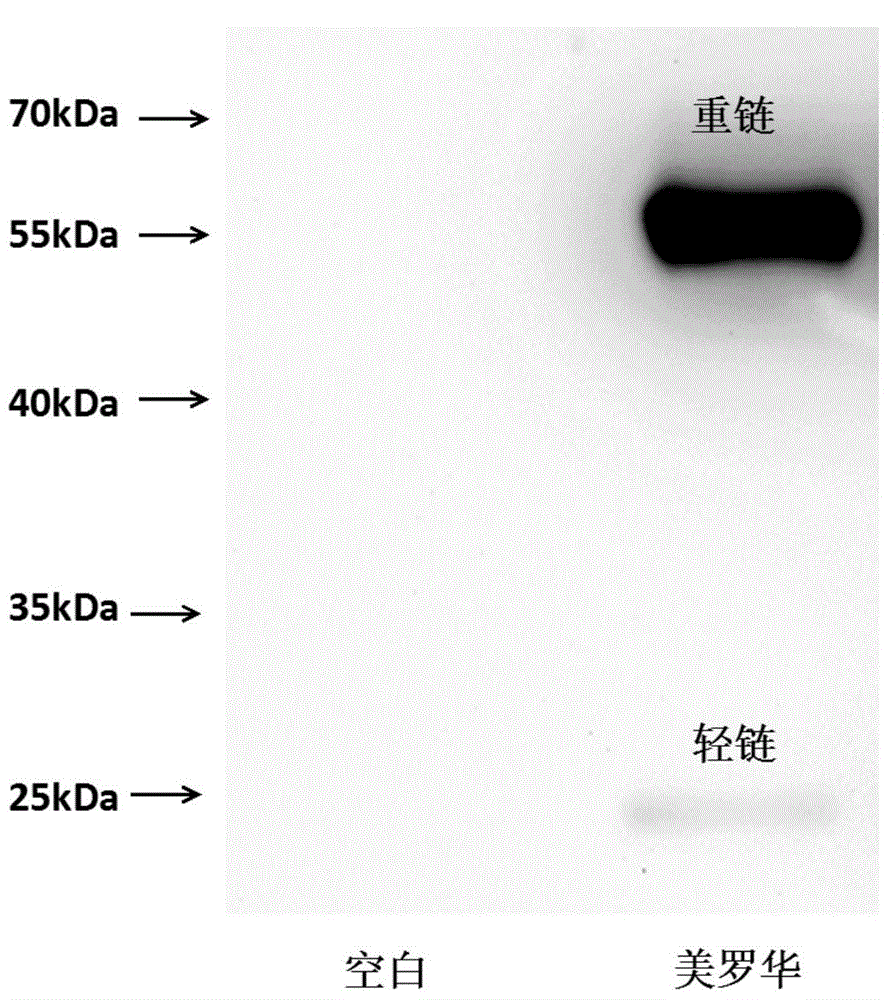
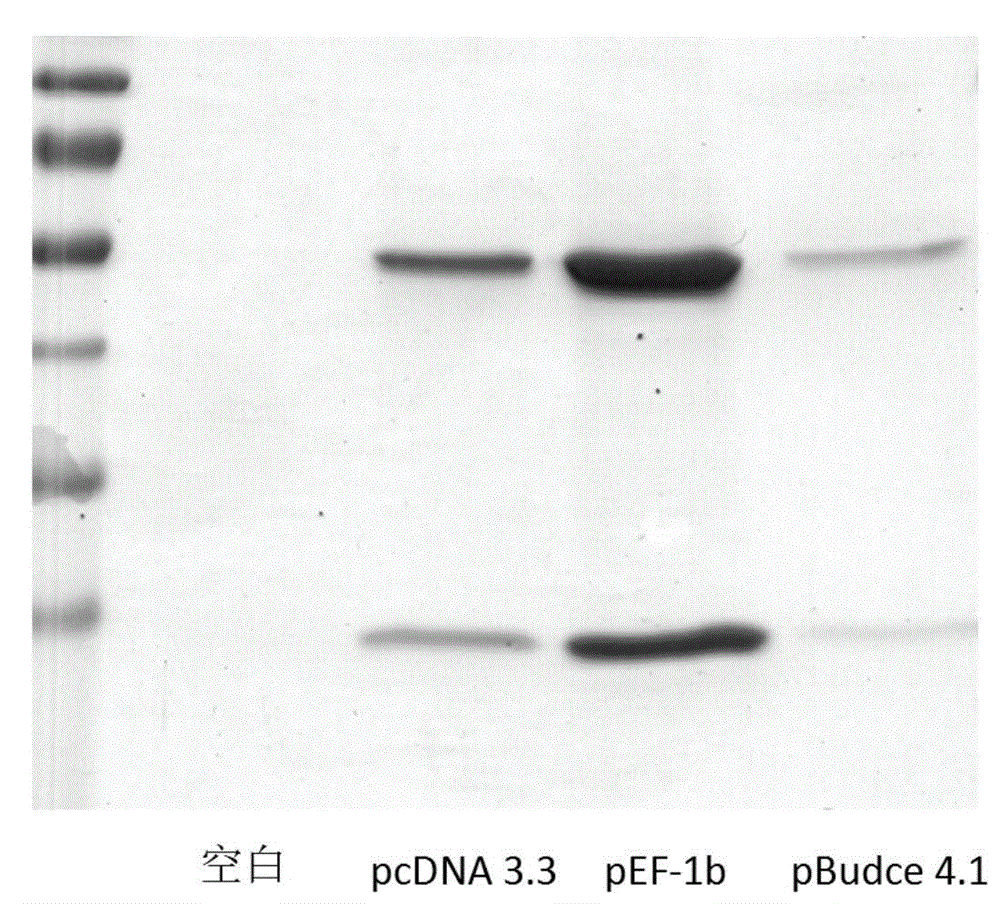
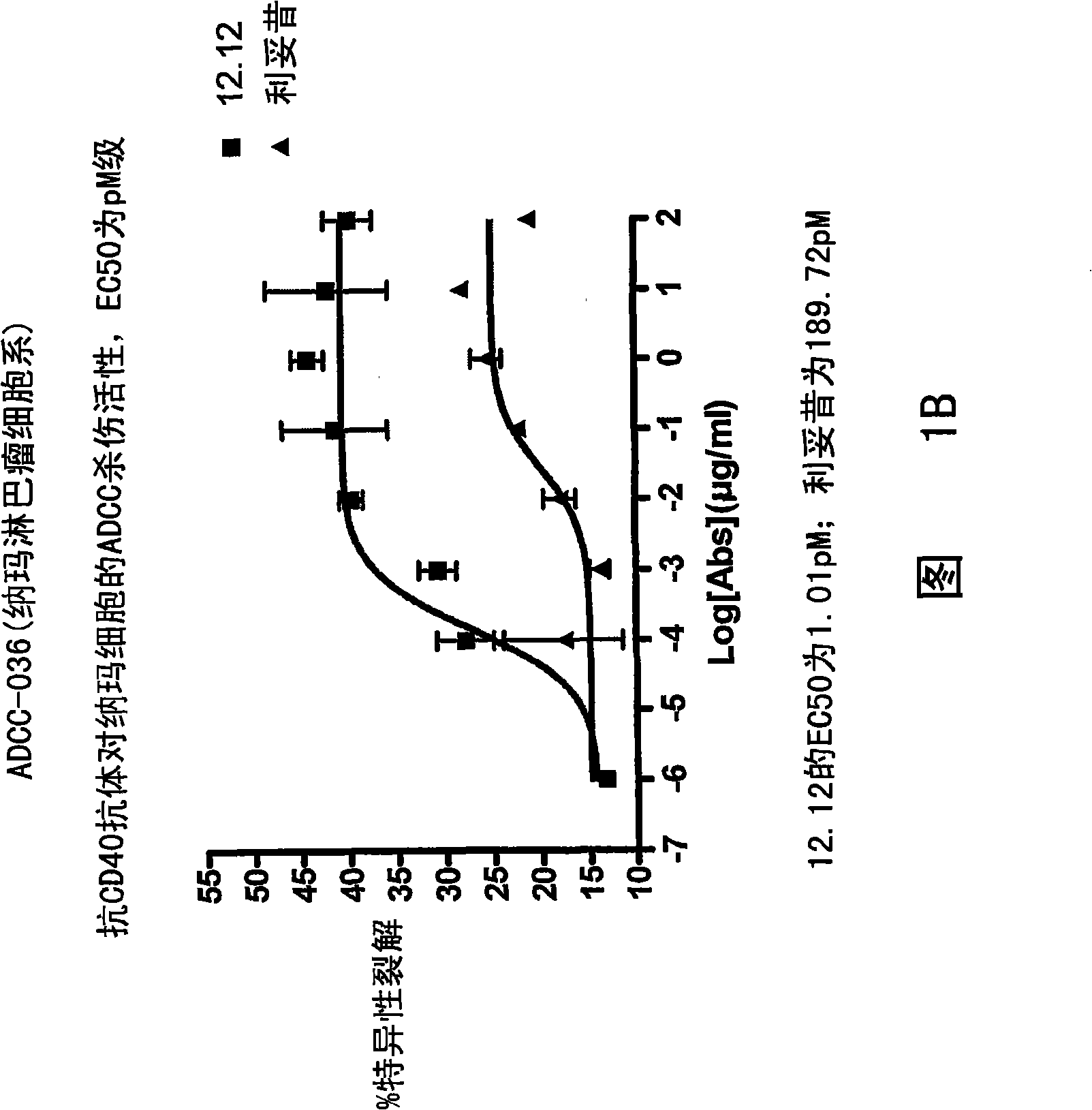
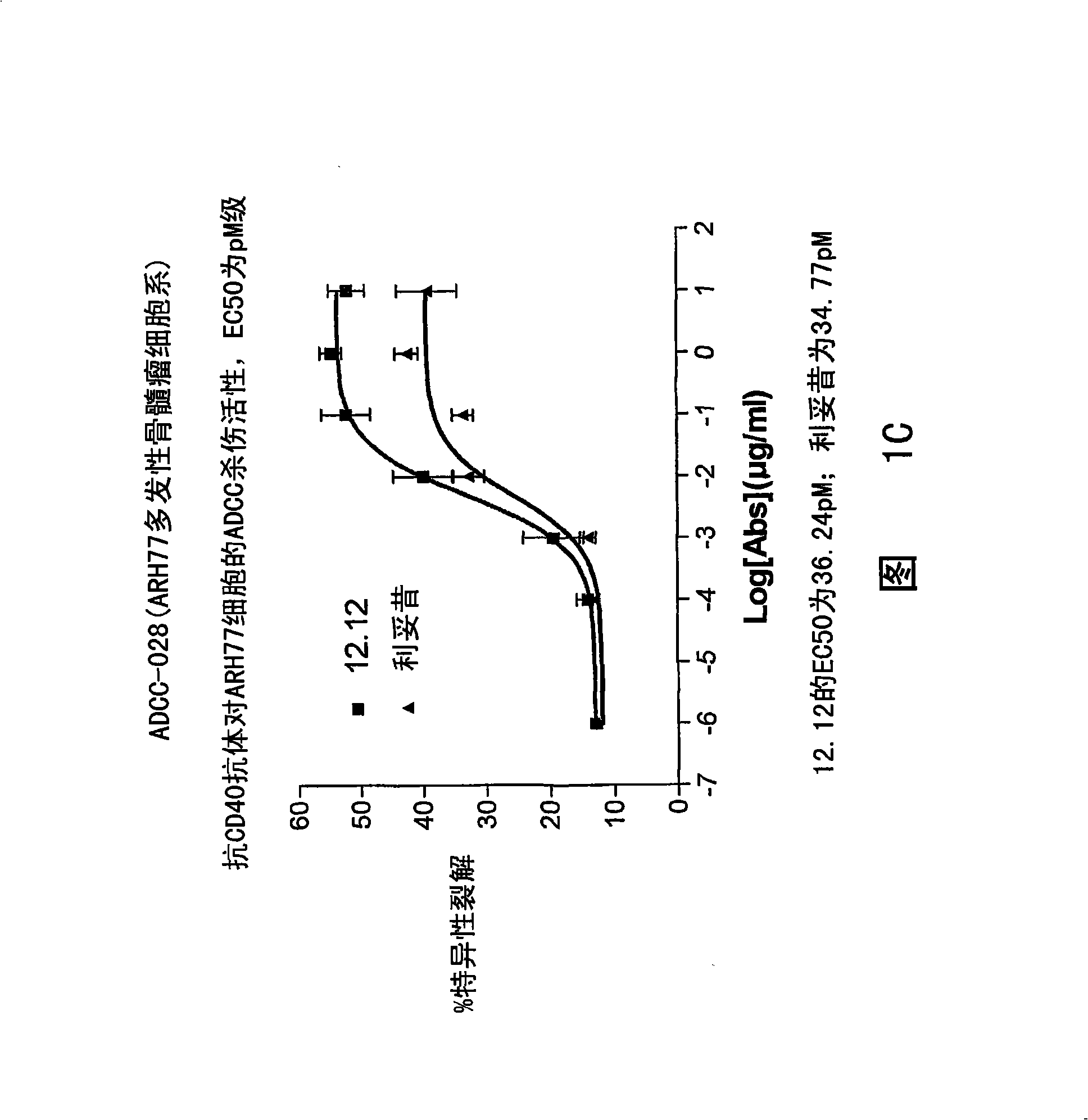
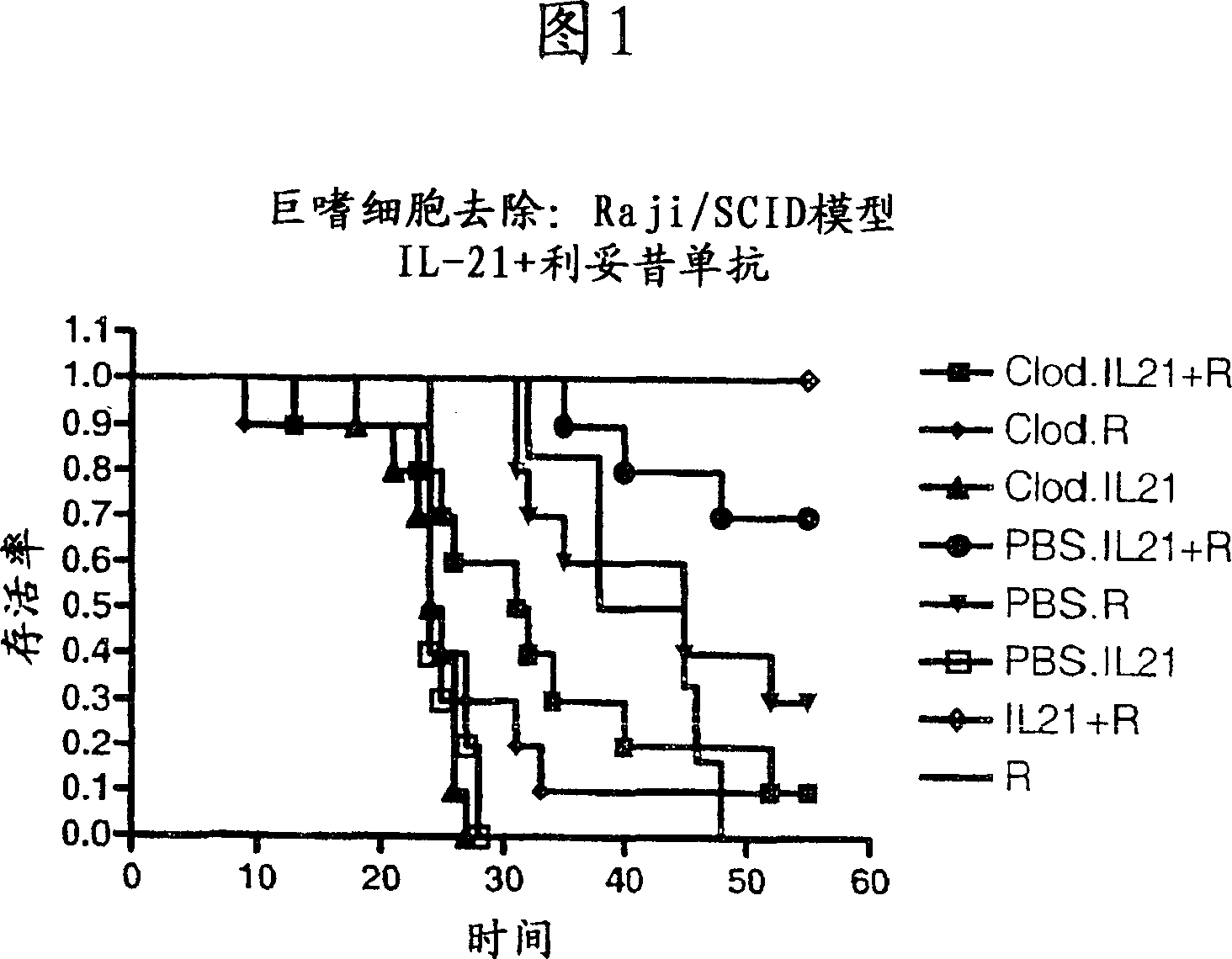
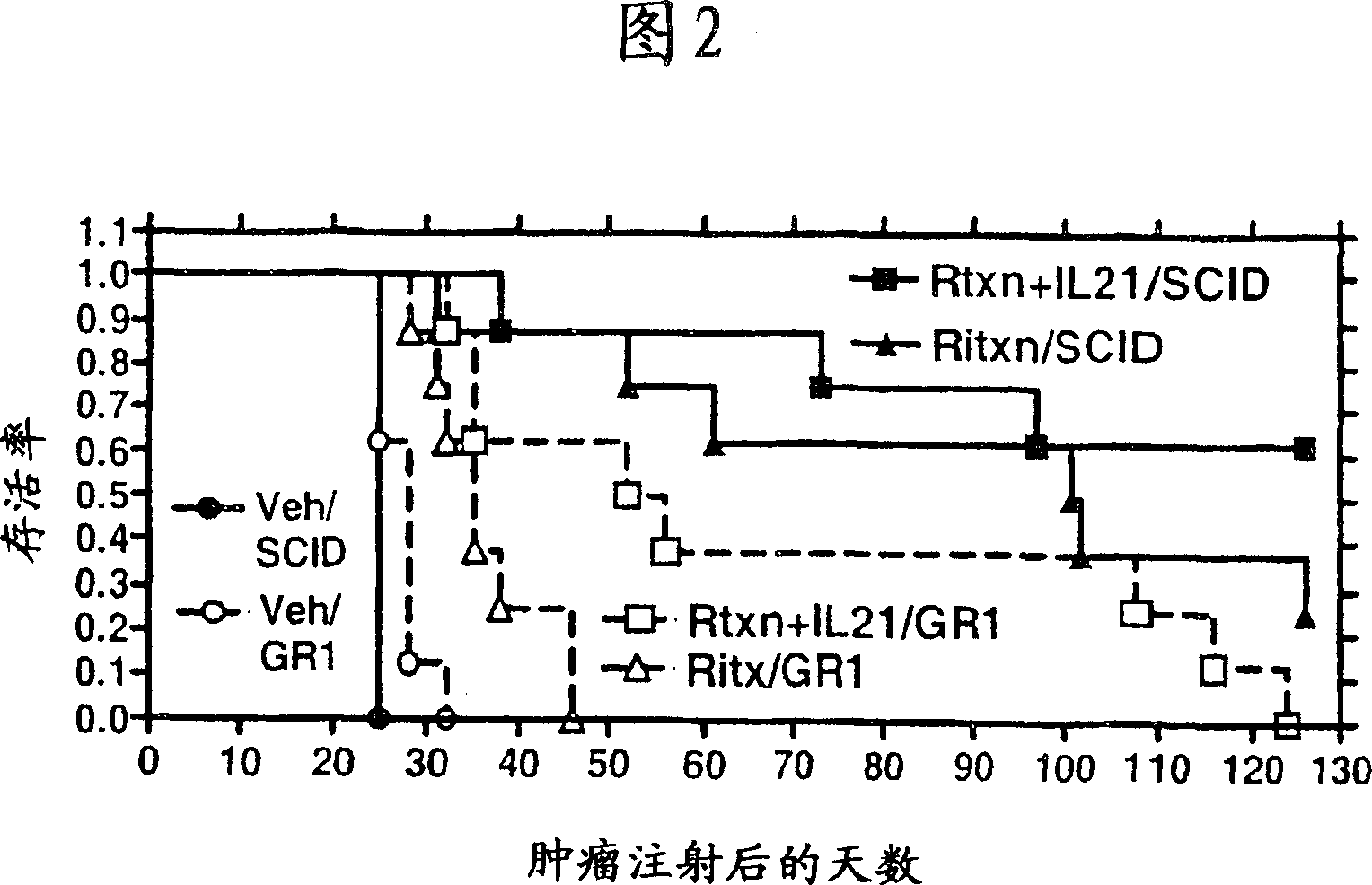
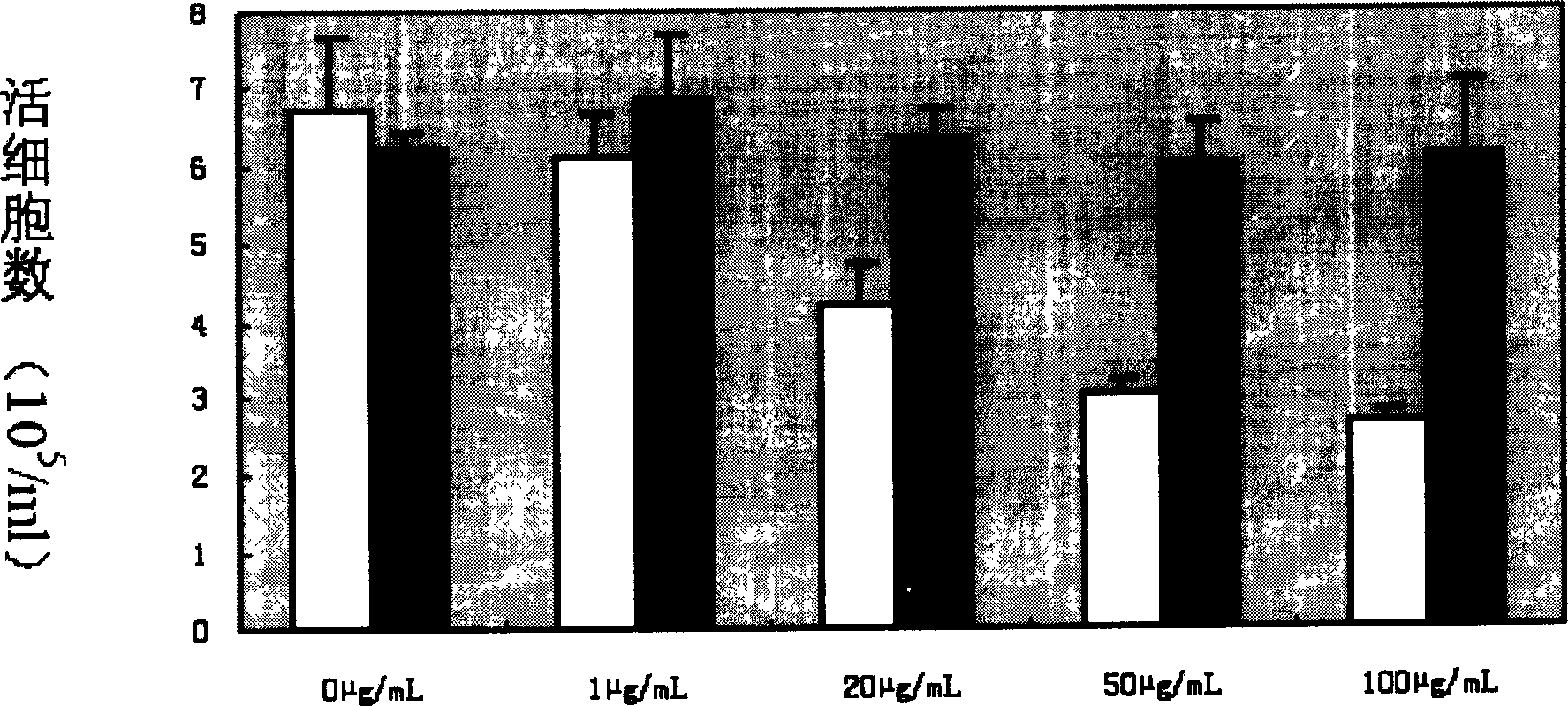
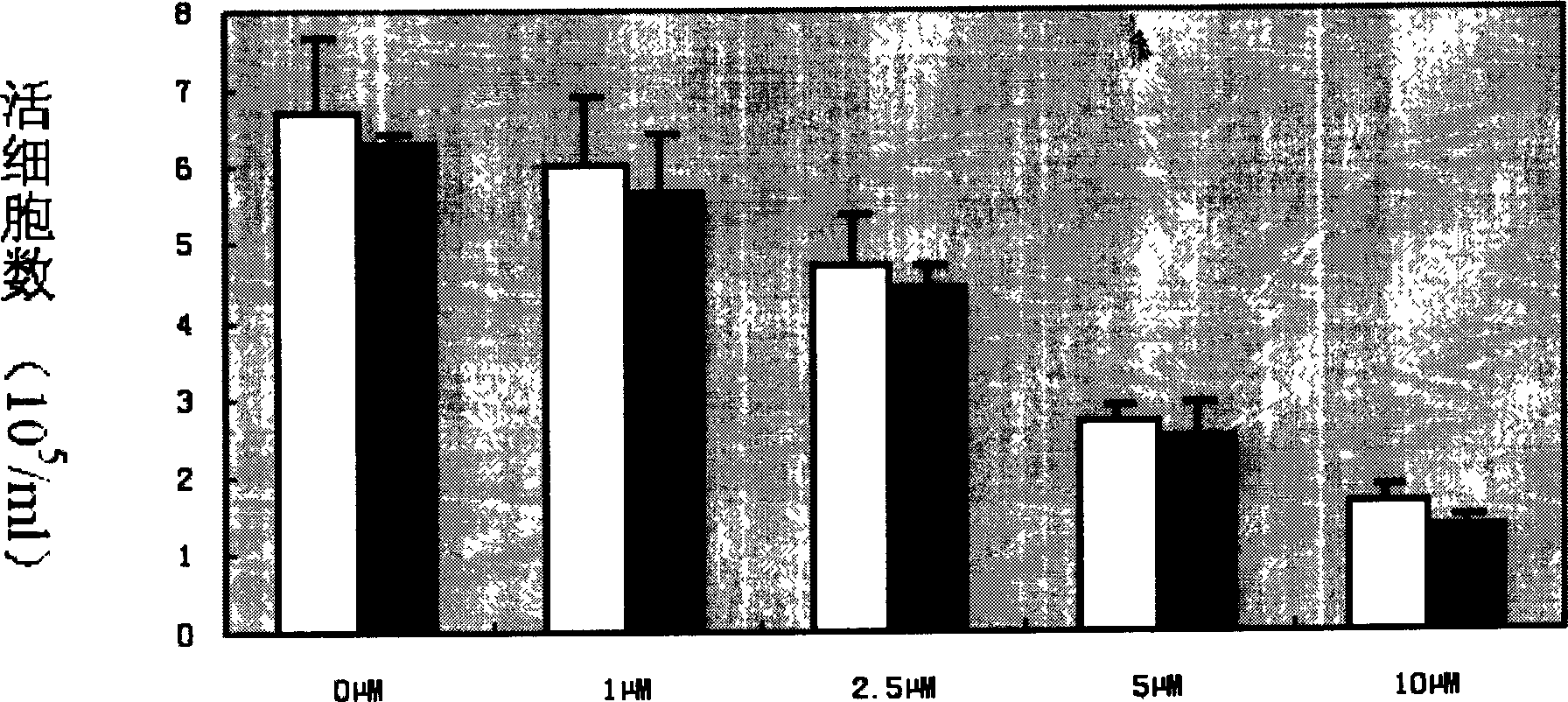
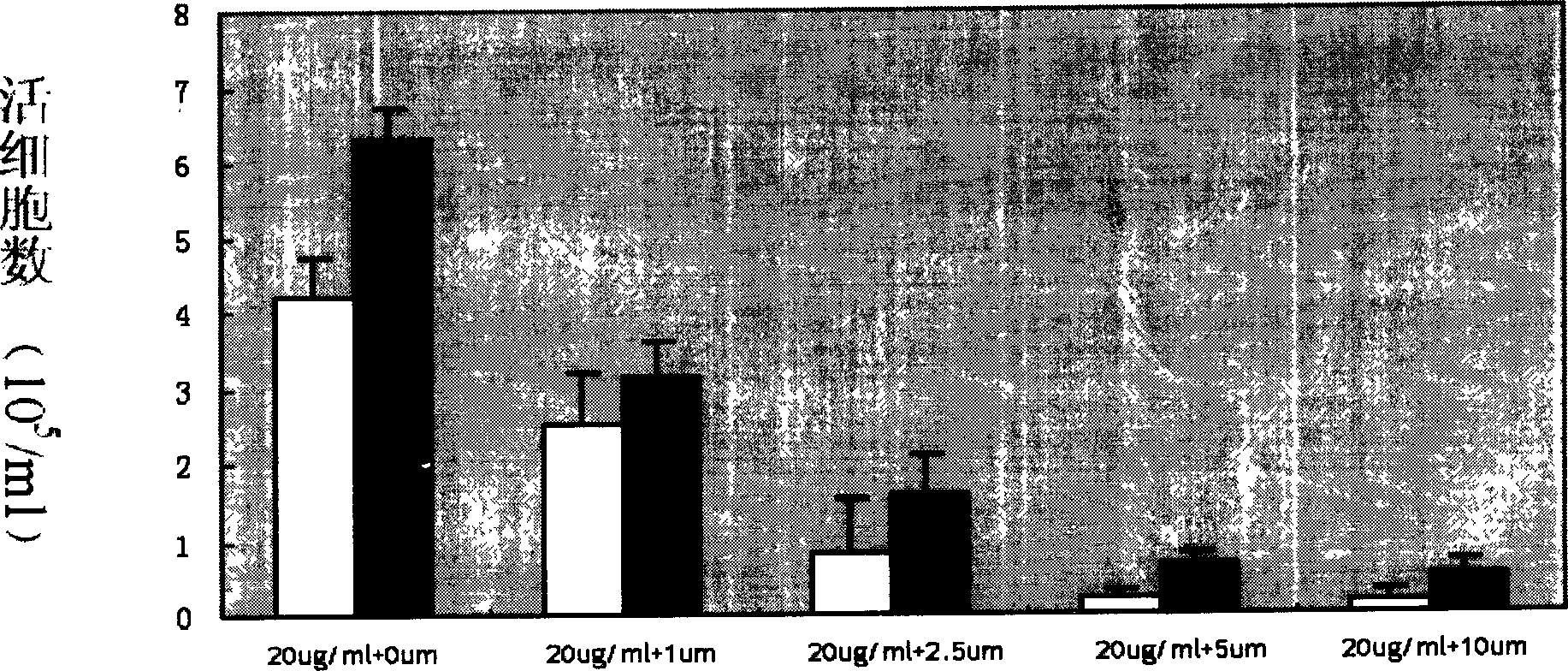
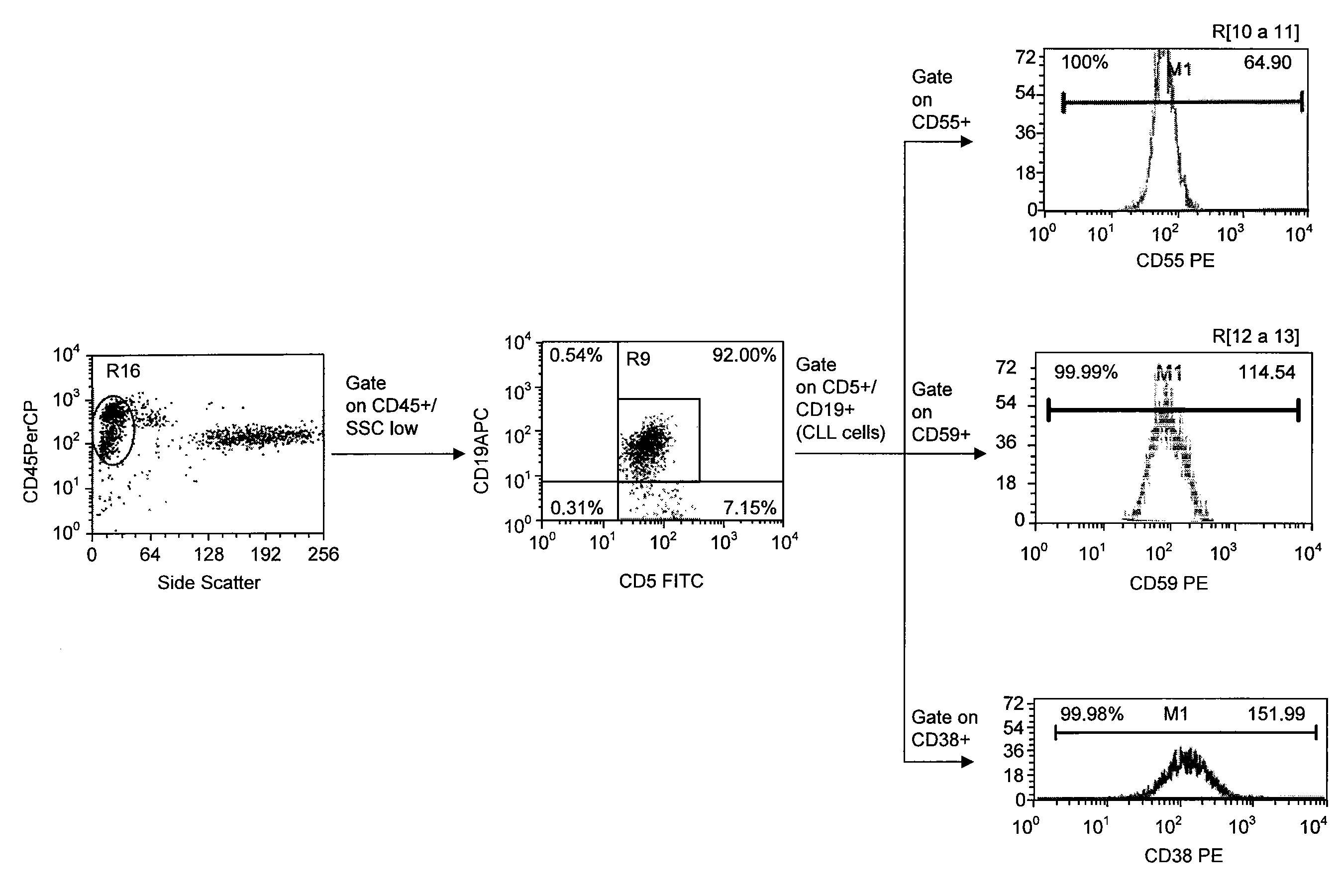
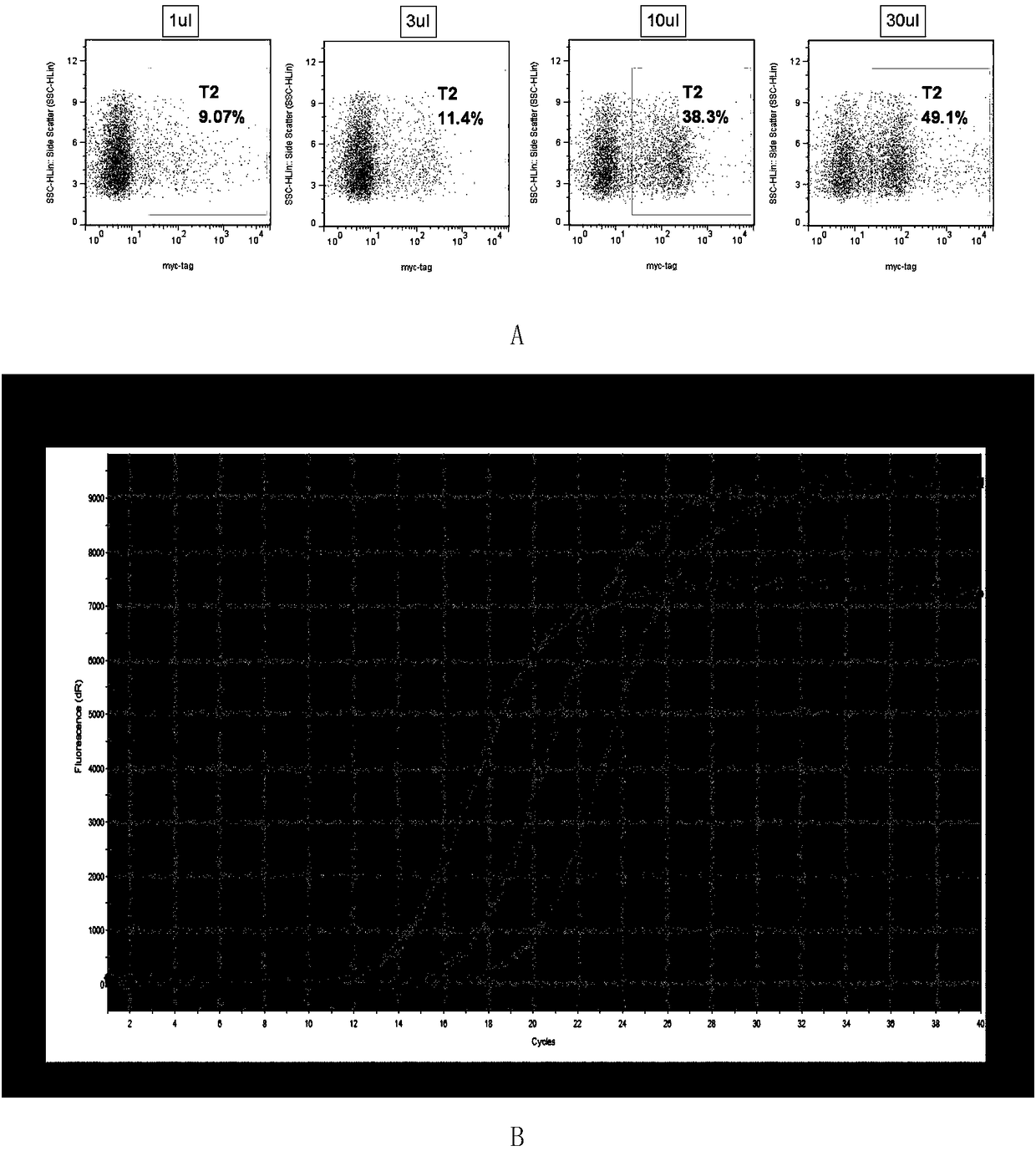
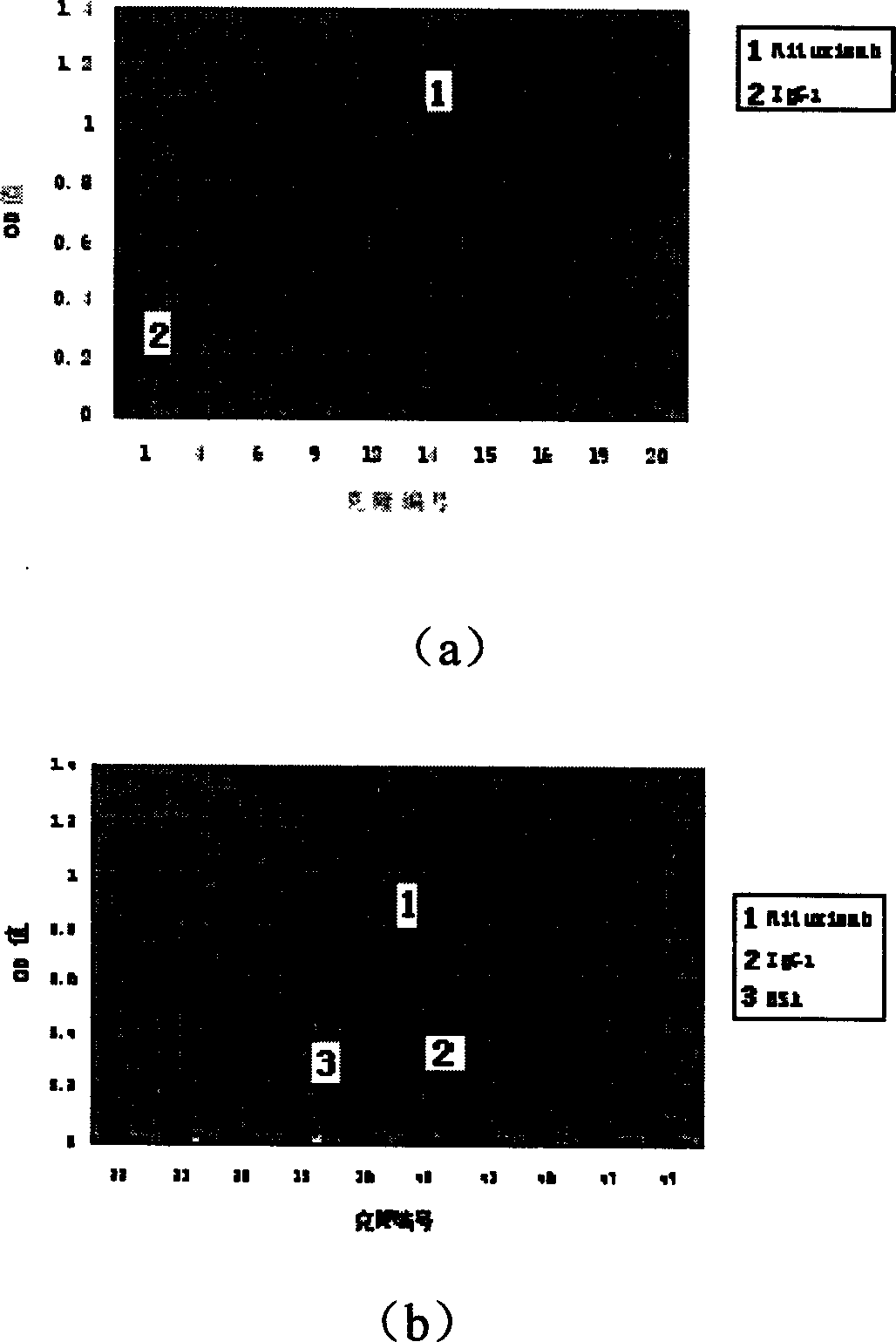
Patents
Literature
78 results about "Rituximab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rituximab is used to treat certain types of cancer (such as non-Hodgkin's lymphoma, chronic lymphocytic leukemia).
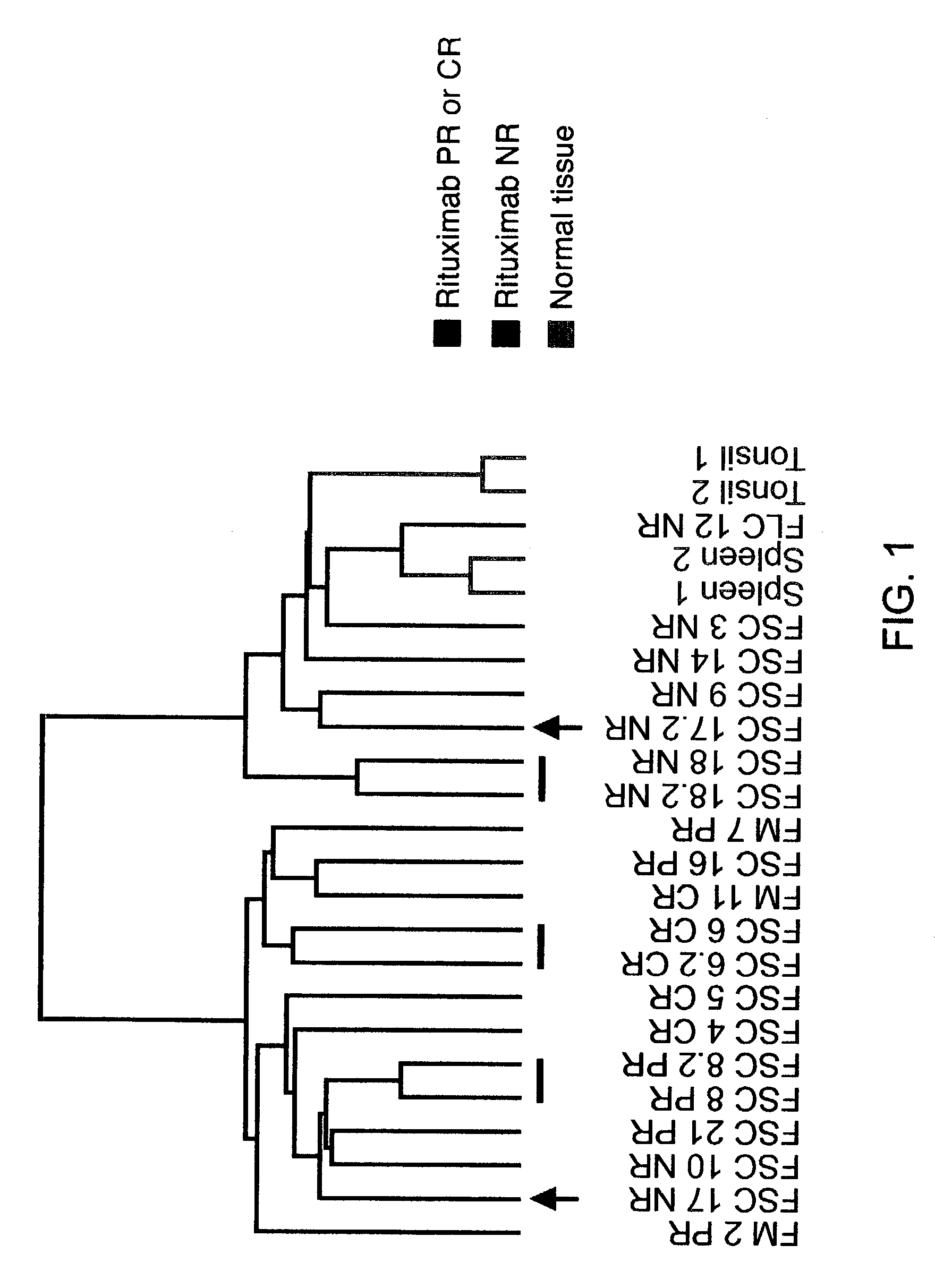
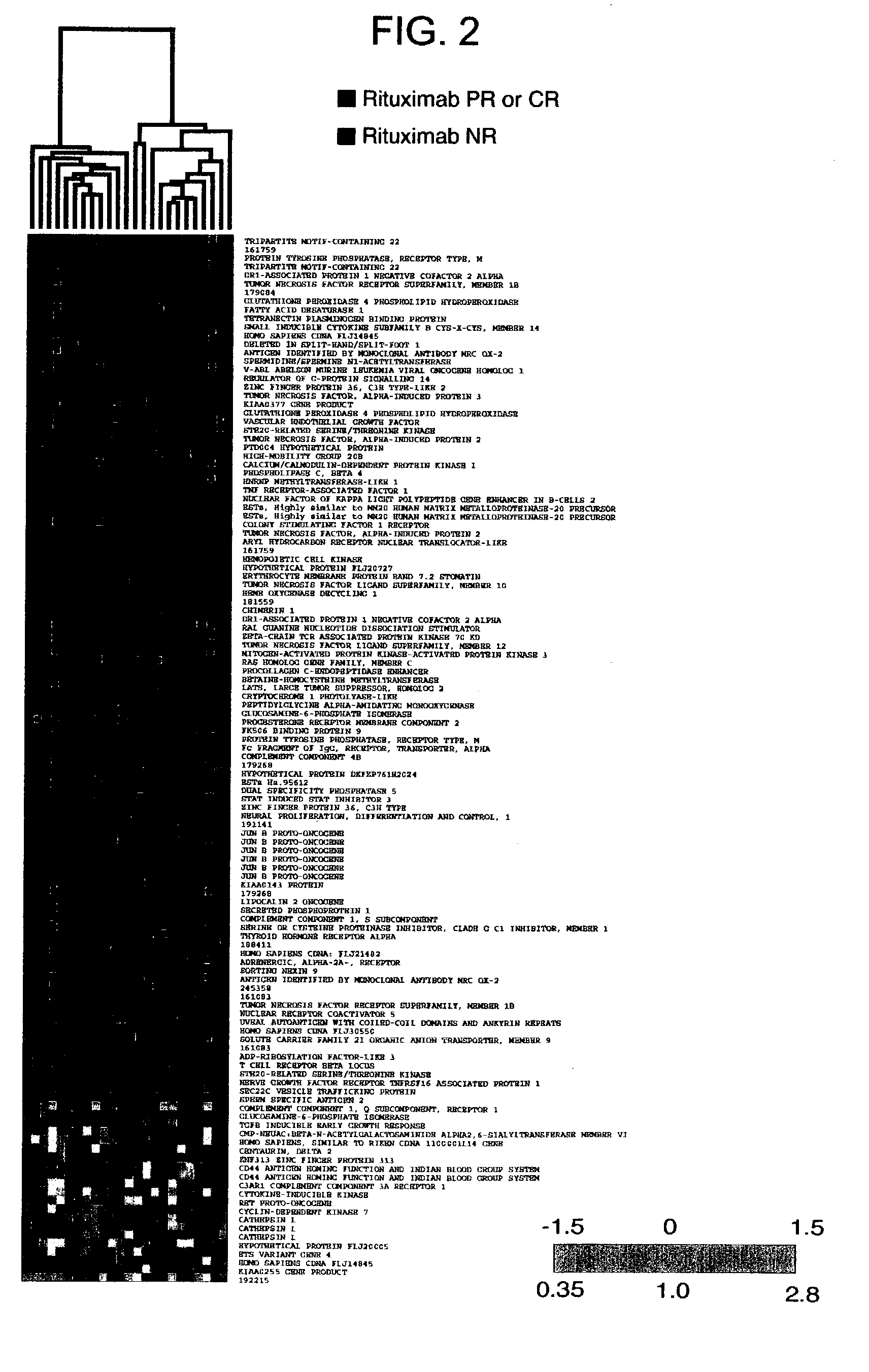
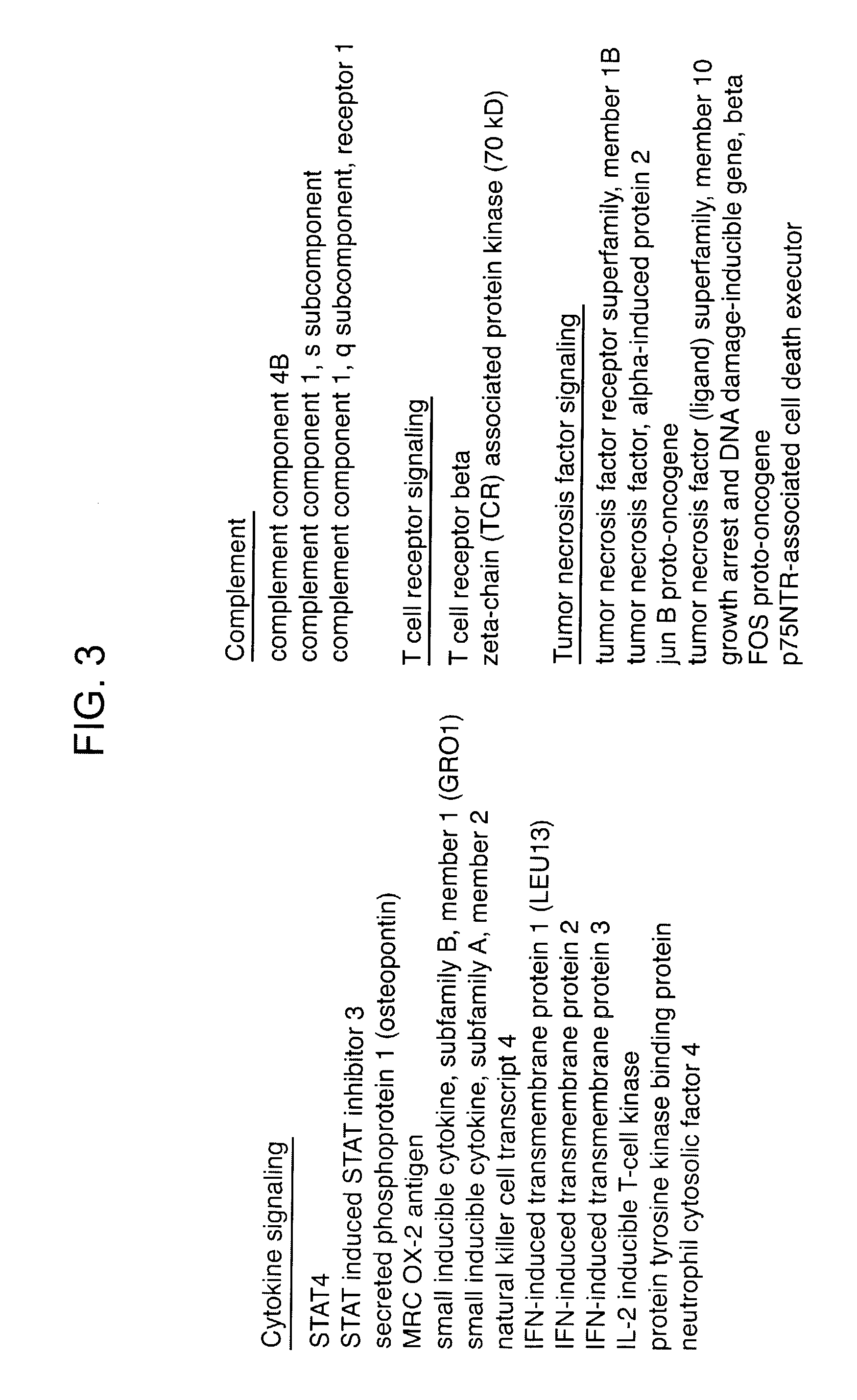
Methods and compositions for determining neoplastic disease responsiveness to antibody therapy
InactiveUS20030219818A1Microbiological testing/measurementDisease diagnosisFollicular lymphoma grade IIWilms' tumor
Methods are provided for determining whether a subject suffering from a neoplastic condition, e.g., non-Hodgkin's lymphoma (NHL), such as follicular lymphoma, is responsive to antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, an expression profile is obtained from the subject suffering from NHL and employed to determine whether the subject is responsive to antineoplastic therapy. In addition, reagents and kits thereof that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapy of rituximab-refractory rheumatoid arthritis patients
A method is disclosed of treating a rituximab-refractory rheumatoid arthritis (RA) patient comprising administering an anti-CD20 antibody other than rituximab to the patient in an amount effective to treat the RA.
Owner:GENENTECH INC
Antineoplastic combinations of CCI-779 and rituximab
InactiveUS20050272758A1Eliminate side effectsIncreased safety marginBiocideAntibody ingredientsPharmacologyNon-Hodgkin's lymphoma
Owner:WYETH
Uses of Anti-cd40 antibodies
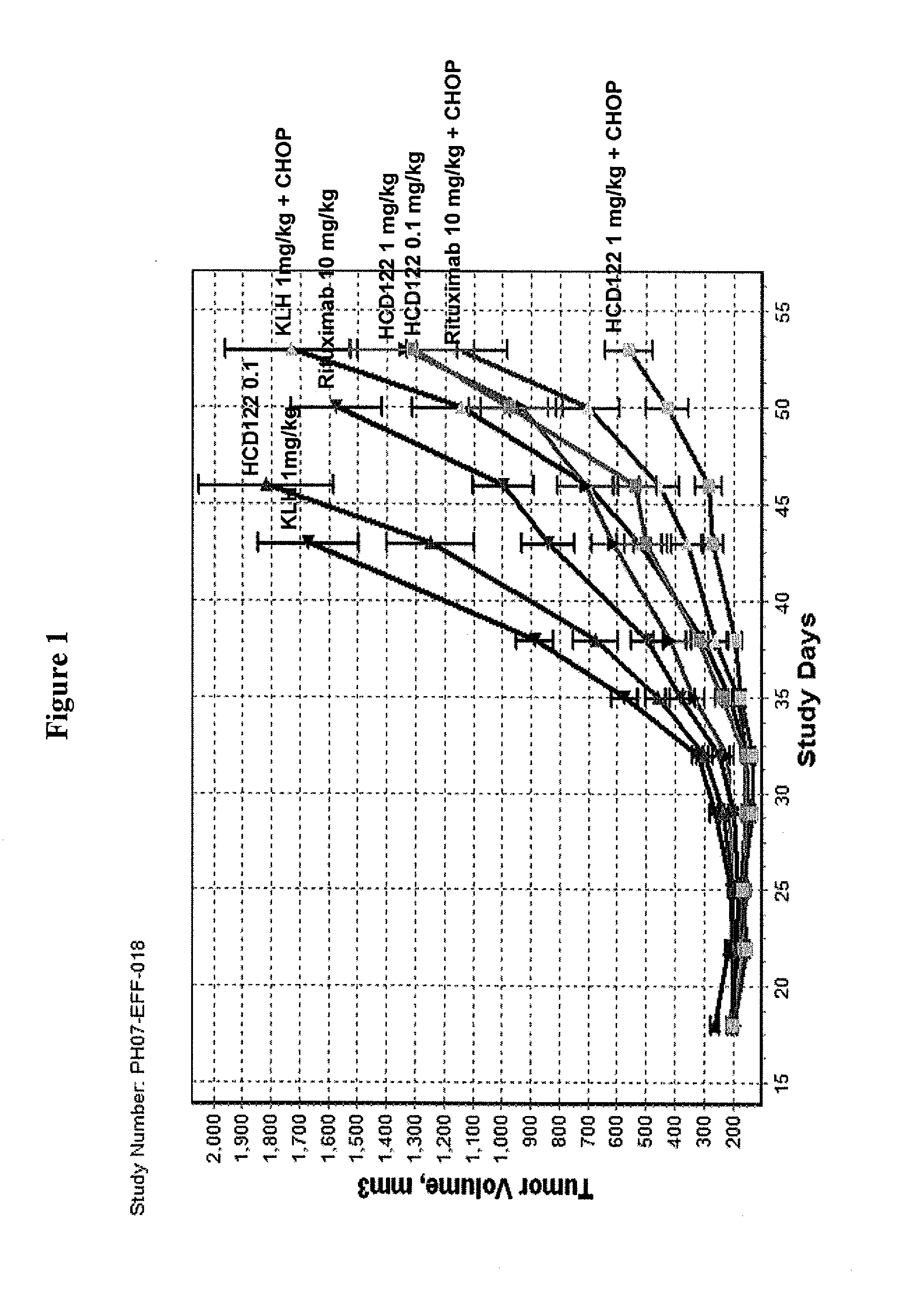
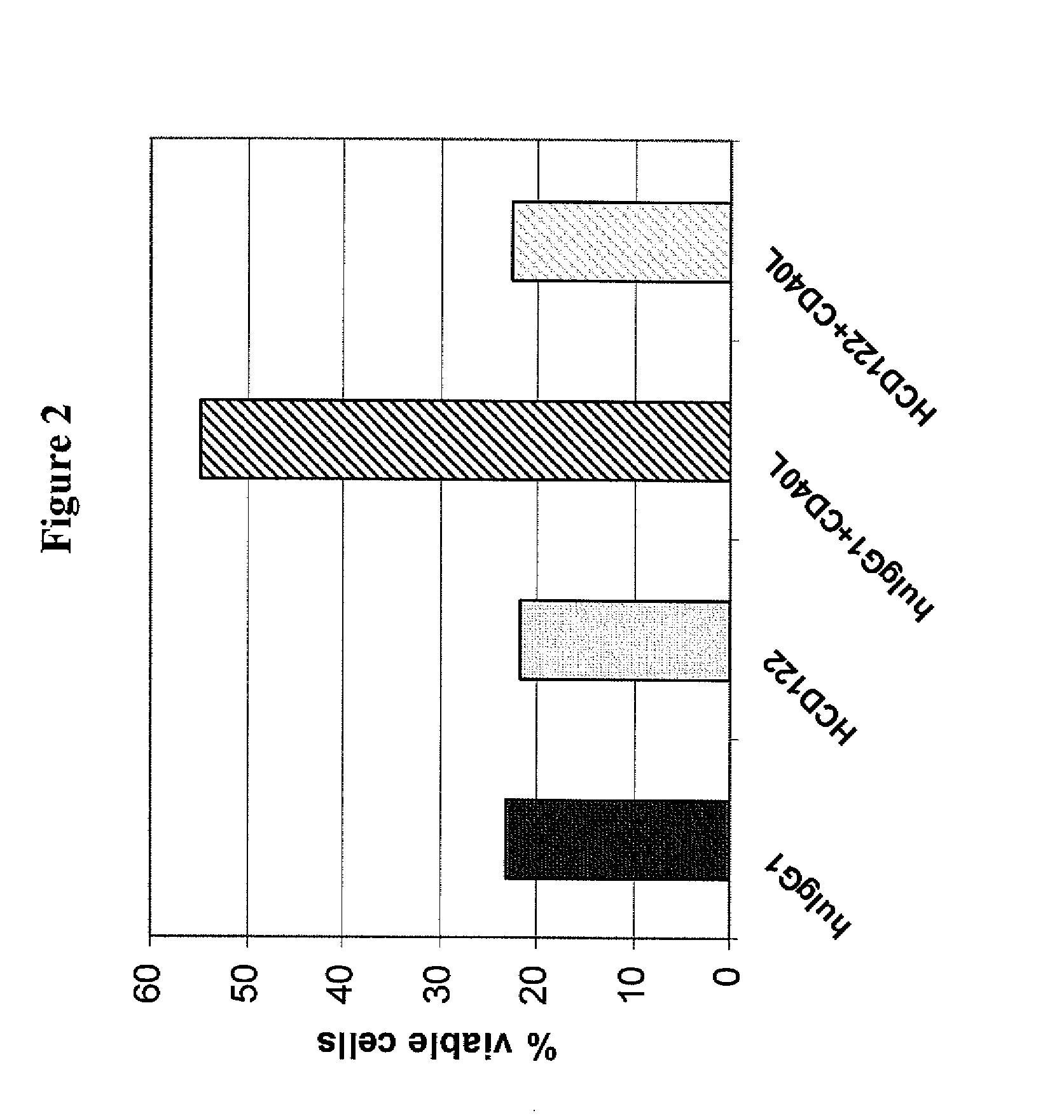
This invention relates to new uses of anti-CD40 antibodies in the treatment of diseases or conditions associated with neoplastic B-cell growth in particular use of anti-CD40 antibodies in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP). The invention is particularly useful for the treatment of patients who have previously been administered (i) CHOP, (ii) the chimeric anti-CD20 monoclonal antibody rituximab, or (iii) combination therapy with CHOP and rituximab.
Owner:XOMA TECH LTD +1
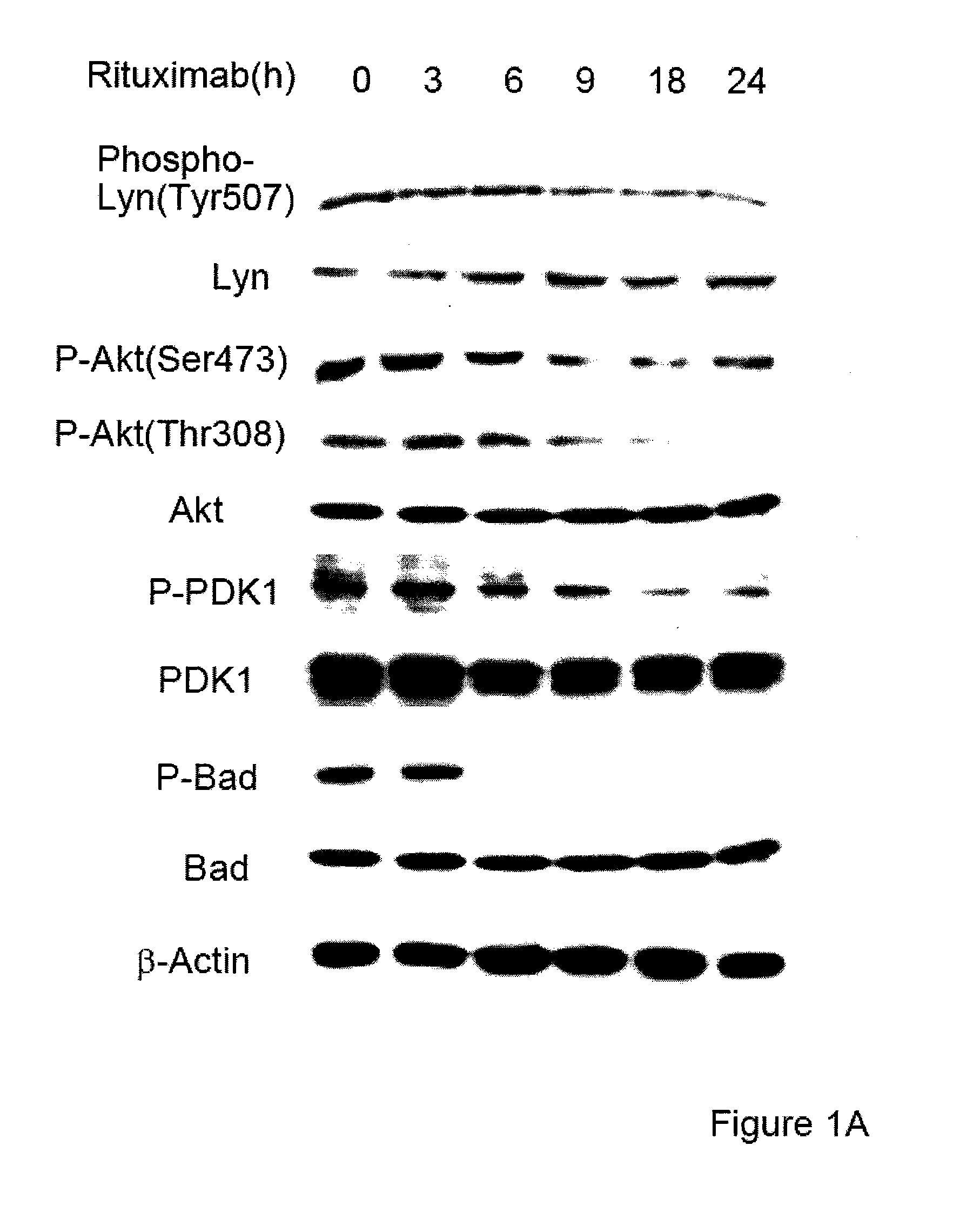
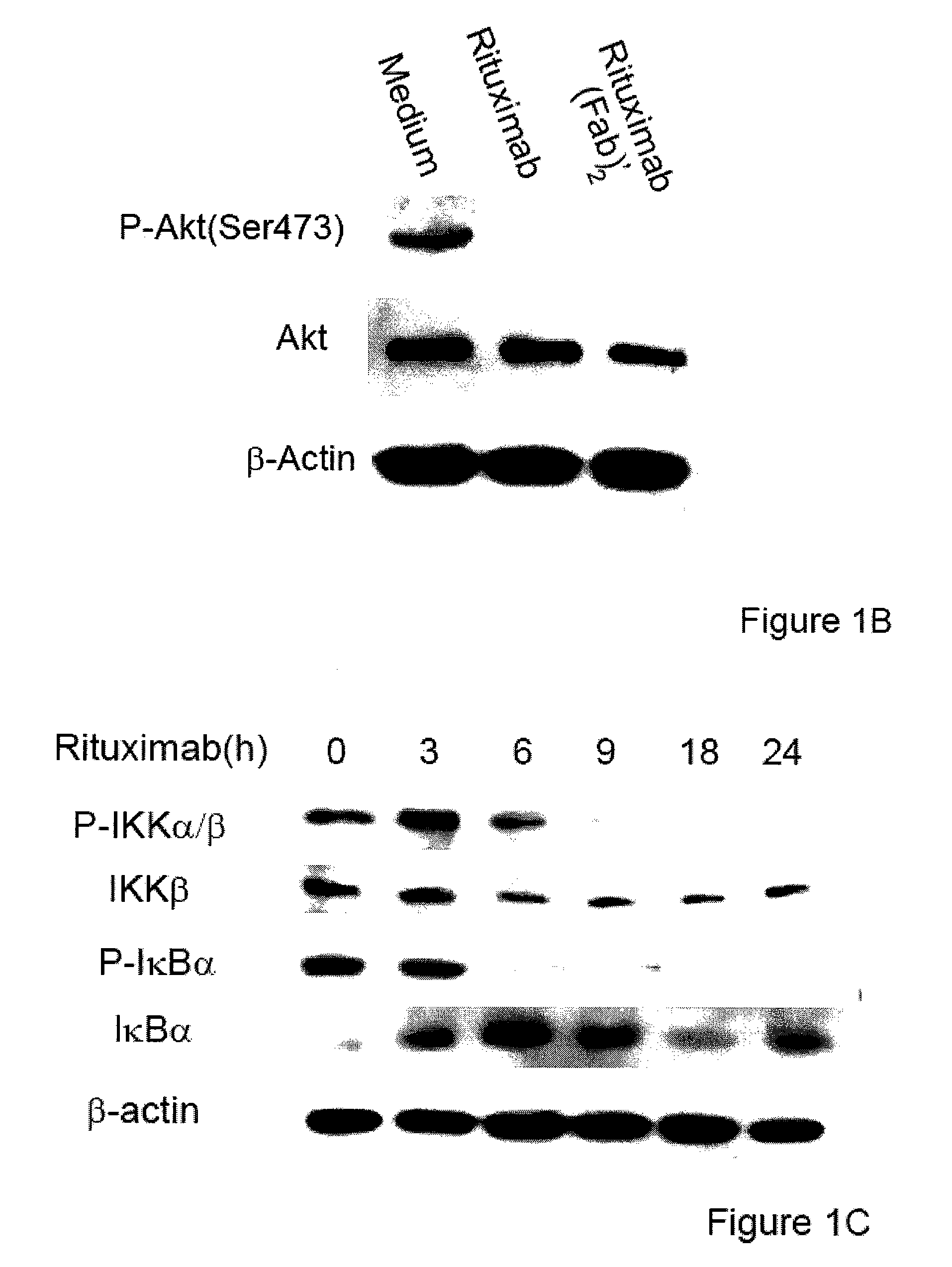
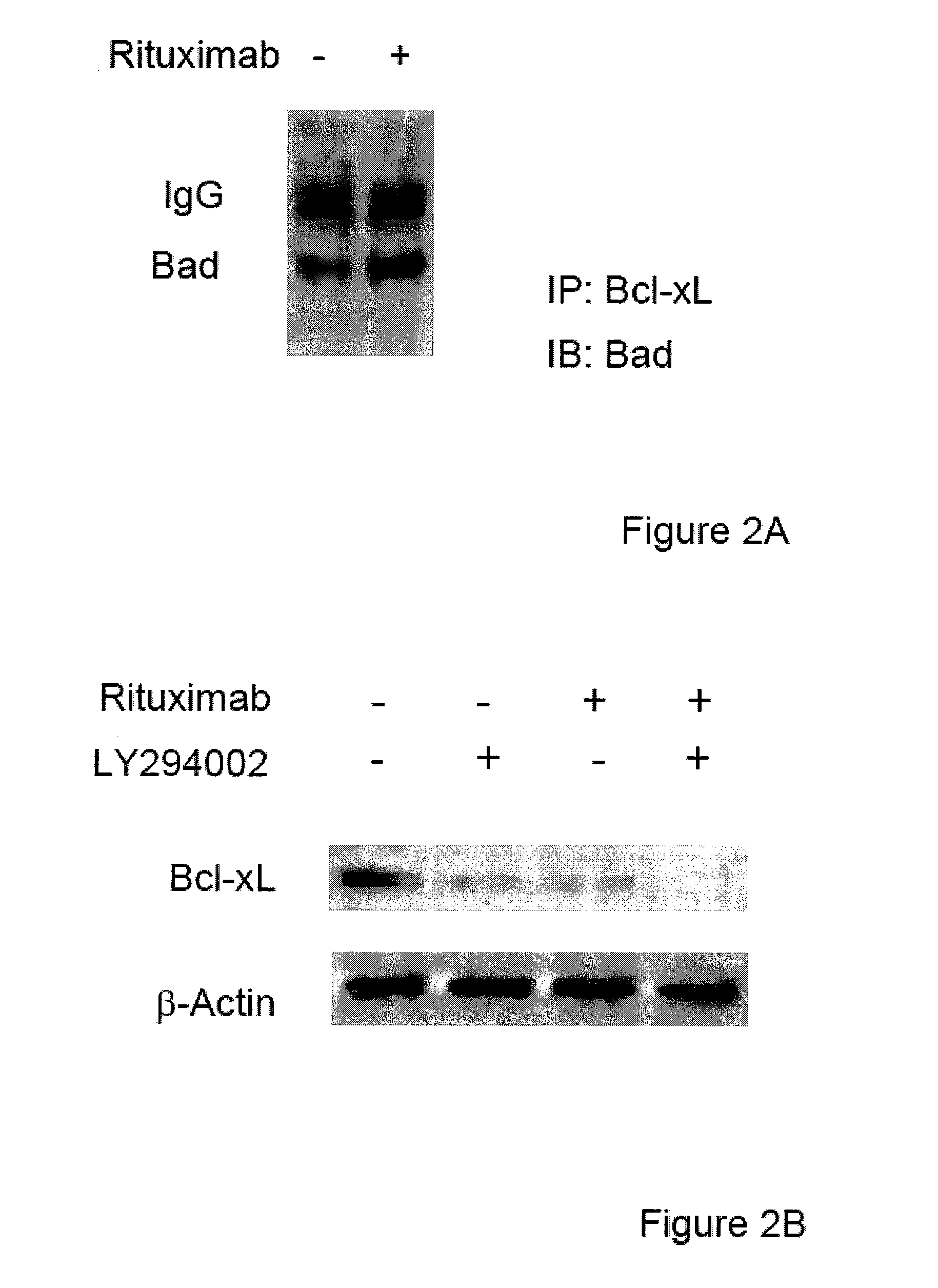
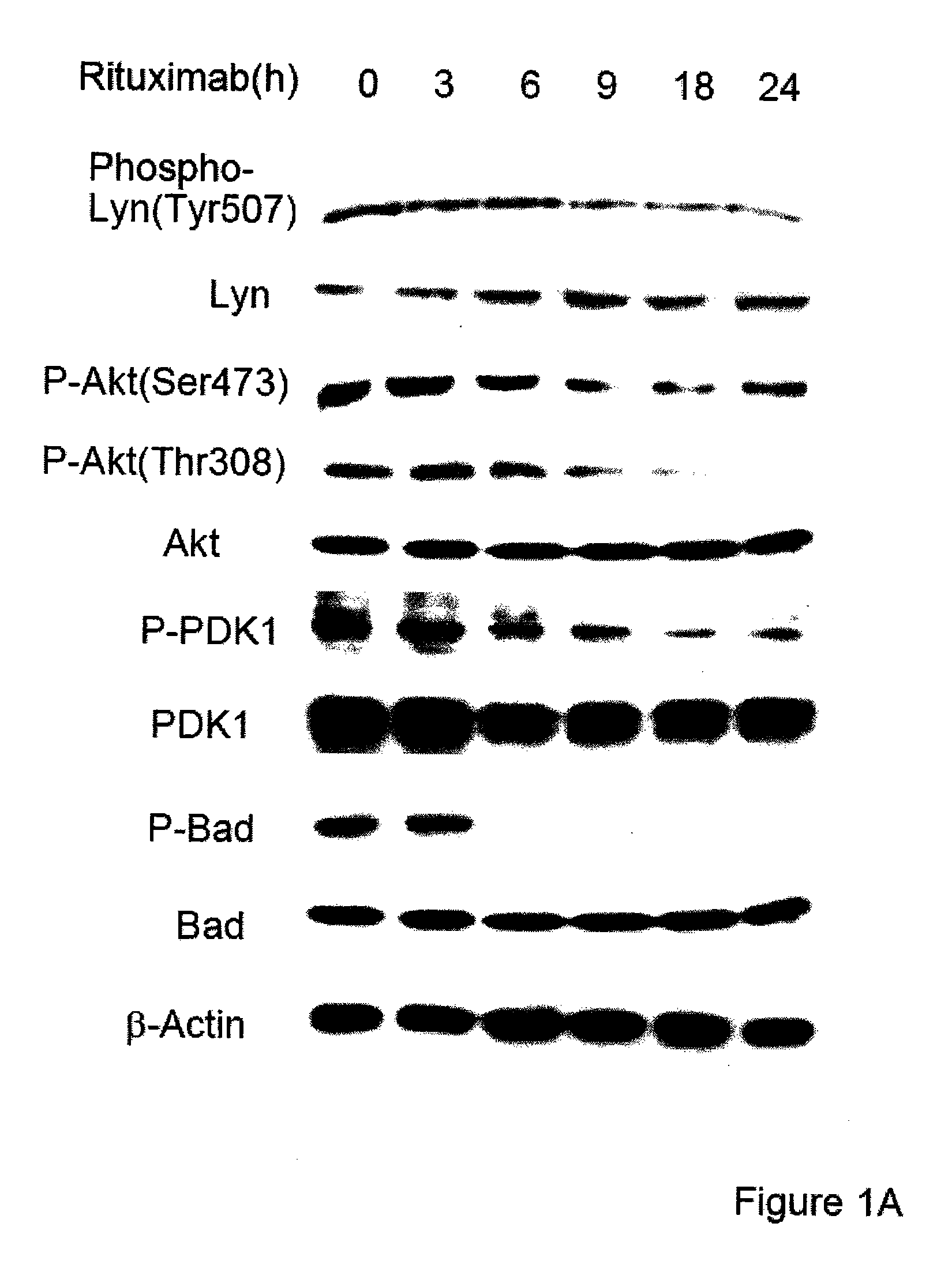
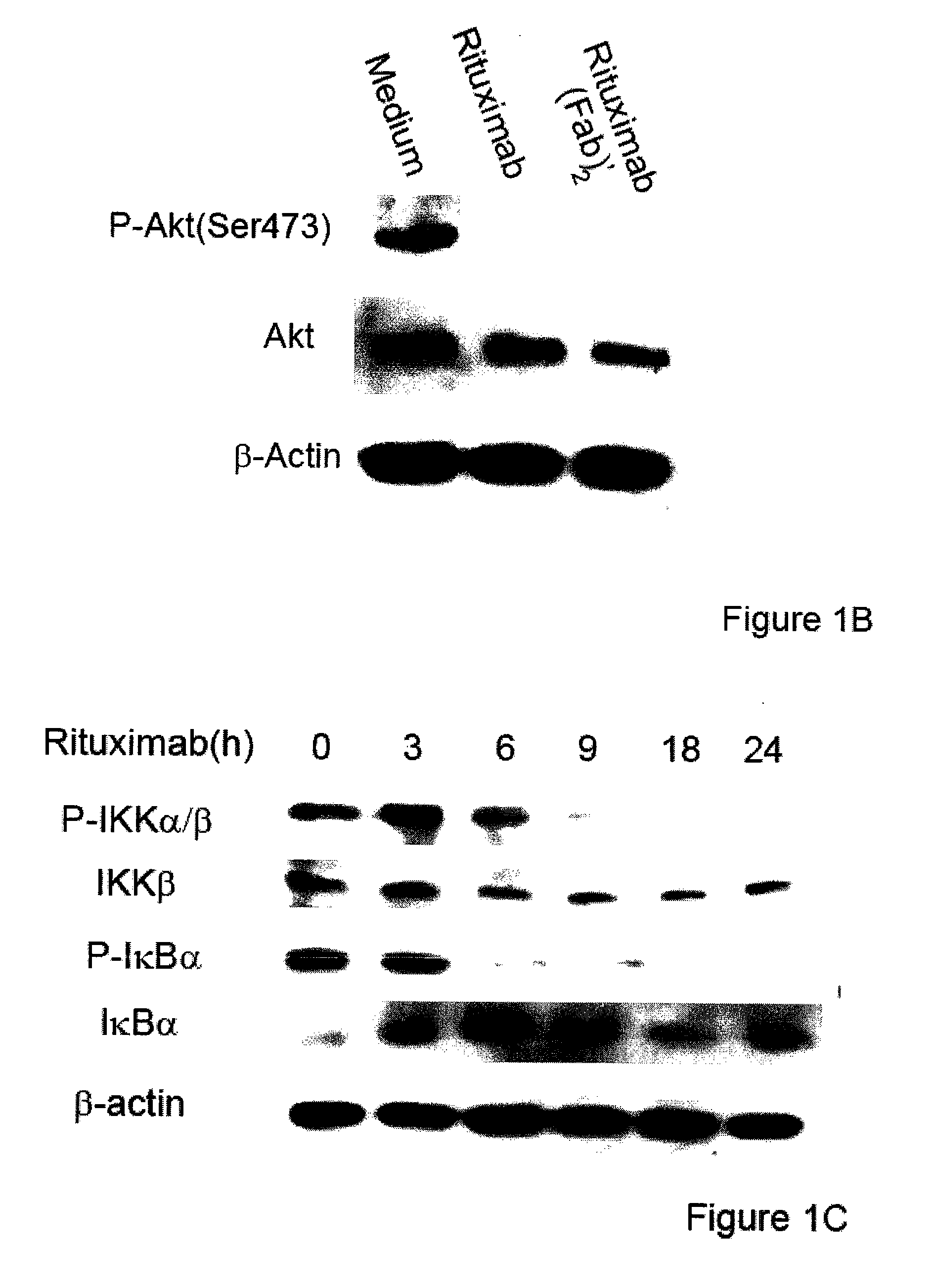
Molecular signaling pathways triggered by rituximab: prognostic, diagnostic, and therapeutic uses
InactiveUS20070172847A1RegulateRegulate the cancer cells' sensitivity to immunotherapyMicrobiological testing/measurementMaterial analysisAutoimmune conditionCancer cell
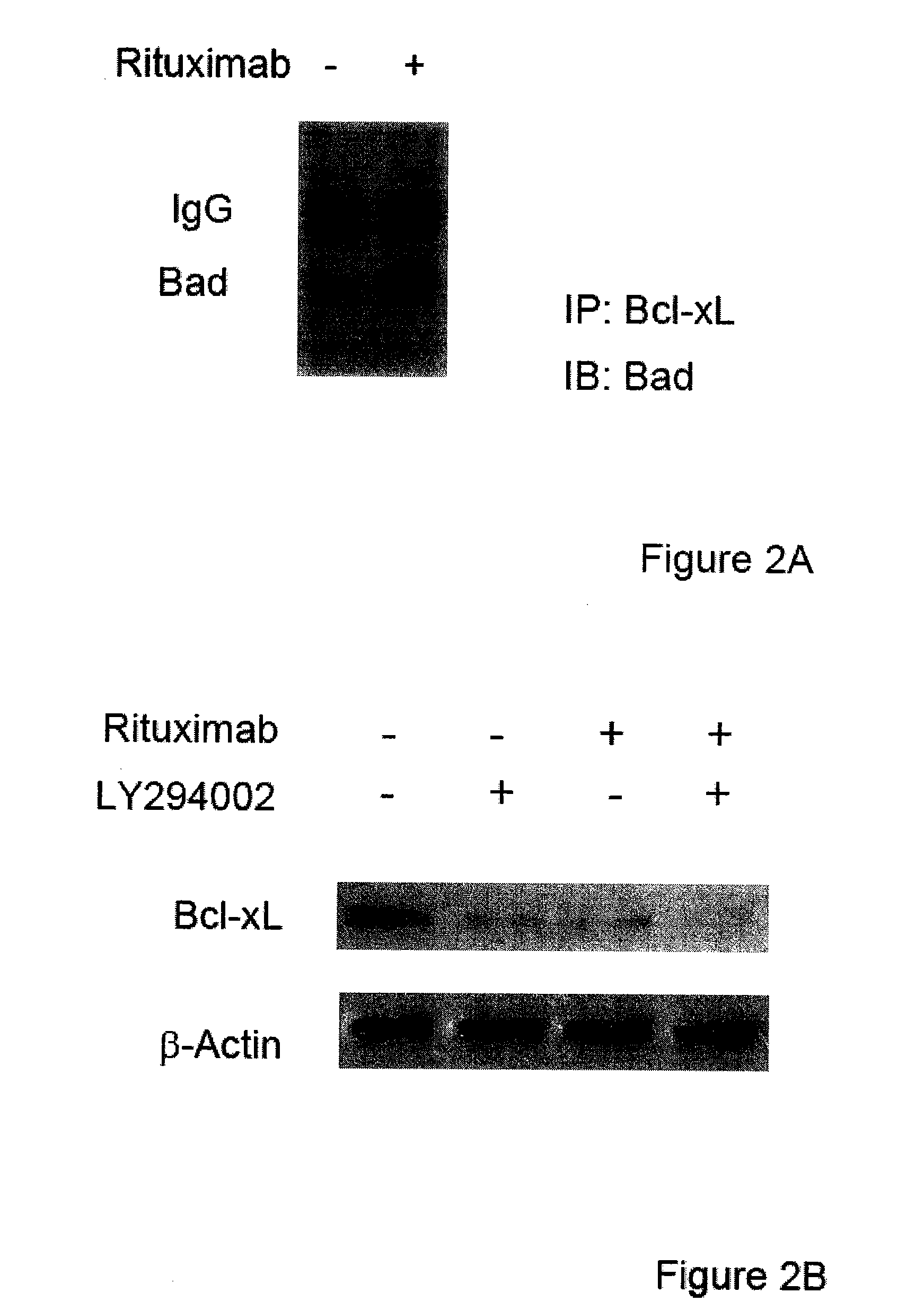
The present invention provides markers associated with activated molecular signaling pathways (example: p38 MAKP, NF-κB, ERK1 / 2, YY-1 and AKT) inhibited by rituximab in cancer cells as well as pathways activated by rituximab (such as death receptors, RKIP, PTEN) all of which are associated with the regulation of chemo and immunoresistance. The present invention provides methods of prognosis and providing a prognosis for cancer such as lymphoma, leukemia, and autoimmune disease, as well as, methods of drug discovery. These markers are also therapeutic targets for treatment of cancer resistant to conventional and experimental cancer therapeutics. Inhibition or activation of expression and / or activity of targeted gene products sensitizes resistant tumor cells to subtoxic doses of cytotoxic treatment including chemotherapy, radiation therapy, or immunotherapy and gene therapy, and the cytotoxic molecules.
Owner:RGT UNIV OF CALIFORNIA
Polypeptide useful in adoptive cell therapy
ActiveUS20150093401A1Easy to packAvoids biological effectAntibody mimetics/scaffoldsGenetic material ingredientsEpitopeAdoptive cellular therapy
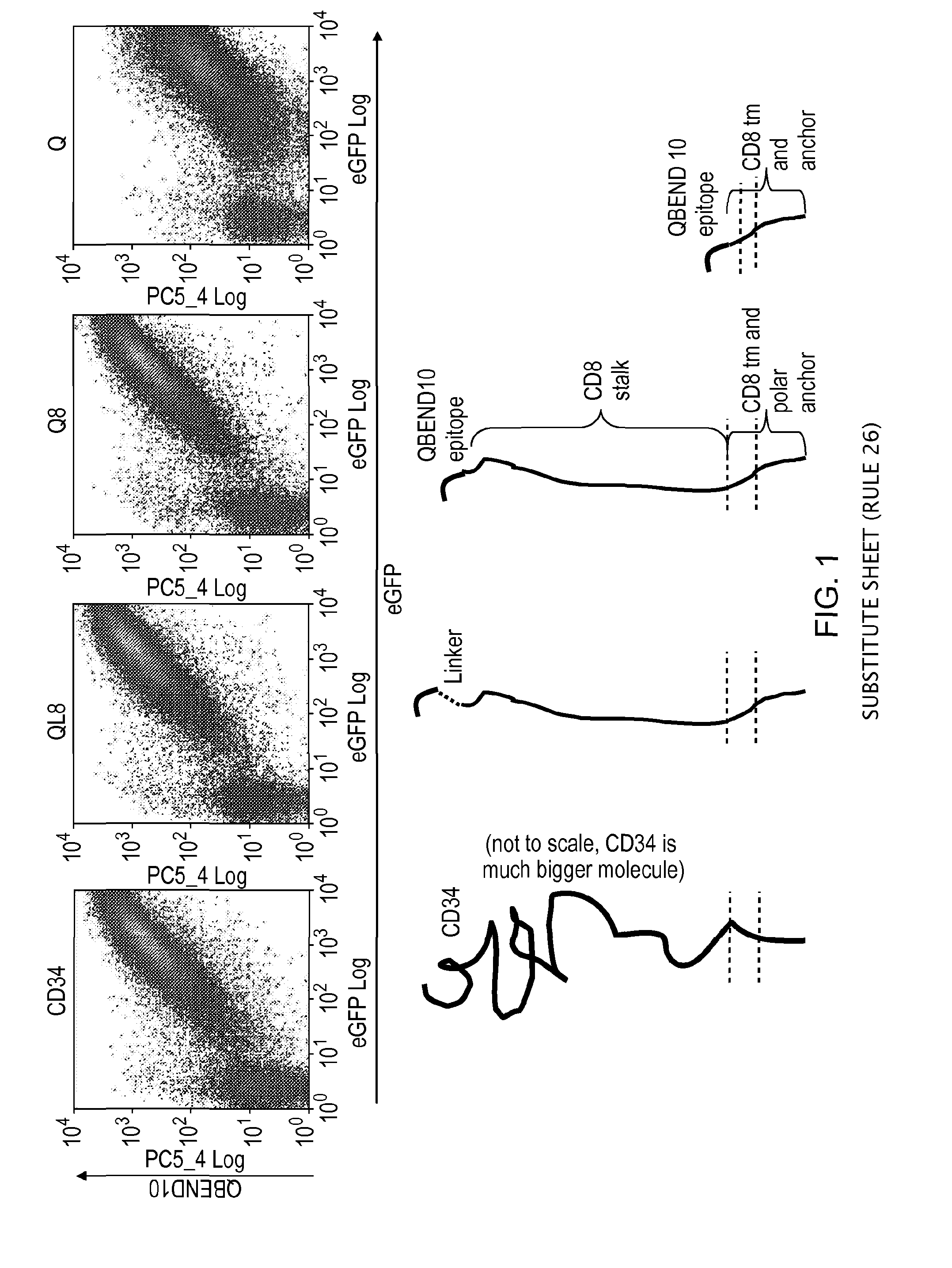
The present invention provides a polypeptide having the formula: St-R1-S1-Q-S2-R2 wherein St is a stalk sequence which, when the polypeptide is expressed at the surface of a target cell, causes the R and Q epitopes to be projected from the cell surface; R1 and R2 are a Rituximab-binding epitopes each having the an amino acid sequence selected from the group consisting of SEQ ID No. 1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16 or a variant thereof which retains Rituximab-binding activity; S1 and S2 are optional spacer sequences, which may be the same or different; and Q is a QBEnd1O-binding epitope having the amino acid sequence shown as SEQ ID No. 2 or a variant thereof which QBEnd1O-binding activity. The invention also provides a nucleic acid sequence encoding such a polypeptide and uses thereof in adoptive cell transfer.
Owner:UCL BUSINESS PLC
Methods and compositions for determining responsiveness to antibody therapy
ActiveUS20060008825A1Microbiological testing/measurementMedical automated diagnosisMedicineGenotyping
Methods and compositions are provided for determining whether a subject suffering from a neoplastic condition is responsive to an antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, the subject is genotyped to determine whether the subject has a least one favorable FcγR polymorphism, e.g., the 131 H / H genotype or the 158 V / V genotype. In addition, reagents, devices and kits thereof, that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Novel unnatural amino acid marked antibody-drug conjugate and preparation thereof
ActiveCN106146663AEfficient modification purposeGood effectOrganic active ingredientsBacteriaFunctional connectivityIsotope
The invention provides a site-directed mutated rituximab containing unnatural amino acid markers and a method for conjugating the rituximab by site-directed mutation and site-directed micro-molecules. The method includes the steps: leading unnatural amino acid into rituximab genes by genetic codon extension technology in a site-directed manner; performing site-directed connection by the aid of the unnatural amino acid and modifiers such as poly-difunctional connecting arms DIBO-DOTA; further realizing site-directed conjugating of the rituximab and radioactive isotope such as Cu64 through DOBO-DOTA. The invention further relates to an application of site-directed conjugated micro-molecule rituximab as development, tracing and therapeutic radio-immunity conjugate.
Owner:PEKING UNIV
Molecular signaling pathways triggered by rituximab: prognostic, diagnostic, and therapeutic uses
InactiveUS20090203050A1Regulate the cancer cells' sensitivity to immunotherapyOrganic active ingredientsMicrobiological testing/measurementAutoimmune conditionCancer cell
The present invention provides markers associated with activated molecular signaling pathways (example: p38 MAKP, NF-κB, ERK1 / 2, YY-1 and AKT) inhibited by rituximab in cancer cells as well as pathways activated by rituximab (such as death receptors, RKIP, PTEN) all of which are associated with the regulation of chemo and immunoresistance. The present invention provides methods of prognosis and providing a prognosis for cancer such as lymphoma, leukemia, and autoimmune disease, as well as, methods of drug discovery. These markers are also therapeutic targets for treatment of cancer resistant to conventional and experimental cancer therapeutics. Inhibition or activation of expression and / or activity of targeted gene products sensitizes resistant tumor cells to subtoxic doses of cytotoxic treatment including chemotherapy, radiation therapy, or immunotherapy and gene therapy, and the cytotoxic molecules.
Owner:RGT UNIV OF CALIFORNIA
Rituximab induction therapy followed by glatiramer acetate therapy
InactiveUS20140271630A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
The present invention provides a method of treating a subject afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject, wherein the amounts are effective to treat the subject. The present invention also provides a method of treating a subject afflicted with an immune disease, comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject wherein the amounts are effective to treat the subject, and wherein the immune disease is an autoimmune disease, an arthritic condition, a demyelinating disease, an inflammatory disease, multiple sclerosis, relapsing-remitting multiple sclerosis, diabetes mellitus, psoriasis, rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, or systemic lupus erythematosus.
Owner:TEVA PHARMA IND LTD
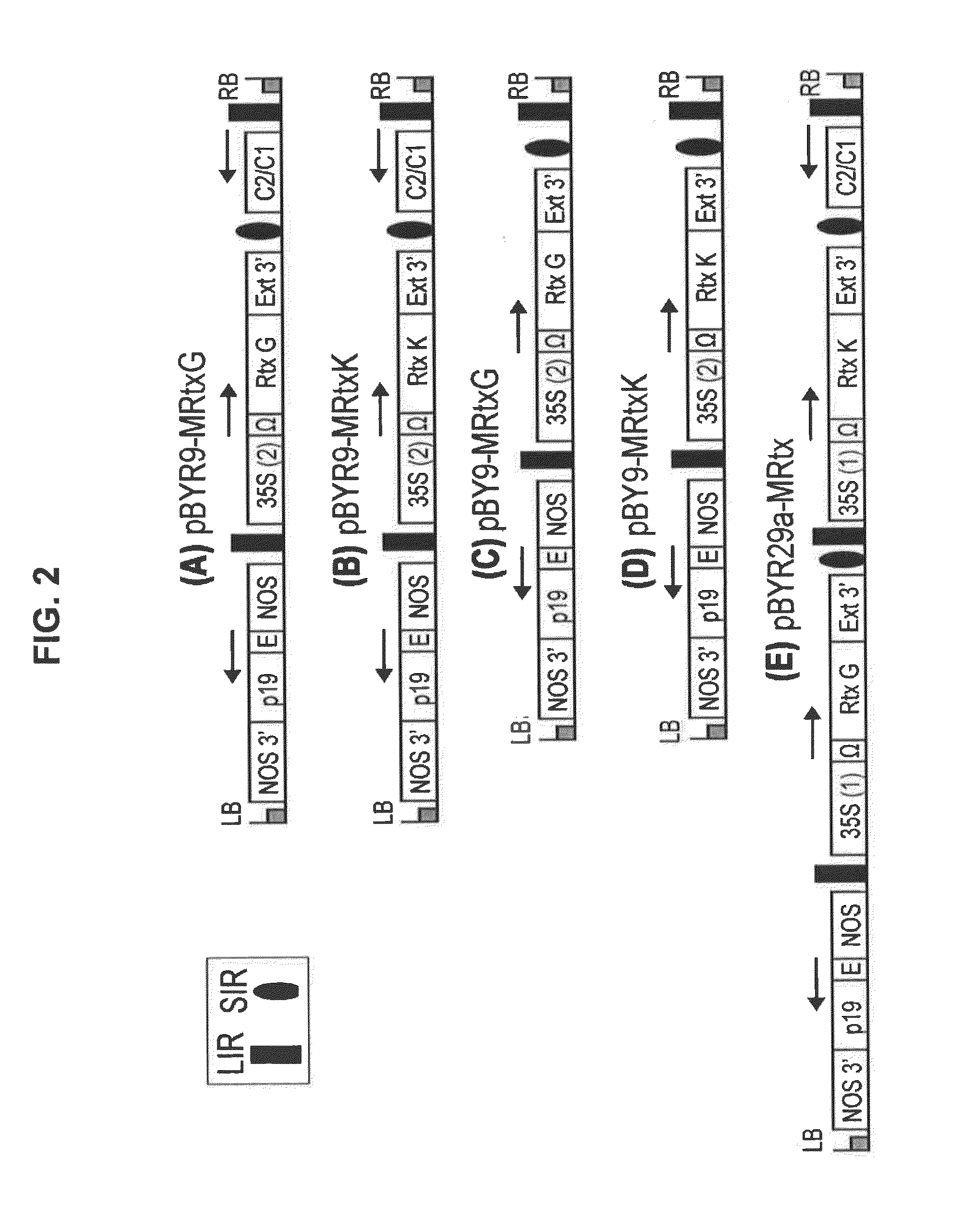
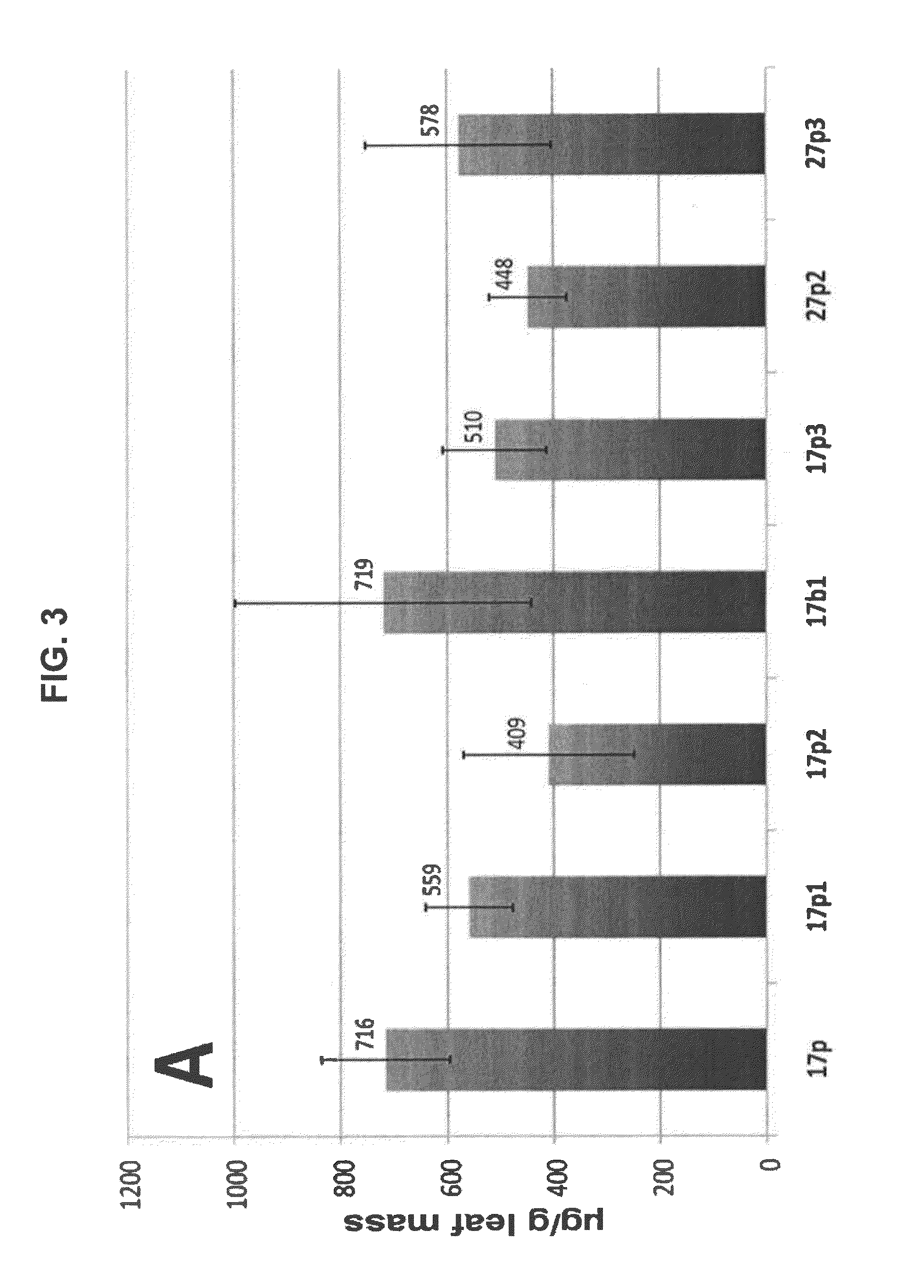
Geminiviral vector for expression of rituximab
ActiveUS10125373B2VaccinesImmunoglobulins against cell receptors/antigens/surface-determinantsNicotiana tabacumHeavy chain
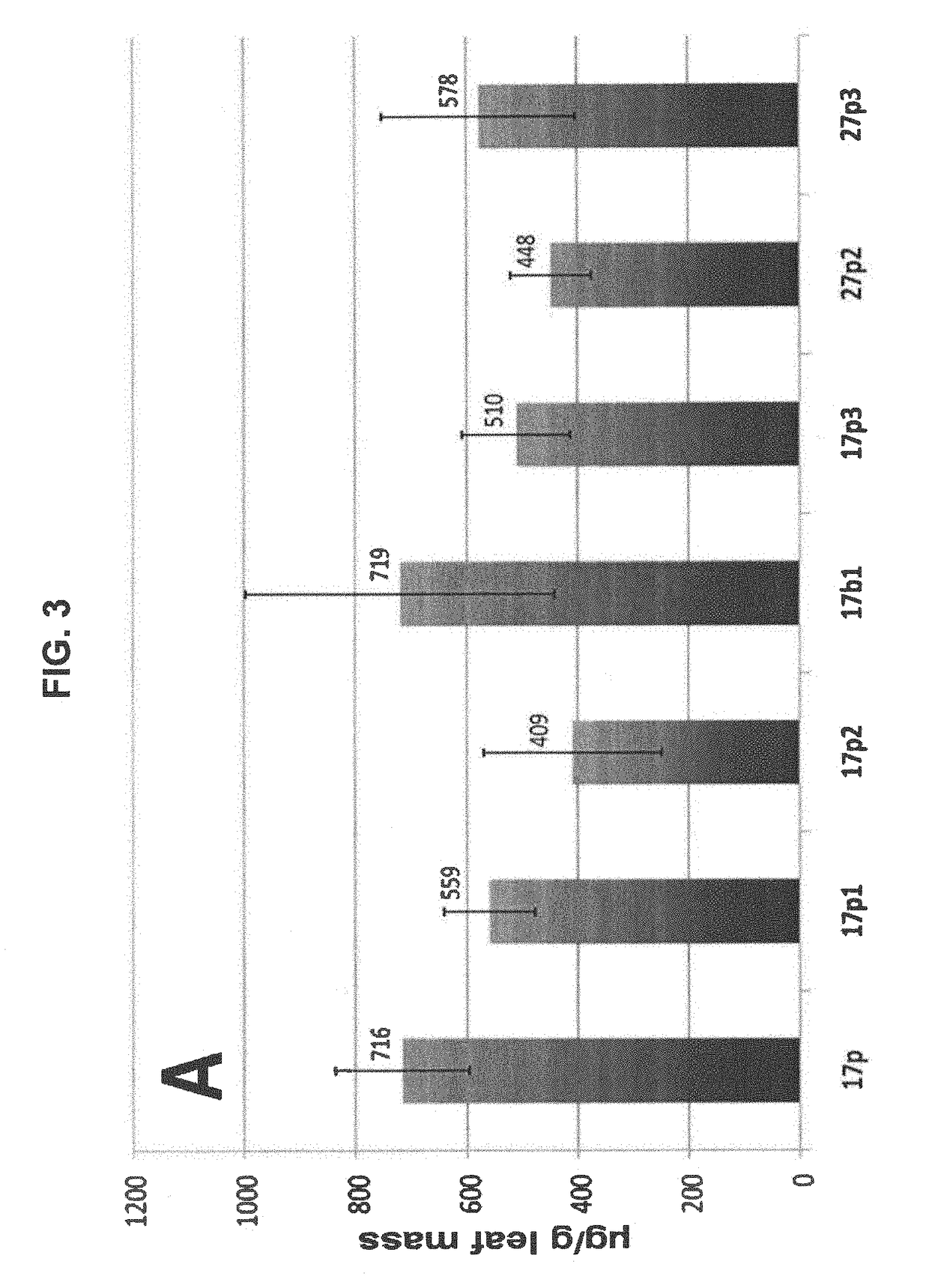
A single vector or multiple separate vectors that contain two or more non-competing replicons for transient expression of the heavy and light chains of Rituximab in Nicotiana benthamiana leaves is described. The correct assembly of these subunit proteins into functional oligomeric structures to optimize the expression is also described. This system advances plant transient expression technology by eliminating the need for non-competing viruses, and thus, enhances the realistic commercial application of the multi-replicon single vector system for producing Rituximab in plant cells.
Owner:ARIZONA STATE UNIVERSITY
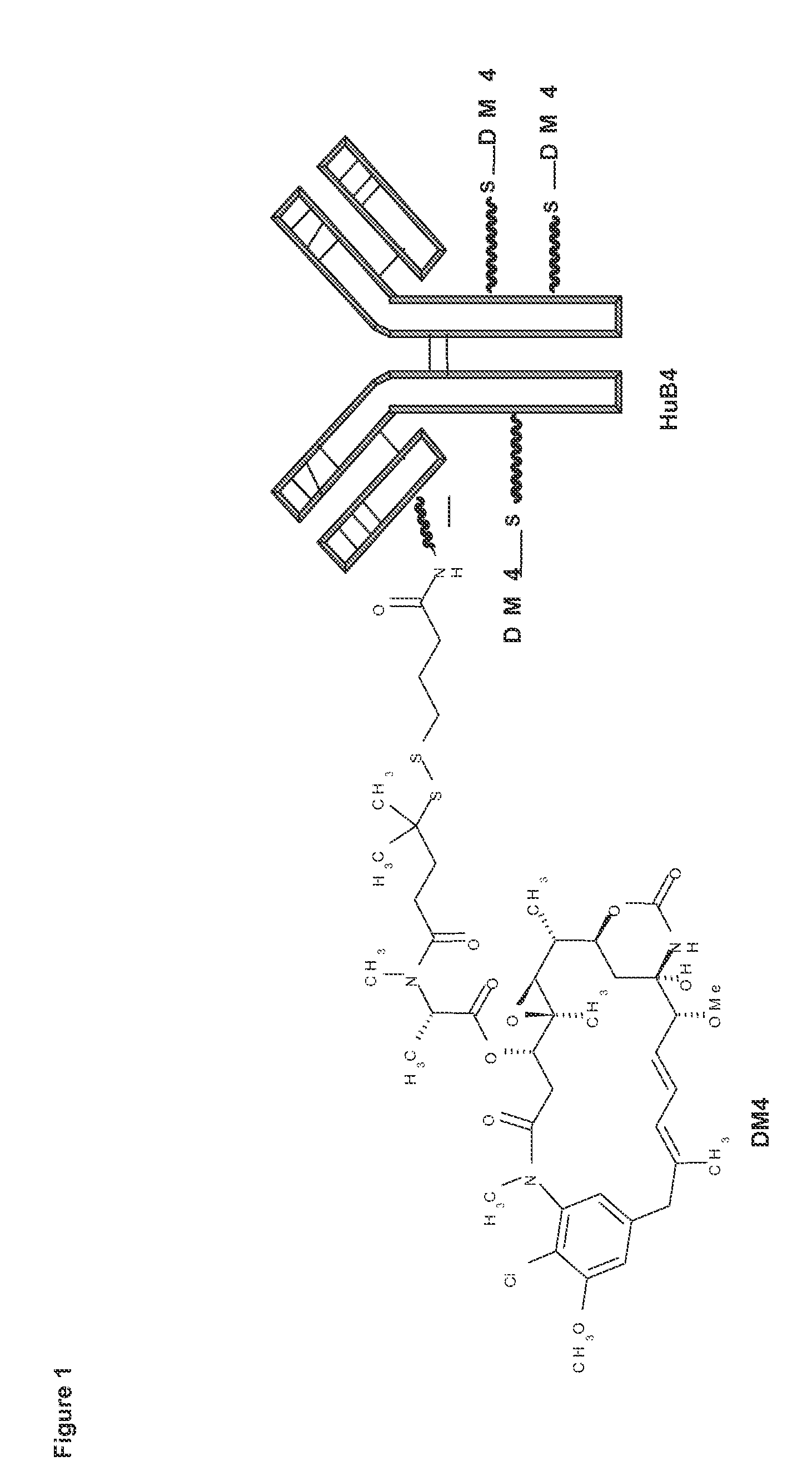
Combination therapy for the treatment of cd19+ b-cell malignancies symptoms comprising an Anti-cd1 maytansinoid immunoconjugate and rituzimab
A combination of an anti-CD19 maytansinoid immunoconjugate and rituximab is used for treating CD19+CD20+ B-cell malignancies symptom, in particular Non-Hodgkin's lymphoma.
Owner:SANOFI SA
Uses of anti-CD40 antibodies
Owner:NOVARTIS AG +1
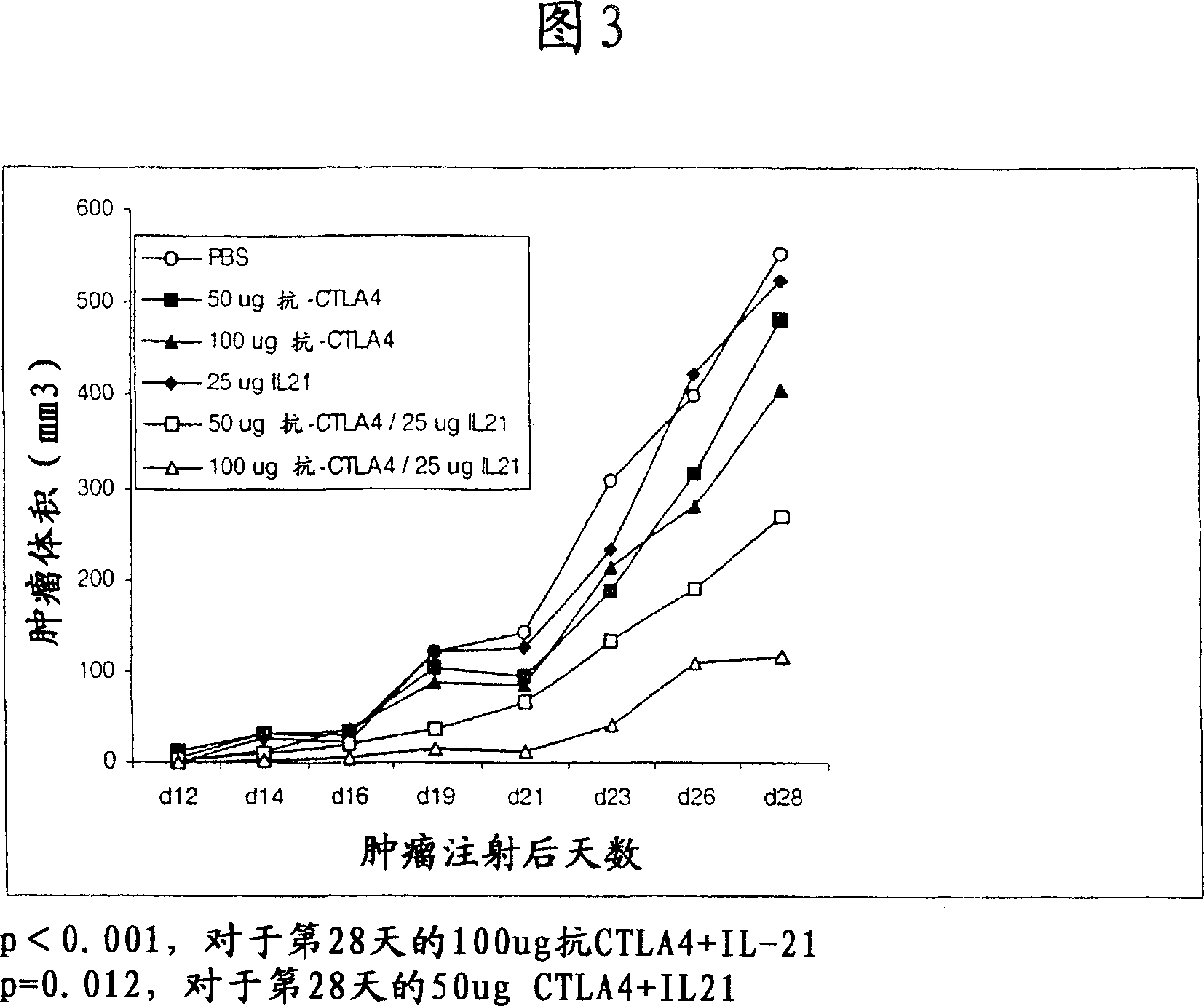
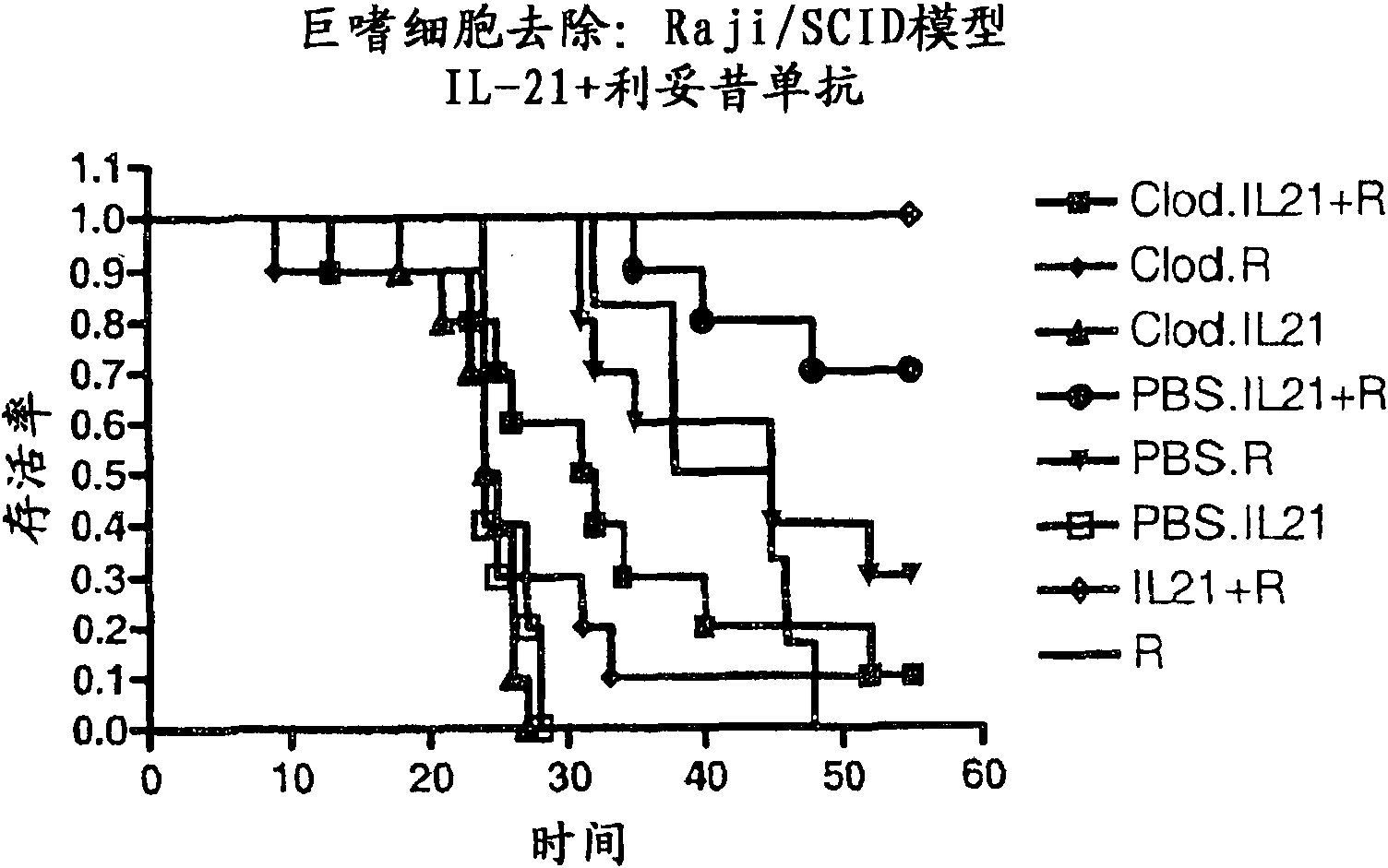
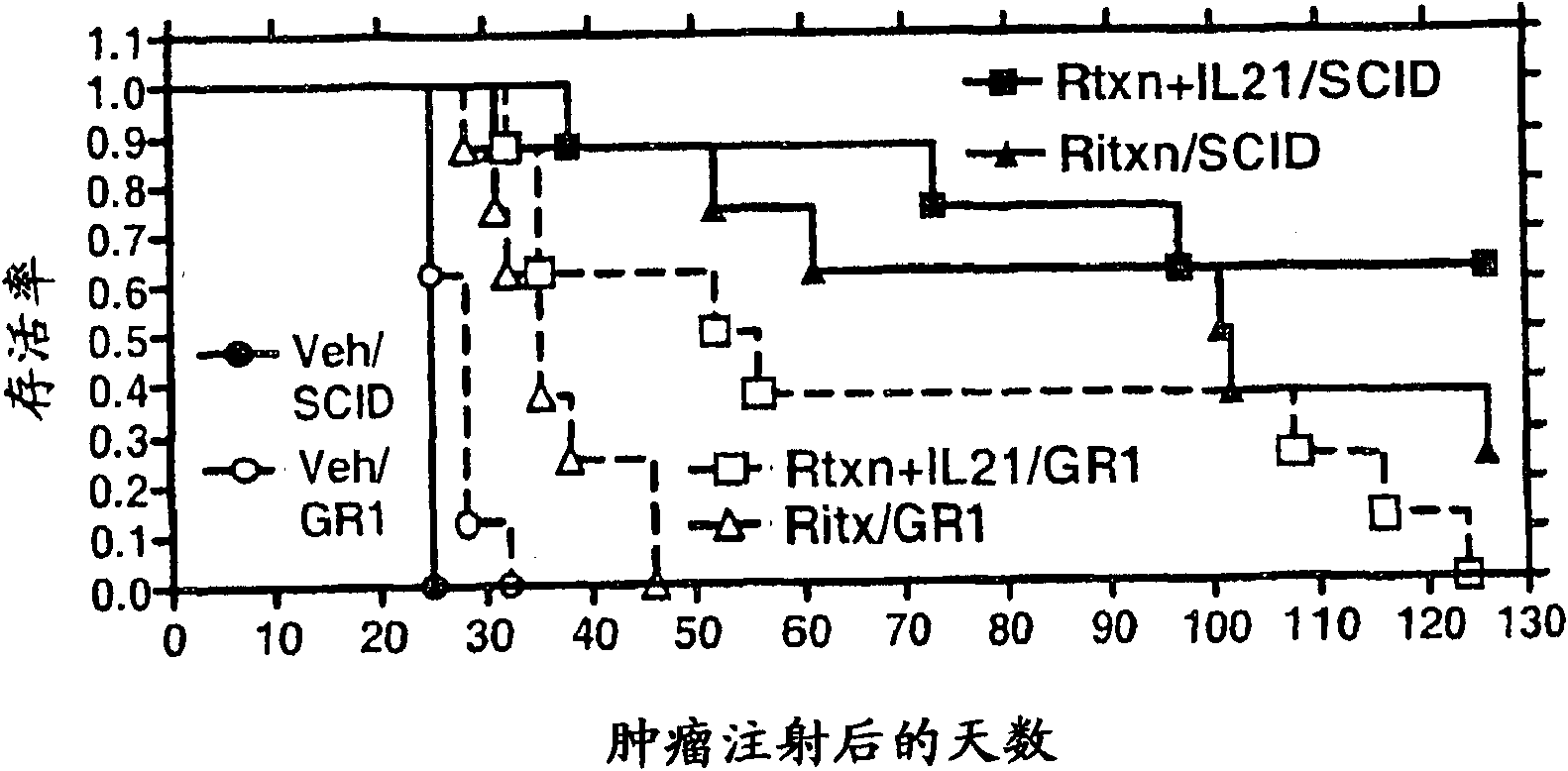
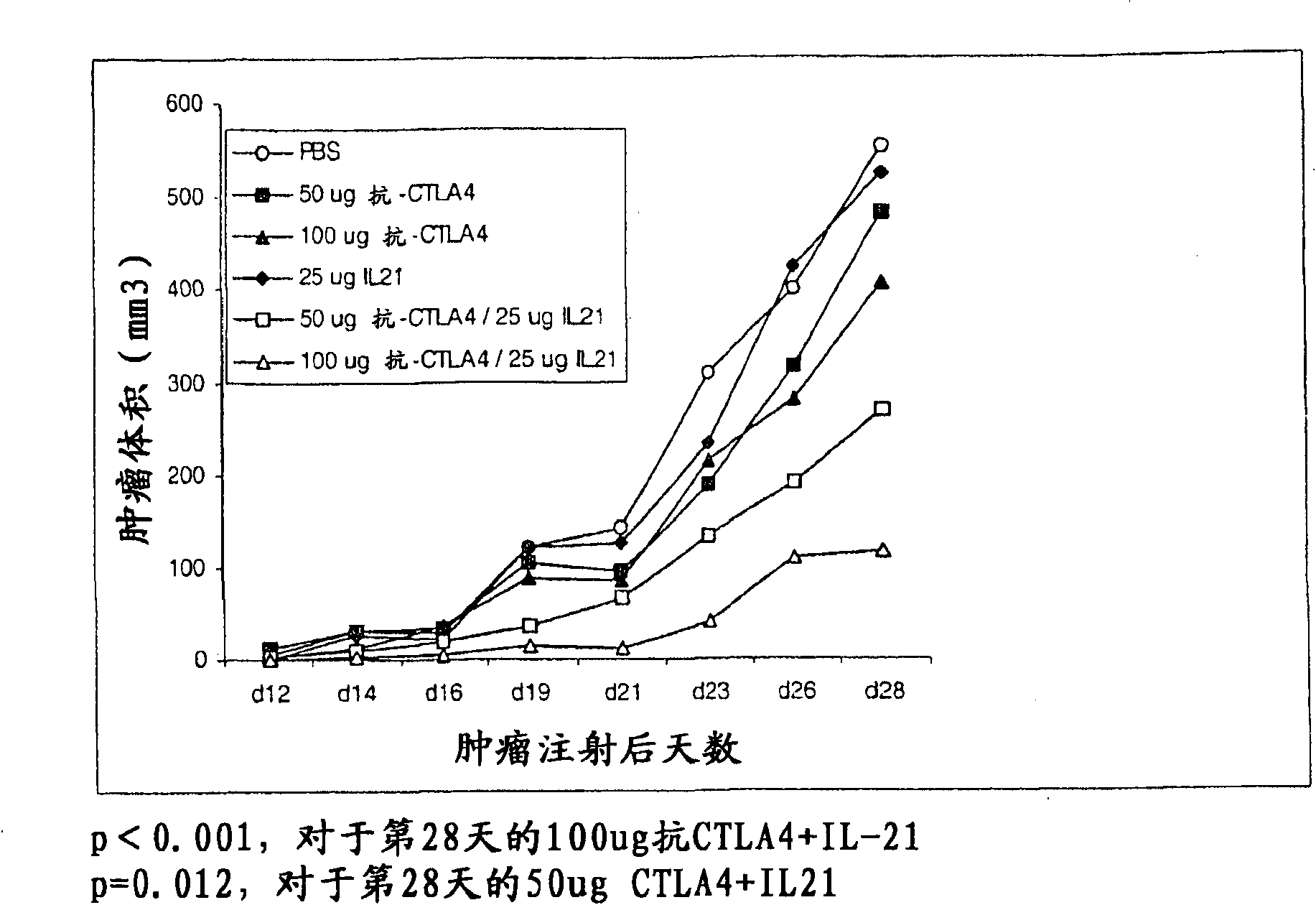
Methods of treating cancer using il-21 and monoclonal antibody therapy
A method of treating cancer by co-administering a therapeutic monoclonal antibody and IL-21 is described. Exemplary monoclonal antibodies that may be used are rituximab, trastuzumab, and anti-CTLA-4 antibodies. For patient populations resistant to monoclonal antibody therapy, relapsed after treatment with monoclonal antibodies, or in which enhanced IL-21 antitumor effects reduce toxicity associated with therapy with monoclonal antibodies, the The enhanced antitumor properties of the combination therapy described above are particularly useful.
Owner:ZYMOGENETICS INC
Methods of treating cancer using IL-21 and monoclonal antibody therapy
InactiveCN102188704APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAfter treatmentTreatment use
Methods for treating cancer by co-administering a therapeutic monoclonal antibody with IL-21 are described. Exemplary monoclonal antibodies that can be used are rituximab, trastuzumab and anti-CTLA-4 antibodies. The enhanced antitumor of the combination therapy is particularly useful for patient populations that are recalcitrant to monoclonal therapy, relapse after treatment with monoclonal antibodies or where the enhanced IL-21 antitumor effect reduces toxicities associated with treatment using the monoclonal antibodies.
Owner:ZYMOGENETICS INC
Medicinal composition for treating B cell lymph tumour
InactiveCN1824307AInduce apoptosisInhibition of growth and proliferationAntibody ingredientsAmine active ingredientsCurative effectCell growth
A composite medicine for treating B-cell leucoma is prepared from rituximab and suberoylanilide hydroxamic acid (SAHA). Its advantages are obvious synergistic effect, high curative effect and low cost.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Use of CD23 Antibodies to Treat Malignancies in Patients with Poor Prognosis
InactiveUS20090252725A1Induce apoptosisTherapeutically effectiveAntibody ingredientsTissue cultureMammalZAP70
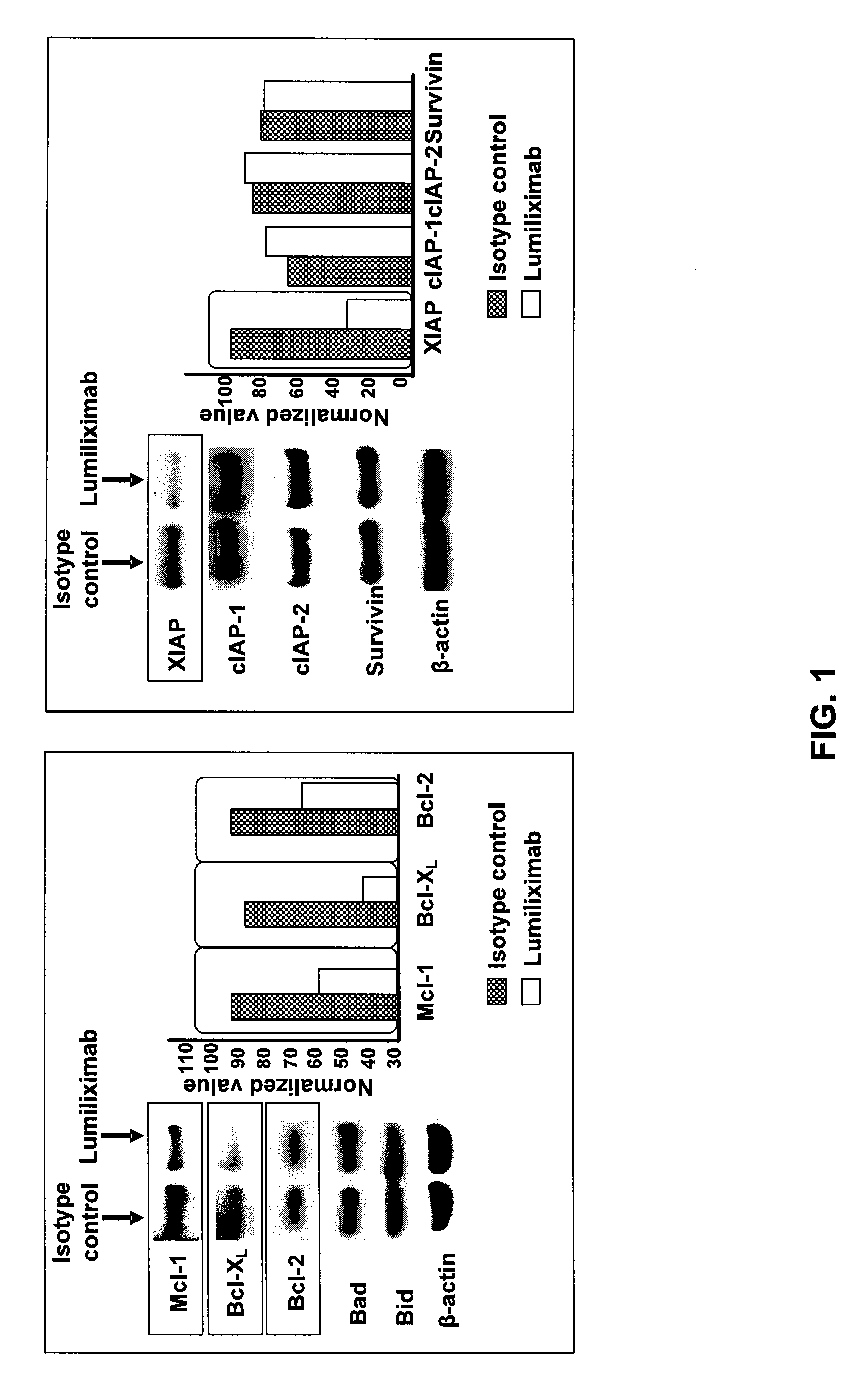
The invention relates to methods of treating B-cell chronic lymphocytic leukemia, and other CD23+ malignancies, in patients with poor prognostic markers. The method comprises administration of an CD23 antibody, including for example, lumiliximab to a mammal that over expresses a poor prognostic marker. The method can also comprise administration of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab. Patients with poor prognostic markers include, for example, patients that overexpress ZAP70, CD38, β2-microglobulin and / or soluble CD23.
Owner:BIOGEN IDEC MA INC
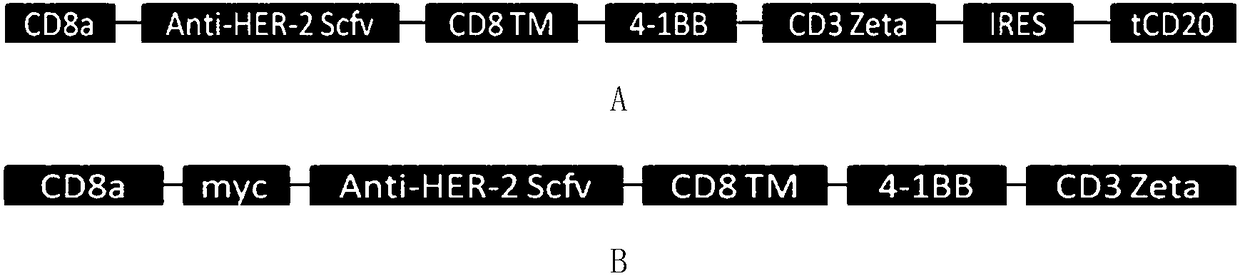
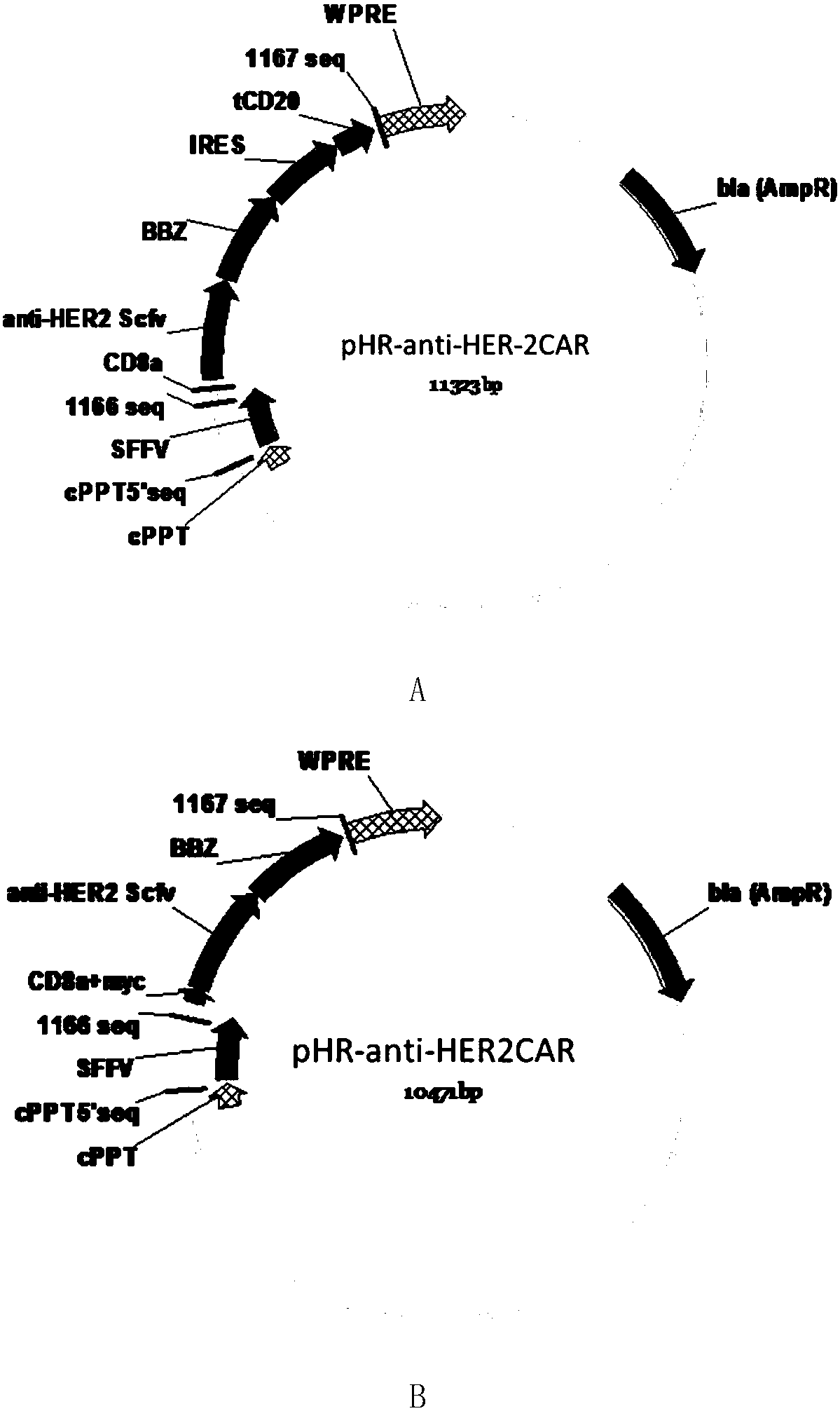
Chimeric antigen receptor containing truncated CD20 molecules and lentiviral vector as well as application
ActiveCN108276498AEfficient killingNo side effectsAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenCD20
The invention discloses a chimeric antigen receptor containing truncated CD20 molecules. The amino acid sequence of the chimeric antigen receptor is shown in SEQ ID NO. 2, and the nucleotide sequenceencoding the chimeric antigen receptor is shown in SEQ ID NO. 1; the chimeric antigen receptor comprises leader peptides, ScFv, a hinge region and a transmembrane region, immunoreceptor tyrosine activation motifs, an internal ribosome entry site (IRES) and tCD20. The invention also discloses a lentiviral vector, which comprises the nucleotide sequence encoding the chimeric antigen receptor. The invention further discloses immune cells capable of expressing the chimeric antigen receptor. The chimeric antigen receptor containing the truncated CD20 molecules can effectively kill antigen-positivetumor cells and does not have toxic and side effects on the negative cells after being expressed in the immune cells; the tCD20 carried by the chimeric antigen receptor not only can be used as tag peptide for detecting CAR expression, but also can be fed with a scavenging antibody such as rituximab after tumor cells are killed, therefore, CAR-T cells are removed, and the clinical use safety is guaranteed.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Geminiviral vector for expression of rituximab
ActiveUS20150368660A1Improve the level ofVaccinesImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNicotiana tabacum
A single vector or multiple separate vectors that contain two or more non-competing replicons for transient expression of the heavy and light chains of Rituximab in Nicotiana benthamiana leaves is described. The correct assembly of these subunit proteins into functional oligomeric structures to optimize the expression is also described. This system advances plant transient expression technology by eliminating the need for non-competing viruses, and thus, enhances the realistic commercial application of the multi-replicon single vector system for producing Rituximab in plant cells.
Owner:ARIZONA STATE UNIVERSITY
12 amino acid analog epi-position of human b cell specificity membrane molecule CD20 and polypeptide epi-position vaccine configurated by said analog epi-position
InactiveCN1786021AEfficient killingReduce the burden onPeptidesAntibody ingredientsChemical synthesisCD20
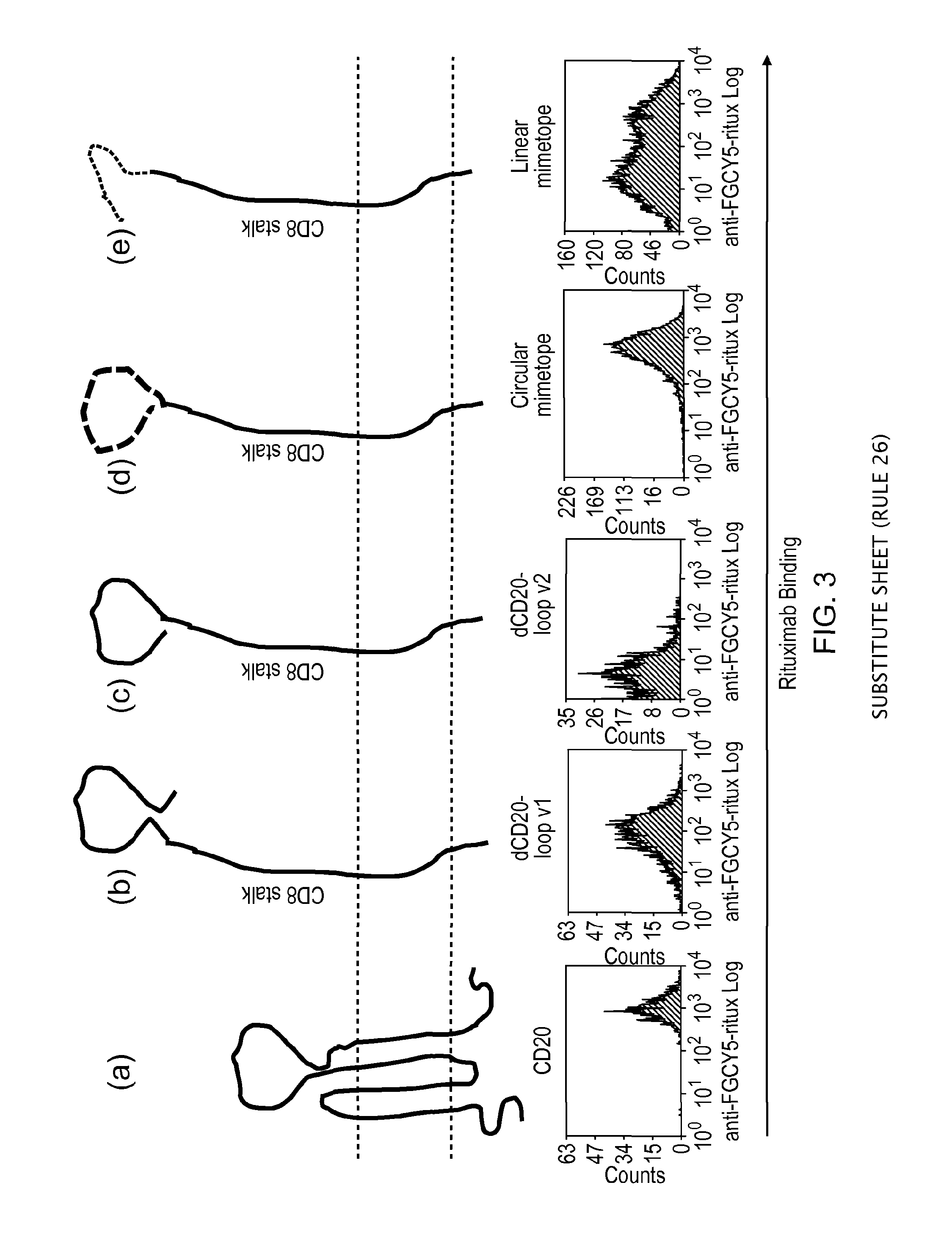
The present invention discloses a simulation epitope of 12 amino acids of human cell B specificity expression membrane molecule CD 20 and its polypeptide epitope vaccine constructed by using said simulation spitope. Said invention also provides a method for screening simulation epitope of CD 20 molecule by using Rituximab as ligand, and provides its amino acid sequence, Gln-Asp-Lys-Leu-Th-Gln-Try-Pro-Lys-Try-Leu-Glu. Said invention also provides a method for chemically-synthesizing simulation epitope of 12 amino acids and making it be chemically-coupled with keyhole limpet hemo cyanin (KLH) to obtain the successfully-constructed vaccine. Besides, said invention also provides the concrete application of said vaccine.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Protein modification open tubular column and application of protein modification open tubular column to monoclonal antibody charge isomer separation
ActiveCN106198780AInhibition of adsorptionEasy to separateComponent separationStationary phaseMonoclonal antibody
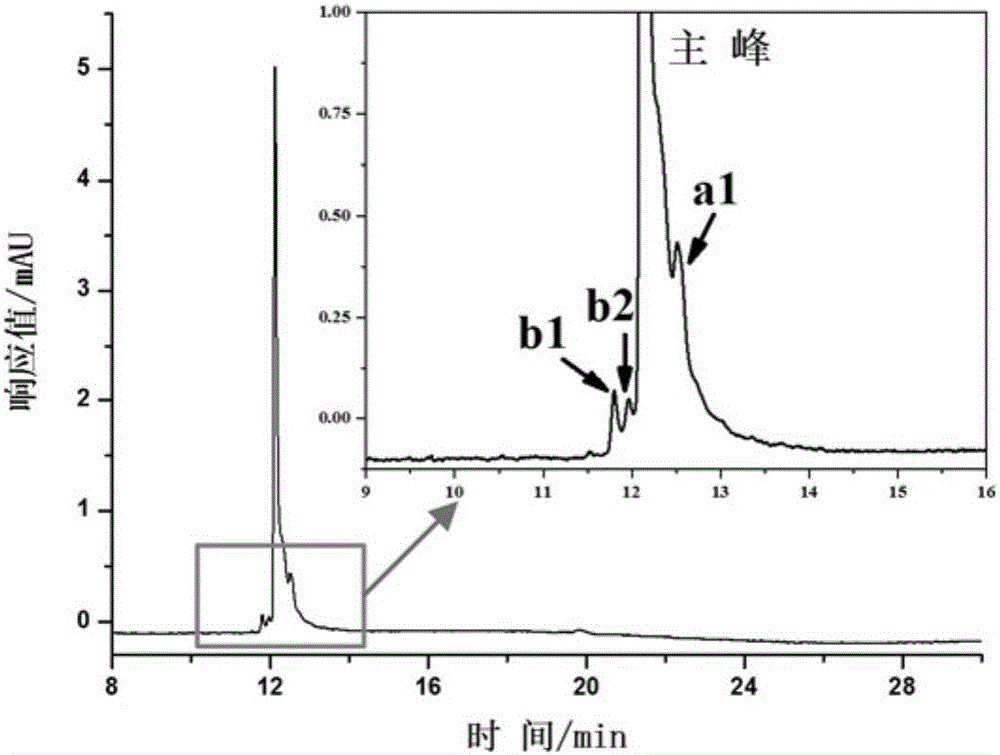
The invention discloses a protein stationary phase modification open tubular column and application of the protein stationary phase modification open tubular column to monoclonal antibody charge isomer separation. The open tubular column is prepared through the following steps that PDDA is fixed on the inner wall of a capillary tube by an electrostatic self-assembly method; then, protein is adsorbed on the PDDA surface through electrostatic self assembly; the protein stationary phase modification open tubular column is obtained. The open tubular column shows specific selectivity on monoclonal antibody charge isomers. Seven kinds of different-form charge isomers of cetuximab are successfully separated; two alkaline isomers and one acid isomer of the rituximab and two alkaline charge isomers and four acid isomers of trastuzumab are successfully separated from main peaks.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Histone Deacetylase (HDAC) Inhibitors for the Treatment of Cancer
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumour cancer, e.g., rectal cancer, colon cancer, ovarian cancer, hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of another chemotherapeutic agent, for example, another chemotherapeutic agent selected from: an antibody against VEGF, AVASTIN® (bevacizumab), an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
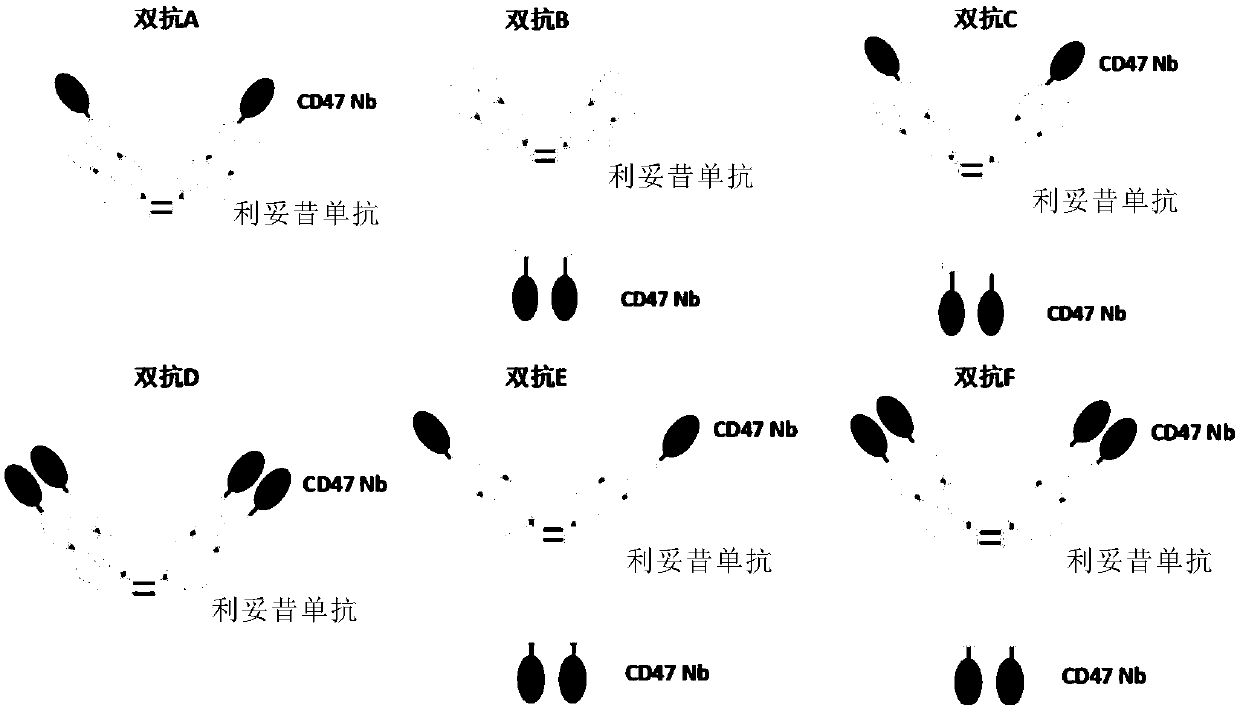
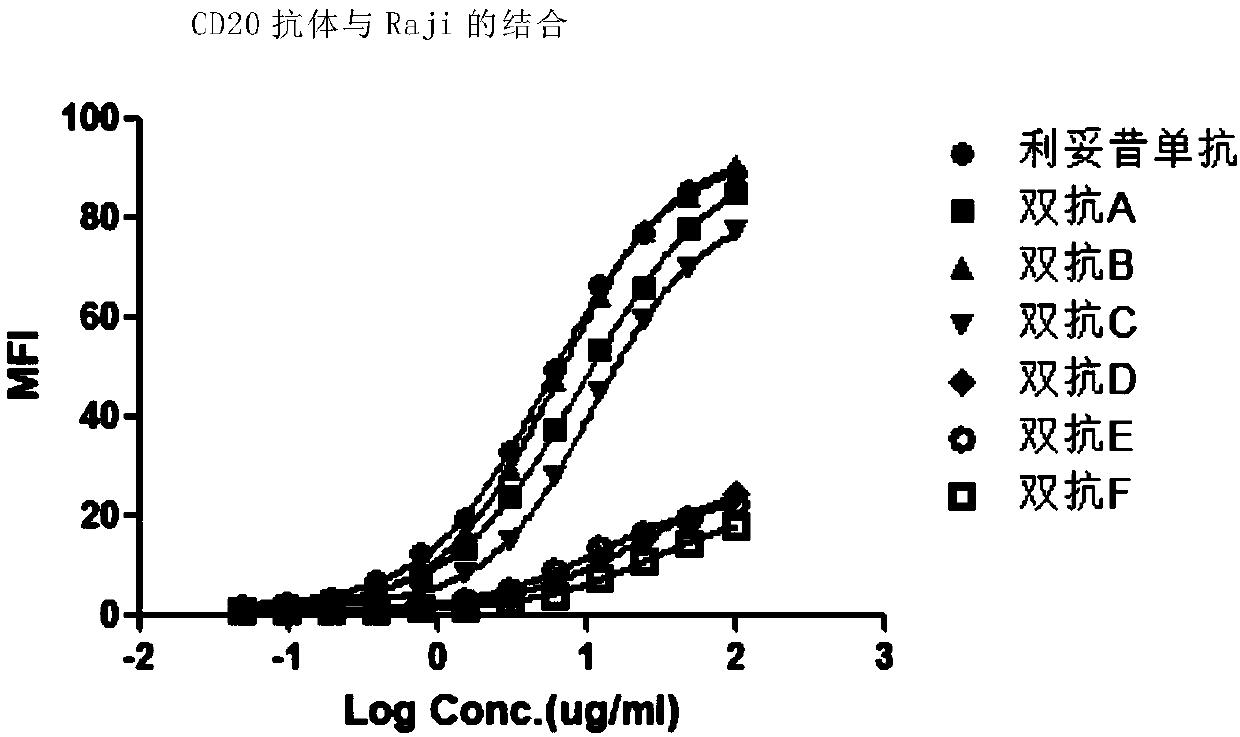
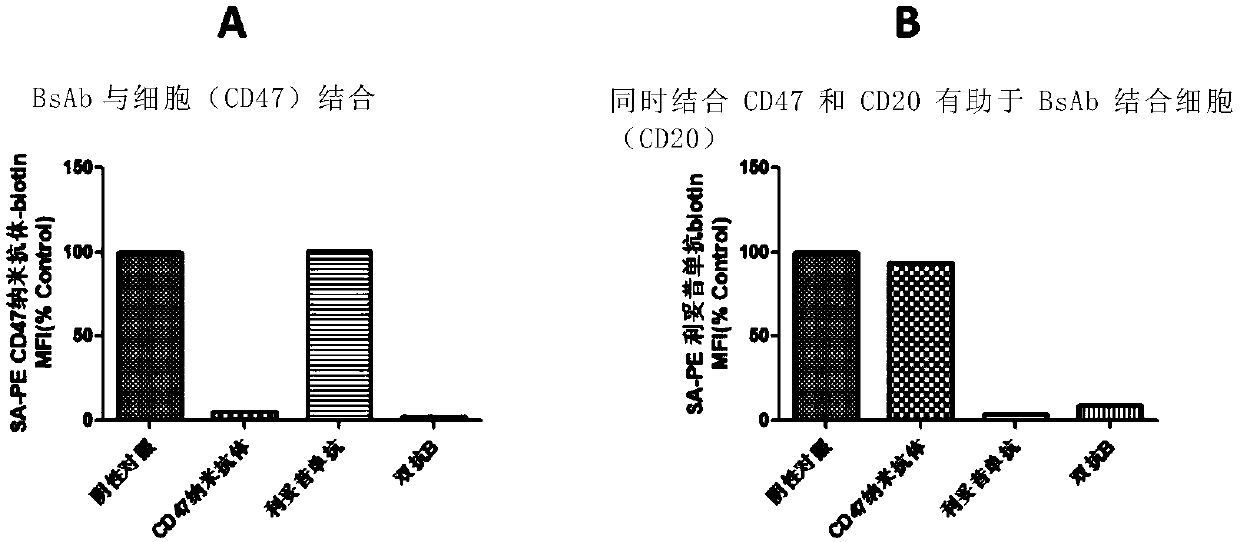
Anti-CD47/CD20 bispecific antibody and application thereof
The invention relates to an anti-CD47 / CD20 bispecific antibody and an application thereof. Specifically, the invention provides the anti-CD47 / CD20 bispecific antibody, which comprises: (a) the anti-CD20 antibody (such as rituximab); and (b) the anti-CD47 nanobody in a monovalent form linked to the anti-CD20 antibody. The bifunctional antibody disclosed by the invention has good stability, the bifunctional antibody has the advantages that CD47 and CD20 can be specifically targeted, the interaction between CD47 and its ligand SIRPa is effectively blocked, CD20 on the cell surface is effectivelycombined, tumor cell proliferation can be remarkably inhibited, the synergistic effect of the bifunctional antibody is achieved, and the bifunctional antibody has a good application prospect.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
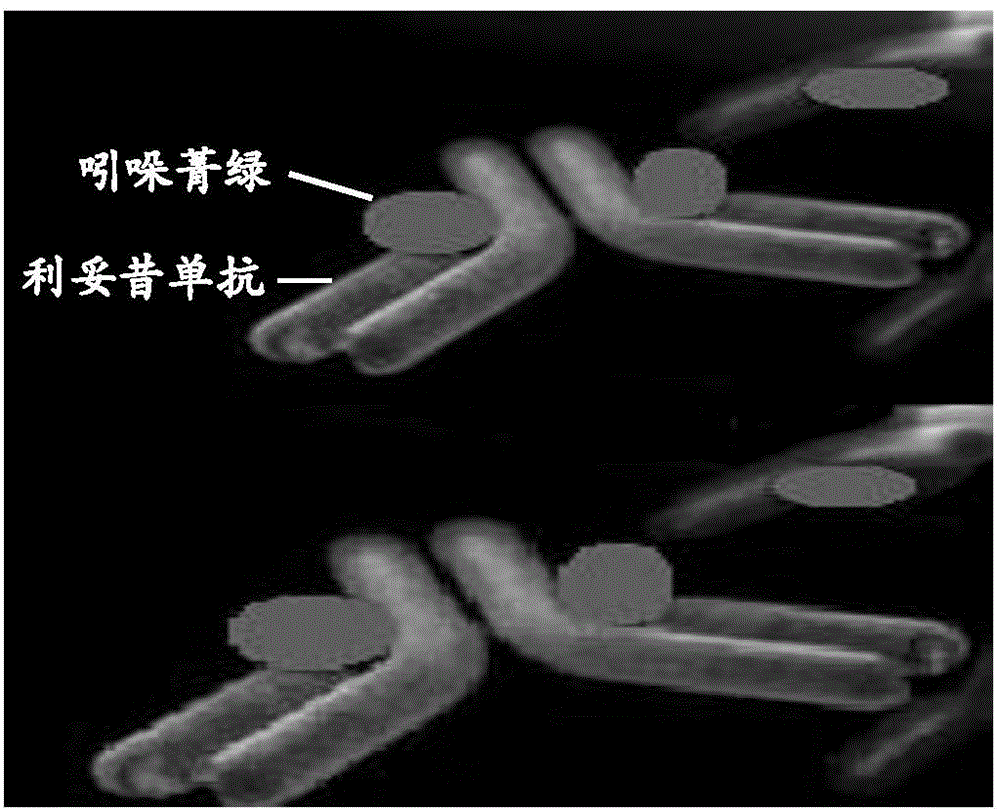
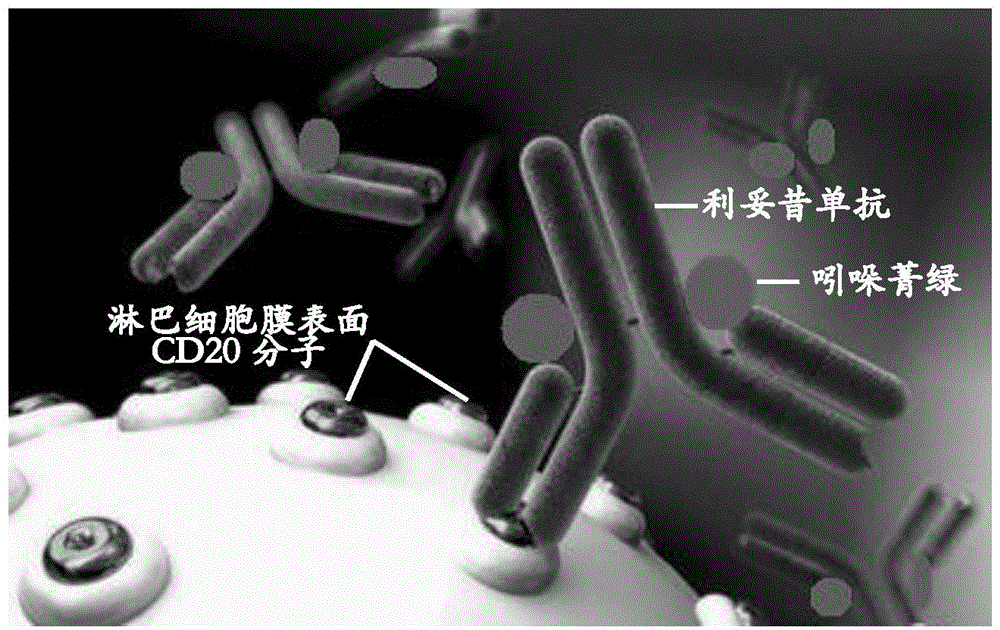
Application of indocyanine green-rituximab in preparation of breast cancer sentinel node tracer
ActiveCN104623695AHigh imaging rateIncreased sensitivityIn-vivo testing preparationsSentinel nodeLymphatic vessel
The invention discloses an application of indocyanine green-rituximab in preparation of a breast cancer sentinel node tracer. When sentinel node biopsy is carried out via an indocyanine green-rituximab coupled specific tracer, since the specific tracer only fluorescently develops the sentinel node and develops no secondary lymph node, the sentinel node does not need to be found along the fluorescently developed lymphatic in the biopsy, the position of the fluorescently developed sentinel node is detected by an infrared fluorescent development system in a surgery, and the sentinel node positioned by fluorescent development is directly dissected and detected after dissecting and dissociating the peripheral tissues of the sentinel node, so as to avoid the situations of leak detection of the sentinel node and fluorescent development pollution of the peripheral tissues.
Owner:王永胜
Rituximab/graphene oxide composite antibody as well as preparation method and application thereof
InactiveCN106039321AEnhanced Biological ResponseAntibody ingredientsPharmaceutical non-active ingredientsOxide compositeInhibitory effect
The invention discloses a rituximab / graphene oxide composite antibody as well as a preparation method and an application thereof. The rituximab / graphene oxide composite antibody consists of rituximab and graphene oxide, wherein the rituximab and the graphene oxide are linked by virtue of a non-covalent bonds, so that the composite antibody is formed; and an antineoplastic biological reaction can be enhanced greatly. Compared with the rituximab which has a slight inhibitory effect in vitro, the rituximab / graphene oxide composite antibody can directly kill lymphoma cells.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
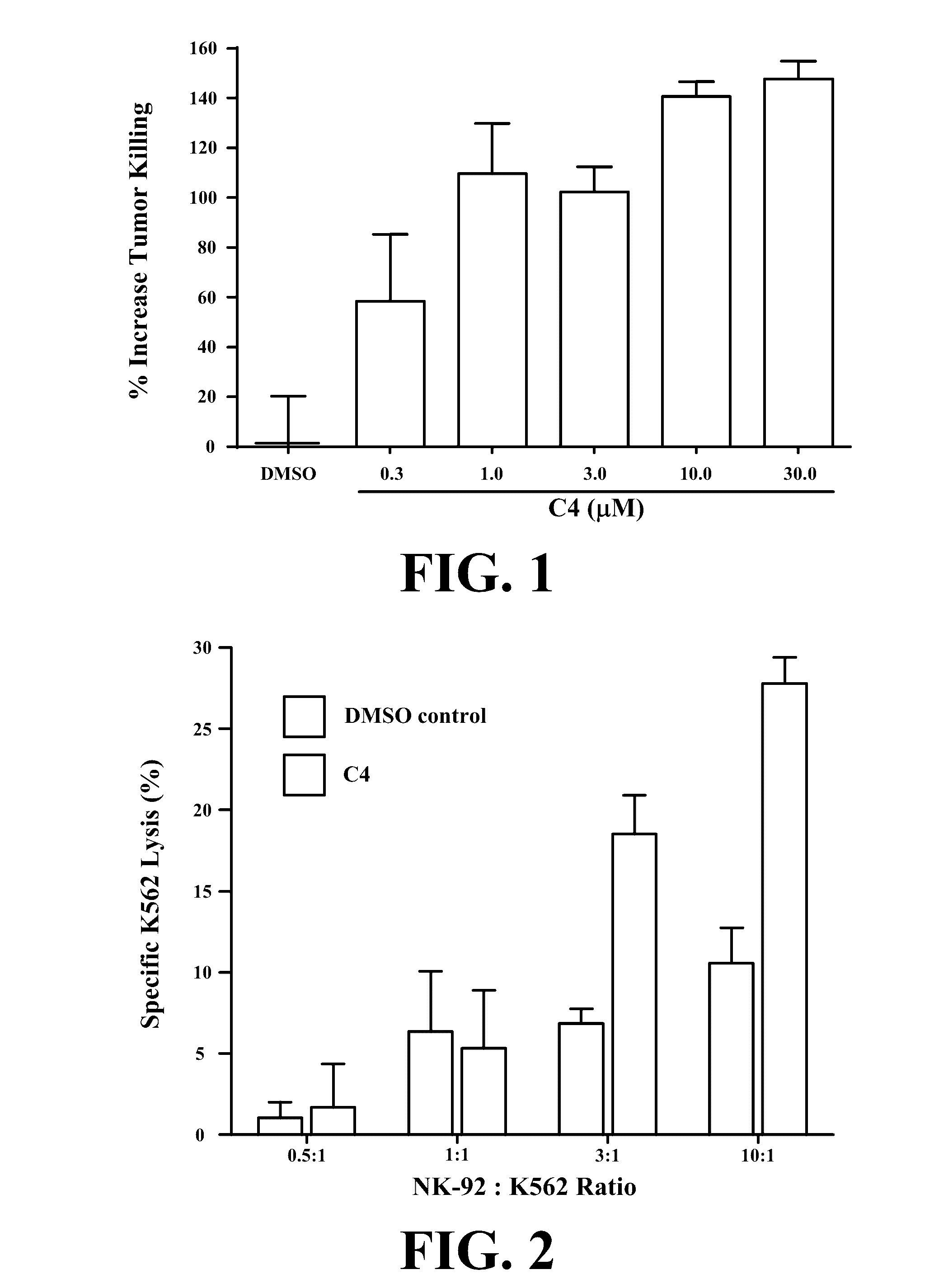
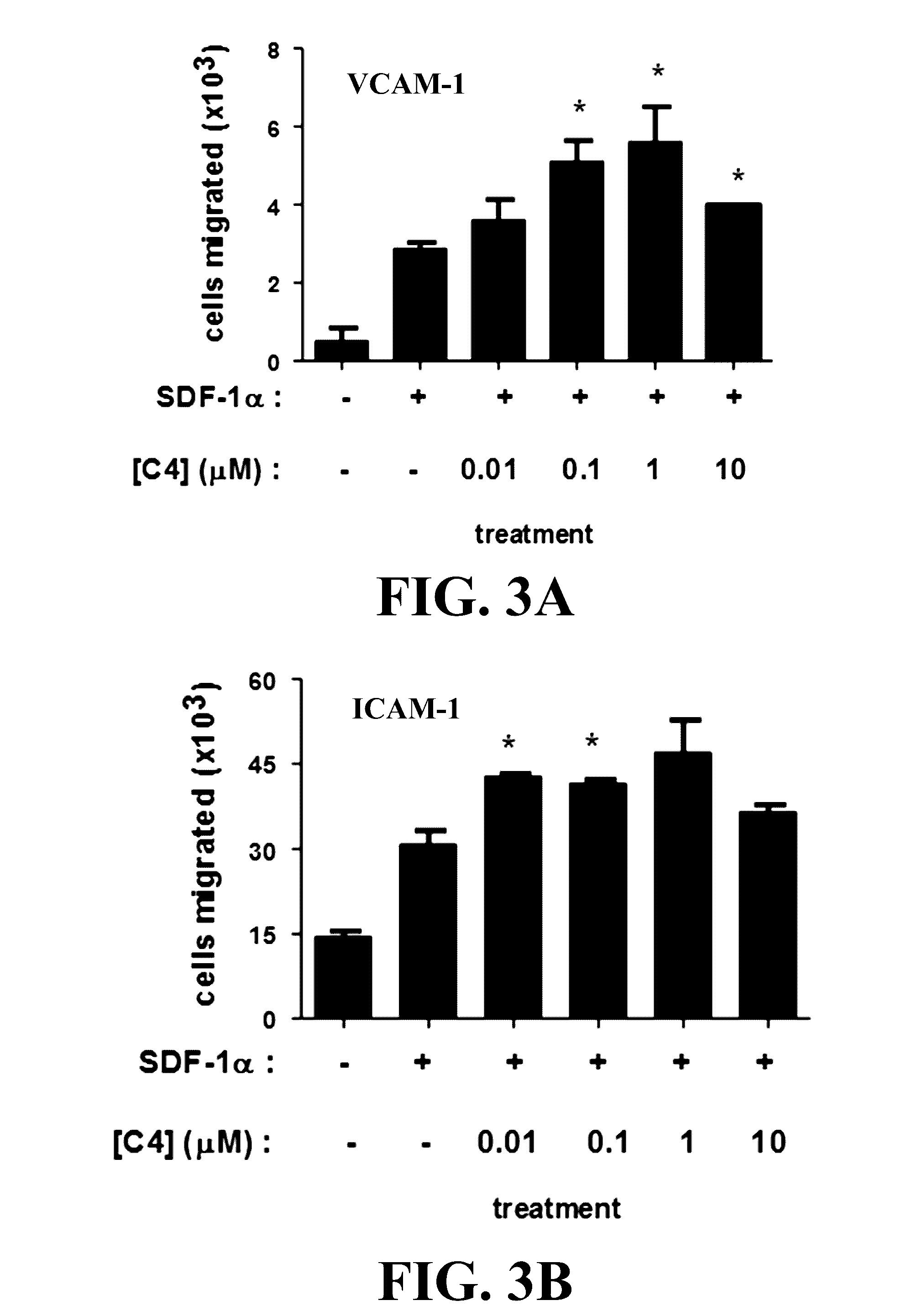
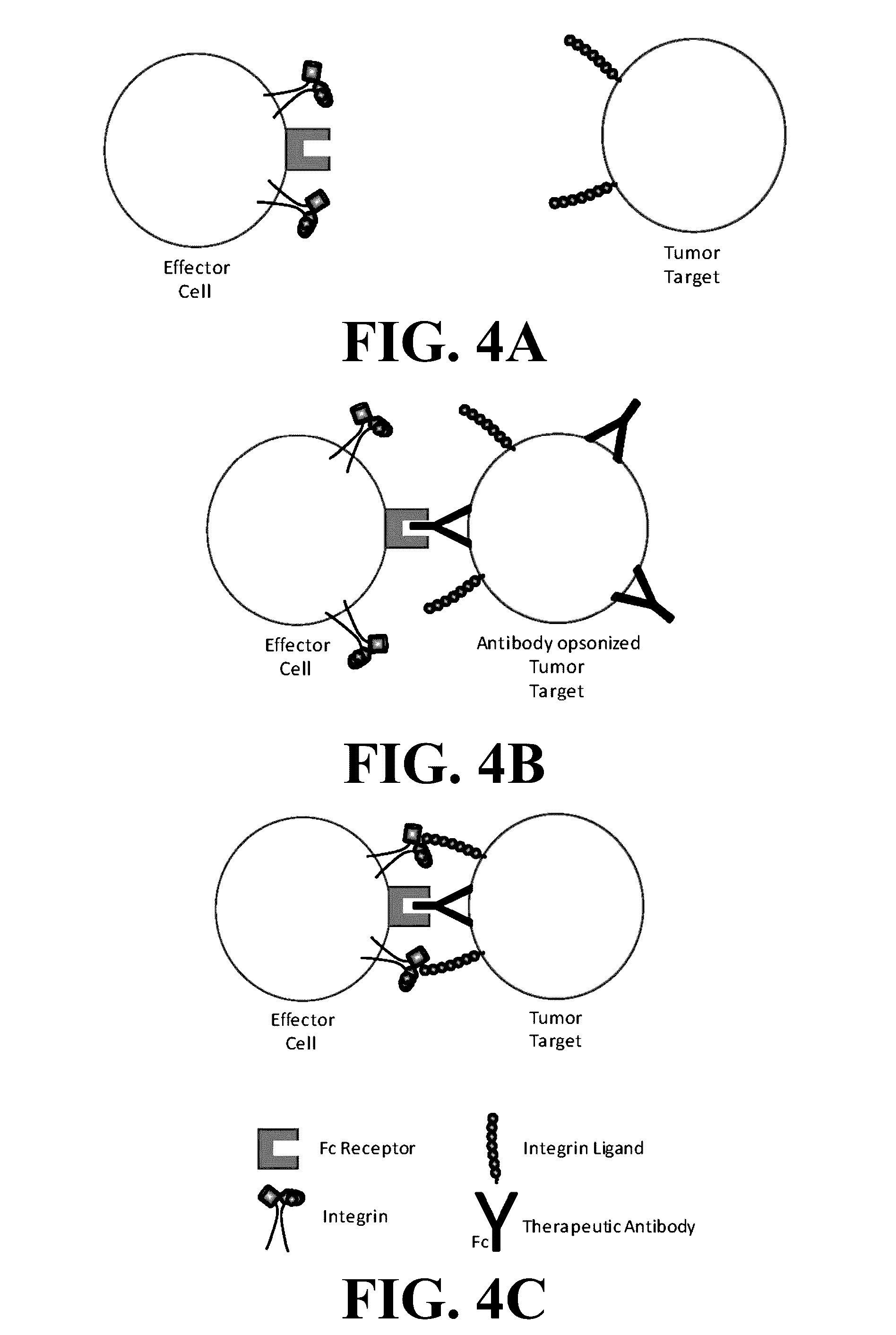
Novel compositions and methods for immunotherapies comprising small molecule integrin receptor-ligand agonist adjuvants
ActiveUS20160339098A1Antibody ingredientsCancer antigen ingredientsAdoptive cellular therapyAdjuvant
Small molecule integrin ligand mimetics facilitate integrin-ligand interactions, which may be used to prepare vaccines, adoptive cell therapies, immunotherapies for cancer, and a variety of other conditions. As integrin mediated cell-cell interactions are critical to antigen presentation and effector cell killing, increasing the efficiency of integrin receptor-ligand interactions will stabilize the immune synapse and improve effector functions. Compositions and methods including the mimetics enhance: (1) the priming of vaccines (including, but not limited to, cancer vaccines); (2) cytolytic activity of adoptive cell therapies (including, but not limited to γδT-cells, CTLs, NK, iNKT); (3) immunotherapies (including, but not limited to, negative checkpoint blockage strategies such as anti-CTLA-4 and anti-PD-1); and (4) biologic therapies (including, but not limited, to trastuzumab and rituxamab), whereby the mechanism-of-action includes antibody dependent cellular cytotoxicity (ADCC).
Owner:TEXAS HEART INST +1
Anticancer sustained-release gel injection containing neonatal blood vessel inhibitor
InactiveCN101283975APharmaceutical delivery mechanismPharmaceutical non-active ingredientsRofecoxibResidual Tumors
A sustained-release gel injection comprises angiogenesis inhibitor, amphiphilic block copolymer, solvent and release regulator, wherein the mixture of amphiphilic block copolymer and solvent has temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The preparation can be injected into or around tumor for treating solid tumors at different stages and metastatic tumors with remarkably reduced systemic toxicity of drug. The angiogenesis inhibitor is selected from alemtuzumab,ibritumomab, bevacizumab, rituximab, gemtuzumab ozogamicin, panitumumab, trastuzumab, celebrex, refecoxib, tositumomab, endostatain, engiostatin, toxitumomab, and cetuximab. The preparation has effects of controlling residual tumor cell relapse after operation and tumor can not be excised via operation, controlling complications at late stage of tumor, and enhancing treatment effect of chemotherapy and radiotherapy (particularly radioactive particles).
Owner:济南基福医药科技有限公司
Compound for Fab fragment of Rituximab and CD20 antigen epitope polypeptide
The invention discloses a compound of Fab segment of anti-cancer drug Rituximab and CD20 antigen epitope peptide, which also provides the preparing method of compound and three-dimensional crystal structure of compound and application, wherein the three-dimensional crystal structure of compound displays the key residue interacted between Rituximab and antigen epitope peptide, which provides theoretical base for higher affinity and specificity antibody; the CD20 antigen epitope peptide displays peculiar ring-shaped structure, which is fit for sieving new antibody of epitope.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Rituximab induction therapy followed by glatiramer acetate therapy
The present invention provides a method of treating a subject afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome comprising periodic administration of an amount of an anti-CD20 antibody at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject, wherein the amounts are effective to treat the subject.
Owner:TEVA PHARMA IND LTD
Protein biomarker for assisting in authenticating Rituximab drug-resistant ABC-DLBCL cells and application thereof
The present invention discloses a protein biomarker for assisting in authenticating Rituximab drug-resistant ABC-DLBCL cells and application thereof. The application of a substance for detecting a specific biomarker to preparing a kit for detecting whether to-be-detected ABC-DLBCL cells are Rituximab drug-resistant ABC-DLBCL cells or not is protected. A biomarker A-1 is any one or a combination of8 proteins. A biomarker A-2 is any one or a combination of 45 proteins. A biomarker B-1 is any one or a combination of 5 proteins. A biomarker B-2 is any one or a combination of 77 proteins. A biomarker C is any one or a combination of 21 proteins. A biomarker D is any one or a combination of 29 proteins. According to the present invention, Rituximab drug-resistant cell strains are successfully constructed, and multiple protein biomarkers are found. The cell model and research results provide a basis for further studying Rituximab drug-resistance and overcoming drug-resistance.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com