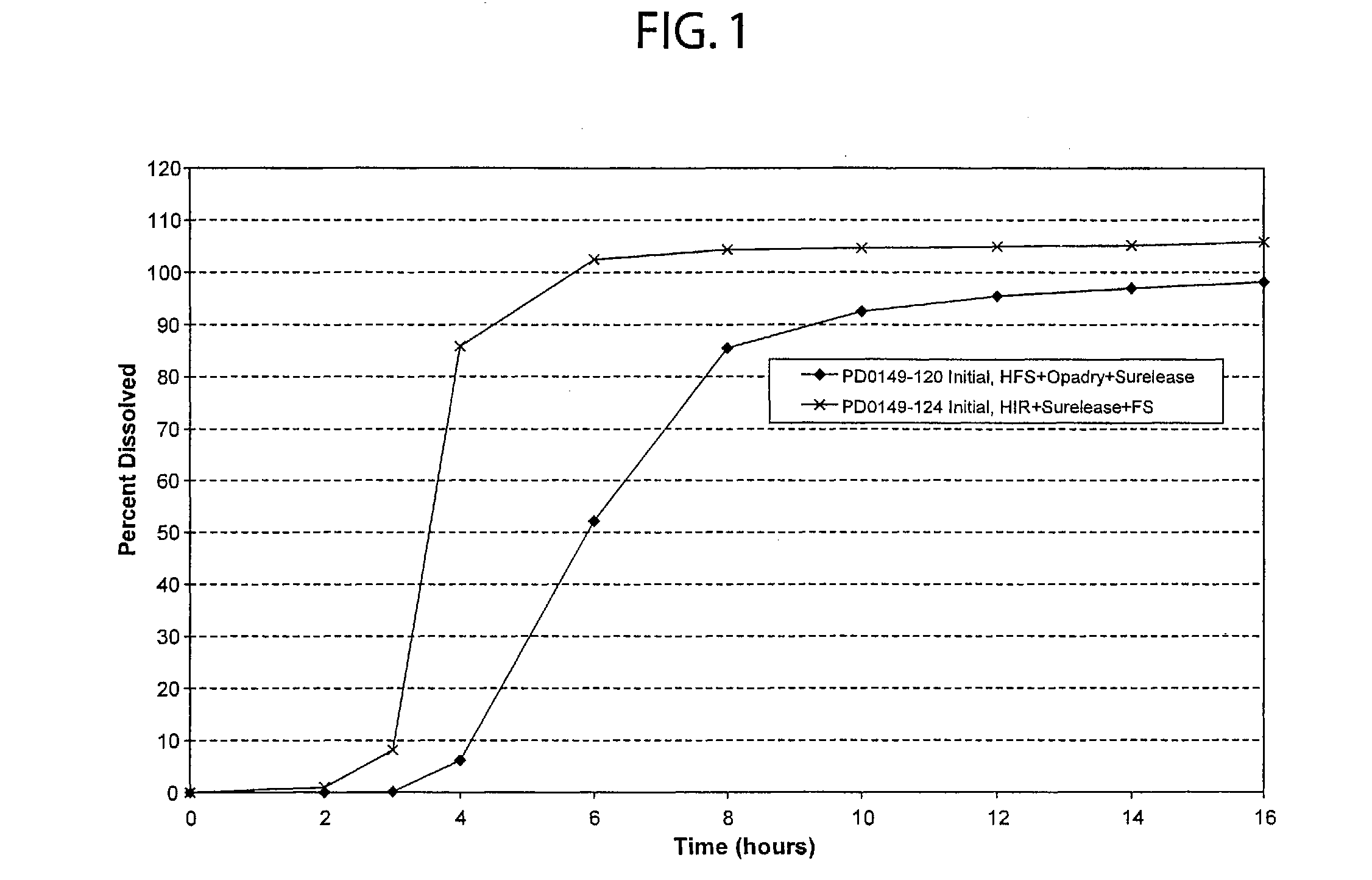
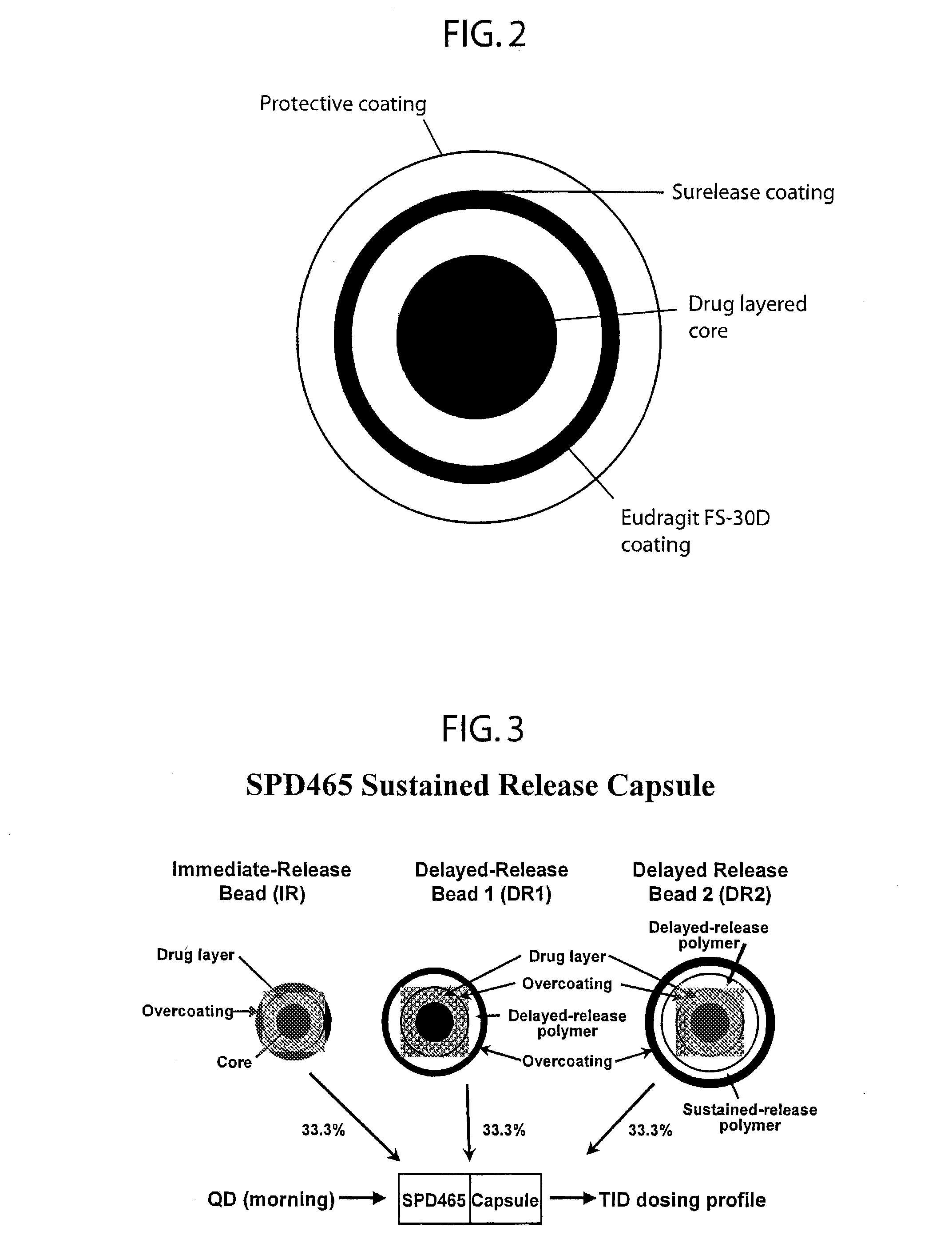
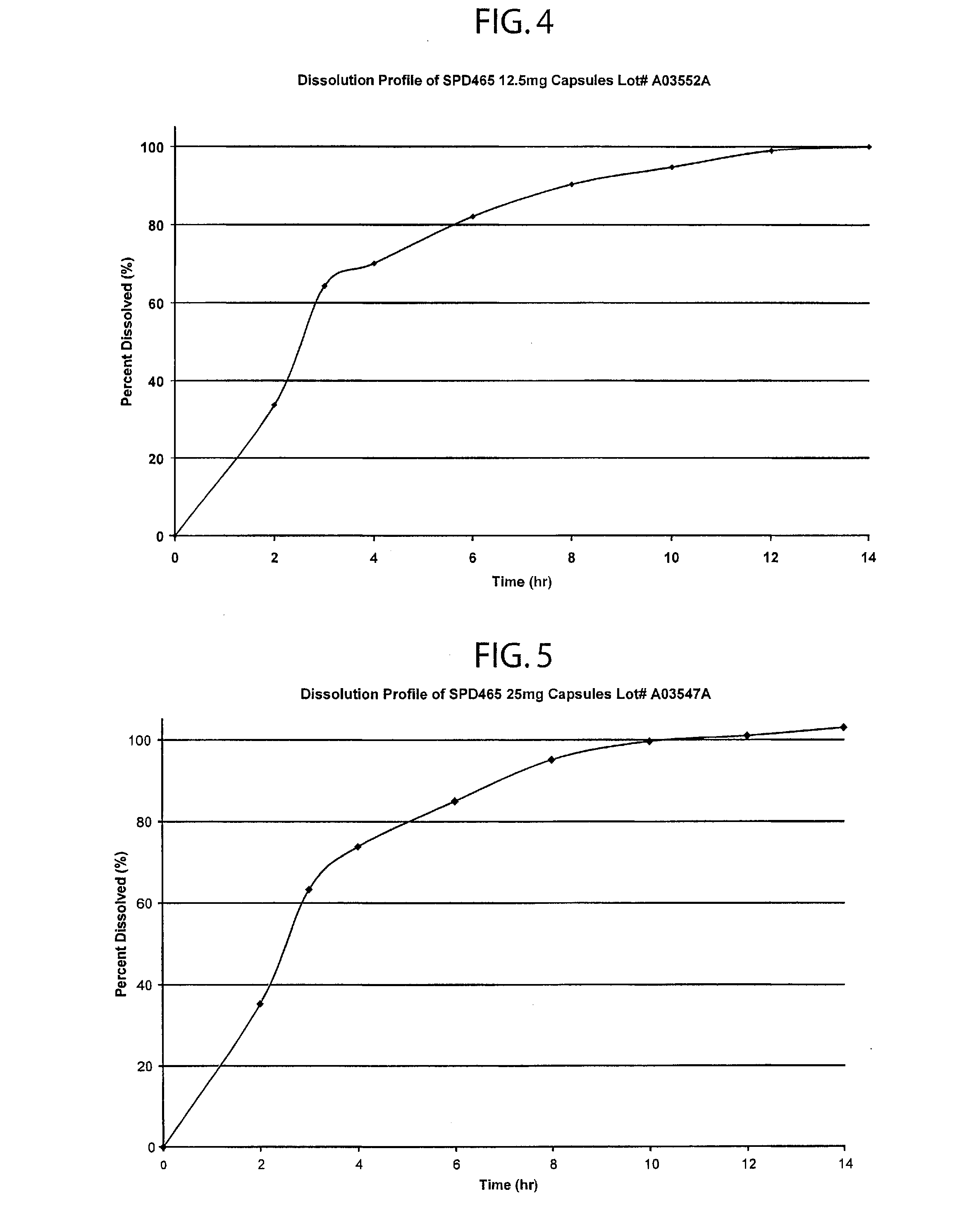
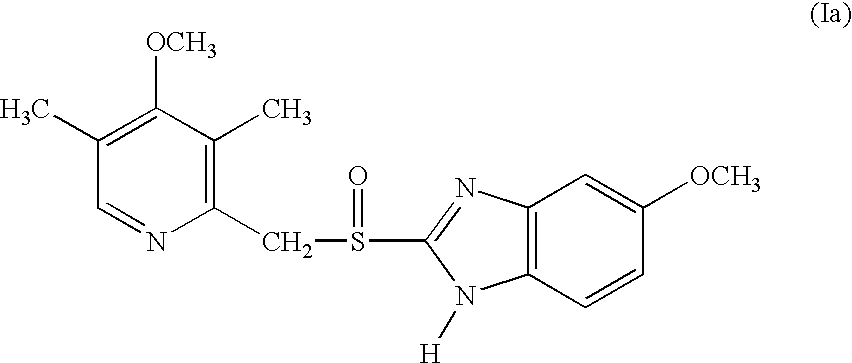
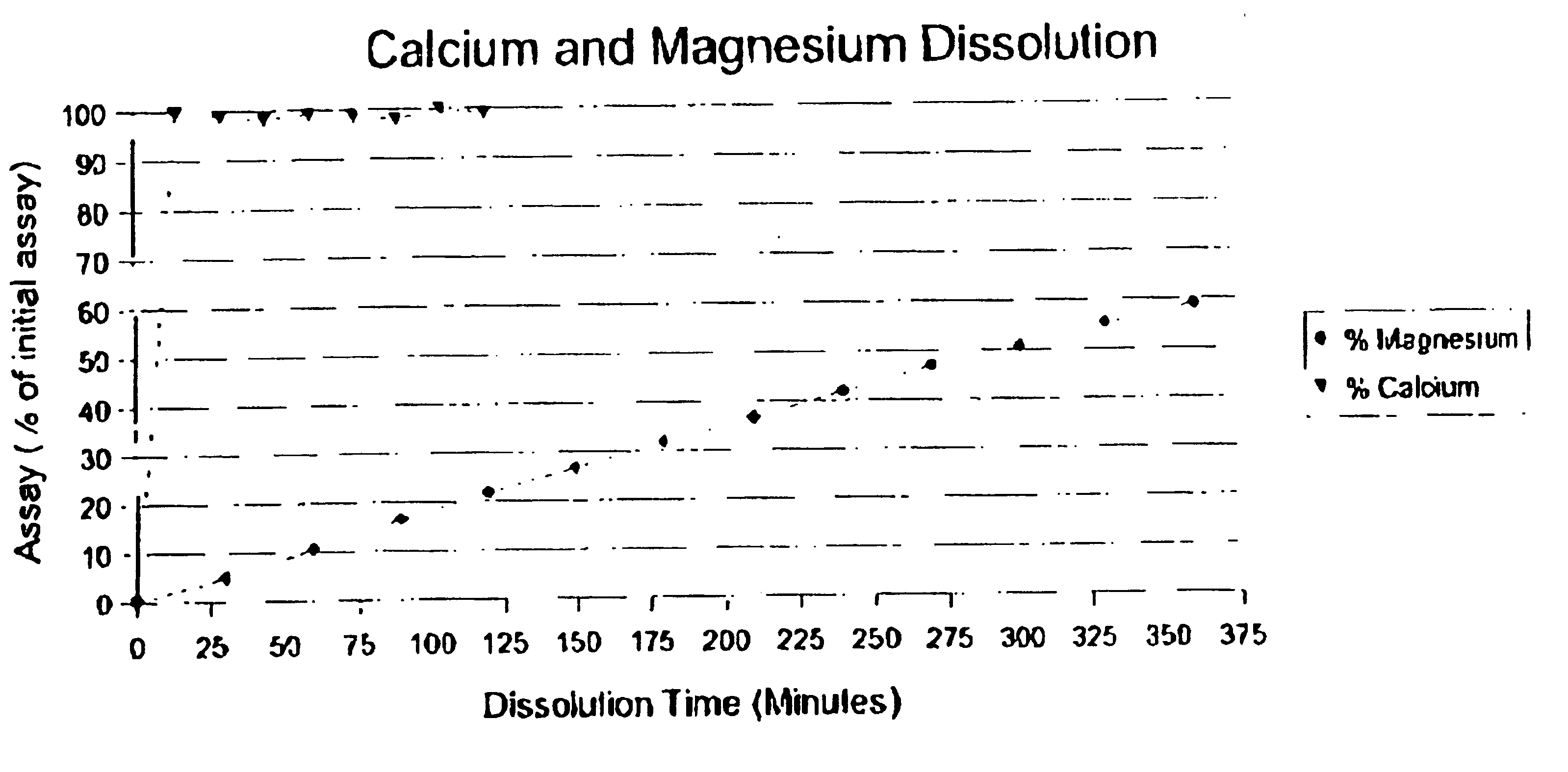
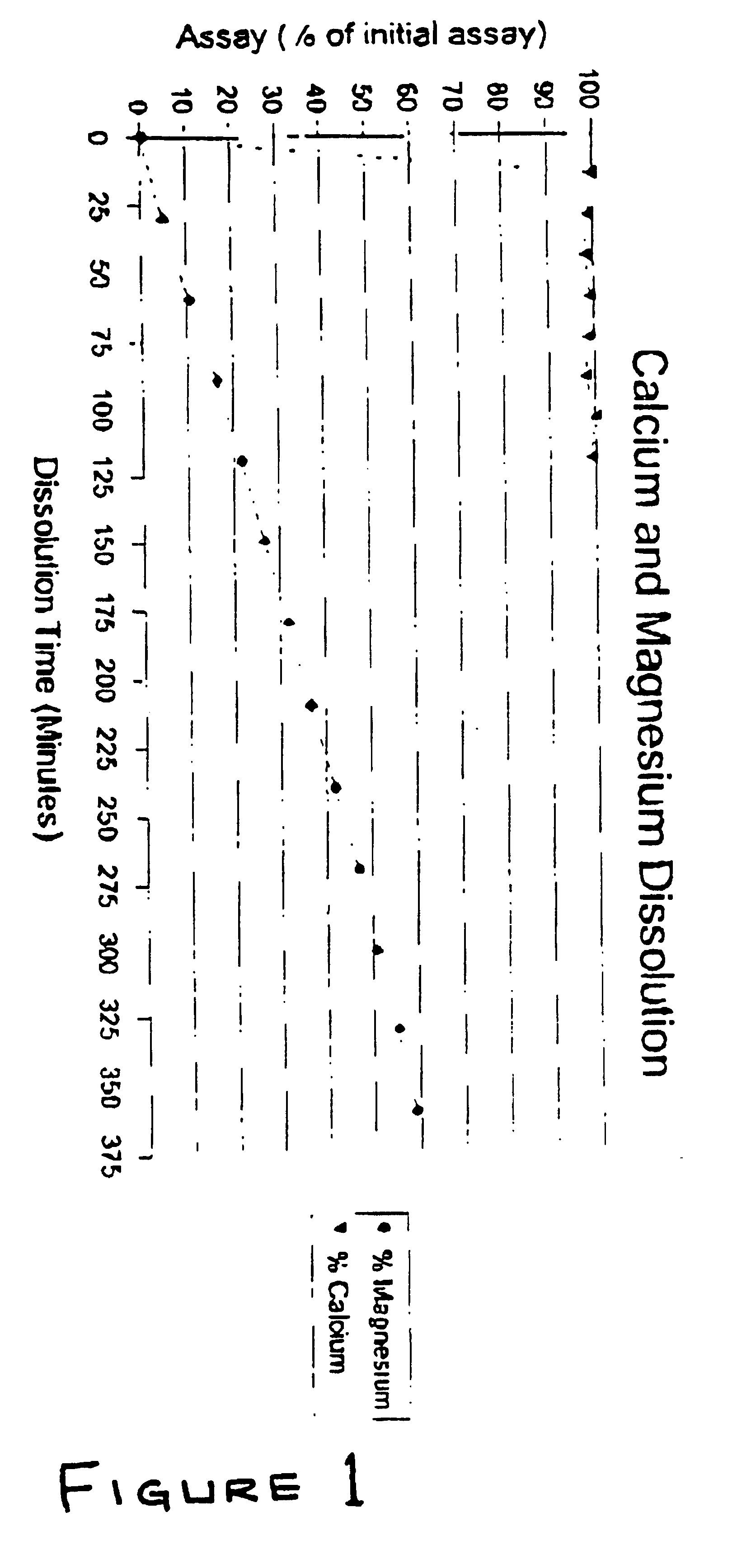
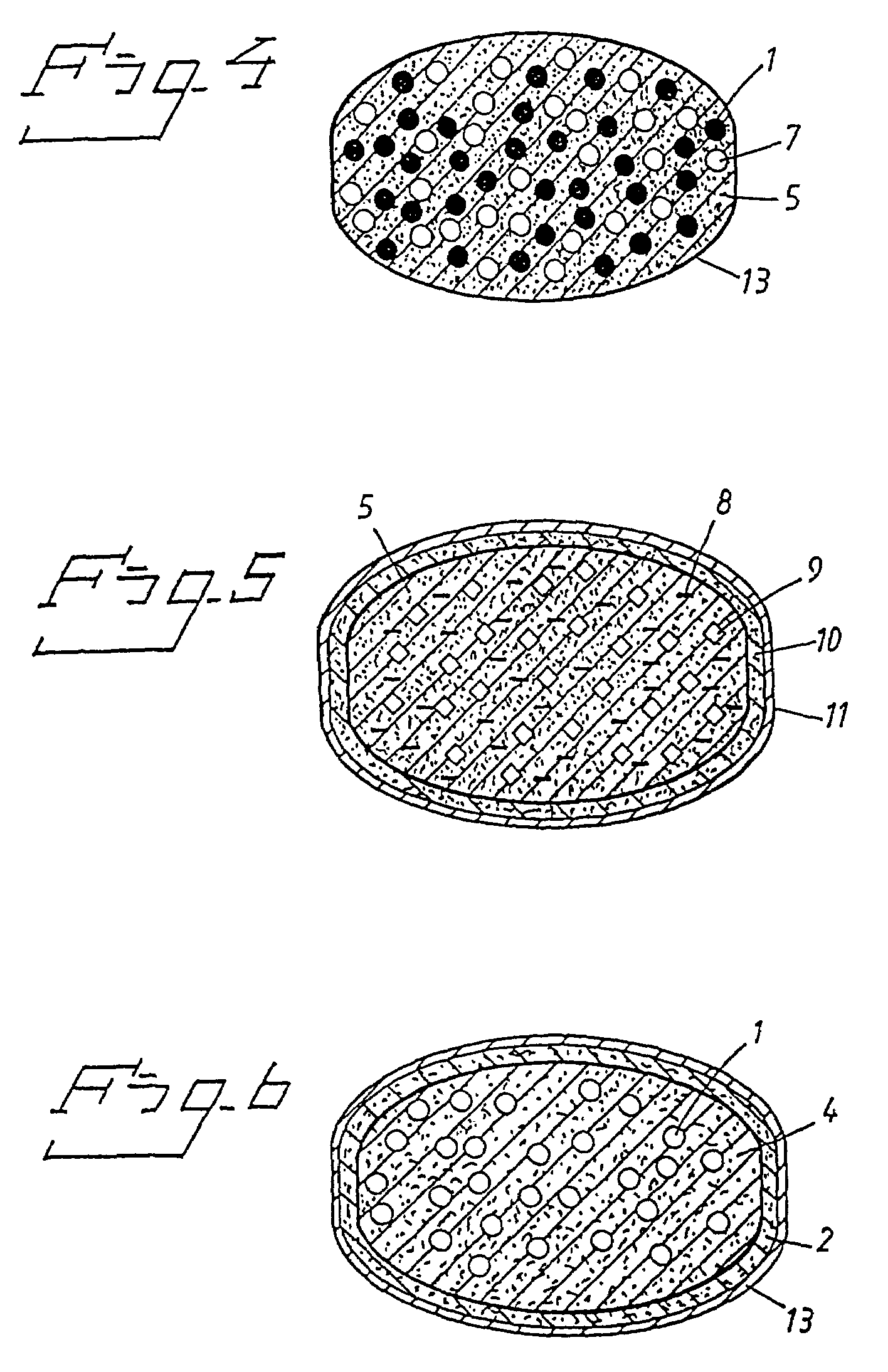
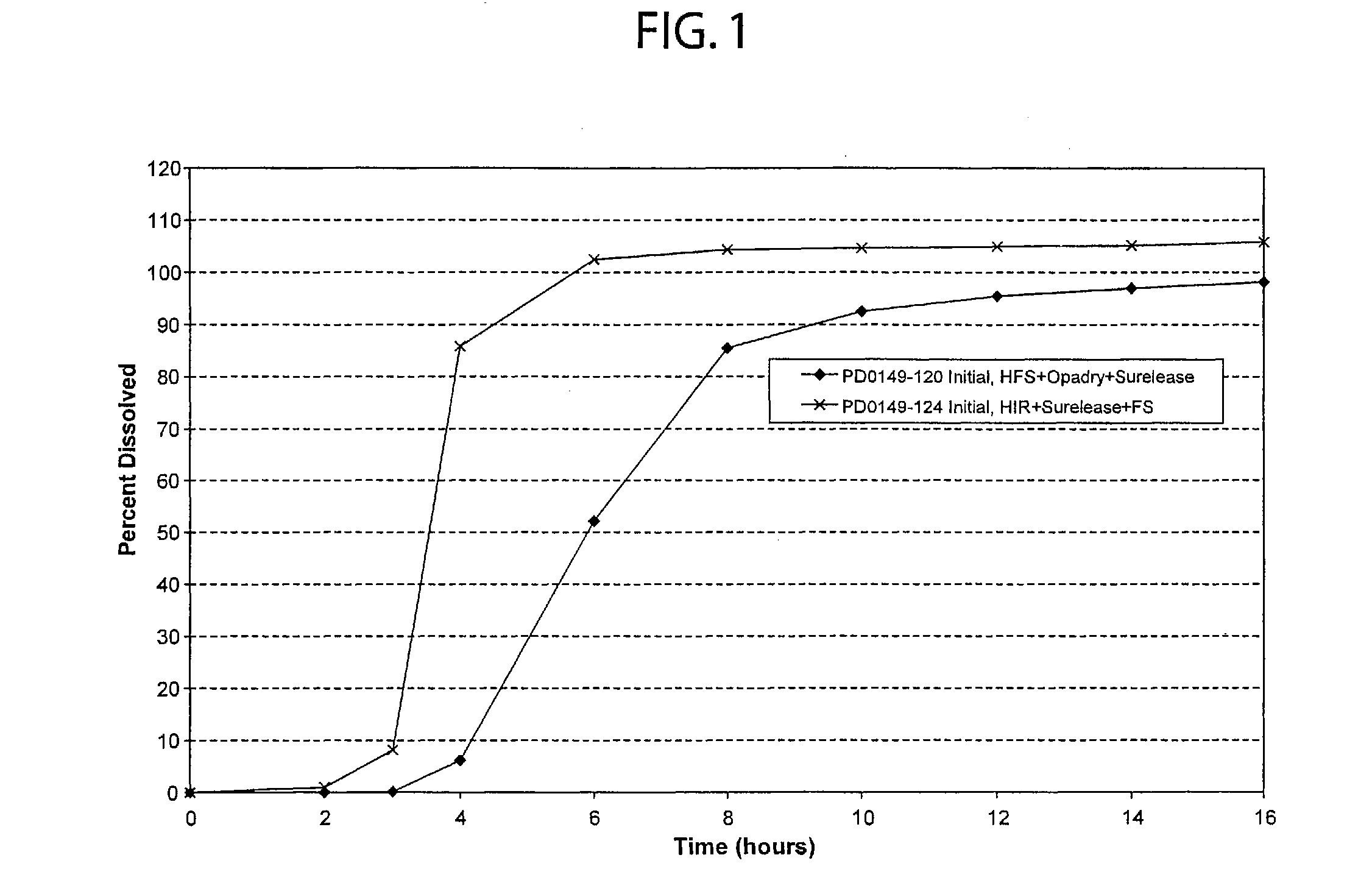
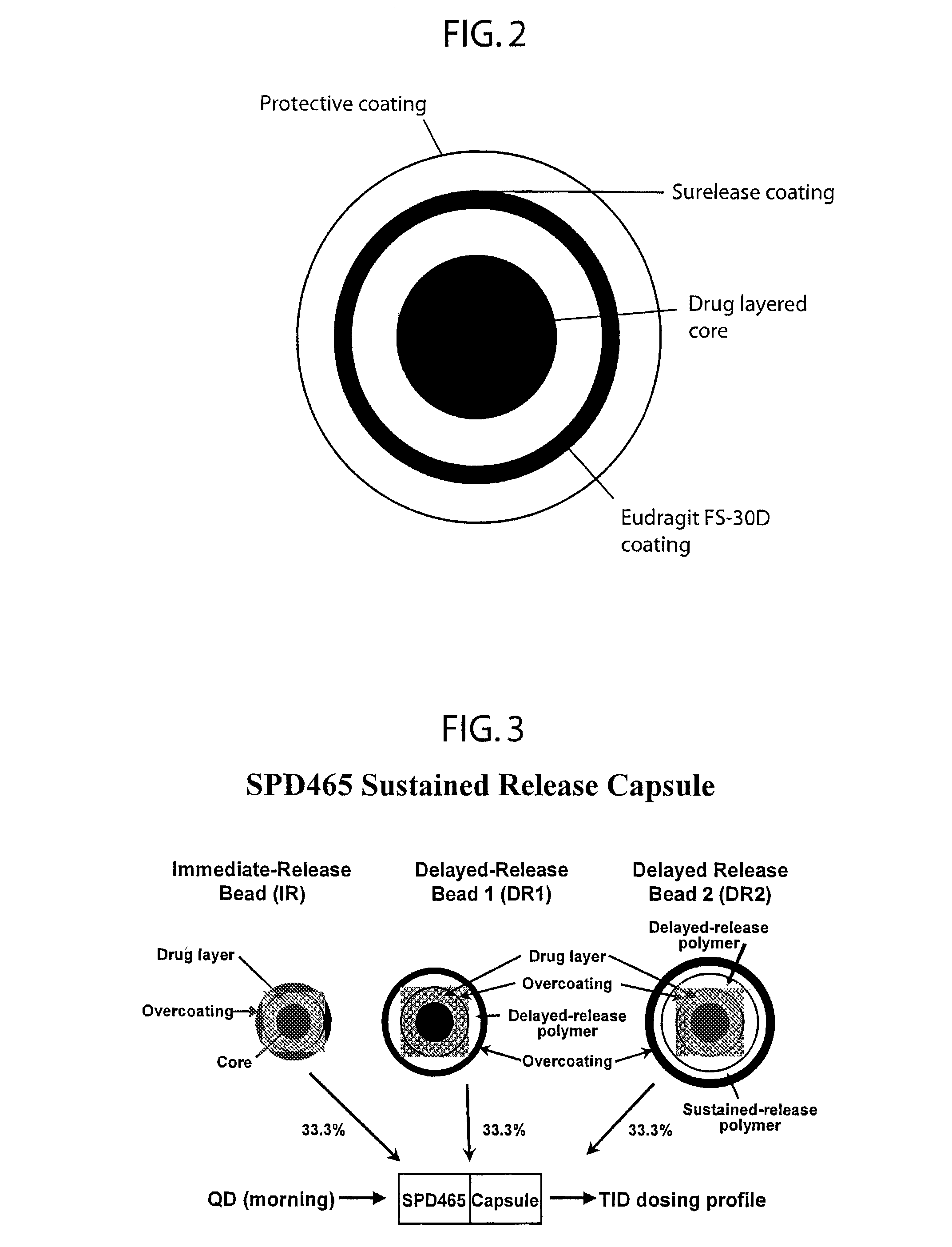
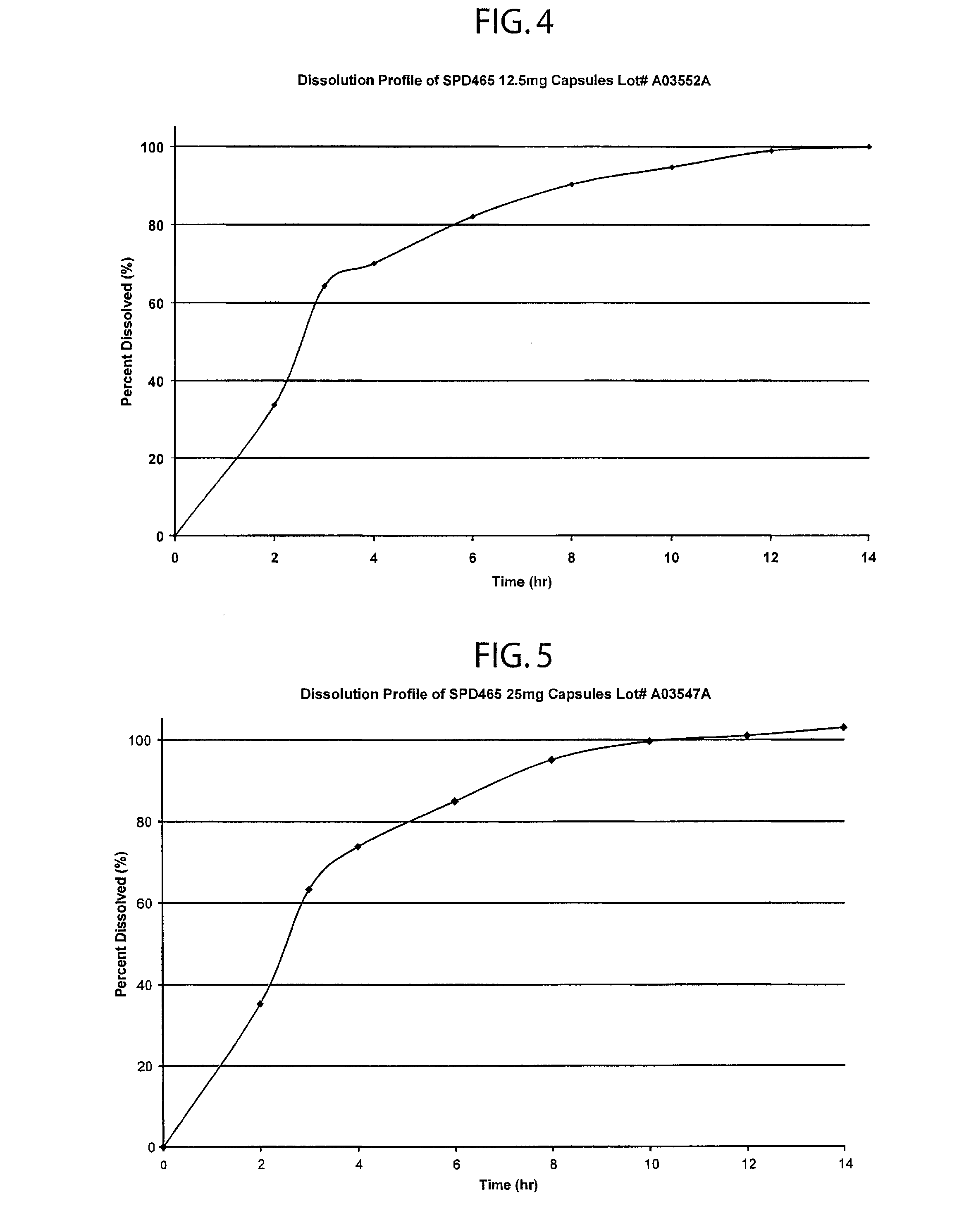
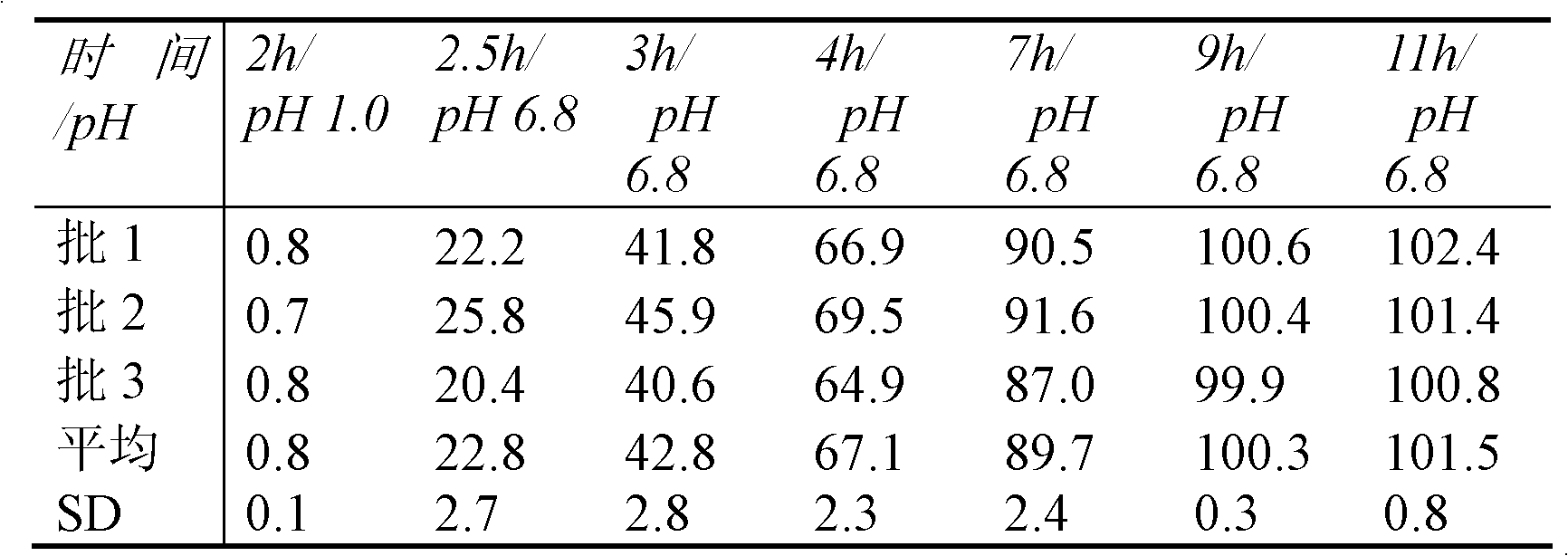Patents
Literature
654 results about "Enteric coating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An enteric coating is a polymer barrier applied on oral medication that prevents its dissolution or disintegration in the gastric environment. This helps by either protecting drugs from the acidity of the stomach, the stomach from the detrimental effects of the drug, or to release the drug after the stomach (usually in the upper tract of the intestine). Some drugs are unstable at the pH of gastric acid, and need to be protected from degradation. Enteric coating is also an effective method to obtain drug targeting (such as gastro-resistant drugs). Other drugs such as some anthelmintics may need to reach a high concentration in a specific part of the intestine. Enteric coating may also be used during studies as a research tool to determine drug absorption. Enteric coated medications pertain to the "delayed action" dosage form category. From a pharmacological point of view the term "enteric coating" is not entirely correct, as gastric resistance can be also obtained by adding enteric polymeric systems to the matrix of the dosage form. Tablets, mini-tablets, pellets and granules (usually filled into capsule shells) are the most common enteric-coated dosage forms.
Colon targeted delivery system
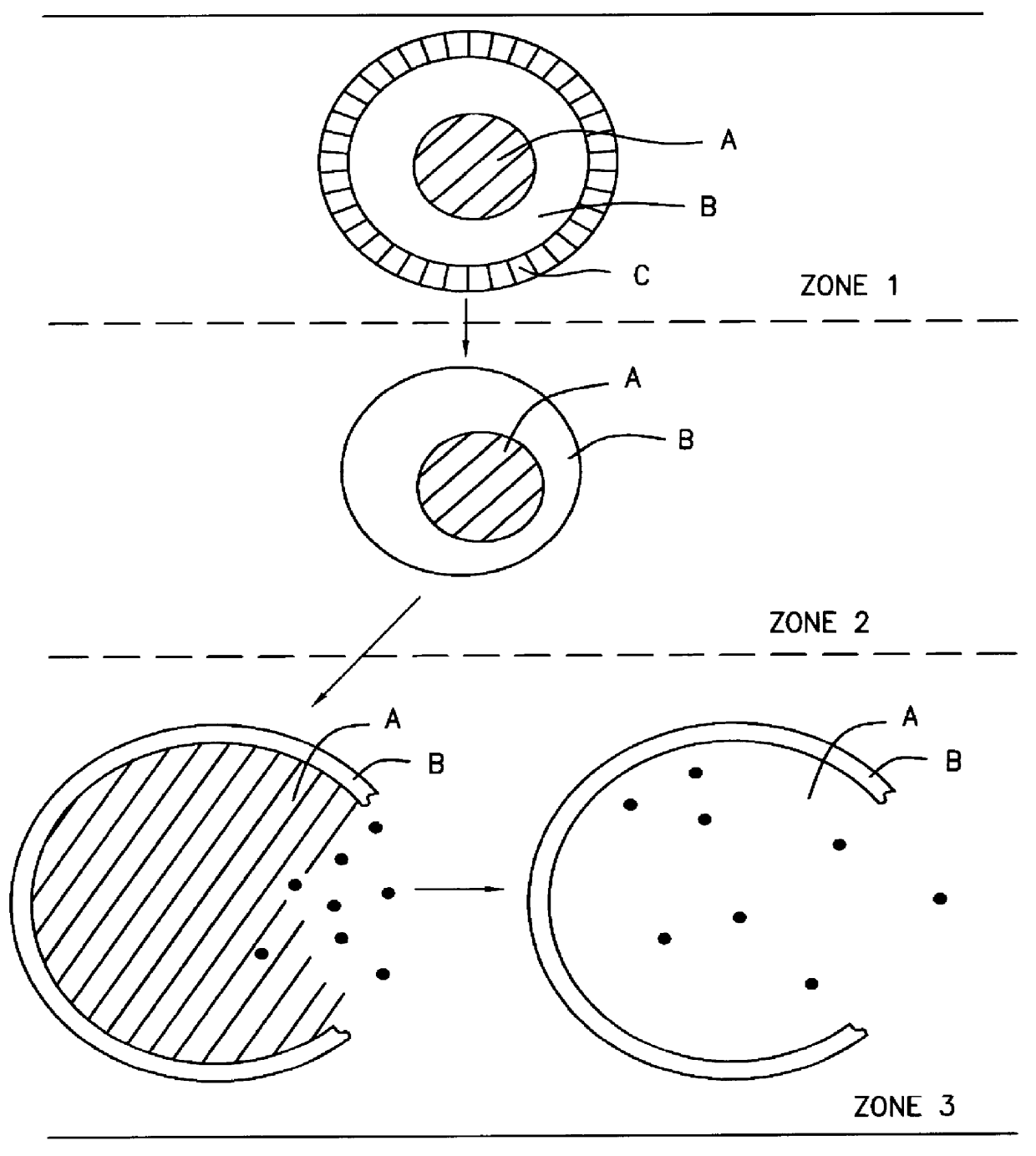
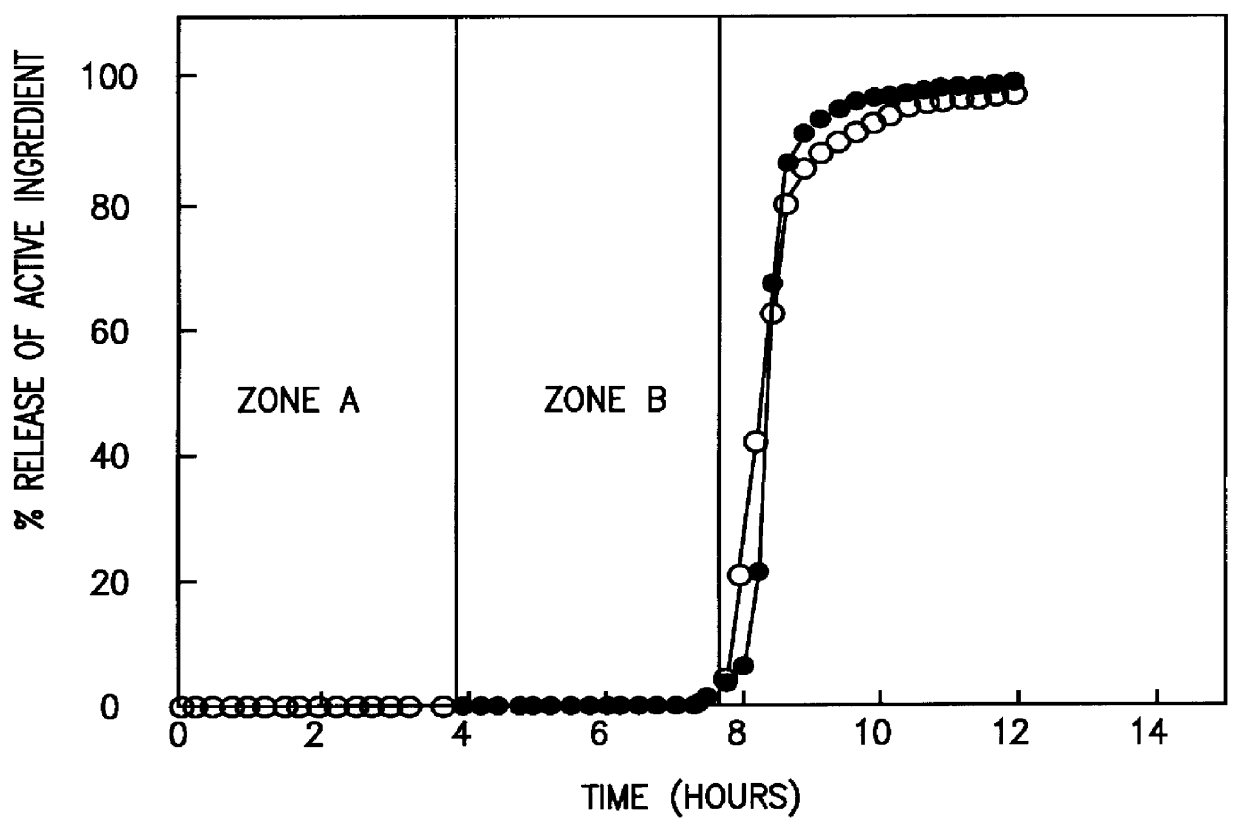
A novel delivery system for targeting drugs to the colon is herein described. The system is a tablet comprised of three parts: 1) an outer enteric coating, 2) an inner semi-permeable polymer membrane containing a plasticizer and 3) a central core comprising swelling excipients and an active ingredient. The novel dosage form described herein will release the drug consistently in the colon by a time-dependent explosion mechanism. This delivery system is particularly suitable for delivering viral protease inhibitors to the colon.
Owner:F HOFFMANN LA ROCHE & CO AG
Orally disintegrable tablets
InactiveUS6328994B1Sufficient oral disintegrabilityHigh strengthOrganic active ingredientsPowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Stable probiotic microsphere compositions and their methods of preparation
The invention relates to viable and stable probiotic formulations for intestinal targeting made of microspheres comprising each a core of one or more probiotic bacteria, microcrystallline cellulose with a degree of polymerization from 165-365 and mean diameter from 45 to 180 μm, a disintegrant and a stabilizer, the core being coated with a non-enteric coating and further coated with an enteric coating. Each probiotic microsphere has a residual moisture level of less than 5% and a water activity (aw) between 0.1 and 0.5. Such a probiotic microsphere shows no reduction in viable bacteria after one hour in simulated gastric fluid. The present invention also relates to the process of preparing such formulation.
Owner:CANACURE CORP
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Controlled dose drug delivery system
ActiveUS20070264323A1Meet needsReduce the amount requiredOrganic active ingredientsBiocideImmediate releaseDose delivery
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
Stable pancreatic enzyme compositions
ActiveUS20080274174A1Stable digestive enzyme compositionThe dosage form is stablePeptide/protein ingredientsHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Enteric coating, comprising alginic acid, for an oral preparation
A composition for forming an enteric coating on a tablet, capsule or pellet for oral ingestion includes a liquid mixture of alginic acid particles dispersed in an aqueous solution of a binding agent of locust bean, gum, gelatine, vegetable hydrocolloids and / or animal protein.
Owner:BIFODAN AS
Methods of producing stable pancreatic enzyme compositions
ActiveUS20080279953A1Minimal loss of activityComposition is stablePowder deliveryPeptide/protein ingredientsDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Analgetic dosage forms that are resistant to parenteral and inhalation dosing and have reduced side effects
InactiveUS20050165038A1Side-effect be reduceEliminate side effectsPowder deliveryBiocideDrugPharmaceutical preservatives
The invention provides a novel solid pharmaceutical dosage form which includes an opiate, an opiate antagonist admixed with the analgetic (opiate agonist) and an amount of a hydrocolloid containing excipient which is effective to form a non-injectable slurry when the dosage form is contacted with water. In addition the dosage form contains pure naloxone in enteric coated form which is designed to release in the colon to prevent or relieve constipation. Thus the formulation, because of the enteric coated naloxone and the hydrocolloid excipient(s), has reduced side effects as compared with formulations which do not contain these features.
Owner:GORDON MAXWELL
Pharmaceutical formulation comprising omeprazole
InactiveUS6428810B1Reduce diffuseHigh film thicknessOrganic active ingredientsOrganic chemistryEnantiomerOmeprazole
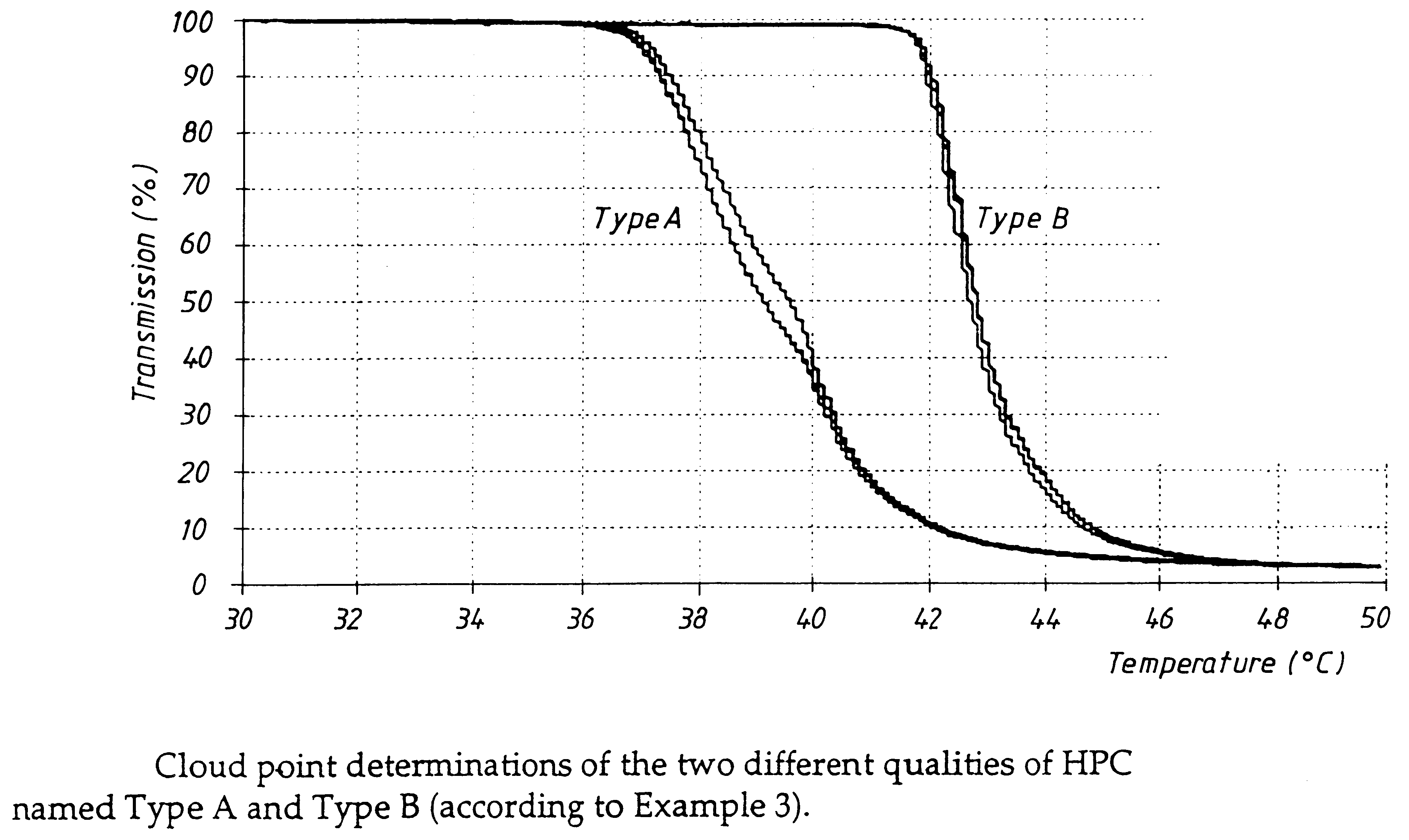
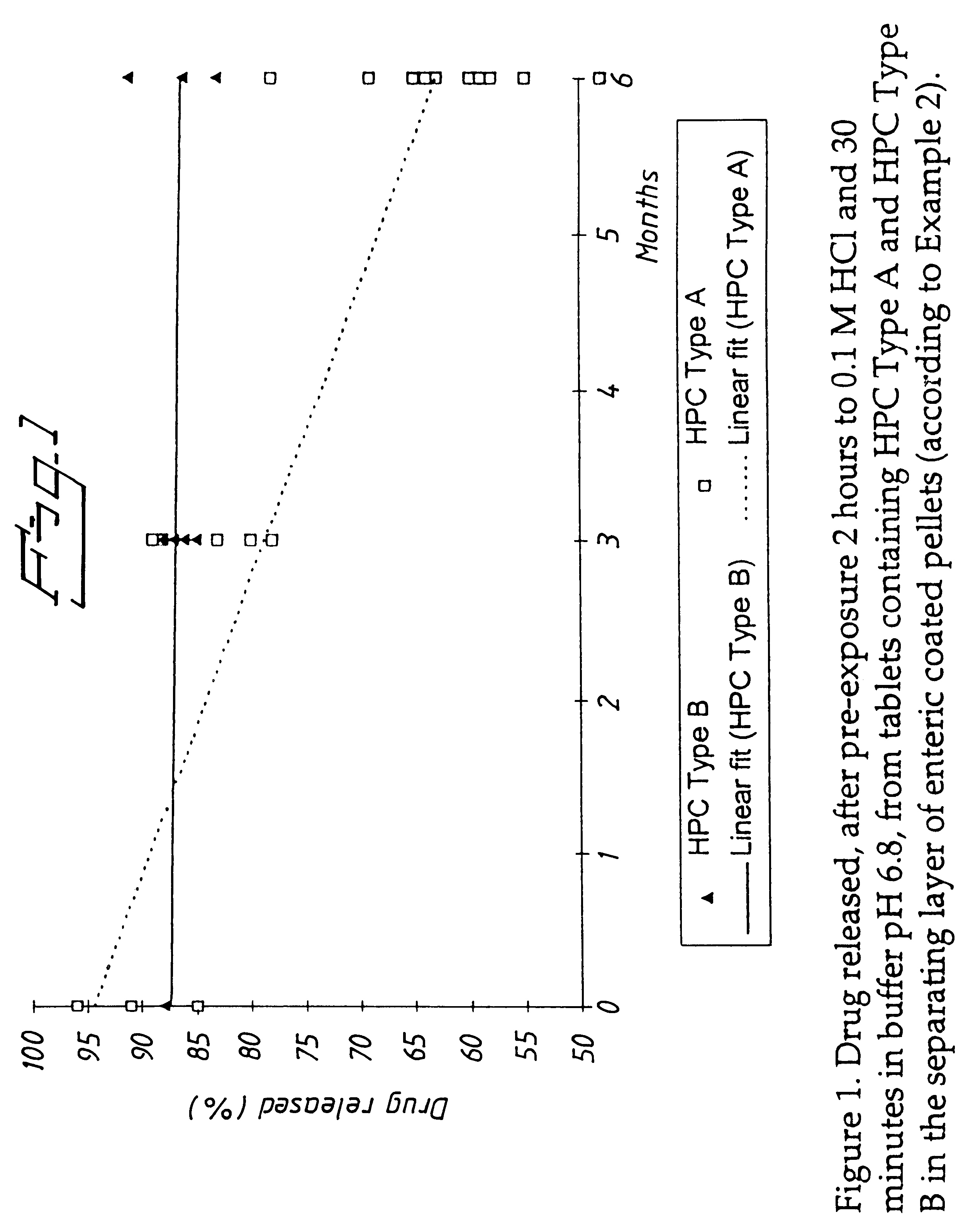
An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, one of the single enantiomers of omeprazole and an alkaline salt of one of the single enantiomers of omeprazole, wherein the formulation comprises a core material that comprises the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl cellulose (HPC) with a specific cloud point is used in the manufacture of the claimed pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formulations in medicine.
Owner:ASTRAZENECA AB
Stable solid preparations
It is intended to provide a process for producing unstable amorphous benzimidazole compounds having a proton pump inhibitor function, and stable solid preparations for medicinal use containing these compounds which are produced by blending such an amorphous benzimidazole compound with a nontoxic base such as a basic inorganic salt, forming an intermediate coating layer on the layer containing the active ingredient and further forming an enteric coating layer or a release-controlling coating layer.
Owner:TAKEDA PHARMA CO LTD
Probiotic composition having acid-resistant enteric coating
InactiveUS20070059296A1Improve digestibilityImproving resistance against diseaseOrganic active ingredientsBiocideBiotechnologyTalc
A probiotic composition essentially comprises 15 to 20 wt % of milk powder, 25 to 30 wt % of corn starch, 8 to 15 wt % of modified starch (capsul), 10 to 15 wt % of ethylcellulose, 5 to 15 wt % of bacterial broth, and 10 to 15 wt % of talc. The probiotic composition is microencapsulated to form a plurality of microencapsule coated with an acid-resistant enteric coating for improving the enteric acid-resistance, the probiotic survival rate, the antimicrobial property, the stability, the moisture-proof property, and the mobility of the probiotic composition preventing from coagulation in a moist environment and for being used as an additive applied to livestock feed.
Owner:BION TECH INC
Drug Delivery Formulations For Targeted Delivery
The size and location of microsphere uptake / delivery are important determinants of the final biodistribution of oral microsphere systems. Formulations, kits, methods of administering the formulations, and using the kits are described herein. The formulations are oral dosage formulations. In one embodiment, the formulations contain microparticles and / or nanoparticles having a homogenous size range selected to optimize uptake in a specific region of the GI tract and target drug delivery to specific organs. In some embodiments, the dosage formulation contains an enteric coating and / or a magnetic material. In a preferred embodiment, the formulation contains a magnetic material and an active agent to be delivered, optionally the active agent is in the form of micro- or nano-particles. In some embodiments metallomucoadhesive materials and / or magnetic materials are employed as magnetic and / or mucoadhesive sources. Formulations containing magnetic materials can be localized using the kits and methods disclosed herein. In one embodiment, the method includes orally administering the formulation and applying an extracorporeal magnet to a site on the outside surface of the patient's body in an area that closely apposes the location in the gastrointestinal tract to which delivery of the formulation is desired. The extracorporeal magnet is applied for a suitable time period to allow for the drug to be released from the formulation and / or to allow for the formulation to adhere to the site. Both magnetic and mucoadhesive forces may be utilized to site-direct and retain the dosage form in the region of the gastrointestinal (GI) tract most suitable for the desired delivery.
Owner:PEROSPHERE INC
Orally disintegrable tablets
InactiveUS20020142034A1High strengthGood disintegrationBiocidePowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Oral peptide pharmaceutical dosage form and method of production
InactiveUS7316819B2Improve bioavailabilityAvoid contactPeptide/protein ingredientsPharmaceutical non-active ingredientsRecombinant salmon calcitoninWater soluble
A pharmaceutical composition for oral delivery of a peptide is in the form of a lamination having at least two layers. The first layer of the lamination includes at least one pharmaceutically acceptable pH-lowering agent. The second layer includes a therapeutically effective amount of the peptide. The composition also includes at least one absorption enhancer effective to promote bioavailability of the peptide, which is preferably in the second layer, and an enteric coating surrounding the lamination. In a preferred dosage form of a tablet, a water-soluble coating is applied between the lamination and enteric coating which substantially prevents contact between the pH-lowering agent and the enteric coating. In a preferred embodiment, the peptide is salmon calcitonin, the pH-lowering agent is citric acid, and the absorption enhancer is lauroyl l-carnitine.
Owner:ENTERIS BIOPHARMA
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
InactiveUS20120148672A1Improved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
Controlled release composition
InactiveUS20050089569A1Controlled diffusionEasy to usePowder deliveryPharmaceutical non-active ingredientsWater dispersiblePolyethylene glycol
A composition for controlled delivery of at least one active substance into an aqueous medium by erosion at a preprogrammed rate of at least one surface of the composition, the composition comprising a matrix which is erodible in the aqueous medium in which the composition is to be used and which allows substantially no diffusion of water into the composition beyond any exposed surface layers of the matrix, the matrix comprising at least one substantially water soluble crystalline polymer, e.g. a polyethylene glycol, with at least one water-dispersible or water-soluble surface active agent, e.g. a non-ionic emulsifier, dispersed therein, at least one release modifier, e.g. an enteric coating material, that functions to regulate erosion of the matrix within a pH range of from about 2 to about 7, and at least one active substance. The composition provides controlled, e.g. substantially zero order, release in both the stomach and the intestines despite different conditions of pH, agitation and absorption.
Owner:EGALET LTD
Orally disintegrable tablets
InactiveUS7431942B2High strengthGood disintegrationPowder deliveryOrganic active ingredientsOrally disintegrating tabletEngineering
An orally disintegrable tablet of the present invention, which comprises (i) fine granules having an average particle diameter of 400 μm or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Dosage form for treating gastrointestinal disorders
InactiveUS20060165797A1Quick reliefPrevent relapseBiocideDigestive systemGastrointestinal disorderSecreted substance
The present invention is directed to drug dosage forms that can be used to treat diseases characterized by abnormal gastric acid secretion. The dosage forms have a core containing a proton pump inhibitor surrounded by an enteric coating or multiple particles containing proton pump inhibitor, each particle being surrounded by an enteric coating. The enteric coating delays the release of drug until the surrounding pH has risen. The tablets also include an outer coating that contains either a proton pump inhibitor or an H2 blocker. The outer coating is designed to rapidly dissolve in a patient's stomach.
Owner:POZEN INC
Enteric coatings for orally ingestible substrates
Enteric film coating systems for orally ingestible substrates such as pharmaceutical tablets and dietary supplements are disclosed. In preferred aspects, the enteric film coatings include an ethylcellulose dispersion and a substantially gastro-insoluble pore former such as sodium alginate.
Owner:BPSI HLDG LLC
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Method for the treatment of obesity, overweight and fluctuations in blood insuline and/or glucose levels
InactiveUS20030113310A1Low calorific valueLowering indexPeptide/protein ingredientsMetabolism disorderIntestinal structureBlood insulin
The present invention relates to a method of treating or preventing obesity, overweight, fluctuations in blood insulin levels and / or fluctuations in blood glucose levels in mammals. The method acording to the invention comprises the enteral administration to a mammal of an effective amount of a preparation containing an enzyme capable of converting an ingested carbohydrate or digestion product thereof into one or more absorbable components, wherein the total metabolic caloric value of the absorbable component(s) is less than the metabolic caloric value of the ingested carbohydrate or digestion product thereof. Thus the present invention effectively provides a method that allows complete digestion of ingested digestible carbohydrates whilst at the same time reducing the actual metabolic caloric value of said ingested carbohydrates. Another aspect of the invention relates to a pill for oral administration provided with an enteric coating and containing 25 to 10.000 IU glucose isomerase per gram.
Owner:NV NUTRICIA
Taste masked pharmaceutical compositions comprising bitter drug and pH sensitive polymer
InactiveUS20050136114A1Improve palatabilityA large amountPowder deliveryDispersion deliveryPH-sensitive polymersLiquid oral
The present invention discloses pharmaceutical compositions comprising of pH sensitive polymers used for taste masking highly bitter drugs. The pH sensitive polymer acts as a reverse enteric coating, which is soluble in the acidic pH range 1.0 to 3.0 normally found in the stomach but is insoluble in the pH range 3.5 to 7 thus inhibiting the release of the bitter drug at the pH of saliva and also at the pH of reconstitution medium in case of liquid orals.
Owner:COUNCIL OF SCI & IND RES
HUMAN SECRETORY IgA FOR THE TREATMENT OF CLOSTRIDIUM DIFFICILE ASSOCIATED DISEASES
A composition for treating a subject is provided. The composition includes dimeric or polymeric IgA therapeutic. Formulating agents are mixed with the dimeric or polymeric IgA to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the sequential modification of monomeric IgA with J chain and secretory component to form a dimeric or polymeric IgA therapeutic. The dimeric or polymeric IgA therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microeneapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R
Use of a mixture of two or more enteric materials to regulate drug release via membrane or matrix for systemic therapeutics
InactiveUS7910128B2Improve bioavailabilitySimple preparation processTetracycline active ingredientsPill deliveryWhole bodyDissolution
Disclosed are pharmaceutical compositions, particularly oral dosage forms, which comprise two or more enteric coating materials, either as a coating or as part of a matrix dosage form, and methods of making and using the same. The compositions are characterized by having a sustained release profile at lower pH and an accelerated dissolution profile at higher pH.
Owner:SUPERNUS PHARM INC
Magnesium plus interactive agent delivery
InactiveUS6887492B2Affect bio-uptakeReduce absorptionBiocideAnimal repellantsControlled releaseOral medication
The present invention is an orally administered pharmaceutical composition that provides for the controlled release of magnesium. The composition has an interactive agent component and a magnesium component which has an enteric coating that controls the release of the magnesium until the composition is in the small intestine or colon. The other component is released in the stomach and is substantially absorbed prior to the release of the magnesium component. Normally, the interactive agent component decreases the absorption of magnesium when the two components are released simultaneously in the gastrointestinal tract.
Owner:NBTY ACQUISITION LLC
Oral pharmaceutical dosage forms comprising a proton pump inhibitor and a NSAID
InactiveUS7488497B2Simplify regimenPatient compliance is goodAntibacterial agentsOrganic chemistrySide effectEnteric coating
An oral pharmaceutical dosage form comprising an acid susceptible proton pump inhibitor and one or more NSAIDs in a fixed formulation, wherein the proton pump inhibitor is protected by an enteric coating layer. The fixed formulation is in the form of an enteric coating layered tablet, a capsule or a multiple unit tableted dosage form. The multiple unit dosage forms are most preferred. The new fixed formulation is especially useful in the treatment of gastrointestinal side-effects associated with NSAID treatment.
Owner:ASTRAZENECA AB
Controlled dose drug delivery system
ActiveUS8846100B2Meet needsReduce the amount requiredOrganic active ingredientsBiocideDose deliveryImmediate release
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
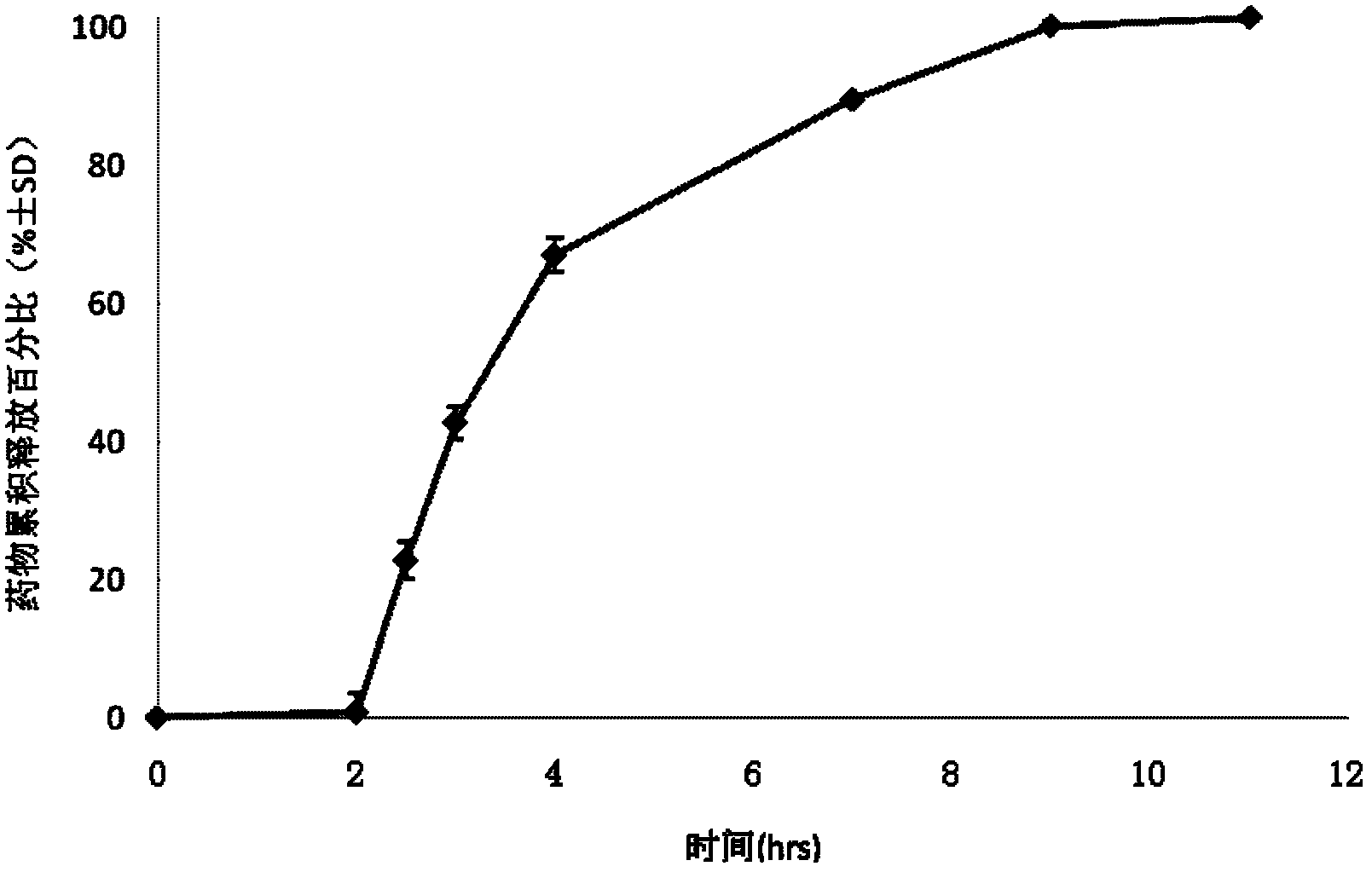
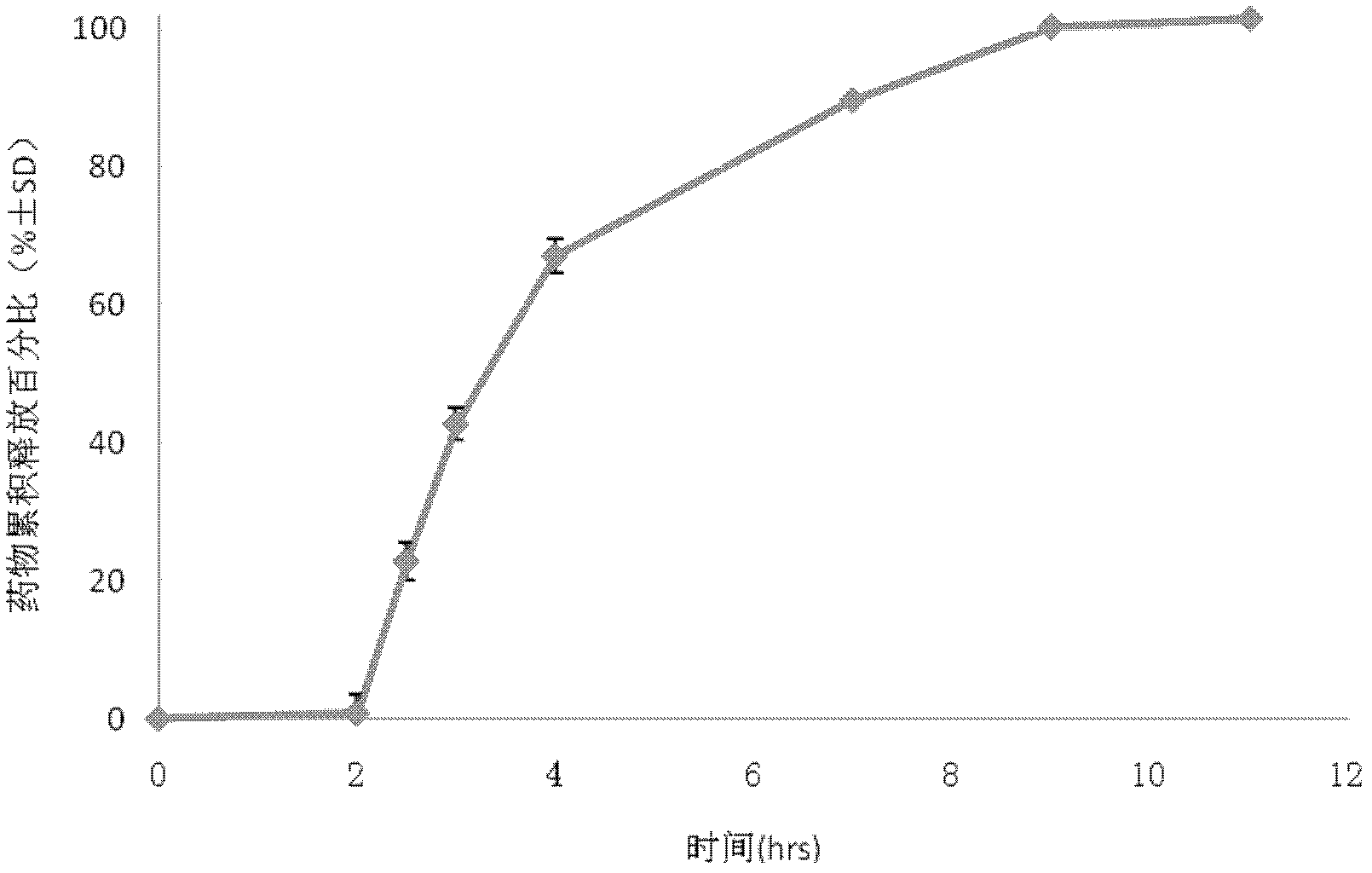
Drug sustained and controlled release microparticle preparation for treating intestinal diseases, and preparation method thereof
ActiveCN102319218AEvenly distributedGuaranteed uniformityPowder deliveryOrganic active ingredientsDiseaseAnti-Adhesion Agent
The present invention discloses a drug sustained and controlled release microparticle preparation for treating intestinal diseases. The preparation comprises: a pill core containing the drug, wherein the pill core contains 5-aminosalicylic acid and an assistant material; an isolation layer for providing a smooth and flat surface for the microparticle and preventing the drug from penetrating into a sustained release coating layer, wherein the penetration of the drug into the sustained release coating layer can affect the release effect, the used material of the isolation layer comprises one or a plurality of materials selected from a water-soluble polymer and an anti-adhesion agent; the sustained release coating layer for slowly releasing the drug, wherein different drug release levels can be achieved through adjusting the thickness of the sustained release coating layer, the used material of the sustained release coating layer mainly adopts a sustained-release material; an enteric-coating layer, the enteric-coating layer is provided for avoiding the early release of the drug in gastric juice, reducing stimulation of the main drug to stomach, increasing the local concentration of the drug in the lesion location, the used material of the enteric-coating layer mainly adopts a polymer enteric material. The invention further discloses a preparation method for the microparticle preparation. According to the present invention, the drug and the sustained release coating material are uniformly dispersed on the surface of the pellet, such that the problem of mixing uniformity of the assistant material and the main drug can be effectively solved.
Owner:PIVOT PHARMA TECH SHANGHAI
Controlled-release pharmaceutical tablets
Controlled-release tablets exhibiting excellent storage stability are achieved by granulating a pharmaceutically active agent with a hydroxyalkylcelluose, blending the resulting granules with an extragranular phase composed of a particulate material that provides a sustained-release matrix, and compressing the blend into a tablet form, which may be optionally coated, such as with an enteric coating composition, to provide delayed release and / or to enhance stability of the active agent.
Owner:INTELGENX CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com