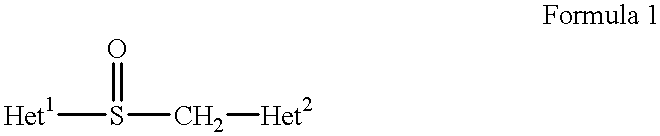Patents
Literature
1617 results about "Hydroxypropyl cellulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
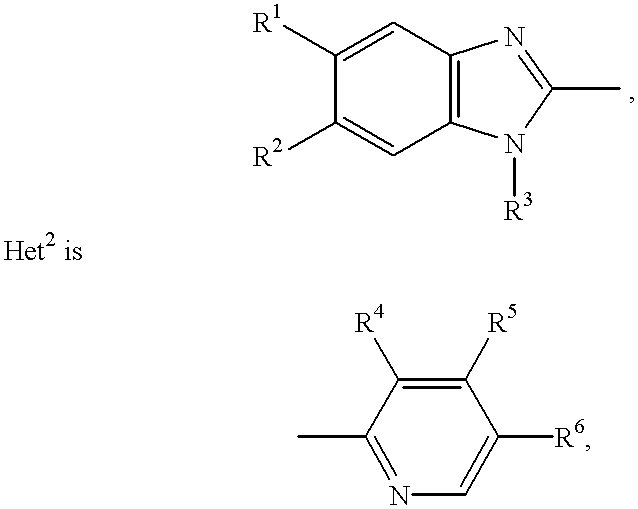
Hydroxypropyl cellulose (HPC) is a derivative of cellulose with both water solubility and organic solubility. It is used as an excipient, and topical ophthalmic protectant and lubricant.
Solid pharmaceutical preparation
InactiveUS6299904B1Good disintegrationPromote dissolutionOrganic active ingredientsBiocideLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohols selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
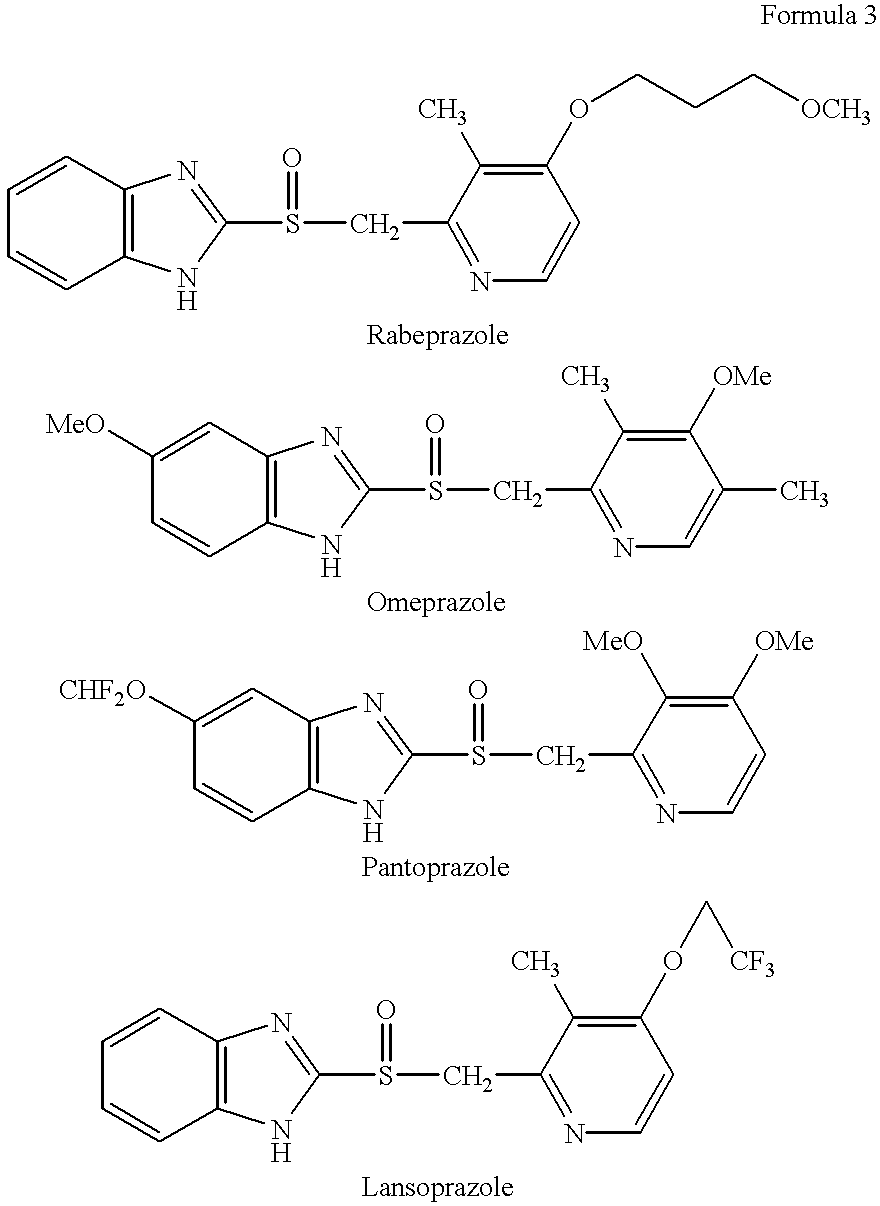
Pharmaceutical formulation comprising omeprazole
InactiveUS6428810B1Reduce diffuseHigh film thicknessOrganic active ingredientsOrganic chemistryEnantiomerOmeprazole
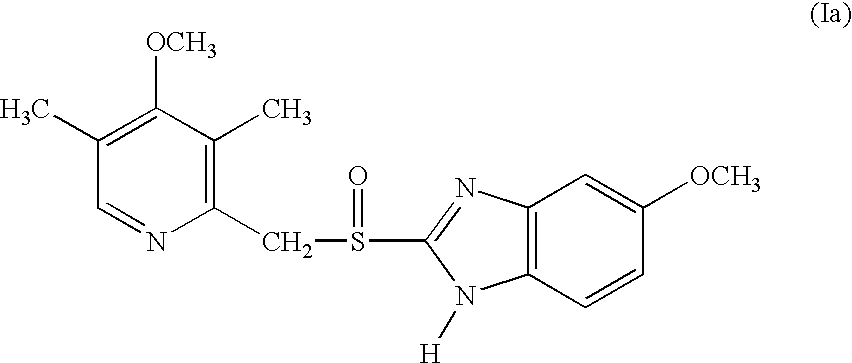
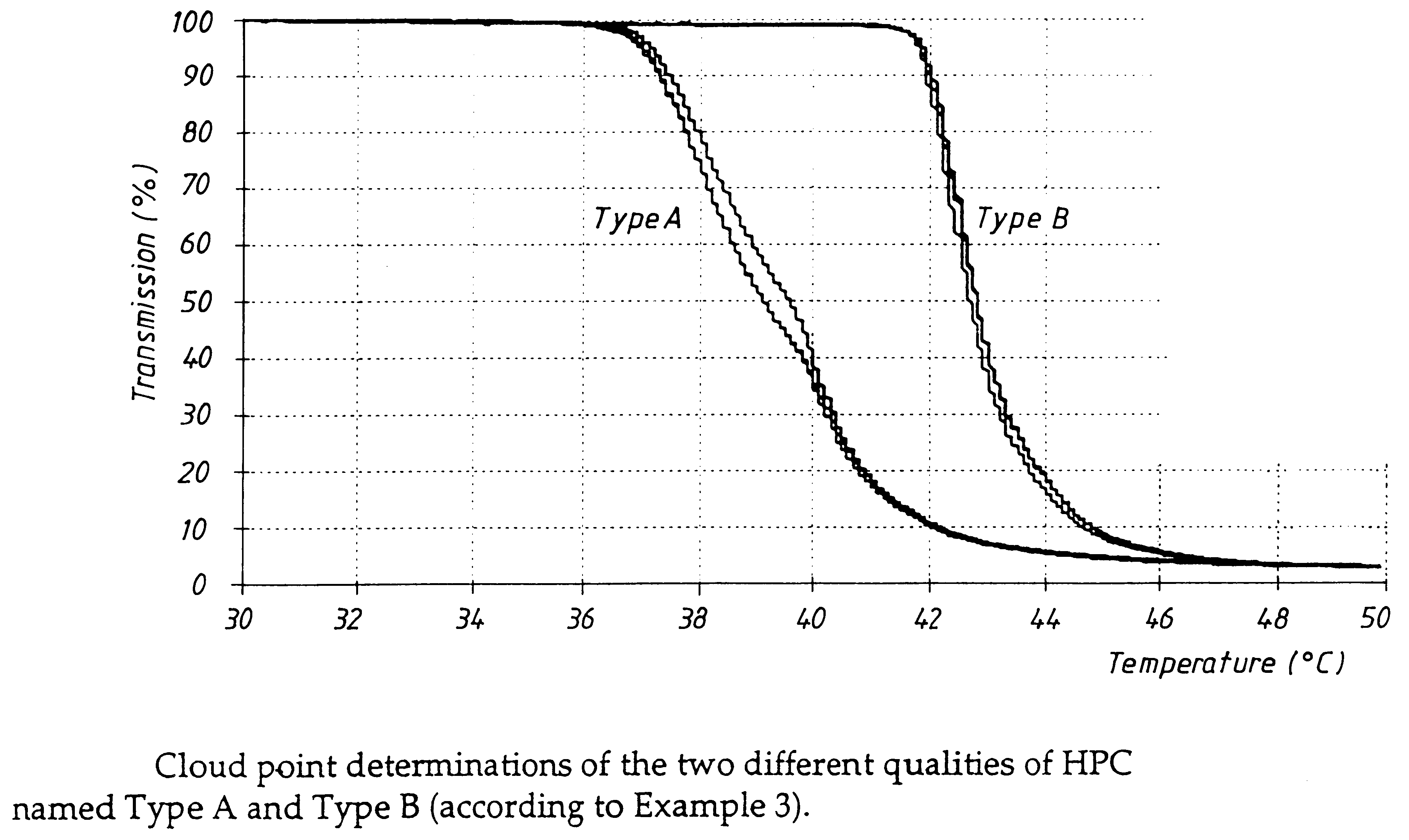
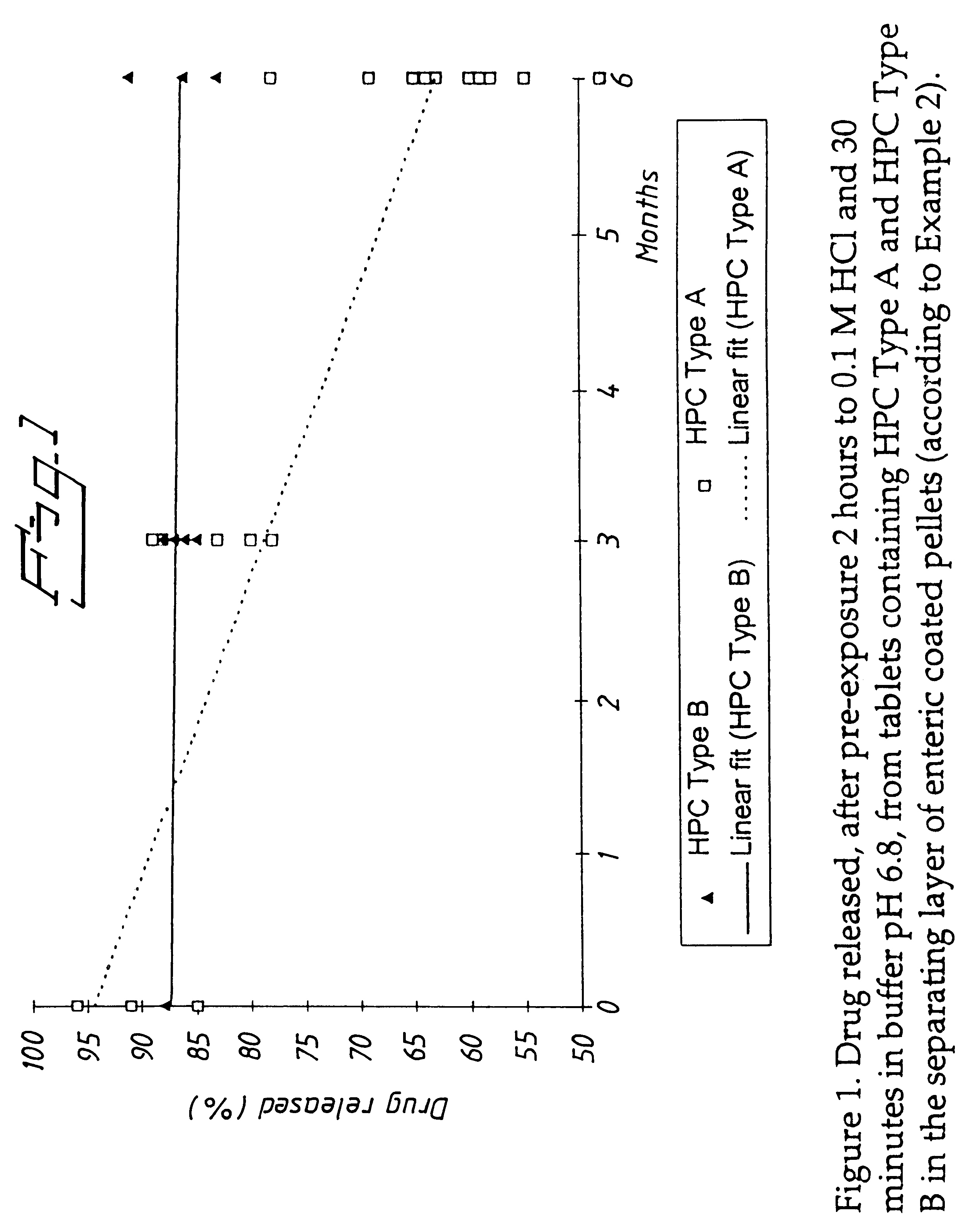
An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, one of the single enantiomers of omeprazole and an alkaline salt of one of the single enantiomers of omeprazole, wherein the formulation comprises a core material that comprises the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl cellulose (HPC) with a specific cloud point is used in the manufacture of the claimed pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formulations in medicine.
Owner:ASTRAZENECA AB
Beta-lactam antibiotic-containing tablet and production thereof
InactiveUS6423341B1Antibacterial agentsOrganic active ingredientsLow-substituted hydroxypropylcelluloseEngineering
This invention provides beta-lactam antibiotic-containing tablets capable of being orally taken either as such owing to their being small-sized, hence still easily swallowable, or, in the case of administration to the aged encountering some difficulty in swallowing, in the form of dispersions resulting from easy self-disintegration upon being dropped into water in a glass as well as a method of producing the same. The tablets of this invention comprise, on the per-tablet basis, 60-85% by weight of a beta-lactam antibiotic, 1-10% by weight of low-substituted hydroxypropylcellulose and / or crosslinked polyvinylpyrrolidone as a disintegrator, and 0.5-2% by weight of a binder. Granules to be compressed for tableting are prepared using water or an aqueous solution of ethanol or the like.
Owner:ASTELLAS PHARMA INC
Methyl ester-based microemulsions for cleaning hard surfaces
A cleaning composition containing: (a) from about 1.0 to about 15.0% by weight of a monoethanolamine salt of an alkyl sulfonic acid; (b) from about 3 to about 50% by weight of a C6-C14 methyl ester primary solvent; (c) from about 1.0 to about 15.0% by weight of a short-chain cosurfactant; (d) from about 1 to about 25% by weight of a polar solvent having a water solubility of from about 1 to 5 g / 100 ml; (e) up to about 10.0% by weight of a nonionic surfactant; (f) from about 0.05 to about 3.0% by weight of a thickening agent selected from the group consisting of hydroxypropyl cellulose, hydroxypropyl methylcellulose, and mixtures thereof; and (g) remainder, water, all weights being based on the total weight of the composition, and wherein the composition is terpene-free.
Owner:COGNIS IP MANAGEMENT GMBH
Stabilized compositions containing benzimidazole-type compounds
InactiveUS20020039597A1Improve stabilityLess color changeDigestive systemPill deliveryCrospovidonesMetallole
The present invention provides a chemically stable pharmaceutical preparation of a benzimidazole type compound. That is, the present invention relates to a composition comprising at least one substance selected from sodium carbonate, potassium carbonate, sodium hydroxide, potassium hydroxide, aminoalkyl methaacrylate copolymer E, arginine aspartate, hydroxypropyl cellulose and crospovidone incorporated into a benzimidazole type compound or an alkali metal salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Stable pharmaceutical compositions containing an ACE inhibitor
InactiveUS6869963B2Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalInstability
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Compound danshen oral disintegrant tablet and its preparation method
The present invention relates to a compound salvia oral disintegrant tablet capable of being disintegrated quickly in oral cavity to release medicine. It composition includes the salvia root extract, notoginseng total saponin, borneol and excipient, and its disintegration time is within 40 sec, in which the excipient includes filling agent, disintegrant, glidant and corrective. Its filling agent is of microcrystalline cellulose, its dose is 40%-90% of total weight of the prescription, and the disintegrant is selected from sodium carboxymethylstarch, cross-linked carboxymethylcellulose sodium, cross-linked polyvinylpyrrolidone and low-substituted hydroxypropyl cellulose, and its dose is 5%-25% of total weight of prescription.
Owner:BEIJING BOERDA BIO TECH DEV
Low-substituted hydroxypropylcellulose powder and method for producing the same
ActiveUS20080039621A1Improve compression performanceGood lowabilityPill deliveryGranular deliveryAqueous sodium hydroxideLow-substituted hydroxypropylcellulose
Provided are a low-substituted hydroxypropylcellulose powder having high compressibility, good flowability and excellent disintegration, and a method for producing the same. More specifically, provided is a method for producing a low-substituted hydroxypropylcellulose powder having a molar substitution number per anhydrous glucose unit of 0.05 to 1.0, which is insoluble in water and swollenable by absorbing water, comprising the steps of: adding an aqueous sodium hydroxide solution to powdered pulp in such a manner that weight ratio of sodium hydroxide with respect to anhydrous cellulose is 0.1 to 0.3 so as to produce alkali cellulose; etherifying the obtained alkali cellulose to obtain a crude product; neutralizing the sodium hydroxide contained in the obtained crude reaction product; washing the resultant; drying; and pulverizing using by compaction-grinding.
Owner:SHIN ETSU CHEM IND CO LTD
Solid preparation
InactiveUS20010009678A1Good disintegrationPromote dissolutionBiocideMetabolism disorderLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohol selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
Stabilized composition comprising a benzimidazole type compound
The present invention provides a chemically stable pharmaceutical preparation of a benzimidazole type compound. That is, the present invention relates to a composition comprising at least one substance selected from sodium carbonate, potassium carbonate, sodium hydroxide, potassium hydroxide, aminoalkyl methaacrylate copolymer E, arginine aspartate, hydroxypropyl cellulose and crospovidone incorporated into a benzimidazole type compound or an alkali metal salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Powdery composition for nasal administration
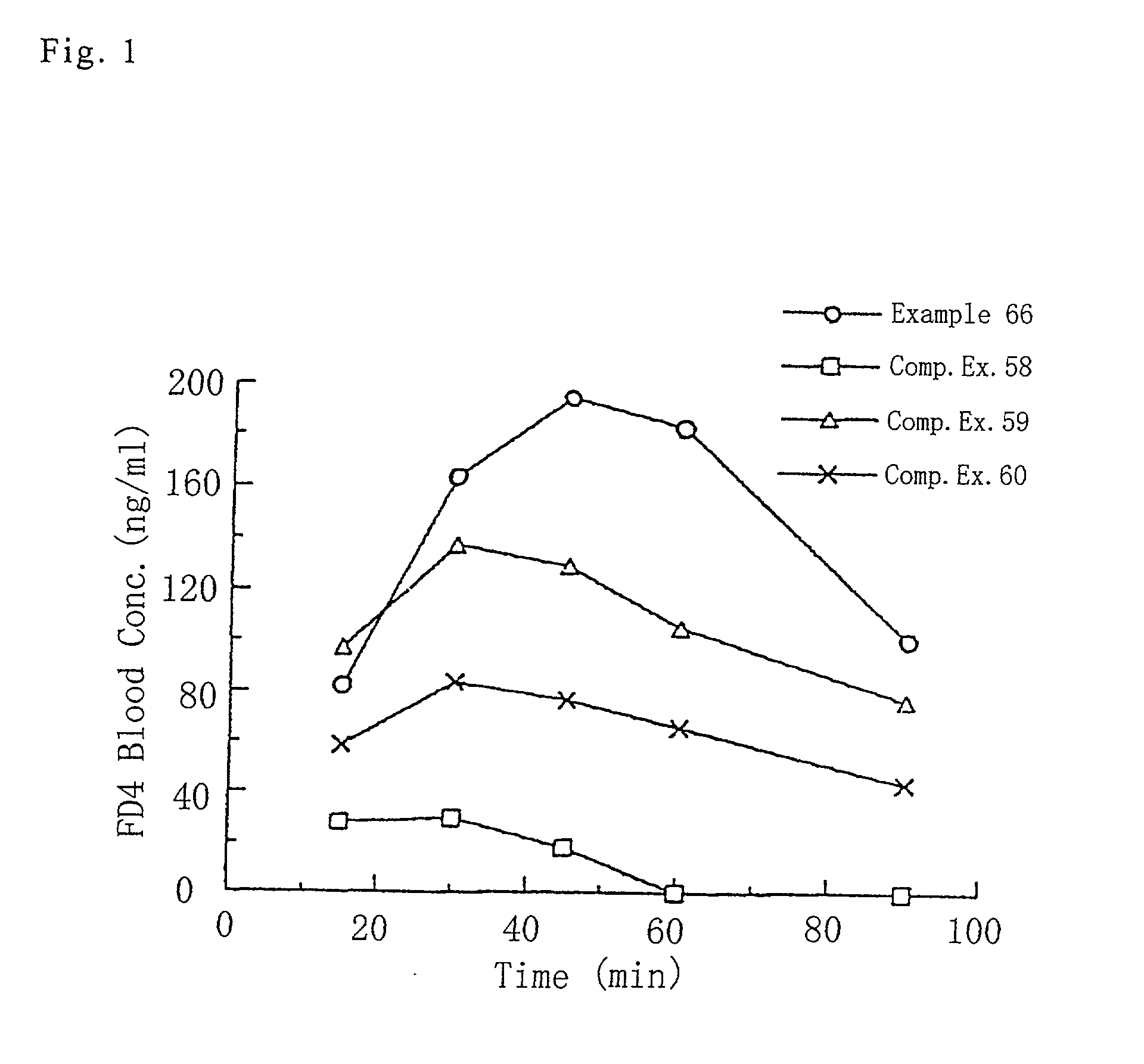
InactiveUS20020012688A1Promote absorptionHigh maximum blood concentrationPowder deliveryMicrocapsulesNasal cavitySolubility
The present invention relates to a powdery composition for nasal administration, which is characterized in that (1) the composition contains (i) a drug, (ii) a water-absorbing and gel-forming base material such as hydroxypropyl cellulose or hydroxypropylmethyl cellulose and (iii) a water-absorbing and water-insoluble base material such as crystalline cellulose or alpha-cellulose, (2) wherein the amount of the water-absorbing and gel-forming base material is about 5-40 wt % based on the total of the water-absorbing and gel-forming base material and the water-absorbing and water-insoluble base material, and (3) wherein the drug is unevenly dispersed more on / in the water-absorbing and water-insoluble base material than on / in the water-absorbing and gel-forming base material. The present invention provides the powdery composition for nasal administration excellent in absorption of the drug from the nasal cavity and having an extremely increased maximum blood concentration comparing a conventional composition for nasal administration even for a drug having a high solubility in water, a drug having a high lipophilicity or a peptide / proteinaceous drug having a large molecular weight.
Owner:TEIJIN LTD
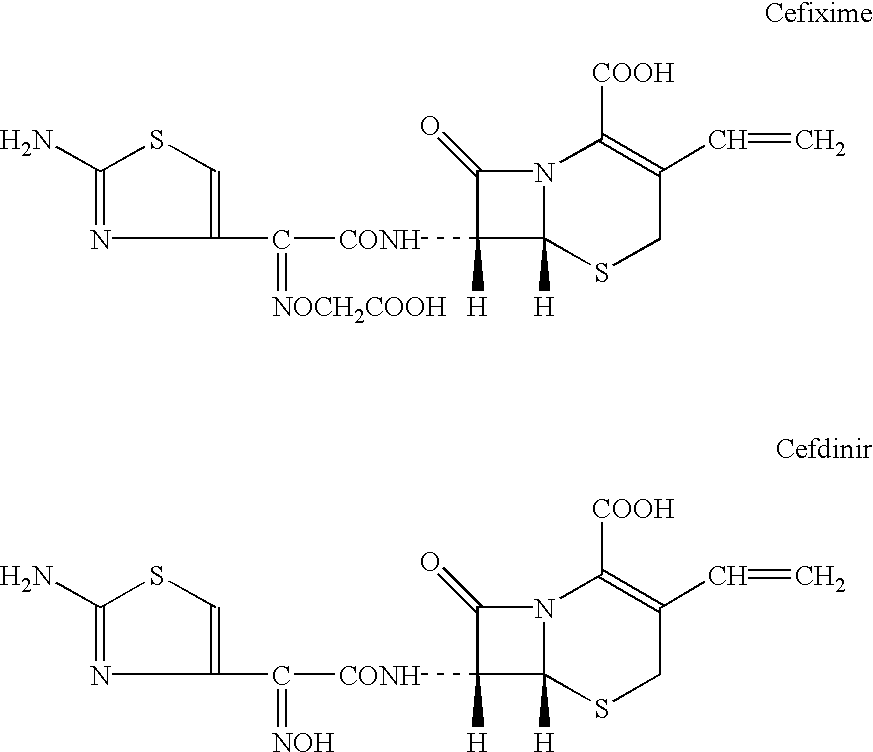
Dispersible tablet containing cefixime and preparation method thereof
The invention belongs to the technical field of pharmaceutical preparation, and in particular relates to a cefixime dispersible tablet and a method for preparing the same. The method comprises the following steps: firstly, weighing cefixime, starch, microcrystalline cellulose, partially cross-linked polyvinylpyrrolidone, partially cross-linked sodium carboxymethyl cellulose, and partially low substituted-hydroxypropyl cellulose, and mixing the components evenly; secondly, dripping 5 percent polyvinylpyrrolidone K30 ethanol solution into the mixture prepare a soft material, and performing granulating through a sieve of between 18 and 24 meshes; thirdly, drying wet particles at a temperature of between 50 and 80 DEG C, palletizing the particles through the sieve of between 18 and 24 meshes, adding the remaining cross-linked polyvinylpyrrolidone, the remaining cross-linked sodium carboxymethyl cellulose, and the remaining low substituted-hydroxypropyl cellulose, magnesium stearate and superfine silica gel powder into the mixture to be mixed evenly, and tabletting the mixture to obtain the required cefixime dispersible tablet. The cefixime dispersible tablet has the characteristics of rapid disintegration with water, even dispersion, high dissolution, and convenient taking and carrying around.
Owner:BEIJING TRADE STAR MEDICAL TECH
Preparation method of ezetimibe tablet
The invention discloses a preparation method of an ezetimibe tablet. The preparation method comprises the following steps of (1) dissolving hydroxypropyl cellulose and ezetimibe into ethanol, and dissolving a carrier material mannitol into a water solution; (2) respectively introducing a supercritical carbon dioxide fluid and the two solutions into a coaxial three-channel nozzle by setting the pressure and temperature of the supercritical carbon dioxide fluid to 10-50 MPa and 35-60 DEG C, and acquiring ultrafine granule mixed powder which contains the ezetimibe, the hydroxypropyl cellulose and the mannitol by utilizing the anti-solvent effect of the supercritical carbon dioxide fluid; (3) directly tabletting the ultrafine granule mixed powder and pharmaceutically acceptable auxiliary materials. The method disclosed by the invention can be used for fast dissolving the ezetimibe, can achieve the dissolution rate of the ezetimibe tablet by 95% for 5 minutes and does not contain the surface active agent.
Owner:QINGDAO UNIV OF SCI & TECH
Color mortar coating
InactiveCN101948278AIncreased durabilityExtended service lifeSolid waste managementUltravioletInorganic pigments
The invention discloses a color mortar coating which comprises the following raw materials in parts by weight: 21-24 parts of cement, 5-10 parts of ash-calcium, 52-58 parts of quartz sand, 12-18 parts of heavy calcium carbonate, 5-10 parts of silica micropowder, 2-4 parts of redispersible emulsion powder, 0.25-0.35 part of water repellent, 0.35-0.45 part of thixotropic lubricant, 0.25-0.3 part of hydroxypropyl cellulose ether and 1-5 parts of iron oxide inorganic pigment. The color mortar coating has the advantages of good weather resistance, long-lasting color and good ultraviolet resistance, has high binding force with various inorganic bottom layer materials, is difficult to crack and has good hydrophobicity.
Owner:赵明
Preparation method of xylitol liver-protecting tablet
InactiveCN101744865AEffective preventionEffective therapeuticAnthropod material medical ingredientsHydroxy compound active ingredientsCross-linkPropolis
The invention relates to a preparation method of a xylitol liver-protecting tablet, belonging to the technical field of medical health care. The formula of the xylitol liver-protecting tablet comprises the following raw materials in proportion: 15-45 parts of kudzu root extract, 2-20 parts of glycyrrhizic acid, 5-35 parts of propolis powder, 20-60 parts of xylitol, 1-10 parts of low-substituted hydroxypropyl cellulose, 1-10 parts of cross-linked sodium carboxymethyl cellulose and 0.5-5 parts of magnesium stearate; and a coating agent comprises 0.5-40 parts of hydroxypropyl methylcellulose, 1-30 parts of polyethylene glycol 6000, 0.5-40 parts of talcum powder, 0.5-30 parts of titanium pigment, 0.5-30 parts of iron oxide brown and 10-75 parts of maltitol. The finished product of the xylitol liver-protecting tablet is prepared by the processing steps of crushing, mixing, tabletting, coating and the like. In the invention, by using the xylitol as a main raw material and adding partial auxiliary materials, the xylitol liver-protecting tablet which is a health food and has auxiliary protection effect on chemical liver injury is prepared. The xylitol liver-protecting tablet can effectively prevent and treat fatty liver; and after patients suffering from the fatty liver take the xylitol liver-protecting tablet, severe fatty liver can be relieved into moderate fatty liver, and moderate fatty liver can be relieved into mild fatty liver. Thus, the xylitol liver-protecting tablet has obvious benefit to improve liver functions of human bodies and has wide market prospects.
Owner:FUTASTE PHARM CO LTD
Non-aqueous thixotropic fragrance gel
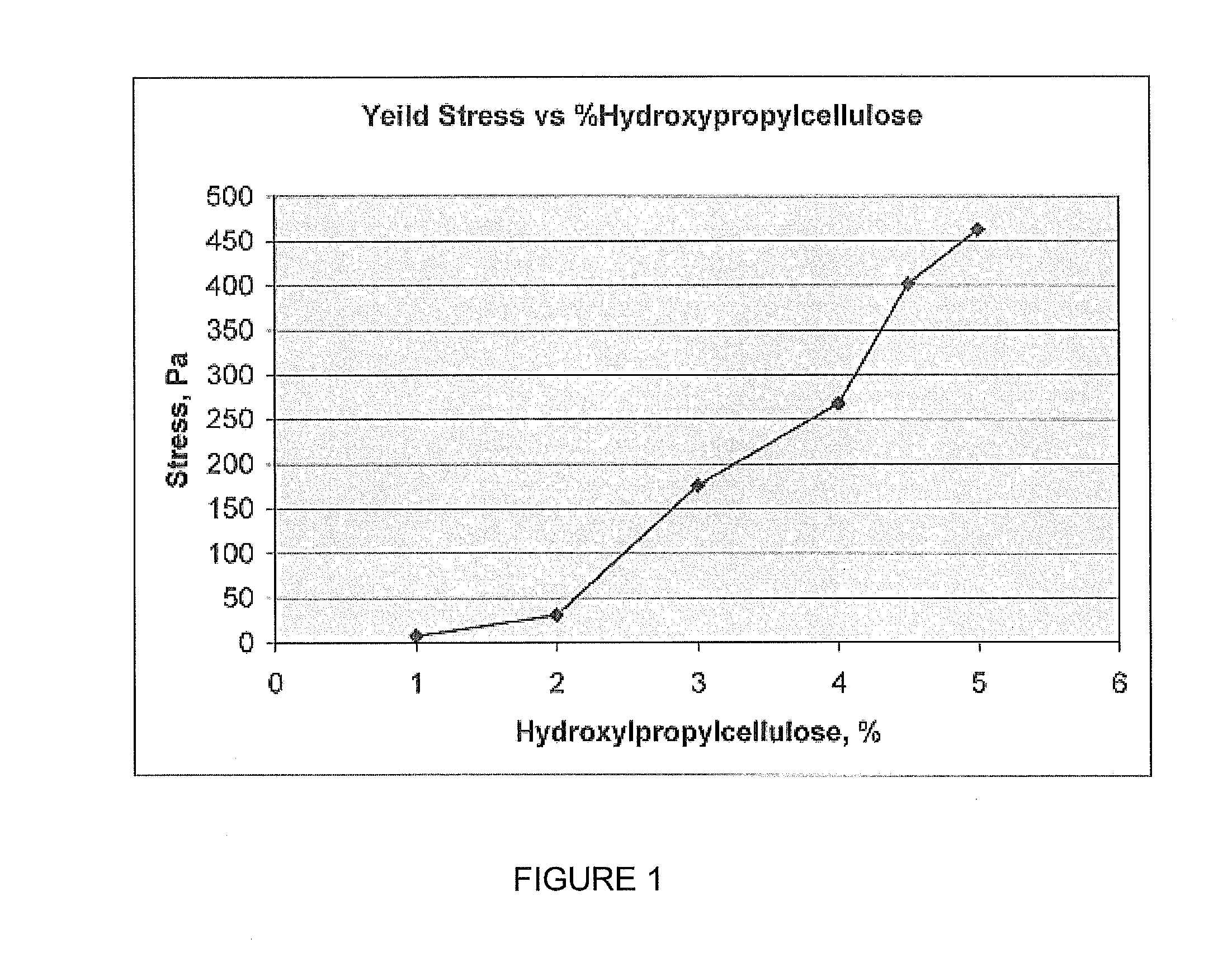
The present invention is a non-aqueous self-standing rigid fragrance gel comprising fragrance oil and a hydroxylalkyl cellulose derivative. In the preferred embodiment of the present invention, about 4.0 weight percent or more of hydroxylpropyl cellulose converts fragrance oil into a rigid gel with pseudoplastic rheology. The pseudoplastic gel shears to allow coating of crystals and objects, with subsequent rigidity restored to at least 80%.
Owner:DIAL CORPORATION
Pharmaceutical preparation containing benzimidazoles
The invention provides a solid dispersion of the benzimidazoles and a method for preparing the same. The solid dispersion comprises benzimidazole drugs, surfactant and a solid dispersion hydrophilic carrier material and can also contain an alkaline compound, wherein the solid dispersion hydrophilic carrier material is selected from or a mixture of polyvinylpyrrolidone, polyethylene glycol, poloxamer, hydroxypropyl methyl cellulose, hydroxypropyl cellulose and sugar alcohols. The invention also provides an enteric-coated micro granule containing the benzimidazoles of the solid dispersion and a method for preparing the same. The structure of the micro granule comprises a drug core and an enteric-coated layer from outside to inside, wherein the solid dispersion is arranged in the drug core, and an insolating layer can also be arranged between the drug core and the enteric-coated layer.
Owner:SHANGHAI HUIDE MEDICINE SCI & TECH
Thickeners for methyl ester microemulsions
A cleaning composition containing: (a) from about 1.0 to about 15.0% by weight of an anionic surfactant; (b) from about 3 to about 50% by weight of a C6–C14 methyl ester primary solvent; (c) from about 1.0 to about 15.0% by weight of a short-chain cosurfactant; (d) from about 1 to about 25% by weight of a polar solvent having a water solubility of from about 1 to 5 g / 100 ml; (e) up to about 10.0% by weight of a nonionic surfactant; (f) from about 0.05 to about 3.0% by weight of a thickening agent selected from the group consisting of hydroxypropyl cellulose, hydroxypropyl methylcellulose, and mixtures thereof; and (g) remainder, water, all weights being based on the total weight of the composition, and wherein the composition is terpene-free.
Owner:COGNIS IP MANAGEMENT GMBH
Butylphthalide dripping pill and preparing method
ActiveCN1943571AFast oral onsetPromote absorptionOrganic active ingredientsDispersion deliveryButylphthalideHypromellose
The invention relates to a drip pills of butylbenzene phthalein and its preparation method and it is characterized by comprising butylbenzene phthalein, base material, dispersing agent and coating materials. PEG4000, PEG6000, PEG20000 and poloxamer are selected as base materials. Dispersing agent is from lightweight aerosol and crospovidone. And coating materials are from hypromellose, hydroxypropyl cellulose, ethyl cellulose and Eudragit E30D.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Thermal insulation textured decorative face brick for building outside wall
InactiveCN102021985AReduce engineering costsMonotonous status quo improvementCovering/liningsBrickCalcium formate
The invention relates to a thermal insulation textured decorative face brick for a building outside wall. The face brick comprises the following raw materials in parts by weight: 25-35 parts of cement, 20-35 parts of quartz sand, 10-15 parts of calcite powder, 10-20 parts of wollastonite powder, 2.0-4.0 parts of latex powder, 0.3-0.4 part of hydroxypropyl cellulose, 0.5-1.0 part of wood fiber, 0.15-0.2 part of short polypropylene fiber, 0.2-0.5 part of calcium formate, 10-20 parts of gray calcium powder, 0.1-0.15 part of thixotropic lubricating agent and 0.25-0.5 part of pigment. After being put on the market, the face brick has great market space, and the current monotonous situation of outside wall exterior thermal insulation textured decorative materials can be improved, thus the face brick has active effects on saving energy of buildings and beautifying the environment of cities.
Owner:张新生
Preparation for composition of vitamin and mineral matter
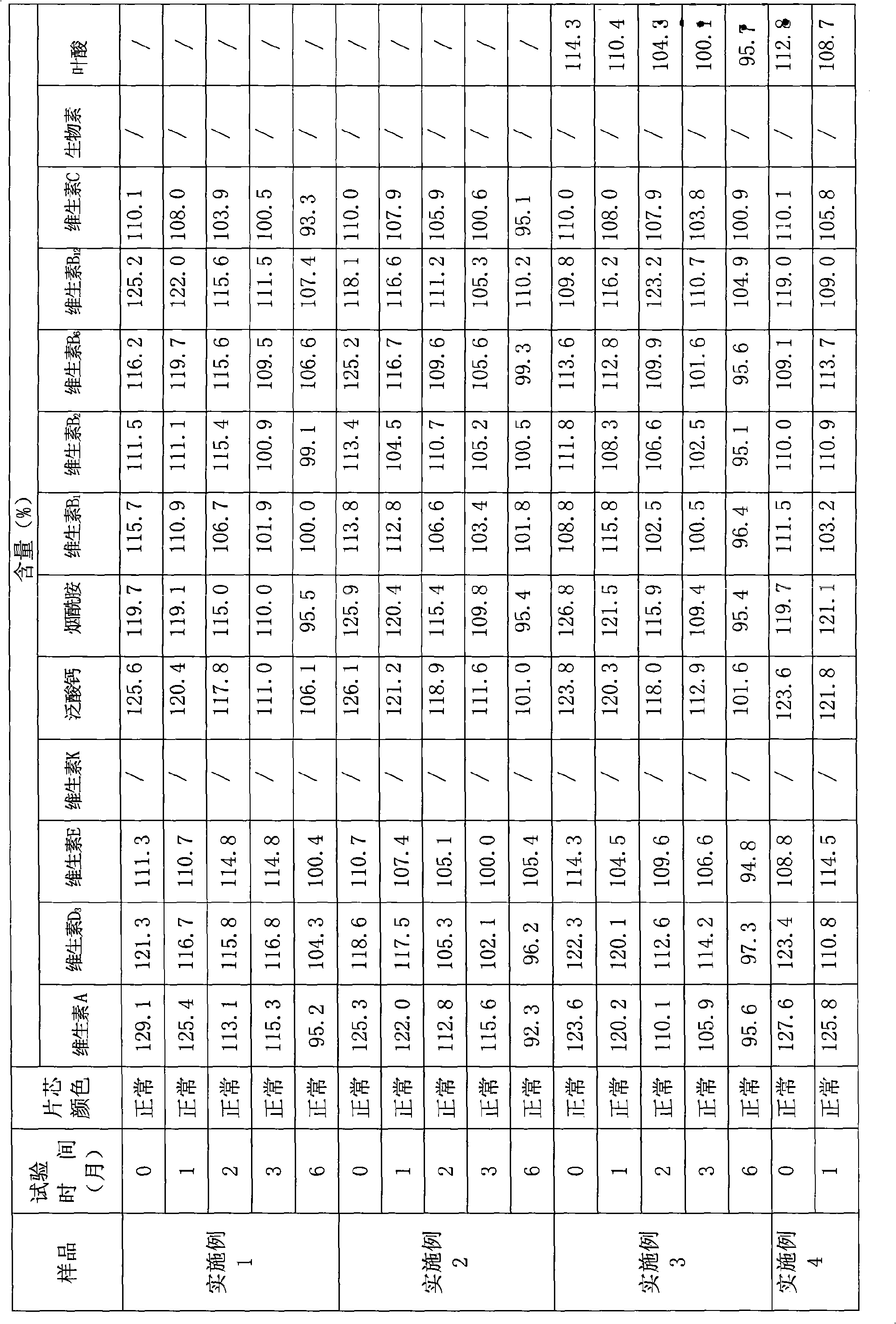
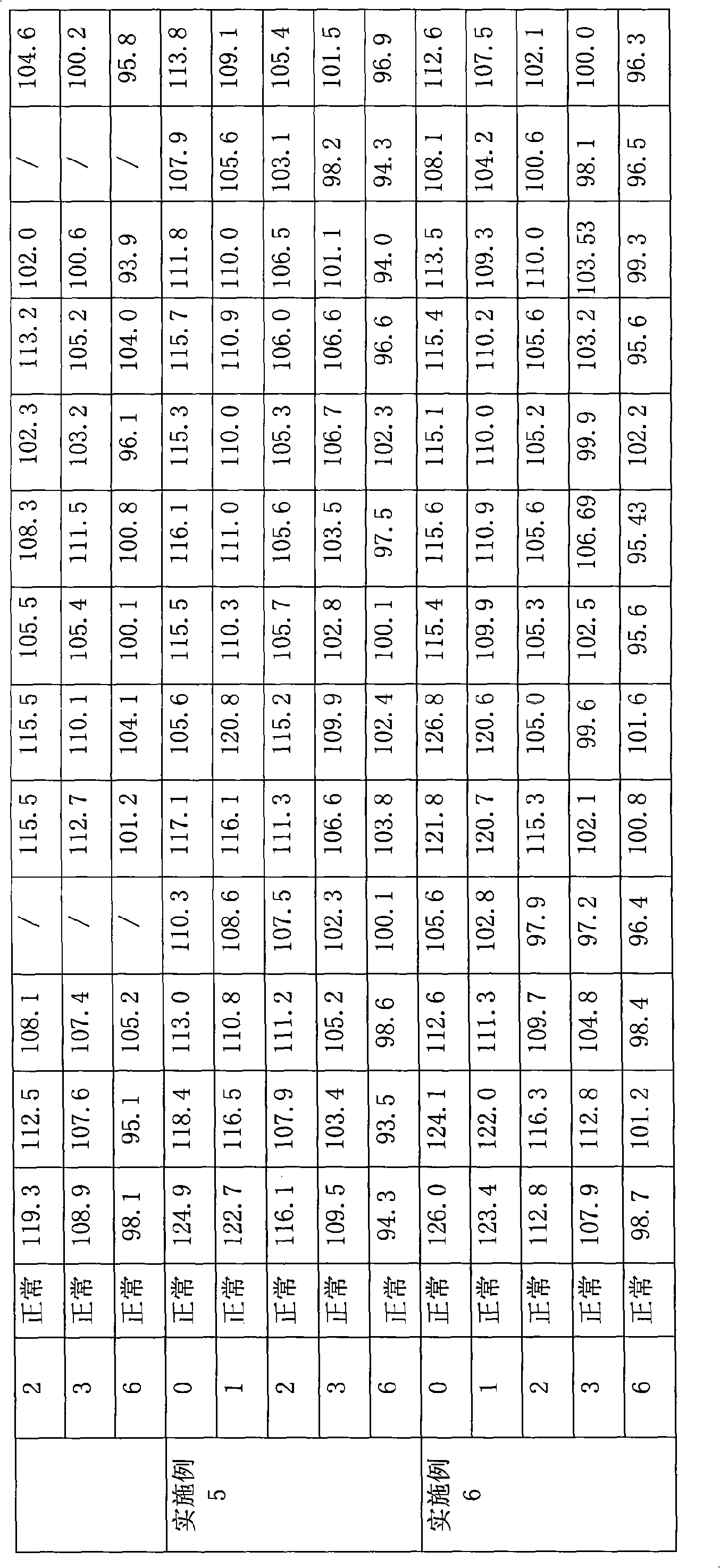
ActiveCN101401809AImprove stabilityNo significant change in colorMetabolism disorderInorganic active ingredientsBeta-CarotenePhosphate
The invention provides a process for preparing a composition of vitamins and minerals, which contains the following components in each 1,000 tablets: 1 to 5 x 10<6>IU of vitamin A, 0 to 2 grams of beta-carotene, 0.75 to 4 x 10<5>IU of vitamin D, 0 to 30 milligrams of vitamin K, 0.5 to 5 grams of vitamin B1, 0.5 to 5 grams of vitamin B2, 0.25 to 1 gram of vitamin B6, 0.5 to 2 milligrams of vitamin B12, 25 to 100 grams of vitamin C, 0 to 0.4 grams of folic acid, 0 to 30 milligrams of biotin, 5 to 15 grams of vitamin E, 5 to 15 grams of nicotinamide, 2.5 to 10 grams of calcium pantothenate, 0 to 50 grams of heavy choline bitartrate, 0 to 100 milligrams of iodine, 0 to 25 grams of L-lysine salt, 0 to 50 grams of inositol, 5 to 10 grams of potassium, 5 to 15 grams of iron, 0.5 to 1 grams of copper, 0 to 50 milligrams of selenium, 0 to 120 grams of magnesium, 0 to 2 grams of manganese, 0.25 to 15 grams of zinc, 0 to 20 milligrams of chromium, 0 to 25 milligrams of molybdenum, 163 to 558 grams of bicalcium phosphate, 0 to 600 grams of calcium carbonate, 50 to 100 grams of starch, 30 to 60 grams of lactose, 100 to 200 grams of microcrystalline cellulose, 20 to 50 grams of dextrin, 15 to 50 grams of cross-linked sodium carboxymethyl cellulose, 0 to 30 grams of low substituted hydroxypropyl cellulose, 8 to 16 grams of tartaric acid, 5 to 20 grams of talcum powder, 8 to 12 grams of superfine silica gel powder, and 6 to 10 grams of magnesium stearate. The process is characterized in that the vitamin C adopts powder coating, and the coating material is methyl acrylate copolymer, preferably a mixture of a methacrylic acid / ethyl acrylate (1 to 1) copolymer and an ethyl acrylate / methyl methacrylate (2 to 1) copolymer, wherien the weight ratio of mixture is between 1 to 4 and 4 to 1. The composition of the vitamins and the minerals has simple preparation process and convenient operation, and can improve the stability of the vitamins.
Owner:HANGZHOU MINSHENG HEALTHCARE CO LTD
Hollow capsule of natural plant gum and its preparing process
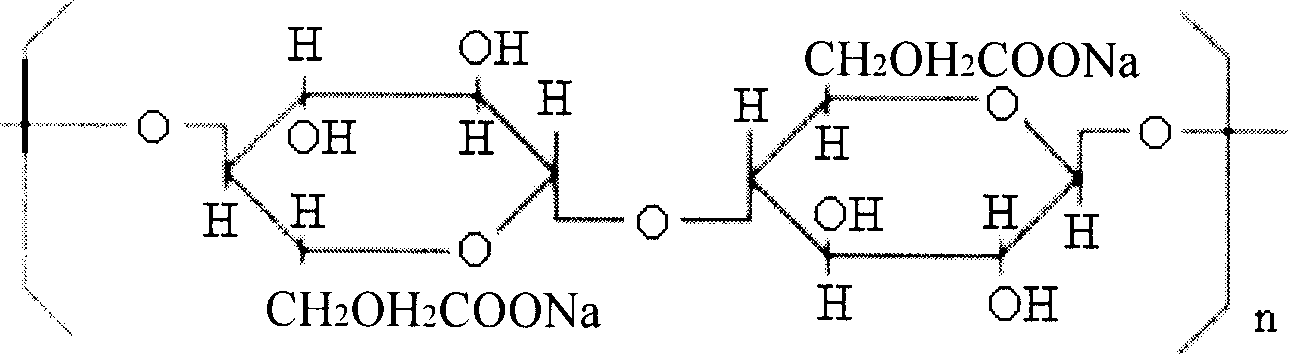
Said invention relates to formula and preparation of plant hollow capsule for packaging powdered preparation, which is composed of 16-25 % of vegetable gelatin, 2.0-5.0 % of intensifier, 0.02-0.026 % of surface active agent, 0.15-0.20 % of moistening agent, the others is water. The said vegetable gelatin is one of or both of pectin and carrageenin, said intensifier is one kind or more than one kind of chitosan, carboxymethyl starch and hydroxyporpyl cellulose. Said surface active agent is sodium dodecyl sulfate. Said moistening agent is glycerol. Said plant hollow capsule has good stability, stored for long time and also can be produced by utilizing the equipment used in producing capsule from geltin.
Owner:ZHEJIANG YAOLIAN CAPSULES
High-toughness inorganic material/polymer composite concrete and preparation method thereof
The invention discloses high-toughness inorganic material / polymer composite concrete and a preparation method thereof. The preparation method of the high-toughness inorganic material / polymer composite concrete comprises the following steps: modifying carbon nanometer tubes through a chemical reaction to obtain modified carbon nanometer tubes of which the surface is chemically grafted with high-substituted hydroxyproxyl cellulose, adding the modified carbon nanometer tubes in polyacrylate emulsion and stirring and mixing with water, a polyacrylate toughening emulsion, concrete, gravel, a fine aggregate, slag powder, fly ash and a water reducer as raw materials, thus obtaining the high-toughness inorganic material / polymer composite concrete. Through detection, the high-toughness concrete provided by the invention can show the significant strain hardening or deformation hardening characteristics under the action of a direct tensile load or a bending load, the bending strength can reach 19-29MPa, the tensile strength can reach 8-16MPa and the compressive strength can reach 78-95MPa. The high-toughness inorganic material / polymer composite concrete can be applied to fields such as bridge floor of large-span cross-sea / cross-river bridges, high-speed rail overhead viaducts and arch walls of various tunnels such as subway.
Owner:FUJIAN JUAN CONSTR ENG CO LTD
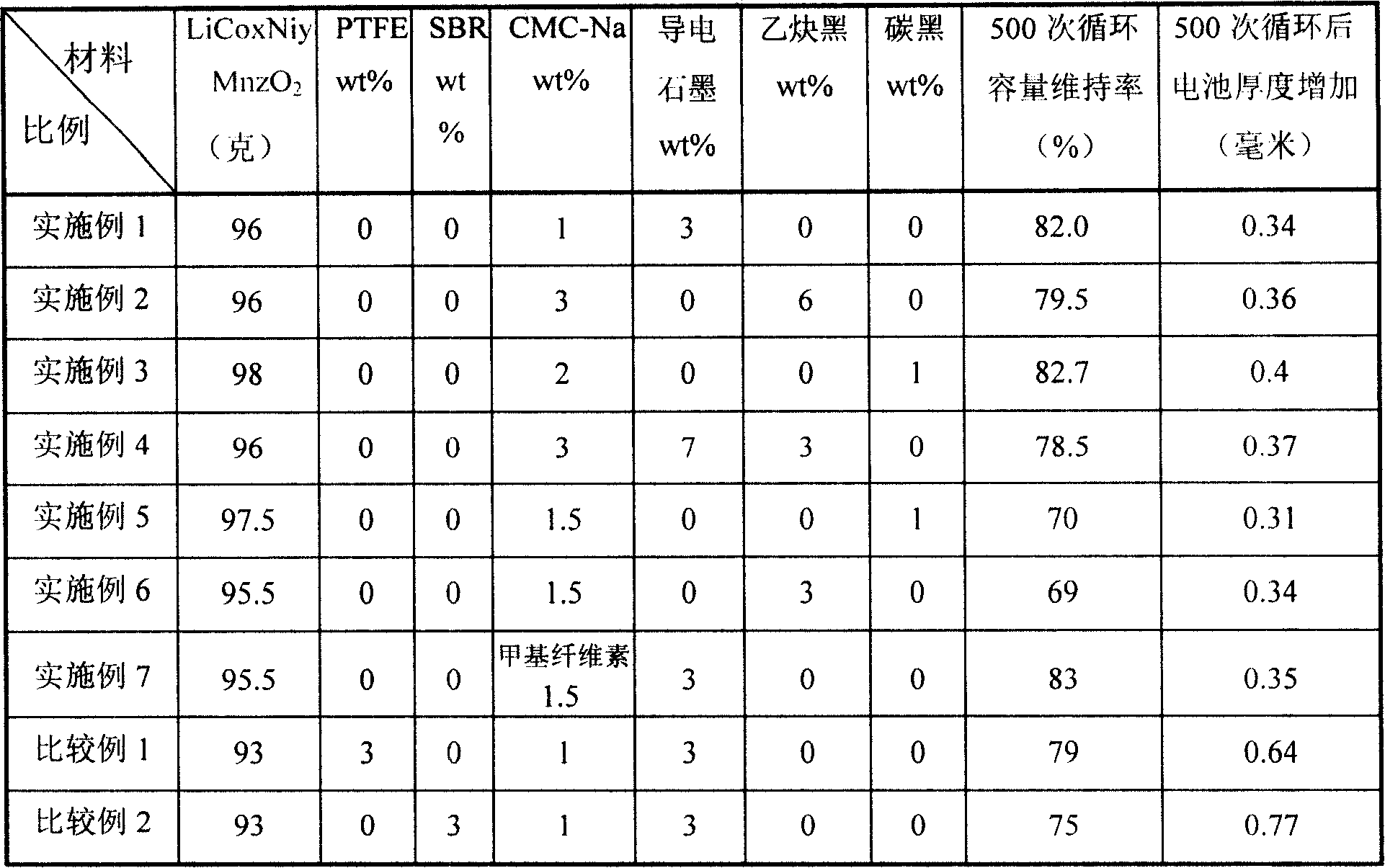
Preparation method of battery positive plate, positive plate and lithium ionic cell
InactiveCN101162773AImprove stabilityReduce distortionElectrode manufacturing processesSecondary cellsCarboxymethyl celluloseFoaming agent
The present invention relates to a method for preparing a lithium-ion battery positive plate, comprising the following procedures: a caking agent is added into the water for dissolution; then an anode active substance is added to the prepared caking agent to mix and prepare a sizing agent; the prepared sizing agent is coated on a collecting fluid; then the lithium-ion battery positive plate is achieved through baking, preforming and slitting; the caking agent is chose from one kind or a plurality of kinds of sodium carboxymethyl cellulose, methylcellulose, hydroxypropyl methylcellulose, carboxymethyl hydroxyethyl cellulose or hydroxypropyl cellulose; the dosage of the caking agent is 0.8 weight percent to 3 weight percent of the battery active substance. The invention also relates to the positive plate made by the method and comprises a lithium-ion battery of the positive plate. The battery achieved by using the positive plate made by the preparation method of the invention has stable electrical property and good cycle performance.
Owner:BYD CO LTD
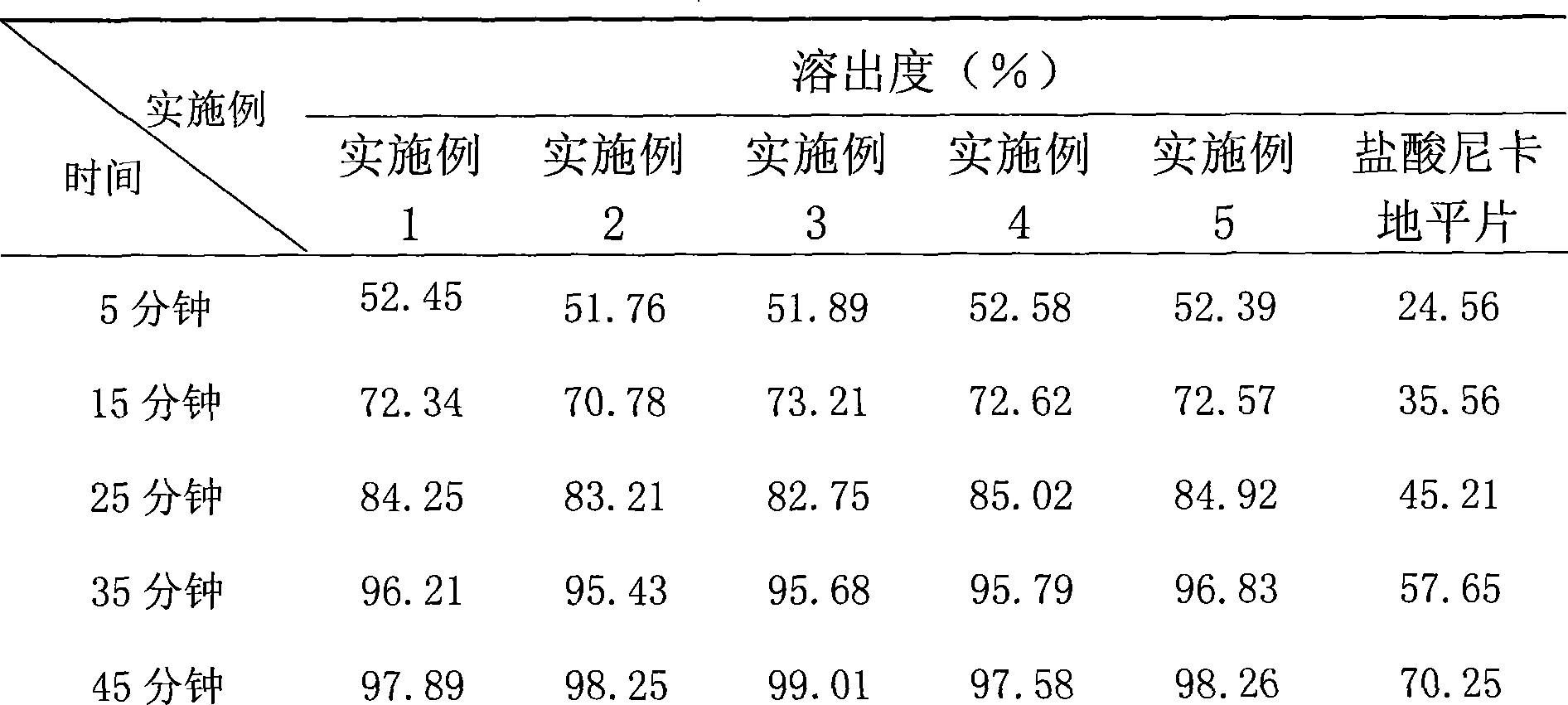
Nicardipine hydrochloride dispersion piece and method for making same
InactiveCN101156851AShort disintegration timeGood dispersionOrganic active ingredientsPill deliveryCross-linked polyethyleneStevioside
The invention discloses a nicardipine hydrochloride dispersible tablet which is characterized in that the nicardipine hydrochloride dispersible tablet is composed of components with the following weight percentage: 8 to 12 percent of nicardipine hydrochloride, 35 to 55 percent of starch, one or more than one of microcrystalline cellulose and lactose, 35 to 50 percent of sodium carboxymethyl starch, one or more than one of hydroxypropyl cellulose, hydroxypropyl methylcellulose, crosslinked polyvinyl pyrrolidone, and crosslinked sodium carboxymethyl cellulose, 5 to 2.5 percent of saccharin sodium or stevioside, and 0.25 to 1 percent of magnesium stearate. The disintegration time of the nicardipine hydrochloride dispersible tablet is short, the dispersed state is good, the drug is dissolved rapidly, the administration is convenient and flexible, the tablet can not only be sucked and swallowed, but also can be taken after being dispersed by adding water. The invention also discloses a preparation method of the nicardipine hydrochloride dispersible tablet.
Owner:刘全胜
Controlled release pharmaceutical compositions of carbidopa and levodopa
The present invention relates to controlled release pharmaceutical compositions of carbidopa and levodopa that include a combination of different molecular weight cellulose ethers and in particular, hydroxypropyl cellulose ether.
Owner:RANBAXY LAB LTD
Multipolymer emulsion, preparation method thereof, and cement mortar prepared from multipolymer emulsion
InactiveCN101591413AImprove compactnessImprove workability(Hydroxyethyl)methacrylatePolyethylene glycol
The invention relates to the technical fields of polymer emulsion preparation and chemical building materials, in particular to a preparation method for multipolymer emulsion, application of the synthesized multipolymer emulsion as an admixture in waterproof mortar, the multipolymer emulsion, and cement mortar prepared from the multipolymer emulsion. The multipolymer emulsion is prepared from raw materials of styrene, methyl methacrylate, butyl acrylate, acrylic acid, beta-hydroxyethyl methacrylate, thermal decomposition initiator and complex emulsifying agent through polymerization by a core-shell emulsion polymerization method; and the cement mortar prepared from the multipolymer emulsion is prepared by mixing raw materials of yellow sand, cement, hydroxypropyl cellulose ether, polyethylene glycol and the multipolymer emulsion, wherein the mass ratio of the yellow sand to the cement to the hydroxypropyl cellulose ether to the polyethylene glycol to the multipolymer emulsion is 600:400:0.2:2:12-20. The multipolymer emulsion solves the problem of poor waterproofness of the prior mortar prepared from acrlate (SA) latex polymer, and improves the workability of the mortar and the compactness of the cement mortar.
Owner:爱福家居(江苏)有限公司
Water dispersible film
InactiveUS20050070501A1Avoid difficultyAntibacterial agentsOrganic active ingredientsDiseaseComposite film
Owner:NEW YORK BLOOD CENT
Chemical sensors using coated or doped carbon nanotube networks
ActiveUS7801687B1Analysing fluids using sonic/ultrasonic/infrasonic wavesNanotechHydrogen halidePolyvinyl alcohol
Methods for using modified single wall carbon nanotubes (“SWCNTs”) to detect presence and / or concentration of a gas component, such as a halogen (e.g., Cl2), hydrogen halides (e.g., HCl), a hydrocarbon (e.g., CnH2n+2), an alcohol, an aldehyde or a ketone, to which an unmodified SWCNT is substantially non-reactive. In a first embodiment, a connected network of SWCNTs is coated with a selected polymer, such as chlorosulfonated polyethylene, hydroxypropyl cellulose, polystyrene and / or polyvinylalcohol, and change in an electrical parameter or response value (e.g., conductance, current, voltage difference or resistance) of the coated versus uncoated SWCNT networks is analyzed. In a second embodiment, the network is doped with a transition element, such as Pd, Pt, Rh, Ir, Ru, Os and / or Au, and change in an electrical parameter value is again analyzed. The parameter change value depends monotonically, not necessarily linearly, upon concentration of the gas component. Two general algorithms are presented for estimating concentration value(s), or upper or lower concentration bounds on such values, from measured differences of response values.
Owner:NASA
Oral disintegrant tablet and its preparation method
InactiveCN1476826AGreat tasteShort disintegration timePill deliveryPharmaceutical non-active ingredientsCross-linkOrally disintegrating tablet
The present invention relates to an oral disintegration tablet and its preparation method. It includes the medicinal active component and auxiliary material. Auxiliary material includes diluting agent and disintegrant, in which the diluting agent is mannite and lactose and the disintegrant is microcrystal cellulose, low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone, the weight of disintegrant is less than 25% of total weight of the auxiliary material, the weight ratio of medicinal active component and auxiliary material is 1:2-75. Said disintegration tablet has enough hardness (strength).
Owner:北京中西经纬释药技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com