Patents
Literature
28257results about "Capsule delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
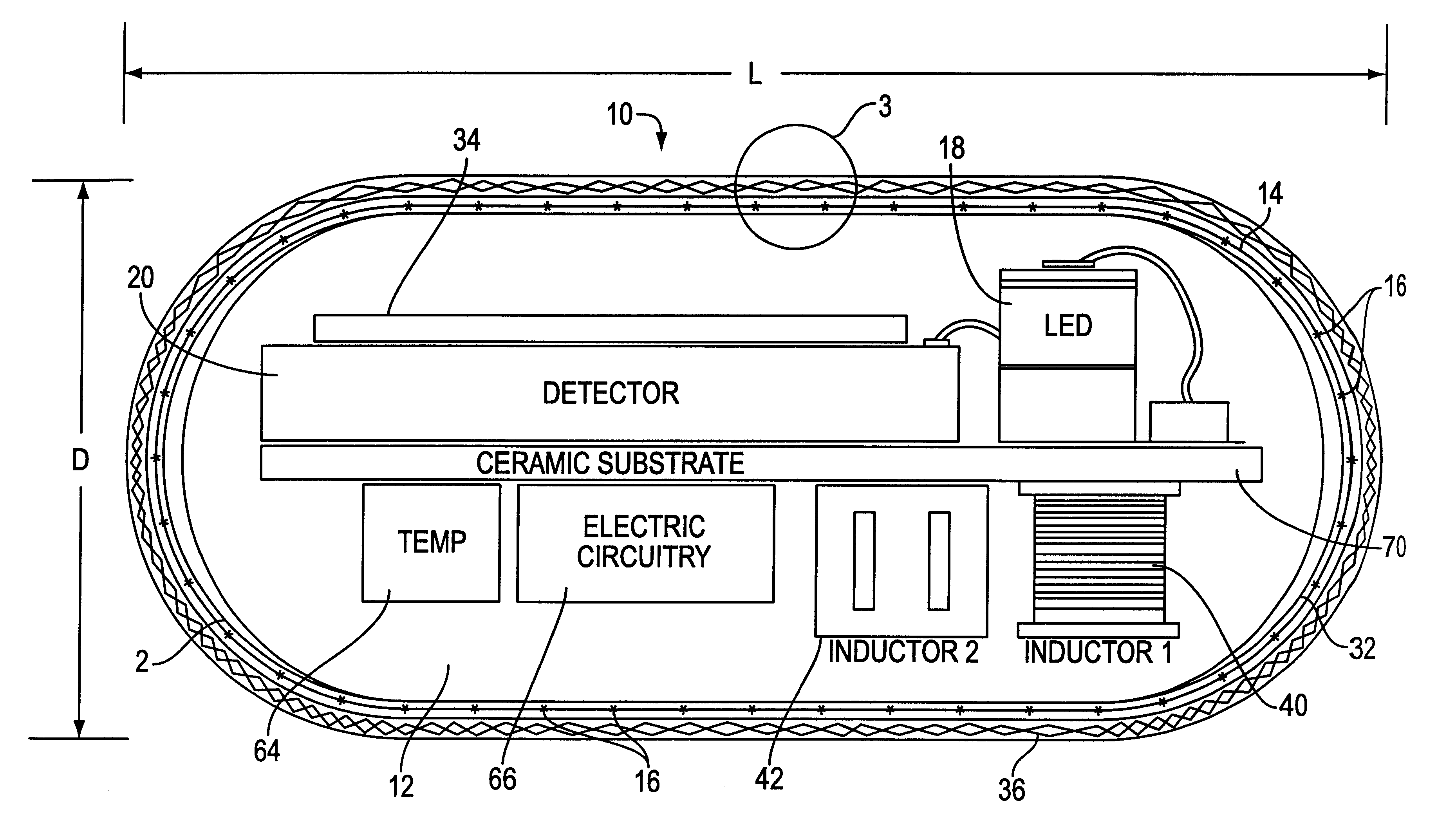
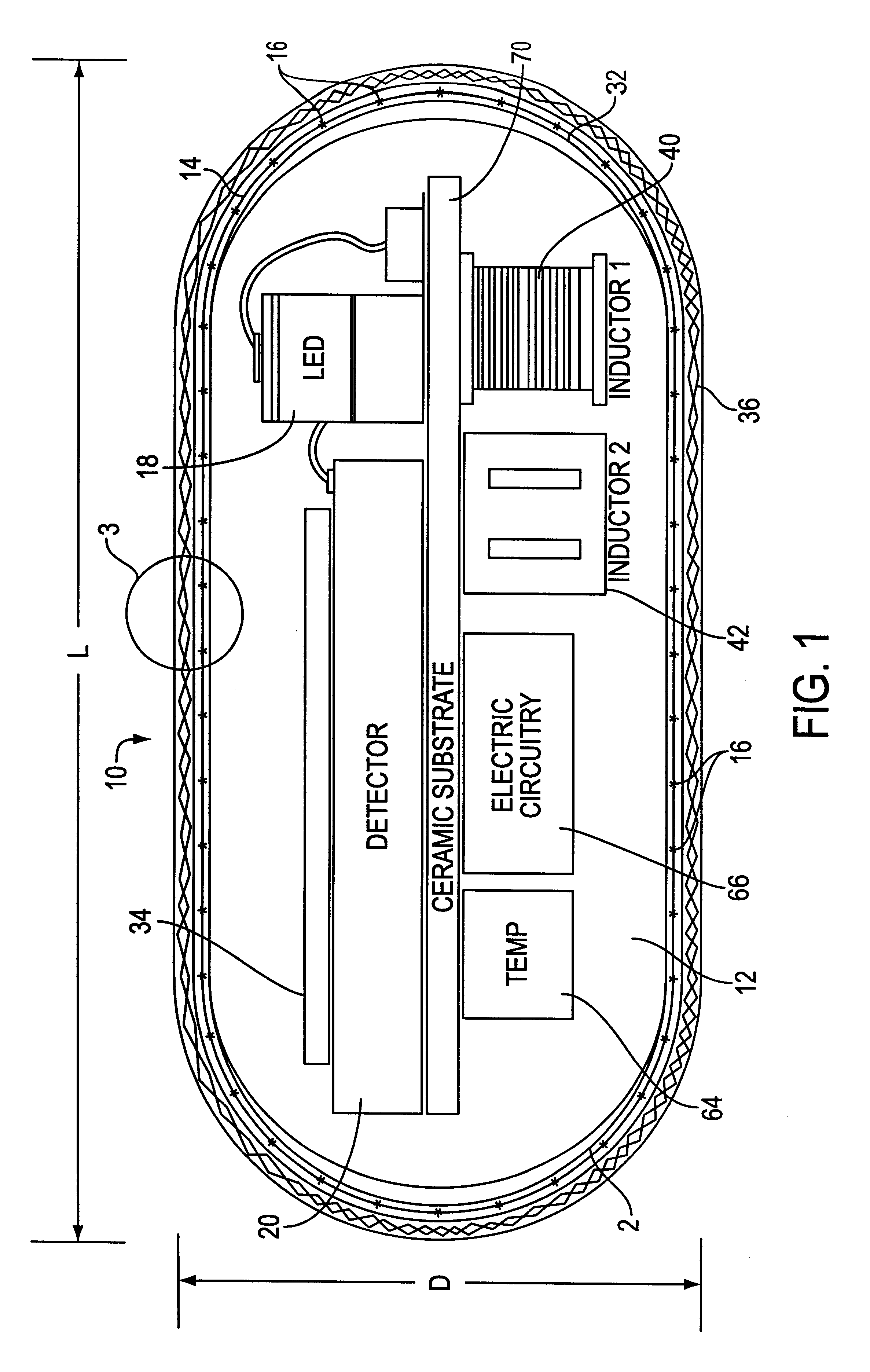
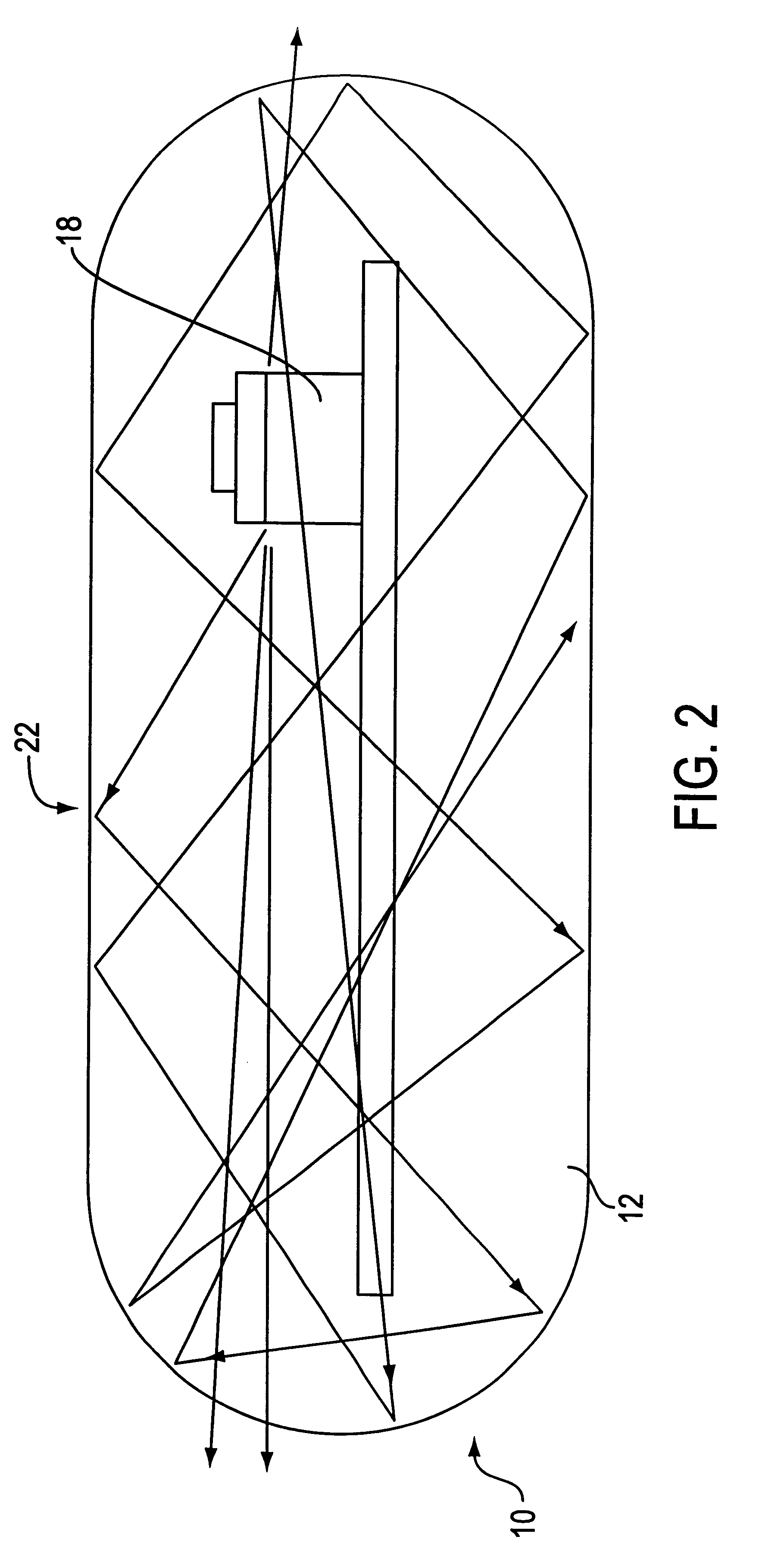
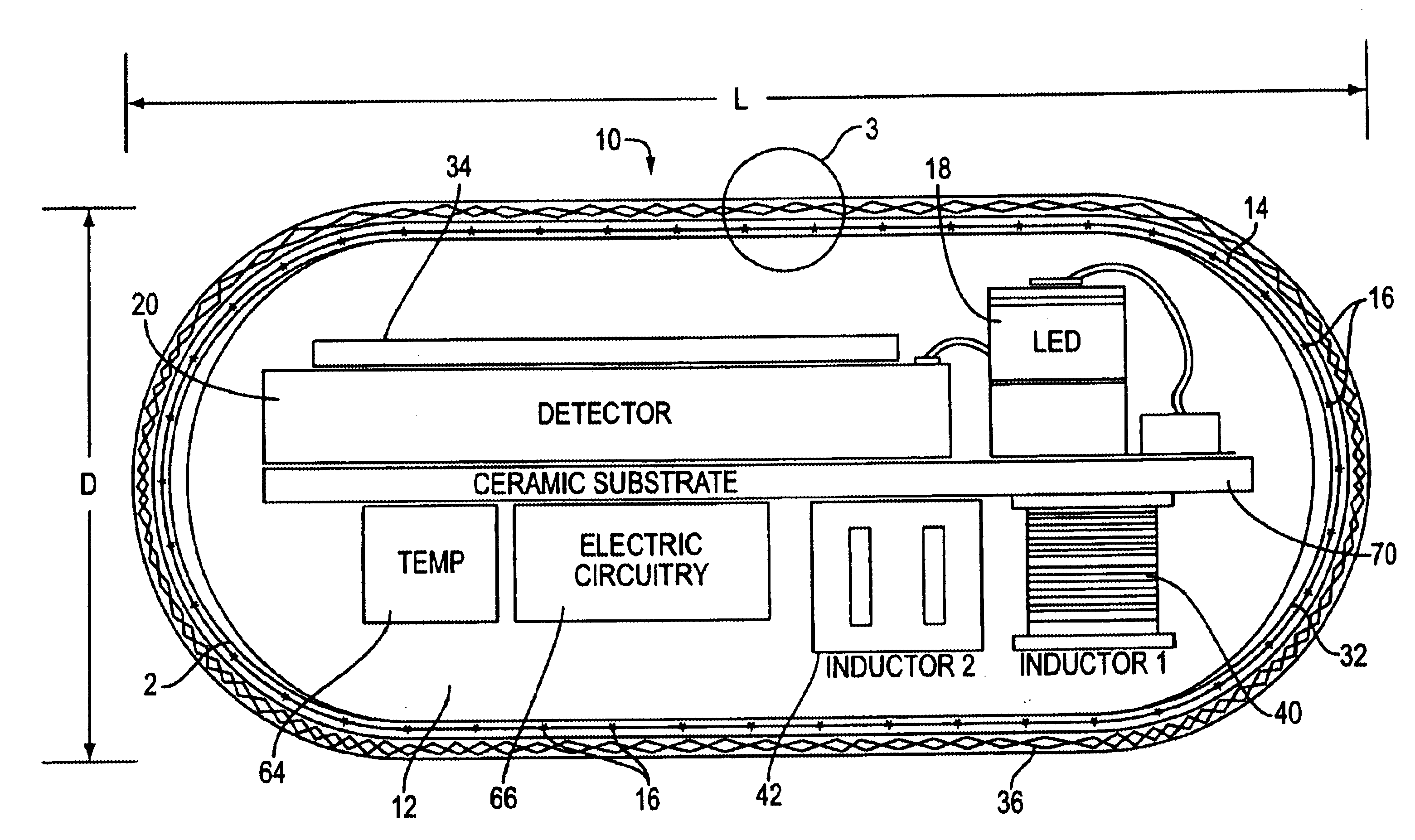
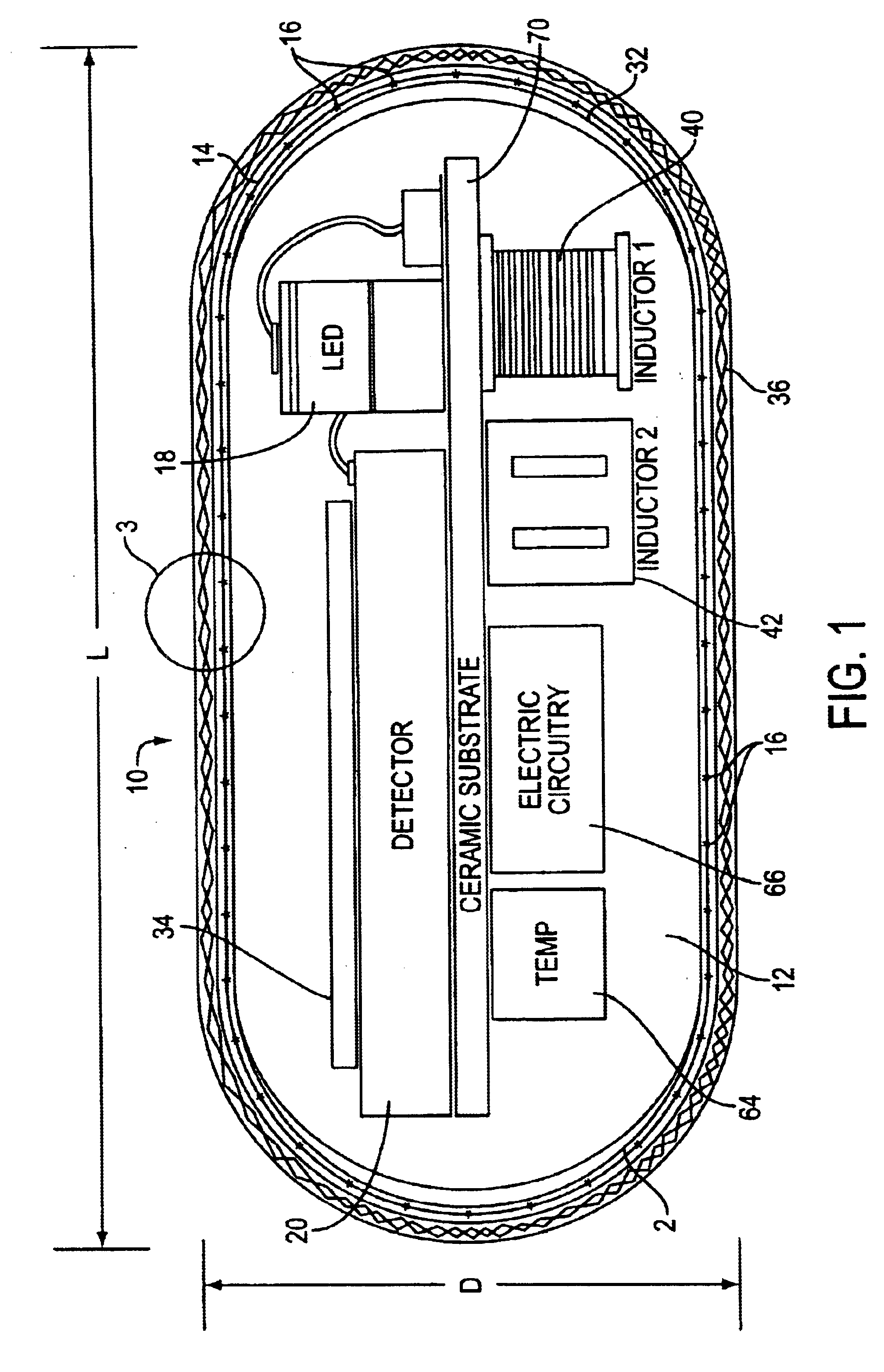
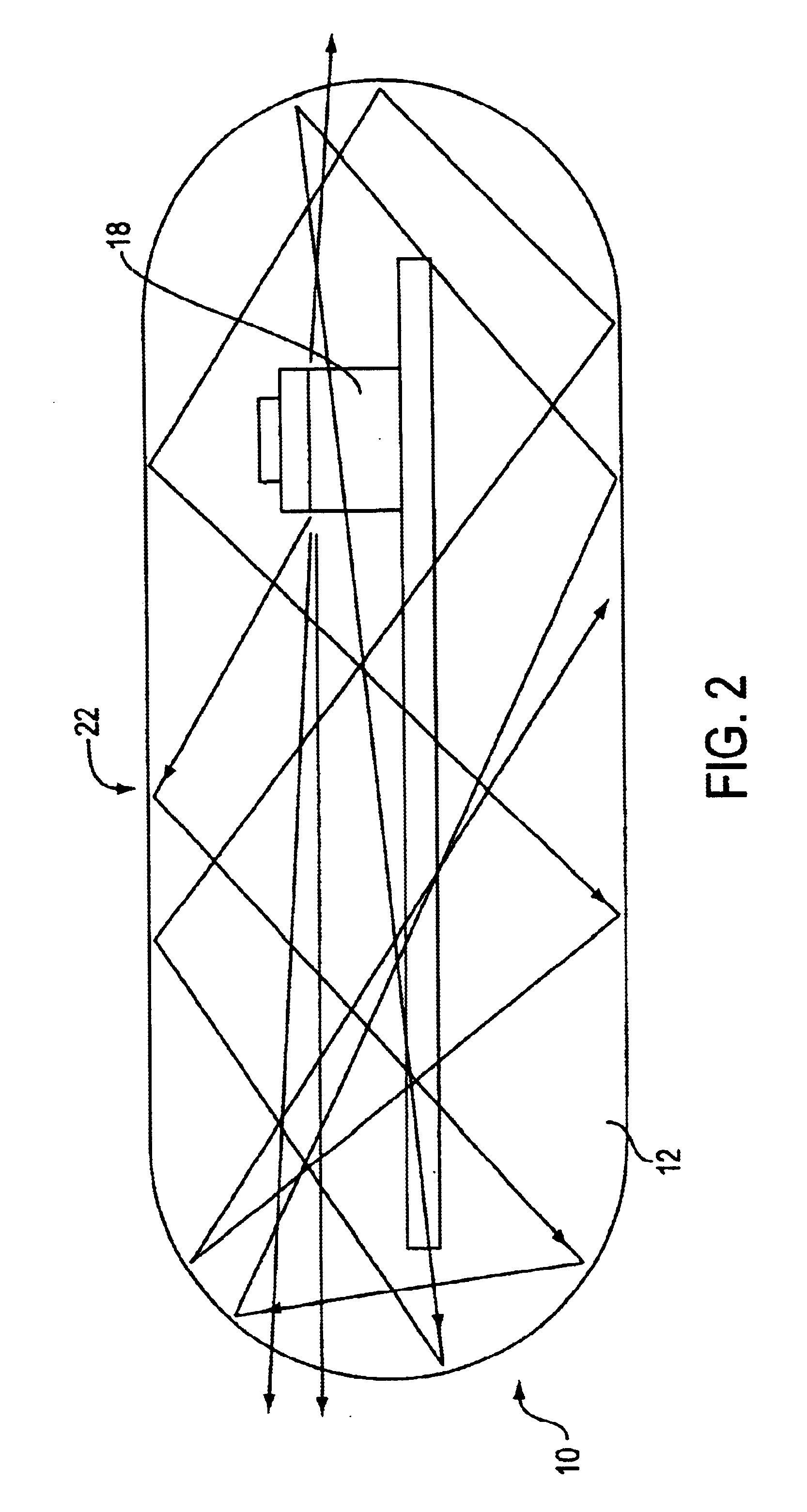
Optical-based sensing devices
InactiveUS6330464B1Material analysis by observing effect on chemical indicatorSurgeryAnalyteFluorescence
An optical-based sensor for detecting the presence or amount of an analyte using both indicator and reference channels. The sensor has a sensor body with a source of radiation embedded therein. Radiation emitted by the source interacts with indicator membrane indicator molecules proximate the surface of the body. At least one optical characteristic of these indicator molecules varies with analyte concentration. For example, the level of fluorescence of fluorescent indicator molecules or the amount of light absorbed by light-absorbing indicator molecules can vary as a function of analyte concentration. In addition, radiation emitted by the source also interacts with reference membrane indicator molecules proximate the surface of the body. Radiation (e.g., light) emitted or reflected by these indicator molecules enters and is internally reflected in the sensor body. Photosensitive elements within the sensor body generate both indicator channel and reference channel signals to provide an accurate indication of the concentration of the analyte. Preferred embodiments are totally self-contained and are sized and shaped for use in vivo in a human being. Such embodiments preferably include a power source, e.g. an inductor, which powers the source of radiation using external means, as well as a transmitter, e.g. an inductor, to transmit to external pickup means the signal representing the level of analyte.
Owner:SENSEONICS INC
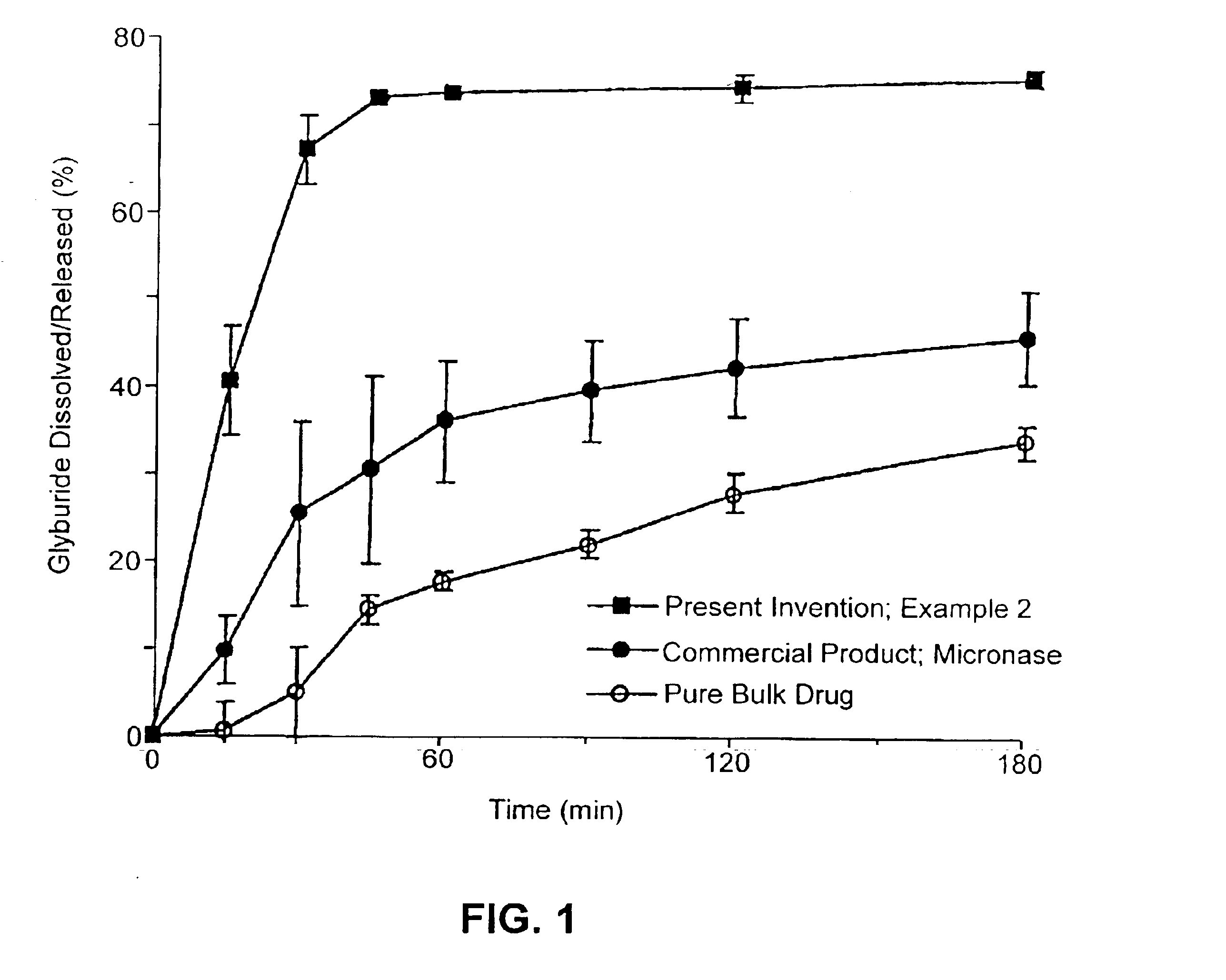
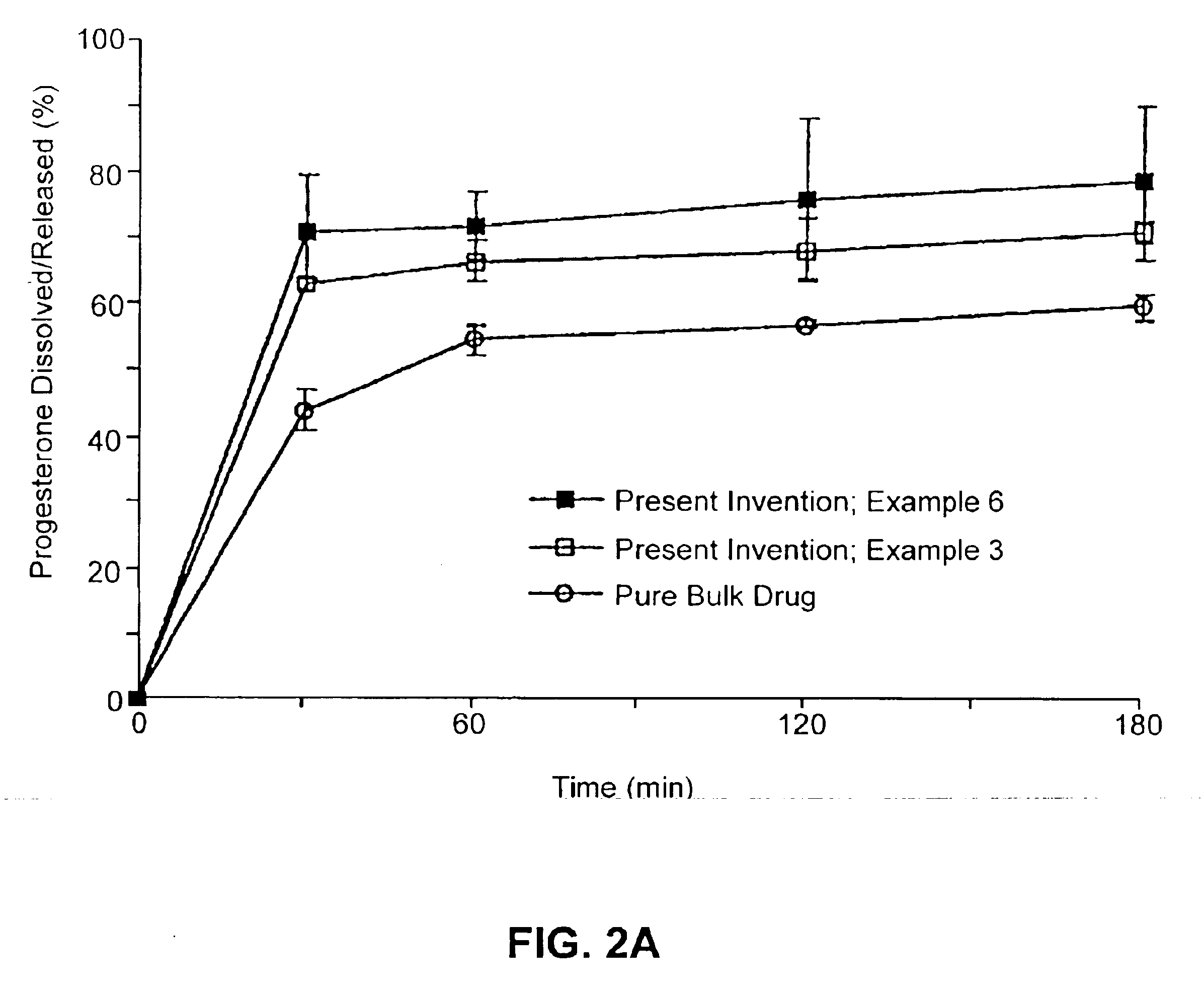
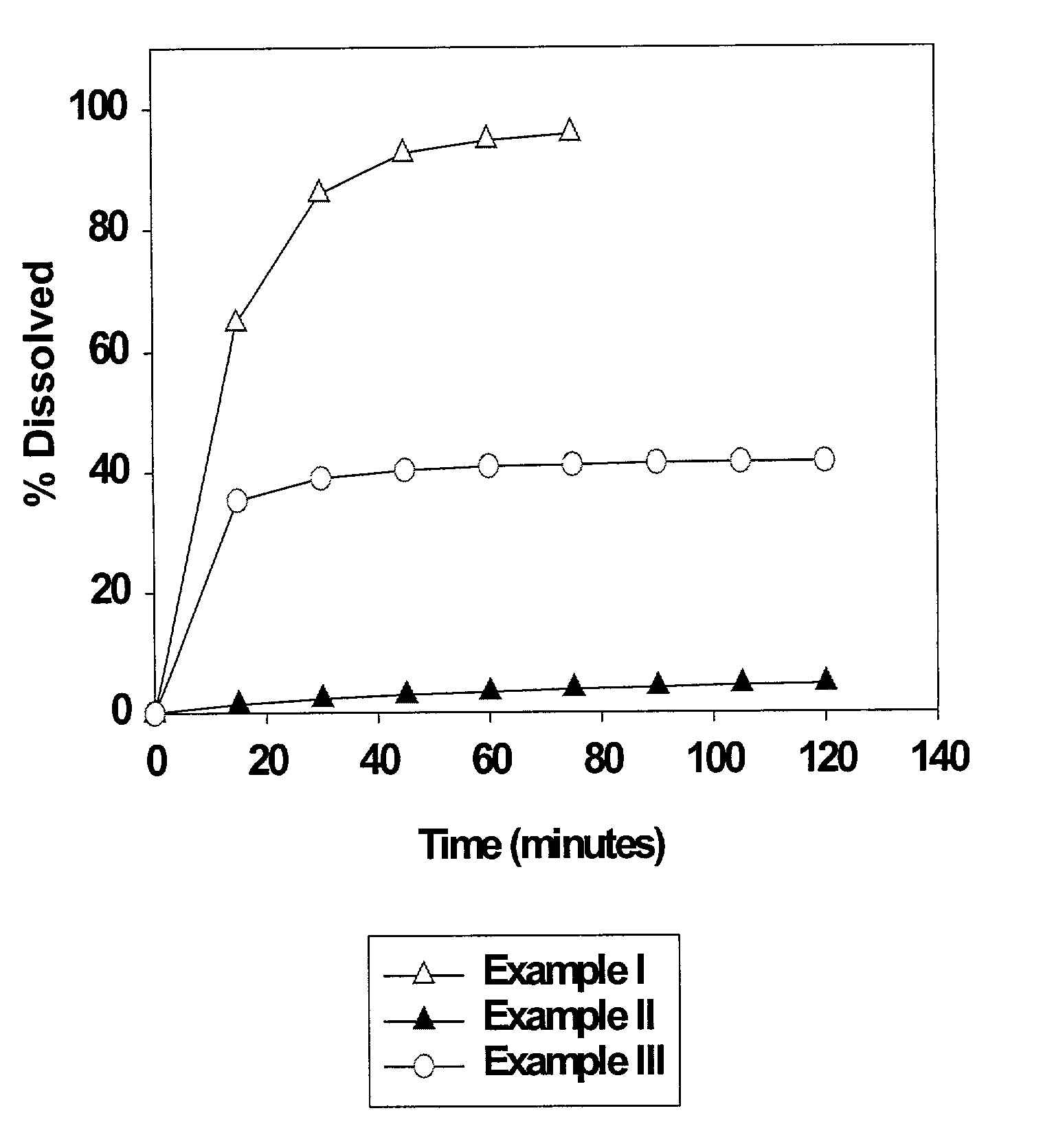
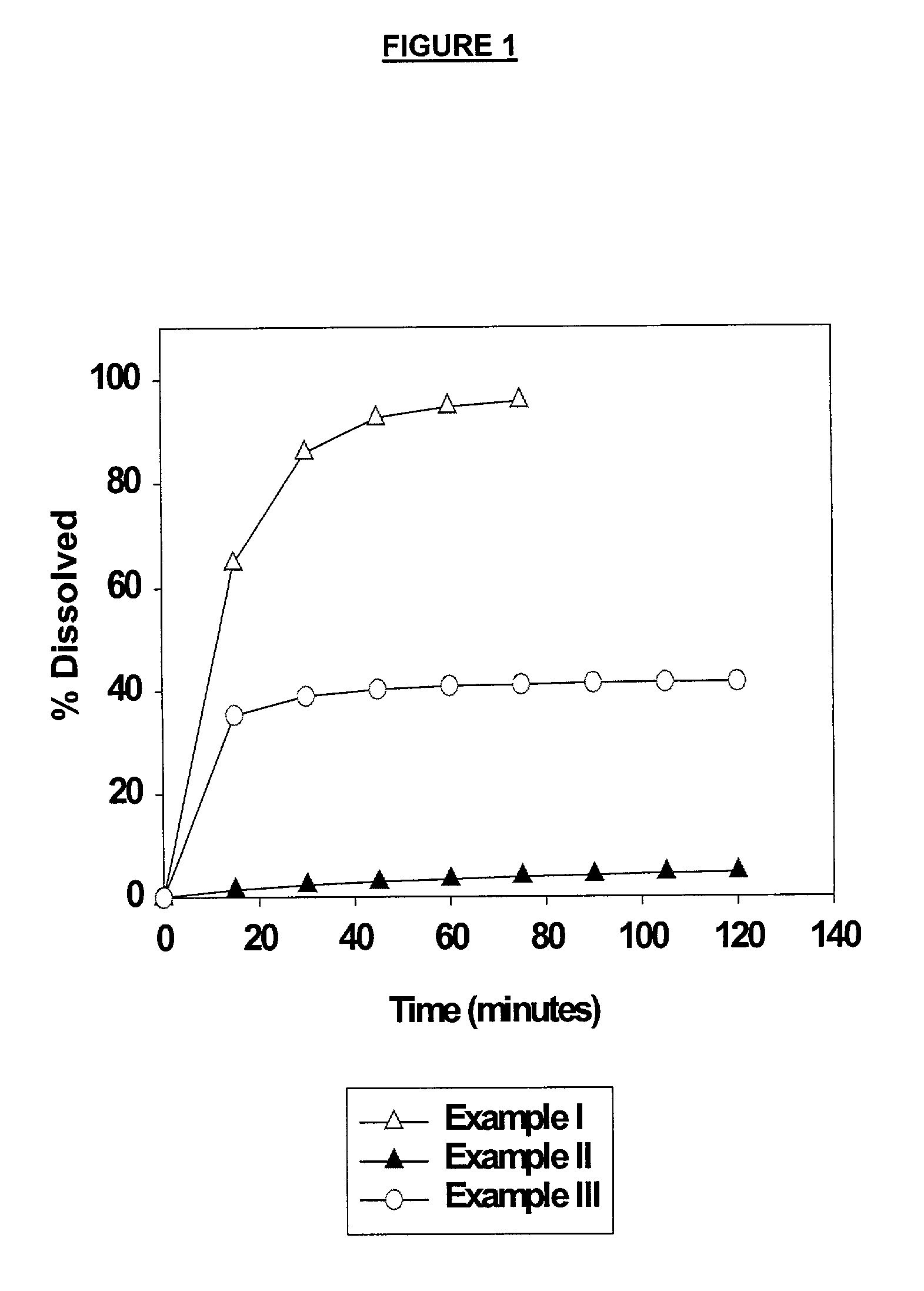
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
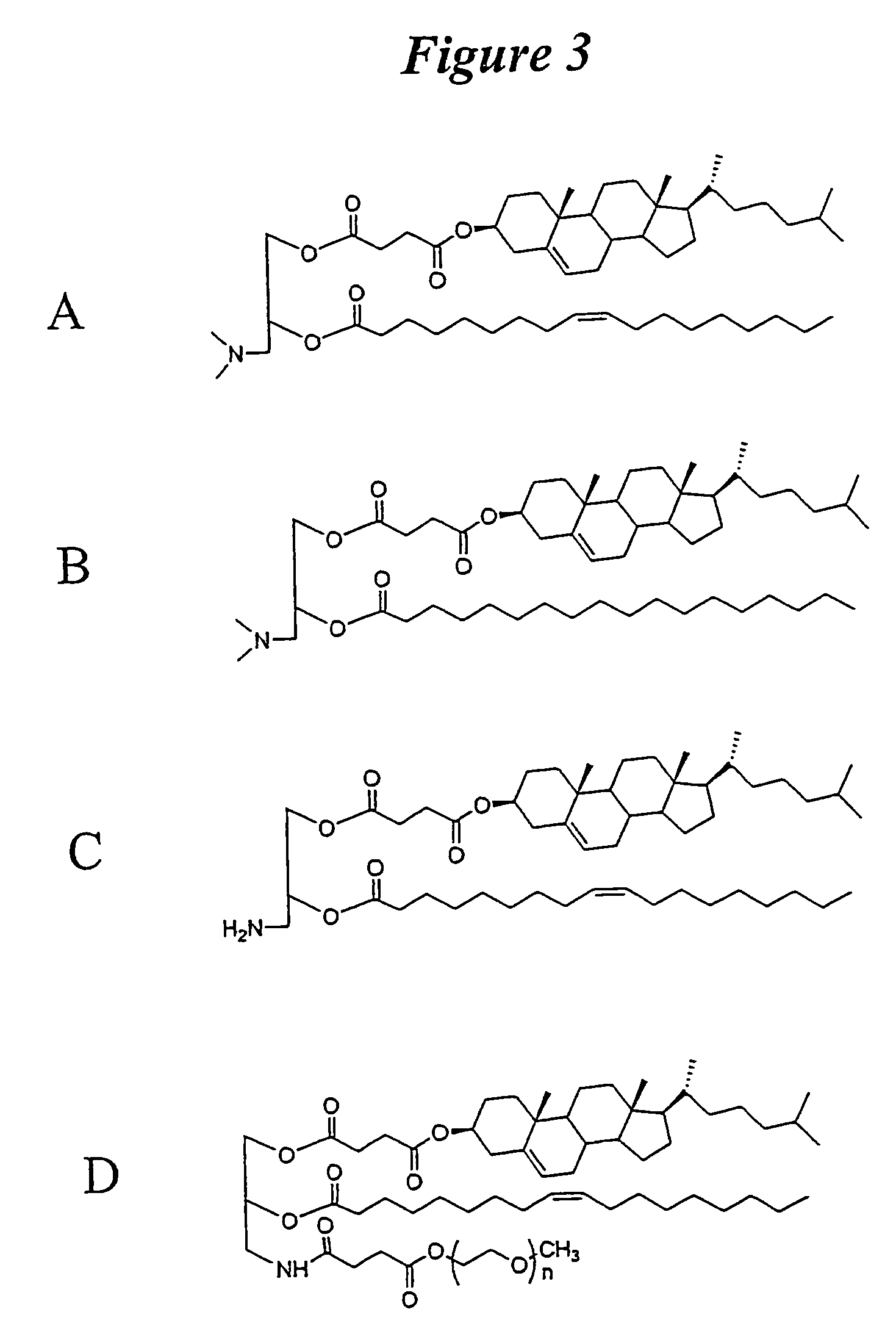
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Methods for producing droplets for use in capsule-based electrophoretic displays
Methods are provided for forming a dispersion of substantially uniform droplets. An internal phase that includes a plurality of particles suspended in a first fluid is provided and an external phase including a second fluid is provided. The internal phase is vibrated and the internal phase is applied to the external phase. Either the internal phase or a combination of the internal and external phases form a series of droplets or complex droplets of substantially uniform size.
Owner:E INK CORPORATION
Drug-eluting medical devices
Owner:ZILBERMAN MEITAL
Drug-eluting medical devices
Composite structures composed of a device as a core structure, being a medical device or article, and a porous polymeric coat and designed capable of encapsulating bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures.
Owner:RAMOT AT TEL AVIV UNIV LTD
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Pharmaceutical dosage forms for highly hydrophilic materials
Pharmaceutical dosage forms having a highly hydrophilic fill material and a shell encapsulating the fill material are disclosed and described. Generally, the shell has at least one plasticizing agent therein in order to provide the shell with an effective plasticity. In one aspect, the shell may have included therein an amount of plasticizing agent that is sufficient to provide the shell with an effective plasticity upon migration of a portion of the plasticizing agent into the fill material. In another aspect, the plasticizing agent may have a solubility in the fill material of less than about 10% w / w. In yet another aspect, a combination of a plasticizing agent, and a plasticizing agent having a solubility in the fill material of less than about 10% w / w, may be presented in a total amount sufficient to provide the shell with an effective plasticity upon migration of plasticizing agent into the fill material.
Owner:LIPOCINE
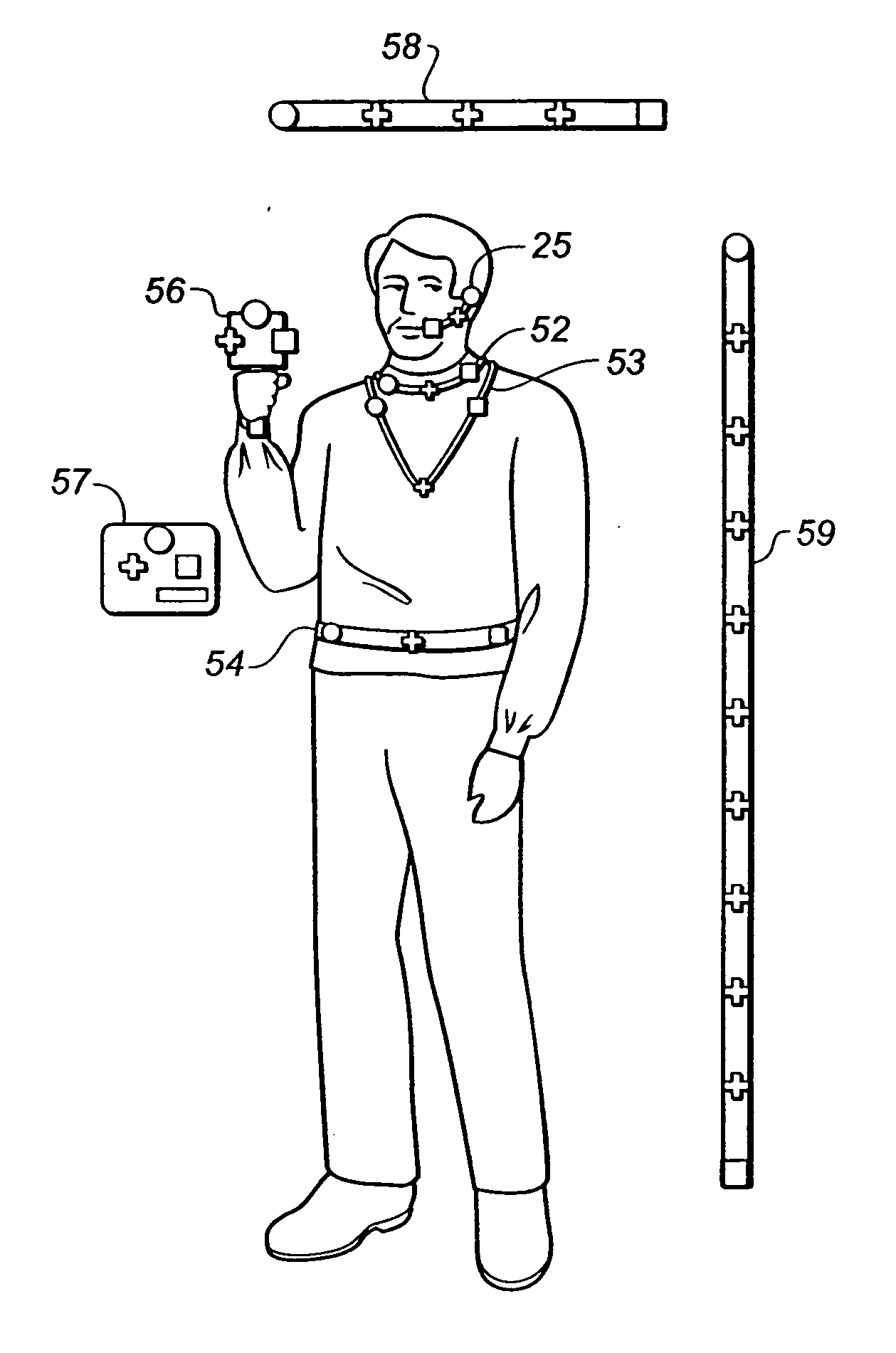
Health monitoring system
A health monitoring system which tracks the state of health of a patient and compiles a chronological health history of the patient uses a multiparametric monitor which periodically and automatically measures and records a plurality of physiological data from sensors in contact with the patient's body. The data collected is not specifically related to a particular medical condition but, instead, provides the information necessary to derive patterns which are characteristic of healthy patients as well as those who are ill. The data collected is periodically uploaded to a database in which it is stored along with similar health histories for other patients. The monitor is preferably self-contained in a chest strap which is located on the patient's torso, and makes use of a controller which controls sampling of the desired data and storage of the data to a local memory device pending uploading to the database. The more voluminous data collected is reduced and compressed prior to storage in the local memory device. Preferably, much of the monitor circuitry is run intermittently to conserve power. The monitor data is supplemented with subjective data (such as psychological and environmental conditions) collected from the patient using a handheld data input device which runs a program to solicit information from the patient. The subjective data collected is chronologically aligned with the monitor data in the database such that the health history of a patient includes both objective and subjective medical data.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Capsule and method for treating or diagnosing the intestinal tract
ActiveUS7160258B2Comprehensive knowledgeElectrotherapyPerson identificationMedicineElectrical stimulations
A device and method for mapping, diagnosing and treating the intestinal tract is provided using a capsule passing through the intestinal tract. Further, a capsule tracking system is provided for tracking a capsule's location along the length of an intestinal tract as various treatment and / or sensing modalities are employed. In one variation, an acoustic signal is used to determine the location of the capsule. A map of sensed information may be derived from the pass of a capsule. Capsules may be subsequently passed through to treat the intestinal tract at a determined location along its length. One variation uses an electrical stimulation capsule to treat and / or diagnose a condition in the intestinal tract.
Owner:ENTRACK INC
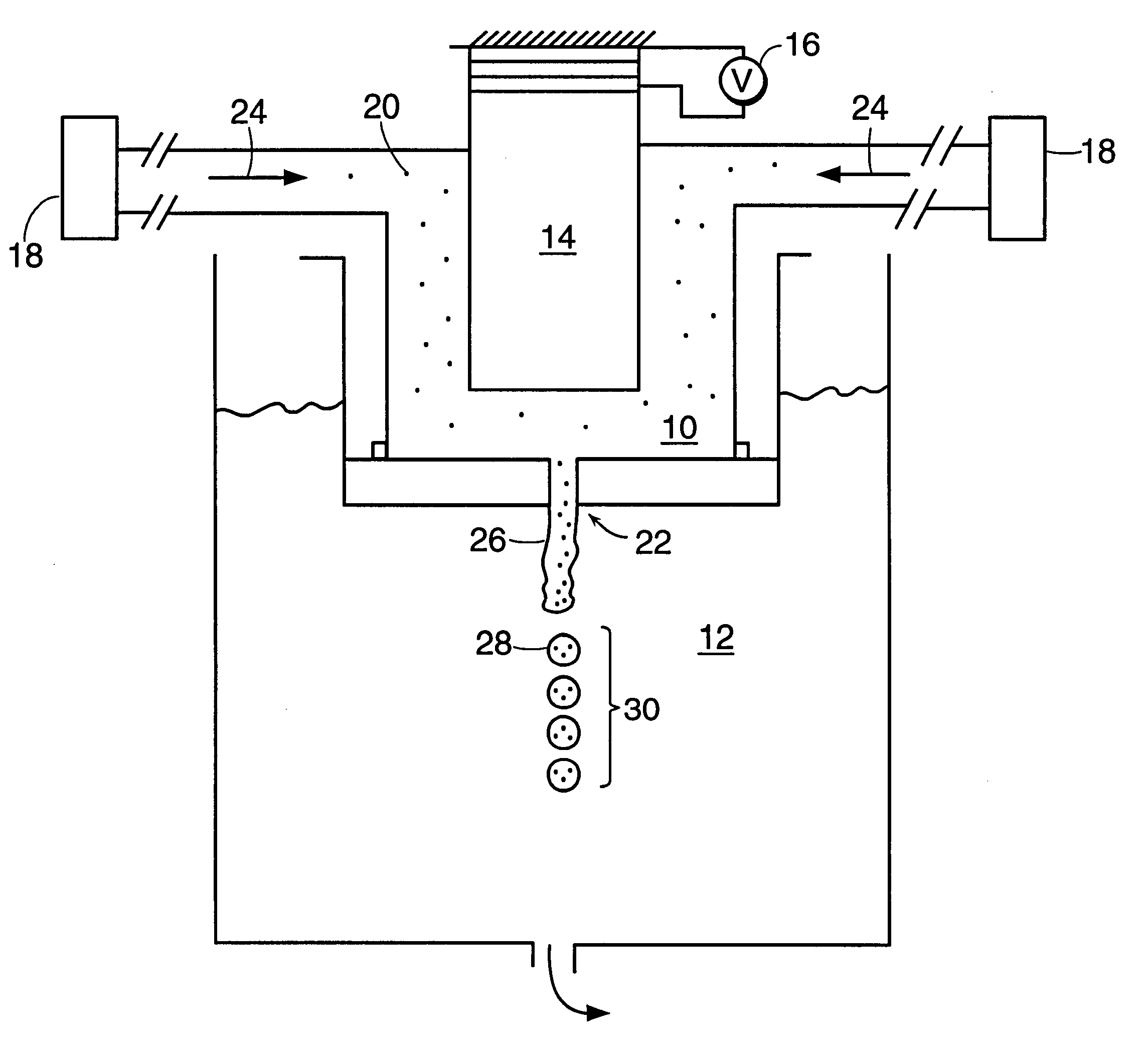
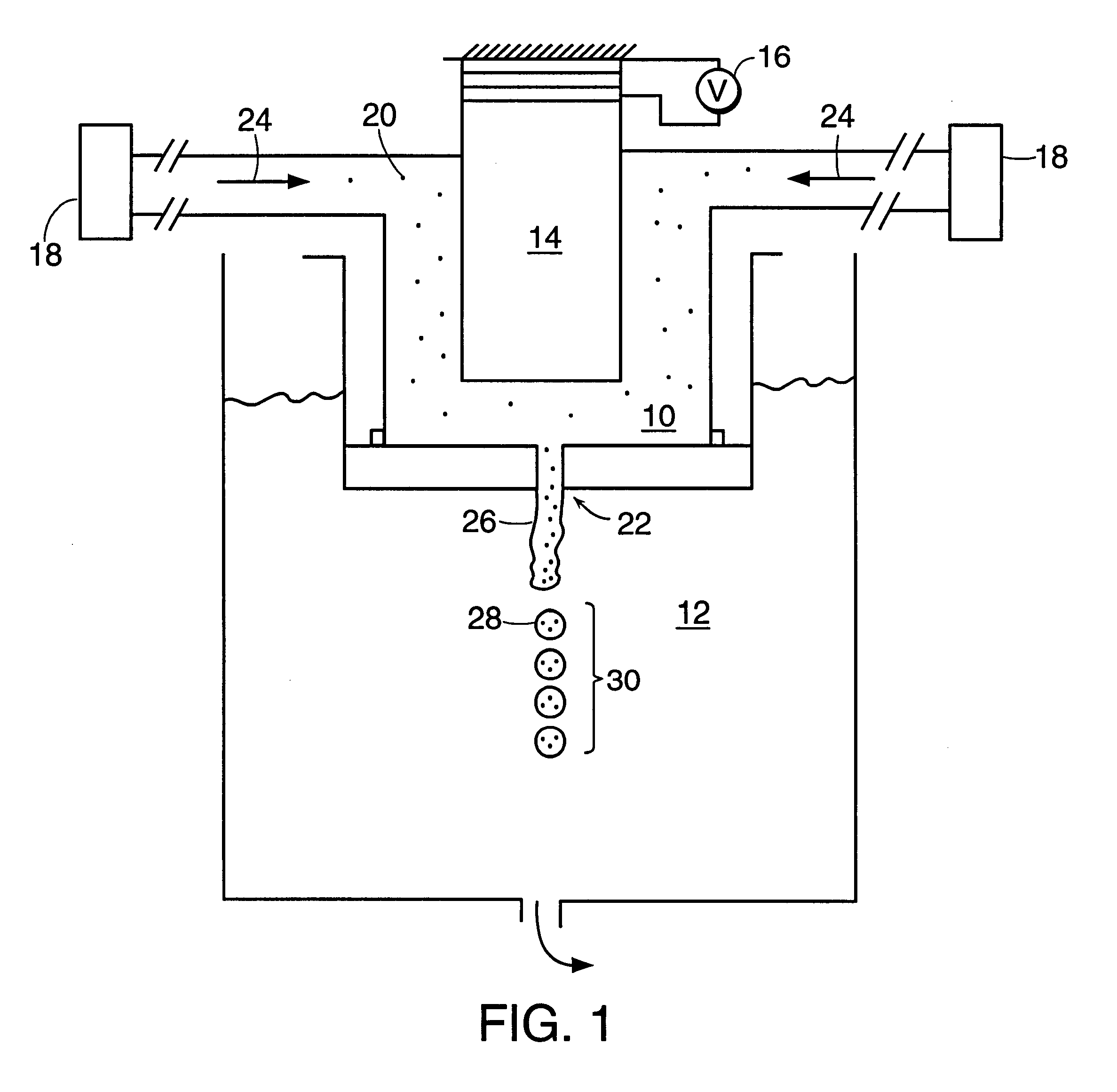
Perfume encapsulates
InactiveUS20040087477A1Improve stabilityImprove retentionGaseous substancesCapsule deliveryFlavorMelamine formaldehyde
A perfume encapsulate comprises an aminoplast capsule, the capsule shell comprising urea-formaldehyde or melamine-formaldehyde polymer and a second polymer comprising a polymer or copolymer of one or more anhydrides, preferably ethylene / maleic anhydride copolymer. The second polymer improves the stability of the capsules with respect to surfactant, thus improving perfume retention properties and enabling use of the capsules in aqueous surfactant-containing products in a way that has not hitherto been possible.
Owner:QUEST INTERNATIONAL
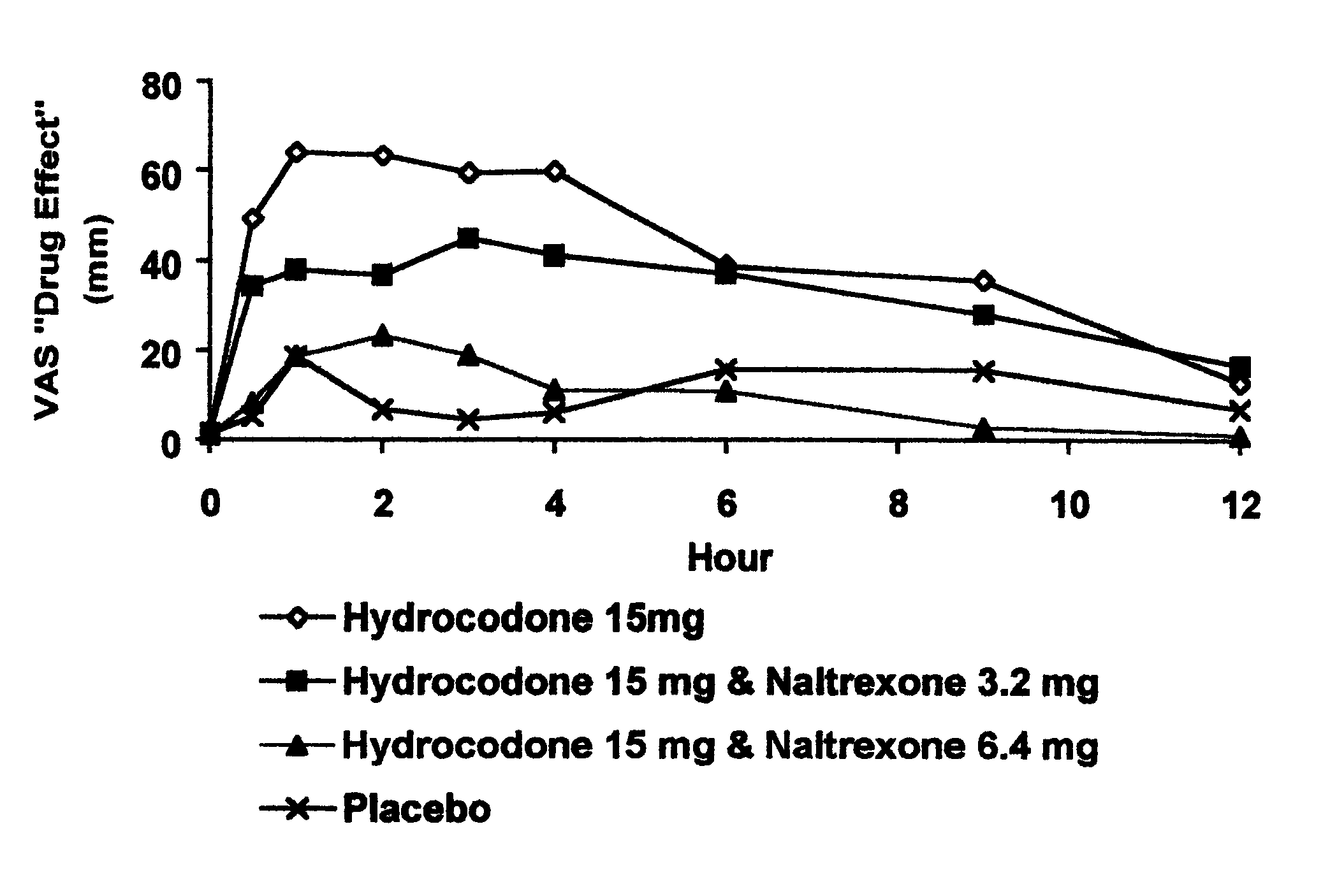
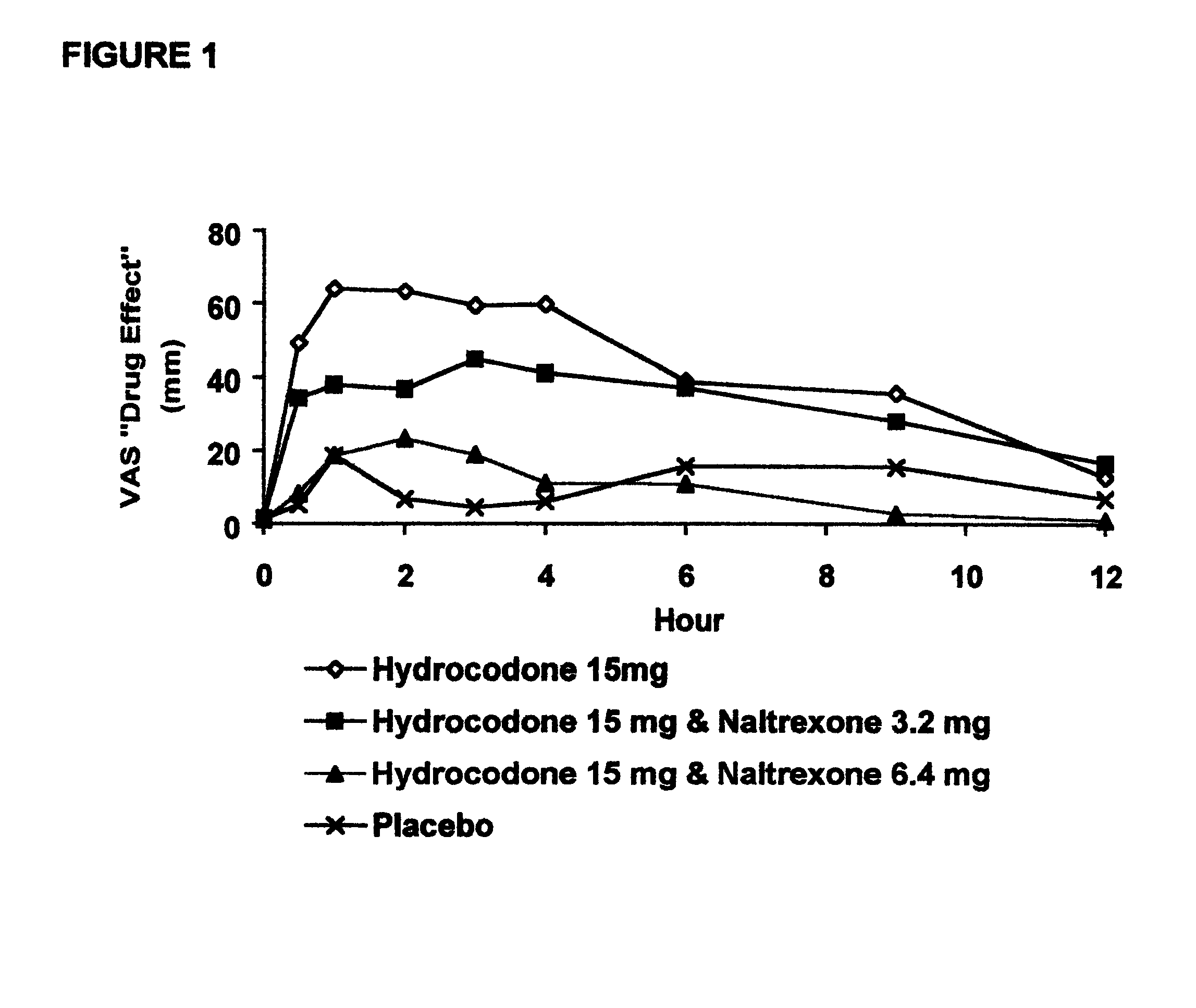
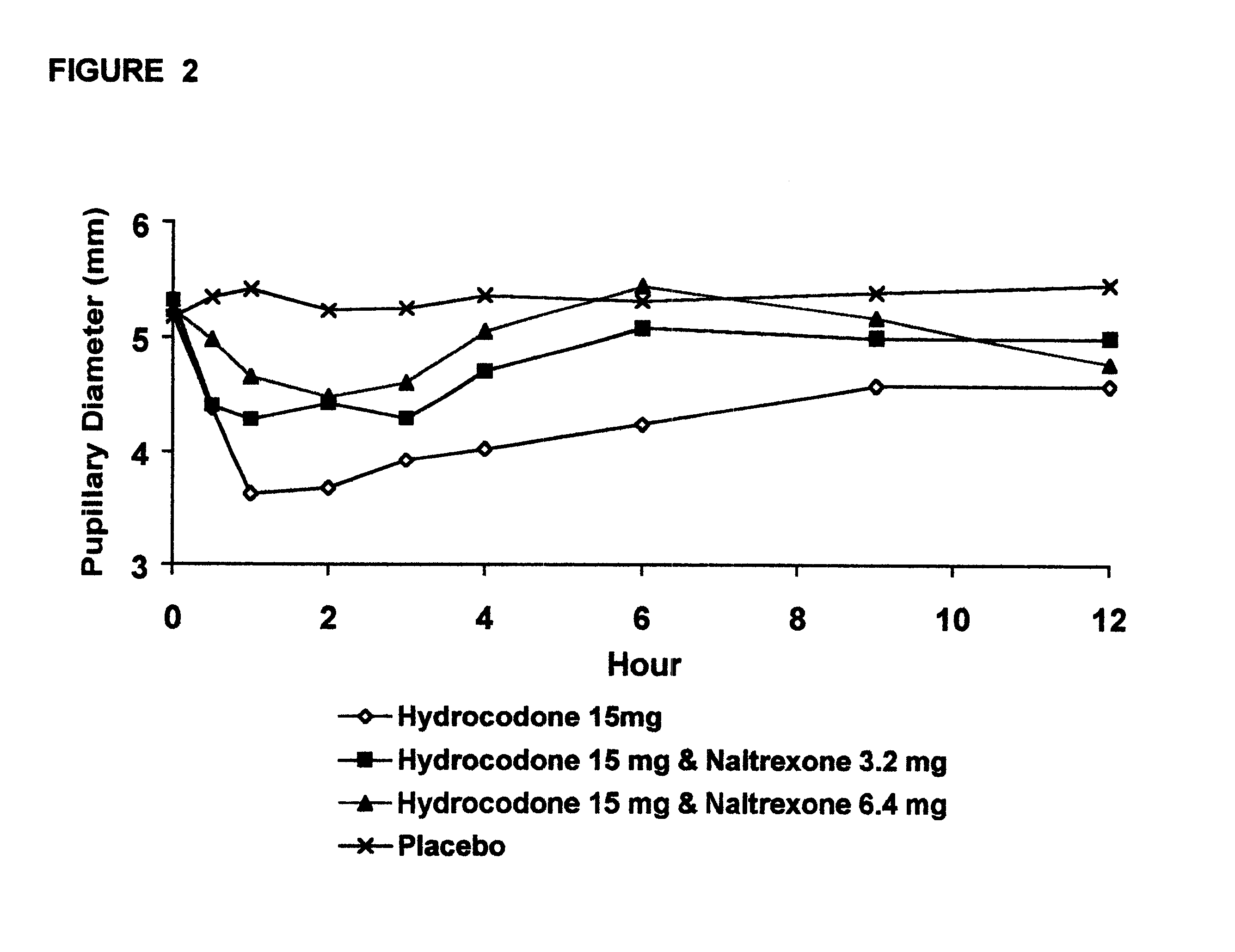
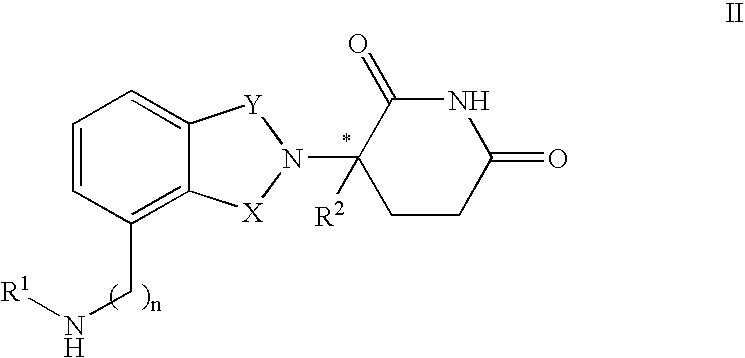
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS7404969B2Reduce deliveryAntibacterial agentsOrganic active ingredientsLipid formationMolecular composition
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver short interfering nucleic acid (siNA). The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
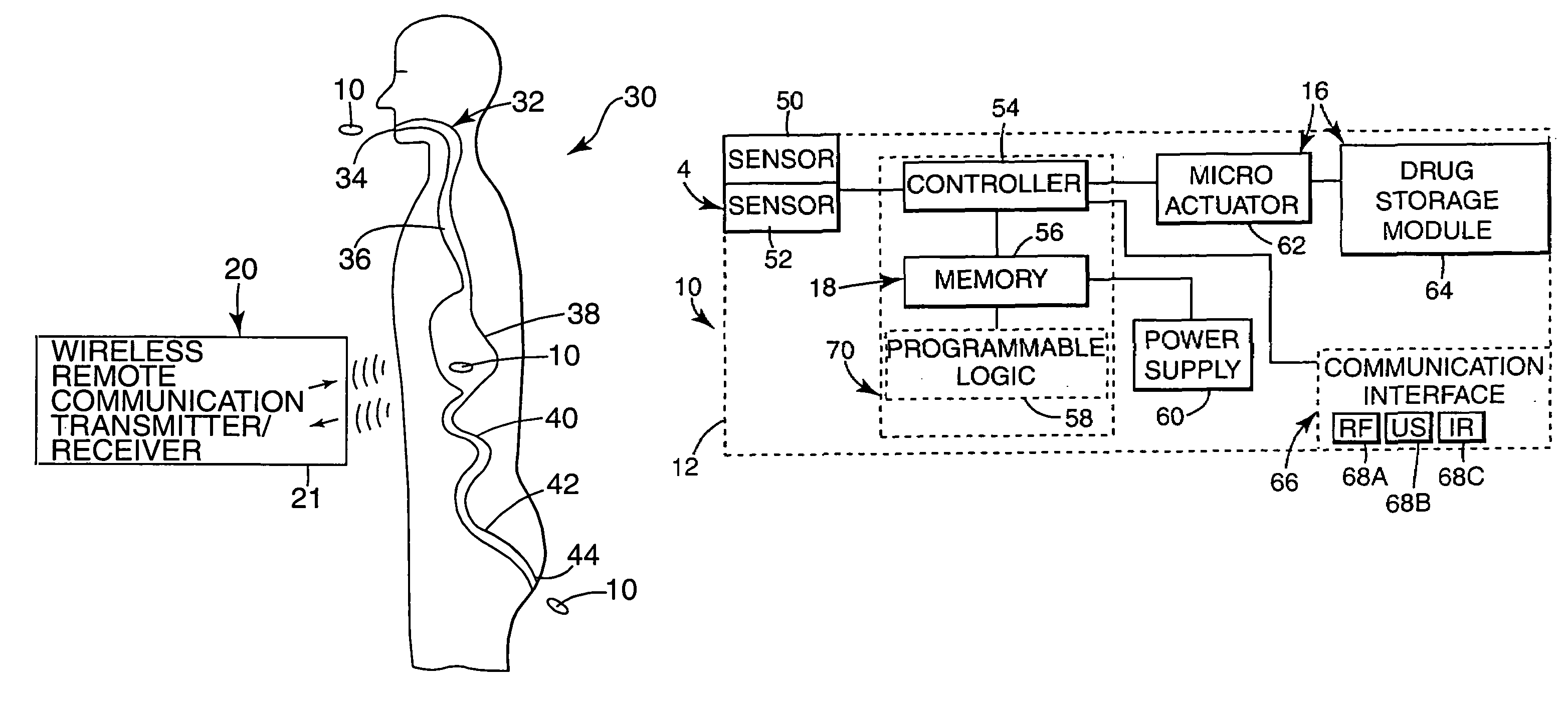
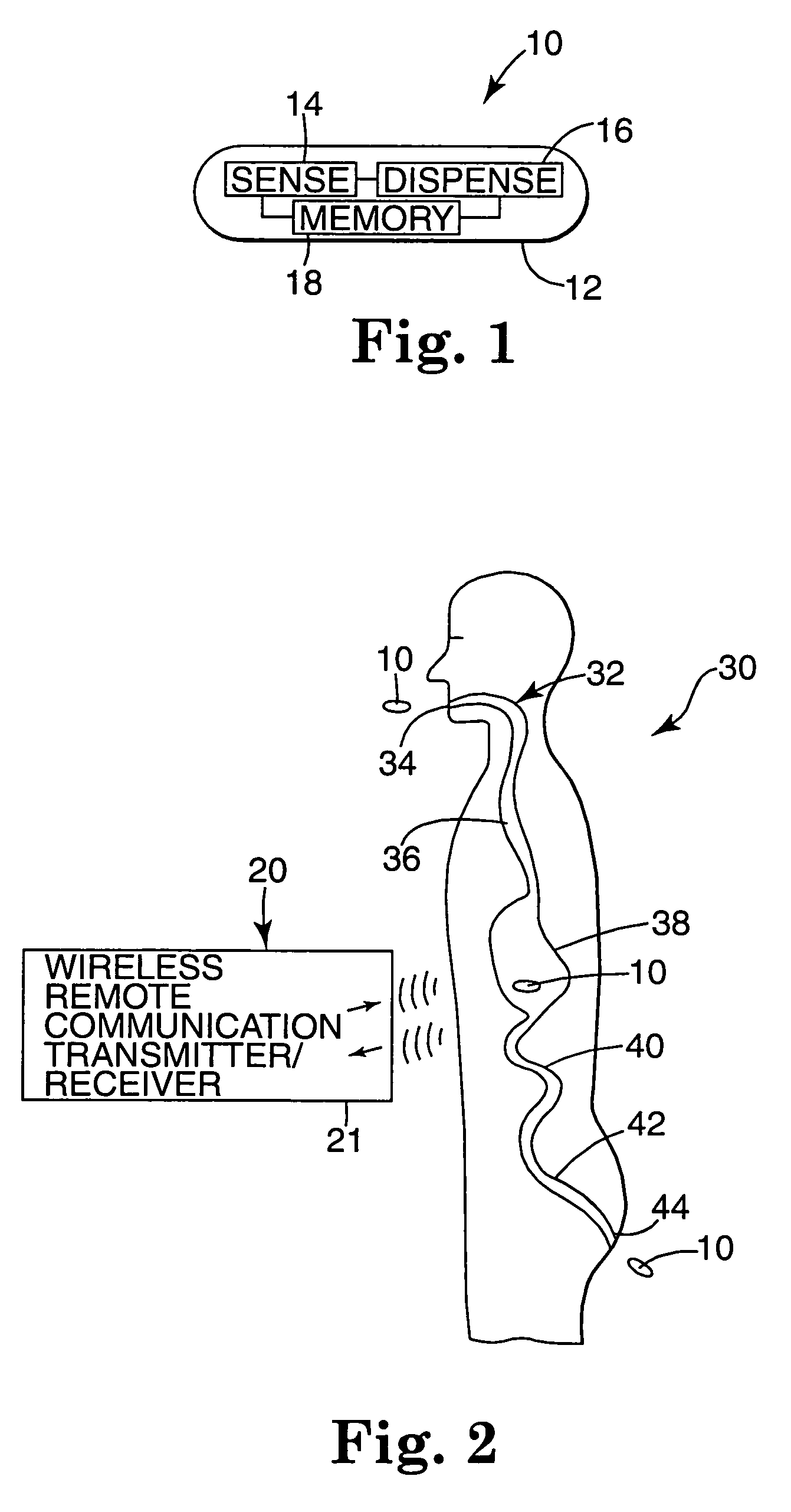
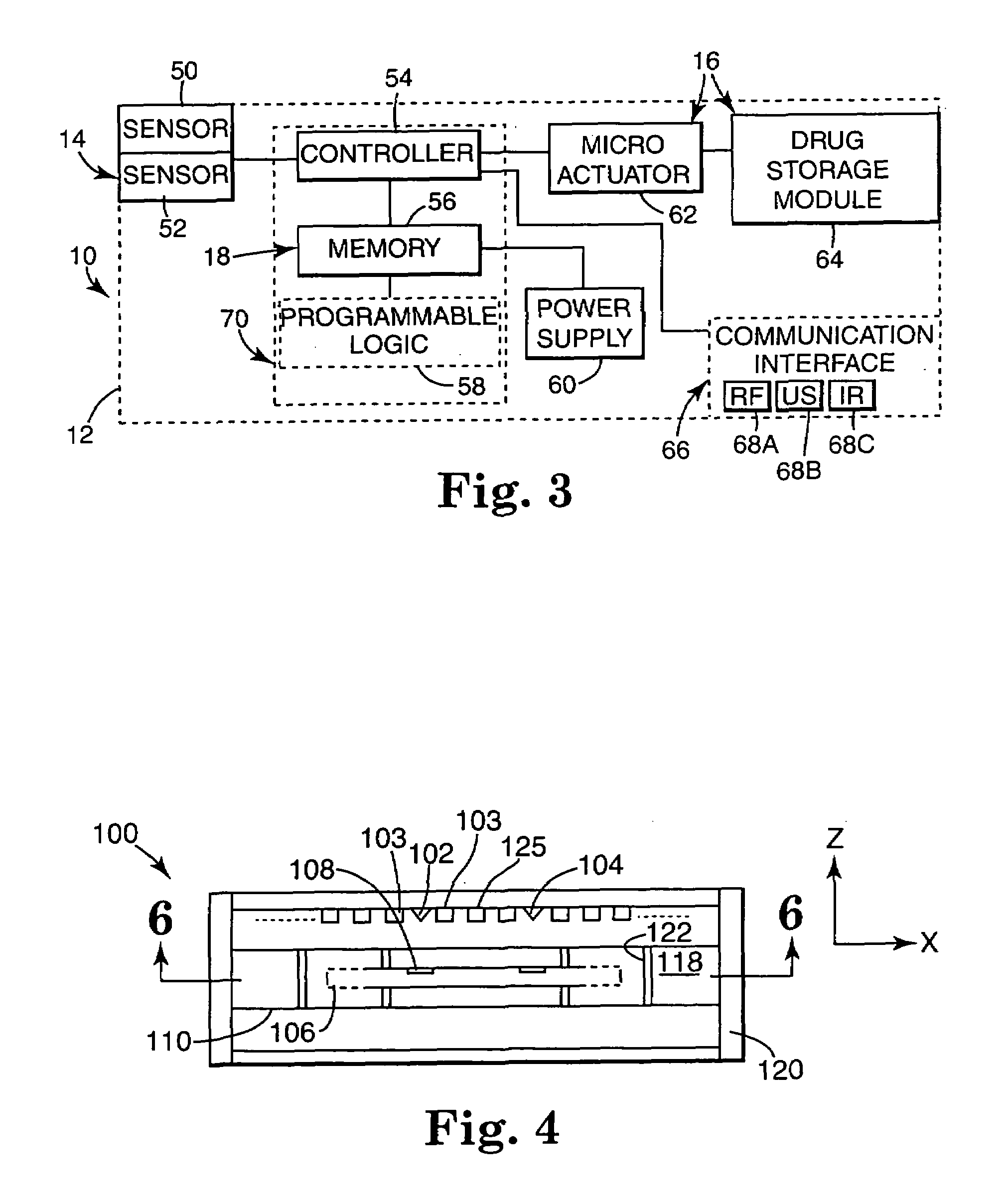
Internal drug dispenser capsule medical device
The present invention provides a swallowable internal drug medical device. The device includes a swallowable capsule. A sensing module is disposed in the capsule. A bioactive substance dispenser is disposed in the capsule. A memory and logic component is disposed in the capsule and in communication with the sensing module and the dispenser.
Owner:HEWLETT PACKARD DEV CO LP
Optical-based sensing devices
InactiveUS6711423B2Material analysis by observing effect on chemical indicatorSurgeryAnalyteFluorescence
An optical-based sensor for detecting the presence or amount of an analyte using both indicator and reference channels. The sensor has a sensor body with a source of radiation embedded therein. Radiation emitted by the source interacts with indicator membrane indicator molecules proximate the surface of the body. At least one optical characteristic of these indicator molecules varies with analyte concentration. For example, the level of fluorescence of fluorescent indicator molecules or the amount of light absorbed by light-absorbing indicator molecules can vary as a function of analyte concentration. In addition, radiation emitted by the source also interacts with reference membrane indicator molecules proximate the surface of the body. Radiation (e.g., light) emitted or reflected by these indicator molecules enters and is internally reflected in the sensor body. Photosensitive elements within the sensor body generate both indicator channel and reference channel signals to provide an accurate indication of the concentration of the analyte. Preferred embodiments are totally self-contained and are sized and shaped for use in vivo in a human being. Such embodiments preferably include a power source, e.g. an inductor, which powers the source of radiation using external means, as well as a transmitter, e.g. an inductor, to transmit to external pickup means the signal representing the level of analyte.
Owner:SENSEONICS INC
System to monitor the ingestion of medicines
InactiveUS20070008113A1Easy to manageEasy to testDigital data processing detailsEndoradiosondesComputer hardwareRadio-frequency identification
Owner:CARESTREAM HEALTH INC
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
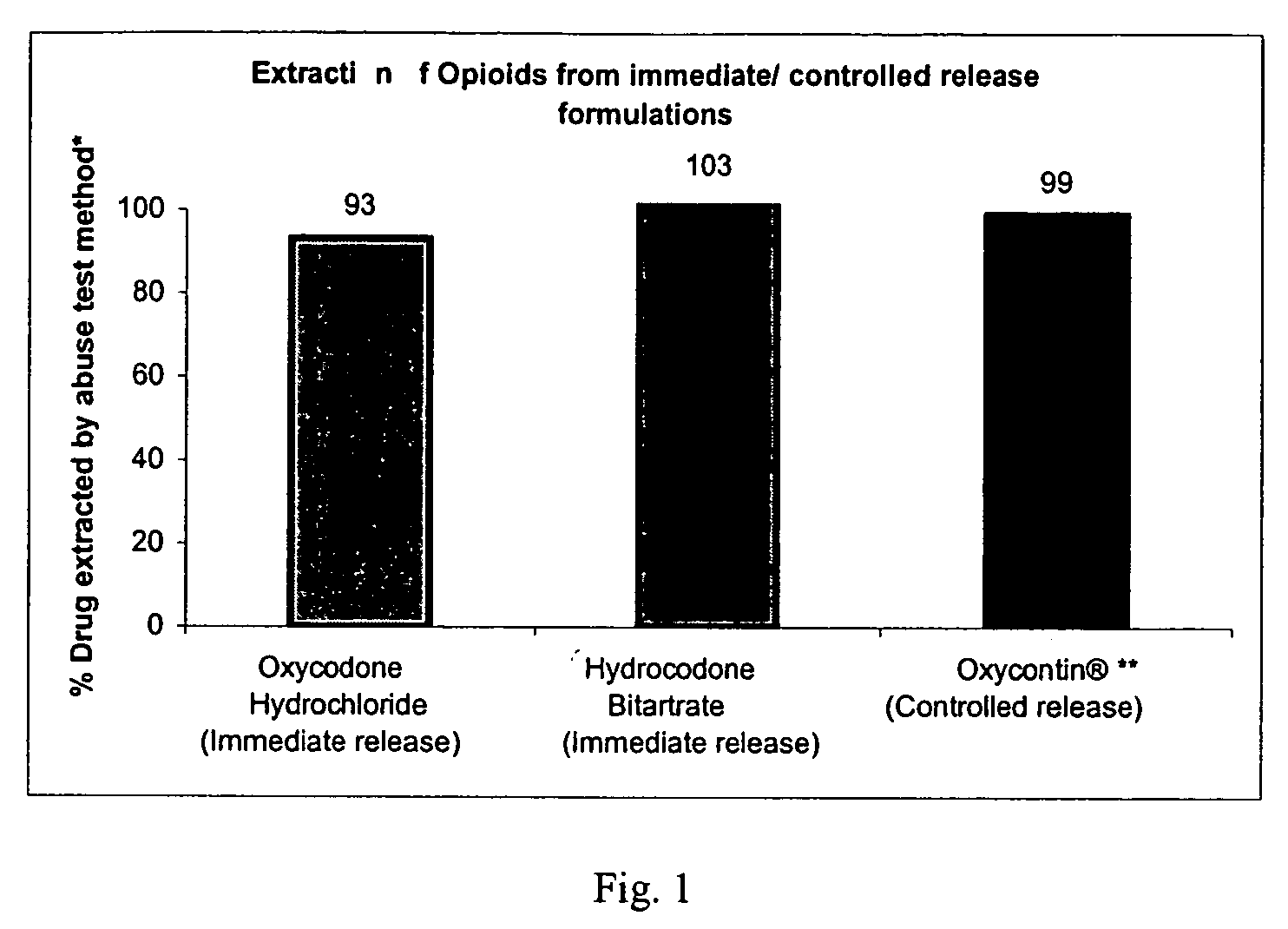
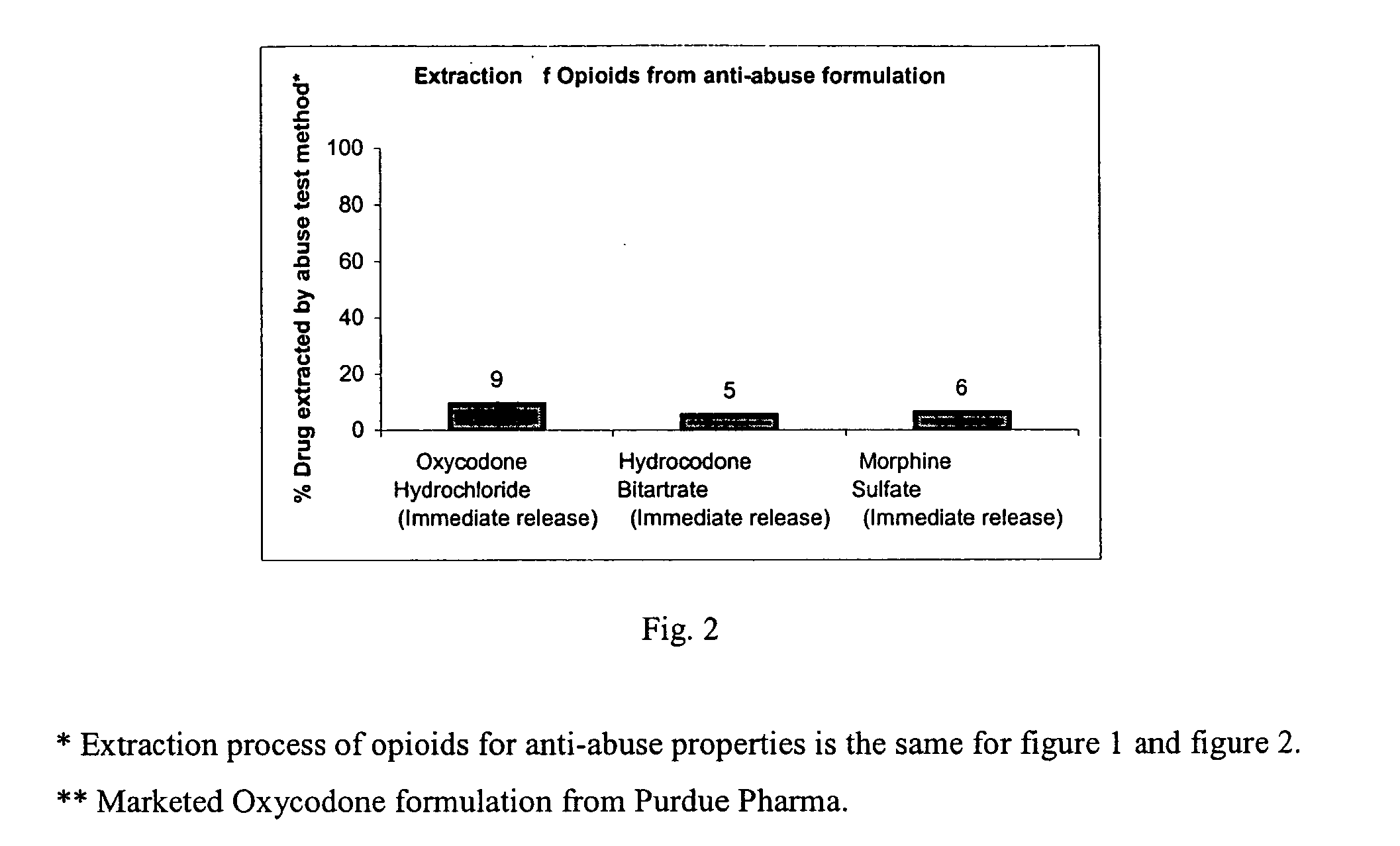
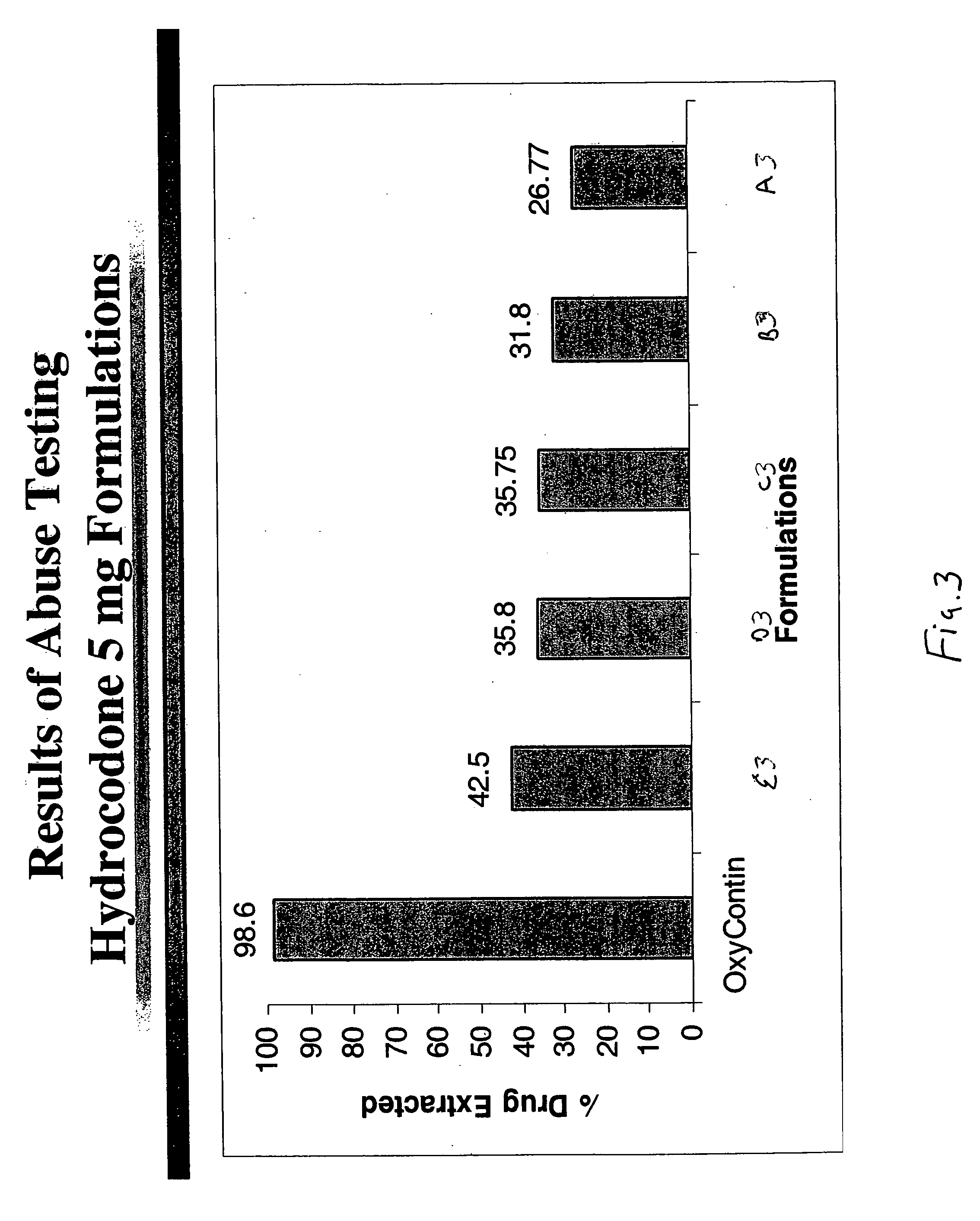
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
Modified polynucleotides for the production of secreted proteins
Owner:MODERNATX INC
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
Lipid encapsulated interfering RNA
The present invention provides lipid-based formulations for delivering, e.g., introducing, nucleic acid-lipid particles comprising an interference RNA molecule to a cell, and assays for optimizing the delivery efficiency of such lipid-based formulations.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Cationic lipids and methods of use
ActiveUS7745651B2Increase flexibilityGood fluidityUltrasonic/sonic/infrasonic diagnosticsBiocideLipid particleLiposome
The present invention provides compositions comprising cationic lipids, liposomes and nucleic acid-lipid particles comprising the cationic lipids, and methods of using such compositions, liposomes, and nucleic acid-lipid particles.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
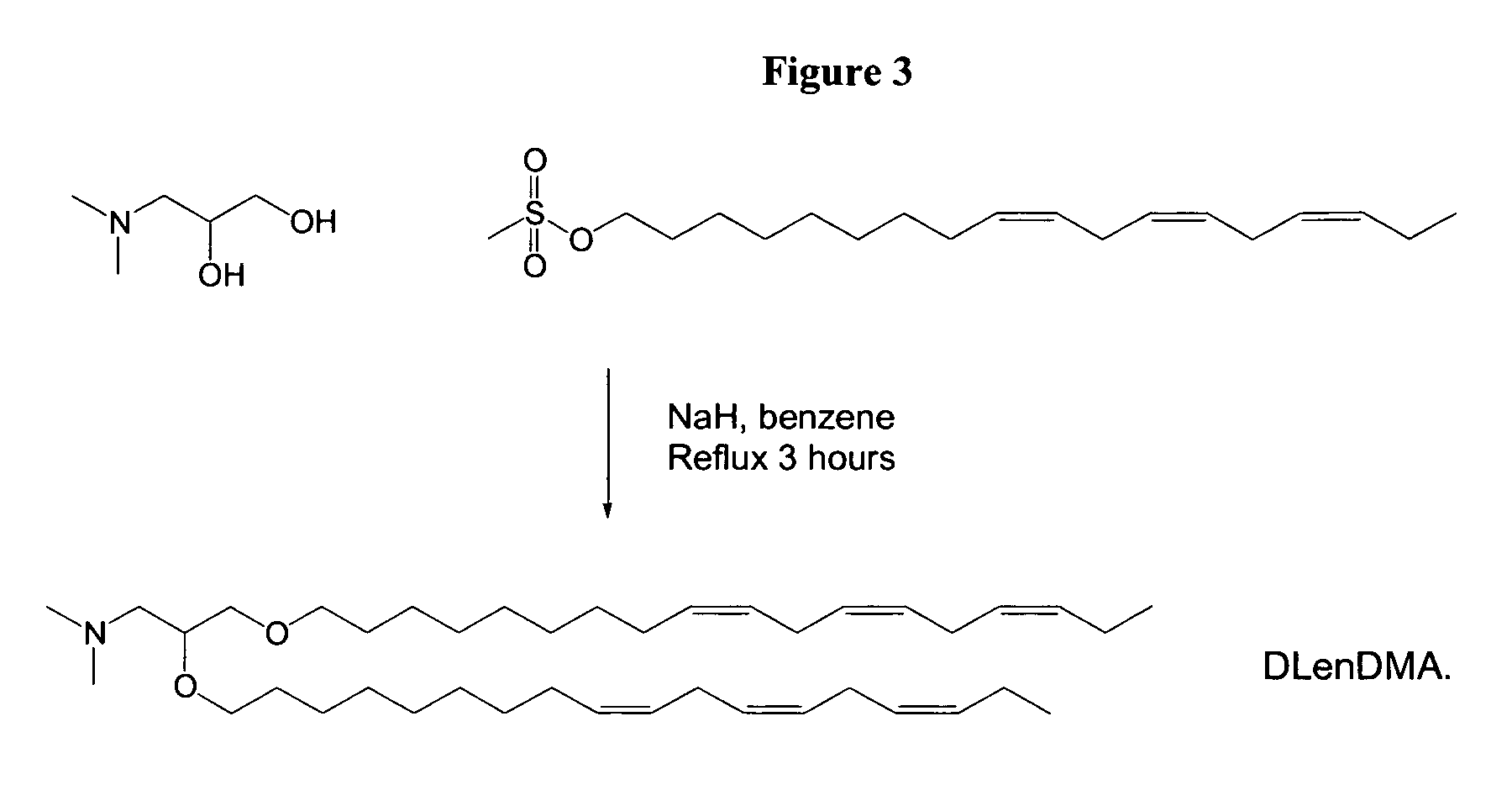
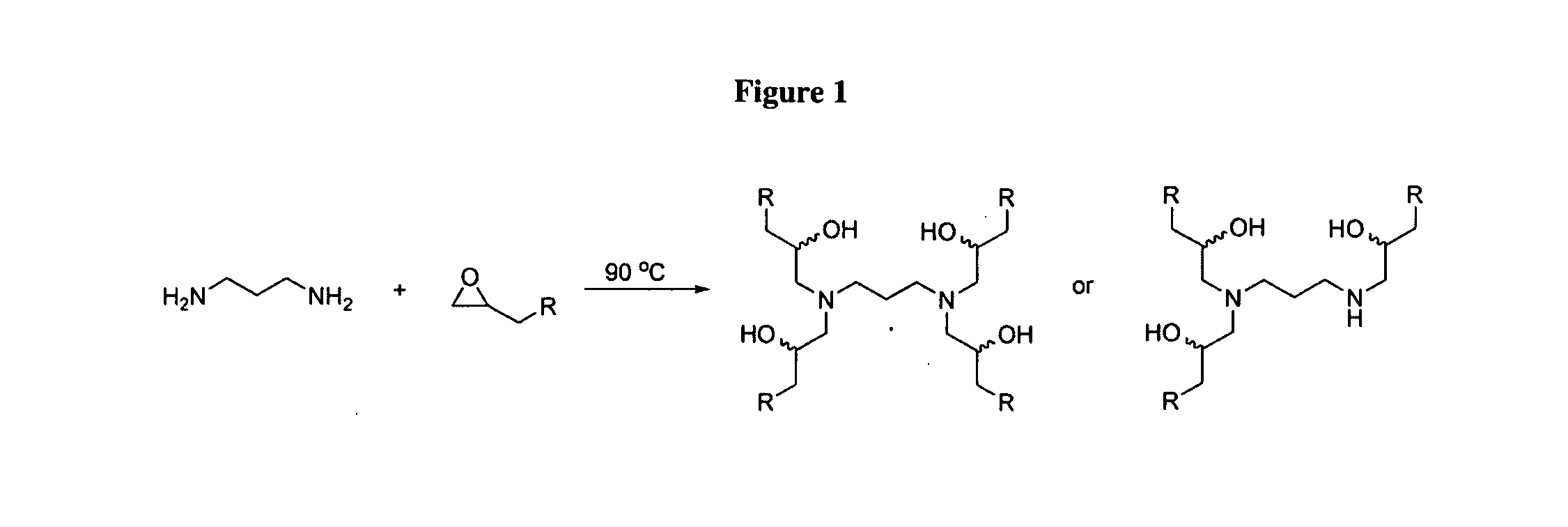
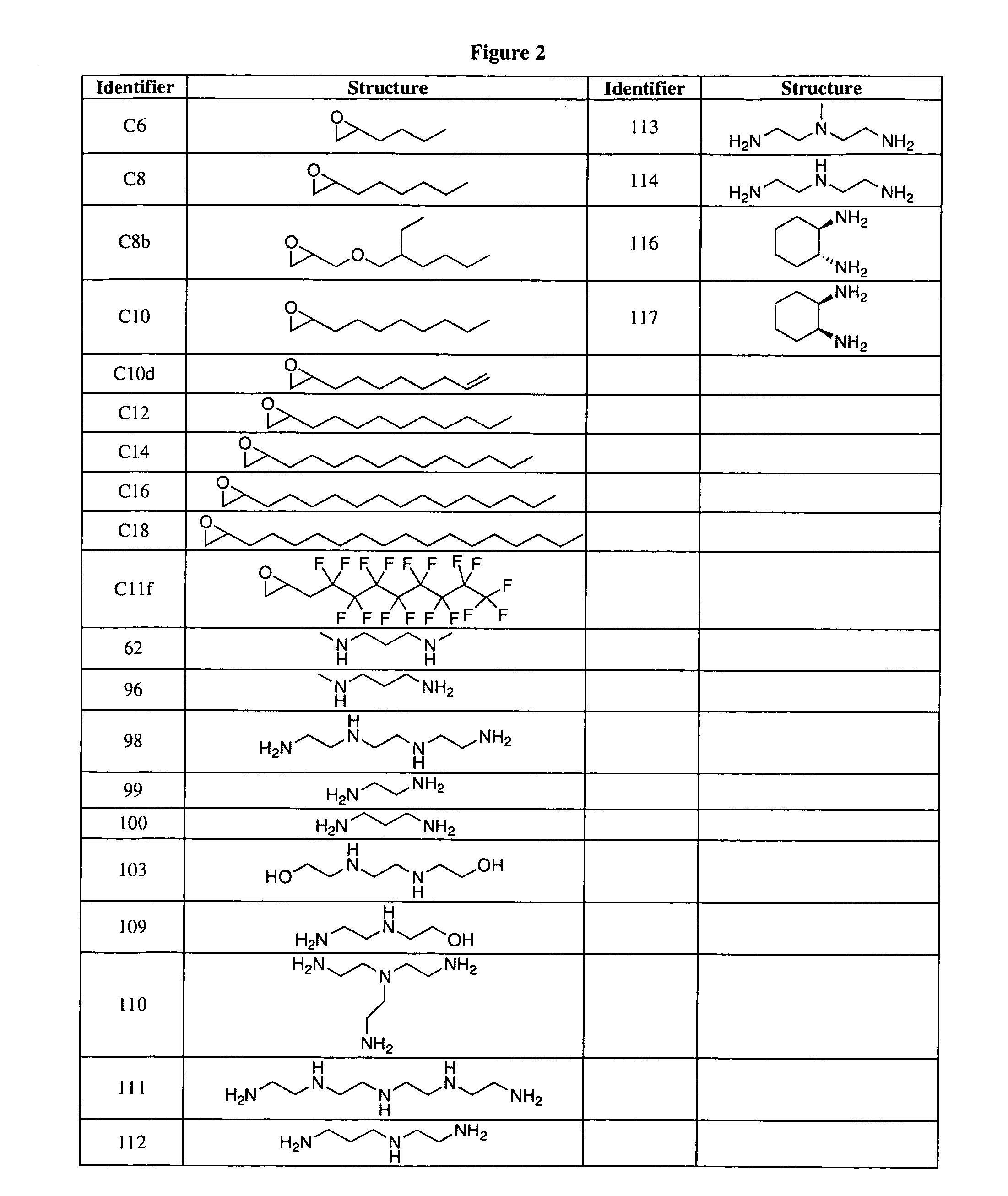
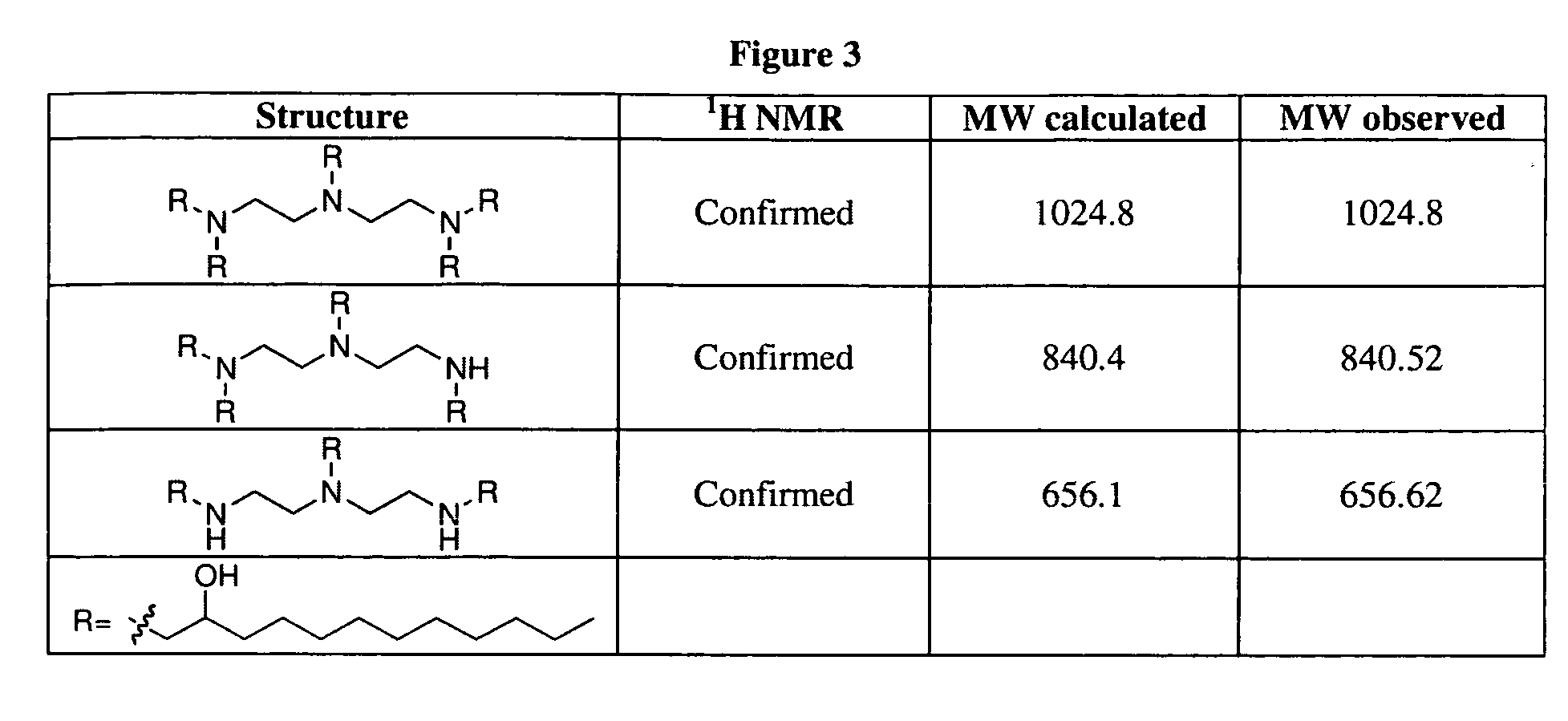
Aminoalcohol lipidoids and uses thereof
Aminoalcohol lipidoids are prepared by reacting an amine with an epoxide-terminated compound are described. Methods of preparing aminoalcohol lipidoids from commercially available starting materials are also provided. Aminoalcohol lipidoids may be prepared from racemic or stereochemically pure epoxides. Aminoalcohol lipidoids or salts forms thereof are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems. Given the amino moiety of these aminoalcohol lipidoid compounds, they are particularly suited for the delivery of polynucleotides. Complexes, micelles, liposomes or particles containing the inventive lipidoids and polynucleotide have been prepared. The inventive lipidoids may also be used in preparing microparticles for drug delivery. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
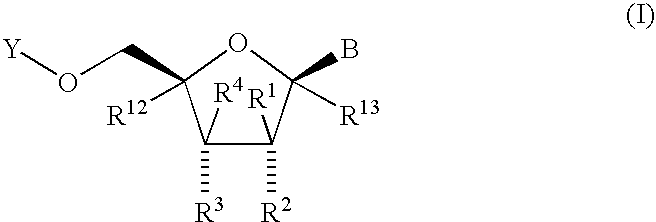
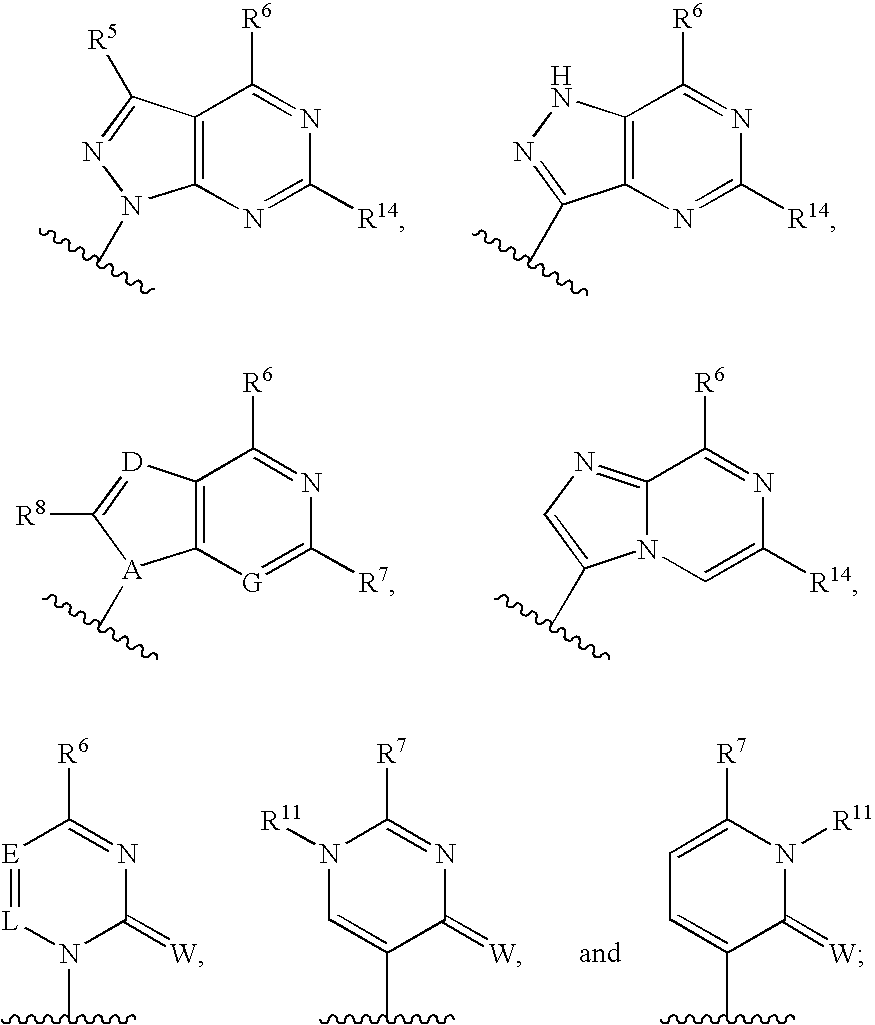
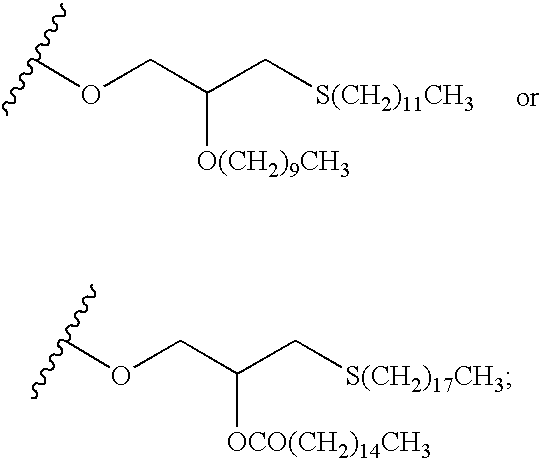
Nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase
The present invention provides nucleoside derivatives which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as inhibitors of hepatitis C virus (HCV) NS5B polymerase, as inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such nucleoside derivatives alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the nucleoside derivatives of the present invention.
Owner:MERCK SHARP & DOHME LLC +1
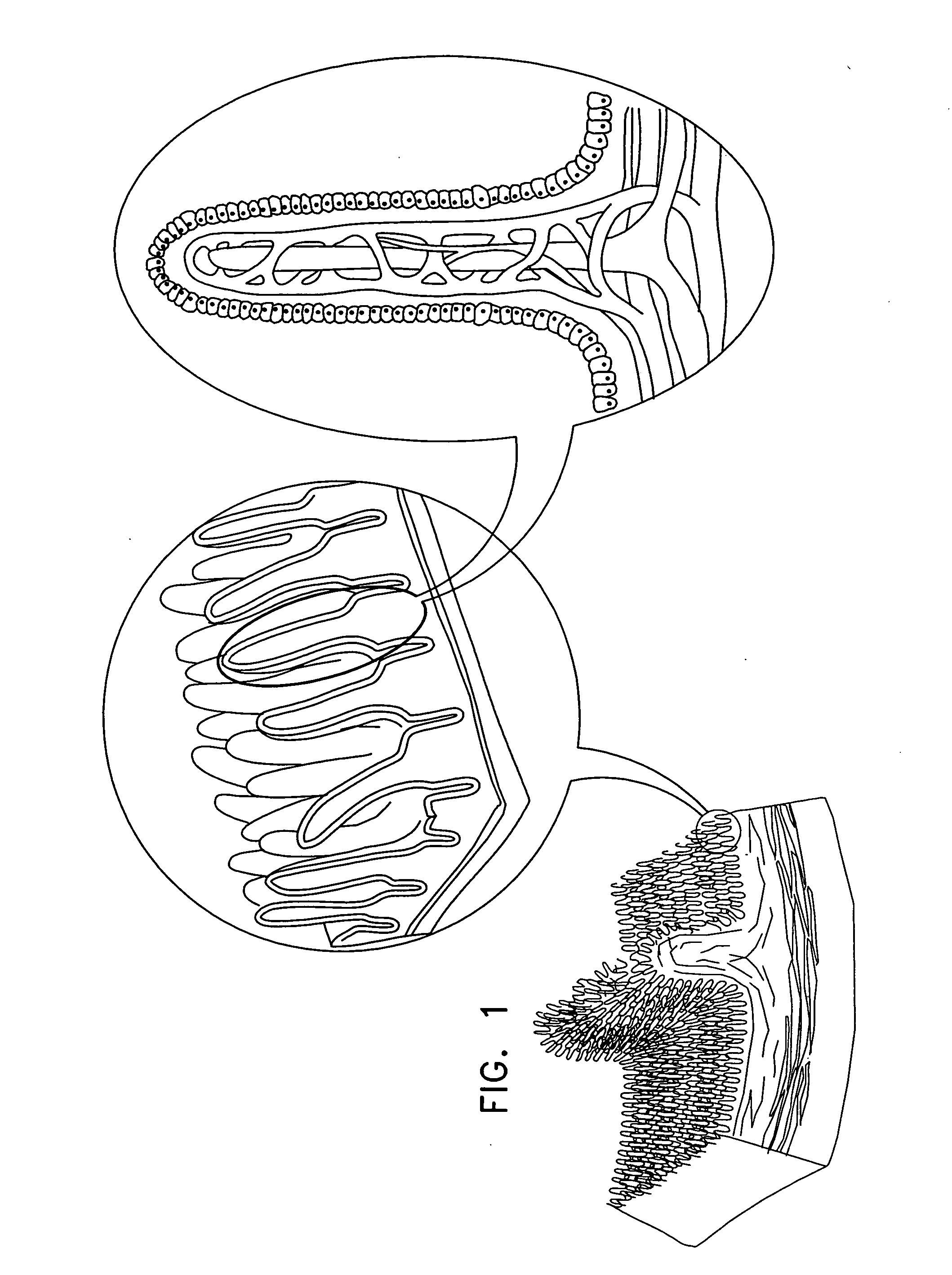
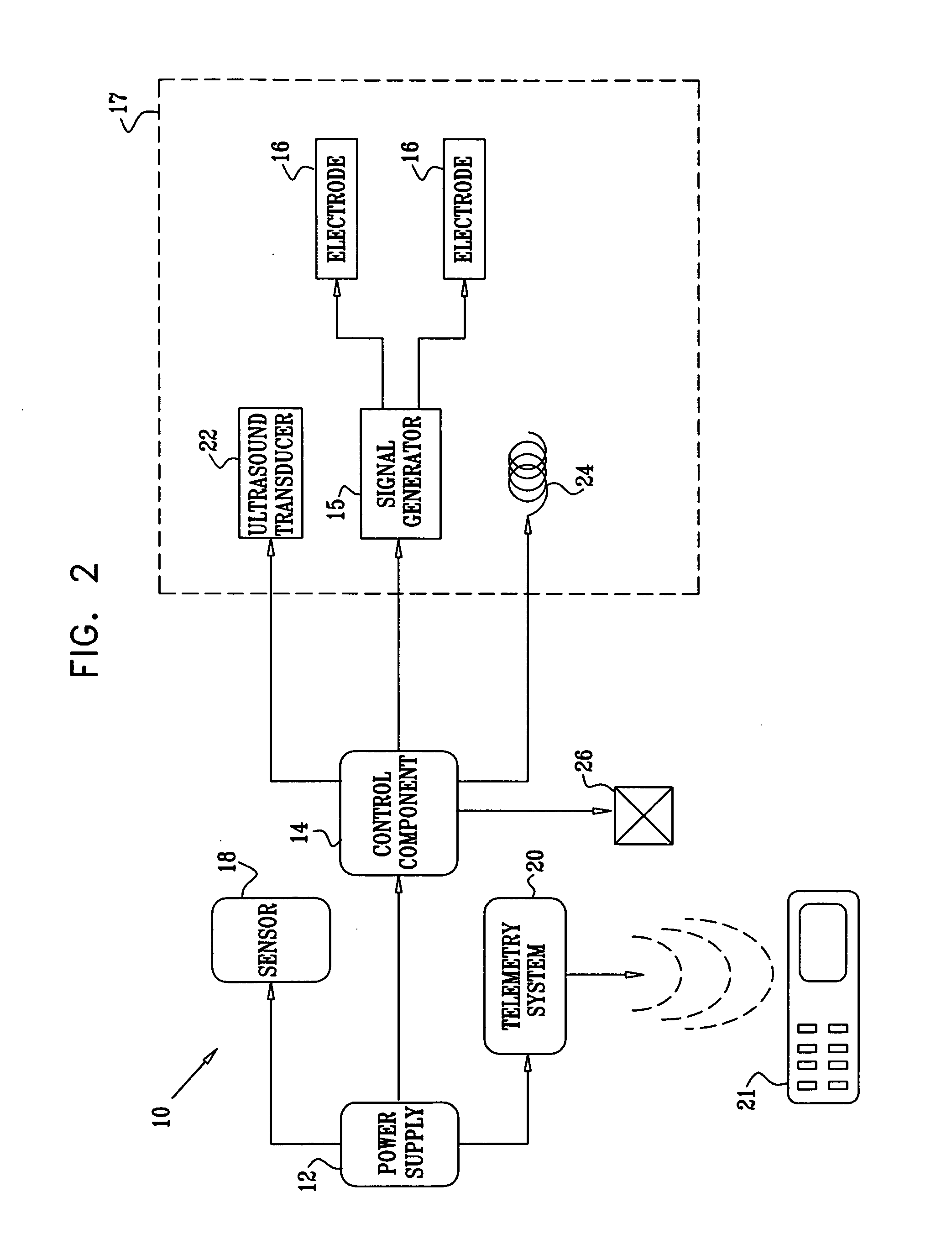
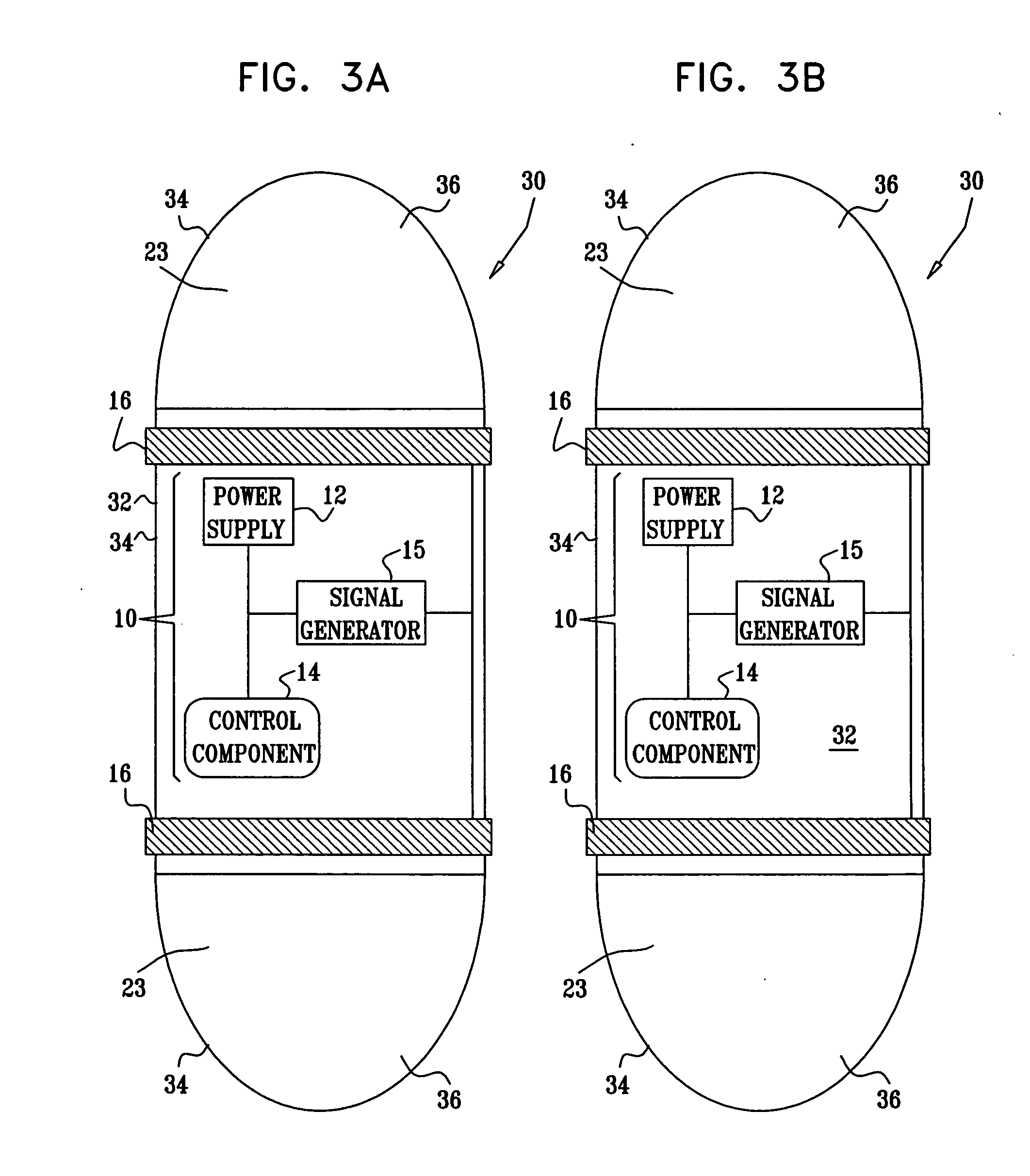
Active drug delivery in the gastrointestinal tract
InactiveUS20050058701A1Easy accessPromote absorptionInternal electrodesBody temperature measurementMedicineDrug administration
Apparatus for drug administration is provided, including an ingestible capsule, which includes a drug, stored by the capsule, and an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject. The capsule further includes first and second electrodes, and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, through an epithelial layer of the GI tract by driving the first and second electrodes to apply a series of pulses at a current of less than about 5 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds.
Owner:E PILL PHARMA
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com