Patents
Literature
211 results about "Fc receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
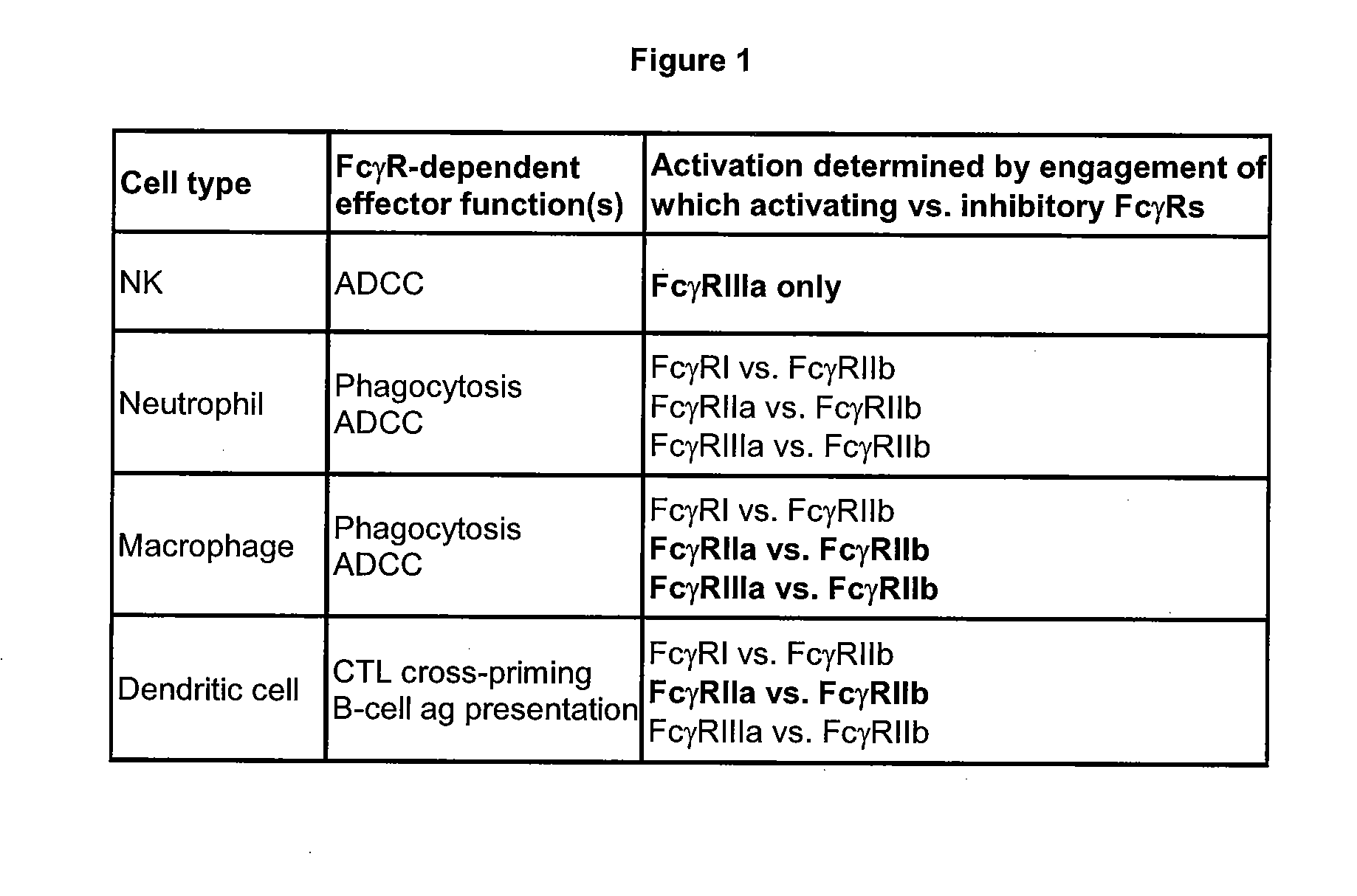
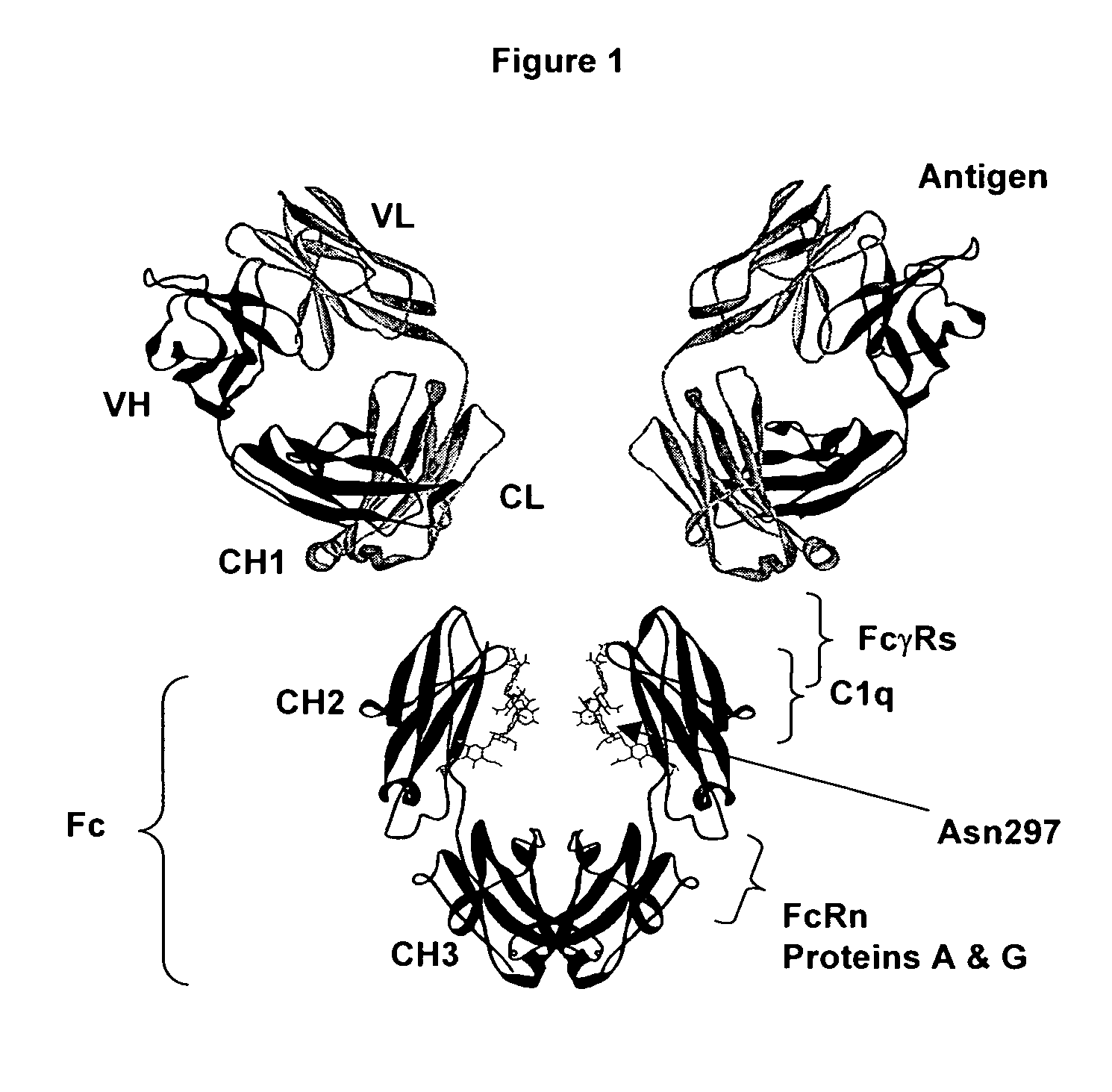
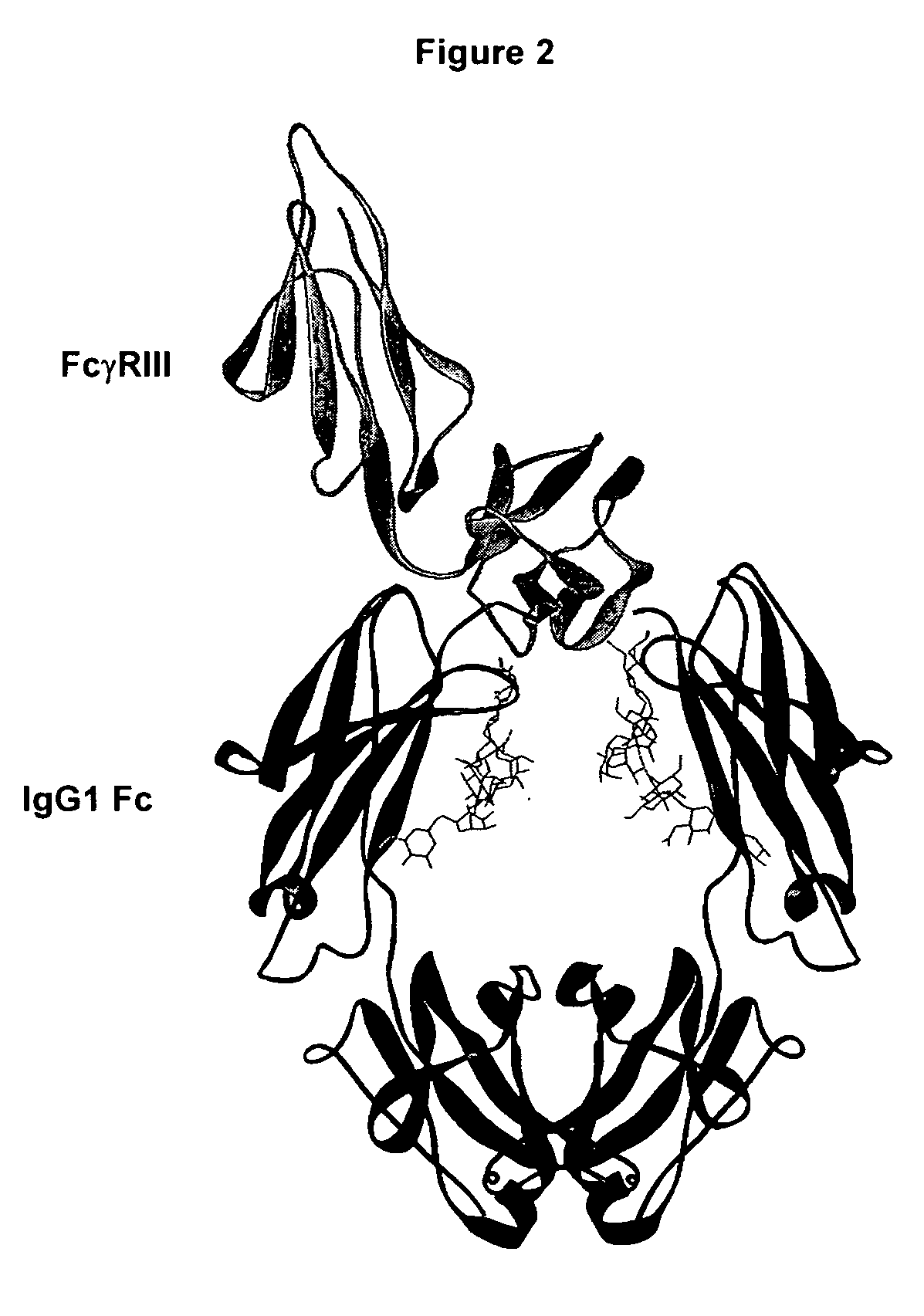
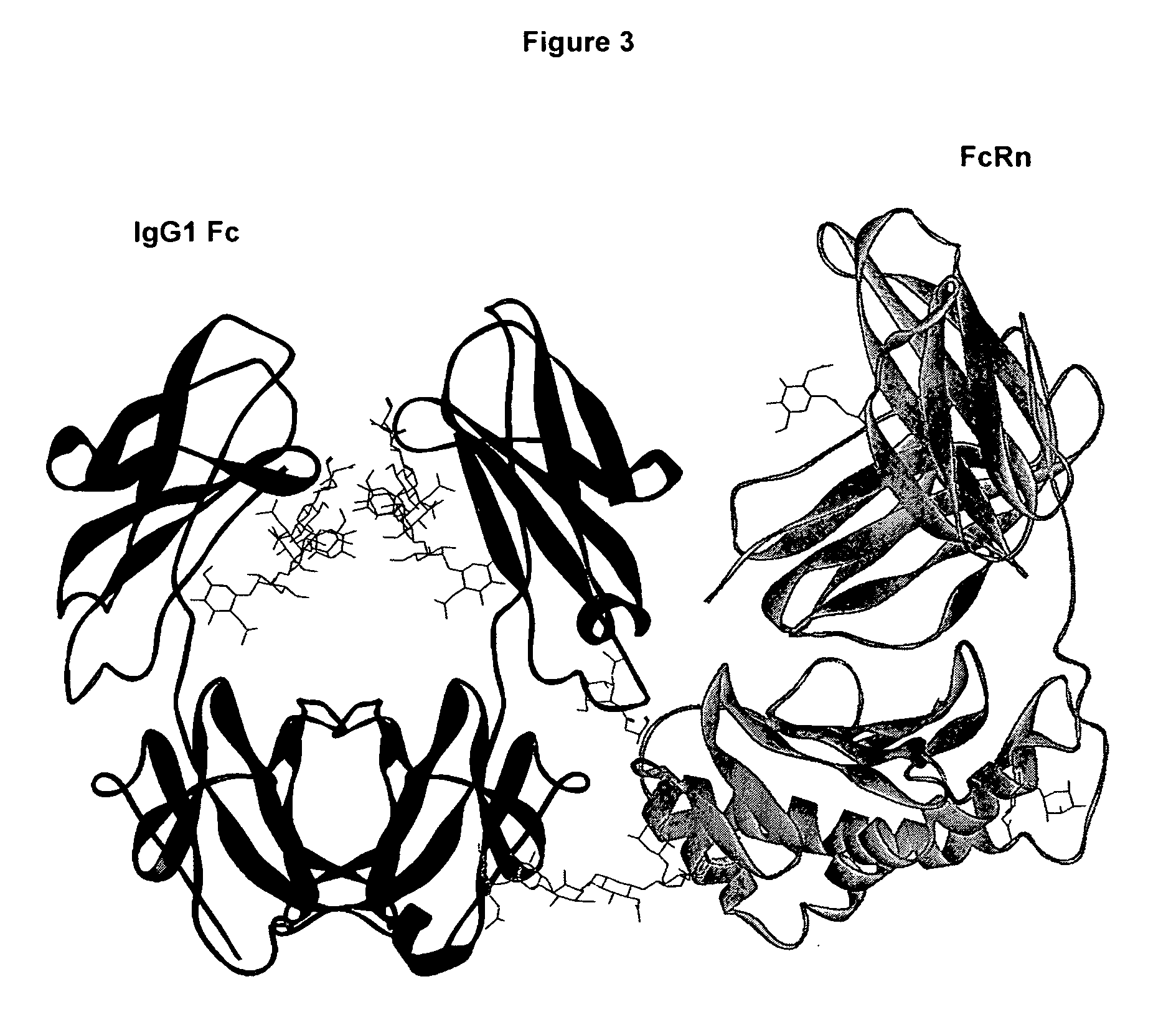
An Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc (Fragment, crystallizable) region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.
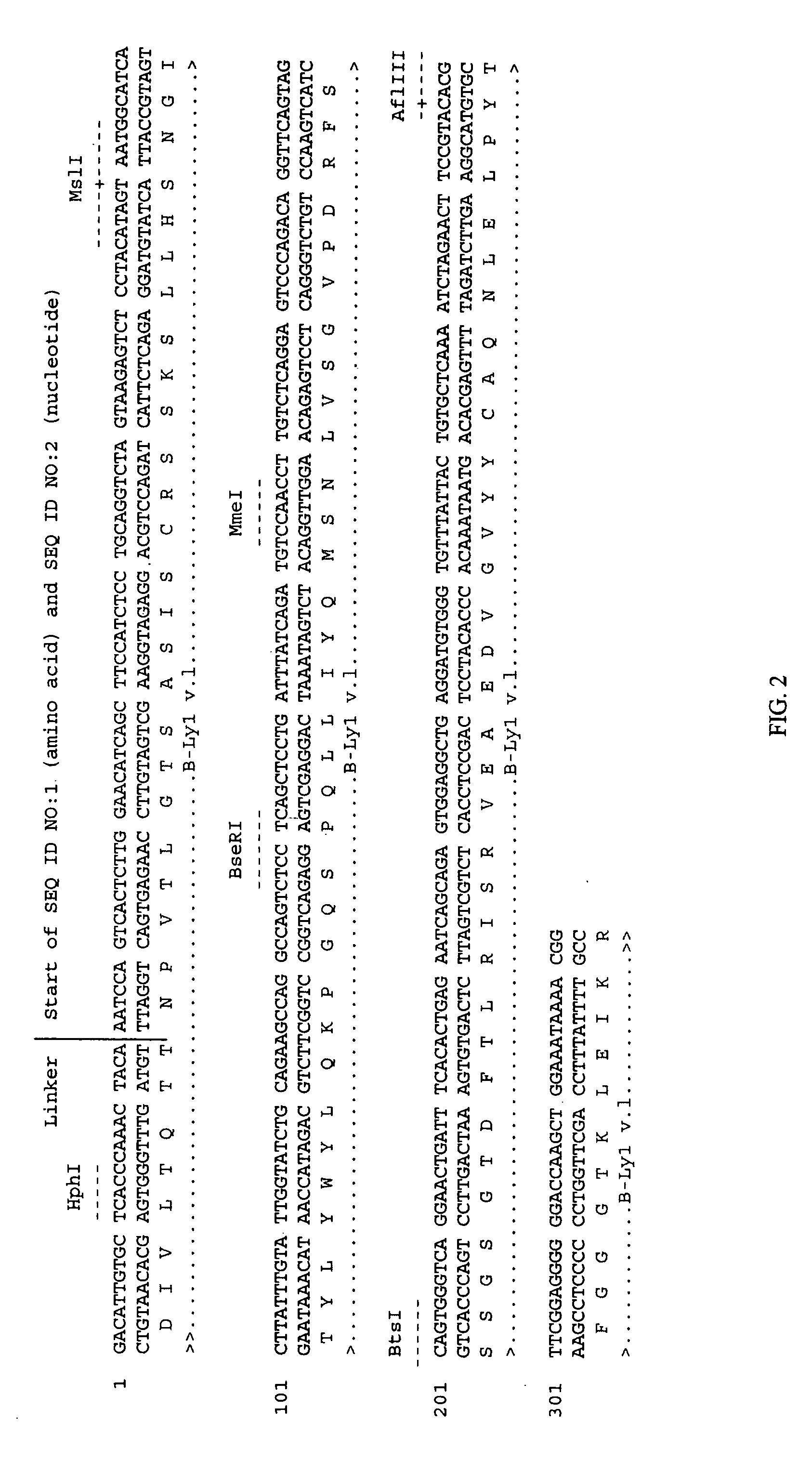
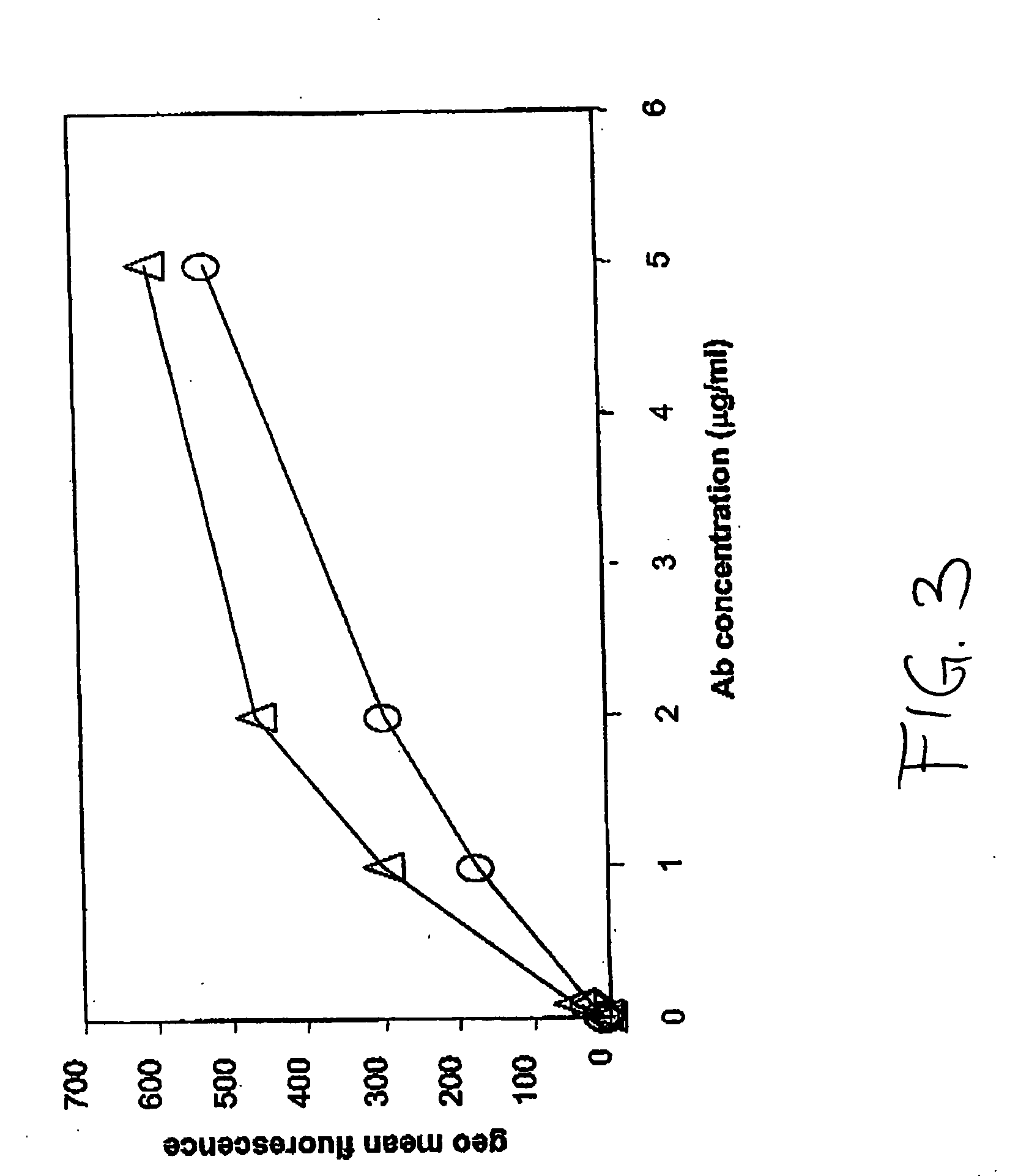
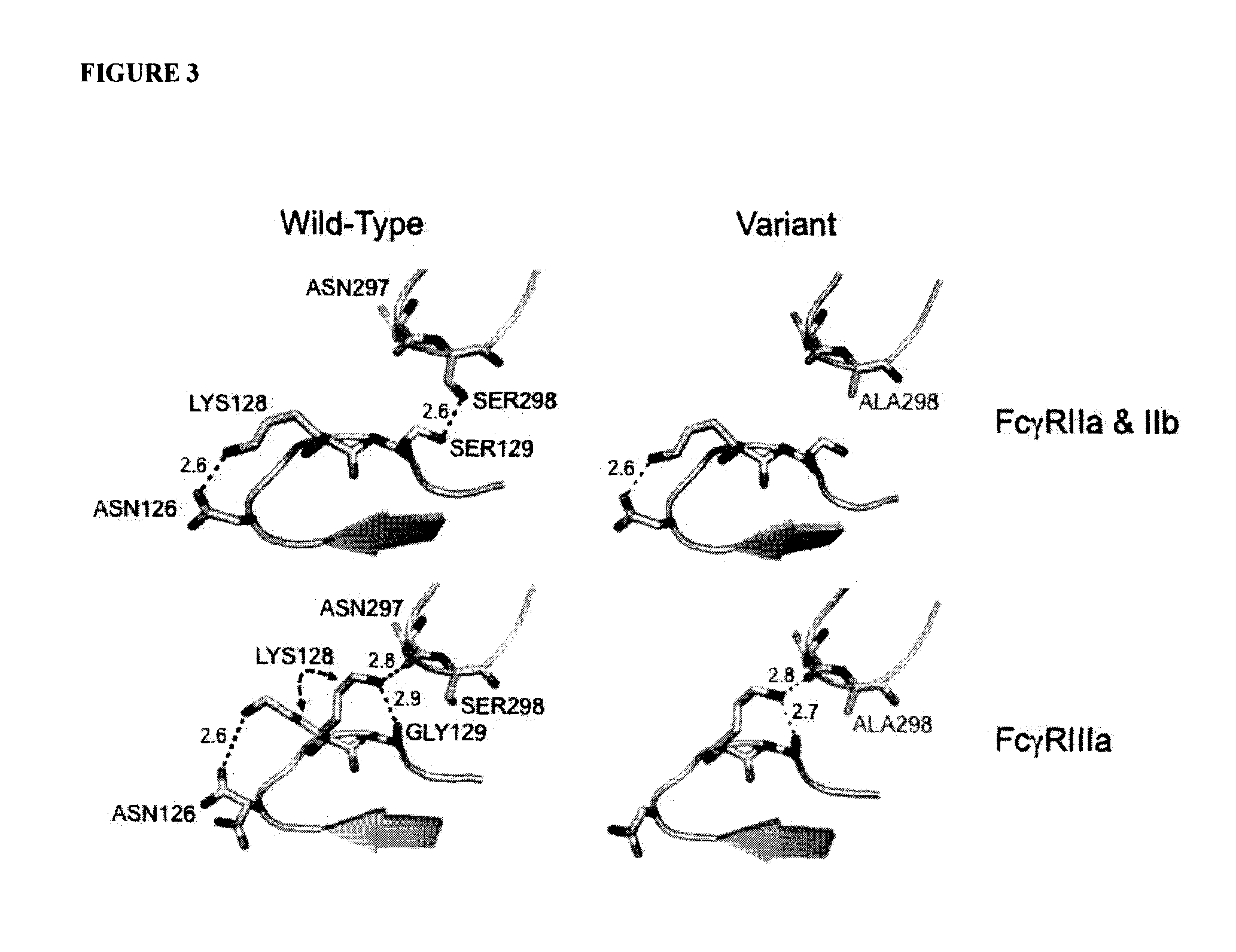
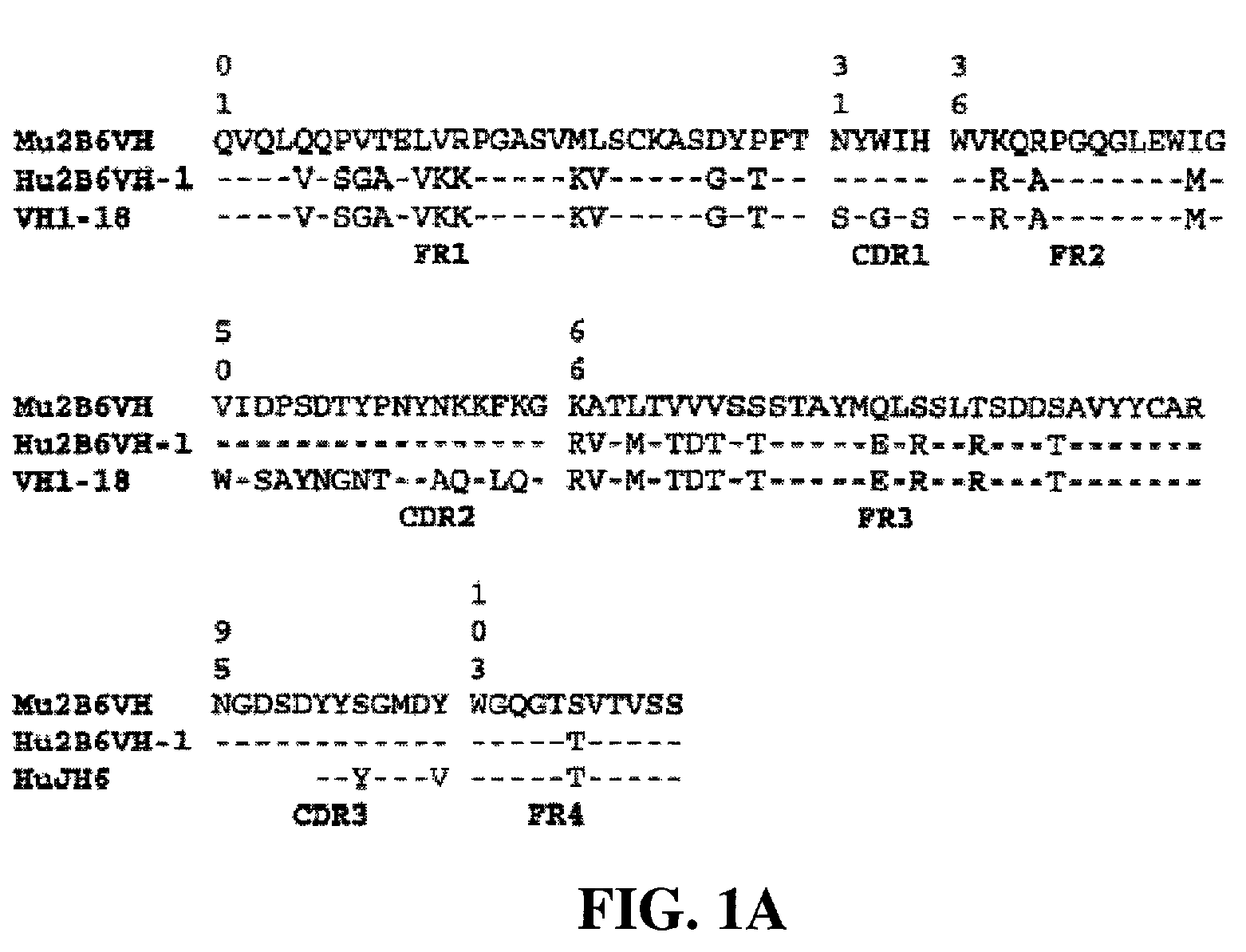
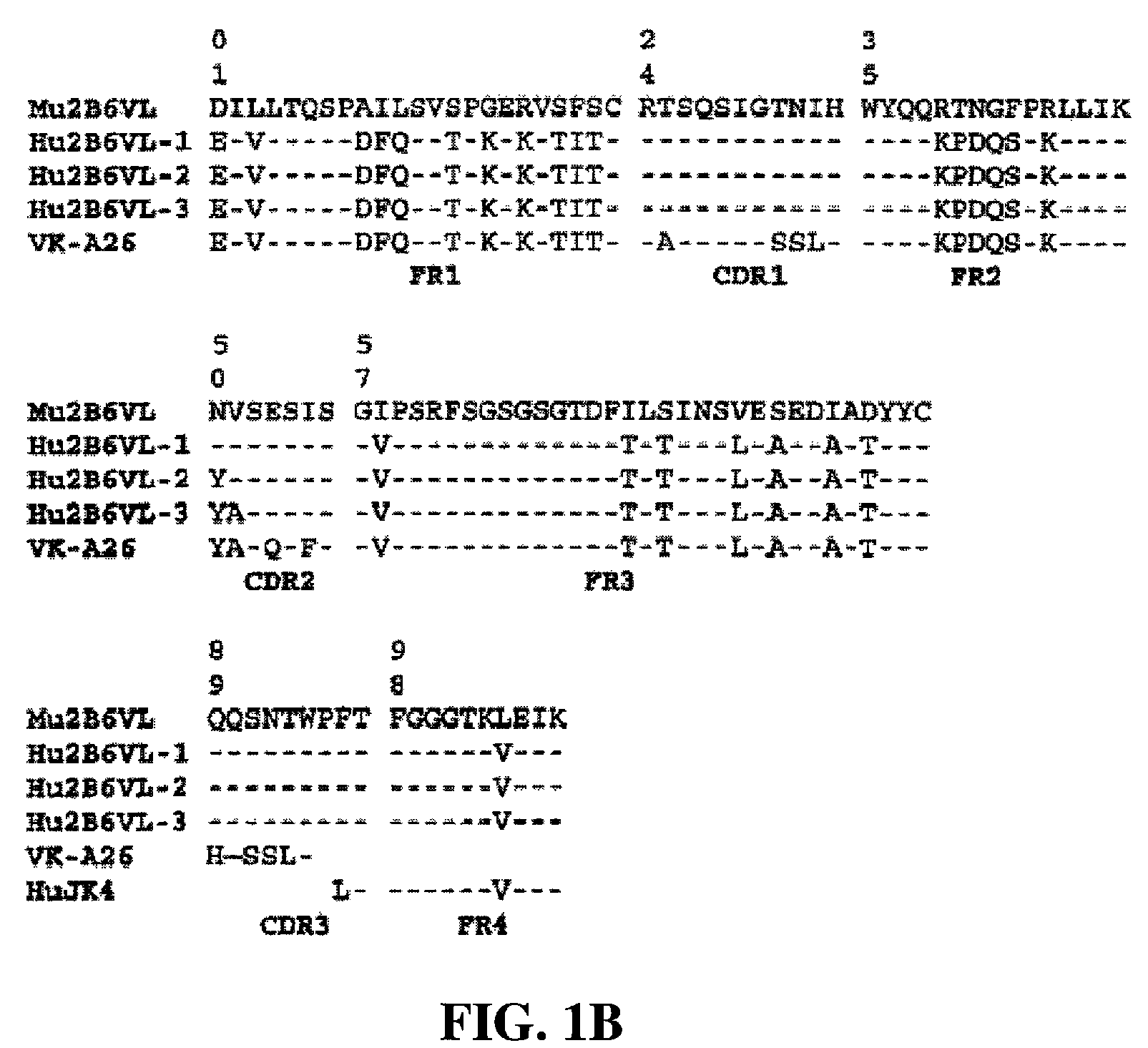
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
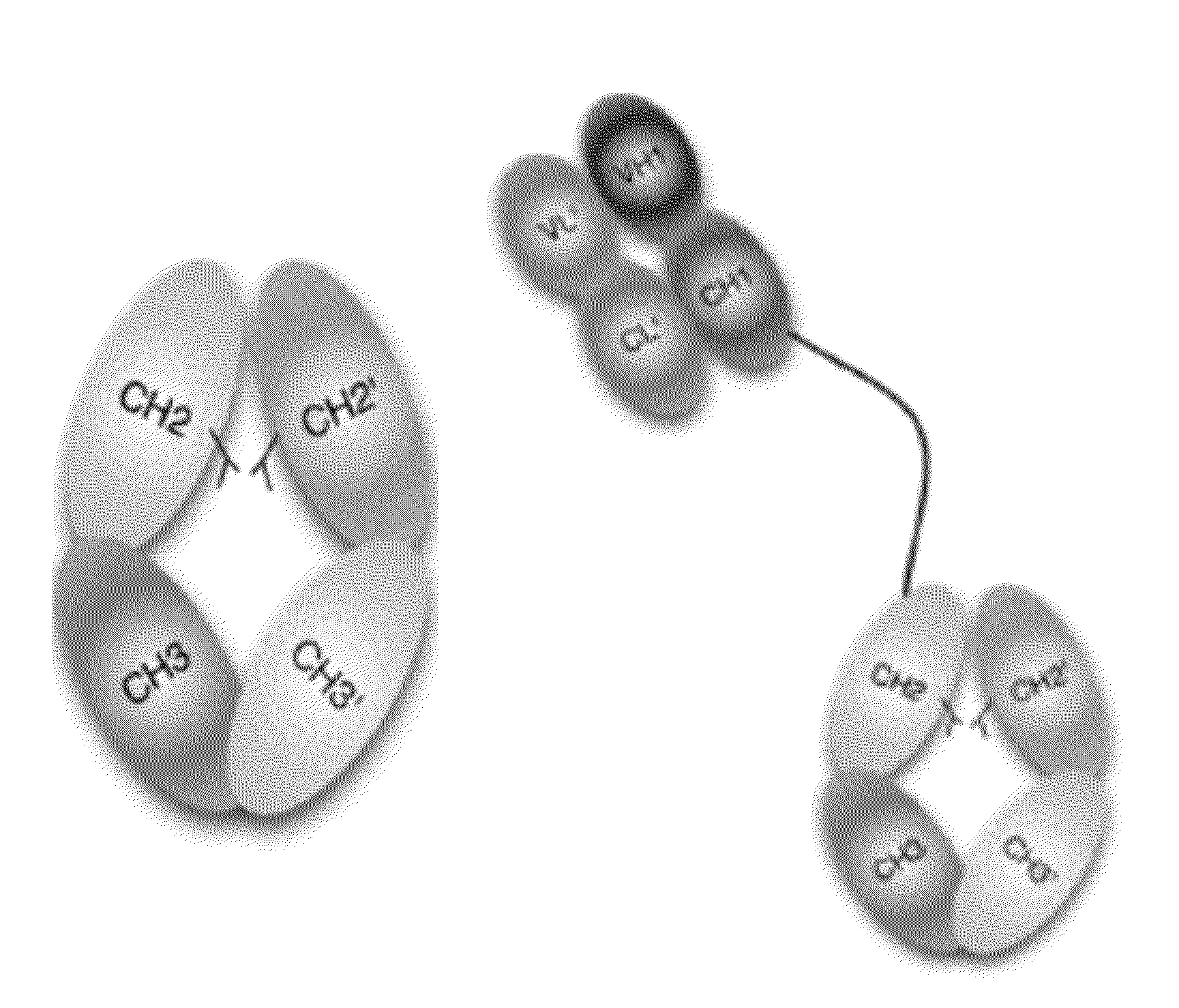
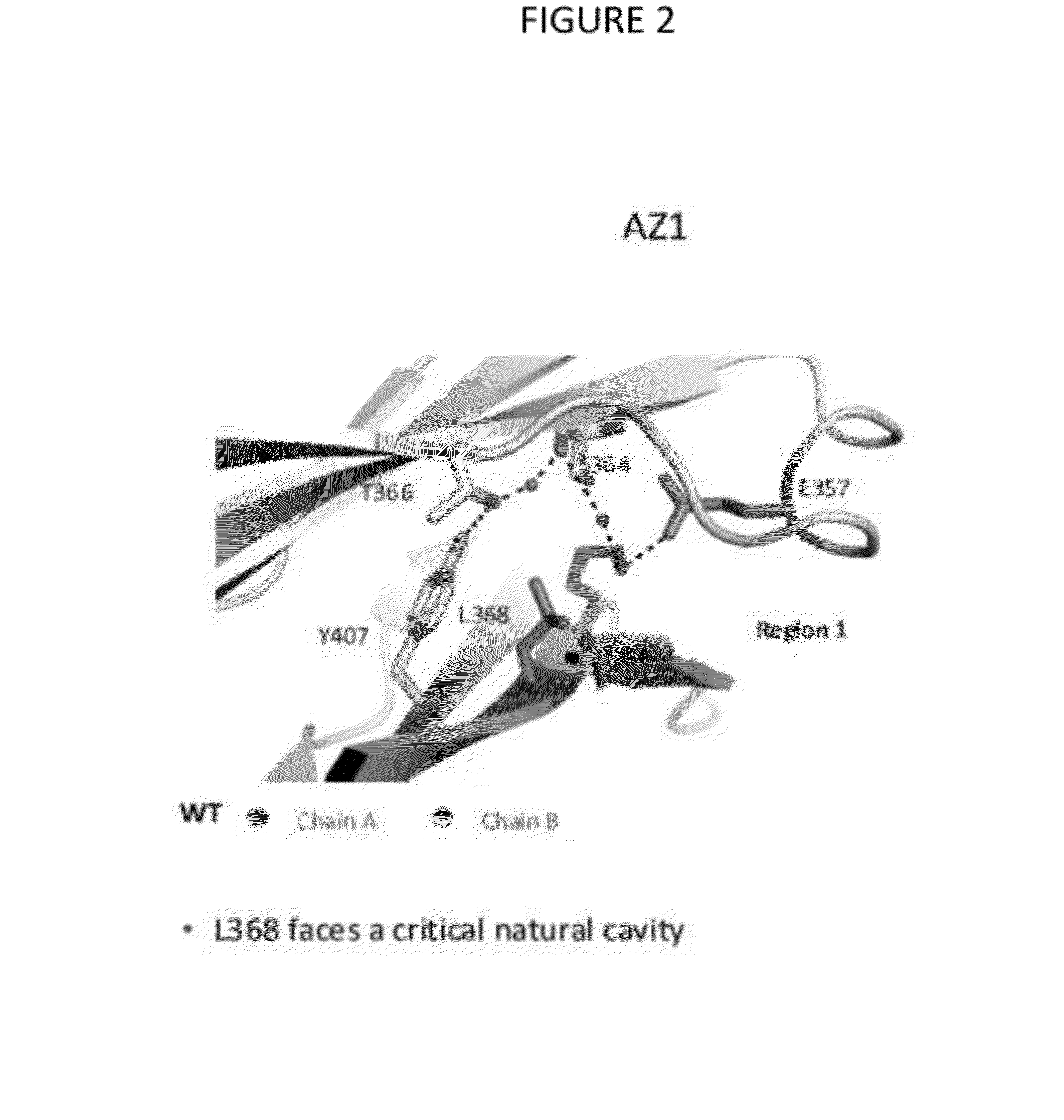
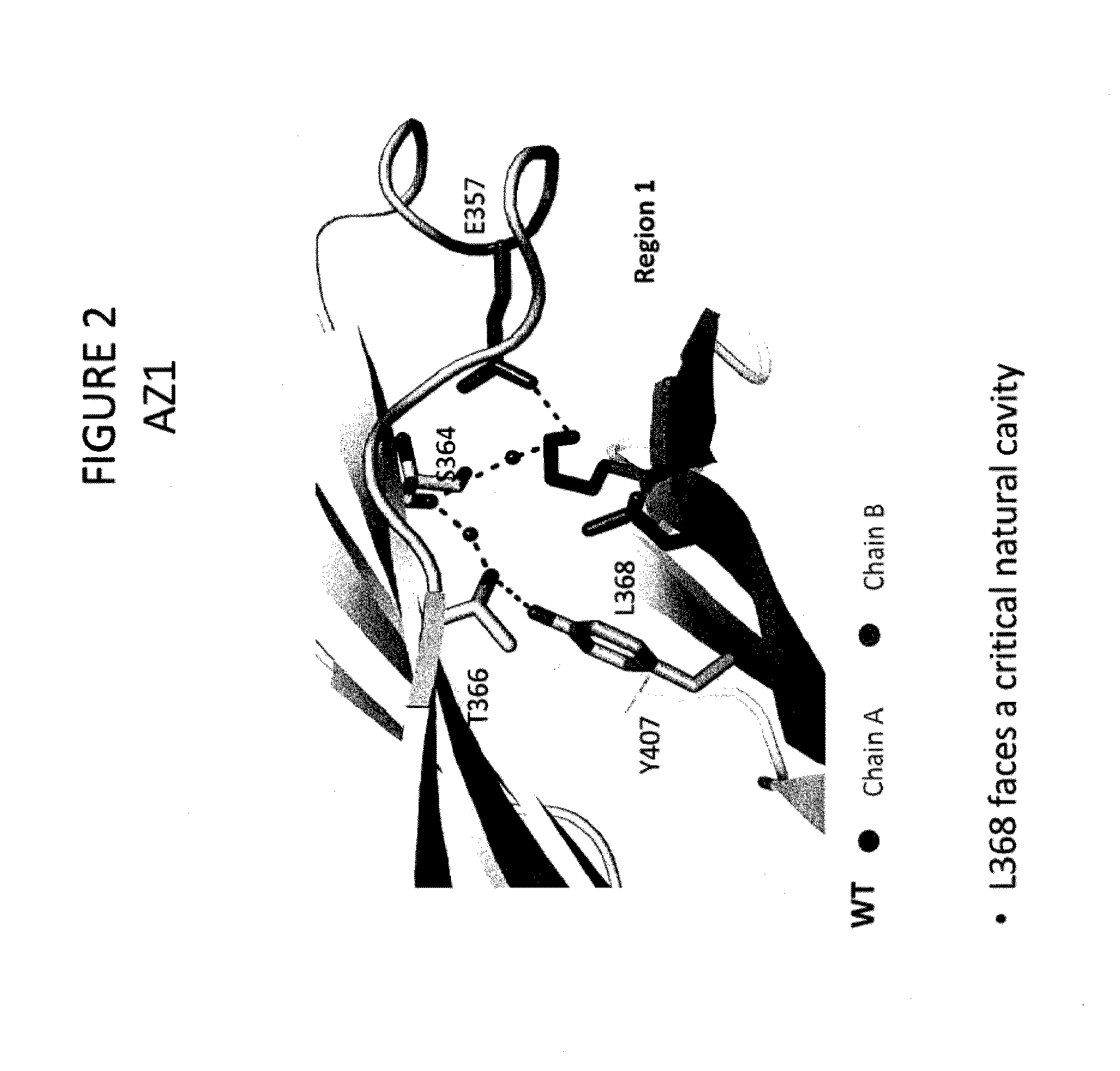
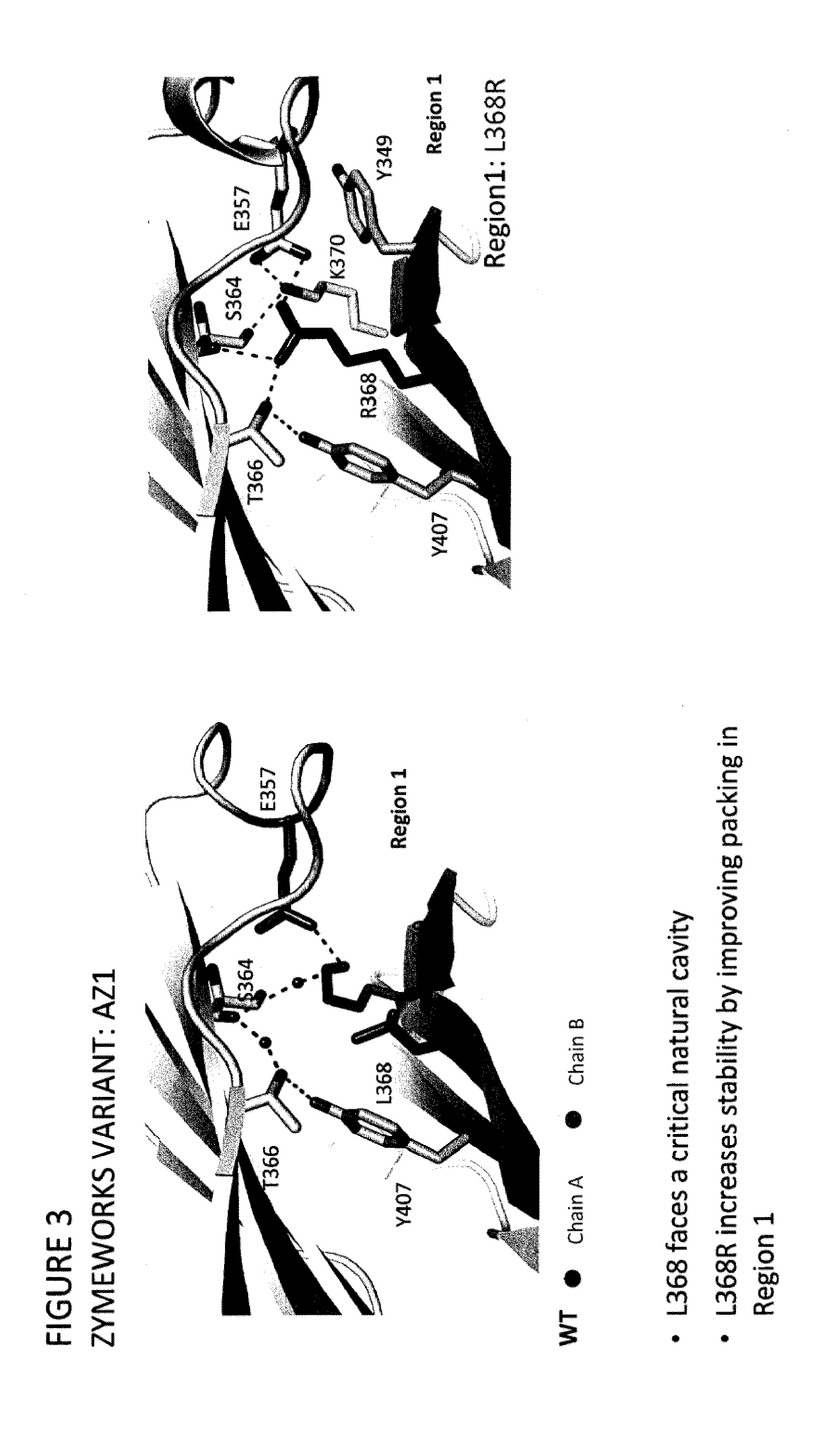
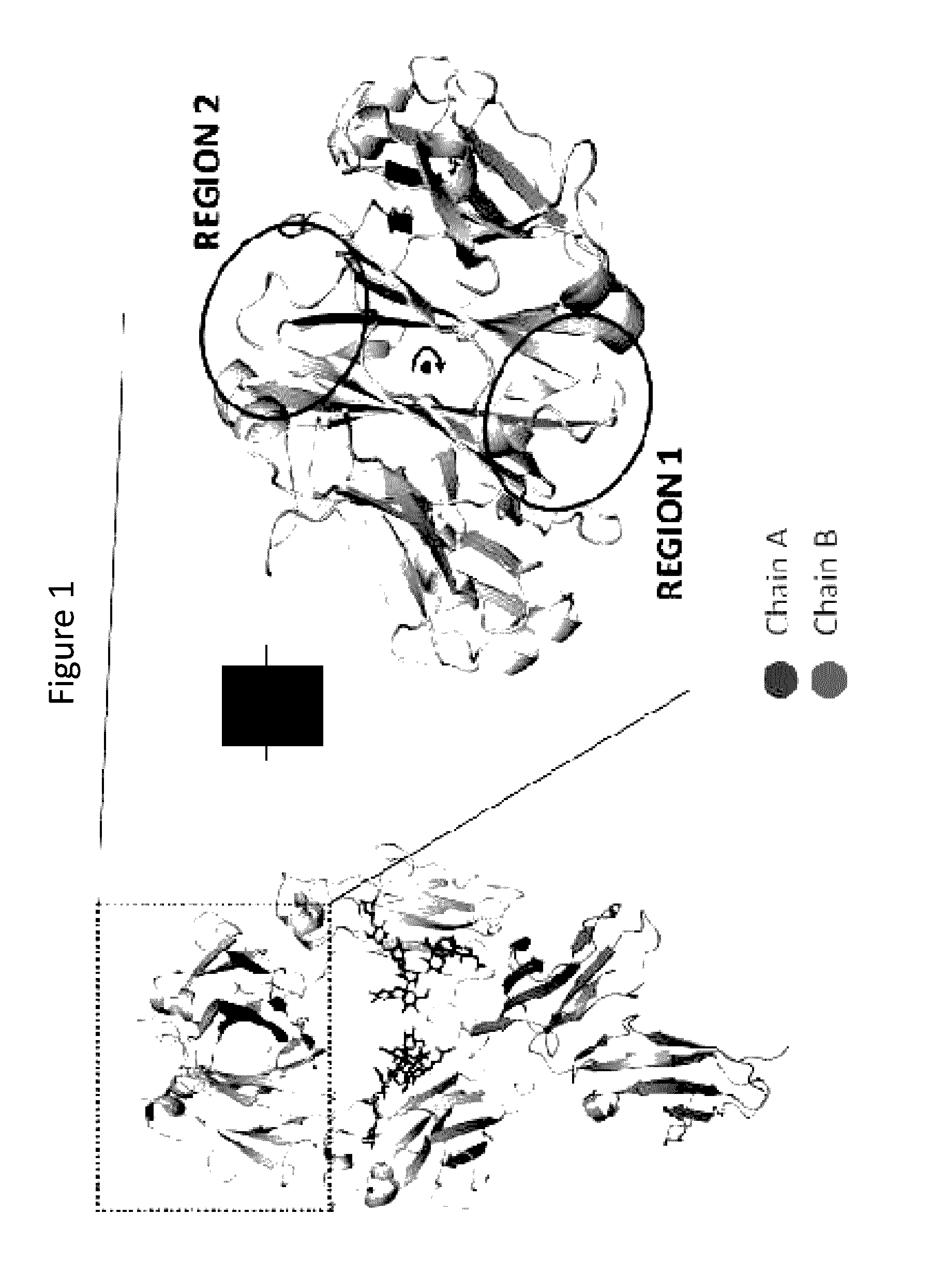
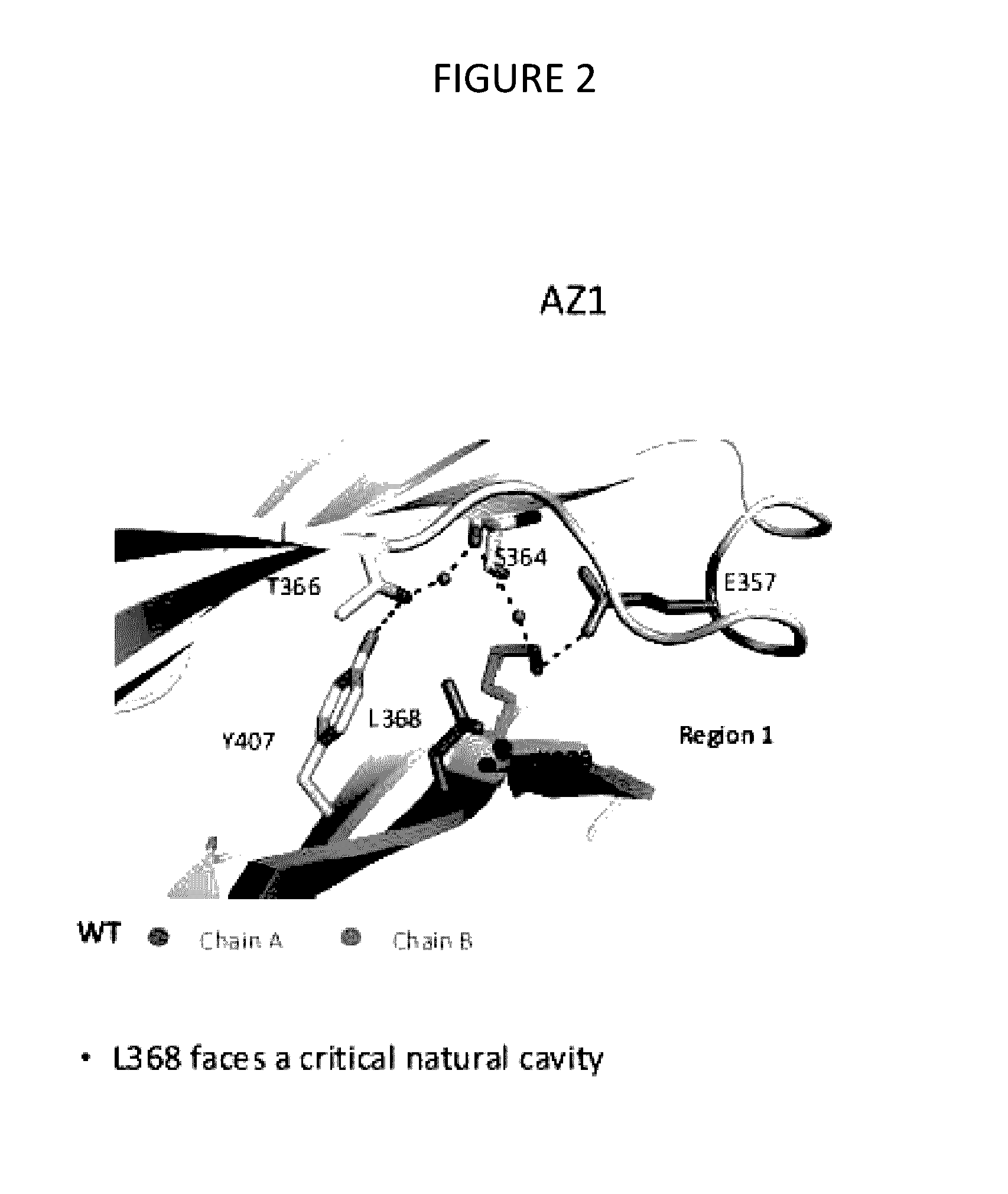
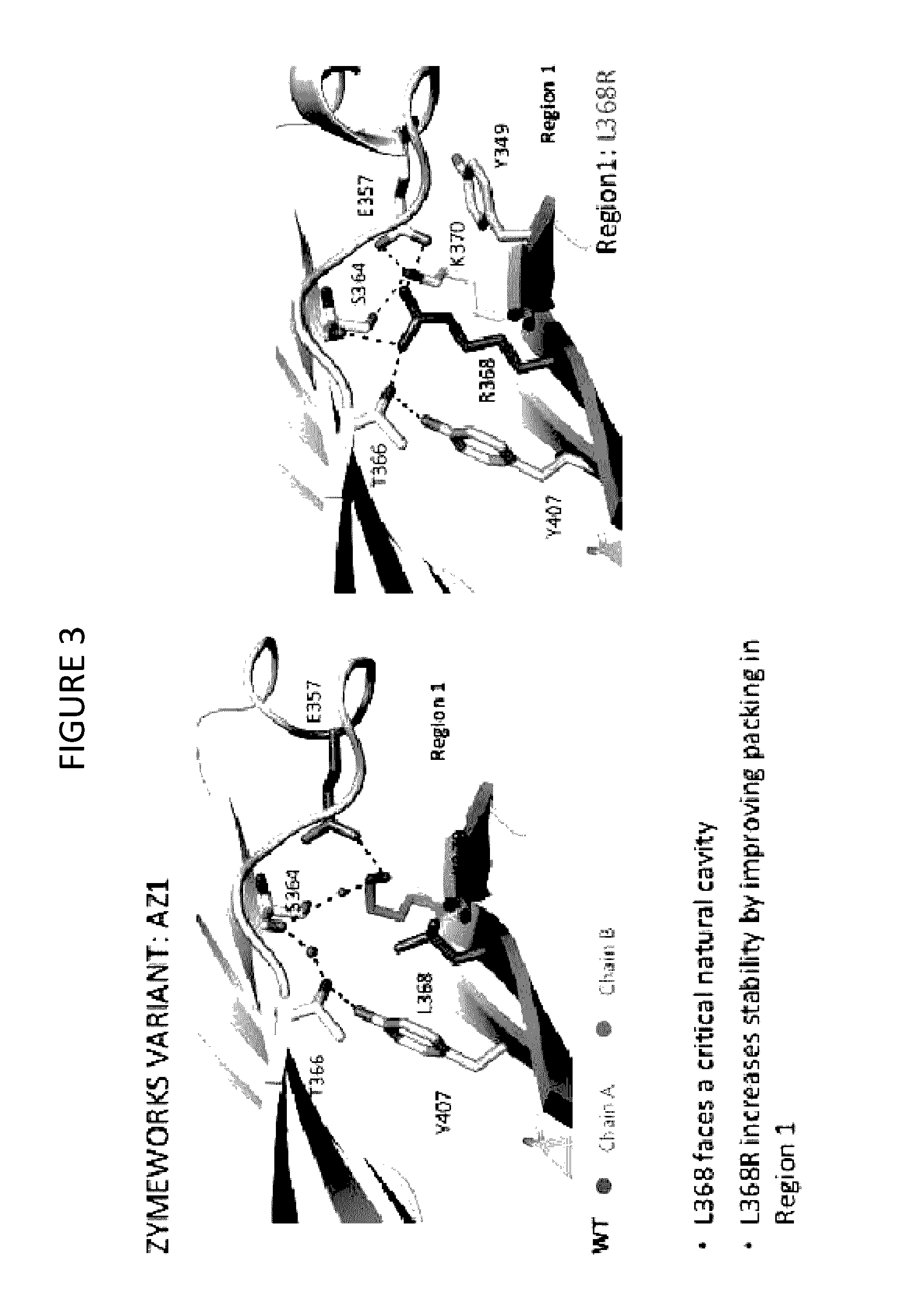
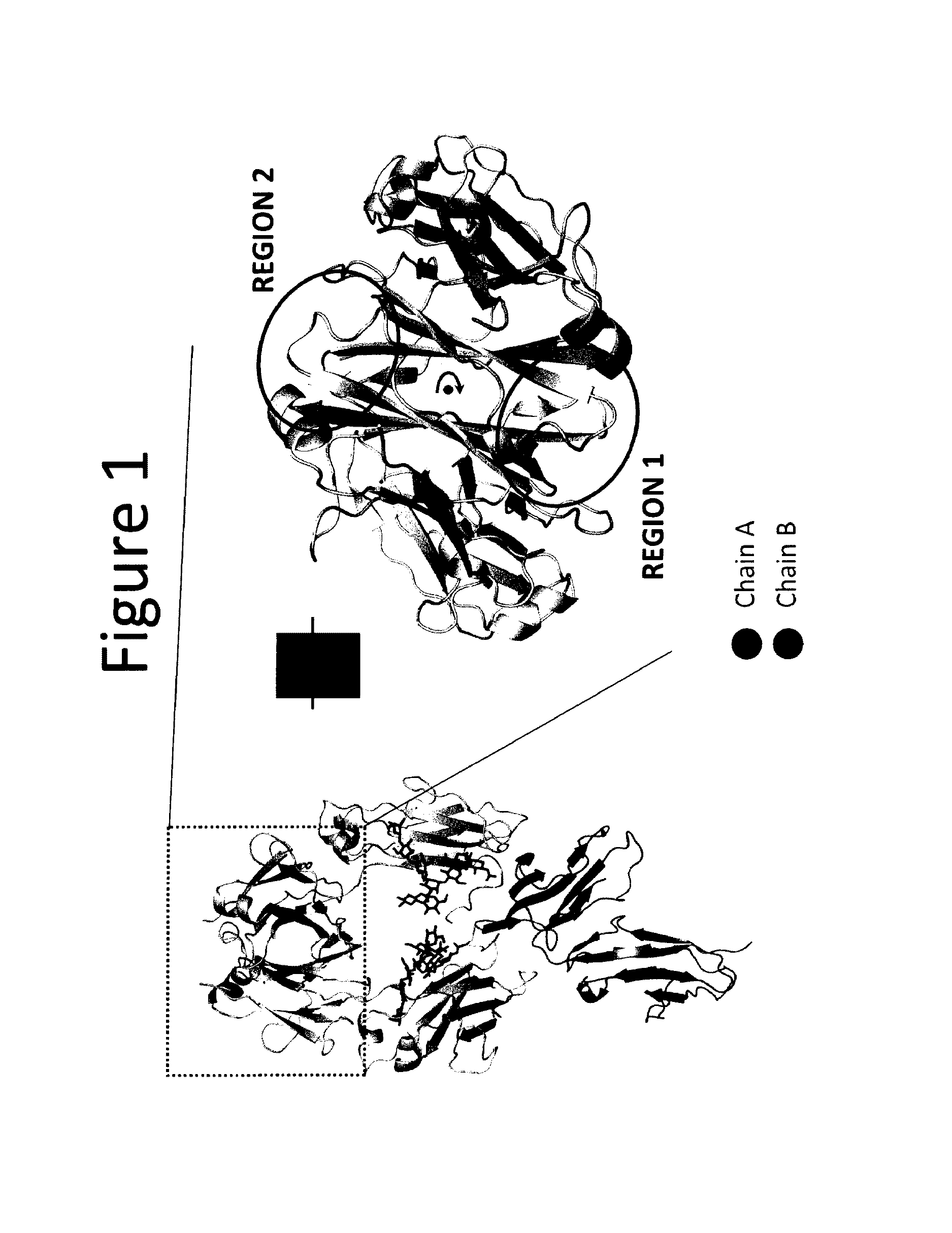
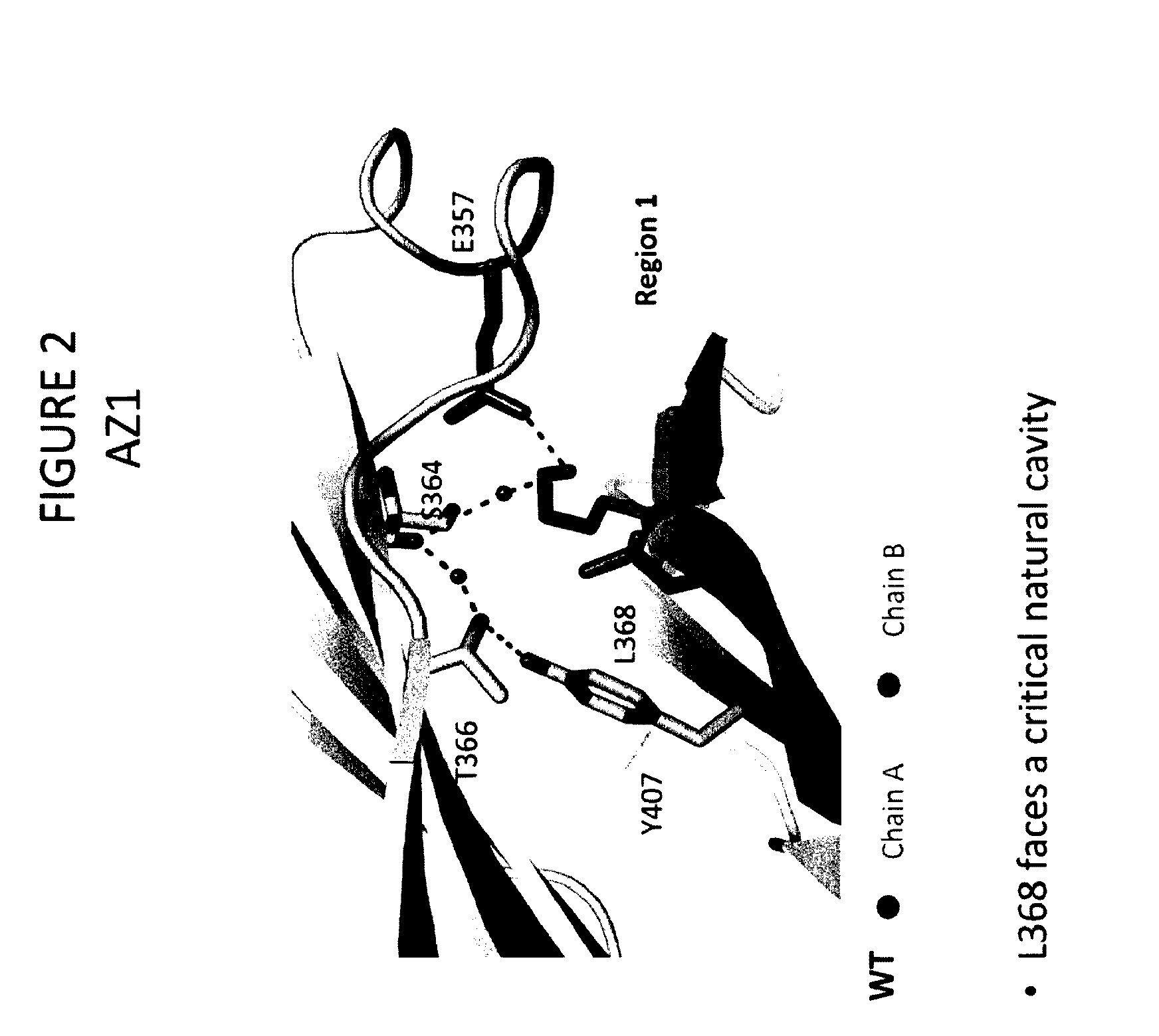
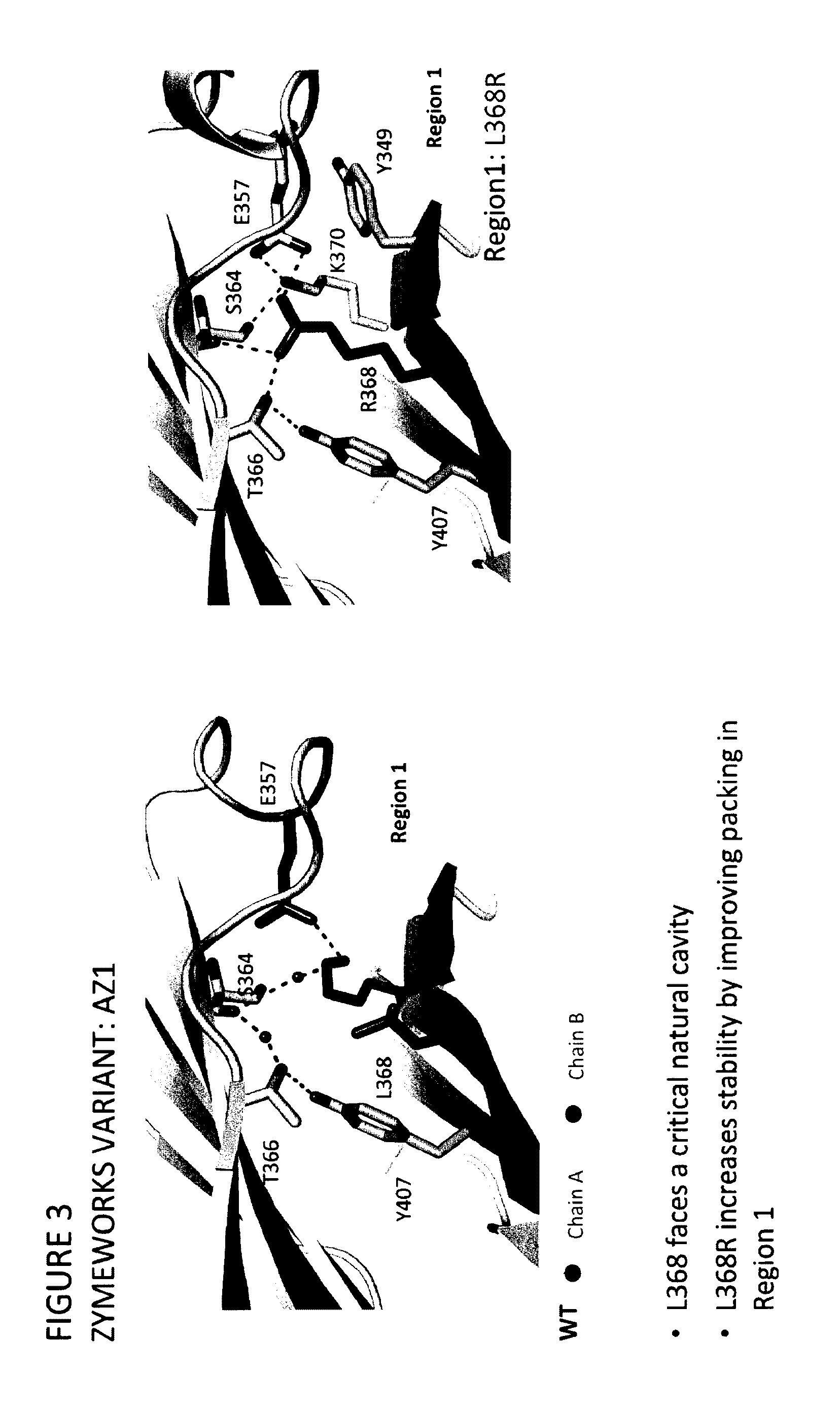
Stable Heterodimeric Antibody Design with Mutations in the Fc Domain
ActiveUS20120149876A1Promote formationImprove stabilityImmunoglobulinsImmunological disordersFc(alpha) receptorFc receptor
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Stable Heterodimeric Antibody Design with Mutations in the Fc Domain
ActiveUS20130195849A1Improve stabilityHybrid immunoglobulinsImmunological disordersFc(alpha) receptorFc receptor
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
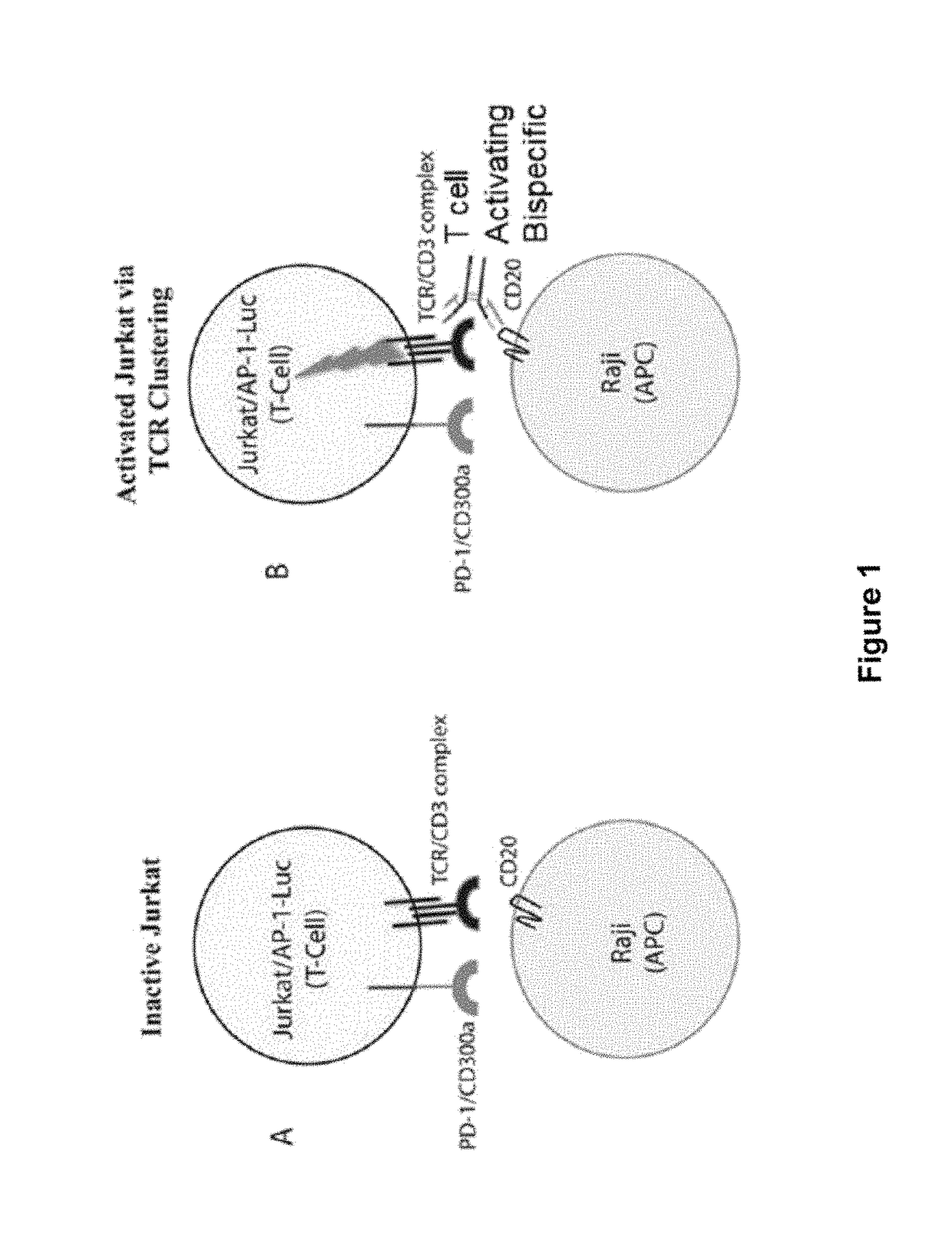
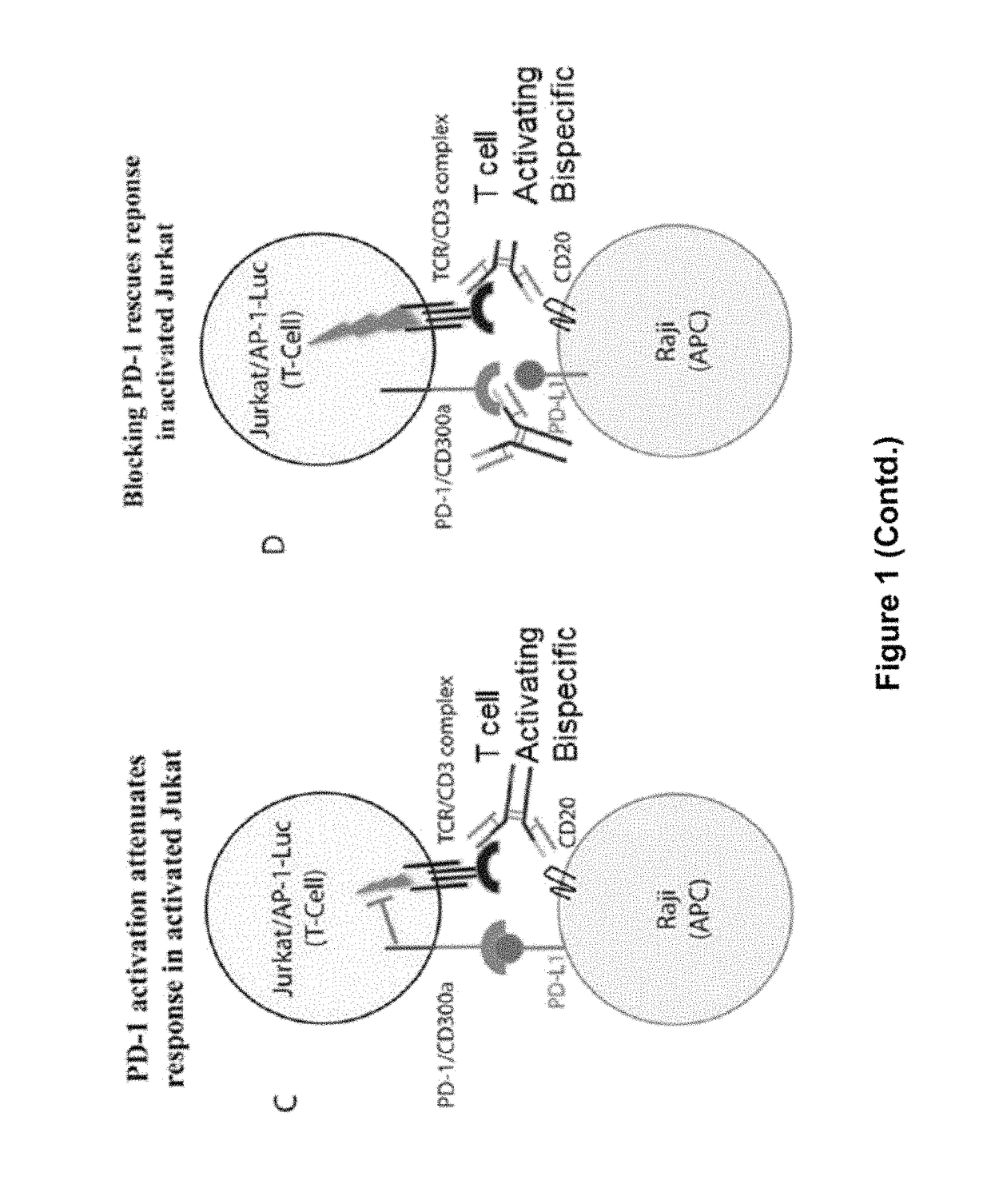
Human Antibodies to PD-1
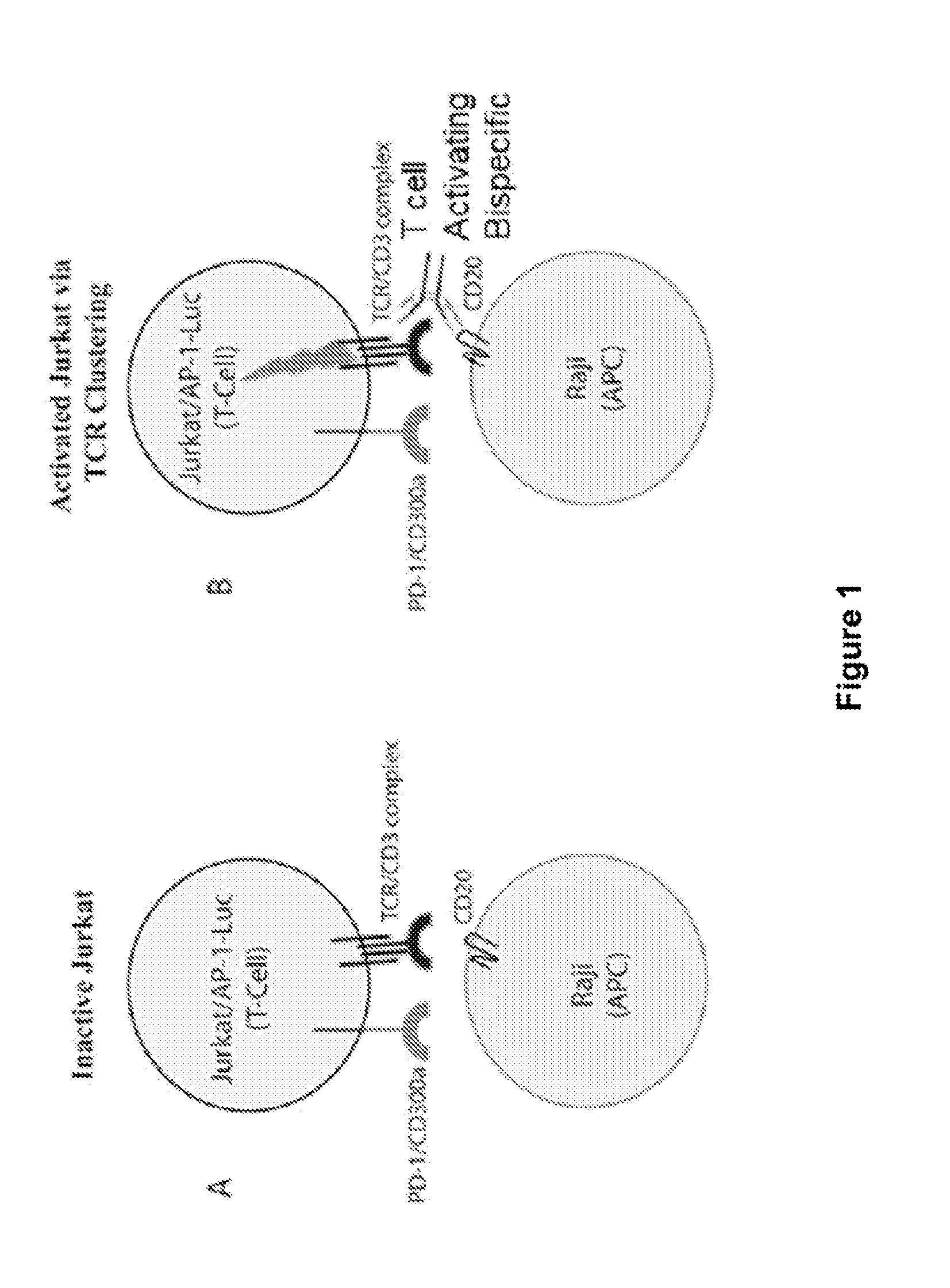
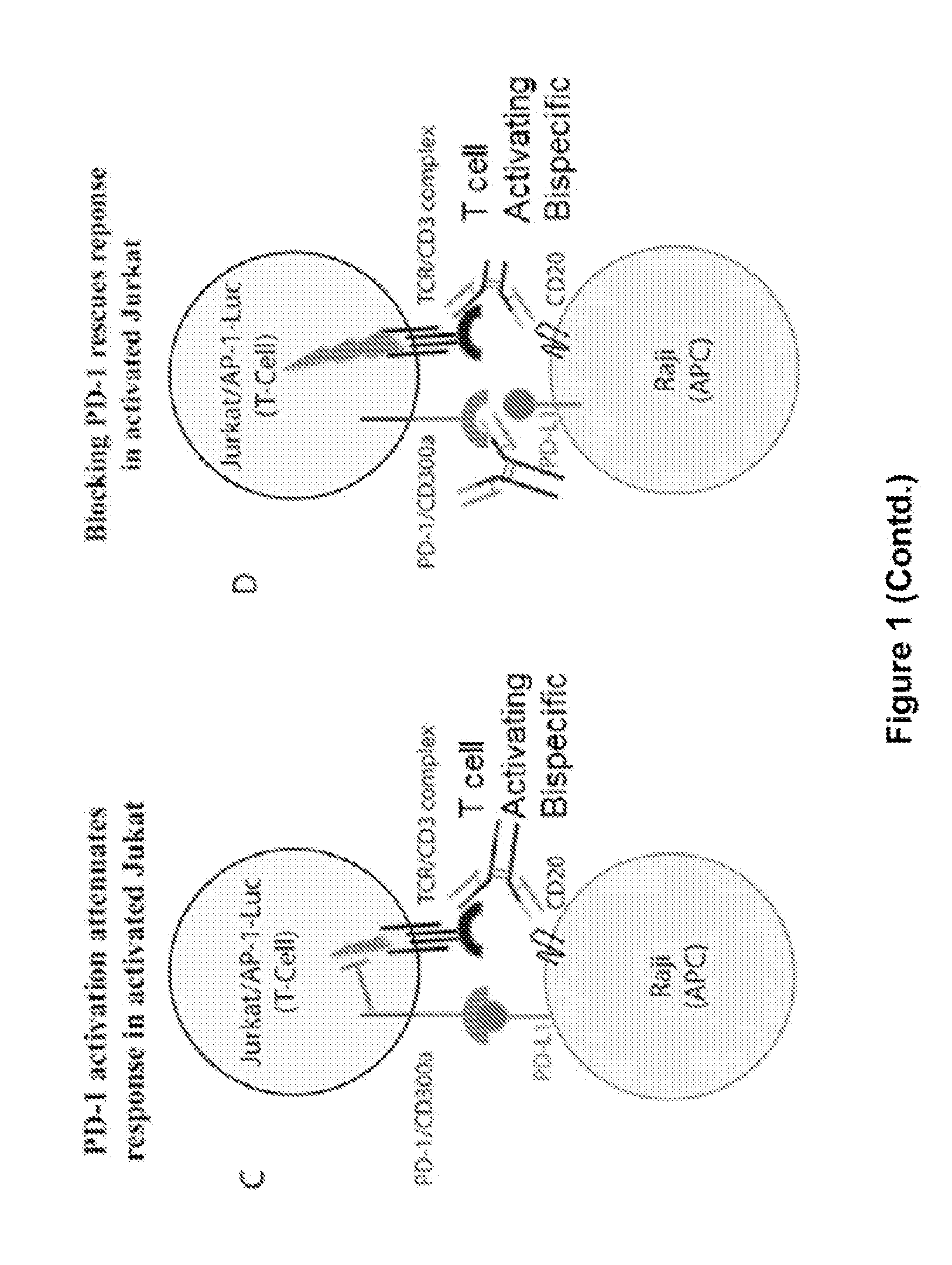
ActiveUS20150203579A1Rescue T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Fc Variants With Optimized Fc Receptor Binding Properties
InactiveUS20070148170A1High affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFc receptorFc(alpha) receptor
The present invention relates to Fc variants with optimized Fc receptor binding properties, methods for their generation, Fc polypeptides comprising Fc variants with optimized Fc receptor binding properties, and methods for using Fc variants with optimized Fc receptor binding properties.
Owner:XENCOR
Antibodies comprising chimeric constant domains
ActiveUS20140243504A1Reduced effector functionAnimal cellsHybrid immunoglobulinsFc(alpha) receptorFc receptor
Antibodies, antigen-binding proteins and Fc-fusion proteins that comprise recombinant polypeptides containing a chimeric heavy chain constant region sequence are provided that bind to certain Fc receptors however have reduced effector functions. Methods of making constructs for expression of such chimeric Fc-containing antibodies, antigen-binding proteins and Fc-fusion proteins in cell systems, and methods of producing and isolating the chimeric Fc-containing proteins are provided.
Owner:REGENERON PHARM INC
Fc polypeptides with novel Fc ligand binding sites
The present invention relates to Fc polypeptides with novel Fc receptor binding sites, and their application, particularly for therapeutic purposes.
Owner:XENCOR
Immunoglobulin constant region fc receptor binding agents
ActiveUS20100239633A1Wide applicationPathological conditionAntibacterial agentsAntibody mimetics/scaffoldsDiseaseFc(alpha) receptor
IVIG replacement compounds are derived from recombinant and / or biochemical creation of immunologically active biomimetic(s). These replacement compounds are then screened in vitro to assess each replacements compound's efficiency at modulating immune function. Particular replacement compounds are selected for further in vivo validation and dosage / administration optimization. Finally, the replacement compounds are used to treat a wide range of diseases, including inflammatory and autoimmune diseases.
Owner:GLIKNIK +1
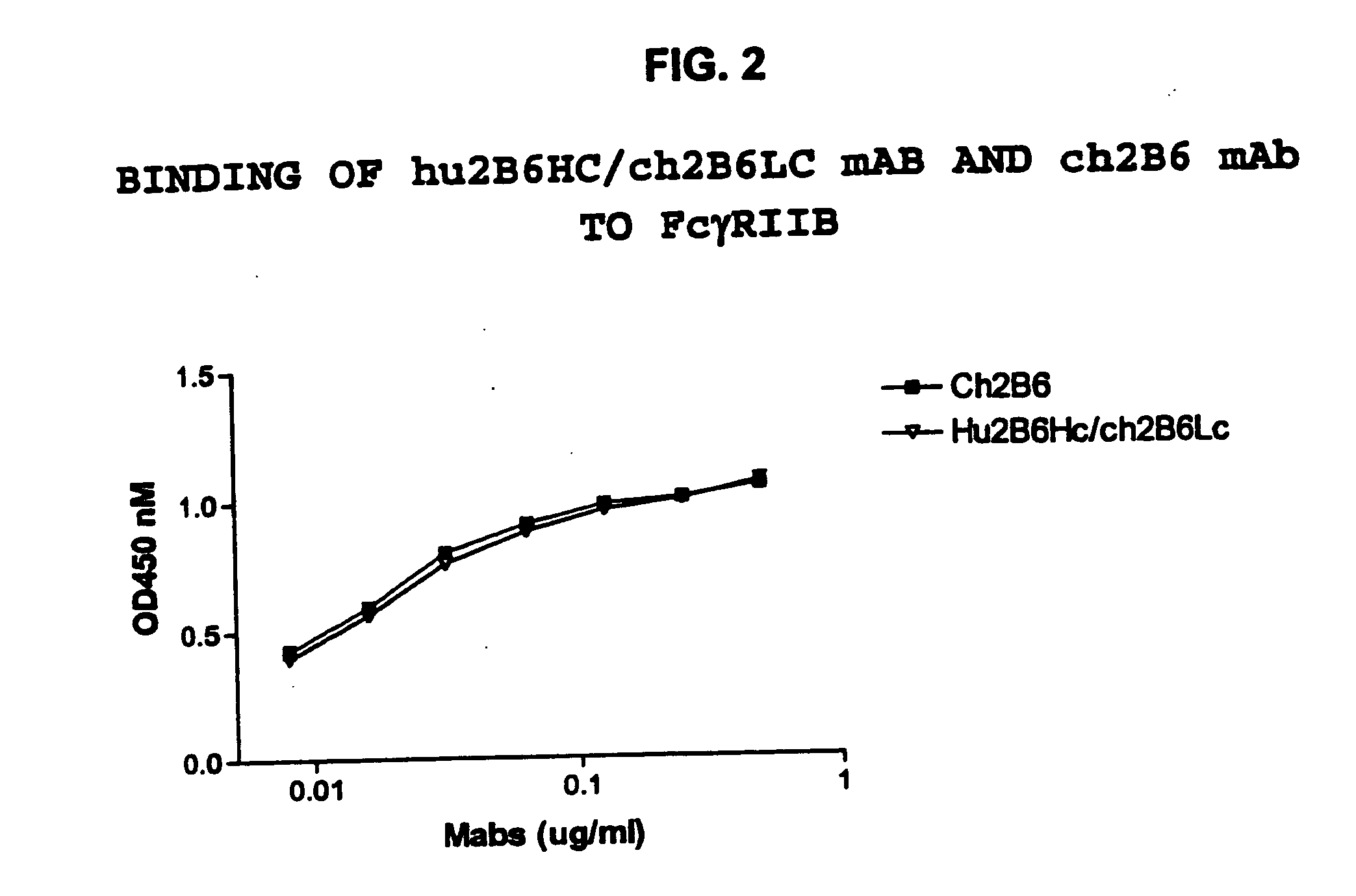
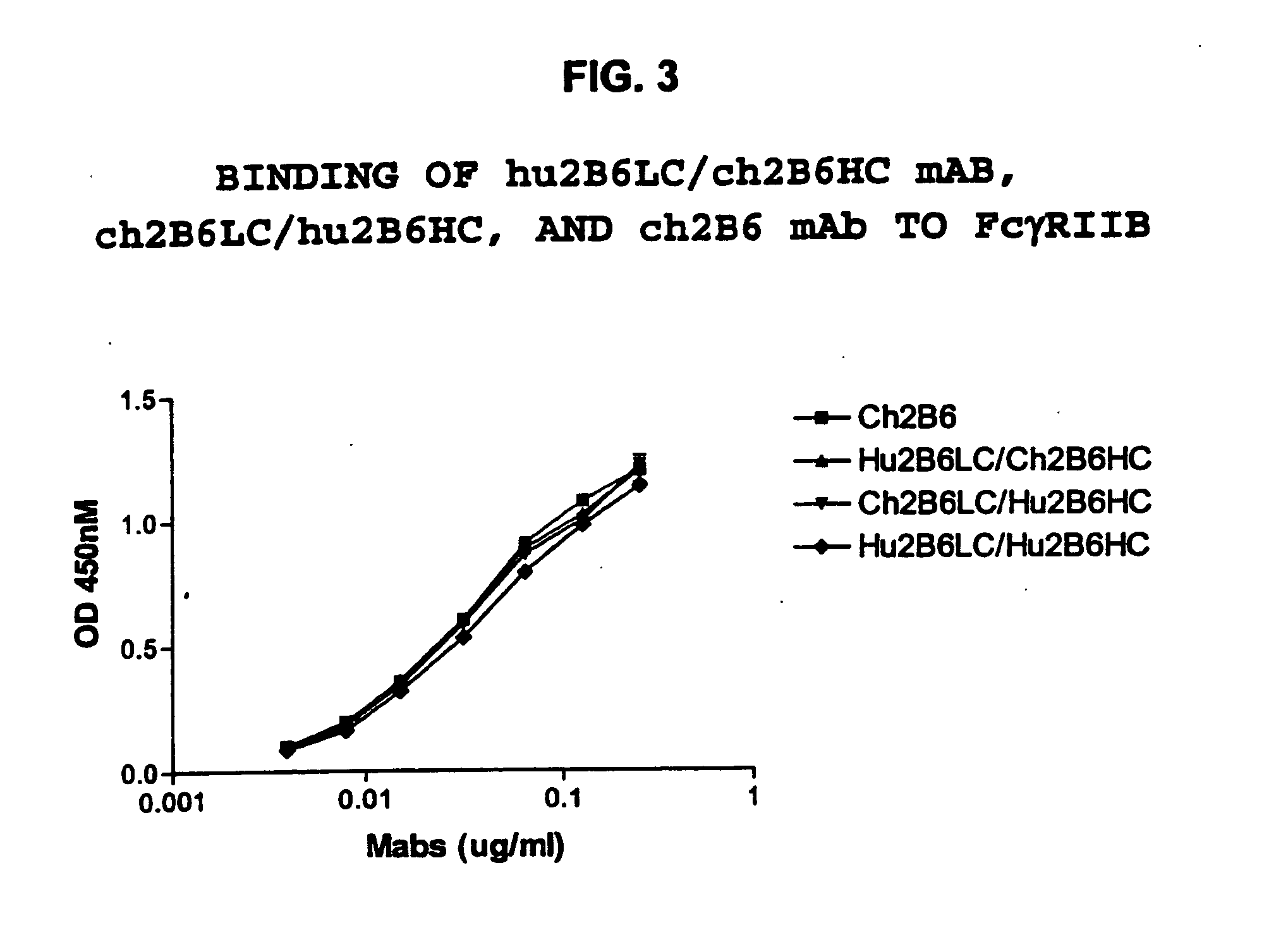
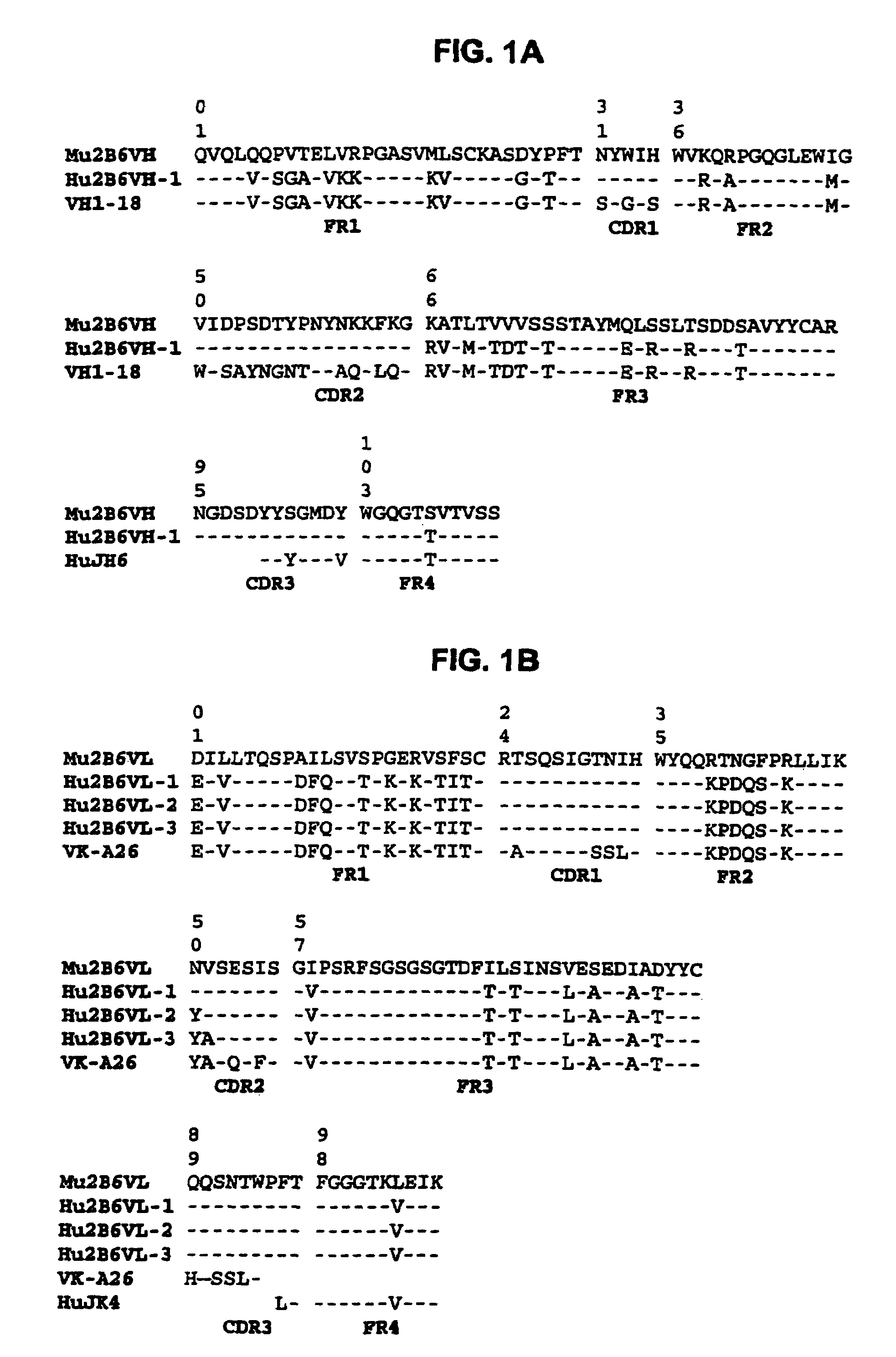
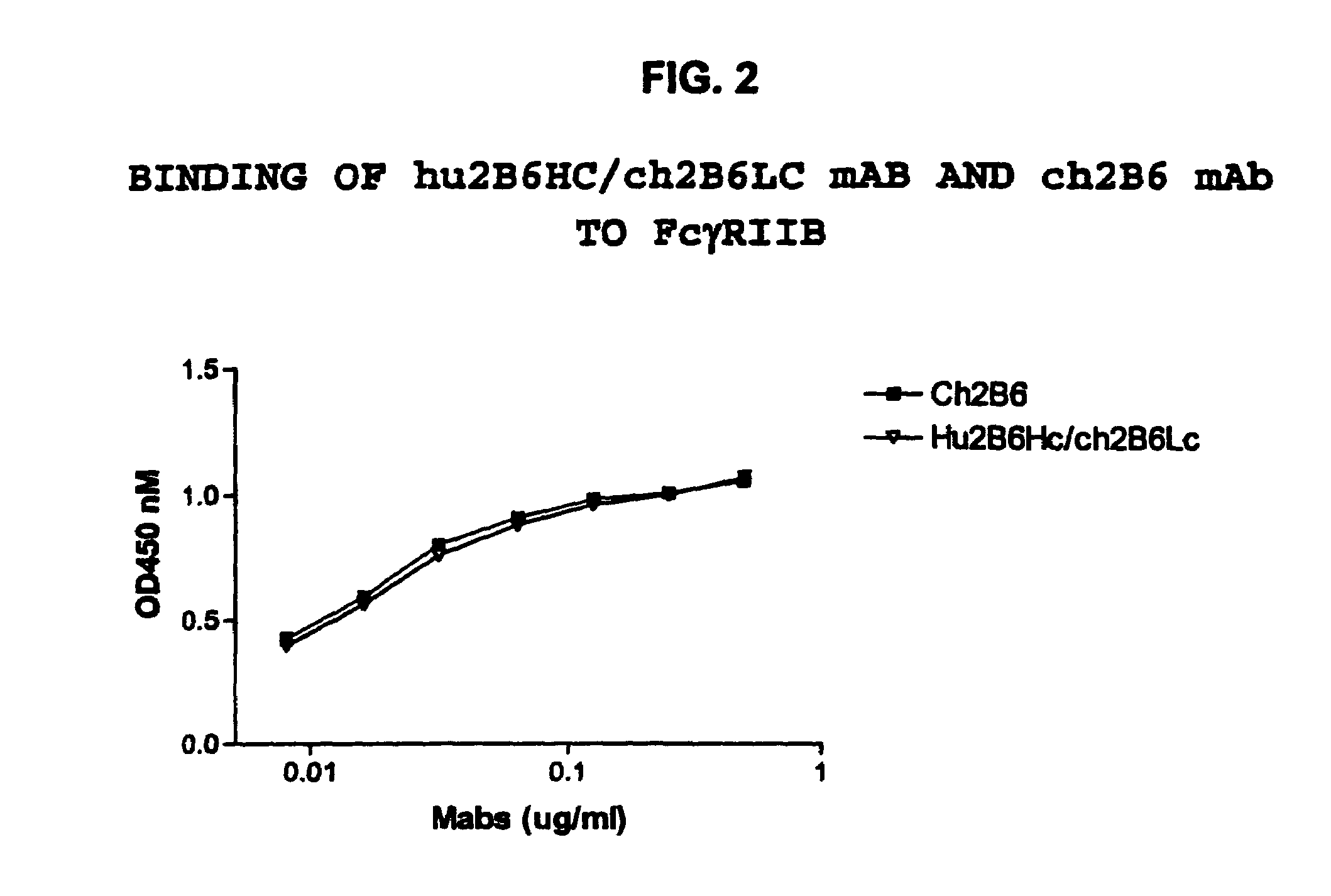
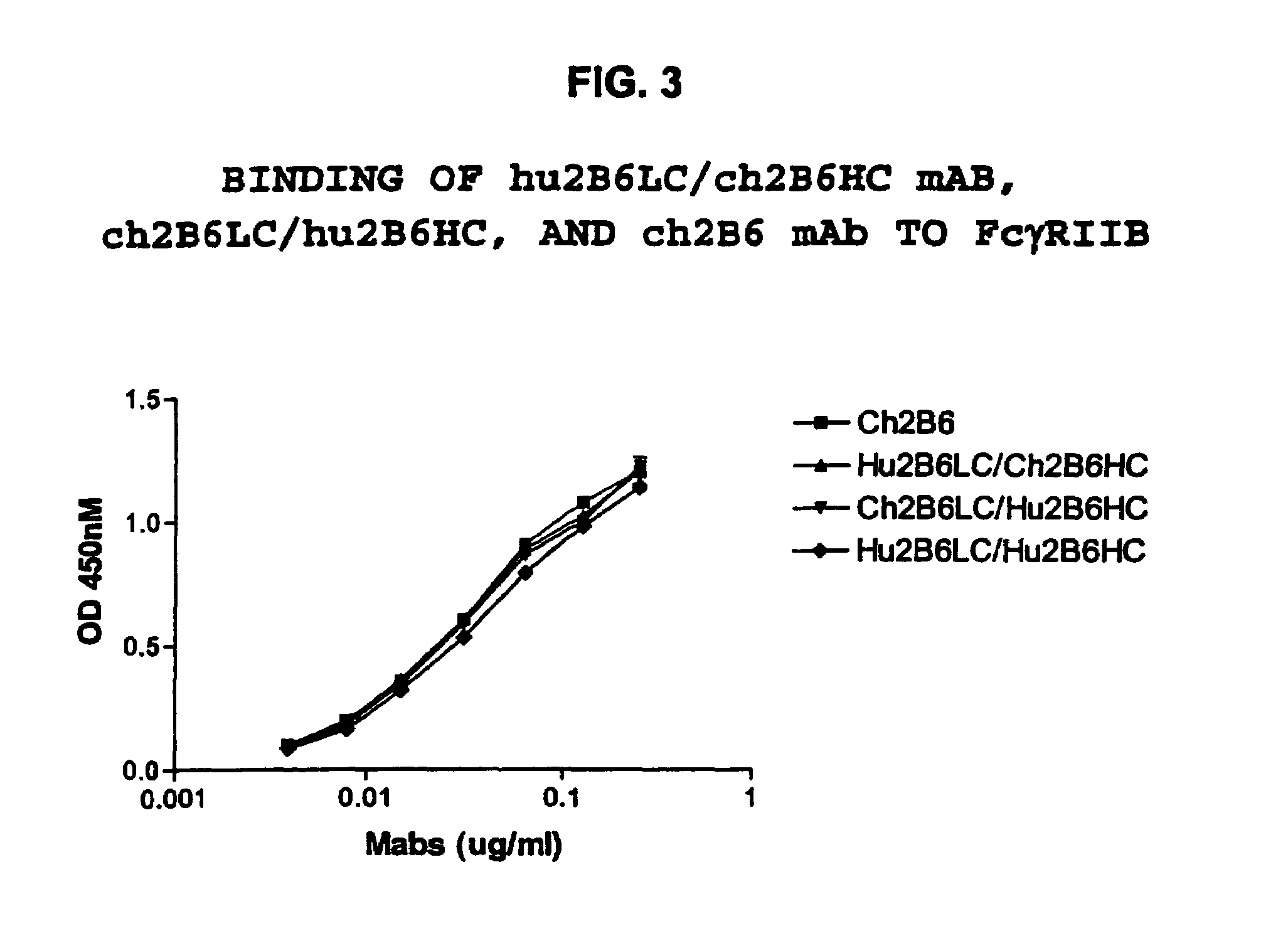
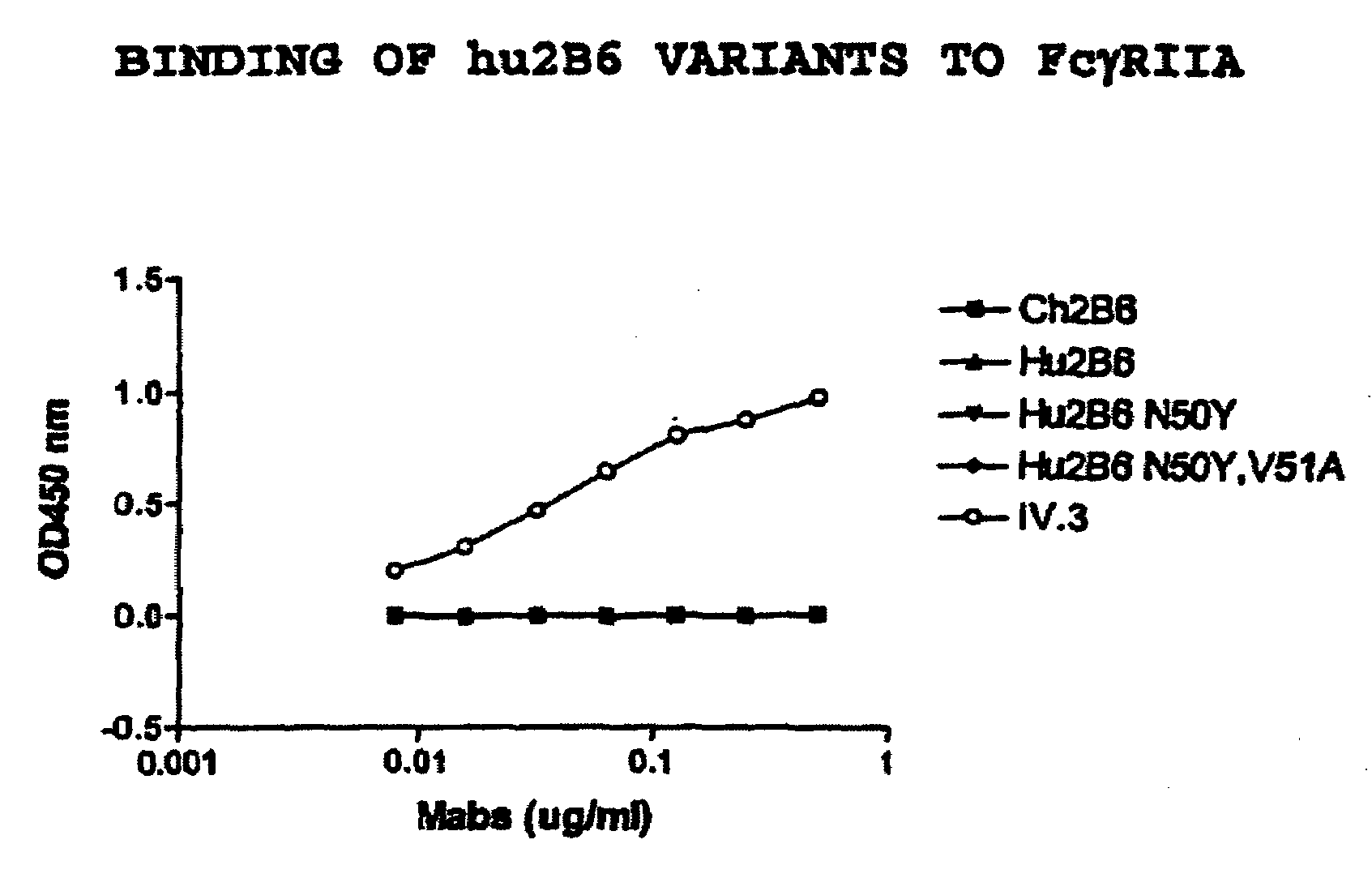
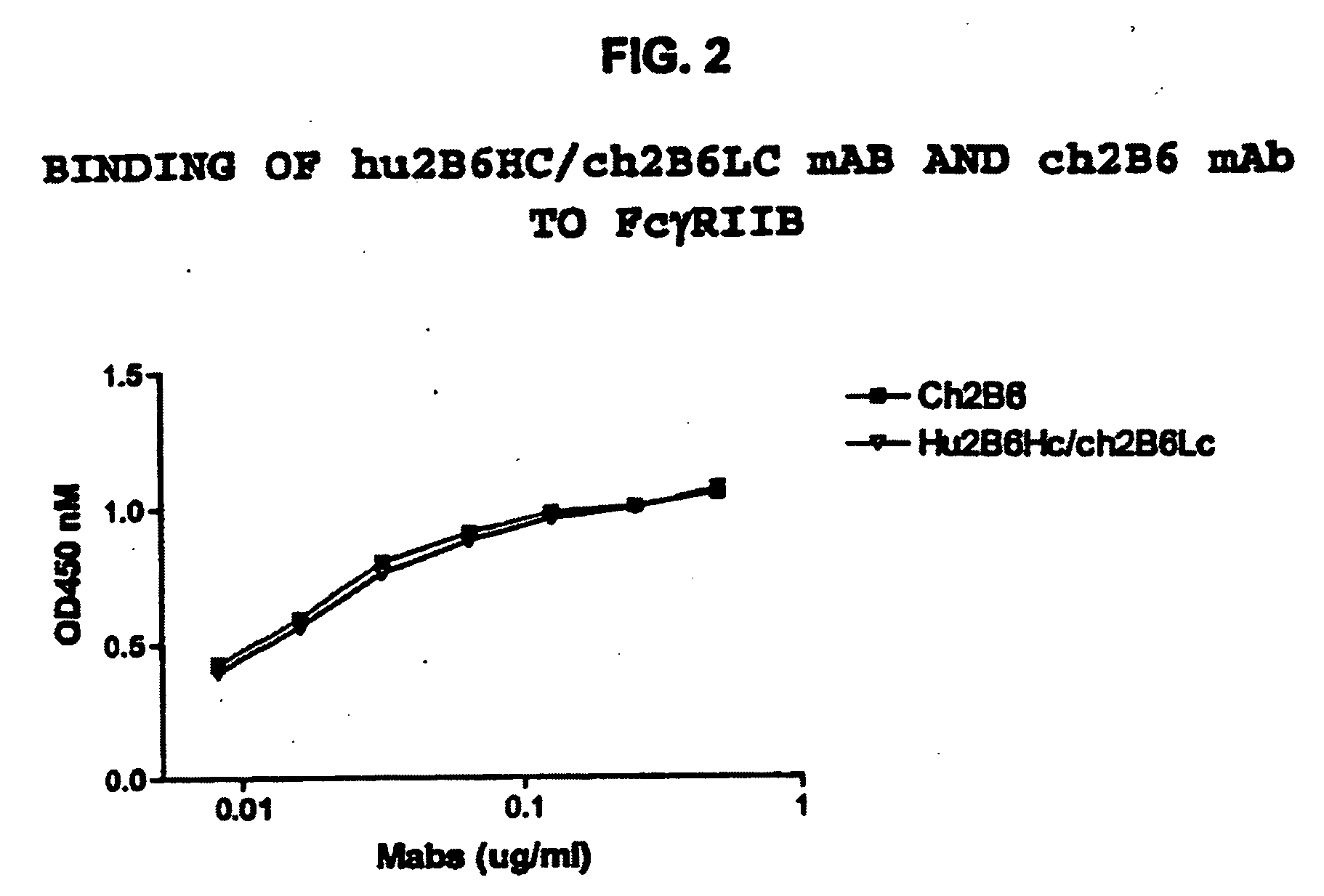
Humanized FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20060013810A1Good curative effectConvenient treatmentSenses disorderNervous disorderFc(alpha) receptorDisease
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Humanized FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS7521542B2Immune responseAvoid immune responseSenses disorderNervous disorderFc(alpha) receptorTherapeutic antibody
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS7067110B1Improving immunogenicityStrengthIn-vivo radioactive preparationsAntibody mimetics/scaffoldsPeptide antigenFc(alpha) receptor
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Humanized Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
InactiveUS20080044417A1Good curative effectEnhanced effector functionDisease diagnosisTissue cultureFc(alpha) receptorFc receptor
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Antibodies with Enhanced or Suppressed Effector Function
ActiveUS20130089541A1Inhibition is effectiveStrong specificityAntipyreticAnalgesicsFc receptorFc(alpha) receptor
Rationally designed antibodies and polypeptides that comprise multiple Fc region amino acid substitutions that synergistically provide enhanced selectivity and binding affinity to a target Fc receptor are provided. The polypeptides are mutated at multiple positions to make them more effective when incorporated in antibody therapeutics than those having wild-type Fc components.
Owner:ZYMEWORKS INC
Fc variants with optimized Fc receptor binding properties
InactiveUS20080206867A1Immunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureFc(alpha) receptorFc receptor
The present invention relates to Fc variants with optimized Fc receptor binding properties, methods for their generation, Fc polypeptides comprising Fc variants with optimized Fc receptor binding properties, and methods for using Fc variants with optimized Fc receptor binding properties.
Owner:DESJARLAIS JOHN R +5
Human antibodies to PD-1
ActiveUS9987500B2Rescues T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Non-human primate Fc receptors and methods of use
InactiveUS20050054046A1Improve stabilityFacilitate oligomerizationAntibody mimetics/scaffoldsImmunoglobulinsFc(alpha) receptorFc receptor
The invention provides isolated non-human primate Fc receptor polypeptides, the nucleic acid molecules encoding the Fc receptor polypeptides, and the processes for production of recombinant forms of the Fc receptor polypeptides, including fusions, variants, and derivatives thereof. The invention also provides methods for evaluating the safety, efficacy and biological properties of Fc region containing molecules using the non-human primate Fc receptor polypeptides.
Owner:GENENTECH INC
Genetically modified human natural killer cell lines
ActiveUS8313943B2Increase rangeHelp studyDrug screeningImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorFc receptor
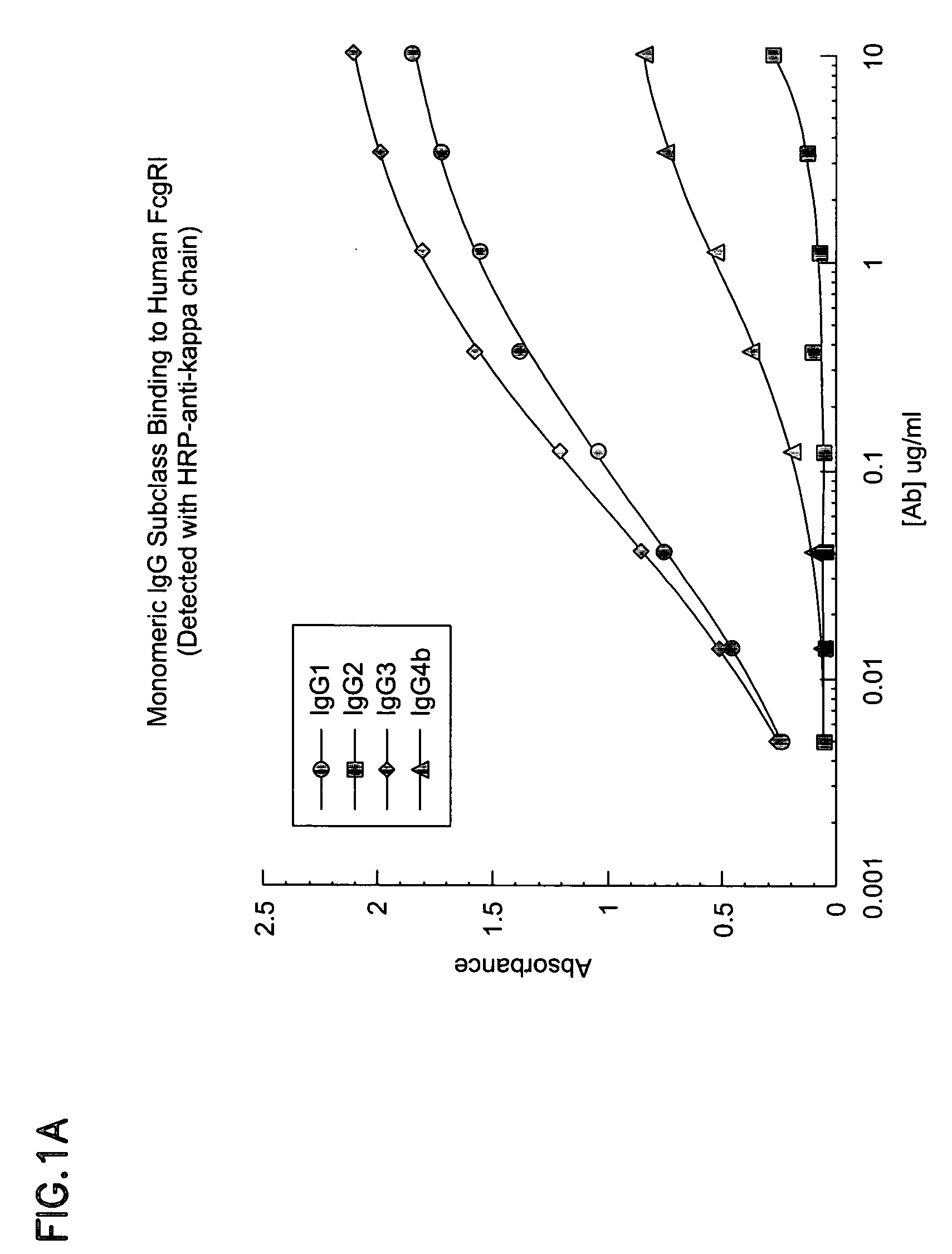
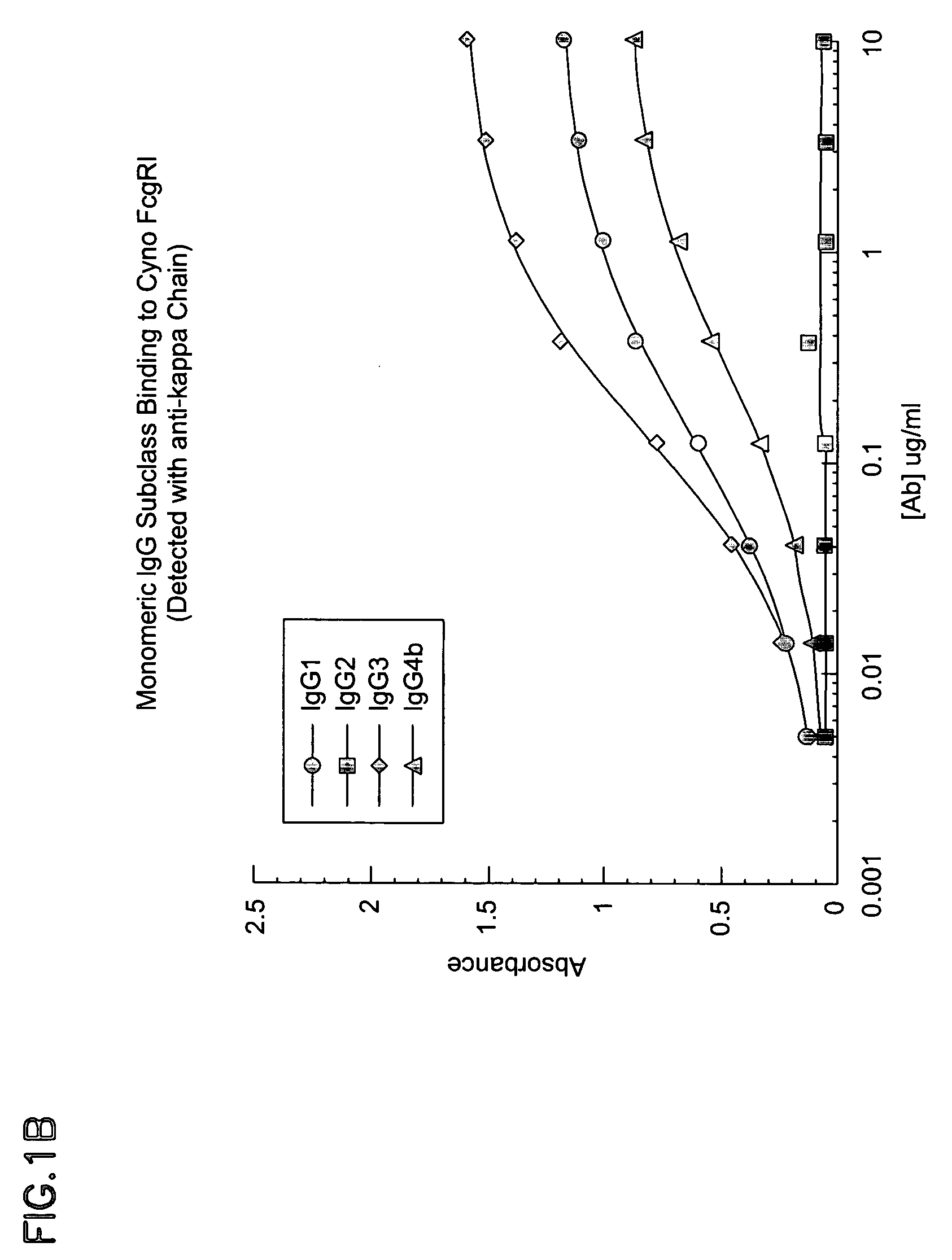
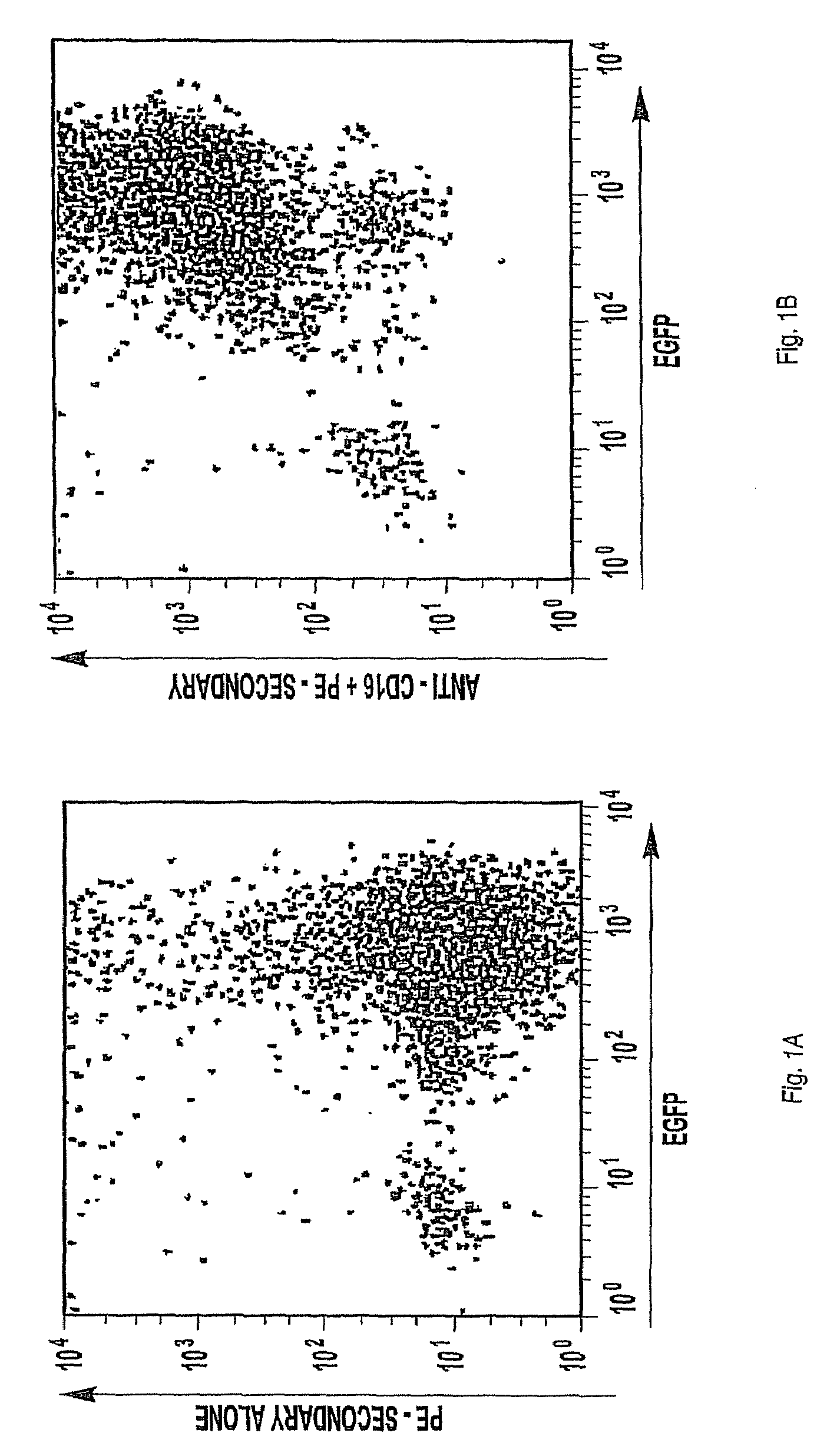
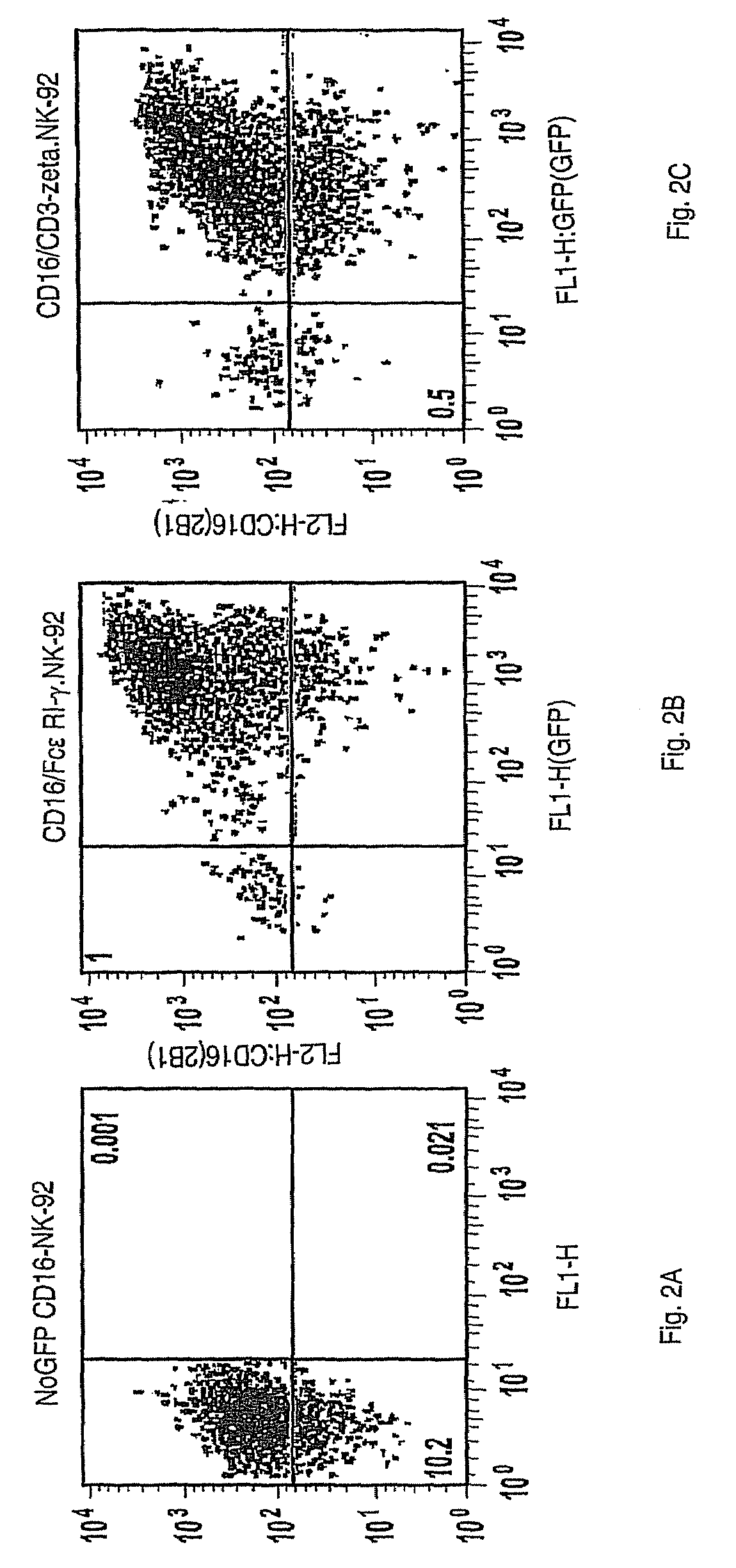
The invention provides a natural killer cell, NK-92, modified to express an Fc receptor on the surface of the cell, such as CD16 (FcγRIII-A), or other Fcγ or Fc receptors. The modified NK-92 cell can be further modified to concurrently express an associated accessory signaling protein, such as FcεRI-γ, TCR-ζ, or to concurrently express interleukin-2 (IL-2) or other cytokines. Additional methods are disclosed for various assays, assessments, and therapeutic treatments with the modified NK-92 cells.
Owner:INST FOR CANCER RES
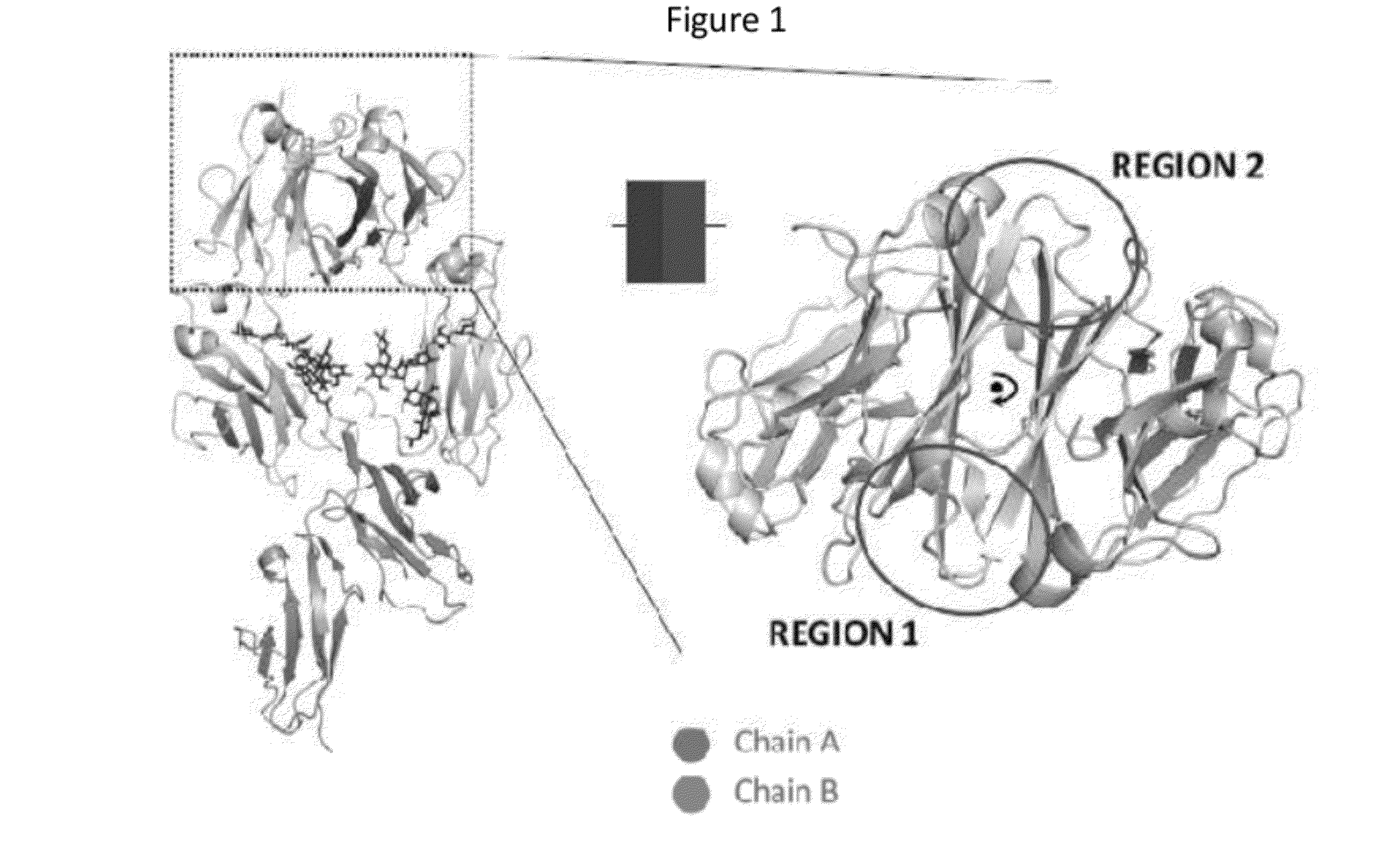
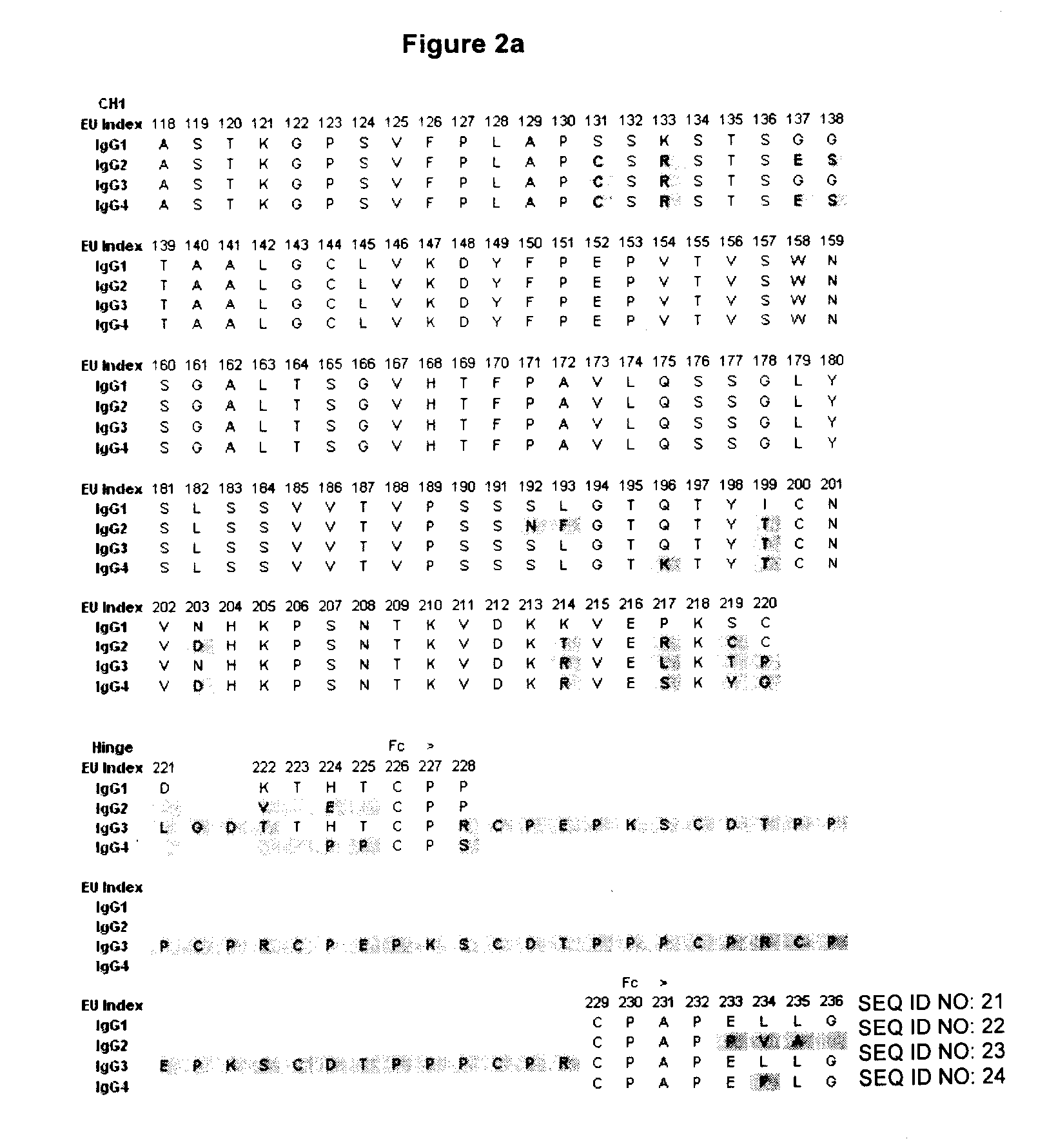
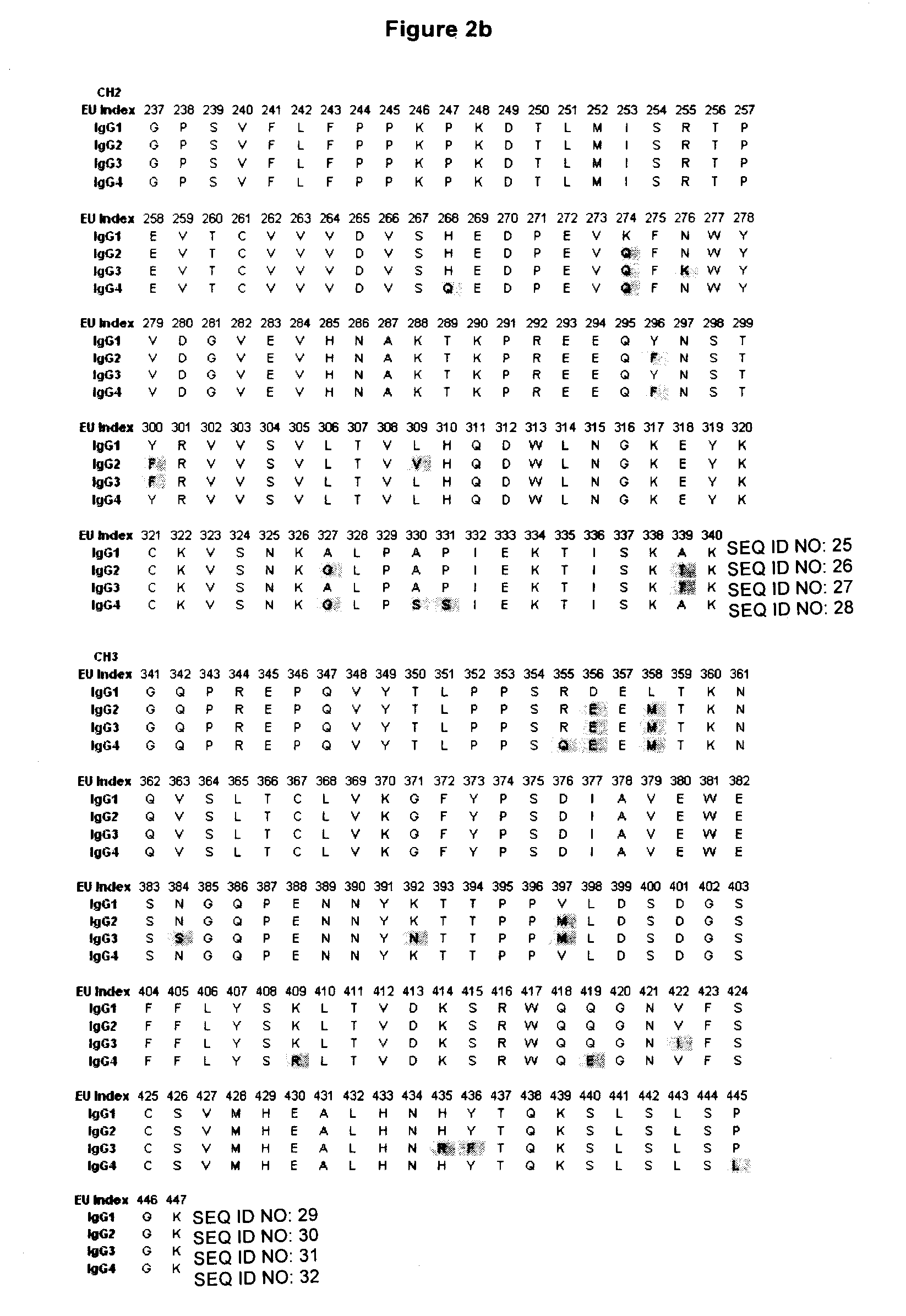
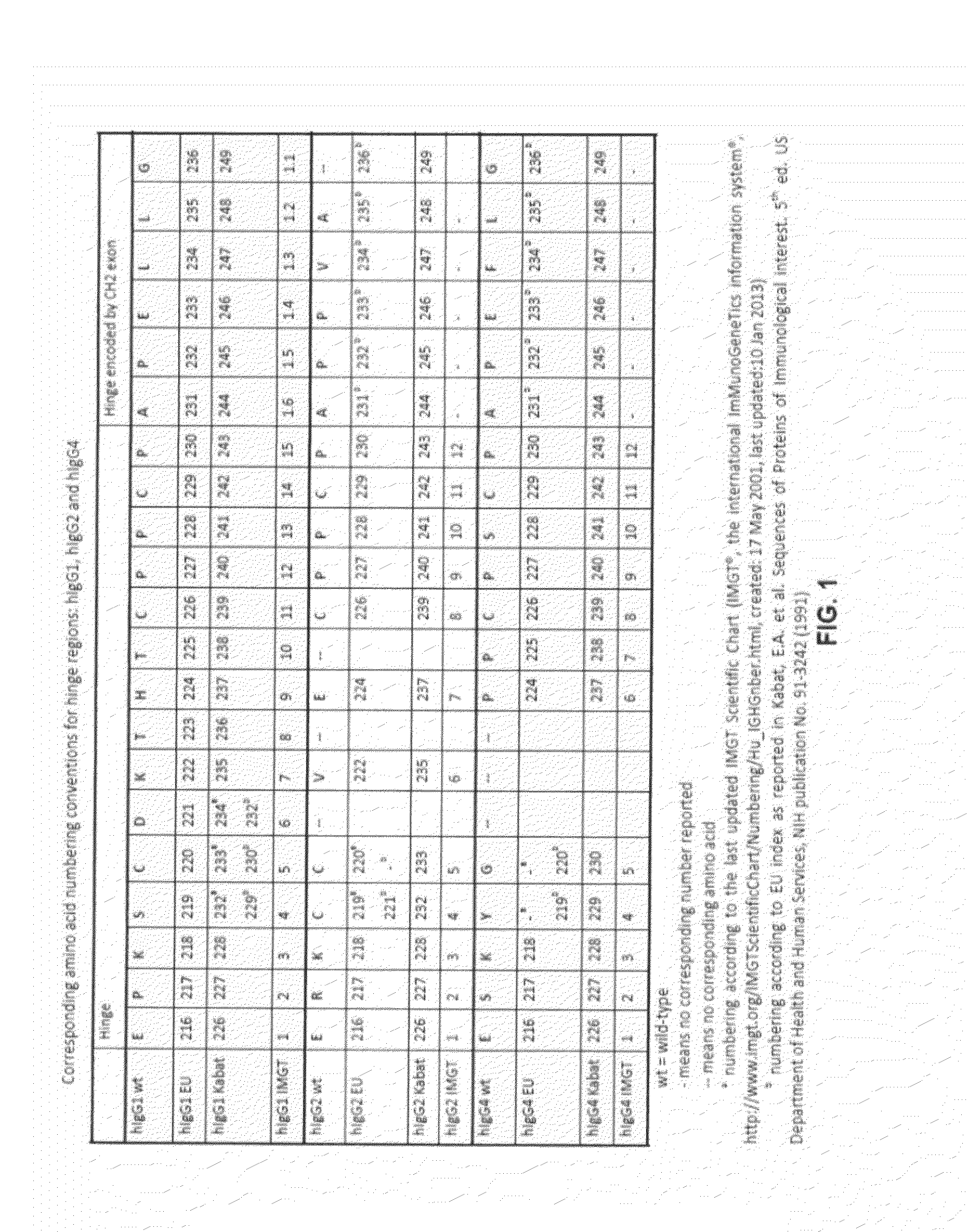
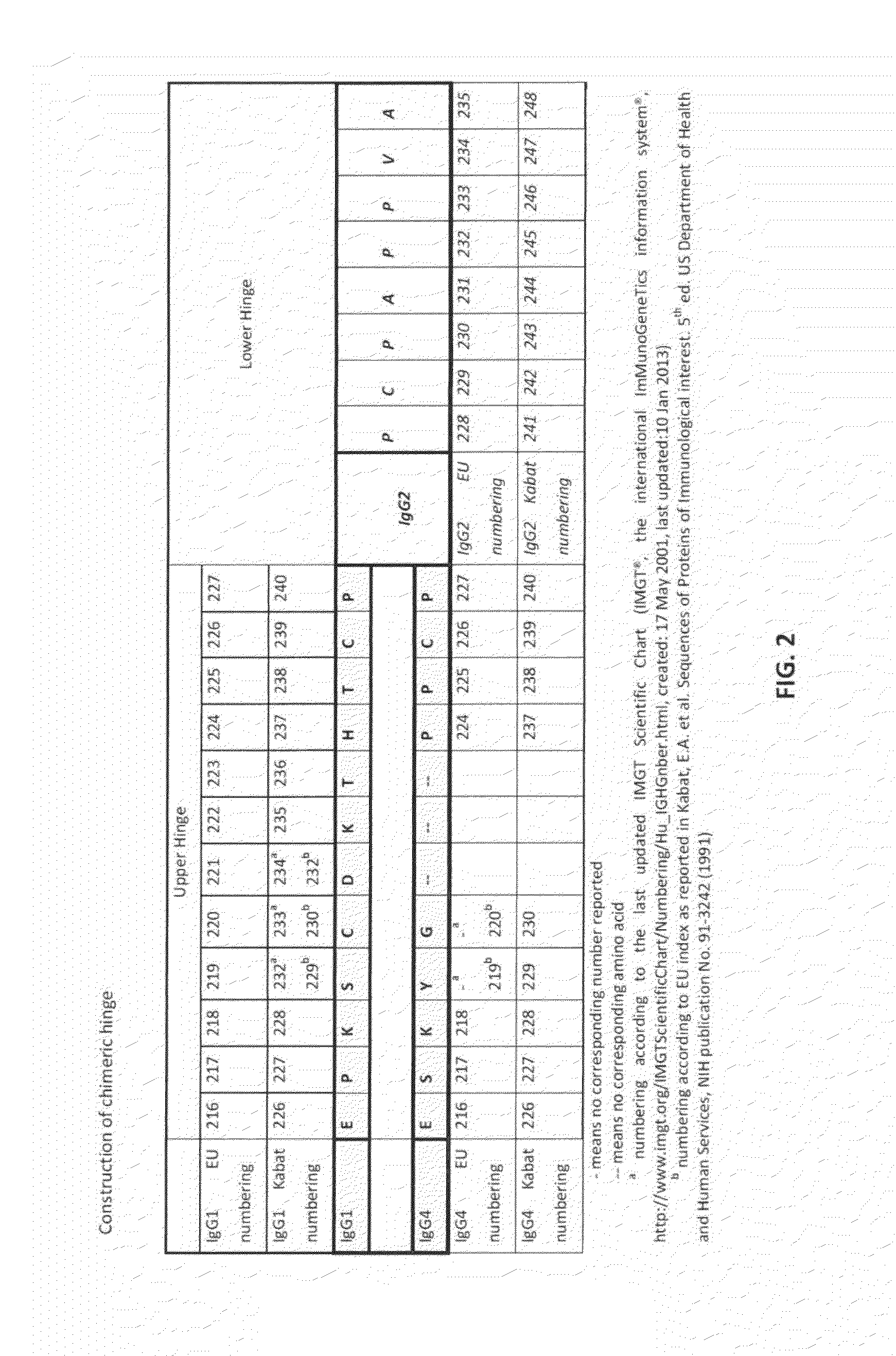
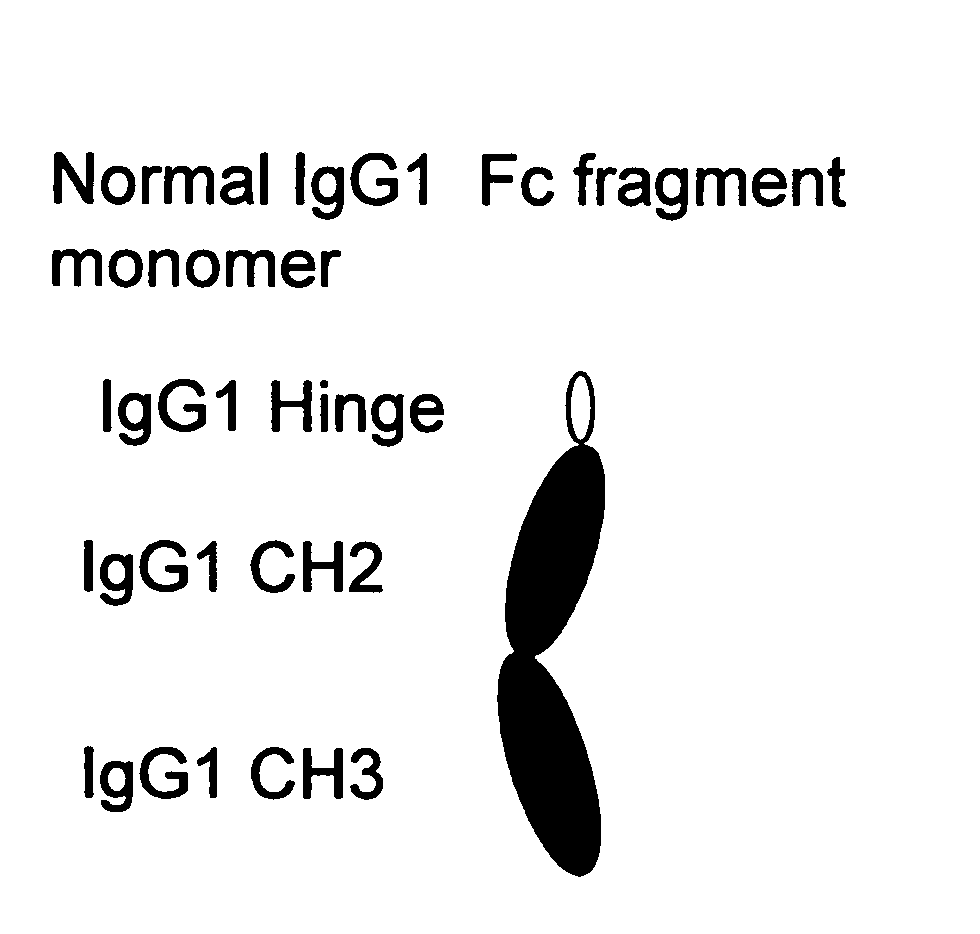
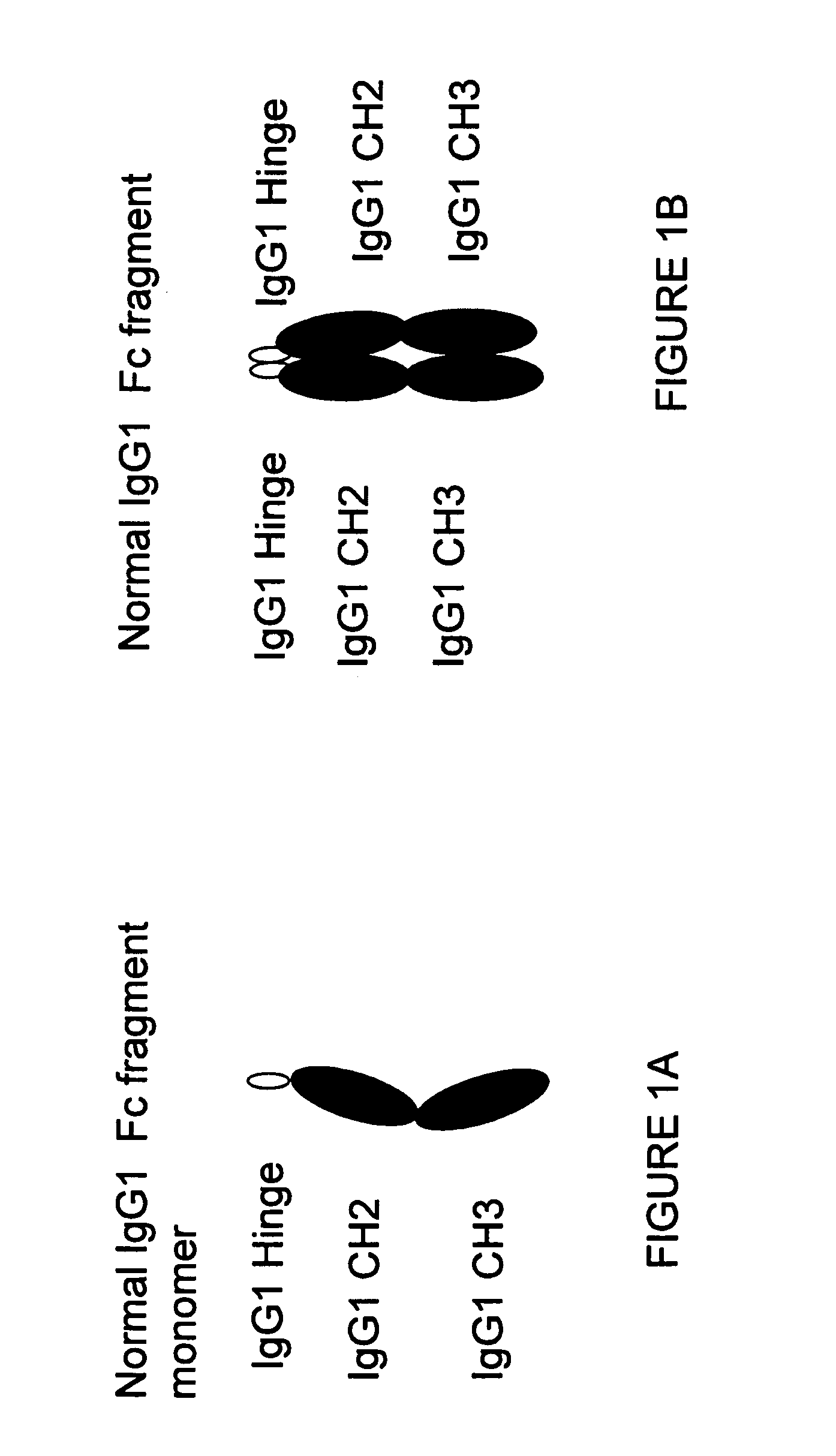
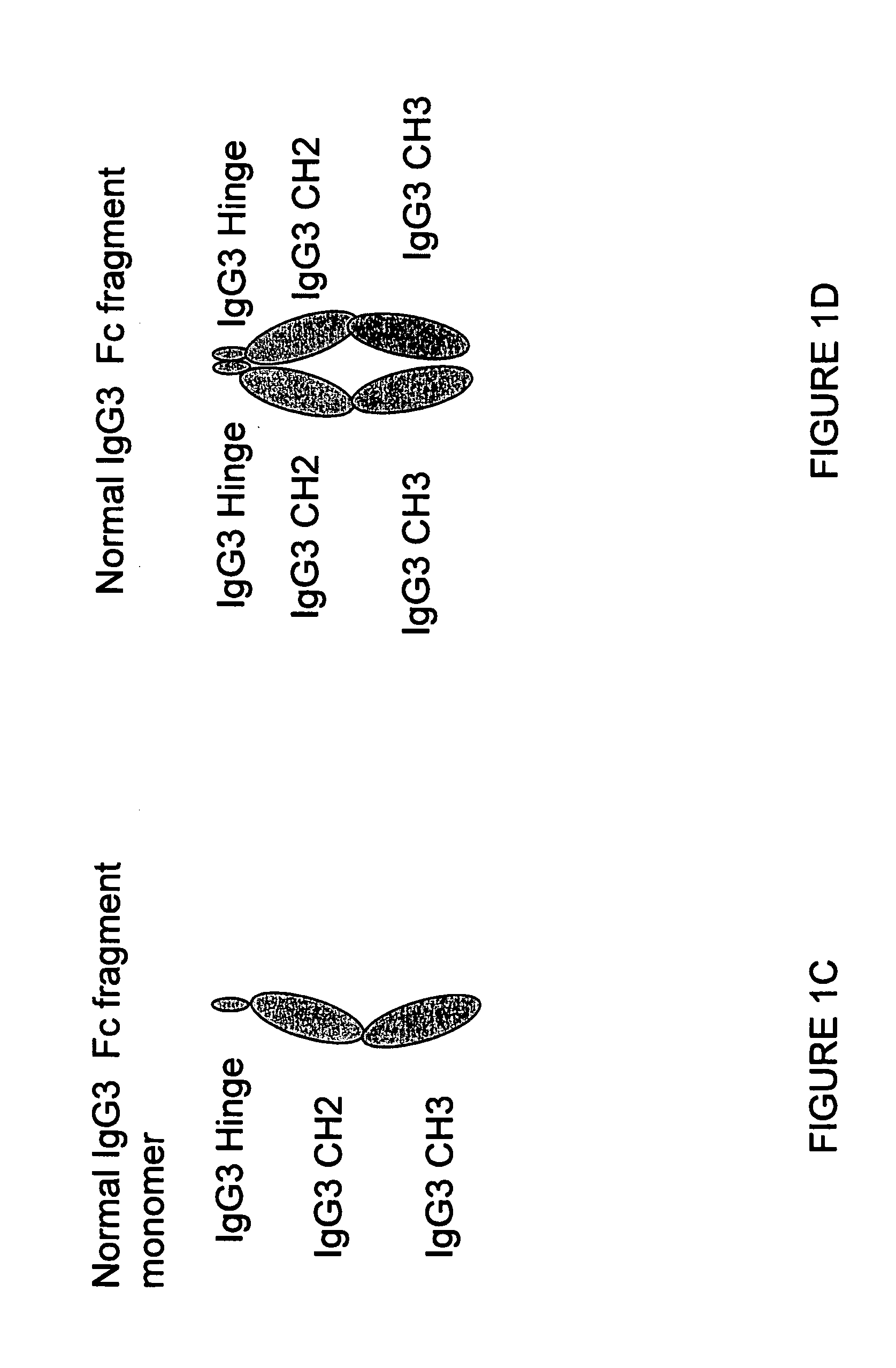
Binding proteins comprising immunoglobulin hinge and fc regions having altered fc effector functions
Provided herein are binding proteins comprising one or more immunoglobulin Fc region hinge, CH2, and / or CH3 domain wherein one or more hinge and / or constant region CH2 and / or CH3 domain is modified to alter the binding protein's binding affinity and / or specificity for a cognate receptor (e.g., an Fc receptor) and / or to impart one or more new binding specificity(ies) to the hinge and / or constant region that the corresponding unmodified immunoglobulin does not possess (e.g., affinity for distinct class of cognate receptor distinct from the class of cognate receptor to which the unmodified binding protein specifically binds). Binding proteins according to the present invention include, for example, modified antibodies, antibody fragments, recombinant binding proteins, and molecularly engineered binding domain-immunoglobulin fusion proteins, including small modular immunopharmaceutical products (SMIP™ products).
Owner:TRUBION PHARM INC
Antibodies comprising chimeric constant domains
ActiveUS9359437B2Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorFc receptor
Owner:REGENERON PHARM INC
Immunosensors: scFv-linker design for surface immobilization
InactiveUS20110201032A1Bioreactor/fermenter combinationsMaterial nanotechnologySingle-Chain AntibodiesSide chain
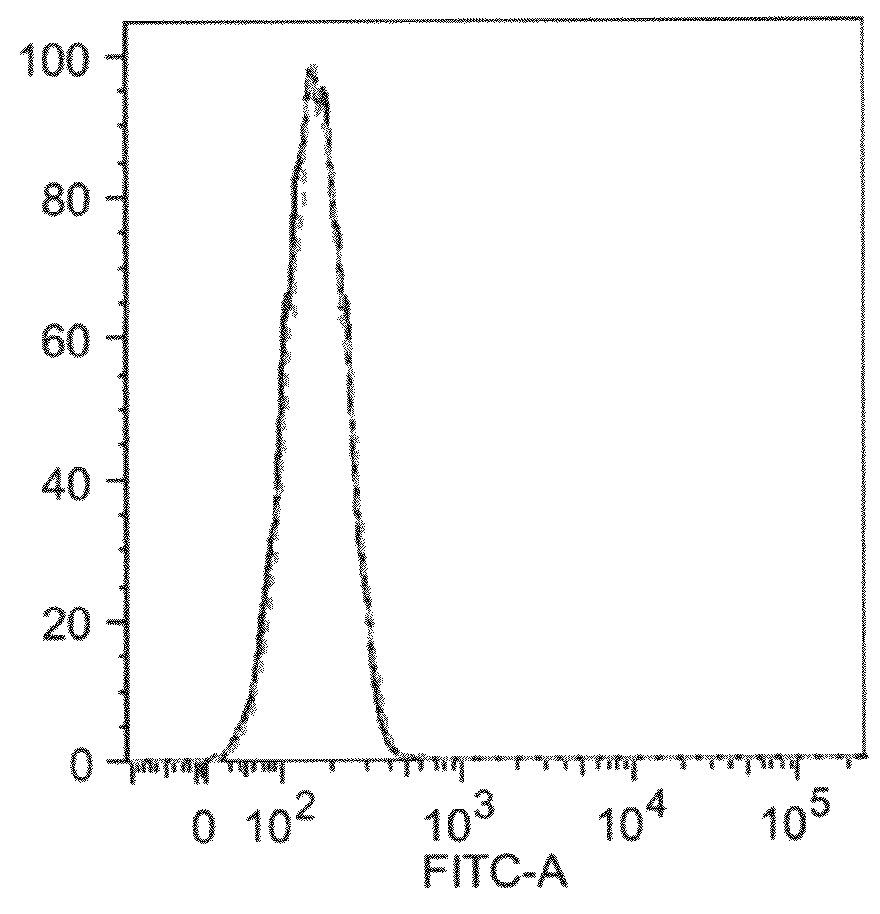
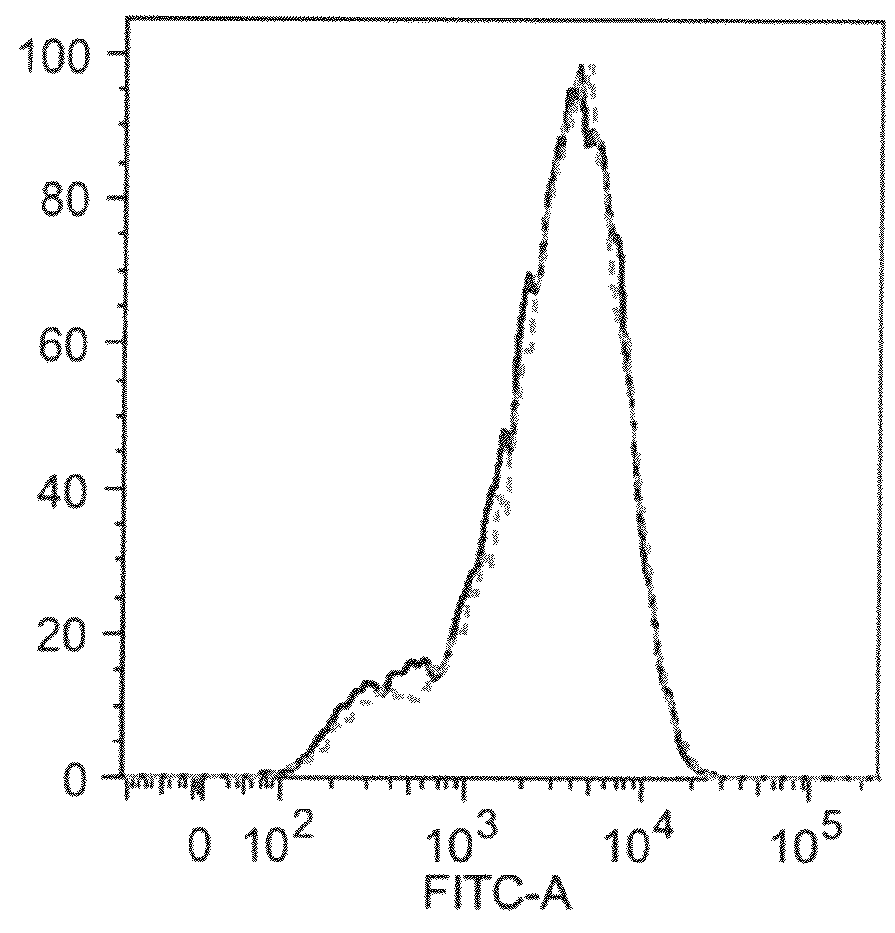
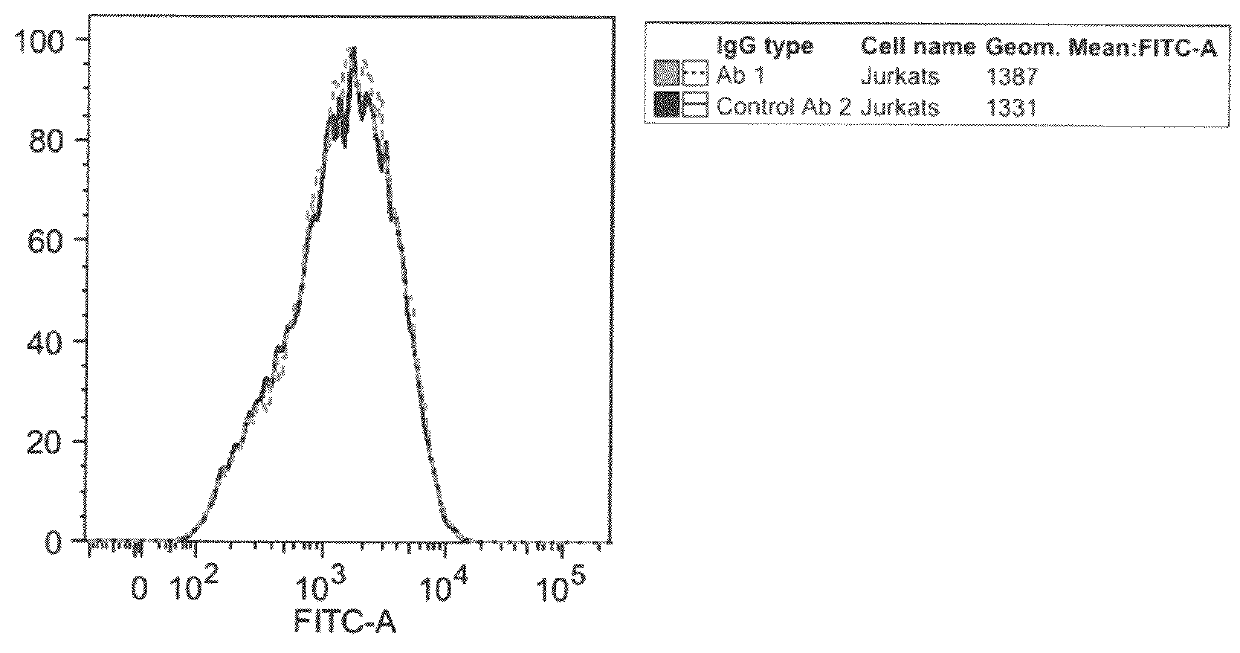
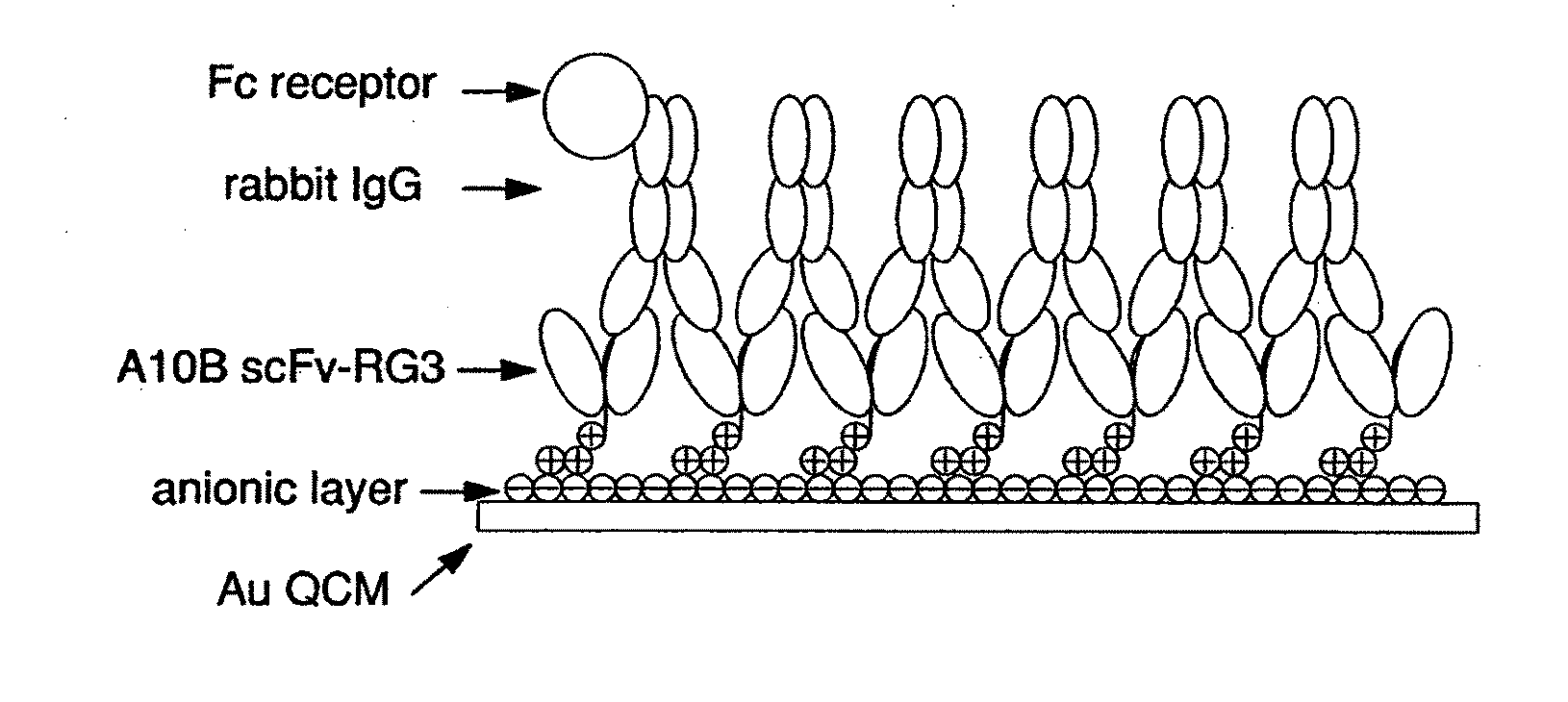
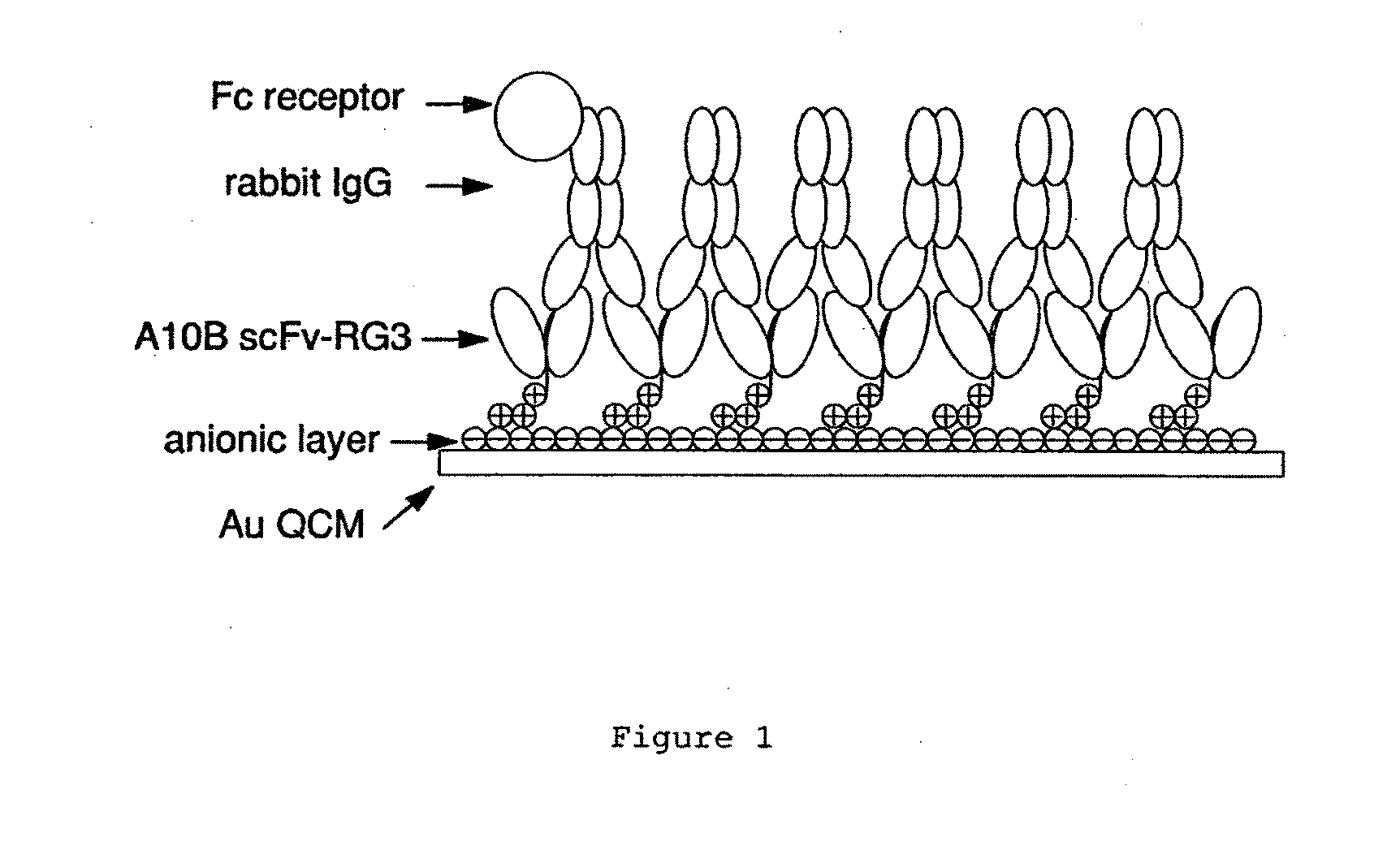
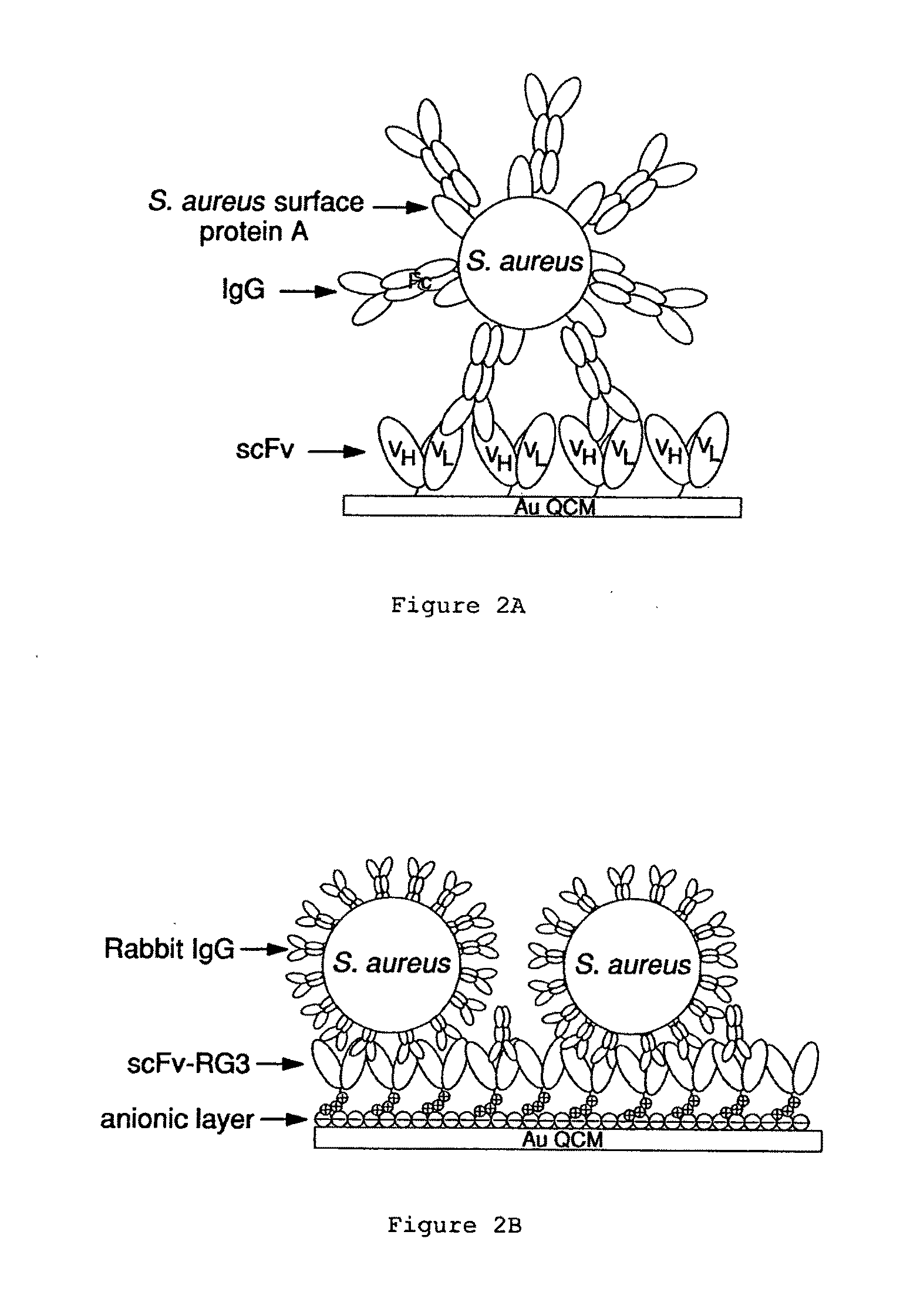
An apparatus and methods for binding an analyte of interest in a sample are provided. The apparatus comprises a substrate with an exposed surface with an compound, that is electrostatically charged or capable of forming hydrogen bonds, provided bound to the solid substrate. A recombinant single chain antibody (scFv) molecule specific for the analyte of interest, having one or more amino acids with charged or hydrogen-bond forming sidechains in a linker polypeptide portion, is bound to the layer on the solid substrate. When the analyte of interest is present in the sample the scFv binds the analyte to the solid substrate. The apparatus can be used with an immunoglobulin layer to detect Fc receptors, so as to detect microorganisms such as Staphylococcus aureus having protein A or protein G.
Owner:OAKLAND UNIVESITY +1
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
Humanized FcγRIIB-specific antibodies and methods of use thereof
InactiveUS7786270B2Immune responseAvoid immune responseDisease diagnosisTissue cultureFc(alpha) receptorCancer cell
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Stable heterodimeric antibody design with mutations in the Fc domain
ActiveUS9562109B2Promote formationImprove stabilityHybrid immunoglobulinsPeptide preparation methodsFc(alpha) receptorFc receptor
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090202537A1Balanced functionImmune responseSenses disorderNervous disorderFc(alpha) receptorImmunologic disorders
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer (preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma), autoimmune disease, inflammatory disease or IgE-mediated allergic disorder. The present invention also encompasses the use of a humanized FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
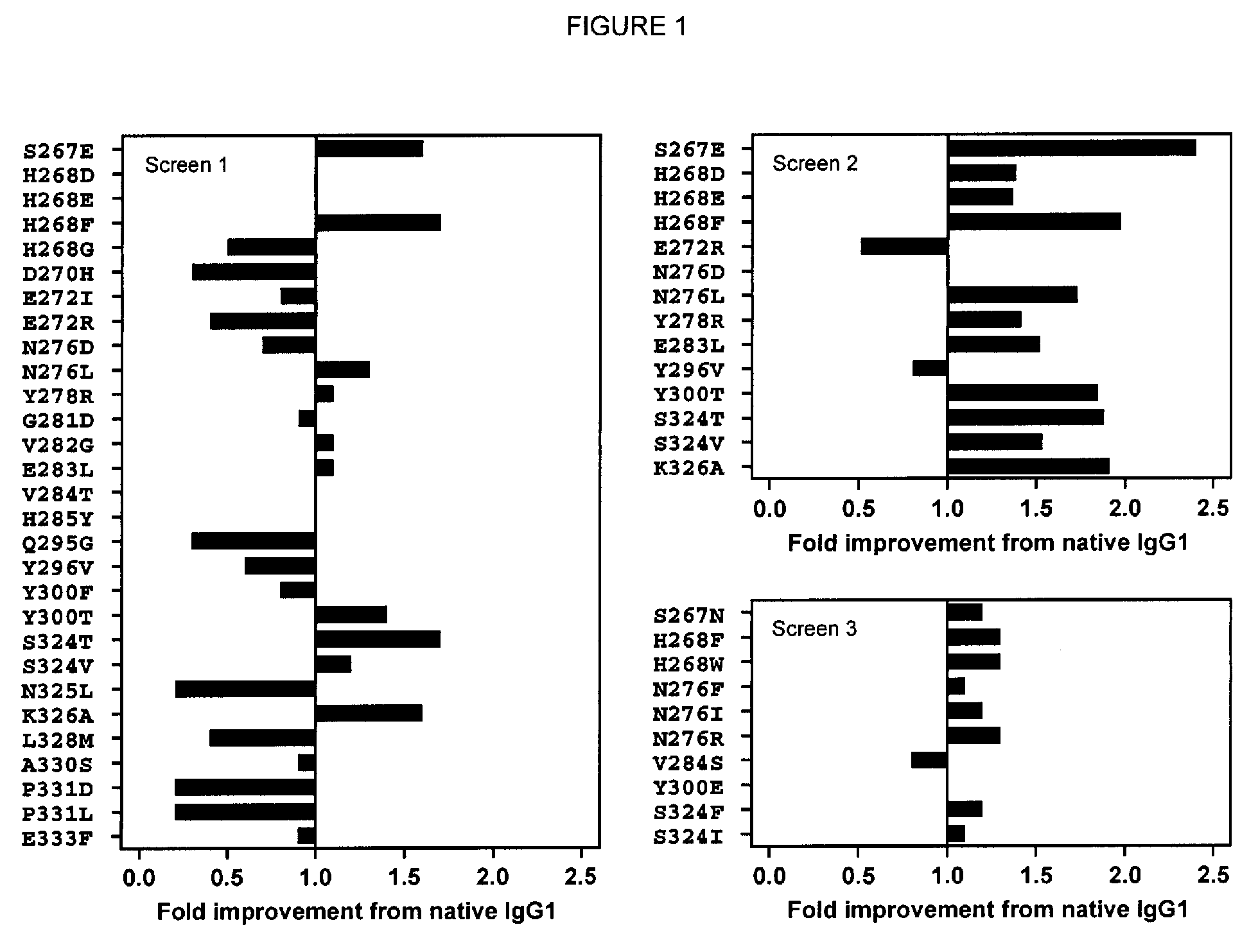
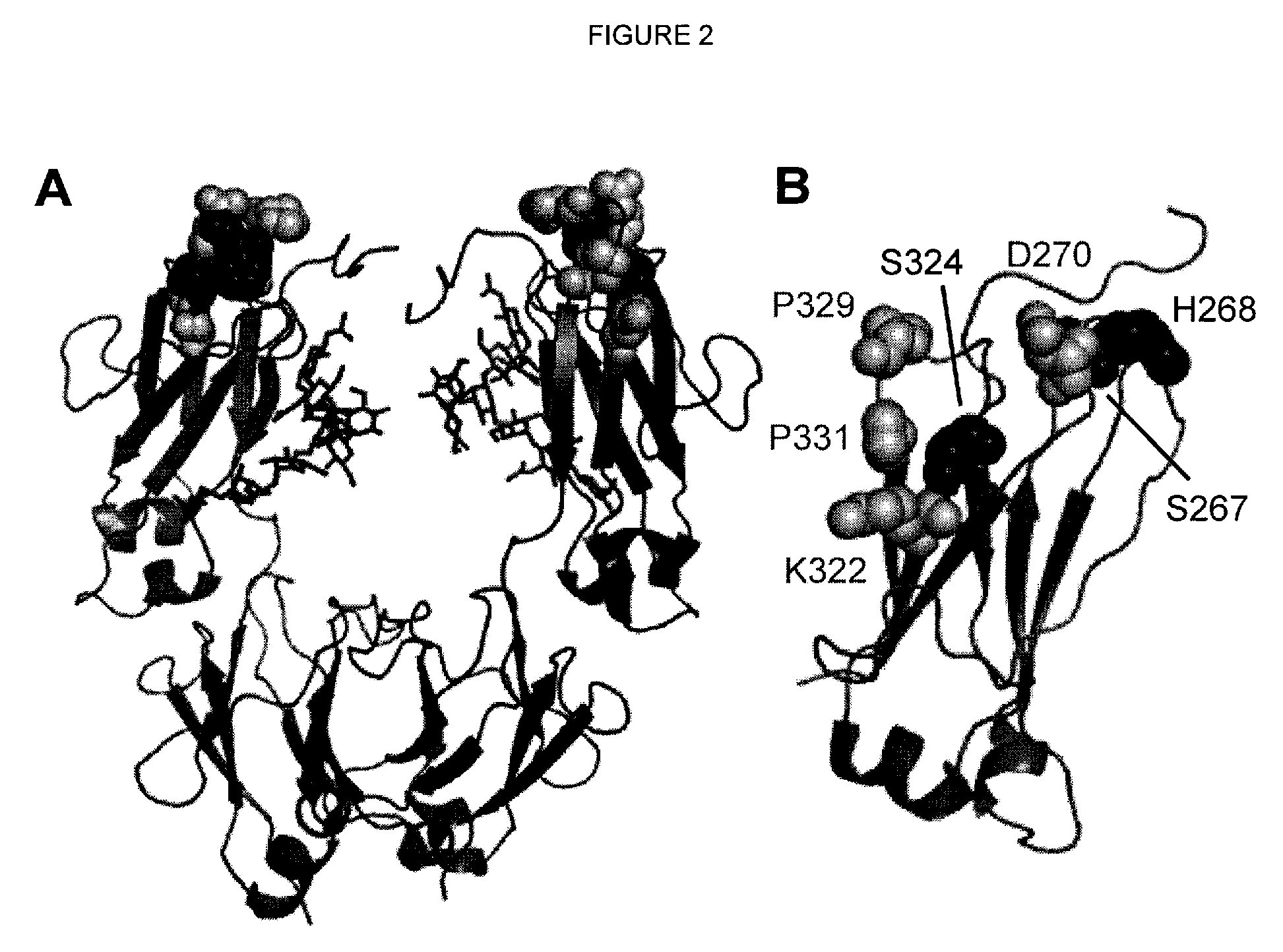
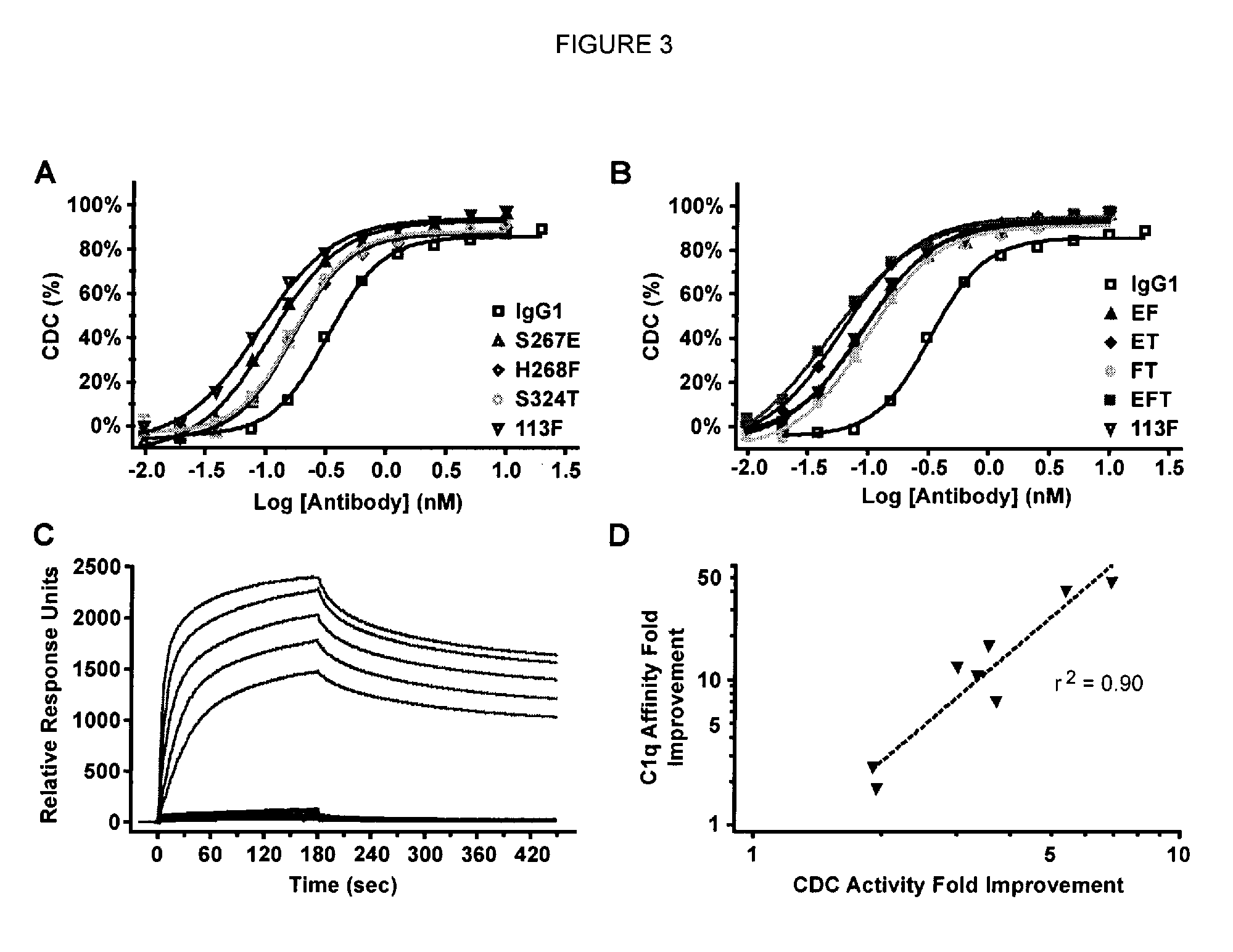
Antibody variants with enhanced complement activity
The present invention relates to novel Fc variants that comprise at least one novel amino acid residue which may provide for enhanced effector function. More specifically, this invention provides Fc variants that have modified binding affinity to one or more Fc receptor or ligand (e.g., Fc gamma R, C1q). Additionally, the Fc variants have altered complement dependent cytotoxicity (CDC) activity and / or antibody-dependent cell-mediated cytotoxicity (ADCC). The invention further provides methods and protocols for the application of said Fc variants, particularly for therapeutic purposes.
Owner:XENCOR INC
Enhancing the circulating half-life of antibody-based fusion proteins
InactiveUS20060194952A1Extended half-lifeReduced binding affinityHybrid immunoglobulinsSugar derivativesFc(alpha) receptorFc receptor
Disclosed are methods for the genetic construction and expression of antibody-based fusion proteins with enhanced circulating half-lives. The fusion proteins of the present invention lack the ability to bind to immunoglobulin Fc receptors, either as a consequence of the antibody isotype used for fusion protein construction, or through directed mutagenesis of antibody isotypes that normally bind Fc receptors. The fusion proteins of the present invention may also contain a functional domain capable of binding an immunoglobulin protection receptor.
Owner:MERCK PATENT GMBH
Anti-cd20 glycoantibodies and uses thereof
InactiveUS20150344585A1Increase healing valueEnhanced ADCC activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFc(alpha) receptorFc receptor
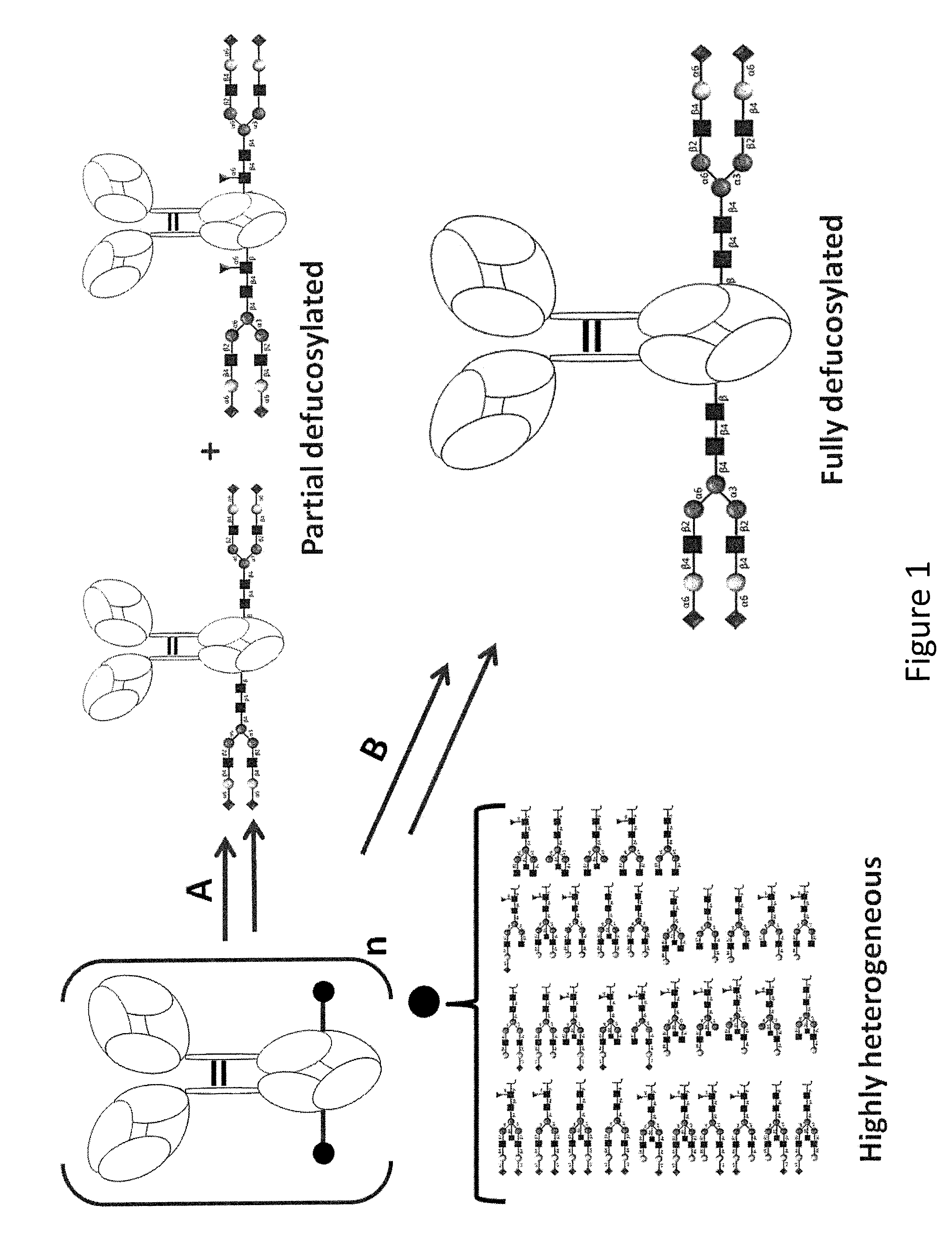
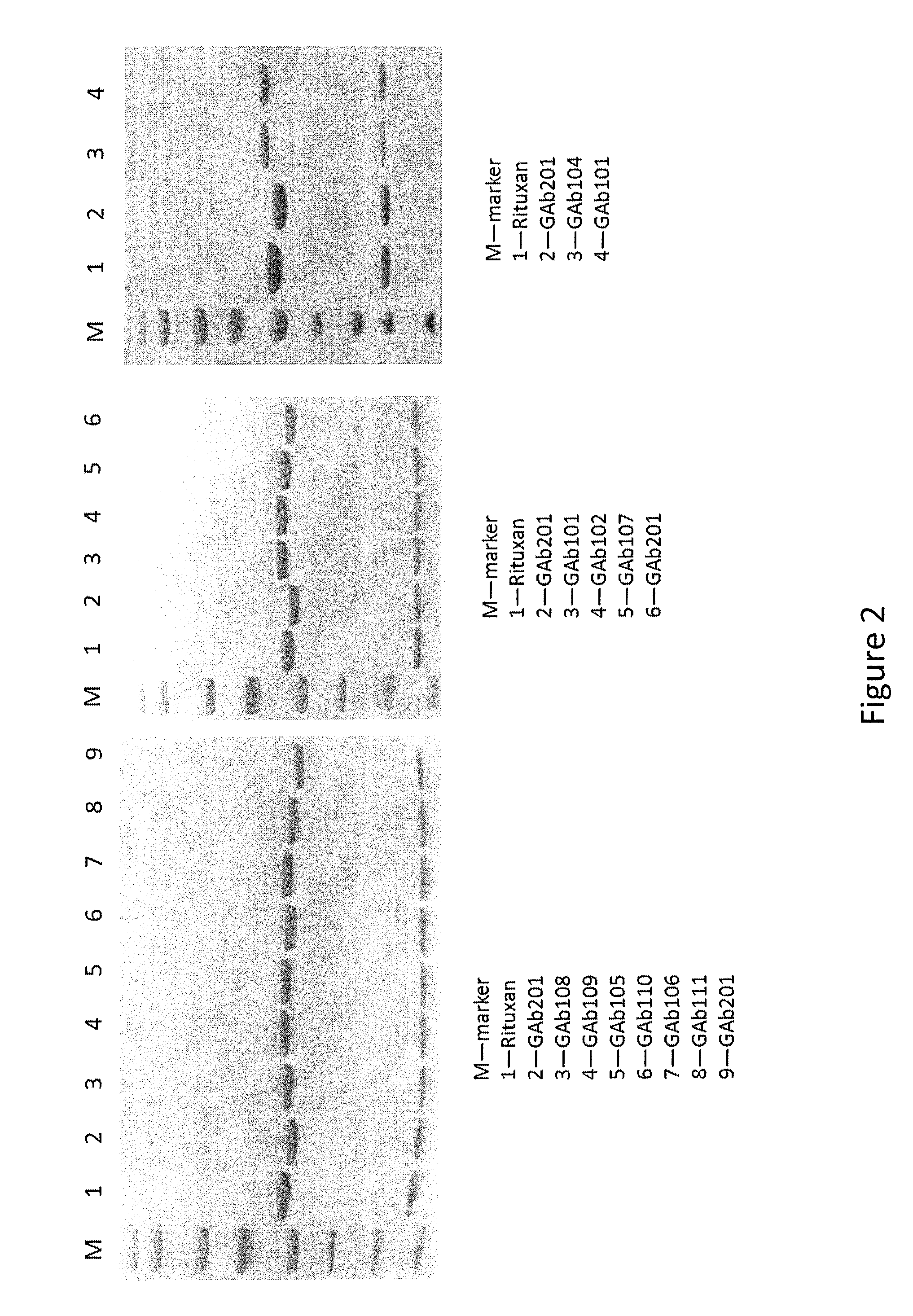
The present disclosure relates to a novel class of anti-CD20 monoclonal antibodies comprising a homogeneous population of anti-CD20 IgG molecules having the same N-glycan on each of Fc. The antibodies of the invention can be produced from anti-CD20 monoclonal antibodies by Fc glycoengineering. Importantly, the antibodies of the invention have improved therapeutic values with increased ADCC activity and increased Fc receptor binding affinity compared to the corresponding monoclonal antibodies that have not been glycoengineered.
Owner:ACAD SINIC
Prevention of brain inflammation as a result of induced autoimmune response
InactiveUS20060008458A1Inhibit bindingIncreased riskImmunoglobulins against animals/humansAntibody ingredientsFc(alpha) receptorFc receptor
A disease characterized by amyloid aggregation in a patient may be prevented or treated by causing antibodies against a peptide component of the amyloid deposit to come into contact with the aggregated or soluble amyloid. In order to decrease the risk of inflammation in such a method, the Fc receptors of the patient are blocked, preferably by administration of an effective amount of IVIg, prior to the procedure of causing the antibodies to come into contact with the amyloid.
Owner:RAMOT AT TEL AVIV UNIV LTD
Antibodies with Enhanced or Suppressed Effector Function
InactiveUS20130108623A1Increase free energyLow affinitySugar derivativesAntipyreticFc receptorAntiendomysial antibodies
Rationally designed antibodies and polypeptides that comprise two Fc region amino acid substitutions that synergistically provide enhanced selectivity and binding affinity to a target Fc receptor are provided. The polypeptides comprise Fc region mutations at two positions that synergistically make the polypeptides more effective when incorporated in antibody therapeutics than those having wild-type Fc components.
Owner:ZYMEWORKS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com