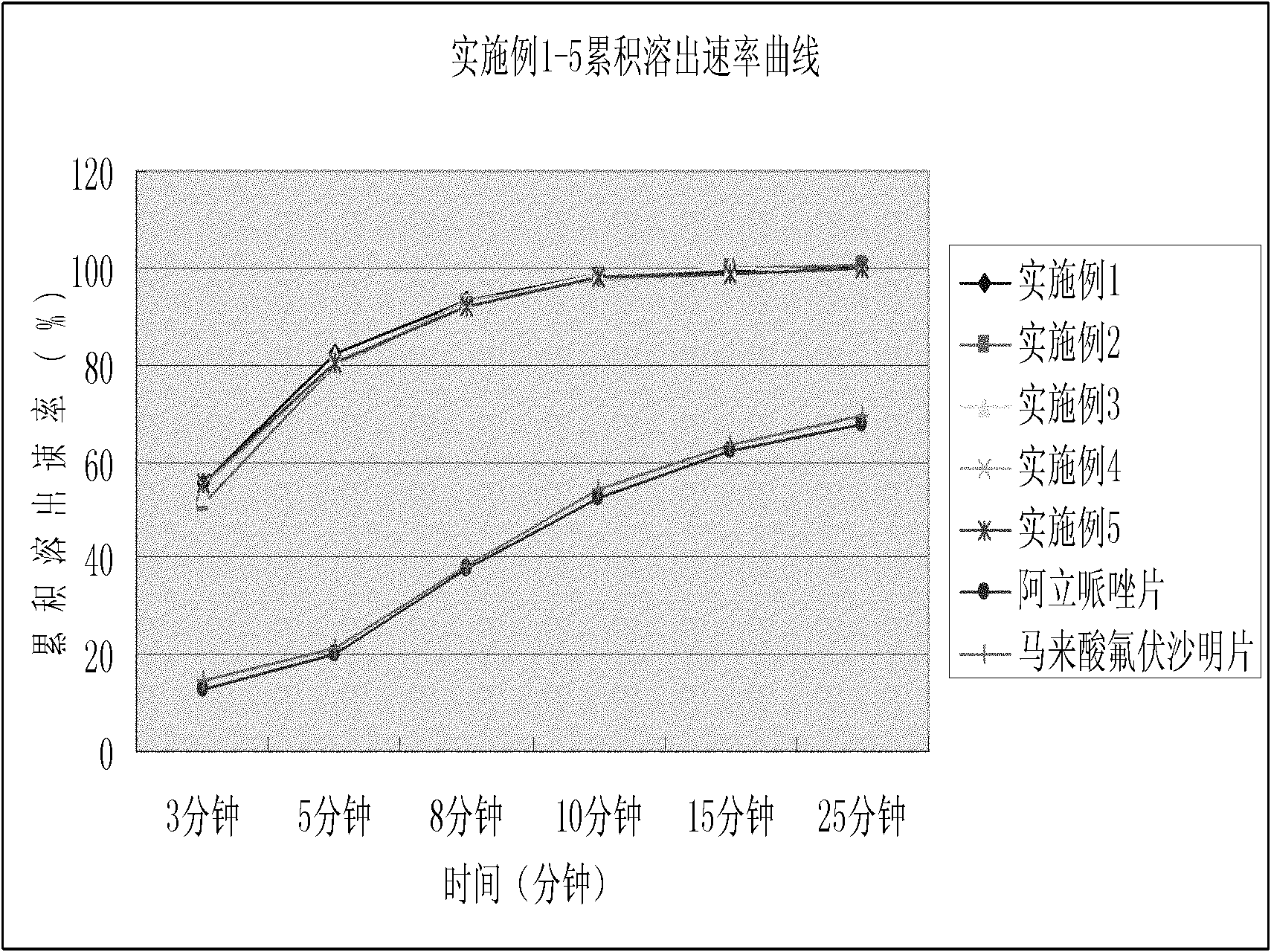
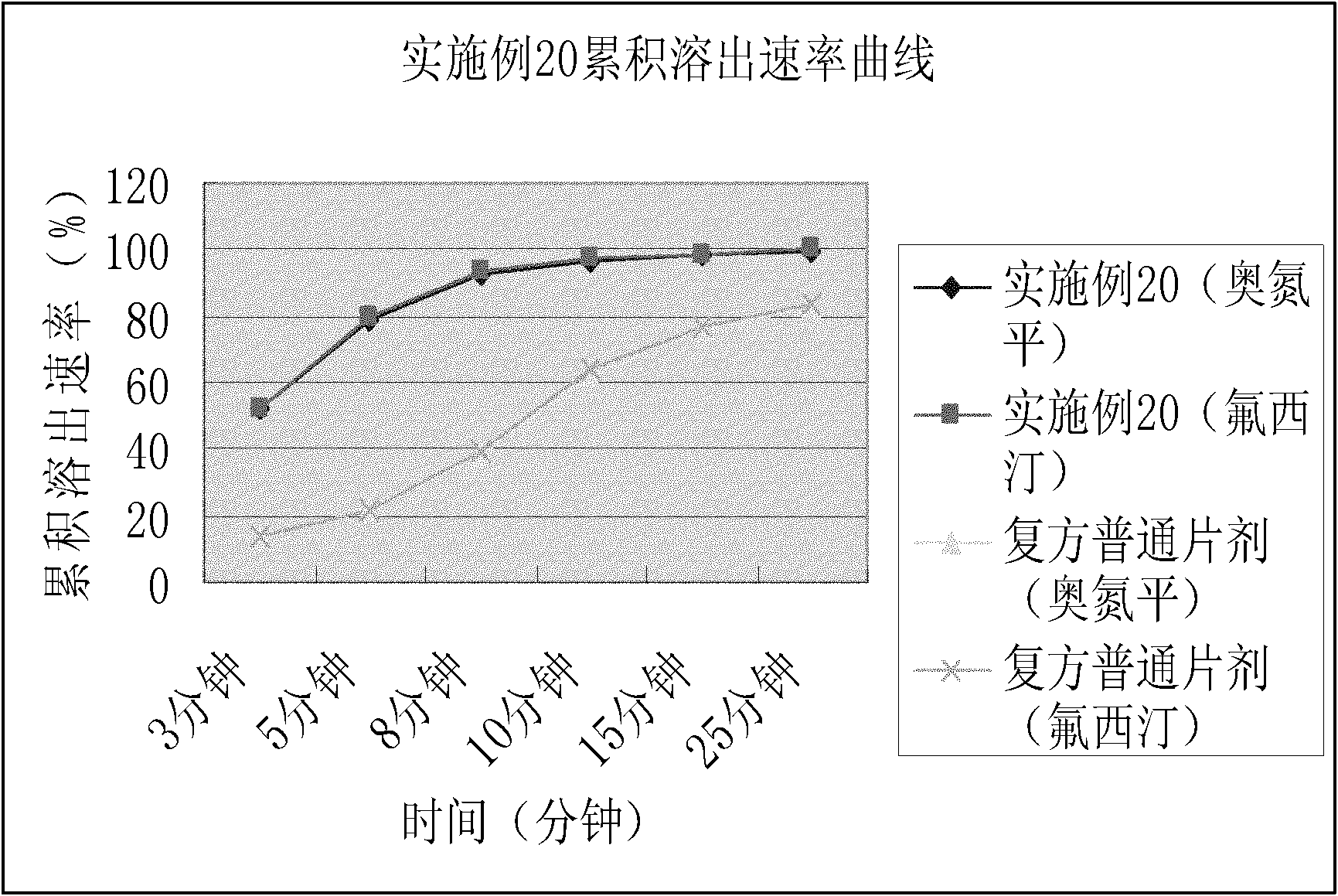
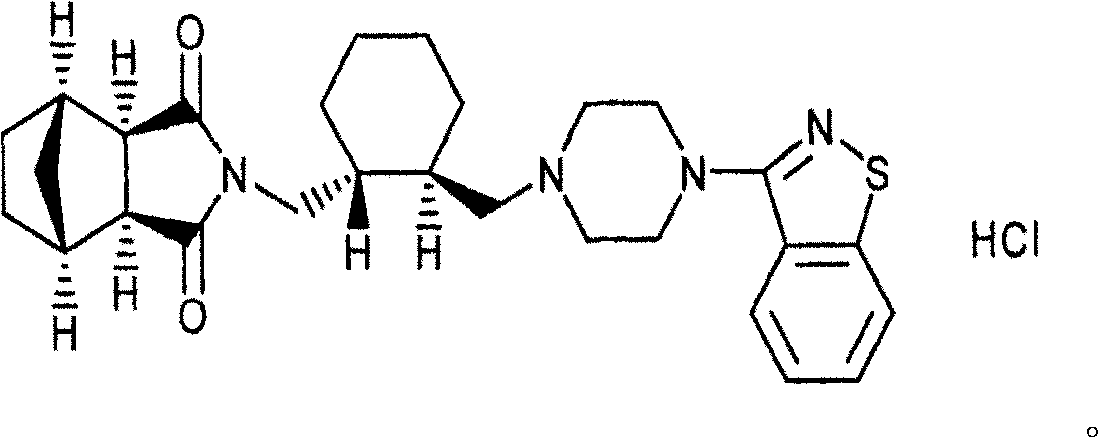
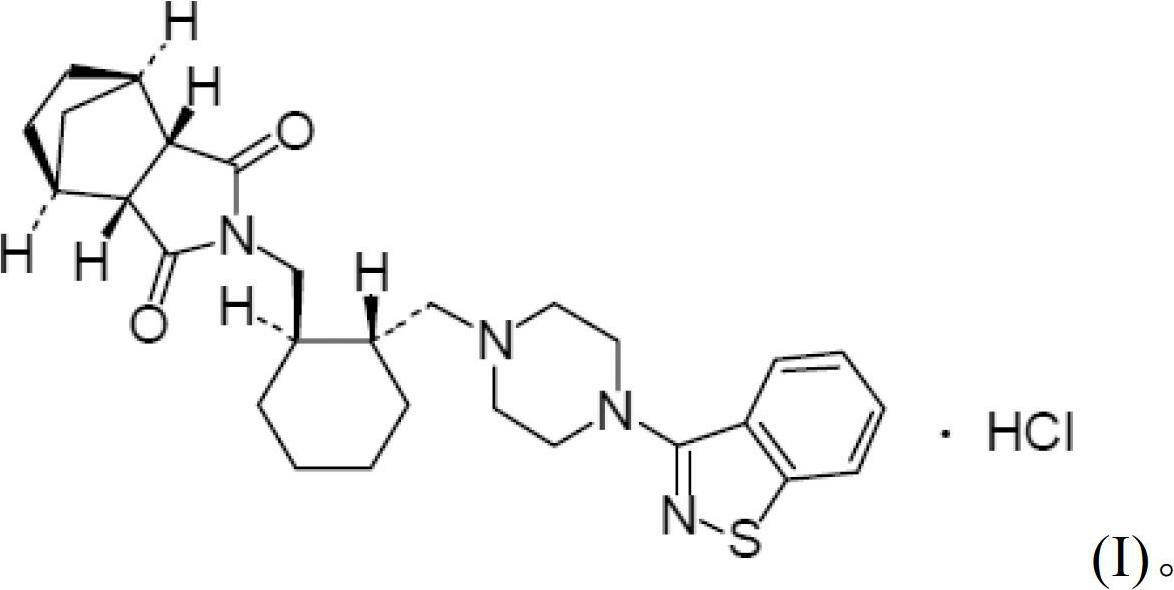
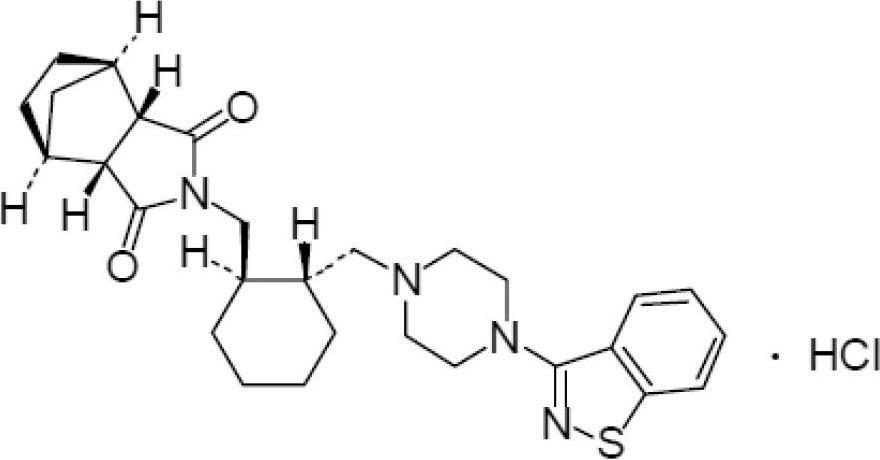
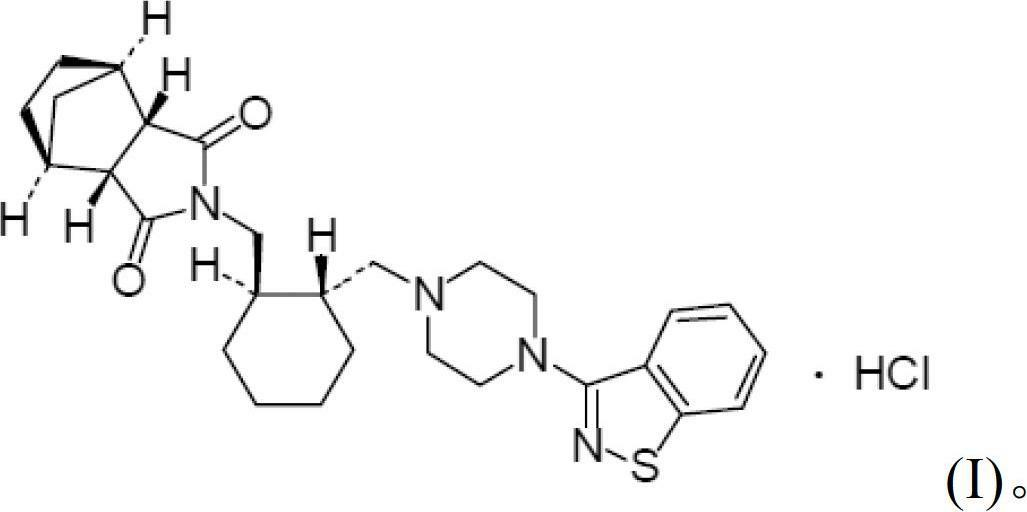
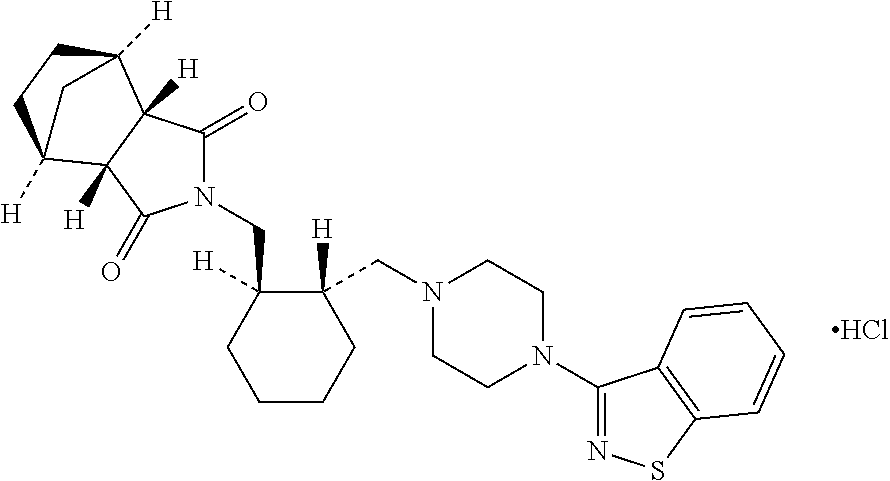
Patents
Literature
72 results about "Lurasidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain mental/mood disorders (such as schizophrenia, depression associated with bipolar disorder).
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
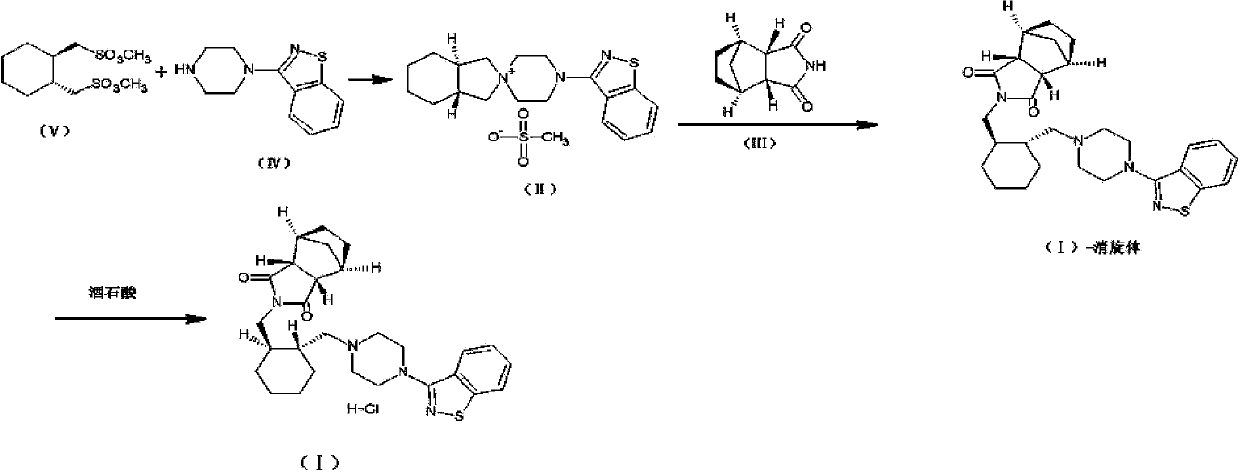
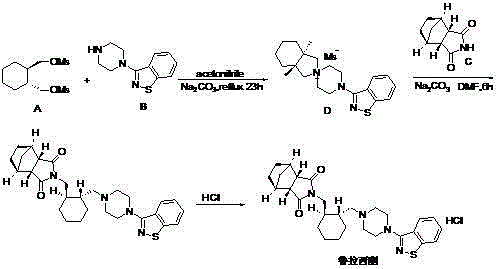
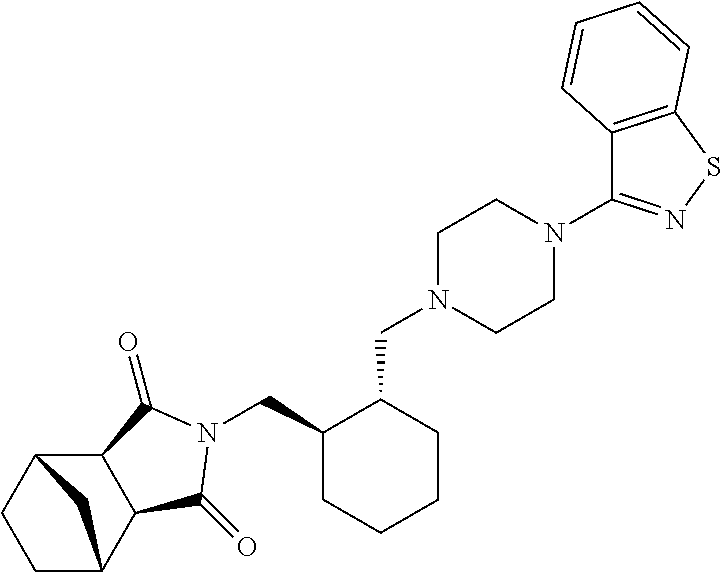
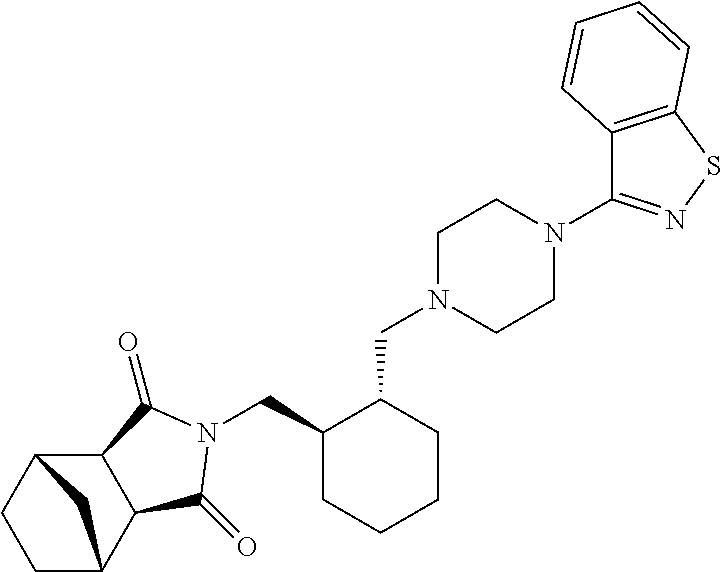
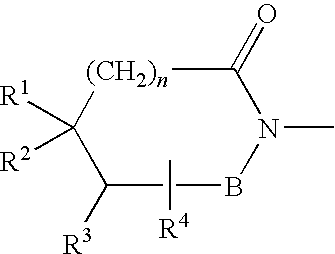
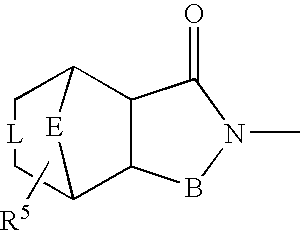
Preparation method of lurasidone intermediate and lurasidone
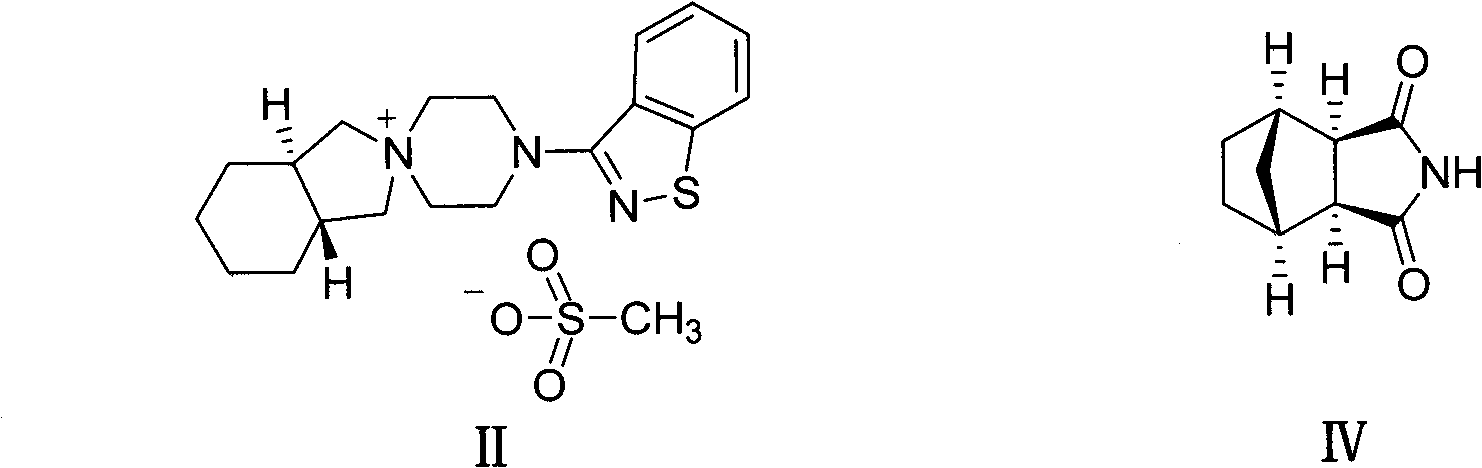
The invention provides a preparation method of a lurasidone intermediate, a compound as shown in formula (II). The method comprises: in the presence of an acid binding agent and a phase transfer catalyst, adding a compound as shown in formula (V) and a compound as shown in formula (IV) into a solvent, conducting heating, refluxing, and water division so as to obtain the compound as shown in formula (II), and in the presence of an acid binding agent, adding the compound as shown in formula (II) and a compound as shown in formula (III) into the solvent for refluxing and water division reaction so as to obtain a formula (I)-racemate, then performing splitting with a tartaric acid enantiomer, thus obtaining the compound lurasidone as shown in formula (I). The preparation method of the lurasidone intermediate and lurasidone of the invention is characterized by short reaction time, simple post-treatment, abolition of column chromatography, and low cost, thus being suitable for industrial production.
Owner:天津泰普制药有限公司
Pharmaceutical composition
ActiveUS20090143404A1Reduce the burden onIncrease the burdenOrganic active ingredientsNervous disorderOral medicationLurasidone
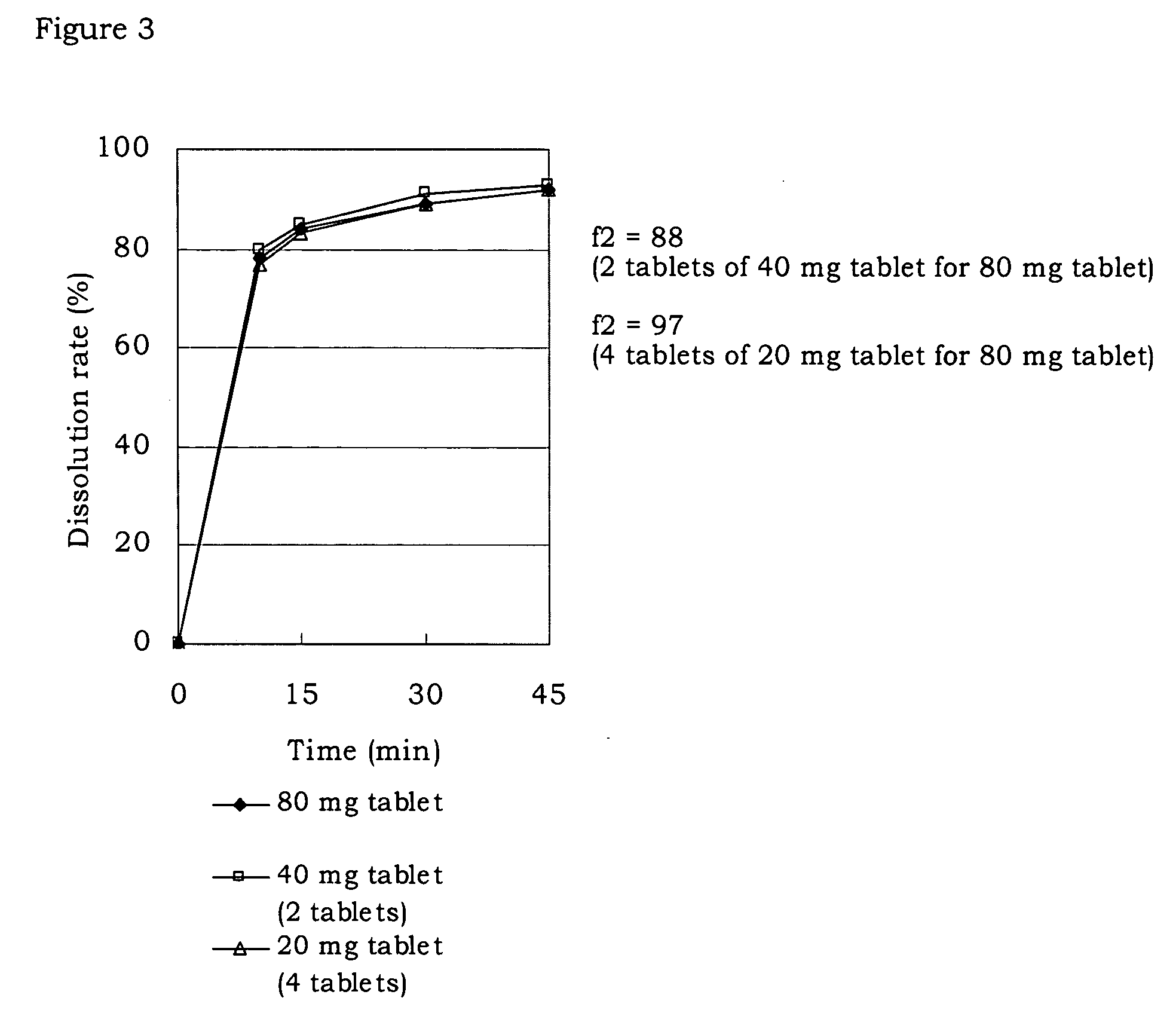
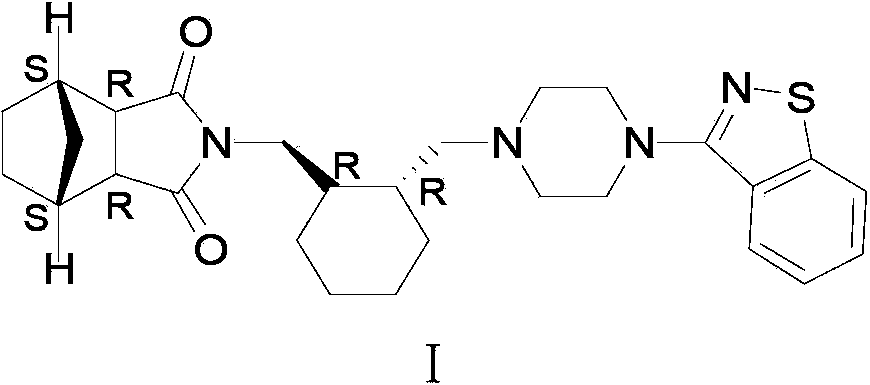
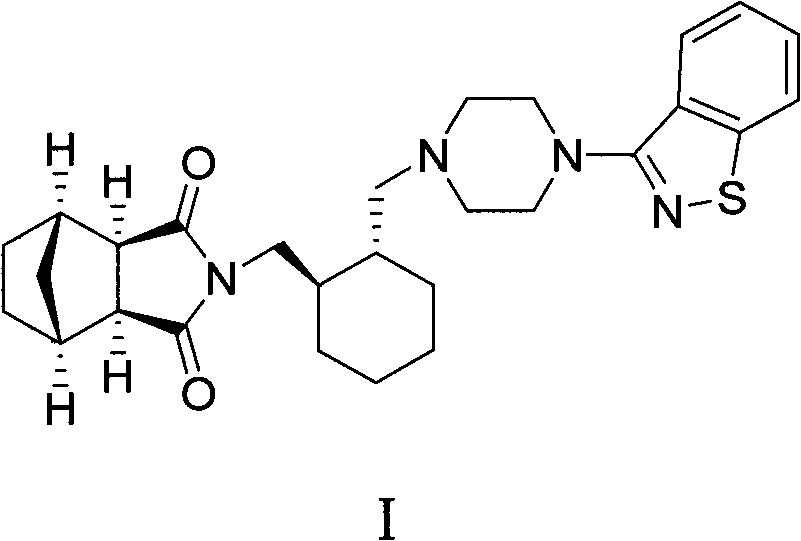
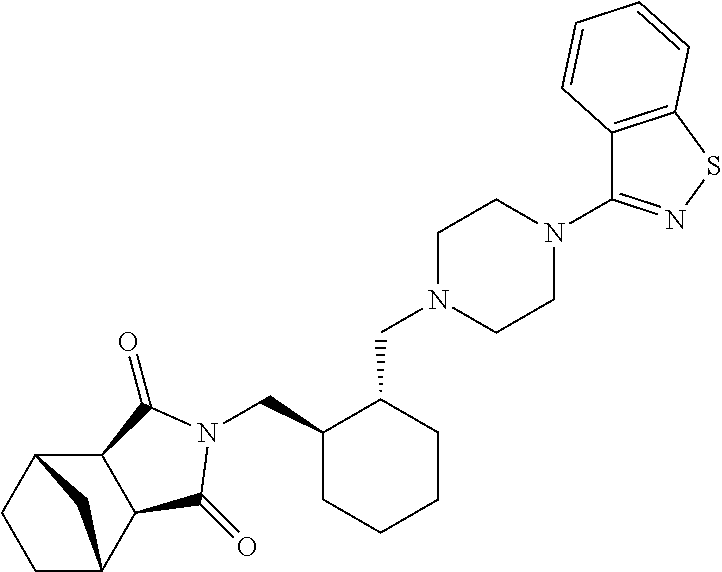
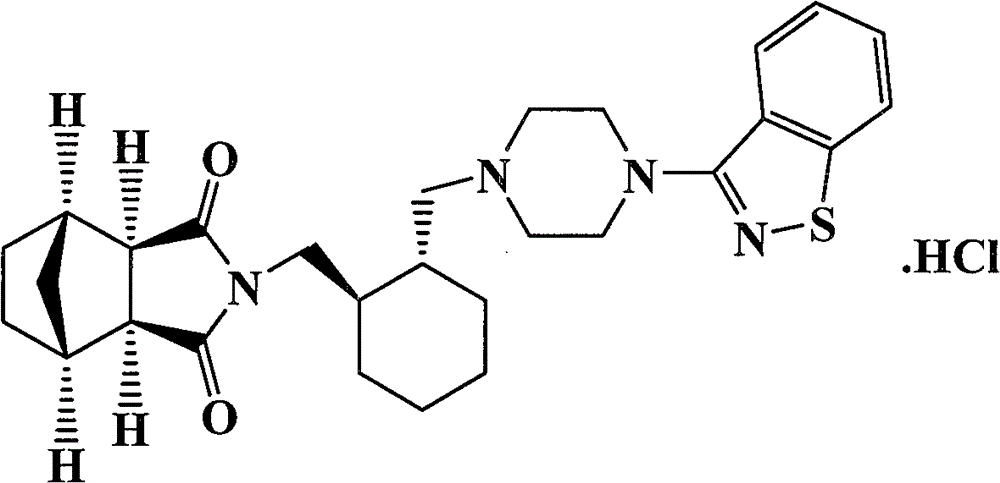
A preparation for oral administration comprising: a pregelatinized starch comprising N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]-(1′R,2′S,3′R,4′S)-2,3-bicyclo[2,2,1]-heptanedicarboxyimide hydrochloride (lurasidone) represented by the formula (1) as an active ingredient; a water-soluble excipient; and a water-soluble polymeric binder, the preparation exhibiting an invariant level of elution behavior even when the content of its active ingredient is varied.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Method for preparing lurasidone
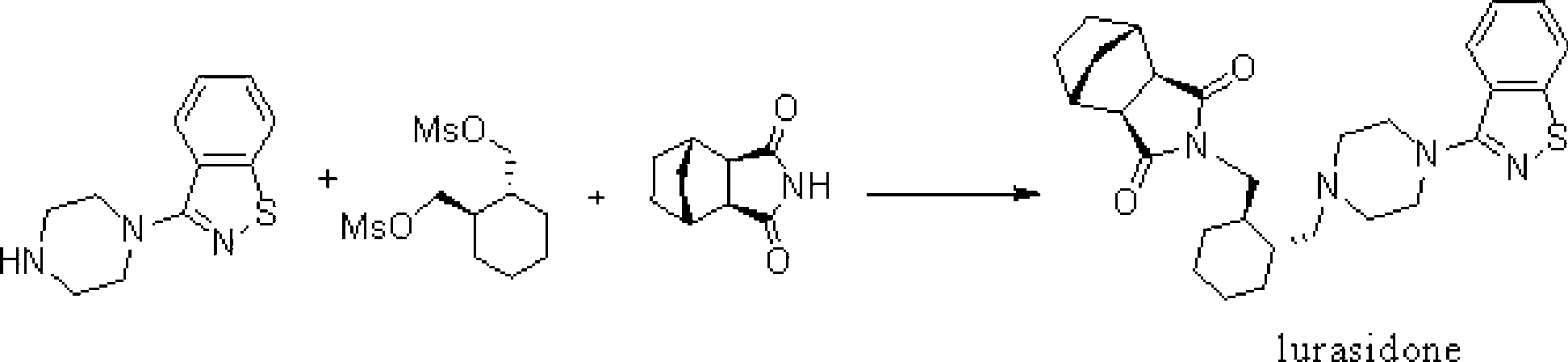
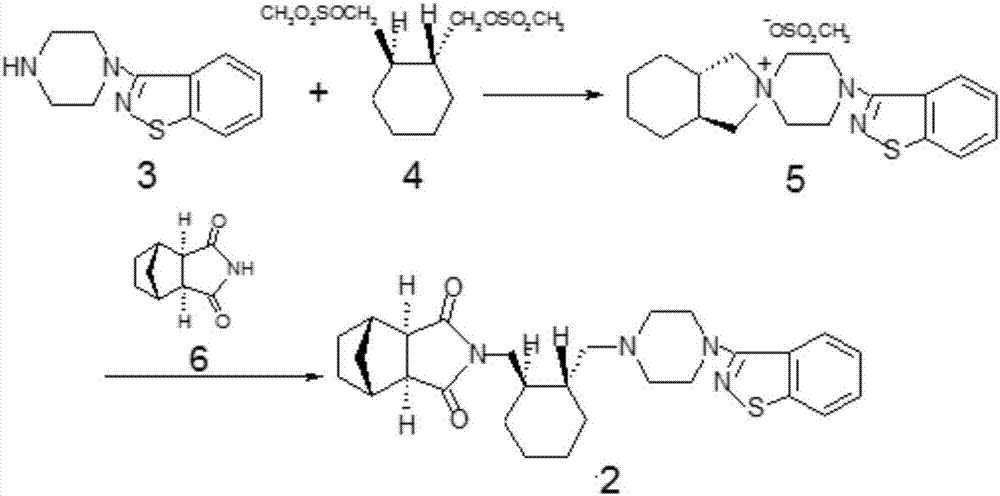
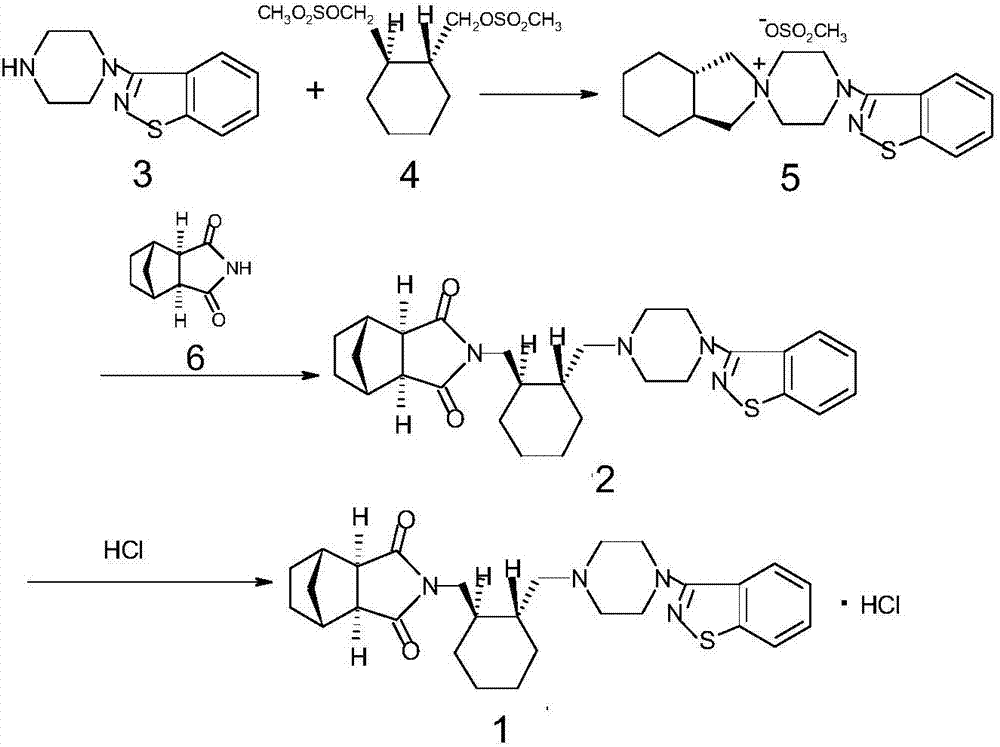
The invention relates to a method for preparing lurasidone and preparation of atypical antipsychotic compound lurasidone, and in particular relates to a method for preparing single salt silicate. The method comprises the following steps of: halogenating hydroxy by using a compound I as an initial raw material to prepare an intermediate compound II; reacting the intermediate compound II with a compound V under an alkalinity condition to generate an intermediate compound VI; and reacting the intermediate compound VI with a compound III under the alkalinity condition to prepare a target product of lurasidone. The method is short in process routine, easy to control and convenient to operate; and the generation of impurities is avoided, the reaction yield is obviously improved, the raw materials are cheap and easy to acquire, and the large-scale production is facilitated.
Owner:BEIJING MEDISAN TECH +1
Solubiliazation preparation
InactiveUS20090286805A1Promote effectiveOrganic active ingredientsNervous disorderLurasidoneBULK ACTIVE INGREDIENT
A solution-type preparation of lurasidone comprising N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]-(1′R,2′S,3′R,4′S)-2,3-bicyclo[2,2,1]heptanedicarboxyimide hydrochloride (lurasidone) as an active ingredient and containing at least one substance selected from benzyl alcohol, N,N-dimethylacetamide, lactic acid and propylene glycol.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Method for preparing lurasidone
ActiveCN103864774AEasy to operateSuitable for industrialized mass productionOrganic chemistryLurasidoneOrganic layer
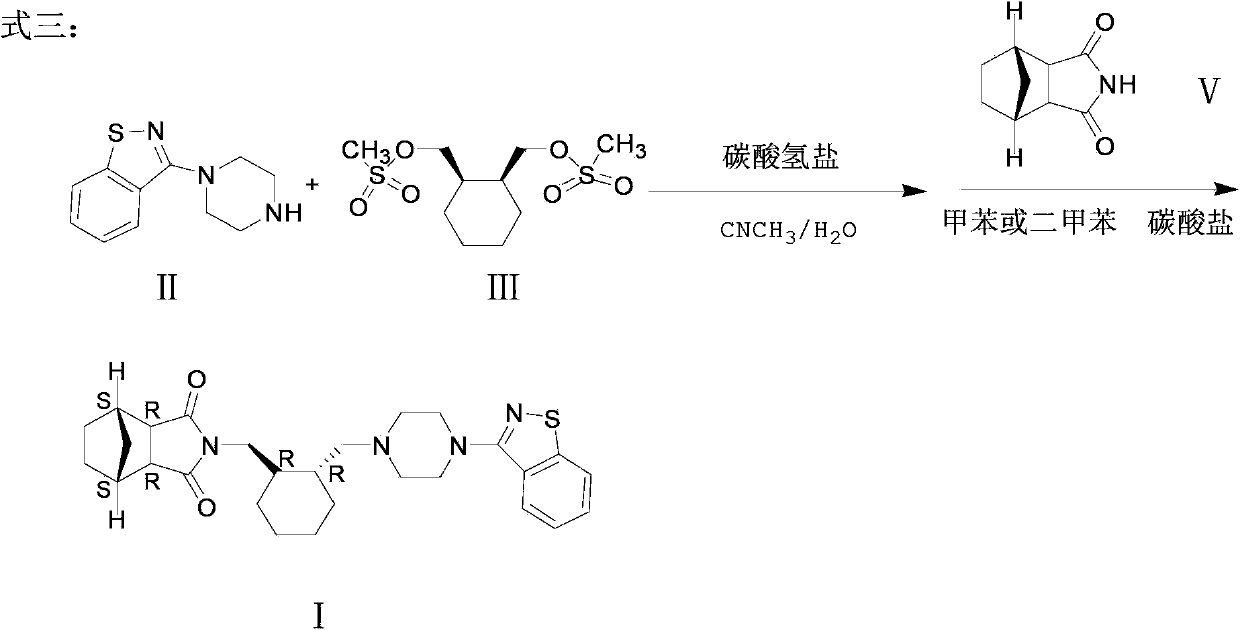
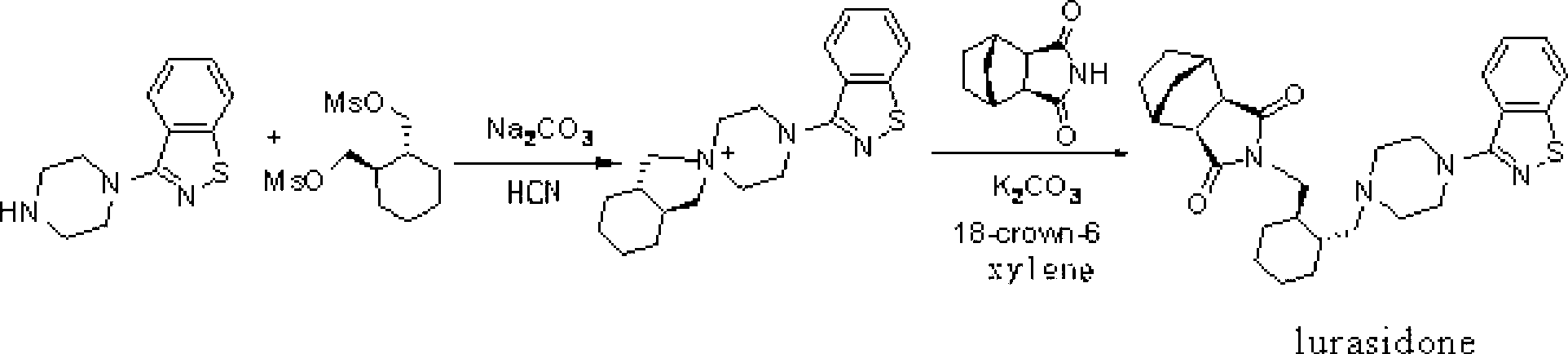
The invention provides a method for preparing lurasidone. The preparation method comprises reacting 1-(1,2-benzisothiazol-3-yl) piperazine with (R, R)-1,2-bis(methylsulfonyl-methoxy) cyclohexane in a mixed solvent of acetonitrile / water in presence of bicarbonate as a base, adding toluene or xylene, separating an organic layer out, adding bicyclo [2.2.1] heptane-2,3-dicarboximide and carbonate into the organic layer and reacting to obtain lurasidone. The conventional separation and purification steps are omitted and the cost of production is greatly reduced; at the same time, the method has the characteristics of high yield and high purity of the product.
Owner:四川弘远药业有限公司
Preparation method of lurasidone
The invention provides a preparation method of lurasidone. On the basis of the existing preparation method of lurasidone, a one-pot method is adopted to replace the method including multiple steps and obtain a target product once. The preparation method comprises the following steps: adding 3-(1-piperazinyl)-1,2-benzisothiazole in toluene, stirring to dissolve; adding (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and an inorganic alkali, heating and carrying out reflux reaction for 12-36 hours; adding (3alpha R,4S,7R,7alpha S)4,7-methano-1H-isoindole-1,3(2H)-dione; heating and refluxing; recycling toluene at reduced pressure; adding ethyl acetate in the residue, stirring to dissolve, washing for 2-3 times with 5% hydrochloric acid, separating out the organic layers, drying for 20-120 minutes, filtering to remove the drying agent, concentrating the obtained ethyl acetate solution, dropwise adding concentrated hydrochloric acid, precipitating the solid, and performing suction filtration to obtain crude lurasidone; and refining crude lurasidone to obtain pure lurasidone. By adopting the preparation method of lurasidone, the solvent can be recycled conveniently and the method is simple in operation.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Pharmaceutical compositions of lurasidone
The present invention relates to pharmaceutical compositions of lurasidone or salts thereof. In particular, the invention relates to pharmaceutical compositions of lurasidone or salts thereof with one or more acids. The invention also relates to processes for the preparation of such compositions and use thereof for treatment of schizophrenia, bipolar disorders or senile dementia.
Owner:CADILA HEALTHCARE LTD
Preparation method of chiral intermediate cyclohexane dimethanol
InactiveCN102952001ALow priceStable in natureOrganic compound preparationHydroxy compound preparationNegative symptomLurasidone
The invention belongs to the technical field of medicine, and specifically relates to a preparation method of a chiral intermediate 1R,2R-cyclohexane dimethanol. The chiral intermediate 1R,2R-cyclohexane dimethanol is an important intermediate of an antischizophrinic medicine lurasidone. Lurasidone has substantially treatment effects against both positive and negative symptoms of mental patients. A reduction agent adopted by the invention has low cost and stable property. The preparation method is suitable for industrialized productions.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Oral lurasidone suspension and preparation method thereof
InactiveCN104606133AQuality improvementGreat tasteOrganic active ingredientsNervous disorderOral suspensionsPreservative
The invention discloses an oral lurasidone suspension and a preparation method thereof. The lurasidone suspension is a pharmaceutical composition which comprises lurasidone or salt thereof, a suspending aid, a wetting agent, a pH regulator, a preservative, a sweetening agent and a corrigent, wherein the particle size range of the lurasidone or salt thereof is 0.1-20 microns. The prepared oral suspension has the characteristics of being stable in quality and favorable in taste, facilitates dose distribution, and is beneficial to the acceptance of schizophrenia patients, simple in preparation method and suitable for industrial production.
Owner:AVENTIS PHARMA HAINAN
Lurasidone pharmaceutical composition and preparation method thereof
ActiveCN104248769AImprove solubilityAvoid wastingOrganic active ingredientsNervous disorderOrganic solventLurasidone
The invention provides a lurasidone pharmaceutical composition, which contains lurasidone or its acid addition salt and cyclodextrin, wherein the lurasidone or its acid addition salt and cyclodextrin are in a mole ratio of 1:1-2. The lurasidone pharmaceutical composition provided by the invention can be prepared into an oral solid preparation, no harsh restriction is needed for the particle size of the raw materials, and waste of raw materials can be avoided. The lurasidone pharmaceutical composition can be prepared into sterile powder for injection, lurasidone can well dissolve, and adding of an organic solvent is avoided.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Lurasidone medicine composition and preparation method
InactiveCN102688210AImprove pharmaceutical propertiesPharmaceutical Performance RealizationOrganic active ingredientsNervous disorderLurasidoneWater insoluble
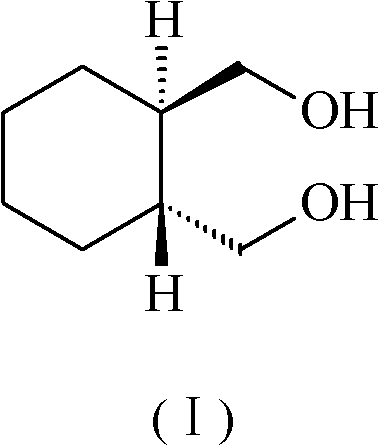
The invention discloses a lurasidone medicine composition and a preparation method. The lurasidone medicine composition comprises the lurasidone shown in the formula (I), sugar alcohol, disintegrant, adhesive, surfactant and optional water-insoluble filler. In the lurasidone medicine composition disclosed by the invention, the contact angle between the surface and water is not greater than 95 degrees. The preparation of lurasidone (particularly tablet) has good release performance and mechanical performance.
Owner:李兴惠
Pharmaceutical compositions of Lurasidone and Process for preparation thereof
Pharmaceutical compositions comprising an atypical antipsychotic as an active agent, process of preparation thereof and method of using the same are provided. Particularly the present invention relates to pharmaceutical compositions comprising lurasidone, process of preparation thereof and method to treat various psychotic disorders such as schizophrenia, positive and negative symptoms of schizophrenia, memory or learning dysfunctions caused by schizophrenia, senile dementia, attention deficit / hyperactivity disorder (ADHD), central nervous system (CNS) disorder responsive to modulation of glutamate levels, major depressive episodes associated with bipolar I disorder and other associated CNS disorders.
Owner:AUROBINDO PHARMA LTD
Lurasidone tablet and preparation method thereof
InactiveCN102688209AImprove pharmaceutical propertiesPharmaceutical Performance RealizationOrganic active ingredientsNervous disorderLurasidoneAlcohol sugars
The invention discloses a lurasidone tablet and a preparation method thereof. The lurasidone tablet comprises lurasidone shown in the formula (I), cellulose derivative and sugar alcohol. The prepared lurasidone preparation, especially lurasidone tablet, has good release performance and excellent mechanical performance.
Owner:李兴惠
Method for preparing lurasidone
The invention discloses a method for preparing lurasidone as shown in format I. The method comprises the following steps: in a polar aprotic solvent, under the action of alkali, a compound II or a compound III and a compound IV are reacted as follows at the temperature of 50-150 DEG C. The method for preparing lurasidone has the advantages of high yield, simplicity and convenience in operation and low cost and is suitable for industrial mass production. A new way is provided for the preparation of lurasidone.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Lurasidone medicine composition and preparation method thereof
ActiveCN102688189AConducive to effective treatmentGood Medication ConvenienceOrganic active ingredientsNervous disorderOld patientsAlcohol
The invention discloses a lurasidone medicine composition and a preparation method thereof. Concretely, the lurasidone medicine composition disclosed by the invention comprises the lurasidone shown in the formula (I), polyhydroxy alcohol and water. The invention also relates to the application of the lurasidone medicine composition in preparing a medicine for psychosis, and a method for preparing the lurasidone medicine composition. The method comprises the following steps of: dissolving the lurasidone in a proper amount of polyhydroxy alcohol; adding water and optional other additives; and adding polyhydroxy alcohol until the total amount of the prescription is obtained; optionally sterilizing or removing bacteria of the liquid composition; and sealing and packing. The lurasidone medicine composition disclosed by the invention is suitable for old patients and patients uncooperative in taking medicines.
Owner:北京鑫开元医药科技有限公司
Lurasidone orally disintegrating tablet
InactiveCN103751139AOrganic active ingredientsNervous disorderOrally disintegrating tabletLurasidone
The invention belongs to the field of pharmaceutical preparations and in particular relates to an orally disintegrating tablet containing lurasidone or a salt thereof and a preparation method thereof. The orally disintegrating tablet is a pharmaceutical composition containing the lurasidone or a pharmaceutically acceptable lurasidone acid addition salt and amorphous silicon dioxide, wherein the particle size range of the lurasidone or the salt thereof is 0.1-10 mu m, the using amount of the amorphous silicon dioxide is 1-30%, and the orally disintegrating tablet has effective storage life. The invention provides the lurasidone orally disintegrating tablet with convenient preparation process, low production cost and effective storage life and aims at providing the preparation containing the lurasidone or the pharmaceutical acceptable salt thereof, which has the advantages of convenience in administration, rapidness in absorption and high bioavailability, for patients.
Owner:BEIJING VENTUREPHARM BIOTECH
Synthetic method of lurasidone
The invention relates to the field of organic synthesis and particularly relates to a synthetic method of lurasidone (lurasidone, CAS#; 367514-87-2). The synthetic method of lurasidone includes the following steps: adding (R,R')-1,2-di(methylsulfonyl-di-oxyl-methyl) cyclohexane, 3-(1-piperazine-base)-1,2-benzo-isothiazolone and alkali into organic solvent, reacting for 6-20 hours under the temperature of 50-150 DEG C, adding bicycle[2,2,2]heptane-2,3-dicarboximide, reacting for 6-20 hours under the temperature of 50-150 DEG C to obtain reaction liquid containing lurasidone, and purifying the reaction liquid to obtain lurasidone. The process line still has the advantages of being high in yield, short in reaction step, simple in post-processing process, good in solvent recycling effect and the like after being amplified, thereby being capable of synthetizing lurasidone in one step.
Owner:SHANGHAI BIRCH CHEM TECH CO LTD
Lurasidone key intermediate preparation method
InactiveCN106946872AHigh yieldSimple and fast operationOrganic chemistry methodsLurasidoneIsoindoles
The present invention relates to a lurasidone key intermediate preparation method, and belongs to the technical field of compound synthesis. According to the method, when 4,-(1,2-benzisothiazol-3-yl)-(3aR,7aR)-octahydrospiro(2H-isoindole-2,1-piperazine)methanesulfonate is generated, 4-(1,2-benzisothiazol-3-yl)-1-piperazine is adopted as a raw material, the 4-(1,2-benzisothiazol-3-yl)-1-piperazine, (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and potassium carbonate are subjected to a reaction in a solvent toluene, and a cyclodextrin phase transfer catalyst is added to the reaction system. According to the present invention, by using the cyclodextrin as the phase transfer catalyst, the incomplete reaction problem is solved, and the yield is substantially improved.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
A method of separating and measuring optical isomers of a lurasidone intermediate by gas chromatography
InactiveCN105467028ARapid and effective separation assayAccurate determination of purityComponent separationCyclohexanedimethanolLurasidone
The invention belongs to the field of analytical chemistry, and discloses a method of measuring optical purity of a lurasidone intermediate that is (1R,2R)-1,2-cyclohexanedimethanol by gas chromatography. The method adopts a cyclodextrin type chiral capillary chromatographic column and a flame ionization detector, can quantificationally measure the optical purity and contents of isomers of the lurasidone intermediate, and can indicate stability of the optical isomers of the lurasidone intermediate. The method is high in specificity, high in accuracy and simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Lurasidone nanosuspension and preparation method thereof
InactiveCN104814926ASmall particle sizeImprove solubilityOrganic active ingredientsNervous disorderFreeze-dryingLurasidone
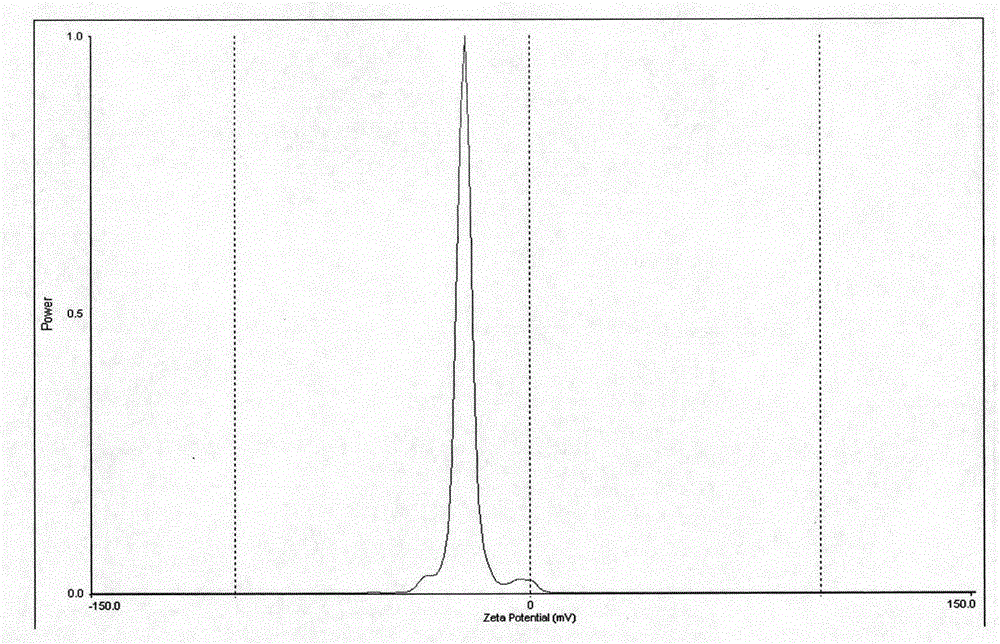
A lurasidone nanosuspension and its preparation method are disclosed. The invention relates to the field of medicinal preparation, specifically to a lurasidone nanosuspension and a preparation method of a freeze-dried powder thereof. The suspension provided by the invention comprises, by weight, 2-20% of lurasidone, 1-36% of a stabilizer and 0-95% of a freeze-drying protective additive. The ratio of lurasidone to the stabilizer is 1:0.5-1:2. Particle size of the suspension is 130-460 nm. The invention also relates to a preparation method of the lurasidone nanosuspension. The lurasidone nanosuspension is prepared by combination of a precipitation method and a high-pressure homogenization method. The preparation method is suitable for industrial production. Quality of the prepared lurasidone nanosuspension is stable, and dissolution speed of lurasidone is raised.
Owner:CHINA PHARM UNIV
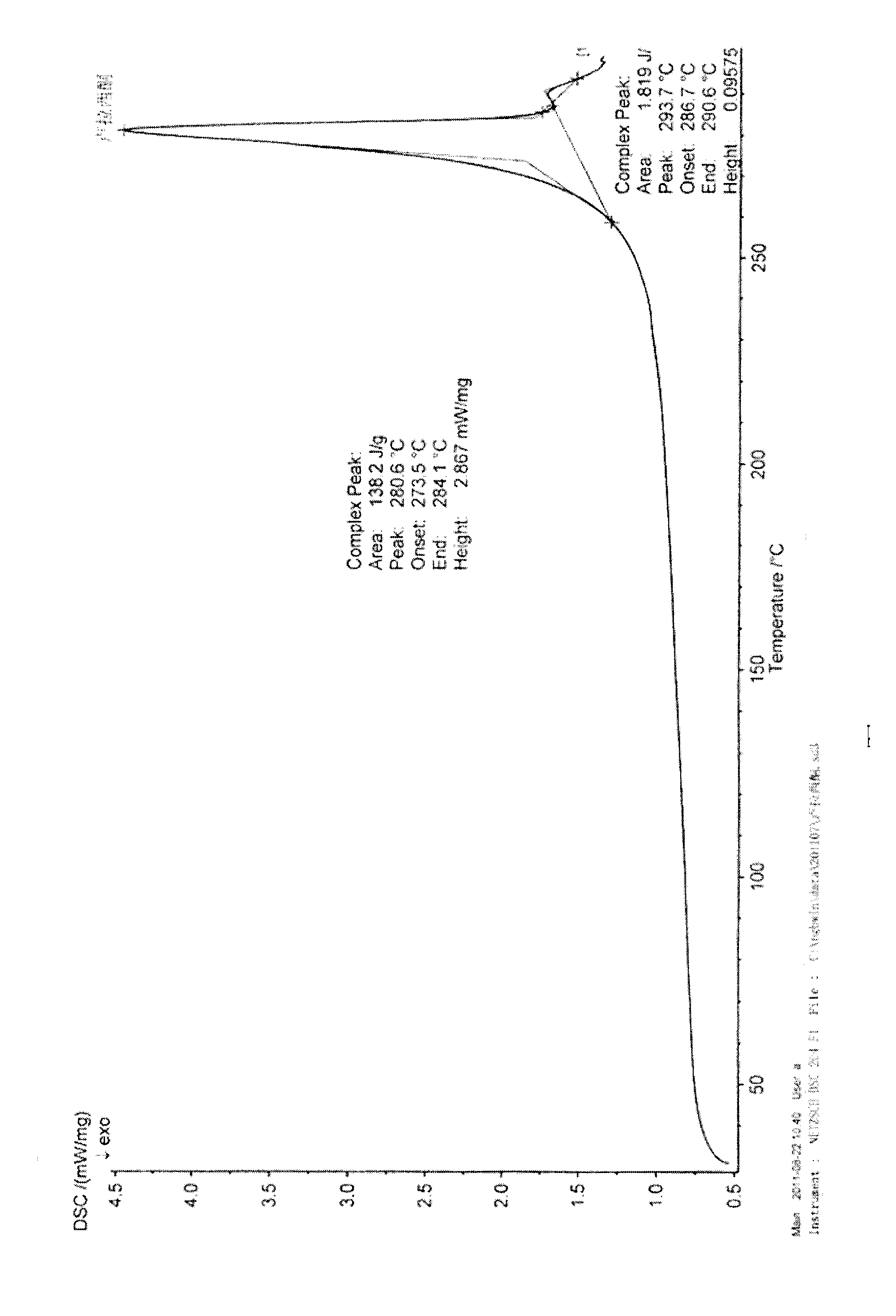
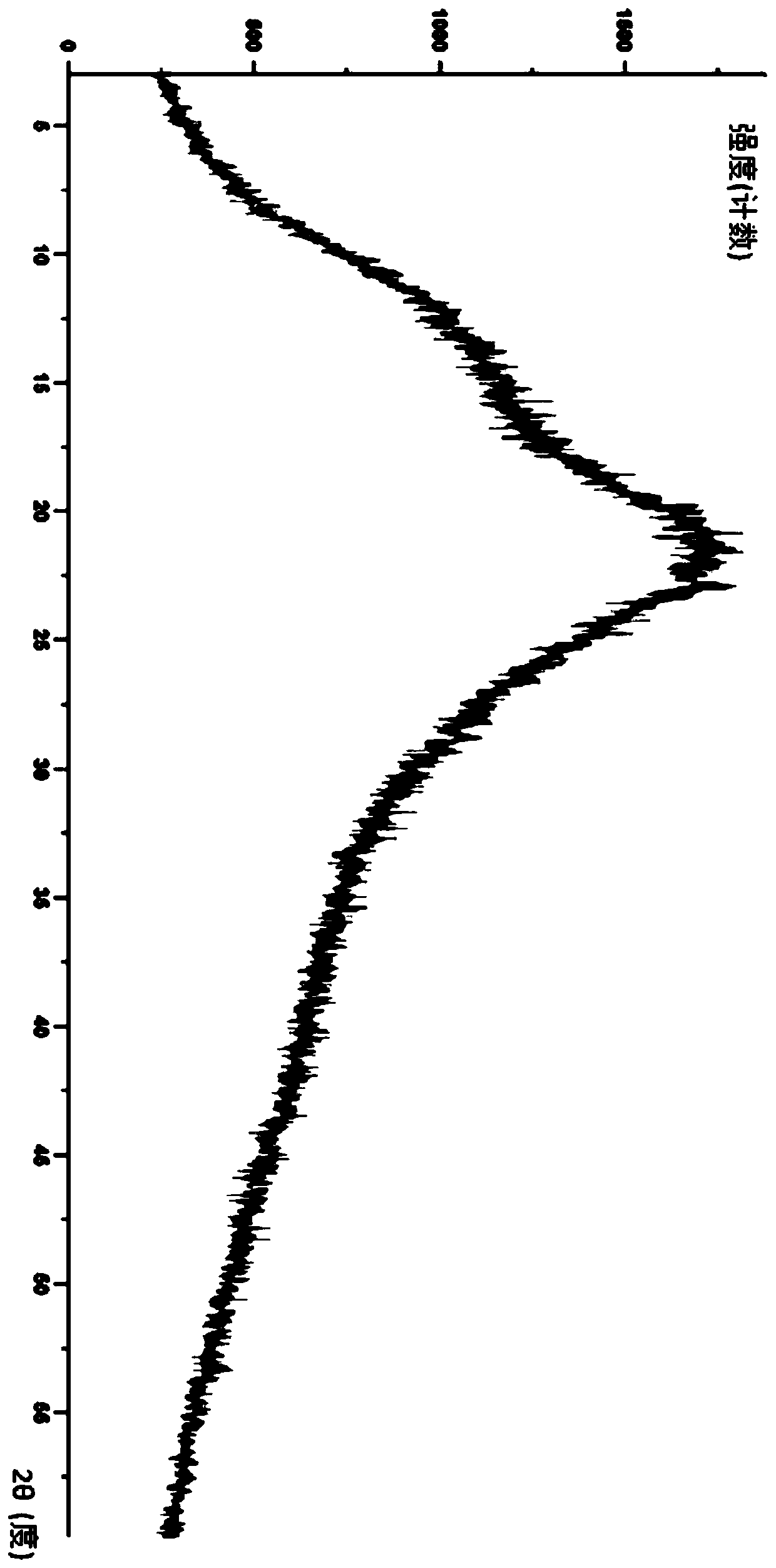
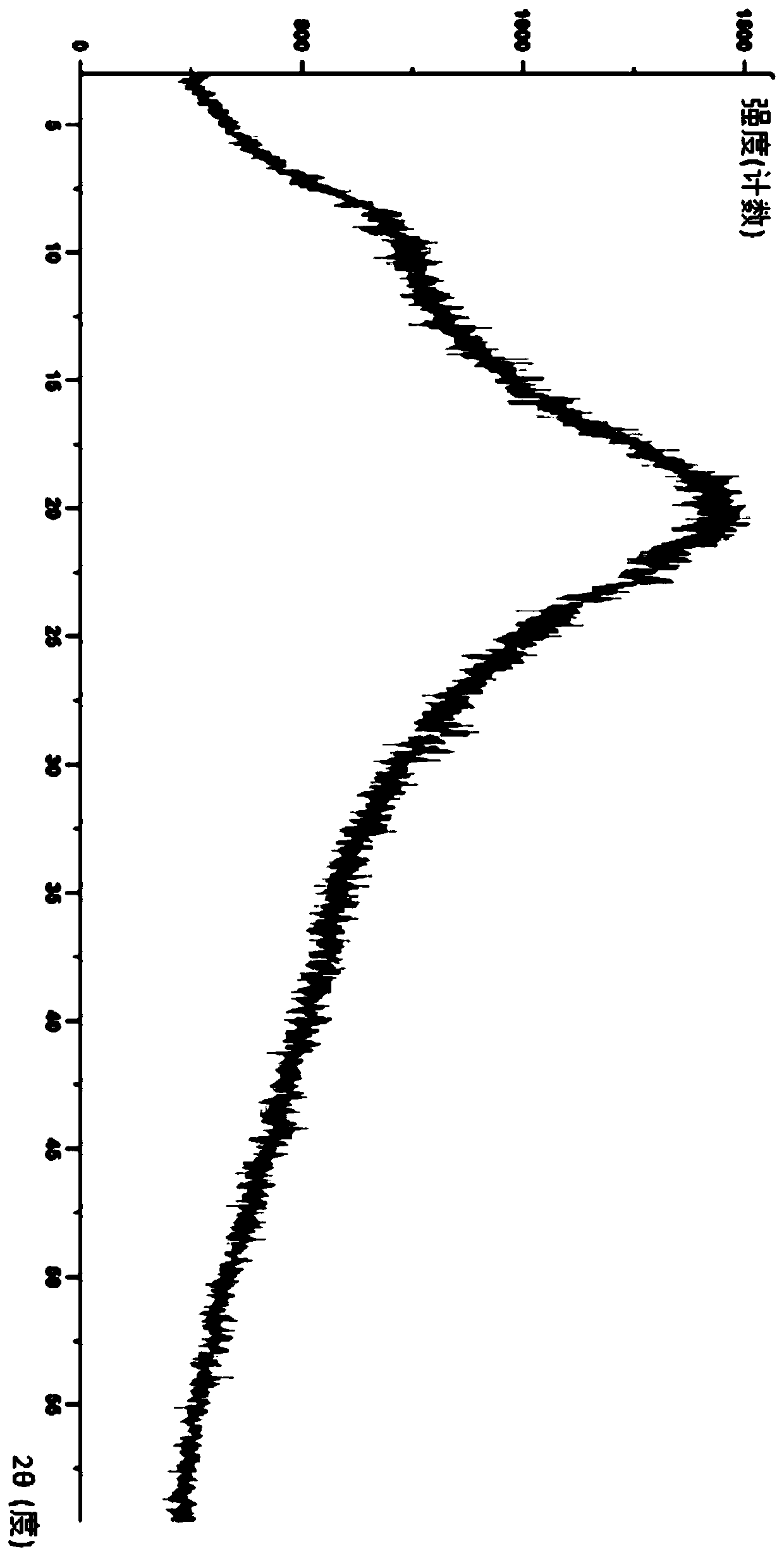
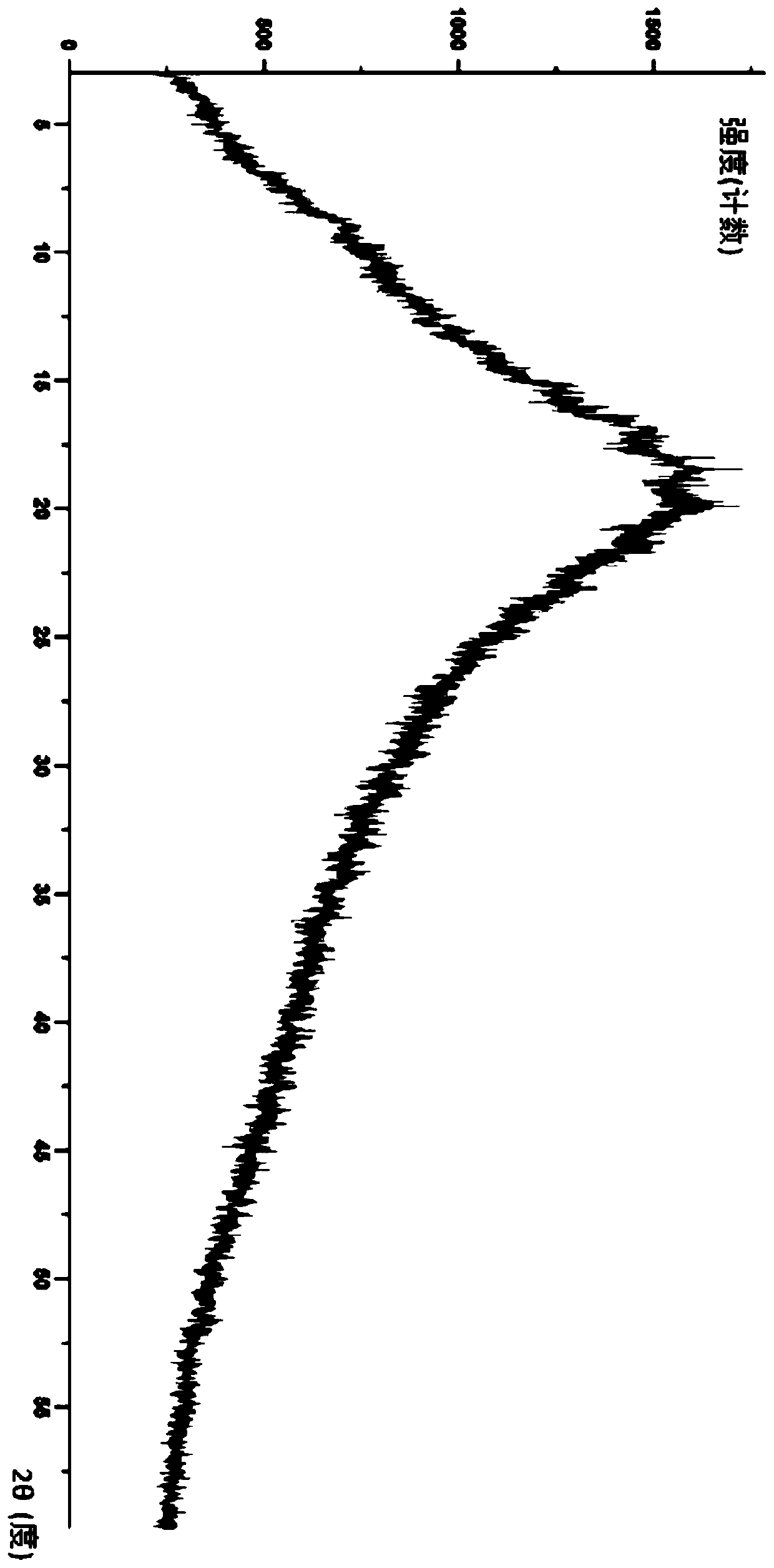
Lurasidone HCl crystal A and purpose thereof
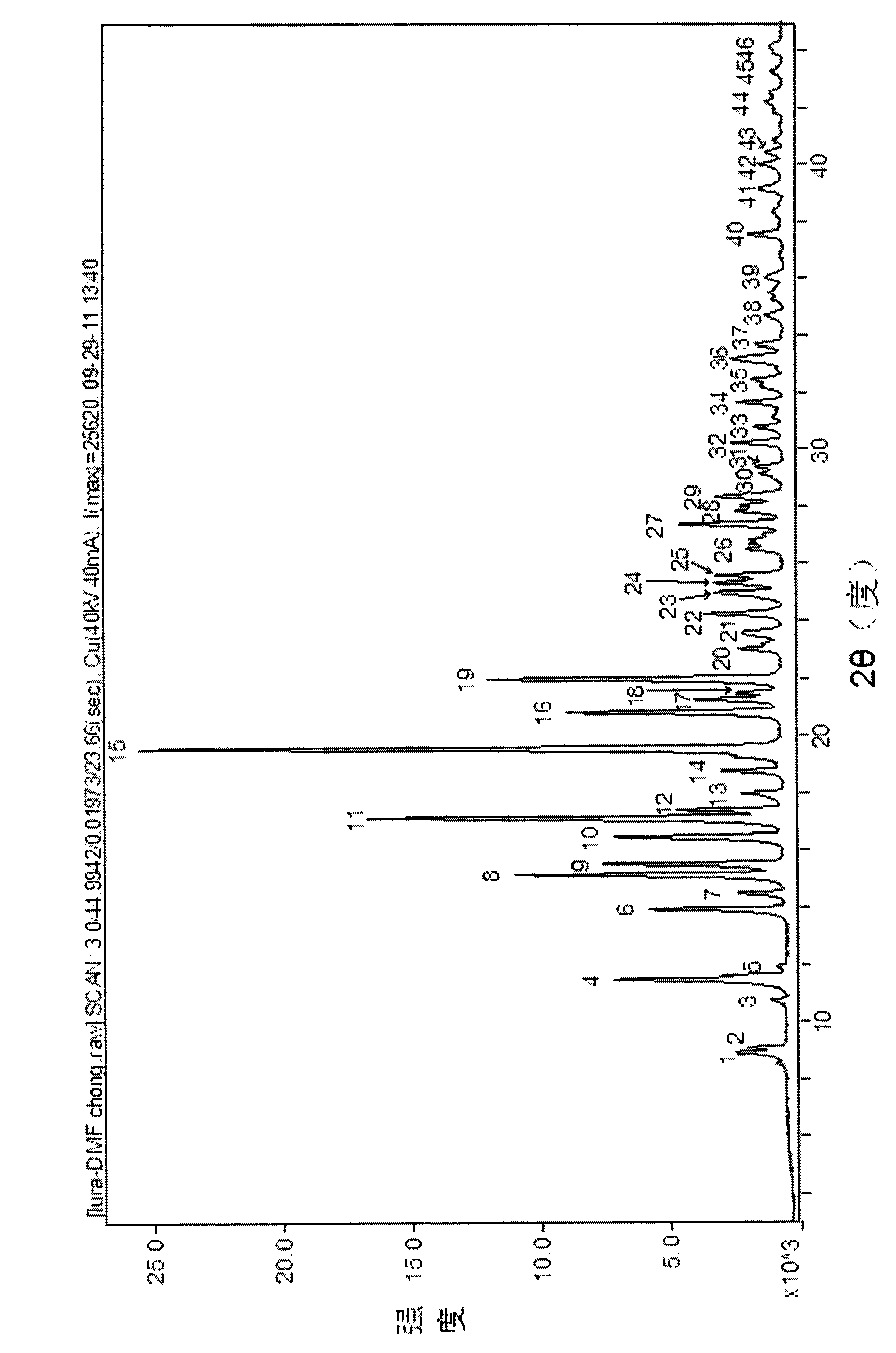
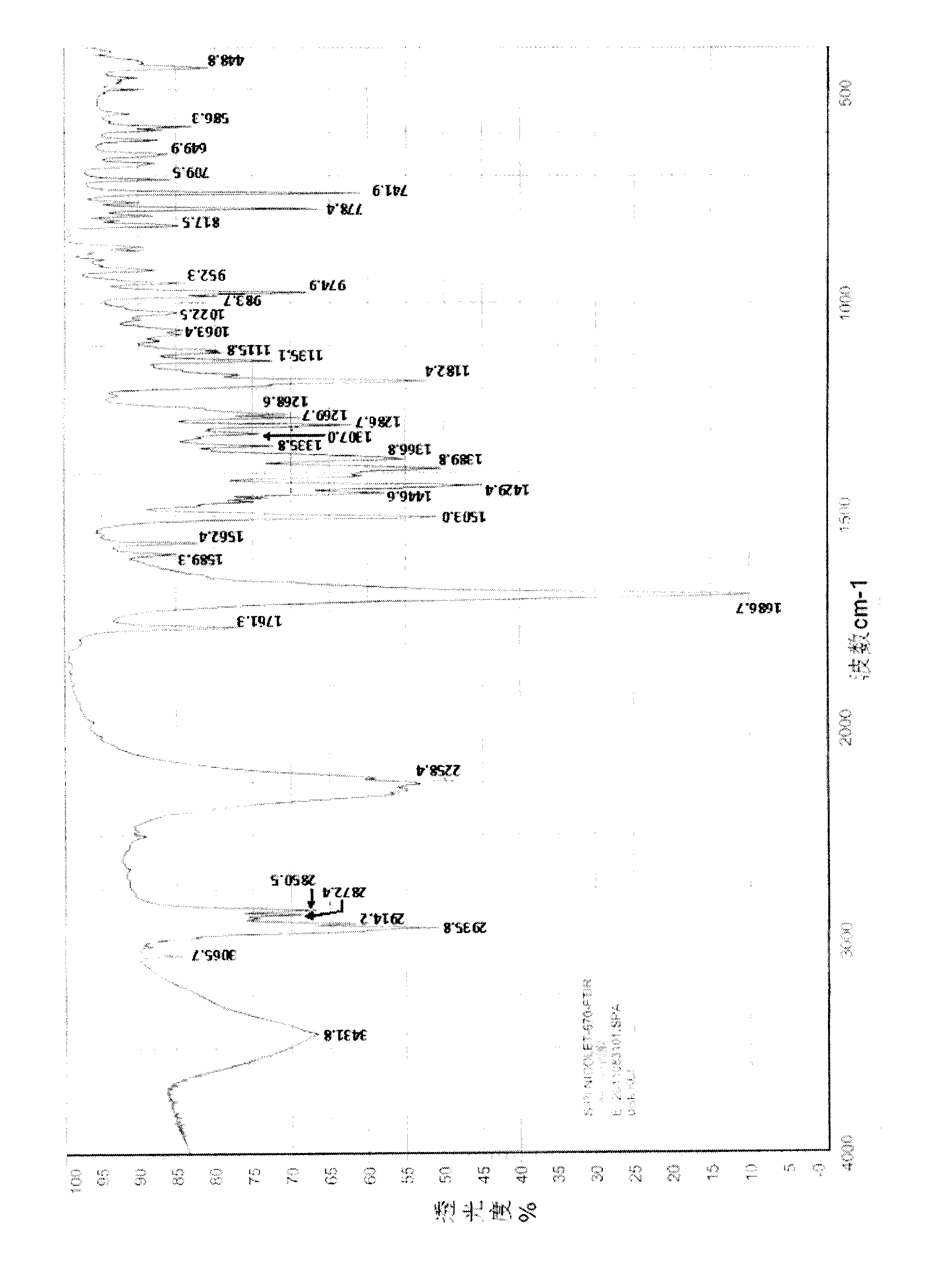
InactiveCN103130795AImprove solubilityToxicOrganic active ingredientsNervous disorderSolubilitySpace group
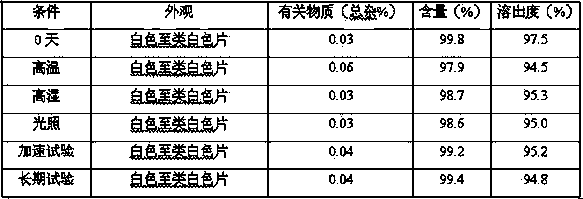
The invention discloses a lurasidone HCl crystal A which is an orthorhombic system. A space group is P212121, cell parameters meet the equation of alpha = gamma = beta = 90.00 degrees. The unsymmetrical unit number Z in a cell is four. The crystal A can be a single crystal. The invention further discloses a purpose when the crystal A is used for preparing medicine for curing schizophrenia. Compared with existing lurasidone HCl, the lurasidone HCl crystal A has good solubility. Total related substances in the lurasidone HCl crystal A (high performance liquid chromatography (HPLC) detection) can be below 0.2%, single impurity is smaller than 0.1%, and high purity is achieved. Solvent with little toxicity is used during preparing, a production preparing process is safe, toxic substances with large toxicity cannot be left in obtained products, and the lurasidone HCl crystal A is suitable for officinal.
Owner:SUZHOU ERYE PHARMA CO LTD +1
Pharmaceutical compositions of lurasidone
The present invention relates to pharmaceutical compositions of lurasidone or salts thereof. In particular, the invention relates to pharmaceutical compositions of lurasidone or salts thereof with one or more water-insoluble pharmaceutical excipients. The invention also relates to processes for the preparation of such compositions and use thereof for treatment of schizophrenia, bipolar disorders or senile dementia.
Owner:CADILA HEALTHCARE LTD
Lurasidone solid dispersion and preparation method thereof
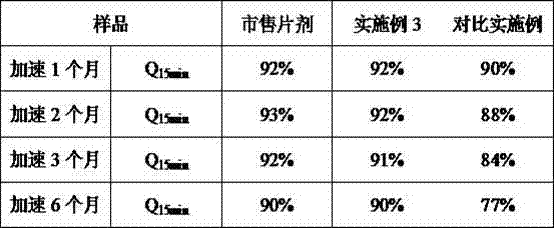
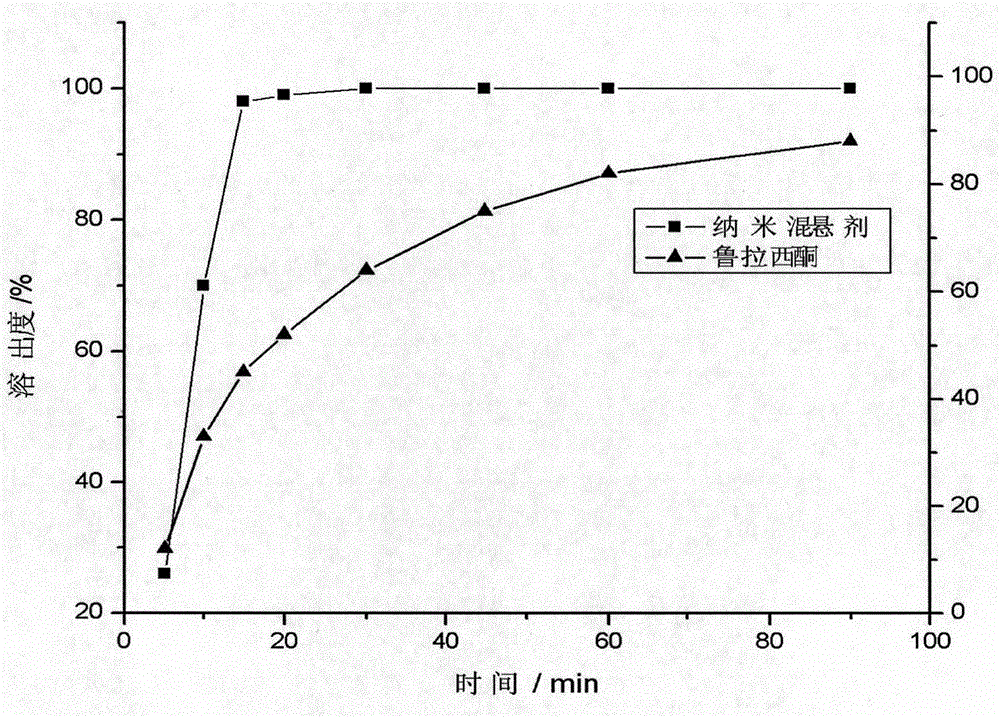
It relates to a lurasidone solid dispersion and a preparation method, wherein the method comprises melting treatment of a mixture containing lurasidone, a medicinal hot melt carrier, optionally an acidic regulator and plasticizer in order to obtain the solid dispersion described herein, and wherein the lurasidone is provided in a form of free base. The lurasidone solid dispersion obtained by the preparation method according to the example of the invention has the characteristics of high dissolution rate (dissolution rate can reach 30%-70%) in a partial neutral medium (e.g. pH 6.0). The bioavailability of lurasidone solid dispersion increased significantly and the food effect of lurasidone solid dispersion prepared from the example decreased remarkably. It overcomes the limitation of too many medication in the prior art and avoids the reduction of curative effect of the improper medication for the patient or even invalid, ensures the normal efficacy, thereby increases the patient's medication flexibility and compliance.
Owner:SUNSHINE LAKE PHARM CO LTD
Method of treating memory/learning dysfunctions caused by schizophrenia with lurasidone
ActiveUS8835438B2High activityImprove cognitive impairmentCompounds screening/testingNervous disorderPsychosis drugTypical antipsychotic
A method of evaluating memory / learning functions with the use of a model with glutamic acid N-methyl-D-aspartate (NMDA) type receptor hypofunction as an animal model for schizophrenia and with the use of reference memory task, wherein there has been found concrete means for detecting any differences in activity between typical anti-psychosis drugs and atypical anti-psychosis drugs is found.An in vivo animal model for screening of a therapeutic agent for improving cognitive dysfunction by schizophrenia is provided.
Owner:SUMITOMO PHARMA CO LTD
Solubilization preparation
InactiveUS8283352B2Promote effectiveOrganic active ingredientsNervous disorderPolymer scienceLurasidone
A solution-type preparation of lurasidone comprising N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]-(1′R,2′S,3′R,4′S)-2,3-bicyclo[2,2,1]heptanedicarboxyimide hydrochloride (lurasidone) as an active ingredient and containing at least one substance selected from benzyl alcohol, N,N-dimethylacetamide, lactic acid and propylene glycol.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Pharmaceutical compositions of lurasidone
The present invention relates to pharmaceutical compositions of lurasidone or salts thereof. In particular, the invention relates to pharmaceutical compositions of lurasidone or salts thereof with one or more water-insoluble pharmaceutical excipients. The invention also relates to processes for the preparation of such compositions and use thereof for treatment of schizophrenia, bipolar disorders or senile dementia.
Owner:CADILA HEALTHCARE LTD
Orally disintegrating tablet containing lurasidone and preparation method thereof
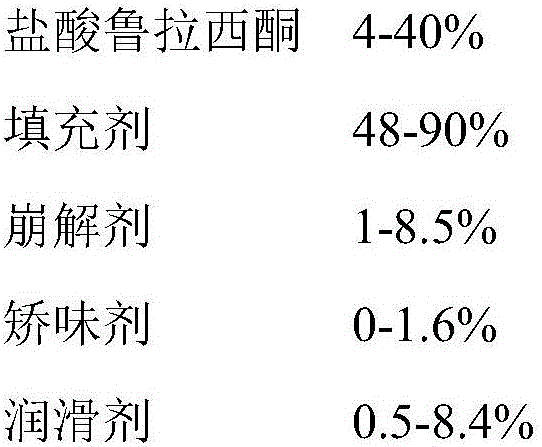
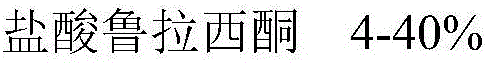
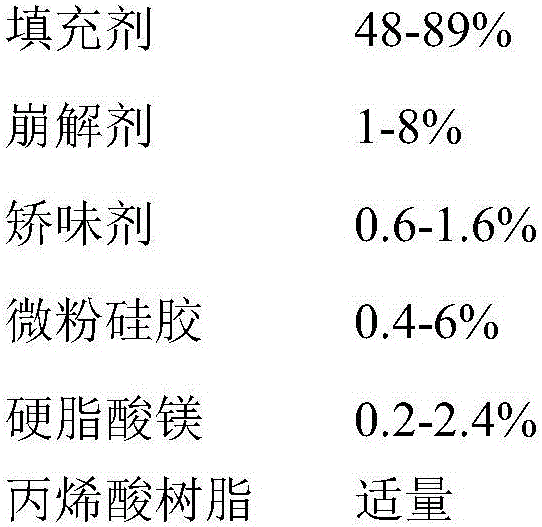
ActiveCN106074414ASolve liquidity problemsSolve the problem of bad tasteOrganic active ingredientsNervous disorderLurasidoneOrally disintegrating tablet
The invention relates to an orally disintegrating tablet containing hydrochloric acid lurasidone and a preparation method thereof. The orally disintegrating tablet contains the hydrochloric acid lurasidone, a filling agent, a disintegrating agent, a flavoring agent and a lubricating agent; meanwhile, the grain diameter D90 of the hydrochloric acid lurasidone is controlled to be smaller than 75mum. The orally disintegrating tablet containing the hydrochloric acid lurasidone has good in-vitro dissolution and good mouthfeel. The orally disintegrating tablet has the advantages that the preparation process is simple; the industrialized mass production can be realized by ordinary pelletizing and tabletting equipment.
Owner:CHENGDU KANGHONG PHARMA GRP
Lurasidone composition
InactiveCN102793701AGood water solubilityOrganic active ingredientsNervous disorderLurasidoneWater soluble
The invention discloses a lurasidone composition, which consists of lurasidone or pharmaceutically acceptable salt and cyclodextrin, wherein the cyclodextrin is hydroxypropyl-beta-cyclodextrin or sulfobutylether-beta-cyclodextrin. By the composition, the water solubility of the lurasidone and the pharmaceutically acceptable salt can be improved; and a feasible preparation method is provided for a lurasidone injection preparation.
Owner:SHANGHAI INST OF PHARMA IND
Pharmaceutical composition
ActiveUS8729085B2Reduce the burden onIncrease the burdenOrganic active ingredientsNervous disorderOral medicationLurasidone
A preparation for oral administration comprising: a pregelatinized starch comprising N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]-(1′R,2′S,3′R,4′S)-2,3-bicyclo[2,2,1]-heptanedicarboxyimide hydrochloride (lurasidone) represented by the formula (1) as an active ingredient; a water-soluble excipient; and a water-soluble polymeric binder, the preparation exhibiting an invariant level of elution behavior even when the content of its active ingredient is varied.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com