Lurasidone key intermediate preparation method
A technology of lurasidone and intermediates, applied in the field of compound synthesis, can solve the problems of low total yield, high cost, and large consumption of resolution reagents, and achieve the effect of easy industrial production and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
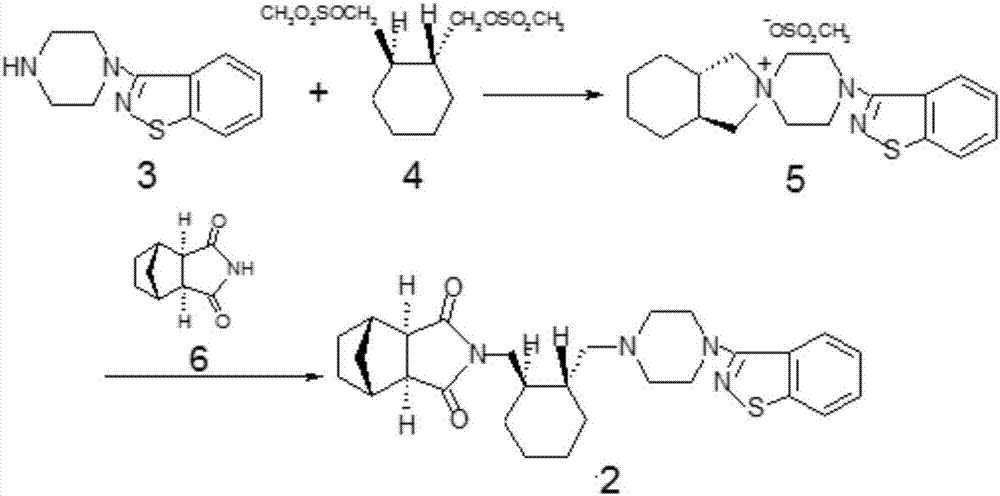
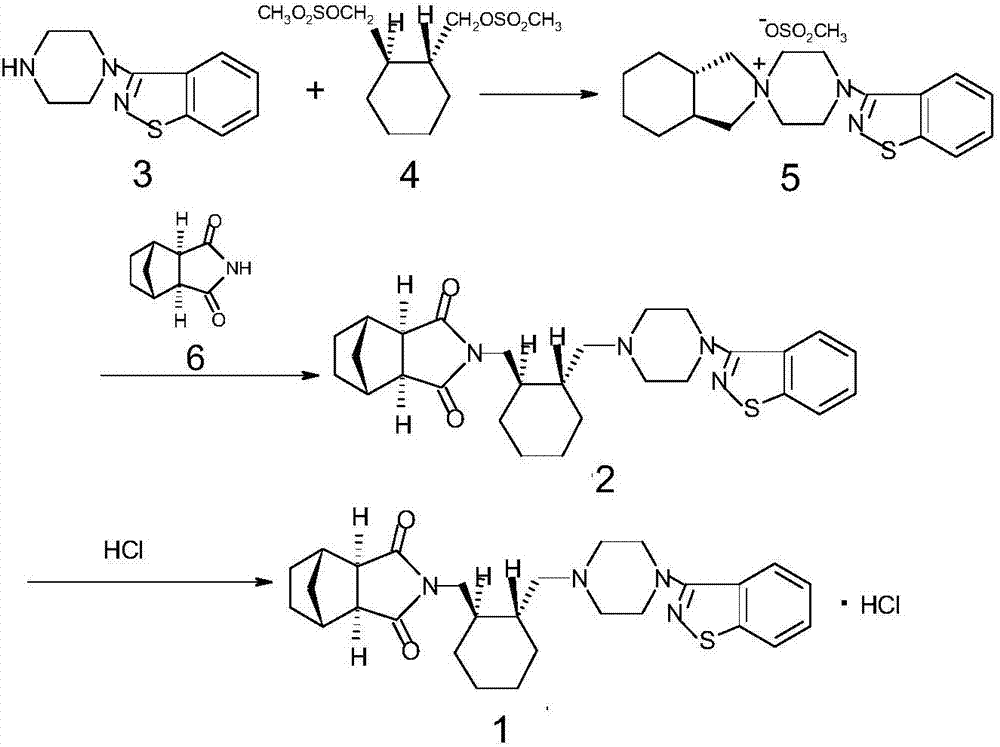
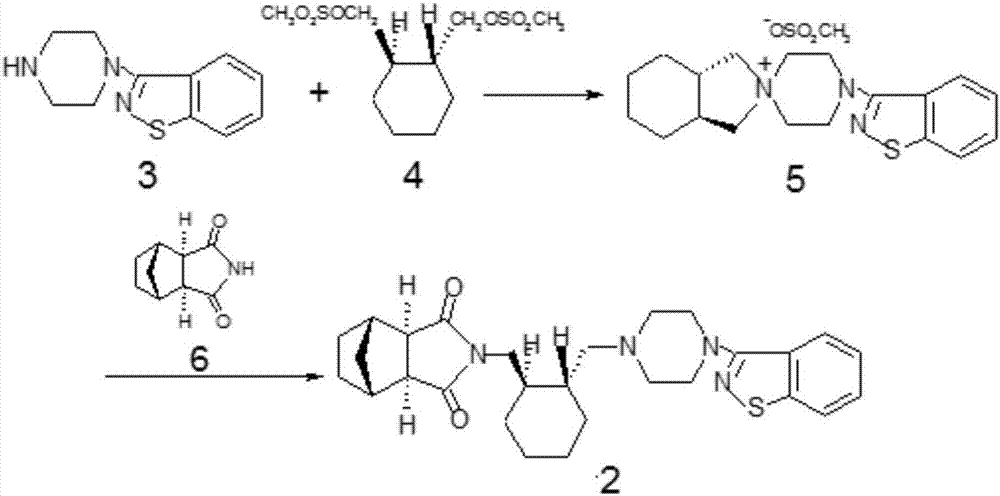
[0022] The synthetic route of compound 1 is as follows:
[0023]
[0024] 4,-(1,2-Benzisothiazol-3-yl)-(3aR,7aR)-octahydrospiro-(2H-isoindole-2,1,-piperazine) methanesulfonate (5) Synthesis: In a reaction flask, add compound 4 (30.0g), compound 3 (20g), anhydrous potassium carbonate (12g), cyclodextrin (0.6g), toluene (200ml), reflux reaction for 4h, HPLC normalization Chemical method detection (detection condition: column Shim-pack VP-ODSC18 150mm*4.6mm / 5um, mobile phase acetonitrile: phosphate buffer = 80; 20, wavelength 230nm, flow velocity 1.0ml / min) raw material compound 4 is less than 0.5%, reaction After finishing, the reaction solution containing the product is directly used in the next step reaction.
[0025] Synthesis of lurasidone (2): Add compound 6 (18.1 g), anhydrous potassium carbonate (15.2 g), and water (1 ml) to the reaction flask containing compound 5 reaction solution, and reflux for 10 h. At the end, add water (300ml), stir, let the layers stand still...
Embodiment 2
[0028] 4,-(1,2-Benzisothiazol-3-yl)-(3aR,7aR)-octahydrospiro-(2H-isoindole-2,1,-piperazine) methanesulfonate (5) Synthesis: In a reaction flask, add compound 4 (30.0g), compound 3 (30g), anhydrous potassium carbonate (18g), cyclodextrin (0.6g), toluene (240ml), reflux reaction for 6h, HPLC normalization The chemical method detects that the raw material compound 4 is less than 0.5%, and the reaction is completed, and the reaction solution containing the product is directly used for the next reaction.
[0029] Synthesis of lurasidone (2): Add compound 6 (18.1 g), anhydrous potassium carbonate (15.2 g), and water (1 ml) to the reaction flask containing compound 5 reaction solution, and reflux for 10 h. Finished, add water (300ml), stir, static layering, the oil layer is adjusted to pH=4.5 with 1% hydrochloric acid, washed with water (200ml), the oil layer is concentrated under reduced pressure to quick dryness, and isopropanol is added for recrystallization, and a white solid 2 (...
Embodiment 3
[0032] 4,-(1,2-Benzisothiazol-3-yl)-(3aR,7aR)-octahydrospiro-(2H-isoindole-2,1,-piperazine) methanesulfonate (5) Synthesis: In a reaction flask, add compound 4 (30.0g), compound 3 (35g), anhydrous potassium carbonate (20g), cyclodextrin (0.6g), toluene (240ml), reflux reaction for 6h, HPLC normalization The chemical method detects that the raw material compound 4 is less than 0.5%, and the reaction is completed, and the reaction solution containing the product is directly used for the next reaction.
[0033]Synthesis of lurasidone (2): Add 6 (18.1g), anhydrous potassium carbonate (15.2g) and water (1ml) to the reaction flask containing the reaction solution of compound 5, reflux for 10h, and the reaction ends , add water (300ml), stir, static layering, the oil layer is adjusted to pH=5.5 with 5% hydrochloric acid, washed with water (200ml), the oil layer is concentrated under reduced pressure to quick dryness, isopropanol is added for recrystallization, and a white solid 2 (46...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com