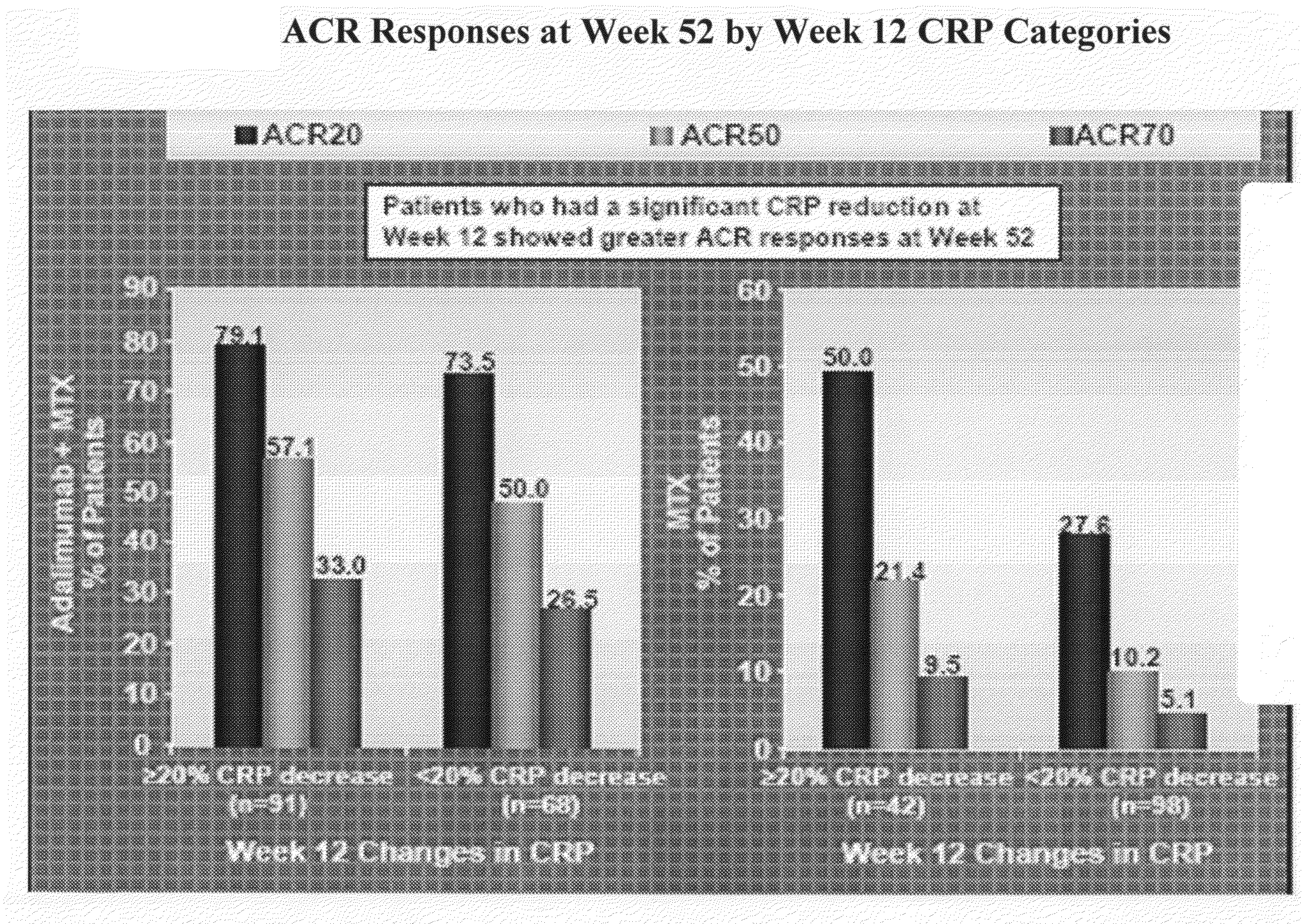Patents
Literature
10587results about "In-vivo testing preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
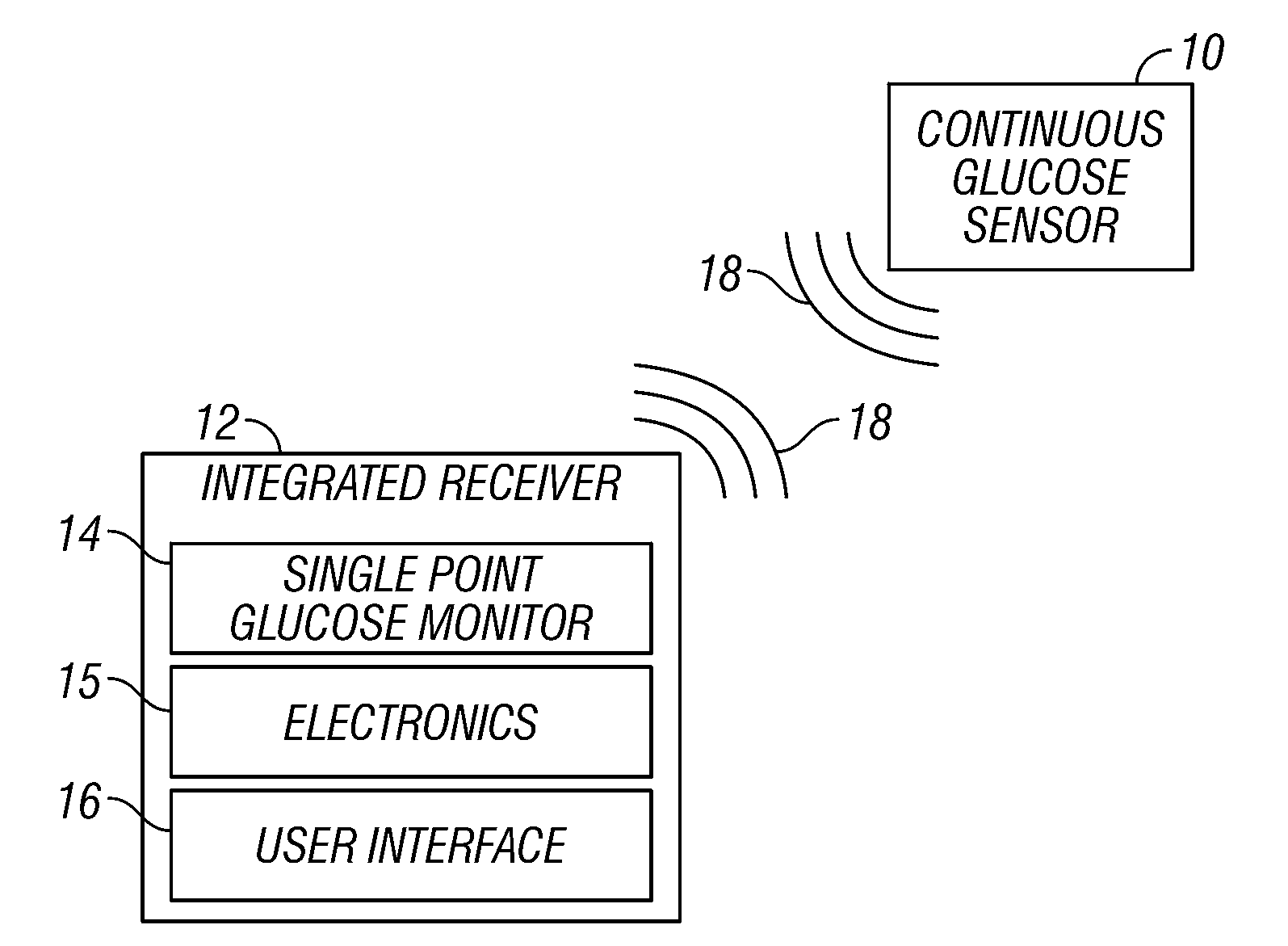
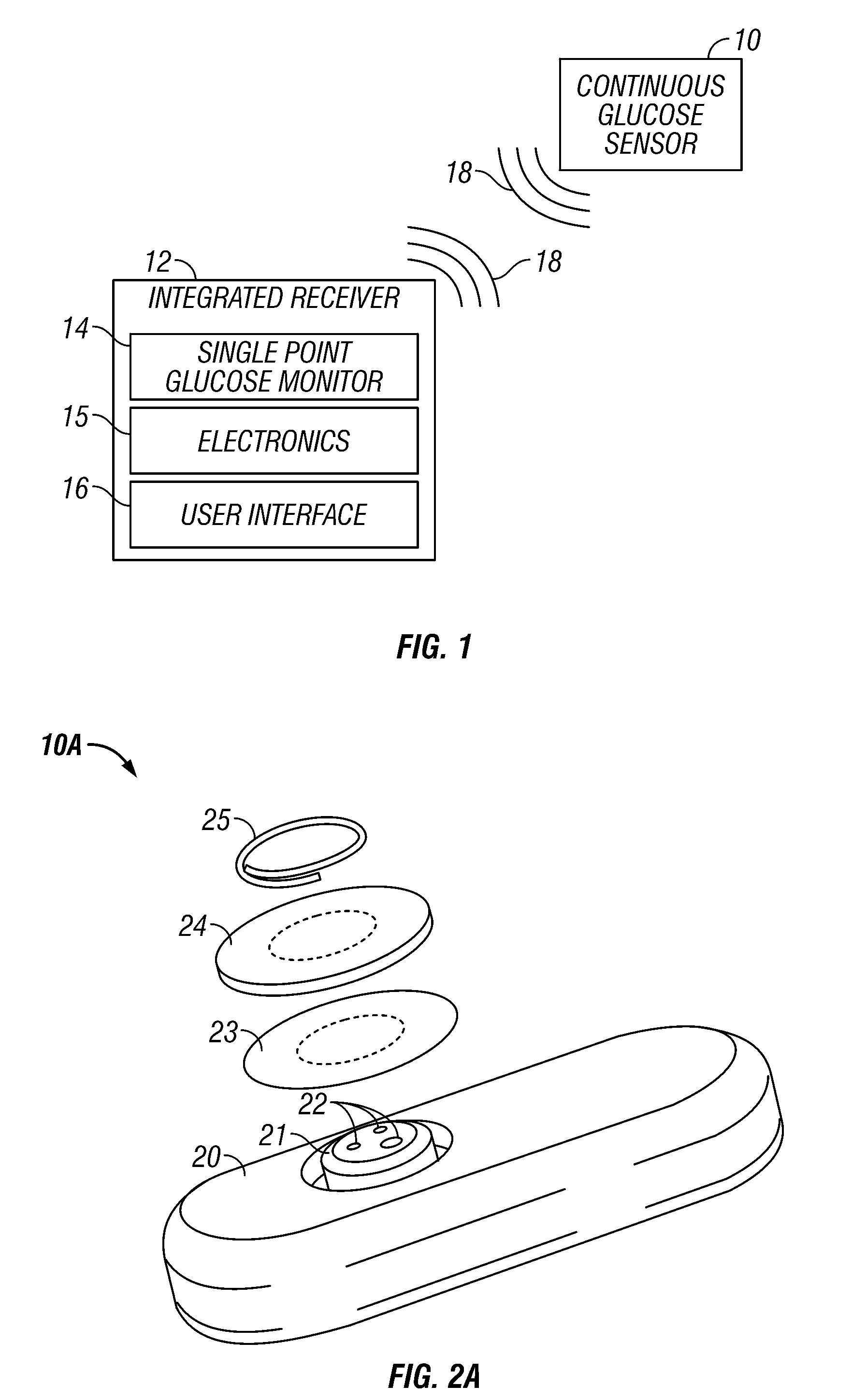
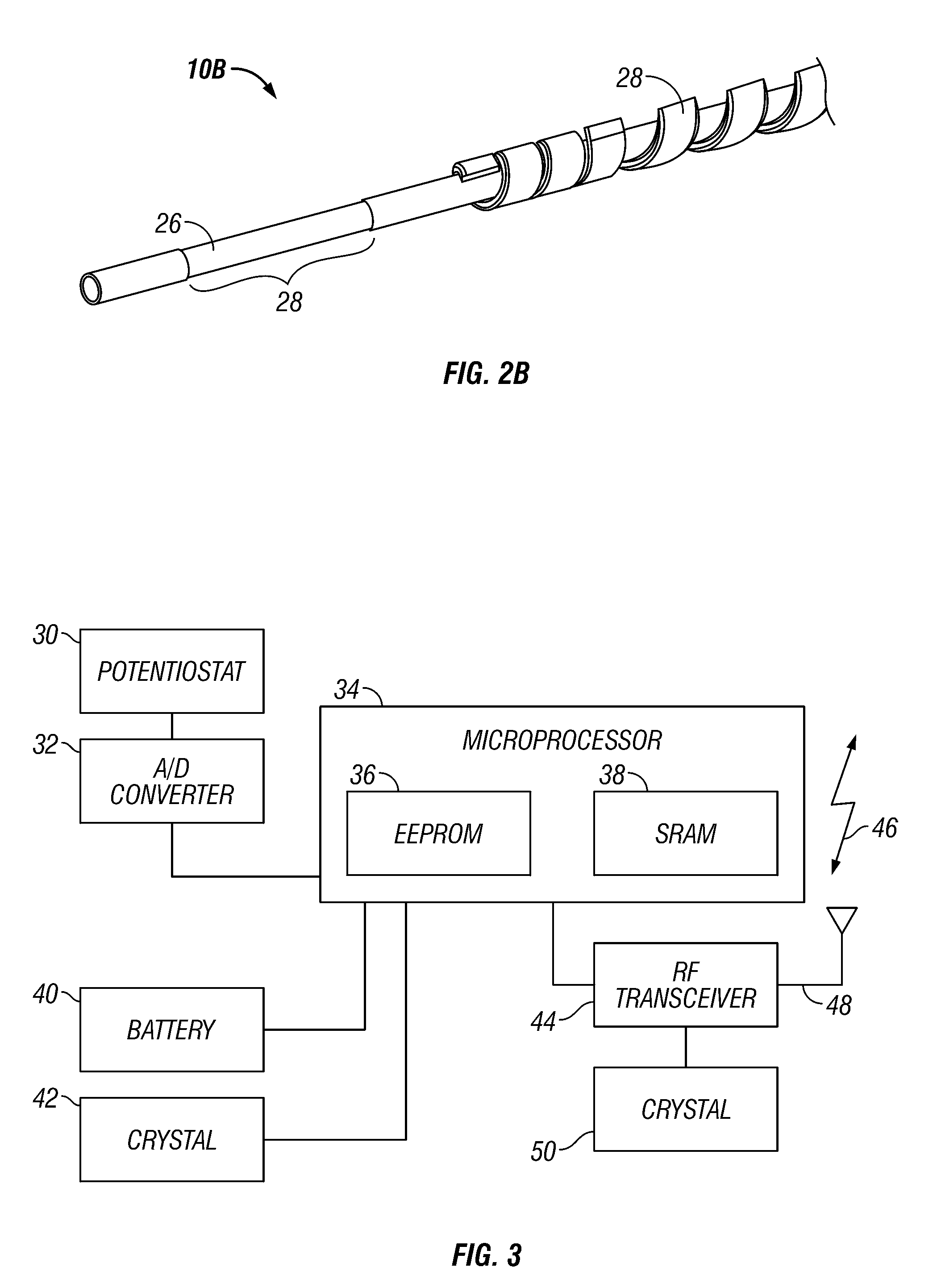
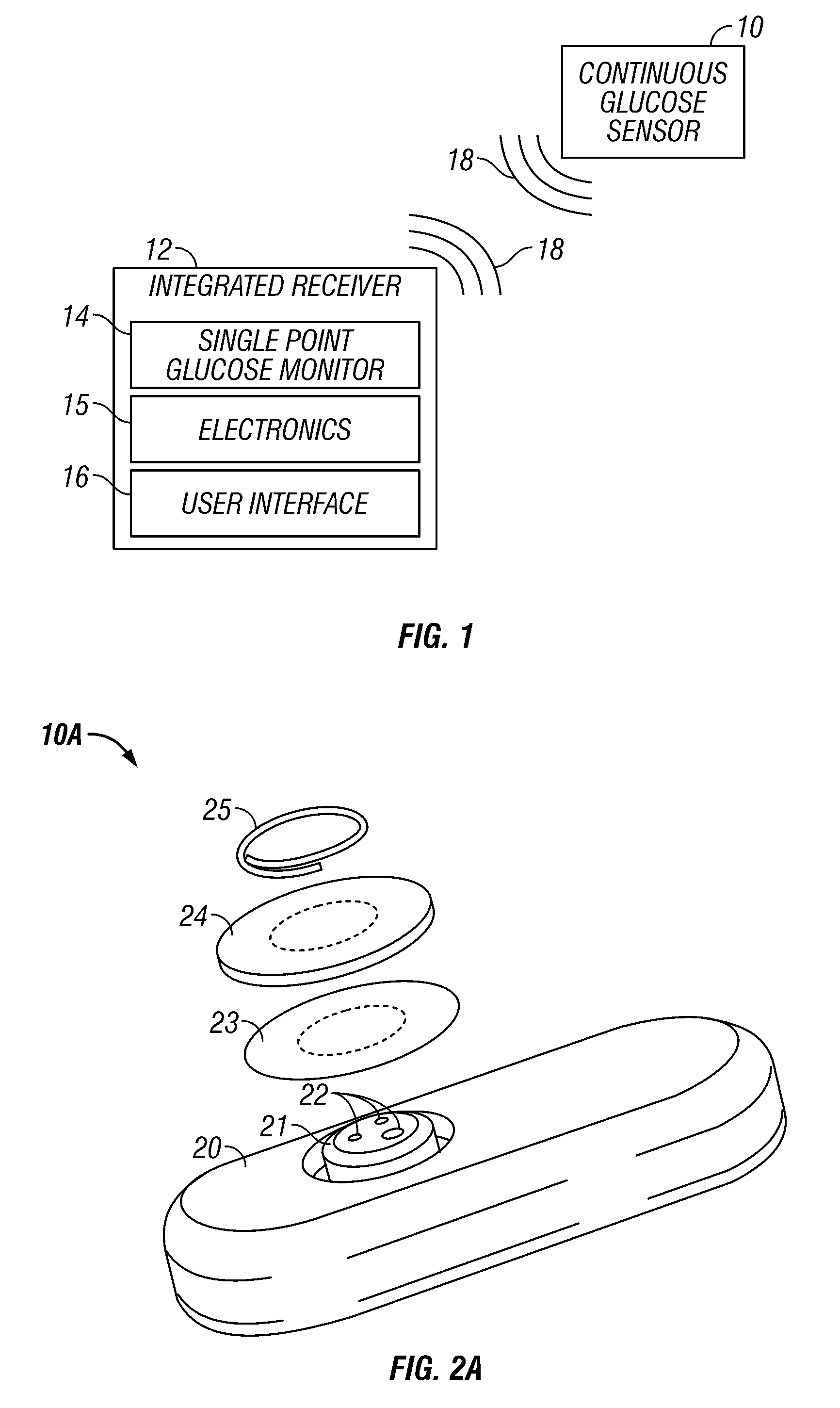
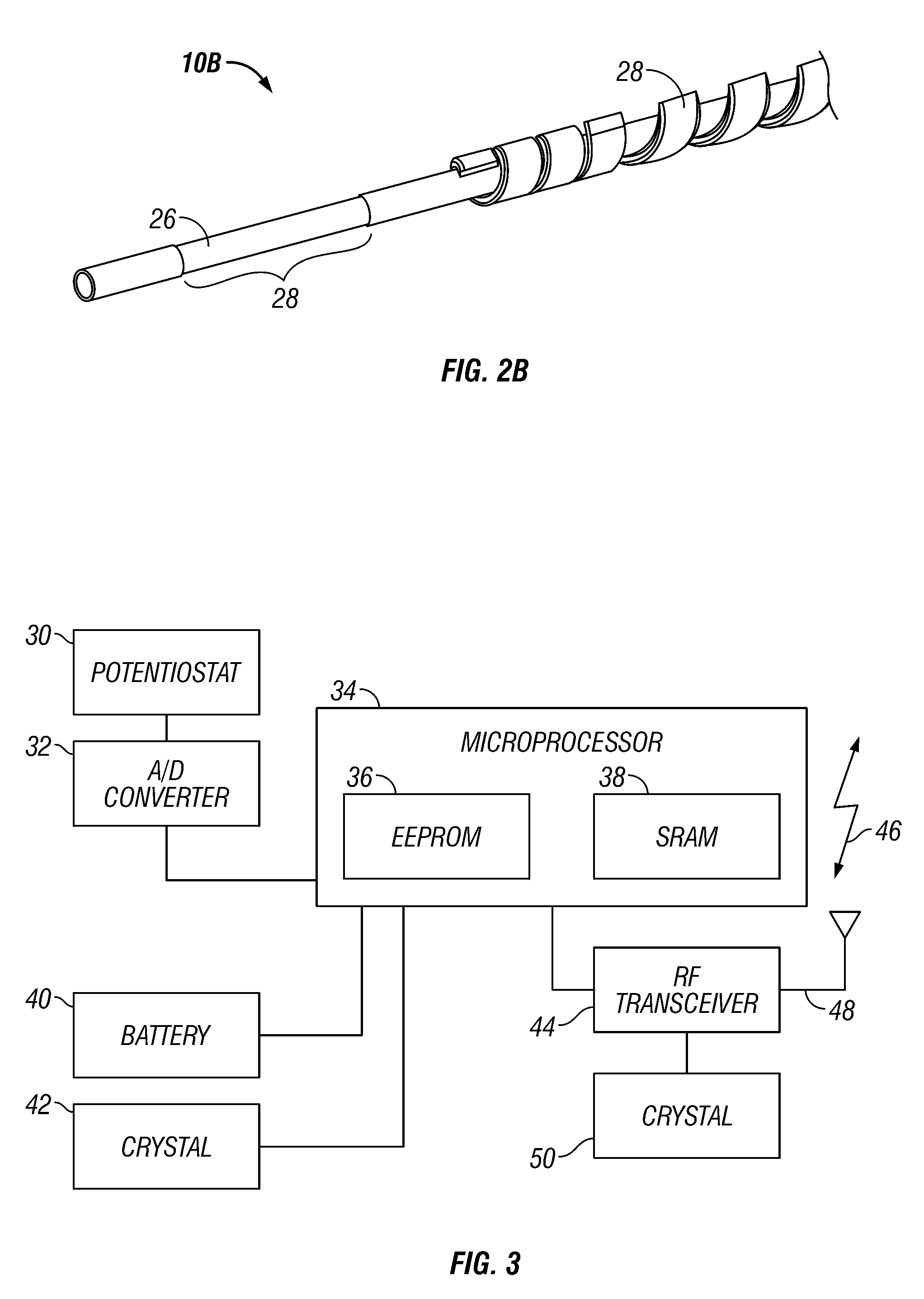
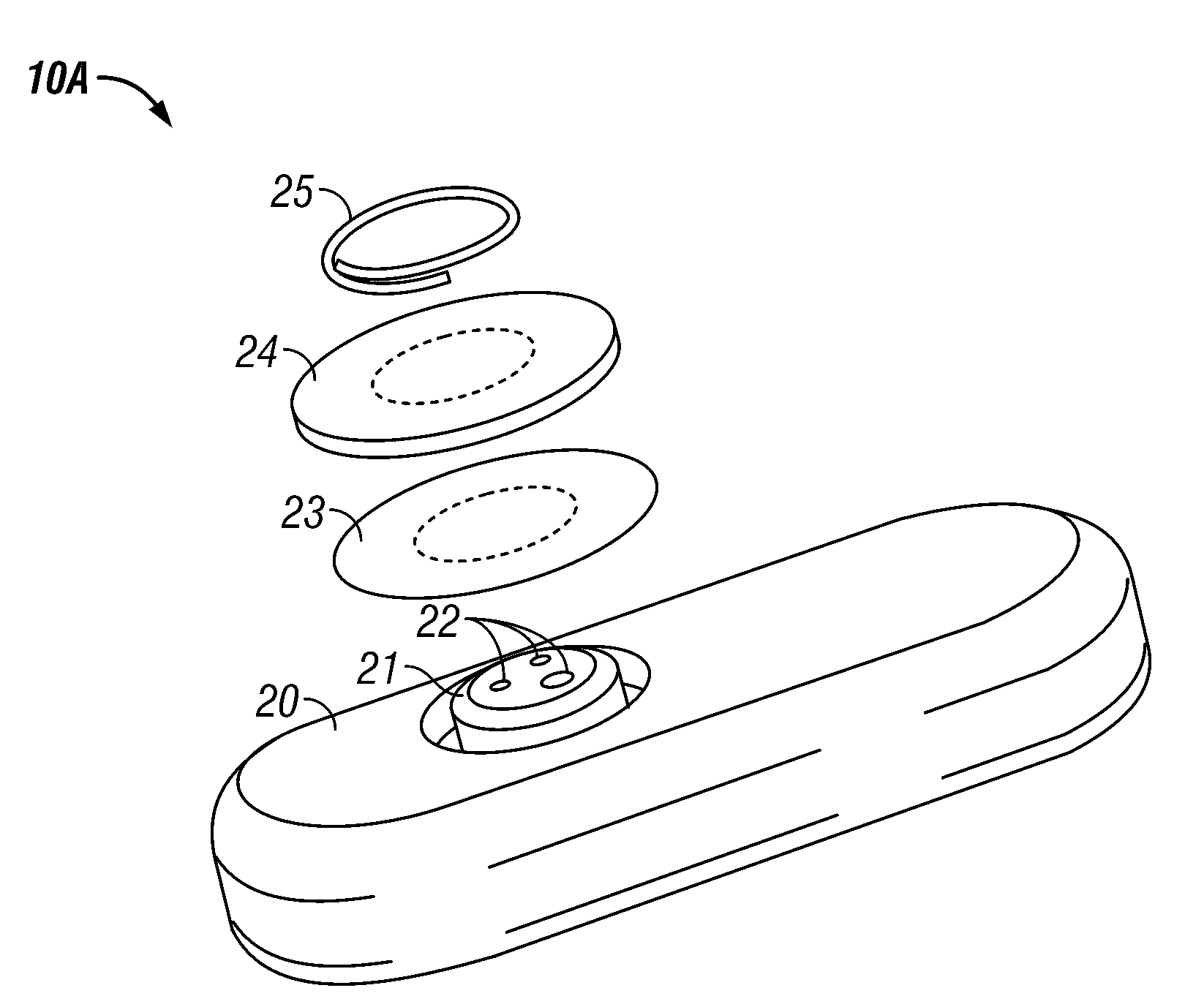
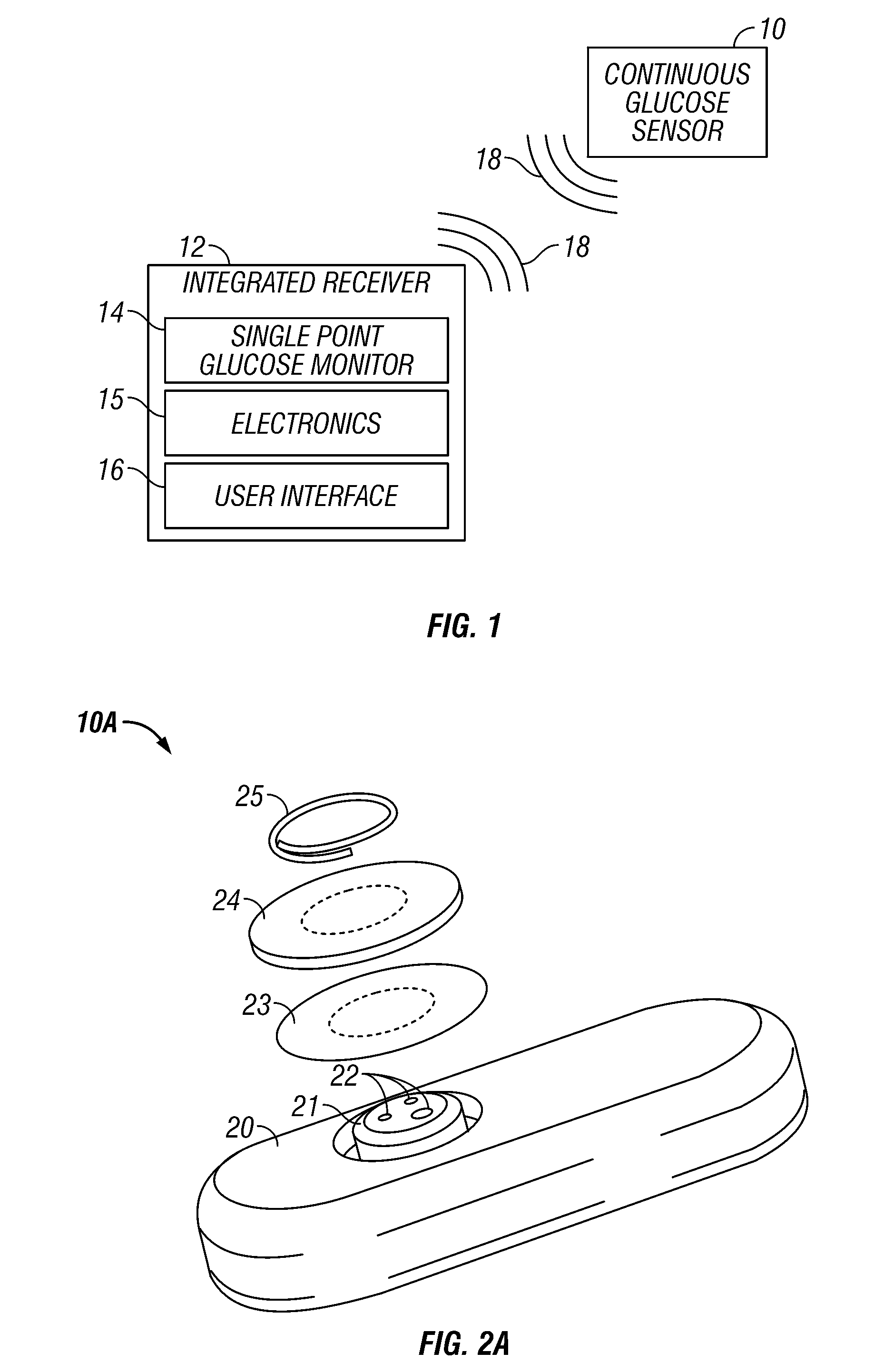
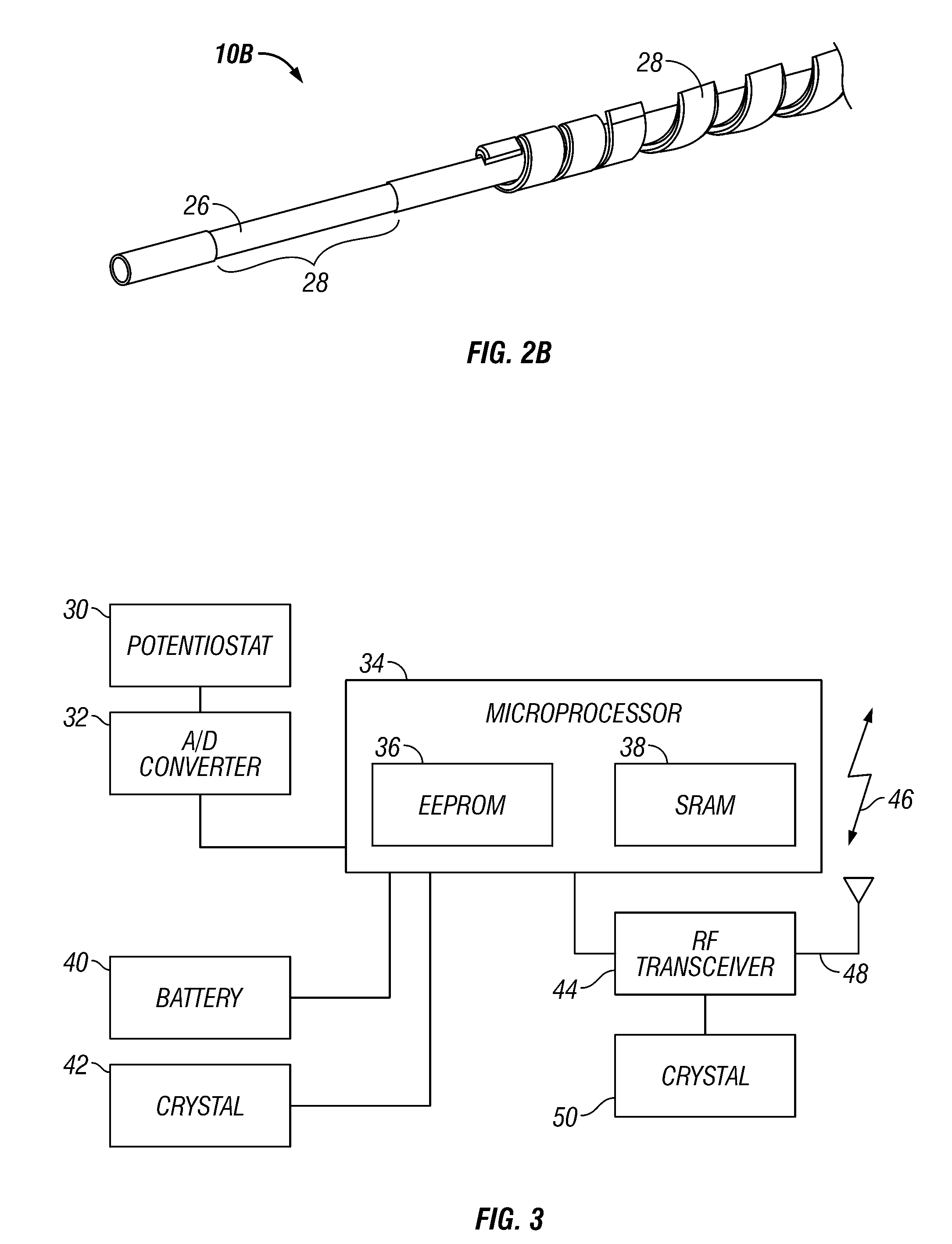
Integrated receiver for continuous analyte sensor
InactiveUS20080287765A1Simpler and few componentReduce errorsEndoradiosondesCatheterGlucose sensorsData stream
A system is provided for monitoring glucose in a host, including a continuous glucose sensor that produces a data stream indicative of a host's glucose concentration and an integrated receiver that receives the data stream from the continuous glucose sensor and calibrates the data stream using a single point glucose monitor that is integral with the integrated receiver. The integrated receiver obtains a glucose value from the single point glucose monitor, calibrates the sensor data stream received from the continuous glucose sensor, and displays one or both of the single point glucose measurement values and the calibrated continuous glucose sensor values on the user interface.
Owner:DEXCOM INC
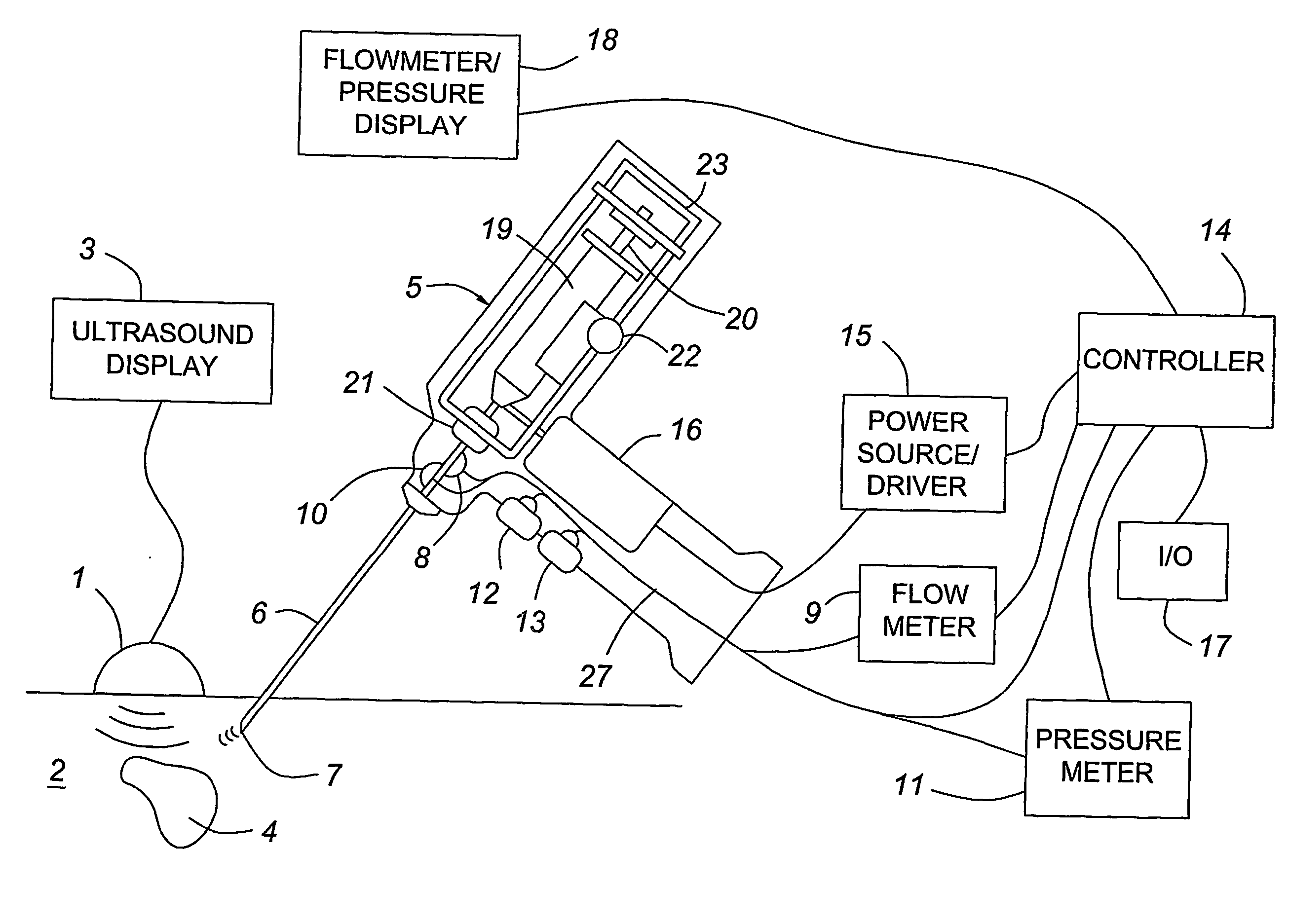
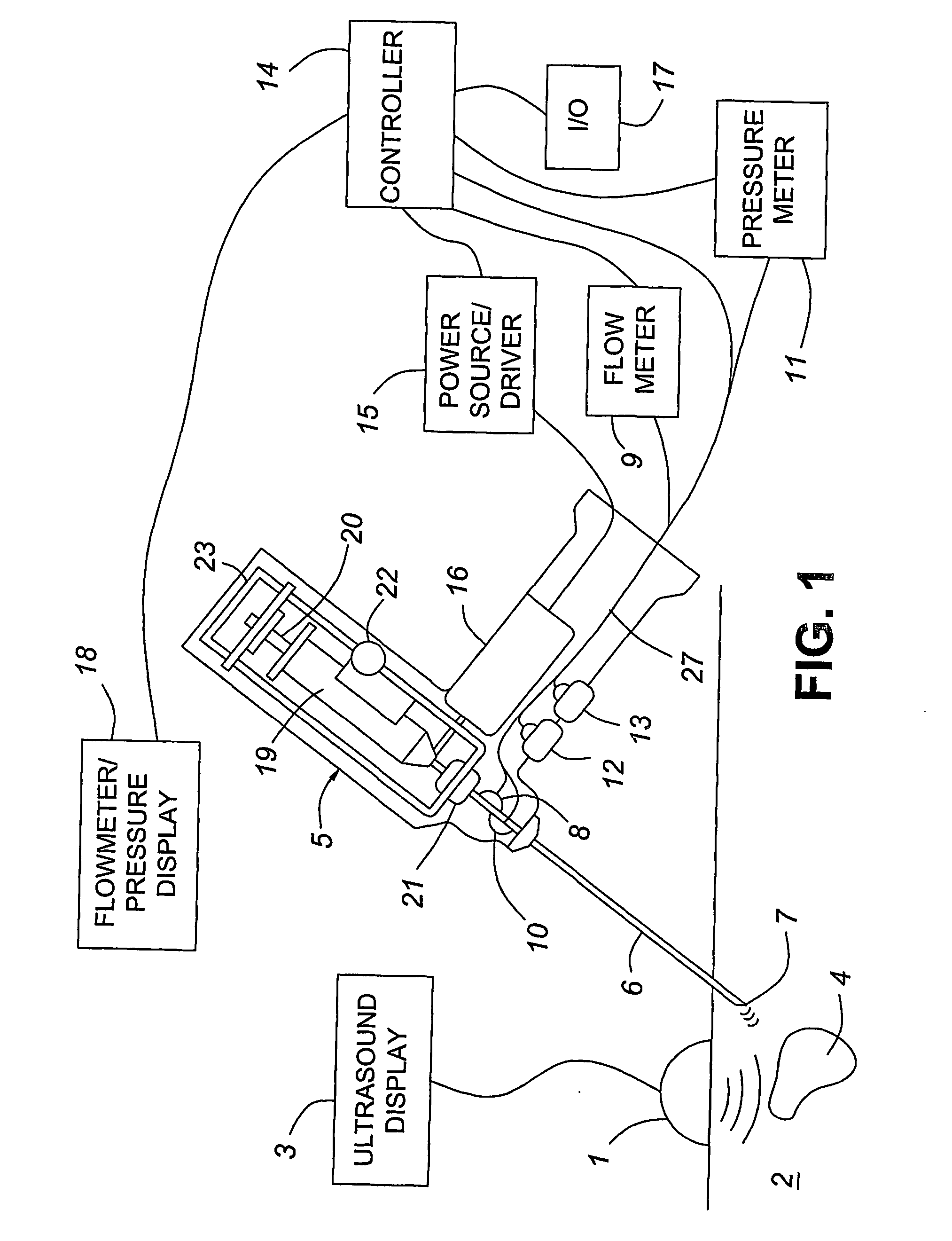
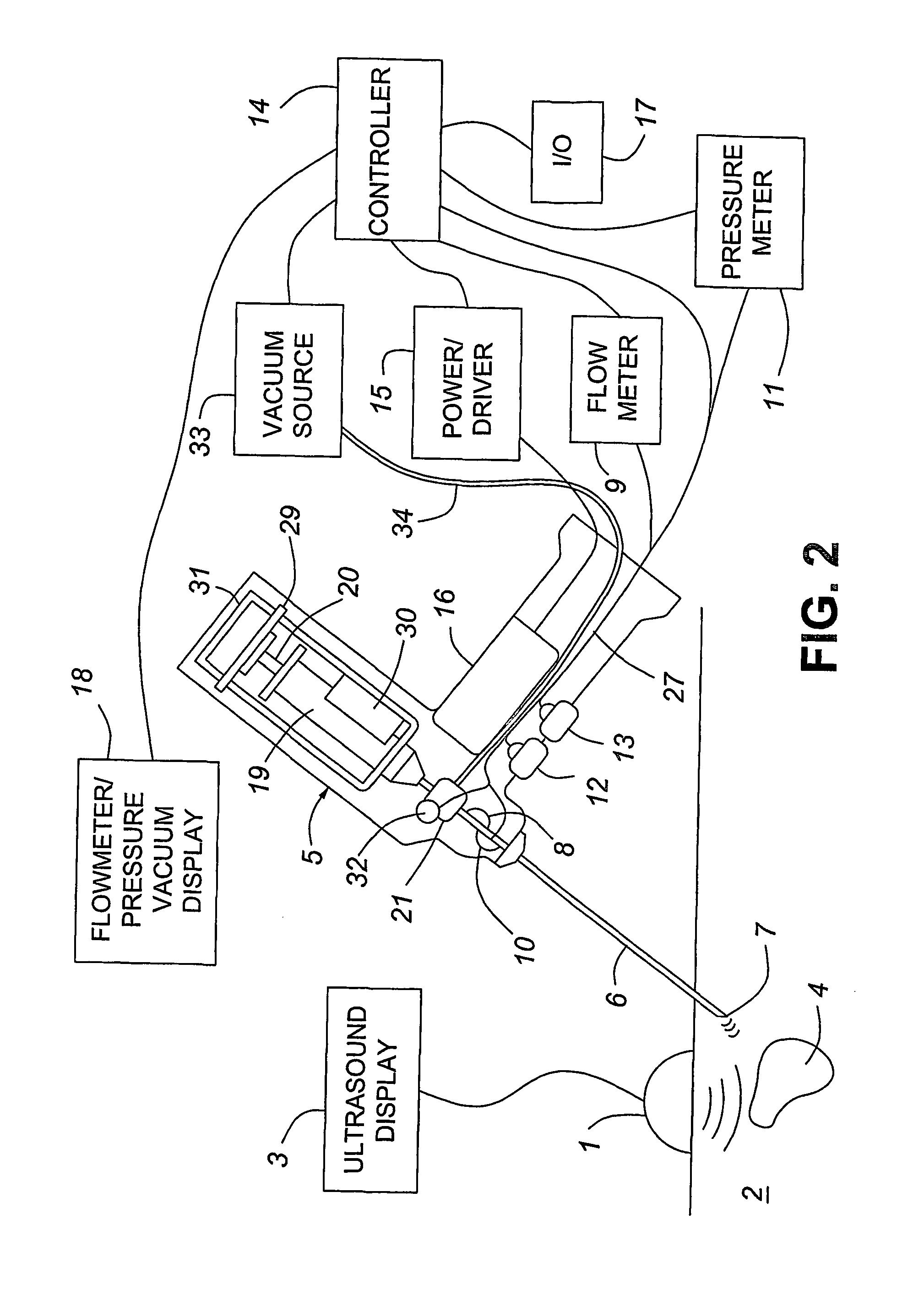
Medical devices with enhanced ultrasonic visibilty
InactiveUS20070197954A1Ultrasonic/sonic/infrasonic diagnosticsElectrotherapyTip positionSolid tissue
A medical device having enhanced ultrasonic visibility is provided. The device permits localized drug delivery, probe positioning, fluid drainage, biopsy, or ultrasound pulse delivery, through the real-time ultrasound monitoring of the needle tip position within a patient. The device permits controlled dispersion of a drug into solid tissue, the lodging of particles into solid tissue, and drug delivery into specific blood vessels. As a needle is inserted, a fluid that contrasts echogenically with the organ environment is injected into the patient. The fluid travels a brief distance before being slowed and stopped by the patient's tissue and this fluid flow will be detectable by ultrasound. The needle position during insertion will be monitored using ultrasound until it is at the desired point of action. A therapeutic drug is then delivered or a probe inserted
Owner:ARTENGA
Integrated receiver for continuous analyte sensor
InactiveUS20080287764A1Simpler and few componentReduce errorsPharmaceutical delivery mechanismEndoradiosondesGlucose sensorsData stream
A system is provided for monitoring glucose in a host, including a continuous glucose sensor that produces a data stream indicative of a host's glucose concentration and an integrated receiver that receives the data stream from the continuous glucose sensor and calibrates the data stream using a single point glucose monitor that is integral with the integrated receiver. The integrated receiver obtains a glucose value from the single point glucose monitor, calibrates the sensor data stream received from the continuous glucose sensor, and displays one or both of the single point glucose measurement values and the calibrated continuous glucose sensor values on the user interface.
Owner:DEXCOM INC
Integrated receiver for continuous analyte sensor
ActiveUS20080287766A1Simpler and few componentReduce errorsEndoradiosondesCatheterGlucose sensorsData stream
A system is provided for monitoring glucose in a host, including a continuous glucose sensor that produces a data stream indicative of a host's glucose concentration and an integrated receiver that receives the data stream from the continuous glucose sensor and calibrates the data stream using a single point glucose monitor that is integral with the integrated receiver. The integrated receiver obtains a glucose value from the single point glucose monitor, calibrates the sensor data stream received from the continuous glucose sensor, and displays one or both of the single point glucose measurement values and the calibrated continuous glucose sensor values on the user interface.
Owner:DEXCOM INC
Non-invasive measurement of analytes
ActiveUS8509867B2Great simplicityHigh sensitivityMicrobiological testing/measurementChemiluminescene/bioluminescenceBiological bodyMetabolite
Owner:CERCACOR LAB INC
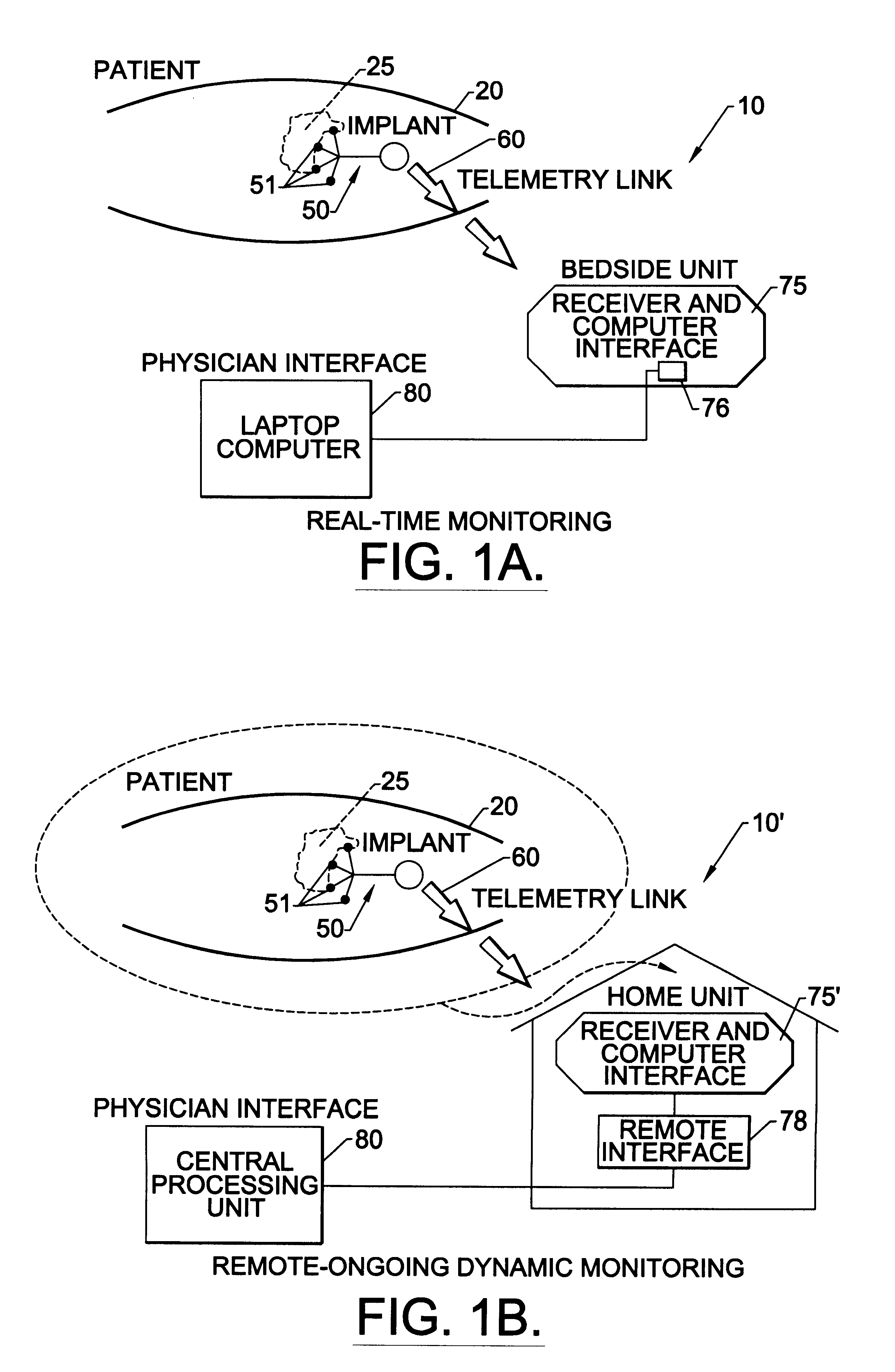
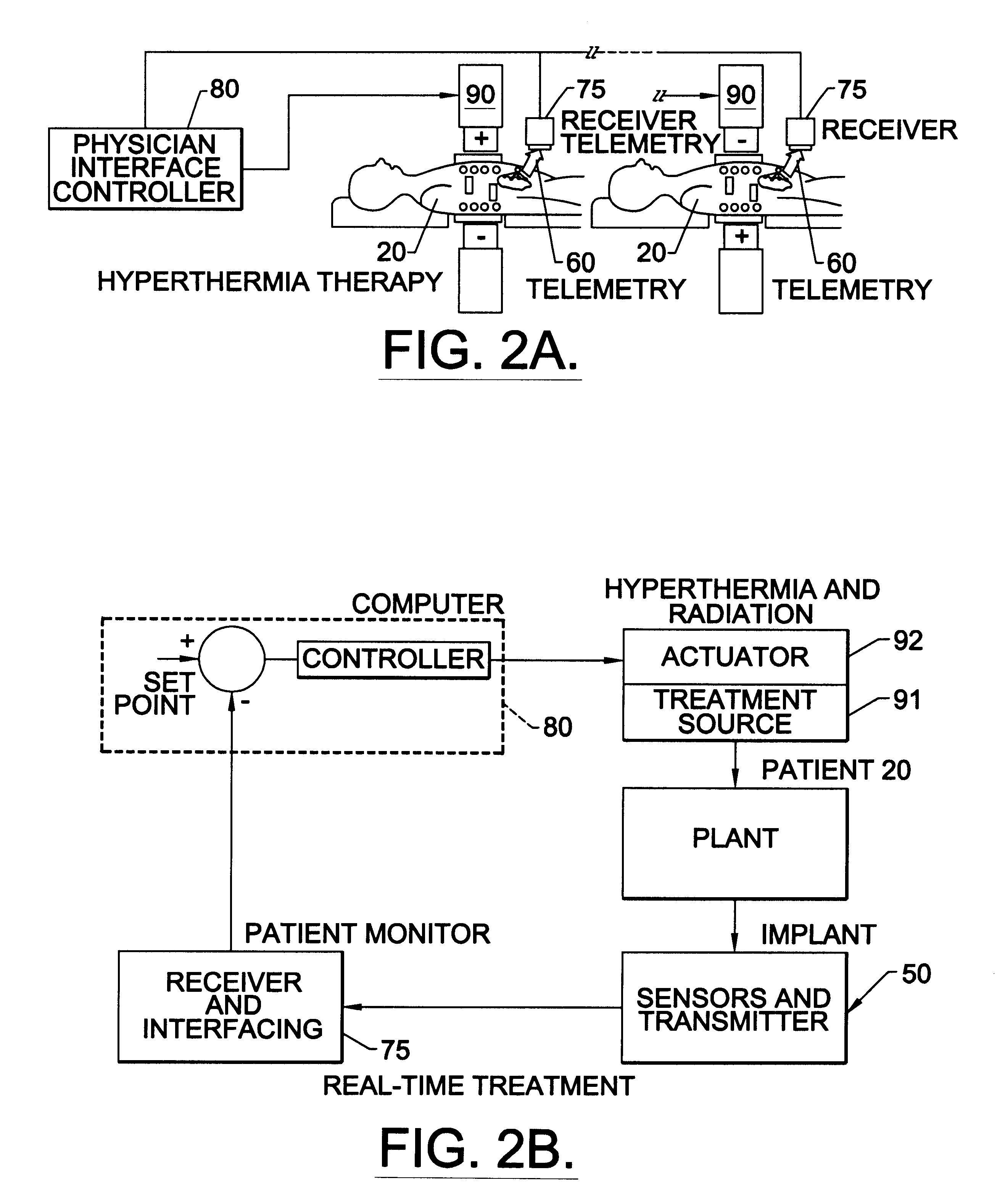
Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors
InactiveUS6402689B1Enhanced and favorable treatmentMinimize couplingMechanical/radiation/invasive therapiesSurgeryDynamic monitoringEngineering
Methods of monitoring and evaluating the status of a tumor undergoing treatment includes monitoring in vivo at least one physiological parameter associated with a tumor in a subject undergoing treatment, transmitting data from an in situ located sensor to a receiver external of the subject, analyzing the transmitted data, repeating the monitoring and transmitting steps at sequential points in time and evaluating a treatment strategy. The method provides dynamic tracking of the monitored parameters over time. The method can also include identifying in a substantially real time manner when conditions are favorable for treatment and when conditions are unfavorable for treatment and can verify or quantify how much of a known drug dose or radiation dose was actually received at the tumor. The method can include remote transmission from a non-clinical site to allow oversight of the tumor's condition even during non-active treatment periods (in between active treatments). The disclosure also includes monitoring systems with in situ in vivo biocompatible sensors and telemetry based operations and related computer program products.
Owner:NORTH CAROLINA STATE UNIV +1
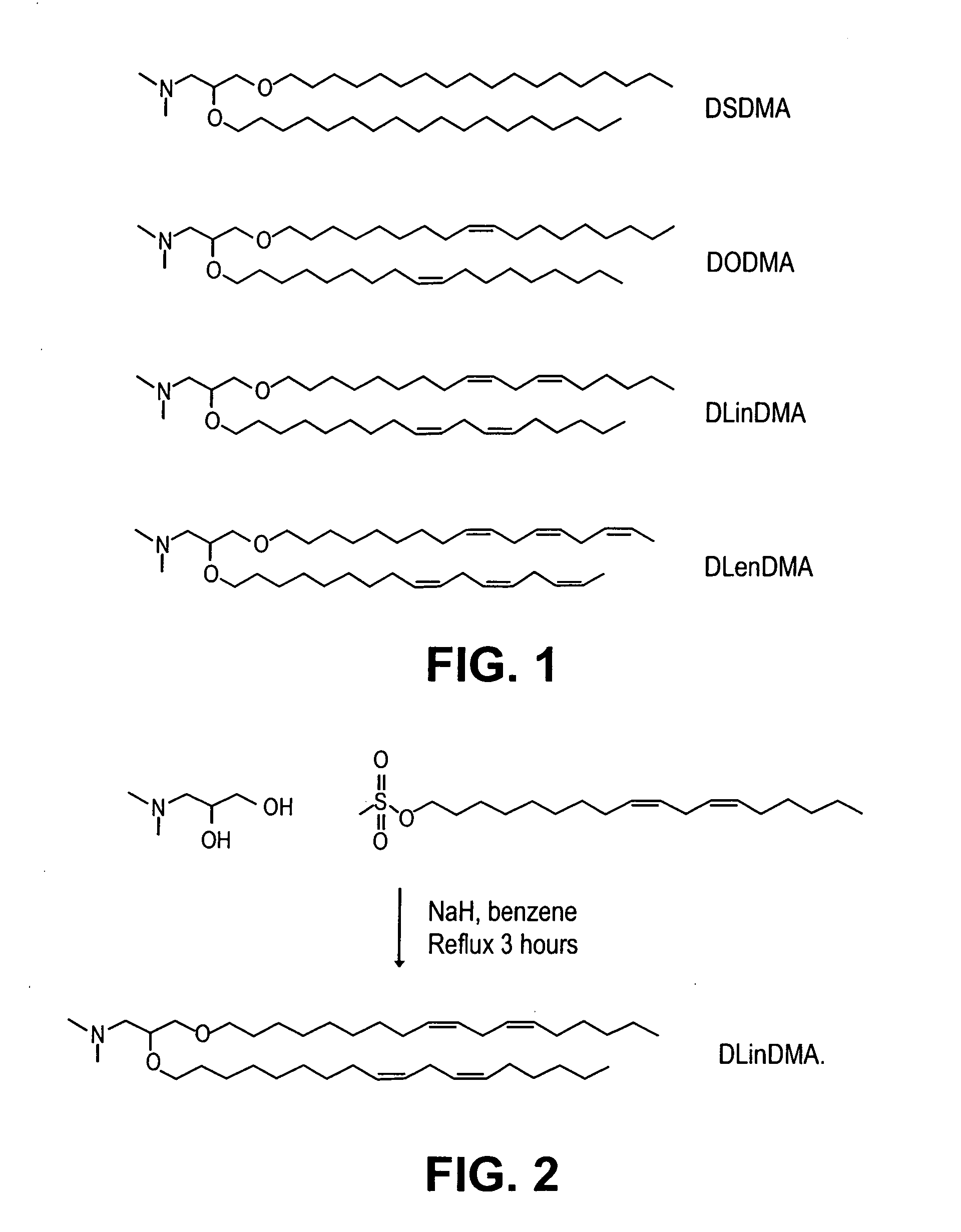
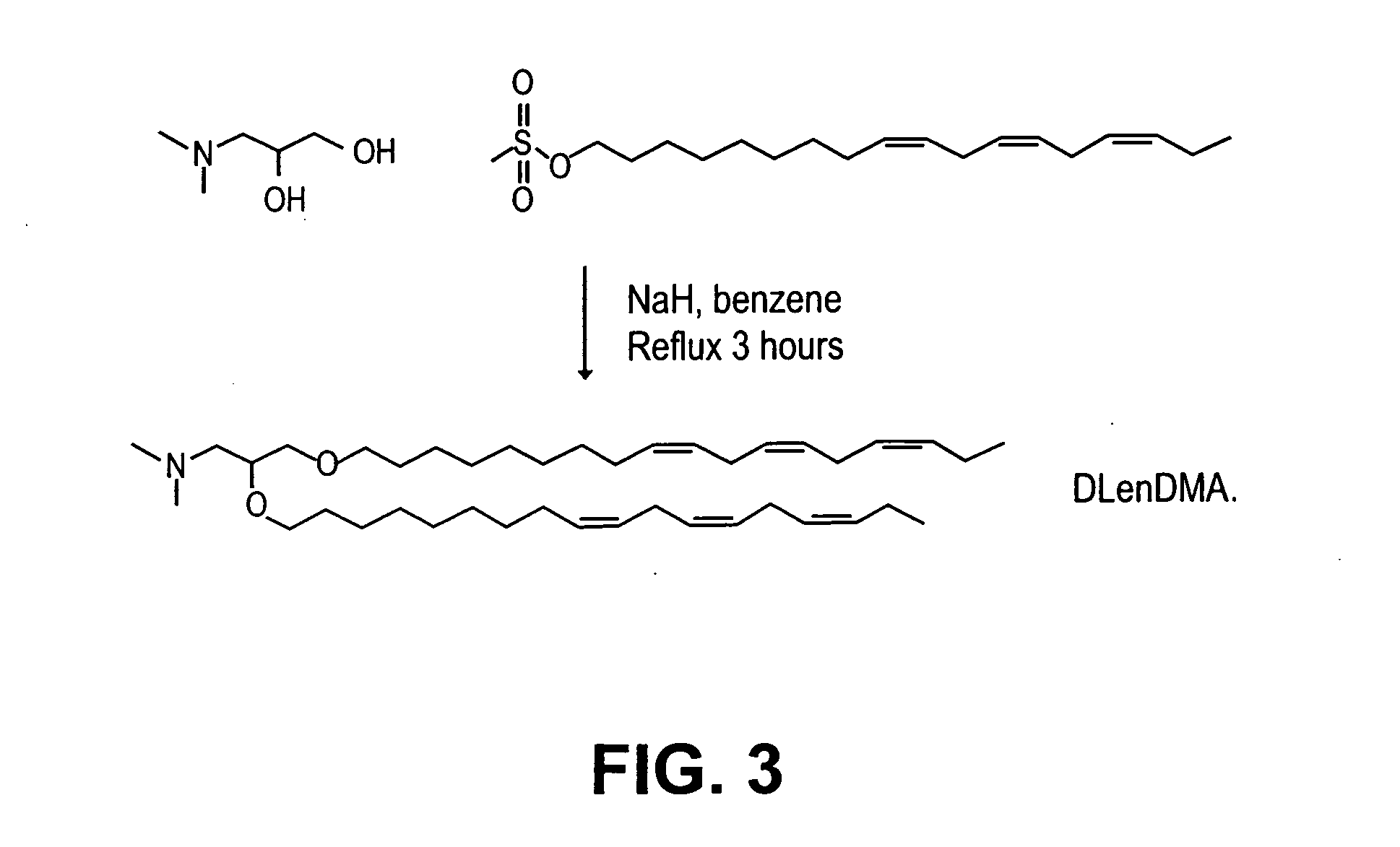
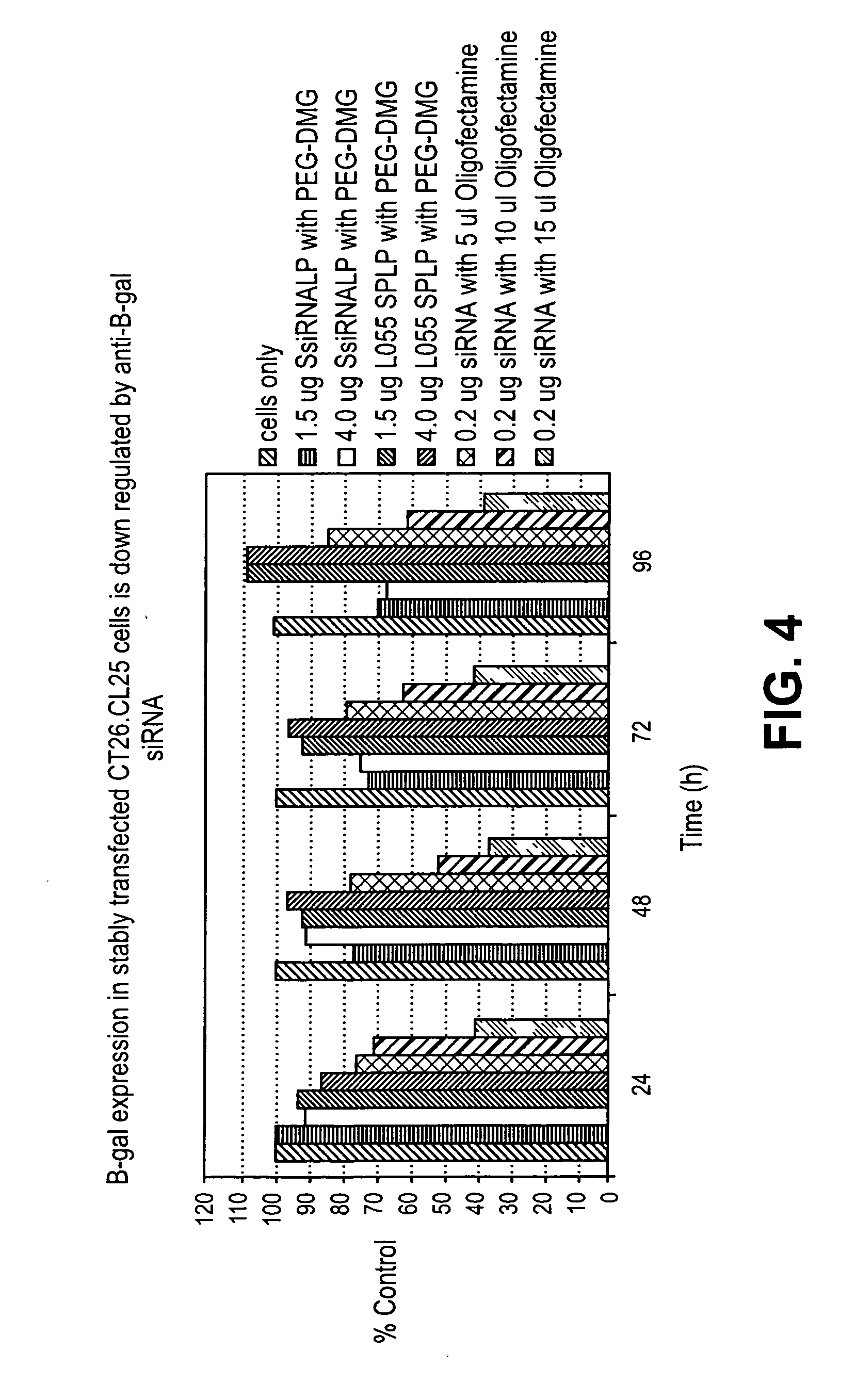
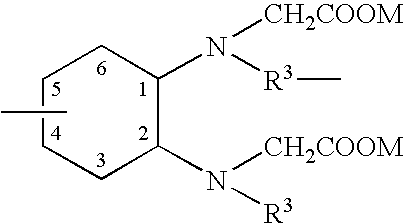
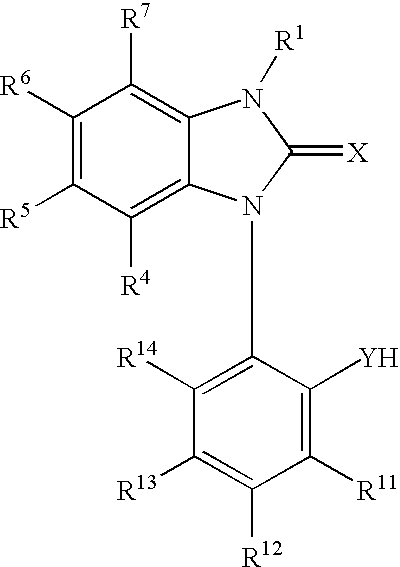
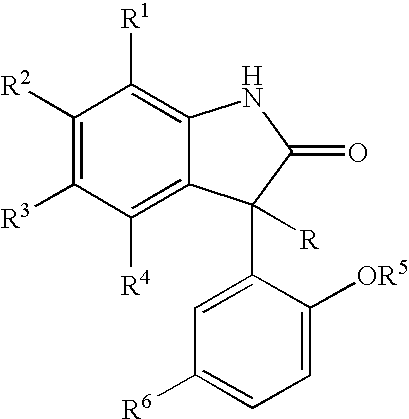
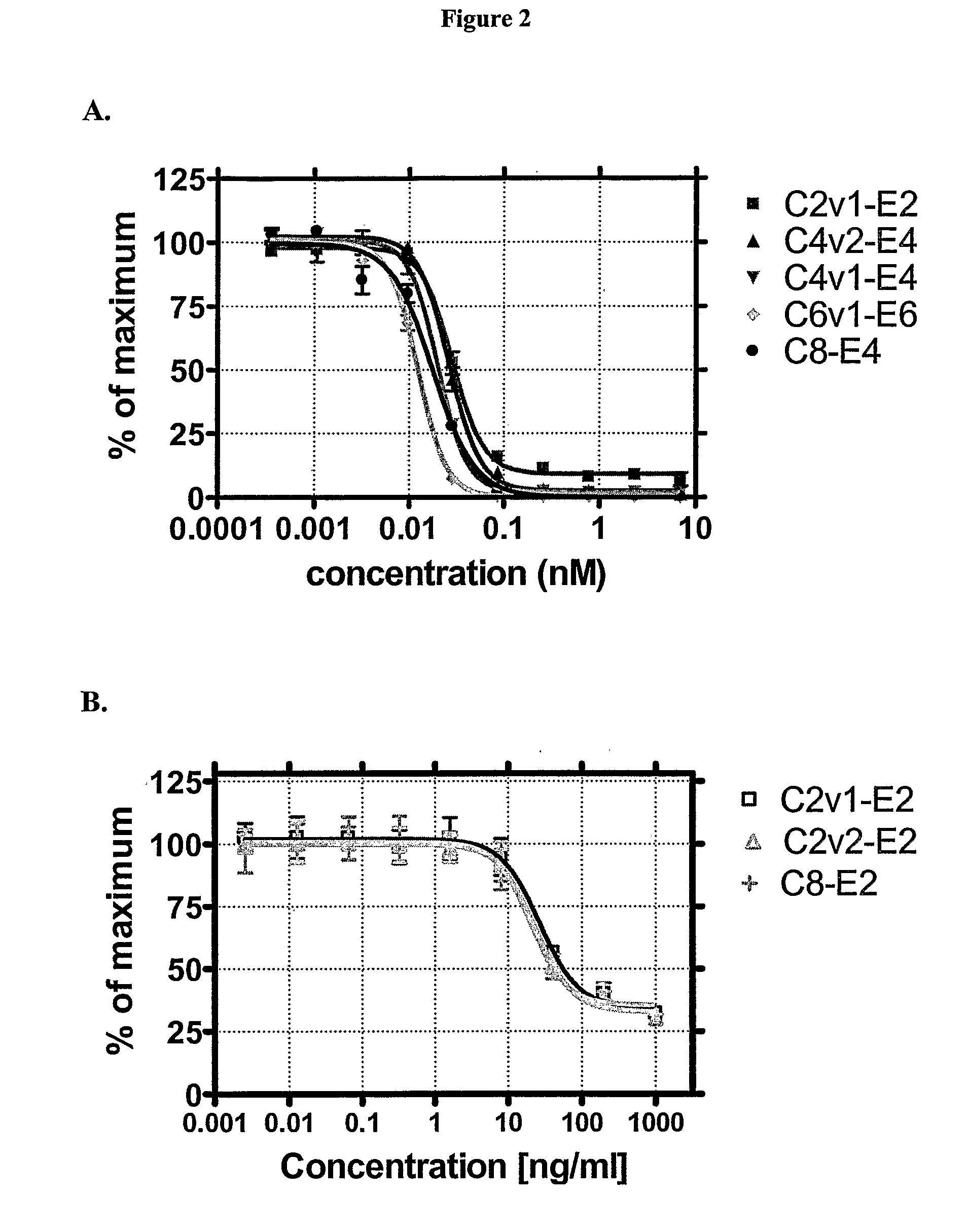
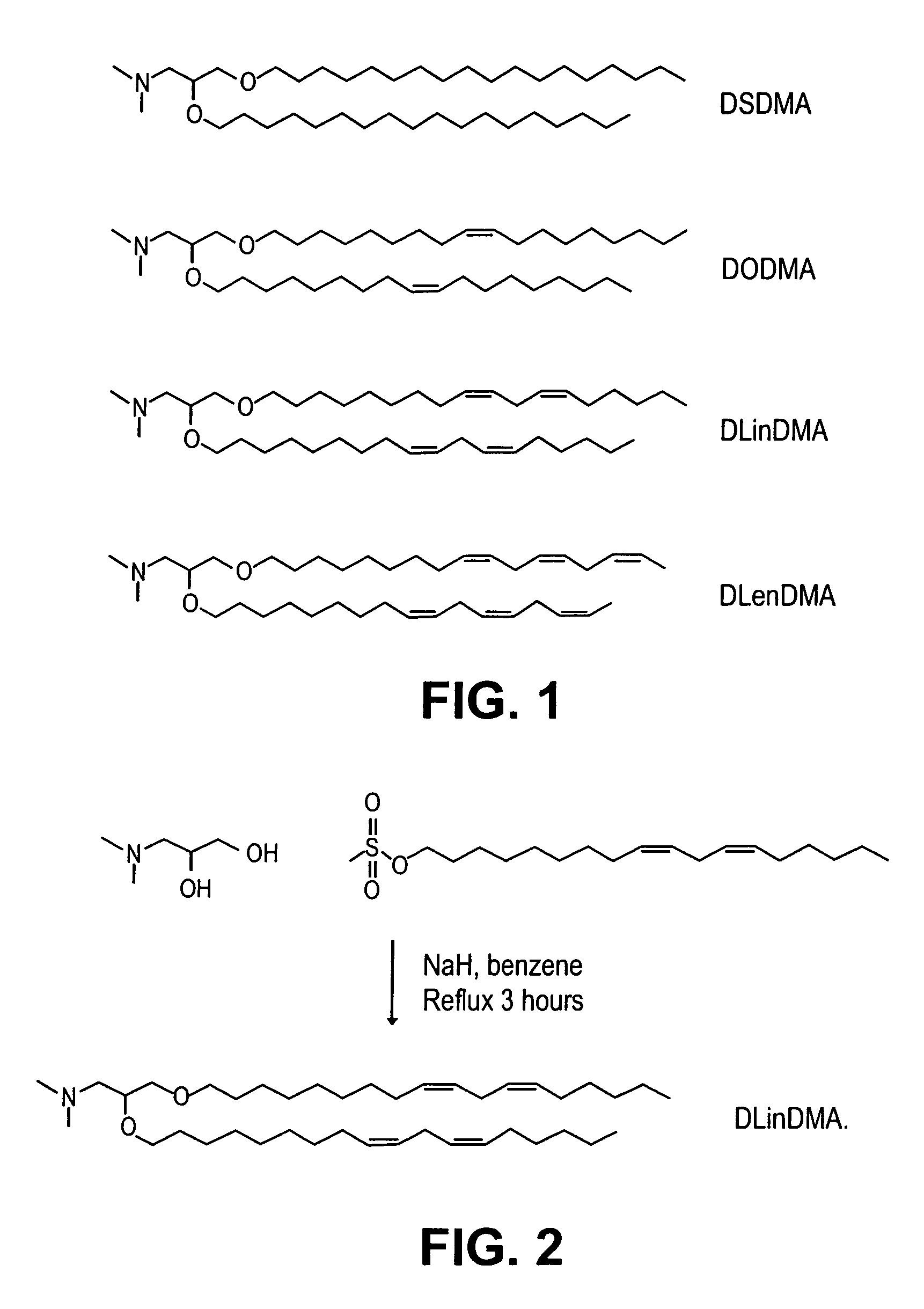
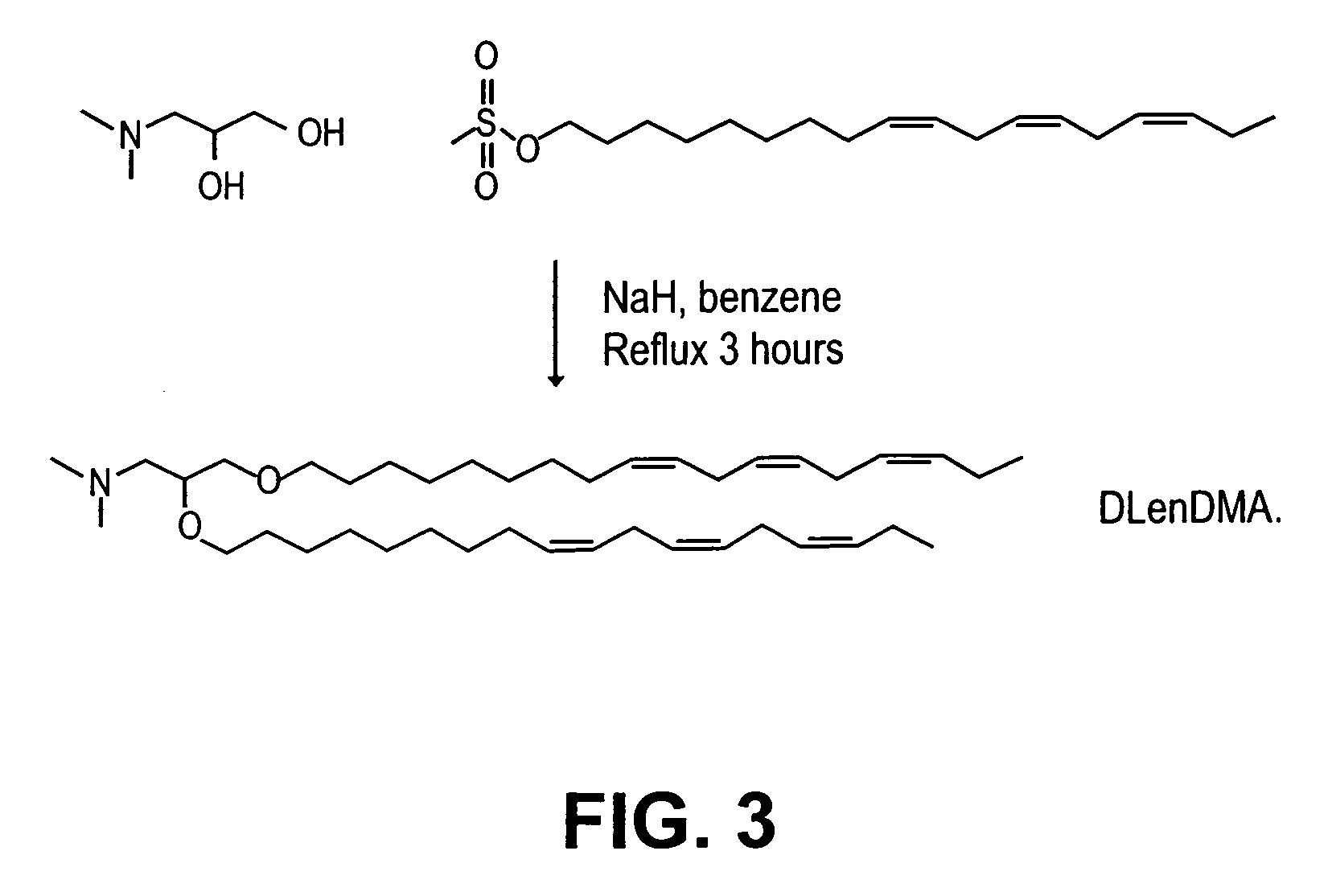
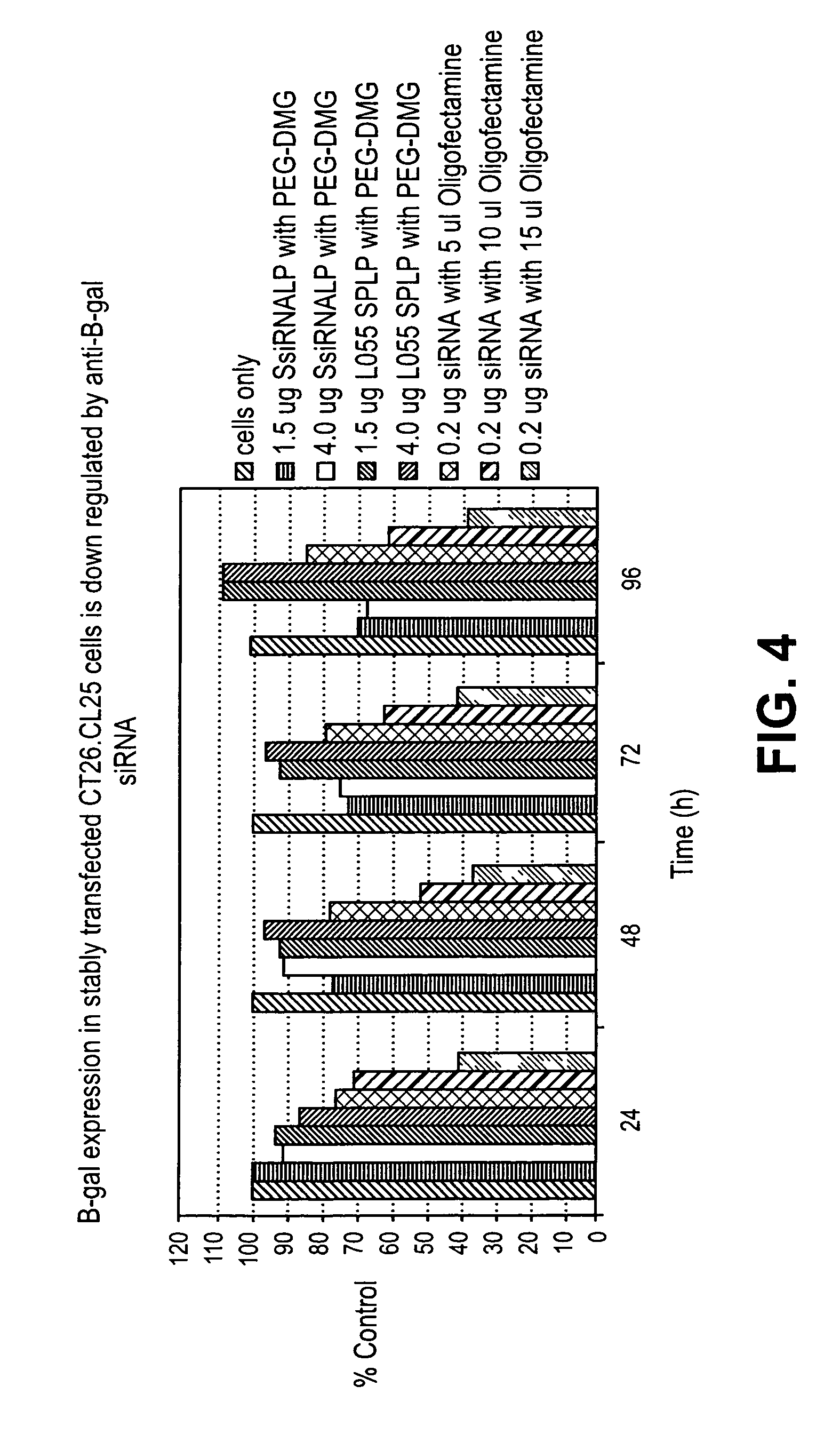
Lipid encapsulated interfering RNA
InactiveUS20060008910A1Inhibit aggregationReduce overexpressionBiocideOrganic active ingredientsLipid formationLipid particle
The present invention provides lipid-based formulations for delivering, e.g., introducing, nucleic acid-lipid particles comprising an interference RNA molecule to a cell, and assays for optimizing the delivery efficiency of such lipid-based formulations.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Potassium channel mediated delivery of agents through the blood-brain barrier
This invention includes pharmaceutical compositions, methods and kits for the treatment or diagnosis of a malignant tumors, including brain tumors, and diseases or disorders characterized by abnormal brain tissue.
Owner:CEDARS SINAI MEDICAL CENT
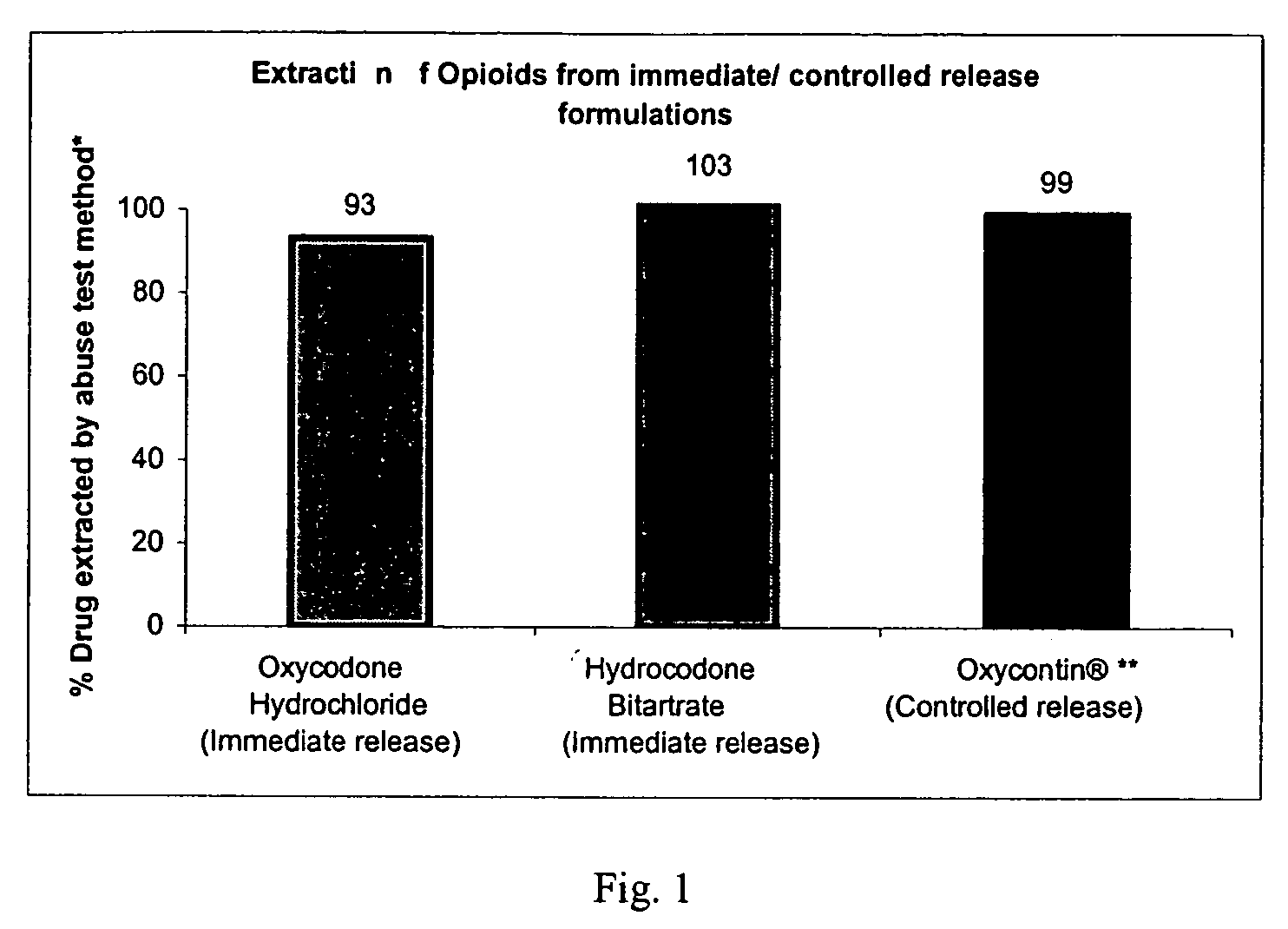
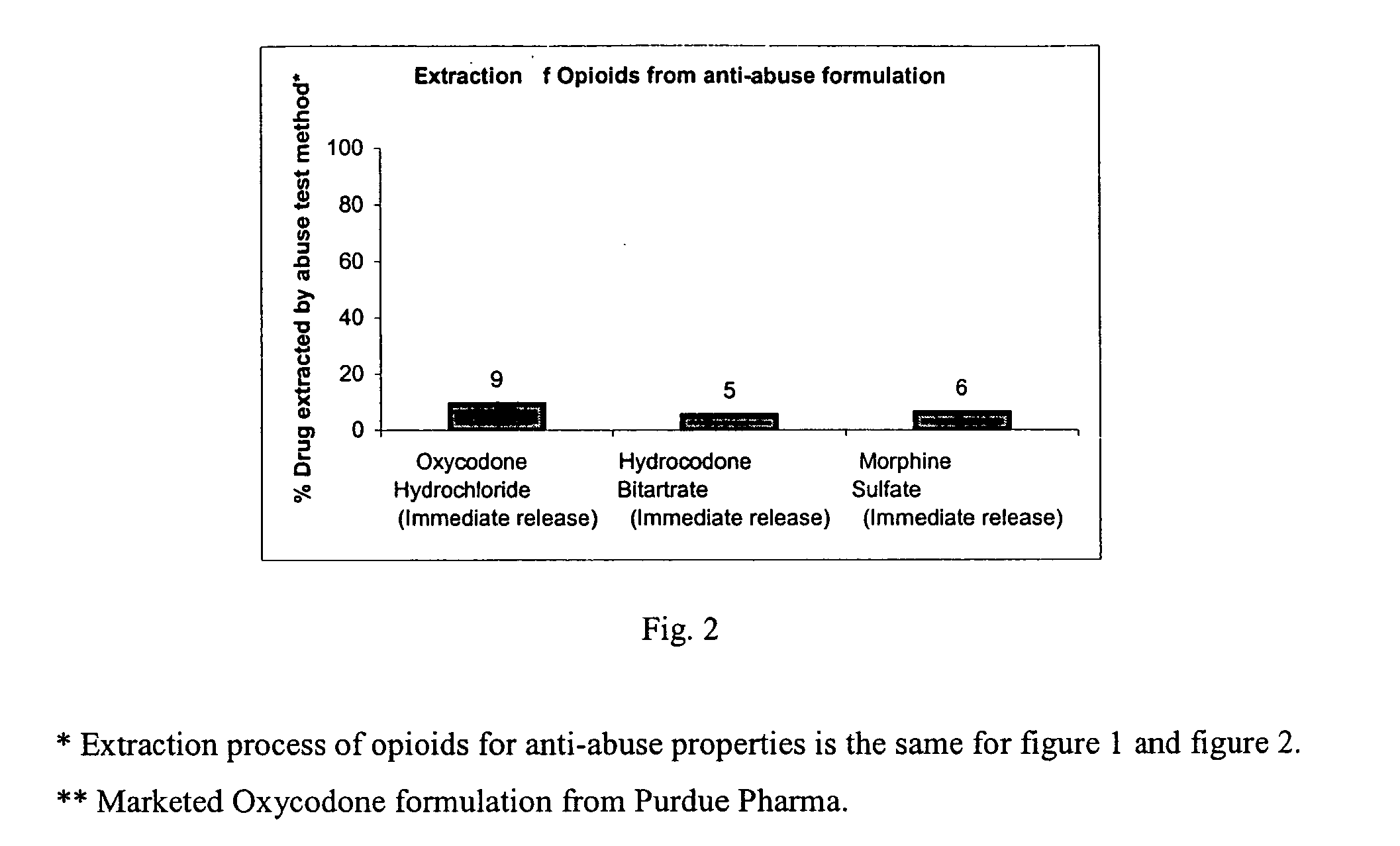
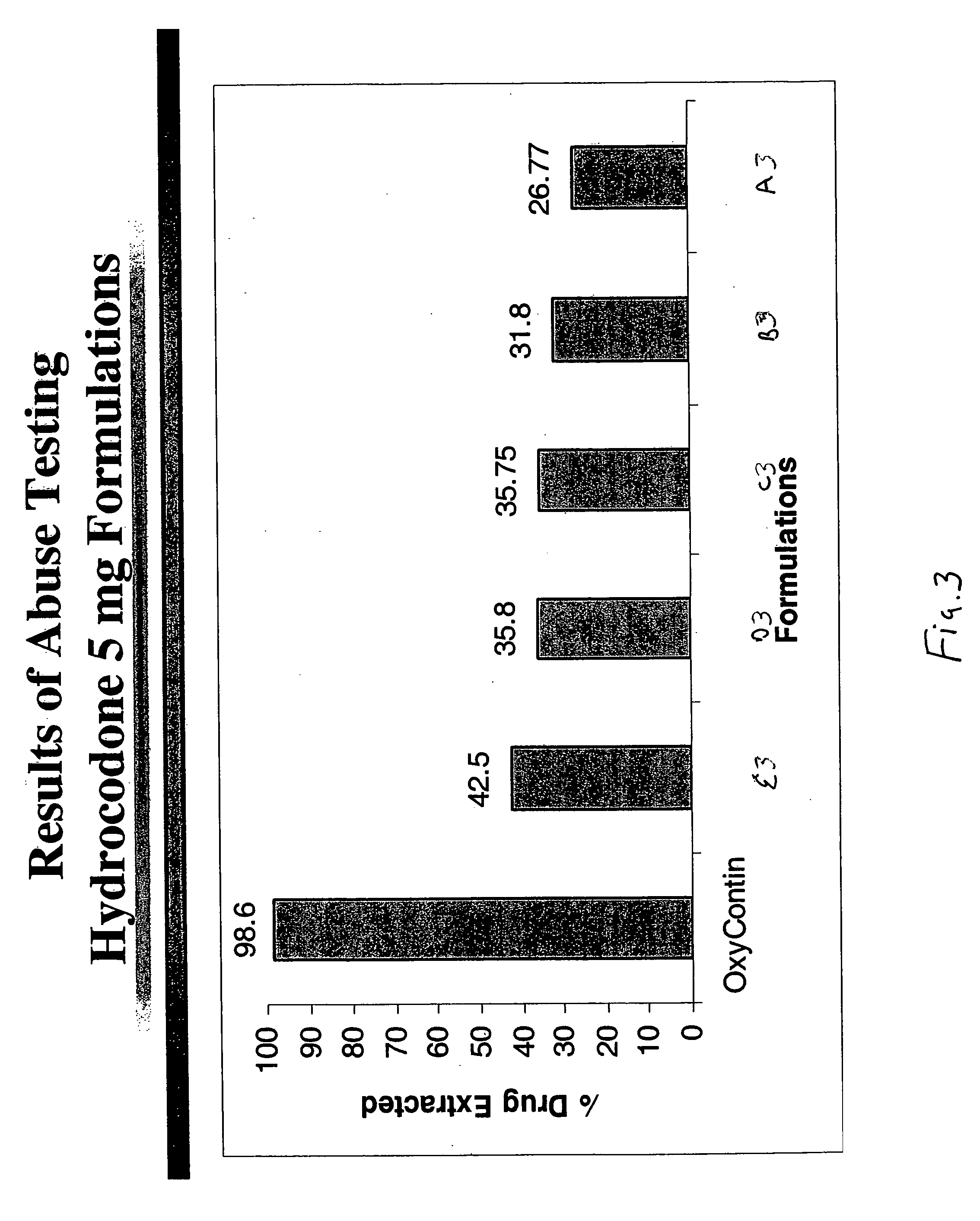
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
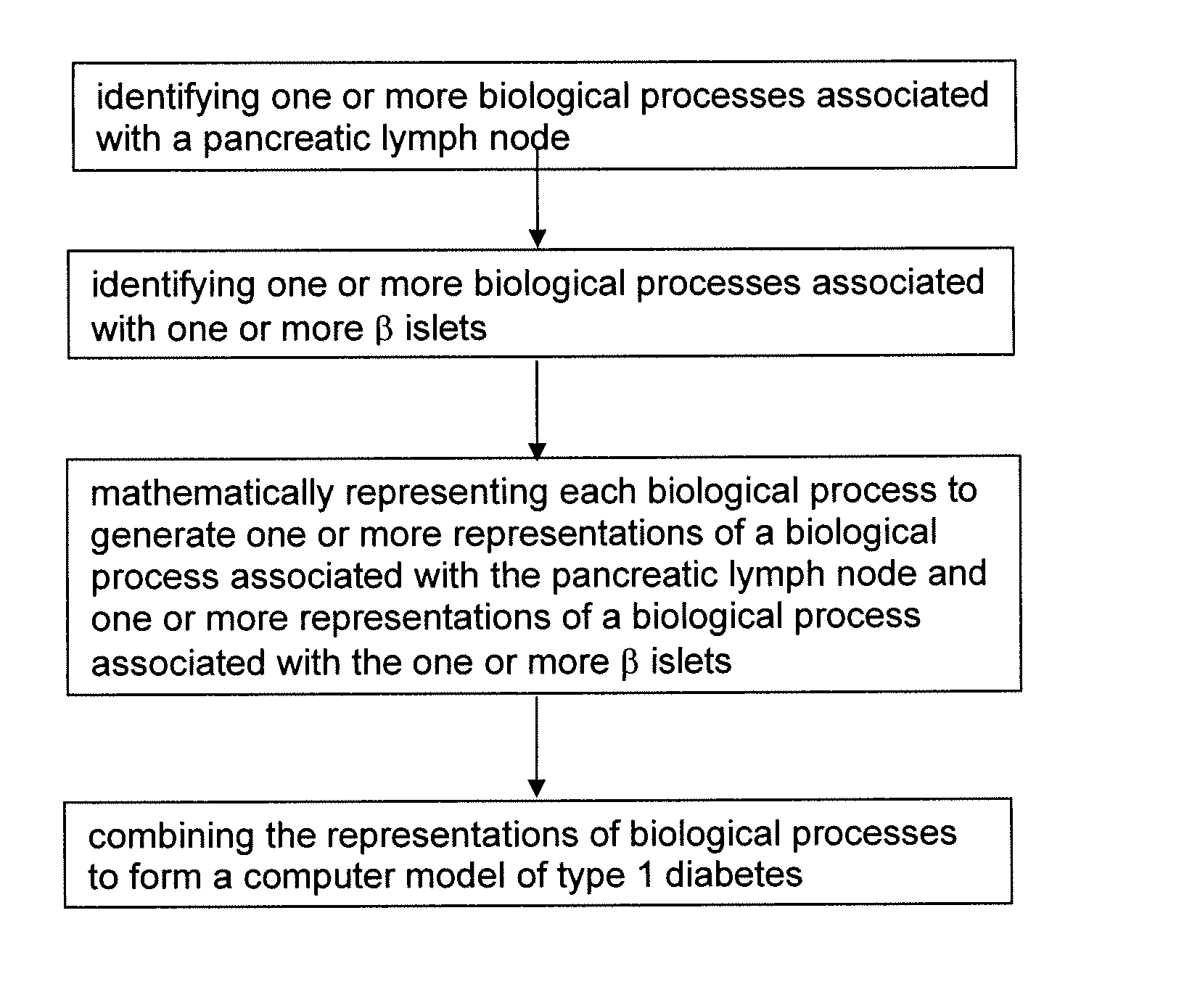
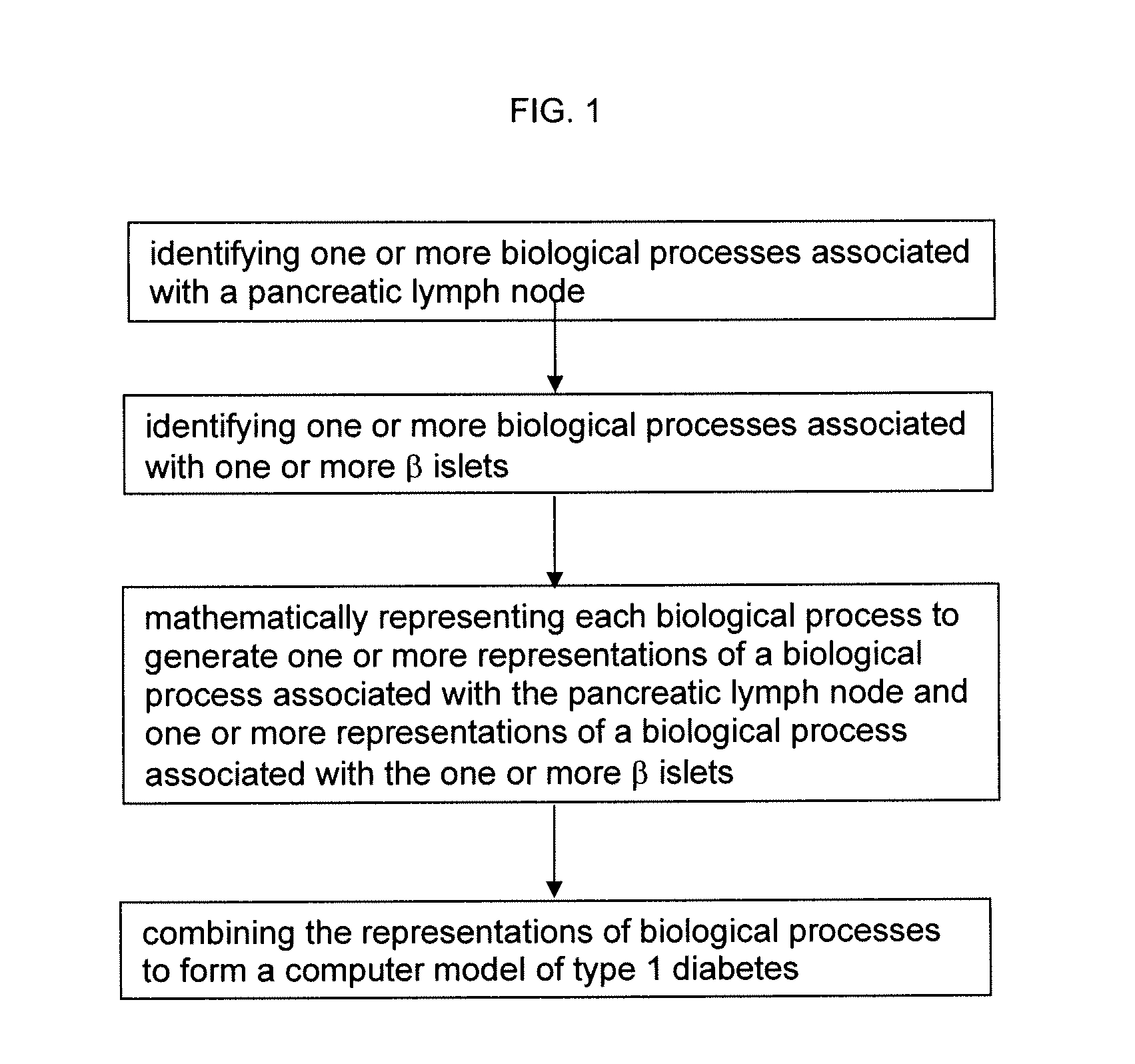
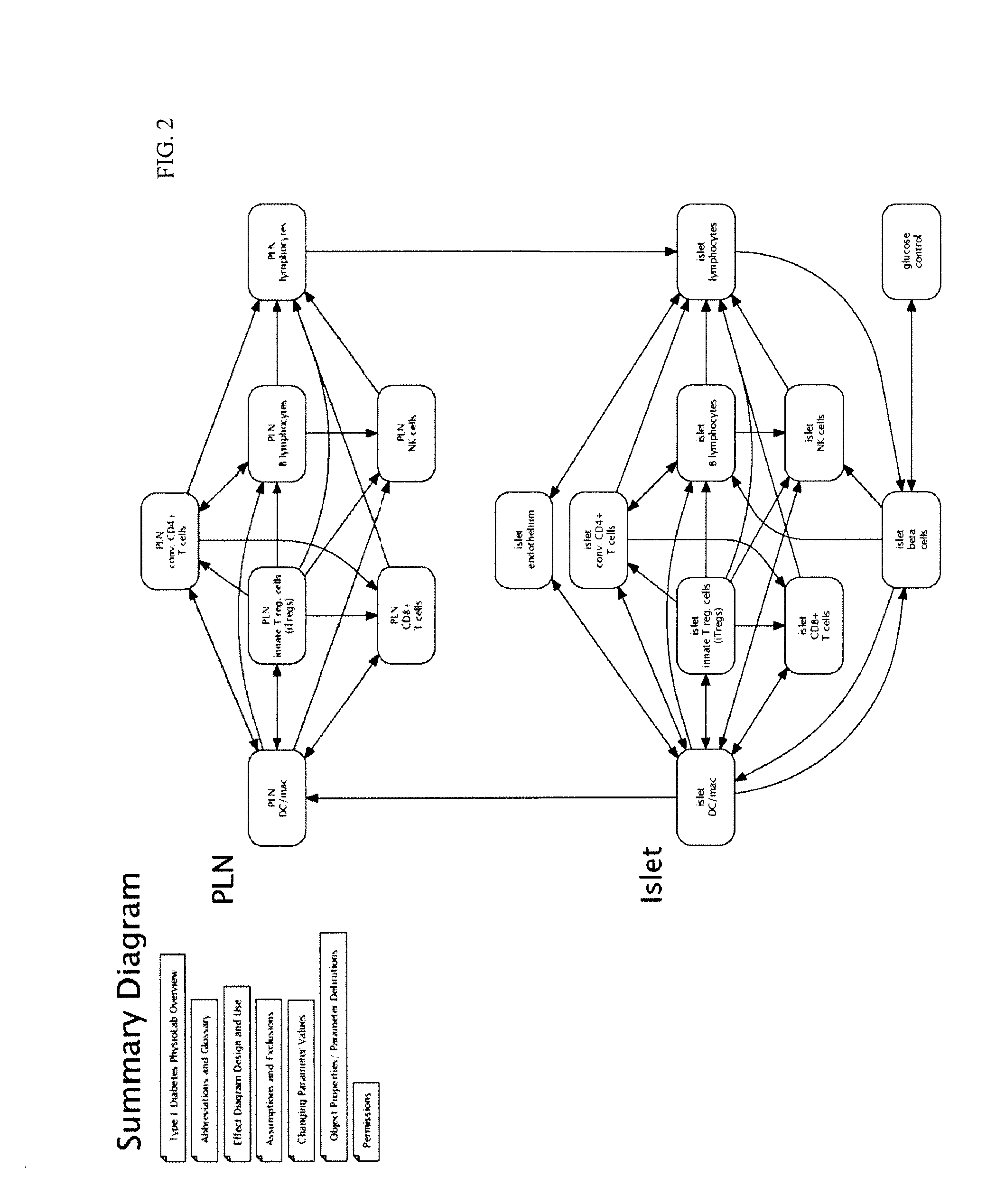
Apparatus and method for computer modeling type 1 diabetes
The invention encompasses novel methods for developing a computer model of type 1 diabetes in a mammal. In particular, the models can include representations of biological processes associated with a pancreatic lymph node and one or more pancreatic islets. Alternatively, the models can include representations of biological processes associated with at least two conditions selected from the group consisting of autoreactive T cell production, autoreactive T cell priming, insulitis and hyperglycemia. The invention also provides methods for developing a computer model of a non-insulin replacement treatment of type 1 diabetes. The invention also encompasses computer models of type 1 diabetes, methods of simulating type 1 diabetes and computer systems for simulating type 1 diabetes and the uses thereof.
Owner:ENTELOS INC
Lipid encapsulated interfering RNA
The present invention provides lipid-based formulations for delivering, e.g., introducing, nucleic acid-lipid particles comprising an interference RNA molecule to a cell, and assays for optimizing the delivery efficiency of such lipid-based formulations.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Non-invasive localization of a light-emitting conjugate in a mammal
InactiveUS6217847B1Accurate measurementEvenly distributedUltrasonic/sonic/infrasonic diagnosticsBacteriaMammalNon invasive
Methods and compositions for detecting and localizing light originating from a mammal are disclosed. Also disclosed are methods for tracking light emission to selected regions, as well as for tracking entities within the mammal. In addition, animal models for disease states are disclosed, as are methods for localizing and tracking the progression of disease or a pathogen within the animal, and for screening putative therapeutic compounds effective to inhibit the disease or pathogen.
Owner:LELAND STANFORD JUNIOR UNIV OF THE BOARD OF TRUSTEES THE
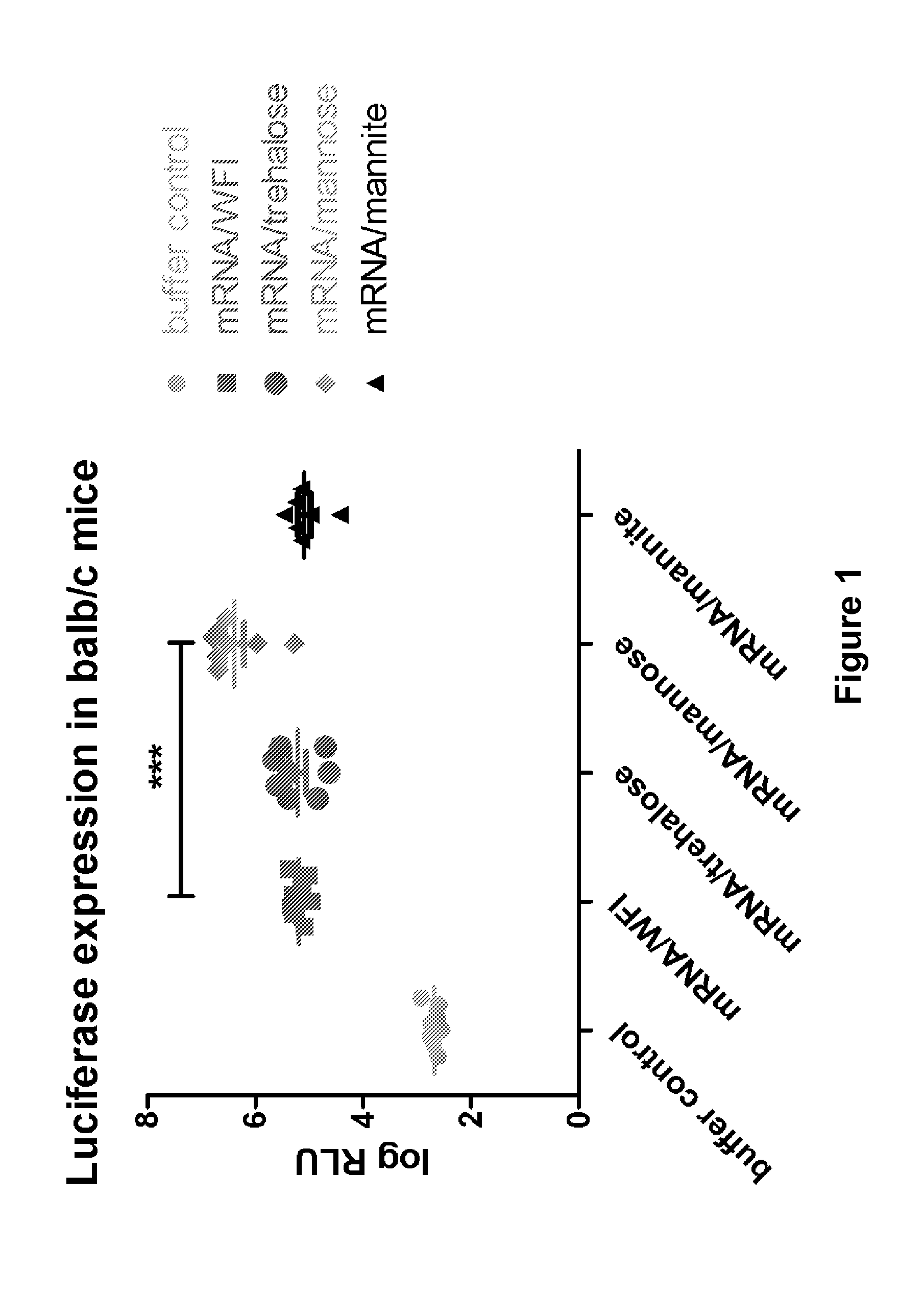
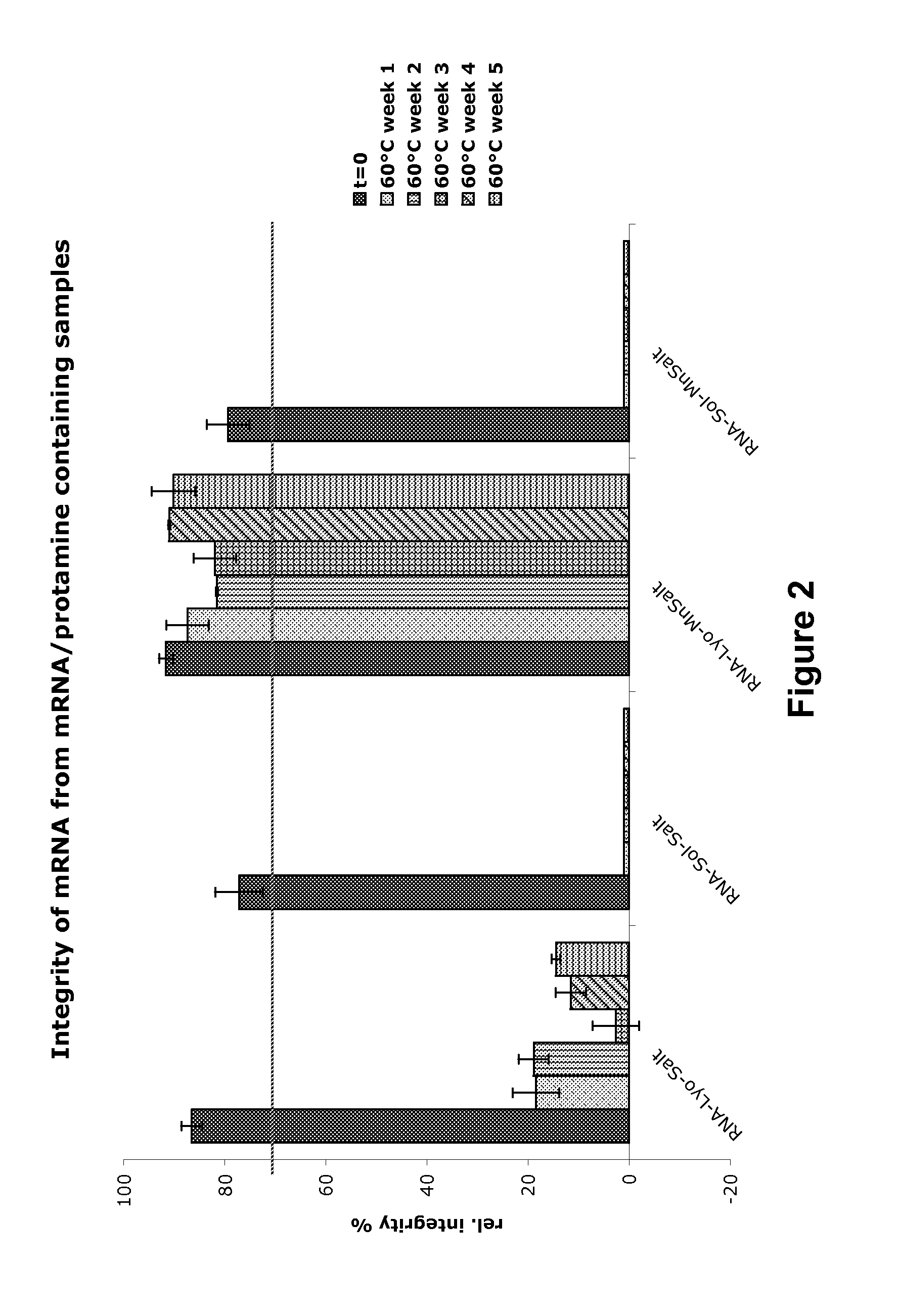
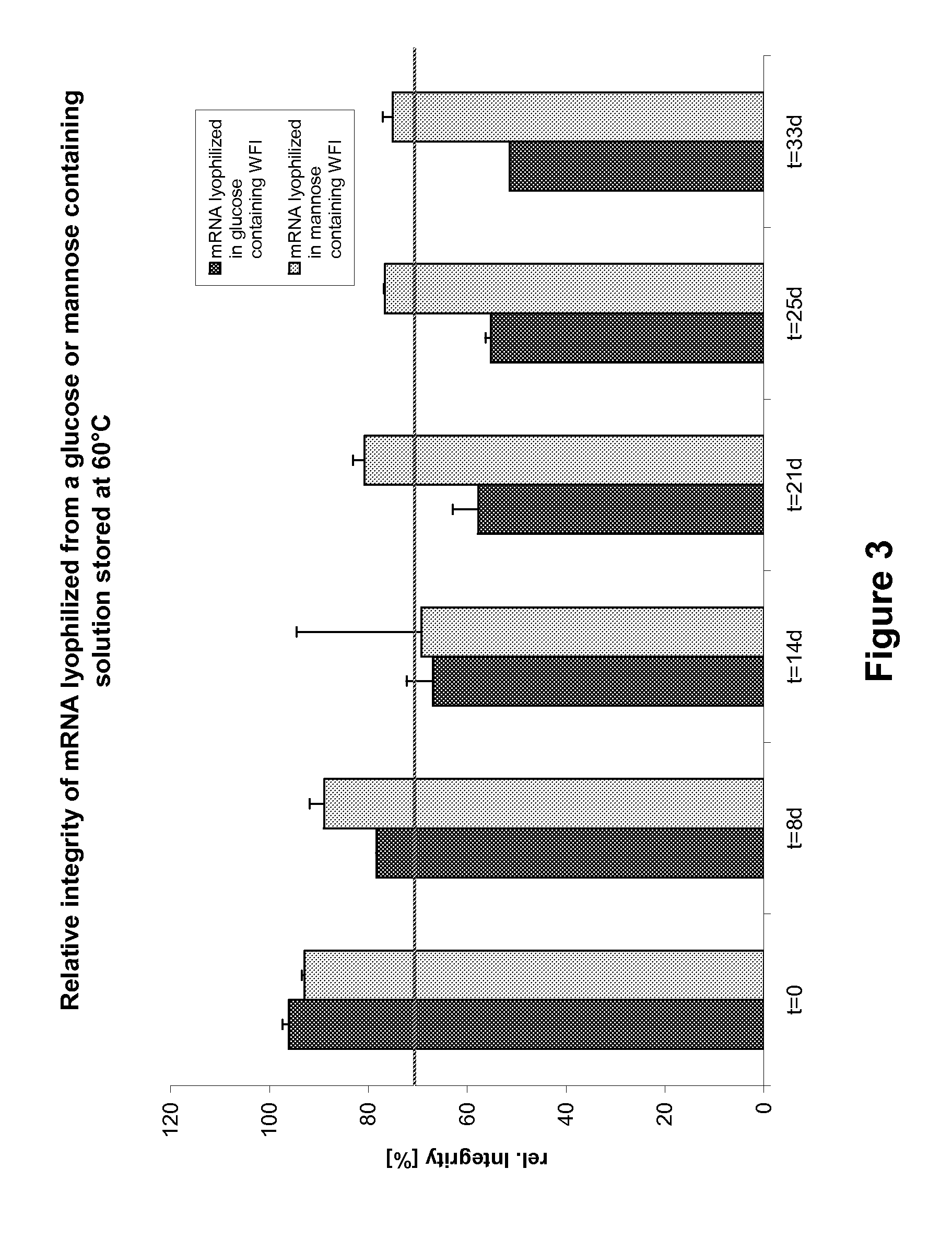
Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids
InactiveUS20120258046A1High transfection efficiencyEnhance protein expressionOrganic active ingredientsGenetic material ingredientsIn vivoTransfection
The present invention is directed to (the use of) a solution containing at least one nucleic acid (sequence) and free mannose for lyophilization, transfection and / or injection, particularly of RNA and mRNA. The inventive solution exhibits a positive effect on stabilization of the nucleic acid (sequence) during lyophilization and storage but also leads to a considerable increase of the transfection efficiency of a nucleic acid. It thus also increases in vivo expression of a protein encoded by such a nucleic acid upon increased transfection rate. The present invention is furthermore directed to a method of lyophilization using the mannose-containing solution, to pharmaceutical compositions, vaccines, kits, first and second medical uses applying such a mannose-containing solution and / or a nucleic acid (sequence) lyophilized or resuspended with such a solution.
Owner:CUREVAC SE
Abuse-proofed dosage form
ActiveUS8114383B2Pulverisation of the dosage form is considerably more difficultComplicating or preventing the subsequent abuseOrganic active ingredientsPowder deliveryBreaking strengthPhysiology
The present invention relates to an abuse-proofed, thermoformed dosage form containing, in addition to one or more active ingredients with abuse potential optionally together with physiologically acceptable auxiliary substances, at least one synthetic or natural polymer with a breaking strength of at least 500 N and to a process for the production thereof.
Owner:GRUNENTHAL GMBH
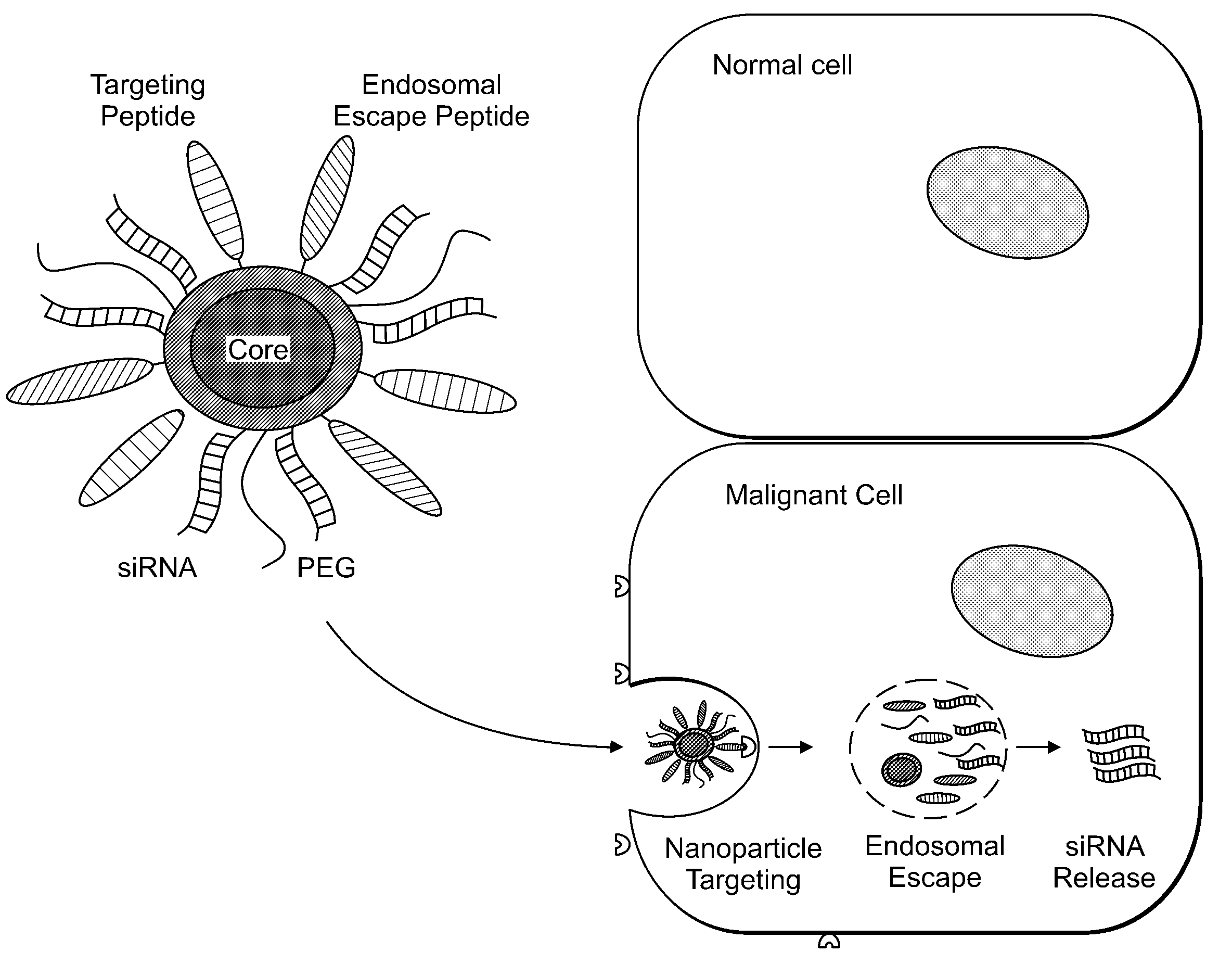
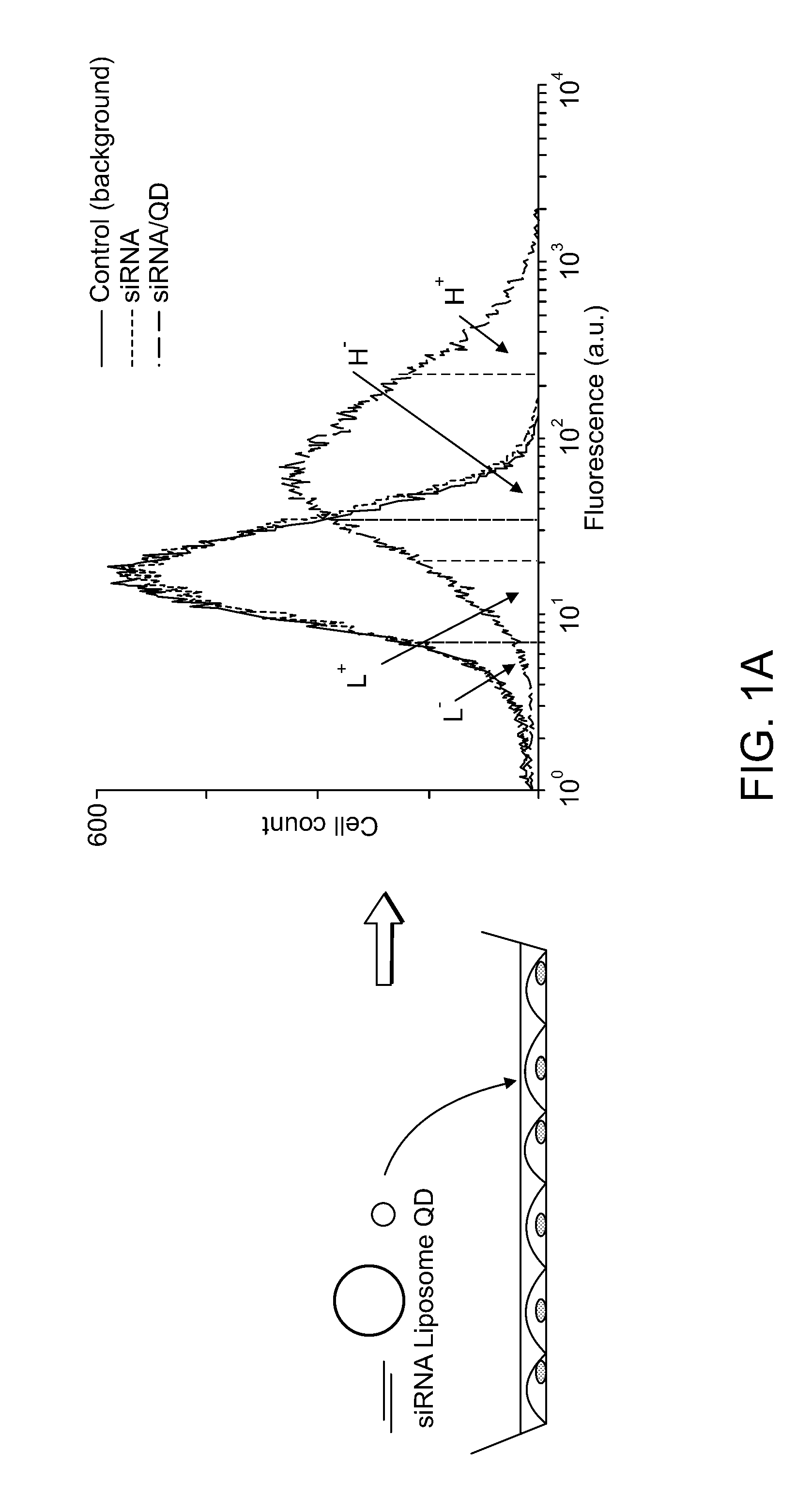
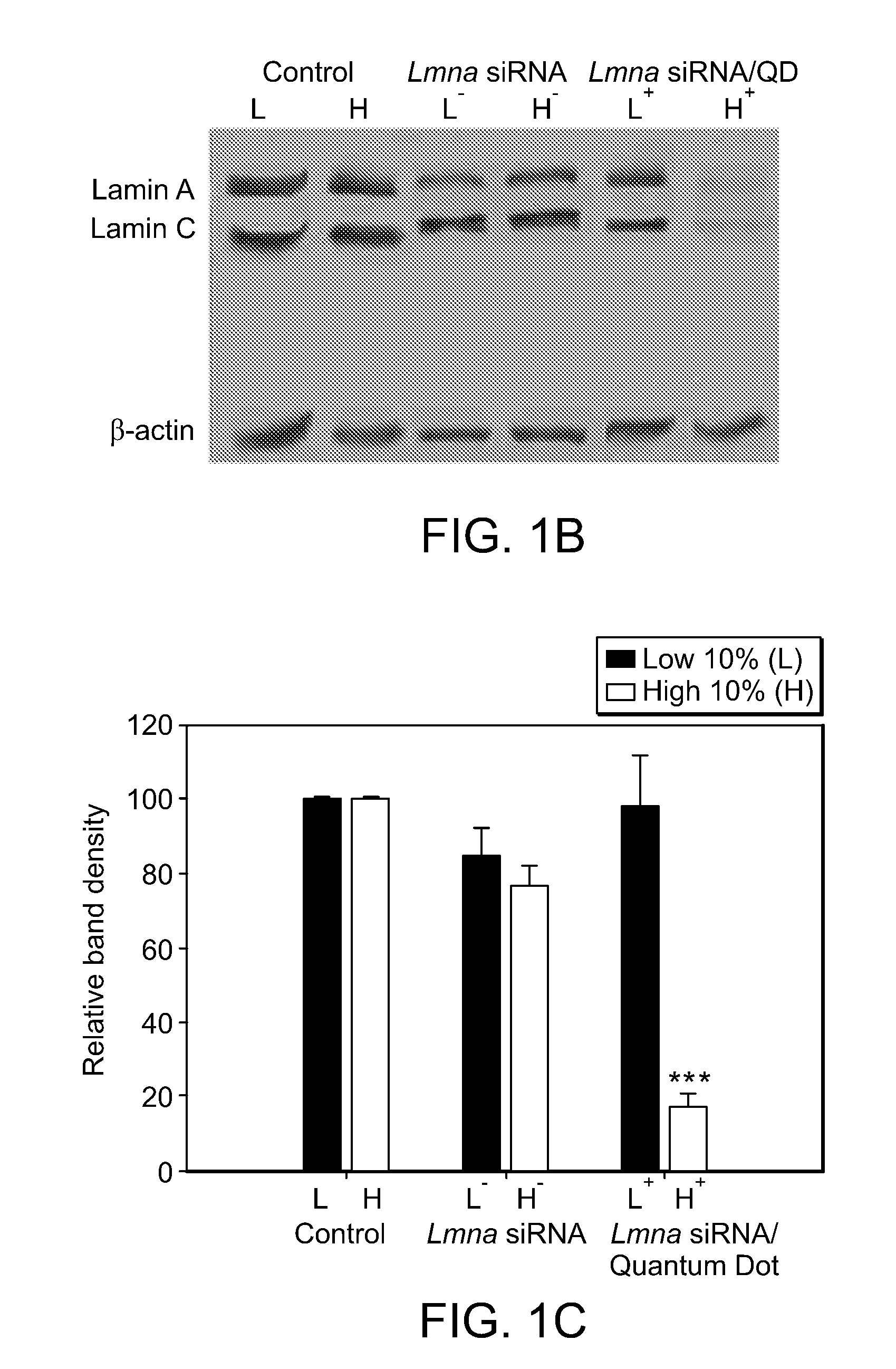
Delivery of Nanoparticles and/or Agents to Cells
InactiveUS20080213377A1Extended circulation timeReduce degradationPowder deliverySugar derivativesDiagnostic agentNanoparticle
The present invention provides systems, methods, and compositions for targeted delivery of nanoparticles and / or agents to tissues, cells, and / or subcellular locales. In general, compositions comprise a nanoparticle (e.g. quantum dot, polymeric particle, etc.), at least one modulating entity (such as a targeting moiety, transfection reagent, protective entity, etc.), and at least one agent to be delivered (e.g. therapeutic, prophylactic, and / or diagnostic agent). The present invention provides methods of making and using nanoparticle entities in accordance with the present invention.
Owner:MASSACHUSETTS INST OF TECH
Fusion of multiple imaging planes for isotropic imaging in MRI and quantitative image analysis using isotropic or near-isotropic imaging
ActiveUS7634119B2Improve imaging resolutionHigh resolutionCharacter and pattern recognitionDiagnostic recording/measuringImaging analysisComputer vision
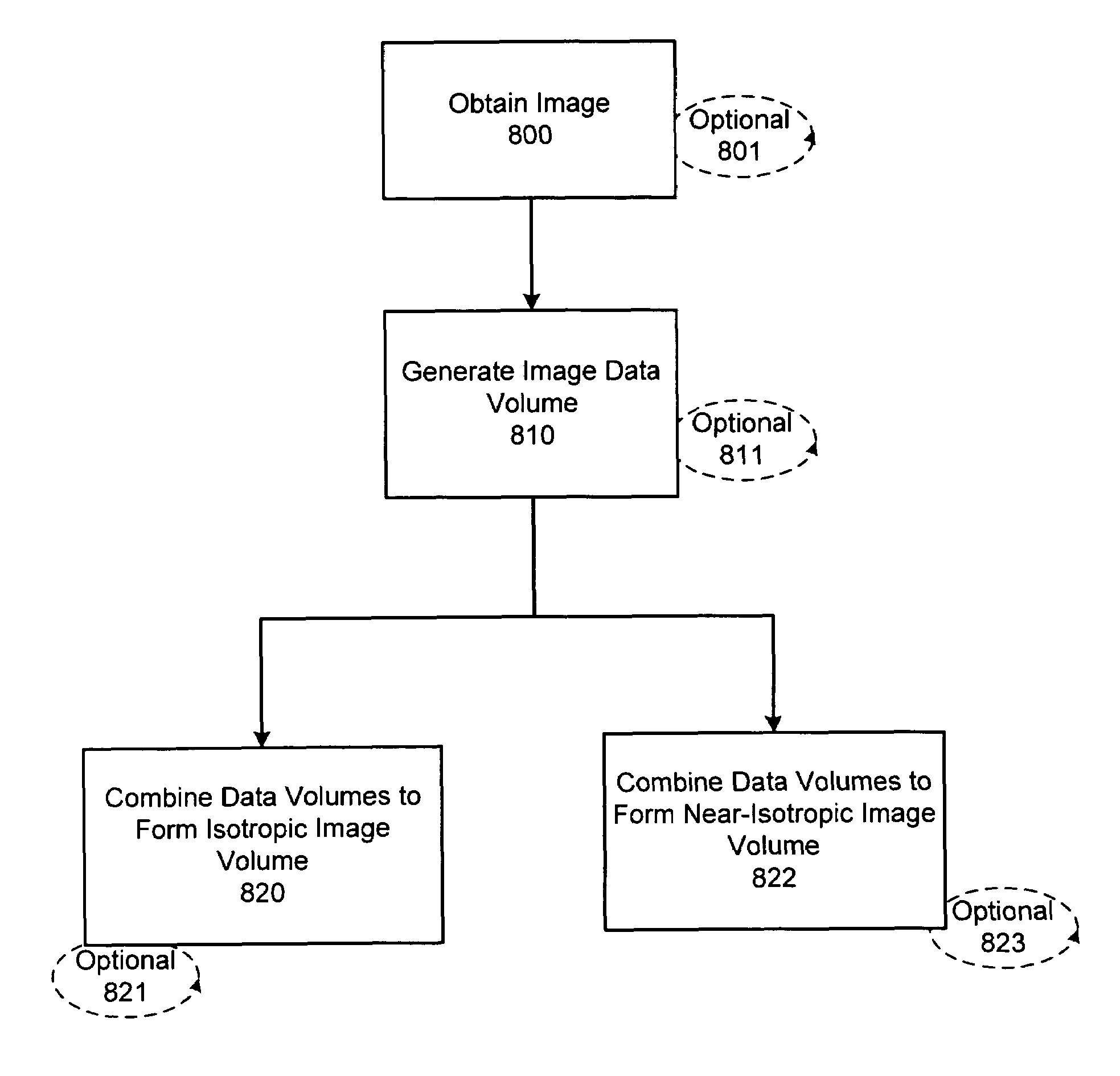
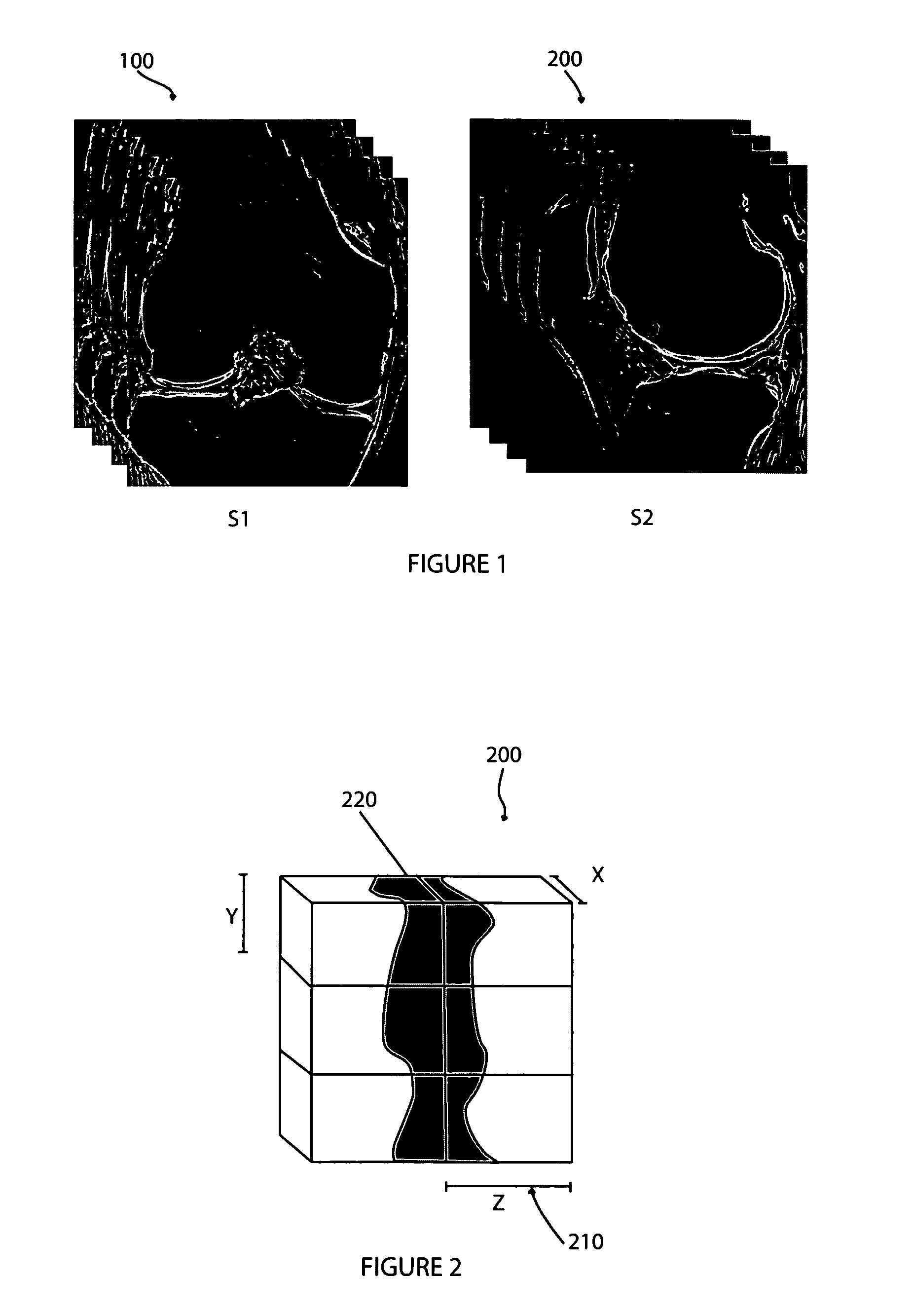
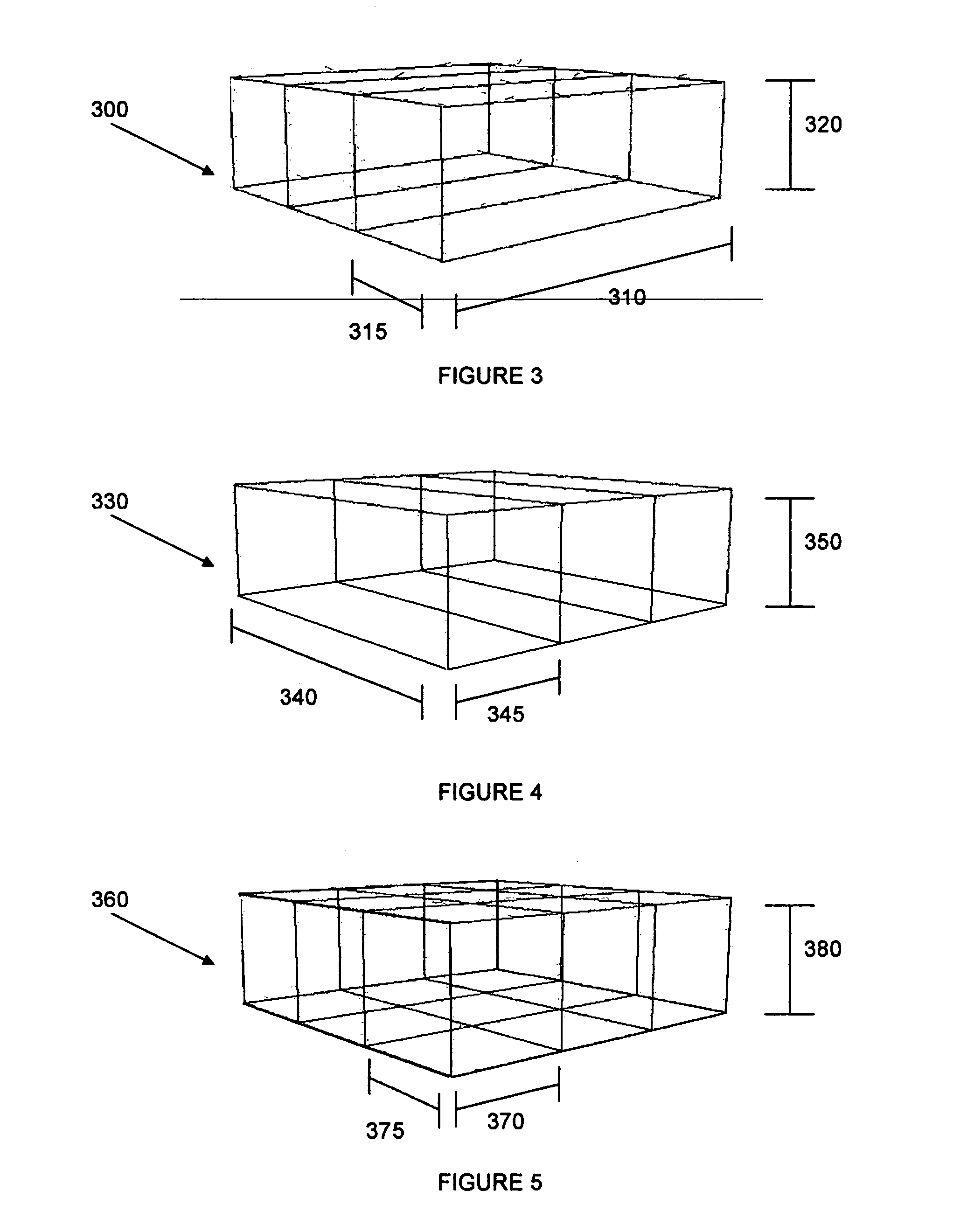
In accordance with the present invention there is provided methods for generating an isotropic or near-isotropic three-dimensional images from two-dimensional images. In accordance with the present invention the method includes, obtaining a first image of a body part in a first plane, wherein the first image generates a first image data volume; obtaining a second image of the body part in a second plane, wherein the second image generates a second image data volume; and combining the first and second image data volumes to form a resultant image data volume, wherein the resultant image data volume is isotropic or near-isotropic.
Owner:CONFORMIS
Antibodies to insulin-like growth factor I receptor
Owner:AMGEN FREMONT INC +1
Rodent HER2 tumor model
The invention concerns HER<HIL><PDAT>2< / BOLD><PDAT>-transgenic non-human mammals, animal models for screening drug candidates for the treatment of diseases and disorders associated with the overexpression of HER<HIL><PDAT>2< / BOLD><PDAT>. In particular, the invention concerns animal models designed to test drug candidates for the treatment of HER<HIL><PDAT>2< / BOLD><PDAT>-overexpressing cancers, including breast cancer, that are not responding or poorly responding to current treatments.< / PTEXT>
Owner:SAN VALLEY SYST +1
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Rapid Diffusion of Large Polymeric Nanoparticles in the Mammalian Brain
ActiveUS20130183244A1Reduce deliveryHigher drug payloadPowder deliveryNervous disorderGene deliveryHydrophilic coating
Non-adhesive particles as large as 110 nm can diffuse rapidly in the brain ECS, if coated with hydrophilic coatings such as PEG coatings and preferably having neutral surface charge. The ability to achieve brain penetration with larger particles will significantly improve drug and gene delivery within the CNS since larger particles offer higher drug payload, improved drug loading efficiency, and significantly longer drug release durations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Nanoparticle conjugates
InactiveUS20060246524A1Easy to detectMaterial nanotechnologyPowder deliveryIn situ hybridisationOrganic chemistry
Conjugate compositions are disclosed that include a specific-binding moiety covalently coupled to a nanoparticle through a heterobifunctional polyalkyleneglycol linker. In one embodiment, a conjugates is provided that includes a specific-binding moiety and a fluorescent nanoparticle coupled by a heterobifunctional PEG linker. Fluorescent conjugates according to the disclosure can provide exceptionally intense and stable signals for immunohistochemical and in situ hybridization assays on tissue sections and cytology samples, and enable multiplexing of such assays.
Owner:VENTANA MEDICAL SYST INC
Abuse-safeguarded dosage form
A pharmaceutical dosage form that is safeguarded against abuse containing at least one active substance that is susceptible to abuse and at least two of the following constituents (a) through (d): (a) at least one substance that irritates the nasal and / or pharyngeal region; (b) at least one viscosity increasing agent that together with a required minimum quantity of an aqueous liquid forms a gel in an extract obtained from the dosage form, which gel can still be discerned after being introduced into an additional quantity of aqueous liquid; (c) at least one antagonist for the at least one active substance that is susceptible to abuse; and (d) at least one emetic.
Owner:GRUNENTHAL GMBH
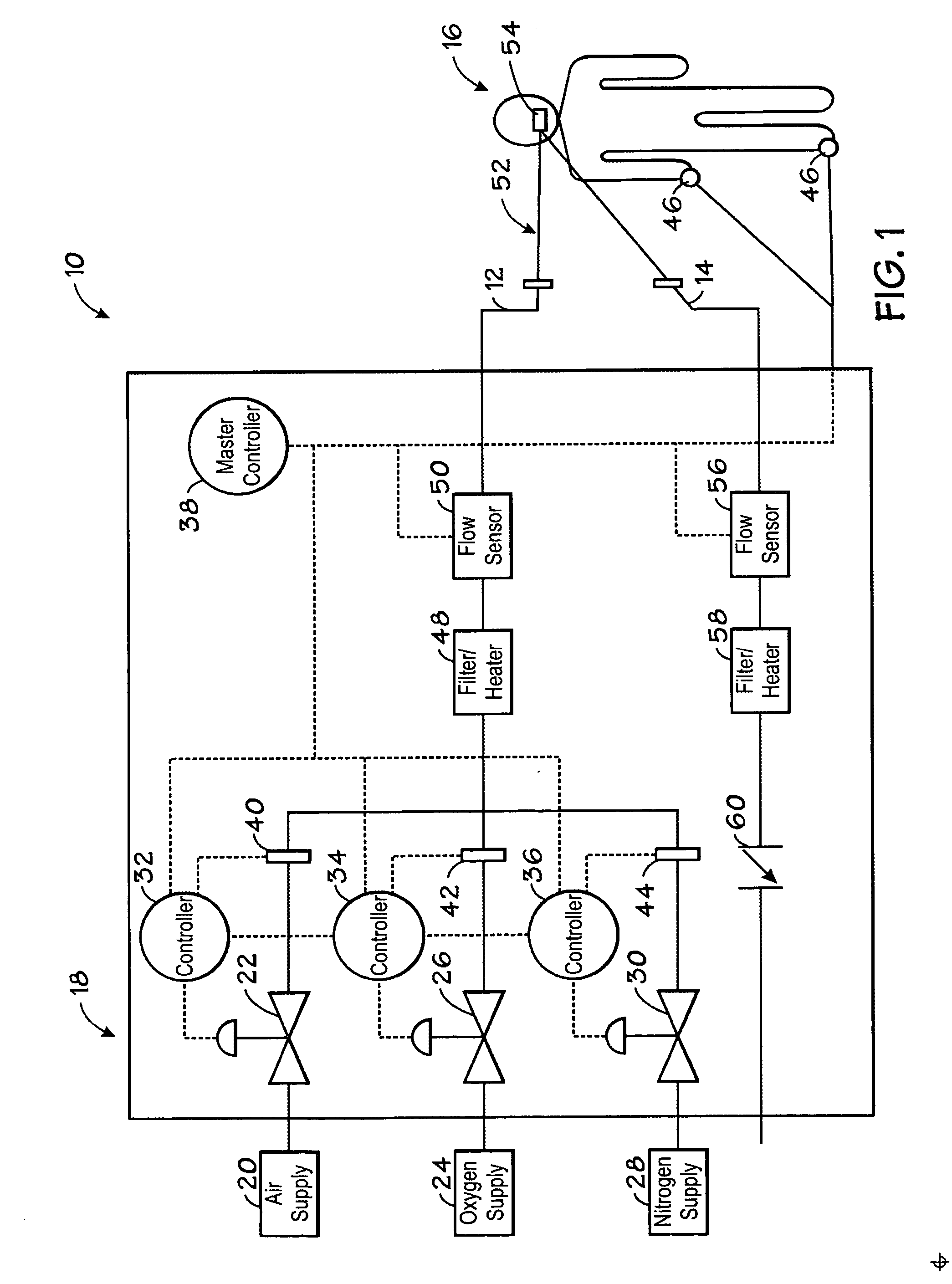
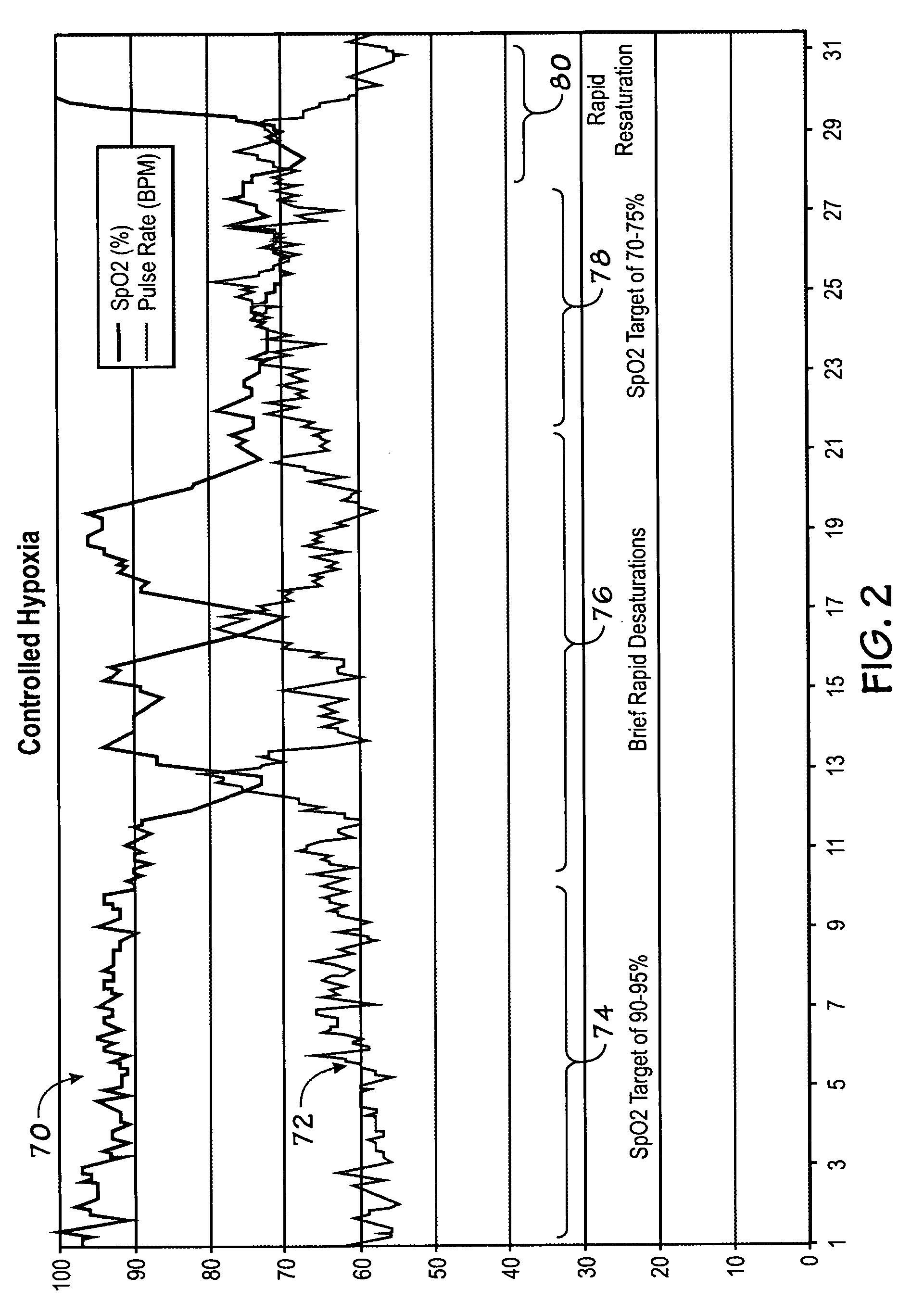
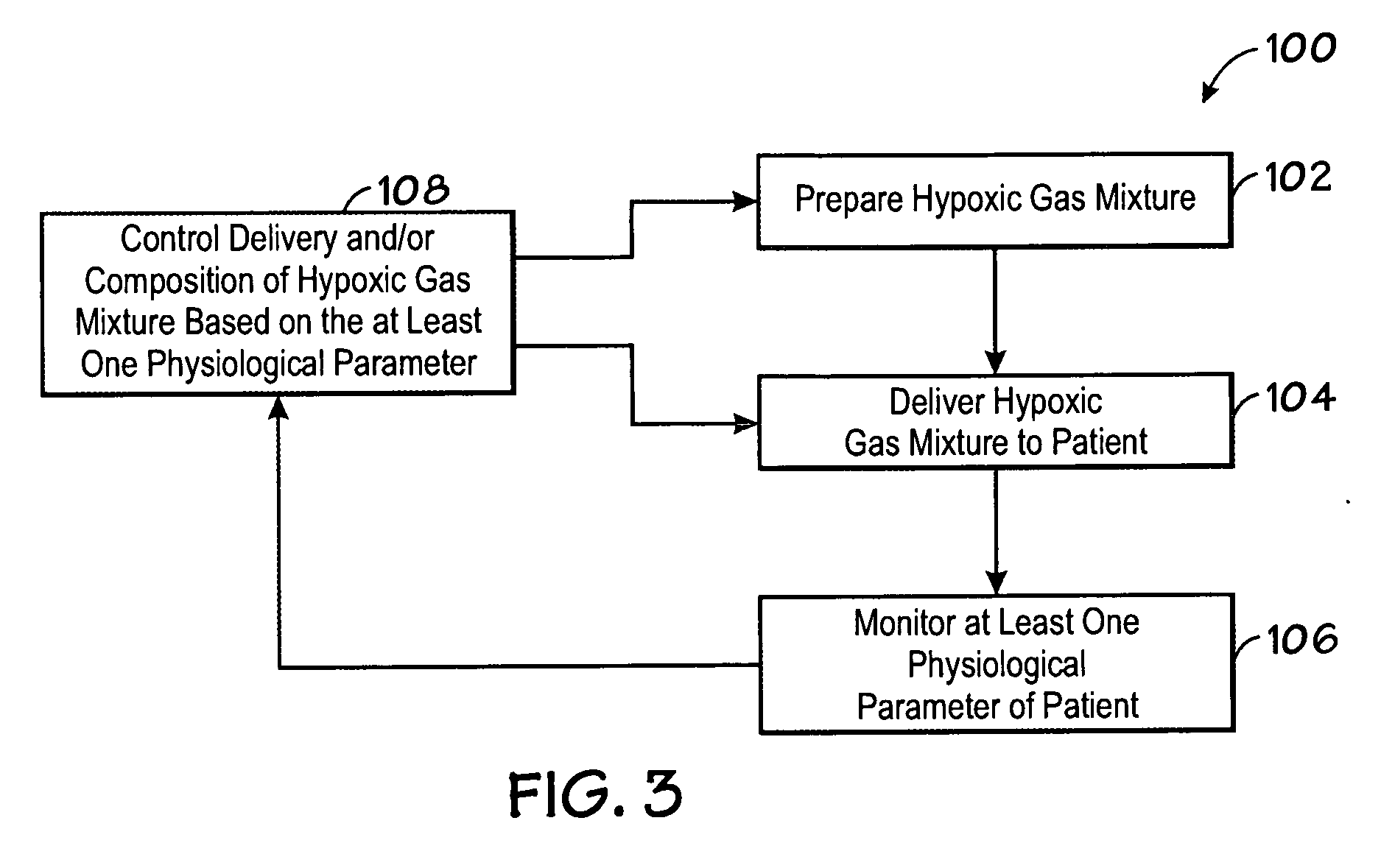
Method and system for controlled maintenance of hypoxia for therapeutic or diagnostic purposes
InactiveUS20070077200A1Safely induce and maintain and control hypoxiaIncrease volumeBiocideInorganic active ingredientsAutomatic controlIntensive care medicine
Embodiments of the present invention relate to a system, device, and method for automatically inducing, maintaining, or controlling hypoxia in a patient. Specifically, embodiments of the present invention relate to delivering a hypoxic gas mixture to a patient, monitoring at least one physiological parameter of the patient, and automatically controlling the delivery of the hypoxic gas mixture based on a value of the physiological parameter.
Owner:TYCO HEALTHCARE GRP LP
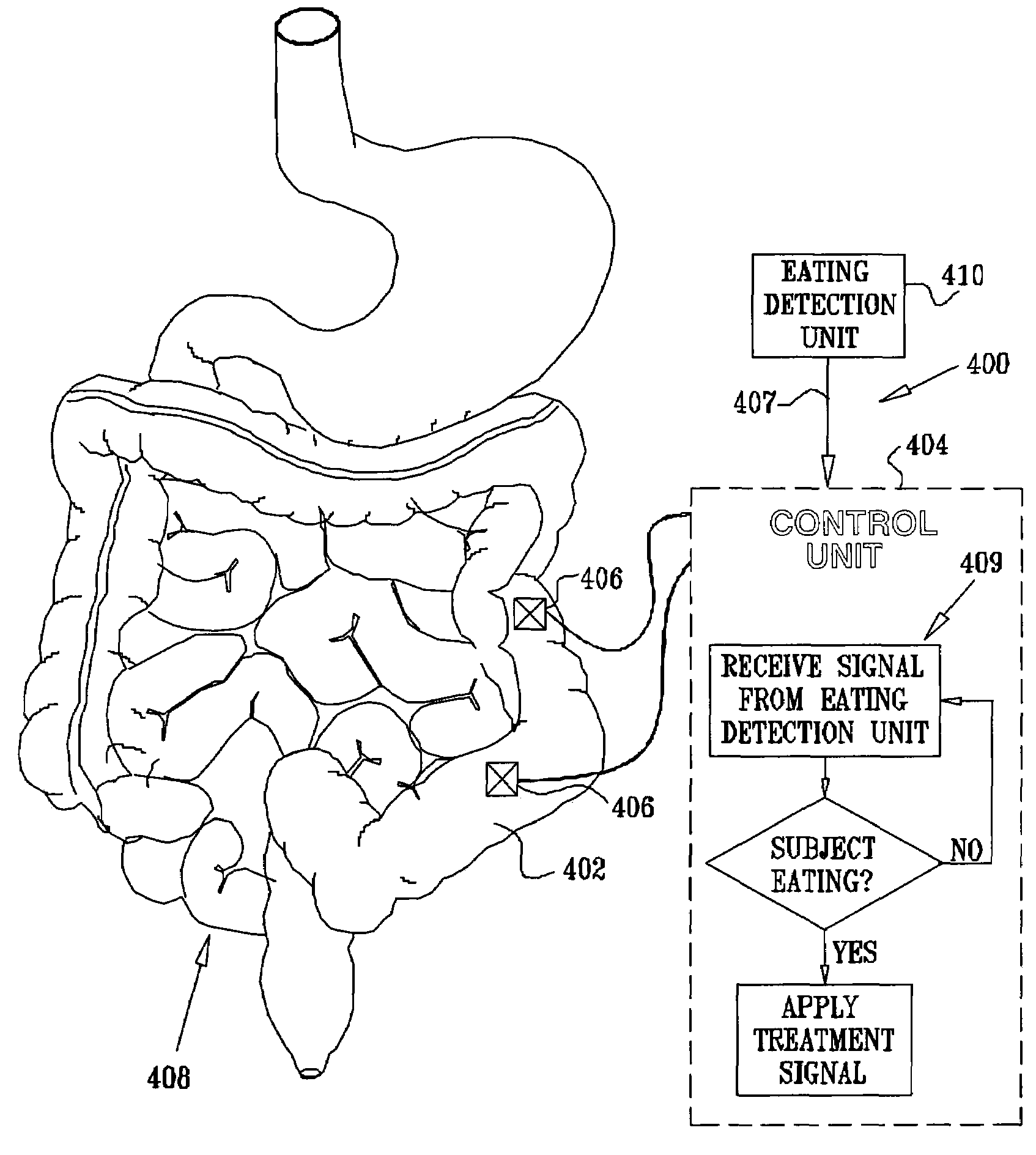
Gastrointestinal methods and apparatus for use in treating disorders
InactiveUS7502649B2Reduce volumeCause a sensation of satiety felt by the patientElectrotherapyMetabolism disorderElectrical resistance and conductanceDisease
A method is provided for detecting a change in posture of a subject. An electrical impedance is measured between two or more sites on a stomach (20) of the subject, and an impedance signal is generated responsive thereto. The change in posture is detected by performing a posture analysis of the impedance signal. A method is also provided for treating a subject. The method includes applying an electrical signal to a site of the subject selected from the list consisting of: a colon (402) of the subject, and a distal small intestine (408) of the subject. The signal is configured to stimulate cells of the subject to increase secretion of glucagon-like-peptide-1 (GLP-1) or PYY, or to decrease secretion of ghrelin, in order to treat the subject.
Owner:TYLERTON INT INC
Method of treating depression using a TNF-alpha antibody
InactiveUS20070041905A1Inhibiting TNFα activityImprove depressionSalicyclic acid active ingredientsBiocideAntiendomysial antibodiesAntigen binding
The invention describes methods of treating depression comprising administering a TNFα antibody, such as a human TNFα antibody. The invention also provides a method for treating depression comprising inhibiting TNFα activity in a subject suffering from depression by systemically administering to the subject a human anti-TNFα antibody, or an antigen-binding portion thereof, such that depression is treated. Also described is a method for the treatment or alleviation of depression or other affective disorders comprising administering an amount of an anti-inflammatory agent effective to treat or alleviate depression or other affective disorder to a subject in need thereof, wherein said anti-inflammatory agent down-regulates peripheral cytokine levels to thereby treat or alleviate depression or other affective disorder.
Owner:HOFFMAN REBECCA S +3
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
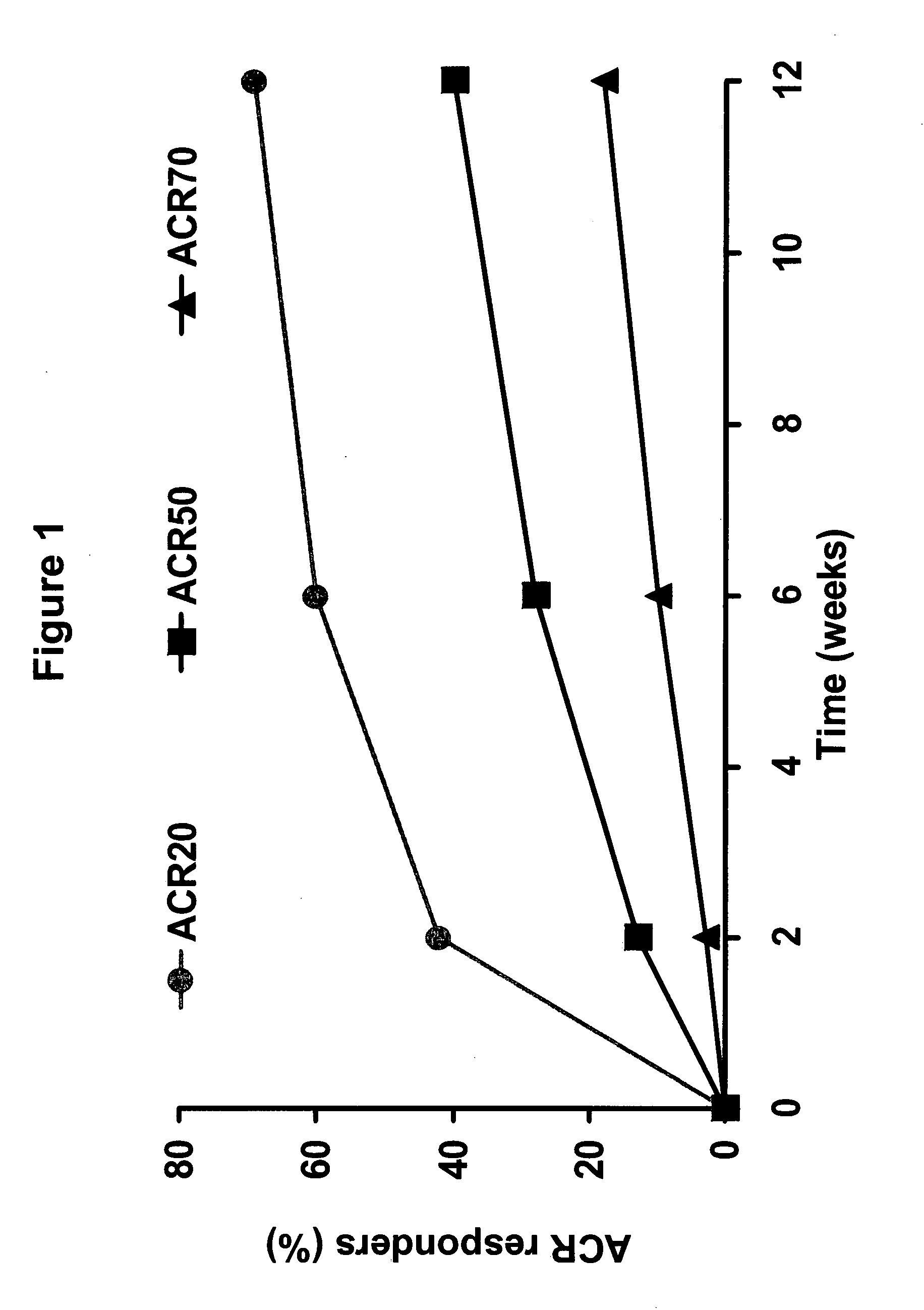
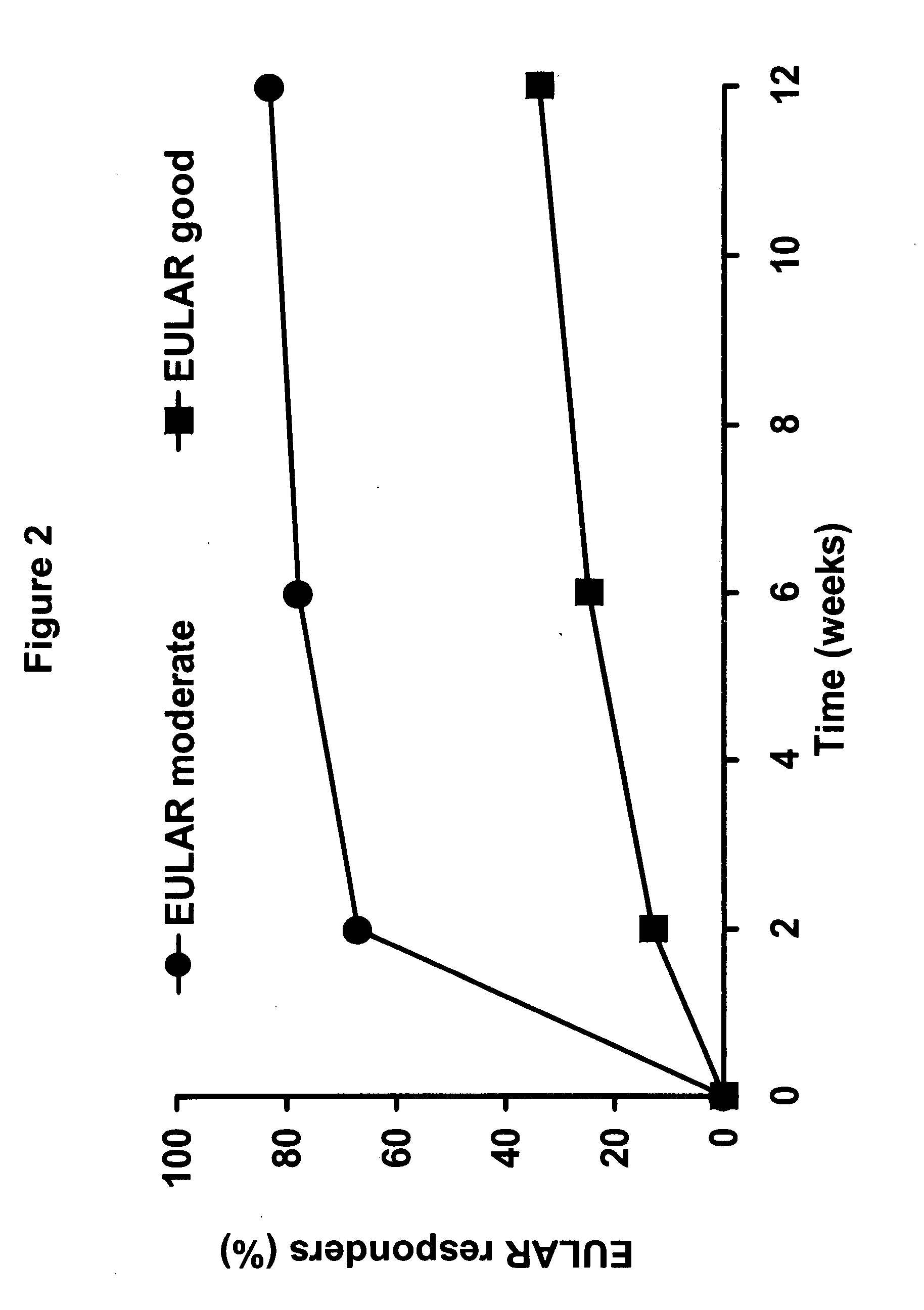
Uses and compositions for treatment of rheumatoid arthritis
InactiveUS20080131374A1Improve the quality of lifeBiocideAntipyreticAntigen bindingEarly rheumatoid arthritis
The invention provides methods, uses and compositions for the treatment of rheumatoid arthritis. The invention describes methods and uses for treating rheumatoid arthritis wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of rheumatoid arthritis in a subject.
Owner:MEDICH JOHN R +7
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
Treatment of renal hypertension or carotid sinus syndrome with adventitial pharmaceutical sympathetic denervation or neuromodulation
ActiveUS20110104061A1Improve concentrationOrganic active ingredientsBacterial antigen ingredientsRenal HypertensionsCvd risk
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict medication regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com