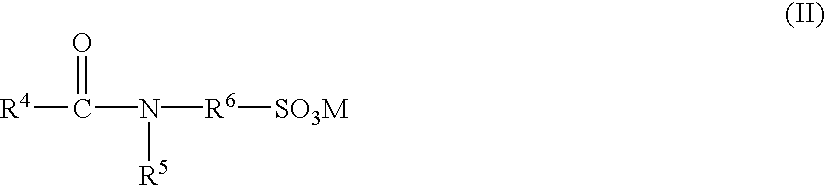Patents
Literature
4391 results about "Carboxylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
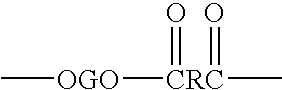
A carboxylate is the conjugate base of a carboxylic acid. Carboxylate salts have the general formula M(RCOO)ₙ, where M is a metal and n is 1, 2,...; carboxylate esters have the general formula RCOOR′ (or RCO₂R′). R and R′ are organic groups; R′ ≠ H.
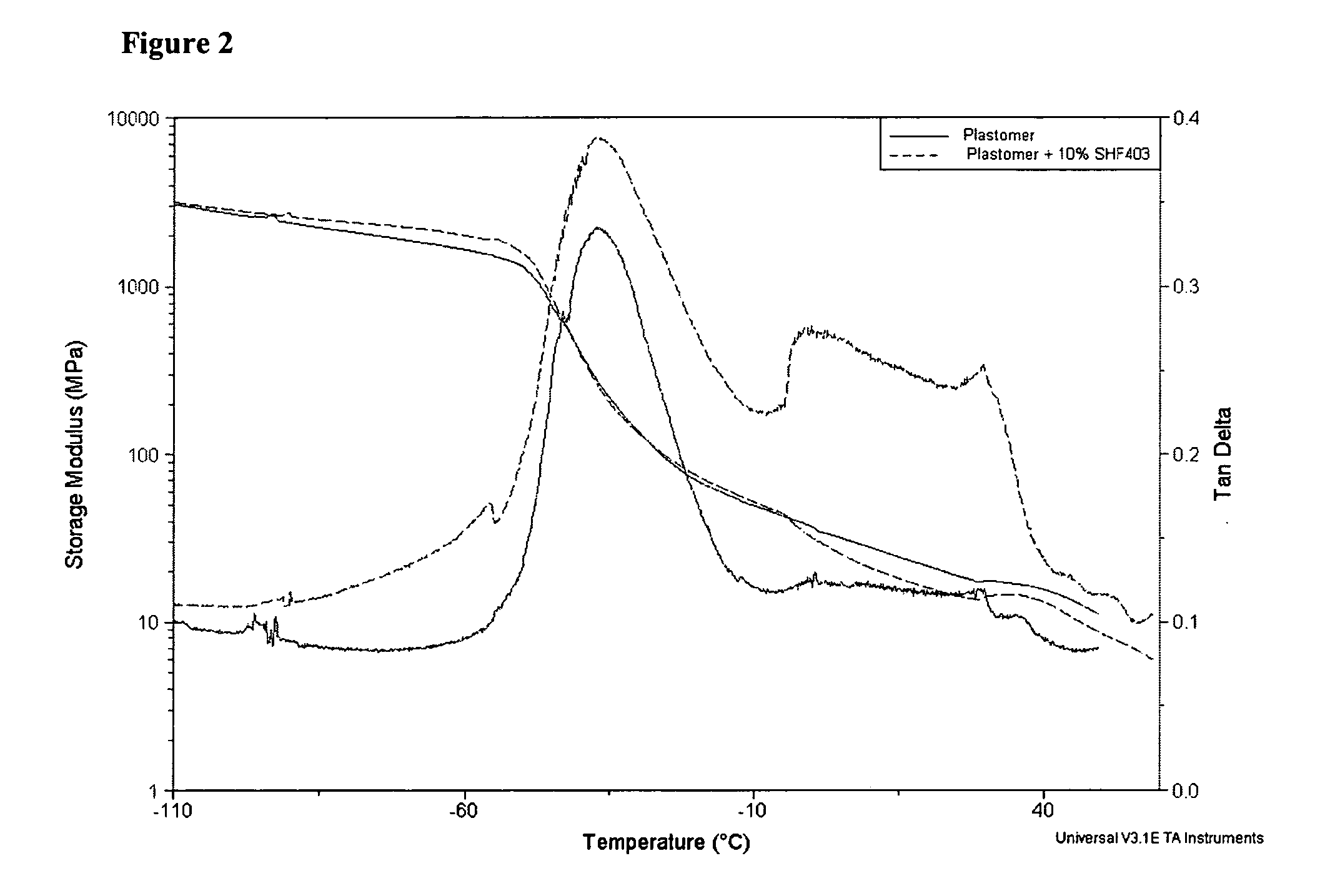
Modified polyethylene compositions
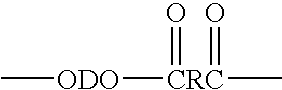
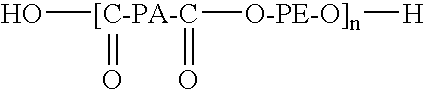
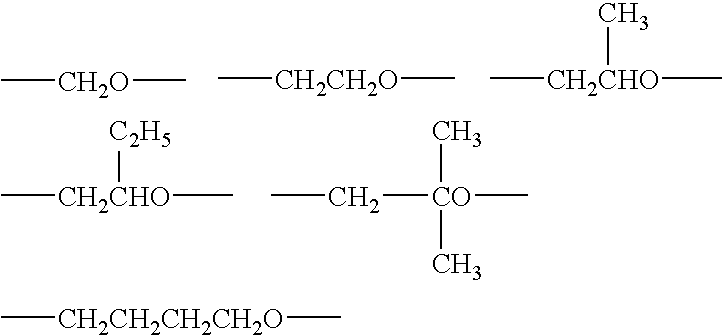
The present invention relates to a composition comprising more than 25 weight % (based on the weight of the composition) of one or more ethylene polymers having an Mw of 20,000 g / mole or more and at least 0.1 weight % of a liquid hydrocarbon modifier where the modifier has: 1) a viscosity index of 120 or more, and 2) an kinematic viscosity of 3 to 3000 cSt at 100° C., and 3) a pour point of −10° C. or less, and 4) a flash point of 200° C. or more; and wherein the modifier contains less than 5 weight % of functional groups selected from hydroxide, aryls, substituted aryls, halogens, alkoxys, carboxylates, esters, acrylates, oxygen, nitrogen, and carboxyl, based upon the weight of the modifier.
Owner:EXXONMOBIL CHEM PAT INC
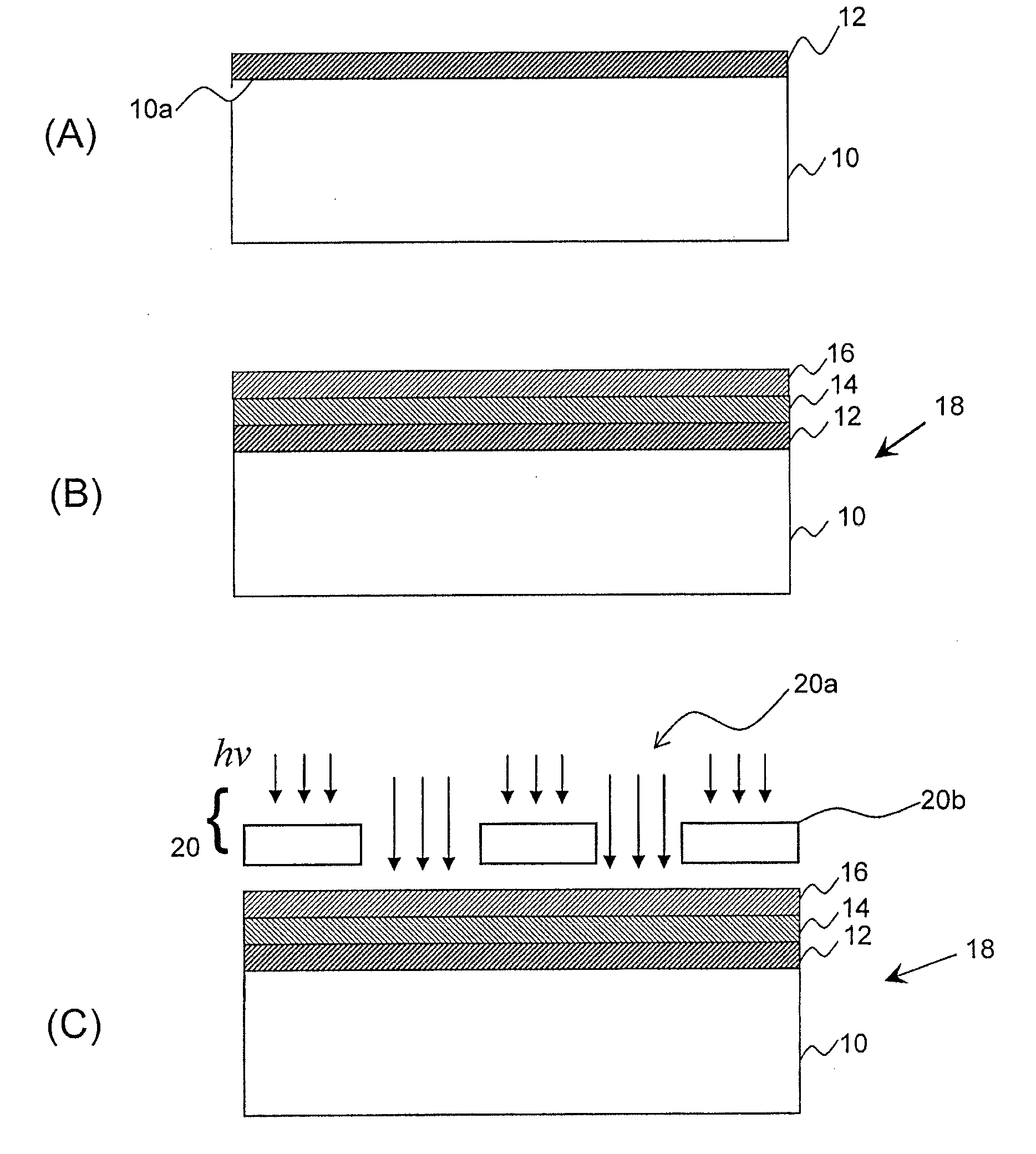
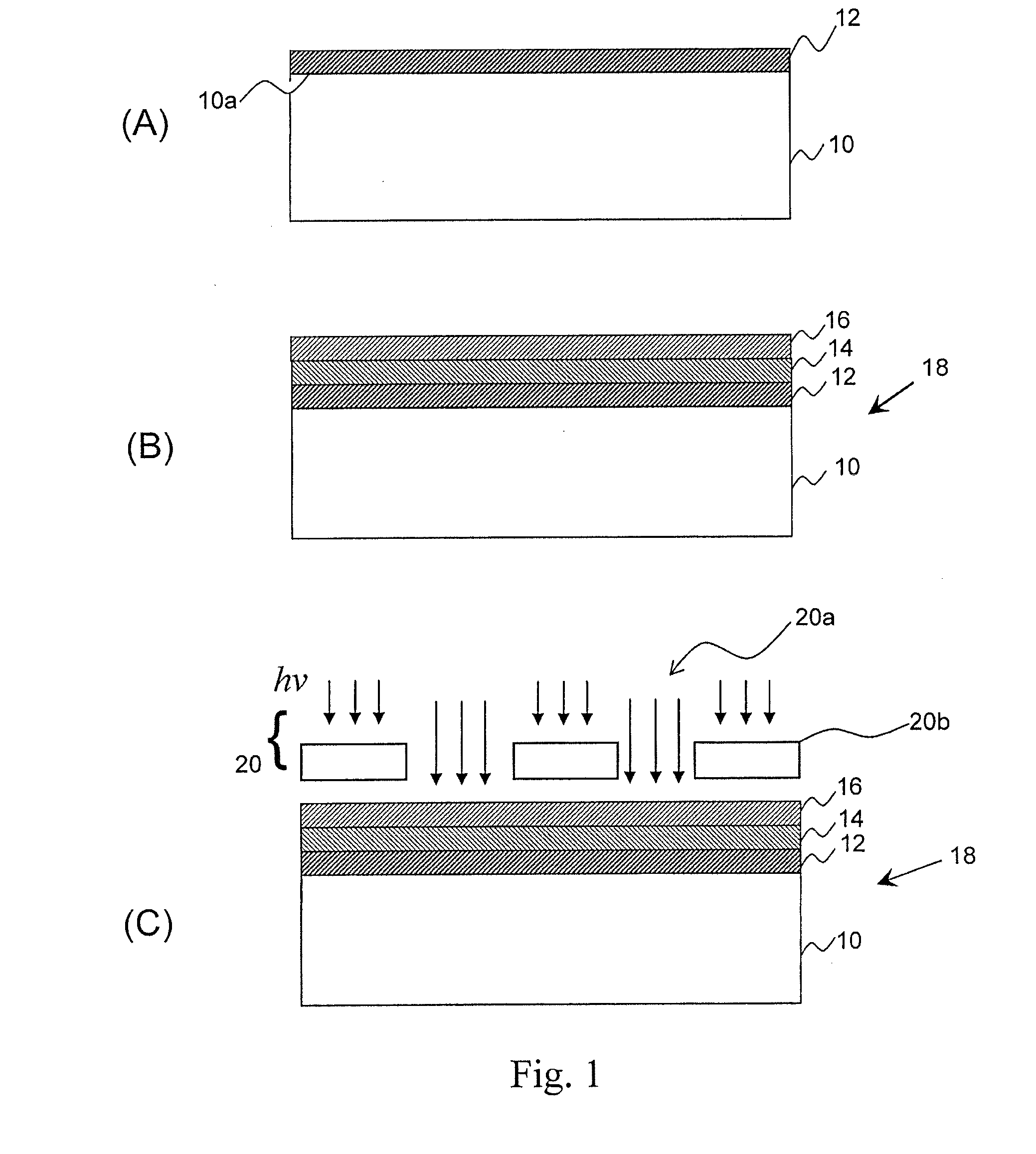
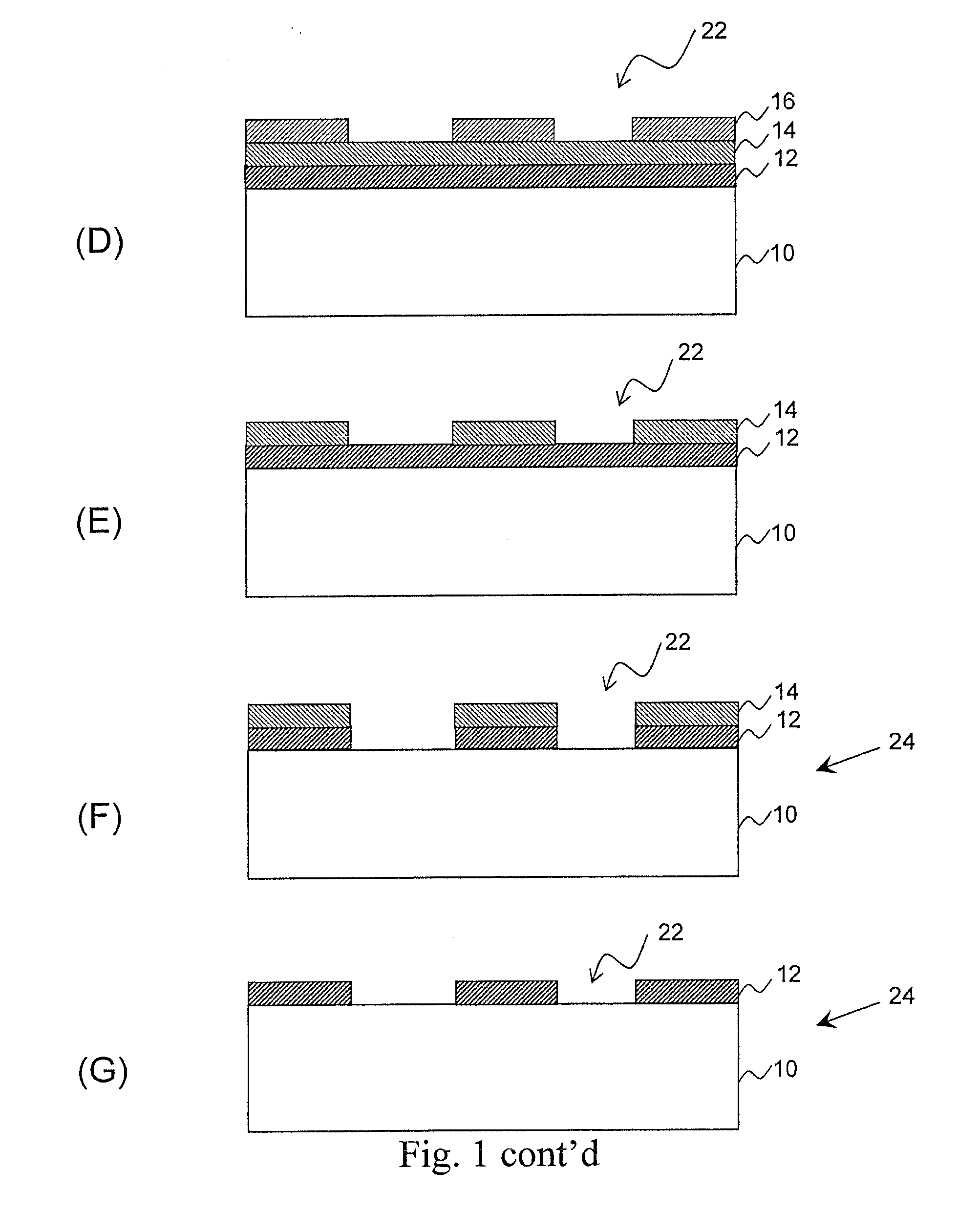
Metal-oxide films from small molecules for lithographic applications
Metal-oxide films for lithographic applications are provided. The films are formed from compositions comprising metal-oxide precursor compounds including metals and metalloids other than silicon. These films are easily produced and can be modified with a variety of ligands, including alkoxides, phenoxides, carboxylates, beta-diketones, and beta-ketoesters.
Owner:BREWER SCI
Thermoplastic vulcanizates and process for making the same
InactiveUS6437030B1Low hygroscopicityReduced halideSynthetic resin layered productsSolid ballsThermoplastic elastomerMetal halides
A process for forming a thermoplastic vulcanizate compromising the steps of dynamically vulcanizing a rubber within a blend that comprises the rubber and a thermoplastic polymer, where said step of vulcanizing is carried out by using a phenolic resin in the presence of a catalyst system formed by combining a metal halide and a metal carboxylate. Also, a thermoplastic vulcanizate having low moisture pick-up with a technologically useful cure.
Owner:ADVANCED ELASTOMER SYST LP
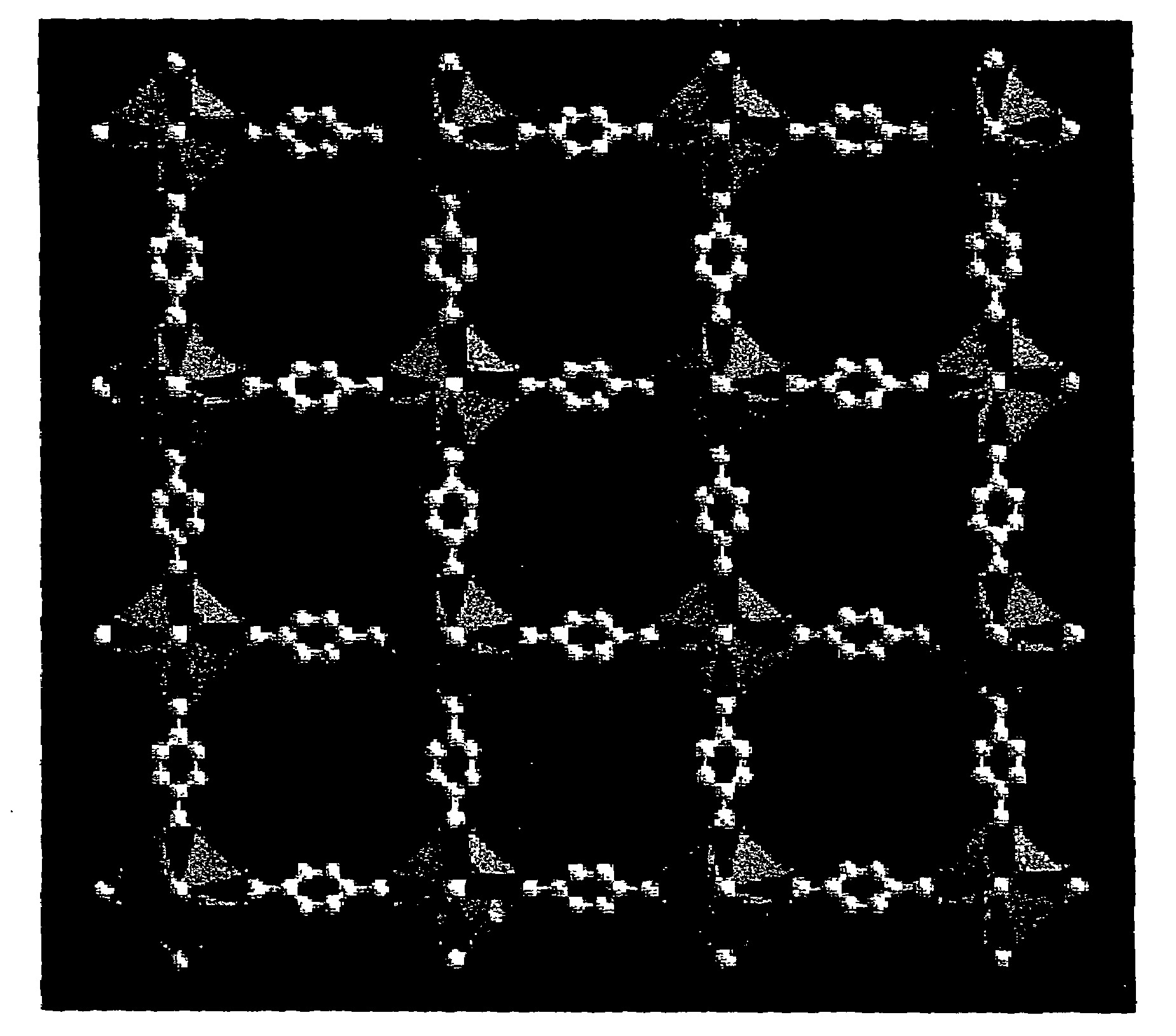
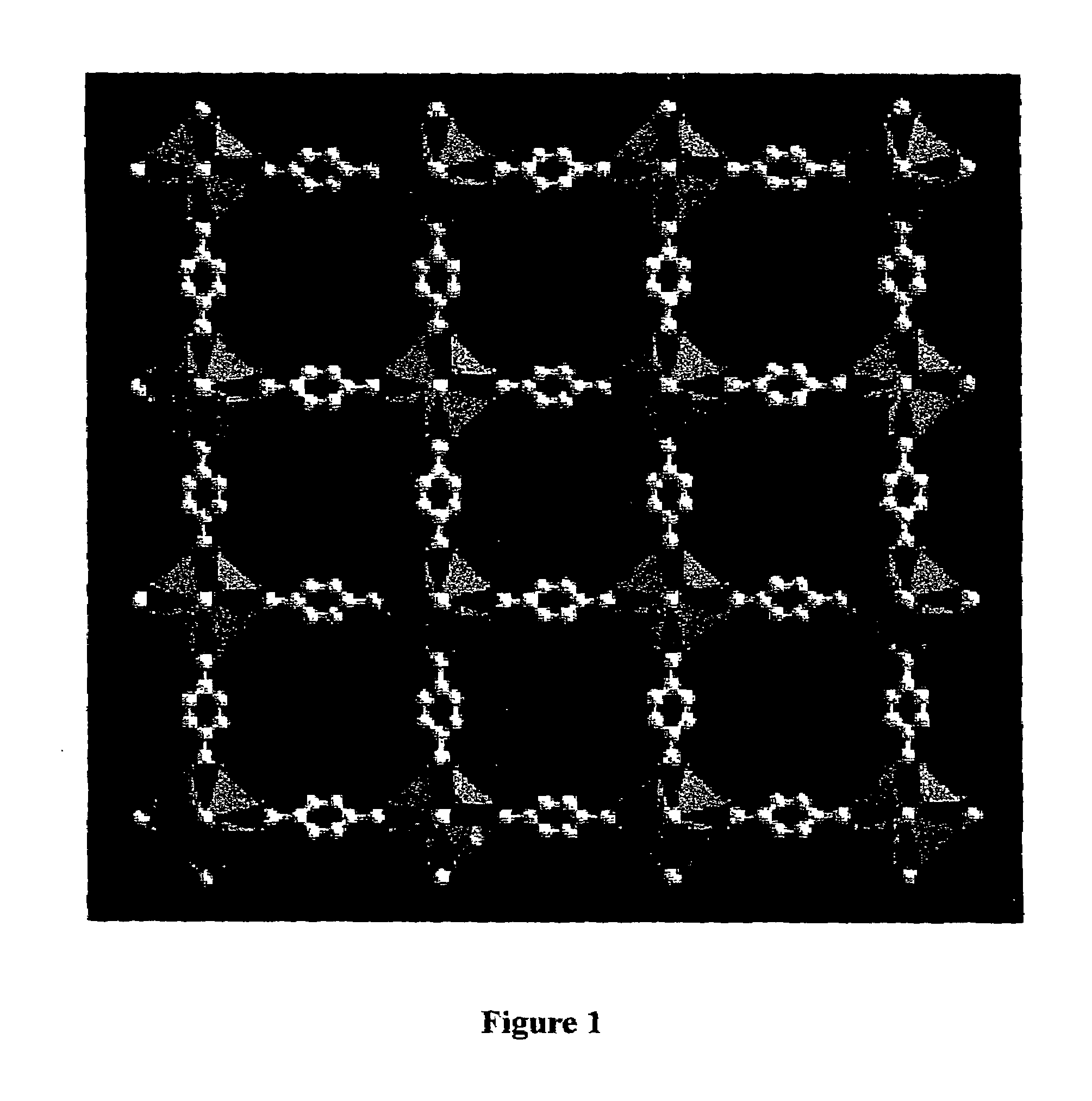
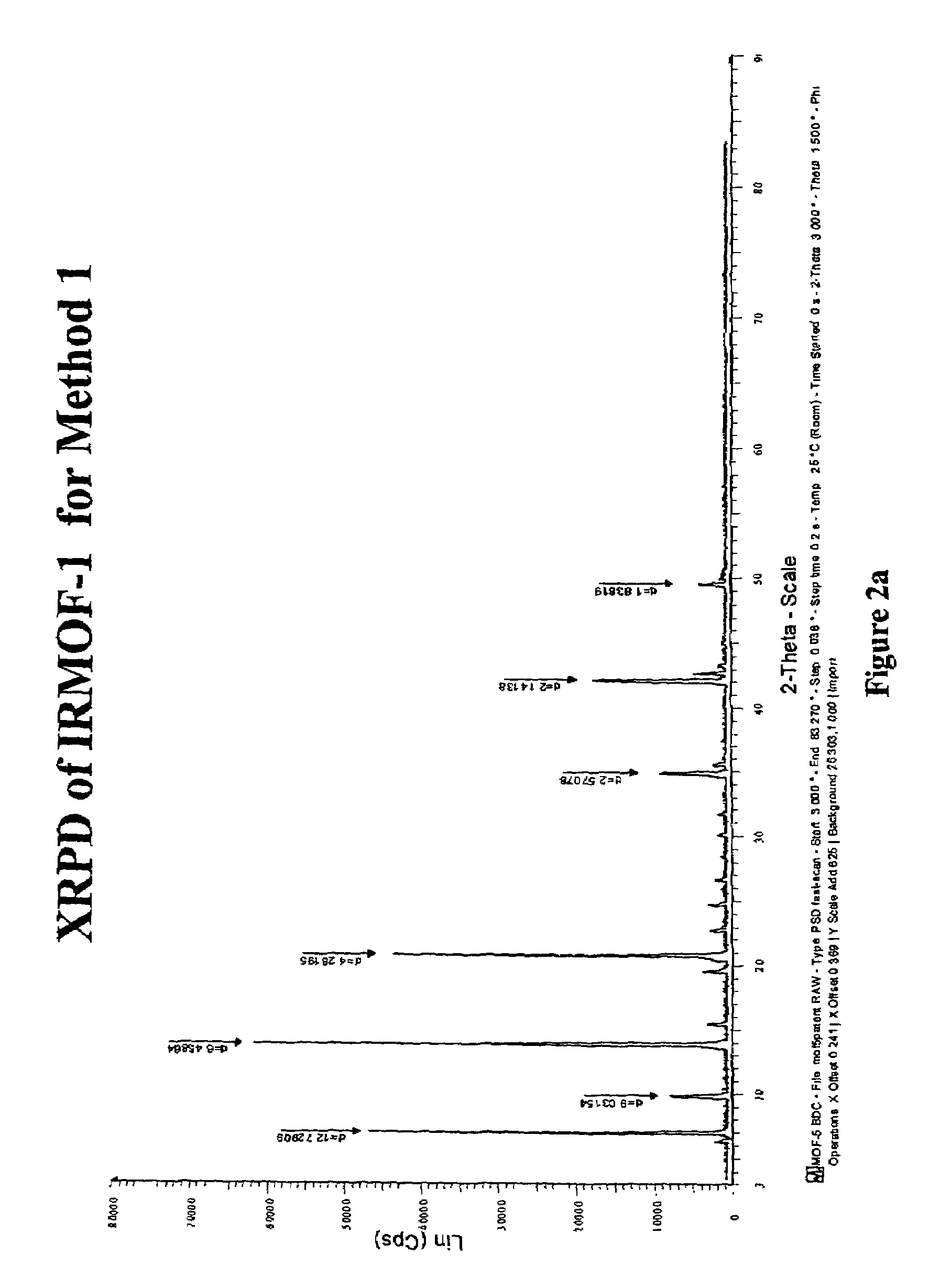
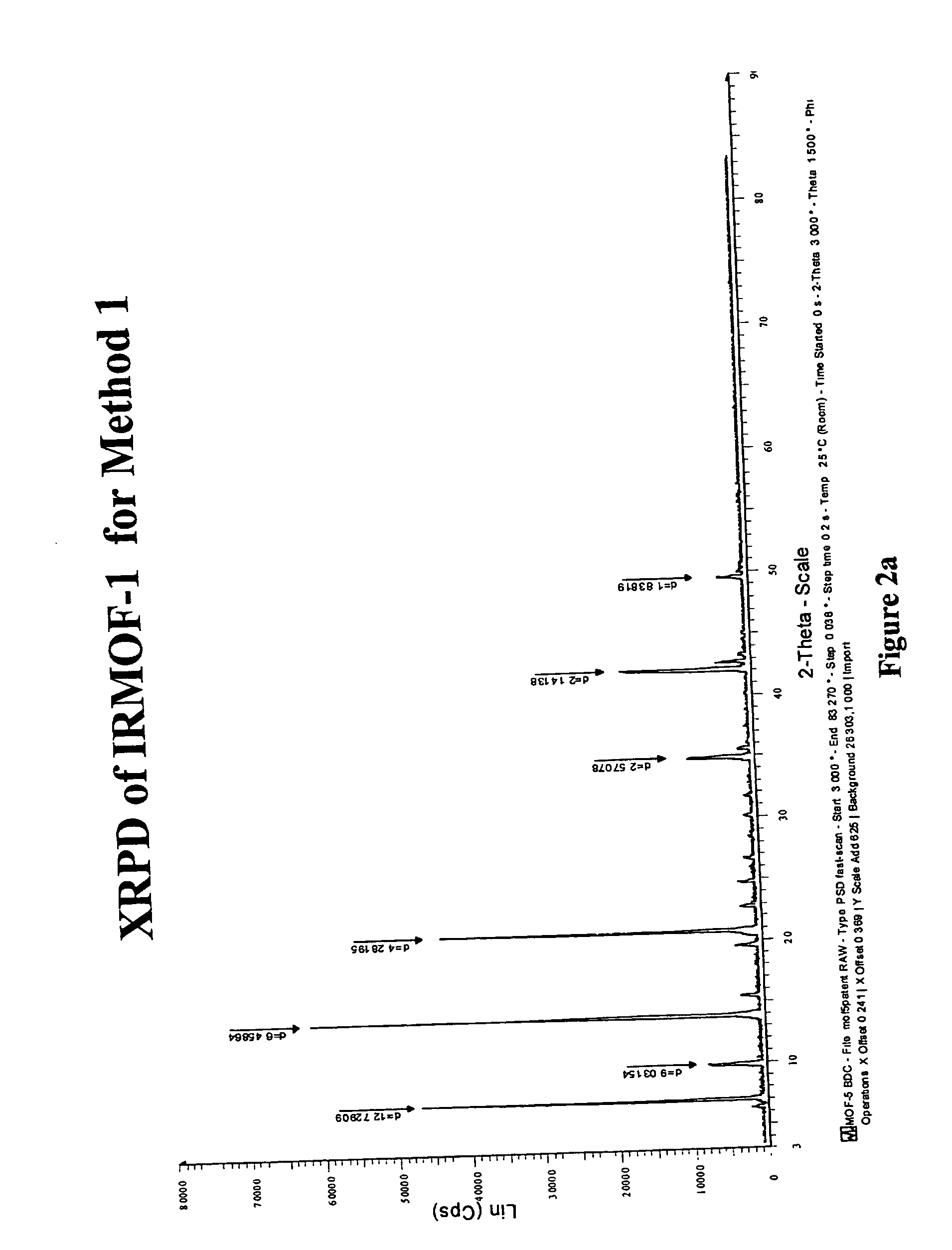
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Dimerized alcohol compositions and biodegradible surfactants made therefrom having cold water detergency
InactiveUS6222077B1Good cold water detergencyOrganic detergent compounding agentsOther chemical processesDouble bondCarboxylic acid
There is provided an alcohol composition obtained by dimerizing an olefin feed comprising C6-C10 linear olefins to obtain C12-C20 olefins, followed by conversion to alcohols, such as by hydroformylation. The composition has an average number of branches ranging from 0.9 to 2.0 per molecule. The linear olefin feed preferably comprises at least 85% of C6-C8-olefins. The primary alcohol compositions are then converted to anionic or nonionic surfactants, preferably sulfated or oxyalkylated or both. The sulfated compositions are biodegradable and possess good cold water detergency. The process for making the dimerized primary alcohol comprises dimerizing, in the presence of a homogeneous dimerization catalyst under dimerization conditions, an olefin feed comprising C6-C10 olefins and preferably at least 85 weight % of linear olefins based on the weight of the olefin feed, to obtain a C12-C20; optionally double bond isomerizing said C12-C20 olefins; and converting the C12-C20 olefins to alcohols, preferably through hydroformylation. The process is preferably a one-step dimerization. The homogenous catalyst comprises a mixture of a nickel carboxylate or a nickel chelate, with an alkyl aluminum halide or an alkyl aluminum alkoxide.
Owner:SHELL OIL CO
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
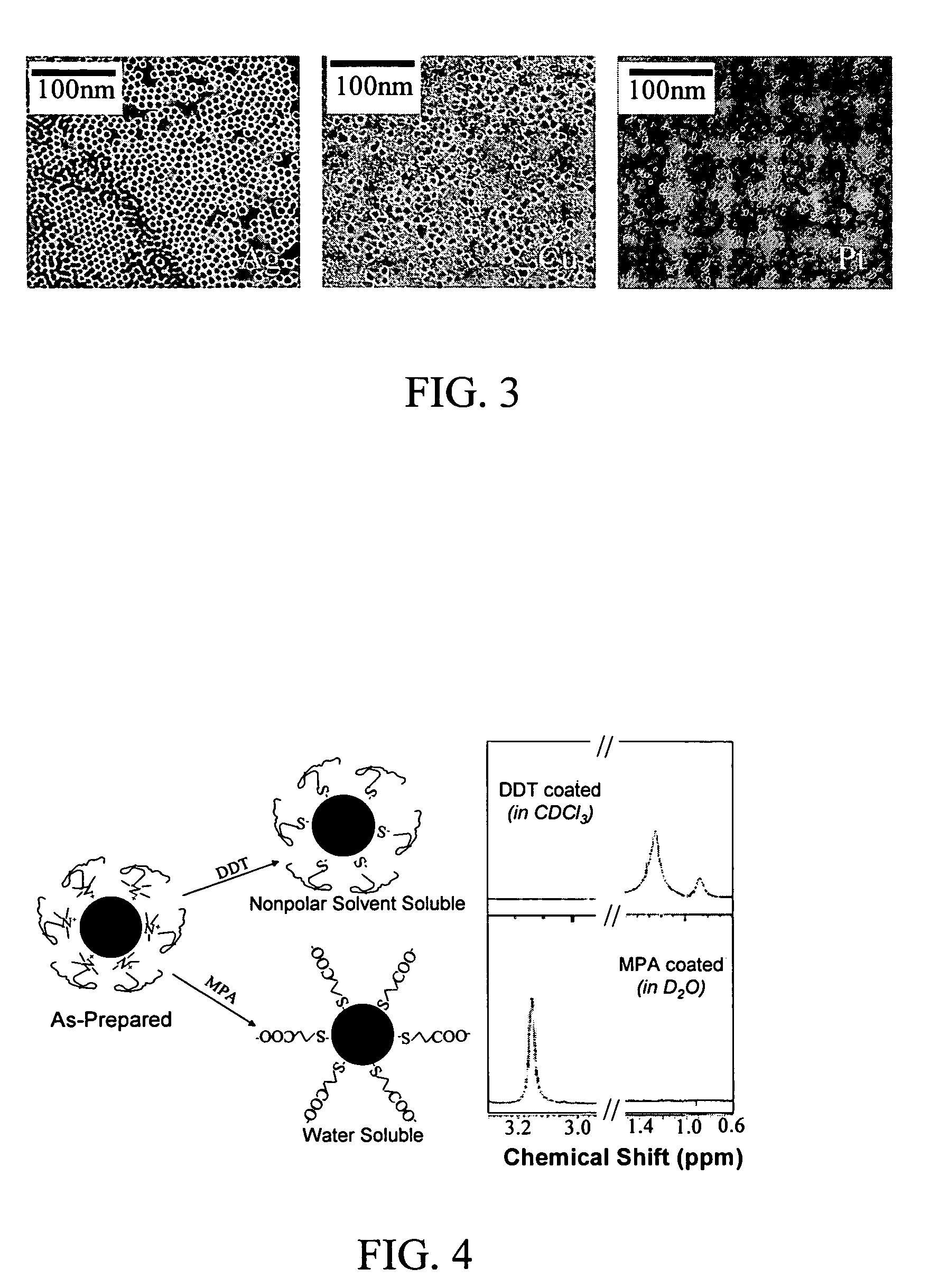
Monodisperse noble metal nanocrystals
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
Catalyst component for olefinic polymerization and its catalyst
This invention provides a catalys conponent and catalyst. Said catalyst comprises titanium, magnesium and at least three electron donor compounds a, b and c, in which is selected from mono-aliphatic-carboxylate or mono-aromatic-carboxylate compounds, b is selected from a special diatomic alkoxide compound, and c is selected from binary binary-aliphatic-carboxylate or binary-aromatic-carboxylate or b is ether compound. Advantages: high polymerization activity and stereospecificity when used for olefin polymerization, in particular for propylene polymerization, wide molecular weight distribution of obtained polymer and good shape of polymer granulars.
Owner:CHINA PETROLEUM & CHEM CORP +1
Resin composition, method of its composition, and cured formulation
InactiveUS20060029811A1Improve flame retardant performanceMaintain good propertiesSemiconductor/solid-state device detailsSynthetic resin layered productsCarboxylic acidMicroparticle
It is an object of the present invention to provide a resin composition which can form cured formulations having various excellent properties such as an insulating property, thermal shock resistance, moldability / formability and strength, and exhibit an excellent appearance in which transparency is enhanced, a resin composition whose cured thin film has excellent flame retardancy, good mechanical property and heat resistance, a dispersing element containing an inorganic microfine particle which can give a flame retardancy to a resin, to which the inorganic microfine particle is added, and can reduce a hygroscopic property to the extent possible, a method for producing the same and a cured formulation obtained by using the resin composition. The present invention relates to a resin composition comprising a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a resin composition comprising three components of a phenolic compound, a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a flame retardant resin composition comprising a polyhydric phenol and an inorganic microfine particle, and a dispersing element containing an inorganic microfine particle obtained by a hydrolysis condensation reaction of alkoxide and / or metal carboxylate in a dispersion medium.
Owner:NIPPON SHOKUBAI CO LTD
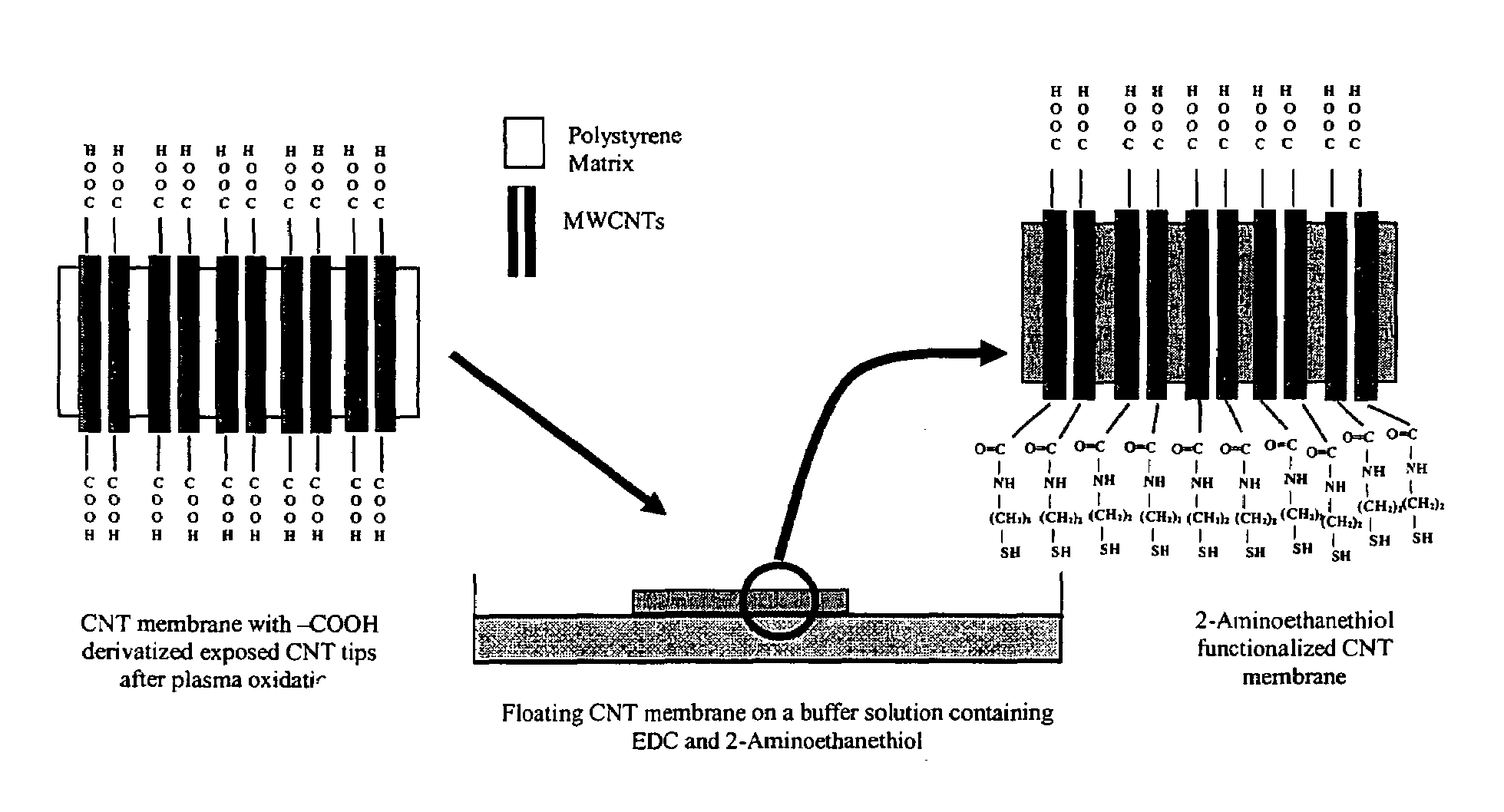
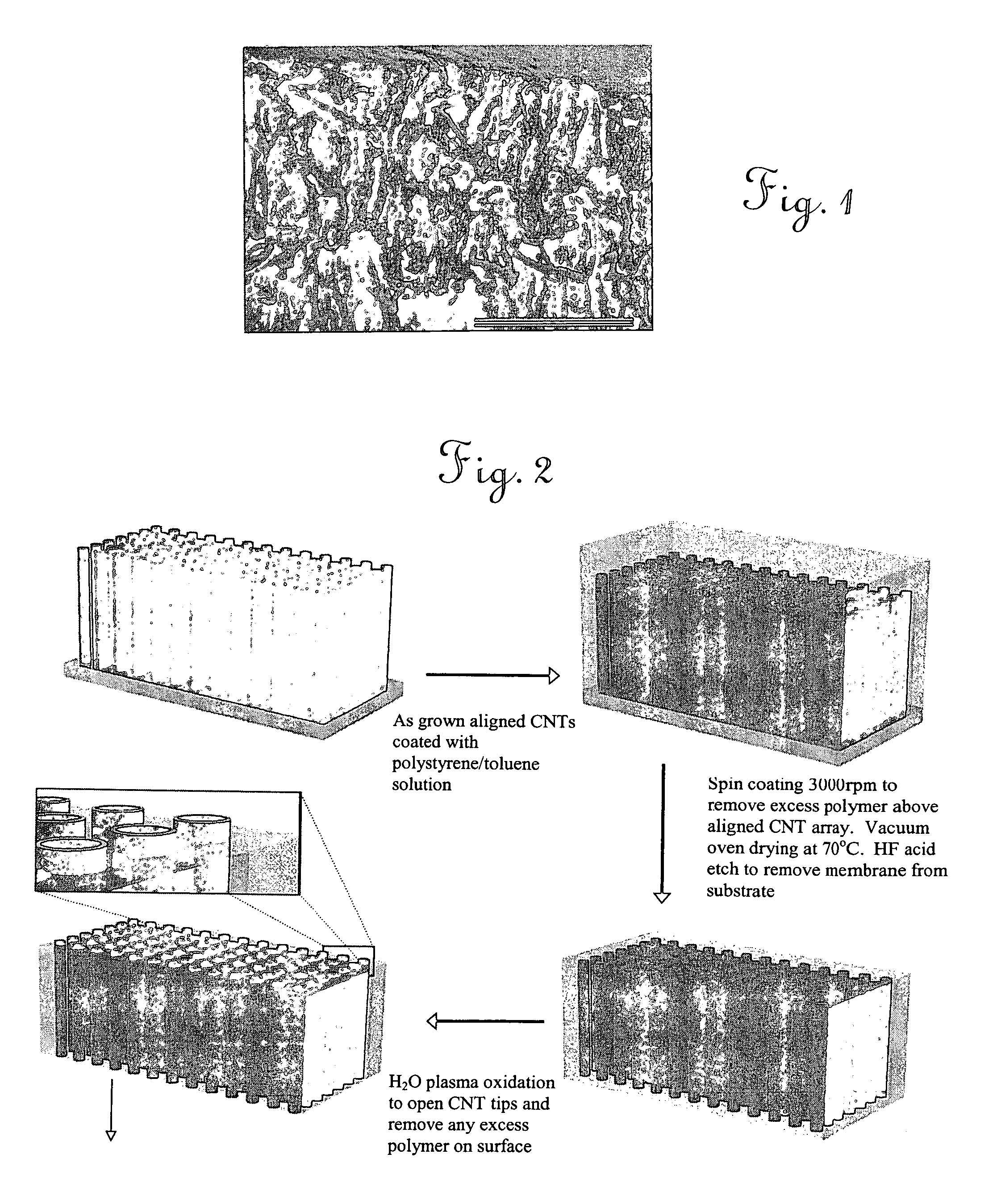
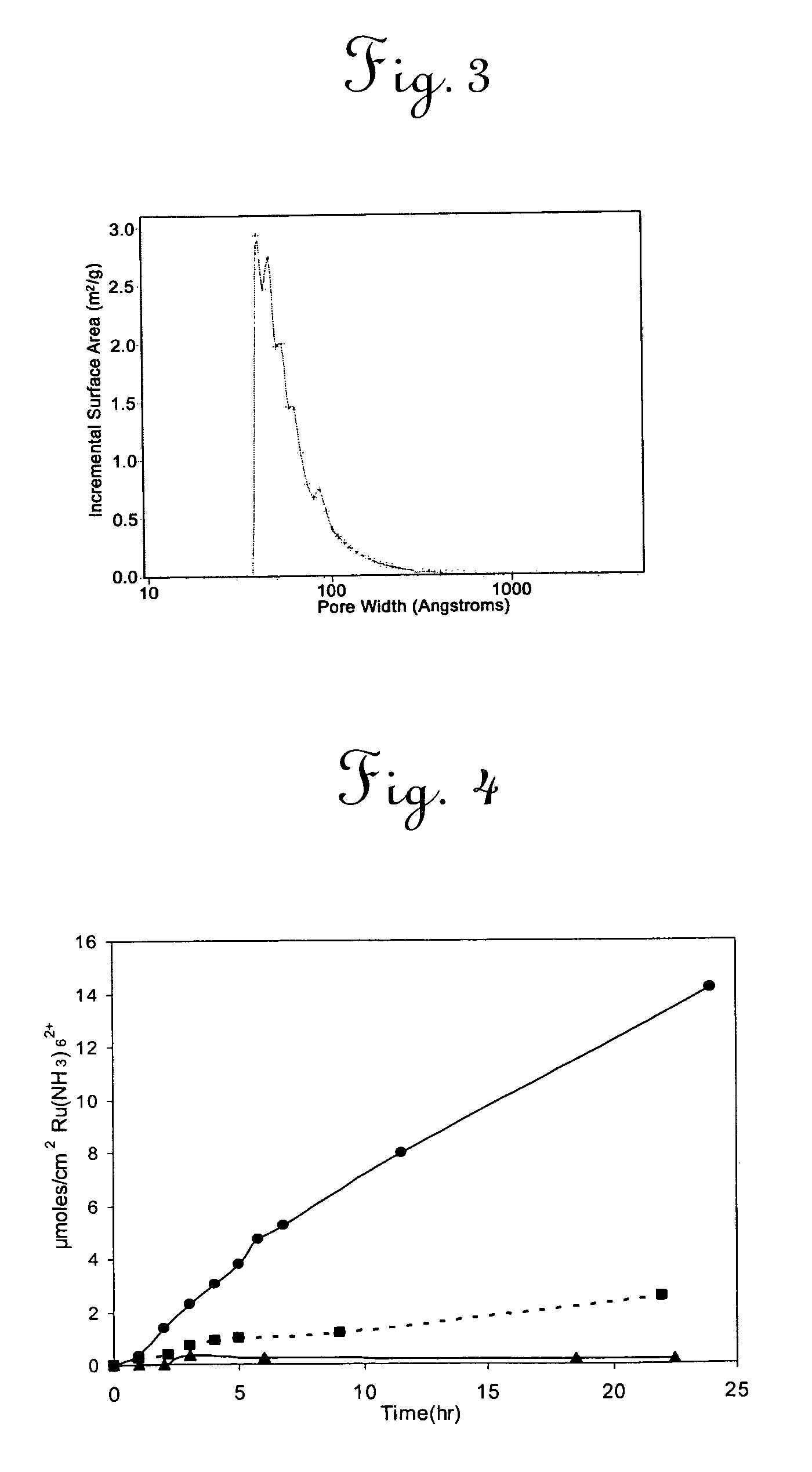
Aligned nanotubule membranes
A method is provided for producing a permeable membrane, comprising the steps of aligning a plurality of hollow nanotubules to form a mat, coating the mat with a continuous polymer matrix to form a membrane. The membrane is etched (a) to open the plurality of hollow nanotubules and form pores and (b) to oxidize the carboxyl groups to carboxylate groups. At least one additional functional unit having at least one available amine group to bind the at least one additional functional unit to the nanotubule end carboxylate group may be provided. Membranes fabricated in accordance with the method of the invention are provided also.
Owner:UNIV OF KENTUCKY RES FOUND
Methods and catalysts for the manufacture of carbon fibrils
An improved catalyst for producing carbon fibrils is made by incorporating an effective yield-enhancing amount of a carboxylate into a fibril-forming catalyst. Alternatively, such a catalyst is made by coprecipitating a compound of a metal having fibril-forming catalytic properties and an aluminum and / or magnesium compound, optionally in the presence of carbon particles or carbon fibril aggregates. The catalyst may also be made by incorporating a compound of a fibril-forming metal onto magnesia particles in carbon particles or carbon fibril aggregates. The catalysts, methods of using them to form carbon fibrils and those carbon fibrils are also disclosed.
Owner:HYPERION CATALYSIS INT
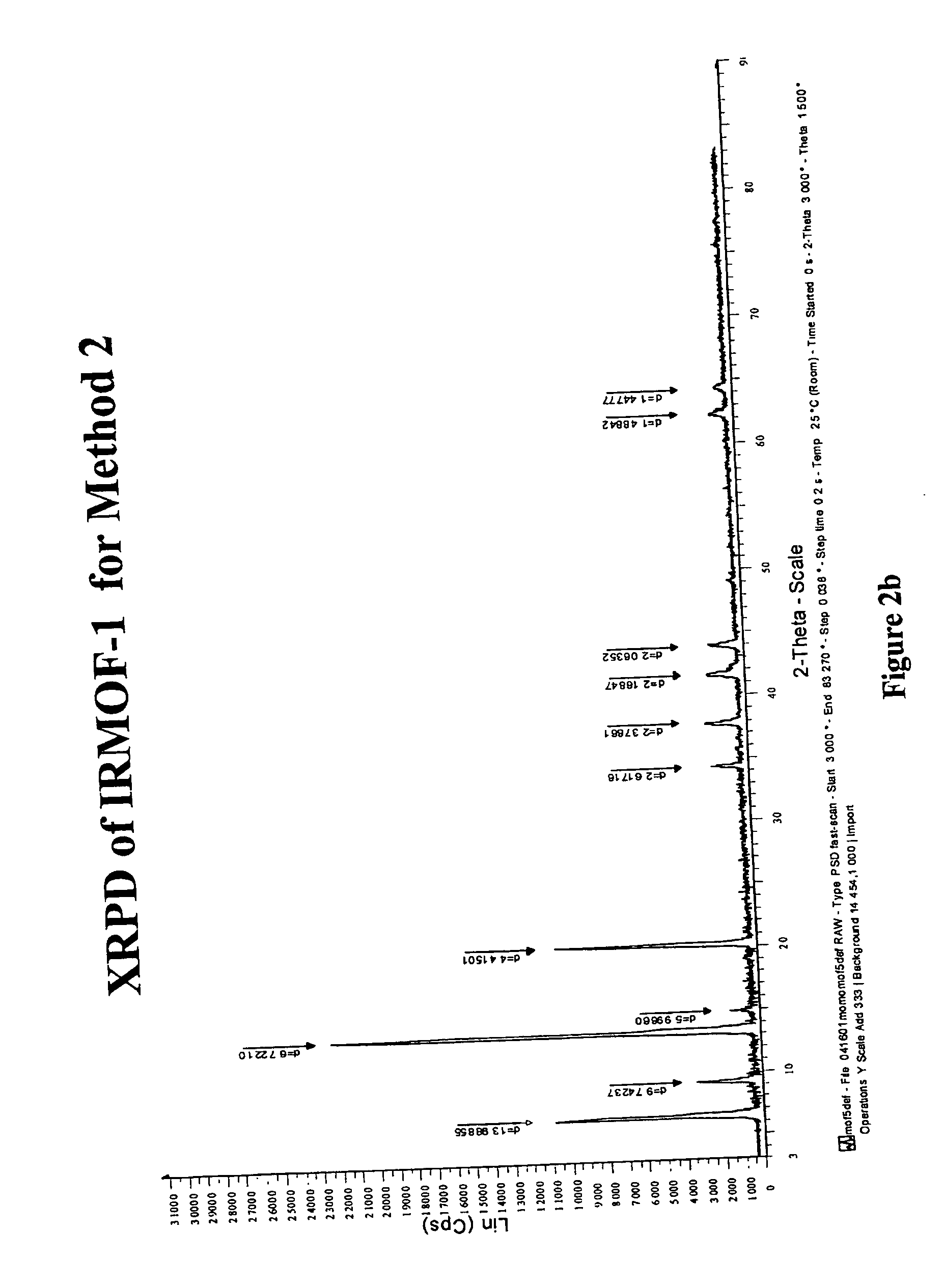
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS20050192175A1Catalyst protectionMolecular sieve catalystsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Releasable corrosion inhibitor compositions
InactiveUS6933046B1Without degrading physical property and performance of coatingReduce impactMaterial nanotechnologyPigmenting treatmentCarboxylic acidMetal
A new class of releasable corrosion inhibiting materials for protective coatings, methods of making the same, methods of using the same, and coatings containing the same are provided. The materials comprise one or more corrosion inhibitors that are chemically anchored to the surface of a particle having an aluminum oxyhydroxide surface through a carboxylate bond. The carboxylate / aluminum-oxyhydroxide-surface bond breaks under corrosion-causing conditions (for example the presence of high levels of hydroxide ions generated by the cathodic oxygen reduction reaction on metals such as iron and aluminum) thereby allowing the corrosion inhibitors to detach from the particle surface when corrosion is present.
Owner:TDA RES
Crosslinked polysaccharide, obtained by crosslinking with substituted polyethylene glycol, as superabsorbant
InactiveUS20030027787A1Improve featuresBiocideOrganic active ingredientsTriflic acidPolyethylene glycol
New crosslinked polysaccharides useful as absorbents or superabsorbents alone or in a mixture are obtained by reacting polysaccharides (preferably containing carboxylates groups) with at least one crosslinker selected in the group constituted by activated polyethylene glycols such as for example halogenated (Cl, Br, I), mesylated, tosylated, or triflated activated polyethylene glycols.
Owner:ARCHER DANIELS MIDLAND CO
Absorbent composition containing molecules with a hindered amine and a metal sulfonate, phosphonate or carboxylate structure for acid gas scrubbing process
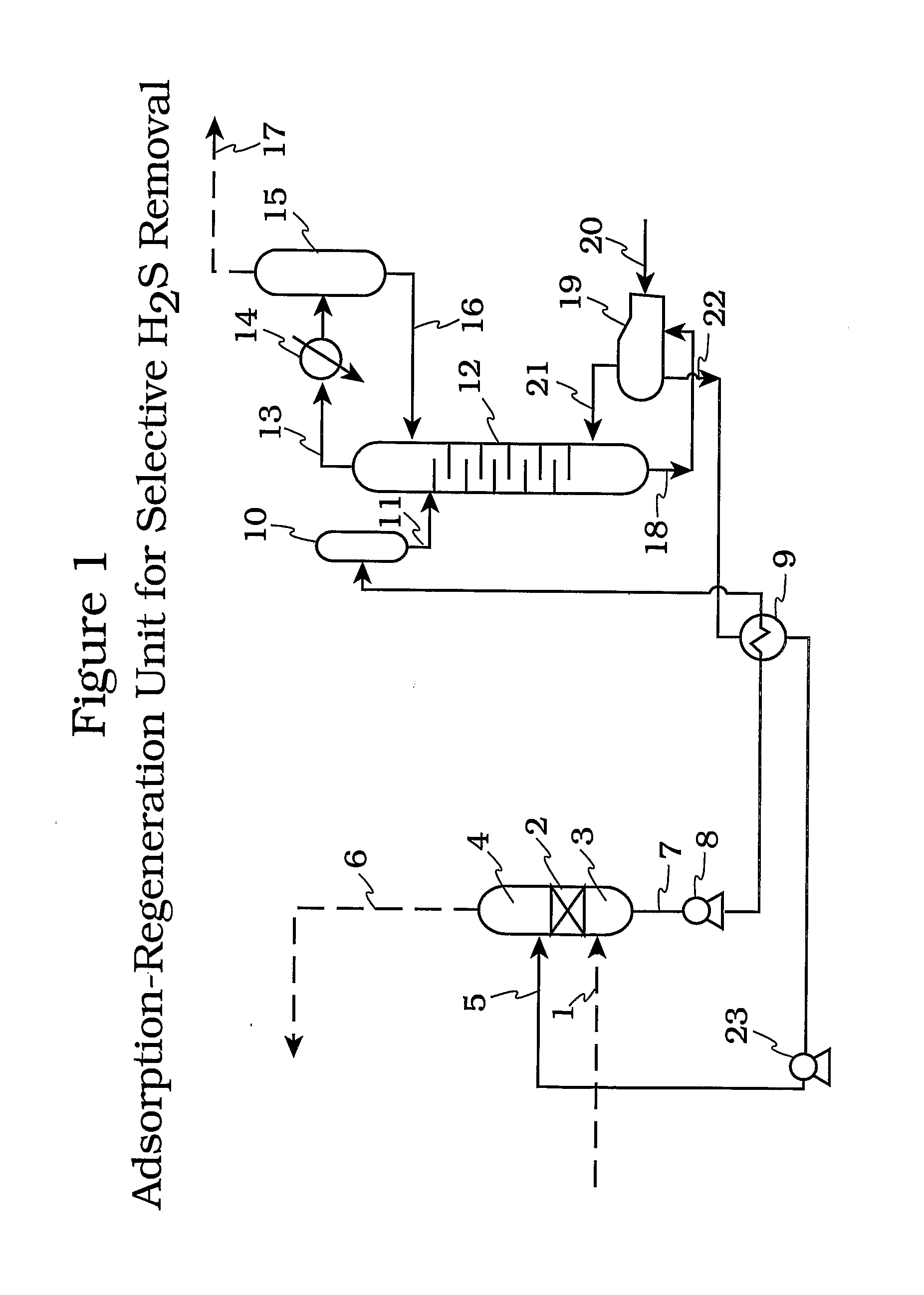
An acid gas absorbent comprising a metal sulfonate, phosphonate or carboxylate of a hindered amine and a process for the selective removal Of H2S as well as other acidic components such as carbon disulfide, carbonyl sulfide and oxygen and sulfur derivatives of C1 to C4 hydrocarbons from mixtures containing such acidic components and CO2 using said absorbent.
Owner:EXXON RES & ENG CO
Polysiloxanes and their preparation
InactiveUS7238768B2Improve catalytic performanceCosmetic preparationsGroup 4/14 element organic compoundsPolymer sciencePtru catalyst
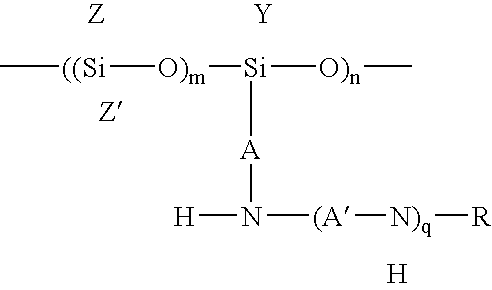
An amino-functional polysiloxane is prepared by reacting an aminosilane (A) which contains an aminoalkyl group and at least one alkoxy group bonded to Si with a carboxylic acid and a silanol-functional polysiloxane (B). The aminosilane (A) is partially converted into its carboxylate salt which acts as a catalyst for the siloxane condensation polymerization reaction between (A) and (B).
Owner:DOW SILICONES CORP
Clear coating composition having improved early hardness and water resistance
InactiveUS6472493B1Improve efficiencyRemove blemishesLiquid surface applicatorsPolyurea/polyurethane coatingsPolyesterOrganic fluid
A fast hardening clear coating composition for repairing a clearcoat / colorcoat finish of a vehicle, which composition is capable of being wet sanded, buffed or polished to a high gloss finish on the same day of application, comprising a film forming binder and an organic liquid carrier, where the binder contains a hydroxyl component comprising a hydroxyl-containing acrylic polymer and a hydroxyl-terminated polyester oligomer, and an organic polyisocyanate crosslinking component, at least portion of which comprises a trimer of isophorone diisocyanate, where the composition further contains, as a combined curing catalyst, at least one dialkyl tin aliphatic carboxylate, at least one tertiary aliphatic mono or diamine, and at least one aliphatic carboxylic acid, in an effective amount such that the clear coating composition on curing at ambient temperatures is in a water spot free and sufficiently hard state for sanding or buffing within about 4 hours after application or on cool down when baked under normal conditions.
Owner:AXALTA COATING SYST IP CO LLC
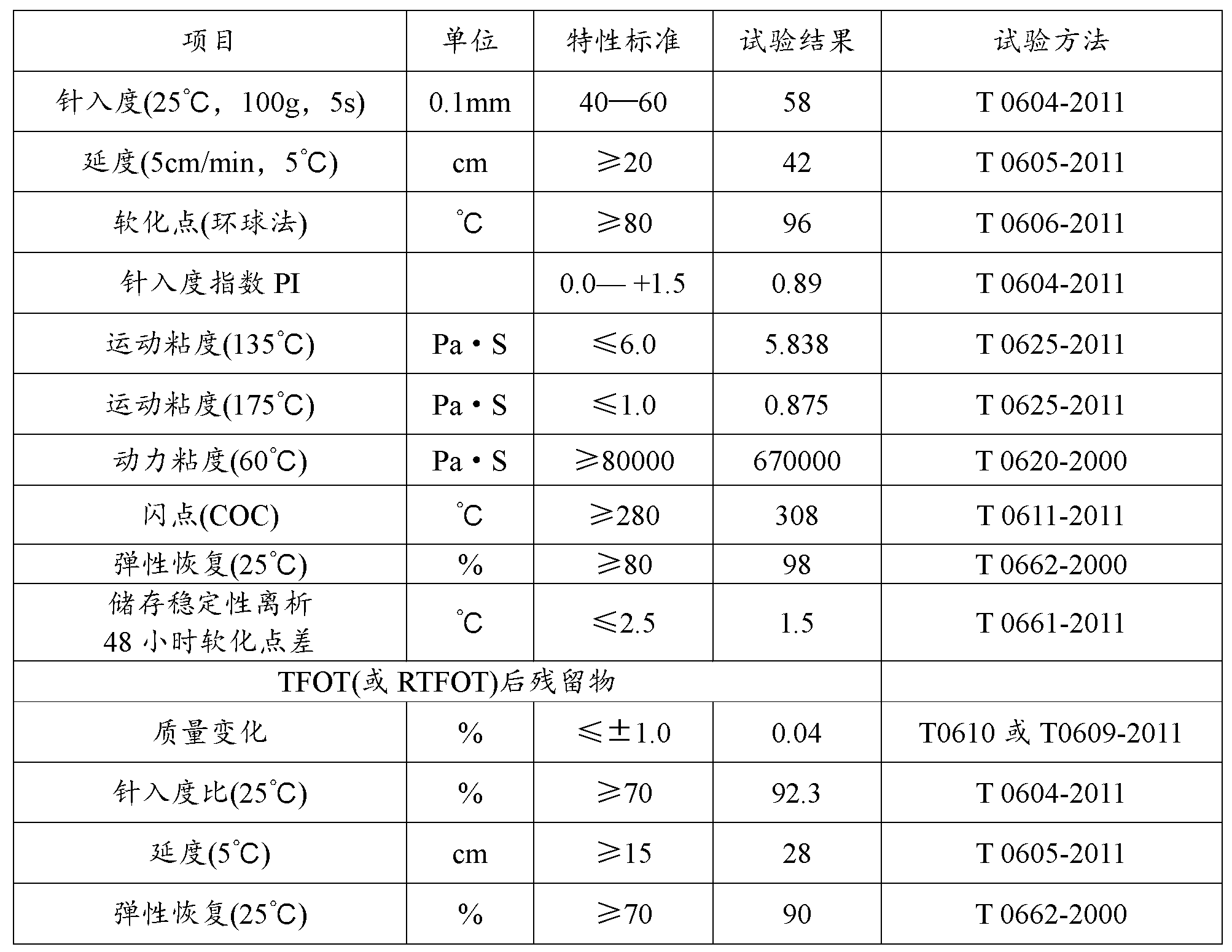
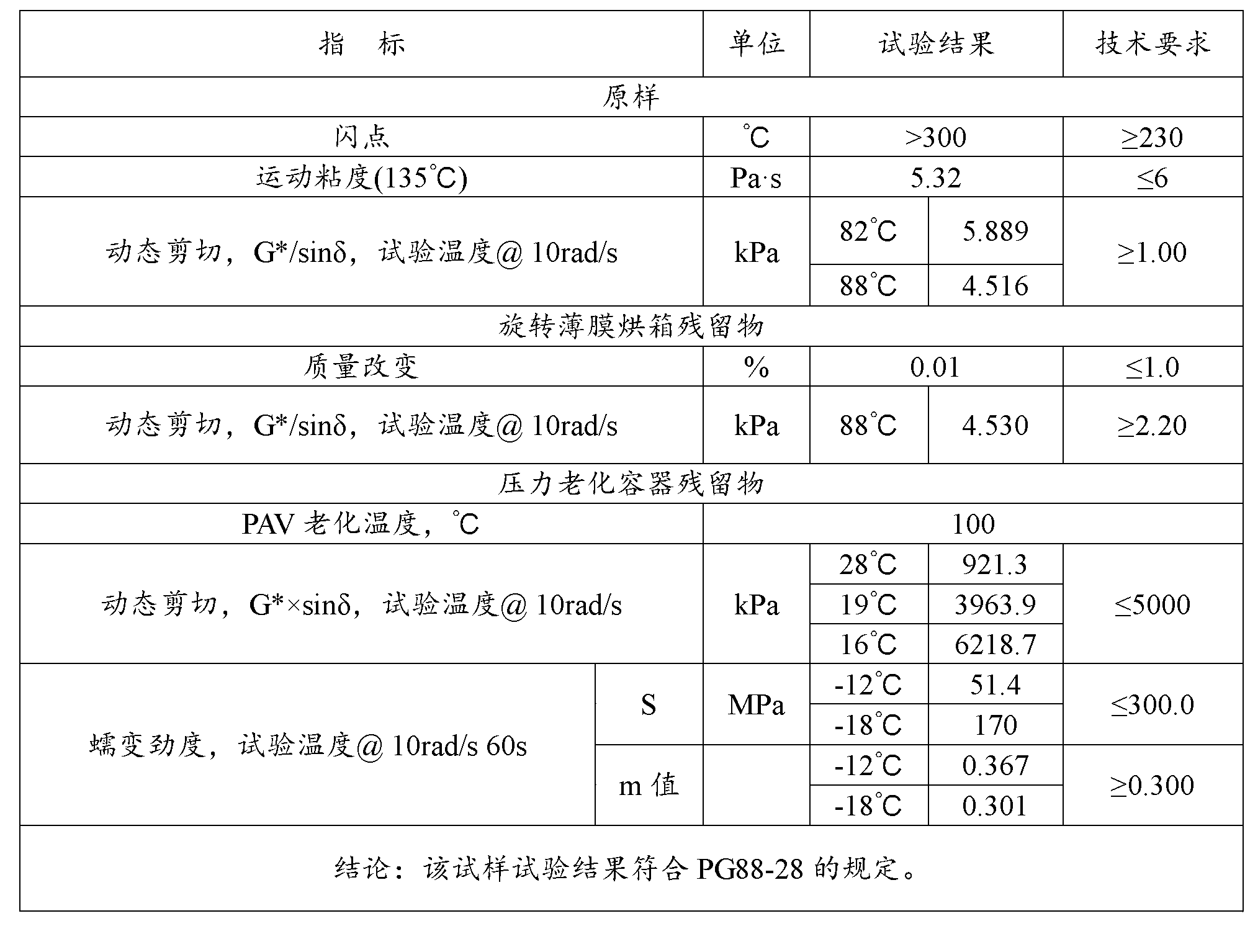
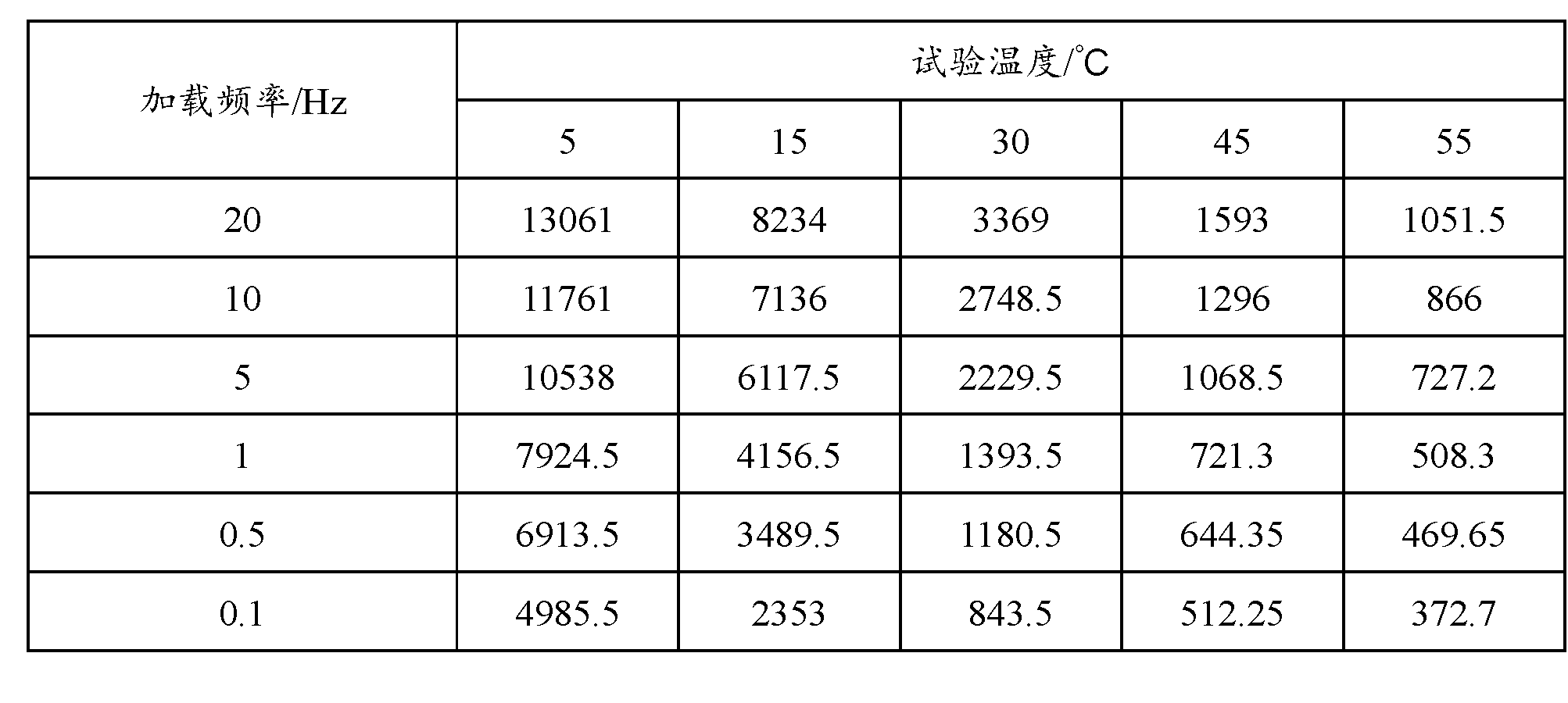
Asphalt modifier, modified asphalt and asphalt mixture
ActiveCN102838874AExcellent Adhesive PropertiesImprove low temperature 60°C viscosityIn situ pavingsBuilding insulationsRoad engineeringBridge deck
The invention belongs to the field of road engineering, and particularly relates to a hyperviscous and high-elastic asphalt modifier applicable to steel bridge deck pavement. The asphalt modifier comprises the following raw materials in parts by weight: 3-10 parts of junked tire rubber powder, 3-10 parts of polyethylene wastes, 3-8 parts of styrene-butadiene-styrene block copolymer, 1-5 parts of styrene-isoprene-styrene block copolymer, 1-5 parts of terpene resin, 5-10 parts of solvent naphtha, and 0.3-0.7 part of alcohol ether carboxylate adhesion agent. In addition, a modified asphalt and amodified asphalt mixture are prepared on the basis of the modifier, the 60 DEG C dynamic viscosity of the modified asphalt prepared by applying the modifier can reach more than 300000Pa.s, and the performance grading reaches PG 88-28; and the asphalt mixture has favorable water stability, higher dynamic modulus, higher track dynamic stability and low temperature failure strain, and can resist complicated mechanics and temperature environment of a steel bridge deck.
Owner:山东高速交通建设集团股份有限公司
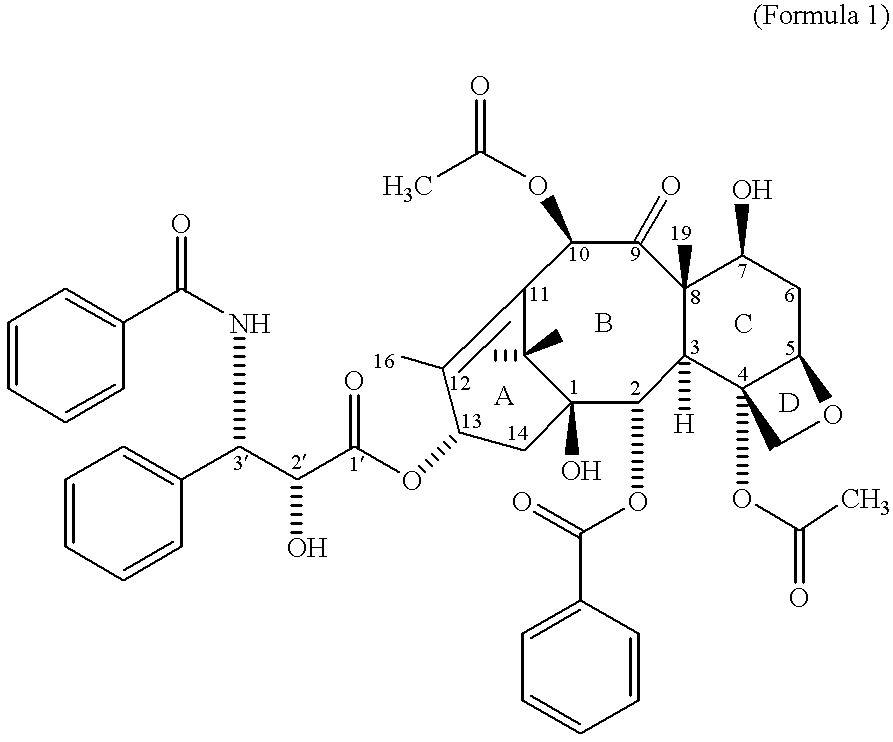
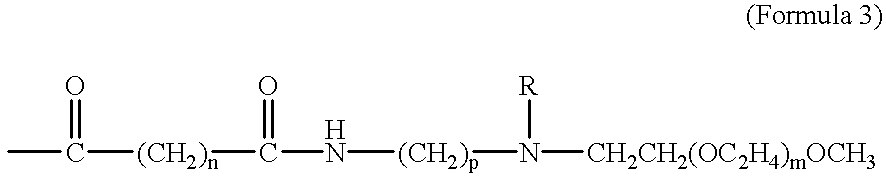
Taxane prodrugs
InactiveUS6380405B1Good water solubilityHigh molecular weightOrganic active ingredientsBiocideOligomerPolyethylene glycol
The present invention generally provides taxane prodrugs comprising at least one taxane joined by hydrolyzable bond(s) to one or more polyethylene glycol oligomer(s) selected from the group consisting of a straight or branched polyethylene glycol oligomer having from 1 to 25 polyethylene glycol units and optionally comprising a salt-forming moiety. The polyethylene glycol oligomer preferably comprises a salt-forming moiety, which is preferably selected from the group consisting of ammonium and carboxylate.
Owner:BIOCON LTD
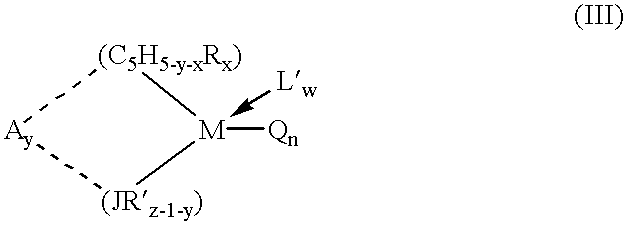
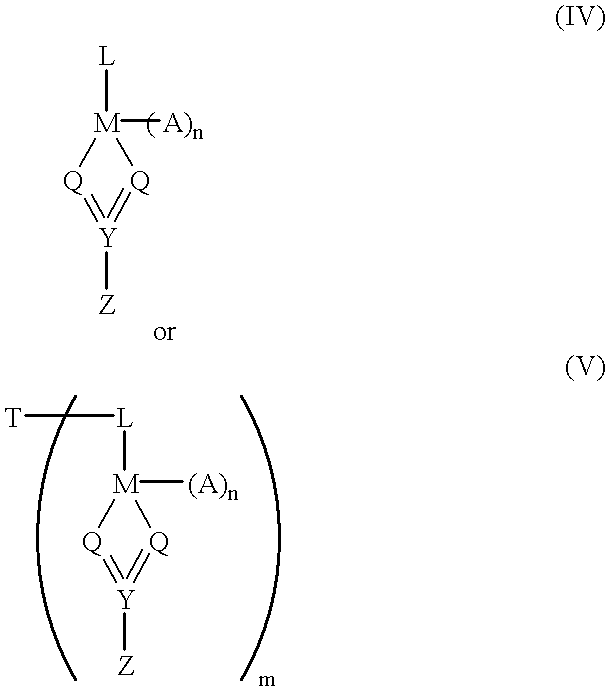
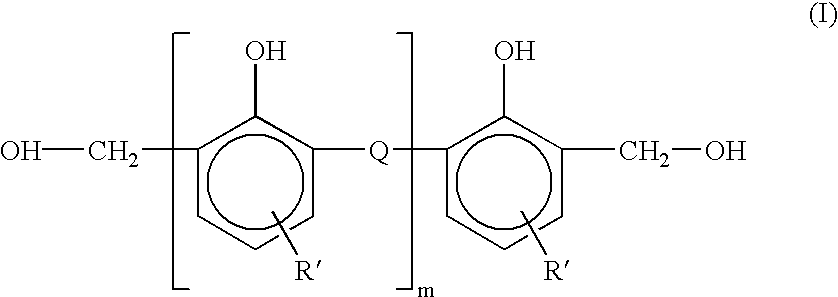
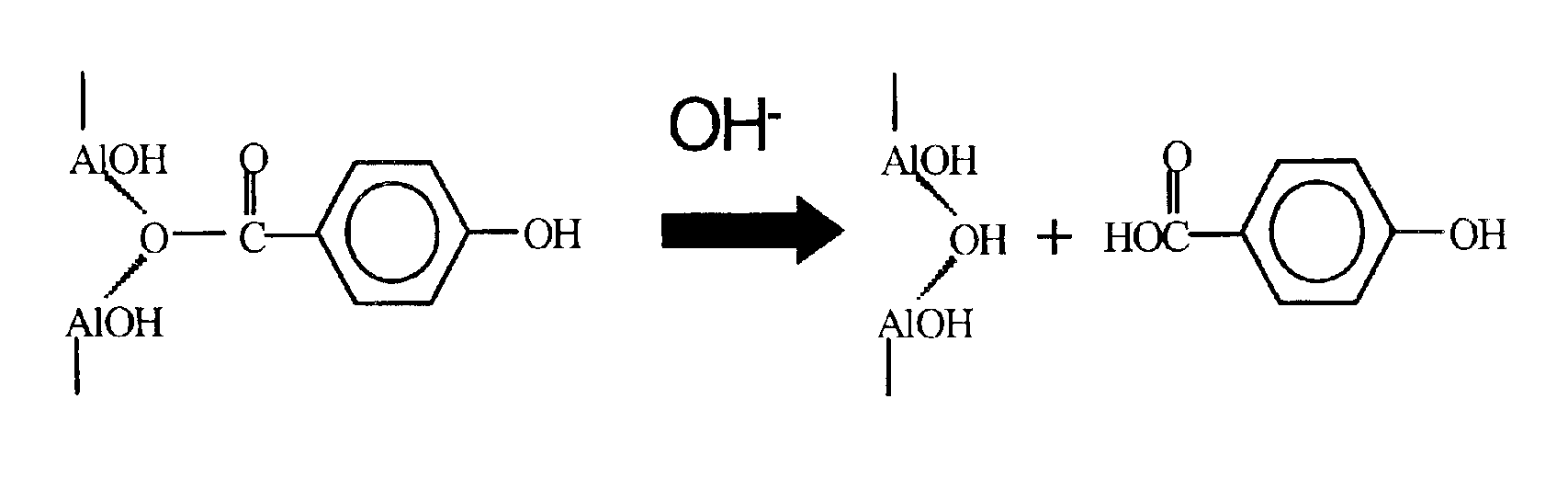
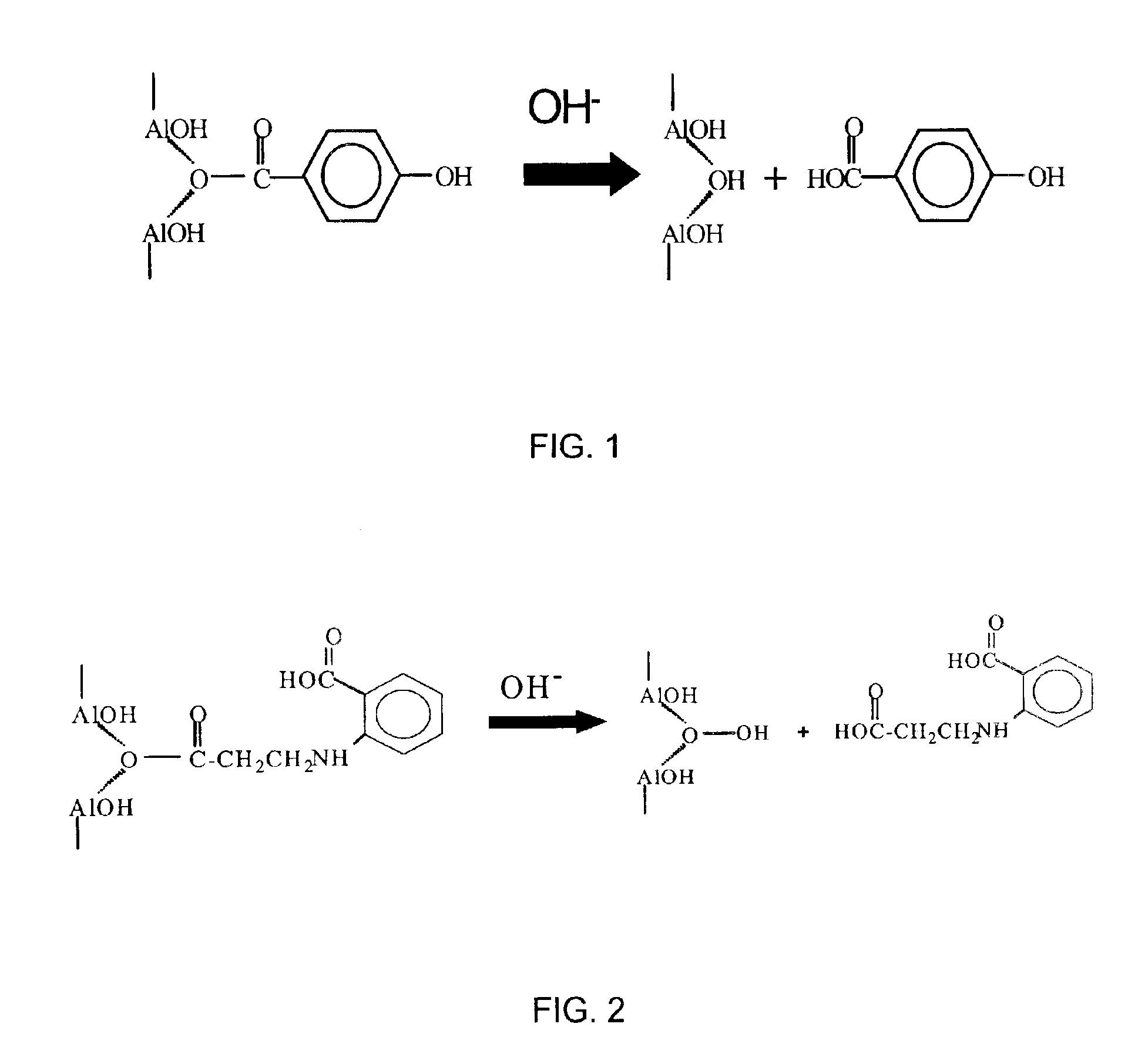
Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease
A compound of formula (I); or a salt or solvate thereof, wherein: the dotted line indicates the optional presence of a double bond between C2 and C3; R2 is selected from —H, —OH, =0, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R7 is selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, nitro, Me3Sn and halo, where R and R′ are independently selected from optionally substituted C1-7 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; R10 and R11 either together form a double bond, or are selected from H and YRY, where Y is selected from O, S and NH and R is H or C1-7 alkyl or H and SOxM, where x is 2 or 3 and M is a monovalent pharmaceutically acceptable cation; each X is independently a heteroarylene group; n is from 1 to 6; and RE is C1-4 alkyl. The compound is useful for the treatment of proliferative diseases.
Owner:MEDIMMUNE LTD
Molecular Adaptors for Dye Conjugates
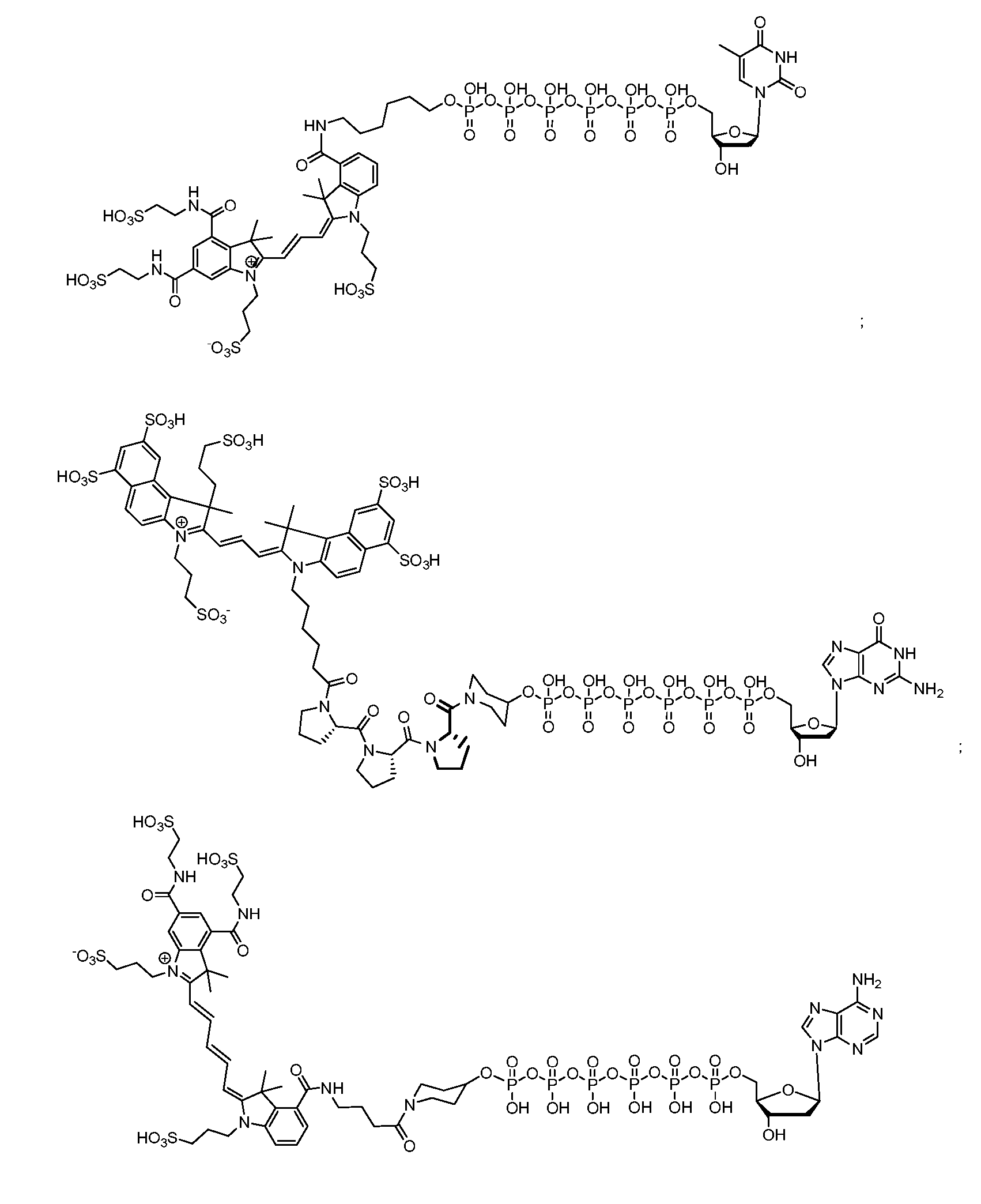
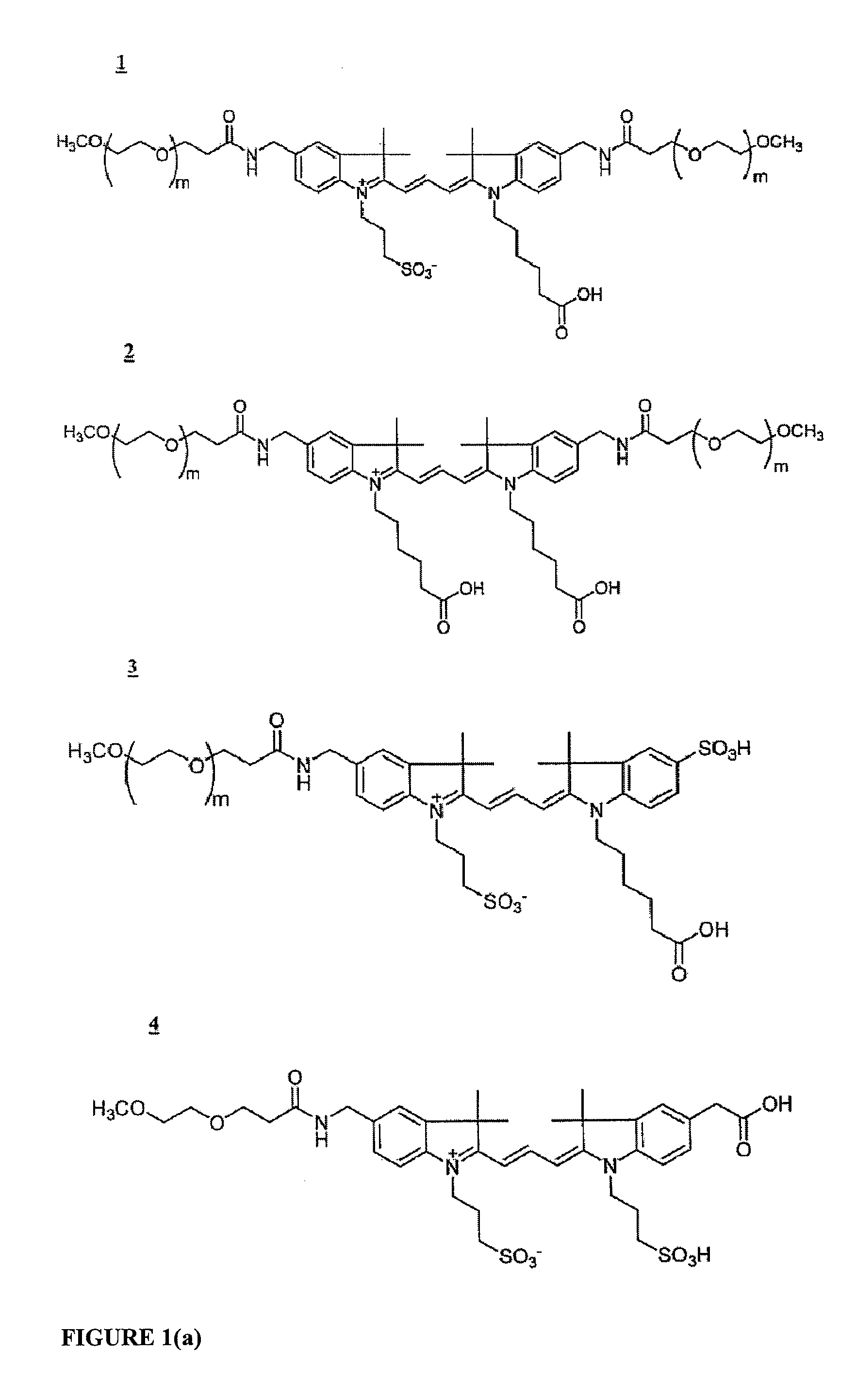
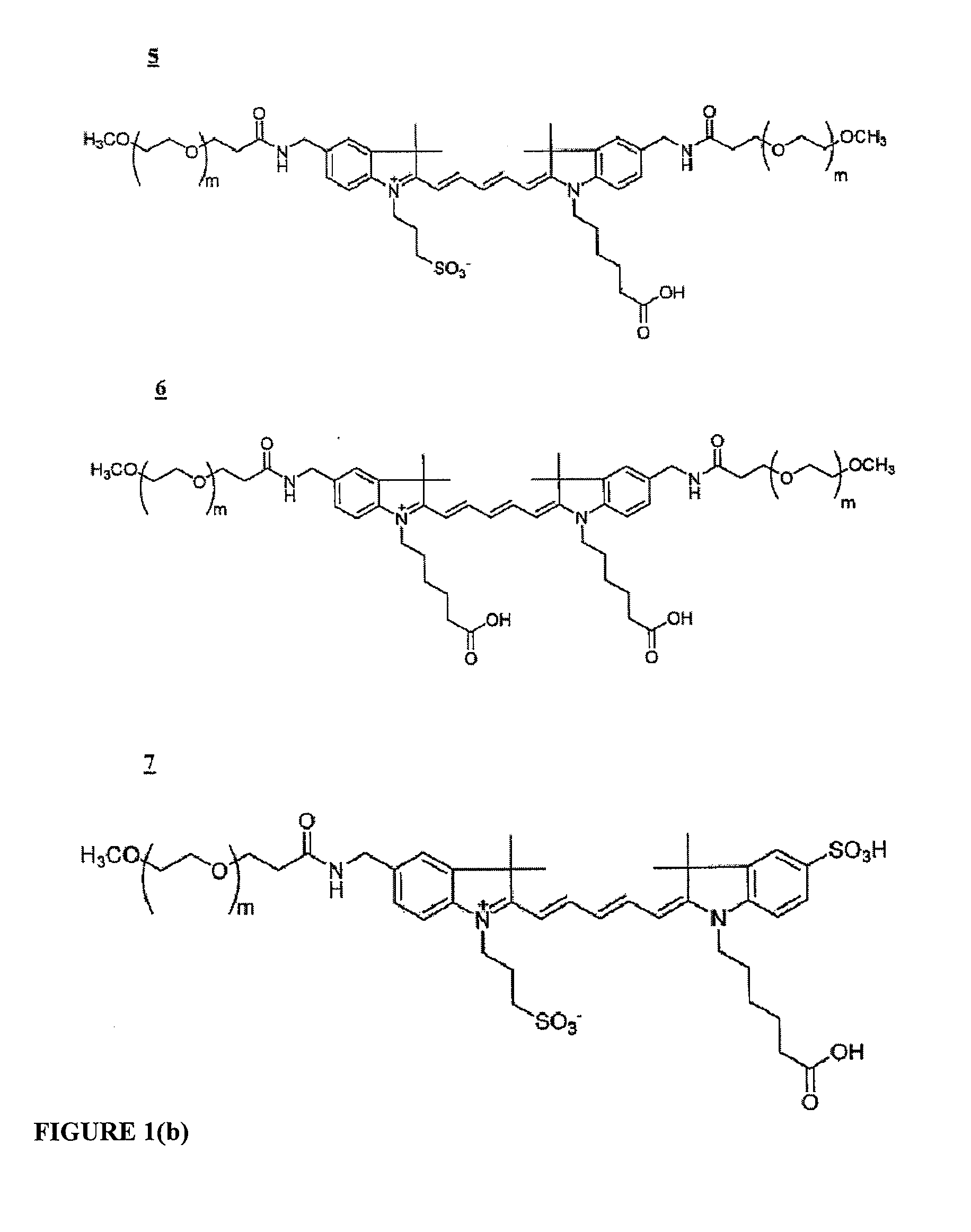
ActiveUS20120058473A1Improve hydrophilicityReduce aggregationMethine/polymethine dyesSugar derivativesFluorescenceCarboxylate
In various embodiments, the present invention provides fluorescent dyes that are linked to another species through an adaptor moiety. In an exemplary embodiment, the dye is linked to a polyphosphate nucleic acid through an adaptor. An adaptor can be a component of a linker. These conjugates find use in single molecule DNA sequencing and other applications. In various embodiments, the dye moiety is a cyanine dye. Cyanine dyes that are highly charged, such as those including multiple sulfonate, alkylsulfonate, carboxylate and / or alkylcarboxylate moieties are examples of cyanine dyes of use in the compounds of the invention.
Owner:PACIFIC BIOSCIENCES
High-performance polycarboxylic acid water reducing agent and preparation method thereof
The invention discloses a preparation method of a high-performance polycarboxylic acid water reducing agent. The preparation method of the high-performance polycarboxylic acid water reducing agent comprises the following steps of: adding an unsaturated polyether macromonomer A and water into a reactor, uniformly stirring and dissolving, and then adding an initiator F hydrogen peroxide; then respectively dropwise adding unsaturated carboxylic acid or an unsaturated carboxylic acid anhydride small monomer B, an unsaturated carboxylate ester small monomer C, an aqueous solution of an initiator D, an aqueous solution of an initiator E and an aqueous solution of a molecular weight regulator, and carrying out reaction for 3-5 hours at the room temperature of 10-40 DEG C, so as to obtain a copolymerization product; carrying out a neutralization reaction, namely regulating the pH value of the copolymerization production to be 6-7 by adopting sodium hydroxide, so as to obtain the high-performance polycarboxylic acid water reducing agent. The invention also discloses a high-performance polycarboxylic acid water reducing agent prepared by adopting the preparation method. By controlling a material adding manner, two initiators are compounded, initiated and synthesized to form the high-performance polycarboxylic acid water reducing agent, the synthesized high-performance polycarboxylic acid water reducing agent is environmentally-friendly, pollution-free and high in reaction conversion ratio, and concrete doped with the high-performance polycarboxylic acid water reducing agent has better water reducing property, slump loss resistant property, dispersibility and workability.
Owner:KZJ NEW MATERIALS GROUP CO LTD
Surfactant composition
InactiveUS6946430B2Improve foamabilityImprove performanceInorganic/elemental detergent compounding agentsCosmetic preparationsSulfonateActive agent
To provide a surfactant composition with an excellent foamability and thickening performance and further an excellent storage stability, it comprises the following components (a) and (b):(a) at least one anionic surfactant selected from (1) sulfonate-type anionic surfactants having amide group or ester group, (2) carboxylate-type anionic surfactants and (3) phosphate-type anionic surfactants and(b) a compound expressed by the formula (I): where R1CO— is an acyl group having 6 to 24 carbon atoms, R2 is an alkyl group having 1 to 3 carbon atoms and R3 is an alkylene group having 1 to 6 carbon atoms or an alkenylene group having 2 to 6 carbon atoms.
Owner:KAO CORP
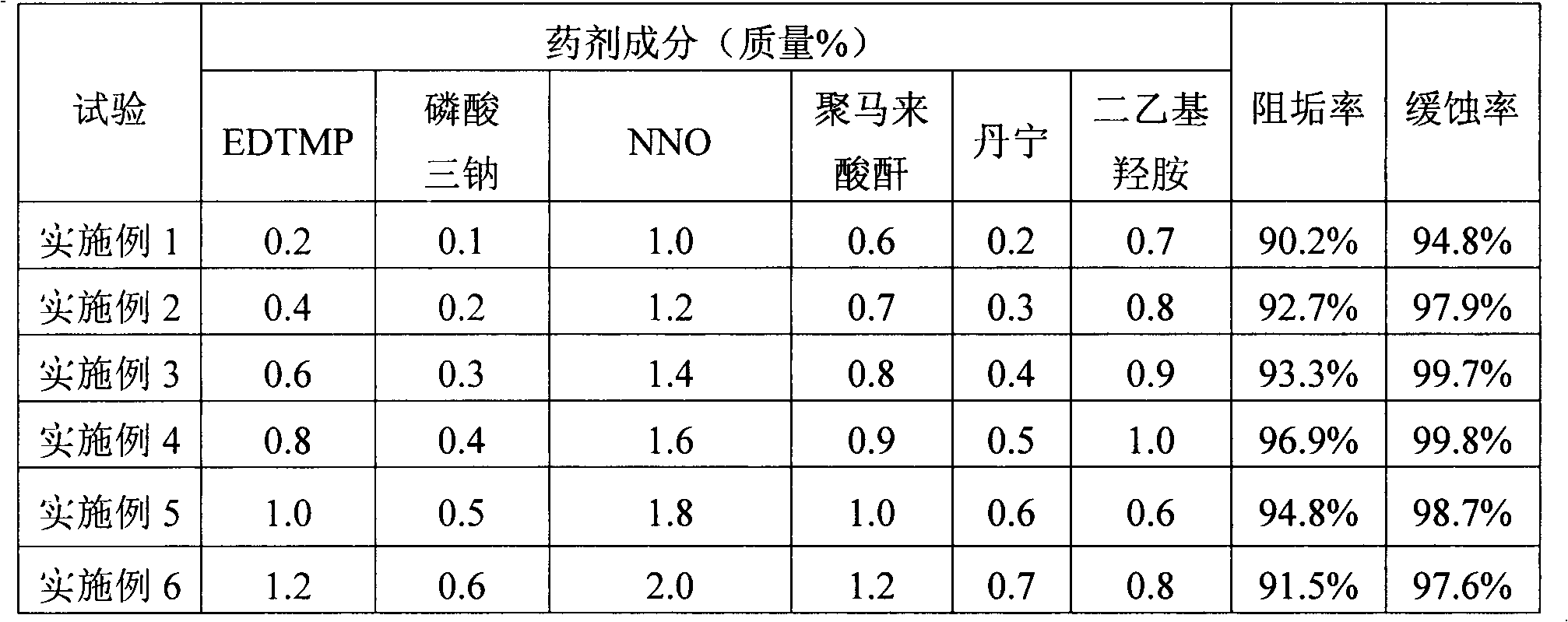
Medicament for treating composite water in hot water boiler as well as preparation method and use method thereof
ActiveCN103183417ADoes not reduce corrosion inhibitionWill not reduce the effect of anti-scalingScale removal and water softeningInorganic phosphatePhosphoric acid
The invention discloses a medicament for treating composite water in a hot water boiler. The medicament comprises the following components by mass percent: 0.1-3% of inorganic phosphate, 0.1-2% of organic phosphoric acid and / or organic phosphate, 0.1-4% of component A, 0.1-3% of component B, 0.6-1.0% of organic amine deoxidizer, 0.1-2% of tannin and / or chitosan and the balance of water, wherein the mass percent of the components is 100%, the component A is one or more of an organic antisludging agent, an organic dispersant and an organic dispersible antisludging agent, and the component B is one or more of an organic carboxylic acid corrosion inhibitor, an organic carboxylate corrosion inhibitor and sodium methylene dinaphthalenesulfonate. The invention further discloses a preparation method and a use method of the medicament, the preparation process is clean, and the medicament is low in toxicity for body health and environment in the using process. The medicament is excellent in corrosion resistance and scale inhibition effects and simple and convenient to store and transport, and the corrosion and scale inhibition effects cannot be reduced after the medicament is stored and transported for a long term.
Owner:SHANGHAI EMPEROR OF CLEANING HI TECH
Catalyst composition and methods for its preparation and use in a polymerization process
InactiveUS7354880B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationCarboxylic acidAlkene
The present invention relates to a catalyst composition and a method for making the catalyst composition of a polymerization catalyst and a carboxylate metal salt. The invention is also directed to the use of the catalyst composition in the polymerization of olefin(s). In particular, the polymerization catalyst system is supported on a carrier. More particularly, the polymerization catalyst comprises a bulky ligand metallocene-type catalyst system.
Owner:UNIVATION TECH LLC
Articles containing bimodal ionomer compositions
Provided are partially or fully neutralized mixtures of carboxylate functionalized ethylene high copolymers or terpolymers (Mw between 80,000 and 500,000 Da) with carboxylate functionalized ethylene low copolymers (Mw between 2,000 and 30,000 Da). The compositions are used in films, multilayer structures and other articles of manufacture. The compositions are preferably used on a surface of the articles.
Owner:PERFORMANCE MATERIALS NA INC
Curing composition with improved heat resistance
The present invention provides a curable composition including: an organic polymer (A) which has on average 1.1 to 50 groups per one molecule thereof each represented by the general formula (1) and has one or more silicon-containing functional groups capable of cross-linking by forming siloxane bonds: —NR1—C(═O)— (1) wherein R1 is a hydrogen atom, or a substituted or unsubstituted monovalent organic group; and a metal carboxylate and / or a carboxylic acid (B), the curable composition giving a cured article excellent in curability and also excellent in heat resistance although a non-organotin catalyst is used.
Owner:KANEKA CORP
Thermoplastic resin compositions suitable for use in laminated safety glass
The present invention is a polymeric resin composition having improved toughness, suitable for use in transparent glazing, comprising an at least partially neutralized ethylene acid copolymer metal carboxylate resin, wherein the metal carboxylate consists essentially of zinc metal counter-ions.
Owner:DOW GLOBAL TECH LLC
Overbased magnesium deposit control additive for residual fuel oils
An overbased magnesium composition deposit control additive for residual fuel oils and turbine fuels is an overbased magnesium sulfonate, carboxylate or phenate or mixtures thereof containing at least 14% and upwards to about 18% by weight of magnesium and containing a succinic anhydride and lower carboxylic acid co-promoter reaction product. The additive when added to fuel oils, such as residual fuel oils containing high asphaltenes, reduces, if not eliminates, magnesium / asphaltene deposits or sediment and the consequential plugging of filters. The additive also reduces, if not eliminates, vanadium caused corrosion in the turbine. The invention is also the process for preparing the overbased composition or deposit control additive, wherein the overbasing reaction incorporates the combination of a lower carboxylic acid, preferably acetic acid and a succinic anhydride, preferably dodecenyl succinic anhydride (DDSA), as the co-promoter.
Owner:CK WITCO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



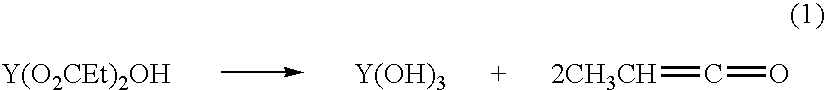



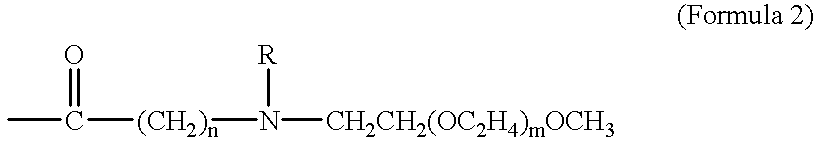
![Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease](https://images-eureka.patsnap.com/patent_img/11c1ed56-14ef-4298-9163-47efb6650e32/US08637664-20140128-D00001.png)
![Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease](https://images-eureka.patsnap.com/patent_img/11c1ed56-14ef-4298-9163-47efb6650e32/US08637664-20140128-D00002.png)
![Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease Alkyl 4- [4- (5-oxo-2,3,5, 11a-tetrahydo-5H-pyrrolo [2, 1-c] [1,4] benzodiazepine-8-yloxy)-butyrylamino]-1H-pyrrole-2-carboxylate derivatives and related compounds for the treatment of a proliferative disease](https://images-eureka.patsnap.com/patent_img/11c1ed56-14ef-4298-9163-47efb6650e32/US08637664-20140128-D00003.png)