Patents
Literature
433 results about "Drug metabolism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms, and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is called pharmacokinetics.
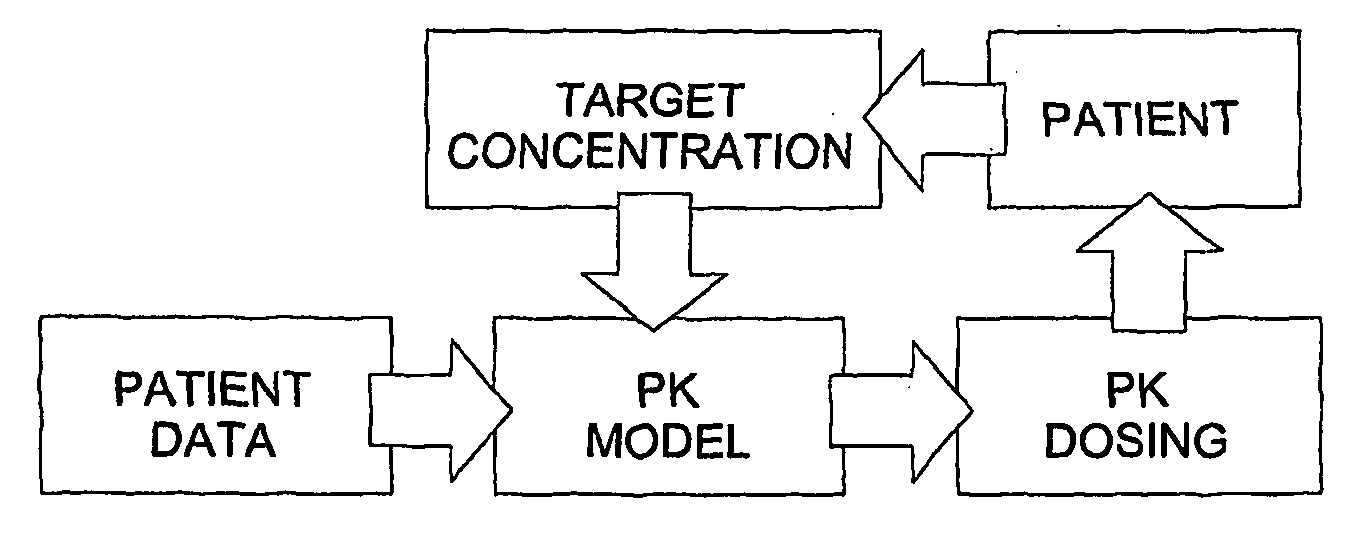
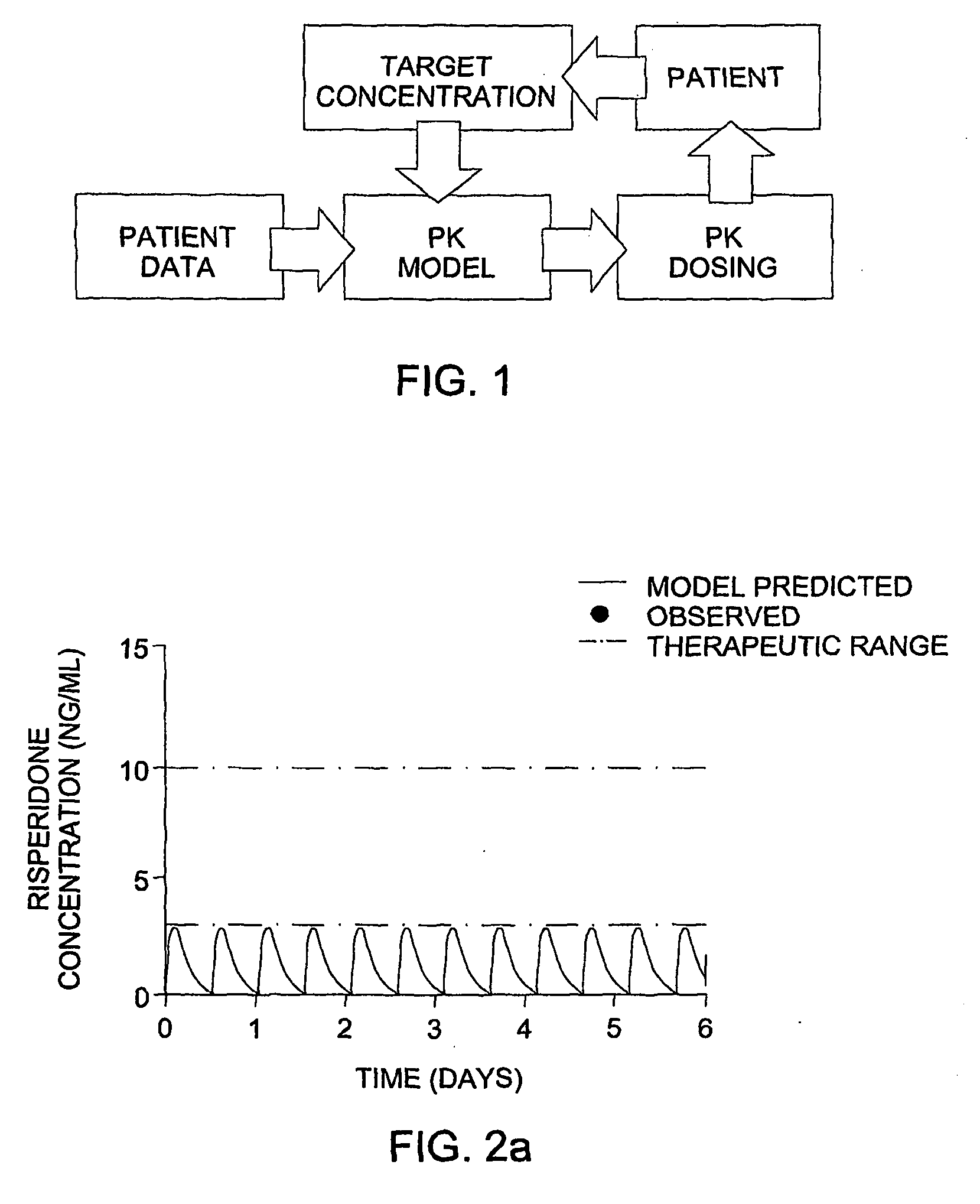
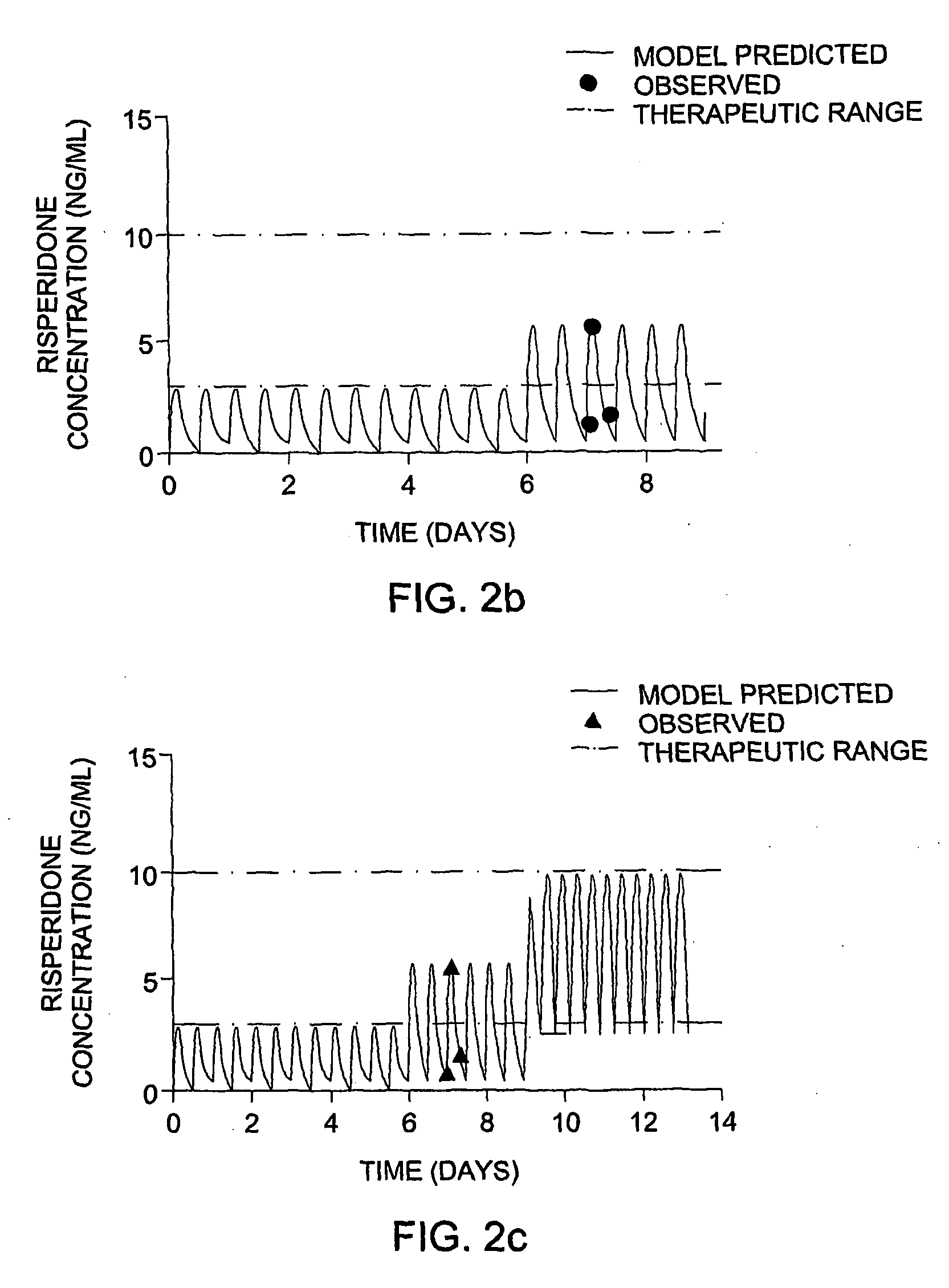
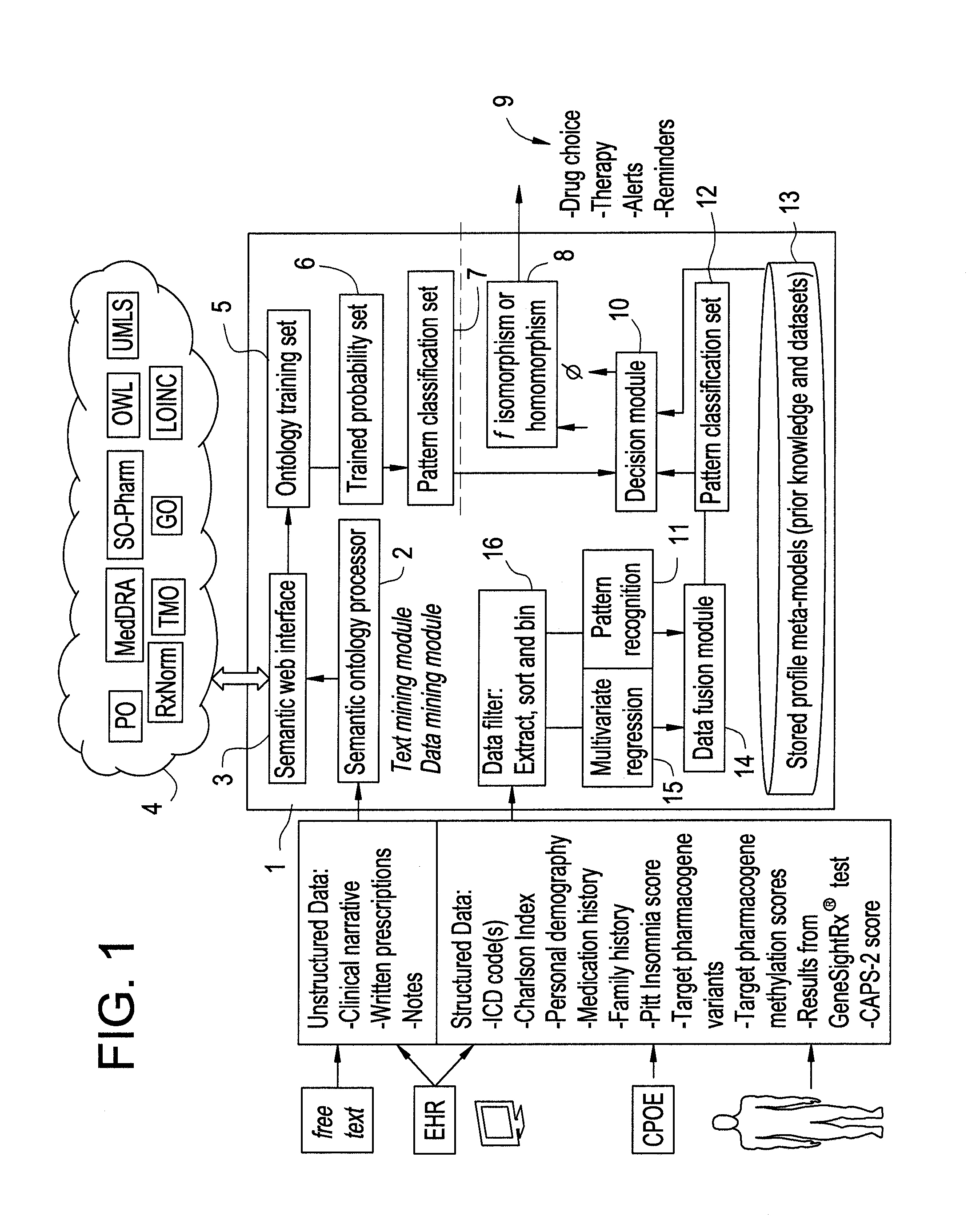
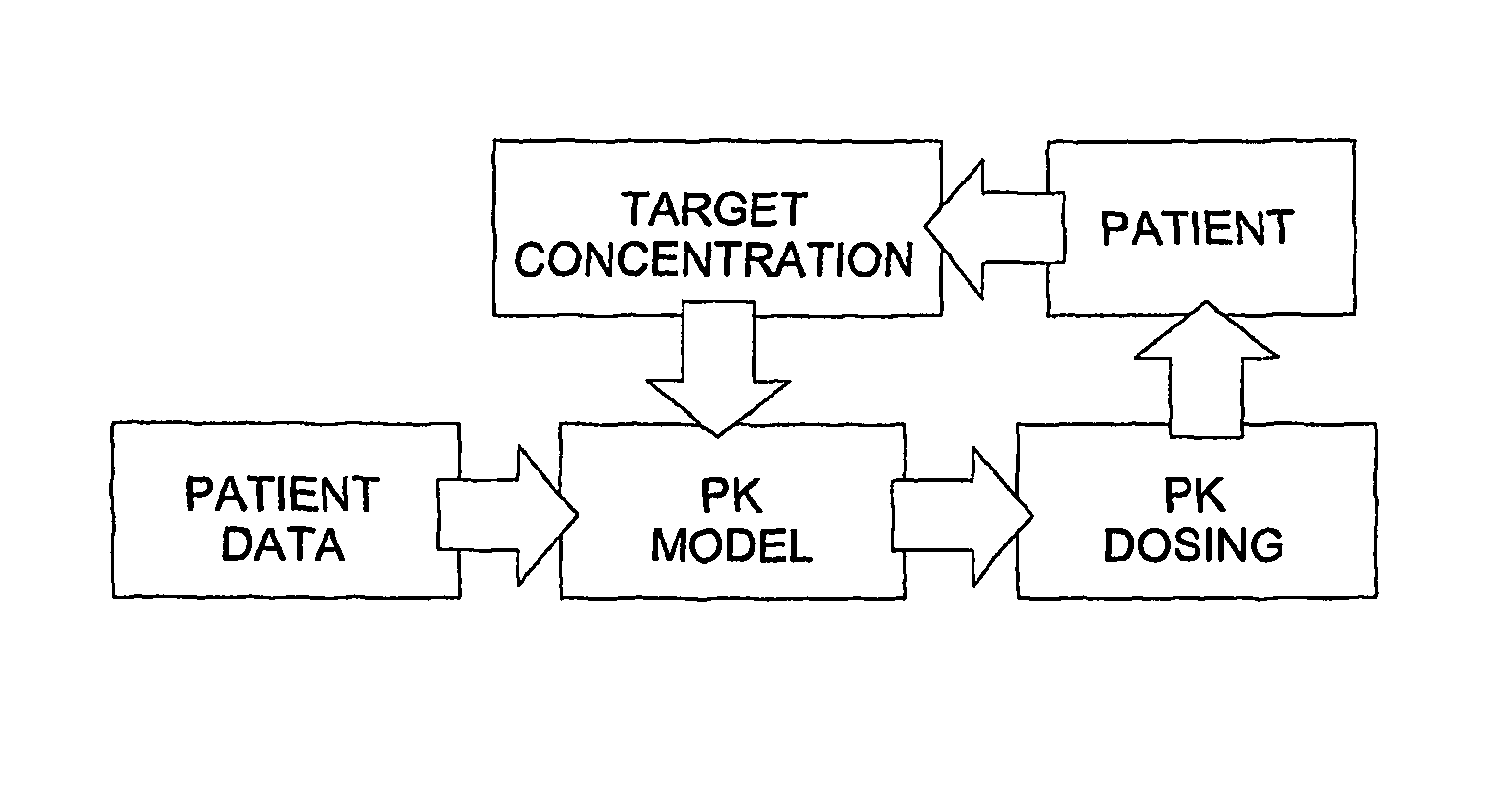
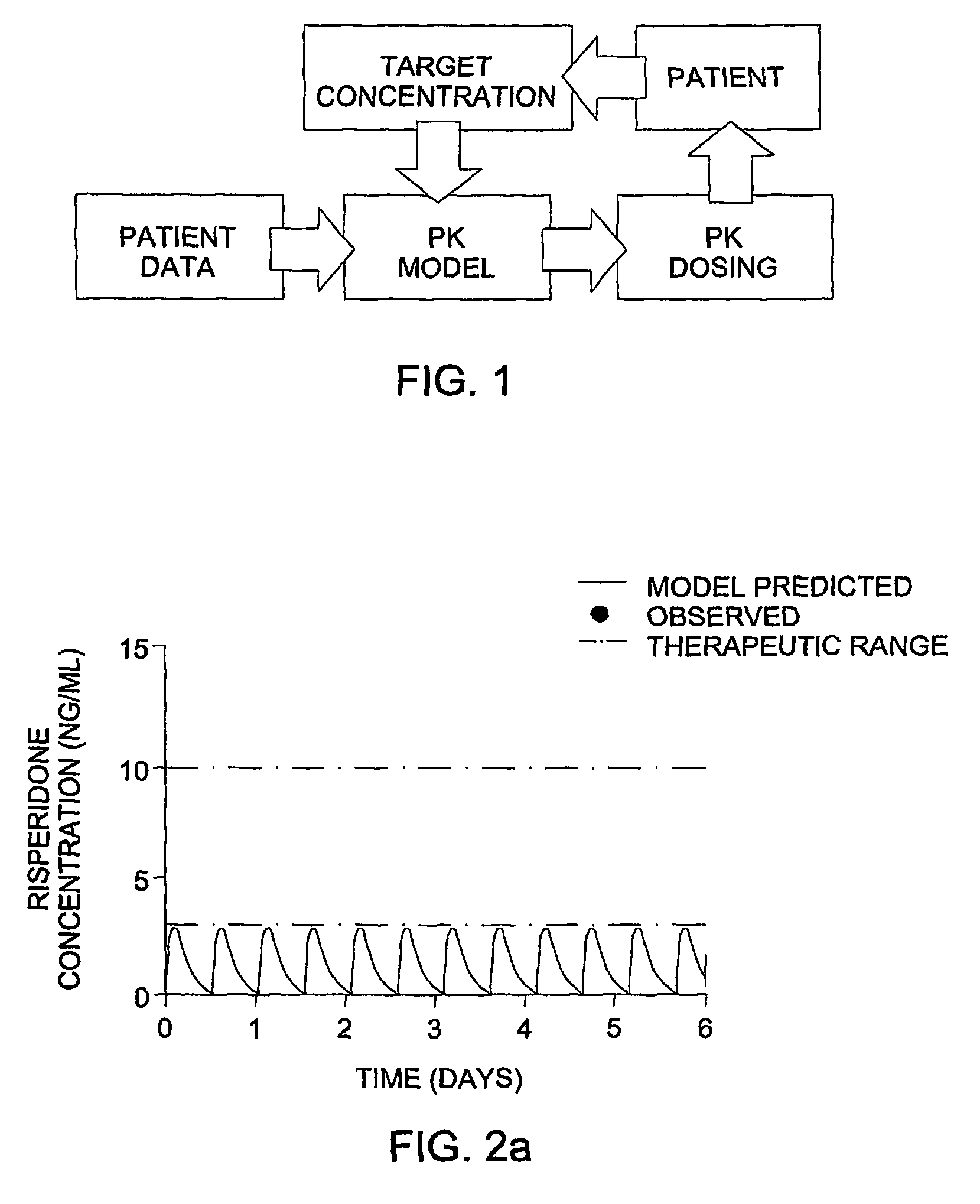
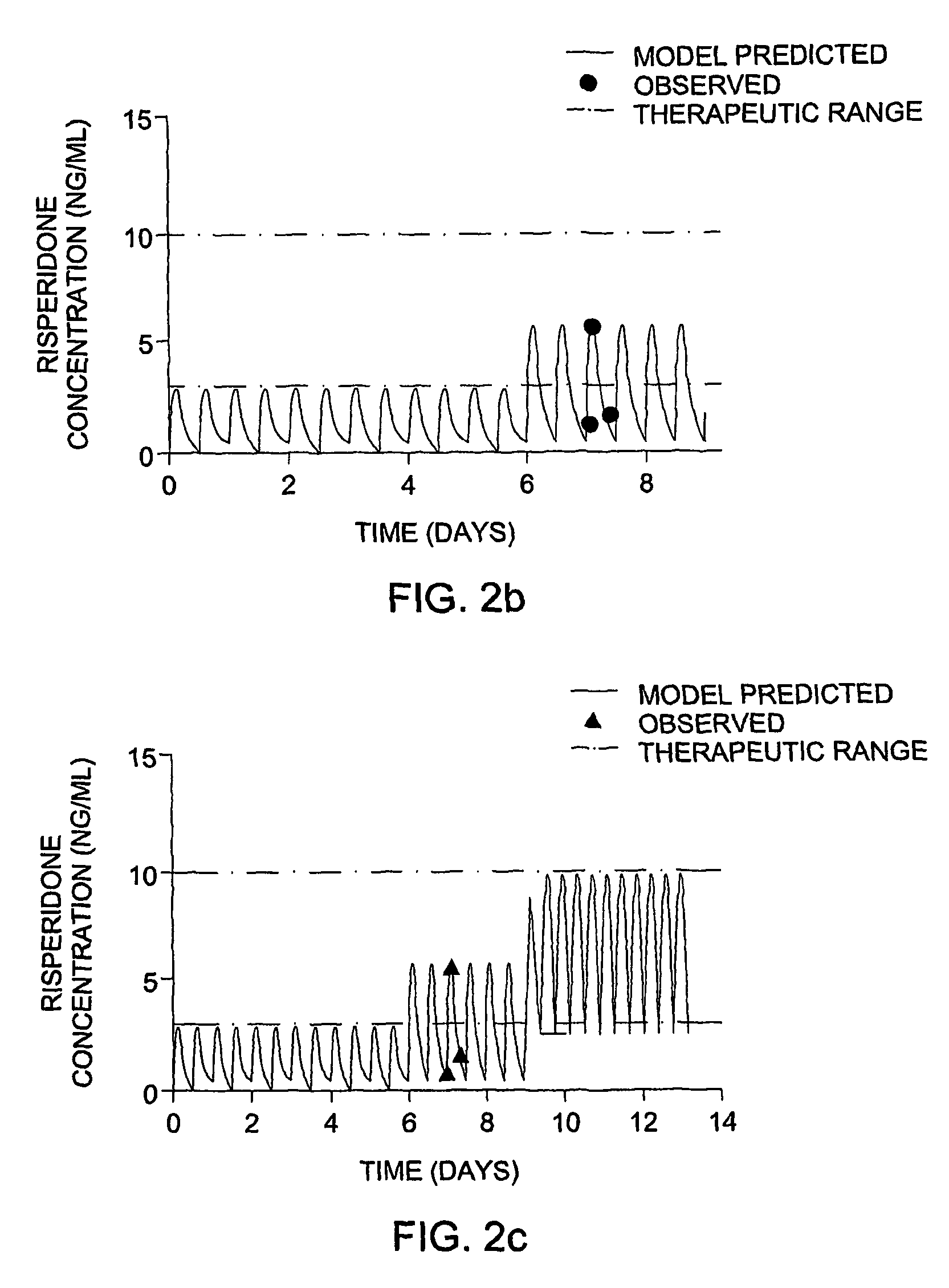
Optimization and Individualization of Medication Selection and Dosing
ActiveUS20090171697A1Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Breath test for detection of drug metabolism
InactiveUS6180414B1Withdrawing sample devicesMaterial analysis by electric/magnetic meansDrug metabolismMetabolite
A breath test for determining the rate of metabolism of a drug is described. First, a safe and effective amount of the drug, preferably appropriately labelled and most preferably isotopically-labelled, is administered to a subject. After a suitable time period, the exhaled breath of the subject is analyzed to determine the concentration of a metabolite. The concentration of the metabolite is then used to determine the rate of metabolism of the drug. A breath test kit is also described. Such a breath test kit would include an item or items necessary for performing at least one of the methods of determining the rate of metabolism of a drug in a subject. For example, such a breath test kit could include an isotopically-labelled drug to be administered to the subject.
Owner:ORIDION MEDICAL
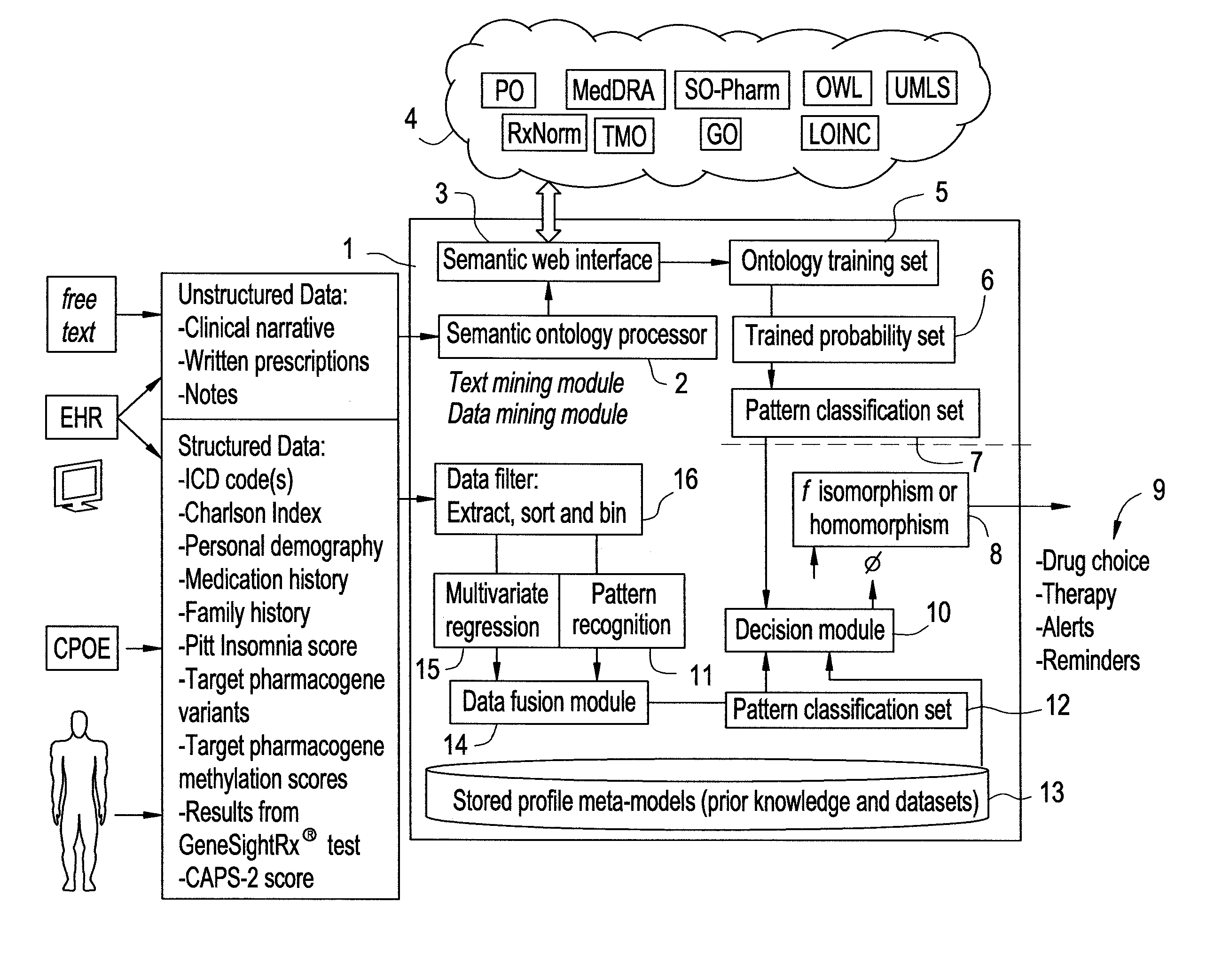
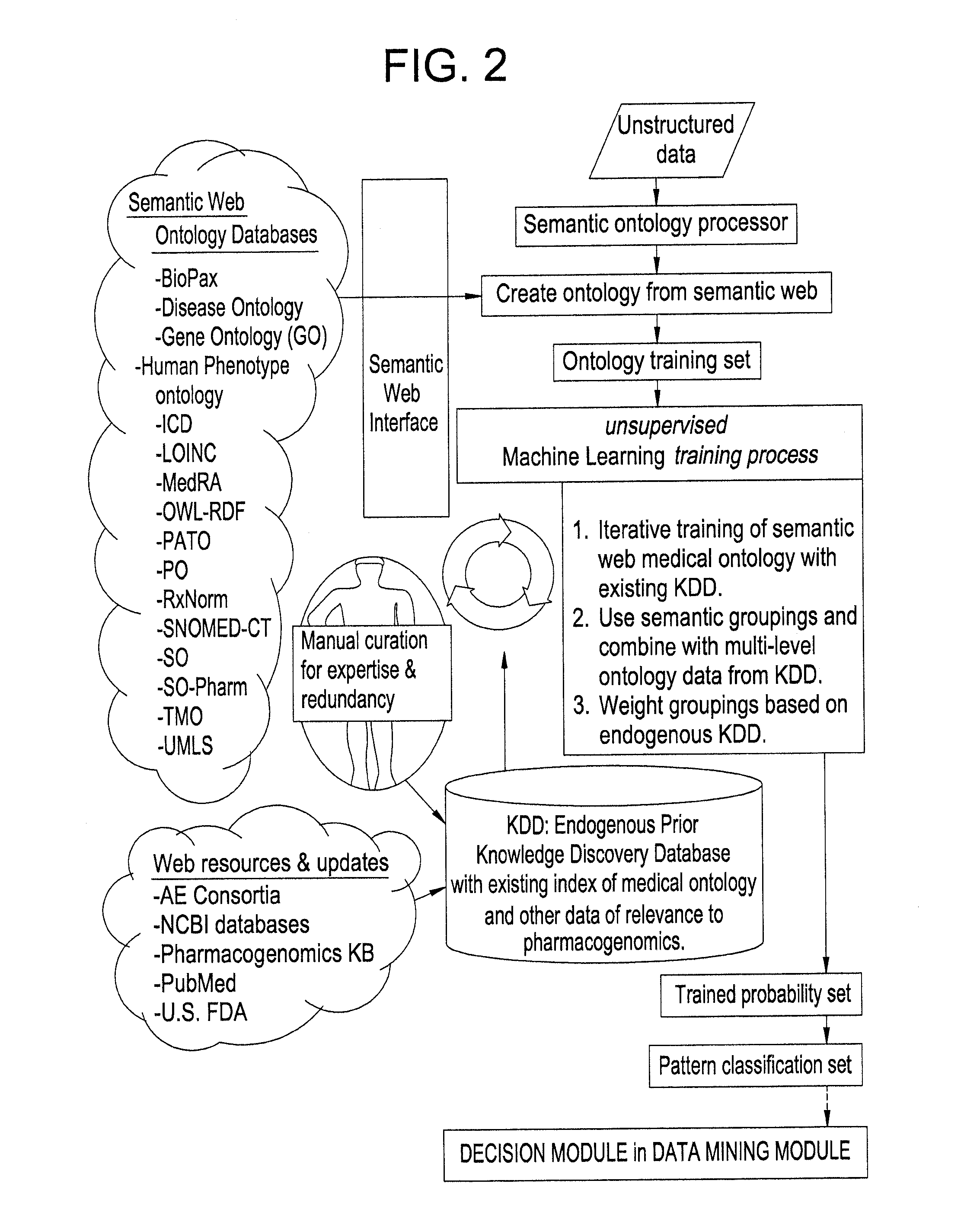
Systems and Methods for Pharmacogenomic Decision Support in Psychiatry
InactiveUS20140046696A1Medical simulationData processing applicationsClinical variablesDecision taking
The present invention provides methods and systems or apparatuses, to analyze multiple molecular and clinical variables from an individual diagnosed with a psychiatric disorder, such as post-traumatic stress disorder (PTSD), in order to optimize medication selection for therapeutic response. Molecular co-variables include polymorphisms in genes including those involved in central control and mediation of the hypothalamic-pituitary axis (HPA) stress response, the density of methylation in regulatory regions of said polymorphic genes, polymorphisms in genes that encode cytochrome P450 enzymes responsible for drug metabolism, and drug-drug and drug-gene interactions. Clinical co-variables include but are not limited to the sex, age and ethnicity of that individual, medication history, family history, diagnostic codes, Pittsburgh insomnia rating score, and Charlson index score. The system makes a determination based on unstructured and structured data types derived from internal and external knowledge resources to determine psychotropic drug choice that best matches the molecular and clinical variation profile of an individual patient. The decision support system provides a therapeutic recommendation for a clinician based on the patient's variation profile.
Owner:ASSUREX HEALTH INC
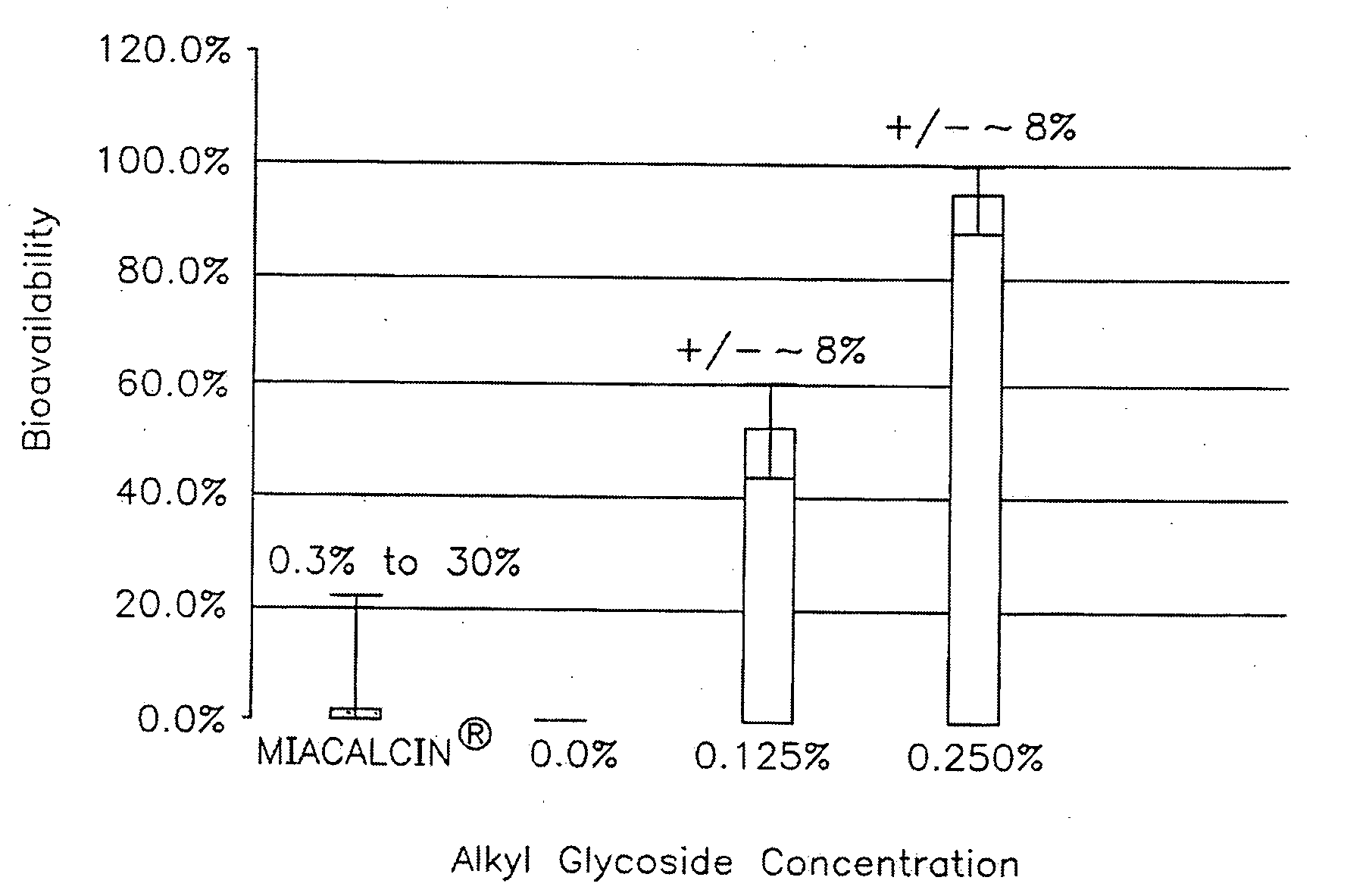
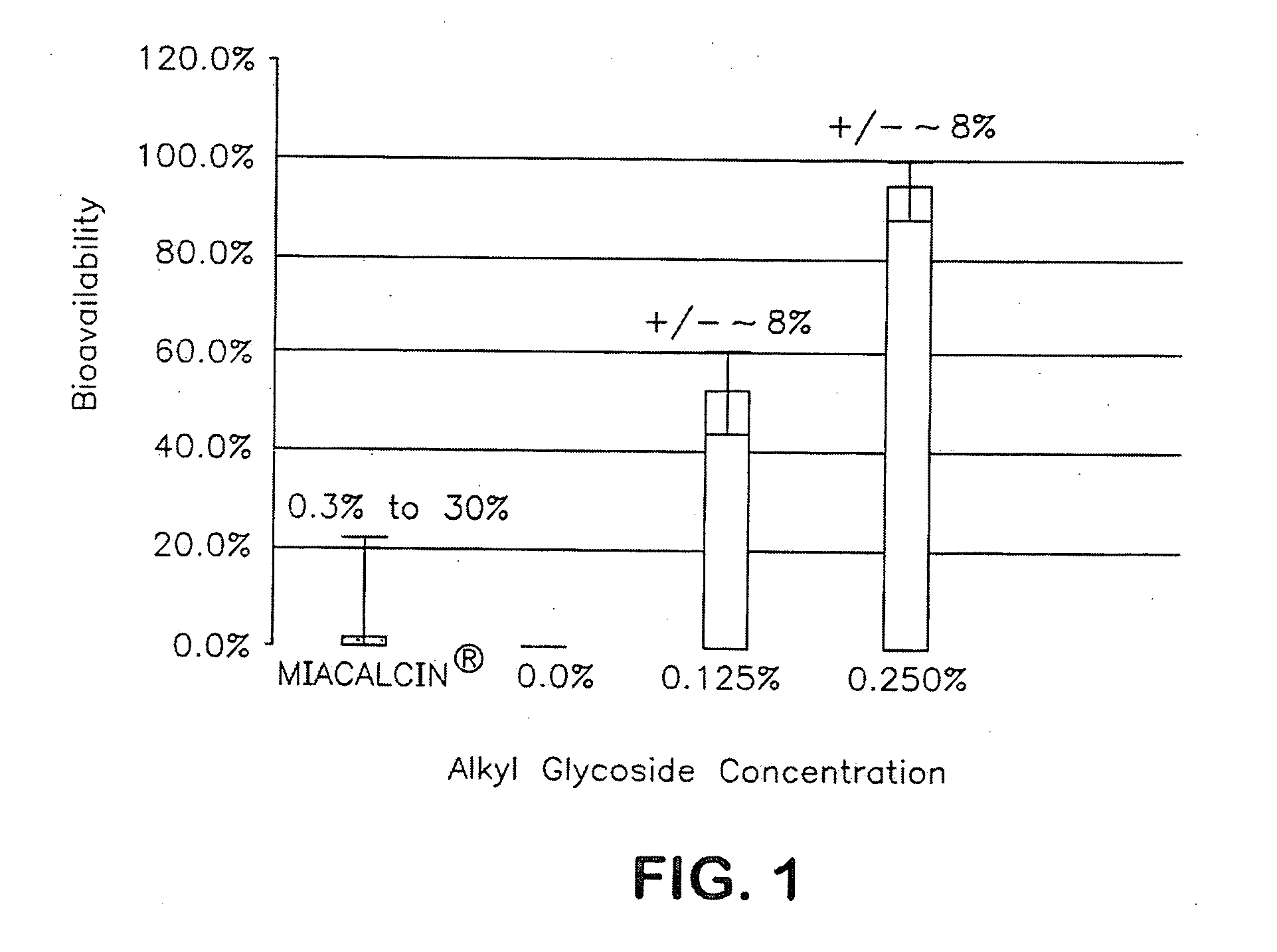
Compositions for Drug Administration
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
Application of compounds in isorhodanic ester classes for treating diseases of prostate and skin cancer
ActiveCN101091705AIncreased ability to remove harmful substancesImprove in vitro dissolutionUrinary disorderEster active ingredientsDiseaseProstate cancer cell
The present invention relates to a method capable of using natural and artificial synthetic isosulfocyanate compound or its derivative to prevent and cure prostatic diseases and skin carcinoma. The internal tests show that various isosulfocyanate compounds or their derivatives can induce prostatic cell II phase drug metabolic detoxication enzyme-glutathione transferase so as to can inhibit the hyperplasia of prostate and inflammation, and can prevent and cure prostatic carcinoma and skin carcinoma.
Owner:JC (WUXI) CO INC
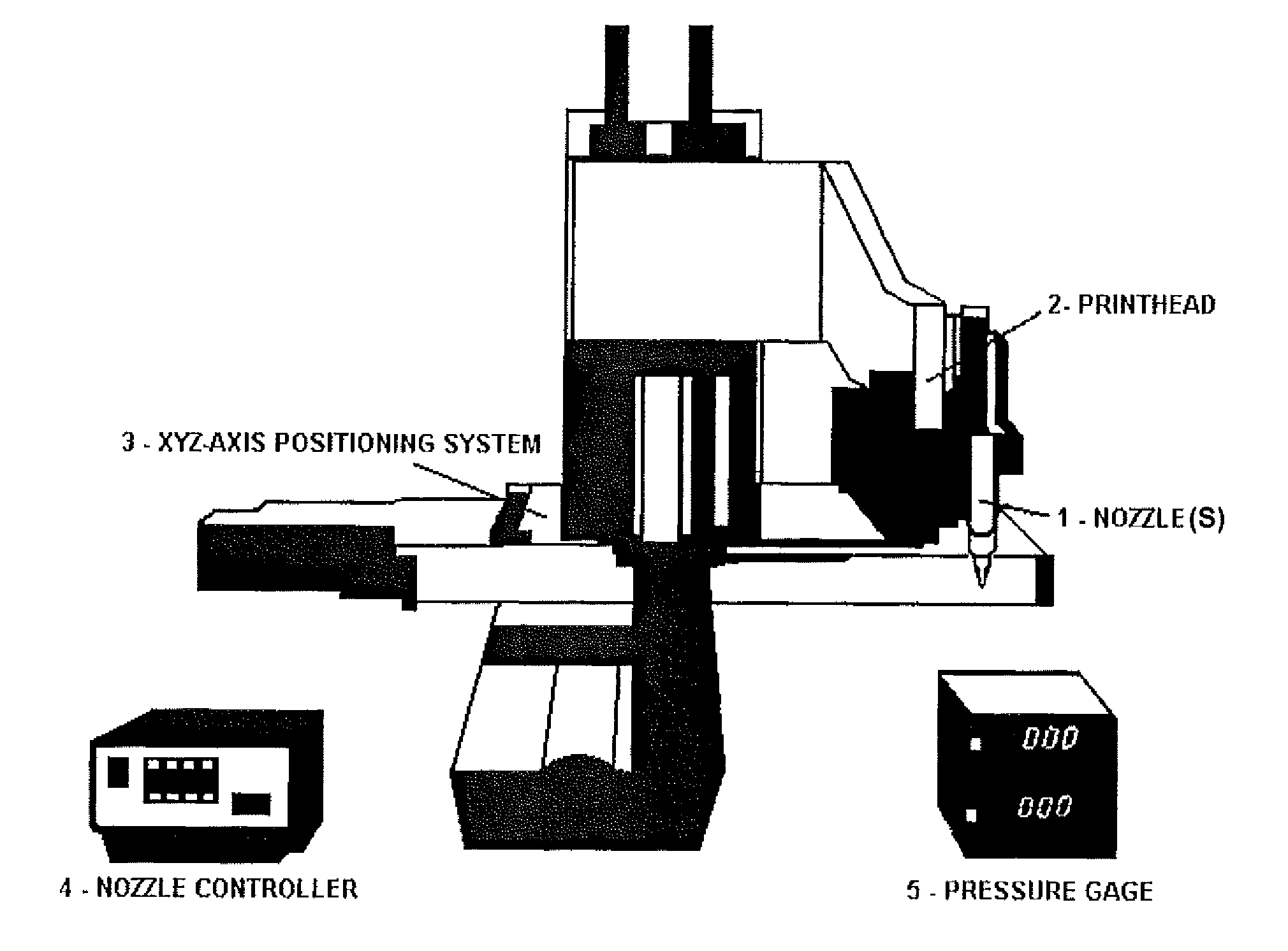
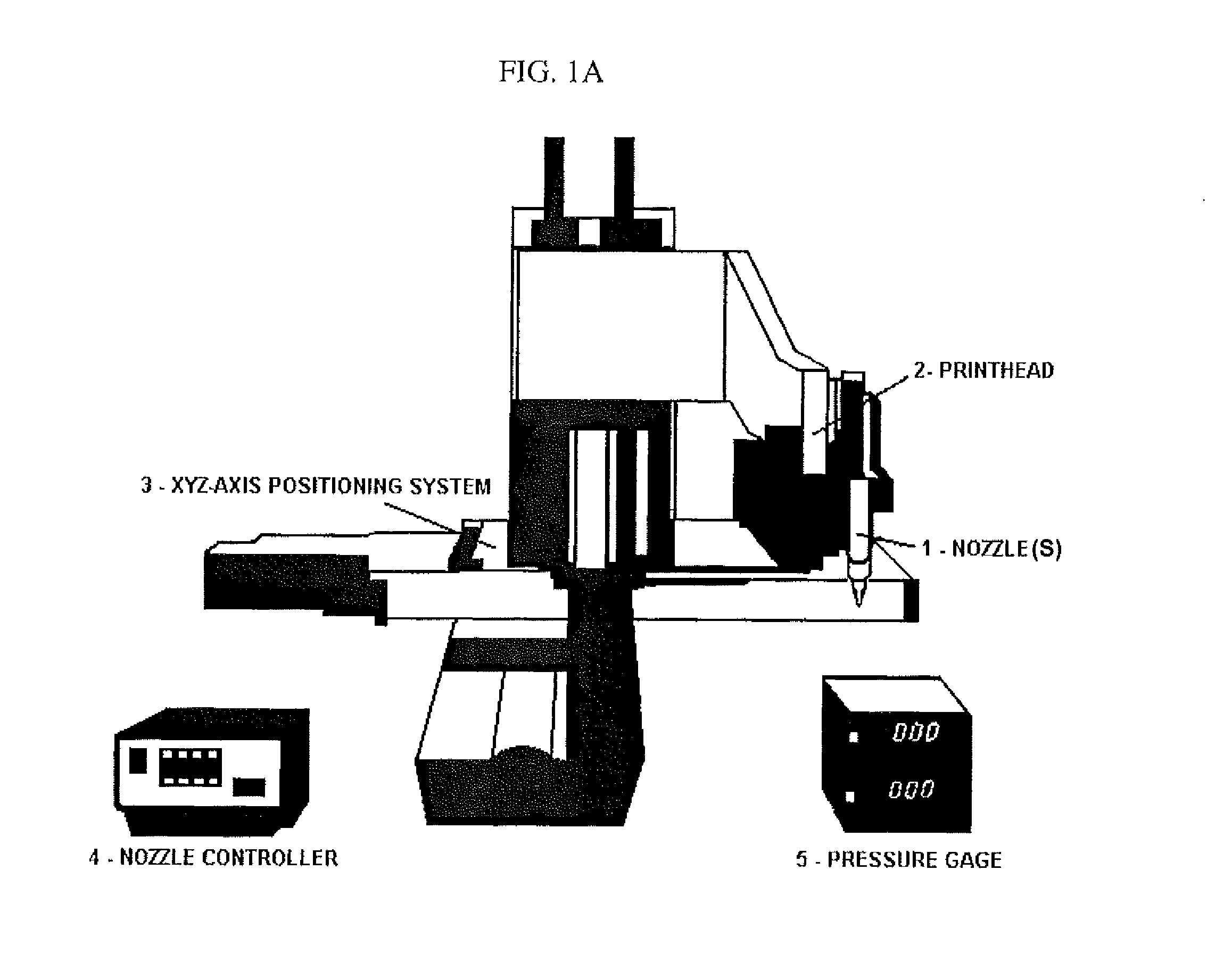
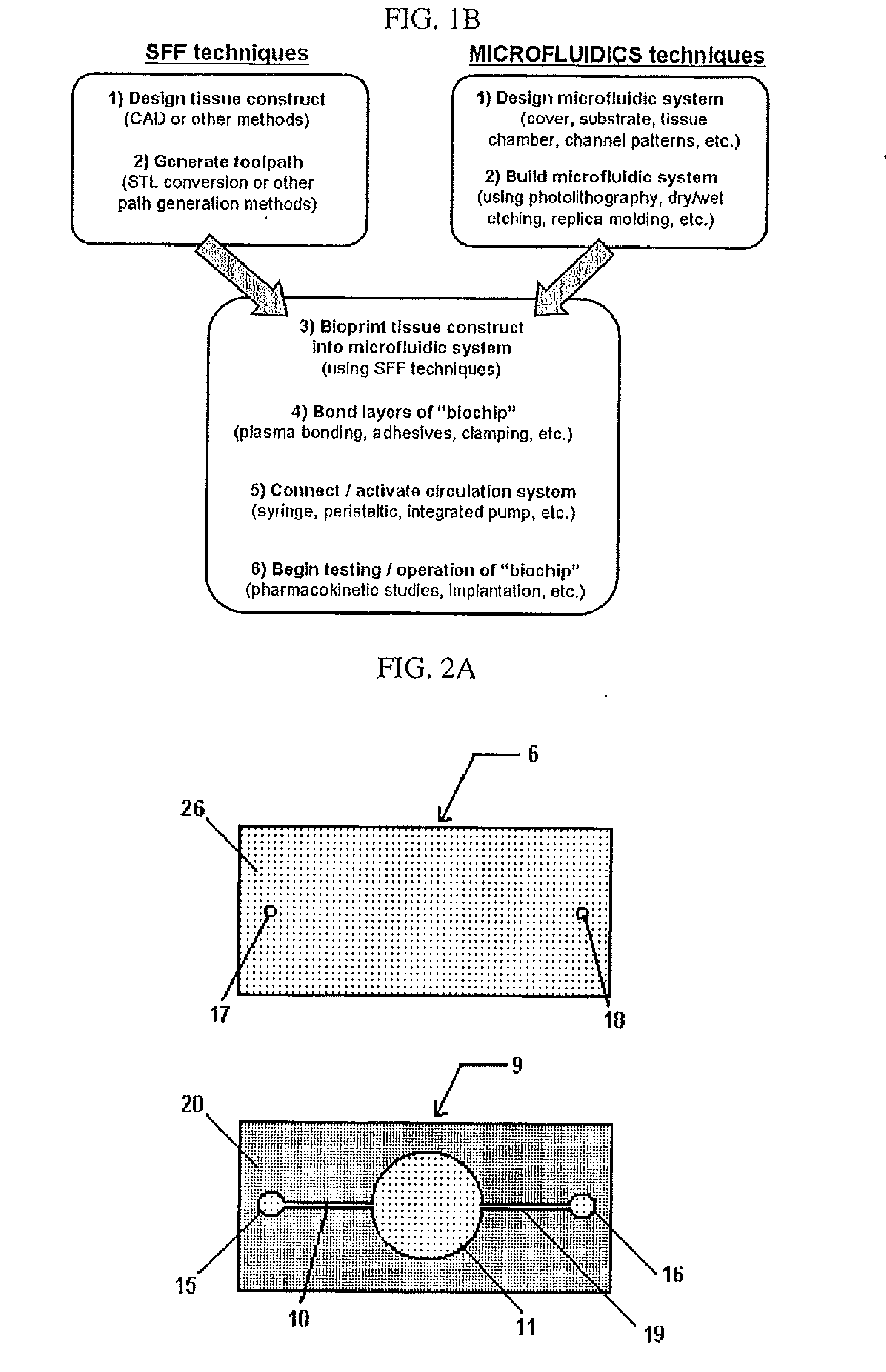
Compositions and Methods for Functionalized Patterning of Tissue Engineering Substrates Including Bioprinting Cell-Laden Constructs for Multicompartment Tissue Chambers
InactiveUS20110136162A1Bioreactor/fermenter combinationsBiological substance pretreatmentsChemical treatmentDrug metabolism
The present invention relates to microfluidic systems and methods for monitoring or detecting a change in a characteristic of an input substance. Specifically, the invention relates to a model for in vitro pharmacokinetic study and other pharmaceutical applications, as well as other uses including computing, sensing, filtration, detoxification, production of chemicals and biomolecules, testing cell / tissue behavior, toxicology, drug metabolism, drug screening, drug discovery, and implantation into a subject. The present invention also relates to systems and methods of a microplasm functionalized surface patterning of a substrate. The present invention represents an improvement over existing plasma systems used to modify the surface of a substrate, as the present invention creates surface patterning without the use of a mask, stamp or a chemical treatment.
Owner:DREXEL UNIV
Pharmacogenetic DME detection assay methods and kits
InactiveUS20060160074A1Increase nucleic acid synthesis reaction rateHeating evenlySugar derivativesMicrobiological testing/measurementDrug metabolismPharmacogenetics
The present invention relates to methods for detecting polymorphisms in enzymes related to drug metabolizm (Drug Metabolizing Enzymes or DMEs) such as uridine diphosphate glucuronosyl transferase (UGT) gene promoter, cytochrome p450, with a non-amplified oligonucleotide detection assays. The present invention also relates to pharmacogenetic DME detection assay kits.
Owner:THIRD WAVE TECH
Optimization and individualization of medication selection and dosing
ActiveUS8589175B2Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
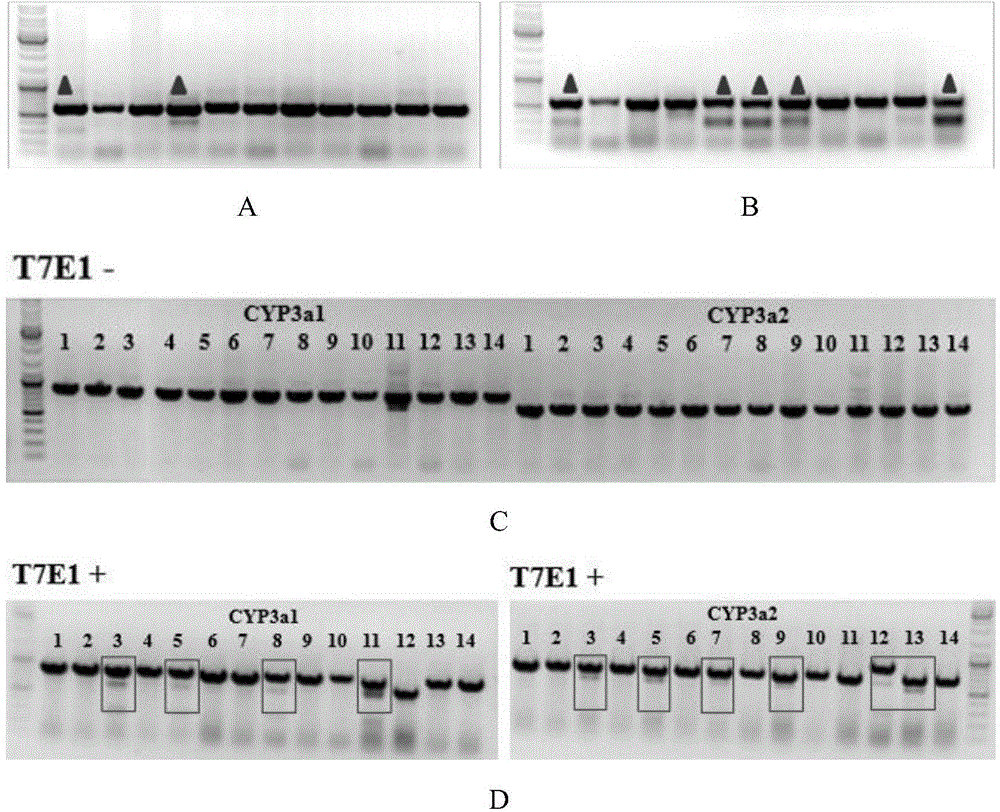
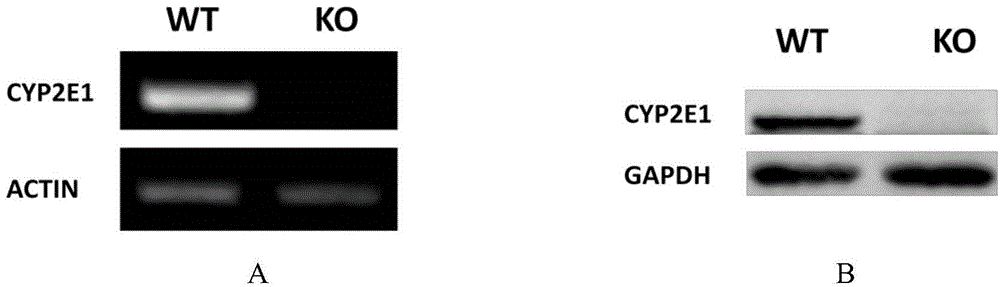
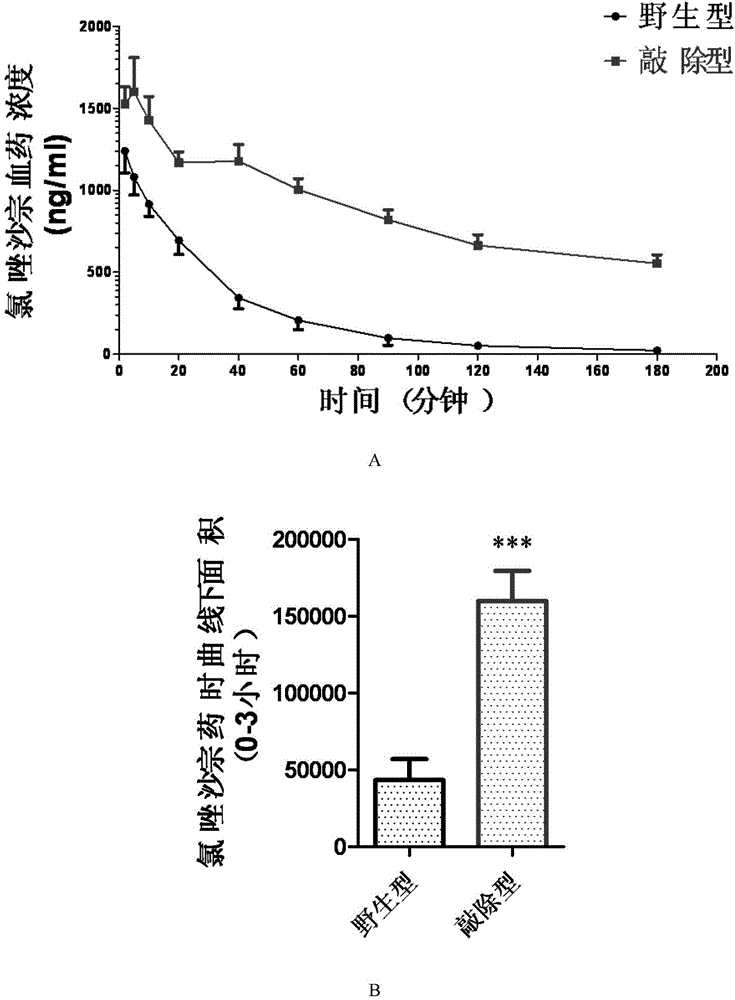
Cultivation method of Cyp gene knocked-out rats, and preparation method of liver microsome of the rats
ActiveCN106148416ADiverse in vitro modelsImprove transfer abilityMicroinjection basedFermentationHepaticaMicro injection
The invention provides a cultivation method of Cyp gene knocked-out rats, and a preparation method of liver microsome of the rats. The Cyp gene knock-out herein includes Cyp single gene knock-out and Cyp multiple gene knock-out. In the method, firstly a Cyp gene knocked-out rat is constructed by means of a CRISPR / Cas system, which includes selection of a knocked-out target site, in-vitro synthesis and transcription of sg RNA and Cas9m RNA, preparation of a pseudopregnant female rat, in-vitro micro-injection and transplanting of a single-cell embryo, and cultivation of the rat, and finally, a homozygote Cyp gene knocked-out rat can be cultured. Furthermore, the liver of the Cyp gene knocked-out rat is extracted and is subjected to homogenization and differential centrifugation to prepare the liver microsome of the rat in Cyp gene deletion. The invention also provides an application of the Cyp gene knocked-out rats and the liver microsome thereof in study on drug metabolism.
Owner:EAST CHINA NORMAL UNIV
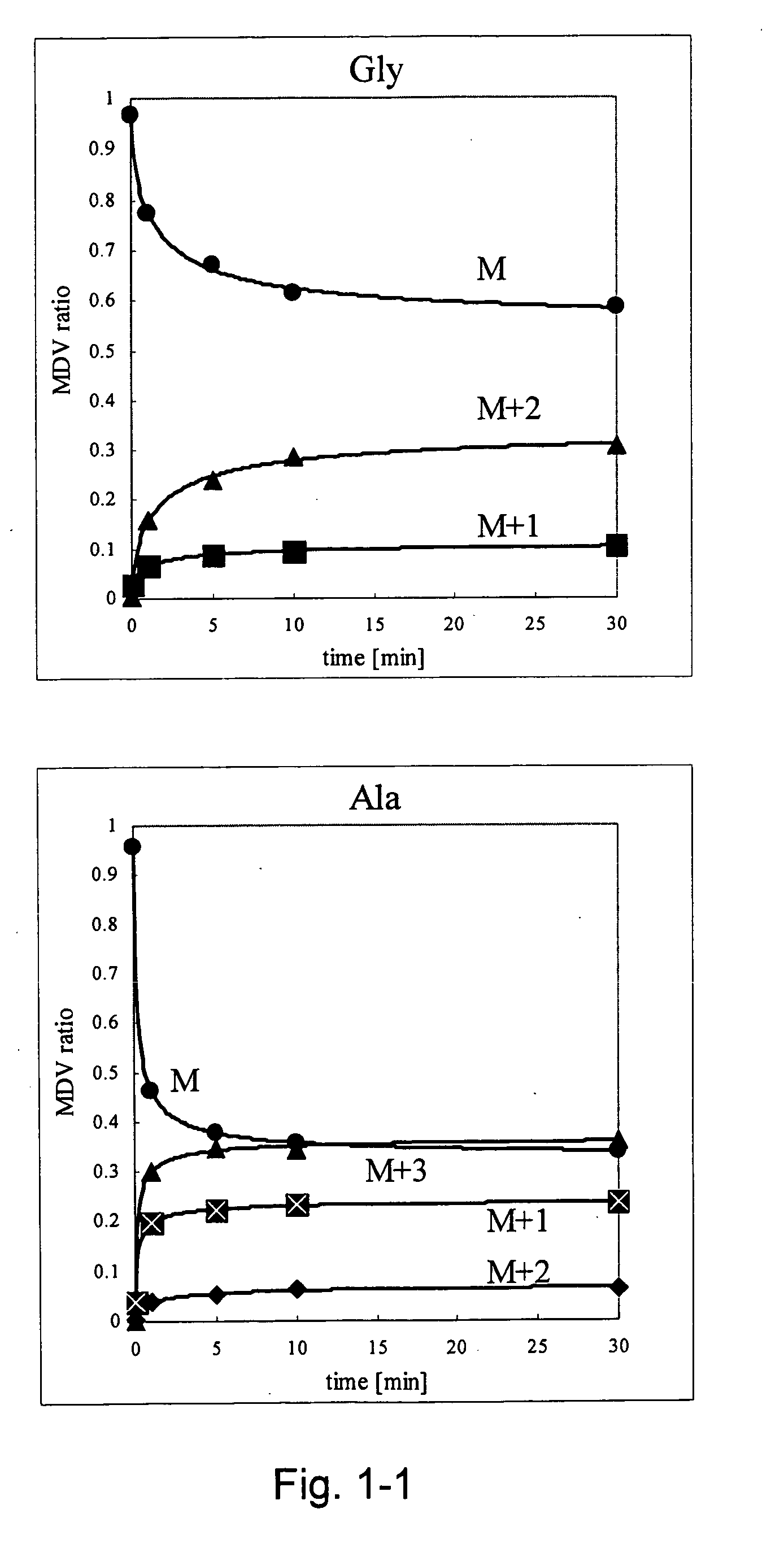
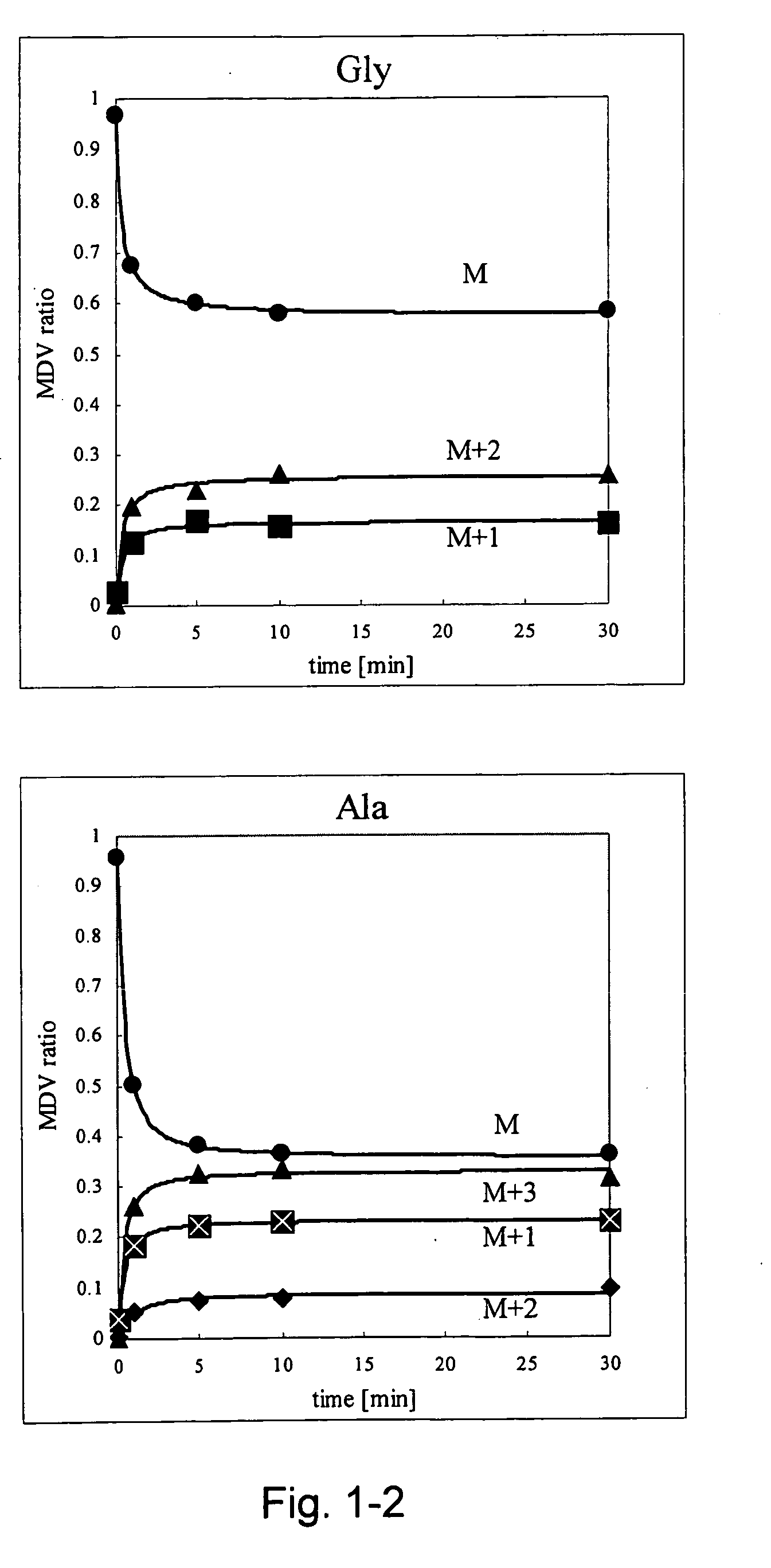
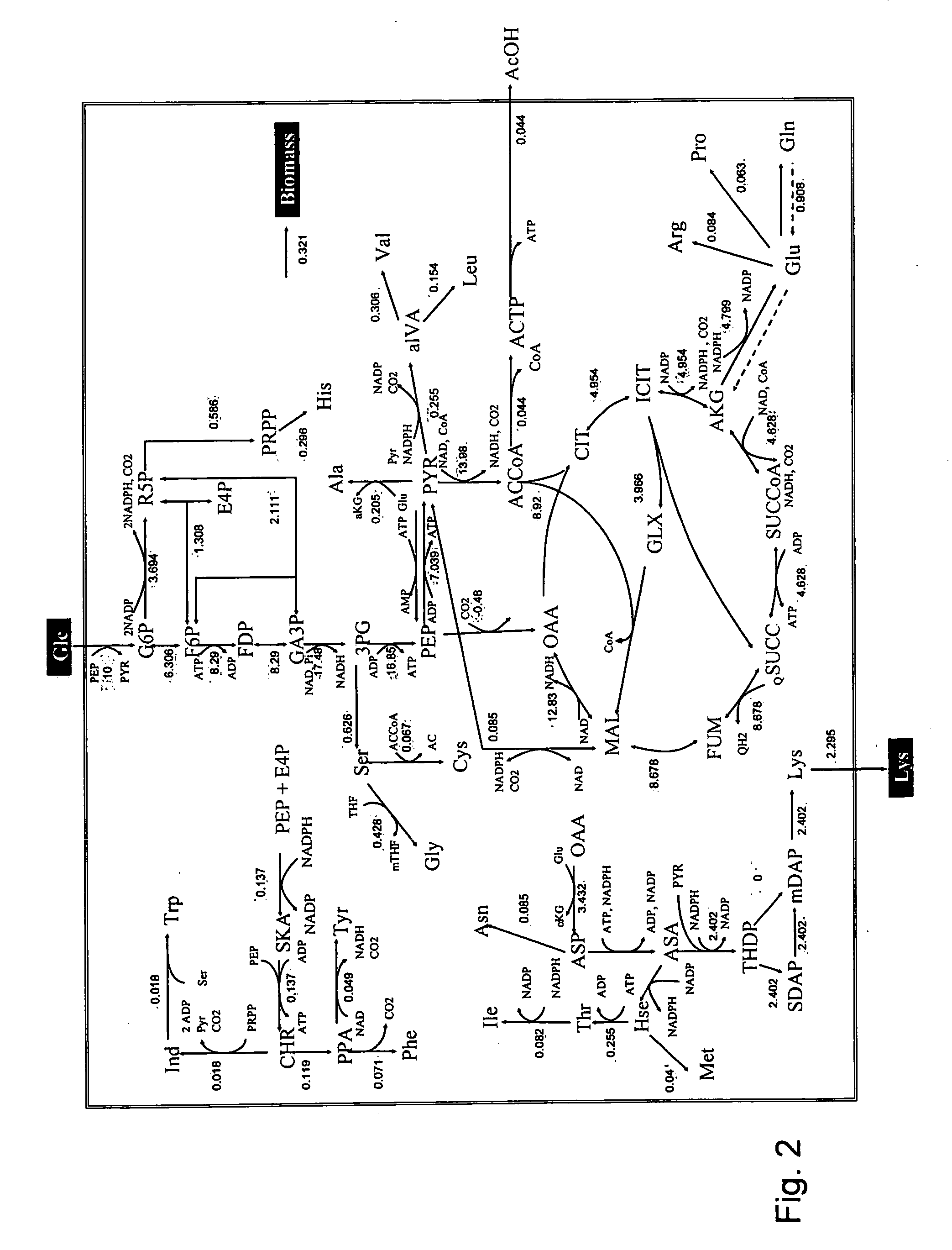
Intracellular metabolic flux analysis method using substrate labeled with isotope
InactiveUS20050175982A1Low costMicrobiological testing/measurementBiological testingDrug metabolismTime course
A method for intracellular metabolic flux analysis comprising (a) culturing cells in a medium not containing any isotope-labeled substrate to a target phase of the metabolic flux analysis, (b) adding an isotope-labeled substrate to the medium, and continuing culture and collecting samples from the medium in time course, (c) measuring isotope distribution in an intracellular metabolite contained in the samples collected in time course, (d) performing a regression analysis for measured data and calculating an isotope distribution ratio in a steady state, and (e) analyzing a metabolic flux of the cultured cells by using the calculated isotope distribution ratio.
Owner:AJINOMOTO CO INC
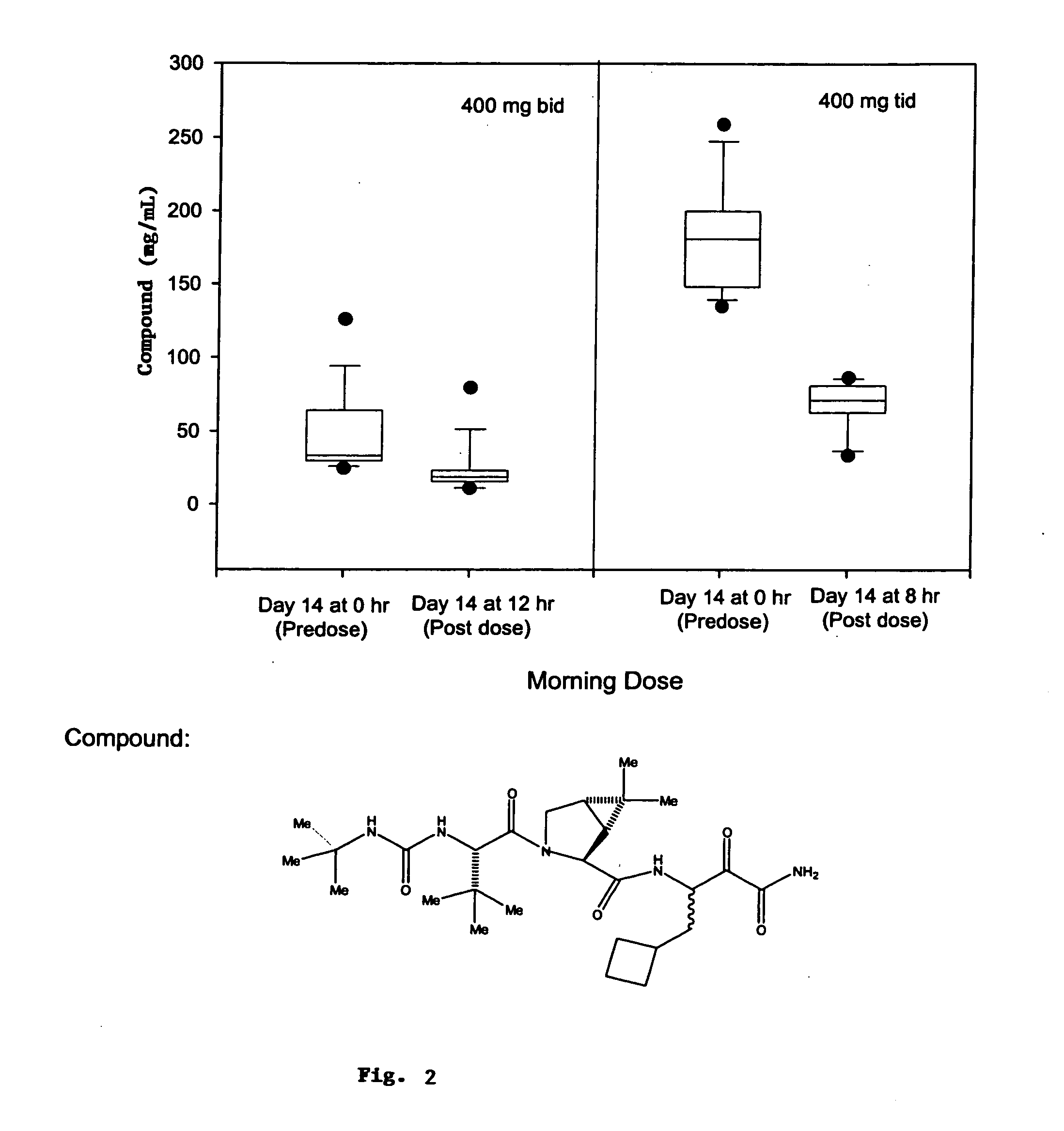
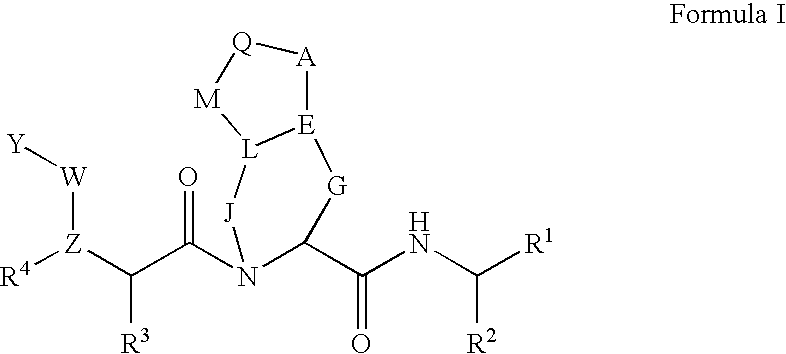
Asymmetric dosing methods
A method of treating, preventing or ameliorating one or more symptoms of hepatitis C, or inhibiting cathepsin activity, in a subject is provided, in which at least one compound (e.g., a HCV protease inhibitor) is administered in one or more discrete dosages over a twenty-four hour time interval in an asymmetric pattern as to dosage amount and / or timing of dosage, wherein the at least one compound is selected from the group consisting of compounds of Formulae I-XXVI, described herein. Methods of modulating the activity of hepatitis C virus protease in a subject are also provided. Asymmetric dosing as to amount of dose and / or timing of dose permits adjustment of dosing to accommodate variations in drug metabolism and / or viral activity caused by viral cell division or a patient's circadian rhythms, thus delivering the maximum amount of dose at the time or times it is most effective.
Owner:SCHERING CORP
Method and compositions for the diagnosis and treatment of non-small cell lung cancer using gene expression profiles
ActiveUS20050260586A1Accurate diagnosisAccurately diagnose lung cancerMicrobiological testing/measurementFermentationDisease progressionBiology
The present invention identifies and quantifies changes in gene expression associated with non-small cell lung cancer NSCLC by examining gene expression in tissue from normal lung and diseased lung. The present invention also identifies and quantifies expression profiles which serve as useful diagnostic markers as well as markers that are useful to monitor disease states, disease progression, drug toxicity, drug efficacy and drug metabolism.
Owner:UNIVERSITY OF TOLEDO
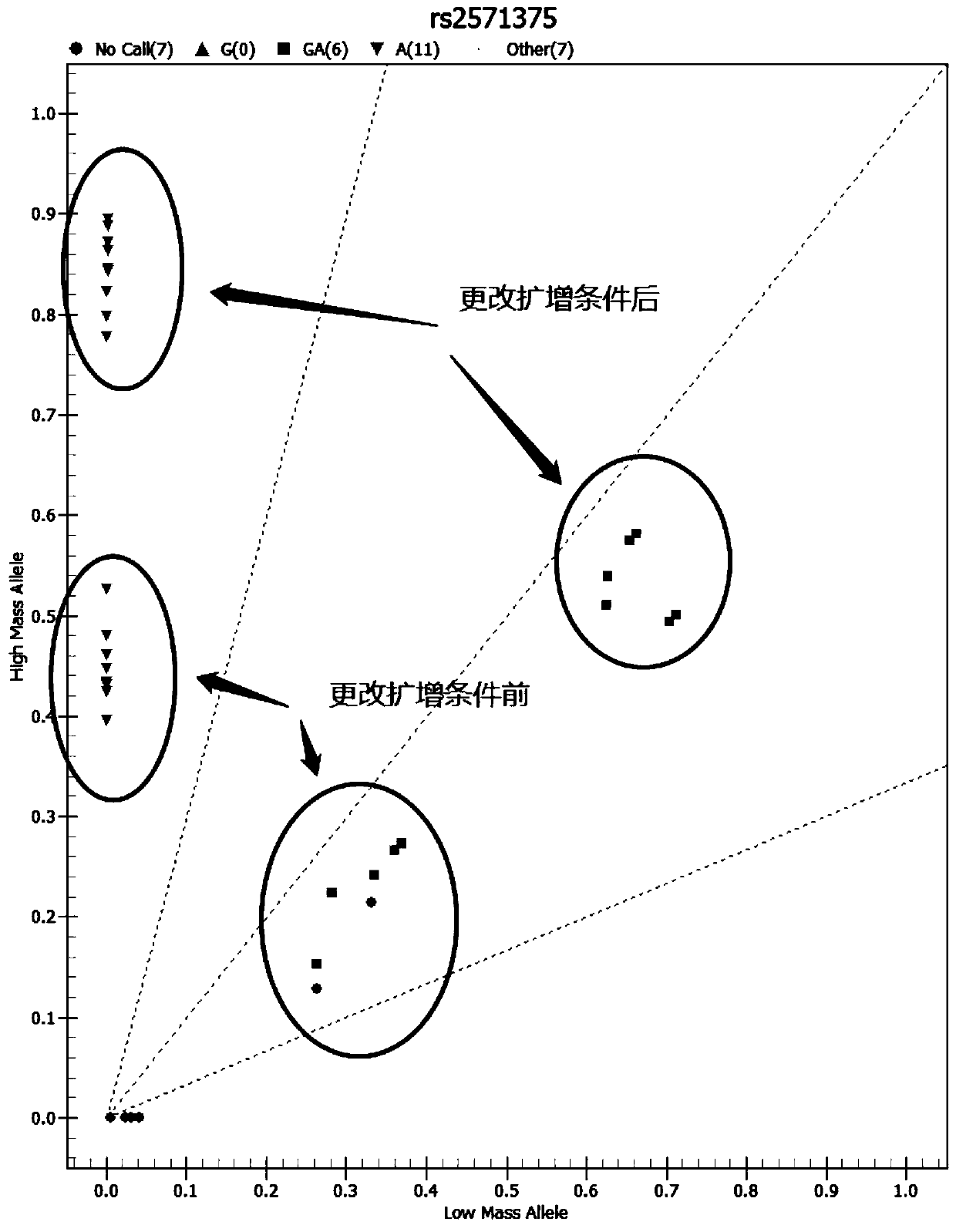
Medicine metabolic relevant loci detection method
InactiveCN101760528AAccurate and reliable metabolic strengthAvoid adverse reactionsMicrobiological testing/measurementDrug metabolismFluorescence
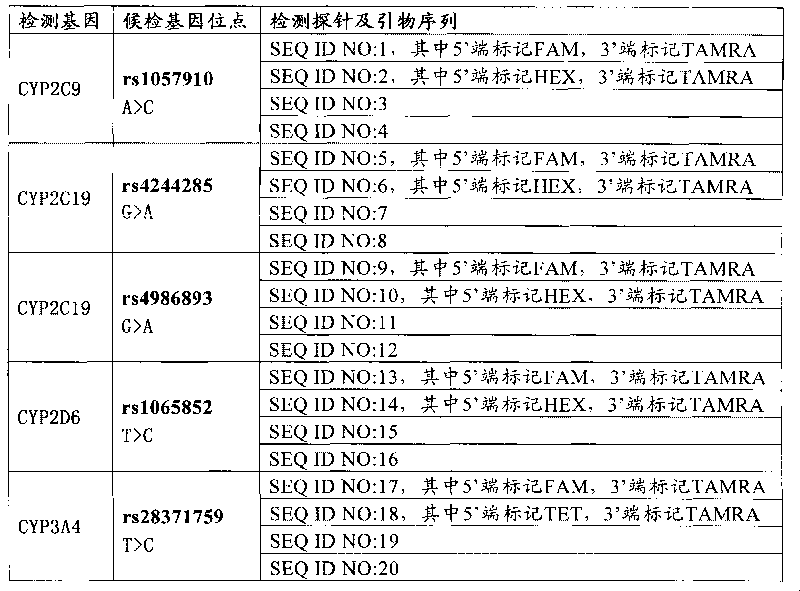
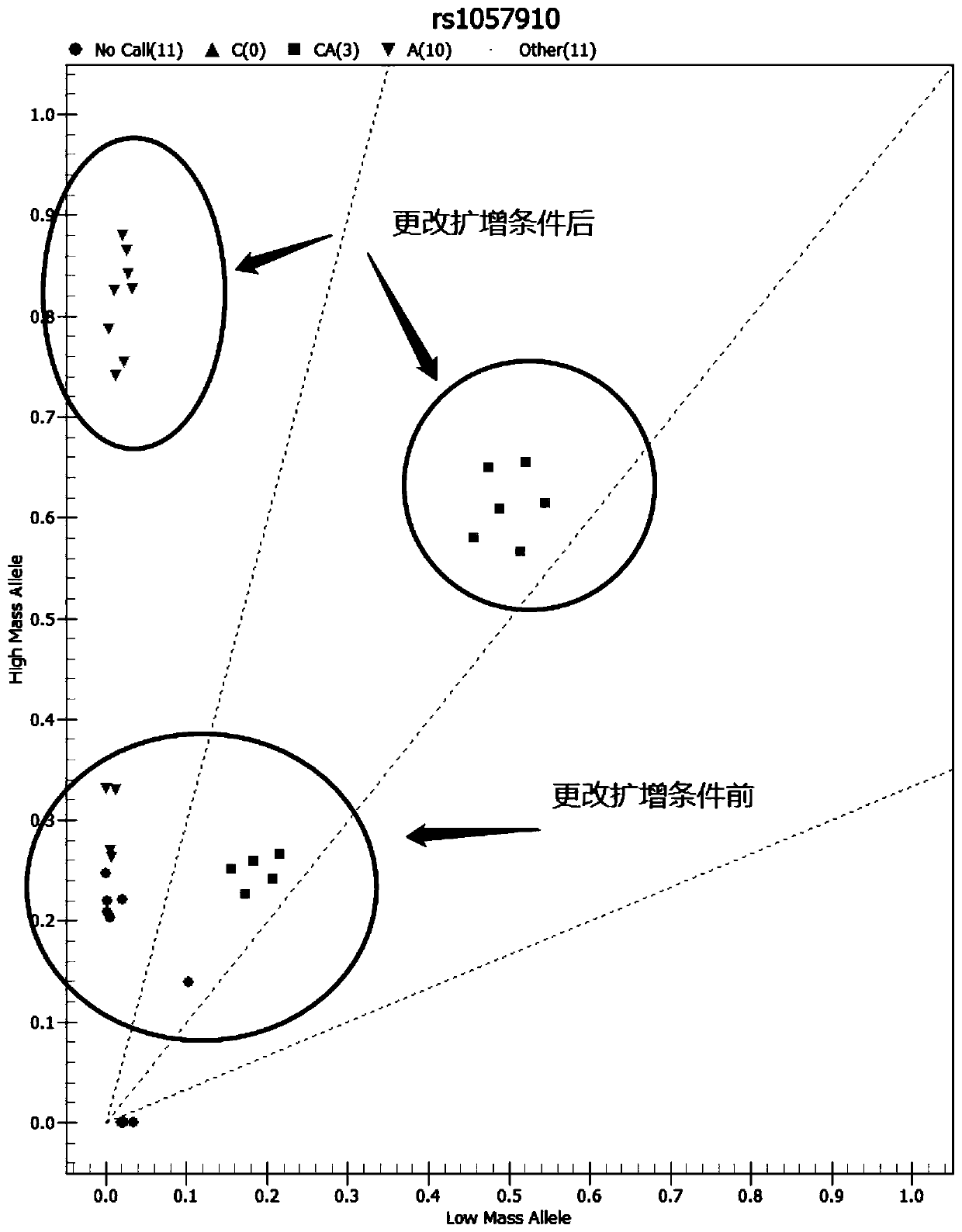
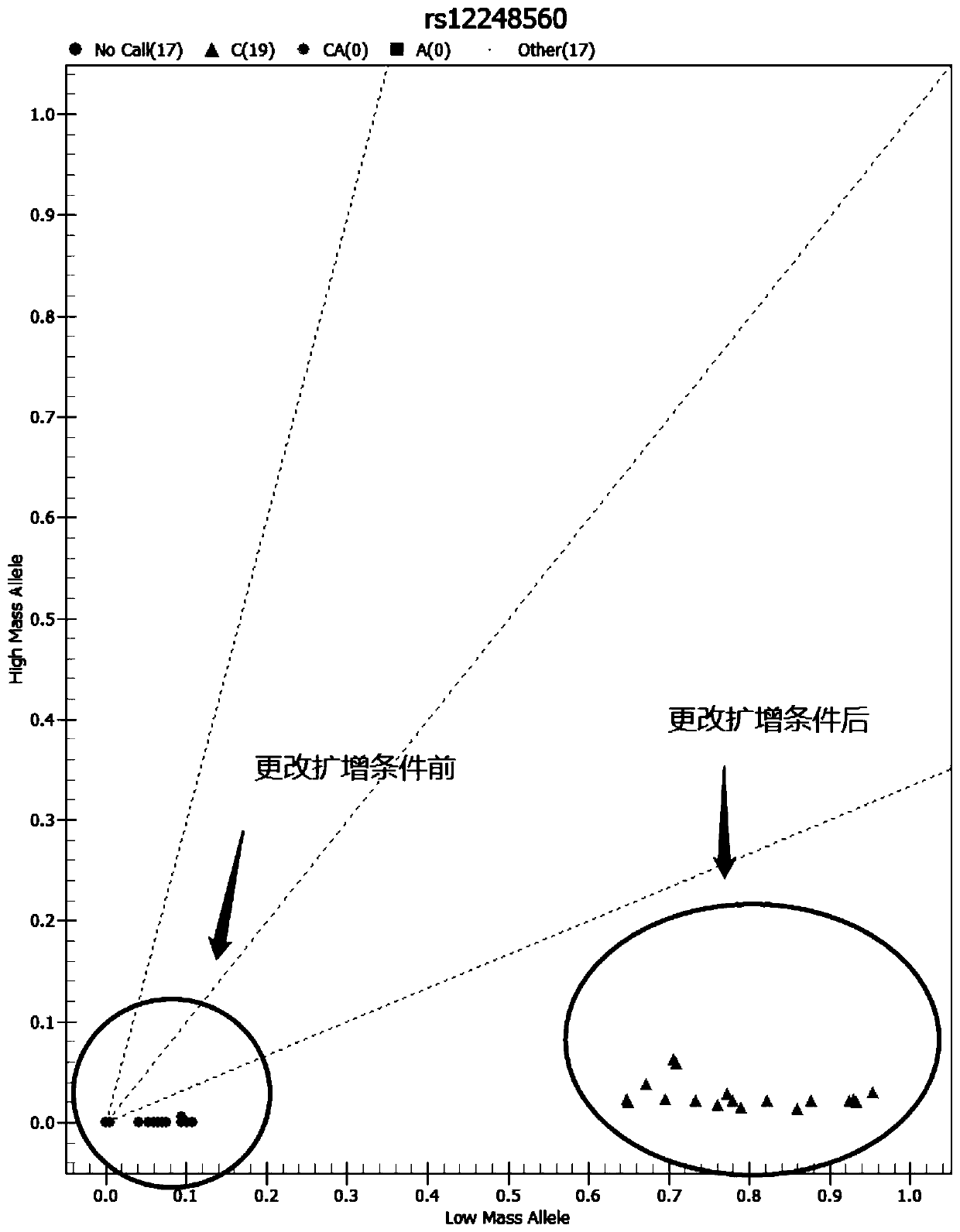
The invention relates to a medicine metabolic relevant loci detection method, which comprises the following steps: extracting genome DNA from human samples; respectively designing a Taqman probe pair and a primer pair according to at least two medicine metabolic relevant genes; respectively marking the 5' end and the 3'end of the Taqman probe pair with fluorescence reporting genes and fluorescence quenching genes; carrying out fluorescence quantitative PCR augmentation on the genome DNA; and judging whether the medicine metabolic relevant genes have the mutation according to the fluorescence quantitative PCR augmentation results. Preferably, the number of the medicine metabolic relevant genes is four, the Taqman probe pair and the primer pair are used for detecting a loci rs1057910 of a gene CYP2C9, a loci rs4244285 of a gene CYP2C19, a loci rs4986893of a gene CYP2C19, a loci rs1065852 of a gene CYP2D6 and a loci rs28371759 of a gene CYP3A4. The invention has the advantages of ingenious design, simple operation and accurate and reliable detection results, and provides the reference frame for determining whether professional doctors are needed to be consulted so as to make sure the medicine can be taken or not or the proper dosage and the like when a certain medicine is taken.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
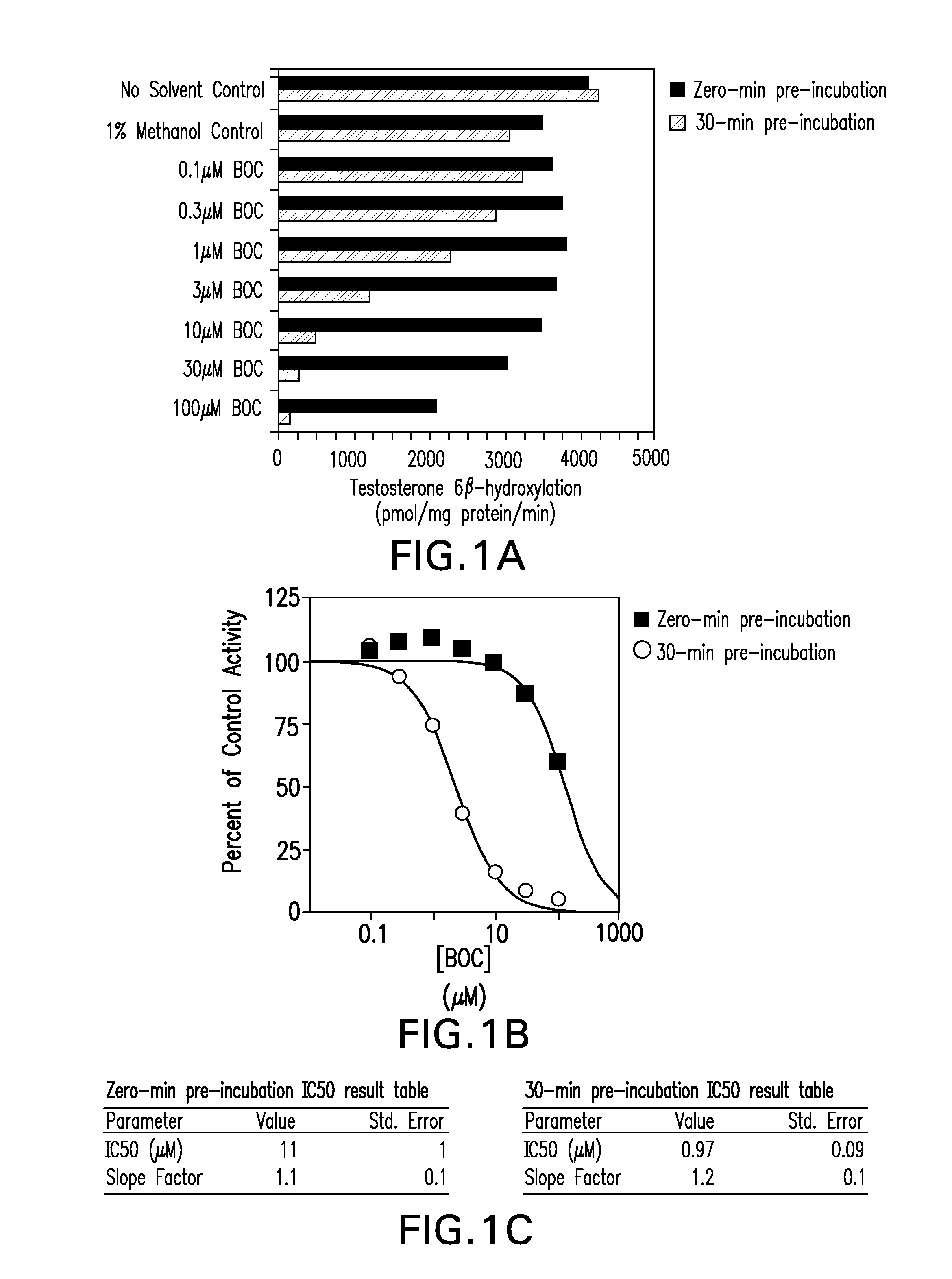
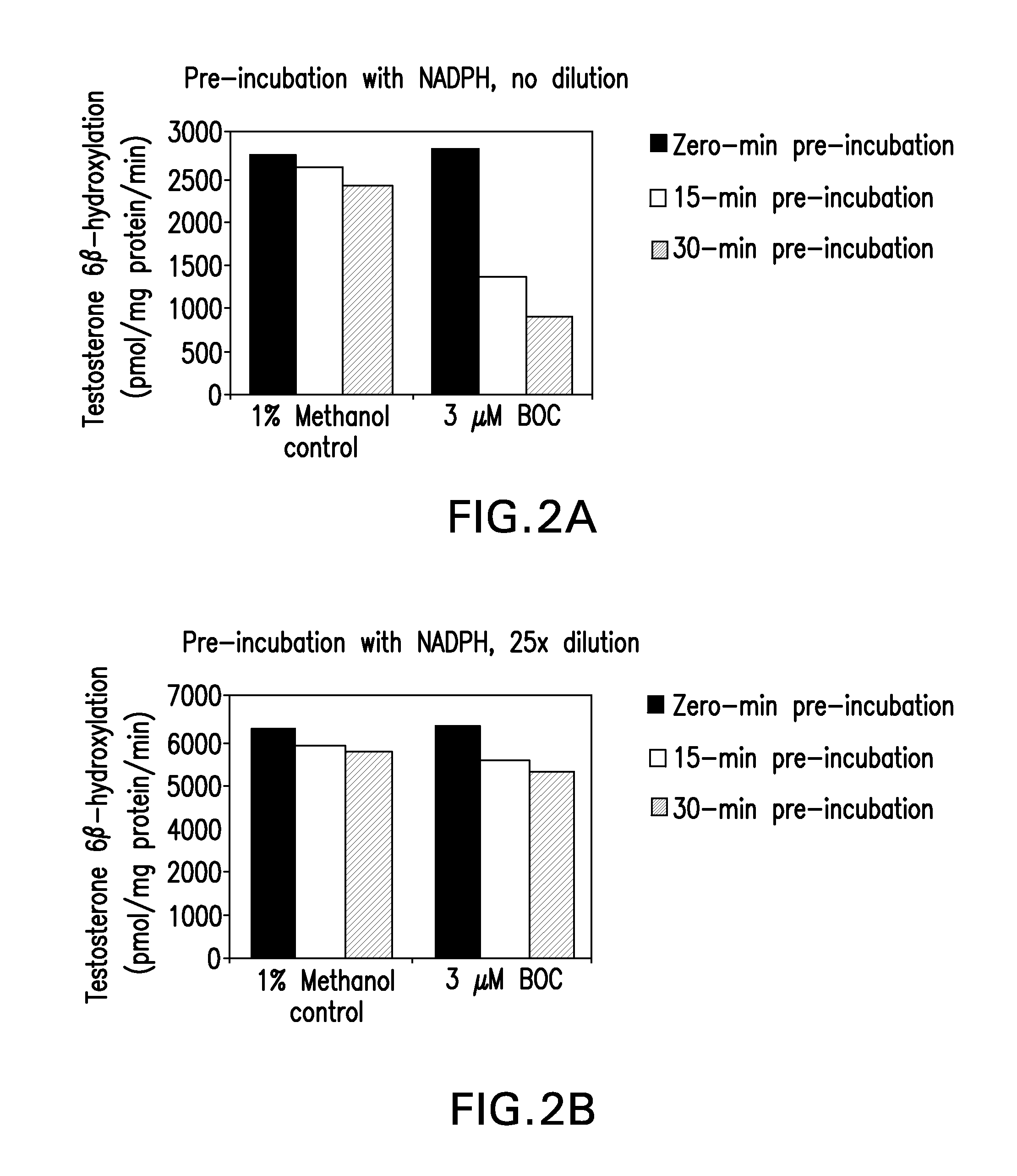
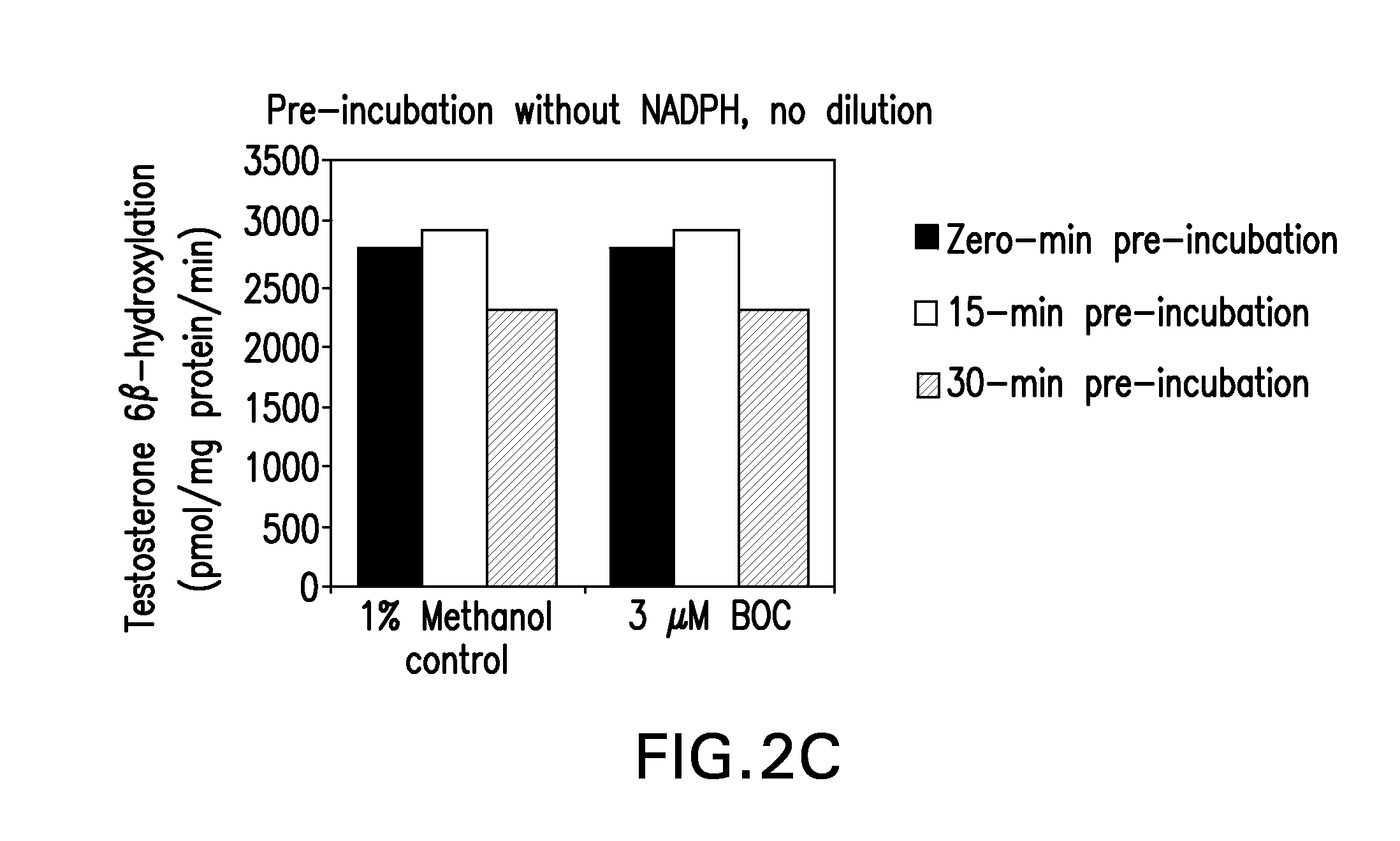
Inhibition of cyp3a drug metabolism
The present invention provides methods, pharmaceutical compositions, medicaments, and pharmaceutical kits that employ the use of boceprevir as a CYP3A4 / 5 inhibitor to improve the pharmacokinetics of therapeutic compounds metabolized by cytochrome P450 3A4 / 5 (CYP3A4 / 5) enzymes.
Owner:MERCK SHARP & DOHME CORP
SNP rs762551 of CYP1A2 gene and application thereof in relevant drug metabolism activity detection
InactiveCN101748196AMicrobiological testing/measurementGenetic engineeringDrug metabolismNormal people
The invention discloses SNP rs762551 of a CYP1A2 gene and application thereof in relevant drug metabolism activity detection, and also provides a method for prejudging drug metabolism activity. The method comprises the steps of detecting whether the cytochrome oxidase P4501A2 gene (CYP1A2) and a transcript of a human individual have variation or not compared with a normal cytochrome oxidase P4501A2 gene and a normal transcript, and if so, showing that the drug metabolism activity of the individual is different from common or normal people, for the individual. The invention also discloses a corresponding detection kit used for prejudging the drug metabolism activity of the individual.
Owner:CHINESE NAT HUMAN GENOME CENT AT SHANGHAI
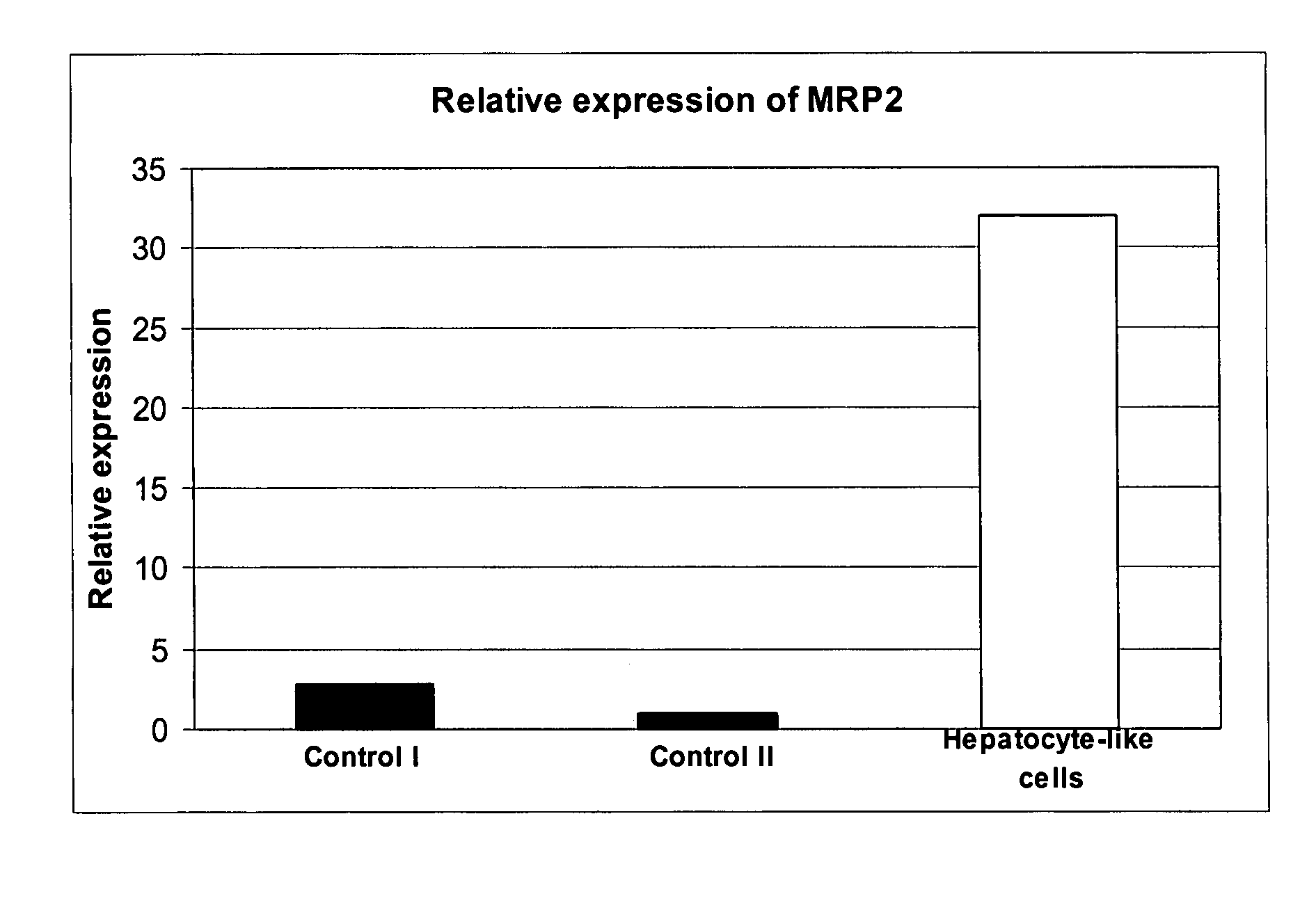
Novel hepatocyte-like cells and hepatoblast-like cells derived from hBS cells
InactiveUS20080019950A1Improve predictabilityReduce needBiocideHepatocytesIn vitro studyMother cells
The present invention relates to a novel hepatocyte-like cell population derived from hBS cells and to the potential use of such heopatocyte-like cells in e.g. medical treatment, drug screening and toxicity testing. Furthermore, the invention relates to hepatoblast-like cells that may have suitable characteristics so that they can be used for the same applications as the hepatocyte-like cells and that furthermore may be used in in vitro studies of hepatogenesis such as early hepatogenesis or hepato-regenerative disorders. Both the hepatocyte-like and the hepatoblast-like cells according to the invention express drug transporter and / or drug metabolising characteristics either at the gene or protein expression level.
Owner:CELLARTIS AB (SE)
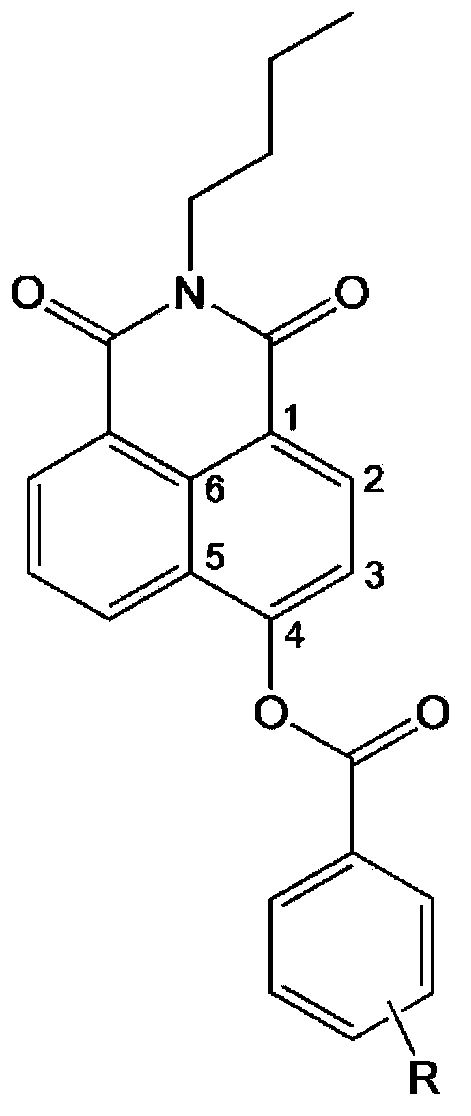
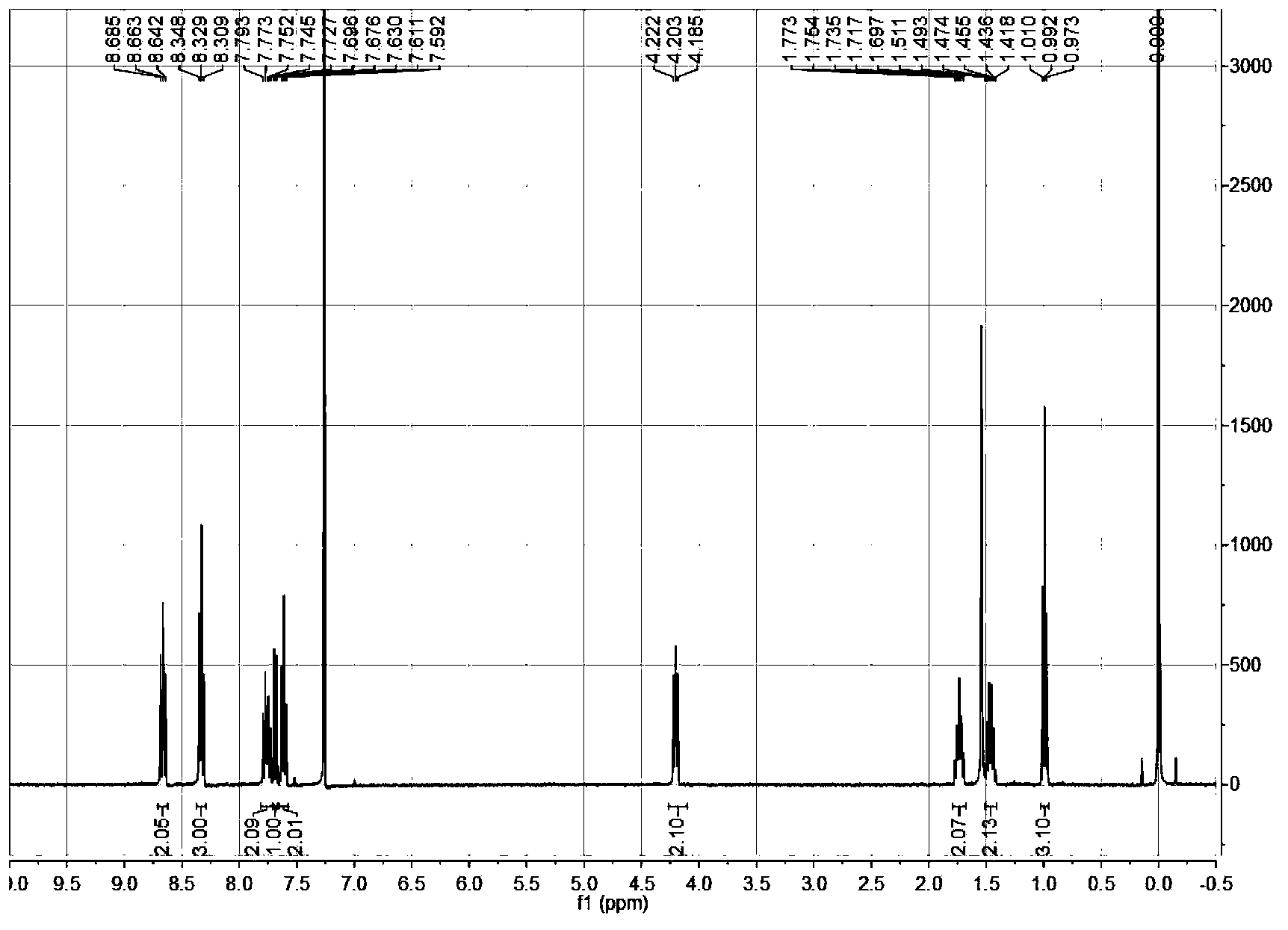
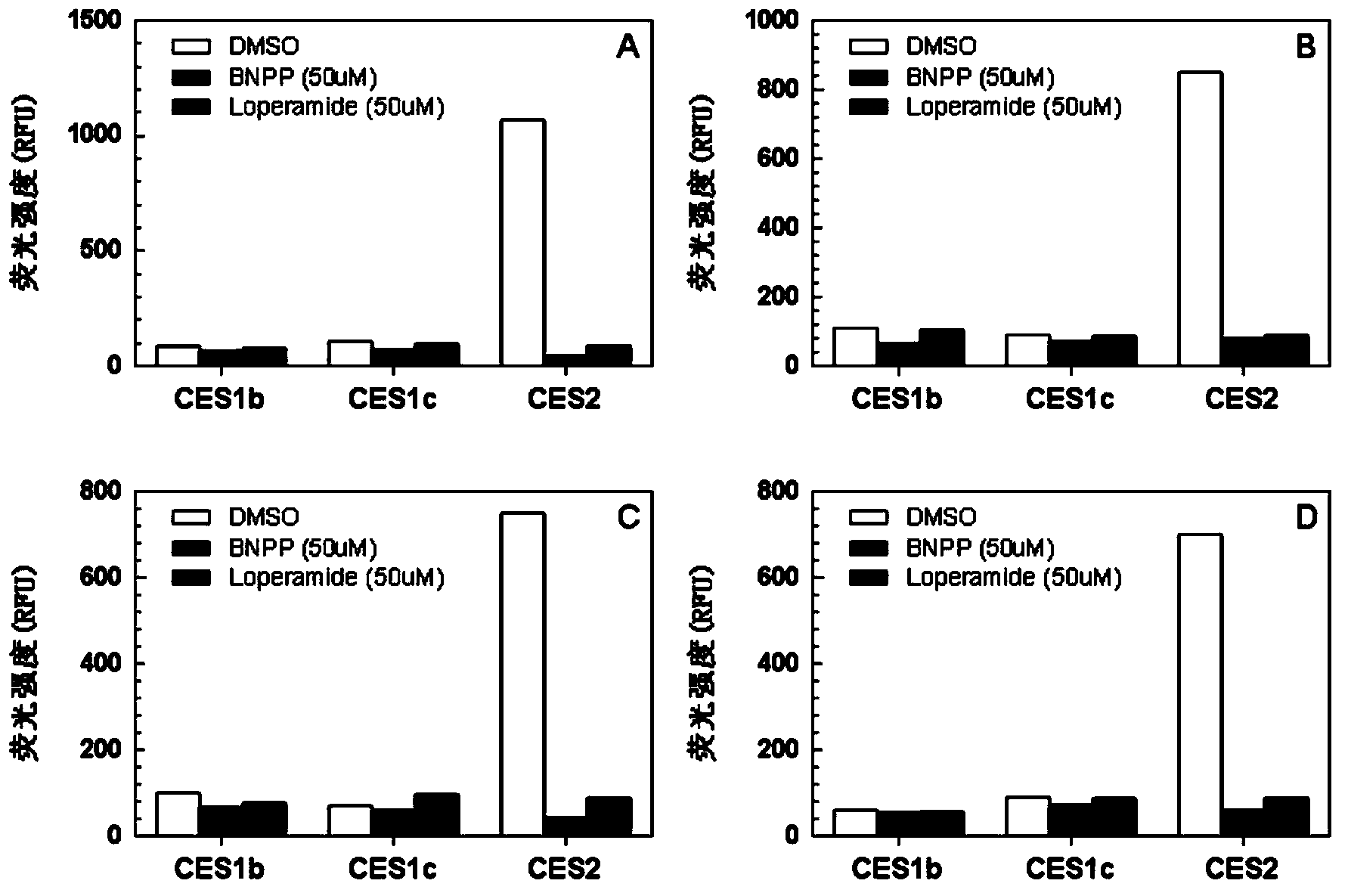
Specific fluorescence probe substrates of human carboxylesterase 2 and application thereof
InactiveCN104120164AEasy to detectThe synthesis process is simpleOrganic chemistryMicrobiological testing/measurementMetaboliteHydrolysis
The invention provides a specific fluorescence probe substrates of human carboxylesterase 2 (CES2) and application thereof. The specific probe substrate is a benzoateb compound of a C4 hydroxyl naphthalimide, and is applicable to determine the enzyme activity of CES2 in a biological system. The CES2 enzyme activity determination flow comprises: selecting a hydrolysis benzoyl-removal reaction of the benzoate compound of the C4 hydroxyl naphthalimide as a probe reaction, and quantitatively determining the generation amount of a hydrolysis metabolite of the compound in a unit time, so as to determine the enzyme activity of CES2 in all biological samples, cells, bodies and integral organs. The probe is applicable to quantitative assessment of CES2 enzyme activity in biological samples of different species and different individual sources, and quantitative determination on CES2 enzyme activity in different sources of animal tissue cell culture fluids and cell preparation substances, so that the probe is expected to help to realize assessment on medicine disposal capability of important drug metablic enzyme CES2. Additionally, the probe also is applicable as an inhibitor for rapidly screening CES2 in vitro by means of the probe reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
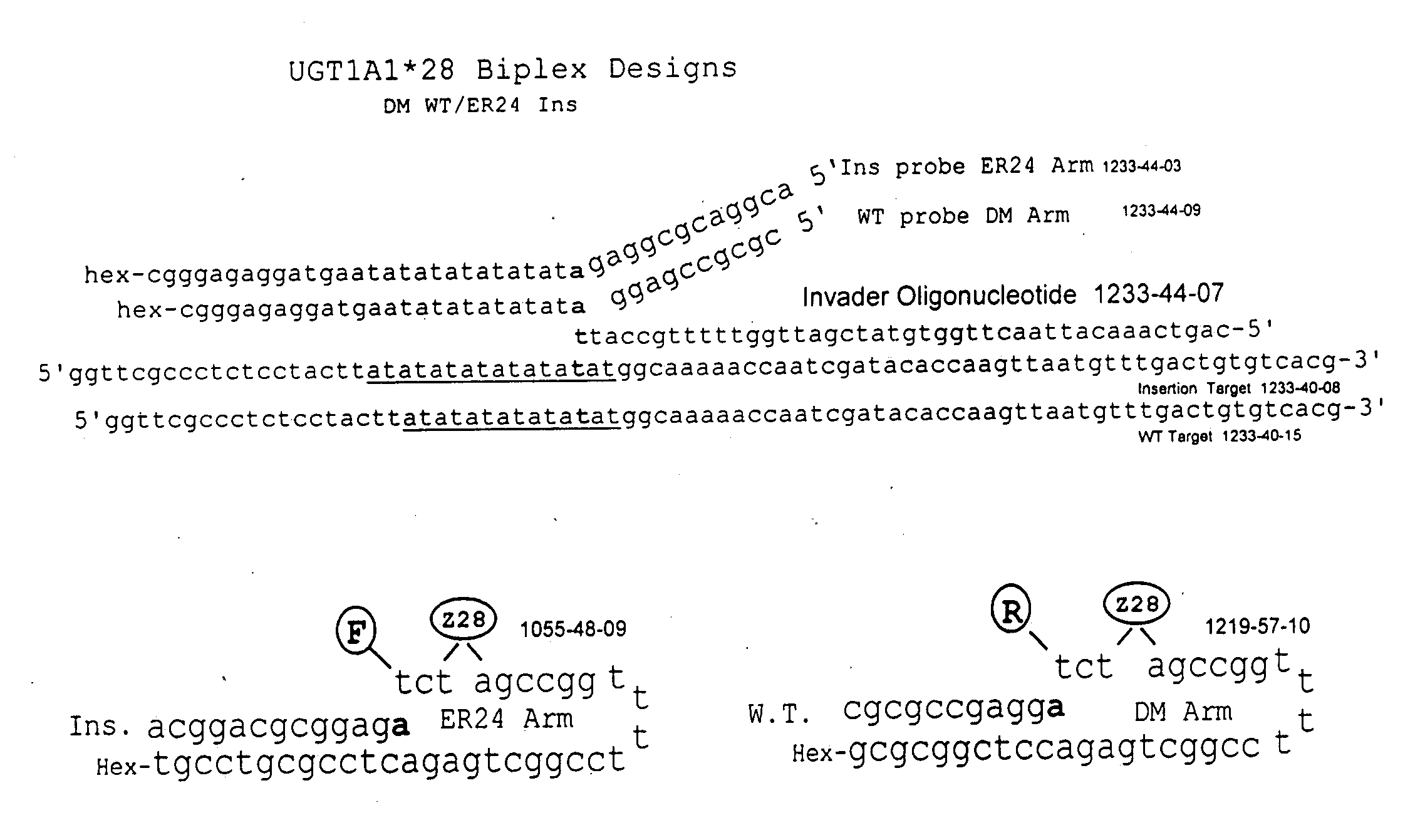
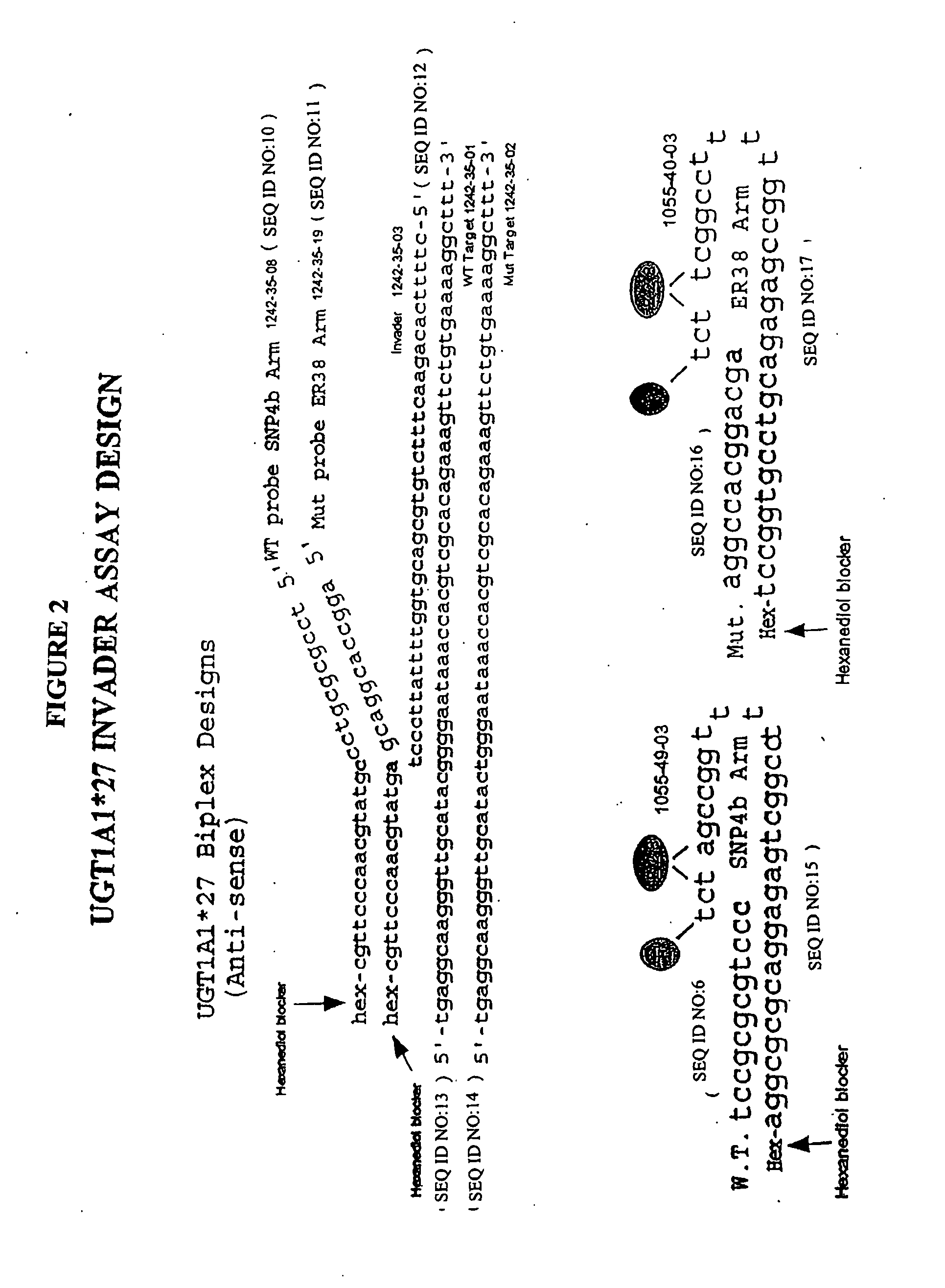
Methods and compositions for analysis of UGT1A1 alleles
InactiveUS20080032305A1Facilitate drug therapyConvenient treatmentMicrobiological testing/measurementDrug metabolismNucleic acid detection
The present invention relates to methods for detecting polymorphisms in enzymes related to drug metabolizm (Drug Metabolizing Enzymes or DMEs) such as uridine diphosphate glucuronosyl transferase (UGT) gene promoter, with nucleic acid detection assays. The present invention also relates to detection assay kits.
Owner:THIRD WAVE TECH
Specific fluorescent probe for UDP-glucuronosyltransferase UGT1A1 and application thereof
ActiveCN104342488AReduce testing costsThe synthesis process is simpleOrganic chemistryMicrobiological testing/measurementDrug metabolismGlucuronosyltransferase
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI +1
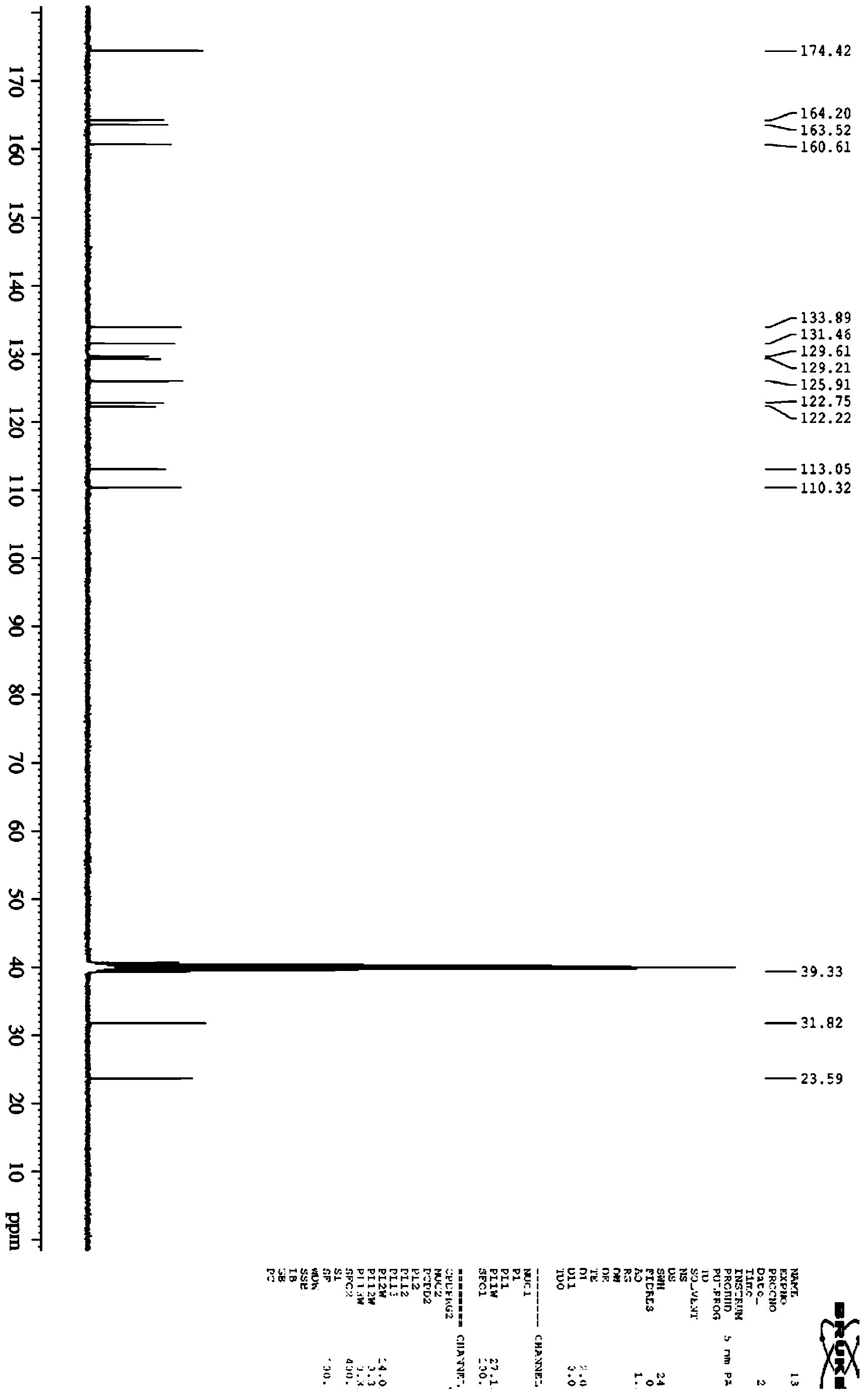
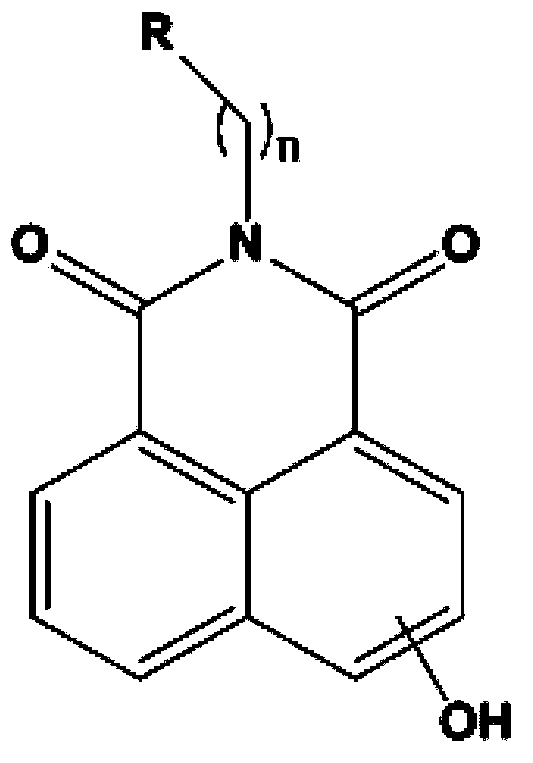
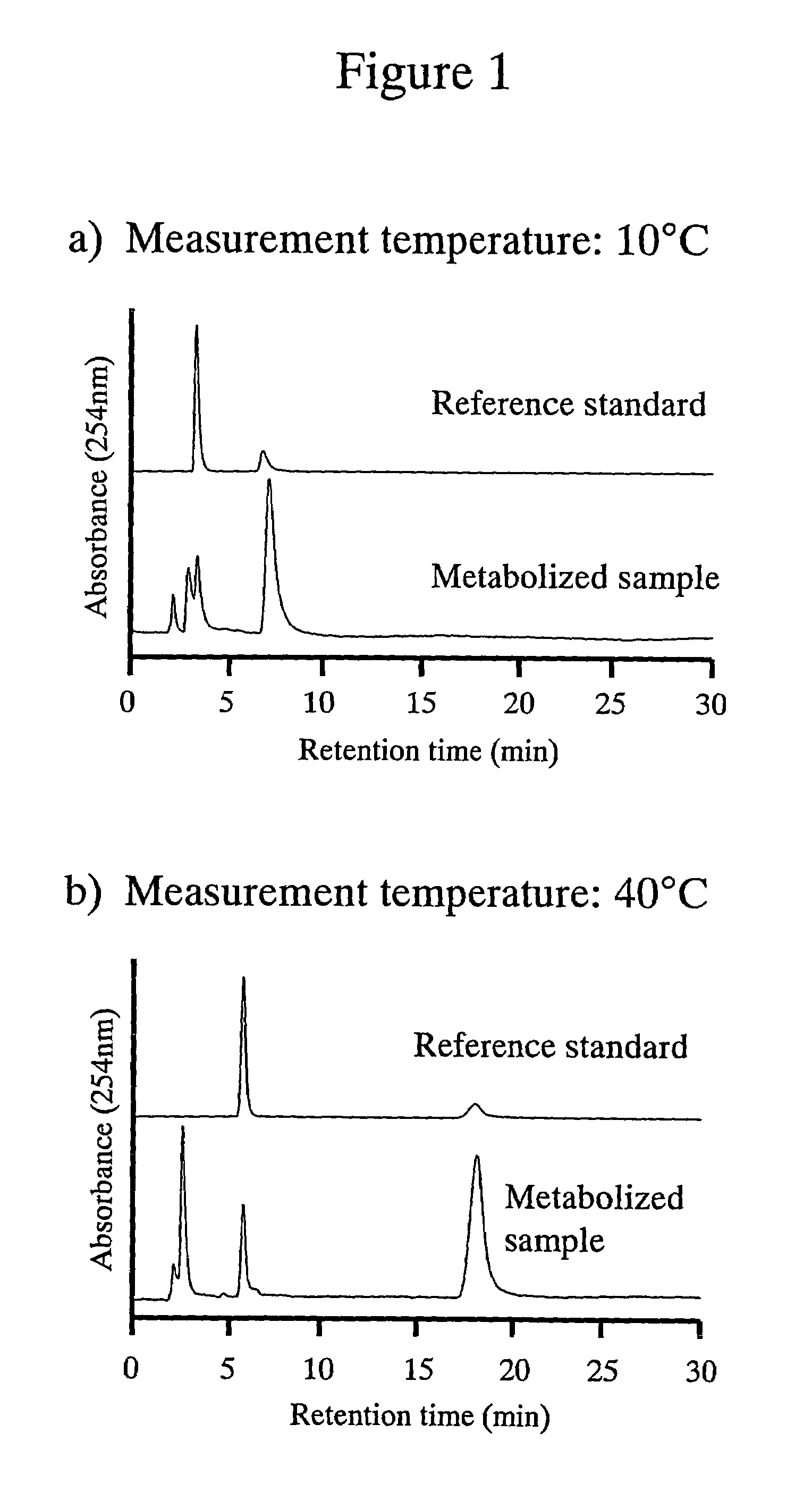
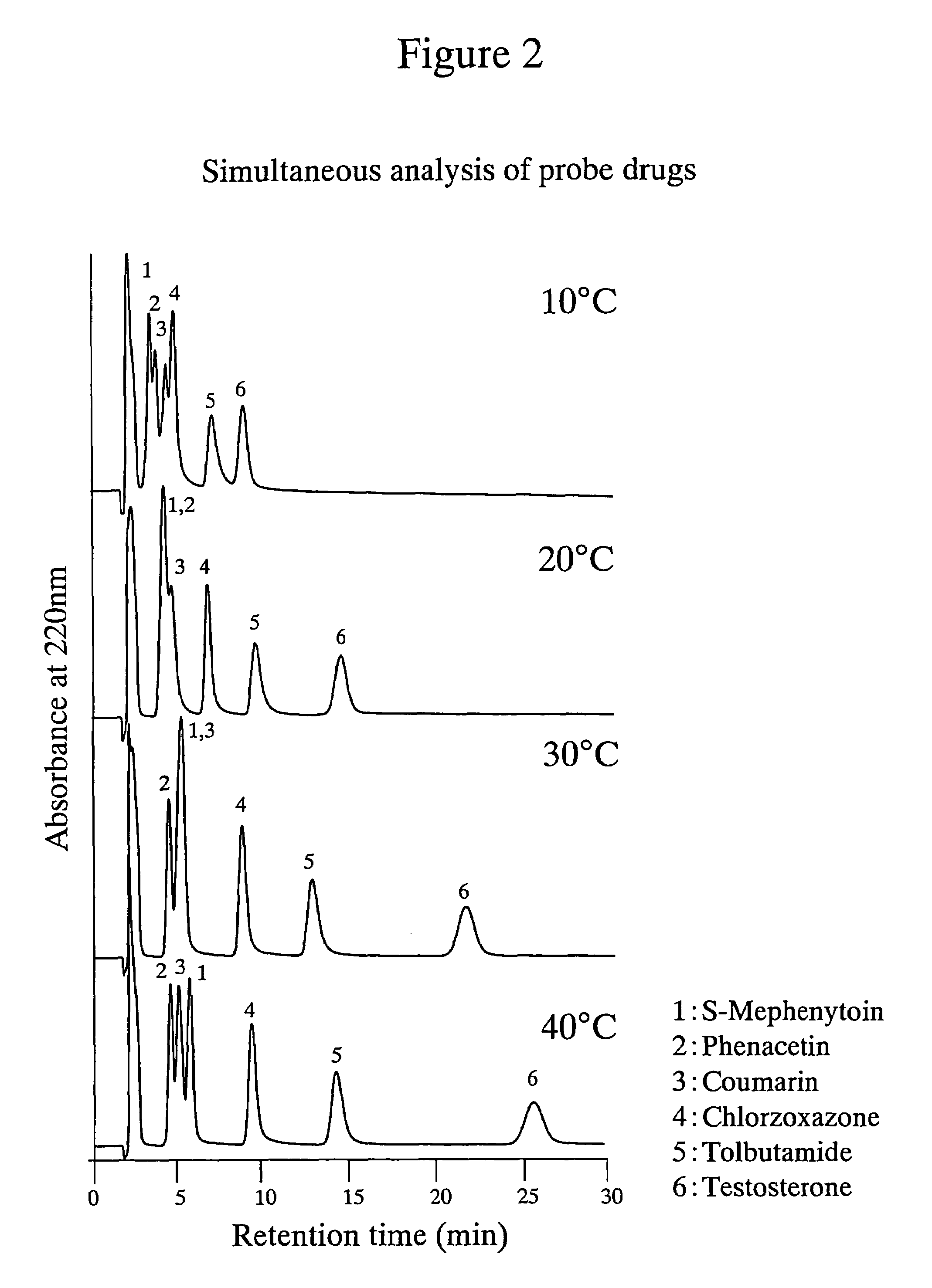
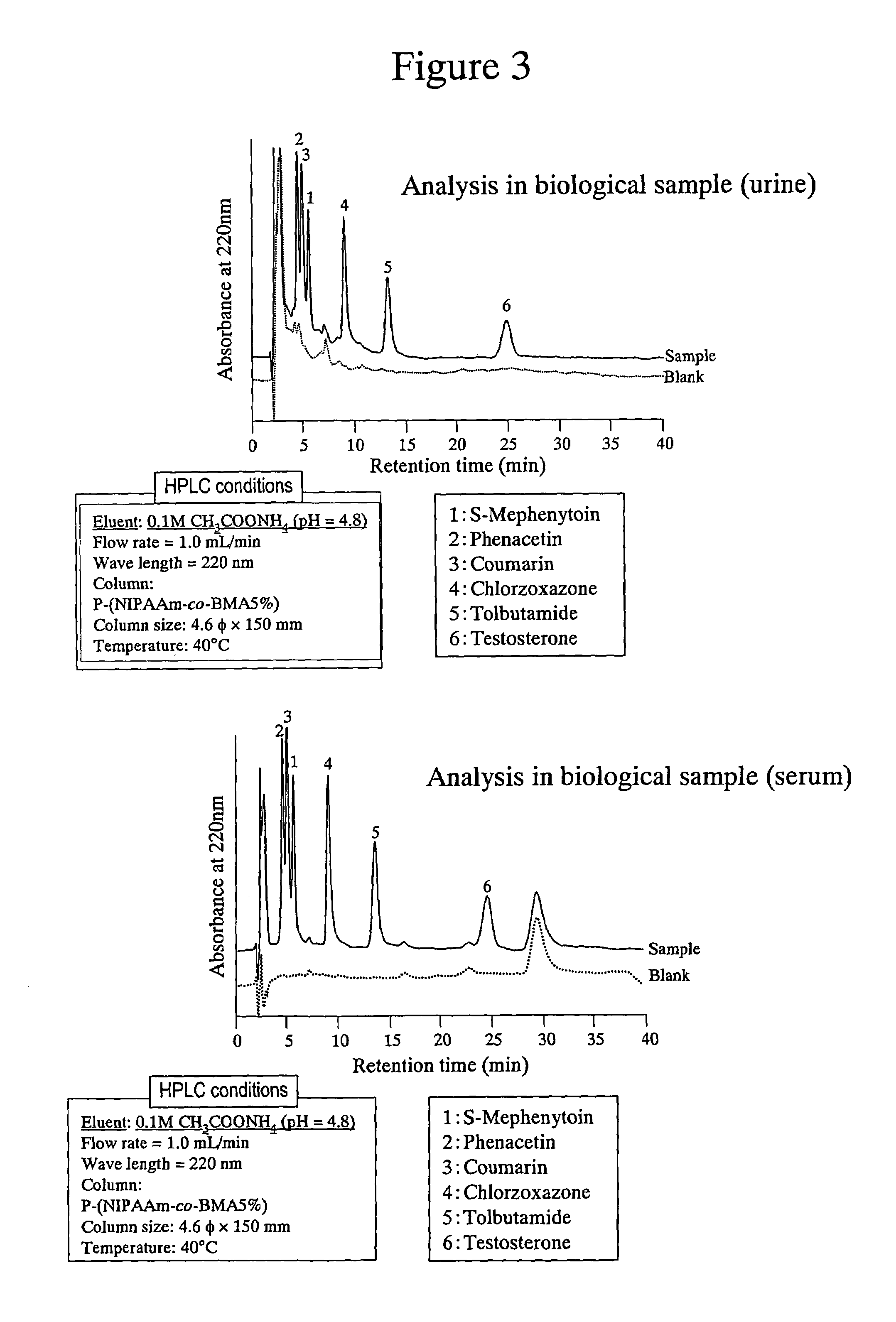
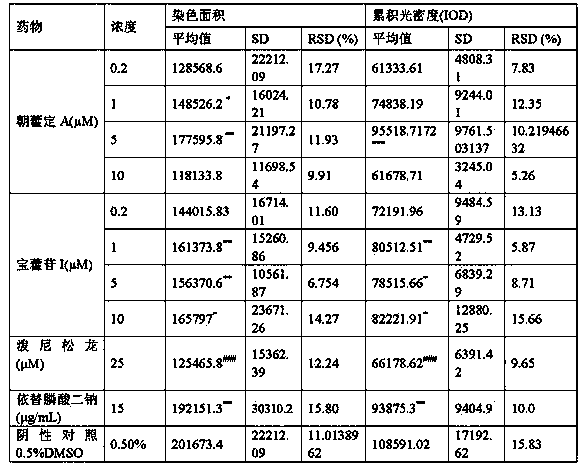
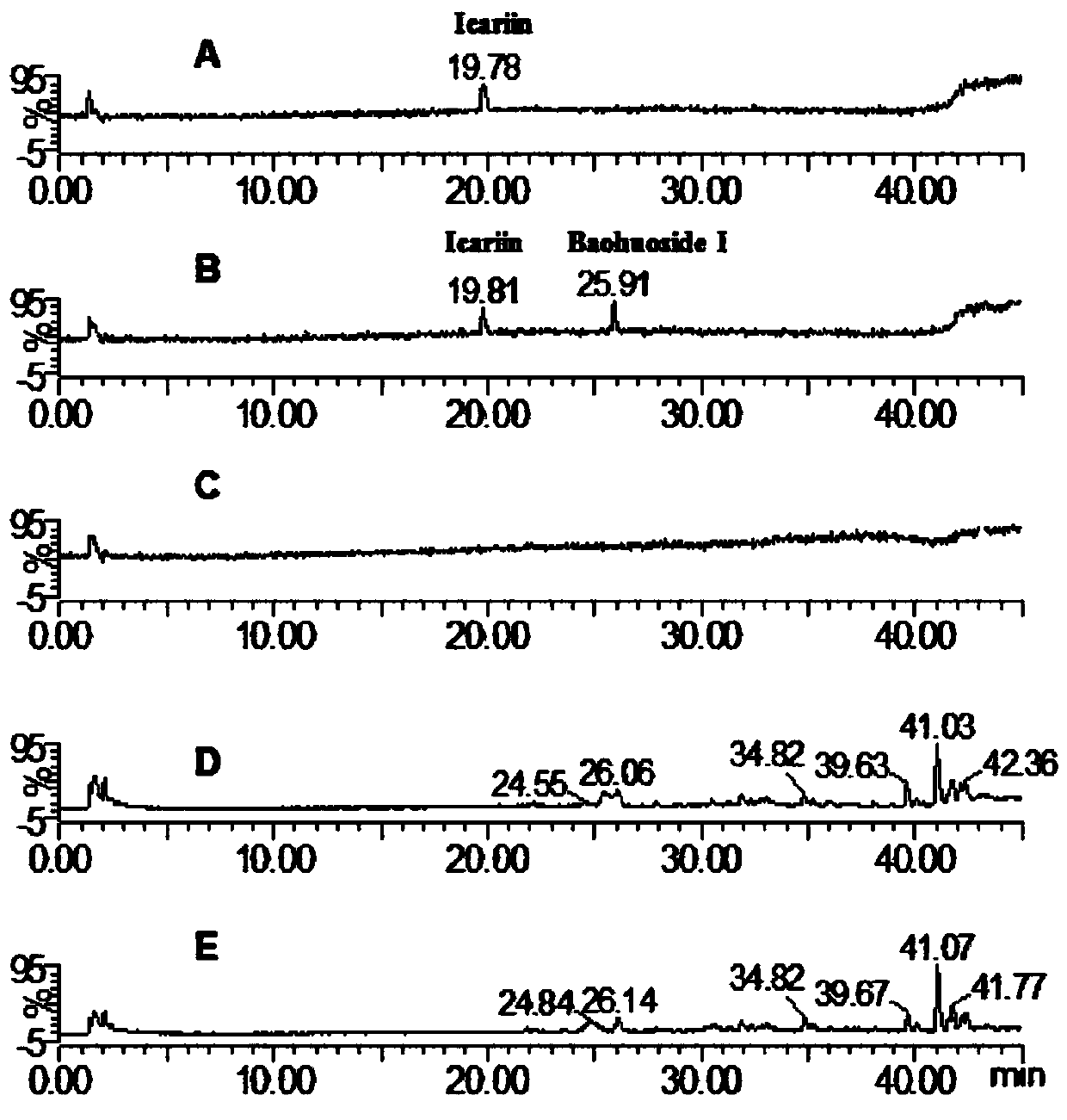
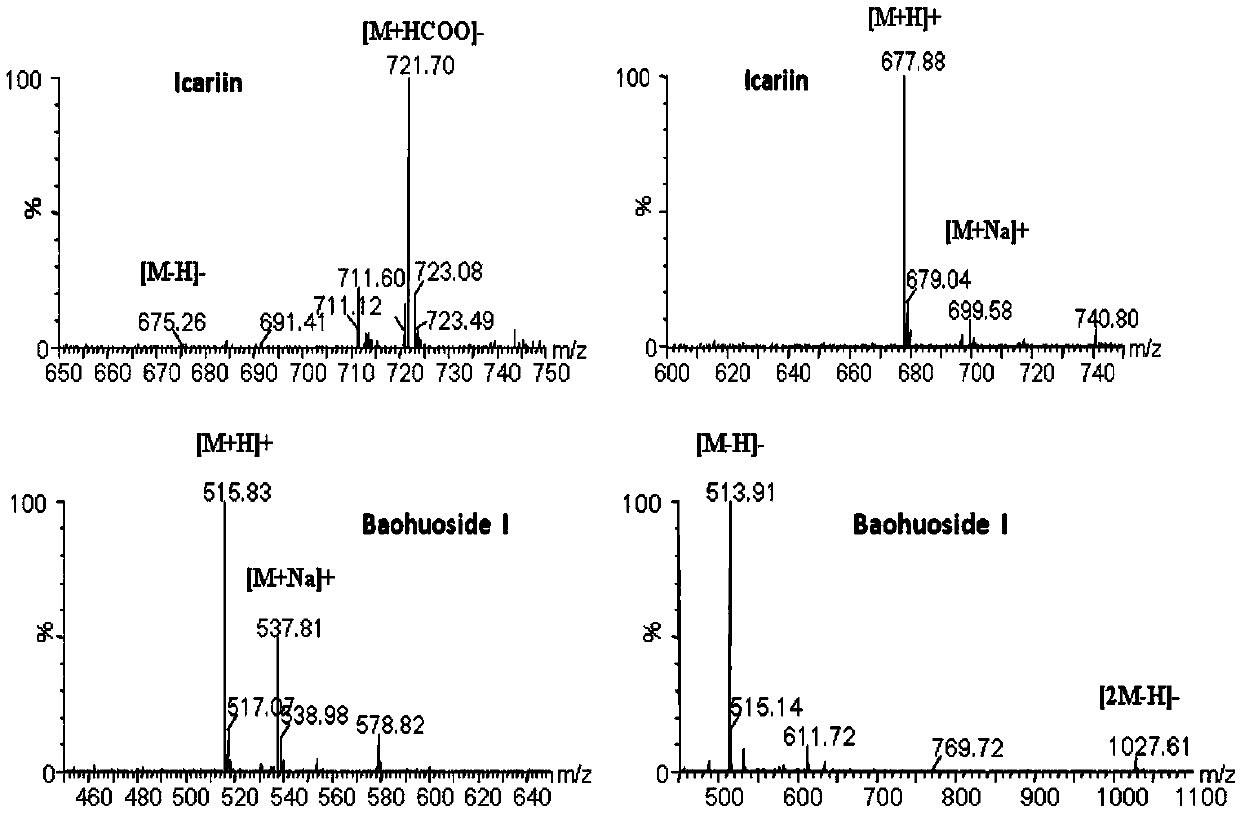
High performance liquid chromatography with an aqueous mobile phase for analysis of drug and its metabolite
InactiveUS7632656B2Easy to useMicrobiological testing/measurementBiological testingDrug metabolismTemperature control
The amount of drug consumption caused by a specific drug-metabolizing enzyme and / or the amount of the resulting metabolite(s) is measured by chromatography with an aqueous mobile phase under temperature control using a packing material whose surface is coated with a polymer having a hydration force varying in the temperature range of 0° C. to 80° C. This system enables proper evaluation of drug-metabolizing capacity through simple means without adversely affecting the environment.
Owner:CELLSEED +1
Modified zebra fish drug metabolism modeling method
InactiveCN103623433AEasy to administerEfficient enrichmentComponent separationPreparing sample for investigationBiotechnologyMetabolite
The invention relates to a modified zebra fish drug metabolism modeling method, which specifically comprises the steps of (1) enriching metabolites by virtue of a zebra fish metabolism model; (2) separating and analyzing the enriched metabolic components of the zebra fish in the last step by using modern chromatography or the hyphenated technique thereof, capturing or knocking out target components or metabolites; (3) collecting zebra fish germ cells; (4) putting zebra fish embryos in three days after fertilization in a culture plate which holds an embryo culture medium and administrating a drug; (5) dyeing the bones of the zebra fish; and (6) detecting and analyzing the dyed bones of the zebra fish. According to the invention, an adolescent zebra fish osteoporosis model is applicable to in-vivo effective evaluation of anti-osteoporosis activity of Chinese herbal medicinal ingredients such as trace ingredients; an adult zebra fish metabolism model is simple in metabolites and efficient in enrichment; the modern chromatography or the hyphenated technique thereof provide powerful guarantee for the separation and analysis of the complex Chinese herbal medicinal system and the metabolic components. The model obtained by the method has the outstanding advantage that the separation and analysis of Chinese herbal medicinal original-form ingredients and the metabolic components are integrated with the in-vivo anti-osteoporosis activity evaluation, and simple and efficient.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Intelligent medicine-taking reminding method and system based on chronopharmacology
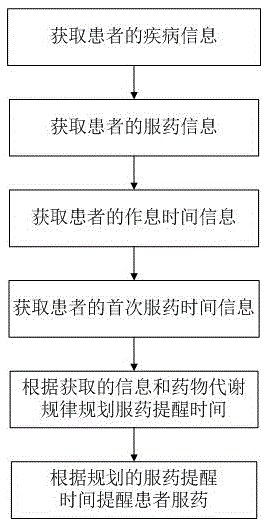
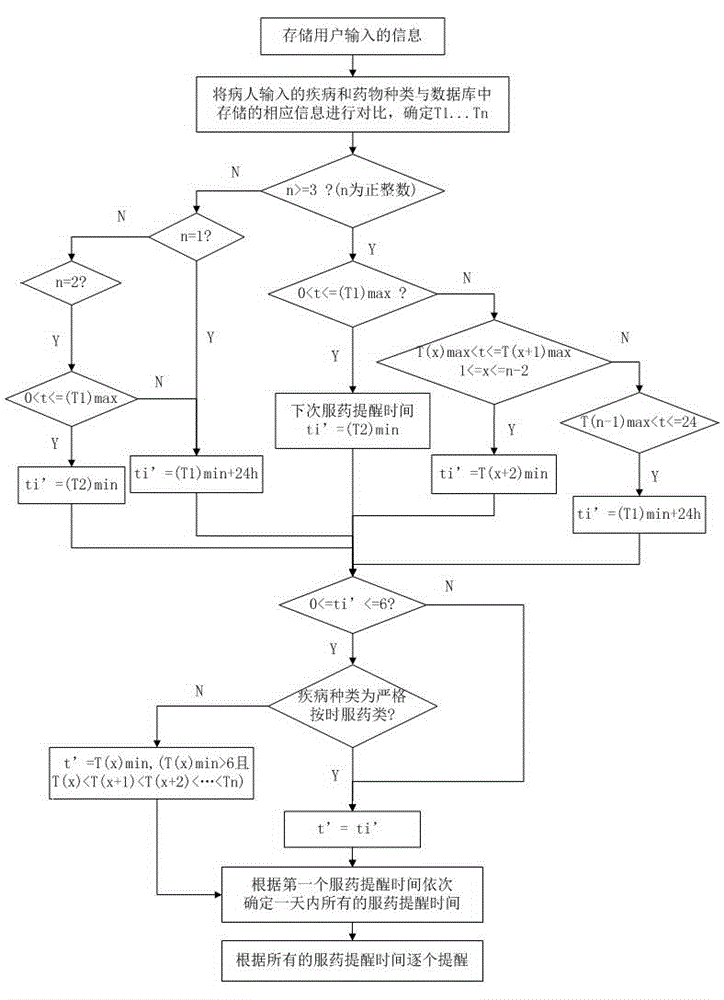
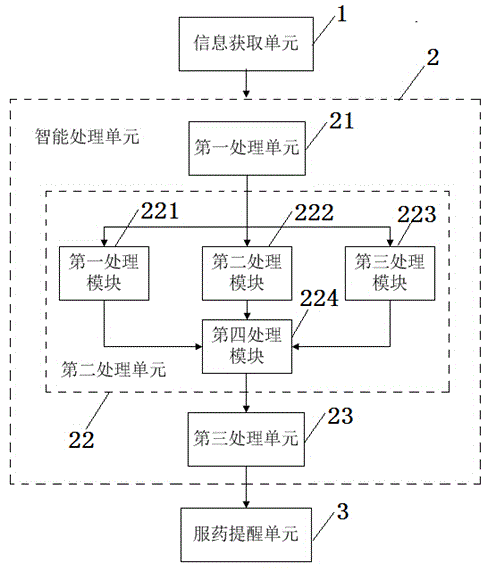
InactiveCN104905975ADoes not affect normal restOral administration deviceDrug metabolismTime information
The invention provides an intelligent medicine-taking reminding method based on chronopharmacology. The method comprises the steps that disease information of a patient is acquired; medicine-taking information of the patient is acquired; work and rest time information of the patient is acquired; first-time medicine-taking time information of the patient is acquired; medicine-taking reminding time is planned according to the acquired information and rules of drug metabolism; the patient is reminded to take medicine according to the planned medicine-taking remind time. The invention further provides an intelligent medicine-taking reminding system based on chronopharmacology. The intelligent medicine-taking remind method and system not only can remind the patient to take medicine scientifically and regularly, but also can make the medicine-taking reminding accord with the rules of the chronopharmacology and pharmacokinetics; the normal rest of the patient cannot be influenced as far as possible.
Owner:ANHUI MEDICAL UNIV
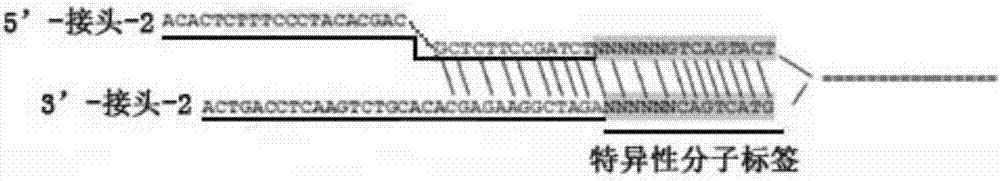
SNP molecular marker for detecting heterogenous cfDNA, detecting method and application
ActiveCN107254514AWide coverageHigh genetic polymorphismBioreactor/fermenter combinationsBiological substance pretreatmentsDrug metabolismBackground noise
The invention discloses an SNP molecular marker for detecting heterogenous cfDNA, a detecting probe based on SNP molecular marker design and a chip, a detecting device for heterogenous cfDNA, a kit and a detecting method. The heterogenous cfDNA in a receptor after kidney transplantation is quantified by the detecting chip and the detecting device, a basis is provided for detection of kidney transplantation rejection, noninvasive early detection with high sensitivity and high specificity on kidney transplantation is realized, and a kidney transplantation patient can be monitored continuously. An SNP molecular marker comprises an SNP site of a drug metabolizing gene, mutation information of a medication gene is provided for a kidney transplantation rejection patient, and individualized medication is realized. The invention further provides a single molecular marker joint. The single molecular marker joint is used for constructing a sequencing library to effectively remove repeated data and error introduced randomly in sequencing and PCR processes, the sequencing background noises are reduced, and the detection accuracy is improved.
Owner:上海奥根诊断技术有限公司
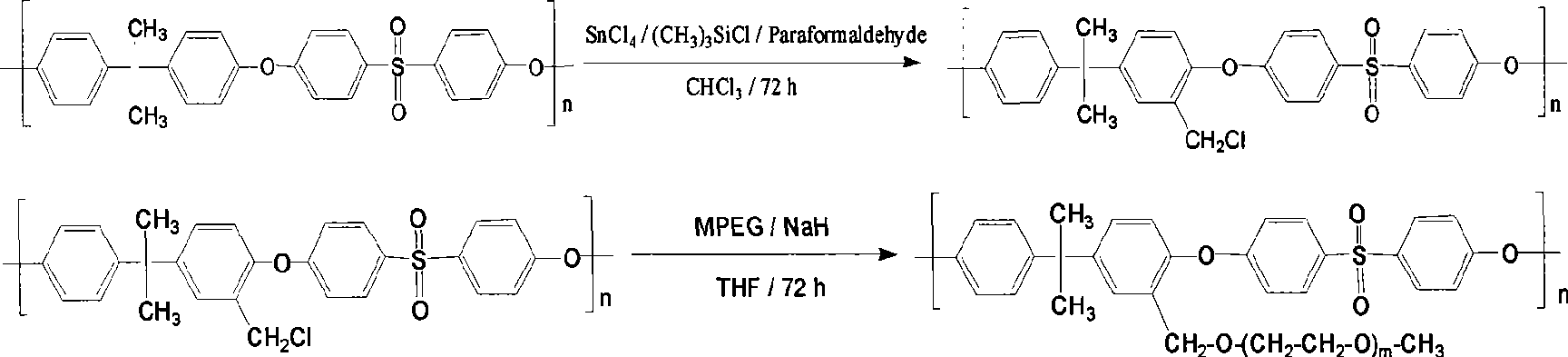
PEG grafted polysulphone or polyether sulphone hollow fibrous membrane, preparation method and application thereof
The invention discloses a PEG grafted polysulphone or polyether sulphone hollow fibrous membrane, a preparation method and application thereof. PEG grafted polysulphone (or polyether sulphone) and polysulphone (or polyether sulphone) are mixed according to a proportion of 1:3-9, and then dissolved in a solvent such as N-methylpyrrolidone and the like by a weight ratio of 15 to 30 percent for 10 to 12 hours under stirring; and after bubbles are removed, a spinning solution is obtained. The spinning solution is extruded by adopting two concentric circular hollow fibrous spinning nozzles at a speed of 5 to 20 milliliters per minute. Nascent fibers are spun with 10 to 30 centimeters in an environment and then solidified and molded in water bath at a temperature between 50 and 80 DEG C, and the fluid line speed is 5 to 40 meters per minute. The hollow fibrous membrane has the beneficial effect of resisting blood platelet, protein and small molecule adsorption, and can be used as a hemodialysis device, or used for researches on biological artificial organs of organization culture of various human or animal cells, drug metabolism, toxicity and pathology.
Owner:ZHEJIANG UNIV
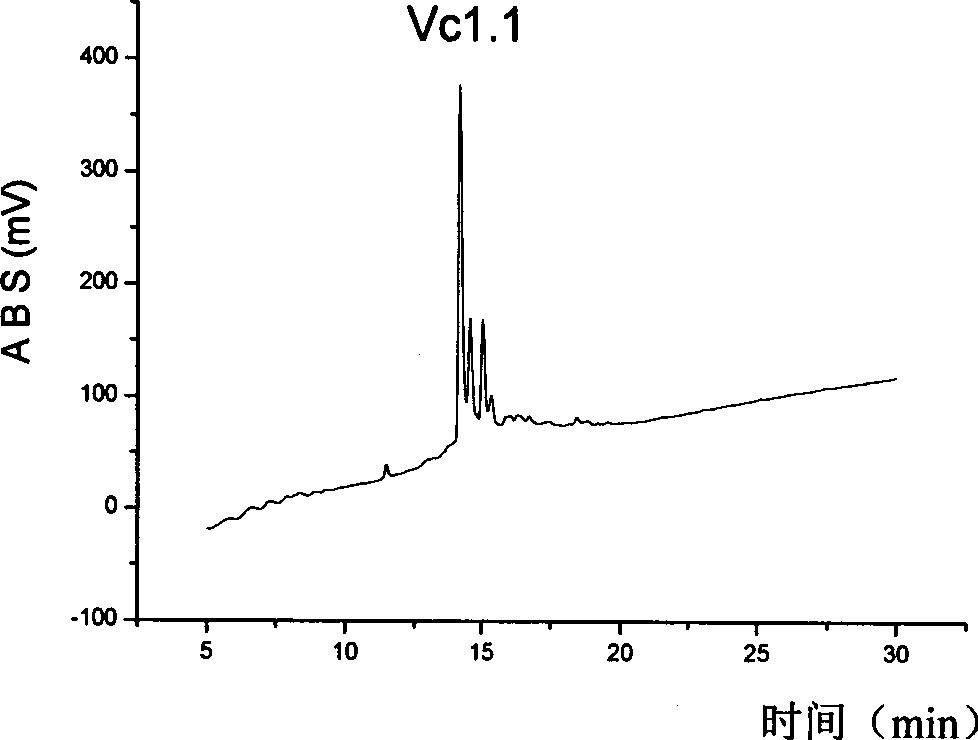
Alpha type conotoxin peptide derivates and use thereof
InactiveCN101381403AStrong analgesic activityHigh analgesic activityNervous disorderPeptide/protein ingredientsConusDrug metabolism
The invention discloses an alpha conus polypeptide derivative, amino acid residues of which are shown in sequence 1 in a sequence table. To effectively resist the degradation digestion of partial protease in a human body, and improve the bioavailability and the drug metabolism performance, an N end of the alpha conus polypeptide derivative can be connected with a benzoyl group. The alpha conus polypeptide derivative can also be subjected to cyclization, fattening or PEG modification to strengthen the treatment effect of the alpha conus polypeptide derivative. A big mouse experiment shows that the alpha conus polypeptide derivative shows strong analgesic activity in a neuropathic pain model of a big mouse, and the analgesic activity is remarkably higher than that of control peptide Vc1.1 and shows dose-dependent relation.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Diagnostic device and method
InactiveUS20100330684A1Microbiological testing/measurementBiological material analysisDrug metabolismStressed state
Diagnostic devices and methods involve comparison of relative levels of first and second components and / or characteristics of a fluid sample (e.g., saliva), preferably using antibodies arranged to interact with selected components, and colorimetric indicators that are bound or released in proportion to relative concentration or amount of the components or characteristics, as indicative of a health condition such as dehydration state, shock state, stress state, disease state, drug consumption, and drug metabolization. Amylase and IgA may be selected as specific salivary components of interest.
Owner:HYDRADX
Tanshinone IIA-polyactic acid/hydroxyacetic acid microsphere and preparation method thereof
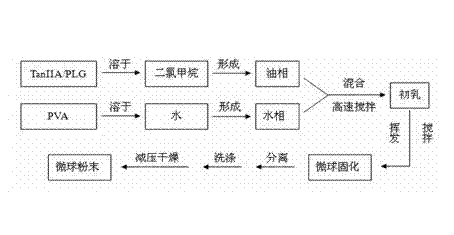
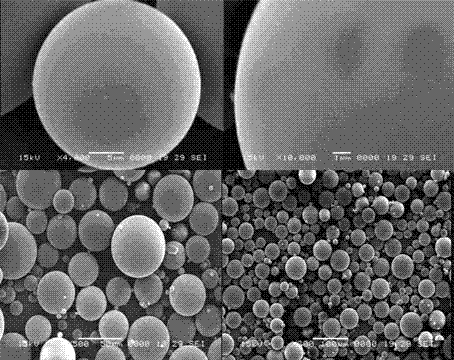
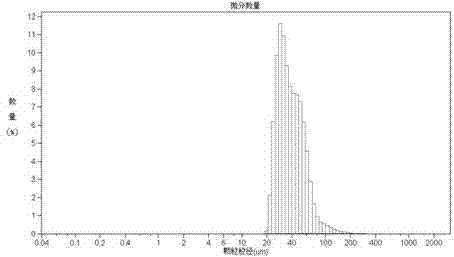
InactiveCN103083250AHigh specific activityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentPolyvinyl alcohol
The invention discloses a tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere and a preparation method thereof. The microsphere is prepared by drying oil-in-water type emulsion, wherein the oil phase is dichloromethane solution of a polyactic acid / hydroxyacetic acid copolymer and the water phase is the water solution of polyvinyl alcohol. The drug content of tanshinone IIA in the microsphere is 1-10% and the entrapment efficiency is 60-90%. The particle size range of the microsphere is 30-200mm. The tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere provided by the invention is suitable for interventional therapy of liver cancers, has a good liver tumor peripheral vascular thrombosis function, has an effective thrombosis time of 7-60 days, can be distributed in tumor tissues in a targeted manner, slowly release drugs, increase the local concentrations of the drugs, prolong the drug metabolism time, obviously inhibit animal liver tumor growth and prolong the animal lifetime and can inhibit expressions of a human hypoxia inducible factor 1alpha and a vascular endothelial growth factor and reduce the tumor tissue microvessel density after thrombosis.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M +1
Primer set, application, product and method for detecting SNP sites related to drug metabolism in children
ActiveCN110511993AImprove accuracyShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationMetabolic powerDrug metabolism
The invention provides a primer set, application, a product and a method for detecting SNP sites related to drug metabolism in children, and relates to the field of biotechnology. The primer set of the SNP sites related to drug metabolism in children provided by the invention can be used to detect the metabolism capacity of thermoanalgesics, metabolism capacity of respiratory system drugs, metabolism capacity of neurological and psychiatric drugs, metabolism capacity of cardiovascular and cerebrovascular drugs, metabolism capacity of anti-infective drugs, metabolism capacity of endocrine drugs, metabolism capacity of digestion system drugs, metabolism capacity of anesthetic drugs and metabolism capacity of chemotherapy and immunosuppressant. Specific detection of site mutation related to common drug metabolism in children can be realized by the primer set, the accuracy is high, the detection cycle can be tremendously shortened, and the detection cost is reduced at the same time. Moreover, the needs of a drug metabolism can be predicted by the detection results, the metabolism capacity of a variety of drugs can also be comprehensively evaluated, and scientific reference is providedfor the individualized medication of children.
Owner:JIANGSU SIMCERE MEDICAL DEVICE CO LTD +2
Ratio type fluorescent probe substrate for cytochrome oxidase CYP1A and application thereof
ActiveCN105219374ANot easy to interfereAdvantages of in vitro activityOrganic chemistryMicrobiological testing/measurementAlkaneDrug metabolism
The invention discloses a ratio type fluorescent probe substrate for cytochrome oxidase CYP1A and an application thereof. The specific probe substrate has a hydroxynaphthalimide alkane acid structure and can be used for determining the CYP1A enzymatic activity in a biosystem. A flow for determining the CYP1A enzymatic activity comprises the following steps: selecting a hydroxynaphthalimide alkane acid demethylation reaction as a probe reaction, and determining the CYP1A enzymatic activity in various biological samples by quantitatively detecting the production amount of demethylation metabolites in a unit time. The ratio type fluorescent probe substrate disclosed by the invention can be used for quantitative evaluation for the CYP1A enzymatic activity in biological samples of different species and different individual sources and for quantitative determination for the CYP1A enzymatic activity in different-source animal tissue cell culture fluids and cell prepared products, so as to realize evaluation for the medicine disposition capacity of the important medicine metabolizing enzyme CYP1A. In addition, the probe reaction can also be used for rapidly screening a CYP1A inhibitor in vitro and evaluating the inhibition capacity of the inhibitor.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI +1
Oil-in-water nanometer perilla seed oil emulsion oral liquid and its prepn process
InactiveCN1931232ALower blood pressureLower blood fatMetabolism disorderDigestive systemIn vivo metabolismOil in water
The oil-in-water nanometer perilla seed oil emulsion oral liquid consists of surfactant, oil, perilla seed oil and distilled water. Its preparation process includes the following steps: weighing surfactant with or without co-surfactant; calculating HLB value and selecting oil phase for reaching emulsifying HLB value near that of the surfactant phase; changing the ratio between the surfactant phase and the oil phase regularly; adding perilla seed oil into surfactant through stirring at 20-25 deg.c; adding distilled water slowly to form clear and flowing O / W type nanometer emulsion liquid. The nanometer perilla seed oil emulsion preparing process results in raised stability, raised bioavailability, delayed in vivo metabolism time, reduced supplementary material amount, lowered production process and wide medicine market foreground.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com