Patents
Literature
1533 results about "Drug administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The U.S. Food and Drug Administration (FDA) is the government agency responsible for reviewing, approving and regulating medical products, including pharmaceutical drugs and medical devices. It also regulates various other products, including food, cosmetics, veterinary drugs, radiation-emitting products, biological products and tobacco.
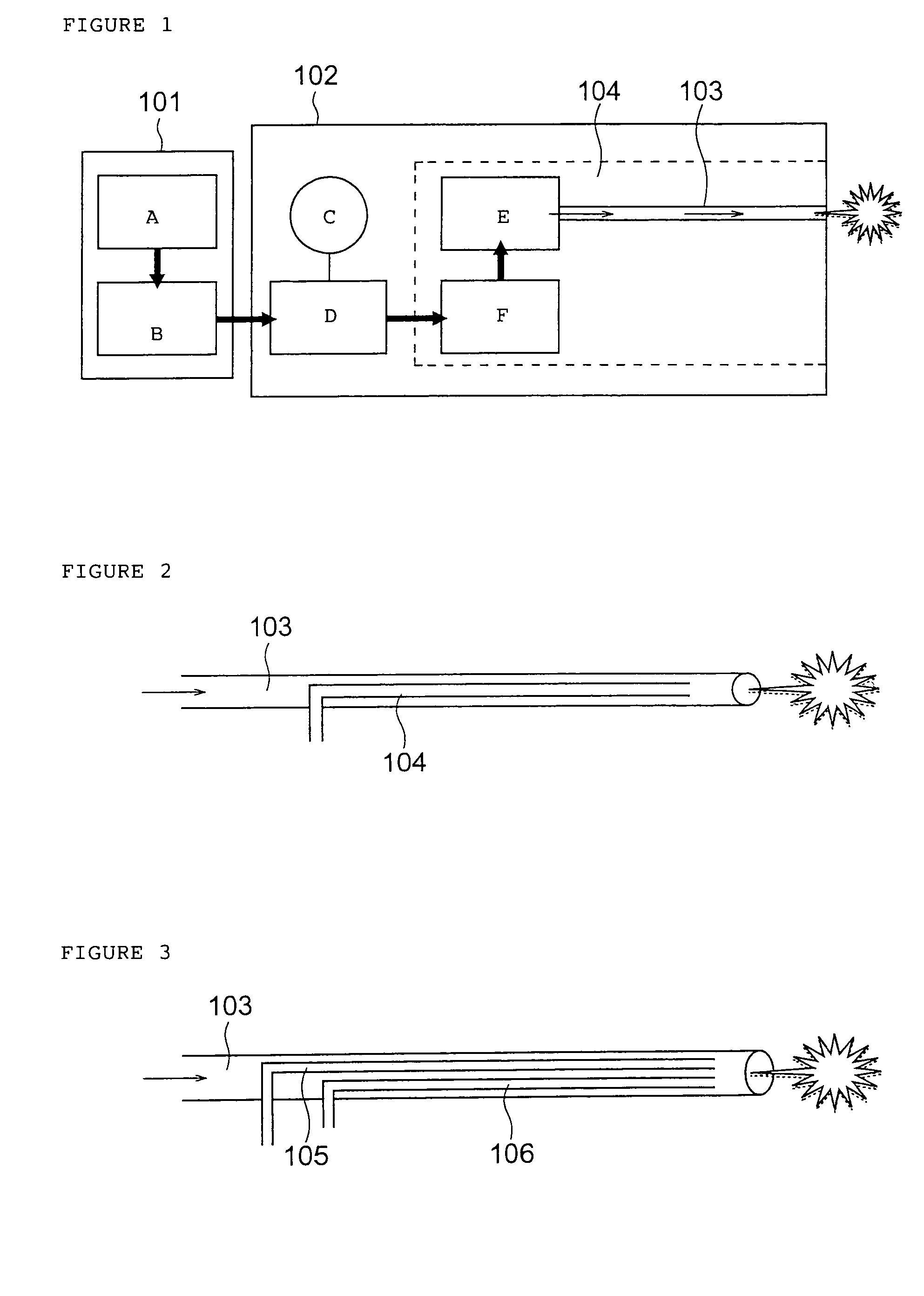
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
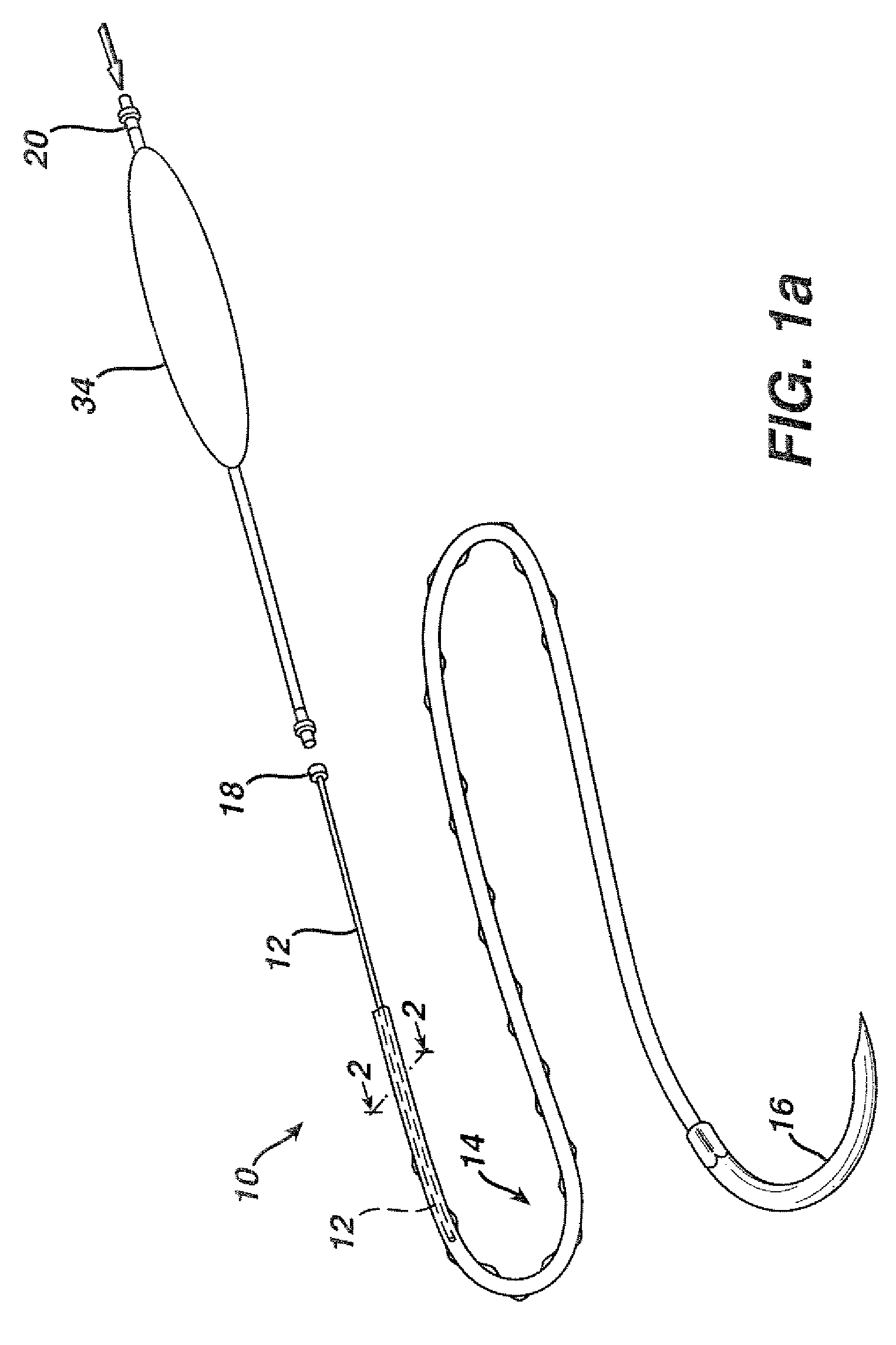
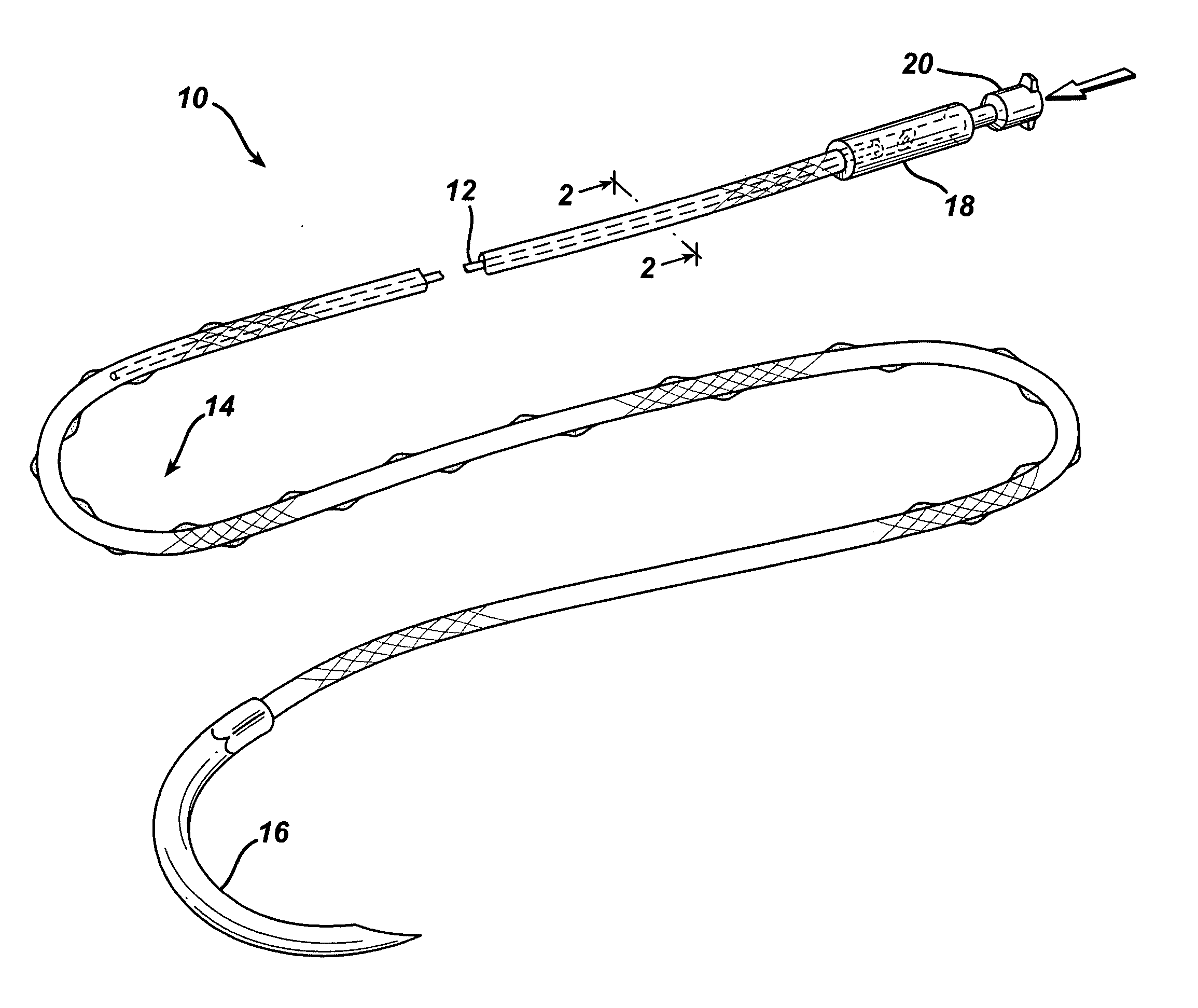
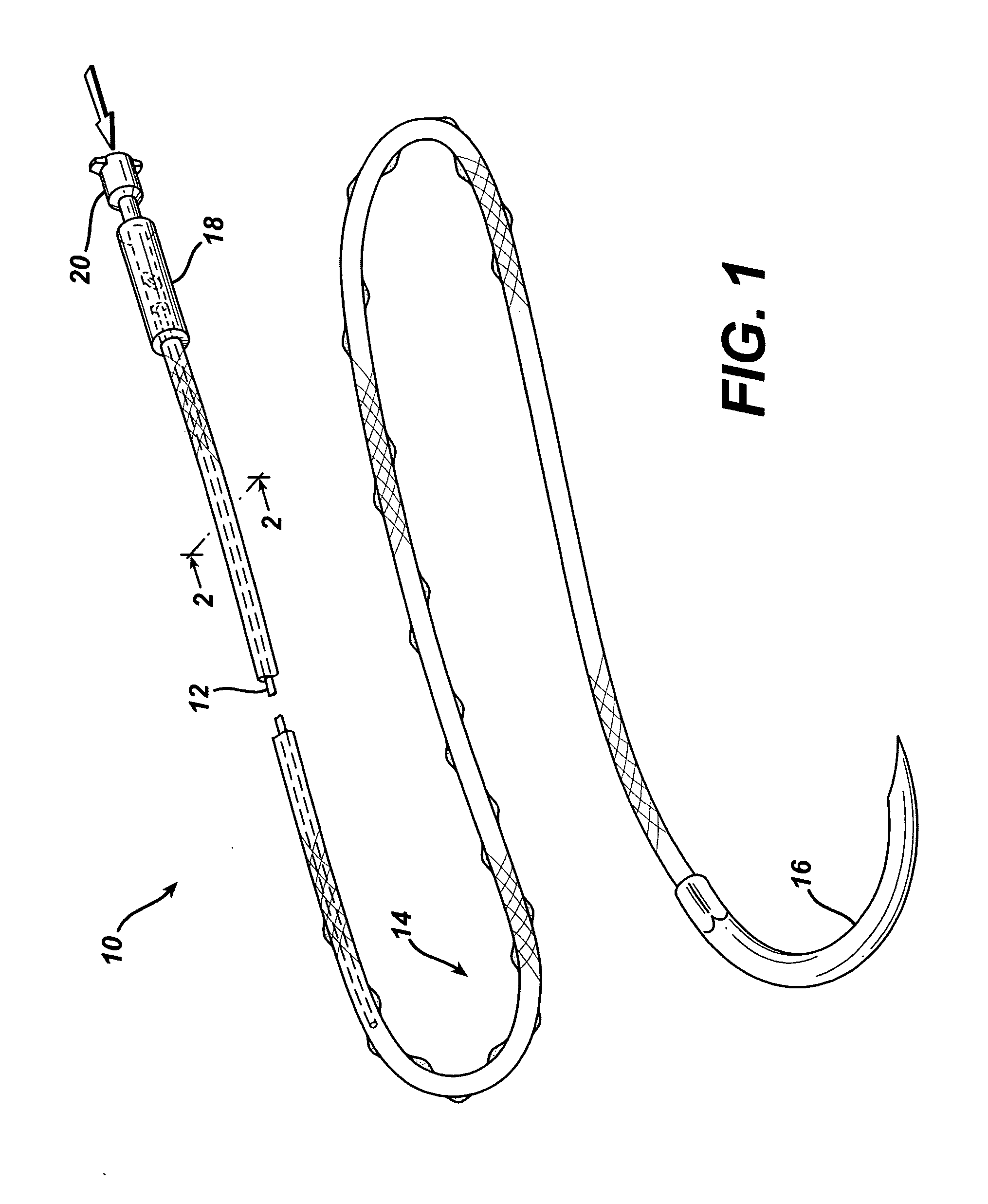
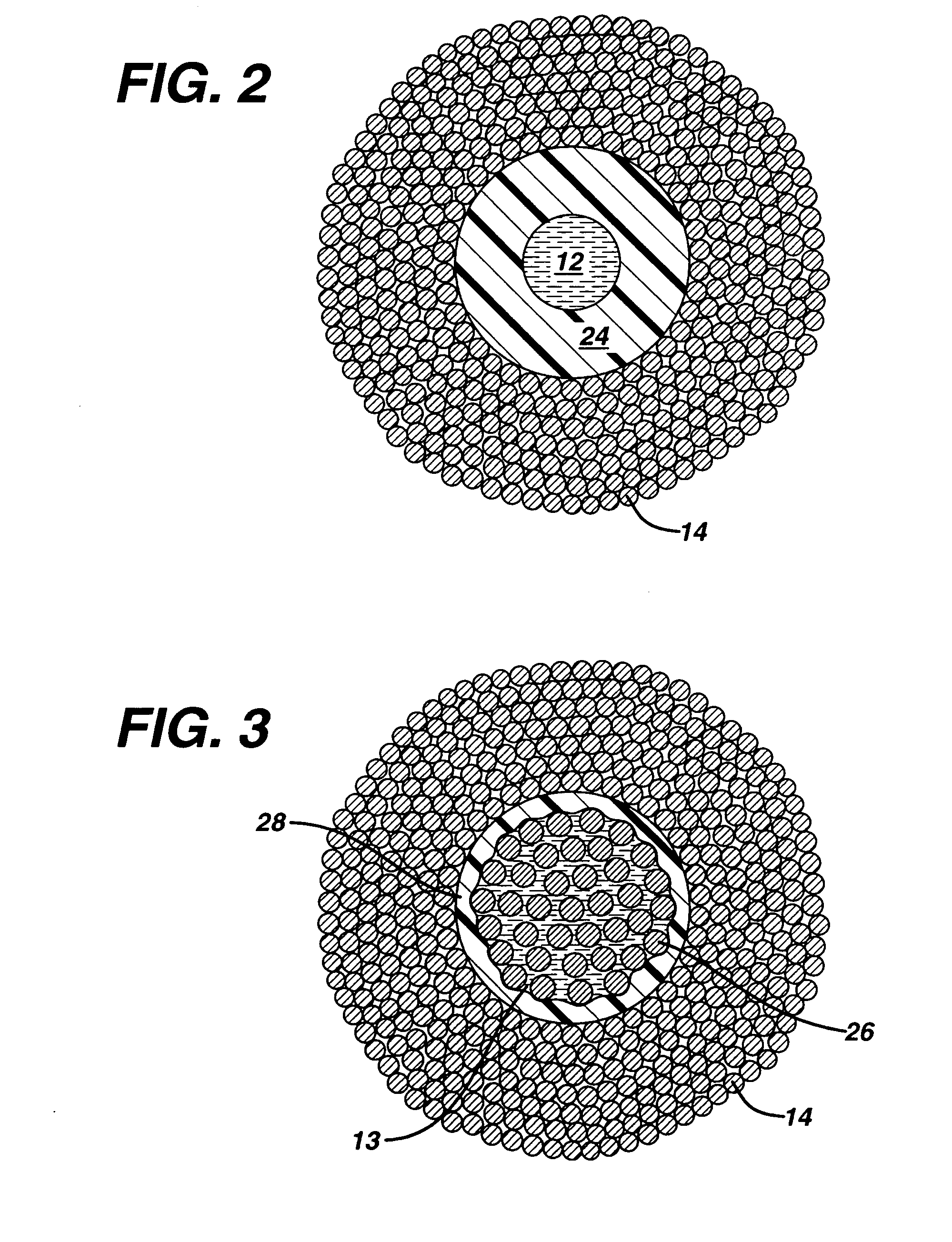
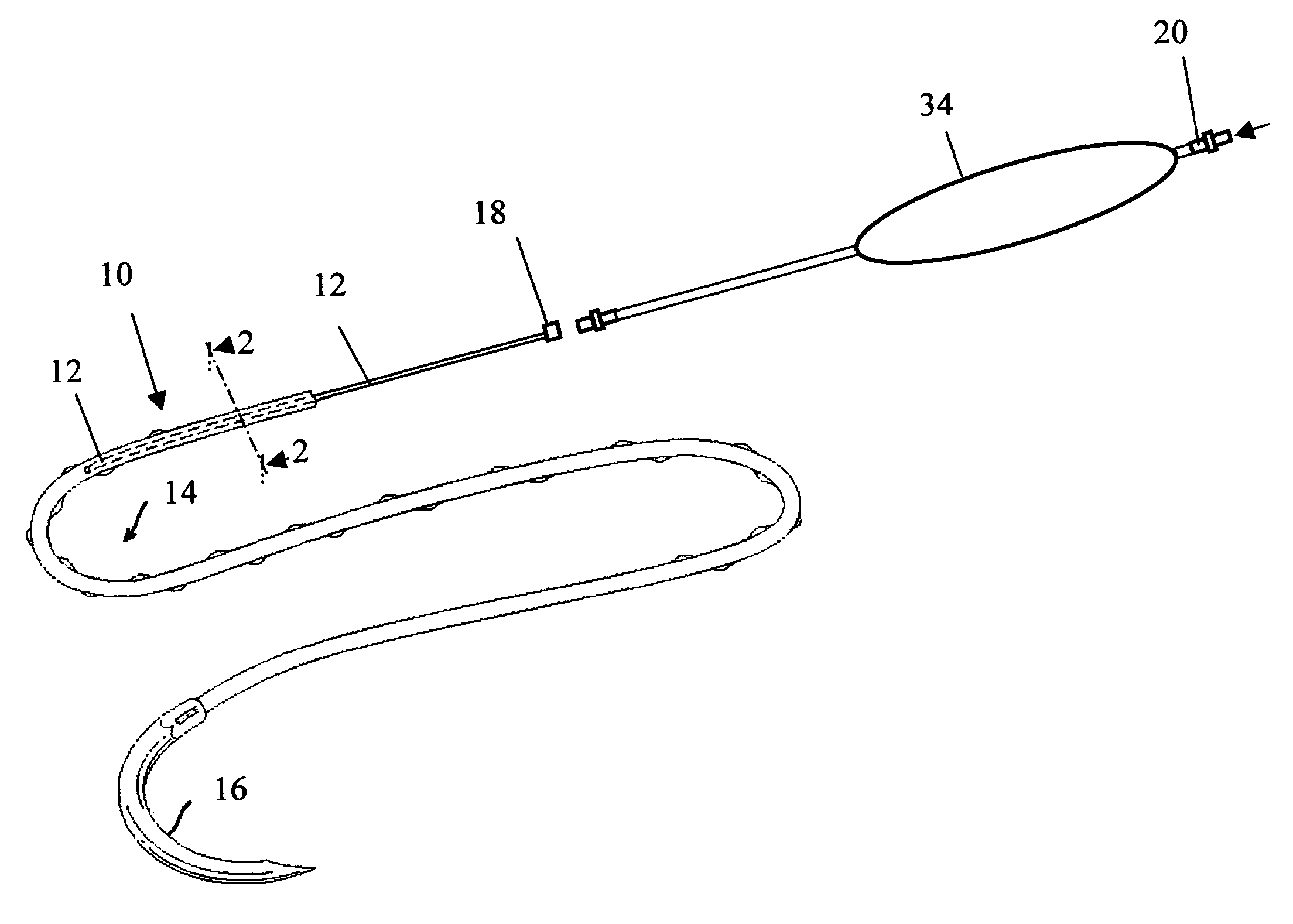
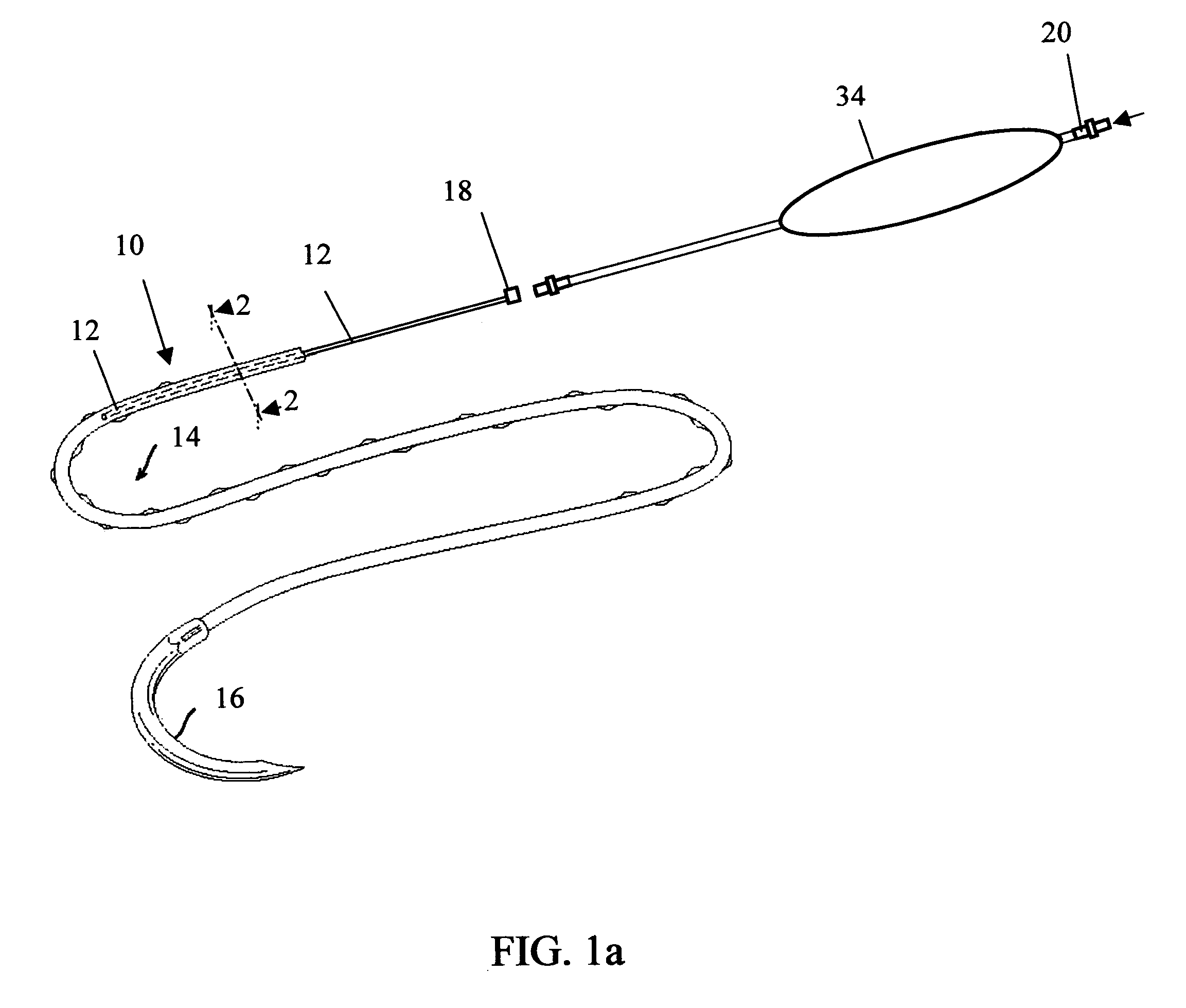
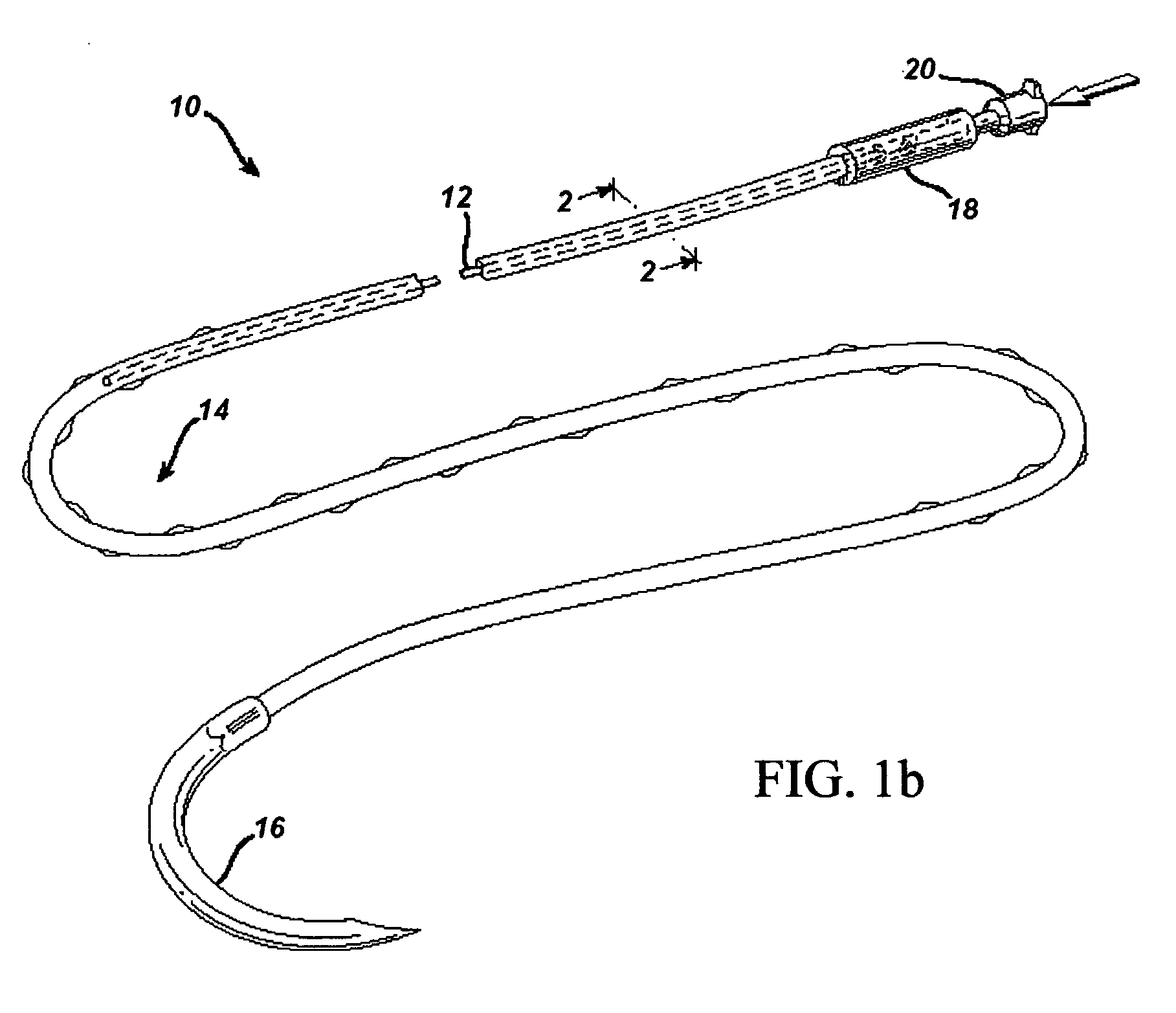
Active suture for the delivery of therapeutic fluids
An active suture that can be used for the delivery of therapeutic fluids to the tissue surrounding a wound is disclosed. The active suture may include a connector designed to join a fluid source, such as a syringe, conventional IV delivery system, or infusion pump to an internal passageway that is embedded within a braided suture. The internal passageway may be comprised of a fine polymeric tube and is capable of conducting and emitting a fluid into at least a portion of the braided suture and surrounding tissue. The invention enables delivery of an efficacious volume of drug bearing solution on the order of milliliters per day, provides a high level of fluid delivery rate control enabling the physician to start or stop drug administration at his / her discretion, and offers a means of providing more than one type of medication that may be selected post-surgically in accord with unexpected patient symptoms that may arise.
Owner:ETHICON INC
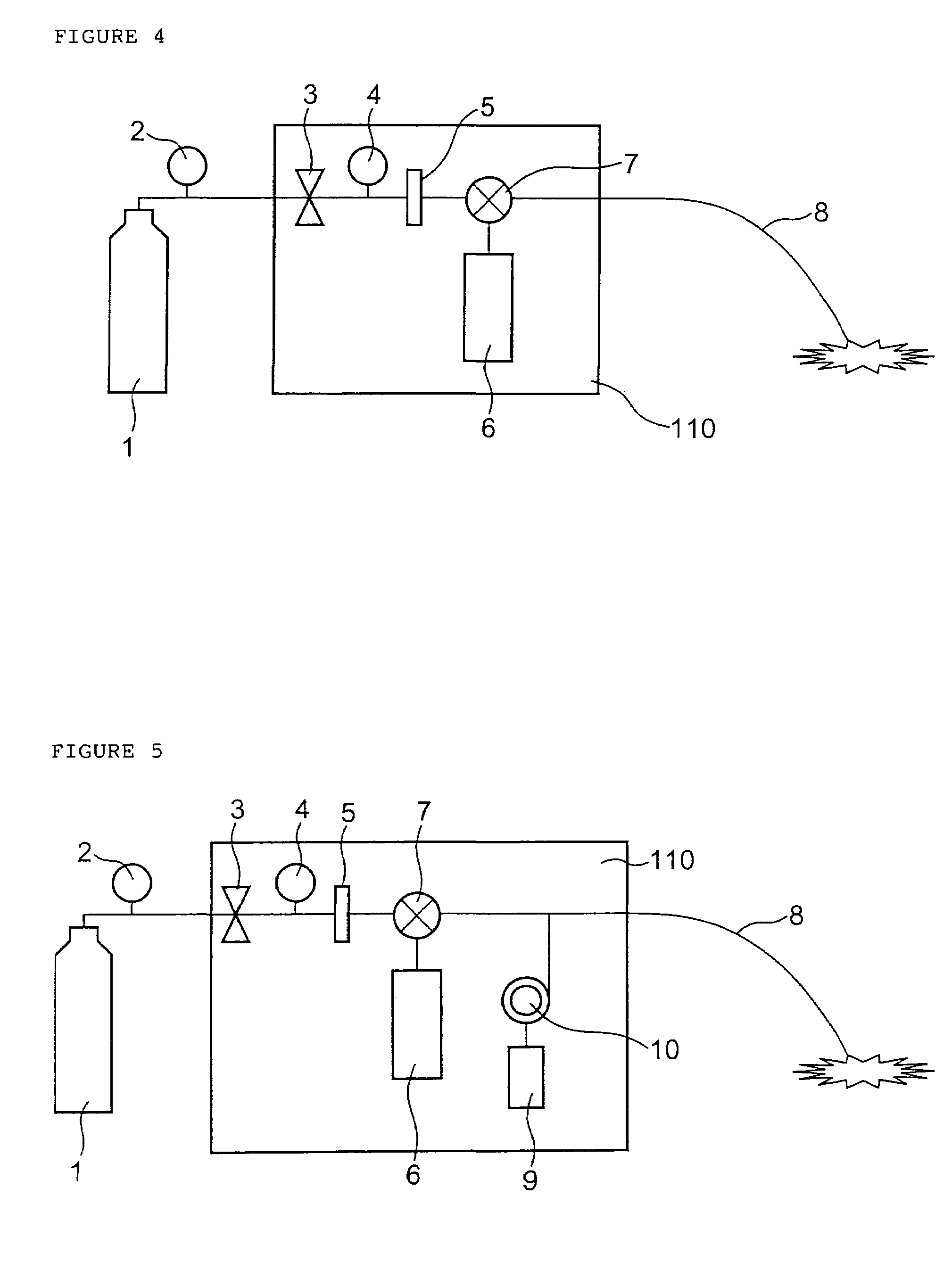
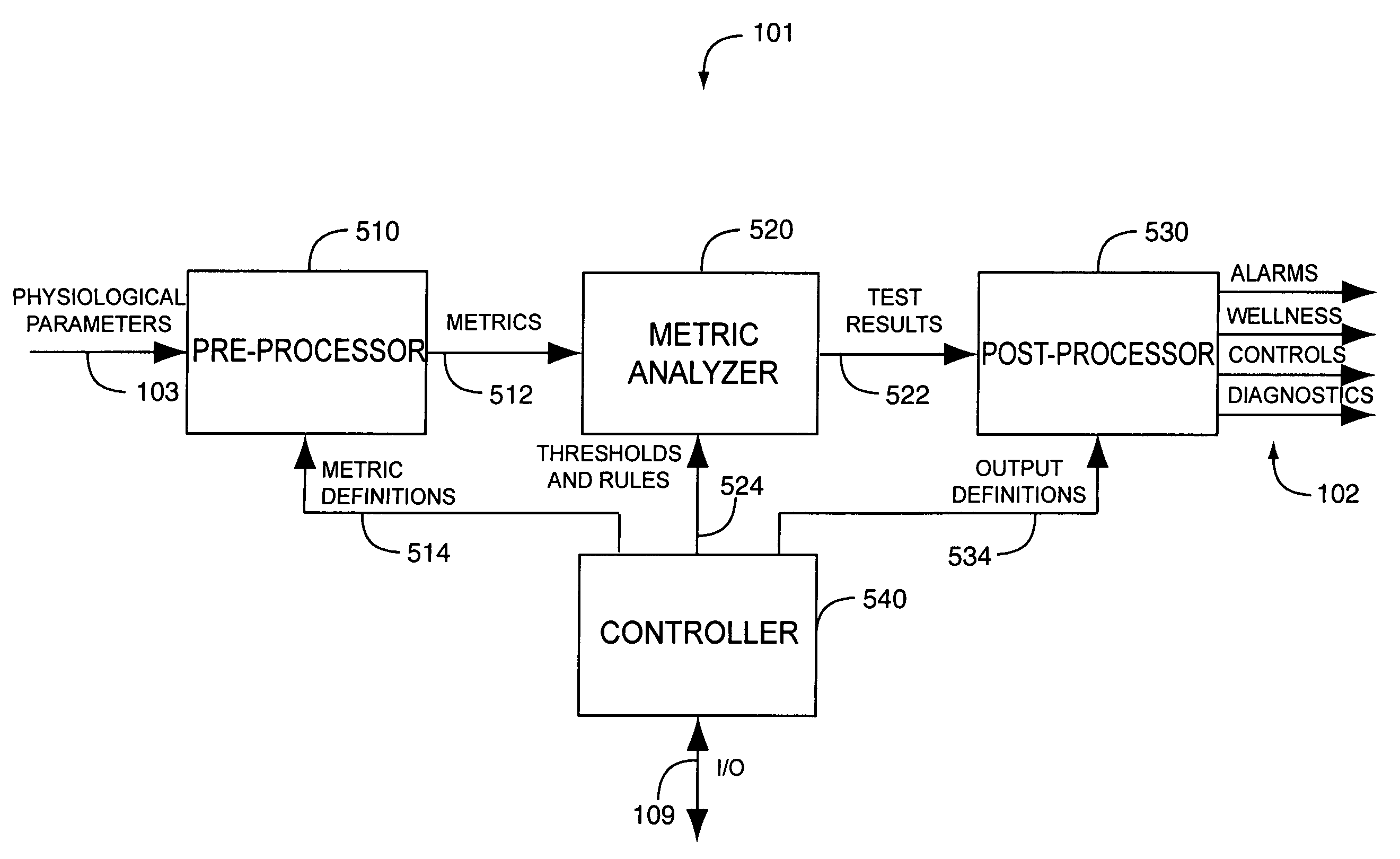
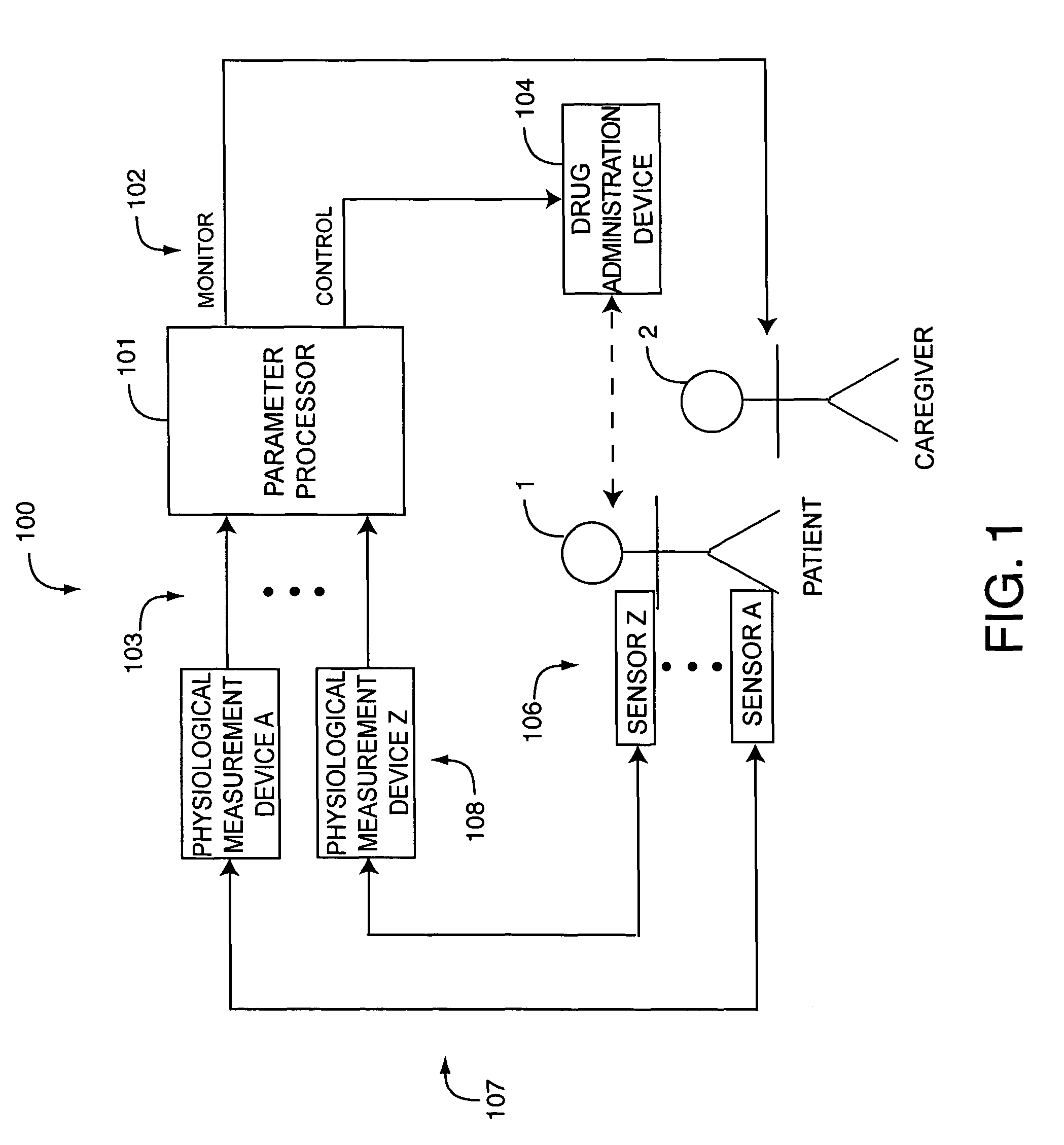
Drug administration controller
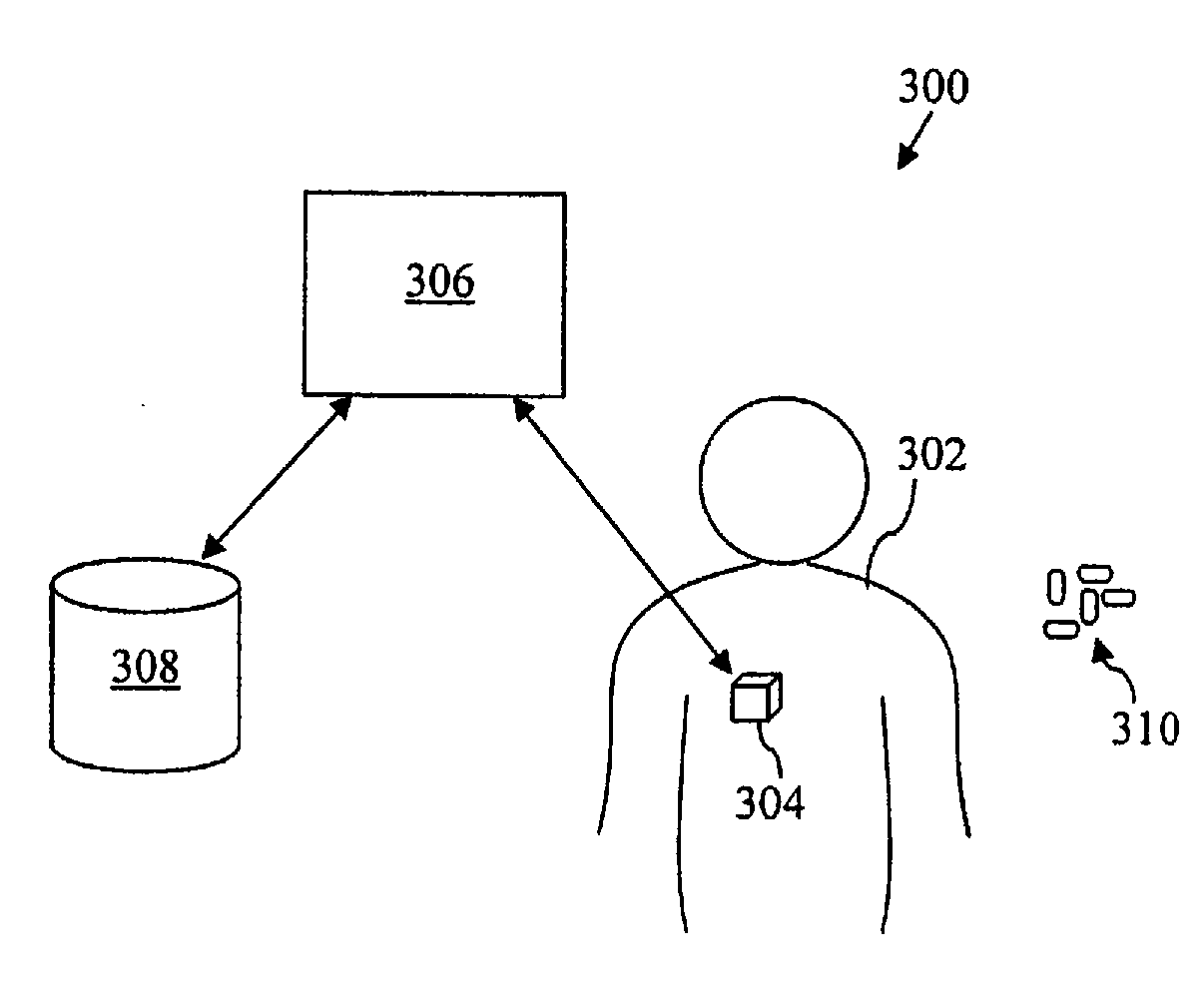
A drug administration controller has a sensor that generates a sensor signal to a physiological measurement device, which measures a physiological parameter in response. A control output responsive to the physiological parameter or a metric derived from the physiological parameter causes a drug administration device to affect the treatment of a person, such as by initiating, pausing, halting or adjusting the dosage of drugs administered to the person.
Owner:JPMORGAN CHASE BANK NA
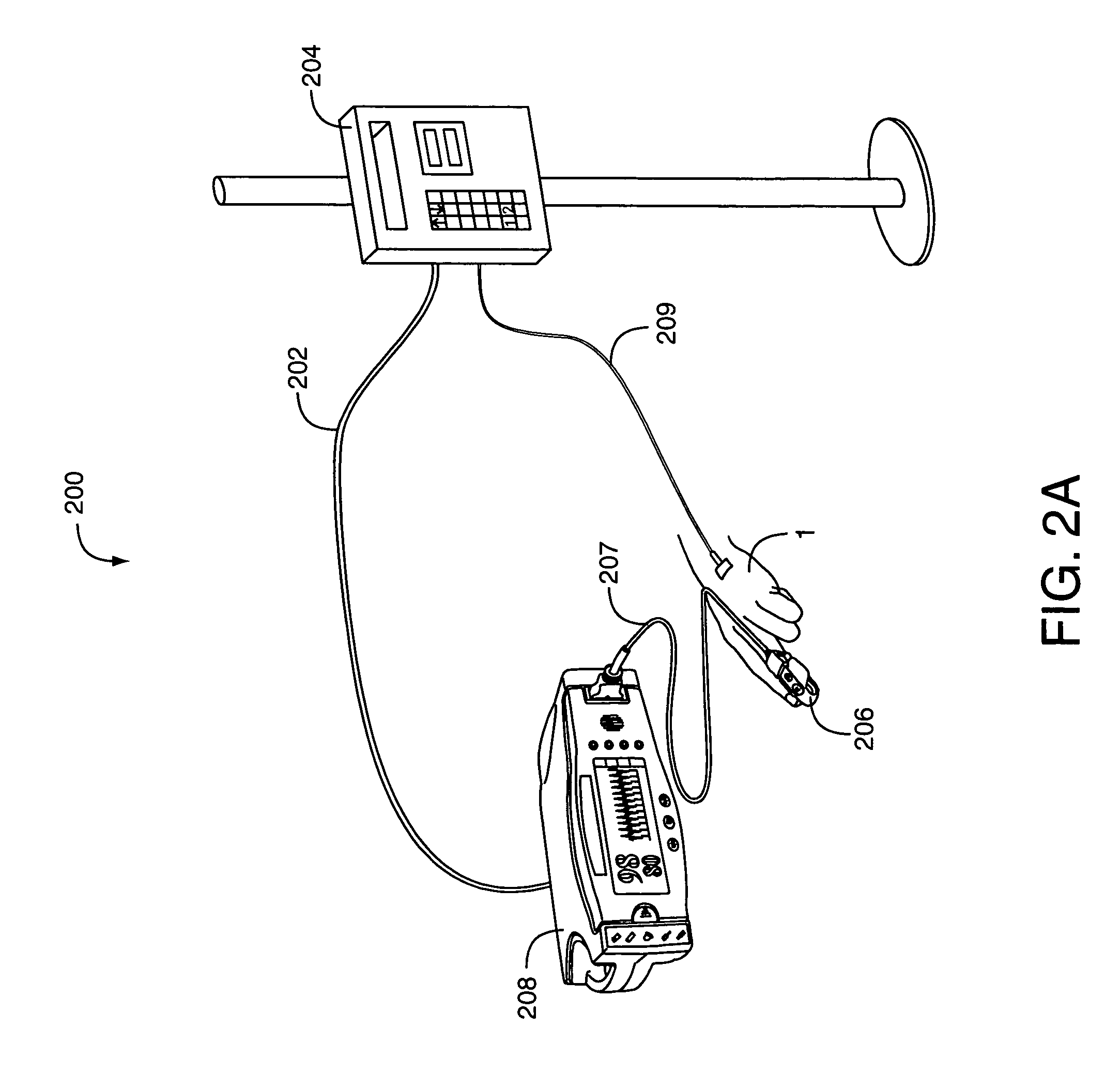
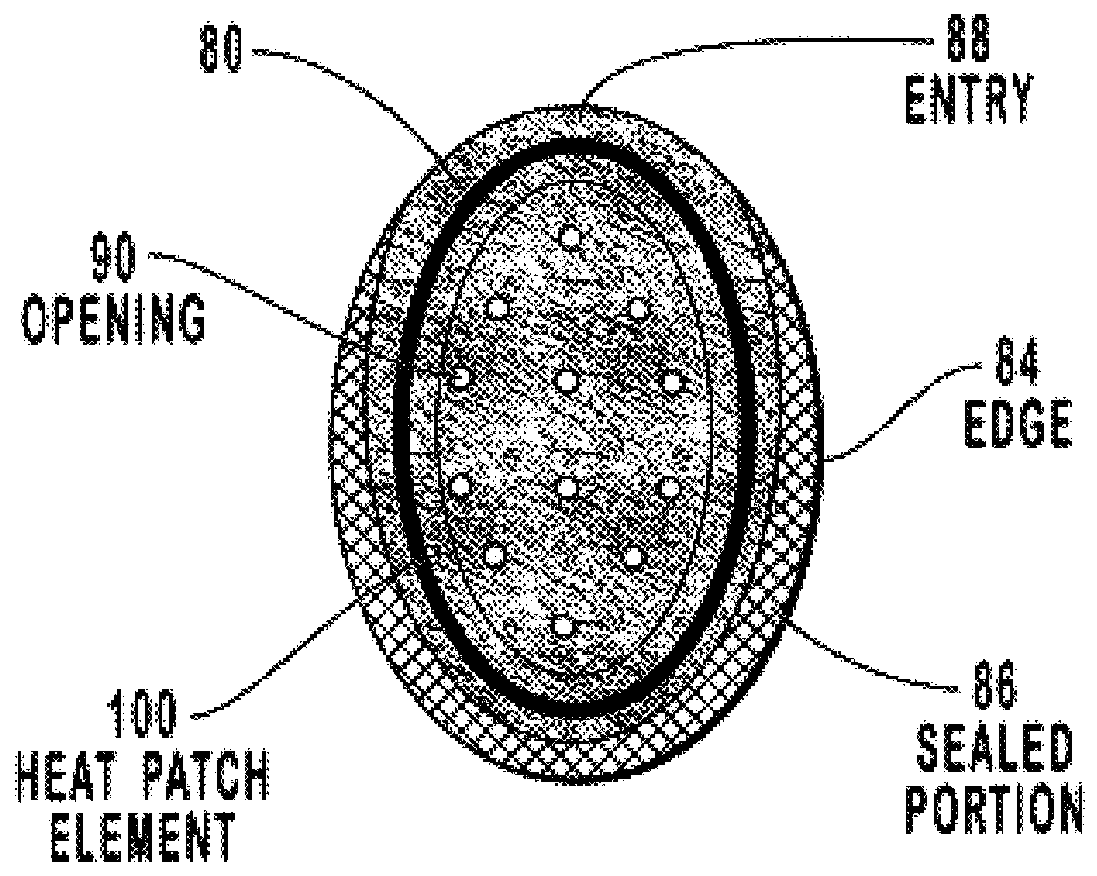
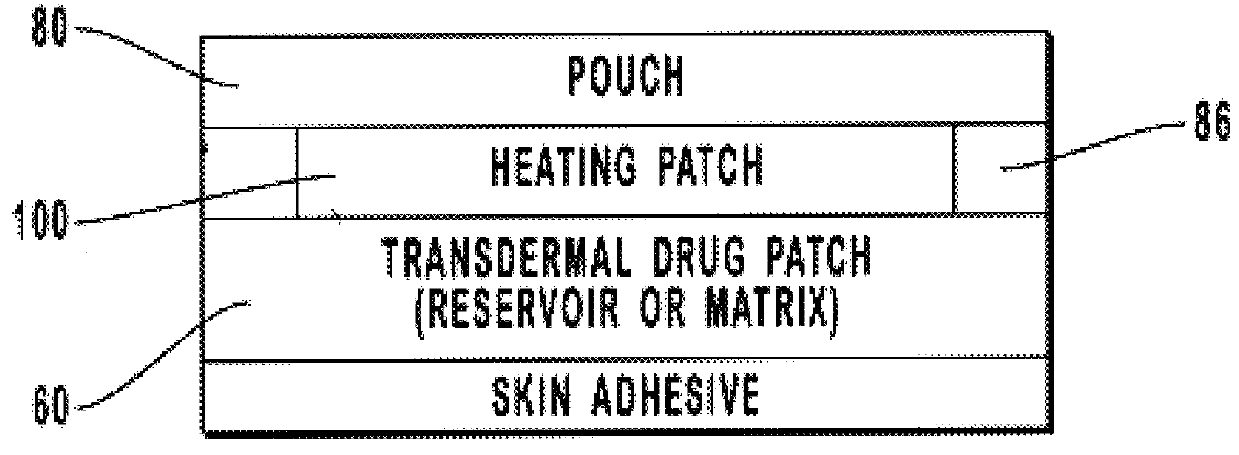
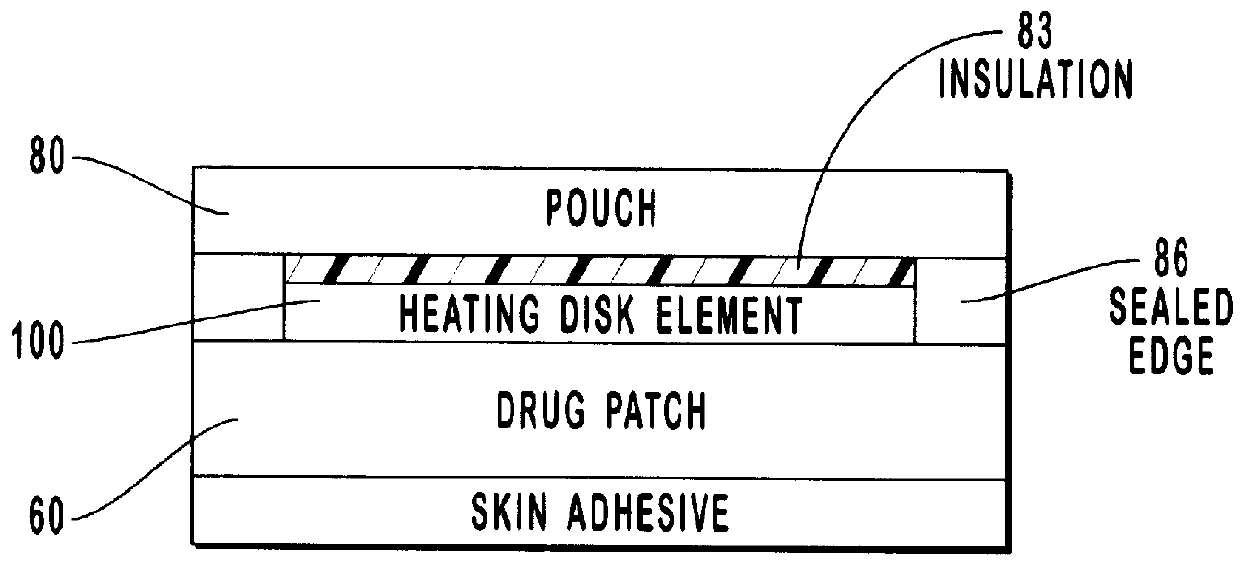
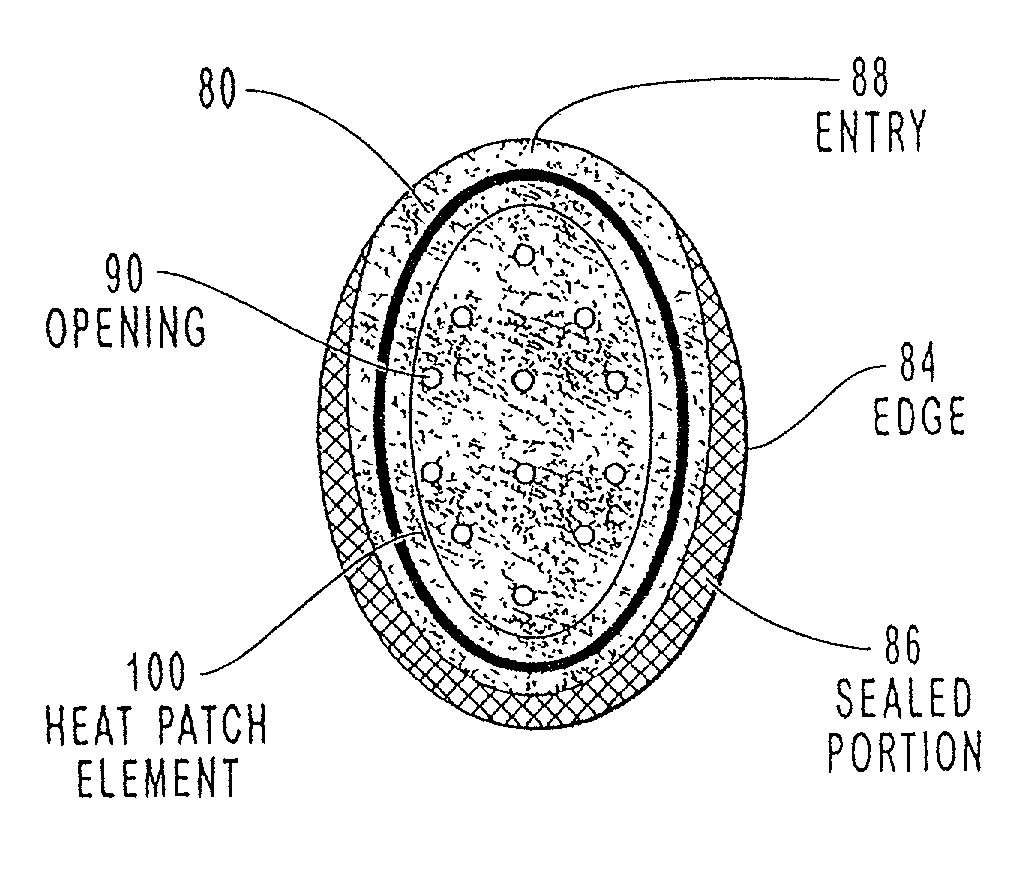
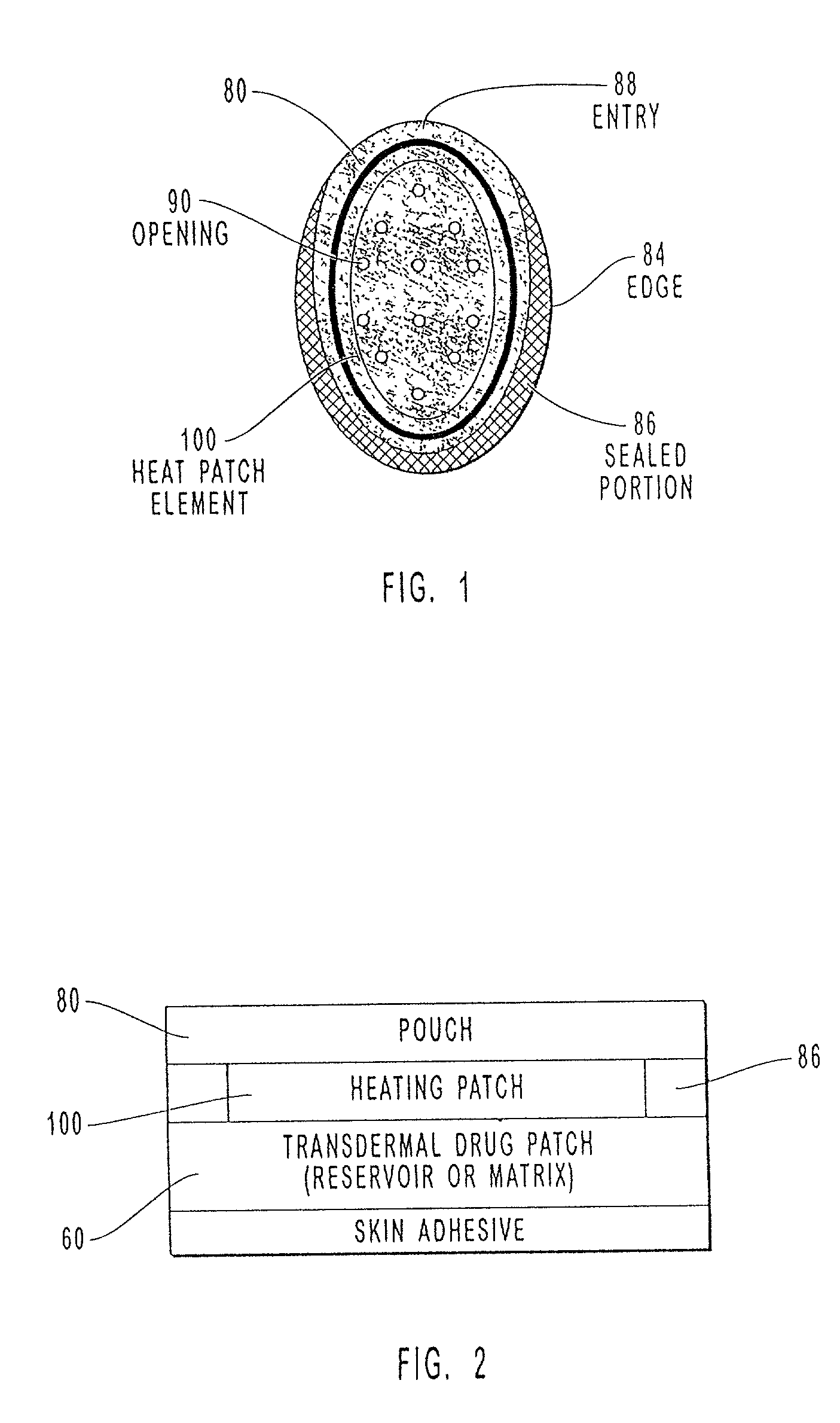
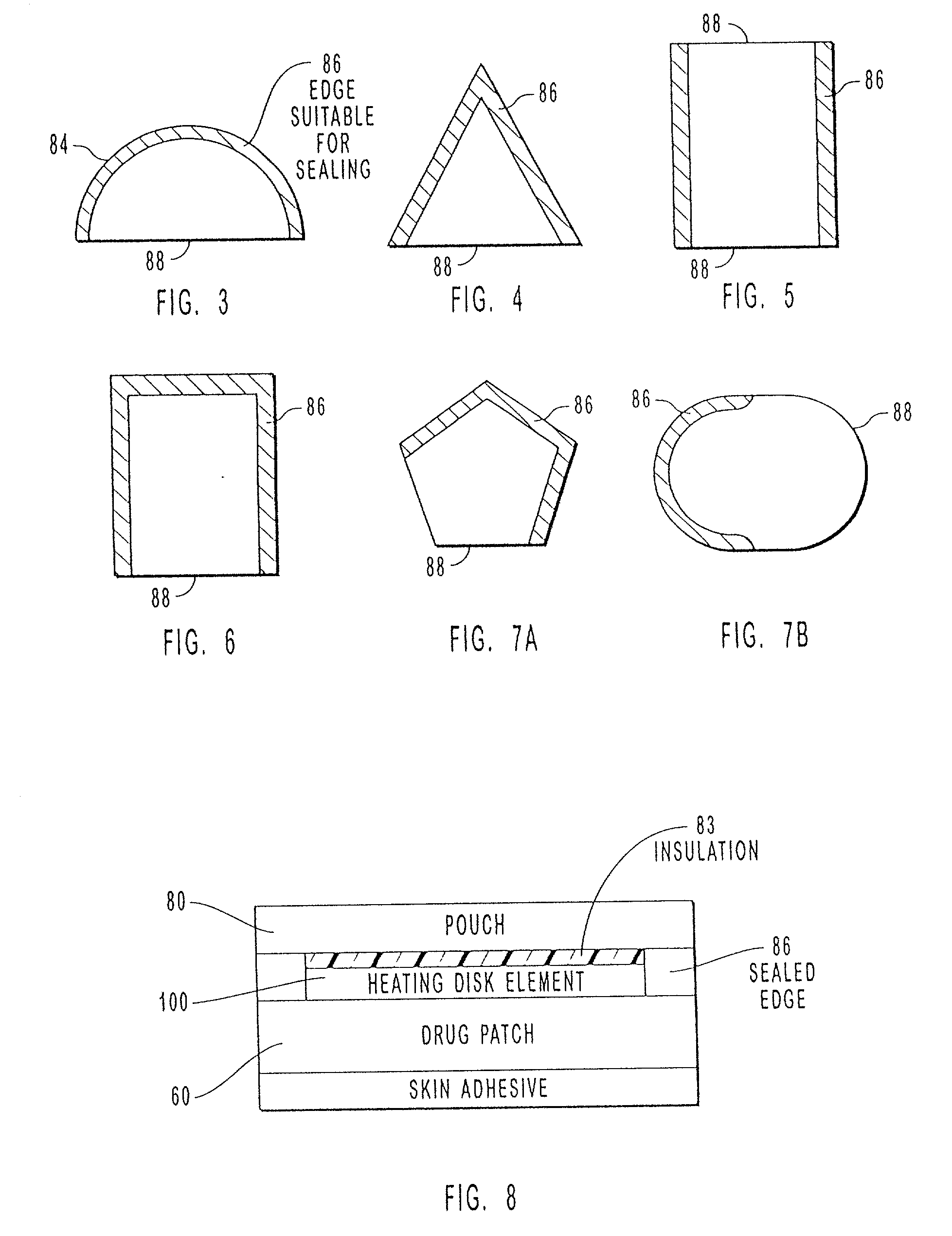
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
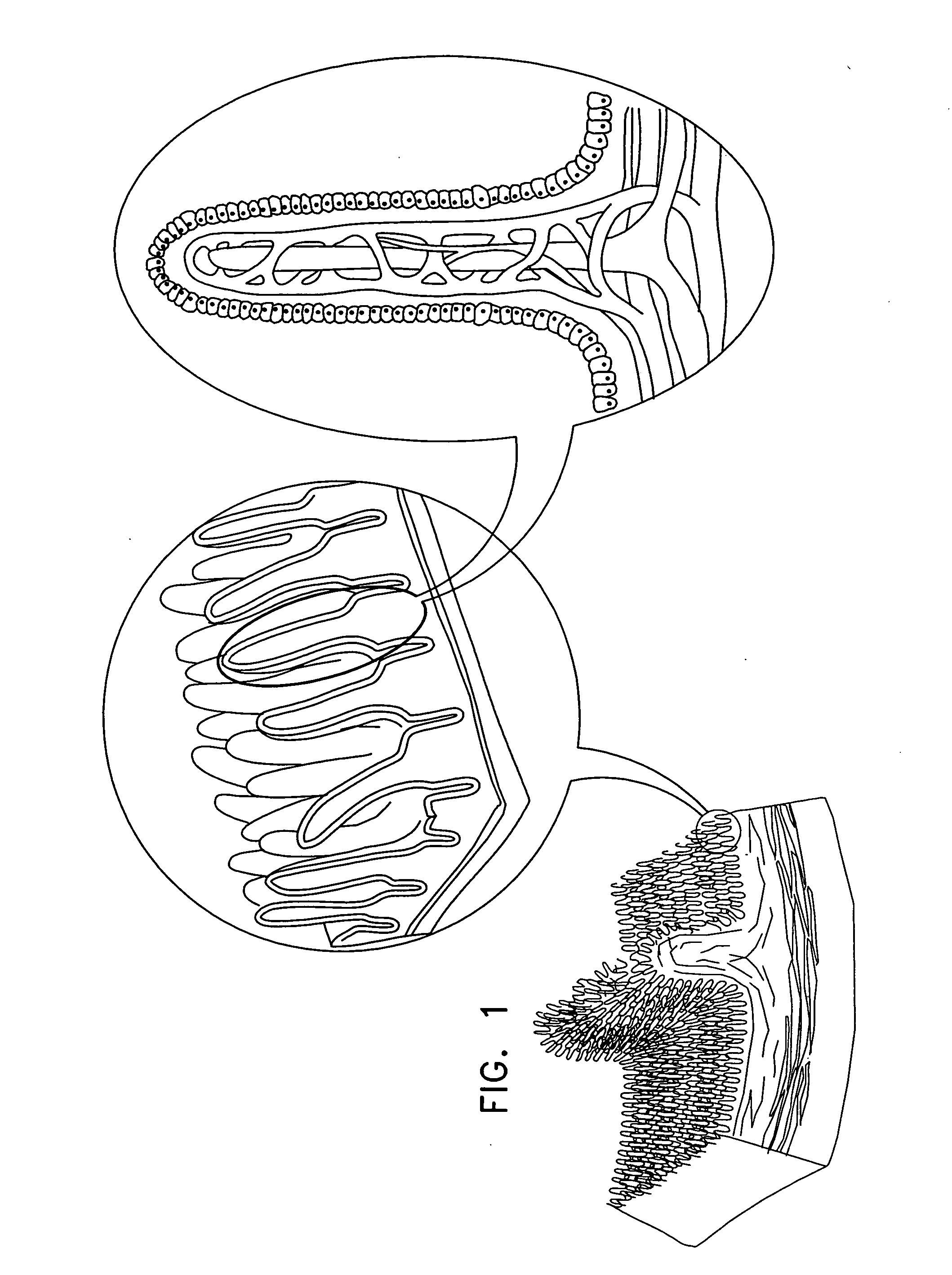
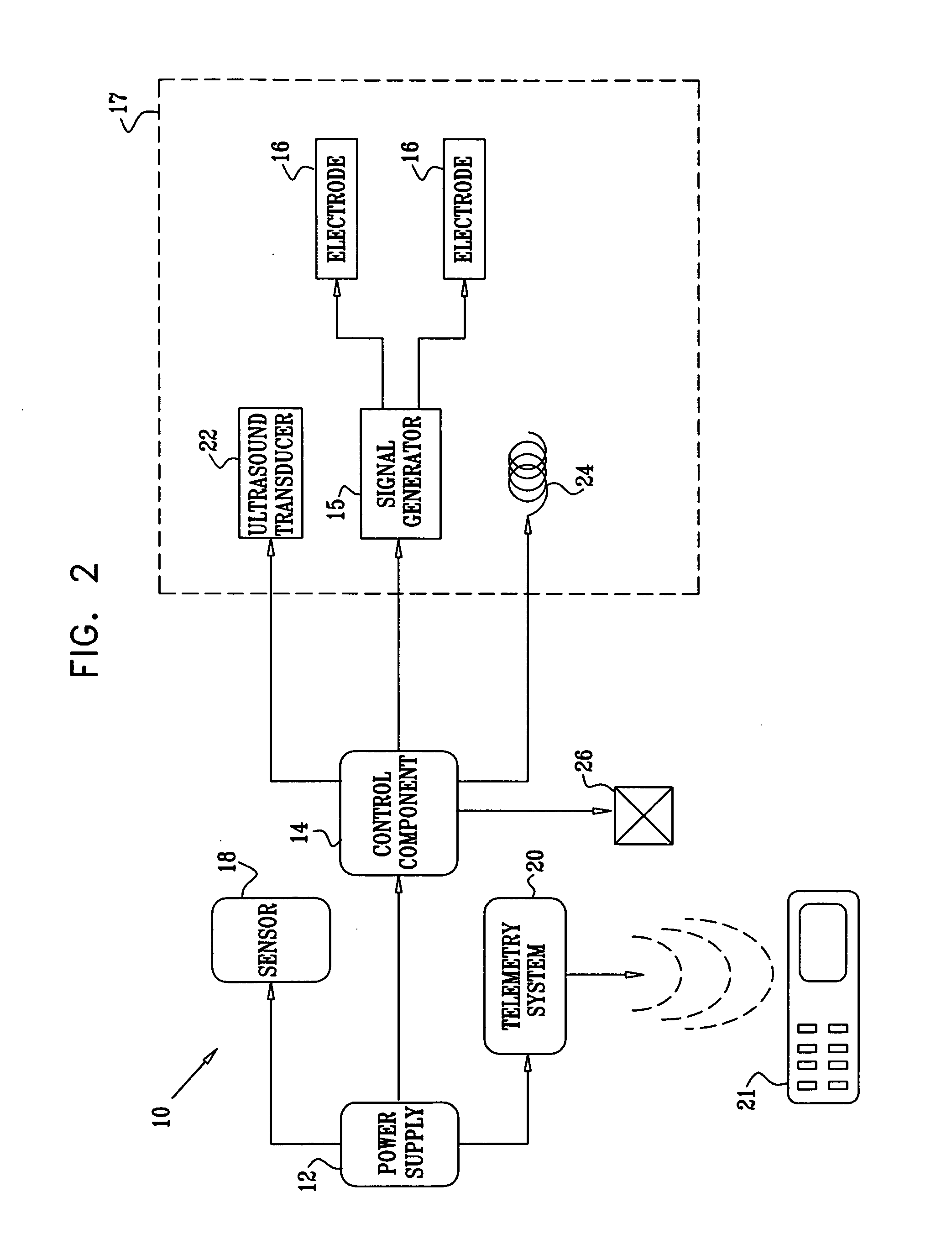
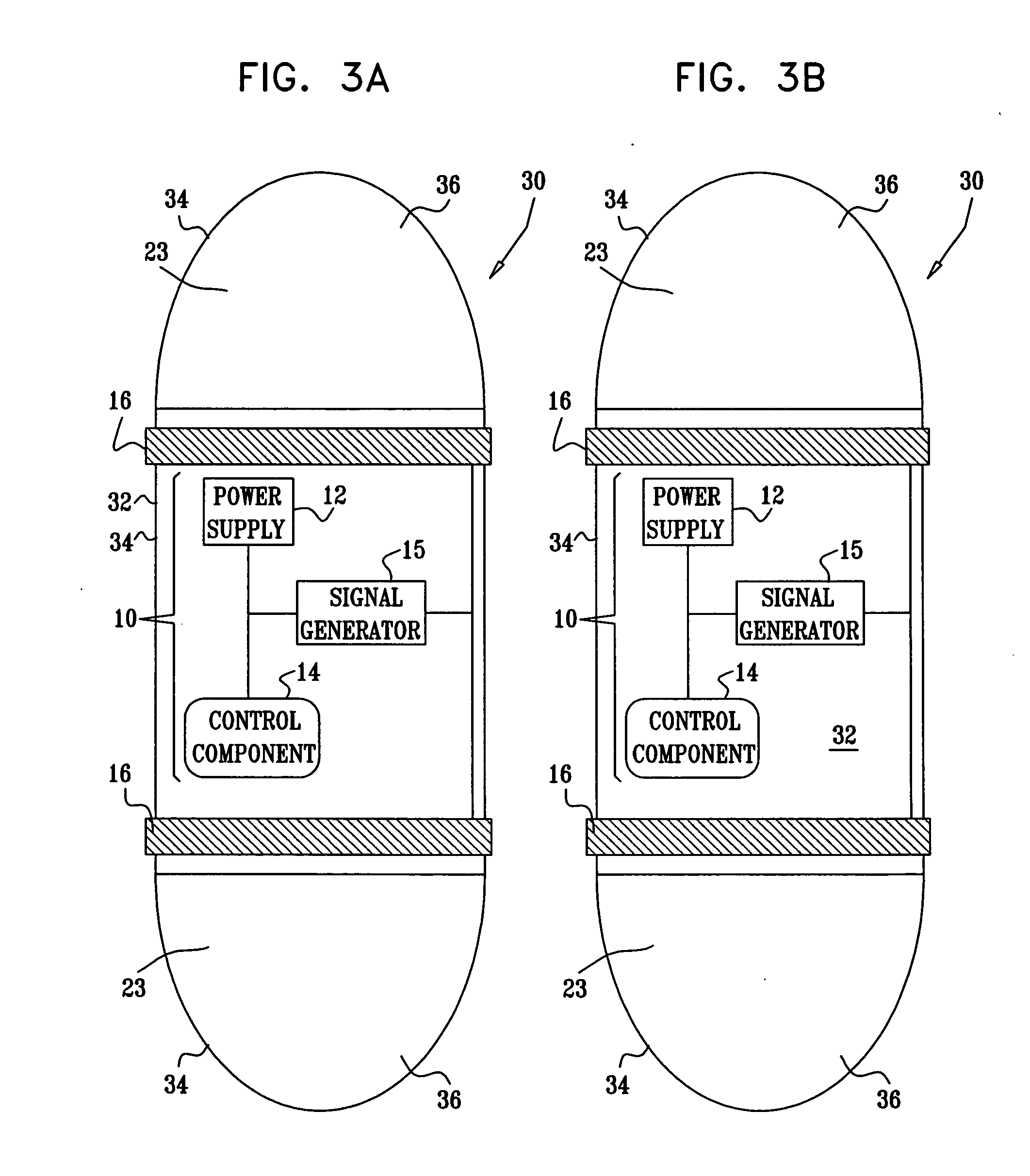
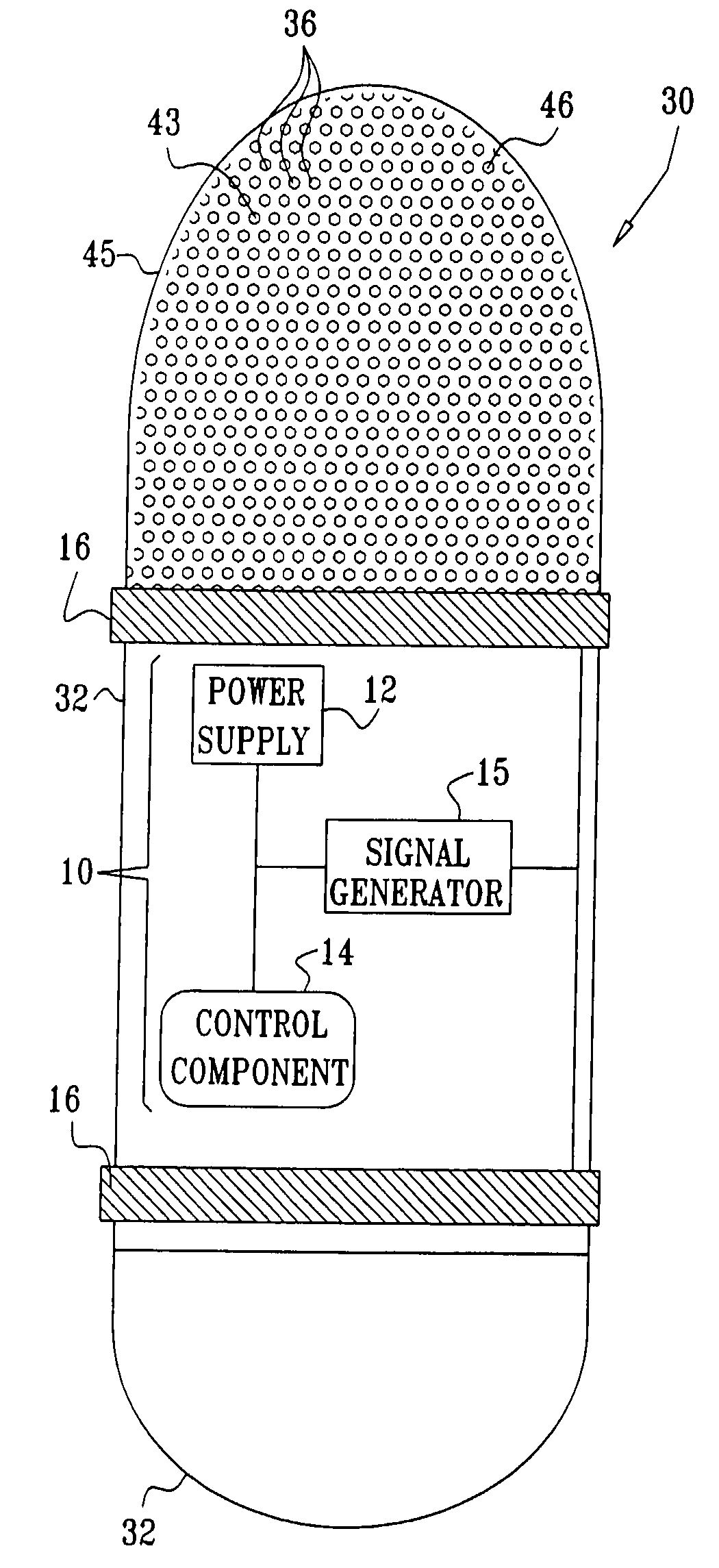
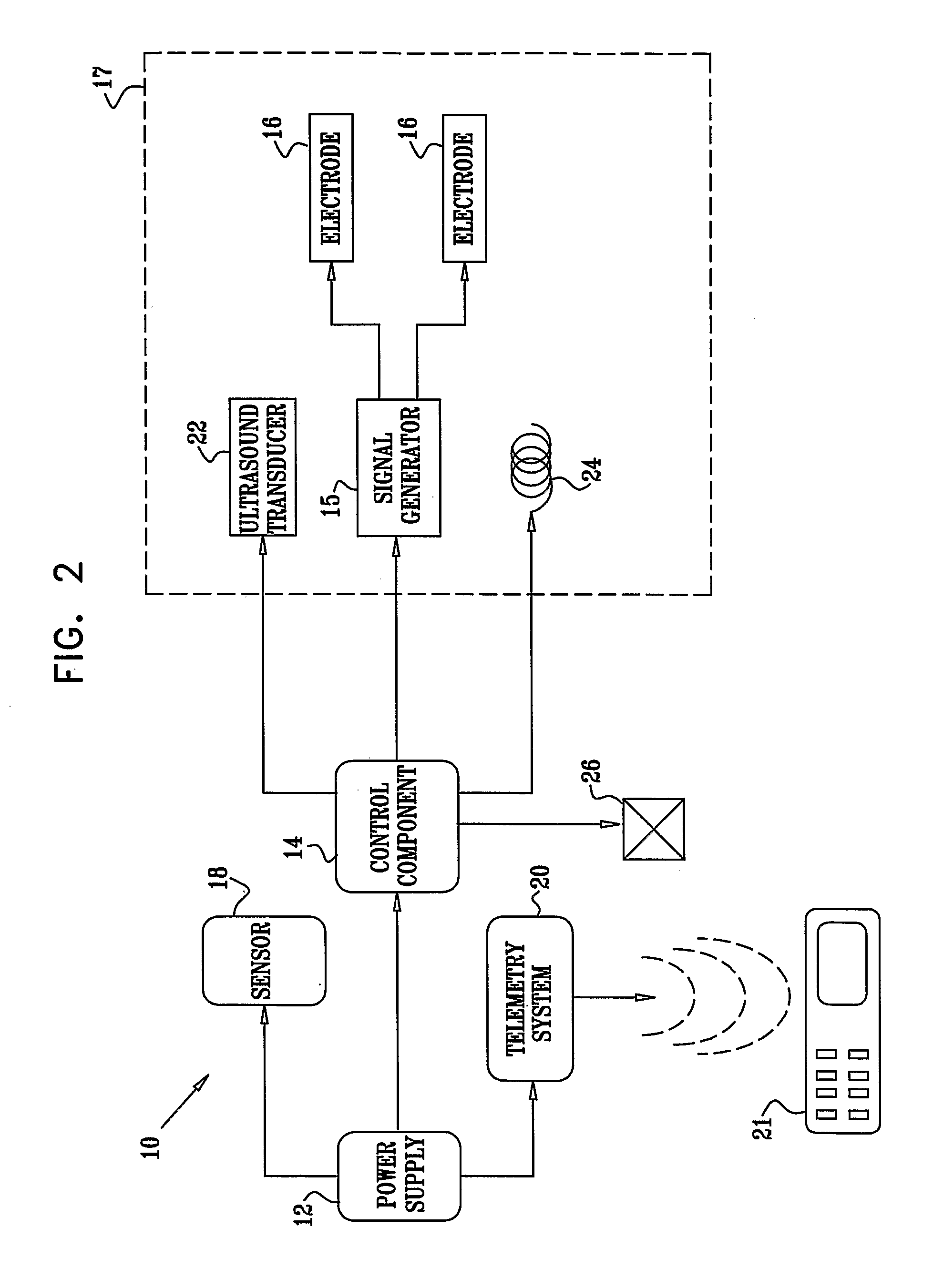
Active drug delivery in the gastrointestinal tract
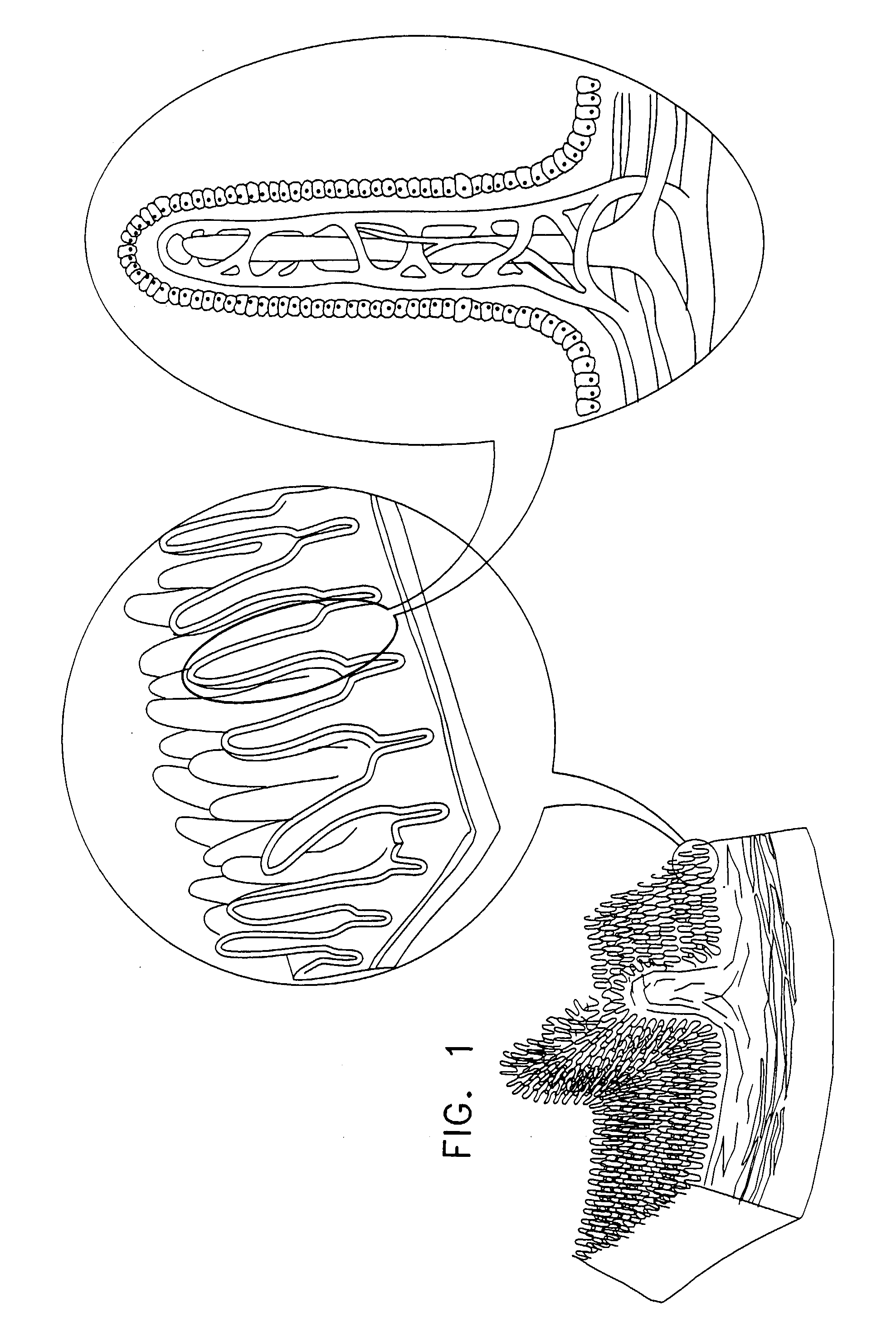
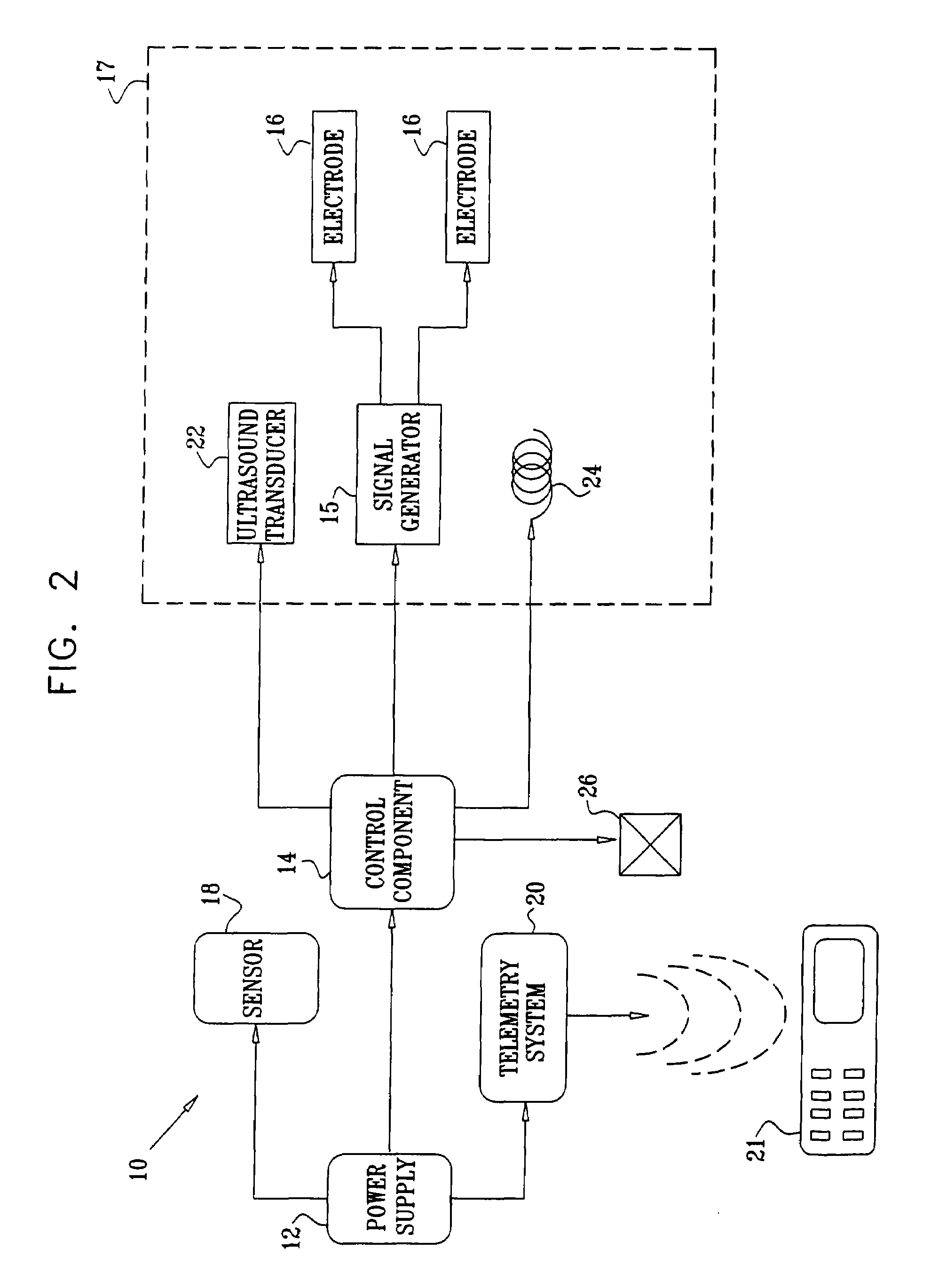
InactiveUS20050058701A1Easy accessPromote absorptionInternal electrodesBody temperature measurementMedicineDrug administration
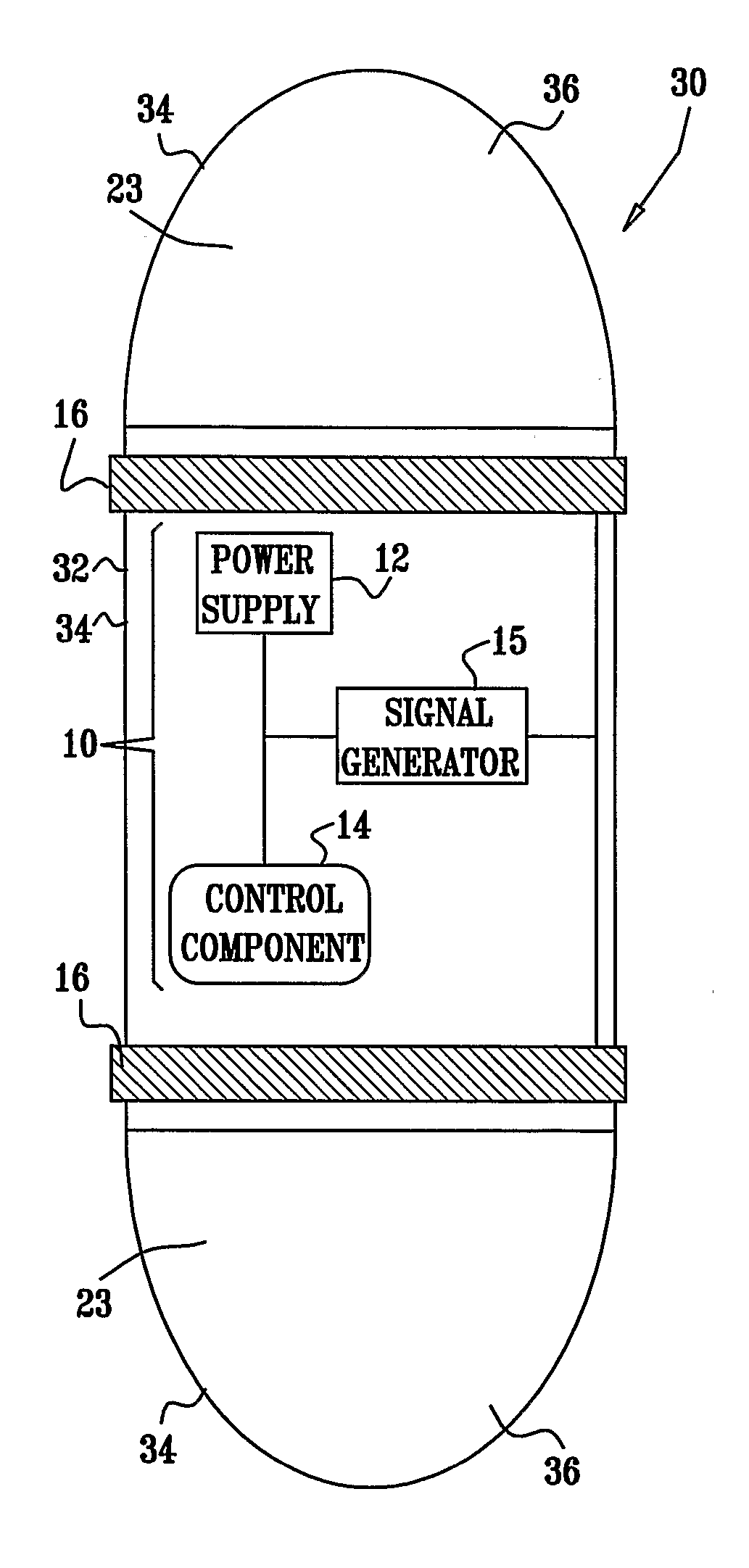
Apparatus for drug administration is provided, including an ingestible capsule, which includes a drug, stored by the capsule, and an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject. The capsule further includes first and second electrodes, and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, through an epithelial layer of the GI tract by driving the first and second electrodes to apply a series of pulses at a current of less than about 5 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds.
Owner:E PILL PHARMA
Device for feeding drug into pulmones
InactiveCN101176805AUniform atomizationAtomized particles are smallMedical atomisersInhalatorsNebulizerMedicine
The invention relates to a medical instrument used for drug absorption of pulmonary alveoli, in particular to an intrapulmonary drug administration device atomizing drugs dissolved in liquid into fine particles reaching the pulmonary alveoli, which comprises a casing and a suction nozzle arranged at one side of the casing. The invention is characterized in that a battery, a liquid supplying bottle and an atomizer are arranged in the casing, wherein, one end of the liquid supplying bottle is communicated with the suction nozzle, while the other end is connected with the atomizer, an air inlet is arranged on the casing above the atomizer, and an air passage is arranged on the outer surface of the liquid supplying bottle; an action switch is arranged on the casing. The invention has the advantages that the drug can be atomized into uniform particles, the particles are small, and the average diameter can reach about 1 micron, which is the best technical parameter for intrapulmonary absorption; a quantitative device arranged in the invention can control the drug administration amount; the invention can be used for drug administration of bronchioles and pulmonary alveoli.
Owner:DAFUBAO INT
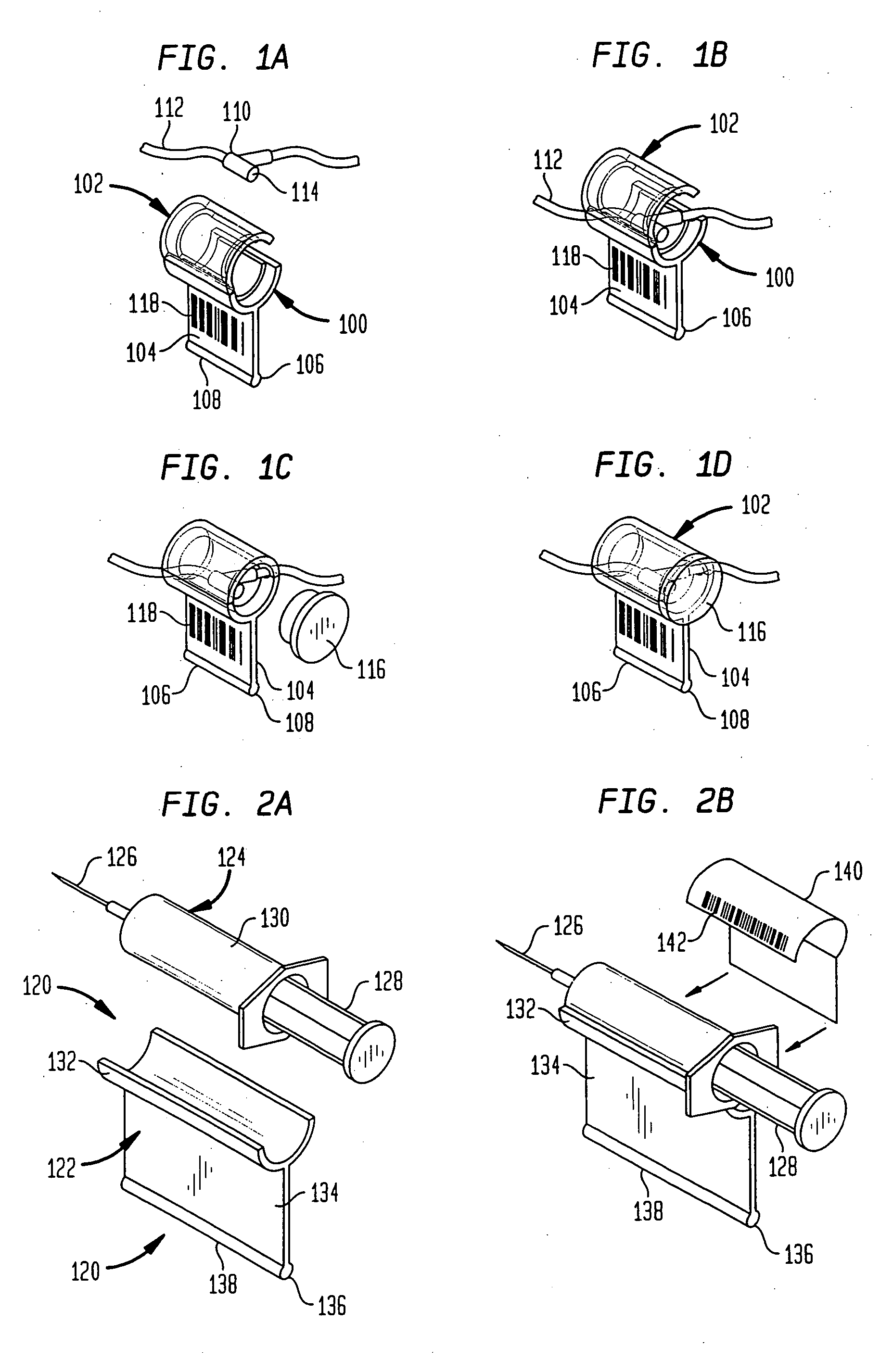
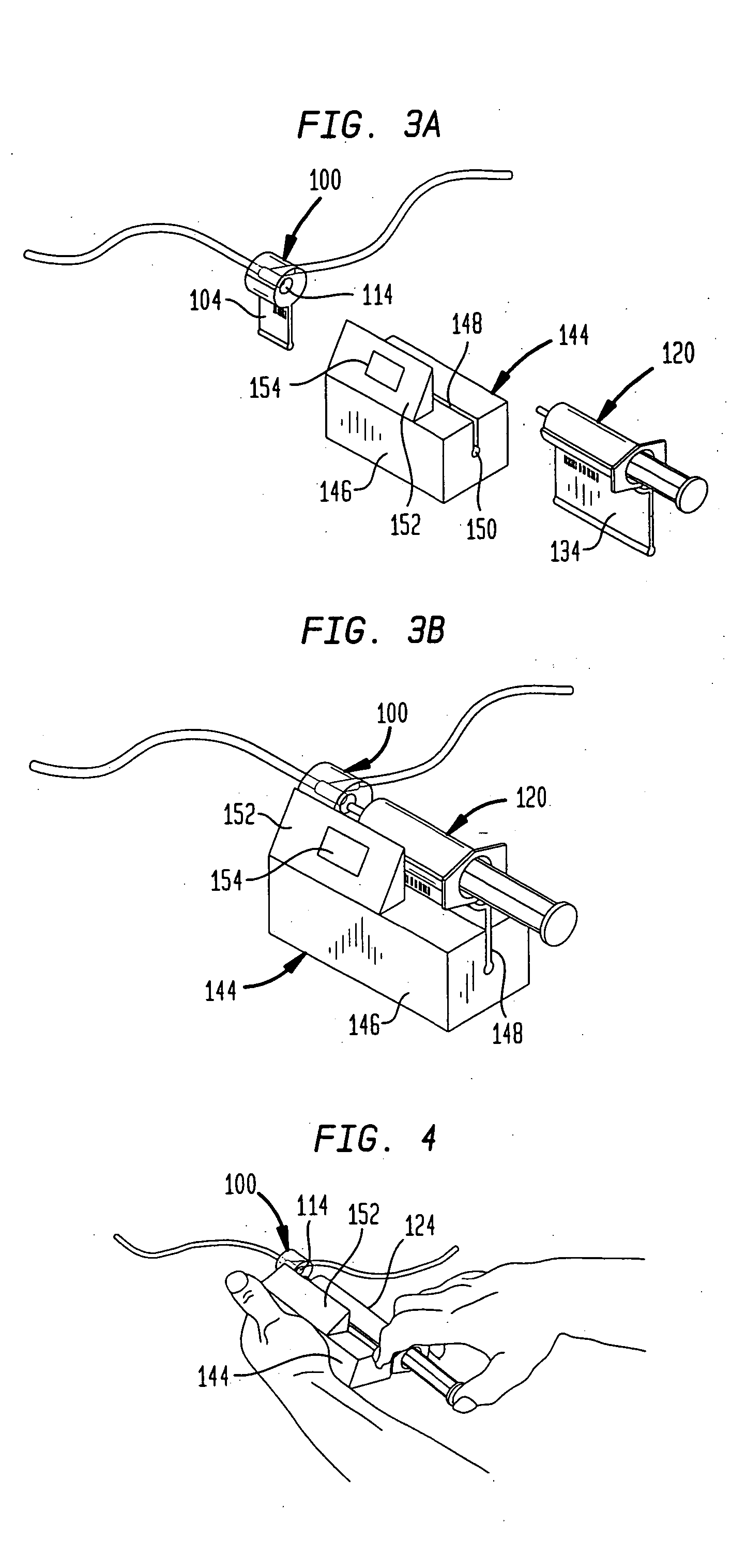
Drug delivery and monitoring system
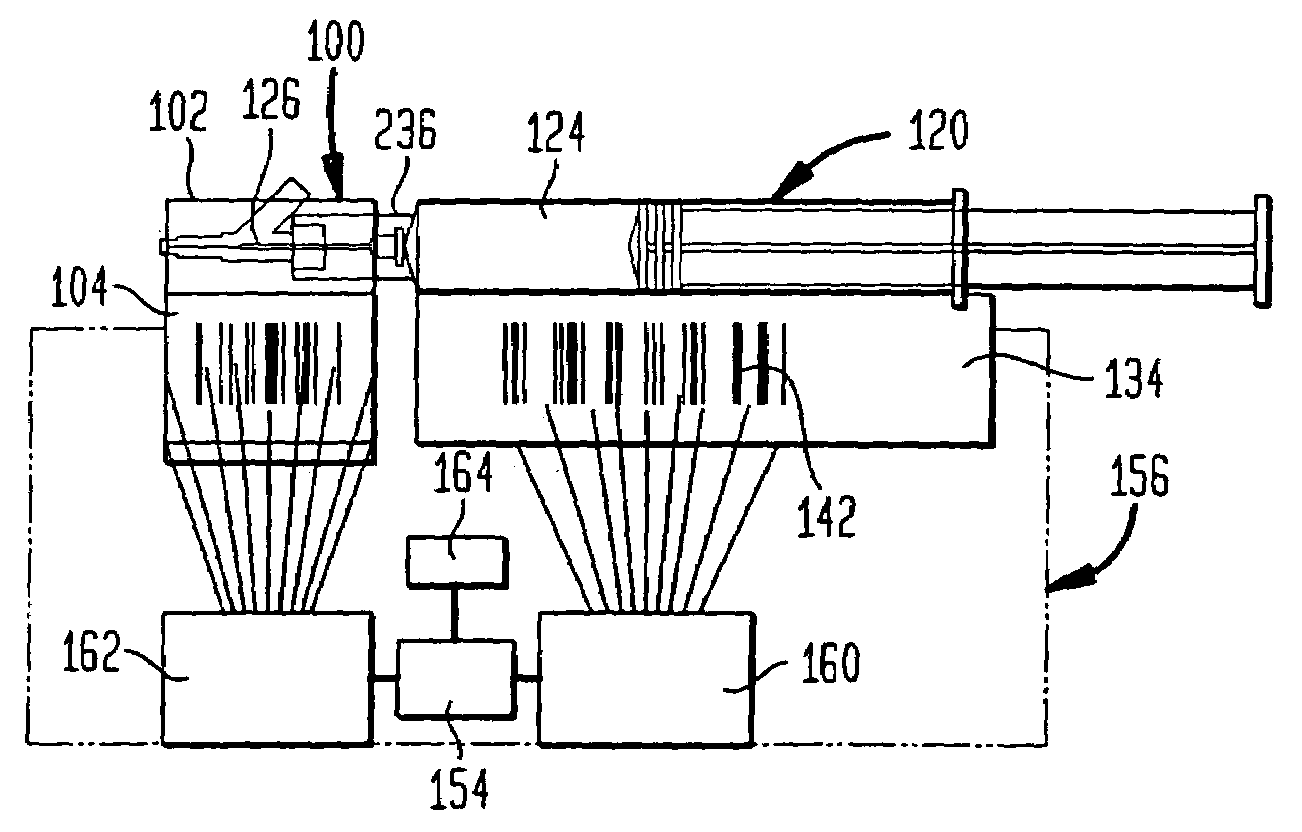
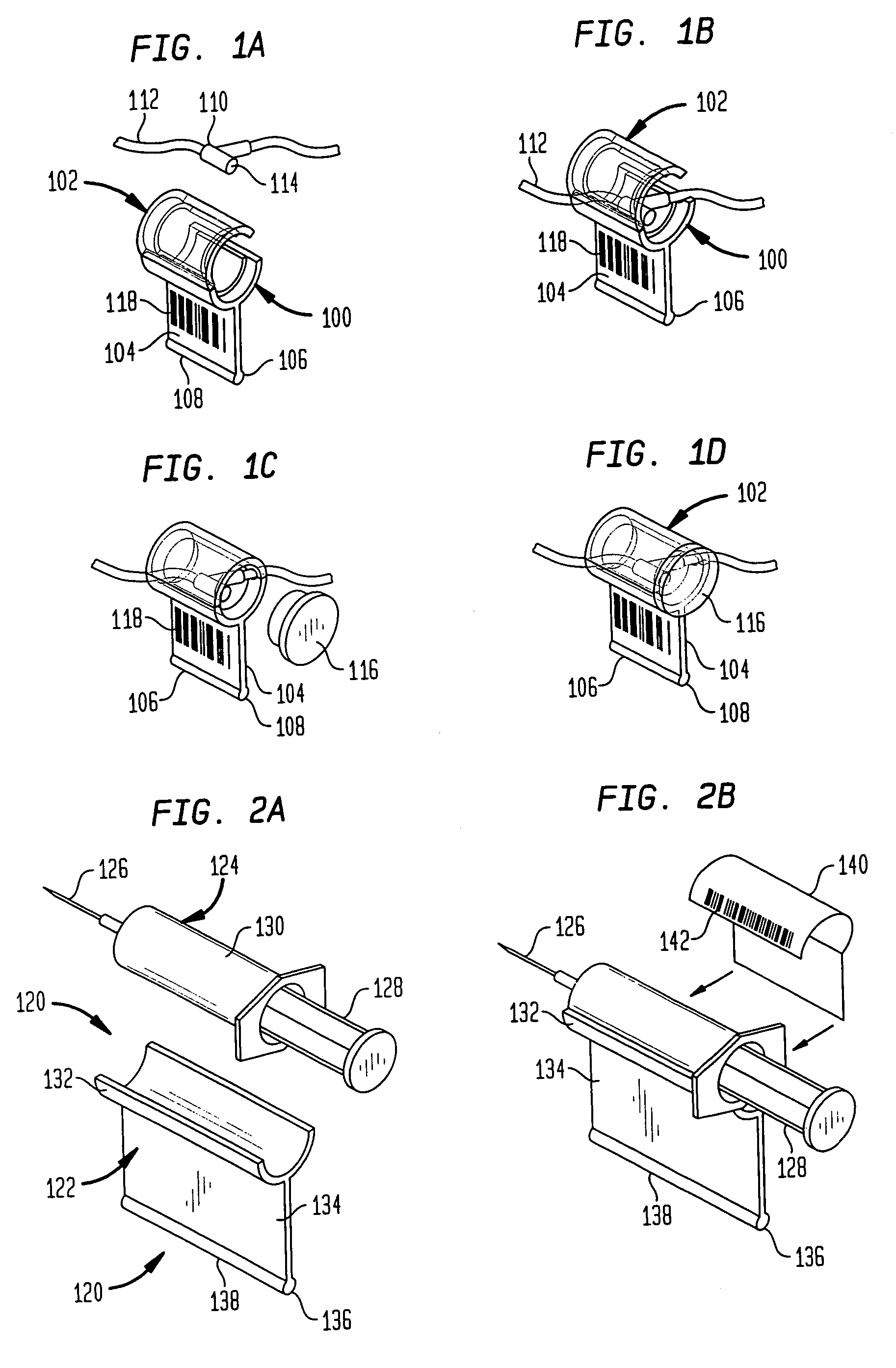
A drug administration system includes a cradle attached about an intravenous injection port having a flange extending therefrom. The cradle supports first drug administration information in the nature of machine and human readable code, for example, barcode. A syringe including a needle includes a flange extending from the syringe. The syringe supports second drug administration information in machine and / or human readable form. A scanner module is constructed to slidably receive the flange of the cradle and syringe whereby the syringe needle is aligned with the intravenous injection port. The module may be provided with an electronic scanning system for identifying the first and second drug administration information, as well as determining the amount of the drug being administered from the syringe to the injection port by monitoring movement of the syringe plunger. The information and data may be stored within the module for uploading to a remote location.
Owner:INT BUSINESS MASCH CORP
Decision information system for drug delivery devices
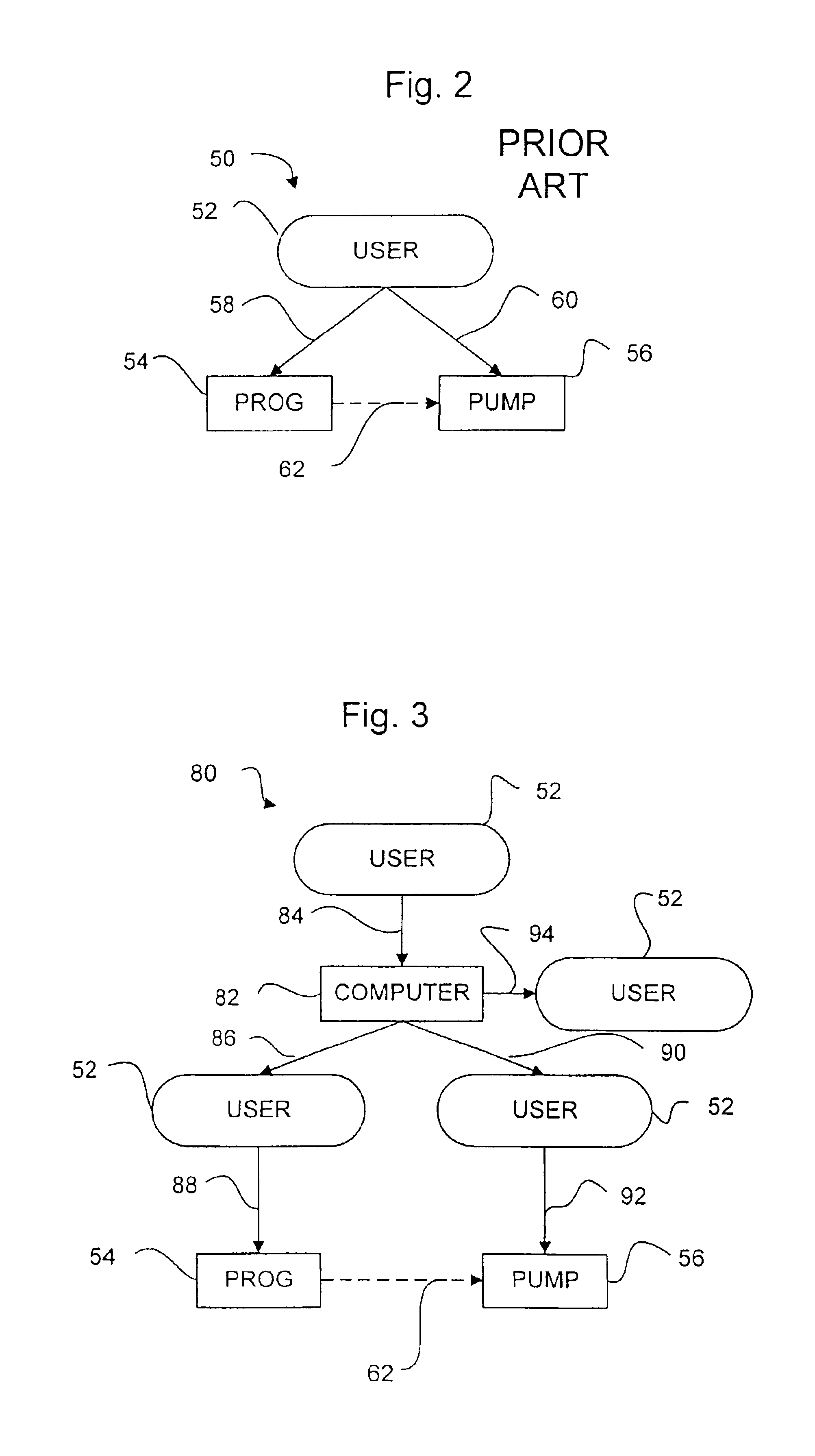
ActiveUS6928338B1Sampled-variable control systemsControlling ratio of multiple fluid flowsDoses rateGeneral purpose computer
Decision information systems, methods, and computer programs for better informing decisions to use multiple drugs in drug delivery devices, including implantable devices, for drug administration. Executable computer programs and logic embodying methods of the invention can calculate consistent multiple drug mixture amounts and drug delivery flow rates. One program accepts user input indicating a desired first drug dose rate, an initial first drug concentration, a desired second drug dose rate, an initial second drug concentration, and the reservoir size of the drug delivery device. The program method calculates a first drug amount and a second drug amount to combine in a mixture as well as a first drug true concentration in the mixture. The drugs can be mixed consistent with the physician's instructions using the program output. The first drug true concentration can be entered into a programmer device as the only drug concentration entered. Another program calculates a consistent first drug, second drug, and diluent amount to be added to a mixture for injection into a fixed flow rate, implantable drug delivery device. Methods preferably output true concentrations and dose rates for all drugs to be added and most preferably show all calculations used to arrive at the flow rate and mixture amount calculations. Yet another program receives a new desired drug dose rate for a previously filled device. The program accepts the existing mixture volume and true drug concentrations for a partially depleted device and calculates a new mixture flow rate to achieve the desired dose rate using the existing mixture. The methods can be implemented as executable computer programs in programmer devices, general purpose computers, servers, handheld computers, and personal digital assistants.
Owner:MEDTRONIC INC
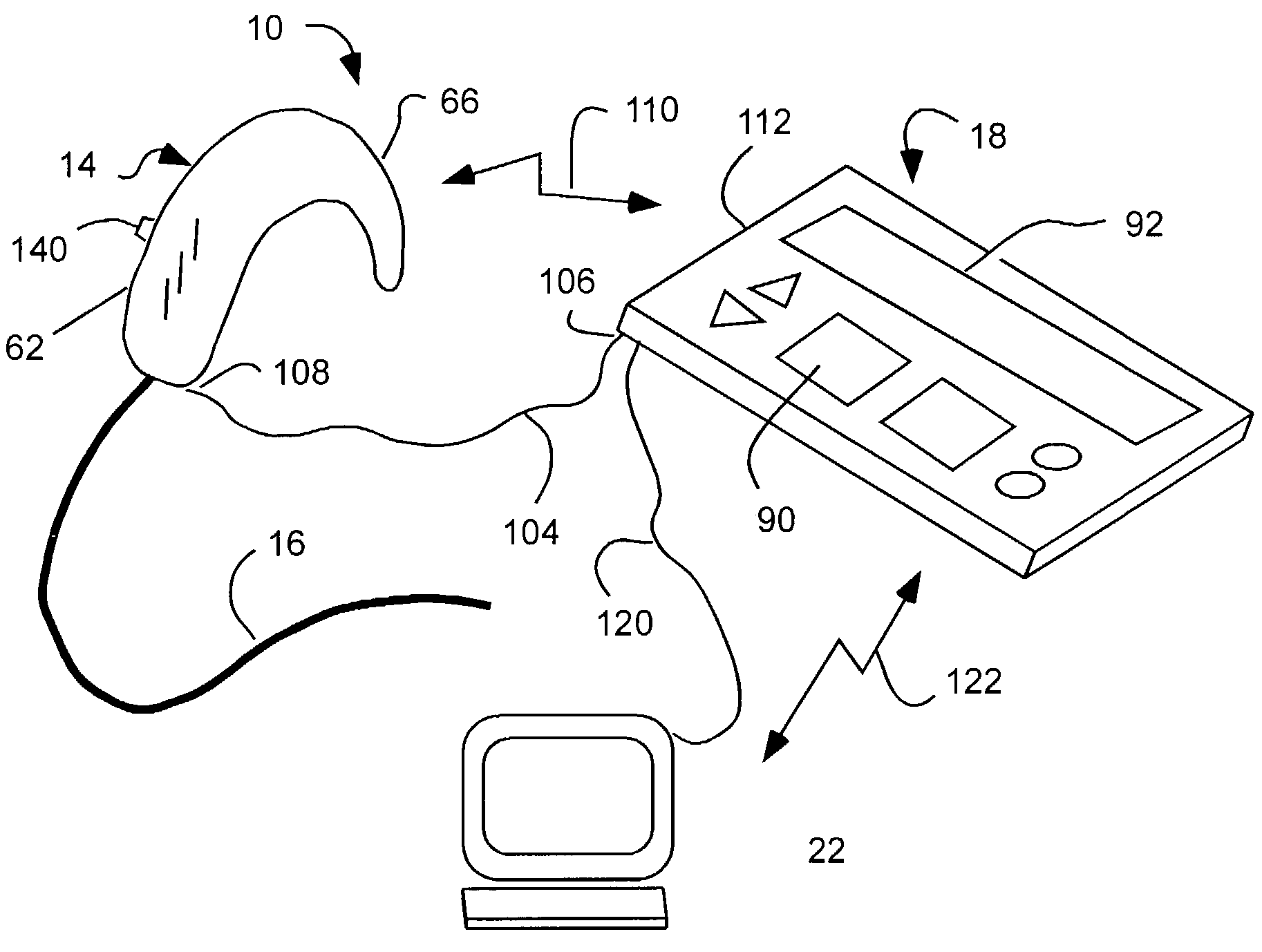
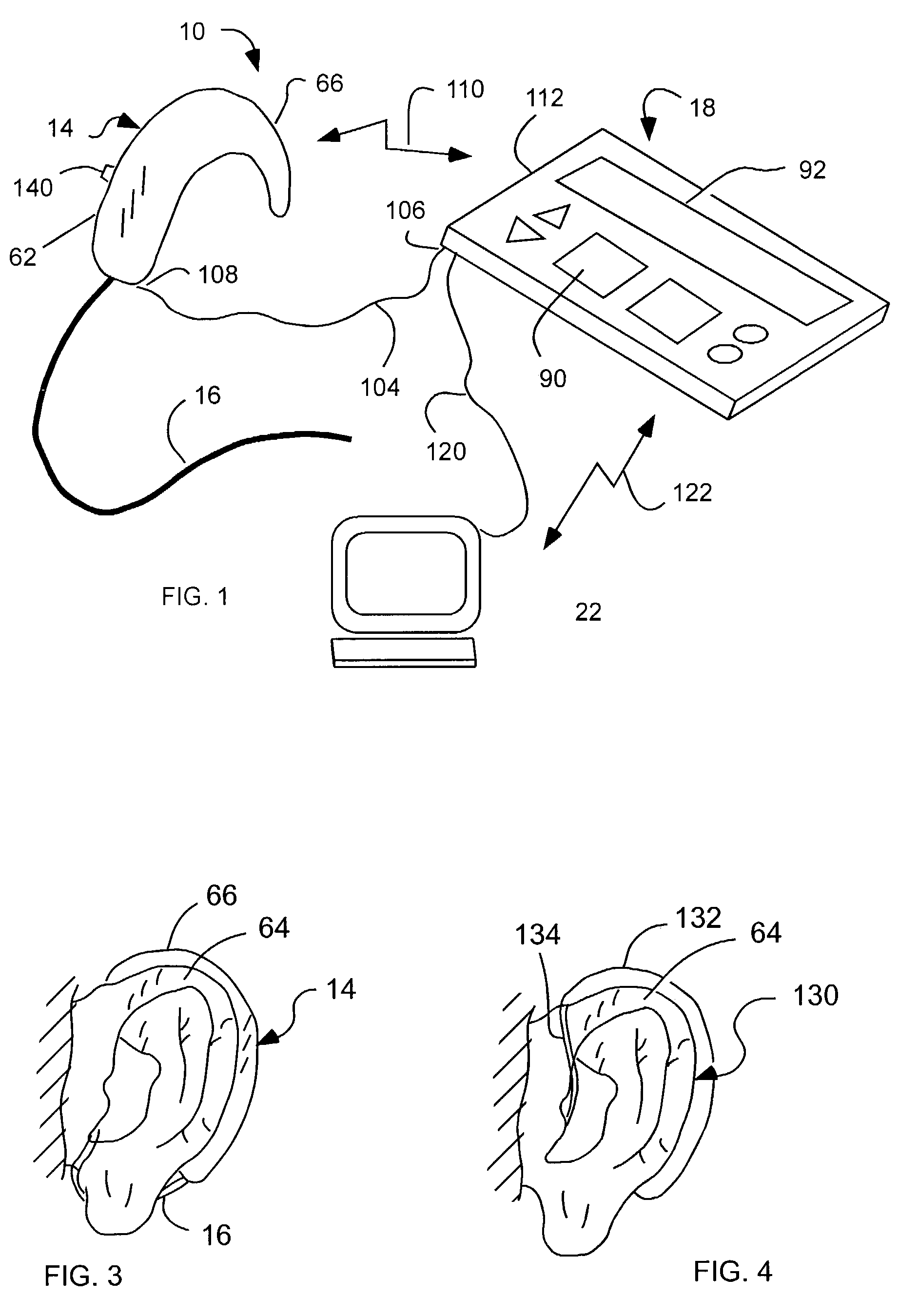
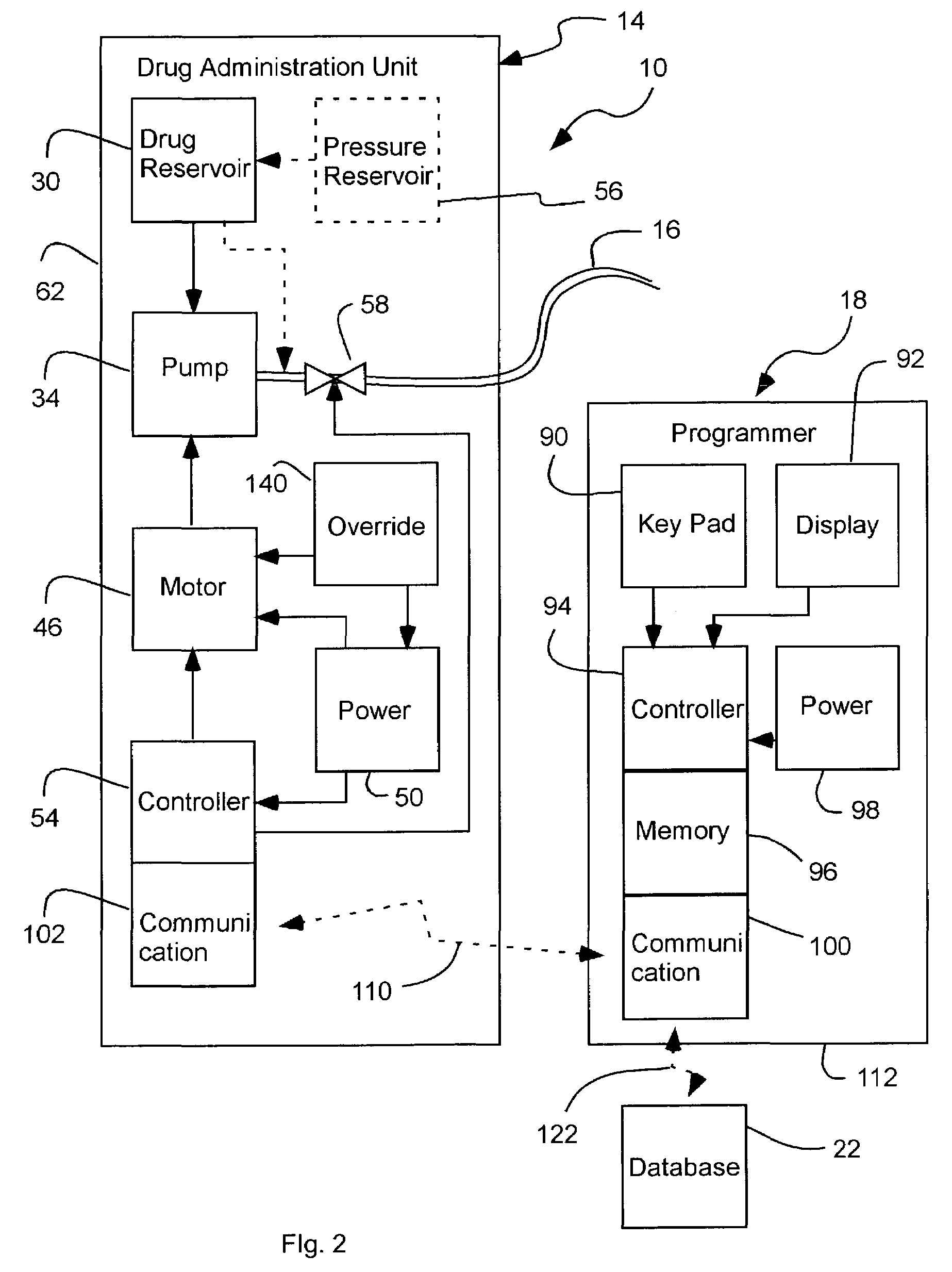
Cochlear drug delivery system and method
InactiveUS7206639B2Convenient, unobtrusive, and inexpensiveElectrotherapyEar treatmentDrug reservoirMiddle ear
A drug administration system configured to administer a drug to a user's ear includes a housing, sized and shaped to substantially fit behind the user's ear, and configured to pump the drug in controlled amounts to the user's middle ear. A drug reservoir is disposed in the housing and includes a drug configured to treat an inner ear condition. A catheter is operatively coupled to the drug reservoir and is sized and shaped to extend from the drug administration unit into the user's middle ear.
Owner:STERLING INVESTMENTS LC
Prolonged Transit Time of Permeability-Enhancing Drug Eluting Pill
InactiveUS20080275430A1Promote absorptionShorten speedMedical devicesMicromachined deliveryPower flowMedicine
Apparatus is provided for drug administration. The apparatus includes an ingestible capsule, which includes a drug, stored by the capsule. The apparatus also includes an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject; one or more drug-passage facilitation electrodes; and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, by driving the drug-passage facilitation electrodes to apply an electrical current. The apparatus further includes a velocity-reduction element adapted to reduce a velocity of the capsule through the GI tract for at least a portion of the time that the control component is facilitating the passage of the drug. Additional embodiments are also described.
Owner:E PILL PHARMA
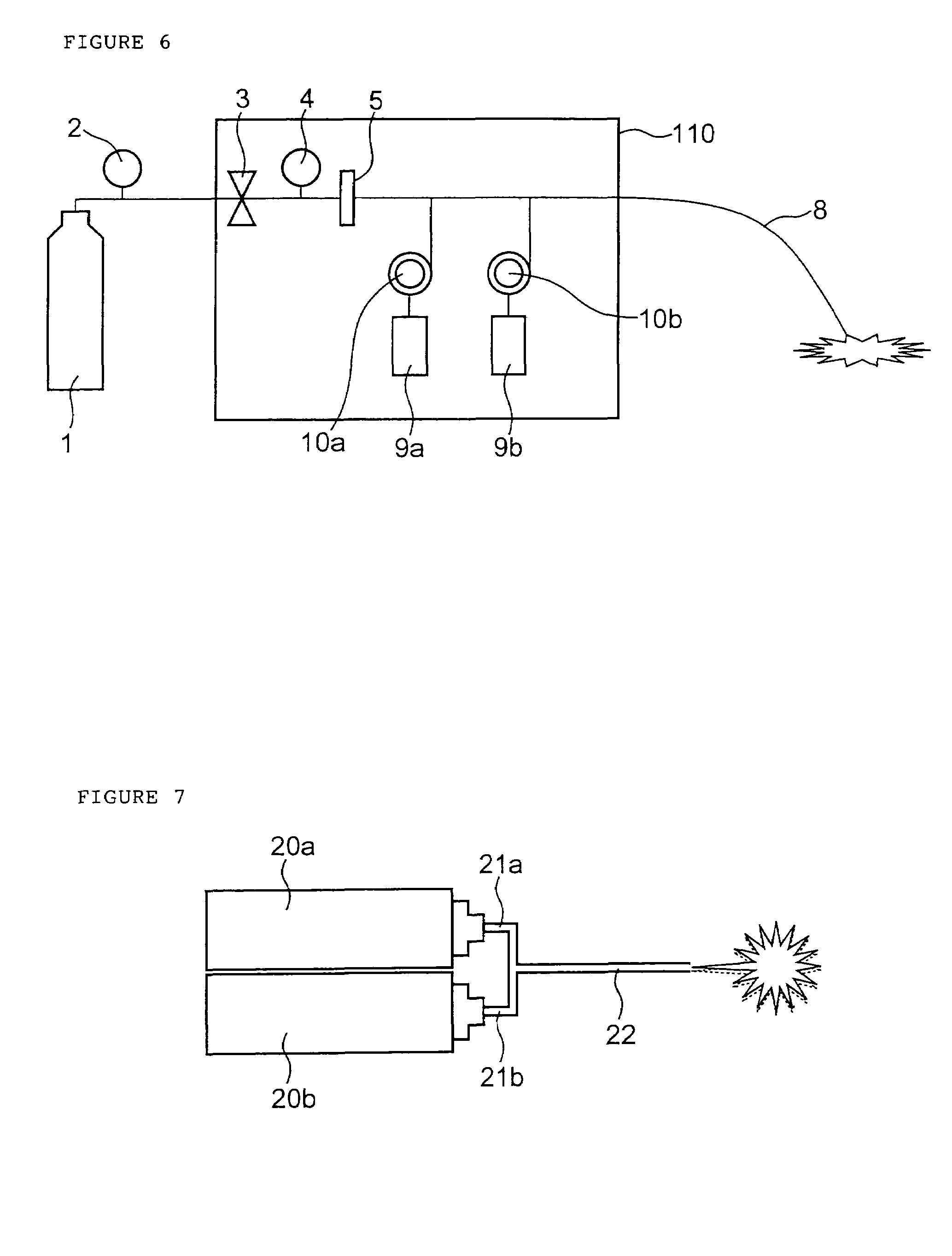
Smart adapter for infusion devices
ActiveUS20160030683A1Improve safety and efficacyInfusion syringesMicroneedlesPen InjectorDrug administration
Smart sensors are employed to determine one or more of drug identification, dose, flow rate, concentration, agglomeration, and degradation and / or other characteristics of drug administration that can be detected via sensing technology. A smart sensor(s) can be coupled to or retrofitted onto injection pen injectors and / or drug delivery cartridges and / or infusion sets or cannulae, enabling infusion sets, pen injector systems or drug delivery cartridges to improve tracking of drug self-administration and stop medication errors that occur primarily through self or automated injection (e.g., due to incorrect or incomplete dosing, excessive dose or rate, incorrect drug, or drug degradation).
Owner:BECTON DICKINSON & CO
Compositions for oral drug administration
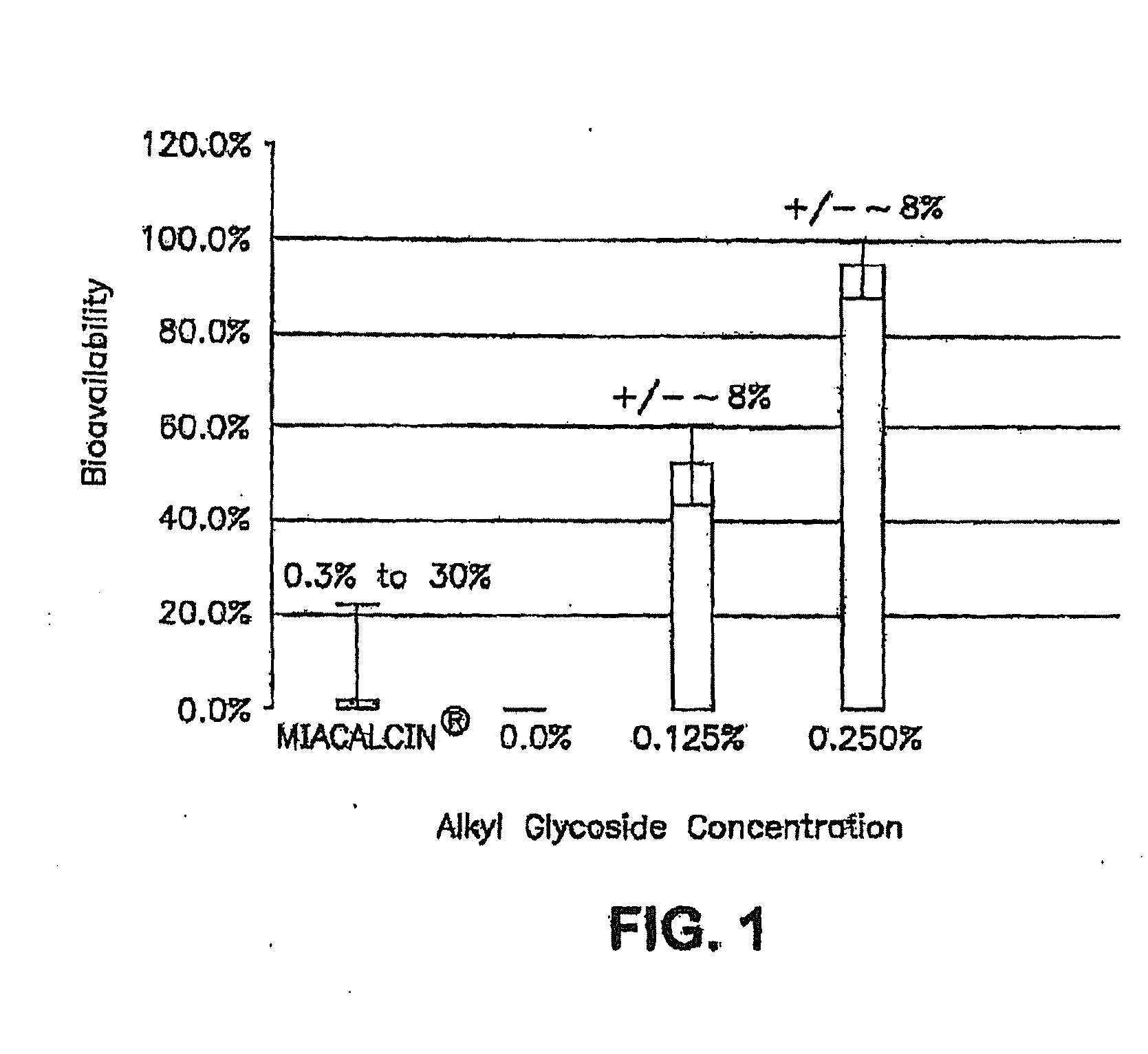
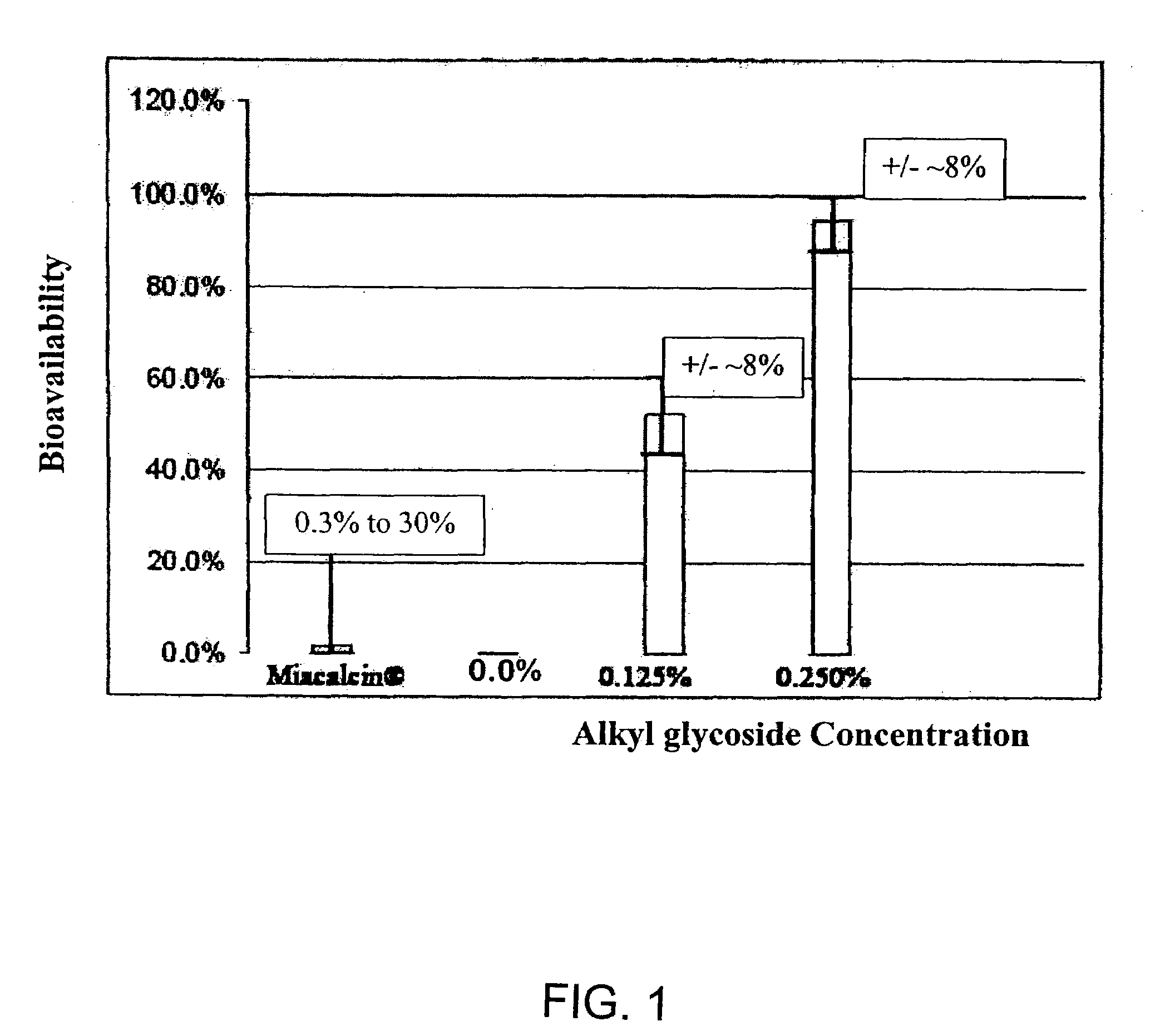
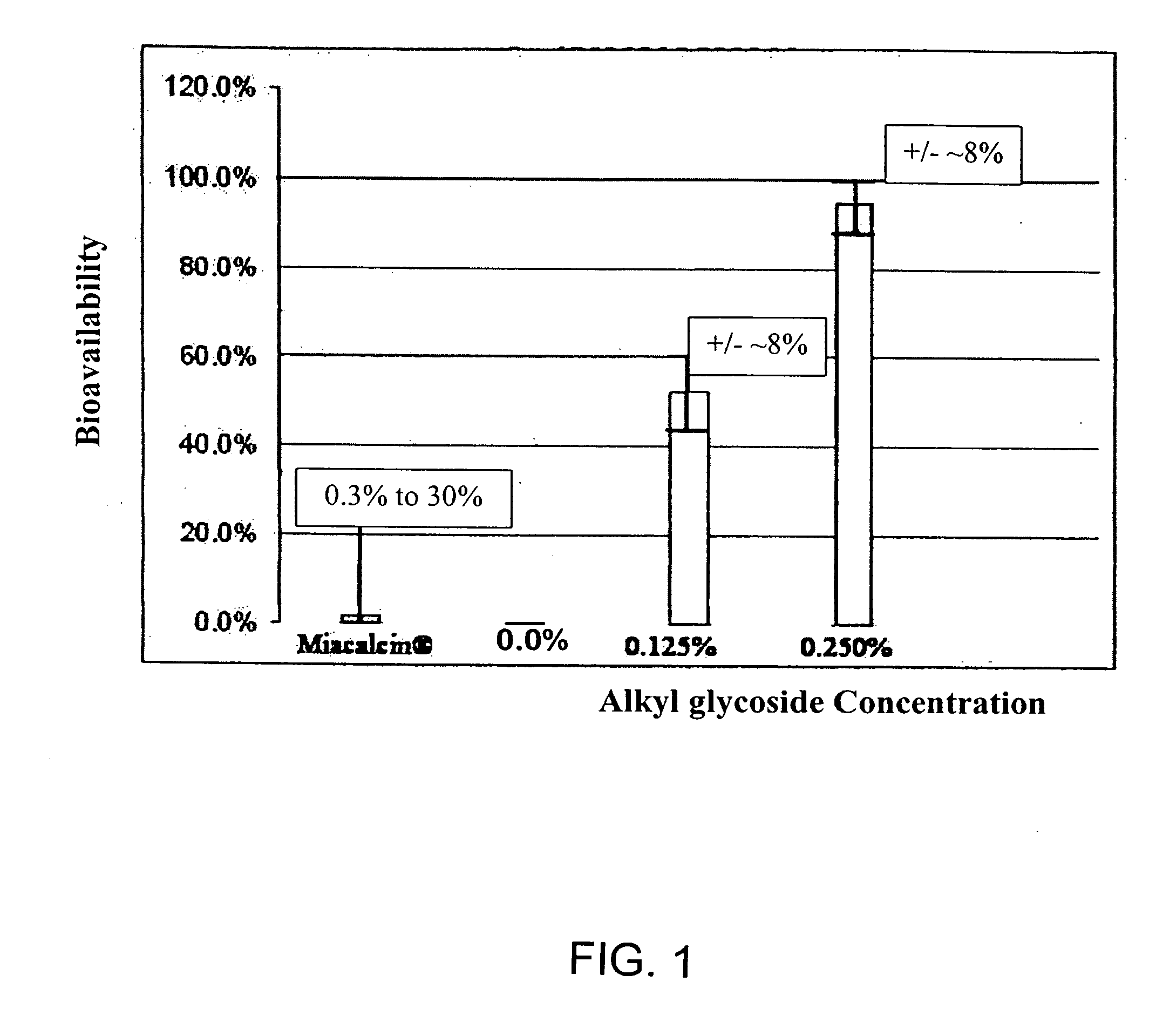
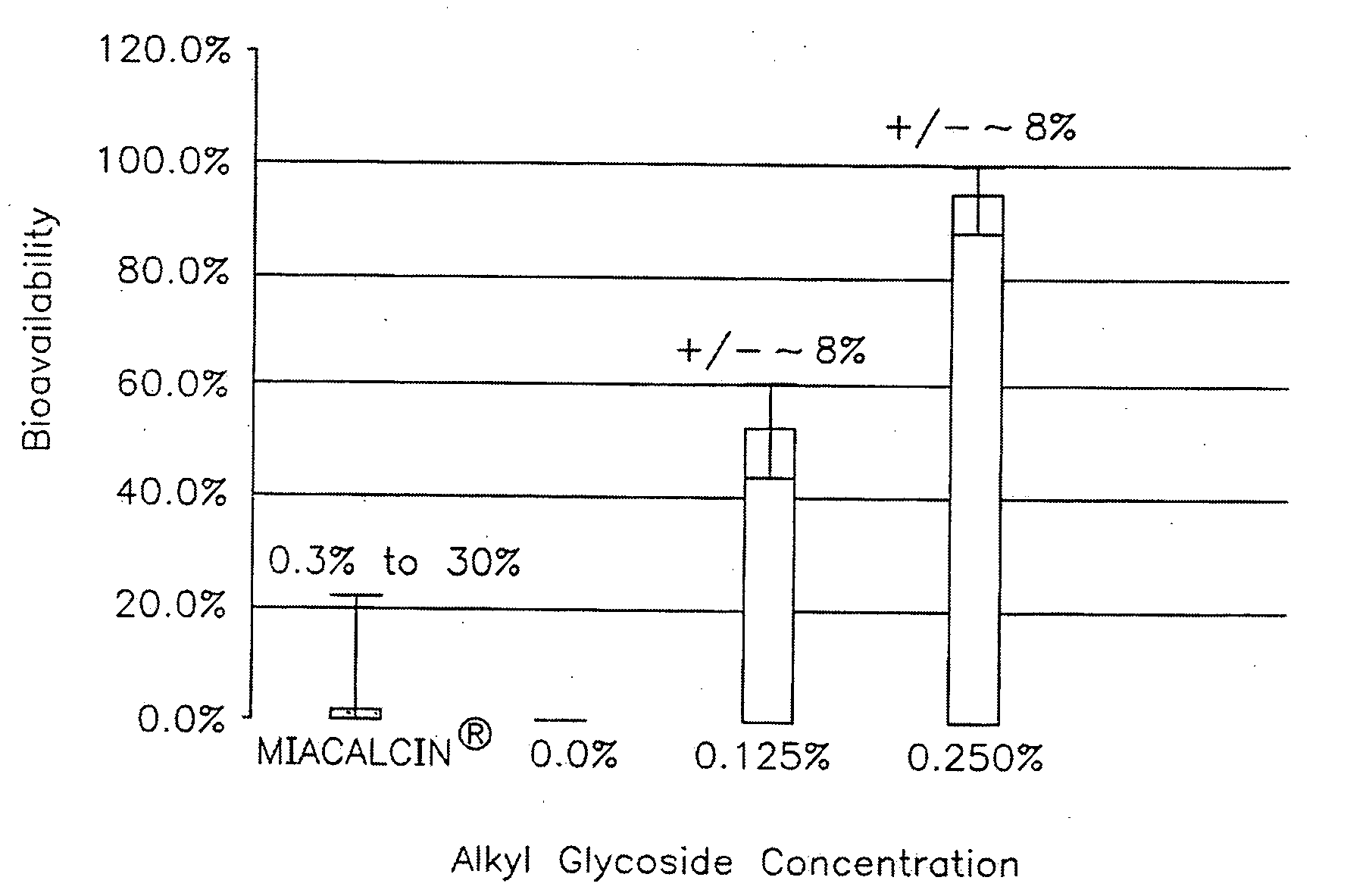
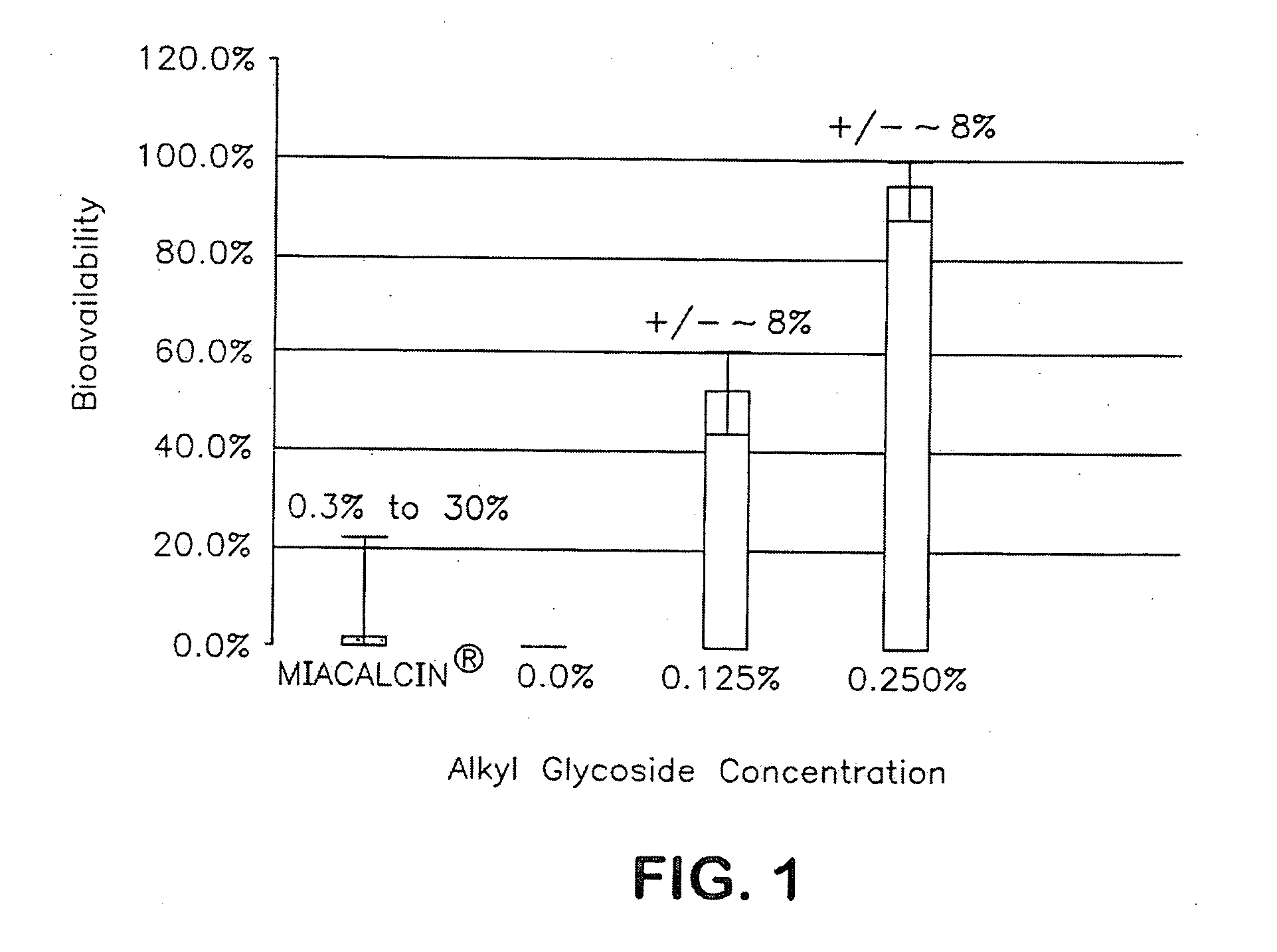
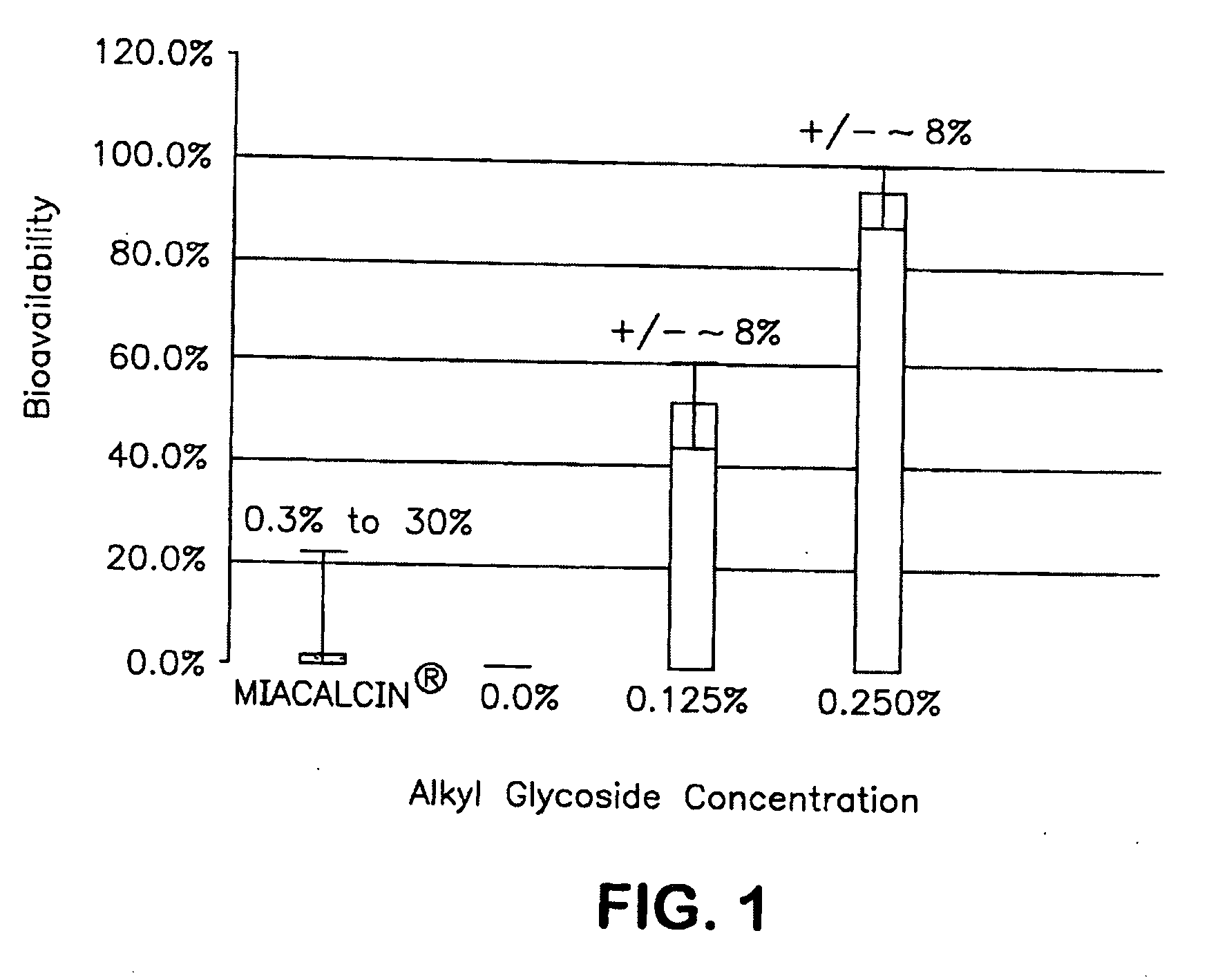
InactiveUS20140162965A1Promote absorptionImprove bioavailabilityBiocideCarbohydrate active ingredientsGlycosideOral medication
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery in the form of a tablet.
Owner:AEGIS THERAPEUTICS LLC
Characterization and modulation of physiologic response using baroreflex activation in conjunction with drug therapy
InactiveUS20080051767A1Good blood pressureLower systolic blood pressureElectrotherapyElectrocardiographyNervous systemPhysiologic States
A method and device for delivering and monitoring baroreflex and drug therapy to manage hypertension. The method includes providing an implanted an implanted medical device configured to automatically detect drug-related effects on the autonomic nervous system including the steps of measuring a physiologic status of the autonomic nervous system at desired intervals, logging the physiologic status of the autonomic nervous system at desired intervals, monitoring the measured and logged physiologic status of the autonomic nervous system for any changes and correlating the changes to a corresponding drug administration time. The device includes an implanted baroreflex activation device capable of administering one or more hypertension treatment drugs including a controller that activates and adjusts therapy delivery, a baroreflex activation therapy delivery device, a drug therapy delivery device and a device that senses physiologic parameters.
Owner:CVRX
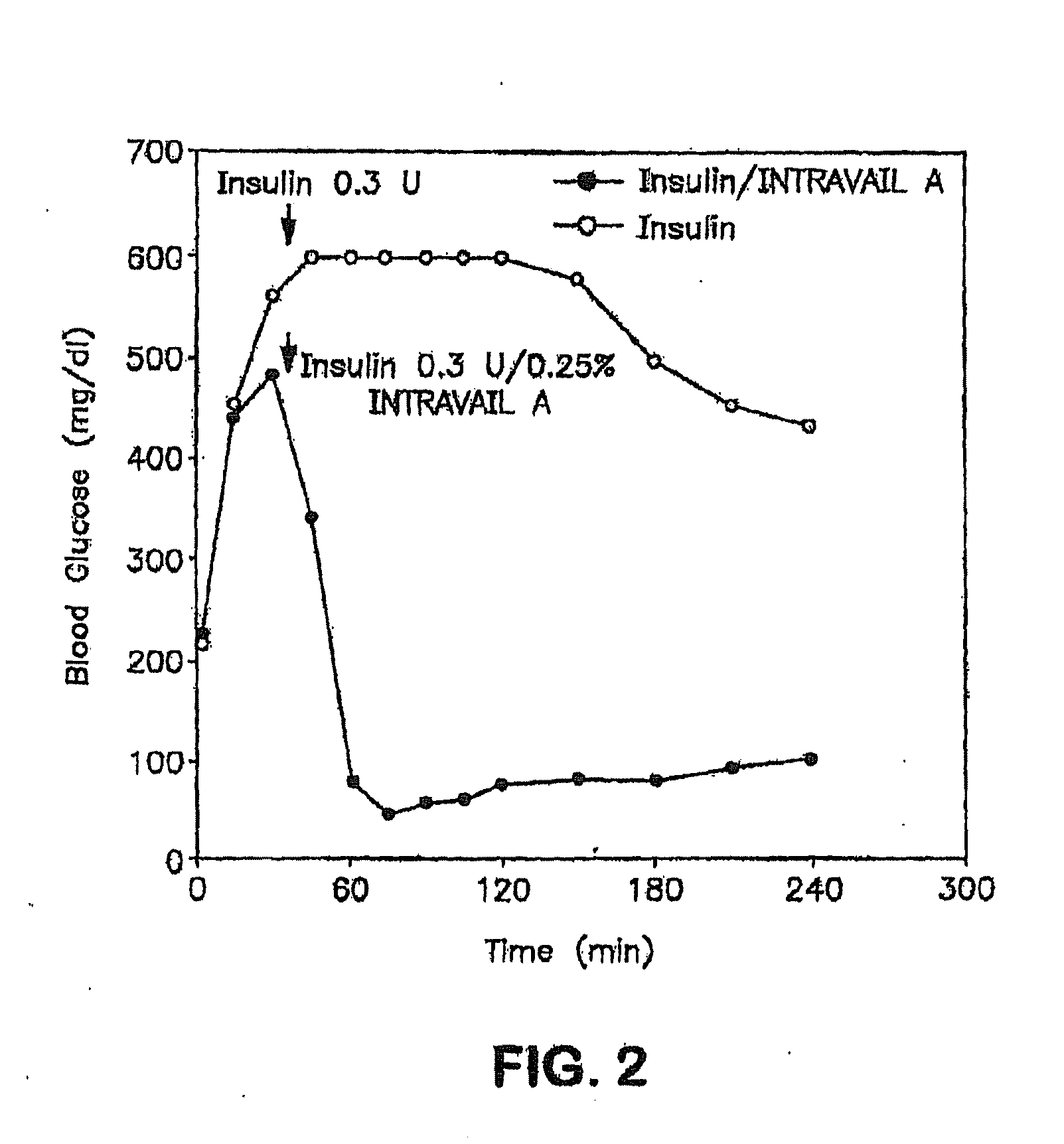
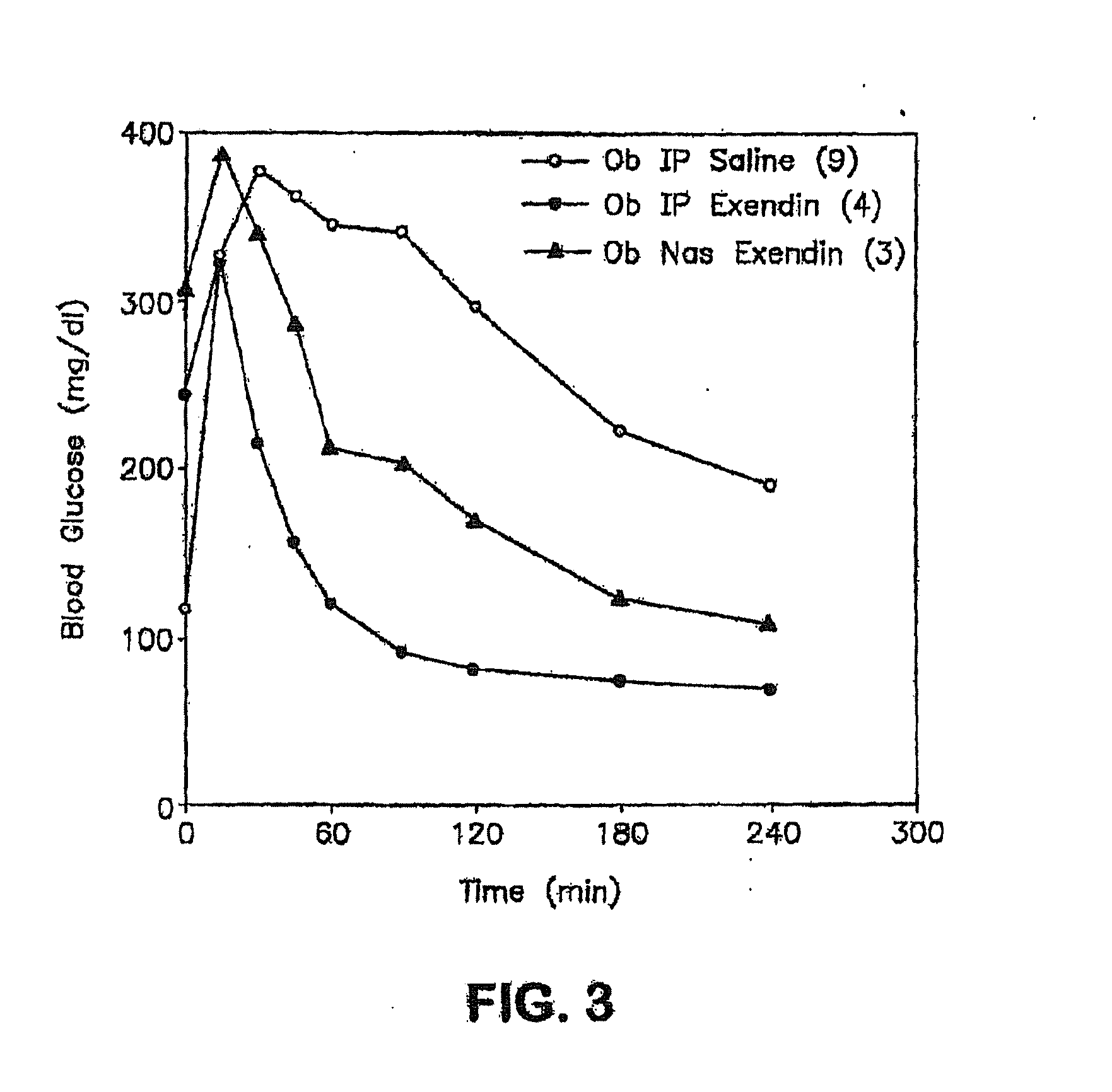
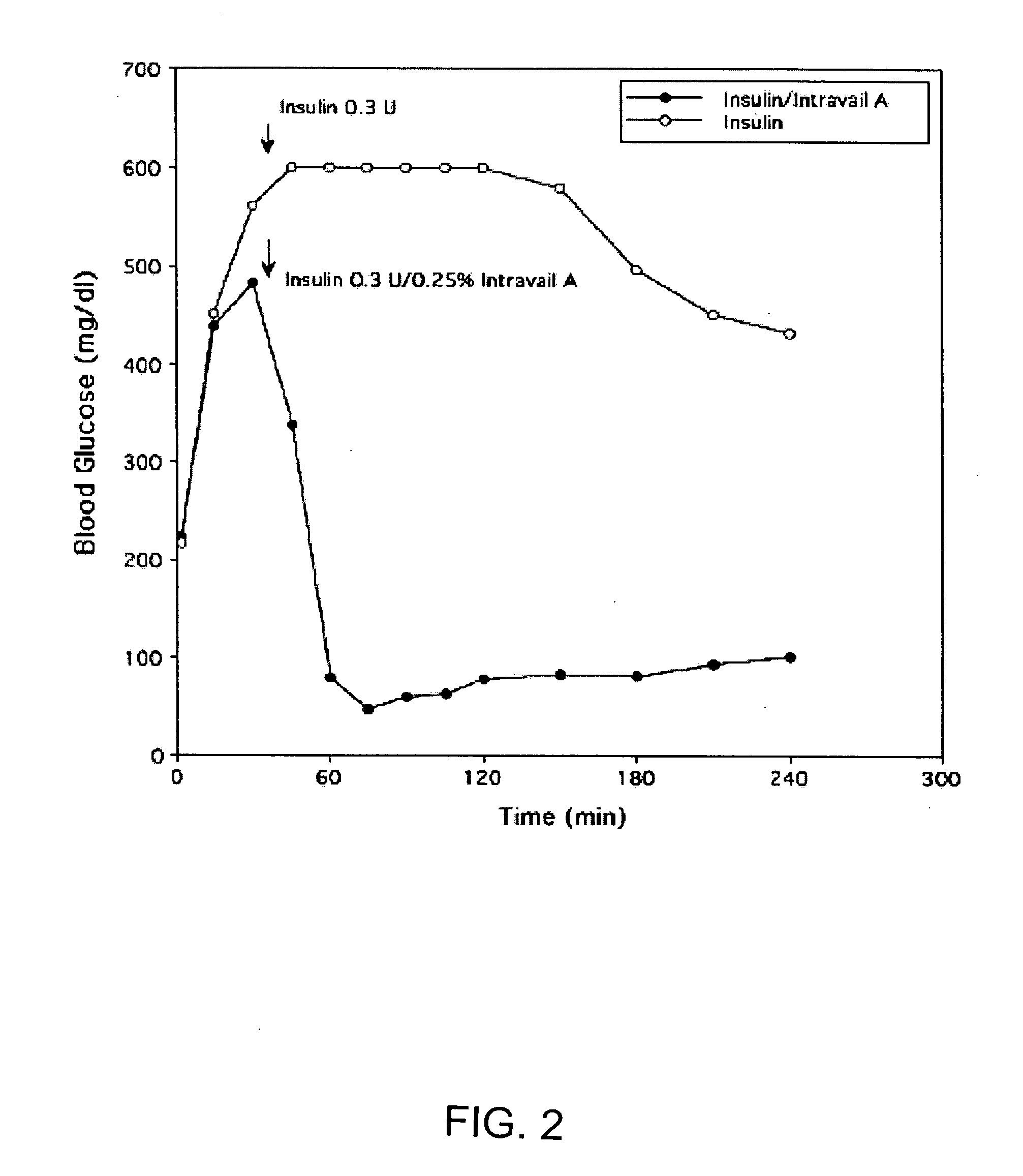
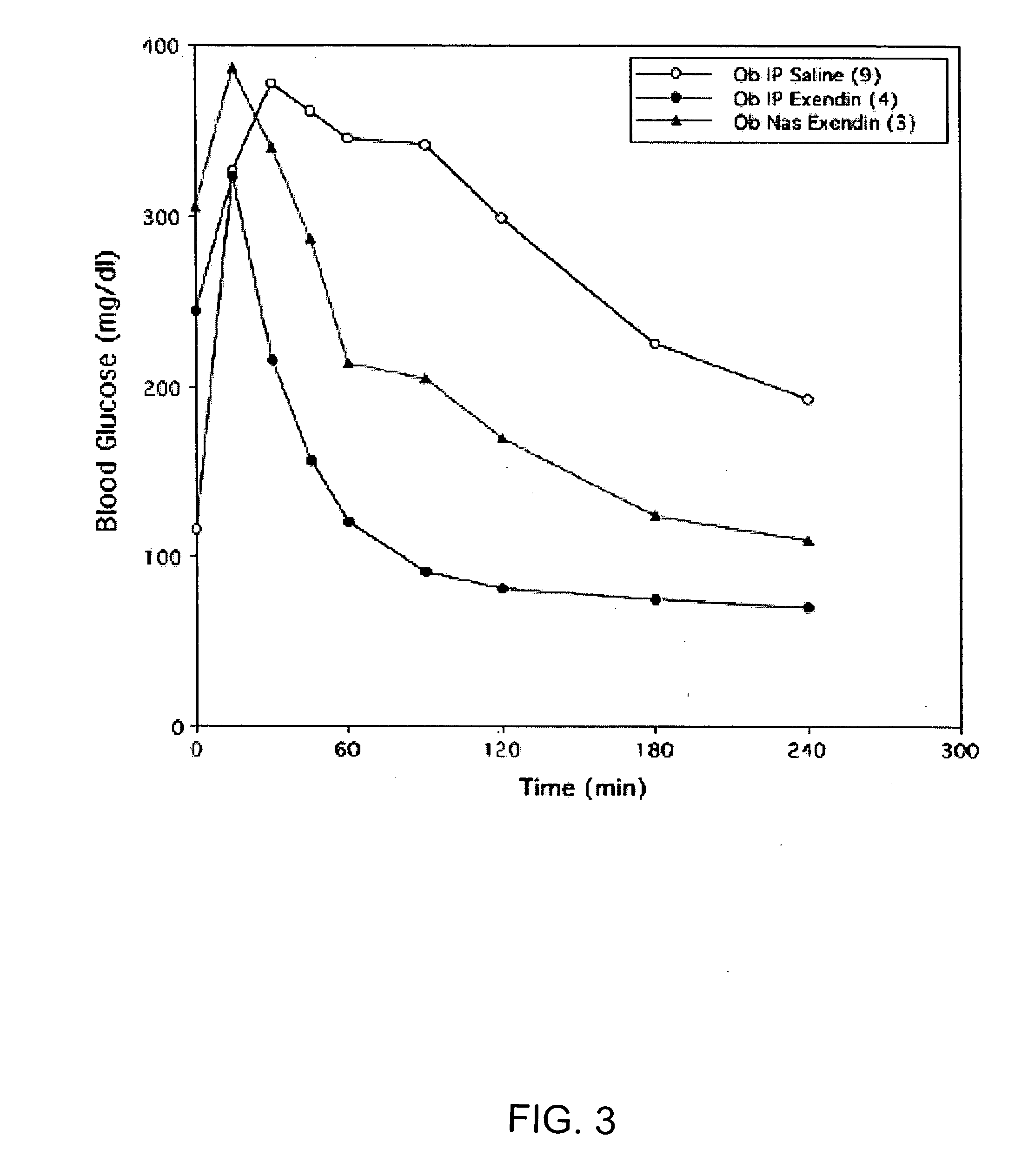
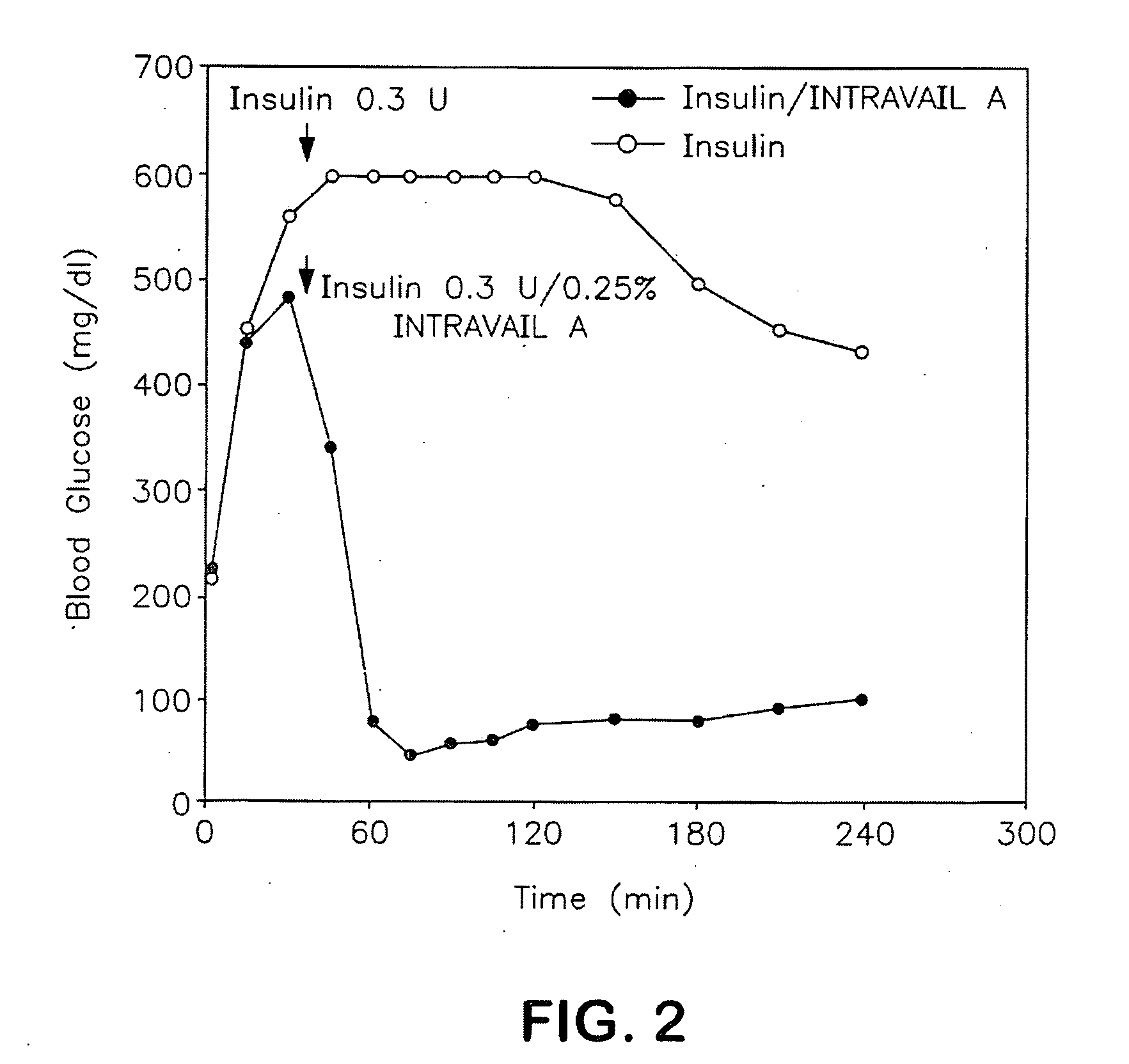
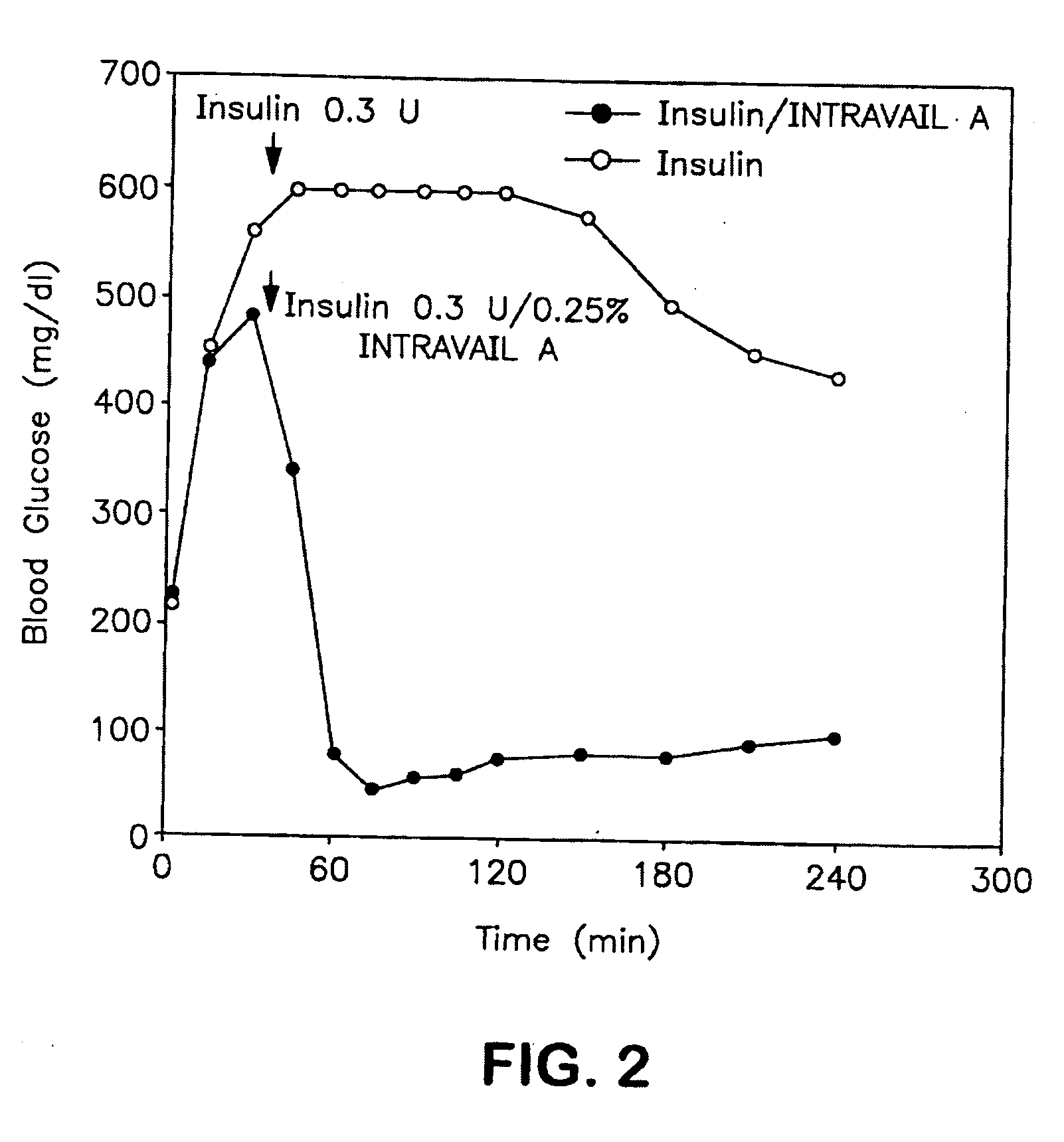
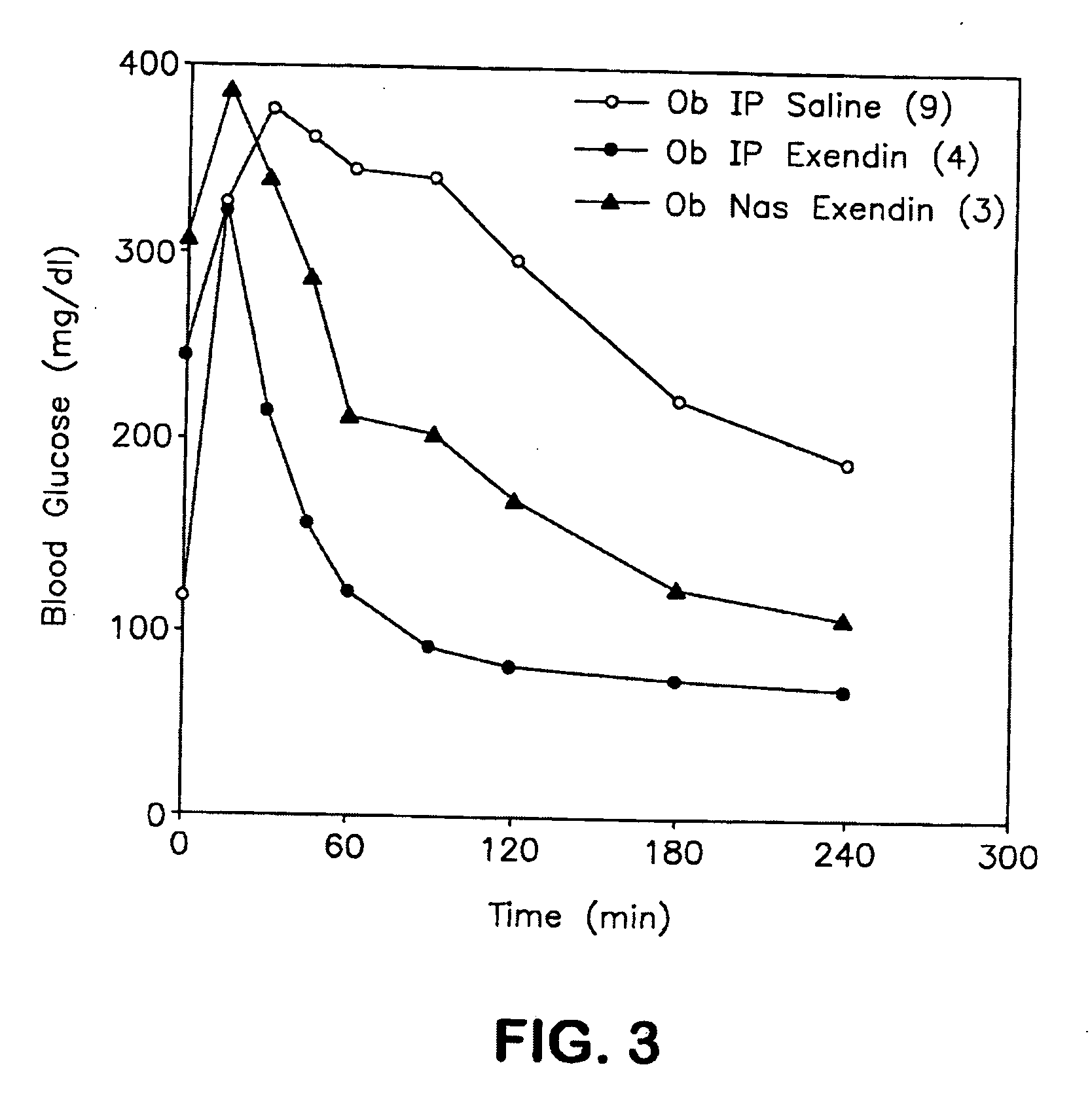
Absorption Enhancers for Drug Administration
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
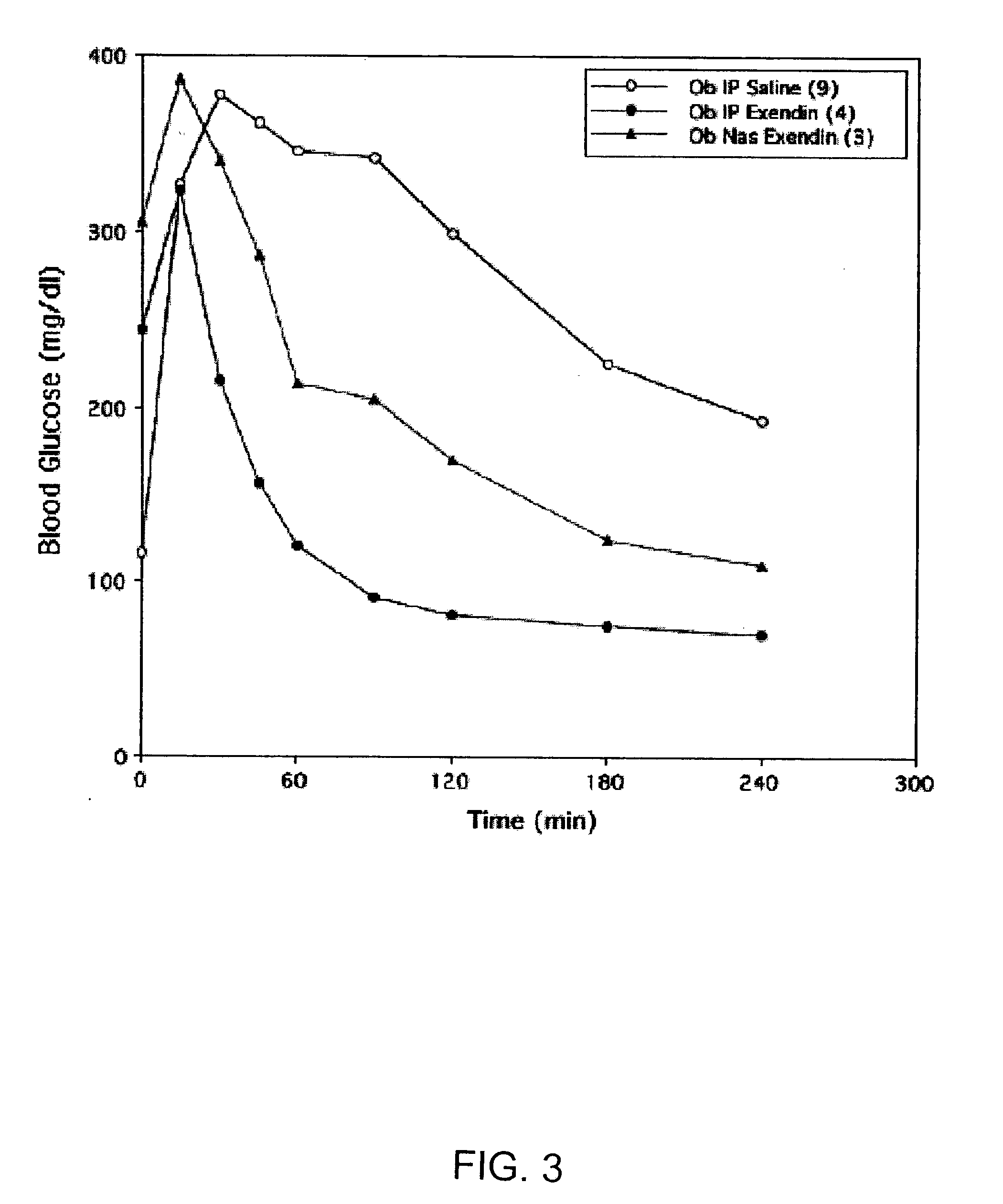
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Active Drug Delivery in the Gastrointestinal Tract
InactiveUS20080063703A1Promote absorptionEasy accessInternal electrodesMedical devicesMedicineDrug administration
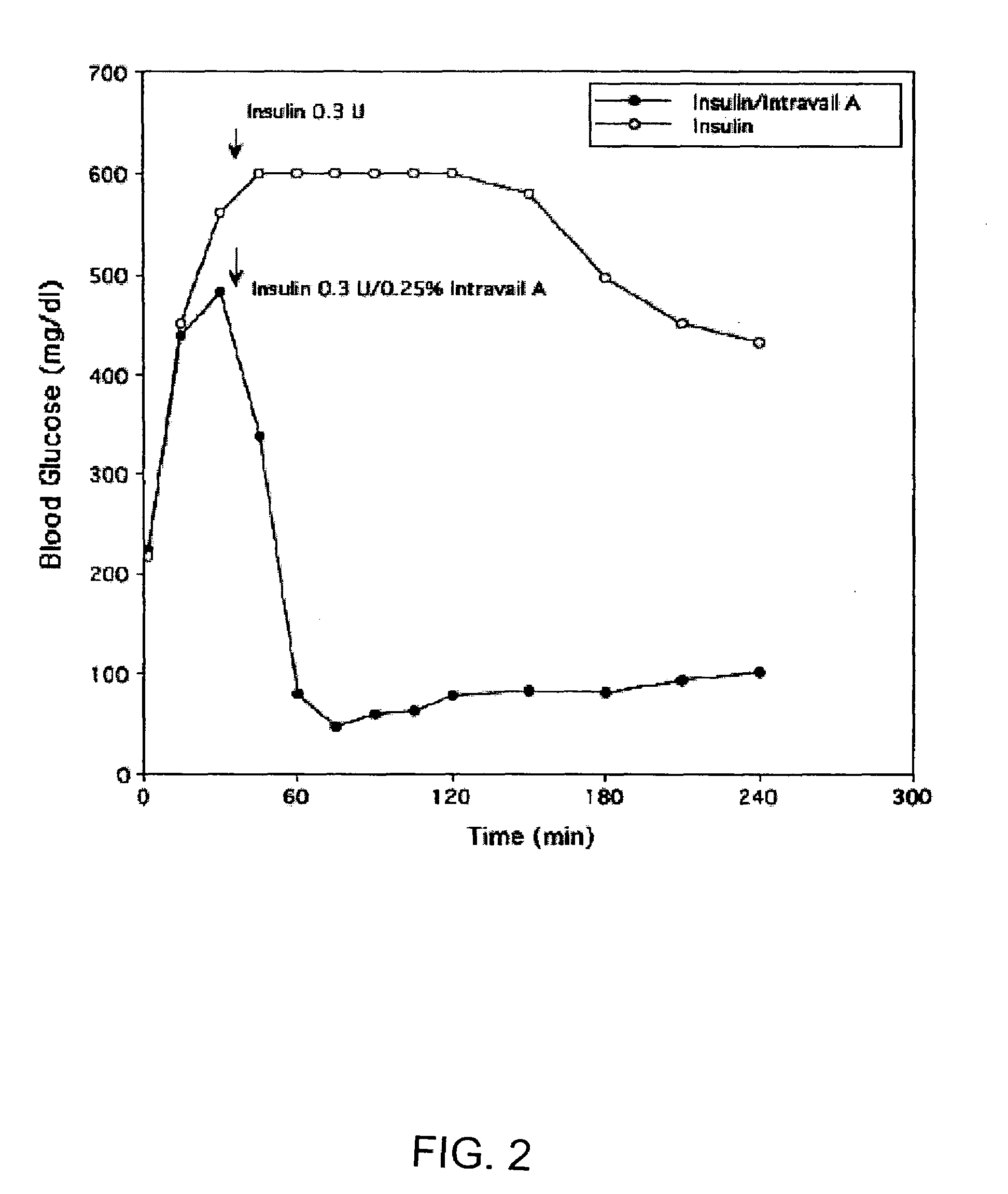
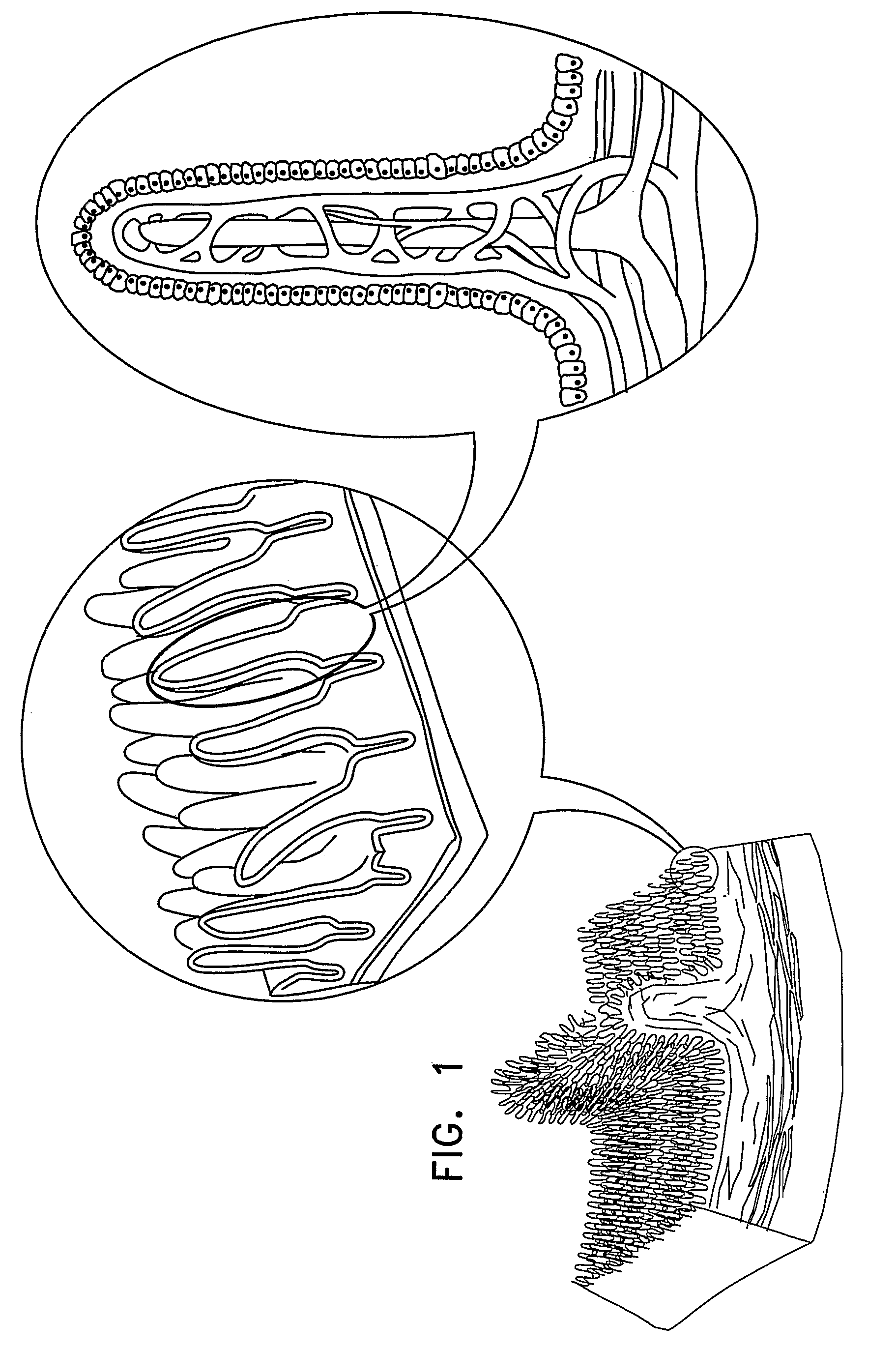
Apparatus (30) for drug administration is provided, including an ingestible capsule (32), which includes a drug (36), stored by the capsule (32), and an environmentally-sensitive mechanism (18), adapted to change a state thereof responsively to a disposition of the capsule (32) within a gastrointestinal (GI) tract (50) of a subject. The capsule (32) further includes first and second electrodes (16), and a control component (14), adapted to facilitate passage of the drug (36), in response to a change of state of the environmentally-sensitive mechanism (18), through an epithelial layer of the GI tract (50) by driving the first and second electrodes (16) to apply a series of pulses at a current of less than about 10 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds. Other embodiments are also described.
Owner:E PILL PHARMA
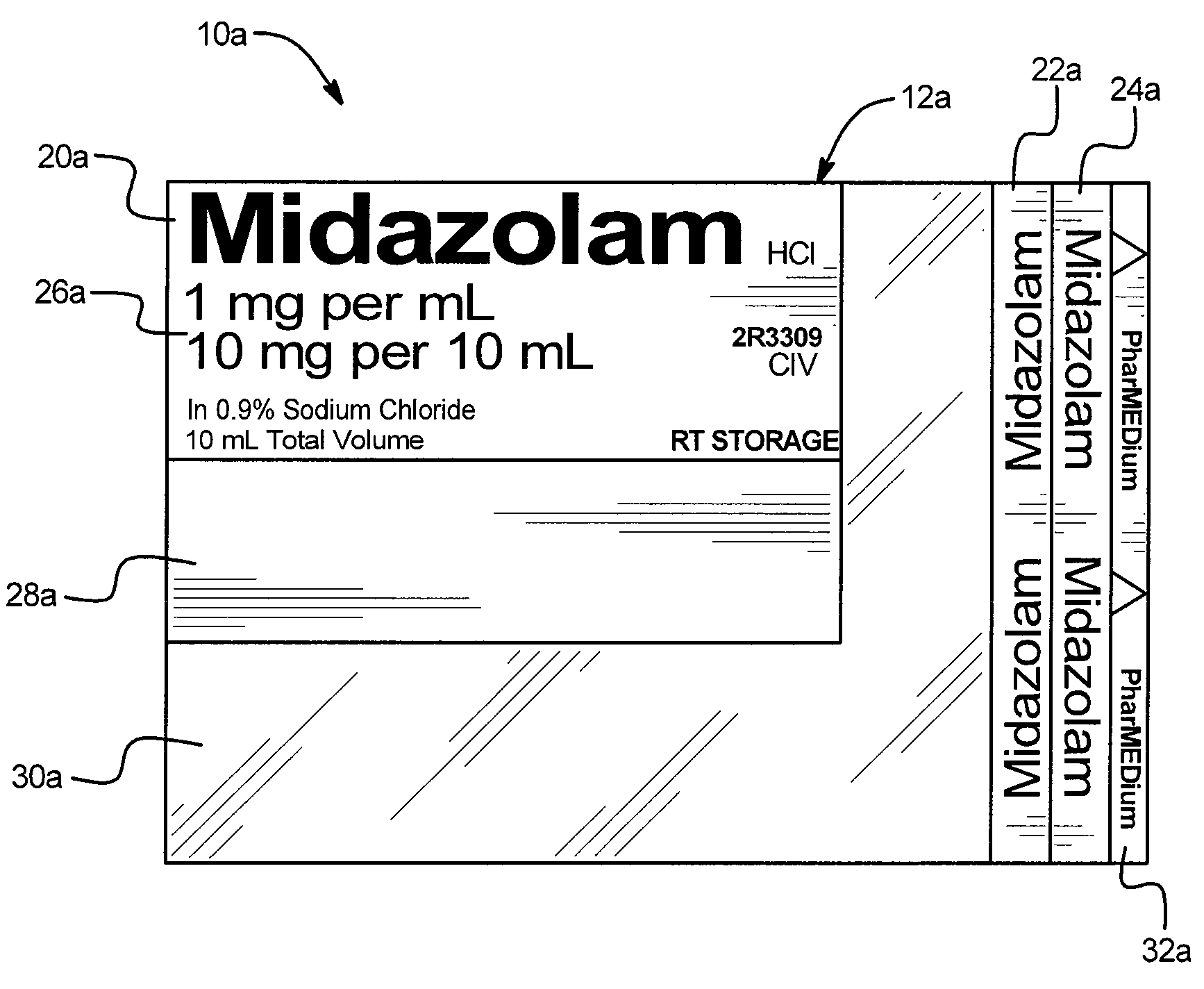
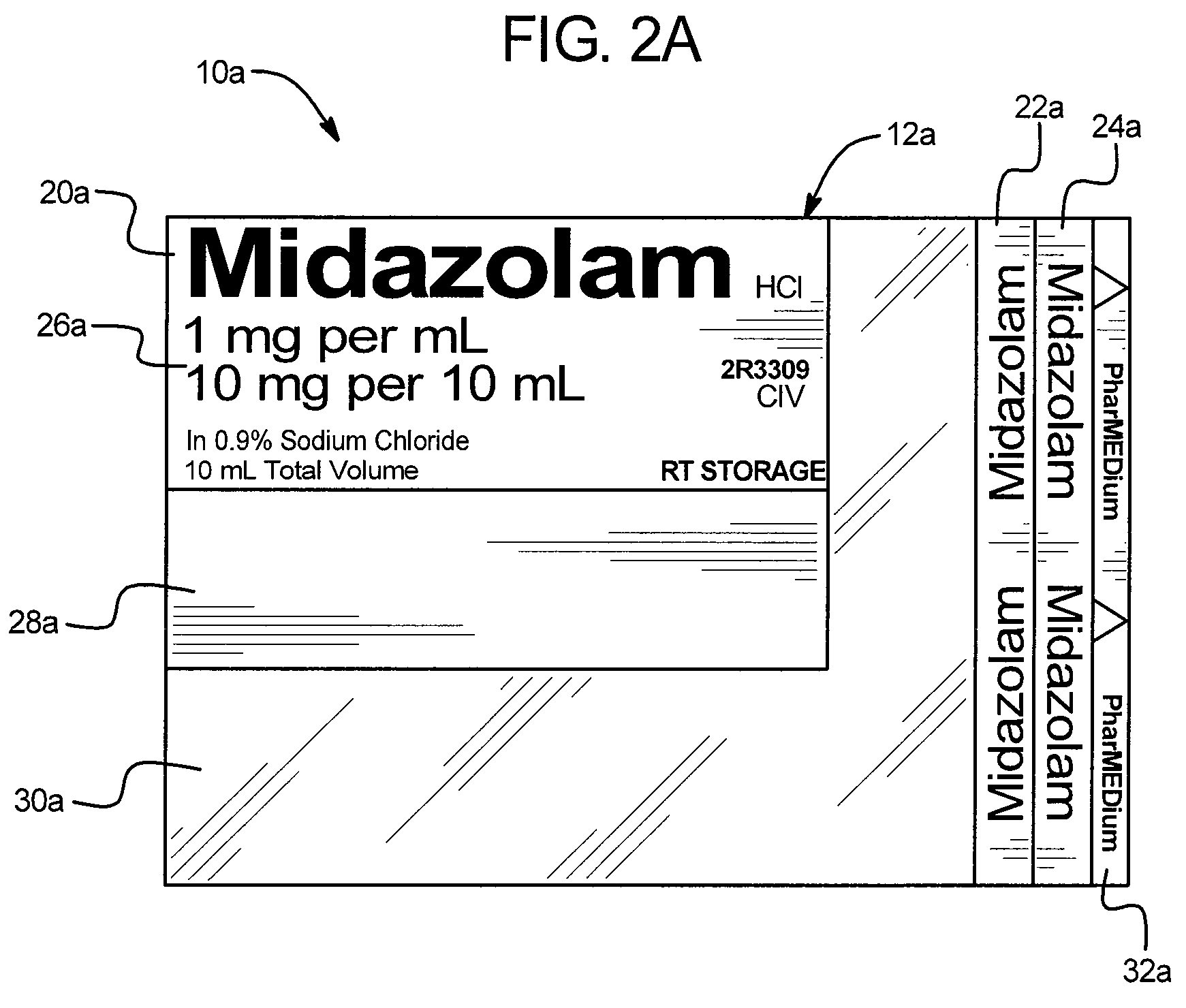
Safety device for drug delivery devices and containers
A drug administration safety device having a label configured to be attached to a drug container such as a syringe or IV bag, an adhesive on the back face of the label, and a backing or substrate for holding the label and protecting the adhesive prior to the application of the label to the drug container. In one embodiment, the label includes a first drug name section in a first orientation, a second drug name section in a second orientation, a third drug name section in a third orientation, a drug concentration section, a variable information section, and a gradiation viewing section. The first orientation, second orientation, and third orientation are different from each other to enable a user to readily see the drug name regardless of the position and orientation of the drug container.
Owner:HIKMA PHARMA USA INC
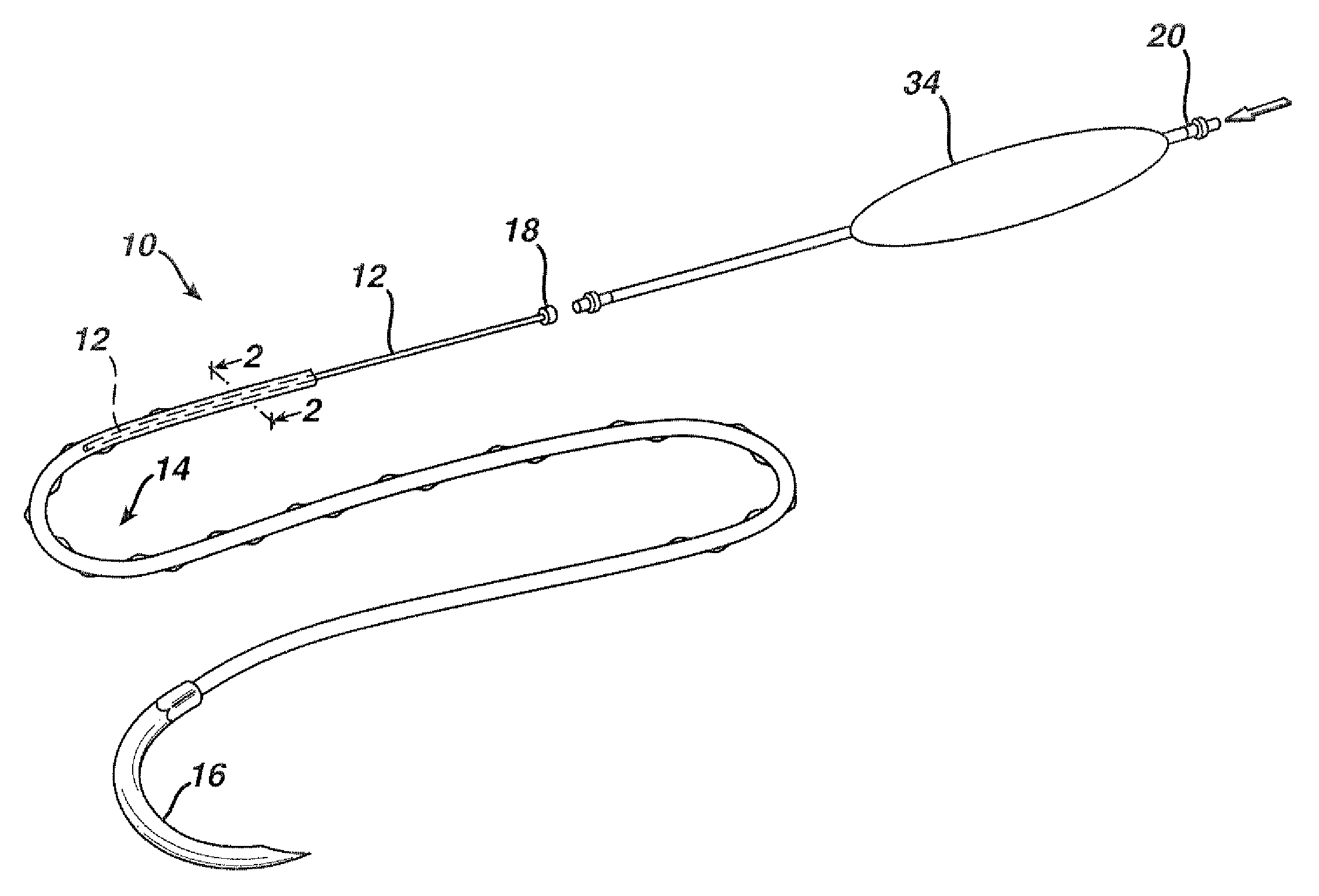
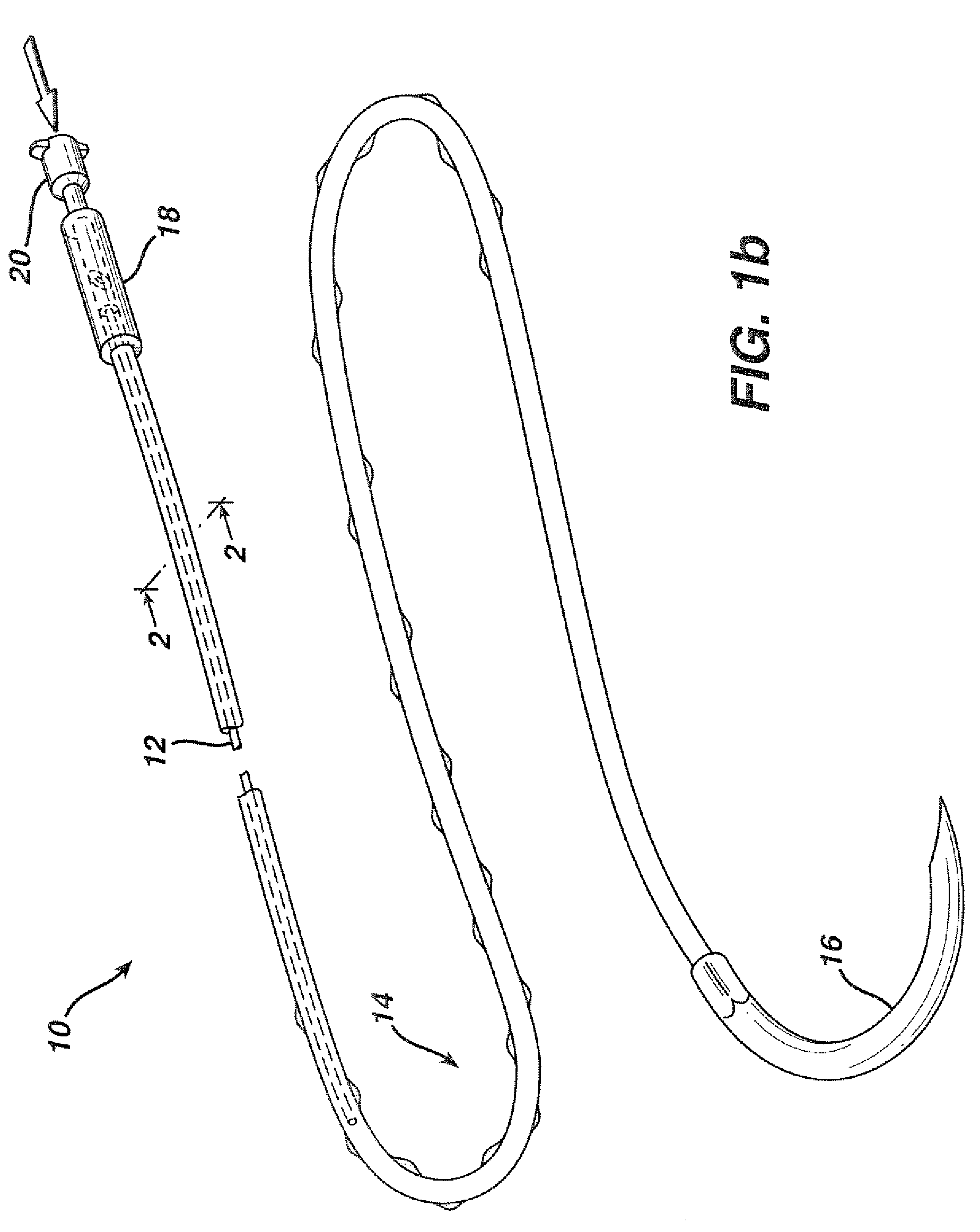
Active suture for the delivery of therapeutic fluids
An active suture that can be used for both wound closure and the delivery of therapeutic fluids to the tissue surrounding a wound is disclosed. The active suture may include a connector designed to join a fluid source, such as a syringe or conventional IV delivery system, to an internal passageway that is embedded within a braided suture. The internal passageway may be comprised of a fine polymeric tube and is capable of conducting and emitting a fluid into at least a portion of the braided suture and surrounding tissue. The invention enables delivery of an efficacious volume of drug bearing solution on the order of milliliters per day, provides a high level of fluid delivery rate control enabling the physician to start or stop drug administration at his / her discretion, and offers a means of providing more than one type of medication that may be selected post-surgically in accord with unexpected patient symptoms that may arise.
Owner:ETHICON INC
Drug delivery and monitoring system
InactiveUS20060144942A1Easy to fillDrug and medicationsMedical devicesInjection portMonitoring system
A drug administration system includes a cradle attached about an intravenous injection port having a flange extending therefrom. The cradle supports first drug administration information in the nature of machine and human readable code, for example, barcode. A syringe including a needle includes a flange extending from the syringe. The syringe supports second drug administration information in machine and / or human readable form. A scanner module is constructed to slidably receive the flange of the cradle and syringe whereby the syringe needle is aligned with the intravenous injection port. The module may be provided with an electronic scanning system for identifying the first and second drug administration information, as well as determining the amount of the drug being administered from the syringe to the injection port by monitoring movement of the syringe plunger. The information and data may be stored within the module for uploading to a remote location.
Owner:IBM CORP
Micelle composition of polymer and passenger drug
InactiveUS20060251710A1Improving micelle encapsulation efficiencyBiocidePowder deliverySolubilitySide effect
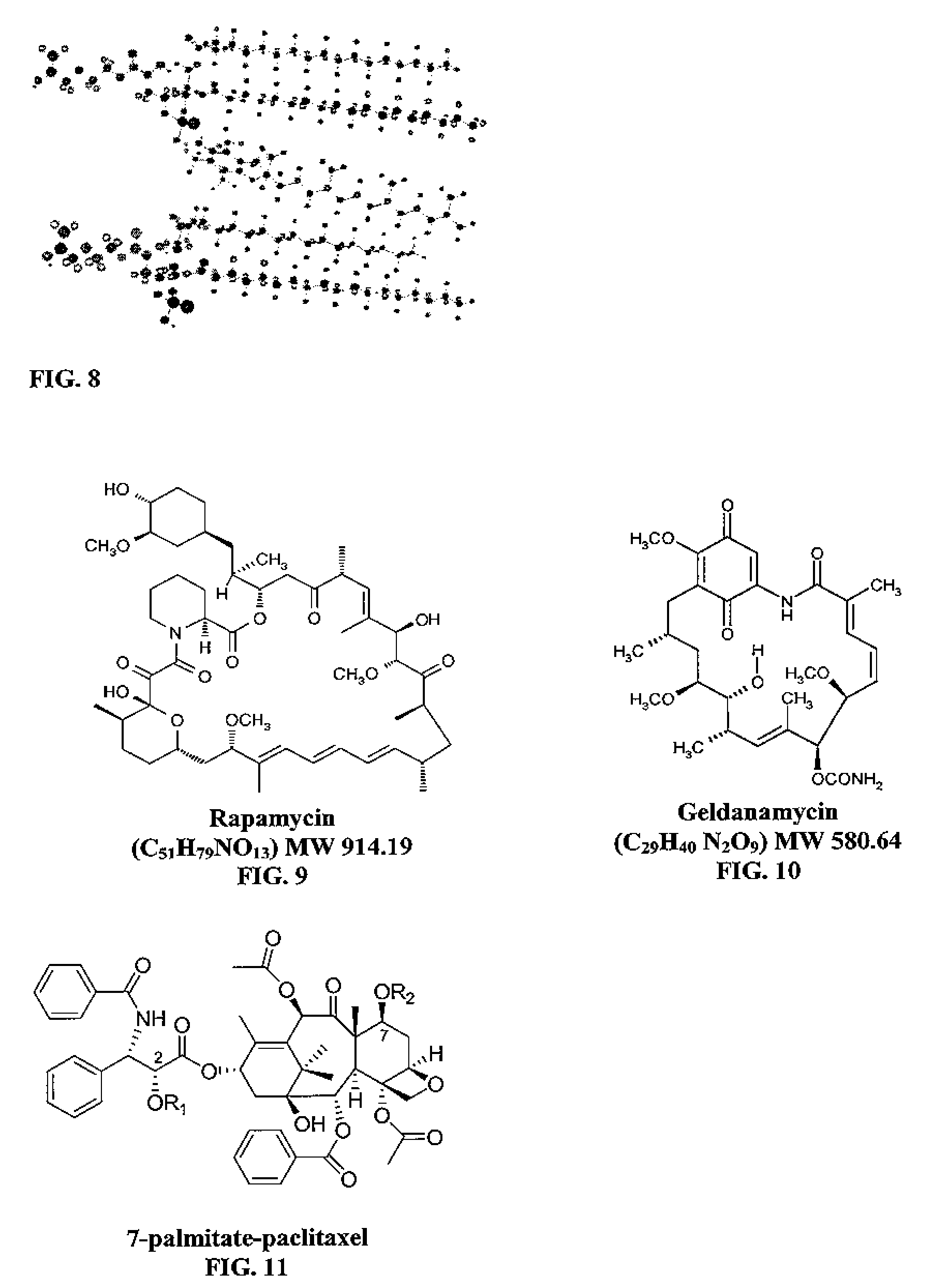
Hydrophobic drugs become more practical for treatments by being encapsulated in micelle compositions for increasing solubility. Micelle compositions may include an excipient tocopherol and / or prodrug formulations of the drug. Micelles extend the time period the drug remains in the micelles to improve drug circulation time and thereby drug delivery. Hydrophobic drugs for micelle encapsulation may include rapamycin, geldanamycin, and paclitaxel. Administration of these micelle compositions does not require Cremophor EL or Tween 80, avoiding serious side effects associated with these products which would previously accompany such drug administration.
Owner:WISCONSIN ALUMNI RES FOUND
Absorption enhancers for drug administration
InactiveUS20060045868A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideInhalationDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Active suture for the delivery of therapeutic fluids
An active suture that can be used for the delivery of therapeutic fluids to the tissue surrounding a wound is disclosed. The active suture may include a connector designed to join a fluid source, such as a syringe, conventional IV delivery system, or infusion pump to an internal passageway that is embedded within a braided suture. The internal passageway may be comprised of a fine polymeric tube and is capable of conducting and emitting a fluid into at least a portion of the braided suture and surrounding tissue. The invention enables delivery of an efficacious volume of drug bearing solution on the order of milliliters per day, provides a high level of fluid delivery rate control enabling the physician to start or stop drug administration at his / her discretion, and offers a means of providing more than one type of medication that may be selected post-surgically in accord with unexpected patient symptoms that may arise.
Owner:ETHICON INC
Compositions for Drug Administration
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
Snap-over clamshell protective port cap
A single-use, clamshell protective cap for use primarily in healthcare settings in order to maintain the integrity of a medication solution. The cap has two halves, a cylindrical skirt, a puncture resistant lid portion, an interior flange, and an interlocking snap. When placed around a flanged injection port such as is found on a conventional fluid container and closed, the lid portion covers an access site on an injection port. After the cap is placed, the interior flange engages the port flange to prevent upward axial movement relative to the port, thereby preventing removal of the cap and thus deterring unwanted or erroneous drug administrations or withdrawals.
Owner:HOSPIRA INC
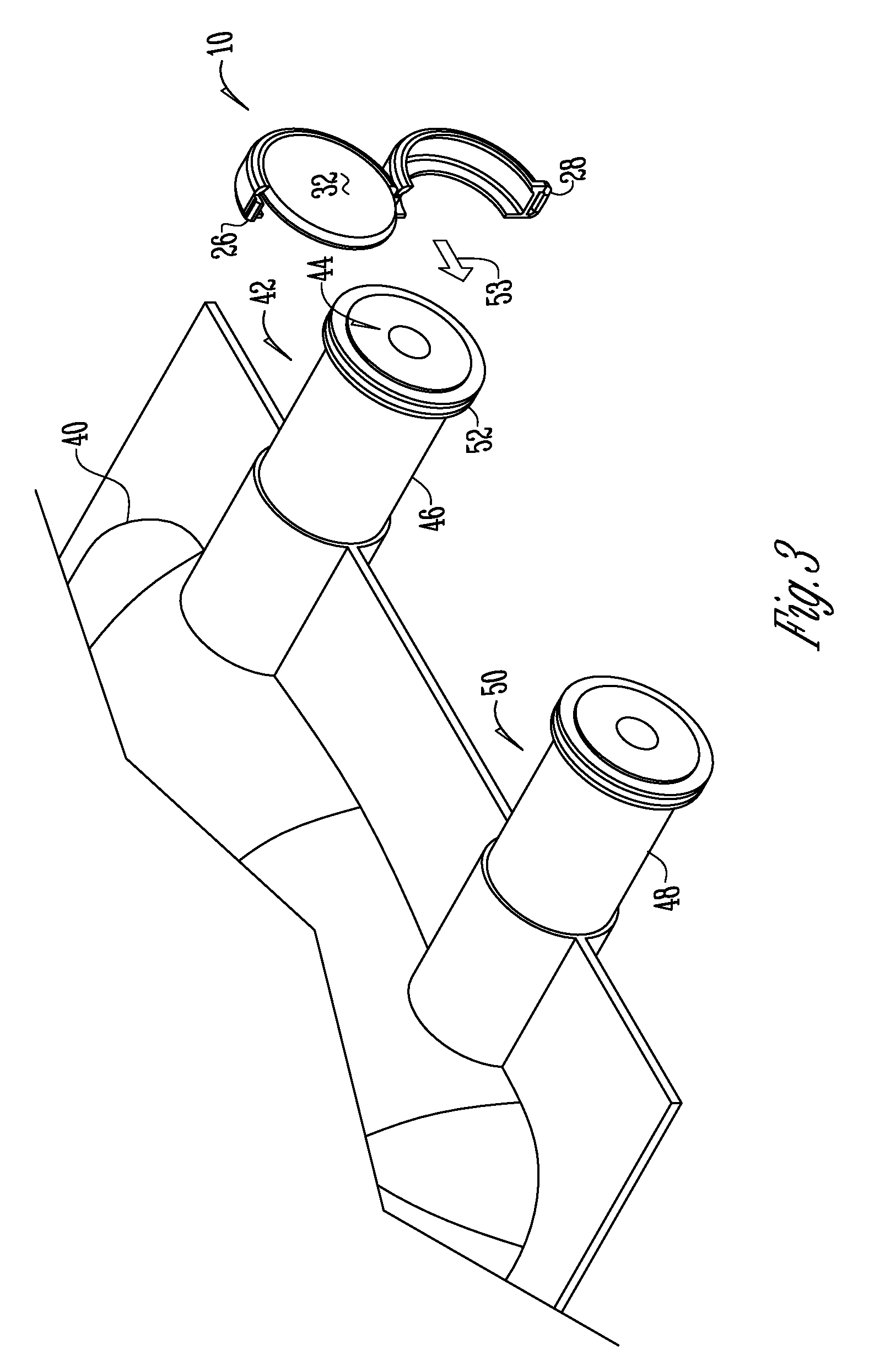
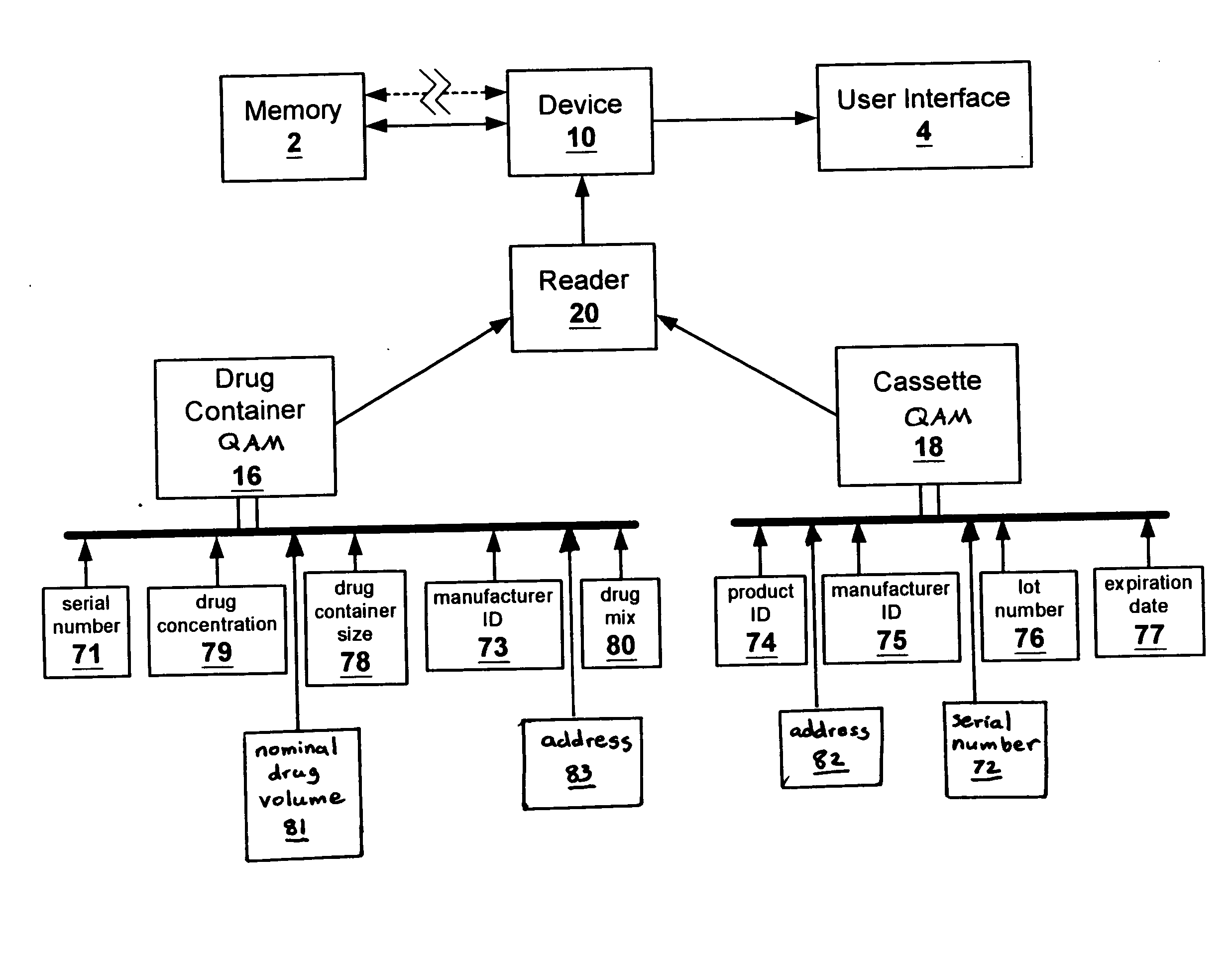
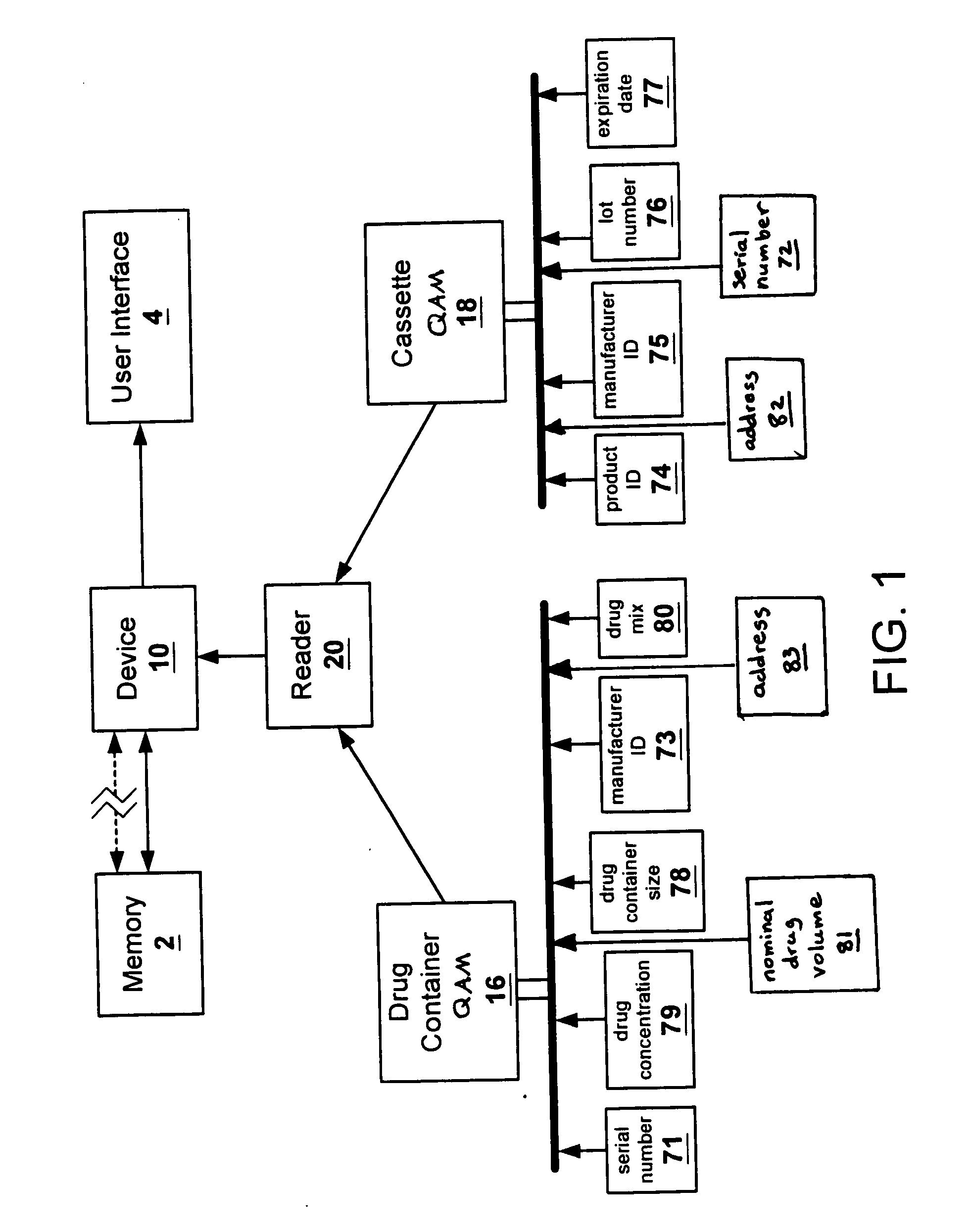
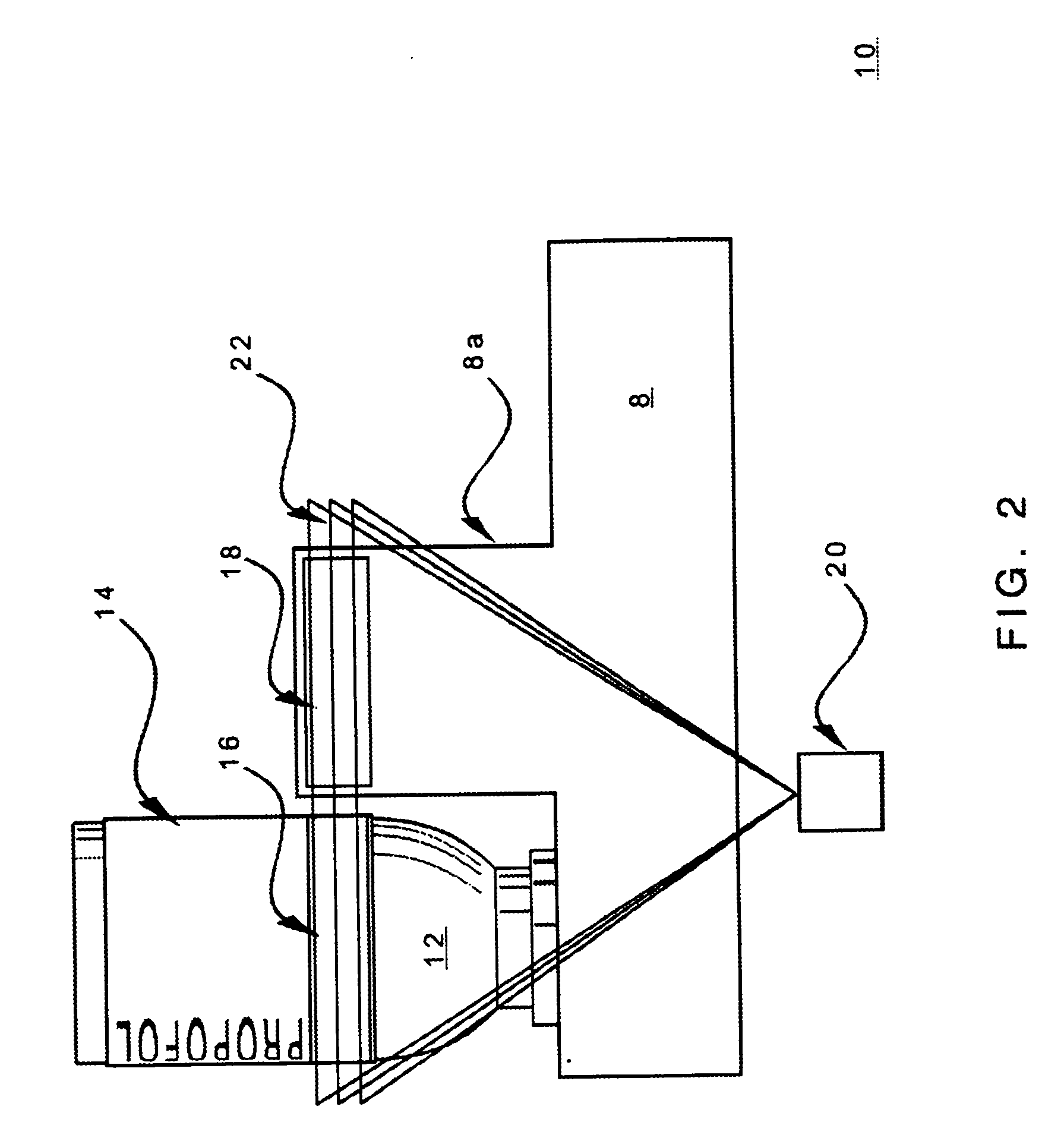
Methods and apparatuses for assuring quality and safety of drug administration and medical products and kits
InactiveUS20070213684A1Prevent unsafe reuseNervous disorderDrug and medicationsQuality assuranceMedical product
The present invention provides apparatuses and methods for marking components, supplies and kits of drug administration devices and other medical systems with quality assurance information. The invention also provides apparatuses and methods for tracking time of use of such components, supplies and kits and various apparatuses and methods for preventing use or reuse of tainted, recalled or unrecognized components, supplies and kits. Quality assurance markers (QAMs) are described which store information regarding the identity and manufacturer of disposable components, supplies and kits. The invention utilizes several QAM modalities, such as, among others, 1-D and 2-D bar codes, 1-D and 2-D symbologies, holograms, written text, radio frequency identification devices (RFIDs), integrated chip smart cards, and EEPROMs.
Owner:SCOTT LAB
NSAID formulations, based on highly adaptable aggregates, for improved transport through barriers and topical drug delivery
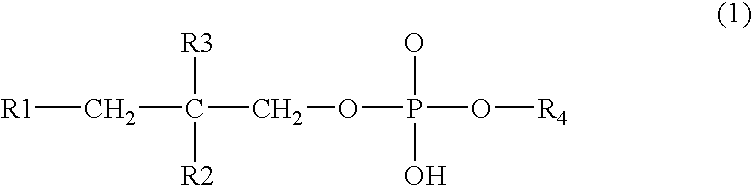
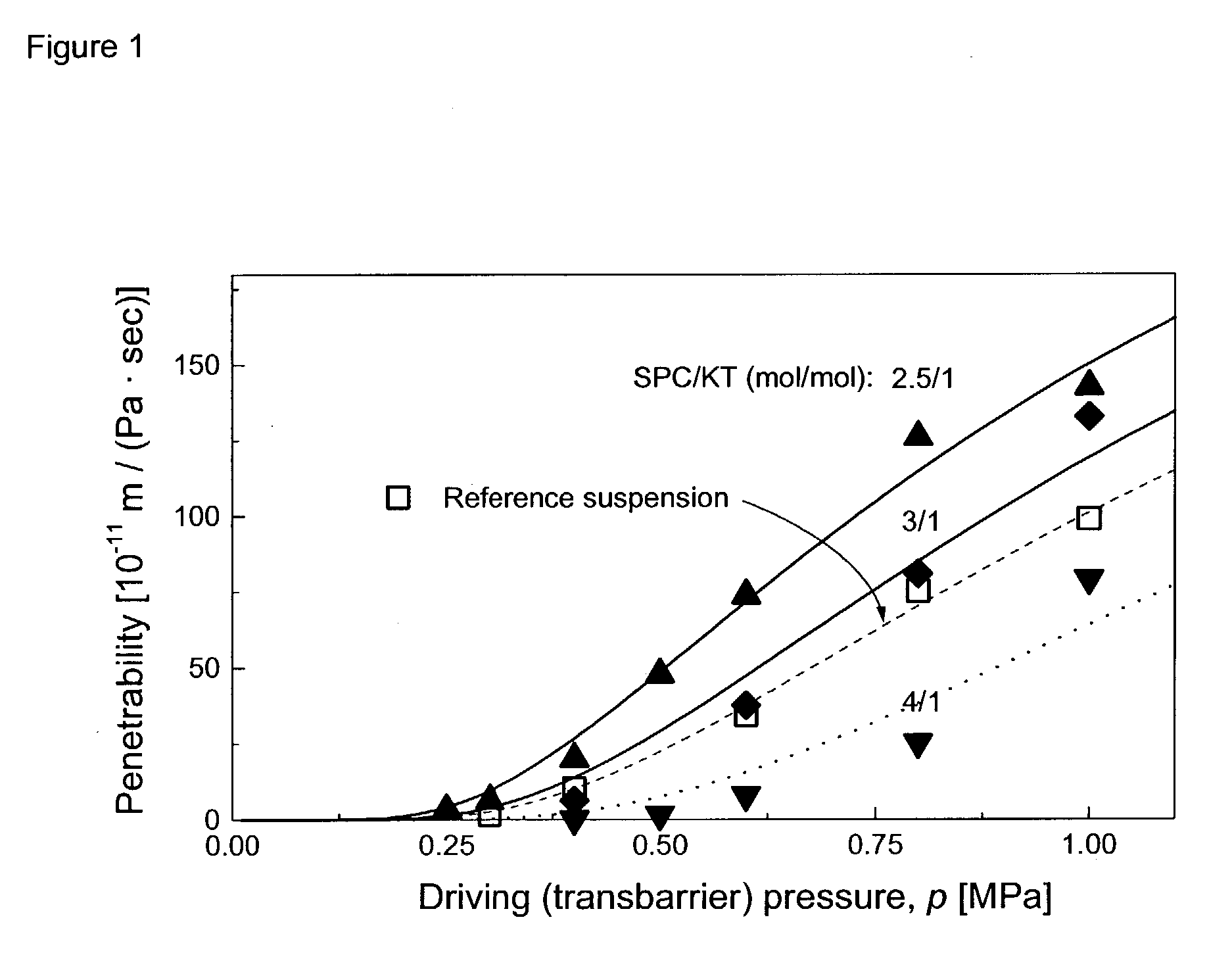
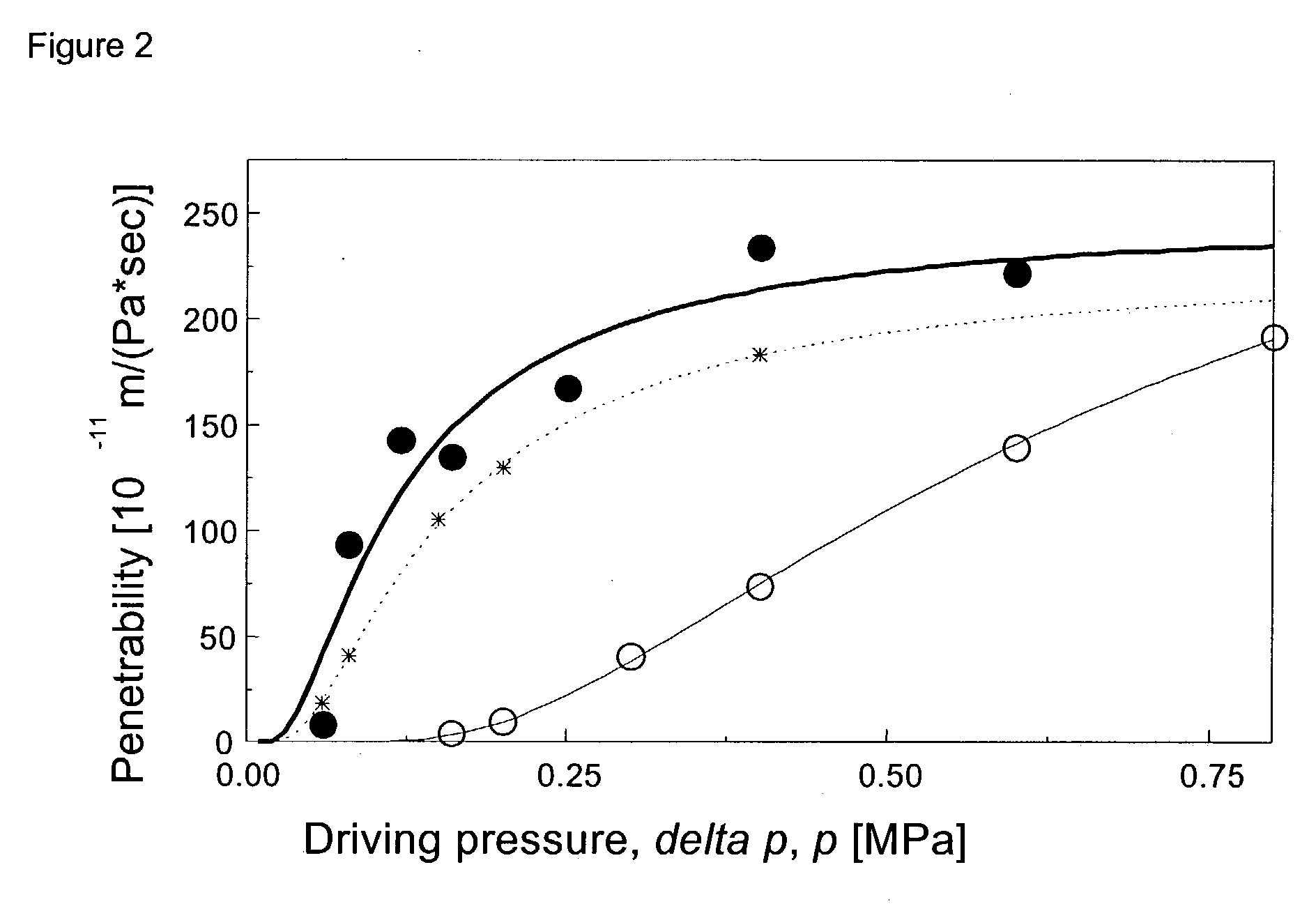
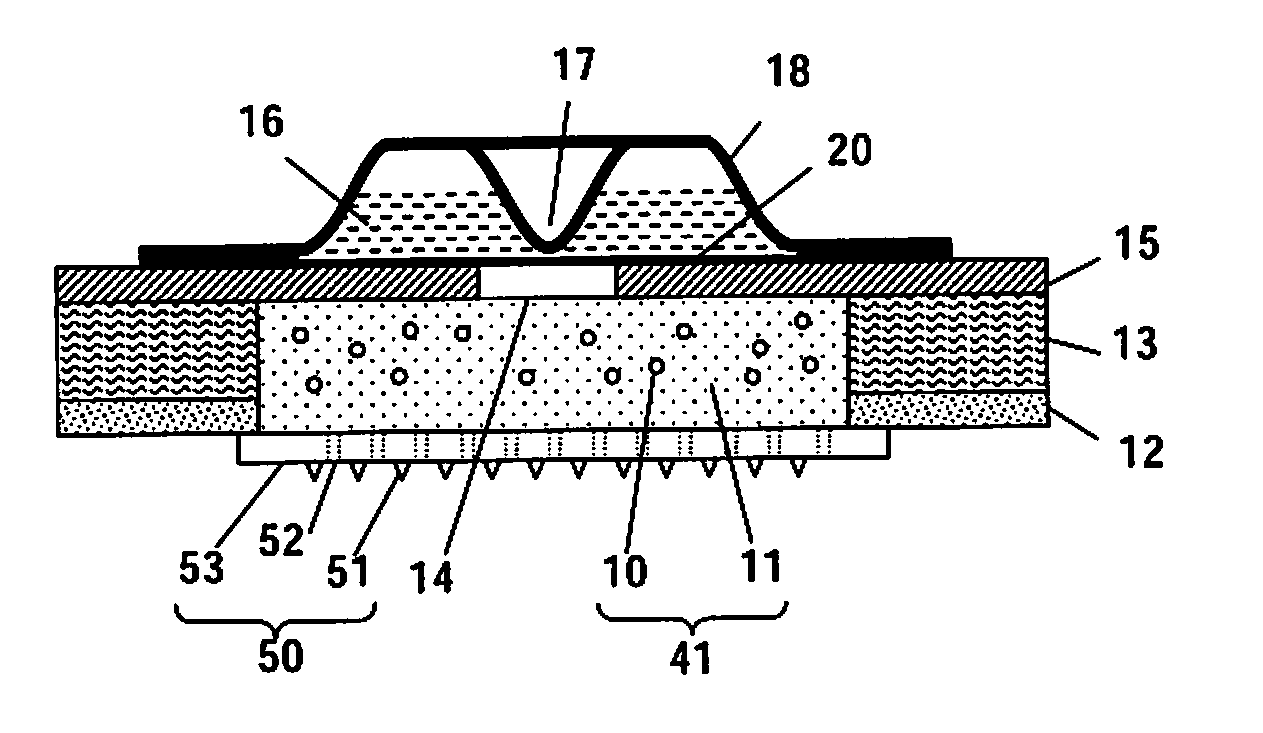
The invention describes novel formulations of nonsteroidal anti-inflammatory drugs (NSAIDs) based on complex aggregates with at least three amphipatic components suspended in a suitable, e.g. pharmaceutically acceptable, polar liquid medium. A suitably ionised NSAID is one of the two, amongst said three, components that tends to destabilise lipid membranes, the other system component with such activity being typically a surfactant. In contrast, the remaining amongst said at least three amphipatic components typically forms a stable lipid membrane on it's own. An essential characteristics of the resulting, relatively large, aggregates is an improved ability to penetrate pores, in a semi-permeable barrier, at least 30%, and often much smaller than the average diameter of the complex aggregate. This enables said aggregates to mediate NSAID transport through semi-permeable barriers including mammalian skin. As a result of the skin penetration by NSAID loaded large aggregates, the drug delivered transcutaneously with such carriers gets deeper into the tissue than the corresponding NSAID from a solution on the skin surface. This is believed to be due to the special ability of suitable large carriers to bypass the local sink of blood capillaries at the epidermal-dermal junction in the skin. The carrier-mediated delivery of locally applied NSAIDs thus allows therapy of deep tissues under the drug administration site, which is medically highly desirable.
Owner:IDEA AG
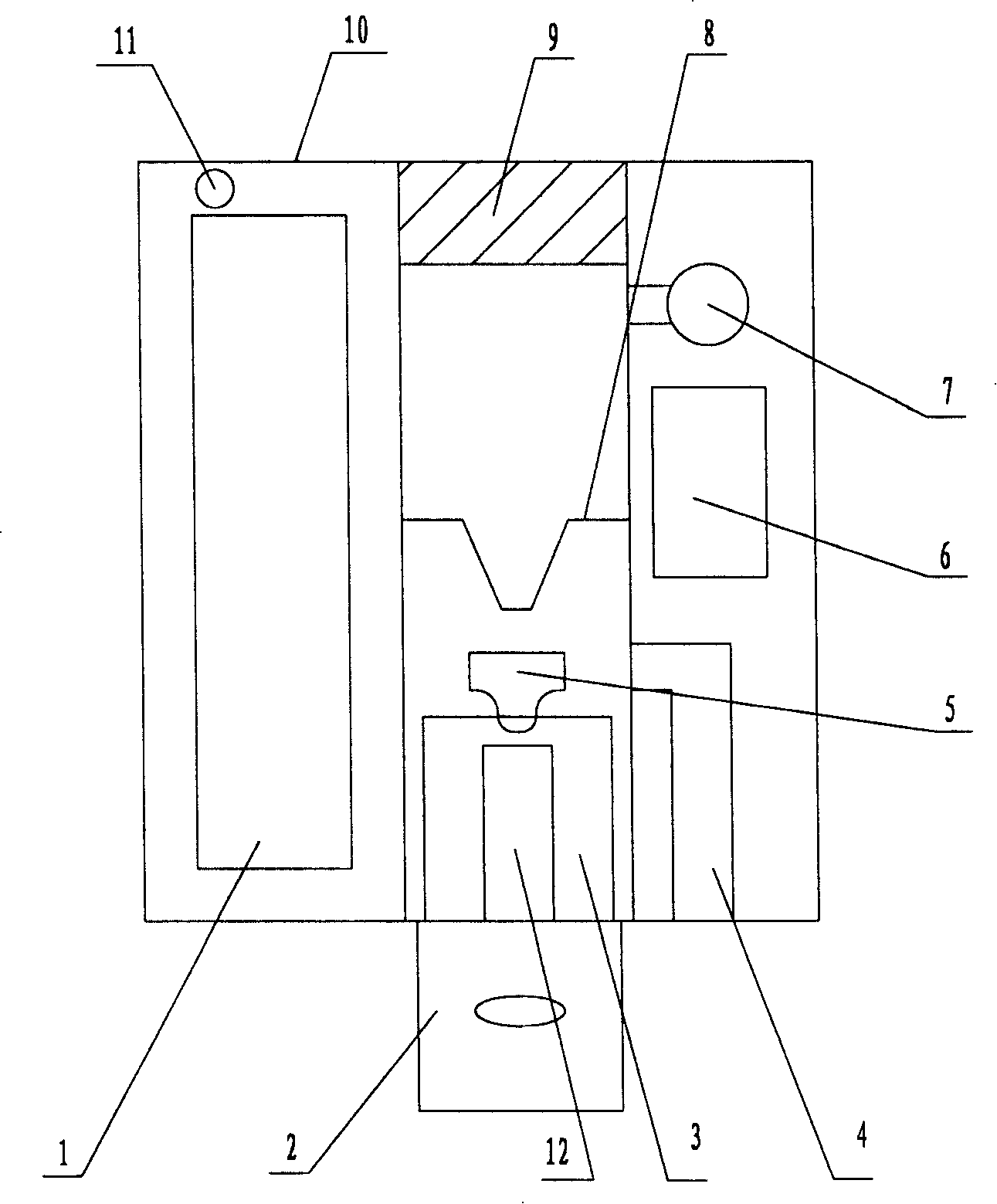
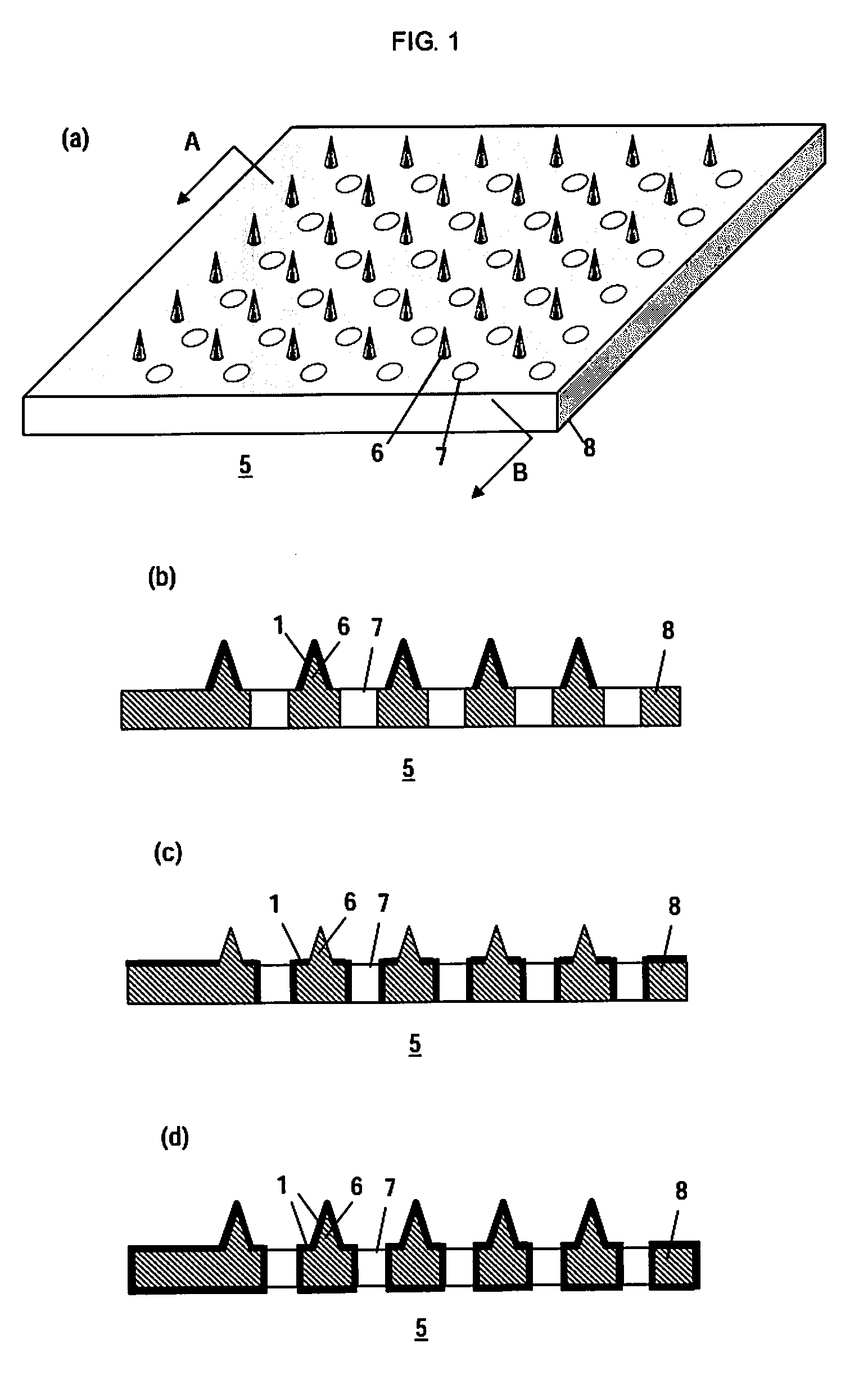
Microneedle Device And Transdermal Administration Device Provided With Microneedles
InactiveUS20090099502A1Good skin permeabilityImprove sustainabilityUltrasound therapyElectrotherapyPolyvinyl alcoholDrug administration
The present invention provides a microneedle device having a coating, which is effective even with a low molecular weight active compound and can sustain the effect of the drug for a long period of time, and a transdermal drug administration apparatus with microneedles. The microneedle device (5) has, on a microneedle substrate (8), a plurality of microneedles (6) that can pierce the skin, wherein the surface of the microneedles (6) and / or the microneedle substrate (8) is partly or entirely coated in fixed state with a coating carrier containing polyvinyl alcohol. The polyvinyl alcohol preferably has a hydrolysis degree of 94.5 mol % or more. Furthermore, the coating carrier can contain a drug.
Owner:HISAMITSU PHARM CO INC
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS20020004066A1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin and thereby improves dermal drug administration.
Owner:ZARS INC
Inhalation device and system for the remote monitoring of drug administration
The present invention is directed to a device for monitoring the usage of inhaled drugs by a patient. The device includes an inhaler, a use sensor, a microprocessor, a wireless transmitter and a battery compartment. These components allow information concerning drug usage to be transmitted to health care personnel that can evaluate the data to determine whether there are changes in drug usage characteristics that are indicative of an impending acute attack. The invention includes not only the device, but also the systems and methods in which the device is employed.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Compositions for Drug Administration
ActiveUS20090163447A1Promote absorptionImprove bioavailabilityBiocideNervous disorderMedicineChain length
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
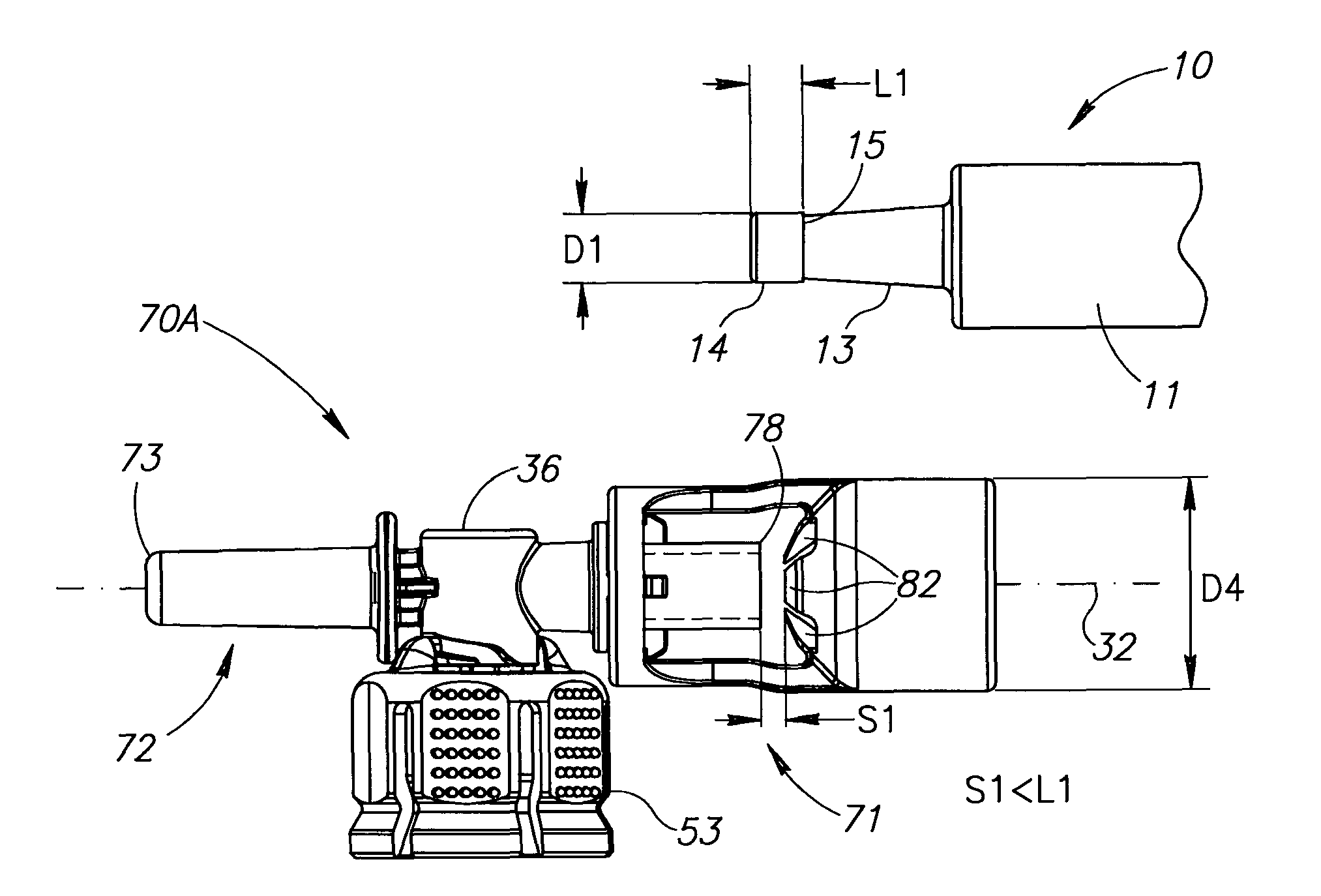
Liquid drug delivery devices for use with syringes with widened distal tips
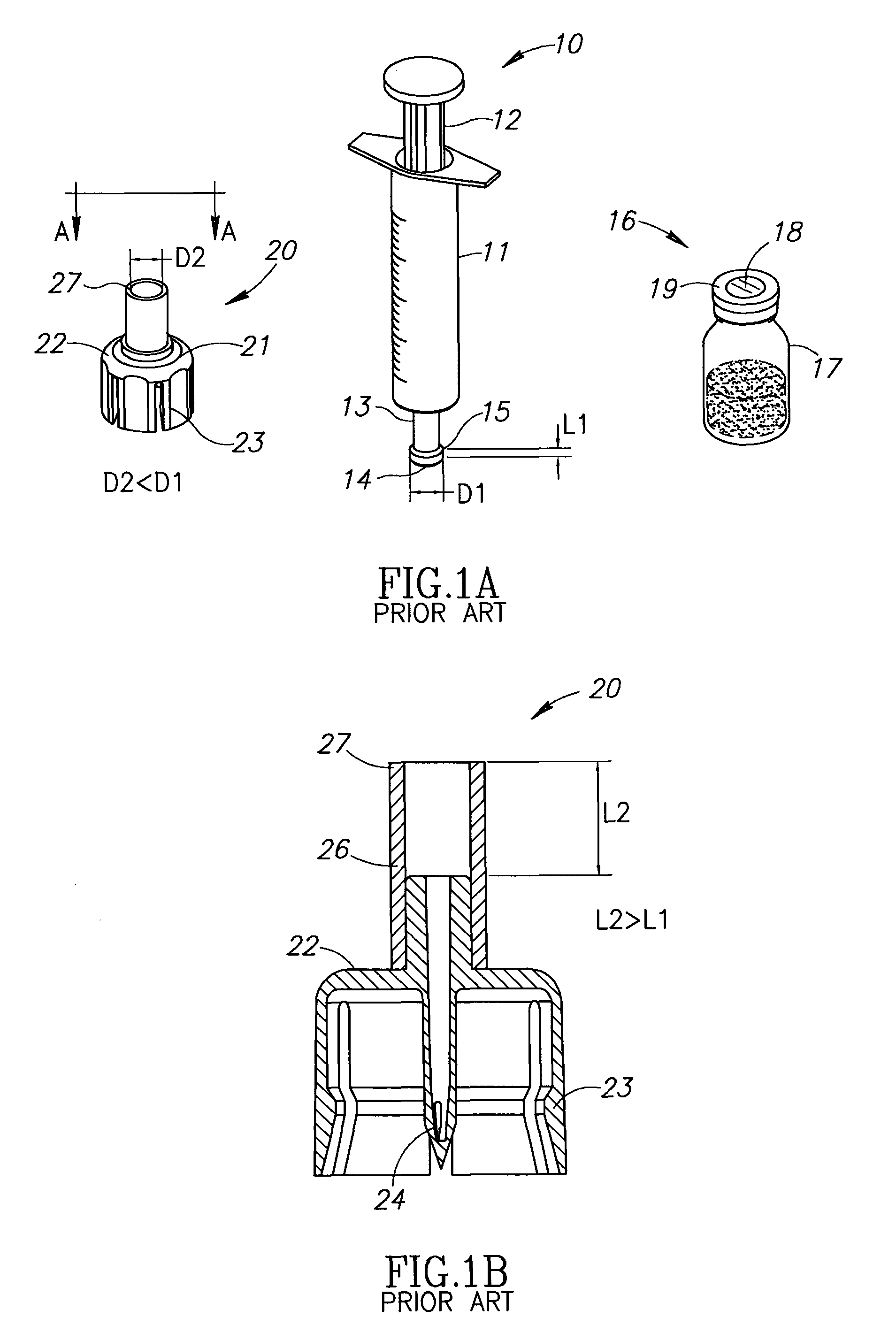
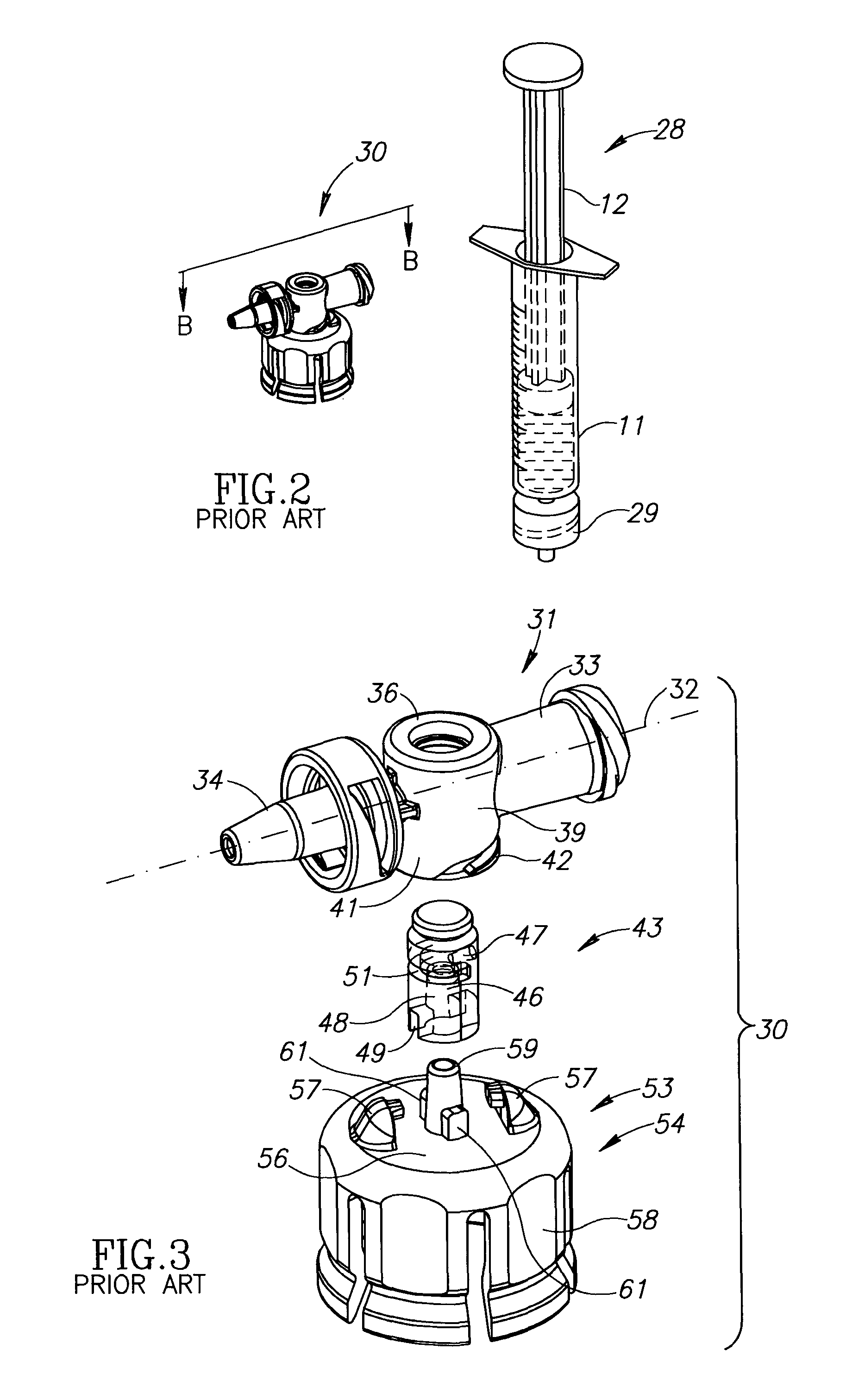
Liquid drug delivery device includes a housing with a syringe port for sealingly receiving a syringe having a syringe tip ending at a widened distal tip, a vial adapter port with a vial adapter for snap fit receiving a vial, and a drug administration port for administering a liquid drug. The vial adapter is intended to be rotationally detached after a mixing procedure for discarding together with a spent vial. The syringe port additionally includes a single use locking mechanism for securing the syringe to preclude inadvertent syringe detachment under normal use including agitation for reconstitution purposes. The drug administration port has a distal tip preferably similar to a syringe's widened distal tip for the same purpose of preventing conventional needles with a female Luer connector being slidingly mounted thereon.
Owner:WEST PHARM SERVICES IL LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com