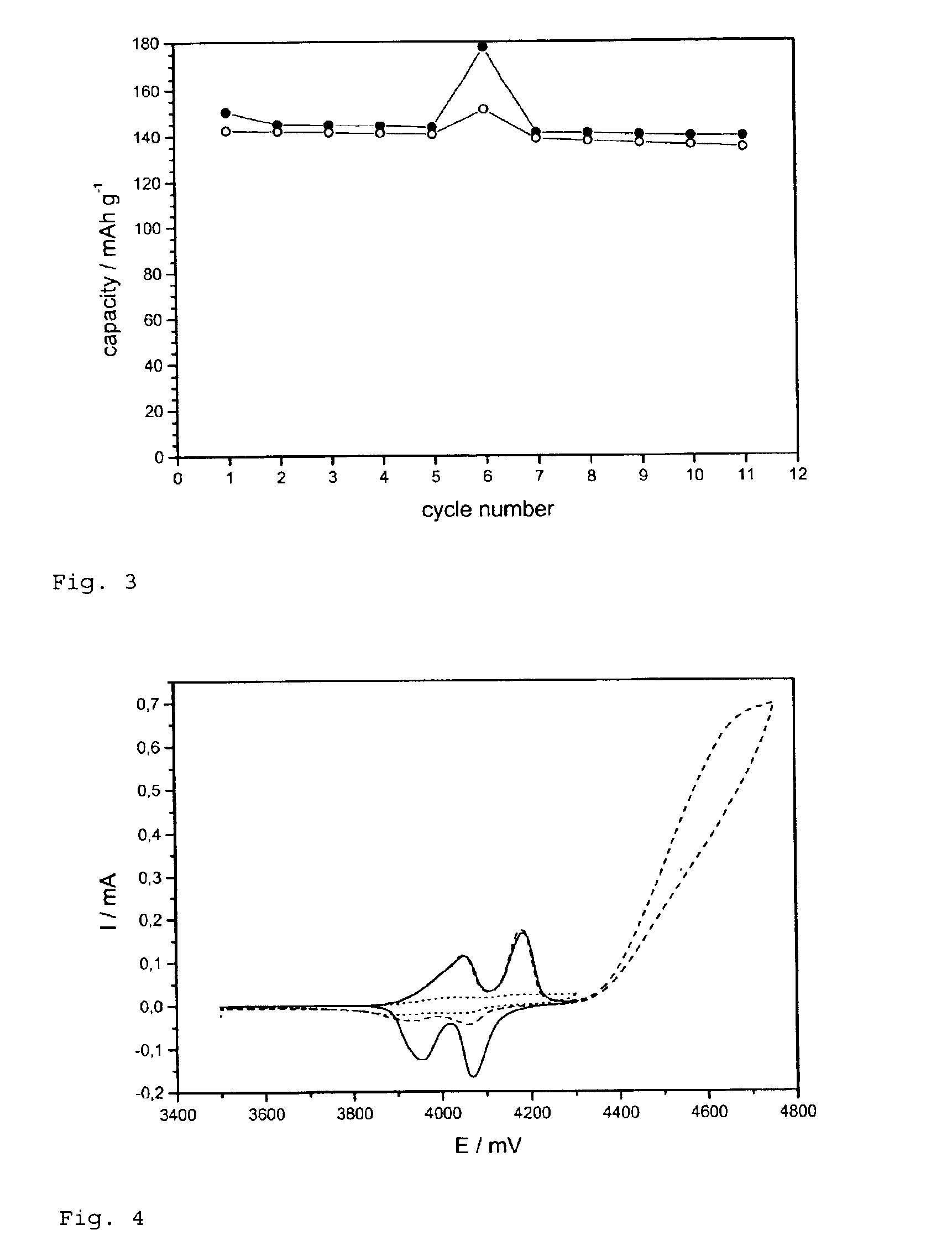
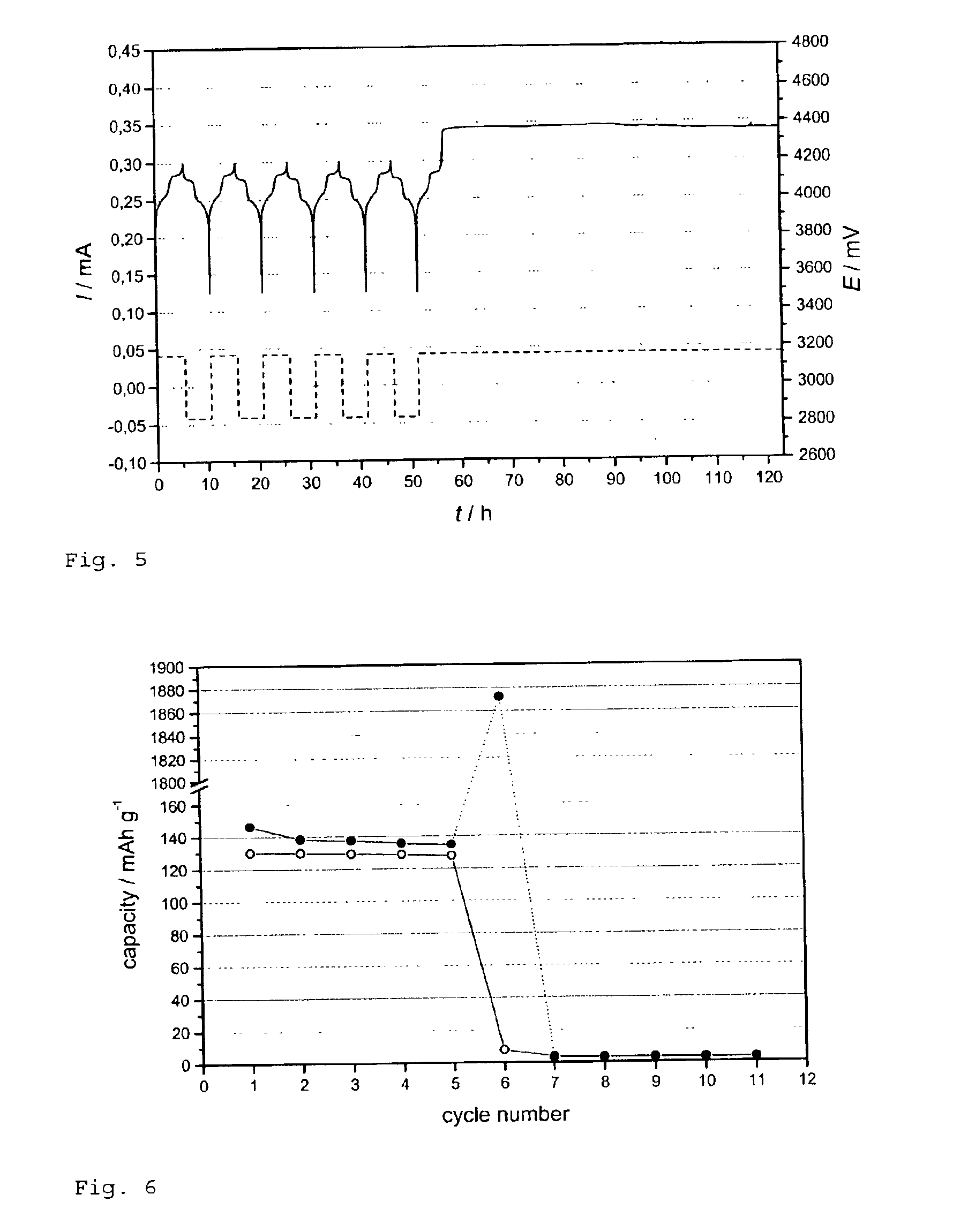
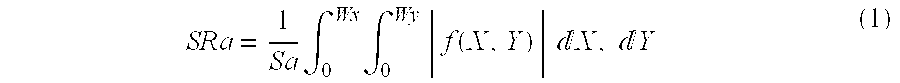Patents
Literature
2225 results about "Aqueous electrolyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
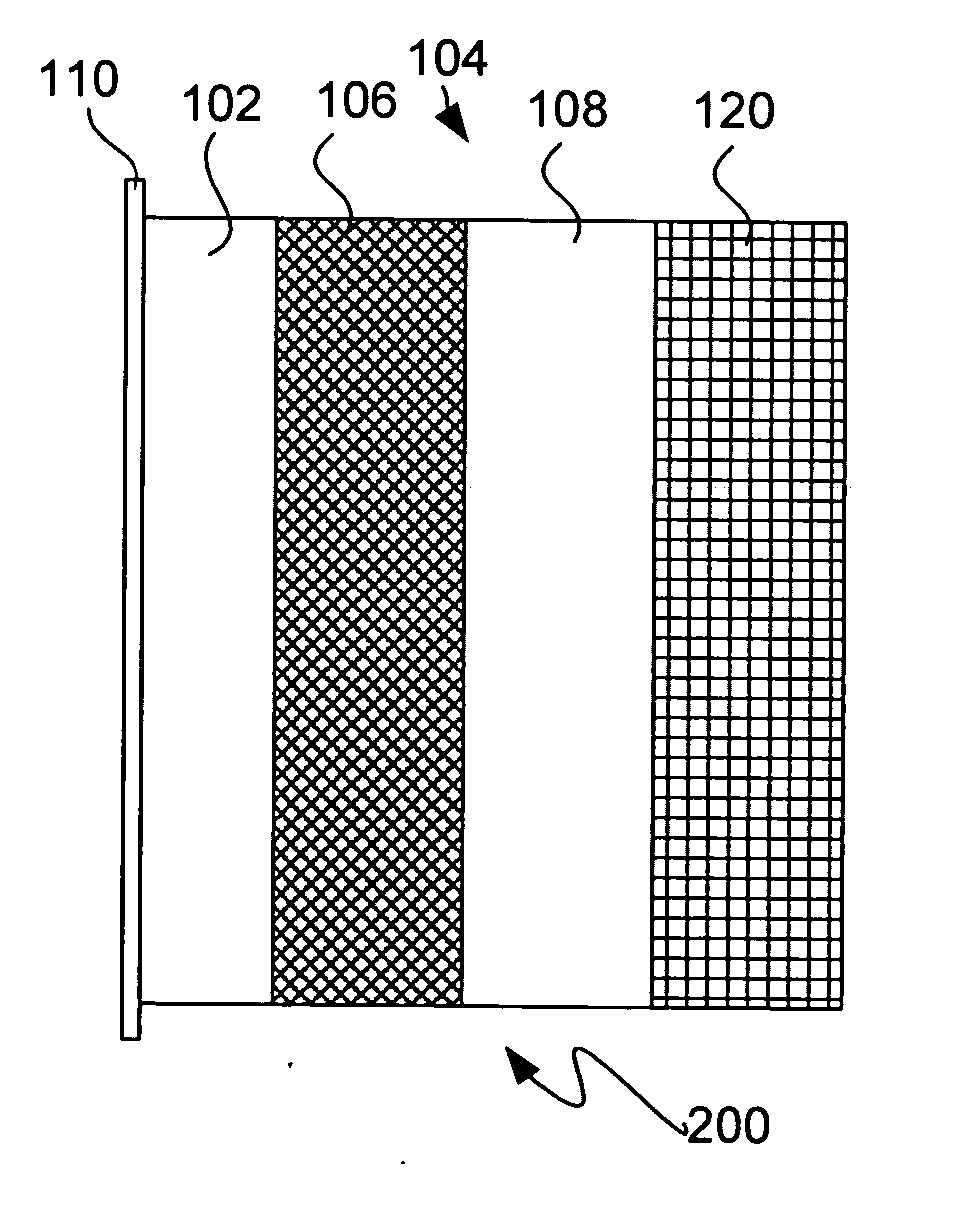
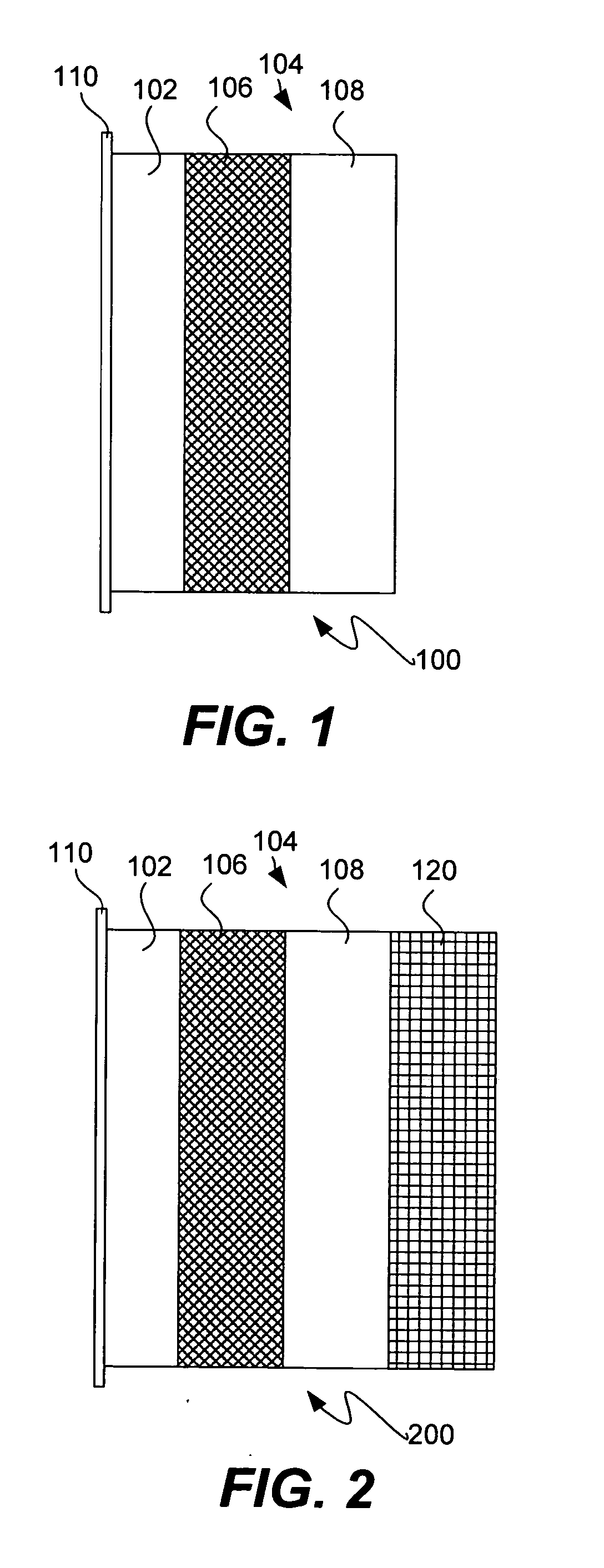
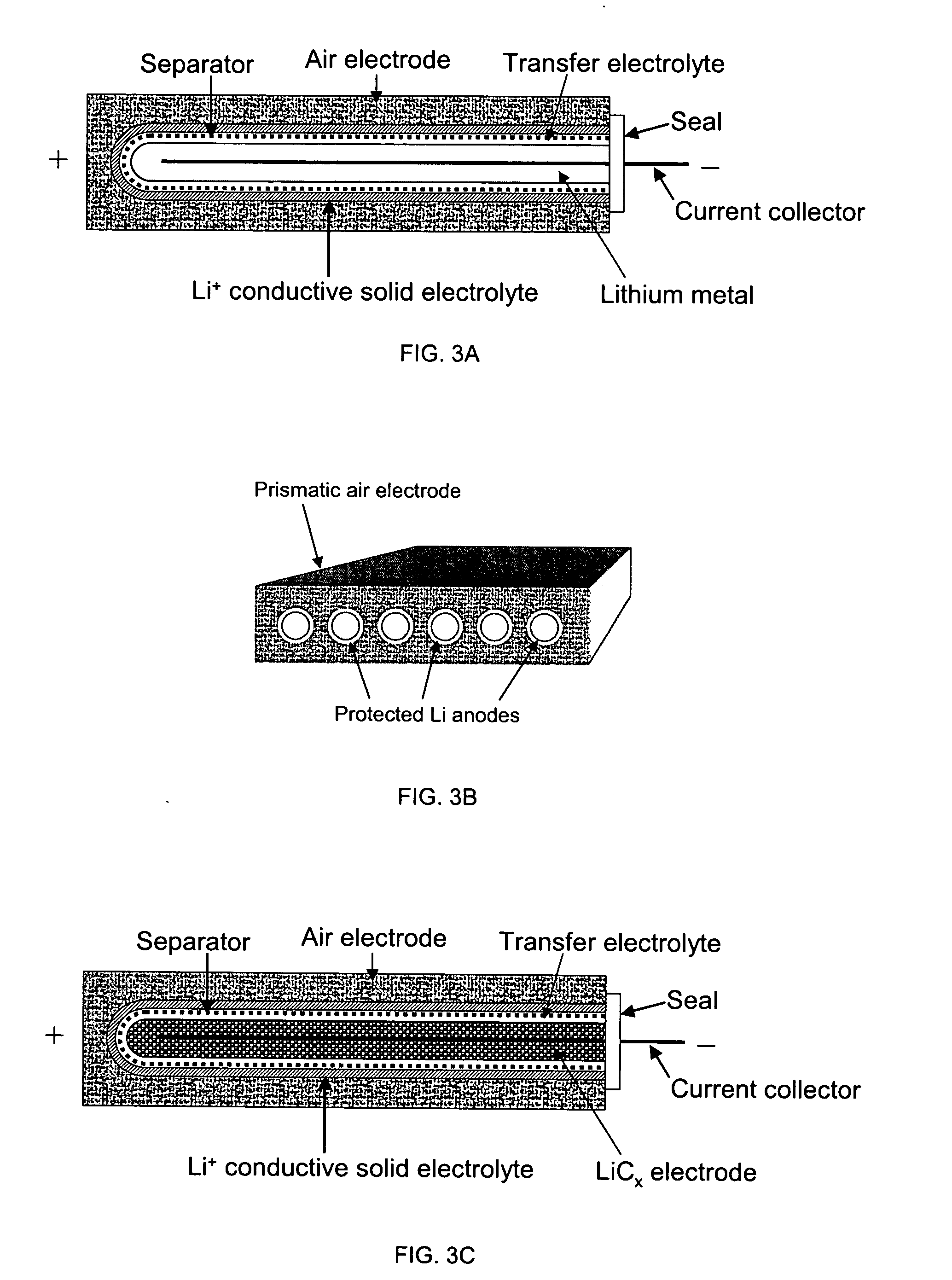
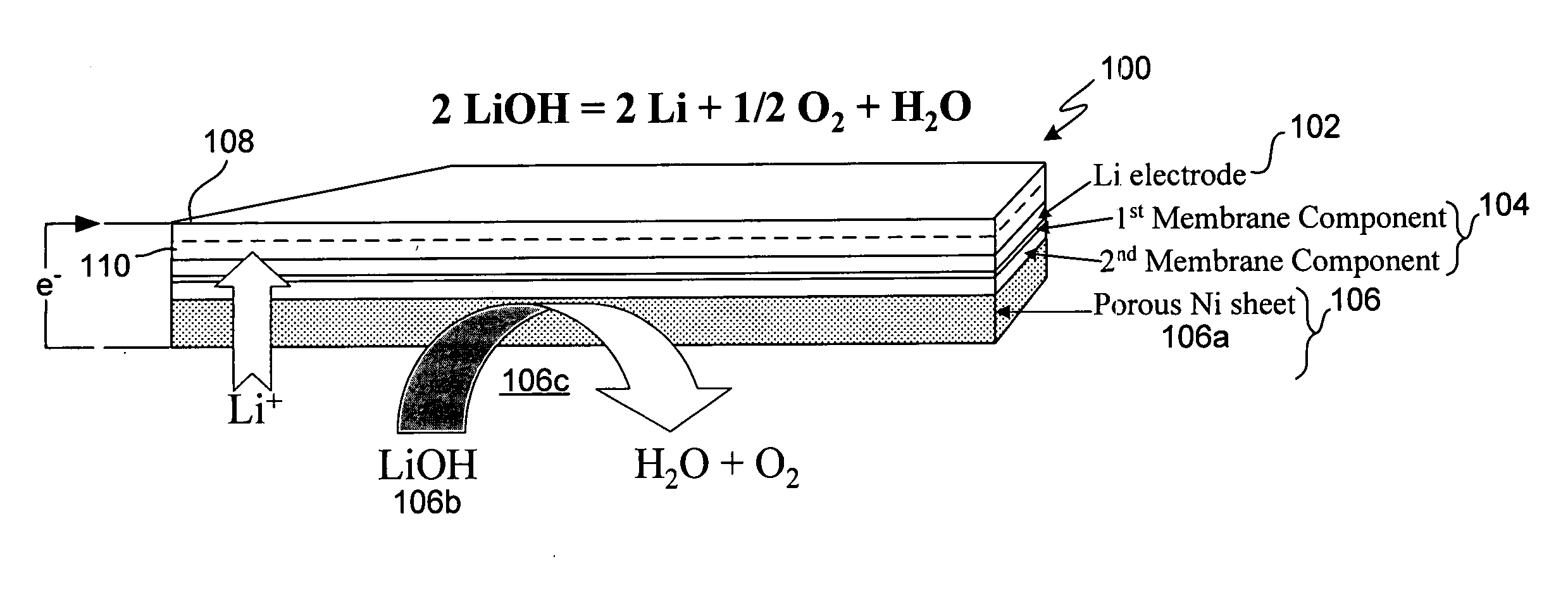
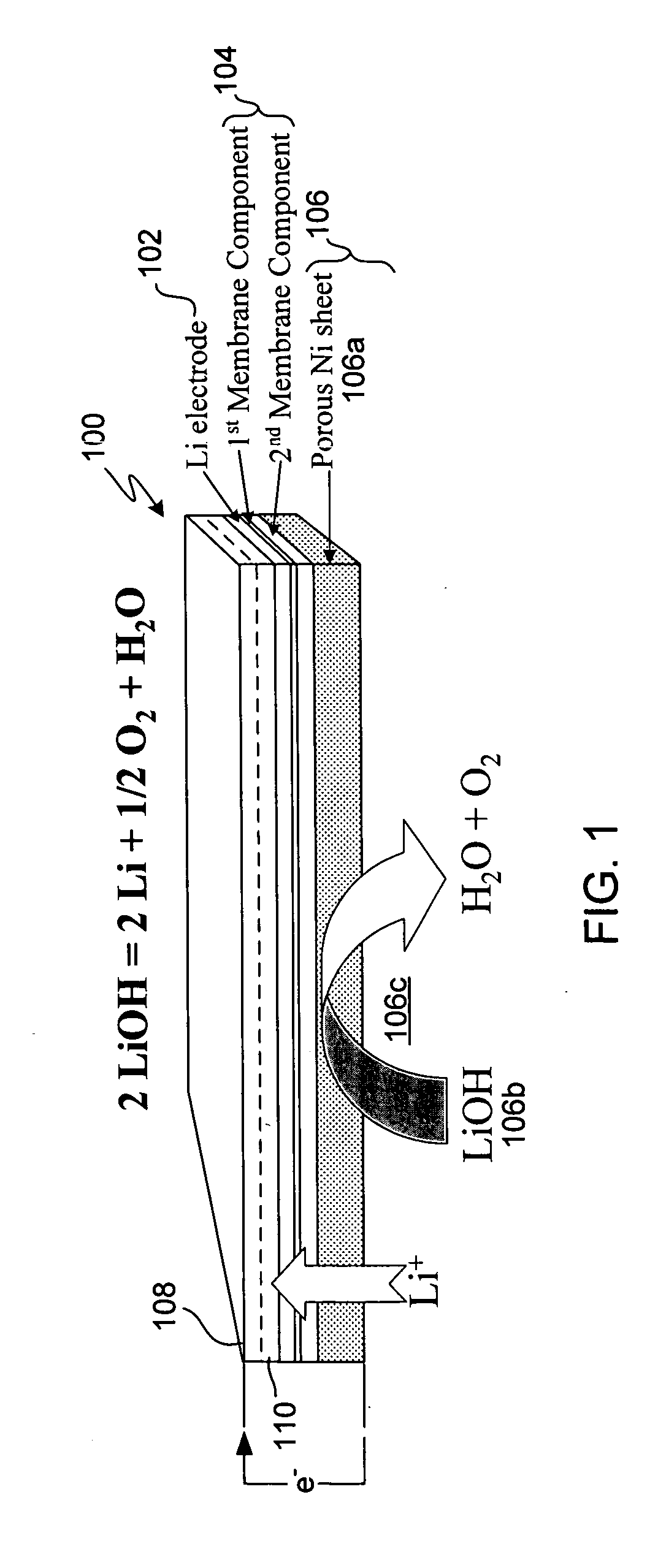
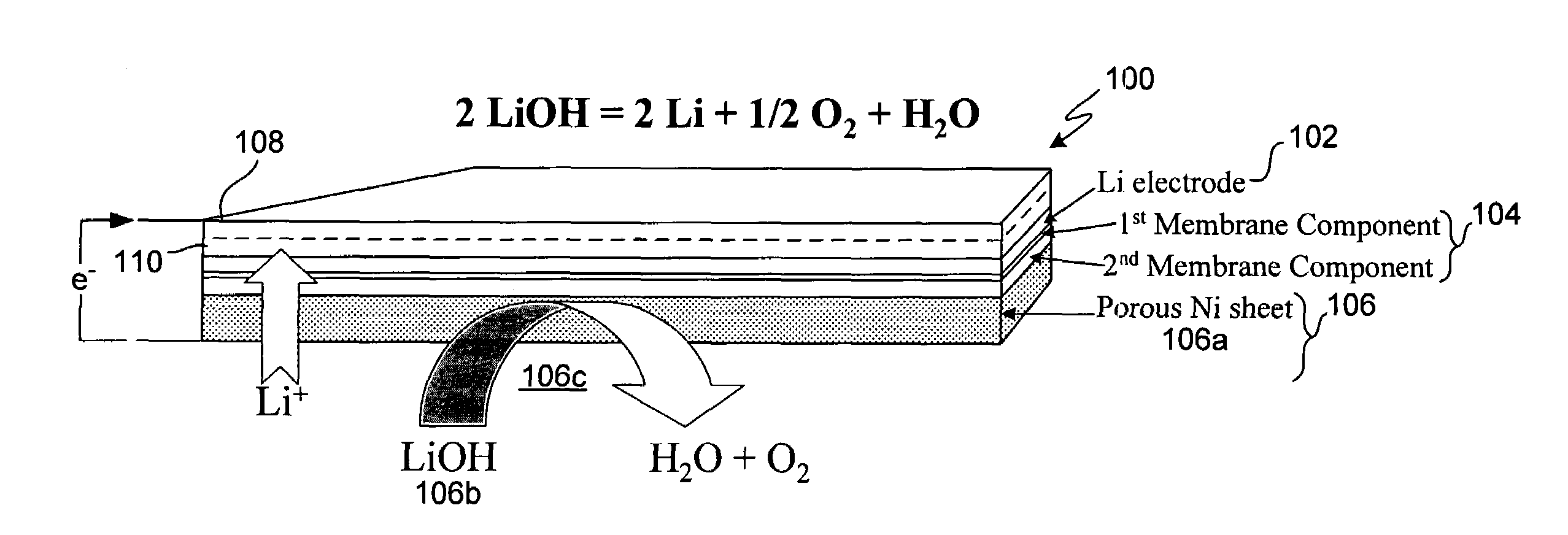
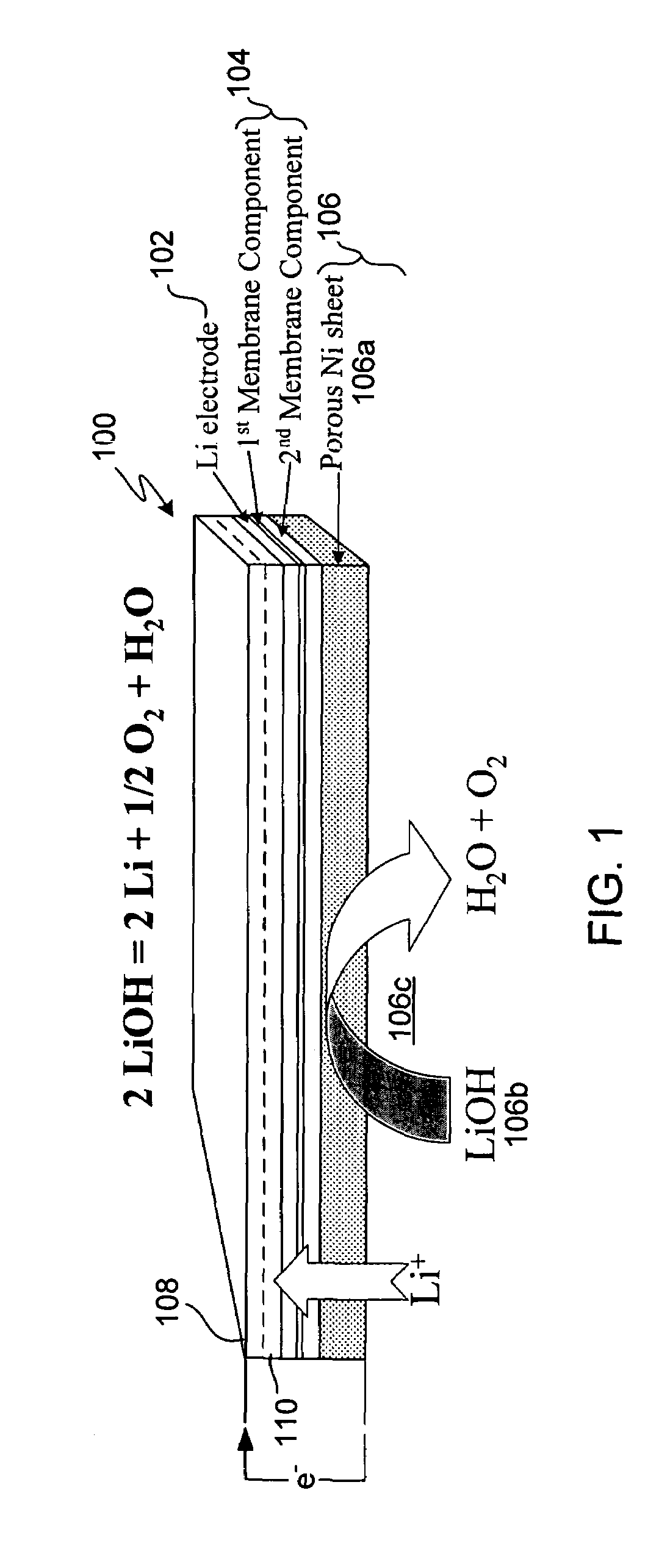
In a cell with an aqueous electrolyte the reduction at the cathode can also produce lithium hydroxide: An aqueous Li–air battery consists of a lithium metal anode, an aqueous electrolyte and a porous carbon cathode. The aqueous electrolyte combines lithium salts dissolved in water.
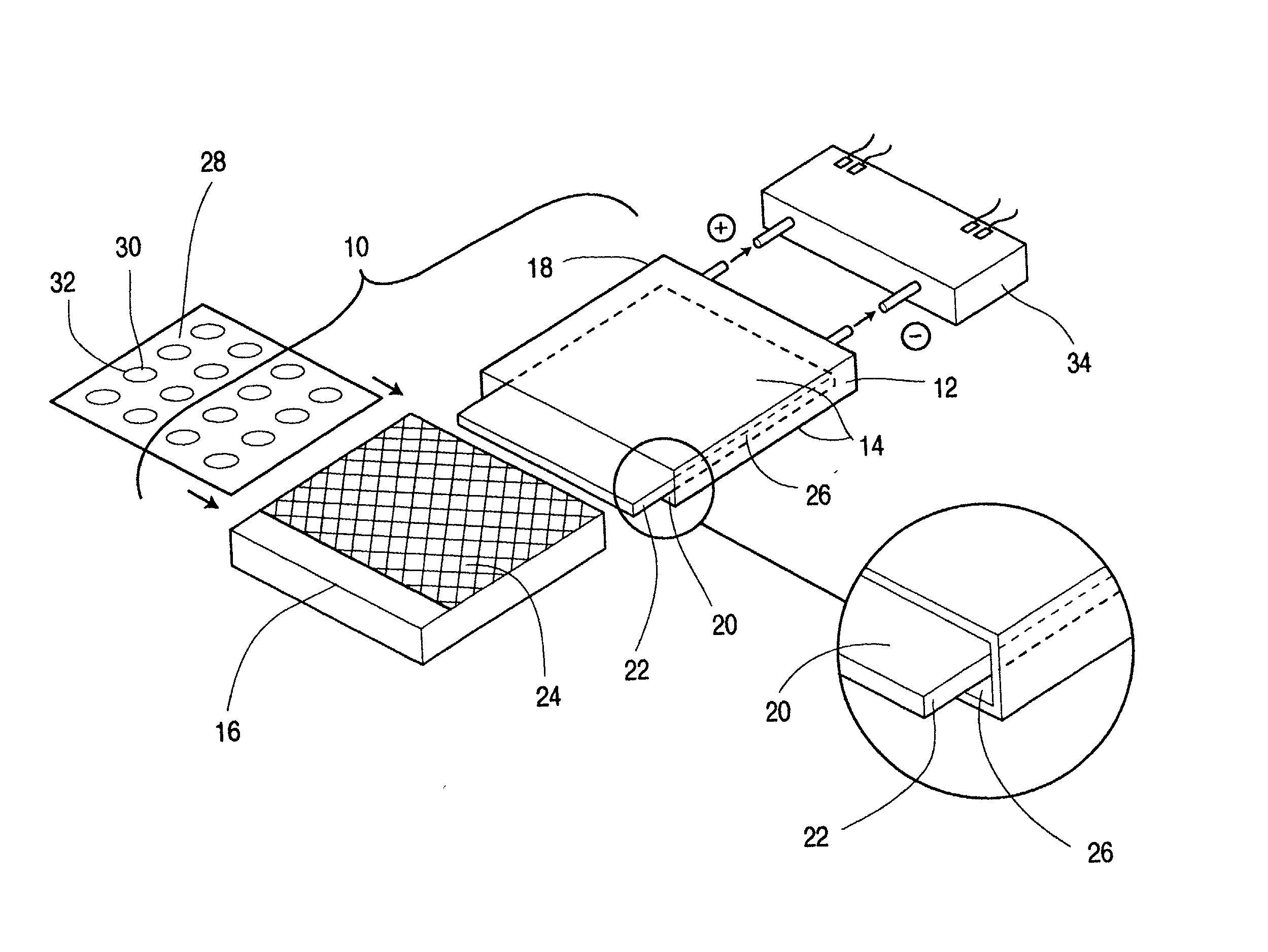
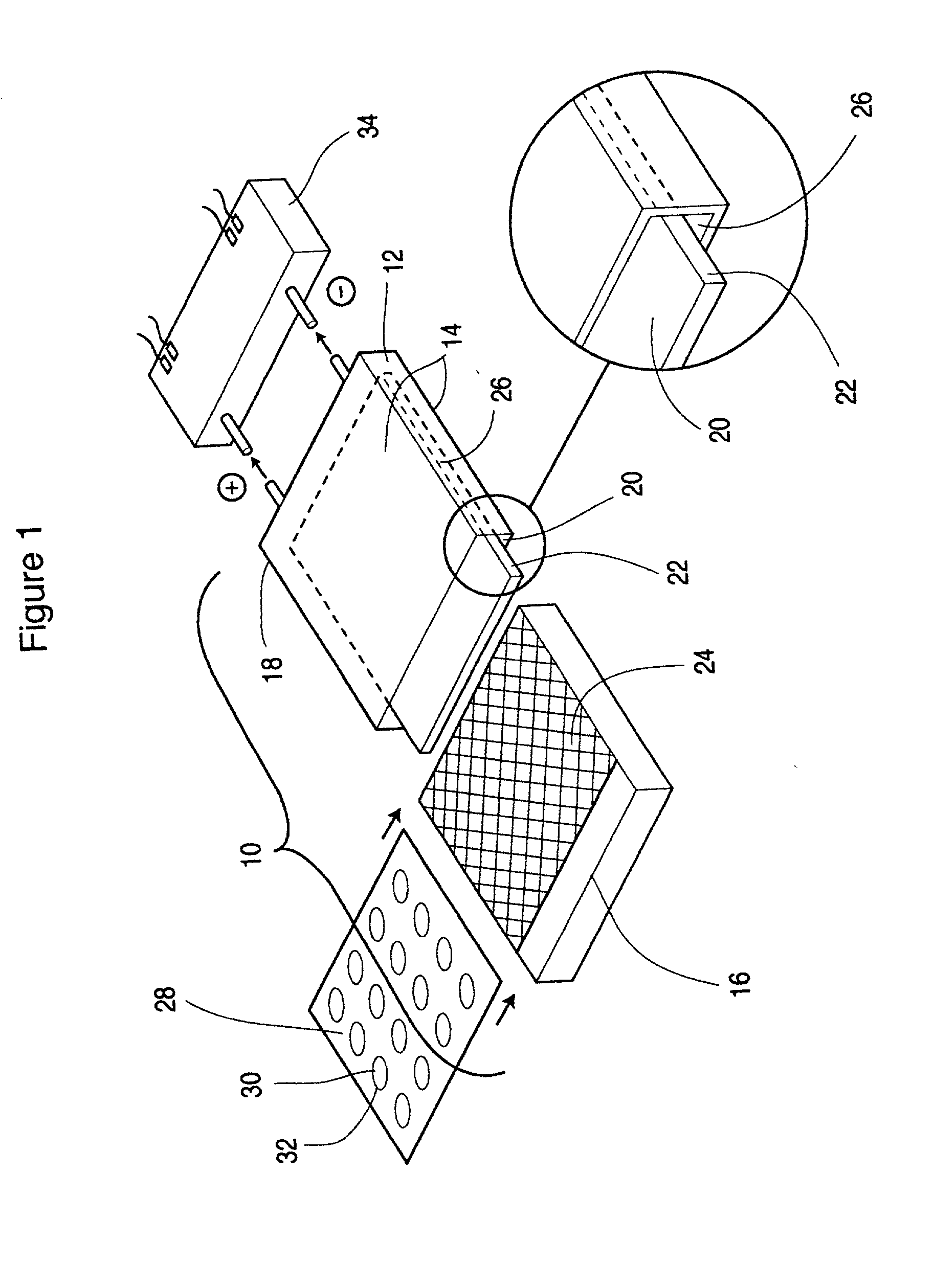
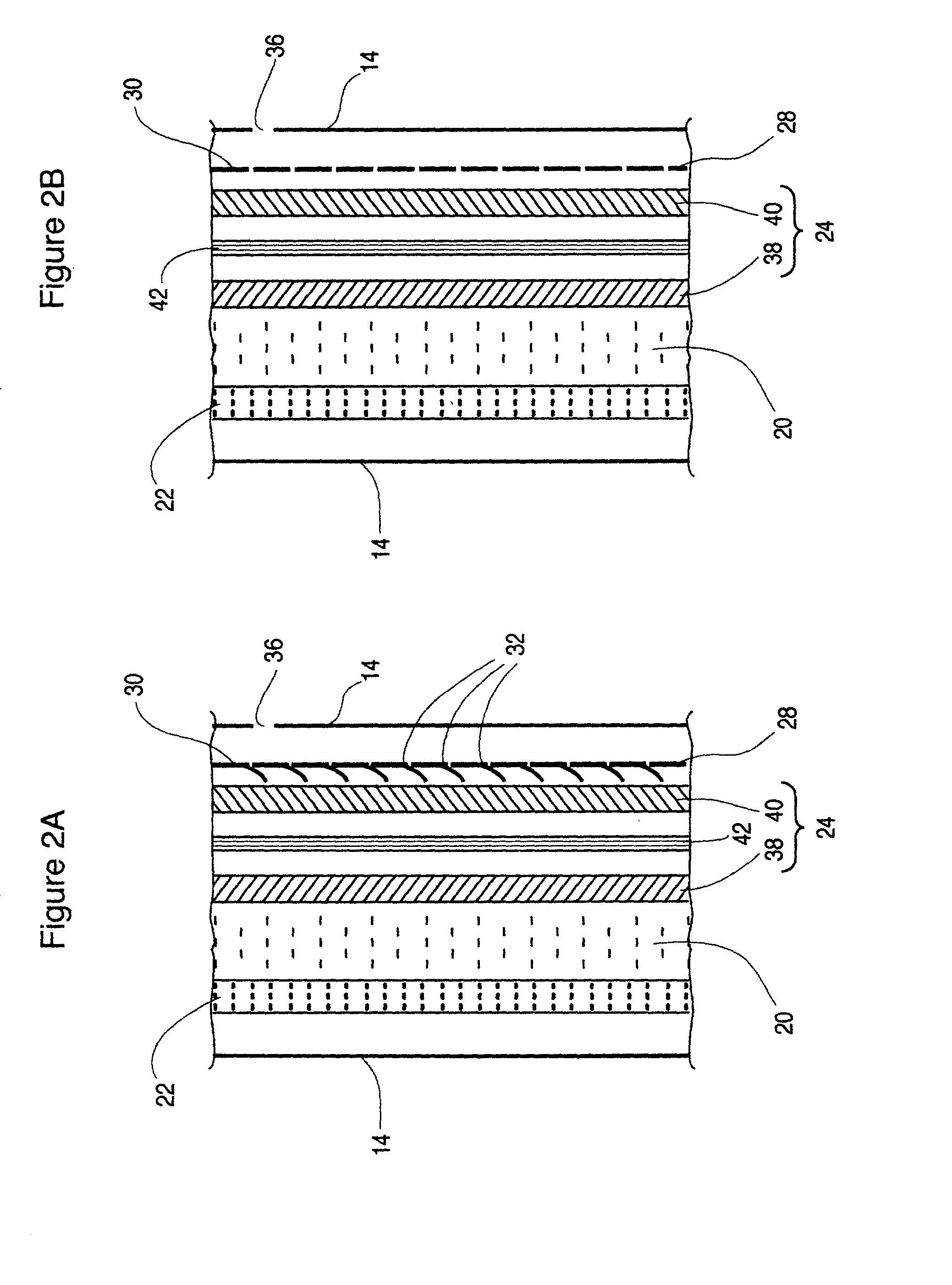
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS20050175894A1Avoid harmful reactionsHybrid capacitor separatorsHybrid capacitor electrolytesMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Non-aqueous electrolyte battery separator
InactiveUS6447958B1Improve securityHigh short-circuit temperatureFinal product manufactureLi-accumulatorsNitrogenPolymer
A non-aqueous electrolyte battery separator comprising a heat-resistant nitrogen-containing aromatic polymer and a ceramic powder.
Owner:SUMITOMO CHEM CO LTD
Rheology modifying copolymer composition
A rheology modifying copolymer composition containing a cross-linked copolymer of unsaturated carboxylic acid, a hydrophobic monomer, a hydrophobic chain transfer agent, a cross linking agent, and, optionally, a steric stabilizer, provides increased viscosity in aqueous electrolyte-containing environments.
Owner:NOVEON IP HLDG CORP
Active metal electrolyzer
InactiveUS20050100793A1Effective isolationReduce environmental pollutionPhotography auxillary processesElectrode manufacturing processesElectrolysisAqueous electrolyte
Electro-winning of active metal (e.g., lithium) ions from a variety of sources including industrial waste, and recycled lithium and lithium-ion batteries is accomplished with an electrolyzer having a protected cathode that is stable against aggressive solvents, including water, aqueous electrolytes, acid, base, and a broad range of protic and aprotic solvents. The electrolyzer has a highly ionically conductive protective membrane adjacent to the alkali metal cathode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the cathode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or anode material in conjunction with the cathode. The electrolyzer can be configured and operated to claim or reclaim lithium or other active metals from such sources.
Owner:POLYPLUS BATTERY CO INC
Sodium ion based aqueous electrolyte electrochemical secondary energy storage device
InactiveUS20090253025A1Preserve electroneutralityHybrid capacitor electrolytesCapacitor and primary/secondary cellsSODIUM CATIONAqueous electrolyte
A secondary hybrid aqueous energy storage device includes an anode electrode, a cathode electrode which is capable of reversibly intercalating sodium cations, a separator, and a sodium cation containing aqueous electrolyte, wherein an initial active cathode electrode material comprises an alkali metal containing active cathode electrode material which deintercalates alkali metal ions during initial charging of the device.
Owner:CARNEGIE MELLON UNIV
Metal gas batteries
InactiveUS20030049508A1Reduce passageEffectively block and closeFuel and primary cellsPrimary cell maintainance/servicingLiquid waterWater vapor
An improved gas-diffusion cathode for use in an electrochemical cell comprising an electrically conductive cathode member having a first side communicable with an aqueous electrolyte and a second side communicable with a gaseous medium; and a water-impermeable membrane adjacent said cathode member second side to reduce passage of liquid water between said cathode member and said gaseous medium and having a membrane first side and a membrane second side wherein said membrane first side faces said cathode member and wherein said water-impermeable membrane comprises one or more portions defining one or more openable and closeable apertures the improvement wherein said apertures are associated with one or more integrally-formed resiliently flexible flaps on said membrane first side to effect said opening and closing. The batteries have reduced unwanted water vapour ingress and egress characteristics in its no-load mode.
Owner:ALUMINUM POWER
Electrically rechargeable, metal-air battery systems and methods
InactiveUS20120021303A1Solution to short lifeAffordable and practicalElectrolyte moving arrangementsFuel and secondary cellsElectricityElectrical battery
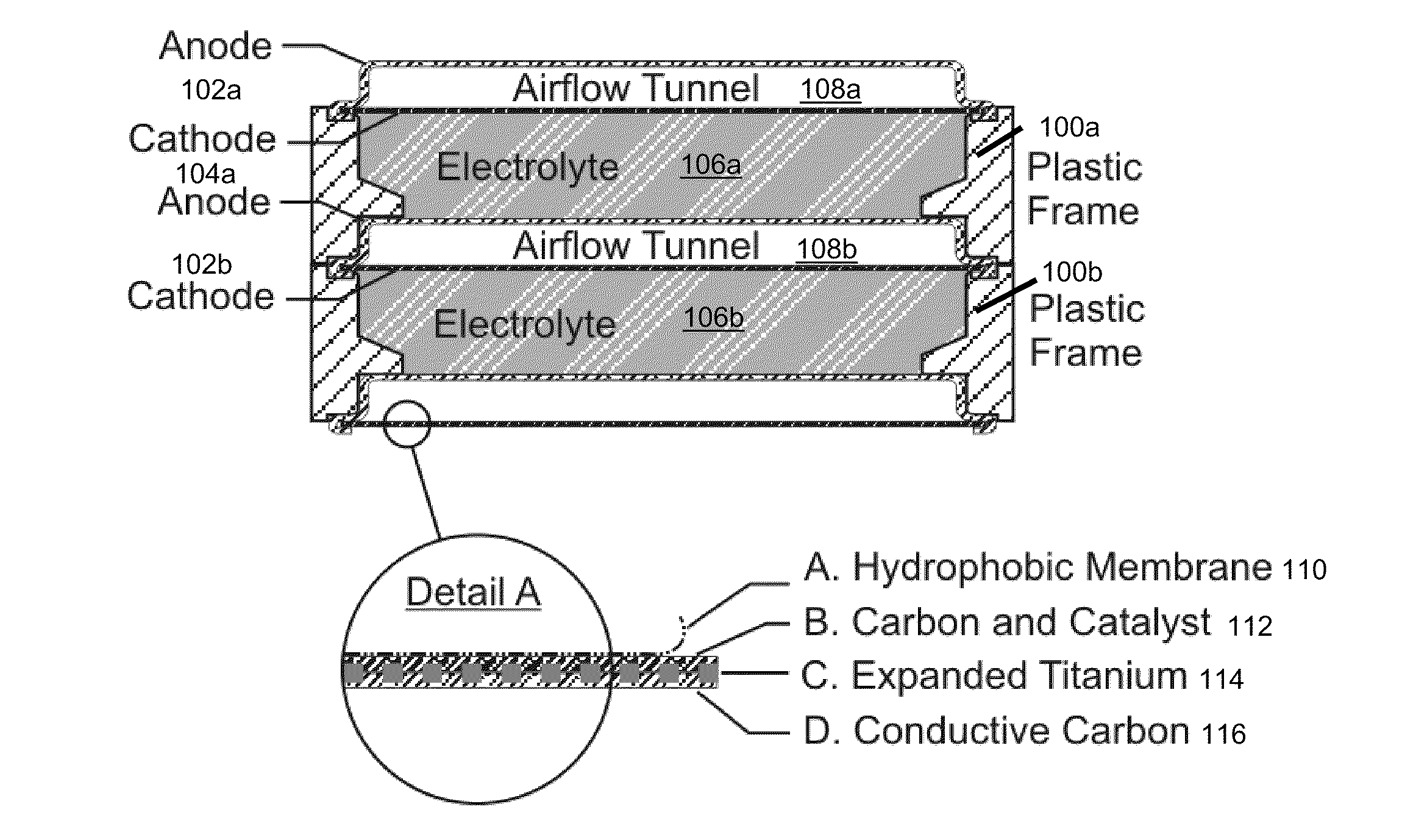
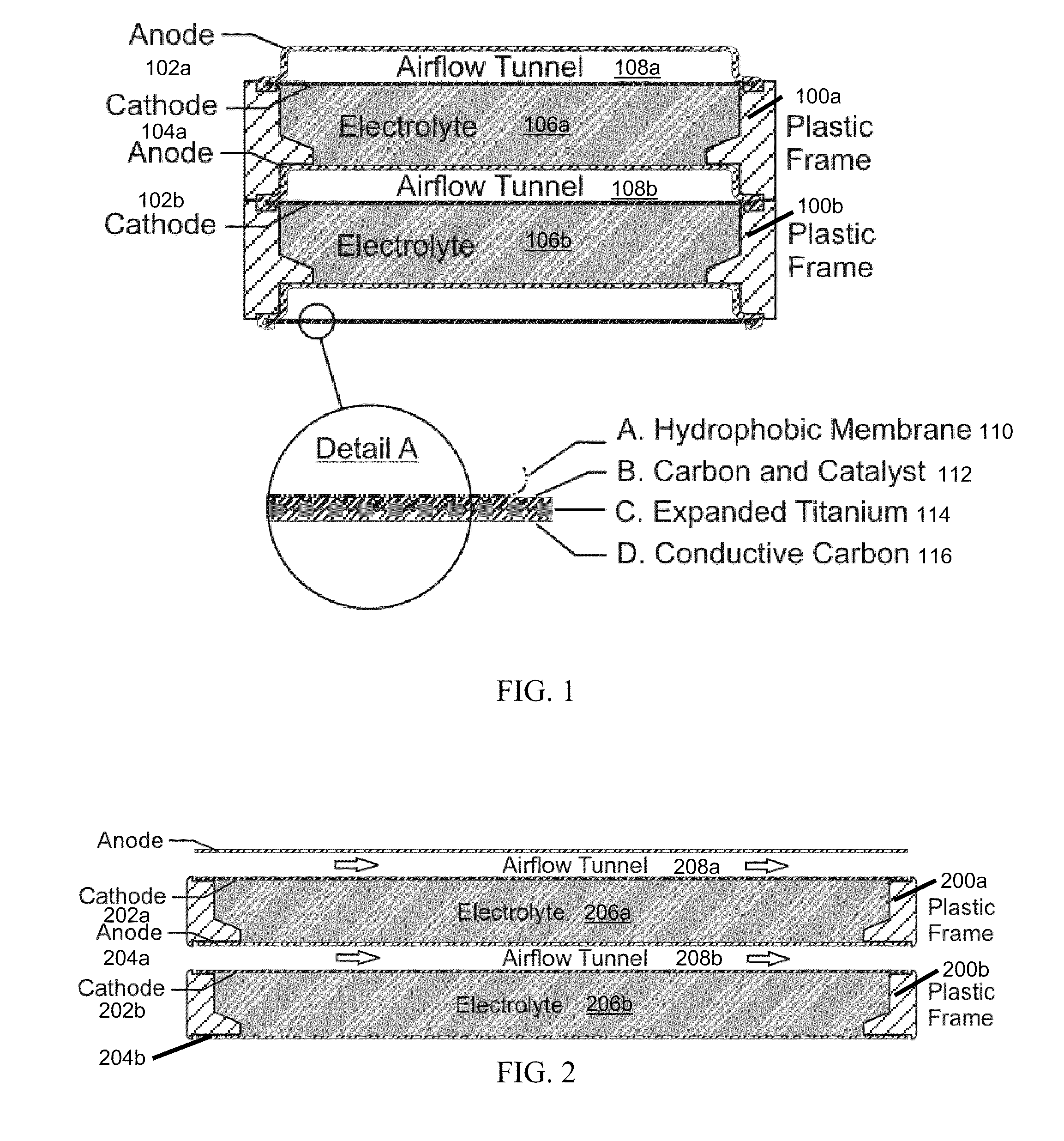
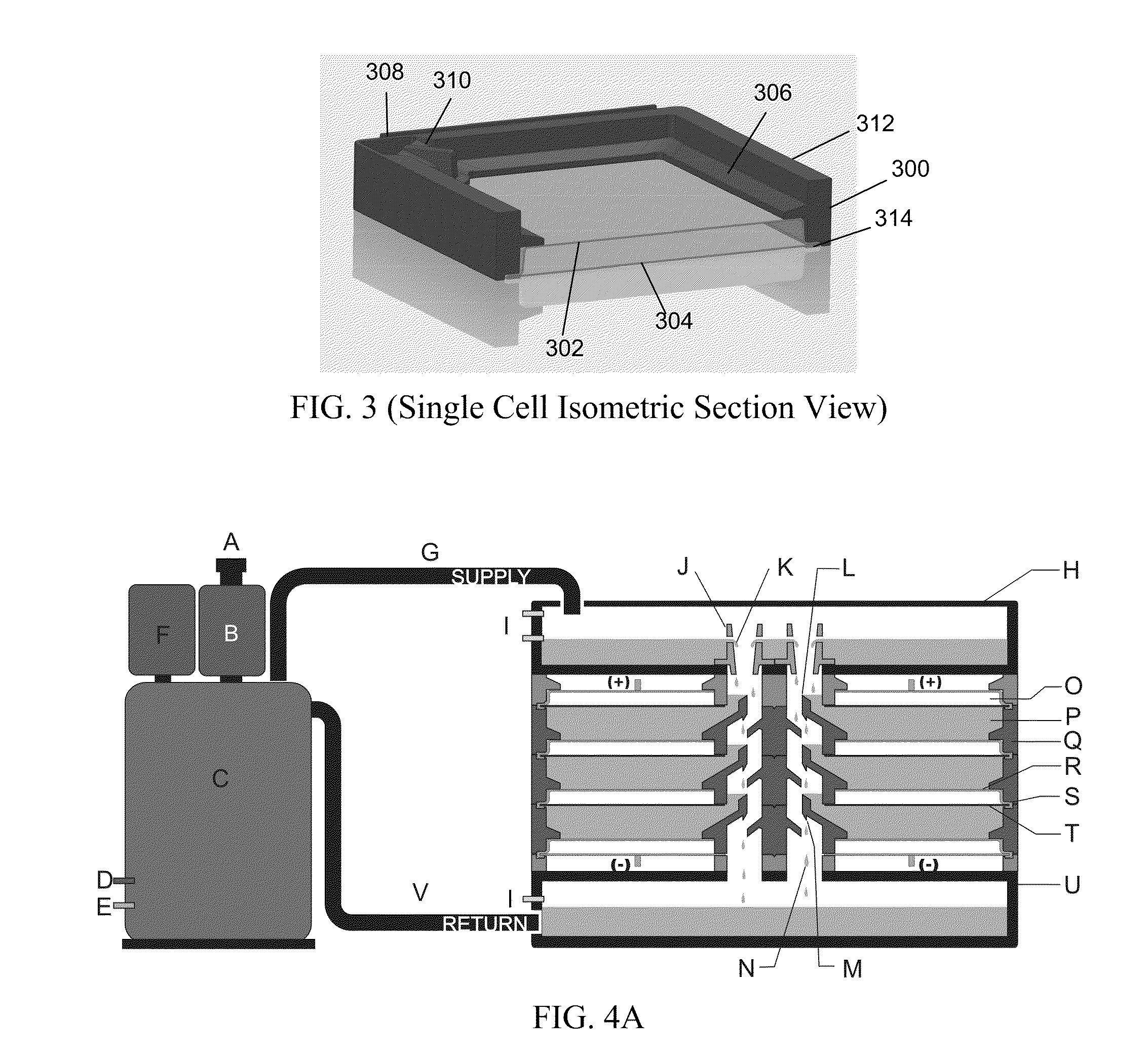
The invention provides for a fully electrically rechargeable metal-air battery systems and methods of achieving such systems. A rechargeable metal air battery cell may comprise a metal electrode an air electrode, and an aqueous electrolyte separating the metal electrode and the air electrode. In some embodiments, the metal electrode may directly contact the electrolyte and no separator or porous membrane need be provided between the air electrode and the electrolyte. Rechargeable metal air battery cells may be electrically connected to one another through a centrode connection between a metal electrode of a first battery cell and an air electrode of a second battery cell. Air tunnels may be provided between individual metal air battery cells. In some embodiments, an electrolyte flow management system may be provided.
Owner:EOS ENERGY STORAGE
Nonwoven fabric for separator of non-aqueous electrolyte battery and non-aqueous electrolyte battery using the same
InactiveUS6200706B1Uniform and efficient productionImprove various performanceOrganic electrolyte cellsPaper/cardboardSurface roughnessEngineering
The object of the present invention is to provide a nonwoven fabric for separators of non-aqueous electrolyte batteries which is superior in adhesion to electrodes, causes no breakage of the separator and neither slippage nor space between electrode and the separator at the time of fabrication of battery, provides superior battery processability such as rollability with electrodes, causes no internal short-circuit due to contact between electrodes caused by shrinking or burning of the nonwoven fabric even when electrodes generate heat owing to external short-circuit, whereby ignition of the battery can be inhibited, has no pin holes and is superior in retention of electrolyte and penetration of electrolyte, and which can give non-aqueous electrolyte batteries superior in capacity, battery characteristics and battery storage characteristics. Specifically, the nonwoven fabric for separators of non-aqueous electrolyte batteries according to the present invention has a thickness non-uniformity index (Rpy) of 1000 mV or less or a center surface average roughness SRa of 6 mum or less in whole wavelength region as measured using a tracer method three-dimensional surface roughness meter.
Owner:MITSUBISHI PAPER MILLS LTD
Steam-generating warming article
InactiveUS7652228B2Exothermal chemical reaction heat productionOther chemical processesFiberEngineering
Owner:KAO CORP
Hydrogen generator for uses in a vehicle fuel system
InactiveUS6866756B2Increase electrode surface areaSimple designCellsPhotography auxillary processesHydrogenElectrolysis
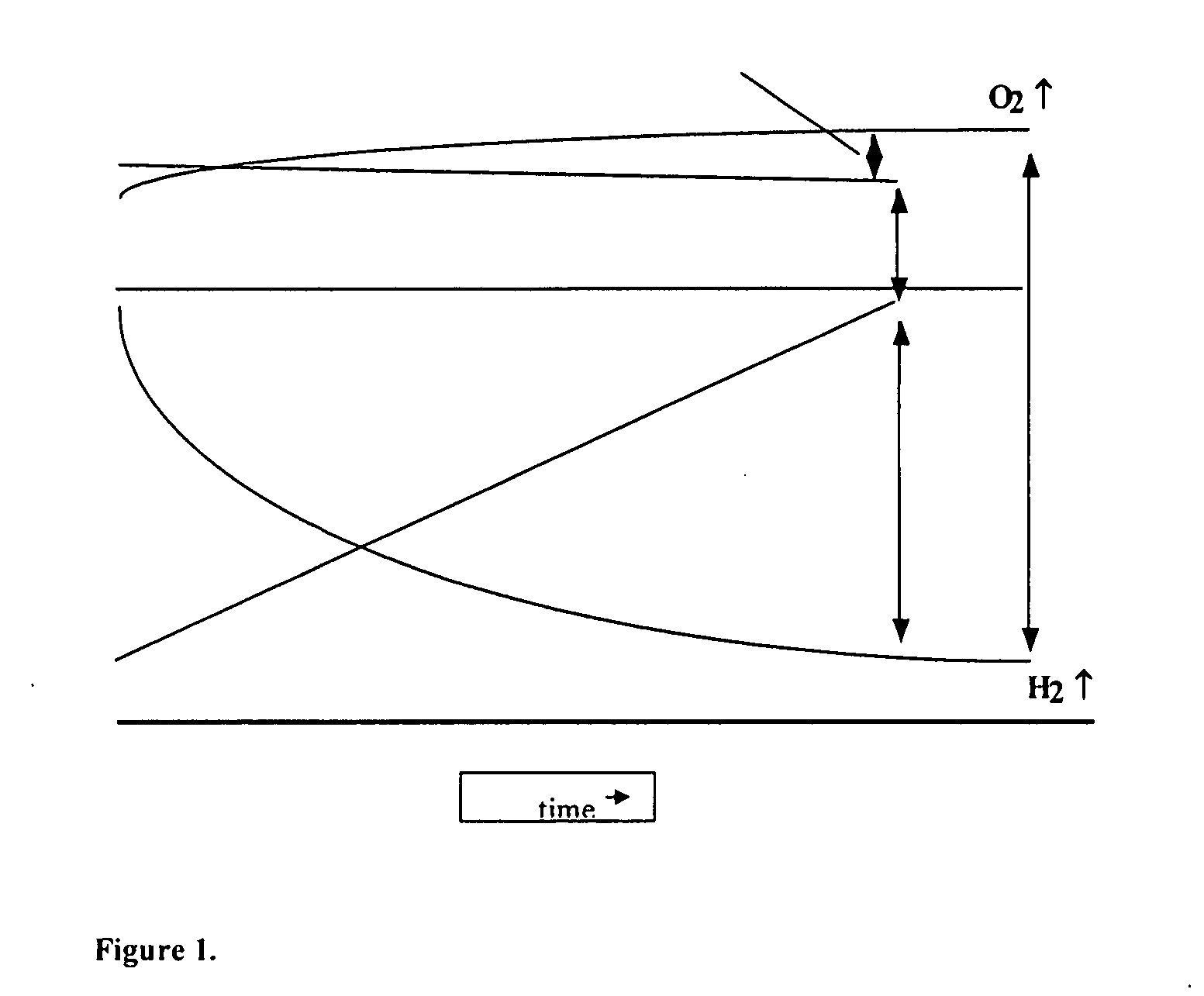
The present invention discloses an electrolyzer for electrolyzing water into a gaseous mixture comprising hydrogen gas and oxygen gas. The electrolyzer is adapted to deliver this gaseous mixture to the fuel system of an internal combustion engine. The electrolyzer of the present invention comprises one or more supplemental electrode at least partially immersed in an aqueous electrolyte solution interposed between two principle electrodes. The gaseous mixture is generated by applying an electrical potential between the two principal electrodes. The electrolyzer further includes a gas reservoir region for collecting the generated gaseous mixture. The present invention further discloses a method of utilizing the electrolyzer in conjunction with the fuel system of an internal combustion engine to improve the efficiency of said internal combustion engine.
Owner:HYDROGEN TECH APPL
Asymmetric electrochemical supercapacitor and method of manufacture thereof
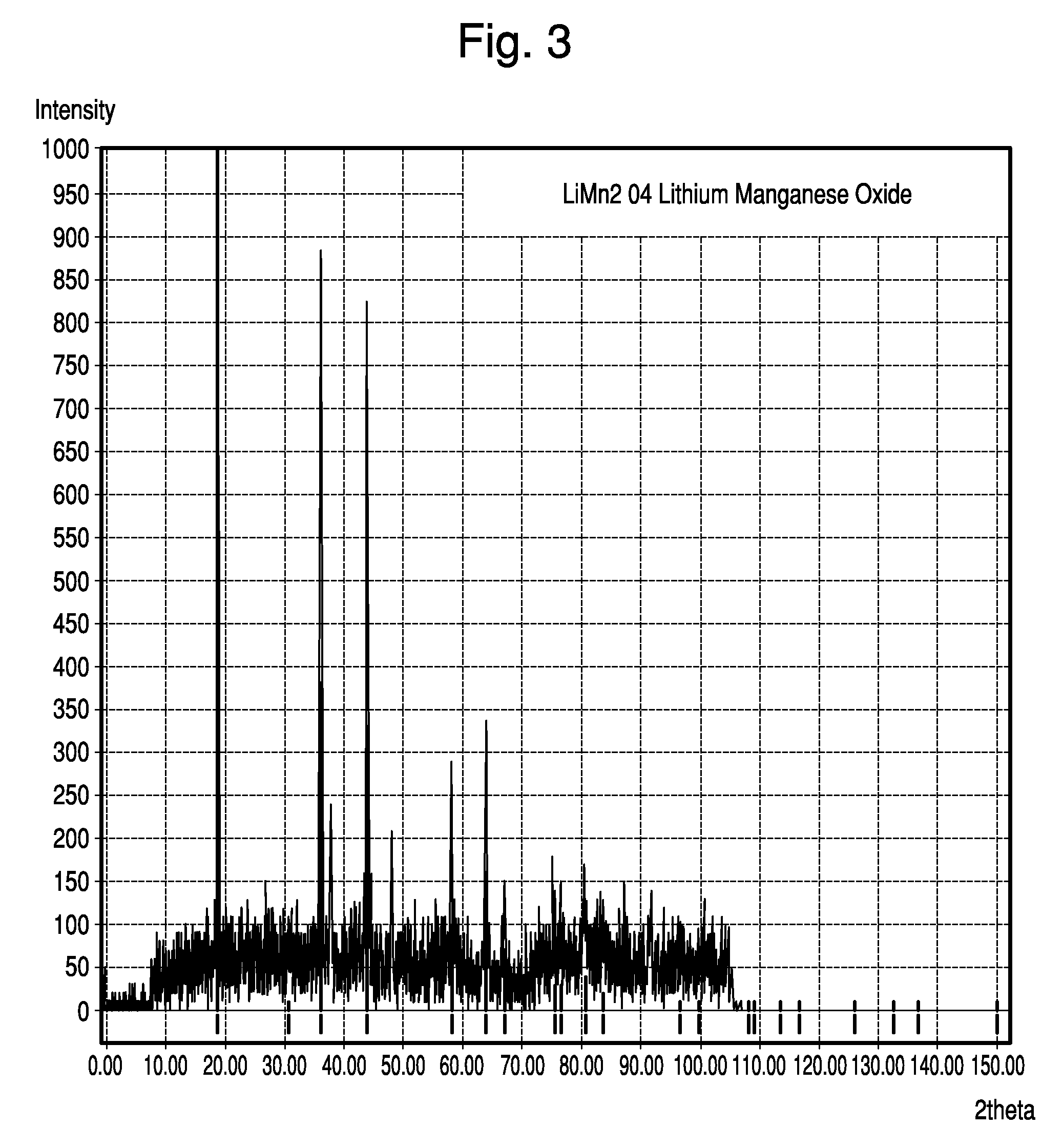
InactiveUS20080158778A1Increase energy densityImprove power densityHybrid capacitor electrodesLiquid electrolytic capacitorsAqueous electrolyteLithium manganese oxide
Asymmetric supercapacitors comprise: a positive electrode comprising a current collector and a first active material selected from the group consisting of manganese dioxide, silver oxide, iron sulfide, lithium manganese oxide, lithium cobalt oxide, lithium nickel oxide, lithium iron phosphate, and a combination comprising at least one of the foregoing active materials; a negative electrode comprising a carbonaceous active material; an aqueous electrolyte solution selected from the group consisting of aqueous solutions of hydroxides of alkali metals, aqueous solutions of carbonates of alkali metals, aqueous solutions of chlorides of alkali metals, aqueous solutions of sulfates of alkali metals, aqueous solutions of nitrates of alkali metals, and a combination comprising at least one of the foregoing aqueous solutions; and a separator plate. Alternatively, the electrolyte can be a non-aqueous ionic conducting electrolyte or a solid electrolyte.
Owner:U S NANOCORP
Functionally improved battery and method of making same
InactiveUS6855441B1Extended service lifePower outputBatteries circuit arrangementsSemiconductor/solid-state device detailsElectrical batteryLiquid state
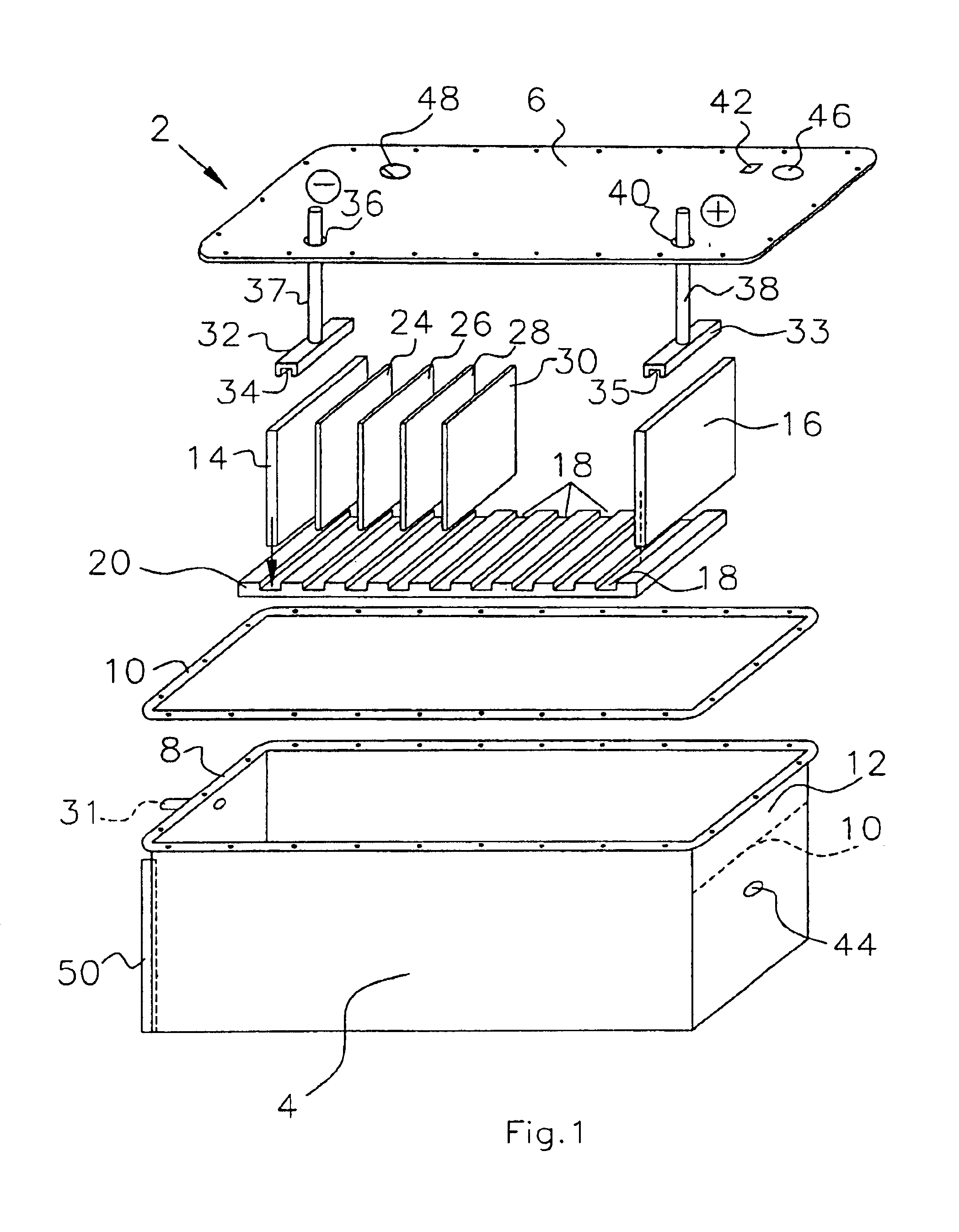
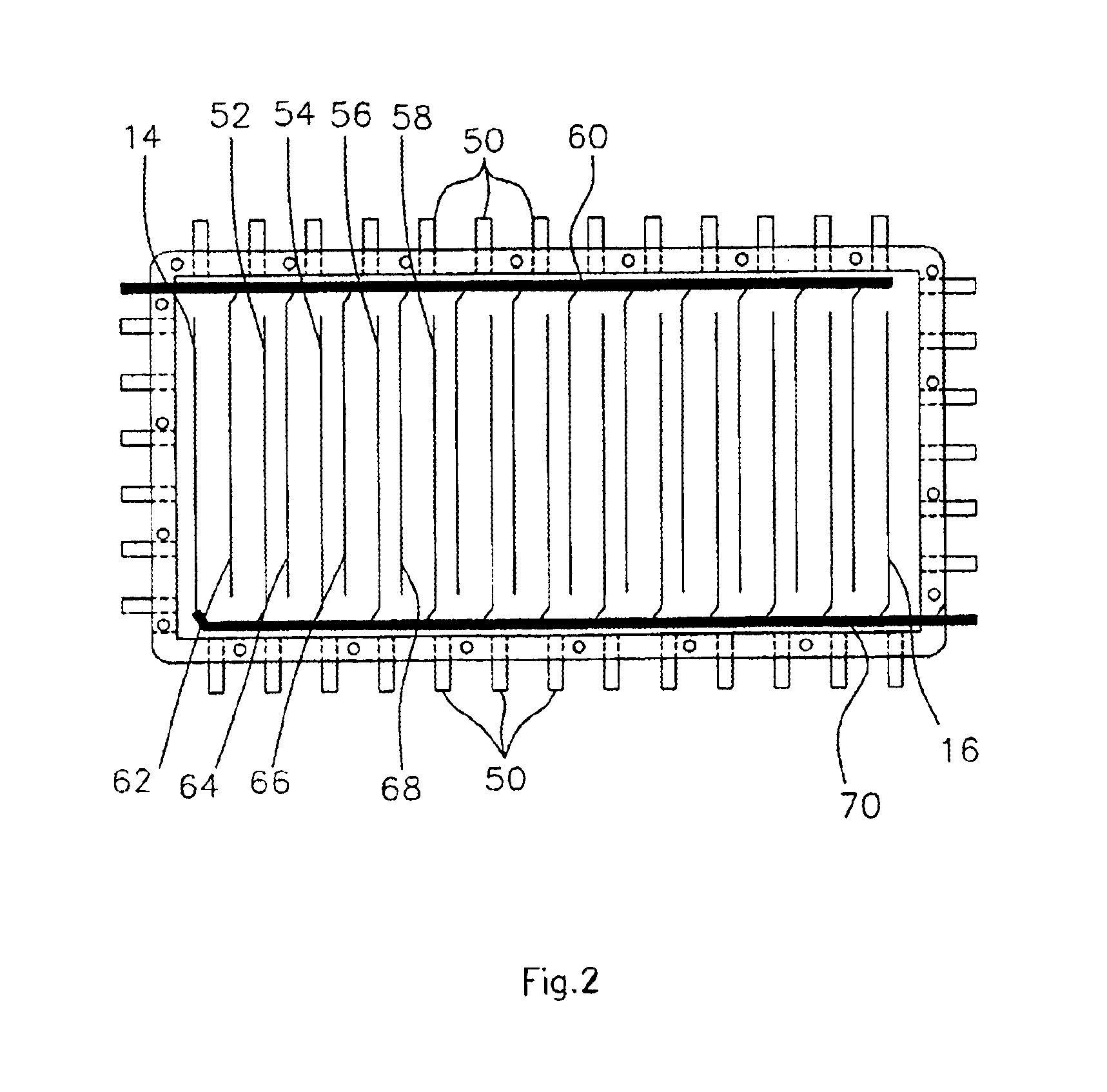
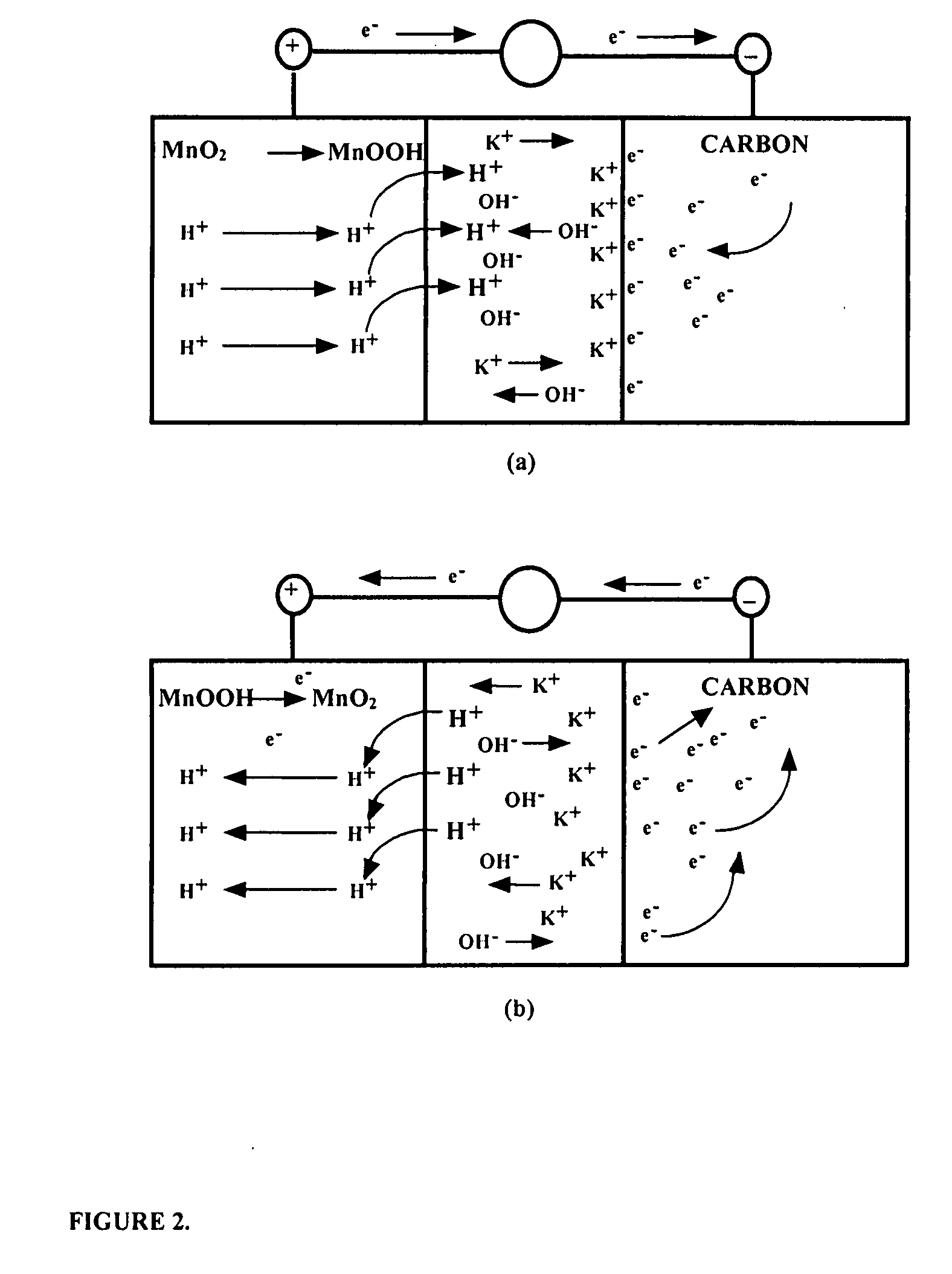
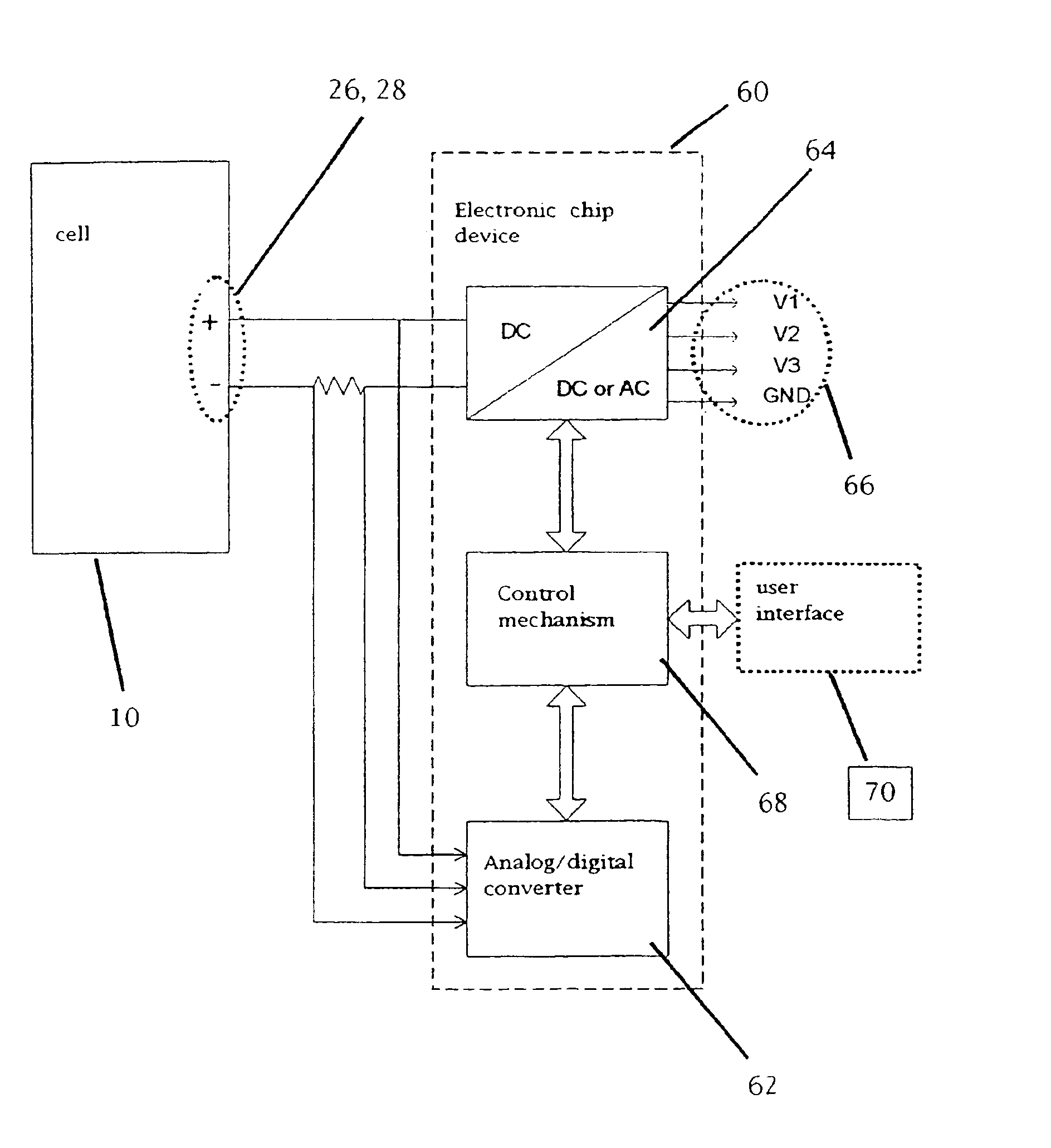
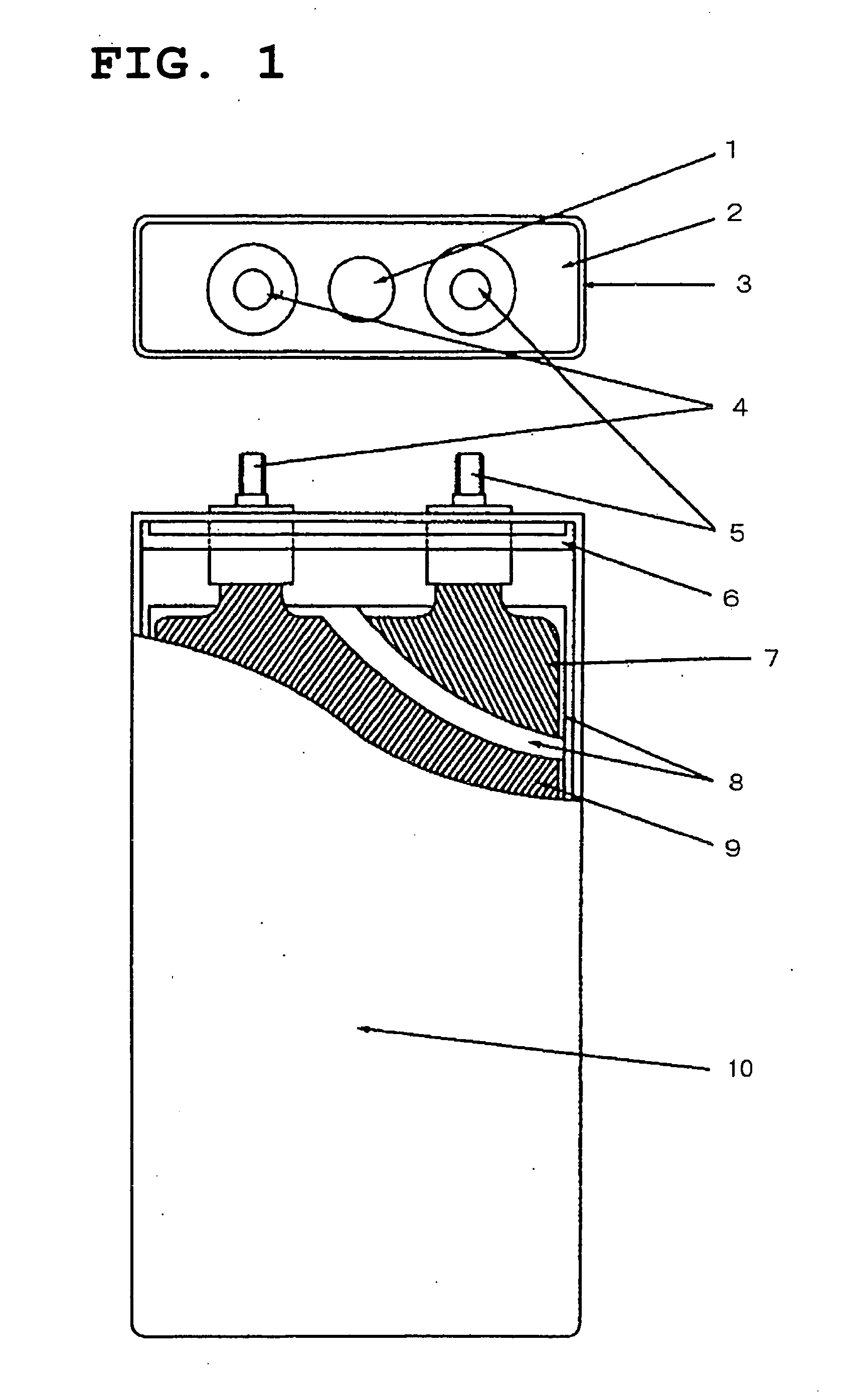
A functionally improved battery is disclosed. The battery includes a flexible thin layer open liquid state electrochemical cell (10) and an electronic chip device (60) integrally formed on or within the electrochemical cell (10). The cell (10) includes a first layer of insoluble negative pole (14), a second layer of insoluble positive pole (16) and a third layer of aqueous electrolyte (12). The third layer (12) is disposed between the first (14) and second (16) layers. The third layer (12) includes a diliquescent material for keeping the cell wet, an electroactive soluble material for ionic conductivity and a watersoluble polymer for viscosity. The viscosity adheres the first (14) and second (16) layer to the third layer (12). The chip (60) serves to improve the functionality of the battery.
Owner:POWER PAPER
Asymmetric electrochemical supercapacitor and method of manufacture thereof
InactiveUS20090290287A1Increase energy densityLarge capacityHybrid capacitor electrodesLiquid electrolytic capacitorsActivated carbonDivalent metal
The disclosure relates to asymmetric supercapacitors containing: a positive electrode comprising a current collector and a first active material selected from a layered double hydroxide of formula [M2+1−xMx3+(OH)2]An−x / n·mH2O where M2+ is at least one divalent metal, M3+ is at least one trivalent metal and A is an anion of charge n−, where x is greater than zero and less than 1, n is 1, 2, 3 or 4 and m is 0 to 10; LiCoO2; LiCoxNiyO2 where x and y are greater than zero and less than 1; LiCoxNiyMn(1−x−y)O2 where x and y are greater than zero and less than 1; CoSx where x is from 1 to 1.5; MoS; Zn; activated carbon and graphite; a negative electrode containing a material selected from a carbonaceous active material, MoO3 and Li1xMoO6−x / 2; an aqueous electrolyte solution or a non-aqueous ionic conducting electrolyte solution containing a salt and a salt and a non-aqueous solution; and a separator plate. Alternatively, the electrolyte can be a solid electrolyte.
Owner:U S NANOCORP
Active substance of positive electrode and nonaqueous electrolyte battery containing the same
ActiveUS20050019659A1Increase energy densityImprove high rate discharge performanceSecondary cellsAlkali metal oxidesHigh rateHigh energy
A positive active material is provided which can give a battery having a high energy density and excellent high-rate discharge performance and inhibited from decreasing in battery, performance even in the case of high-temperature charge. Also provided is a non-aqueous electrolyte battery employing the positive active material. The positive active material contains a composite oxide which is constituted of at least lithium (Li), manganese (Mn), nickel (Ni), cobalt (Co), and oxygen (O) and is represented by the following chemical composition formula: LiaMnbNicCodOe (wherein 0<a≦1.3, |b−c|≦0.05, 0.6≦d<1, 1.7≦e≦2.3, and b+c+d=1). The non-aqueous electrolyte battery has a positive electrode containing the positive active material, a negative electrode, and a non-aqueous electrolyte.
Owner:GS YUASA INT LTD
Redox flow cell rebalancing
A redox cell rebalance system is provided. In some embodiments, the rebalance system includes electrochemical cell and a photochemical cell. In some embodiments, the photochemical cell contains a source of ultraviolet radiation for producing HCl from H2 and Cl2 generated by the system. The HCl product may be collected or circulated back through the system for the rebalancing of electrolytes. A rebalance cell for use in a rebalance system is also provided. In some embodiments, the rebalance cell is the combination of an electrochemical cell and a photochemical cell. In some embodiments, a source of ultraviolet radiation is housed in the cathode compartment of the rebalance cell. In some embodiments, the source of ultraviolet radiation is used to effect the formation of HCl from H2 and Cl2 present in the rebalance cell. The HCl is dissolved in aqueous electrolytes contained in the rebalance cell, which can subsequently be circulated through a rebalance system for the rebalancing of redox cells.
Owner:IMERGY POWER SYST
Method of formation and charge of the negative polarizable carbon electrode in an electric double layer capacitor
InactiveUS6706079B1Protecting/adjusting hybrid/EDL capacitorDouble layer capacitorsCapacitanceHydrogen
A method of formation and charge of a negative polarizable electrode of an electric double layer capacitor. The method can be used for manufacturing of high capacitance capacitors utilizing the energy of the electric double layer. The methods achieve hydrogen evolution on carbonaceous materials using very negative potentials. The methods provide an EDL capacitor, employing an aqueous electrolyte, with improved specific energy. The methods may also ensure the hermeticity of the capacitor. The methods include pretreating the electric double layer capacitor by keeping the negative polarizable electrode at a desired minimum potential prior to use. Desirably, the minimum potential ranges from about -0.25 to about -1.2 V vs. a reference hydrogen electrode.
Owner:WAINWRIGHT D WALKER
Non-aqueous electrolyte for high-voltage lithium ion batteries
ActiveCN103268956ASimple compositionPromote circulationSecondary cellsHigh voltage batteryPropylene carbonate
The invention relates to a non-aqueous electrolyte for high-voltage lithium ion batteries, which is prepared from the following raw materials in percentage by weight: 70-85% of carbonate, 3-20% of functional additive and 11-17% of lithium hexafluorophosphate. The carbonate is one or mixture of more of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, dipropyl carbonate, methylethyl carbonate, methyl propyl carbonate and methyl butyl carbonate; and the functional additive is one or mixture of more of 0.5-10% of negative pole film-forming additive, 0.5-10% of high-temperature additive, 0.5-10% of positive pole film-forming additive, 0.5-10% of high-voltage additive and 0.001-2% of stability additive. The invention solves the problem of adaptation of the lithium ion battery electrolyte to the 4.35V high-voltage battery positive / negative pole, and provides an electrolyte for high-voltage batteries, which has the advantages of high cycle life, low inflation rate and favorable high-temperature properties.
Owner:广东金光高科股份有限公司
Additives for non-aqueous electrolyte and lithium secondary battery using the same
ActiveUS20070166609A1Improved overcharge stabilityCell electrodesOrganic electrolyte cellsOrganic solventLithium-ion battery
Disclosed is an electrolyte for batteries, comprising: (a) an electrolyte salt; (b) an organic solvent; (c) a first compound having an oxidation initiation voltage (vs. Li / Li+) higher than the operating voltage of a cathode; and (d) a second reversible compound having an oxidation initiation voltage higher than the operating voltage of the cathode, but lower than the oxidation initiation voltage of the first compound. Also disclosed is a lithium secondary battery comprising said electrolyte. In the lithium secondary battery, two compounds having different safety improvement actions at a voltage higher than the operating voltage of the cathode are used in combination as electrolyte components. Thus, the safety of the secondary battery in an overcharged state can be ensured, and at the same time, the deterioration of the battery can be prevented from occurring when it is repeatedly cycled, continuously charged and stored at high temperature for a long time.
Owner:LG ENERGY SOLUTION LTD
Non-aqueous electrolytic secondary cell
InactiveUS6436577B1Improve cycle performanceLow viscosityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsSolventTitanium oxide
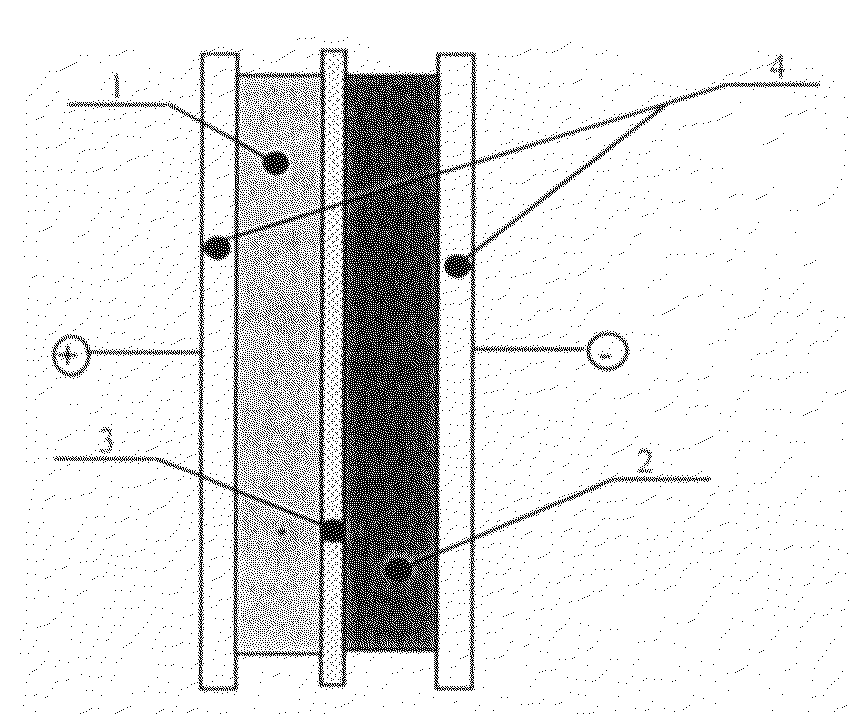
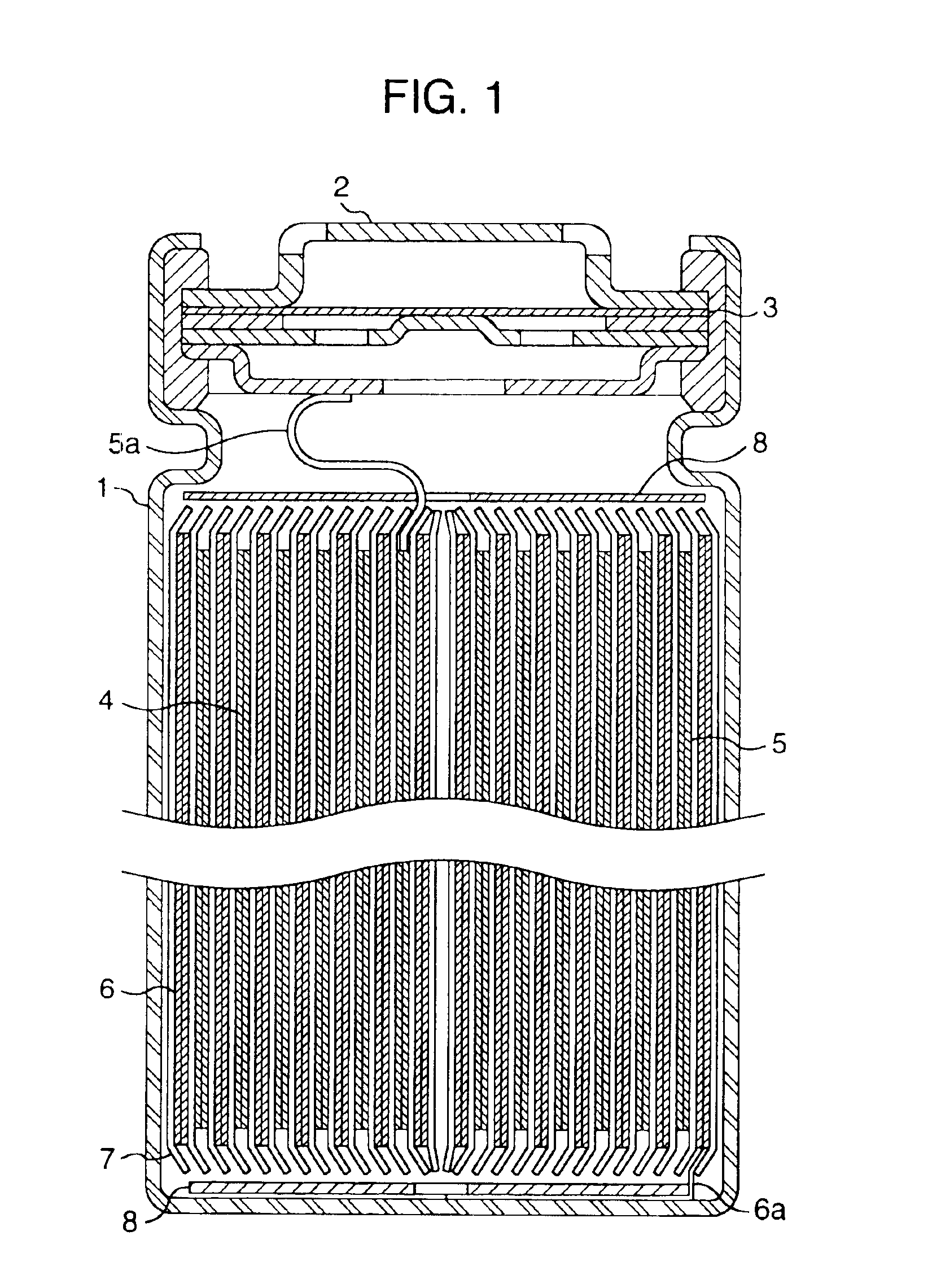
In a non-aqueous electrolyte secondary battery provided with a positive electrode 1, a negative electrode 2, and a non-aqueous electrolyte solution, a lithium-containing composite nickel oxide is used as a chief component of the positive electrode material for the positive electrode, a lithium-containing titanium oxide is used as a chief component of the negative electrode material for the negative electrode, and the solvent of the non-aqueous electrolyte solution contains a cyclic carbonic ester and a chain carbonic ester in such a manner that the cyclic carbonic ester and chain carbonic ester are contained in amounts of not less than 10% by volume of the whole solvent, respectively, and the total content of the cyclic carbonic ester and the chain carbonic ester is not less than 60% by volume of the whole solvent.
Owner:SANYO ELECTRIC CO LTD
Additive for non-aqueous liquid electrolyte, non-aqueous liquid electrolyte secondary cell and non-aqueous liquid electrolyte electric double layer capacitor
InactiveUS20030170548A1Improve the immunityImprove securityLight-sensitive devicesCell electrodesElectrolytic agentPhysical chemistry
The present invention provides an additive for a non-aqueous electrolyte comprising a phosphazene derivative represented by the following formula (1): (PNR2)n formula (1) wherein R represents a fluorine-containing substituent or fluorine, at least one of all R's is a fluorine-containing substituent, and n represents 3 to 14. More particularly, the present invention provides a non-aqueous electrolyte secondary cell and a non-aqueous electrolyte electric double layer capacitor comprising the additive for a non-aqueous electrolyte which exhibit good low temperature characteristics, good resistance to deterioration, and good incombustibility, and accordingly are significantly high in safety.
Owner:BRIDGESTONE CORP
Positive electrode active material, positive electrode and non-aqueous electrolyte secondary battery using thereof
ActiveUS7026070B2Large capacityIncrease energy densityElectrode manufacturing processesSecondary cellsHigh energyManganese
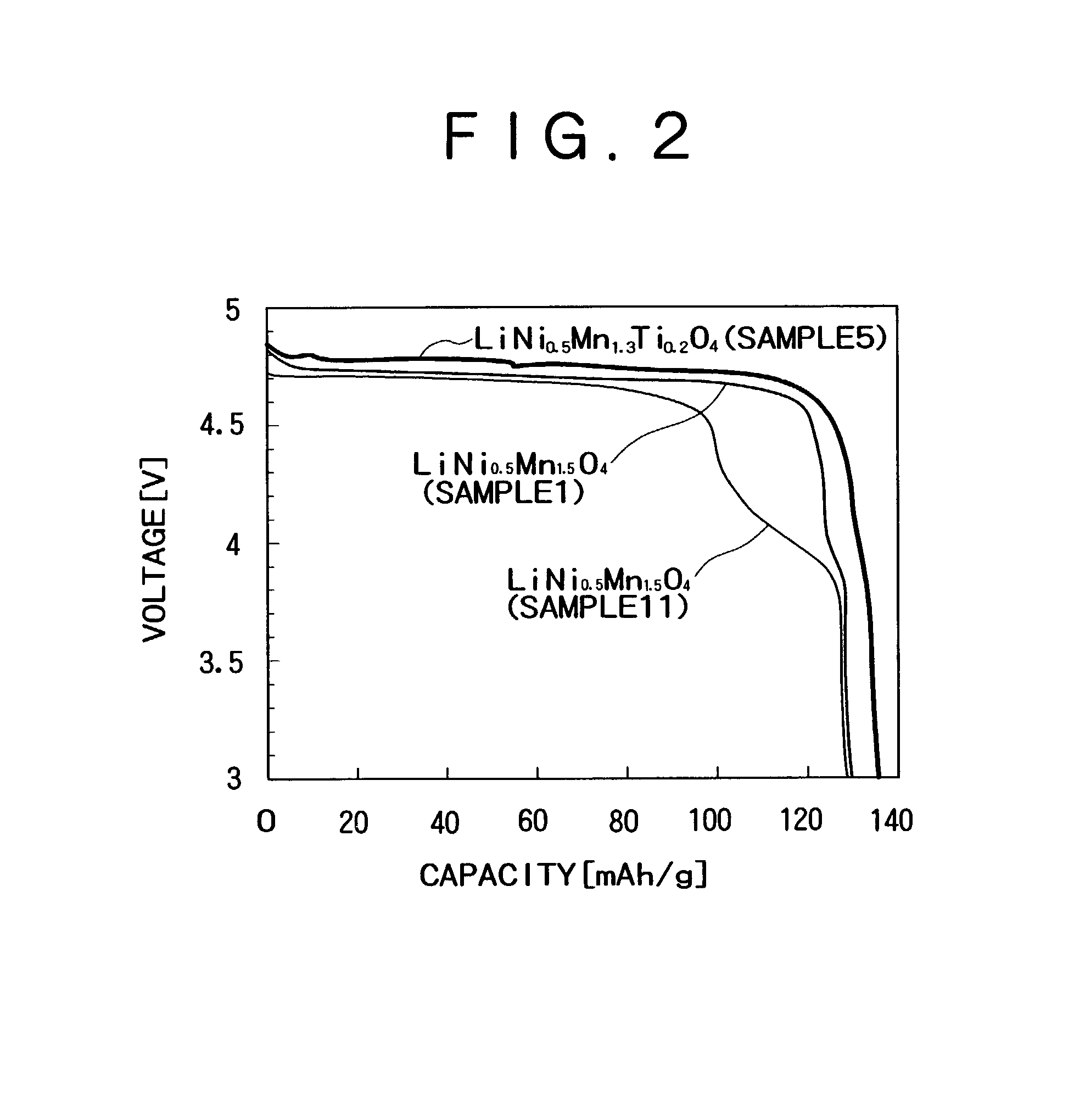
A positive electrode active material for a secondary battery contains a spinel lithium manganese composite oxide expressed by a general formula of Lia(MxMn2−x−yAy)O4 where x and y are positive values which satisfy 0.4<x, 0<y, x+y<2, and 0<a<1.2. “M” denotes Ni and at least one metal element selected from the group consisting of Co, Fe, Cr and Cu. “A” denotes at least one metal element selected from the group consisting of Si and Ti. The ratio y of A has a value of 0.1<y in case where A includes only Ti. Accordingly, it is possible to acquire a material for the positive electrode of a lithium ion secondary battery, which has a high capacity and a high energy density with a high voltage of 4.5 V or higher with respect to Li.
Owner:NEC CORP
Nonaqueous electrolytic solution type secondary battery
InactiveUS6919145B1Deterioration of battery performanceHigh viscosityElectrolytic capacitorsOrganic electrolyte cellsElectrolytic agentElectrical battery
A non-aqueous electrolyte secondary battery comprising a negative electrode, a positive electrode and an electrolyte having a lithium salt dissolved in a non-aqueous solvent characterized in that said non-aqueous solvent contains a vinylethylene carbonate compound represented by the general formula (I) in an amount of from 0.01 to 20% by weight is subject to minimized decomposition of the electrolyte and can provide a high capacity as well as exhibits excellent storage properties and cycle life performance. wherein R1, R2, R3, R4, R5 and R6 each independently represent a hydrogen atom or an alkyl group having 1 to 4 carbon atoms.
Owner:MITSUBISHI CHEM CORP
Electrically rechargeable, metal anode cell and battery systems and methods
ActiveUS20150010833A1Increased operating lifeAffordable and practicalAlkaline accumulatorsFuel and secondary cellsElectricityElectrical battery
The invention provides for a fully electrically rechargeable metal anode battery systems and methods of achieving such systems. An electrically rechargeable metal anode cell may comprise a metal electrode, an air contacting electrode, and an aqueous electrolyte separating the metal electrode and the air contacting electrode. In some embodiments, the metal electrode may directly contact the liquid electrolyte and no separator or porous membrane is needed between the air contacting electrode and the electrolyte. Rechargeable metal anode cells may be electrically connected to one another through a centrode connection where a metal electrode of one cell and an air contacting electrode of a second cell are electrically connected. Air tunnels or pathways may be provided between individual metal anode cells arranged in a stack. In some embodiments, an electrolyte flow management system may also be provided to maintain liquid electrolyte at constant levels during charge and discharge cycles.
Owner:EOS ENERGY TECH HLDG LLC
Method of forming colored film, driving device and liquid crystal display device
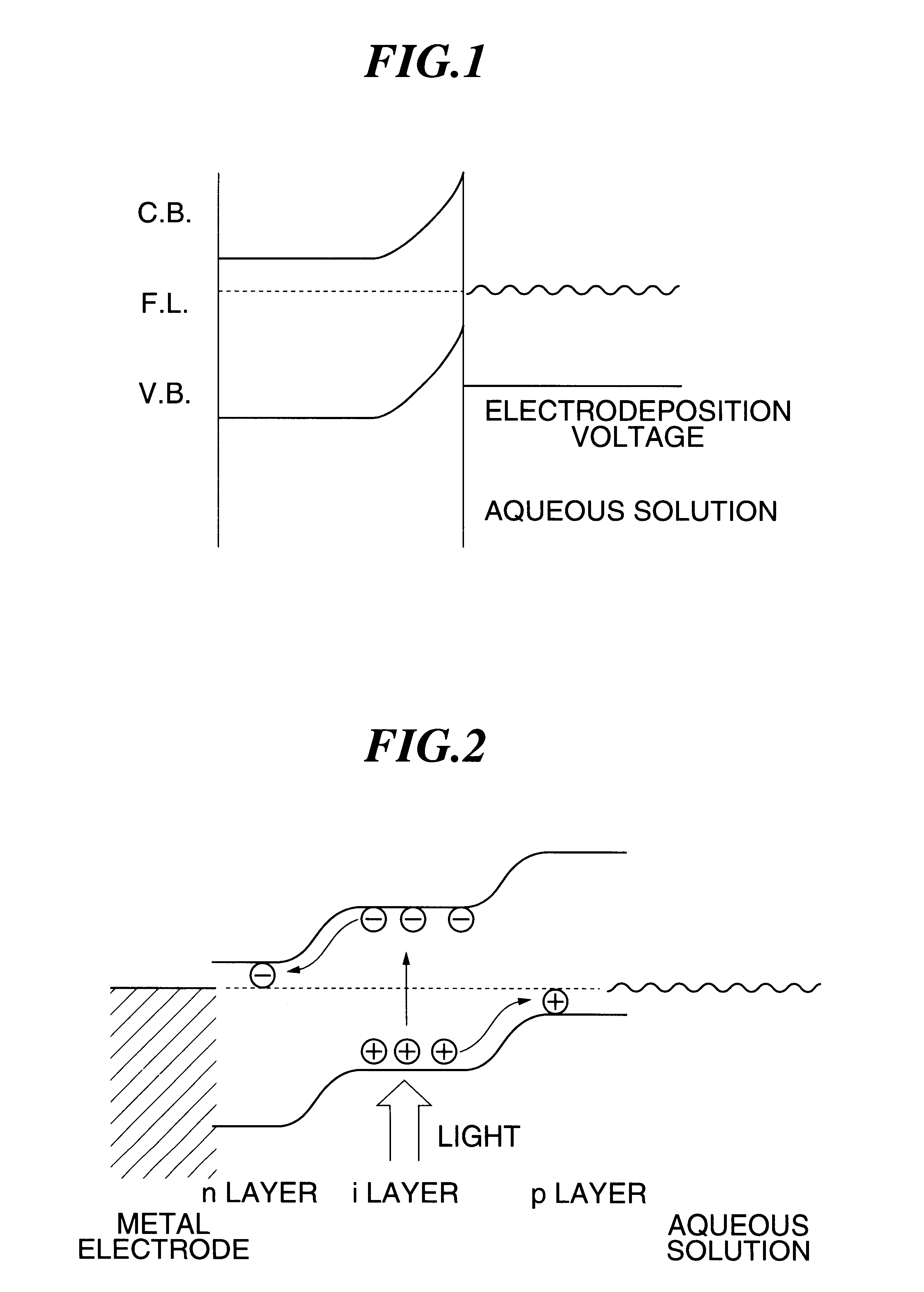
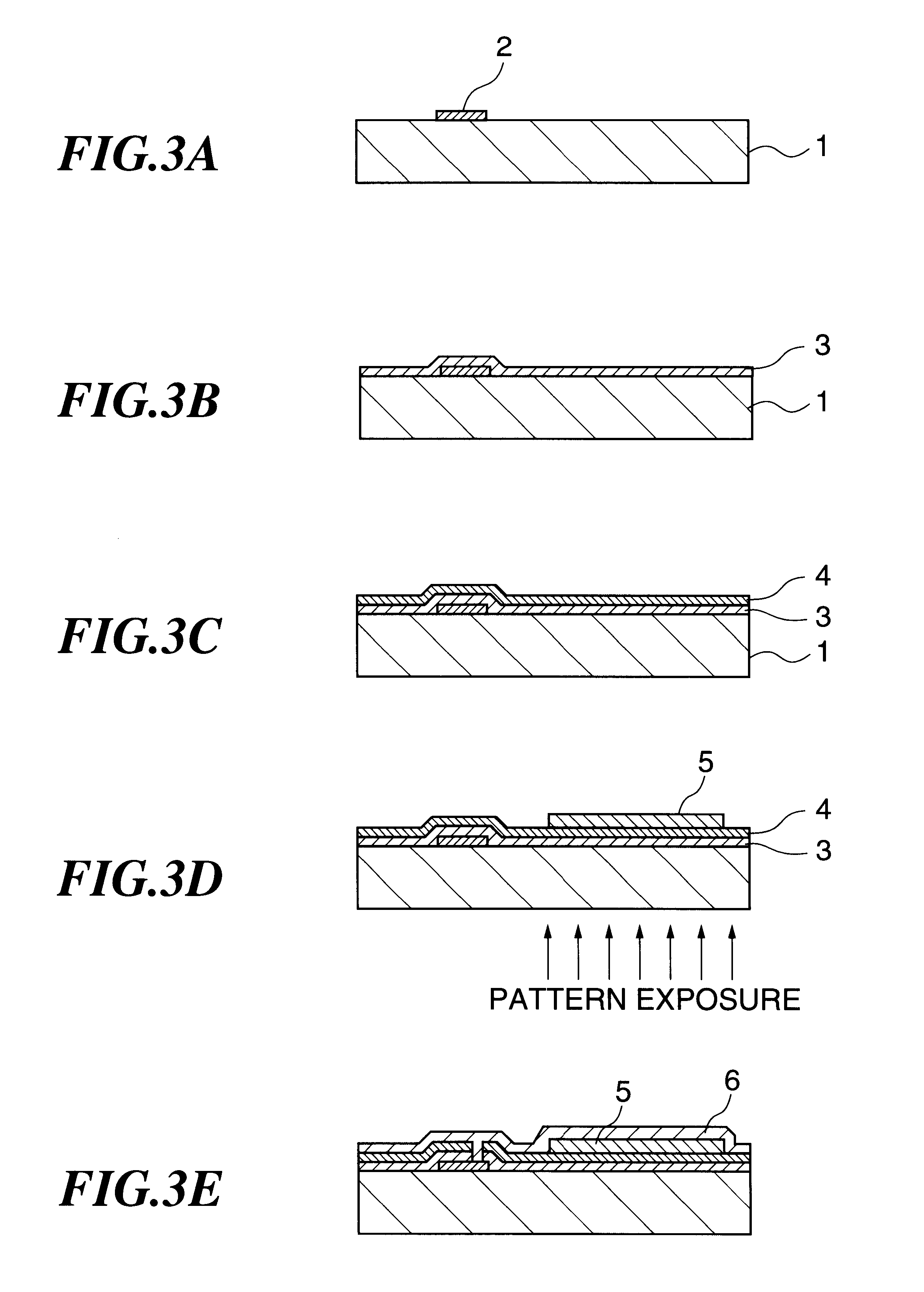
A method of forming a colored film includes a step of forming a substrate for electrodeposition by laminating at least a transparent conductive film and a thin transparent photosemiconductor film having a photovoltaic function in this order on the surface of a support having an electronic circuit materialthereon, and a step of irradiating with a light at least a thin photosemiconductor film of the substrate for electrodeposition while bringing the same into contact with an aqueous electrolyte containing an electrodeposition material containing a colorant, thereby selectively generating a photoelectromotive force to an irradiated area of the thin photosemiconductor film and electrochemically depositing the electrodeposition material to form a colored electrodeposition film; an electronic driving device containing the colored film and a liquid crystal display device having the electronic driving device, the method capable of forming the colored film of high quality and excellent surface smoothness being formed directly on a substrate having thin film transistor and capable of providing a high quality electronic driving device and a liquid crystal display device at low cost.
Owner:FUJIFILM BUSINESS INNOVATION CORP
Photo-electrolytic catalyst systems and method for hydrogen production from water
InactiveUS20050051439A1Reduce probabilityIncrease chanceCellsMaterial nanotechnologyElectron holeHigh rate
A photo-electrolytic catalyst system which comprises two materials: (a) a semiconductor material with a non-zero energy gap Eg which, in response to an incident radiation having an energy greater than Eg, generates electron-hole pairs as charge carriers; and (b) a facilitating material in electronic contact with the semiconductor material to facilitate separation of the radiation-generated electrons from the holes to reduce the probability of charge carrier recombinations The catalyst makes use of both majority and minority charge carriers to promote photo-electrolysis reactions for producing hydrogen directly from water or an aqueous electrolyte at higher rates and improved efficiencies.
Owner:JANG BOR Z
Non-aqueous electrochemical apparatus
InactiveUS6958198B2Improve featuresImprove wettabilityNon-aqueous electrolyte accumulatorsFinal product manufactureFree energiesDyne
The invention relates to a non-aqueous electrochemical apparatus in which the difference (γl−γse) between the surface tension γl of non-aqueous electrolyte and the surface free energy γse of electrode is not more than 10 dynes / cm.
Owner:PANASONIC CORP +1
Non-aqueous electrolyte solution for lithium ion battery and corresponding lithium ion battery
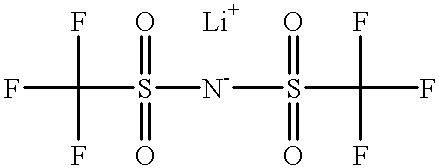
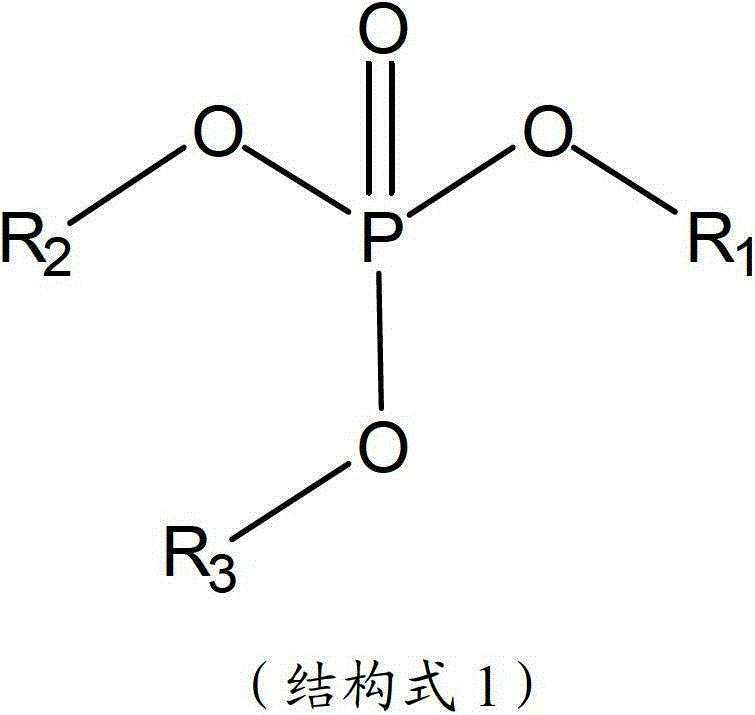
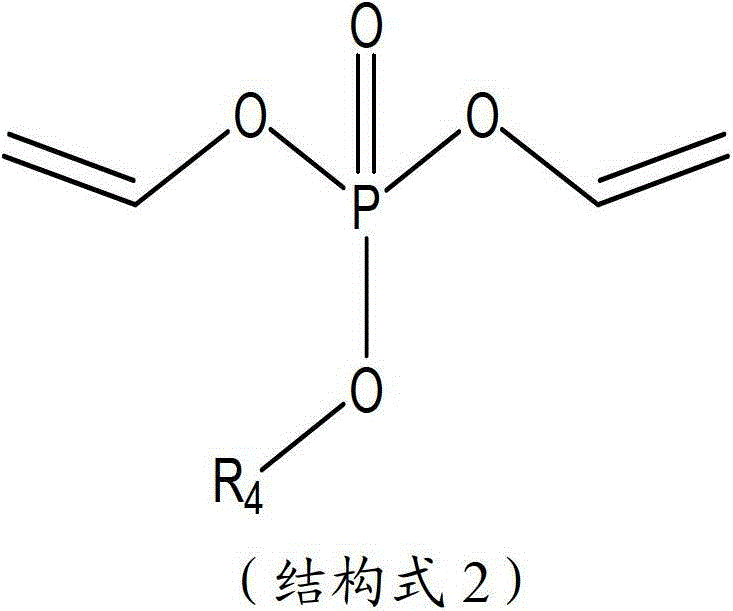
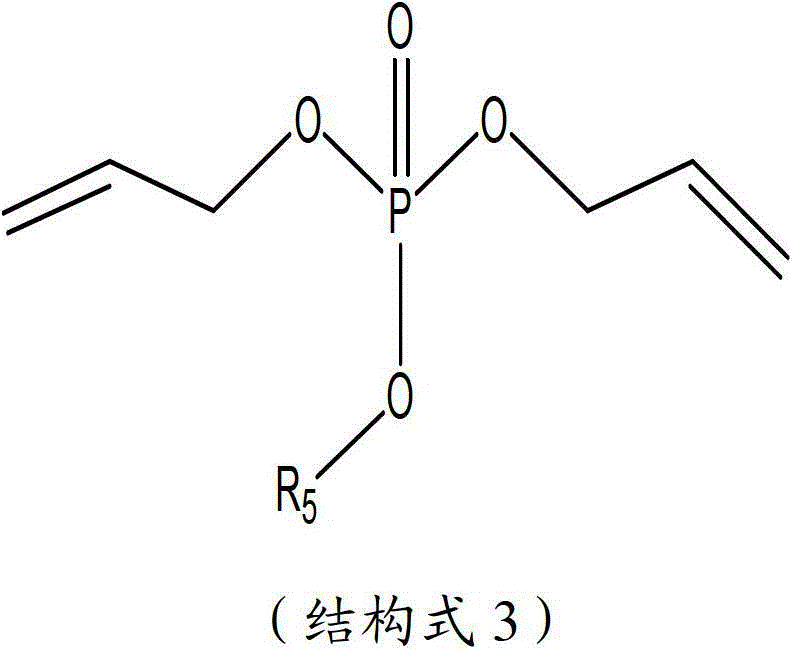
The invention aims to provide a high performance non-aqueous electrolyte solution used for a lithium ion battery. The non-aqueous electrolyte solution includes: a lithium salt; an organic solvent; and an unsaturated phosphate compound. The unsaturated phosphate compound is in favor of forming a stable and compact passivation film (SEI (solid electrolyte interface)) on an electrode surface so as to prevent further decomposition of solvent molecules. The electrolyte solution obtained according to the scheme involved in the invention can improve the high-temperature storage performance and cycle performance of the battery.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Active metal electrolyzer
InactiveUS7608178B2Effective isolationReduce environmental pollutionPhotography auxillary processesElectrode manufacturing processesElectrolysisAqueous electrolyte
Owner:POLYPLUS BATTERY CO INC
Current collector for non-aqueous electrolyte secondary battery, electrode for non-aqueous electrolyte secondary battery, production methods thereof, and non-aqueous electrolyte secondary battery
InactiveUS20110111277A1Increase flexibilityAvoid problemsFinal product manufactureElectrode carriers/collectorsMetal foilEngineering
A current collector includes a metal foil and protrusions formed on one face or both faces of the metal foil in a predetermined arrangement. The protrusions are substantially rhombic and aligned in a zigzag. Also, both end portions of each protrusion in each of two orthogonal axial directions protrude outward. Middle portions between the end portions are recessed inward. When columnar blocks of an active material are formed on the protrusions to form an active material layer, the gaps between the protrusions can be increased at portions where the interval between the protrusions is the smallest. As a result, internal stress of the active material layer created by charge / discharge of the battery can be alleviated, and the battery life can be increased.
Owner:PANASONIC CORP
Rechargeable lithium electrochemical cell
InactiveUS6942949B2Organic electrolyte cellsNon-aqueous electrolyte accumulator electrodesMethyl carbonatePhosphate
A secondary battery is comprised of a positive electrode, a negative electrode formed from a lithium storage material, and a non-aqueous electrolyte. The non-aqueous electrolyte includes a lithium salt, non-aqueous aprotic solvent(s), such as ethylen carbonate, propylene carbonate, dimethyl carbonate, ethymethyl carbonate and diethyl carbonate, and a small percentage of at least one organic additive. The negative electrode may comprise a carbon such as graphite, and the positive electrode may comprise a lithiated metal oxide or phosphate, such as LiCoO2, LiNiO2, LiMn2O4, LiFePO4, or mixtures thereof. The organic additives have one or more unsaturated bonds activated with respect to oxidation by electron-pushing alkyl groups. They are in most cases known to be able to undergo polymerization reactions, such as an anodically induced polymerization especially under certain conditions. The additives are oxidized at the cathode at a potential of more than 4.3 V vs. Li / Li+. With these additives in amounts of 0.001 to 10%, a passivation layer is formed on the cathodes, and the sensitiveness of the battery against overcharge is reduced. The electrolyte mixtures do not deteriorate the properties of the battery anodes.
Owner:LG ENERGY SOLUTION LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com