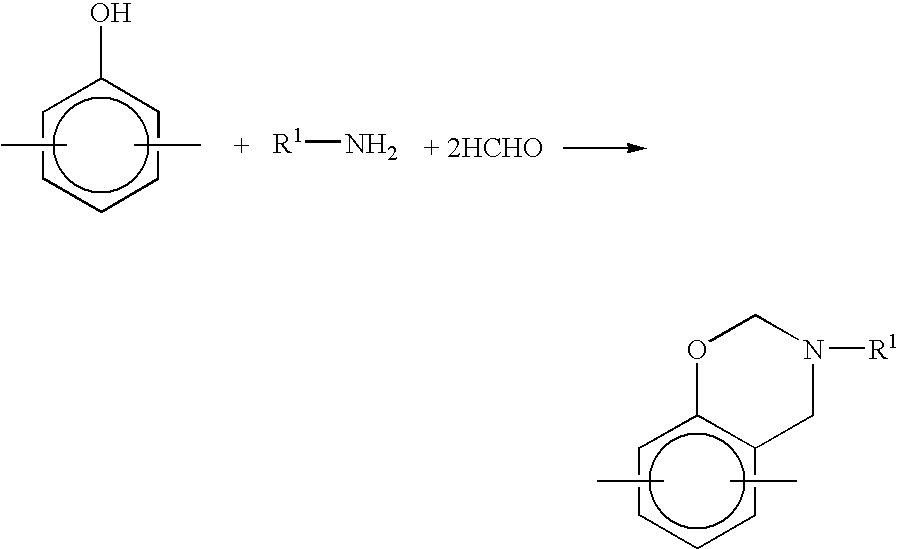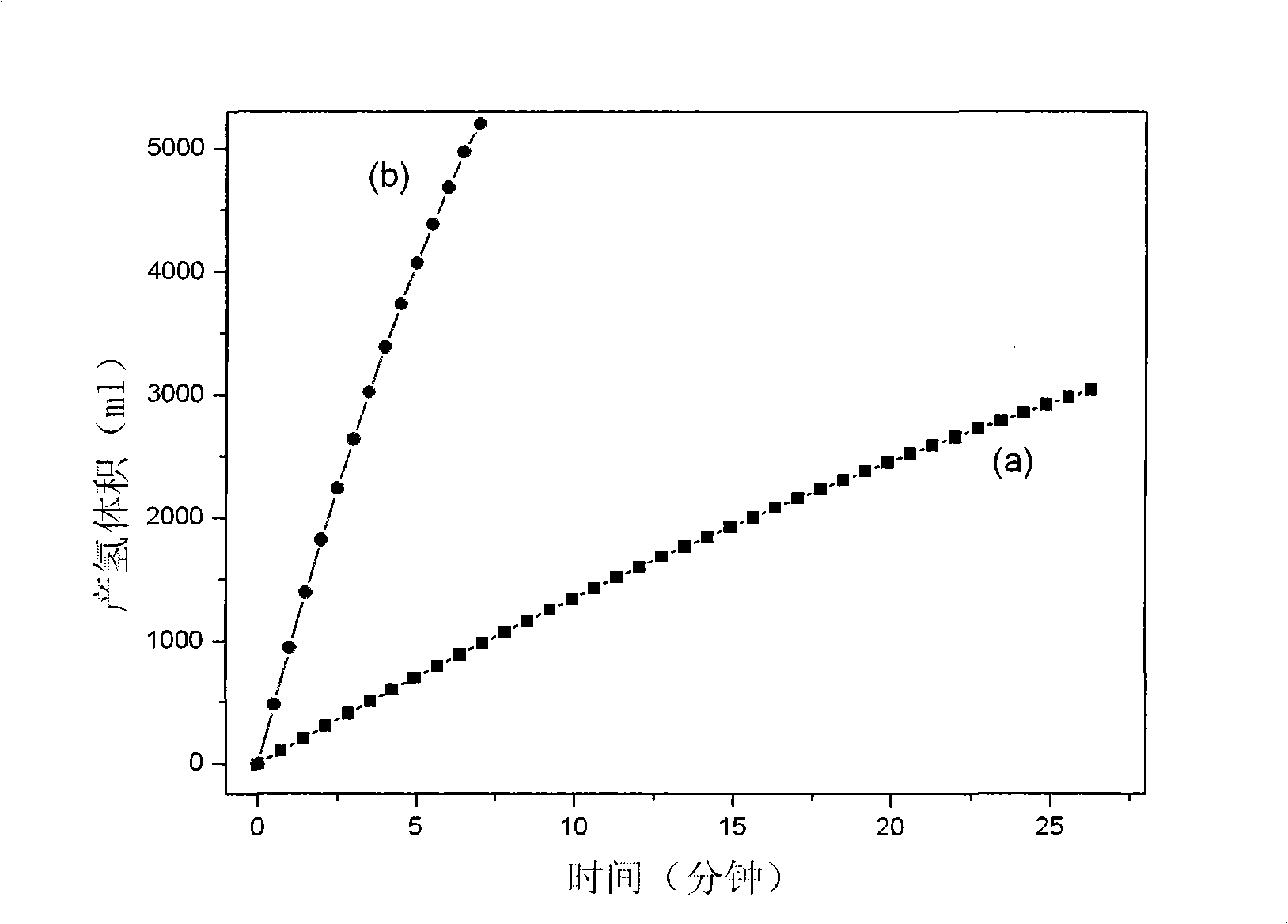Patents
Literature
15786 results about "Reaction system" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reaction system. The Reaction system implant artificially augmented the user's nervous system by allowing impulses to travel faster and farther along the nerve bundle pathways. This resulted in an enhanced reaction time and increased accuracy from the improvement of fine motor control.
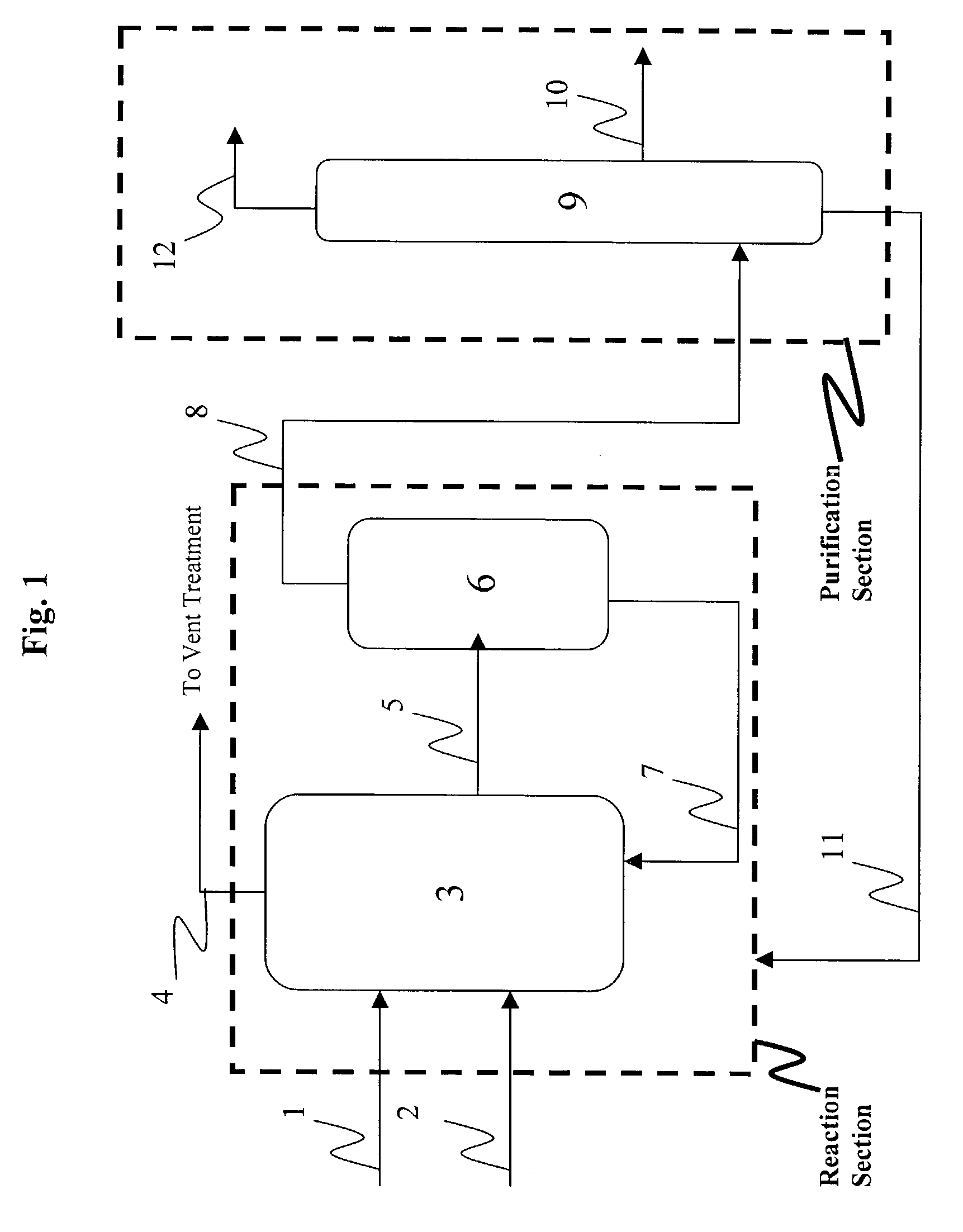
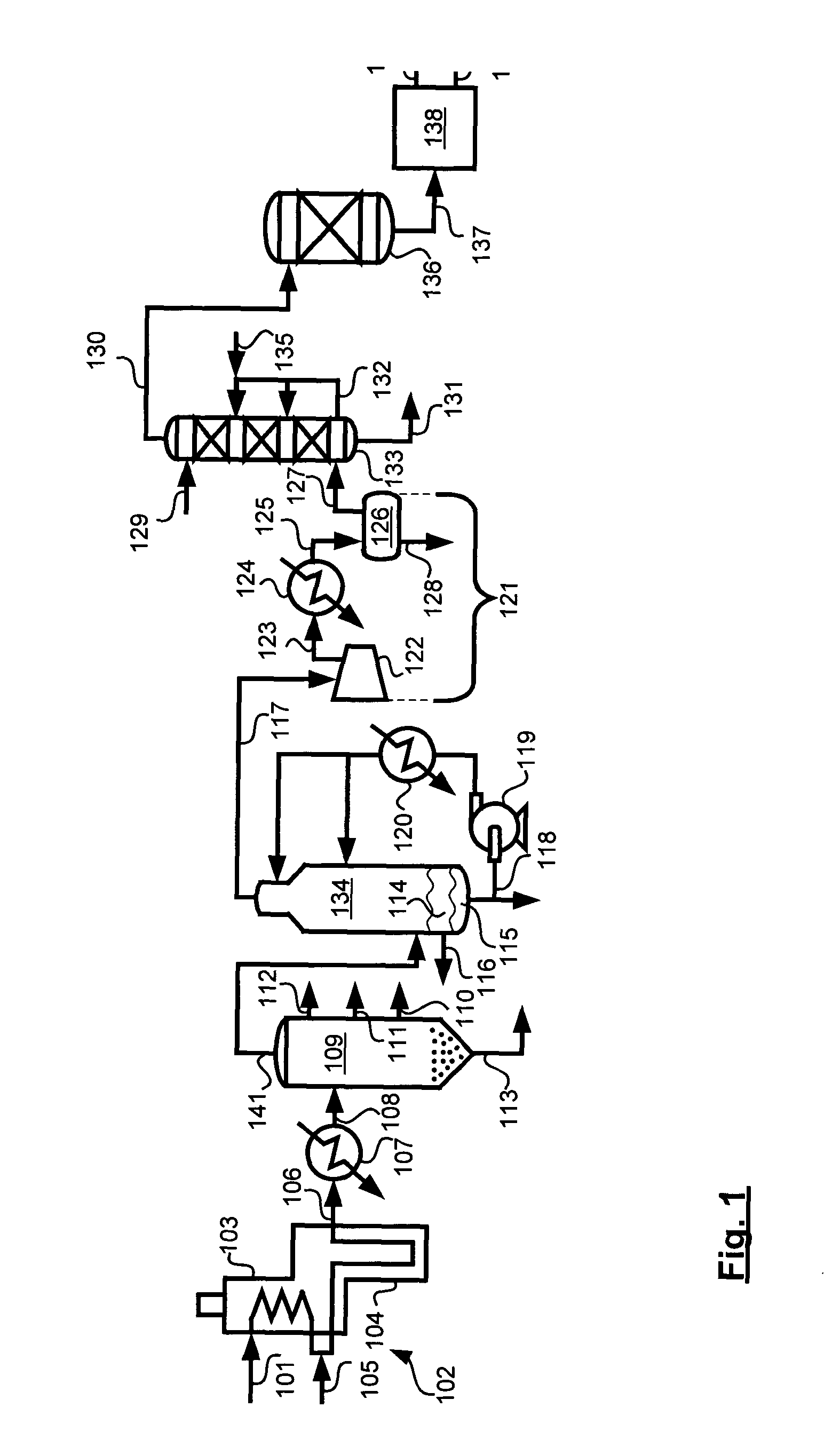
Low water methanol carbonylation process for high acetic acid production and for water balance control
ActiveUS7005541B2High acetic acid production rateIncrease chanceOrganic compound preparationCarboxylic preparation from carbon monoxide reactionWater methanolAcetic anhydride
The invention relates to a process for the production of acetic acid by carbonylation of methanol, and reactive derivatives thereof, in a reaction mixture using a rhodium-based catalyst in low water conditions. The process is used to achieve reaction rates of at least 15 g mol / l / hr. The high rate reactions proceed at water concentrations of less than 2.0 wt. %. Under certain conditions, the water concentration in the reaction mixture of the process is maintained at a desired concentration by at least one process step including adding a compound such as methyl acetate, dimethyl ether, acetic anhydride, or mixtures of these compounds to the reaction system. The process step of adding the components to the reaction mixture may be combined with other process steps for controlling water concentrations in reaction mixtures for the carbonylation of methanol.
Owner:CELANESE INT CORP
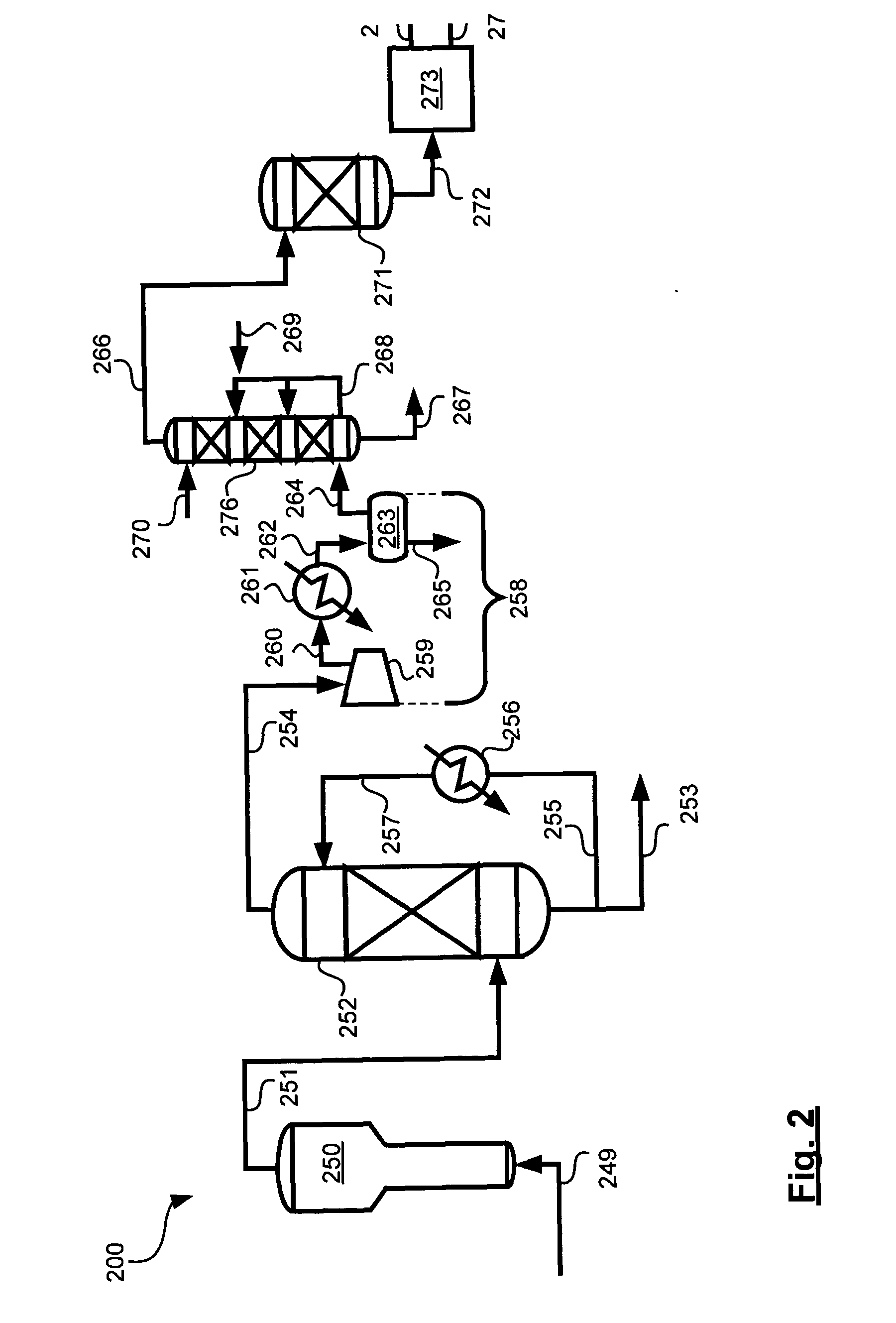
Reaction system for growing a thin film
ActiveUS20060266289A1Easy to cleanEliminates and significantly reduces dead pocketAfter-treatment apparatusSemiconductor/solid-state device manufacturingEngineeringReaction system
An atomic deposition (ALD) thin film deposition apparatus includes a deposition chamber configured to deposit a thin film on a wafer mounted within a space defined therein. The deposition chamber comprises a gas inlet that is in communication with the space. A gas system is configured to deliver gas to the gas inlet of the deposition chamber. At least a portion of the gas system is positioned above the deposition chamber. The gas system includes a mixer configured to mix a plurality of gas streams. A transfer member is in fluid communication with the mixer and the gas inlet. The transfer member comprising a pair of horizontally divergent walls configured to spread the gas in a horizontal direction before entering the gas inlet.
Owner:ASM IP HLDG BV
Reaction system for growing a thin film
ActiveUS20050241176A1Extension of timeLong stepDrying using combination processesDrying solid materials with heatControl systemDiffusion barrier
A reactor defines a reaction chamber for processing a substrate. The reactor comprises a first inlet for providing a first reactant and to the reaction chamber and a second inlet for a second reactant to the reaction chamber. A first exhaust outlet removes gases from the reaction chamber. A second exhaust outlet removes gases from the reaction chamber. A flow control system is configured to alternately constrict flow through the first and second exhaust outlets. The reactor chamber is configured to for a diffusion barrier within the reaction chamber.
Owner:ASM IP HLDG BV
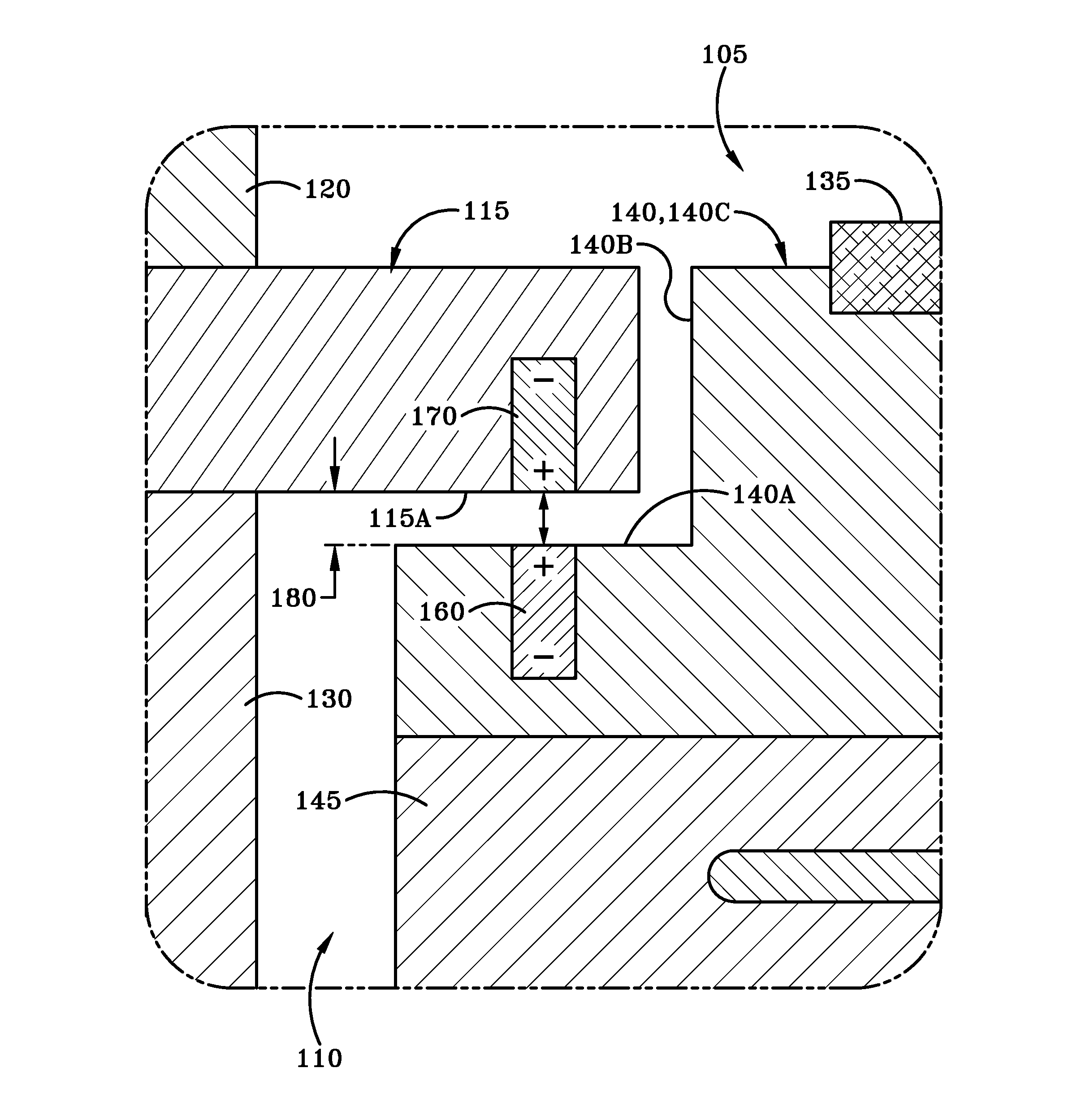
Variable adjustment for precise matching of multiple chamber cavity housings
A vertical adjustment assembly is disclosed in order to provide for matching vertical positions of two substrates within separate chambers or cavities of a reaction system for processing of semiconductor substrates. The vertical adjustment assembly, in cooperation with a main lift driver, can provide for a more accurate positioning of the substrates to account for a tolerance stack-up error.
Owner:ASM IP HLDG BV
Variable adjustment for precise matching of multiple chamber cavity housings
A vertical adjustment assembly is disclosed in order to provide for matching vertical positions of two substrates within separate chambers or cavities of a reaction system for processing of semiconductor substrates. The vertical adjustment assembly, in cooperation with a main lift driver, can provide for a more accurate positioning of the substrates to account for a tolerance stack-up error.
Owner:ASM IP HLDG BV
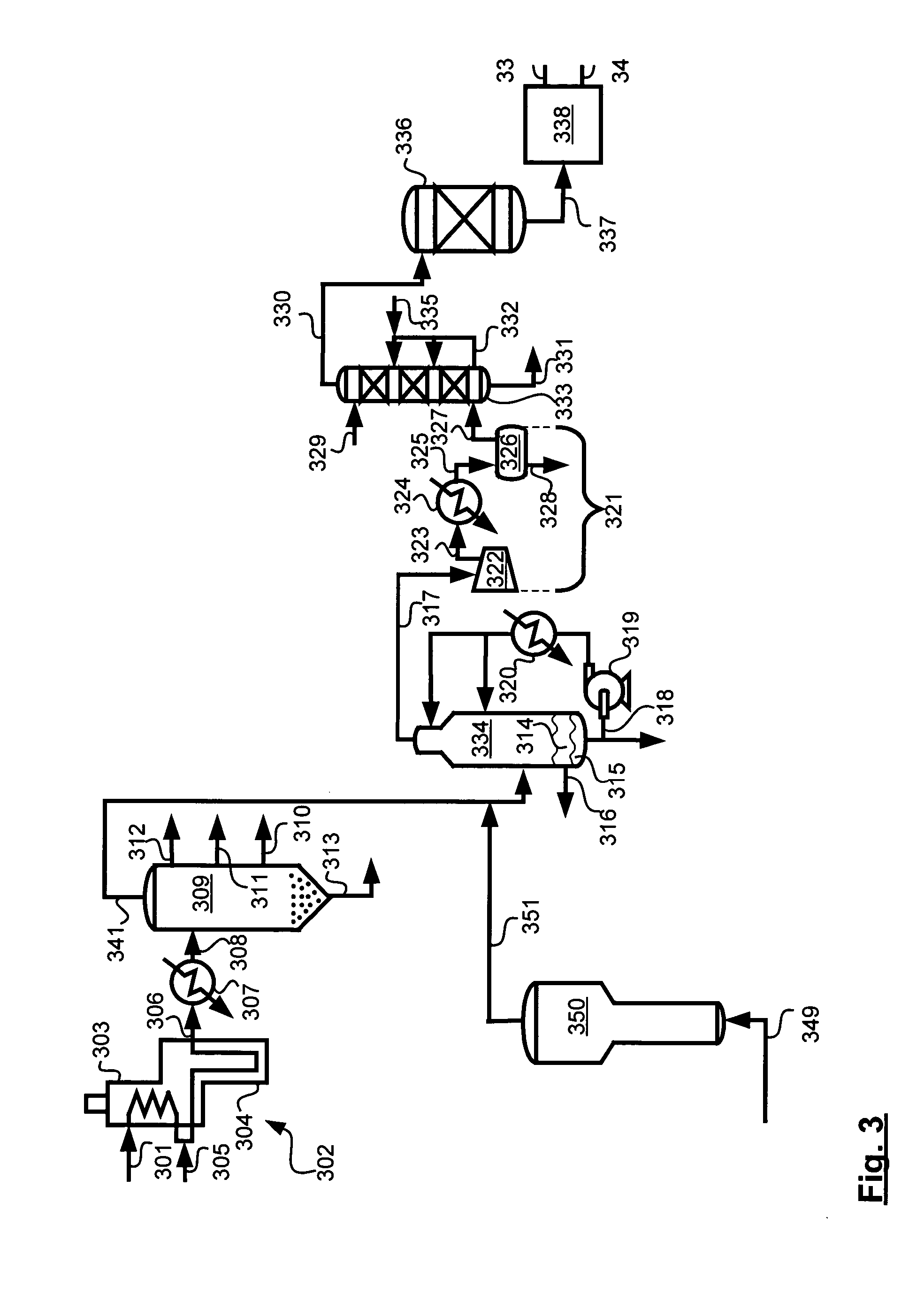
Pre-passivation process for a continuous reforming apparatus, and passivation process for a continuous reforming apparatus during the initial reacation
ActiveUS20100282645A1Reduce operational riskReduce contentThermal non-catalytic crackingPhysical/chemical process catalystsLiquid productReaction temperature
The present invention relates to a pre-passivation process for a continuous reforming apparatus prior to the reaction, or a passivation process for a continuous reforming apparatus during the initial reaction, comprising loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, injecting sulfide into the gas at a reactor temperature ranging from 100-650° C., controlling the sulfur amount in the recycle gas within a range of 0.5-100×10−6 L / L so as to passivate the apparatus.The process of the present invention may also comprise the following steps:(1) loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, feeding the reforming feedstock into the reaction system when the temperature of the reactor is increased to 300-460° C., introducing sulfide into the reaction system while or after the reforming feedstock is fed, controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.5 μg / g-50 μg / g, reducing the content of sulfide introduced into the system when hydrogen sulfide concentration in the recycle gas reaches to 2.0 μL / L˜30 μL / L; and(2) maintaining the reforming reactor at a temperature of 460-490° C., controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.2 μg / g-0.5 μg / g, adjusting the amount of the reforming feedstock to the design value of the apparatus, increasing the reforming reaction temperature to 490-545° C. according to the requirements on the octane number of the liquid product, and letting the reforming apparatus run under normal operating conditions.
Owner:CHINA PETROCHEMICAL CORP +1
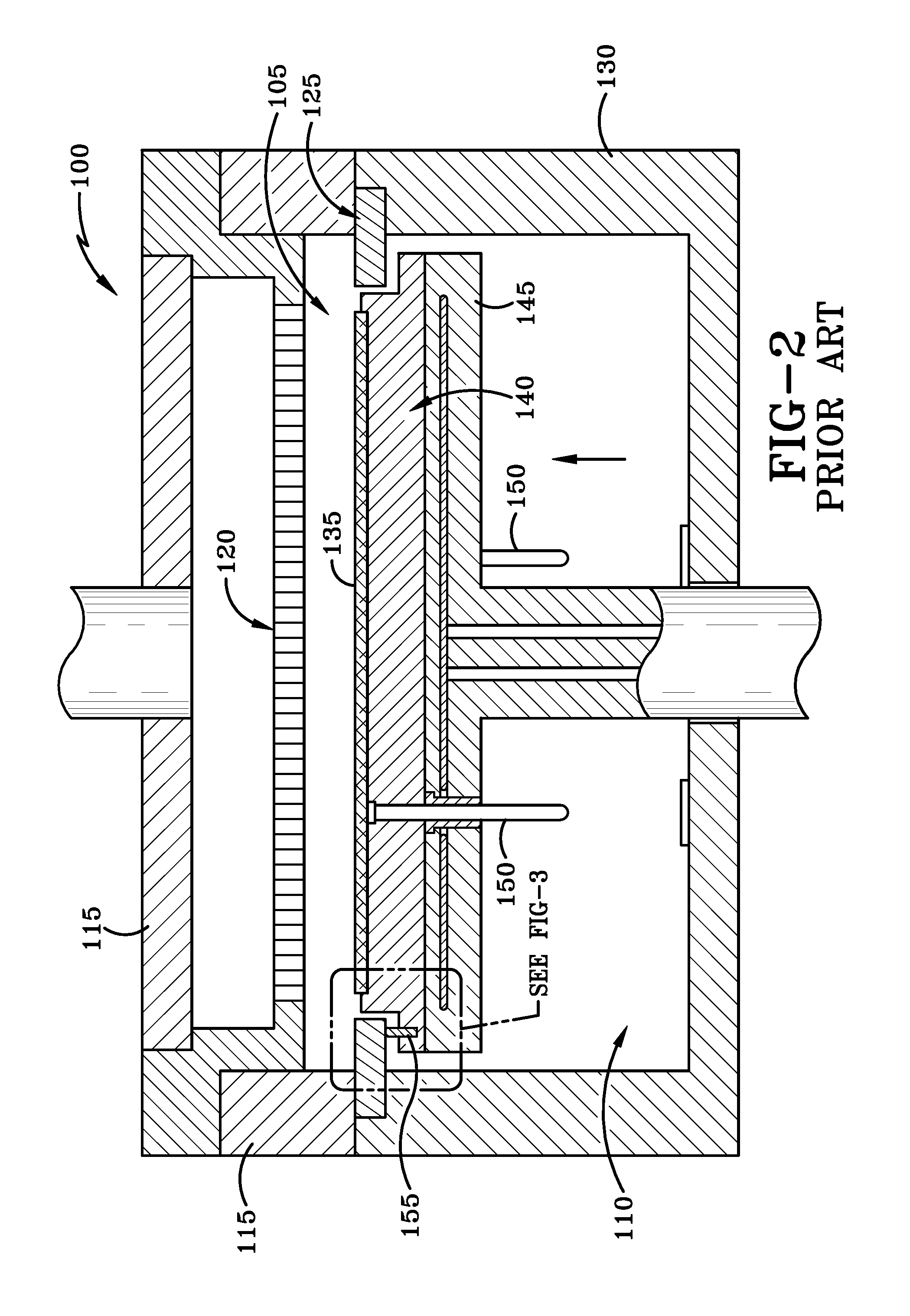
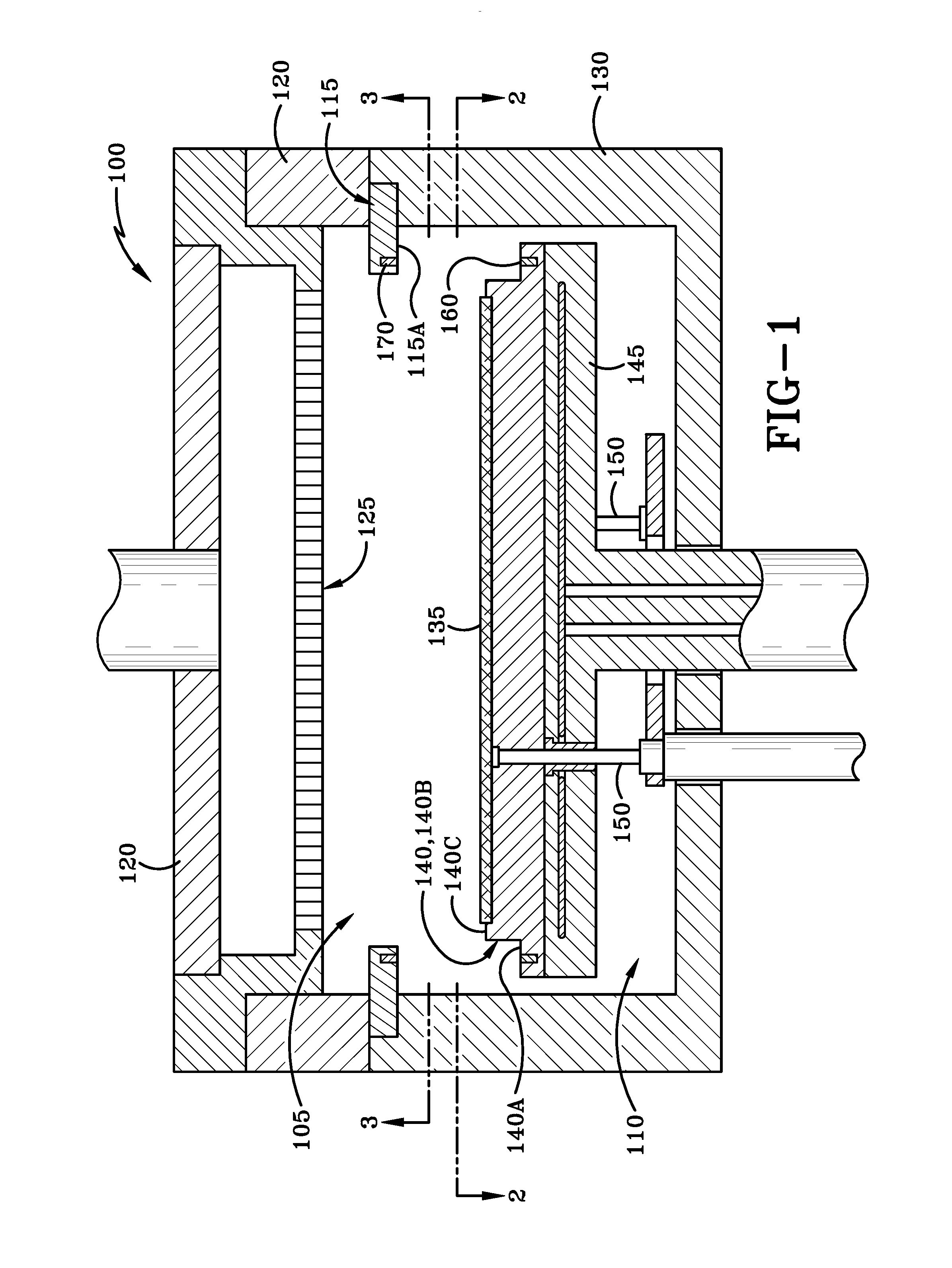
Variable gap hard stop design
ActiveUS10087525B2Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSusceptorEngineering
A reaction system for processing semiconductor substrates is disclosed. The reaction system includes a susceptor for holding the substrate as well as a baseplate as a part of housing for the reaction system. A pin located on the susceptor can interact with a baseplate feature located on the baseplate to result in a variable gap between the susceptor and the baseplate. The baseplate feature may take the form of a series of steps, a wedge, or a milled-out feature.
Owner:ASM IP HLDG BV
Variable adjustment for precise matching of multiple chamber cavity housings
A vertical adjustment assembly is disclosed in order to provide for matching vertical positions of two substrates within separate chambers or cavities of a reaction system for processing of semiconductor substrates. The vertical adjustment assembly, in cooperation with a main lift driver, can provide for a more accurate positioning of the substrates to account for a tolerance stack-up error.
Owner:ASM IP HLDG BV
Variable gap hard stop design
ActiveUS20170040206A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSusceptorEngineering
A reaction system for processing semiconductor substrates is disclosed. The reaction system includes a susceptor for holding the substrate as well as a baseplate as a part of housing for the reaction system. A pin located on the susceptor can interact with a baseplate feature located on the baseplate to result in a variable gap between the susceptor and the baseplate. The baseplate feature may take the form of a series of steps, a wedge, or a milled-out feature.
Owner:ASM IP HLDG BV
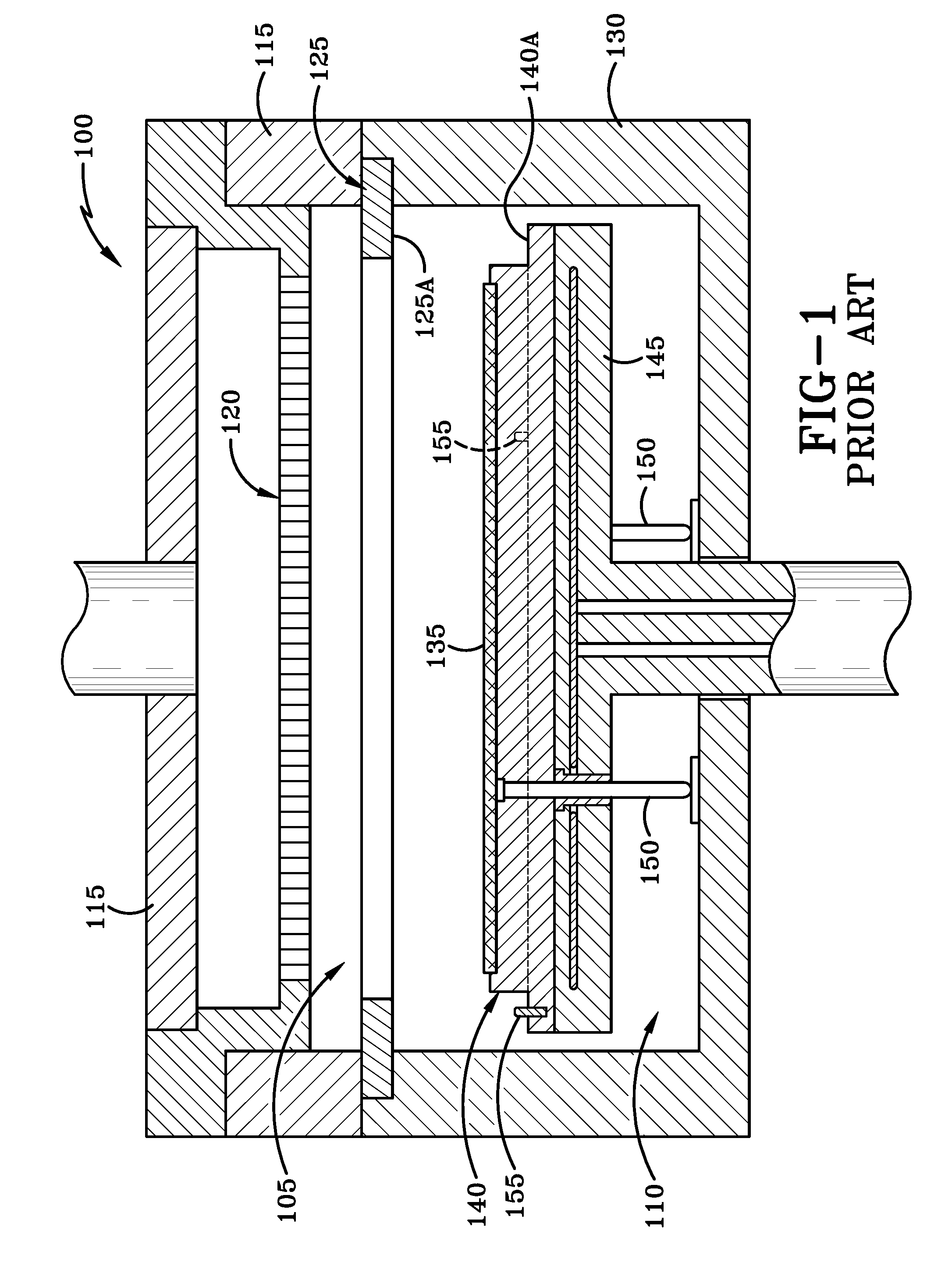
Magnetic susceptor to baseplate seal
ActiveUS20170011950A1Maintaining gapSolid-state devicesSemiconductor/solid-state device manufacturingSusceptorEngineering
A reaction system for processing semiconductor substrates is disclosed. In particular, the invention discloses an arrangement of a susceptor and a baseplate for when a substrate is placed into a reaction region. Magnets are embedded into the susceptor and the baseplate in order to create a gap between the two. As a result of the gap, the invention prevents an accumulation of gaseous materials that would exist in prior art systems as well as particle generation due to physical contact between parts.
Owner:ASM IP HLDG BV
High melting thermoplastic elastomeric alpha-olefin polymers (PRE/EPE effect) and catalysts therefor
InactiveUS6559262B1Activity of fluxional unbridged metallocene polymerization catalystsHigh molecular weightGroup 4/14 element organic compoundsMetallocenesElastomerEthylene Homopolymers
This invention relates generally to low ethylene insertions into I-olefin polymers and processes for production of such polymers using unbridged fluxional metallocenes, primarily substituted aryl indenyl metallocenes, and more particularly to use of unbridged, fluxional, cyclopentadienyl or indenyl metallocene catalyst systems in methods of production of high melting point I-olefin homo- and co-polymers, particularly elastomeric crystalline and amorphous block homo- and co-polymers of I-olefins. The activity of fluxional unbridged metallocene polymerization catalysts containing at least one 2-arylindene ligand is increased 10x or more by the addition of small (typically 0.1-10 wt. %) amounts of ethylene to the polymerization system, which increase is termed the Polymerization Rate-Enhancement effect (PRE), which is measured in terms of an Ethylene Enhancement Factor (EEF) as a dimensionless ratio in the range of from about 1.1 to about 10 or above. The amount of ethylene included in the reaction system can be selected and controlled to be so small as to result in essentially minimal (<2 mole %) incorporation of ethylene units into the resulting elastomeric polymer and the molecular weight may be increased. Amounts of ethylene to generate the PRE effect may be greater than 0.1 wt. % and preferably range up to about 2 wt. %. However, if a polymer with more ethylene is desired, additional ethylene may be incorporated into the polymerization feed, including up to 10 to about 50 mole % based on olefin units. A second important aspect of this invention is the ability to use a PRE activity-enhancing amount of ethylene in an olefin polymerization without substantially affecting the physical properties of the elastomer. In a third important aspect of this invention, I-olefin elastomers are produced through incorporation of ethylene using unbridged fluxional catalyst systems which may not otherwise produce acceptable elastomeric homopolymers. This effect is termed the EPE effect, for Elastomeric Property-Enhancing effect. The EPE amount of ethylene required to produce such elastomers typically overlaps the PRE activity-enhancing amount. Incorporation of up to about 5 mole % or more of ethylene typically will produce an elastomeric polymer using such catalyst systems. Typical useful amounts of incorporated ethylene include about 1 to 3 mole %. Preferred polymers of this invention retain sufficient crystallinity to provide a high melting point (by DSC) of about 80° C., preferably above 100° C., including in the range of from about 120° C. to about 140° C. and above. Novel flexible alpha-olefin homo and copolymers having elongation in excess of 600% and substantially no retained force are disclosed.
Owner:BP CORP NORTH AMERICA INC
Gas distribution device for a wafer processing apparatus
ActiveUS20200056282A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingWaferingElectrical polarity
A reaction system is disclosed that may be used to prevent formation of contaminants. The reaction system includes a showerhead that may be configured with a gated nanochannel grid to prevent particular gaseous precursors from passing through depending on whether a voltage is applied. The gated nanochannel grid may allow for both polar and non-polar molecules to pass, or may be configured to allow just non-polar or just polar molecules to pass.
Owner:CANADAVFD CORP LTD +1
Parallel flow process optimization reactors
InactiveUS20020048536A1Extreme flexibilityAdvantageously and flexibly employedProcess control/regulationSequential/parallel process reactionsProcess optimizationDistribution system
Owner:FREESLATE
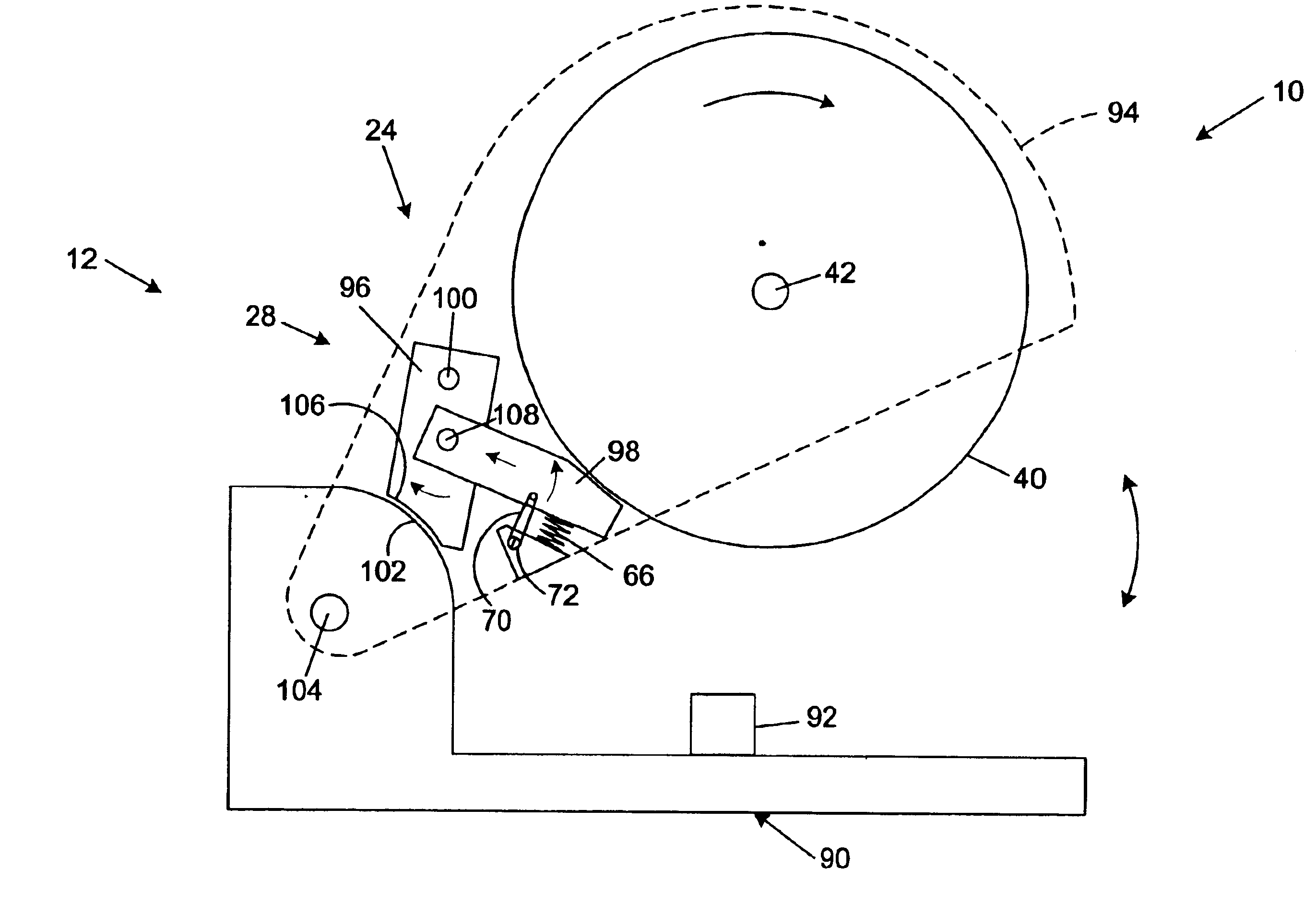
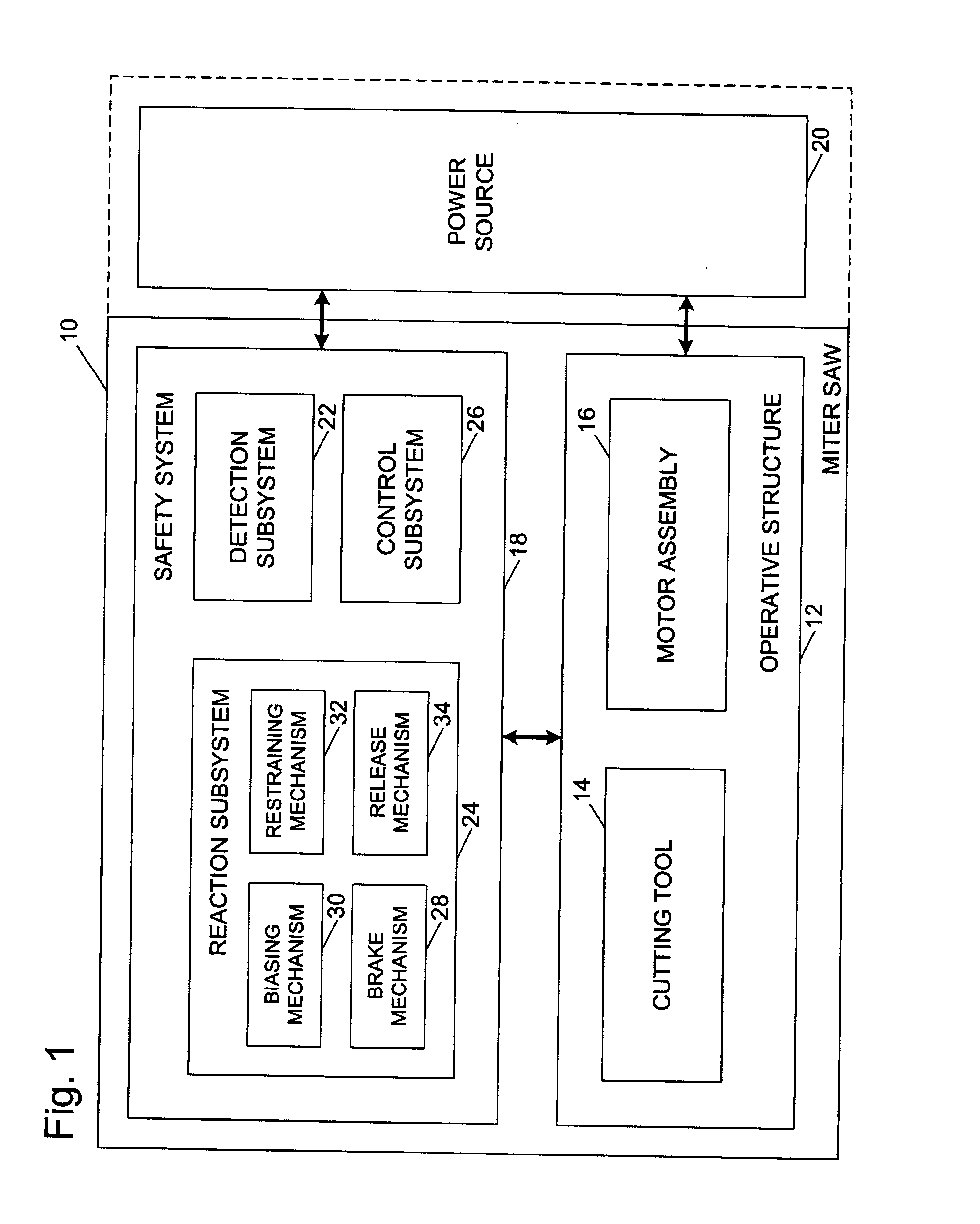
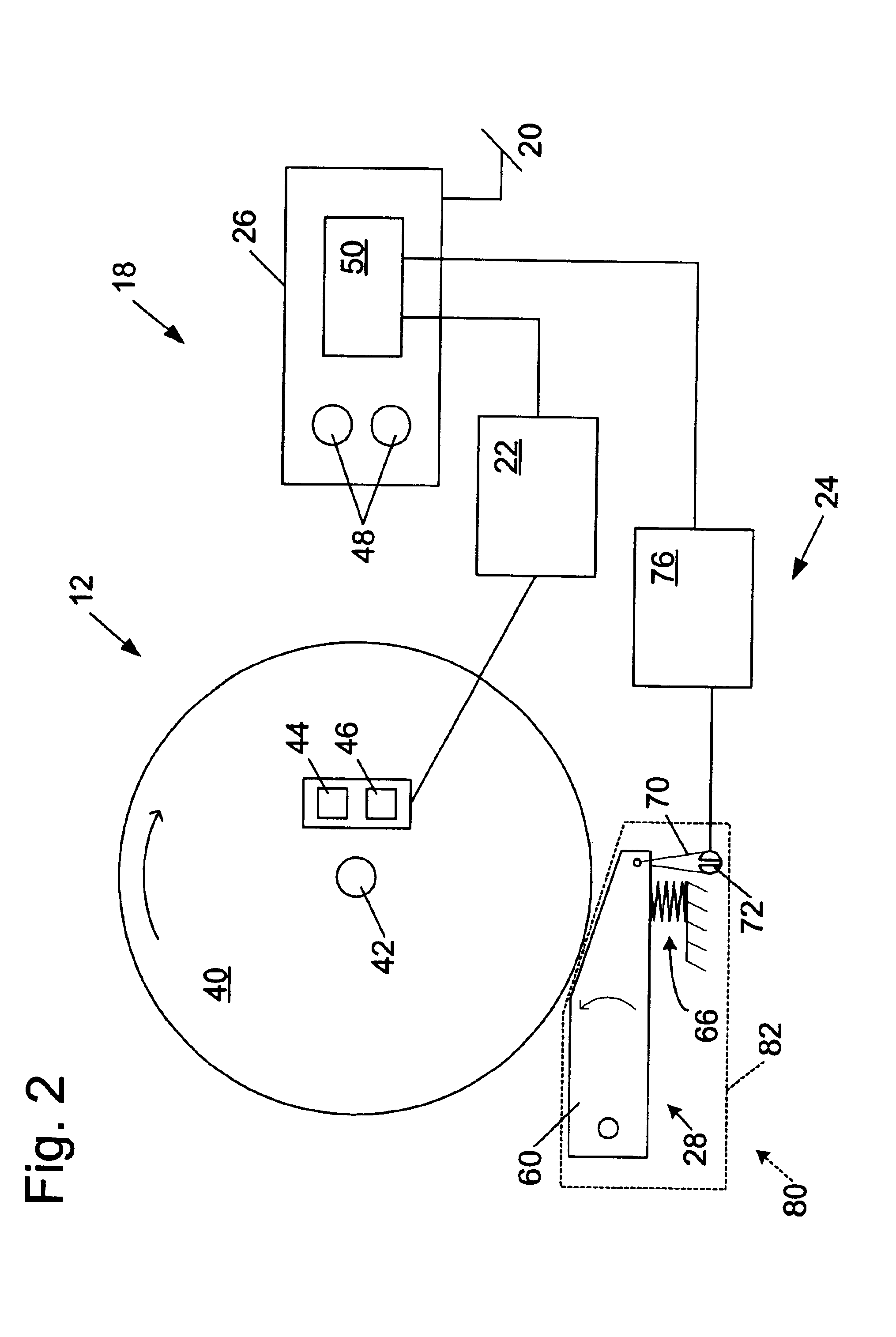
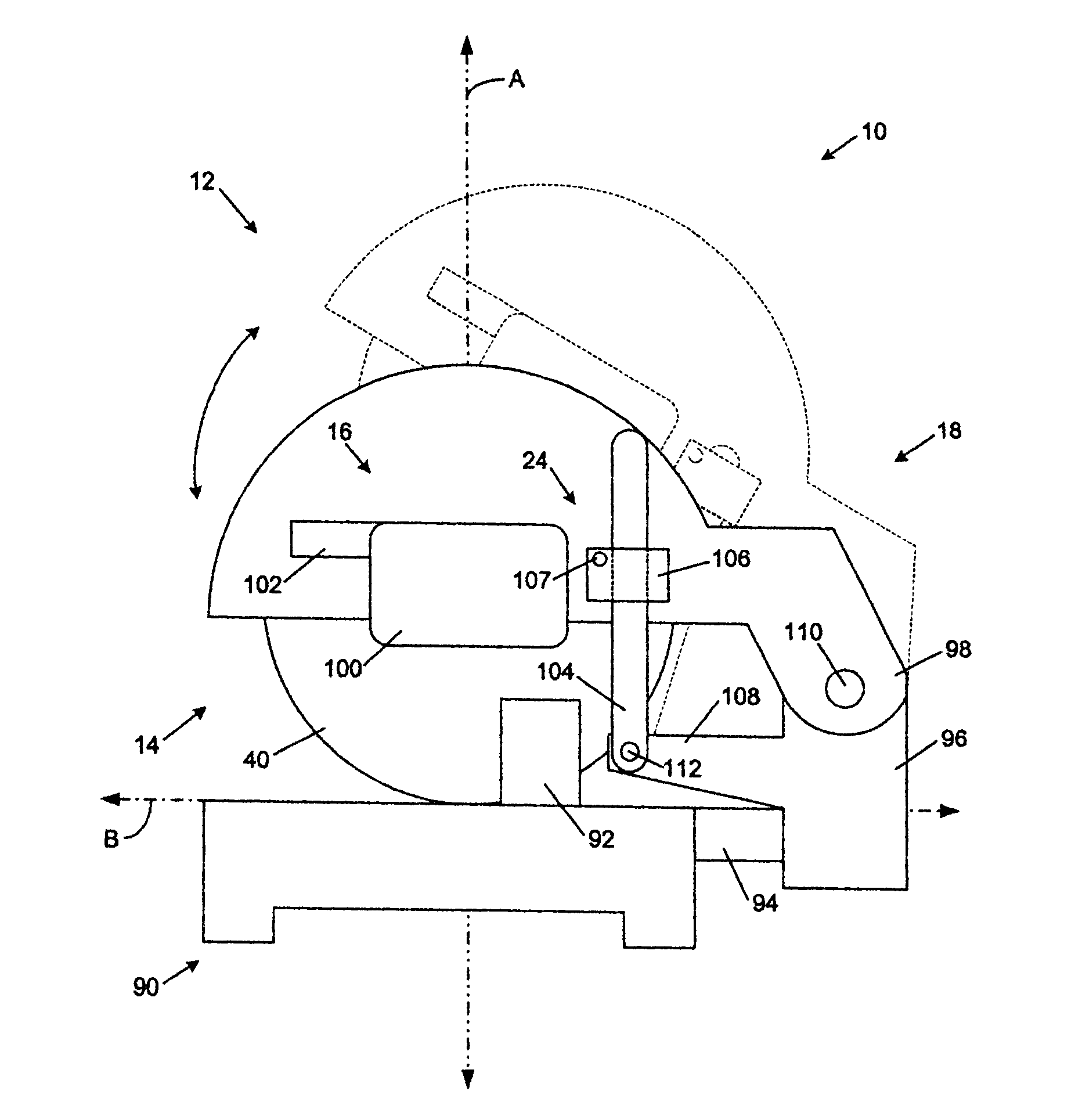
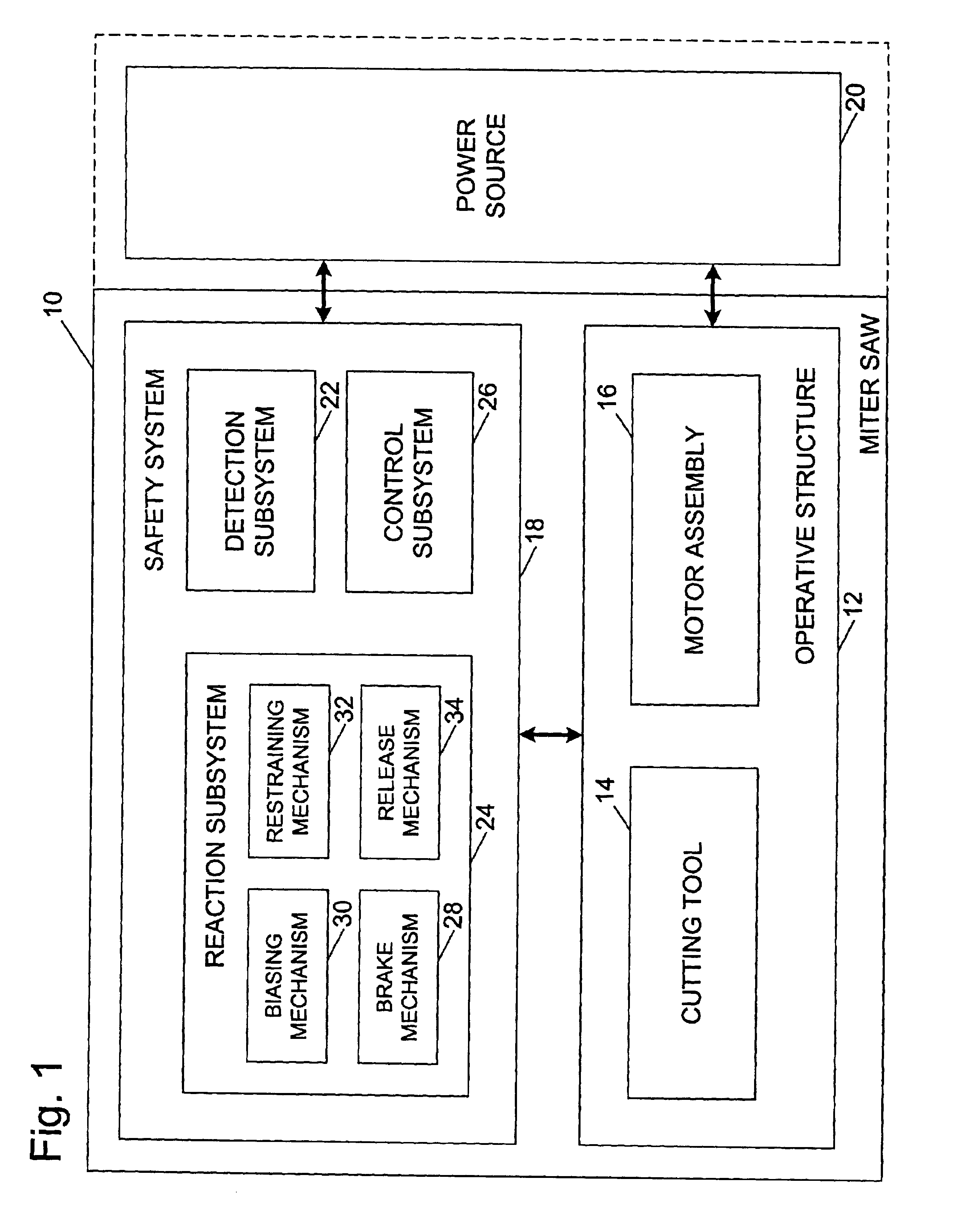
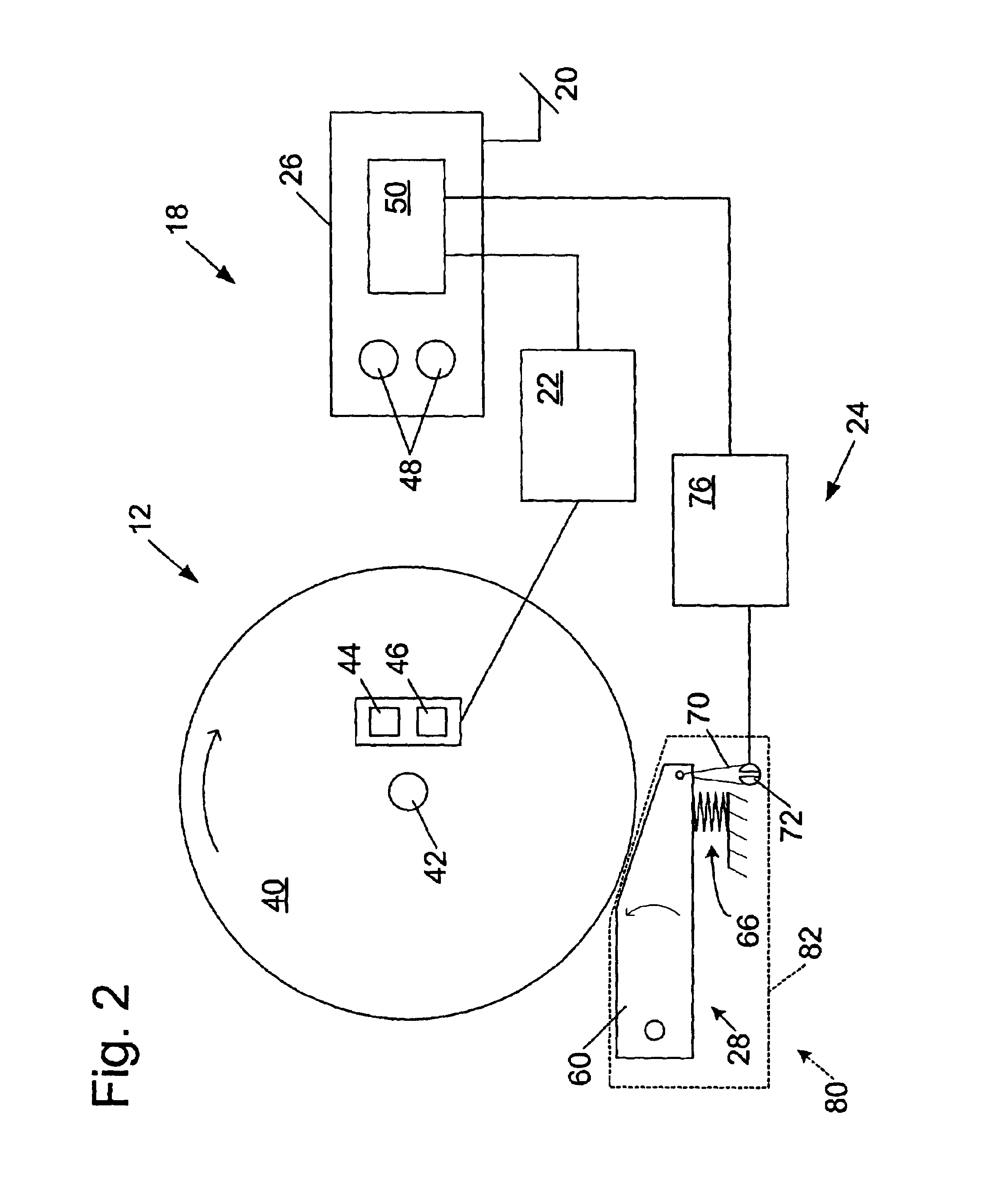
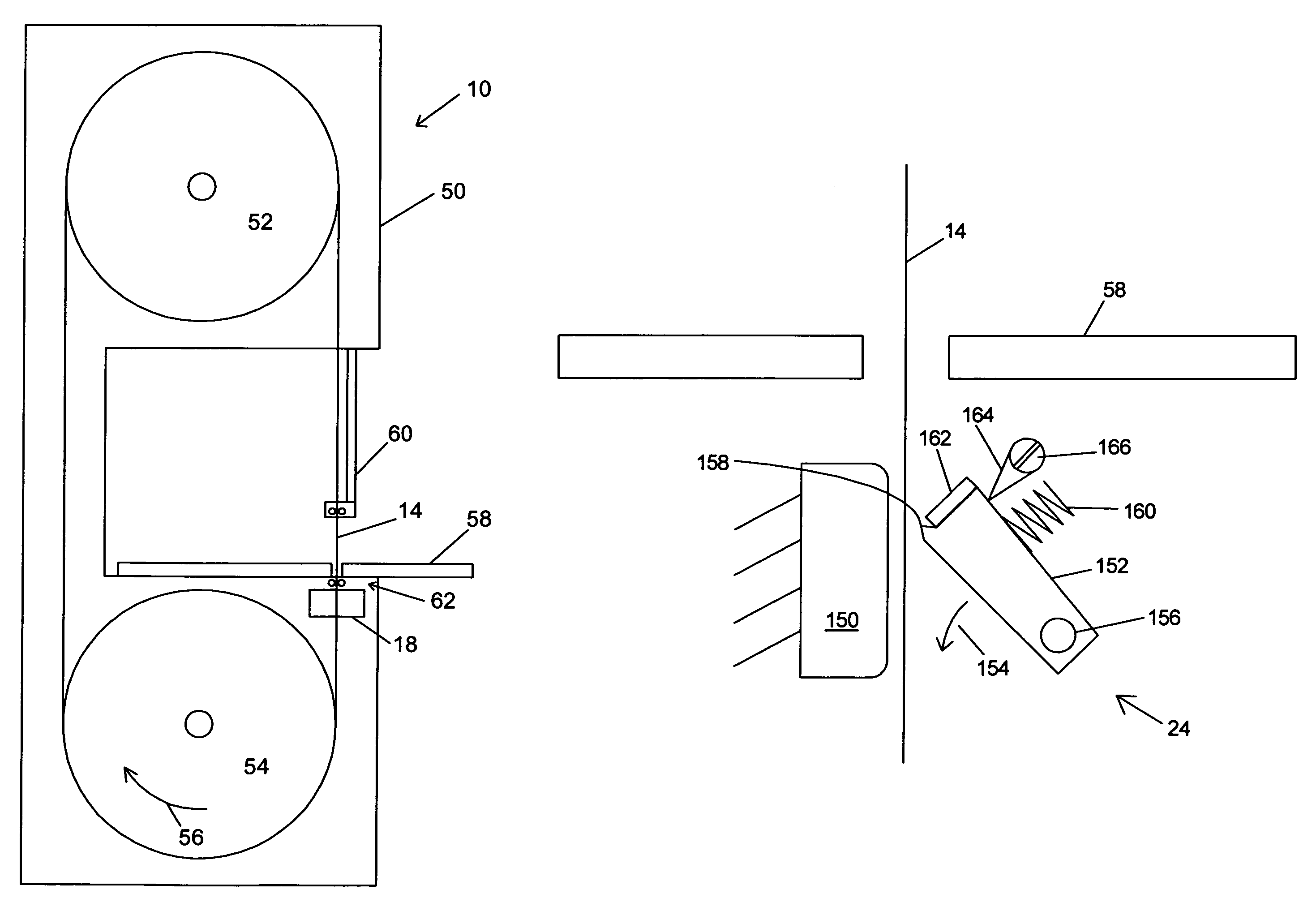
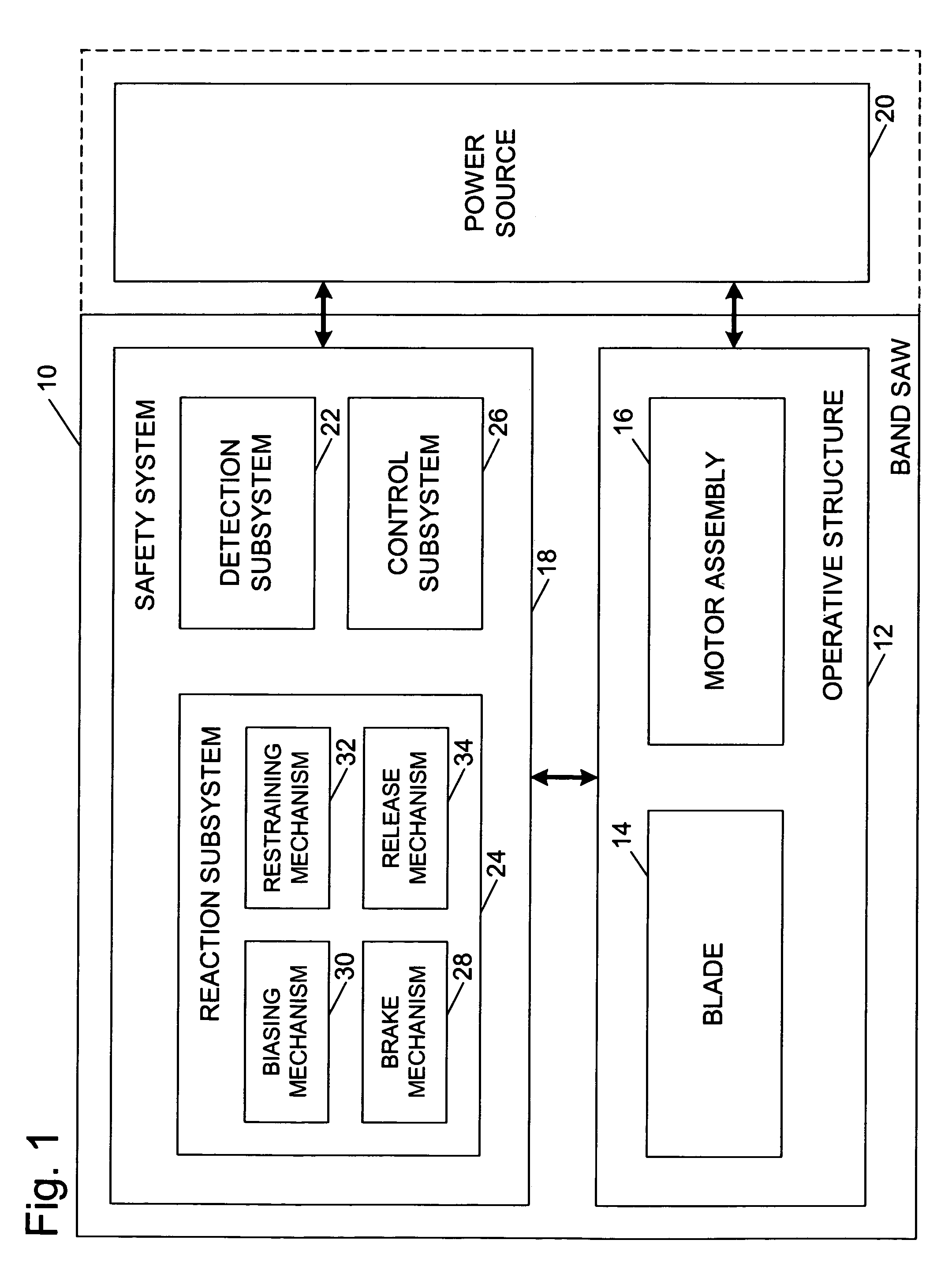
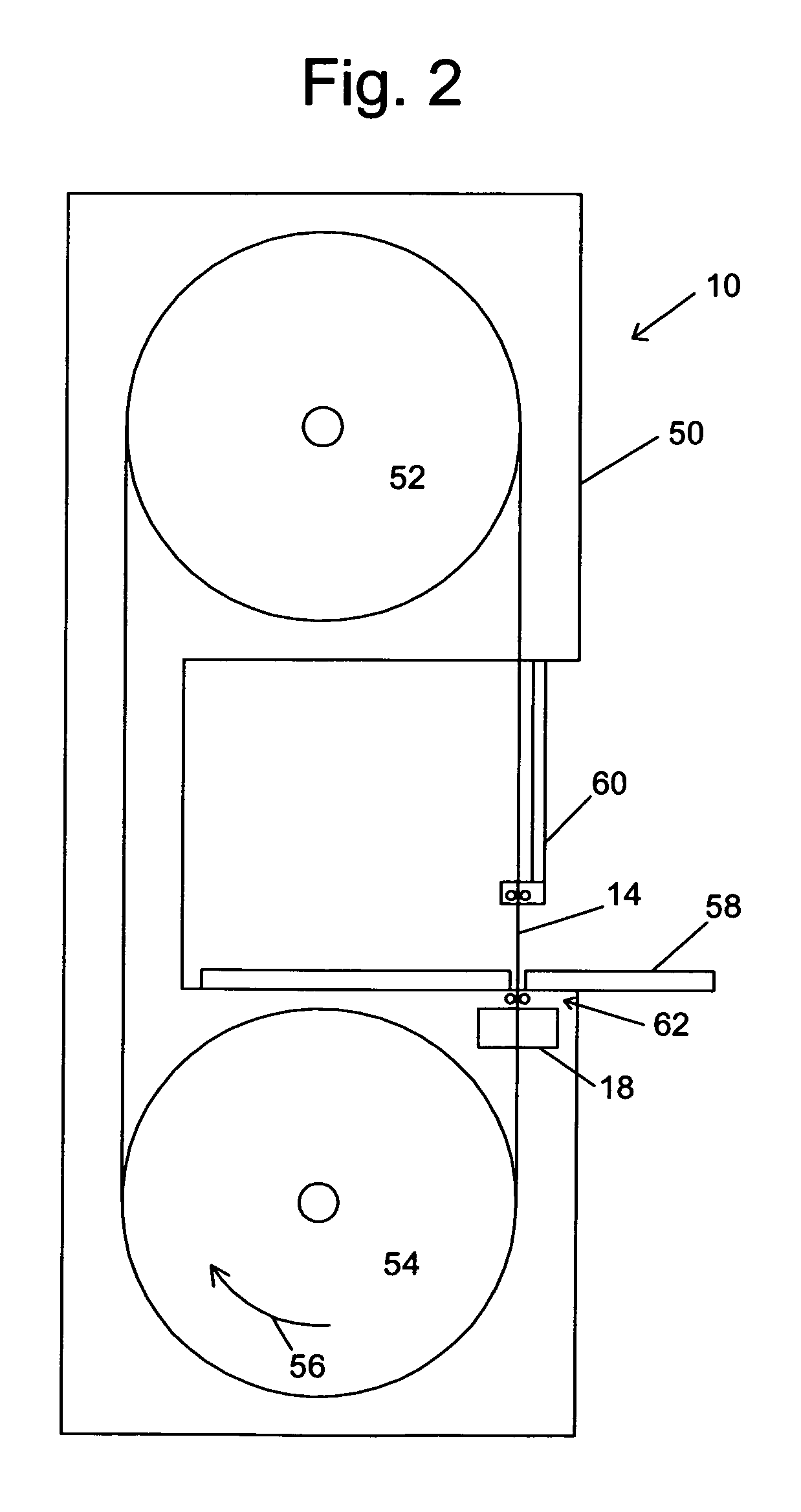
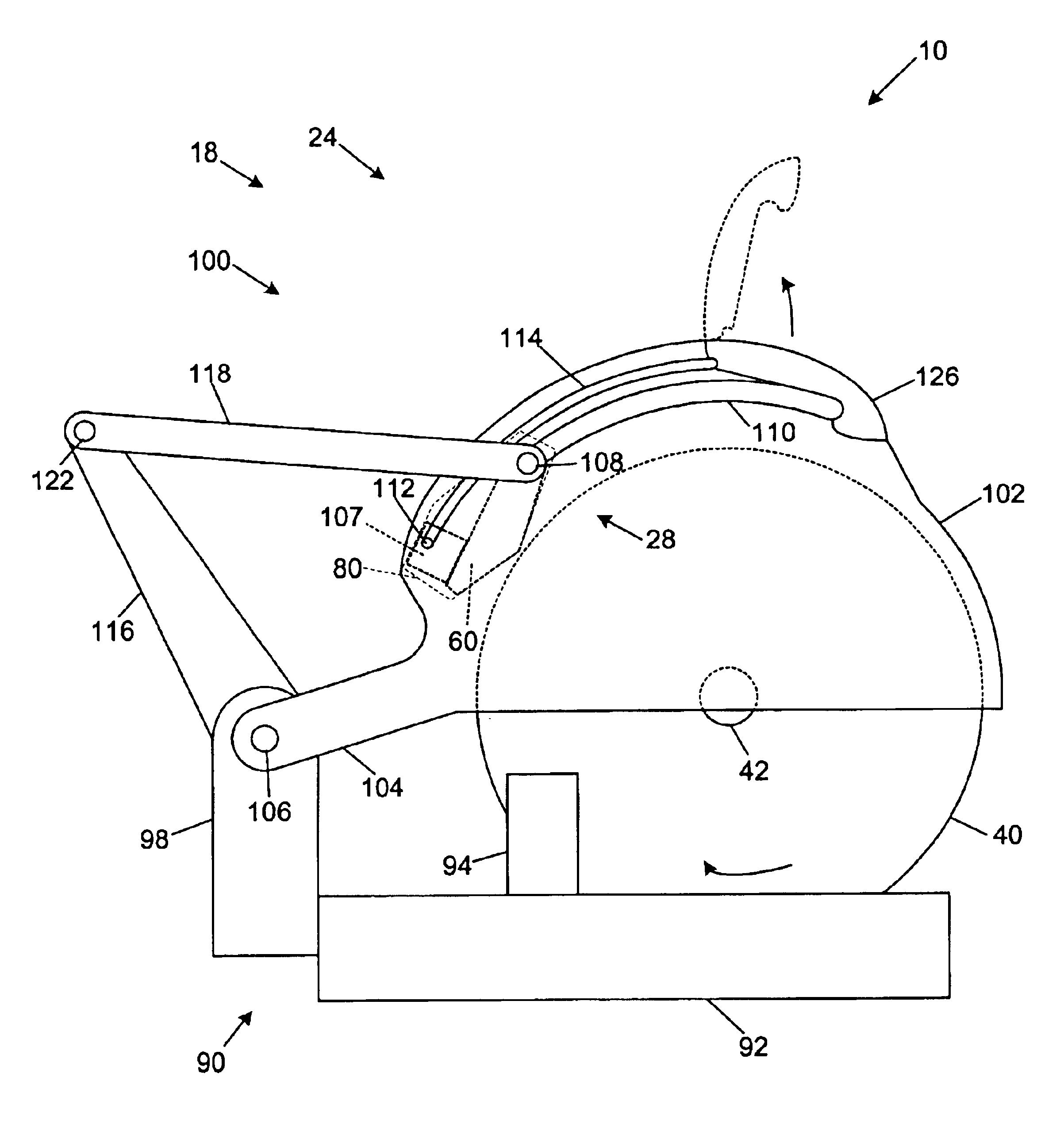
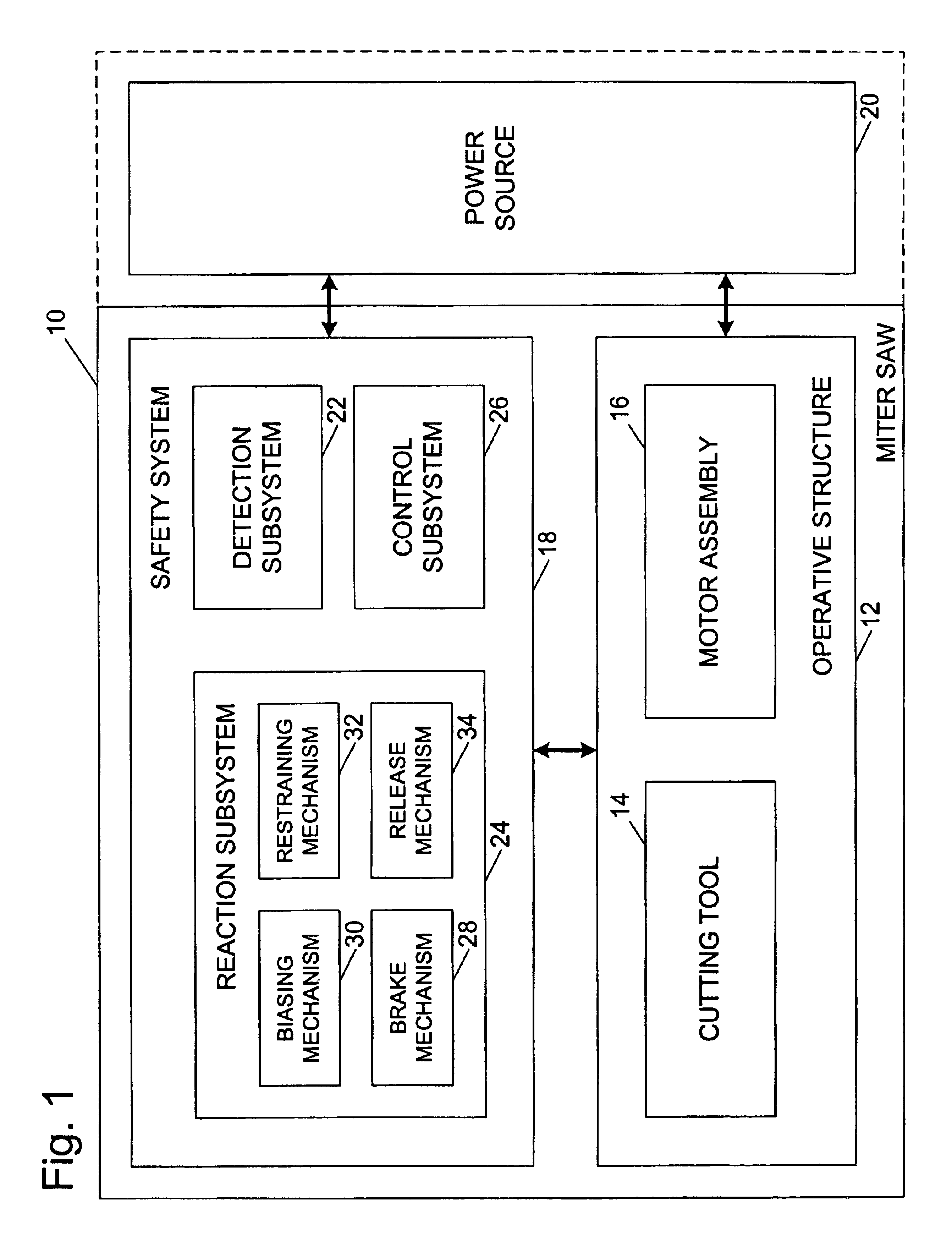
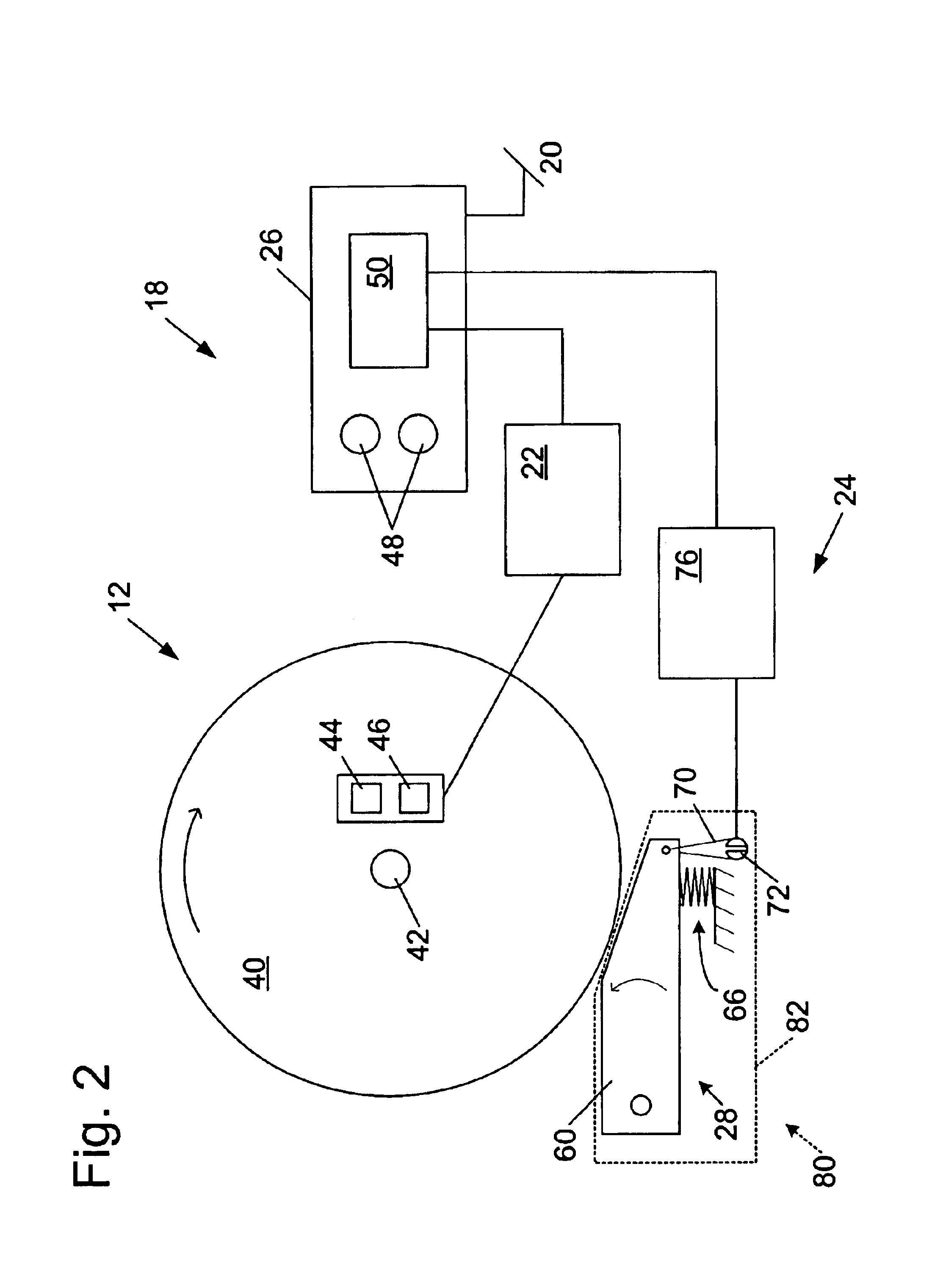
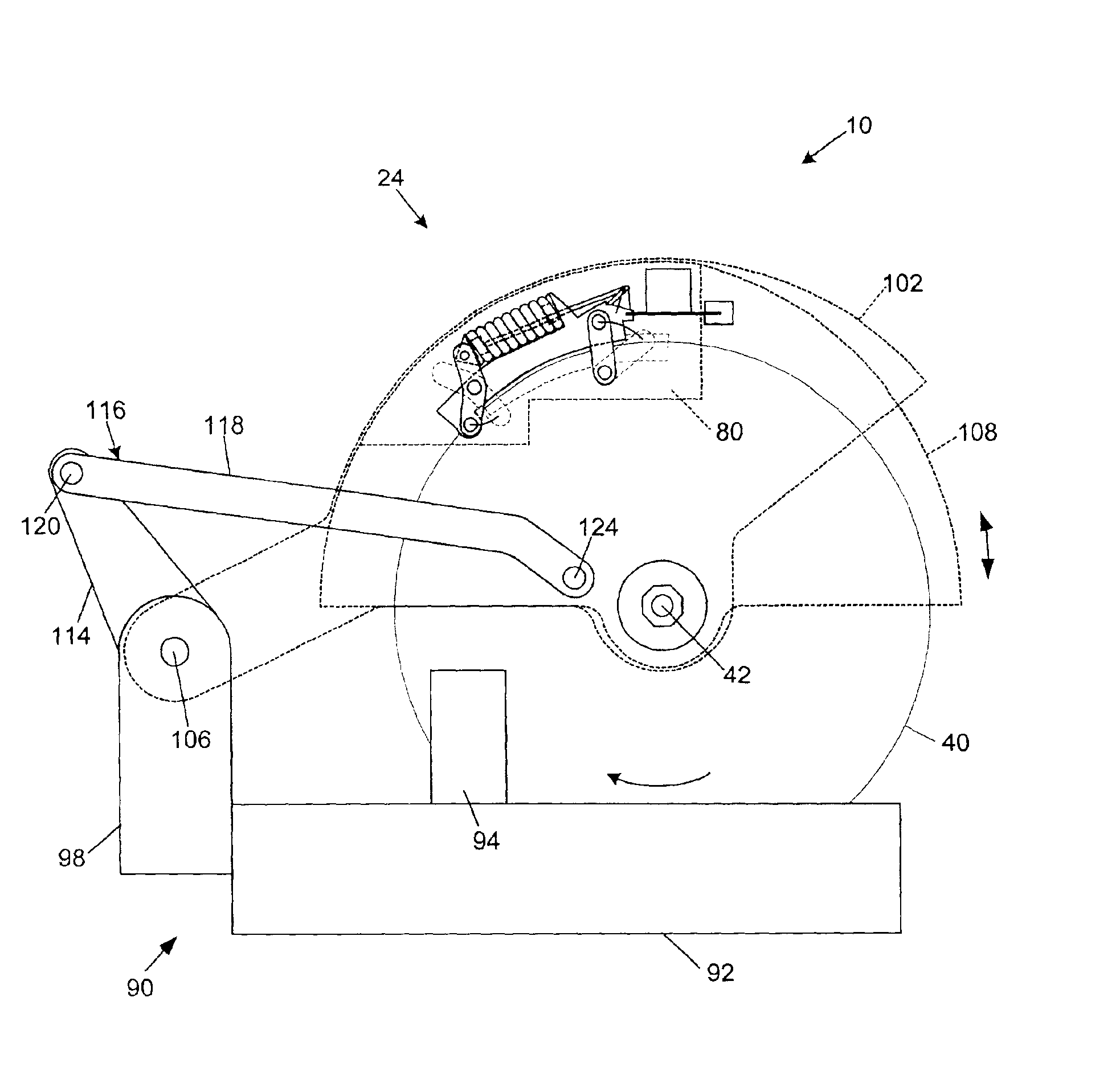
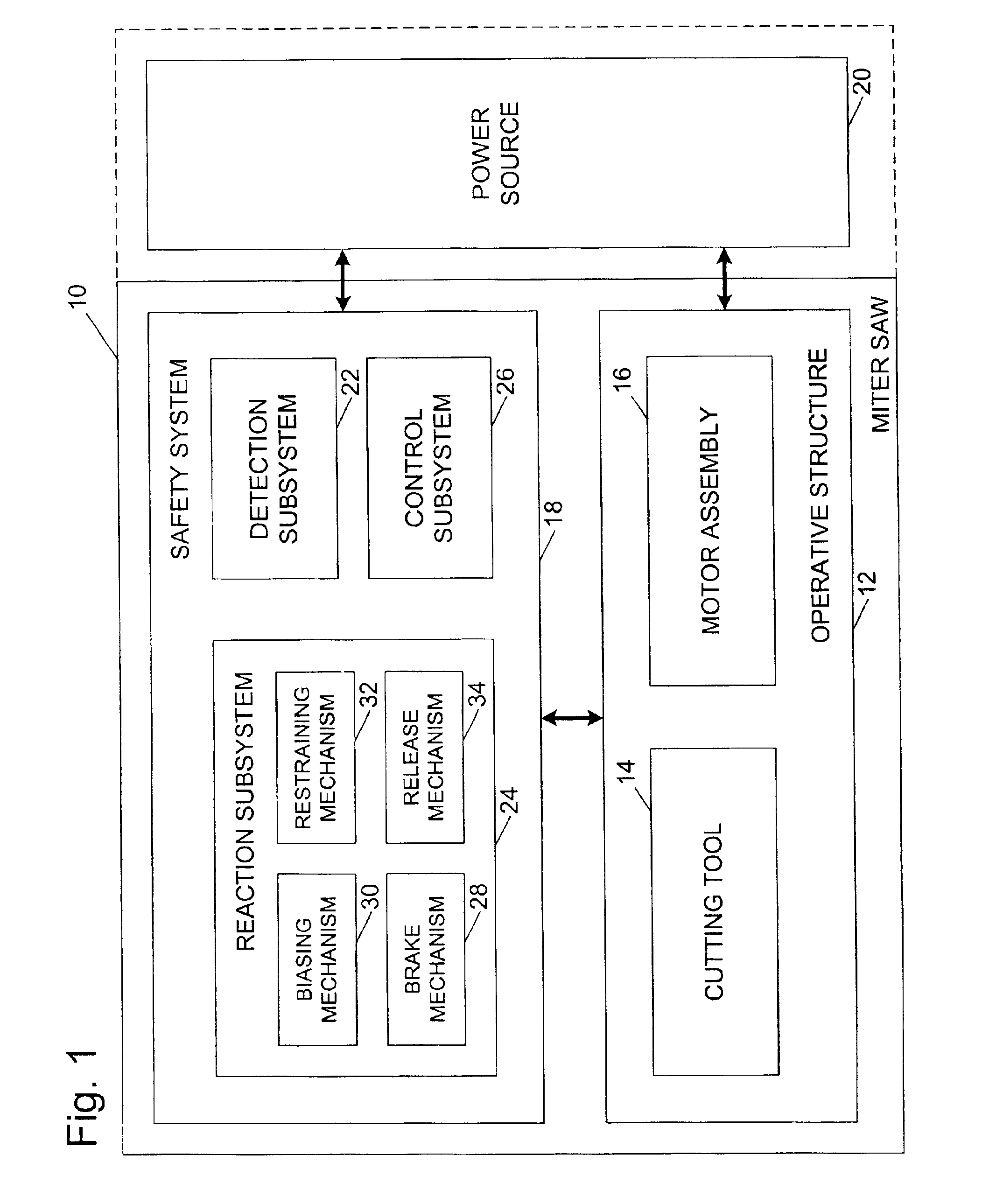
Miter saw with improved safety system
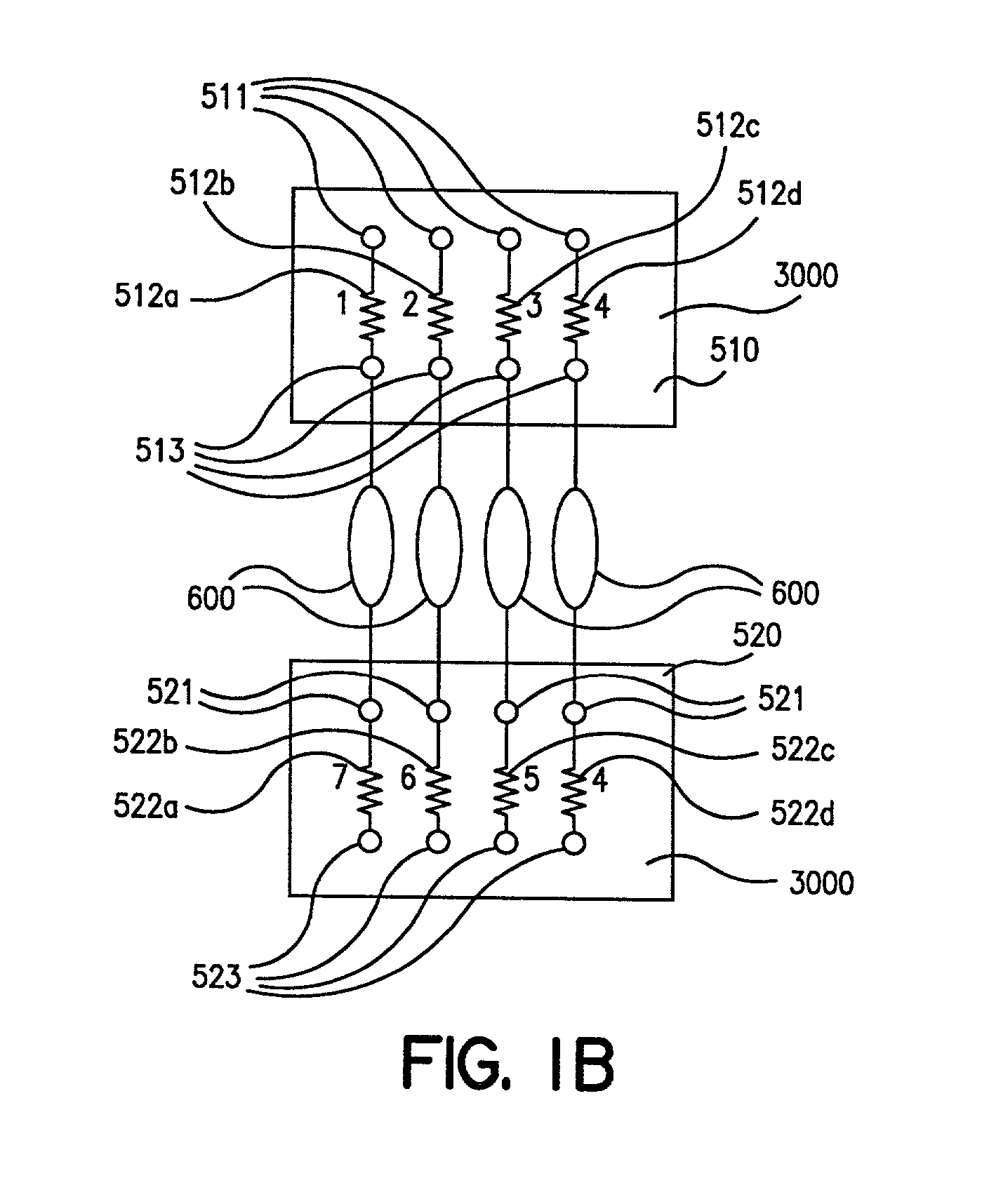
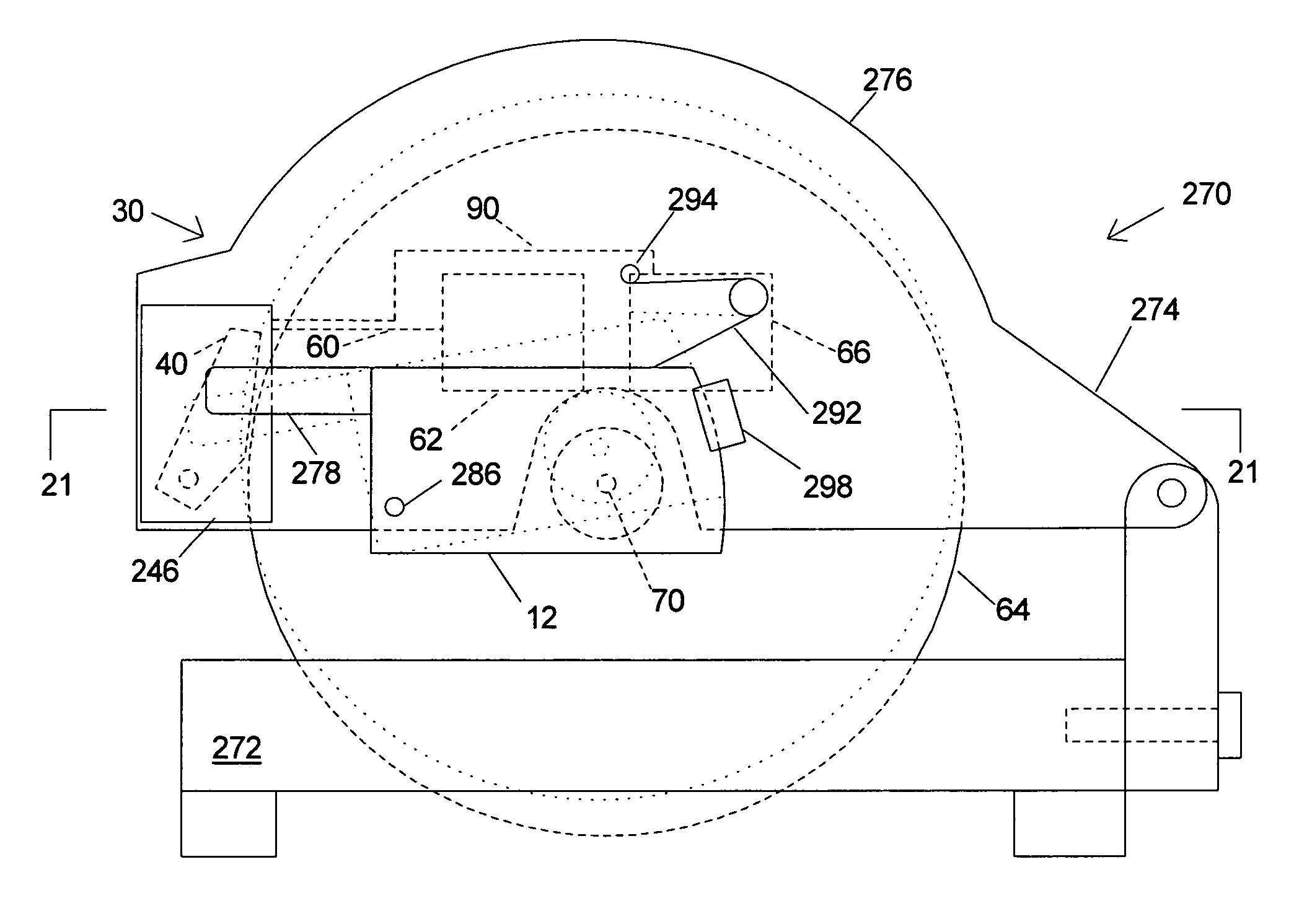
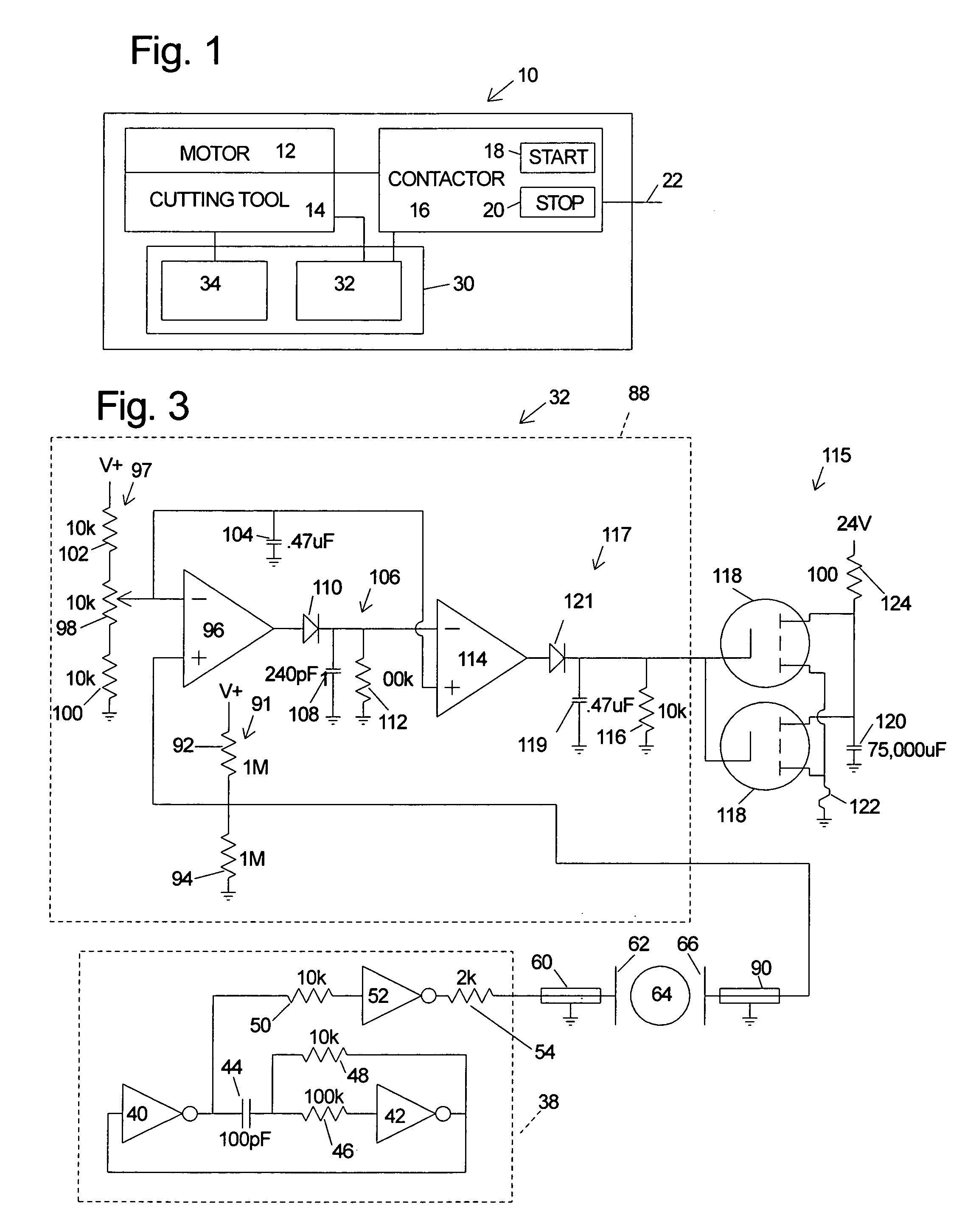
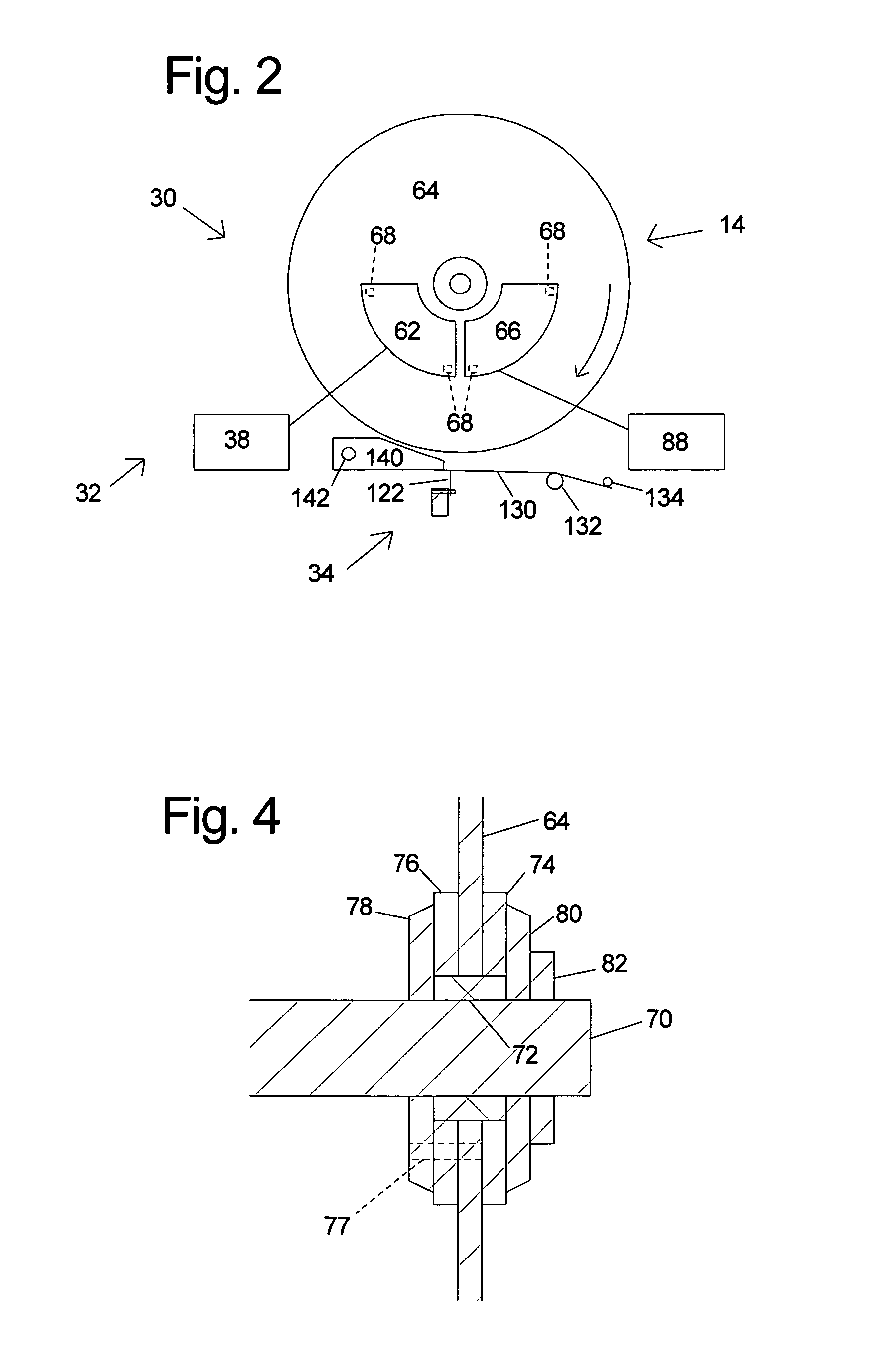
InactiveUS6826988B2Increase opportunitiesLimit and even prevent injuryEmergency protective circuit arrangementsEngineering safety devicesEngineeringWoodworking machine
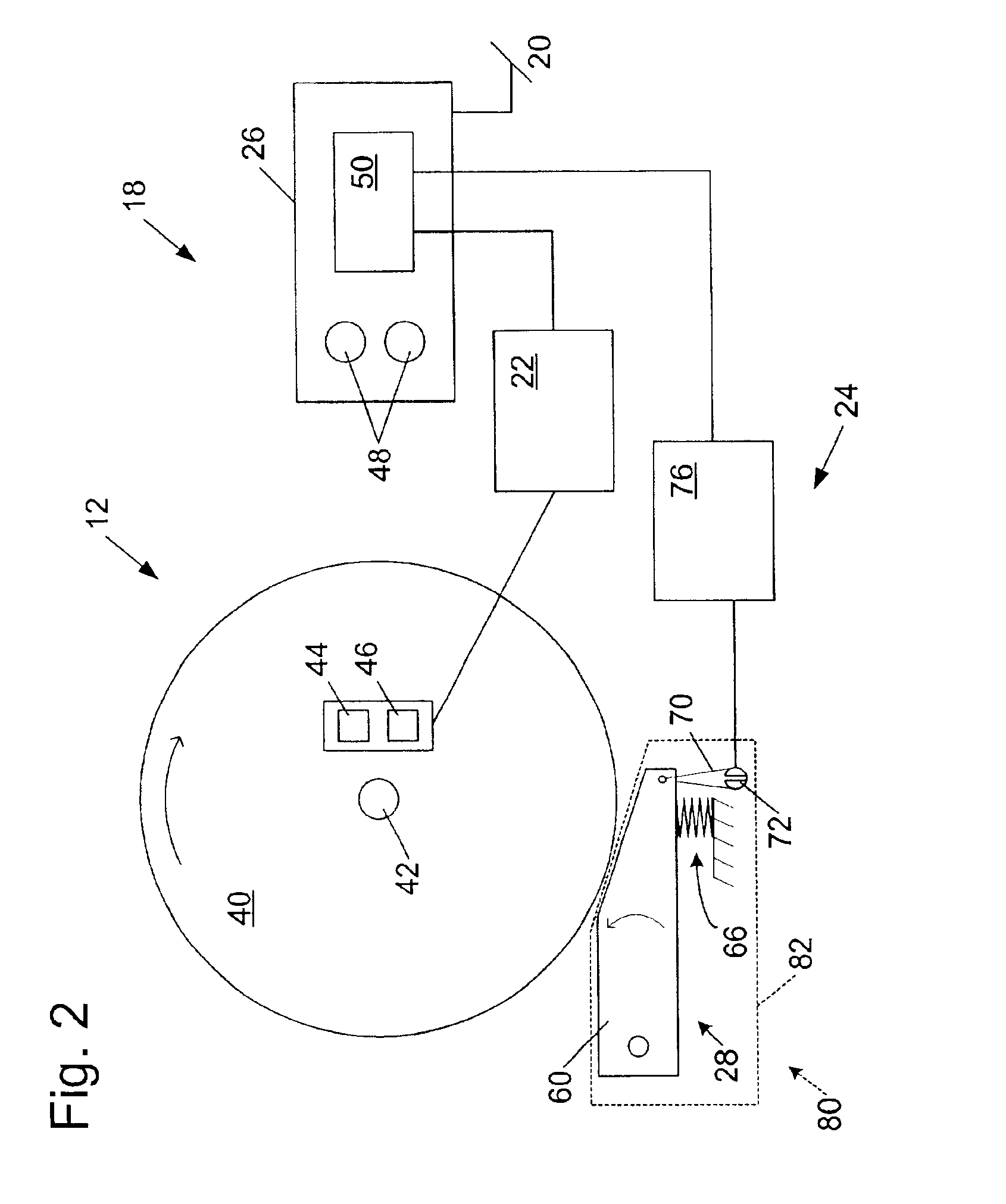
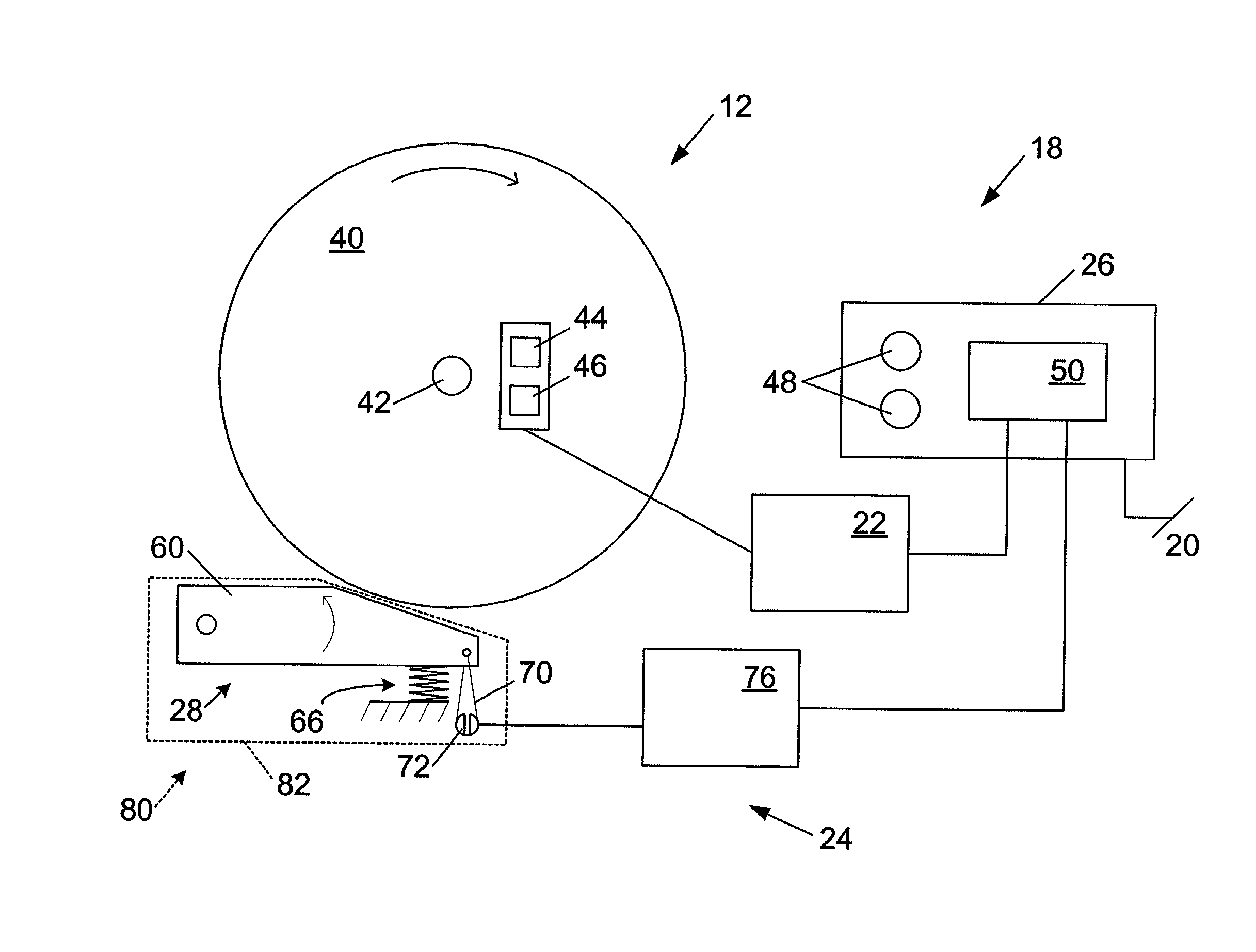
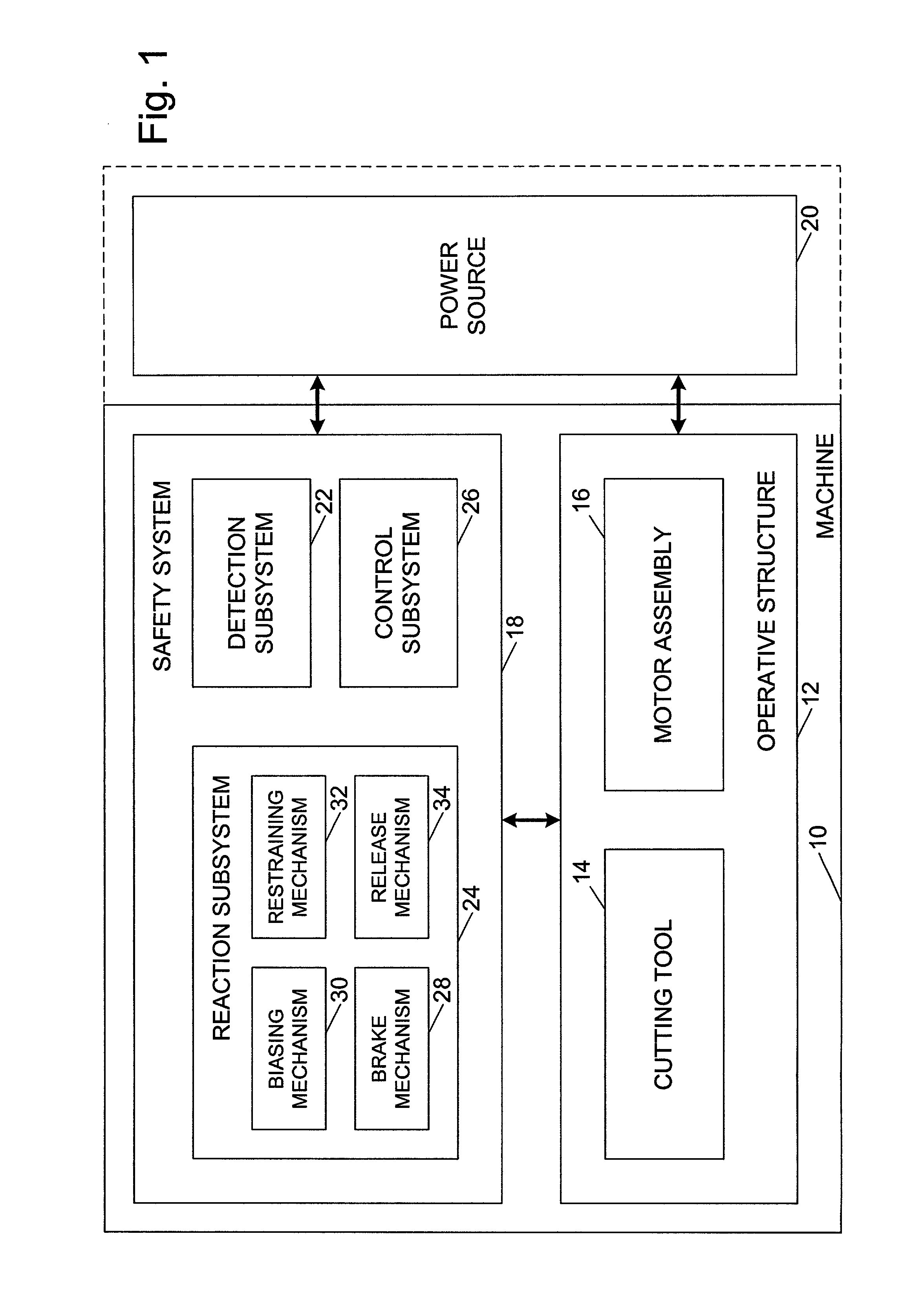
A woodworking machine is disclosed having a base, a blade, a detection system adapted to detect a dangerous condition between a person and the blade, and a reaction system associated with the detection system to cause a predetermined action to take place upon detection of the dangerous condition. The blade is rotatable, and moves into a cutting zone to cut a workpiece. The predetermined action may be to stop the blade from rotating and / or to stop movement of the blade toward the cutting zone.
Owner:SAWSTOP HLDG LLC
Miter saw with improved safety system
InactiveUS6880440B2Limit and even prevent injuryImprove the security systemEmergency protective circuit arrangementsEngineering safety devicesEngineeringMechanical engineering
A miter saw having a base and an arm that pivots toward the base is disclosed. A blade is supported by the arm, and is designed to cut workpieces resting on the base when the arm and blade pivot downward. The saw includes a detection system configured to detect one or more dangerous conditions between a person and the blade, such as when a person accidentally touches the spinning blade, and the saw includes a reaction system to stop the downward movement of the blade and arm when the dangerous condition is detected.
Owner:SAWSTOP HLDG LLC
Parallel flow reactor having variable composition
InactiveUS20020045265A1Extreme flexibilityAdvantageously and flexibly employedProcess control/regulationSequential/parallel process reactionsDistribution systemEngineering
Owner:FREESLATE
Safety system for power equipment
Safety systems for power equipment are disclosed. The safety systems include a detection system adapted to detect contact between a person and a working portion of a machine, where the detection system is adapted to capacitively impart an electric charge on the working portion and to detect when that charge drops; and a reaction system associated with the detection system to cause a predetermined action to take place relative to the working portion upon detection of contact between the person and the working portion by the detection system. Machines equipped with safety systems are also disclosed, such as saws, jointers, etc. The machines include a working portion, such as a cutter or blade, a detection system adapted to detect a dangerous condition between a person and the working portion, and a reaction system associated with the detection system to cause a predetermined action to take place upon detection of the dangerous condition, such as a brake system to stop the working portion, a retraction system to retract the working portion, or a system to cover the working portion. The machines may include a control system adapted to control the operability of one or more of the working portion, the detection system and the reaction system.
Owner:SAWSTOP HLDG LLC
Safety systems for band saws
Owner:SAWSTOP HLDG LLC
Miter saw with improved safety system
InactiveUS6945148B2Increase opportunitiesLimit and even prevent injuryOther plywood/veneer working apparatusMechanically actuated brakesEngineeringMechanical engineering
A miter saw is disclosed having a base, a blade supported by the base, a detection system adapted to detect a dangerous condition between a person and the blade, and a reaction system associated with the detection system to cause a predetermined action to take place upon detection of the dangerous condition. The blade is rotatable, and moves into a cutting zone to cut a workpiece. The predetermined action may be to stop the blade from rotating, to create an impulse against movement of the blade into the cutting zone, or to cause the blade to move away from the cutting zone.
Owner:SAWSTOP HLDG LLC
Miter saw with improved safety system
InactiveUS6877410B2Increase opportunitiesLimit and even prevent injuryOther plywood/veneer working apparatusMetal sawing devicesMechanical engineeringReaction system
A miter saw is disclosed having a base, a blade supported by the base, a detection system adapted to detect a dangerous condition between a person and the blade, and a reaction system associated with the detection system to cause a predetermined action to take place upon detection of the dangerous condition. The blade is rotatable, and moves into a cutting zone to cut a workpiece. The predetermined action may be to stop the blade from rotating, to create an impulse against movement of the blade into the cutting zone, or to cause the blade to move away from the cutting zone.
Owner:SAWSTOP HLDG LLC
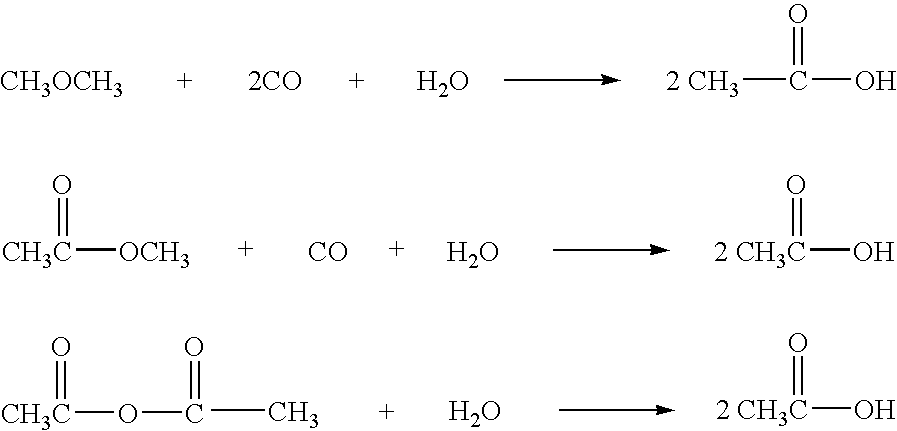
Method for preparing alcohol by acetic acid gas phase hydrogenation
ActiveCN102229520ALow reaction pressureReduce reaction energy consumptionOrganic compound preparationHydroxy compound preparationACETIC ACID LIQUIDGas phase
The invention relates to a method for preparing alcohol by acetic acid gas phase hydrogenation. A reaction system comprises acetic acid, hydrogen and a catalyst; the reaction temperature is 120-300 DEG C; the reaction pressure is 1.0-20.0MPa; the space velocity of acetic acid liquid is 0.5-10.5h<-1>; the molar ratio of H2 to acetic acid is 1-250; the catalyst takes activated carbon as a carrier; and main active components comprise one or two of transitional metals such as W and Mo. The addition agent is one of or more of precious metals such as Pd, Re, Pt, Rh and Ru and the like; and the acetic acid and the hydrogen can be converted into alcohol with high activity and selectivity under the action of the catalyst.
Owner:JIANGSU SOPO CHEM +1
Process for producing peptides by using in vitro transcription/translation system
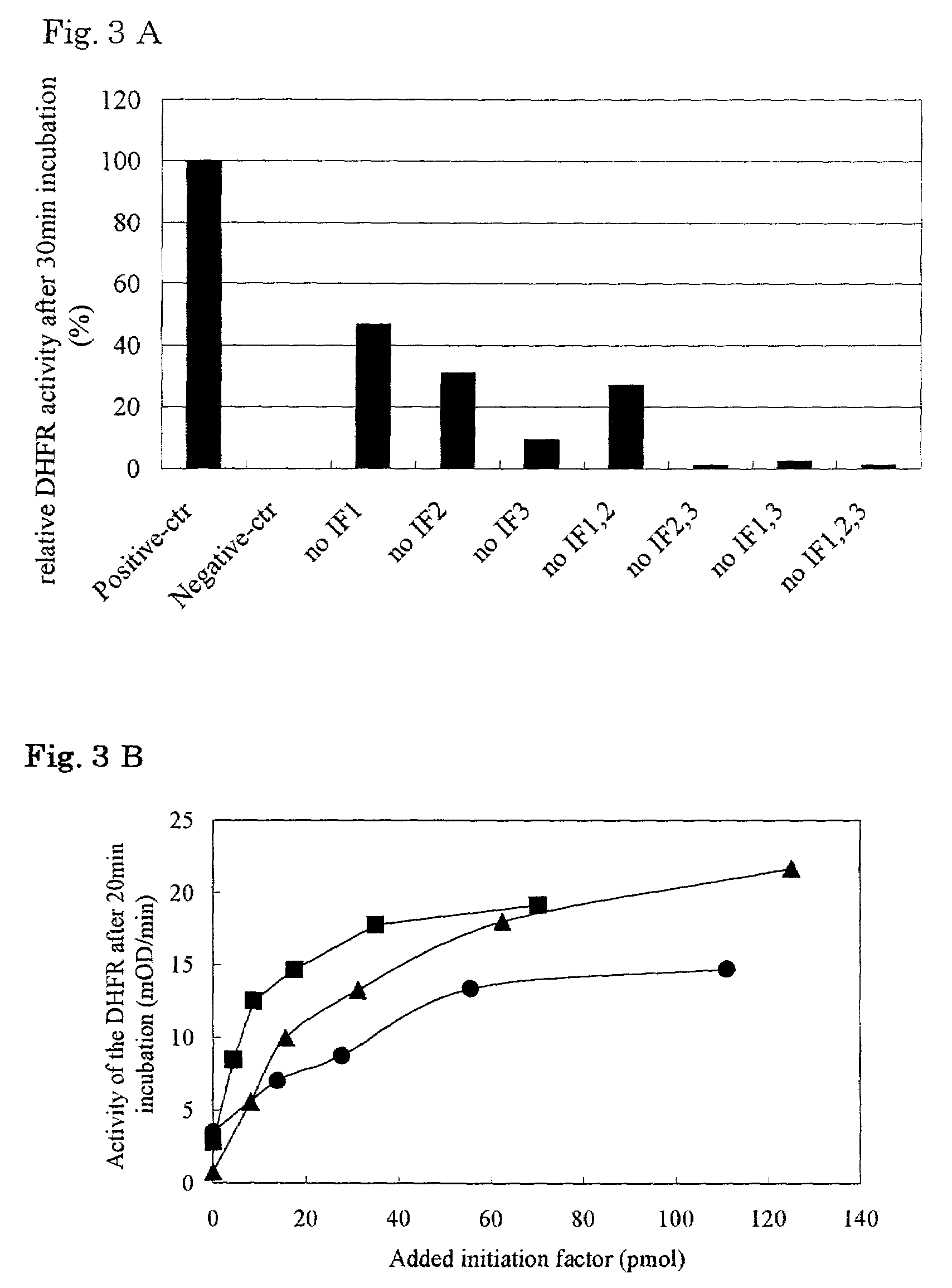
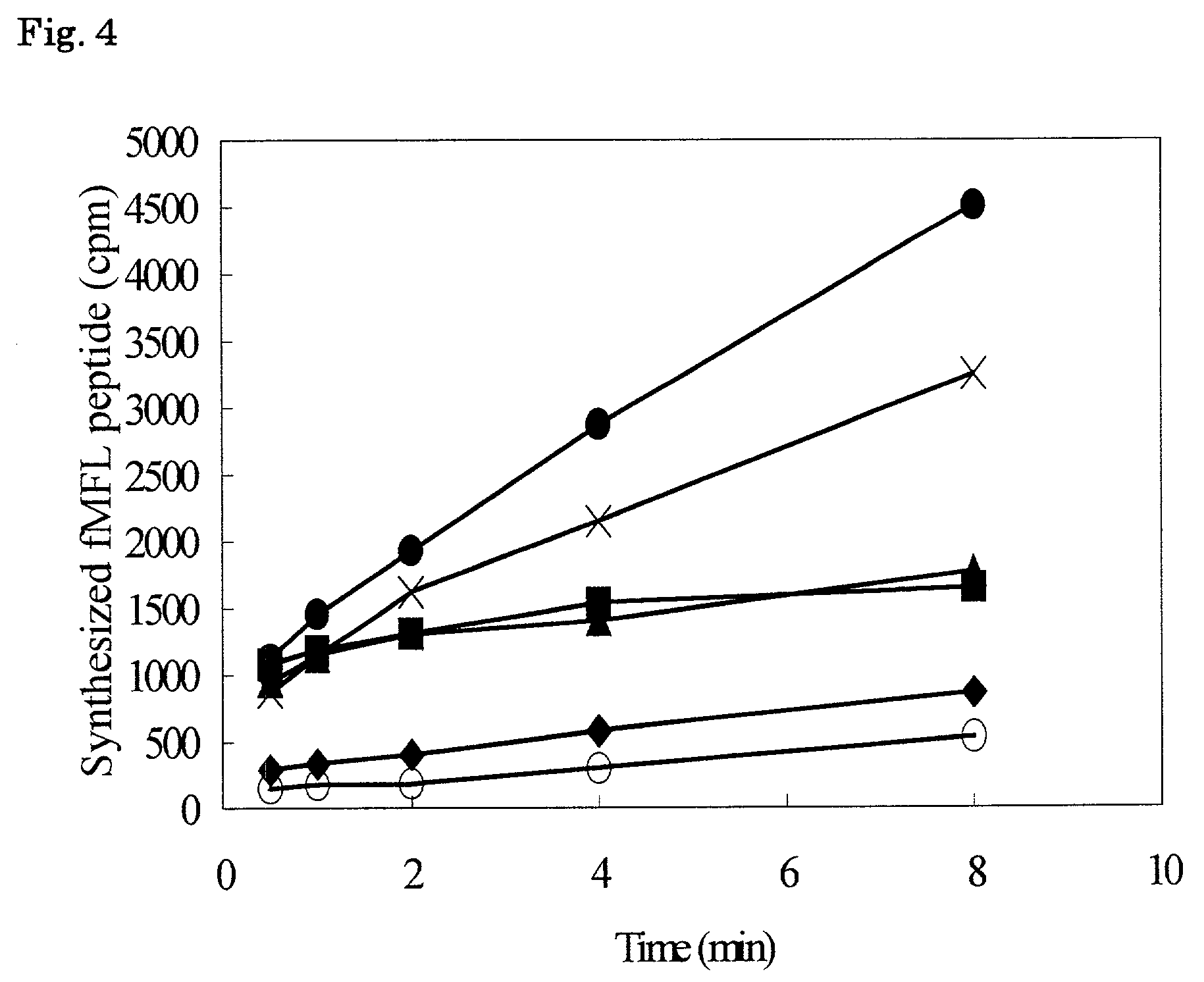
The present invention relates to a reaction system whereby a peptide produced in an in vitro peptide synthesis system can be efficiently isolated at a high purity from the reaction system. Thus the present invention is a process for producing a peptide or a peptide derivative by using a reaction system of transcribing a DNA into an RNA and then translating the RNA produced or a reaction system of translating an RNA in vitro wherein at least one protein component of the reaction system is labeled with a first substance which adheres to a second substance, and said second substance is used as an adsorbent for capturing said labeled protein components after translating.
Owner:BIOCOMBER
Detection system for power equipment
Woodworking machines including conductive cutters adapted to cut workpieces, and motors adapted to drive the cutters are disclosed. The machines also include a contact detection system adapted to detect contact between a person and the cutter, and to distinguish contact between the person and the cutter from contact between the workpiece and the cutter. The machines further include a reaction system adapted to cause a predetermined action to take place upon detection of contact between the person and the cutter by the contact detection system.
Owner:SAWSTOP HLDG LLC
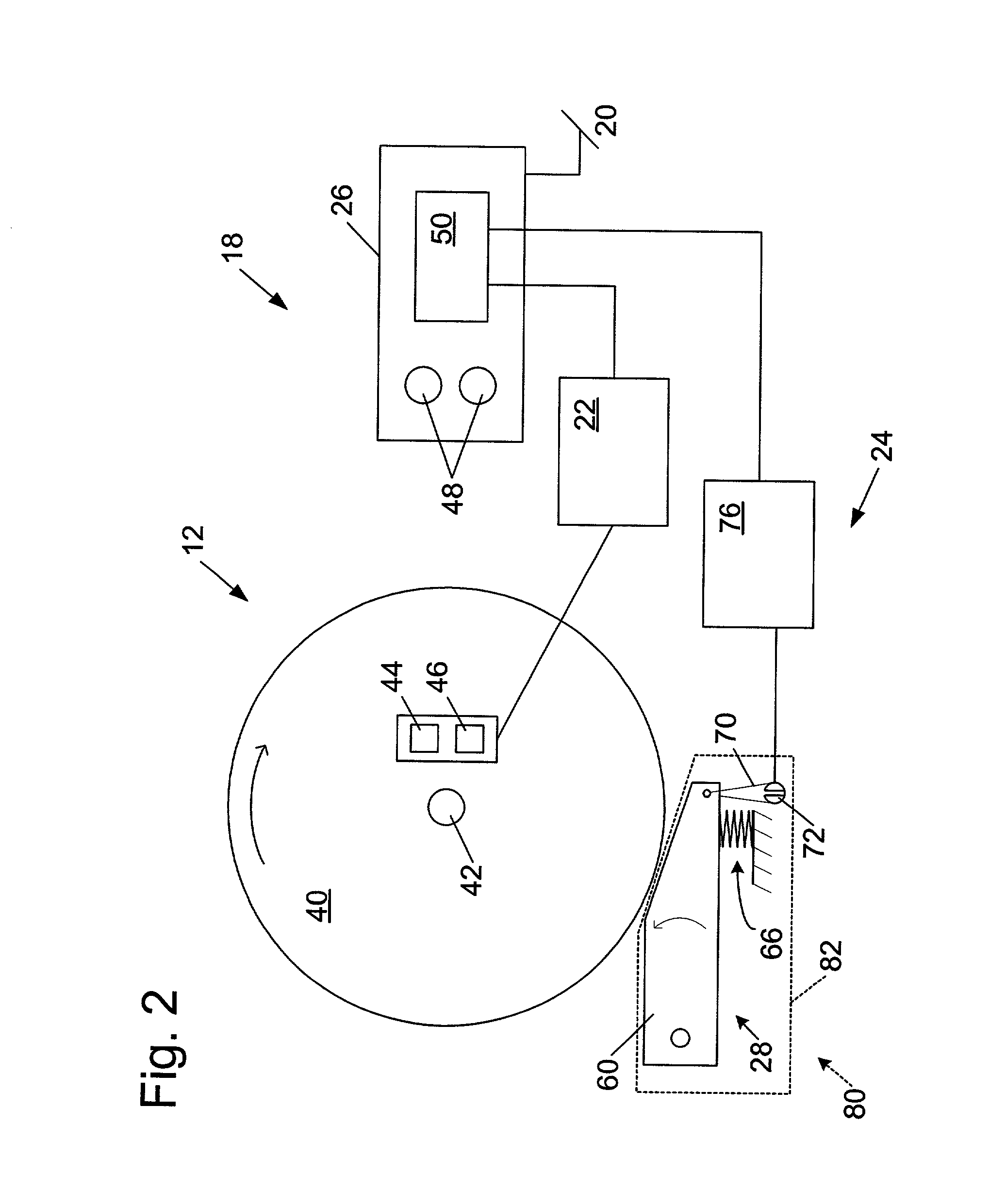
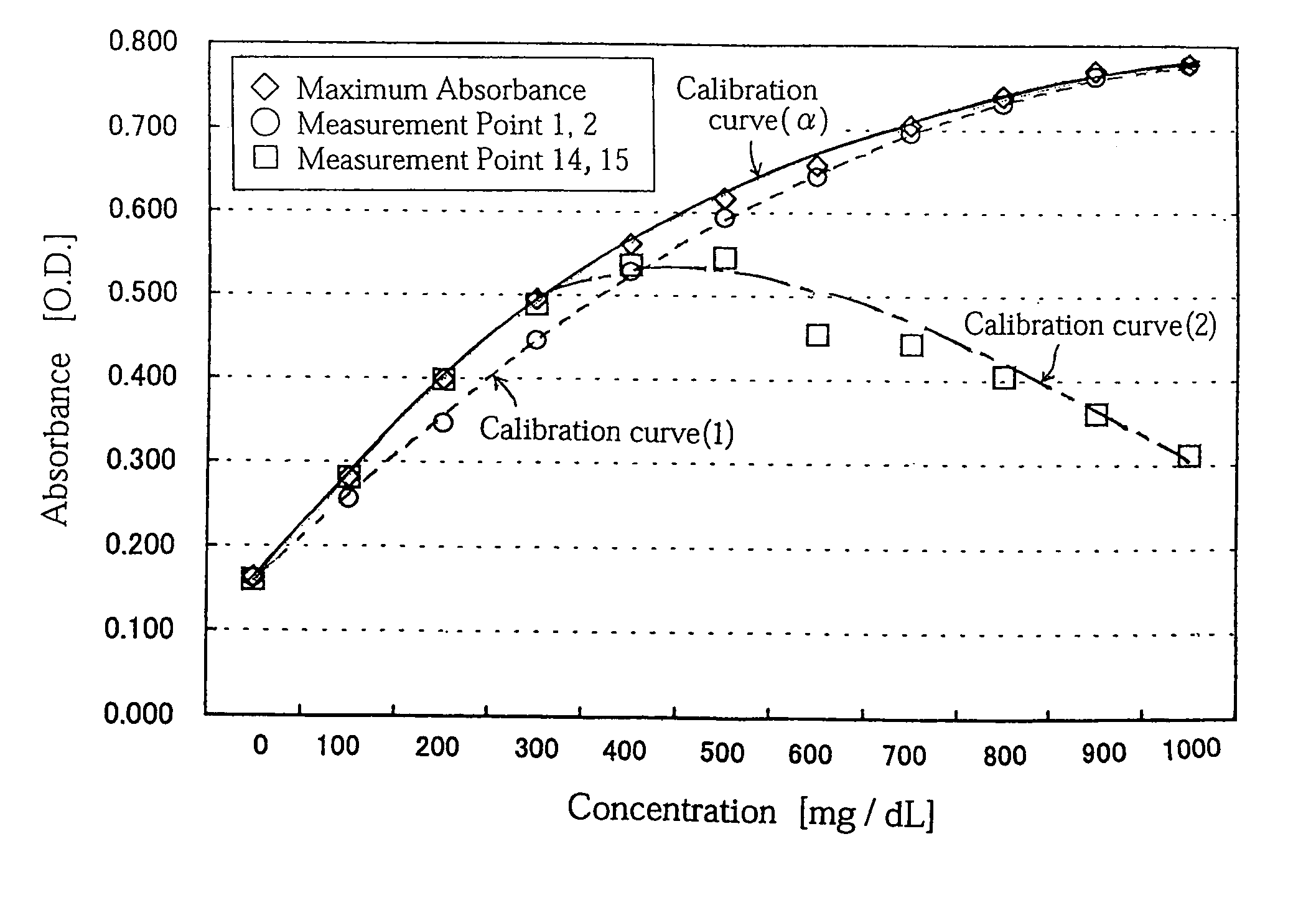
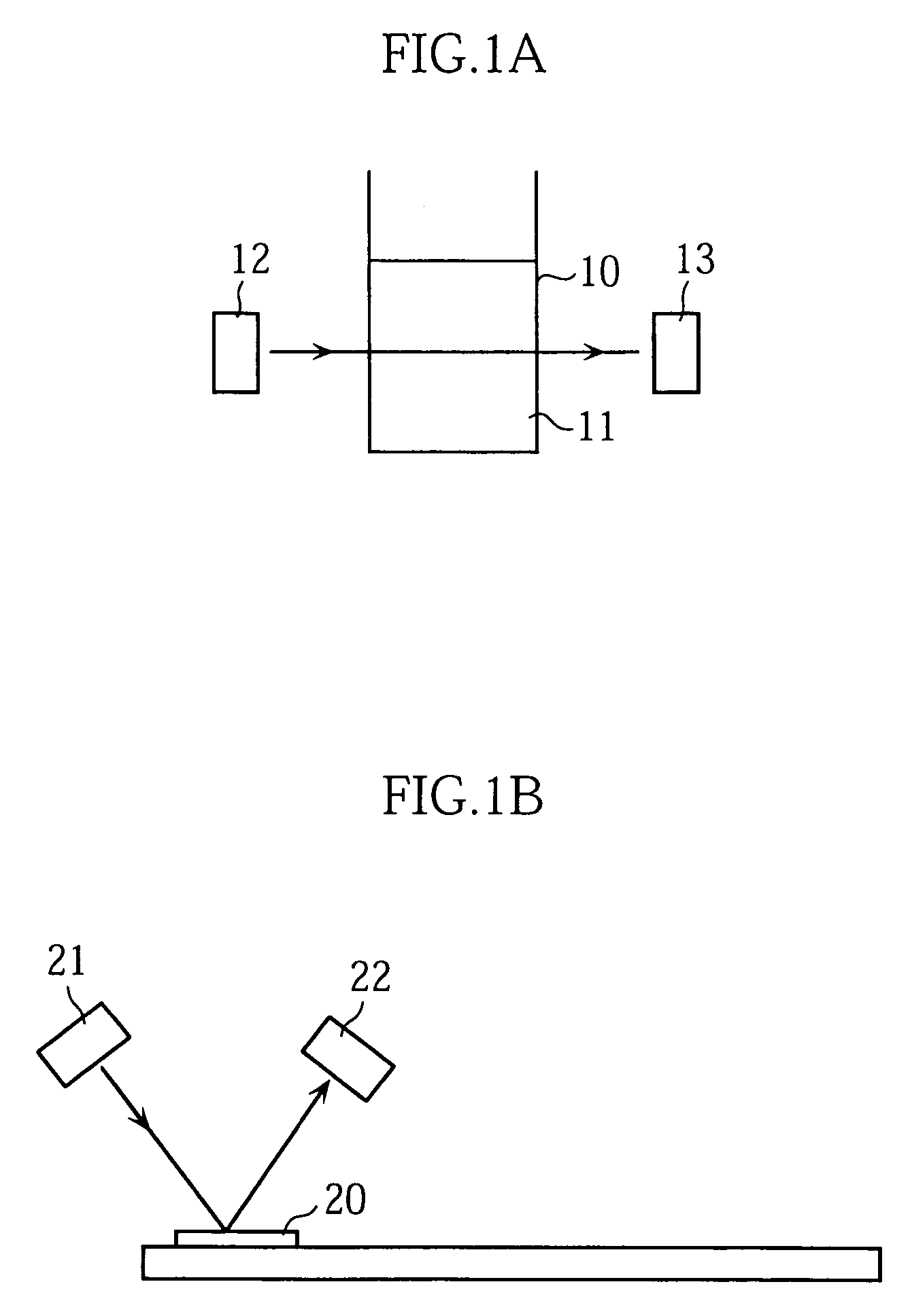
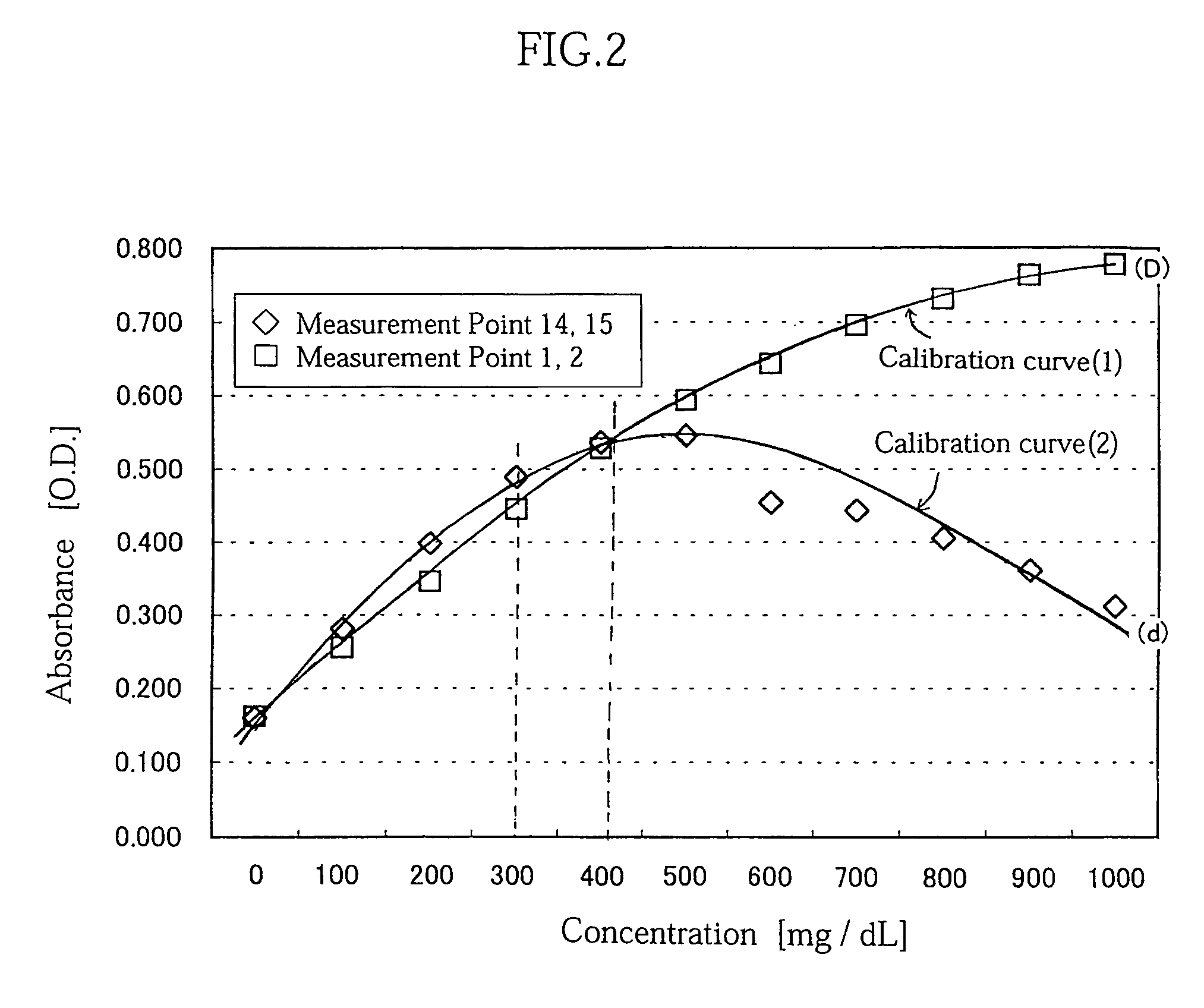
Concentration measuring method
InactiveUS7054759B2Easily and inexpensively prevent deteriorationReduce resolutionBioreactor/fermenter combinationsBiological substance pretreatmentsCalibration curveReaction system
A concentration measuring method includes selecting a calibration curve optimum for computing the concentration of a measurement target substance from a plurality of calibration curves based on an output from a reaction system containing the target substance and a reactant capable of reacting with the target substance, and computing the concentration of the target substance based on the optimum calibration curve and the output. Each of the calibration curves is prepared based on a plurality of outputs generated upon lapse of a same reaction time from a plurality of standard reaction systems each containing a standard reagent of a known different concentration and the reactant. The plurality of calibration curves differ from each other in reaction time based on which the calibration curves are prepared.
Owner:ARKRAY INC
Method for measuring substance and testing piece
InactiveUS7153696B2High measurement accuracyHigh measurement sensitivityAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorDiffusionChemical reaction
A method of measuring an analyte, comprising a step of measuring a detectable substance by using a reaction system including a formation reaction of the detectable substance based on a chemical reaction of the analyte contained in a sample, wherein a layered inorganic compound is caused to exist in the reaction system including the formation reaction of the detectable substance, whereby high-sensitivity measurement is made possible, the detectable substance can be stabilized to improve accuracy of the measurement, a rate of a chemical reaction is increased to enable quick measurement, and high-sensitivity measurement is made possible even in a reaction system which forms an insoluble substance. Also, it can be provided an analytical testing piece for measuring an analyte, by measuring a detectable substance by using a reaction system including a formation reaction of the detectable substance based on a chemical reaction of the analyte contained in a sample, wherein the testing piece comprises at least one test portion having a detection portion for detecting the detectable substance and contains a layered inorganic compound at least in the test portion, whereby diffusion and elution of a dyestuff or the like is prevented, more sensitive and accurate simple analysis is made possible, and easy handling is possible.
Owner:ARKRAY INC
Process for preparing alpha -hydroxy acids using microorganism and novel microorganism
PCT No. PCT / JP97 / 00578 Sec. 371 Date Aug. 11, 1998 Sec. 102(e) Date Aug. 11, 1998 PCT Filed Feb. 27, 1997 PCT Pub. No. WO97 / 32030 PCT Pub. Date Sep. 4, 1997A process for preparing alpha -hydroxy acids represented by the general formula (II): RCH(OH)COOH (wherein R represents a hydrogen atom, an optionally substituted C1-C6 alkyl group, an optionally substituted C2-C6 alkenyl group, an optionally substituted C1-C6 alkoxy group, an optionally substituted aryl group, an optionally substituted aryloxy group, or an optionally substituted heterocyclic group) by allowing a microorganism to act on alpha -hydroxy nitriles (I): RCH(OH)CN (wherein R is as defined above) to hydrolyze and convert the alpha -hydroxy nitrites to alpha -hydroxy acids (II), wherein the alpha -hydroxy acids (II) are produced and accumulated in an aqueous solvent by a microorganism having the concentration resistance to the alpha -hydroxy nitrites (I) and / or alpha -hydroxy acids (II) and durability preferably in the presence of a cyanide, and harvested. According to this process, the use of the microorganism having the concentration resistance to the alpha -hydroxy nitriles (I) and / or alpha -hydroxy acids (II) and durability high enough to permit the activity to persist for a long period of time enables alpha -hydroxy acids (II) to be accumulated in high concentrations and cell bodies to be repeatedly used, and hence enables alpha -hydroxy acids (II) to be efficiently prepared. The addition of a cyanide to the reaction system results in more efficient preparation of alpha -hydroxy acids (II).
Owner:NIPPON SODA CO LTD
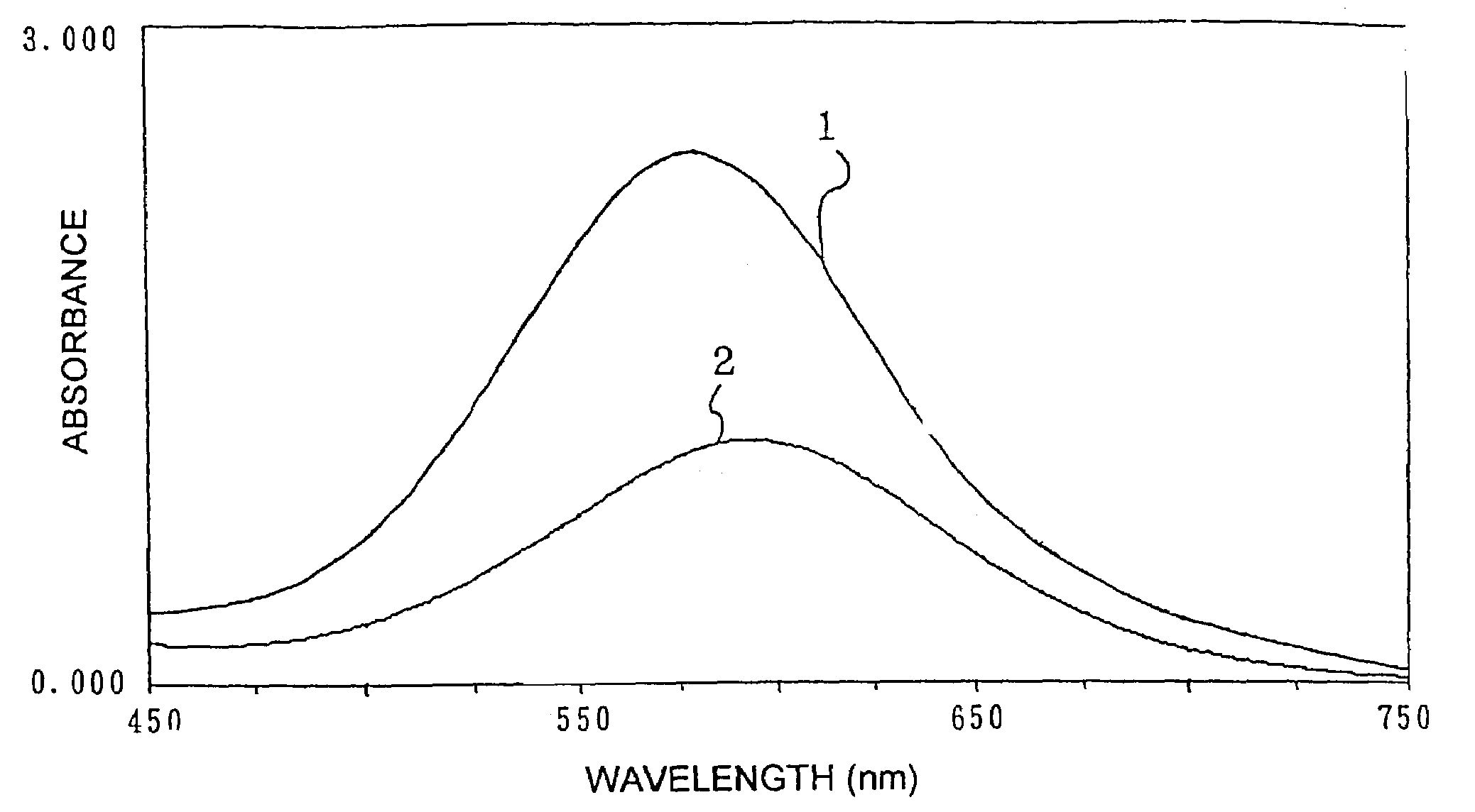
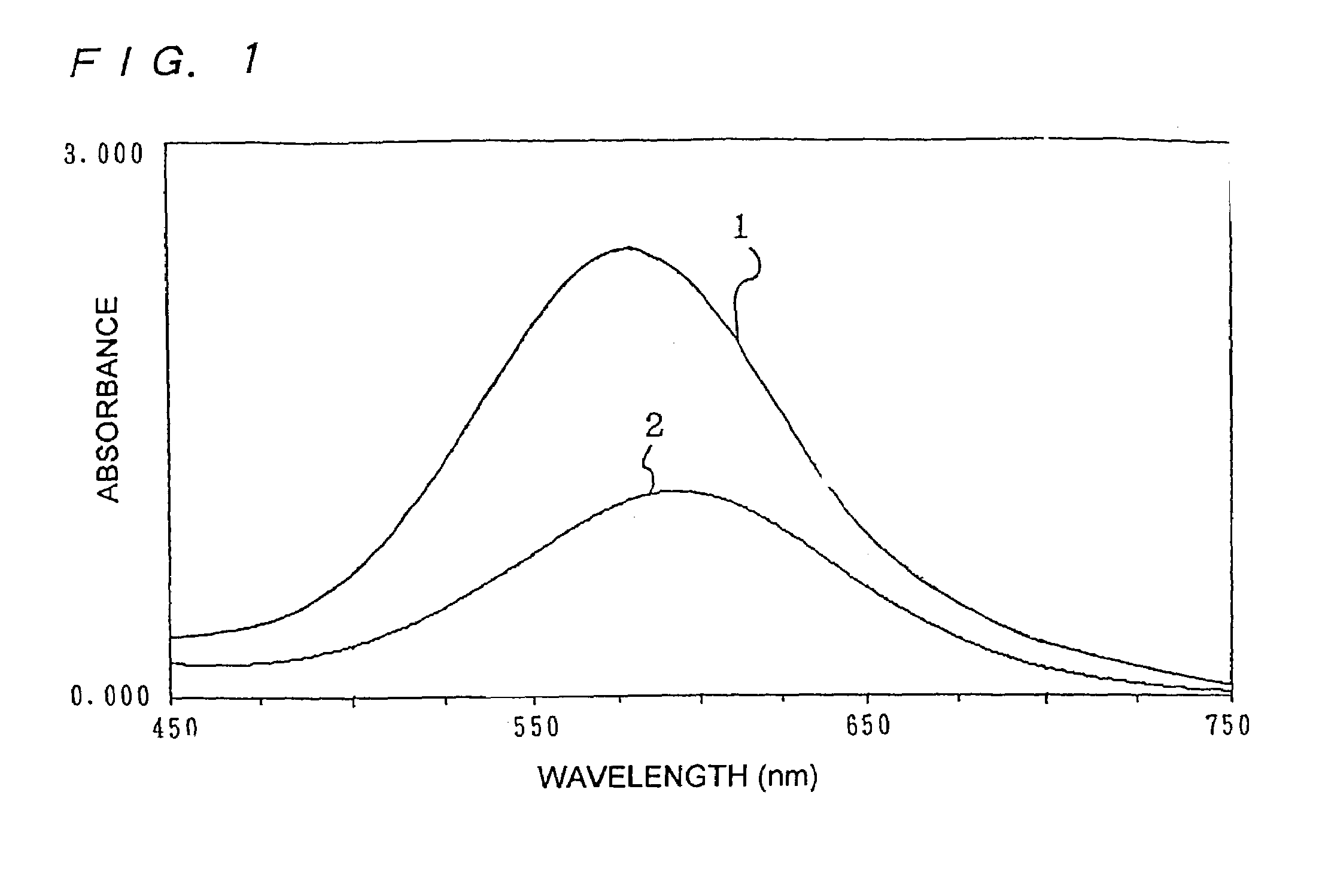
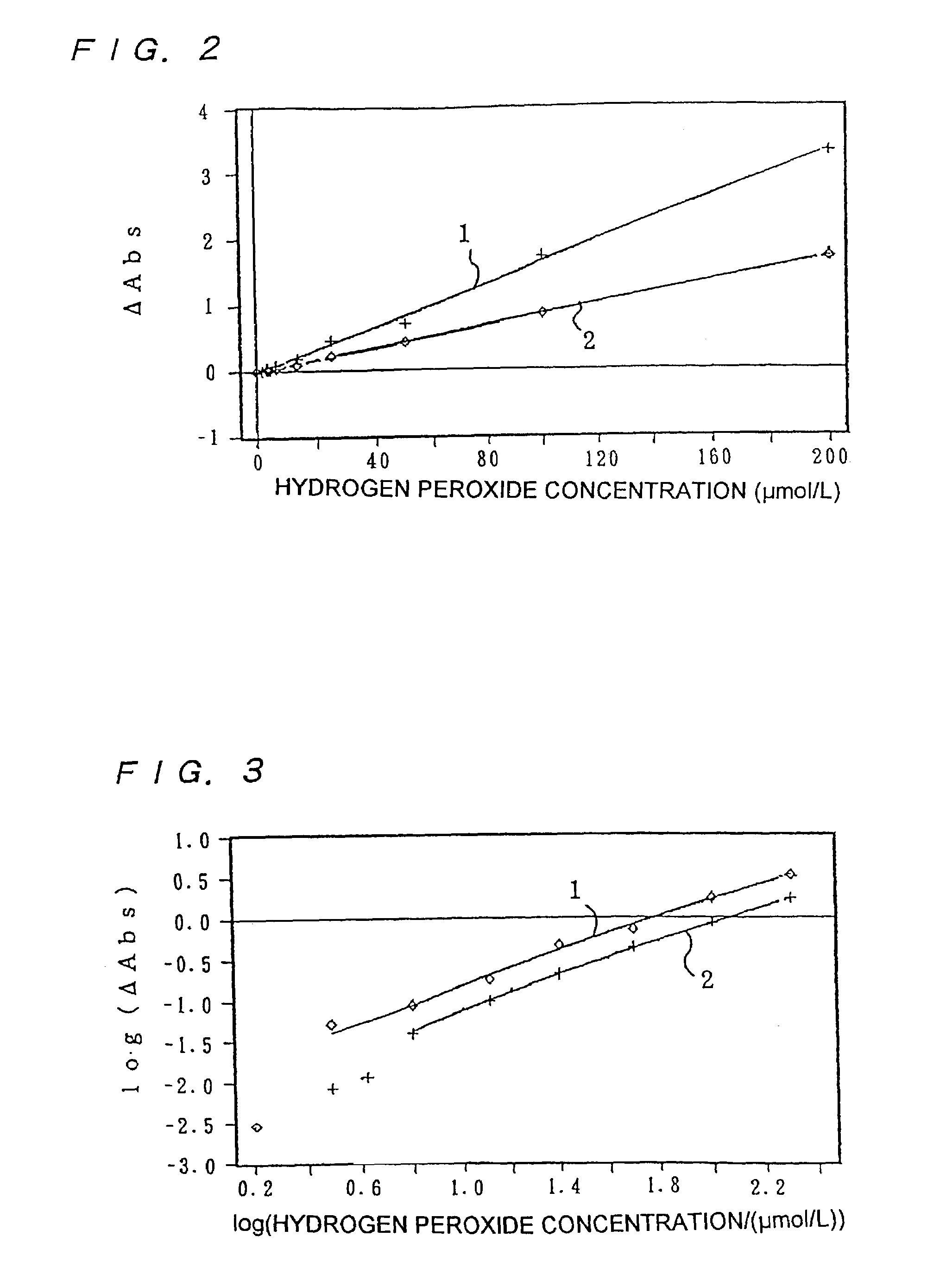
Method for producing benzoxazine resin
InactiveUS7041772B2Improve efficiencyPromote safe productionNatural resin processChemical/physical/physico-chemical stationary reactorsOrganic solventReaction system
The present invention discloses a process for producing a benzoxazine resin which comprises the steps of reacting a phenol compound, an aldehyde compound and a primary amine in the presence of an organic solvent to synthesize a benzoxazine resin and removing generated condensation water and the organic solvent from a system under heating and a reduced pressure, wherein a pressure in the reaction system at the time of removal is set to 260 mmHg or higher.
Owner:HITACHI CHEM CO LTD
Integrating a methanol to olefin reaction system with a steam cracking system
InactiveUS20050038304A1Shorten the counting processReduce the amount requiredThermal non-catalytic crackingMolecular sieve catalystOxygenateFractionating column
The present invention provides an integrated system for producing ethylene and propylene from an oxygenate to olefin (OTO) reaction system and a steam cracking system. In a preferred embodiment, at least a portion of an effluent stream from a steam cracking furnace is combined with at least a portion of an effluent stream from an OTO reaction system. Preferably the combined effluent stream is processed by one or more quench units, compression units, and / or fractionation columns. By integrating a steam cracking system with an OTO reaction system, equipment count can be reduced at a significant commercial savings. Compressor efficiency per pound of ethylene and propylene can also be advantageously increased over conventional steam cracking systems. Moreover, the amount of pollutants produced per pound of ethylene and propylene produced can be significantly reduced over the amount of pollutants produced per pound of ethylene and propylene produced in a steam cracking system.
Owner:EXXONMOBIL CHEM PAT INC
Compositions and methods for use in analytical reactions
ActiveUS8252911B2Rate of reactionSugar derivativesMicrobiological testing/measurementSequence analysisPolyphosphate
Compositions, methods, substrates and systems for use in analysis of single molecule reactions and particularly single molecule nucleic acid sequence analysis. Compositions that include non-reactive, distinguishable or undetectable competitive substrates for the reaction system of interest are provided, as well as their use in systems and substrates for such applications, such compounds typically preferably polyphosphate chains or analogous structures.
Owner:PACIFIC BIOSCIENCES
Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
InactiveCN101347736AFast deposition rateIncrease concentrationMetal/metal-oxides/metal-hydroxide catalystsMetal hydridesChemical platingRare earth
The invention relates to hydrogen production and hydrogen storage technologies and materials, in particular to a catalyst for catalytic hydrolysis of borane for the hydrogen production and a preparation method thereof, thereby solving the problems that the direct application of powder catalyst in a catalytic hydrolysis solid-liquid reaction system can cause the loss of the catalyst, the catalytic hydrolysis reaction is difficult to control and the hydrolysis by-products are difficult to be recovered, etc. The catalyst is composed of an active component and a carrier; the active component is a binary, ternary or multinary alloy or a single precious metal or the combination thereof which is composed of one or more transition metals, rare earth metals or precious metals and metalloids; the active component is deposited on the carrier through the improved chemical plating technology, the surface thereof is rough and porous, and the structure of the prepared catalyst is the amorphous or the nanocrystalline structure. The preparation method has simple preparation process, high preparation efficiency and convenient large-scale preparation; the sources of the used raw materials are rich; the catalytic activity of the prepared supported catalyst is high, the real-time control of the catalytic hydrolysis reaction of the borane can be realized, the catalytic performance is stable, and the catalyst can be repeatedly used for a plurality of times.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com