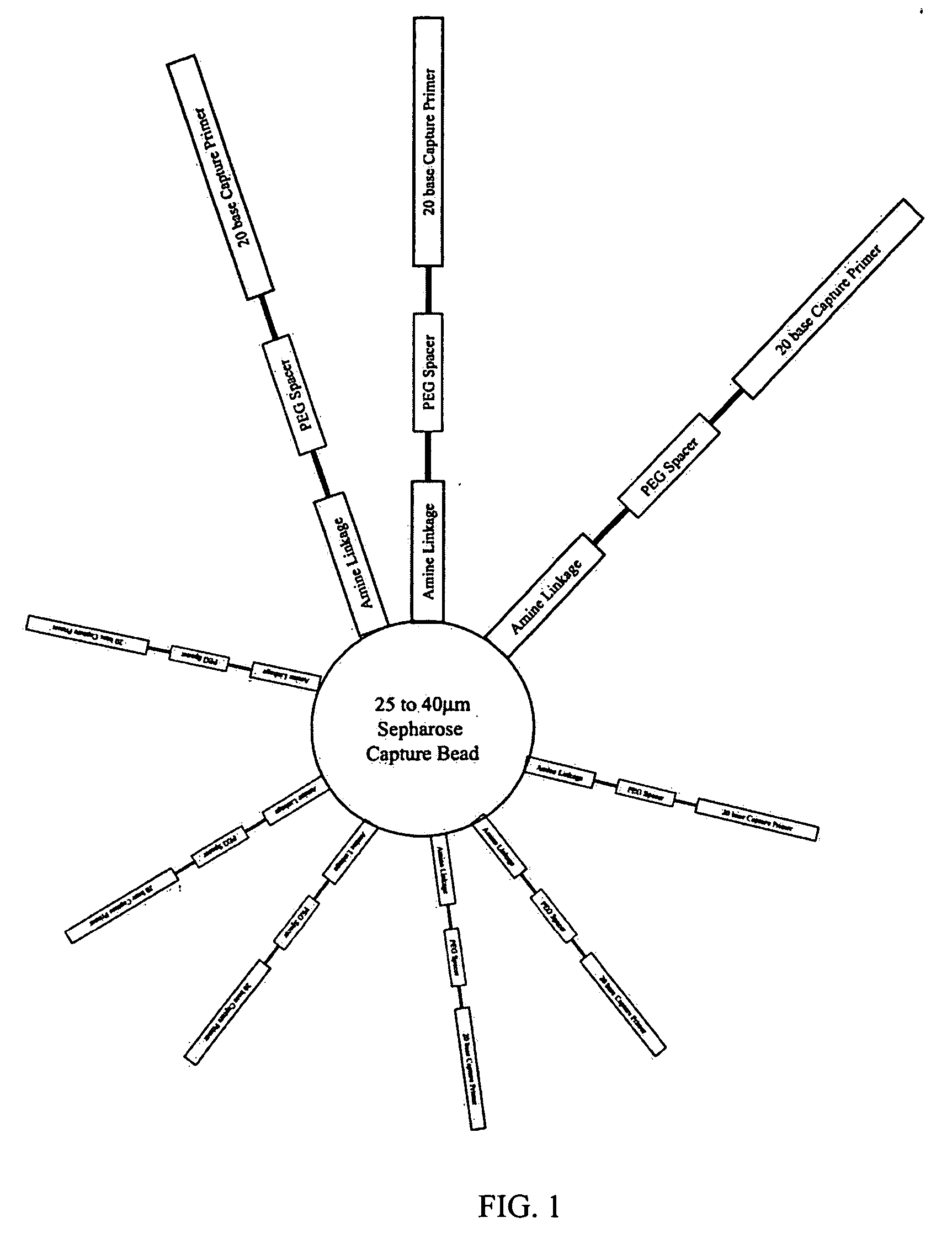
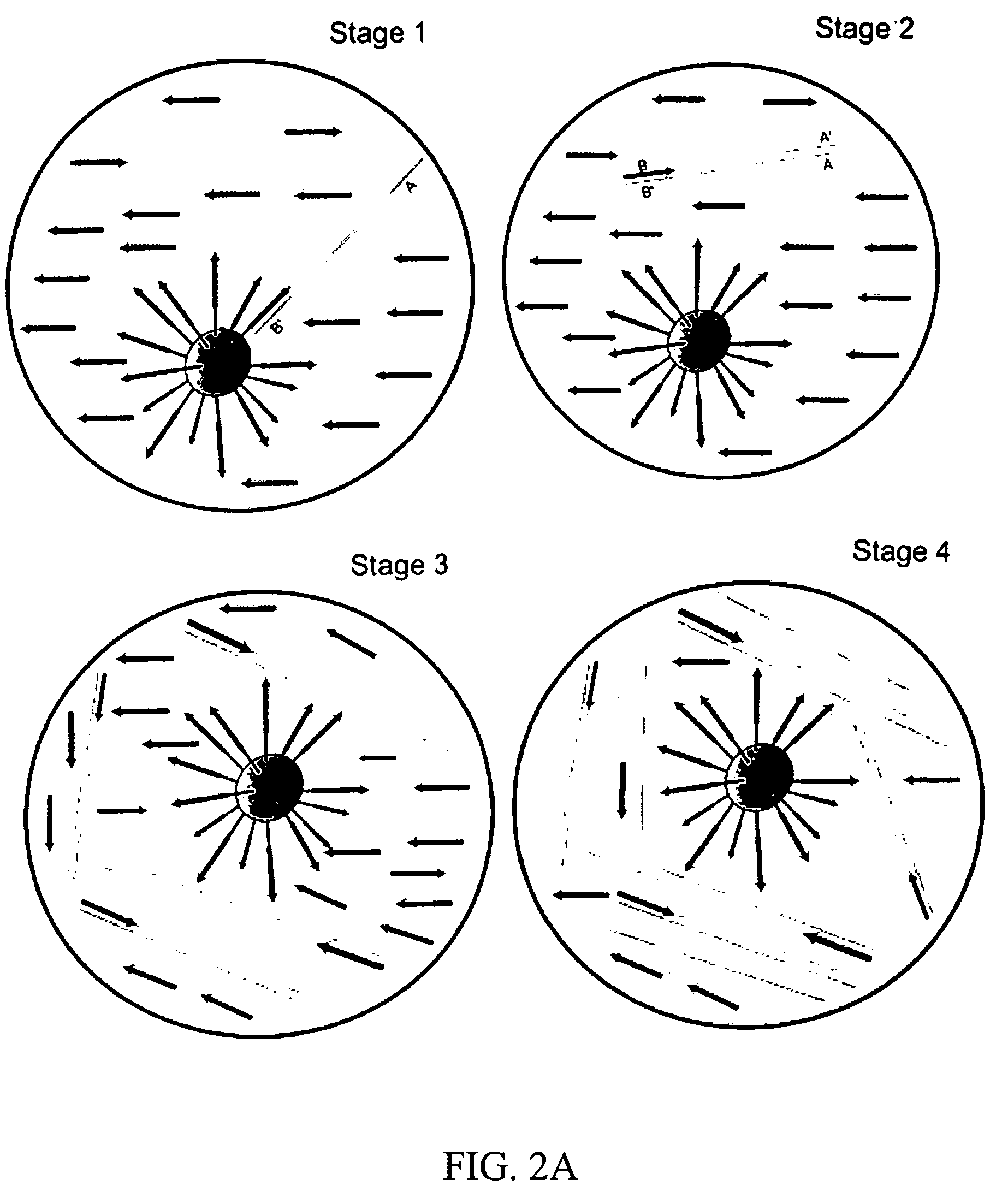
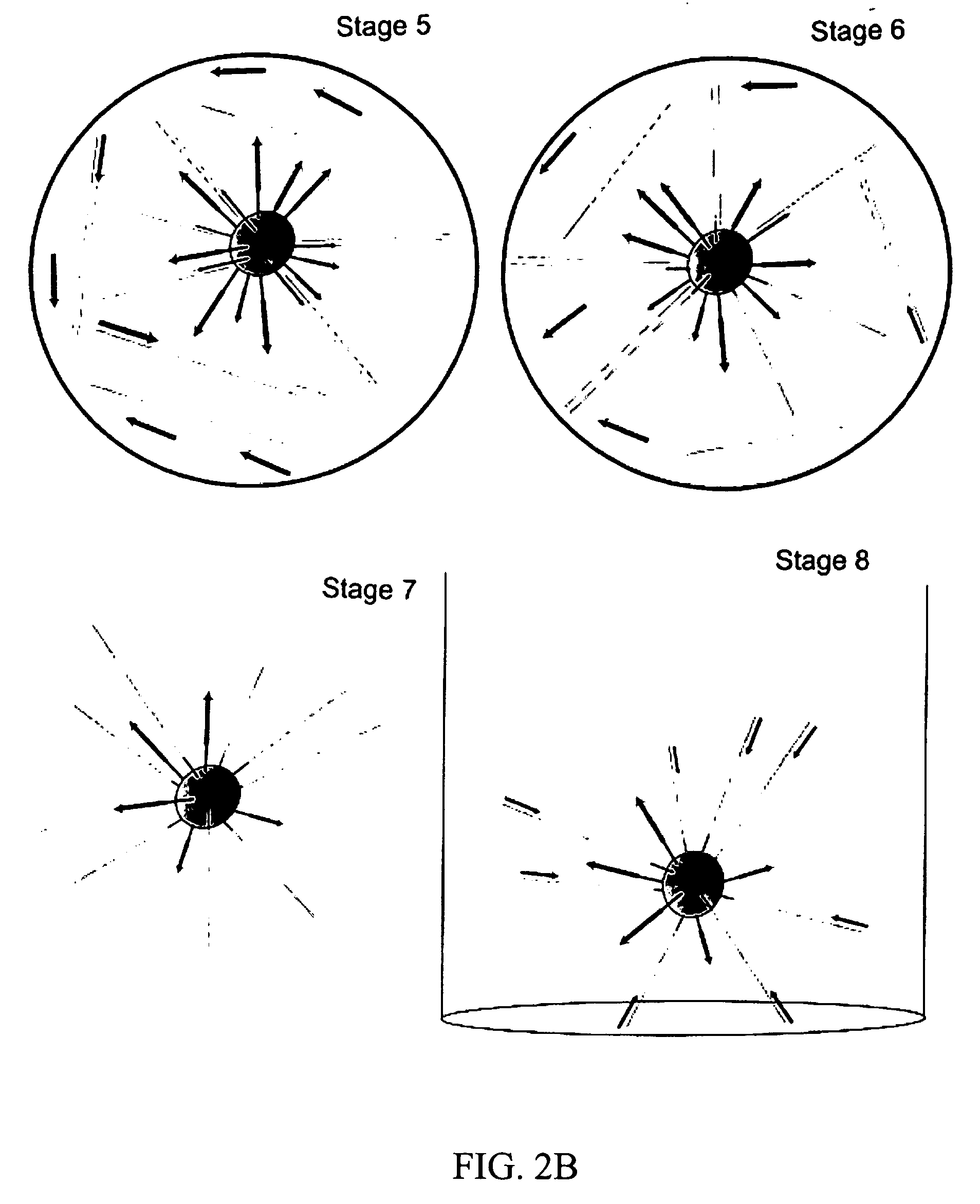
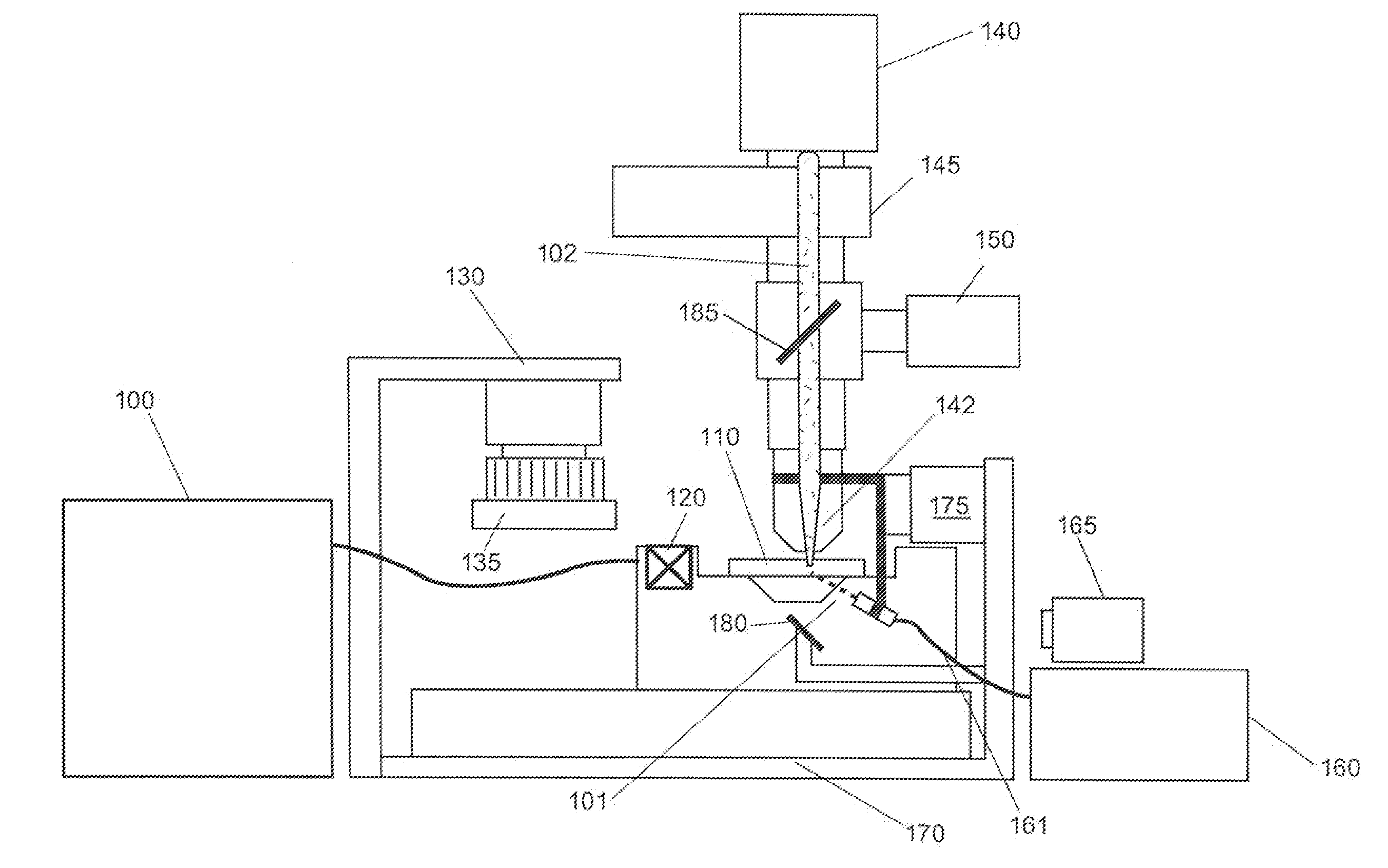
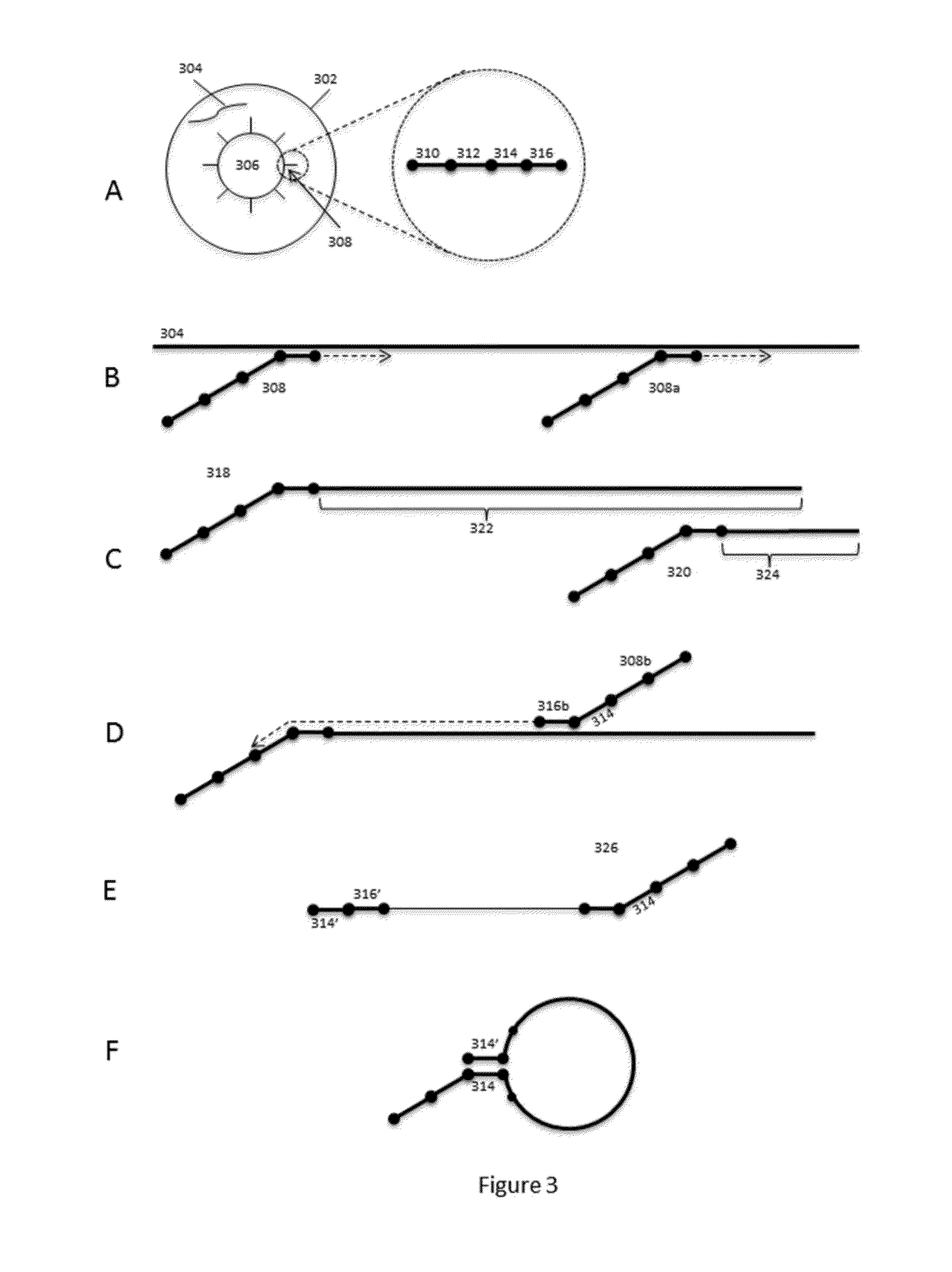
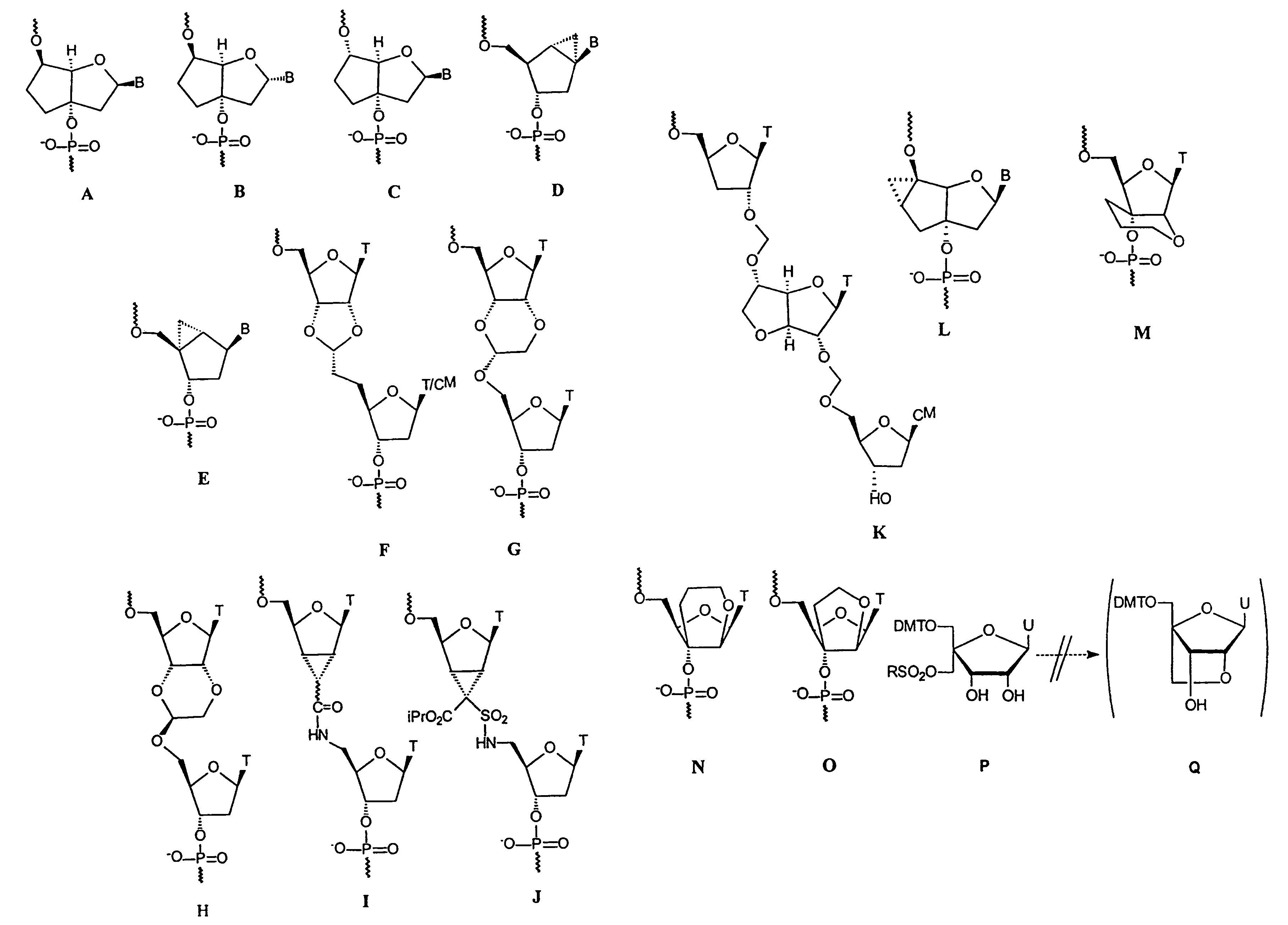Patents
Literature
31181 results about "Nucleic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nucleic acids are the biopolymers, or small biomolecules, essential to all known forms of life. The term nucleic acid is the overall name for DNA and RNA. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. If the sugar is a compound ribose, the polymer is RNA (ribonucleic acid); if the sugar is derived from ribose as deoxyribose, the polymer is DNA (deoxyribonucleic acid).
Anti-PD-L1 antibodies, compositions and articles of manufacture
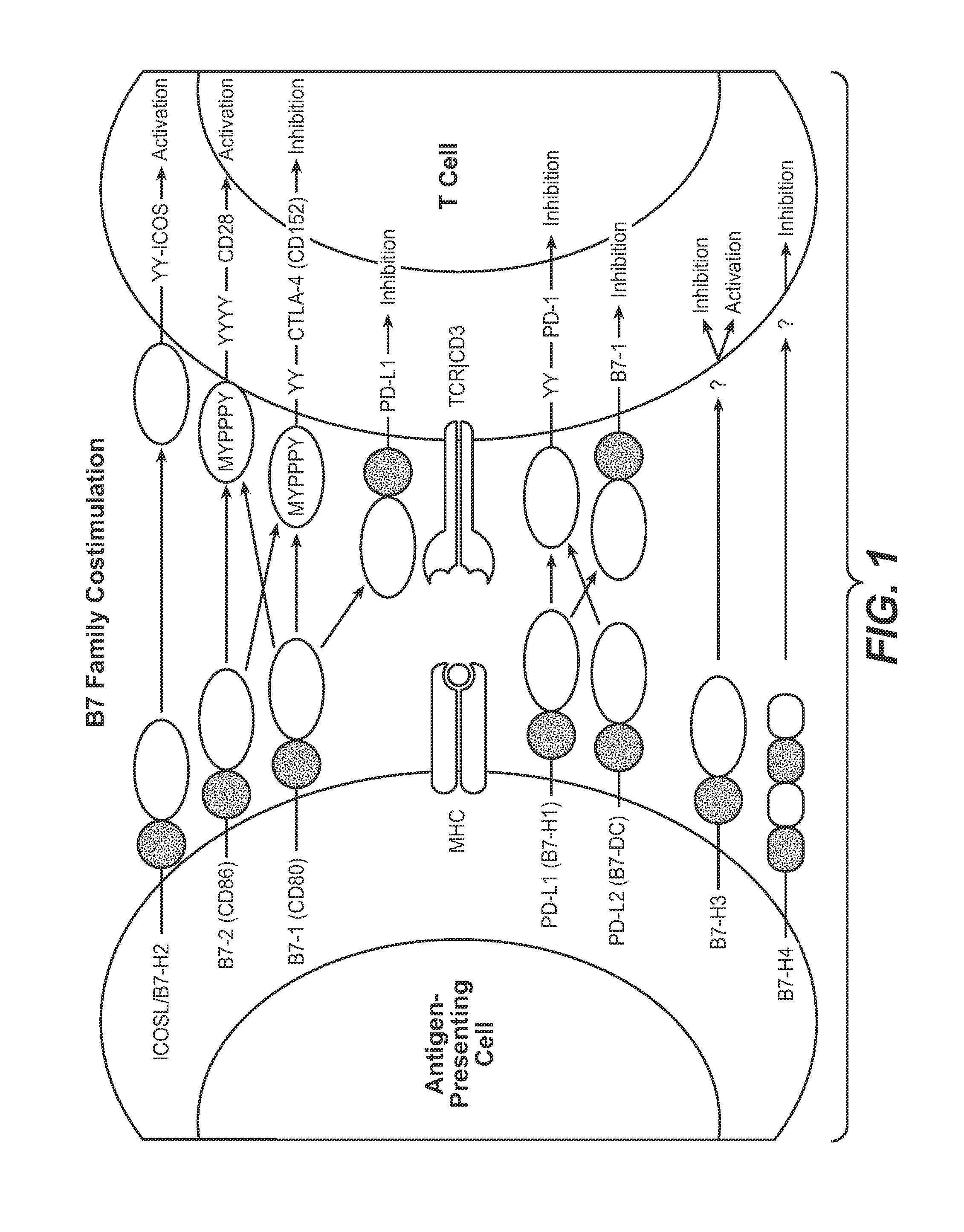
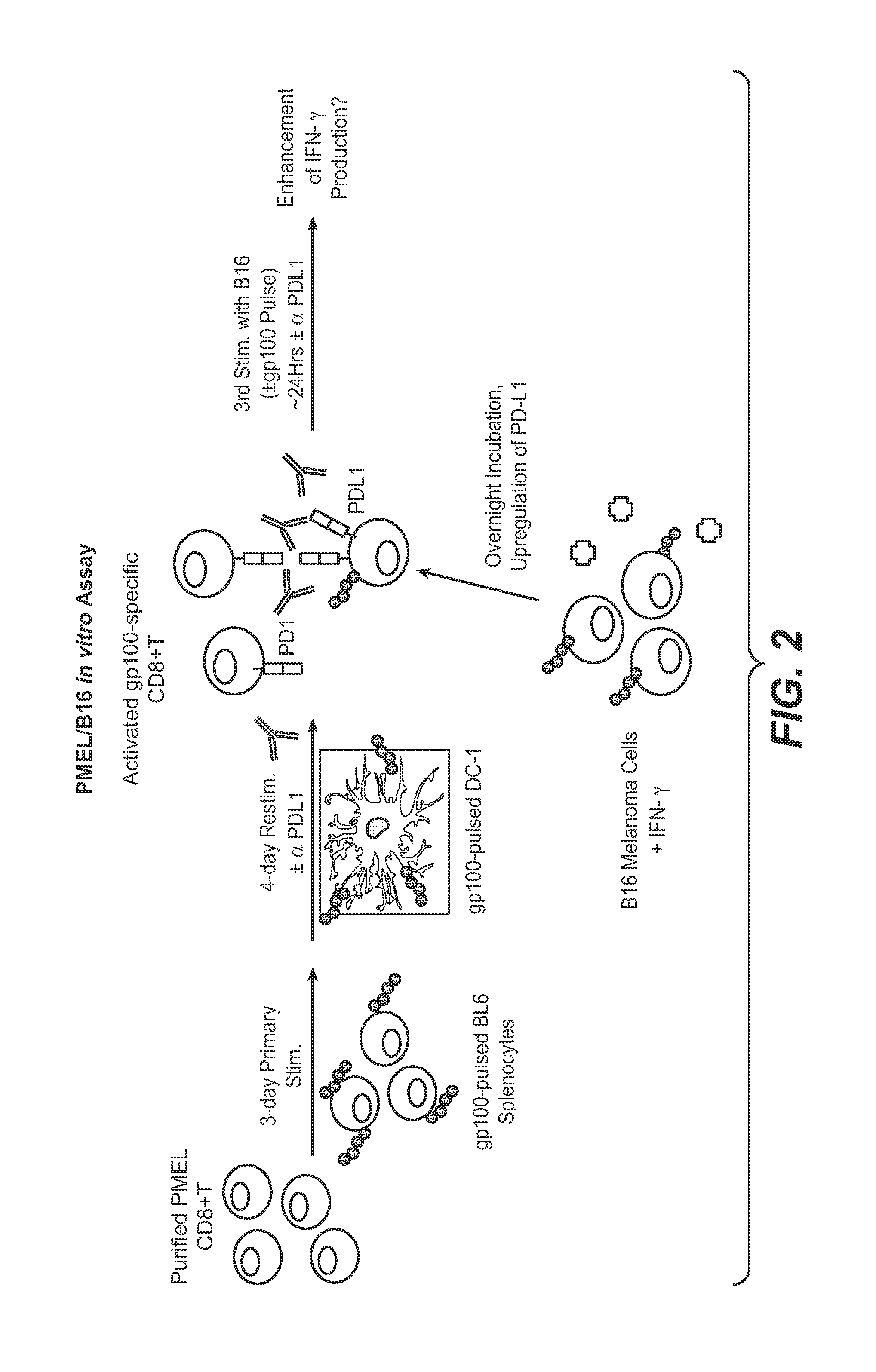
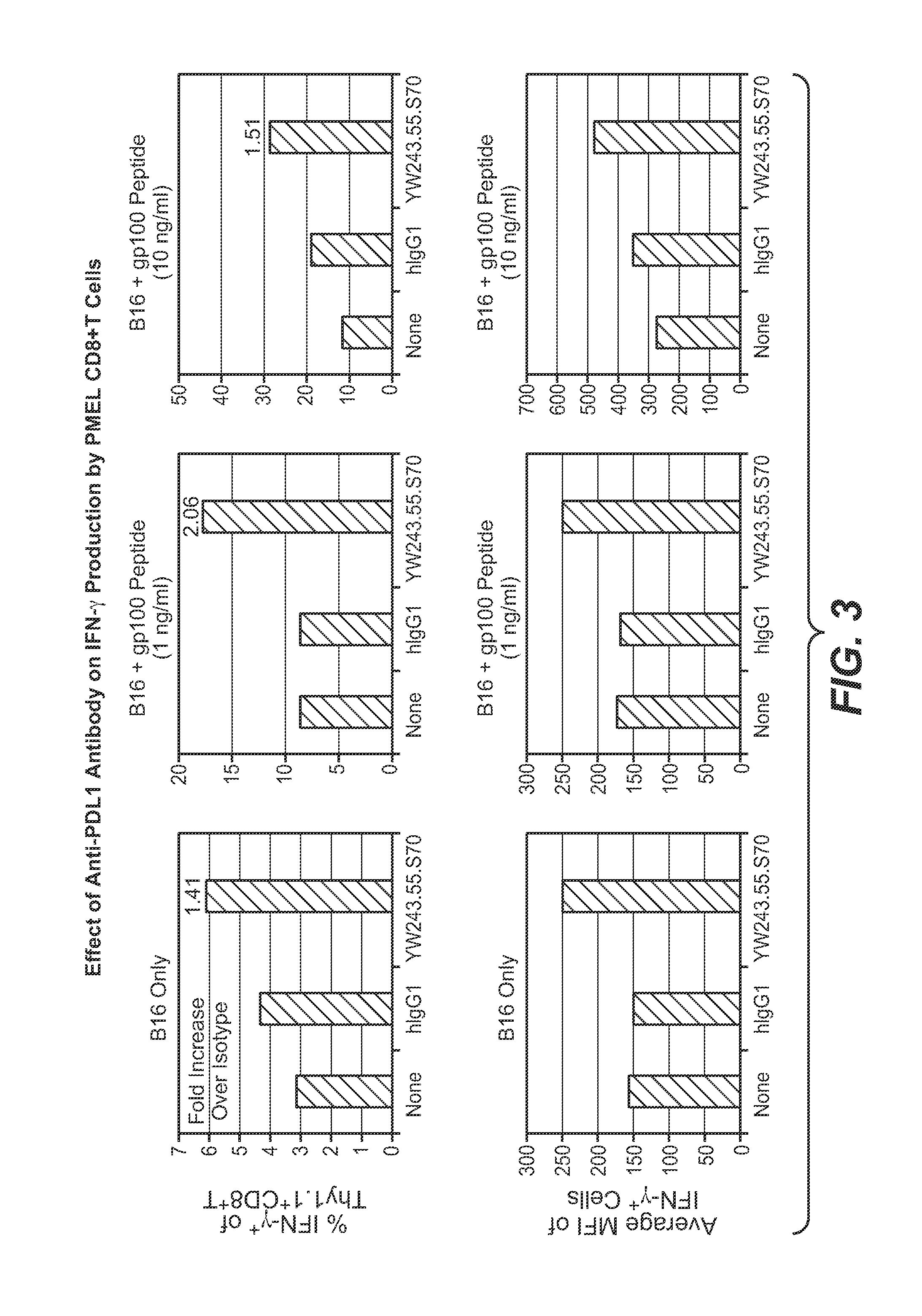
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
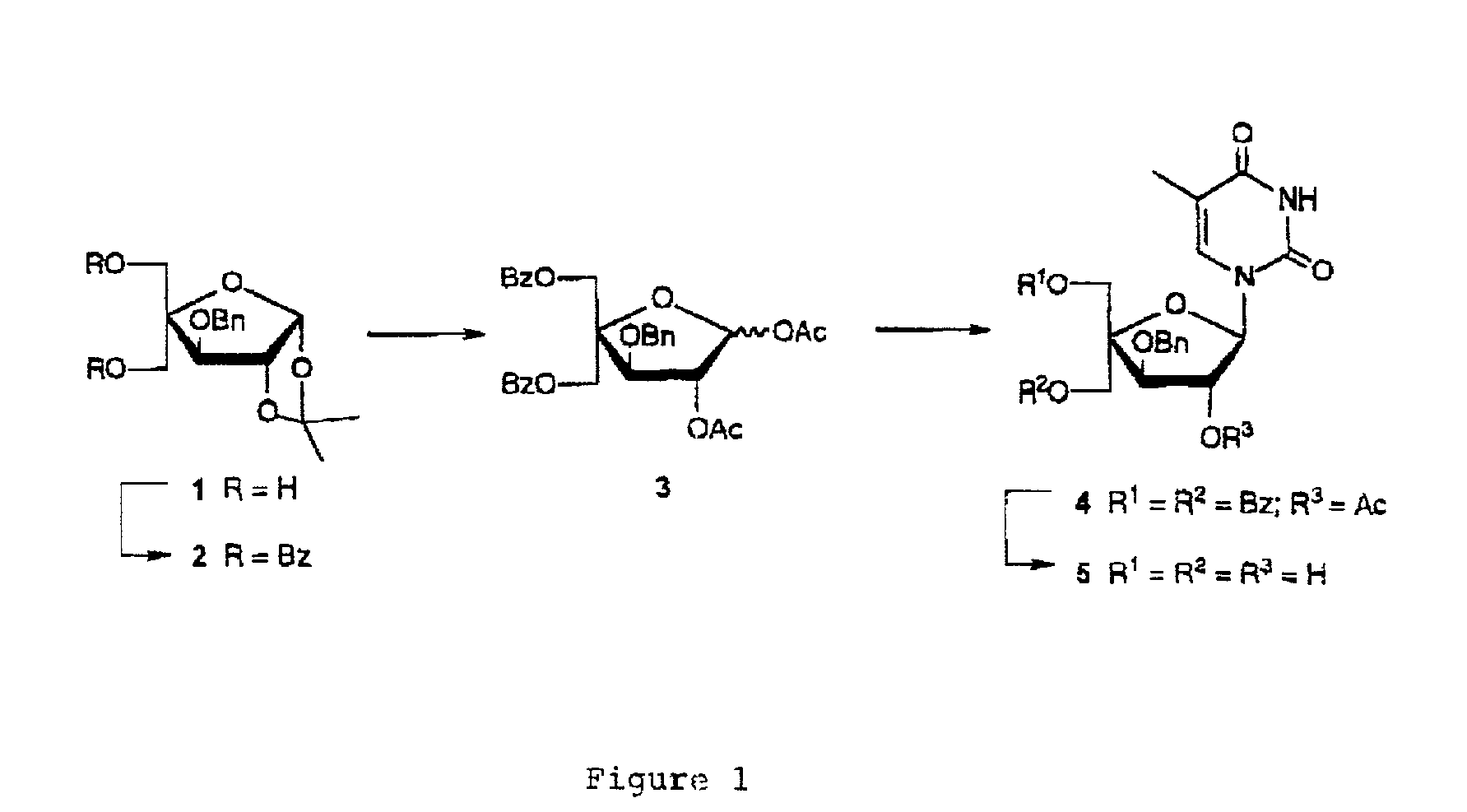
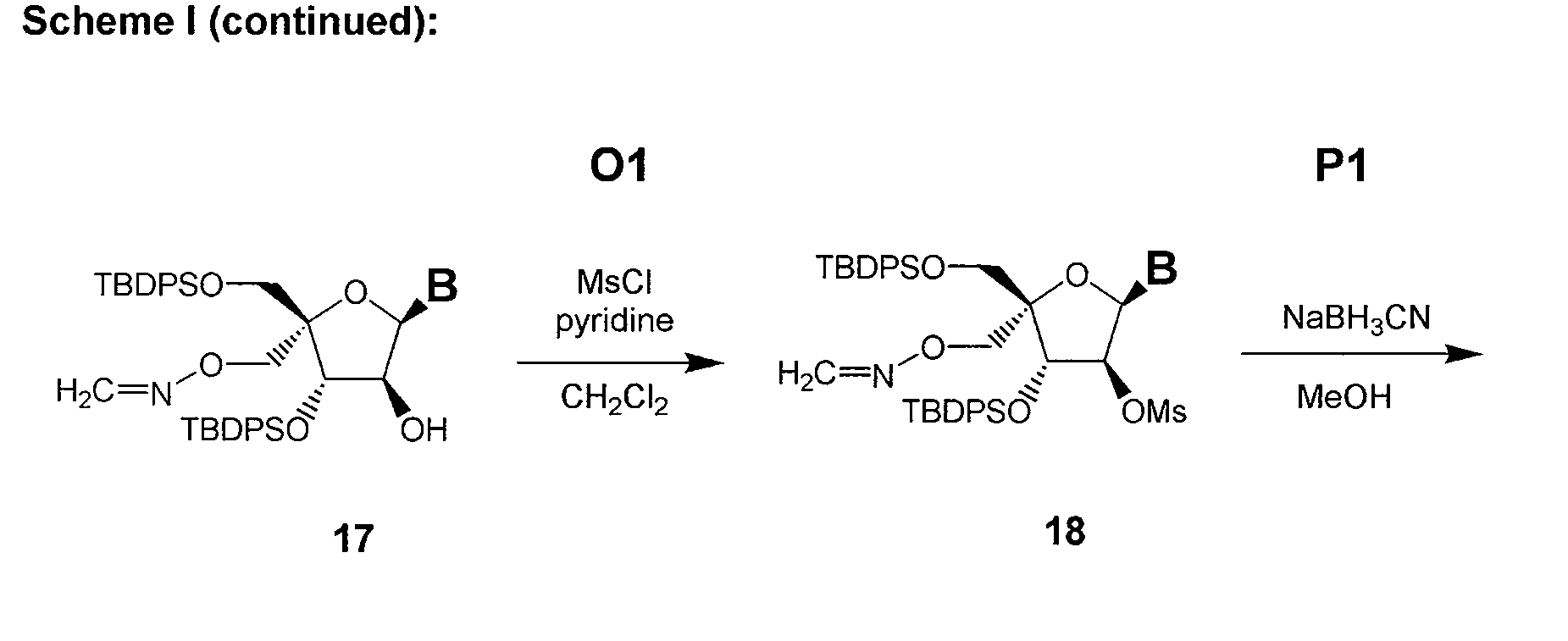
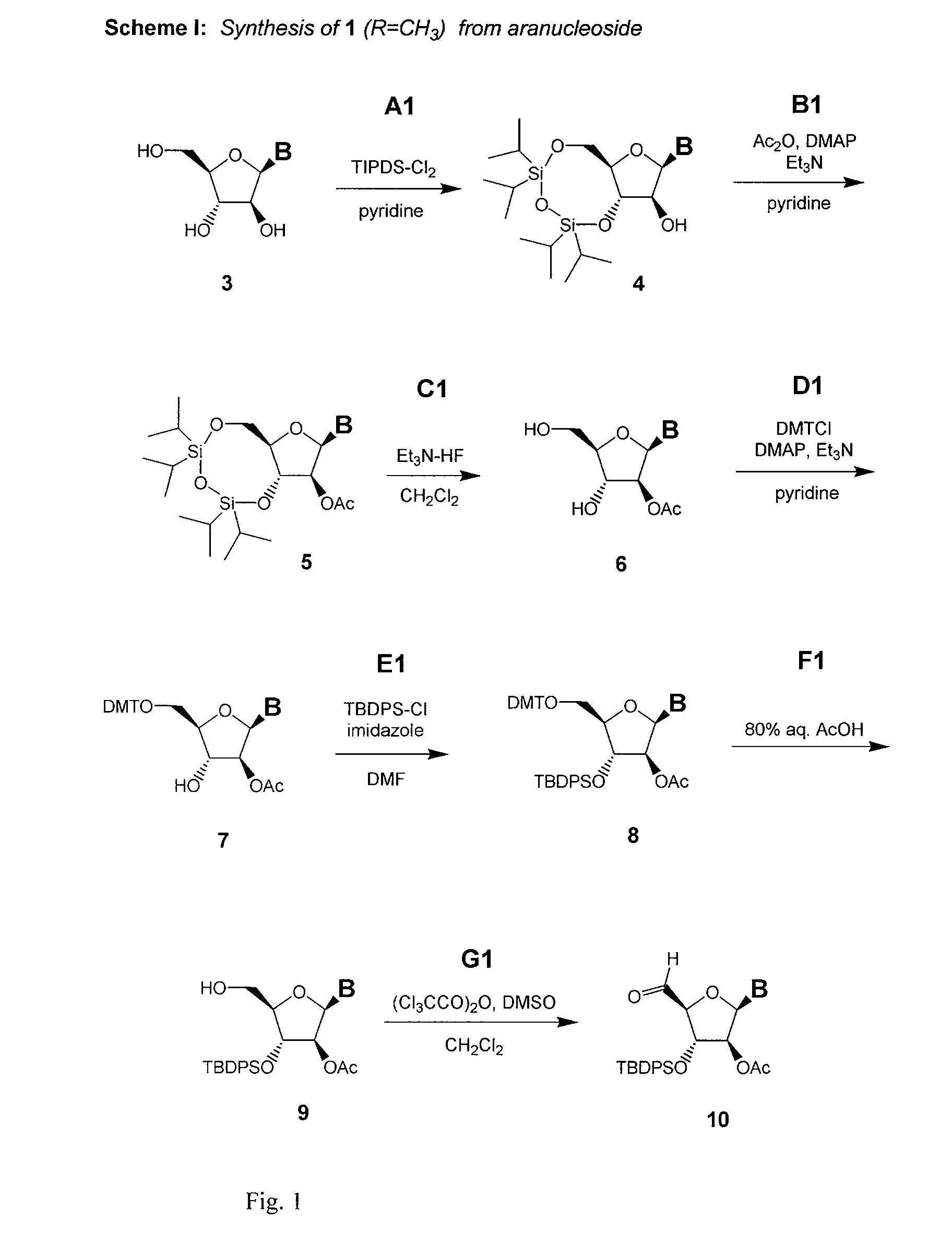
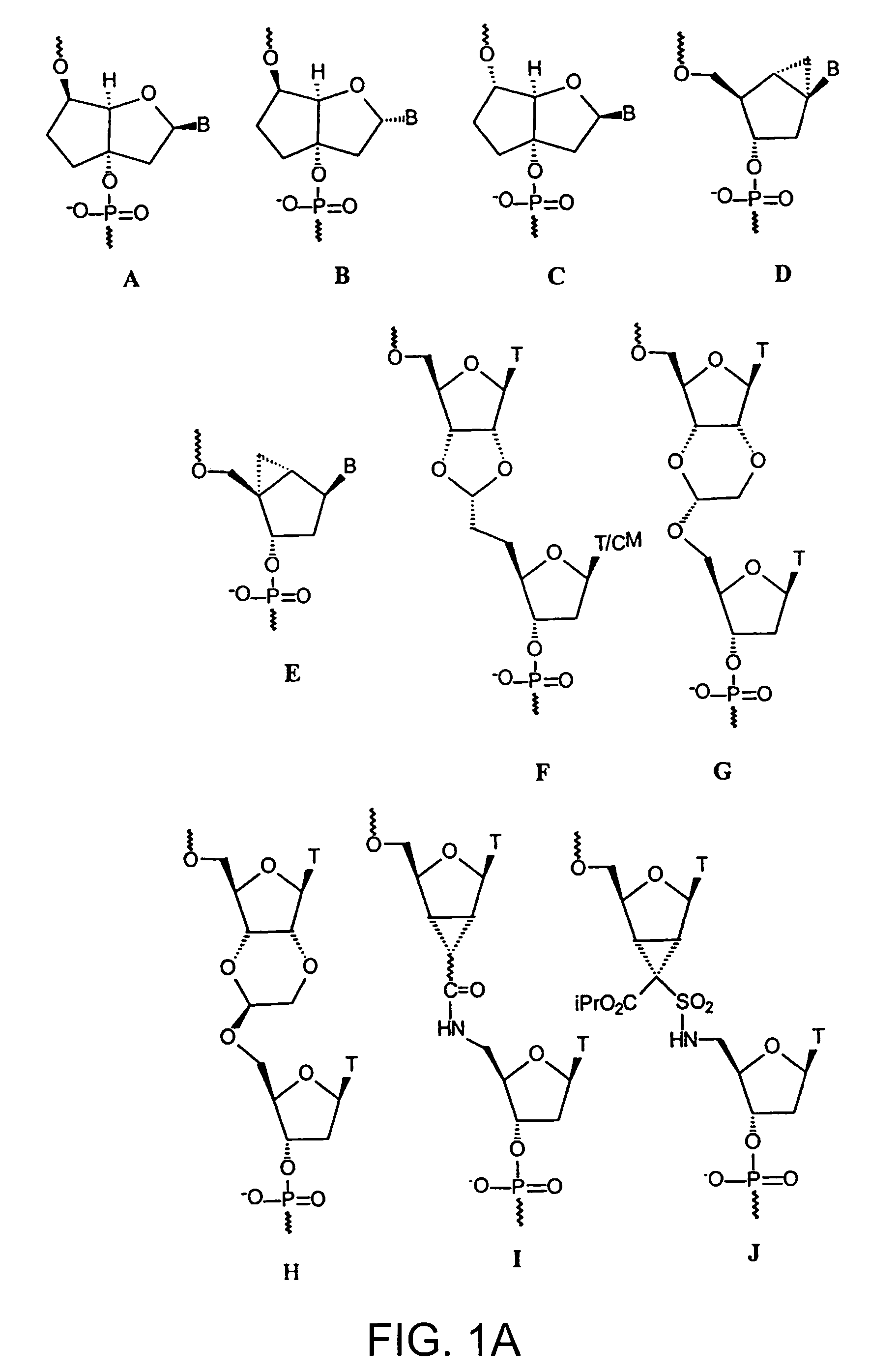
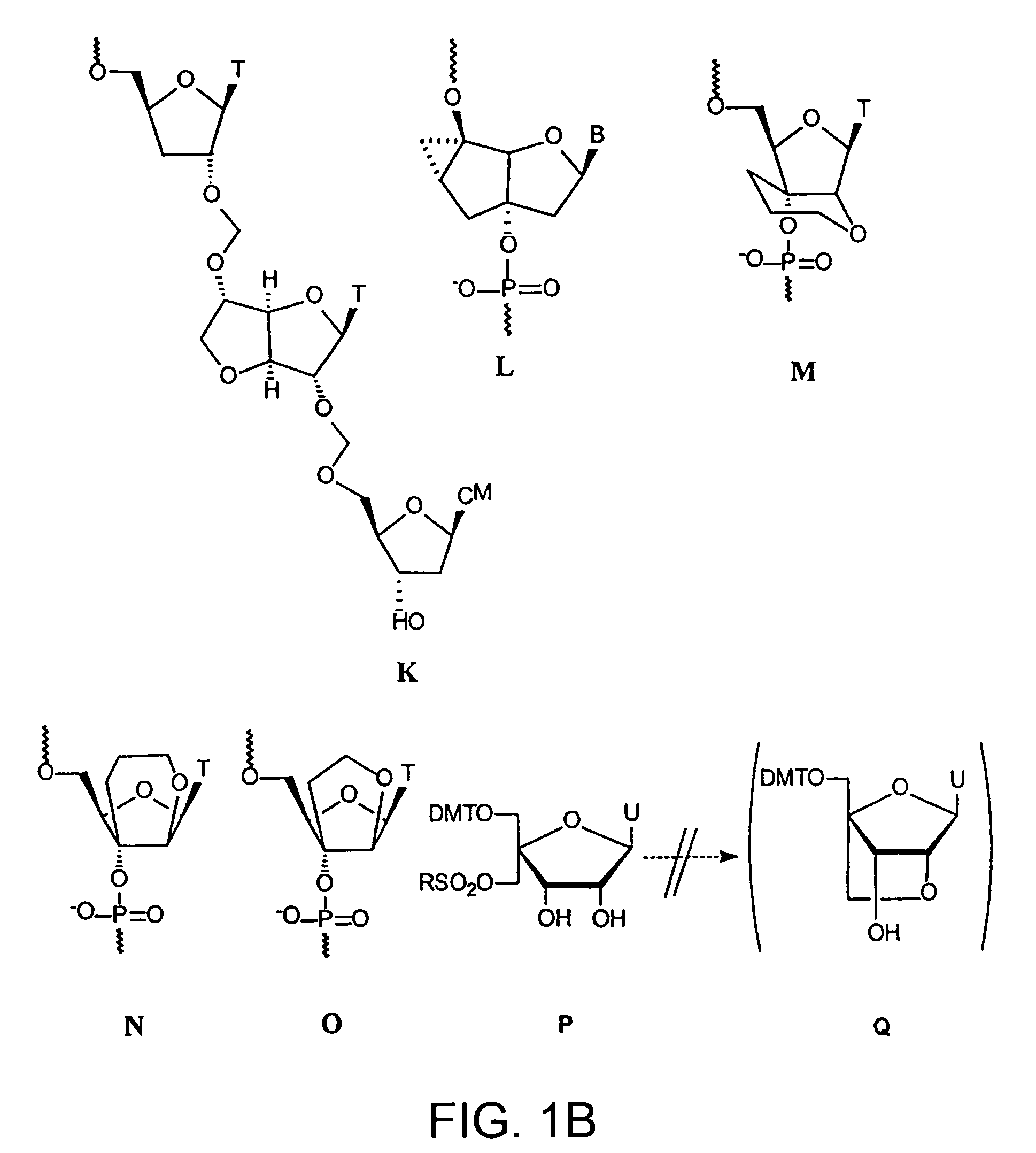
L-ribo-LNA analogues
Provided are L-ribo bicyclic nucleotide compounds as well as syntheses of such compounds. The nucleoside compounds of the invention are useful in forming oligonucleotides that can produce nucleobase specific duplexes with complementary single stranded and double stranded nucleic acids.
Owner:SANTARIS PHARMA AS
Oligonucleotide analogues
Novel oligomers, and synthesis thereof, comprising one or more bi-, tri, or polycyclic nucleoside analogues are disclosed herein. The nucleoside analogues have a “locked” structure, termed Locked Nucleoside Analogues (LNA). LNA's exhibit highly desirable and useful properties. LNA's are capable of forming nucleobase specific duplexes and triplexes with single and double stranded nucleic acids. These complexes exhibit higher thermostability than the corresponding complexes formed with normal nucleic acids. The properties of LNA's allow for a wide range of uses such as diagnostic agents and therapeutic agents in a mammal suffering from or susceptible to, various diseases.
Owner:EXIQON AS
Artificial nucleic acids of n-o bond crosslinkage type
ActiveUS7427672B2High sensitivity analysisConfirmed its usefulnessBiocideSugar derivativesSingle strandDouble strand
Owner:RIKEN GENESIS
Enzymatic nucleic acid synthesis: compositions and methods for altering monomer incorporation fidelity
InactiveUS7211414B2Extent of pyrophosphorolysis of a primer extension product is reducedImprove fidelityBiocideSugar derivativesPhosphatePhosphoric acid
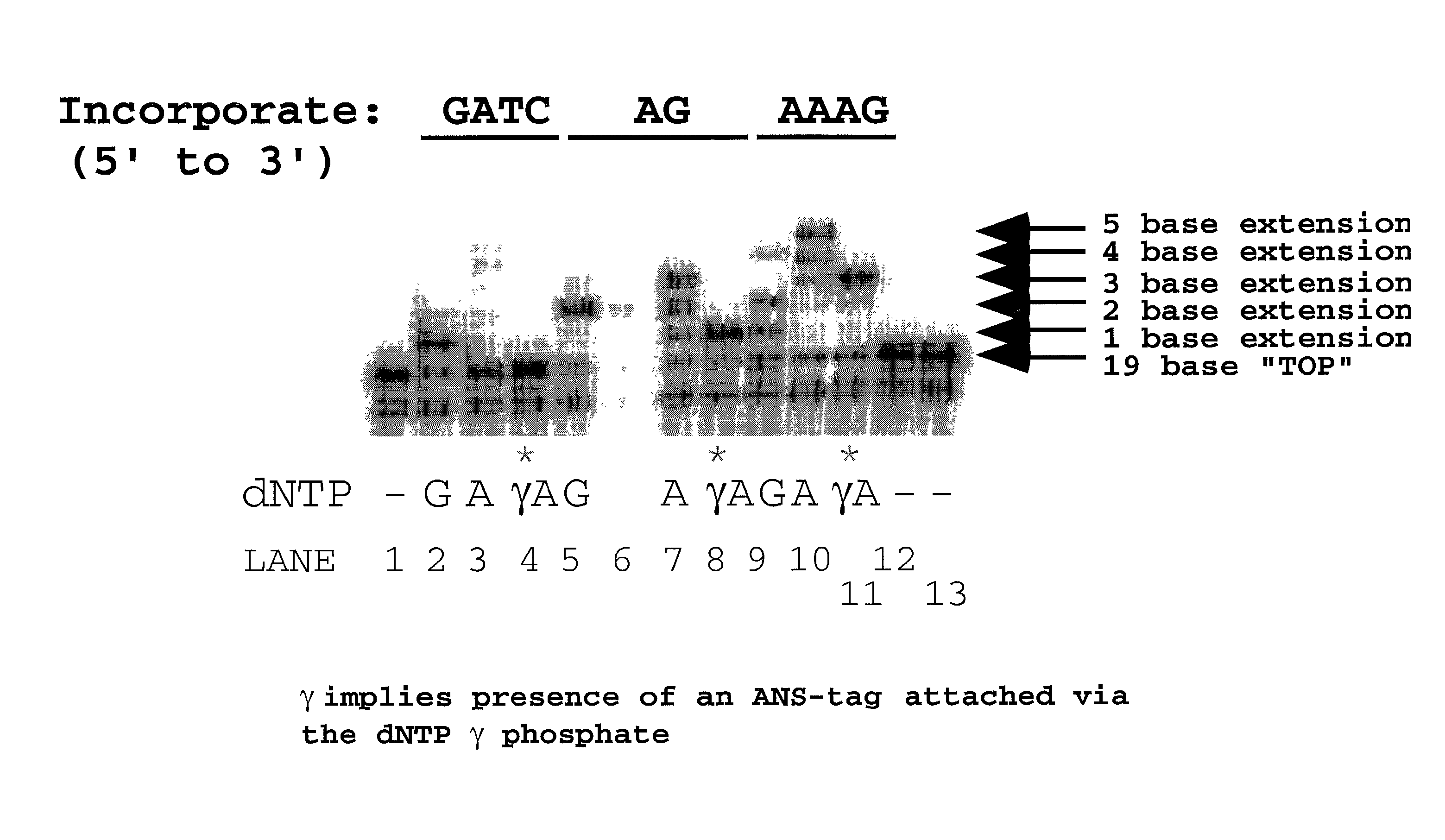
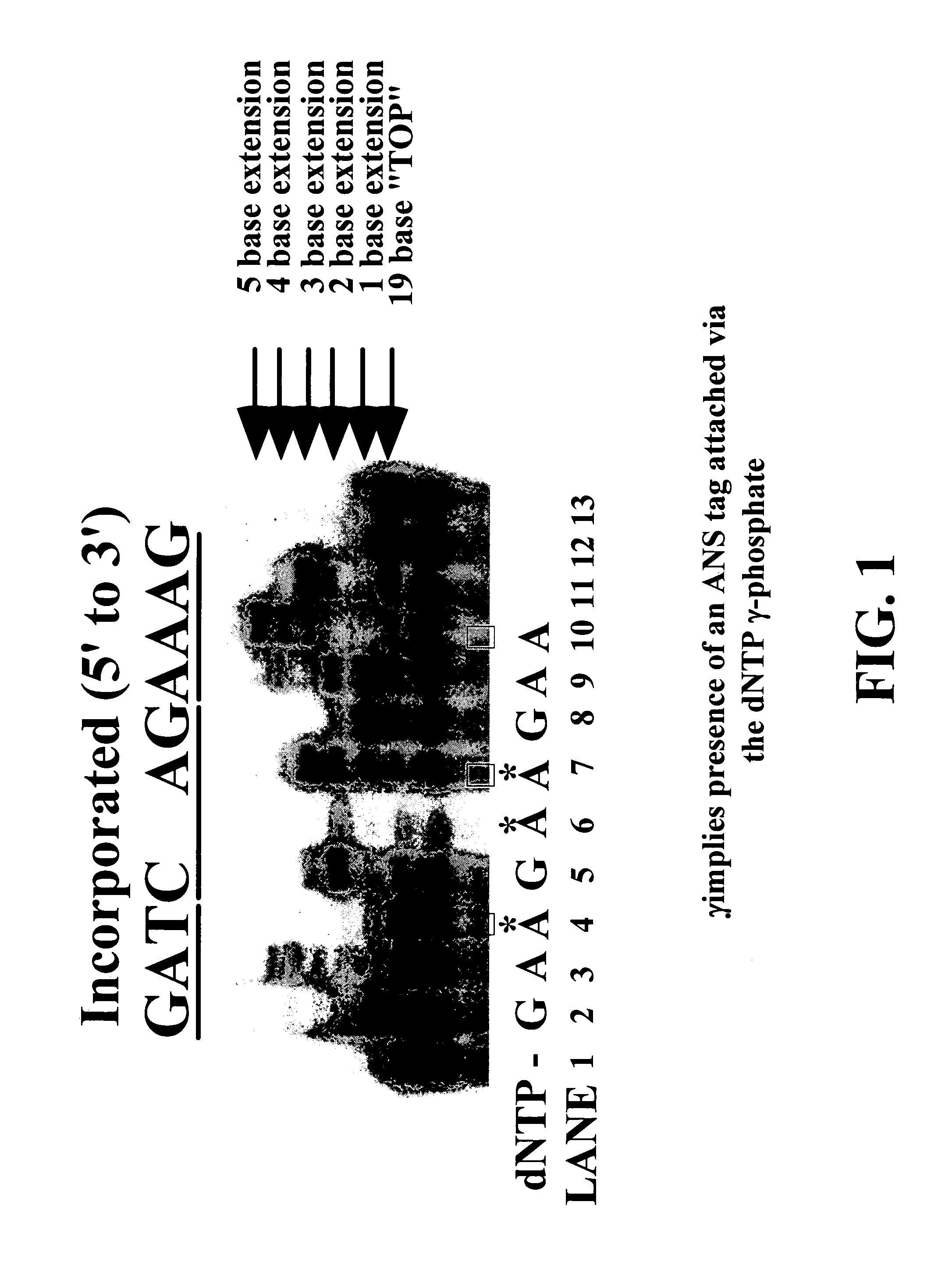
Nucleotide triphosphate probes containing a molecular and / or atomic tag on a a γ and / or β phosphate group and / or a base moiety having a detectable property are disclosed, and kits and method for using the tagged nucleotides in sequencing reactions and various assay. Also, phosphate and polyphosphate molecular fidelity altering agents are disclosed.
Owner:LIFE TECH CORP
Methods of amplifying and sequencing nucleic acids
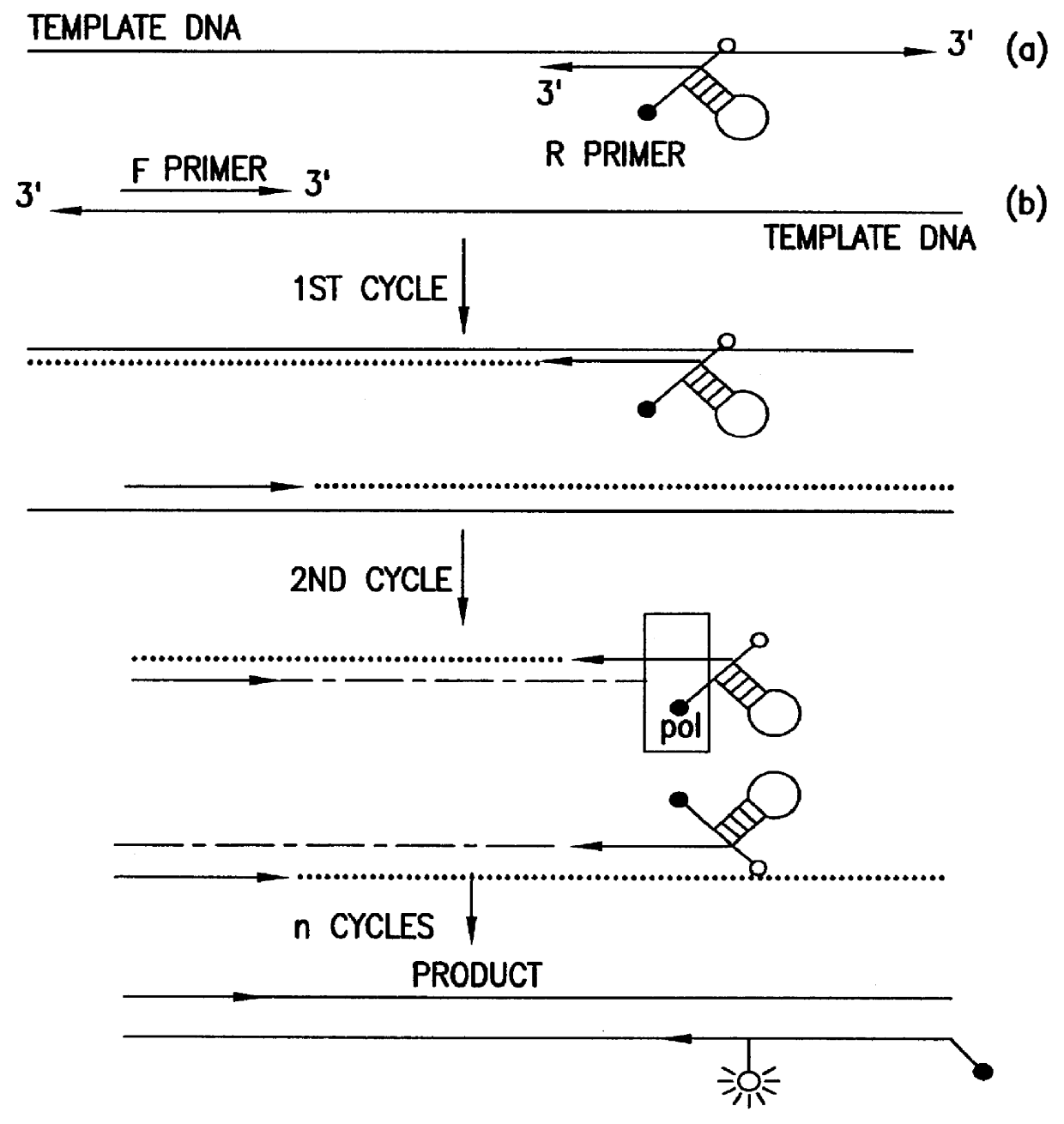
An apparatus and method for performing rapid DNA sequencing, such as genomic sequencing, is provided herein. The method includes the steps of preparing a sample DNA for genomic sequencing, amplifying the prepared DNA in a representative manner, and performing multiple sequencing reaction on the amplified DNA with only one primer hybridization step.
Owner:454 LIFE SCIENCES CORP
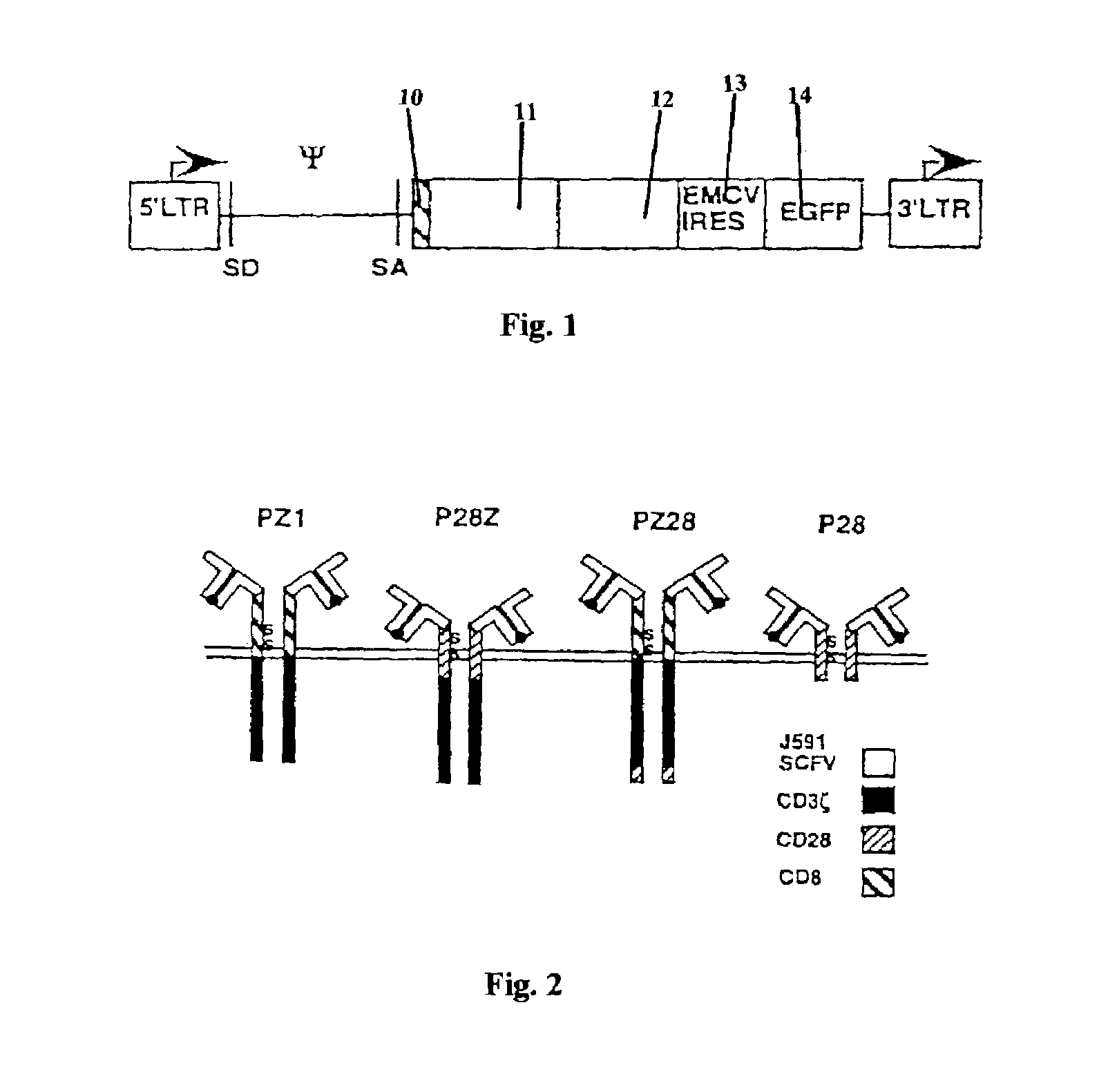
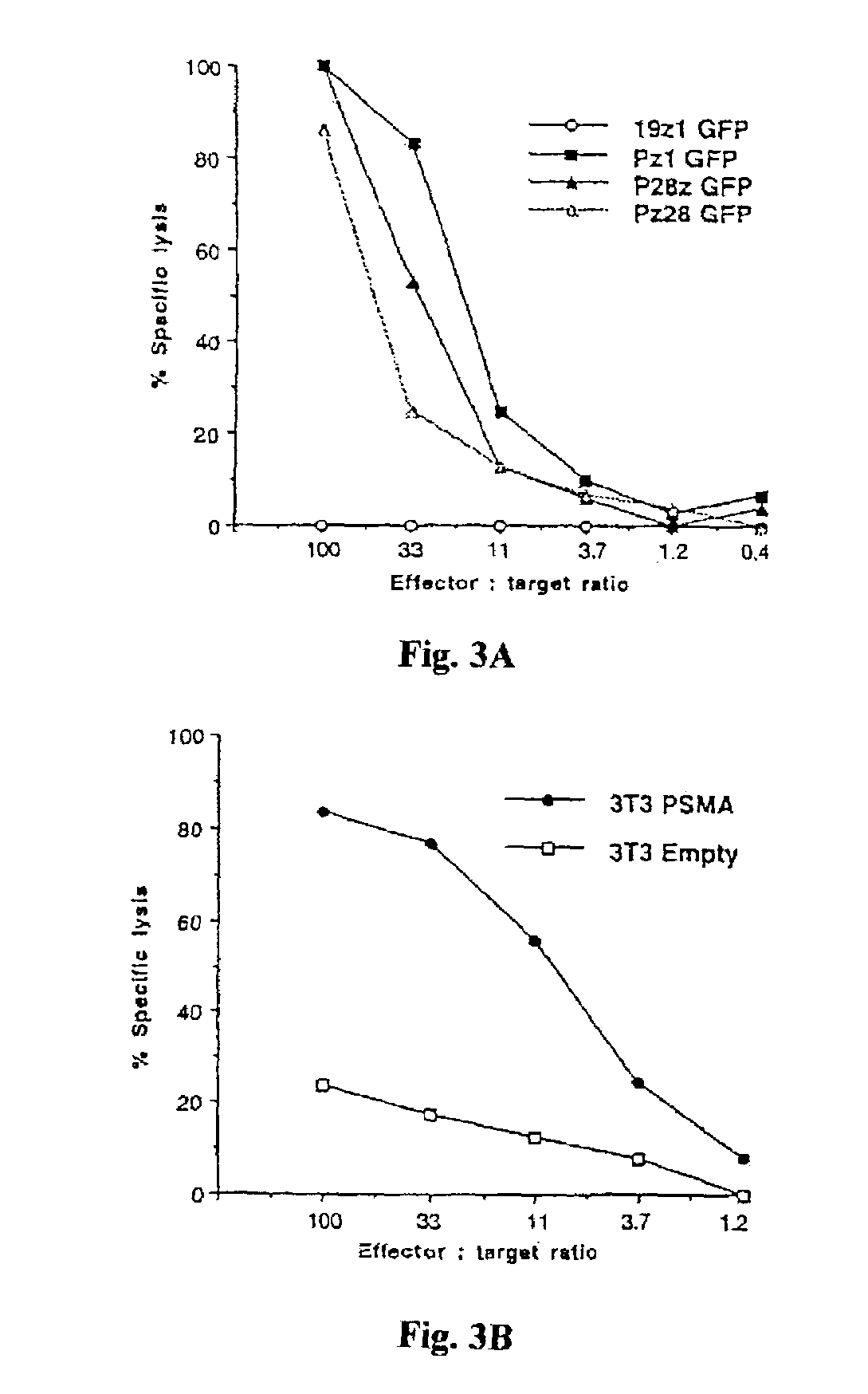
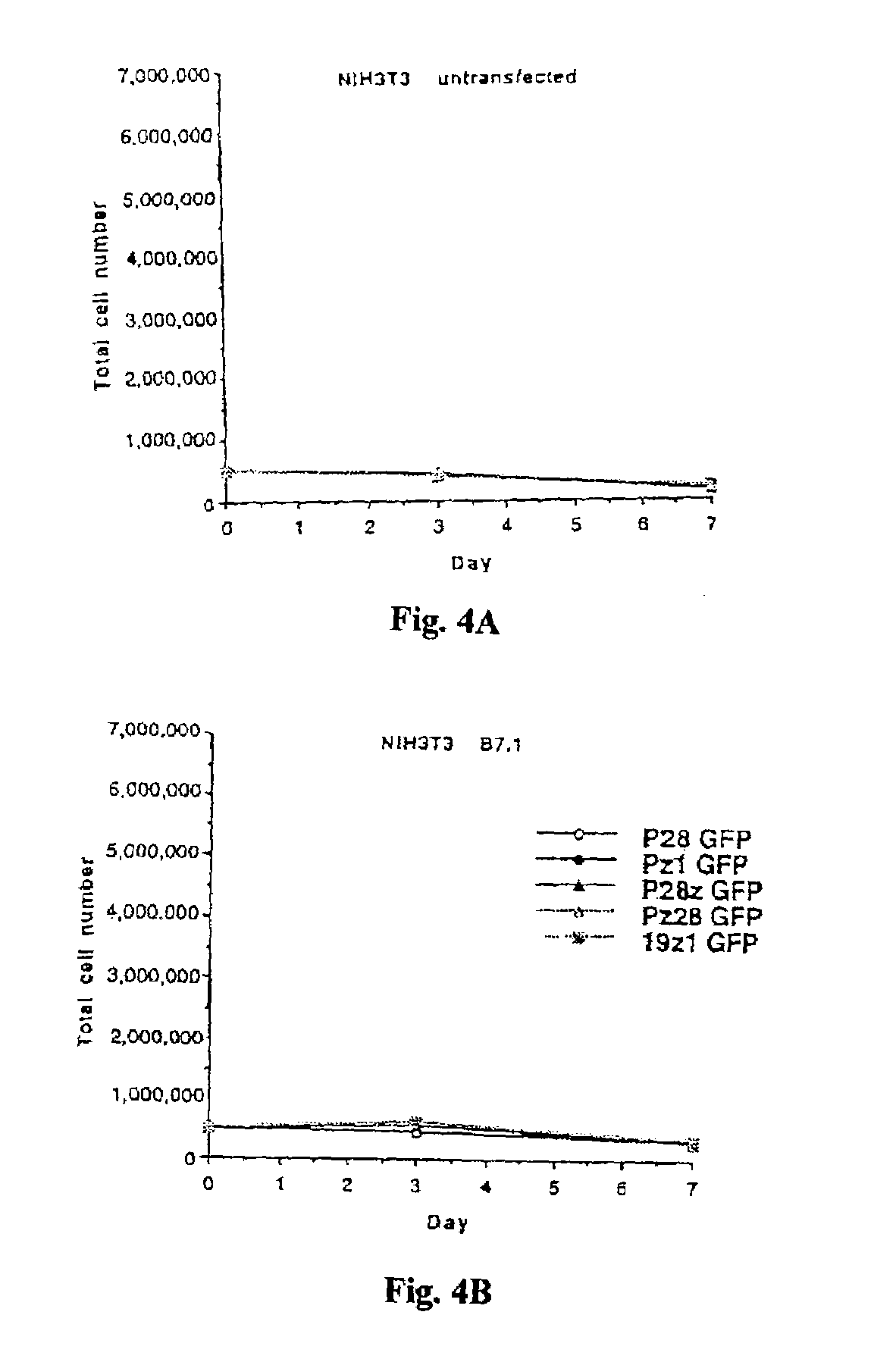
Nucleic acids encoding chimeric T cell receptors
ActiveUS7446190B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCytotoxicityBiological activation
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 ζ-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
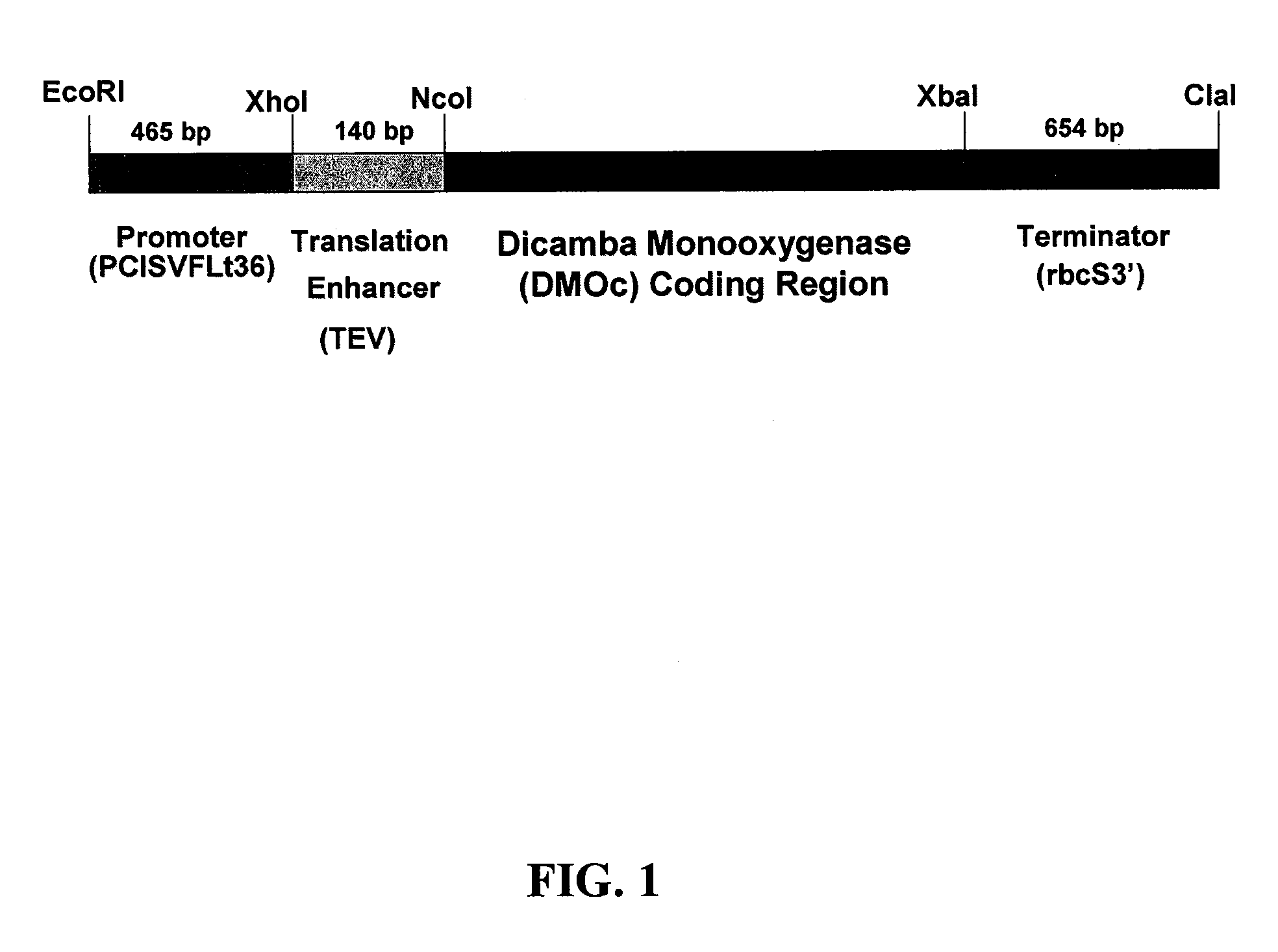
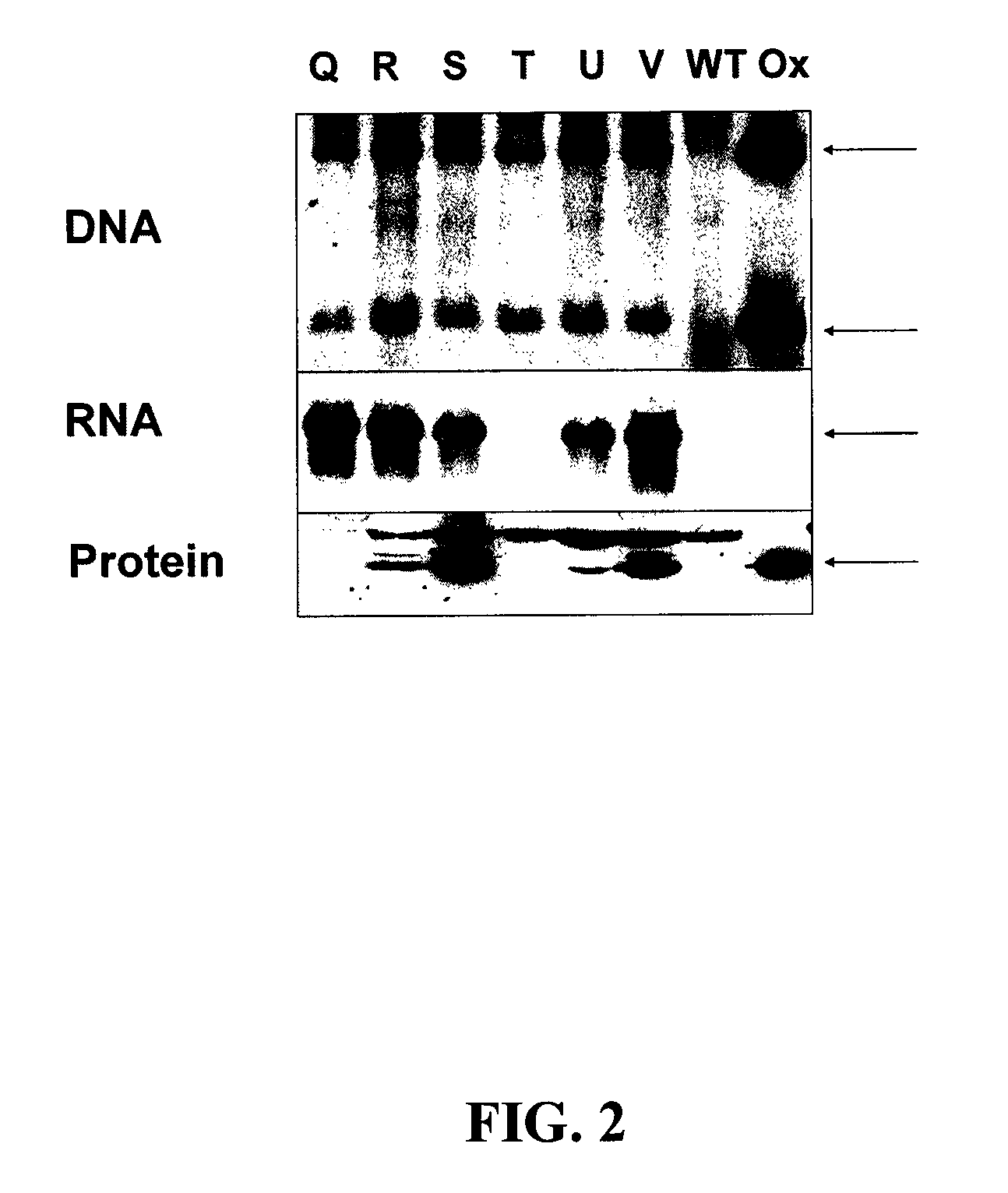
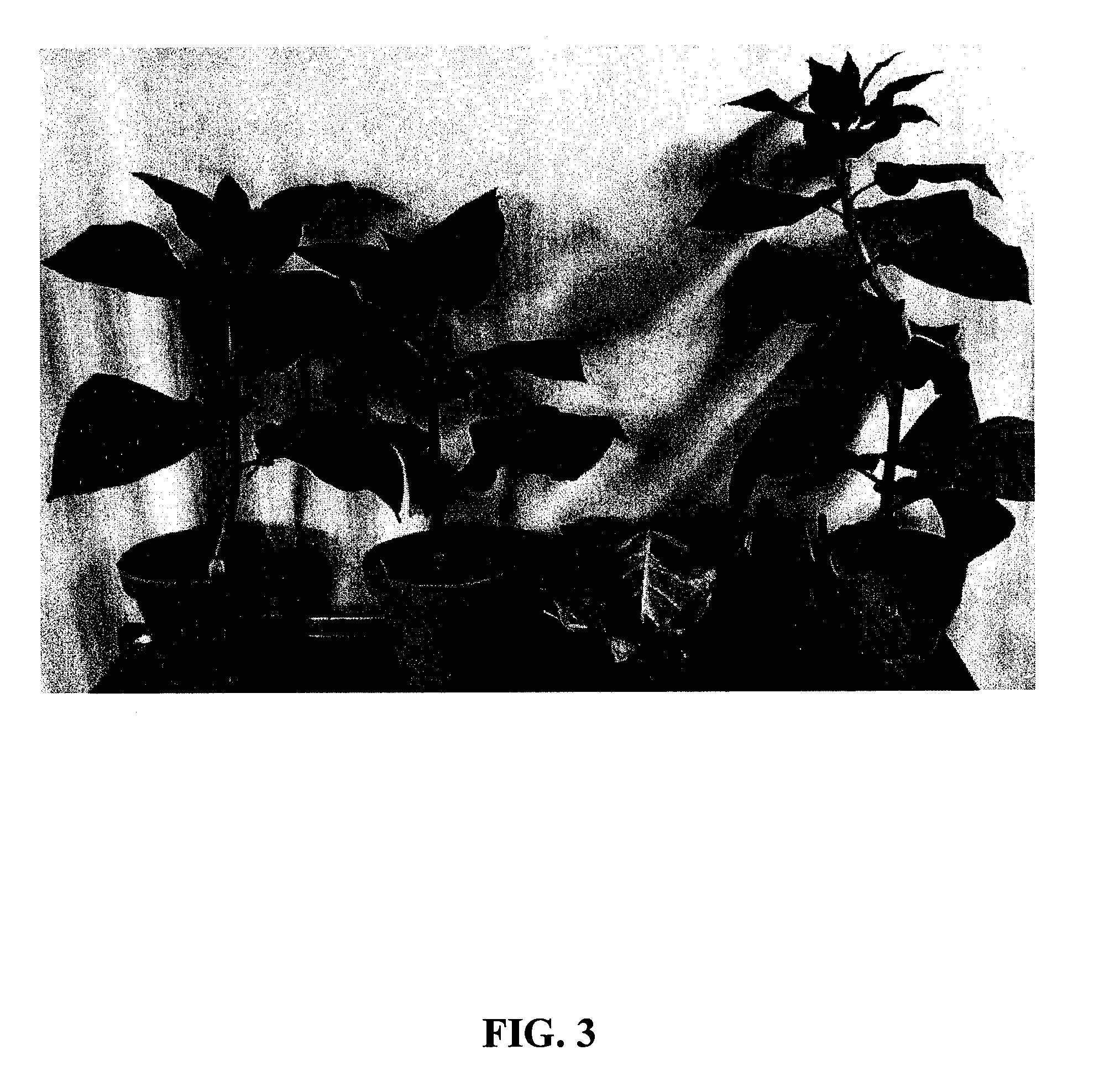
Modified DMO enzyme and methods of its use
Owner:MONSANTO TECH LLC +1
Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation
Compositions comprising first and second oligomers are provided wherein at least a portion of the first oligomer is capable of hybridizing with at least a portion of the second oligomer, at least a portion of the first oligomer is complementary to and capable of hybridizing to a selected target nucleic acid, and at least one of the first or second oligomers includes a modification comprising a polycyclic sugar surrogate. Oligomer / protein compositions are also provided comprising an oligomer complementary to and capable of hybridizing to a selected target nucleic acid and at least one protein comprising at least a portion of an RNA-induced silencing complex (RISC), wherein at least one nucleoside of the oligomer has a polycyclic sugar surrogate modification.
Owner:ALLERSON CHARLES +6
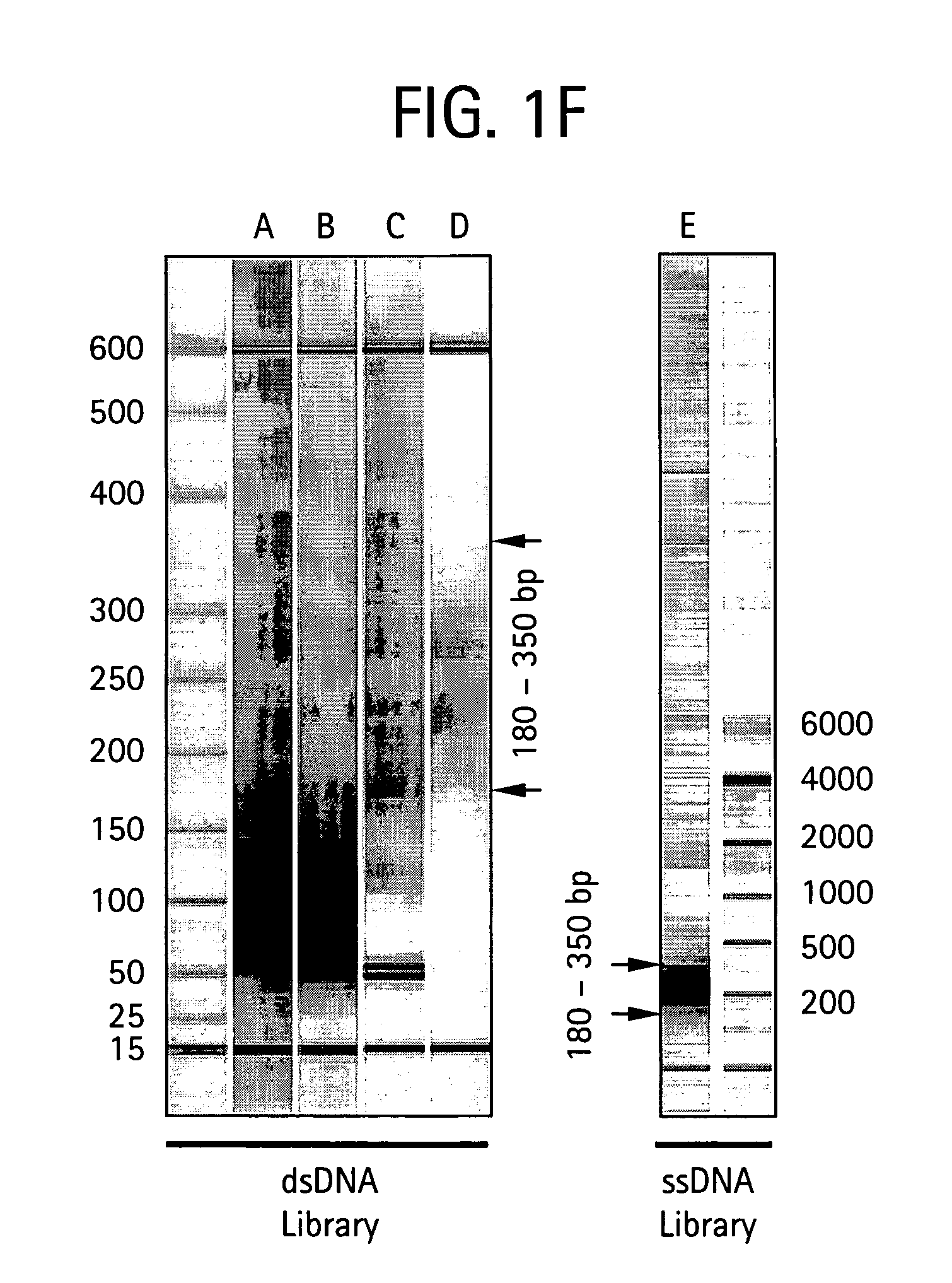
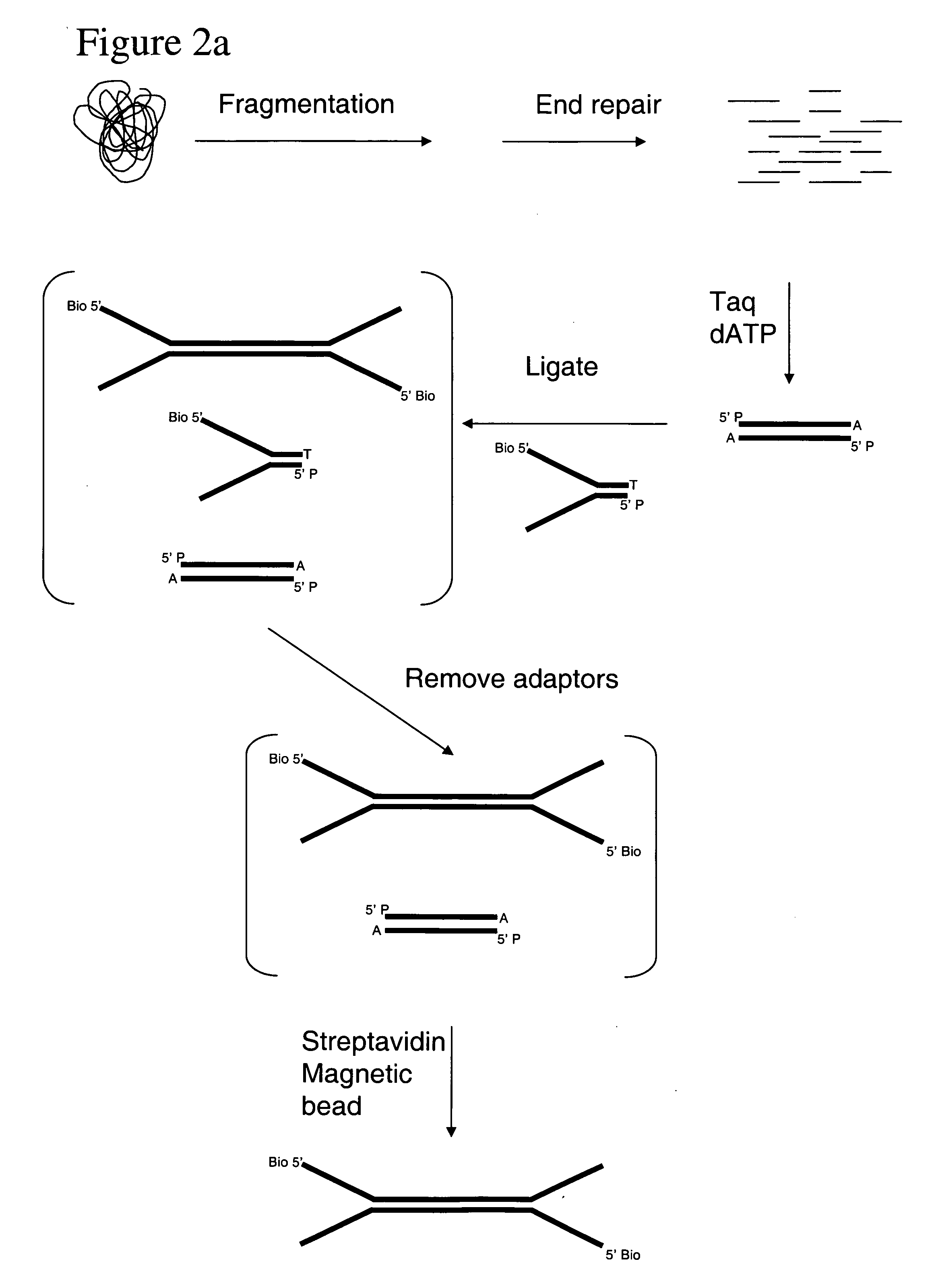
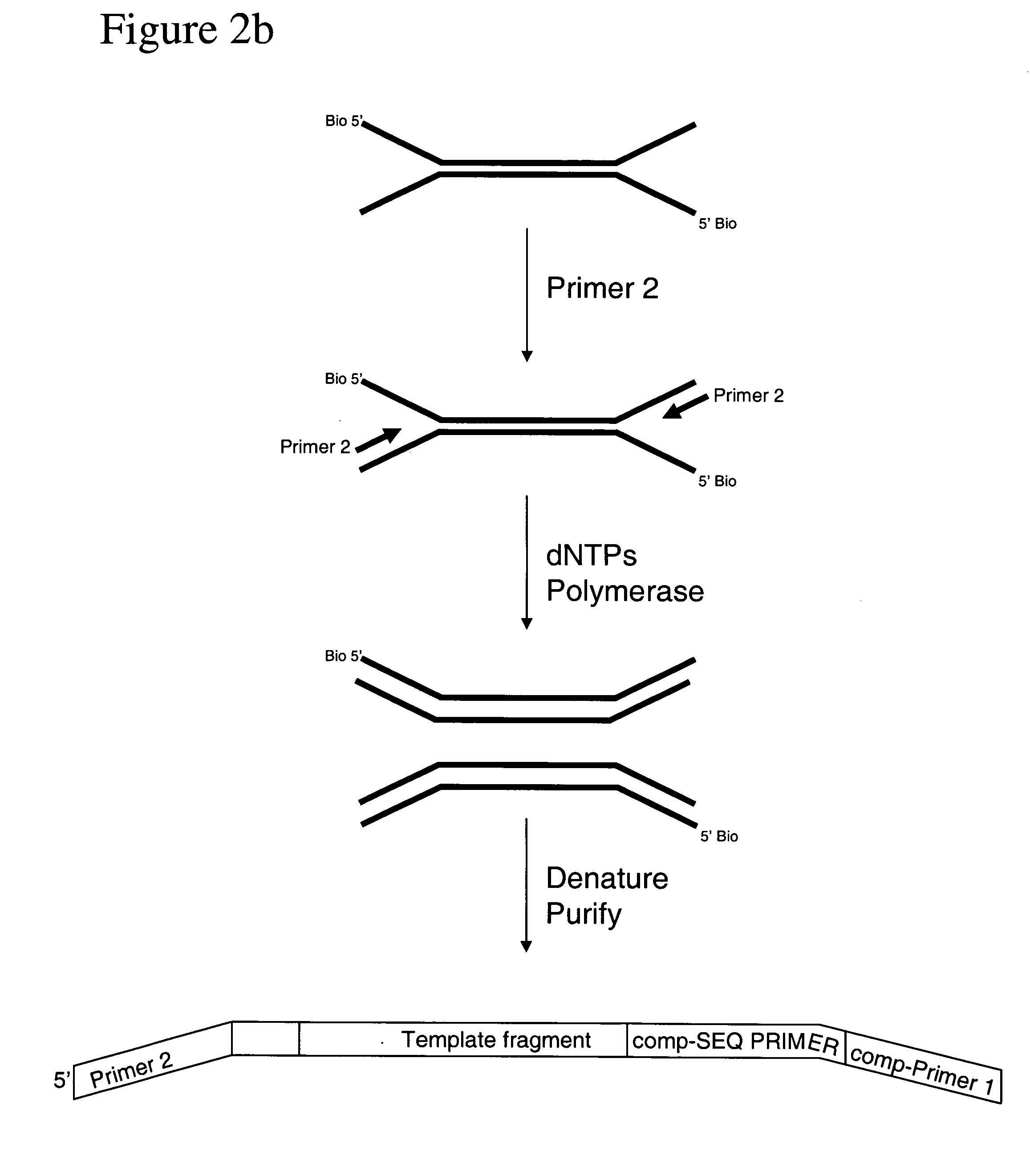
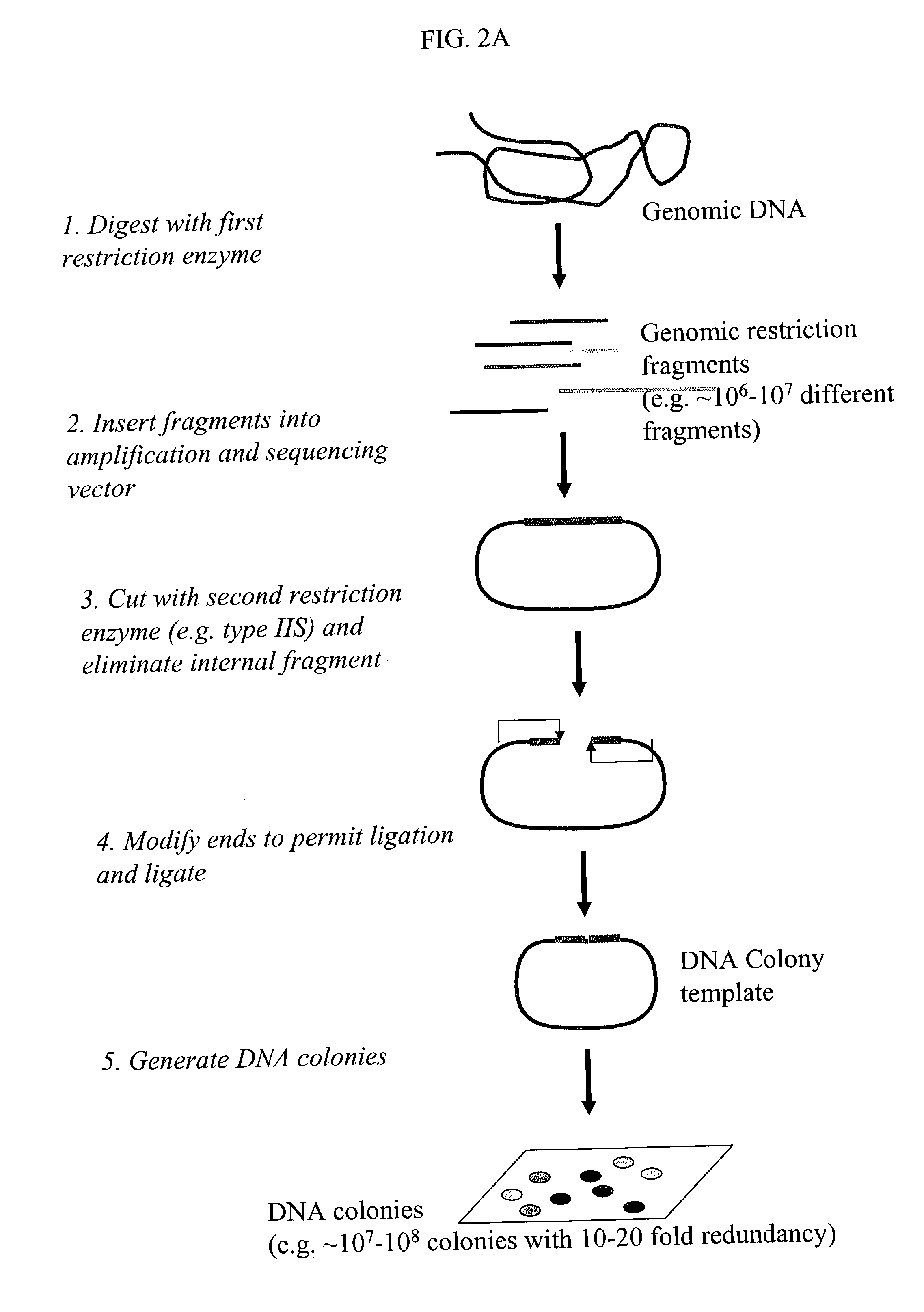
Method of preparing libraries of template polynucleotides
The present invention relates to a method for preparing a library of template polynucleotides and use thereof in methods of solid-phase nucleic acid amplification. More specifically, the invention relates to a method for preparing a library of template polynucleotides that have common sequences at their 5′ ends and at their 3′ ends.
Owner:ILLUMINA CAMBRIDGE LTD
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
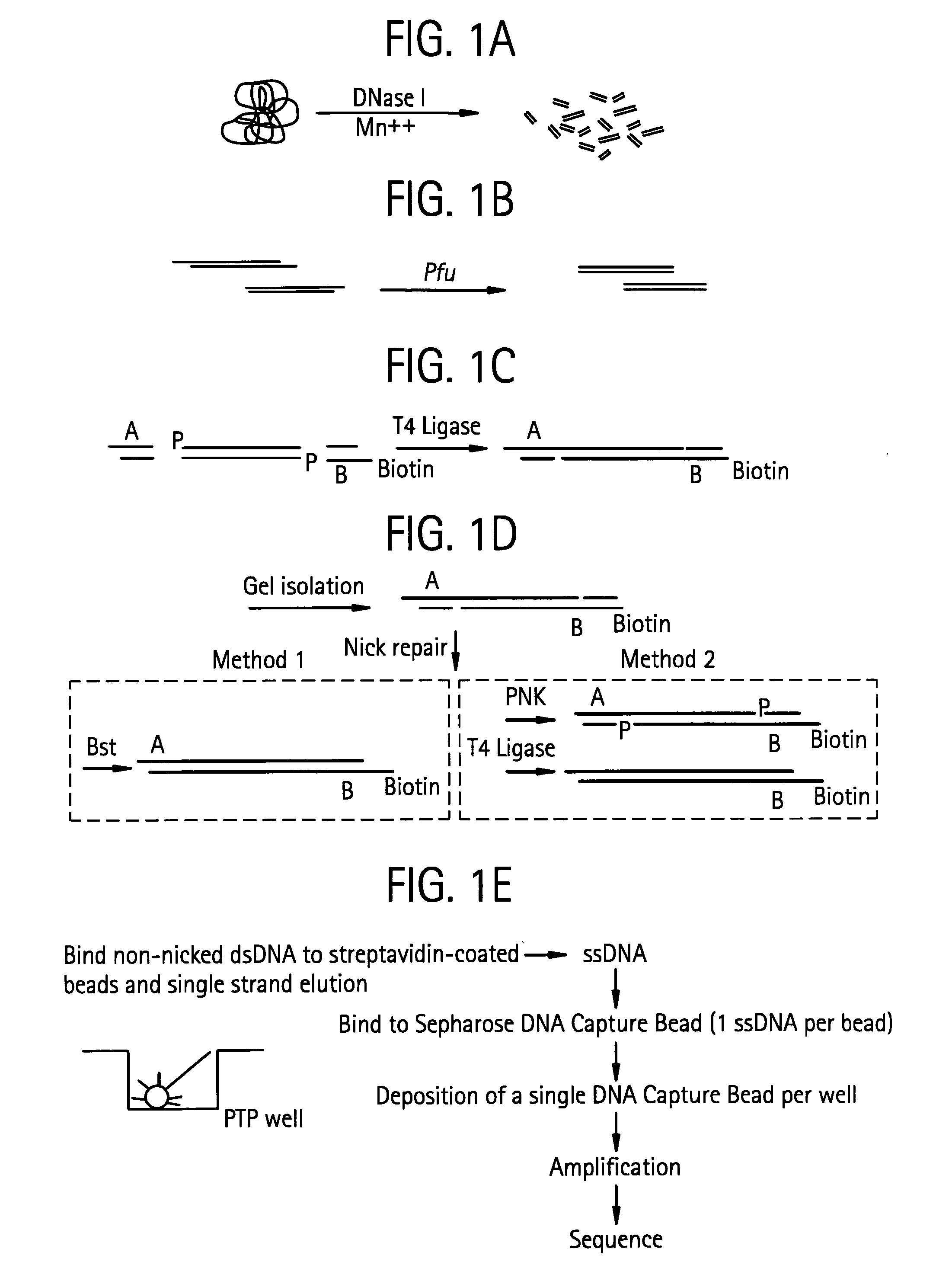
Bead emulsion nucleic acid amplification
Disclosed are methods for nucleic acid amplification wherein nucleic acid templates, beads, and amplification reaction solution are emulsified and the nucleic acid templates are amplified to provide clonal copies of the nucleic acid templates attached to the beads. Also disclosed are kits and apparatuses for performing the methods of the invention.
Owner:454 LIFE SCIENCES CORP
Systems and devices for sequence by synthesis analysis
ActiveUS20100111768A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDNAComputational biology
The present invention comprises systems and devices for sequencing of nucleic acid, such as short DNA sequences from clonally amplified single-molecule arrays.
Owner:ILLUMINA INC
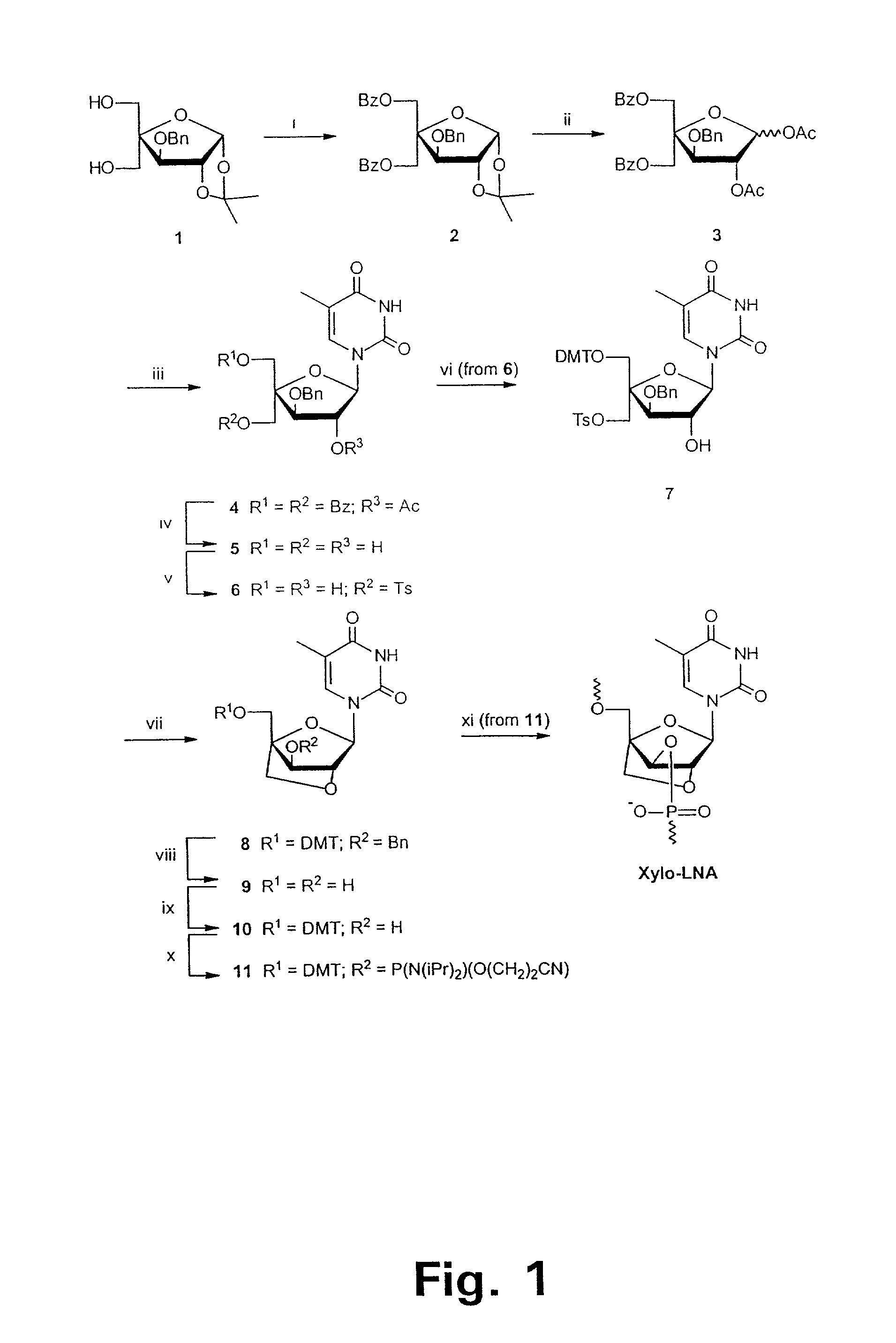
Xylo-LNA analogues
Based on the above and on the remarkable properties of the 2′-O,4′-C-methylene bridged LNA monomers it was decided to synthesise oligonucleotides comprising one or more 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer(s) as the first stereoisomer of LNA modified oligonucleotides. Modelling clearly indicated the xylo-LNA monomers to be locked in an N-type furanose conformation. Whereas the parent 2′-deoxy-β-D-xylofuranosyl nucleosides were shown to adopt mainly an N-type furanose conformation, the furanose ring of the 2′-deoxy-β-D-xylofuranosyl monomers present in xylo-DNA were shown by conformational analysis and computer modelling to prefer an S-type conformation thereby minimising steric repulsion between the nucleobase and the 3′-O-phopshate group (Seela, F.; Wömer, Rosemeyer, H. Helv. Chem. Acta 1994, 77, 883). As no report on the hybridisation properties and binding mode of xylo-configurated oligonucleotides in an RNA context was believed to exist, it was the aim to synthesise 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer and to study the thermal stability of oligonucleotides comprising this monomer. The results showed that fully modified or almost fully modified Xylo-LNA is useful for high-affinity targeting of complementary nucleic acids. When taking into consideration the inverted stereochemistry at C-3′ this is a surprising fact. It is likely that Xylo-LNA monomers, in a sequence context of Xylo-DNA monomers, should have an affinity-increasing effect.
Owner:QIAGEN GMBH
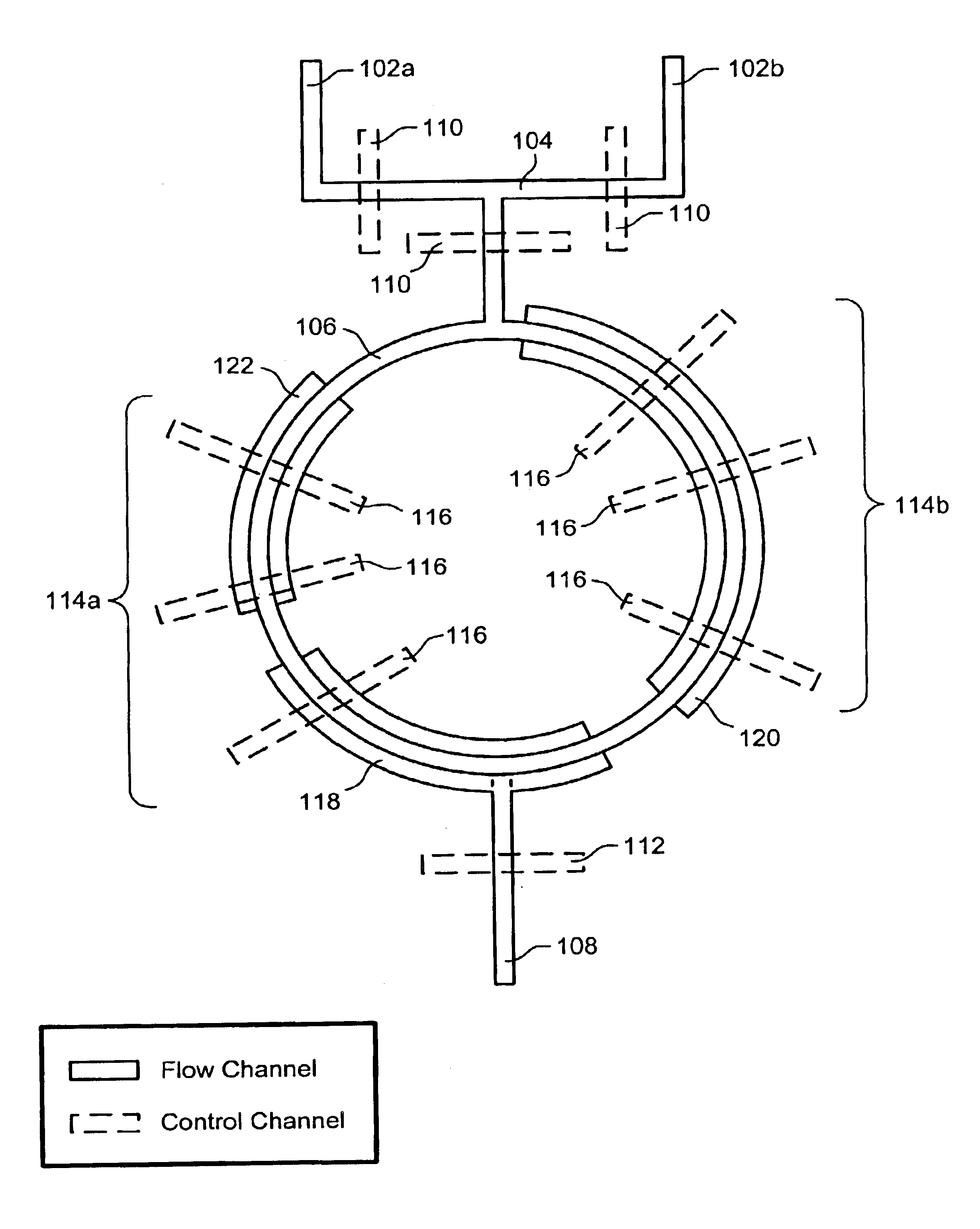
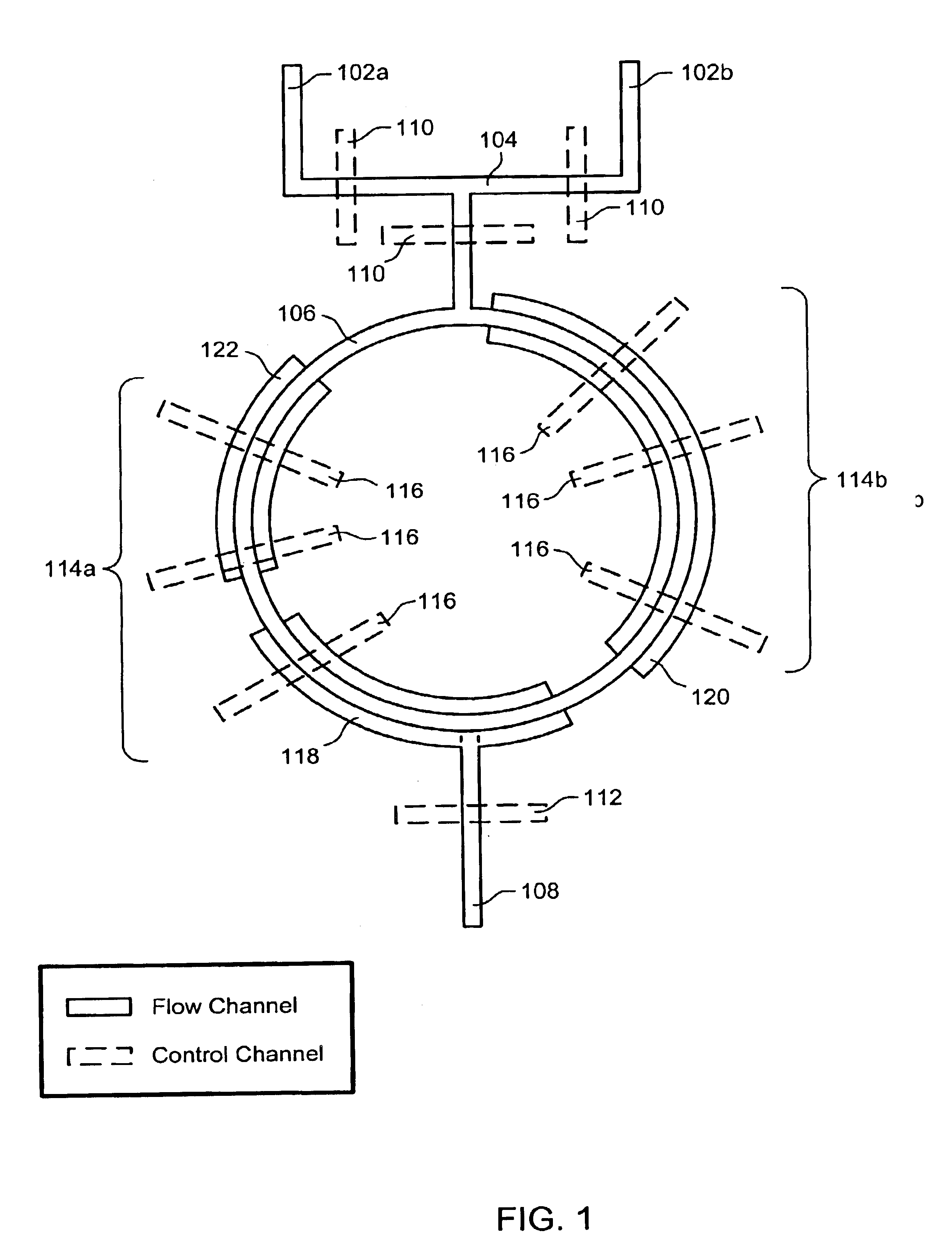
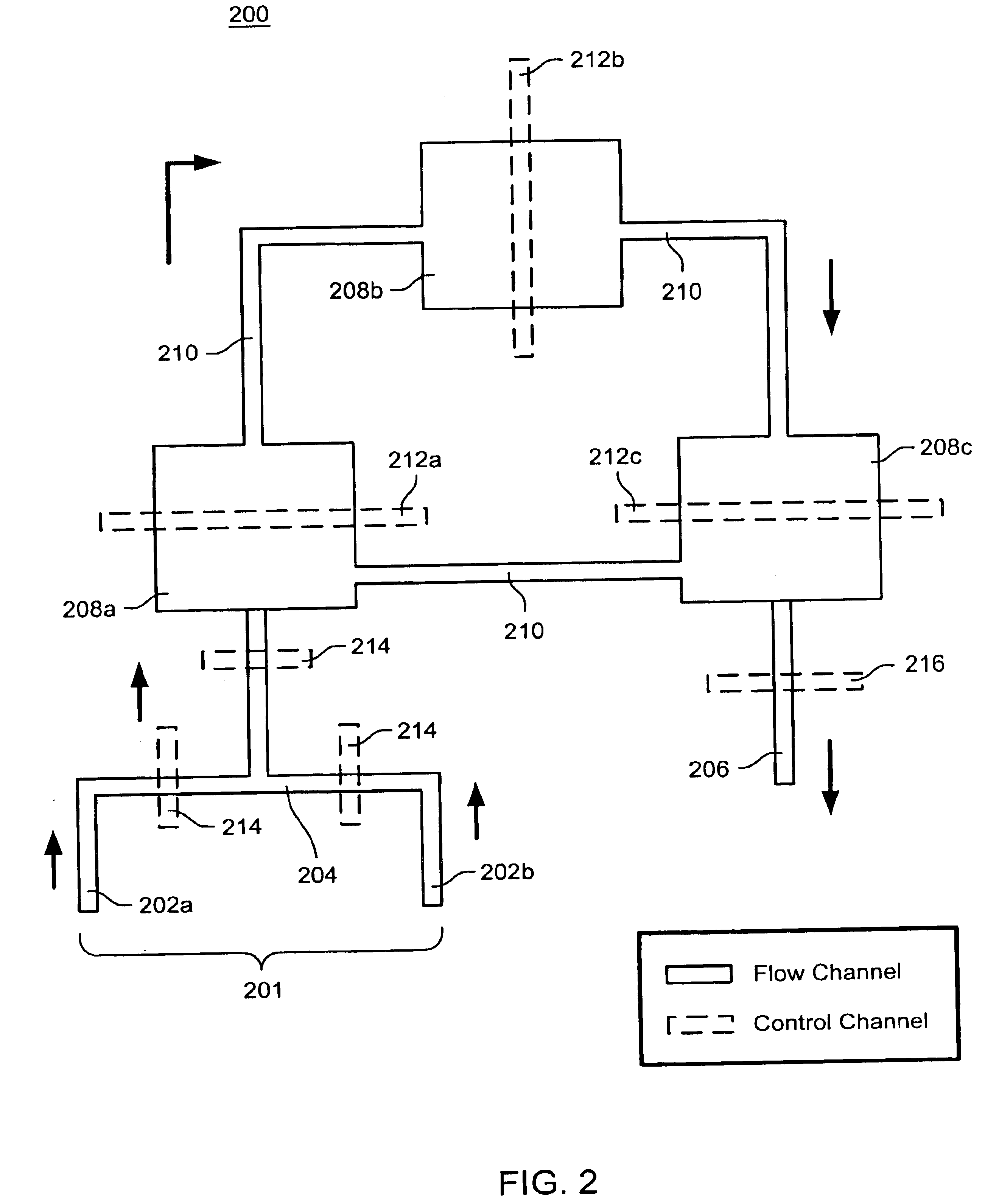
Nucleic acid amplification utilizing microfluidic devices
InactiveUS6960437B2Bioreactor/fermenter combinationsBiological substance pretreatmentsRegulation temperatureEngineering
The present invention provides microfluidic devices and methods using the same in various types of thermal cycling reactions. Certaom devices include a rotary microfluidic channel and a plurality of temperature regions at different locations along the rotary microfluidic channel at which temperature is regulated. Solution can be repeatedly passed through the temperature regions such that the solution is exposed to different temperatures. Other microfluidic devices include an array of reaction chambers formed by intersecting vertical and horizontal flow channels, with the ability to regulate temperature at the reaction chambers. The microfluidic devices can be used to conduct a number of different analyses, including various primer extension reactions and nucleic acid amplification reactions.
Owner:CALIFORNIA INST OF TECH
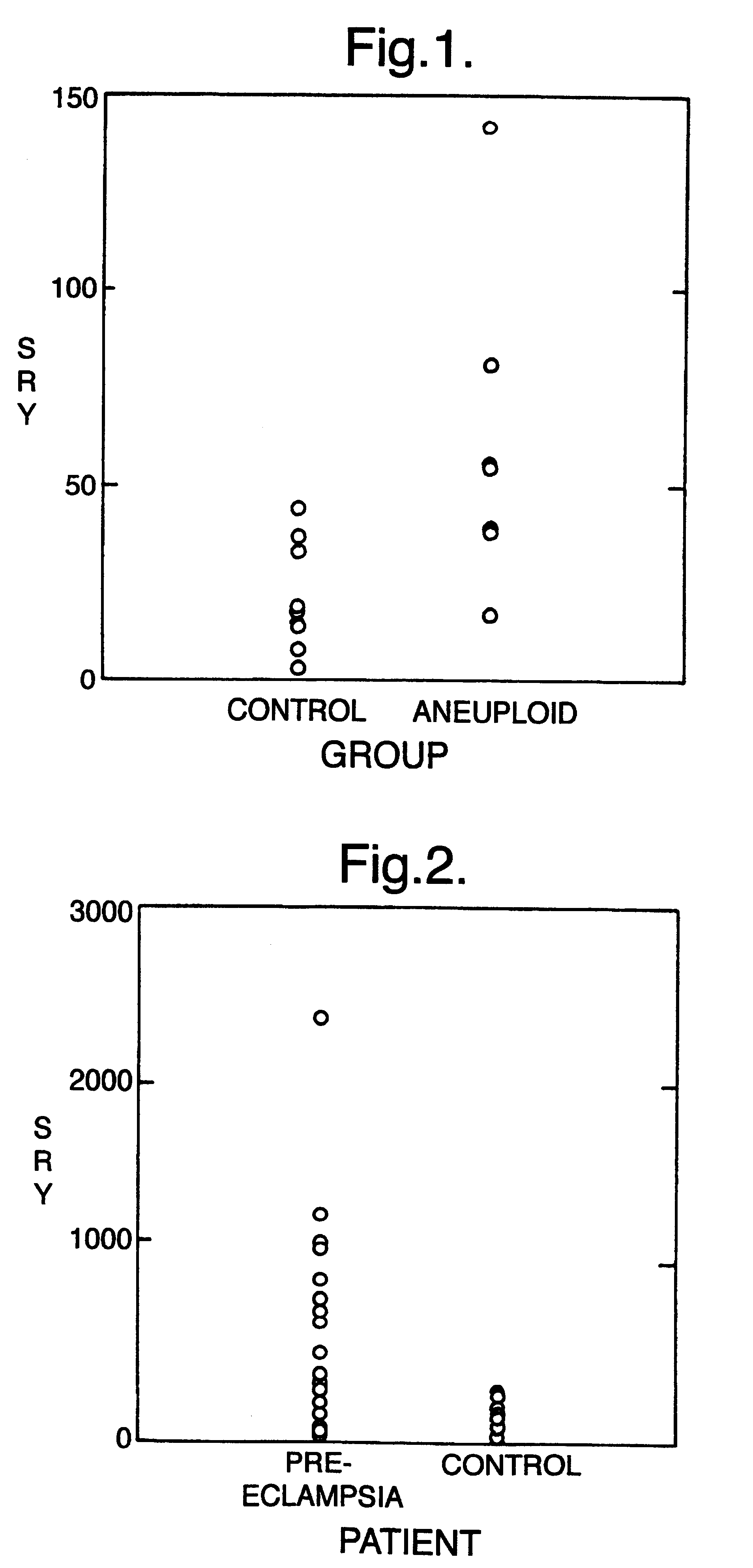
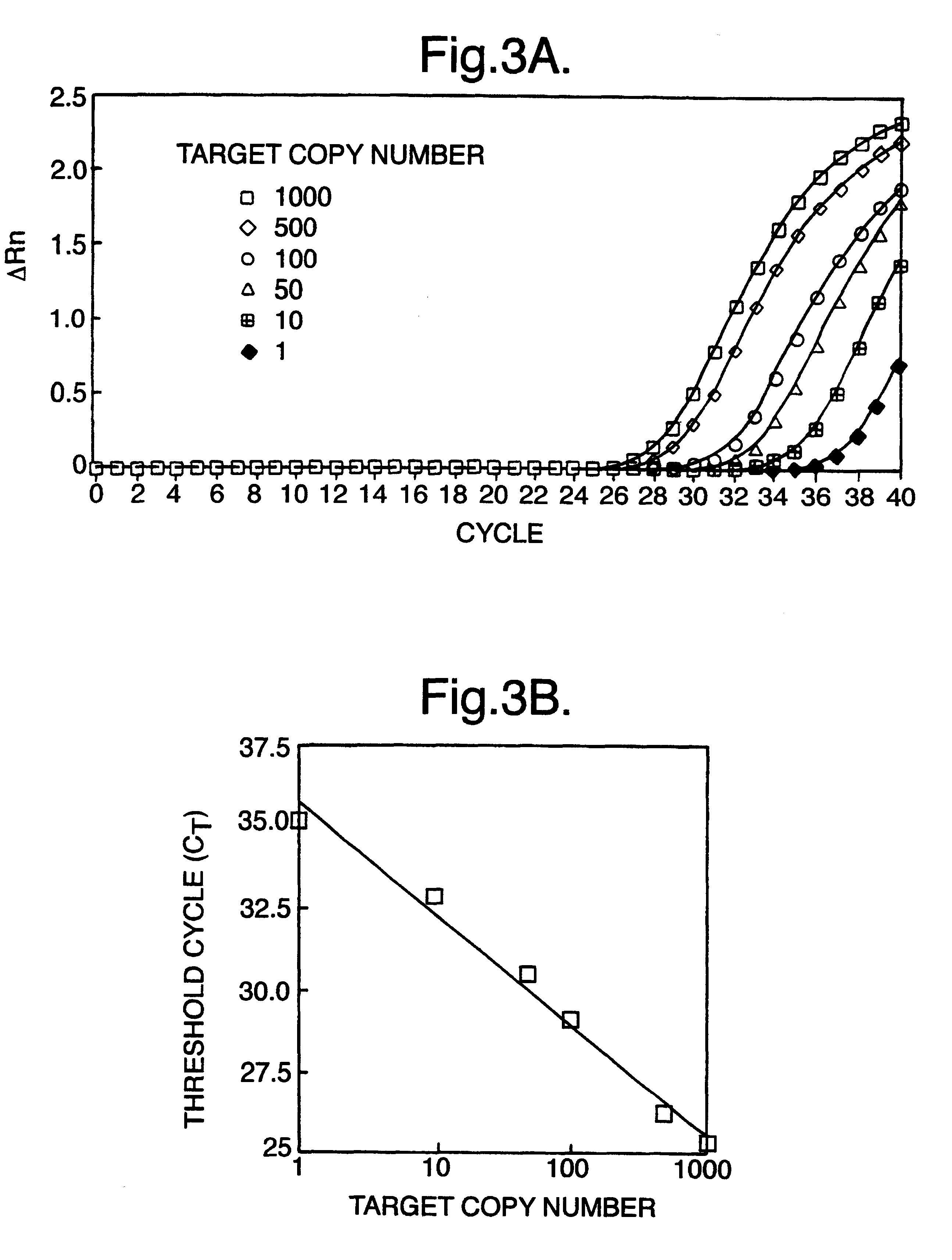
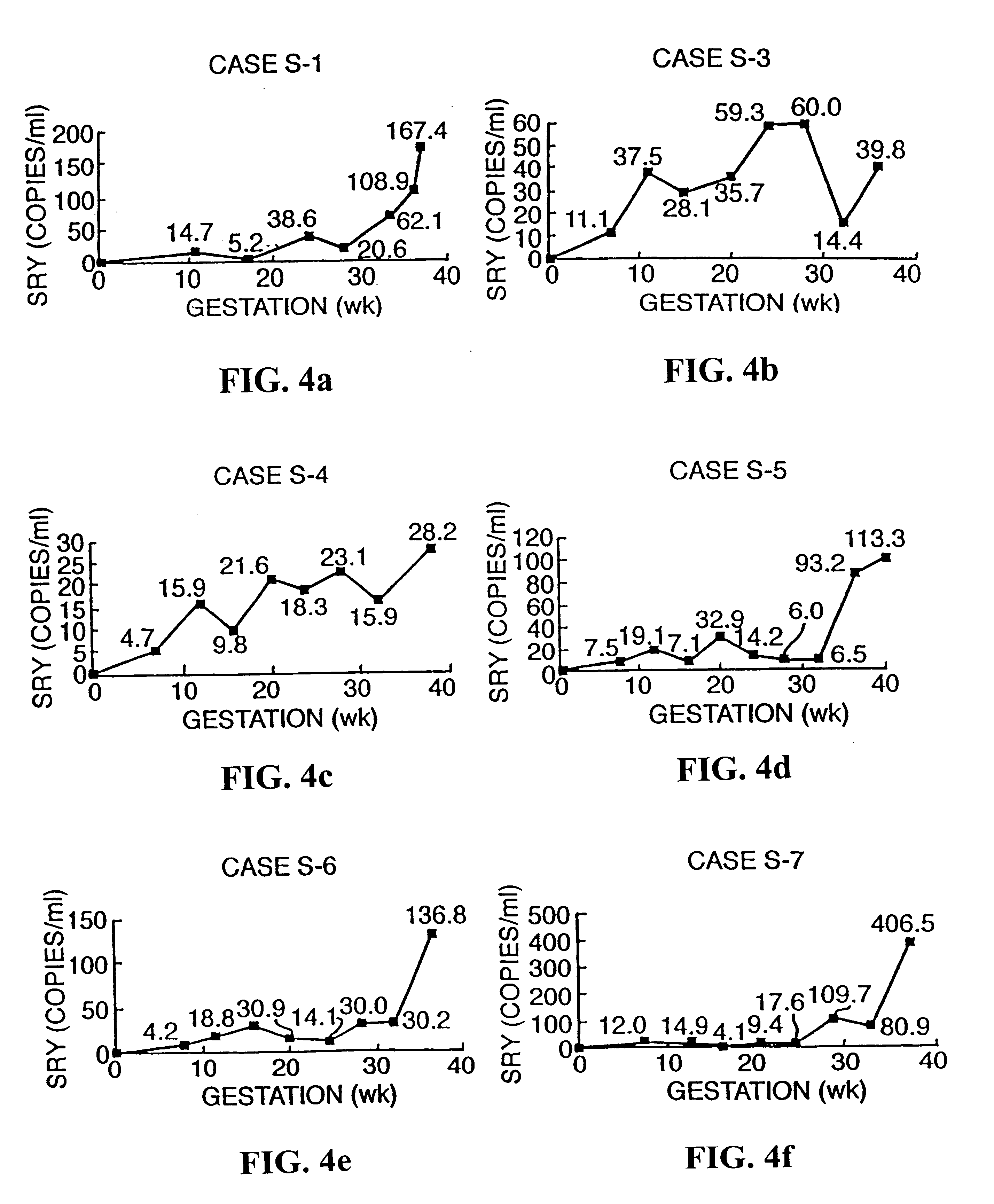
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
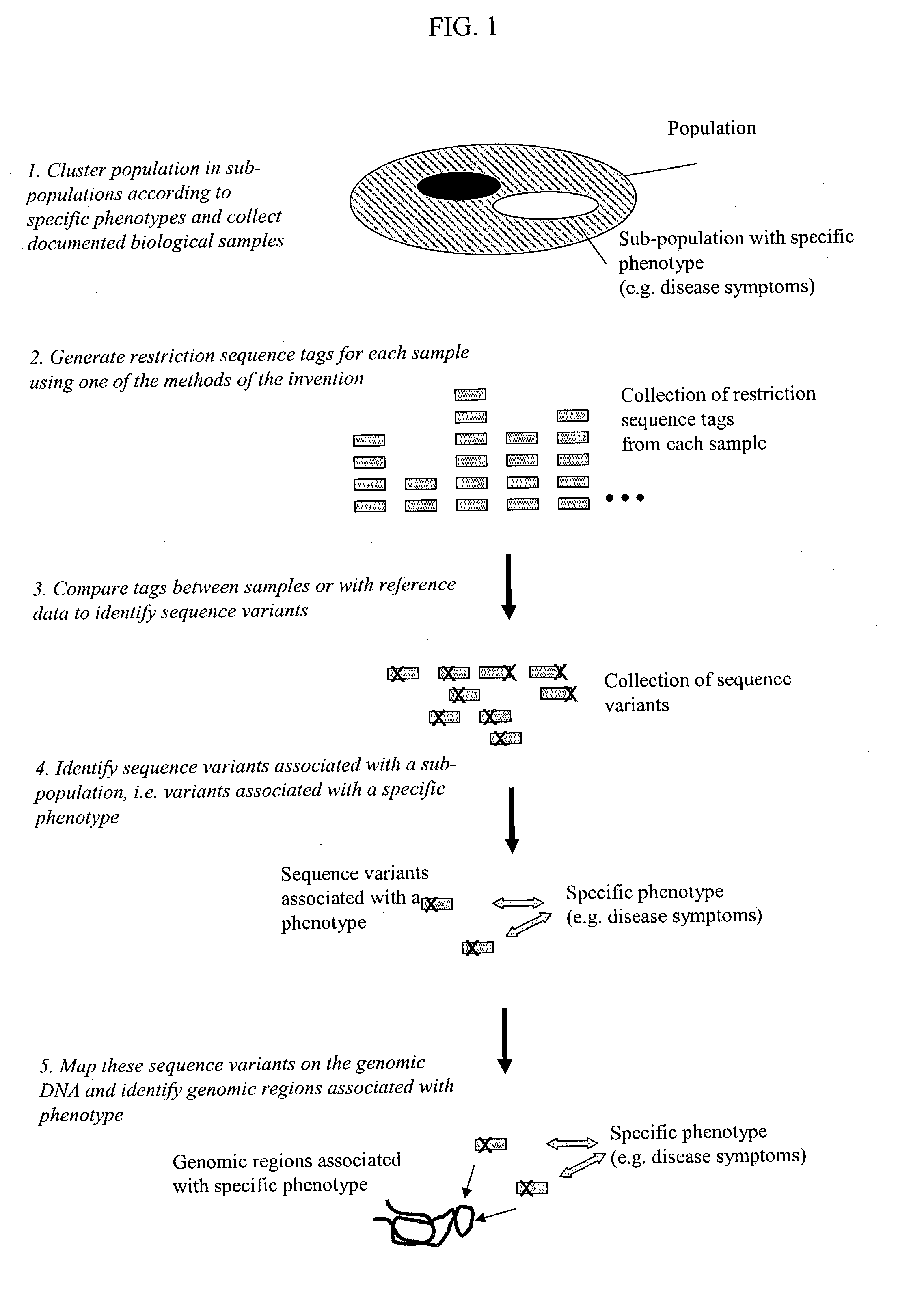
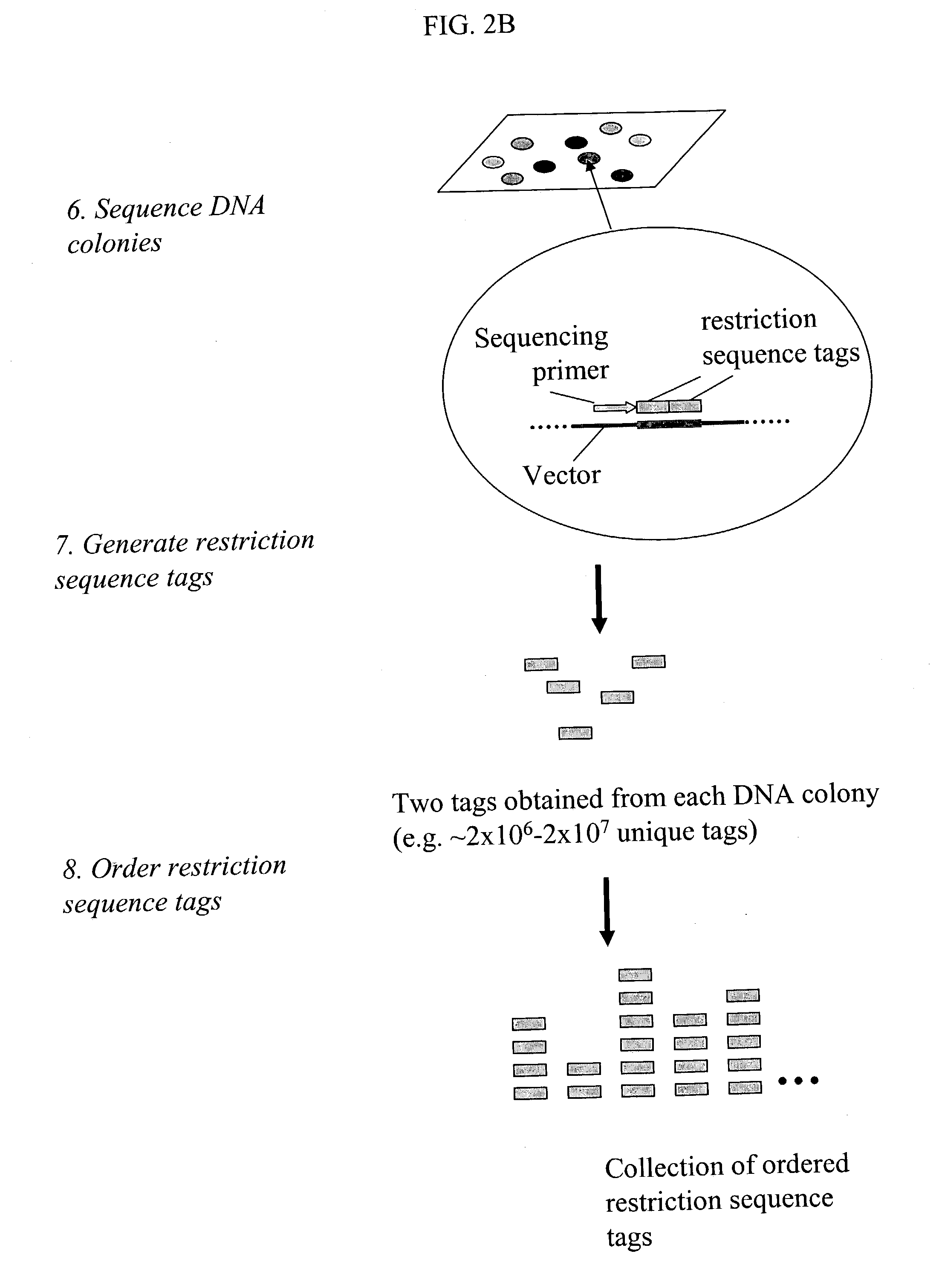
Methods for detecting genome-wide sequence variations associated with a phenotype
InactiveUS20040002090A1Microbiological testing/measurementFermentationSub populationsGenetic risk factor
The invention provides methods for determining genome-wide sequence variations associated with a phenotype of a species in a hypothesis-free manner. In the methods of the invention, a set of restriction fragments for each of a sub-population of individuals having the phenotype are generated by digesting nucleic acids from the individual using one or more different restriction enzymes. A set of restriction sequence tags for the individual is then determined from the set of restriction fragments. The restriction sequence tags for the sub-population of organisms are compared and grouped into one or more groups, each of which comprising restriction sequence tags that comprise homologous sequences. The obtained one or more groups of restriction sequence tags identify the sequence variations associated with the phenotype. The methods of the invention can be used for, e.g., analysis of large numbers of sequence variants in many patient samples to identify subtle genetic risk factors.
Owner:SOLEXA
Nucleic acid amplification oligonucleotides with molecular energy transfer labels and methods based thereon
InactiveUS6117635AReduce the possibilityRapid and sensitive and reliableSugar derivativesMicrobiological testing/measurementEnergy transferFluorophore
The present invention provides labeled nucleic acid amplification oligonucleotides, which can be linear or hairpin primers or blocking oligonucleotides. The oligonucleotides of the invention are labeled with donor and / or acceptor moieties of molecular energy transfer pairs. The moieties can be fluorophores, such that fluorescent energy emitted by the donor is absorbed by the acceptor. The acceptor may be a fluorophore that fluoresces at a wavelength different from the donor moiety, or it may be a quencher. The oligonucleotides of the invention are configured so that a donor moiety and an acceptor moiety are incorporated into the amplification product. The invention also provides methods and kits for directly detecting amplification products employing the nucleic acid amplification primers. When labeled linear primers are used, treatment with exonuclease or by using specific temperature eliminates the need for separation of unincorporated primers. This "closed-tube" format greatly reduces the possibility of carryover contamination with amplification products, provides for high throughput of samples, and may be totally automated.
Owner:MILLIPORE CORP
Systems for in vivo site-directed mutagenesis using oligonucleotides
InactiveUS20040171154A1Large deletionImprove applicabilitySugar derivativesMicrobiological testing/measurementHeterologousSite-directed mutagenesis
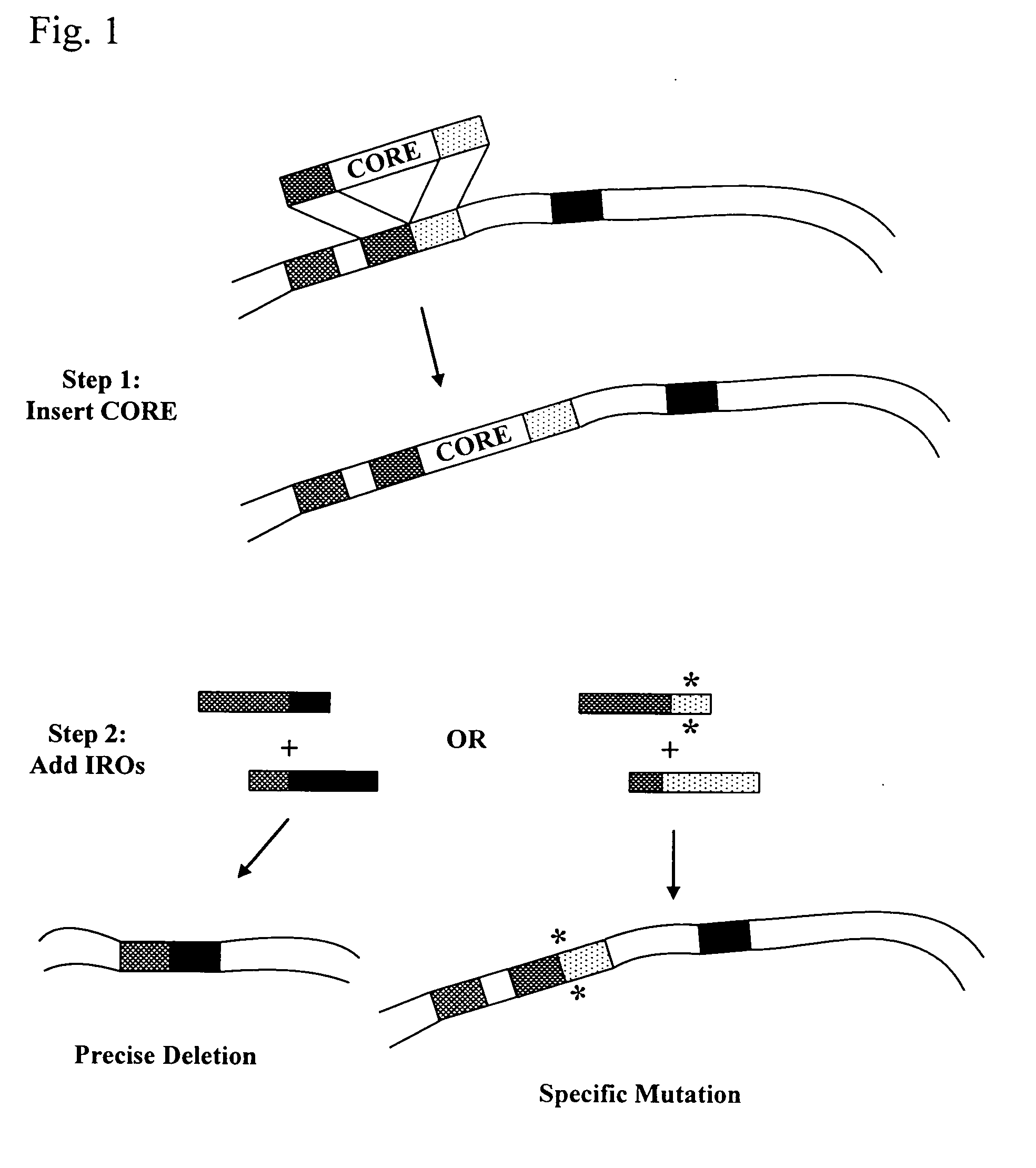
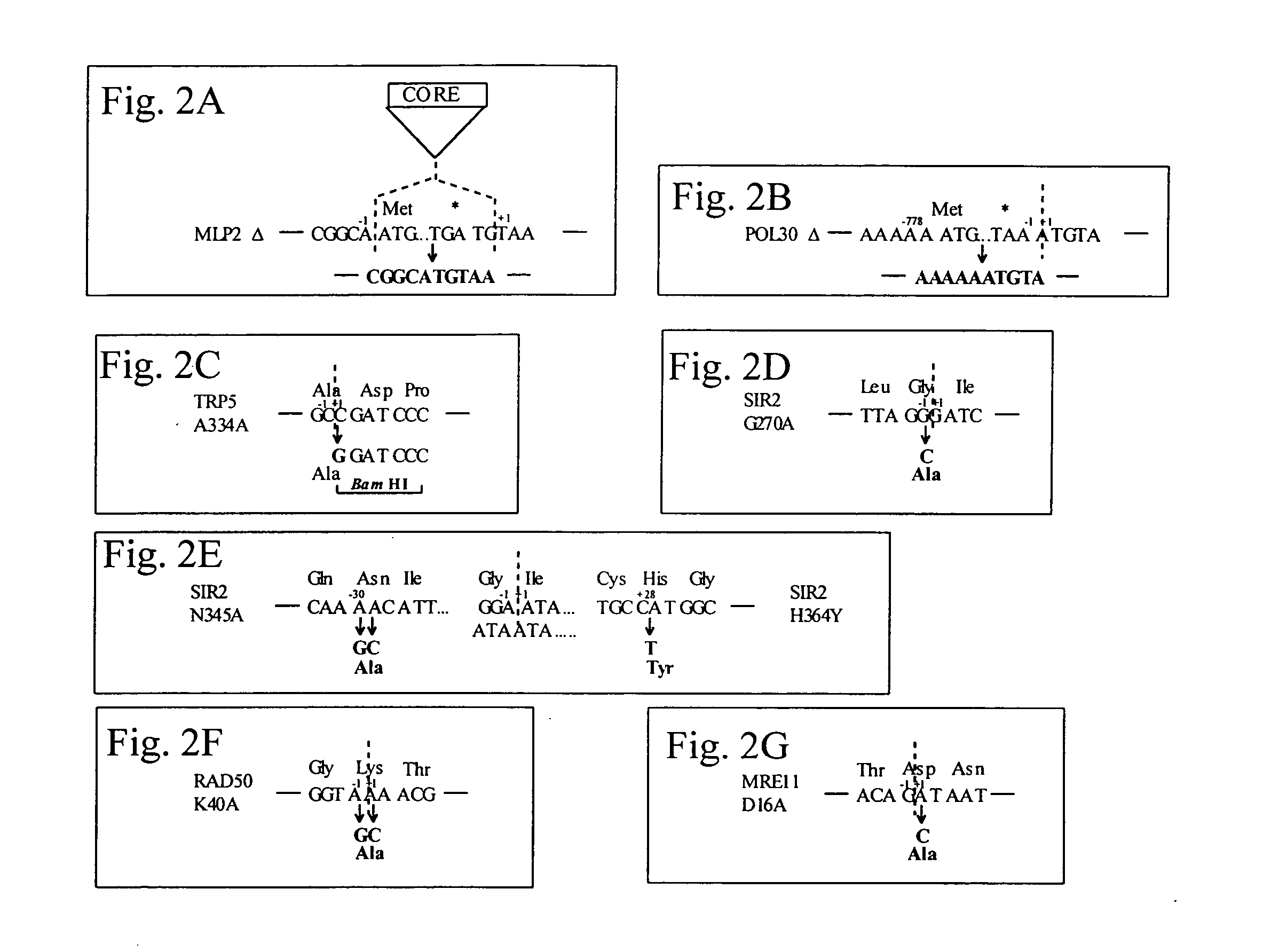
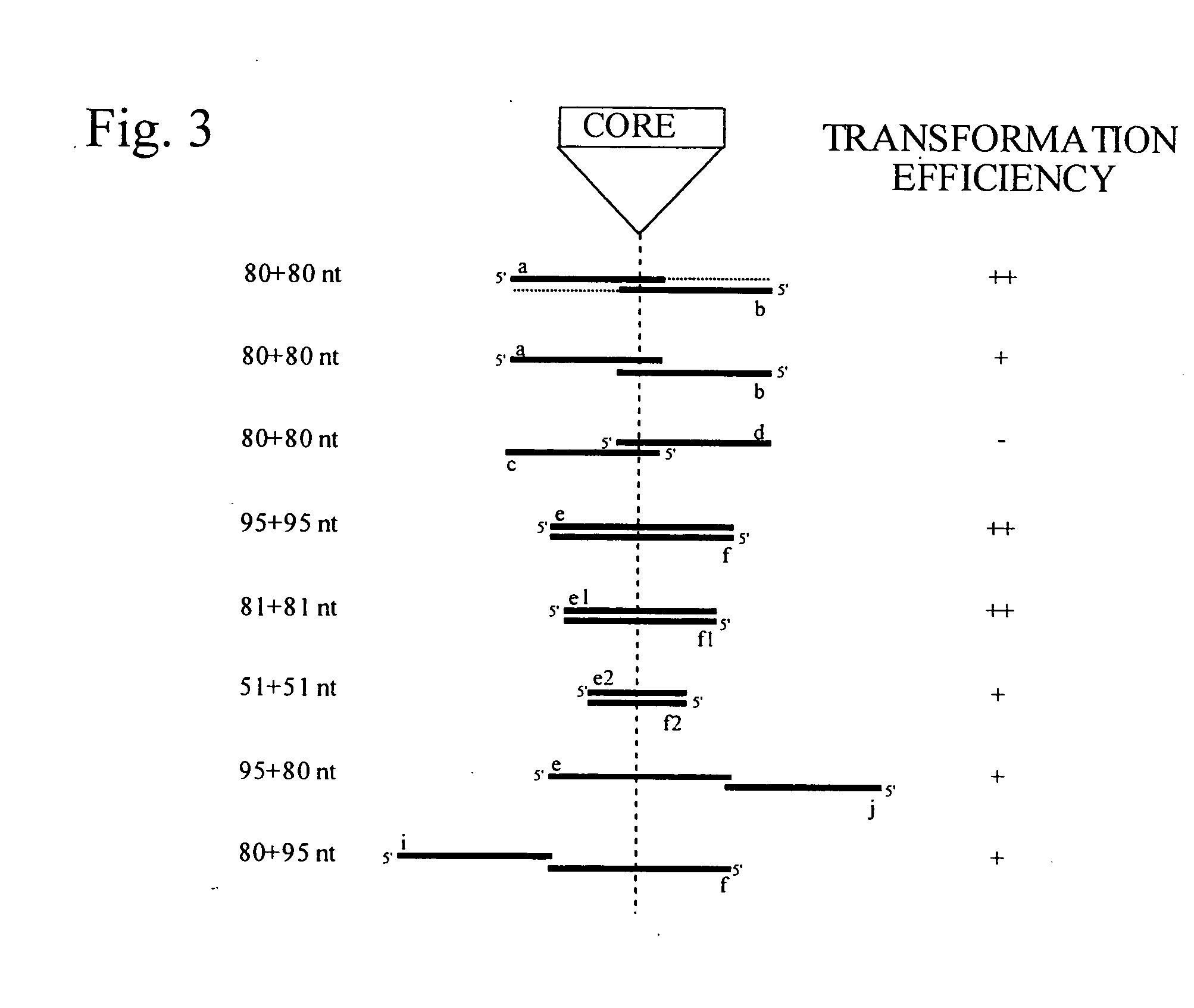
This disclosure provides several methods to generate nucleic acid mutations in vivo, for instance in such a way that no heterologous sequence is retained after the mutagenesis is complete. The methods employ integrative recombinant oligonucleotides (IROs). Specific examples of the described mutagenesis methods enable site-specific point mutations, deletions, and insertions. Also provided are methods that enable multiple rounds of mutation and random mutagenesis in a localized region. The described methods are applicable to any organism that has a homologous recombination system.
Owner:HEALTH & HUMAN SERVICES DEPT OF THE GOVERNMENT OF THE US SEC THE
Non-planar microstructures for manipulation of fluid samples
This invention comprises an apparatus and method for the manipulation of materials, including particles, cells, macromolecules, such as proteins, nucleic acids and other moieties, in fluid samples. The apparatus comprises an enclosed chamber on a chip having an internal microstructure with surface area substantially greater than the facial surface area of the internal structure. Generally the internal microstructure comprises a continuous network of channels, each of which has a depth substantially greater than its width. The network may comprise a single channel, a single channel with multiple branches, multiple channels, multiple channels with multiple branches, and any combination thereof. The internal structure may present an inert, non-reactive surface, or be coated with a reactive ligand, or be electrically conductive and optionally be coated with an electrical insulator. Discrete portions of the internal structure may differ in structural and surface properties. Multiple chips may be linked together to create a multiplexed array of chambers, optionally linked to other analytical devices.
Owner:CEPHEID INC
Scaffolded nucleic acid polymer particles and methods of making and using
ActiveUS20100304982A1Bioreactor/fermenter combinationsSequential/parallel process reactionsParticle compositionPolynucleotide
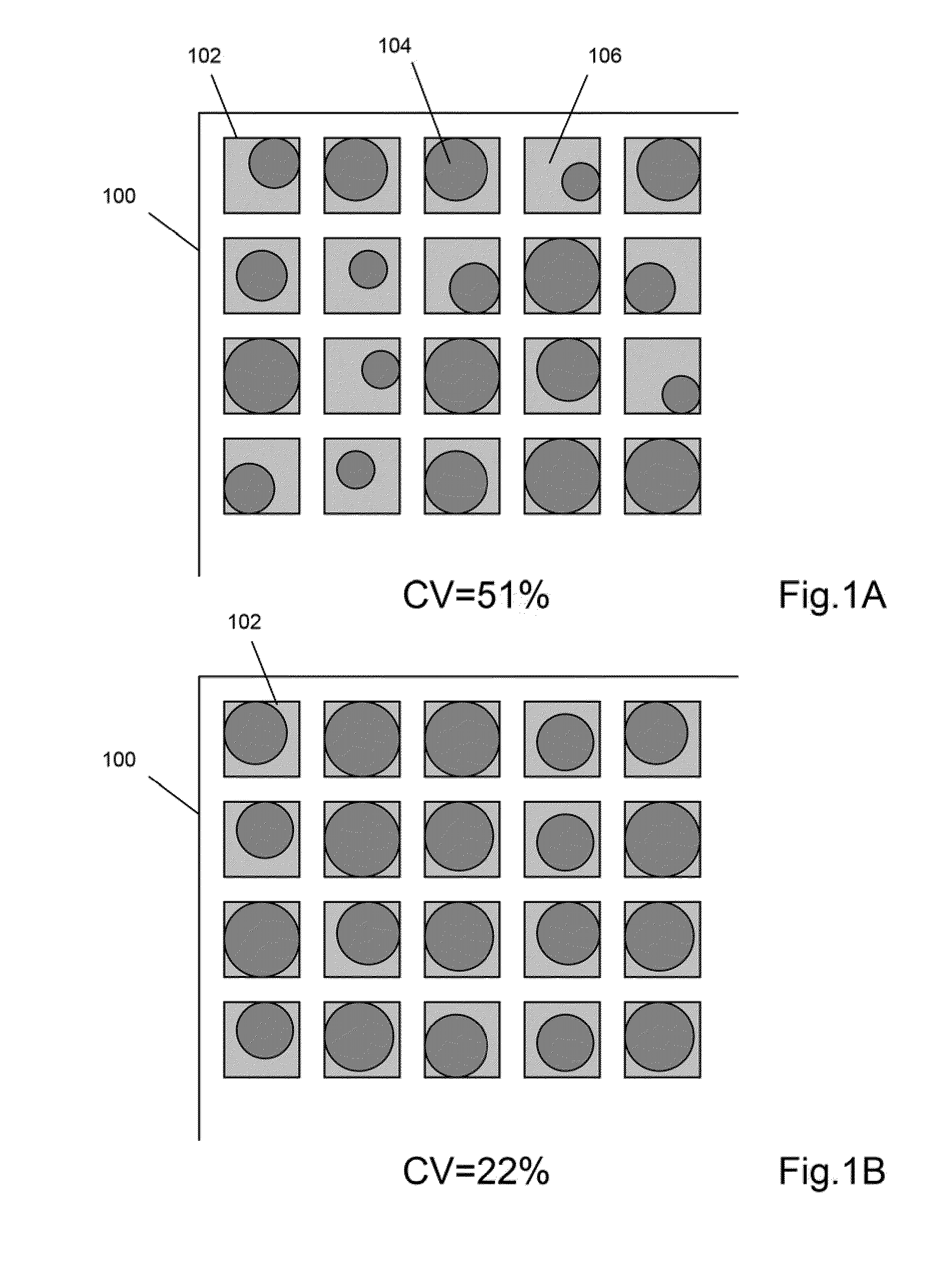
The invention provides particle compositions having applications in nucleic acid analysis. Nucleic acid polymer particles of the invention allow polynucleotides to be attached throughout their volumes for higher loading capacities than those achievable solely with surface attachment. In one aspect, nucleic acid polymer particles of the invention comprise polyacrylamide particles with uniform size distributions having low coefficients of variations, which result in reduced particle-to-particle variation in analytical assays. Such particle compositions are used in various amplification reactions to make amplicon libraries from nucleic acid fragment libraries.
Owner:LIFE TECH CORP
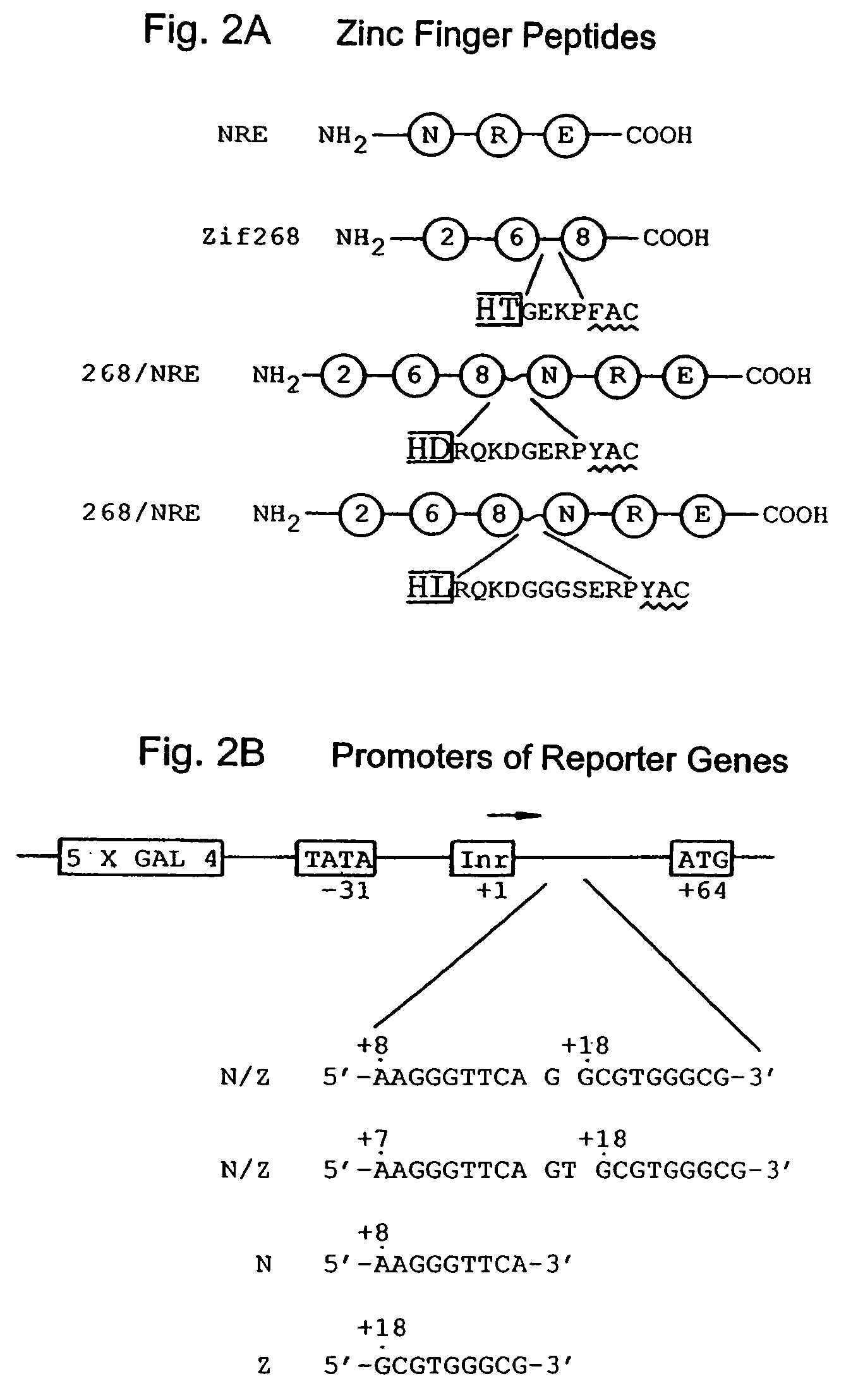
Nucleic acid encoding poly-zinc finger proteins with improved linkers
InactiveUS7153949B2Enhanced affinity and specificityImprove the level ofPeptide/protein ingredientsAntibody mimetics/scaffoldsDNA-binding domainNucleotide
Polynucleotides encoding chimeric proteins, and methods for their production and use are disclosed. The chimeric proteins comprise a flexible linker between two zinc finger DNA-binding domains, wherein the linker contains eight or more amino acids between the second conserved histidine residue of the carboxy-terminal zinc finger of the first domain and the first conserved cysteine residue of the amino-terminal zinc finger of the second domain.
Owner:MASSACHUSETTS INST OF TECH
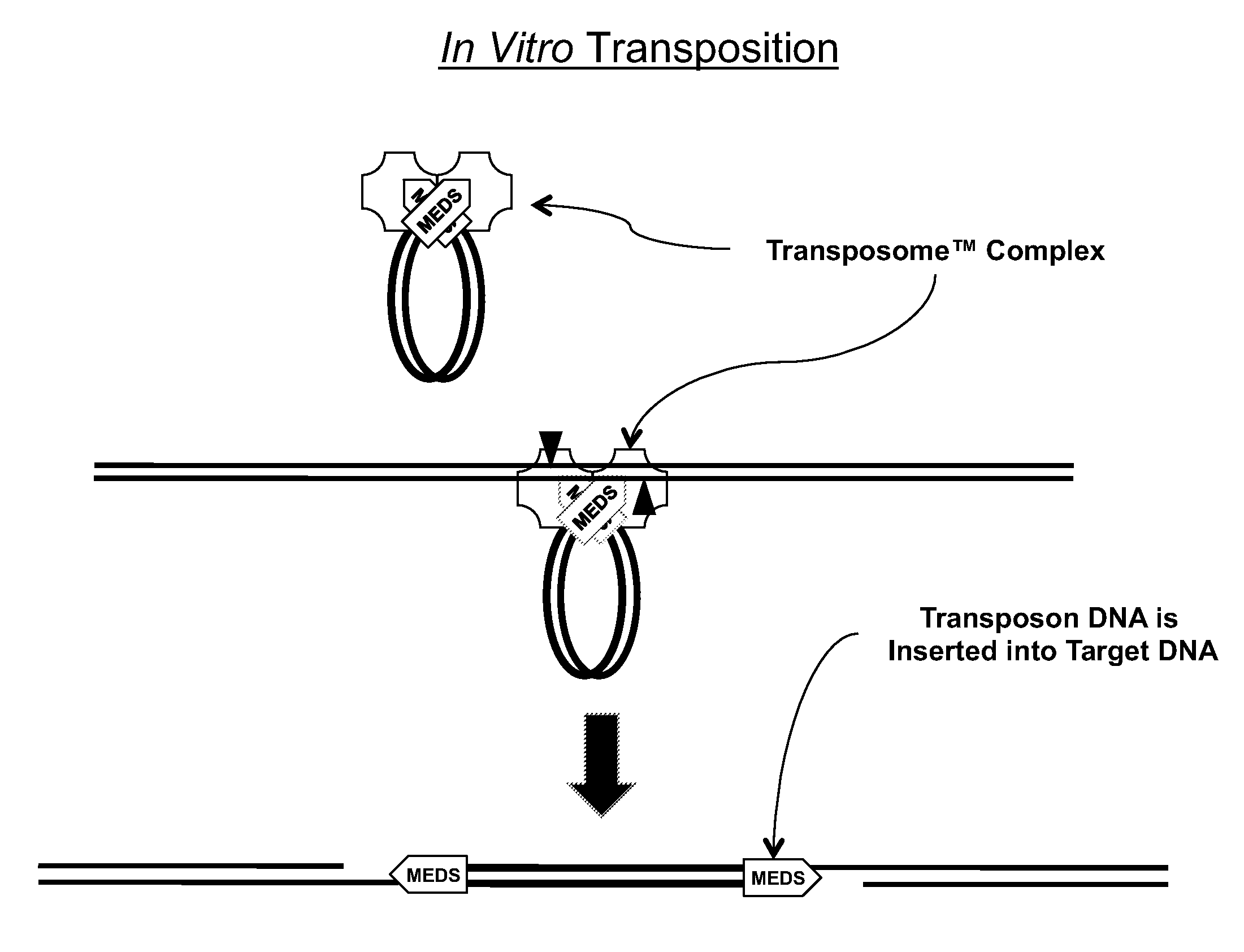
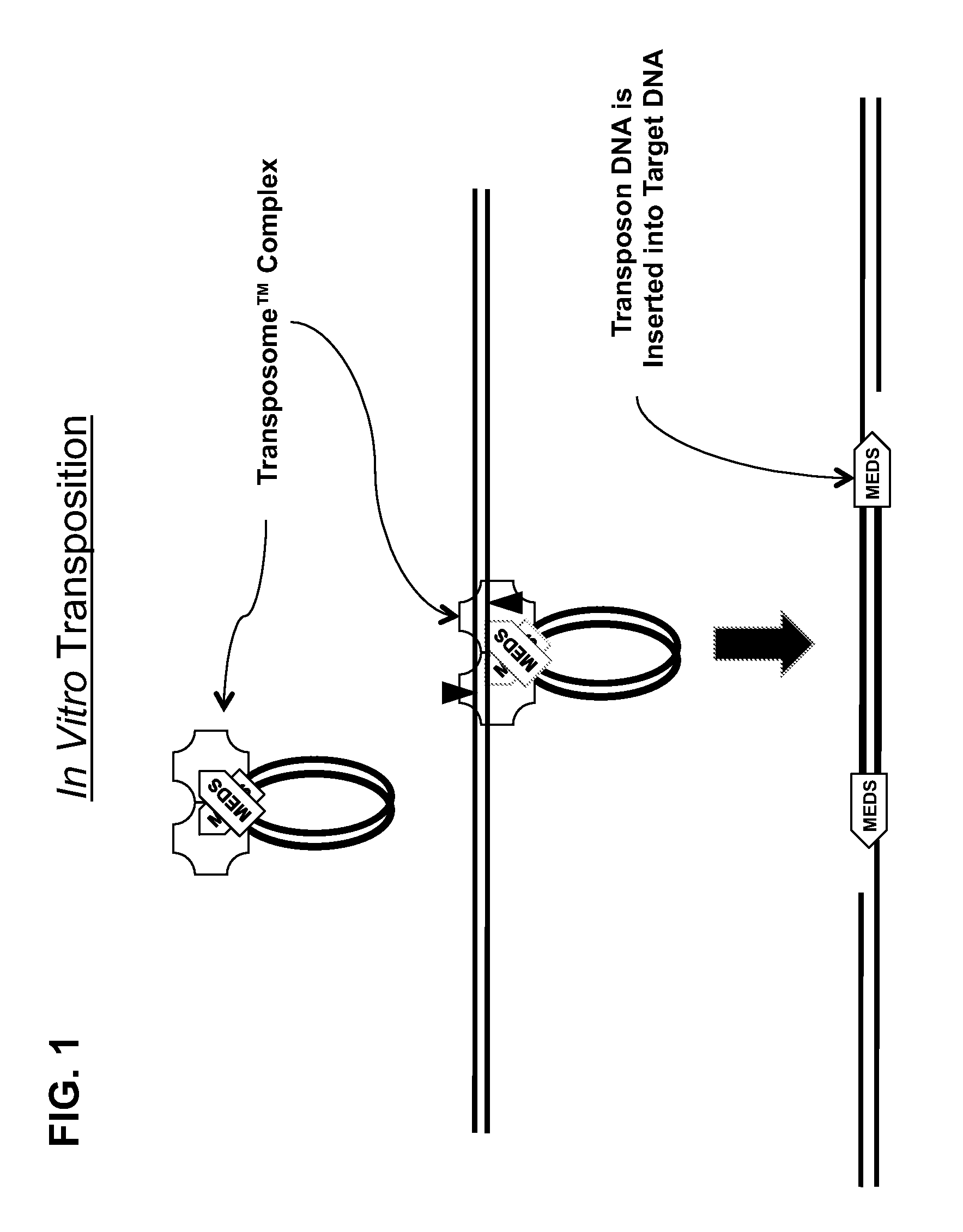
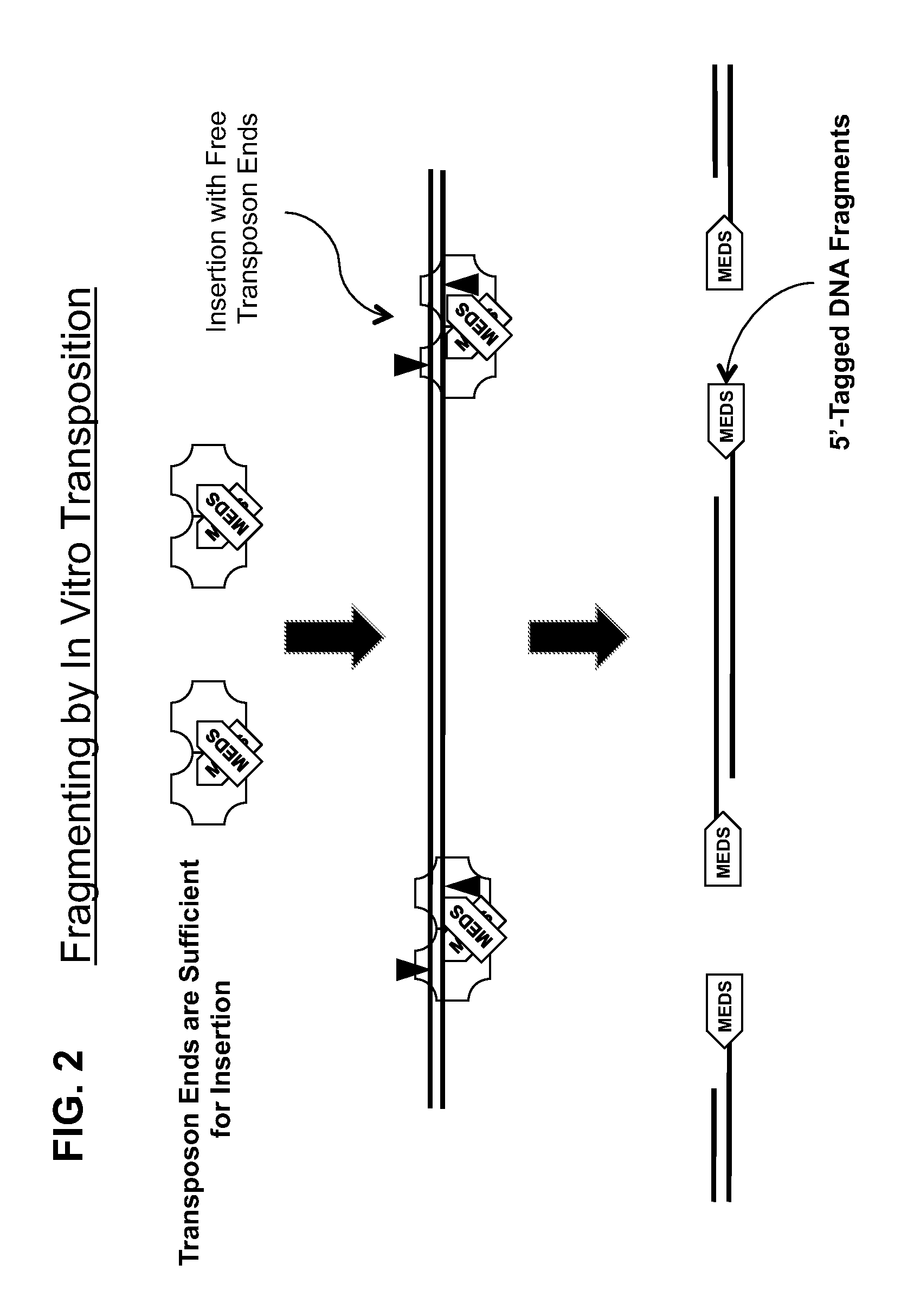
Transposon end compositions and methods for modifying nucleic acids
ActiveUS20100120098A1Sugar derivativesMicrobiological testing/measurementGenomic sequencingPolymerase L
The present invention provides methods, compositions and kits for using a transposase and a transposon end for generating extensive fragmentation and 5′-tagging of double-stranded target DNA in vitro, then using a DNA polymerase for generating 5′- and 3′-tagged single-stranded DNA fragments without performing a PCR amplification reaction, wherein the first tag on the 5′-ends exhibits the sequence of the transferred transposon end and optionally, an additional arbitrary sequence, and the second tag on the 3′-ends exhibits a different sequence from the sequence exhibited by the first tag. The method is useful for generating 5′- and 3′-tagged DNA fragments for use in a variety of processes, including processes for metagenomic analysis of DNA in environmental samples, copy number variation (CNV) analysis of DNA, and comparative genomic sequencing (CGS), including massively parallel DNA sequencing (so-called “next-generation sequencing.)
Owner:ILLUMINA INC
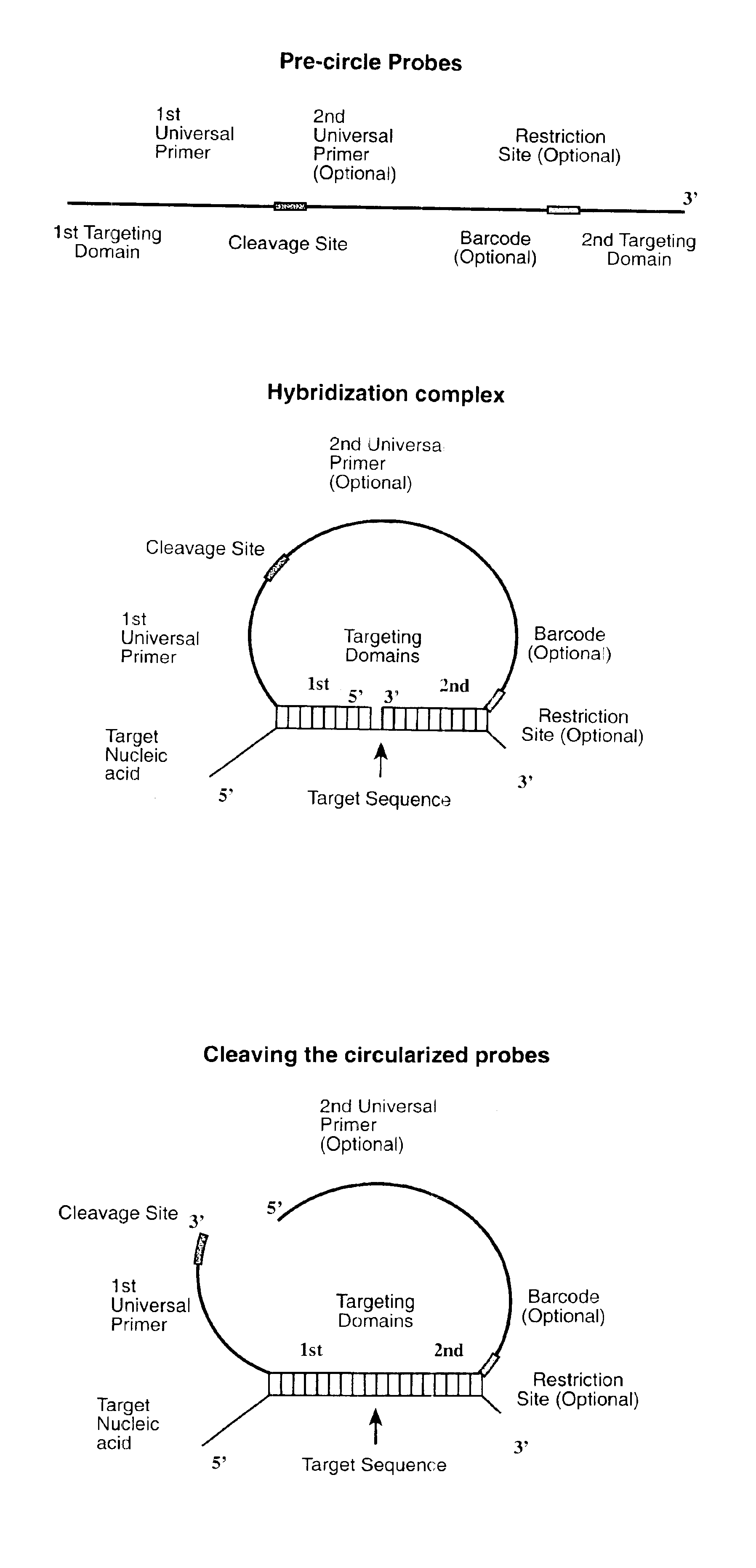
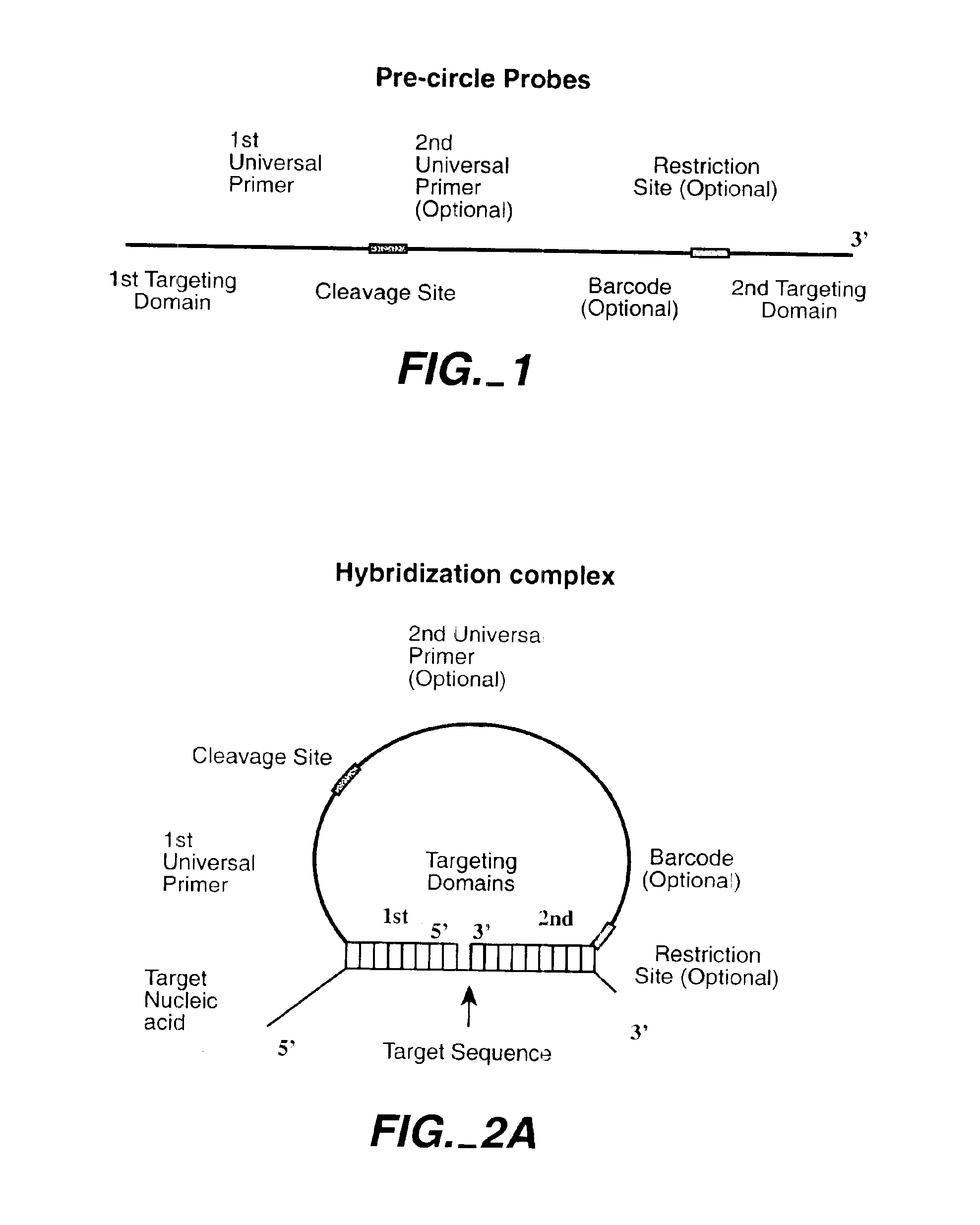
Direct multiplex characterization of genomic DNA
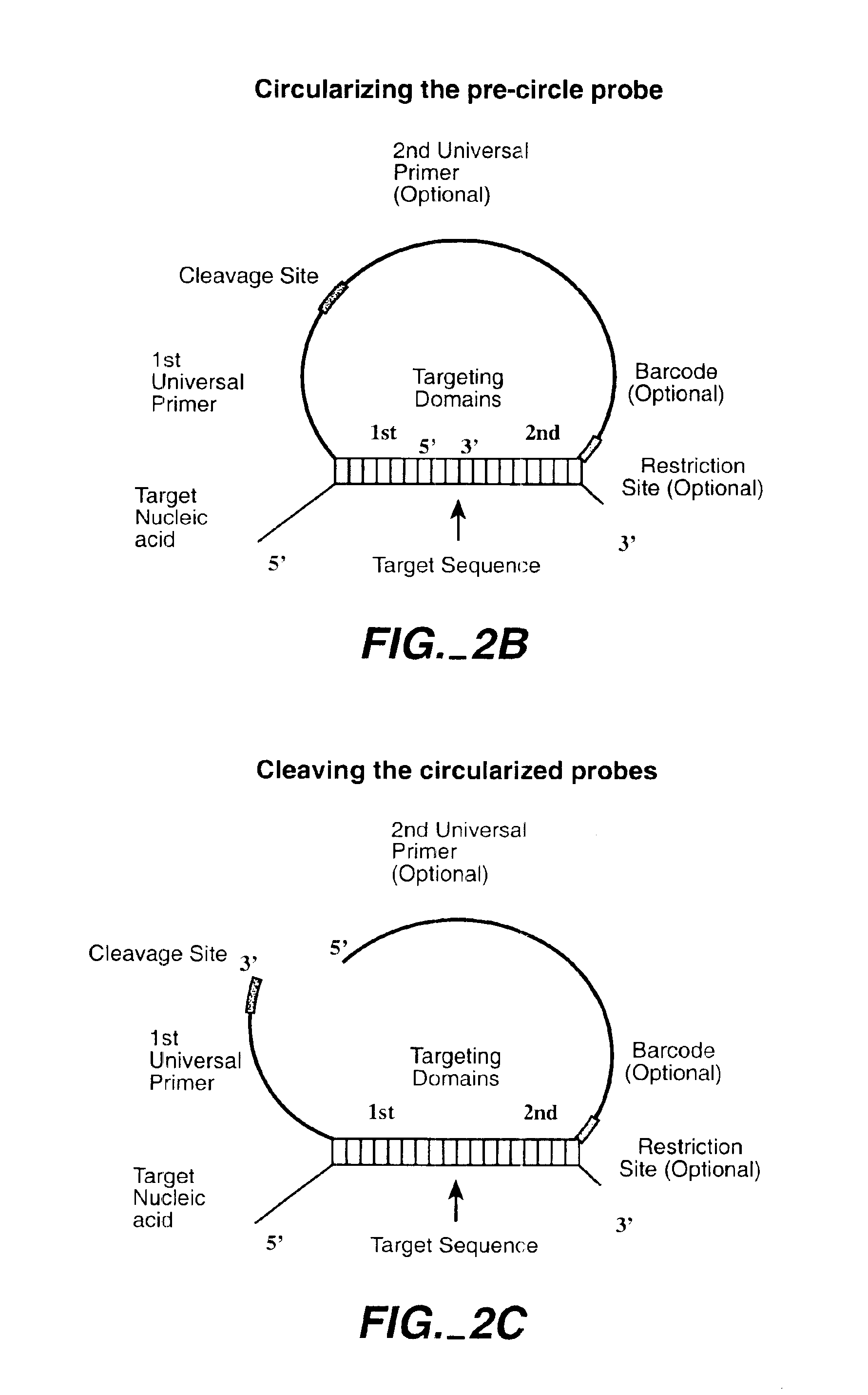
The invention is directed to novel methods of multiplexing nucleic acid reactions, including amplification, detection and genotyping. The invention relies on the use of precircle probes that are circularized in the presence of the corresponding target nucleic acids, cleaved, and then amplified.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
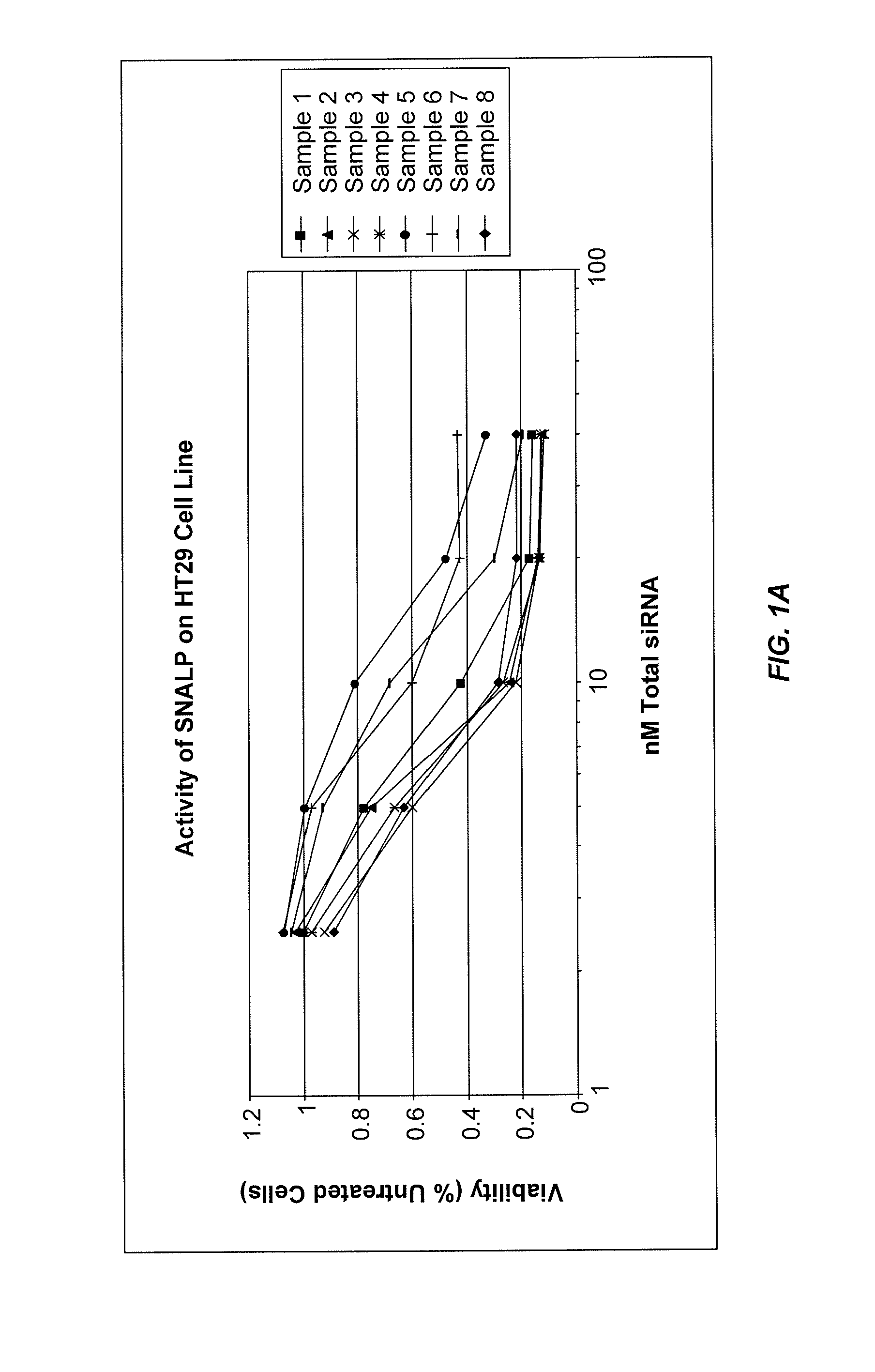
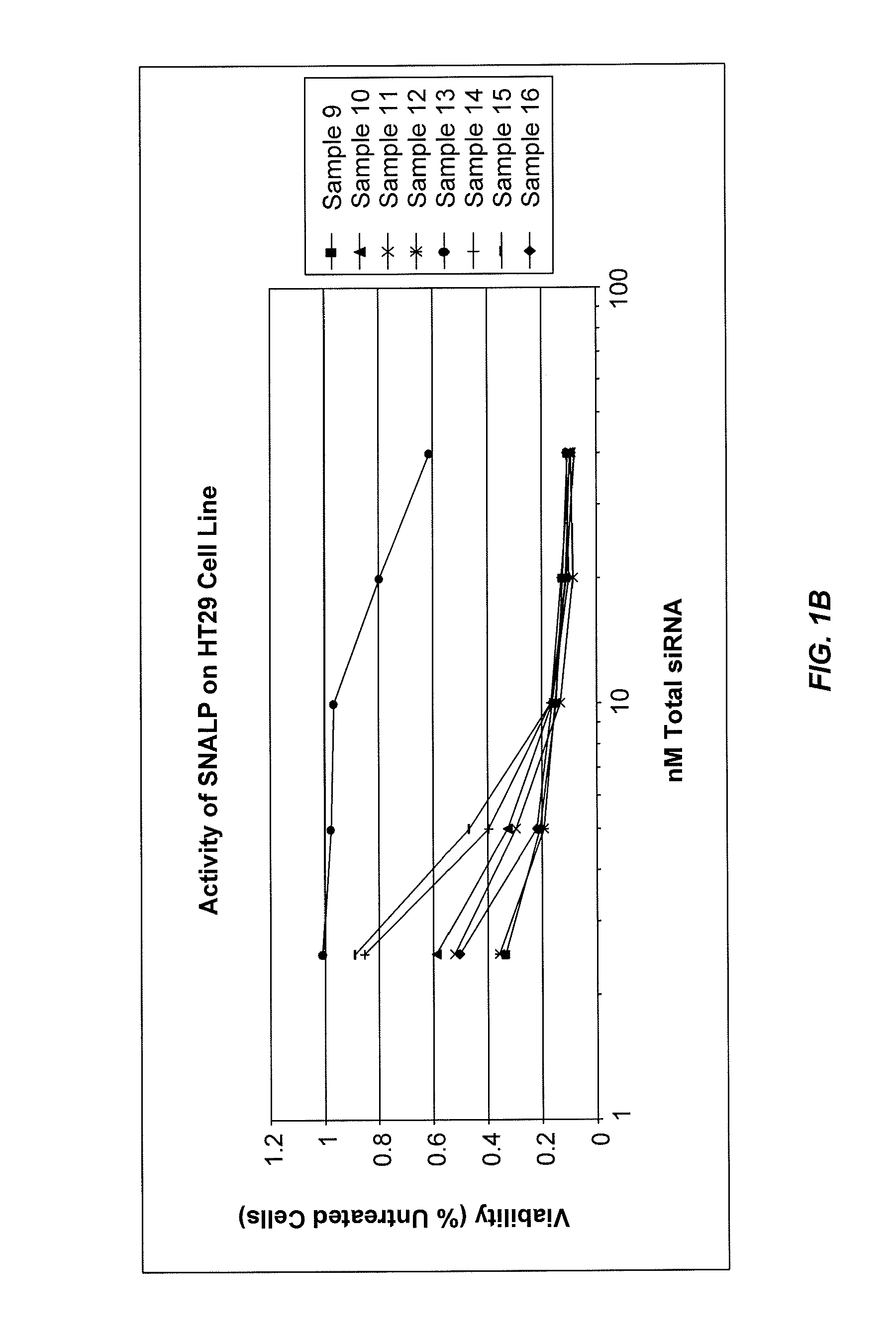
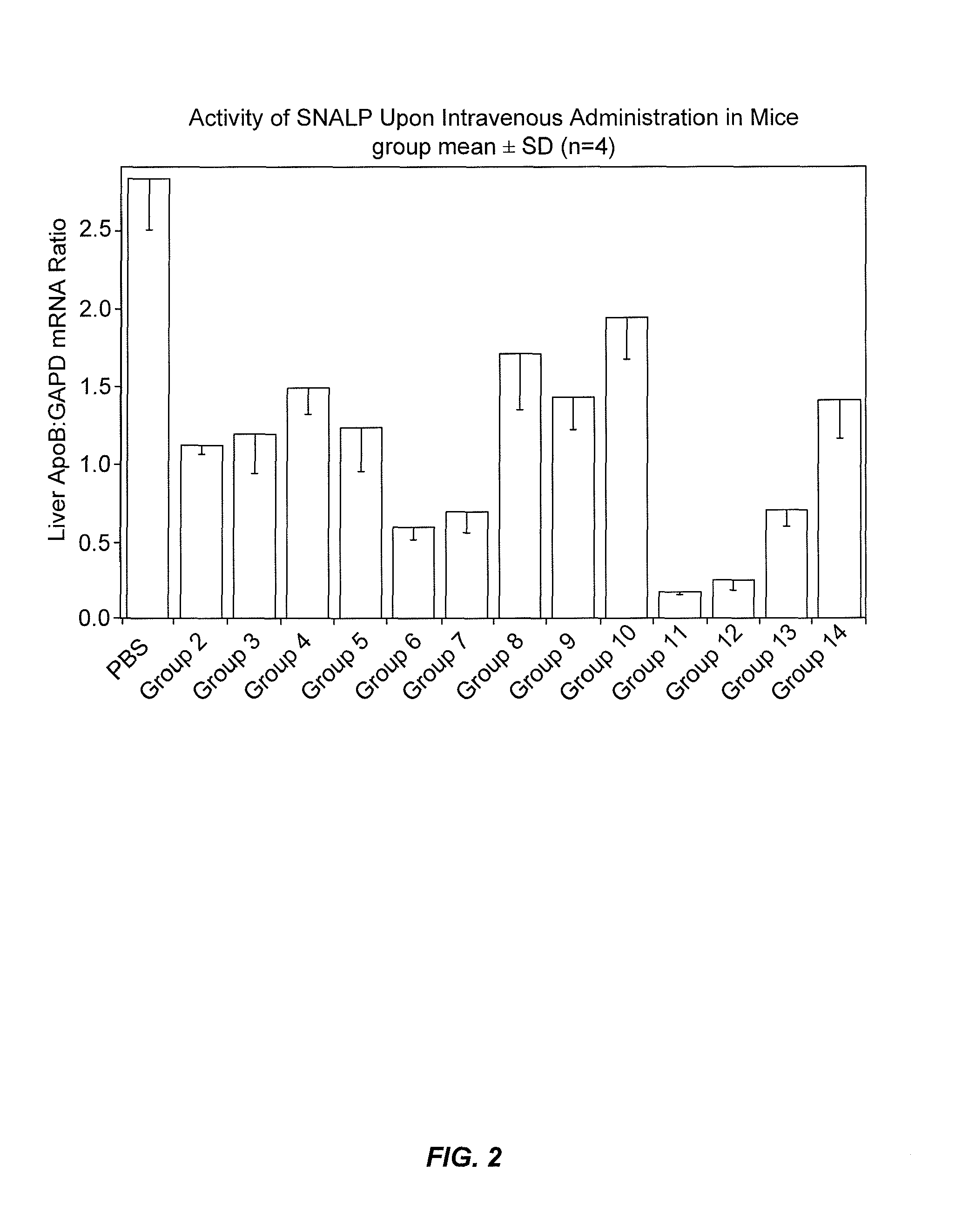
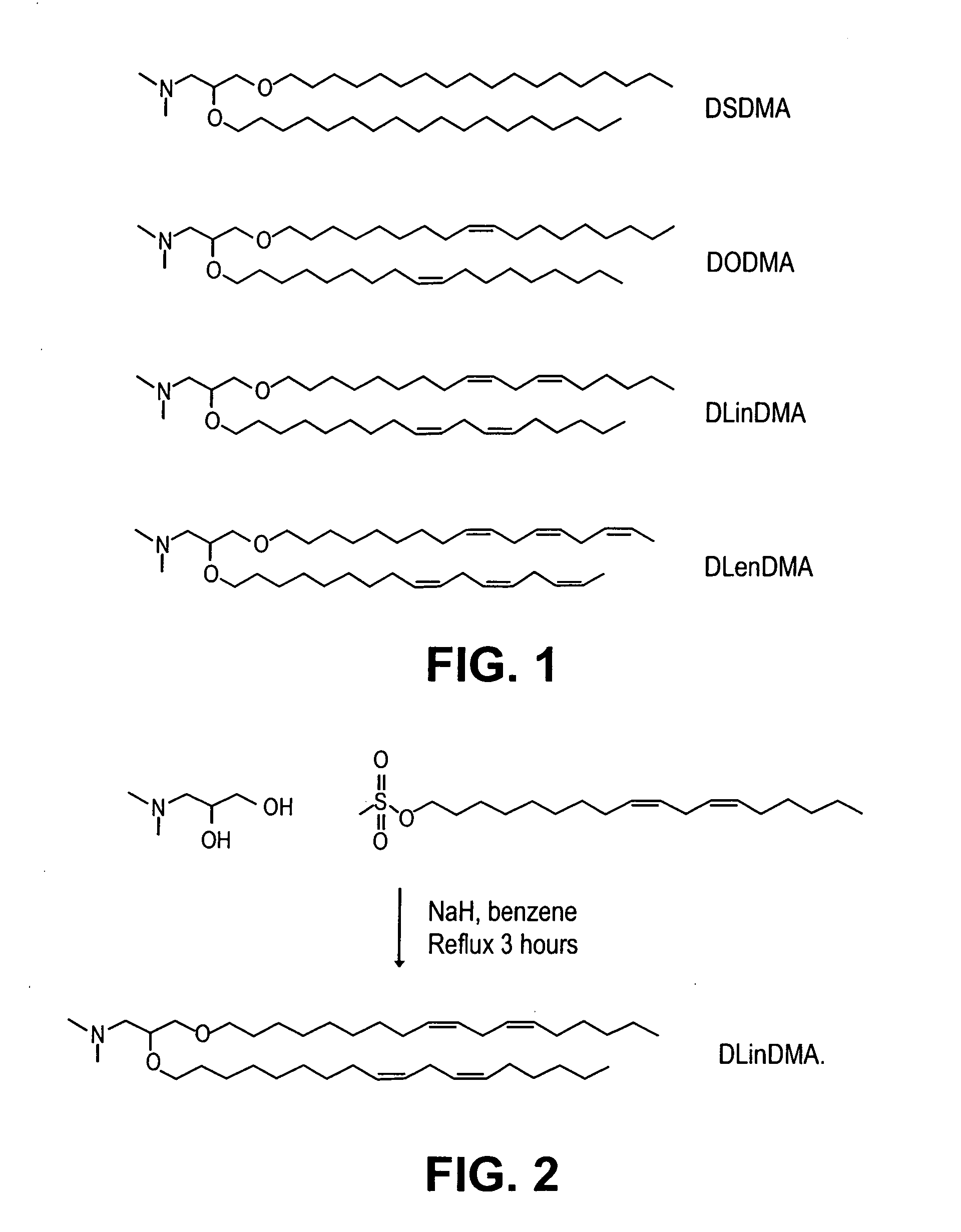
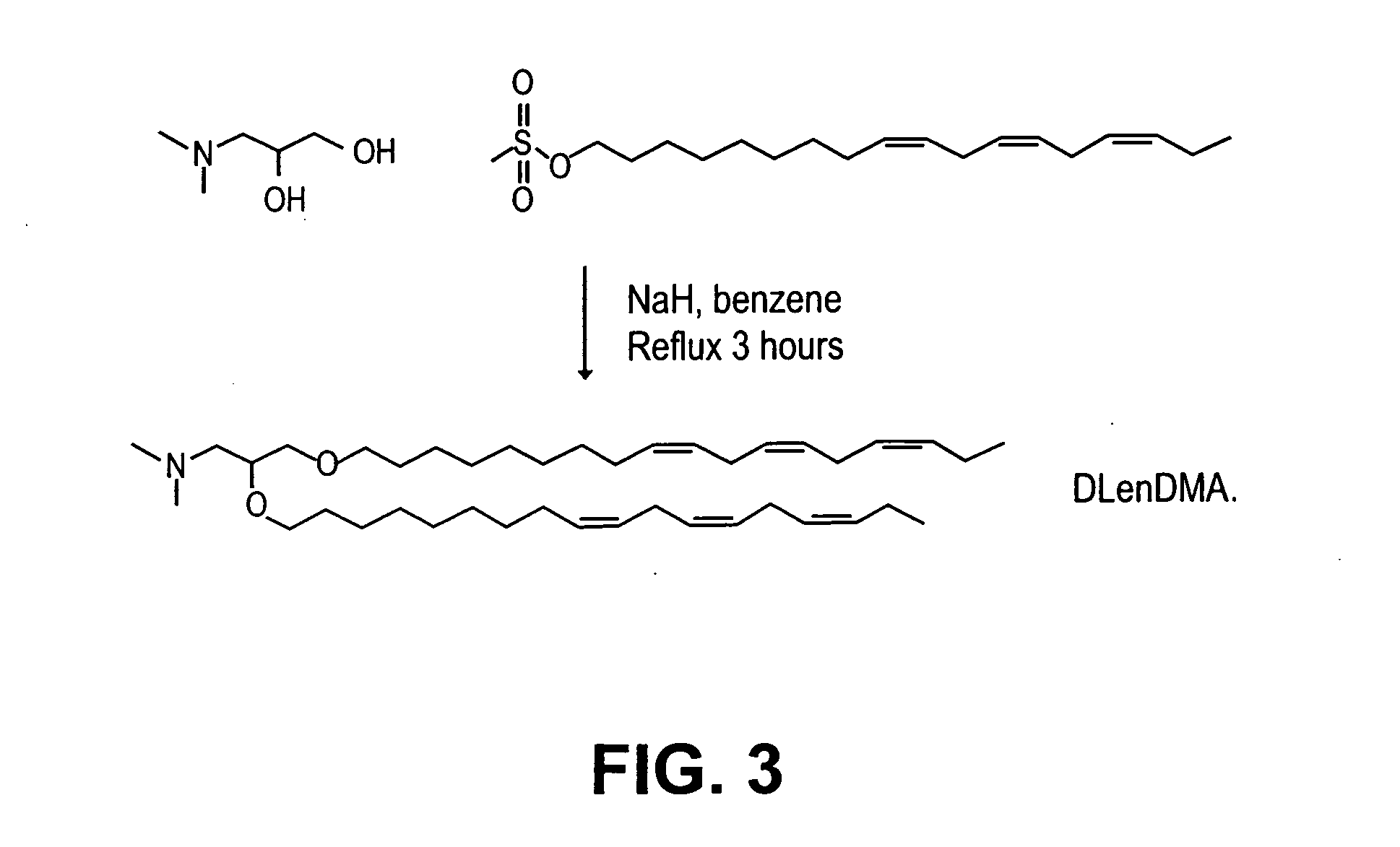
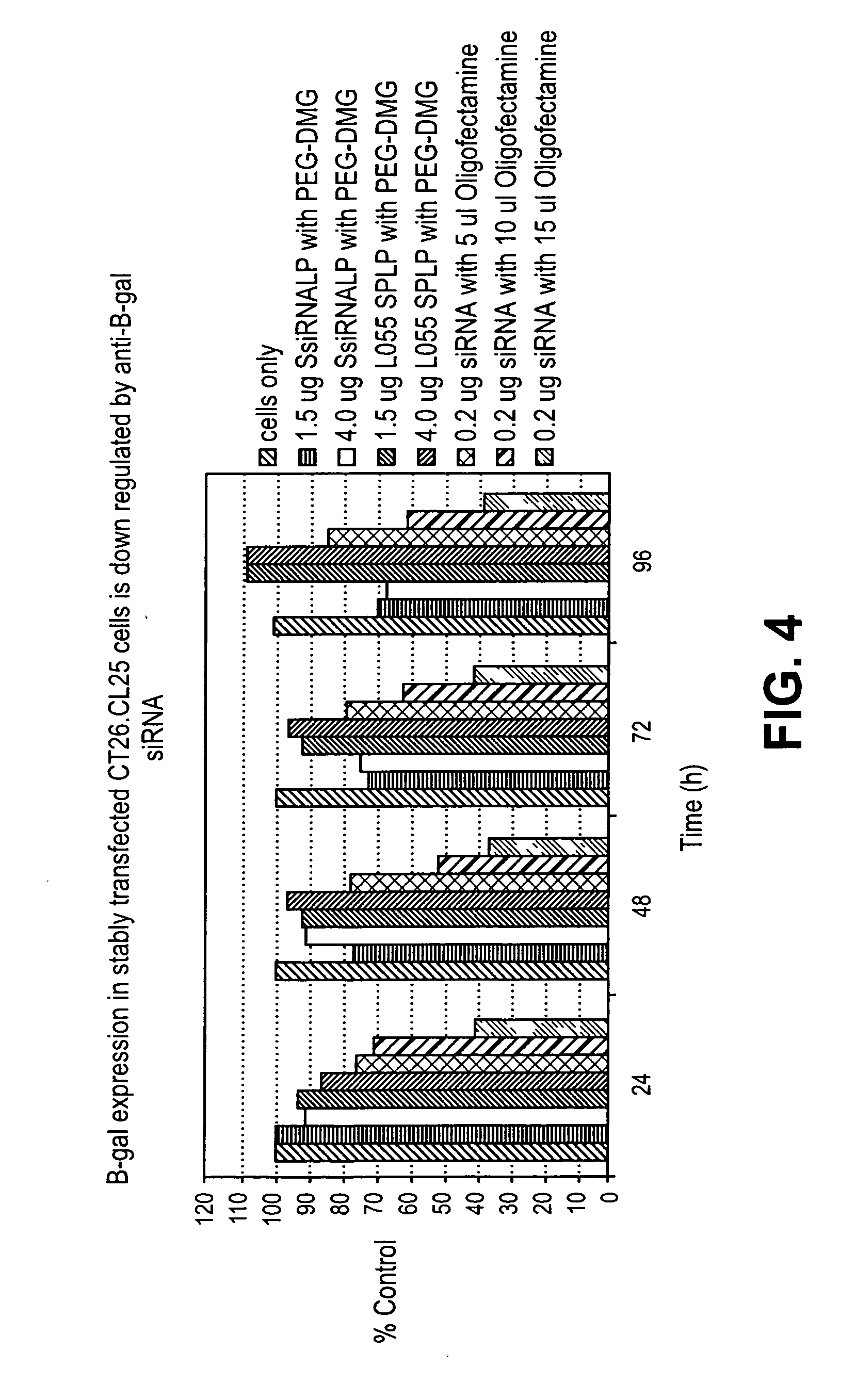
Lipid formulations for nucleic acid delivery
The present invention provides novel, stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (such as one or more interfering RNA), methods of making the SNALP, and methods of delivering and / or administering the SNALP.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
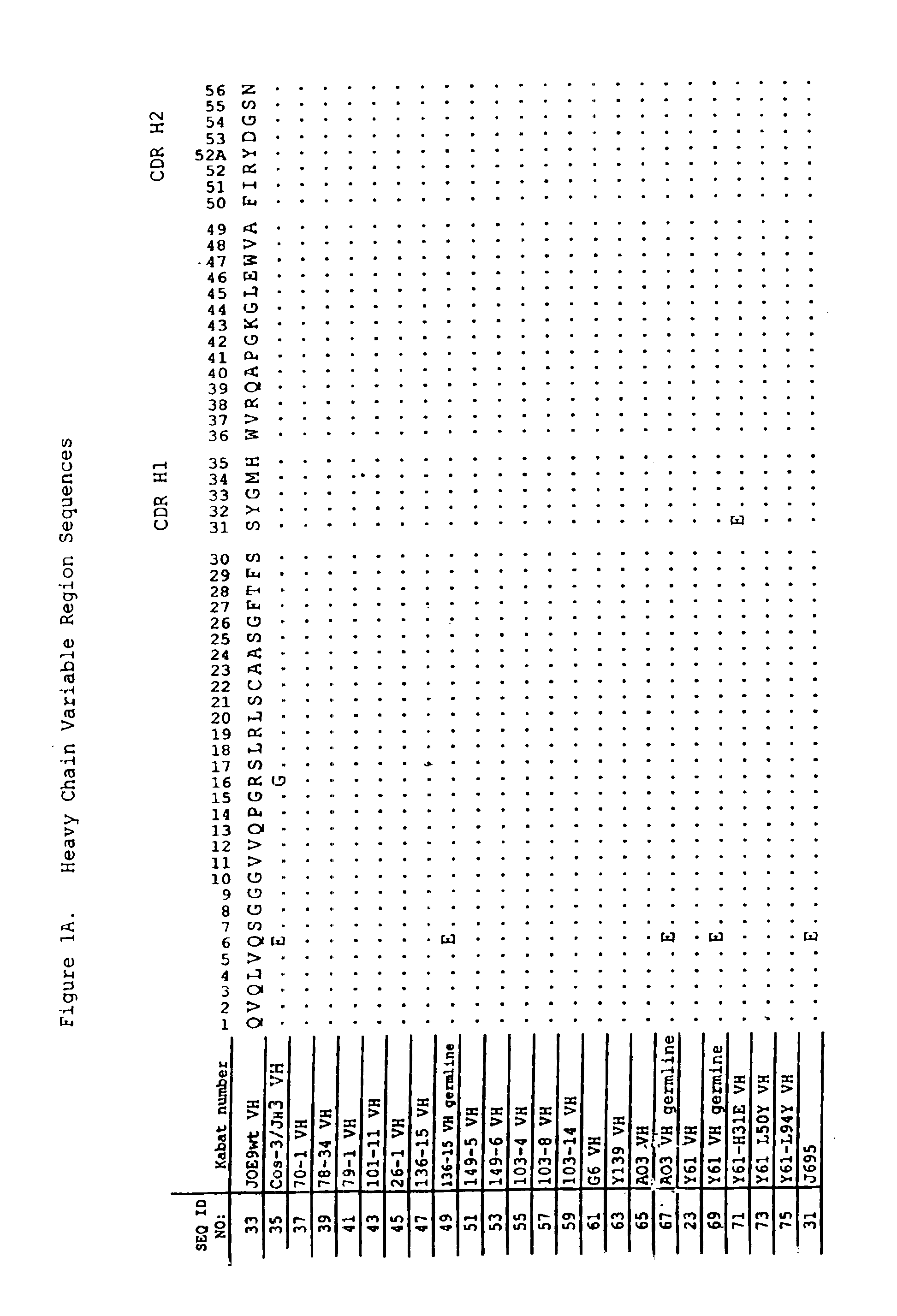
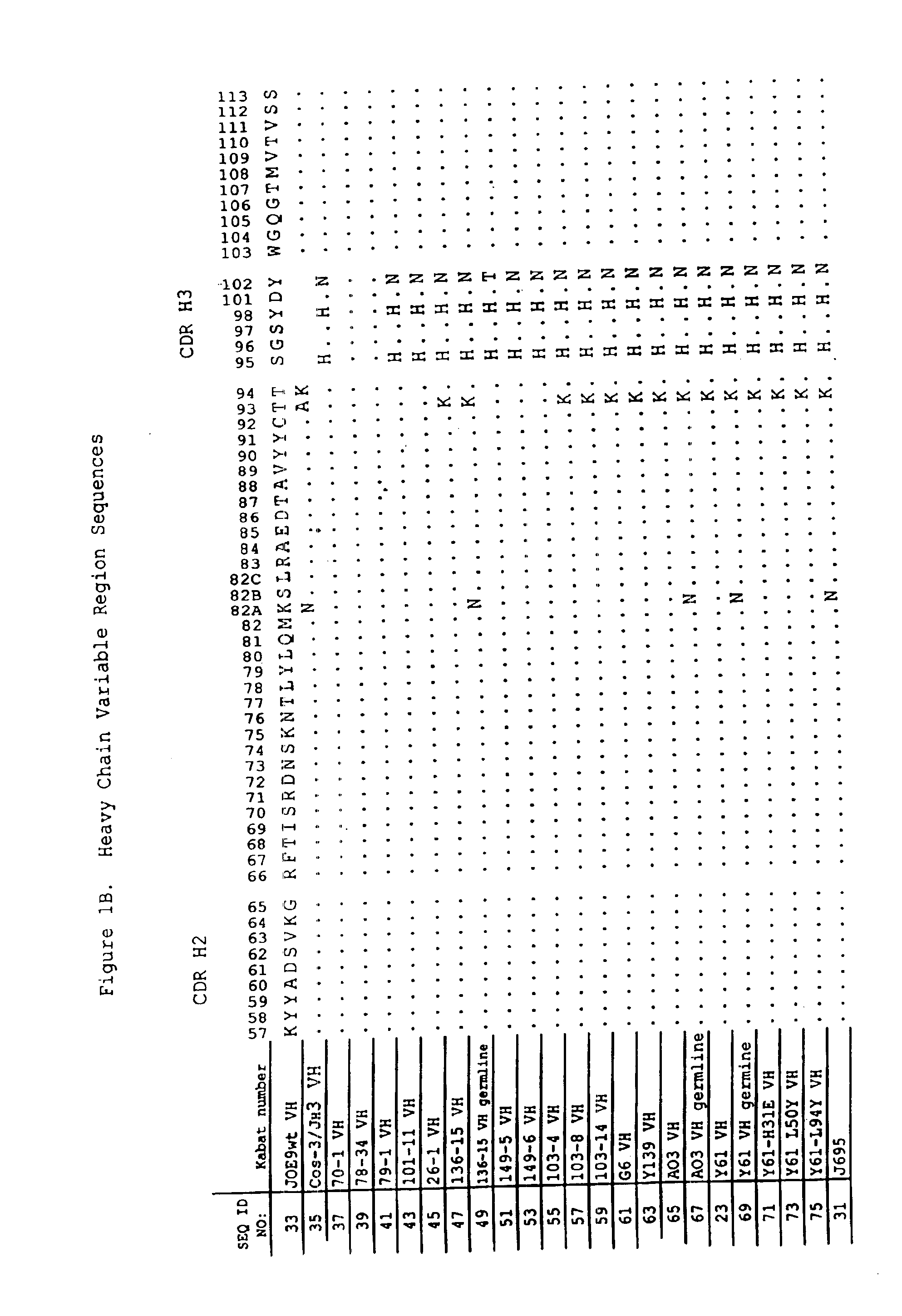
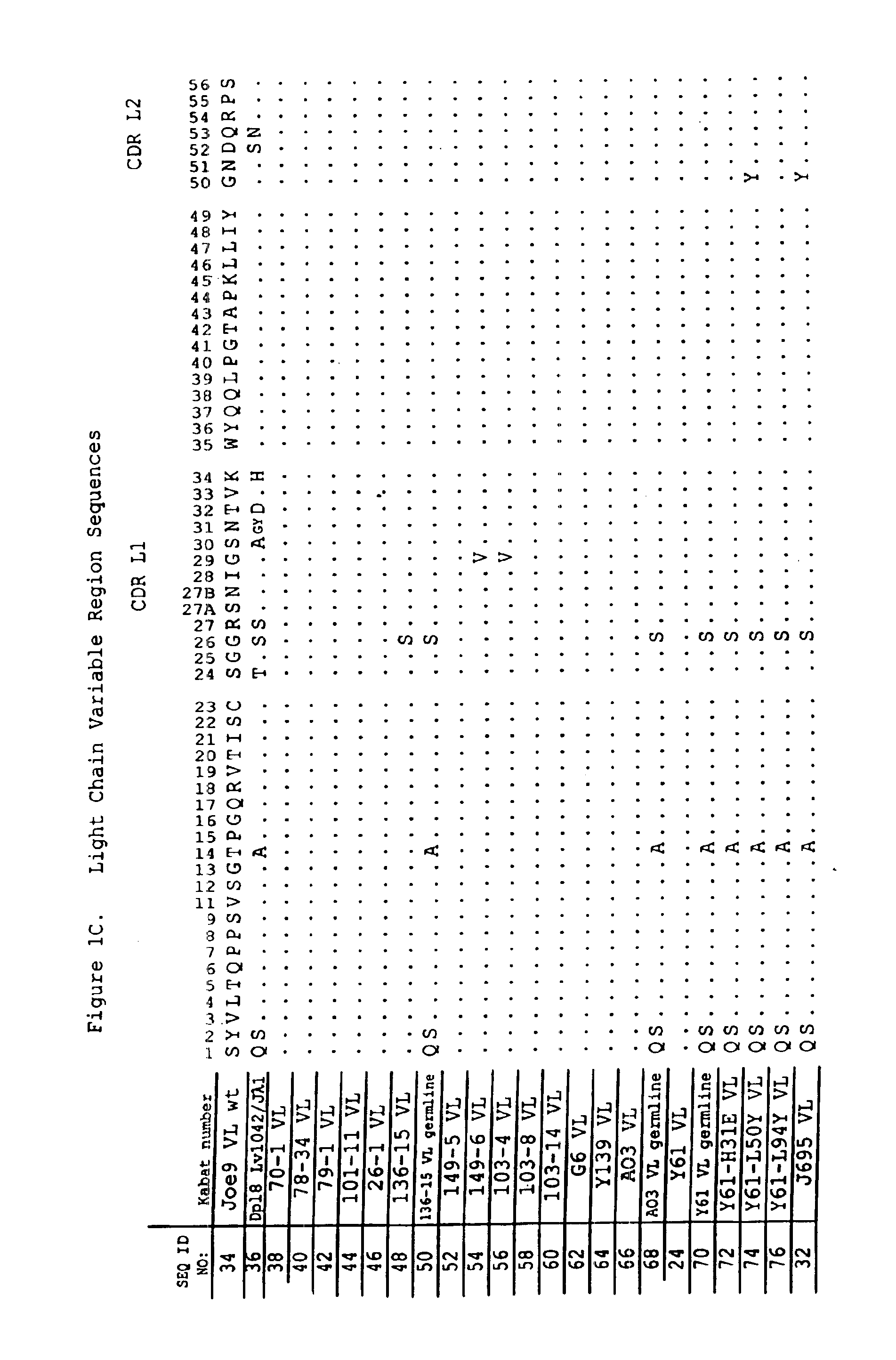
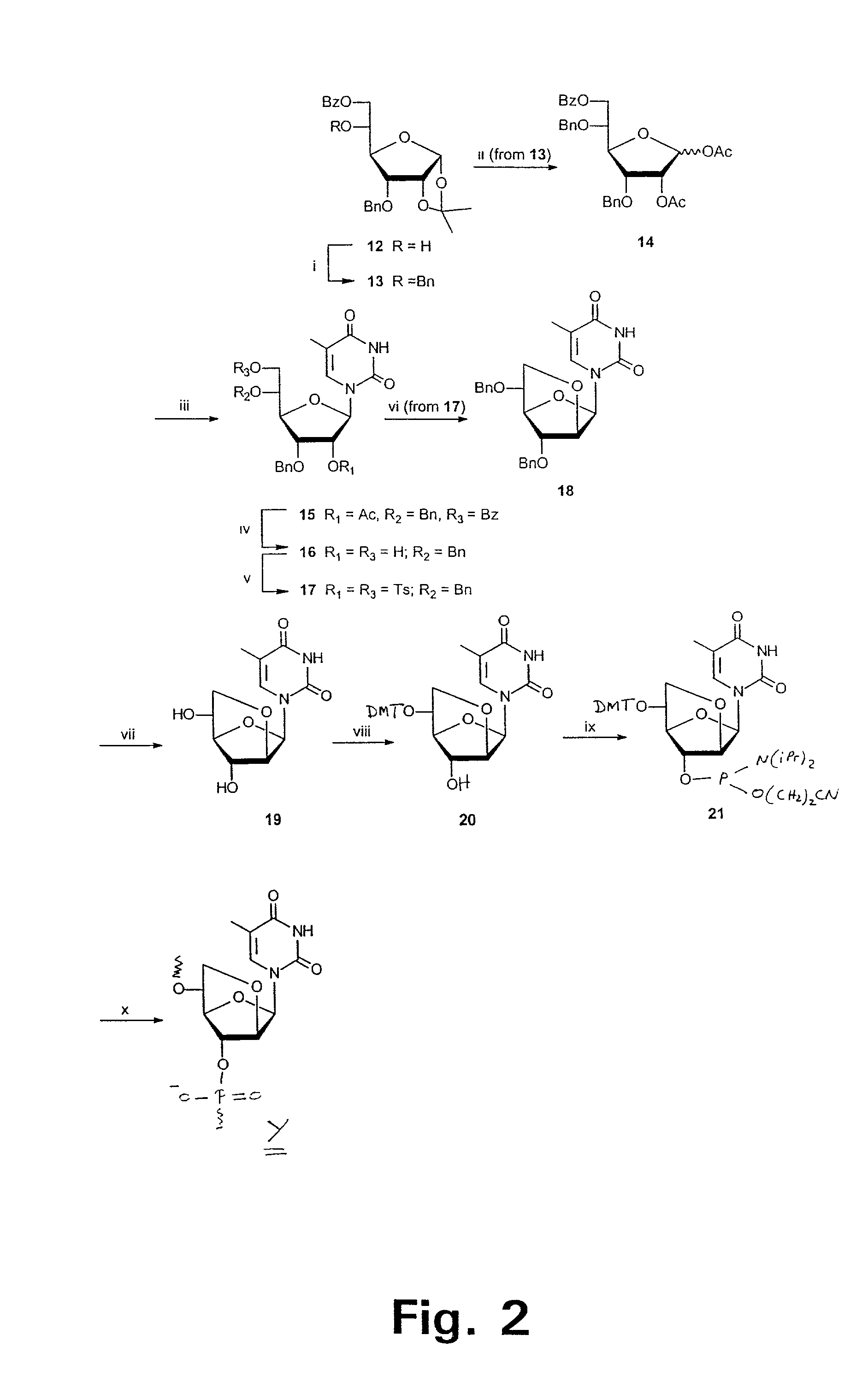
Oligonucleotide analogues
The present invention relates to novel bicyclic and tricyclic nucleoside and nucleotide analogues as well as to oligonucleotides comprising such elements. The nucleotide analogues, LNAs (Locked Nucleoside Analogues), are able to provide valuable improvements to oligonucleotides with respect to affinity and specificity towards complementary RNA and DNA oligomers. The novel type of LNA modified oligonucleotides, as well as the LNAs as such, are useful in a wide range of diagnostic applications as well as therapeutic applications. Among these can be mentioned antisense applications, PCR applications, strand displacement oligomers, as substrates for nucleic acid polymerases, as nucleotide based drugs, etc. The present invention also relates to such applications.
Owner:QIAGEN GMBH
Methods of analyzing polymers using a spatial network of fluorophores and fluorescence resonance energy transfer
InactiveUS6263286B1Easy to analyze and useMicrobiological testing/measurementLaboratory glasswaresEnergy transferResonance
The present invention relates to methods and apparatuses for analyzing molecules, particularly polymers, and molecular complexes with extended or rod-like conformations. In particular, the methods and apparatuses are used to identify repetitive information in molecules or molecular ensembles, which is interpreted using an autocorrelation function in order to determine structural information about the molecules. The methods and apparatuses of the invention are used for, inter alia, determining the sequence of a nucleic acid, determining the degree of identity of two polymers, determining the spatial separation of specific sites within a polymer, determining the length of a polymer, and determining the velocity with which a molecule penetrates a biological membrane.
Owner:U S GENOMICS INC
Methods of Analyzing Nucleic Acids from Individual Cells or Cell Populations
InactiveUS20150376609A1Facilitate hybridizationMicrobiological testing/measurementLibrary member identificationReagentNucleic acid
Methods, compositions and systems for analyzing individual cells or cell populations through the partitioned analysis of contents of individual cells or cell populations. Individual cells or cell populations are co-partitioned with processing reagents for accessing cellular contents, and for uniquely identifying the contents of a given cell or cell population, and subsequently analyzing the cell's contents and characterizing it as having derived from an individual cell or cell population, including analysis and characterization of the cell's nucleic acids through sequencing.
Owner:10X GENOMICS
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
Lipid encapsulated interfering RNA
InactiveUS20060008910A1Inhibit aggregationReduce overexpressionBiocideOrganic active ingredientsLipid formationLipid particle
The present invention provides lipid-based formulations for delivering, e.g., introducing, nucleic acid-lipid particles comprising an interference RNA molecule to a cell, and assays for optimizing the delivery efficiency of such lipid-based formulations.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com