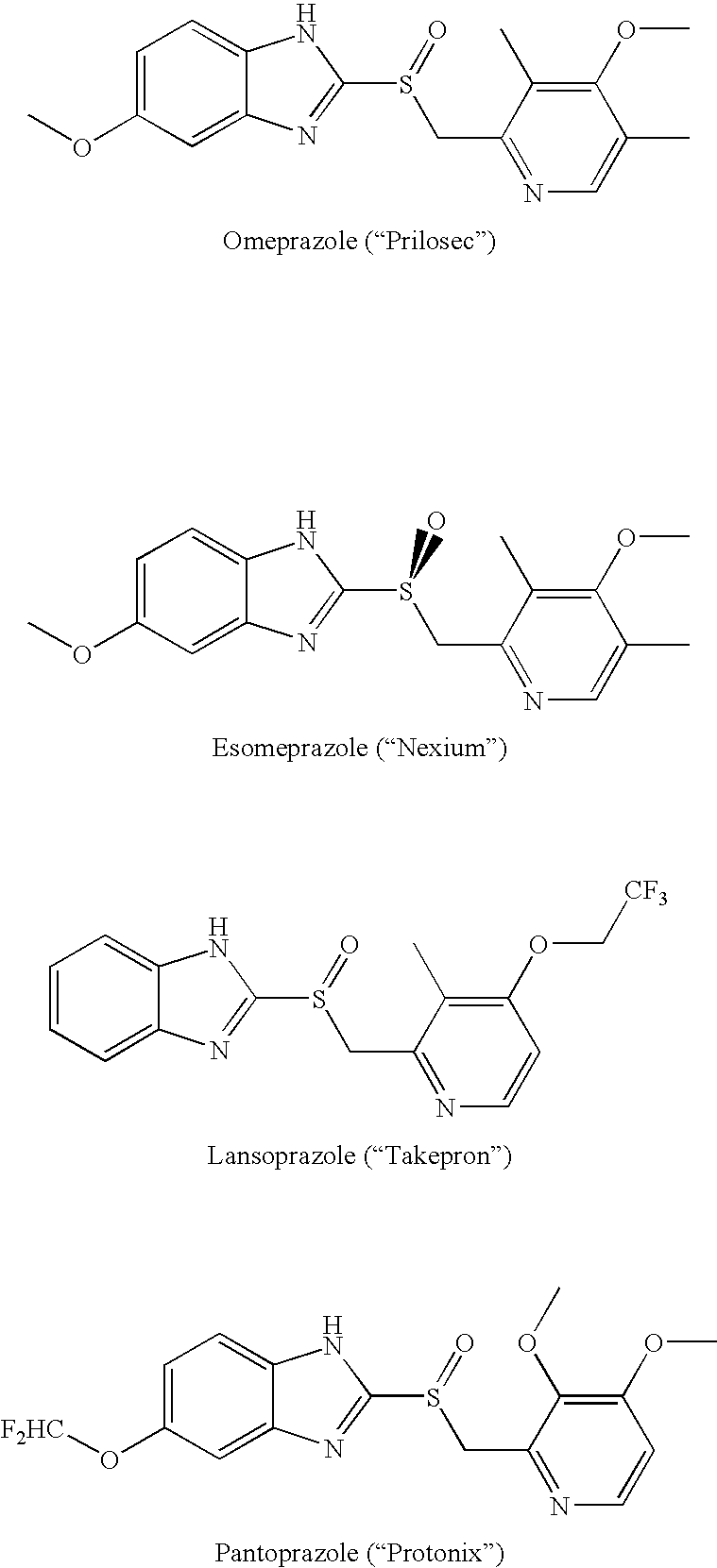
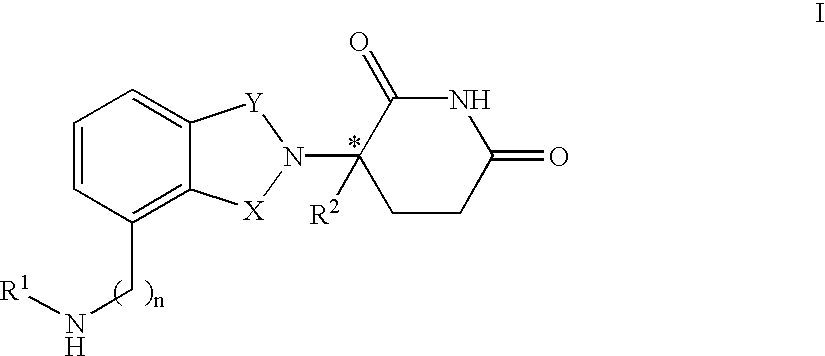
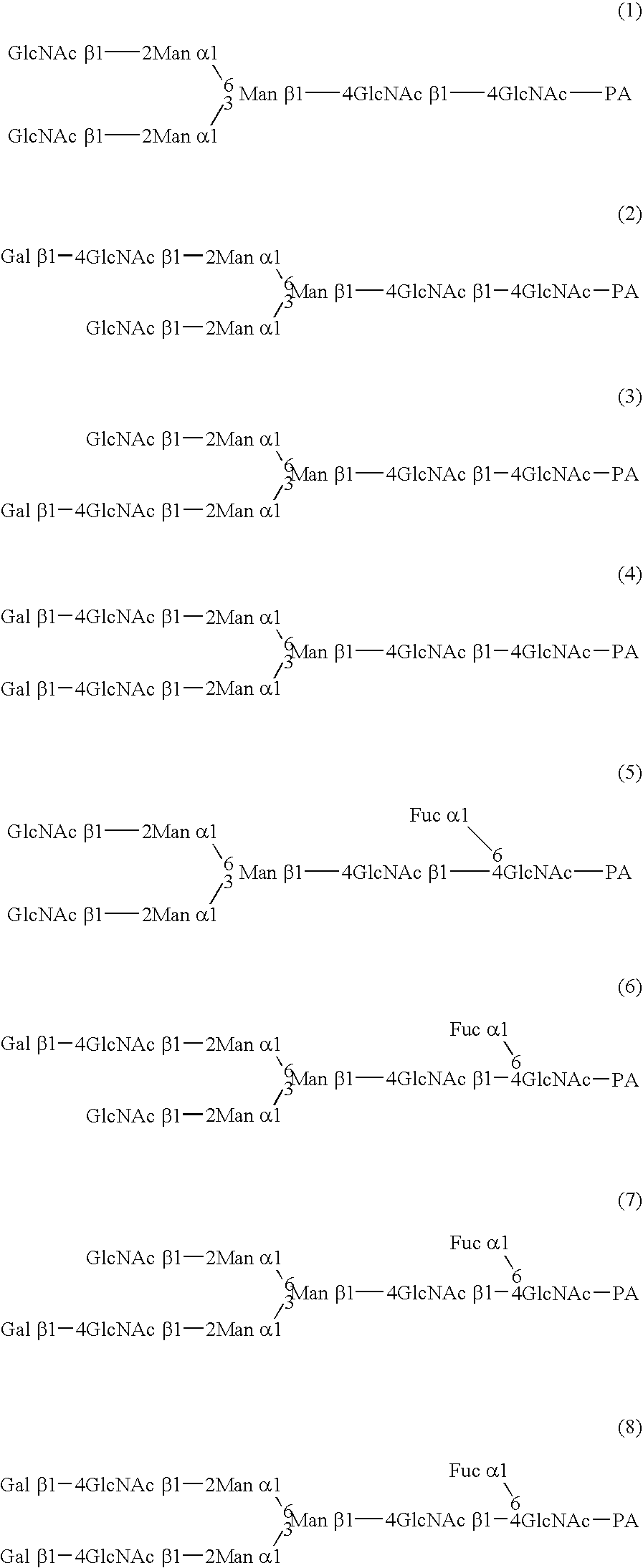Patents
Literature
85234results about "Antipyretic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cells of which genome is modified
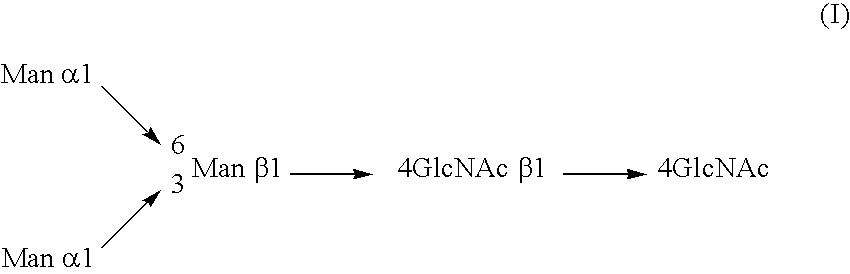
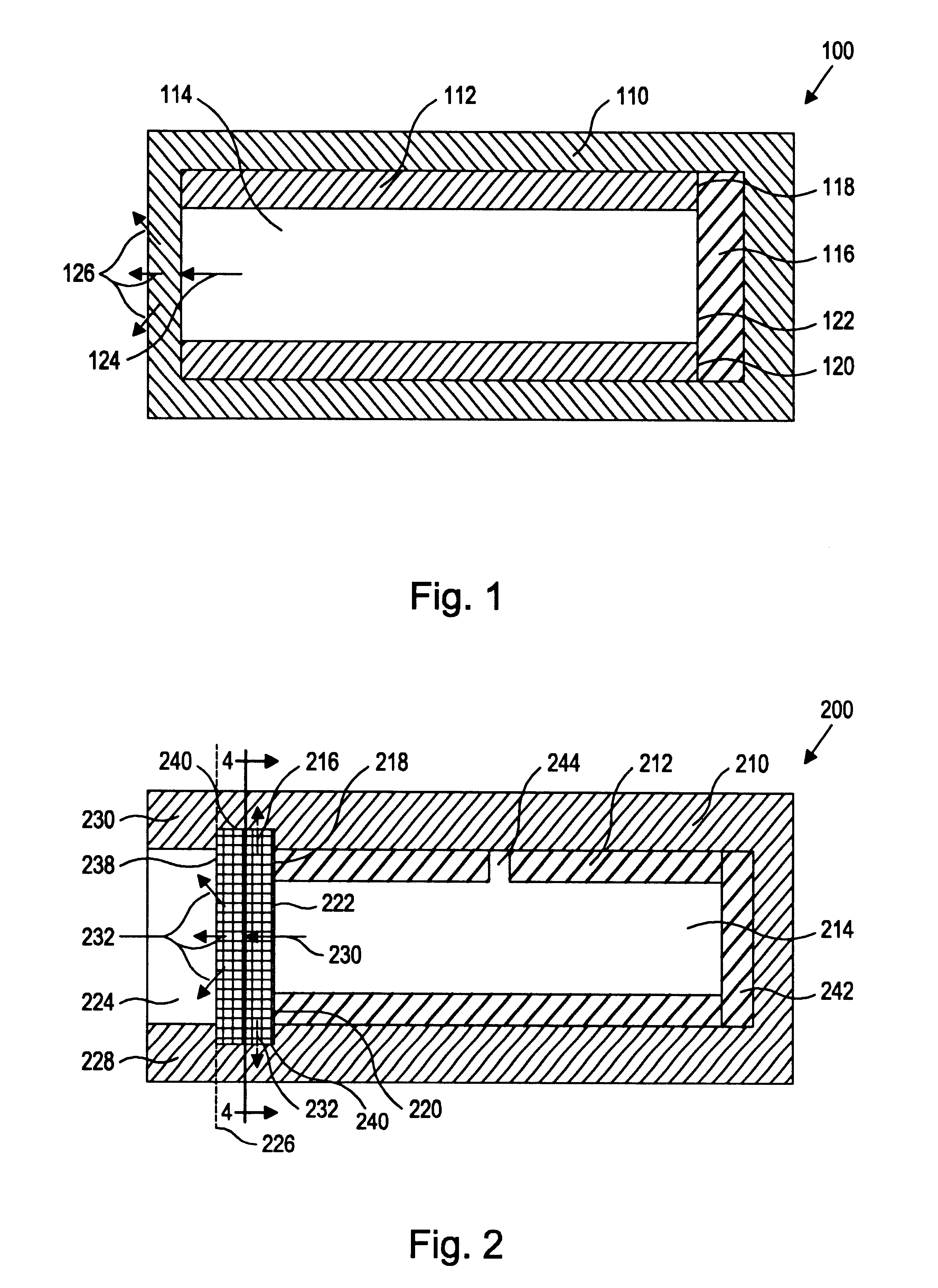
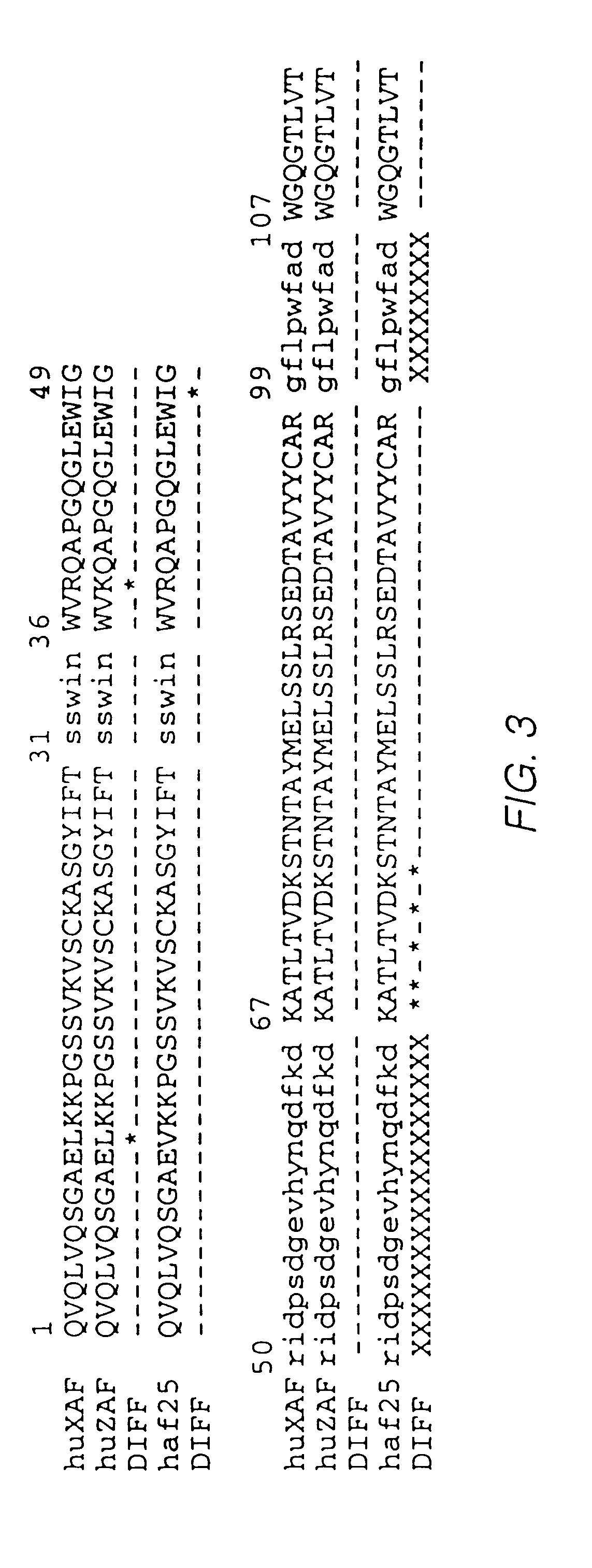
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
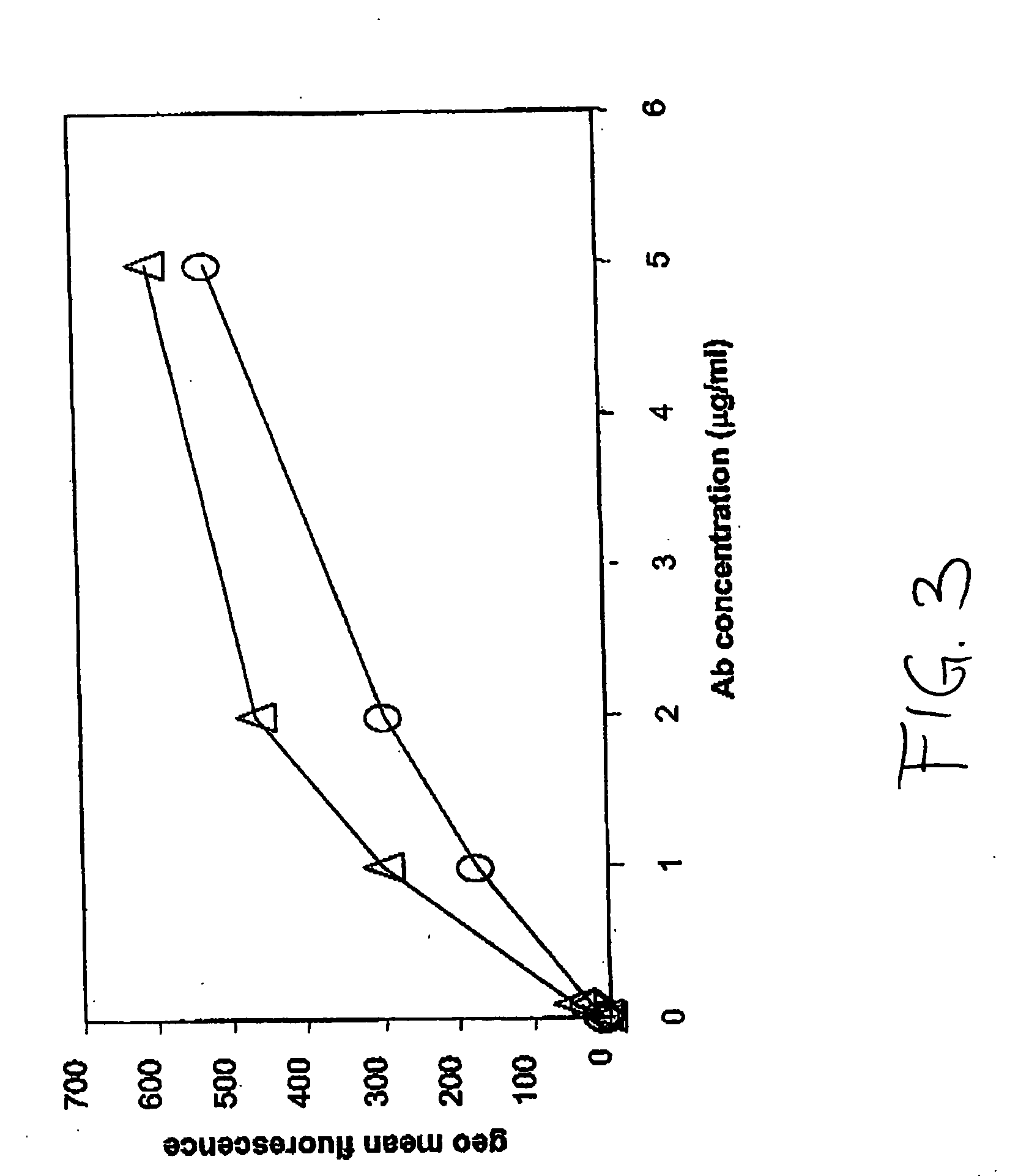
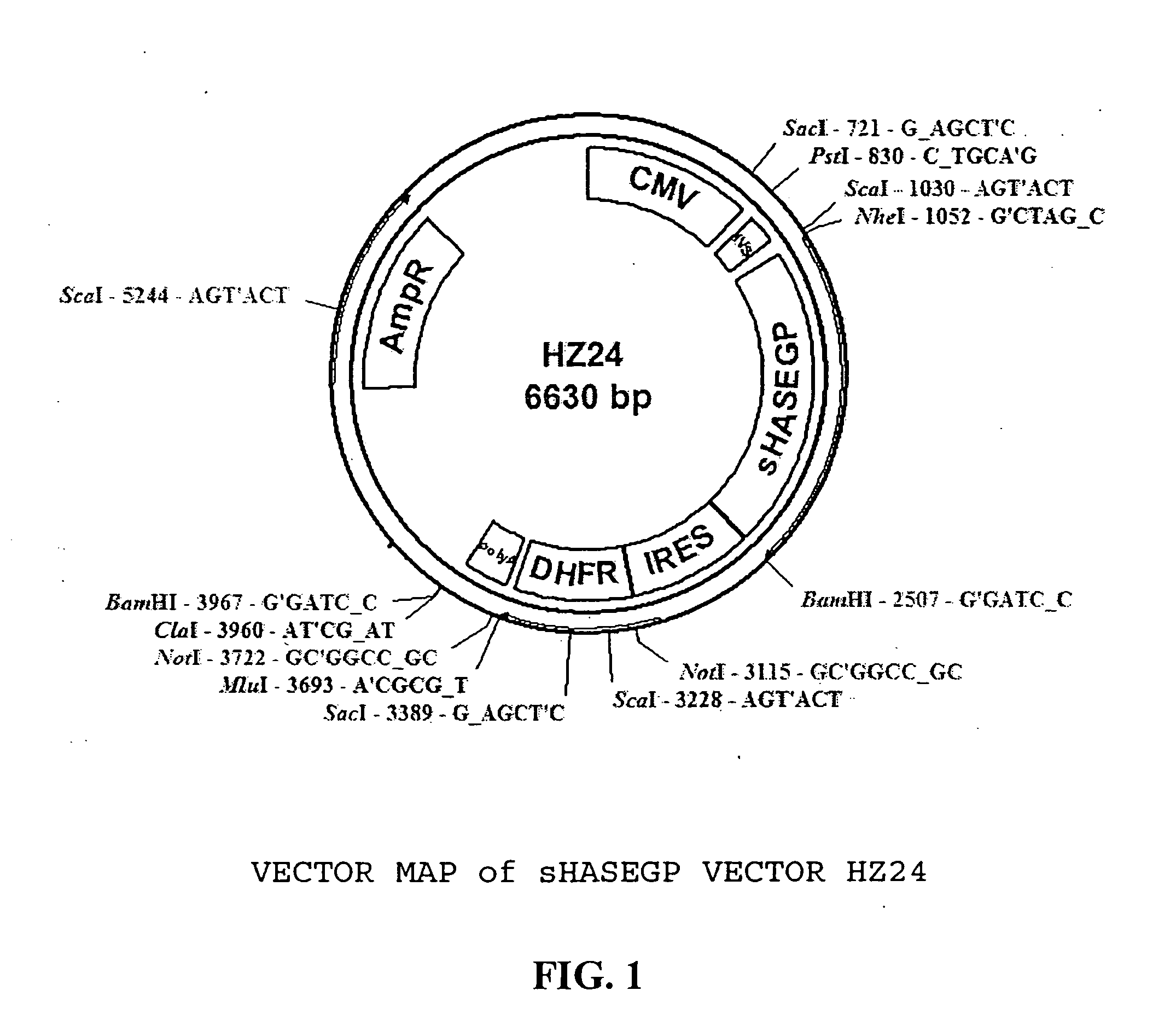
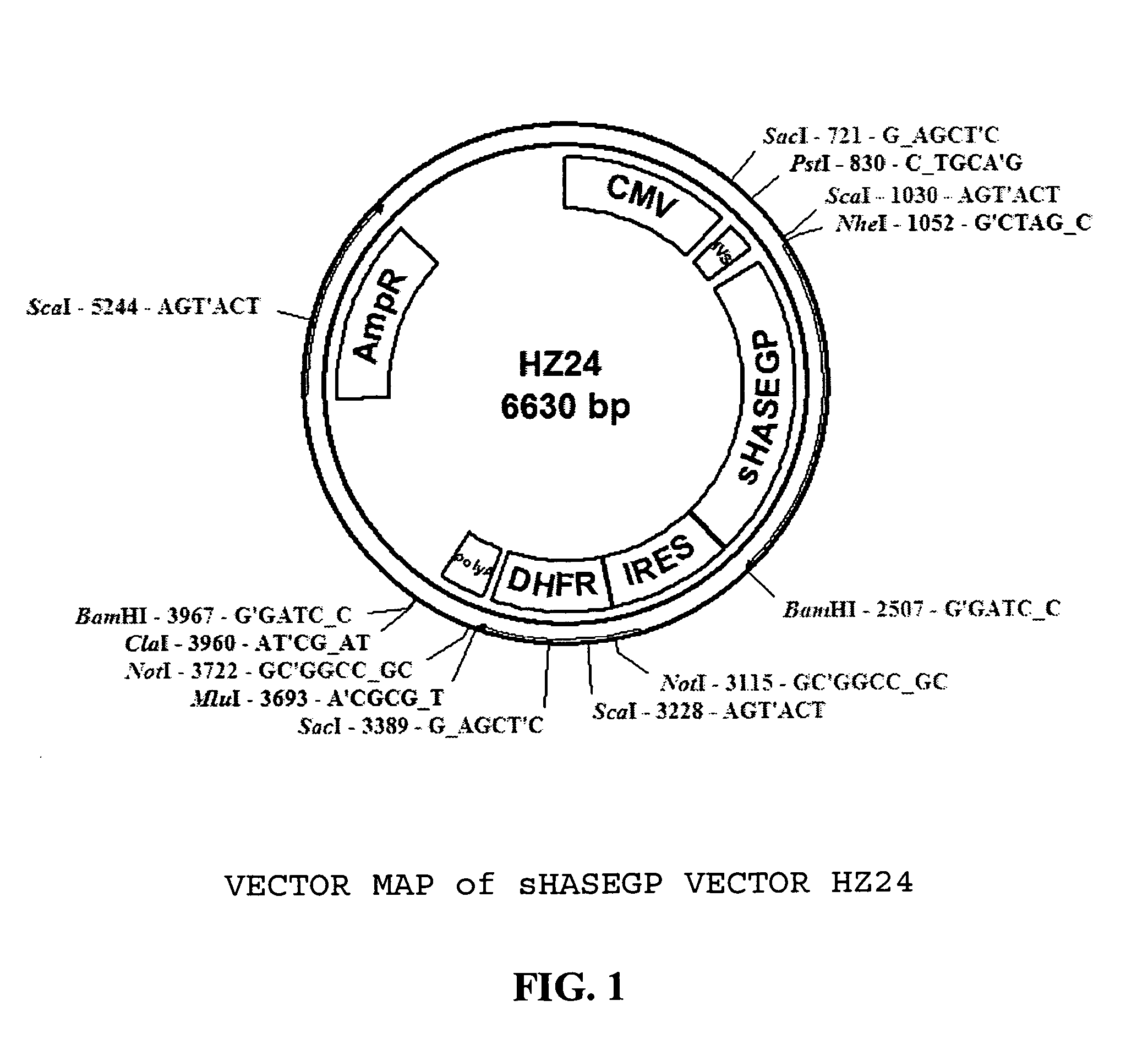
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
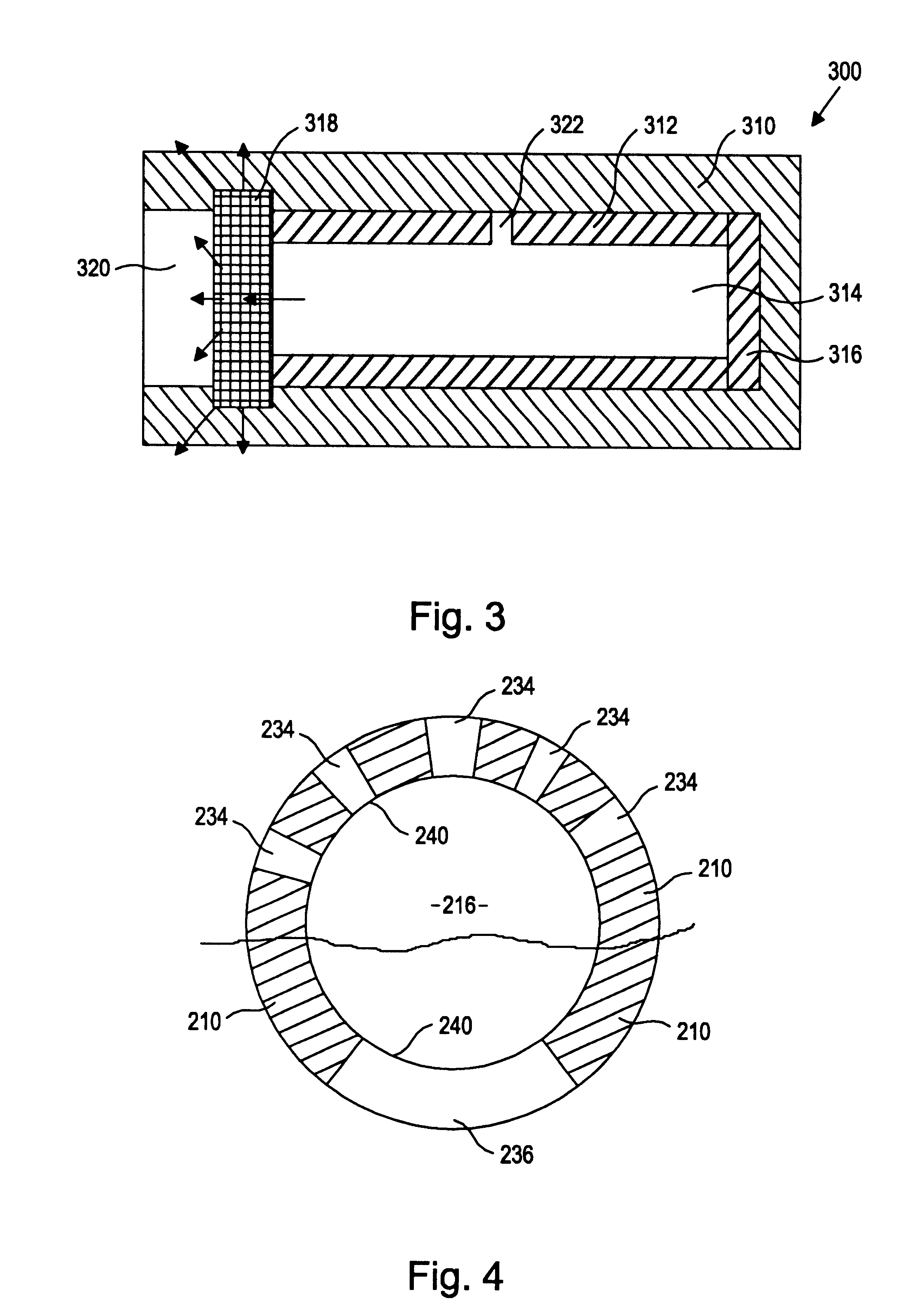
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
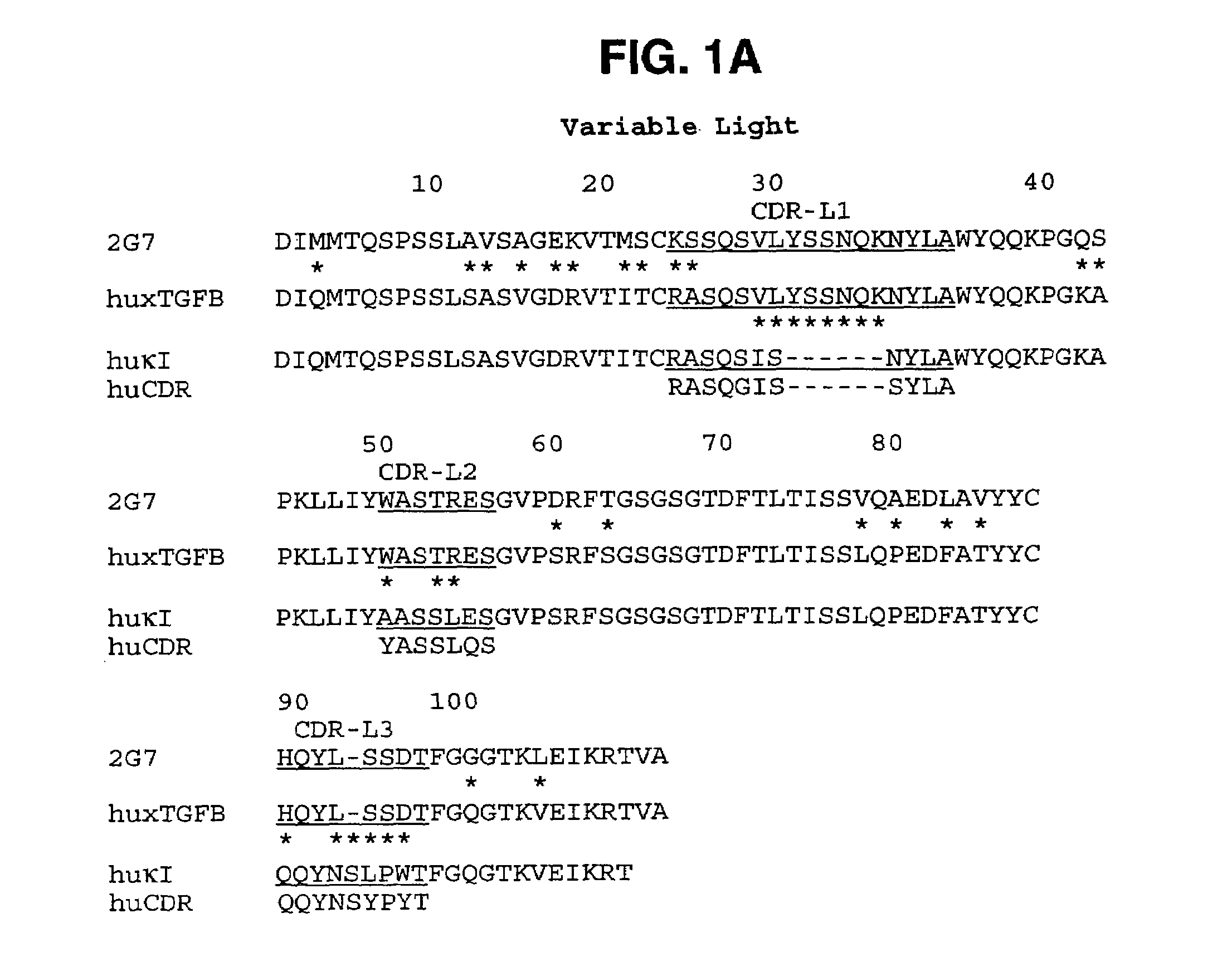
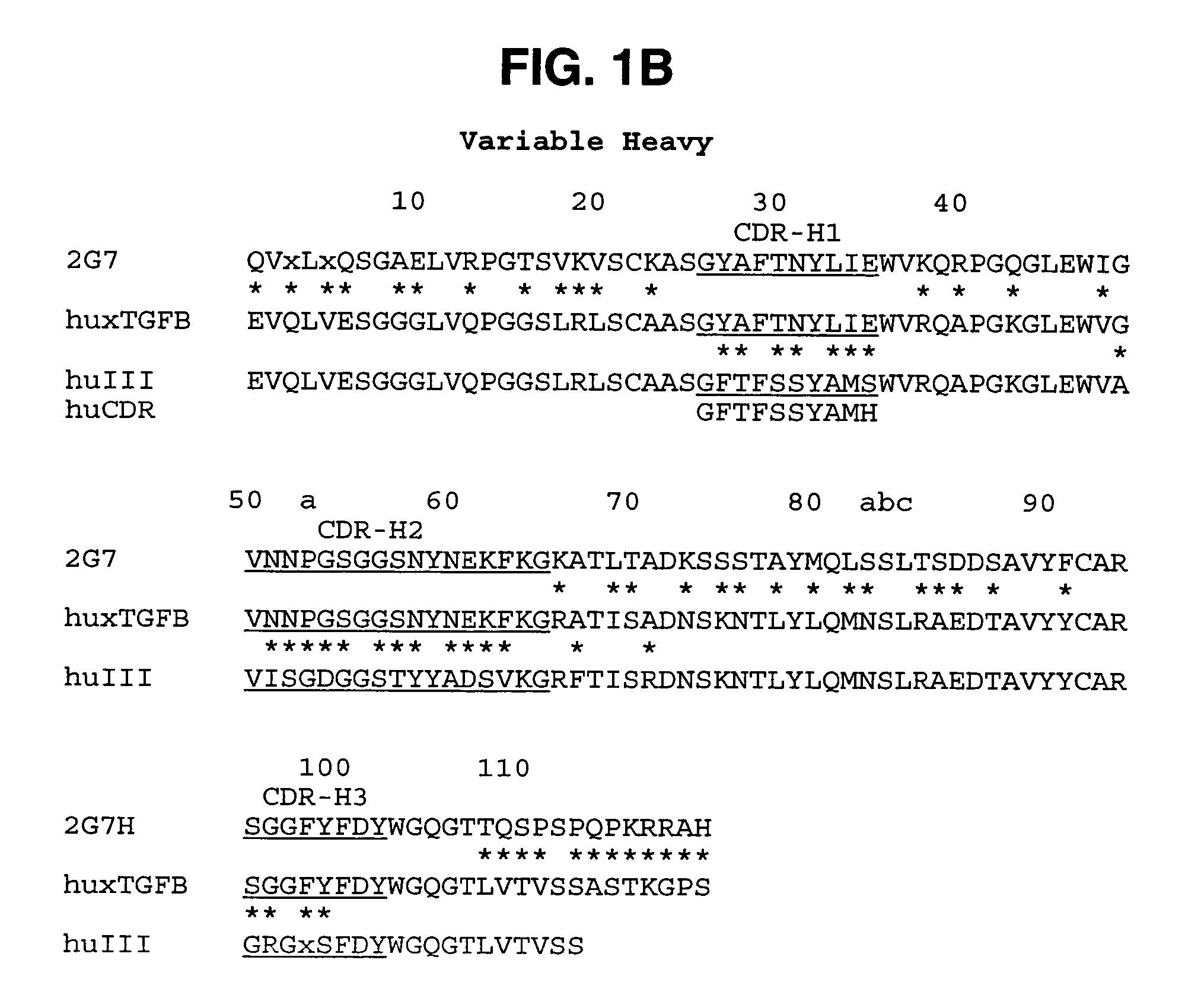
Humanized anti-TGF-beta antibodies
Humanized anti-TGF-beta antibodies are provided, as well as methods for their preparation and use, including methods for treating TGF-beta disorders, for example, cancer. Also provided are articles of manufacture designed for various uses that contain the humanized antibodies.
Owner:GENENTECH INC
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
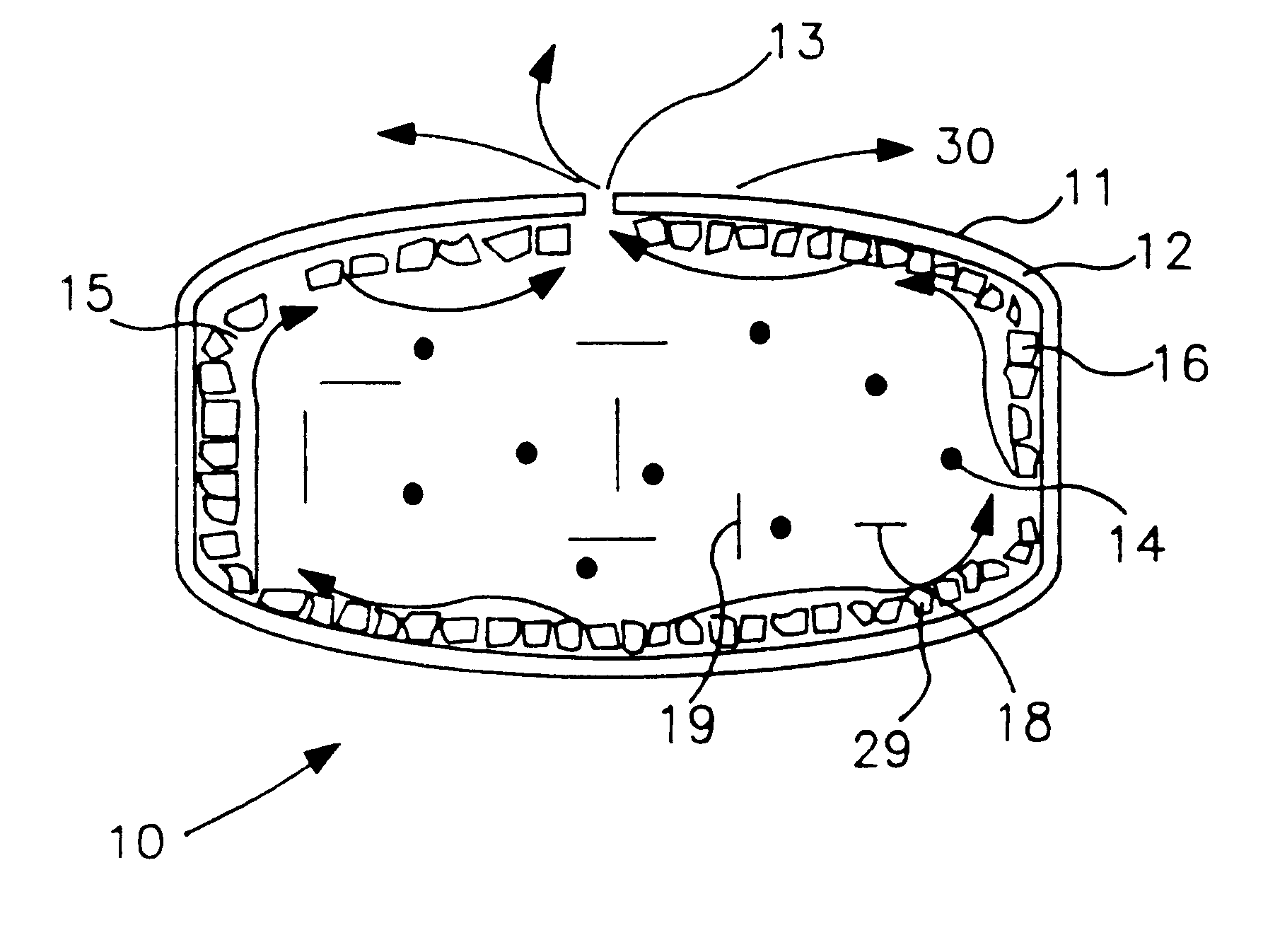
Composite self-cohered web materials
InactiveUS20070026039A1High porositySuitable for useAntipyreticAnalgesicsBiomedical engineeringPorosity
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
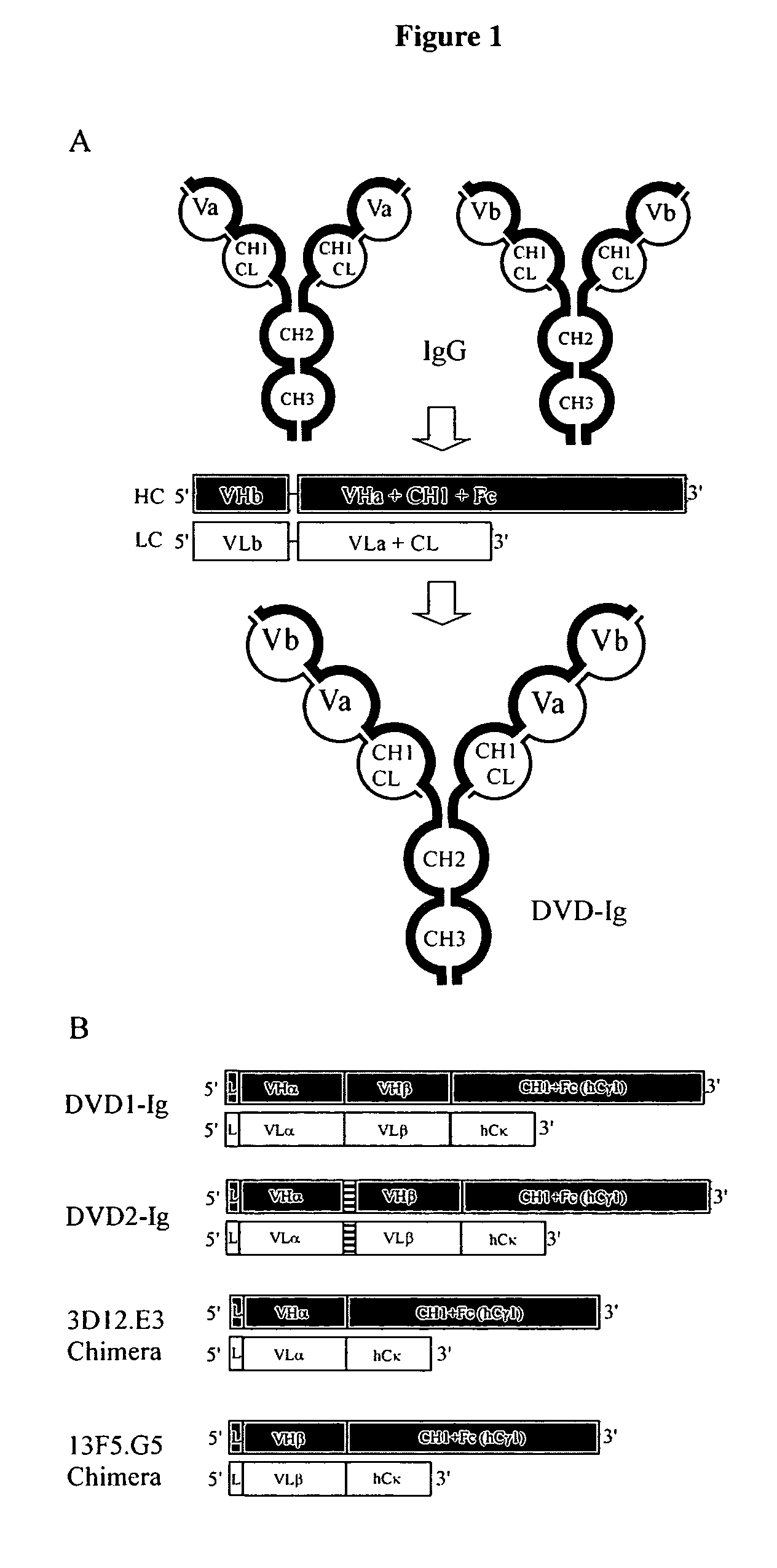
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
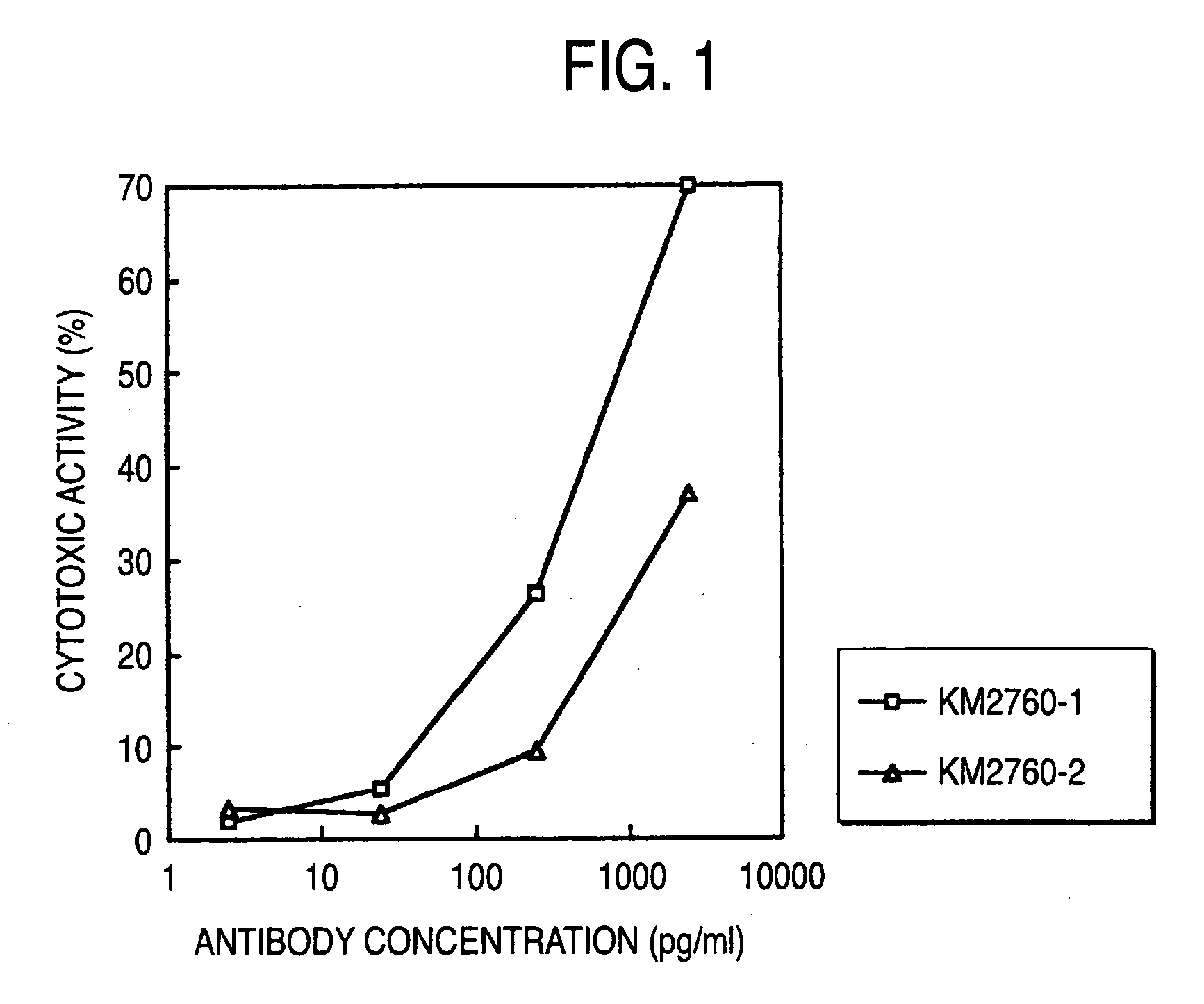
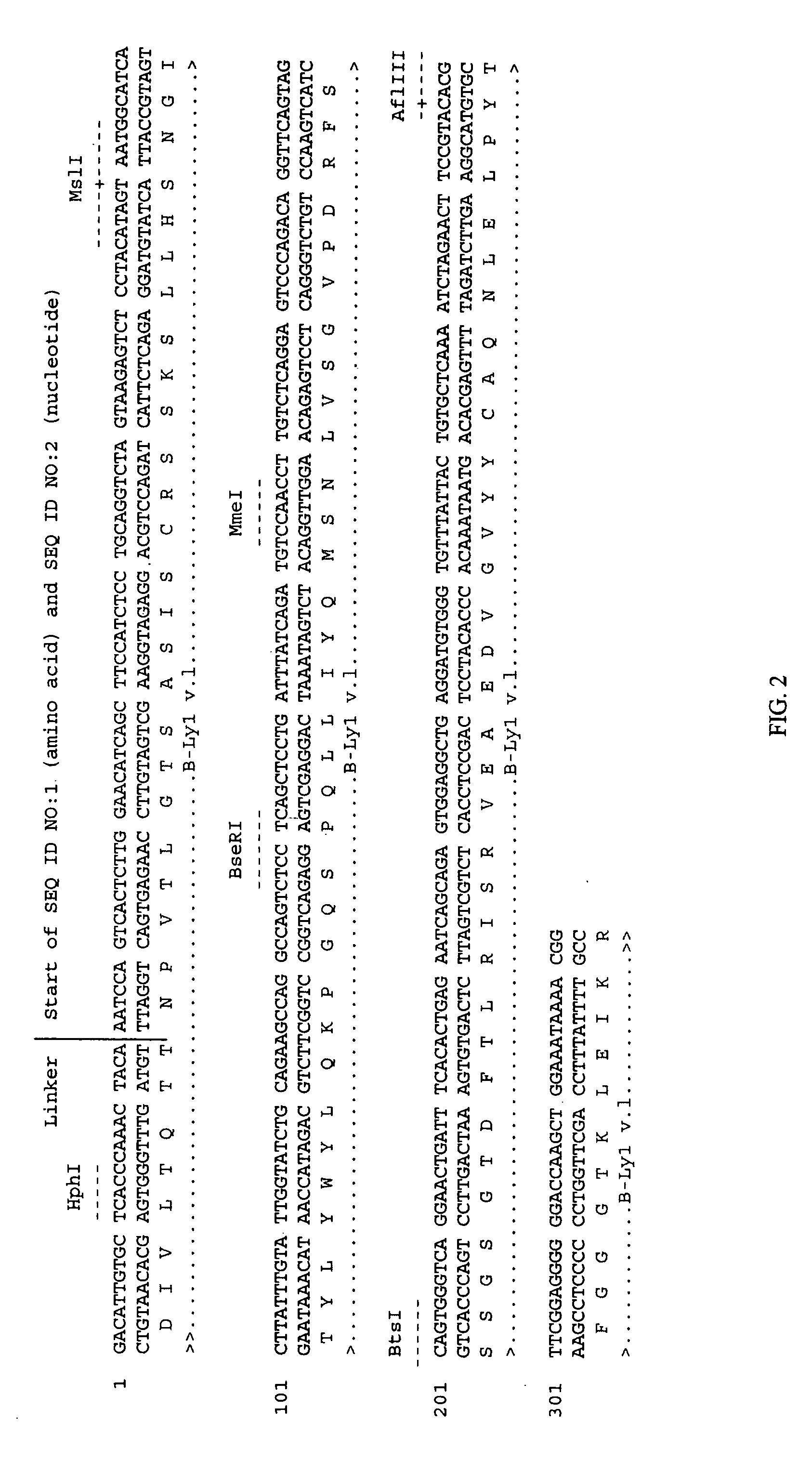
Cells producing antibody compositions with increased antibody dependent cytotoxic activity
The present invention relates to a cell for the production of an antibody molecule such as an antibody useful for various diseases having high antibody-dependent cell-mediated cytotoxic activity, a fragment of the antibody and a fusion protein having the Fc region of the antibody or the like, a method for producing an antibody composition using the cell, the antibody composition and use thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
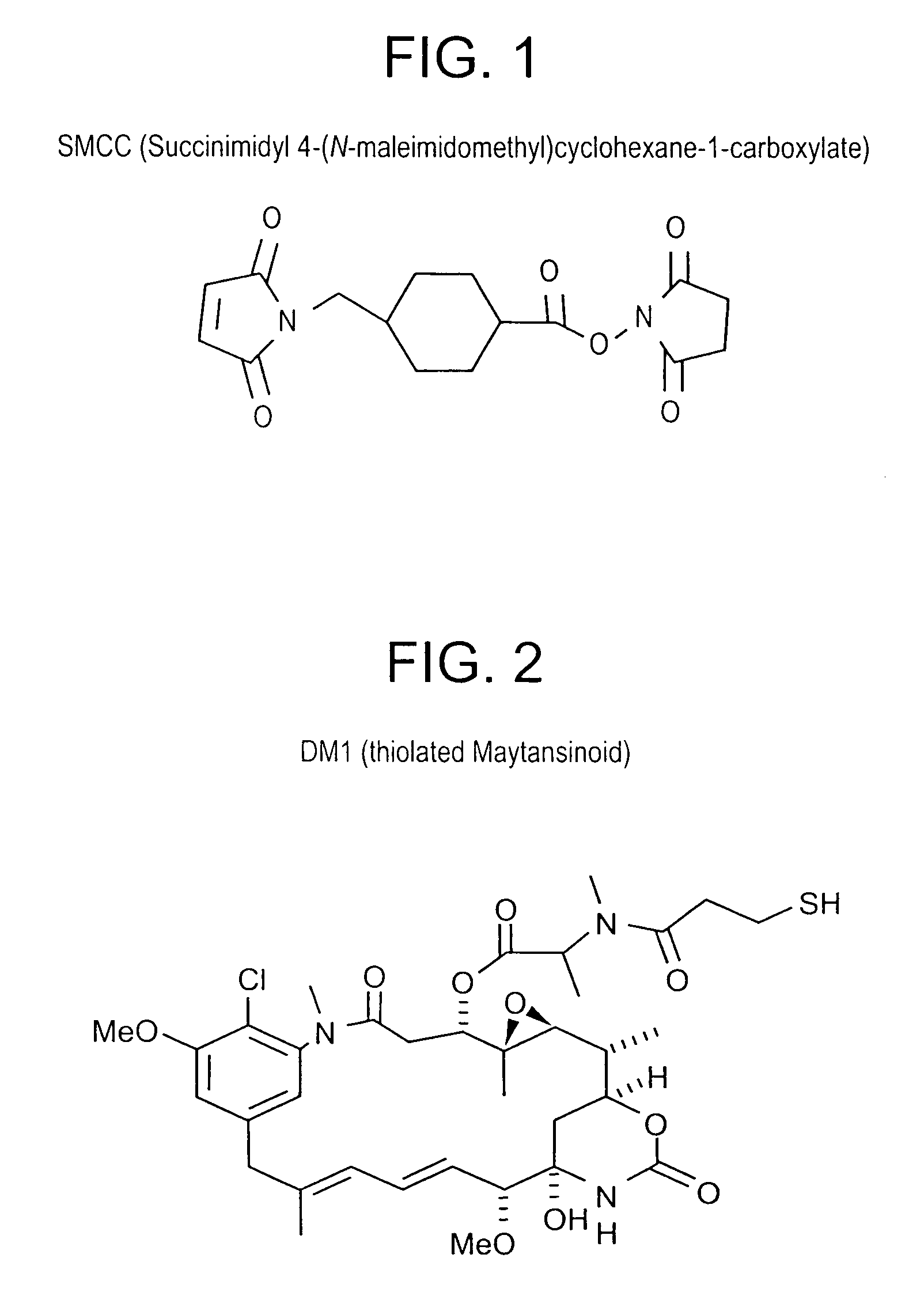
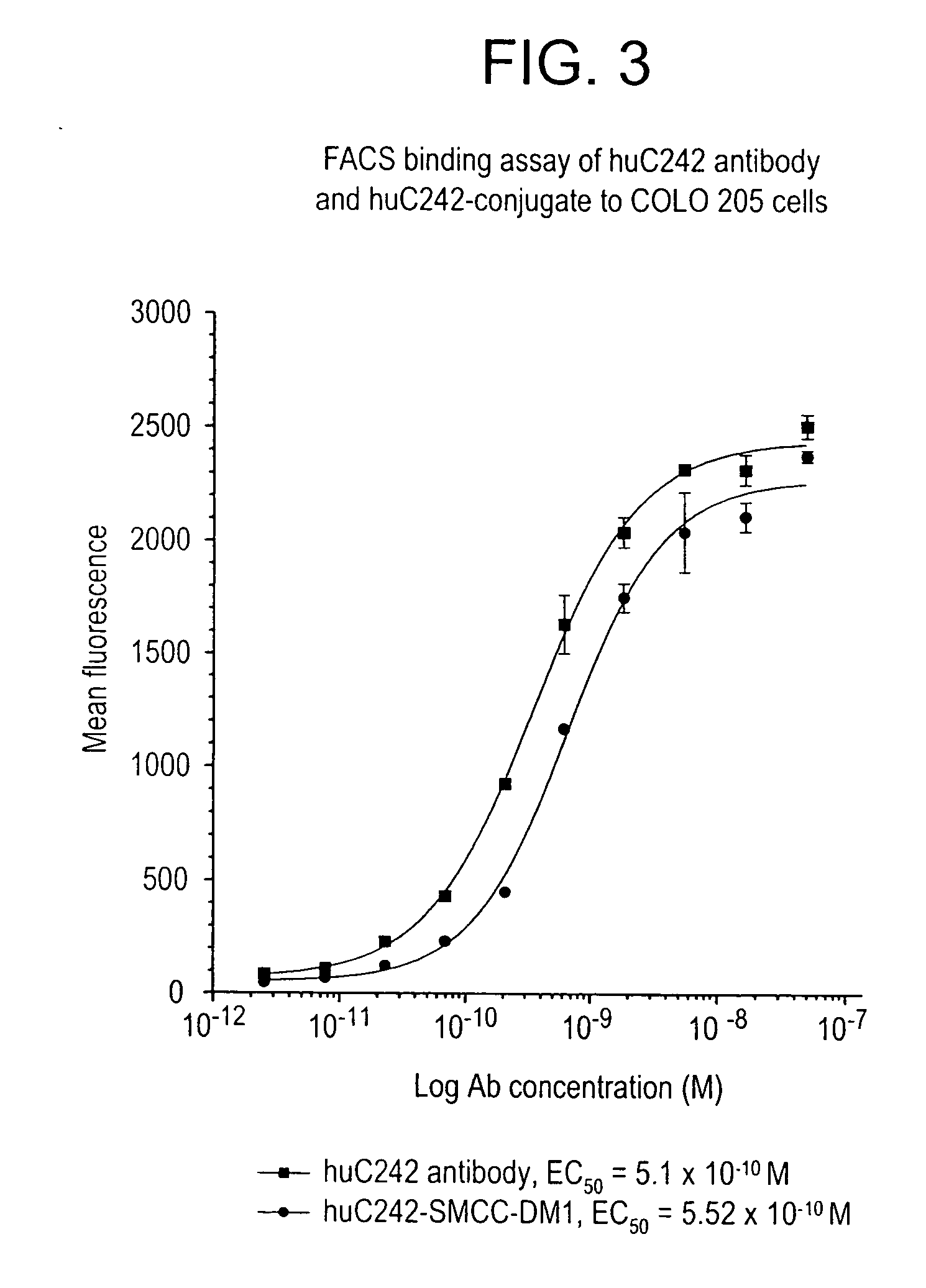
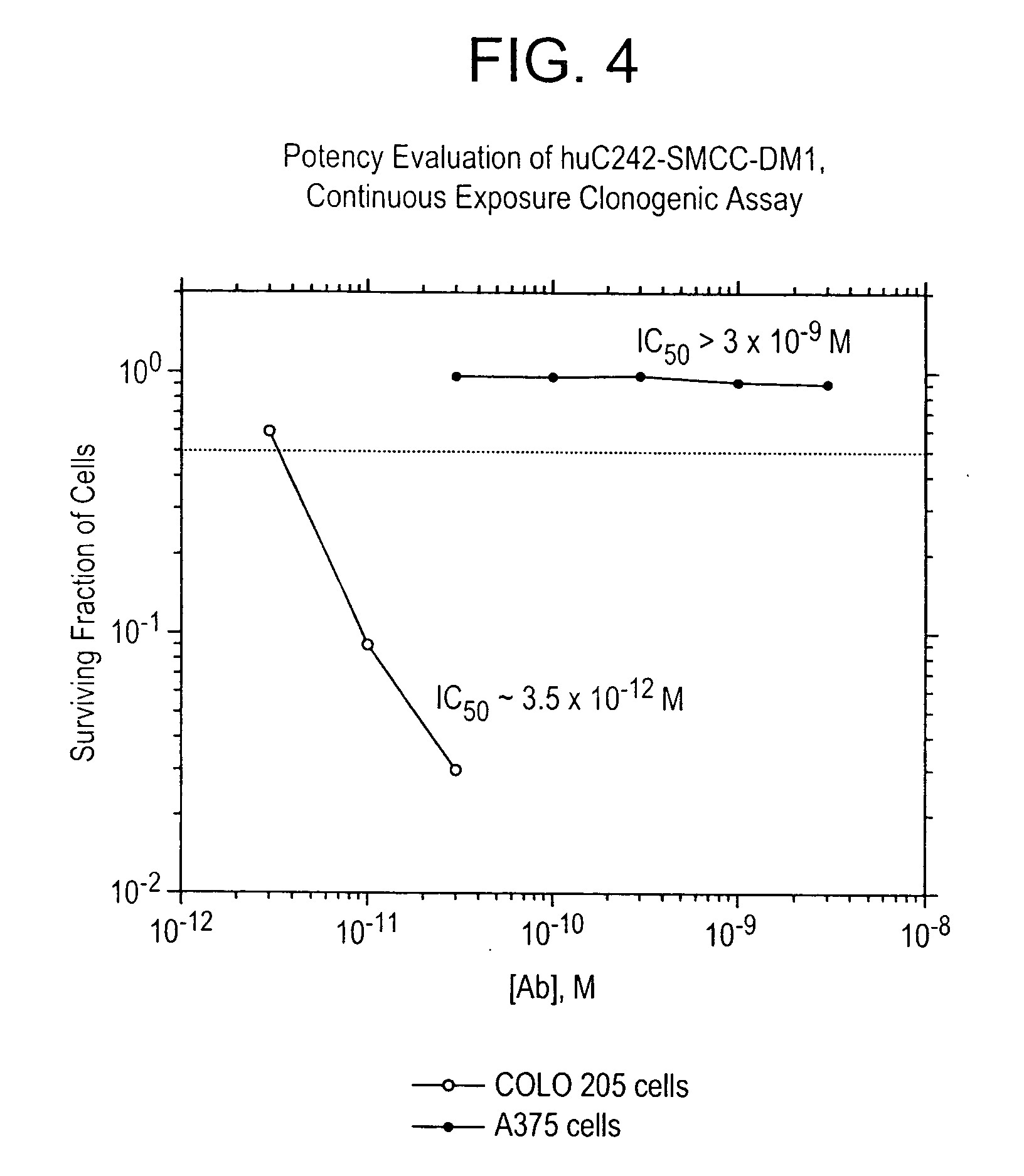
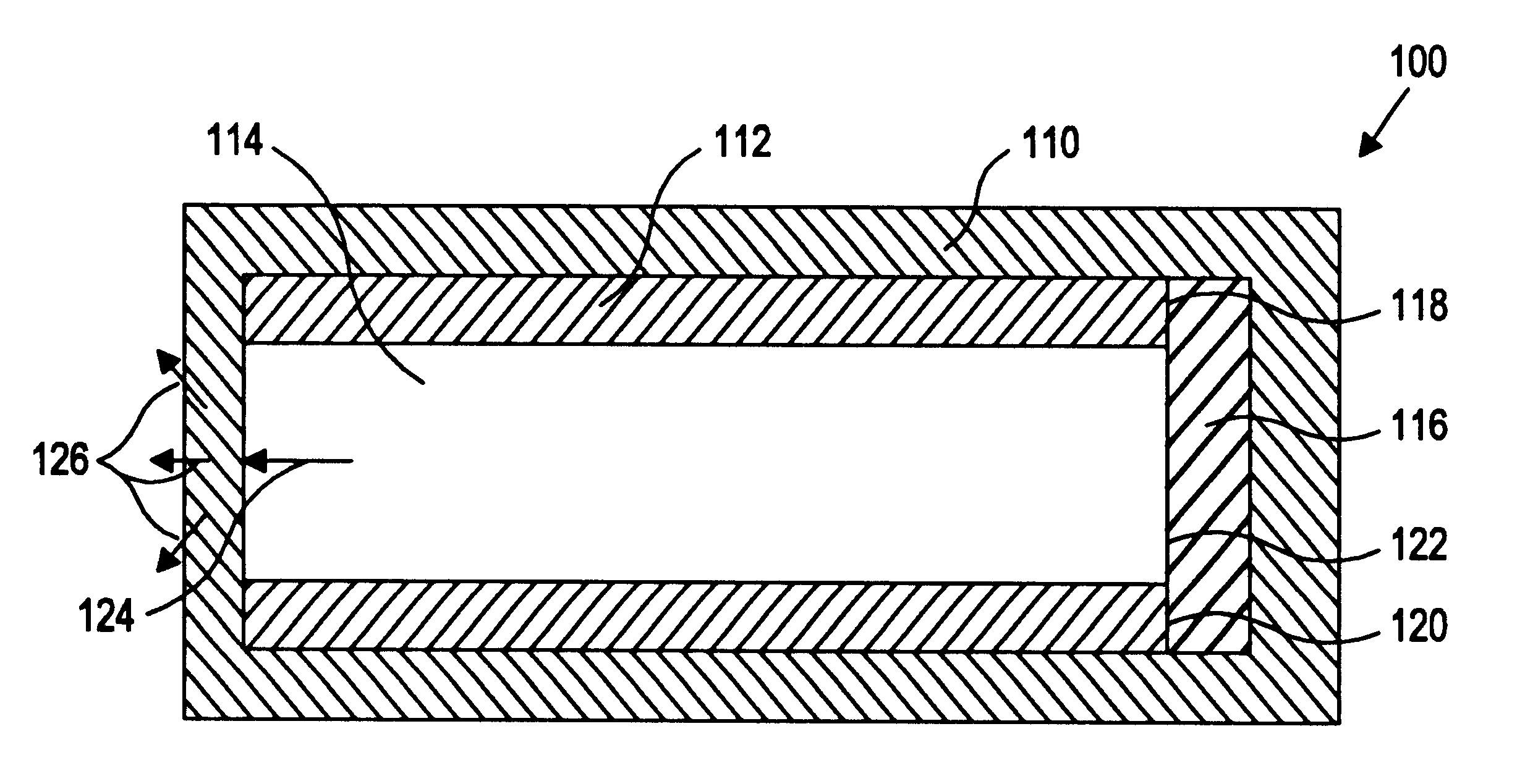
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof
InactiveUS6375972B1Without of effectWithout riskOrganic active ingredientsSenses disorderBiological bodySustained release drug
A method and device for treating a mammalian organism to obtain a desired local or systemic physiological or pharmacological effect is provided. The method includes administering a sustained release drug delivery system to a mammalian organism in need of such treatment at an area wherein release of an effective agent is desired and allowing the effective agent to pass through the device in a controlled manner. The device includes an inner core or reservoir including the effective agent, an impermeable tube which encloses portions of the reservoir, and a permeable member at an end of the tube.
Owner:EYEPOINT PHARMA INC
Extended release dosage form
InactiveUS6245357B1Free to changeOrganic active ingredientsNervous disorderExtended Release Dosage Form
A dosage form comprising a composition comprising a drug surrounded by an interior and an exterior wall with an exit for administering the drug to a patient; and a method of using the dosage form are disclosed for an indicated therapy.
Owner:ALZA CORP
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
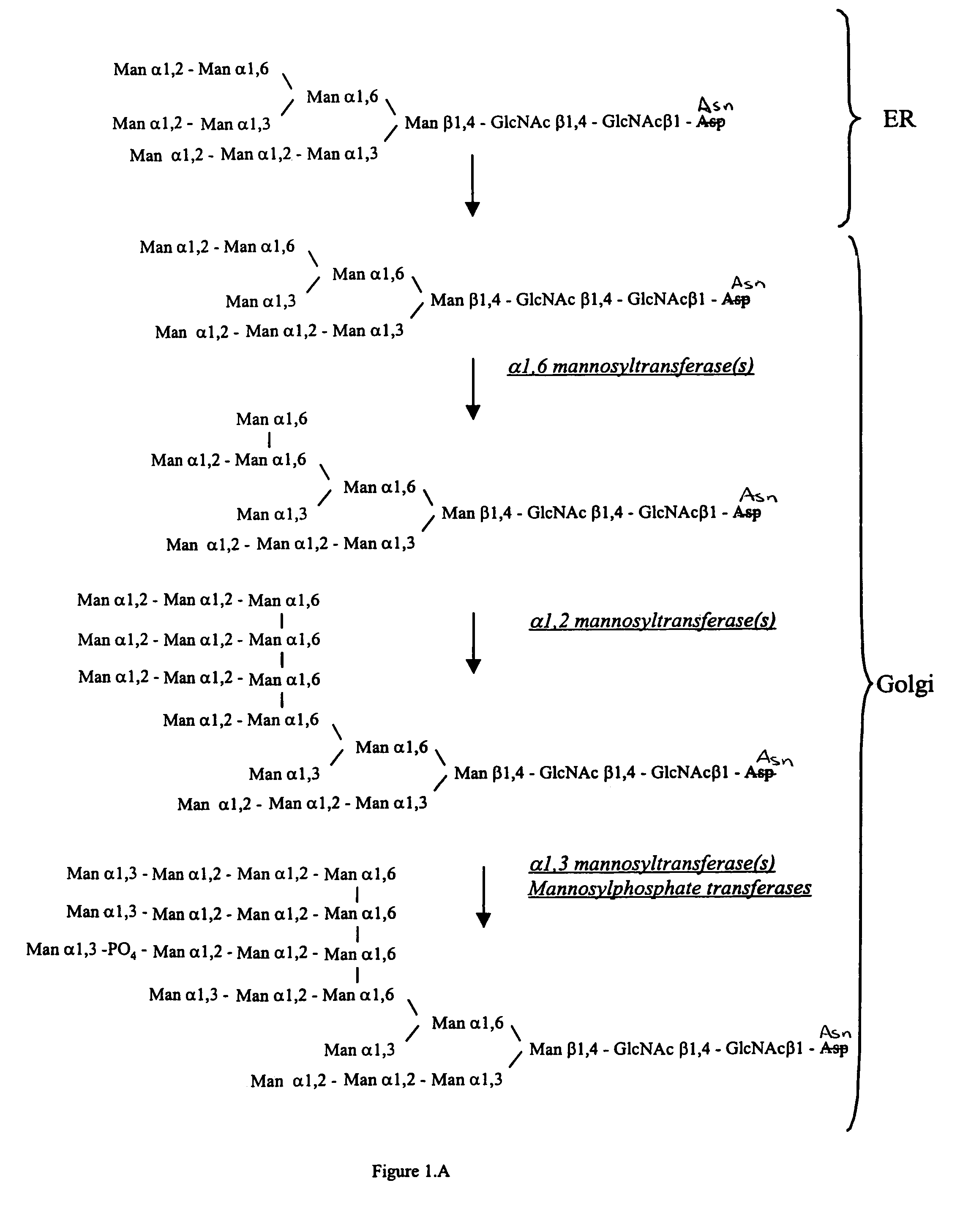
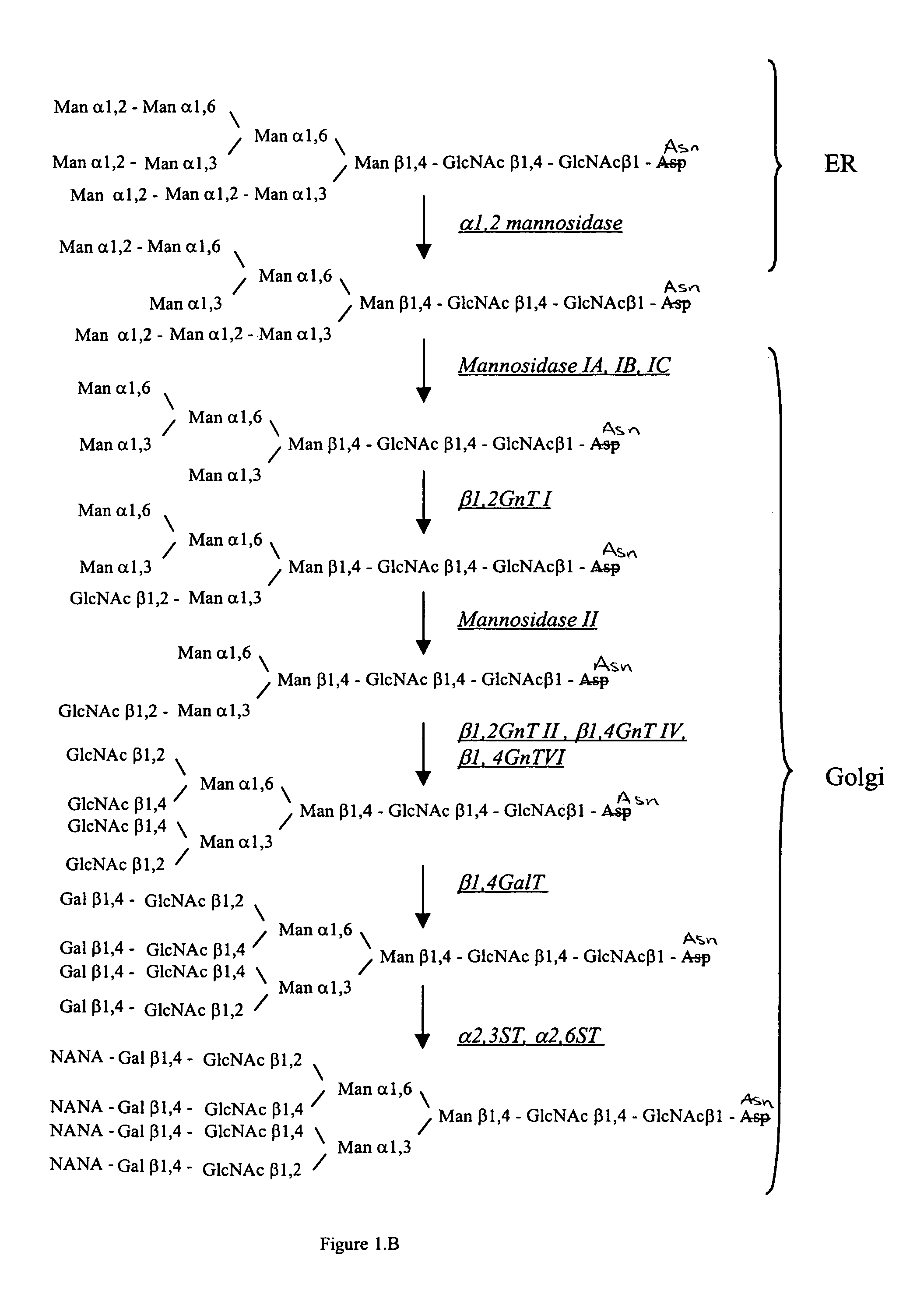
Methods for producing modified glycoproteins
Cell lines having genetically modified glycosylation pathways that allow them to carry out a sequence of enzymatic reactions, which mimic the processing of glycoproteins in humans, have been developed. Recombinant proteins expressed in these engineered hosts yield glycoproteins more similar, if not substantially identical, to their human counterparts. Thelower eukaryotes, which ordinarily produce high-mannose containing N-glycans, including unicellular and multicellular fungi are modified to produce N-glycans such as Man5GlcNAc2 or other structures along human glycosylation pathways. This is achieved using a combination of engineering and / or selection of strains which: do not express certain enzymes which create the undesirable complex structures characteristic of the fungal glycoproteins, which express exogenous enzymes selected either to have optimal activity under the conditions present in the fungi where activity is desired, or which are targeted to an organelle where optimal activity is achieved, and combinations thereof wherein the genetically engineered eukaryote expresses multiple exogenous enzymes required to produce “human-like” glycoproteins.
Owner:GLYCOFI
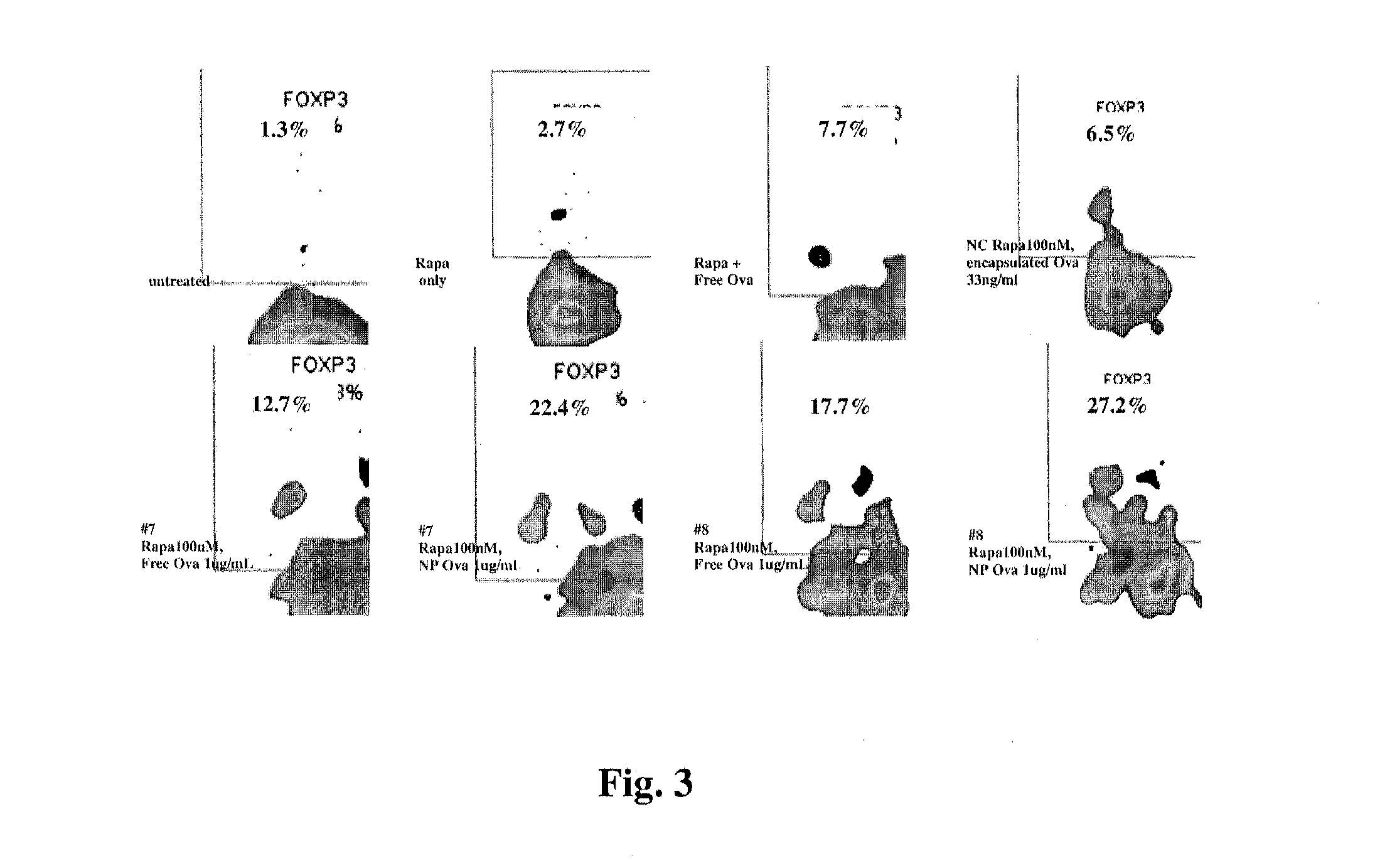
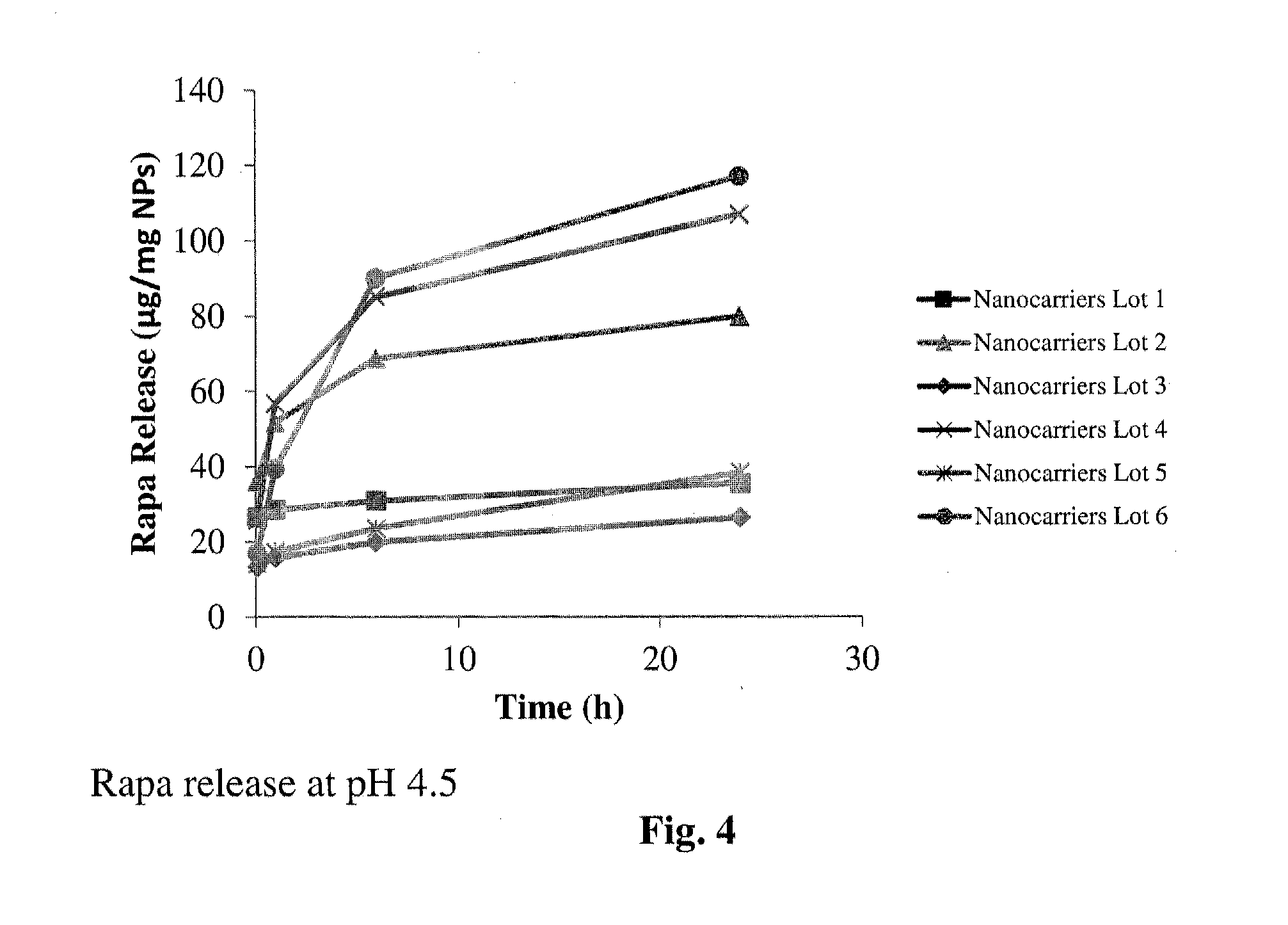
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
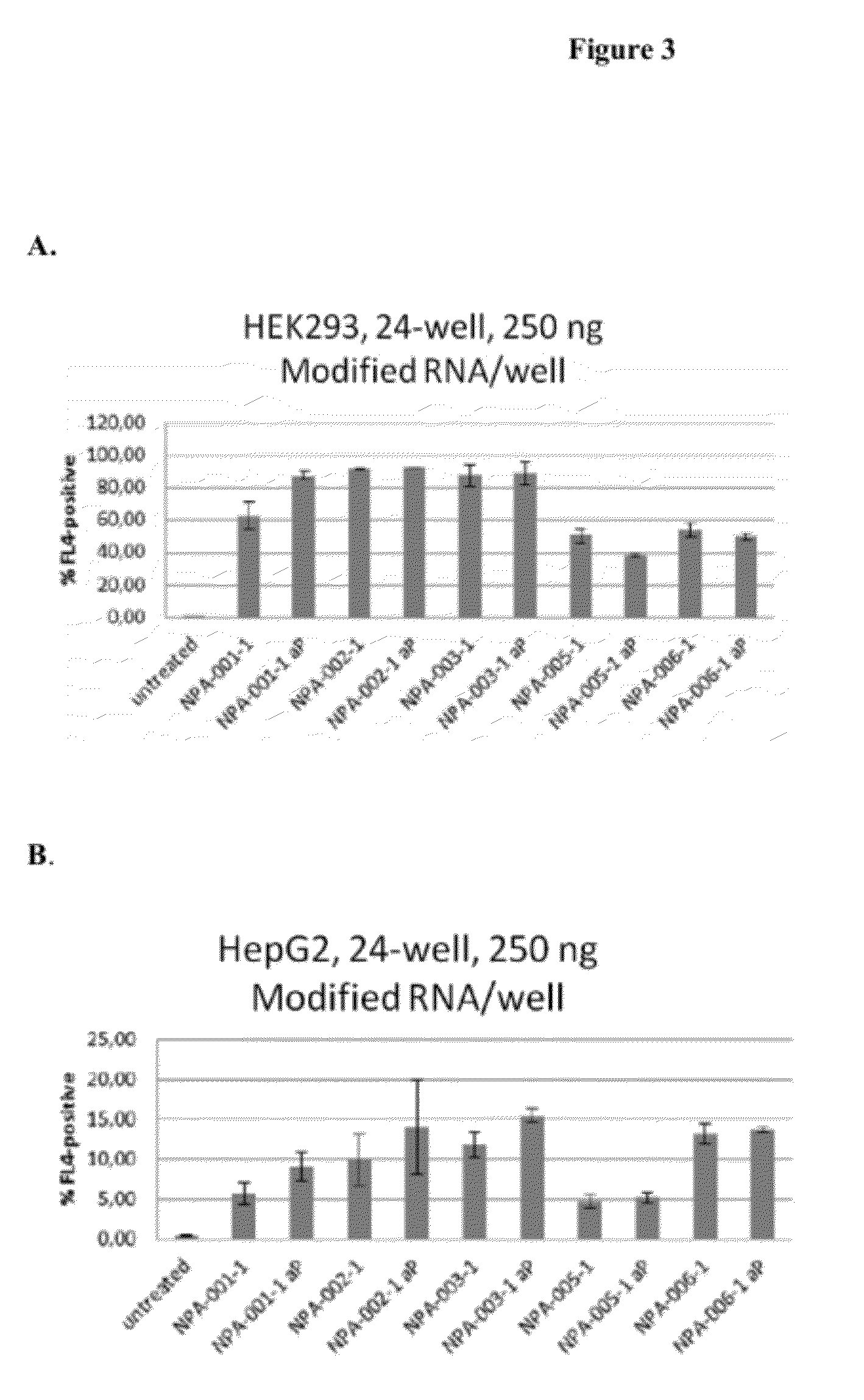
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
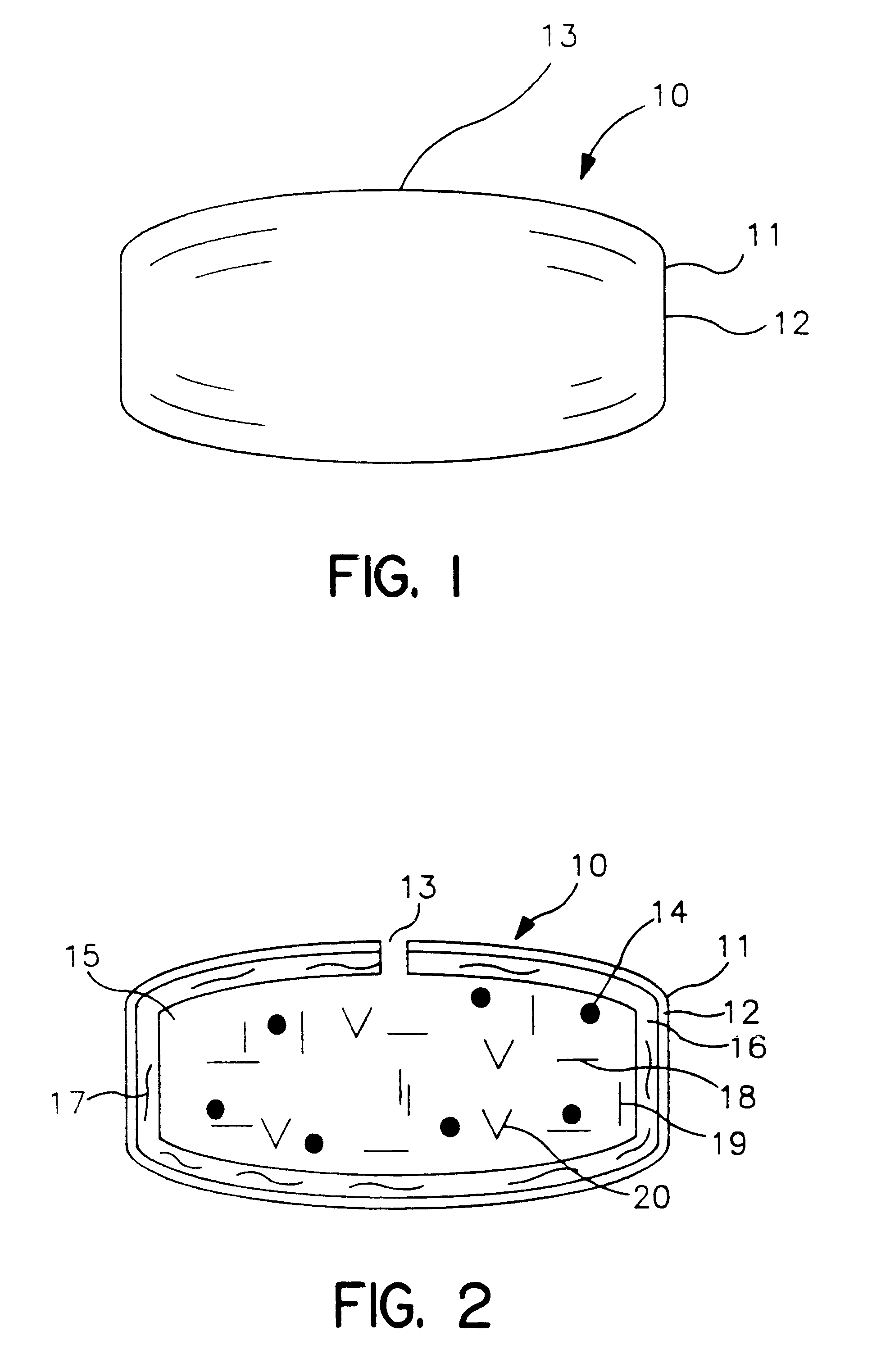
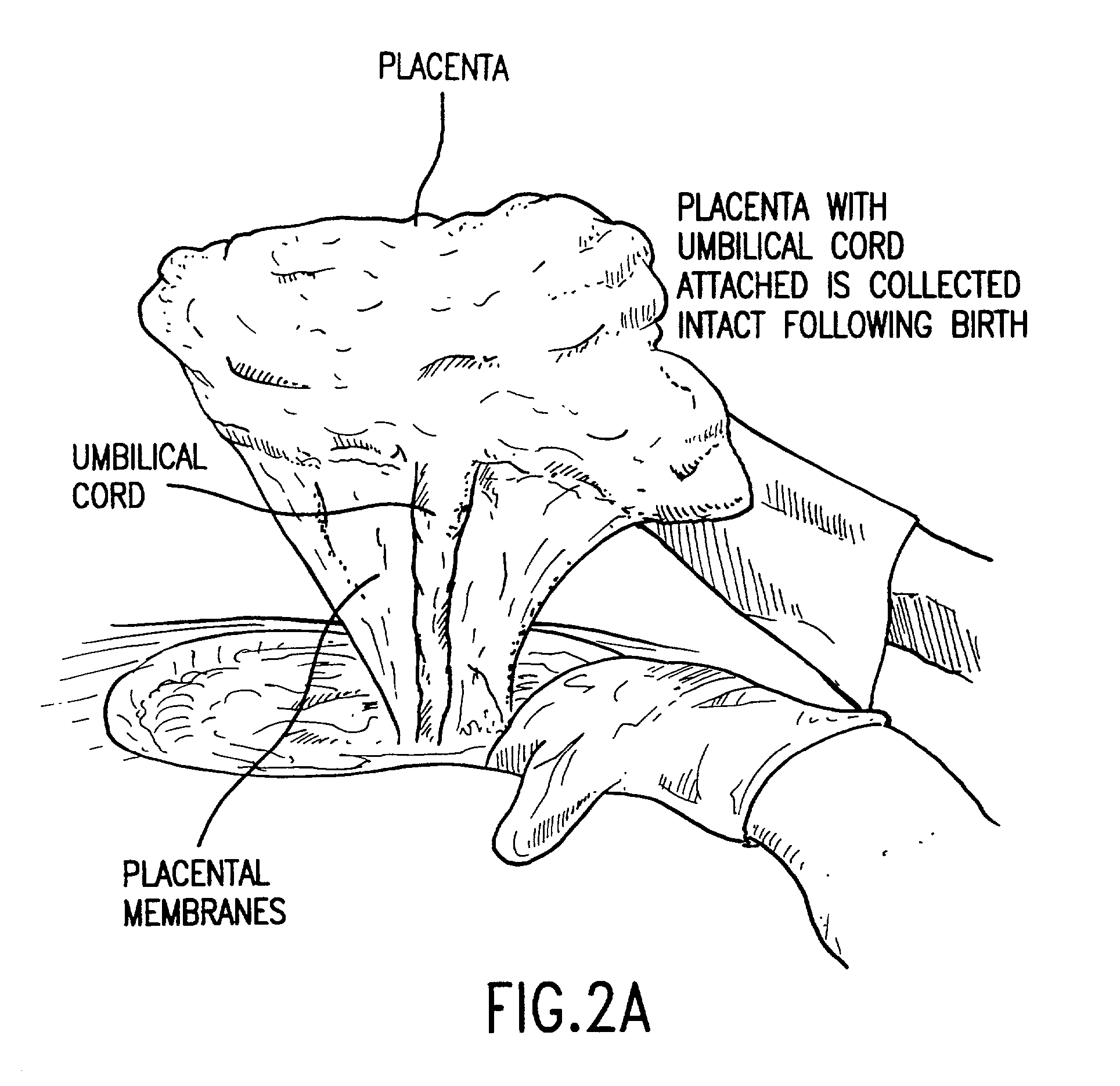
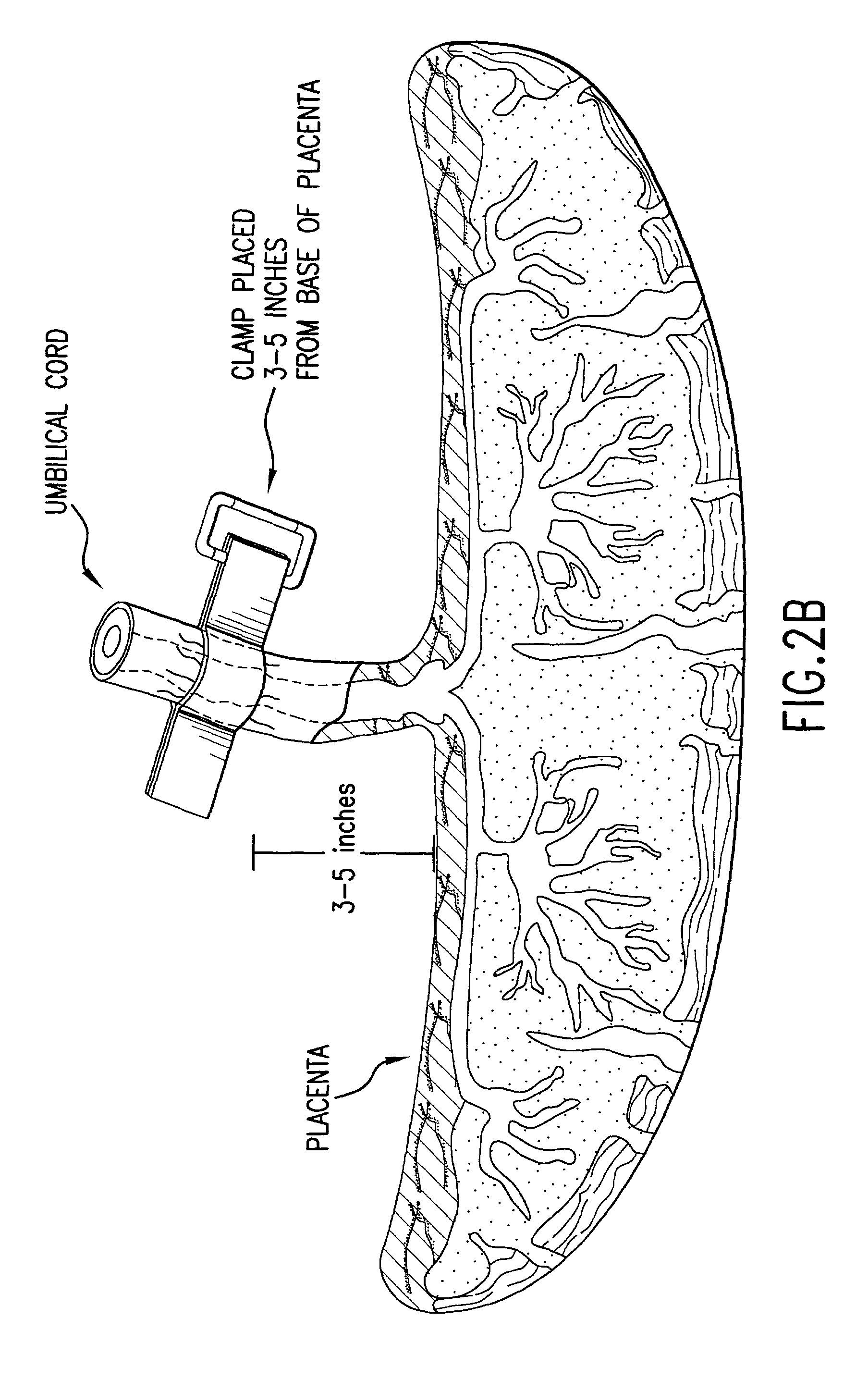
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
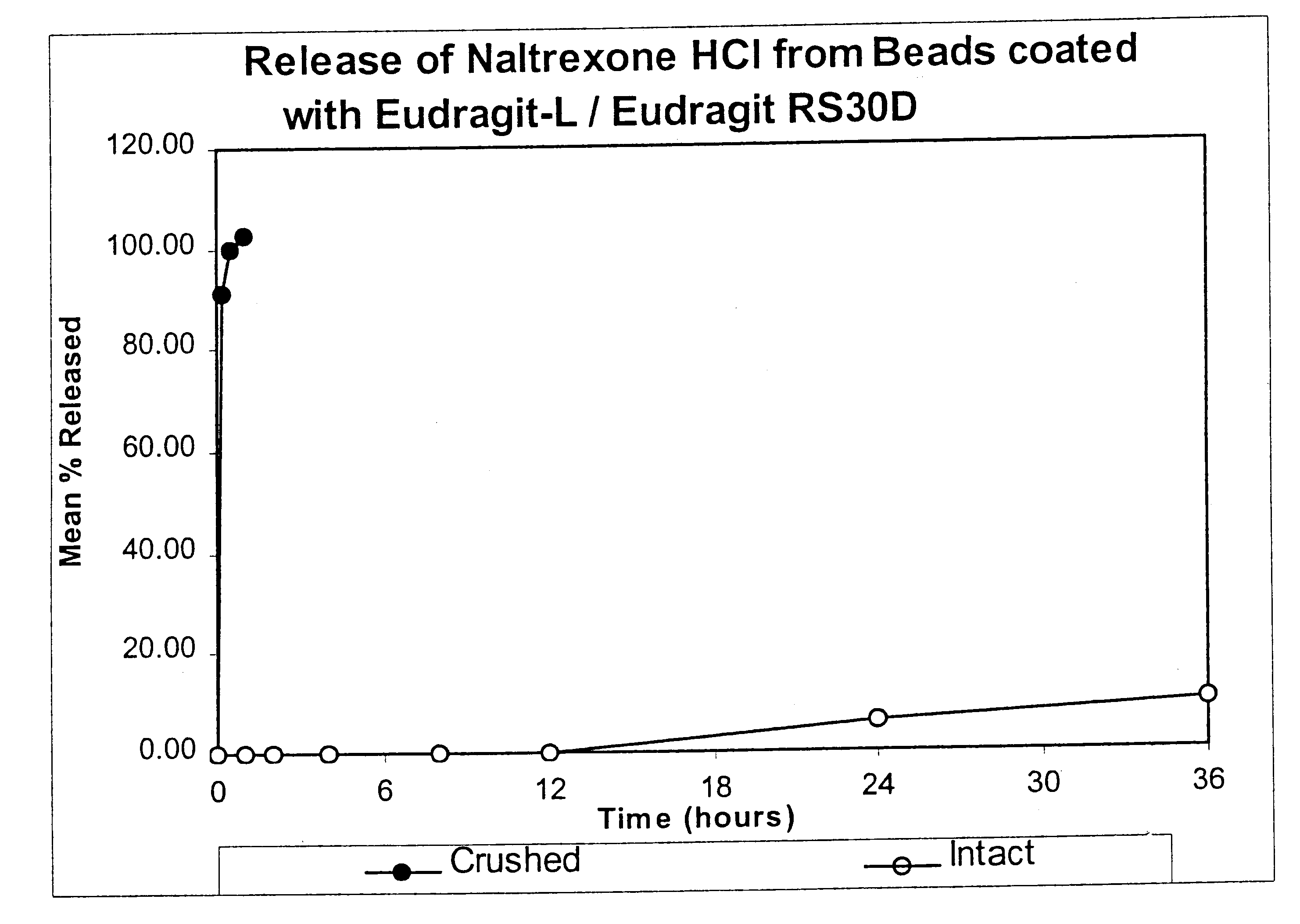
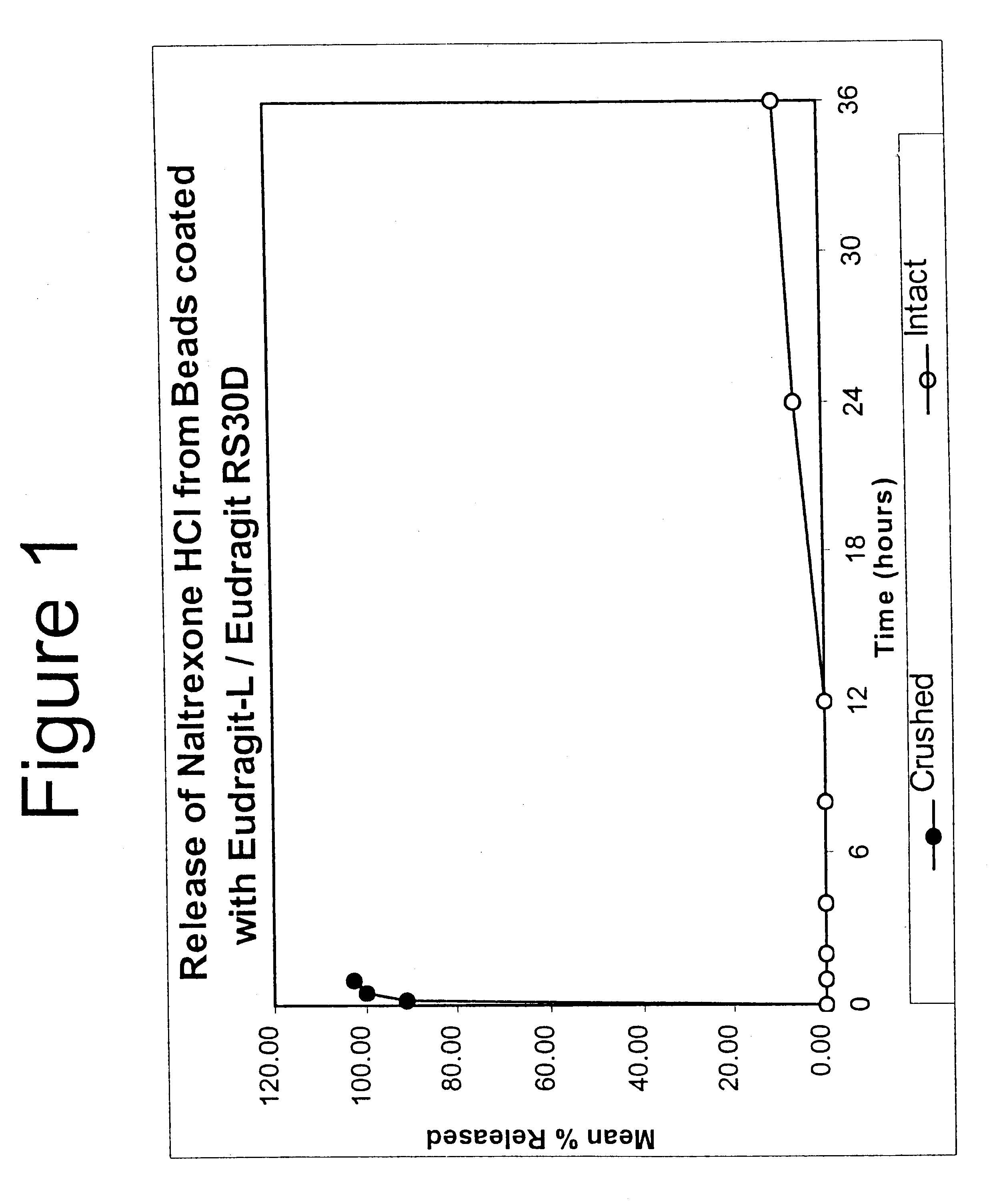
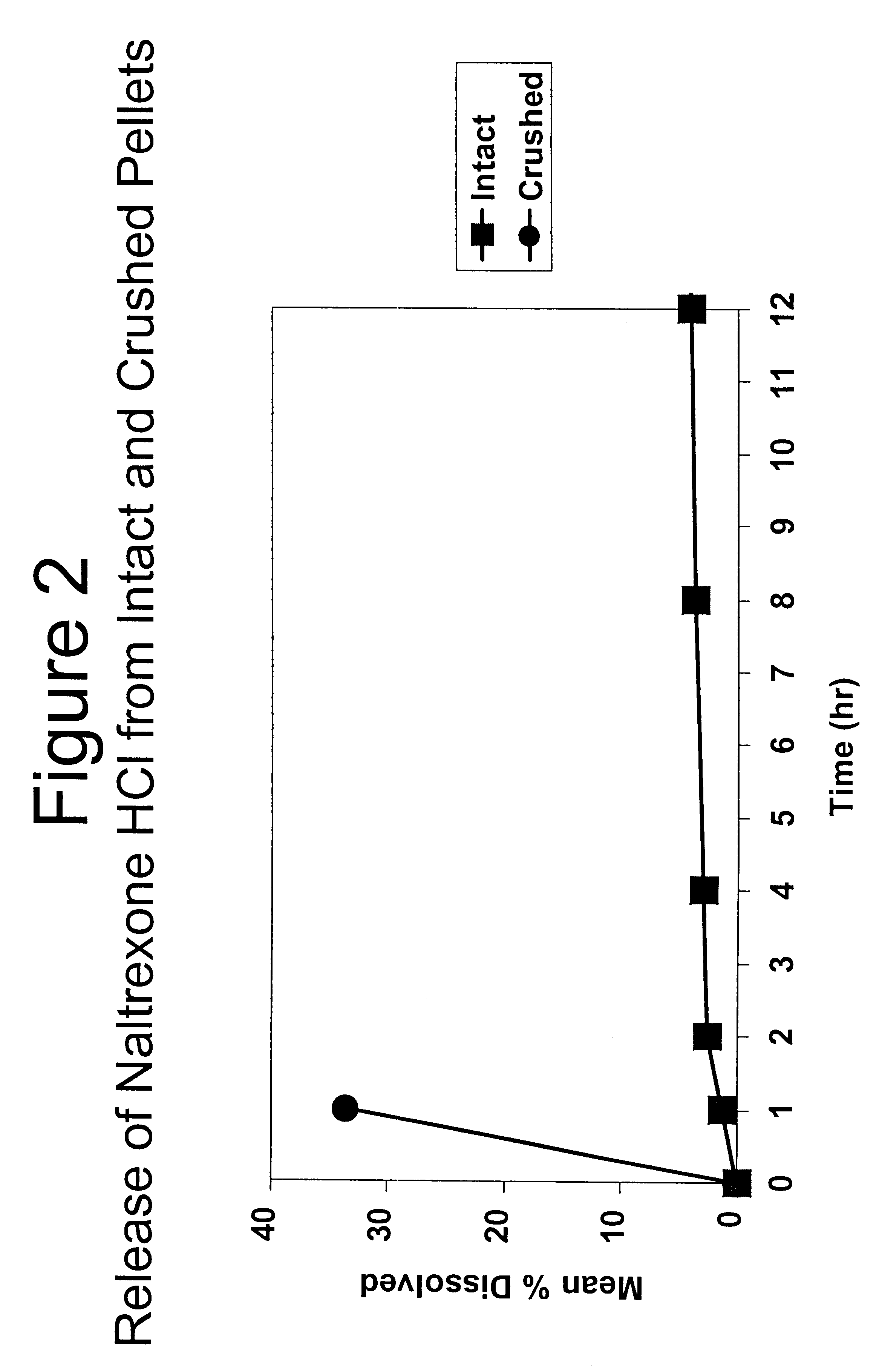
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
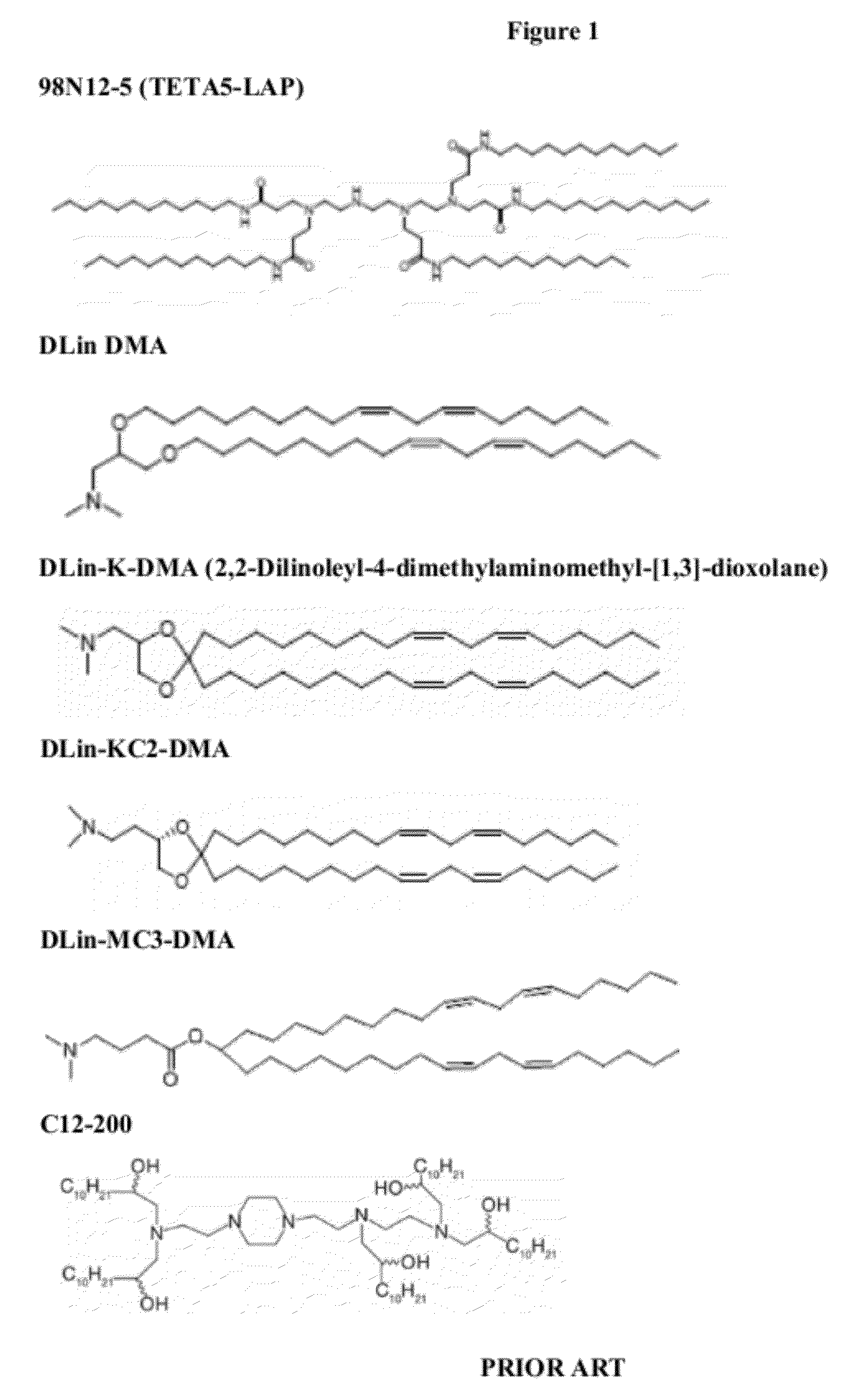
Cationic lipids and methods of use
ActiveUS20060083780A1Increase flexibilityImprove propertiesUltrasonic/sonic/infrasonic diagnosticsNervous disorderLipid formationLipid particle
The present invention provides compositions comprising cationic lipids, liposomes and nucleic acid-lipid particles comprising the cationic lipids, and methods of using such compositions, liposomes, and nucleic acid-lipid particles.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Methods and systems for annotating biomolecular sequences
A method of annotating biomolecular sequences. The method comprises (a) computationally clustering the biomolecular sequences according to a progressive homology range, to thereby generate a plurality of clusters each being of a predetermined homology of the homology range; and (b) assigning at least one ontology to each cluster of the plurality of clusters, the at least one ontology being: (i) derived from an annotation preassociated with at least one biomolecular sequence of each cluster; and / or (ii) generated from analysis of the at least one biomolecular sequence of each cluster thereby annotating biomolecular sequences.
Owner:COMPUGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com