Anti-cd20 glycoantibodies and uses thereof
a glycoantibody and anti-cd20 technology, applied in the field of anti-cd20 glycoantibodies, can solve the problems of reducing the life span of the circulatory system of the drug, affecting the effector function of the therapeutic antibody, and provoking immunogenicity, so as to improve the therapeutic value, increase the binding affinity of the fc receptor, and increase the activity of the adcc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for Analysis of N-Glycosylation of Anti-CD20 Antibodies
[0233]We developed a mass spectrometric method to monitor the yield of oligosaccharide-derived fragment ions (oxonium ions) over a collision induced dissociation (CID) energy applied to a glycopeptides precursor. Multiple Reaction Monitoring (MRM) of oxonium ions method could fulfill the regulatory requirement on the routine quality control analysis of forthcoming biosimilar therapeutics.
[0234]5 ug of Rituximab (purchased from Genentech) was dissolved in 25 ul of 2M Guanidine-HCl, and dithiothreitol (DTT) were added to a final concentration of 5 mM. After 10 minutes incubation in 110° C., reduced cysteine residues were alkylated in 10 mM Iodoacetamide (IAA) at 37° C. for 1 hour. Add 5 mM DTT to quench excess IAA at RT for 10 minutes. The product was diluted 15 times in 50 mM ammonium bicarbonate before microcentrifugation with spin column (10 kDa protein MW cut-off). The trypsin digestion was performed for 4 ho...
example 2
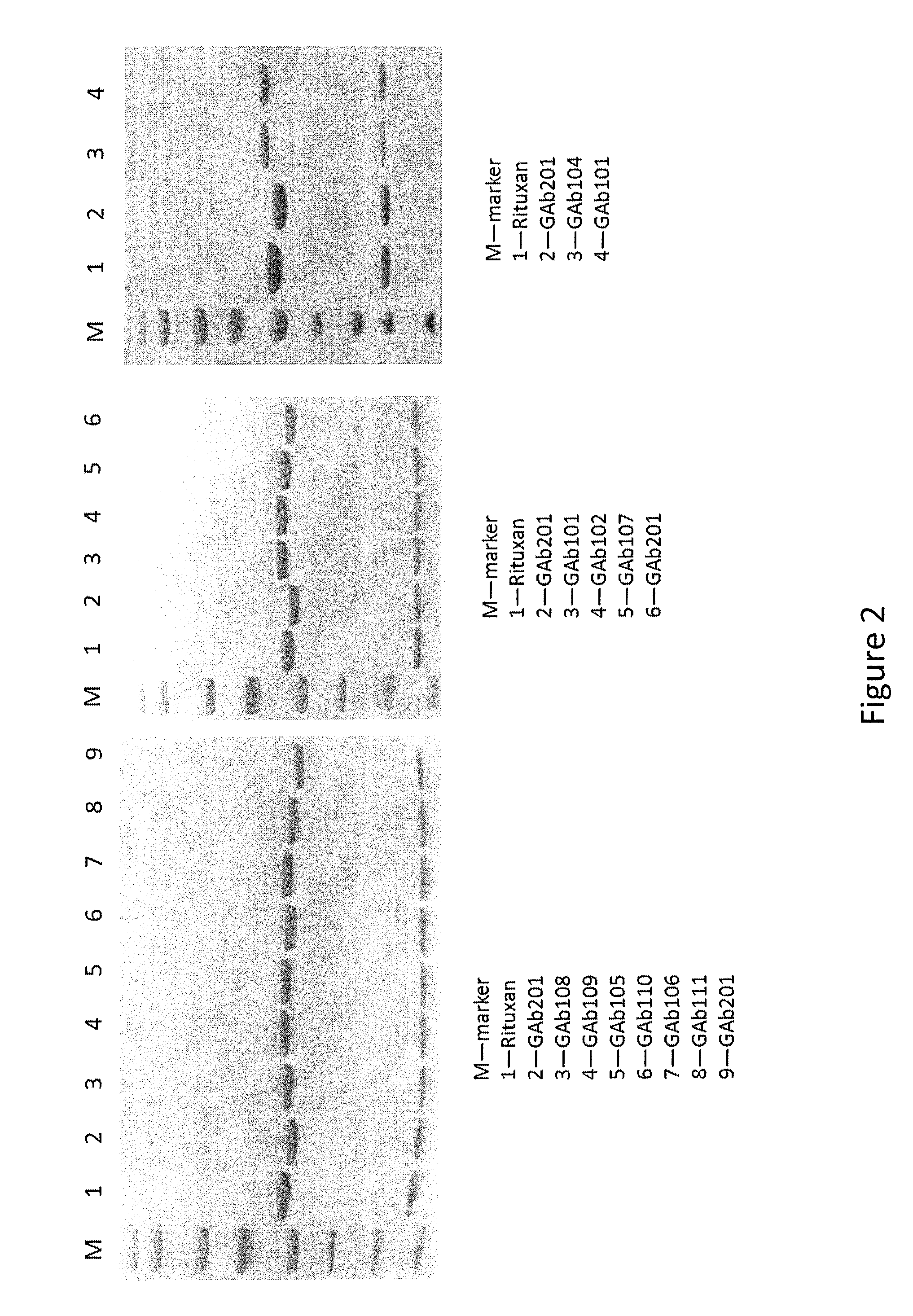
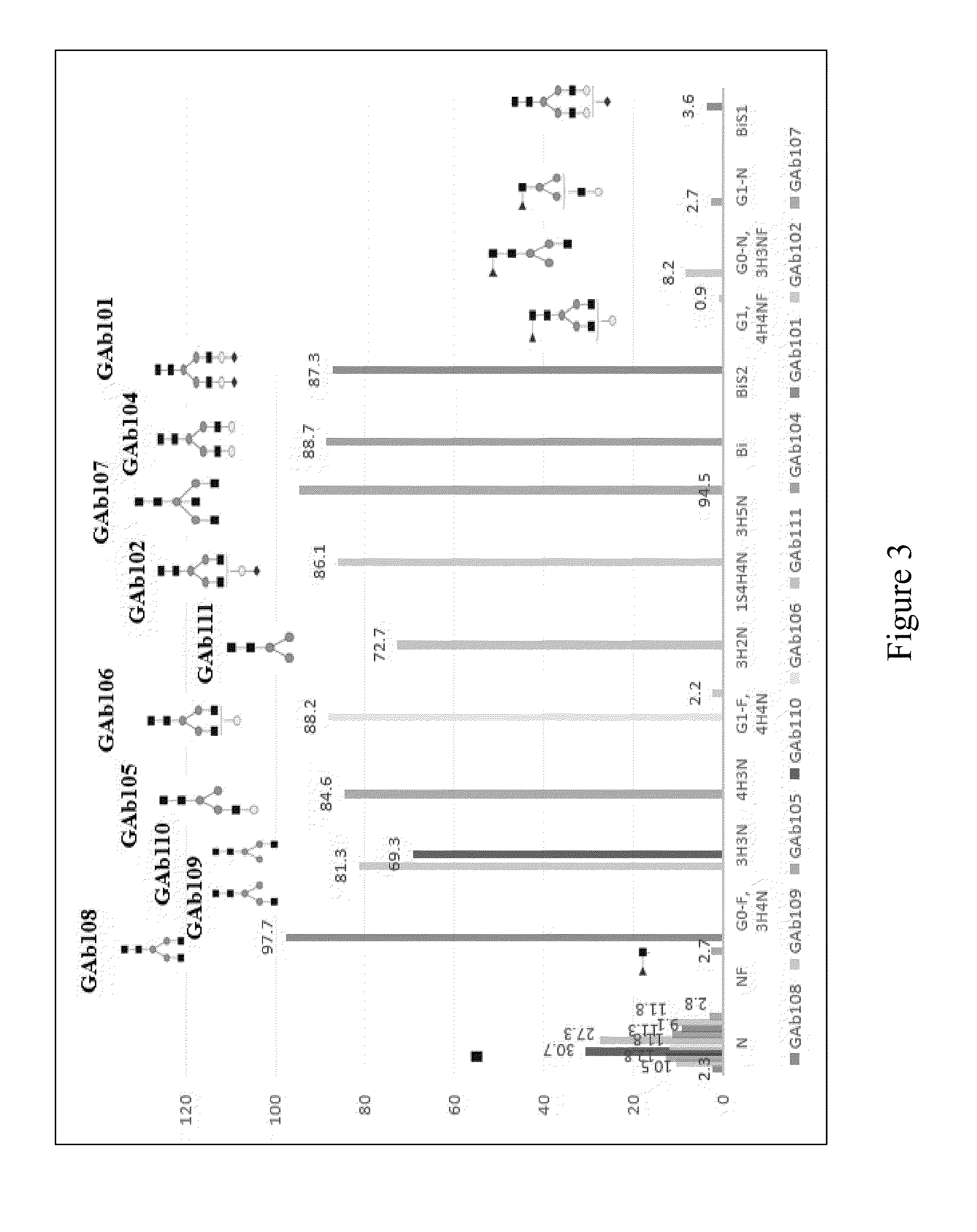
Generation of Anti-CD20 GAbs
Anti-CD20 GAb301
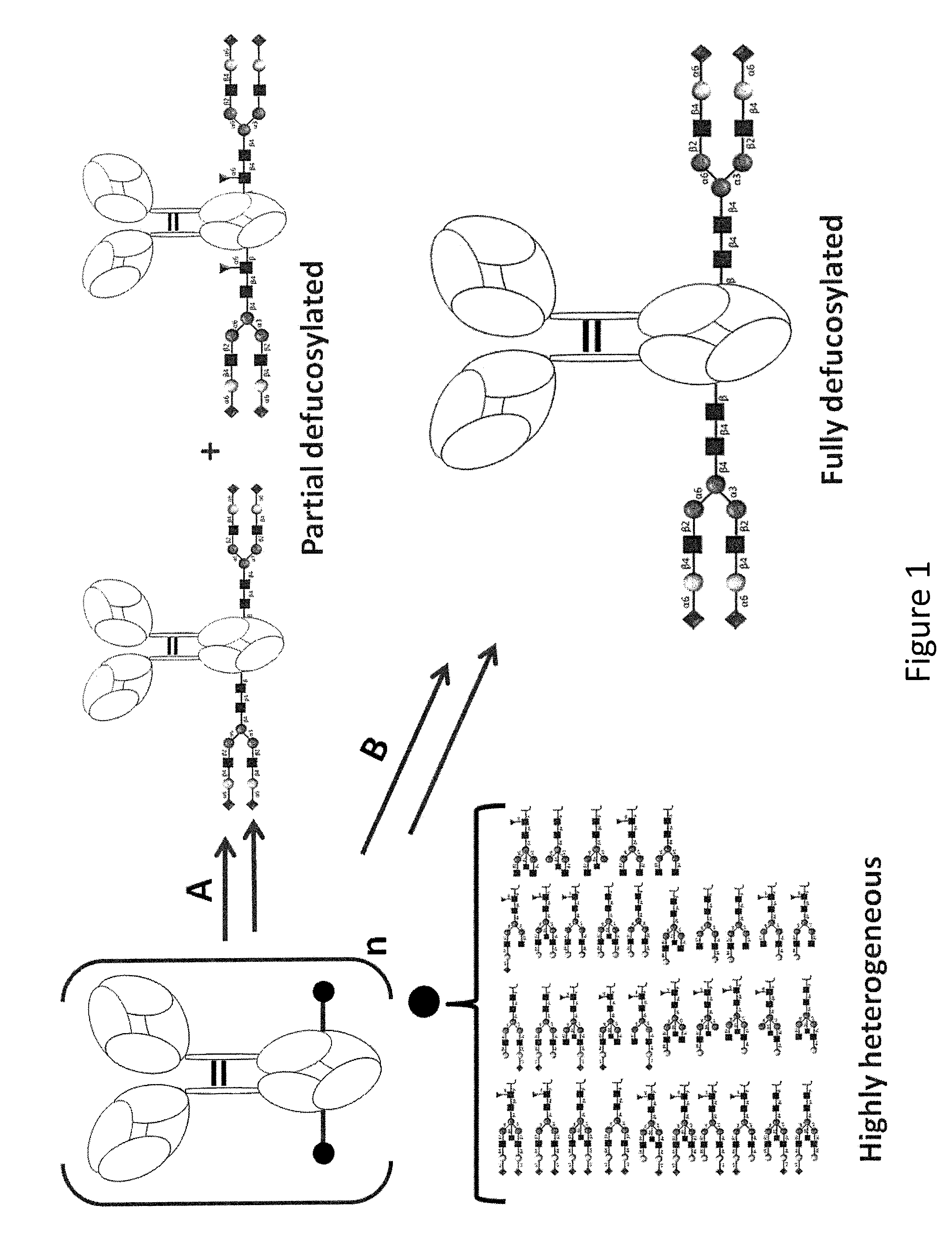
[0238]The complete removal of N-linked glycan at Asn297 from Fe region of Rituximab (Rituxan) is achieved by means of PNGase F, and evaluated with 4-12% Bis-Tris NeuPAGE and LC-MS / MS analysis of tryptic glycopeptides from modified and unmodified IgG. The molecular weights of tryptic glycopeptides helps to determine the potential site of N-linked glycosylation at each asparagine and to elucidate the species of predominant glycans.
Anti-CD20 GAb200
[0239]Commercial or home-made heterogeneous mAb was used as the starting material and modified with selected glycosidases. Application of Endoglycosidase (Endo F2, Endo F3, or Endo H) can yield a homogenous di-sugar mAb of GlcNAc-Fuc at its Fc N-linked glycosylation site (GAb200). Subsequently a homogeneous mono-sugar mAb can be obtained with application of fucosidase; or the mono-sugar species can also be obtained with combination of Endo F3 and fucosidase in one step as shown with Rituximab. The p...
example 3
Characterization of GAb301
[0264]Anti-CD20 GAb301 was tested for its antigenic binding and induced functions using B-lymphoma Ramos cells. The sugar-free Rituximab variant retained a full strength in both CD20 binding activity and induction of apoptosis, and reserved a 35% of CDC effect as compared to the Rituximab's maximum values; however, GAb301 lost almost completely the ADCC effect. These results indicate the presence of carbohydrates is essential to the induction of ADCC; the CDC activity is much impaired.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com