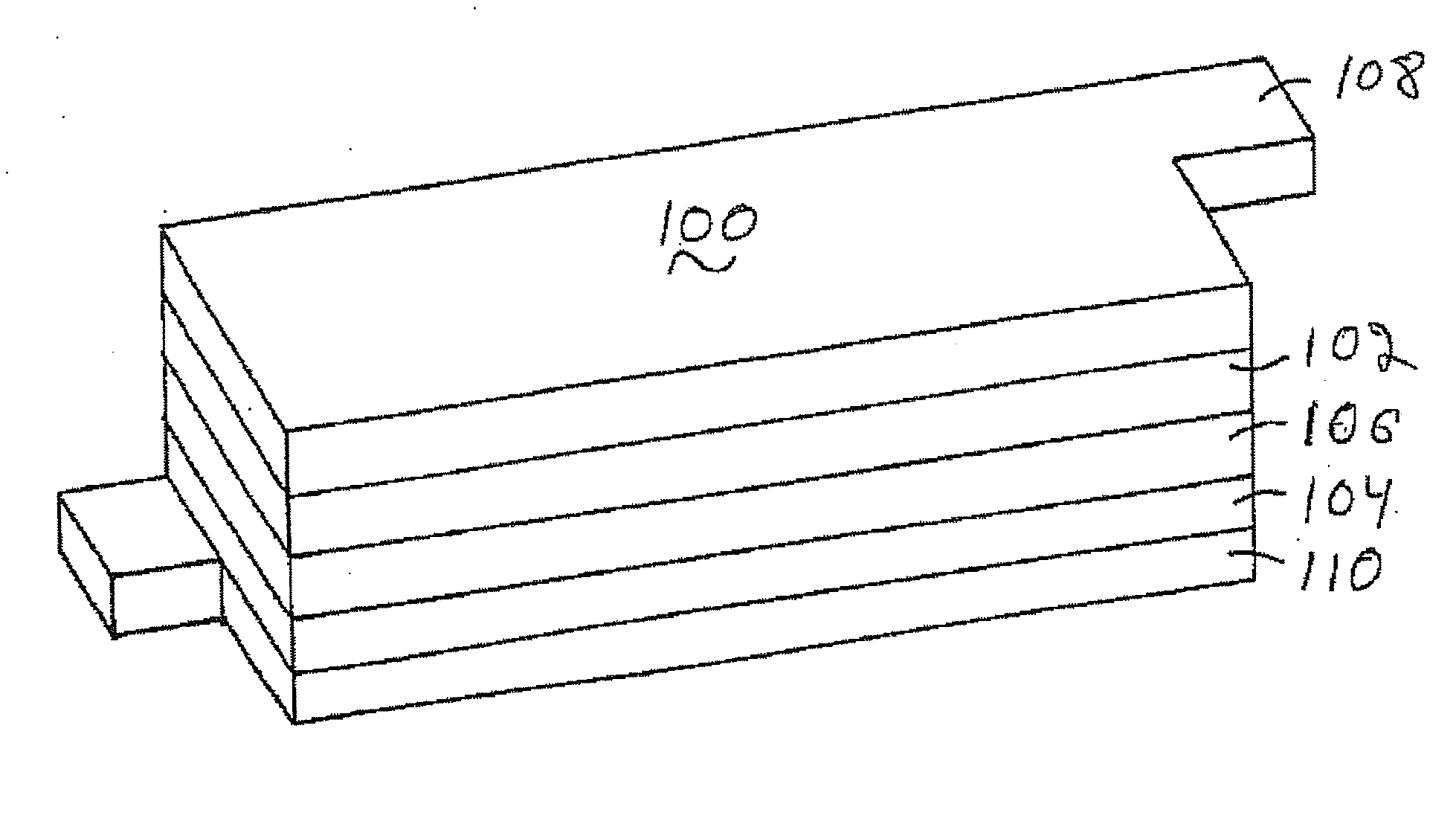
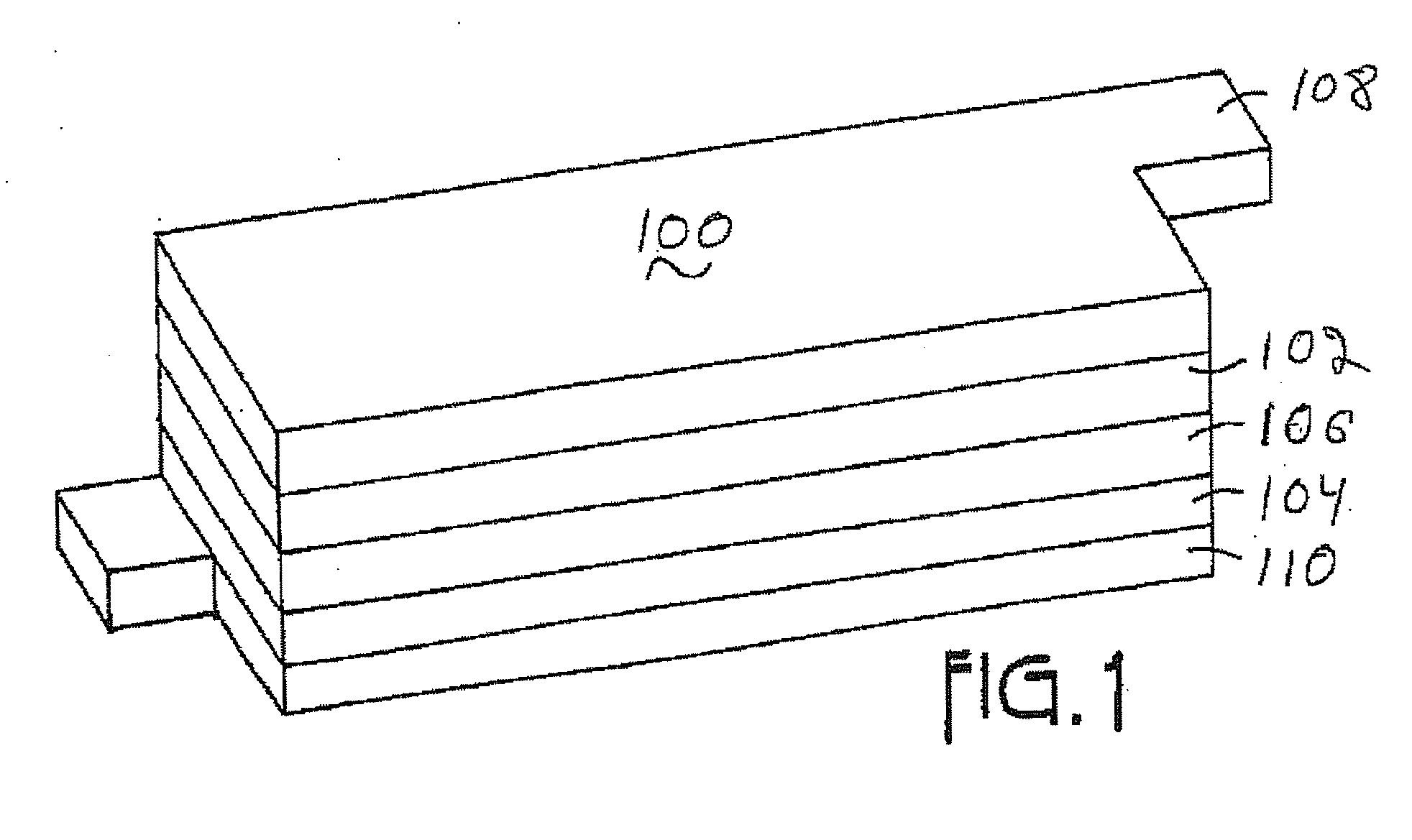
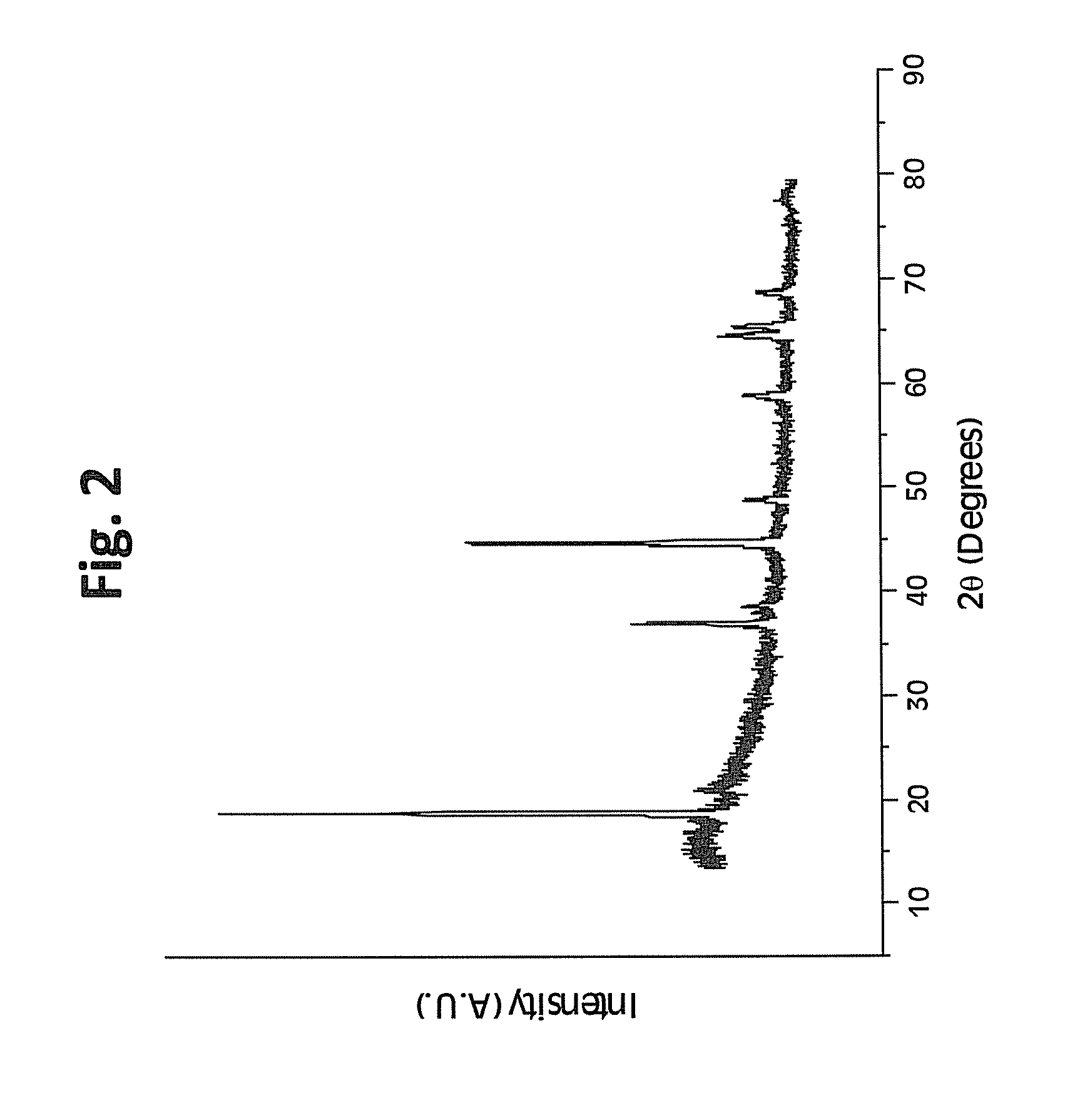
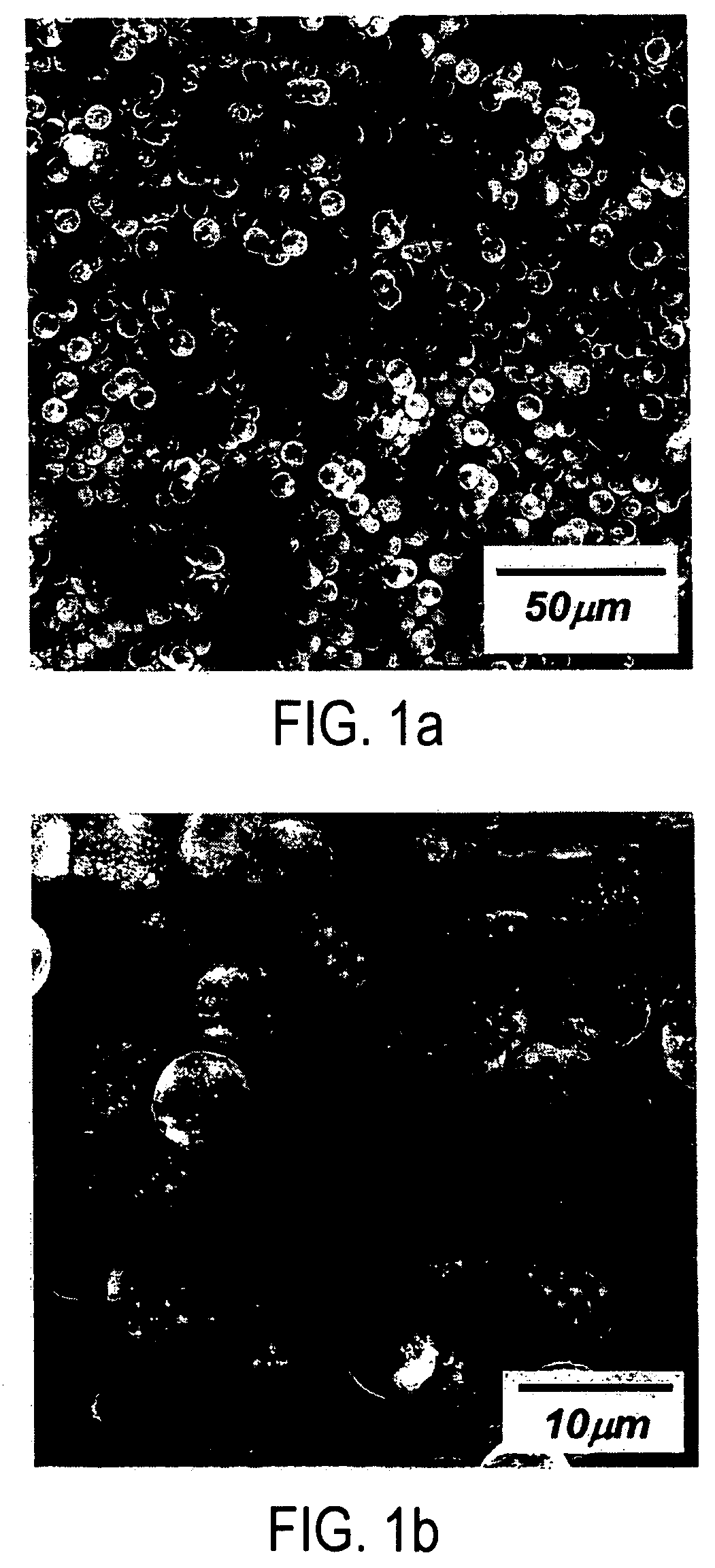
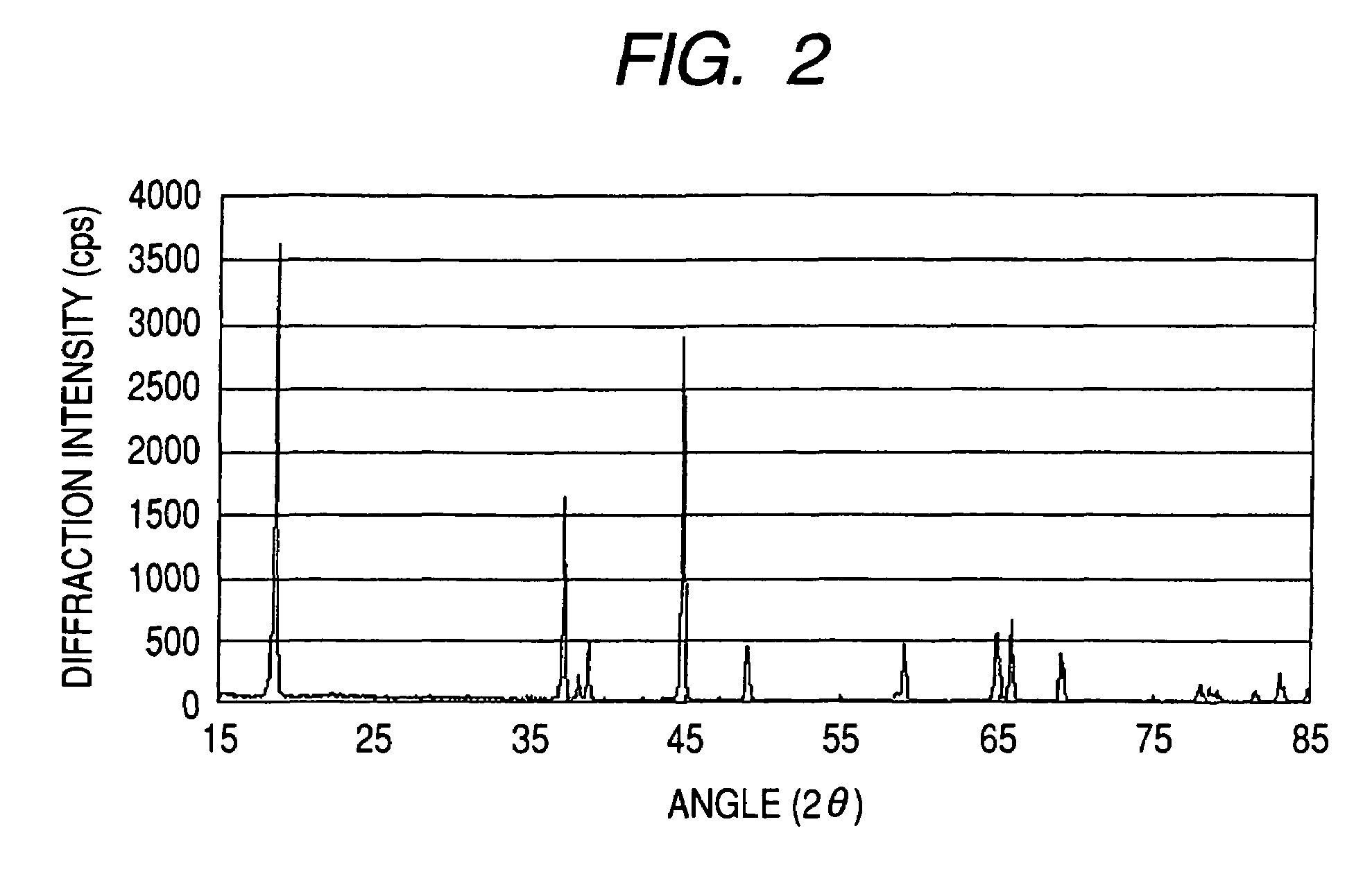
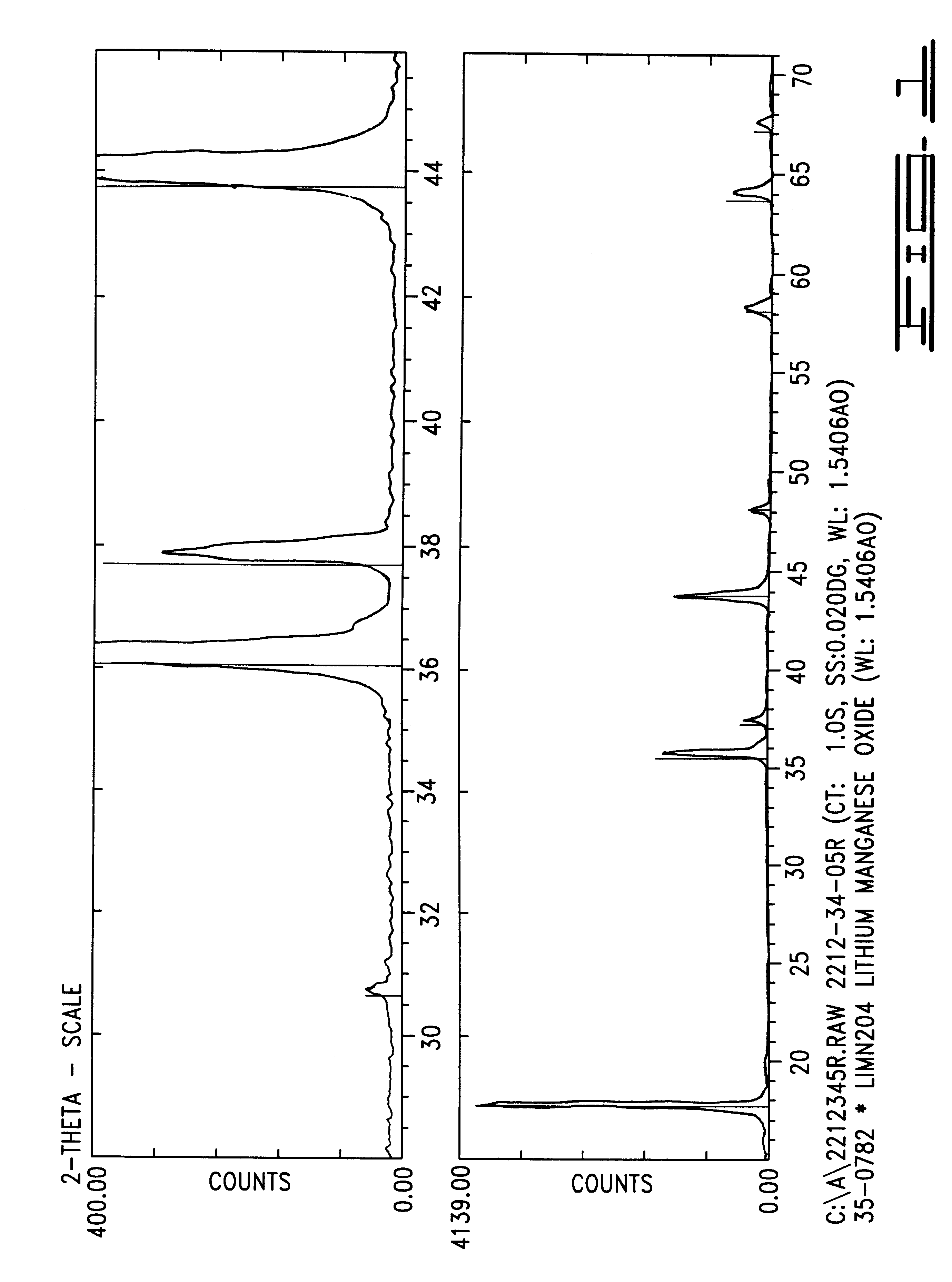
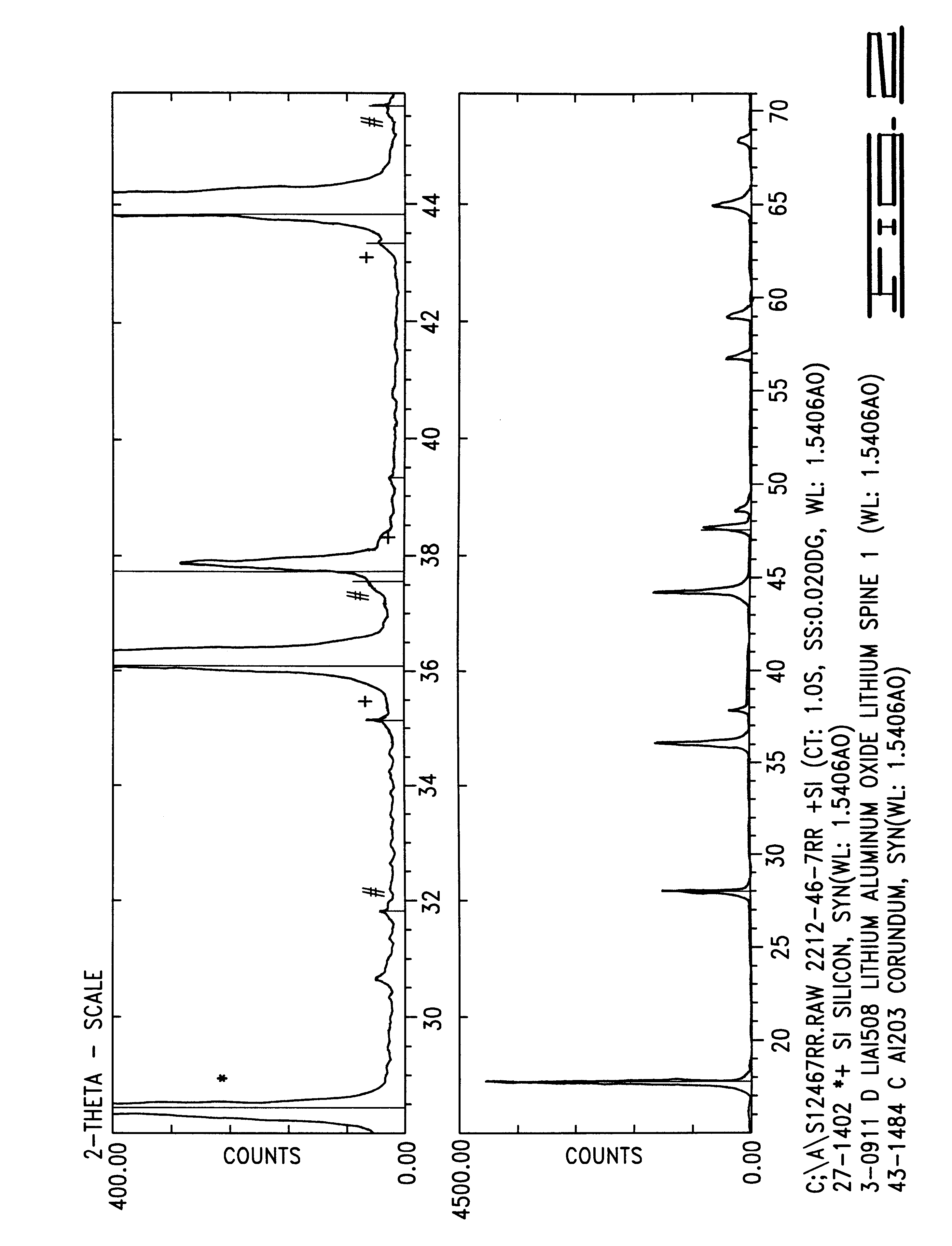
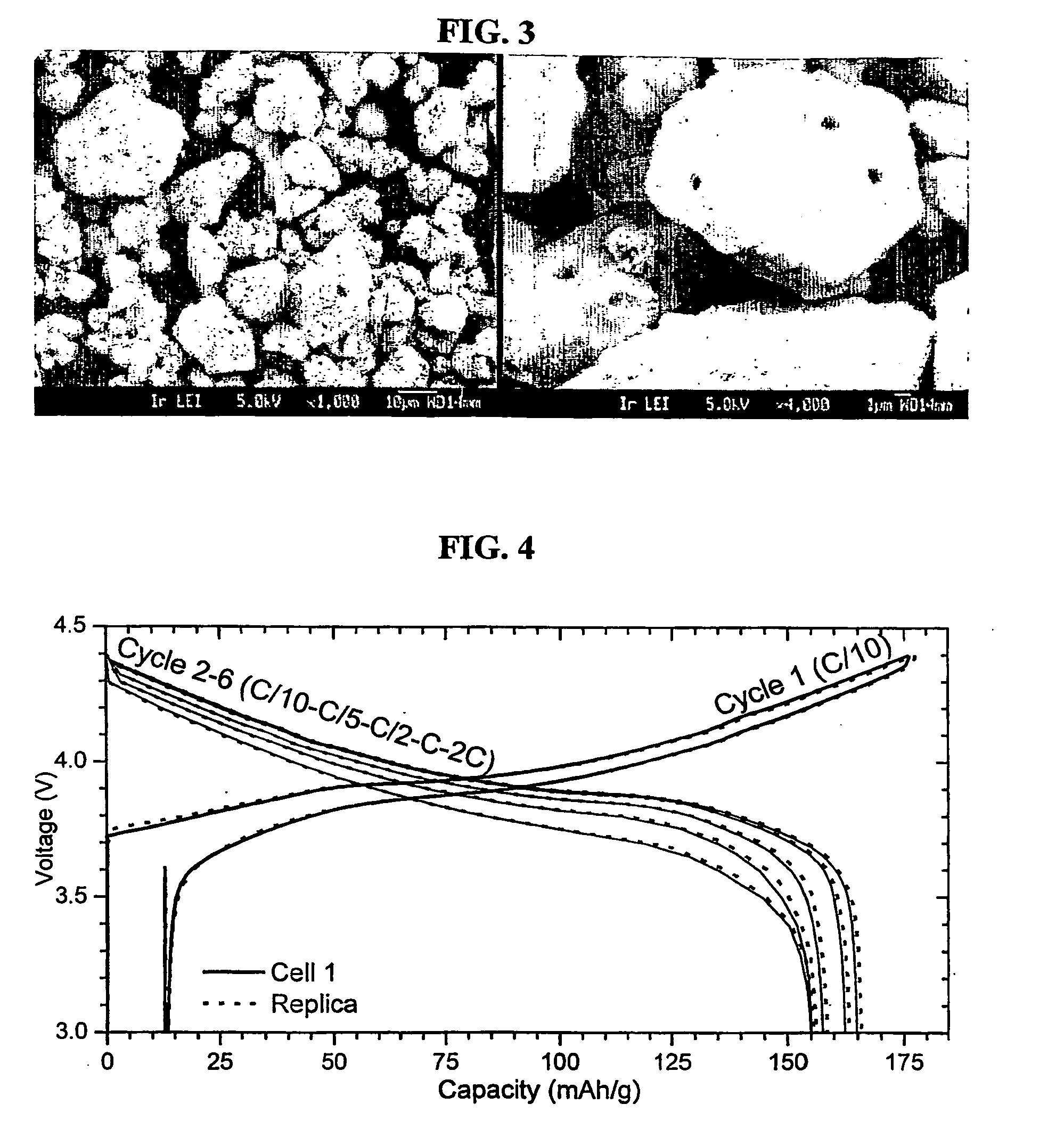
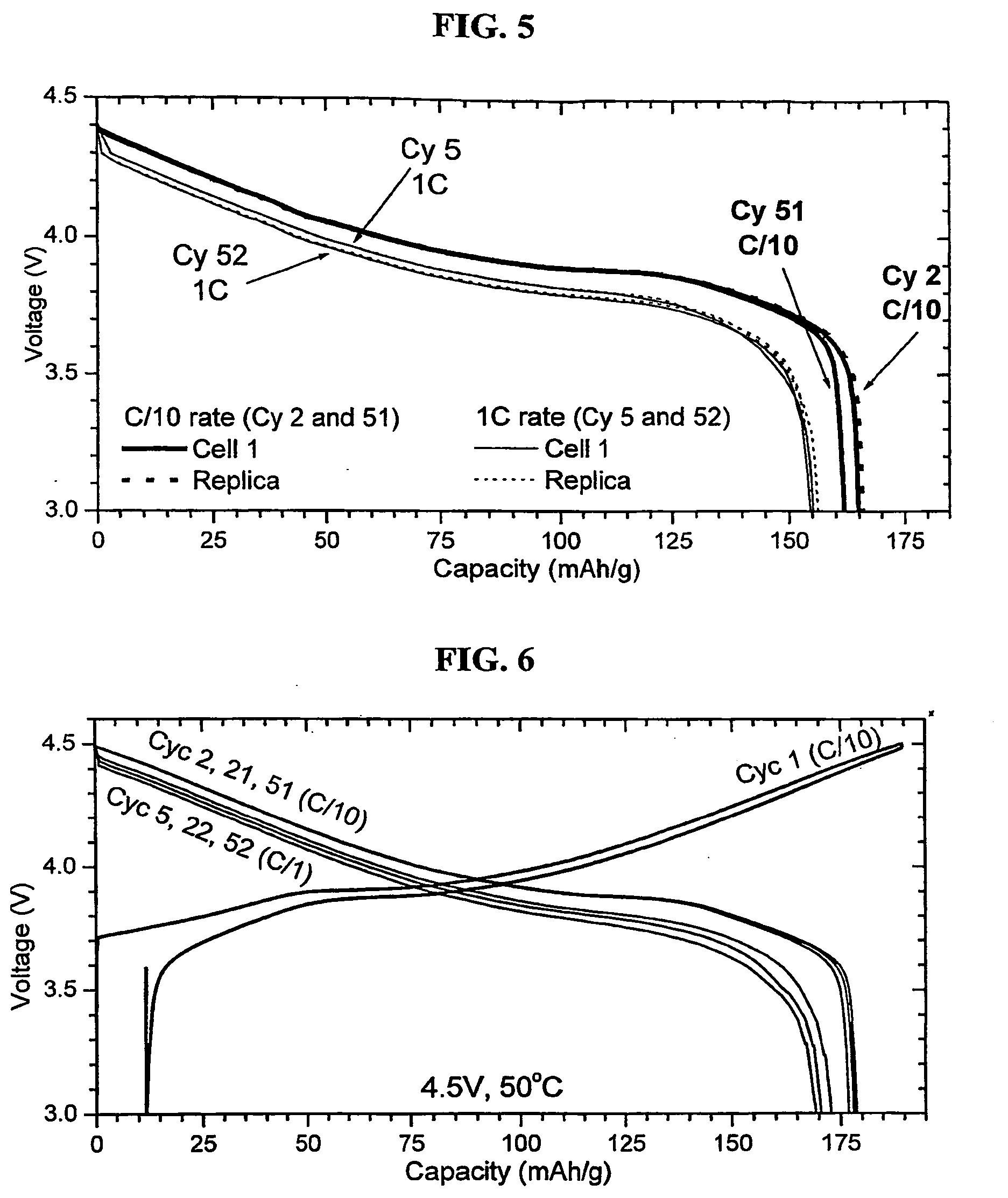
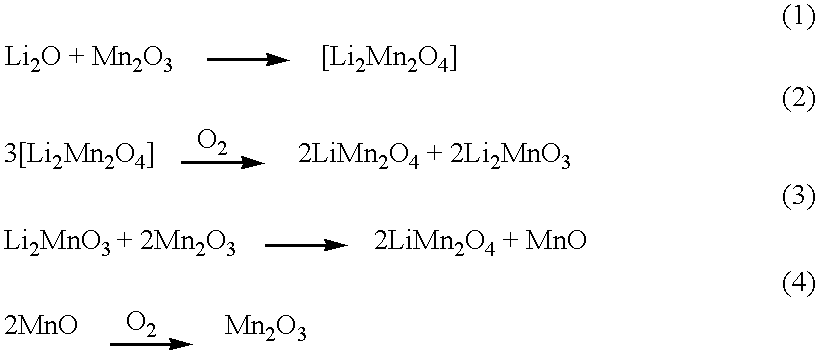Patents
Literature
682results about "Alkali metal oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
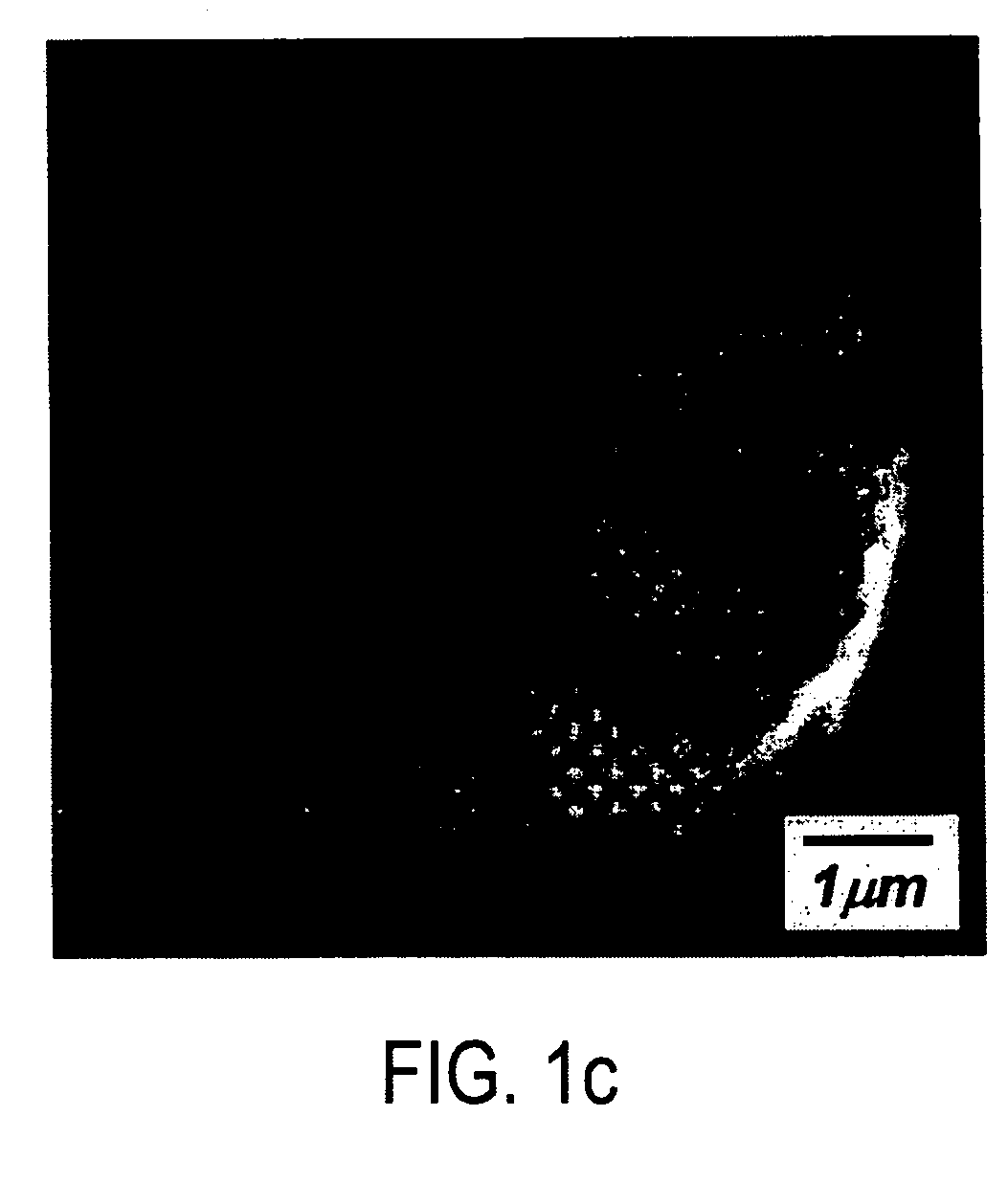
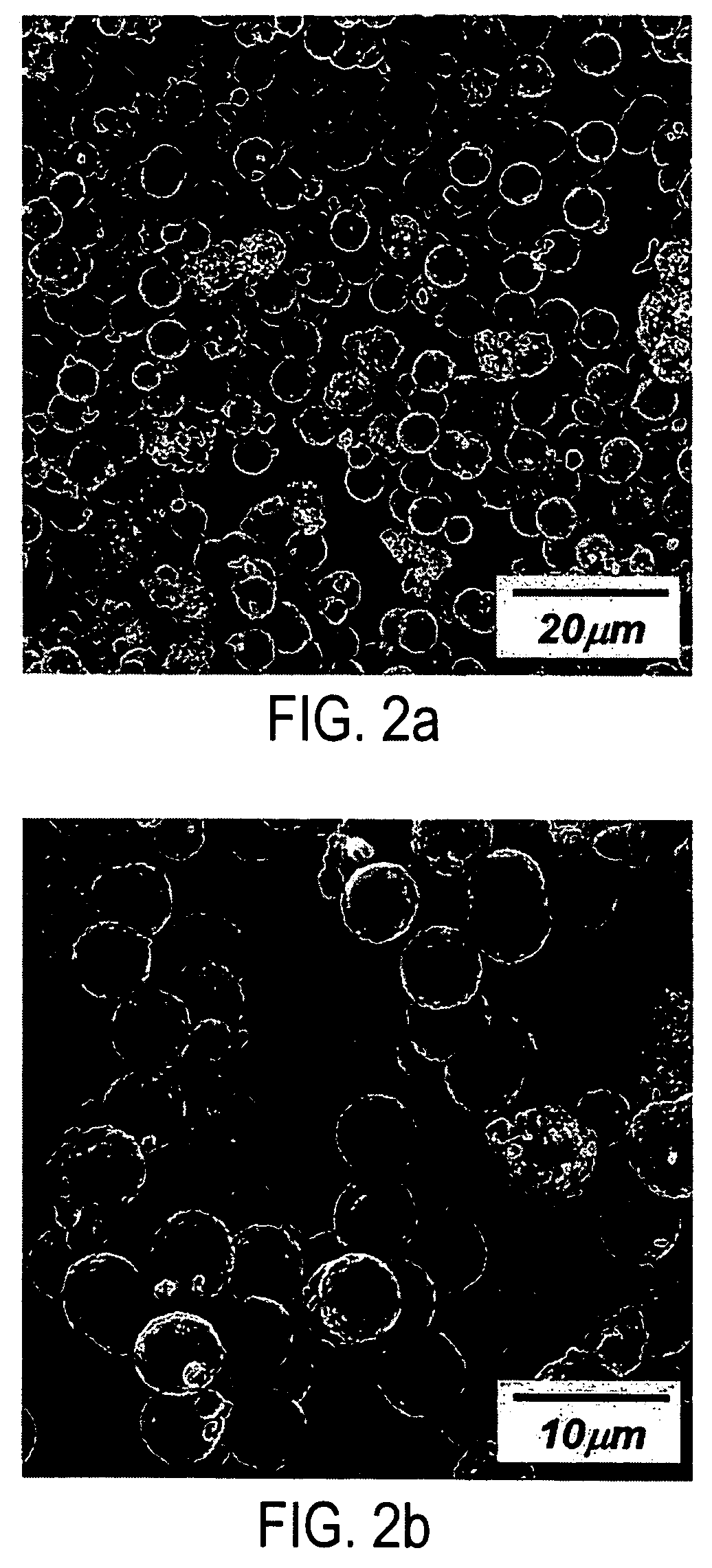
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS20100086853A1Electrode manufacturing processesAlkali metal oxidesDischarge rateLithium-ion battery
Owner:IONBLOX INC
Layered cathode materials for lithium ion rechargeable batteries
ActiveUS7205072B2Improve impedance characteristicsImprove stabilityAlkali metal oxidesNon-aqueous electrolyte accumulator electrodesDopantRechargeable cell
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling.
Owner:UCHICAGO ARGONNE LLC +2
Thin film battery and electrolyte therefor
A solid amorphous electrolyte composition for a thin-film battery. The electrolyte composition includes a lithium phosphorus oxynitride material containing a sulfide ion dopant wherein the atomic ratio of sulfide ion to phosphorus ion (S / P) in the electrolyte ranges greater than 0 up to about 0.2. The composition is represented by the formula: where 2x+3y+2z=5+w, x ranges from about 3.2 to about 3.8, y ranges from about 0.13 to about 0.46, z ranges from greater than zero up to about 0.2, and w ranges from about 2.9 to about 3.3. Thin-film batteries containing the sulfide doped lithium oxynitride electrolyte are capable of delivering more power and energy than thin-film batteries containing electrolytes without sulfide doping.
Owner:OAK RIDGE MICRO ENERGY
Ternary oxide nanostructures and methods of making same
InactiveUS20070138459A1Suitable for preparationFrom gel stateAlkaline earth titanatesNanostructureDislocation
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Ti, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Flame made metal oxides
ActiveUS7211236B2Add featureWell mixedMaterial nanotechnologyZirconium oxidesSpray pyrolysisCarboxylic acid
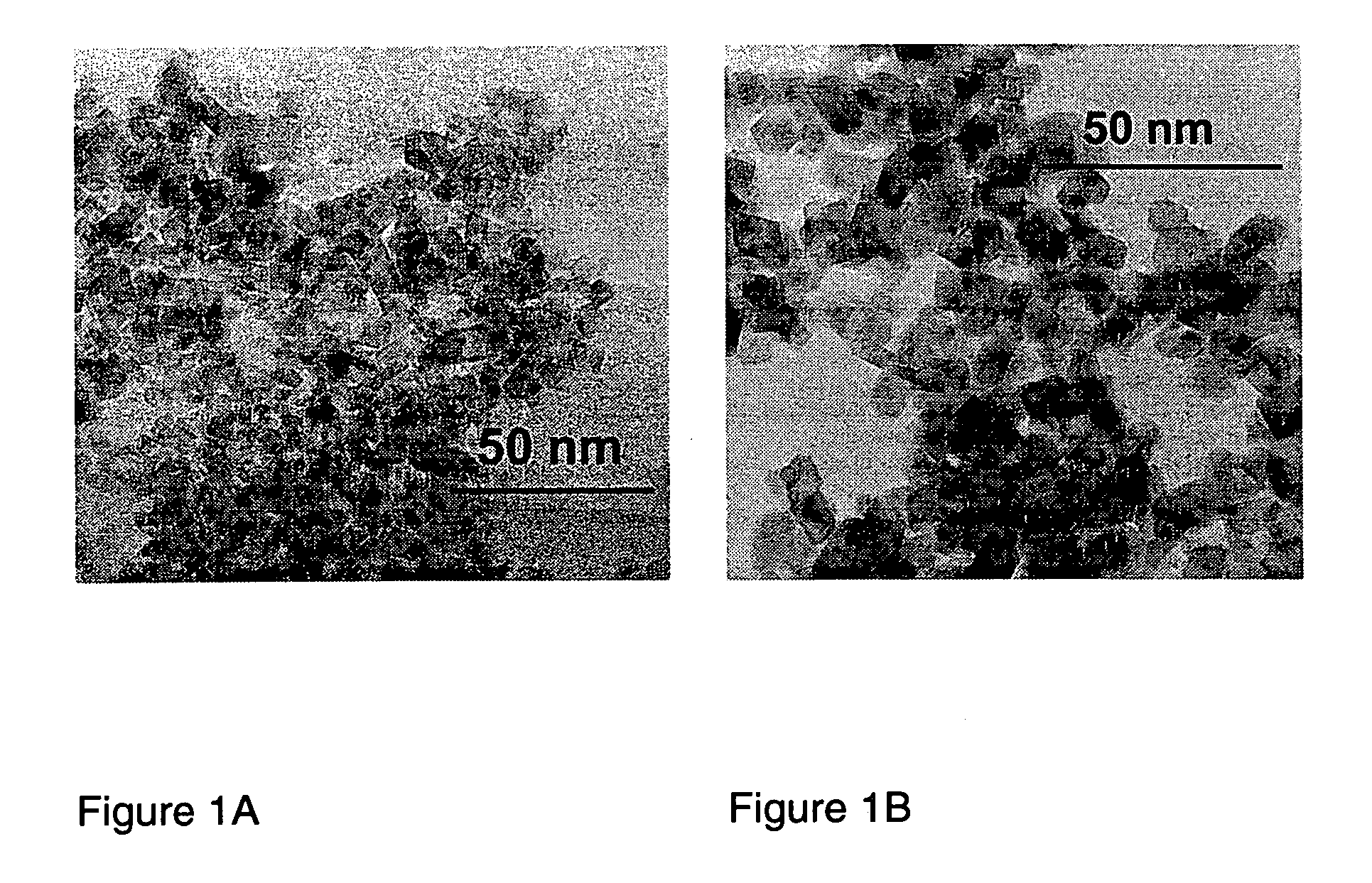
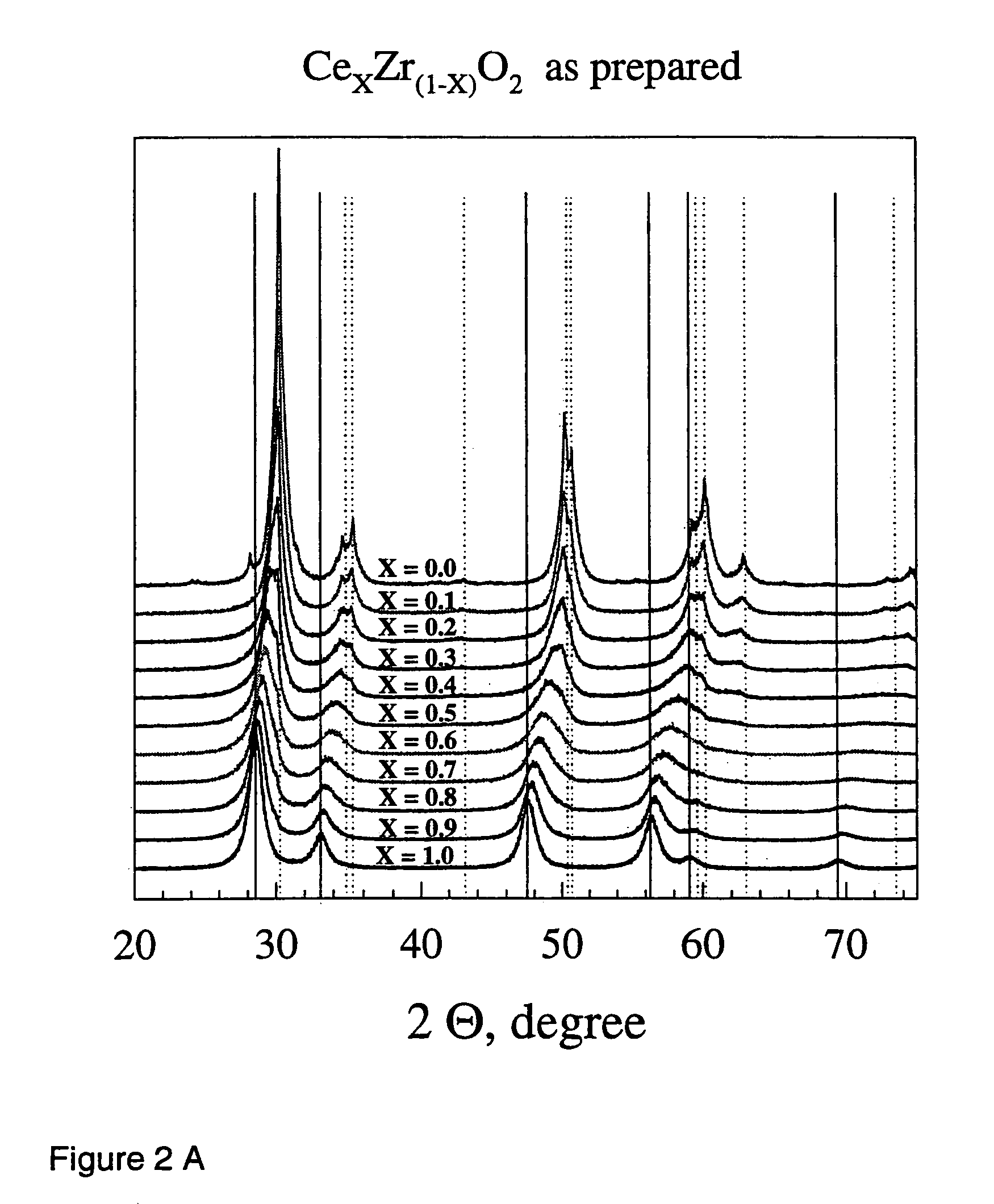
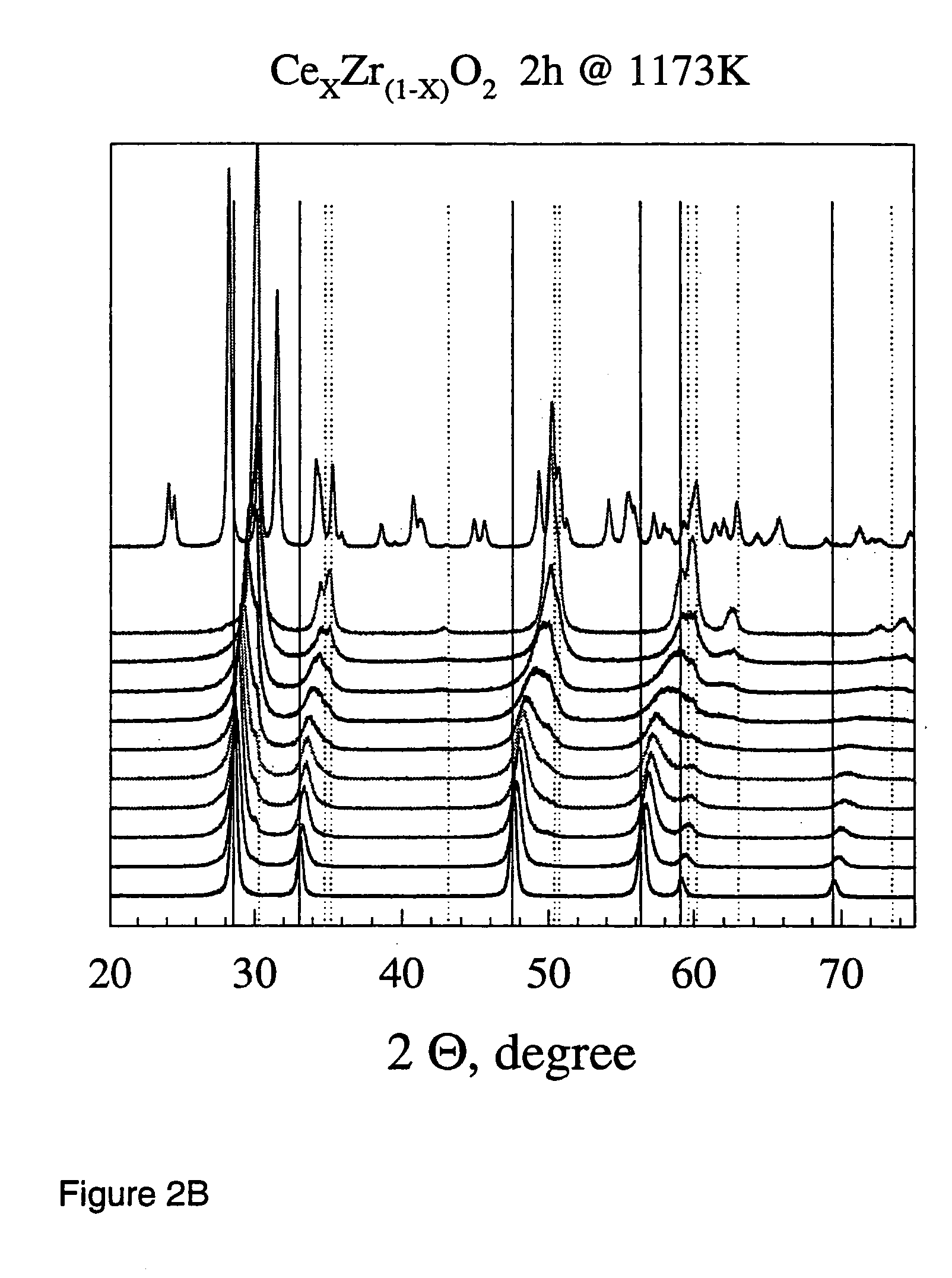
Described is a method for the production of metal oxides by flame spray pyrolysis, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Positive electrode active material precursor for lithium secondary battery, positive electrode active material manufactured by using thereof, and lithium secondary battery including same
ActiveUS20140158932A1Large capacityEasy to insertFinal product manufactureAlkali metal oxidesLithiumEngineering
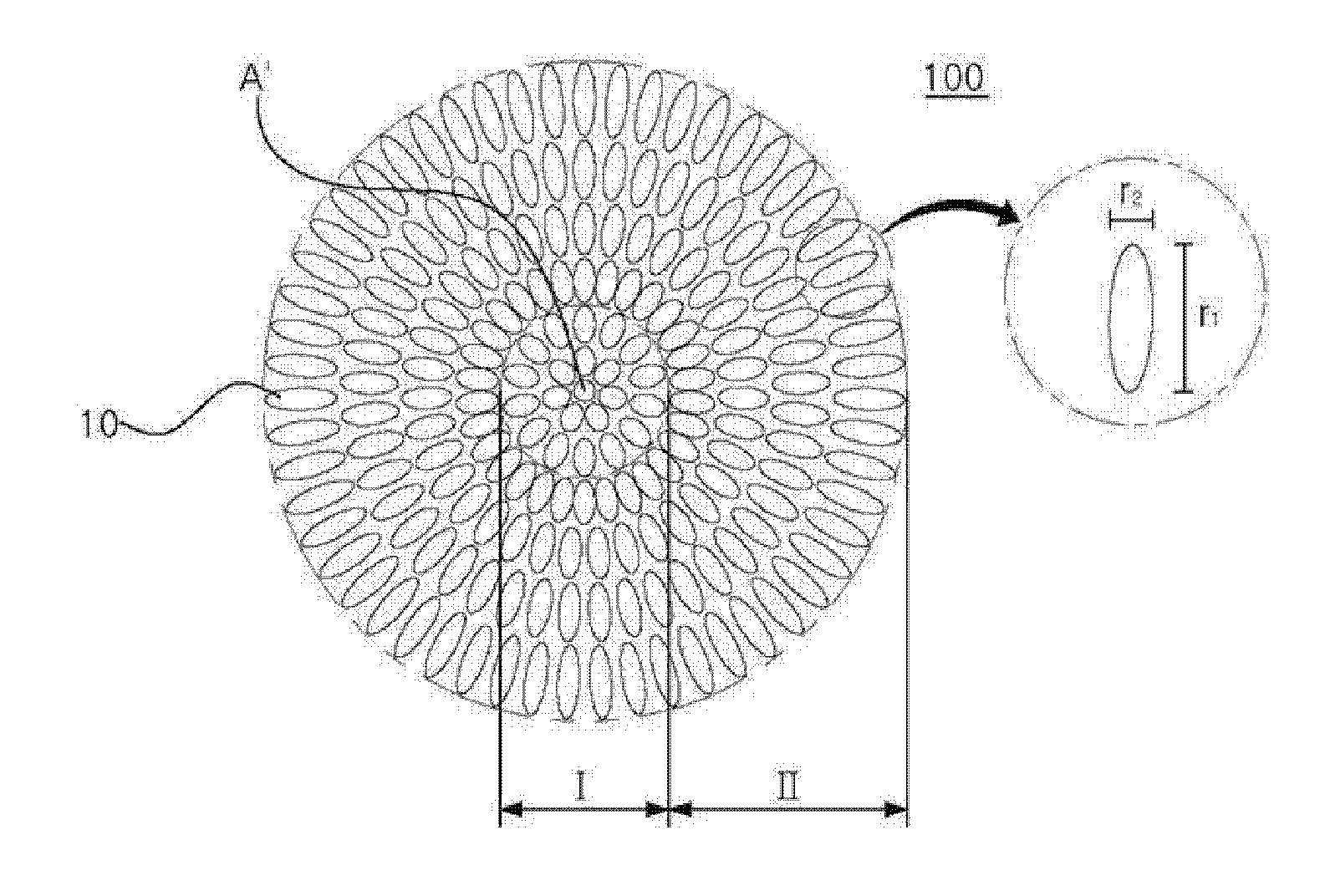
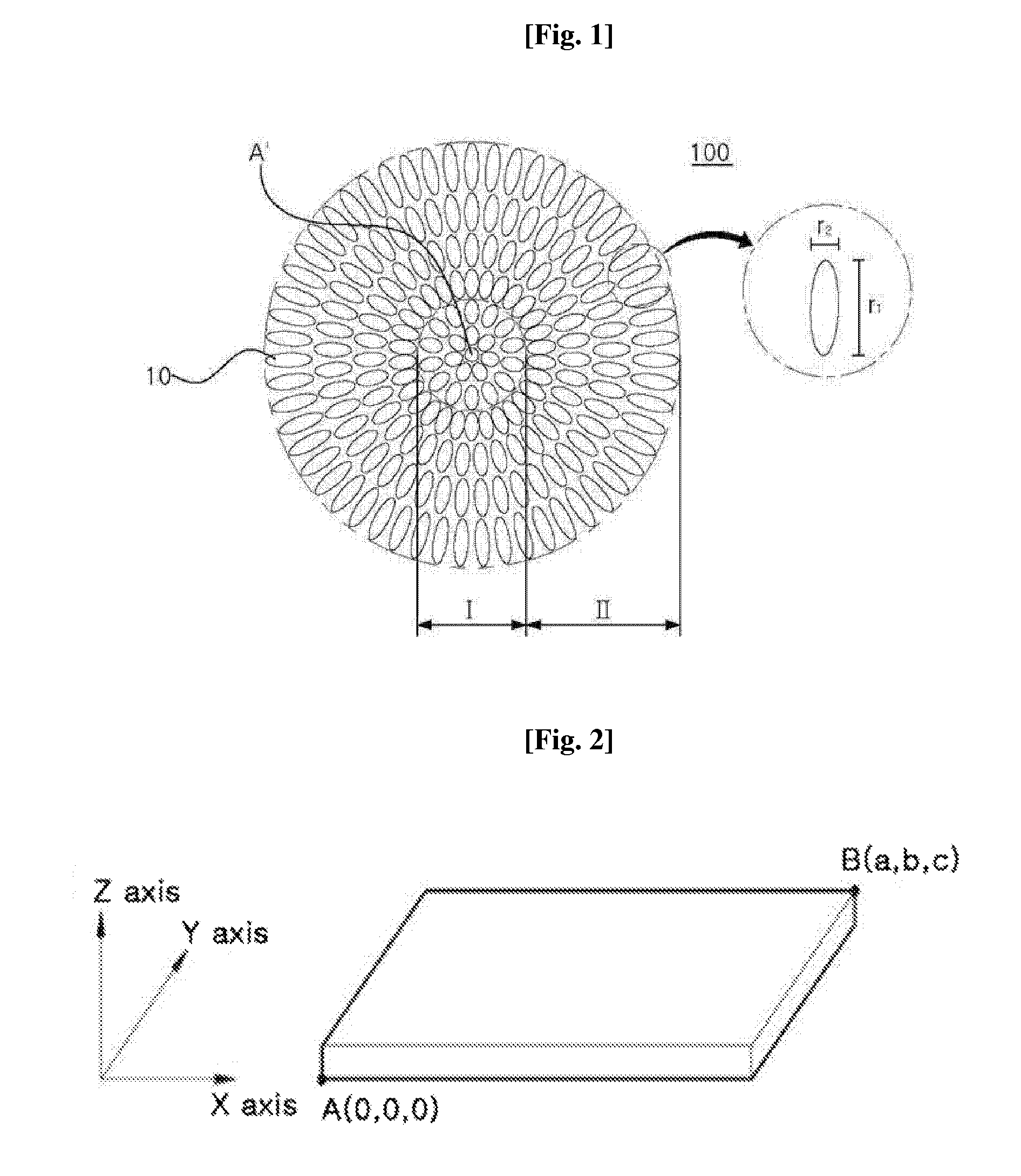
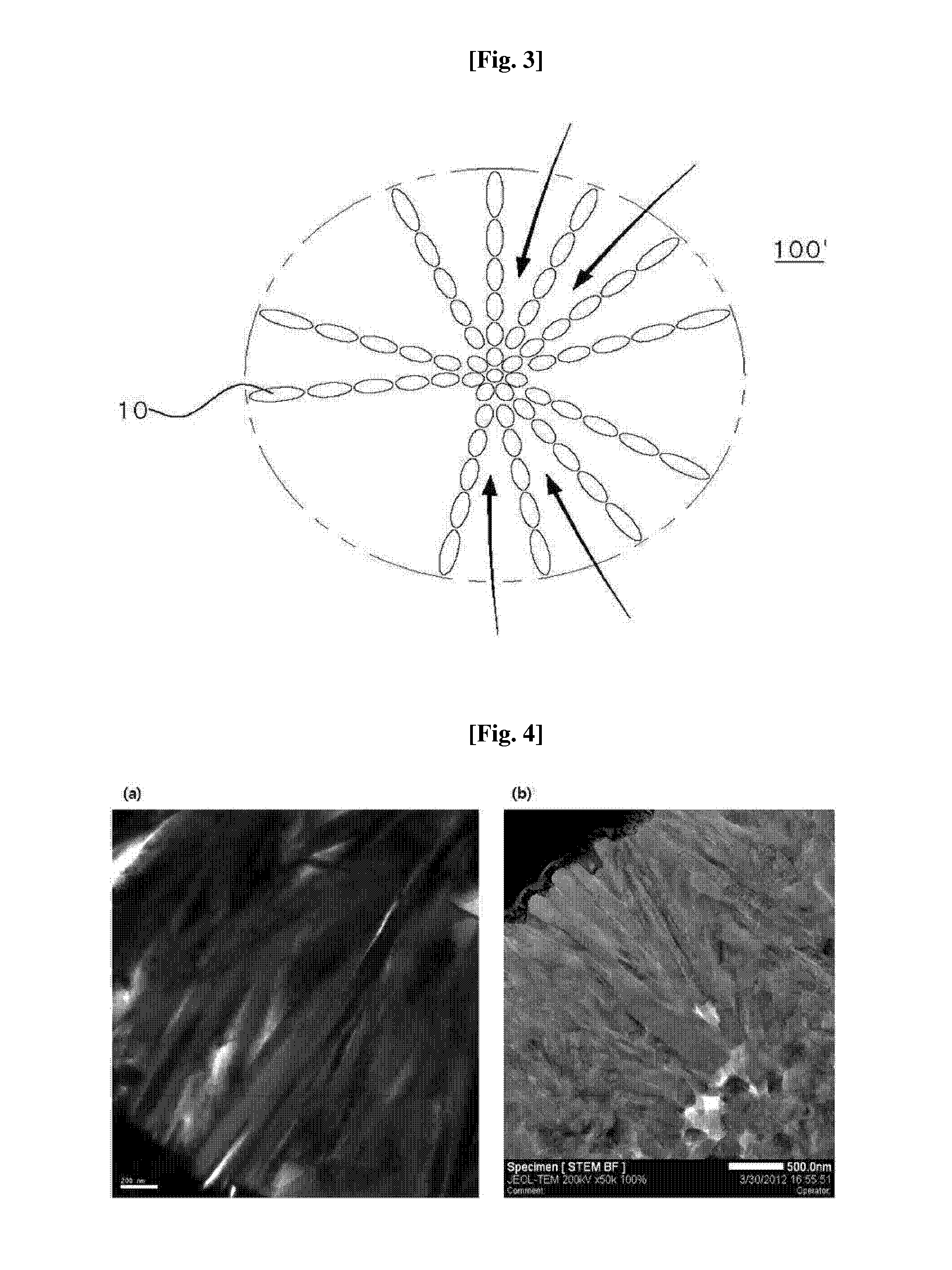
The present disclosure relates to a positive electrode active material precursor for a lithium secondary battery, a positive electrode active material manufactured by using thereof, and a lithium secondary battery comprising the same. More specifically, it relates to a positive electrode active material precursor for a lithium secondary battery as a secondary particle comprising transition metals, and formed by gathering of a plurality of primary particles having different a-axis direction length to c-axis direction length ratio, wherein the a-axis direction length to c-axis direction length ratio of the primary particle making up the secondary particle is increased from the center to the surface of the secondary particle; a positive electrode active material; and a lithium secondary battery comprising the same.
Owner:LG CHEM LTD
Ni-based lithium transition metal oxide
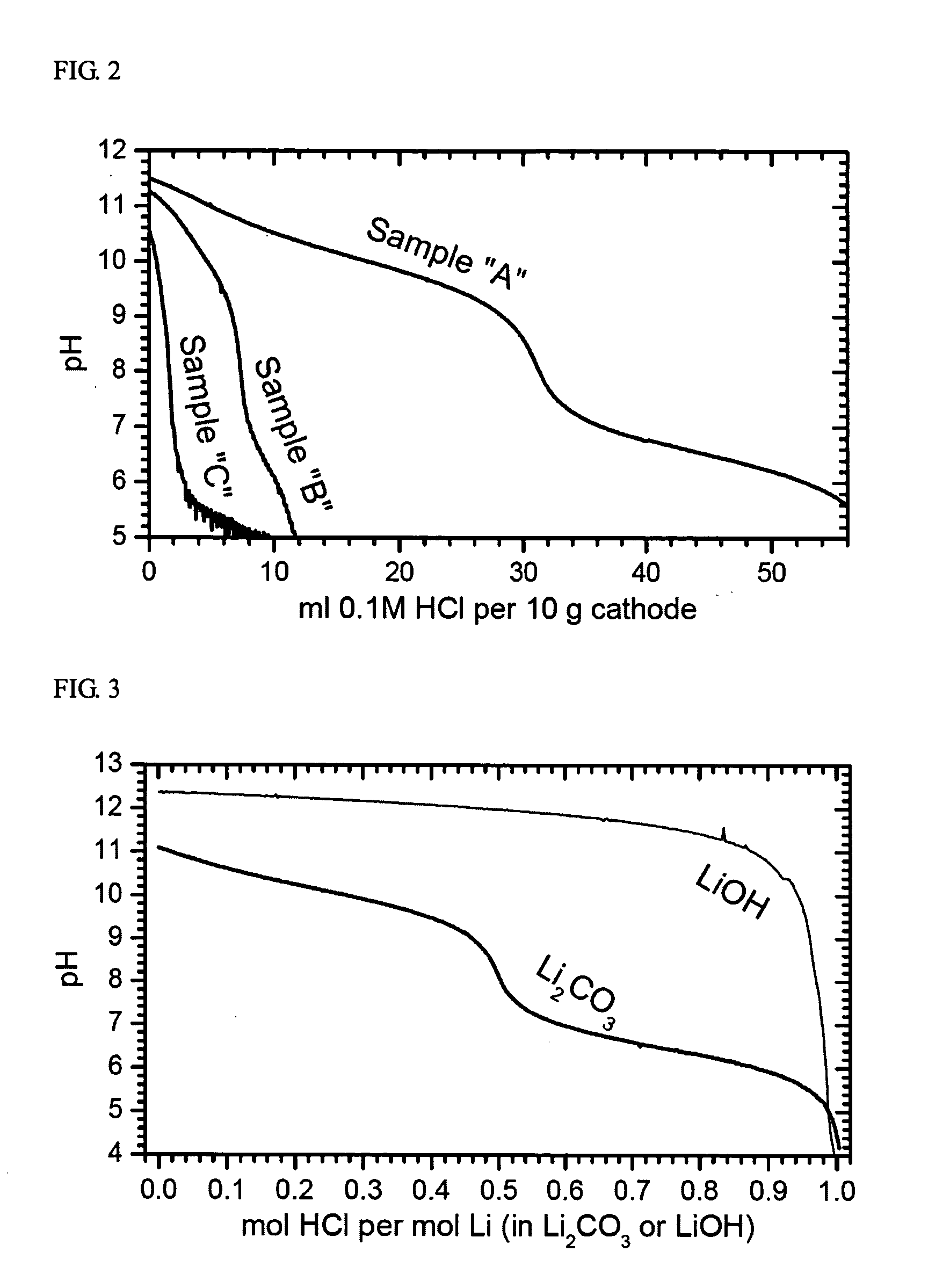
The present invention provides a powderous lithium transition metal oxide with the composition as represented by the below Formula and prepared by solid state reaction in air from a mixed transition metal precursor and Li2CO3, with being practically free of Li2CO3 impurity: LixMyO2 wherein M=M′l-kAk, where M′=Nil-a-b(Ni1 / 2Mn1 / 2)aCob on condition of 0.65≦a+b≦0.85 and 0.1≦b≦0.4; A is a dopant; and 0≦k≦0.05; and x+y=2 on condition of 0.95≦x≦1.05. The Ni-based lithium transition metal oxide according to the present invention has a well-layered structure, and also improved safety, cycling stability and stability against aging and low gas evolution during storage, when used as an active material for cathode of lithium secondary batteries, because it has a high sintering stability and is substantially free of soluble bases. Moreover, the lithium transition metal oxide of the present invention can be prepared by a low-cost process using a mixed transition metal precursor and Li2CO3 as raw stocks and under relatively unrestricted conditions.
Owner:LG ENERGY SOLUTION LTD
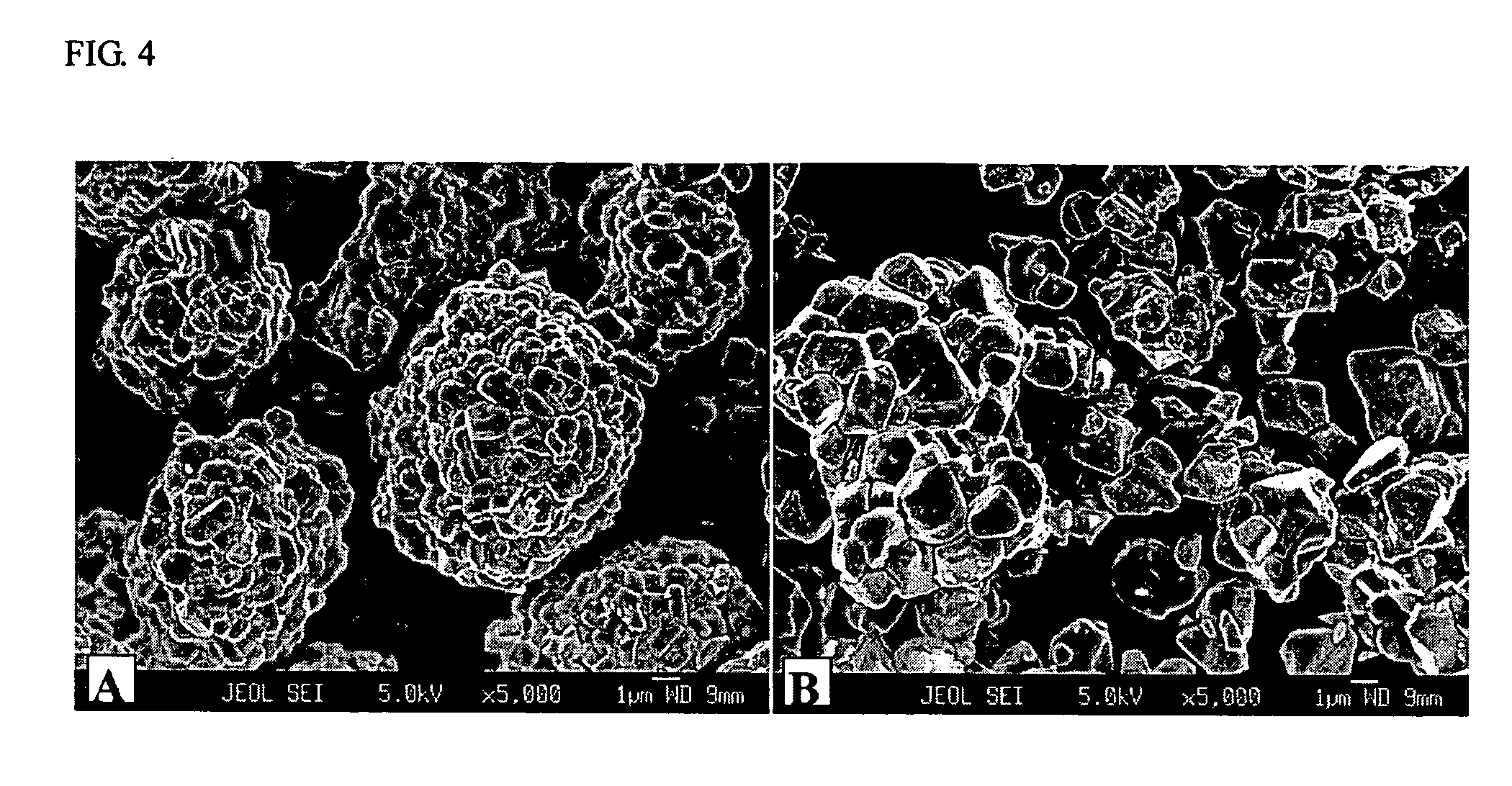
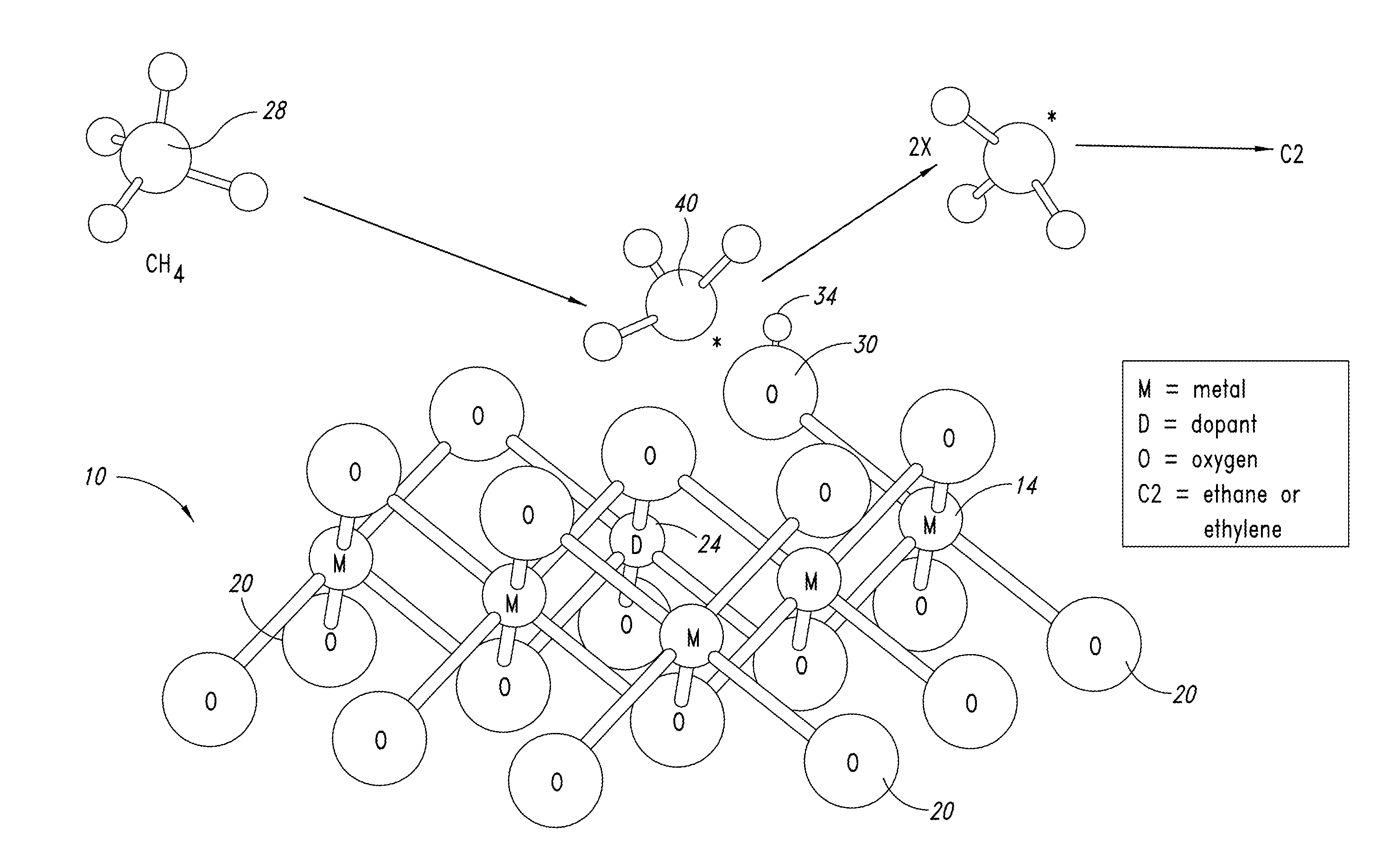
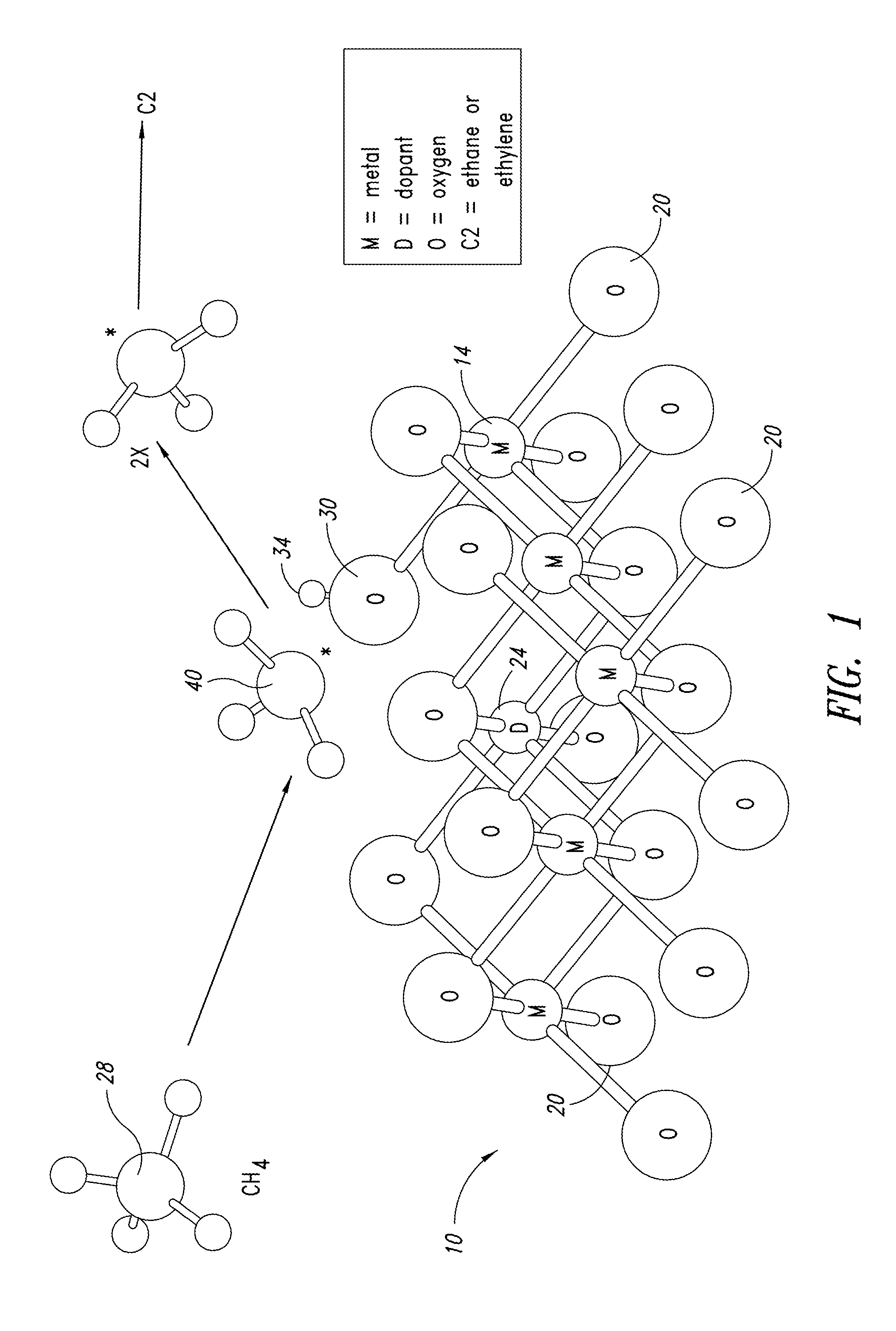
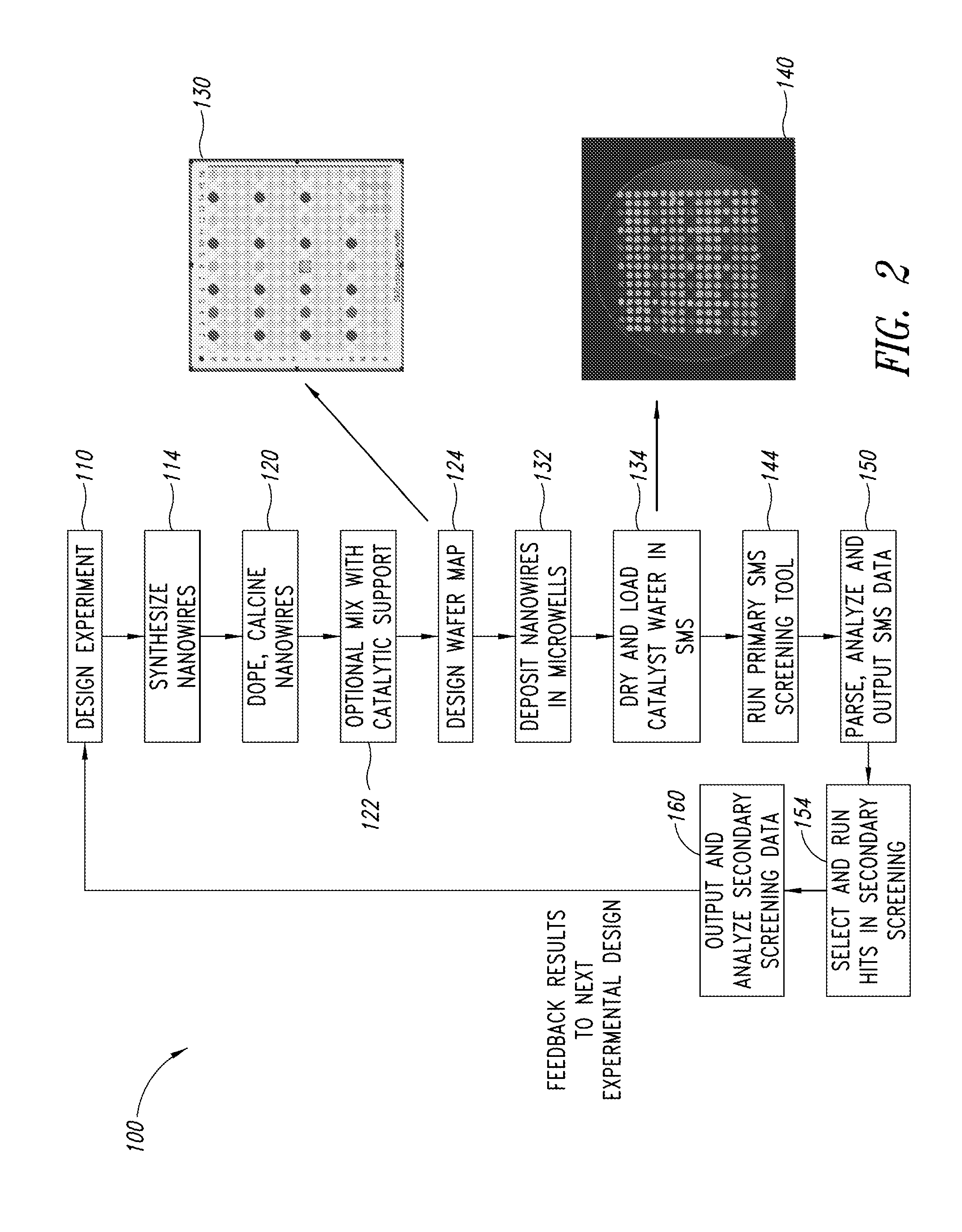
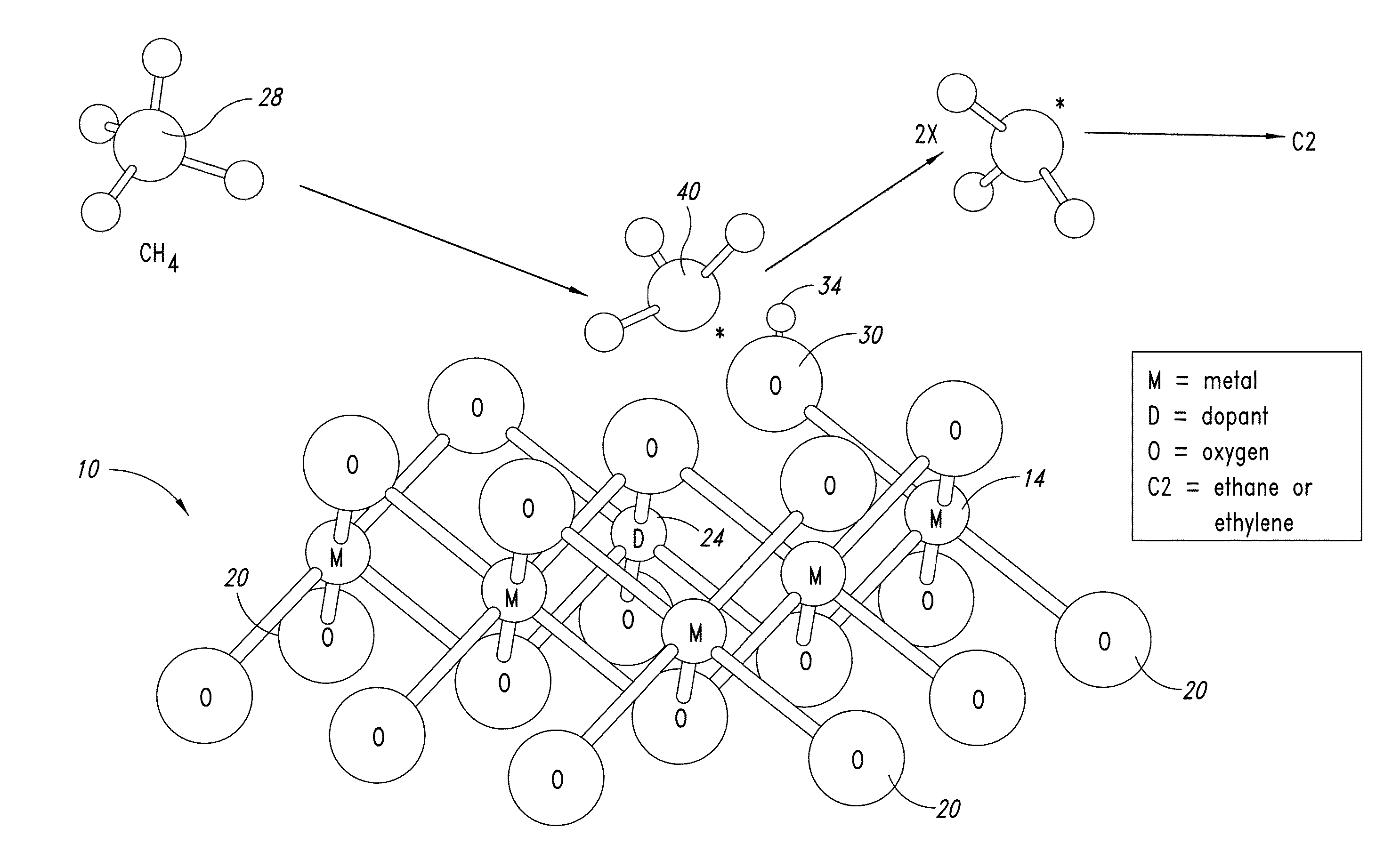
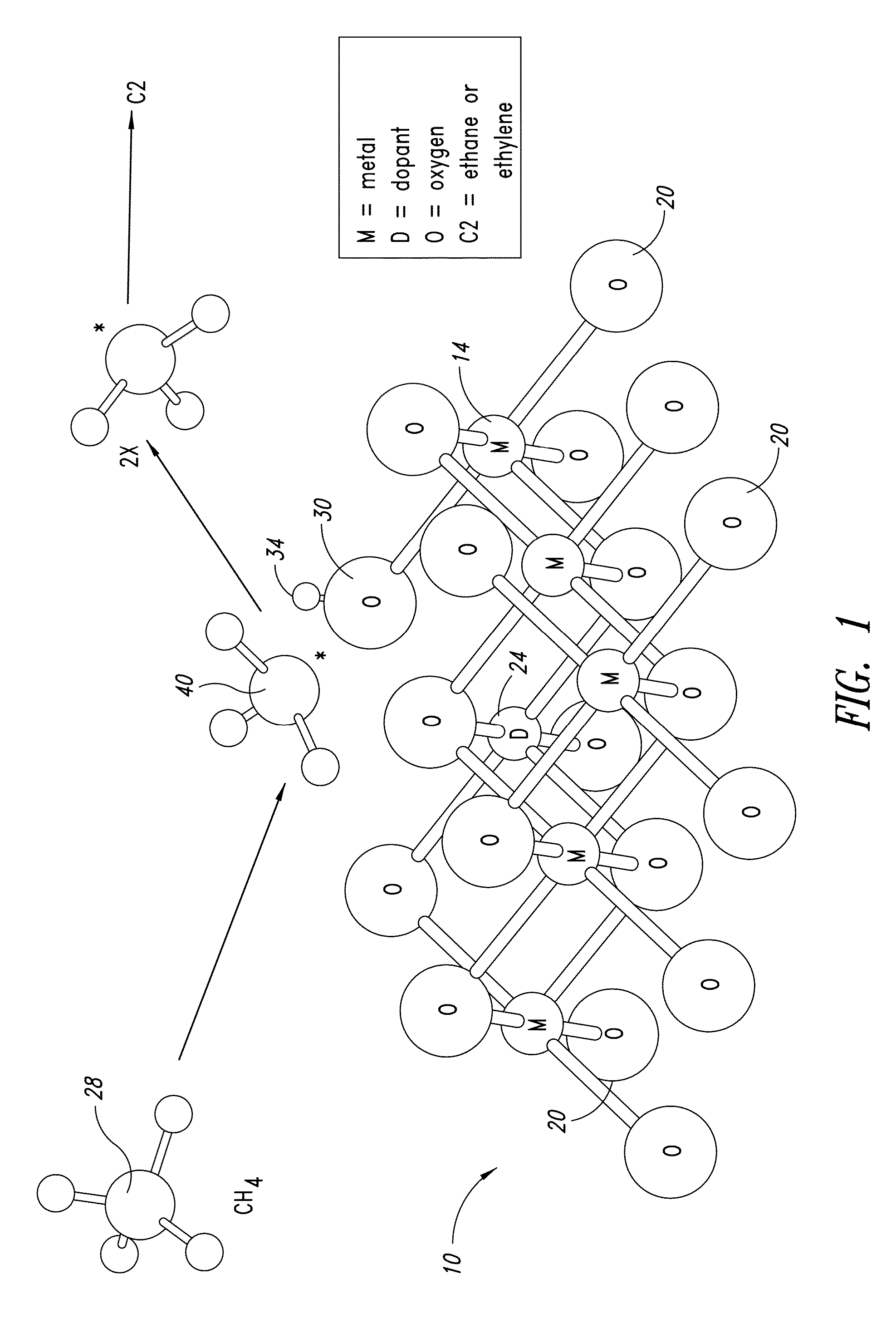
Nanowire catalysts and methods for their use and preparation
ActiveUS20130165728A1Material nanotechnologyManganese oxides/hydroxidesNanowireOxidative coupling of methane
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons. Related methods for use and manufacture of the same are also disclosed.
Owner:LUMMUS TECH LLC
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
Process for the production of ultrafine particles
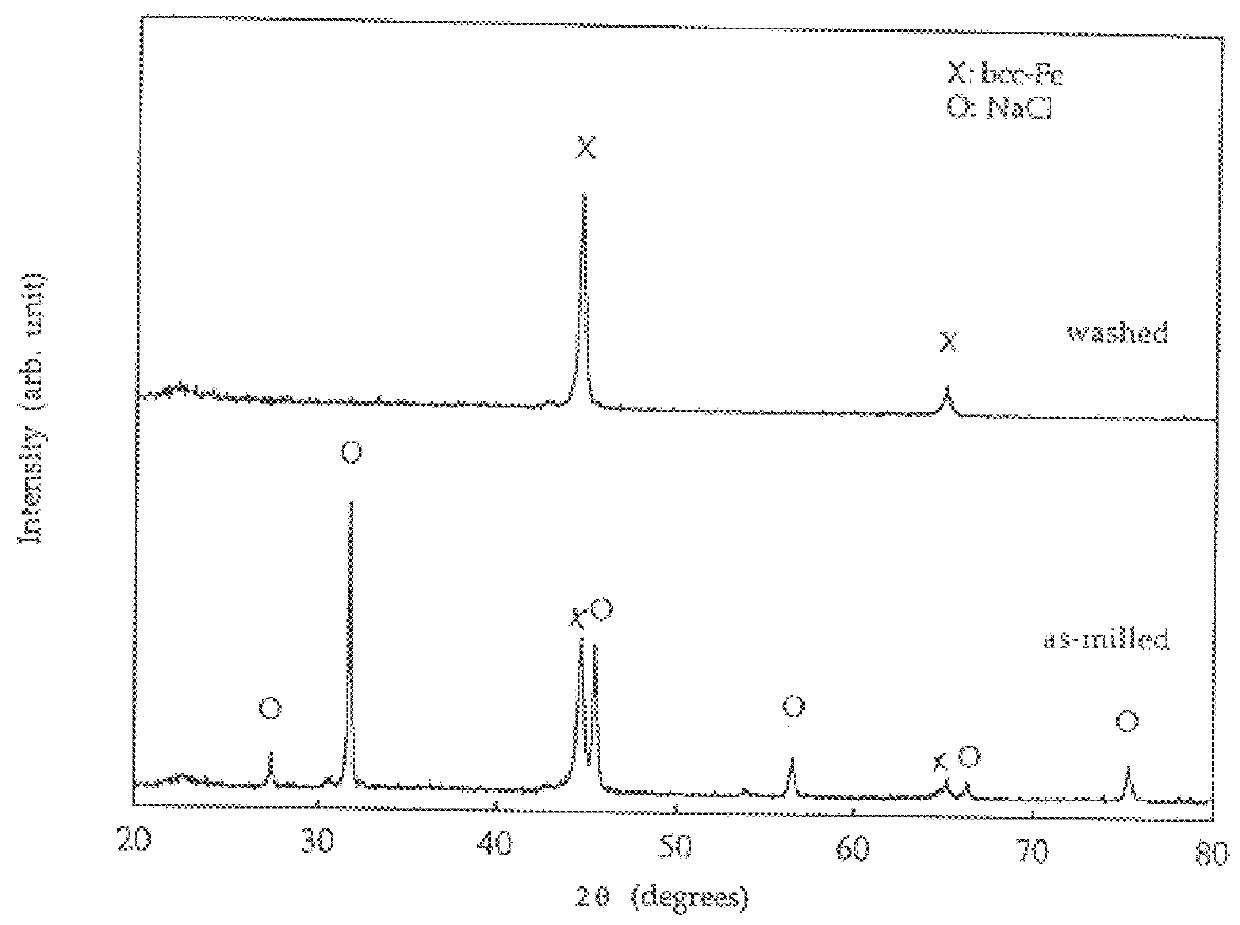
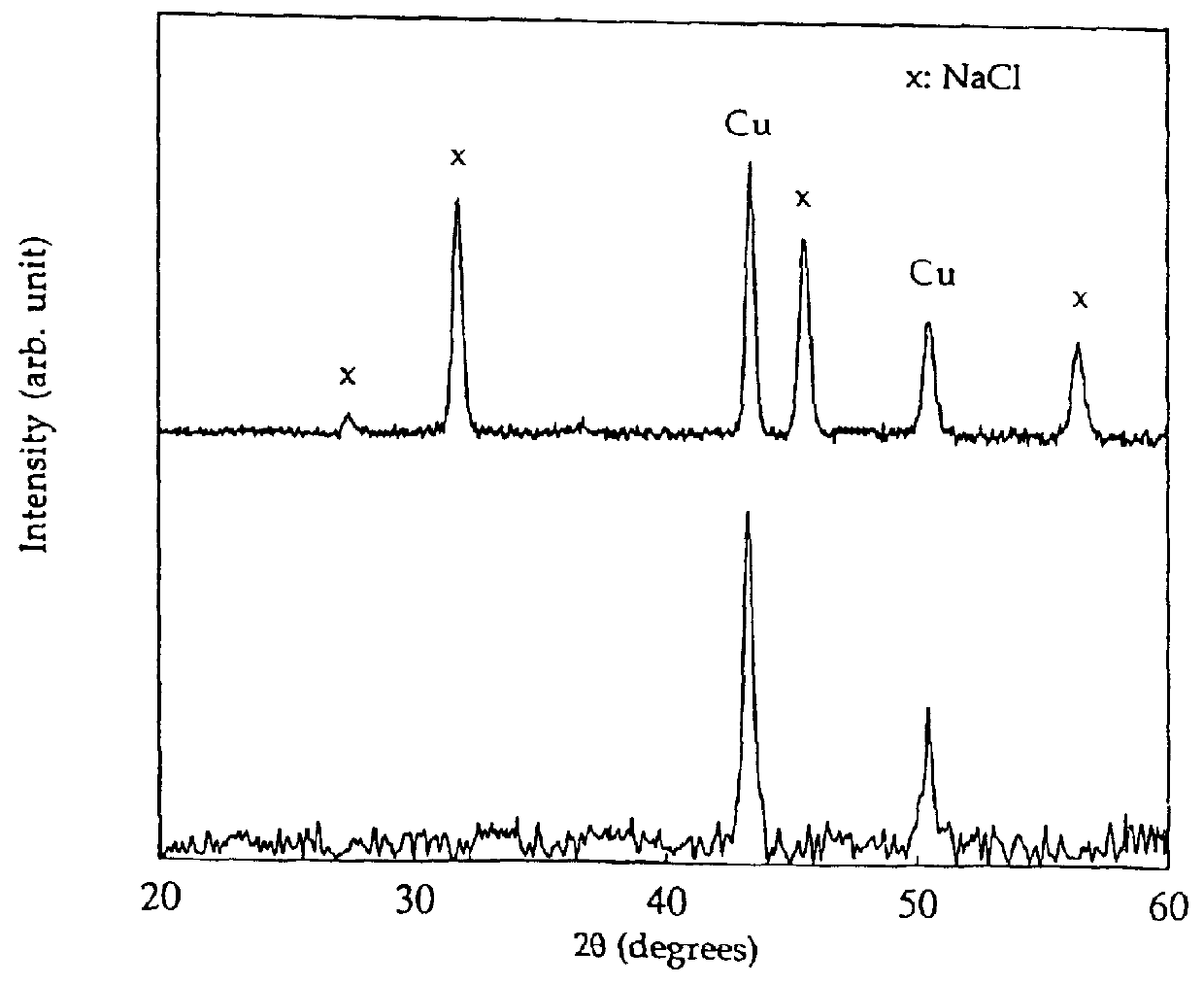
A new, cost effective process for the production of ultrafine particles which is based on mechanically activated chemical reaction of a metal compound with a suitable reagent. The process involves subjecting a mixture of a metal compound and a suitable reagent to mechanical activation to increase the chemical reactivity of the reactants and / or reaction kinetics such that a chemical reaction can occur which produces a solid nano-phase substance. Concomitantly, a by-product phase is also formed. This by-product phase is removed so that the solid nano-phase substance is left behind in the form of ultrafine particles. During mechanical activation a composite structure is formed which consists of an intimate mixture of nano-sized grains of the nano-phase substance and the reaction by-product phase. The step of removing the by-product phase, following mechanical activation, may involve subjecting the composite structure to a suitable solvent which dissolves the by-product phase, while not reacting with the solid nano-phase substance. The process according to the invention may be used to form ultrafine metal powders as well as ultrafine ceramic powders. Advantages of the process include a significant degree of control over the size and size distribution of the ultrafine particles, and over the nature of interfaces created between the solid nano-phase substance and the reaction by-product phase.
Owner:WESTERN AUSTRALIA UNIV OF THE
Polymer templated nanowire catalysts
InactiveUS20130158322A1Improve drawing legibilityMaterial nanotechnologyManganese oxides/hydroxidesNanowirePolymer science
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are prepared by polymer templated methods and are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethane and / or ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
Layered Lithium Nickel Manganese Cobalt Composite Oxide Powder For Material Of Positive Electrode Of Lithium Secondary Battery, Process For Producing The Same, Positive Electrode Of Lithium Secondary Battery Therefrom, And Lithium Secondary Battery
ActiveUS20070202405A1Improve securityImprove battery performanceNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsManganeseCobalt
A powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is provided which, when used as a positive-electrode material for lithium secondary battery, enables a cost reduction and higher safety to be reconciled with improved battery performances. The powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is composed of secondary particles formed by the aggregation of primary particles. It has a composition represented by the following formula (I), has a volume resistivity of 5×105 Ω·cm or lower in the state of being compacted at a pressure of 40 MPa, and has a value of C / S, wherein C is the concentration of carbon contained therein (% by weight) and S is the BET specific surface area thereof (m2 / g), of 0.025 or smaller. The powder has been regulated so as to have a volume resistivity not higher than the specified value and a considerably reduced carbon content while having a composition in a limited range, whereby a cost reduction and higher safety can be reconciled with improved battery performances. Li1+zNixMnyCo1−x−yOδ (I) (0<z≦0.91, 0.1≦x≦0.55, 0.20≦y≦0.90, 0.50≦x+y≦1, 1.9≦δ≦3)
Owner:MITSUBISHI CHEM CORP
Positive electrode active material for lithium secondary cell and lithium secondary cell
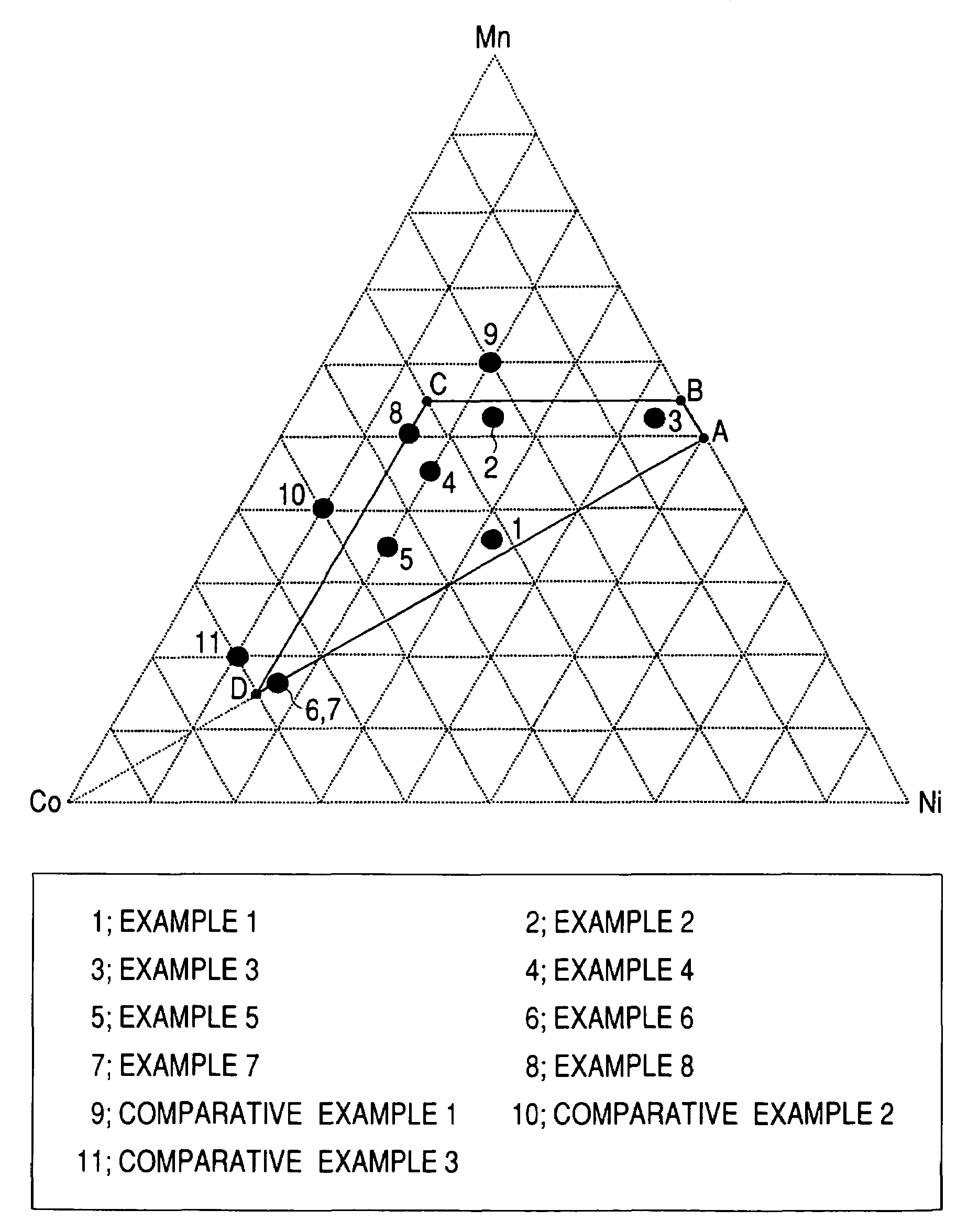
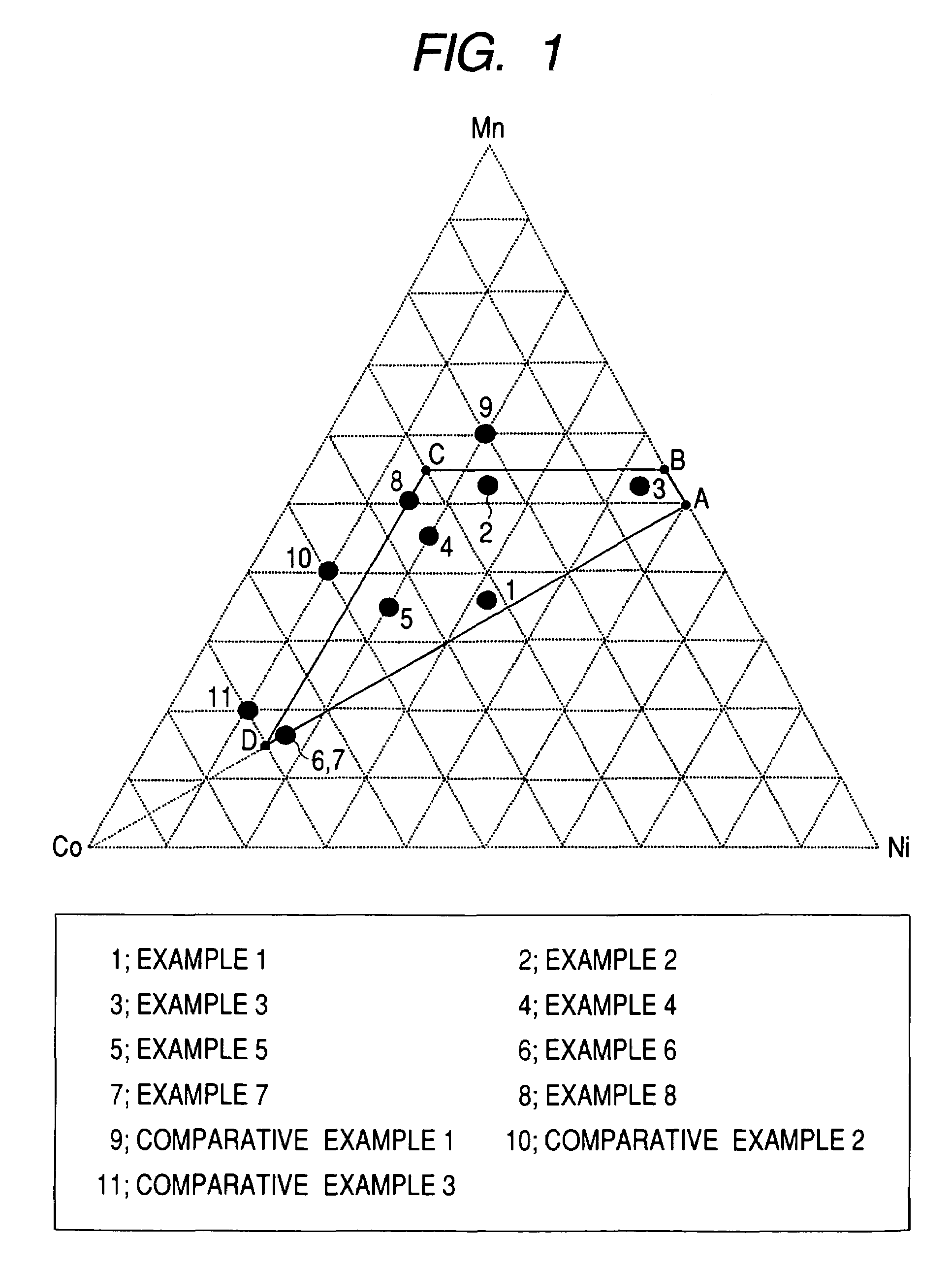
ActiveUS7393476B2Improve cycle performanceIncrease energy densityMaterial nanotechnologyCell temperature controlCrystallographyComposite oxide
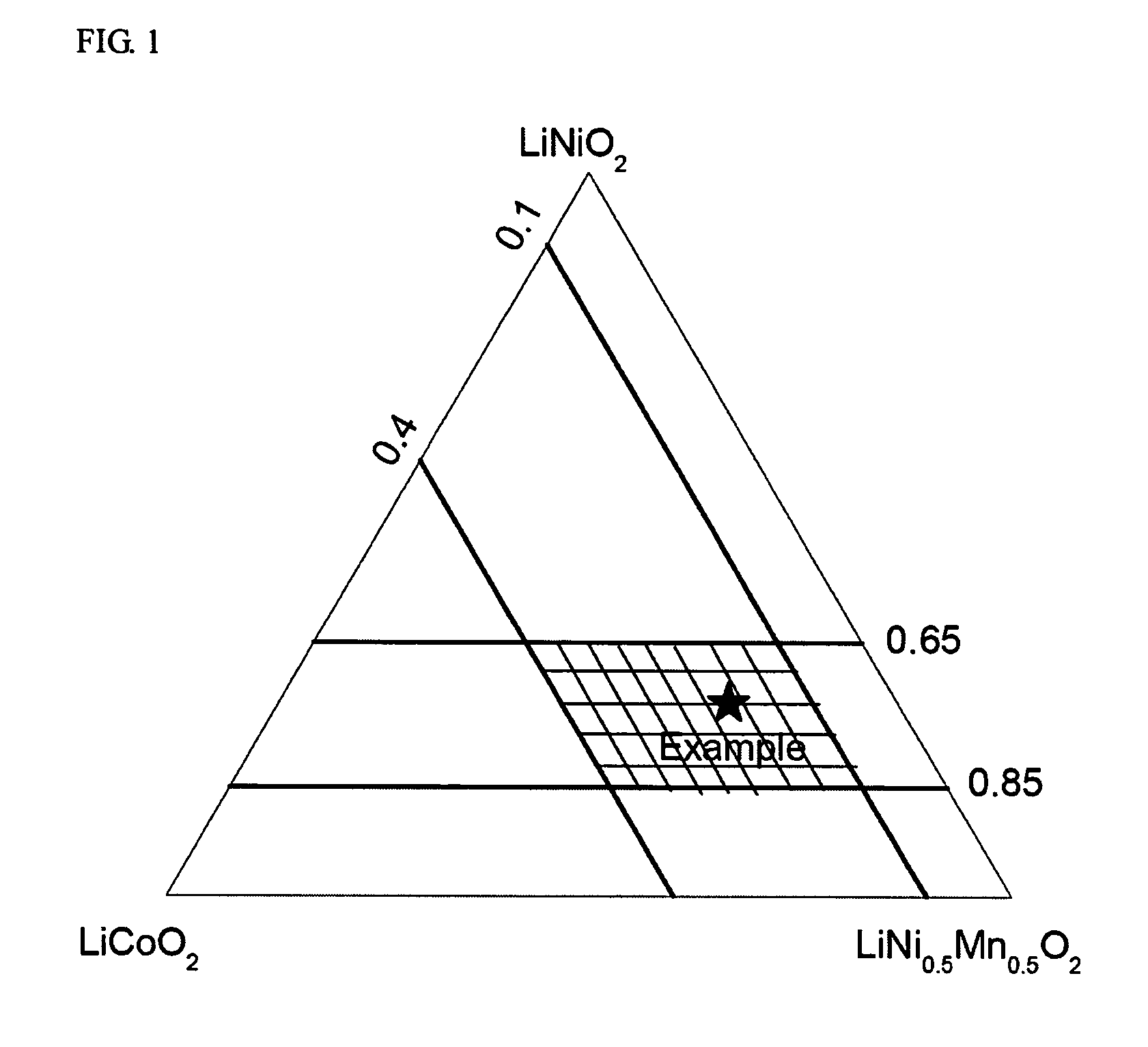
A positive active material for lithium secondary batteries, includes a composite oxide including an oxide which is represented by the composite formula LixMnaNibCocO2 and has an α-NaFeO2 structure, and an impurity phase including Li2MnO3. The values a, b, and c are within such a range that in a ternary phase diagram showing the relationship among these, (a, b, c) is present on the perimeter of or inside the quadrilateral ABCD defined by point A (0.5, 0.5, 0), point B (0.55, 0.45, 0), point C (0.55, 0.15, 0.30), and point D (0.15, 0.15, 0.7) as vertexes, and 0.95<x / (a+b+c)<1.35.
Owner:GS YUASA INT LTD
Cathode intercalation compositions, production methods and rechargeable lithium batteries containing the same
InactiveUS6248477B1Easy to adaptOxygen/ozone/oxide/hydroxideElectrode thermal treatmentMetalSpinel group
Intercalation compositions having spinel structures with crystallites of metal oxides (M2O3) dispersed throughout the structure are provided having the general formula Li1+xMyMn2-x-yO4. Methods of producing the intercalation compositions and rechargeable lithium batteries containing the compositions are also provided.
Owner:EMD ACQUISITION LLC
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
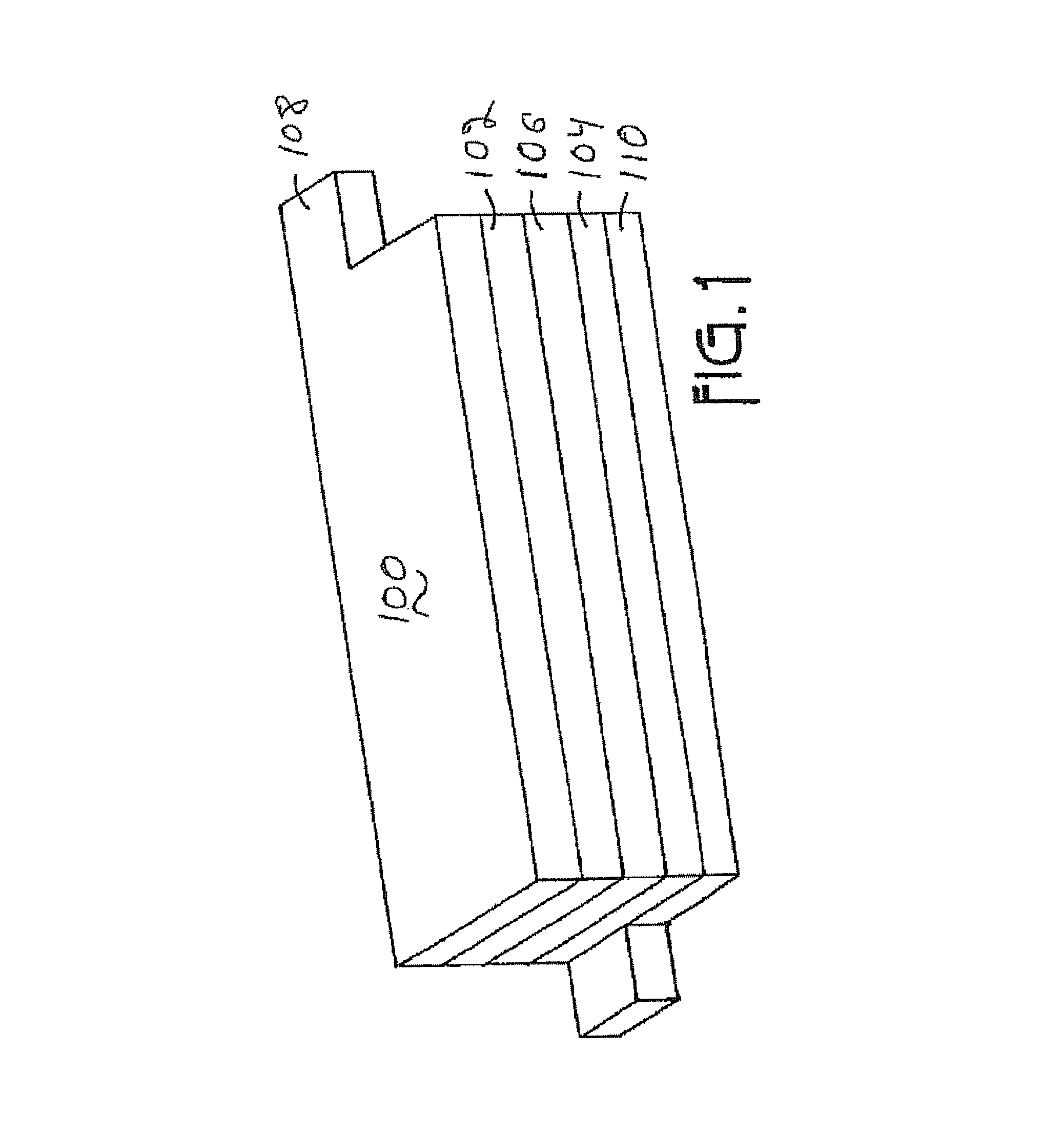
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS8389160B2Electrode manufacturing processesAlkali metal oxidesElectrical batteryDischarge rate
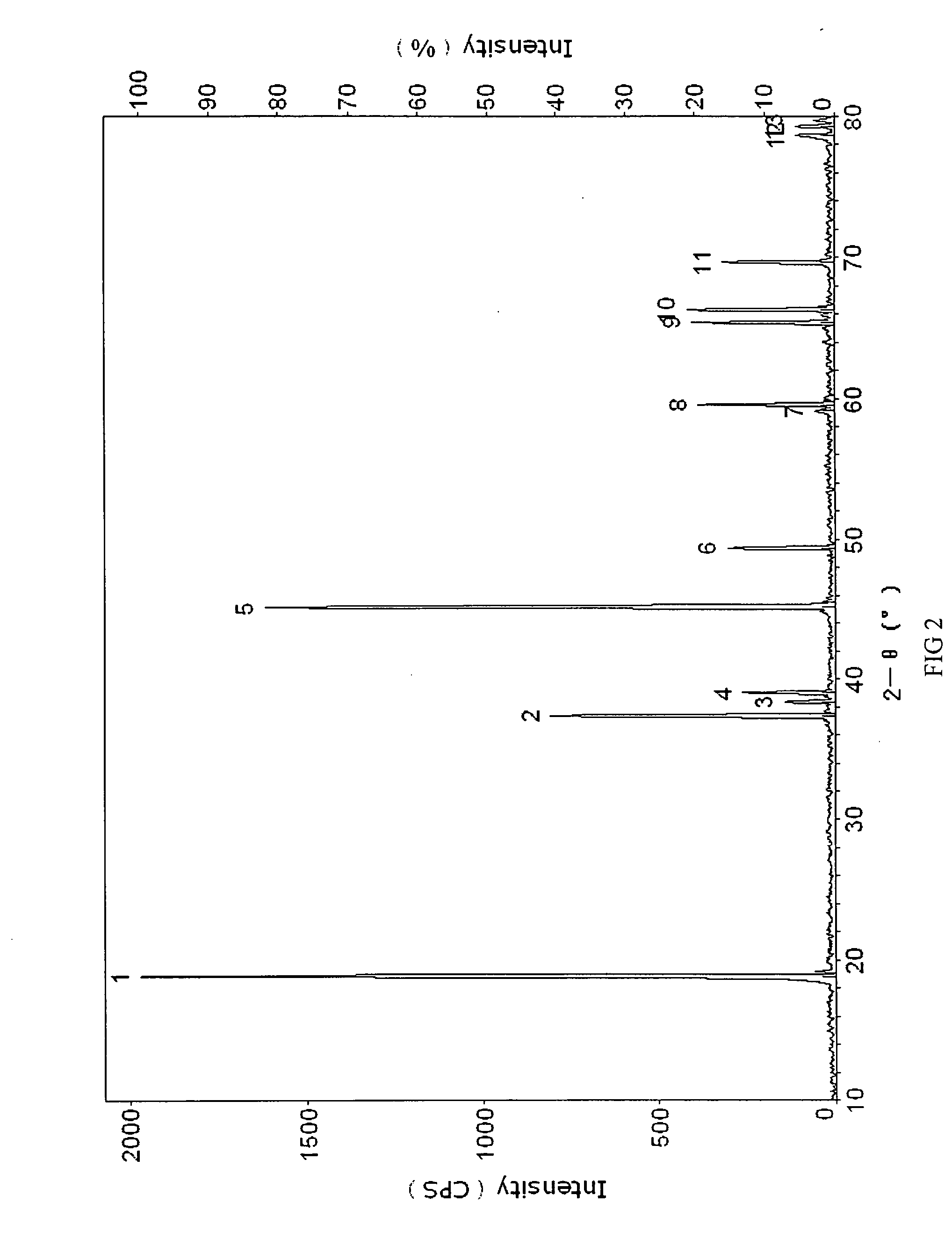
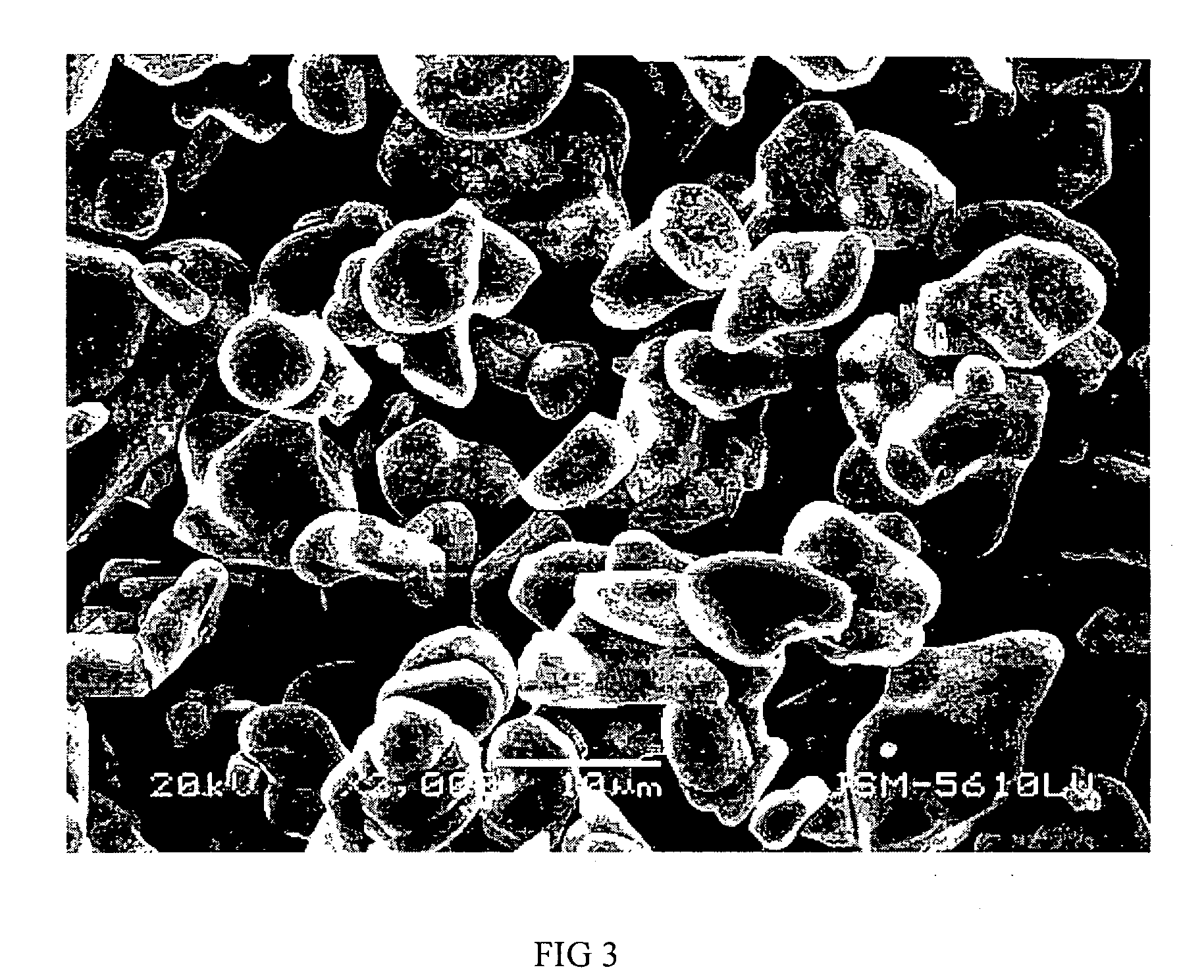
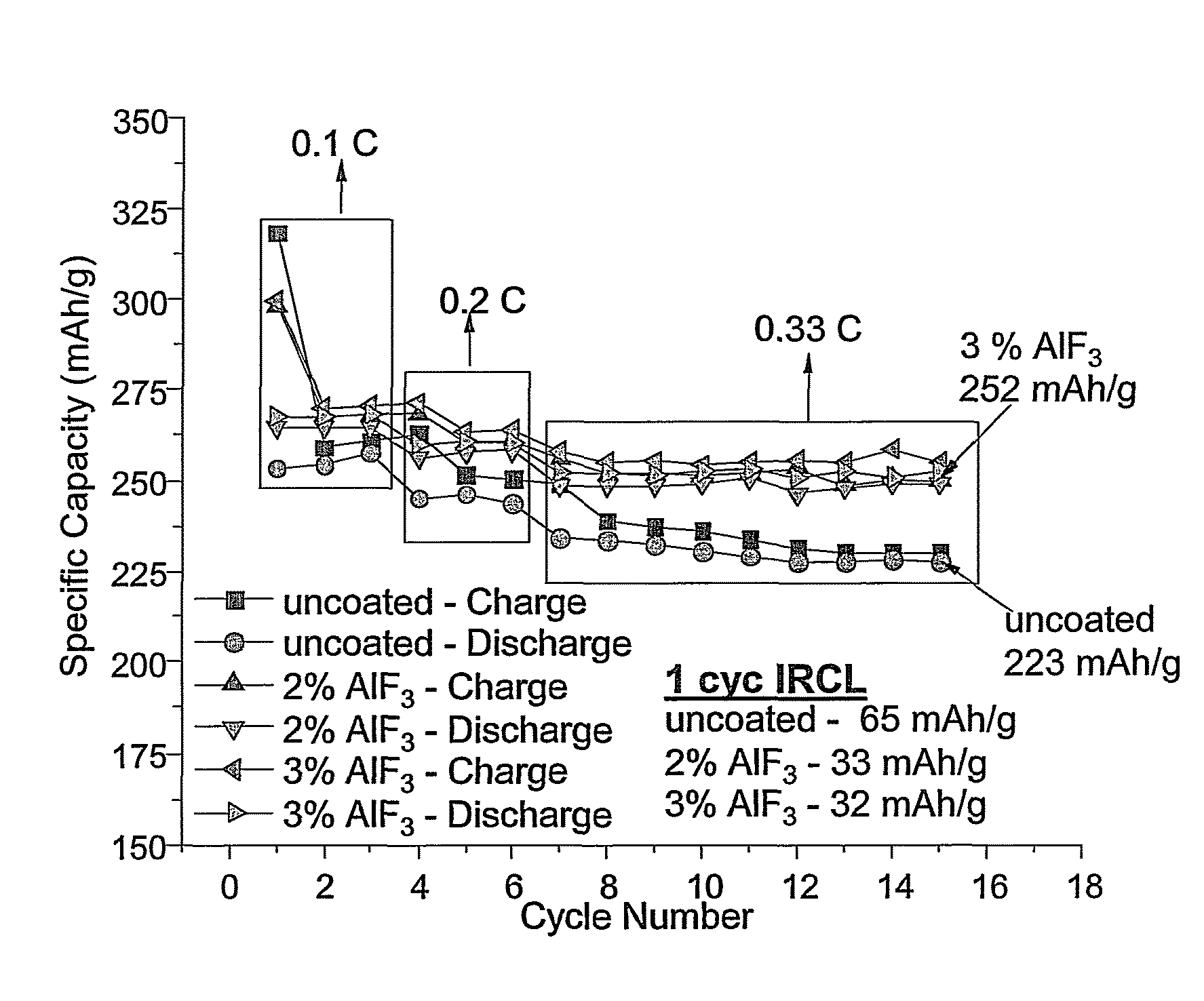
Positive electrode active materials are described that have a very high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβCOγO2, where x ranges from about 0.05 to about 0.25, α ranges from about 0.1 to about 0.4, β ranges from about 0.4 to about 0.65, and γ ranges from about 0.05 to about 0.3. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. Also, the coated materials can exhibit a very significant decrease in the irreversible capacity lose upon the first charge and discharge of the cell. Methods for producing these materials include, for example, a co-precipitation approach involving metal hydroxides and sol-gel approaches.
Owner:IONBLOX INC
Lithium metal oxide electrodes for lithium batteries
InactiveUS20060188781A1Increase capacityImprove cycle stabilityActive material electrodesAlkali metal oxidesLithium metalOxidation state
An uncycled preconditioned electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn, and an uncycled electrode of the formula xLi2M′O3.(1−x)LiMO2, in which 0≦x<1, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more first-row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first- and second-row transition metal elements and Sn, the electrode being preconditioned in a proton-containing medium with a pH<7.0. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC +1
Lithium metal oxide electrodes for lithium batteries
InactiveUS7314682B2Electrode thermal treatmentActive material electrodesLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Lithium metal oxide materials and methods of synthesis and use
ActiveUS7381496B2Large capacityImprove cycle lifeOxide/hydroxide preparationNickel compounds preparationLithium metalElectrochemical cell
A composition having a formula LixMgyNiO2 wherein 0.9<x<1.3, 0.01<y<0.1, and 0.91<x+y<1.3 can be utilized as cathode materials in electrochemical cells. A composition having a core, having a formula LixMgyNiO2 wherein 0.9<x<1.3, 0.01<y<0.1, and 0.9<x+y<1.3, and a coating on the core, having a formula LiaCobO2 wherein 0.7<a<1.3, and 0.9<b<1.2, can also be utilized as cathode materials in electrochemical cells.
Owner:TIAX LLC
Positive electrode active material and nonaqueous electrolyte secondary cell including the same
InactiveUS20050079416A1Large capacityImprove charge and discharge efficiencyOxygen/ozone/oxide/hydroxideIron oxides/hydroxidesMetallic lithiumManganese
A nonaqueous electrolytic secondary cell produced at low cost and having a large capacity comprises a negative electrode having an active material mainly composed of a material that at least absorbs and releases lithium ions or metallic lithium, a positive electrode, and an electrolyte. The active material of the positive electrode is an oxide containing nickel, manganese, and cobalt, and the contents of the elements are substantial the same.
Owner:PANASONIC CORP +1
Active substance of positive electrode and nonaqueous electrolyte battery containing the same
ActiveUS20050019659A1Increase energy densityImprove high rate discharge performanceSecondary cellsAlkali metal oxidesHigh rateHigh energy
A positive active material is provided which can give a battery having a high energy density and excellent high-rate discharge performance and inhibited from decreasing in battery, performance even in the case of high-temperature charge. Also provided is a non-aqueous electrolyte battery employing the positive active material. The positive active material contains a composite oxide which is constituted of at least lithium (Li), manganese (Mn), nickel (Ni), cobalt (Co), and oxygen (O) and is represented by the following chemical composition formula: LiaMnbNicCodOe (wherein 0<a≦1.3, |b−c|≦0.05, 0.6≦d<1, 1.7≦e≦2.3, and b+c+d=1). The non-aqueous electrolyte battery has a positive electrode containing the positive active material, a negative electrode, and a non-aqueous electrolyte.
Owner:GS YUASA INT LTD
Cathode active material for lithium secondary battery, process for preparing the same and reactor for use in the same process
ActiveUS20070111098A1Large capacityHigh tap densityElectrode manufacturing processesPhosphatesLithiumHigh rate
The present invention relates to a cathode active material for a lithium secondary battery and a process for preparing the same. In accordance with the present invention, the cathode active material having a high packing density was designed and synthesized and thus provided is a cathode active material for a lithium secondary battery exhibiting structural stability such as improved characteristics for charge / discharge, service life and high-rate and thermal stability, by modifying surface of the electrode active material with amphoteric or basic compounds capable of neutralizing acid produced around the cathode active material.
Owner:LG CHEM LTD
Method of synthesizing electrochemically active materials from a slurry of precursors
InactiveUS6913855B2Quality improvementHigh-quality materialPhosphatesElectrode thermal treatmentCompound (substance)Slurry
A method for making an active material comprises the steps of forming a slurry, spray drying the slurry to form a powdered precursor composition, and heating the powdered precursor composition at a temperature and for a time sufficient to form a reaction product. The slurry has a liquid phase and a solid phase, and contains at least an alkali metal compound and a transition metal compound. Preferably the liquid phase contains dissolved alkali metal compound, and the solid phase contains an insoluble transition metal compound, an insoluble carbonaceous material compound, or both. Electrodes and batteries are provided that contain the active materials.
Owner:RIL USA INC +1
Lithium composite oxide particle for positive electrode material of lithium secondary battery, and lithium secondary battery positive electrode and lithium secondary battery using the same
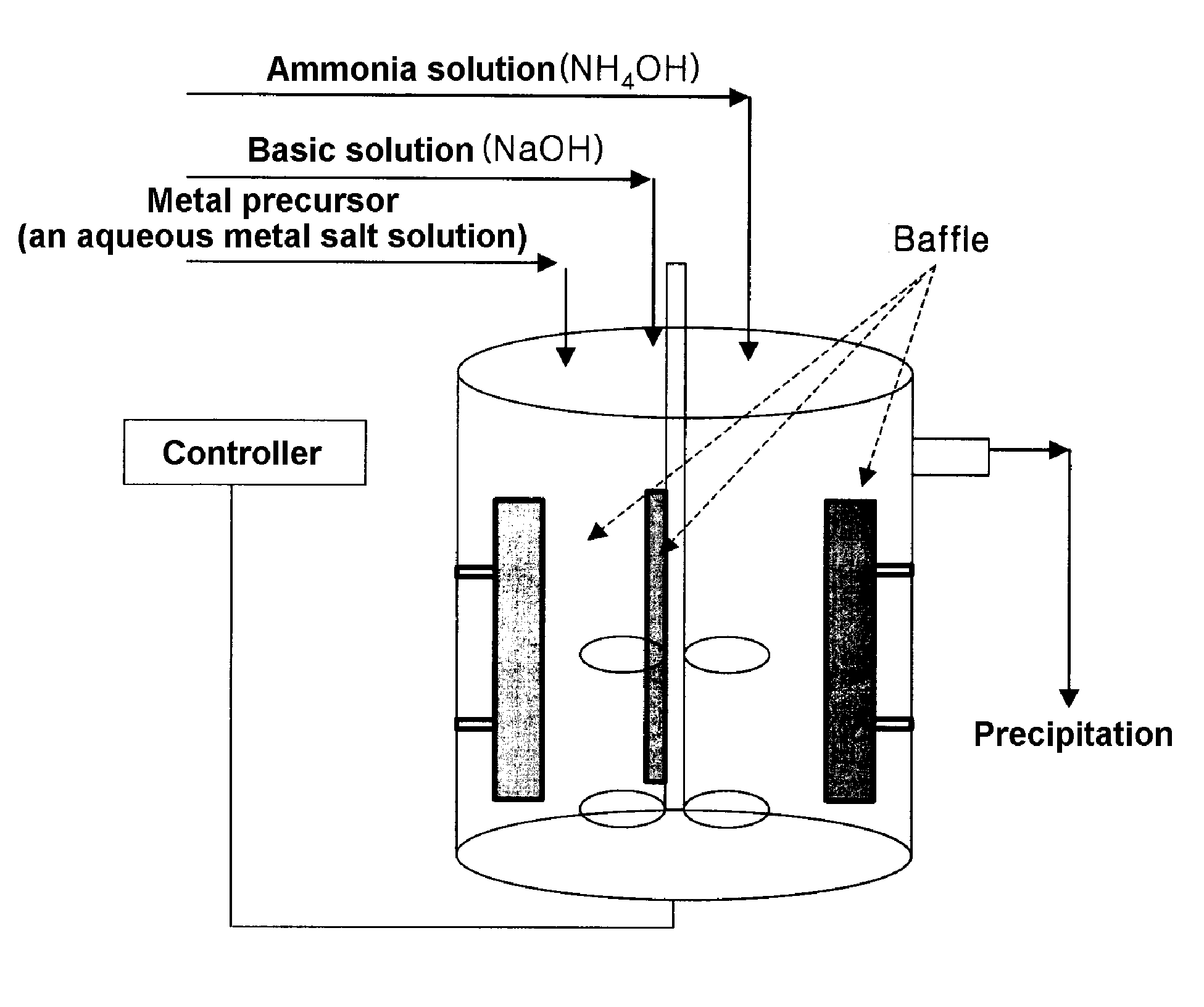
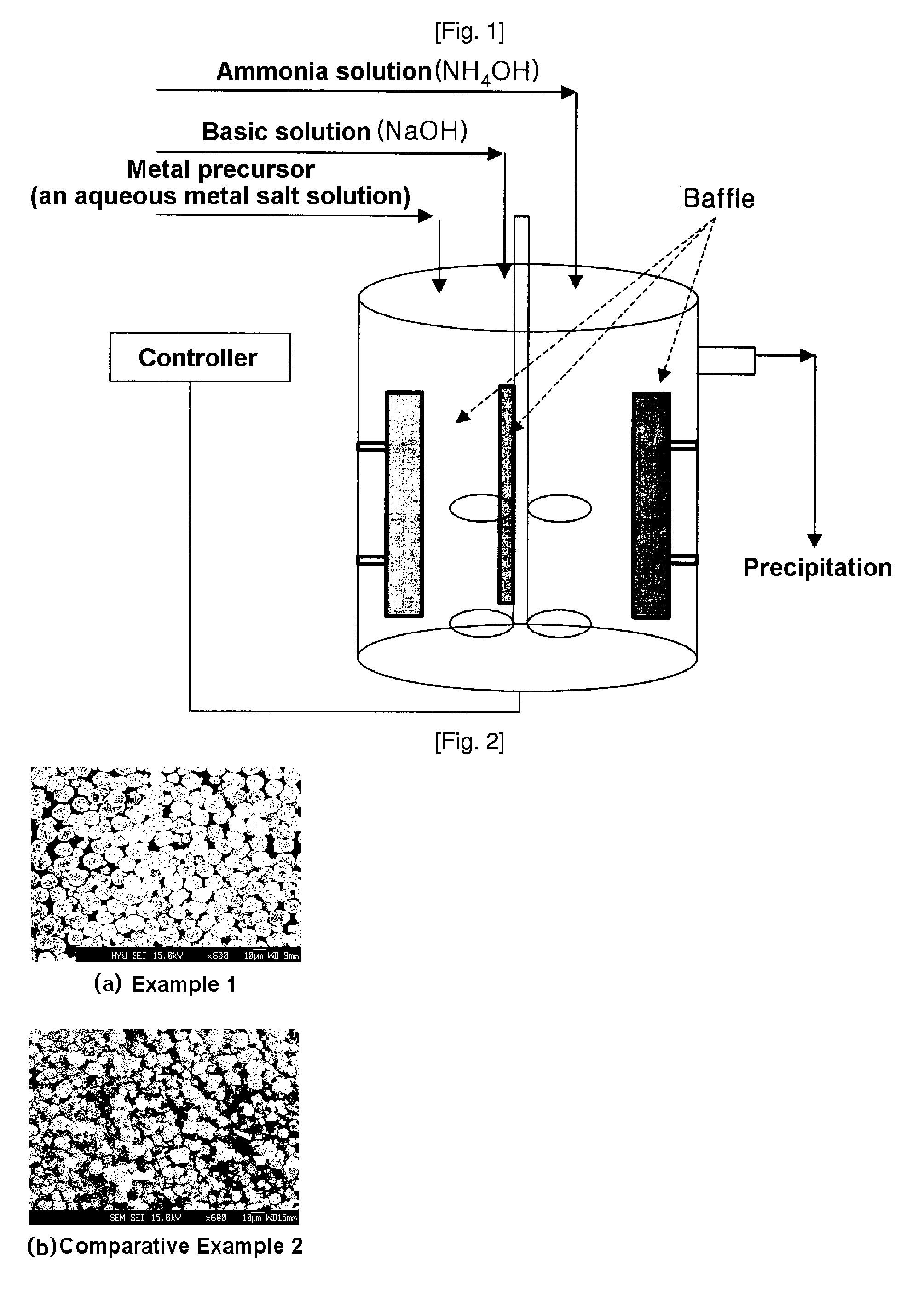
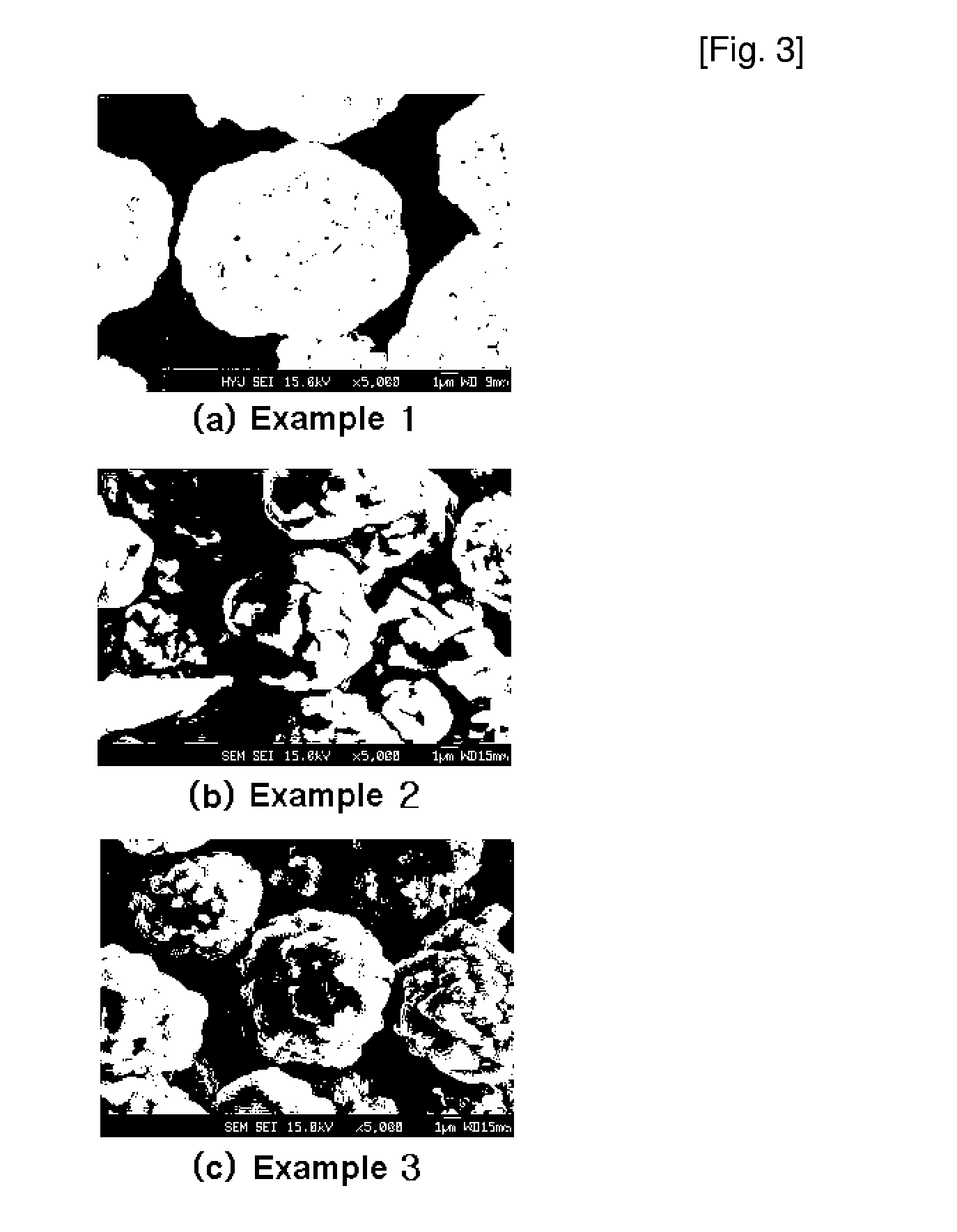
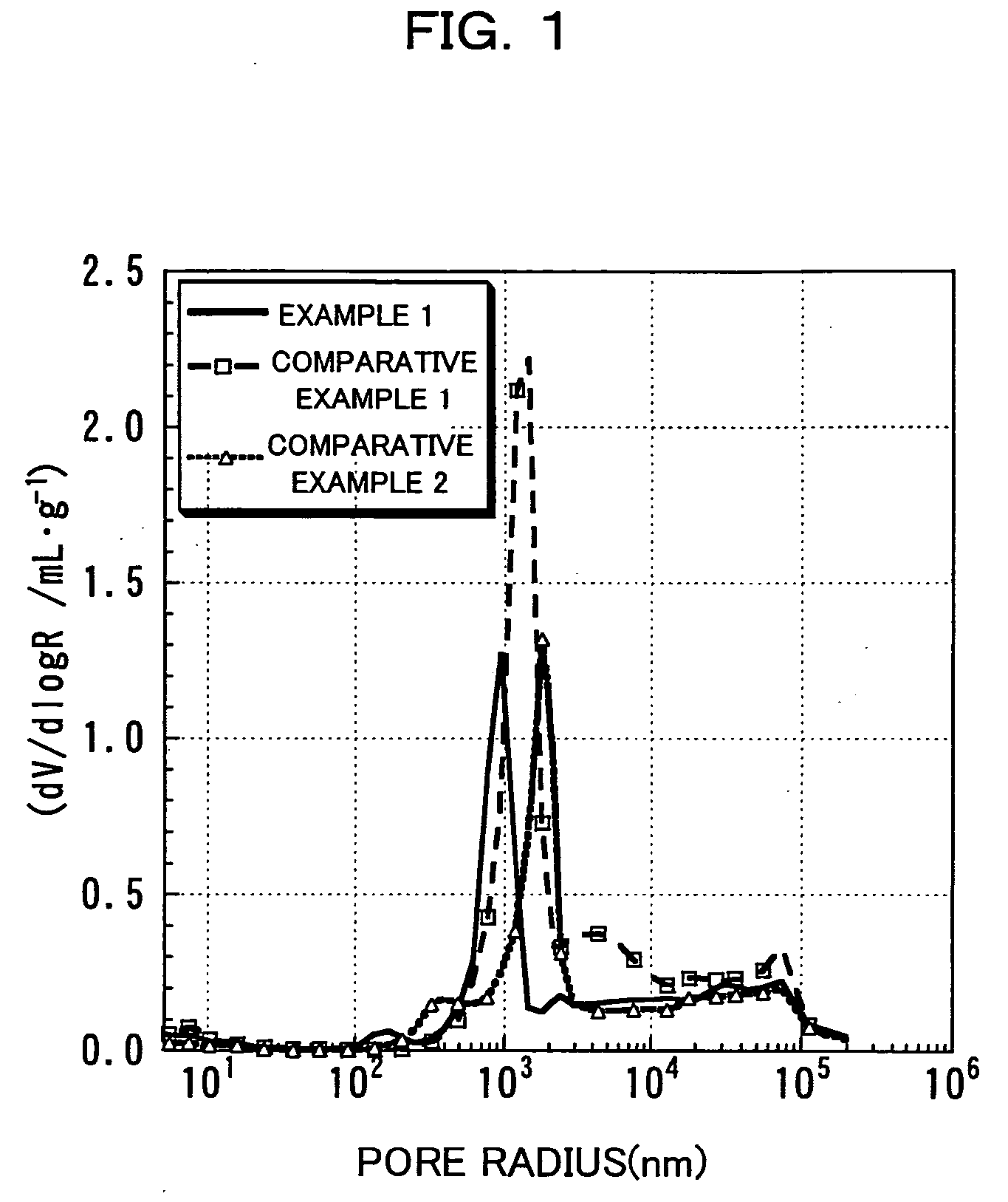
InactiveUS20060134521A1Improving low-temperature load characteristicGood paintabilityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsAlkali metal oxidesElectrical batteryComposite oxide
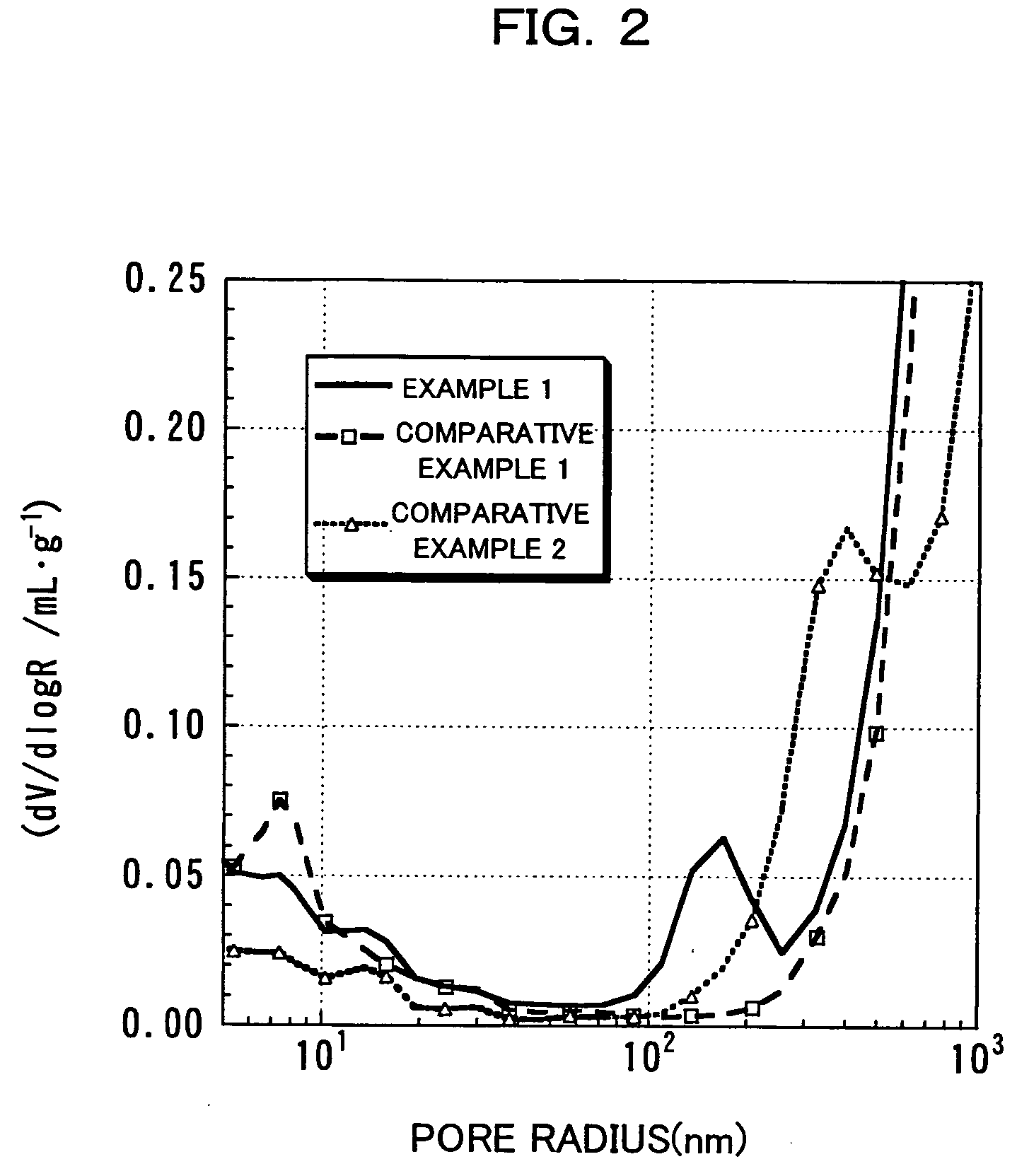
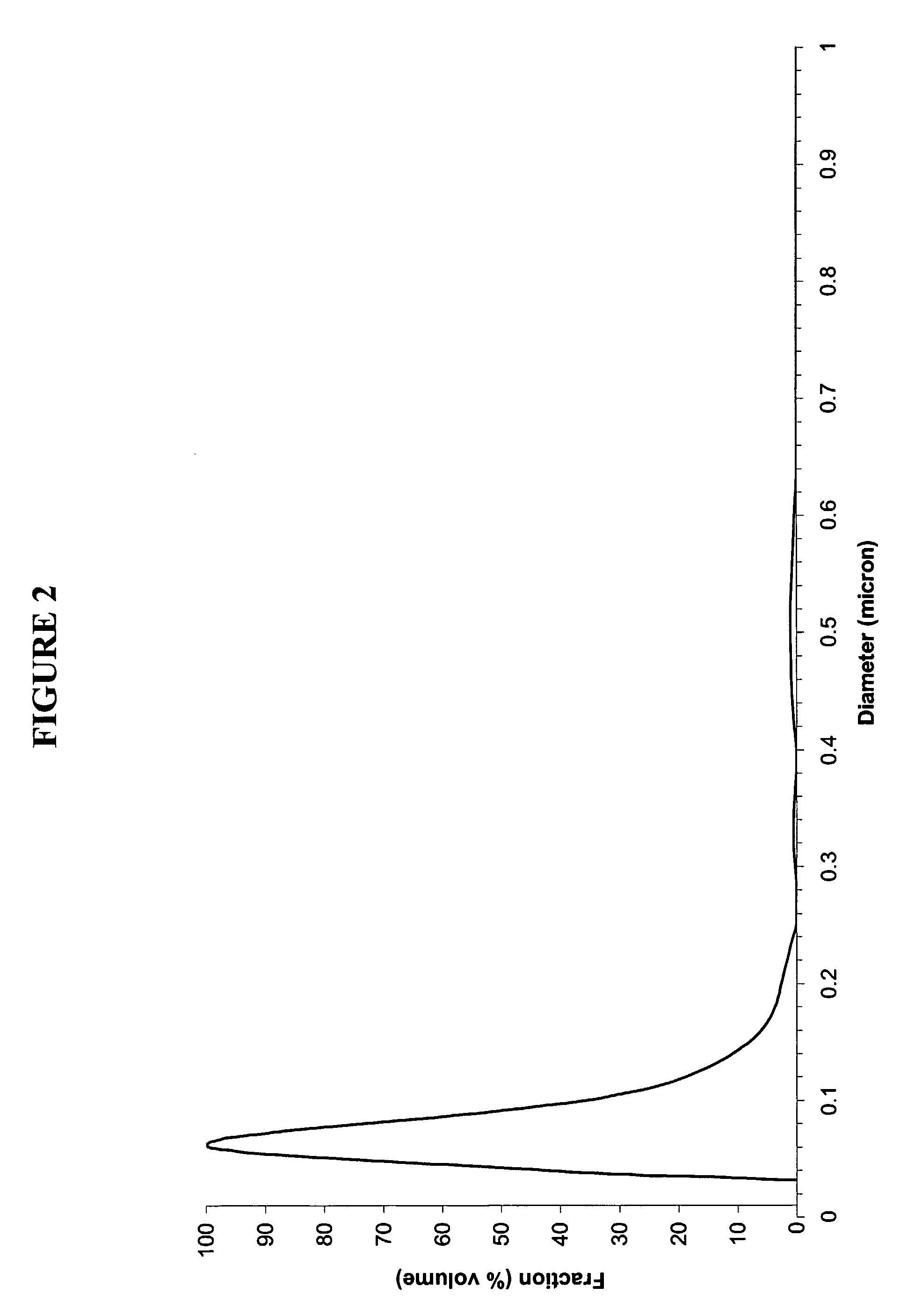
An excellent positive electrode material for a lithium secondary battery is provided that can increase low-temperature load characteristics of the battery as well as improving coatability. When measured by mercury intrusion porosimetry, the material meets Condition (A) and at least either Condition (B) or Condition (C). Condition (A) : on a mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.02 cm3 / g or smaller. Condition (B): on the mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.01 cm3 / g or larger. Condition (C): the average pore radius is within 10-100 nm, and the pore-size distribution curve has a main peak (with peak top at a pore radius of within 0.5-50 μm) and a sub peak (with peak top at a pore radius of within 80-300 nm).
Owner:MITSUBISHI CHEM CORP
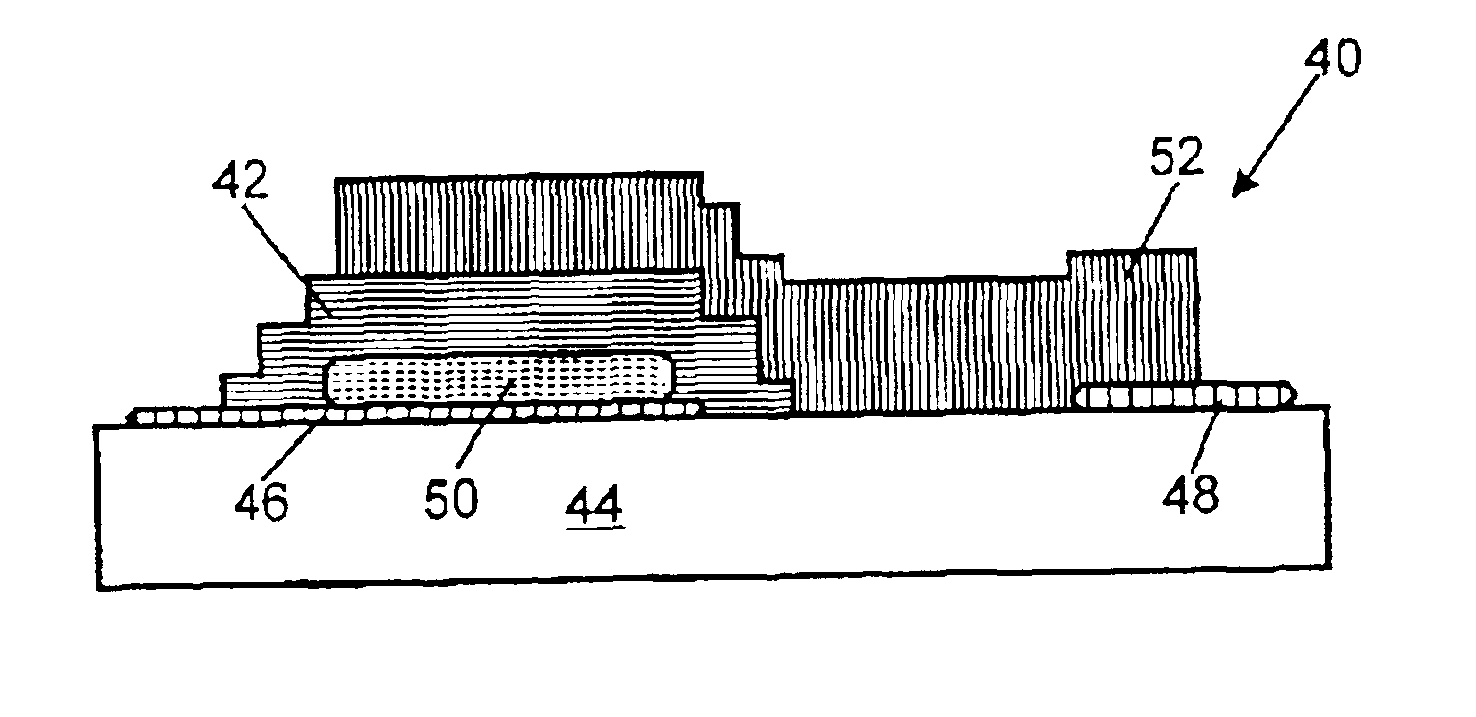
Lithium ion battery comprising nanomaterials
InactiveUS20040131937A1Material nanotechnologyNon-aqueous electrolyte accumulatorsCarbon nanotubeAlloy
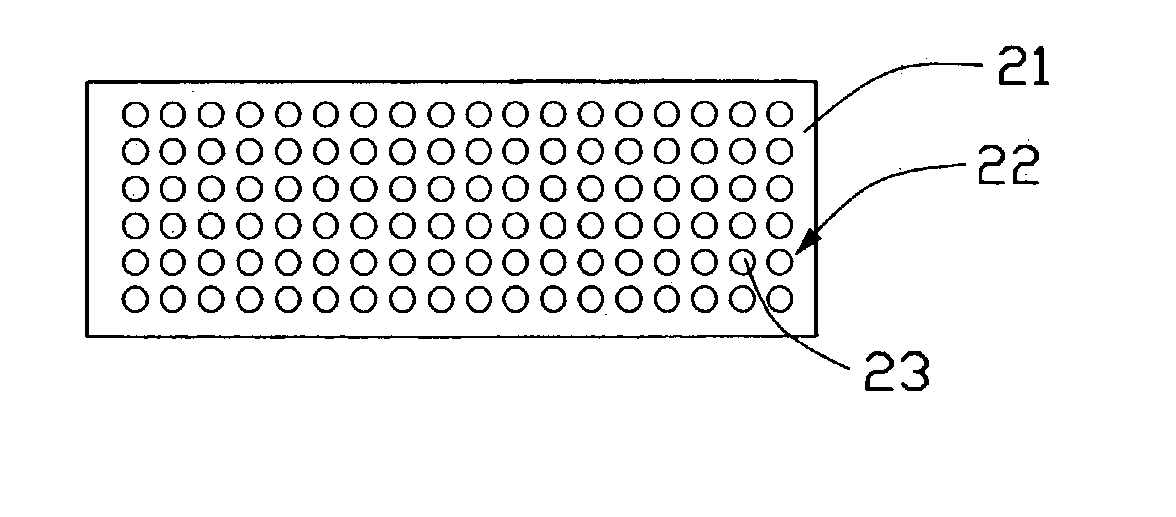
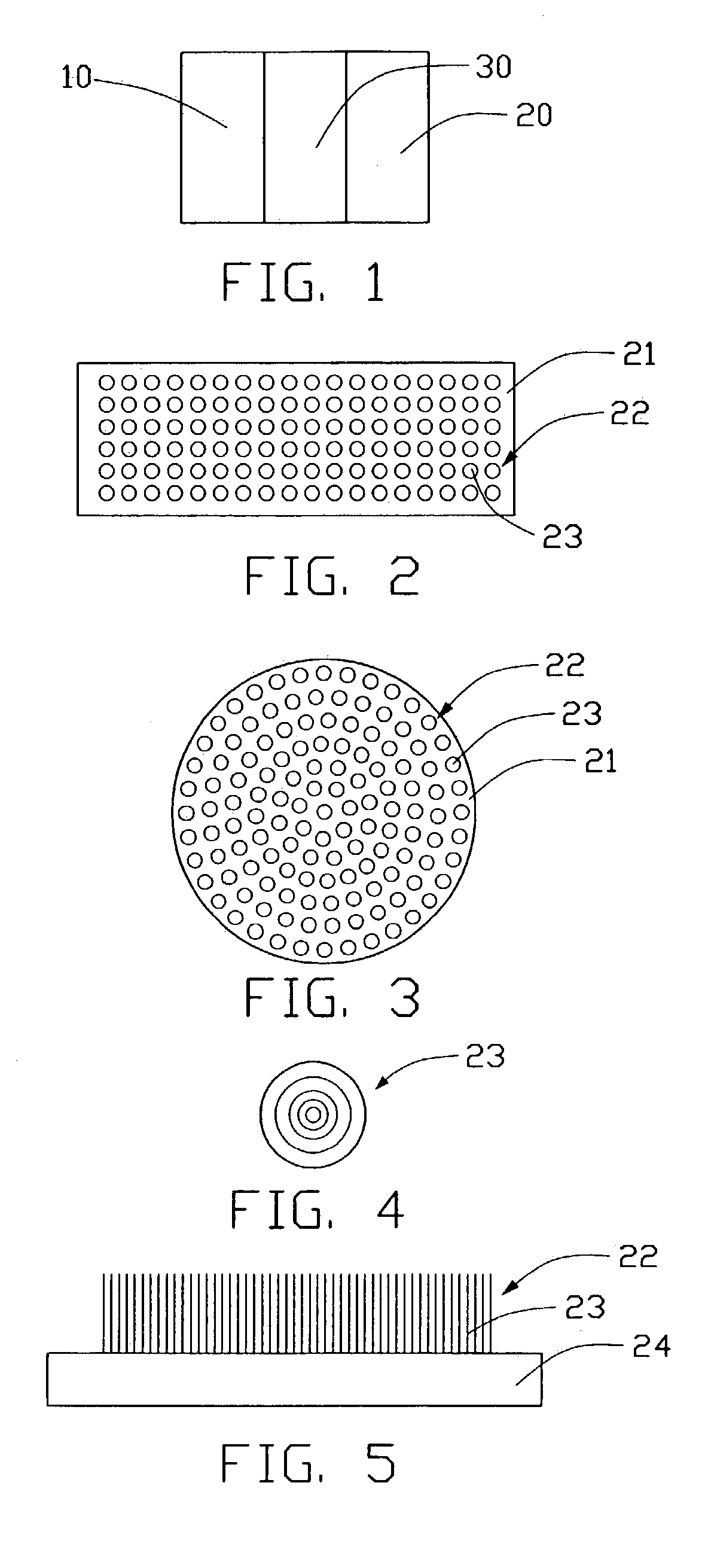
A lithium ion battery includes a cathode (10) having a plurality of nanoparticles of lithium doped transition metal alloy oxides represented by the formula LixCoyNizO2, an anode (20) having at least one carbon nanotube array (22), an electrolyte, and a membrane (30) separating the anode from the cathode. The carbon nanotube array includes a plurality of multi-walled carbon nanotubes (23). Preferably, an average diameter of an outermost wall of the multi-walled carbon nanotubes is in the range from 10 to 100 nanometers, and a pitch between adjacent multi-walled carbon nanotubes is in the range from 20 to 500 nanometers. In the carbon nanotube array, the lithium ions are able to intercalate not only inside the multi-walled carbon nanotubes, but also in the interstices between adjacent multi-walled carbon nanotubes. Thus a density of intercalation of the carbon nanotube array is significantly higher than that of graphite.
Owner:HON HAI PRECISION IND CO LTD
Production Of Barium Titanate Compounds
InactiveUS20070202036A1High purityLow costMaterial nanotechnologyAlkaline earth titanatesBarium titanateSpherical shaped
An ultrafine powder of barium titanate including solid solutions and doped compounds that meets up to specific characteristics is produced by method comprising two main steps. The first step is a reaction, typically in a Segmented Flow Tubular Reactor, between reactants to produce cubic-structure barium titanate composed of non-agglomerated ultrafine particles having a shape of given aspect ratio, usually a generally spherical shape, of low density corresponding at most to 90% of the intrinsic density, all particles being smaller than 1 micron and having a narrow particle size distribution and wherein the ratio of Ba:Ti including substitutents and dopants is very close to the ideal stoichiometry. This is followed by subjecting the powder produced in the first step to a second stage solvothermal post treatment typically in an autoclave at temperature less than 400° C. to convert the cubic-structure particles of low density to ultrafine tetragonal particles of increased density corresponding to at least 90% of the intrinsic density while maintaining the same aspect ratio, and maintaining the size of all particles below 1 micron, the narrow particle size distribution span, and the given ideal stoichiometry. The produced particles can have a non-spherical facetted shape such as cube-like.
Owner:JONGEN NATHALIE +1
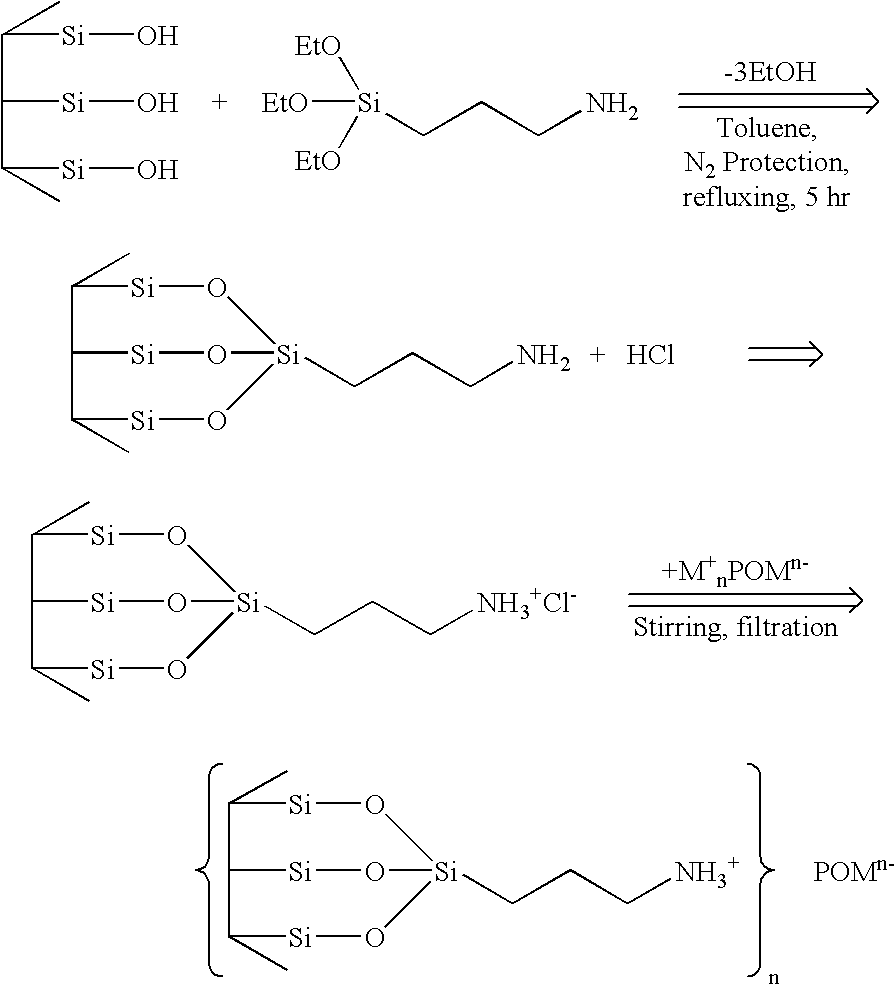
Supported polyoxometalates and process for their preparation
InactiveUS7417008B2Group 4/14 element organic compoundsOxygen/ozone/oxide/hydroxideCatalytic oxidationMesoporous silica
Owner:EXXONMOBIL CHEM PAT INC
Electrode active material powder with size dependent composition and method to prepare the same
ActiveUS20070122705A1Increased gravimetric energy densityLow costElectrode thermal treatmentActive material electrodesLithiumPhysical chemistry
The present invention relates to a powderous electrode active material of lithium transition metal oxide LiaMbO2, wherein 0.9 < a < 1.1, 0.9 < b < 1.1 and M is dominantly transition metal chosen from Mn, Co and Nickel, having particles with a distribution of sizes, where the composition M varies with the size of the particles, and a preparation method thereof. The present invention also relates to an electrochemical cell, particularly rechargeable lithium battery, using the powderous electrode active material.
Owner:LG ENERGY SOLUTION LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com