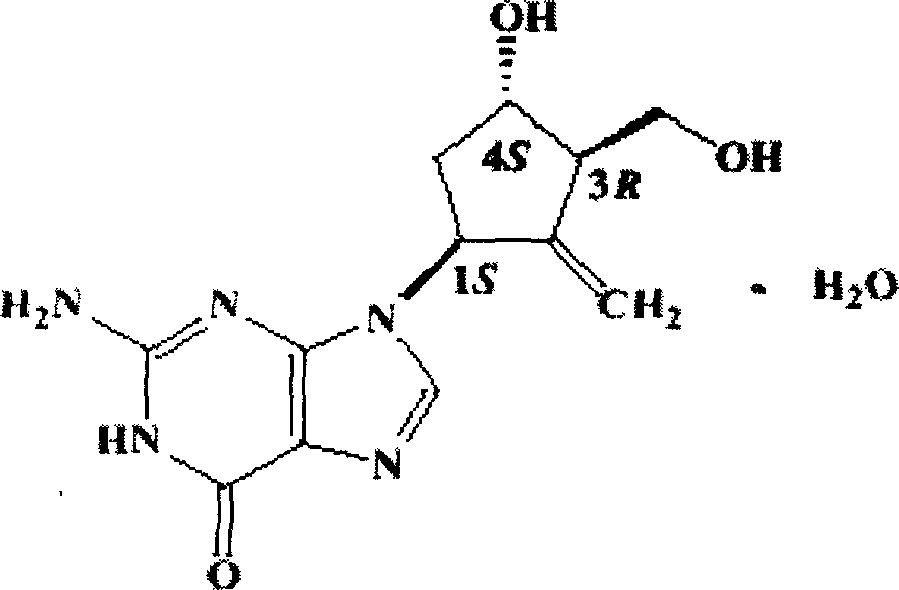
Patents
Literature
1278 results about "Dispersible tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
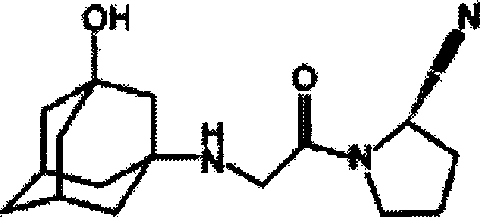
Pharmaceutical composition containing diabetosan and vildagliptin and preparation thereof
InactiveCN101234105AInhibits non-enzymatic glycation reactionsControl fluctuationsMetabolism disorderPill deliveryEffervescent tabletAdditive ingredient
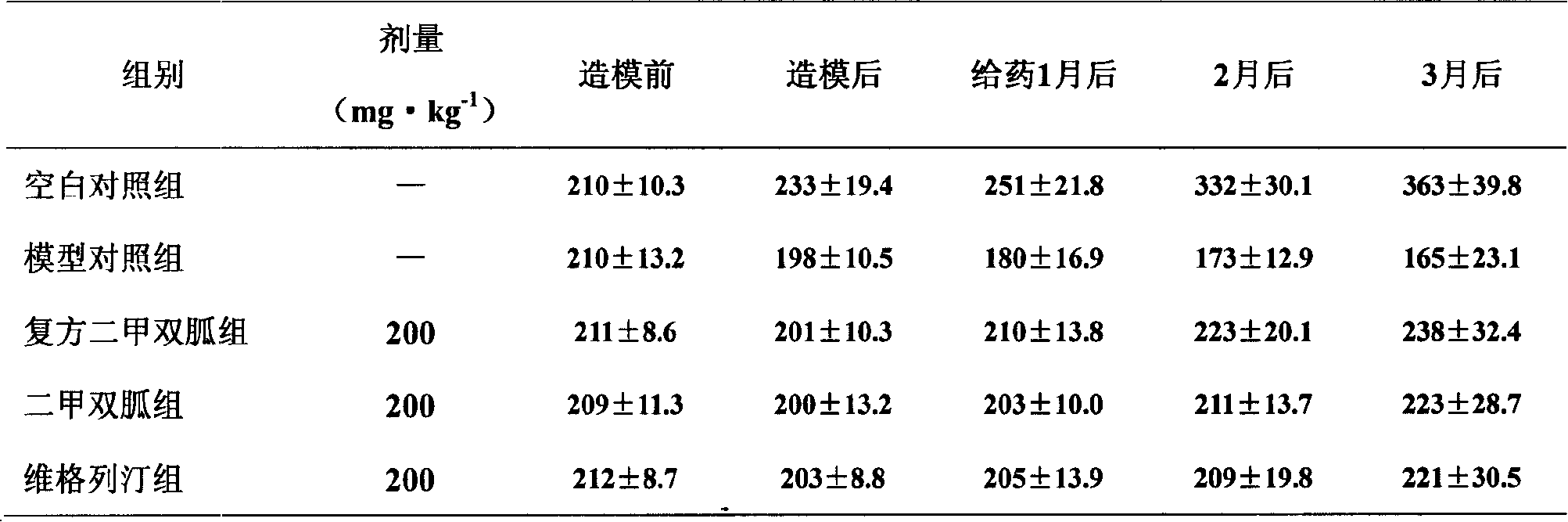
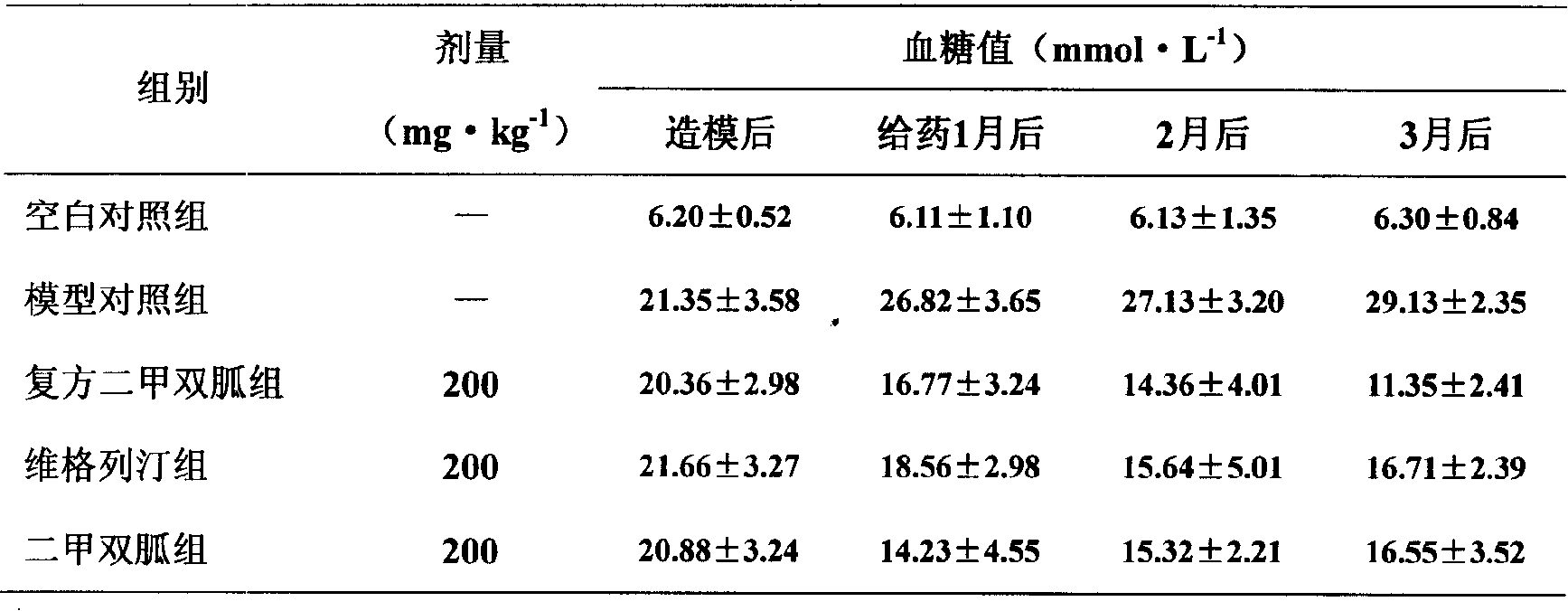
The invention relates to medical composition containing metformin and Vildagliptin and a preparation method thereof. The medical composition is formed by taking metformin chloride and Vildagliptin as medical active components and blending with pharmaceutically acceptable auxiliary materials; the invention adopts the metformin chloride and Vildagliptin as materials and adds auxiliary materials with some special kinds and proportions to prepare and develop diversified oral preparations such as tablets, capsules, granules, dispersible tablets, chewable tablets, buccal tablets, effervescent tablets, effervescent granules according to the technical method of the invention. The medical composition of the invention can be used for treating diabetes type II that can not be properly cured by alimentary control and sports and also for diabetes type II that can not be controlled by simply using metformin chloride or Vildagliptin.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Novel Dispersible Tablet Composition
ActiveUS20080312168A1Reduce sedimentation rateDisintegrates quicklyBiocideCarbohydrate active ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to a novel dispersible tablet composition, which comprises of a pharmacologically active ingredient and at least one excipient, which reduces the sedimentation rate of active ingredient. This invention further relates to a process for the preparation of a dispersible tablet of a pharmacologically active ingredient.
Swertia mileensis dispersible tablet and preparation method thereof
ActiveCN102600234AThe active ingredients are fully extractedHigh content of active ingredientsDigestive systemAntiviralsSwertia mileensisHigh energy
The invention discloses a swertia mileensis dispersible tablet and a preparation method thereof. The preparation method is characterized by comprising the following steps: extracting swertia mileensis by adopting a carbon dioxide supercritical extraction method; drying at reduced pressure; crushing the swertia mileensis into nano-paste by using a high-energy nano-impact mill; adding a functional auxiliary material to prepare the swertia mileensis dispersible tablet. By adopting the preparation method, the contents of effective components are remarkably improved, the disintegration time is remarkably shortened, the curative effect is remarkably superior to the swertia mileensis tablet, and a positive effect is obtained.
Owner:GUIPING PRODIVITY PROMOTION CENT
Taste-masked oral formulations of influenza antivirals
The present invention relates to taste-masked oral formulations of influenza antivirals. The taste-masked pharmaceutical formulations for oral administration comprise one or more influenza antivirals, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. Further, the taste-masked influenza antiviral formulations of the present invention are provided in the form of dispersible tablets, effervescent tablets, orally disintegrating tablets, chewable tablets, bite-dispersion tablets or the like, wherein the bitter taste of influenza antivirals is masked thereby providing palatable formulations.
Owner:RUBICON RES PTY LTD
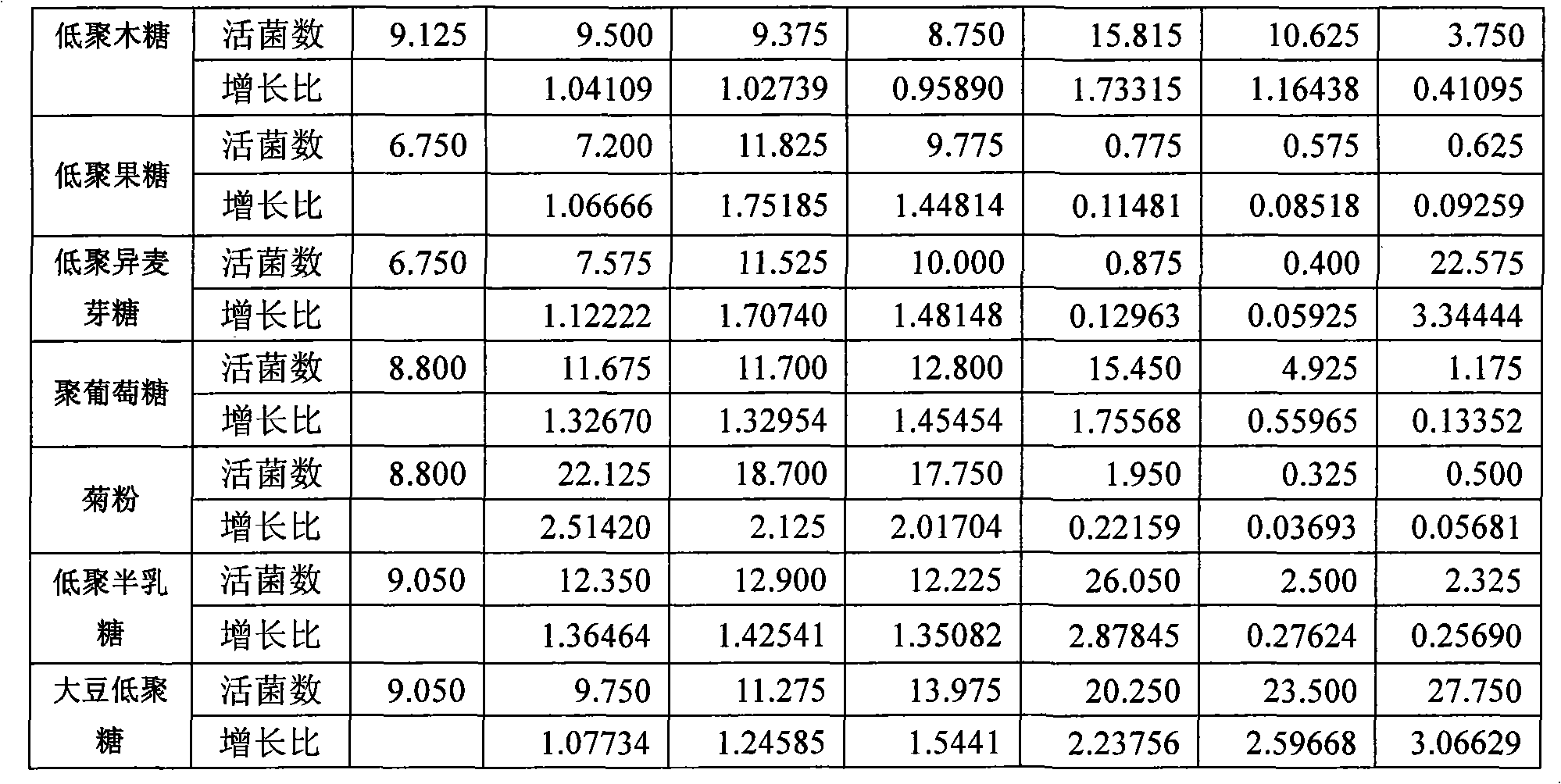
Synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and composition and formulation thereof
ActiveCN101537020APromote growth and reproductionStrong antagonistic effectOrganic active ingredientsBacteria material medical ingredientsBacillus licheniformisSynbiotics
The invention relates to a synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and a composite and a formulation thereof, being characterized in that the synbiotics comprises bacillus licheniformis medical live bacterial powder and the oligosaccharide class prebiotics, wherein the bacillus licheniformis medical live bacterial powder contains live bacteria about 30 billion / gram; the weight ratio of the bacillus licheniformis medical live bacterial powder to the oligosaccharide class prebiotics is 1:1 to 76; the weight ratio of the medical live bacterial powder of the composite, the oligosaccharide class prebiotics and auxiliary materials is as follows: 1.25-5 percent of the bacillus licheniformis medical live bacterial powder, 5-95 percent of the oligosaccharide class prebiotics, 20-50 percent of preferable oligosaccharide class prebiotics, 30-65 percent of diluting agents, 1-20 percent of bonding agents, 1-15 percent of disintegrating agents, 0.1-3 percent of lubricating agents, 1-10 percent of coating agents, 0.01-0.1 percent of flavoring agents, 0.0001-0.001 percent of coloring agents, and 0.1-15 percent of suspending agents. The synbiotics composite is prepared into oral common tablets, oral cavity disintegration tablets, dispersing tablets, enteric-coated tablets, granular formulation, enteric-coated granular formulation, capsules, enteric-coated capsules, dry supensoid agents and external tablets according to a conventional process; and an in vitro test shows that each formulation can promote the growth and the reproduction of the synbiotics of bacillus licheniformis, restrain the growth of harmful bacteria, enhance the immunizing power of parasitifers, reduce diarrhea and enhance the health care.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
A kind of gefitinib dispersible tablet and its preparation method and application
ActiveCN102266300AImprove bioavailabilityPromote dissolutionOrganic active ingredientsPill deliveryMedicineActive agent
The invention discloses a gefitinib dispersible tablet, a preparation method and an application thereof. The gefitinib dispersible tablet of the invention comprises the following components by weight: 10-65% of gefitinib, 1-30% of fillers, 10-50% of disintegrants, 1-60% of acidifiers, 0.1-20% of adhesives, and 0.1-30% of lubricants and glidants. According to the invention, gefitinib is wrapped bythe acidifier or gefitinib and the acidifier are wrapped with each other so as to reach the embedding effect. Compared with commercially available common tablets, the gefitinib dispersible tablet of the invention does not contain surfactants, has good dissolvability, dispersibility and disintegrability, and can be disintegrated completely within one minute. The gefitinib dispersible tablet prepared by the method of the invention has a high dissolution rate, good bioavailability, rapid distribution in vivo, and stable quality, and the preparation method is simple and practical, and is applicable to industrial production.
Owner:GUANGDONG PHARMA UNIV
Dispersion tablet containing ginkgo leaf extract
ActiveCN1528350AImproved rheologySignificant improvementUnknown materialsPill deliveryAdhesiveCuring diseases
The present invention relates to a dispersion tablet preparation containing ginkgo leaf extract and its preparation method, in which for one weight portion of extract said dispersion tablet also contains 0.5-10 weight portions of filling agent, 0.1-5 weight portions of disintegrant, 0.01-0.1 weight portion of lubricant and 0.01-0.1 weight portion of adhesive. Said invented medicine dispersion tablet mainly is used for curing diseases of cardiovascular system, and it is characterized by that it is placed in the warm water with 37 deg.C, it can be completely disintegrated within 3 min.
Owner:JIANGSU CHENPAI PHARM GRP CO LTD
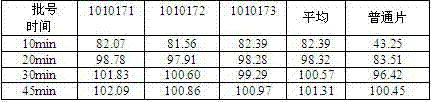
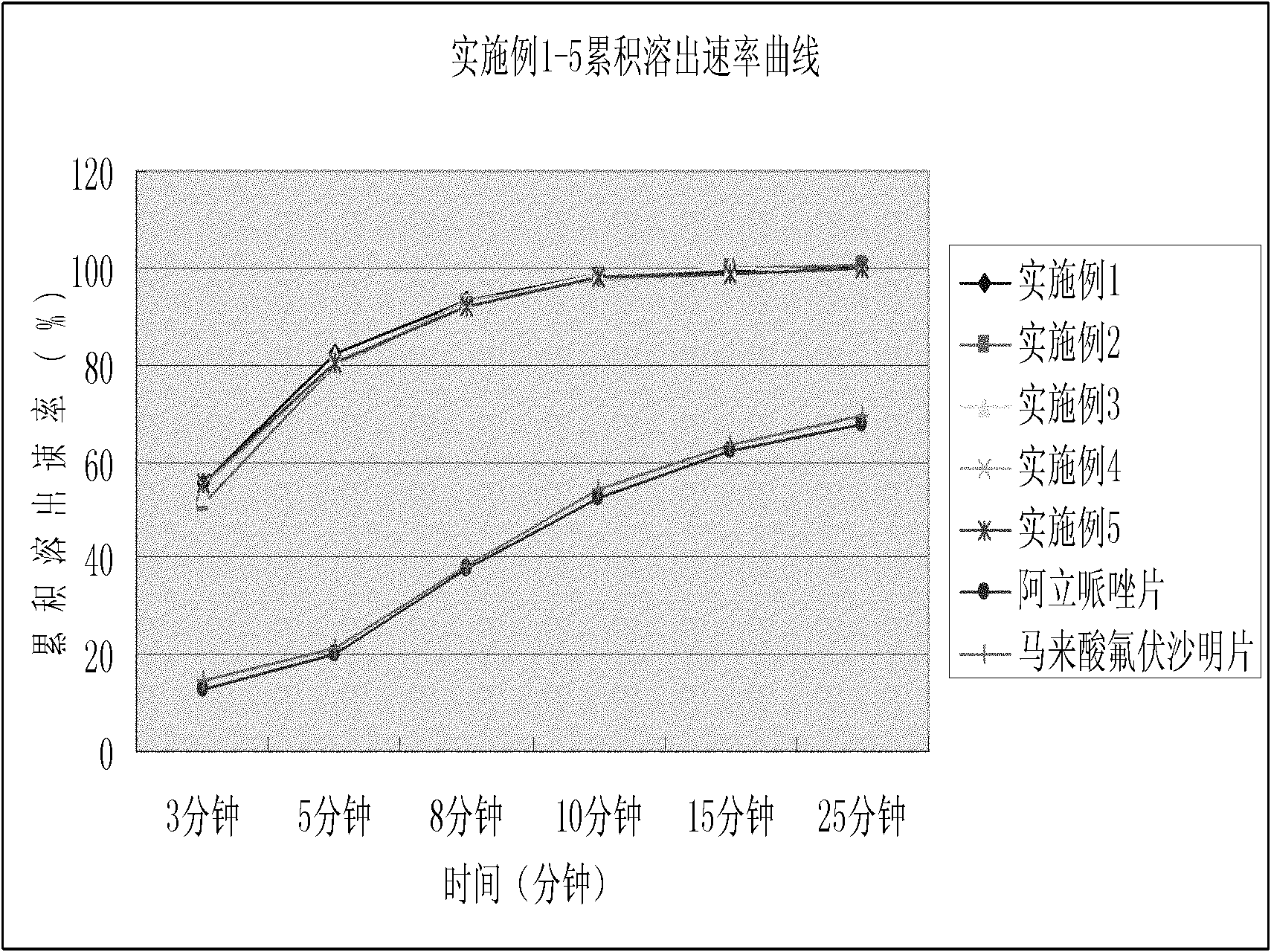
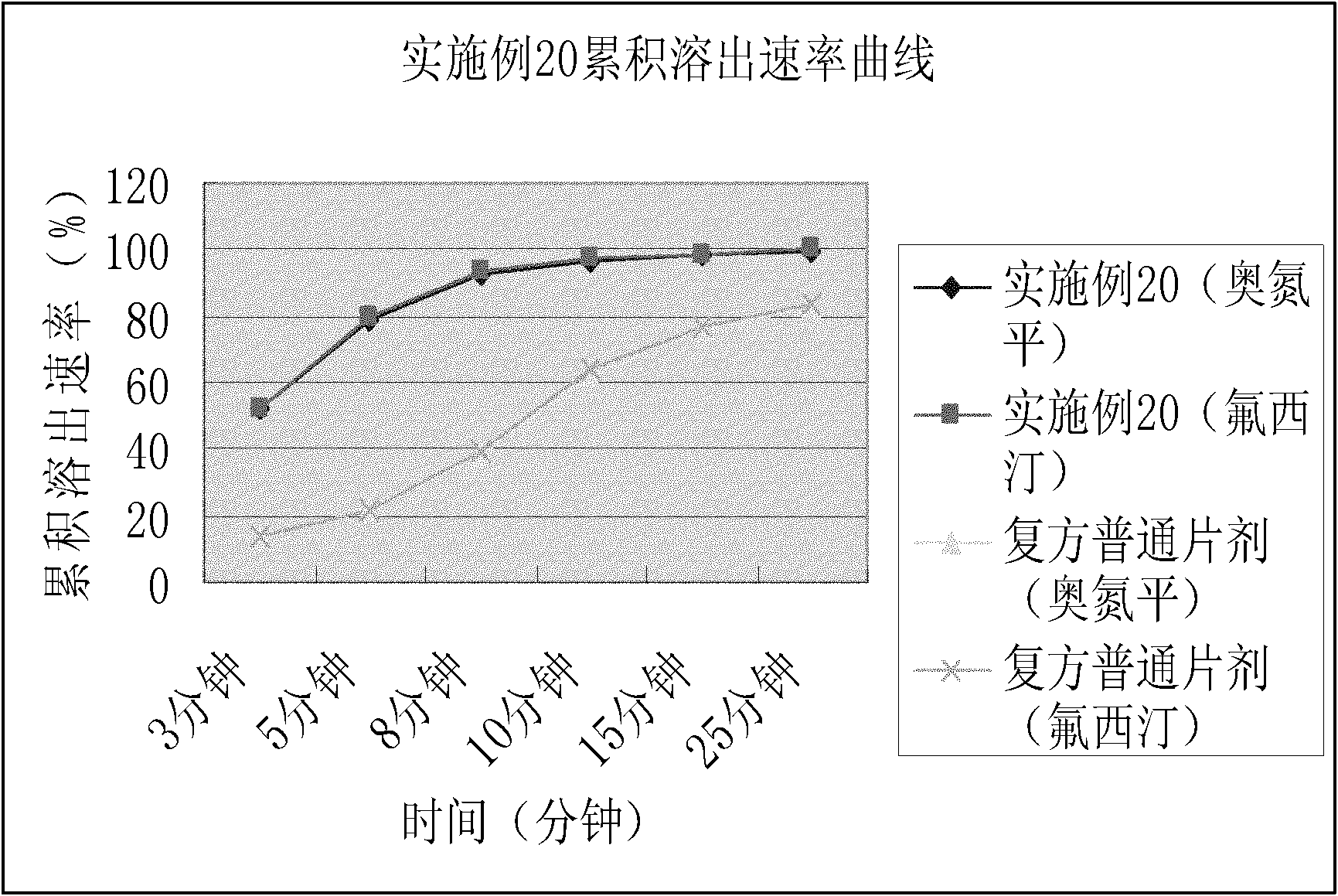
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
Cefixime dispersing tablet and preparation methods thereof
ActiveCN101606913AIncrease contentEasy to takeAntibacterial agentsOrganic active ingredientsMagnesium stearatePharmaceutical preservatives
The invention discloses a cefixime dispersing tablet and preparation methods thereof, belonging to the technical field of pharmaceutical preparation. The cefixime dispersing tablet comprises 40-420 mg of cefixime, 0-100 mg of starch, 0-250 mg of amylum pregelatinisatum, 10-80 mg of mannite, 0-150 mg of microcrystalline cellulose, 10-60 mg of carboxyrnethyl starch sodium, 2-20 mg of polyvidone K30, 0.4-10 mg of magnesium stearate, 0-10 mg of Steviosin and 0-10 mg of orange compound perfume. The preparation method comprises the following steps: evenly mixing basic remedies and excipients; adding adhesive to prepare granulates; drying; sorting and then adding with other excipients; and tabletting. The other preparation method is described as follows: the basic remedies are added after granulating. The invention aims to solve the technical problem that cefixime preparation has shorter disintegration time, better leachability, higher content of the basic remedies, production cost reduction, quality detection time reduction and production environment pollution reduction.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Febuxostat dispersible tablet drug and preparing method thereof
ActiveCN101780073AEasy to prepareLow costOrganic active ingredientsSkeletal disorderAdditive ingredientEther
The invention relates to a febuxostat dispersible tablet drug and a preparing method. The drug is prepared from febuxostat as the active drug ingredient, and acceptable auxiliary ingredients in the dispersible tablet preparation. The febuxostat dispersible tablet drug is characterized in that at least one of polyoxyethylene 40 monostearate ingredient, polyethenoxy ether castor oil ingredient and hydrogenated castor oil polyoxyl ingredient in the auxiliary ingredients is used as a solubilizing agent ingredient and has the usage amount of 0.1-5 times the weight of febuxostat. The drug can obviously enhance the dissolution rate of the insoluble effective drug ingredient of febuxostat, and has the advantages of high drug dispersion degree, high dissolution rate, quick absorption and effect taking, high biological utilization degree, and the like.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Salt of leonurine and its preparation
InactiveCN1415603ALess irritatingGuaranteed stabilityOrganic active ingredientsOrganic chemistryLeonurineOrganic acid
Owner:李晓祥
Injection containing burufen
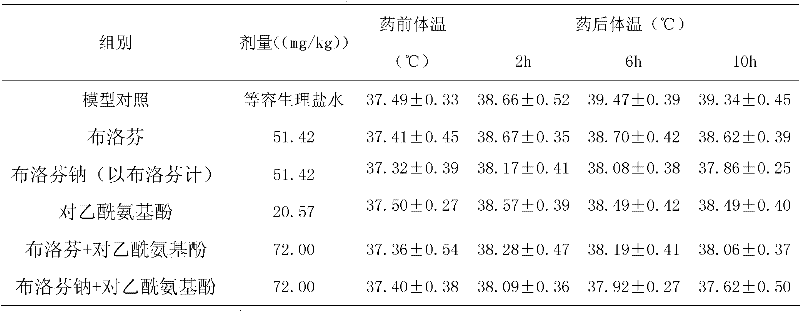
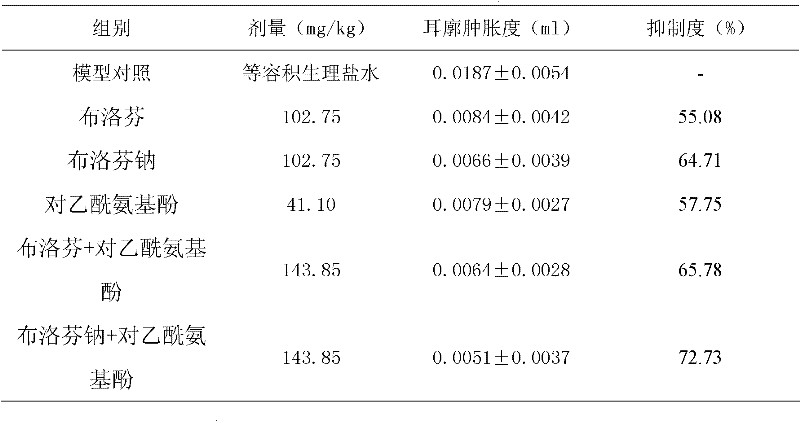
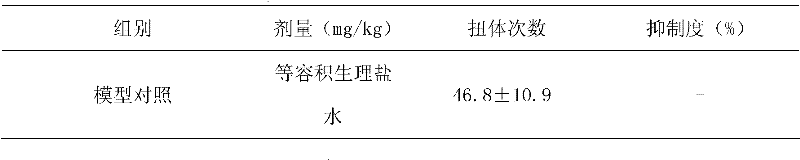
InactiveCN101069681AReduce solubilitySlow absorptionOrganic active ingredientsAntipyreticSolubilityIbuprofen Injection
The present invention provides an injection preparation containing ibuprofen and its preparation method. Said invention provides the concrete steps of its preparation method. Said ibuprofen injection preparation has high solubility and bioavailability, and its product quality is stable.
Owner:汪洪湖
Dispersible tablet containing cefixime and preparation method thereof
The invention belongs to the technical field of pharmaceutical preparation, and in particular relates to a cefixime dispersible tablet and a method for preparing the same. The method comprises the following steps: firstly, weighing cefixime, starch, microcrystalline cellulose, partially cross-linked polyvinylpyrrolidone, partially cross-linked sodium carboxymethyl cellulose, and partially low substituted-hydroxypropyl cellulose, and mixing the components evenly; secondly, dripping 5 percent polyvinylpyrrolidone K30 ethanol solution into the mixture prepare a soft material, and performing granulating through a sieve of between 18 and 24 meshes; thirdly, drying wet particles at a temperature of between 50 and 80 DEG C, palletizing the particles through the sieve of between 18 and 24 meshes, adding the remaining cross-linked polyvinylpyrrolidone, the remaining cross-linked sodium carboxymethyl cellulose, and the remaining low substituted-hydroxypropyl cellulose, magnesium stearate and superfine silica gel powder into the mixture to be mixed evenly, and tabletting the mixture to obtain the required cefixime dispersible tablet. The cefixime dispersible tablet has the characteristics of rapid disintegration with water, even dispersion, high dissolution, and convenient taking and carrying around.
Owner:BEIJING TRADE STAR MEDICAL TECH
Dispersible tablet of colloid petcin
InactiveCN1634132AHeavy metal active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseCross-linked polyethylene
The invention discloses a dispersible tablet of colloid pectin, which comprises (by weight ratio, in mg) colloid pectine bismuth 25-100 (by bismuth), filling agent 60-400, crumbling agent 42-280, flow aid 1-6, lubricant 0.25-5, the filling agent can be selected from lactose, white dextrine, pregelatine starch or / and starch. The crumbling agent can be selected from cross bonding polyvinylpyrrolidone, crystalline cellulose, cross-linked sodium carboxymethylstarch, low substituted methylcellulose propylene glycol ether, sodium carboxymethylstarch, the glidant can be selected from miropowdered silica gel, the lubricant can be selected from magnesium stearate or / and talcum powder.
Owner:HUNAN WARRANT PHARMA
Dispersible tablets containing valsartan and amlodipine besylate and preparation method thereof
InactiveCN101843615AAvoid stabilityAvoid the disadvantages of inconvenient storage and transportationPill deliveryHeterocyclic compound active ingredientsValsartanActive agent
The invention discloses to dispersible tablets containing valsartan and amlodipine besylate and a preparation method thereof, and relates to medicament dispersible tablets and the preparation method thereof, solving the problem of poor stability, slow disintegration and dissolution, low oral bioavailability and high cost existing in the traditional medicine containing valsartan and amlodipine besylate. The dispersible tablets are prepared from valsartan, amlodipine besylate, disintegrant, diluent, adhesive, lubricant, fluidizer, surfactant and flavoring agent. The method comprises the following steps of: weighting and screening materials; mixing the valsartan, disintegrant and diluent to obtain mixed powder A; mixing amlodipine besylate and the mixed powder A to obtain mixed powder B; mixing the adhesive, surfactant and the mixed powder B to prepare soft material; screening, granulating and drying the soft material; mixing and screening and the dried granules with the fluidizer, lubricant, disintegrant and flavoring agent; and finally carrying out size stabilization and tabletting. The medicine prepared by the method has good stability, fast disintegration and dissolution, high oral bioavailability and low cost.
Owner:包丽昕
Rapidly-dispersible tablet and preparation method thereof
InactiveCN107823150AOvercoming stratificationAccurate doseHydrocarbon active ingredientsHydroxy compound active ingredientsWater insolubleActive component
The invention relates to a rapidly-dispersible tablet and a preparation method thereof, in particular to a rapidly-dispersible tablet composition containing no disintegrating agent and a preparation process thereof, which belong to the field of drugs and healthcare products. The invention discloses a rapidly-dispersible tablet. The rapidly-dispersible tablet is prepared from 0.5 to 900 parts by weight of active components, 200 to 900 parts by weight of excipient and 5 to 30 parts by weight of lubricating agents, wherein the active component is a water-insoluble active component. The rapidly-dispersible tablet has the advantages that the rapidly-dispersible tablet composition containing insoluble active component is prepared into the tablet rather than a liquid dosage form, so that a complex process for preparing the insoluble active component into the liquid preparation is not needed, the layering phenomenon of the product liquid during a long-term storage period can also be avoided, and the rapidly-dispersible tablet has accurate dose and stable quality, is convenient to store and carry, can be swallowed and can also be administered after being dissolved.
Owner:北京素维生物科技有限公司
Pesticide water effervescent dispersible tablet and preparation method thereof
InactiveCN102823589AAvoid degradation of active ingredientsReduce the use effectBiocideFungicidesAmmonium Hydrogen CarbonateAmmonia gas
The invention discloses a pesticide water effervescent dispersible tablet and a preparation method thereof. According to the pesticide water effervescent dispersible tablet and the preparation method thereof, ammonium hydrogen carbonate is used as a gas source, and an acid source or alkali source is selected according to the physical property of different raw materials, i.e. the acid source and the ammonium hydrogen carbonate are added into the acid-resistant raw materials, and tablets are put into water to produce carbon oxide during use; and the alkali source and the ammonium hydrogen carbonate are added into the alkali-resistant raw materials, and tablets are put into water to produce ammonia gas. The pesticide water effervescent dispersible tablet can rapidly boil and disintegrate in water and therefore avoid the reduction of application effect caused by degradation of effective components of the pesticide effervescent dispersible tablet, so that the pesticide water effervescent dispersible tablet with stable chemical properties is obtained.
Owner:FIVESTAR NANTONG CHEM
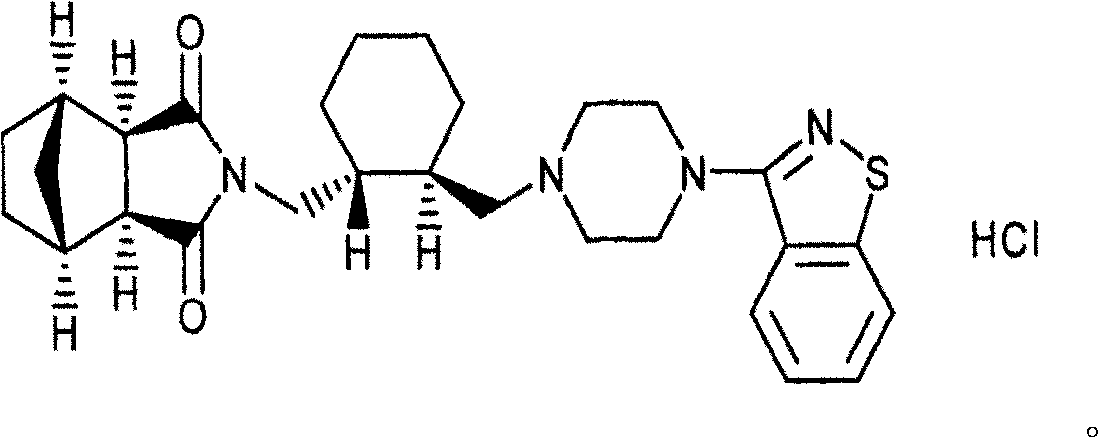
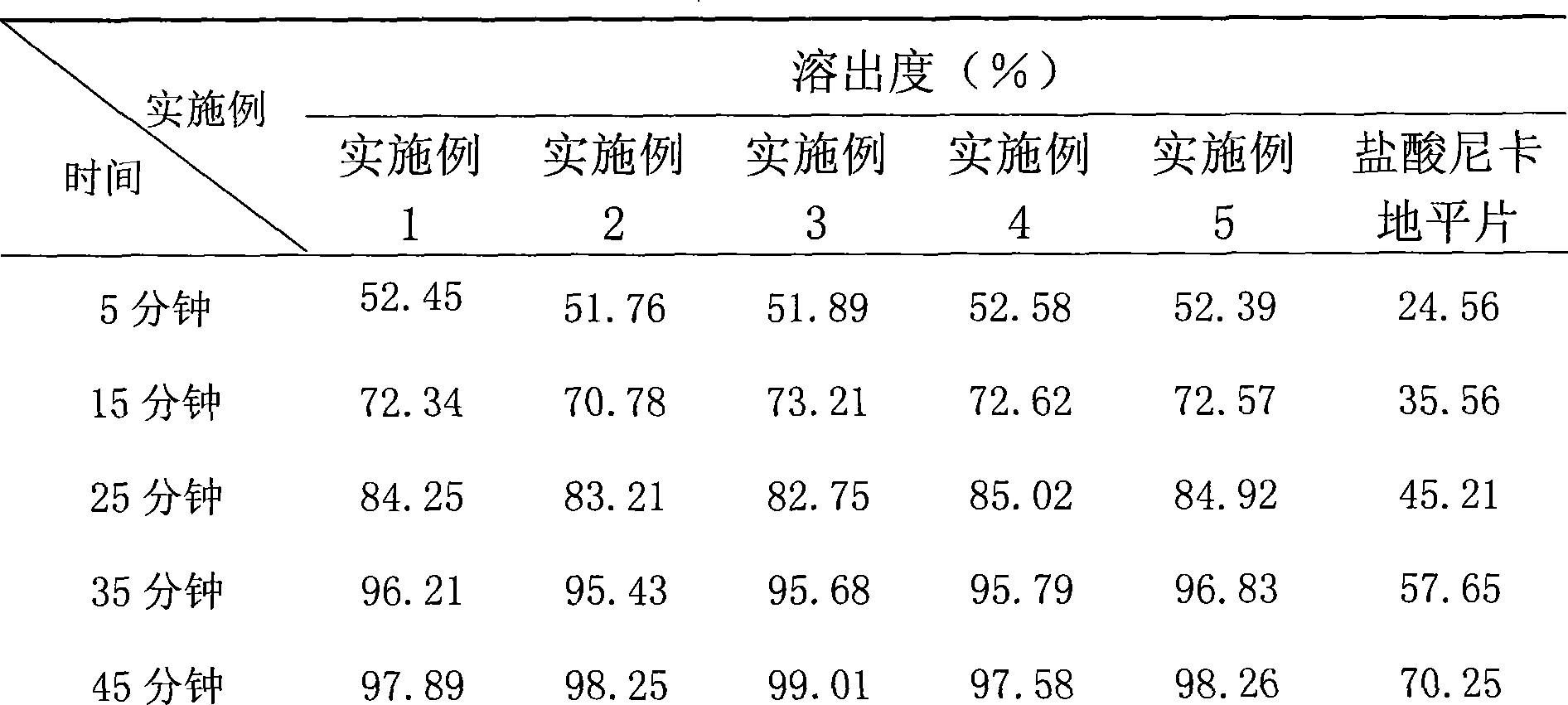
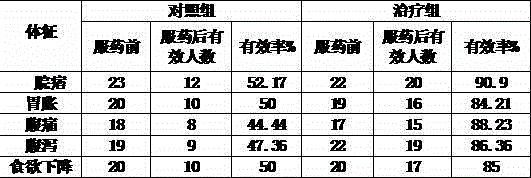
Nicardipine hydrochloride dispersion piece and method for making same
InactiveCN101156851AShort disintegration timeGood dispersionOrganic active ingredientsPill deliveryCross-linked polyethyleneStevioside
The invention discloses a nicardipine hydrochloride dispersible tablet which is characterized in that the nicardipine hydrochloride dispersible tablet is composed of components with the following weight percentage: 8 to 12 percent of nicardipine hydrochloride, 35 to 55 percent of starch, one or more than one of microcrystalline cellulose and lactose, 35 to 50 percent of sodium carboxymethyl starch, one or more than one of hydroxypropyl cellulose, hydroxypropyl methylcellulose, crosslinked polyvinyl pyrrolidone, and crosslinked sodium carboxymethyl cellulose, 5 to 2.5 percent of saccharin sodium or stevioside, and 0.25 to 1 percent of magnesium stearate. The disintegration time of the nicardipine hydrochloride dispersible tablet is short, the dispersed state is good, the drug is dissolved rapidly, the administration is convenient and flexible, the tablet can not only be sucked and swallowed, but also can be taken after being dispersed by adding water. The invention also discloses a preparation method of the nicardipine hydrochloride dispersible tablet.
Owner:刘全胜
Compound dispersible tablet for treating hypertension
The invention relates to a medicinal oral preparation containing Valsartan and Hydrochlorothiazide, more specifically the dispersible tablets for enhancing the beneficial effects of Valsartan and Hydrochlorothiazide.
Owner:江苏万高药业股份有限公司
'Yi Kang Bu Yuan' formulation for benefiting qi, moving blood, invigorating spleen and tonifying kidney, its preparation method and quality control method
InactiveCN1961929AImprove bioavailabilityGood disintegrationDigestive systemPharmaceutical delivery mechanismDiseaseRhizome
The invention relates to a preparation having the functions of promoting blood circulation, enlivening spleen and tonifying kidney, the process for preparation and quality control method, wherein the preparation is produced mainly from astragalus root, Chinese ephedra, Chinese angelica root, herba ecliptae, atractylodes rhizome, safflower, radix paeoniae rubrathe, peach kernels, achyranthes and cyathula root, Ligusticum wallichii, bitter orange and root of balloonflower. The preparation can be made into injections and various oral administration preparations including dripping pills, mini-pills and dispersible tablets. The invention also provides the method for determining and identifying contents of the preparation.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Yueju Baohe dispersible tablets and preparing method thereof
InactiveCN106728634AWith industrial productionImprove solubilityDigestive systemPill deliveryMaterials preparationTraditional medicine
The invention discloses Yueju Baohe dispersible tablets and a preparing method thereof. The Yueju Baohe dispersible tablets are prepared from, by weight, 10-50% of traditional Chinese medicine extract, 10-80% of filler, 5-50% of internally added disintegrant, 5-15% of externally added disintegrant, 0.1-10% of corrigent, 0.1-20% of wetting agent and 0.05-20% of lubricant. The traditional Chinese medicine extract comprises, by weight, 60-120 parts of ginger-processed fructus gardeniae, 60-120 parts of medicated leaven stir-fried with bran, 60-120 parts of vinegar-processed rhizoma cyperi, 60-120 parts of rhizoma chuanxiong, 60-120 parts of rhizoma atractylodis, 60-120 parts of radix aucklandiae and 60-120 parts of semen arecae. The preparing method comprises the steps of material preparation, extraction, tablet preparation and packaging. The Yueju Baohe dispersible tablets can soothe liver and dispel melancholy, whet the appetite and promote digestion, and are used for treating symptoms like dyspepsia, qi depression and stasis, poor appetite and thoracico-abdominal swelling pain.
Owner:HARBIN SHENGJI PHARMA
Entecavir dispersible tablet and its preparation process
ActiveCN1732944ASlow disintegrationImprove bioavailabilityDigestive systemAntiviralsPharmaceutical industryEntecavir
The invention relates to an Entecavir dispersible tablet for treating hepatitis B and process for its preparation by using Entecavir as raw material, charging auxiliary materials of specific species and proportions, and preparing tablet through the conventional techniques in the pharmaceutical industry.
Owner:HAINAN ZHONGHE PHARM CO LTD
Medicinal composition containing ibuprofen sodium salt
InactiveCN102389423AOrganic active ingredientsNervous disorderSustained Release TabletBULK ACTIVE INGREDIENT
The invention relates to a medicinal composition containing ibuprofen sodium salt, which is prepared by taking ibuprofen sodium salt and other one or a plurality of medicinal components as active ingredients, and combing the active ingredients with pharmaceutically appropriate auxiliary materials. The medicinal composition can be made into oral preparations, including conventional tablets, dispersible tablets, sustained release tablets, capsules, sustained release capsules, soft capsules, particles, dry suspension, suspension and the like, and used for symptomatic treatment of being antipyretic, antiphlogistic, analgesic and anti-allergic, and improving sleeping.
Owner:FUKANGREN BIO PHARMA
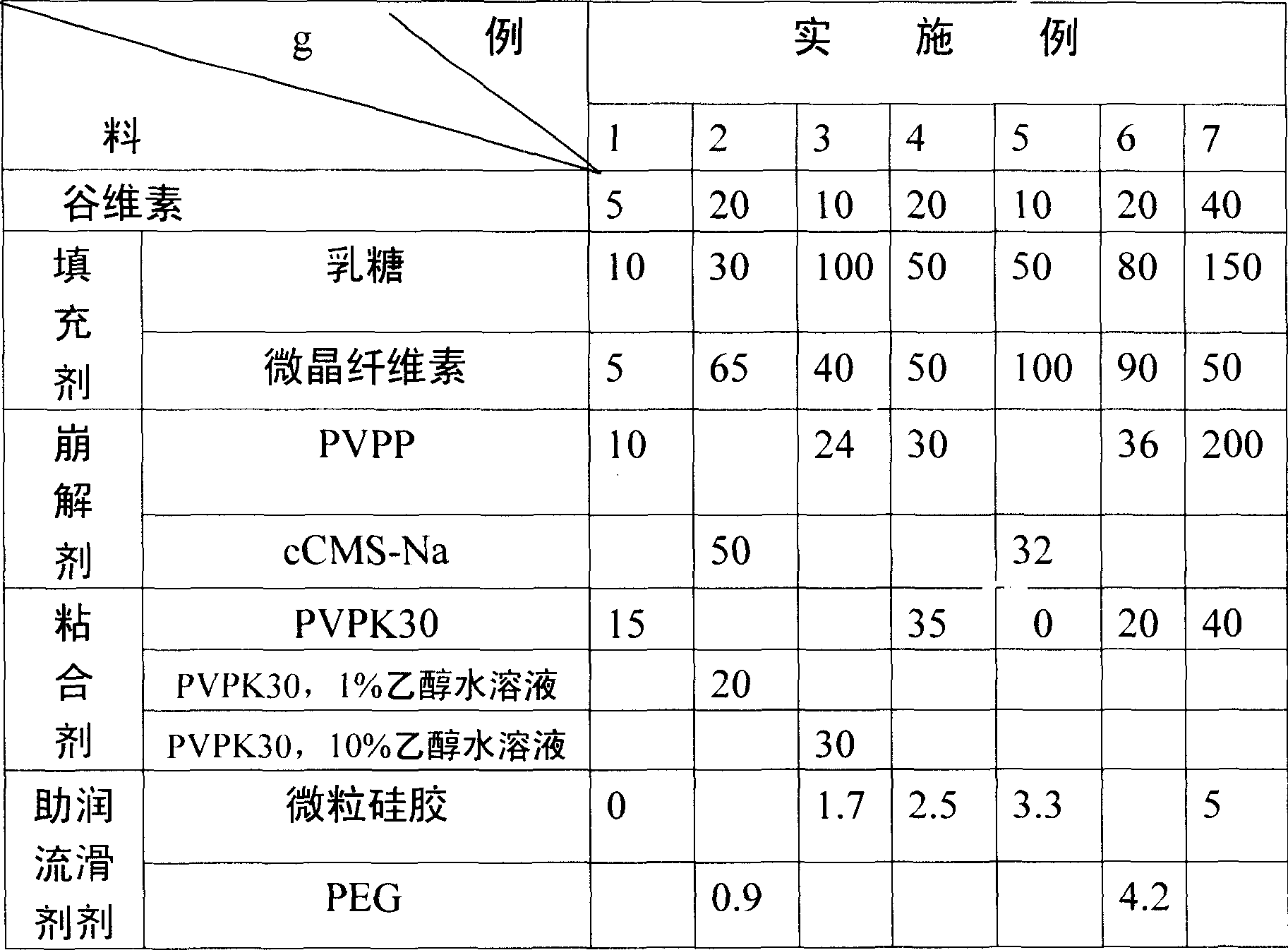
Dispersion tablet medicine containing oryzanol and its preparation method
InactiveCN1839850AImprove complianceMeet medication needsOrganic active ingredientsNervous disorderDiseaseAdjuvant
The invention relates to a dispersible tablet containing oryzanol for treating neurological system diseases and its preparing process, wherein the tablet comprises (by weight parts): oryzanol 5-200, filling agent 20-200, crumbling agent 15-40, bonding agent 1-70, flowing adjuvant or lubricating agent 0-5.
Owner:马志梅
Hydrochloric acid cefetamet pivoxil dispersible tablet and method for preparing the same
ActiveCN101219124AThe content of the main drug is uniformGood content uniformityAntibacterial agentsOrganic active ingredientsDissolutionBULK ACTIVE INGREDIENT
The invention discloses a cefetamet pivoxil hydrochloride tablet and a preparation method thereof. On the premise of specific active ingredients of the cefetamet pivoxil hydrochloride, the invention takes into account the varieties and dosages of disintegrating agents and joint usage thereof, suitable components and ratios of bonding agents and other filling agents, and the micronization treatment of the raw and auxiliary materials and selection of corresponding optimized process conditions. Experiment results indicate that compared with the prior art, the product of the invention has quick disintegration and dissolution and stable quality.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
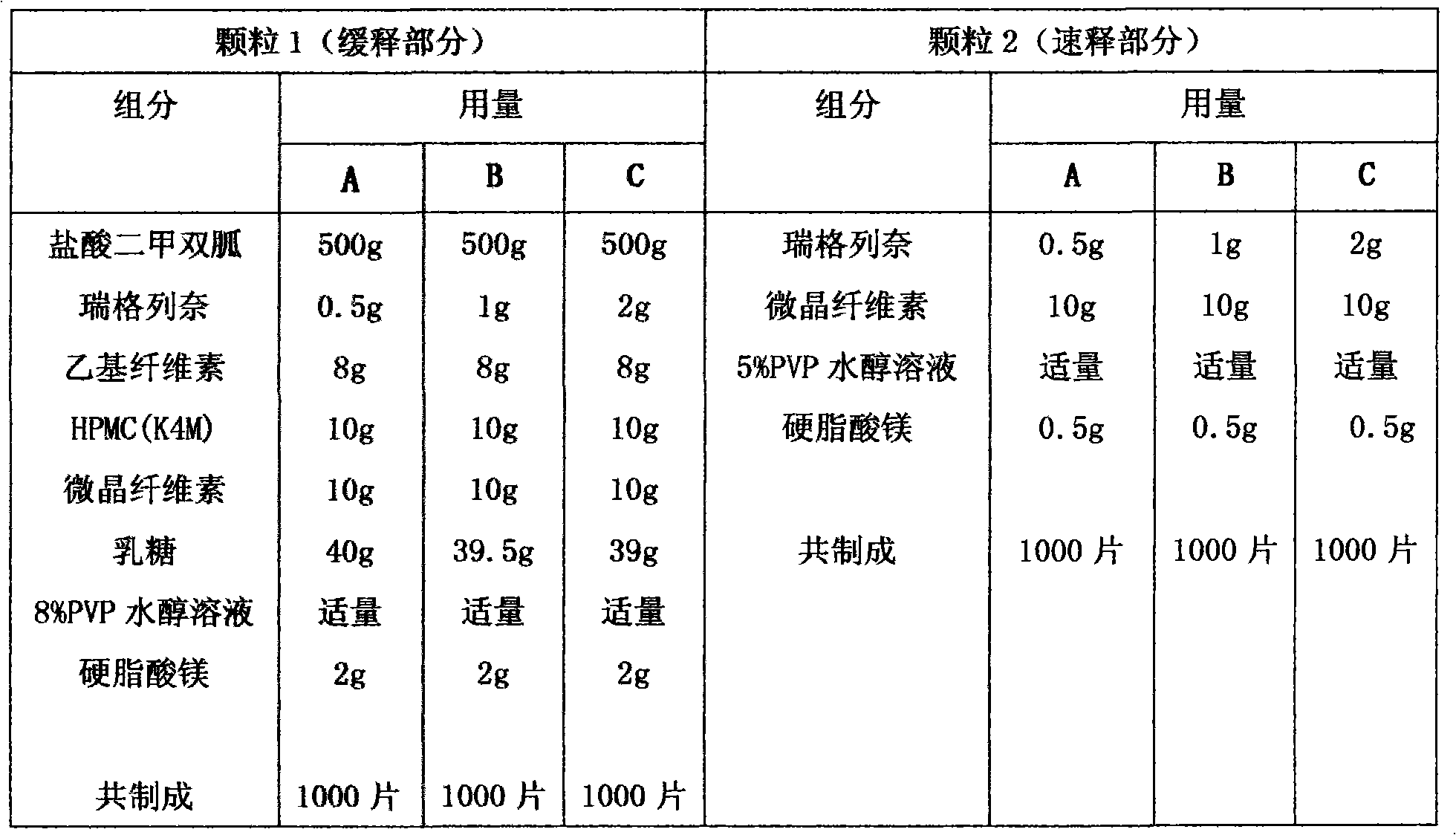
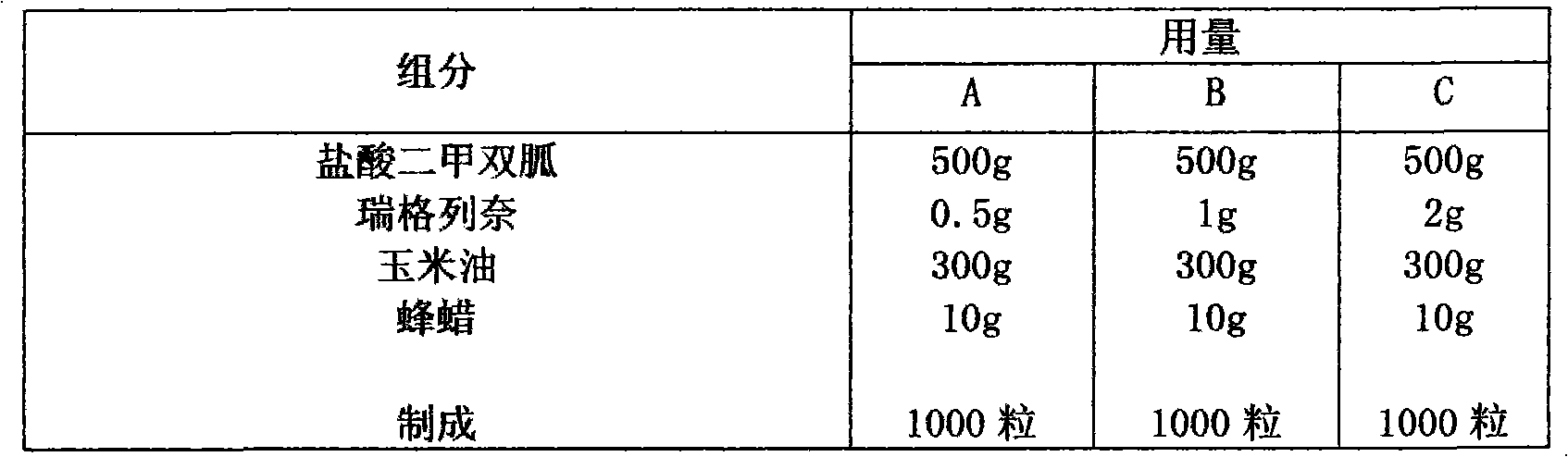
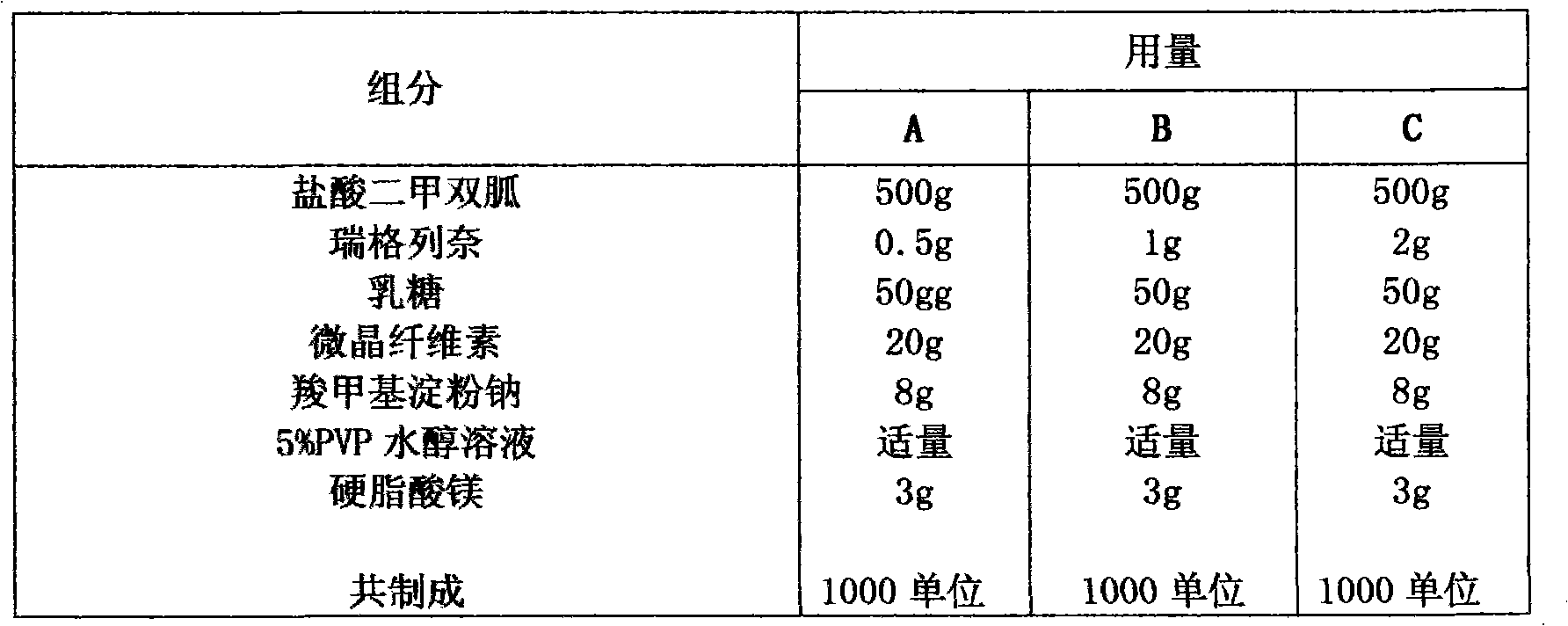
Compound with metformin and repaglinide, preparation method thereof and application thereof
InactiveCN101822672ALower glucose toleranceLower natural responsesOrganic active ingredientsMetabolism disorderInsulin dependent diabetesOrally disintegrating tablet
The invention relates to a composite composition with metformin hydrochloride and repaglinide as active ingredients, a preparation method thereof and application thereof and belongs to the technical field of medicaments. The composite composition is a medicinal composition which is mixed by using the metformin hydrochloride and repaglinide as the active ingredients and by using a carrier and can be prepared into sustained-release tablets, sustained-release granules, sustained-release capsules, common troches and capsules, granules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, liquid capsules, soft capsules, drop pills and other oral preparations. The composite composition is used for treating patients with I-type diabetes or II-type diabetes (non-insulin-dependent diabetes) and has synergistic effect on controlling blood sugar.
Owner:深圳南方盈信制药有限公司 +1
Tablets of mosapride citrate and preparation method thereof
ActiveCN101816639ADissolution rate is fastReduce solubilityOrganic active ingredientsDigestive systemDissolutionIn vivo
The invention belongs to the field of medicaments and in particular relates to tablets containing mosapride citrate and a preparation method thereof. Because the mosapride citrate is almost insoluble, defects of slow dissolution and low in-vitro and in-vivo bioavailability are present in the mosapride citrate tablets prepared by the common method. In order to improve the dissolution and bioavailability of the mosapride citrate, the invention provides dispersible tablets containing the mosapride citrate and a preparation method thereof. The prepared mosapride citrate dispersible tablets have high dissolution speed and low dissolution difference. The accumulated dissolution in five minutes is close to 100 percent, and the dispersible tablets are almost dissolved completely.
Owner:LUNAN PHARMA GROUP CORPORATION
Effervescence dispersible tablet
InactiveCN101138554AEvenly dispersedUniform disintegrationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsEffervescent tabletOral medication
The present invention relates to a new preparation of a Chinese medicine. The present invention particularly relates to a novel preparation of a drug with the effervescent tablet property and the dispersing agent property. In the effervescent dispersing tablet of the present invention, the weight ratio of each components are as following, which comprises 5 percentage to 60 percentage of effervescent agent, 3 percentage to 30 percentage of effervescing agent, 3 percentage to 30 percentage of disintegrant, 3 percentage to 30 percentage of excipient of the hydrophilic medicine, 1 percentage to 5 percentage of correctant. The effervescent dispersing tablet of the present invention is a novel preparation of the Chinese medicine, which has the effervescent tablet property and the dispersing agent property. The present medicine is a tablet, which can produce the gas in the water, which can disaggregate quickly and disperse evenly. The present invention is a novel preparation of the medicine, which can generate the gas; as a result the medicine can disaggregate more quickly. The drug loading dosage of the agent is large, which is beneficial for improving the dispersion uniformity, the dissolution and the bioavailability of the medicine. The present invention has the characteristics of convenience for oral administration and small dose. The present invention can sufficiently display the drug efficacy in order to meet the drug requirement of the patients.
Owner:YUNNAN BAIYAO GROUP
Indian bread polysaccharide formulation and its preparation method
InactiveCN1762378AImprove immunityIncrease profitOrganic active ingredientsSolution deliveryAcetic acidEffervescent tablet
The invention provides a pachyman preparation, wherein the dosage forms include oral liquid, capsule, pill, tablet, pelletized granule, dispersible tablet, effervescent tablet, and injection, the main composition is pachyman (C6H1206)n no less than 90% calculated by glucose. The preparing process for the pachyman is also disclosed.
Owner:戴甲木
Clopidogrel hydrogen sulfate dispersible tablets and preparation method thereof
InactiveCN101396350AReduce manufacturing costGood disintegrationOrganic active ingredientsPharmaceutical non-active ingredientsAnti plateletDrug administration
The invention discloses an anti-platelet aggregation drug-clopidogrel hydrogen sulfate tablet dispersible tablet and a preparation method thereof. The clopidogrel hydrogen sulfate tablet dispersible tablet consists of 30 percent to 55 percent of clopidogrel hydrogen sulfate and 45 percent to 70 percent of auxiliary materials according to the weight percentage. The auxiliary materials comprise disintegrant, loading agent, lubricant and taste-modifying agent, wherein, the disintegrant accounts for 5 percent to 20 percent of the total weight percentage of the prescription. The preparation method of the clopidogrel hydrogen sulfate tablet dispersible tablet is wet granulation tabletting press method or direct powder pressing method. Compared with other forms of preparation, the invention has the advantages of good dispersing state, short disintegration time, fast drug dissolvability, convenient drug administration, low production cost, good portability, high stability, etc.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com