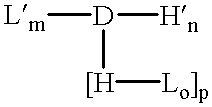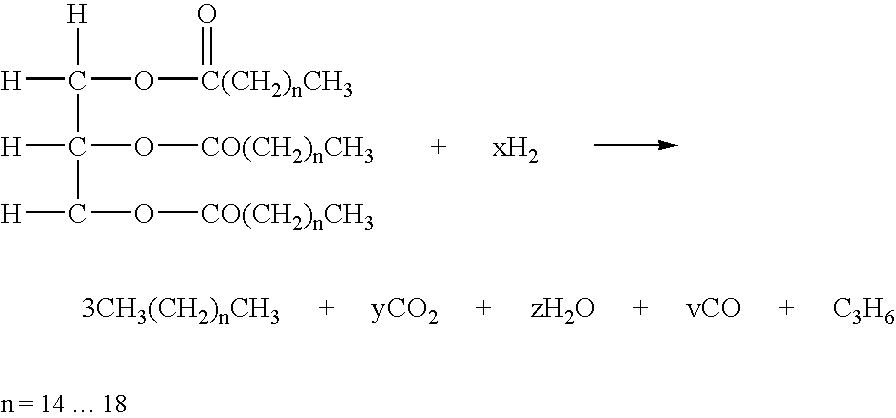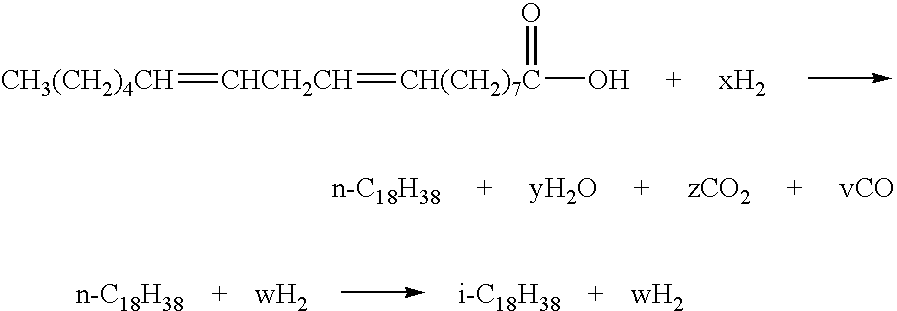Patents
Literature
16986 results about "Fatty acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually not found in organisms, but instead as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and they are important structural components for cells.
Antimicrobial composition
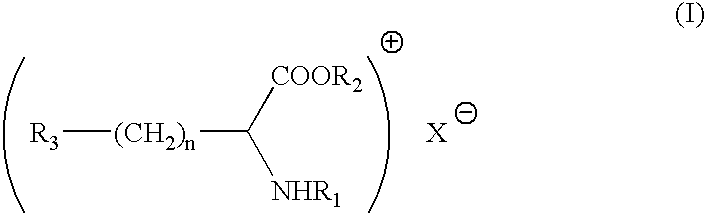
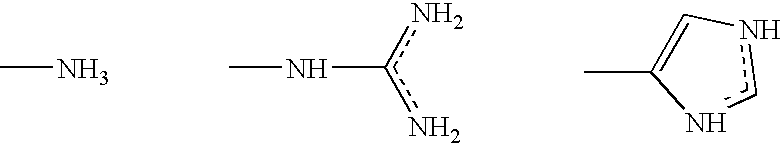
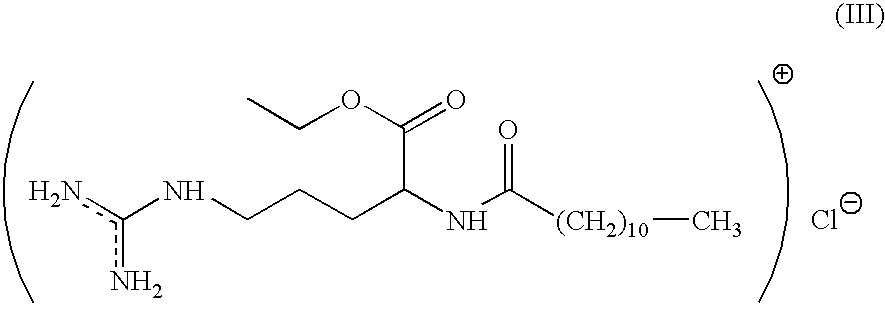
An antimicrobial composition comprising (a) a cationic surfactant derived from the condensation of fatty acids and esterified dibasic amino acids, such as lauric arginate, and (b) an antimicrobial metal, such as elemental silver or alloys thereof or silver compounds. The composition may be used as a stand alone antimicrobial formulation, or in combination with medical articles or medical devices.
Owner:ETHICON INC
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
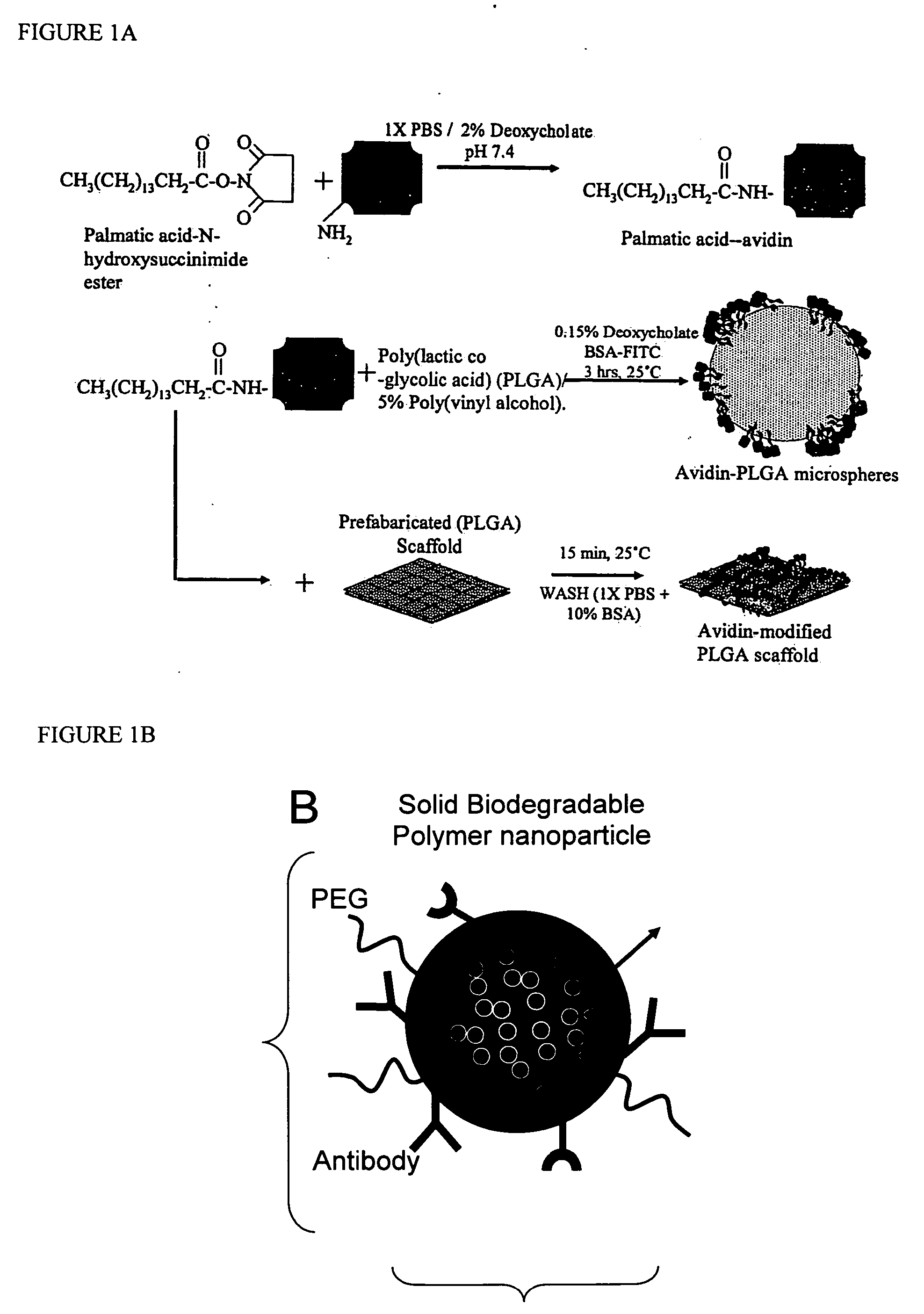
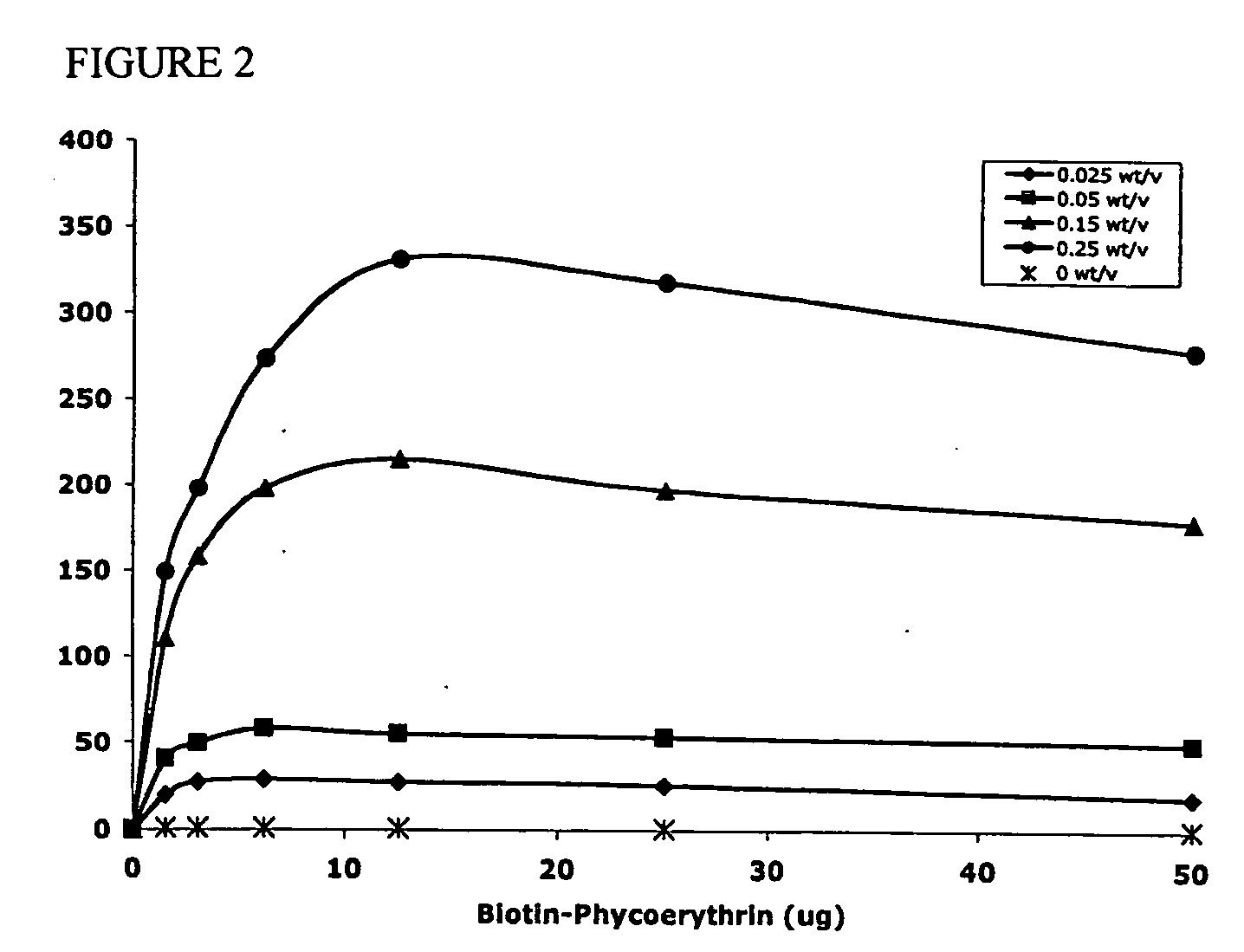
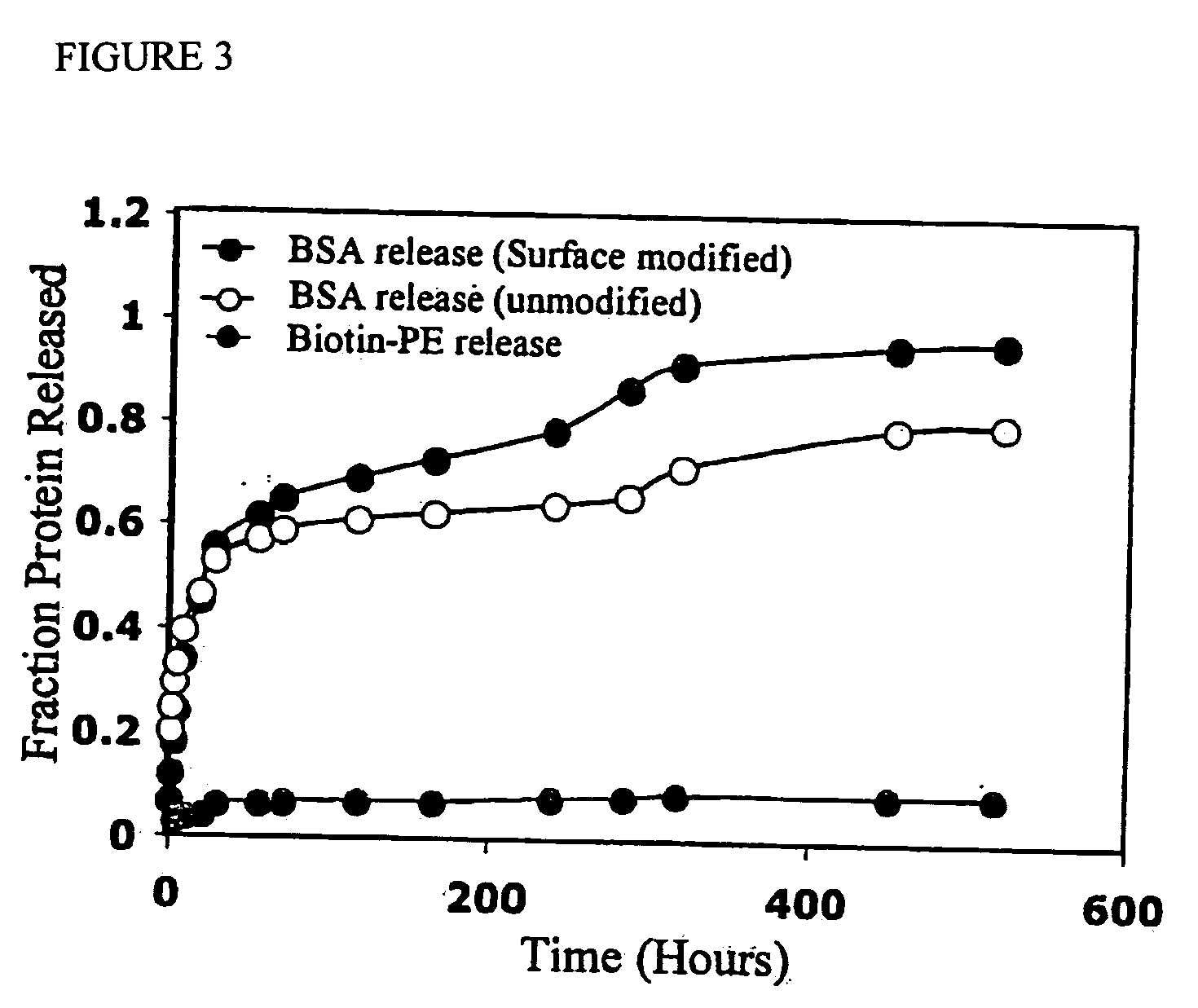
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Product and process for transformation of Thraustochytriales microorganisms
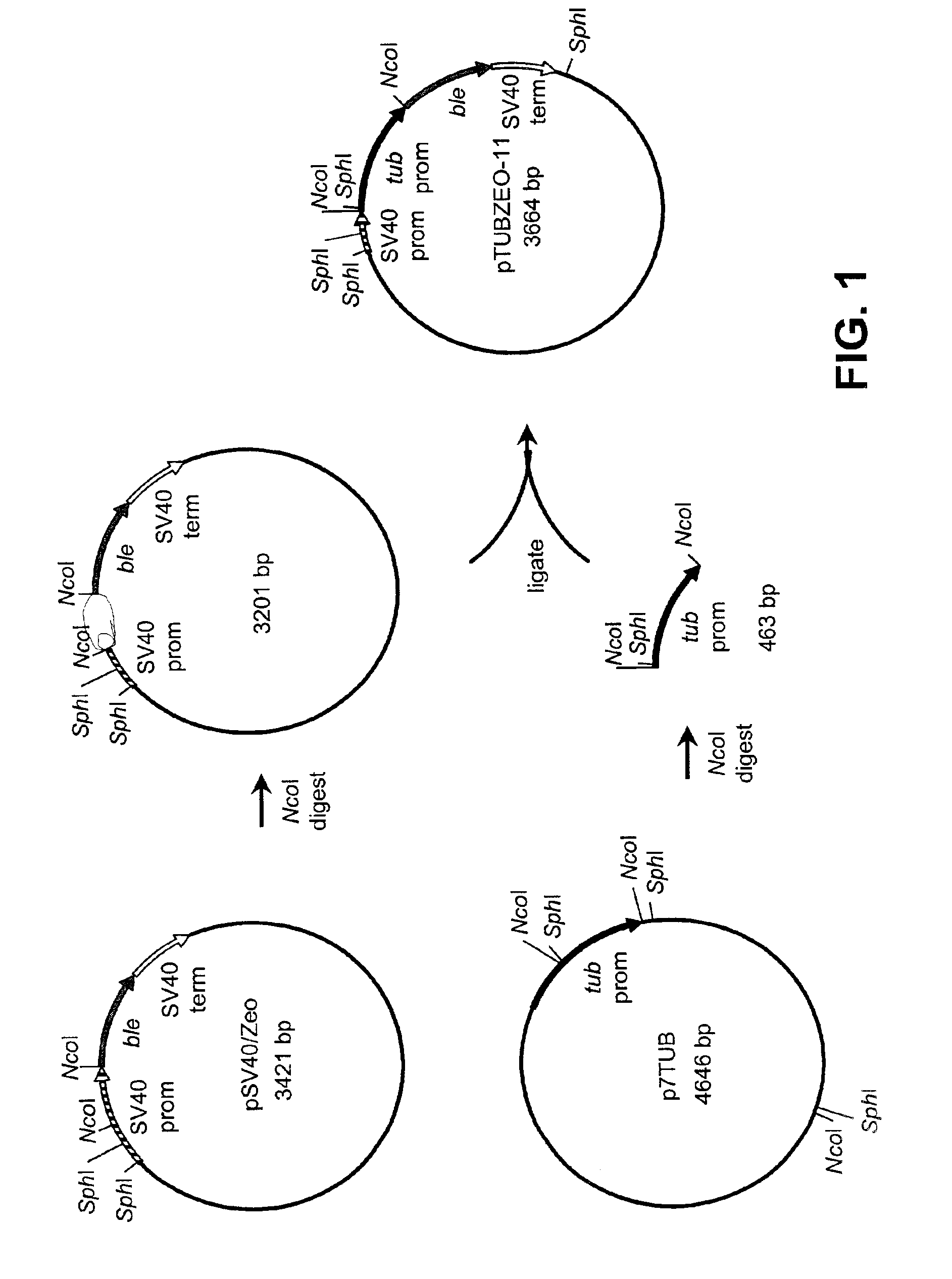
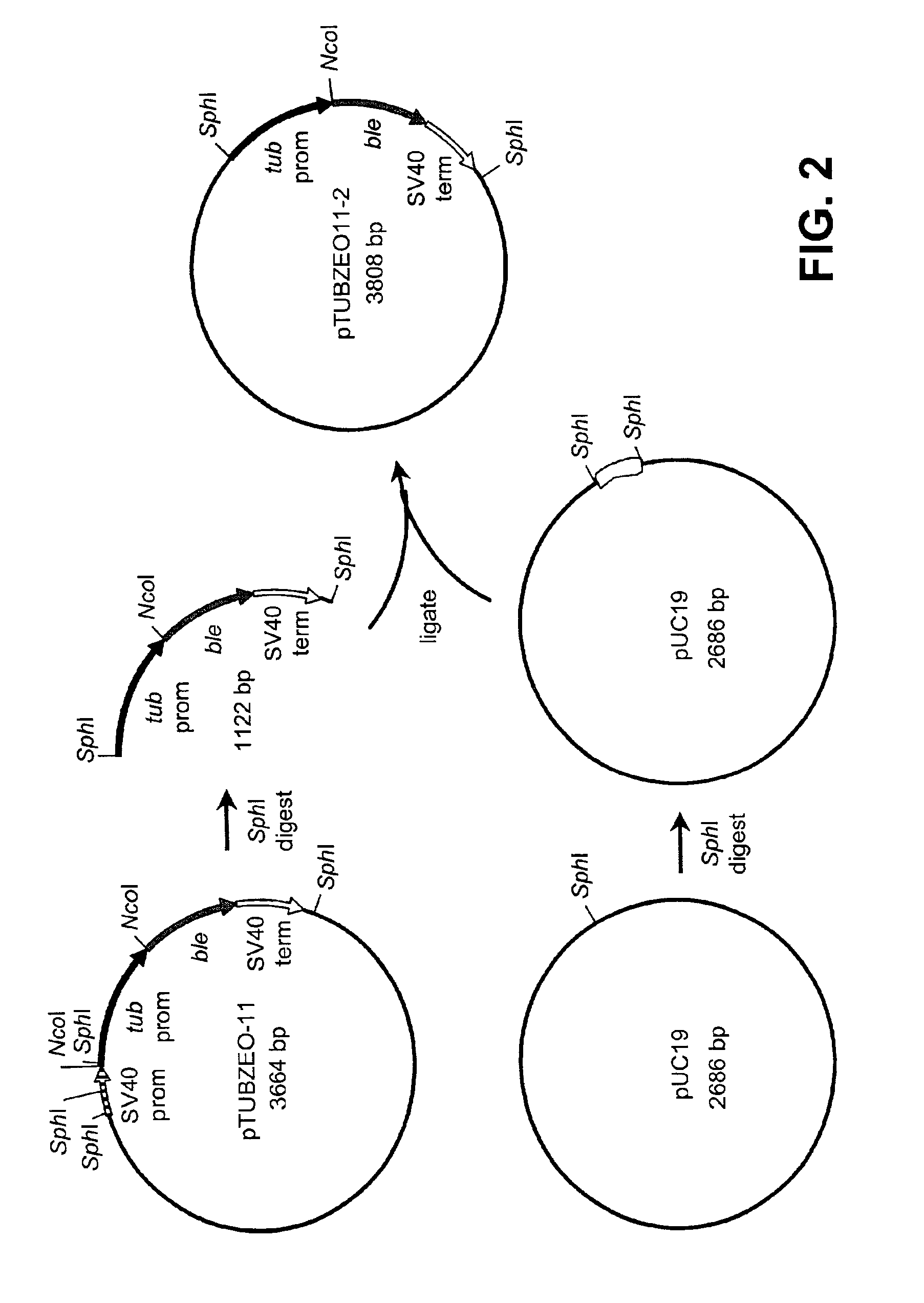
Disclosed are nucleic acid and amino acid sequences for acetolactate synthase, acetolactate synthase regulatory regions, α-tubulin promoter, a promoter from a Thraustochytriales polyketide synthase (PKS) system, and fatty acid desaturase promoter, each from a Thraustochytriales microorganism. Also disclosed are recombinant vectors useful for transformation of Thraustochytriales microorganisms, as well as a method of transformation of Thraustochytriales microorganisms. The recombinant nucleic acid molecules of the present invention can be used for the expression of foreign nucleic acids in a Thraustochytriales microorganism as well as for the deletion, mutation, or inactivation of genes in Thraustochytriales microorganisms.
Owner:DSM IP ASSETS BV
Process for the manufacture of diesel range hydrocarbons
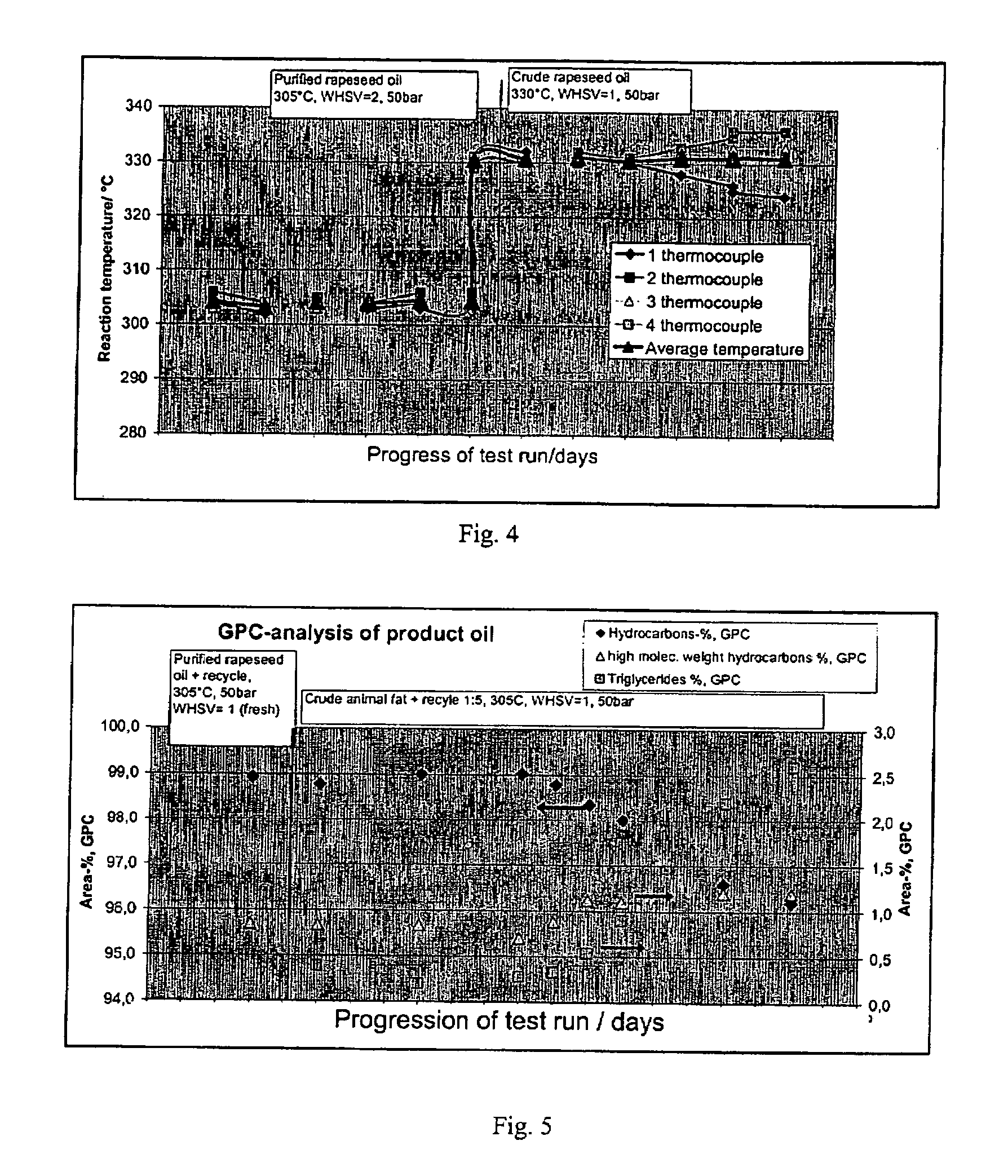
ActiveUS20070010682A1Fatty oils/acids recovery from wasteHydrocarbon by isomerisationIsomerizationReaction temperature
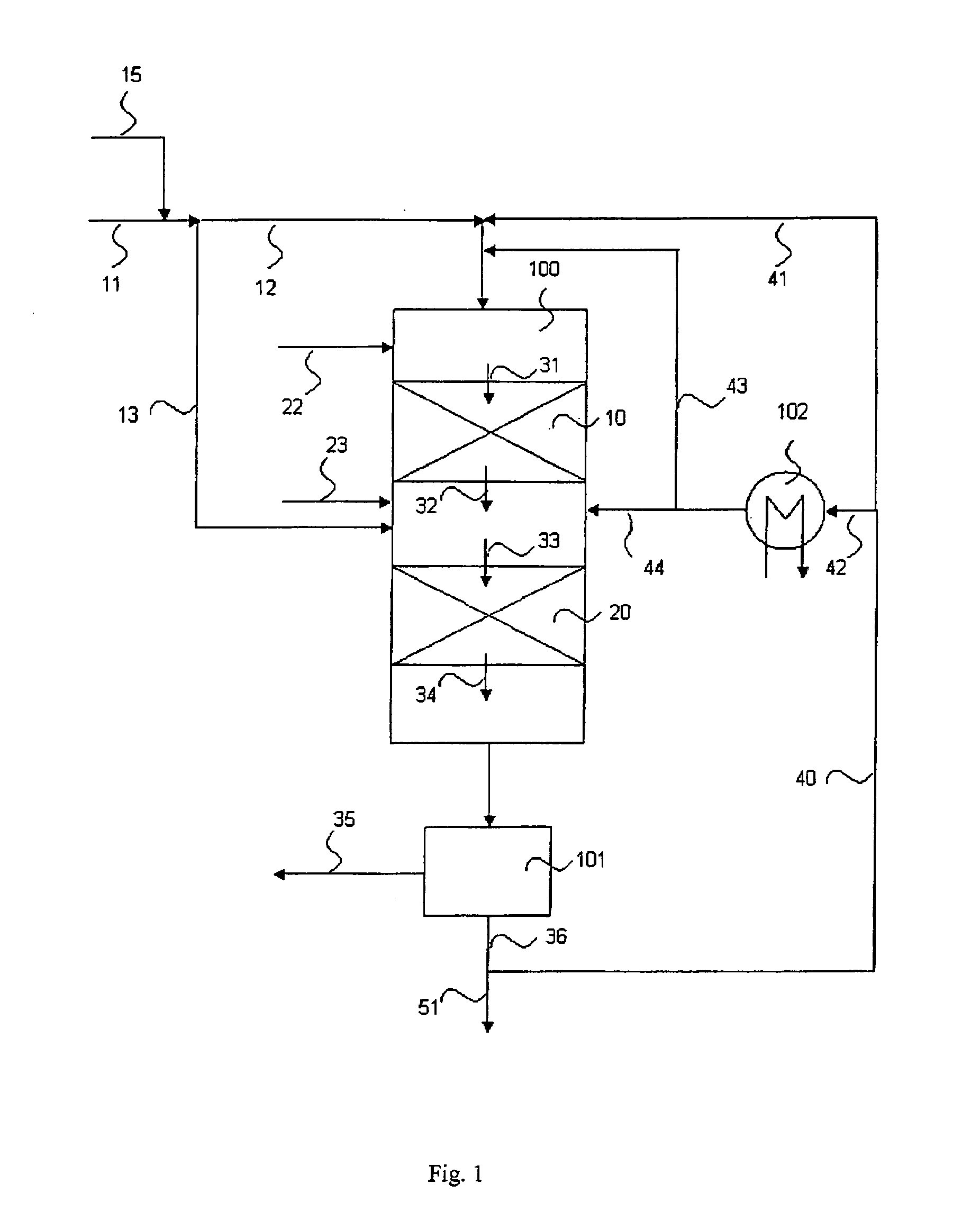
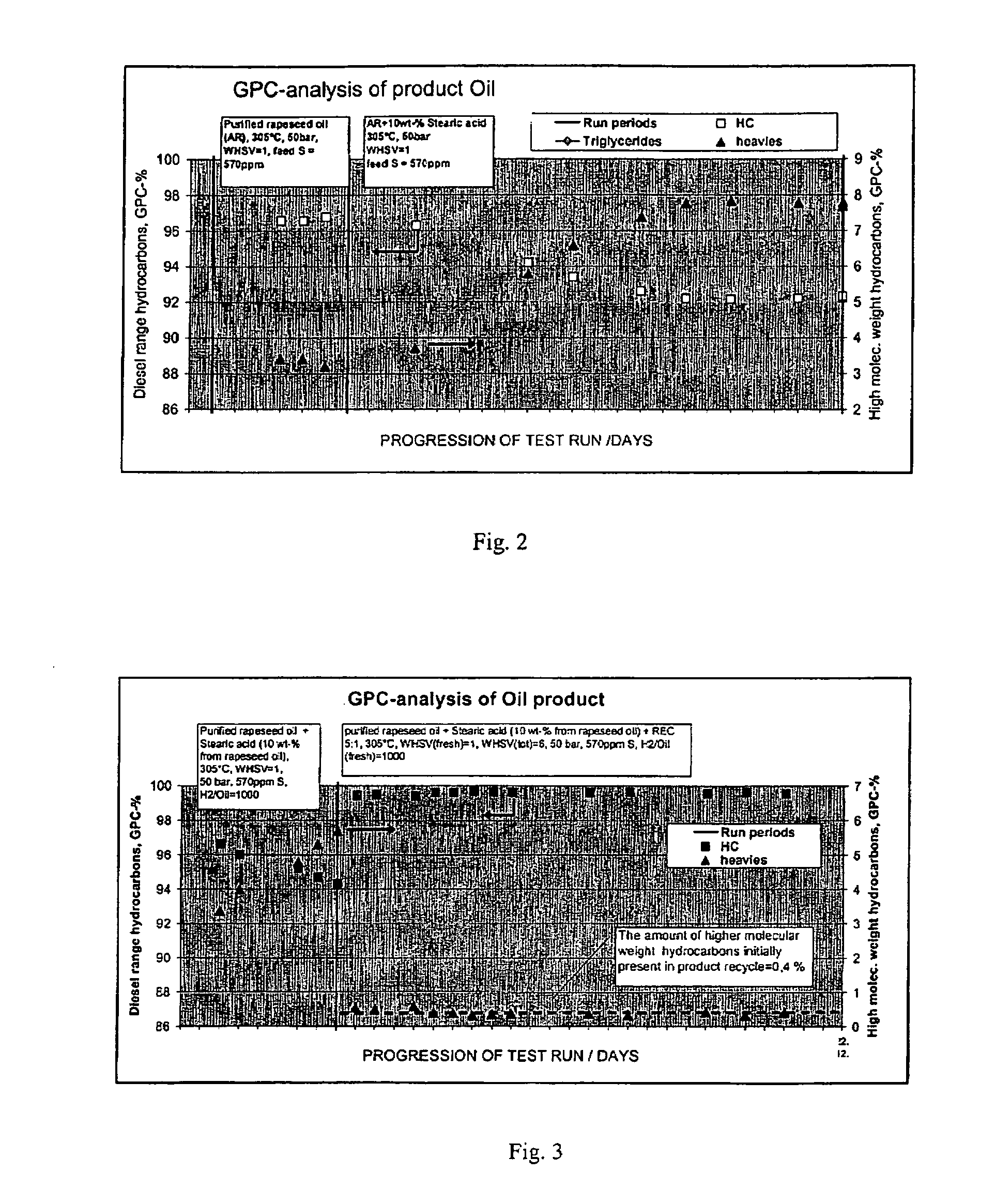
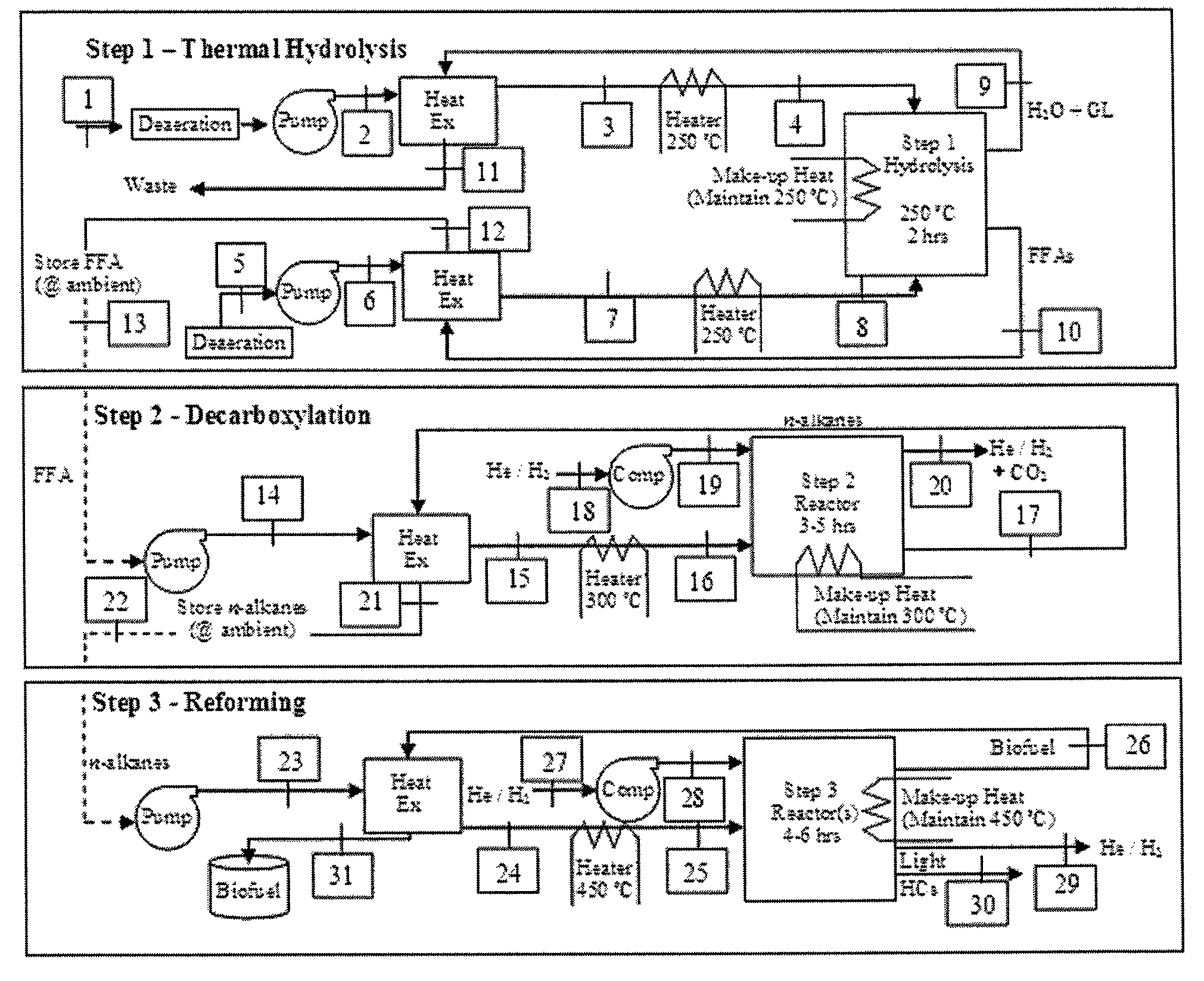
The invention relates to a process for the manufacture of diesel range hydrocarbons wherein a feed is hydrotreated in a hydrotreating step and isomerised in an isomerisation step, and a feed comprising fresh feed containing more than 5 wt % of free fatty acids and at least one diluting agent is hydrotreated at a reaction temperature of 200-400° C., in a hydrotreating reactor in the presence of catalyst, and the ratio of the diluting agent / fresh feed is 5-30:1.
Owner:NESTE OIL OY
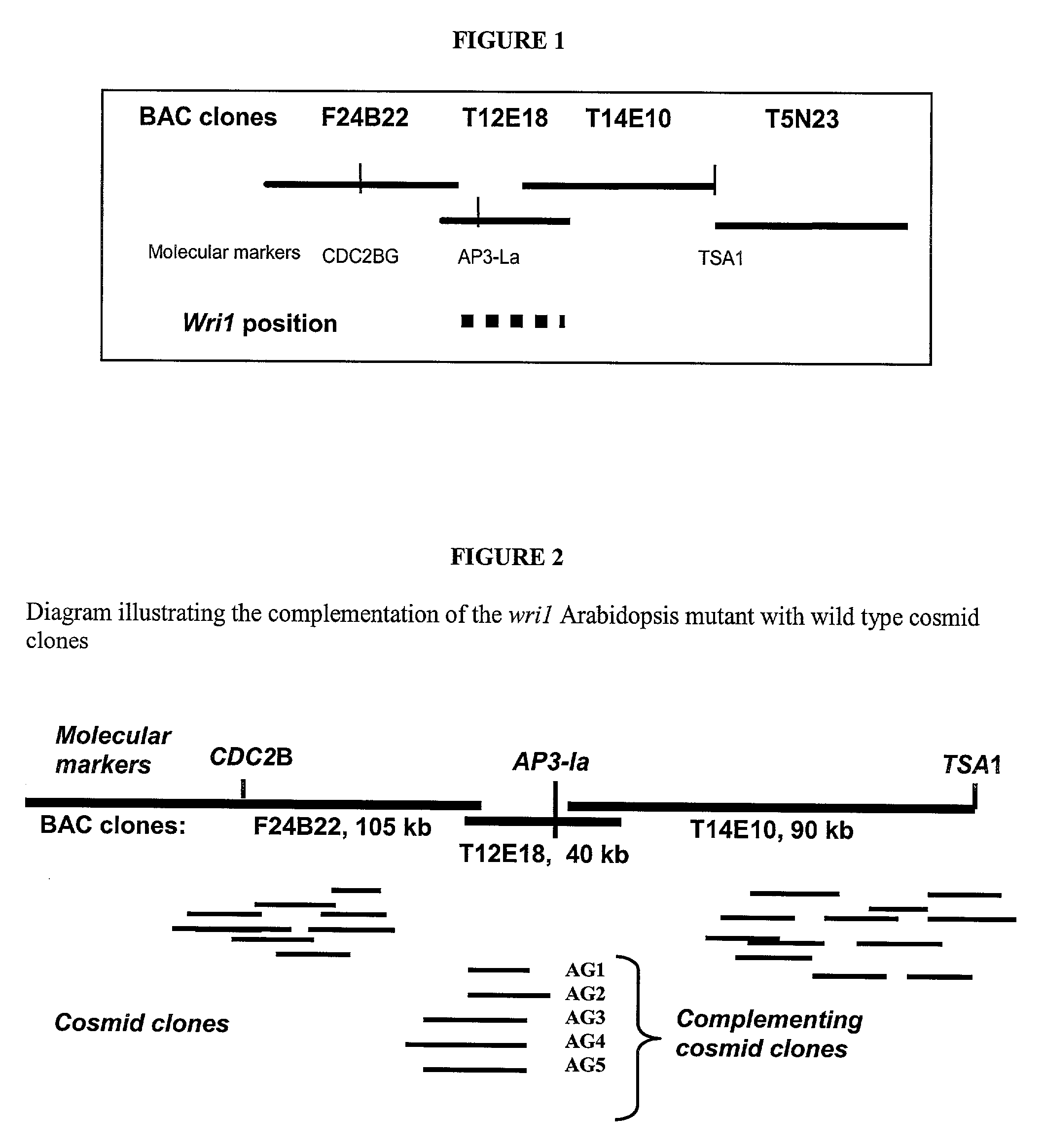
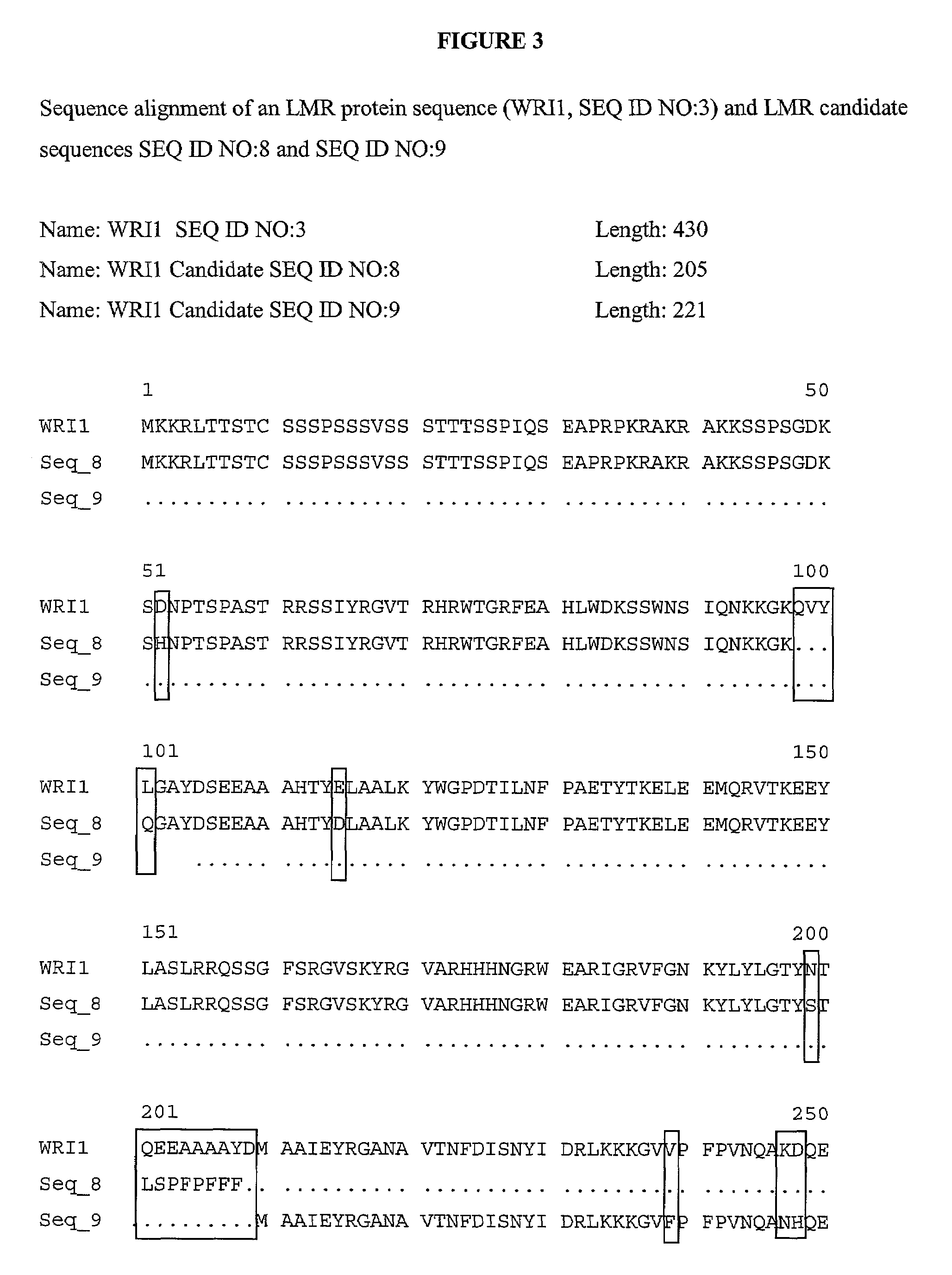
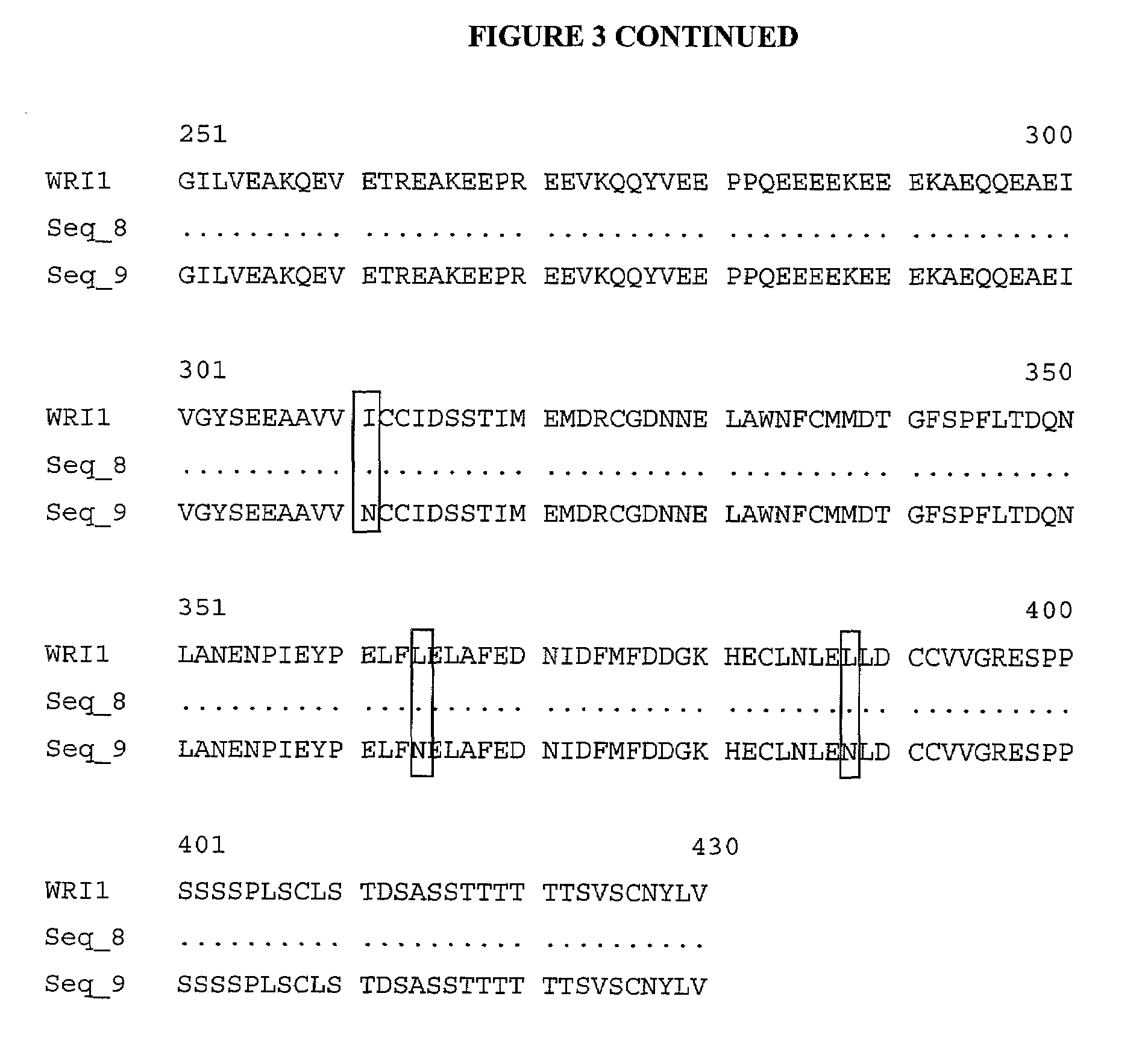
Lipid metabolism regulators in plants
The present invention is directed to novel nucleic acid and amino acid sequences associated with the metabolism of seed storage compounds in plants. A novel discovery described herein lies in the identification of the nucleic acid sequences that encode the wri1 genetic locus in Arabidopsis thaliana, and lipid metabolism regulator (LMR) polynucleotide sequences contained therein. Preferably, the seed storage compounds are lipids, fatty acids, starches or seed storage proteins.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Omega-3 fatty acids and dyslipidemic agent for lipid therapy
InactiveUS20060211762A1Effective treatmentBiocideMetabolism disorderDyslipidemiaCoronary artery disease
A method and composition for blood lipid therapy by administering to the subject an effective amount of a dyslipidemic agent and omega-3 fatty acids. The method utilizes a single administration or a unit dosage of a combination of dyslipidemic agent and omega-3 fatty acids for the treatment of patients with hypertriglyceridemia, hypercholesterolemia, mixed dyslipidemia, coronary heart disease (CHD), vascular disease, artherosclerotic disease and related conditions, and the prevention or reduction of cardiovascular and vascular events.
Owner:RELIANT PHARMACEUTICALS INC +1
Statin and omega-3 fatty acids for lipid therapy
InactiveUS20070191467A1Lower triglyceride levelsBiocideMetabolism disorderLipid formationLow-density lipoprotein
A method of lipid therapy, comprising providing a subject group having a baseline triglyceride level of 200 to 499 mg / dl and being at or near its low-density lipoprotein cholesterol (LDL-C) level goal, and reducing the triglyceride level and the non-high-density lipoprotein cholesterol (non-HDL-C) level of the subject group as compared to treatment with a 3-hydroxy-3-methyl glutaryl coenzyme A (HMG CoA) inhibitor alone, by administering to the subject group an effective amount of an HMG CoA inhibitor and a composition comprising omega-3 fatty acids.
Owner:RELIANT PHARMACEUTICALS INC +1
Method for supplementing the diet
InactiveUS6579544B1Increased susceptibilityPrevent diseaseHeavy metal active ingredientsBiocideDietary supplementAlpha-Lipoic Acid
A dietary supplement blend composition is disclosed, the basic formulation of the composition containing vitamins, minerals, and carotenoids. The composition can also contain bioflavonoids, cartilage protectors such as glucosamine or chondroitin, alpha-lipoic acid, coenzyme Q10, and a source of omega-3 fatty acids such as flax seed oil. The composition is beneficial for improving health and preventing disease, particularly for degenerative conditions. A method for supplementing the diet is also disclosed, wherein the quantity of daily rations of the dietary supplement blend composition is determined based on the person's age, body weight, and quality of diet.
Owner:NUTRIEX
Barrier layer with underlying medical device and one or more reinforcing support structures
A barrier layer device is formed of an underlying biocompatible structure having a barrier layer coating that can exhibit anti-inflammatory properties, non-inflammatory properties, and / or adhesion-limiting properties, as well as generate a modulated healing effect on injured tissue. As implemented herein, the barrier layer is a non-polymeric cross-linked gel derived at least in part from a fatty acid compound, and may include a therapeutic agent. The underlying structure can be in the form of a surgical mesh. The barrier device is further provided with anchoring reinforcements to aid with the fastening of the barrier device for implantation purposes and reinforcing truss sections or portions that prohibit or substantially reduce the occurrence of excessive stretching and tearing. The barrier device is implantable in a patient for short term or long term applications, and can include controlled release of the therapeutic agent.
Owner:ATRIUM MEDICAL
Treatment with Statin and Omega-3 Fatty Acids and a Combination Product Thereof
A pharmaceutical composition in unit dose form, comprising an essentially homogeneous solution comprising a statin essentially dissolved in solvent system comprising natural or synthetic omega-3 fatty acids or pharmaceutically acceptable esters, derivatives, conjugates, precursors or salts thereof, or mixtures thereof, wherein less than 10% of the statin is undissolved in the solvent system.
Owner:RELIANT PHARMACEUTICALS INC
Compositions for treatment of diabetic complications
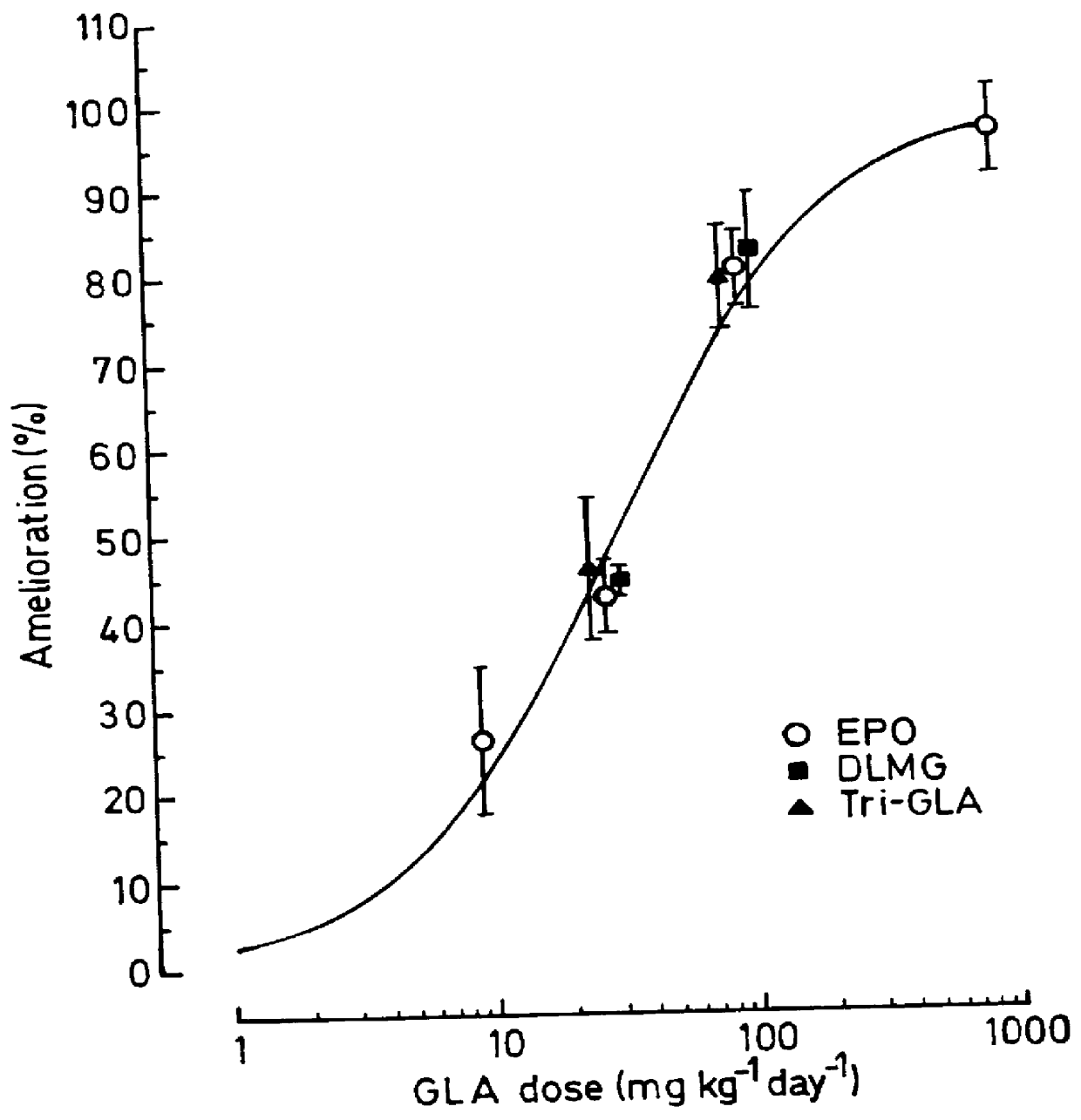
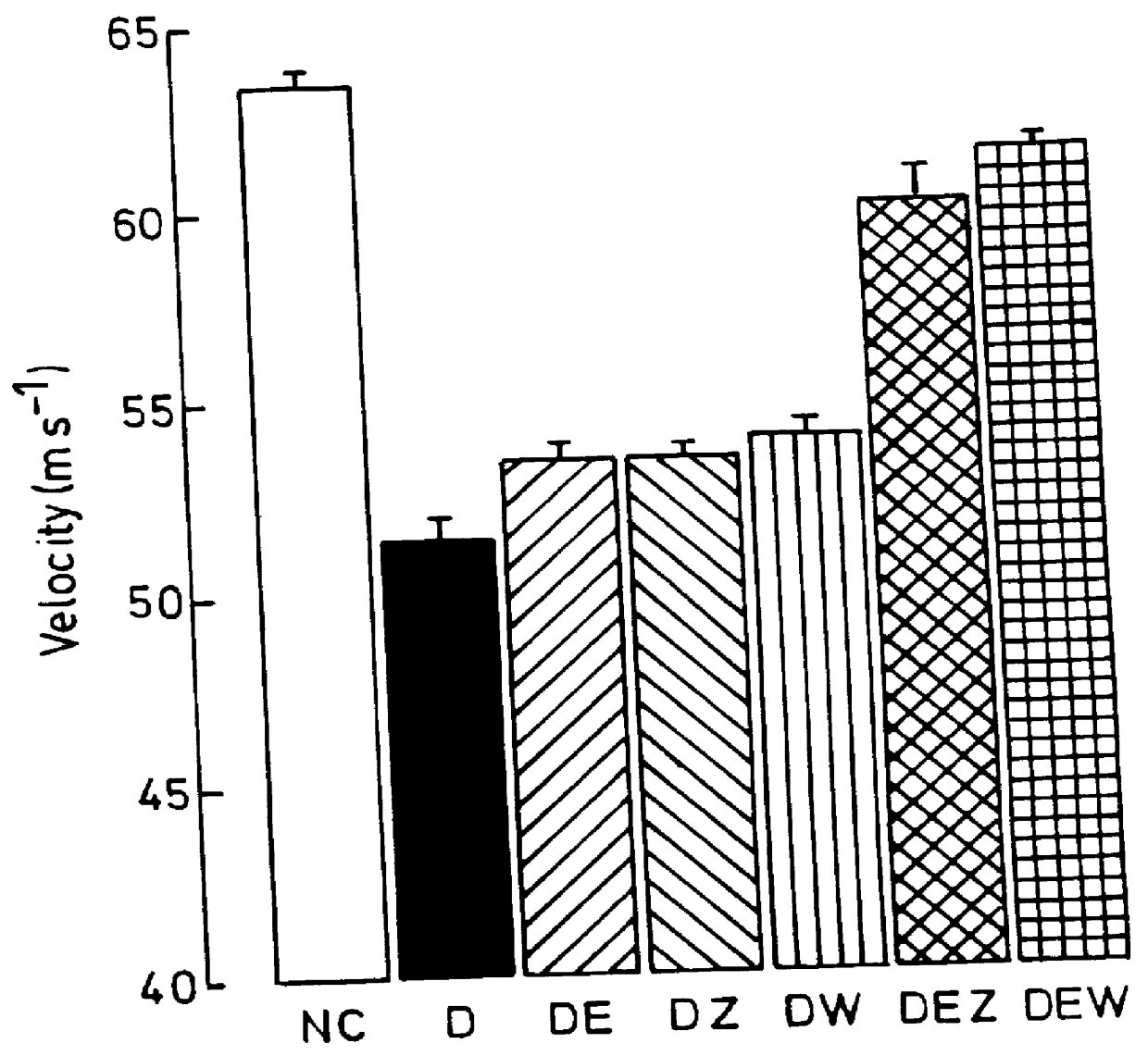
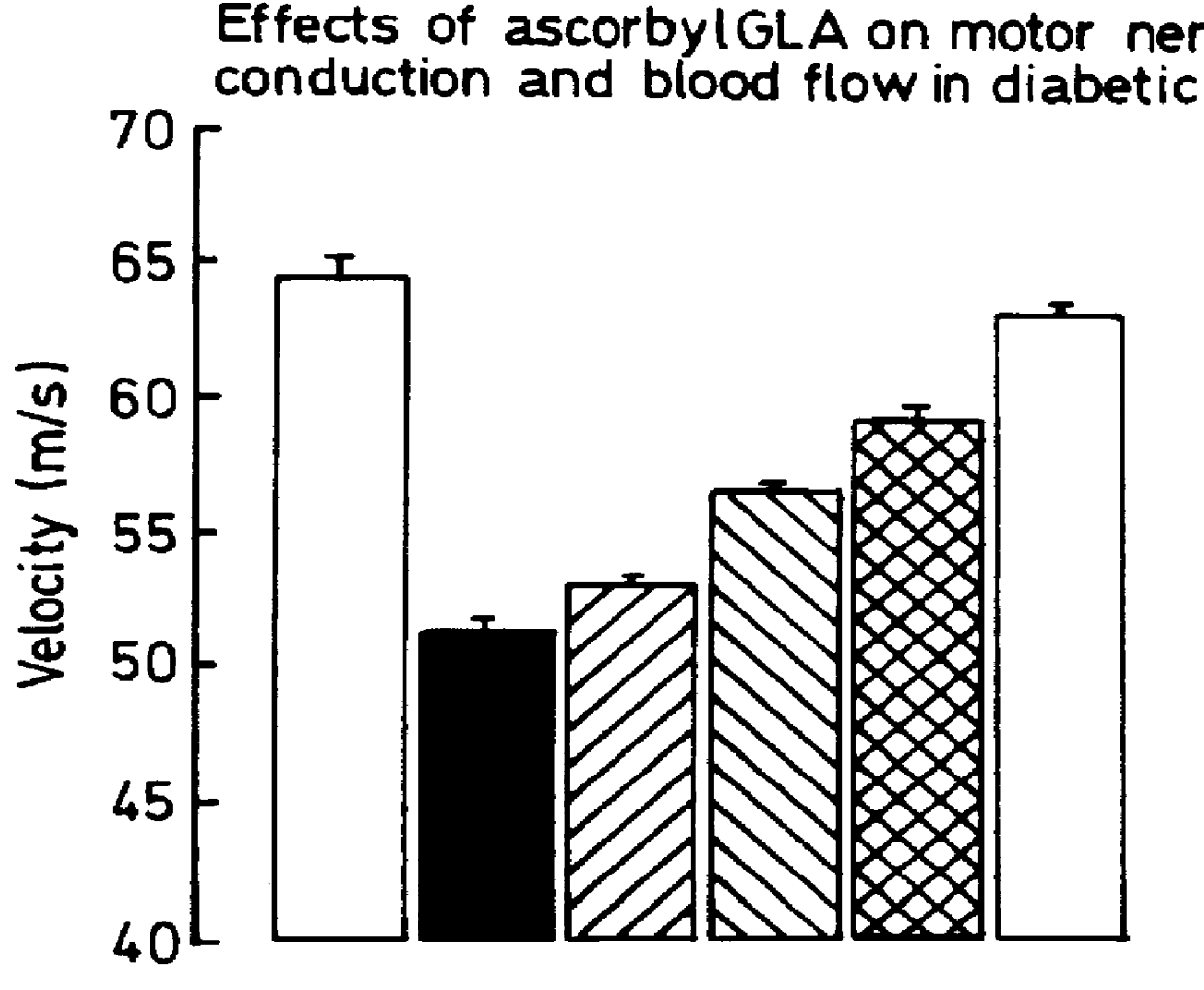
Use of 6-desaturated n-6 fatty acids, especially gammalinolenic acid (GLA), dihomogammalinolenic acid (DGLA) or arachidonic acid (AA), together with a pharmaceutically acceptable material reducing intracellular levels of sorbitol in the body, particularly an aldose reductase inhibitor, in the treatment of (including prophylactic treatment), and in the preparation of medicaments for the treatment of (including prophylactic treatment), the long-term complications of diabetes mellitus. Pharmaceutical compositions of said materials. The ascorbate esters of 6-desaturated n-6 fatty acids (other than GLA or DGLA) per se.
Owner:SCOTIA HLDG
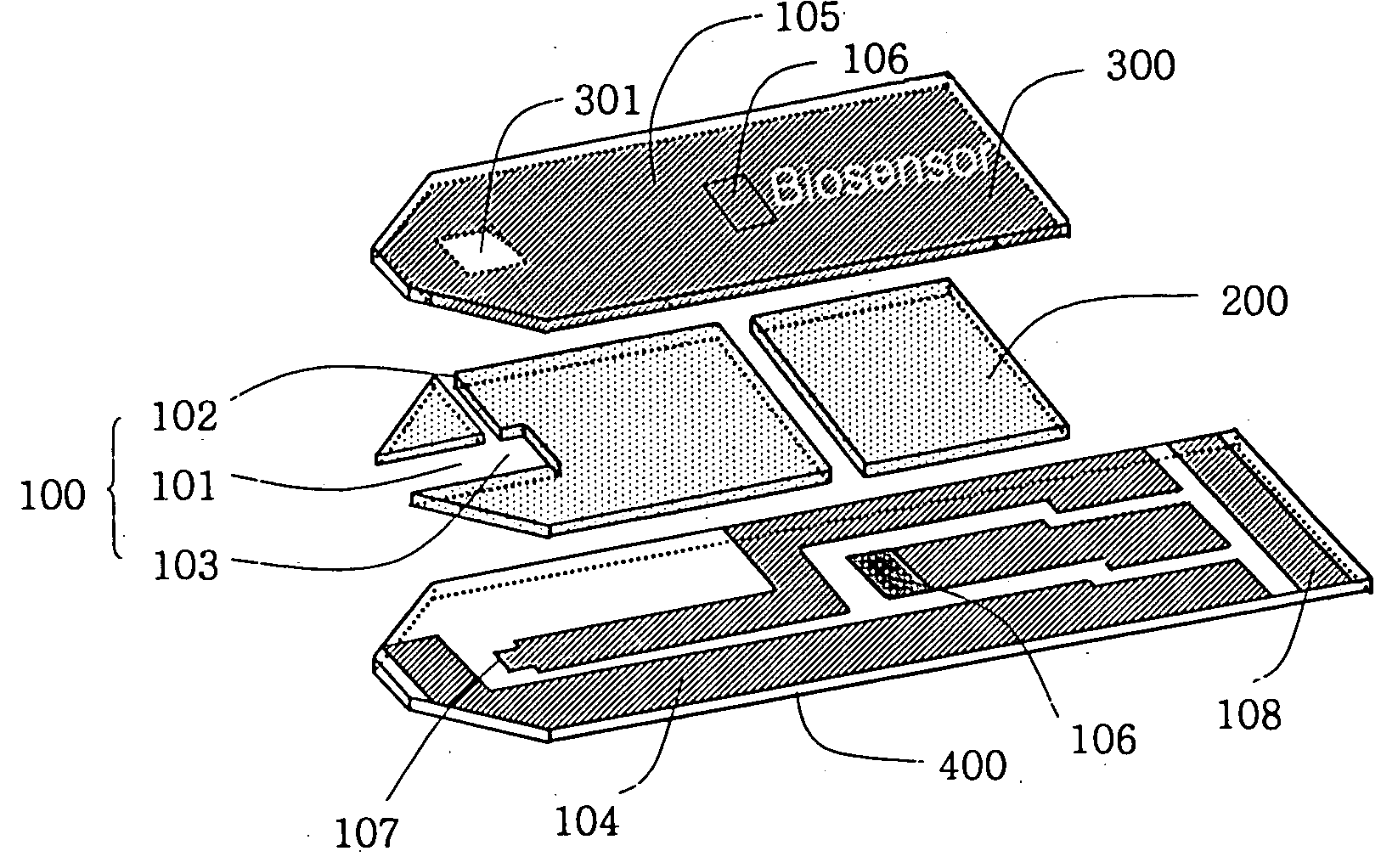
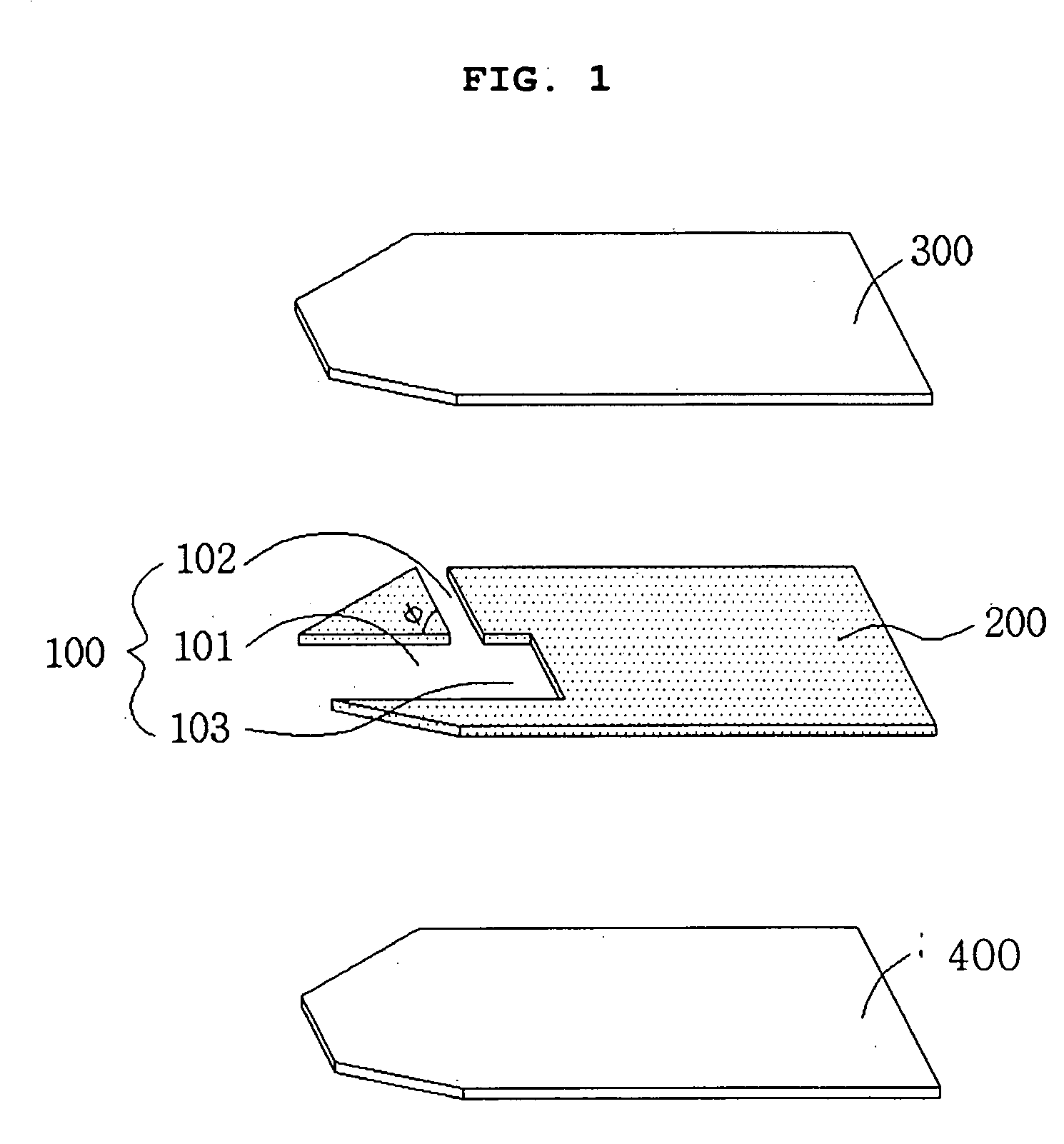
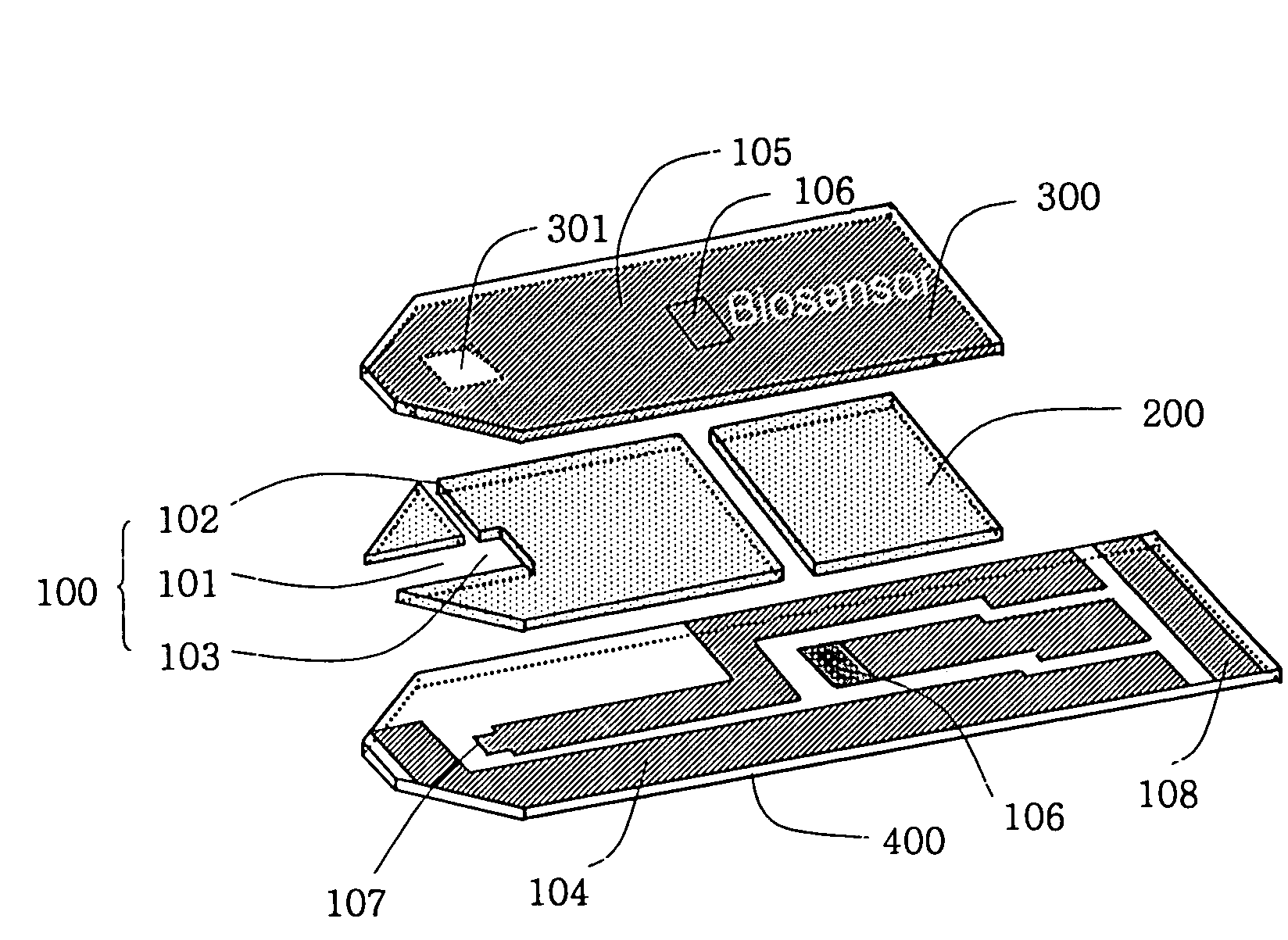
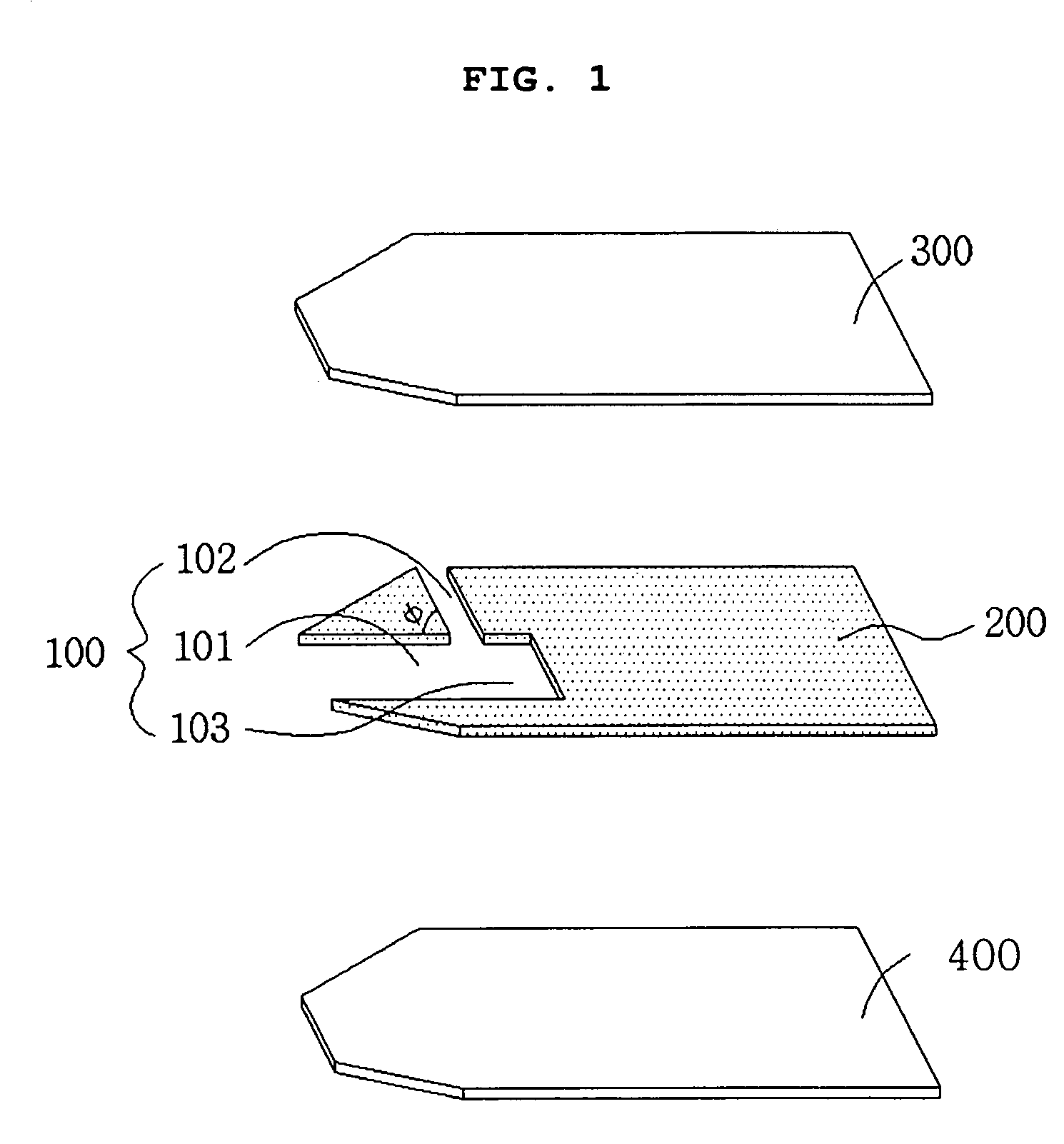
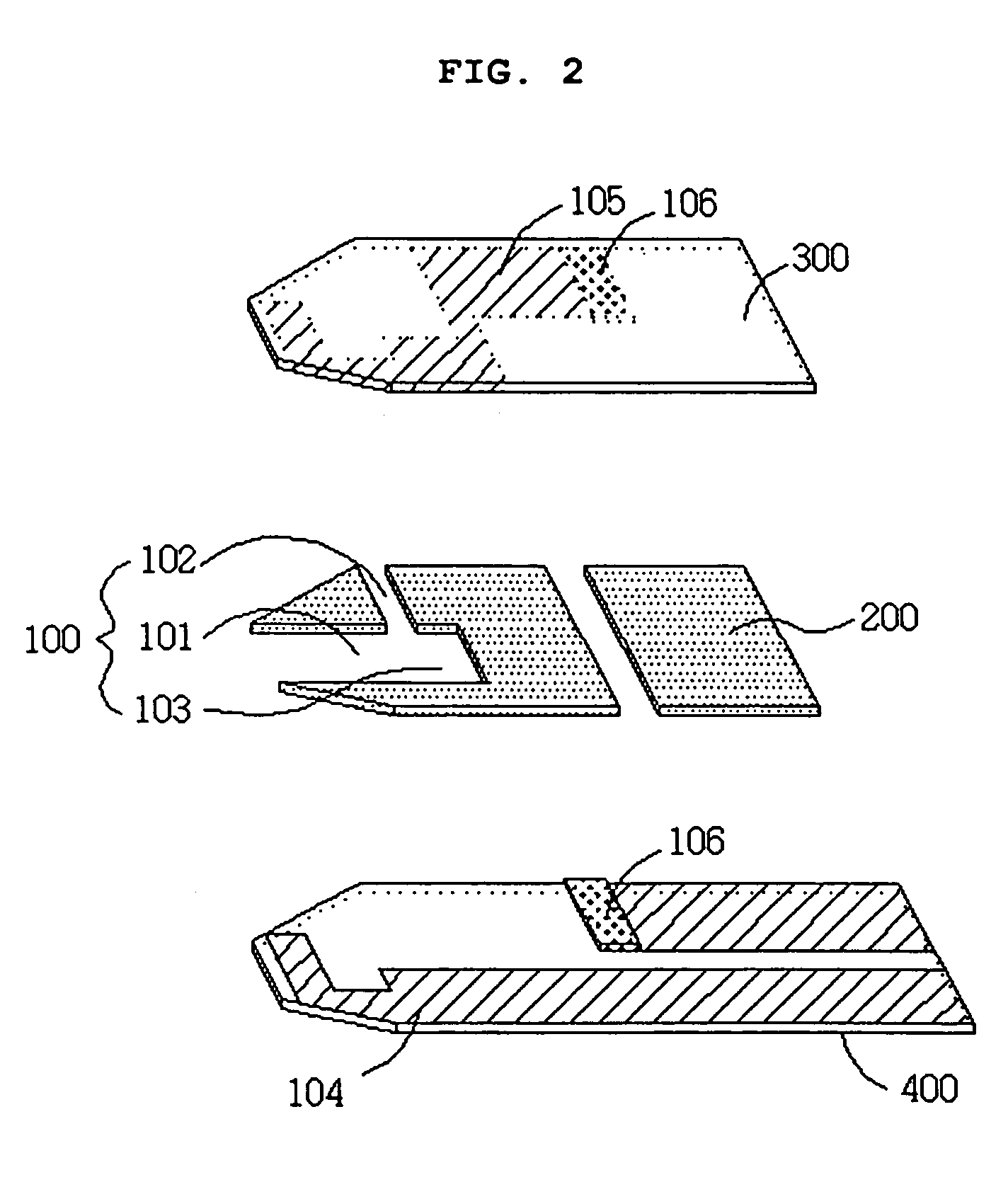
Electrochemical biosensor
ActiveUS20050000808A1Reduced hematocrit level-dependent biasImprove manufacturabilityImmobilised enzymesBioreactor/fermenter combinationsElectron transfer mediatorElectrochemical biosensor
There is provided the reagent layer composition that can substantially reduce the measurement bias arising from hematocrits. The addition of fatty acid (4-20 carbons) and quaternary ammonium salt to a commonly used reagent layer composition composed of an enzyme, an electron transfer mediator, and several water soluble polymers not only reduce the hematocrit level-dependent bias but also provide very stable performance for an extended period of time. Disclosed are also various types of sub microliter sample volume electrochemical biosensors that are suitable to use with the reagent layer composition of present invention.
Owner:I SENS INC
Process for the manufacture of pharmaceutical composition with modified release of active principle comprising the matrix
The invention relates to a process for the manufacture of a pharmaceutical composition with modified release of active principle comprising at least one active principle, a lipid matrix agent composed of ester of alcohol with at least one fatty acid and at least one adjuvant.This process is characterized in that:a powder composed of at least one component selected in the group comprising the active principle and the adjuvant, is mixed, while heating and fluidizing, in order to obtain individual grains;the said lipid matrix agent is liquefied separately under warm conditions;the said powder is then coated under warm conditions by spraying the said lipid matrix agent over the individual grains;finally, the temperature of the combined product is lowered in order to allow the lipid matrix agent to solidify.
Owner:GATTEFOSSE HLDG
Infant formulas for early brain development
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis, at least about 5 mg / L of gangliosides, at least about 150 mg / L of phospholipids, at least about 70 mg / L of total sialic acid with at least about 2.5% as lipid-bound sialic acid, at least about 0.13% docosahexaenoic acid by weight of total fatty acids, and at least about 0.25% arachidonic acid by weight of total fatty acids. Also disclosed are methods of accelerating brain development, neural migration, and cognitive development in an infant by administering the infant formulas during the first 2-4 months of life, preferably as a sole source of nutrition.
Owner:ABBOTT LAB INC
Process for the manufacture of diesel range hydro-carbons
ActiveUS20070006523A1Improve low temperature performanceBig ratioBiofuelsLiquid carbonaceous fuelsChemical industryAlkane
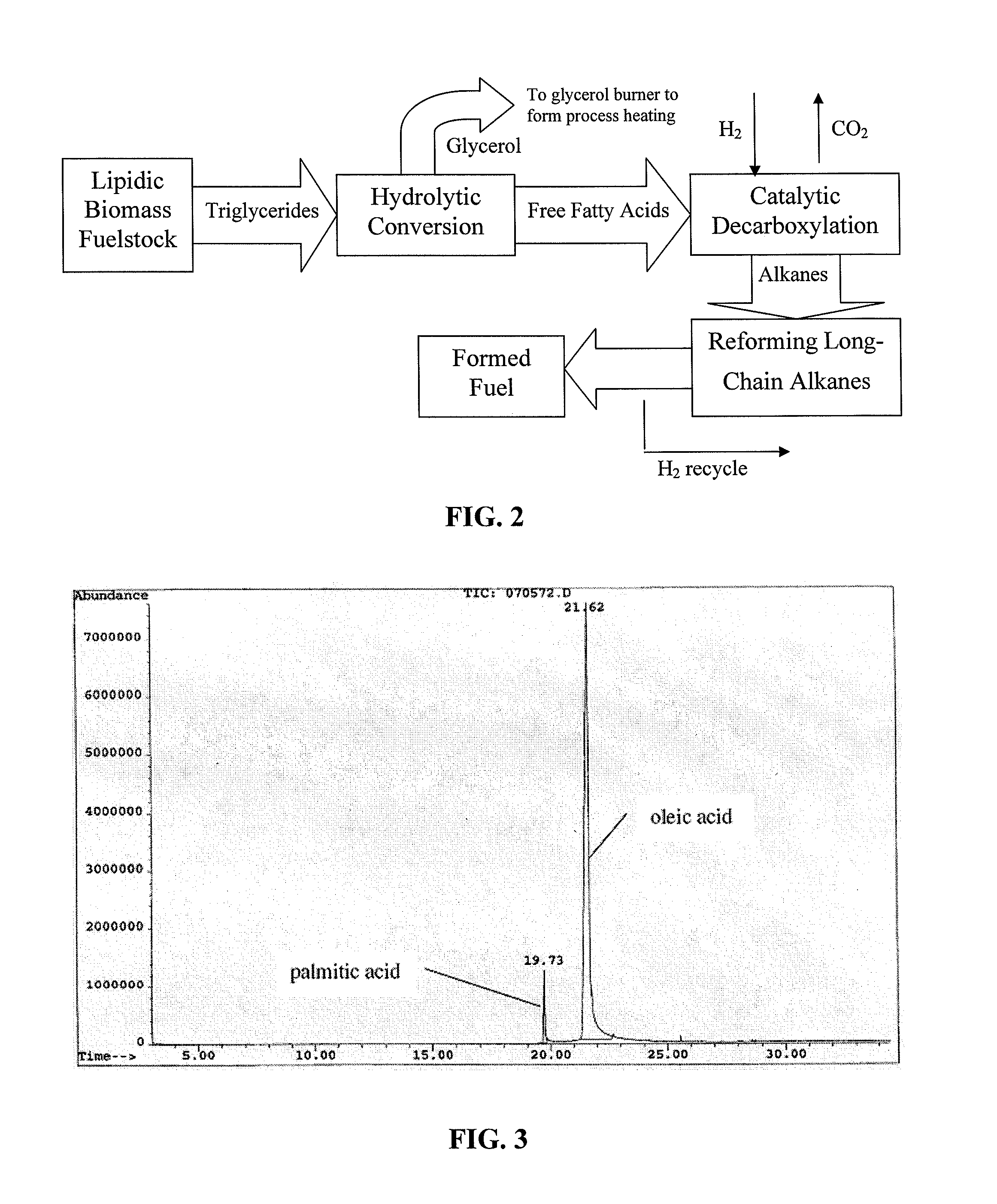
The invention relates to chemical industry and is directed to the production of middle distillate from vegetable oils. In the first step of the production method, the fatty acids or triglycerides of said vegetable oils are hydrogenated to give n-paraffins, and in the second step, the n-paraffins are catalytically converted to paraffins with branched chains. Using this process having two steps, a high-quality middle distillate useful as a component of diesel fuels without any particular specifications may be produced.
Owner:NESTE OIL OY
Process for conversion of biomass to fuel
ActiveUS7816570B2Meet growth needsEasy to processFatty acid esterificationRefining to change hydrocarbon structural skeletonAlkaneChain length
Owner:NORTH CAROLINA STATE UNIV
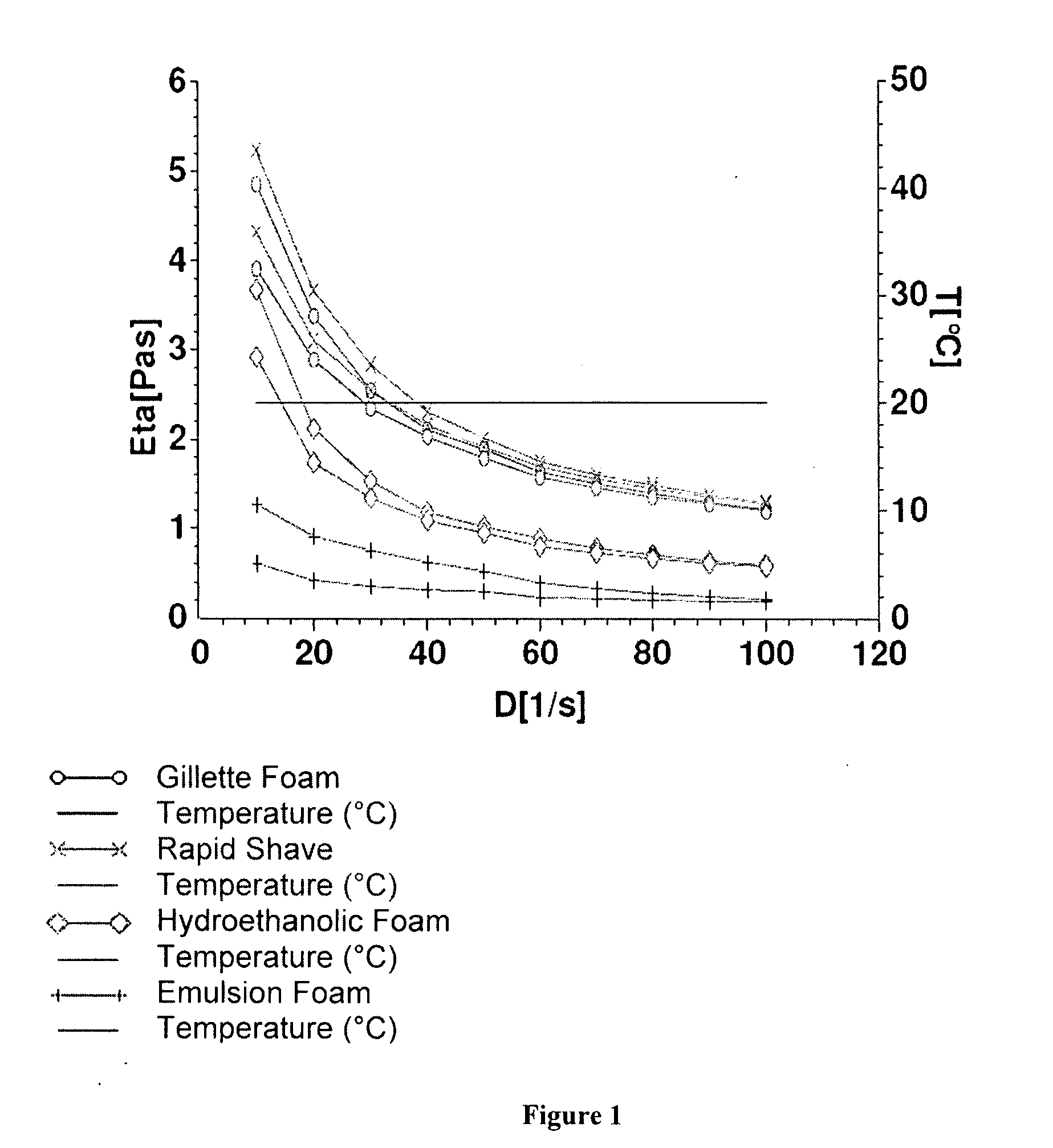
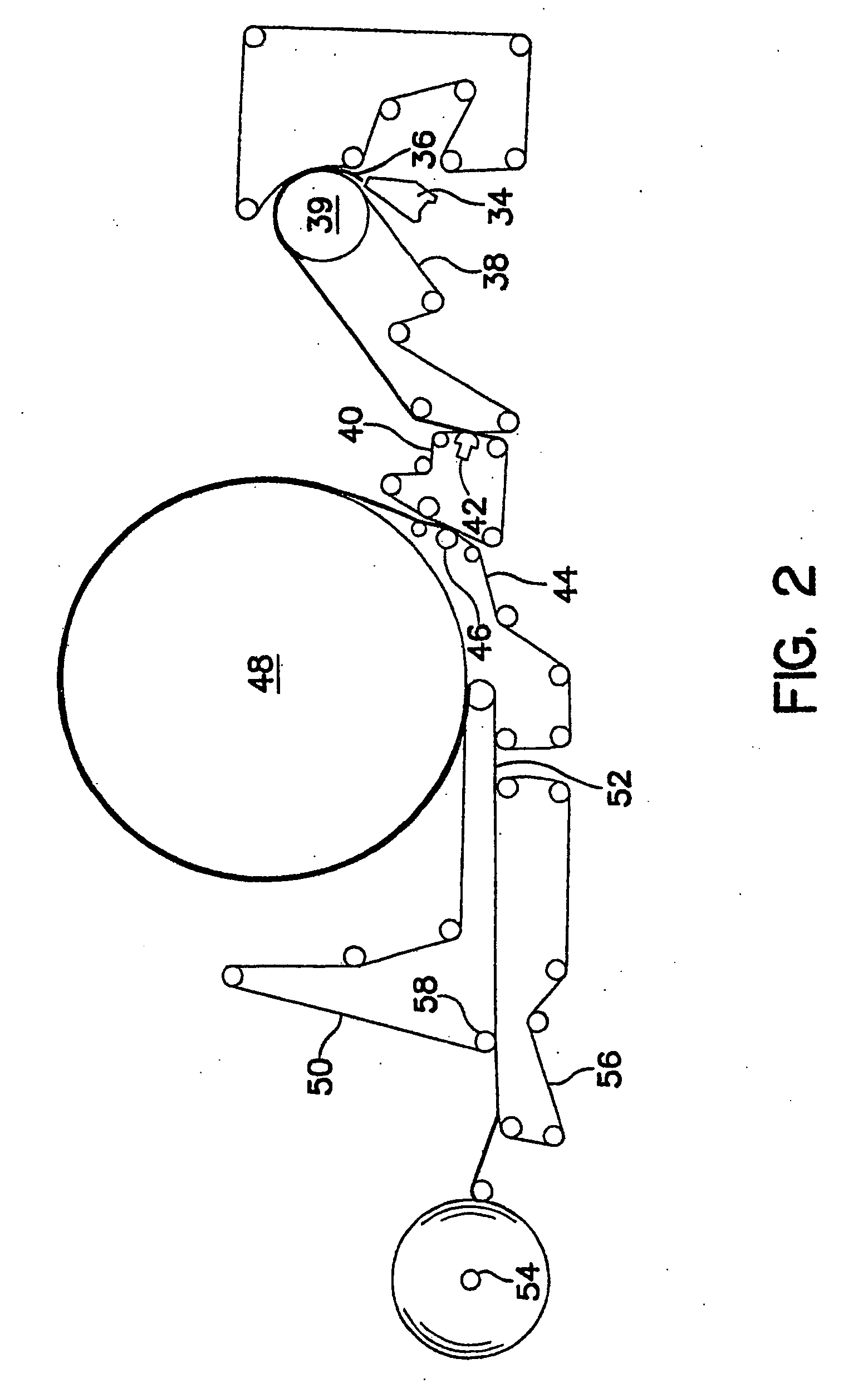
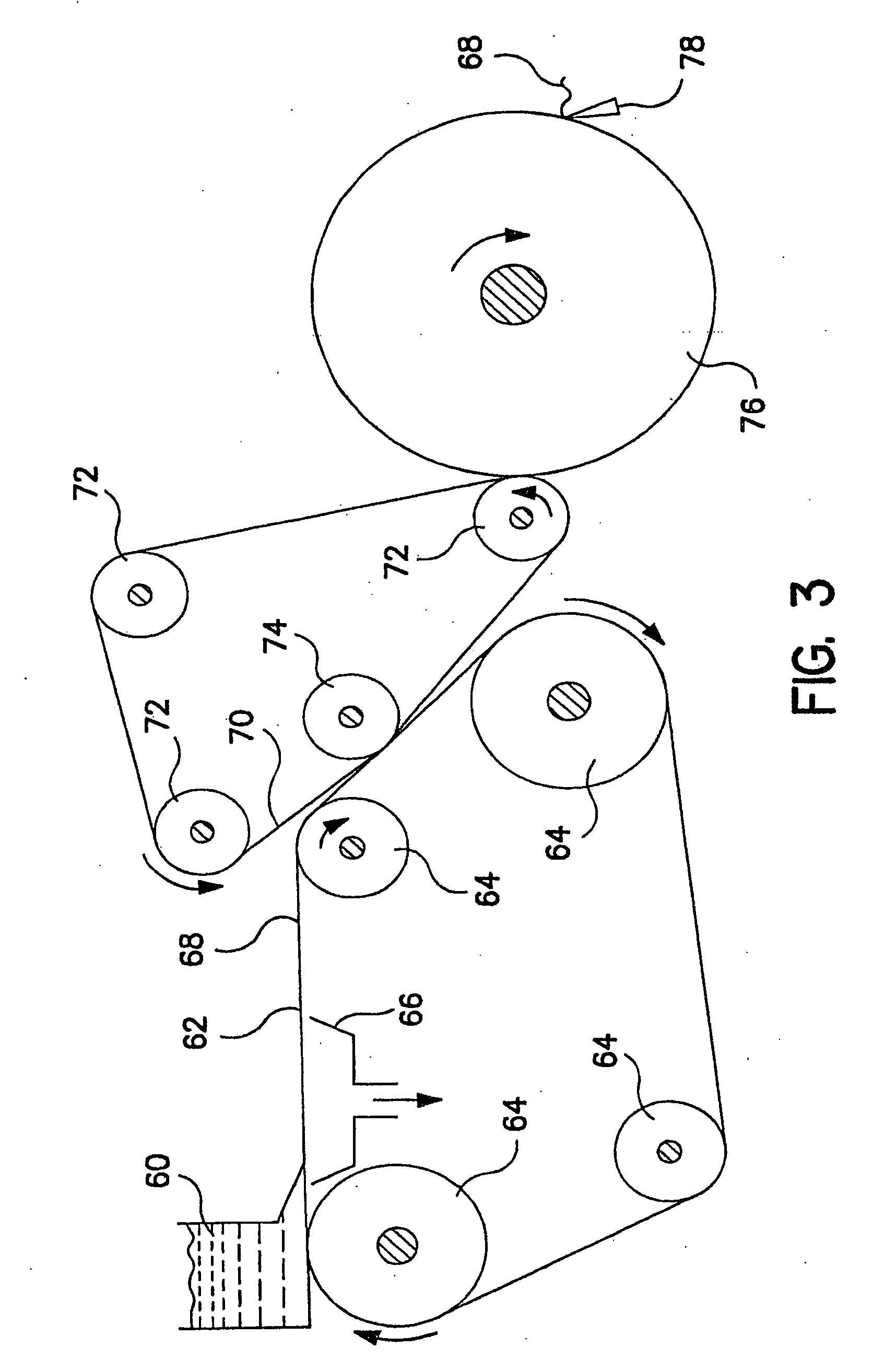
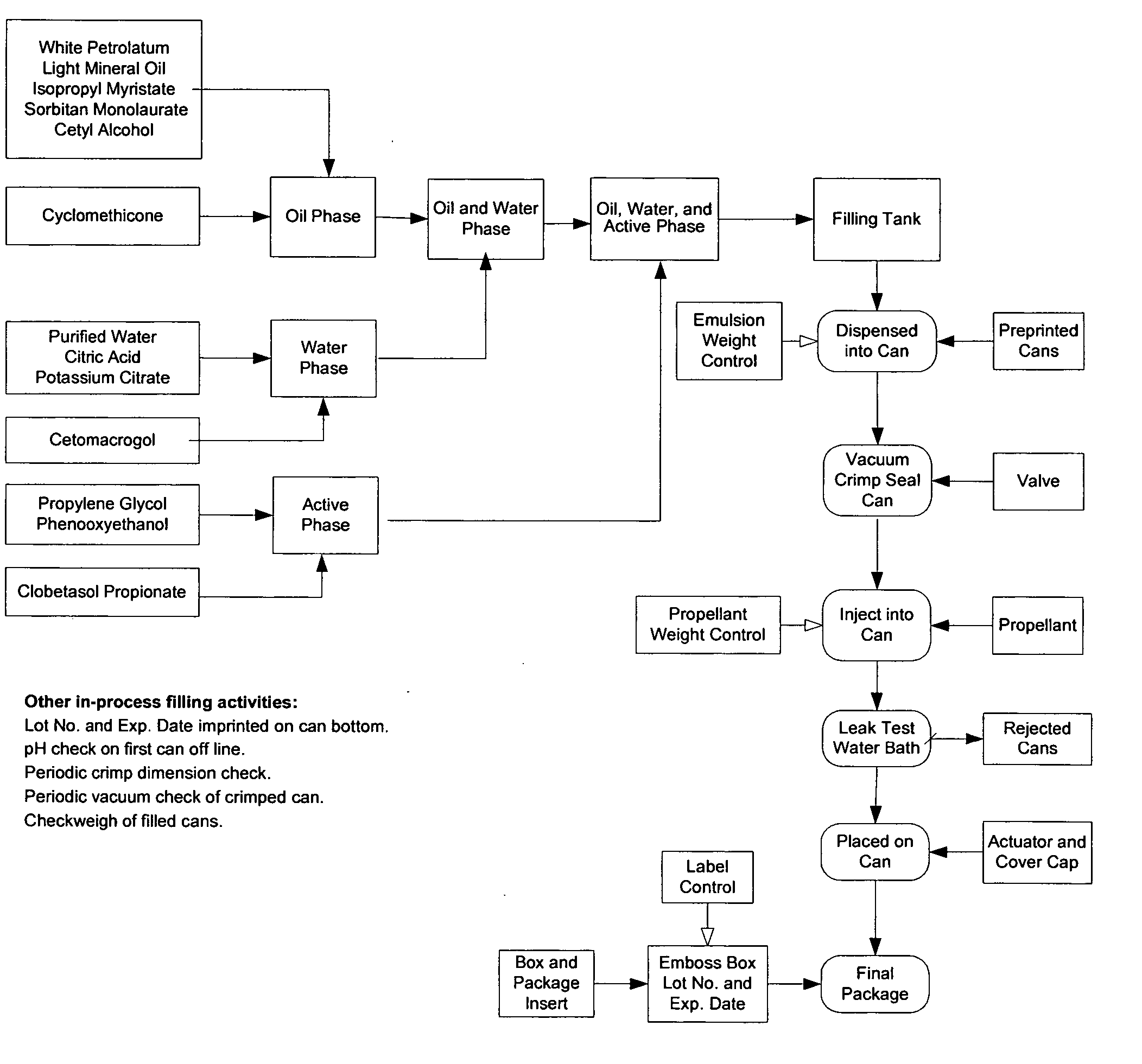
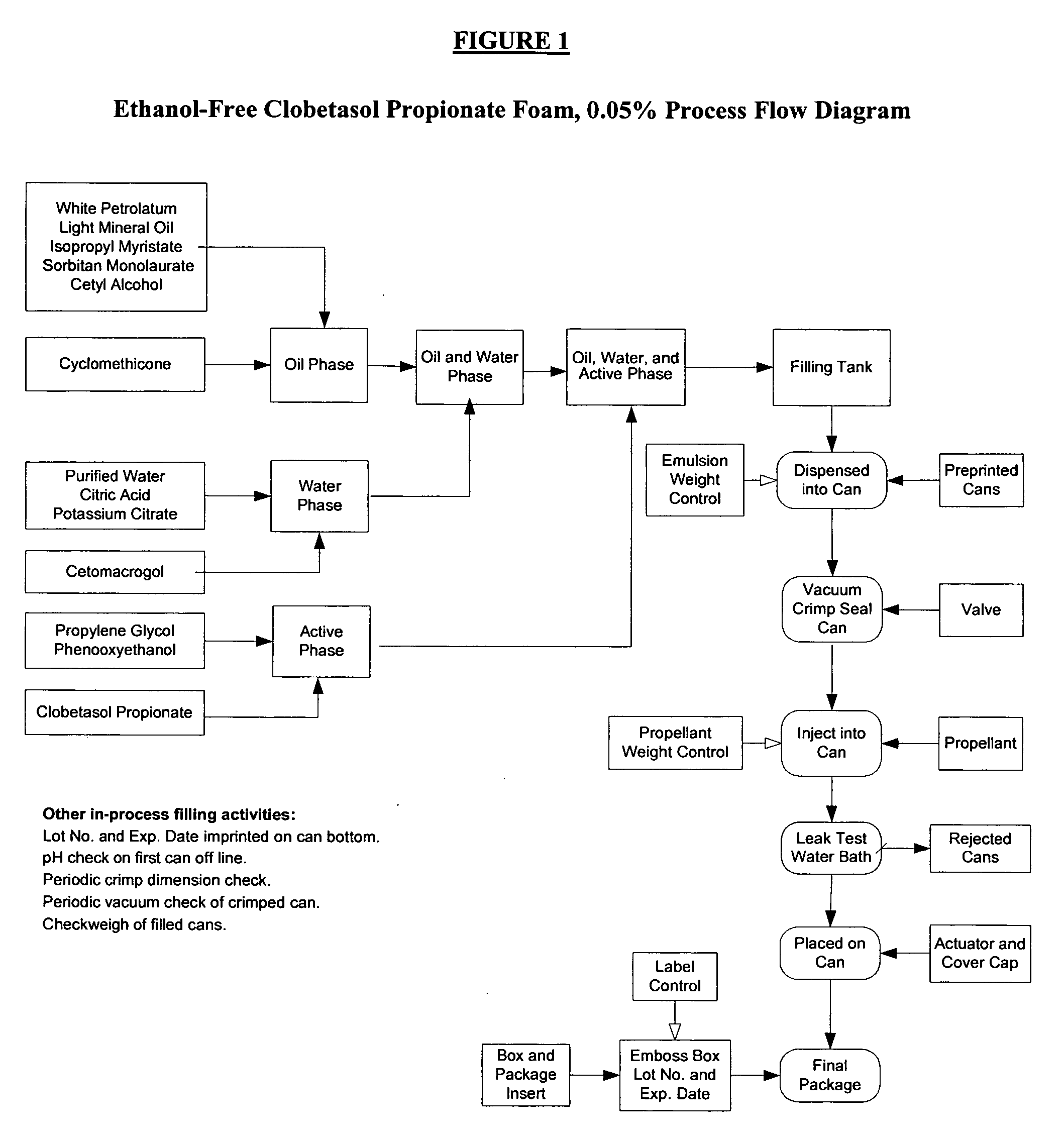
Fatty acid pharmaceutical foam
The present invention provides a foamable composition comprising water and an organic solvent, wherein the organic solvent comprises a fatty acid. The composition may further comprise a pharmaceutically active agent. The composition of the invention is also useful for the treatment of a dermatological disorder in a mammal by the topical administration of the composition.
Owner:STIEFEL RESEARCH AUSTRALIA PTY LTD
Omega-3 Fatty Acids and Dyslipidemic Agent for Lipid Therapy
InactiveUS20090012167A1Effective treatmentBiocideAnimal repellantsDyslipidemiaCoronary artery disease
Owner:RELIANT PHARMACEUTICALS INC +1
Analyte test system for determining the concentration of an analyte in a physiological or aqueous fluid
InactiveUS20050196747A1Cheap productionReliable resultsBioreactor/fermenter combinationsBiological substance pretreatmentsSmall sampleQuality control system
This invention provides a device for determining the concentration of an analyte like glucose, cholesterol, free fatty acids, triglycerides, proteins, ketones, phenylalanine or enzymes, in a physiological or aqueous fluid like blood, serum, plasma, saliva, urine, interstitial and / or intra-cellular fluid, the device having an integrated calibration and quality control system suitable for dry reagent test strips with a very small sample volume of about 0.5 μL based on to a new sample distribution system. The production of the inventive analyte test element involves only a small number of uncomplicated production steps enabling an inexpensive production of the strips.
Owner:EGOMEDICAL SWISS
Process for producing bio-derived fuel with alkyl ester and iso-paraffin components
InactiveUS20080163543A1Improve low temperature performanceImprove the lubrication effectBiofuelsLiquid carbonaceous fuelsAlkaneParaffin oils
A process for producing a diesel fuel of biological origin. The process includes a biological component to be trans-esterified into a fatty acid alkyl ester. A fraction of the fatty acid alkyl ester is hydrodeoxygenated and hydroisomerized to produce an iso-paraffinic hydrocarbon. The fatty acid alkyl ester and the iso-paraffin components are combined into a middle distillate product suitable for direct use as diesel or jet fuel.
Owner:REG SYNTHETIC FUELS LLC
Electrochemical biosensor
ActiveUS7288174B2Reduced hematocrit level-dependent biasImprove manufacturabilityImmobilised enzymesBioreactor/fermenter combinationsElectron transfer mediatorElectrochemical biosensor
There is provided the reagent layer composition that can substantially reduce the measurement bias arising from hematocrits. The addition of fatty acid (4-20 carbons) and quaternary ammonium salt to a commonly used reagent layer composition composed of an enzyme, an electron transfer mediator, and several water soluble polymers not only reduce the hematocrit level-dependent bias but also provide very stable performance for an extended period of time. Disclosed are also various types of sub microliter sample volume electrochemical biosensors that are suitable to use with the reagent layer composition of present invention.
Owner:I SENS INC
Composition Containing Statins and Omega-3 Fatty Acids
InactiveUS20080089876A1Hydroxy compound active ingredientsPeptide/protein ingredientsFatty acidStatine
A combination is described comprising at least one omega-3 fatty acid, optionally esterified or salified, at least one statin, Coenzyme Q10, resveratrol, at least one policosanol, pantethine, selenium, and zinc. This combination is endowed with a synergistic effect and is useful in the treatment of disease forms due to insulin resistance and in cardiovascular diseases.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Treatment and prevention of major adverse cardiovascular events or major coronary evens by administering Omega-3 fatty acids
InactiveUS20080306154A1Effective treatmentReduce generationBiocideMetabolism disorderDyslipidemiaCoronary event
Omega-3 fatty acid compositions comprising eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are provided, where the compositions are useful for treating, reducing the occurrence of, or preventing major adverse cardiovascular events or major coronary events in patients who have established cardiovascular disease without prior myocardial infarction, preventing their further progression, and treating underlying risk factors for CVD such as hypertension, dyslipidemia, obesity and / or diabetes.
Owner:PRONOVA BIOCARE AS
Embossed tissue products
InactiveUS20070137813A1Preserve strengthHigh strengthNon-fibrous pulp additionNatural cellulose pulp/paperFiberPolymer science
Tissue products are disclosed containing an additive composition. The additive composition, for instance, comprises an aqueous dispersion containing an olefin polymer, an ethylene-carboxylic acid copolymer, or mixtures thereof. The olefin polymer may comprise an interpolymer of ethylene and octene, while the ethylene-carboxylic acid copolymer may comprise ethylene-acrylic acid copolymer. The additive composition may also contain a dispersing agent, such as a fatty acid. The additive composition may be incorporated into the tissue web by being combined with the fibers that are used to form the web. Alternatively, the additive composition may be topically applied to the web after the web has been formed. After the additive composition is applied to the web or otherwise incorporated into the tissue web, the tissue web is embossed. During embossing, the additive composition forms well defined embossments in the web that are water resistant. In one embodiment, the additive composition may also be used to bond multiple tissue webs together to form a multiple ply product during the embossing operation.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Microemulsion process and composition
ActiveUS20060057168A1Quality improvementCosmetic preparationsOrganic active ingredientsVegetable oilSilanes
There is provided a process for the preparation of an oil in water (O / W) microemulsion or sub-micron emulsion composition for dermal delivery of at least one pharmaceutically active ingredient, the method including the steps of a) Admixing a first part including at least one of the group consisting of animal, mineral or vegetable oils, silanes, siloxanes, esters, fatty acids, fats, halogen compounds or alkoxylated alcohols; and one or more lipophilic surfactants, and a second part including water and at least one hydrophilic surfactant to achieve homogeneity, b) heating the mix of step a) to a phase assembly temperature in the range of 40-99° C., preferably 45-95° C., more preferably 65-85° C. with continuous mixing to obtain a microemulsion or sub-micron emulsion, c) allowing said microemulsion or sub-micron emulsion to cool, and d) adding a third part to said microemulsion or sub-micron emulsion at a temperature between 2° C. and said phase assembly temperature, said third part if necessary being premixed and heated until the components are dissolved and including at least one component selected from the group consisting of non-surfactant amphiphilic type compound, surfactant and water with the proviso that when the third part includes water it also includes a non-surfactant amphiphilic type compound and / or surfactant. The phase assembly temperature can be determined visually by the achievement of translucence in the composition or by measures such as conductivity which peaks and then is maintained at a plateau whilst phase assembly occurs. It has been found that whilst if a non-surfactant amphiphilic type compound such as the polyol is added together with the second part as would conventionally be the case, a microemulsion or sub-micron emulsion is not formed, by adding the so called third part, phase assembly occurs at a lower temperature than would be expected and moreover, this phase appears to assist in maintaining the microemulsion or sub-micron emulsion characteristics of the formulation during storage at normal temperatures.
Owner:STIEFEL WEST COAST
Fuel Composition
ActiveUS20080229654A1Maximize product yieldYield maximizationFatty acid isomerisationFatty acid oxidationIsomerizationVegetable oil
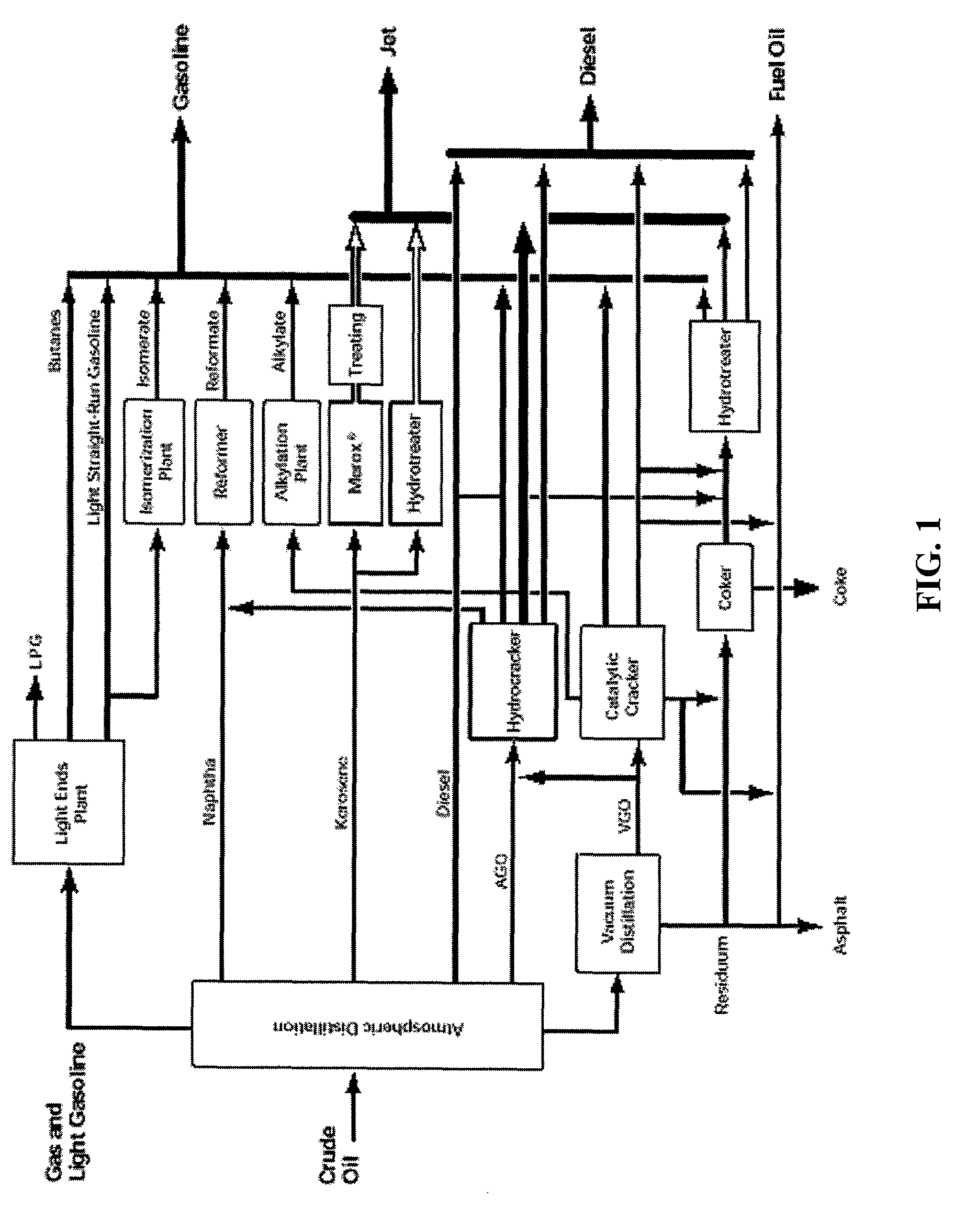
Compositions and methods for forming hydrocarbon products from triglycerides are disclosed. In one aspect, the methods involve the thermal decomposition of fatty acids, which can be derived from the hydrolysis of triglycerides. The thermal decomposition products can be combined with low molecular weight olefins, such as Fischer-Tropsch synthesis products, and subjected to molecular averaging reactions. Alternatively, the products can be subjected to hydrocracking reactions, isomerization reactions, and the like. The products can be isolated in the gasoline, jet and / or diesel fuel ranges. Thus, vegetable oils and / or animal fats can be converted using water, catalysts, and heat, into conventional products in the gasoline, jet and / or diesel fuel ranges. These products are virtually indistinguishable from those derived from their petroleum-based analogs, except that they can have virtually no aromatic, sulfur or nitrogen content, they are derived, in whole or in part, from renewable resources, and can also be derived from domestically available coal and / or natural gas.
Owner:BRADIN DAVID
Statin and omega-3 fatty acids for reduction of apo-b levels
InactiveUS20080085911A1Reduce additionalAvoid loweringBiocideMetabolism disorderDyslipidemiaApolipoproteins E
Methods of utilizing a combined administration or a unit dosage of a combination of an HMG-CoA inhibitor and omega-3 fatty acids for the reduction of apolipoprotein-B levels. The methods are especially useful in the treatment of patients with hypertriglyceridemia or hypercholesterolemia or mixed dyslipidemia, coronary heart disease (CHD), vascular disease, atherosclerotic disease and related conditions, and for the prevention or reduction of cardiovascular, cardiac, and vascular events.
Owner:RELIANT PHARMACEUTICALS INC
Treatment with omega-3 fatty acids and PPAR agonist and/or antagonist and a combination product thereof
InactiveUS20060211749A1Reduced dosages of PPAR agonistEffective treatmentBiocideMetabolism disorderDyslipidemiaFasting glucose
A method and composition for blood lipid therapy that comprises administering to the subject an effective amount of a PPAR agonist and / or antagonist and an omega-3 fatty acid. The methods and compositions include combination products or concomitant therapy for the treatment of subjects with hypertriglyceridemia, hypercholesteremia, mixed dyslipidemia, vascular disease, artherosclerotic disease and related conditions, obesity, the prevention or reduction of cardiovascular and vascular events, the reduction of insulin resistance, fasting glucose levels and postprandial glucose levels, and / or the reduction of incidence and / or the delay of onset of diabetes.
Owner:RELIANT PHARMACEUTICALS INC
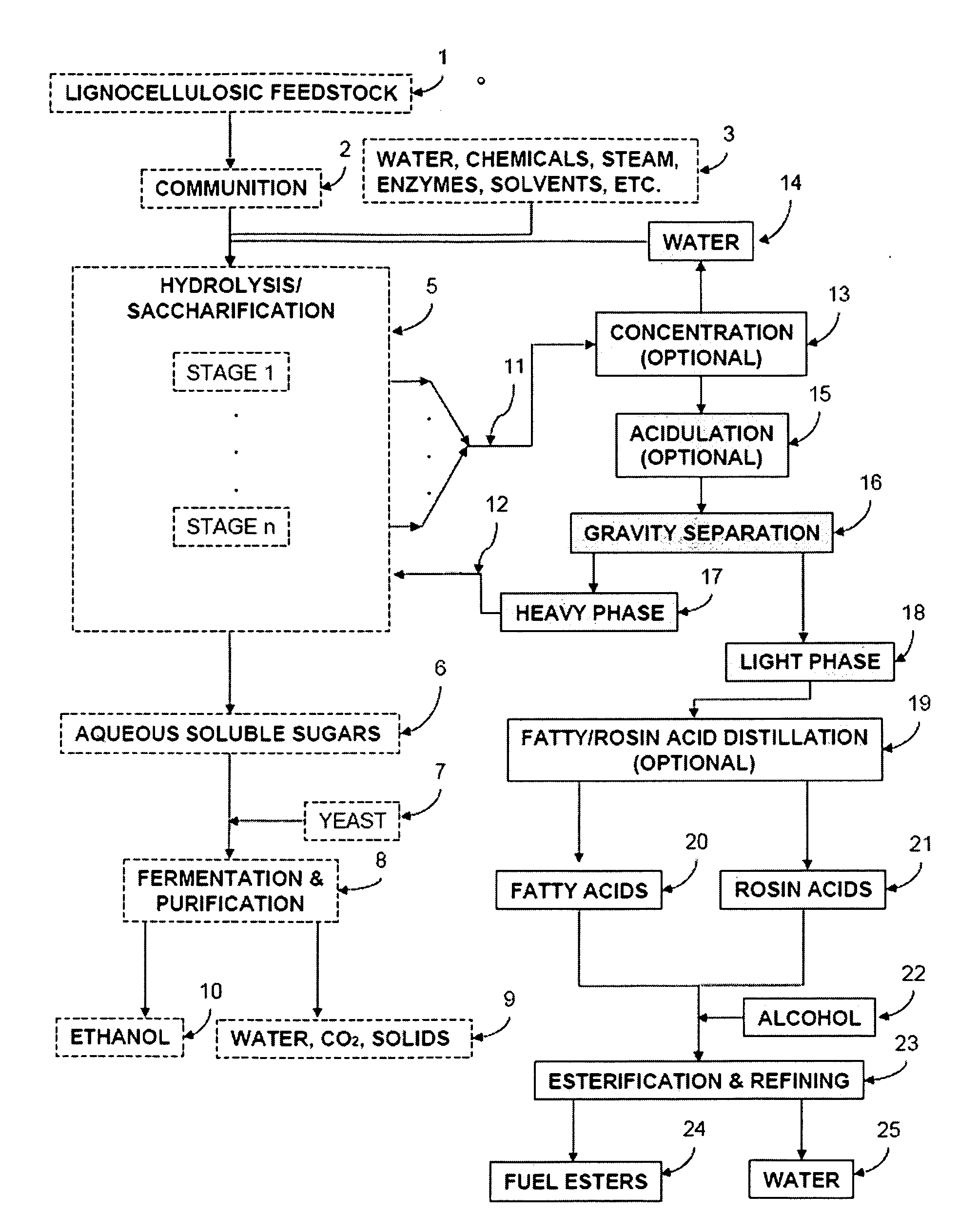
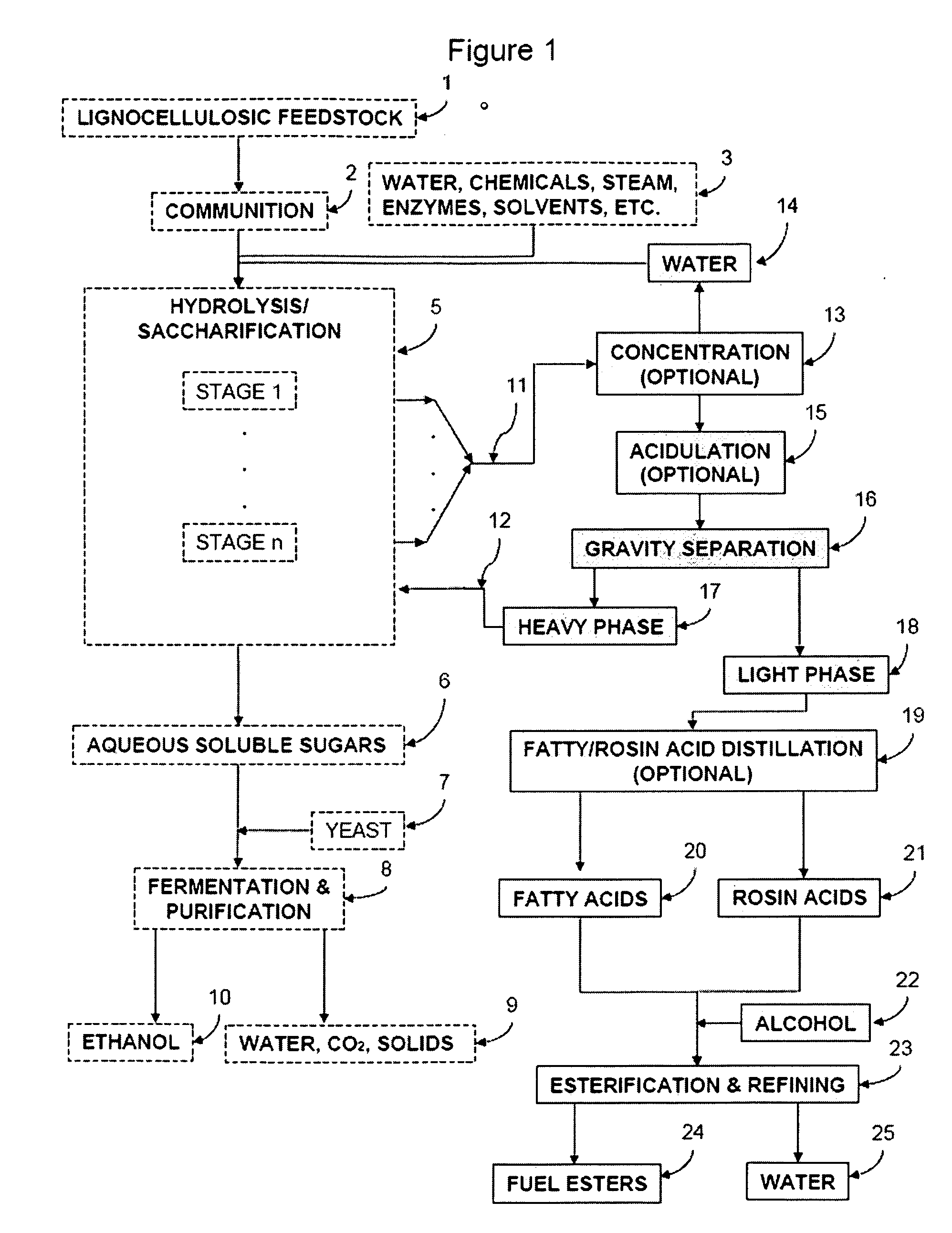
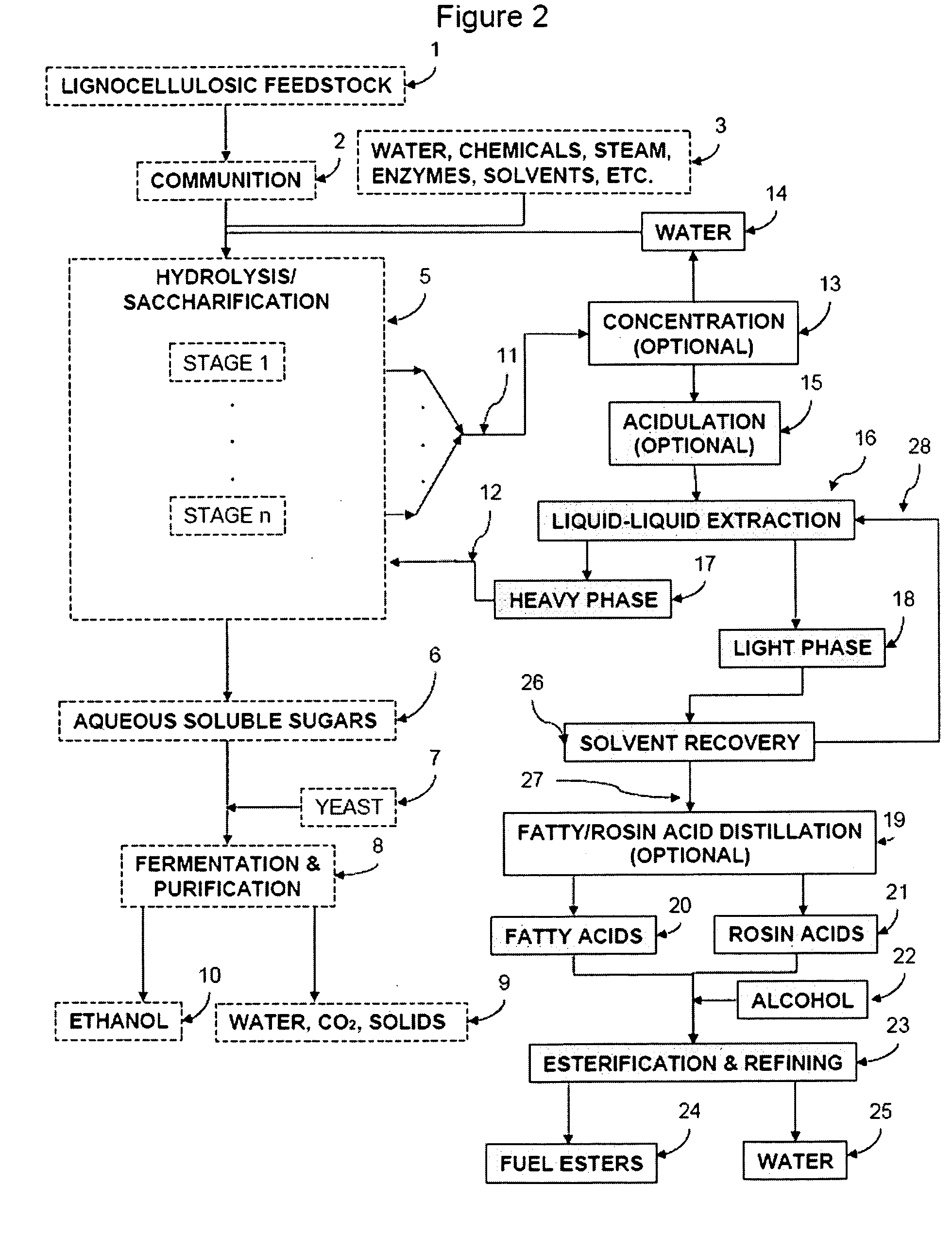
Production of Ester-based Fuels Such As Biodiesel From Renewable Starting Materials
ActiveUS20090056201A1Fatty oils/acids recovery from wasteFatty acid esterificationCelluloseBiodiesel
Production of ester-based fuels such as biodiesel or jet fuel from renewable starting materials such as lignocellulosic material or algae is disclosed. Pulping and saccharification of the renewable starting materials produces carboxylic acids such as fatty acids or rosin acids, which are esterified via a gas sparged, slurry form of heterogeneous reactive distillation to yield ester-based fuels.
Owner:ENDICOTT BIOFUELS II
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com