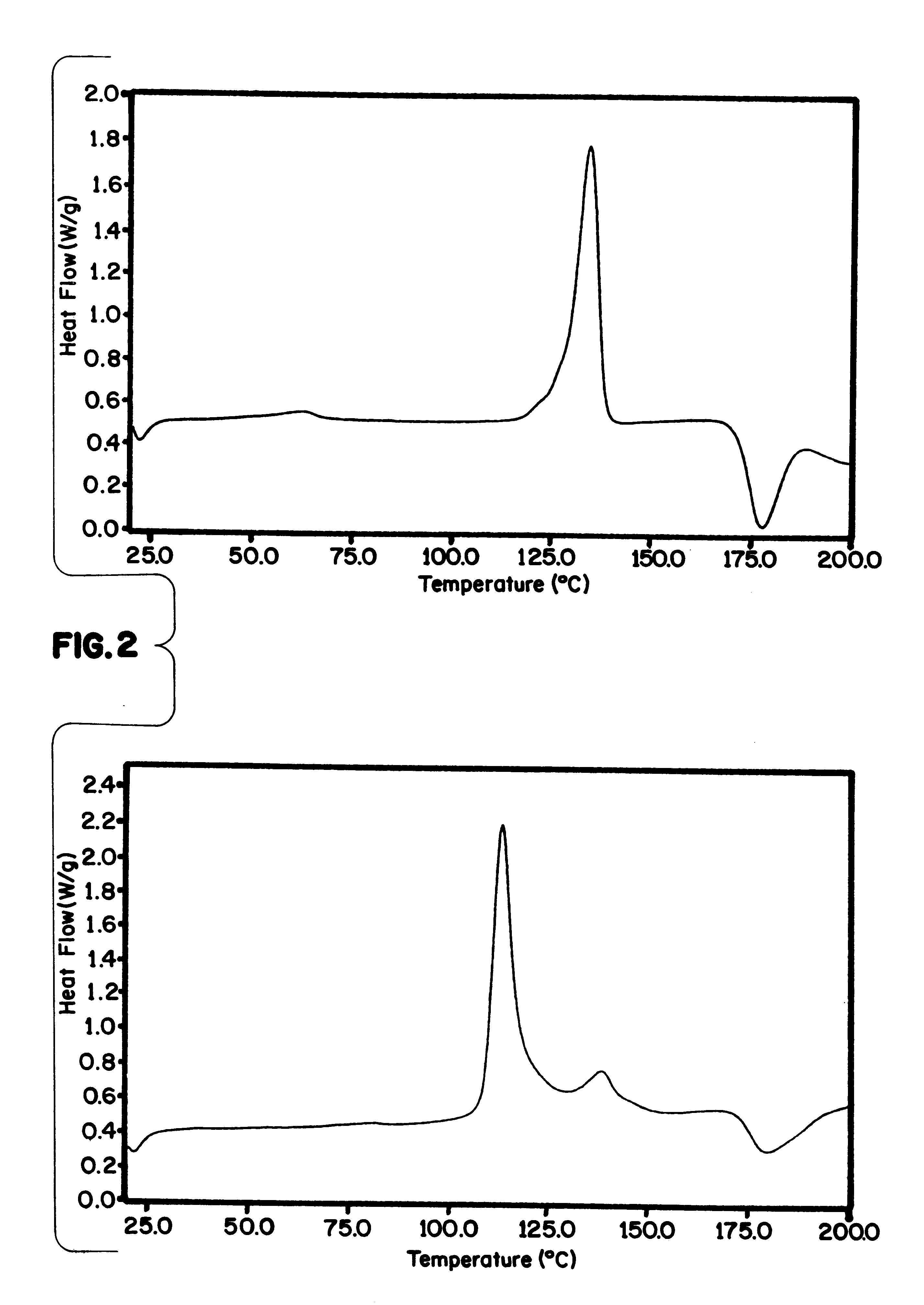
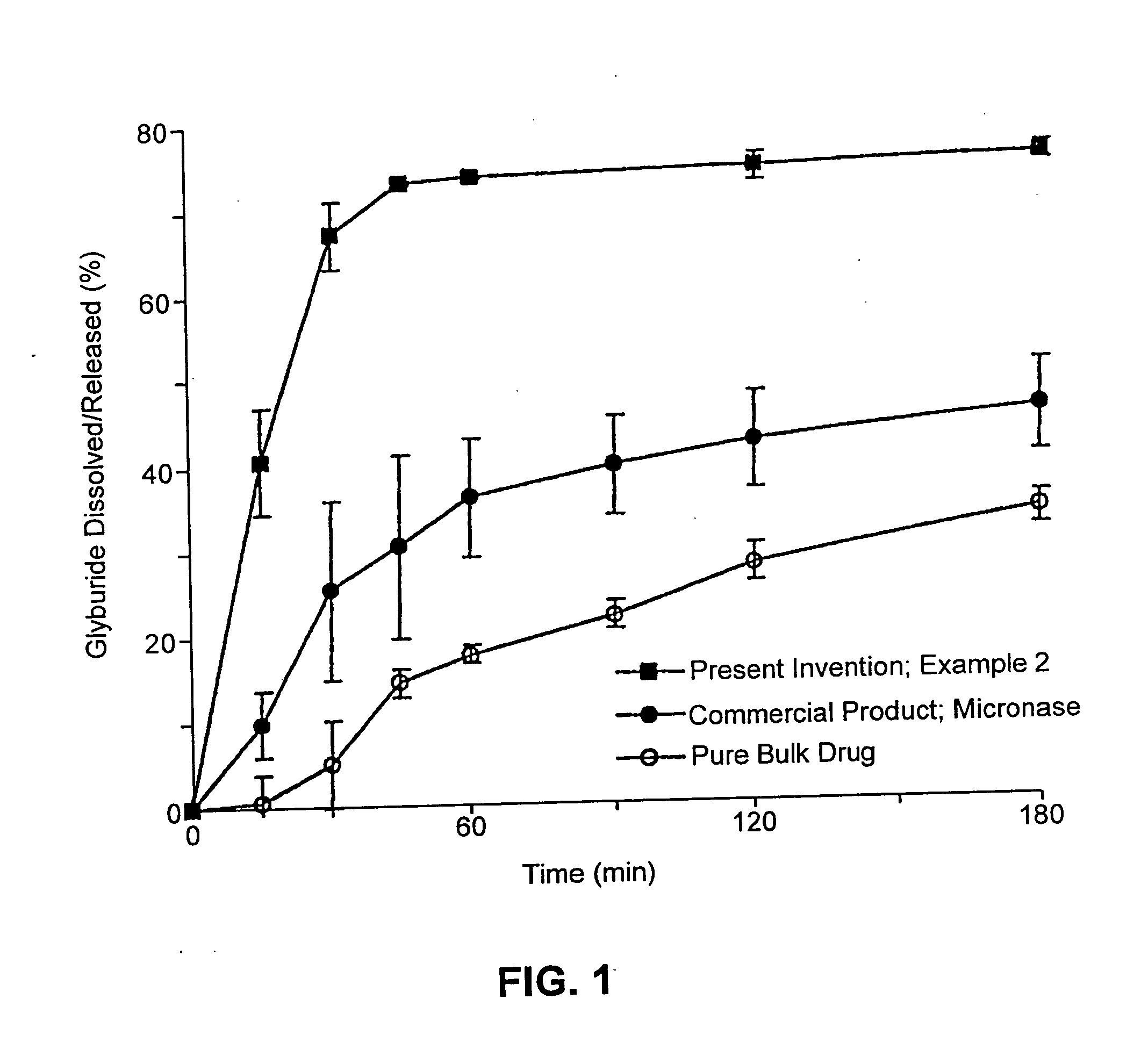
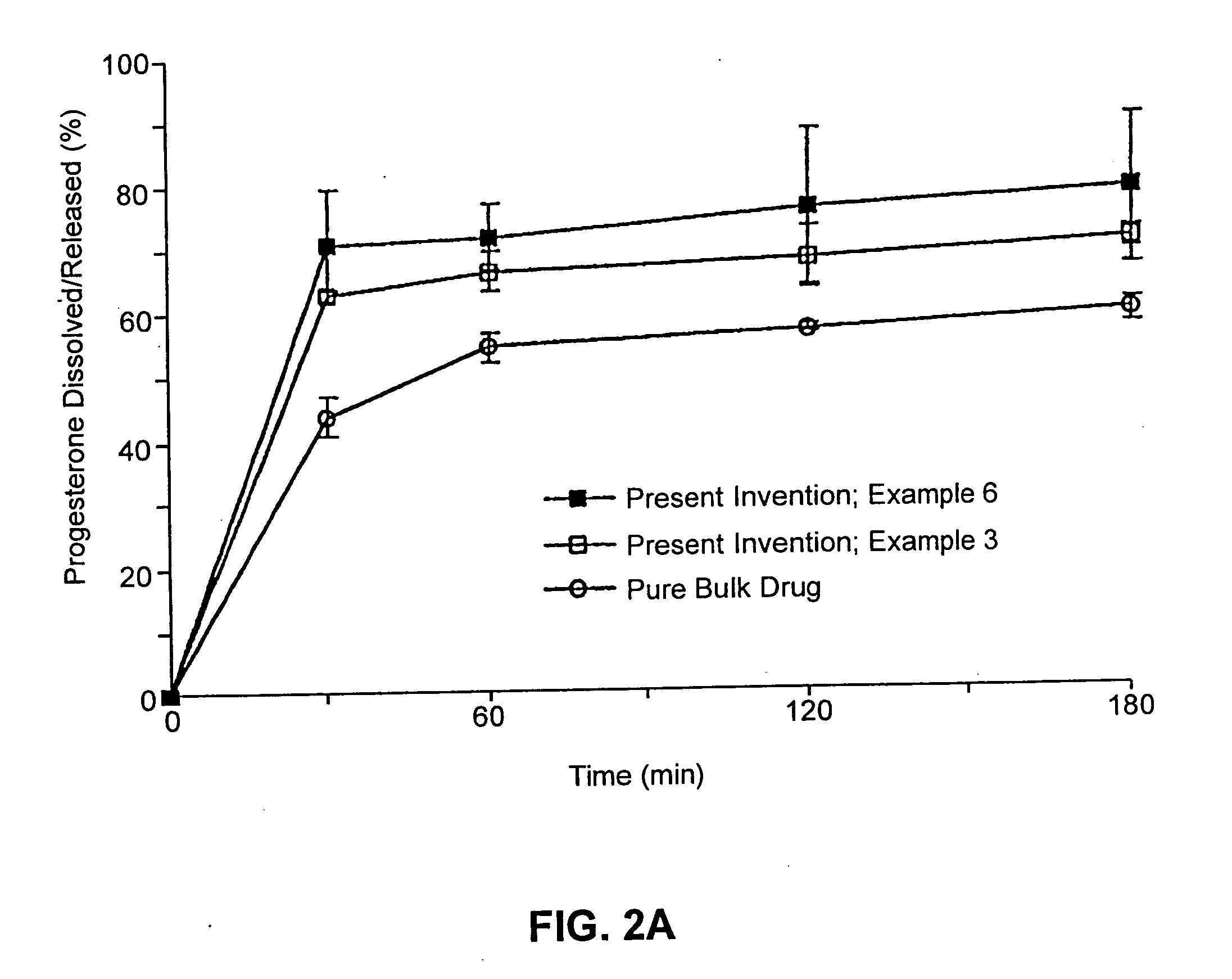
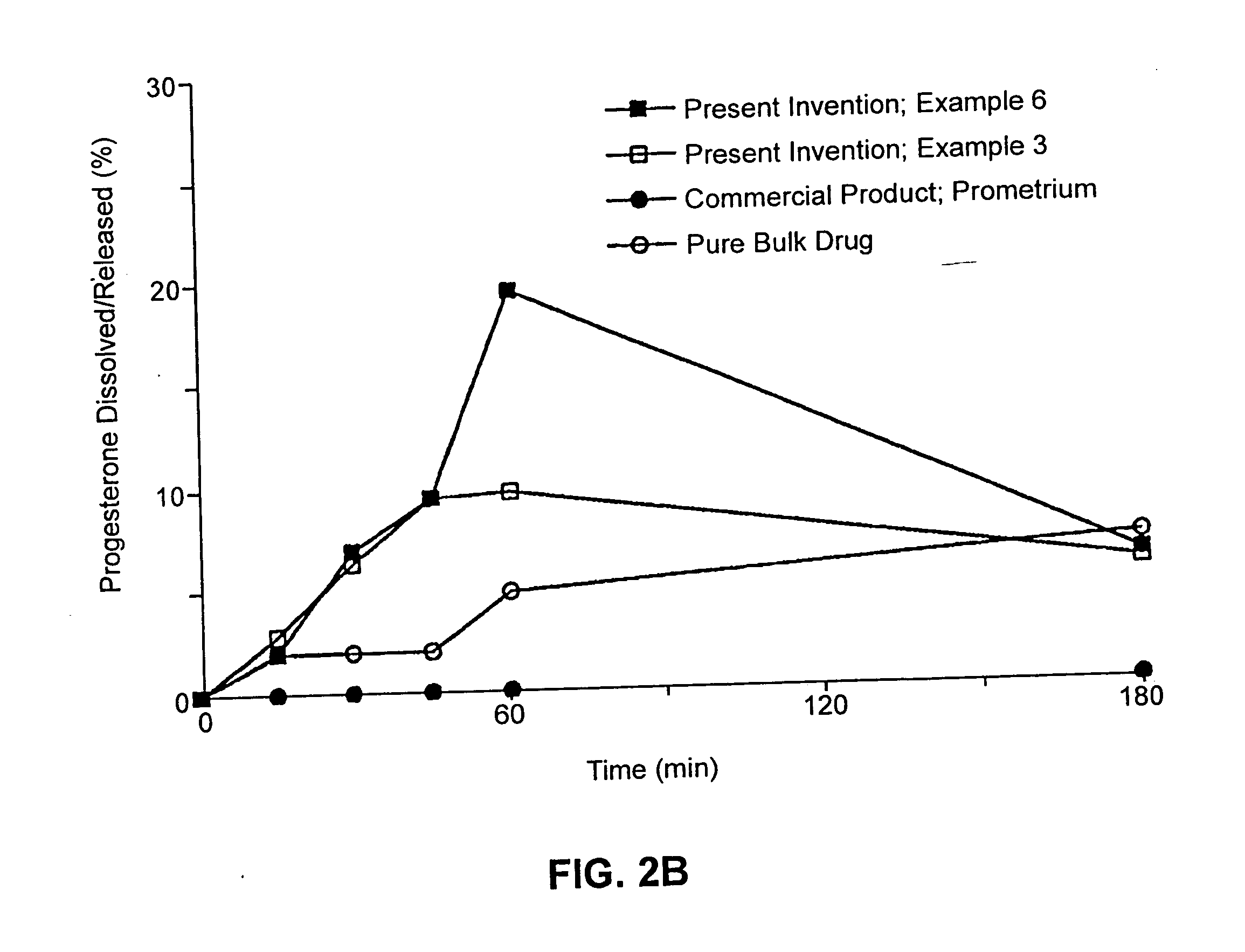
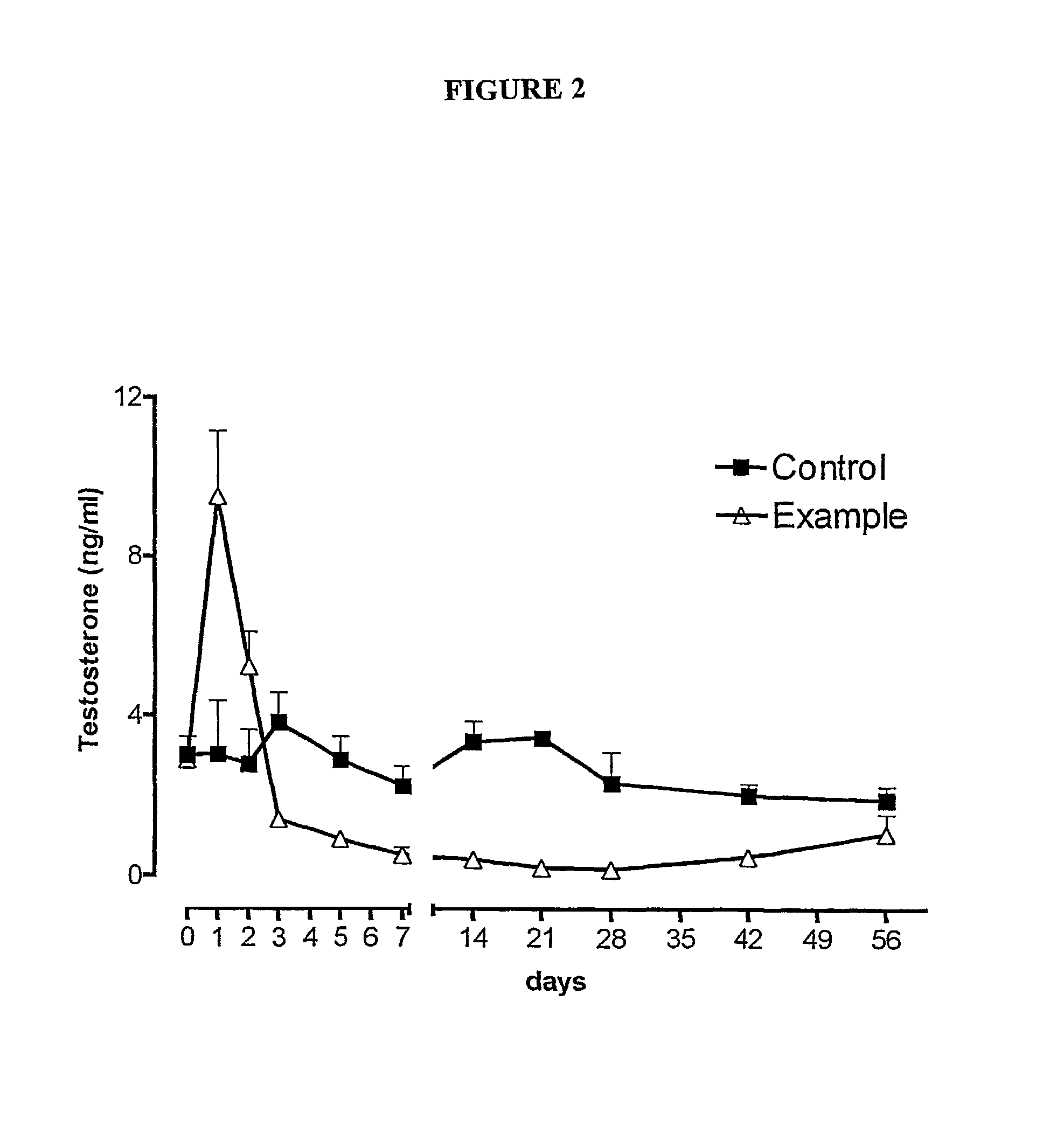
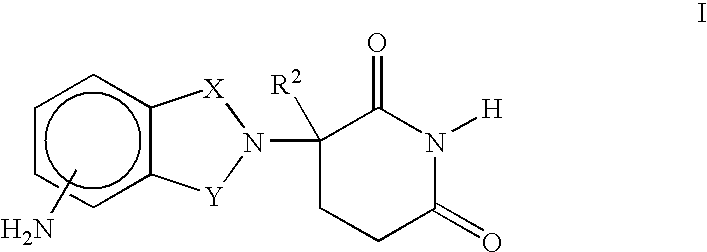
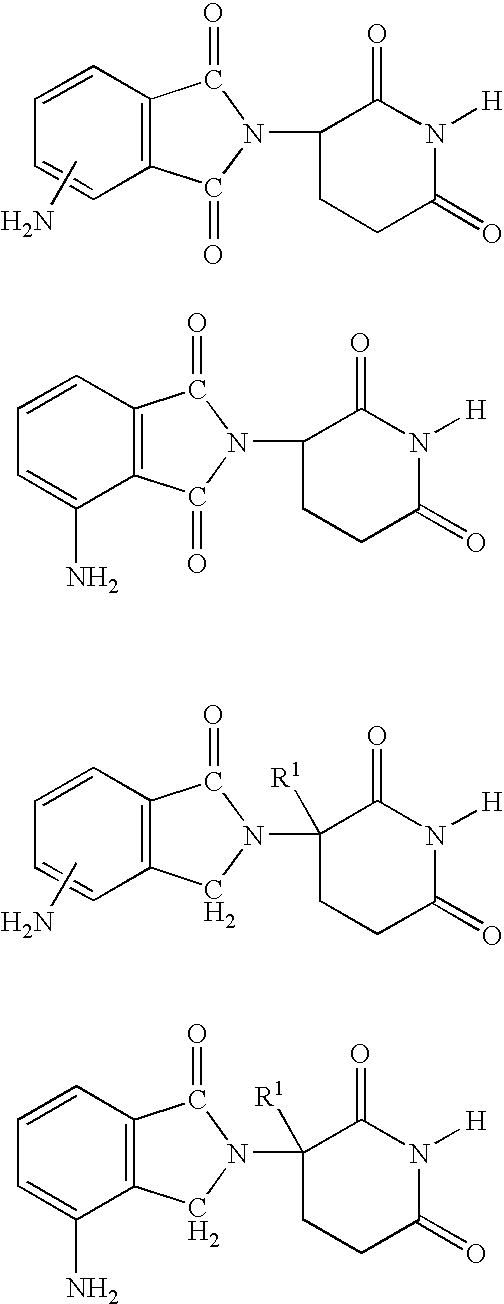
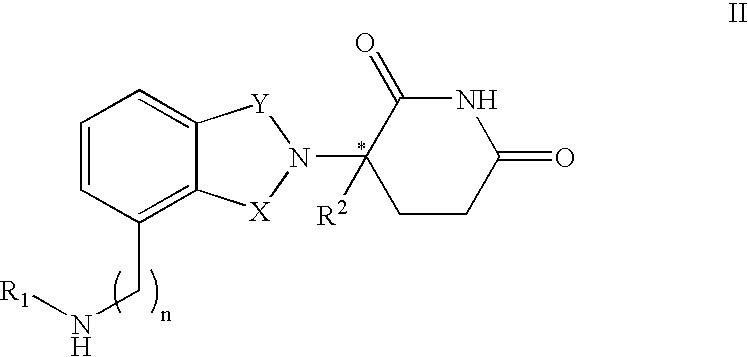
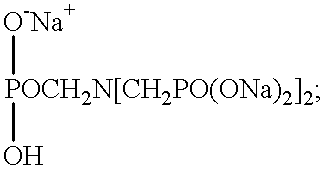Patents
Literature
69325 results about "Active ingredient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An active ingredient (AI) is the ingredient in a pharmaceutical drug or pesticide that is biologically active. The similar terms active pharmaceutical ingredient (API) and bulk active are also used in medicine, and the term active substance may be used for natural products. Some medication products may contain more than one active ingredient. The traditional word for the API is pharmacon or pharmakon (from Greek: φάρμακον, adapted from pharmacos) which originally denoted a magical substance or drug.
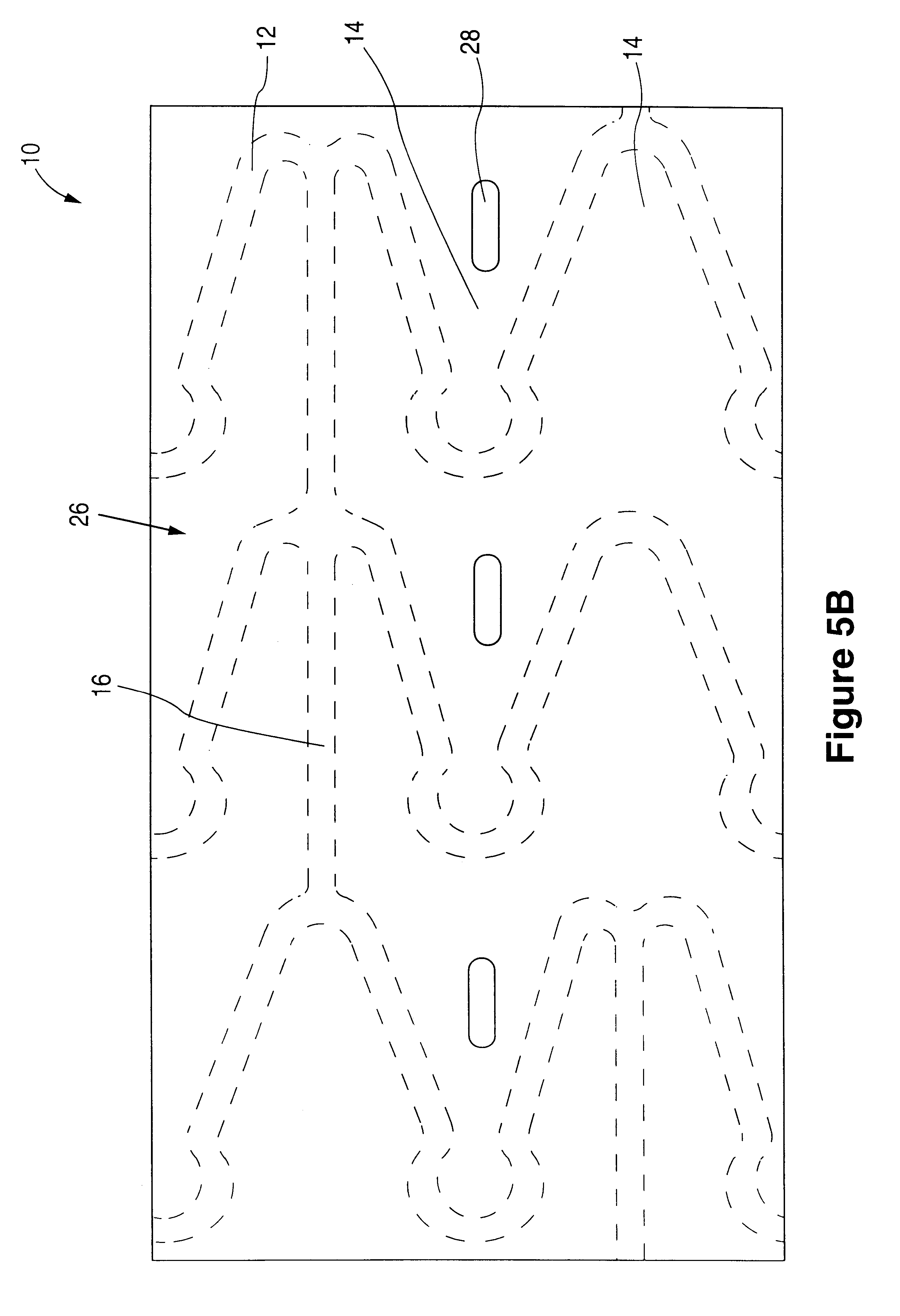
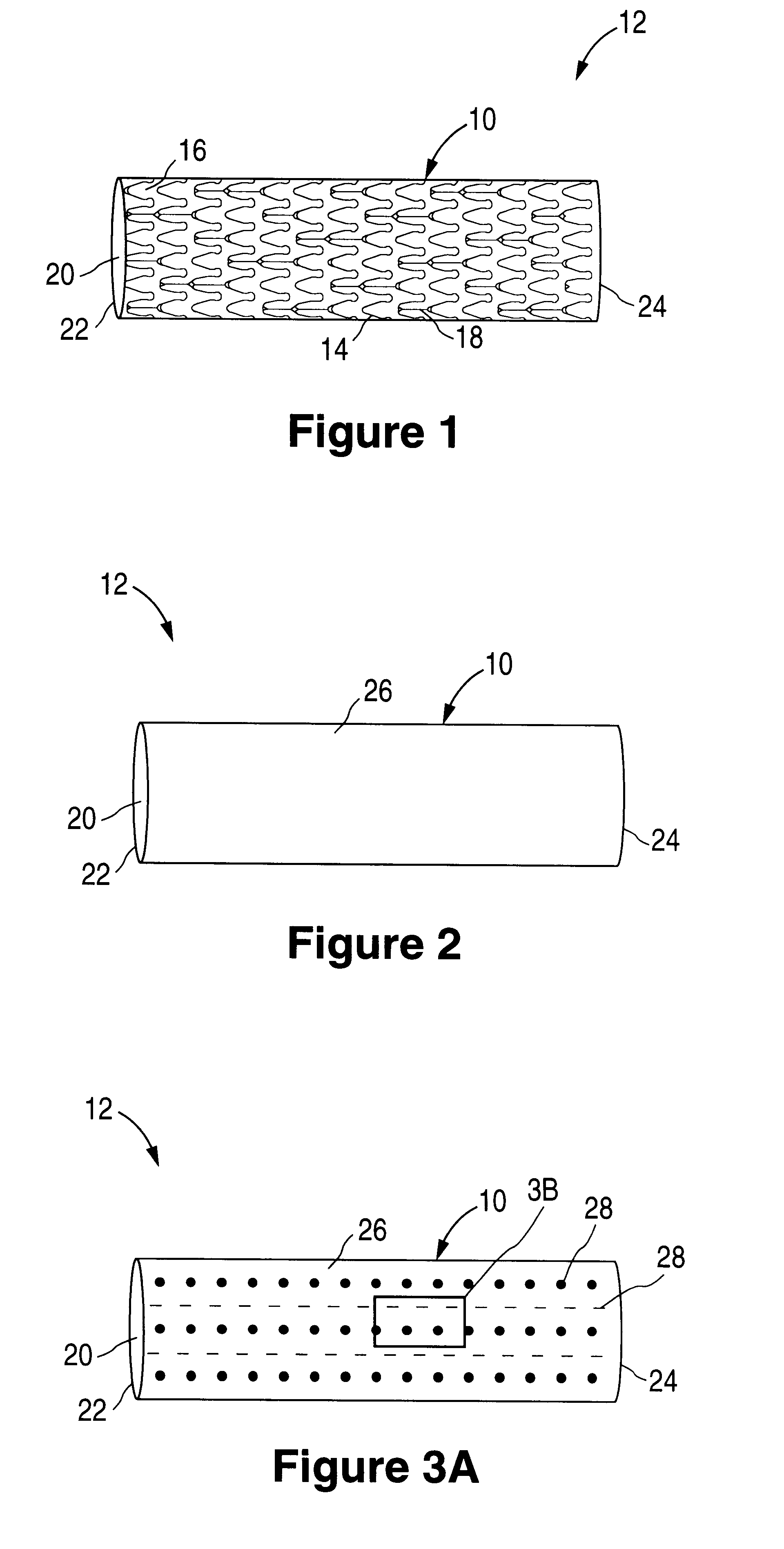
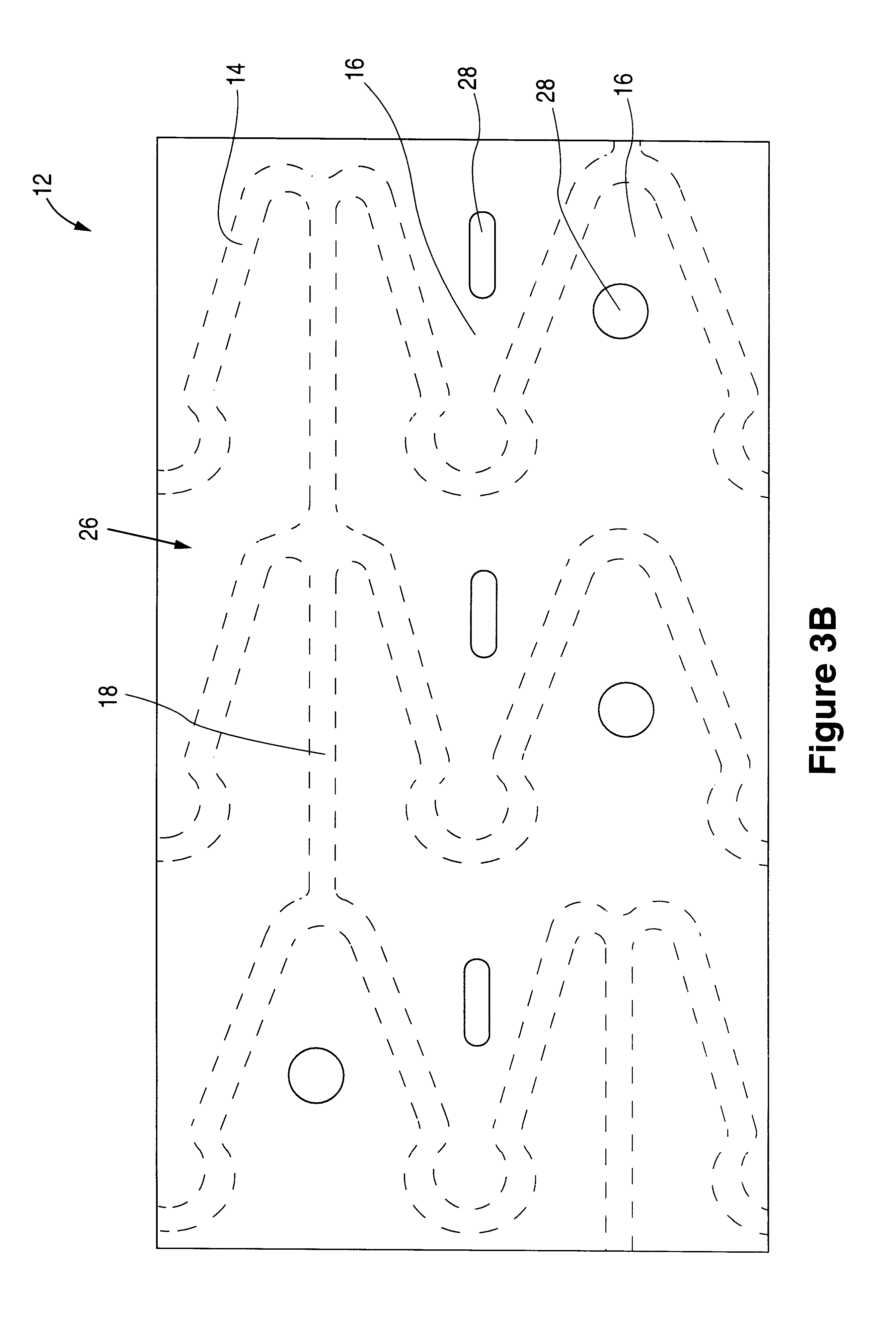
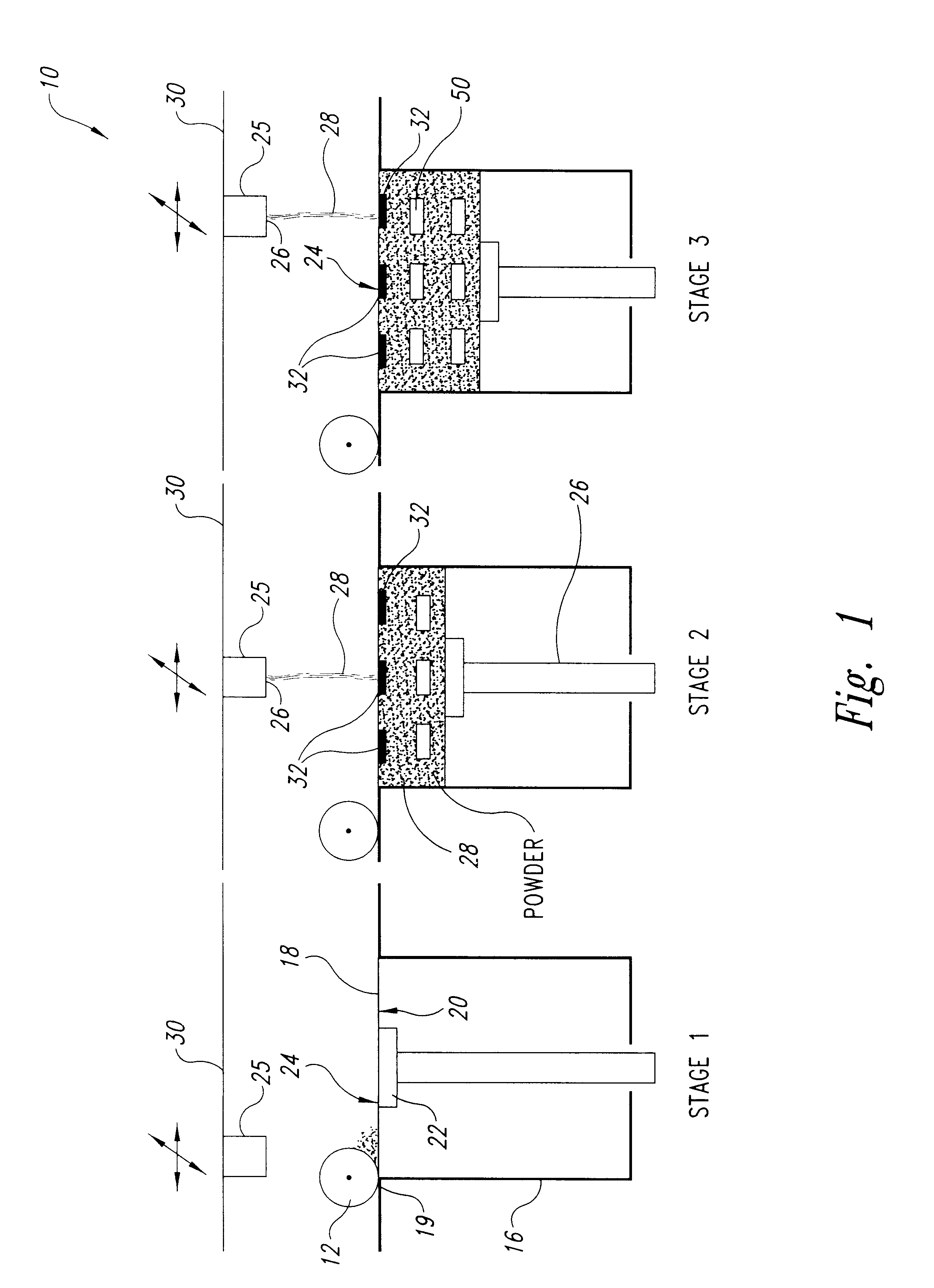
Tear and abrasion resistant expanded material and reinforcement
InactiveUS20070207186A1Increase flexibilityLess complex manufacturing processStentsSurgeryDiseaseEngineering
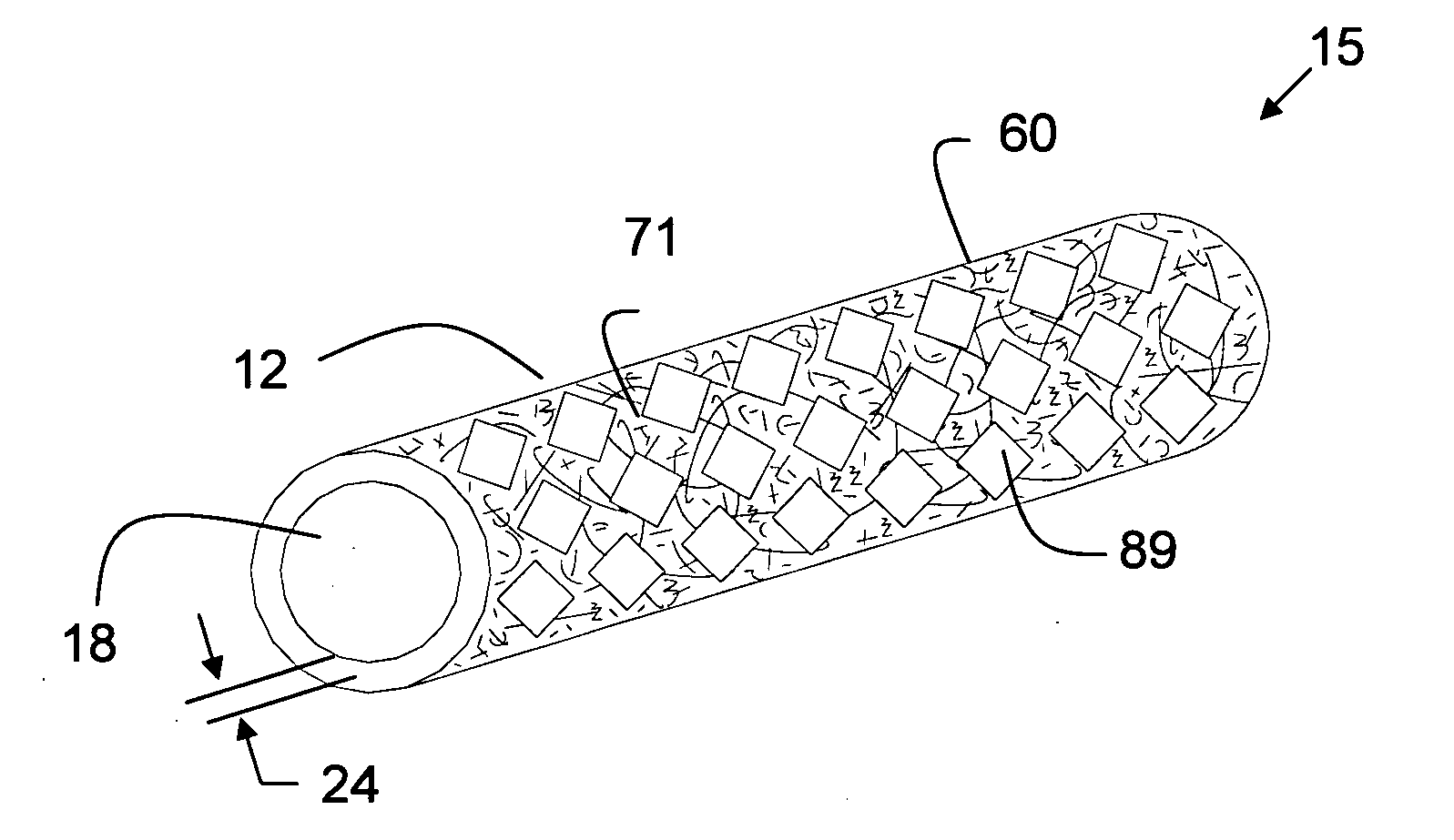
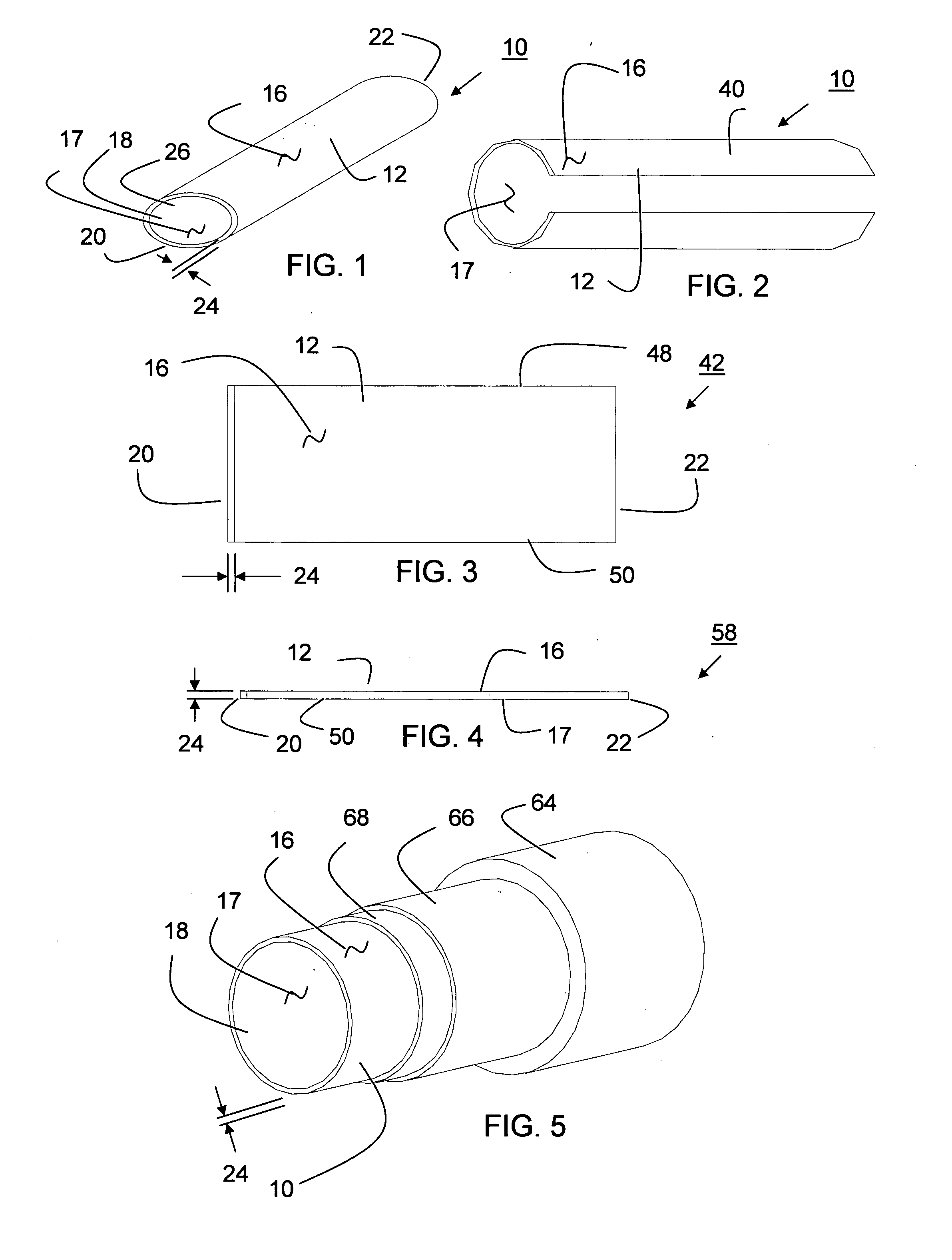
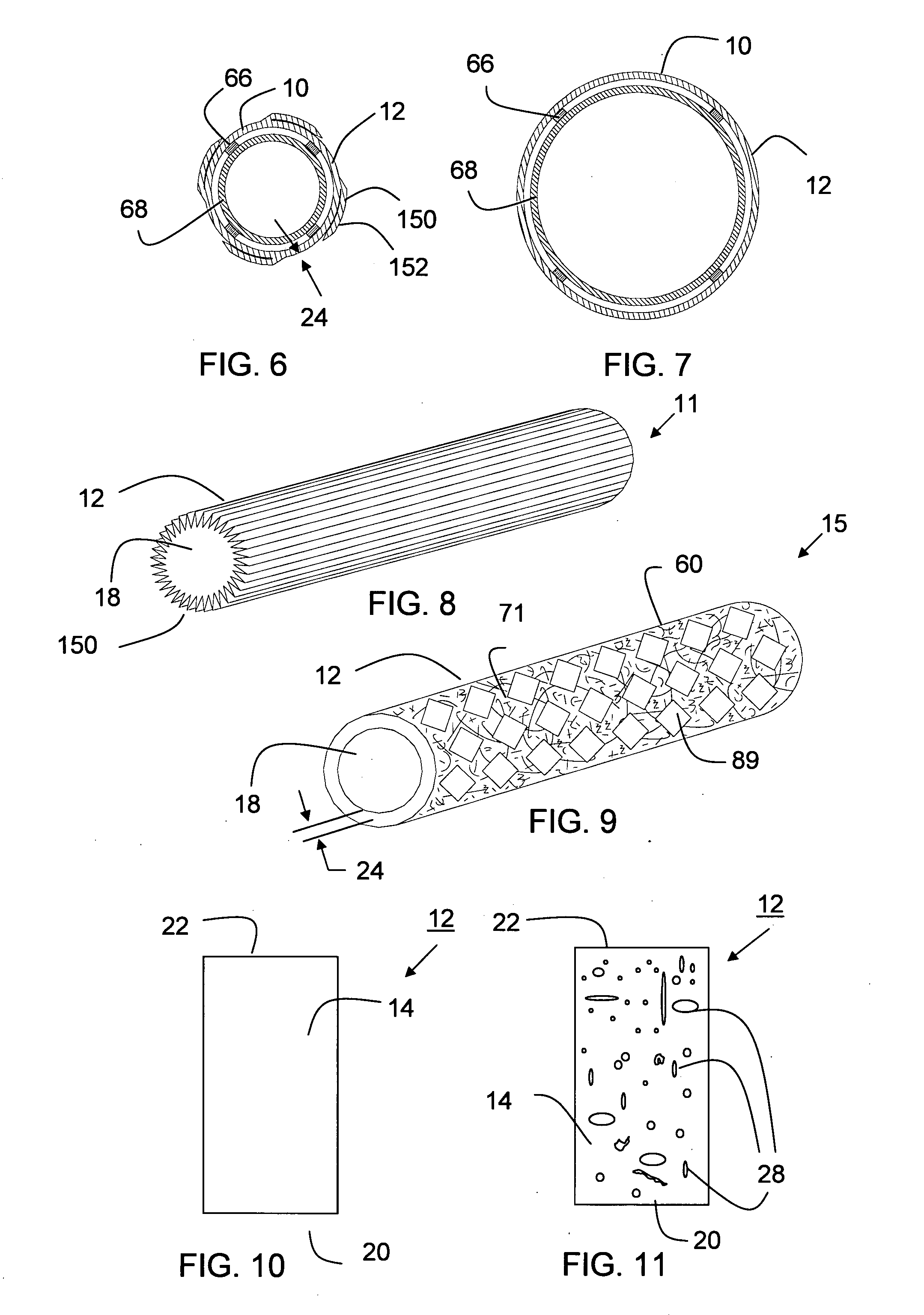
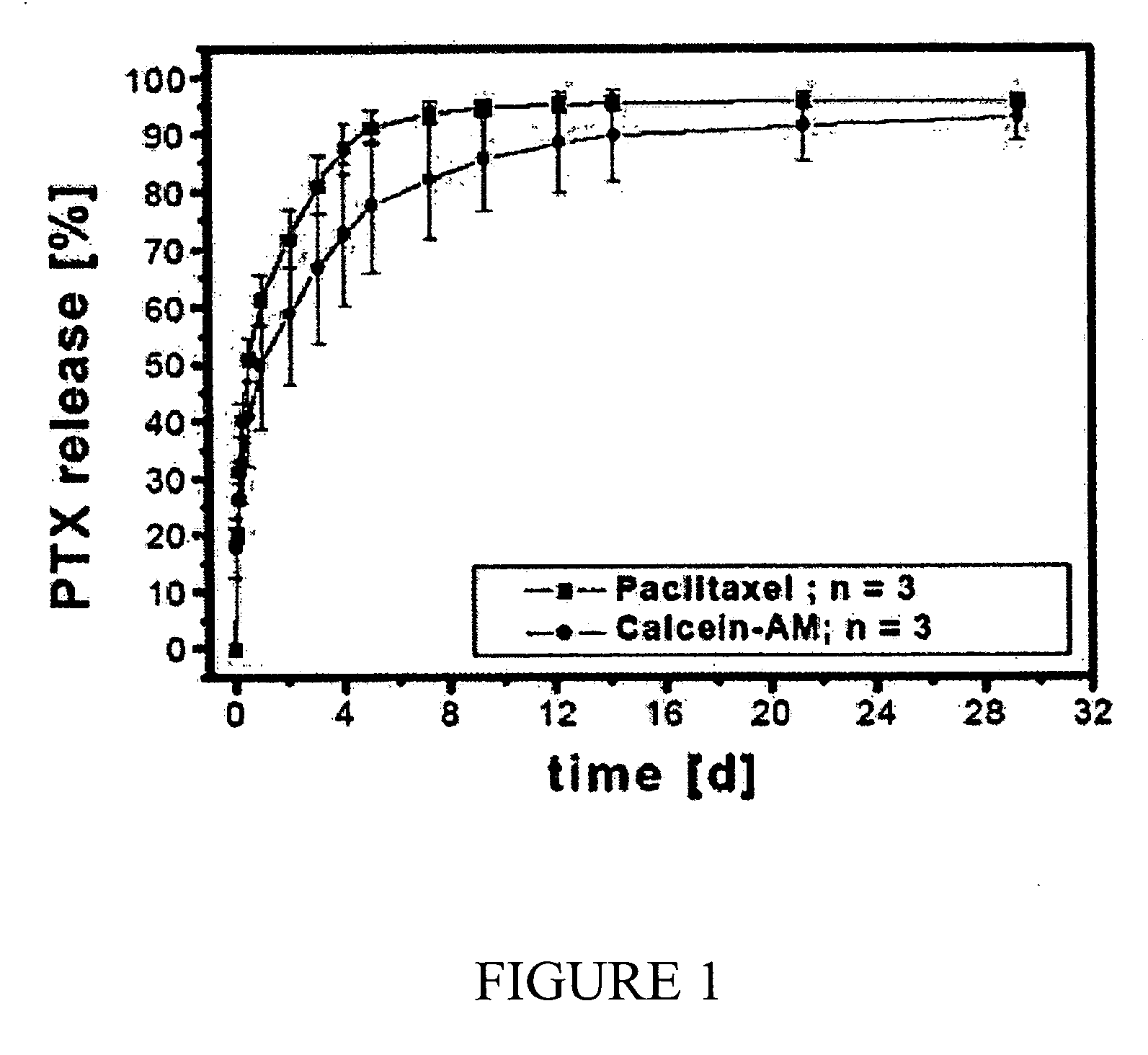
The present invention is a more durable expanded material that enables thinner wall thicknesses and a more flexible reinforcement suitable for stenting. The present invention is especially useful in the construction of grafts, stents, and stent-grafts which are used, for example, in repairing or replacing blood vessels that are narrowed or occluded by disease, aneurismal blood vessels, or other medical treatments. The inventive material and configurations allow expansion or contraction in size or adjustment in size in an incremental manner so that the optimum size, shape, and fit with other objects can be obtained. The present invention is also optionally capable of more accurately delivering one or more active ingredients such as drugs over longer periods of time. The present invention optionally includes surface modifications and additives that increase the surface adhesion of active ingredients, coatings, or combinations thereof. Finally, the present invention optionally includes growing cells on the inventive material so that the expanded material, reinforcement, or combinations thereof are useful, for example, in producing lab-grown blood vessels or organs.
Owner:SCANLON JOHN JAMES +1
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
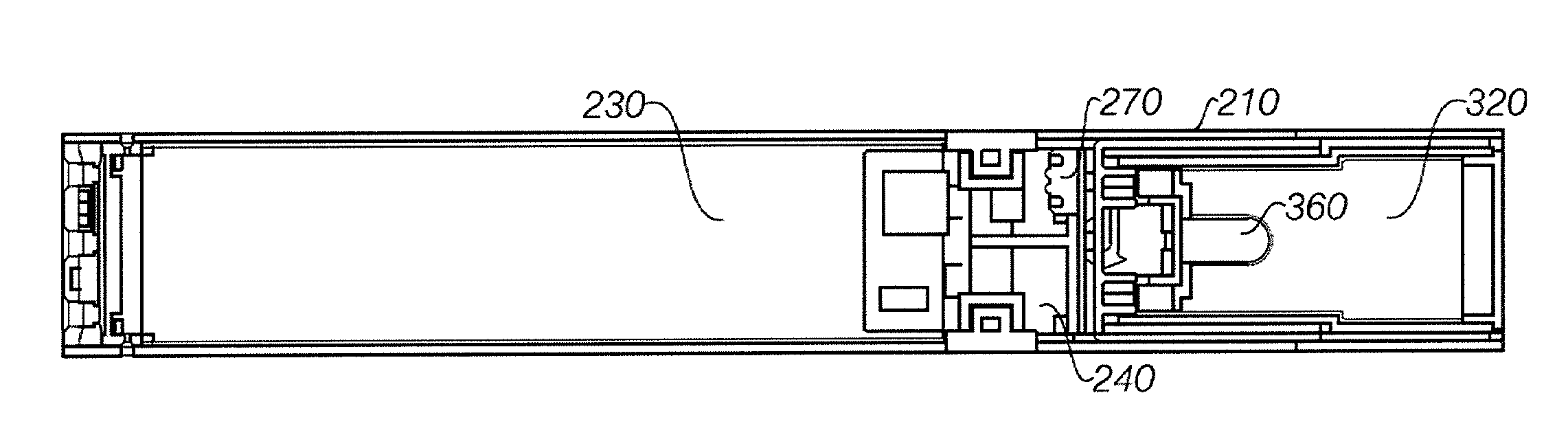
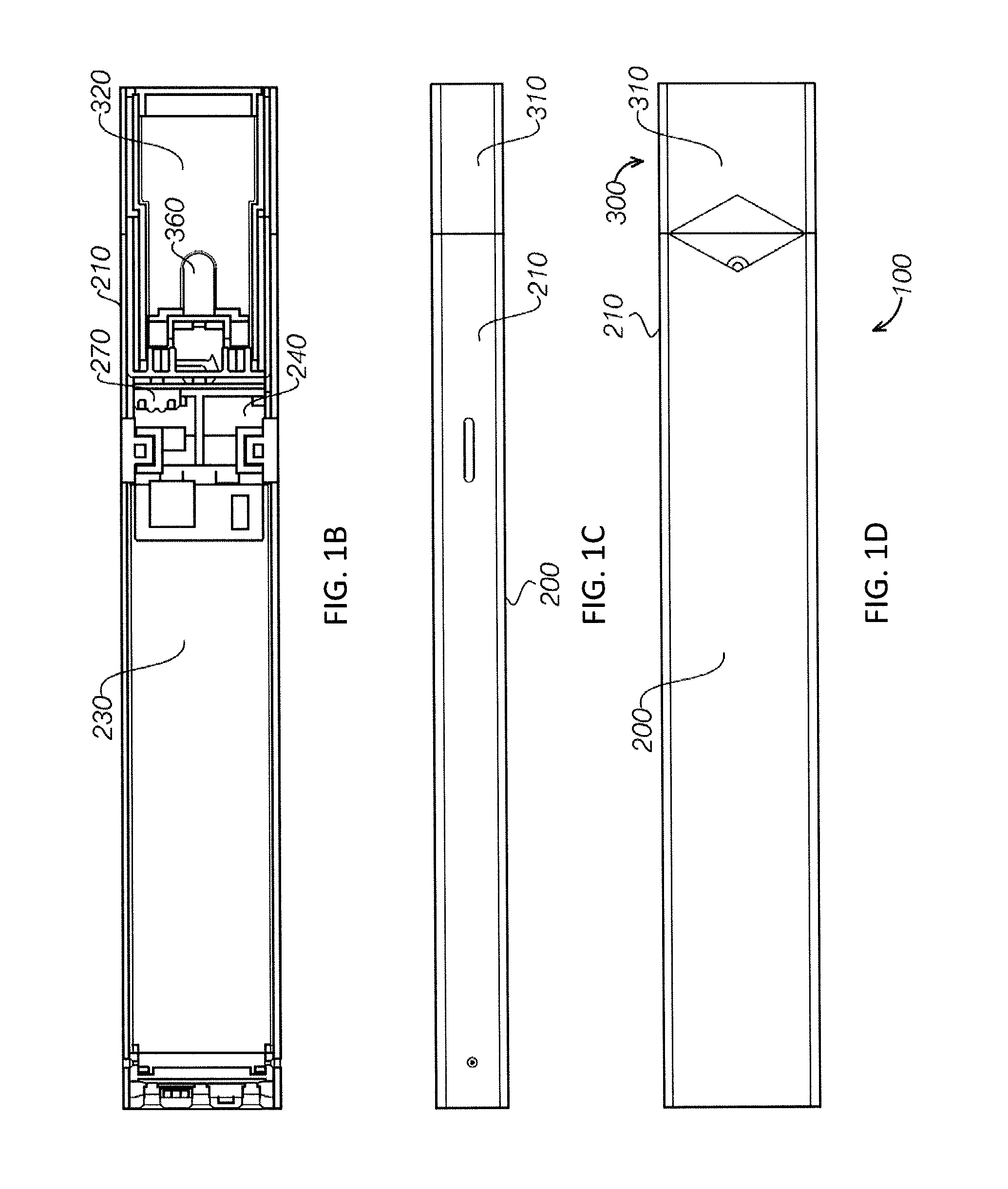
Electronic vaporizing device and methods for use
InactiveUS20130298905A1Increase heatImproved vaporizing capabilityTobacco devicesInhalatorsCelluloseBrick
Devices and methods for vaporizing active ingredients of a selected substance for inhalation using a portable vaporization device are provided herein. In certain aspects, the device includes a portable power source, a heating portion, an inhalation sensor, a temperature sensor, a distal light source, and a grinding portion. In response to an inhalation by a user, the power source energizes a heating element of the heating portion so as to heat air flow to a desired vaporization temperature within a few seconds of detecting inhalation, using convection and radiative heating. The device may include a receptacle for receiving a cartridge containing a pre-prepared substance, such as a liquid, gel, powder, or solid brick, and a grinding portion to allow a user to grind intact portions of cellulose-based material into smaller pieces to facilitate vaporization by manually rotating portions of the device relative to each other.
Owner:UPTOKE
Colon targeted delivery system
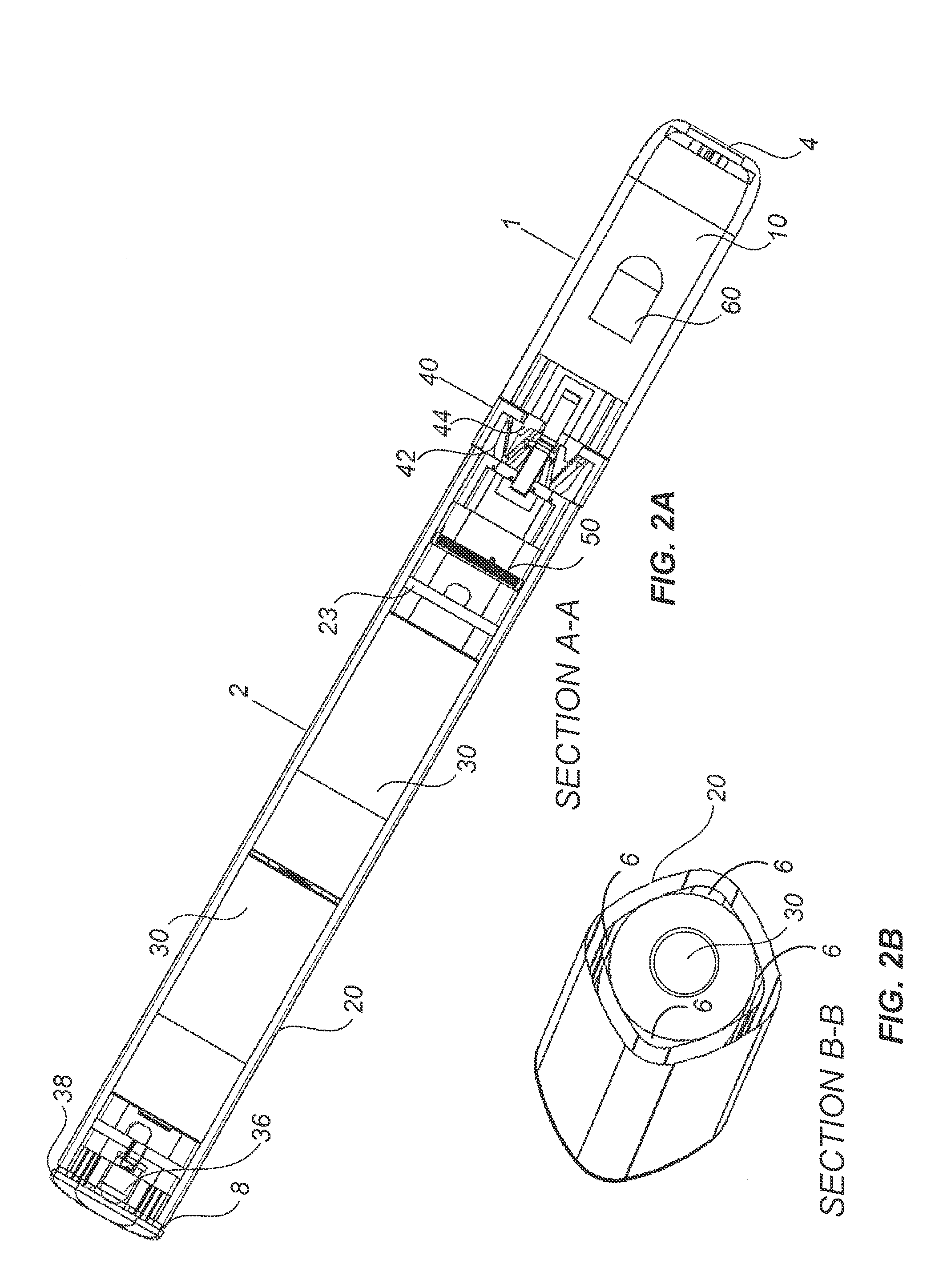
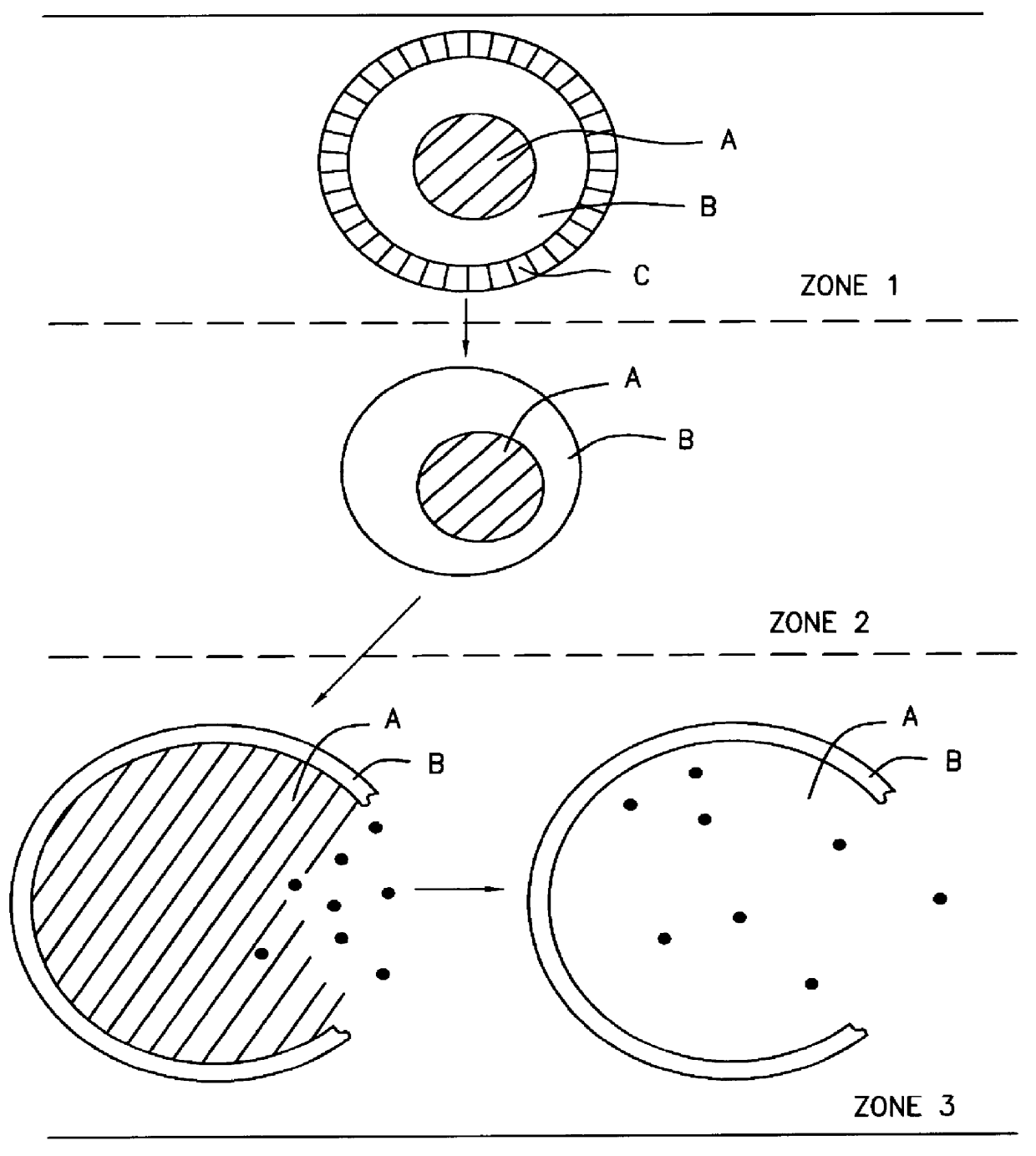
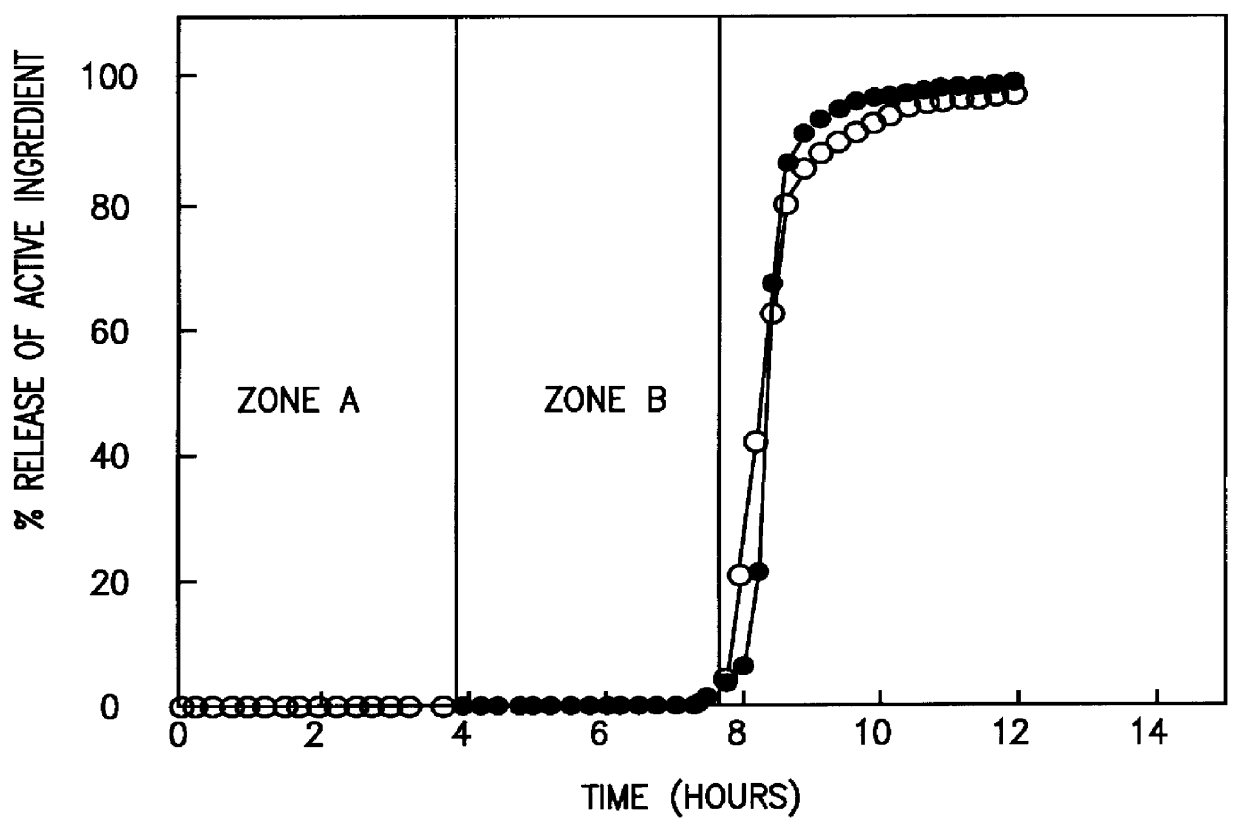
A novel delivery system for targeting drugs to the colon is herein described. The system is a tablet comprised of three parts: 1) an outer enteric coating, 2) an inner semi-permeable polymer membrane containing a plasticizer and 3) a central core comprising swelling excipients and an active ingredient. The novel dosage form described herein will release the drug consistently in the colon by a time-dependent explosion mechanism. This delivery system is particularly suitable for delivering viral protease inhibitors to the colon.
Owner:F HOFFMANN LA ROCHE & CO AG
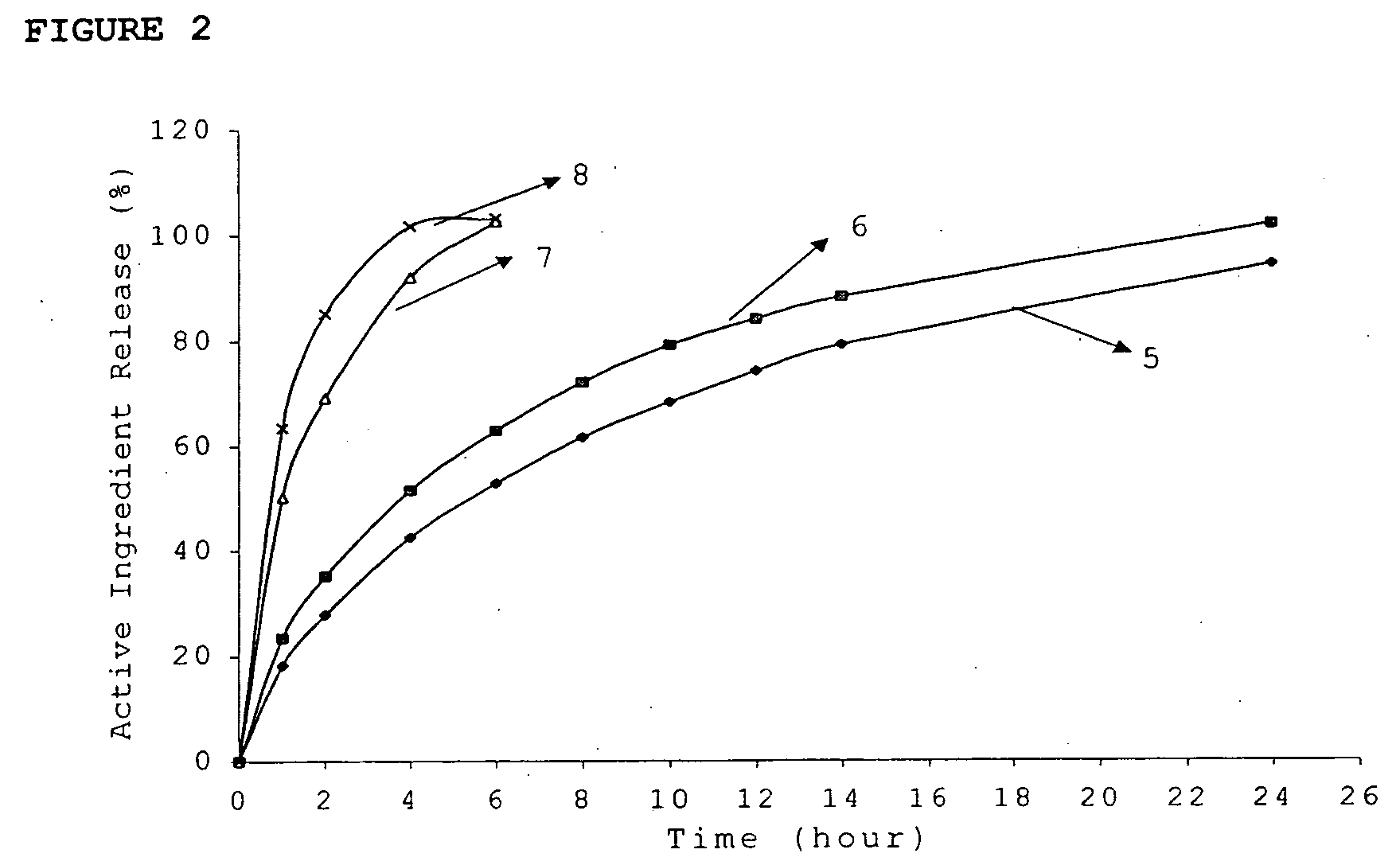
Multiparticulate modified release composition
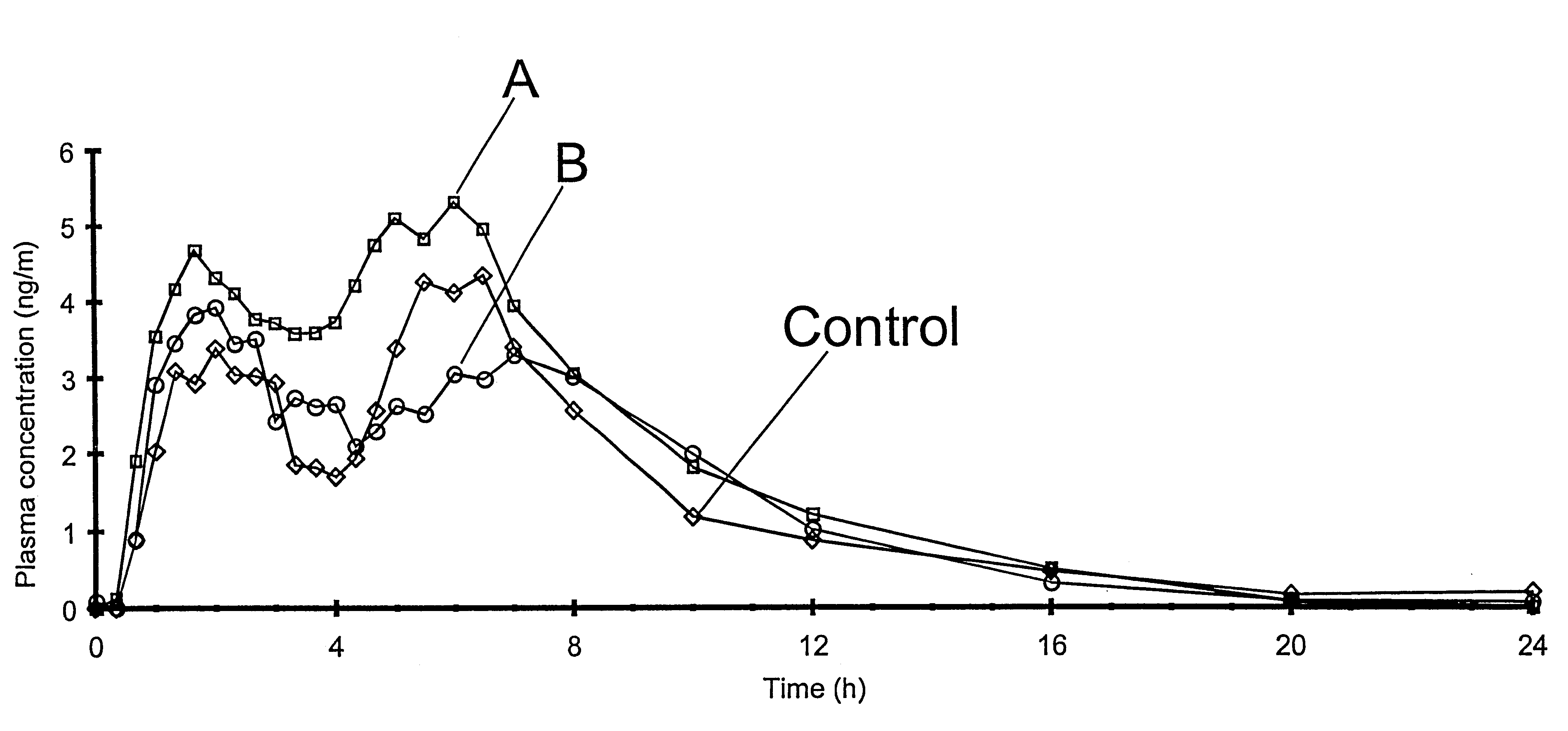
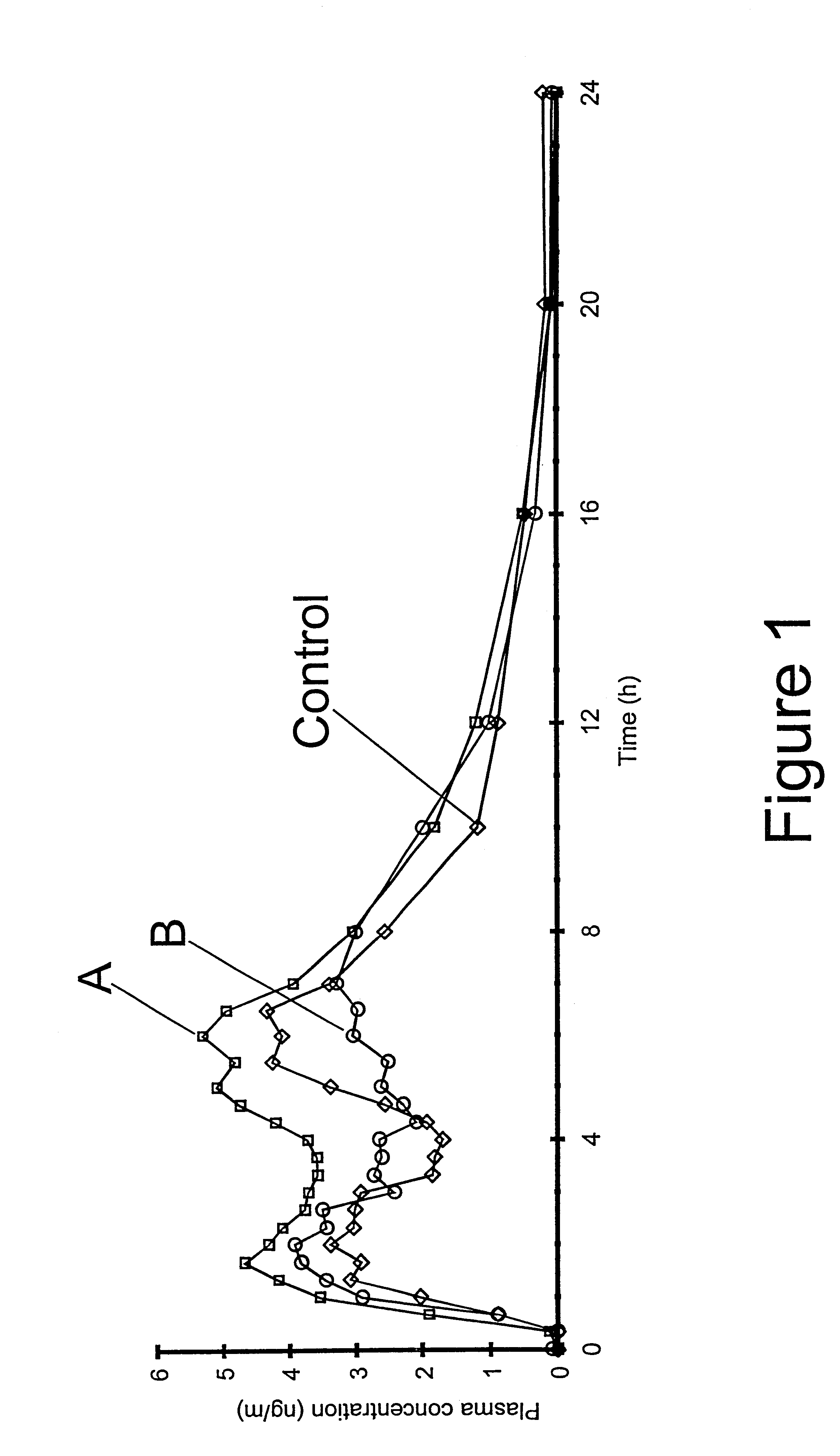
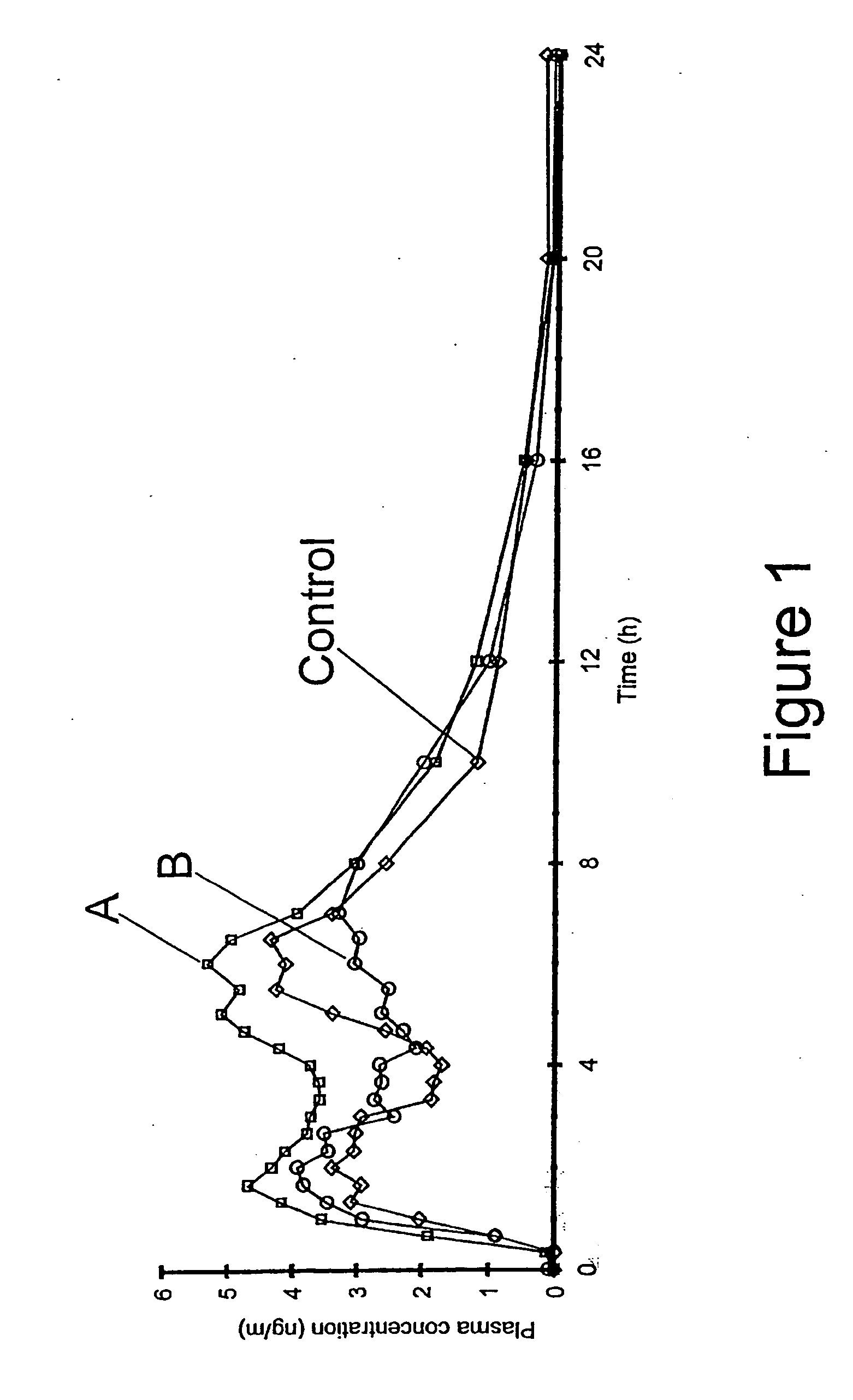
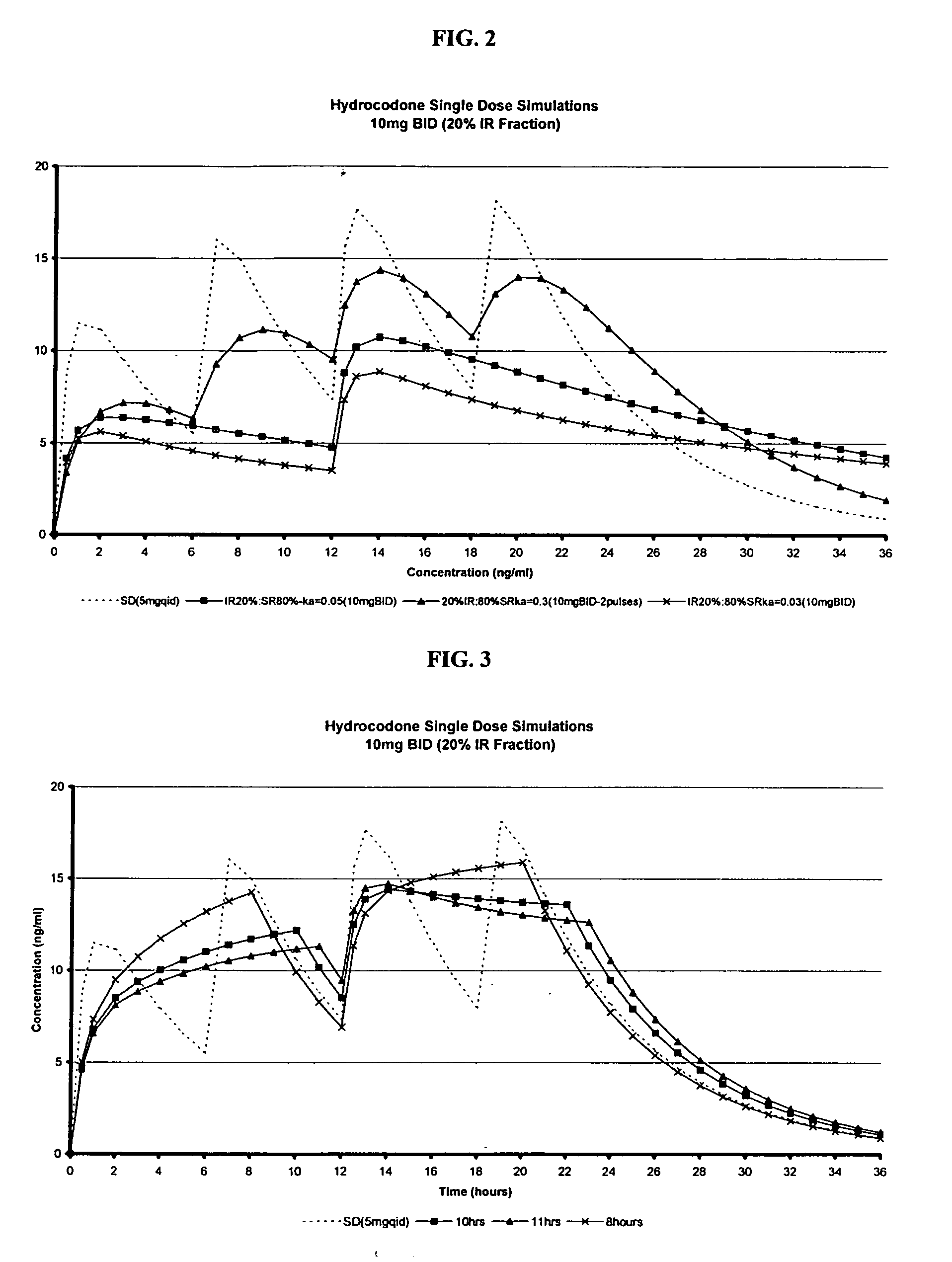
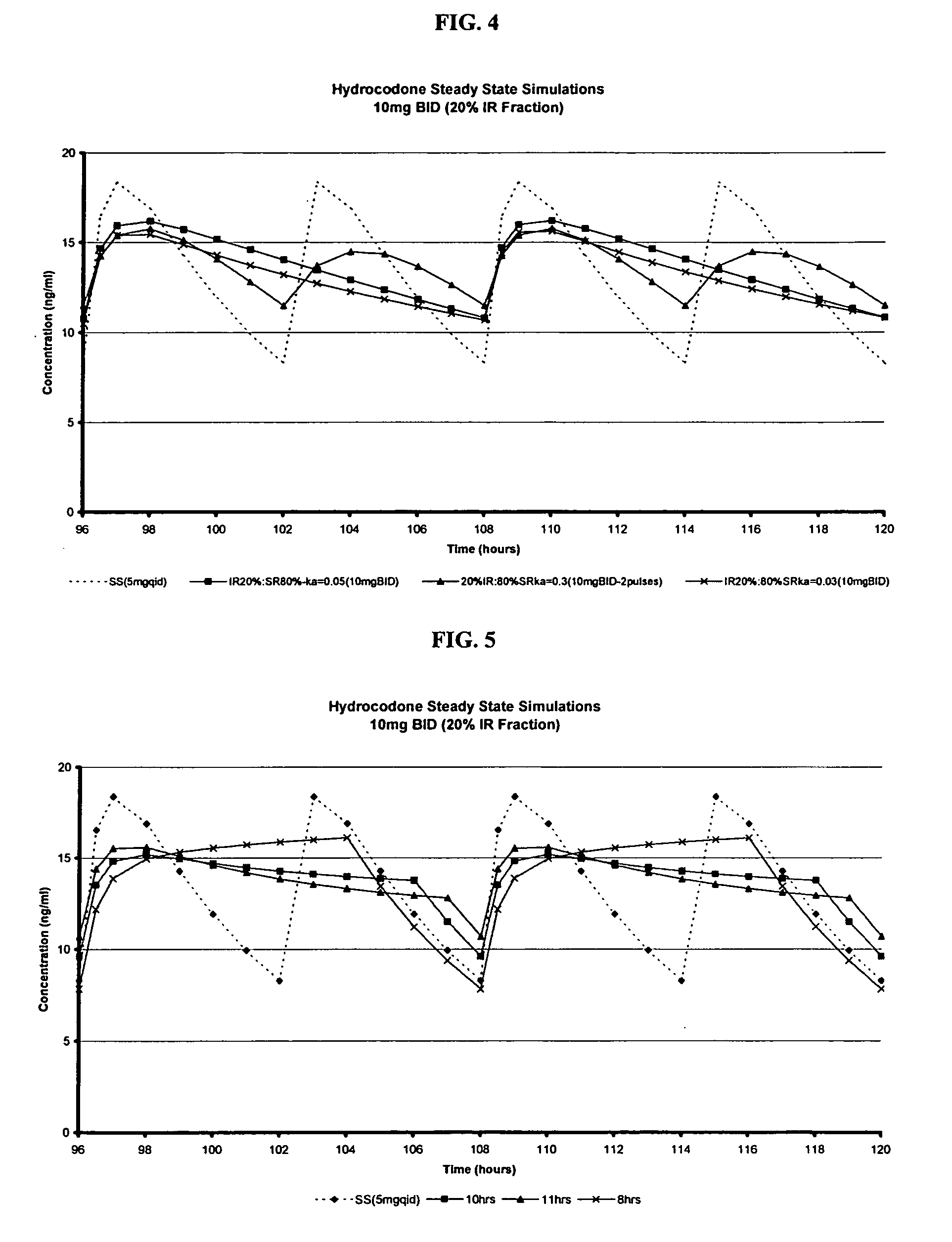
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Methods of forming a coating for a prosthesis
InactiveUS6503556B2Increase the amount addedIncrease the number ofRadiation applicationsGlovesProsthesisImplanted device
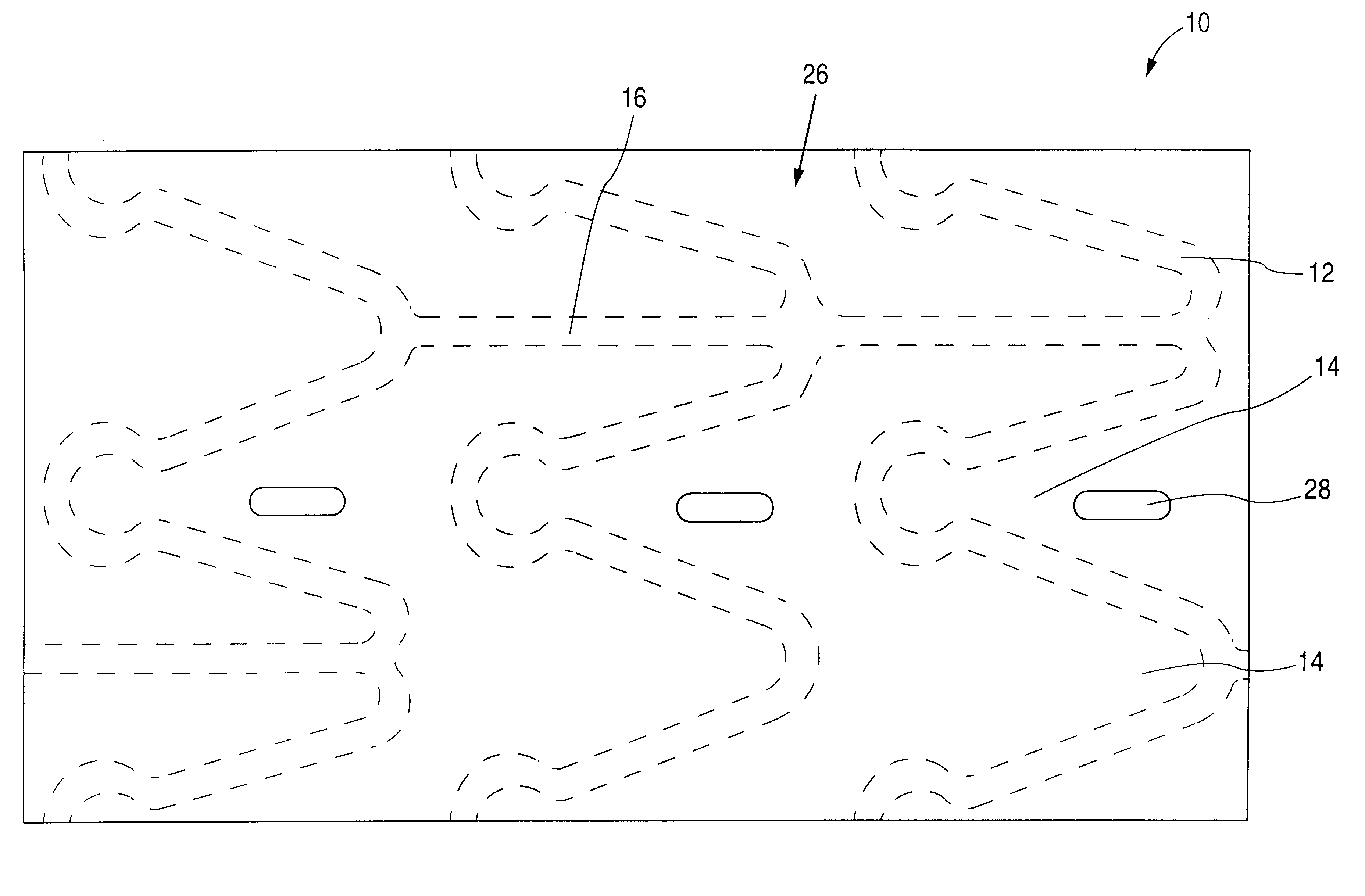
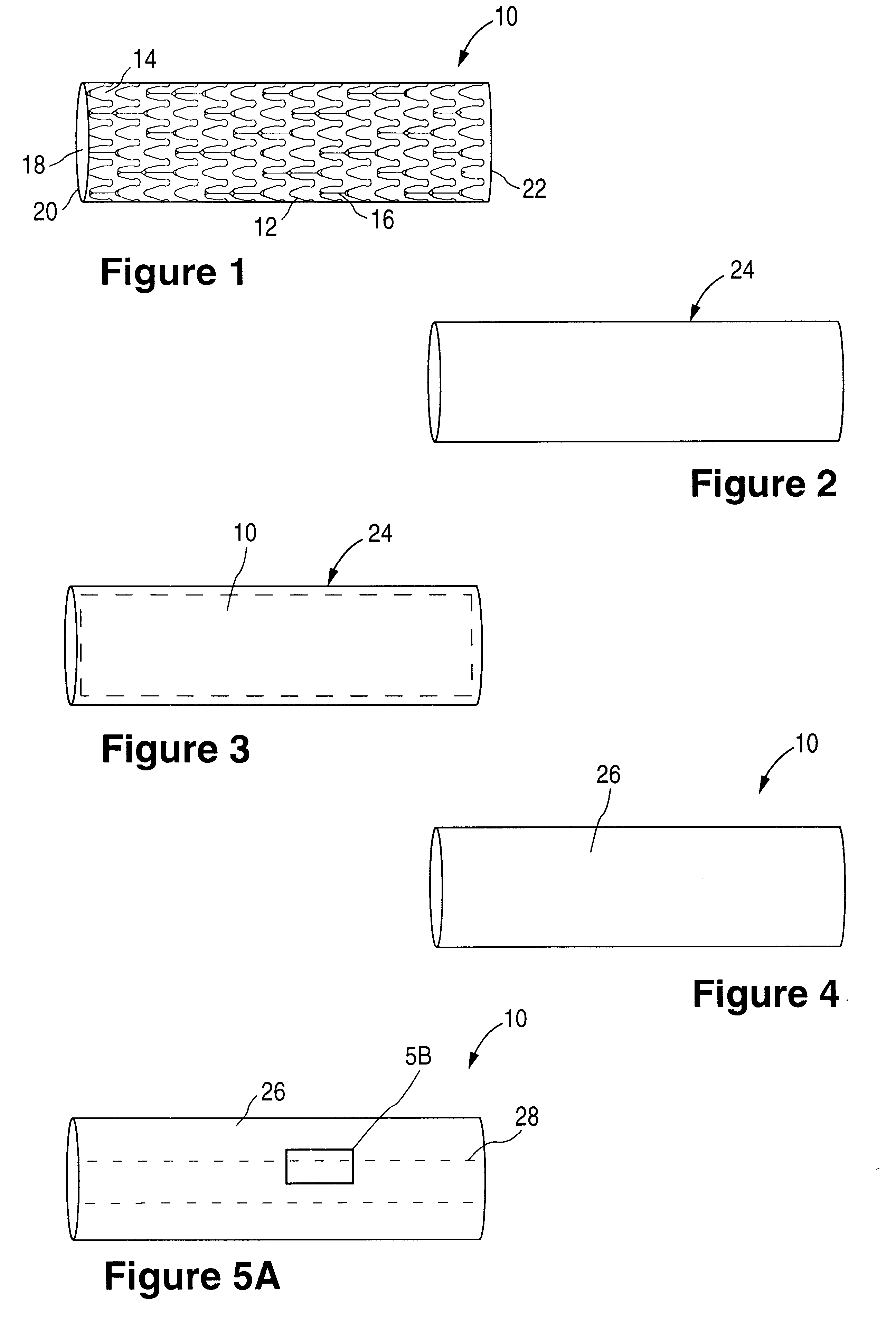
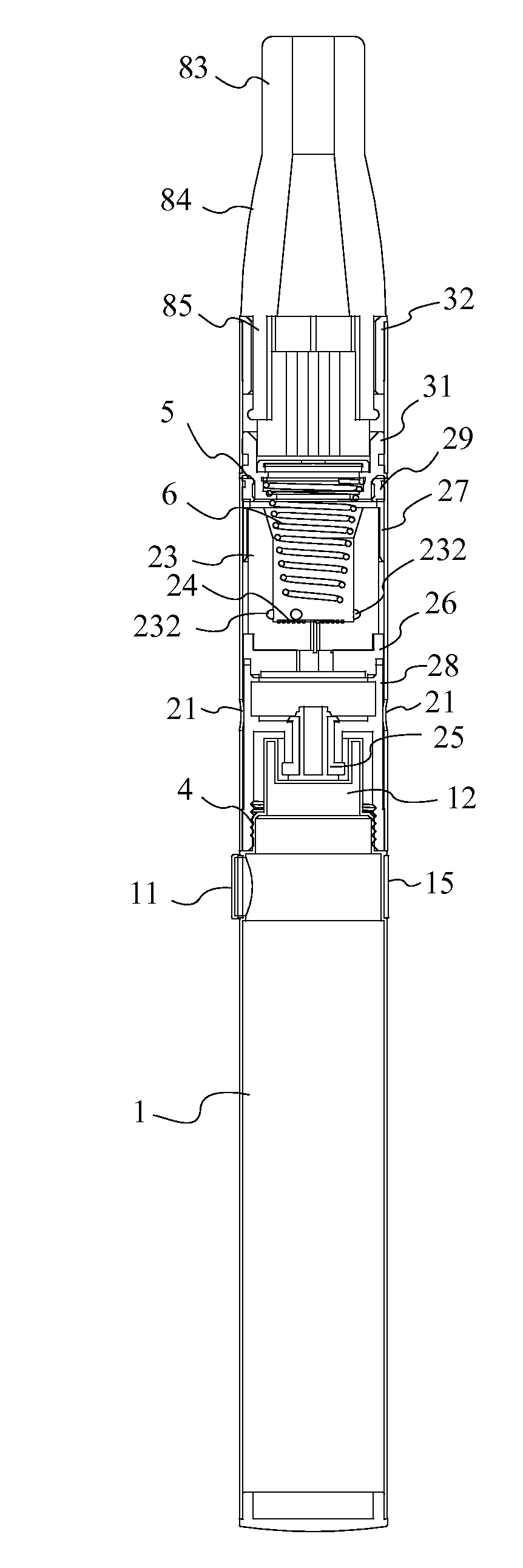
Methods of forming a coating onto an implantable device or endoluminal prosthesis, such as a stent, are provided. The coating may be used for the delivery of an active ingredient. The coating may have a selected pattern of interstices for allowing a fluid to seep through the coating in the direction of the pattern created.
Owner:ABBOTT CARDIOVASCULAR
N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
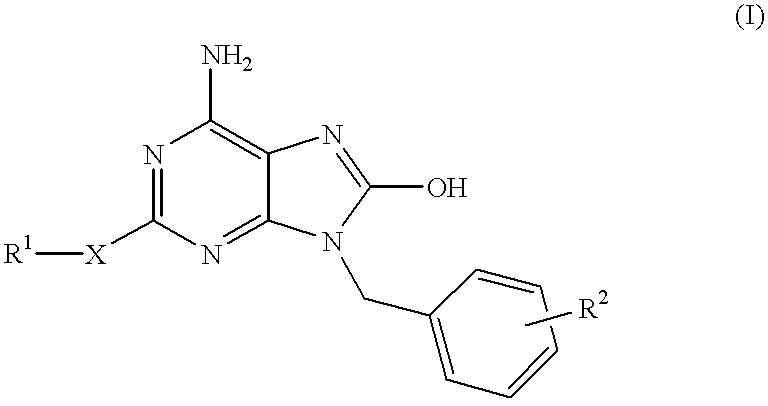
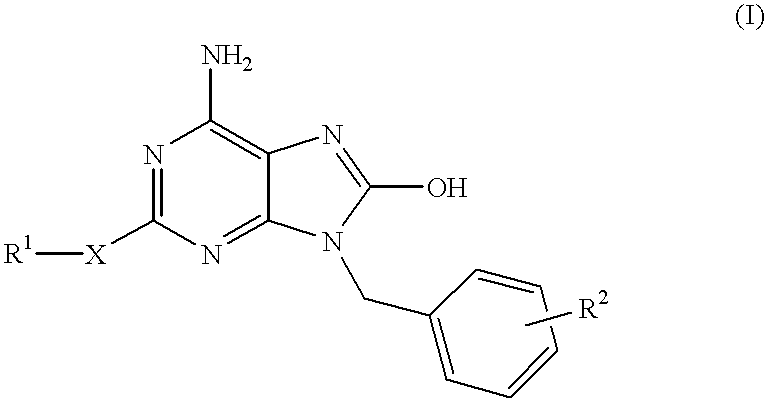
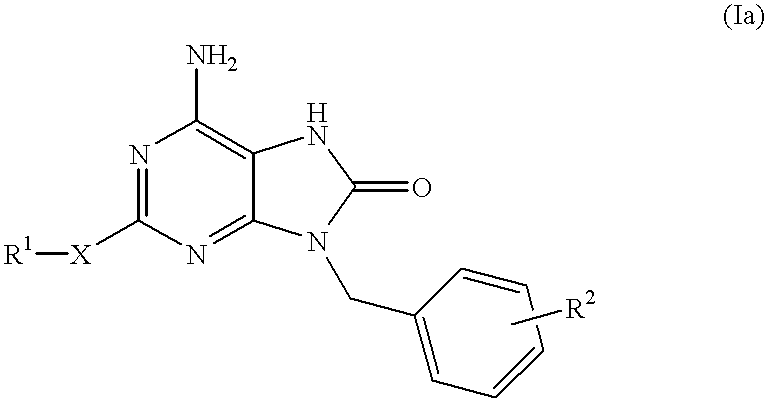
Heterocyclic compounds
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Sheath for a prosthesis and methods of forming the same
InactiveUS6540776B2Increase the amount addedIncrease the number ofStentsPharmaceutical containersProsthesisImplanted device
Owner:ABBOTT CARDIOVASCULAR
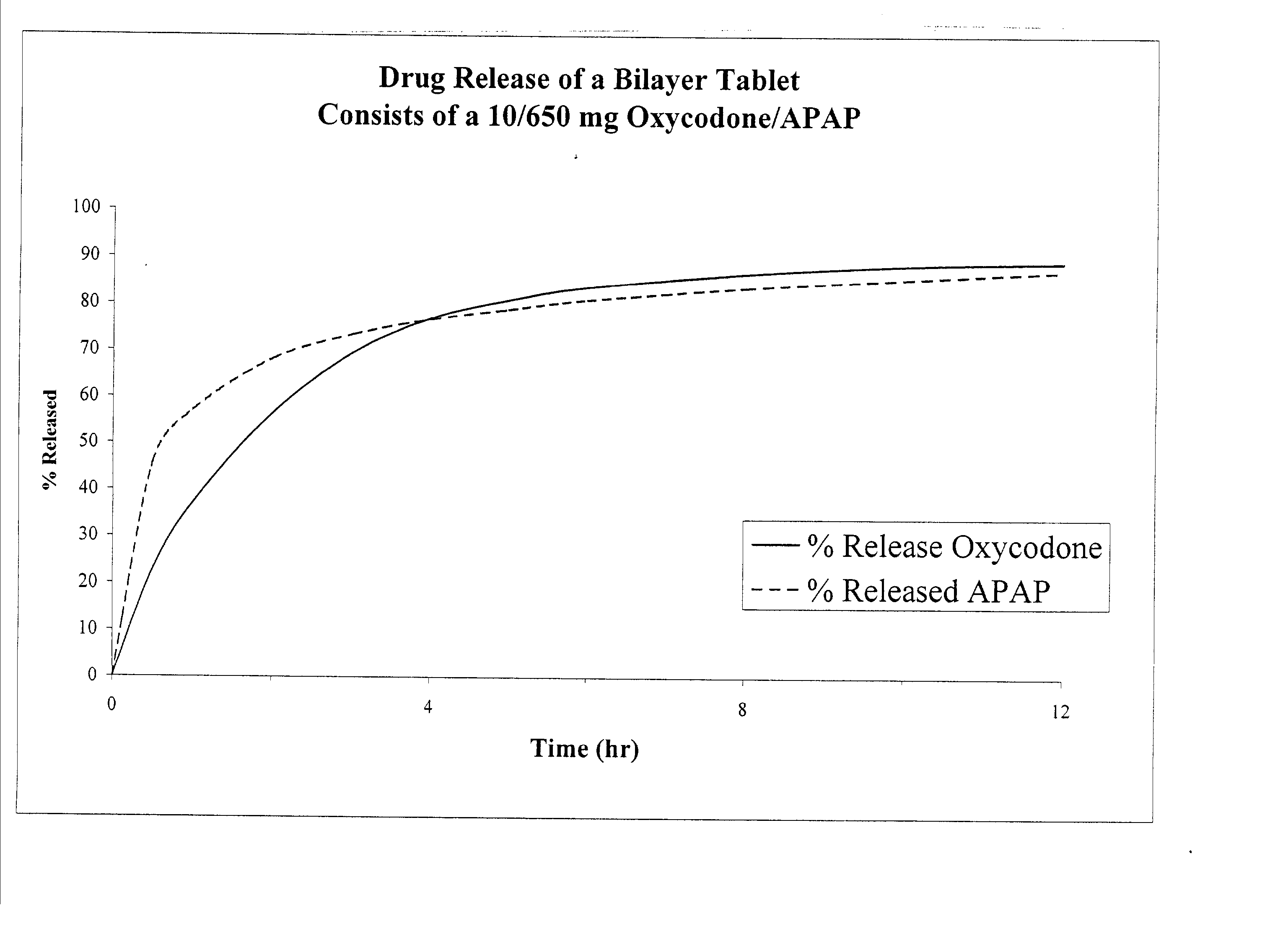
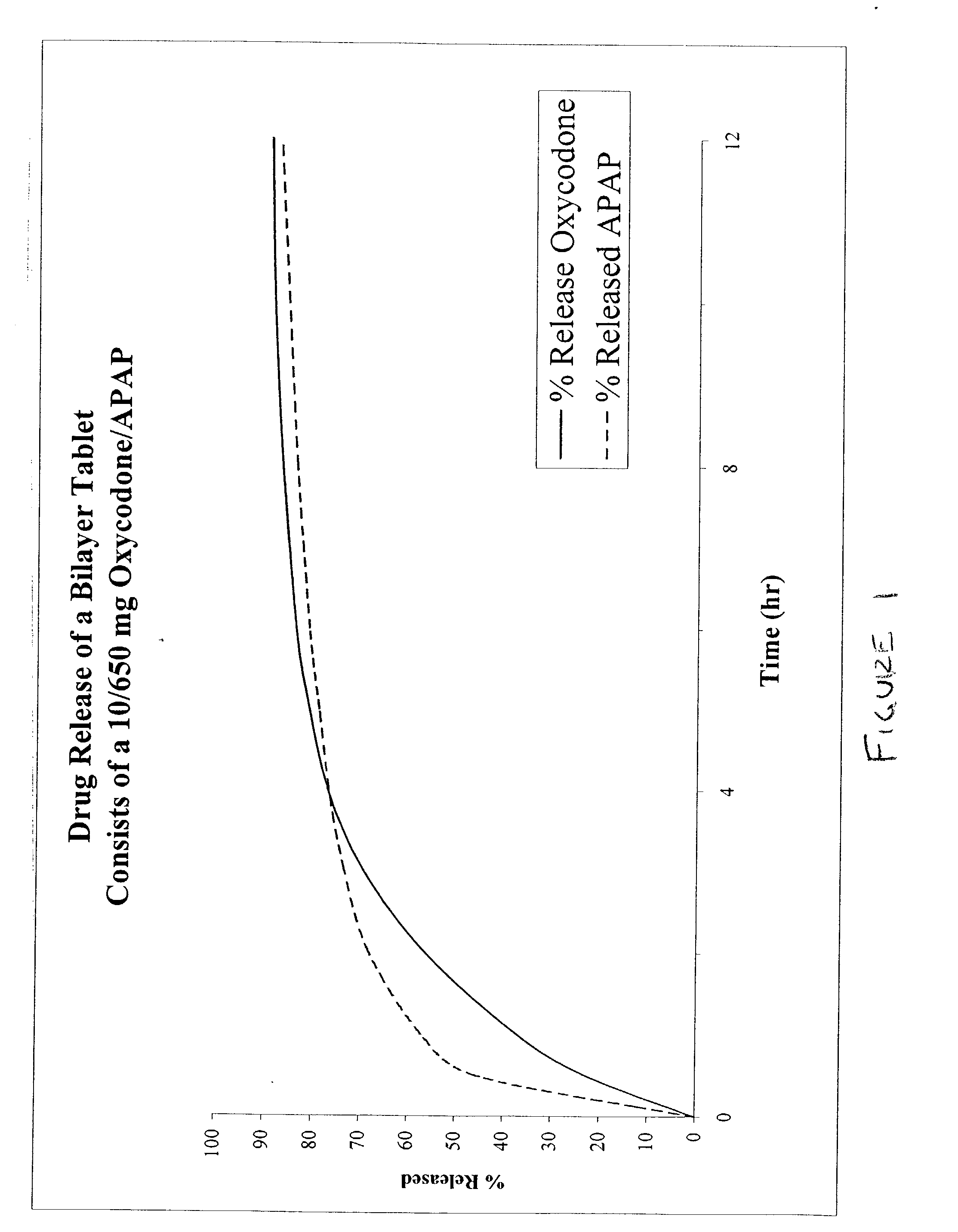
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
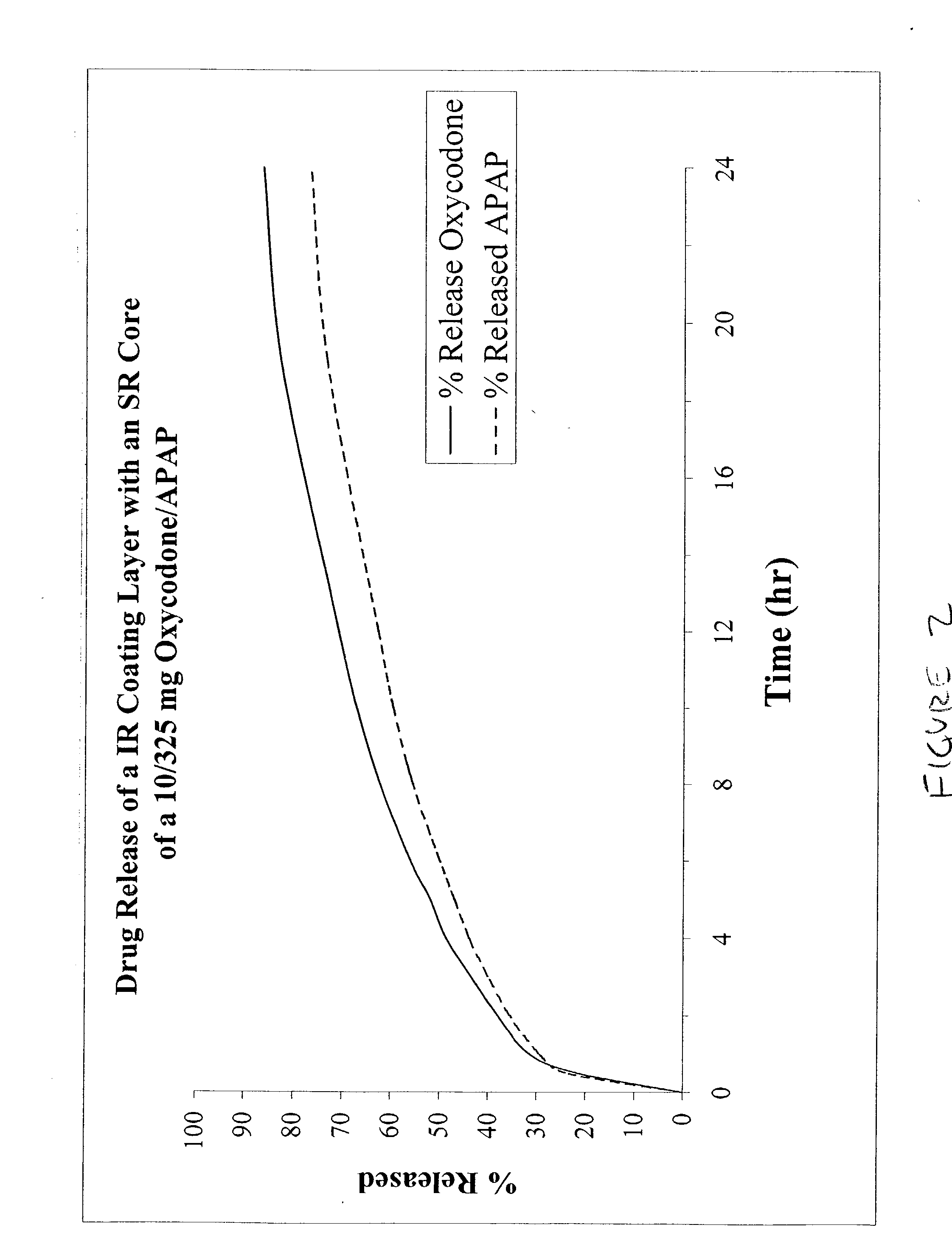
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Anti-TIM-3 antibody
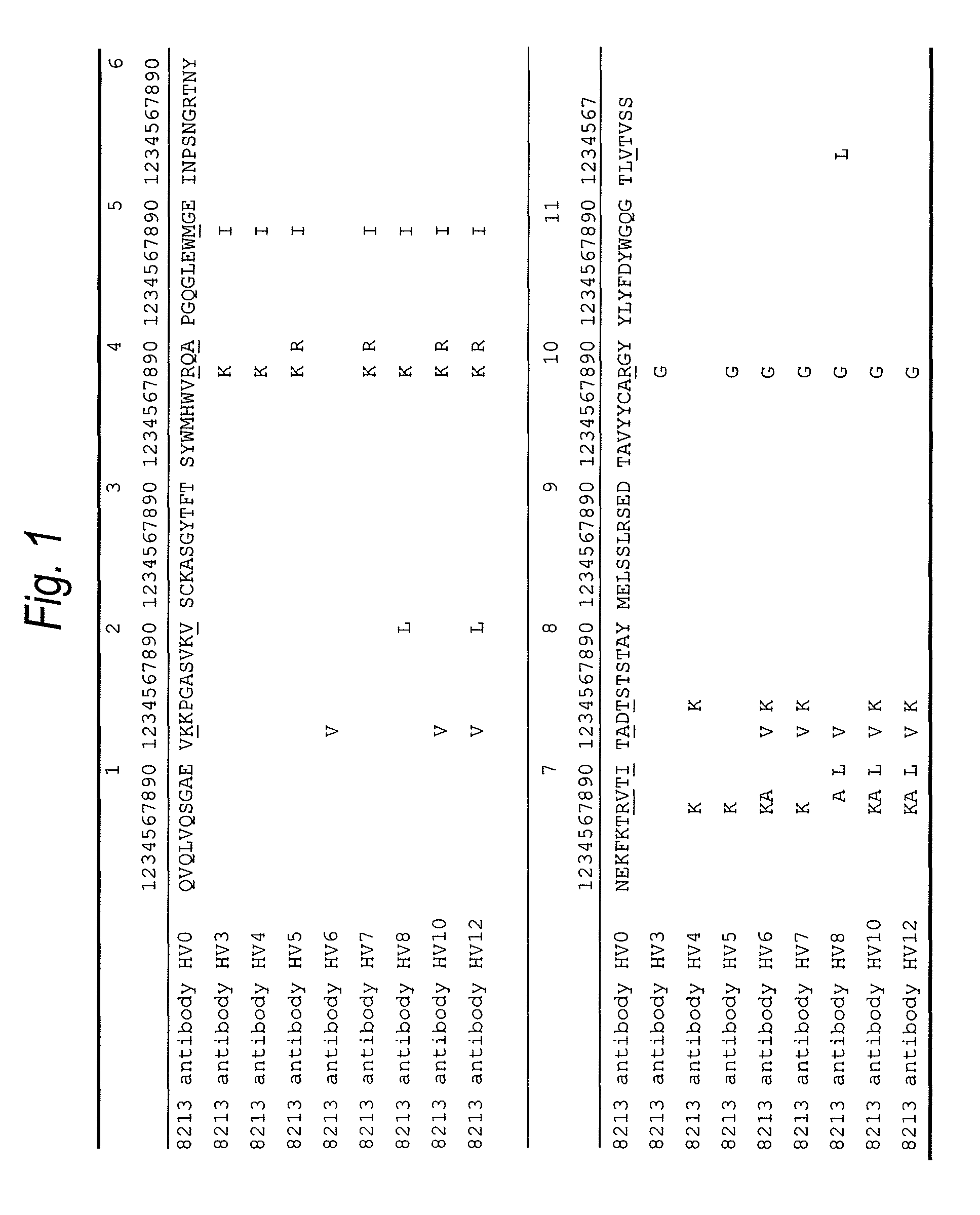
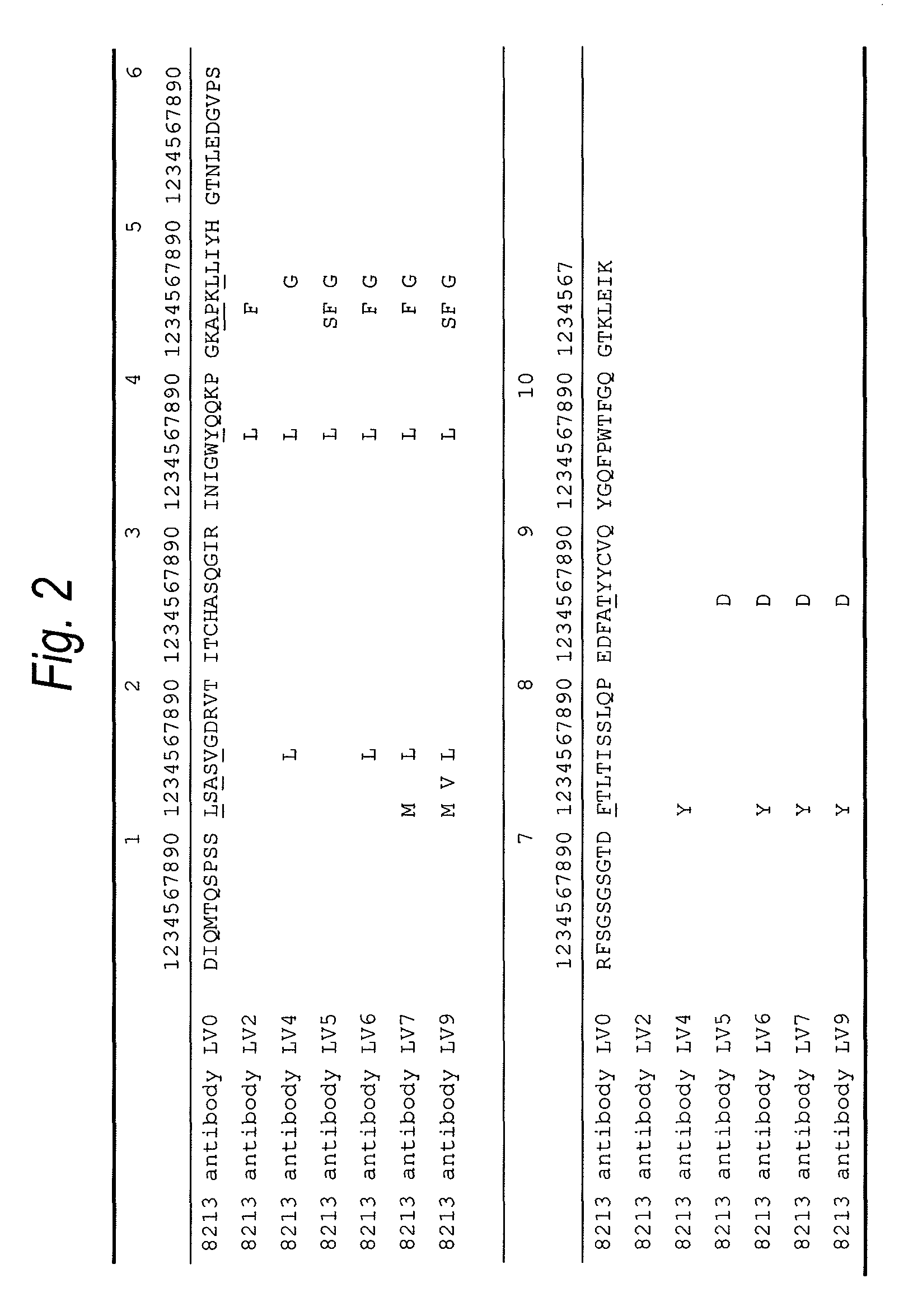
The present invention provides an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYUSHU UNIV +1
Binding agent for solid block functional material
InactiveUS6258765B1Fit tightlyEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsBULK ACTIVE INGREDIENTActive ingredient
A solid functional material comprises a functional agent such as a cleaning composition, a sanitizing agent, where a rinse agent, etc. in a solid block format. The solid block is formed by a binding agent that forms the active ingredients into a solid block. The binding agent comprises a phosphonate or amino acetate sequestrant, a carbonate salt and water in an E-Form hydrate. These materials at a specific mole ratio form a novel binding agent that can form functional materials into a solid matrix form.
Owner:ECOLAB USA INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
Photobleach speckle and laundry detergent compositions containing it
InactiveUS20030087790A1Little and no stainingLittle or no stainingOrganic detergent compounding agentsDetergent dyesParticulatesBleach
A speckle composition for use in particulate laundry detergent compositions comprising a porous granular carrier, and at least 0.01 wt % photobleach, preferably at least 0.05 wt %, more preferably at least 0.1 wt %, based on the active ingredient the composition being layered with a finely divided high carrying capacity particulate material and / or a water-soluble material. The most preferred photobleach is a blend of Zn and Al sulphonated phthalocyanine.
Owner:HENKEL IP & HOLDING GMBH
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
Abuse-proofed dosage form
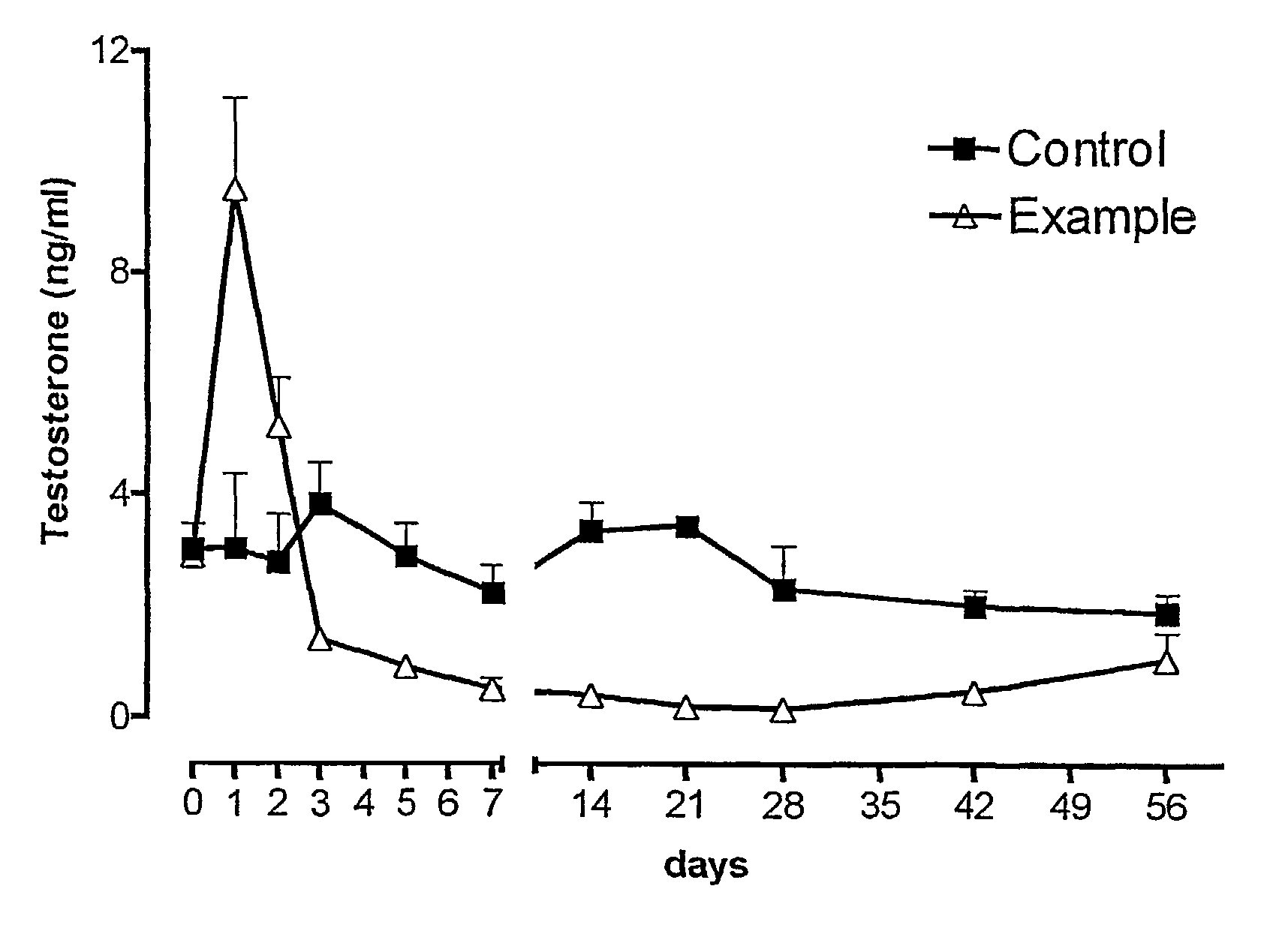
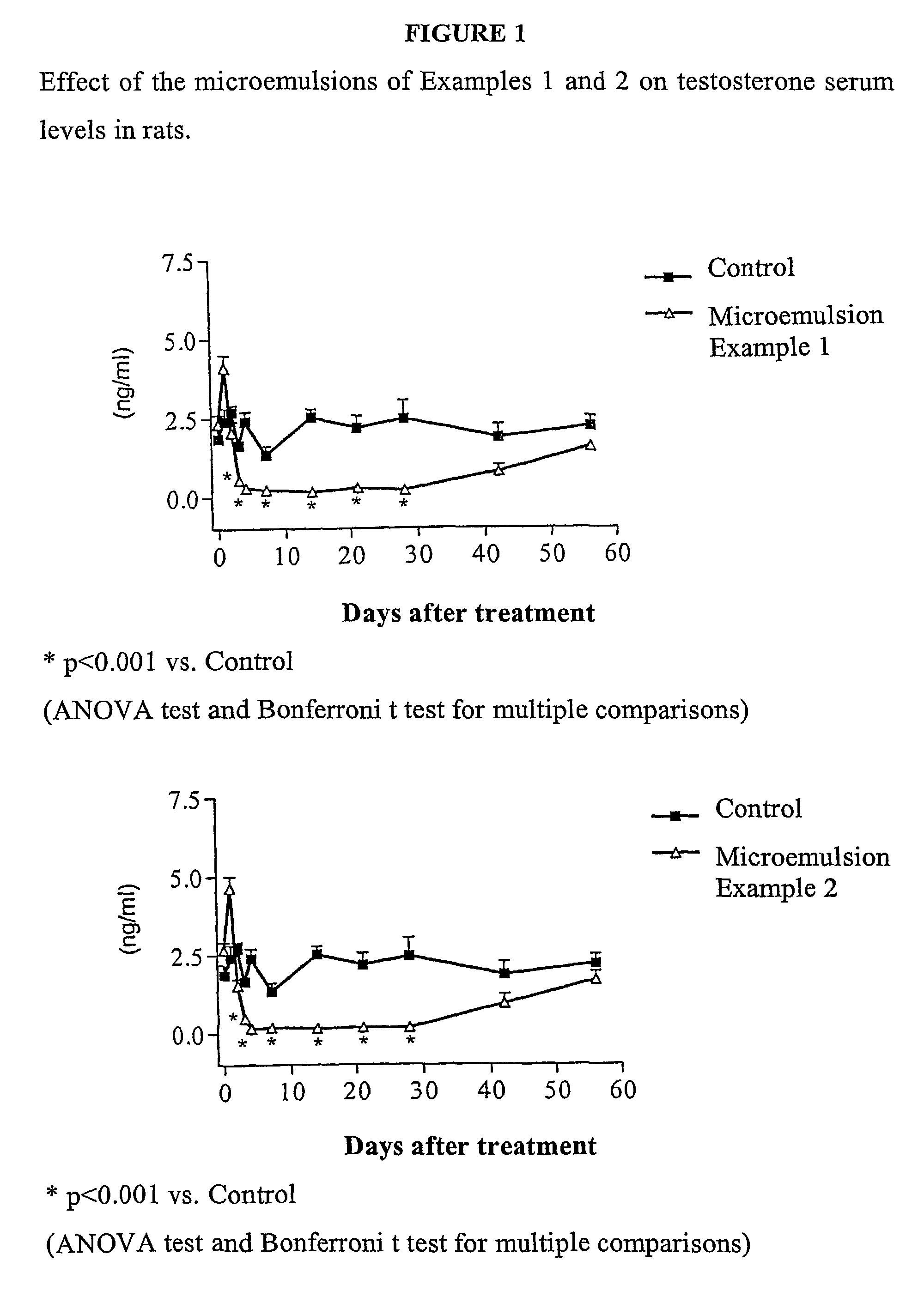
ActiveUS8114383B2Pulverisation of the dosage form is considerably more difficultComplicating or preventing the subsequent abuseOrganic active ingredientsPowder deliveryBreaking strengthPhysiology
The present invention relates to an abuse-proofed, thermoformed dosage form containing, in addition to one or more active ingredients with abuse potential optionally together with physiologically acceptable auxiliary substances, at least one synthetic or natural polymer with a breaking strength of at least 500 N and to a process for the production thereof.
Owner:GRUNENTHAL GMBH
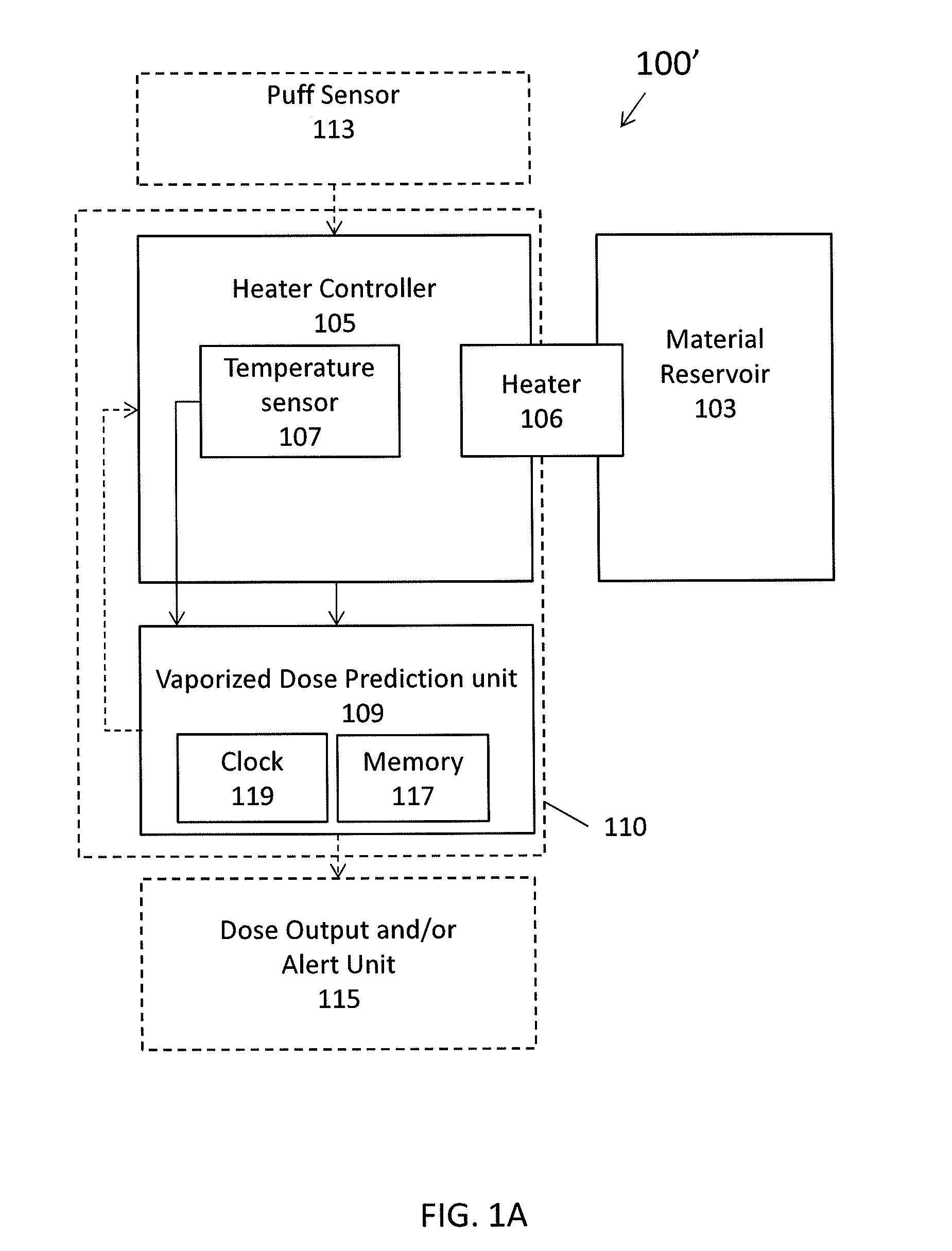
Calibrated dose control
Methods and vaporizer apparatuses that estimate, measure and / or predict the amount of vapor and / or material (including active ingredients) released by the vaporizer apparatus. In particular, described herein are electronic vaporizers and methods of using them that determine a dose / amount of vapor and / or a material in the vapor based primarily or exclusively on the electrical and thermal properties, e.g., power or energy applied to the vaporizing element (e.g., heating coil) and the temperature of the material immediately before and as it is vaporized. Dose information may be used to control operation of the device and / or reported to the user.
Owner:JLI NAT SETTLEMENT TRUST
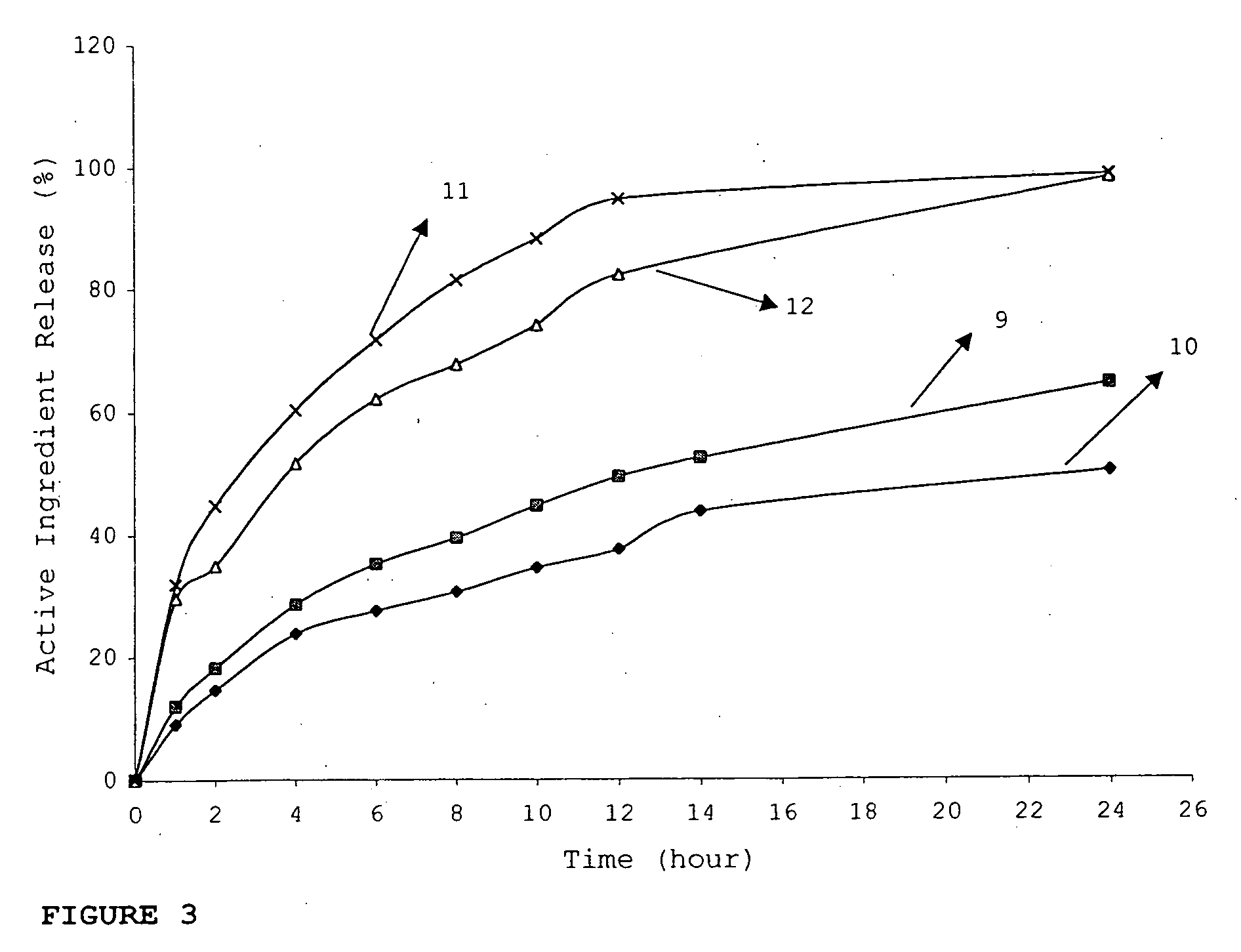
Multiparticulate modified release composition
InactiveUS20060240105A1Reduce dosing frequencyReduce frequencyBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
The invention relates to a multiparticulate modified release composition that, upon administration to a patient, delivers at least one active ingredient in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of active ingredient containing particles and the at least one subsequent component comprising a second population of active ingredient containing particles wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition.
Owner:ALKERMES PHARMA IRELAND LTD
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
InactiveUS7157099B2Easy to prepareOrganic active ingredientsPeptide/protein ingredientsParenteral nutritionBULK ACTIVE INGREDIENT
Owner:ITALFARMACO SPA
Method of treating inflammatory intestinal diseases containing as the ingredient IL-6 receptors antibodies
A preventive or therapeutic agent for treating bowel disease, including Crohn's disease and ulcerative colitis, where the agent has as an active ingredient an antibody directed against IL-6 receptor which is an interleukin-6 antagonist.
Owner:CHUGAI PHARMA CO LTD +1
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myelodysplastic syndromes
InactiveUS20040220144A1Extension of timeBiocidePeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Portable Pen Sized Electric Herb Vaporizer with Ceramic Heating Chamber
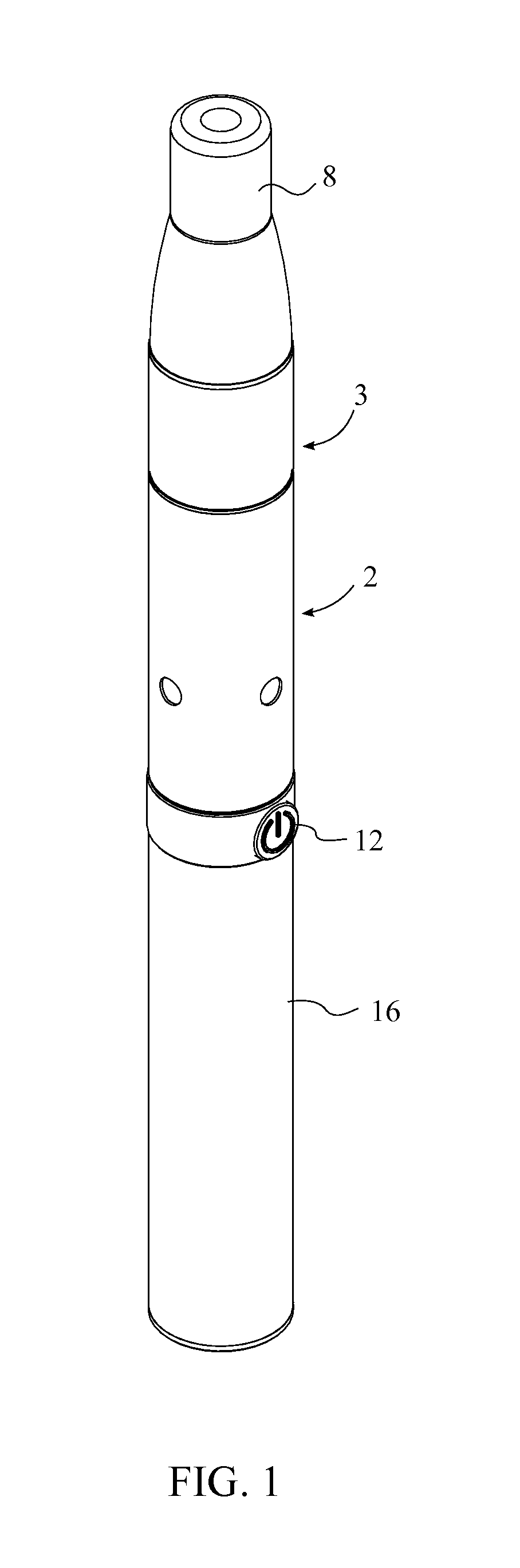
A portable pen sized electric herb vaporizer with ceramic heating chamber is an elongated and cylindrical device used to vaporize the active ingredient of or burn herbs, having a battery, a heating compartment, a chamber connector, and a mouthpiece. Two threaded screw interfaces attach the battery to the heating compartment and the heating compartment to the chamber connector. The mouthpiece holds a ceramic filter and is inserted into the chamber connector. The heating compartment has a ceramic heating chamber within which herbs are placed, and a heating coil connected to the battery within the ceramic heating chamber heats the herbs. The user presses an activator button and inhales through the mouthpiece, drawing heated air over the herbs, through the chamber connector and into the user's lungs. The chamber connector has a disk filter to catch ash and a spring that presses on the herbs for efficient vaporization.
Owner:ATMOS TECH
Composition comprising an agent providing a signal, an implant material and a drug
InactiveUS20060177379A1Facilitated releaseAvoid impairment of material compositionMaterial nanotechnologySurgeryTreatment effectBULK ACTIVE INGREDIENT
The present invention relates to compositions or combinations of materials for non-degradable and degradable implantable medical devices with regard to the setup of their signal generating properties and control of their therapeutic effectiveness, as well as to a method for the control of degradation of degradable or partially degradable medical devices composed like this, based on their signal generation, and to a method for supervision of their therapeutic effectiveness and / or the release of therapeutically active ingredients from such devices.
Owner:CINVENTION AG
Dosage form exhibiting rapid disperse properties, methods of use and process for the manufacture of same
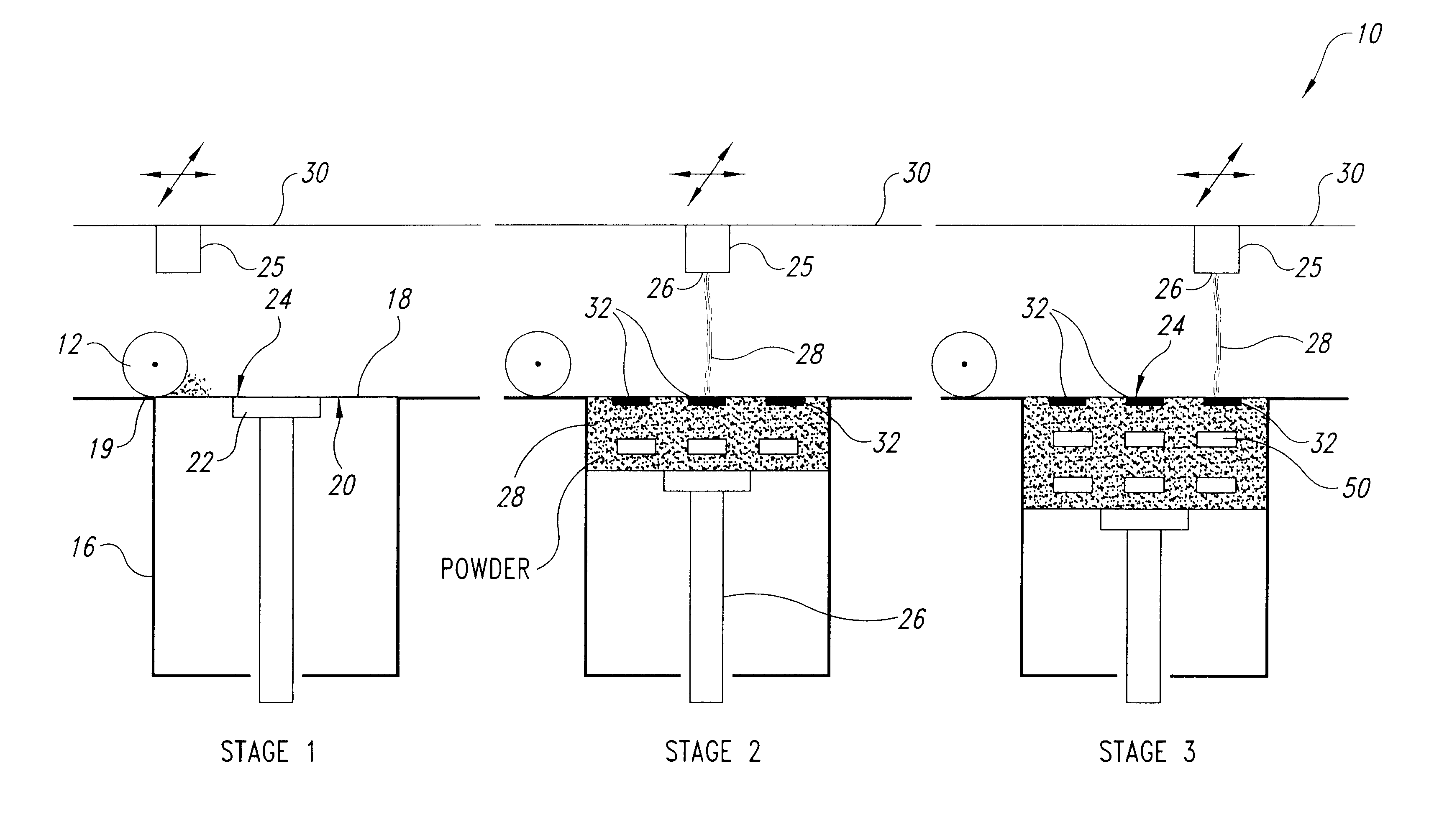
InactiveUS6471992B1Disperse fastHigh hardnessPowder deliveryAdditive manufacturing apparatusThree dimensional shapeBULK ACTIVE INGREDIENT
A rapidly dispersing dosage form is described, which releases its active ingredients within a period of less than about ninety seconds. These dosage forms exhibit a three-dimensional shape that is retained for adequate storage but is readily dispersed in the presence of excess moisture. Also disclosed are methods of administration of a medicament and a process for the preparation of rapidly dispersing dosage forms.
Owner:MASSACHUSETTS INST OF TECH +2
Novel drug delivery system
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
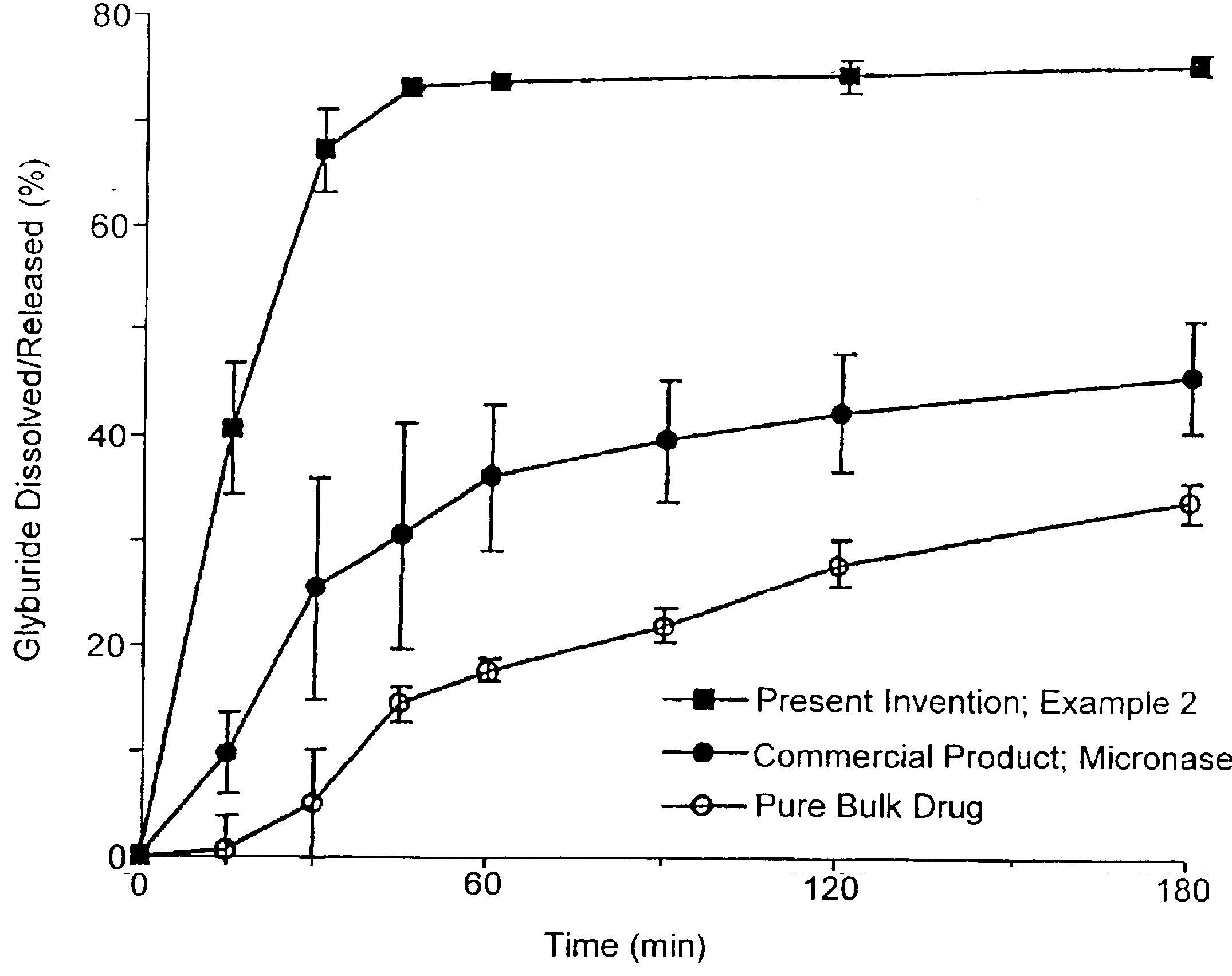
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Immunosuppression modulating compounds
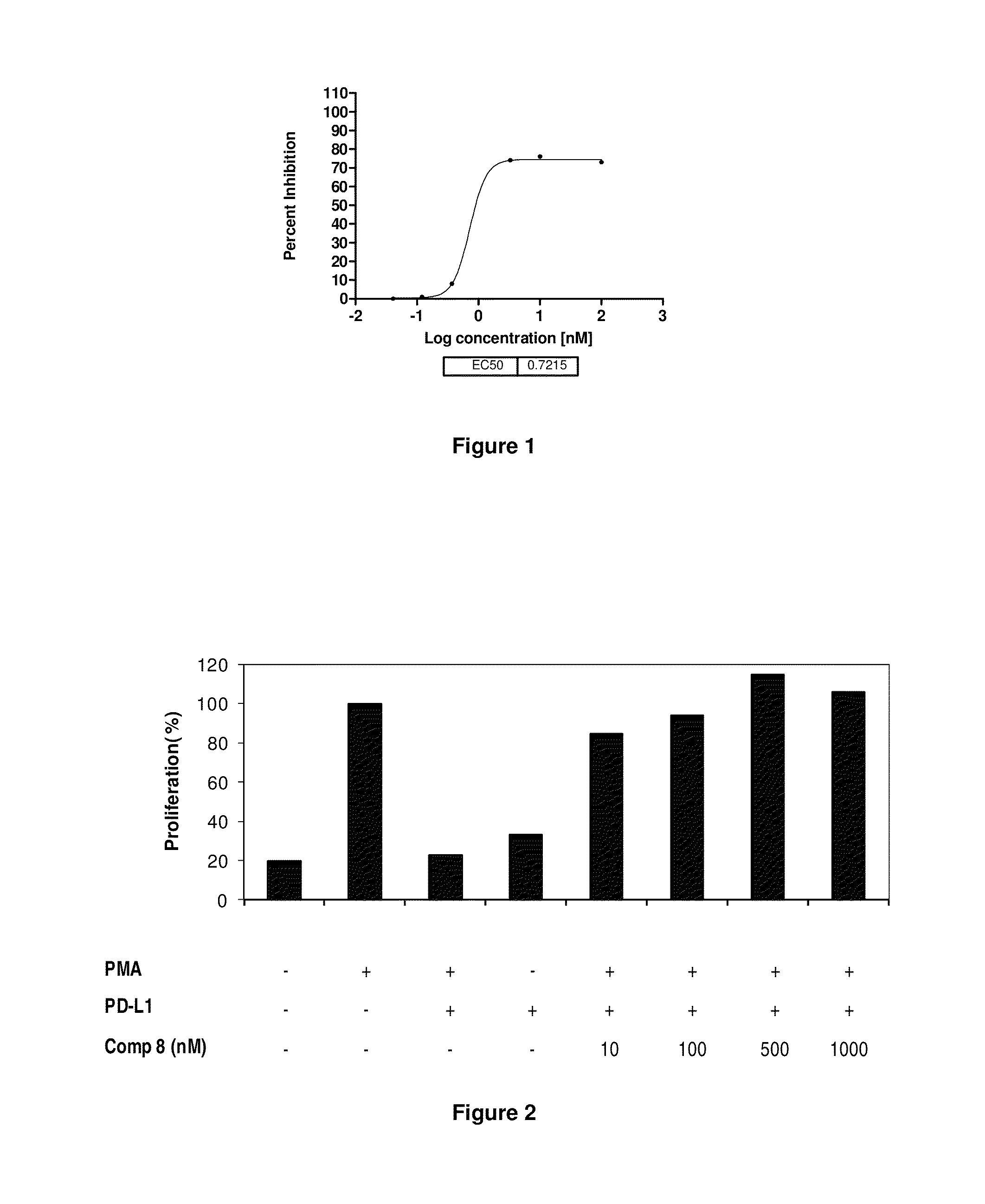
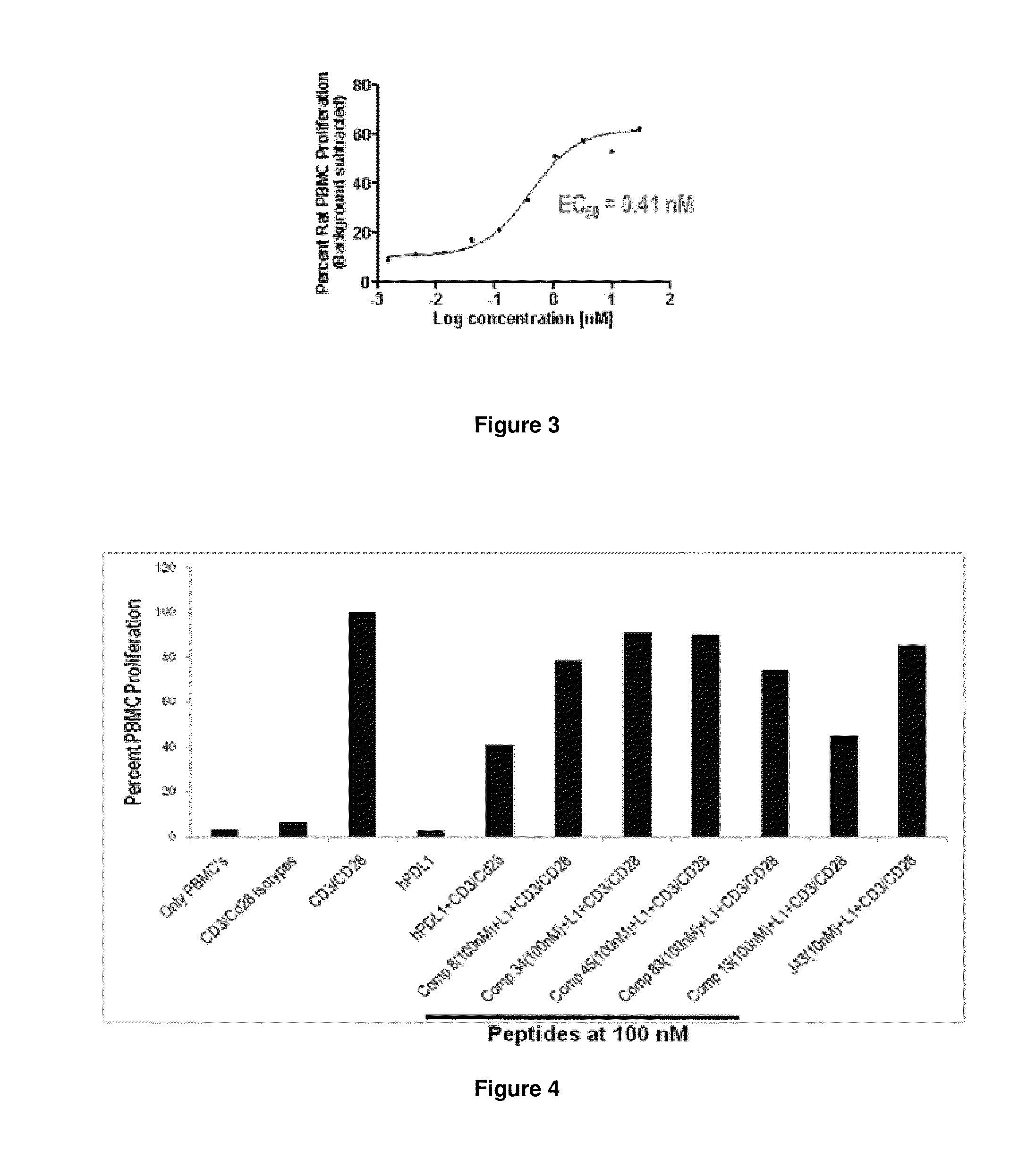
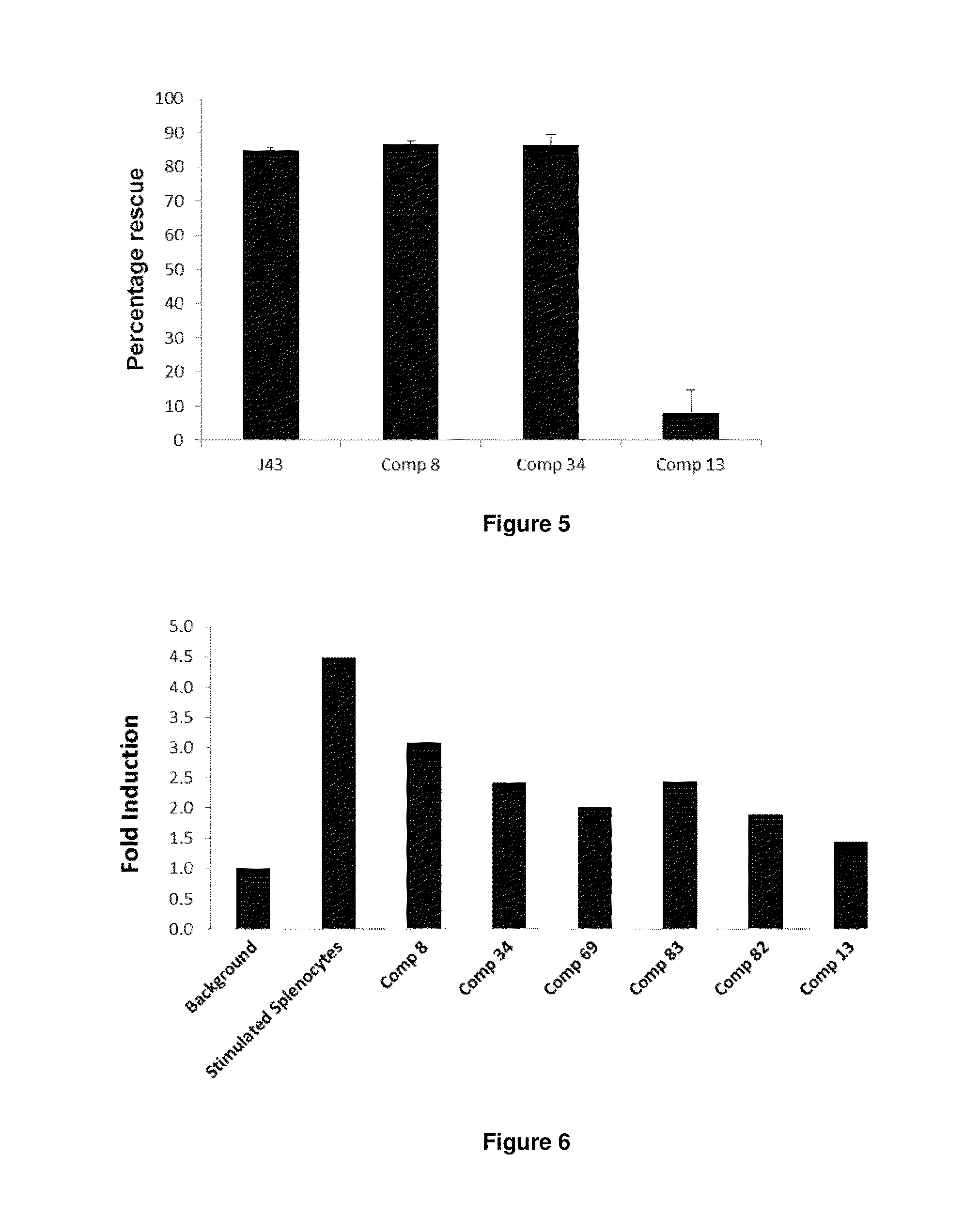
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
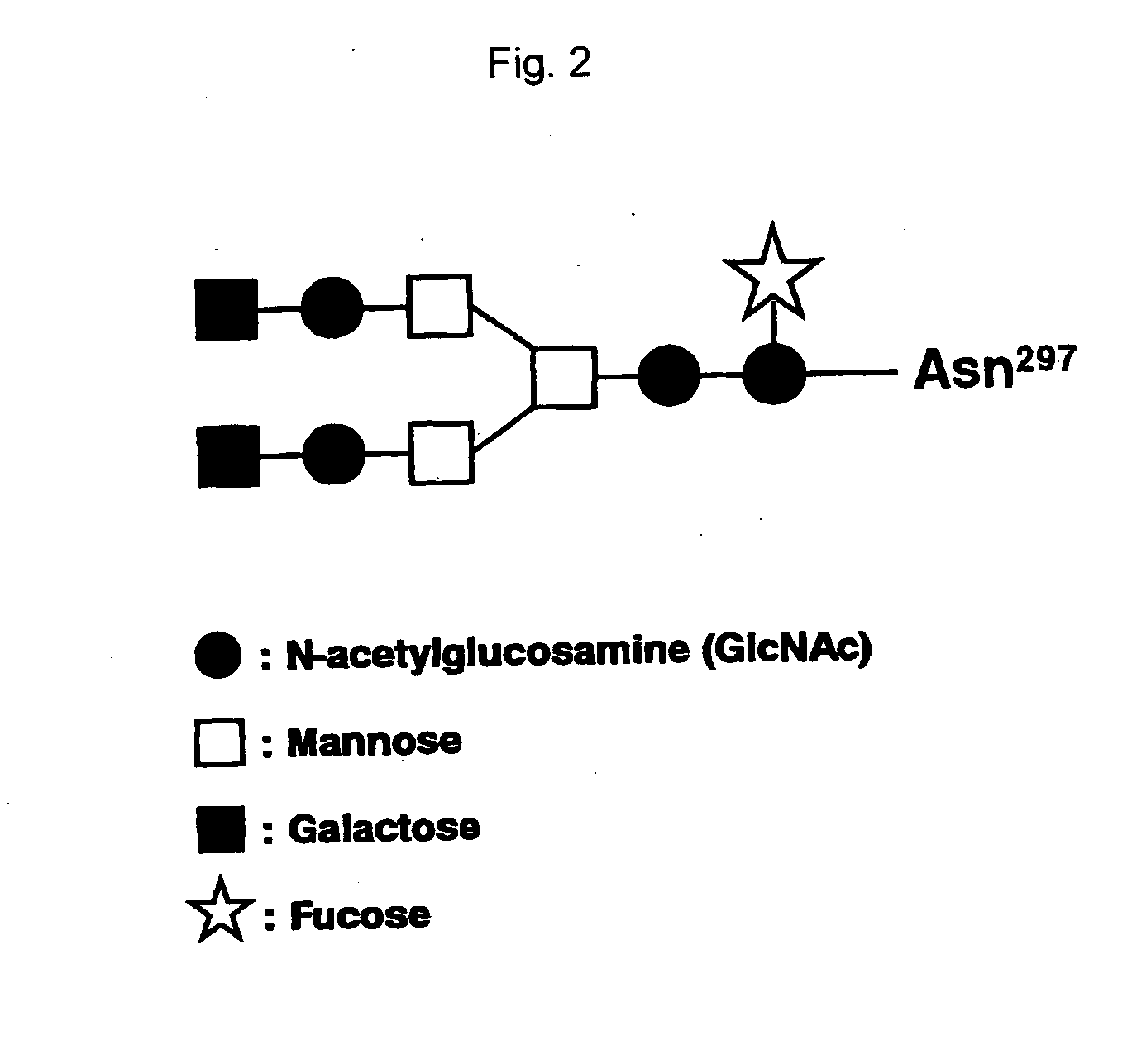
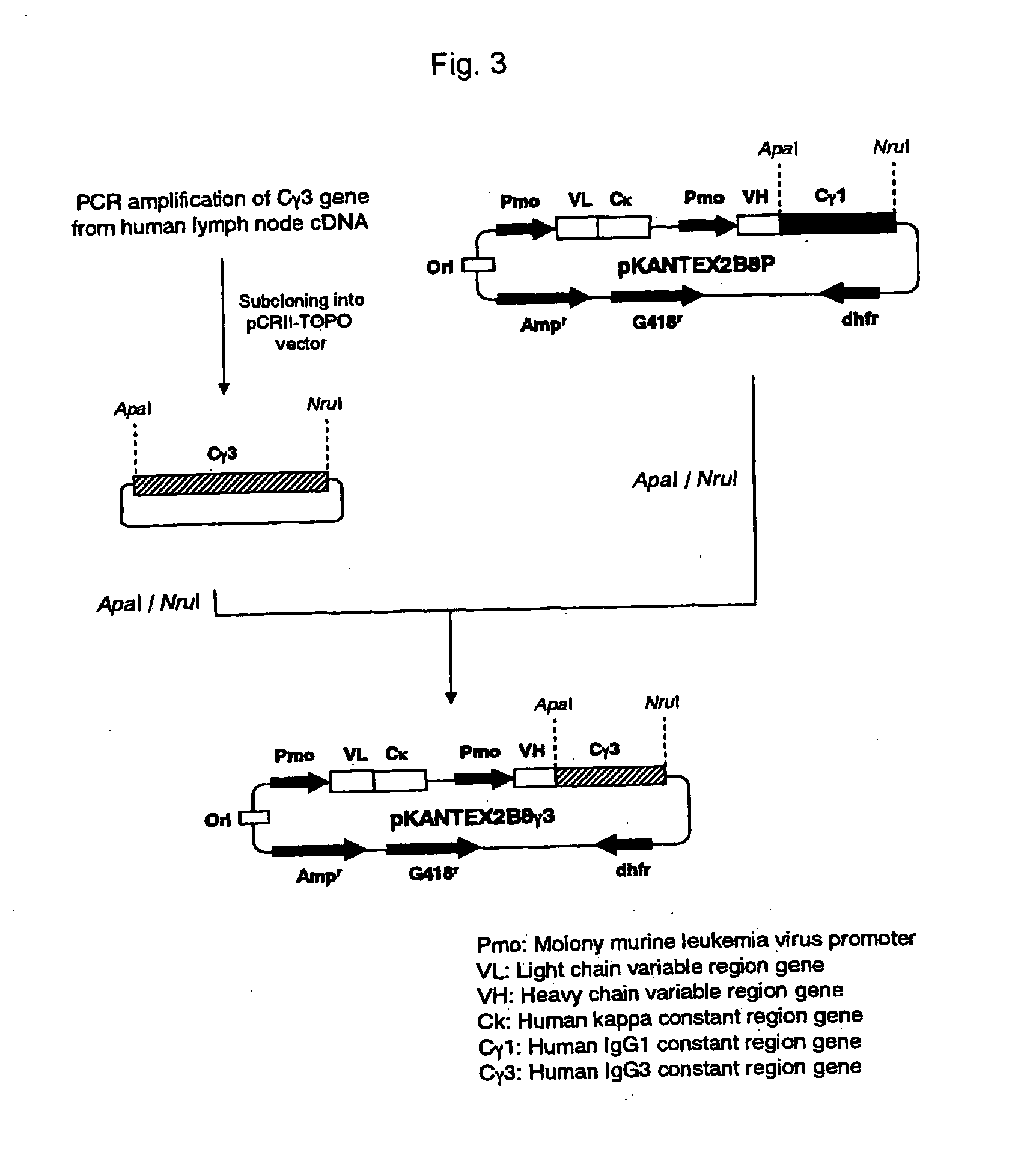
Recombinant antibody composition
ActiveUS20070148165A1Enhanced effector functionGood treatment effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsA-DNABULK ACTIVE INGREDIENT
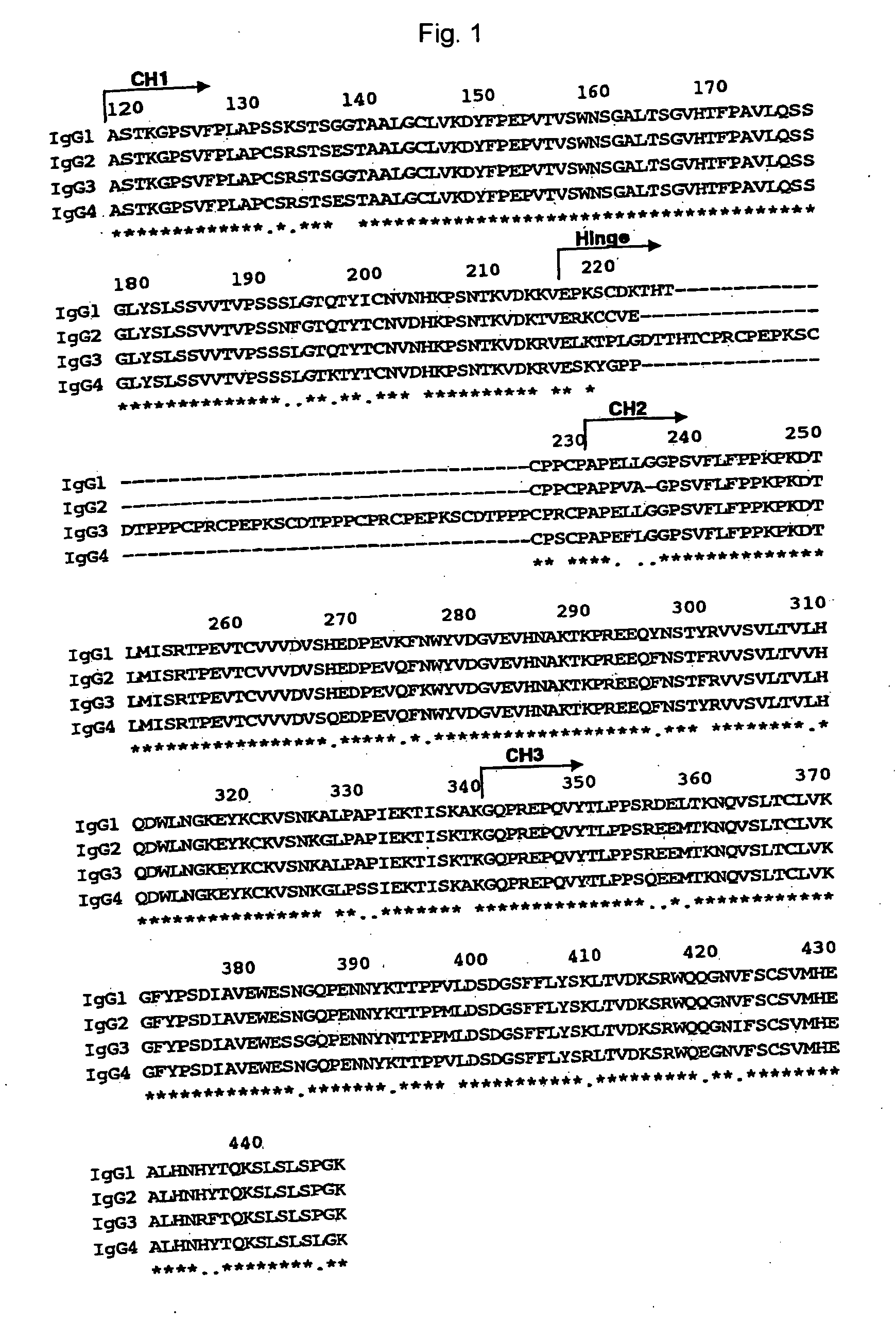
The present invention relates to a recombinant antibody composition having higher complement-dependent cytotoxic activity than a human IgG1 antibody and a human IgG3 antibody, wherein a polypeptide comprising a CH2 domain in the Fc region of a human IgG1 antibody is replaced by a polypeptide comprising an amino acid sequence which corresponds to the same position of a human IgG3 antibody indicated by the EU index as in Kabat, et al.; a DNA encoding the antibody molecule or a heavy chain constant region of the antibody molecule contained in the recombinant antibody composition; a transformant obtainable by introducing the recombinant vector into a host cell; a process for producing the recombinant antibody composition using the transformant; and a medicament comprising the recombinant antibody composition as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Methods and compositions for increasing the efficacy of biologically-active ingredients
The invention provides methods and compositions for modulating the sensitivity of cells to cytotoxic compounds and other active agents. In accordance with the invention, compositions are provided comprising combinations of ectophosphatase inhibitors and active agents. Active agents include antibiotics, fungicides, herbicides, insecticides, chemotherapeutic agents, and plant growth regulators. By increasing the efficacy of active agents, the invention allows use of compositions with lowered concentrations of active ingredients.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com