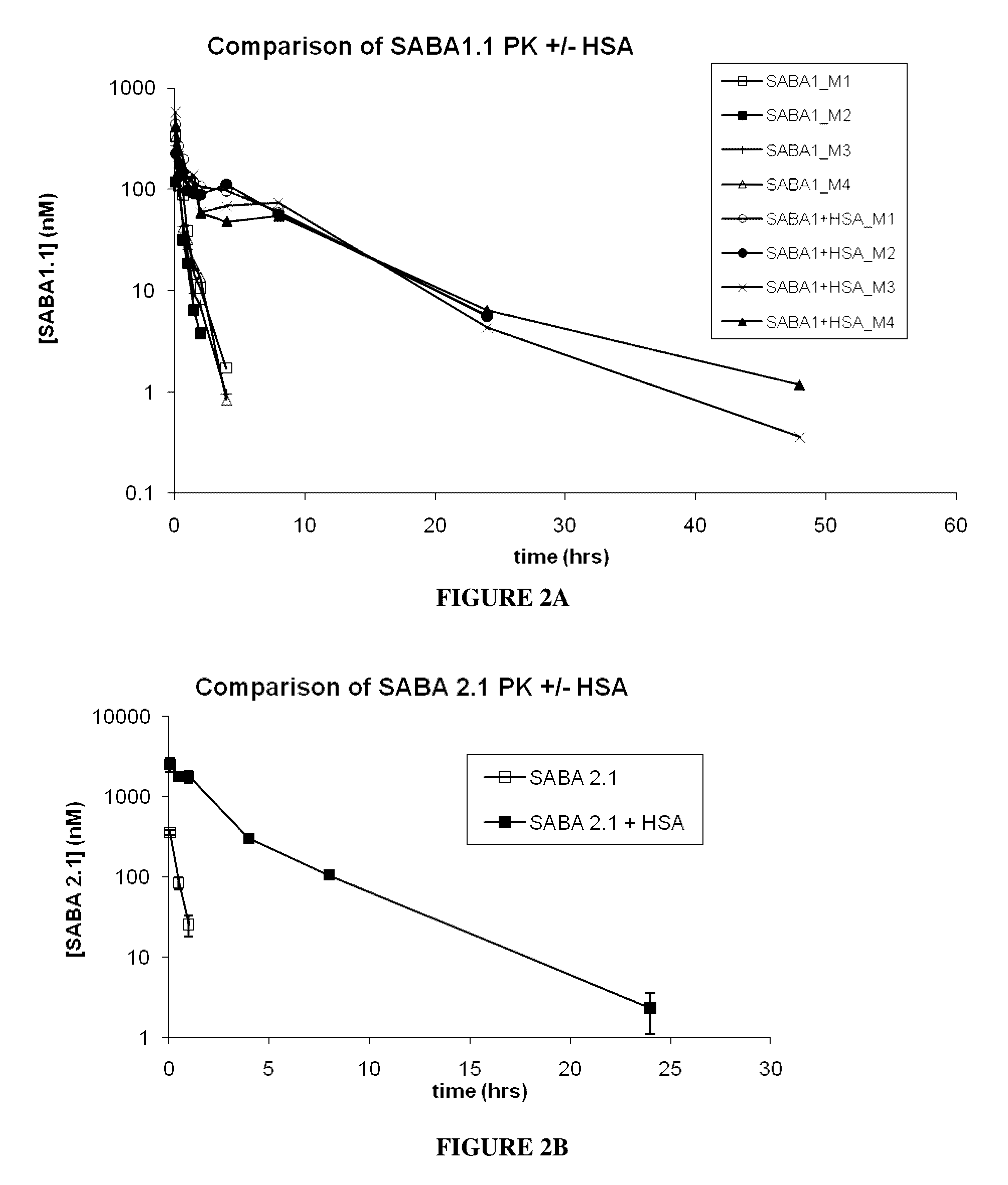
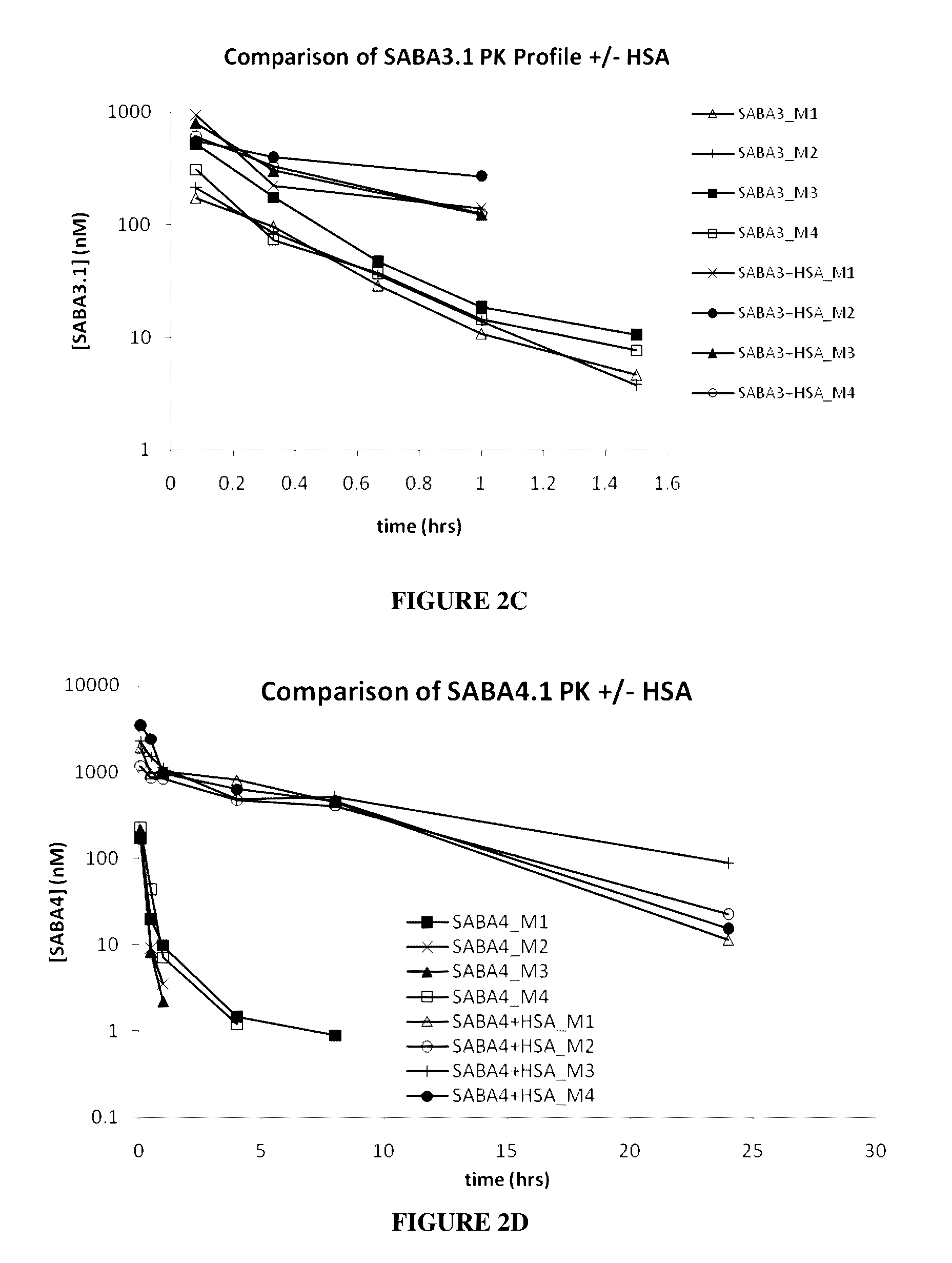
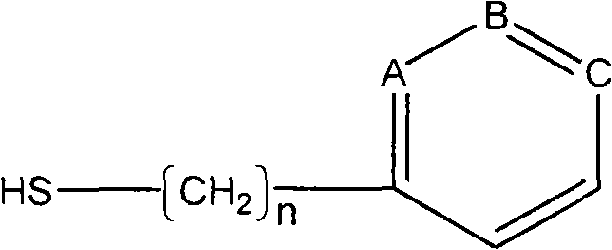
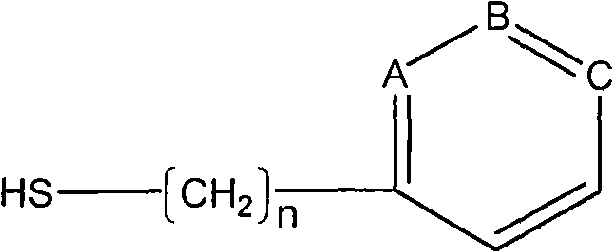
Patents
Literature
951 results about "Serum albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
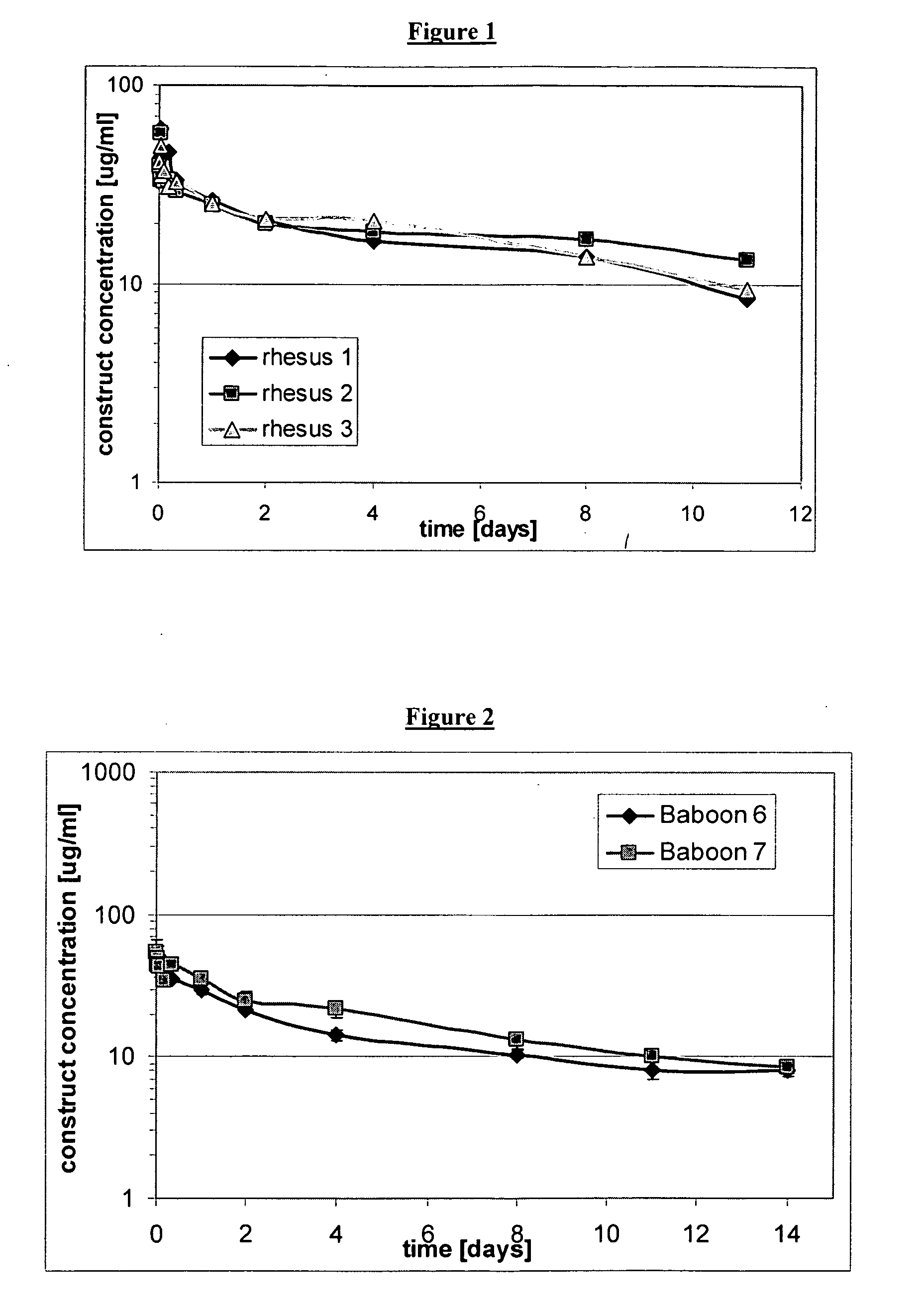
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
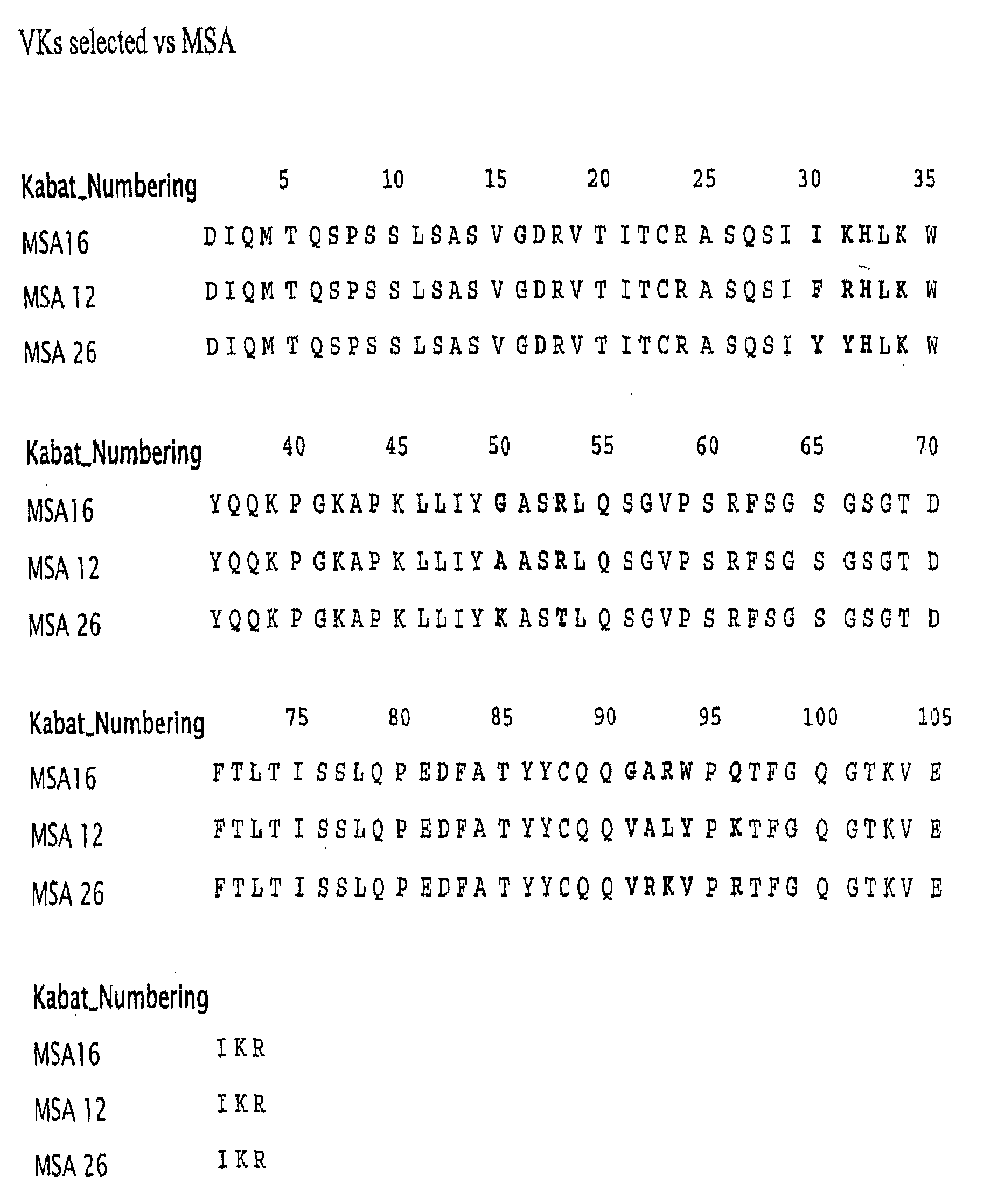
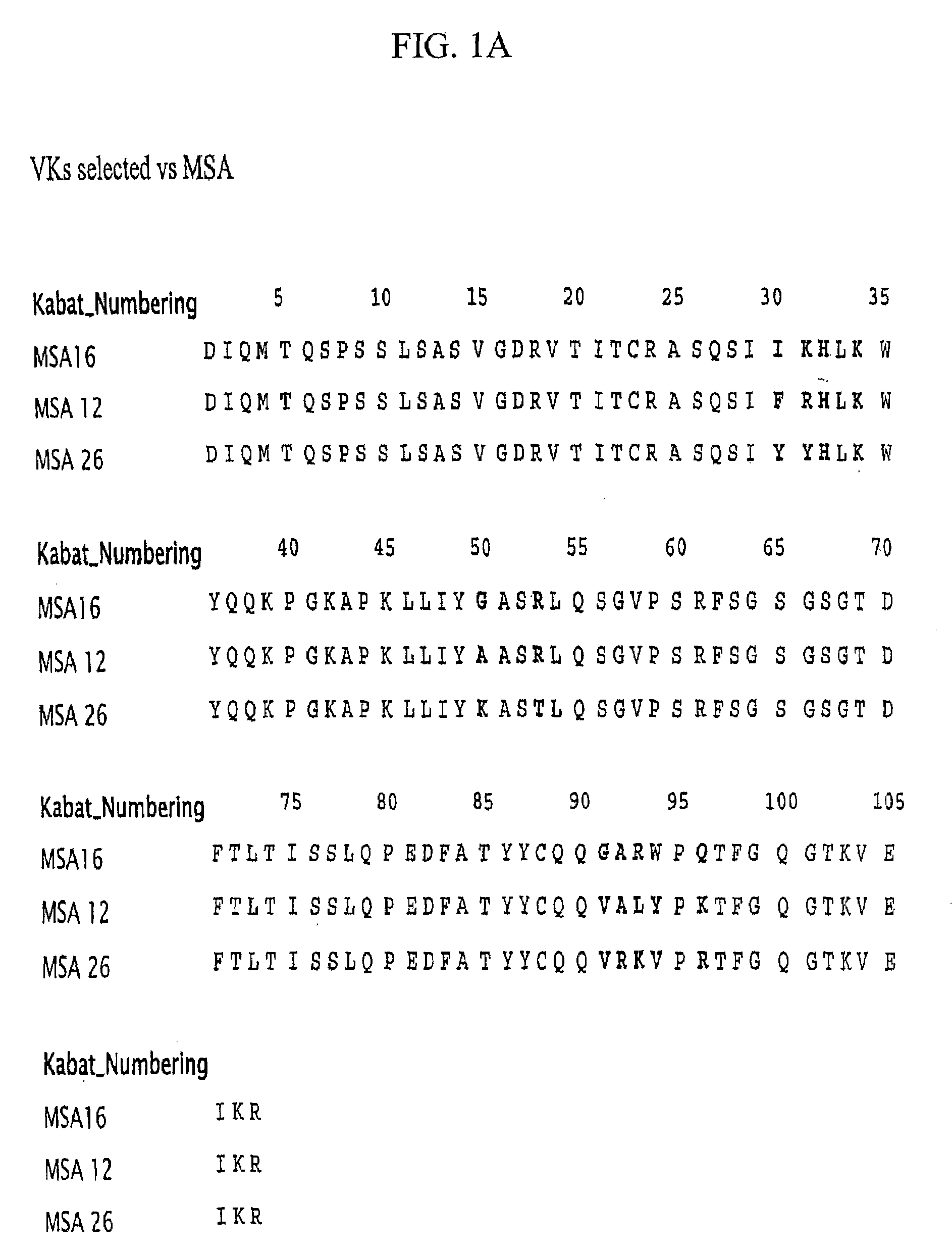
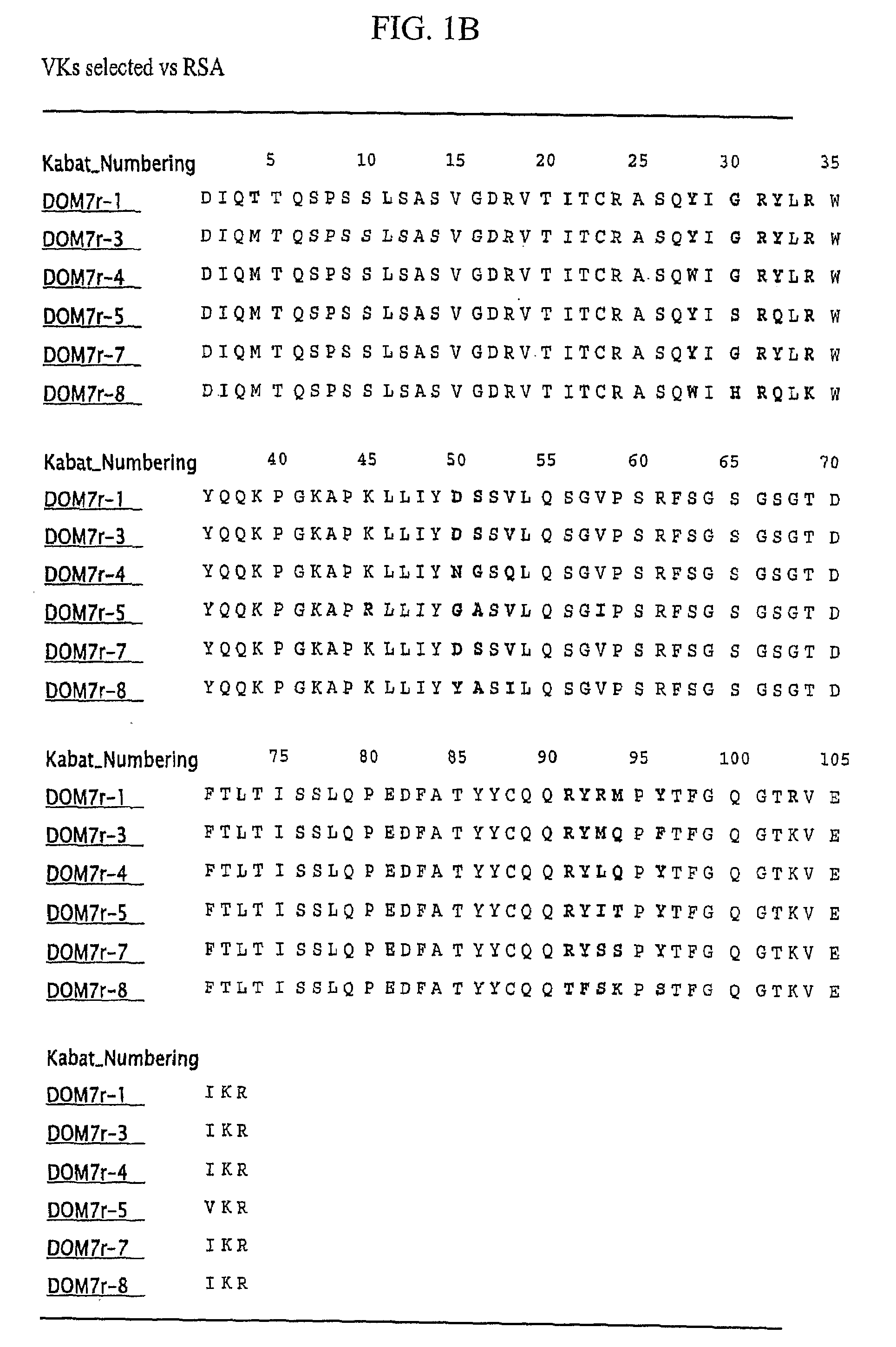
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
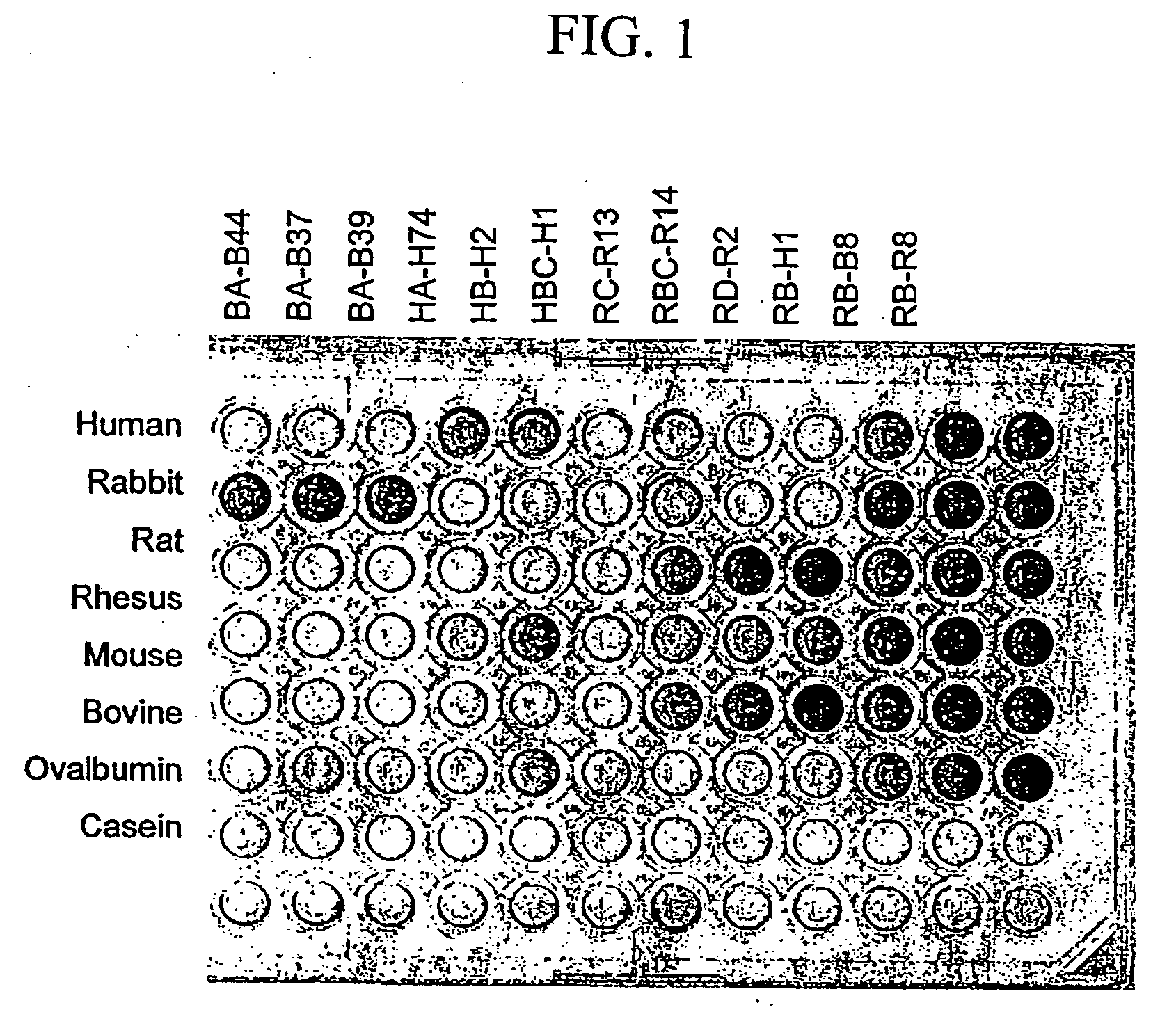
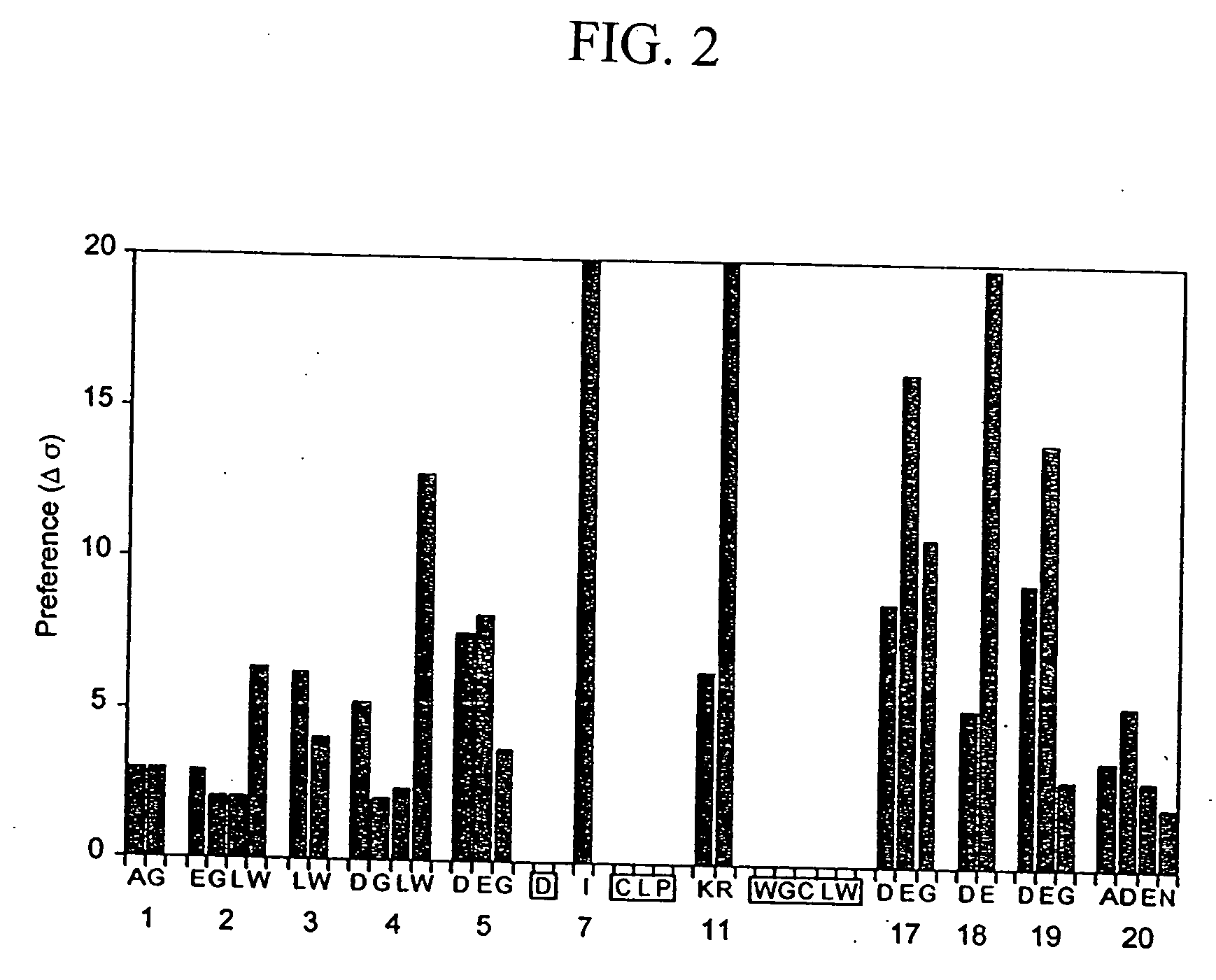
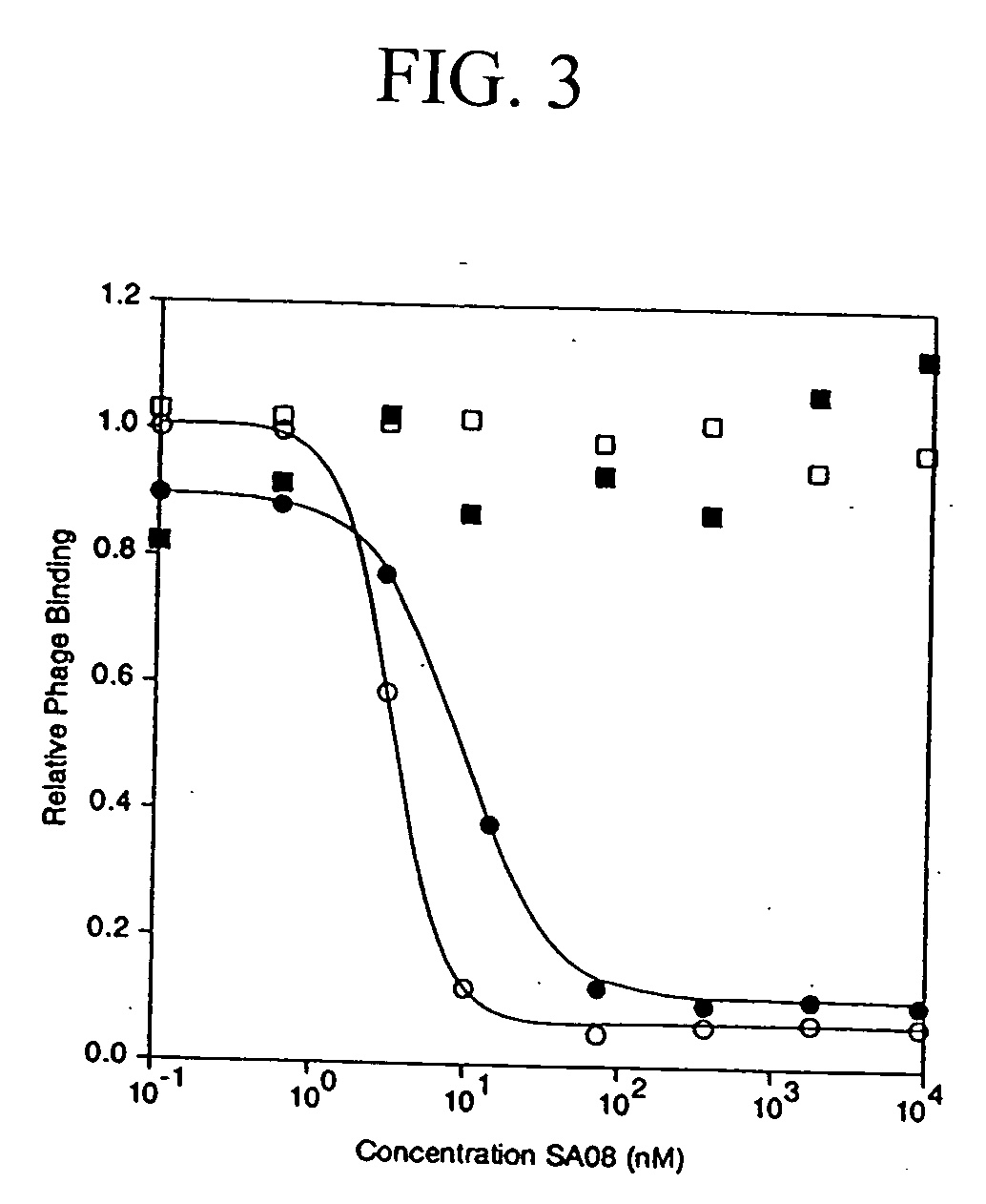
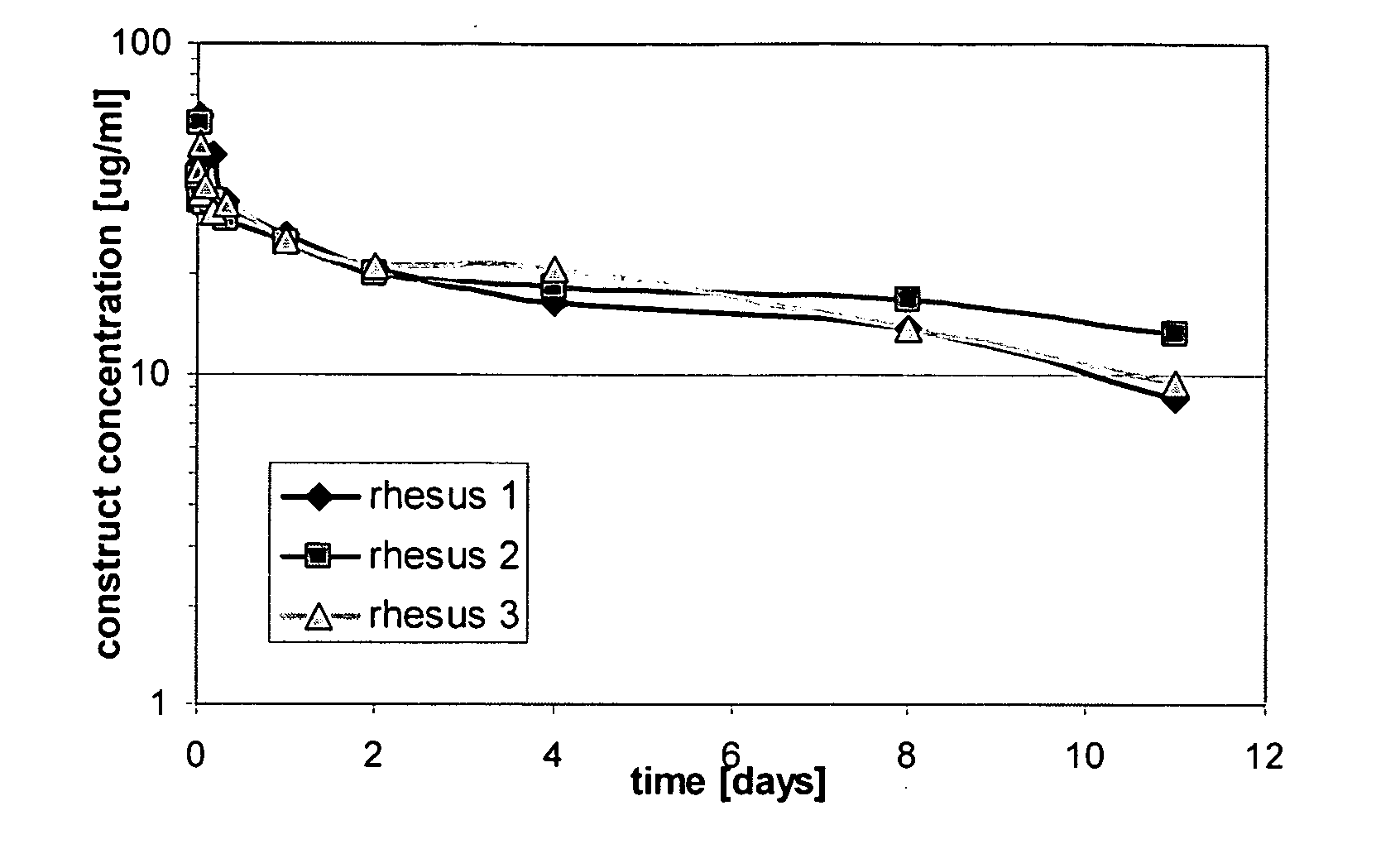
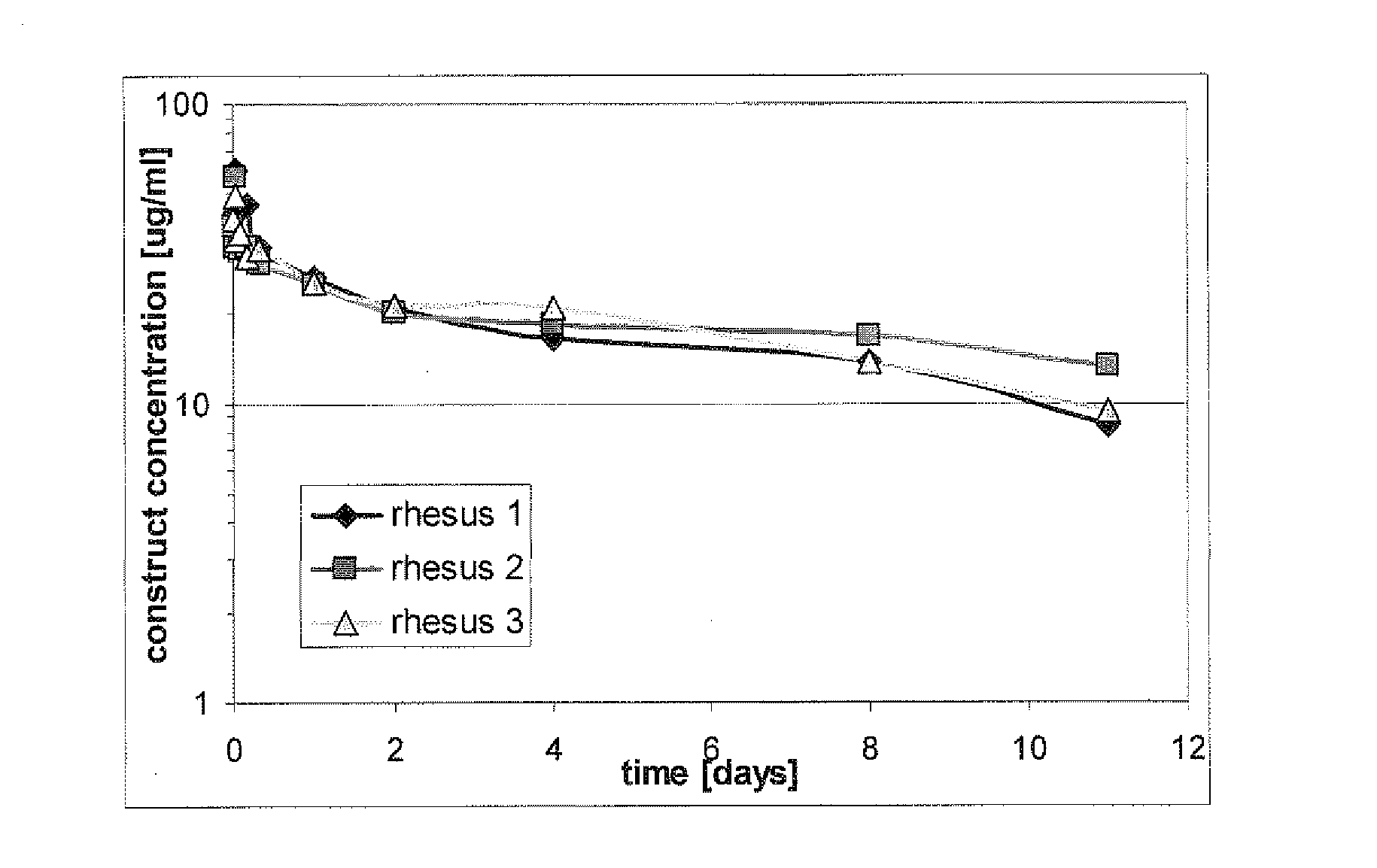
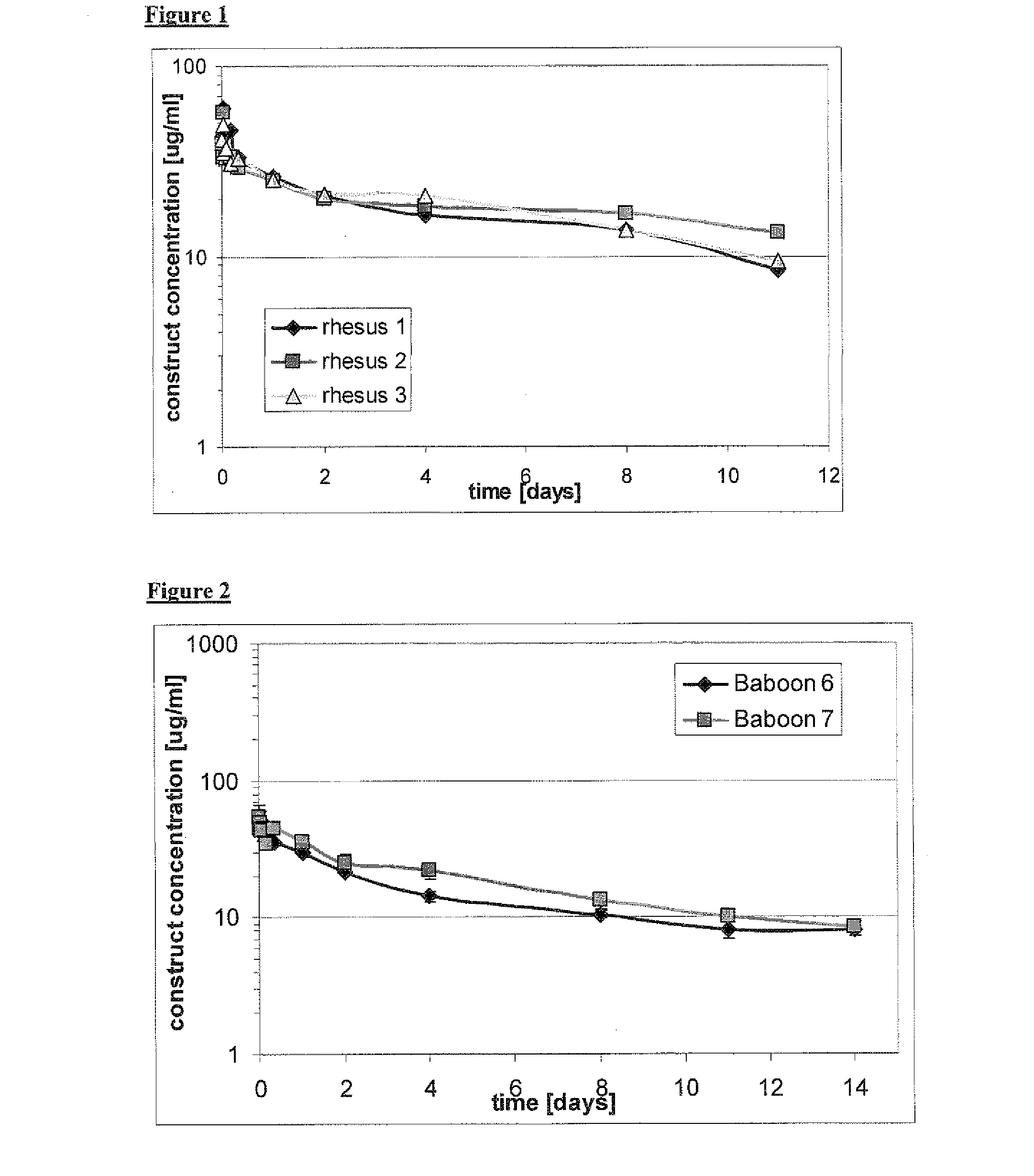
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
Chondroitinase, process for preparing the same, and pharmaceutical composition comprising the same
InactiveUS6184023B1Avoid stickingInhibit productionBacteriaHydrolasesChondroitinase ABCConcentration gradient
A crystallizable, purified chondroitinase ABC having a molecular weight of about 100,000 dalton by the measurement of the SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the measurement by the gel permeation chromatography method, having alanine as the N-terminal amino acid and proline as the C-terminal amino acid. A process for the purification of the crystallizable purified chondroitinase ABC comprising removing nucleic acid from an surfactant solution extract obtained from cells of chondroitinase ABC-producing microorganisms and chromatographically treating by concentration gradient elution using a weak cation exchange resin or a strong cation exchange resin. A composition comprising a chondroitinase and serum albumin, gelatin, or a nonionic surfactant.
Owner:SEIKAGAKU KOGYO CO LTD
Bispecific Fusion Antibodies With Enhanced Serum Half-Life
Drug compositions, fusions and conjugates are provided. The drug fusions and conjugates contain a therapeutic or diagnostic agent that is fused or conjugated to an antigen-binding fragment of an antibody that binds serum albumin. The drug compositions, fusions and conjugates have a longer in vivo half-life in comparison with the unconjugated or unfused therapeutic or diagnostic agent.
Owner:DORMANTIS LTD
Serum albumin binding proteins with long half-lives
InactiveUS20100113339A1Increased serum half-lifeReduce dosing frequencyNervous disorderPeptide/protein ingredientsHalf-lifeSerum albumin
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides.
Owner:ABLYNX NV
Cryopreservation of Adipose Tissue for the Isolation of Mesenchymal Stem Cells
The present invention relates to a method and composition for the cryopreservation of adipose tissue with the intention to use this tissue in the culturing of stem and / or progenitor cells. The method uses a specific cryoprotection medium to prevent damage of the original tissue during the cryopreservation while still maintaining a high viability of the stem and / or progenitor cells obtained from the cryopreserved adipose tissue. Furthermore the cryoprotection medium of the present invention does not contain any kind of xenogeneic sera, a critical factor since it is the intention of that the cryopreserved tissue is used for obtaining stem and / or progenitor cells that can be used in medicine. The cryoprotection medium is characterized in that it is a solution of physiological water comprising glycerol and sucrose and / or trehalose and optionally serum albumin.
Owner:CRYO SAVE
Serum albumin binding peptides for tumor targeting
InactiveUS20060228364A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsPeptide-nucleic acidsAbnormal tissue growthTumor targeting
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Diagnostic Methods Of Multiple Organ Amyloidosis
InactiveUS20080038192A1In-vivo radioactive preparationsMicrobiological testing/measurementElevated C-reactive proteinPredictive biomarker
Described are methods of assessing whether a subject has or is at risk of having multiple organ amyloidosis (MOA) The method includes detecting a diagnostically predictive collection of biomarkers of multiple organ amyloidosis, wherein the detection of a diagnostically predictive collection of biomarkers indicates the subject has or is at risk of having multiple organ amyloidosis Also described are methods of monitoring treatment of subjects with multiple organ amyloidosis and evaluating therapeutic compounds Representative biomarkers for use in the methods may be selected from variant serum amyloid A (SAA) allele, elevated SAA level, elevated C-reactive protein (CRP) level, depressed glycosammoglycan (GAG) level, elevated interleukin-18 (IL-18) level, elevated macrophage-colony stimulating factor (M-CSF) level, elevated hepatocyte growth factor (HGF) level, presence of an antibody against citrullmated vimentm (Sa), presence of a monoclonal immunoglobulin light chain, increased serum albumin, and increased creatinine clearance
Owner:NEUROCHEM INT
Adhesive sealant composition
InactiveUSRE38158E1High bonding strengthOrganic active ingredientsNon-fibrous pulp additionAqueous bufferWater soluble
This invention is related to an adhesive composition which may be used to bond or seal tissue in vivo. The adhesive composition is readily formed from a two component mixture which includes a first part of a protein, preferably a serum albumin protein, in an aqueous buffer having a pH in the range of about 8.0-11.0 and a second part of a water-compatible or water-soluble bifunctional crosslinking agent. When the two parts of the mixture are combined, the mixture is initially a liquid which cures in vivo on the surface of tissue in less than about one minute to give a strong, flexible, pliant substantive composition which bonds to the tissue and is absorbed in about four to sixty days. The adhesive composition may be used either to bond tissue, to seal tissue or to prevent tissue adhesions caused by surgery.
Owner:NEOMEND INC
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
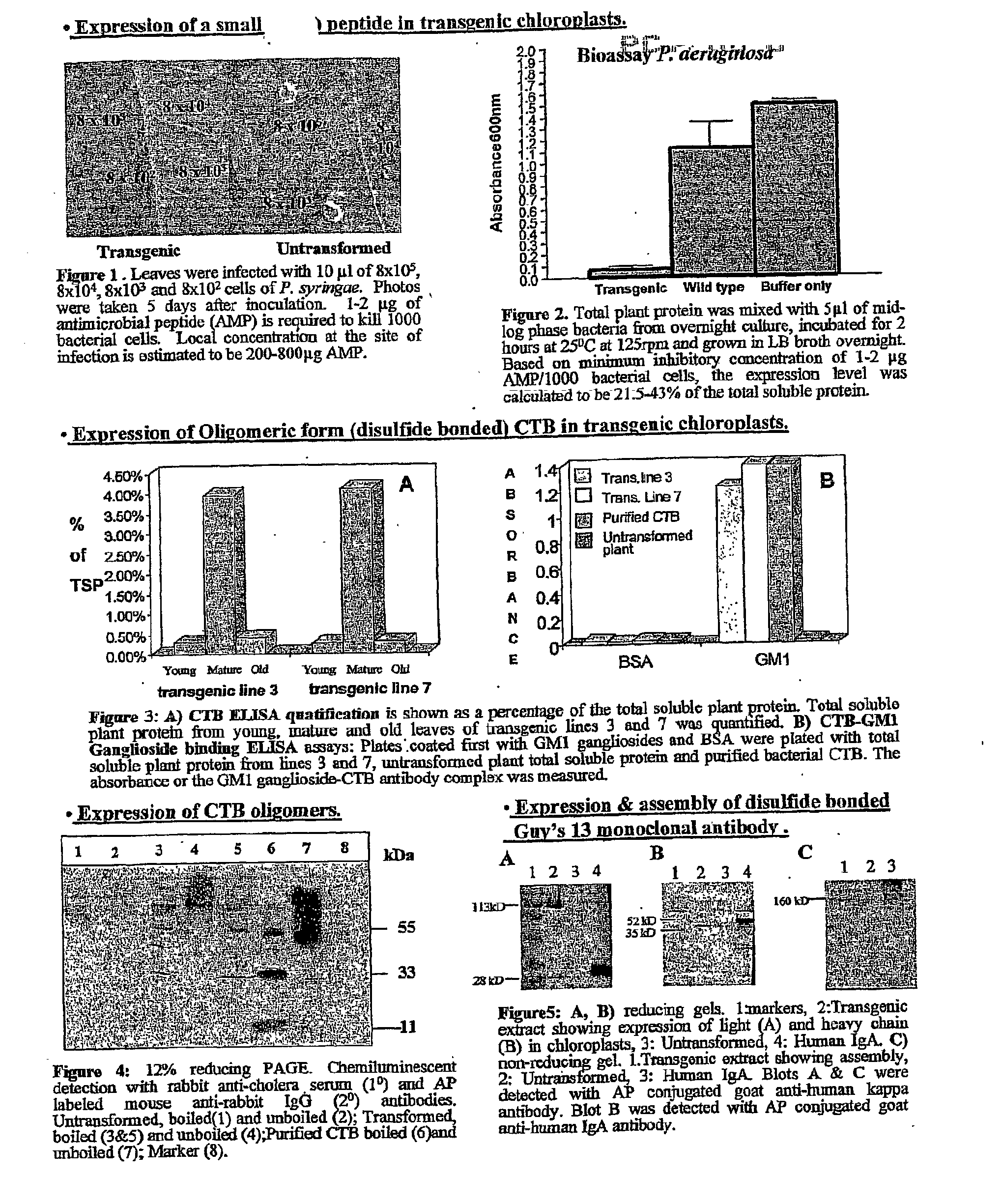
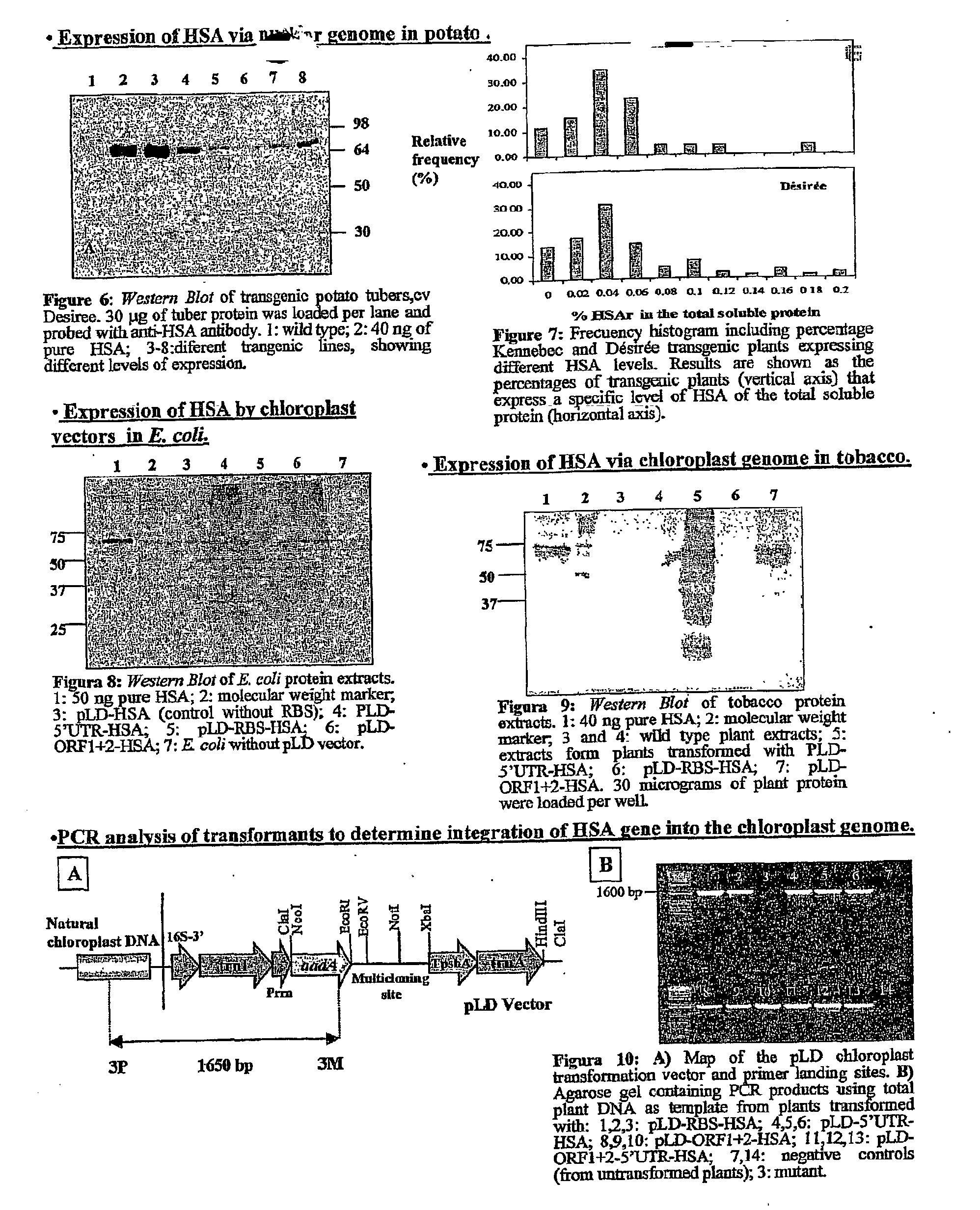
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and application thereof
The invention relates to an aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and an application thereof. The kit comprises a fluorescent test strip and a sample reaction bottle containing an europium-labeled anti-aflatoxin B1 monoclonal antibody lyophilized product, wherein the fluorescent test strip comprises a cardboard, a water absorption pad, a detection pad and a sample pad are sequentially pasted on one surface of the cardboard from top to bottom, adjacent pads are connected at the connection in an overlapping manner, the detection pad treats a cellulose nitrate membrane as a base pad, a transverse quality control line and a detection line are arranged on the cellulose nitrate membrane from top to bottom, the quality control line is coated with a rabbit anti-mouse polyclonal antibody, and the detection line is coated with an aflatoxin B1 bovine serum albumin conjugate; and the anti-aflatoxin B1 monoclonal antibody is secreted by a hybridoma cell strain having a preservation number of CCTCC NO.C201015. The kit can be used for the quantitative determination of the content of the aflatoxin B1, and has the advantages of simple operation, rapidness and high accuracy.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Recombinant human albumin fusion proteins with long-lasting biological effects
ActiveUS7244833B2Good for healthImprove stabilityBacteriaPeptide/protein ingredientsDiseaseHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
Serum albumin binding peptides for tumor targeting
InactiveUS20070202045A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAbnormal tissue growthTumour targeting
Owner:GENENTECH INC
Protein containing serum albumin domain
InactiveUS7253259B2Improve functional activityImprove antioxidant capacityFungiBacteriaSerum igeADAMTS Proteins
A protein produced by gene recombinant technology, including at least one domain selected from domains I, II, and III of serum albumin but having a different structure from that of native albumin; and a method of producing the protein. The protein has an enhanced functional activity or activities selected from among various functional activities or serum albumin including antibacterial activity, antioxidative effect, inflammation inhibitory effect, in vivo substance transporting action, and enzymatic activity.
Owner:NIPRO CORP
Preparation method and uses of carboxymethyl chitosan and sodium alginate blend microcapsule
InactiveCN1546557AGood drug release functionIncrease release rateCapsule deliveryMicroballoon preparationFlavorWax
The invention discloses the preparation method and uses of carboxymethyl chitosan and sodium alginate blend microcapsule wherein the method comprises, using water as solvent to blend the water solution of carboxymethyl chitosan and sodium alginate into blending liquid I, dissolving the contained medicament bovine serum albumin into the blended solvent I and mixing homogeneously, obtaining blended liquid II, dispersing the blended liquid II into fluid wax containing emulsifying agent, dropping CaCl2 coagulated liquid into the system, charging 10-30 mL alcohol isopropylicum and hardening to obtain micro-encapsulation, which exhibits sensitive response function to the pH changes.
Owner:WUHAN UNIV
Methods and systems for increasing protein stability
InactiveUS20130129727A1High expressionEasy to purifyBacteriaAntibody mimetics/scaffoldsSerum protein albuminHalf-life
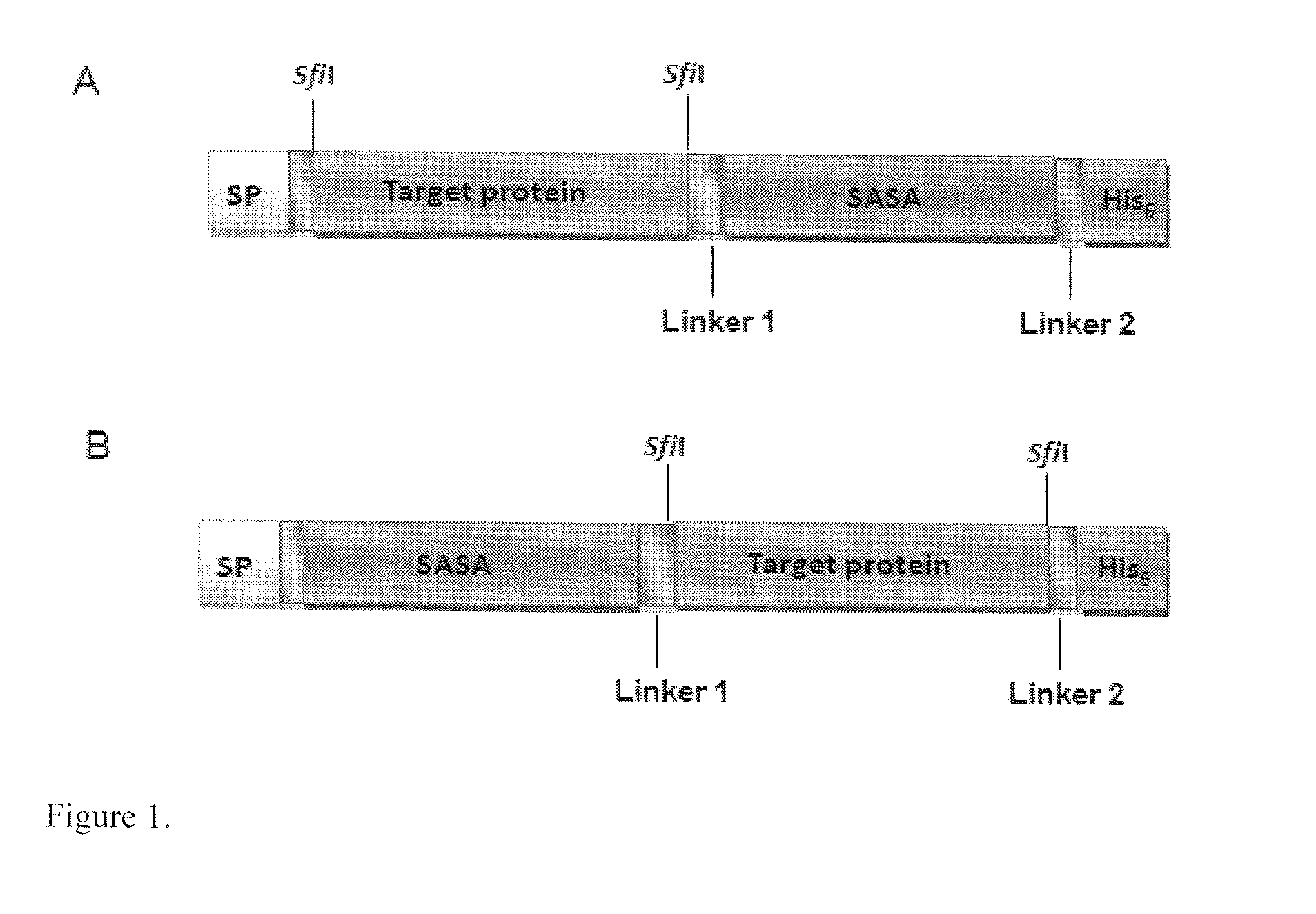
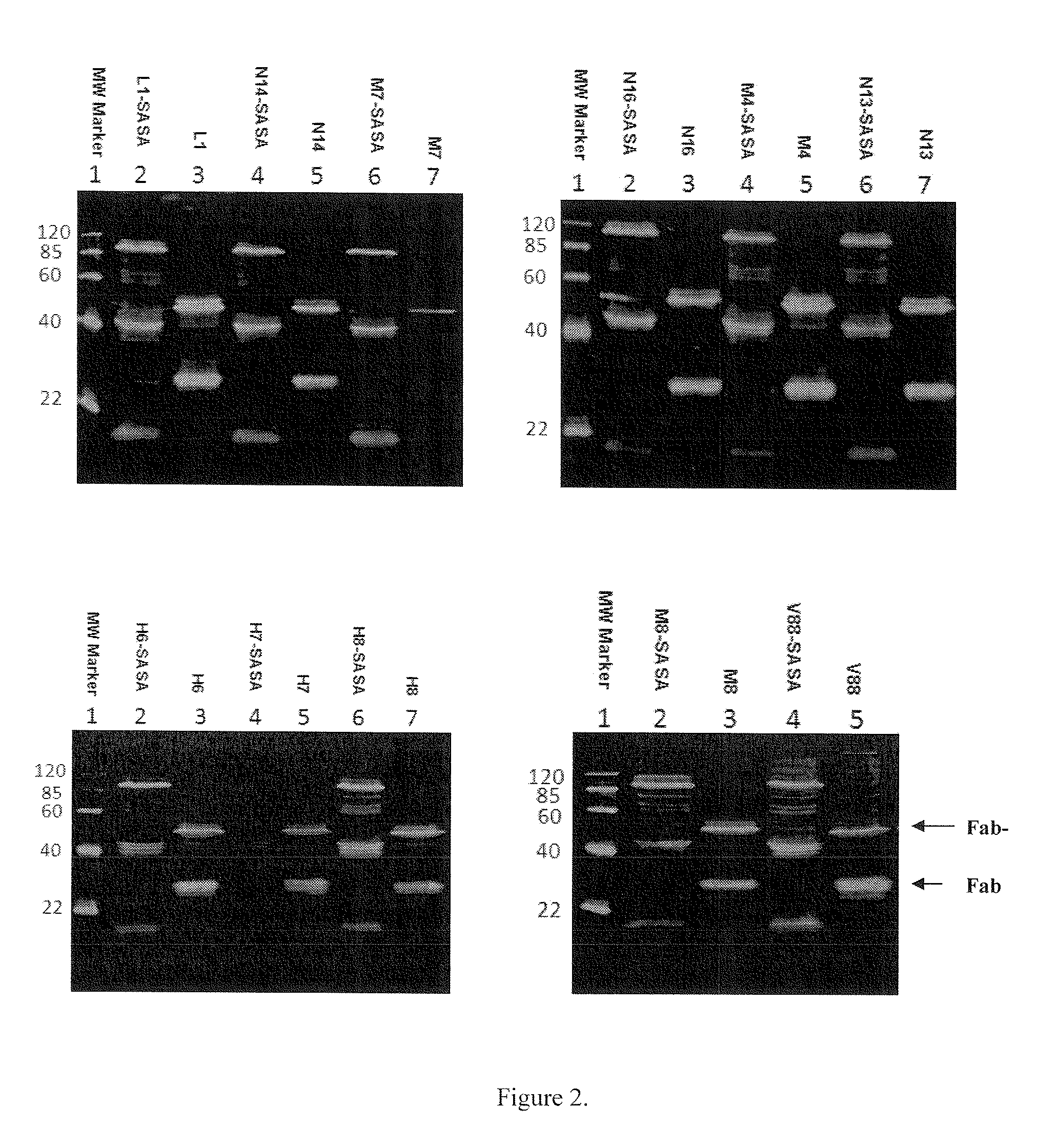
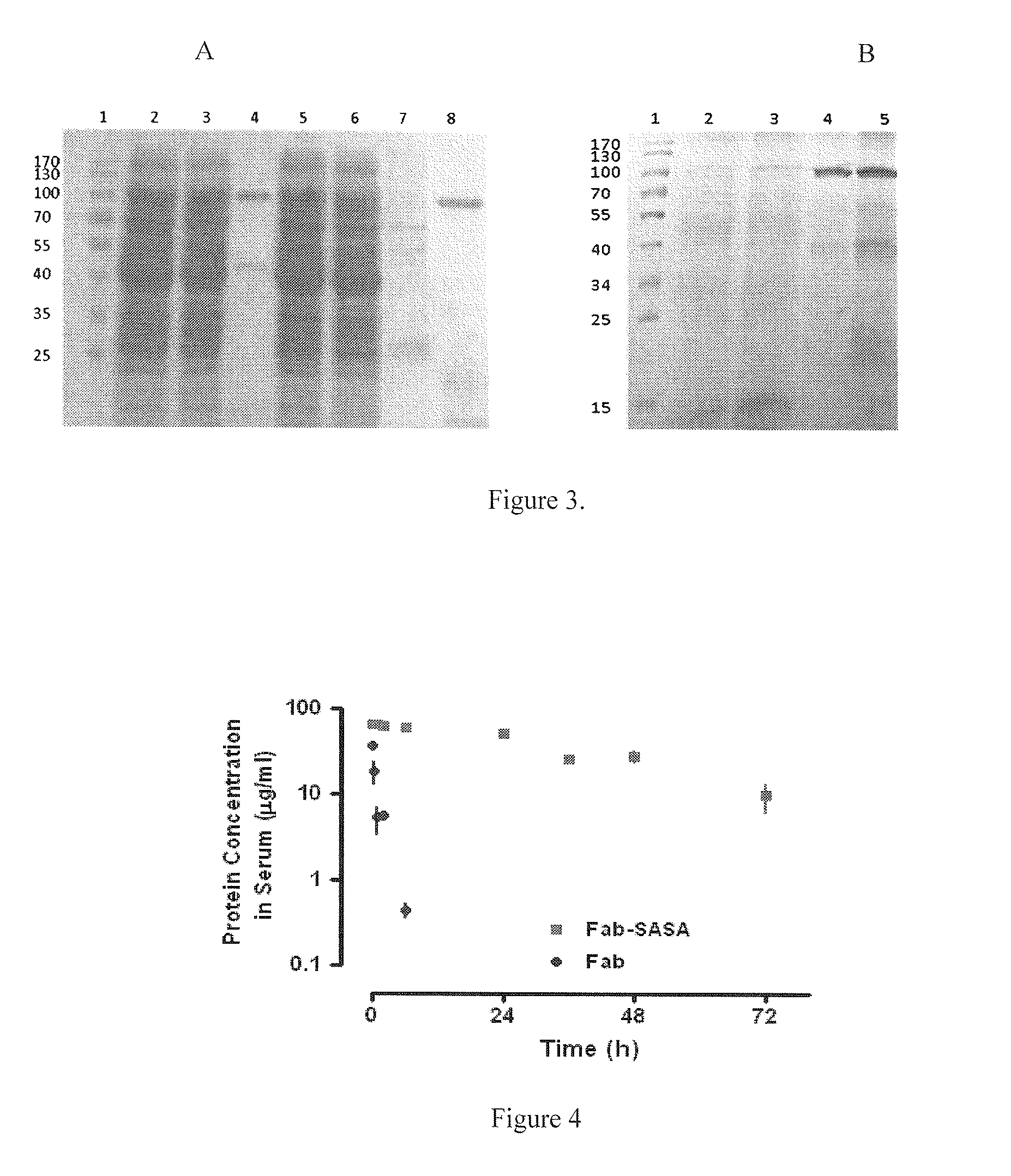
Methods and systems for increasing stability of a target polypeptide in a serum are described. The methods and systems utilize a fusion protein comprising a single-domain antibody against a serum albumin (SASA), the target polypeptide and optionally a linker. The fusion protein has a significantly prolonged serum half life in comparison with the target polypeptide alone. The SASA fusion tag also facilitates the expression and purification of the fusion protein. This allows direct in vivo screening or utilization of the target polypeptide for its biological activity or efficacy regardless of its intrinsic serum half life, which has significantly increased the number of candidates for the development of novel protein based diagnosis or treatment.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Bovine serum albumin-platinum composite nanomaterial mimetic peroxidase
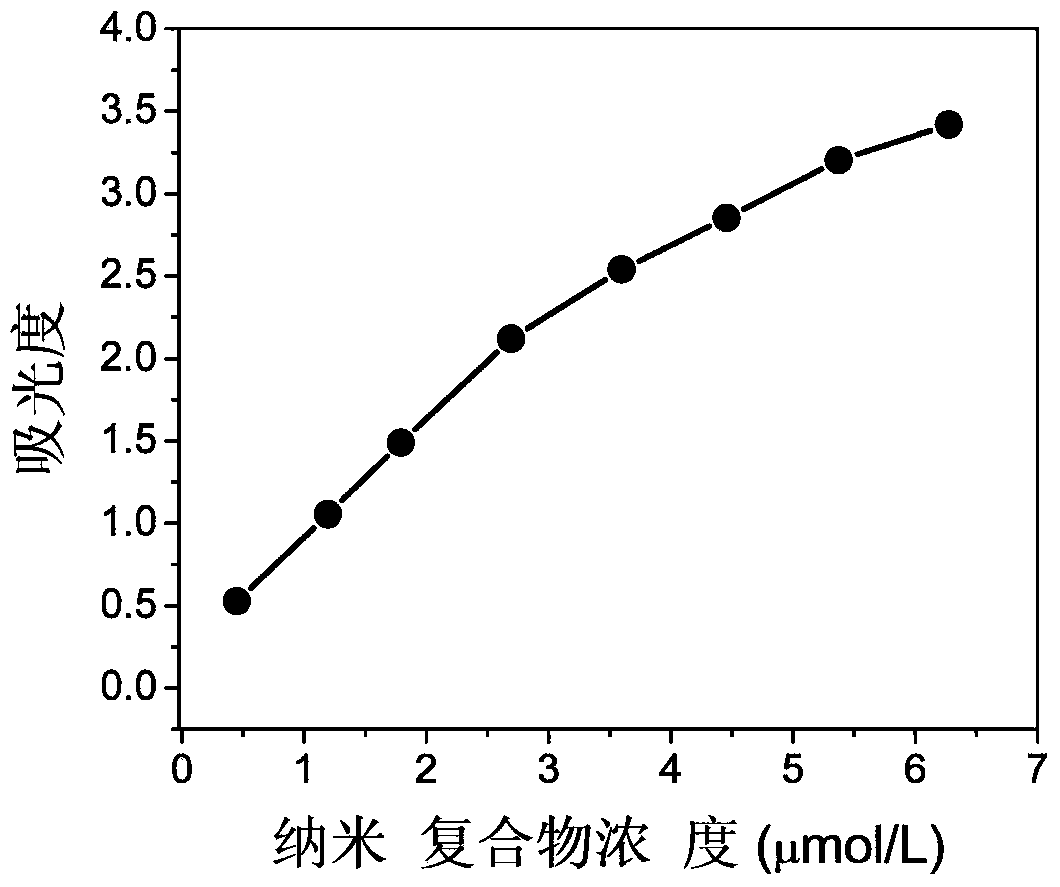
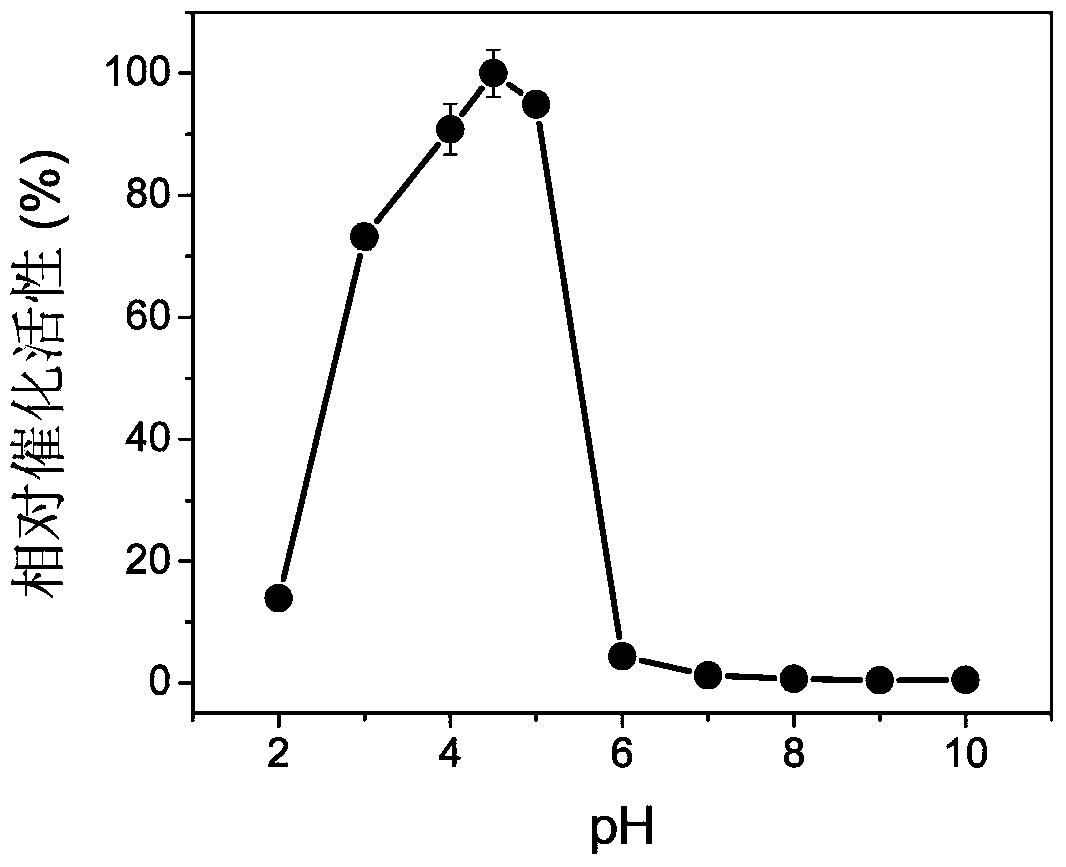
InactiveCN103433484ANo significant change in catalytic activityImprove stabilitySerum albuminPeroxidaseUltrafiltration
The invention discloses bovine serum albumin-platinum composite nanomaterial mimetic peroxidase as well as a preparation method thereof and application. Bovine serum albumin is used as a template, and the bovine serum albumin-platinum composite nanomaterial mimetic peroxidase is prepared through biomineralization. Bovine serum albumin-platinum composite nanomaterials are prepared through the following method that chloroplatinic acid aqueous solutions are added to bovine serum albumin aqueous solutions and are mixed, sodium hydroxide aqueous solutions are added to obtain mixed solutions, and water bath heating is carried out; ultrafiltration is carried out on the solutions, then the solutions are washed, and bovine serum albumin-platinum composite nanomaterial aqueous solutions are obtained. The bovine serum albumin-platinum composite nanomaterials have excellent peroxidase activity, and can catalyze hydrogen peroxide oxidation 3, 3', 5, 5'-tetramethyl benzidine hydrochloride to be in color development. Meanwhile, the mimetic peroxidase resists acid and base, high temperature and high salinity, and has excellent short-term indoor temperature stability and long-term indoor temperature stability.
Owner:FUJIAN MEDICAL UNIV
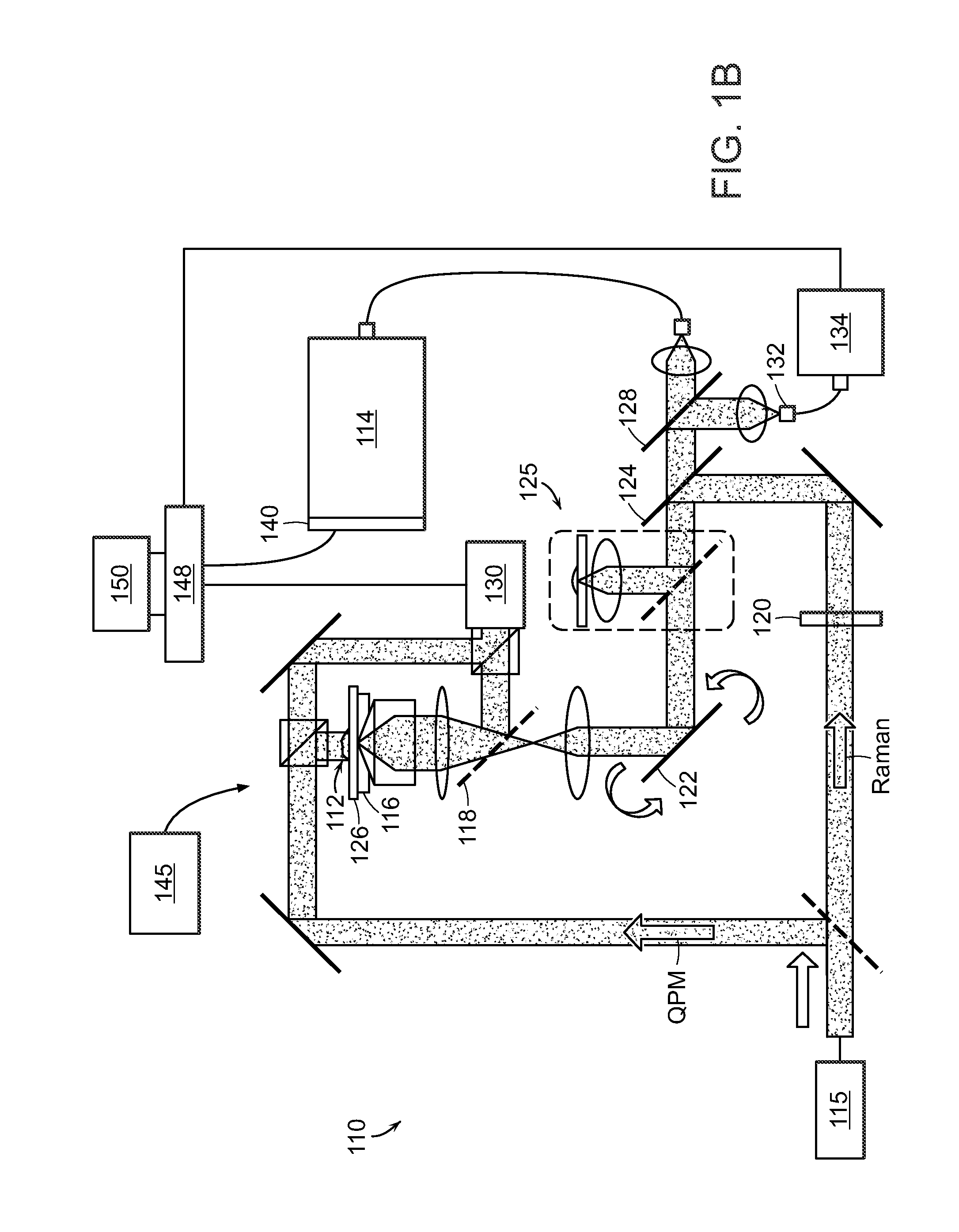
Raman spectroscopy for detection of glycated analytes
ActiveUS20140349337A1Enhanced signalGood reproducibilityBioreactor/fermenter combinationsBiological substance pretreatmentsOptical measurementsGlycated hemoglobin
The present invention relates to the optical measurement of blood analytes, such as glycated hemoglobin (HbA1c) and serum albumin as a functional metric of mean blood glucose in the diagnosis of diabetic patients. Non-enhanced Raman spectroscopy is employed as the analytical method for quantitative detection of blood analytes. Using processing techniques, non-enzymatic glycosylation (glycation) of the analytes results in measurable and highly reproducible changes in the acquired spectral data, which enable the accurate measurements and classification of glycated and unglycated analytes.
Owner:MASSACHUSETTS INST OF TECH
Dried blood factor composition comprising trehalose
A stable blood factor composition contains a stabilising amount of trehalose in the absence of human serum albumin to provide a product stable at up to 60° C.
Owner:QUANDRANT HLDG CAMBRIDGE LTD
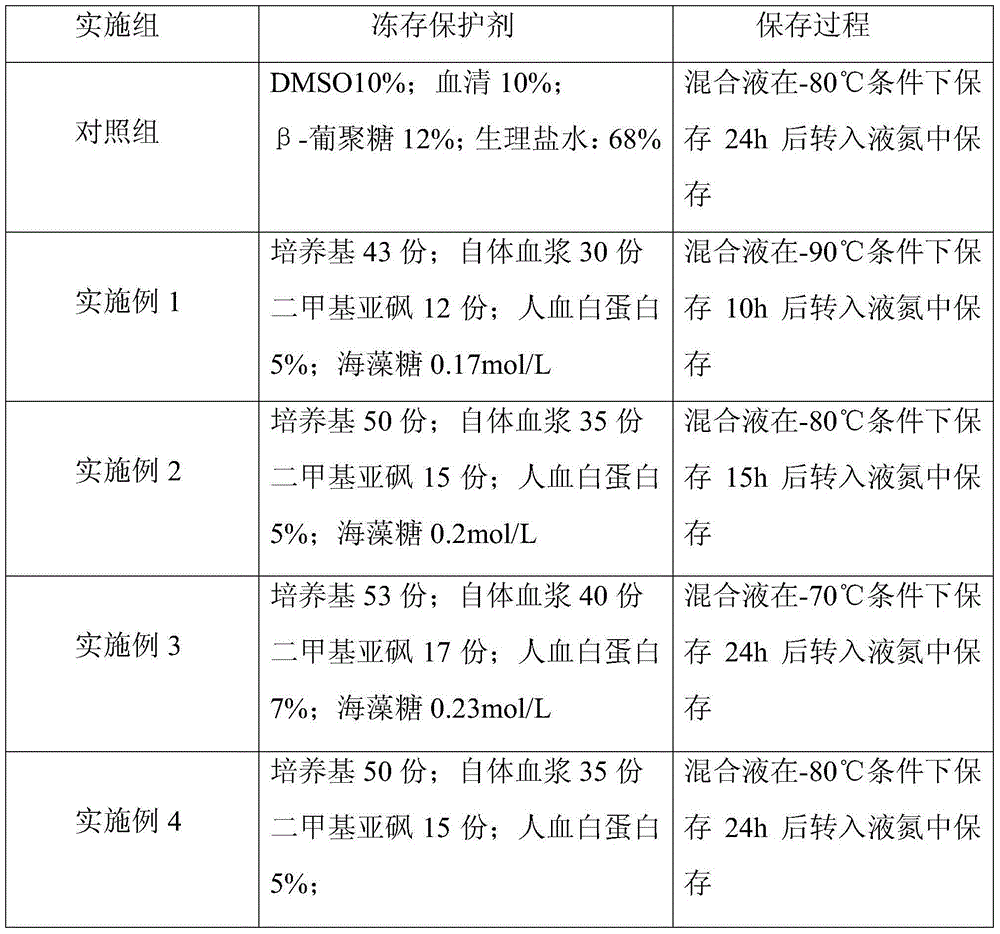
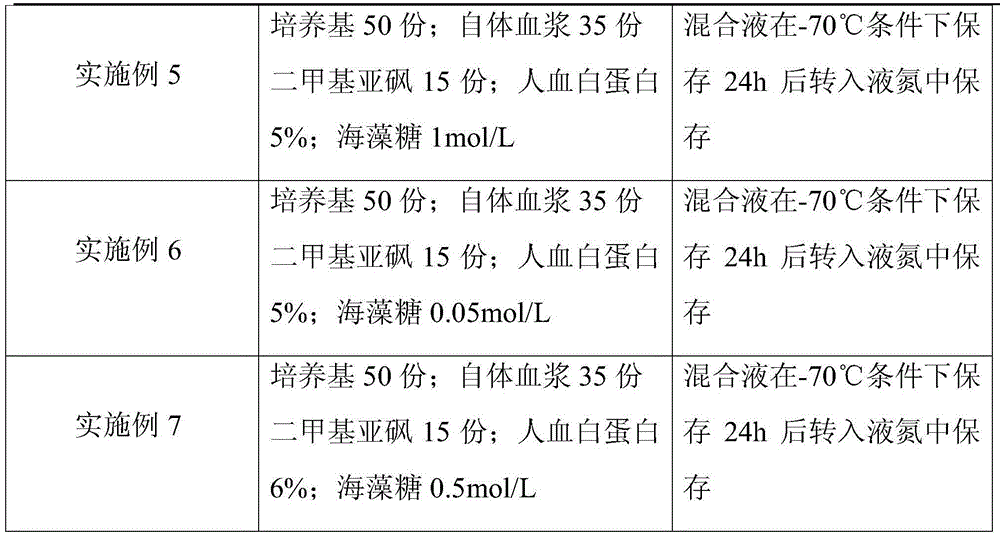
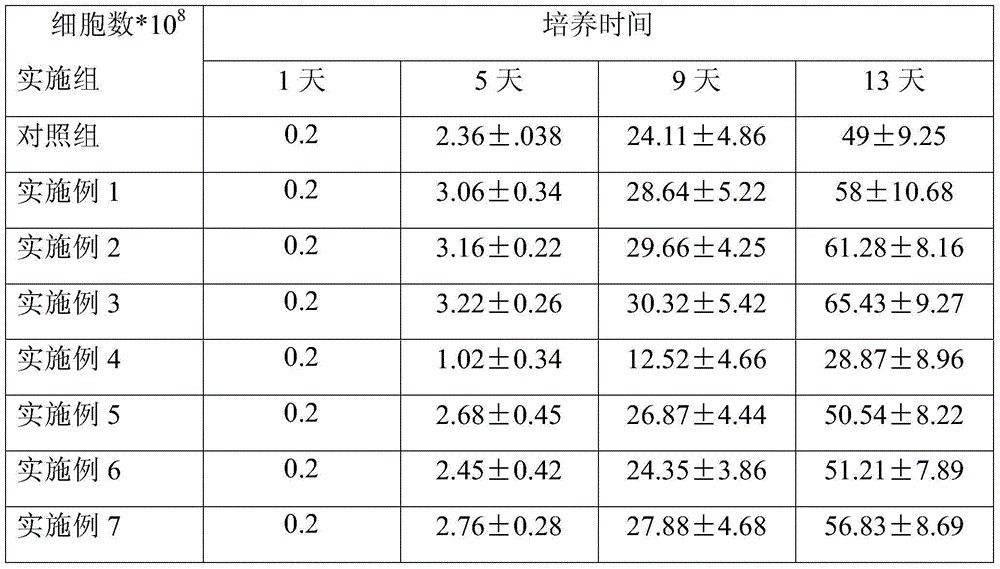
Cryoprotective agent of peripheral blood mononuclear cells and preservation method of cryoprotective agent
ActiveCN104082277AImprove survival rateImprove cell activityDead animal preservationPeripheral blood mononuclear cellMotility
The invention discloses a cryoprotective agent of peripheral blood mononuclear cells. The cryoprotective agent is prepared from the following ingredients in parts by weight: 43-53 parts of a culture medium, 30-40 parts of auto-plasma, 12-17 parts of dimethyl sulfoxide, 5-7% of human serum albumin, and the balance being 0.17-0.23mol / L trehalose. The invention discloses a preservation method of the cryoprotective agent. The preservation method comprises the steps of collecting fresh anticoagulation blood, preparing peripheral mononuclear cells into cell suspension by using human serum albumin, adding the cryoprotective agent with the same volume as that of the cell suspension, mixing uniformly, preserving for 10-20h at below 90-below-70-below DEG C, and preserving in liquid nitrogen. According to the cryoprotective agent and the preservation method thereof, a cryopreserved cell has the high motility rate and the strong cell activity and can shorten the Cytokine Induced Killer (CIT) cell induced amplification period.
Owner:CHENGDU QINGKE BIOTECH
Fusion protein of Exendin-4 tandem polypeptide and human serum albumin, preparation and application thereof
ActiveCN101525386AGenetic stabilityNot easy to losePeptide/protein ingredientsMetabolism disorderHalf-lifeSerum albumin
The invention relates to a fusion protein of Exendin-4 tandem polypeptide and human serum albumin, a preparation method and application thereof. The fusion protein of the Exendin-4 tandem polypeptide and the human serum albumin is expressed by a genetic engineering method. The fusion protein shows long-acting controlling blood sugar activity in a body, solves the problem that the half life period of the Exendin-4 tandem polypeptide in the body is short and has the advantage of convenient preparation. The fusion protein can be used for preparing diabetes medicaments and weight-losing products.
Owner:浙江昂大久力生物制药有限公司
Modified human serum albumin with reduced or eliminated affinity to chemical or biological contaminants at Cys 34
InactiveUS20060018859A1Reducing a wide variety of undesired chemical reactionsReducing and eliminating undesirable incorporationCosmetic preparationsPeptide/protein ingredientsAmino acid deficiencyHypersensitive response
A modified serum albumin is provided which has been modified at position cysteine 34 of human serum albumin, or at equivalent locations in other mammalian serum albumins, with an amino acid which lacks a sulphydryl group and will thus be less reactive with biological or chemical molecules such as trace metals at this site. Particularly suitable amino acids to substitute for cysteine at position 34 include methionine and other amino acids which are less reactive than cysteine and which maintain the same folding and antigenicity of the native serum albumin. The modified albumin of the present invention is advantageous in that it is binding to trace metals and other contaminants is reduced or eliminated, and it can thus be used more safely and effectively than unmodified albumin with a reduced or eliminated likelihood of causing an allergic reaction in the human being treated with the albumin composition.
Owner:CARTER DANIEL C
Serum albumin binding molecules
ActiveUS9540424B2Extended half-lifeReduced potencyPeptide/protein ingredientsAntibody mimetics/scaffoldsSerum igeSerum albumin
The present invention relates to an antibody-like protein based on the tenth fibronectin type III domain (10Fn3) that binds to serum albumin. The invention further relates to fusion molecules comprising a serum albumin-binding 10Fn3 joined to a heterologous protein for use in diagnostic and therapeutic applications.
Owner:BRISTOL MYERS SQUIBB CO
A sodium alginate microsphere vascular embolizing agent containing water soluble drug, its preparation and application
ActiveCN1879607AStrong hydrationImprove curative effectOrganic active ingredientsPharmaceutical non-active ingredientsSodium hyaluronateIon
The invention relates to a sodium alginate micro ball vessel suppository which contains soluble medicine, and relative preparation and application, wherein said agent can be divided into two kinds as wet ball and dry ball made from degradable biological material; said carrier comprises sodium alginate, blood serum albumin, chitose, or transparent sodium solution, via the static to be solidified with calcium ion, to be made into the micro ball at 20 mum-1000 mum. The inventive material has high mechanical strength, biological compatibility, biological degradability and stability. The invention can be used to cure the vessel suppository of variable cancers.
Owner:BEIJING SHENGYIYAO SCI & TECH DEV
Blood-purifying adsorbing agent for cleaning antibody
InactiveCN101279242AHigh adsorption selectivityImprove stabilityOther blood circulation devicesOther chemical processesImmune complex depositionSorbent
A blood purification sorbent for antibody removal belongs to the technical field of biomedicine, which consists of the two parts of solid-phase carrier material and a petunidin fixed on the carrier by chemical coupling. The molecular structure of the petunidin is shown as above, wherein, one atom among A, B and C is N, the others are C; n is 0-2. The blood purification sorbent can adsorb the antibody component in plasma and autoantibodies such as rheumatoid factor, antinuclear antibody, etc. in heavy load, has limited nonspecific adsorption to other plasma components such as seralbumin, etc., and also has low preparation cost and stable physicochemical property. The material can be used as adsorption filler of a blood purification device for removing the autoantibody and immune complex in the plasma.
Owner:DALIAN UNIV OF TECH
Expression of human serum albumin (HSA) in monocot seeds
A method of producing human serum albumin (HSA) in monocot plant seeds, by transforming a monocot plant cell with a chimeric gene containing a promoter from the gene of a maturation-specific monocot plant storage protein, a first DNA sequence operably linked to the promoter and encoding a signal sequence, and a second DNA sequence linked in translation frame with the first DNA sequence and encoding HSA, where the first DNA sequence and the second DNA sequence together encode a fusion protein containing an N-terminal signal sequence and the HSA; growing a monocot plant from the transformed monocot plant cell for a time sufficient to produce seeds containing the HSA; and harvesting the seeds from the plant.
Owner:VENTRIA BIOSCIENCE
High encapsulation rate curcumin albumin nano pharmaceutical composition
InactiveCN103054810AHigh encapsulation efficiencyImprove bioavailabilityKetone active ingredientsGranular deliveryBovine serum albuminSerum albumin
The invention provides a pharmaceutical composition of curcumin and albumin nano particles, and a preparation method of the pharmaceutical composition. The albumin is made from human serum albumin or bovine serum albumin, or is prepared by taking ovalbumin as a raw material; and preferentially, the albumin is made from human serum albumin. The pharmaceutical composition prepared by the invention has better encapsulation rate.
Owner:CHANGZHOU TARGET MEDICINE TECH CO LTD
Immunochromatography reagent strip and sealing agent composition used for same
ActiveCN102426231AExtended service lifeEasy to useMaterial analysisReagent stripBovine serum albumin
The invention provides a sealing agent composition used for an immunochromatography reagent strip, which comprises protein, protein protective agent, surface active agent and first buffer solution, wherein the protein is casein, casein treatment solution, methylation bovine serum albumin (BSA) and human serum albumin; and the protein protective agent is saccharide. The sealing agent composition provided by the invention is capable of effectively reducing the background of the immunochromatography reagent strip, and is long in service life and high in detection sensitivity. The invention also provides the immunochromatography reagent strip.
Owner:SINOCARE
Serum albumin binding proteins with long half-lives
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides.
Owner:ABLYNX NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com